Abstract
Background
Disease‐related malnutrition has been reported in 10% to 55% of people in hospital and the community and is associated with significant health and social‐care costs. Dietary advice (DA) encouraging consumption of energy‐ and nutrient‐rich foods rather than oral nutritional supplements (ONS) may be an initial treatment.
Objectives
To examine evidence that DA with/without ONS in adults with disease‐related malnutrition improves survival, weight, anthropometry and quality of life (QoL).
Search methods
We identified relevant publications from comprehensive electronic database searches and handsearching.
Last search: 01 March 2021.
Selection criteria
Randomised controlled trials (RCTs) of DA with/without ONS in adults with disease‐related malnutrition in any healthcare setting compared with no advice, ONS or DA alone.
Data collection and analysis
Two authors independently assessed study eligibility, risk of bias, extracted data and graded evidence.
Main results
We included 94, mostly parallel, RCTs (102 comparisons; 10,284 adults) across many conditions possibly explaining the high heterogeneity. Participants were mostly older people in hospital, residential care and the community, with limited reporting on their sex. Studies lasted from one month to 6.5 years.
DA versus no advice ‐ 24 RCTs (3523 participants)
Most outcomes had low‐certainty evidence. There may be little or no effect on mortality after three months, RR 0.87 (95% confidence interval (CI) 0.26 to 2.96), or at later time points. We had no three‐month data, but advice may make little or no difference to hospitalisations, or days in hospital after four to six months and up to 12 months. A similar effect was seen for complications at up to three months, MD 0.00 (95% CI ‐0.32 to 0.32) and between four and six months. Advice may improve weight after three months, MD 0.97 kg (95% CI 0.06 to 1.87) continuing at four to six months and up to 12 months; and may result in a greater gain in fat‐free mass (FFM) after 12 months, but not earlier. It may also improve global QoL at up to three months, MD 3.30 (95% CI 1.47 to 5.13), but not later.
DA versus ONS ‐ 12 RCTs (852 participants)
All outcomes had low‐certainty evidence. There may be little or no effect on mortality after three months, RR 0.66 (95% CI 0.34 to 1.26), or at later time points. Either intervention may make little or no difference to hospitalisations at three months, RR 0.36 (95% CI 0.04 to 3.24), but ONS may reduce hospitalisations up to six months. There was little or no difference between groups in weight change at three months, MD ‐0.14 kg (95% CI ‐2.01 to 1.74), or between four to six months. Advice (one study) may lead to better global QoL scores but only after 12 months. No study reported days in hospital, complications or FFM.
DA versus DA plus ONS ‐ 22 RCTs (1286 participants)
Most outcomes had low‐certainty evidence. There may be little or no effect on mortality after three months, RR 0.92 (95% CI 0.47 to 1.80) or at later time points. At three months advice may lead to fewer hospitalisations, RR 1.70 (95% CI 1.04 to 2.77), but not at up to six months. There may be little or no effect on length of hospital stay at up to three months, MD ‐1.07 (95% CI ‐4.10 to 1.97). At three months DA plus ONS may lead to fewer complications, RR 0.75 (95% CI o.56 to 0.99); greater weight gain, MD 1.15 kg (95% CI 0.42 to 1.87); and better global QoL scores, MD 0.33 (95% CI 0.09 to 0.57), but this was not seen at other time points. There was no effect on FFM at three months.
DA plus ONS if required versus no advice or ONS ‐ 31 RCTs (3308 participants)
Evidence was moderate‐ to low‐certainty. There may be little or no effect on mortality at three months, RR 0.82 (95% CI 0.58 to 1.16) or at later time points. Similarly, little or no effect on hospitalisations at three months, RR 0.83 (95% CI 0.59 to 1.15), at four to six months and up to 12 months; on days in hospital at three months, MD ‐0.12 (95% CI ‐2.48 to 2.25) or for complications at any time point. At three months, advice plus ONS probably improve weight, MD 1.25 kg (95% CI 0.73 to 1.76) and may improve FFM, 0.82 (95% CI 0.35 to 1.29), but these effects were not seen later. There may be little or no effect of either intervention on global QoL scores at three months, but advice plus ONS may improve scores at up to 12 months.
DA plus ONS versus no advice or ONS ‐ 13 RCTs (1315 participants)
Evidence was low‐ to very low‐certainty. There may be little or no effect on mortality after three months, RR 0.91 (95% CI 0.55 to 1.52) or at later time points. No study reported hospitalisations and there may be little or no effect on days in hospital after three months, MD ‐1.81 (95% CI ‐3.65 to 0.04) or six months. Advice plus ONS may lead to fewer complications up to three months, MD 0.42 (95% CI 0.20 to 0.89) (one study). Interventions may make little or no difference to weight at three months, MD 1.08 kg (95% CI ‐0.17 to 2.33); however, advice plus ONS may improve weight at four to six months and up to 12 months. Interventions may make little or no difference in FFM or global QoL scores at any time point.
Authors' conclusions
We found no evidence of an effect of any intervention on mortality. There may be weight gain with DA and with DA plus ONS in the short term, but the benefits of DA when compared with ONS are uncertain. The size and direction of effect and the length of intervention and follow‐up required for benefits to emerge were inconsistent for all other outcomes. There were too few data for many outcomes to allow meaningful conclusions. Studies focusing on both patient‐centred and healthcare outcomes are needed to address the questions in this review.
Plain language summary
Advice on diet for adults with malnutrition that is the result of disease
Review question
Can dietary advice with or without oral nutritional supplements (ONS) improve disease‐related malnutrition in adults?
Background
Ill people often have a poor appetite or feel sick because of medicines or other treatments and eat less than usual. Eating less over a longer time can cause weight loss, malnutrition, more health problems and death. Healthcare professionals may offer advice about dietary changes to help people to re‐establish good eating habits. They might recommend high‐protein and high‐energy foods so that these people can gain weight and improve their nutrition and general health. It is common for sick people to be offered ONS with or without advice about changing their food intake.
To find the best answer to our review question, we looked for studies that compared five different treatment options: dietary advice compared with no advice; dietary advice compared with ONS; dietary advice plus ONS compared with dietary advice; dietary advice plus ONS if appropriate compared with no dietary advice; and dietary advice plus ONS compared with no dietary advice and no ONS. To make these comparisons fair, we looked for randomised controlled trials (RCTs), where the people taking part had an equal chance (like the flip of a coin) of being in either group that was being compared.
Search date
The evidence is current to: 01 March 2021.
Study characteristics
We found 94 studies (with a total of 10,284 people) that we could include in our review. Although older people have a higher risk of malnutrition, the people in these studies ranged from 17 to over 80 years of age and they were living either at home, in the community, or in hospital. They had a wide range of health conditions, including cancer, dementia and kidney disease. The studies reported on the participants for the length of their hospital stay or in some people in the community for up to six and a half years.
Key results
There is no evidence that any of the treatments affected how long many of the people in the studies lived. They did report some positive changes in energy intake (measured in calories), protein intake, weight, muscle bulk and quality of life. There were some reductions in complications and the length of time spent in hospital. However, there is no clear evidence about which treatment is the most helpful or the time it takes to achieve any benefit. Few studies reported results separately for men and women and so we cannot comment on whether there were any overall differences by sex. No studies recorded information about adverse events (harms) so we cannot offer a summary about possible harms.
More research is needed to work out the best ways to help people who are losing weight because of illness in order to improve their clinical outcomes and quality of life.
Certainty of the evidence
Overall we rated the certainty of the evidence as low for most results, which means that we cannot be confident about the findings we report. There were several reasons for this. Some of the treatment comparisons that we looked at had only a few studies and some of those had small numbers of participants. There were problems with the design of some studies that may have affected the results. Some people knew which treatment they were receiving. We think this may influence the way that they reported some changes, e.g. their energy and protein intake, body weight and quality of life. We think that the way the decision about which group a person went into at the start of the study may have affected the results for some outcomes, e.g. change in weight, change in muscle bulk and mortality.
We needed to see particular results to help us understand whether adults living with disease‐related malnutrition can improve their survival, weight and general quality of life if they receive advice about diet with or without ONS. None of the studies reported all of the results that we needed to do this. We were not able to estimate whether participants gain any benefits from the treatments, such as shortening the length of hospital stay, lowering the risk of readmission to hospital or developing complications. The low certainty of evidence, with no evidence in many areas, means we cannot make statements about any benefits and the possible disadvantages of these treatments despite the fact they are being used extensively in clinical practice. We recommend that future studies should be designed to measure these important patient‐centred and healthcare outcomes as well as any potential harms.
Summary of findings
Summary of findings 1. Dietary advice compared with no advice for disease‐related malnutrition in adults.
| Dietary advice compared with no advice for disease‐related malnutrition in adults | ||||||
|
Patient or population: adults with disease‐related malnutrition Settings: all healthcare settings Intervention: dietary advice Comparison: no advice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No advice | Dietary advice | |||||
|
Mortality Follow‐up: up to 3 months |
67 per 1000 | 58 per 1000 (17 to 198) |
RR 0.87 (0.26 to 2.96) | 574 (7 studies) | ⊕⊕⊝⊝ lowa,b | The results at all other time points also suggest there may be little or no difference between dietary advice and no advice. |
|
Number of people admitted or readmitted to hospital Follow‐up: up to 3 months |
See comments. | NA | NA | NA | The results at 4 to 6 months and 12 months and over suggest there may be little or no difference between dietary advice and no advice. | |
|
Length of hospital stay (days) Follow‐up: up to 3 months |
The mean length of hospital stay in the no dietary advice group was 13.5 days. | The mean length of hospital stay in the dietary advice group was 1.10 days lower (1.35 days lower to 0.85 days lower). | NA | 148 (1 study) | ⊕⊕⊝⊝ lowc,d | The results at 4 to 6 months and 12 months and over suggest there may be little or no difference between dietary advice and no advice. |
|
Complications Follow‐up: up to 3 months |
The mean number of complications in the no dietary advice group was 1.2. | The mean difference in the number of complications in the dietary advice group was 0.00 higher (0.32 lower to 0.32 higher). | NA | 148 (1 study) | ⊕⊕⊝⊝ lowc,d | The results at 4 to 6 months suggest there may be little or no difference between dietary advice and no advice. |
|
Change in weight (kg) Follow‐up: up to 3 months |
The mean change in weight in the no dietary advice group ranged from ‐2.0 kg to 1.32 kg. | The mean change in weight in the dietary advice group was 0.97 kg higher (0.06 kg higher to 1.87 kg higher). | NA | 802 (10 studies) | ⊕⊕⊕⊝ lowe,f | The results at all other time points also suggest dietary advice may improve weight gain. |
|
Change in fat‐free mass (kg) Follow‐up: up to 3 months |
The mean change in fat‐free mass in the no dietary advice group was ‐0.14 kg. | The mean change in fat‐free mass in the dietary advice group was 0.29 kg higher (0.11 kg lower to 0.69 kg higher). | NA | 98 (2 studies) | ⊕⊕⊝⊝ lowd,g | The results at 4 to 6 months also suggest there may be little or no difference between dietary advice and no advice. However, results at 12 months and over suggest that dietary advice may increase fat‐free mass. |
|
Change in global QoL score Follow‐up: up to 3 months |
The mean change in global QoL score in the no dietary advice group ranged from ‐19.0 to 2.9. | The mean change in global QoL score in the dietary advice group was 3.30 higher (1.47 higher to 5.13 higher). | NA | 421 (5 studies) | ⊕⊕⊝⊝ lowg,h | The results at all other time points suggest there may be little or no difference between dietary advice and no advice. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; QoL: quality of life; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision caused by low event rates.
b. Downgraded once due to indirectness; the studies included in this outcome look at mortality in different disease groups. Most of the deaths occurred in one study where the disease was cancer of the gastro‐intestinal tract and the results may be not be applicable across different diseases.
c. Downgraded once due to risk of bias in the single included trial for this outcome particularly across the domains of sequence generation and allocation concealment.
d. Downgraded once due imprecision caused by small sample size which doesn't meet the optimal information size.
e. Downgraded once due to indirectness: the studies included in this outcome look at different disease groups and the results of these studies may not be generalisable to other disease groups.
f. Downgraded once due to heterogeneity: I2 value was 88%.
g. Downgraded once due to risk of bias across several domains but particularly around randomisation and allocation concealment.
h. Downgraded once due to risk of bias within the included trials from concerns around blinding. Although it is not possible to blind this kind of intervention, knowledge of allocation could affect how participants score themselves with regard to QoL.
Summary of findings 2. Dietary advice compared with oral nutritional supplements for disease‐related malnutrition in adults.
| Dietary advice compared with nutritional ONS for disease‐related malnutrition in adults | ||||||
|
Patient or population: adults with disease‐related malnutrition Settings: all healthcare settings Intervention: dietary advice Comparison: nutritional ONS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nutritional ONS | Dietary advice | |||||
|
Mortality Follow‐up: up to 3 months |
74 per 1000 | 49 per 1000 (25 to 93) |
RR 0.66 (0.34 to 1.26) | 576 (8 studies) |
⊕⊕⊝⊝ lowa,b | The results at all other time points also suggest there may be little or no difference between dietary advice and nutritional ONS. |
|
Number of people admitted or re‐admitted to hospital Follow‐up: up to 3 months |
115 per 1000 | 41 per 1000 (5 to 373) |
RR: 0.36 (0.04 to 3.24) | 50 (1 study) |
⊕⊕⊝⊝ lowa,c | The results for 4 to 6 months suggest nutritional ONS may reduce the number of people admitted or re‐admitted to hospital. |
|
Length of hospital stay (days) |
Not reported. | NA | NA | NA | ||
|
Complications |
Not reported. | NA | NA | NA | ||
|
Change in weight (kg) Follow‐up: up to 3 months |
The mean change in weight in the nutritional ONS group ranged from 0 kg to 3.2 kg. | The mean change in weight in the dietary advice group was 0.14 kg lower (2.01 kg lower to 1.74 kg higher). | NA | 517 (9 studies) |
⊕⊕⊝⊝ lowd,f | The results for 4 to 6 months also suggest there may be little or no difference between the 2 groups. |
|
Change in fat‐free mass (kg) Follow‐up: up to 3 months |
Not reported. | NA | NA | NA | ||
|
Change in global QoL score Follow‐up: up to 3 months |
The mean change in global QoL score in the nutritional ONS group ranged from ‐0.66 to 20. | The mean change in global QoL score in the dietary advice group was 1.26 higher (0.32 lower to 2.85 higher). | NA | 283 (4 studies) |
⊕⊕⊝⊝ lowd,e | The results for 12 months and over suggest dietary advice may improve global QoL scores. The results at all other time points suggest there may be little or no difference between dietary advice and nutritional ONS. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ONS: oral nutritional supplements; QoL: quality of life; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to imprecision caused by low event rates or small sample size or a combination of both.
b. Downgraded once due to indirectness; the studies included in this outcome look at mortality in different disease groups. Most of the deaths occurred in one study where the disease was cancer of the gastro‐intestinal tract and the results may be not be applicable across different diseases.
c. Downgraded due to indirectness; it is not clear whether the results of this single study would be generalisable to other disease groups.
d. Downgraded once due to indirectness; the studies included in this outcome look at different disease groups and the results of these studies may not be generalisable to other disease groups.
e. Downgraded once due to risk of bias within the included trials from concerns around blinding. Although it is not possible to blind this kind of intervention, knowledge of allocation could affect how participants score themselves with regard to QoL.
f. Downgraded once due to heterogeneity: I2 value was 94%.
Summary of findings 3. Dietary advice compared with dietary advice plus oral nutritional supplements for disease‐related malnutrition in adults.
| Dietary advice compared with dietary advice plus nutritional ONS for disease‐related malnutrition in adults | ||||||
|
Patient or population: adults with disease‐related malnutrition Settings: all healthcare settings Intervention: dietary advice plus nutritional ONS Comparison: dietary advice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dietary advice | Dietary advice plus nutritional ONS | |||||
|
Mortality Follow‐up: up to 3 months |
74 per 1000 | 68 per 1000 (35 to 133) |
RR 0.92 (0.47 to 1.80) | 777 (10 studies) |
⊕⊕⊝⊝ lowa,b | The results for all other time points also suggest there may be little or no difference between the 2 groups. |
|
Number of people admitted or re‐admitted to hospital Follow‐up: up to 3 months |
283 per 1000 | 481 per 1000 (294 to 784) |
RR 1.70 (1.04 to 2.77) | 114 (1 study) |
⊕⊕⊝⊝ lowc,d | The results for 4 to 6 months suggest there is probably no difference between dietary advice with or without nutritional ONS. |
|
Length of hospital stay (days) Follow‐up: up to 3 months |
The mean length of hospital stay in the dietary advice group was 17.5 days. | The mean length of hospital stay in the dietary advice plus nutritional ONS group was 1.07 days lower (4.10 days lower to 1.97 days higher). | NA | 202 (2 studies) |
⊕⊕⊝⊝ lowc,d | |
|
Complications Follow‐up: up to 3 months |
417 per 1000 | 313 per 1000 (234 to 413) |
RR 0.75 (0.56 to 0.99) | 317 (3 studies) |
⊕⊕⊝⊝ lowd,e | The results for 4 to 6 months suggest there may be little or no difference between the 2 groups. |
|
Change in weight (kg) Follow‐up: up to 3 months |
The mean change in weight in the dietary advice group ranged from ‐5.86 kg to 2.2 kg. | The mean change in weight in the dietary advice plus nutritional ONS group was 1.15 kg higher (0.42 kg higher to 1.87 kg higher). | NA | 931 (14 studies) |
⊕⊕⊝⊝ lowa,d | The results for all other time points suggest there may be little or no difference between the 2 groups. |
|
Change in fat‐free mass (kg) Follow‐up: up to 3 months |
The mean change in fat‐free mass in the dietary advice group ranged from ‐0.1 kg to 0.9 kg. | The mean change in fat‐free mass in the dietary advice plus nutritional ONS group was 0.10 higher (0.18 lower to 0.39 higher). | NA | 187 (3 studies) |
⊕⊕⊝⊝ lowc,d | |
|
Change in global QoL score Follow‐up: up to 3 months |
The mean change in global QoL score in the dietary advice group ranged from ‐9.55 to 2.0. | The mean change in global QoL score in the dietary advice plus nutritional ONS group was 0.33 higher (0.09 higher to 0.57 higher). | NA | 321 (4 studies) |
⊕⊕⊝⊝ lowd,f | The results for 4 to 6 months suggest there may be little or no difference between the two groups. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ONS: oral nutritional supplements; QoL: quality of life; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to risk of bias within the included trials; in 4 out of the 10 trials there were concerns around the randomisation process or allocation concealment, or both. All studies had concerns around blinding of outcome assessment.
b. Downgraded once due to indirectness; the studies included in this outcome look at mortality in different disease groups. Most of the deaths occurred in one study where the disease was cancer of the gastro‐intestinal tract and the results may be not be applicable across different diseases.
c. Downgraded once due to imprecision caused by small sample size which does not reach the optimum information size.
d. Downgraded once due to indirectness as it is unclear whether the results are generalisable to other disease groups.
e. Downgraded once due to inconsistency; there is some heterogeneity in both the magnitude and direction of effect (I² = 58%).
f. Downgraded once due to risk of bias within the included trials from concerns around blinding. Although it is not possible to blind this kind of intervention, knowledge of allocation could affect how participants score themselves with regard to QoL.
Summary of findings 4. Dietary advice plus supplements if required compared with no advice for disease‐related malnutrition in adults.
| Dietary advice plusONS if required compared with no advice for disease‐related malnutrition in adults | ||||||
|
Patient or population: adults with disease‐related malnutrition Settings: all healthcare settings Intervention: dietary advice plus ONS Comparison: no advice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No advice | Dietary advice plus ONS | |||||
|
Mortality Follow‐up: up to 3 months |
105 per 1000 | 86 per 1000 (61 to 122) |
RR 0.82 (0.58 to 1.16) | 1261 (15 studies) |
⊕⊕⊝⊝ lowa,b | The results for all other time points also suggest there may be little or no difference between groups. |
|
Number of people admitted or re‐admitted to hospital Follow‐up: Up to 3 months |
385 per 1000 | 320 per 1000 (227 to 443) |
RR 0.83 (0.59 to 1.15) | 673 (7 studies) |
⊕⊕⊕⊝ moderatec | The results for all other time points also suggest there may be little or no difference between groups. |
|
Length of hospital stay (days) Follow‐up: up to 3 months |
The mean length of hospital stay in the no dietary advice group ranged from 2.5 days to 18.6 days. | The mean length of hospital stay in the dietary advice plus ONS group was 0.12 days lower (2.48 days lower to 2.25 days higher). | NA | 400 (3 studies) | ⊕⊕⊝⊝ lowc,d | |
|
Complications Follow‐up: up to 3 months |
265 per 1000 | 148 per 1000 (58 to 387) |
RR 0.56 (0.22 to 1.46) | 280 (2 studies) |
⊕⊕⊝⊝ lowc,d | The results for 7 to 12 months also suggest there may be little or no difference between groups |
|
Change in weight (kg) Follow‐up: up to 3 months |
The mean change in weight in the no dietary advice group ranged from ‐4.7 kg to 1.6 kg. | The mean change in weight in the dietary advice plus ONS group was 1.25kg higher (0.73 kg higher to 1.76 kg higher). | NA | 1192 (17 studies) |
⊕⊕⊕⊝ moderatec | The results for all other time points suggest there may be little or no difference between groups. |
|
Change in fat‐free mass (kg) Follow‐up: up to 3 months |
The mean change in fat‐free mass in the no dietary advice group ranged from ‐1.4 kg to 0.092 kg. | The mean change in fat‐free mass in the dietary advice plus ONS group was 0.82 kg higher (0.35 kg higher to 1.29 kg higher). | NA | 262 (4 studies) |
⊕⊕⊝⊝ lowc,e | The results for 4 to 6 months suggest there may be little or no difference between the two groups. |
|
Change in global QoL score Follow‐up: up to 3 months |
The mean change in global QoL score in the no dietary advice group ranged from ‐12.6 to 62. | The mean change in global QoL score in the dietary advice plus ONS group was 0.15 higher (0.18 lower to 0.48 higher). | NA | 389 (7 studies) |
⊕⊕⊝⊝ lowd,f | The results for 7 to 12 months suggest dietary advice plus ONS may improve global QoL scores. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ONS: oral nutritional supplements; QoL: quality of life; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to risk of bias within the included trials; in 7 out of the 14 trials there were concerns around the randomisation process or allocation concealment, or both. All studies had concerns around blinding of outcome assessment.
b. Downgraded once due to indirectness; the studies included in this outcome look at mortality in different disease groups. Most of the deaths occurred in one study where the disease was cancer of the gastro‐intestinal tract and the results may be not be applicable across different diseases.
c. Downgraded once due to indirectness as it is unclear whether the results are generalisable to other disease groups.
d. Downgraded once due to risk of bias in the included trials for this outcome; there were particular concerns across the domains of randomisation and allocation concealment.
e. Downgraded once due to small sample size that does not reach the optimum information size.
f. Downgraded once due to high risk of bias within the included trials from lack of blinding. Although it is not possible to blind for this type of study, we felt that for this outcome the allocation may have an effect on the subjective QoL.
Summary of findings 5. Dietary advice plus supplements compared with no dietary advice plus no supplements for disease‐related malnutrition in adults.
| Dietary advice plus ONS compared with no dietary advice plus no ONS for disease‐related malnutrition in adults | ||||||
|
Patient or population: adults with disease‐related malnutrition Settings: all healthcare settings Intervention: dietary advise plus ONS Comparison: no dietary advice plus no ONS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No dietary advice plus no ONS | Dietary advice plus ONS | |||||
|
Mortality Follow‐up: up to 3 months |
76 per 1000 | 69 per 1000 (42 to 116) |
RR 0.91 (0.55 to 1.52) | 797 (7 studies) |
⊕⊕⊝⊝ lowa,b | The results for all other time points also suggest there may be little or no difference between groups. |
|
Number of people admitted or re‐admitted to hospital Follow‐up: up to 3 months |
Outcome not reported. | NA | NA | NA | ||
|
Length of hospital stay (days) Follow‐up: up to 3 months |
The mean length of hospital stay in the no dietary advice plus no ONS group was 13.25 days. | The mean length of hospital stay in the dietary advice plus ONS group was 1.81 days lower (3.65 days lower to 0.04 days higher). | NA | 258 (2 studies) |
⊕⊕⊝⊝ lowb,c | The results from a further study at 4 to 6 months also suggest there may be little or no difference between groups |
|
Complications Follow‐up: up to 3 months |
643 per 1000 | 270 per 1000 (128 to 572) |
RR 0.42 (0.20 to 0.89) | 50 (1 study) | ⊕⊕⊝⊝ lowb,c | |
|
Change in weight (kg) Follow‐up: up to 3 months |
The mean change in weight in the no dietary advice plus no ONS group ranged from ‐0.9 kg to 3.4 kg. | The mean change in weight in the dietary advice plus ONS group was 1.08 kg higher (0.17 kg lower to 2.33 kg higher). | NA | 620 (8 studies) |
⊕⊕⊝⊝ lowa,b | The results for all other time points suggest that dietary advice plus ONS group may increase weight. |
|
Change in fat‐free mass (kg) Follow‐up: up to 3 months |
The mean change in fat‐free mass in the no dietary advice plus no ONS group ranged from ‐1.01 kg to 2.8 kg. | The mean change in fat‐free mass in the dietary advice plus ONS group was 0.26 kg higher (0.09 kg lower to 0.62 kg higher). | NA | 130 (3 studies) |
⊕⊝⊝⊝ very lowa,b,c | The results for all other time points also suggest there may be little or no difference between groups. |
|
Change in global QoL score Follow‐up: up to 3 months |
The mean change in global QoL score in the no dietary advice plus no ONS group ranged from 1.86 to 25. | The mean change in global QoL score in the dietary advice plus ONS group was 0.32 higher (0.33 lower to 0.96 higher). | NA | 357 (4 studies) |
⊕⊝⊝⊝ very lowa,b,c | The results for all other time points also suggest there may be little or no difference between groups. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ONS: oral nutritional supplements; QoL: quality of life; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
a. Downgraded once due to risk of bias within the included trials, particularly across the domains of randomisation or allocation concealment, or both.
b. Downgraded once due to indirectness as it is unclear whether the results are generalisable to different disease groups.
c. Downgraded once due to imprecision from a small sample size which does not meet the optimum information size.
d. Downgraded twice due to risk of bias within the included trials, particularly across the domains of randomisation or allocation concealment, or both; but for this outcome the effect of not being able to blind participants is more significant as knowledge of the allocation may alter the way they perceive their QoL.
Background
Description of the condition
Malnutrition can be defined as “a state resulting from lack of intake or uptake of nutrition that leads to altered body composition (decreased fat‐free mass) and body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease” (de van der Schueren 2019). Malnutrition can arise from a single cause such as the disease or from a combination of psychological and social conditions which act as co‐factors in the development or exacerbation of ill health. The general diagnosis of malnutrition has subgroups of aetiology‐based types of malnutrition: disease‐related malnutrition with inflammation, disease‐related malnutrition without inflammation, and malnutrition or undernutrition without disease (Cederholm 2016). Subclassifications of malnutrition are crucial for the understanding of the related complexities and to plan treatment.
Clinically significant malnutrition consists of nutritional deficits that have serious adverse effects on the treatment and outcome of disease (Cederholm 2016; Jensen 2010). Malnutrition or being at risk of malnutrition is associated with increased morbidity, mortality and increased length of stay in hospital (Kubrak 2007; McWhirter 1994; Naber 1997; Norman 2008a). In addition, in a large cross‐sectional study in older people living in Norway, malnutrition was associated with significant reductions in health‐related quality of life (HRQoL) assessed by EQ‐5D, with impacts in all the five dimensions seen in men and in usual activities and anxiety and depression in women (Kvamme 2011).
Only recently, consensus‐based diagnostic criteria were proposed to facilitate recording of the occurrence of malnutrition in adults. These criteria are based on the minimum phenotypic and etiologic criteria of: significant weight loss; or low body mass index (BMI); or low muscle mass and reduced food intake or its assimilation; or inflammation (Cederholm 2019; Jensen 2019).
In practice, malnutrition varies along a spectrum from mild to severe. The difficulties in defining malnutrition are reflected in the variation in reported prevalence which ranges from 9% to 55% (Braunschweig 1999; Elia 2009; Hanger 1999; Kubrak 2007; McWhirter 1994; Norman 2008a; Peake 1998a; Prieto 1996; Watson 1998; Weekes 1998). Although malnutrition is present in individuals from all disease backgrounds, all ages and in all healthcare settings, older people are more likely to be malnourished than younger people. Those over the age of 80 have a five times higher prevalence of malnutrition than those under 50 years old (Age Concern 2006). A recent systematic review of the prevalence of the nutritional risk among older adults varied by country, by method of defining malnutrition risk, and by healthcare setting (Leij‐Halfwerk 2019). For hospital, residential care and community settings high malnutrition risk among older adults was prevalent in 28.0% 17.5% and 8.5% of individuals, respectively.
A substantial proportion of disease‐related malnutrition occurs and is managed in a community setting. Although the prevalence of malnutrition in the community is lower than in institutions, at any one time the greatest number of malnourished people are living in their own home. Between 5% and 10% of older people are malnourished and malnutrition prevalence rates may increase to 35% in older people receiving home care (Guigoz 1997; McCormack 1997; Schilp 2012).
The management of disease‐related malnutrition is likely to be different in areas of food security from its management in poorer parts of the world where there may be less food security, although the mechanisms of any effects seen may be similar. The focus of this review is the management of disease‐related malnutrition in high‐income countries, where food insecurity is less likely to be an issue for sectors of the population. In this update of our review we have included studies from low‐ and middle‐income countries, where malnutrition was studied in relation to disease. When the term malnutrition is used throughout this review, it is intended to refer to undernutrition and not overnutrition or obesity.
Description of the intervention
Malnutrition is largely unrecognised despite the potentially adverse consequences for individuals and the implications for healthcare resources (Bavelaar 2008; Khalatbari‐Soltani 2018; McWhirter 1994). There are no internationally accepted protocols for nutritional intervention in the management of disease‐related malnutrition. People who are identified as malnourished in hospital and in the community may be considered for referral to a dietitian. In routine clinical practice the poor nutritional status of many individuals is not recognised and many do not receive any advice (McWhirter 1994; Peake 1998a; Volkert 2010). Dietitians are uniquely qualified to provide appropriate interventions such as diet instruction and intensive nutritional support, but there is no theoretical reason to believe that other health professionals could not give effective dietary advice. The provision of dietary advice is a core dietetic skill, but it is not known whether it is effective at increasing nutrient intake and weight or influencing function and outcome. A range of dietetic strategies is commonly used to promote weight gain and improve the nutritional status of a malnourished individual. These include:
advice to increase food intake (e.g. increase portion size, add food items in the form of snacks, nourishing drinks, side dishes and desserts);
advice to modify food constituents to increase the energy density (food fortification);
the provision of ONS without dietary advice; and
a combination of advice to increase to food intake and the provision of ONS.
How the intervention might work
We think that the intervention will work by increasing nutritional intake which will then translate to improvements in nutritional status and other outcomes. There is an assumption underpinning the use of interventions to increase nutritional intake that they have equal efficacy, but in practice their use might be influenced by other factors. ONS are usually nutritionally complete, available on prescription and easy to use. However, individuals' willingness to incorporate these frequently sweet‐tasting drinks in their daily intake may be adversely influenced by the monotony of taste and sensory‐specific satiety. A number of studies highlight problems with the use of ONS and the monitoring of people taking them (Bruce 2003; Gosney 2003; Keele 1997; Munro 1998; Peake 1998b); adherence to ONS has been reported to vary (Hubbard 2012). In addition, ONS are expensive to the healthcare system.
Food is often preferred in the first line management of malnutrition. Food is considered to have practical advantages because it is familiar to patients, can be varied in type, texture and flavour and is cheaper to healthcare providers. However, advice to change aspects of food intake might represent a significant burden to people who are ill and their carers. It is reasonable to presume that any benefits from ONS reflect their functional contribution to an increased nutrient intake (or balance of nutrients). It follows that if a similar increase in nutrient intake can be achieved by dietary means rather than using ONS, it is reasonable to expect similar clinical benefits. However, we do not know which nutrient or combination of nutrients in ONS is responsible for any benefits (protein, energy, vitamins, trace elements) and it may not be possible to modify food intake to produce comparable changes to those achieved with ONS. It is infeasible to replicate interventions because studies of dietary advice rarely report the details of specific foods and combinations of foods used to increase nutrient intake. It is commonly overlooked that individuals may need support from health professionals to implement the necessary behaviour change to achieve an increase in intake, whether from food or ONS. We urge future authors to include a full description of any behaviour change models in their publications, along with any underpinning mechanisms.
Why it is important to do this review
The health and social care costs for people with malnutrition are thought to be three to four times higher than for non‐malnourished individuals(Guest 2011), estimated to be GBP 19.6 billion in the UK (Elia 2015), with at least GBP 5 billion of that directly attributed to healthcare costs (Wilson 2013), USD 157 billion in the USA (Snider 2014) and EUR 120 billion in Europe (Ljungqvist 2010). Fuller implementation of the NICE guideline (NICE 2006) and accompanying quality standard (QS24) have been estimated to have the potential to result in cost savings, with effective recognition and treatment of malnutrition and continuity of management across healthcare being key to achieving these goals (Elia 2015). In the UK, National Health Service expenditure on nutritional prescribing is growing. In 2018/19 the cost of nutriitonal prescribing in primary care was around GBP 358 million of which GBP 150 million (42%) was accounted for by ONS (NHS BSA). Increased awareness of nutrition and active marketing by manufacturers may have contributed to the increased use of ONS. Additional or increased food intake resulting from targeted dietary advice to increase nutritional intake and weight has potential advantages in that it offers greater variety, can be tailored to individual eating habits and additional costs are not met by the health services, although people who are unwell may have some difficulties with shopping and the preparation of food. The increasing costs of ONS in the UK have resulted in enhanced scrutiny of prescribing practices and the encouragement of a 'food first' policy in many areas (NICE 2012).
There is limited evidence to support the hypothesis that food‐based interventions and ONS have equal efficacy in managing disease‐related malnutrition. Although more than 30 systematic reviews have examined the efficacy of ONS in the management of illness‐related malnutrition, there is considerable discordance in the findings for similar outcomes (Baldwin 2021) and there remain uncertainties about whether ONS in routine care can improve outcomes. A retrospective data analysis of the impact of ONS on inpatient outcomes across multiple clinical conditions, including 1.2 million episodes of ONS use, found that ONS was associated with decreased length of hospital stay, episode cost, and 30‐day risk for readmission. Cost savings were impressive, with a return on investment of USD 52.63 in immediate net episode cost savings and USD 2.56 net savings due to averted 30‐day readmissions for every USD spent on ONS in the matched sample (Philipson 2013). A serious limitation of this analysis was its retrospective nature and the fact that detailed health and nutrition information was not available.
The evidence for food‐based approaches to the management of malnutrition is limited. A systematic review of use of fortified foods and snacks in hospitalised older patients suggested benefits to energy and protein intake, as well as finding good acceptability and evidence of cost‐effectiveness (Mills 2018). A recent pooled data‐analysis of dietary counselling, ONS, or both in older adults with disease‐related malnutrition suggested that dietary counselling combined with ONS was the most effective way to achieve a positive effect on body weight and energy intake (Reinders 2019). No effects were shown, however, for other relevant clinical outcomes. The data in this analysis were from only nine out of 38 eligible studies and should be interpreted with caution. It is widely recommended that improving nutritional intake using foods and beverages is the first step in the process of providing nutritional support and that ONS are a second step in the process which may be appropriate for some people. The evidence base for ONS has been extensively reviewed, whereas that relating to dietary advice given with or without ONS has received relatively little attention. It may be possible to increase oral nutritional intake in a number of different ways and it is important to clarify the role and efficacy of each method as the service, staffing and financial resource implications differ.
Objectives
To examine the effects of dietary advice alone given by a dietitian or other healthcare professional to adults at nutritional risk of or living with disease‐related malnutrition compared with:
no advice;
the prescription of ONS; or
dietary advice ONS.
Also to examine the effects of:
dietary advice plus ONS if required compared with no advice and no prescription of ONS; or
dietary advice plus ONS compared with no advice and no prescription of ONS.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Adults over 16 years of age with disease‐related malnutrition or described as at nutritional risk by the study investigator or judged to be at nutritional risk by the review authors due to their clinical condition or clinical treatment or both. We considered studies conducted in all healthcare settings
We excluded studies carried out in pregnant women or people with eating disorders and in conditions of food insufficiency (inadequate availability of food in a whole or part of a country meaning that the population are at risk of famine).
Types of interventions
Dietary advice was defined as instruction in the modification of food intake given with the aim of improving nutritional intake by a dietitian or other healthcare professional; ONS was defined as a whole protein enteral food supplement which is marketed as a clinical product for the management of disease‐related malnutrition and taken for any period of time
dietary advice compared with no advice;
dietary advice compared with ONS;
dietary advice compared with dietary advice plus ONS;
dietary advice plus ONS if required compared with no advice and no ONS
dietary advice and prescription of an ONS compared with no advice and no ONS.
The second comparison includes studies that examined the efficacy of the two different active interventions .
The third comparison includes studies that aimed to explore whether there was additional benefit to giving ONS with dietary advice.
We added a fourth comparison post hoc in response to an additional group of studies that we identified during searching and study identification for the 2004 update. We consider these studies relevant to our review as they examine dietary advice compared with no advice, but the dietary advice includes information on using ONS if considered necessary. In our experience, this style of providing dietary advice most closely reflects how dietary advice is given in practice.
We added the fifth comparison post hoc at the 2021 update and in response to closer scrutiny of the studies of dietary advice plus ONS if required. It became clear that studies for this comparison were falling into two distinct groups. In the first group we saw that ONS were used in addition to dietary advice in only some participants, and this intervention was distinguished by the frequent use of the phrase "if judged appropriate". We identified a second group of studies, where dietary advice and ONS were given to all participants from the start. These two groups comprise the fifth comparison that we have added to this updated review.
We excluded studies of ONS with novel ingredients, e.g. arginine, glutamine and omega‐3 fatty acids and elemental and semi‐elemental ONS, where the constituents are present in their simplest form. These products are address specialised situations and were judged to be beyond the scope of this review.
Where studies of dietary advice also included escalation of intervention to include enteral and parenteral feeding, studies have only been included if additional feeding was received by 10% or fewer of included participants or if results for participants receiving dietary advice with or without ONS were presented seperately.
Types of outcome measures
We have assessed the following primary and secondary outcome measures.
Primary outcomes
Mortality
Morbidity (assessed by risk of hospital admission or readmission and length of hospital stay and complications)
-
Measures of nutritional status and body composition
change in weight
BMI
fat‐free mass (FFM)
mid‐arm circumference (MAC)
mid‐arm muscle circumference (MAMC)
triceps skinfold thickness (TSF)
Secondary outcomes
Nutritional intake before and after the intervention
Measures of clinical function (e.g. immune function, cardiac function, respiratory function and other indices of nutritional status)
Quality of life (QoL) assessed using validated scales (e.g. EORTC, EQ‐5D, SF‐36)
Cost
Search methods for identification of studies
The authors searched for all relevant published or unpublished studies irrespective of publication status (e.g. abstract or online study report) or language. Full text articles describing the results of RCTs were included in the review.
Electronic searches
The Cochrane Cystic Fibrosis and Genetic Disorders Group's Information Specialist conducted a search of the Group's Trials Register for relevant studies using the following terms: ((diet* OR malnutrition* OR nutrition* OR food* OR feed* OR eat*):TI,KW,AB,MH,EMT) AND ((behavio* OR supplement* OR advice OR advise* OR counsel* OR educat* OR guide OR guidance OR personal* OR program OR programme OR support*):TI,KW,AB,MH,EMT).
The Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching through the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group website.
Date of the most recent search of the Group's Trials Register: 03 March 2021.
We also searched the following databases and study registries; please see the appendices for the previous and current search strategies (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5):
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 3) in the Cochrane Library (searched 01 March 2021);
MEDLINE Ovid (1946 to 10 January 2020);
Embase Ovid (1974 to 10 January 2020);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 10 January 2020);
AMED Ovid (Allied and Complementary Medicine; 1985 to 30 June 2005), not searched for this update as authors do not have access;
National Cancer Institute CancerLit (1999 to 30 June 2005), database no longer available;
ISI Web of Science (1898 to 10 January 2020);
Scopus (1823 to 10 January 2020);
ERIC (searched 1966 to 1998), not searched for this update as authors do not have access;
Dissertation Abstracts (1861 to July 2000), not searched for this update as authors do not have access;
US National Institutes of Health Ongoing Trials Register Clinicaltrials.gov (www.clinicaltrials.gov; searched 03 March 2021);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch; not searched in 2021 as unavailable due to Covid‐19).
Searching other resources
We assessed the bibliographies of all retrieved studies and any relevant systematic reviews identified for additional reports of relevant studies. We identified all articles that met the inclusion criteria on PubMed and using the 'Related articles' feature, we carried out a further search out for additional articles, including newly published articles (Snowball searching).
We identified additional studies from electronic searches carried out by the National Collaborating Centre for Acute Care undertaken in the production of a guideline on nutrition support in adults (NICE 2006).
We sought unpublished work by contacting experts in clinical nutrition and the membership of the British Dietetic Association in 1999. We contacted the manufacturers of ONS for information on additional studies in 1999. In 1999, we contacted the group of dietitians conducting handsearching of nutrition‐based journals to identify RCTs for inclusion in the Cochrane Library, before undertaking any additional handsearching.
The authors did not undertake any additional handsearching for the 2021 update.
Data collection and analysis
Selection of studies
Until the update in 2007, one author (CB) reviewed the titles and abstracts from each search on‐screen and two authors (CB, TP) obtained the full‐text of any potentially relevant studies and assessed these independently against the inclusion criteria. They resolved their differences by discussion and where necessary by consultation with a third author (SL). For the 2007 and 2011 updates, two authors (CB, EW) carried out the study selection. For the 2021 update, four authors (CB, MdvdS, HK, EW) reviewed titles and abstracts from searches on‐screen, obtained potentially relevant studies and assessed these against the review's inclusion criteria.
The authors recorded abstracts that described the findings of potentially relevant RCTs as 'Studies awaiting classification' to be added once data are available from study investigators or a full‐text paper is available.
Data extraction and management
Until the update in 2007 two authors (CB, TP) independently extracted data from all papers obtained. They resolved their differences by discussion and where necessary by consultation with a third author (SL). For the updates in 2007 and 2011, two authors (CB, EW) carried out independent data extraction as for previous updates. For the 2021 update, four authors (CB, MdvdS, HK, EW) carried out the data extraction process.
Since the authors sought papers with no restriction on language, they obtained the translation of any relevant non‐English papers. They assessed data from inclusion in the study to the end of intervention at the following time points: up to three months; four to six months; seven to 12 months and over 12 months.
For data to be entered into meta‐analyses of continuous outcomes (weight, energy intake, etc.), the review authors require sufficient information to allow the derivation of a mean change with standard deviation (SD) for both the intervention and comparison groups; for meta‐analyses of dichotomous outcomes (death, hospital admissions) they require the number of participants who experienced the event of interest and the total number in the group. These data have either been available from the paper or the authors have obtained these from the study investigators where possible. Unfortunately for a number of outcomes the review authors have not been able to obtain data in a format that they can enter into a meta‐analysis. The review authors performed calculations to obtain the data they required (see Description of studies for full details).
Six studies reported data for more than one intervention group that met the inclusion criteria for this review or the authors subdivided them according to characteristics of participants. In order to facilitate the inclusion of these data in the meta‐analyses, they created duplicate study IDs (Macia 1991a; Macia 1991b; Macia 1991c; Pedersen 2016a; Pedersen 2016b; Sharma 2002a; Sharma 2002b).
Assessment of risk of bias in included studies
In earlier versions of the review, the review authors assessed the methodological quality of the included studies based on a method described by Schulz (Schulz 1995). This assessment included an examination of the method of randomisation, whether the study was blinded and whether investigators recorded the number of participants lost to follow‐up or excluded from the study.
In the current version of the review, the review authors have assessed the risk of bias for each study for each of the criteria below as high risk of bias, unclear risk of bias or low risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). They assessed the generation of the randomisation sequence and allocation concealment as low risk of bias, unclear risk of bias, or high risk of bias; they recorded the blinding of outcome assessment as reported (low risk of bias), unclear (unclear risk of bias) or not reported (high risk of bias) separately for clinical, functional and nutritional outcomes and as an overall judgement for all outcomes. Other sources of bias that the review authors considered were the reporting of complete outcome data (accounting for all participants randomised in the study), avoidance of selective reporting of outcome variables and the inclusion of a comparison of baseline variables as well as recording information on any variables not similar at baseline. See 'Risk of bias tables' for details of individual studies (Characteristics of included studies) (Figure 1).
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
For a number of studies the review authors have set up duplicate study IDs for analysis purposes, i.e. where they present multiple data sets from a single study within the same comparison (Macia 1991a; Macia 1991b; Macia 1991c; Pedersen 2016a; Pedersen 2016b; Sharma 2002a; Sharma 2002b), below they report on just one of these study IDs in the text but the risk of bias judgements apply to the whole study.
In the original review and updates up until 2007, two authors (CB, TP) independently assessed the methodological quality of each study. For the 2011 and 2021 updates respectively, two authors (CB, EW) and four authors (CB, MdvdS, HK, EW) respectively assessed the risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The authors recorded the results of this assessment in the risk of bias tables for the following domains:
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective reporting;
other potential sources of bias.
The authors additionally scrutinised cluster RCTs for recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised studies or different types of clusters and recorded the findings under “other potential sources of bias”.
For incomplete outcome data, where it was possible the review authors assessed the attrition rates in the different intervention or control groups and if the difference was at least 20%, they considered this to constitute a high risk of bias.
Measures of treatment effect
For continuous outcomes, such as change in weight, the authors combined the data across studies using a mean difference (MD) and 95% confidence intervals (CIs) (Review Manager 2014). When different measurement scales were used, then the review authors considered whether a meaningful combined analysis was possible, e.g. by using standardised mean difference (SMD). They used the SMD to combine data on the following outcomes:
Complications — in Group 1 (dietary advice compared with no advice) as different validated tools were used to collect data for this outcome (Analysis 1.4);
FFM — in Group 1, Group 3 (dietary advice compared with dietary advice plus an ONS) and Group 5 (dietary advice and prescription of an ONS compared with no advice and no ONS) as data on both FFM and body cell mass were combined in the same analysis (Analysis 1.7; Analysis 3.7; Analysis 5.5); and
QoL for all groups as data were collected using different validated questionnaires (Analysis 1.16; Analysis 1.17; Analysis 1.18; Analysis 1.19; Analysis 1.20; Analysis 1.21; Analysis 1.22; Analysis 2.11; Analysis 2.12; Analysis 2.13; Analysis 2.14; Analysis 2.15; Analysis 2.16; Analysis 2.17; Analysis 3.15; Analysis 3.16; Analysis 3.17; Analysis 3.18; Analysis 3.19; Analysis 3.20; Analysis 3.21; Analysis 4.16; Analysis 4.17; Analysis 4.18; Analysis 4.19; Analysis 4.20; Analysis 4.21; Analysis 4.22; Analysis 4.23; Analysis 5.13; Analysis 5.14; Analysis 5.15; Analysis 5.16; Analysis 5.17; Analysis 5.18; Analysis 5.19).
1.4. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 4: Complications
1.7. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 7: Change in fat‐free mass (kg)
3.7. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 7: Change in fat free mass (kg)
5.5. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 5: Change in fat free mass
1.16. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 16: Change in global QoL
1.17. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 17: QoL ‐ change in physical function
1.18. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 18: QoL ‐ change in mental function
1.19. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 19: QoL ‐ change in social function
1.20. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 20: QoL ‐ change in cognitive function
1.21. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 21: QoL ‐ change in pain
1.22. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 22: QoL ‐ change in energy/fatigue
2.11. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 11: Change in global QoL
2.12. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 12: QoL ‐ change in physical function
2.13. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 13: QoL ‐ change in mental function
2.14. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 14: QoL ‐ change in social function
2.15. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 15: QoL ‐ change in cognitive function
2.16. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 16: QoL ‐ change in pain
2.17. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 17: QoL ‐ change in energy/fatigue
3.15. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 15: Change in global QoL
3.16. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 16: QoL ‐ change in physical function
3.17. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 17: QoL ‐ change in mental function
3.18. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 18: QoL ‐ change in social function
3.19. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 19: QoL ‐ change in cognitive function
3.20. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 20: QoL ‐ change in pain
3.21. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 21: QoL ‐ change in energy/fatigue
4.16. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 16: Change in global QoL
4.17. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 17: Final global QoL
4.18. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 18: QoL ‐ change in physical function
4.19. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 19: QoL ‐ change in mental function
4.20. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 20: QoL ‐ change in social function
4.21. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 21: QoL ‐ change in cognitive function
4.22. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 22: QoL ‐ change in pain
4.23. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 23: QoL ‐ change in energy/fatigue
5.13. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 13: Change in global QoL
5.14. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 14: QoL ‐ change in physical function
5.15. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 15: QoL ‐ change in mental function
5.16. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 16: QoL ‐ change in social function
5.17. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 17: QoL ‐ change in cognitive function
5.18. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 18: QoL ‐ change in pain
5.19. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 19: QoL ‐ change in energy/fatigue
Cohen's rules have been used to interpret the magnitude of the SMD as follows: small SMD = 0.2; medium SMD = 0.5; and a large SMD = 0.8 as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For binary outcomes, such as mortality, the authors combined the data from the studies using risk ratios (RR) and 95% CIs.
Unit of analysis issues
Where the review authors identified studies with non‐standard designs such as cross‐over RCTs and cluster RCTs, they took into account the level at which randomisation occurred. They could not recalculate data taking into account the design effect for cluster RCTs because they did not have reliable information about intra‐cluster correlation coefficients for the substantially heterogeneous populations in the included studies. Therefore, where meta‐analyses included both parallel and cluster RCTs the authors have taken this into consideration when accounting for any heterogeneity identified. For cross‐over studies, they included data in the meta‐analyses from baseline to the end of phase 1 of the cross‐over. They did not use data from phase 2 of cross‐over studies because of the anticipation of substantial carryover effects.
Dealing with missing data
In order to allow an intention‐to‐treat analysis, the review authors sought data on the number of participants, by allocated treatment group for each outcome, irrespective of adherence to group allocation and whether or not the participant was later thought to be ineligible.
Where the published study reports did not present relevant data, the review authors contacted study investigators for these data.
Where data were available on baseline and follow‐up measurements, the review authors calculated the mean change and then imputed SDs from studies judged to be similar for the mean change using a correlation coefficient of 0.8 assuming there was a strong correlation between baseline and follow‐up measurements or where calculation was not possible (Higgins 2011).
Assessment of heterogeneity
The authors examined differences between the results of the studies for heterogeneity using the Chi² test, by inspecting the results of the meta‐analysis and by using the I² statistic (Higgins 2003). The authors used a P value of less than 0.1 rather than less than 0.05 as evidence of statistical heterogeneity. The I² statistic describes the percentage of total variation across studies that is due to heterogeneity rather than chance (Higgins 2003). The values of I² lie between 0% and 100%, and a simplified categorisation of heterogeneity that the authors used is:
0 to 30% — no heterogeneity;
30% to 40% — no to moderate heterogeneity;
40% to 50% — moderate heterogeneity;
50% to 60% — moderate to substantial heterogeneity;
60% to 75% — substantial heterogeneity;
over 75% — considerable heterogeneity.
In all analyses with a heterogeneity of 50% or greater the authors planned to explore the clinical characteristics of studies included in the analysis for an explanation and report on this in the results section.
Assessment of reporting biases
The review authors scrutinised studies to ensure that all the outcome variables stated in the 'Methods' section were presented in the 'Results' section of the published reports. They also compared final papers to published protocols where these were available.
They assessed risk of publication bias and considered that there were sufficient studies (greater than 10) to undertake this for the outcome change in weight at up to three months. Using STATA, they assessed the risk of bias using the asymmetry of the funnel plot, the regression asymmetry test (Egger 1997) and the adjusted rank correlation (Begg 1994).
Data synthesis
The review authors judged that the included studies addressed a range of different participants and interventions, but these were related by a common aim of the intention to improve nutritional intake, therefore, the authors utilised the random‐effects model using the Mantel‐Haenszel method for all analyses to account for these differences.
Subgroup analysis and investigation of heterogeneity
In order to investigate any heterogeneity where the I² value is greater than 50%, when the review authors are able to include sufficient studies in this review, they planned to conduct subgroup analyses based on clinical judgement of the factors likely to account for differences in outcome within and between groups as follows:
underlying clinical condition (e.g. cancer, lung disease, gastrointestinal disease);
age (under 65 years and over 65 years);
nutritional status at inclusion in the study (percentage of malnourished participants versus participants at risk of malnutrition); and
study setting (hospital versus community and mixed).
Malnutrition is a multifactorial condition and previous analyses have demonstrated that no one factor is seminal in making a difference to outcomes. For this reason, in the 2021 updated version of this review, the authors did not conduct formal subgroup analyses but attempted to explain any heterogeneity in individual analyses from scrutiny of the clinical characteristics of studies included in the analyses. Most of the included studies did not report on outcomes by sex or gender and so the authors did not undertake any formal analyses by sex.
Sensitivity analysis
When the review authors were able to combine a sufficient number of studies (10 studies or more) (Higgins 2011), they planned to test the robustness of their results based on the risk of bias of the studies, e.g. according to rigour of randomisation method or RCTs versus quasi‐RCTs, and the potential impact of including studies where they had imputed SDs.
Summary of findings and assessment of the certainty of the evidence
In a post hoc change to bring this review in line with current (2021) Cochrane guidance, the authors have introduced summary of findings information for the outcomes listed below, where there was at least one study assessing their chosen outcomes.
Mortality
Length of stay
Hospital readmissions
Complications
Change in weight
FFM
QoL
For each outcome they have reported the illustrative risk with and without the intervention, magnitude of effect (RR or MD), numbers of studies and participants addressing each outcome and a grade of the overall certainty of the body of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) with comments (Schunemann 2006). They have created separate tables for each separate comparison they present.
Results
Description of studies
The review authors detail the studies they identified in several tables (Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies).
Results of the search
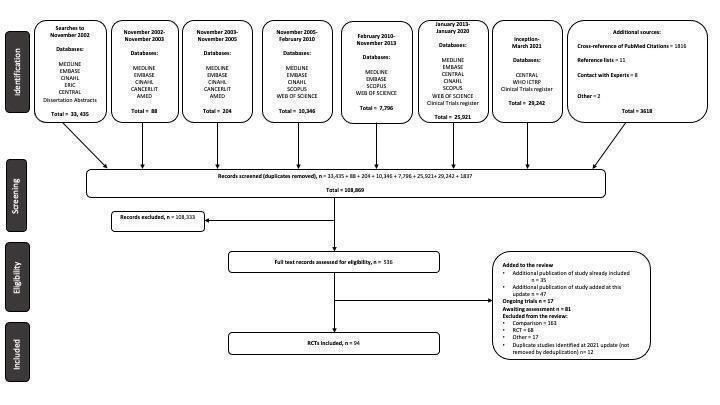
The searches conducted to 2021 identified 536 records which the review authors scrutinised against the inclusion criteria; the process is illustrated in a PRISMA diagram (Figure 2). The authors have included 94 studies (102 comparisons), with 10,284 randomised participants which fulfilled the inclusion criteria for this review (of which 49 studies are new at the 2021 update); they excluded 248 studies (Characteristics of excluded studies), 81 studies are awaiting classification (Studies awaiting classification) and they have listed 17 studies potentially relevant to this review as ongoing (Characteristics of ongoing studies). Searches undertaken for this update identified 35 additional records related to studies already included in the review, mainly clinical study records and conference abstracts, which have been added to the review and 47 additional records related to studies added at this update (Figure 2).
2.
The review authors have requested additional data on outcomes of interest and on aspects of study quality from 55 study investigators and obtained replies from 45 investigators. For 17 of the studies the investigators were unable to provide the data and information requested (Andersson 2017; Banks 2016; Beck 2015; Berneis 2000; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Evans 1987; Jensen 1997; Kendell 1982; Murphy 1992; Olejko 1984; Ovesen 1993; Schilp 2013; Sharma 2002a; Silvers 2014; Uster 2013). The review authors did not receive a reply from the investigators of a further nine studies (Arnold 1989; Chandra 1985; Dixon 1984; Macia 1991a; Moloney 1983; Pedersen 2016a; Pedersen 2016b; Rabeneck 1998; Rogers 1992; Wilson 2001).
Six studies reported data for more than one intervention group that met the inclusion criteria for this review or were subdivided according to characteristics of participants. In order to facilitate inclusion of these data in the meta‐analyses, duplicate IDs were created as follows (Macia 1991a; Macia 1991b; Macia 1991c; Pedersen 2016a; Pedersen 2016b; Sharma 2002a; Sharma 2002b).
Included studies
Please also see the additional tables which provide summaries of additional clinical outcomes (Table 6), additional functional outcomes (Table 7) and QoL assessments (Table 8) for all included studies across all interventions.
1. Summary of additional clinical outcomes reported in included studies.
| Study | Clinical measures (generic) | Clinical measures (disease specific) |
| Dietary advice versus no advice ‐ group 1 | ||
| Alo 2014 | Haemoglobin concentration | |
| Baldwin 2011 | ||
| Campbell 2008 | Estimated glomerular filtration rate, albumin, C‐reactive protein | |
| Cano‐Torres 2017 | Serum biochemistry (U&Es, FBC, glucose, creatinine, lipids) | |
| Casals 2015 | Serum biochemistry (protein, albumin, cholesterol, lymphocytes) | |
| Dixon 1984 | ||
| Fernandez‐Barres 2017 | Nutritional biochemistry (albumin, haemoglobin, haematrocrit, cholesterol) | |
| Forster 2012 | Symptoms & illness (diary), infections, GP visits, hospital visits, prescribed medication, temperature, nutritional biochemistry | |
| Gu 2015 | Complications score | |
| Imes 1988 | Crohn's Disease Activity Index Need for medication Need for surgery Number of work days lost due to Crohn's |
|
| Kunvik 2018 | ||
| Locher 2013 | ||
| Macia 1991 | Clinical observation of symptoms Days of suspended treatment |
|
| Manguso 2005 | Disease severity (Childs Score) | |
| Ollenschlager 1992 | No. days with temperature > 38.5 C | Number of complete remissions Clinical symptoms LAS |
| Pivi 2011 | Biochemical status (total protein, albumin, total lymphocyte count) | |
| Ravasco 2005a (C/R) | Symptom‐induced morbidity | |
| Ravasco 2005b (H&N) | Symptom‐induced morbidity | |
| Rydwik 2008 | ||
| Salva 2011 | Caregiver burden (Zarit), Use of healthcare and social resources (Resource Utilisation in Dementia (RUD) scale) | Clinical Dementia Rating scale Neuropschiatric Inventory scale |
| Stow 2015 | Adverse events, healthcare resource usage | |
| Tu 2013 | ||
| Weekes 2009 | Need for medication | |
| Wong 2004 | ||
| Dietary advice versus supplements ‐ group 2 | ||
| Akpele 2004 | rate of change of serum albumin | |
| Baldwin 2011 | ||
| Gray‐Donald 1995 | Number of falls | |
| Hernandez 2014 | biochemical status % of patients malnourished (defined as serum albumin < 3.5 g/dL) |
|
| Kalnins 2005 | Faecal balance studies | |
| Parsons 2016 | ||
| Pivi 2011 | Biochemical status (total protein, albumin, total lymphocyte count) | |
| Ravasco 2005a (C/R) | Symptom‐induced morbidity | |
| Ravasco 2005b (H&N) | Symptom‐induced morbidity | |
| Schwenk 1999 | ||
| Singh 2008 | Creatinine/height index, nitrogen balance | Abdominal pain score (not validated) Faecal fat Endocrine and exocrine function |
| Stow 2015 | Adverse events, healthcare resource usage | |
| Dietary advice versus dietary advice and supplements ‐ group 3 | ||
| Arnold 1989 | Serum albumin, serum transferrin | Tumour response Treatment interruptions Radiation side effects |
| Baldwin 2011 | ||
| Beattie 2000 | Need for medication Number of wound and chest infections |
|
| Burden 2011 | Number of participants with infections, need for antibiotics, self‐reported adverse events, compliance | |
| Burden 2017 | Number of participants with surgical infections, total complications | |
| de Luis 2003 | Serum albumin, prealbumin and transferrin | Viral load, CD4 |
| de Sousa 2012 | Albumin, total protein, total cholesterol, vit B12, folic acid | MMSE, clock drawing test |
| Diouf 2016 | Haemoglobin, zinc, % anaemic | |
| Dixon 1984 | ||
| Fuenzalida 1990 | Biochemical status (full range of tests), Creatinine Height Index, urinary creatinine and urea nitrogen. | Skin antigen testing Lymphocyte count |
| Gonzalez‐Espinoza 2005 | Biochemical status (full range of tests) | Number of episodes of peritonitis |
| Huynh 2015 | SGA, prealbumin, albumin, hemoglobin, total protein, CRP | |
| Kapoor 2017 | Sum of skinfolds, PG‐SGA, food frequency questionnaire, adherence to dietary supplements | |
| Kendell 1982 | Biochemical status (full range of tests), albumin, transferrin, total lymphocyte count, urinary nitrogen, Creatinine Height Index | |
| Le Cornu 2000 | LOS in ICU, hours on ventilatory support, septic complications, major noninfectious complications, rejections, changes in immunosuppression | |
| McCarthy 1999 | ||
| Murphy 1992 | ||
| Norman 2008b | Comorbidity count, number of drugs at discharge, albumin, CRP, haemoglobin, white blood cell count, platelets, costs |
|
| Olejko 1984 | Comorbidity count | |
| Rabeneck 1998 | ||
| Sharma 2002a | Renal‐related outcomes (protein catabolic rate, protein nitrogen appearance, albumin, potassium, phosphate) | Karnofsky Index, Self‐reported adverse effects |
| Swaminathan 2010 | Haemoglobin, albumin, total cholesterol, triglycerides, | CD 4 cell count |
| Wilson 2001 | Albumin number of days spent in hospital |
Time to nutritional repletion |
| Dietary advice plus supplements if required versus no advice ‐ group 4 | ||
| Andersson 2017 | Appetite (DRAQ), self perceived state of health | |
| Banks 2016 | Serum albumin, protein, C‐reactive protein, urea, glucose, HbA1C, TIBC, serum iron, serum transferrin, haemoglobin, neutrophil count, lymphocyte count, zinc, magnesium, ascorbate, creatinine | PU change, from baseline, in score (PUSH) and area (VISITRAC), length of stay to heal or discharge, early discharge, PU healed, PU worsened, discharged not healed |
| Beck 2012 | Risk of re‐admissions, need of social services (home care, home nursing, meals‐on‐wheels). | |
| Beck 2015 | The economic analysis of time spent by the dietitian, use of oral nutritional supplements (ONS) and number of hospitalisation days | |
| Bonilla‐Palomas 2016 | A composite of all‐cause death or readmission for worsening of HF. | |
| Bourdel‐Marchasson 2014 | Hospitalisation for reasons other than chemotherapy, MMS, depression (GDS) | Chemotherapy toxicity |
| Caccialanza 2015 | MAMC | |
| Carey 2013 | SGA | GI symptoms |
| Endevelt 2011 | Biochemical measurements, health care costs | cognition (MMSE), depression (GDS‐sf) |
| Evans 1987 | Tumour response to chemotherapy | |
| Feldblum 2011 | Albumin, total lymphocyte count, haemoglobin, transferrin, total cholesterol, mortality | Depression (GDS), cognition (MMSE) |
| Forli 2001 | Respiratory function | |
| Ganzoni 1994 | Respiratory function | |
| Hampson 2003 | Bone mineral density | |
| Holyday 2012 | 1 month and 6 months emergency frequency | |
| Isenring 2004 | Change in PG‐SGA score | |
| Jensen 1997 | Appetite, fatigue, work capacity | |
| Kiss 2016 | Fatigue, PG‐SGA score | |
| Lovik 1996 | Serum chemistry (albumin, transferrin) | |
| Moloney 1983 | Micronutrient intake | |
| Ovesen 1993 | Tumour response to chemotherapy | |
| Pedersen 2016a and Pedersen 2016b | ||
| Persson 2002 | ||
| Rogers 1992 | Sickness impact profile | |
| Schilp 2013 | Healthcare costs | |
| Sharma 2017 | PG‐SGA | complications |
| Silvers 2014 | PG‐SGA | |
| Starke 2011 | Number of antibiotic therapies, vit D, vit C, glutathione, compliance to ONS | complications |
| Suominen 2015 | ||
| Terp 2018 | Self rated health | |
| Uster 2013 | Appetite (DRAQ), self‐perceived state of health (VAS; scores 0‐100). |
|
| Vivanti 2015 | LOS, number of falls | depression |
| Dietary advice plus supplements versus no advice and no supplements ‐ group 5 | ||
| Anbar 2014 | Cumulative energy balance | New pressure sores; complications |
| Baldwin 2011 | ||
| Berneis 2000 | Lean body mass, fat mass | CD4 and CD8 countsbiochemical measurements and inflammatory markers (TNF R55, TNF R75, ILR2), |
| Calegari 2011 | Lean body mass and fat mass, biochemical measurements. Tolerance of ONS | |
| Chandra 1985 | Serum prealbumin levels and number achieving seroconversion | |
| Jahnavi 2010 | Sputum conversion and treatment completion rates | |
| Neelemaat 2011 | Adherence; fat free mass | |
| Paton 2004 | Lean body mass and fat mass | |
| Payette 2002 | ||
| Persson 2007 | Peak expiratory flow | |
| Um 2014 | Nutritional status (PG‐SGA) | |
| Wyers 2013 | Post‐operative complications | |
CD4: (cluster differentiation 4) cells of T‐mediated immune system C/R: colorectal FBC: GDS: H&N: head and neck HbA1C: ILR2: interlukin R2 LAS: lymphadenopathy syndrome LOS: MMS: MMSE: ONS: PG‐SGA: PU: SGA: TIBC: TNF R55: Tumour necrosis factor R55 TNF R75: Tumour necrosis factor R75 U&Es:
2. Summary of additional functional outcomes reported in included studies.
| Study | Functional measures (physical) | Functional measures (status) | notes |
| Dietary advice versus no advice ‐ group 1 | |||
| Alo 2014 | |||
| Baldwin 2011 | |||
| Campbell 2008 | |||
| Cano‐Torres 2017 | |||
| Casals 2015 | Barthel index | mean change scores for intervention and control | |
| Dixon 1984 | Karnofsky scale | Pre‐ and post‐intervention (0 and 4 months) | |
| Fernandez‐Barres 2017 | Degree of dependency (Barthel) Cognitive function (Pfieffer's) Mood (Yesavage Depression Scale) |
mean(SD) baseline and end of intervention (6 months) | |
| Forster 2012 | Geriatric Depression Score | Data reported as not different between groups | |
| Gu 2015 | |||
| Imes 1988 | |||
| Kunvik 2018 | |||
| Locher 2013 | |||
| Macia 1991 | |||
| Manguso 2005 | |||
| Ollenschlager 1992 | |||
| Pivi 2011 | |||
| Ravasco 2005a (C/R) | |||
| Ravasco 2005b (H&N) | |||
| Rydwik 2008 | Timed up and go Number of step‐ups in 30 seconds Walking speed over 10 m Modified figure of 8 test |
Functional independence measure Instrumental activities measure |
Between and within group differences in domain scores |
| Salva 2011 | ADL & iADL MMSE |
||
| Stow 2015 | |||
| Tu 2013 | |||
| Weekes 2009 | Respiratory muscle function (Pimax, Pe max) Respiratory function (FEV1 & FVC) |
ADL score Dyspnoea score |
|
| Wong 2004 | |||
| Dietary advice versus supplements ‐ group 2 | |||
| Akpele 2004 | |||
| Baldwin 2011 | |||
| Gray‐Donald 1995 | |||
| Hernandez 2014 | |||
| Kalnins 2005 | Respiratory function (FEV1) | ||
| Parsons 2016 | |||
| Pivi 2011 | |||
| Ravasco 2005a (C/R) | |||
| Ravasco 2005b (H&N) | |||
| Schwenk 1999 | |||
| Singh 2008 | |||
| Stow 2015 | |||
| Dietary advice versus dietary advice and supplements ‐ group 3 | |||
| Arnold 1989 | |||
| Baldwin 2011 | |||
| Beattie 2000 | |||
| Burden 2011 | |||
| Burden 2017 | |||
| de Luis 2003 | |||
| de Sousa 2012 | Barthel index | ||
| Diouf 2016 | |||
| Dixon 1984 | Karnofsky scale | Pre‐ and post‐intervention (0 and 4 months) | |
| Fuenzalida 1990 | Respiratory function (FEV1 & FVC) | Self‐assessment of lung function | |
| Gonzalez‐Espinoza 2005 | |||
| Huynh 2015 | |||
| Kapoor 2017 | Physical activity questionnaire, MET | ||
| Kendell 1982 | |||
| Le Cornu 2000 | |||
| McCarthy 1999 | |||
| Murphy 1992 | |||
| Norman 2008b | Respiratory function (PEF) | ||
| Olejko 1984 | |||
| Rabeneck 1998 | Cognitive function (Buschke selective reminding test) | ||
| Sharma 2002a | |||
| Swaminathan 2010 | |||
| Wilson 2001 | |||
| Dietary advice plus supplements if required versus no advice ‐ group 4 | |||
| Andersson 2017 | |||
| Banks 2016 | |||
| Beck 2012 | Chair stand | Mobility (DEMMI), rehabilitation capacity, Functional Recovery Score | from baseline to 3 months |
| Beck 2015 | Chair stand | Mobility and ADL | from baseline to 3 months |
| Bonilla‐Palomas 2016 | |||
| Bourdel‐Marchasson 2014 | |||
| Caccialanza 2015 | Performance status (ECOG) | from baseline to 12 months | |
| Carey 2013 | |||
| Endevelt 2011 | ADL (Barthel) | from baseline 6 months | |
| Evans 1987 | |||
| Feldblum 2011 | ADL (Barthel) | from baseline 6 months | |
| Forli 2001 | |||
| Ganzoni 1994 | 6 minute walking distance | ||
| Hampson 2003 | |||
| Holyday 2012 | |||
| Isenring 2004 | |||
| Jensen 1997 | Respiratory function (FEV1 & FVC) | Ordinal fatigue scale Lambert disability screening questionnaire |
Mean scores at baseline and 4 months |
| Kiss 2016 | Functional status | ||
| Lovik 1996 | |||
| Moloney 1983 | |||
| Ovesen 1993 | |||
| Persson 2002 | ADL, cognitive function, peak expiratory flow | ||
| Pedersen 2016a and Pedersen 2016b | Chair stand test | Geriatric Depression Score, ADL, Avlund mobility tiredness score | |
| Rogers 1992 | Respiratory muscle function (Pimax, Pe max) 12 minute walking distance |
Perceived dyspnoea (Borg) | |
| Schilp 2013 | Short Physical Performance Battery |
from baseline to 3 and 6 months | |
| Sharma 2017 | |||
| Silvers 2014 | |||
| Starke 2011 | |||
| Suominen 2015 | Rate of falls | ||
| Terp 2018 | Barthel index | ||
| Uster 2013 | Performance status | ||
| Vivanti 2015 | Performance status (ECOG) Global Depression Score |
From baseline to 3 and 6 months | |
| Dietary advice plus supplements versus no advice and no supplements ‐ group 5 | |||
| Anbar 2014 | |||
| Baldwin 2011 | |||
| Berneis 2000 | |||
| Calegari 2011 | 6‐minute walk test | ||
| Chandra 1985 | |||
| Jahnavi 2010 | Sit‐to‐stand test | ||
| Neelemaat 2011 | LASA Functional Limitations Questionnaire and LASA Physical Activity Questionnaire; Short Physical Performance Battery | ||
| Paton 2004 | Sit‐to‐stand test | ||
| Payette 2002 | Sit‐to‐stand test; knee and elbow extensor tests | ||
| Persson 2007 | ADL (Katz) Cognitive function (MMSE) |
||
| Um 2014 | |||
| Wyers 2013 | |||
ADL: activities of daily living FEV1: forced expiratory volume at one second FVC: forced expiratory capacity MMSE: Mini mental state examination Pe max: maximal expiratory mouth pressure PEF: peak expiratory flow Pimax: maximal inspiratory mouth pressure
3. Summary of quality of life assessments made in included studies.
| Study | QOL instrument | notes |
| Dietary advice versus no advice ‐ group 1 | ||
| Alo 2014 | ||
| Baldwin 2011 | EORTC FAACT |
Mean change from baseline to 6 and 26 weeks |
| Campbell 2008 | KDQOL‐SF | Data provided by author on mean (SD) at baseline and 12 weeks for each component |
| Cano‐Torres 2017 | ||
| Casals 2015 | SF‐12 | Mean change from baseline to 6 months |
| Dixon 1984 | ||
| Fernandez‐Barres 2017 | ||
| Forster 2012 | SF‐36 | Mean change from baseline to 3 months obtained from author |
| Gu 2015 | ||
| Imes 1988 | ||
| Kunvik 2018 | ||
| Locher 2013 | ||
| Macia 1991 | ||
| Manguso 2005 | ||
| Ollenschlager 1992 | Subjective well‐being using Linear Analogue Self Assessment questionnaire (LASA) |
assessed in intervention group only |
| Pivi 2011 | ||
| Ravasco 2005a | EORTC | Mean change from baseline to 12 weeks plus additional data for longer term follow‐up provided in 2012 publication |
| Ravasco 2005b | EORTC | Mean change from baseline to 12 weeks plus additional data for longer term follow‐up provided in 2012 publication |
| Rydwik 2008 | ||
| Salva 2011 | ||
| Stow 2015 | EQ5D‐5: Dartmouth COOP Quality of life chart |
not reported because of completion by too few residents |
| Tu 2013 | ||
| Weekes 2009 | SF‐36 SGRQ |
Mean change from baseline to 6 and 12 months |
| Wong 2004 | ||
| Dietary advice versus supplements ‐ group 2 | ||
| Akpele 2004 | ||
| Baldwin 2011 | EORTC FAACT |
Mean change from baseline to 6 and 26 weeks |
| Gray‐Donald 1995 | General self‐perceived health question General well‐being schedule |
Mean scores for both groups at baseline and 12 weeks |
| Hernandez 2014 | ||
| Kalnins 2005 | ||
| Parsons 2016 | EuroQol/EQ‐5d | scores reported as baseline scores for both intervention groups combined and mean (SE) after 6 weeks and 12 weeks by intervention group. |
| Pivi 2011 | ||
| Ravasco 2005a (C/R) | EORTC | Mean change from baseline to 12 weeks plus additional data for longer term follow‐up provided in 2012 publication |
| Ravasco 2005b (H/N) | EORTC | Mean change from baseline to 12 weeks plus additional data for longer term follow‐up provided in 2012 publication |
| Schwenk 1999 | ||
| Singh 2008 | ||
| Stow 2015 | EQ5D‐5: Dartmouth COOP Quality of life chart |
not reported because of completion by too few residents |
| Dietary advice versus dietary advice and supplements ‐ group 3 | ||
| Arnold 1989 | ||
| Baldwin 2011 | EORTC FAACT |
Mean change from baseline to 6 and 26 weeks |
| Beattie 2000 | SF‐36 | Summary and mean change scores for physical and mental health at baseline and final assessment |
| Burden 2011 | ||
| Burden 2017 | ||
| de Luis 2003 | ||
| de Sousa 2012 | ||
| Diouf 2016 | ||
| Dixon 1984 | ||
| Fuenzalida 1990 | ||
| Gonzalez‐Espinoza 2005 | ||
| Huynh 2015 | ||
| Kapoor 2017 | EORTC‐C30 | mean change from baseline to 3 months and 6 months |
| Kendell 1982 | ||
| Le Cornu 2000 | ||
| McCarthy 1999 | ||
| Murphy 1992 | ||
| Norman 2008b | SF‐36 | Mean scores for QALYs for all domains at baseline and 3 months |
| Olejko 1984 | ||
| Rabeneck 1998 | 30‐item QOL instrument (not validated) | |
| Sharma 2002a | ||
| Swaminathan 2010 | ||
| Wilson 2001 | ||
| Dietary advice plus supplements if required versus no advice ‐ group 4 | ||
| Andersson 2017 | EQ‐5D | Mean change from baseline to 3 months in all 5 domains |
| Banks 2016 | ||
| Beck 2012 | ||
| Beck 2015 | EQ‐5D | Mean change from baseline to 3 months |
| Bonilla‐Palomas 2016 | ||
| Bourdel‐Marchasson 2014 | ||
| Caccialanza 2015 | SF‐36 | Mean change from baseline to 12 months for PCS and MCS |
| Carey 2013 | EORTC‐QLQ‐C30 | Mean change from baseline to 3 months and 6 months |
| Endevelt 2011 | ||
| Evans 1987 | ||
| Feldblum 2011 | ||
| Forli 2001 | ||
| Ganzoni 1994; | ||
| Hampson 2003 | ||
| Holyday 2012 | ||
| Isenring 2004 | EORTC QLQ‐C30 | Mean change from baseline to 12 weeks |
| Jensen 1997 | QOL index | Means values at baseline and 4 months |
| Kiss 2016 | FACT‐L | From baseline to 3 months following completion of radiation therapy |
| Lovik 1996 | ||
| Moloney 1983 | ||
| Ovesen 1993 | QOL index (modified) | Mean scores at baseline and 3 and 5 months |
| Pedersen 2016a; Pedersen 2016b | SF‐36 | Mean change of subscores from baseline to eight weeks |
| Persson 2002 | EORTC | Mean scores at baseline and 24 months |
| Rogers 1992 | Sickness Impact Profile | Change after 4 months |
| Schilp 2013 | EQ‐5D | Results reported as QALY |
| Sharma 2002a | EQ‐5D | Mean change from baseline to 3 months |
| Silvers 2014 | EORTC‐QLQ‐C30 and EQ‐5D | Mean change from baseline to 6 months |
| Starke 2011 | ||
| Suominen 2015 | HRQoL | Mean change from baseline to 12 months |
| Terp 2018 | ||
| Uster 2013 | EORTC‐C30 | Mean change from baseline to 3 and 6 months |
| Vivanti 2015 | EQ‐5D | Mean change from baseline to 12 weeks |
| Dietary advice plus supplements versus no advice and no supplements ‐ group 5 | ||
| Anbar 2014 | Not reported | |
| Baldwin 2011 | EORTC FAACT |
Mean change from baseline to 6 and 26 weeks |
| Berneis 2000 | Medical Outcomes Study Instrument (adapted for use in patients with HIV infection) | Summary scores (physical function, social role, mental health and pain) at baseline and 12 weeks. Scores range from 1 to 6 with 6 being the optimal score. |
| Calegari 2011 | SF‐36 | Baseline and end of Phase 1 (3 months) scores for all eight domains (physical role functioning, bodily pain, physical functioning, general health, vitality, social functioning, role emotional and mental health). |
| Chandra 1985 | Not reported | |
| Hampson 2003 | Not reported | |
| Jahnavi 2010 | 36‐item Medical Outcomes Study Short form (adapted for use in patients with HIV infection) i.e. SF‐36 (modified) | Baseline scores and mean changes at 3 months for all eight domains (physical role functioning, bodily pain, physical functioning, general health, vitality, social functioning, role emotional and mental health) |
| Neelemaat 2011 | SF‐12 and 3‐level EuroQol‐5D (EQ‐5D) | EQ‐5D was used in the cost effectiveness analysis; QALYs at baseline and difference between groups at 3 months |
| Paton 2004 | 30‐item Medical Outcomes Study Short form (adapted for use in patients with HIV infection) i.e. SF‐36 (modified) | Baseline scores and mean changes at 6, 12 and 24 weeks for all domains |
| Payette 2002 | 36‐item SF‐36 | Scores at baseline and 16 weeks for three domains (physical role functioning, emotional role functioning and vitality) |
| Persson 2007 | SF‐36 | Summary scores at baseline and 4 months for physical and mental domains.Graphical presentation of changes in all domain scores over time. |
| Um 2014 | 30‐item EORTC | Scores at baseline, end of radiotherapy and 1 month follow‐up for function and symptom scales plus global health status |
| Wyers 2013 | 3‐level EQ‐5D | Used in the cost effectiveness analysis; intervention effect for change in QALYs |
EORTC: European organisation for research and treatment of cancer FAACT: functional assessment anorexia‐cancer therapy KDQOL‐SF: Kidney Disease Quality of Life Short Form QOL: quality of life SF‐36: 36‐item Short Form Medical Outcomes Study Instrument SGRQ: St George respiratory questionnaire
Five studies included comparisons in two parts of the review (Dixon 1984; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Stow 2015) and one study included comparisons in four parts of the review (Baldwin 2011). The participants in the studies were from a variety of clinical backgrounds. The length of intervention varied between studies; 55 (58.6%) of the 94 included studies presented interventions that were given for up to three months, 30 (32%) studies gave the intervention for up to six months and seven (3%) studies gave an active intervention for seven months or longer. In two of the studies the length of intervention was unclear (Macia 1991a; Tu 2013). The study by Persson appears to describe an intervention that lasts for up to two years (Persson 2002). Data at 3, 6, 12 and 24 months have been used in this review.
28 studies provided data on additional follow‐up beyond the intervention for some outcomes for between six months and five years (Arnold 1989; Baldwin 2011; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014 Burden 2011; Burden 2017; Cano‐Torres 2017; de Sousa 2012; Evans 1987; Feldblum 2011; Forster 2012; Holyday 2012; Jahnavi 2010; Kalnins 2005; Le Cornu 2000; Moloney 1983; Neelemaat 2011; Olejko 1984; Paton 2004; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Starke 2011; Weekes 2009; Wilson 2001; Wyers 2013).
Across the studies, it was originally unclear how grip strength had been measured as the units of measurement were described slightly differently. After consultation with a Professor of Applied Physiology, the review authors have decided that the studies have all reported kg, with some calling it force and others kg force. They have therefore decided to present these data in the analysis with the unit of measurement denoted as kg force.
1. Dietary advice compared with no advice
The review authors identified 24 studies (3523 participants) for this comparison (Alo 2014; Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Macia 1991a; Manguso 2005; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Tu 2013; Weekes 2009; Wong 2004), with a paper by Ravasco published in 2012 describing additional follow‐up to a median of 6.5 (range 4.9 to 8.1) years from the 2005 study by Ravasco (Ravasco 2005a).
Study design
There were 18 RCTs of parallel design (Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Imes 1988; Kunvik 2018; Locher 2013; Macia 1991a; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Weekes 2009; Wong 2004), two were quasi‐RCTs of parallel design (Alo 2014; Gu 2015), one was a cross‐over RCT (Manguso 2005), two were cluster‐RCTs (Salva 2011; Stow 2015); the review authors were unable to determine the design of one study because there was insufficient detail (Tu 2013). All but one of the included studies were conducted in a single centre; the Baldwin study was multicentre (Baldwin 2011). Studies were globally diverse; the majority (14 out of 24 studies (58%)) were conducted in Europe (Baldwin 2011; Casals 2015; Fernandez‐Barres 2017; Forster 2012; Kunvik 2018; Macia 1991a; Manguso 2005; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Weekes 2009), three studies were conducted in Asia (Gu 2015; Tu 2013; Wong 2004), three in North America (Dixon 1984; Imes 1988; Locher 2013), two studies in South America (Cano‐Torres 2017; Pivi 2011), and one each in Africa (Alo 2014) and Australia (Campbell 2008). The source of funding was reported in 16 studies (Baldwin 2011; Campbell 2008; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Salva 2011; Stow 2015; Weekes 2009). Of these, 12 obtained funding from educational or charitable grants and four included some commercial funding. Three studies declared some funding from a company making commercial nutritional products (Forster 2012; Pivi 2011; Stow 2015) and one study was funded by Nestec Ltd. (Salva 2011). The duration of the intervention ranged from the length of hospital stay (Cano‐Torres 2017; Gu 2015), up to three months (Baldwin 2011; Campbell 2008; Forster 2012; Locher 2013; Ravasco 2005a; Ravasco 2005b; Rydwik 2008), four to six months (Alo 2014; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Imes 1988; Kunvik 2018; Manguso 2005; Ollenschlager 1992; Pivi 2011; Stow 2015; Weekes 2009; Wong 2004) and 12 months (Salva 2011). Two studies did not report clear information on the length of the intervention (Macia 1991a; Tu 2013). Seven studies included an additional follow‐up period beyond the end of the intervention (Baldwin 2011; Cano‐Torres 2017; Forster 2012; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Weekes 2009); these ranged from three months (Forster 2012; Ravasco 2005a; Ravasco 2005b) to a median of 6.5 (range 4.9 to 8.1) years (Ravasco 2005a).
Participants
The largest study included 946 participants (Salva 2011) and the smallest enrolled 19 participants (Dixon 1984).
The majority of studies included older participants with a mean age ranging from 57 years (Cano‐Torres 2017) to 76 years (Wong 2004). Three studies included younger adults; the participants in one study had a mean age of 34 to 35 years (Alo 2014) and two studies involved participants as young as 17 years (Imes 1988; Ollenschlager 1992).
20 studies reported the sex of participants; in four of these there were fewer (less than 45%) females than males overall (Baldwin 2011; Campbell 2008; Dixon 1984; Gu 2015) and in two studies this was the case in the intervention group only (Forster 2012; Manguso 2005). In 10 studies there were more (over 55%) females than males (Alo 2014; Cano‐Torres 2017; Fernandez‐Barres 2017; Kunvik 2018; Locher 2013; Pivi 2011; Ravasco 2005a; Salva 2011; Stow 2015; Wong 2004); in three of these there were over 82% females (Locher 2013; Stow 2015; Wong 2004). The remaining studies included approximately equal numbers of males and females (45% to 55%). The age of participants was reported in 22 out of 24 studies as either mean (SD) age, median (interquartile (IQR) range), a range or as a single figure (over 75 years in one study (Rydwik 2008)).
The participants in studies had a wide variety of clinical conditions. Six studies were in people with cancer (Baldwin 2011; Dixon 1984; Macia 1991a; Ravasco 2005a; Ravasco 2005b; Tu 2013), five were in older people (Fernandez‐Barres 2017; Forster 2012; Locher 2013; Rydwik 2008: Stow 2015), in one of which participants were in residential care (Stow 2015). Three studies enrolled participants from mixed clinical backgrounds (Cano‐Torres 2017; Casals 2015; Gu 2015), two were in people with Alzheimer's disease or dementia (Pivi 2011; Salva 2011), one was in people living with HIV infection (Alo 2014), one was in people with Crohn's disease (Imes 1988), one in people at risk of osteoporotic fractures (Wong 2004), one in people with chronic obstructive pulmonary disease (COPD) (Weekes 2009), one in people with liver cirrhosis (Manguso 2005), one in people with chronic kidney disease (Campbell 2008) and one in carers (Kunvik 2018). The study setting varied with three studies beginning the intervention in hospital and continuing it into the community (Cano‐Torres 2017; Tu 2013; Wong 2004), two were conducted in hospital only (Gu 2015; Ollenschlager 1992), and the remainder were in outpatients or people living in the community. Three studies involved advice provided to groups of carers and participants via an educational intervention (Fernandez‐Barres 2017; Pivi 2011; Salva 2011).
The nutritional status of participants at baseline was assessed in 18 of 24 studies, but the method of assessment varied. Some investigators used validated tools including the SGA, which was used in four studies (Campbell 2008; Ravasco 2005a; Ravasco 2005b; Tu 2013), the Nutrition Risk Score (NRS)‐ 2002 which was used in two studies (Cano‐Torres 2017; Gu 2015), the MNA which was used in three studies (Fernandez‐Barres 2017; Kunvik 2018; Salva 2011) and the MUST which was used in two studies (Casals 2015; Stow 2015). The remaining studies assessed nutritional status using combinations of weight loss, BMI and changes in food intake (Alo 2014; Baldwin 2011; Dixon 1984; Locher 2013; Ollenschlager 1992; Rydwik 2008; Weekes 2009). 10 studies reported the BMI of participants at baseline (Alo 2014; Campbell 2008; Cano‐Torres 2017; Gu 2015; Kunvik 2018; Manguso 2005; Salva 2011; Stow 2015; Weekes 2009; Wong 2004); the mean BMI was in the normal range in five studies (Alo 2014; Gu 2015; Stow 2015; Weekes 2009; Wong 2004), in the overweight range for four studies (Campbell 2008; Kunvik 2018; Manguso 2005; Salva 2011) and in one study the mean BMI in the intervention (advice) group was in the normal range but participants in the no advice group had a mean BMI in the overweight range (Cano‐Torres 2017). Eight studies assessed all participants as malnourished or at nutritional risk (Baldwin 2011; Casals 2015; Dixon 1984; Gu 2015; Ollenschlager 1992; Rydwik 2008; Stow 2015; Weekes 2009), and six studies included some malnourished or at risk participants (Campbell 2008; Kunvik 2018; Locher 2013; Ravasco 2005a; Ravasco 2005b; Salva 2011). The remaining studies did not report how many participants were malnourished.
Interventions
All participants in the intervention group received dietary instruction, but the nature and intensity varied. In 15 out of 24 studies, authors specify that a dietitian delivered the dietary intervention (Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Dixon 1984; Imes 1988; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Weekes 2009; Wong 2004) or a trained nutritionist (Alo 2014; Kunvik 2018). In three studies nurses gave the intervention (Casals 2015; Dixon 1984; Fernandez‐Barres 2017), in one study it was given by doctors from the nutrition and dietetics department (Macia 1991a) and in another study by a postdoctoral researcher (Forster 2012). In three studies the role or occupation of the person delivering the intervention was not stated (Gu 2015; Pivi 2011; Tu 2013). In 14 out of 24 studies, investigators described the intervention as either individualised or personalised (Alo 2014; Campbell 2008; Cano‐Torres 2017; Casals 2015; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Macia 1991a; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Tu 2013; Weekes 2009) and one study included an individualised component following group sessions (Fernandez‐Barres 2017).
Description of arrangements for follow‐up varied with 18 studies incorporating plans for review or monitoring, or both, of participants but the frequency varied from daily follow‐up in hospitalised participants with acute leukaemia (Ollenschlager 1992) to three‐monthly follow‐up in outpatients with Crohn's disease (Imes 1988) and older individuals in residential care (Stow 2015). One study described bi‐weekly monitoring (Dixon 1984), three studies used a weekly review (Baldwin 2011; Ravasco 2005a; Ravasco 2005b), three studies monitored on a monthly basis (Alo 2014; Fernandez‐Barres 2017; Manguso 2005) and four studies described monitoring and review at varying time intervals throughout the study (Campbell 2008; Casals 2015; Locher 2013; Weekes 2009). Forster stated that investigators conducted follow‐up and monitoring at the same interval in both the intervention and control group, but did not report the interval (Forster 2012); and in the study by Wong there was just one additional review following the initial consultation lasting 15 minutes (Wong 2004). Six studies did not describe any plans for review and monitoring (Cano‐Torres 2017; Gu 2015; Macia 1991a; Pivi 2011; Tu 2013; Rydwik 2008). One of the studies employed an educational intervention to deliver the dietary advice described a voluntary sign‐up system for monitoring (Salva 2011). In one study, follow‐up occurred via optional attendance at group discussion and cooking sessions (Kunvik 2018) and in a further study, where the participants received the intervention from case‐manager nurses, there was a protocol for monitoring with a decision‐tree approach to identify participants who needed referral to a dietitian (Casals 2015).
Outcomes
Not all studies contributed data on all outcomes and data for only some outcomes were available for review authors to enter into the analyses; no study reported on costs. Two studies were unable to contribute data on any outcomes to this review because of how investigators reported data (Dixon 1984; Tu 2013). Additional data were obtained on request from the study investigators or have been derived by imputation (Table 9).
4. Dietary advice compared with no advice: outcomes with additional data provided or imputed.
| Study ID | Outcomes where the author provided data | Outcomes where data were imputed |
| Alo 2014 | BMI | |
| Baldwin 2011 | Mortality, weight, energy intake, handgrip strength, QoL scores | |
| Campbell 2008 | Weight, BCM, protein intake, QoL scores | SD of change for global QoL and protein intake. |
| Cano‐Torres 2017 | BMI, MAC | |
| Fernandez‐Barres 2017 | mean change (SD) weight, BMI, energy and protein intake | |
| Forster 2012 | Mean change (SD) weight, MAC, TSF, QoL and infections | |
| Kunvik 2018 | BMI, protein intake | |
| Locher 2013 | mean change (SD) calculated from individual patient data reported in the paper. | |
| Macia 1991 | weight, BMI, MAC, MAMC, TSF; SD of change imputed using a correlation co‐efficient of 0.8. | |
| Manguso 2005 | Weight, MAMC, TSF, energy intake | |
| Pivi 2011 | ||
| Ravasco 2005a | Mortality, weight, QoL scores | |
| Ravasco 2005b | Mortality, weight, QoL scores | |
| Rydwik 2008 | Weight, energy intake | |
| Stow 2015 | Mortality, hospital admissions | |
| Weekes 2009 | Weight, MAC, MAMC, FFM, TSF, handgrip strength, SGRQ, SF‐36 | |
| Wong 2004 | Mortality |
BCM: body cell mass BMI: body mass index FFM: fat‐free mass MAC: mid‐arm circumference MAMC: mid‐arm muscle circumference QoL: quality of life SD: standard deviation SF‐36: Short‐Form 36 SGRQ: St George's Respiratory Questionnaire TSF: triceps skinfold thickness
Primary outcomes
Mortality
17 out of 24 studies reported mortality data (Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Imes 1988; Manguso 2005; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Weekes 2009; Wong 2004); one study reported longer‐term follow‐up data in a separate paper (Ravasco 2005a). One study did not report mortality by group, so is not included in the analyses (Dixon 1984) and there were no events in five studies (Imes 1988; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Wong 2004).
Morbidity
Investigators used hospital readmissions, length of stay and complications to assess morbidity. Data on the number of people admitted to hospital were available from five studies (Casals 2015; Fernandez‐Barres 2017; Imes 1988; Stow 2015; Weekes 2009). However, the study by Casals reported data as mean (SD) admissions per group and mean (SD) days of admission and so the review authors could not combine this with the number of admissions per group data from the other studies. Data on length of hospital stay were available from four studies (Cano‐Torres 2017; Casals 2015; Gu 2015; Weekes 2009). Data on complications were available from two studies (Forster 2012; Gu 2015). Complications were self‐reported in a symptom diary in one study (Forster 2012) and as a complications score which is part of the NRS‐2002 in a further study (Gu 2015). The review authors combined these data using the SMD.
Nutritional status
Data on change in weight were available from 21 studies (Baldwin 2011; Campbell 2008; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Locher 2013; Macia 1991a; Manguso 2005; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Tu 2013; Weekes 2009; Wong 2004), for change in BMI from 11 studies (Alo 2014; Cano‐Torres 2017; Casals 2015; Fernandez‐Barres 2017; Forster 2012; Kunvik 2018; Macia 1991a; Pivi 2011; Salva 2011; Stow 2015; Wong 2004), data on change in FFM from three studies (Campbell 2008; Rydwik 2008; Weekes 2009), data on MAC from six studies (Cano‐Torres 2017; Forster 2012; Macia 1991a; Pivi 2011; Stow 2015; Weekes 2009), change in MAMC from five studies (Macia 1991a; Manguso 2005: Pivi 2011; Stow 2015; Weekes 2009) and data on change in TSF from six (Forster 2012; Macia 1991a; Manguso 2005: Pivi 2011; Stow 2015; Weekes 2009). The Pivi study reports data as mean and median change with a range; the review authors have not been able to obtain additional data from investigators and the data are not similar enough to other studies for imputation and so are reported narratively (Pivi 2011).
Secondary outcomes
Nutritional outcomes
17 studies provided data on energy intake (Baldwin 2011; Campbell 2008; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Manguso 2005; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Stow 2015; Tu 2013; Weekes 2009; Wong 2004). Two studies do not report data in a format that allows entry into a meta‐analysis (Ollenschlager 1992; Tu 2013); one study reported data as a composite measure (Tu 2013) and the second study reported intake in the intervention group only (Ollenschlager 1992). Furthermore, the Ravasco study reported follow‐up data as medians in the 2012 paper (Ravasco 2005a). 12 studies reported the change in energy intake (Baldwin 2011; Campbell 2008; Fernandez‐Barres 2017; Forster 2012; Kunvik 2018; Locher 2013; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Stow 2015; Wong 2004) and three studies analysed final intake values (Gu 2015; Imes 1988; Weekes 2009).
10 studies provided data on the change in protein intake (Campbell 2008; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Kunvik 2018; Manguso 2005; Stow 2015; Weekes 2009; Wong 2004). Manguso reported protein intake as a percentage of energy intake and original data were not available from the author (Manguso 2005). Five studies report the change in protein intake (Fernandez‐Barres 2017; Forster 2012; Kunvik 2018; Stow 2015; Wong 2004) and four studies presented final intake values (Campbell 2008; Gu 2015; Imes 1988; Weekes 2009). Ravasco reported the median intake in the 2012 follow‐up paper and so these are not included in the meta‐analysis (Ravasco 2005a).
Clinical and physical functional outcomes
For the 2021 update, the review authors have summarised the data on handgrip strength in meta‐analyses as this is the most frequently reported functional outcome across studies. For this group, two studies provided data on handgrip strength (Stow 2015; Weekes 2009). A summary of other clinical and functional outcomes reported is provided in the additional tables (Table 6; Table 7).
QoL
Seven studies provided data on QoL (Baldwin 2011; Campbell 2008; Casals 2015; Forster 2012; Ravasco 2005a; Ravasco 2005b; Weekes 2009), with one study reporting data collected using both the Short Form‐36 (SF‐36) and the St George's Respiratory Questionnaire (SGRQ) (Weekes 2009). The review authors have only entered data for global QoL scores into a meta‐analysis.
2. Dietary advice compared with ONS
The review authors identified 12 studies (852 participants) for this comparison and obtained additional data from all investigators (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015). The study by Kalnins includes 13 participants, of whom only five are older than 16 years of age; the review authors obtained individual patient data from the lead investigator for inclusion in this review (Kalnins 2005). The 2012 paper by Ravasco described additional study follow‐up to a median of 6.5 (range 4.9 to 8.1) years on participants described in an earlier paper (Ravasco 2005a).
Study design
The review authors included 10 RCTs (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008), one quasi‐RCT (Kalnins 2005), and one was a cluster‐RCT (Stow 2015). 11 studies were single‐centre and one was multicentre (Baldwin 2011). The majority of studies (seven out of 12 (54%) were conducted in Europe (Baldwin 2011; Hernandez 2014; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Stow 2015), three were conducted in North America (Akpele 2004; Gray‐Donald 1995; Kalnins 2005), one in South America (Pivi 2011) and one in India (Singh 2008). The source of funding was reported in 11 studies (Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015); seven obtained funding from educational or charitable grants, of which three included some commercial funding or provision of nutritional products from a company making ONS (Baldwin 2011; Pivi 2011; Stow 2015). Companies making nutritional products funded three studies (Kalnins 2005; Parsons 2016; Schwenk 1999).
The duration of the intervention ranged from up to three months (Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008) to four to six months (Akpele 2004; Hernandez 2014; Pivi 2011; Stow 2015). Three studies included an additional follow‐up period beyond the end of intervention which varied from three months (Ravasco 2005a; Ravasco 2005b) to 11 months (46 weeks) (Baldwin 2011). Additional follow‐up data from one of the studies reported follow up to a median of 6.5 (range 4.9 to 8.1) years (Ravasco 2005a).
Participants
The largest study in this comparison included 176 participants (Baldwin 2011) and the smallest five participants (Kalnins 2005).
In 11 of 12 studies, the age of participants was reported as either mean (SD) age, median (IQR range), a range or as an average without SD or range. Stow did not report age, but the study was conducted in a residential care home, so participants were likely to be in their eighties (Stow 2015). The majority of studies included older participants with a mean (SD) age ranging from 58 (15) years (Ravasco 2005a) to 89.6 (6.9) years (Wong 2004). Three studies involved younger adults (Kalnins 2005; Schwenk 1999; Singh 2008); two studies had participants with a mean age of 28 (10) years to 39.5 (10.2) years (Schwenk 1999; Singh 2008) and the third study included young adults (over 16 years) (Kalnins 2005).
Sex was reported in 10 of 12 studies. In four studies there were fewer females than males overall (less than 45%) (Baldwin 2011; Hernandez 2014; Schwenk 1999; Singh 2008). In the remaining six studies, there were more females than males (over 55%) (Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Stow 2015) with two studies including over 85% females (Parsons 2016; Stow 2015) and a third study had solely female participants (Kalnins 2005).
The participants in studies had a wide variety of clinical conditions. Three studies were in people with cancer (Baldwin 2011; Ravasco 2005a; Ravasco 2005b), two in people with chronic kidney disease (Akpele 2004; Hernandez 2014), three were in older people (Gray‐Donald 1995; Parsons 2016; Stow 2015), of which two were in older people in residential care (Parsons 2016; Stow 2015), the study by Gray‐Donald was in older people living at home. One study was in people with Alzheimer's disease or dementia (Pivi 2011), one was in people living with HIV/AIDS (Schwenk 1999), one was in people with cystic fibrosis (Kalnins 2005) and one in people with chronic pancreatitis (Singh 2008). Most (11 out of 12) studies were in outpatients or people living in the community and one study was in a hospital dialysis unit (Hernandez 2014). One study involved advice provided to groups of carers and participants via an educational intervention (Pivi 2011).
In 12 out of 13 studies, investigators assessed the nutritional status of participants at baseline; the Pivi study did not report the nutritional status of participants at baseline (Pivi 2011). The method of assessment varied. Some investigators used validated tools including the Patient Generated Subjective Global Assessment (PG‐SGA) which was used in two studies (Ravasco 2005a; Ravasco 2005b), MNA in one study (Akpele 2004) and the MUST in two studies (Parsons 2016; Stow 2015). The seven remaining studies used combinations of weight loss and BMI to assess nutritional status (Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Schwenk 1999; Singh 2008), with two studies in people with chronic kidney disease using serum albumin (Akpele 2004; Hernandez 2014). Five studies reported BMI of participants at baseline (Gray‐Donald 1995; Hernandez 2014; Schwenk 1999; Singh 2008; Stow 2015); the mean BMI was in the normal range for all groups in two studies (Gray‐Donald 1995; Schwenk 1999), in the overweight range in one study (Hernandez 2014) and in the underweight range in one study (Singh 2008). In one study the mean BMI in the dietary counselling group was in the normal range, but participants in the ONS group had a mean BMI in the underweight range (Stow 2015). All participants were assessed as malnourished or at nutritional risk in eight studies (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Schwenk 1999; Singh 2008; Stow 2015), and three studies included some malnourished or at risk participants (Hernandez 2014; Ravasco 2005a; Ravasco 2005b). One study did not report on how many participants were malnourished (Pivi 2011).
Interventions
All participants in the intervention groups received either dietary instruction or an ONS but the nature, amount and intensity of support varied. In 11 of 12 studies, the authors specified that the dietary intervention was given by a dietitian and one study did not specify the role or occupation of the person who delivered the training that comprised the dietary instruction (Pivi 2011). Two out of 12 studies described the intervention as either individualised or personalised (Ravasco 2005a; Ravasco 2005b) and one study encouraged nursing staff in the care home to take the preferences of residents into consideration (Stow 2015). Three studies used a specially designed diet sheet to provide information on increasing intake from food (Baldwin 2011; Parsons 2016; Schwenk 1999) and two studies used education sessions to provide dietary information (Hernandez 2014; Pivi 2011). The remaining four studies described encouragement and counselling, but not whether this was standardised or individualised (Akpele 2004; Gray‐Donald 1995; Kalnins 2005; Singh 2008). Prescription of ONS varied in terms of the type of supplement used and the amount prescribed. Only two studies failed to specify the amount prescribed, as the prescription was individually tailored to the participants' requirements (Kalnins 2005; Schwenk 1999). The amounts in the remaining studies varied from 360 kcal/day (supplied as two cans of Nepro® three days a week during dialysis) in one study (Hernandez 2014) to up to 800 kcal/day (prescribed as one to two cans of Nepro® daily) in a further study (Akpele 2004). The majority of studies provided an average of 600 kcal/day from a range of different ONS.
The description of arrangements for follow‐up varied, with nine studies incorporating plans for review or monitoring of participants, or both, but the frequency ranged from weekly follow‐up (Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Ravasco 2005a; Ravasco 2005b) to three‐monthly review of food record charts in people living in residential care (Stow 2015). One study described monitoring at one and three months (Kalnins 2005), another at six weeks (Parsons 2016), and in a further study the monitoring arrangements likely varied by dialysis unit but “dietitians were encouraged to spend extra time each week with their patients reviewing dietary records” (Akpele 2004). Three studies did not describe any plans for review and monitoring (Pivi 2011; Schwenk 1999; Singh 2008).
Outcomes
Not all studies contributed data on all outcomes and data for only some outcomes were available to enter into the analyses; no study reported on costs. Two studies reported no data relevant to the outcomes of this review, with their primary outcome being change in serum albumin (Akpele 2004; Hernandez 2014). Additional data were obtained on request from the study investigators or have been derived by imputation for some outcomes (Table 10).
5. Dietary advice compared with oral nutritional supplements: outcomes with additional data provided or imputed.
| Study ID | Outcomes where the author provided data | Outcomes where data were imputed |
| Baldwin 2011 | Mortality, weight, energy intake, handgrip strength, QoL scores | |
| Gray‐Donald 1995 | Energy intake, handgrip strength | |
| Kalnins 2005 | Weight, energy intake | |
| Ravasco 2005a | Mortality, weight, QoL scores | |
| Ravasco 2005b | Mortality, weight, QoL scores | |
| Schwenk 1999 | Energy intake | |
| Singh 2008 | Weight, BMI, MAC, TSF, protein intake | |
| Stow 2015 | Mortality, hospital admissions |
BMI: body mass index; MAC: mid‐arm circumference; MAMC: mid‐arm muscle circumference; QoL: quality of life; SD: standard deviation; TSF: triceps skinfold thickness.format?
Primary outcomes
Mortality
Nine of 12 studies reported mortality data (Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Stow 2015). There were no events in four studies (Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999).
Morbidity
In this comparison, investigators only assessed morbidity by hospital readmissions and two studies provided data on the number of people admitted to hospital (Schwenk 1999; Stow 2015). While one study mentions collecting data on number of admissions, it does not report the data and the review authors have not been able to contact this investigator (Akpele 2004).
Nutritional status
10 studies provided data on change in weight (Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015), three studies reported change in BMI (Pivi 2011; Singh 2008; Stow 2015), one study provided data on change in FFM (Schwenk 1999), three studies provided data on MAC (Pivi 2011; Singh 2008; Stow 2015), three studies provided data on MAMC (Gray‐Donald 1995; Pivi 2011; Stow 2015) and three studies provided data on change in TSF (Gray‐Donald 1995; Pivi 2011; Stow 2015). One study reports data as mean and median change with a range and the review authors have not been able to obtain additional data from the study investigators; the data are not similar enough to other studies for imputation and so the review authors report them narratively (Pivi 2011).
Secondary outcomes
Nutritional outcomes
Nine studies provided data on energy intake (Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015) and six studies on change in protein intake (Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015). One study describes the collection of data on energy intake and protein intake, but did not report the results (Akpele 2004). Ravasco reported the 2012 follow‐up data for both energy and protein intake as medians which could not be combined in a meta‐analysis (Ravasco 2005a).
Clinical and physical functional outcomes
For the 2021 update, the review authors summarised the data on handgrip strength in meta‐analyses as this is the most frequently reported functional outcome across studies. Two studies provided data on handgrip strength for this comparison (Gray‐Donald 1995; Stow 2015). The review authors have presented a summary of other clinical and functional outcomes reported in the additional tables (Table 6; Table 7).
QoL
Six studies provided data on QoL (Baldwin 2011; Gray‐Donald 1995; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Stow 2015); however, Stow did not report the data collected because too few residents completed the assessment. The review authors have entered data for only global QoL scores into a meta‐analysis.
3. Dietary advice compared with dietary advice plus ONS
The review authors identified 22 studies (1286 participants) for this comparison (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Kendell 1982; Le Cornu 2000; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a; Wilson 2001).
Study design
There were 21 RCTs (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Kendell 1982; Le Cornu 2000; McCarthy 1999; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a; Wilson 2001), and one quasi‐RCT (Murphy 1992).
16 studies were conducted in a single centre (Arnold 1989; Beattie 2000; Burden 2011; de Luis 2003; de Sousa 2012; Diouf 2016; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kapoor 2017; Kendell 1982; Le Cornu 2000; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Sharma 2002a) and six were multicentre (Baldwin 2011; Burden 2017; Dixon 1984; Huynh 2015; Rabeneck 1998; Wilson 2001). Studies were globally diverse; nine out of 22 were conducted in Europe (Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Dixon 1984; Le Cornu 2000; Norman 2008b) and nine in North America (Arnold 1989; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kendell 1982; McCarthy 1999; Murphy 1992; Olejko 1984; Rabeneck 1998; Wilson 2001), three studies were conducted in India (Huynh 2015; Kapoor 2017; Sharma 2002a) and one in Africa (Diouf 2016). There were no studies from South America or Australia. The source of funding was declared in 16 studies (Beattie 2000; Burden 2011; Burden 2017; de Sousa 2012; Diouf 2016; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Le Cornu 2000; McCarthy 1999; Norman 2008b; Rabeneck 1998; Sharma 2002a; Wilson 2001). Of these five obtained funding from educational or charitable grants (Burden 2011; Burden 2017; Diouf 2016; Gonzalez‐Espinoza 2005; Wilson 2001) and one study was reported to be self‐funded (Kapoor 2017). Five studies declared funding from a company making commercial nutritional products (Beattie 2000; Huynh 2015; Norman 2008b; Rabeneck 1998; Sharma 2002a), and a further three studies declared that products were provided by the industry (de Sousa 2012; Le Cornu 2000; McCarthy 1999). The duration of intervention ranged from up to three months (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Huynh 2015; Kendell 1982; Le Cornu 2000; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a), to four to six months (Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kapoor 2017; Murphy 1992; Wilson 2001). Eight studies included an additional follow‐up period beyond the end of the intervention (Arnold 1989; Baldwin 2011; Burden 2011; Burden 2017; de Sousa 2012; Le Cornu 2000; Olejko 1984; Wilson 2001), which ranged from 90 days (de Sousa 2012) to over 12 months (Baldwin 2011). Five studies involved pre‐operative or pre‐transplant patients awaiting hospital admission (Burden 2011; Burden 2017; Kendell 1982; Le Cornu 2000; Olejko 1984), four were initiated at hospital admission and continued post‐discharge (Beattie 2000; Fuenzalida 1990; Huynh 2015; Norman 2008b), and 12 were set in outpatient departments for participants undergoing radiotherapy, chemotherapy, haemodialysis or HIV treatment (Arnold 1989; Baldwin 2011; de Luis 2003; Diouf 2016; Dixon 1984; Gonzalez‐Espinoza 2005; Kapoor 2017; McCarthy 1999; Murphy 1992; Rabeneck 1998; Sharma 2002a; Wilson 2001). Although not entirely clear, it seems that only one study was fully completed while the participants were hospitalised (de Sousa 2012).
Participants
The largest study included 212 participants (Huynh 2015) and the smallest nine participants (Fuenzalida 1990).
All studies reported the age of participants as either mean (SD) age, median (IQR range) or as a range. The majority of studies included middle‐aged participants with a mean age ranging from 23 years (Olejko 1984) to 79 years (de Sousa 2012).
All studies reported sex. In seven studies there were fewer females than males (less than 45%), with three studies only including males (Fuenzalida 1990; Murphy 1992; Rabeneck 1998). In 13 studies there were more females than males (over 55%) with one study including only females (Kapoor 2017). One study included 50% males and 50% females (Olejko 1984).
The participants in studies had a wide variety of clinical conditions. Five studies were in people with cancer (Arnold 1989; Baldwin 2011; Dixon 1984; Kapoor 2017; McCarthy 1999), five were in people undergoing surgery (pre‐operative or post‐operative) (Beattie 2000; Burden 2011; Burden 2017; Kendell 1982; Olejko 1984), four were in people living with HIV infection (de Luis 2003; Diouf 2016; Murphy 1992; Rabeneck 1998), one study was in people with COPD (Fuenzalida 1990), one in people with benign gastrointestinal disease (Norman 2008b), one in people with Alzheimer's disease (de Sousa 2012), one in people with end‐stage liver disease (Le Cornu 2000), three studies were in people with renal failure (Gonzalez‐Espinoza 2005; Sharma 2002a; Wilson 2001) and one in people from various medical and surgical wards (non‐cancer) (Huynh 2015).
The nutritional status of participants was assessed at baseline in 21 of 23 studies but the method of assessment varied. Investigators in two studies used the MNA (Beattie 2000, de Sousa 2012), in three studies the SGA (Burden 2011; Gonzalez‐Espinoza 2005; Norman 2008b), and a modified SGA in one study (Huynh 2015). One study used both PG‐SGA and the MUST (Burden 2017). The remaining studies used weight loss, ideal body weight and BMI or a combination of these to assess nutritional status (Baldwin 2011; de Luis 2003; Dixon 1984; Diouf 2016; Fuenzalida 1990; Kapoor 2017; Kendell 1982; Le Cornu 2000; Murphy 1992; Olejko 1984; Rabeneck 1998; Sharma 2002a; Wilson 2001). Eight studies reported the BMI of participants at baseline; the mean BMI was below 22 in seven out of nine studies (de Sousa 2012; Diouf 2016; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a). Values for BMI between 25 and 27 were reported in two studies (Burden 2011; Burden 2017). There were 14 studies which only included malnourished people or individuals who had at least experienced (any) weight loss (Baldwin 2011; de Luis 2003; de Sousa 2012; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Le Cornu 2000; Murphy 1992; Norman 2008b; Rabeneck 1998; Sharma 2017; Wilson 2001), four studies included both well‐nourished and malnourished participants (Beattie 2000; Burden 2011; Burden 2017; Diouf 2016). Two studies did not report the proportion of malnourished participants (Arnold 1989; McCarthy 1999) and two studies included well‐nourished participants only, who were suspected to become malnourished due to the nature of their disease (orthognathic surgery) (Kendell 1982; Olejko 1984).
Intervention
Participants in both groups received dietary instructions, and participants in the intervention groups also received ONS or other supplements made from local ingredients and judged to be comparable to the use of ONS (Diouf 2016; Gonzalez‐Espinoza 2005; Kapoor 2017). The nature and intensity of the dietary advice varied. In four studies, dietary advice consisted of pre‐operative written and verbal advice (Burden 2011; Burden 2017; Kendell 1982; Olejko 1984). It remained unclear whether nutritional or dietary advice was individualised or not as no further specifications were given in five studies, which described interventions as routine nutritional management (Beattie 2000), standard dietetic advice (de Sousa 2012), individually‐planned hospital diets (Fuenzalida 1990), or individual meal plans (Murphy 1992); in one study authors did not provide details (de Luis 2003). Other studies further specified dietetic advice as intensive nutritional counselling on a weekly basis (Arnold 1989), study dietitians giving verbal and written advice and follow‐up at outpatients appointments alongside weekly telephone calls (Baldwin 2011), bi‐weekly visits, during which a research nurse gave nutritional counselling (Dixon 1984), nutritional counselling to reach or increase individualized (protein and energy) goals (Gonzalez‐Espinoza 2005; Huynh 2015; Le Cornu 2000; Rabeneck 1998; Sharma 2002a; Wilson 2001), 30‐minutes dietary counselling sessions (Kapoor 2017), weekly meetings with the research nurse or dietitian to review dietary intake (McCarthy 1999) and standard dietary counselling session (Norman 2008b). In 11 studies, the authors specified that the dietary intervention was delivered by a dietitian or nutritionist (Baldwin 2011; de Sousa 2012; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; McCarthy 1999; Norman 2008b; Olejko 1984; Rabeneck 1998; Wilson 2001). In four studies, the intervention was given by nurses (Dixon 1984) or research assistants (Burden 2011; Burden 2017) or doctors (Diouf 2016). It remains unclear who gave the advice in seven studies (Arnold 1989; Beattie 2000; de Luis 2003; Kendell 1982; Le Cornu 2000; Murphy 1992; Sharma 2002a). In all studies, the focus of the intervention was on consumption of the supplements, which varied from ONS (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Dixon 1984; Fuenzalida 1990; Huynh 2015; Kendell 1982; Le Cornu 2000; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a; Wilson 2001) to a mixture of rice and porridge (Diouf 2016), a mixture of different flours (Kapoor 2017) or a dried‐egg albumin‐based supplement (Gonzalez‐Espinoza 2005). The intake of ONS varied from one can of the supplement (Burden 2017; de Sousa 2012) to approximately 1000 kcal/day (Arnold 1989; Rabeneck 1998) or to 50% of caloric requirements (Kendell 1982; Olejko 1984).
Outcomes
Not all studies contributed data on all outcomes and data were available to enter into the analyses for only some outcomes. Four studies reported no data relevant to the outcomes of this review or data were reported in a format meaning no outcomes could be included in the meta‐analyses (Dixon 1984; Kendell 1982; Le Cornu 2000; Olejko 1984). Some additional data were obtained on request from the study authors or have been derived by imputation (Table 11).
6. Dietary advice versus dietary advice plus oral nutritional supplements: outcomes with additional data provided or imputed.
| Study ID | Outcomes where the author provided data | Outcomes where data were imputed |
| Arnold 1989 | Weight read from a graph and SD of change derived by imputation | |
| Baldwin 2011 | Mortality, weight, grip strength, QoL scores | |
| Burden 2011 | Energy intake, protein intake | |
| Burden 2017 | Energy intake, protein intake, weight, grip strength | |
| de Luis 2003 | Energy intake, weight, MUAC, TSF | SD of change in FFM imputed from the study by Campbell 2008 |
| Diouf 2016 | Weight, FFM, body fat, BMI | |
| Fuenzalida 1990 | SD of change in weight, TSF, MAC derived by calculation from reported P values | |
| Gonzalez‐Espinoza 2005 | Energy intake, weight, MAC, MAMC | SD of change in BMI derived by imputation from Sharma 2002a. SD for TSF imputed using a correlation coefficient of 0.8 |
| Huynh 2015 | Weight, BMI, grip strength, energy intake, protein intake | |
| Kapoor 2017 | Weight, QoL scores, energy intake, protein intake, MUAC | |
| McCarthy 1999 | Energy & protein intake | |
| Murphy 1992 | SD of change in weight & energy intake calculated from reported P values | |
| Norman 2008b | Weight, MAMC, TSF | Data on body cell mass were used in the analysis of FFM and combined using the SMD |
| Sharma 2002a | SD of change in weight and BMI calculated from reported P values for one intervention group and then used to impute the SD of change for the second intervention group. Duplicate IDs have been used to include the two groups in the analysis. |
SD: standard deviation; BMI: body mass index; FFM: fat‐free mass; MAMC: mid‐arm muscle circumference; MAC: mid‐arm circumference; MUAC: mid‐upper arm circumferences; QoL: quality of life; TSF: triceps skinfold thickness. format?
Three studies presented data in a format that did not allow us to derive mean change with a SD (Dixon 1984; Kendell 1982; Olejko 1984).
Primary outcomes
Mortality
13 studies reported mortality data (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kapoor 2017; Le Cornu 2000; Murphy 1992; Norman 2008b).
Morbidity
Review authors assessed morbidity by hospital readmissions, length of stay and complications. Two studies provided data on the number of people admitted to hospital (Gonzalez‐Espinoza 2005; Norman 2008b). Six studies provided data on the length of hospital stay (Beattie 2000; Burden 2011; Burden 2017; Huynh 2015; Norman 2008b; Wilson 2001). Four studies provided data on complications (Beattie 2000; Burden 2011; Burden 2017; Gonzalez‐Espinoza 2005).
Nutritional status
A total of 14 studies reported change in weight (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kapoor 2017; Murphy 1992; Norman 2008b; Rabeneck 1998; Sharma 2002a), five studies reported the change in BMI (de Sousa 2012; Diouf 2016; Huynh 2015; Norman 2008b; Sharma 2002a), three studies reported the change in FFM (de Luis 2003; Diouf 2016; Norman 2008b), three studies reported MAC (de Luis 2003; Kapoor 2017; Murphy 1992), five studies reported MAMC (Beattie 2000; de Luis 2003; de Sousa 2012; Gonzalez‐Espinoza 2005; Kapoor 2017), and seven studies reported TSF (Beattie 2000; de Luis 2003; de Sousa 2012; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Norman 2008b; Rabeneck 1998).
Secondary outcomes
Nutritional outcomes
Nine studies reported the change in energy intake (Baldwin 2011; Burden 2011; Burden 2017; de Luis 2003; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; McCarthy 1999; Murphy 1992) and three studies provided data on final energy intake (Arnold 1989; Norman 2008b; Sharma 2002a). Three studies reported data on the change in protein intake (Burden 2017; Huynh 2015; Kapoor 2017) and five studies on final protein intake (Arnold 1989; de Luis 2003; McCarthy 1999; Norman 2008b; Sharma 2002a).
QoL
Five studies reported this outcome; two used the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires (Baldwin 2011, Kapoor 2017), two used the SF‐36 (in the study by Norman SF‐36 was used to calculate quality‐adjusted life years (QALYs)) (Norman 2008b; Beattie 2000), and one used a self‐developed, non‐validated questionnaire (Rabeneck 1998). The review authors only entered data into a meta‐analysis for global QoL scores.
Clinical and functional outcomes
For the 2021 update, the review authors have summarised data on handgrip strength in meta‐analyses as this is the most frequently reported functional outcome across studies. Six studies provided data on handgrip strength for this comparison (Beattie 2000; Burden 2017; de Sousa 2012; Huynh 2015; Norman 2008b; Rabeneck 1998). The review authors have provided a summary of other clinical and functional outcomes in the additional tables (Table 6; Table 7).
Costs
Only one study reported on costs, using QALYs and incremental cost‐effectiveness ratio (ICER) (Norman 2008b).
4. Dietary advice plus ONS, if required, compared with no advice and no ONS
The review authors identified 31 unique studies* (3308 participants) for this comparison (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Endevelt 2011; Evans 1987; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Jensen 1997; Kiss 2016; Lovik 1996; Moloney 1983; Ovesen 1993; Pedersen 2016a; Pedersen 2016b; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Uster 2013; Vivanti 2015).
*for analysis purposes they have set up a duplicate study ID for the 2016 Pedersen study (Pedersen 2016b).
Study design
All 31 RCTs had a parallel design and most were single‐centre studies; two studies were multicentre (Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014). The majority of studies (n = 20, 63%) were conducted in Europe (Andersson 2017; Baldwin 2011; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Forli 2001; Ganzoni 1994; Jensen 1997; Lovik 1996; Moloney 1983; Ovesen 1993; Pedersen 2016a; Pedersen 2016b; Persson 2002; Schilp 2013; Suominen 2015; Starke 2011; Terp 2018; Uster 2013), eight in Australia (Banks 2016; Holyday 2012; Isenring 2004; Kiss 2016; Sharma 2017; Silvers 2014; Vivanti 2015), two in North America (Evans 1987; Rogers 1992) and two in Asia (Endevelt 2011; Feldblum 2011). 27 studies reported the source of funding, all obtained funding from educational or charitable grants (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Endevelt 2011; Feldblum 2011; Hampson 2003; Holyday 2012; Isenring 2004; Jensen 1997; Kiss 2016; Lovik 1996; Ovesen 1993; Persson 2002; Rogers 1992; Schilp 2013; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Uster 2013; Vivanti 2015). Eight studies declared some funding from a company making commercial nutritional products (Holyday 2012; Isenring 2004; Jensen 1997; Rogers 1992; Starke 2011; Suominen 2015; Uster 2013).
The study setting varied with 22 studies beginning the intervention in hospital and continuing it into the community (Andersson 2017; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Carey 2013; Evans 1987; Feldblum 2011; Forli 2001; Holyday 2012; Isenring 2004; Jensen 1997; Kiss 2016; Pedersen 2016a; Pedersen 2016b; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002; Rogers 1992; Sharma 2017; Silvers 2014; Terp 2018), one was conducted in hospital only (Banks 2016 ) and the remaining eight studies were in outpatients or people living in the community (Caccialanza 2015; Endevelt 2011; Ganzoni 1994; Schilp 2013; Suominen 2015; Starke 2011; Uster 2013; Vivanti 2015).
The duration of intervention ranged from the length of the hospital stay (Banks 2016; Holyday 2012; Starke 2011), one to three months (Andersson 2017; Beck 2012; Beck 2015; Evans 1987; Forli 2001; Isenring 2004; Jensen 1997; Kiss 2016; Pedersen 2016a; Pedersen 2016b; Lovik 1996; Moloney 1983; Sharma 2017; Terp 2018; Uster 2013; Vivanti 2015), four to six months (Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Carey 2013; Endevelt 2011; Feldblum 2011; Ovesen 1993; Rogers 1992; Schilp 2013; Silvers 2014), one year (Caccialanza 2015; Ganzoni 1994; Suominen 2015) and up to two years (Persson 2002). Nine studies included an additional follow‐up period beyond the end of the intervention which ranged from three months (Feldblum 2011) to six months (Beck 2012; Beck 2015; Holyday 2012; Starke 2011), 12 months (Bonilla‐Palomas 2016; Moloney 1983), two years (Bourdel‐Marchasson 2014) and three to five years (Evans 1987).
Participants
The largest study included 336 participants (Bourdel‐Marchasson 2014) and the smallest 21 participants relevant to this comparison (Silvers 2014).
All but one study reported the age of participants as either mean (SD) age, median (IQR range) or as a range. The majority of studies included older participants with a mean age ranging from 49 years (Forli 2001) to 87 years (Terp 2018).
Sex was reported in 29 out of 31 studies. In 13 studies there were fewer females than males (less than 45%) (Banks 2016; Caccialanza 2015; Carey 2013; Endevelt 2011; Evans 1987; Isenring 2004; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002; Silvers 2014; Terp 2018; Uster 2013), in 11 studies there were more females than males (over 55%) (Andersson 2017; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Feldblum 2011; Holyday 2012; Pedersen 2016a; Pedersen 2016b; Sharma 2017; Schilp 2013; Suominen 2015; Vivanti 2015). Two studies included over 80% males (Isenring 2004; Lovik 1996). The remaining four studies included approximately equal numbers of males and females (45% to 55%) (Bourdel‐Marchasson 2014; Forli 2001; Jensen 1997; Kiss 2016).
The participants in studies had a wide variety of clinical conditions. Participants in nine studies were older adults (Beck 2012; Beck 2015; Endevelt 2011; Feldblum 2011; Holyday 2012; Pedersen 2016a; Pedersen 2016b; Schilp 2013; Sharma 2017; Terp 2018), nine studies were in people with cancer (Bourdel‐Marchasson 2014; Evans 1987; Isenring 2004; Kiss 2016; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002; Silvers 2014), three were in groups with mixed clinical backgrounds (Starke 2011; Uster 2013; Vivanti 2015), two in people with COPD (Ganzoni 1994; Rogers 1992), two in post‐surgical participants (Carey 2013; Jensen 1997), one in people with Alzheimer's disease (Suominen 2015), one was in people undergoing rehabilitation from a wide variety of chronic conditions (Andersson 2017), one was in people with heart failure (Bonilla‐Palomas 2016), one was in people with amyloidosis (Caccialanza 2015), one was in people with pressure ulcers (Banks 2016) and one in people undergoing lung transplantation (Forli 2001).
In 28 out of 31 studies, investigators assessed the nutritional status of participants at baseline, but the method of assessment varied. Seven studies used the MNA (Bourdel‐Marchasson 2014; Bonilla‐Palomas 2016; Endevelt 2011; Feldblum 2011; Holyday 2012; Pedersen 2016a; Suominen 2015), three studies used the SGA (Banks 2016; Carey 2013; Vivanti 2015), three studies used the PG‐SGA (Isenring 2004; Kiss 2016; Silvers 2014), six studies used the NRS‐2002 (Andersson 2017; Beck 2012; Beck 2015; Starke 2011; Terp 2018; Uster 2013) and one study used the Short Nutritional Assessment Questionnaire 65+ (SNAQ65+) (Schilp 2013). The remainder of studies used combinations of weight loss, ideal body weight and BMI to assess nutritional status (Caccialanza 2015; Evans 1987; Forli 2001; Ovesen 1993; Persson 2002; Rogers 1992). 25 studies reported the BMI of participants at baseline and the mean BMI was in the normal range for all groups (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Endevelt 2011; Feldblum 2011; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Pedersen 2016a; Pedersen 2016b; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015; Uster 2013) and in two studies the baseline BMI was low (Forli 2001; Terp 2018).
Seven studies included only malnourished participants (Bonilla‐Palomas 2016; Endevelt 2011; Feldblum 2011; Forli 2001; Holyday 2012; Schilp 2013; Vivanti 2015) (only data of malnourished participants in the Holyday study were included in this review), nine studies included participants assessed as malnourished or at nutritional risk (Andersson 2017; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Pedersen 2016a; Pedersen 2016b; Persson 2002; Rogers 1992; Sharma 2017; Terp 2018), and 12 studies included well‐nourished, malnourished or at risk participants (Banks 2016; Caccialanza 2015; Carey 2013; Evans 1987; Ganzoni 1994; Isenring 2004; Kiss 2016; Ovesen 1993; Silvers 2014; Suominen 2015; Starke 2011; Uster 2013). Three studies did not report how many participants were malnourished (Jensen 1997; Lovik 1996; Moloney 1983).
Interventions
All participants in the intervention group received dietary instruction but the nature and intensity varied. In 25 studies, the authors specified that the dietary intervention was given by a dietitian (Andersson 2017; Banks 2016; Bourdel‐Marchasson 2014; Carey 2013; Endevelt 2011; Evans 1987; Feldblum 2011; Forli 2001; Holyday 2012; Isenring 2004; Jensen 1997; Kiss 2016; Lovik 1996; Moloney 1983; Ovesen 1993; Pedersen 2016a; Pedersen 2016b; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Suominen 2015; Uster 2013; Vivanti 2015). In two studies a multidisciplinary team of a dietitian, GP and nurse gave the intervention (Beck 2012; Beck 2015), in one study a dietitian and physician (Bonilla‐Palomas 2016) and in two studies a dietitian and a nurse (Caccialanza 2015; Terp 2018).
The intervention was described as either individualised or personalised in 28 of 31 studies (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Endevelt 2011; Evans 1987; Feldblum 2011; Forli 2001; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Moloney 1983; Ovesen 1993; Pedersen 2016a; Pedersen 2016b; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Suominen 2015; Terp 2018; Uster 2013; Vivanti 2015). Description of arrangements for follow‐up varied with 29 studies describing plans for review and monitoring of participants, but the frequency varied from only at admission (Banks 2016) to whenever necessary during six months (Schilp 2013). Seven studies described monthly monitoring (Bonilla‐Palomas 2016; Endevelt 2011; Forli 2001; Rogers 1992; Sharma 2017; Uster 2013; Vivanti 2015), 13 studies monitored once per two to three weeks (Andersson 2017; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Feldblum 2011; Isenring 2004; Kiss 2016; Lovik 1996; Moloney 1983; Pedersen 2016a; Pedersen 2016b; Terp 2018) and one study reviewed weekly (Silvers 2014). Follow‐up and monitoring were conducted at the same interval in both the intervention and control groups. The remaining studies either did not describe arrangements for review and monitoring or characterised these as when necessary (Evans 1987; Ganzoni 1994; Holyday 2012; Jensen 1997; Persson 2002; Schilp 2013; Starke 2011; Suominen 2015).
Prescription of ONS varied both in terms of the type of supplement used and the amount prescribed. In all studies, ONS were prescribed “when necessary” and participants' personal preferences guided the ONS selection. In five studies, there were fewer than five options in the choice of types of supplement (Evans 1987; Forli 2001; Ganzoni 1994; Jensen 1997; Starke 2011). Seven studies reported the proportion of participants who used ONS (Banks 2016; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Suominen 2015; Starke 2011; Uster 2013) and varied from 25% (Bourdel‐Marchasson 2014) to 88% (Starke 2011). The remaining studies did not report the proportion of participants who used ONS during the study period.
Outcomes
Not all studies contributed data on all outcomes and data were available to enter into the analyses for only some outcomes. One study reported no data relevant to the outcomes of this review or data were reported in a format meaning no outcomes could be included in the meta‐analyses (Jensen 1997). Some additional data were obtained on request from the study investigators or have been derived by imputation (Table 12).
7. Dietary advice plus supplements, if required, compared with no advice and no supplements: outcomes with additional data provided or imputed.
| Study ID | Outcomes where the author provided data | Outcomes where data were imputed |
| Andersson 2017 | Weight | |
| Banks 2016 | Weight | |
| Beck 2015 | Weight, grip strength, energy and protein intake | |
| Bourdel‐Marchasson 2014 | Weight | |
| Forli 2001 | weight, energy intake | |
| Ganzoni 1994 | Weight | |
| Holyday 2012 | Re‐admission | |
| Isenring 2004 | Weight, energy & protein intake | SD of change in FFM from 2 studies (Kiss 2016; Ovesen 1993) |
| Kiss 2016 | FFM, weight | |
| Persson 2002 | Weight, energy, QoL | |
| Rogers 1992 | SD of change in weight, TSF, MAMC, handgrip strength from 1 study (Weekes 2009). | |
| Schilp 2013 | FFM, weight, grip strength, protein intake, energy intake | |
| Uster 2013 | Mortality, grip strength, protein intake, energy intake | |
| Vivanti 2015 | Information on methodology |
FFM: fat‐free mass; SD: standard deviation; QoL: quality of life. format?
Primary outcomes
Mortality
26 studies reported mortality data (Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Evans 1987; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Moloney 1983; Ovesen 1993; Pedersen 2016a; Persson 2002; Schilp 2013; Sharma 2017, Silvers 2014; Starke 2011; Suominen 2015; Terp 2018, Uster 2013; Vivanti 2015).
Morbidity
Review authors assessed morbidity by hospital readmissions, length of stay and complications. Nine studies provided data on the number of people admitted to hospital (Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Holyday 2012; Pedersen 2016a; Pedersen 2016b; Sharma 2017; Starke 2011; Terp 2018). Five studies provided data on the length of hospital stay (Banks 2016; Beck 2012; Holyday 2012; Sharma 2017; Starke 2011). Three studies provided data on complications (Bourdel‐Marchasson 2014; Sharma 2017; Starke 2011).
Nutritional status
A total of 24 studies reported data on the change in weight (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Ovesen 1993; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Terp 2018; Uster 2013; Vivanti 2015), six studies reported data on BMI (Carey 2013; Forli 2001; Persson 2002; Sharma 2002a; Starke 2011; Suominen 2015), four studies reported data on change in FFM (Isenring 2004; Kiss 2016; Ovesen 1993; Schilp 2013), two studies reported data on MAC (Rogers 1992; Sharma 2017), two studies reported data on MAMC (Caccialanza 2015; Sharma 2017) and three studies reported data on TSF (Ovesen 1993; Rogers 1992; Sharma 2017).
Secondary outcomes
Nutritional outcomes
Data on the change in energy intake were available in nine studies (Beck 2012; Beck 2015; Caccialanza 2015; Forli 2001; Isenring 2004; Moloney 1983; Ovesen 1993; Schilp 2013; Uster 2013), and final energy intake from three studies (Carey 2013; Feldblum 2011; Starke 2011). Data on change in protein intake were available in eight studies (Beck 2012; Beck 2015; Isenring 2004; Moloney 1983; Ovesen 1993; Schilp 2013; Suominen 2015; Uster 2013).
Clinical and functional outcomes
For the 2021 update, the review authors have summarised data on handgrip strength in meta‐analyses as this is the most frequently reported functional outcome across studies. Nine studies provided data on handgrip strength (Beck 2012; Beck 2015; Carey 2013; Pedersen 2016a; Pedersen 2016b; Rogers 1992; Schilp 2013; Sharma 2017; Terp 2018; Uster 2013). The authors have presented a summary of other clinical and functional outcomes reported in the additional tables (Table 6; Table 7).
QoL
18 studies provided data on QoL (Andersson 2017; Beck 2015; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Isenring 2004; Jensen 1997; Kiss 2016; Ovesen 1993; Pedersen 2016a; Pedersen 2016b; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015;Uster 2013; Vivanti 2015). The review authors have only entered data for the global QoL scores into the meta‐analysis.
Costs
Three studies provided data on costs (Beck 2015; Endevelt 2011; Schilp 2013).
5. Dietary advice plus ONS compared with no advice and no ONS
We identified 13 studies (1315 participants) in this comparison (Anbar 2014; Baldwin 2011; Berneis 2000; Calegari 2011; Chandra 1985; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013).
Study design
There were 12 RCTs with a parallel design and one with a cross‐over design (Calegari 2011). Six (46%) studies were conducted in Europe (Baldwin 2011; Berneis 2000; Hampson 2003; Neelemaat 2011; Persson 2007; Wyers 2013), three (23%) in Southeast Asia and India (Jahnavi 2010; Paton 2004; Um 2014), two (15%) in Canada (Chandra 1985; Payette 2002) and one each in the Middle East (Anbar 2014) and South America (Calegari 2011). All but two studies reported the source of funding (Anbar 2014; Um 2014). 10 studies obtained funding from educational or charitable grants (Baldwin 2011; Berneis 2000; Calegari 2011; Chandra 1985; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Paton 2004; Persson 2007; Wyers 2013) and five studies declared some funding from companies making commercial nutritional products (Chandra 1985; Paton 2004; Payette 2002; Persson 2007; Wyers 2013).
The study setting varied: one study was conducted in hospital (Anbar 2014); three studies began the intervention in hospital and continued it into the community (Neelemaat 2011; Persson 2007; Wyers 2013); six studies were conducted in outpatients (Baldwin 2011; Berneis 2000; Calegari 2011; Chandra 1985, Paton 2004; Um 2014); and three studies were solely in the community (Hampson 2003; Jahnavi 2010; Payette 2002).
The duration of the intervention ranged from the length of hospital stay (Anbar 2014), one to three months (Baldwin 2011; Berneis 2000; Chandra 1985; Jahnavi 2010; Neelemaat 2011; Paton 2004; Um 2014; Wyers 2013), four to six months (Payette 2002; Persson 2007) and 12 months (Hampson 2003). The cross‐over study lasted seven to 12 months; however, we only used data from phase 1, i.e. baseline to three months (Calegari 2011). Five studies included an additional follow‐up period beyond the end of the intervention which varied from three months (Wyers 2013), four to six months (Paton 2004), and seven to 12 months (Baldwin 2011; Jahnavi 2010). One study reported survival data up to four years post intervention (Neelemaat 2011).
Participants
The largest study included 210 participants (Neelemaat 2011) and the smallest enrolled 18 participants (Calegari 2011).
In 12 of 13 studies, the age of participants was reported as either mean (SD), median (IQR range), a range with most studies reporting age separately by intervention group. One study did not report age (Berneis 2000). The majority of studies included older participants with a mean (SD) age ranging from 56.4 (15.6) years (Calegari 2011) to 85.0 (6.1) years (Persson 2007). Two studies involved younger adults, with participants having a mean age of 38.4 (19.3) years to 41.0 (14.2) years (Jahnavi 2010; Paton 2004).
Sex was reported in 12 of 13 studies, only one study did not report on this (Persson 2007). In five studies there were fewer females than males overall (less than 45%) (Baldwin 2011; Berneis 2000; Calegari 2011; Jahnavi 2010; Um 2014). In four studies, there were more females than males (over 55%) (Anbar 2014; Neelemaat 2011; Payette 2002; Wyers 2013). One study included approximately similar numbers of males and females (Paton 2004), one study included only males (Chandra 1985) and another included only females (Hampson 2003).
The participants in studies had a wide variety of clinical conditions. Participants in seven studies were older people, in two studies they had a hip fracture (Anbar 2014; Wyers 2013), in two studies the participants were transitioning from hospital to the community (Neelemaat 2011; Persson 2007), two studies were in older people living in the community (Chandra 1985; Payette 2002), and one study was in older underweight women with osteoporosis (Hampson 2003). Two studies were in people with cancer (Baldwin 2011; Um 2014), two were in people with tuberculosis (Jahnavi 2010; Paton 2004), one was in people with stable HIV infection (Berneis 2000) and one was in people with renal failure on haemodialysis (Calegari 2011).
The nutritional status of participants at baseline was assessed in 11 of 13 studies, but the method of assessment varied. One investigator used a validated tool, the MNA (Wyers 2013) and two studies used weight loss in the previous three to six months (Baldwin 2011; Neelemaat 2011). The remaining eight studies reported the BMI of participants at baseline (Anbar 2014; Calegari 2011; Hampson 2003; Jahnavi 2010; Paton 2004; Payette 2002; Persson 2007; Um 2014); the mean BMI was in the normal range in six studies (Anbar 2014; Calegari 2011; Hampson 2003; Payette 2002; Persson 2007; Um 2014), and in the underweight range for two studies (Jahnavi 2010; Paton 2004). Seven studies assessed all participants as malnourished or at nutritional risk (Baldwin 2011; Chandra 1985; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007) and one study included some malnourished or at risk participants (Wyers 2013). One study a BMI below 21 kg/m2 or 5% weight loss in six months was an inclusion criterion (Berneis 2000), while in a further study a low BMI was an inclusion criterion (Hampson 2003). The remaining studies did not report how many participants were malnourished or specify malnutrition as an inclusion criteria (Anbar 2014; Calegari 2011; Um 2014).
Interventions
All participants in the intervention group received dietary instruction and ONS, but the nature and intensity of the interventions varied. In nine studies authors reported that a dietitian provided the intervention (Baldwin 2011; Berneis 2000; Calegari 2011; Hampson 2003; Neelemaat 2011; Payette 2002; Persson 2007; Um 2014; Wyers 2013) and in one study Anganwadi workers (i.e. paid, part‐time women selected from the community who are trained in various aspects of health, nutrition and child development) gave the intervention (Jahnavi 2010). In three studies the authors did not specify the role or occupation of the person who provided the intervention (Anbar 2014; Chandra 1985; Paton 2004). In all but one study the intervention was tailored to individuals' habitual intake or preferences, or both (Chandra 1985). Two studies did not describe follow‐up and review arrangements (Calegari 2011; Chandra 1985) and in two studies follow‐up arrangements were unclear (Anbar 2014; Jahnavi 2010). In those studies where follow‐up and review arrangements were described these varied considerably; three studies reported that follow‐up and review occurred during outpatient visits (Berneis 2000; Hampson 2003; Um 2014) and six studies described a combination of face‐to‐face sessions in hospital or outpatient visits and phone calls, or a combination of these (Baldwin 2011; Neelemaat 2011, Paton 2004; Payette 2002; Persson 2007; Wyers 2013).
Outcomes
Not all studies contributed data on all outcomes and data were available to enter into the analyses for only some outcomes. One study reported no data relevant to the outcomes of this review or data were reported in a format meaning no outcomes could be included in the meta‐analyses (Chandra 1985). Some additional data were obtained on request from the study authors or have been derived by imputation (Table 13).
8. Dietary advice plus supplements compared with no advice and no supplements: outcomes with additional data provided or imputed.
| Study ID | Outcomes for which data received from author | Outcomes for which data derived by imputation |
| Baldwin 2011 | Mortality, weight, grip strength, QoL scores | |
| Berneis 2000 | Weight change and SD | |
| Calegari 2011 | SD of change in weight imputed from 2 studies (Campbell 2008; Sharma 2002a), SD of change in FFM imputed from 1 study (Campbell 2008). | |
| Paton 2004 | Energy intake, n for weight, FFM and handgrip strength | |
| Payette 2002 | Handgrip strength (nb. data on global QoL and MAMC not available) | |
| Persson 2007 | Weight, handgrip | |
| Um 2014 | SD of change in weight imputed from 1 study (Baldwin 2011). | |
| Wyers 2013 | Weight, QoL, energy‐ and protein intake |
FFM: fat‐free mass; MAMC: mid‐arm muscle circumference; QoL: quality of life; SD: standard deviation. format?
Primary outcomes
Mortality
Nine studies reported data on mortality (Anbar 2014; Baldwin 2011; Calegari 2011; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Persson 2007; Um 2014; Wyers 2013).
Morbidity
The review authors assessed morbidity by hospital readmissions, length of stay and complications. No studies in this group reported data on the number of participants admitted to hospital. Three studies reported data on length of hospital stay (Anbar 2014; Neelemaat 2011; Wyers 2013) and one study provided data on complications (Anbar 2014).
Nutritional status
11 studies reported data on weight change (Baldwin 2011; Berneis 2000; Calegari 2011; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013). Four studies reported data on BMI (Calegari 2011; Persson 2007; Um 2014; Wyers 2013), five studies reported data on change in FFM (Berneis 2000; Calegari 2011; Hampson 2003; Neelemaat 2011; Paton 2004), one study reported data on MAC (Wyers 2013), one study reported data on MAMC (Payette 2002) and one study reported data on TSF (Payette 2002).
Secondary outcomes
Nutritional intake
Nine studies reported data on changes in energy intake (Anbar 2014; Baldwin 2011; Berneis 2000; Hampson 2003; Neelemaat 2011; Paton 2004; Payette 2002; Um 2014; Wyers 2013). Only two studies reported on protein intake (Neelemaat 2011; Wyers 2013).
Clinical and functional outcomes
For the 2021 update, the review authors summarised data on handgrip strength in meta‐analyses as this is the most frequently reported functional outcome across studies. Four studies provided data on handgrip strength (Jahnavi 2010; Neelemaat 2011; Paton 2004; Persson 2007); and one study reported data graphically which could not be extracted for meta‐analysis (Payette 2002). The review authors have provided a summary of other clinical and functional outcomes reported in the additional tables (Table 6; Table 7). One study reported data on the number of post‐operative and infectious complications in people with hip fracture (Anbar 2014), one study reported data on the response to influenza vaccine in older people (Chandra 1985) and one study reported data on sputum conversion and treatment completion rates in individuals with tuberculosis (Jahnavi 2010). One study reported data on the six‐minute walk test (Calegari 2011), one study reported data on the timed sit‐to‐stand test (Jahnavi 2010) and one study reported data on activities of daily living assessed using the Katz score (Persson 2007).
QoL
Nine studies reported QoL data (Baldwin 2011; Berneis 2000; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013); however, various tools were used and data were reported in different ways, e.g. total QoL scores and different domain scores. Five studies used versions of the Medical Outcomes Study Instrument which is designed as a generic tool for use across different clinical conditions: three studies used versions of the instrument adapted for use with people with HIV infection (Berneis 2000; Jahnavi 2010; Paton 2004) and two studies used the SF‐36 (Payette 2002; Persson 2007). Two studies used the three‐level EQ‐5D in cost‐effectiveness analyses (Neelemaat 2011; Wyers 2013). Two studies used QoL tools designed for use in people with cancer; the EORTC (Baldwin 2011; Um 2014) and FAACT (Baldwin 2011).
Costs
Two studies reported cost‐effectiveness analyses (Neelemaat 2011; Wyers 2013).
Excluded studies
The review authors excluded a total of 248 studies for the reasons detailed in the tables (Characteristics of excluded studies). They excluded 68 studies because after scrutiny they were not RCTs and a further 163 studies because the comparison did not fulfil the inclusion criteria. They excluded 17 studies for other reasons as follows. In eight studies participants were not malnourished or at nutritional risk (Antila 1993; Bauer 1994; Hansra 2017; Jacka 2017; Kiss 2014; Rollo 2020; Sartorelli 2005; Zweers 2020). In one study the majority of participants were children and all participants in the control group were children (Williams 1989a). In one study the routine care arm was not consistent with others in this review (Orell 2019). Four old clinical study records were excluded because there was no evidence of publication (ISRCTN11132850; NCT00136253; NCT00417508; NCT01116947) and one record was excluded because the study was withdrawn after difficulty with recruitment (NCT01190969). In one study there was insufficient information to identify the study ( Zhao 1995) and one study has remained unavailable on the journal website or through contact with authors (Margare 2002).
Studies awaiting assessment
Review authors have listed 81 studies as awaiting assessment.
58 studies were listed for the following reasons. 10 studies require translation (Cong 2016; Cui 2017; Jia 2019; Kwon 2004; Liu 2018; Liu 2019; Park 2012; Sui 2020; Zhang 2018b; Zhou 2011). In 47 studies, there was insufficient detail about the intervention to enable a judgement to be made about eligibility for inclusion (Abdelsalam 2019; Banda 2017; Britton 2019; Camere 2016; Cawood 2017; Chewaskulyong 2015; ChiCTR1800014842; ChiCTR‐IOR‐17013151; Collins 2014; Cramon 2019; CTRI/2012/05/002698; CTRI/2018/10/015882; CTRI/2018/11/016369; Gaitan 2017; Hansen 2020; Hebuterne 2019; Hoekstra 2005; Hubbard 2009; Kalal 2016; Kandel 2014; Kang 2013; Kuhlmann 1999; Lin 2017; Movahed 2020; NCT01171495; NCT02975089; NCT03631537; NCT03632200; NCT03944161; NCT04217564; Norshariza 2018; Otten 2016; Pinto 2021; Qui 2020; RBR‐35kjvg; RBR‐3shhxs; Salem 2020; Sathiaraj 2020; Shadid 2019; Shatenstein 2017; Stratton 2007; Tharun 2020; Touger Decker 1997 UMIN000032234; Vazquez‐Sanchez 2019; Verho 2017; Wu 2018). One clinical study record is eligible for inclusion and is listed as completed online, but no full‐text publication has been identified (NCT02051777).
In total, 45 of these 58 studies are described as RCTs. Of the remaining studies, one is a stepped‐wedge RCT (Britton 2019), one is described as a two‐group study (ChiCTR1800014842), one is described as a controlled study (CTRI/2018/11/016369) and one is described as a prospective clinical study (Lin 2017). One study is described as a randomised study (Abdelsalam 2019), a further study is described as having a randomised factorial design (Banda 2017) and in six studies it is unclear whether participants were randomly allocated (ChiCTR‐IOR‐17013151; Kuhlmann 1999; Kwon 2004; Park 2012; Shatenstein 2017; UMIN000032234). The smallest study reports on 18 participants (Kuhlmann 1999) and the largest includes 308 participants (Cawood 2017).
The participants in the studies are from a variety of clinical backgrounds; 19 studies are in people with cancer (Britton 2019; Chewaskulyong 2015; Cong 2016; Cui 2017; Hebuterne 2019; Lin 2017; Movahed 2020; NCT03631537; NCT03632200; Norshariza 2018; Park 2012; Pinto 2021; Qui 2020; RBR‐35kjvg; RBR‐3shhxs; Sathiaraj 2020; Shadid 2019; Sui 2020; Zhang 2018b), 12 in malnourished or frail older people (Cawood 2017; Cramon 2019; Hubbard 2009; Kandel 2014; Kwon 2004; NCT02051777; NCT02975089; NCT03944161; Otten 2016; Vazquez‐Sanchez 2019; Verho 2017; Wu 2018), six are in people with renal disease (Abdelsalam 2019; Gaitan 2017; Kuhlmann 1999; Salem 2020; UMIN000032234; Zhou 2011), four are in people with COPD (Camere 2016; Collins 2014; Hoekstra 2005; Jia 2019), three are in people with liver disease (CTRI/2018/11/016369; Kalal 2016; Tharun 2020), three are in older people with Alzheimer's disease or dementia (Liu 2018; Liu 2019; Shatenstein 2017), two in people with diseases of the gastrointestinal tract (ChiCTR1800014842; CTRI/2018/10/015882), two studies are in people hospitalised with a hip fracture (Kang 2013; Stratton 2007), two are in people with tuberculosis (Banda 2017; ChiCTR‐IOR‐17013151), two are in people with HIV infection (CTRI/2012/05/002698; NCT01171495), one study is in people with multiple sclerosis (NCT04217564) one study is in people with pneumonia (Hansen 2020) and one is in people undergoing denture fitting (Touger Decker 1997).
The remaining 23 studies which are listed as awaiting assessment have been identified as eligible for inclusion in the review, but could not be included at this update (Abdollahi 2019; Cereda 2019; Ha 2010; Hsieh 2019; Jabbour 2019; Limwannata 2021; Loser 2021; Maharshi 2016; Meng 2021; Molassiotis 2021; Nyguyen 2020; Reinders 2020; Sahathevan 2018; Schuetz 2019; Smith 2020; Soderstrom 2020; Sudarsanam 2011; Torbergsen 2019; van der Werf 2020; Wills 2019; Yang 2019; Yang 2020; Zhu 2019). All these studies are described as RCTs. The smallest study reports on 32 participants (Molassiotis 2021) and the largest 2088 participants (Schuetz 2019). The participants are from a variety of clinical backgrounds. Nine studies are in people with cancer (Abdollahi 2019; Cereda 2019; Jabbour 2019; Loser 2021; Meng 2021; Molassiotis 2021; van der Werf 2020; Yang 2020; Zhu 2019), five in older people (Hsieh 2019; Reinders 2020; Smith 2020; Soderstrom 2020; Yang 2019), two are in people with renal disease on replacement therapy (Limwannata 2021; Sahathevan 2018), one in medical inpatients at nutritional risk (Schuetz 2019), one is in people who have suffered an acute stroke (Ha 2010), one is in people with liver cirrhosis (Maharshi 2016), one is in people with COPD (Nyguyen 2020), one is in people with tuberculosis (Sudarsanam 2011), one is in people hospitalised with a hip fracture (Torbergsen 2019) and one is in people with Amyotrophic Lateral Sclerosis (Wills 2019).
The interventions in these studies varied. Seven studies are of dietary counselling interventions compared with routine are and are eligible for inclusion in Comparison 1 of the review (Abdollahi 2019; Hsieh 2019; Loser 2021; Maharshi 2016; Nyguyen 2020; Reinders 2020; Wills 2019). One study is of dietary counselling compared with an ONS and is eligible for inclusion in Comparison 2 of the review (Yang 2019). Eight studies are of dietary counselling plus and ONS compared with dietary counselling alone and are eligible for inclusion in Comparison 3 of the review (Cereda 2019; Limwannata 2021; Meng 2021; Sahathevan 2018; Smith 2020; Sudarsanam 2011; Yang 2020; Zhu 2019). Five studies are of dietary counselling interventions plus ONS if required compared with routine care and are eligible for inclusion in Comparison 4 of the review (Ha 2010; Jabbour 2019; Molassiotis 2021; Schuetz 2019; van der Werf 2020). One study is of dietary counselling plus ONS compared with routine care and is eligible for inclusion in Comparison 5 of the review (Torbergsen 2019). One study has four arms dietary counselling, ONS, dietary counselling plus ONS and routine care and can be included in Comparisons 1,2,3 and 5 of the review (Soderstrom 2020).
Ongoing studies
The review authors identified 17 ongoing studies which might be eligible for inclusion in this review in the future; 13 from searches of Clintrials.gov (Munk 2020; NCT02440165; NCT02763904; NCT02892747; NCT03075189; NCT03114202; NCT03191253; NCT03315195; NCT03352388; NCT03519139; NCT03540784; NCT03995303; NCT04628117) and four by searching other databases (ACTRN12612001253897; ChiCTR2000028963; CTRI/2019/05/019387; PACTR201108000303396).
14 of the ongoing studies are RCTs (ACTRN12612001253897; ChiCTR2000028963; Munk 2020; NCT02763904; NCT02892747; NCT03075189; NCT03114202; NCT03191253; NCT03315195; NCT03352388; NCT03519139; NCT03540784; NCT03995303; NCT04628117), one is a multicentre cluster‐RCT (NCT02440165), one describes the method as a two‐group study with no details of any randomisation (CTRI/2019/05/019387) and the description of the assignment to groups in one study implies it is a quasi‐RCT (PACTR201108000303396).
14 studies report their planned recruitment numbers and the smallest study aims to recruit 22 participants (NCT04628117) and the largest 295 participants (NCT02892747).
The participants in the studies are from a variety of clinical backgrounds. Five are in older adults (ACTRN12612001253897; NCT03075189; NCT03352388; NCT03519139; NCT03995303), two are in people with cancer (ChiCTR2000028963; NCT03114202), two are in people undergoing surgery (NCT02440165; NCT03315195), two in people with HIV infection (NCT03191253; PACTR201108000303396), one in hospitalised patients at nutritional risk (NCT02763904), one in people with cirrhosis, frailty and sarcopenia (CTRI/2019/05/019387), one in people with inflammatory bowel disease (NCT03540784), one in adults with chronic heart failure (NCT02892747), one in people with end‐stage kidney disease receiving peritoneal dialysis (NCT04628117) and one in hospitalised patients with mixed clinical conditions (oncology, gastrointestinal and medical patients)(Munk 2020).
The interventions and comparisons in the ongoing studies also varied. Five studies are of dietary counselling interventions compared with routine care and are potentially eligible for inclusion in Comparison 1 of the review (NCT03075189; NCT03114202; NCT03352388; NCT03519139; NCT02892747), five studies are of dietary counselling plus an ONS compared with dietary counselling alone and are potentially eligible for inclusion in Comparison 3 (ACTRN12612001253897; NCT02763904; NCT03315195; NCT04628117; PACTR201108000303396), three studies were of dietary advice with ONS (if required) compared with routine care and potentially eligible for inclusion in Comparison 4 (NCT02440165; NCT03191253; NCT03995303), one study was of dietary advice plus ONS compared with routine care and potentially eligible for inclusion in Comparison 5 (ChiCTR2000028963) and one study was of dietary counselling with ONS,but it was not possible from the study report to determine whether this might be eligible for inclusion in Comparison 4 or 5 (Munk 2020). For two studies it is not possible from the study record to fully evaluate the intervention (CTRI/2019/05/019387; NCT03540784).
Risk of bias in included studies
The review authors used the original Cochrane risk of bias tool to assess the risk of bias in the included studies.
Allocation
Generation of sequence
Dietary advice compared with no advice
There are 24 studies included in this comparison, the review authors judged 14 studies to have a low risk of bias for sequence generation (Alo 2014; Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Fernandez‐Barres 2017; Forster 2012; Kunvik 2018; Macia 1991a; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Stow 2015; Weekes 2009; Wong 2004). Methods of generation were mainly the use of computer‐generated randomisation lists or random number tables. They judged 10 studies to have an unclear risk of bias; in one study the author could not recall how the sequence was generated (Imes 1988) and the remaining nine studies did not report any details of how the sequence was generated (Casals 2015; Dixon 1984; Locher 2013; Ollenschlager 1992; Pivi 2011; Rydwik 2008; Salva 2011; Tu 2013). The review authors judged one study in this comparison to have a high risk of bias as participants were assigned a number based on time of admission and group allocation was dependent on whether the number was odd or even (Gu 2015).
Dietary advice compared with ONS
The review authors judged nine of the 12 studies in this comparison to have a low risk of bias for sequence generation (Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015). Again, studies mainly used computer‐generated sequences or random number tables. A further two studies did not provide any information regarding sequence generation and were judged to have an unclear risk of bias (Akpele 2004; Pivi 2011). The final study used alternation to randomise participants and is judged to have a high risk of bias (Kalnins 2005).
Dietary advice versus dietary advice plus ONS
The authors judged 11 of the 22 studies in this comparison to have a low risk of bias for sequence generation as the methods used (mostly computer‐generated lists and random number tables) were appropriate (Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; Diouf 2016; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; McCarthy 1999; Norman 2008b). 10 studies did not provide any information on how the sequence was generated and the review authors judged these to have an unclear risk of bias (Arnold 1989; de Sousa 2012; Dixon 1984; Fuenzalida 1990; Kendell 1982; Le Cornu 2000; Olejko 1984; Rabeneck 1998; Sharma 2002a; Wilson 2001). One study used alternation and the review authors judged this to have a high risk of bias for randomisation (Murphy 1992).
Dietary advice plus ONS, if required, compared with no advice and no ONS
The review authors judged 23 out of 31 studies in this comparison to have a low risk of bias for sequence generation as the methods used were appropriate (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Evans 1987; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Lovik 1996; Ovesen 1993; Pedersen 2016a; Persson 2002; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Vivanti 2015). Seven studies did not provide sufficient information to make a judgement and had an unclear risk of bias (Bonilla‐Palomas 2016; Endevelt 2011; Jensen 1997; Kiss 2016; Moloney 1983; Rogers 1992; Uster 2013). One study allocated participants according to month and ward of hospitalisation and the review authors judged this to have a high risk of bias for the generation of randomisation sequence (Feldblum 2011).
Dietary advice plus ONS compared with no advice and no ONS
Six of the 13 studies in this comparison used appropriate methods of sequence generation and the review authors judged these to have a low risk of bias (Anbar 2014; Baldwin 2011; Berneis 2000; Neelemaat 2011; Paton 2004; Wyers 2013). The remaining seven studies did not provide sufficient information on generation of randomisation sequence and the authors judged them to have an unclear risk of bias (Calegari 2011; Chandra 1985; Hampson 2003; Jahnavi 2010; Payette 2002; Persson 2007; Um 2014).
Allocation concealment
Dietary advice compared with no advice
The authors judged 12 out of the 24 studies in this comparison to have a low risk of bias for this domain as investigators adequately concealed the allocation of participants prior to study start (Baldwin 2011; Campbell 2008; Fernandez‐Barres 2017; Forster 2012; Imes 1988; Kunvik 2018; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Stow 2015; Weekes 2009; Wong 2004); 11 studies failed to provide sufficient information on this domain and have an unclear risk of bias (Cano‐Torres 2017; Casals 2015; Dixon 1984; Gu 2015; Locher 2013; Macia 1991a; Ollenschlager 1992; Pivi 2011; Rydwik 2008; Salva 2011; Tu 2013). The authors judged one study to have a high risk of bias (Alo 2014).
Dietary advice compared with ONS
The review authors judged eight of the 12 studies in this comparison to have a low risk of bias due to allocation concealment (Baldwin 2011; Gray‐Donald 1995; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015). Three studies did not provide sufficient information to allow the authors to make a judgement and have an unclear risk of bias (Akpele 2004; Hernandez 2014; Pivi 2011). The review authors judged one study, which used alternate allocation, to have a high risk of bias due to a lack of allocation concealment (Kalnins 2005).
Dietary advice versus dietary advice plus ONS
The authors judged that 10 of the 22 studies in this comparison sufficiently concealed group allocation and these have a low risk of bias (Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; Diouf 2016; Gonzalez‐Espinoza 2005; Huynh 2015; Le Cornu 2000; Norman 2008b). A further 11 studies did not provide information on allocation concealment and have an unclear risk of bias (Arnold 1989; de Sousa 2012; Dixon 1984; Fuenzalida 1990; Kapoor 2017; Kendell 1982; McCarthy 1999; Olejko 1984; Rabeneck 1998; Sharma 2002a; Wilson 2001). One study did not conceal allocation and has a high risk of bias (Murphy 1992).
Dietary advice plus ONS, if required, compared with no advice and no ONS
The review authors judged most of the studies in this comparison (19 out of 31 studies) to have adequately concealed allocation and to have a low risk of bias (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Evans 1987; Ganzoni 1994; Isenring 2004; Jensen 1997; Lovik 1996; Ovesen 1993; Pedersen 2016a; Persson 2002; Schilp 2013; Sharma 2017; Silvers 2014; Terp 2018; Vivanti 2015). There were 11 studies which did not provide sufficient information to allow the review authors to make a judgement and which have an unclear risk of bias (Caccialanza 2015; Carey 2013; Endevelt 2011; Forli 2001; Holyday 2012; Kiss 2016; Moloney 1983; Rogers 1992; Starke 2011; Suominen 2015; Uster 2013). One study did not conceal allocation and has a high risk of bias (Feldblum 2011).
Dietary advice plus ONS compared with no advice and no ONS
Just five of the 13 studies in this comparison had a low risk of bias from allocation concealment (Anbar 2014; Baldwin 2011; Hampson 2003; Neelemaat 2011; Paton 2004). The remaining eight studies did not provide information about allocation concealment and the review authors judged these to have an unclear risk of bias (Berneis 2000; Calegari 2011; Chandra 1985; Jahnavi 2010; Payette 2002; Persson 2007; Um 2014; Wyers 2013).
Blinding
Blinding of assessment of clinical outcomes
Dietary advice compared with no advice
The review authors judged 22 studies in this comparison to have a low risk of bias (Alo 2014; Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Macia 1991a; Manguso 2005; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Tu 2013; Weekes 2009; Wong 2004). Two of the studies did not assess clinical outcomes and the authors judged these to have an unclear risk of bias (Kunvik 2018; Locher 2013).
Dietary advice compared with ONS
The review authors judged all 12 studies to have a low risk of bias (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015).
Dietary advice versus dietary advice plus ONS
The authors judged 18 of the 22 studies in this comparison to have a low risk of bias (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Le Cornu 2000; Murphy 1992; Norman 2008b; Olejko 1984; Sharma 2002a; Wilson 2001). The review authors judged four studies to have an unclear risk of bias: two studies did not report clinical outcomes (Diouf 2016; Kendell 1982) and two studies did not measure clinical outcomes (McCarthy 1999; Rabeneck 1998).
Dietary advice plus ONS, if required, compared with no advice and no ONS
The review authors judged 28 of the 31 studies in this comparison to have a low risk of bias (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Endevelt 2011; Evans 1987; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Jensen 1997; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Uster 2013; Vivanti 2015). They judged three studies to have an unclear risk of bias as these studies did not measure clinical outcomes (Carey 2013; Kiss 2016; Pedersen 2016a).
Dietary advice plus ONS compared with no advice and no ONS
The review authors judged 12 out of 13 studies in this comparison to have a low risk of bias (Baldwin 2011; Berneis 2000; Calegari 2011; Chandra 1985; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013). In the remaining study the assessment of clinical outcomes (post‐operative complications) was not blinded and review authors judged that lack of blinding might have influenced assessment of this outcome; therefore this study was judged to be at high risk of bias (Anbar 2014).
Blinding of assessment of functional outcomes
Dietary advice compared with no advice
The authors judged just one study out of 24 to have a low risk of bias from blinding of functional outcomes (Casals 2015). They judged 15 studies to have an unclear risk of bias (Alo 2014; Cano‐Torres 2017; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Macia 1991a; Manguso 2005; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Tu 2013; Wong 2004) and eight studies to have a high risk of bias from a lack of blinding for functional outcomes (Baldwin 2011; Campbell 2008; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Salva 2011; Stow 2015; Weekes 2009).
Dietary advice compared with ONS
The review authors judged two of the 12 studies in this comparison to have a low risk of bias of blinding of functional outcomes (Gray‐Donald 1995; Singh 2008). They judged six studies to have an unclear risk of bias (Akpele 2004; Hernandez 2014; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999) and four studies to have a high risk of bias because the lack of blinding might have influenced assessment of functional outcomes (Baldwin 2011; Kalnins 2005; Parsons 2016; Stow 2015).
Dietary advice versus dietary advice plus ONS
The authors judged two out of 22 studies in this comparison to have a low risk of bias for the blinding of functional outcomes (Burden 2017; Gonzalez‐Espinoza 2005). They judged seven studies to have an unclear risk of bias (Arnold 1989; Beattie 2000; Burden 2011; Huynh 2015; Kendell 1982; McCarthy 1999; Wilson 2001) and the 13 studies to have a high risk of bias (Baldwin 2011; de Luis 2003; de Sousa 2012; Diouf 2016; Dixon 1984; Fuenzalida 1990; Kapoor 2017; Le Cornu 2000; Murphy 1992; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a).
Dietary advice plus ONS, if required, compared with no advice and no ONS
The authors judged eight out of 31 studies to have a low risk of bias for this domain (Andersson 2017; Carey 2013; Feldblum 2011; Ganzoni 1994; Holyday 2012; Pedersen 2016a; Sharma 2017; Starke 2011) and a further four studies to have an unclear risk of bias (Bonilla‐Palomas 2016; Moloney 1983; Uster 2013; Vivanti 2015). They judged the remaining 19 studies to have a high risk of bias because the lack of blinding might have influenced assessment of functional outcomes (Banks 2016; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Caccialanza 2015; Endevelt 2011; Evans 1987; Forli 2001; Isenring 2004; Jensen 1997; Kiss 2016; Lovik 1996; Ovesen 1993; Persson 2002; Rogers 1992; Schilp 2013; Silvers 2014; Suominen 2015; Terp 2018).
Dietary advice plus ONS compared with no advice and no ONS
The authors judged just one of the 13 studies in this comparison to have a low risk of bias for the blinding of functional outcomes (Payette 2002). They judged six studies to have an unclear risk of bias (Berneis 2000; Calegari 2011; Jahnavi 2010; Paton 2004; Um 2014; Wyers 2013) and the six studies to have a high risk of bias (Anbar 2014; Baldwin 2011; Chandra 1985; Hampson 2003; Neelemaat 2011; Persson 2007).
Blinding of assessment of nutritional outcomes
Dietary advice compared with no advice
The authors judged seven out of 24 studies to have a low risk of bias from blinding of nutritional outcomes (Alo 2014; Cano‐Torres 2017; Casals 2015; Gu 2015; Manguso 2005; Pivi 2011; Salva 2011). Four studies had an unclear risk of bias (Locher 2013; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b) and 13 studies had a high risk of bias (Baldwin 2011; Campbell 2008; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Imes 1988; Kunvik 2018; Macia 1991a; Rydwik 2008; Stow 2015; Tu 2013; Weekes 2009; Wong 2004).
Dietary advice compared with ONS
The review authors judged two out of 12 studies to have a low risk of bias from blinding of nutritional outcomes (Pivi 2011; Singh 2008) and two further studies had an unclear risk of bias (Ravasco 2005a; Ravasco 2005b). However, they judged eight studies in this comparison to have a high risk of bias (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Parsons 2016; Schwenk 1999; Stow 2015).
Dietary advice versus dietary advice plus ONS
The authors judged two out of 22 studies in this comparison to have a low risk of bias from blinding of nutritional outcomes (Burden 2017; Gonzalez‐Espinoza 2005). Three studies had an unclear risk of bias (Fuenzalida 1990; Kendell 1982; Wilson 2001) and 17 studies had a high risk of bias (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; de Luis 2003; de Sousa 2012; Diouf 2016; Dixon 1984; Huynh 2015; Kapoor 2017; Le Cornu 2000; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a).
Dietary advice plus ONS, if required, compared with no advice and no ONS
The review authors judged seven out of 31 studies in this comparison to have a low risk of bias from blinding of nutritional outcomes (Andersson 2017; Bonilla‐Palomas 2016; Carey 2013; Feldblum 2011; Ganzoni 1994; Pedersen 2016a; Sharma 2017). They judged five studies to have an unclear risk of bias (Holyday 2012; Ovesen 1993; Persson 2002; Rogers 1992; Uster 2013) and 18 studies to have a high risk of bias (Banks 2016; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Caccialanza 2015; Endevelt 2011; Evans 1987; Forli 2001; Isenring 2004; Jensen 1997; Kiss 2016; Lovik 1996; Moloney 1983; Schilp 2013; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Vivanti 2015).
Dietary advice plus ONS compared with no advice and no ONS
No study in this comparison had a low risk of bias from blinding of nutritional outcomes. Six of the 13 studies had an unclear risk of bias (Berneis 2000; Calegari 2011; Jahnavi 2010; Paton 2004; Um 2014; Wyers 2013) and seven had a high risk of bias (Anbar 2014; Baldwin 2011; Chandra 1985; Hampson 2003; Neelemaat 2011; Payette 2002; Persson 2007).
Overall blinding of participants and personnel (performance bias)
Dietary advice compared with no advice
The authors judged 23 out of 24 studies to have an overall high risk of performance bias ( Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Macia 1991a; Manguso 2005; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Tu 2013; Weekes 2009; Wong 2004). One study had a low risk of performance bias as although the study participants and personnel were not blinded to the group allocation, this is unlikely to have affected the assessment of outcomes (Alo 2014).
Dietary advice compared with ONS
The review authors judged all 12 studies to have an overall high risk of performance bias (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015).
Dietary advice versus dietary advice plus ONS
The review authors judged one study in this comparison to have an overall low risk of performance bias; although group allocation was unblinded, we considered the assessment of outcomes was unlikely to be affected by lack of blinding (Holyday 2012). The authors judged the remaining 21 studies in this comparison to have an overall high risk of performance bias (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Kendell 1982; Le Cornu 2000; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a).
Dietary advice plusONS, if required, compared with no advice and no ONS
All 31 studies had an overall high risk of performance bias (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016;Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Endevelt 2011; Evans 1987; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Jensen 1997; Kiss 2016; Lovik 1996; Moloney 1983; Ovesen 1993; Pedersen 2016a; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Uster 2013; Vivanti 2015).
Dietary advice plus ONS compared with no advice and no ONS
The review authors judged all 13 studies in this comparison to have an overall high risk of performance bias (Anbar 2014; Baldwin 2011; Berneis 2000; Calegari 2011; Chandra 1985; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013).
Overall blinding of outcome assessors (detection bias)
Dietary advice compared with no advice
The review authors judged three out of 24 studies in this comparison to have an overall low risk of detection bias (Alo 2014; Casals 2015; Pivi 2011); Casals reported the blind assessment of all outcomes (clinical, functional and nutritional) (Casals 2015). The remaining 21 studies had an unclear risk of detection bias (Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Macia 1991a; Manguso 2005; Ollenschlager 1992; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Tu 2013; Weekes 2009; Wong 2004).
Dietary advice compared with ONS
The review authors judged two out of 12 studies in this comparison to have an overall low risk of detection bias (Pivi 2011; Singh 2008) and 10 studies to have an unclear risk of bias (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Stow 2015).
Dietary advice versus dietary advice plus ONS
The review authors judged two out of 22 studies in this comparison to have an overall low risk of detection bias (Gonzalez‐Espinoza 2005; Wilson 2001). They judged 17 studies to have an unclear risk of detection bias (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Dixon 1984; Fuenzalida 1990; Huynh 2015; Kapoor 2017; Kendell 1982; Le Cornu 2000; Murphy 1992; Norman 2008b; Olejko 1984; Sharma 2002a) and three studies to have a high risk of detection bias (Diouf 2016; McCarthy 1999; Rabeneck 1998).
Dietary advice plus ONS, if required, compared with no advice and no ONS
The review authors judged seven out of 31 studies in this comparison to have an overall low risk of detection bias (Andersson 2017; Carey 2013; Feldblum 2011; Ganzoni 1994; Holyday 2012; Pedersen 2016a; Sharma 2017) and 22 studies to have an unclear risk of bias (Banks 2016; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Endevelt 2011; Evans 1987; Forli 2001; Isenring 2004; Jensen 1997; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002; Rogers 1992; Schilp 2013; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Uster 2013; Vivanti 2015). They judged two studies to have a high risk of detection bias (Beck 2012; Kiss 2016).
Dietary advice plus ONS compared with no advice and no ONS
The review authors judged that none of the 13 studies in this comparison had an overall low risk of detection bias. They judged 12 studies to have an overall unclear risk of bias (Baldwin 2011; Berneis 2000; Calegari 2011; Chandra 1985; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013) and one study to have an overall high risk of bias (Anbar 2014).
Incomplete outcome data
Dietary advice compared with no advice
The review authors judged 14 of the 24 studies in this comparison to have a low risk of attrition bias (Alo 2014; Campbell 2008; Cano‐Torres 2017; Casals 2015; Fernandez‐Barres 2017; Forster 2012; Manguso 2005; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Weekes 2009). In three of these studies, all participants completed the study (Alo 2014; Ravasco 2005a; Ravasco 2005b). In five studies attrition rates were less than 15% with numbers of dropouts equal across groups or reasons for withdrawal given, or both (Casals 2015; Forster 2012; Manguso 2005; Ollenschlager 1992; Pivi 2011). In the remaining six studies attrition rates ranged from 19% to 44%, but again investigators provided reasons for withdrawal and numbers were similar across groups (Campbell 2008; Cano‐Torres 2017; Fernandez‐Barres 2017; Rydwik 2008; Salva 2011; Weekes 2009).
The review authors judged 10 studies to have an unclear risk of attrition bias (Baldwin 2011; Dixon 1984; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Macia 1991a; Stow 2015; Tu 2013; Wong 2004). Three studies did not report attrition rates (Gu 2015; Macia 1991a; Tu 2013). In five studies attrition rates ranged from 9% to 37%, but investigators either provided no information about which group the withdrawals were in (Dixon 1984; Stow 2015) or they specified the groups but failed to provide reasons (Imes 1988) or they did not describe either the groups or the reasons for withdrawal (Locher 2013; Wong 2004). One study reported an attrition rate of 14% with reasons, but investigators reported only on 55 participants with protein intake below 1.2 g/kg body weight per day and did not report on the 24 participants with protein intake over 1.2 g/kg body weight per day (Kunvik 2018). In the final study 2% withdrew (reasons not given) and only 43% were still alive at 12 months, but death rates were similar across groups (Baldwin 2011).
Dietary advice compared with ONS
We included 12 studies (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015).
Nine studies had a low risk of attrition bias (Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015). Two studies lost no participants to follow‐up (Ravasco 2005a; Ravasco 2005b). Five studies had an attrition rate below 15% with similar rates across groups and fully reported reasons for withdrawal (Gray‐Donald 1995; Kalnins 2005; Pivi 2011; Schwenk 1999; Singh 2008). Two studies had attrition rates over 15%, but again with similar rates across groups and reasons for withdrawal fully reported (Parsons 2016; Stow 2015).
Two studies were judged to have an unclear risk of bias; one study did not report attrition (Akpele 2004) and in the second while 2% withdrew (reasons not given) and only 43% were still alive at 12 months, death rates were similar across groups (Baldwin 2011).
The remaining study had a high risk of attrition bias with 28% overall attrition, but there was a difference in rates between groups of 10% in the advice group compared to 45% in the supplement group (Hernandez 2014).
Dietary advice versus dietary advice plus ONS
The review authors judged 15 out of the 22 studies in this comparison to have a low risk of bias (Arnold 1989; Beattie 2000; Burden 2011; de Luis 2003; de Sousa 2012; Diouf 2016; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Kendell 1982; Le Cornu 2000; Norman 2008b; Olejko 1984; Rabeneck 1998). Three studies reported no loss to follow‐up (Fuenzalida 1990; Kendell 1982; Olejko 1984). A further seven studies reported an attrition rate below 15% (Arnold 1989; Beattie 2000; Burden 2011; de Luis 2003; de Sousa 2012; Gonzalez‐Espinoza 2005; Le Cornu 2000); in one study all the withdrawals were from the control group (de Sousa 2012) and in a second study the attrition rate was 5% in the intervention group and 17.5% in the control group (Le Cornu 2000). Five studies reported attrition rates over 15%, sometimes much higher, but with similar numbers across groups (Diouf 2016; Huynh 2015; Kapoor 2017; Norman 2008b; Rabeneck 1998).
The review authors judged three studies to have an unclear risk of bias; one study reported less than 15% attrition, but did not provide reasons for withdrawals (Sharma 2002a) and in the second while 2% withdrew (reasons not given) and only 43% were still alive at 12 months, death rates were similar across groups (Baldwin 2011). The third study reported an attrition rate of 37%, but did not state which groups these were from (Dixon 1984).
The review authors judged four studies to have a high risk of attrition bias (Burden 2017; McCarthy 1999; Murphy 1992; Wilson 2001). One study reported less than 15% attrition with reasons for how many participants dropped out of which groups, but for some outcome measures data are provided for fewer participants than originally included in the study (Burden 2017). Investigators do not report the reasons why data were missing for these outcomes. One study reported 15% attrition, but gave no details on which group the withdrawals were from (Wilson 2001). Two studies reported high rates of attrition that were not balanced across groups; in one there was an overall rate of 40% with 10% withdrawing from the advice only group and 30% from the advice plus ONS group (McCarthy 1999) and in the second an overall rate of 27% broke down to 45% withdrawals in the advice only group and 9% in the advice plus ONS group (Murphy 1992).
Dietary advice plus ONS, if required, compared with no advice and no ONS
The review authors judged 21 out of the 31 studies included in this comparison to have a low risk of bias (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Carey 2013; Evans 1987; Forli 2001; Ganzoni 1994; Isenring 2004; Kiss 2016; Lovik 1996; Persson 2002; Rogers 1992; Sharma 2017; Schilp 2013; Starke 2011; Suominen 2015; Terp 2018; Uster 2013; Vivanti 2015). In nine studies, 15% or less dropped out with reasons given and similar numbers across groups (Andersson 2017; Beck 2015; Carey 2013; Forli 2001; Holyday 2012; Isenring 2004; Lovik 1996; Schilp 2013; Starke 2011; Suominen 2015). In two further studies fewer than 3% dropped out, but all were in the control group (Bourdel‐Marchasson 2014; Rogers 1992). In six studies attrition rates ranged from 18% to 43%, but numbers were balanced across groups (Banks 2016; Beck 2012; Kiss 2016; Sharma 2017; Terp 2018; Uster 2013). Similarly, two studies reported attrition rates of 45% to 55% but with similar numbers across groups (Ganzoni 1994; Persson 2002). One study reported more than 20% of participants dropped out but gave reasons why (Vivanti 2015). The final low‐risk study reported a large number of deaths during the study (87%), but to put this into context, all participants had cancer and the numbers of deaths were approximately equal across groups (Evans 1987).
The review authors judged five studies to have an unclear risk of attrition bias (Endevelt 2011; Holyday 2012; Moloney 1983; Ovesen 1993; Pedersen 2016a). Two of these did not report on attrition (Endevelt 2011; Moloney 1983); in two studies attrition rates ranged from 25% to 35% and while the numbers were similar across groups there were no reasons given for withdrawals (Ovesen 1993; Pedersen 2016a). In the final study there was a low number of dropouts (all due to death), but data on weight change was only available 48% of participants and no information was reported to explain why (Holyday 2012).
The remaining five studies had a high risk of attrition bias (Bonilla‐Palomas 2016; Caccialanza 2015; Feldblum 2011; Jensen 1997; Silvers 2014). In one study, mortality in the control group was more than double that in intervention group (Bonilla‐Palomas 2016). In the remaining four studies, overall attrition rates ranged from 25.8% to 58%, but were not balanced across groups. In one study an overall rate of 25.8% was calculated from 11.5% in the advice plus ONS group compared to 32% in the no advice or supplements group (Feldblum 2011); in a further study overall attrition of 28.6% broke down to 10% in the advice plus ONS group and 45% in the no advice or ONS group (Silvers 2014). Conversely, Jensen reported 33.5% attrition overall with 50% withdrawals in the advice and ONS group compared to 17% in the no advice or ONS group (Jensen 1997) and finally Caccialanza showed an overall attrition rate of 58% due to 71% in the advice plus ONS group and 46% in the no advice or ONS group (Caccialanza 2015).
Dietary advice plus ONS compared with no advice and no ONS
The review authors judged eight of the 13 studies in this comparison to have a low risk of attrition bias (Anbar 2014; Jahnavi 2010; Hampson 2003; Neelemaat 2011; Paton 2004; Payette 2002; Um 2014; Wyers 2013). The first study reported that all participants completed the study (Anbar 2014). Five studies reported less than 15% attrition and provided reasons for dropouts (Hampson 2003; Jahnavi 2010; Payette 2002; Um 2014; Wyers 2013). The remaining two studies had almost 30% attrition, but provided reasons for this and numbers were similar across groups (Neelemaat 2011; Paton 2004).
Three studies had an unclear risk of bias; one study did not report any information on attrition (Chandra 1985). A further study had 16% attrition, but did not report which group withdrawals were from (Berneis 2000). Finally, in the Baldwin study, while 2% withdrew (reasons not given) and only 43% were still alive at 12 months, death rates were similar across groups (Baldwin 2011).
Two studies had a high risk of attrition bias (Calegari 2011; Persson 2007). In one study there was 17% attrition, but all from the control group (Calegari 2011); while in the second there was a 50% attrition rate with 43% in the intervention group and 56% in the control group (Persson 2007).
Selective reporting
Dietary advice compared with no advice
Seven out of the 24 studies included in this comparison had a low risk of selective reporting bias; for five studies a protocol was identified and all specified outcomes were reported (Campbell 2008; Fernandez‐Barres 2017; Forster 2012; Salva 2011; Stow 2015). For a further two studies there was no published protocol, but a review author was an investigator on the study and provided all requisite data (Baldwin 2011; Weekes 2009).
The review authors judged 14 studies to have an unclear risk of selective reporting bias (Alo 2014; Cano‐Torres 2017; Casals 2015; Dixon 1984; Gu 2015; Imes 1988; Locher 2013; Macia 1991a; Manguso 2005; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Wong 2004). They were unable to identify a pre‐registered or pre‐published protocol for 12 studies; however, in 11 of these studies, all the outcomes described in the methods section were reported in the results (Alo 2014; Cano‐Torres 2017; Casals 2015; Gu 2015; Imes 1988; Macia 1991a; Manguso 2005; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Wong 2004). In a further study, all outcomes described in the methods section except one were reported in results (Rydwik 2008). In one study, the review authors identified the study protocol, but one outcome was not reported at all and the available results for the other outcomes only reported at the earlier of two planned time points (Locher 2013).
The remaining three studies had a high risk of selective reporting bias (Kunvik 2018; Ollenschlager 1992; Tu 2013). For one study, review authors identified the pre‐registered protocol but not all the main aims of the study were reported (Kunvik 2018). The review authors did not identify the protocols for the remaining two studies; for one study additional data for outcomes were provided by the investigators, but there were still some missing data for some outcomes (Ollenschlager 1992) and in the second study investigators reported outcomes using a composite score and not the original data (Tu 2013).
Dietary advice compared with ONS
The review authors judged two of 12 studies reporting data for this comparison to have a low risk of selective reporting bias (Baldwin 2011; Stow 2015). In one study the pre‐registered protocol was identified and all outcomes were reported (Stow 2015). The review authors did not identify a pre‐registered protocol for the remaining study, but a review author was an investigator on the study and provided all requisite data (Baldwin 2011).
The review authors judged nine studies to have an unclear risk of selective reporting bias. For eight studies they were not able to identify a pre‐registered protocol, but investigators reported in the results section all the outcomes listed in the methods sections (Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008); for one of these the results were not in an analysable format, but the investigator provided the data (Schwenk 1999). In the remaining study, the review authors identified a protocol, but this only records the primary outcome; other outcomes listed in the methods section of the full paper are reported in results section (Parsons 2016).
The review authors judged one study to have a high risk of bias as they could not identify a study protocol and some outcomes listed in the methods section were not reported in the results section (Akpele 2004).
Dietary advice versus dietary advice plus ONS
The review authors judged three out of 22 studies in this comparison to have a low risk of selective reporting (Baldwin 2011; Diouf 2016; Huynh 2015). For two of these the review authors identified a study protocol and investigators reported all outcome measures (Diouf 2016; Huynh 2015). For the remaining study, the review authors did not identify a protocol, but one of the investigators is a review author and provided all requisite data (Baldwin 2011).
The review authors judged 16 studies to have an unclear risk of selective reporting bias. They did not identify a pre‐registered protocol for 15 studies, so they were not able to judge if all outcomes were reported by investigators, although some investigators did provide additional details for some outcomes on request (Arnold 1989; Beattie 2000; Burden 2011; de Luis 2003; de Sousa 2012; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kapoor 2017; Le Cornu 2000; McCarthy 1999; Murphy 1992; Norman 2008b; Rabeneck 1998; Sharma 2002a). The review authors identified a published protocol for one study, but it did not report on all pre‐specified secondary outcome measures (Burden 2017).
The review authors judged the remaining three studies to have a high risk of bias (Kendell 1982; Olejko 1984; Wilson 2001). One study lacked a published protocol and did not report results for all the outcomes that were listed in the methods section (Wilson 2001), while two further studies reported all outcomes, but only using general narrative statements and no data (these were also not available from the investigators) (Kendell 1982; Olejko 1984).
Dietary advice plus ONS, if required, compared with no advice and no ONS
The review authors deemed eight studies to have a low risk of selective reporting bias as investigators reported all outcomes that were specified in the protocol in the results section (Beck 2012; Bonilla‐Palomas 2016; Caccialanza 2015; Endevelt 2011; Kiss 2016; Pedersen 2016a; Schilp 2013; Terp 2018). Investigators for one study reported most outcome measures (some secondary outcomes missing) (Schilp 2013).
Review authors were unable to identify a protocol for 22 studies and judged there to be an unclear risk of selective reporting bias as they were not sure whether investigators reported all planned outcomes (Andersson 2017; Banks 2016; Beck 2015; Bourdel‐Marchasson 2014; Carey 2013; Evans 1987; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Jensen 1997; Lovik 1996; Moloney 1983; Ovesen 1993; Persson 2002; Rogers 1992; Sharma 2017; Silvers 2014; Starke 2011; Uster 2013; Vivanti 2015).
The review authors judged the remaining study to have a high risk of selective reporting bias (Suominen 2015). While they identified a published protocol for the study, in which all outcomes were reported, the study investigators only provided narrative text and no data were available for the primary outcome (Suominen 2015).
Dietary advice plus ONS compared with no advice and no ONS
The review authors judged one study to have a low risk of selective outcome reporting (Baldwin 2011). This study did not have a published protocol, but one of the investigators is a review author and provided all requisite data (Baldwin 2011).
Nine studies had an unclear risk of selective reporting bias as the review authors did not identify the relevant published protocols (Anbar 2014; Berneis 2000; Calegari 2011; Hampson 2003; Jahnavi 2010; Paton 2004; Payette 2002; Persson 2007; Um 2014); in seven studies the lack of a published protocol meant that review authors were unable to judge if investigators reported all planned outcomes (Anbar 2014; Berneis 2000; Calegari 2011; Hampson 2003; Jahnavi 2010; Payette 2002; Um 2014). In a further two of these studies the data were not always in a format that allowed analysis and some additional data were made available from the investigators (Paton 2004; Persson 2007).
The review authors judged three studies to have a high risk of bias as they identified a protocol, but the investigators did not report all the outcomes (Chandra 1985; Neelemaat 2011; Wyers 2013).
Other potential sources of bias
This domain was mainly assessed on the basis of whether the baseline characteristics revealed any imbalances between the intervention and control groups.
Dietary advice compared with no advice
Investigators reported baseline variables to be similar between the groups in nine of the 24 studies in this comparison and the review authors considered these studies to be at low risk of bias (Alo 2014; Casals 2015; Fernandez‐Barres 2017; Kunvik 2018; Manguso 2005; Ollenschlager 1992; Rydwik 2008; Weekes 2009; Wong 2004). One study reported similar baseline characteristics, but was stopped prematurely which led to an overall unclear risk of bias from other sources (Baldwin 2011). One study reported no differences in baseline characteristics, but investigators did not report nutritional status as this was not an inclusion criterion of their study, so the review authors judged this study to have an unclear risk of bias (Pivi 2011).
In seven studies there were differences in the baseline characteristics (Campbell 2008; Cano‐Torres 2017; Forster 2012; Gu 2015; Imes 1988; Salva 2011; Stow 2015). In one of the studies, there was additional clustering bias which led to a judgement of a high risk of bias (Stow 2015). Details are presented in the additional tables (Table 14).
9. Dietary advice versus no advice: differences at baseline characteristics.
| Study | Differences at baseline | Risk of bias judgement |
| Campbell 2008 | The numbers of participants malnourished at baseline differed between groups. | Unclear risk |
| Cano‐Torres 2017 | Difference in haemoglobin levels between groups, but unlikely to affect outcomes | Low risk |
| Forster 2012 | Intervention group 1 (food group) had significantly higher alcohol intake than micronutrient or control groups. | Unclear risk |
| Gu 2015 | Gender imbalance (more males than females), but unlikely to affect outcomes | Low risk |
| Imes 1988 | Participants in the no advice group were younger and in better clinical condition than those in the group receiving dietary advice. | Unclear risk |
| Salva 2011 | Intervention group were frailer and more of these participants were malnourished or at risk of malnourishment.This trial also has potential sources of bias related to being cluster‐randomised | High risk |
| Stow 2015 | Control group (food‐based) were heavier, had higher energy, protein and fluid intake as well as higher VAS score.This trial also has potential sources of bias related to being cluster‐randomised. | High risk |
Six studies did not supply details of baseline characteristics and the review authors judged all of these to have an unclear risk of bias (Dixon 1984; Locher 2013; Macia 1991a; Ravasco 2005a; Ravasco 2005b; Tu 2013). In two of these studies baseline characteristics were not shown, but one study reported them to be statistically equivalent (Dixon 1984) and the second reported successful balancing for sex and BMI (Locher 2013).
Dietary advice compared with ONS
Reported baseline variables were similar between the groups in four of the 12 studies that compared dietary advice to ONS and the review authors consider three of these studies to be at a low risk of bias (Akpele 2004; Schwenk 1999; Singh 2008). One study reported similar baseline characteristics, but was stopped prematurely which led to an overall unclear risk of bias from other sources (Baldwin 2011). A further study reported no differences in baseline characteristics, but did not report nutritional status as this was not an inclusion criterion of the study, so the review authors judged this study to have an unclear risk of bias (Pivi 2011).
Four studies reported differences between some characteristics of the groups at baseline (Gray‐Donald 1995; Hernandez 2014; Parsons 2016; Stow 2015). In one of the studies, there was additional clustering bias which led to a judgement of a high risk of bias (Stow 2015). Details are presented in the additional tables (Table 15).
10. Dietary advice compared with oral nutritional supplements: differences in baseline characteristics.
| Study | Differences at baseline | Risk of bias judgement |
| Gray‐Donald 1995 | Appetite was better in the advice group than in the supplements group. | Unclear risk |
| Hernandez 2014 | SIgnificantly higher total serum protein and creatinine in control group. | Unclear risk |
| Parsons 2016 | Visual analogue score in EQ5D higher in supplement group. | Unclear risk |
| Stow 2015 | Control group (food‐based) were heavier, had higher energy, protein and fluid intake as well as higher VAS score. This study also has potential sources of bias related to being cluster randomised. |
High risk |
In three studies investigators provided no details of baseline characteristics and the review authors judged these to have an unclear risk of bias (Kalnins 2005; Ravasco 2005a; Ravasco 2005b).
Dietary advice versus dietary advice plus ONS
Investigators reported baseline variables to be similar between the groups in eight of the 22 studies in this comparison and the review authors consider these studies to be at a low risk of bias (Arnold 1989; Burden 2017; de Luis 2003; de Sousa 2012; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Le Cornu 2000; Rabeneck 1998). The review authors note that in one of these studies there were more participants in the intervention group and that at the point of recruitment it was undecided if surgery would be open or laparoscopic; the default used was open‐surgery stratum for randomisation, but the groups seem to be well‐balanced (Burden 2017).
Eight studies reported differences in baseline characteristics (Beattie 2000; Diouf 2016; Huynh 2015; Kapoor 2017; McCarthy 1999; Murphy 1992; Sharma 2002a; Wilson 2001); these characteristics are presented in the additional tables (Table 16).
11. Dietary advice versus dietary advice plus oral nutritional supplements: differences in baseline characteristics.
| Study | Differences at baseline | Risk of bias judgement |
| Beattie 2000 | Participants in the advice plus supplements group were significantly younger than those in the advice only group. | Unclear risk |
| Diouf 2016 | Some non‐significant minor differences in variables at baseline. | Low risk |
| Huynh 2015 | Baseline characteristics comparable except weight (control group heavier). | Unclear risk |
| Kapoor 2017 | Several differences in baseline characteristics, but baseline parameters adjusted to observe the overall difference between groups using a generalised estimating equation | Low risk |
| McCarthy 1999 | The group receiving nutritional supplements were lighter and received a smaller amount of radiation. | Unclear |
| Murphy 1992 | The group receiving dietary advice plus nutritional supplements were 5 kg heavier at the start of the study than the group receiving dietary advice alone. | Unclear risk |
| Sharma 2002a | Baseline characteristics only compared for the participants who completed the study and five participants crossed over from the control group to the intervention group. | High risk |
| Wilson 2001 | The dietary counselling and supplement group were significantly older than the dietary group. | Unclear risk |
The review authors judged six studies to have an unclear risk of bias; one study reported similar baseline characteristics, but was stopped prematurely which led to an overall unclear risk of bias from other sources (Baldwin 2011). Five studies did not present baseline characteristics (Burden 2011; Dixon 1984; Kendell 1982; Norman 2008b; Olejko 1984). While two of these did not detail baseline characteristics, investigators reported these to be similar (Dixon 1984; Norman 2008b); the review authors judged both studies to have an unclear risk as the available information was insufficient to assess whether an important risk of bias exists. One study also measured dietary intake using an unreliable outcome measure and the reported SDs indicated that data for this outcome might be skewed; the review authors judged this study to have an unclear risk of bias overall (Burden 2011).
Dietary advice plus ONS, if required, compared with no advice and no ONS
Baseline variables were similar between the groups in 23 of the 31 studies in this comparison and the review authors considered these studies to have a low risk of bias (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Endevelt 2011; Evans 1987; Feldblum 2011; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Ovesen 1993; Pedersen 2016a; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Starke 2011; Suominen 2015; Terp 2018).
There were differences between some characteristics of the groups at baseline in six studies (Carey 2013; Forli 2001; Jensen 1997; Moloney 1983; Silvers 2014; Uster 2013); these are presented in the additional tables (Table 17).
12. Dietary advice plus supplements, if required, compared with no advice and no supplements: differences in baseline characteristics.
| Study | Differences at baseline | RIsk of bias judgement |
| Carey 2013 | Participants in intervention groups weighed less and had lower BMI and MAMC and pre‐op weight loss was higher. | Unclear risk |
| Forli 2001 | Some of the assessments of lung function differed significantly between groups. | Unclear risk |
| Jensen 1997 | The participants in the no advice group were significantly older and heavier than those in the advice group. | Unclear risk |
| Moloney 1983 | The treatment group were older than the no treatment group. | Unclear risk |
| Silvers 2014 | Intervention group older and with a higher BMI. | Unclear risk |
| Uster 2013 | Performance status lower in intervention group, comparison of tumour types between groups indicated that patients with head and neck tumours were all randomised to the intervention group, with none in the control group. | Unclear risk |
BMI: body mass index
Two studies did not report details of baseline characteristics and the review authors judged both of these studies to have an unclear risk of bias (Ganzoni 1994; Vivanti 2015).
Dietary advice plus ONS compared with no advice and no ONS
Baseline variables were similar between the groups in seven of the 13 studies that compared data and the review authors considered these studies to have a low risk of bias (Anbar 2014; Calegari 2011; Jahnavi 2010; Neelemaat 2011; Paton 2004; Um 2014; Wyers 2013). One study reported similar baseline characteristics, but was stopped prematurely which led to an overall unclear risk of bias from other sources of bias (Baldwin 2011).
Two studies reported some differences between groups at baseline (Hampson 2003; Payette 2002). Details are presented in the additional tables (Table 18).
13. Dietary advice plus supplements compared with no advice and no supplements: differences in baseline characteristics.
| Study | Differences at baseline | RIsk of bias judgement |
| Hampson 2003 | Treatment group were significantly lighter and had a lower fat mass than the control group. | Unclear risk of bias |
| Payette 2002 | Baseline characteristics similar apart from age (the control group was significantly younger than the intervention group), but this was judged to be unlikely to affect the outcomes as the mean difference was only 3 years. | Low risk of bias |
Three studies did not report details of baseline characteristics and the review authors judged all of these to have an unclear risk of bias (Berneis 2000; Chandra 1985; Persson 2007). In one study, investigators described the groups as similar in the text, but data are not shown (Persson 2007).
Sensitivity analyses
As no studies were at overall low risk of bias, it was not possible to test the robustness of the results by producing sensitivity analyses exploring the potential impact of study quality.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
All comparisons
Review authors collected data on a variety of outcome measures encompassing clinical and functional status and QoL. However, because the study investigators used different measures to assess the outcomes or reported the data in such a way that they could not be analysed, it has only been possible to pool some of these data within a meta‐analysis. The review authors have summarised the types of data collected and the tools used in the additional tables (Table 6; Table 7; Table 8). They have analysed available data on the change in handgrip strength and included data on several domains of QoL in the analyses.
In the summary of findings tables, the review authors have graded the certainty of the evidence for pre‐defined outcomes (see above) and provided definitions of these gradings (Table 1; Table 2; Table 3; Table 4; Table 5).
The review authors only report results below in the text for time points for which data or information are available. If investigators did not report data or information at a specific time point, then the authors have not included any text.
Group 1 — Dietary advice compared with no advice
The comparison includes 24 studies (3523 randomised participants) (Alo 2014; Baldwin 2011; Campbell 2008; Cano‐Torres 2017; Casals 2015; Dixon 1984; Fernandez‐Barres 2017; Forster 2012; Gu 2015; Imes 1988; Kunvik 2018; Locher 2013; Macia 1991a; Manguso 2005; Ollenschlager 1992; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Salva 2011; Stow 2015; Tu 2013; Weekes 2009; Wong 2004), but there were no usable data from two of these studies (Dixon 1984; Tu 2013). Authors of the 2005 Ravasco study published additional data in 2012 which described additional follow‐up to a median of 6.5 (range 4.9 to 8.1) years (Ravasco 2005a; Ravasco 2012). The study by Macia reports data according to disease site (head and neck, breast and abdominopelvic). The review authors have added these data to the analyses by group for weight, BMI, MAC, MAMC and TSF using different identifiers for head and neck (Macia 1991a) for breast cancer (Macia 1991b) and for abdominopelvic cancers (Macia 1991c).
Please refer to the summary of findings table for the explanations of judgements (Table 1). Note that GRADE judgements are for specific outcomes at the three‐months time point and are not provided for all outcomes at each time point.
Primary outcome
1. Mortality
Zero to three months
Data were available from seven studies (574 participants) at up to three months (Baldwin 2011; Campbell 2008; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Stow 2015). There was no difference in mortality between the participants who received dietary advice and those who received no advice, RR 0.87 (95% CI 0.26 to 2.96) (low‐certainty evidence). Moderate to substantial heterogeneity was observed (I² = 52%) (Analysis 1.1). Removal of one study reduces heterogeneity (I² = 28%) (Stow 2015) and there remains no difference in mortality between groups, RR 1.54 (95% CI 0.39 to 6.17). The Stow study is a cluster‐RCT and it was not possible to recalculate data taking into consideration the design effect for clusters which might account for the difference between this and other studies in the analysis (Stow 2015).
1.1. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 1: Mortality
Four to six months
Data were available from 10 studies (1028 participants) at the time point from four to six months (Baldwin 2011; Cano‐Torres 2017; Casals 2015; Fernandez‐Barres 2017; Imes 1988; Ollenschlager 1992; Pivi 2011; Stow 2015; Weekes 2009; Wong 2004). There was no difference in mortality between the participants who received dietary advice and those who received no advice, RR 0.88 (95% CI 0.61 to 1.27). There was no heterogeneity (I² = 6%) (Analysis 1.1). One study with four intervention groups and a control group reported that overall 23 of 88 (26%) participants died during the four‐month study, but mortality is not reported according to group allocation, so it is not possible to include these results in the analysis (Dixon 1984).
12 months and over
Data were available from five studies (1445 participants) that assessed mortality at 12 months and over (Baldwin 2011; Fernandez‐Barres 2017; Ravasco 2005a; Salva 2011; Weekes 2009). There was no difference in mortality between the participants who received dietary advice and those who received no advice, RR 1.07 (95% CI 0.59 to 1.91), but there was considerable heterogeneity in this analysis (I² = 79%) (Analysis 1.1). The authors are unable to explain this heterogeneity as removal of each study individually did not reduce the heterogeneity noticeably.
The review authors did not undertake a combined total analysis as a number of studies report mortality at several time points.
2. Morbidity
a. Hospital admissions
Four studies provided hospital admission data for inclusion in the meta‐analysis (Fernandez‐Barres 2017; Imes 1988; Stow 2015; Weekes 2009).
Four to six months
Three studies (259 participants) reported on interventions at between four and six months (Imes 1988; Stow 2015; Weekes 2009); there was no difference between the two groups, RR 1.33 (95% CI 0.55 to 3.18) but substantial heterogeneity (I² = 75%) (Analysis 1.2). Removal of the Stow study reduces heterogeneity (I² = 0%) and there remains no difference in hospital readmissions between groups, RR 0.86 (95% CI 0.54 to 1.36) ; the study by Stow is a cluster‐RCT and it was not possible to recalculate data taking into consideration the design effect for clusters which might account for the difference between this and other studies in the analysis (Stow 2015).
1.2. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 2: Number of people admitted or readmitted to hospital
12 months and over
Two studies (230 participants) reported on interventions at 12 months and over (Fernandez‐Barres 2017; Weekes 2009). Again there was no difference between the two groups, RR 0.64 (95% CI 0.36 to 1.13) and no heterogeneity (I² = 0%) (Analysis 1.2).
One study in 106 hospitalised participants, reported significantly fewer mean (SD) hospital admissions per participant after six months in the group receiving dietary advice compared with the group receiving no advice, 0.25 (0.4) admissions and 0.62 (1.11) admissions respectively (P = 0.04) (Casals 2015). Since investigators reported data as mean (SD) admissions, these could not be combined with data from the other studies reporting this outcome.
b. Length of hospital stay
Four studies provided data on length of hospital stay (Cano‐Torres 2017; Casals 2015; Gu 2015; Weekes 2009).
Zero to three months
Hospital stay was significantly shorter in 148 participants receiving dietary advice compared with no advice at up to three months (Gu 2015), MD ‐1.10 days (95% CI ‐1.35 to ‐0.85) (low‐certainty evidence) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 3: Length of hospital stay (days)
Four to six months
Data were available from three studies at over four and up to six months (Cano‐Torres 2017; Casals 2015; Weekes 2009). There was no difference between the two groups, MD 1.93 (95% CI ‐3.42 to 7.28), but considerable heterogeneity (I² = 81%) (Analysis 1.3). Removal of one study reduced heterogeneity (I² = 4%) and length of stay was significantly shorter in the groups receiving no advice compared to the groups receiving advice, MD 4.52 days (95% CI 0.80 to 8.25) (Cano‐Torres 2017). The review authors find it difficult to account for the different effect seen in the study by Cano‐Torres, as both the Casals and the Cano‐Torres studies enrolled hospital inpatients (Cano‐Torres 2017; Casals 2015). In the study by Cano‐Torres, a dietitian provided the dietary advice and in the study by Casals nurses did so; but in the Weekes study, participants also received dietary advice from a dietitian and yet the effect on length of hospital stay is different.
12 months and over
There was no difference in length of hospital stay in 57 participants receiving dietary advice compared with no advice at 12 months (Weekes 2009), MD ‐3.00 days (95% CI ‐11.58 to 5.58) (Analysis 1.3).
The review authors did not undertake a combined analysis as one study reported length of hospital stay at several time points. (Analysis 1.3).
c. Complications
Data on complications were available from two studies (288 participants), but investigators used different tools and so the review authors combined results using the SMD (Forster 2012; Gu 2015).
There were no differences in complications between groups for a study at the three‐month time point, SMD 0.00 (95% CI ‐0.32 to 0.32) (low‐certainty evidence) (Gu 2015) or for the second study at six months, SMD ‐0.21 (95% CI ‐0.55 to 0.12) (Forster 2012). Analysis of both studies combined showed no difference in complications between the two groups, SMD ‐0.10 (95% CI ‐0.34 to 0.13) and no heterogeneity (I² = 0%). There was no heterogeneity between subgroups (I² = 0%) (Analysis 1.4).
3. Measures of nutritional status
a. Change in weight
Zero to three months
10 studies (802 participants) reported data on weight change for interventions lasting up to three months (Baldwin 2011; Campbell 2008; Forster 2012; Gu 2015; Locher 2013; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Stow 2015). Groups receiving dietary advice had a significantly greater weight gain compared with groups receiving no advice, MD 0.97 kg (95% CI 0.06 to 1.87) (low‐certainty evidence). Heterogeneity was considerable (I² = 88%). Removal of one study resulted in no weight change between groups and reduced the heterogeneity to moderate (MD) 0.34 kg (95% CI ‐0.14 to 0.81)(Ravasco 2005a) (Analysis 1.5). It is difficult to explain why the effect size from dietary advice in one study by Ravasco is so much greater than other studies in the group with participants with similar disease backgrounds (Baldwin 2011; Ravasco 2005b). Neither study by Ravasco reported baseline characteristics of participants making comparison across studies difficult; the investigators provided the data included in the meta‐analysis.
1.5. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 5: Change in weight (kg)
Four to six months
Six studies (573 participants) reported data on weight change for interventions that lasted from four to six months (Baldwin 2011; Casals 2015; Fernandez‐Barres 2017; Stow 2015; Weekes 2009; Wong 2004). Groups receiving dietary advice had a significantly greater weight gain compared with groups receiving no advice, MD 1.61 kg (95% CI 0.09 to 3.13). Heterogeneity was considerable, (I² = 83%) (Analysis 1.5). The removal of one study resulted in no difference in weight change between groups (Casals 2015) and reduced the heterogeneity to moderate, MD 0.69 kg (95% CI ‐0.14 to 1.52) (Analysis 1.5). This study was in acutely ill hospitalised participants (Casals 2015), whereas the other studies in this analysis were in participants with chronic conditions living at home or in residential care and the different aetiology of malnutrition might account for the differences seen in effects of interventions.
The study by Pivi reported a higher mean weight change (group body weight range) in participants receiving education on diet compared with a control group, 1.19 kg (54.29 to 50kg) and ‐2.20 kg (61.87 to 60.65kg) respectively (Pivi 2011).
12 months and over
Five studies (1215 participants) reported data on weight change after 12 months of the intervention (Baldwin 2011; Fernandez‐Barres 2017; Macia 1991a; Macia 1991b; Macia 1991c; Salva 2011; Weekes 2009). There was a statistically significant benefit to receiving dietary advice compared with no advice, MD 2.95 kg (95 % CI 0.75 to 5.16), heterogeneity was substantial (I² = 70%). The removal of one study resulted in a significantly greater weight gain in groups receiving dietary advice compared with groups receiving no advice (Salva 2011) and reduced the heterogeneity to zero per cent, MD 3.88 kg (95% CI 2.34 to 5.43) (Analysis 1.5). The study by Salva is a cluster‐RCT and it was not possible to recalculate data taking into consideration the design effect for clusters which might account for the difference between this and other studies in the analysis (Salva 2011).
The review authors did not undertake any combined analysis as several studies report data at more than one time point (Analysis 1.5).
Sensitivity analysis
The review authors imputed the SD of weight change for one study reporting data at 12 months and over (Macia 1991a; Macia 1991b; Macia 1991c). There were too few studies in the analysis at this time point to examine the impact of this on the overall result.
The funnel plot examination, the Egger regression asymmetry test and the Begg's adjusted rank correlation suggested no evidence of small study bias (P = 0.824 and P = 0.348, respectively) (Figure 3).
3.

Funnel plot of comparison: 1 Dietary advice compared with no advice, outcome: 1.5.1 Change in weight (kg).
b. BMI
Zero to three months
Two studies (181 participants) reported the change from baseline in BMI for interventions lasting up to three months (Forster 2012; Stow 2015). There was no difference between groups receiving dietary advice and groups receiving no advice, MD 0.34 kg/m² (95% CI ‐0.24 to 0.92). Heterogeneity was substantial (I² = 61%) (Analysis 1.6). One study was a cluster‐RCT and it was not possible to recalculate data taking into consideration the design effect for clusters which might account for the difference between this and the other study in the analysis (Stow 2015).
1.6. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 6: Change in BMI (kg/m²)
Four to six months
Seven studies (596 participants) reported the change from baseline in BMI for interventions lasting from four to six months (Alo 2014; Cano‐Torres 2017; Casals 2015; Fernandez‐Barres 2017; Kunvik 2018; Stow 2015; Wong 2004). There were no differences between groups receiving dietary advice compared with no advice MD 0.26 kg/m² (95% CI ‐0.08 to 0.60). Heterogeneity was considerable, (I² = 78%). Removal of one study reduced heterogeneity to moderate (I² = 43%) (Casals 2015), but it is not possible to explain how this study might be different from others in the analysis (Analysis 1.6).
One study reported a higher mean change in BMI (group BMI range) in participants receiving education on diet compared with a control group, 1.19 kg/m² (22.71 to 22.84 kg/m²) and ‐2.21 kg/m² (24.81 to 24.32kg/m²) respectively (Pivi 2011).
12 months and over
Three studies (1148 participants) reported data on the change in BMI after 12 months of the intervention (Fernandez‐Barres 2017; Macia 1991a; Macia 1991b; Macia 1991c; Salva 2011). There was a significant benefit to receiving dietary advice compared with no advice, MD 2.17 kg/m² (95 % CI 0.25 to 4.09), but heterogeneity was considerable, (I² = 87%). Removal of each study in the analysis did not reduce the heterogeneity significantly and so it is not possible to explain why the effect sizes differ between studies.
The review authors did not undertake any combined analysis as several studies report data at more than one time point (Analysis 1.6).
c. FFM
In total, three studies (138 participants) reported on this outcome (Campbell 2008; Rydwik 2008; Weekes 2009). One study reported data as body cell mass derived from total body potassium (Campbell 2008) and two studies calculated FFM from the sum of four skinfolds (Rydwik 2008; Weekes 2009); therefore, the review authors used the SMD to combine data (Analysis 1.7).
Zero to three months
Two studies (98 participants) reported data for the change from baseline in FFM at up to three months (Campbell 2008; Rydwik 2008). There was no difference between groups receiving dietary advice and groups receiving no advice, SMD 0.29 kg (95% CI ‐0.11 to 0.69) (low‐certainty evidence) and there was no heterogeneity (I² = 0%) (Analysis 1.7).
Four to six months
One study (40 participants) reported data on the change from baseline in FFM at four to six months (Weekes 2009). There was no difference between groups receiving dietary advice compared with no advice, SMD 0.62 kg (95% CI ‐0.02 to 1.26) (Analysis 1.7).
12 months and over
The Weekes study (40 participants) also reported data for the change from baseline in FFM at 12 months and over (Weekes 2009) and found a large increase in FFM in groups receiving dietary advice compared with no advice, SMD 0.83 kg (95 % CI 0.14 to 1.53) (Analysis 1.7).
The review authors did not undertake any combined analysis as one study reported data at more than one time point (Analysis 1.7).
d. MAC
Zero to three months
Two studies (176 participants) reported the change in MAC at up to three months (Forster 2012; Stow 2015). There was no difference between groups receiving dietary advice and groups receiving no advice, MD 0.23 cm (95% CI ‐0.61 to 1.07). Heterogeneity was substantial (I² = 62%) (Analysis 1.8). The difference between results might be explained by the fact that the Stow study is a cluster‐RCT and it was not possible to recalculate the data taking into consideration the design effect for clusters (Stow 2015).
1.8. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 8: Change in mid‐arm circumference (cm)
Four to six months
Three studies (120 participants) reported the change in MAC from baseline at four to six months (Cano‐Torres 2017; Stow 2015; Weekes 2009). There were no differences between groups receiving dietary advice compared with no advice, MD 0.14 cm (95% CI ‐0.37 to 0.65). There was no heterogeneity, (I² = 0%) (Analysis 1.8).
One study reported a higher mean change in MAC (group MAC range) in participants receiving education on diet compared with a control group, 1.87 cm (24.72 to 25.11cm) and ‐0.41 cm (26.14 to 26.07cm) respectively (Pivi 2011).
12 months and over
Two studies (126 participants) reported data on change in MAC after 12 months of intervention (Macia 1991a; Macia 1991b; Macia 1991c; Weekes 2009); one of the studies reported data according to site of disease (Macia 1991a; Macia 1991b; Macia 1991c). There was no difference between groups receiving dietary advice compared with no advice, MD 0.65 cm (95 % CI ‐0.20 to 1.50) and there was no heterogeneity (I² = 0%).
The review authors did not undertake any combined analysis as several studies report data at more than one time point (Analysis 1.8).
e. MAMC
Zero to three months
Two studies (109 participants) reported the change from baseline in MAMC at up to three months (Manguso 2005; Stow 2015). There was a significant improvement in MAMC in participants receiving dietary advice, MD 1.05 cm (95% CI 0.71 to 1.39) and no heterogeneity (Analysis 1.9).
1.9. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 9: Change in mid‐arm muscle circumference (cm)
Four to six months
Two studies (66 participants) reported the change in MAMC at four to six months (Stow 2015; Weekes 2009). There was a significant increase in MAMC in groups receiving dietary advice compared with no advice, MD 0.56 cm (95% CI 0.07 to 1.04). There was no heterogeneity (I² = 0%) (Analysis 1.9).
One study reported a higher mean change in MAMC (group range for MAMC) in participants receiving education on diet compared with a control group, ‐1.27 cm (20.25 to 19.96 cm) and ‐0.19 cm (21.21 to 21.60 cm), respectively (Pivi 2011).
12 months and over
Two studies (128 participants) reported data for this outcome at 12 months (Macia 1991a; Macia 1991b; Macia 1991c; Weekes 2009), with one study reporting data according to site of disease (Macia 1991a; Macia 1991b; Macia 1991c). There was no difference in MAMC between groups MD 2.04 cm (95 % CI ‐0.07 to 4.15), but heterogeneity was considerable (I² = 91%). The two studies in this analysis include participants from different disease backgrounds, COPD (Weekes 2009) and cancer (Macia 1991a; Macia 1991b; Macia 1991c), and the different aetiology of malnutrition might explain the different effects of intervention. In addition, the review authors derived the data for the Macia study by imputation using an assumed correlation co‐efficient of 0.8 and assumptions may have been incorrect resulting in use of incorrect SDs of change (Macia 1991a; Macia 1991b; Macia 1991c).
The review authors did not undertake any combined analysis as several studies report data at more than one time point (Analysis 1.9).
f. TSF
Zero to three months
Three studies (254 participants) reported the change from baseline in TSF at up to three months (Forster 2012; Manguso 2005; Stow 2015). There was a significant reduction in TSF in participants receiving dietary advice compared to groups receiving no advice, MD ‐0.95 mm (95% CI ‐1.83 to ‐0.08) and no heterogeneity (Analysis 1.10).
1.10. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 10: Change in triceps skinfold thickness (mm)
Four to six months
Two studies (67 participants) reported the change in TSF at four to six months (Stow 2015; Weekes 2009). There were no differences between groups receiving dietary advice compared with no advice, MD 0.89 mm (95% CI ‐0.25 to 2.04). There was low heterogeneity (I² = 5%) (Analysis 1.10).
One study reported a higher mean change in TSF (group range for TSF) in participants receiving education on diet compared with a control group, 2.32 mm (14.20 to 16.40 mm) and 2.20 mm (15.67 to 15.85 mm), respectively (Pivi 2011).
12 months and over
Two studies (128 participants) reported data on change in TSF after 12 months of intervention (Macia 1991a; Macia 1991b; Macia 1991c; Weekes 2009), with one study reporting data according to site of disease (Macia 1991a; Macia 1991b; Macia 1991c). There was no difference in TSF between groups, MD 1.24 mm (95 % CI ‐0.84 to 3.31) and heterogeneity was moderate (I² = 50%).
The review authors did not undertake a combined analysis as several studies report data at more than one time point (Analysis 1.10).
Secondary outcomes
1. Nutritional intake before and after the intervention
a. Change in energy intake
Zero to three months
Nine studies (536 participants) reported changes in energy intake at up to three months (Baldwin 2011; Campbell 2008; Forster 2012; Locher 2013; Manguso 2005; Ravasco 2005a; Ravasco 2005b; Rydwik 2008; Stow 2015). There was no difference between those who received dietary advice and those who received no advice, MD 242.63 kcal (95% CI ‐40.31 to 525.56), but heterogeneity was considerable (I² = 97%) (Analysis 1.11). Removal of each study individually did not reduce the heterogeneity noticeably, so the review authors are unable to explain the high level of heterogeneity.
1.11. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 11: Change in energy intake (kcal)
Four to six months
Three studies (356 participants) measured energy intake in participants receiving dietary advice at four to six months (Fernandez‐Barres 2017; Stow 2015; Wong 2004). Results showed a significantly higher energy intake in those receiving dietary advice compared with no advice, MD 97.17 kcal (95% CI 20.22 to 174.12) and heterogeneity was substantial (I² = 61%) (Analysis 1.11). Removal of one study reduced heterogeneity to zero (I² = 0%) (Stow 2015), and there remained a significantly higher energy intake in groups receiving dietary advice, MD 63.63 kcal (95% CI 55.25 to 72.01). This study is a cluster‐RCT and it was not possible to recalculate data taking into consideration the design effect for clusters which might account for the difference between this and other studies in the analysis (Stow 2015).
12 months and over
One study (111 participants) measured energy intake up to 12 months (Fernandez‐Barres 2017) and reported no difference between groups, MD 52.00 kcal (95% CI ‐64.88 to 168.88).
The review authors did not undertake a combined analysis as several studies report data at more than one time point (Analysis 1.11).
b. Final energy intake
Zero to three months
Two studies (276 participants) reported the final energy intake for participants at up to three months (Gu 2015; Imes 1988). There was no difference between those who received dietary advice and those who received no advice, MD ‐45.91 kcal (95% CI ‐390.74 to 298.92), but heterogeneity was considerable (I² = 80%) (Analysis 1.12). One study was in acutely ill hospital inpatients (Gu 2015) and the second study was in people with Crohn's disease living at home (Imes 1988). The different aetiology of malnutrition in those with acute and chronic disease might account for the differences between these groups as the acute malnutrition might be expected to resolve as the patient gets better and eating return to normal pre‐illness levels.
1.12. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 12: Final energy intake (kcal)
Four to six months
One study (124 participants) measured the final energy intake in participants receiving dietary advice at four to six months (Imes 1988). Investigators reported no difference between those receiving dietary advice compared with no advice, MD ‐20.00 kcal (95% CI ‐320.10 to 280.10) (Analysis 1.12).
12 months and over
One study (37 participants) measured the final energy intake after 12 months (Weekes 2009) and reported a significantly higher intake in the group receiving dietary advice compared with the group receiving no advice, MD 194 kcal (95% CI 34.33 to 353.67) (Analysis 1.12).
The review authors did not undertake a combined analysis as several studies report data at more than one time point (Analysis 1.12).
The 2012 paper by Ravasco presented follow‐up data and reported median energy intakes after a median follow‐up of 6.5 years; the review authors were not able to analyse these in RevMan (Ravasco 2005a). Ravasco reported the median final energy intake was significantly higher in the group receiving dietary advice, 2482 kcal (95% CI 2210 to 2685) than the group receiving no advice 1332 kcal (95% CI 1098 to 1426) (Ravasco 2005a).
c. Change in protein intake
Zero to three months
Five studies (345 participants) reported changes in protein intake at up to three months (Campbell 2008; Ravasco 2005a; Ravasco 2005b; Forster 2012; Stow 2015). Protein intake was significantly higher in those who received dietary advice than those who received no advice, MD 12.50 g (95% CI 2.80 to 22.19), but heterogeneity was considerable (I² = 83%). Removal of the two studies by Ravasco and reporting just the results from the remaining two studies reduced the heterogeneity to zero, and there was no difference in protein intake between the groups, MD 3.15 g (95% CI ‐0.36 to 6.66) (Analysis 1.13). The two Ravasco studies are both in people with cancer (Ravasco 2005a; Ravasco 2005b), whereas the remaining two studies are in older people living at home or in residential care (Forster 2012; Stow 2015). The different clinical background of participants might explain some of the differences in effect size, but it is unlikely to be responsible for all the differences as the final study is in individuals with chronic kidney disease (Campbell 2008), which includes participants with significant illness similar to the Ravasco studies. The studies by Ravasco consistently achieve effects that are substantially different from other similar studies and there is no obvious reason to explain this.
1.13. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 13: Change in protein intake (g)
Four to six months
Four studies (356 participants) measured protein intake in participants receiving dietary advice for four to six months (Fernandez‐Barres 2017; Kunvik 2018; Stow 2015; Wong 2004) and reported a significantly higher protein intake in those receiving dietary advice compared with no advice, MD 3.02 g (95% CI 2.60 to 3.43); there was no heterogeneity (Analysis 1.13).
12 months and over
One study measured protein intake up to 12 months and reported no difference between groups MD 4.00 g (95% CI ‐0.51 to 8.51) (Fernandez‐Barres 2017).
The review authors did not undertake a combined analysis as several studies report data at more than one time point (Analysis 1.13).
d. Final protein intake
Zero to three months
Four studies (426 participants) reported the final protein intake at up to three months (Campbell 2008; Gu 2015; Imes 1988; Manguso 2005). Final protein intake was significantly higher in those who received dietary advice than those who received no advice, MD 8.29 g (95% CI 1.24 to 15.34), but heterogeneity was considerable (I² = 89%) (Analysis 1.14). Removal of each study individually did not reduce the heterogeneity noticeably, so we are unable to explain the high level of heterogeneity.
1.14. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 14: Final protein intake (g)
Four to six months
One study (124 participants) measured protein intake in participants receiving dietary advice for six months (Imes 1988). Investigators reported no significant difference between those receiving dietary advice compared with no advice, MD 5.00 g (95% CI ‐7.32 to 17.32) (Analysis 1.14).
12 months and over
One study (50 participants) measured protein intake at 12 months (Weekes 2009) and reported a significantly higher intake in the group receiving dietary advice compared with the group receiving no advice, MD 11.8 g (95% CI 10.73 to 12.87) (Analysis 1.14).
The 2012 follow‐up paper to the Ravasco study reported protein intake after a median follow‐up of 6.5 years and found this to be significantly higher in the group receiving dietary advice, 74 g (95% CI 69 to 77) than the group receiving no advice 40 g (95% CI 38 to 42.5) (Ravasco 2005a).
The review authors did not undertake a combined analysis as several studies report data at more than one time point (Analysis 1.14).
2. Measures of functional status
a. Handgrip strength
Zero to three months
One study (24 participants) reported the change in handgrip strength at up to three months (Stow 2015). There was no difference between those receiving dietary advice and those receiving no advice, MD ‐0.98 kg force (95% CI ‐3.38 to 1.42) (Analysis 1.15).
1.15. Analysis.

Comparison 1: Dietary advice compared with no advice, Outcome 15: Change in grip strength (kg force)
Four to six months
Two studies (57 participants) reported on the change in handgrip strength from baseline at four to six months (Stow 2015; Weekes 2009). No difference was observed between the groups, MD ‐0.86 kg force (95% CI ‐3.32 to 1.59) and there was substantial heterogeneity (I² = 63%) (Analysis 1.15). The Stow study is a cluster‐RCT and it was not possible to recalculate data taking into consideration the design effect for clusters which might account for the difference between this and the other study in the analysis (Stow 2015).
12 months and over
One study (37 participants) reported on this outcome at 12 months and over (Weekes 2009). There was no difference in handgrip strength between those receiving dietary advice and those receiving no advice, MD 0.30 kg force (95% CI ‐1.32 to 1.92) (Analysis 1.15).
The review authors did not undertake a combined analysis as several studies report data at more than one time point (Analysis 1.15).
3. QoL
Eight studies reported this outcome; three used the EORTC questionnaires (Baldwin 2011; Ravasco 2005a; Ravasco 2005b), one used the FAACT questionnaire (presented separately in the analysis where data for both questionnaires from the study were available) (Baldwin 2011), three used the SF‐12 or SF‐36 (Casals 2015; Forster 2012; Weekes 2009), one used the Kidney Disease Quality of Life Short Form (Campbell 2008), one used the Linear Analogue Self‐ Assessment Questionnaire (Ollenschlager 1992) and one used the SGRQ (Weekes 2009). One study only reported data for the intervention group and so has not been included in any of the analyses (Ollenschlager 1992). A further study reported collecting data on QoL, but does not report any results because of completion of questionnaires by too few participants (Stow 2015).
The review authors have entered data into a meta‐analysis for global QoL scores, physical function, mental function, social function, cognitive function, pain and energy or fatigue using the SMD to combine data using different QoL questionnaires.
a. Global QoL
Zero to three months
Five studies (545 participants) reported on change in global QoL scores (Baldwin 2011; Campbell 2008; Forster 2012; Ravasco 2005a; Ravasco 2005b). There was a large improvement in global QoL in groups receiving advice compared with no advice, SMD 3.30 (95% CI 1.47 to 5.13) (low‐certainty evidence) but there was considerable heterogeneity (I² = 98%) (Analysis 1.16). Removal of each study individually did not reduce the heterogeneity noticeably, however we expect the heterogeneity likely arises from the differences in QoL instruments used, and the differences in clinical backgrounds and care settings. The studies by Ravasco consistently achieve effects that are substantially larger than other similar studies and there is no obvious reason to explain this.
Additionally, one study reported on a second QoL questionnaire (FAACT) (Baldwin 2011); data showed no difference between groups, SMD 0.03 (95% CI ‐0.32 to 0.38) (Analysis 1.16).
Four to six months
Three studies (278 participants) reported on the change in global QoL scores (Baldwin 2011; Casals 2015; Weekes 2009). One of these used two different QoL questionnaires, but the review authors only report one (SGRQ) here (Weekes 2009); a second study also used two questionnaires and these are presented separately (Baldwin 2011). There was no difference in change in global QoL at four to six months between the groups receiving advice and the groups receiving no advice, SMD 0.52 (95% CI ‐0.00 to 1.04). There was substantial heterogeneity (I² = 69%) (Analysis 1.16). Removal of one study reduced heterogeneity (I² = 0%) (Casals 2015) and there remained no difference in results between groups, SMD 0.24 (95% CI ‐0.15 to 0.63). This study randomised acutely ill hospitalised participants (Casals 2015), whereas the other studies in this analysis were in individuals with chronic disease; therefore the different disease backgrounds and aetiology of malnutrition might explain some of the differences.
Additionally, one study reported on a second QoL questionnaire (FAACT) (Baldwin 2011); data showed no difference between groups, SMD ‐0.05 (95% CI ‐0.52 to 0.42) (Analysis 1.16).
12 months and over
Two studies (97 participants) reported on a change in QoL at 12 months or longer (Ravasco 2005a; Weekes 2009); one of these used two different QoL questionnaires, but review authors report only one (SGRQ) here (Weekes 2009). There was a large improvement in global QoL in groups receiving advice compared with groups receiving no advice, SMD 3.79 (95% CI 0.33 to 7.25); however, there was considerable heterogeneity (I² = 98%). Removal of one study reduced heterogeneity (I² = 0%) (Ravasco 2005a) and there remained a large improvement in QoL in the group receiving advice, SMD 0.73 (95% CI 0.24 to 1.21). As in previous analyses, it is difficult to explain the large effect sizes seen in this study, but some of the differences may simply be explained by the different disease background of participants (cancer and COPD) as well as the use of different QoL instruments (Ravasco 2005a).
Review authors did not undertake a combined analysis as several studies reported data at more than one time point.
b. QoL ‐ physical function
Zero to three months
Five studies (429 participants) reported on change in physical function at up to three months (Baldwin 2011; Campbell 2008; Forster 2012; Ravasco 2005a; Ravasco 2005b). There was a large improvement in physical function in groups receiving advice compared with no advice alone, SMD 3.38 (95% CI 1.54 to 5.23). However, there was considerable heterogeneity (I² = 98%). Removal of two studies reduced heterogeneity (I² = 0%) (Ravasco 2005a; Ravasco 2005b), but there was no difference between the groups, SMD ‐0.03 (95% ‐0.26 to 0.19). As in previous analyses, it is difficult to explain the large effect sizes seen in the Ravasco studies, but some of the differences may simply be explained by the different disease background of participants (cancer and COPD) as well as the use of different QoL instruments (Analysis 1.17). The studies by Ravasco consistently achieve effects that are substantially larger than other similar studies and there is no obvious reason to explain this.
Four to six months
Two studies (147 participants) reported on change in physical function at six months (Casals 2015; Weekes 2009), one of which used two different QoL questionnaires, which the review authors did not combine (Weekes 2009). There was no difference between the groups receiving advice and the groups receiving no advice, SMD 0.44 (95% CI ‐0.47 to 1.35) (Analysis 1.17); however, there was considerable heterogeneity between subgroups (I² = 84%). Differences may be due to the different disease backgrounds and aetiology of malnutrition; one study being in acutely ill hospitalised participants (Casals 2015), whereas the other study in this analysis was in outpatients with COPD (Weekes 2009).
The second QoL questionnaire (SGRQ) reported by Weekes showed no difference between groups, SMD 0.38 (95% CI ‐0.24 to 1.01) (Analysis 1.17).
12 months and over
One study (35 participants) using two different QoL questionnaires reported change in physical function at 12 months or over (Weekes 2009). There was no difference between the group receiving advice and the group receiving no advice, either with the SF‐36, SMD 0.65 (95% CI ‐0.03 to 1.33) or with the SGRQ, SMD 0.04 (95% CI ‐0.64 to 0.71) (Analysis 1.17).
Review authors did not undertake a combined analysis as several studies reported data at more than one time point.
c. QoL — mental function
Zero to three months
Five studies (421 participants) reported on change in mental function at up to three months (Baldwin 2011; Campbell 2008; Forster 2012; Ravasco 2005a; Ravasco 2005b). There was a large improvement in mental function in groups receiving advice compared with no advice, SMD 2.99 (95% CI 1.30 to 4.67); however, there was considerable heterogeneity (I² = 98%) (Analysis 1.18). Removal of two studies reduced heterogeneity (I² = 0%) (Ravasco 2005a; Ravasco 2005b) and there remained a significant but small to moderate improvement in mental function in the groups receiving advice compared with the groups receiving no advice, SMD 0.36 (95% 0.13 to 0.59). As in previous analyses, it is difficult to explain the large effect sizes seen in the studies by Ravasco, but some of the differences may simply be explained by the different disease background of participants (cancer, kidney disease and older people) as well as the use of different QoL instruments.
Four to six months
Two studies (106 participants) reported on change in physical function at six months (Casals 2015; Weekes 2009). There was no difference between the groups receiving advice and the groups receiving no advice, SMD 0.49 (95% CI ‐0.61 to 1.59); however, there was considerable heterogeneity (I² = 89%) (Analysis 1.18). One study was in people who were acutely ill and hospitalised (Casals 2015) whereas the other study in this analysis was in outpatients with COPD (Weekes 2009), therefore, the different disease backgrounds and aetiology of malnutrition might explain some of the differences.
12 months and over
One study (35 participants) reported change in mental function at 12 months or over (Weekes 2009). There was no difference between the group receiving advice and the group receiving no advice, SMD ‐0.64 (95% CI ‐0.05 to 1.34) (Analysis 1.18).
Review authors did not undertake a combined analysis as one study reported data at more than one time point.
d. QoL — social function
Zero to three months
Five studies (419 participants) reported on change in social function at up to three months (Baldwin 2011; Campbell 2008; Forster 2012; Ravasco 2005a; Ravasco 2005b). There was a large improvement in social function in groups receiving advice compared with no advice, SMD 3.52 (95% CI 1.71 to 5.32); however, there was considerable heterogeneity (I² = 98%) (Analysis 1.19). Removal of two studies reduced heterogeneity (I² = 0%) (Ravasco 2005a; Ravasco 2005b) and there remained a significant but small to moderate improvement in social function in the groups receiving advice compared with the groups receiving no advice, SMD 0.38 (95% 0.15 to 0.61). As in previous analyses, it is difficult to explain the large effect sizes seen in the studies by Ravasco, but some of the differences may simply be explained by the different disease background of participants (cancer, kidney disease and older people) as well as the use of different QoL instruments.
Four to six months
One study (40 participants) using two different QoL questionnaires to report on change in social function at six months (Weekes 2009). There was no difference between the group receiving advice and the group receiving no advice, either with the SF‐36 SMD 0.10 (95% CI ‐0.52 to 0.72) or with the SGRQ, SMD 0.11 (95% CI ‐0.50 to 0.73) (Analysis 1.19).
12 months and over
One study (34 participants) using two different QoL questionnaires reported change in social function at 12 months or over (Weekes 2009). There was no difference between the group receiving advice and the group receiving no advice using the SF‐36, SMD 0.36 (95% CI ‐0.32 to 1.04); however, results from the SGRQ favoured advice, SMD 1.17 (95% CI 0.44 to 1.91) (Analysis 1.19).
Review authors did not undertake a combined analysis as one study reported data at more than one time point.
e. QoL — cognitive function
Zero to three months
Four studies (287 participants) reported on change in cognitive function at up to three months (Baldwin 2011; Campbell 2008; Ravasco 2005a; Ravasco 2005b). There was a large improvement in cognitive function in groups receiving advice compared with no advice, SMD 3.43 (95% CI 0.79 to 6.07), however, there was considerable heterogeneity (I² = 98%) (Analysis 1.20). Removal of each study individually did not reduce the heterogeneity noticeably, however we expect the heterogeneity likely arises from the differences in QoL instruments used, and the differences in clinical backgrounds and care settings. The studies by Ravasco consistently achieve effects that are substantially larger than other similar studies and there is no obvious reason to explain this.
f. QoL — pain
Zero to three months
Four studies (376 participants) reported on change in pain at up to three months (Baldwin 2011; Campbell 2008; Ravasco 2005a; Ravasco 2005b). There was a large reduction in pain in groups receiving advice compared with no advice, SMD ‐5.48 (95% CI ‐8.13 to ‐2.84) (Analysis 1.21); however, there was considerable heterogeneity (I² = 99%). Removal of two studies reduced heterogeneity (I² = 29%) (Ravasco 2005a; Ravasco 2005b) and there was no difference in pain between the groups receiving advice compared with the groups receiving no advice, SMD ‐0.15 (95% ‐0.14 to 0.45) (P = 0.32). As in previous analyses, it is difficult to explain the large effect sizes seen in the studies by Ravasco, but some of the differences may simply be explained by the different disease background of participants (cancer and older people) as well as the use of different QoL instruments. The studies by Ravasco consistently achieve effects that are substantially larger than other similar studies and there is no obvious reason to explain this.
Four to six months
One study (40 participants) reported on change in pain at six months (Weekes 2009). There was no difference between the group receiving advice and the group receiving no advice, SMD 0.30 (95% CI ‐0.32 to 0.93) (Analysis 1.21).
12 months and over
One study (34 participants) reported change in pain at 12 months or over (Weekes 2009). There was no difference between the group receiving advice and the group receiving no advice, SMD 0.22 (95% CI ‐0.46 to 0.90) (Analysis 1.21).
Review authors did not undertake a combined analysis as one study reported data at more than one time point.
g. QoL ‐ energy/fatigue
Zero to three months
Four studies (375 participants) reported on change in energy/fatigue at up to three months (Baldwin 2011; Forster 2012; Ravasco 2005a; Ravasco 2005b). There was a large improvement in energy/fatigue in groups receiving advice compared with no advice, SMD ‐5.95 (95% CI ‐8.65 to ‐3.25) (Analysis 1.22), however, there was considerable heterogeneity (I² = 99%). Removal of each study individually did not reduce the heterogeneity noticeably, however we expect the heterogeneity likely arises from the differences in QoL instruments used, and the differences in clinical backgrounds and care settings. The studies by Ravasco consistently achieve effects that are substantially larger than other similar studies and there is no obvious reason to explain this.
Four to six months
One study (40 participants) reported on change in energy/fatigue at six months (Weekes 2009). There was no difference between the group receiving advice and the group receiving no advice, SMD 0.06 (95% CI ‐0.56 to 0.69) (Analysis 1.22).
12 months and over
One study (34 participants) reported change in energy/fatigue at 12 months or over (Weekes 2009). There was no difference between the group receiving advice and the group receiving no advice, SMD 0.34 (95% CI ‐0.33 to 1.01) (Analysis 1.22).
Review authors did not undertake a combined analysis as one study reported data at more than one time point.
4. Cost
The lead investigator on the Weekes study provided unpublished data on cost‐effectiveness (Weekes 2009). In this study of outpatients with COPD (59 participants), the total costs and subcategories of costs were not significantly different between the groups at six or 12 months. However, based on bootstrapping of incremental cost‐effectiveness ratios (ICERs), the probability for dietary advice being cost‐effective for QALYs gained was 93% at 12 months when the willingness to pay was GBP 20,000 per QALY (a conservative ceiling value often used in England) and 90% when the willingness to pay was zero GBP, mainly resulting from the cost savings associated with hospital admissions.
One study reported there was no difference in the mean (SD) costs of hospitalisation in those that received dietary advice, CNY 16,099 (1243) and those that received no advice CNY 17,834 (1823) (Gu 2015).
Group 2 — Dietary advice compared with ONS
This comparison includes 12 studies (852 participants) (Akpele 2004; Baldwin 2011; Gray‐Donald 1995; Hernandez 2014; Kalnins 2005; Parsons 2016; Pivi 2011; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015).
Please refer to the summary of findings table for the explanations of judgements (Table 2). Note that GRADE judgements are for specific outcomes at the three‐months time point and are not provided for all outcomes at each time point.
Primary outcome
1. Mortality
Zero to three months
Eight studies (576 participants) provided data at up to three months (Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Stow 2015). There was no difference in mortality between the participants who received dietary advice and those who received ONS, RR 0.66 (95% CI 0.34 to 1.26) (low‐certainty evidence) (Analysis 2.1). There was no heterogeneity (I² = 0%).
2.1. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 1: Mortality
Four to six months
Three studies (302 participants) provided data at between four and six months (Baldwin 2011; Pivi 2011; Stow 2015). There was difference in mortality between the participants who received dietary advice and those who received ONS, RR 0.98 (95% CI 0.65 to 1.47). There was no heterogeneity (I² = 0%) (Analysis 2.1).
12 months and over
Two studies (256 participants) assessed mortality at 12 months and over (Baldwin 2011; Ravasco 2005a). There was no difference in mortality between the participants who received dietary advice and those who received ONS, RR 0.56 (95% CI 0.15 to 2.06), but there was considerable heterogeneity in this analysis (I² = 80%) (Analysis 2.1). Participants in both studies had cancer, but one study was in those receiving palliative treatment (Baldwin 2011), whereas in the second study a higher proportion of individuals had potentially curable disease (Ravasco 2005a).
Review authors did not undertake a combined analysis as a number of studies report mortality at several time points (Analysis 2.1).
2. Morbidity
a. Hospital admissions
Two studies (111 participants) provided data on hospital admissions (Schwenk 1999; Stow 2015). No studies reported data on length of hospital stay or complications.
Zero to three months
At up to three months, there was no difference between the two groups, RR 0.36 (95% CI 0.04 to 3.24) (low‐certainty evidence) (Schwenk 1999) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 2: Number of people admitted or readmitted to hospital
Four to six months
One study reported at four to six months (Stow 2015) and found that those receiving ONS had significantly fewer hospital admissions that those receiving dietary advice, RR 3.63 (95% CI 1.37 to 9.60) (Analysis 2.2).
A combined analysis of both studies showed significantly fewer hospital admissions in those receiving ONS, RR 2.30 (95% CI 1.02 to 5.15), but substantial heterogeneity (I² = 72%) (Analysis 2.2). The two studies were undertaken in different populations, i.e. one studied people with HIV infection (Schwenk 1999) and the second was in older people in residential care (Stow 2015). The two studies used different methodologies; one study was a parallel RCT (Schwenk 1999), whereas the other was a cluster‐RCT (Stow 2015).
3. Measures of nutritional status
a. Change in weight
Zero to three months
Nine studies (517 participants) reported the change in weight from baseline at up to three months (Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Parsons 2016; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015), but one study reported no change in weight with a SD of zero in the group receiving ONS and so the MD for this study was not estimable (Ravasco 2005b). There was no difference between groups receiving dietary advice and groups receiving ONS, MD ‐0.14 kg (95% CI ‐2.01 to 1.74) (low‐certainty evidence), but heterogeneity was considerable (I² = 94%) (Analysis 2.3). Removal of one study reduced the heterogeneity to zero (Ravasco 2005a); and the result then showed significantly greater weight gain in the groups receiving ONS, MD ‐0.81 kg (95% CI ‐1.37 to ‐0.24). It is not possible to explain the effect on heterogeneity on removing this study on the basis of differences in clinical condition of the participants. The lead investigator on this study provided these data on request (Ravasco 2005a).
2.3. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 3: Change in weight (kg)
Four to six months
One study (44 participants) reported analysable data on weight change at four to six months (Stow 2015). There was no difference between groups receiving dietary advice compared with ONS, MD 0.03 kg (95% CI ‐1.72 to 1.78) (Analysis 2.3).
One study reported a lower mean weight change (group range for weight) in participants receiving education on diet compared with participants receiving ONS, 1.19 kg (54.29 to 50 kg) and 6.66 kg (54.70 to 57.93 kg) respectively (Pivi 2011).
Review authors did not undertake a combined analysis as one study reports data at more than one time point (Analysis 2.3).
The funnel plot examination, the Egger regression asymmetry test and the Begg's adjusted rank correlation suggested evidence of significant small study bias (P = 0.049 and P = 0.266, respectively), although there were only eight studies in this analysis, therefore, the test may be invalid (Figure 4).
4.

Funnel plot of comparison: 2 Dietary advice compared with ONS, outcome: 2.3.1 Change in weight (kg).
b. BMI
Zero to three months
Two studies (97 participants) reported the change in BMI from baseline to up to three months (Singh 2008; Stow 2015). There was no difference between groups receiving dietary advice and groups receiving ONS, MD 0.21 kg/m² (95% CI ‐0.64 to 0.23). There was no heterogeneity (I² = 0%) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 4: Change in BMI (kg/m²)
Four to six months
One study (44 participants) reported analysable data for the change in BMI from baseline to four to six months (Stow 2015). There was no difference between groups receiving dietary advice compared with ONS, MD ‐0.01 kg/m² (95% CI ‐0.72 to 0.70) (Analysis 2.4).
One study reported a lower mean change in BMI (group range for BMI) in participants receiving education on diet compared with participants receiving ONS, 1.19 kg/m² (22.71 to 22.84 kg/m²) and 6.55 kg/m² (21.66 to 22.98 kg/m²) respectively (Pivi 2011).
Review authors did not undertake a combined analysis as one study reports data at more than one time point (Analysis 2.4).
c. FFM
One study (50 participants) reported data on change in FFM at up to three months (Schwenk 1999). After eight weeks of intervention there was no difference in the mean (SD) change in FFM between those receiving dietary advice (3.8% (6.2)) and those receiving ONS (1.6% (4.5)).
d. MAC
Zero to three months
Two studies (91 participants) reported data on change in MAC from baseline to up to three months (Singh 2008; Stow 2015). There was no difference between groups receiving dietary advice and groups receiving ONS, MD ‐0.17 cm (95% CI ‐0.72 to 0.38). There was no heterogeneity (I² = 0%) (Analysis 2.6).
2.6. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 6: Change in mid‐arm circumference (cm)
Four to six months
One study (20 participants) reported data on change in MAC from baseline to four to six months (Stow 2015). There was no difference between groups, MD ‐0.15 cm (95% CI ‐4.30 to 4.00) (Analysis 2.6).
One study reported a lower mean change in MAC (group range for MAC) in participants receiving education on diet compared with participants receiving ONS, 1.87 cm (24.72 to 25.11 cm) and 5.44 cm (23.99 to 25.20 cm), respectively (Pivi 2011).
Review authors did not undertake a combined analysis as one study reports data at more than one time point (Analysis 2.6).
e. MAMC
Zero to three months
Two studies (81 participants) reported the change from baseline in MAMC at up to three months (Gray‐Donald 1995; Stow 2015). There was no difference in MAMC between groups, MD 0.43 cm (95% CI ‐0.36 to 1.22) and no heterogeneity (Analysis 2.5).
2.5. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 5: Change in mid‐arm muscle circumference (cm)
Four to six months
One study (29 participants) reported the change in MAMC from baseline to four to six months (Stow 2015). There was no difference between groups receiving dietary advice compared with ONS, MD ‐0.11 cm (95% CI ‐1.07 to 0.85) (Analysis 2.5).
One study reported a lower mean change in MAMC (group range for MAMC) in participants receiving education on diet compared with a control group, ‐1.27 cm (20.25 to 19.96 cm) and 3.43 cm (20.36 to 21.01 cm), respectively (Pivi 2011).
Review authors did not undertake a combined analysis as one study reports data at more than one time point (Analysis 2.5).
f. TSF
Zero to three months
Three studies (129 participants) reported the change in TSF from baseline up to three months (Gray‐Donald 1995; Singh 2008; Stow 2015). There was no difference in TSF between groups, MD ‐0.75 mm (95% CI ‐1.55 to 0.05) and no heterogeneity (Analysis 2.7).
2.7. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 7: Change in triceps skinfold thickness (mm)
Four to six months
One study (17 participants) reported the change from baseline in TSF to four to six months (Stow 2015). There was no difference between groups receiving dietary advice compared with those receiving ONS, MD ‐0.99 mm (95% CI ‐8.96 to 6.98) (Analysis 2.7).
One study reported a higher mean change in TSF (group range for TSF) in participants receiving education on diet compared with those receiving ONS, 2.32 mm (14.20 to 16.40 mm) and 1.44 mm (11.54 to 13.55 mm), respectively (Pivi 2011).
Review authors did not undertake a combined analysis as one study reports data at more than one time point (Analysis 2.7).
Secondary outcomes
1. Nutritional intake before and after the intervention
a. Change in energy intake
Zero to three months
Eight studies (327 participants) reported analysable data for the changes in energy intake at up to three months (Baldwin 2011; Gray‐Donald 1995; Kalnins 2005; Ravasco 2005a; Ravasco 2005b; Schwenk 1999; Singh 2008; Stow 2015). There was no difference between those who received dietary advice and those who received ONS, MD ‐1.52 kcal (95% CI ‐206.23 to 203.20), but heterogeneity was considerable (I² = 78%) (Analysis 2.8). Removal of the two studies by Ravasco reduced the heterogeneity (I2 = 0%), and participants receiving ONS had a significantly higher energy intake than participants receiving dietary advice (Ravasco 2005a; Ravasco 2005b).
2.8. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 8: Change in energy intake (kcal)
Parsons studied 70 participants in a residential care setting and reported that mean (SE) energy intake was higher in the ONS group (39 participants), than in the dietary advice group (31 participants), 1646 (75) kcal compared to 1223 (94) kcal, but the differences were not statistically significant (Parsons 2016).
Four to six months
One study (25 participants) reported analysable data for the change in energy intake at six months (Stow 2015); investigators found no difference between those receiving dietary advice compared with ONS, MD ‐145.00 kcal (95% CI ‐598.85 to 308.85) (Analysis 2.8).
12 months and over
The follow‐up paper to the Ravasco study reported that the median energy intake after a median follow‐up of 6.5 years was significantly higher in the group receiving dietary advice, 2482 kcal (95% CI 2210 to 2685) than in the group receiving ONS, 1335 kcal (95% CI 1150 to 1569) (Ravasco 2005a) (Analysis 2.8).
Review authors did not undertake a combined analysis as one study reports data at more than one time point (Analysis 2.8).
b. Change in protein intake
Zero to three months
Five studies (221 participants) reported the change from baseline in protein intake at up to three months (Parsons 2016; Ravasco 2005a; Ravasco 2005b; Singh 2008; Stow 2015). There was a statistically higher increase in protein intake in the ONS group compared to the dietary advice group, MD ‐13.09 g (95% CI ‐19.23 to ‐6.96), with no heterogeneity (I² = 0%) (Analysis 2.9).
2.9. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 9: Change in protein intake (g)
Four to six months
Only one study (25 participants) provided analysable data for the change in protein intake at four to six months (Stow 2015) and reported a significantly higher protein intake in the ONS group compared with the dietary advice group, MD ‐6.00 g (95% CI ‐9.91 to ‐2.09) (Analysis 2.9).
12 months and over
Ravasco (63 participants) reported that participants' median protein intake after a median follow‐up of 6.5 years was significantly higher in the dietary advice group (74 g (95% CI 69 to 77)), than in the ONS group (42 g (95% CI 39 to 44)) (Ravasco 2005a) (Analysis 2.9).
Review authors did not undertake a combined analysis as one study reports data at more than one time point (Analysis 2.9).
2. Measures of functional status
a. Handgrip strength
Zero to three months
Two studies (69 participants) provided data for change in handgrip strength at up to three months (Gray‐Donald 1995; Stow 2015) and found no difference between those receiving dietary advice and those receiving ONS, MD 0.32 kg force (95% CI ‐1.10 to 1.74) with no heterogeneity (Analysis 2.10).
2.10. Analysis.

Comparison 2: Dietary advice compared with nutritional supplements, Outcome 10: Change in grip strength (kg force)
Four to six months
One study (17 participants) provided data on the change in grip strength from baseline to between four and six months (Stow 2015). No difference was observed between the groups, MD ‐0.07 kg force (95% CI ‐2.35 to 2.21)(Analysis 2.10).
Review authors did not undertake a combined analysis as one study reports data at more than one time point (Analysis 2.10).
3. QoL
Five studies reported on this outcome; three used the EORTC questionnaires (Baldwin 2011; Ravasco 2005a; Ravasco 2005b), one used the FAACT questionnaire (Baldwin 2011), one used the EQ‐5D (Parsons 2016) and one used the General Perceived Health Questionnaire (Gray‐Donald 1995). Another study reports collecting data on QoL, but does not report any results because of completion by too few participants (Stow 2015).
The review authors have entered data into meta‐analyses for global QoL scores, physical function, mental function, social function, cognitive function, pain and energy/fatigue using the SMD to combine data using different QoL questionnaires.
a. Global QoL
Zero to three months
Four studies (290 participants) reported on change in global QoL scores (Baldwin 2011; Gray‐Donald 1995; Ravasco 2005a; Ravasco 2005b). There was no difference in global QoL between groups receiving advice compared with groups receiving ONS, SMD 1.26 (95% CI ‐0.32 to 2.85) (low‐certainty evidence). There was considerable heterogeneity (I² = 97%) (Analysis 2.11). Removal of two studies (Ravasco 2005a; Ravasco 2005b) reduced heterogeneity (I² = 0%) and there remained no difference between the groups, SMD ‐0.12 (95% CI ‐0.43 to 0.19). When assessing changes in global QoL, it is difficult to explain the effect sizes three to four times larger in the studies by Ravasco than in other studies using EORTC QLQ‐C30 to report outcomes in people with cancer (King 1996; Ravasco 2005a; Ravasco 2005b). The studies by Ravasco consistently achieve effects that are substantially larger than other similar studies and there is no obvious reason to explain this.
One study (68 participants) additionally reported QoL using FAACT (Baldwin 2011) and found no difference between groups, SMD ‐0.04 (95% CI ‐0.40 to 0.31) (Analysis 2.11).
Four to six months
One study (68 participants), using two different QoL questionnaires, reported on change in global QoL scores (Baldwin 2011). There was no difference in change in global QoL at four to six months between the group receiving advice and the group receiving ONS, either with the EORTC, SMD 0.07 (95% CI ‐0.43 to 0.57) or with FAACT, SMD ‐0.15 (95% CI ‐0.63 to 0.33) (Analysis 2.11).
12 months and over
One study (63 participants) reported on change in QoL at 12 months or more (Ravasco 2005a). There was a large improvement in global QoL in groups receiving advice compared with groups receiving ONS, SMD 10.68 (95% CI 8.69 to 12.67) (Analysis 2.11).
Review authors did not undertake a combined analysis as studies reported data at more than one time point.
b. QoL — physical function
Zero to three months
Three studies (236 participants) reported on change in physical function at up to three months (Baldwin 2011; Ravasco 2005a; Ravasco 2005b). There was no difference in physical function between groups receiving advice compared with groups receiving ONS, SMD 2.41 (95% CI ‐0.79 to 5.61) (Analysis 2.12). However, there was considerable heterogeneity (I² = 98%). Removal of one study reduced heterogeneity (I² = 0%) (Baldwin 2011), leaving only the two studies by Ravasco in the analysis (Ravasco 2005a; Ravasco 2005b). Since the review authors have some concerns about the effect sizes reported in these studies i.e. three to four times larger than those reported in other studies in people with cancer using EORTC QLQ‐C30 (King 1996), they feel it is prudent not to report an overall effect at this time.
c. QoL — mental function
Zero to three months
Three studies (232 participants) reported on change in mental function at up to three months (Baldwin 2011; Ravasco 2005a; Ravasco 2005b). There was no difference in mental function between groups receiving advice compared with groups receiving ONS, SMD 3.45 (95% CI ‐0.24 to 7.15) (Analysis 2.13). However, there was considerable heterogeneity (I² = 98%). Removal of each study individually did not reduce the heterogeneity noticeably; however, the review authors expect the heterogeneity reflects the use of different QoL instruments and the differences in clinical backgrounds and care settings. Since they have some concerns about the effect sizes reported in these studies i.e. three to four times larger than those reported in comparable studies in people with cancer using EORTC QLQ‐C30 (King 1996), they feel it is prudent not to report an overall effect at this time.
d. QoL — social function
Zero to three months
Three studies (232 participants) reported on change in social function at up to three months (Baldwin 2011; Ravasco 2005a; Ravasco 2005b). There was no difference in social function between groups receiving advice compared with groups receiving ONS, SMD 3.13 (95% CI ‐0.21 to 6.48) (Analysis 2.14). However, there was considerable heterogeneity (I² = 98%). Removal of one study reduced heterogeneity (I² = 13%) (Baldwin 2011), leaving only the two studies by Ravasco in the analysis (Ravasco 2005a; Ravasco 2005b). Since the review authors have some concerns about the effect sizes reported in these studies i.e. three to four times larger than those reported in other studies in people with cancer using EORTC QLQ‐C30 (King 1996), they feel it is prudent not to report an overall effect at this time.
e. QoL — cognitive function
Zero to three months
Three studies (234 participants) reported on change in cognitive function at up to three months (Baldwin 2011; Ravasco 2005a; Ravasco 2005b). There was a large improvement in cognitive function in groups receiving advice compared with groups receiving ONS, SMD 4.23 (95% CI 0.05 to 8.42) (Analysis 2.15), however, there was considerable heterogeneity (I² = 99%). Removal of each study individually did not reduce the heterogeneity noticeably, however, we expect the heterogeneity reflects the differences in QoL instruments used, and the differences in clinical backgrounds and care settings. Since the review authors have some concerns about the effect sizes reported in these studies i.e. three to four times larger than those reported in other studies in people with cancer using EORTC QLQ‐C30 (King 1996), they feel it is prudent not to report an overall effect at this time.
f. QoL ‐ pain
Zero to three months
Three studies (236 participants) reported on change in pain at up to three months (Baldwin 2011; Ravasco 2005a; Ravasco 2005b). There was no difference in pain between groups receiving advice compared with groups receiving ONS, SMD ‐5.42 (95% CI ‐11.40 to 0.56) (Analysis 2.16). However, there was considerable heterogeneity (I² = 99%). Removal of each study individually did not reduce the heterogeneity noticeably, however, we expect the heterogeneity reflects the differences in QoL instruments used, and the differences in clinical backgrounds and care settings. Since the review authors have some concern about the effect sizes reported in these studies i.e. three to four times larger than those reported in other studies in people with cancer using EORTC QLQ‐C30 (King 1996), they feel it is prudent not to report an overall effect at this time.
g. QoL — energy/fatigue
Zero to three months
Three studies (232 participants) reported on change in energy/fatigue at up to three months (Baldwin 2011; Ravasco 2005a; Ravasco 2005b). There was no difference in energy/fatigue between groups receiving advice compared with groups receiving ONS, SMD ‐8.41 (95% CI ‐18.21 to 1.39) (Analysis 2.17). However, there was considerable heterogeneity (I² = 99%). Removal of each study individually did not reduce the heterogeneity noticeably; however, the review authors expect the heterogeneity reflects the differences in QoL instruments used, and the differences in clinical backgrounds and care settings. Since they have some concern about the effect sizes reported in these studies i.e. three to four times larger than those reported in other studies in people with cancer using EORTC QLQ‐C30 (King 1996), they feel it is prudent not to report an overall effect at this time.
4. Cost
None of the studies in this comparison reported cost data.
Group 3 — Dietary advice compared with dietary advice plus ONS
This comparison includes 22 studies (1498 participants) (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2011; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Dixon 1984; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Kendell 1982; Le Cornu 2000; McCarthy 1999; Murphy 1992; Norman 2008b; Olejko 1984; Rabeneck 1998; Sharma 2002a; Wilson 2001). There were no usable data for three of these studies (Dixon 1984; Kendell 1982; Olejko 1984).
Please refer to the summary of findings table for the explanations of judgements (Table 3). Note that GRADE judgements are for specific outcomes at the three‐months time point and are not provided for all outcomes at each time point.
Primary outcome
1. Mortality
13 studies reported mortality data (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Kapoor 2017; Le Cornu 2000; Murphy 1992; Norman 2008b).
Zero to three months
Data were available from 10 studies (777 participants) at up to three months (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Fuenzalida 1990; Kapoor 2017; Norman 2008b). There was no difference in mortality between the participants who received dietary advice plus ONS versus participants who received dietary advice only, RR 0.92 (95% CI 0.47 to 1.80) (low‐certainty evidence). There was no heterogeneity (I² = 29%) (Analysis 3.1). Four studies (285 participants) reported no deaths (Beattie 2000; de Luis 2003; Fuenzalida 1990; Norman 2008b).
3.1. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 1: Mortality
Four to six months
Five studies (373 participants) provided data at the time point from four to six months (Baldwin 2011; Gonzalez‐Espinoza 2005; Kapoor 2017; Le Cornu 2000; Murphy 1992). There was no difference in mortality between the dietary advice plus ONS group compared to the dietary advice only group, RR 1.03 (95% CI 0.53 to 2.00). There was moderate heterogeneity (I² = 38%) (Analysis 3.1).
Seven to 12 months
Only one study (176 participants) reported mortality at seven to 12 months (Baldwin 2011), RR 0.96 (95% CI 0.58 to 1.58) (Analysis 3.1).
Review authors did not undertake a combined analysis as two studies report data at more than one time point.
2. Morbidity
a. Hospital admissions
Two studies reported hospital admission data (Gonzalez‐Espinoza 2005; Norman 2008b).
Zero to three months
One study (114 participants) reported on hospital readmissions between zero and three months (Norman 2008b). The group receiving dietary advice plus ONS had a significantly lower number of readmissions compared to the dietary advice only group, RR 1.70 (95% CI 1.04 to 2.77) (low‐certainty evidence) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 2: Number of people admitted or readmitted to hospital
Four to six months
One study (28 participants) reported on hospital readmissions between four and six months (Gonzalez‐Espinoza 2005). There was no difference between the two groups, RR 0.84 (95% CI 0.50 to 1.42) (Analysis 3.2).
Analysis of both studies combined showed no difference in hospital admissions between the two groups, RR 1.20 (95% CI 0.58‐2.48).
Heterogeneity between subgroups was substantial (I² = 75%). Heterogeneity may be explained by the difference in number of participants in the studies; one study included more than 100 participants (Norman 2008b) and the second study less than 30 (Gonzalez‐Espinoza 2005). Disease background was comparable between the groups (non‐malignant disease), as was nutritional status (both confirmed by SGA).
b. Length of hospital stay
Zero to three months
Data on length of hospital stay were available for analysis from two studies (202 participants) at up to three months (Beattie 2000; Norman 2008b). There was no difference in the length of hospital stay in the group receiving dietary advice plus ONS compared with the dietary advice alone group, MD ‐1.07 days (95% CI ‐4.10 to 1.97) (low‐certainty evidence) (Analysis 3.3). There was no heterogeneity (I² = 0%).
3.3. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 3: Length of hospital stay (days)
An additional three studies reported data on length of hospital stay, but could not be included in the meta‐analysis (Burden 2011; Burden 2017; Huynh 2015). The first study reported no difference in median length of stay in pre‐operative participants with colorectal cancer receiving dietary advice plus ONS compared with dietary advice alone; 13.5 versus 14.0 days respectively (Burden 2011). The second study also reported no difference in median (IQR) length of stay in pre‐operative participants with colorectal cancer receiving dietary advice plus ONS compared with dietary advice alone; 7.0 days (4.0 to 10.5) versus 7.0 days (4.0 to 10.0) respectively, (P = 0.630) (Burden 2017). The third study reported was no difference in median (IQR) length of stay in a mixed population of participants on discharge from hospital receiving dietary advice plus ONS compared with dietary advice alone; 4.0 days (3.0 to 6.0) versus 4.5 days (3.0 to 7.0) respectively, (P = 0.1694) (Huynh 2015).
Seven to 12 months
One study (46 participants) in people on haemodialysis reported data on the total number of days of hospitalisation at up to nine months (Wilson 2001). Those receiving dietary advice plus ONS were hospitalised for 71 days and those receiving dietary advice alone were hospitalised for 107 days.
c. Complications
Data on the number of participants with complications were available from four studies (Beattie 2000; Burden 2011; Burden 2017; Gonzalez‐Espinoza 2005).
Zero to three months
Three studies reported data on complications at up to three months (Beattie 2000; Burden 2011; Burden 2017). There were significantly fewer complications in groups receiving dietary advice plus ONS versus dietary advice alone, RR 0.75 (95% CI 0.56 to 0.99) (low‐certainty evidence); however, heterogeneity was moderate to substantial (I² = 58%) (Analysis 3.4). Removal of one study reduced heterogeneity to zero (Burden 2011) and groups receiving dietary advice plus ONS had significantly fewer complications than groups receiving dietary advice alone, RR 0.57 (95% CI 0.38 to 0.84). We have not been able to explain the reason for heterogeneity.
3.4. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 4: Complications
Four to six months
One study (28 participants) provided data on the number of participants with complications (Gonzalez‐Espinoza 2005). There was no difference in the incidence of peritonitis in individuals with renal disease receiving continuous ambulatory peritoneal dialysis (CAPD) between groups receiving dietary advice plus ONS and dietary advice alone, RR 1.92 (95% CI 0.57 to 6.54) (Analysis 3.4).
There was moderate to substantial heterogeneity between subgroups (I² = 54%). Analysis of all studies combined showed no differences in complications between the two groups, RR 0.79 (95% CI 0.60‐1.05); (Analysis 3.4).
3. Measures of nutritional status
a. Change in weight
Data on weight change were available in 15 studies (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; Murphy 1992; Norman 2008b; Rabeneck 1998; Sharma 2002a).
Zero to three months
At this time point, 14 studies (931 participants) reported on weight change (Arnold 1989; Baldwin 2011; Beattie 2000; Burden 2017; de Luis 2003; de Sousa 2012; Diouf 2016; Fuenzalida 1990; Huynh 2015; Kapoor 2017; Norman 2008b; Rabeneck 1998; Sharma 2002a; Sharma 2002b). Weight change was significantly higher in the groups receiving dietary advice plus ONS compared with dietary advice alone, MD 1.15 kg (95% CI 0.42 to 1.87) (low‐certainty evidence) with substantial heterogeneity (I² = 71%) (Analysis 3.5). Removal of three studies reduced heterogeneity (I2 = 0%) (Beattie 2000; Diouf 2016; Kapoor 2017) and there was significantly greater weight gain in the groups receiving dietary advice plus ONS, MD 0.52 (95% CI 0.16 to 0.88). We have not been able to explain the reason for heterogeneity.
3.5. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 5: Change in weight (kg)
Four to six months
Four studies (209 participants) reported data on weight change between four and six months (Baldwin 2011; Gonzalez‐Espinoza 2005; Kapoor 2017; Murphy 1992). There was no difference between the two groups, MD 2.27 kg (95% CI ‐0.44 to 4.98) and considerable heterogeneity (I² = 76%) (Analysis 3.5). One study stood out of the others with very positive results (Kapoor 2017). This study was different from the other studies as it was performed in India in 63 participants with cancer undergoing palliative treatment; these participants had very low body weight and very low baseline intakes. The intervention product was a macronutrient and flour mixture that could be used to make bread, this being quite different from ONS to be taken in addition to other meals. The review authors assume that the participants in this study had greater potential to improve their nutritional status, given their poor nutritional status at the start of the study, while, in addition, the product may have fulfilled basic nutritional needs (Kapoor 2017). After removing this study from the analysis, the differences between groups were smaller, but there remained no significant difference between groups, MD 0.67 kg (95% CI ‐0.79 to 2.13) and no heterogeneity (I² = 0%).
Seven to 12 months
One study (31 participants) reported on weight change between seven and 12 months (Baldwin 2011). There was no difference between the two groups, MD 0.14 kg (95% CI ‐4.24 to 4.52) (Analysis 3.5).
Review authors did not undertake a combined analysis as two studies report data at more than one time point.
Sensitivity analysis
The SD of weight change was imputed for three studies at up to three months (Arnold 1989; Fuenzalida 1990; Sharma 2002a; Sharma 2002b). The removal of these studies from the analysis had no impact on the results, MD 1.48 kg (95% CI 0.65 to 2.32) and heterogeneity remained substantial (I² = 77%).
The funnel plot examination, the Egger regression asymmetry test and the Begg's adjusted rank correlation suggested no evidence of small study bias (P = 0.245 and P = 0.511 respectively) (Figure 5).
5.

Funnel plot of comparison 3: dietary advice plus ONS compared with dietary advice alone. Outcome 3.5.1 change in weight (kg)
b. BMI
Six studies (seven data sets) reported data on the change in BMI (de Sousa 2012; Diouf 2016; Gonzalez‐Espinoza 2005; Huynh 2015; Norman 2008b; Sharma 2002a; Sharma 2002b).
Zero to three months
Five studies (six data sets; 350 participants) reported on this outcome between zero and three months (de Sousa 2012; Diouf 2016; Huynh 2015; Norman 2008b; Sharma 2002a; Sharma 2002b). There was no difference in the change in BMI between groups receiving dietary advice plus ONS and groups receiving dietary advice alone, MD 0.50 kg/m² (95% CI ‐0.30 to 1.33) with considerable heterogeneity (I² = 95%) (Analysis 3.6). Studies took place in a wide variety of different clinical conditions, settings and age ranges and removal of any single study did not alter heterogeneity.
3.6. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 6: Change in BMI (kg/m²)
Four to six months
One study (18 participants) in people on continuous ambulatory peritoneal dialysis reported on the change in BMI at four to six months (Gonzalez‐Espinoza 2005). There was no difference between the group receiving dietary advice and ONS and those receiving advice alone, MD ‐0.10 (95% CI ‐0.47 to 0.27).
There was moderate heterogeneity between subgroups (I² = 44.4%). Analysis of all studies combined showed no differences in change in BMI between the two groups, MD 0.42 (95% CI ‐0.31 to 1.16). Heterogeneity was considerable (I² = 95%).
c. FFM
Zero to three months
Three studies (187 participants) reported on change in FFM at up to three months and data have been combined using the SM (de Luis 2003; Diouf 2016; Norman 2008b). There was no difference between the two groups, SMD 0.10 kg (95% CI ‐0.18 to 0.39) (low‐certainty evidence) with no heterogeneity (I² = 0%) (Analysis 3.7).
d. MAC
This outcome was reported in three studies (de Luis 2003; Kapoor 2017; Murphy 1992); however, the data were not presented in a format that was amenable to meta‐analysis.
In 70 participants living with HIV infection, there was no difference in change in MAC between the groups receiving dietary advice plus ONS and dietary advice alone; mean change ‐0.1 cm versus ‐0.7 cm, respectively (de Luis 2003). In a further study of people with HIV infection (n = 16), there was no difference in change in MAC between the groups receiving dietary advice plus ONS and dietary advice alone, mean change 1.3 cm versus 1.2 cm, respectively (Murphy 1992). In 15 participants with cancer cachexia undergoing palliative care in India, there was a statistically significant reduction in MAC in the group receiving dietary advice alone (P = 0.006), but investigators did not report data relating to the 17 participants who received dietary advice plus ONS (Kapoor 2017).
e. MAMC
Data on MAMC were available from five studies (Beattie 2000; de Luis 2003; de Sousa 2012; Gonzalez‐Espinoza 2005; Kapoor 2017).
Zero to three months
Four studies (241 participants) reported on change in MAMC up to three months (Beattie 2000; de Luis 2003; de Sousa 2012; Kapoor 2017). There was a significantly greater improvement in MAMC in groups receiving dietary advice plus ONS compared with dietary advice alone, MD 0.78 cm (95% CI 0.37‐1.18), with zero to moderate heterogeneity (I² = 37%) (Analysis 3.8).
3.8. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 8: Change in mid‐arm muscle circumference (cm)
Four to six months
Two studies (60 participants) reported on change in MAMC between four and six months (Gonzalez‐Espinoza 2005; Kapoor 2017). There was no difference between the groups, MD 1.20 cm (95% CI ‐0.63 to 3.03), with considerable heterogeneity (I² = 74%) (Gonzalez‐Espinoza 2005; Kapoor 2017) (Analysis 3.8). The heterogeneity most likely reflects the two very different populations; participants with renal disease receiving CAPD and participants with cancer cachexia receiving palliative care.
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
c. TSF
Seven studies reported data on TSF (Beattie 2000; de Luis 2003; de Sousa 2012; Fuenzalida 1990; Gonzalez‐Espinoza 2005; Norman 2008b; Rabeneck 1998).
Zero to three months
Six studies (393 participants) reported on the change in TSF up to three months (Beattie 2000; de Luis 2003; de Sousa 2012; Fuenzalida 1990; Norman 2008b; Rabeneck 1998). While analysis showed a beneficial effect in favour of dietary advice and ONS, MD 1.06 mm (95% CI 0.14 to 1.97), heterogeneity was considerable (I² = 78%) (Analysis 3.9). Removal of any single study did not reduce heterogeneity. There was a wide variation in the clinical backgrounds of participant populations.
3.9. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 9: Change in triceps skinfold thickness (mm)
Four to six months
One study in 28 renal patients receiving CAPD reported data at four to six months (Gonzalez‐Espinoza 2005). There was no difference in TSF between the group receiving dietary advice plus ONS and the group receiving dietary advice alone, mean change 1.6 mm versus 0.5 mm respectively.
Secondary outcomes
1. Nutritional intake before and after the intervention
a. Change in energy intake
Data on change in energy intake was available in nine studies (Baldwin 2011; Burden 2011; Burden 2017; de Luis 2003; Gonzalez‐Espinoza 2005; Huynh 2015; Kapoor 2017; McCarthy 1999; Murphy 1992).
Zero to three months
Seven studies (464 participants) reported on change in energy intake between zero and three months (Baldwin 2011; Burden 2011; Burden 2017; de Luis 2003; Huynh 2015; Kapoor 2017; McCarthy 1999). There was a significantly greater increase in energy intake in groups receiving dietary advice plus ONS, MD 344.46, (95% CI 164.21 to 524.71). Heterogeneity was moderate to substantial (I² = 59%). Removal of one study in participants receiving perioperative supplementation reduced heterogeneity to zero (Burden 2011), while the effect of dietary advice plus ONS continued to result in significantly greater energy intake than dietary advice alone MD 284.75 kcal (95% CI 163.02 to 406.49). Investigators collected data on energy intake using unstructured dietary recalls and the lack of accuracy of this method might have contributed to large SD of change and suggests that data were skewed.
Four to six months
Three studies (75 participants) reported on change in energy intake between four and six months (Gonzalez‐Espinoza 2005; Kapoor 2017; Murphy 1992). The group receiving dietary advice and ONS had a significantly greater energy intake than the group receiving dietary advice along, MD 362.75 kcal (95% CI 128.53 to 596.97) and no heterogeneity (I² = 0%) (Analysis 3.10).
3.10. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 10: Change in energy intake (kcal)
The review authors did not undertake a combined analysis as several studies report data at more than one time point.
b. Final energy intake
Three studies reported data on final energy intake (Arnold 1989; Norman 2008b; Sharma 2002a; Sharma 2002b).
Zero to three months
Data were available from all three studies (140 participants) at up to three months, final energy intake was significantly higher in groups receiving dietary advice and ONS compared with dietary advice alone, MD 303.81 (95% CI 110.58 to 497.03), but heterogeneity was substantial (I² = 84%). Studies took place in a wide variety of different clinical conditions, settings and age ranges and removal of any single study did not alter heterogeneity.
c. Change in protein intake
Data on change in protein intake was available in three studies (Burden 2017; Huynh 2015; Kapoor 2017).
Zero to three months
Three studies (230 participants) reported on change in protein intake between zero and three months (Burden 2017; Huynh 2015; Kapoor 2017). There was a statistically significant difference between the two groups, MD 12.21 (95% CI 6.39 to 18.03) and no heterogeneity (I² = 0%) (Analysis 3.12).
3.12. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 12: Change in protein intake (g)
Four to six months
One study (32 participants) reported on change in protein intake between four and six months (Kapoor 2017. There was a significantly greater increase in protein intake in the group receiving dietary advice plus ONS compared with dietary advice alone, MD 16.20 g (95% CI 4.83 to 27.57) (Analysis 3.12).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
d. Final protein intake
Five studies provided data on final protein intake (Arnold 1989; de Luis 2003; McCarthy 1999; Norman 2008b; Sharma 2002a; Sharma 2002b).
Zero to three months
Data were available from all five studies (299 participants) at up to three months, final protein intake was significantly higher in groups receiving dietary advice and ONS compared with dietary advice alone, MD 11.76 g (95% CI 5.59 to 17.93), but heterogeneity was substantial (I² = 81%). Studies took place in a wide variety of different clinical conditions, settings and age ranges and removal of any single study did not alter heterogeneity.
2. Measures of functional status
a. Handgrip strength
Data on change in handgrip strength were available from six studies (Beattie 2000; Burden 2017; de Sousa 2012; Huynh 2015; Norman 2008b; Rabeneck 1998).
Zero to three months
All six studies (537 participants) reported on change in handgrip strength between zero and three months (Beattie 2000; Burden 2017; de Sousa 2012; Huynh 2015; Norman 2008b; Rabeneck 1998). There was no difference between the two groups, MD 1.07 kg force (95% CI ‐0.22 to 2.37) and considerable heterogeneity (I² = 82%) (Analysis 3.14). Studies took place in a wide variety of different clinical conditions, settings and age ranges and removal of any single study did not alter heterogeneity.
3.14. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 14: Change in grip strength (kg force)
3. QoL
Five studies reported this outcome; two used the EORTC questionnaires (Baldwin 2011; Kapoor 2017), one used the FAACT questionnaire (Baldwin 2011) and in two used the SF‐36 (in the Norman study SF‐36 was used to calculate quality‐adjusted life years (QALYs)) (Norman 2008b; Beattie 2000), and one used a self‐developed, non‐validated 30‐item QoL questionnaire designed specifically for the study (Rabeneck 1998).
The review authors have entered data into the meta‐analyses for global QoL scores, physical function, mental function, social function, cognitive function, pain and energy/fatigue using the SMD to combine data using different QoL questionnaires.
a. Global QoL
Zero to three months
Four studies (334 participants) reported on change in global QoL scores (Baldwin 2011, Kapoor 2017, Norman 2008b; Rabeneck 1998). There was a small to moderate improvement in global QoL in groups receiving advice plus ONS compared with advice alone, SMD 0.33 (95% CI 0.09 to 0.57) (low‐certainty evidence). There was zero to moderate heterogeneity (I² = 13%) (Analysis 3.15).
One study (113 participants) additionally reported QoL using a second questionnaire (FAACT) (Baldwin 2011) and found no difference between groups, SMD ‐0.02 (95% CI ‐0.39 to 0.35) (Analysis 3.15).
Four to six months
Two studies (94 participants) reported on change in global QoL scores (Baldwin 2011, Kapoor 2017). There was no difference between the two groups, SMD 0.35 (95% CI ‐0.55 to 1.24) but there was substantial heterogeneity (I² = 75%) (Analysis 3.15). Both studies were in people with cancer undergoing palliative care and the sample sizes were comparable. However, the studies were conducted in different parts of the world, UK (Baldwin 2011) and India (Kapoor 2017). The differences in the results might reflect cultural and national differences in response to QoL questions.
One study (62 participants) additionally reported QoL using a second questionnaire (FAACT) (Baldwin 2011) and found no difference between groups, SMD ‐0.40 (95% CI ‐0.90 to 0.10) (Analysis 3.15).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
b. QoL — physical function
Zero to three months
Four studies (324 participants) reported on change in physical function at up to three months (Baldwin 2011; Beattie 2000; Kapoor 2017; Norman 2008b). There was a moderate improvement in physical function in groups receiving advice plus ONS compared with advice alone, SMD 0.52 (95% CI 0.08 to 0.95) (Analysis 3.16). However, there was substantial heterogeneity (I² = 72%). Removal of one study reduced heterogeneity (I2 = 0%) (Baldwin 2011) and a moderate to large effect remained, SMD 0.74 (95% 0.47 to 1.02). Both studies were in people with cancer undergoing palliative care and the sample sizes were comparable. However, the studies were conducted in different parts of the world, UK (Baldwin 2011) and India (Kapoor 2017). The differences in the results might reflect cultural and national differences in response to QoL questions.
Four to six months
One study (32 participants) reported on change in physical function at six months (Kapoor 2017). There was no difference between the group receiving advice plus ONS and the group receiving advice alone, SMD 0.08 (95% CI ‐0.62 to 0.77) (Analysis 3.16).
The review authors did not undertake a combined analysis as two studies reported data at more than one time point.
c. QoL ‐ mental function
Zero to three months
Four studies (316 participants) reported on change in mental function at up to three months (Baldwin 2011; Beattie 2000; Kapoor 2017; Norman 2008b). There was no difference between the group receiving advice plus ONS and the group receiving advice alone, SMD 0.29 (95% CI ‐0.25 to 0.83) (Analysis 3.17); however, there was considerable heterogeneity (I² = 82%). Removal of one study reduced heterogeneity to zero to moderate (I² = 35%) (Baldwin 2011) and there was a moderate improvement in mental function in groups receiving advice plus ONS compared with advice alone, SMD 0.55 (95% CI 0.21 to 0.89). It is difficult to explain how the removal of this study affected heterogeneity as the participants were similar to those in the Indian study (Kapoor 2017) in that they were patients with cancer receiving palliative chemotherapy and the sample sizes were comparable. However, the studies were conducted in different parts of the world, UK (Baldwin 2011) and India (Kapoor 2017). The differences in the results might reflect cultural and national differences in response to QoL questions.
Four to six months
One study (32 participants) reported on change in physical function at six months (Kapoor 2017). There was no difference between the group receiving advice plus ONS and the group receiving advice alone, SMD 0.27 (95% CI ‐0.42 to 0.97) (Analysis 3.17).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
d. QoL — social function
Zero to three months
Three studies (214 participants) reported on change in social function at up to three months (Baldwin 2011; Kapoor 2017; Norman 2008b). There was no difference between the groups receiving advice plus ONS and the groups receiving advice alone, SMD 0.06 (95% CI ‐0.33 to 0.45) (Analysis 3.18). Heterogeneity was moderate (I² = 49%).
Four to six months
One study (32 participants) reported on change in social function at six months (Kapoor 2017). The group receiving advice plus ONS had a large improvement in social function compared with the group receiving advice alone, SMD 0.87 (95% CI 0.14 to 1.60) (Analysis 3.18).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
e. QoL — cognitive function
Zero to three months
Two studies (137 participants) reported on change in cognitive function (Baldwin 2011; Kapoor 2017). There was no difference between the groups receiving advice plus ONS and the groups receiving advice alone, SMD 0.11 (95% CI ‐0.23 to 0.45) with no heterogeneity (I² = 0%) (Analysis 3.19).
Four to six months
One study (32 participants) reported on change in social function at six months (Kapoor 2017). There was no difference between the group receiving advice plus ONS and the group receiving advice alone, SMD 0.40 (95% CI ‐0.30 to 1.10) (Analysis 3.19).
Review authors did not undertake a combined analysis as one study reported data at more than one time point.
f. QoL — pain
Zero to three months
Three studies (219 participants) reported on change in pain at up to three months (Baldwin 2011; Kapoor 2017; Norman 2008b). There was no difference between the groups receiving advice plus ONS and the groups receiving advice alone, SMD ‐0.07 (95% CI ‐0.36 to 0.23) with no heterogeneity (I² = 14%) (Analysis 3.20).
Four to six months
One study (32 participants) reported on change in pain at six months (Kapoor 2017). There was no difference between the group receiving advice plus ONS and the group receiving advice alone, SMD 0.13 (95% CI ‐0.57 to 0.82) (Analysis 3.20).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
g. QoL — energy/fatigue
Zero to three months
Three studies (218 participants) reported on change in energy/fatigue at up to three months (Baldwin 2011; Kapoor 2017; Norman 2008b). There was no difference between the groups receiving advice plus ONS and the groups receiving advice alone, SMD ‐0.16 (95% CI ‐0.84 to 0.51) however, there was considerable heterogeneity (I² = 83%) (Analysis 3.21). Removal of one study reduced heterogeneity to zero (Kapoor 2017) and there remained no difference between groups in energy/fatigue, SMD 0.21 (95% CI ‐0.09 to 0.50) (P = 0.17) (Analysis 3.21). It is difficult to explain how the studies by Kapoor and Baldwin differ. Both studies were in people with cancer undergoing palliative care and the sample sizes were comparable. However, the studies were conducted in different parts of the world, UK (Baldwin 2011) and India (Kapoor 2017). The differences in the results might reflect cultural and national differences in response to QoL questions.
Four to six months
One study (32 participants) reported on change in pain at six months (Kapoor 2017). There was no difference between the group receiving advice plus ONS and the group receiving advice alone, SMD ‐0.31 (95% CI ‐1.10 to 0.38) (Analysis 3.21).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
4. Cost
Only one study carried out in Germany (101 participants) reported on cost‐effectiveness (Norman 2008b). Investigators concluded that the intervention was cost‐effective according to international benchmarks. After three months, the mean (SD) health status utilities were significantly higher in the intervention group than in the control group, 0.731 (0.015) compared to 0.671 (0.016) (P = 0.028). The intervention was associated with significantly higher costs, the ICER was EUR 9497 and EUR 12,099 per additional QALY, respectively, but deemed cost‐effective according to international thresholds (under EUR 50,000 per QALY).
Group 4 — Dietary advice plus ONS if required compared with no advice and no ONS
This comparison includes 31 studies (32 data sets; 3308 participants) (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Endevelt 2011; Evans 1987; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Jensen 1997; Kiss 2016; Lovik 1996; Moloney 1983; Ovesen 1993; Pedersen 2016a; Pedersen 2016b; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Uster 2013; Vivanti 2015). There were no usable data for one of these studies (Jensen 1997).
Please refer to the summary of findings table for the explanations of judgements (Table 4). Note that GRADE judgements are for specific outcomes at the three‐months time point and are not provided for all outcomes at each time point.
Primary outcome
1. Mortality
Mortality data were available from 26 studies (Beck 2012; Beck 2015; Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Evans 1987; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Moloney 1983; Ovesen 1993; Pedersen 2016a; Persson 2002; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018, Uster 2013; Vivanti 2015).
Zero to three months
Data were available from 15 studies (1261 participants) at up to three months (Beck 2015; Caccialanza 2015; Carey 2013; Forli 2001; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Pedersen 2016a; Persson 2002; Schilp 2013; Sharma 2017, Terp 2018, Uster 2013; Vivanti 2015). There was no difference in mortality between the participants who received dietary advice plus ONS if required and those who received usual care, RR 0.82 (95% CI 0.58 to 1.16) (low‐certainty evidence). There was no heterogeneity (I² = 0%) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 1: Mortality
Four to six months
Data were available from 10 studies (1140 participants) at the time point from four to six months (Beck 2012; Beck 2015; Feldblum 2011; Ovesen 1993; Persson 2002; Silvers 2014; Starke 2011; Suominen 2015; Terp 2018; Uster 2013). There was no difference in mortality between the participants receiving dietary advice plus ONS if required and those receiving no advice or ONS, RR 1.03 (95% CI 0.69 to 1.55). There was no heterogeneity (I² = 21%) (Analysis 4.1).
Seven to 12 months
Data were available from six studies (851 participants) at this time point (Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Caccialanza 2015; Ganzoni 1994; Moloney 1983; Persson 2002). There was no difference in mortality between those receiving dietary advice plus ONS if required and those receiving no advice or ONS, RR 0.87 (95% CI 0.63 to 1.19). There was substantial heterogeneity (I² = 64%) (Analysis 4.1). Removal of one study in individuals with heart failure reduced heterogeneity to approaching moderate (Bonilla‐Palomas 2016), RR 0.98 (95% CI 0.77 to 1.24) (I² = 35%). Due to the number and variety of clinical conditions in this analysis, it is not possible to explain the heterogeneity.
12 months and over
Data were available from five studies (900 participants) at 12 months and over (Bourdel‐Marchasson 2014; Caccialanza 2015; Evans 1987; Persson 2002; Sharma 2017). There was no difference in mortality between those receiving dietary advice plus ONS if required and those receiving no advice or ONS, RR 0.93 (95% CI 0.81 to 1.08). There was moderate heterogeneity (I² = 44%) (Analysis 4.1).
The review authors did not undertake a combined analysis as a number of studies report data at more than one time point.
2. Morbidity
a. Hospital admissions
Hospital admission data were available from nine studies (Beck 2012; Beck 2015, Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014; Holyday 2012; Pedersen 2016a; Pedersen 2016b; Sharma 2017; Starke 2011; Terp 2018).
Zero to three months
Six studies (673 participants) reported on hospital readmissions at up to three months (Beck 2012; Beck 2015; Holyday 2012; Pedersen 2016a; Pedersen 2016b; Sharma 2017; Terp 2018). There was no difference between the those receiving dietary advice plus ONS if required and those receiving no advice or ONS, RR 0.83 (95% CI 0.59 to 1.15) and moderate to substantial heterogeneity (I² = 58%) (moderate‐certainty evidence) (Analysis 4.2). Removal of two studies reduced heterogeneity (I² = 0%) (Beck 2012; Terp 2018) and resulted in a significant reduction in readmissions in the groups receiving dietary advice plus ONS if required, RR 0.67 (95% CI 0.52 to 0.86). In these two studies, in contrast to the others, there were more readmissions in the intervention group than in the control group (Beck 2012; Terp 2018). It is difficult to explain this difference since all studies were performed in an older population and had a sufficient sample size.
4.2. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 2: Number of people admitted or readmitted to hospital
Four to six months
Five studies (456 participants) reported on hospital readmissions between four and six months (Beck 2012; Beck 2015; Holyday 2012; Sharma 2017; Starke 2011). There was no difference between those receiving dietary advice plus ONS if required and those receiving no advice or ONS, RR 0.79 (95% CI 0.58 to 1.03) and moderate to substantial heterogeneity (I² = 55%) (Analysis 4.2). Removal of one study reduced heterogeneity (I² = 25%) (Beck 2012) and resulted in a significant reduction in readmissions in the groups receiving dietary advice plus ONS if required, RR 0.71 (95% CI 0.53 to 0.94). In this study, in contrast to the others, there were more readmissions in the intervention group than in the control group (Beck 2012). It is difficult to explain this difference since all studies were performed in an older population and had a sufficient sample size.
Seven to 12 months
Two studies (456 participants) reported on hospital readmissions between four and six months (Bonilla‐Palomas 2016; Bourdel‐Marchasson 2014). There was no difference in hospital admissions between the participants who received dietary advice plus ONS if required and those who received usual care, RR 0.52 (95% CI 0.18 to 1.55) and high heterogeneity (I² = 83%) (Analysis 4.2). This heterogeneity might be explained by the different clinical backgrounds (participants with heart failure and participants with cancer).
The review authors did not undertake a combined analysis as a number of studies report data at more than one time point
b. Length of hospital stay
Data on the length of hospital stay were available from five studies (Banks 2016; Beck 2012; Holyday 2012; Sharma 2017; Starke 2011).
Zero to three months
Data on the length of hospital stay to enter into meta‐analysis were available from three studies (400 participants) (Beck 2012; Holyday 2012; Starke 2011). There was no difference in length of hospital stay in participants receiving dietary advice plus ONS if required compared with no advice or ONS, MD ‐0.12 days (95% CI ‐2.48 to 2.45) (low‐certainty evidence) (Analysis 4.3). There was low heterogeneity (I² = 2%).
4.3. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 3: Length of hospital stay (days)
An additional two studies reported data on the length of hospital stay in a format that authors could not include in the meta‐analysis (Banks 2016; Sharma 2017). In people with pressure ulcers there was no difference in median (IQR) length of stay between the group receiving dietary advice plus ONS if required compared with no advice or ONS; 14.5 days (6.2 to 23.5) versus 14.0 days (10 to 31), respectively (Banks 2016). There was a significantly shorter median (IQR) length of stay in older acute medical patients receiving dietary advice plus ONS if required compared with no advice or ONS; 5.0 days (3.0 to 8.4) versus 8.8 days (4.1 to 13.9), respectively, (P = 0.007) (Sharma 2017).
c. Complications
Data on the number of participants with complications were available from three studies (Bourdel‐Marchasson 2014; Sharma 2017; Starke 2011).
Zero to three months
Data were available from two studies (280 participants) at up to three months (Sharma 2017; Starke 2011). There were no differences in complications between those receiving dietary advice plus ONS if required and those receiving no advice or ONS, RR 0.56 (95% CI 0.22 to 1.46) (low‐certainty evidence) with substantial heterogeneity (I² = 64%) (Analysis 4.4). Participants in both studies were older acute medical patients and it is not possible to explain the heterogeneity.
4.4. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 4: Complications
Seven to 12 months
Data were available from one study (336 participants) (Bourdel‐Marchasson 2014). There were no differences in complications between those receiving dietary advice plus ONS if required and those receiving no advice or ONS, RR 0.88 (95% CI 0.35 to 2.22) (Analysis 4.4).
Analysis of all studies combined showed no difference in complications between those receiving dietary advice plus ONS if required and those receiving no advice or ONS, RR 0.68 (95% CI 0.40 to 1.18) with no heterogeneity (I² = 32%). There was no heterogeneity between subgroups (I² = 0%) (Analysis 4.4).
3. Measures of nutritional status
a. Change in weight
Data on weight change were available from 24 studies (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Feldblum 2011; Forli 2001; Ganzoni 1994; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Ovesen 1993; Persson 2002; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Terp 2018; Uster 2013; Vivanti 2015).
Zero to three months
17 studies (1192 participants) reported on weight change at up to three months (Andersson 2017; Banks 2016; Beck 2012; Beck 2015; Carey 2013; Forli 2001; Holyday 2012; Isenring 2004; Kiss 2016; Lovik 1996; Persson 2002; Schilp 2013; Sharma 2017; Starke 2011; Terp 2018; Uster 2013; Vivanti 2015). Weight gain was greater in groups receiving dietary advice plus ONS if required compared with no advice and no ONS, MD 1.25 kg (95% CI 0.73 to 1.76) (moderate‐certainty evidence) and there was moderate heterogeneity (I² = 44%) (Analysis 4.5).
4.5. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 5: Change in weight (kg)
Four to six months
10 studies (961 participants) reported on weight change after four to six months (Beck 2012; Bourdel‐Marchasson 2014; Carey 2013; Feldblum 2011; Ovesen 1993; Persson 2002; Rogers 1992; Schilp 2013; Silvers 2014; Uster 2013). There was no difference between those receiving dietary advice plus ONS if required and those receiving no advice or ONS, MD 0.58 kg (95% CI ‐0.30 to 1.45) with substantial heterogeneity (I² = 61%) (Analysis 4.5). Removal of two studies reduced heterogeneity (I² = 28%) (Silvers 2014; Uster 2013) and there was a significantly greater weight gain in the groups receiving advice plus ONS if required compared with those that received no advice or ONS, MD 0.63 (95% CI 0.02 to 1.24). One study was underpowered with a high risk of attrition bias due to a higher mortality rate in the control group (Silvers 2014). Investigators from one study provided original data which, on closer inspection, appeared to be skewed by the inclusion of a participant in the intervention group who lost more than 20 kg body weight (Uster 2013).
Seven to 12 months
Two studies (107 participants) reported on weight change between seven and 12 months (Caccialanza 2015; Persson 2002). There was no difference between the two groups, MD 0.94 kg (95% CI ‐0.35 to 2.23) (P = 0.15) and no heterogeneity (I² = 0%) (Analysis 4.5).
12 months and over
Two studies (77 participants) reported on weight change from baseline up to 12 months and over (Ganzoni 1994; Persson 2002). There was no difference between the two groups, MD 2.17 kg (95% CI ‐1.20 to 5.54) and moderate heterogeneity (I² = 44%) (Analysis 4.5).
The review authors did not undertake a combined analysis as several studies report data at more than one time point (Analysis 4.5).
Sensitivity analysis
The review authors imputed the SD of weight change for one study reporting data at four to six months (Rogers 1992). Removal of this study from the analysis had no impact on the results, MD 0.41 kg (95% CI ‐0.47 to 1.28) and heterogeneity remained moderate to substantial, (I² = 60%).
The funnel plot examination, the Egger regression asymmetry test and the Begg's adjusted rank correlation suggested no evidence of small study bias (P = 0.133 and P = 0.303 respectively) (Figure 6).
6.

Funnel plot of comparison 4: dietary advice plus ONS if required compared with no advice and no ONS. Outcome 4.5.1 change in weight (kg)
b. BMI
Four studies report data on change in BMI (Carey 2013; Persson 2002; Sharma 2017; Suominen 2015).
Zero to three months
Two studies (130 participants) reported the change in BMI from baseline for interventions lasting up to three months (Carey 2013; Sharma 2017). The increase in BMI was significantly greater in those receiving dietary advice and ONS if required compared with no advice or ONS, MD 0.72 kg/m² (95% CI 0.06 to 1.37). There was no heterogeneity (I² = 0%) (Analysis 4.6).
4.6. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 6: Change in BMI (kg/m²)
Four to six months
One study (27 participants) reported the change in BMI from baseline for interventions lasting from four to six months (Carey 2013). There was no difference between groups receiving dietary advice and ONS if required compared with no advice or ONS, MD 0.80 kg/m² (95% CI ‐1.12 to 2.72) (Analysis 4.6).
Seven to 12 months
One study (78 participants) reported data on the change in BMI after 12 months of intervention (Suominen 2015). There was no difference between the group receiving dietary advice and ONS if required compared with no advice or ONS, MD ‐0.10 kg/m² (95% CI ‐0.61 to 0.41) (Analysis 4.6). In one study BMI increased in the intervention group at 12 months from 25.4 to 25.8 kg/m² and at 24 months from 25.4 to 26.0 kg/m² (Persson 2002). No data were reported for the control group.
Review authors did not undertake a combined analysis as one study reported data at more than one time point (Analysis 4.6).
Two studies report data on final values for BMI (Forli 2001; Starke 2011).
Zero to three months
Two studies (169 participants) reported final BMI measurements for interventions lasting up to three months (Forli 2001; Starke 2011). The final BMI was significantly greater in those receiving dietary advice and ONS if required compared with no advice or ONS, MD 1.19 kg/m² (95% CI 0.18 to 2.20). There was no heterogeneity (I² = 0%) (Analysis 4.7).
4.7. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 7: Final BMI (kg/m²)
c. FFM
Data on change in FFM was available from four studies (Isenring 2004; Kiss 2016; Ovesen 1993; Schilp 2013).
Zero to three months
Four studies (262 participants) reported on change in FFM at up to three months (Isenring 2004; Kiss 2016; Ovesen 1993; Schilp 2013). There was a significantly greater change in FFM in the groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 0.82 kg (95% CI 0.35 to 1.29) (low‐certainty evidence) but with substantial heterogeneity (I² = 73%) (Analysis 4.8). Removal of one study reduced heterogeneity (I² = 18%) (Isenring 2004) and there was no difference between groups in FFM, MD 0.34 kg (95% CI ‐0.23 to 0.91). The SD of change was imputed for this study from two other studies in this group, but it is possible the assumptions were incorrect.
4.8. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 8: Change in fat free mass (kg)
Four to six months
Two studies (184 participants) reported on the change in FFM between four and six months (Ovesen 1993; Schilp 2013). There was no difference between the two groups, MD 0.15 kg (95% CI ‐0.52 to 0.82) and moderate heterogeneity (I² = 42%) (Analysis 4.8).
The review authors did not undertake a combined analysis as two studies report data at more than one time point.
d. MAC
Two studies report data on change in MAC (Rogers 1992; Sharma 2017).
Zero to three months
One study (103 participants) reported data on change in MAC from baseline to three months (Sharma 2017). There was no difference between groups receiving dietary advice and ONS if required and groups receiving no advice or ONS, MD 0.13 cm (95% CI ‐0.68 to 0.94) (Analysis 4.9).
4.9. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 9: Change in mid‐arm circumference (cm)
Four to six months
One study (27 participants) reported data on change in MAC from baseline to four months (Rogers 1992). There was no difference between groups, MD 0.30 cm (95% CI ‐0.84 to 1.44) (Analysis 4.9).
There was no heterogeneity between subgroups (I² = 0%). In a combined analysis there was no difference in change in MAC between groups, MD 0.19 (95% CI ‐0.47 to 0.85) with no heterogeneity (I² = 0%) (Analysis 4.9).
e. MAMC
Two studies report data on change in MAMC (Caccialanza 2015; Sharma 2017).
Zero to three months
One study (103 participants) reported the change from baseline in MAMC to three months (Sharma 2017). There was no difference in MAMC between groups, MD ‐0.14 cm (95% CI ‐0.71 to 0.43) (Analysis 4.10).
4.10. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 10: Change in mid‐arm muscle circumference (cm)
Seven to 12 months
One study (144 participants) reported the change in MAMC from baseline to seven to 12 months (Caccialanza 2015). There was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 0.60 cm (95% CI ‐0.17 to 1.37) (Analysis 4.10).
There was moderate to substantial heterogeneity between subgroups (I² = 56.7%). In a combined analysis there was no difference in change in MAMC between groups, MD 0.18 (95% CI ‐0.54 to 0.90) with moderate to substantial heterogeneity (I² = 57%). The studies recruited participants with very different clinical backgrounds (acute medical older people and individuals with chronic amyloidosis) and investigators measured the outcome at very different time points (up to three months and at 12 months).
f. TSF
Three studies reported data on change in TSF (Ovesen 1993; Rogers 1992; Sharma 2017).
Zero to three months
Only one study (103 participants) contributed data on change in TSF following an intervention lasting up to three months (Sharma 2017). There was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 0.89 mm (95% CI ‐1.15 to 2.93) (Analysis 4.11).
4.11. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 11: Change in triceps skinfold thickness (mm)
Four to six months
Two studies (132 participants) contributed data on change in TSF following an intervention lasting four to six months (Ovesen 1993; Rogers 1992). There was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 0.60 mm (95% CI ‐0.57 to 1.77) with no heterogeneity (I² = 0%) (Analysis 4.11).
There was no heterogeneity between subgroups (I² = 0%). In a combined analysis there was no difference in change in TSF between groups, MD 0.67 mm (95% CI ‐0.34 to 1.69) with no heterogeneity (I² = 0%).
Secondary outcomes
1. Nutritional intake before and after the intervention
a. Change in energy intake
Data on change in energy intake was available from eight studies (Beck 2012; Beck 2015; Forli 2001; Isenring 2004; Moloney 1983; Ovesen 1993; Schilp 2013; Uster 2013).
Zero to three months
Eight studies (645 participants) reported on the change in energy intake up to three months (Beck 2012; Beck 2015; Forli 2001; Isenring 2004; Moloney 1983; Ovesen 1993; Schilp 2013; Uster 2013). There was a significantly greater improvement in energy intake in the groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 147.01 kcal/day, 95% CI 21.55 to 272.47 kcal with moderate to substantial heterogeneity (I² = 58%) (Analysis 4.12). Removal of one study reduced heterogeneity to zero (Moloney 1983) and the difference in change in energy intake remained statistically significant in favour of the groups receiving advice plus ONS if required, MD 222.89 kcal/day (95% CI 142.67 to 303.11). This is the only study in this group where energy intake decreased in both groups; however, it is not possible to explain why the results of this study differ from those undertaken in a similar population, i.e. people with cancer receiving radiotherapy (Isenring 2004; Ovesen 1993; Uster 2013).
4.12. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 12: Change in energy intake (kcal)
Four to six months
Three studies (290 participants) reported on change in energy intake between four and six months (Ovesen 1993; Schilp 2013; Uster 2013). There was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 50.31 kcal/day (95% CI ‐154.15 to 254.76) and zero to moderate heterogeneity (I² = 39%) (Analysis 4.12). One study in 336 older people with cancer receiving chemotherapy reported an increase in energy intake between visits 1 and 2 in both groups, but the change was significantly higher (P < 0.01) in the group receiving dietary advice plus ONS if required (intervention 328 kcal/day; control 132 kcal/day) (Bourdel‐Marchasson 2014). In a study of older people living in the community (n = 68), after six months the intervention group (dietary advice plus ONS if required) showed a significant improvement in dietary intake compared with the control group (no advice or ONS) (Endevelt 2011).
Seven to 12 months
A study of 144 outpatients with amyloidosis reported that dietary advice and ONS if required significantly improved total daily energy intake compared with no advice and no ONS, OR 2.18 (95% CI 1.04 to 4.57) (Caccialanza 2015).
The review authors did not undertake a combined analysis as three studies report data at more than one time point.
b. Final energy intake
Three studies reported final energy intake (Carey 2013; Feldblum 2011; Starke 2011).
Zero to three months
Three studies (327 participants) reported final energy intake at up to three months (Carey 2013; Feldblum 2011; Starke 2011). There was no difference between groups receiving dietary advice plus ONS if required and those receiving no advice or ONS, MD 215.17 kcal/day (95% CI ‐55.09 to 485.43) with considerable heterogeneity (I² = 83%). Removal of one study reduced heterogeneity (I2 = 12%) (Starke 2011) and there remained no difference between the two groups, MD 109.58 kcal/day (95% CI ‐71.29 to 290.44). All three studies were conducted in similar populations (hospitalised older people); however, in one study the duration of the intervention was for the length of hospital stay only (two to three weeks) (Starke 2011), whereas the intervention continued for three to six months after hospital discharge in the other two studies (Carey 2013; Feldblum 2011).
Four to six months
Two studies (195 participants) reported final energy intake at up to three months (Carey 2013; Feldblum 2011). There was no difference between groups receiving dietary advice plus ONS if required and those receiving no advice or ONS, MD ‐8.62 kcal/day (95% CI ‐154.63 to 137.39) with no heterogeneity (I² = 0%).
No combined analysis was undertaken as several studies reported data at more than one time point.
c. Change in protein intake
Data on change in protein intake were available in eight studies (Beck 2012; Beck 2015; Isenring 2004; Moloney 1983; Ovesen 1993; Schilp 2013; Suominen 2015; Uster 2013).
Zero to three months
Seven studies (610 participants) reported on the change in protein intake up to three months (Beck 2012; Beck 2015; Isenring 2004; Moloney 1983; Ovesen 1993; Schilp 2013; Uster 2013). There was a significantly higher intake in the between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 7.76 g/day, 95% CI 0.47 to 15.05 with considerable heterogeneity (I² = 79%) (Analysis 4.14). Removal of two studies reduced heterogeneity to zero (Moloney 1983; Uster 2013) and there was a significantly higher protein intake in groups receiving dietary advice and ONS if required, MD 13.04 g/day (95% CI 9.65 to 16.43). In these two studies protein intake decreased in both groups in contrast with the other studies. It is not possible to explain why the results of these studies differ from the others conducted in similar populations, i.e. individuals with cancer receiving radiotherapy (Isenring 2004; Ovesen 1993).
4.14. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 14: Change in protein intake (g)
Four to six months
Three studies (290 participants) reported on the change in protein intake between four and six months (Ovesen 1993; Schilp 2013; Uster 2013). There was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 3.10 g/day (95% CI ‐7.41 to 13.61) and substantial heterogeneity (I² = 60%) (Analysis 4.14). Removal of one study reduced heterogeneity to zero (Uster 2013) and protein intake was significantly greater in groups receiving advice plus ONS if required, MD 8.31 g/day (95% CI 1.33 to 15.30). In contrast to the other studies, in this study the increase in protein intake was higher in the control group (Uster 2013).
12 months and over
Only one study (78 participants) contributed data on the change in protein intake between baseline and 12 months and over (Suominen 2015); there was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 5.60 g (95% CI ‐3.00 to 14.20) (Analysis 4.14).
Review authors did not undertake a combined analysis as several studies report data at more than one time point.
2. Measures of functional status
Data on change in handgrip strength was available in nine studies (Beck 2012; Beck 2015; Carey 2013; Pedersen 2016a; Pedersen 2016b; Rogers 1992; Schilp 2013; Sharma 2017; Terp 2018; Uster 2013).
Zero to three months
Eight studies (801 participants) reported the change in handgrip strength at up to three months (Beck 2012; Beck 2015; Carey 2013; Pedersen 2016a; Pedersen 2016b; Schilp 2013; Sharma 2017; Terp 2018; Uster 2013). There was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 0.18 kg (95% CI ‐0.36 to 0.72) and no heterogeneity (I² = 0%) (Analysis 4.15).
4.15. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 15: Change in grip strength (kg force)
Four to six months
Three studies (224 participants) reported analysable data for the change in handgrip strength between four and six months (Carey 2013; Schilp 2013; Uster 2013). There was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, MD 0.28 kg (95% CI ‐1.02 to 1.59) with no heterogeneity (I² = 0%) (Analysis 4.15).
The review authors did not undertake a combined analysis as several studies report data at more than one time point.
A further study of 17 outpatients with COPD reported a significantly greater increase in handgrip strength in the group receiving dietary advice and ONS if required compared with no advice, 5.5 kg versus ‐6.0 kg (P = 0.01) (Rogers 1992).
3. QoL
There were 18 studies (19 data sets) which assessed QoL, but used a variety of QoL instruments (Andersson 2017; Beck 2015; Bourdel‐Marchasson 2014; Caccialanza 2015; Carey 2013; Isenring 2004; Jensen 1997; Kiss 2016; Ovesen 1993; Pedersen 2016a; Pedersen 2016b; Rogers 1992; Schilp 2013; Sharma 2017; Silvers 2014; Starke 2011; Suominen 2015; Uster 2013; Vivanti 2015). Five studies used the EQ‐5D (Andersson 2017; Beck 2015; Schilp 2013; Sharma 2017; Vivanti 2015) and four studies used the EORTC questionnaire (Bourdel‐Marchasson 2014; Carey 2013; Isenring 2004; Uster 2013). One study used both the EQ‐5D and EORTC‐C‐30 (Silvers 2014 ), three studies (four data sets) used the SF‐36 (Caccialanza 2015; Pedersen 2016a; Pedersen 2016b; Starke 2011), two studies used the QoL Index (Jensen 1997; Ovesen 1993), one study used FACT‐L (Kiss 2016), one study used the Sickness Impact Profile (Rogers 1992) and one study used the HR‐QoL (Suominen 2015).
Review authors entered data into the meta‐analyses for global QoL scores, physical function, mental function, social function, cognitive function, pain and energy/fatigue using the SMD to combine data using different QoL questionnaires.
a. Global QoL
Zero to three months
Seven studies (389 participants) reported on change in global QoL scores (Beck 2015; Carey 2013; Isenring 2004; Kiss 2016; Persson 2002; Sharma 2017; Vivanti 2015). There was no difference in global QoL between groups receiving advice plus ONS if required compared with no advice and no ONS, SMD 0.15 (95% CI ‐0.18 to 0.48) (low‐certainty evidence) with moderate to substantial heterogeneity (I² = 57%) (Analysis 4.16). Removal of one study reduced heterogeneity (I2 = 39%) (Isenring 2004) and there remained no effect of intervention on global QoL, SMD 0.05 (95% CI ‐0.25 to 0.35). All studies took place in a variety of different clinical conditions, settings and age ranges, but it is not possible to explain how the removal of this study affected heterogeneity as the participants in the Isenring study were similar to those in the Persson study (Isenring 2004; Persson 2002).
Four to six months
Two studies (156 participants) reported on change in global QoL scores (Carey 2013; Schilp 2013). There was no difference between groups receiving dietary advice plus ONS if required compared with no advice or ONS, SMD 0.04 (95% CI ‐0.28 to 0.36) with no heterogeneity (I² = 0%) (Analysis 4.16).
Seven to 12 months
One study (78 participants) reported on change in global QoL at up to 12 months (Suominen 2015) and the group receiving advice plus ONS if required had a small improvement in global QoL compared with the group receiving no advice and no ONS, SMD 0.60 (95% CI 0.14 to 1.05) (Analysis 4.16).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
b. QoL — physical function
Zero to three months
Five studies (458 participants) reported on change in physical function at up to three months (Isenring 2004; Kiss 2016; Pedersen 2016a; Persson 2002; Schilp 2013). There was no difference in change in physical function between groups receiving dietary advice plus ONS if required compared with no advice or ONS, SMD 0.02 (95% CI ‐0.22 to 0.25) with zero to moderate heterogeneity (I² = 31%) (Analysis 4.18).
Four to six months
Two studies (147 participants) reported on change in physical function at six months (Kiss 2016; Schilp 2013). There was no difference between the groups receiving advice plus ONS if required and the groups receiving no advice and no ONS, SMD ‐0.08 (95% CI ‐0.40 to 0.25) with no heterogeneity (I² = 0%) (Analysis 4.18).
Seven to 12 months
One study (144 participants) reported on change in physical function at up to 12 months (Caccialanza 2015). There was no difference between the group receiving advice plus ONS if required and the group receiving no advice and no ONS, SMD 0.02 (95% CI ‐0.31 to 0.35) (Analysis 4.18).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
c. QoL — mental function
Zero to three months
Four studies (435 participants) reported on change in mental function at up to three months (Isenring 2004; Pedersen 2016a; Persson 2002; Schilp 2013). Groups receiving advice plus ONS if required had a small improvement in mental function compared with groups receiving no advice and no ONS, SMD 0.29 (95% CI 0.10 to 0.48) with no heterogeneity (I² = 0%) (Analysis 4.19).
Four to six months
One study (123 participants) reported on change in mental function at six months (Schilp 2013). The group receiving advice plus ONS if required had a small to moderate improvement in mental function compared with the group receiving no advice and no ONS, SMD 0.42 (95% CI 0.07 to 0.78) (Analysis 4.19).
Seven to 12 months
One study (144 participants) reported on change in mental function at up to 12 months (Caccialanza 2015). The group receiving advice plus ONS if required had a small to moderate improvement in mental function compared with the group receiving no advice and no ONS, SMD 0.46 (95% CI 0.13 to 0.79) (Analysis 4.19).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
d. QoL ‐ social function
Zero to three months
Two studies (156 participants) reported on change in social function at up to three months (Isenring 2004; Persson 2002). There was no difference between the groups receiving advice plus ONS if required and the groups receiving no advice and no ONS, SMD 0.02 (95% CI ‐0.35 to 0.40) with no heterogeneity (I² = 27%) (Analysis 4.20).
e. QoL — cognitive function
Zero to three months
Two studies (156 participants) reported on change in cognitive function at up to three months (Isenring 2004; Persson 2002). There was no difference between the groups receiving advice plus ONS if required and the groups receiving no advice and no ONS, SMD 0.35 (95% CI ‐0.23 to 0.92) with substantial heterogeneity (I² = 66%) (Analysis 4.21). It is not possible to explain the heterogeneity between these two studies since the populations were similar and they used the same QoL tool.
f. QoL — pain
Zero to three months
Two studies (156 participants) reported on change in pain at up to three months (Isenring 2004; Persson 2002). There was no difference between the groups receiving advice plus ONS if required and the groups receiving no advice and no ONS, SMD ‐0.48 (95% CI ‐1.03 to 0.07) with substantial heterogeneity (I² = 62%) (Analysis 4.22). It is not possible to explain the heterogeneity between these two studies since the populations were similar and they used the same QoL tool.
g. QoL — energy/fatigue
Zero to three months
Two studies (155 participants) reported on change in energy/fatigue at up to three months (Isenring 2004; Persson 2002). There was no difference between the groups receiving advice plus ONS if required and the groups receiving no advice and no ONS, SMD ‐0.58 (95% CI ‐1.61 to 0.46) with considerable heterogeneity (I² = 89%) (Analysis 4.23). It is not possible to explain the heterogeneity between these two studies since the populations were similar and they used the same QoL tool.
4. Cost
Zero to three months
One study (71 participants) estimated that the cost for the discharge liaison team plus a dietitian and ONS if required in the intervention group (n = 34) as being EUR 9416 compared to EUR 1150 just for the discharge liaison team with no dietitian and no ONS in the control group (n = 37) (Beck 2015). The estimated cost of hospitalisations was EUR 92,020 in the intervention group and EUR 220,025 in the control group; cost savings added up to EUR 3048 per participant in the intervention group.
Four to six months
Two studies reported at this time point (Endevelt 2011; Schilp 2013). The Endevelt study (68 participants) reported that the costs of visits by a primary care physician were significantly lower in the intervention group; investigators also reported a trend of decreased cost in hospital admissions and prescribed medications but these differences did not reach statistical significance (Endevelt 2011). The Schlip study (146 participants) reported that dietetic treatment in undernourished older adults living in the community was not cost‐effective compared with usual care (Schilp 2013).
Group 5 — Dietary advice with ONS compared with no advice and no ONS
This comparison 13 studies (1315 participants) (Anbar 2014; Baldwin 2011; Berneis 2000; Calegari 2011; Chandra 1985; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013).
Please refer to the summary of findings table for the explanations of judgements (Table 5). Note that GRADE judgements are for specific outcomes at the three‐months time point and are not provided for all outcomes at each time point.
Primary outcome
1. Mortality
Mortality data were available from nine studies (Anbar 2014; Baldwin 2011; Calegari 2011; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Persson 2007; Um 2014; Wyers 2013).
Zero to three months
Data were available from seven studies (797 participants) at up to three months (Anbar 2014; Baldwin 2011; Calegari 2011; Jahnavi 2010; Neelemaat 2011; Um 2014; Wyers 2013). There was no difference in mortality between the dietary advice with ONS group and the no advice and no ONS group, RR 0.91 (95% CI 0.55 to 1.52) (low‐certainty evidence). No heterogeneity was evident (I² = 0%) (Analysis 5.1).
5.1. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 1: Mortality
Four to six months
Data were available from four studies (650 participants) at this time point (Baldwin 2011; Neelemaat 2011; Persson 2007; Wyers 2013). There was no difference in mortality between the dietary advice with ONS group and the no advice and no ONS group, RR 0.85 (95% CI 0.62 to 1.17). There was no heterogeneity (I² = 0%) (Analysis 5.1).
Seven to 12 months
Data were available from three studies (461 participants) at the time point from seven to 12 months (Baldwin 2011; Hampson 2003; Neelemaat 2011). There was no difference in mortality between the dietary advice with ONS group and the no advice and no ONS group, RR 0.99 (95% CI 0.76 to 1.29). There was low heterogeneity (I² = 13%) (Analysis 5.1).
12 months and over
Data were available from three studies (542 participants) at the time point of 12 months and over (Baldwin 2011; Neelemaat 2011; Wyers 2013). There was no difference in mortality between the dietary advice with ONS group and the no advice and no ONS group, RR 1.07 (95% CI 0.96 to 1.20). There was no heterogeneity (I² = 0%) (Analysis 5.1).
The review authors did not undertake a combined analysis as a number of studies report data at more than one time point.
2. Morbidity
b. Length of hospital stay
Three studies reported data on the length of hospital stay (Anbar 2014; Neelemaat 2011; Wyers 2013).
Zero to three months
Data were available from two studies (258 participants) on the length of hospital stay at up to three months (Anbar 2014; Neelemaat 2011). There was no difference in length of hospital stay between the dietary advice with ONS group and the no advice and no ONS group, MD ‐1.81 days (95% CI ‐3.65 to 0.04) (low‐certainty evidence). There was no heterogeneity (I² = 0%) (Analysis 5.2).
5.2. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 2: Length of hospital stay (days)
Four to six months
Data were available from one study (147 participants) on the length of hospital stay at four to six months (Wyers 2013). There was no difference in length of hospital stay between the dietary advice with ONS group and the no advice and no ONS group, MD 1.10 days (95% CI ‐12.46 to 14.66) (Analysis 5.2).
There was no heterogeneity between subgroups (I² = 0%). In a combined analysis there was no difference in the length of hospital stay between groups, MD ‐1.75 days (95% CI ‐3.58 to 0.08) with no heterogeneity (I² = 0%) (Analysis 5.2).
c. Complications
Data on complications were available from one study (Anbar 2014).
Zero to three months
One study (50 participants) reported on complications at up to three months (Anbar 2014). There were significantly fewer complications in the group receiving advice plus ONS compared with the group receiving no advice or ONS, RR 0.42 (95% CI 0.20 to 0.89) (low‐certainty evidence) (Analysis 5.3).
5.3. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 3: Complications
3. Measures of nutritional status
a. Change in weight
Data on weight change were available in 11 studies (Baldwin 2011; Berneis 2000; Calegari 2011; Hampson 2003; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013).
Zero to three months
Eight studies (620 participants) reported on weight change at up to three months (Baldwin 2011; Berneis 2000; Jahnavi 2010; Neelemaat 2011; Payette 2002; Persson 2007; Um 2014; Wyers 2013). There was no difference in weight change between the dietary advice with ONS group and the no advice and no ONS group, MD 1.08 kg (95% CI ‐0.17 to 2.33) (low‐certainty evidence) and considerable heterogeneity (I² = 80%) (Analysis 5.4). Removal of two studies removed heterogeneity (I² = 1%) (Calegari 2011; Um 2014) and there was significantly greater weight gain the groups receiving dietary advice plus ONS compared with the groups receiving no advice and no ONS, MD 2.02 kg (95% CI 1.46 to 2.57). In these two studies weight gain was greater in the control group than in the group receiving dietary advice and ONS. It is difficult to explain this difference since it contrasts with the results of other studies in similar populations (Baldwin 2011). The review authors note that they imputed the SD for change in both these studies.
5.4. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 4: Change in weight (kg)
Four to six months
Five studies (450 participants) reported on weight change between four and six months (Baldwin 2011; Paton 2004; Payette 2002; Persson 2007; Wyers 2013). There was a significantly greater weight gain in the dietary advice with ONS group compared with the no advice and no ONS group, MD 1.88 kg (95% CI 0.90 to 2.87) and substantial heterogeneity (I² = 54%) (Analysis 5.4). Heterogeneity might be explained by a greater increase in weight in one study (Persson 2007). Removing this study still results in a significant increase in weight, MD 1.47 kg (95% CI 0.88 to 2.06), but with no heterogeneity (I² = 0%). Persson included older participants admitted onto acute elderly care and trauma wards (Persson 2007). A further study also included older participants, but these participants were living in the community (Payette 2002). Two studies were in participants with cancer and tuberculosis respectively (Baldwin 2011; Paton 2004) and the final study was in older people admitted for surgery for a hip fracture (Wyers 2013). It is likely that at baseline participants in the study by Persson were in a more acute situation, but had a better recovery once this passed (Persson 2007).
Seven to 12 months
Two studies (110 participants) reported data on weight change at this time point (Baldwin 2011; Hampson 2003). There was a significantly greater weight gain in the dietary advice with ONS group than the no advice and no ONS group, MD 2.60 kg (95% CI 1.42 to 3.78) and no heterogeneity (I² = 0%) (Analysis 5.4).
The review authors did not undertake a combined analysis as several studies provided data at more than one time point.
Sensitivity analysis
The review authors imputed the SD of weight change for three studies (Berneis 2000; Calegari 2011; Um 2014). There were too few studies in the analysis at this time point to examine the impact of this on the overall result.
Funnel plot examination, the Egger regression asymmetry test and the Begg's adjusted rank correlation suggested no evidence of small study bias (P = 0.377 and P = 0.386 respectively) although there were only eight studies in this analysis therefore the test may be invalid (Figure 7).
7.

Funnel plot of comparison 5: dietary advice plus ONS compared with no advice and no ONS. Outcome 5.4.1 change in weight (kg)
b. BMI
Data on the change in BMI were available from four studies (Calegari 2011; Persson 2007; Um 2014; Wyers 2013).
Zero to three months
One study (137 participants) reported data on change in BMI at up to three months (Wyers 2013). There was a significantly greater increase in BMI in the dietary advice plus ONS group compared with the no advice and no ONS group, MD 0.66 kg/m² (95% CI 0.19 to 1.13) (Analysis 5.6).
5.6. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 6: Change in BMI (kg/m²)
A second study (15 participants) in people on haemodialysis reported the change in BMI was significantly greater in the no advice and no ONS group (0.6 kg/m²) compared to the dietary advice plus ONS group (0.37 kg/m²) (Calegari 2011). Similarly, a further study (87 participants) of people with cancer receiving radiotherapy also reported a greater change in BMI in the no advice and no ONS group (1.1 kg/m²)compared with the dietary advice plus ONS group (0.2 kg/m²) (Um 2014).
Four to six months
Data on change in BMI were available to enter into a meta‐analysis from one study (131 participants) between four and six months (Wyers 2013). There was no difference in change in BMI between the dietary advice plus ONS group and the no advice and no ONS group, MD 0.44 kg/m² (95% CI ‐0.09 to 0.98). A study of older people at discharge from hospital (108 participants) reported that the change in BMI was greater in the dietary advice plus ONS group (0.3 kg/m²) compared with the no advice and no ONS group (‐ 0.7 kg/m²) (P < 0.001) (Persson 2007).
The review authors did not undertake a combined analysis as the included study provided data at more than one time point.
Data on final values for BMI were available from four studies (Calegari 2011; Persson 2007; Um 2014; Wyers 2013).
Zero to three months
Three studies (254 participants) reported data on final BMI at up to three months (Calegari 2011; Um 2014; Wyers 2013). There was no difference in final BMI between the dietary advice plus ONS groups compared with the no advice and no ONS groups, MD 0.64 kg/m² (95% CI ‐0.76 to 2.04) with moderate heterogeneity (I² = 46%) (Analysis 5.7).
5.7. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 7: Final BMI (kg/m²)
Four to six months
Two studies (242 participants) reported data on final BMI between four and six months (Persson 2007; Wyers 2013). There was no difference in final BMI between the groups receiving dietary advice plus ONS and the groups receiving no advice and no ONS, MD 0.71 kg/m² (95% CI ‐0.45 to 1.87) with moderate heterogeneity (I² = 40%) (Analysis 5.7).
The review authors did not undertake a combined analysis as one study provided data at more than one time point.
c. FFM
Data on change in FFM were available from five studies and the review authors combined these in a meta‐analysis using the SMD because the data consisted of estimates of FFM using different methods (Berneis 2000; Calegari 2011; Hampson 2003; Neelemaat 2011; Paton 2004).
Zero to three months
Three studies (130 participants) reported on the change in FFM at up to three months (Calegari 2011; Neelemaat 2011; Paton 2004). There was no difference in the change in FFM between the dietary advice with ONS group and the no advice and no ONS group, SMD 0.26 kg (95% CI ‐0.09 to 0.62) (very low‐certainty evidence) and no heterogeneity (I² = 0%) (Analysis 5.5). In a study of 15 people with HIV infection, mean (SD) FFM as a % of total body weight increased in the dietary advice plus ONS group from 83.5% (1.8) to 86.3% (1.7) (P < 0.05), but there was no change in FFM in the no advice and no ONS group (Berneis 2000).
Four to six months
One study (26 participants) reported on the change in FFM at six months (Paton 2004). There was no difference between the dietary advice with ONS groups and the no advice and no ONS groups, SMD 0.21 kg (95% CI ‐0.57 to 0.99) (Analysis 5.5).
Seven to 12 months
A further study (71 participants) reported the change in FFM at 12 months (Hampson 2003). There was no difference between the dietary advice with ONS groups and the no advice and no ONS groups, SMD 0.29 kg (95% CI ‐0.18 to 0.75) (Analysis 5.5).
The review authors did not undertake a combined analysis as one study provided data at more than one time point.
d. MAC
One study (152 participants) which recruited frail older people after hip fracture provided data for the change in MAC (Wyers 2013).
Zero to three months
The study reported no difference in change in MAC at three months between the dietary advice plus ONS group and the no advice and no ONS group, MD 0.01 cm (95% CI ‐0.75 to 0.76) (Wyers 2013).
Four to six months
After six months there was no difference in change in MAC between the dietary advice plus ONS group and the no advice and no ONS group, MD 0.38 cm (95% CI ‐0.33 to 1.10) (Wyers 2013).
e. MAMC
One study (83 participants) of frail older people living at home reported on the change in MAMC, but presented data in narrative as no SDs of change were available (Payette 2002).
Four to six months
There was no difference in the mean (SD) change in MAMC at four months between the dietary advice plus ONS group (from 21.0 (2.4) cm at baseline to 21.0 (2.0) cm at four months, change = 0.0 cm) and the no advice and no ONS group (from 21.3 (2.0) cm at baseline to 21.1 (2.5) cm at four months, change = ‐0.2 cm) (Payette 2002).
f. TSF
Data on change in TSF were available from one study (83 participants) of frail older people living at home (Payette 2002), but the review authors present the data in narrative. While the mean (SD) is reported at baseline and at the end of the intervention, the change data has no SD available.
Four to six months
Data on TSF were available from one study (83 participants) in older people living in the community (Payette 2002). There was a greater increase in TSF in the dietary advice and ONS group (reported as mean (SD)), from 13.5 (5.3) mm at baseline to 14.4 (5.6) mm at 16 weeks (the end of intervention) (change = 0.9 mm) compared with the no advice and no ONS group which increased from 13.3 (6.5) mm at baseline to 13.6 (6.6) mm at 16 weeks (change = 0.3 mm).
Secondary outcomes
1. Nutritional intake before and after the intervention
a. Change in energy intake
Data on change in energy intake were available from seven studies (Baldwin 2011; Berneis 2000; Hampson 2003; Neelemaat 2011; Paton 2004; Payette 2002; Wyers 2013).
Zero to three months
Five studies (347 participants) reported on the change in energy intake at up to three months (Baldwin 2011; Berneis 2000; Neelemaat 2011; Paton 2004; Wyers 2013). There was a significantly higher energy intake in the groups receiving dietary advice and ONS compared with the no advice and no ONS group, MD 319.78 kcal/day (95% CI 152.83 to 486.73). Moderate heterogeneity was evident (I² = 50%) (Analysis 5.8).
5.8. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 8: Change in energy intake (kcal)
Four to six months
Three studies (244 participants) reported on the change in energy intake between four and six months (Paton 2004; Payette 2002; Wyers 2013). There was a significantly greater intake in the groups receiving dietary advice plus ONS compared with the groups receiving no advice and no ONS, MD 239.83 kcal/day (95% CI 38.74 to 440.92) and moderate to substantial heterogeneity (I² = 54%) (Analysis 5.8). Removal of one study reduced heterogeneity (I2 = 0%) (Wyers 2013) and the effect remained significant, MD 318.79 kcal/day (95% CI 179.93 to 457.64). Heterogeneity might be explained by the fact that in two studies the participants were recovering from an acute illness (Paton 2004; Payette 2002), while in the third study the participants were frail, older people living at home (Wyers 2013).
Seven to 12 months
One study (63 participants) reported data on the change in energy intake at up to 12 months (Hampson 2003). There was a significantly greater energy intake in the dietary advice and ONS group and the no advice and no ONS group, MD 464.00 kcal/day (95% CI 270.07 to 657.93) (Analysis 5.8).
The review authors did not undertake a combined analysis as two studies report data at more than one time point.
b. Final energy intake
Data on final energy intake were available from three studies (Anbar 2014; Berneis 2000; Um 2014).
Zero to three months
All three studies (152 participants) reported data at up to three months (Anbar 2014; Berneis 2000; Um 2014). The dietary advice and ONS groups had a significantly higher energy intake compared with the no advice and no ONS groups, MD 399.11 kcal/day (95% CI 123.00 to 675.22) with considerable heterogeneity (I² = 76%) (Analysis 5.9). Removal of one study reduced heterogeneity (I2 = 25% ) (Berneis 2000) and the effect remained statistically significant, MD 281.16 kcal/day (95% CI 111.55 to 450.76). Heterogeneity is likely explained by the different disease backgrounds (older participants compared with younger adults with HIV infection) and small study sample sizes.
5.9. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 9: Final energy intake (kcal)
c. Change in protein intake
Data on the change in protein intake were available from two studies (Neelemaat 2011; Wyers 2013).
Zero to three months
Both studies (285 participants) reported on the change in protein intake at up to three months (Neelemaat 2011; Wyers 2013). There was no difference between the dietary advice and ONS group and the no advice and no ONS group, MD 7.14 g/day (95% CI ‐0.46 to 14.74) with no heterogeneity (I² = 25%) (Analysis 5.10).
5.10. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 10: Change in protein intake
Four to six months
Only one study (135 participants) reported the change in protein intake at six months (Wyers 2013). There was no difference in protein intake between the dietary advice and ONS group and the no advice and no ONS group, MD 0.92 g/day (95% CI ‐8.93 to 10.76) (Analysis 5.10).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
d. Final protein intake
Data on final protein intake were available from four studies (Anbar 2014; Berneis 2000; Hampson 2003; Um 2014).
Zero to three months
Three studies (152 participants) reported data at up to three months (Anbar 2014; Berneis 2000; Um 2014). The dietary advice and ONS group had a significantly higher final protein intake than the no dietary advice and no ONS group, MD 18.15 g/day (95% CI 9.37 to 26.93) with no heterogeneity (I² = 17%).
Seven to 12 months
Data were available from one study (71 participants) at this time point (Hampson 2003). The dietary advice and ONS group had a significantly higher final protein intake than the no dietary advice and no ONS group, MD 17.00 g/day (95% CI 7.18 to 26.82).
There was no heterogeneity between subgroups (I² = 0%). In an analysis of all studies combined, the dietary advice and ONS groups had a significantly higher final protein intake than the no dietary advice and no ONS groups, MD 17.67 g/day (95% CI 11.80 to 23.55) with no heterogeneity (I² = 0%) (Analysis 5.11).
5.11. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 11: Final protein intake (g/day)
2. Measures of functional status
a. Handgrip strength
Data on change in handgrip strength were available from six studies (Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Wyers 2013).
Zero to three months
Three studies (244 participants) reported data for analysis on the change in handgrip strength at up to three months (Jahnavi 2010; Neelemaat 2011; Paton 2004). There was no difference between the dietary advice and ONS group and the no advice and no ONS group, MD 0.99 kg force (95% CI ‐0.42 to 2.40) with moderate heterogeneity (I² = 49%) (Analysis 5.12). In a further study of frail older people after hip fracture (152 participants), there was no difference in change in handgrip strength at three months between the dietary advice plus ONS group and the no advice and no ONS group, MD 0.13 kg force (95% CI ‐1.35 to 1.62) (Wyers 2013).
5.12. Analysis.

Comparison 5: Dietary advice plus supplements compared with no advice and no supplements, Outcome 12: Change in handgrip strength (kg)
Four to six months
Three studies (200 participants) reported data for analysis on the change in handgrip strength between four and six months (Paton 2004; Payette 2002; Persson 2007). There was no statistically significant difference between the dietary advice and ONS group and the no advice and no ONS groups, MD 0.72 kg force (95% CI ‐0.88 to 2.31) and no heterogeneity (I² = 10%) (Analysis 5.12). In a further study of frail older people after hip fracture (152 participants), there was no difference in change in handgrip strength at six months between the dietary advice plus ONS group and the no advice and no ONS group, MD 0.12 kg force (95% CI ‐1.63 to 1.86) (Wyers 2013).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
3. QoL
The 10 studies reporting data on QoL used a variety of different tools and reported data in different ways e.g. total QoL scores and different domain scores (Baldwin 2011; Berneis 2000; Calegari 2011; Jahnavi 2010; Neelemaat 2011; Paton 2004; Payette 2002; Persson 2007; Um 2014; Wyers 2013).
Two studies used the EORTC questionnaire (Baldwin 2011; Um 2014), one used the FAACT (Baldwin 2011), four used the SF‐36 (Calegari 2011; Jahnavi 2010; Payette 2002; Persson 2007), one used the SF‐12 (Neelemaat 2011), two used the EuroQol‐5D (Neelemaat 2011; Wyers 2013) and one used the Medical Outcomes Instrument adapted for use in people with HIV (Berneis 2000).
The review authors entered data into meta‐analyses for global QoL scores, physical function, mental function, social function, cognitive function, pain and energy/fatigue using the SMD to combine data using different QoL questionnaires.
a. Global QoL
Zero to three months
Four studies (367 participants) reported on change in global QoL (Baldwin 2011; Jahnavi 2010; Paton 2004; Wyers 2013). There was no difference in the change in global QoL between the dietary advice plus ONS group compared with the no advice or ONS group, SMD 0.32 (95% CI ‐0.33 to 0.96) (very low‐certainty evidence) and there was considerable heterogeneity (I² = 88%) (Analysis 5.13). Removal of one study reduced heterogeneity to zero (Jahnavi 2010) but there remained no difference in global QoL between the two groups, SMD ‐0.04 (95% CI ‐0.29 to 0.20). Although the intent of intervention was the same for all studies, they took place in a variety of different populations, countries and healthcare systems and used different QoL tools and one or more of these factors likely explains the observed heterogeneity.
One study (117 participants) reporting global QoL using FAACT also found no difference between groups (Baldwin 2011), SMD 0.01 (95% CI ‐0.35 to 0.38) (Analysis 5.13).
Four to six months
Three studies (214 participants) reported on change in global QoL scores (Baldwin 2011; Paton 2004; Wyers 2013). There was no difference in global QoL between the dietary advice and ONS group and the no advice and no ONS groups, SMD 0.04 (95% CI ‐0.24 0.31) with no heterogeneity (I² = 0%) (Analysis 5.13).
One study (64 participants) reporting global QoL using FAACT also found no difference between groups (Baldwin 2011), SMD ‐0.30 (95% CI ‐0.80 to 0.19) (Analysis 5.13).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
b. QoL — physical function
Zero to three months
Three studies (242 participants) reported on change in physical function at up to three months (Baldwin 2011; Jahnavi 2010; Paton 2004). There was no difference in change in physical function between the dietary advice plus ONS group compared with no advice or ONS group, SMD 0.37 (95% CI ‐0.11 to 0.84); however, there was substantial heterogeneity (I² = 67%) (Analysis 5.14). Removal of one study reduced heterogeneity to zero (Baldwin 2011) and dietary advice and ONS groups had a moderate improvement in physical function compared with the no advice and no ONS groups, SMD 0.58 (95% CI 0.23 to 0.93). Although the intent of intervention was the same for all studies, they took place in a variety of different populations, countries and healthcare systems and used different QoL tools and one or more of these factors likely explains the observed heterogeneity.
Four to six months
Two studies (90 participants) reported on change in physical function at six months (Paton 2004; Persson 2007). The dietary advice and ONS groups had a moderate to large improvement in physical function compared with the no advice and no ONS groups, SMD 0.63 (95% CI 0.18 to 1.09) with no heterogeneity (I² = 11%).
The review authors did not undertake a combined analysis as two studies reported data at more than one time point.
c. QoL — mental function
Zero to three months
Three studies (239 participants) reported on change in mental function at up to three months (Baldwin 2011; Jahnavi 2010; Paton 2004). There was no difference in change in mental function between the dietary advice plus ONS groups compared with the no advice or ONS groups, SMD 0.39 (95% CI ‐0.16 to 0.93); however, there was considerable heterogeneity (I² = 75%) (Analysis 5.15). Removal of one study reduced heterogeneity to zero (Jahnavi 2010) and there remained no difference between the two groups, SMD 0.12 (95% CI ‐0.21 to 0.46). Although the studies all shared the same objectives, they were undertaken in a variety of different populations, countries and healthcare systems and used different QoL tools and one or more of these factors likely explains the observed heterogeneity.
Four to six months
Two studies (90 participants) reported on change in mental function at six months (Paton 2004; Persson 2007). There was no difference in change in mental function between the dietary advice plus ONS group compared with the no advice or ONS group, SMD 0.04 (95% CI ‐0.38 to 0.45) with no heterogeneity (I² = 0%).
The review authors did not undertake a combined analysis as two studies reported data at more than one time point.
d. QoL — social function
Zero to three months
Three studies (235 participants) reported on change in social function at up to three months (Baldwin 2011,Jahnavi 2010; Paton 2004). The dietary advice plus ONS group had a small to moderate improvement in social function compared with the no advice and no ONS group, SMD 0.47 (95% CI 0.02 to 0.91) with substantial heterogeneity, (I² = 62%) (Analysis 5.16). Removal of one study reduced heterogeneity to zero (Baldwin 2011) and the effect remained significant with the dietary advice plus ONS group showing a small improvement in social function compared with the no advice and no ONS group, SMD 0.71 (95% CI 0.36 to 1.06). Although the objective was the same for all studies, they took place in a variety of different populations, countries and healthcare systems and used different QoL tools and one or more of these factors likely explains the observed heterogeneity.
Four to six months
One study (36 participants) reported on change in social function at six months (Paton 2004). There was no difference in change in social function between the dietary advice plus ONS group compared with the no advice or ONS group, SMD 0.40 (95% CI ‐0.26 to 1.06) (Analysis 5.16).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
e. QoL — cognitive function
Zero to three months
Two studies (141 participants) reported on change in cognitive function (Baldwin 2011; Paton 2004). There was no difference between the groups receiving advice plus ONS and the groups receiving advice alone, SMD 0.21 (95% CI ‐0.13 to 0.54) with no heterogeneity (I² = 0%) (Analysis 5.17).
Four to six months
One study (36 participants) reported on change in cognitive function at six months (Paton 2004). There was no difference in change in cognitive function between the dietary advice plus ONS group compared with the no advice or ONS group, SMD 0.29 (95% CI ‐0.37 to 0.95) (Analysis 5.17).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
f. QoL — pain
Zero to three months
Three studies (238 participants) reported on change in pain scores at up to three months (Baldwin 2011; Jahnavi 2010; Paton 2004). There was no difference between the dietary advice plus ONS groups and the no advice and no ONS groups, SMD 0.46 (95% CI ‐0.24 to 1.16) with considerable heterogeneity (I² = 85%) (Analysis 5.18). Removal of one study reduced heterogeneity to zero (Jahnavi 2010) and there remained no difference in the change in pain scores between the two groups, SMD 0.08 (95% CI ‐0.25 to 0.42). Although the objective was the same for all studies, they took place in a variety of different populations, countries and healthcare systems and used different QoL tools and one or more of these factors likely explains the observed heterogeneity.
Four to six months
One study (36 participants) reported no difference in the change in pain scores at six months between the dietary advice plus ONS group compared with the no advice or ONS group, SMD 0.28 (95% CI ‐0.37 to 0.94) (Paton 2004) (Analysis 5.18).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
g. QoL — energy or fatigue
Zero to three months
Three studies (238 participants) reported on change in energy or fatigue at up to three months (Baldwin 2011,Jahnavi 2010; Paton 2004). There was no difference between the dietary advice plus ONS group and the no advice and no ONS groups, SMD 0.46 (95% CI ‐0.24 to 1.16) with no heterogeneity, (I² = 20%) (Analysis 5.19).
Four to six months
One study (36 participants) reported no difference in change in energy or fatigue between the dietary advice plus ONS group compared with the no advice or ONS group, SMD ‐0.01 (95% CI ‐0.66 to 0.64) (Paton 2004) (Analysis 5.19).
The review authors did not undertake a combined analysis as one study reported data at more than one time point.
4. Cost
Two studies reported cost‐effectiveness analyses (Neelemaat 2011; Wyers 2013).
One study (210 participants, 105 in each group) showed that after three months there were no differences in costs between groups (Neelemaat 2011). Cost‐effectiveness for QALYs and physical activities could not be demonstrated because there was no effect on QoL and physical activity. For functional limitations investigators reported a 0.95 probability that the intervention is cost‐effective in comparison with usual care for ceiling ratios over EUR 6500.
A second study (152 participants) showed that the mean costs of the nutritional intervention were EUR 613 (Wyers 2013). Total costs and subcategories of costs were not significantly different between groups. Based on bootstrapping of ICERs, the nutritional intervention was likely to be cost‐effective for weight as measured over the three‐month intervention period, regardless of nutritional status at baseline. When assessing QALYs, the probability for the nutritional intervention being cost‐effective was relatively low, except in people aged below 75 years.
Discussion
This update is the third substantial update of the original review (Baldwin 2001) and the review now includes 94 studies and 10,284 participants.
The results of the 2021 update confirm the results from the previous versions. In general, dietary advice, with or without ONS, elicited an improvement in energy intake, and to a lesser extent protein intake, and an increase in weight. Results were most positive for the first three months and attenuated at later time points. It is important to note that in the majority of studies (59%) included in this review the intervention lasted for three months and the majority of benefits observed also occurred in the first three months but in the absence of agreed cut‐offs for recognition of clinically meaningful benefits it is difficult to draw conclusions on their clinical significance. For the smaller number of studies reporting longer intervention and follow‐up (32% for four to six months and 3% for seven to 12 months or more), there was insufficient evidence to draw conclusions on any benefits. To date there is insufficient information to determine whether three months is the optimal length of intervention of this kind and indeed whether it represents a realistic goal in clinical practice.
This Cochrane Review includes nutritional, clinical, functional, economic and patient‐centred outcomes. The authors found some evidence for improvements in nutritional intake and body weight; however, the evidence for estimates of body composition and physical function such as MAMC, FFM, TSF thickness and grip strength was lacking as few studies reported these outcomes. It can be questioned whether these parameters are as sensitive to improved dietary intake as weight, especially when the changes in weight are only small (in this review the observed MDs in weight were around 1 kg with CIs indicating all differences were less than 2 kg). The majority of participants in this review were older people and understanding of the molecular and cellular mechanisms involved in the recovery of physical function in this group is unclear (Munk 2016). It has been demonstrated that recovery of habitual strength and physical function in healthy people after exposure to semi‐starvation took more than six months (Keys 1950). It is possible that the length of intervention and follow‐up in studies in this review was too short to capture changes to these outcomes
For the first time, the review authors have included QoL scores in this review, with no consistency in effect for either global scores or individual domain scores. QoL measures are intended to measure what really matters to the patient. It has been shown that self‐assessed health status may be an even more powerful predictor of mortality and morbidity than many objective measures of health (DeSalvo 2006; Dominick 2002). QoL measures are increasingly used to assess the effectiveness of interventions because they are thought to reflect how patients feel in addition to objective outcomes such as mortality. Whilst it might be hoped that improved nutrition resulted in improvements to patient‐centred outcomes such as participation in family life, reduced reliance on formal and informal carers and activities of daily living, studies mostly failed to measure these types of outcomes or used a range of different tools making comparisons difficult to draw. A better QoL is associated with less use of medical services, and with decreased healthcare costs (Ferris 2009). The studies included in this review mostly used generic QoL measures to provide an assessment of an individual's overall health. This allows comparison between different health conditions which is in contrast to disease‐specific QoL measures that have the advantage of being able to detect changes specific to the impact of treatment of a particular disease, but prevents comparisons across different patient groups. QoL measures may involve scores for separate domains (e.g. pain, energy or fatigue, social functioning, cognitive functioning, physical functioning) or combine different domains into a composite score. In the studies included in this review there was inconsistent reporting of domain or composite scores at different time points, which may partially explain the lack of consistency in findings. A disadvantage of the general QoL scales is that usually there is no weighting of the importance of the separate domains. Also, factors seemingly important to a patient, e.g. financial security or ability to work are often not incorporated in the QoL scales (Carr 2001).
Temel showed that intensive supportive care (without nutrition) improved QoL and clinical outcomes in people with advanced lung cancer and raised the suggestion that attention, frequent visits and early treatment of symptoms may be as important as the intervention itself (Temel 2010). In the studies in this review it was not always possible to distinguish between benefits resulting from the nutritional intervention, or the improved level of care.
With regard to clinically relevant endpoints, this Cochrane Review does not provide consistent evidence for positive effects of dietary advice, with or without ONS, on mortality, complications, length of stay or readmissions. As this review assessed adults with disease‐related malnutrition, it must be accepted that a nutritional intervention is only one of multiple interventions, that may affect clinical outcomes. This is particularly relevant when considering people with cachexia, or for whom inflammation is a key contributor to their disease‐related malnutrition, such that treatment of the underlying disease or inflammation is critical to an improvement in clinical outcomes. It must, therefore, be questioned whether it is realistic to expect that optimal treatment of malnutrition by nutritional interventions alone would improve clinical outcomes.
There was statistical and clinical heterogeneity across all studies contributing to the findings of this Cochrane Review, apart from the effects on mortality. The review authors combined studies for each intervention and therefore the findings of this review must be interpreted with caution. The possibility that the effects of interventions vary according to factors which it has not been possible to identify must be borne in mind, since the authors cannot assume that the effects of different interventions will be the same in all clinical groups, care settings and participants of different ages. Until there are more homogenous studies in different patient groups, the review authors can not fully evaluate the effects of dietary advice given with or without ONS in individuals and patient populations.
In conclusion, although this Cochrane Review has summarised the findings of 94 separate studies of dietary advice there remains a lack of good‐quality evidence for all reported outcomes and in particular there is a need for more evidence of the effects of dietary advice on clinically‐relevant endpoints, patient‐centred outcomes and cost‐effectiveness.
Summary of main results
The aim of this review was to assess whether adults with disease‐related malnutrition (or at risk of malnutrition) can improve their survival, weight, and general health‐related QoL if they receive dietary advice and ONS. The review authors identified 94 studies (10,284 participants) which included a heterogeneous group of participants from a variety of healthcare settings and represented five different intervention types. All studies were at risk of bias for one or more elements, statistical heterogeneity was frequently high and the certainty of evidence was low for the majority of analyses. The review authors undertook pooled analyses by intervention for the outcomes specified and explained heterogeneity where possible when the I² statistic exceeded 50%.
Dietary advice compared with no advice (usual diet)
The review includes 24 studies comparing dietary advice with no dietary advice, but not all studies contributed data on all outcomes and data were available to enter into the meta‐analyses for only some outcomes. Except for mortality, few studies provided data for inclusion in these analyses.
There was no effect of dietary advice observed on the review's primary outcomes of mortality and two estimates of morbidity (hospital readmissions and complications). There was low‐certainty evidence from one study of significantly shorter hospitalisation in people receiving dietary advice (Analysis 1.3). For all time points there was low‐certainty evidence of an effect on weight change, but this did not translate into consistent improvements in other measures of body composition. One study with data at the 12‐month time point reported a significant improvement in FFM (low‐certainty evidence; Analysis 1.7); and data demonstrated an increase in MAMC at up to three months and at four to six months (Analysis 1.8) in addition to a decrease in TSF measurements at up to three months (Analysis 1.10). There was inconsistent evidence for an effect of dietary advice on energy and protein intake (Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14). There was no effect of dietary advice observed on handgrip strength at any time point (Analysis 1.15), but there was evidence of an improvement in global QoL scores at up to three months (five studies) and at 12 months and over (two studies; low‐certainty evidence; Analysis 1.16). There was no effect seen on cost.
Dietary advice compared with ONS
The review includes 12 studies comparing dietary advice with ONS, but not all studies contributed data on all outcomes and data were available to enter into the meta‐analyses for only some outcomes.
The review authors observed no effect on mortality at any time point. There was no difference between groups in the number of hospital admissions at three months (low‐certainty evidence); however there were fewer hospital admissions in one study at four to six months in the group receiving ONS (Analysis 2.2). No data were reported on the effects of intervention on complications and length of hospital stay. There were no differences between groups in weight change (low‐certainty evidence) or changes in body composition. However, there was evidence of higher energy intake (six studies) and protein intake (five studies) in groups receiving ONS after three months, which persisted to four to six months for protein (Analysis 2.8; Analysis 2.9). One study with an extended follow‐up (median 6.5 years) reported higher energy and protein intakes in the dietary advice group (Ravasco 2005a). Similarly, there was no difference in handgrip strength or in global QoL score between groups at three months, but significantly better global QoL scores from one study at 12 months and over in the group receiving dietary advice (low‐certainty evidence; Analysis 2.11). No studies assessed cost‐effectiveness.
Dietary advice compared with dietary advice plus ONS
The review includes 22 studies comparing dietary advice with dietary advice plus ONS, but not all studies contributed data on all outcomes and data were available to enter into the meta‐analyses for only some outcomes.
As for previous comparisons, there was no difference between groups in mortality at any time point. There was no difference between groups in length of hospital stay at up to three months (low‐certainty evidence; Analysis 3.3); however, in the group receiving dietary advice alone there were fewer hospital admissions at that time point (low‐certainty evidence; Analysis 3.2). There was low‐certainty evidence of fewer complications in the groups receiving dietary advice plus ONS at up to three months (three studies); however, there were no differences at other time points (Analysis 3.4). There was low‐certainty evidence of a greater improvement in weight in the group receiving dietary advice plus ONS up to three months, but there was no difference in weight beyond three months (Analysis 3.5) and minimal evidence of any impact on changes in body composition (Analysis 3.7; Analysis 3.8; Analysis 3.9). There was evidence of higher energy intake (six studies) and protein intake (three studies) in the groups receiving dietary advice plus ONS at up to three months (three studies) and four to six months (one study) (Analysis 3.10; Analysis 3.12). Again, there was no difference in handgrip strength (Analysis 3.14). There was low‐certainty evidence of an improvement in global QoL in the group receiving dietary advice plus ONS at up to three months (four studies) and no difference at other time points (Analysis 3.15). Data from one study suggested that dietary advice plus an ONS was cost‐effective up to three months (Norman 2008b).
Dietary advice plus ONS if required compared with no advice and no ONS
The review includes 31 studies comparing dietary advice plus ONS if required with no advice and no ONS, but not all studies contributed data on all outcomes and data were available to enter into the meta‐analyses for only some outcomes.
There was no difference between groups for clinical outcomes (mortality, hospital readmissions, length of hospital stay and complications) (moderate to low‐certainty evidence; Analysis 4.2; Analysis 4.3; Analysis 4.4). There were improvements in weight and FFM in the groups receiving dietary advice plus ONS at up to three months (moderate‐certainty evidence, Analysis 4.5; low‐certainty evidence, Analysis 4.8), but there were no differences at other time points or for other measures of body composition (Analysis 4.9; Analysis 4.10; Analysis 4.11). At three months, there was evidence of an increase in energy intake (eight studies) and protein intake (seven studies) (Analysis 4.12; Analysis 4.14). At four to six months there was no difference in energy intake (three studies), but there was a higher protein intake in groups receiving dietary advice plus ONS (three studies) (Analysis 4.12; Analysis 4.14). The review authors found no difference in handgrip strength at any time point. There was no difference in global QoL up to six months, but there was greater improvement in global QoL scores at seven to 12 months in the groups receiving dietary advice plus ONS if required (low‐certainty evidence; Analysis 4.16). One study with data at up to three months demonstrated cost savings associated with dietary advice and ONS if required compared with no advice and no ONS. Two studies with data up to six months showed no difference in cost‐effectiveness between groups (Endevelt 2011; Schilp 2013).
Dietary advice and ONS compared with no advice and no ONS
The review includes 13 studies comparing dietary advice plus ONS with no advice and no ONS, but not all studies contributed data on all outcomes and data were available to enter into the meta‐analyses for only some outcomes.
There was no difference in mortality between groups at any time point (Analysis 5.1). There were no data on hospital readmissions and low‐certainty evidence of no difference in length of hospital stay at up to three months or four to six months (Analysis 5.2). There were significantly fewer complications in the groups receiving dietary advice plus ONS at up to three months (one study), but data were not reported at other time points (low‐certainty evidence; Analysis 5.3). There was no difference in weight change at up to three months (low‐certainty evidence), however, there was an improvement in weight in the groups receiving dietary advice plus ONS at all other time points (eight studies; Analysis 5.4). There were no changes in measures of body composition (FFM, MAMC, TSF) (very low‐certainty evidence, Analysis 5.5). There was evidence of an increase in energy intake (five studies) and protein intake (three studies) in groups receiving dietary advice plus ONS at up to three months and at seven to 12 months (one study each) (Analysis 5.8; Analysis 5.10). Energy intake was also improved in the group receiving dietary advice plus ONS at four to six months (three studies; Analysis 5.8). There was no difference in handgrip strength at any time point (Analysis 5.12). There was very low‐certainty evidence of no difference between the two groups in global QoL scores at any time point (four studies; Analysis 5.13). Intervention with dietary advice and ONS was probably cost‐effective for functional limitations after three months (Neelemaat 2011) and likely to be cost‐effective for weight (Wyers 2013).
Overall completeness and applicability of evidence
There was statistical and clinical heterogeneity across all groups of studies in this review. The review authors addressed this statistically by using a random‐effects model for all the analyses presented in the review. Where heterogeneity was moderate or high (I² ≥ 50%), they removed the studies one by one to allow examination of their effect on heterogeneity. When the authors derived a homogeneous effect, they attempted to identify the characteristics of studies that might have contributed to the heterogeneity. The authors made their decisions to remove studies on statistical grounds and these may not necessarily be clinically justified. The heterogeneity could be explained by a number of factors including clinical condition, stage and disease severity of the participants, healthcare setting, frequency and intensity of intervention and other yet to be identified factors. The identified studies represented a wide range of clinical conditions, but the numbers of participants with any one condition were small with the possible exception of studies conducted in those with cancer and in older people. Even amongst these groups of studies, there was wide variation in co‐morbidities in older people and site, stage and treatment modality in people with cancer. In the majority of included studies the mean age of participants was over 65 years, but a wide age range was represented overall. The majority of studies were conducted in outpatients or community, a smaller number involved individuals who spent some time in hospital.
The review authors assume that the mode, duration and the intensity of the intervention may have influenced the effects, but due to lack of reporting of relevant data they were unable to undertake further analyses to explore their assumptions. Whilst all of these studies included some type of dietary advice, the investigators rarely fully described the nature, intensity and content of the intervention. Furthermore, health literacy of participants may have influenced their understanding of information or their experience of attempting to implement it. Interpretation of dietary advice differed hugely amongst studies. In some, investigators provided the participants with a written leaflet, while in others they used face‐to‐face or telephone contact, or a combination of both. In many cases the review authors were unable to identify how (written or verbal), how often and by whom the dietary advice was delivered. Studies also varied in amount and timing of contact (frequency and intensity) from one meeting at the start of the study through to bi‐weekly meetings over a period of months. Sometimes a trained dietitian or nutritionist gave the dietary advice, but sometimes it was a nurse, a doctor, a research assistant, or a community worker. In addition, investigators rarely reported the details of the experience and training of the healthcare worker giving the advice.
In the majority of studies, the intervention lasted for three months. To date there is insufficient information to determine whether this is the optimal length of intervention of this kind and indeed whether it represents a realistic goal in clinical practice. These variations are reflected in clinical practice, where these interventions might consist of only one or two visits by a dietitian to an inpatient through to regular repeated dietetic outpatient visits in people with long‐term disease, e.g. renal failure or COPD. A shorter and less intensive intervention is often seen as more feasible in clinical practice, but we do not know about its effectiveness in comparison to a longer, more intensive intervention. Whilst it may seem that a longer, more intensive dietary intervention might be more effective, in the absence of formal cost‐effectiveness analyses it is not possible to say whether these are more effective than shorter and less intensive interventions. However, in all studies there was a consistent aim of improving nutritional intake with the goal of minimising weight loss or promoting weight gain. Dietitians receive referrals to provide nutritional support to individuals from a variety of clinical backgrounds in different healthcare settings. It is not possible from the findings of this review to be specific about the effect size that can be achieved in any one patient group and indeed it is likely that the effect size will vary according to all the above variables. This review suggests that it is possible to achieve an increase in energy intake and weight gain with dietary advice with or without ONS and in some cases the increase in weight gain may be accompanied by beneficial changes in body composition. However, it remains to be determined whether these improvements in weight and body composition translate consistently into clinical benefits and patient‐related outcomes.
Quality of the evidence
The certainty of evidence in this review is low for the majority of analyses (Table 1; Table 2; Table 3; Table 4; Table 5), with only studies of dietary advice plus supplements if required compared with no advice having some moderate‐certainty evidence (Table 4). The main issues were the high risk of bias due to a lack of blinding of outcome assessors, selection bias resulting from failures in the randomisation process and some studies were downgraded because of differences in baseline characteristics between groups. Furthermore, only two of the 94 included studies were able to adequately blind participants and personnel to the intervention (Alo 2014; Holyday 2012). Whilst this is a considerable source of potential bias, it is important to bear in mind that it is difficult to conceive of an adequate placebo for dietary advice. It is impossible to prevent some participants in the control arm from seeking other sources of dietary advice which act as confounders. Whilst dietary advice can be compared with usual care, this has varied enormously in terms of quality and duration between studies; and in fact was rarely defined or fully described. Furthermore, only one study reported the behaviour change model underpinning their intervention (Locher 2013). Whilst in theory it might be possible to design a study where outcome assessment may be blinded, this was not the case in many of the included studies and a possible reason for this is inadequate funding for this type of research.
Potential biases in the review process
The protocol for this review specified three comparison groups. An additional group was added after the first searches conducted in 1999 when review authors identified a comparison that they had not anticipated and most closely represented actual dietetic practice. Since they included this comparison in the first version of the review the potential for bias at this stage is minimal. The authors included a fifth comparison in the 2021 update of this review, as they identified many studies in which investigators provided dietary advice with ONS to all participants, rather than ‘if judged to be appropriate’. The addition of the two extra groups are logical and consistent with changes in routine clinical practice. The addition of the fifth group at a later stage in the review process means that the review authors cannot rule out the possibility that they have missed studies that might have been identified from earlier searches. After the inclusion of this comparison, they scrutinised the list of studies excluded from earlier searches again and they might also have identified additional studies through the Snowball searching process.
The original search strategy for this review and the current update was comprehensive in that eight databases were searched including databases other than the most commonly used (Avenell 2001); there was no language restriction on papers retrieved and two review authors have selected studies independently. From the 2021 update, the authors have also included searches of the online database Clinicaltrials.gov and WHO ICTRP to identify ongoing studies and recently finished studies that may not yet have been published (Appendix 5). The review authors did not undertake any formal handsearching and searching of the grey literature was not possible because of time constraints.
Using the asymmetry of the funnel plot, the Egger’s test and the Begg’s test suggested no evidence of small study bias for four out of five comparisons. There was evidence of significant small study bias in the comparison of dietary advice with ONS; however, it is worth noting that since there were only eight studies in the analysis the test may be invalid. Interestingly, 22 of the 80 studies that are listed as 'Awaiting classification' would be eligible for inclusion in this group; these are currently listed as such because they are reported in abstract form only and the review authors have not been able to obtain data and full details of interventions to enter into the analysis. An additional 23 full‐text publications have been identified as eligible for inclusion in final searches, but it has not been possible to include these in the 2021 update because of time constraints.
Agreements and disagreements with other studies or reviews
The review authors are unaware of any other systematic reviews that have addressed the potential benefits of dietary advice given with or without ONS as comprehensively as this one. Two recent syntheses have addressed ONS interventions in different healthcare settings (Feinberg 2017; Reinders 2019) and one large RCT has evaluated the impact of individualised dietary advice with ONS, enteral or parenteral nutrition if required in hospitalised patients (Schuetz 2019).
Reinders presented a pooled analysis of individual patient data in older adults across a range of healthcare settings and suggested that dietary counselling with ONS results in a significant increase in energy intake and that dietary counselling with or without ONS results in significant benefits to weight gain; with dietary counselling combined with ONS seeming to be more effective (Reinders 2019). Drawbacks to this review are that individual patient data from only nine out of 38 eligible studies provided data for analysis; therefore it remains unknown whether the results are representative. Nonetheless, Reinders' results are in agreement with this Cochrane Review in so far as the review authors found beneficial effects on weight from dietary advice given with or without ONS over usual diet. They also found improvements in energy and protein intake in groups receiving dietary advice with or without ONS; however, the timing of the improvements varied. In both the review by Reinders and in this Cochrane Review there are a number of limitations to the evidence. In both reviews, the data for each of these outcomes are drawn from a limited number of studies and may not be representative of the real effect had data been available from all studies.
In a systematic review of nutritional support in hospitalised patients there was no effect of a general nutrition intervention (defined as aiming to increase normal food consumption and including, but not limited to, dietary counselling) on weight or BMI (Feinberg 2017). These findings are in contrast to those in this Cochrane Review, but this review includes studies across care settings and the studies have not been analysed according to care setting.
Since the completion of this updated Cochrane Review, a large RCT in hospitalised patients has been published (Schuetz 2019). In this study, 2088 participants were randomised to receive protocol‐guided individualised nutritional support provided by a dietitian or standard hospital food for the duration of hospital stay. By 30 days those in the intervention group reported significant improvements to energy and protein intake compared to those in the control group and experienced significantly fewer adverse outcomes (infections, major complications, major cardiovascular disease event, respiratory failure, acute kidney failure), lower mortality, a significant improvement in QoL and a significantly reduced decline in functional status. These findings are in partial agreement with this Cochrane Review. The improvements in energy and protein intake concur with the review's findings and the review authors found improvement to clinical and functional outcomes for some comparisons; however, they found no effect on mortality. This is an important RCT because it is adequately‐powered, outcomes were assessed blinded to group allocation and it reflects current clinical practice. It is difficult to fully assess whether the RCT's findings are in agreement with those of this Cochrane Review as the RCT was limited to one healthcare setting and this review spans all care settings.
Authors' conclusions
Implications for practice.
This Cochrane Review has summarised the findings of 94 separate studies and found largely low‐certainty evidence (but individual gradings ranged from moderate to very low) to suggest that dietary advice given with or without oral nutritional supplements (ONS) may improve nutritional intake, weight, and quality of life in some adults with disease‐related malnutrition or at nutritional risk. The results were inconsistent and there were no clear trends related to which intervention might be the most beneficial or the length time needed for the intervention to be effective.
This review found no evidence for a beneficial effect of dietary advice on mortality and little evidence of benefit to clinical and functional outcomes. Furthermore, there was very little evidence regarding cost benefits. For many outcomes there were too few data to draw firm conclusions. There is no reason for these interventions not to be made available to adults who have experienced weight loss that is secondary to disease. However, in the context of shared decision‐making discussions, it is currently not possible to specify any benefits that individuals or their families might reasonably expect.
Implications for research.
In this review there was moderate to very low‐certainty evidence that dietary advice given with or without nutritional ONS may improve nutritional intake, weight, and quality of life in some people with disease‐related malnutrition or at nutritional risk. The results were inconsistent and there were no clear trends related to which intervention might be the most beneficial or the length time needed for the intervention to be effective. The review authors found no evidence for a beneficial effect of dietary advice on mortality and limited evidence of benefit to clinical and functional outcomes. There were almost no data on cost‐effectiveness. The heterogeneity of findings may result from variation in the interventions themselves, the healthcare settings, patient populations and the underlying mechanisms by which the intervention might operate. Any future randomised controlled trials (RCTs) and well‐designed non‐RCTs should consider the following points.
Population
Research is needed in populations:
homogeneous for severity of malnutrition at study inclusion, defined using standard assessment tools;
with a shared aetiology of the underlying malnutrition and which groups similar participants, where possible;
with a range of clinical conditions where the stage and treatment intent of the condition are as homogeneous as possible and clearly described; and
in a range of community, health and social care settings.
Intervention
Interventions in this review were extremely varied in terms of nature, intensity, length of intervention, healthcare professional involved and their level of expertise whilst all being considered as dietary advice with or without ONS. A more consistent approach is required to define and describe different dietary interventions for nutritionally vulnerable individuals. The healthcare background of people delivering interventions should be fully described as well as their relevant expertise in nutrition. Interventions should be underpinned by a behaviour change model and this should be valid and appropriate to the aims of the intervention and fully described.
Comparison
It is widely accepted that participants in the control groups should be offered an intervention. However, both active and other interventions should be standardised across studies and fully specified as for the interventions.
Outcomes
There are three key points in relation to assessment of outcomes:
outcomes should be measured using tools that have been validated in the relevant population;
outcomes should be measured at consistent time points that reflect the realities of clinical practice eg. 30‐day mortality or hospital readmissions; and
outcomes should be assessed by assessors blinded to group allocation.
We suggest the following relevant outcomes.
-
Clinical
Mortality
Measures of morbidity, e.g. length of hospital stay, complications
-
Nutritional
Weight and change in body composition
Dietary intake
-
Functional
Functional changes which are relevant to the population under consideration, e.g. Barthel Index, activities of daily living (ADL) scores, handgrip strength
-
Patient‐centred outcomes
Quality of life
Patient satisfaction
Patient experience
Cost effectiveness
Adherence to both food‐based and ONS interventions
Adverse events
The most pressing research priority is to strengthen the evidence base for clinical practice. If further syntheses are undertaken in this area, it might be more meaningful to follow the principles of realist reviews which might inform which interventions are more likely to be beneficial in which patient groups and under which conditions (Pawson 2005).
What's new
| Date | Event | Description |
|---|---|---|
| 21 December 2021 | Amended | In the 'Dates and Events' section, the spelling of the name of one of the new co‐authors (Marian de van der Schueren) has been corrected. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 21 December 2021 | New citation required and conclusions have changed | Two new review authors have joined the team (Marian de van der Schueren and Hinke Kruizenga). A new comparison of 'dietary advice and prescription of an oral nutritional supplement compared with no advice and no oral nutritional supplement' has been added to the review allowing conclusions to be drawn about this comparison. The authors have added 'complications' as a measure of morbidity and report on this outcome. |
| 21 December 2021 | New search has been performed | Following updated searches we added 49 new studies (7098 participants) to the review at this update. A new comparison of 'dietary advice and prescription of an oral nutritional supplement compared with no advice and no oral nutritional supplement' has been added to the review. We have added a summary of findings tables to the review (one for each comparison presented). |
| 22 May 2012 | Amended | Contact details updated. |
| 19 July 2011 | New citation required but conclusions have not changed | The title of the review has been changed from 'Dietary advice for illness‐related malnutrition in adults' to 'Dietary advice with or without oral nutritional supplements for disease‐related malnutrition in adults' following advice from the peer reviewers and the editor. |
| 8 June 2011 | New search has been performed | In total 12 new studies have been included in the review (Baldwin 2011; Campbell 2008; Chandra 1985; Gonzalez‐Espinoza 2005; Manguso 2005; Norman 2008b; Persson 2007; Ravasco 2005b; Rydwik 2008; Sharma 2002a; Singh 2008; Stratton 2007a). Whilst updating this review, a separate group of studies of supportive interventions to enhance nutritional intake have been identified. These studies will be included in a new review. Two studies originally included in this review meet the inclusion criteria of the new review and therefore have been removed from this review at this update and will be included in the new review (Hickson 2004; Turic 1998). After consideration by both authors, 30 new studies have been excluded from the review (Arutiunov 2009; Beck 2008; Botella‐Carretero 2008; Carlsson 2005; Duncan 2006; Forli 2006; Idilman 2009; Jie 2009; Krasnoff 2006; Kruizenga 2005; Lejeune 2005; Manders 2009; Nijs 2006; Olofsson 2007; Parrott 2006; Pedersen 2005; Planas 2005; Plank 2008; Rabinovitch 2006; Rassmussen 2006; Rüfenacht 2010; Salas‐Salvado 2005; Simmons 2008; Smoliner 2008; Solerte 2008; Swanenburg 2007; Tatsumi 2009; Taylor 2006; Watson 2008; Wouters‐Wessling 2005). There are three studies 'Awaiting classification' (Margare 2002a; Penalva 2009a; Shatenstein 2008). |
| 12 November 2008 | Amended | Converted to new review format. |
| 14 November 2007 | New citation required and conclusions have changed | Substantive amendment. Tessa Parsons and Stuart Logan have stepped down as authors on this review. A new co‐author, Elizabeth Weekes, has been recruited. |
| 14 November 2007 | New search has been performed | The latest search did not identify any studies eligible for inclusion in the review.
Two papers previously listed as 'Awaiting Assessment' have now been moved to 'Included studies' (Kalnins 2005; Weekes 2006). The Kalnins 2005 paper is the primary paper for the previously included study (abstracts) ‐ Kalnins 1996.
In the previous version of the review it was unclear how the different studies measured grip strength and so we removed the graphs showing these data and presented the reported means and standard deviations in an additional table. We have now been able to clarify this issue and the data for this outcome is again presented in the analysis. The plain language summary has been updated in light of the current guidance from The Cochrane Collaboration. |
| 15 November 2006 | New search has been performed | Eleven studies have been added to the 'Included studies' section and there are now two studies listed as 'Awaiting Assessment'. It is unclear how the different studies have measured grip strength. Until this has been clarified, we have removed the graphs showing these data and presented the reported means and standard deviations in an additional table. The previous version of this review suggested that nutritional supplements were associated with significantly greater short‐term weight gains. The addition of data at this update has challenged this finding, although it has not been possible to combine the new data in a meta‐analysis. Additionally, this review demonstrates significant improvements in weight in people receiving dietary advice with nutritional supplements rather than dietary advice alone or no intervention. This review has still failed to find any evidence for clinical benefits, such as improved survival, rate of complications and reductions in numbers of hospital admissions and length of stay, of dietary advice. |
| 19 February 2004 | New search has been performed | Two studies (McCarthy 1999; Persson 2002) have been added to the 'Included studies' section. Data are not currently available from these studies, but are being sought from the authors. The reviewers aim to incorporate these data into the next update of the review. Data from a study previously included in 'Studies awaiting assessment' has been obtained from the author and this study is now incorporated into the review (Hickson 2002). |
| 27 February 2002 | New search has been performed | This includes the addition of one study into the "Studies Awaiting Assessment" section of the review. The Hickson 2002 study has not been published in full, but has been submitted for publication and will be incorporated into a future update of this review. |
Acknowledgements
We acknowledge the valuable input from Professor Stuart Logan and Dr Tessa Parsons in the development of the protocol and the full review and also with updating the review until January 2007, when they stepped down from the review team.
We are grateful to the following authors who have provided additional data and information for the included studies:
Dr Chihurumnanya Alo, Ebonyi State University, Nigeria Dr Joanna Andersson, Klinikk Bærum Sykehus, Gjettum, Norway Dr. Abou Badiane, Laboratoire de Nutrition, Université Cheikh Anta Kiop Dakar, Senegal Dr. Christine Baldwin, King’s College, London, UK Professor Peter Ballmer, Kantonsspital, Winterthur, Switzerland Dr Merrilyn Banks, Royal Brisbane & Woman's Hospital, Herston, Australia Dr Alison Beattie, Ninewells Hospital, Dundee, UK Dr Anne Marie Beck, Herlev hospital, Herlev, Denmark Dr Isabelle Bourdel‐Marchasson, Hospital Xavier Arnozan, Bordeaux, France Dr Brandli, Surcher Hohenklinik Wald, Lausanne, Switzerland Dr Sorrel Burden, University of Manchester, Manchester, UK Dr Sharon Carey, Royal Prince Alfred Hospital, Sydney, Australia Dr Katrina Campbell, King's College, London, UK Professor T Cederholm, Uppsala University Hospital, Uppsala, Sweden Dr Silvia Fernandez‐Barres, Universitat Rovira I Virgili (URV), Tarragona, Spain Dr R Fonseca, Dean of School of Dental Medicine, Michigan, USA Dr Liv Forli, Rikshospitalet, Oslo, Norway Dr Liliana Gonzalez‐Espinoza, Hospital de Especialidades, Tamaulipas, Mexico Dr Margaret Holyday, Prince of Wales Hospital, Sydney, Austratia Dr Catherine Huggins, Monash University, Clayton, Victoria, Australia Dr D.T.T Huynh, Abbott Nutrition, Singapore, Singapore Sharleen Imes, University of Alberta, Alberta, Canada Dr Elizabeth Isenring, Flinders University, Adelaide, Australia Dr Per Ole Iversen, University of Oslo, Denmark Dr Martin Jensen, Denmark Daina Kalnins, The Hospital for Sick Children,Toronto, Canada Dr. Neha Kapoor, The All India Institute of Medical Sciences, New Delhi, India Professor Ulrich Keller, University Hospital, Basel, Switzerland Dr Nicole Kiss, Deakin University, Victoria, Australia Dr Hinke Kruizenga, Amsterdam UMC, location VUmc, Amsterdam, the Netherlands Dr Susanna Kunvik, The Social Services & Healthcare Centre of Pori, Finland Dr Julie L Locher, University of Alabama, Birmingham, USA Astrid Lovik, Oslo University Hospital, Oslo, Norway Dr Daniel de Luis, Hospital Universitario Rio Hortega, Valladolid, Spain Dr Francesco Manguso, Fredico II University of Naples, Naples, Italy Kathleen Mayer, Ross Laboratories, Colombus, USA Professor Donna McCarthy, University of Wisconsin Hospitals, Madison, USA Joseph Murphy, Ottawa Hospital, Ottawa, Canada Professor Kristina Norman, Charité, , UKBerlin, Germany Dr Guenter Ollenschlager, Cologne, Germany Dr Nick Paton, St Georges Hospital, London Prof Helene Payette, University of Sherbrooke, Sherbrooke, Canada Professor Joeri Pen, Vrije Universiteit Brussel, Brussels, Belgium Dr Christina Persson, Akademiska Sjukhuset, Uppsala, Sweden Dr Margareta Persson, Uppsala University Hospital, Uppsala, Sweden Dr Thomas Petty, Presbyterian St. Luke's Hospitals, Chicago, USA Dr Paula Ravasco, Santa Maria University Hospital, Lisbon,Portugal Dr Robert Rogers, University of Pittsburgh, Pittsburgh, USA Dr Elizabeth Rydwik, Karolinska Institutet, Solna, Sweden Dr Achim Schwenk, St Georges Hospital, London, UK Dr Luis E. Simental‐Mendia, Mexican Social Security Institute, Durango, Mexico Dr Pierre Singer, Rabin Medical Center, Israel Dr Siddharth Singh, All India Institute of Medical Sciences, New Delhi, India Dr Ruth Stow, Birmingham City University, Birmingham, UK Dr Merja Suominen, University of Helsinki, Helsinki, Finland Dr Marian van Bokhorst de van der Schuren on behalf of Dr Floor Neelemaat VU Medical Center, The Netherlands Dr Angela Vivanti, University of Queensland, Brisbane, Australia Dr Lena Vogt, Kantonsspital, Winterthur, Switzerland Dr Liz Williams, University of Sheffield, Sheffield, UK Dr Samuel Y Wong, Prince of Wales Hospital, Hong Kong, Hong Kong Dr Caroline Wyers,VieCuri Medical Center, The Netherlands Judith van Zwienen ‐ Pot Msc, Amsterdam UMC, location VUmc, Amsterdam, the Netherlands Additionally we are grateful to Professor Marinos Elia, Institute of Human Nutrition, Southampton General Hospital, UK for his expert advice and guidance on clinical nutrition for this review. Also to Reinhard Wentz, Dipl. Bibl., Campus Library Manager, Imperial College London, UK and Nick Woolley, Information Specialist King's College, University of London, UK for their help with developing the search strategy and Mr Alex Cheok, King’s College London, UK for translation of a paper included in the review.
Many thanks to The Systematic Review Training Unit who provided support, funded by the London Regional Health Authority.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies used up to 2005
| Database | Search terms | Date of latest search |
| CENTRAL The Cochrane Library |
1. NUTRITION*:ME 2. NUTR* 3. DIET*:ME 4. DIET* 5. FOOD*:ME 6. FOOD 7. EATING*:ME 8. EAT* 9. ENERGY‐INTAKE*:ME 10. (ENERGY and (NEAR5 and INTAKE)) 11. (ENERGY and INTAKE)DIET‐THERAPY*:ME 12. DIETARY‐SERVICES*:ME 13. DIETETICS*:ME 14. FOOD‐HABITS*:ME 15. FEEDING‐BEHAVIOR*:ME 16. (#1 or #2) or #3) or #4) or #5) or #6) or #7) or #8) or #9) or #10) or #11) or #12) or #13) or #14) or #15) or #16) or #17) 17. DIETARY‐SUPPLEMENTS*:ME 18. FOOD‐FORMULATED*:ME 19. SUPPLEM* 20. (NUTR* and SUPPLEM*) 21. DIET* and SUPPLEM*) 22. (FOOD* and SUPPLEM*) 23. (#19 or #20) or #21) or #22) or #23) or #24) 24. WEIGHT‐GAIN*:ME 25. (WEIGHT and GAIN) 26. (WEIGHT and INCREASE) 27. (WEIGHT and CHANGE) 28. (WEIGHT and FLUCTUATION) 29. NUTRITIONAL‐STATUS*:ME 30. (NUTR* and STATUS) 31. ANTHROPOMETRY*:ME 32. ANTHROPOMET* 33. #26 or #27) or #28) or #29) or #30) or #31) or #32) or #33) or #34) 34. ((#18 and #25) and #35) 35. (#18 and #25) |
Dates between 2002 and 2005 |
| MEDLINE Silver Platter | 1. explode "Nutrition"/ all subheadings 2. nutr* 3. explode "Diet"/ all subheadings 4. diet* 5. explode "Food"/ all subheadings 6. food* 7. "Eating"/ all subheadings 8. eat* 9. "Energy‐Intake"/ all subheadings 10. energy 11. intake 12. energy intake 13. explode "Diet‐Therapy"/ all subheadings 14. explode "Dietary‐Services"/ all subheadings 15. "Dietetics"/ all subheadings 16. "Food‐Habits"/ all subheadings 17. explode "Feeding‐Behavior"/ all subheadings 18. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #12 or #13 or #14 or #15 or #16 or #17 19. energy 20. intake 21. energy near5 intake 22. diet* 23. advice 24. diet* near5 advice 25. diet* 26. therapy 27. diet* near5 therapy 28. diet* 29. service* 30. #28 near5 service* 31. counsel* 32. #28 near5 counsel* 33. educat* 34. #2 near5 educat* 35. habit* 36. #6 near5 habit* 37. feed* 38. behav* 39. feed* near5 behav* 40. #21 or #24 or #30 or #33 or #35 or #37 or #39 or #41 or #44 or #1 or #3 or #5 or #7 or #9 or #13 or #14 or #15 or #16 or #17 41. TG=ANIAL 42. TG=HUMAN 43. TG=ANIMAL 44. (TG=ANIMAL)not ((TG=HUMAN)and (TG=ANIMAL)) 45. RANDOMIZED‐CONTROLLED‐TRIAL in PT 46. CONTROLLED‐CLINICAL‐TRIAL in PT 47. RANDOMIZED‐CONTROLLED‐TRIALS 48. RANDOM‐ALLOCATION 49. DOUBLE‐BLIND‐METHOD 50. SINGLE‐BLIND‐METHOD 51. "DIETARY‐SUPPLEMENTS"/ all subheadings 52. "FOOD,‐FORMULATED"/ all subheadings 53. SUPPLEM* 54. #4 near5 #53 55. #2 near5 #53 56. #6 near5 #53 57. #51 or #52 or #53 or #54 or #55 or #56 58. "Weight‐Gain"/ all subheadings 59. weight 60. gain 61. weight near5 gain 62. increas* 63. #59 near5 increas* 64. "Nutritional‐Status"/ all subheadings 65. status 66. #2 near5 status 67. improv* 68. intake* 69. (improv* near5 #2) near intake* 70. #58 or #61 or #63 or #64 or #66 or #69 71. explode "Child"/ all subheadings 72. explode "Adult"/ all subheadings 73. #71 not (#71 and (#72)) 74. #45 or #46 or #47 or #48 or #49 or #50 75. #18 not #44 76. #18 not #73 77. #75 and #76 and #74 78. #40 not #44 79. #40 not #73 80. #78 and #74 81. #79 and #74 82. #77 and #70 83. #80 and #70 84. #81 and #70 85. #82 and #57 86. #83 and #57 87. #84 and #57 |
Dates between 2002 and 2005 |
| Embase Silver Platter | 1. explode "nutrition"/ all subheadings 2. nutr* 3. explode "diet"/ all subheadings 4. diet* 5. explode "food"/ all subheadings 6. food* 7. "eating"/ all subheadings 8. eat* 9. caloric intake 10. "caloric‐intake"/ all subheadings 11. energy intake 12. explode "diet‐therapy"/ all subheadings 13. explode "health‐service"/ all subheadings 14. "dietetics"/ all subheadings 15. explode "feeding‐behavior"/ all subheadings 16. feed* near5 behav* 17. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 18. "weight‐gain"/ all subheadings 19. weight near5 gain 20. weight near5 increas* 21. #18 or #19 or #20 22. explode "nutritional‐status"/ all subheadings 23. nutr* near5 status 24. (improv* near5 nutr*) near intake* 25. #22 or #23 or #24 26. #18 or #19 or #20 or #22 or #23 or #24 27. #17 and #26 |
Dates between 2002 and 2005 |
| AMED Ovid | A. nutrition$ or nutritive or diet or diet therapy or (energy and intake) or dietary service$ or dietary or eating or food or feeding or feeding behaviour or feeding behavior or food habit$ or diet advice or dietary advice or dietetics or dietician$ or caloric intake or calorie intake or (dietary and supplement$) or (formula$ and food) or food supplements or elemental).af or dh.fs B. weight gain or (weight adj5 gain) or nutrition$ status or (nutrition$ adj5 status) or ((improv$ or gain$ or increase$) adj5 (weight or intake)).af C. (random$ or rct$ or double blind or single blind or treble blind or triple blind or (control$ and trual$) or (clinical adj5 trial$) or trial or trials or systematic$ review$ or metaanal$ or meta‐analys$).af ((A.ti and B) or (A and B.ti)) and C |
Dates between 2002 and 2005 |
| CINAHL Silver Platter |
1. nutr* 2. "Nutrition‐Management‐(Iowa‐NIC)"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 3. explode "Nutrition"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 4. explode "Nutrition‐Care‐(Saba‐HHCC)"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 5. explode "Diet"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 6. diet* 7. explode "Food"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 8. food* 9. "Eating"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 10. eat* 11. "Caloric‐Intake"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 12. caloric 13. intake 14. caloric intake 15. explode "Diet‐Therapy"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 16. "Nutrition‐Therapy‐(Iowa‐NIC)"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 17. explode "Nutrition‐Services"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 18. "Dietetics"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 19. "Research,‐Dietetics"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 20. "Education,‐Dietetics"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 21. explode "Eating‐Behavior"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 22. feed* 23. behav* 24. feed* near5 behav* 25. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #14 or #15 or #16 or #17 or #18 or #21 or #24 26. explode "Weight‐Gain"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 27. weight 28. gain 29. weight near5 gain 30. weight 31. increas* 32. weight near5 increas* 33. "Nutritional‐Status"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 34. nutr* 35. status 36. nutr* near5 status 37. improv* 38. nutr* 39. intake 40. improv* near5 nutr* intake 41. "Nutritional‐Assessment"/ all topical subheadings / in‐adulthood , in‐old‐age, in‐middle‐age 42. #26 or #29 or #32 or #33 or #36 or #40 or #41 43. #25 and #42 |
Dates between 2002 and 2005 |
| National Cancer Institute CancerLit | A. nutrition$ or nutritive or diet or diet therapy or (energy and intake) or dietary service$ or dietary or eating or food or feeding or feeding behaviour or feeding behavior or food habit$ or diet advice or dietary advice or dietetics or dietician$ or caloric intake or calorie intake or (dietary and supplement$) or (formula$ and food) or food supplements or elemental).af or dh.fs B. weight gain or (weight adj5 gain) or nutrition$ status or (nutrition$ adj5 status) or ((improv$ or gain$ or increase$) adj5 (weight or intake)).af C. (random$ or rct$ or double blind or single blind or treble blind or triple blind or (control$ and trual$) or (clinical adj5 trial$) or trial or trials or systematic$ review$ or metaanal$ or meta‐analys$).af ((A.ti and B) or (A and B.ti)) and C |
Dates between 2002 and 2005 |
| ERIC SilverPlatter | 1. explode "NUTRITION" 2. nutr* 3. diet* 4. explode "FOOD" 5. food* 6. eat* 7. energy 8. intake 9. energy intake 10. "EATING‐HABITS" in DE 11. feed* 12. behav* 13. feed* near5 behav* 14. "FOODS‐INSTRUCTION" in DE 15. "OCCUPATIONAL‐HOME‐ECONOMICS" in DE 16. "NUTRITION‐INSTRUCTION" in DE 17. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #9 or #10 or #13 or #14 or #15 or #16 18. explode "BODY‐WEIGHT" 19. weight 20. gain 21. weight near5 gain 22. weight 23. increas* 24. weight near5 increas* 25. improv* 26. nutr* 27. intake 28. (improv* near5 nutr*) near intake 29. nutr* 30. status 31. nutr* near5 status 32. #18 or #21 or #24 or #31 33. #17 and #32 |
Dates between 2002 and 2005 |
| Dissertation Abstracts Silver Platter | 1. diet* 2. food* 3. eat* 4. energy 5. intake 6. energy near5 intake 7. caloric 8. intake 9. caloric near5 intake 10. feed* 11. behav* 12. feed* near5 behav* 13. #1 or #2 or #3 or #4 or #5 or #8 or #11 or #14 14. weight 15. gain 16. weight near5 gain 17. weight 18. increas* 19. weight near5 increas* 20. nutr* 21. status 22. nutr* near5 status 23. improv* 24. status 25. intake* 26. (improv* near5 status) near intake* 27. #18 or #21 or #24 or #28 28. #15 and #29 |
July 2000 |
Appendix 2. Search strategies 2005 to 2010
| Database | Search terms | Latest date searched |
| CENTRAL | not available | |
| MEDLINE Ovid | 1 nutrit*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (197693) 2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/ (167285) 3 diet*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (374221) 4 Diet/ or exp *Diet Therapy/ (96580) 5 eat*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (70476) 6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/(35764) 7 food.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (269212) 8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/ (37268) 9 feed*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (231101) 10 exp *Eating/ (17444) 11 calori*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (47662) 12 exp *Energy Intake/ (7820) 13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/ (13550) 14 energy.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (250080) 15 oral nutritional supplement.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (25) 16 exp *Dietary Supplements/ (10700) 17 exp *Nutritional Support/ (21933) 18 sip feed.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (17) 19 suppl*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (287335) 20 exp *Adult/ or exp *Dietary Supplements/ (35396) 21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or 20 or 14 or 15 or 8 or 4 or 19 or 10 or 5 (1362303) 22 weight.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (626210) 23 weight.mp. (626210) 24 weight gain.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (34990) 25 exp *Weight Gain/ (4196) 26 nutrit* status.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (24171) 27 exp *Nutritional Status/ (6871) 28 27 or 25 or 22 or 24 or 26 or 23 (642887) 29 28 and 21 (180107) 30 limit 29 to yr="2005 ‐ 2008" (34527) 31 limit 30 to humans (22734) 32 limit 31 to controlled clinical trial (200) 33 from 32 keep 1‐200 (200) 34 from 33 keep 1‐200 (200) 35 from 33 keep 1‐200 (200) | February 2010 |
| Embase Ovid | 1 nutrit*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (197693)
2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/ (167285)
3 diet*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (374221)
4 Diet/ or exp *Diet Therapy/ (96580)
5 eat*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (70476)
6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/(35764)
7 food.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (269212)
8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/ (37268)
9 feed*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (231101)
10 exp *Eating/ (17444)
11 calori*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (47662)
12 exp *Energy Intake/ (7820)
13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/ (13550)
14 energy.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (250080)
15 oral nutritional supplement.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (25)
16 exp *Dietary Supplements/ (10700)
17 exp *Nutritional Support/ (21933)
18 sip feed.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (17)
19 suppl*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (287335)
20 exp *Adult/ or exp *Dietary Supplements/ (35396)
21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or
20 or 14 or 15 or 8 or 4 or 19 or 10 or 5 (1362303)
22 weight.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (626210)
23 weight.mp. (626210) 24 weight gain.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (34990) 25 exp *Weight Gain/ (4196) 26 nutrit* status.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (24171) 27 exp *Nutritional Status/ (6871) 28 27 or 25 or 22 or 24 or 26 or 23 (642887) 29 28 and 21 (180107) 30 limit 29 to yr="2005 ‐ 2008" (34527) 31 limit 30 to humans (22734) 32 limit 31 to controlled clinical trial (200) 33 from 32 keep 1‐200 (200) 34 from 33 keep 1‐200 (200) 35 from 33 keep 1‐200 (200) |
February 2010 |
| Cinahl EBSCO | (TX+(weight)+OR+(TX+(nutrit*) +AND+ (TX+(nutrit*) +OR+(TX+(diet*) +OR+(TX+(eat) +OR+(TX+(food) +OR+(TX+(feed*) +OR+(TX+(calori*) +OR+(TX+(energy) +OR+(TX+(oral+nutritional+supplement) +OR+(TX+(sip+feed) +OR+(TX+(suppl*) +OR+(TX+(educat*) +OR+(TX+(behav*) +OR+(TX+(snack) NOTE: TX denotes a word in the full text |
February 2010 |
| ISI Web of Science (Searched on dates between 2005‐2010) |
(nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav*) AND (nutrit* OR weight gain) AND (random* OR RCT OR control* OR clinical) NOT (child* OR infant OR paediatric) NOT (animal OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog) |
February 2010 |
| Scopus (Searched on dates between 2005‐2010) |
TITLE‐ABSKEY ( nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* KEY ( nutrit* OR weight gain ) ) AND ( TITLE‐ABS‐KEY ( random* OR rct OR control* OR clinical ) ) AND ( TITLE‐NOT ( ABS ( child* ) ) AND NOT ( ABS ( pregnan* ) ) AND NOT ( ABS ( animal* OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog ) ) AND NOT ( ABS NOT ( ABS ( adolescent* ) ) AND NOT ( ABS ( starv* ) ) AND NOT ( ABS ( infan* ) ) AND NOT ( ABS ( matern* ) ) AND NOT NOT ( ABS ( baby ) ) AND NOT ( ABS ( babies* ) ) AND NOT ( ABS ( neonat* ) ) AND ( LIMIT‐TO ( PUBYEAR , 2016 ) OR 2017 ) OR LIMIT‐TO ( PUBYEAR, 2018 ) ) |
Appendix 3. Search strategies 2013 to 2016
| Database | Search strategy | Date searched |
| CENTRAL | #1 nutrit*
#2 MeSH descriptor: [Enteral Nutrition] explode all trees
#3 MeSH descriptor: [Nutrition Assessment] explode all trees
#4 MeSH descriptor: [Nutrition Therapy] explode all trees
#5 MeSH descriptor: [Nutrition Disorders] explode all trees
#6 diet*
#7 MeSH descriptor: [Diet] this term only
#8 MeSH descriptor: [Diet Therapy] explode all trees
#9 eat*
#10 MeSH descriptor: [Food Services] explode all trees
#11 MeSH descriptor: [Feeding Behavior] explode all trees
#12 MeSH descriptor: [Food Habits] explode all trees
#13 food
#14 MeSH descriptor: [Food] explode all trees
#15 MeSH descriptor: [Food, Fortified] explode all trees
#16 MeSH descriptor: [Food, Formulated] explode all trees
#17 MeSH descriptor: [Food Habits] explode all trees
#18 MeSH descriptor: [Food Services] explode all trees
#19 feed*
#20 MeSH descriptor: [Eating] explode all trees
#21 calori*
#22 MeSH descriptor: [Energy Intake] explode all trees
#23 MeSH descriptor: [Protein‐Energy Malnutrition] explode all trees
#24 MeSH descriptor: [Energy Intake] explode all trees
#25 energy
#26 oral nutritional supplement
#27 MeSH descriptor: [Dietary Supplements] explode all trees
#28 MeSH descriptor: [Nutritional Support] explode all trees
#29 sip feed
#30 suppl*
#31 MeSH descriptor: [Adult] explode all trees
#32 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31)
#33 weight
#34 weight gain
#35 MeSH descriptor: [Weight Gain] explode all trees
#36 nutrit* status
#37 MeSH descriptor: [Nutritional Status] explode all trees
#38 (#33 or #34 or #35 or #36 or #37)
#39 (#32 and #38)
#40 (child* or pregnan* or starv* or baby* or babies or pediatric or paediatric or adolescen* or infant or mother* or matern* or obes* or overweight)
#41 (#39 not #40) Publication Year from 2010 to 2016, in Trials Search Notes: Ran search strategy on Cochrane Library 25/9/16 Limits: 1. Publication Year 2010‐2016 2. Trials |
25 September 2016 |
| MEDLINE Ovid | 1 nutrit*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/
3 diet*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
4 Diet/ or exp *Diet Therapy/
5 eat*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/
7 food.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/
9 feed*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
10 exp *Eating/
11 calori*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
12 exp *Energy Intake/
13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/
14 energy.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
15 oral nutritional supplement.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
16 exp *Dietary Supplements/
17 exp *Nutritional Support/
18 sip feed.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
19 suppl*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
20 exp *Adult/ or exp *Dietary Supplements/
21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or 20 or 14 or 15 or 8 or 4 or 19 or 10 or 5
22 weight.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
23 weight.mp.
24 weight gain.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
25 exp *Weight Gain/
26 nutrit* status.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
27 exp *Nutritional Status/
28 27 or 25 or 22 or 24 or 26 or 23
29 28 and 21
30 limit 29 to yr="2010 ‐ 2016"
31 limit 30 to humans
32 limit 31 to (controlled clinical trial or randomized controlled trial)
33 limit 32 to "all adult (19 plus years)"
34 (obes* or overweight).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
35 33 not 34 Search Notes: Ran search strategy on OVID platform. Includes all of Medline: ePub ahead of print, in‐process & other non‐indexed citations, OVID medline (R) daily and OVID medline (R) 1946‐present) 25/09/16 Limits:
|
25 September 2016 |
| Embase Ovid | 1 nutrit*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/
3 diet*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
4 Diet/ or exp *Diet Therapy/
5 eat*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/
7 food.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/
9 feed*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
10 exp *Eating/
11 calori*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
12 exp *Energy Intake/
13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/
14 energy.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
15 oral nutritional supplement.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
16 exp *Dietary Supplements/
17 exp *Nutritional Support/
18 sip feed.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
19 suppl*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
20 exp *Adult/ or exp *Dietary Supplements/
21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or 20 or 14 or 15 or 8 or 4 or 19 or 10 or 5
22 weight.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
23 weight.mp.
24 weight gain.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
25 exp *Weight Gain/
26 nutrit* status.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
27 exp *Nutritional Status/
28 27 or 25 or 22 or 24 or 26 or 23
29 28 and 21
30 limit 29 to yr="2010 ‐ 2016"
31 limit 30 to human
32 limit 31 to (exclude medline journals and (randomized controlled trial or controlled clinical trial) and (adult <18 to 64 years> or aged <65+ years>))
33 (obes* or overweight).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
34 32 not 33 Search Notes: Ran search strategy on OVID platform. Embase 1974 – 2016 September 23 Limits:
|
25 September 2016 |
| CINAHL EBSCO | S1 TX nutrit* OR diet* OR eat OR food OR feed* OR calori* OR energy OR (oral nutritional supplement) OR (sip feed) OR suppl* OR educat* OR behav* OR snack*
S2 TX weight OR nutrit*
S3 S1 AND S2
S4 (SU Pregnancy) OR (TI pregnan*)
S5 (SU child*) OR (TI child*)
S6 (SU Infant*) OR (TI infant*)
S7 (SU Paediatric*) OR (TI Paediatric*)
S8 (SU matern*) OR (TI matern*)
S9 (SU mother*) OR (TI mother*)
S10 (SU adolescen*) OR (TI adolescen*)
S11 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10
S12 S3 NOT S11
S13 (SU obes*) OR (SU overweight)
S14 S12 NOT S13 Search Notes: Ran search strategy on EBSCHO host platform. Limits:
|
25 September 2016 |
| ISI Web of Science | #1 TS=(nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav*)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016
#2 TS=(nutrit* OR weight gain)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016
#3 TS=(random* OR rct OR control* OR clinical)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016
#4 TS=(adult*)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016
#5 #1 AND #2 AND #3 AND #4
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016
#6 ((TS=(child*)) or (TS=(pregnan*)) OR (TS=(animal* OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog)) OR (TS=(paediatric)) OR (TS=(adolescent*)) OR (TS=(starv*)) OR (TS=(infan*)) OR (TS=(matern*)) OR (TS=(mother*)) OR (TS=(baby)) OR (TS=(babies*)) OR (TS=(neonat*)))
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016
#7 #5 NOT #6
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016
#8 TS=(obes* OR overweight)
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016
#9 #7 NOT #8
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2010‐2016 Search Notes: Ran search strategy on Web of Science (Core collection). Limits: 1. 2010 – 2016 timespan |
25 September 2016 |
| Scopus | TITLE‐ABS‐KEY ( nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav* ) AND ( TITLE‐ABS‐KEY ( nutrit* OR weight gain ) ) AND ( TITLE‐ABS‐KEY ( random* OR rct OR control* OR clinical ) ) AND ( TITLE‐ABS ( adult* ) ) AND NOT ( ABS ( child* ) ) AND NOT ( ABS ( pregnan* ) ) AND NOT ( ABS ( animal* OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog ) ) AND NOT ( ABS ( paediatric ) ) AND NOT ( ABS ( adolescent* ) ) AND NOT ( ABS ( starv* ) ) AND NOT ( ABS ( infan* ) ) AND NOT ( ABS ( matern* ) ) AND NOT ( ABS ( mother* ) ) AND NOT ( ABS ( baby ) ) AND NOT ( ABS ( babies* ) ) AND NOT ( ABS ( neonat* ) ) AND NOT ( ABS ( obes* ) ) AND NOT ( ABS ( overweight ) ) AND ORIG‐LOAD‐DATE AFT 20160925 AND ( LIMIT‐TO ( PUBYEAR , 2010 ) OR EXCLUDE ( PUBYEAR , 2016 OR limit‐to PUBYEAR ) OR EXCLUDE ( PUBYEAR , 2014 OR limit‐to PUBYEAR ) OR EXCLUDE ( PUBYEAR , 2012 OR limit‐to PUBYEAR ) ) Search Notes: Ran search strategy on Scopus. Limits:
|
25 September 2016 |
Appendix 4. Search strategies 2016 to 2018
| Database | Search terms | Date searched |
| CENTRAL | #1 nutrit*
#2 MeSH descriptor: [Enteral Nutrition] explode all trees
#3 MeSH descriptor: [Nutrition Assessment] explode all trees
#4 MeSH descriptor: [Nutrition Therapy] explode all trees
#5 MeSH descriptor: [Nutrition Disorders] explode all trees
#6 diet*
#7 MeSH descriptor: [Diet] this term only
#8 MeSH descriptor: [Diet Therapy] explode all trees
#9 eat*
#10 MeSH descriptor: [Food Services] explode all trees
#11 MeSH descriptor: [Feeding Behavior] explode all trees
#12 MeSH descriptor: [Food Habits] explode all trees
#13 food
#14 MeSH descriptor: [Food] explode all trees
#15 MeSH descriptor: [Food, Fortified] explode all trees
#16 MeSH descriptor: [Food, Formulated] explode all trees
#17 MeSH descriptor: [Food Habits] explode all trees
#18 MeSH descriptor: [Food Services] explode all trees
#19 feed*
#20 MeSH descriptor: [Eating] explode all trees
#21 calori*
#22 MeSH descriptor: [Energy Intake] explode all trees
#23 MeSH descriptor: [Protein‐Energy Malnutrition] explode all trees
#24 MeSH descriptor: [Energy Intake] explode all trees
#25 energy
#26 oral nutritional supplement
#27 MeSH descriptor: [Dietary Supplements] explode all trees
#28 MeSH descriptor: [Nutritional Support] explode all trees
#29 sip feed
#30 suppl*
#31 MeSH descriptor: [Adult] explode all trees
#32 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31)
#33 weight
#34 weight gain
#35 MeSH descriptor: [Weight Gain] explode all trees5
#36 nutrit* status
#37 MeSH descriptor: [Nutritional Status] explode all trees
#38 (#33 or #34 or #35 or #36 or #37)
#39 (#32 and #38)
#40 (child* or pregnan* or starv* or baby* or babies or pediatric or paediatric or adolescen* or infant or mother* or matern* or obes* or overweight)
#41 (#39 not #40) Publication Year from 2016 to 2018 Search Notes: Ran search strategy on Cochrane Library. Limits: 1. Publication Year 2016‐2018 2. Trials |
10 January 2018 |
| MEDLINE Ovid | 1 nutrit*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/ 3 diet*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 4 Diet/ or exp *Diet Therapy/ 5 eat*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/ 7 food.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/ 9 feed*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 10 exp *Eating/ 11 calori*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 12 exp *Energy Intake/ 13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/ 14 energy.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 15 oral nutritional supplement.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 16 exp *Dietary Supplements/ 17 exp *Nutritional Support/ 18 sip feed.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 19 suppl*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 20 exp *Adult/ or exp *Dietary Supplements/ 21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or 20 or 14 or 15 or 8 or 4 or 19 or 10 or 5 22 weight.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 23 weight.mp. 24 weight gain.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 25 exp *Weight Gain/ 26 nutrit* status.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 27 exp *Nutritional Status/ 28 27 or 25 or 22 or 24 or 26 or 23 29 28 and 21 30 limit 29 to yr="2016 ‐ 2018" 31 limit 30 to humans 32 limit 31 to (controlled clinical trial or randomized controlled trial) 33 limit 32 to "all adult (19 plus years)" 34 (obes* or overweight).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 35 33 not 34 Search notes: Ran search strategy on OVID platform. Includes all of Medline: ePub ahead of print, in‐process & other non‐indexed citations, OVID medline (R) daily and OVID medline (R) 1946‐present) Limits:
|
10 January 2018 |
| Embase Ovid | 1 nutrit*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/
3 diet*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
4 Diet/ or exp *Diet Therapy/
5 eat*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/
7 food.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/
9 feed*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
10 exp *Eating/
11 calori*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
12 exp *Energy Intake/
13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/
14 energy.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
15 oral nutritional supplement.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
16 exp *Dietary Supplements/
17 exp *Nutritional Support/
18 sip feed.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
19 suppl*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
20 exp *Adult/ or exp *Dietary Supplements/
21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or 20 or 14 or 15 or 8 or 4 or 19 or 10 or 5
22 weight.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
23 weight.mp.
24 weight gain.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
25 exp *Weight Gain/
26 nutrit* status.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
27 exp *Nutritional Status/
28 27 or 25 or 22 or 24 or 26 or 23
29 28 and 21
30 limit 29 to yr="2013 ‐ 2016"
31 limit 30 to human
32 limit 31 to (exclude medline journals and (randomized controlled trial or controlled clinical trial) and (adult <18 to 64 years> or aged <65+ years>))
33 (obes* or overweight).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading]
34 32 not 33 Search notes: Ran search strategy on OVID platform. Embase 1974 – 2018 Week 2 Limits:
|
10 January 2018 |
| CINAHL EBSCO |
S1 TX nutrit* OR diet* OR eat OR food OR feed* OR calori* OR energy OR (oral nutritional supplement) OR (sip feed) OR suppl* OR educat* OR behav* OR snack* S2 TX weight OR nutrit* S3 S1 AND S2 S4 (SU Pregnancy) OR (TI pregnan*) S5 (SU child*) OR (TI child*) S6 (SU Infant*) OR (TI infant*) S7 (SU Paediatric*) OR (TI Paediatric*) S8 (SU matern*) OR (TI matern*) S9 (SU matern*) OR (TI matern*) S10 (SU adolescen*) OR (TI adolescen*) S11 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 S12 S3 NOT S11 S13 (SU obes*) OR (SU overweight) S14 S12 NOT S13 Search notes: Ran search strategy on EBSCHO host platform. 10/1/18 @ 3:30pm Limits:
|
10 January 2018 |
| ISI Web of Science | #1 TS=(nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2016‐2018 #2 TS=(nutrit* OR weight gain) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2016‐2018 #3 TS=(random* OR rct OR control* OR clinical) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2016‐2018 #4 TS=(adult*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2016‐2018 #5 #1 AND #2 AND #3 AND #4 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2016‐2018 #6 ((TS=(child*)) or (TS=(pregnan*)) OR (TS=(animal* OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog)) OR (TS=(paediatric)) OR (TS=(adolescent*)) OR (TS=(starv*)) OR (TS=(infan*)) OR (TS=(matern*)) OR (TS=(mother*)) OR (TS=(baby)) OR (TS=(babies*)) OR (TS=(neonat*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2016‐2018 #7 #5 NOT #6 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2016‐2018 Search notes: Ran search strategy on Web of Science (Core collection). Limits:
|
10 January 2018 |
| Scopus | TITLE‐ABS‐KEY ( nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav* ) AND ( TITLE‐ABS‐KEY ( nutrit* OR weight gain ) ) AND ( TITLE‐ABS‐KEY ( random* OR rct OR control* OR clinical ) ) AND ( TITLE‐ABS ( adult* ) ) AND NOT ( ABS ( child* ) ) AND NOT ( ABS ( pregnan* ) ) AND NOT ( ABS ( animal* OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog ) ) AND NOT ( ABS ( paediatric ) ) AND NOT ( ABS ( adolescent* ) ) AND NOT ( ABS ( starv* ) ) AND NOT ( ABS ( infan* ) ) AND NOT ( ABS ( matern* ) ) AND NOT ( ABS ( mother* ) ) AND NOT ( ABS ( baby ) ) AND NOT ( ABS ( babies* ) ) AND NOT ( ABS ( neonat* ) ) AND ( LIMIT‐TO ( PUBYEAR , 2016 ) OR LIMIT‐TO ( PUBYEAR , 2017 ) OR LIMIT‐TO ( PUBYEAR , 2018 ) ) Search notes: Ran search strategy on Scopus. Limits:
|
10 January 2018 |
| ClinicalTrials.gov | nutrit* OR diet* OR eat OR food OR feed* OR calori* OR energy OR (oral nutritional supplement) OR (sip feed) OR suppl* OR educat* OR behav* OR snack* | Interventional Studies | (dietary advice) OR (oral nutritional supplement*) OR (dietary counselling) OR (nutrition* counselling) OR (nutrition* supplement*) OR (oral nutrition supplement*) OR dietitian OR dietician OR (sip feed) OR (food fortification) OR (increase* intake) | Adult, Senior | First posted from 11/10/2016 to 01/10/2018 Search notes: Ran search strategy on Clinical Trials.gov. Limits:
|
10 January 2018 |
Appendix 5. Search strategies current version (2021)
| Database | Search terms | Date searched |
| CENTRAL | #1 Malnutrition:ti,ab,kw 4782
#2 Nutrition*:ti,ab,kw 42988
#3 Food*:ti,ab,kw 48413
#4 Diet*:ti,ab,kw 92912
#5 Snack*:ti,ab,kw 2563
#6 Fortifi*:ti,ab,kw 3255
#7 Supplement*:ti,ab,kw 71499
#8 MeSH descriptor: [Diet] explode all trees 18618
#9 MeSH descriptor: [Dietetics] explode all trees 96
#10 MeSH descriptor: [Diet Therapy] explode all trees 5953
#11 MeSH descriptor: [Dietary Supplements] explode all trees 12689
#12 MeSH descriptor: [Nutrition Therapy] explode all trees 9471
#13 MeSH descriptor: [Nutritional Status] explode all trees 2506
#14 MeSH descriptor: [Nutritional Support] explode all trees 3401
#15 MeSH descriptor: [Nutritional Requirements] explode all trees 682
#16 MeSH descriptor: [Nutrition Disorders] explode all trees 19150
#17 MeSH descriptor: [Malnutrition] explode all trees 4294
#18 MeSH descriptor: [Eating] explode all trees 3593
#19 MeSH descriptor: [Food] explode all trees 34794
#20 MeSH descriptor: [Enteral Nutrition] explode all trees 1857
#21 MeSH descriptor: [] explode all trees and with qualifier(s): [diet therapy ‐ DH] 8040
#22 #1 or #2 or #3 #4 or #5 #6 #7 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 96488
#23 (Appointment* or Meet* or Session* or Seminar* or Discuss* or Advise* or Advice or Liais* or Verbal* or Class or Counsel* or Visit*):ti,ab,kw 311638
#24 ("Face to face" or face‐to‐face or Leaflet* or Booklet* or written or Phone* or Telephone* or Guide* or Guidance or Inform* or Document* or Program or programme):ti,ab,kw 353041
#25 (Recommend* or Monitor* or Manage* or Support* or Educat* or Instruct* or Teach* or Taught):ti,ab,kw 434232
#26 (Personal* or Individual* or Devise* or Prescription* or Prescribe* or Tailor*):ti,ab,kw 192492
#27 ((Prepared NEAR/2 food*) or (Prepared NEAR/2 diet*)):ti,ab,kw 247
#28 MeSH descriptor: [Dietary Services] explode all trees 95
#29 MeSH descriptor: [Feeding Behavior] explode all trees 8947
#30 MeSH descriptor: [Nutrition Assessment] explode all trees 688
#31 MeSH descriptor: [Counseling] explode all trees 5526
#32 #23 or #24 or #25 or #26 or #27 #28 or #29 or #30 or #31 811785
#33 #22 and #32 57014
#34 (breastfed* or breastfeed* or pregnant or baby or babies or caesarean or cesarean or C‐section or matern* or mother* or overweight or bariatric or obese or obesity or starv*):ti,ab,kw 105862
#35 #33 not #34 36843
#36 (#33 not #34) not ((children or paediatric* or pediatric*):ti not ((children or paediatric* or pediatric*) and adult*):ti) 33944
eSH descriptor: [Nutrition Assessment] explode all trees 680 #4 MeSH descriptor: [Nutrition Therapy] explode all trees 8971 #5 MeSH descriptor: [Nutrition Disorders] explode all trees 17148 #6 diet* 87645 #7 MeSH descriptor: [Diet] this term only 6603 #8 MeSH descriptor: [Diet Therapy] explode all trees 5621 #9 eat* 17506 #10 MeSH descriptor: [Food Services] explode all trees 357 #11 MeSH descriptor: [Feeding Behavior] explode all trees 8519 #12 MeSH descriptor: [Feeding Behavior] explode all trees 8519 #13 food 45141 #14 MeSH descriptor: [Food] explode all trees 32218 #15 MeSH descriptor: [Food, Fortified] explode all trees 1373 #16 MeSH descriptor: [Food, Formulated] explode all trees 1336 #17 MeSH descriptor: [Feeding Behavior] explode all trees 8519 #18 MeSH descriptor: [Food Services] explode all trees 357 #19 feed* 40437 #20 MeSH descriptor: [Eating] explode all trees 3448 #21 calori* 13812 #22 MeSH descriptor: [Energy Intake] explode all trees 5177 #23 MeSH descriptor: [Protein‐Energy Malnutrition] explode all trees 243 #24 MeSH descriptor: [Energy Intake] explode all trees 5177 #25 energy 33189 #26 oral nutritional supplement 914 #27 MeSH descriptor: [Dietary Supplements] explode all trees 11667 #28 MeSH descriptor: [Nutritional Support] explode all trees 3264 #29 sip feed 51 #30 suppl* 187237 #31 MeSH descriptor: [Adult] explode all trees 3442 #32 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31) 334844 #33 weight 106965 #34 weight gain 13574 #35 MeSH descriptor: [Weight Gain] explode all trees 2399 #36 nutri* status 15152 #37 MeSH descriptor: [Nutritional Status] explode all trees 2361 #38 (#33 or #34 or #35 or #36 or #37) 116911 #39 (#32 and #38) 62672 #40 (child* or pregnan* or starv* or baby* or babies or pediatric or paediatric or adolescen* or infant or mother* or matern* or obes* or overweight) 352233 #41 (#39 not #40) with Publication Year from 2018 to 2020, in Trials 4000 Search Notes: Ran search strategy on Cochrane library. 10/1/20 @ 5:00pm Limits: 1. Publication Year 2018‐2020 2. Trials Results: 4000 (imported into Endnote 9) |
01 March 2021 |
| MEDLINE Ovid | #1 nutrit*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/ #3 diet*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #4 Diet/ or exp *Diet Therapy/ #5 eat*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/ #7 f ood.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/ #9 feed*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #10 exp *Eating/ #11 calori*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #12 exp *Energy Intake/ #13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/ #14 energy.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #15 oral nutritional supplement.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #16 exp *Dietary Supplements/ #17 exp *Nutritional Support/ #18 sip feed.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #19 suppl*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #20 exp *Adult/ or exp *Dietary Supplements/ #21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or 20 or 14 or 15 or 8 or 4 or 19 or 10 or 5 #22 weight.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #23 weight.mp. #24 weight gain.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #25 exp *Weight Gain/ #26 nutrit* status.mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #27 exp *Nutritional Status/ #28 27 or 25 or 22 or 24 or 26 or 23 #29 28 and 21 #30 (obes* or overweight).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] #31 29 not 30 #32 limit 31 to (humans and yr="2018 ‐ 2020" and "adult (19 to 44 years)" and (controlled clinical trial or randomized controlled trial)) Ran search strategy on OVID platform. Includes all of Medline: ePub ahead of print, in‐process & other non‐indexed citations, OVID medline (R) daily and OVID medline (R) 1946‐present) 10.1.2020 @ 2:00pm Limits: 1. 2018‐2020 2. Humans 3. Randomised controlled trials & controlled clinical trials 4. Adult aged 19+ years |
10 January 2020 |
| Embase Ovid | 1 nutrit*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 2 exp *Enteral Nutrition/ or exp *Nutrition Assessment/ or exp *Nutrition Therapy/ or exp *Nutrition Disorders/ 3 diet*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 4 Diet/ or exp *Diet Therapy/ 5 eat*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 6 exp *Food Services/ or exp *Feeding Behavior/ or exp *Food Habits/ 7 food.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 8 exp *Food, Fortified/ or Food/ or exp *Food, Formulated/ or exp *Food Habits/ or exp *Food Services/ 9 feed*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 10 exp *Eating/ 11 calori*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 12 exp *Energy Intake/ 13 exp *Protein‐Energy Malnutrition/ or exp *Energy Intake/ 14 energy.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 15 oral nutritional supplement.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 16 exp *Dietary Supplements/ 17 exp *Nutritional Support/ 18 sip feed.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 19 suppl*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 20 exp *Adult/ or exp *Dietary Supplements/ 21 11 or 7 or 17 or 2 or 1 or 18 or 16 or 13 or 6 or 3 or 9 or 12 or 20 or 14 or 15 or 8 or 4 or 19 or 10 or 5 22 weight.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 23 weight.mp. 24 weight gain.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 25 exp *Weight Gain/ 26 nutrit* status.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 27 exp *Nutritional Status/ 28 27 or 25 or 22 or 24 or 26 or 23 29 28 and 21 30 (obes* or overweight).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 31 limit 29 to (human and exclude medline journals and (randomized controlled trial or controlled clinical trial) and yr="2018 ‐ 2020" and (adult <18 to 64 years> or aged <65+ years>)) 32 31 not 30 Ran search strategy on OVID platform. Embase 1974 – 2018 Week 1 Limits:
|
10 January 2020 |
| CINAHL EBSCO |
S1 TX nutrit* OR diet* OR eat OR food OR feed* OR calori* OR energy OR (oral nutritional supplement) OR (sip feed) OR suppl* OR educat* OR behav* OR snack* S2 TX weight OR nutrit* S3 S1 AND S2 S4 (SU Pregnancy) OR (TI pregnan*) S5 (SU child*) OR (TI child*) S6 (SU Infant*) OR (TI infant*) S7 (SU Paediatric*) OR (TI Paediatric*) S8 (SU matern*) OR (TI matern*) S9 (SU mother*) OR (TI mother*) S10 (SU adolescen*) OR (TI adolescen*) S11 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 S12 S3 NOT S11 S13 (SU obes*) OR (SU overweight) S14 S12 NOT S13 Limiters ‐ Published Date: 20180101‐20200131; Exclude MEDLINE records; Human; Publication Type: Randomized Controlled Trial; Age Groups: All Adult |
10 January 2020 |
| ISI Web of Science | #1 TS=(nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2018‐2020 #2 TS=(nutrit* OR weight gain) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2018‐2020 #3 TS=(random* OR rct OR control* OR clinical) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2018‐2020 #4 TS=(adult*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2018‐2020 #5 1 AND #2 AND #3 AND #4 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2018‐2020 #6 ((TS=(child*)) OR (TS=(pregnan*)) OR (TS=(obes*)) OR (TS=(animal* OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog)) OR (TS=(paediatric)) OR (TS=(adolescent*)) OR (TS=(starv*)) OR (TS=(infan*)) OR (TS=(matern*)) OR (TS=(mother*)) OR (TS=(baby)) OR (TS=(babies*)) OR (TS=(neonat*))) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2018‐2020 #7 #5 NOT #6 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI Timespan=2018‐2020 Ran search strategy on Web of Science (Core collection). 10.1.2020 @ 4:30pm Limits: 1. 2018 – 2020 timespan Results: 2156 (imported into Endnote 9) |
10 January 2020 |
| Scopus | TITLE‐ABS‐KEY ( nutrit* OR diet* OR eat* OR food OR feed* OR calori* OR energy* OR sip OR suppl* OR snack OR educat* OR behav* ) AND ( TITLE‐ABS‐KEY ( nutrit* OR weight gain ) ) AND ( TITLE‐ABS‐KEY ( random* OR rct OR control* OR clinical ) ) AND ( TITLE‐ABS ( adult* ) ) AND NOT ( ABS ( child* ) ) AND NOT ( ABS ( pregnan* ) ) AND NOT ( ABS ( animal* OR rat OR mouse OR guinea pig OR primate OR monkey OR cat OR dog ) ) AND NOT ( ABS ( paediatric ) ) AND NOT ( ABS ( adolescent* ) ) AND NOT ( ABS ( starv* ) ) AND NOT ( ABS ( infan* ) ) AND NOT ( ABS ( matern* ) ) AND NOT ( ABS ( mother* ) ) AND NOT ( ABS ( baby ) ) AND NOT ( ABS ( babies* ) ) AND NOT ( ABS ( neonat* ) ) AND ( LIMIT‐TO ( PUBYEAR , 2018 ) OR LIMIT‐TO ( PUBYEAR , 2019 ) OR LIMIT‐TO ( PUBYEAR, 2020 ) ) Search Notes: Ran search strategy on Scopus. 10/1/2020 @ 4:00 pm Limits: 1. Publication year 2018 – 2020 Results: 534 (imported into Endnote 9) |
10 January 2020 |
| ClinicalTrials.gov | [Advanced Search Form] Condition or disease: NOT (obesity OR pregnancy) Study type: Interventional Studies (Clinical Trials) Age Group: Adult (18‐64) Older Adult (65+) Intervention/ treatment: (nutrition OR nutritional OR malnutrition OR dietary OR diet OR food OR eating OR snack) AND (advice OR counseling OR counselling OR education OR educate OR guidance OR guide OR personalised OR personalized OR program OR programme OR support OR information) (4082 results found) |
03 March 2021 |
| WHO ICTRP | Database not available in 2020 ‐ 21 due to pandemic. | |
Data and analyses
Comparison 1. Dietary advice compared with no advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Up to 3 months | 7 | 574 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.26, 2.96] |
| 1.1.2 4 to 6 months | 10 | 1028 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.27] |
| 1.1.3 12 months and over | 5 | 1445 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.59, 1.91] |
| 1.2 Number of people admitted or readmitted to hospital | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 4 to 6 months | 3 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.55, 3.18] |
| 1.2.2 12 months and over | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.13] |
| 1.3 Length of hospital stay (days) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Up to 3 months | 1 | 148 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.35, ‐0.85] |
| 1.3.2 4 to 6 months | 3 | 212 | Mean Difference (IV, Random, 95% CI) | 1.93 [‐3.42, 7.28] |
| 1.3.3 12 months and over | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐11.58, 5.58] |
| 1.4 Complications | 2 | 288 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.34, 0.13] |
| 1.4.1 Up to 3 months | 1 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.32, 0.32] |
| 1.4.2 4 to 6 months | 1 | 140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.55, 0.12] |
| 1.5 Change in weight (kg) | 18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 Up to 3 months | 10 | 802 | Mean Difference (IV, Random, 95% CI) | 0.97 [0.06, 1.87] |
| 1.5.2 4 to 6 months | 6 | 573 | Mean Difference (IV, Random, 95% CI) | 1.61 [0.09, 3.13] |
| 1.5.3 12 months and over | 7 | 1216 | Mean Difference (IV, Random, 95% CI) | 2.95 [0.75, 5.16] |
| 1.6 Change in BMI (kg/m²) | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 Up to 3 months | 2 | 181 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.24, 0.92] |
| 1.6.2 4 to 6 months | 7 | 596 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.08, 0.60] |
| 1.6.3 12 months and over | 5 | 1148 | Mean Difference (IV, Random, 95% CI) | 2.17 [0.25, 4.09] |
| 1.7 Change in fat‐free mass (kg) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 Up to 3 months | 2 | 98 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.11, 0.69] |
| 1.7.2 4 to 6 months | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.02, 1.26] |
| 1.7.3 12 months and over | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.83 [0.14, 1.53] |
| 1.8 Change in mid‐arm circumference (cm) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 Up to 3 months | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.61, 1.07] |
| 1.8.2 4 to 6 months | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.37, 0.65] |
| 1.8.3 12 months and over | 4 | 126 | Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.20, 1.50] |
| 1.9 Change in mid‐arm muscle circumference (cm) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 Up to 3 months | 2 | 119 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.71, 1.39] |
| 1.9.2 4 to 6 months | 2 | 66 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.07, 1.04] |
| 1.9.3 12 months and over | 4 | 128 | Mean Difference (IV, Random, 95% CI) | 2.04 [‐0.07, 4.15] |
| 1.10 Change in triceps skinfold thickness (mm) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 Up to 3 months | 3 | 254 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.83, ‐0.08] |
| 1.10.2 4 to 6 months | 2 | 67 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐0.25, 2.04] |
| 1.10.3 12 months and over | 4 | 128 | Mean Difference (IV, Random, 95% CI) | 1.24 [‐0.84, 3.31] |
| 1.11 Change in energy intake (kcal) | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.11.1 Up to 3 months | 9 | 536 | Mean Difference (IV, Random, 95% CI) | 242.63 [‐40.31, 525.56] |
| 1.11.2 4 to 6 months | 4 | 356 | Mean Difference (IV, Random, 95% CI) | 97.17 [20.22, 174.12] |
| 1.11.3 12 months and over | 1 | 111 | Mean Difference (IV, Random, 95% CI) | 52.00 [‐64.88, 168.88] |
| 1.12 Final energy intake (kcal) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.12.1 Up to 3 months | 2 | 276 | Mean Difference (IV, Random, 95% CI) | ‐45.91 [‐390.74, 298.92] |
| 1.12.2 4 to 6 months | 1 | 124 | Mean Difference (IV, Random, 95% CI) | ‐20.00 [‐320.10, 280.10] |
| 1.12.3 12 months and over | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 194.00 [34.33, 353.67] |
| 1.13 Change in protein intake (g) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.13.1 Up to 3 months | 5 | 354 | Mean Difference (IV, Random, 95% CI) | 12.50 [2.80, 22.19] |
| 1.13.2 4 to 6 months | 4 | 356 | Mean Difference (IV, Random, 95% CI) | 3.02 [2.60, 3.43] |
| 1.13.3 12 months and over | 1 | 111 | Mean Difference (IV, Random, 95% CI) | 4.00 [‐0.51, 8.51] |
| 1.14 Final protein intake (g) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.14.1 Up to 3 months | 4 | 416 | Mean Difference (IV, Random, 95% CI) | 8.29 [1.24, 15.34] |
| 1.14.2 4 to 6 months | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 5.00 [‐7.32, 17.32] |
| 1.14.3 6 to 12 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 11.80 [10.73, 12.87] |
| 1.15 Change in grip strength (kg force) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.15.1 Up to 3 months | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐3.38, 1.42] |
| 1.15.2 4 to 6 months | 2 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐3.32, 1.59] |
| 1.15.3 12 months and over | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐1.32, 1.92] |
| 1.16 Change in global QoL | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.16.1 Up to 3 months | 5 | 421 | Std. Mean Difference (IV, Random, 95% CI) | 3.30 [1.47, 5.13] |
| 1.16.2 Up to 3 months (FAACT) | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.32, 0.38] |
| 1.16.3 4 to 6 months | 3 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.00, 1.04] |
| 1.16.4 4 to 6 months (FAACT) | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 1.16.5 12 months and over | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | 5.71 [‐3.70, 15.12] |
| 1.17 QoL ‐ change in physical function | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.17.1 Up to 3 months | 5 | 429 | Std. Mean Difference (IV, Random, 95% CI) | 3.38 [1.54, 5.23] |
| 1.17.2 4 to 6 months | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.47, 1.35] |
| 1.17.3 4 to 6 months (SGRQ) | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.24, 1.01] |
| 1.17.4 12 months and over (SF‐36) | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.03, 1.33] |
| 1.17.5 12 months and over (SGRQ) | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.64, 0.71] |
| 1.18 QoL ‐ change in mental function | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.18.1 Up to 3 months | 5 | 421 | Std. Mean Difference (IV, Random, 95% CI) | 2.99 [1.30, 4.67] |
| 1.18.2 4 to 6 months | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.61, 1.59] |
| 1.18.3 12 months and over | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.05, 1.34] |
| 1.19 QoL ‐ change in social function | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.19.1 Up to 3 months | 5 | 419 | Std. Mean Difference (IV, Random, 95% CI) | 3.52 [1.71, 5.32] |
| 1.19.2 4 to 6 months (SF‐36) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.52, 0.72] |
| 1.19.3 4 to 6 months (SGRQ) | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.50, 0.73] |
| 1.19.4 12 months and over (SF‐36) | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.32, 1.04] |
| 1.19.5 12 months and over (SGRQ) | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 1.17 [0.44, 1.91] |
| 1.20 QoL ‐ change in cognitive function | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.20.1 Up to 3 months | 4 | 284 | Std. Mean Difference (IV, Random, 95% CI) | 3.43 [0.79, 6.07] |
| 1.21 QoL ‐ change in pain | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.21.1 Up to 3 months | 4 | 376 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.48 [‐8.13, ‐2.84] |
| 1.21.2 4 to 6 months | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.32, 0.93] |
| 1.21.3 12 months and over | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.46, 0.90] |
| 1.22 QoL ‐ change in energy/fatigue | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.22.1 Up to 3 months | 4 | 375 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.95 [‐8.65, ‐3.25] |
| 1.22.2 4 to 6 months | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.56, 0.69] |
| 1.22.3 12 months and over | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.33, 1.01] |
Comparison 2. Dietary advice compared with nutritional supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mortality | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Up to 3 months | 8 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.26] |
| 2.1.2 4 to 6 months | 3 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.65, 1.47] |
| 2.1.3 12 months and over | 2 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.06] |
| 2.2 Number of people admitted or readmitted to hospital | 2 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.02, 5.15] |
| 2.2.1 Up to 3 months | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 2.2.2 4 to 6 months | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [1.37, 9.60] |
| 2.3 Change in weight (kg) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 Up to 3 months | 9 | 517 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐2.01, 1.74] |
| 2.3.2 4 to 6 months | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.72, 1.78] |
| 2.4 Change in BMI (kg/m²) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 Up to 3 months | 2 | 97 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.64, 0.23] |
| 2.4.2 4 to 6 months | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.72, 0.70] |
| 2.5 Change in mid‐arm muscle circumference (cm) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 Up to 3 months | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.36, 1.22] |
| 2.5.2 4 to 6 months | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐1.07, 0.85] |
| 2.6 Change in mid‐arm circumference (cm) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 Up to 3 months | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.72, 0.38] |
| 2.6.2 4 to 6 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐4.30, 4.00] |
| 2.7 Change in triceps skinfold thickness (mm) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 Up to 3 months | 3 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.55, 0.05] |
| 2.7.2 4 to 6 months | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐8.96, 6.98] |
| 2.8 Change in energy intake (kcal) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 Up to 3 months | 8 | 327 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐206.23, 203.20] |
| 2.8.2 4 to 6 months | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐145.00 [‐598.85, 308.85] |
| 2.9 Change in protein intake (g) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.9.1 Up to 3 months | 4 | 221 | Mean Difference (IV, Random, 95% CI) | ‐13.09 [‐19.23, ‐6.96] |
| 2.9.2 4 to 6 months | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐6.00 [‐9.91, ‐2.09] |
| 2.10 Change in grip strength (kg force) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.10.1 Up to 3 months | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐1.10, 1.74] |
| 2.10.2 4 to 6 months | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐2.35, 2.21] |
| 2.11 Change in global QoL | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.11.1 Up to 3 months | 4 | 283 | Std. Mean Difference (IV, Random, 95% CI) | 1.26 [‐0.32, 2.85] |
| 2.11.2 Up to 3 months (FAACT) | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.40, 0.31] |
| 2.11.3 4 to 6 months | 1 | 62 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.43, 0.57] |
| 2.11.4 4 to 6 months (FAACT) | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.63, 0.33] |
| 2.11.5 12 months and over | 1 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 10.68 [8.69, 12.67] |
| 2.12 QoL ‐ change in physical function | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.12.1 Up to 3 months | 3 | 236 | Std. Mean Difference (IV, Random, 95% CI) | 2.41 [‐0.79, 5.61] |
| 2.13 QoL ‐ change in mental function | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.13.1 Up to 3 months | 3 | 232 | Std. Mean Difference (IV, Random, 95% CI) | 3.45 [‐0.24, 7.15] |
| 2.14 QoL ‐ change in social function | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.14.1 Up to 3 months | 3 | 232 | Std. Mean Difference (IV, Random, 95% CI) | 3.13 [‐0.21, 6.48] |
| 2.15 QoL ‐ change in cognitive function | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.15.1 Up to 3 months | 3 | 234 | Std. Mean Difference (IV, Random, 95% CI) | 4.23 [0.05, 8.42] |
| 2.16 QoL ‐ change in pain | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.16.1 Up to 3 months | 3 | 236 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐11.40, 0.56] |
| 2.17 QoL ‐ change in energy/fatigue | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.17.1 Up to 3months | 3 | 232 | Std. Mean Difference (IV, Random, 95% CI) | ‐8.41 [‐18.21, 1.39] |
Comparison 3. Dietary advice compared with dietary advice plus nutritional supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mortality | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Up to 3 months | 10 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.47, 1.80] |
| 3.1.2 4 to 6 months | 5 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.53, 2.00] |
| 3.1.3 7 to 12 months | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.58, 1.58] |
| 3.2 Number of people admitted or readmitted to hospital | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.58, 2.48] |
| 3.2.1 Up to 3 months | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.04, 2.77] |
| 3.2.2 4 to 6 months | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.50, 1.42] |
| 3.3 Length of hospital stay (days) | 2 | 202 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐4.10, 1.97] |
| 3.3.1 Up to 3 months | 2 | 202 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐4.10, 1.97] |
| 3.4 Complications | 4 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.05] |
| 3.4.1 Up to 3 months | 3 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 0.99] |
| 3.4.2 4 to 6 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.57, 6.54] |
| 3.5 Change in weight (kg) | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.5.1 Up to 3 months | 14 | 931 | Mean Difference (IV, Random, 95% CI) | 1.15 [0.42, 1.87] |
| 3.5.2 4 to 6 months | 4 | 209 | Mean Difference (IV, Random, 95% CI) | 2.27 [‐0.44, 4.98] |
| 3.5.3 7 to 12 months | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐4.24, 4.52] |
| 3.6 Change in BMI (kg/m²) | 7 | 378 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.31, 1.16] |
| 3.6.1 Up to 3 months | 6 | 350 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.30, 1.33] |
| 3.6.2 Four to six months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.47, 0.27] |
| 3.7 Change in fat free mass (kg) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.7.1 Up to 3 months | 3 | 187 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.18, 0.39] |
| 3.8 Change in mid‐arm muscle circumference (cm) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.8.1 Up to 3 months | 4 | 241 | Mean Difference (IV, Random, 95% CI) | 0.78 [0.37, 1.18] |
| 3.8.2 4 to 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.63, 3.03] |
| 3.9 Change in triceps skinfold thickness (mm) | 6 | 393 | Mean Difference (IV, Random, 95% CI) | 1.06 [0.14, 1.97] |
| 3.9.1 Up to 3 months | 6 | 393 | Mean Difference (IV, Random, 95% CI) | 1.06 [0.14, 1.97] |
| 3.10 Change in energy intake (kcal) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.10.1 Up to 3 months | 7 | 464 | Mean Difference (IV, Random, 95% CI) | 344.46 [164.21, 524.71] |
| 3.10.2 4 to 6 months | 3 | 75 | Mean Difference (IV, Random, 95% CI) | 362.75 [128.53, 596.97] |
| 3.11 Final energy intake (kcal/day) | 4 | 140 | Mean Difference (IV, Fixed, 95% CI) | 303.81 [110.58, 497.03] |
| 3.11.1 Up to 3 months | 4 | 140 | Mean Difference (IV, Fixed, 95% CI) | 303.81 [110.58, 497.03] |
| 3.12 Change in protein intake (g) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.12.1 Up to 3 months | 3 | 230 | Mean Difference (IV, Random, 95% CI) | 12.21 [6.39, 18.03] |
| 3.12.2 4 to 6 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 16.20 [4.83, 27.57] |
| 3.13 Final protein intake (g/day) | 6 | 239 | Mean Difference (IV, Fixed, 95% CI) | 11.76 [5.59, 17.93] |
| 3.13.1 Up to 3 months | 6 | 239 | Mean Difference (IV, Fixed, 95% CI) | 11.76 [5.59, 17.93] |
| 3.14 Change in grip strength (kg force) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.14.1 Up to 3 months | 6 | 537 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐0.22, 2.37] |
| 3.15 Change in global QoL | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.15.1 Up to 3 months | 4 | 321 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.09, 0.57] |
| 3.15.2 Up to 3 months (FAACT) | 1 | 113 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.39, 0.35] |
| 3.15.3 4 to 6 months | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.55, 1.24] |
| 3.15.4 4 to 6 months (FAACT) | 1 | 62 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.90, 0.10] |
| 3.16 QoL ‐ change in physical function | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.16.1 Up to 3 months | 4 | 324 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.08, 0.95] |
| 3.16.2 4 to 6 months | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.62, 0.77] |
| 3.17 QoL ‐ change in mental function | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.17.1 Up to 3 months | 4 | 316 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.25, 0.83] |
| 3.17.2 4 to 6 months | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.42, 0.97] |
| 3.18 QoL ‐ change in social function | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.18.1 up to 3 months | 3 | 214 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.33, 0.45] |
| 3.18.2 4 to 6 months | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.87 [0.14, 1.60] |
| 3.19 QoL ‐ change in cognitive function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.19.1 Up to 3 months | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.23, 0.45] |
| 3.19.2 4 to 6 months | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.30, 1.10] |
| 3.20 QoL ‐ change in pain | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.20.1 Up to 3 months | 3 | 219 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.36, 0.23] |
| 3.20.2 4 to 6 months | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.57, 0.82] |
| 3.21 QoL ‐ change in energy/fatigue | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.21.1 Up to 3 months | 3 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.84, 0.51] |
| 3.21.2 4 to 6 months | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.01, 0.38] |
3.11. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 11: Final energy intake (kcal/day)
3.13. Analysis.

Comparison 3: Dietary advice compared with dietary advice plus nutritional supplements, Outcome 13: Final protein intake (g/day)
Comparison 4. Dietary advice plus supplements if required compared with no advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mortality | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Up to 3 months | 15 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.16] |
| 4.1.2 4 to 6 months | 10 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.69, 1.55] |
| 4.1.3 7 to 12 months | 6 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.63, 1.19] |
| 4.1.4 12 months and over | 5 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.08] |
| 4.2 Number of people admitted or readmitted to hospital | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Up to 3 months | 7 | 673 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.15] |
| 4.2.2 4 to 6 months | 5 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.58, 1.09] |
| 4.2.3 7 to 12 months | 2 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.18, 1.55] |
| 4.3 Length of hospital stay (days) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.3.1 Up to 3 months | 3 | 400 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐2.48, 2.25] |
| 4.4 Complications | 3 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.40, 1.18] |
| 4.4.1 Up to 3 months | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.22, 1.46] |
| 4.4.2 7 to 12 months | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.35, 2.22] |
| 4.5 Change in weight (kg) | 24 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.5.1 Up to 3 months | 17 | 1192 | Mean Difference (IV, Random, 95% CI) | 1.25 [0.73, 1.76] |
| 4.5.2 4 to 6 months | 10 | 976 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐0.30, 1.45] |
| 4.5.3 7 to 12 months | 2 | 107 | Mean Difference (IV, Random, 95% CI) | 0.94 [‐0.35, 2.23] |
| 4.5.4 12 months and over | 2 | 77 | Mean Difference (IV, Random, 95% CI) | 2.17 [‐1.20, 5.54] |
| 4.6 Change in BMI (kg/m²) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.6.1 Up to 3 months | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.72 [0.06, 1.37] |
| 4.6.2 4 to 6 months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐1.12, 2.72] |
| 4.6.3 7 to 12 months | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.61, 0.41] |
| 4.7 Final BMI (kg/m²) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.7.1 Up to 3 months | 2 | 169 | Mean Difference (IV, Random, 95% CI) | 1.19 [0.18, 2.20] |
| 4.8 Change in fat free mass (kg) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.8.1 Up to 3 months | 4 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [0.35, 1.29] |
| 4.8.2 4 to 6 months | 2 | 184 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.52, 0.82] |
| 4.9 Change in mid‐arm circumference (cm) | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.47, 0.85] |
| 4.9.1 Up to 3 months | 1 | 103 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.68, 0.94] |
| 4.9.2 4 to 6 months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.84, 1.44] |
| 4.10 Change in mid‐arm muscle circumference (cm) | 2 | 247 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.54, 0.90] |
| 4.10.1 Up to 3 months | 1 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.71, 0.43] |
| 4.10.2 7 to 12 months | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.17, 1.37] |
| 4.11 Change in triceps skinfold thickness (mm) | 3 | 235 | Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.34, 1.69] |
| 4.11.1 Up to 3 months | 1 | 103 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐1.15, 2.93] |
| 4.11.2 4 to 6 months | 2 | 132 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.57, 1.77] |
| 4.12 Change in energy intake (kcal) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.12.1 Up to 3 months | 8 | 645 | Mean Difference (IV, Random, 95% CI) | 147.01 [21.55, 272.47] |
| 4.12.2 4 to 6 months | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 50.31 [‐154.15, 254.76] |
| 4.13 Final energy intake (kcal) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.13.1 Up to 3 months | 3 | 327 | Mean Difference (IV, Random, 95% CI) | 215.17 [‐55.09, 485.43] |
| 4.13.2 4 to 6 months | 2 | 195 | Mean Difference (IV, Random, 95% CI) | ‐8.62 [‐154.63, 137.39] |
| 4.14 Change in protein intake (g) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.14.1 Up to 3 months | 7 | 610 | Mean Difference (IV, Random, 95% CI) | 7.76 [0.47, 15.05] |
| 4.14.2 4 to 6 months | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 3.10 [‐7.41, 13.61] |
| 4.14.3 7 to 12 months | 1 | 78 | Mean Difference (IV, Random, 95% CI) | 5.60 [‐3.00, 14.20] |
| 4.15 Change in grip strength (kg force) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.15.1 Up to 3 months | 9 | 801 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.36, 0.72] |
| 4.15.2 4 to 6 months | 3 | 214 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.02, 1.59] |
| 4.16 Change in global QoL | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.16.1 Up to 3 months | 7 | 389 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.18, 0.48] |
| 4.16.2 4 to 6 months | 2 | 153 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.28, 0.36] |
| 4.16.3 7 to 12 months | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.14, 1.05] |
| 4.17 Final global QoL | 9 | 797 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.06, 0.77] |
| 4.17.1 Up to 3 months | 8 | 526 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.36, 0.86] |
| 4.17.2 4 to 6 months | 4 | 271 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.04, 0.81] |
| 4.18 QoL ‐ change in physical function | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.18.1 Up to 3 months | 6 | 458 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.22, 0.25] |
| 4.18.2 4 to 6 months | 2 | 147 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.25] |
| 4.18.3 7 to 12 months | 1 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.31, 0.35] |
| 4.19 QoL ‐ change in mental function | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.19.1 Up to 3 months | 5 | 435 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.10, 0.48] |
| 4.19.2 4 to 6 months | 1 | 123 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.07, 0.78] |
| 4.19.3 7 to 12 months | 1 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.13, 0.79] |
| 4.20 QoL ‐ change in social function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.20.1 Up to 3 months | 2 | 156 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.35, 0.40] |
| 4.21 QoL ‐ change in cognitive function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.21.1 Up to 3 months | 2 | 156 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.23, 0.92] |
| 4.22 QoL ‐ change in pain | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.22.1 Up to 3 months | 2 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.03, 0.07] |
| 4.23 QoL ‐ change in energy/fatigue | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.23.1 Up to 3 months | 2 | 155 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.61, 0.46] |
4.13. Analysis.

Comparison 4: Dietary advice plus supplements if required compared with no advice, Outcome 13: Final energy intake (kcal)
Comparison 5. Dietary advice plus supplements compared with no advice and no supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mortality | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Up to 3 months | 7 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.55, 1.52] |
| 5.1.2 4 to 6 months | 4 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.17] |
| 5.1.3 7 to 12 months | 3 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.29] |
| 5.1.4 12 months and over | 3 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.96, 1.20] |
| 5.2 Length of hospital stay (days) | 3 | 405 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐3.58, 0.08] |
| 5.2.1 Up to 3 months | 2 | 258 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.65, 0.04] |
| 5.2.2 4 to 6 months | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐12.46, 14.66] |
| 5.3 Complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3.1 Up to 3 months | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.20, 0.89] |
| 5.4 Change in weight (kg) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.4.1 Up to 3 months | 8 | 620 | Mean Difference (IV, Random, 95% CI) | 1.08 [‐0.17, 2.33] |
| 5.4.2 4 to 6 months | 5 | 450 | Mean Difference (IV, Random, 95% CI) | 1.88 [0.90, 2.87] |
| 5.4.3 7 to 12 months | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 2.60 [1.42, 3.78] |
| 5.5 Change in fat free mass | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5.1 Up to 3 months | 3 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.09, 0.62] |
| 5.5.2 4 to 6 months | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.57, 0.99] |
| 5.5.3 7 to 12 months | 1 | 71 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.18, 0.75] |
| 5.6 Change in BMI (kg/m²) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.6.1 Up to 3 months | 1 | 137 | Mean Difference (IV, Random, 95% CI) | 0.66 [0.19, 1.13] |
| 5.6.2 4 to 6 months | 1 | 131 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.09, 0.98] |
| 5.7 Final BMI (kg/m²) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.7.1 Up to 3 months | 3 | 254 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.76, 2.04] |
| 5.7.2 4 to 6 months | 2 | 242 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐0.45, 1.87] |
| 5.8 Change in energy intake (kcal) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.8.1 Up to 3 months | 5 | 347 | Mean Difference (IV, Random, 95% CI) | 319.78 [152.83, 486.73] |
| 5.8.2 4 to 6 months | 3 | 244 | Mean Difference (IV, Random, 95% CI) | 239.83 [38.74, 440.92] |
| 5.8.3 7 to 12 months | 1 | 63 | Mean Difference (IV, Random, 95% CI) | 464.00 [270.07, 657.93] |
| 5.9 Final energy intake (kcal) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.9.1 Up to 3 months | 3 | 152 | Mean Difference (IV, Random, 95% CI) | 399.11 [123.00, 675.22] |
| 5.10 Change in protein intake | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.10.1 Up to 3 months | 2 | 285 | Mean Difference (IV, Random, 95% CI) | 7.14 [‐0.46, 14.74] |
| 5.10.2 4 to 6 months | 1 | 135 | Mean Difference (IV, Random, 95% CI) | 0.92 [‐8.93, 10.76] |
| 5.11 Final protein intake (g/day) | 4 | 223 | Mean Difference (IV, Random, 95% CI) | 17.67 [11.80, 23.55] |
| 5.11.1 Up to 3 months | 3 | 152 | Mean Difference (IV, Random, 95% CI) | 18.15 [9.37, 26.93] |
| 5.11.2 Up to 12 months | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 17.00 [7.18, 26.82] |
| 5.12 Change in handgrip strength (kg) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.12.1 Up to 3 months | 3 | 244 | Mean Difference (IV, Random, 95% CI) | 0.99 [‐0.42, 2.40] |
| 5.12.2 4 to 6 months | 3 | 200 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐0.88, 2.31] |
| 5.13 Change in global QoL | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.13.1 Up to 3 months | 4 | 357 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.33, 0.96] |
| 5.13.2 Up to 3 months (FAACT) | 1 | 117 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.35, 0.38] |
| 5.13.3 4 to 6 months | 3 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.24, 0.31] |
| 5.13.4 4 to 6 months (FAACT) | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.80, 0.19] |
| 5.14 QoL ‐ change in physical function | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.14.1 Up to 3 months | 3 | 242 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.11, 0.84] |
| 5.14.2 4 to 6 months | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.18, 1.09] |
| 5.15 QoL ‐ change in mental function | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.15.1 Up to 3 months | 3 | 239 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.16, 0.93] |
| 5.15.2 4 to 6 months | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.38, 0.45] |
| 5.16 QoL ‐ change in social function | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.16.1 Up to 3 months | 3 | 235 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.02, 0.91] |
| 5.16.2 4 to 6 months | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.26, 1.06] |
| 5.17 QoL ‐ change in cognitive function | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.17.1 Up to 3 months | 2 | 141 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.13, 0.54] |
| 5.17.2 4 to 6 months | 1 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.37, 0.95] |
| 5.18 QoL ‐ change in pain | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.18.1 Up to 3 months | 3 | 238 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.24, 1.16] |
| 5.18.2 4 to 6 months | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.37, 0.94] |
| 5.19 QoL ‐ change in energy/fatigue | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.19.1 Up to 3 months | 3 | 239 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.16, 0.43] |
| 5.19.2 4 to 6 months | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.66, 0.64] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akpele 2004.
| Study characteristics | ||
| Methods | Pilot RCT. Duration: mean 7.5 months (longest follow‐up 14 months). Location: USA. |
|
| Participants |
Inclusion criteria: adults (> 18 years), receiving haemodialysis three times weekly for a minimum of 6 months, serum albumin < 3.5g/dL, MNA score < 23.5. Exclusioncriteria: co‐morbidities or medical illness that reduced life expectancy to less than 6 months. Number randomised: 40; attrition: none described. Diagnosis: CKD receiving haemodialysis. Age (mean): dietary advice group 61.5 years; supplement group 66.6 years. Gender split: not reported. Nutritional status: serum albumin <3.5g/dL, MNA score <23.5. |
|
| Interventions |
Intervention (intervention group 1): participants received dietary advice in the form of intensive dietary counselling. Intervention (intervention group 2): particpants received ONS in the form of 1 ‐ 2 cans of Nepro daily in addition to usual diet. |
|
| Outcomes | Rate of change of serum albumin. | |
| Publication details |
Language: English. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | No outcomes usable for this review reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information/detail. Quote: "subjects were randomised". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information/detail. Quote: "subjects were randomised". |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not described but unlikely that assessment of clinical outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not reported but likely that assessment of nutritional outcomes would be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes were influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described and likely that assessment of some outcomes would be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition reported. |
| Selective reporting (reporting bias) | High risk | No protocol identified. Some outcomes described in the methods not reported (nutritional intake, hospital admissions, missed treatments and weight). |
| Other bias | Low risk | Baseline characteristics compared and Chi² tests used for difference. No statistically significant differences between groups. |
Alo 2014.
| Study characteristics | ||
| Methods | RCT ("quasi randomised" ‐ no further details). Parallel design with 2 arms. Duration: intervention and follow‐up: 6 months. Location: Nigeria. |
|
| Participants |
Inclusion criteria: diagnosed with HIV and receiving HAART, providing consent, resident with Abakaliki town (Nigeria), without opportunistic infection. Exclusion criteria: pregnancy. Number randomised: 84 (intervention group 42, control group 42); attrition: not described. Gender split: 26 (31%) males, 58 (69%) females. Age: mean (SD) years: intervention group 33.8 (7.7) years; control group 35.3 (10.2) years. Nutritional status: BMI (kg/m²) at baseline: intervention group 23.1 in males and 21.9 in females; control group 23.3 in males and 20.3 females. |
|
| Interventions |
Intervention: participants received dietary advice in the form of dietary counselling and individualised food prescriptions based on locally available food and counselling on food hygiene. Presription based on easily available and affordable foods, to contain all food groups and assessment of requirements. Control: participants received no dietary advice but the details were not described. |
|
| Outcomes | BMI, haemoglobin. | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer reviewed journal. |
|
| Notes | Emailed authors for details of how randomisation carried out, any attrition, mean change in BMI and weight data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study described as quasi‐experimental. No information on random sequence generation in the paper. Quote: "Participants were selected using a simple random sampling technique and were then randomized" Additional information from authorsQuote: "Two nurses representing the intervention group and the control group respectively picked patients enrolment numbers from the ballot bag. All the patients enrolment numbers picked by the nurse representing intervention group automatically became the intervention group and the same with the control group." Therefore judged as low risk. |
| Allocation concealment (selection bias) | Low risk | No information on allocation concealment in the paper. Information provided by authors indicated that 'drawing lots' was used, therefore judged as high risk. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not described but assessment of clinical outcomes unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Not described but only BMI assessed which is unlikely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is unlikely that assessment of outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described but unlikely that assessment of the 2 outcomes reported would be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition, (information provided by authors). Quote: "We did not record any attrition by death or withdrawal from study or lost to follow up. Patients were ambulatory patients and so not chronically ill. There was available grant to trace lost to follow up patients to their homes by treatment supporters, so it was easy to follow up patients even when they did not come to clinic on their monthly appointments". |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes described in the methods reported in the results. |
| Other bias | Unclear risk | Baseline characteristics compared (gender, age and BMI). No differences noted between groups. No comparison of stage of disease and treatment characteristics which might have had an impact on the outcomes of interest. |
Anbar 2014.
| Study characteristics | ||
| Methods | Prospective RCT (unblinded). Parallel design. Duration: data collected up to 14 days or discharge (including pre‐op period). Location: single centre (Petah Tikva, Israel). |
|
| Participants |
Inclusion criteria: aged > 65 years and admitted within 48 hours of injury to an orthogeriatric unit following hip fracture; orthopedic surgery considered the treatment of choice. Exclusion criteria: presented to hospital > 48 hours after injury, receiving steroids and/or immunosuppression therapy; presence of active oncologic disease, multiple fractures, diagnosed dementia or in the event that participants required supplemental nasal oxygen which precludes the measurement of REE. Number randomised: 50 participants: intervention group n = 22; control group n = 28. Gender split: intervention group 6 (27%) male and 16 (73%) female; control group 11 (39%) male and 17 (61%) female. Age: mean (SD) intervention group 82.3 (6.1) years; control group 83.7 (6.4) years. Nutritional status: BMI, mean (SD): intervention group 25.2 (3.2) kg/m²; control group 24.7 (4.4) kg/m². |
|
| Interventions |
Intervention: participants received dietary advice plus ONS in the form of a hospital‐prepared diet plus ONS (355 kcal/237 mL and 13.5 g protein or 237 kcal/237 ml and 9.9 g protein/237 mL), adjusted to meet energy goals which were determined by repeated resting energy expenditure measurements using indirect calorimetry; participants, family and caregivers educated regarding the importance of nutritional support and more attention was given to personal food preferences. Control: participants received no dietary advice and no ONS in the form of usual hospital food (standard or texture adapted) and a fixed dose of oral nutritional supplement if already prescribed prior to hospitalisation. |
|
| Outcomes |
Primary outcomes Postoperative complications (i.e. surgical, infectious, cardio‐vascular, gastro‐intestinal, deep vein thrombosis and new pressure sores). Length of hospital stay. Secondary outcomes Energy intake. Calculated energy balance. |
|
| Publication details |
Language: English. Funding: not stated. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomisation was performed using a concealed, computer‐generated program. |
| Allocation concealment (selection bias) | Low risk | Quote: use of a consecutively numbered opaque envelope. |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Quote: Patients were examined daily by the research nurse and attending physician for the presence of postoperative complications. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not blinded and lack of blinding might have affected assessment of outcomes. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Quote: The nutrient intake of each patient was monitored by the research team on a daily basis. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcome assessors were aware of group allocation and this might have affected assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study with no drop‐outs. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar in both groups. |
Andersson 2017.
| Study characteristics | ||
| Methods | Open‐label RCT. Parallel design with 2 arms. Duration: 3 months. Location: Oslo, Norway. |
|
| Participants |
Inclusion criteria: resident in the capital Oslo or the nearby municipalities of Asker or Bærum, able to communicate in Norwegian and to provide written informed consent. Exclusion criteria: lack of rehabilitation potential (decided by the treating physician), duration of stay < 10 days or > 30 days at Godthaab Health and Rehabilitation Institution, not planned to return home after their stay, expected survival < 1 year, and refusal to participate. Number randomised: 100 adults (with musculoskeletal disorders, cancer, lymphedema, cardiovascular disease, chronic pulmonary disease, stroke or neurodegenerative diseases) admitted to Godthaab Health and Rehabilitation Institution. Gender split: 28 men and 72 women. Age: mean (SD) intervention group 75.2 (7.8) years, control group 75.5 (9.4) years. Nutritional status: undernourished or at risk of disease‐related malnutrition (scored > 3 on NRS‐2002). |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of an individual nutritional plan (developed by a clinical nutritionist in collaboration with the participant) with documentation of nutritional status, nutrient requirements, and nutrient intake. Nutritional plan: information on swallowing function, bowel function, appetite, food preferences, and personal habits such as eating patterns, dietary intake, and estimated energy and protein requirements according to national guidelines*. The swallowing function, bowel function, appetite, food preferences, and personal habits were self‐reported by the participants. Participants received repetition (and individual adjustments if needed) of this counselling during 3 telephone calls of 0.5 h duration at 1, 7 and 10 weeks after discharge and at 1 home visit (1 h duration) 4 weeks after discharge. Control: participants received no dietary advice and no ONS in the form of no specific nutritional advice at the time of discharge. Both groups received the same standard care including general advice on nutrition during their stay at the rehabilitation centre. * Guidelines Energy: ‐ bedridden participants: 29 kcal/kg per day; ‐ ambulatory participants: 33 kcal/kg per day; ‐ participants in recovery phase: 40 kcal/kg per day; ‐ age > 70 years: reduce by 10%; ‐ overweight (BMI > 25 kg/m²): reduce by 10%. Protein: ‐ healthy participants: 0.8‐1.5 g/kg per day; ‐ with disease: 1.5 ‐ 2.0 g/kg per day. |
|
| Outcomes | Body weight*, QoL EQ‐5D (only sub‐scores), appetite (DRAQ), self‐perceived state of health (VAS; scores 0 ‐ 100). | |
| Publication details |
Language: English. Funding: Throne Holst Foundation and The Directorate of Health, Norway. Publication status: peer‐reviewed journal. |
|
| Notes | Few of the participants were undernourished (NRS 2002 score > 4); whereas most of them had a NRS 2002 score of 3 or 4, thus being at risk of disease‐related malnutrition. The data for weight change and change in EQ 5D were obtained from the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was performed by a person not involved in the study with the software program www.randomization.com." |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes were used. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Quote: the assessors of the study outcomes were blinded to the allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Quote: the assessors of the study outcomes were blinded to the allocation. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Quote: the assessors of the study outcomes were blinded to the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: the assessors of the study outcomes were blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15/100 (15%) (intervention group: 6; control group: 9) were lost to follow‐up at 3 months due to death (n = 1), too tired (n = 3) and no reason was given (n = 11). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Low risk | Baseline variables stated, groups similar at baseline. |
Arnold 1989.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 6 months, intervention to 10 weeks and follow‐up to 6 months for some outcomes. Location: single centre in the USA. |
|
| Participants |
Inclusion: living at home, planned to receive potentially curative radiotherapy for cancers of head and neck Exclusion: both chemotherapy and radiotherapy planned for treatment Number randomised: 50 adults. Gender split: 29 males and 21 females. Age: 34 ‐ 88 years. Nutritional status: mean weight in treatment and comparison groups was 1 ‐ 2 kg below usual weight at study entry. |
|
| Interventions |
Intervention: participants (n = 23) received dietary advice and ONS in the form of intensive dietary counselling and the prescription of nutritional supplements to provide an additional 960 ‐ 1080 kcal/day. Control: participants (n = 27) received dietary advice alone in the form of intensive dietary counselling. |
|
| Outcomes | Survival*, number having a complete response to therapy, radiation side‐effects, tumour status, body weight*, serum albumin, transferrin, change in dietary energy*, protein intake. | |
| Publication details |
Language: English. Funding: not mentioned. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details of method. |
| Allocation concealment (selection bias) | Unclear risk | No details of method of allocation concealment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The trial was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes reported. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. 3 (6%) deaths in the dietary counselling and supplement group, no deaths in the control group. |
| Selective reporting (reporting bias) | High risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Data on mortality obtained from the paper. Data on weight change obtained by extrapolation from Figure 3. Energy intake data presented in a figure with no SDs or SEs, therefore risk of bias. No response received from author to request for data. |
| Other bias | Low risk | Baseline variables stated, groups similar at baseline. |
Baldwin 2011.
| Study characteristics | ||
| Methods | RCT. Design: 2 x 2 factorial trial. Duration: 12 months; intervention 6 weeks and follow‐up to 12 months. Location: started with a single centre in the UK; ended with 6 centres (5 UK and 1 Australia). |
|
| Participants |
Inclusion: adults with histologically proven, metastatic or locally advanced tumours of the GI tract, non‐small lung cancer or mesothelioma with weight loss in the previous 3 months and planned to undergo palliative chemotherapy. Exclusion: unable or unwilling to provide consent, had a clinical condition precluding oral nutrition, were unable to tolerate milk or it was considered that they should receive immediate enteral or parenteral nutrition. Number randomised: 358 adults (group 1, n = 96; group 2, n = 90; group 3, n = 86; group 4, n = 86). Gender split: 256 males and 102 women. Age: median (range) 66 (24 ‐ 88 years). Disease status: locally advanced or metastatic cancers of the gastrointestinal tract (n = 277) or non‐small‐cell lung cancer or mesothelioma. All participants had lost weight at the start of the trial (mean (SD) 9.8% (6%) in lung participants and 11.2% (6.4%) GI participants)). Nutritional status: any amount of weight loss in the previous 3 months. 153 participants were alive at 12 months: No intervention group: 47 deaths and 2 withdrawals. Dietary advice group: 52 deaths and 2 withdrawals. Nutritional supplements group: 55 deaths and 2 withdrawals. Dietary advice and supplements group: 44 deaths and 1 withdrawal. |
|
| Interventions |
Control: (trial group 1) participants received no dietary advice and no ONS in the form of no additional intervention. Intervention: (trial group 2) participants received dietary advice in the form of standardised dietary counselling and an information booklet to increase intake by 600 kcal/day Intervention: (trial group 3) participants received ONS in the form of an oral nutritional supplement providing 588 kcal/day. Intervention: (Trial group 4) participants received dietary advice and ONS in the form of standardised dietary counselling and an information booklet to increase intake by 600 kcal/day and an oral nutritional supplement providing 588 kcal/d. |
|
| Outcomes | Survival*, QoL*, weight*, handgrip strength*, energy intake*. | |
| Publication details |
Language: English. Funding: Henry Smith Charity and The Special Trustees Chelsea and Westminster Hospital, London. Publication status: peer‐reviewed journal. |
|
| Notes | Trial was stopped prematurely on advice of the independent data monitoring committee. Data, specified per group, were received from the authors. Data on handgrip strength were not normally distributed and so have not been included in the meta‐analyses. Both FAACT and EORTC QoL data were provided. For meta‐analyses we only used the EORTC QoL data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an independent trials centre using a computer‐generated list. Participants were stratified for performance status and site of disease. |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed until participants had signed consent to participate. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The outcome assessors were not blinded but unlikely that these outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The outcome assessors were not blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The outcome assessors were not blinded and assessment of some nutritional outcomes might be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 153/358 (43%) participants were alive at 12 months: No intervention group: 49 (51 %) including 47 deaths. Dietary advice group: 54 (60 %) including 52 deaths. Nutritional supplements group: 57 (66 %) including 55 deaths. Dietary advice and supplements group: 45 (52 %) including 44 deaths. 7 (2 %) participants withdrew but reasons for withdrawal were not reported. |
| Selective reporting (reporting bias) | Low risk | No published protocol but the trial principal investigator is one of the review authors. The data on survival, weight, QoL and grip strength were not presented by group and not fully reported in the paper, therefore original data have been provided by the authors for this review. The numbers of participants completing assessment of energy intake was only 31 of 358 and so these data should be interpreted with caution. Data on grip strength were judged to be unsafe because of difficulties carrying out this assessment according to standard protocols and so are not included. |
| Other bias | Unclear risk | Baseline characteristics for the 4 groups were similar. Trial was stopped prematurely on advice of the independent data monitoring committee. Unclear how this may have influenced results. |
Banks 2016.
| Study characteristics | ||
| Methods | Pilot RCT. Parallel design with 2 arms. Duration: during hospital admission, follow up on day 5, 10, 15, 22 and then weekly or until discharge. Location: a tertiary referral hospital in Brisbane, Australia. |
|
| Participants |
Inclusion criteria: participants with a stage II–IV pressure ulcers (PU), either existing on admission or acquired during admission. Exclusion criteria: unable to receive nutrition support via the enteral route (on parenteral nutrition); inappropriate for intensive nutrition support (receiving palliative care or medically deteriorating); unable to follow nutrition support advice (cognitively impaired, language barriers); previously enrolled in the study. Number randomised: 50 adults. Gender split: intervention group 14 males, 11 females; control group 19 males; 6 females. Age: mean (SD): intervention group 62.3 (20.7) years; control group 65.8 (15.8) years. Nutritional status: assessed by SGA; intervention group ‐ well nourished n = 5, mild to moderate malnutrition n = 13, severe malnutrition n = 7; control group ‐ well nourished n = 4, mild to moderate malnutrition n = 15, severe malnutrition n = 6. |
|
| Interventions | All participants received evidence‐based PU care according to local hospital practices. Intervention: participants received dietary advice plus ONS if required in the form of intensive individualised nutritional care provided by a research dietitian including a high protein/energy diet and/or supplements aimed at meeting estimated nutritional requirements of 1.2g protein/kg body weight/day and 30 kcal/kg per day and the prescription of a 'wound healing' nutritional formula, enriched with arginine, vitamin C and zinc; participants offered the choice of 2 different brands of the supplement based on their preference and prescription based on recommended daily dosage by manufacturer. It was expected that participants would be reviewed by the research dietitian at least 3 times/week. Control: participants received no dietary advice and no ONS in the form of standard nutritional care provided by the clinical team which usually included a dietitian and may have included a standard hospital diet or high protein/energy diet and/or nutritional supplements and/or enteral tube feeding. |
|
| Outcomes | PU change, from baseline, in score (PUSH) and area (measured using Visitrak system), adequacy of intake of protein and calories, length of stay to heal or discharge, participant outcome (early discharge, PU healed, PU worsened, discharged not healed). | |
| Publication details |
Language: English. Funding: by a grant from the Queensland Health, Health Practitioner Research Scheme. Publication status: peer reviewed journal. |
|
| Notes | The data for weight change data were obtained from the author. Weight change was over any time within the study period, not just the end of the study, but was at least 1 week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: After recruitment, a computer‐generated randomised list was used to determine the group allocation sequence. Randomisation was stratified by PU stage (stage ll or stage III and lV) to ensure equal representation of PU severity across groups. Where more than one PU existed, the highest stage PU was chosen as the primary PU for data collection purposes. |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by telephoning an independent researcher. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The trial was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The trial was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition: (36% overall) intervention group 10/25 (40%); control group 8/25 (32%) due to early discharge. No differences in the baseline characteristics between the measured and lost to follow‐up groups. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Only the PUSH tool score and the PU area change was reported in detail. Data on weight change were obtained from the author. Data on energy and protein intake were not in a format suitable for this review, and were not provided by the author. |
| Other bias | Low risk | Groups were comparable at baseline. |
Beattie 2000.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 10 weeks. Location: single centre in the UK. |
|
| Participants |
Inclusion criteria: adults resuming oral food intake after surgery with BMI < 20 kg/m², TSF or MAMC < 15th percentile or > 5% weight loss. All participants had an MNA score 20 or less. Exclusion criteria: requiring parenteral nutrition, pregnant or lactating, with terminal illness, decompensated liver or renal disease. Number randomised: 101 adults (both men and women). Gender split: 41 females, 60 males. Age: mean (SD); intervention group 54.4 (19.4) years; control group 62.4 (10.9) years. Nutritional status: on inclusion to study defined as mild (BMI <20 kg/m2), moderate (BMI <18 kg/m2), severe (BMI <16 kg/m2) normal (BMI 20‐25 kg/m2), overweight (BMI >25 kg/m2); intervention group severe 1, moderate 5, mild 29, normal 13, overweight 4; control group severe 2, moderate 9, mild 19, normal 16, overweight 3. nb. participants with normal or overweight BMI had weight loss >5% at inclusion. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of routine nutritional management and 400 mL of a 1.5 kcal/mL nutritional supplement. Control: participants received dietary advice alone in the form of routine nutritional management. |
|
| Outcomes | Survival*, weight*, BMI*, MAMC*, TSF*, handgrip strength*, complication rate, wound infection, chest infection, antibiotic use, QoL. | |
| Publication details |
Language: English. Funding: this study was funded by Abbott Laboratories. Publication status: peer‐reviewed journal. |
|
| Notes | Routine nutritional management provided by more than one dietitian and not described in the paper. Information on quality obtained from authors. Information on QoL not added to meta‐analyses as only scores for physical and mental QoL were provided in the manuscript ‐ global QoL missing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | The allocation was not concealed physically but the list of numbers was not consulted until the participant was recruited. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The paper states that assessors were not blinded to treatment. However, it unlikely that morbidity is influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes reported. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The paper states that assessors were not blinded to treatment. Nutritional status and QoL outcomes can be influenced by assessors knowing group allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for nutritional and QoL outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/101 (8%) attrition, 3 drop outs in intervention group (advice plus supplements) (transferred to intensive care unit n = 1, required artificial nutritional support n = 2) and 5 dropouts in control group (routine nutritional management group) (lost to follow‐up n = 2, required artificial nutritional support n = 3). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Data for analysis were extracted from the paper. Additional information on study quality obtained from authors. QoL data were not complete, only physical and mental health data provided |
| Other bias | Unclear risk | Baseline variables provided, but groups not similar ‐ group receiving advice plus supplements was younger by almost 10 years than the advice only group. Routine nutritional management provided by more than one dietitian and not described in the paper. |
Beck 2012.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 8 weeks intervention with 26 weeks follow‐up. Location: University Hospital of Herlev in Denmark (but participants drawn from 3 municipalities). |
|
| Participants |
Inclusion criteria: identified as at nutritional risk according to the level 1 screen NRS 2002; were 65+ years of age, living in three municipalities (Herlev, Roedovre or Gladsaxe), hospitalised for a minimum of 2 days in the geriatric medicine wards of the University Hospital of Herlev. Exclusion criteria: suffered from senile dementia or terminal disease; could not understand the Danish language; resident in nursing homes; or unable to or willing to give informed consent. Number randomised:152 elderly participants (over 65 years of age) randomised, 124 completed study. Gender split: intervention group 54 females (74%) and 19 males (26%); control group 57 females (72%) and 22 males (28%). Age: data not reported (all greater than 65 years). Nutritional status: all at nutritional risk assessed using NRS 2002. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of comprehensive nutritional assessment by a dietitian, followed by 3 home visits with individualised nutritional counselling by a registered dietitian complemented with 3 follow‐up visits conducted by the GP. Control: participants received no dietary advice and no ONS in the form of 3 follow‐up visits by GPs alone. |
|
| Outcomes |
Primary outcome: risk of readmissions*. Secondary outcomes: functional status (hand grip strength*, chair stand, mobility, disability and tiredness in daily activities, rehabilitation capacity), nutritional status (weight*, BMI, energy* and protein intake*), need of social services (home care, home nursing, meals‐on‐wheels) and mortality*. |
|
| Publication details |
Language: English. Funding: grants from the Health Insurance Foundation, the Tryg Foundation and the General Practitioners’ Foundation for Development of General Practice. These are all non‐commercial and had no role in study design, or in the collection, analysis, interpretation and publication of the data. TDC provided cell phones for scientific research assistants and registered dietitians and, as the others, had no role in study design, or in the collection, analysis, interpretation and publication of data. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from paper: "Participants were randomized at discharge. Each allocation using generated random numbers was written on paper and concealed in a serially numbered, opaque envelope. The scientific research assistants opened the next envelope after recruiting each participant and then contacted the GP and, if relevant, the registered dietitians. Hence the scientific research assistants, who collected the outcome data, knew which group a participant was in. The principal investigator was the only one blinded for the intervention". Randomisation ratio not described. |
| Allocation concealment (selection bias) | Low risk | Quote from paper: "Each allocation using generated random numbers was written on paper and concealed in a serially numbered, opaque envelope." |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Comment: Not blinded. Scientific research assistants were aware of group assignment. Outcome not likely influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Comment: Not blinded. Hence the scientific research assistants, who collected the outcome data, knew which group a participant was in. Outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Comment: Not blinded. Hence the scientific research assistants, who collected the outcome data, knew which group a participant was in. Outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded, most of the outcomes were functional and nutrition parameters; knowing the group allocation could have influenced outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 34/152 (22%) attrition. Withdrawals were balanced across groups: 18 (23%) in control group, and 16 (22%) in intervention group. Otherwise no missing data. It was even possible to obtain follow‐up data from some of those who withdrew. |
| Selective reporting (reporting bias) | Low risk | Study protocol identified Clintrials.gov. All specified outcomes reported. |
| Other bias | Low risk | Comment: baseline characteristics were similar between groups. |
Beck 2015.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 12 weeks, follow‐up at 6 months. Location: single centre in Denmark. |
|
| Participants |
Inclusion criteria: > 70 years of age, hospitalised at the wards of Geriatric Medicine and Orthopaedic Surgery, at nutritional risk according to the level 2 screening in NRS 2002 and planned to be discharged to their private home assisted by the discharge Liaison‐Team. Exclusion criteria: dementia or terminal disease, impaired renal function (GFR < 30 mL/min/1.73 m²), unable to understand the Danish language, nursing homes or rehabilitation homes, incapable of performing hand‐grip test, planning a weight‐reducing diet, no informed consent. Number randomised: 71 adults. Gender split: 23 males, 48 females. Age: data not reported (all greater than 70 years). Nutritional status: all at nutritional risk assessed using NRS 2002. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of visits from the Liaison‐Team in cooperation with a dietician (who made 3 home visits over a period of 12 weeks). Control: participants received no dietary advice and no ONS in the form of visits from the Liaison‐Team without dietitian. |
|
| Outcomes | Nutritional status (weight*, and dietary intake*), muscle strength (hand‐grip strength*, chair stand), functional status (mobility, and activities of daily living), QoL (EQ‐5D), use of social services, rehospitalisation and mortality*, costs. The economic analysis of time spent by the dietitian, use of oral nutritional supplements and number of hospitalisation days was described by Pohju et al (2016). |
|
| Publication details |
Language: English. Funding: a grant from the Danish Regions and the Danish Health Cartel. Publication status: peer‐reviewed journal. |
|
| Notes | Outcomes were presented as median change scores in the article. In the review we used the mean change scores which were provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Simple randomisation was used, i.e. each allocation was written on paper, concealed in an opaque envelope. The opaque envelopes were gathered in a jar from which the patients drew a lot after recruitment. |
| Allocation concealment (selection bias) | Low risk | Quote: ... each allocation was written on paper, concealed in an opaque envelope. The opaque envelopes were gathered in a jar from which the patients drew a lot after recruitment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 63 (89%) participants completed the second data collection; 8 participants died (2 (6%) in the intervention group and 6 (16%) in the control group). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Berneis 2000.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 12 weeks. Location: Basel, Switzerland. |
|
| Participants |
Inclusion criteria: adults with HIV infection and weight loss 5% or more in 6 months or BMI < 21 or CD4 cell count < 500/mm‐3 in a stable condition without acute infectious complications. Exclusion criteria: not specified. Number randomised: 18 adults but 3 not included because of non‐adherence and severe disease complications, so 15 participants. Gender split: 14 males and 1 female. Age: not reported. Nutritional status: not reported |
|
| Interventions |
Intervention: participants (n = 8) received dietary advice and ONS in the form of dietary advice and nutritional supplements (target unspecified). Control: participants (n = 7) received no dietary advice and no ONS in the form of no nutritional therapy. |
|
| Outcomes | Weight*, lean and fat mass, macronutrient intake, energy intake*, immune function, QoL. | |
| Publication details |
Language: English. Funding: grant from the Swiss Federal Office of Health (Grant no 94–7189), Novartis Nutrition (Bern, Switzerland), and the Swiss National Science Foundation. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information on quality requested from authors. Received a reply to say information no longer available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from author states that randomisation performed by pharmacy using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | The author could not supply details about allocation concealment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Although not specifically mentioned, the trial must have been unblinded due to the nature of the intervention. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | The trial was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | The trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Original group consisted of 18 participants, but 3 (17%) not included because of non‐adherence and severe disease complications. Author unable to provide details of which groups the drop‐outs were in. |
| Selective reporting (reporting bias) | High risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes were reported, as mean change at baseline and end of follow‐up therefore change scores were calculated and SDs imputed. |
| Other bias | Unclear risk | Baseline variables not stated, don't know if groups similar at baseline. |
Bonilla‐Palomas 2016.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: intervention began at hospital admission and lasted 6 months, follow‐up to a maximum of 12 months. Location: multicentre (2 centres) in Spain. |
|
| Participants |
Inclusion criteria: adults with acute heart failure (whether chronic and uncompensated or of new onset), in a state of malnutrition (score on the MNA < 17 points). Exclusion criteria: pregnancy, chronic renal failure in dialysis, already receiving nutritional treatment, concomitant disease with life expectancy < 1 year regardless of heart failure itself, participation in other clinical trials, surgery or percutaneous coronary intervention during hospital stay to correct the cause of acute heart failure, clinical status meaning it is impossible to perform the nutritional assessment as established in the study protocol, lack of consent for such procedures. Number randomised: 120 participants. Gender split: 45 males and 75 females. Age: mean (SD) 79.2 (7) years. Nutritional status: all malnourished (MNA < 17 points). |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of heart failure treatment with follow‐up by cardiologist combined with an individualized nutritional intervention performed by a physician specialist in nutrition assisted by a nutritionist and based on diet optimisation, specific recommendations and nutritional supplement prescriptions in cases in which nutritional goals were not reached; supplements chosen according to the requirements and co‐morbidities of the participant. Control: participants received no dietary advice and no ONS in the form of standard heart failure treatment with follow‐up by cardiologist. |
|
| Outcomes | A composite of all‐cause death or readmission for worsening of heart failure, weight, nutritional intake. | |
| Publication details |
Language: English. Funding: Spanish Society of Cardiology as a Project of the Spanish Society of Cardiology for Clinical Research in Cardiology. Publication status: peer‐reviewed journal. |
|
| Notes | The design paper states that a sample size of 182 participants is needed; in the paper reporting study outcomes only 120 participants were included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using a simple randomization process, 120 patients were assigned to the control or intervention group. The randomization sequence has been generated and deposited in the secretariat of the Internal Medicine Department of Hospital Juan de la Cruz". There was no description of how the randomisation sequence was generated e.g. computer‐generated or random number tables. |
| Allocation concealment (selection bias) | Low risk | Quote: "A randomization sequence has been generated and deposited in the secretariat of the Internal Medicine Department of Hospital Juan de la Cruz. The investigators are in contact with the staff of the secretariat by telephone". |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded. However, this is unlikely to influence mortality rate and readmissions. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No data on functional outcomes collected. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was only caused by mortality (47% in the control group and 20% in the intervention group). |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the protocol article are reported. |
| Other bias | Low risk | Baseline characteristics are similar between groups. |
Bourdel‐Marchasson 2014.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: interviews were performed 6 times during the chemotherapy sessions for 3 to 6 months with two‐year follow‐up. Location: multicentre in France. |
|
| Participants |
Inclusion criteria: aged 70 years or older and being treated with chemotherapy for solid tumour, at risk of malnutrition (MNA < 17 or > 23.5). Exclusion criteria: Karnofsky index < 50%, under chemotherapy process. Number randomised: 336 participants (randomised 341, dropouts 5). Gender split: 51% males, 49% females. Age: mean (SD), intervention group 77.7 (5.2) years; control group 78.3 (4.7) years. Nutritional status: all at risk of malnutrition (MNA < 17 or > 23.5 |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of dietary counselling (face‐to‐face discussion targeting the main nutritional symptoms) to achieve an energy intake of 30 kcal/kg body weight/day and 1.2 g protein/kg/day. Control: participants received no dietary advice and no ONS in the form of usual nutritional care routinely given in the cancer treatment settings with no restrictions for dietary advice, oral supplements or prescription of artificial nutrition. |
|
| Outcomes |
Primary outcome: 1‐year mortality. Secondary outcomes: 2‐year mortality, toxicities and chemotherapy outcomes, weight change, nutritional intake, prescription of enteral and parenteral nutrition, hospitalisation for reasons other than chemotherapy, QoL (EORTC‐C30) , MNA, ADL, IADL, MMS and GDS. |
|
| Publication details |
Language: English. Funding: supported by the National Hospital Program of Clinical Research (Programme Hospitalier de Recherche Clinique 2006) (46%), La Ligue contre le cancer (52%) and AMGEN (2%) and sponsored by the university hospital of Bordeaux (CHU Bordeaux). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Publication status: peer‐reviewed journal. |
|
| Notes | Quote: "The trial was stopped before completion of the planned inclusions. However, it is unlikely that the lack of an observed effect of intervention is due to lack of power. In the whole sample, a trend of an increase in two‐year mortality in the intervention group was seen. However, even if we had reached the planned sample size, such mortality rates in both arms would not have provided a significant difference in survival. This absence of effect on mortality and other outcomes was not due to unbalanced characteristics of patients between groups according to cancer disease, chemotherapy or nutritional baseline assessment." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was prepared by the clinical trial unit biostatistician. Randomization was centralized by internet, with a 1:1 ratio, stratified by recruitment centre. |
| Allocation concealment (selection bias) | Low risk | Randomisation was centralized by internet and the randomization list stored by the clinical trial unit biostatistician. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The trial was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The trial was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None of the staff in cancer treatment centres were aware of the content of the nutritional intervention. Patients were unblinded to group assignment. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 341 were randomised, 336 were analyzed. Attrition n = 5 (less than 1%) (1 consent withdrawal, 4 with exclusion criteria) all in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol identified Clinicaltrials NCT00459589. Some secondary outcomes not reported (function, QoL, MNA and some biochemical outcomes). |
| Other bias | Low risk | Baseline characteristics presented and groups were similar. Study was stopped early. However, it is unlikely that the lack of an observed effect of intervention is due to lack of power. |
Burden 2011.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: pre‐operative intervention (mean (range, SD) time to surgery was 37 (7 – 371 days, 54.7 days); participants followed up until 3 months after operation. Location: single centre in the UK. |
|
| Participants |
Inclusion criteria: diagnosed with colorectal cancer and scheduled for surgery with pre‐operative weight loss > 1 kg/3 ‐ 6 months. Exclusion criteria: pregnant, enrolled in another trial, unable to give informed consent, had an inoperable tumour. Number randomised: 125 participants (intervention group n = 59 (analysed n = 54), control group n = 66 (analysed n = 62). Gender split: intervention group 63% male; control group 61% male. Age: mean (SD), intervention group 64.5 (13.9) years; control group 65.3 (2.7) years. Nutritional status: 83 (71%) participants had lost weight in 3 – 6 months preceding surgery; the % of usual body weight lost was in the range 1% – 31% (mean (SD) 5.8% (6.5%)). |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of 400 mL of oral supplement and dietary advice (consisting of increasing energy and protein from food, based on an information leaflet*). Control: participants received dietary advice alone in the form of dietary advice as described above. *The author explained: "The leaflet was Build yourself up: for patients undergoing surgical procedures". It included the eat well plate, foods to include in your diet going through the food groups, There was a section on increasing energy and protein content of the diet. At the end it included high calorie snacks. Dietary counselling was not provided in the sense of dietetic practice i.e. assessment of habitual intake and tailored advice. The research assistant talked the patient through the written leaflet. So this advice was usual care as it is usually given out in pre‐op clinics by clinic nurses". |
|
| Outcomes |
Primary outcome: number of post‐operative complications. Secondary outcomes: use of postoperative antibiotics, length of hospital stay, energy and protein intake, complications, grip strength. |
|
| Publication details |
Language: English. Funding: NHS fellowship award and Central Manchester foundation trust small awards. Publication status: peer‐reviewed journal. |
|
| Notes | Of the 54 participants randomised to the treatment group, 50 completed a diary to record compliance to the intervention (2 full cartons of supplement daily). It was reported that 36 (72%) of participants managed 100% of the intervention, 8 (16%) managed 50% (at least 1 carton daily) and 6 (12%) managed < 25% of the intervention. Tha author provided change scores for energy intake and protein intake on our request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numeral sequence of random blocks generated by independent statistician. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered brown opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Incomplete blinding, personnel blinded, participants not blinded. It is unlikely that post‐op complications is influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Functional outcomes were not measured in this study. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | It is possible that there is bias associated with dietary intake recorded by 24 h recall because participants were unblinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Incomplete blinding, personnel blinded, participants not blinded. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 of 54 (9%) in the intervention group and 4 of 62 (6%) in the control group did not have surgery so were excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Data on change in grip strength were not reported. |
| Other bias | Unclear risk | Baseline characteristics were compared and were broadly similar in the two groups. Dietary intake was measured by unstructured dietary recalls; this method is not very reliable. The SD of the mean change in energy intake was twice as high as the mean change, indicating a skewed distribution. |
Burden 2017.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: oral nutritional supplements were administered from diagnosis to the day preceding surgery for a minimum of 5 days. Location: multicentre study in the UK. |
|
| Participants |
Inclusion criteria: diagnosis of colorectal cancer, scheduled for surgery with pre‐operative weight loss > 1 kg/3 – 6 months. Exclusion criteria: pregnant, had a pacemaker, already taking a similar ONS, with insulin dependent diabetes Number randomised: 101 participants (intervention group n = 55; control group n = 46). Gender split: intervention 64% male, control 70% male. Age: mean (SD) intervention group 70.5 (11.7) years; control group 68.9 (11.5) years. Nutritional status: median (IQR) % weight loss, intervention 4.9 kg (2.2 ‐ 8.8); control 6.8 kg (3.4 ‐ 12.1) |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of 250 mL/day oral nutritional supplements (10.1 KJ and protein 0.096 g/mL) and dietary advice. Control: participants received dietary advice alone in the form of dietary advice. |
|
| Outcomes |
Primary outcome: 1 or more surgical site infection or chest infection. Secondary outcomes: % weight loss, total complications, and body composition measurements. |
|
| Publication details |
Language: English. Funding: Macmillan Cancer Support and British Dietetic Association. Publication status: peer‐reviewed journal. |
|
| Notes | Non‐normally distributed data are displayed as median and IQRs. In this format, the data are not in a format sufficient for meta‐analyses. Data on handgrip strength are reported as mean (SD) and thus included in meta‐analyses. On request, change scores of weight, kcal intake, and protein intake were obtained from the author, and included in the meta‐analyses. Outcomes are reported at 2 time‐points, preoperatively and post‐operatively. As the intervention was given preoperatively only, we have chosen to only report the preoperative outcome measures in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly allocated on a 1:1 ratio by using blocks of two ensuring equal numbers in each group. Allocation was stratified according to tumour site (rectal versus colon) and surgical approaches (open versus laparoscopic). 4 lists of random numbers were produced by a statistician, and an independent researcher set up the randomization procedure for each of the strata. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque sealed envelopes were used, which allowed block randomization sequence allocation to be implemented and ensured sequence allocation concealment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Described as single blind. For the purposes of blinding, control participants were given sealed cardboard boxes of identical weight and appearance as the supplement group at the time of group allocation. These boxes contained bottled water in 125 mL bottles. Thus research team was blind to the intervention, but the participants were not. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Described as single blind. For the purposes of blinding, control participants were given sealed cardboard boxes of identical weight and appearance as the supplement group at the time of group allocation. These boxes contained bottled water in 125 mL bottles. Thus the research team was blind to the intervention, but the participants were not. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Described as single blind. For the purposes of blinding, control participants were given sealed cardboard boxes of identical weight and appearance as the supplement group at the time of group allocation. These boxes contained bottled water in 125 mL bottles. Thus research team was blind to the intervention, but the participants were not. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The research team was blind to the intervention, but the participants were not. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The research team was blind to the intervention, but the participants were not. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 of 55 (2%) in the intervention group died; 6 of 46 (13%) in the control group failed to complete (4 died, 1 participant withdrew from the control group and 1 further participant from the control group was excluded from the analysis). For the outcome measures: handgrip strength, % weight loss, PG‐SGA, energy intake and protein intake data are provided for fewer participants than originally included. The reasons why data were missing for these outcomes is not reported. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol identified. All outcomes reported apart from hospital readmissions and quality of life (secondary outcomes). Many outcomes reported as median and IQR, thus not in a format sufficient for meta‐analysis. |
| Other bias | Unclear risk | Baseline variables reported. Overall, there were more participants in the intervention group (n = 55) than in the control group (n = 46). At the point of recruiting participants, if it was undecided if surgery was open or laparoscopic, the default used was open‐surgery stratum for randomization. The two arms of the trial were well‐matched with similar proportions of participants within each stratum, site of cancer, and type of operation (laparoscopic or open). |
Caccialanza 2015.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 12 months. Location: single centre in Italy. |
|
| Participants |
Inclusion criteria: amyloidosis adult outpatients who were treatment naïve and able to provide written informed consent. Exclusion criteria: indication to autologous stem cell transplantation, in need of starting or ongoing dialysis or ongoing artificial nutrition, presence of ascites or relevant peripheral oedema. Number randomised: 144 participants; intervention n = 72, control n = 72. Gender split: intervention group 62% male; control group 60% male. Age: mean (SD) intervention 65.4 (10.5) years; control 64.8 (9.7) years. Nutritional status: BMI at baseline, mean (SD): intervention group 24.6 (3.3) and control group 25.4 (4.1). Unintentional weight loss, median (IQR): intervention group 3.0 (0.0 – 6.0) kg and control group 4.0 (0.3 – 7.0) kg. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form off personalized dietary prescription (modelled on personal eating patterns and preferences, to satisfy estimated protein–calorie requirements* and based primarily on the use of regular foods; oral nutrition supplements were prescribed when necessary) plus dietetic advice from a registered dietitian every 3 weeks by telephone and every 3 months face‐to‐face. Control: participants received no dietary advice and no ONS in the form of written general nutritional advice aimed at maintaining or recovering body weight, participants were allowed to ad libitum food intake, without fixed prescription of oral nutritional supplements; if participants had questions concerning nutrition, they were advised by the Amyloidosis Center’s attending physician or the nurses, but not by the professional dietitian. *Energy requirements: Harris‐Benedict equation x 1.5, protein requirements 1.1 g/kg of actual body weight in case of normal kidney function; in the presence of kidney involvement, adjustments were performed according to available guidelines and laboratory data. |
|
| Outcomes | Body weight*, MAMC, energy intake (% of participants reaching requirements), QoL (SF‐36, only subscales), mortality*. | |
| Publication details |
Language: English. Funding: the Fondazione IRCCS Policlinico San Matteo (Pavia,Italy) and partly supported by grants from "Associazione Italiana per la Ricerca sul Cancro ‐ Special Program Molecular Clinical Oncology 5 per mille" (grant n. 9965) and Cariplo Foundation (grant no. 2013‐0964). Publication status: peer‐reviewed journal. |
|
| Notes | Not all participants were malnourished at baseline. QoL data could not be used because only subscales were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: a computer‐generated randomization list (randomized blocks) was used. Accordingly, eligible participants were randomized (1:1) to either the nutritional counselling (NC) or usual care (UC) group." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Stated as open‐label, meaning that both researchers and participants know which treatment is being administered. However, this is unlikely to influence mortality rate. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Stated as open‐label, meaning that both researchers and participants know which treatment is being administered. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Stated as open‐label, meaning that both researchers and participants know which treatment is being administered. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition 84/144 (58%) participants; intervention group 51/72 (71%), control group 33/72 (46%), therefore amounts judged to be unbalanced between groups. High mortality rates due to complexity of the group. Assessed at 3 months: intervention n = 56, control n = 47. Assessed at 12 months: intervention n = 39, control n = 21. 41 did not attend the first follow‐up visit at 3 months (intervention group n = 16, control group n = 25). Most drop outs were caused by death: intervention n = 20, control n = 33. |
| Selective reporting (reporting bias) | Low risk | Protocol identified Clintrials NCT02055534 all planned outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Calegari 2011.
| Study characteristics | ||
| Methods | RCT. Duration: 2 phases (separated by a 1 month wash‐out). Phase 1: 3‐month intervention compared with control. Phase 2: Control group also received the intervention. Only data from Phase 1 included. Location: single centre study in Porto Alegre, Brazil. |
|
| Participants |
Inclusion criteria: on haemodialysis. Exclusion criteria: clinically unstable, with infectious or inflammatory diseases, neoplasias, scheduled for transplant or death before inclusion in the study. Number randomised: 18 participants; intervention group n = 9, control group n = 9. Attrition: 15 (83%) completed the first phase. 2 participants died and 1 moved to peritoneal dialysis, all from control group. Gender split: 15 (83%) males, 3 (17%) females. Age: mean (SD) and range 56.4 (15.6) years, 26 years to 88 years. Nutritional status: BMI, mean (SD): intervention group 22.3 (2.3) kg/m², control group 20.9 (2.1) kg/m². |
|
| Interventions |
Intervention: participants received dietary advice plus ONS in the form of oral nutritional supplementation (355 kcal, 53% carbohydrates, 10 g of proteins, 15 g of lipids, 257 mg of calcium, 271 mg of phosphorus, 313 mg of potassium, and 106 mg of sodium) during each haemodialysis session for 3 months; supplementation was offered to participants in the period between the beginning and mid‐dialysis. In addition, they were provided with special attention, such as nutritional guidance, family counselling, and dental assessment. Control: participants received no dietary advice and no ONS in the form of "routine nutritional guidance". |
|
| Outcomes | Nutritional status (dry weight, BMI, SGA); body composition (MAC, MAMA, lean mass, fat mass), QoL (SF‐36 domain scores); functional status (6‐minute walking test). | |
| Publication details |
Language: English. Funding: Instituto de Doenças Renais provided ingredients for the intervention formula. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not described but mortality unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not described and likely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not described and likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the outcome assessors were blinded and some outcome assessment might be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition data fully reported, however, reasons were unbalanced between groups: 3/18 (17 %) in did not complete the first phase: intervention group 0/9 (0 %) and control group 3/9 (33 %); of these 2/9 (22 %) died and 1/9 (11 %) moved to peritoneal dialysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes mentioned in the methods are reported. |
| Other bias | Low risk | Intervention and control groups were similar at baseline. |
Campbell 2008.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 12 weeks. Location: Australia. |
|
| Participants |
Inclusion criteria: adults with stage 4 chronic kidney disease, not previously seen by a dietitian, not expected to require renal replacement therapy within 6 months. Exclusion criteria: not specified. Number randomised: 62 participants randomised, 56 participants started study, 50 participants completed the study. Gender split: (at baseline) males n = 34, females n = 22. Age: mean (SD) intervention group 69.5 (11.7) years; control group 70.9 (11.6) years. Nutritional status: (assessed using SGA) intervention group 24% malnourished (SGA B); control group 11% malnourished (SGA B). |
|
| Interventions |
Intervention: participants (n = 24) received dietary advice in the form of dietary counselling to increase energy intake, maintain protein intake and information on appropriate nutritional choices for people with renal disease. Control: participants (n = 26) received no dietary advice in the form of usual care (generic information on nutrition). |
|
| Outcomes | Dietary intake*, body weight*, nutritional status (SGA), body composition (total body potassium), survival * biochemistry. | |
| Publication details |
Language: English. Funding: Royal Brisbane and Women's Hospital Foundation seeding grant, Queensland University of technology PG Research Award and an Institute of Health and Biomedical Innovation Research Scholarship. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number sequence. |
| Allocation concealment (selection bias) | Low risk | Described as concealed to recruiting officer until after baseline assessment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | It is not stated whether the outcome assessors were blinded but unlikely that these outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not stated and likely that some of these outcomes might have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not stated and likely that some of these outcomes might have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the outcome assessors were blinded and some outcome assessment might be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 62 participants randomised in the study, attrition fully described with reasons: attrition 12 (I: 8/32(27%); C:4/30(13%): 6 participants did not receive the intervention; 6 did not complete (4 deaths (all in the intervention group) and 2 participants received dialysis). |
| Selective reporting (reporting bias) | Low risk | Protocol identified on ANZCTR database. All specified outcomes reported. Data on mortality, change in weight, energy and protein intake, body cell mass and QoL were used in this review. The data are reported in the paper but the weight and body cell mass data are presented as a mean (SD) at baseline and at 12 weeks and therefore the mean change with SD has been obtained from the authors. The energy intake data reported in kJ/kg, and protein as g/kg, therefore mean change (SD) obtained from the author. |
| Other bias | Unclear risk | Baseline variables stated, groups similar at baseline apart from amounts of malnutrition (see above) and it is unclear what influence this might have had on outcomes assessed. |
Cano‐Torres 2017.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: intervention given for duration of hospital stay; follow‐up for 6 months (data collection at 2, 4, 6 months). Location: Mexico. |
|
| Participants |
Inclusion criteria: adults over 20 years of age with malnutrition admitted to Dept. Internal Medicine. Exclusion criteria: expected length of stay less than 48h, disturbance of consciousness, psychiatric disease, pregnant or breastfeeding, chronic kidney disease, parenteral or enteral nutrition, needing ventilation, liver disease, CVD alcohol‐related disease, malignancy. Number randomised: 55 participants (intervention group n = 28, control group n = 27). Attrition: total n = 7 (intervention group n = 2, control group n = 5). Gender split: 22 (40%) males, 33 (60%) females. Age: mean (SD) 57.1 (20.7) years. Diagnoses: various, intervention group 61.5% chronic disease; control group 59% chronic disease. Nutritional status: BMI (kg/m²) at baseline: intervention group 24.7 (7.7) and control group 27.3(8.8). NRS score, mean (SD): intervention group 4.1 (0.8) and control group 4.2 (1.2). |
|
| Interventions |
Intervention: participants received dietary advice in the form of an individualised nutrition plan from a clinical dietitian provided daily including, estimation of energy and protein requirements, motivation to adhere to diet, assessment of intake, nutritional counselling. Control group: participants received no dietary advice in the form of standard nutritional management in the hospital. |
|
| Outcomes | Length of stay, mortality, BMI, arm circumference. | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Email to authors, allocation concealment, confirm that length of stay and BMI are expressed as mean(SD)? mean change for BMI and arm circumference. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated (using a list of random numbers generated by computer". |
| Allocation concealment (selection bias) | Unclear risk | Judged as unclear because insufficient information about how group allocation was concealed from participants. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | It is not stated whether the outcome assessors were blinded to group allocation but unlikely that these outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Not described but nutritional intake not assessed and other nutritional outcomes unlikely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the outcome assessors were blinded and some outcome assessment might be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition fully described with reasons. 7 (intervention group n = 2/28 (7%) (1 death and 1 withdrawal), control group n = 5/27 (19%) (5 deaths)) drop outs out of 55 participants. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. The stated outcomes of the study are mortality and length of stay and both of these are reported. A number of additional outcomes are also reported but not referred to explicitly in the methods, therefore judged as unclear risk of bias. |
| Other bias | Low risk | At baseline the control group had a lower haemoglobin level than the intervention group ‐ 10.2 (2.3) g/dL compared to 12.3 (2.7) g/dL; otherwise groups well‐balanced for all characteristics. This has been judged to be unlikely to affect the outcomes of interest. |
Carey 2013.
| Study characteristics | ||
| Methods | RCT. Duration: 6 months. Location: Sydney, Australia |
|
| Participants |
Inclusion criteria: adults with major upper GI surgery with Roux‐en‐y reconstructive surgery, defined as a gastrectomy (total or partial), esophagectomy or pancreoduodenectomy. Exclusion criteria: known active disease, pyloric preserving surgery, inability to consent or living more than 2 hours from the centre. Number randomised: 27 participants (intervention group n = 14 and control group n = 13). Gender split: 21 males, 6 females. Age: mean (SD) intervention group 65 (11) years, control group 66 (7) years. Nutritional status: assessed by SGA, intervention group status A n = 4, B n = 7, C n = 3; control group status A n = 6, B n = 6, C n = 1. *SGA A (well nourished), B (mild to moderate malnutrition), C (severe malnutrition) |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of 45‐minute dietary education session* from the ward dietitian prior to discharge from hospital (usual care) followed up with regular phone review by the clinical dietitian every 2 weeks 6 months and face‐to‐face follow‐up when deemed appropriate by the dietitian to discuss current weight and oral dietary intake (assessed by patient report and individualized nutritional counselling to improve intakes as required) and GI symptoms (such as reflux, bloating/wind, anorexia, early satiety, vomiting and bowel habits with advice to alleviate the symptoms provided) with additional written advice and oral or enteral nutrition supplementation provided as necessary. Control group: participants received no dietary advice and no ONS in the form of 45‐minute dietary education session* from the ward dietitian prior to discharge from hospital (usual care), discharged with the ward dietitian’s contact details but no dietitian follow‐up was arranged. * Education included written information and advice regarding the use of oral nutritional supplements if appropriate. |
|
| Outcomes | Weight, triceps skinfold, MAMC, mid‐arm muscle mass, hand‐grip strength, SGA, dietary intake, QoL (EORTC QLQ‐C30), GI symptoms (Gastro Intestinal Symptom Rating Scale) | |
| Publication details |
Language: English. Funding: not stated. Publication status: peer‐reviewed journal. |
|
| Notes | Not all participants were malnourished according to the SGA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomization consisted of a pre‐determined randomization table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not measured. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Quote: The research dietitian performed nutritional assessment and data collection for all patients, and was blinded to the group allocations. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Quote: The research dietitian performed nutritional assessment and data collection for all patients, and was blinded to the group allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: The research dietitian performed nutritional assessment and data collection for all patients, and was blinded to the group allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In both groups, 1 participant was lost to follow‐up because of moving and death. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Unclear risk | Participants in the intervention groups weighed 66.6 kg, compared to participants in the control group 82.7 kg P=0.022), and also BMI, arm muscle circumference were less (non‐significant) and pre‐operative weight loss was higher (non‐significant). |
Casals 2015.
| Study characteristics | ||
| Methods | RCT. Duration: intervention and follow‐up 6 months. Location: Spain. |
|
| Participants |
Inclusion criteria: hospitalised, medium/high risk of malnutrition (MUST), over 18 years of age, willing to participate, resident in the healthcare district. Exclusion criteria: treatment with oral food supplements, enteral or parenteral nutrition during admission, chemotherapy or radiotherapy during admission, presence of malabsorption. Number randomised: 106 participants randomised (intervention group n = 52, control group n = 54). Attrition: 13 participants dropped out (intervention group n = 6, control group n = 7). Diagnosis: mixed clinical conditions. Gender split: 53 (50%) males, 53 (50%) females. Age: mean (SD) intervention group 73 (13) years; control group 73 (12) years. Nutritional status: all at medium/high risk (MUST) mean (SD) score: intervention group 2.6 (1.27) and control group 2.4 (1.27). |
|
| Interventions |
Intervention: participants received dietary advice in the form of individualised nutritional counselling provided by case manager nurses which began in hospital and continued at home; review and monitoring varied according to whether participants were at medium or high risk. Control: participants received no dietary advice in the form of standard hospital discharge procedures (report to community team and follow‐up phone call). |
|
| Outcomes | BMI, number of participants at medium/high risk, biochemical measures (total protein, albumin, cholesterol, total lymphocytes), hospital readmissions, length of stay, QoL (SF‐12), functional independence (Barthel), satisfaction (CSQ‐8). | |
| Publication details |
Language: English. Funding: Andalucian Government. Publication status: peer‐reviewed journal. |
|
| Notes | Email authors, detail of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Quote: "once the patient had been included, they were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk |
Quote: "to minimize potential biases, the final assessment and data analysis were performed by professionals other than those who conducted the intervention and follow‐up". Judged low risk because blinding unlikely to affect clinical outcomes |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk |
Quote: "to minimize potential biases, the final assessment and data analysis were performed by professionals other than those who conducted the intervention and follow‐up". Judged as low risk. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Quote: "to minimize potential biases, the final assessment and data analysis were performed by professionals other than those who conducted the intervention and follow‐up". Judged as low risk because outcome assessment blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Final assessment and analyses performed blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition fully described with reasons. 13 out of 106 participants dropped out. (intervention group: 6/52 (12%) all deaths; control group: 7/54 (13%) 6 deaths and 1 loss to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes mentioned in the methods are reported. |
| Other bias | Low risk | Groups well balanced at baseline for all characteristics. |
Chandra 1985.
| Study characteristics | ||
| Methods | RCT.
Duration: 4 weeks. Location: Newfoundland, Canada |
|
| Participants |
Inclusion criteria: elderly men with clinical and biochemical parameters suggesting malnutrition. Exclusion criteria: not specified. Number randomised: 30 participants (intervention group n = 15, control group n = 15). Gender split: all male Age: range 70 ‐ 84 years. Nutritional status: not reported. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of dietary advice and supplements. Control: participants received no dietary advice and no ONS in the form of no intervention. |
|
| Outcomes | Weight*, TSF*, biochemistry. | |
| Publication details |
Language: English. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | It is not stated whether the trial was blinded or not, but the nature of the intervention suggest that the trial was unblinded. However, this is unlikely to influence biochemistry outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | It is not stated whether the trial was blinded or not. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | It is not stated whether the trial was blinded or not. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on attrition. |
| Selective reporting (reporting bias) | High risk | No study protocol identified. Not all outcome data reported. Data presented on change in weight and TSF and pre‐albumin for the intervention group only. No data extracted and no response to request for data from the author. |
| Other bias | Unclear risk | Baseline characteristics not stated. |
de Luis 2003.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 3 months. Location: single centre in Spain. |
|
| Participants |
Inclusion criteria: adults with HIV infection, absence of chronic fever, digestive conditions, drug consumption that might affect nutritional intake, with normal renal and hepatic function and 5% or more weight loss in previous 6 months. Exclusion criteria: not specified. Number randomised: 70 participants, 6 participants withdrew between randomisation and baseline (intervention group n = 33, control group n = 33). Gender split: intervention group 71.4% males, control group 82.8 % males. Age: mean (SD) intervention group 37.5 (11) years, control group 39.9 (9) years. Nutritional status: not reported. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of dietary advice to increase energy and protein intake and 3 x 250 mL supplement (Ensure). Control: participants received dietary advice alone in the form of dietary advice to increase energy and protein intake. |
|
| Outcomes | Survival*, weight*, BMI*, TSF*, MUAC*, energy intake*, immune function, cardiac function. | |
| Publication details |
Language: Spanish. Funding: not reported. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from the author indicated that a random number series was used to generate a sequence. |
| Allocation concealment (selection bias) | Low risk | Information from the author indicated that sealed envelopes were used to conceal allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded. The trial was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Information from the author indicated that outcome assessment was not blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Information from the author indicated that outcome assessment was not blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants (2 in each group) withdrew as they relocated due to work commitments |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes reported not all in a format usable for meta‐analysis. Change in weight, TSF, MAMC and energy intake are reported as mean (SD) at baseline and end of intervention. Mean change (SD) obtained from authors. |
| Other bias | Low risk | Baseline variables stated, groups similar at baseline. |
de Sousa 2012.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 3 weeks intervention when ONS were stopped, with follow‐up to 90 days Location: single centre in Portugal (Geriatric Unit of a Psychiatric Hospital in Porto (Hospital Magalha ~es Lemos)). |
|
| Participants |
Inclusion criteria: aged 60 years and older admitted to hospital with recently diagnosed probable mild Alzheimer disease on the basis of the Diagnostic Statistics Disorders‐ IV and International Classification of Diseases criteria, who presented weight loss higher than 5% of body weight in the previous year. Exclusion criteria: having severe acute illness, receiving terminal care, cancer, receiving enteral or parenteral nutrition, receiving dietary advice or ONS in the previous month. Number randomised: 35 participants. Gender split: 9 males, 26 females. Age: mean (SD) intervention group 79.4 (6.9) years; control group 78.4 (5.2) years. Nutritional status: mean (SD) MNA score, intervention 11.6 (3.8); control 13 (1.8). |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of standard dietetic advice (usual care) with once daily 200 mL high protein energy‐dense liquid oral nutritional supplement. Control: participants received dietary advice alone in the form of standard dietetic advice (usual care). |
|
| Outcomes | MNA (score), weight (kg), BMI, TSF (mm), MAMC (cm), Albumin (%), total protein (g/dL), total cholesterol (mg/dL), vitamin B12 (pg/mL), folic acid (ng/mL), MMSE (score), clock‐drawing test (score), Barthel index (score). | |
| Publication details |
Language: English. Funding: no funding, products were provided by Novartis. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The trial was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | No blinding. This may have influenced functional outcomes. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | No blinding. This may have influenced nutritional outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear as low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 17 (11%) participants in the control group died and were excluded from the analyses. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Low risk | Groups were comparable at baseline. |
Diouf 2016.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: intervention during hospitalisation and then 9 weeks following discharge. Location: single centre in Senegal. |
|
| Participants |
Inclusion criteria: people living with HIV/AIDS (any stage, taking or not‐taking antiretroviral treatment). Exclusion criteria: psychiatric illness, diabetes, physical disability, unable to eat. Number randomised: 65 participants (intervention group n = 32, control group n = 33). Gender split: 32% males, 68% females. Age: mean (SD) intervention 40 (12) years; control 42 (12) years. Nutritional status: severe malnutrition (BMI < 16 kg/m²): intervention group n = 34% and control group n = 24%. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of standard hospital diet supplemented with supplement of 200 g/day* plus dietary counselling to improve diet at home (after discharge); supplementation was continued for 9 weeks at home. Control: participants received dietary advice alone in the form of standard hospital diet alone plus dietary counselling to improve diet at home (after discharge). *200 g of supplement made up of 100 g ready to use food mixed with 100 g of rice porridge. Ready to use food is composed of peanut butter and skimmed milk powder fortified with a vitamin‐mineral complex commercialised by Nutriset. The rice porridge (9.1 g rice flour per 100 mL water) was prepared extemporaneously, mixed with the ready to use food and served immediately. |
|
| Outcomes | Body weight, fat‐free mass, % body fat, dietary intake. Individual dietary intakes were measured and compared to the Recommended Dietary Allowances. Body composition was determined using Bio‐Impedance Analysis. |
|
| Publication details |
Language: English. Funding: Universite Cheikh Anta Dio de Dakar, Senegal. UNICEF Senegal provided the products. Publication status: peer‐reviewed journal. |
|
| Notes | Both well‐nourished and malnourished participants were included. At our request, authors provided change scores for weight, fat‐free mass and body fat. Energy and protein intakes were only measured during 7 consecutive days in 10 randomly selected participants from each group during hospitalization; therefore results on nutritional intake are not presented in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation and assignment to group was performed by the senior researcher using a computer‐generated random number list (EPI INFO 6.0; Centers for Disease Control and Prevention, Atlanta). |
| Allocation concealment (selection bias) | Low risk | The randomisation and assignment to group was performed by the senior researcher using a computer‐generated random number list (EPI INFO 6.0; Centers for Disease Control and Prevention, Atlanta). |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | No clinical outcomes reported. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was unblinded. Outcomes could have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High drop‐out rates, but same numbers of dropouts across both groups (intervention group n = 12, control group n = 16). During hospitalisation, 6 participants died in the intervention group and 8 in the control group so 51 completed this stage (intervention group n = 26, control group n = 25). After 9 weeks of home monitoring, 6 participants dropped out of the intervention group (3 participants withdrew and 3 died) and 8 dropped out of the control group (4 participants lost to follow‐up and 4 died). Final analysis of 37 participants, 20 in intervention group and 17 in control group. |
| Selective reporting (reporting bias) | Low risk | Study protocol identified; all outcomes reported. |
| Other bias | Low risk | Trend towards less females, lower CD4 counts and lower Hb (all non‐significant) in the intervention group. Nutritional intake from the standard hospital diet was comparable between groups. |
Dixon 1984.
| Study characteristics | ||
| Methods | RCT. Parallel design with 4 arms. Duration: 4 months. Location: USA. |
|
| Participants |
Inclusion criteria: adults with more than 5% weight loss in previous 2 months or persistent change to eating habits or problems interfering with eating, undergoing palliative treatment or chemotherapy for cancer affecting a variety of sites (main sites colorectal (27%) lymphoma (16%)). Exclusion criteria: people with breast cancer. Number randomised: 88 participants, 63% of participants completed (23 deaths and 10 dropouts, groups not specified). Gender split: 50 males and 38 females. Age: mean (SD) 59.6 (13.7) years. Nutritional status: mean (SD) number of problems interfering with eating 6.6 (3.4); mean (SD) number of changes to eating patterns 1.6 (1.3). |
|
| Interventions |
Intervention group 1 participants (n = 9) received dietary advice and ONS in the form of nutritional counselling and a range of nutritional supplements. Intervention group 2 (n = 14): nutritional counselling, nutritional supplements and relaxation training. Intervention group 3 (n = 13): nutritional counselling and relaxation training. Intervention group 4 participants (n = 9 ) received dietary advice alone in the form of nutritional counselling during bi‐weekly visits. Control group participants (n = 10) received no dietary advice and no ONS in the form of no home visits. Intervention groups 1 and 4 and the control group were included in the review. |
|
| Outcomes | Survival*, body weight*, TSF*, MAMC*, performance status (Karnofsky scale), subjective evaluation of helpfulness. | |
| Publication details |
Language: English. Funding: PHS grant 5R18 CA22619. Publication status: peer‐reviewed journal. |
|
| Notes | Data from 2 interventions used. 1. nutritional counselling versus no dietary advice; and 2. nutritional counselling versus nutritional counselling and nutritional supplements. Nutritional counselling provided by nurses No data usable for analysis because data are presented as mean change from desirable weight and change from standard for TSF and analysed using ANOVA. No response received from author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | It is not stated whether the outcome assessors were blinded but unlikely that these outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The trial was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 63% of participants completed the study. 23 deaths and 10 dropouts, groups not specified therefore insufficient information to judge risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. All outcomes mentioned in the methods reported. No data usable for analysis because data are presented as mean change from desirable weight and change from standard for TSF and analysed using ANOVA. No response received from author. |
| Other bias | Unclear risk | Baseline variables not shown, but reported to be statistically equivalent, therefore, insufficient information to make a judgement. |
Endevelt 2011.
| Study characteristics | ||
| Methods | RCT. Parallel design with 3 arms. Duration: 6 months. Location: Haifa, Israel |
|
| Participants |
Inclusion criteria: adults aged 75 and older, community dwelling at nutritional risk ((serum cholesterol below 160 mg/dL or serum albumin below 3.5 mg/dL or total lymphocyte count of less than 1800) AND (MNA‐sf below 10 or more than 10% weight loss in 6 months)). Exclusion criteria: diagnosis of cancer or liver disease, clinical depression, cognitive impairment (MMSE < 23) and inability or unwillingness to sign and informed consent. Number randomised: 127 people agreed to participate, 68 participants randomised but 59 people not randomised due to communication and language difficulties or unwillingness to have home visits by a dietitian. Total cohort, n = 127; Group 1, n = 59; Group 2, n = 35; Group 3, n = 33. Gender split: Group 1, 24 male, 35 female; Group 2, 13 male, 22 female; Group 3, 12 male, 21 female. Age: mean (SD) Group 1, 84.5 (5.6) years; Group 2, 84.2 (6.0) years; Group 3, 84.7 (4.7) years. Nutritional status: mean (SD) BMI: Group 1, 27.4 (5.2) kg/m²; Group 2, 27.3 (5.0) kg/m²; Group 3, 27.0 (5.2) kg/m². |
|
| Interventions |
Intervention group 1: participants received dietary advice plus ONS if required in the form of dietetic intervention treatment where each participant had 5 meetings with the clinic dietitian in their homes over a period of 6 months (baseline, 2 weeks, 1 month, 2 months, 6 months); the intensity varied according to the severity of the undernutrition and the content of the meetings was protocolled. Intervention group 2: advice by the primary care physician and a booklet on nutrition. Intervention group 3: participants received no dietary advice and no ONS in the form of standard care. |
|
| Outcomes | MNA, food frequency questionnaire (vitamins, minerals, protein), biochemical measurements, health care costs*, cognition (MMSE), depression (GDS‐sf), ADL (Barthel). | |
| Publication details |
Language: English. Funding: grant from the National Institute for Health Policy Israel (NIHP). Publication status: peer‐reviewed journal. |
|
| Notes | The sample size of intervention group 2 was too small according to the sample size calculation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The procedure of randomisation was not reported. From the 127 individuals who agreed to participate, only 68 participants were randomised to either dietetic intervention or medical intervention. The other 59 individuals were not included in the randomisation process due to communication and language difficulties or unwillingness to have home visits by a dietitian. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Assessments were performed by trained interviewers. Details on blinding are not reported. The design suggests that the study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Assessments were performed by trained interviewers. Details on blinding are not reported. The design suggests that the study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Low risk | Study protocol identified Clintrials NCT00316966. All specified endpoints reported but not all endpoints were reported in a way that made them usable for meta‐analysis. |
| Other bias | Low risk | Baseline characteristics were similar, except for a trend for higher education in group 1. |
Evans 1987.
| Study characteristics | ||
| Methods | RCT. Parallel design with 3 arms. Duration: 12 weeks (all outcomes) and follow‐up between 3 and 5 years for survival. Location: Toronta, Canada |
|
| Participants |
Inclusion criteria: adults receiving chemotherapy for advanced colorectal and non‐small‐cell lung cancer. Exclusion criteria: obstruction of the superior vena cava, CNS metastases or chronic systemic illness Number randomised: 180 participants, 156 deaths in the 3 study groups. Gender split: 109 males, 71 females. Age: range intervention group 23 ‐ 79 years; control group 33 ‐ 78 years. Nutritional status: more than 5% weight loss at study entry: 46% of participants. |
|
| Interventions |
Intervention 1: participants (n = 51) received dietary advice plus ONS if required in the form of nutritional counselling to achieve a target caloric intake (using supplements if required). Intervention 2 (n = 60): nutritional counselling to achieve target caloric intake but including 25% of calories as protein (using food and protein supplements) plus a supplement of zinc and magnesium. Control: participants (n = 69) received no dietary advice and no ONS in the form of ad lib food intake. |
|
| Outcomes | Body weight, energy intake, mortality*, tumour response to chemotherapy. | |
| Publication details |
Language: English. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | Data from only the first intervention group and the control group (120 participants) will be used: 1. nutritional counselling to achieve target caloric intake 2. ad lib food intake. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a central office. Participants were stratified and randomisation blocked. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by using a central office. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Although not mentioned, the trial was likely unblinded, due to the nature of the intervention. However, this is unlikely to influence clinical outcomes Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | We assume the trial was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | We assume the trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Follow‐up assessments were performed by the study dietitian either in person or by telephone. We assume this was not blinded and it is likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 156 deaths in the 3 study groups: 88 due to lung cancer and 68 due to colorectal cancer; 94/111 (85%) in both intervention groups combined and 62/69 (90%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Data presented as median % change and therefore not in a usable format and author unable to supply data. |
| Other bias | Low risk | Baseline variables stated, groups similar at baseline. |
Feldblum 2011.
| Study characteristics | ||
| Methods | RCT. Parallel design with 3 arms. Duration: 6 months intervention and 3 months follow‐up. Location: Israel. |
|
| Participants |
Inclusion criteria: adults aged 65 and older admitted to internal medicine department with a positive malnutrition screening score (MNA‐sf or > 10% weight loss in 6 months). Exclusion criteria: a current diagnosis of cancer, cognitive impairment (MMSE score < 23), an inability to be interviewed, language difficulties, or an unwillingness to provide informed consent. Number randomised: 259 participants. Gender split: 113 males, 146 females. Age: mean (SD) intervention 1, 75.3 (5.8) years; intervention 2, 75.2 (5.6) years; control 75.1(5.8) years. Nutritional status: mean (SD) MNA score, intervention 1, 19.3 (2.3); intervention 2, 19.7 (2.3); control 19.4 (2.9). |
|
| Interventions |
Intervention 1: participants received dietary advice plus ONS if required in the form of individualised nutritional treatment from a dietitian in the hospital and 3 home visits (1 week, 1 month and 1 month after discharge). Intervention 2: participants received no dietary advice and no ONS in the form of 1 meeting with a dietitian in the hospital. Control: participants received no dietary advice and no ONS in the form of standard care. Groups 2 and 3 were combined into a single group that served as the control group in the analysis. |
|
| Outcomes | MNA, weight, albumin, total lymphocyte count, haemoglobin, transferrin, total cholesterol, nutritional intake (energy, carbohydrate, fat, protein), ADL (Barthel Index), depression (GDS), cognition (MMSE), mortality. | |
| Publication details |
Language: English. Funding: the Israel National Institute for Health Policy and Health Services Research. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The intervention groups were allocated according to month and ward of hospitalization, with an equal proportion of winter and summer months being maintained. In each month, the internal medicine departments were randomly assigned a treatment group. |
| Allocation concealment (selection bias) | High risk | Not concealed. Participants who were admitted to these departments were given the assigned treatment of that month. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Trained interviewers blinded to treatment group allocation performed the measurements. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Trained interviewers blinded to treatment group allocation performed the measurements. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Trained interviewers blinded to treatment group allocation performed the measurements. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained interviewers blinded to treatment group allocation performed the measurements. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The overall dropout rate (people who refused the 6‐month visit) was 25.8%.The main causes for withdrawal were subjective health deterioration and ‘not feeling good'. The attrition was not balanced between groups (intervention group: 11.5% and control group: 32%) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Low risk | Baseline results were similar between groups, except from educational level. This was lower in the control group. |
Fernandez‐Barres 2017.
| Study characteristics | ||
| Methods | RCT. Duration: 12 months. Location: multicentre in Spain. |
|
| Participants |
Inclusion criteria: participation in Home Care Program, over 65 years, MNA score 17 ‐ 23.5, difficulty performing ADLs. Exclusion criteria: MNA score outside the range specified, enteral feed, severe dysphagia, serious illness that progresses to malnutrition, taking vitamin or mineral supplements. Diagnosis: older people receiving Home Care. Number randomised: 173 (intervention group n = 101, control group n = 72). Attrition fully described, intervention group 30/101 (38%), control group 24/72 (33%). Gender split: 32% male, 68% female. Age: mean (SD): intervention group 84.3 (6.7) years; control group 85.4 (7.6) years. Nutritional status: BMI mean (SD): intervention group 27 (5) kg/m²; control group 26.9 (6.3) kg/m². |
|
| Interventions |
Intervention group: participants received dietary advice in the form of individual and group sessions for carers and further individual dietary monitoring of participants in the presence of caregivers. Control group: participants received no dietary advice in the form of routine care from nurses and doctors. |
|
| Outcomes | Mortality, MNA (3 dimensions), weight, BMI, MUAC, calf circumference, food intake (12 groups), biochemical status, dependency (Barthel), cognitive function (Pfeiffers test), mood (Yesavage Depression scale). Caregiver knowledge also assessed. |
|
| Publication details |
Language: English. Funding: Instituto Salud, Madrid & Generalitat de Catlyunya, Barcelona. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Subjects were classified randomly, individually and stratified by Primary Health Care Centre. From a common database subjects were computer‐assigned to the intervention group and non‐intervention group." Judgement: implies computer used to facilitate random assignment, but poorly described. |
| Allocation concealment (selection bias) | Low risk | Judgement: implies computer used to allocate groups, but poorly described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk |
Quote: "participants, nurses and researchers were not blinded because of the practical impossibilities" but unlikely that assessment of clinical outcomes would be influenced by lack of blinding. Quote: Lab technicians analysing biochemical parameters were blinded to group assignment but these outcomes are not included in the review. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Quote: "participants, nurses and researchers were not blinded because of the practical impossibilities", and it is likely that assessment of this outcome would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Quote: "participants, nurses and researchers were not blinded because of the practical impossibilities", and it is likely that assessment of this outcome would be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding for the majority of outcomes assessed and likely that assessment of some outcomes would be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully described. Intervention group 30/101(38%); control group 24/72 (33%). Reasons: mortality n = 12 (intervention group n = 10, control group n = 2), withdrew n = 7 (intervention group n = 1, control group n = 6), institutionalisation or hospitalisation n = 9 (intervention group n = 5, control group n = 4). |
| Selective reporting (reporting bias) | Low risk | Protocol identified on Clinicaltrials.gov. All primary outcomes described in the protocol are reported and several secondary outcomes. Continuous data reported as mean at baseline and end of intervention. Mean change requested from the author. |
| Other bias | Low risk | Groups well balanced at baseline for all characteristics. |
Forli 2001.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 10 ‐ 18 days. Location: Norway. |
|
| Participants |
Inclusion criteria: adults with end‐stage lung disease awaiting transplantation; all participants malnourished defined as BMI < 18.7 kg/m². Exclusion criteria: people with cystic fibrosis, pulmonary hypertension or with BMI over 25 kg.m2. Number randomsied: 37 participants; intervention group, n = 20; control group, n = 22. Attrition: 2 participants withdrew from each group. Gender split: 18 males, 19 females. Age: mean (range) intervention group, 49 (44 ‐ 53) years; control group, 48 (44 ‐ 52) years. Nutritional status: mean (95% CI) BMI intervention group, 17.5 (16.8 ‐ 18.3) kg/m²; control group, 17.0 (16.1 ‐ 17.9) kg/m². |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of dietary advice to take an energy‐rich diet and supplements if wanted. Control: participants received no dietary advice and no ONS in the form of normal hospital diet. |
|
| Outcomes | Survival*, weight*, BMI, TSF*, MAMC*, MUAC*, respiratory function*. | |
| Publication details |
Language: English. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described in the paper as using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Stated in the paper as assessed blind to intervention group. Quote: None of the investigators who performed the measurements was aware of the group allocation, apart from the dietitian who, for practical reasons, was responsible for assessments of dietary intake, allocation of dietary counselling, measurements of weight, skinfold and hand grip strength. When food records prior to the first hospital stay were made, everyone was blinded to group affiliation. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | All assessments of nutritional status performed by the study dietitian who was not blinded to intervention group. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | All assessments of nutritional status performed by the study dietitian who was not blinded to intervention group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical and functional outcomes and high risk for nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrew from each group, the reasons for withdrawals are clearly stated. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. Data not reported for clinical and functional outcomes (secondary outcomes), but stated as not significantly different. Data on weight reported as median change with no SD, therefore mean change (SD) obtained from author. Data on energy intake reported as median intake KJ/kg therefore obtained from authors. |
| Other bias | Unclear risk | Baseline variables given, but one assessment of lung function was significantly different. |
Forster 2012.
| Study characteristics | ||
| Methods | RCT. Parallel design with 3 treatment arms. Duration: total 6 months ‐ intervention 3 months and follow‐up 3 months. Location: UK. |
|
| Participants |
Inclusion criteria: adults aged 65 ‐ 85 years, living in the community, no history of hospitalisation in the previous year, self‐reported daily fruit and vegetable intake less than 3 portions. Exclusion criteria: use of micronutrient or fish oil supplements in the last 3 months, active GI disease, malabsorption, previous gastric surgery, BMI < 18 kg/m², psychiatric illness, unable to understand, IDDM or illness known to influence immune status. Diagnosis: chronic diseases mean (SD) 2.6 (1.6), prescribed medications 4 (2.9). Number randomised: 217 participants randomised (intervention group 1, n = 73; intervention group 2, n = 73; control group, n = 71). Attrition: total 8 (intervention group 1, n = 1; intervention group 2, n = 3; control group, n = 4). Gender split: intervention group 1, 57.5% males; intervention group 2, 45.2% males; control group, 43.7% males. Age: mean (SD) intervention group 1, 72.5 (5) years; intervention group 2, 72.6 (5.2) years; control group, 72.6 (5.5) years. Nutritional status: BMI mean (SD), intervention group 2, 8.0 (4.4) kg/m²; control group, 29.2 (5.7) kg/m². |
|
| Interventions |
Intervention 1: participants received dietary advice in the form of provision of a selection of foods designed to meet individualised prescription to include 5 portions fruit and vegetables per day, whole grain bread, fish 2x weekly and nuts at least once a week. Intervention 2: micronutrient supplement. Control: participants received no dietary advice in the form of placebo micronutrient supplement and no dietary advice. |
|
| Outcomes | Self‐reported infections (symptom diary), nutritional biochemistry, dietary intake, weight, BMI, MAC, TSF, QoL (SF‐36), GDS. | |
| Publication details |
Language: English. Funding: UK Food Standards Agency and some funding to one author from Nutricia. Publication status: peer‐reviewed journal. |
|
| Notes | Intervention group 1 and control used in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated block randomization was used". Stratified according to age. |
| Allocation concealment (selection bias) | Low risk | Quote:"a distant email randomization service was used to assign treatment group & study number". |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Only one clinical outcome (self‐reported infections); judged to have adequate blinding. Quote: "study physician who was blinded to randomisation" interpreted information provided in the symptom diaries. The principal investigator, lab staff and statistician were blinded to group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Functional outcomes: QoL and GDS. Insufficient information on how these data were collected but likely that they could be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | No information available on who measured nutritional outcomes, dietary intake, weight, BMI, MAC, TSF but likely that lack of blinding could have influenced assessment of some. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of the intervention and possible for participants in other groups to obtain the intervention. Quote: Partial blinding achieved with micronutrient supplement but not possible to blind participants or study researchers to dietary intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available on who measured nutritional outcomes, dietary intake, weight, BMI, MAC, TSF, therefore not possible to make a judgement. Quote: "the researcher responsible for implementation of the intervention was aware of treatment allocation but laboratory personnel, principal investigators and trial statistician were all blinded to participants identity and group allocation until completion of analyses". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully reported. 5 participants withdrew, 1/73 (0.01%) from the advice group and 4/71 (0.06%) from the no advice group. |
| Selective reporting (reporting bias) | Low risk | Reported outcomes checked against the protocol. All outcomes other than immune function included in this paper. Author contacted for more information. Response indicated that immune function outcomes will be reported in a separate paper. |
| Other bias | Unclear risk | At baseline intervention group 1 (food group) had a significantly higher alcohol intake than the micronutrient group or control group, otherwise groups were well balanced for all characteristics. Judged as unclear because it is not possible to judge the impact that this might have had on the outcomes assessed |
Fuenzalida 1990.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 42 days total (21 days in hospital and 21 days at home). Location: single centre in the USA. |
|
| Participants |
Inclusion criteria: adults with COPD with FEV1 30% ‐ 50% of predicted and more than 5% weight loss, mean (SD) % IBW at study entry 78.5% (9.6%). Exclusion criteria: not living in the area of the study, medical problems confounding thier nutritional or lung status, home oxygen therapy, cancer, use of oral corticosteroids, alcoholism, previous lobectomy, dementia, azotemia, CVD, liver disease, nonambulatory, chemotherapy use, interstitial lung disease, pneumonia in the past month, diabetes, malabsorption, use of ONS, and intestinal resection. Number randomised: 9 participants (intervention group, n = 4, control group, n = 5). Gender split: all males. Age: mean (SD) 62.4 (5.6) years. Nutritional status: per cent (SD) loss of usual weight in previous year 8.3 (1.54) %. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of individually planned diet and 1080 kcal of a nutritional supplement. Control: participants received dietary advice alone in the form of individualised diet planned by dietitian to provide 100% of recommended daily intake. |
|
| Outcomes | Survival*, weight*, BMI*, TSF*, MAMC*, MUAC*, energy intake*, measures of pulmonary function (FEV1*), measures of immune function. | |
| Publication details |
Language: English. Funding: grant from the National Institute of Arthritis, Metabolism, Kidney and Digestive Diseases and grant from the National Institutes of Health. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not reported, but must have been an unblinded trial due to the nature of the intervention. However, this is unlike to have influenced clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not reported, but must have been an unblinded trial due to the nature of the intervention. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not reported, but must have been an unblinded trial due to the nature of the intervention. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear as low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses occurred during the study. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes reported not in a format suitable for direct entry into a meta‐analysis. Data in the paper on weight have been used to derive mean change (SD). Information on study quality obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Ganzoni 1994.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 12 months. Location: Germany. |
|
| Participants |
Inclusion criteria: adults with COPD. Exclusion criteria: not reported/translated. Number randomised: 29 participants (intervention group, n = 15, control group, n = 14). 20 participants completed the study, there were 5 deaths (3 in the intervention group and 2 in the control group), 3 participants could not be followed up at 12 months and 1 participant did not have a baseline assessment completed (information provided by author). Gender split: not reported/translated. Age: average age 66 years. Nutritional status: body weight: average 52 kg (range 38 ‐ 68 kg); lung function (FEV1):average 0.81 (range 0.4 ‐ 1.51). |
|
| Interventions |
Intervention: participants (n = 15) received dietary advice plus ONS if required in the form of nutritional counselling to use a high‐calorie diet using a variety of methods including nutritional supplements if required. Control: participants (n = 14) received no dietary advice and no ONS in the form of no individual nutritional counselling. Participants may have attended a group session where diet was discussed. |
|
| Outcomes | Body weight*, 4‐site skinfold measurements (summed), survival*, energy intake*, respiratory function (FEV1* and 6‐minute walking distance). | |
| Publication details |
Language: German. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information obtained from author, randomisation performed using a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Information from the author, a person not involved in the study administered the random allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Information obtained from author confirmed blind assessment of outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Information obtained from author confirmed blind assessment of outcomes. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Information obtained from author confirmed blind assessment of outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessments were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 participants completed the study, there were 5 deaths, 3 in the intervention group and 2 in the control group and an additional 4 participants had missing assessment information. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes reported but not in a format suitable for entry into meta‐analysis. Data on mortality obtained from the author as the detail in the paper was unclear. Data on mean change for weight are reported without a SD and therefore the original data have been obtained from the authors. Data on energy intake are reported as mean and range with no SD at baseline and end of follow‐up. Data requested from authors but no detail available. |
| Other bias | Unclear risk | Baseline variables not given, and not known if groups similar at baseline. |
Gonzalez‐Espinoza 2005.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 6 months. Location: single centre in Mexico. |
|
| Participants |
Inclusion criteria: adults receiving continuous peritoneal dialysis for at least 1 month. Exclusion criteria: peritonitis within 6 weeks of the study, allergy to egg albumin, decompensated heart failure, nephrotic syndrome, liver disease, malabsorption, cancer or AIDS. Number randomised: 30 participants. 28 in final study group (intervention group n = 13; control group n = 15), as 2 participants not included in the intervention group because of a deterioration in health. Gender split: 19 males and 9 females. Age: mean (SD) intervention group 45.7 (14.4) years; control group 47.6 (17.4) years. Nutritional status: all participants malnourished according to SGA. |
|
| Interventions |
Intervention group: participants received dietary advice and ONS in the form of nutritional counselling plus a dried egg‐albumin‐based supplement added to milk or sprinkled on food. Control group: participants received dietary advice alone in the form of nutritional counselling alone. |
|
| Outcomes | Survival, weight*, energy intake* BMI, MAMC*, TSF* hospital admission. | |
| Publication details |
Language: English. Funding: partially funded by a grant from Laboratorios Pisa, SA de CV, Guadalajara, Mexico. Publication status: peer‐reviewed journal. |
|
| Notes | Information on nutritional supplement from www.inovaalimentos.com/#Ultrashock | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. |
| Allocation concealment (selection bias) | Low risk | Performed by a person external to the study once the individual had provided informed consent. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Information from author, outcomes assessed blinded to intervention. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Information from author, outcomes assessed blinded to intervention. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Information from author, outcomes assessed blinded to intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Information from author, outcomes assessed blinded to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 15 participants in the dietary advice and supplements group were not included in the analysis because of deterioration in health. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes reported not in a format suitable for entry into meta‐analysis. Data on change in weight, energy intake, TSF and MAMC are presented as mean (SD) at baseline and at end of intervention. Data on mean change (SD) from baseline were obtained from the authors for weight, energy and MAMC. SDs for change in TSF were imputed. Data on hospital admissions obtained from the authors. |
| Other bias | Low risk | Baseline characteristics reported, groups similar at baseline. |
Gray‐Donald 1995.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 12 weeks. Location: Canada. |
|
| Participants |
Inclusion criteria: elderly people living at home at nutritional risk (defined as the presence of 2 of the following conditions) (i) involuntary weight loss of over 5% in last month, over 7.5% in last 3 months, over 10% in last 6 months and (ii) BMI < 27 or BMI < 24. Exclusion criteria: receiving palliative care, alcoholic, cancer, with any illness requiring a therapeutic diet not compatible with nutritional supplementation. Number randomised: 50 participants (intervention group, n = 25; control group, n = 25). 4 deaths, 3 in the intervention group and 1 in the control group. Gender split: intervention group 26% males; control group 33% males. Age: mean 78 years. Nutritional status: BMI mean (SD), intervention group 19 (3) kg/m²; control group 19 (3) kg/m². |
|
| Interventions |
Intervention (group 1): particpants received ONS in the form of weekly visits from a dietitian and 2 x 235 mL of a nutritional supplement. Intervention (group 2): participants received dietary advice in the form of weekly visits from a dietitian with dietary counselling. |
|
| Outcomes | Survival*, body weight*, MAMC*, MUAC skinfold (triceps*, subscapular, suprailliac), energy intake*, handgrip strength*, perception of health, general well‐being score, number of falls. | |
| Publication details |
Language: English. Funding: National Health Research & Development Program, Health Canada. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "separate randomization lists were prepared for each group by a person outside the study". Insufficient information on how sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Information from author indicates that sealed envelopes were used. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Clinical outcomes assessed blinded to group allocation. Quote: "the other research dietitian, who was unaware of the subject's treatment, completed measurements of functional and health status outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Functional outcomes assessed blinded to group allocation. Quote: "the other research dietitian, who was unaware of the subject's treatment, completed measurements of functional and health status outcomes. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Quote: "one research dietitian was responsible for recruitment, collection of baseline data and follow‐up nutritional data". Assessment not blinded to group allocation and might have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Some outcomes collected without blinding of group allocation and lack of blinding might have affected the assessment of these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 deaths, 3/25 (12%) in the supplement group and 1/25 (4%) in the dietary counselling group. Fully reported with reasons and similar between groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes specified in the methods reported and data on mortality, change in weight, TSF, MAMC were extracted from the paper. Data on energy intake are presented as mean change in daily intake averaged over 3 months, therefore mean change (SD) from baseline to 3 months has been obtained from the authors. Data on grip strength are presented as a mean (SD) at baseline and at end of intervention, therefore mean change (SD) obtained from the author. |
| Other bias | Unclear risk | Baseline variables given, participants reporting a good appetite was significantly better in advice group than supplement group. |
Gu 2015.
| Study characteristics | ||
| Methods | Quasi‐RCT. Parallel design with 2 treatment arms. Duration: length of hospitalisation. Location: China. |
|
| Participants |
Inclusion criteria: aged 18 ‐ 80 years, able to give consent, hospitalised for longer than 5 days. Exclusion criteria: severe liver and kidney disease, expected life expectancy of > 1 month, receiving enteral or parenteral nutrition. Diagnosis: mixed clinical backgrounds. Number randomised: 148 participants (intervention group, n = 73; control group, n = 75); no attrition reported. Gender split: 92 (62%) males, 56 (38%) females. Age: mean (SD) total cohort 63.6 (13.7) years; intervention group 64.6 (1.5) years; control group 62.7 (1.6) years. Nutritional status: all at nutritional risk assessed by NRS‐2002. |
|
| Interventions |
Intervention group: participants received dietary advice in the form of regular care and treatment plus individualised nutritional support*. Control group: participants received no dietary advice in the form of regular care and treatment. *Requirements assessed using Harris Benedict formula, intake below 75% of requirements for energy and protein resulted in 3 different dietary interventions to promote increased food intake (education, tailoring of food provision to meet individual preferences and provision of snacks). |
|
| Outcomes | Energy and protein intake (total intake and % achieving requirements), weight, complications (infectious and severe), length of stay, cost (hospital expenses). | |
| Publication details |
Language: Chinese. Funding: The State's scientific advancement program (Wenzhou State Science & Technology Programme fund (Y20120030). Publication status: peer‐reviewed journal. |
|
| Notes | Translation completed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients at nutritional risk on hospital admission were assigned a number based on time of admission. Patients with odd numbers were placed in the control group; patients with even numbers were placed in the intervention group." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a judgement. Quote: "the researchers/risk assessors were blinded and had no knowledge of the arrangement of patient's numbers" |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blinding of study personnel implied. Quote: "the researchers/risk assessors were blinded and had no knowledge of the arrangement of patient's numbers". This outcome is unlikely to be affected by blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Functional outcomes not measured. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Food intake assessed by dietary recall and blinding of study personnel implied. Judged to be unclear, as awareness of group allocation and study aims might influence how the participants recall intake. Quote: "the researchers/risk assessors were blinded and had no knowledge of the arrangement of patient's numbers". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of personnel implied but because this is a dietary intervention it is not possible to blind participants. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment. Quote: "the researchers/risk assessors were blinded and had no knowledge of the arrangement of patient's numbers". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition reported, therefore insufficient information to make a judgement. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes discussed in the methods section are fully reported in the results. |
| Other bias | Low risk | Baseline characteristics compared and groups similar for all elements with the exception of gender. More males than females included but this is unlikely to affect the outcomes assessed. |
Hampson 2003.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 12 months. Location: UK. |
|
| Participants |
Inclusion criteria: adult women with osteoporosis at the femoral neck and or total hip and a low BMI (≤ 21 kg/m2). Exclusion criteria: evidence of any progressive wasting disease, severe renal impairment, cardiorespiratory disease, endocrine disease, drug herapy known to interfere with bone metabolism. Number randomised: 71 participants (intervention group, n = 36; control group, n = 35). 6 participants withdrew from the study (5 in the intervention group and 1 in control group). Gender split: all female. Age: mean (SD) intervention group 76 (4.2) years; control group 76.7 (5.7) years. Nutritional status: BMI mean (SD), intervention group 19.9 (1.9) kg/m²; control group 20.7 (1.8) kg/m². |
|
| Interventions |
Intervention group: participants received dietary advice and ONS in the form of dietary advice to increase intake and 2x 200mL supplement (Nutricia) plus 1 g calcium and 800 units cholecalciferol. Control group: participants received no dietary advice and no ONS in the form of no dietary advice plus 1 g calcium and 800 units cholecalciferol. |
|
| Outcomes | Survival*, weight*, bone mineral density, fat mass, lean mass, energy intake*. | |
| Publication details |
Language: English. Funding: the medical charity Action Research. Nutricia Clinical Care, Nutricia Ltd and Shire Pharmaceuticals Ltd provided the nutritional and calcium/vitamin D supplements. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data on outcomes requested from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generated in a department external to the study (Department of Public Health). |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not stated, but we assume the trial was unblinded given the nature of the intervention. However, this is unlikely to influence clinical outcomes |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not stated, but we assume the trial was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not stated, but we assume the trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/71 (8%) participants did not complete the study; 5/36 (14%) in the dietary advice and supplement group and 2/35 (6%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes reported not in a format suitable for entry into meta‐analysis. Data on energy intake reported as mean (SD) for groups at baseline and end of intervention, therefore mean change data obtained from the authors. Data on weight change reported as % change. Data requested from authors but not received. |
| Other bias | Unclear risk | Baseline variables given, treatment group were significantly lighter and had lower fat mass than the control group. |
Hernandez 2014.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 4 months (intervention and follow‐up). Location: Spain. |
|
| Participants |
Inclusion criteria: over 18 years of age, receiving haemodialysis for more than 6 months, stable haemodynamic condition. Exclusion criteria: transfer to another haemodialysis unit, renal drug therapy that would interfere with plasma metabolite concerns, cognitive impairment or psychiatric disorder, active inflammatory or infectious disease, pregnancy or hospitalisation. Diagnosis: end‐stage kidney failure, undergoing haemodialysis. Number randomised: 120 randomised (intervention group, n = 60; control group, n = 60). Attrition: 33 (28%) participants, 87 participants completed. Gender split: (for the 87 completing the study) intervention group 37 males and 17 females; control group 20 males and 13 females. Age: mean (SD) intervention group 70 (2) years; control group 72 (2) years. Nutritional status: (assessed as % with serum albumin below 3.5 g/dL) intervention group, n = 31 (57%); control group, n = 19 (57%). |
|
| Interventions |
Intervention (intervention group 1): particpants received dietary advice in the form of 12‐session education program (weekly for 2 months and fornightly for the next 2 months ) on a range of nutrition topics inline with National Kidney Foundation guidelines. Control (intervention group 2): particpants received ONS in the form of 470 mL Nepro taking during dialysis or at home if full amount not tolerated. |
|
| Outcomes | Biochemical (large range of serum biochemistry), % malnourished (albumin status), nutrition knowledge. | |
| Publication details |
Language: English. Funding: grant from the Catholic University of Murcia (a member of the private Fresenius Medical Care Clinic). Publication status: peer‐reviewed journal. |
|
| Notes | No data reported on outcomes of interest for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: randomization was performed through a computer‐generated number sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: the number sequence was concealed from the researchers until after baseline assessment. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not described but unlikely that assessment of clinical outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not described but no functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not described and likely that assessment of some nutritional outcomes would be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described and likely that assessment of some outcomes would be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition fully reported. 33 (28%) participants dropped out of the study; 6 (10%) of 60 in the dietary advice group and 27 (45%) of 60 in the ONS group. Significantly more participants withdrew from the ONS group (n = 20 (33%)) compared with the dietary advice group (n = 0) because of unwillingness to continue with the study, and the imbalance might have influenced outcome assessment. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes discussed in the methods section are fully reported in the results. |
| Other bias | Unclear risk | Baseline characteristics compared between groups remaining in the study. Statistically significant differences between total serum protein and creatinine (significantly higher in supplement group). No significant differences for knowledge or serum biochemistry between those who remained in the study and those that dropped out. The influence of these differences on outcomes unclear. |
Holyday 2012.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: follow‐up at the end of admission to acute geriatric medicine ward (at least 72 hours) up to 6 months following discharge. Location: single centre in Australia (acute geriatric medicine wards of the Prince of Wales Hospital, Sydney). |
|
| Participants |
Inclusion critieria: adults admitted to acute geriatric medicine wards by a geriatrician with an expected length of stay of at least 72 hours. Exclusion criteria: expected length of stay less than 72 hours, palliative treatment, not able to be nutritionally assessed, already seen by a dietitian. Number randomised: 143 individuals were screened and randomised. Gender split: 61 males and 82 females. Age: mean (SD) intervention group 83.7 (0.8) years; control group 83.4 (0.9) years. Nutritional status: intervention group MNA well‐nourished n = 12, MNA at risk of malnutrition n = 47, MNA malnourished n = 12; control group MNA well‐nourished n = 12, MNA at risk of malnutrition n = 40, MNA malnourished n = 20. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of individually tailored hospital meals (with texture modification and fortification), nutrition supplements, assistance with meals by ward‐based staff, education of individuals and their carers, referral to other health professionals for discharge planning. Control: participants received no dietary advice and no ONS in the form of usual nutritional care: the dietitian on the ward was not informed on the screening outcome. |
|
| Outcomes | 1 month and 6 months emergency frequency, readmissions, weight change during admission, in‐hospital death. | |
| Publication details |
Language: English. Funding: The Gut Foundation (Randwick, Australia); Pharmatel Fresenius Kabi Pty Ltd provided a unrestricted research grant to support this study. They had no other involvement in the work. Publication status: peer‐reviewed journal. |
|
| Notes | Only the data of the malnourished group were included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from paper: Patients were randomly allocated using a computerised random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Screening and randomisation was performed by the research dietitian. It is unclear whether the dietitian had access to the randomisation list. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | All data were extracted from the hospital charts. The medical personnel was blinded. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | All data were extracted from the hospital charts. The medical personnel was blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not measured. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dietitian and participants were aware of the intervention but this is unlikely to have influenced assessment of outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were extracted from the hospital charts. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was 1 death in the control group and 4 in the intervention group. Weight change was only obtained for 69 (48%) participants and no information was reported to explain why, therefore judged as unclear risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Low risk | All baseline characteristics were similar in both group. The percentage of malnourished patients was 28% in the control group and 17% in the intervention group. |
Huynh 2015.
| Study characteristics | ||
| Methods | Prospective RCT. Parallel design. Duration: 12 weeks. Location: multicentre trial at 9 private and 4 public hospitals across India. |
|
| Participants |
Inclusion criteria: aged ≥ 18 years, males and non‐pregnant, non‐lactating females, admitted within 36 hours to either the medical or the surgical wards, and who were diagnosed with moderate or severe malnutrition based on the modified SGA. Exclusion criteria: active tuberculosis, acute hepatitis, HIV infection, diabetes, dementia, brain metastases, active malignancy, severe renal or liver failure, burn injury,, clinically significant ascites, oedema, eating disorders or psychological condition interfering with nutritional intake, taking progesterone, steroids or growth hormone. Diagnosis groups: neurological disorders, respiratory medicine, cardiovascular medicine, gastrointestinal disorders, genitourinary and haematological, trauma and orthopaedic diseases, various internal medicine (infection, malaria), others. Number randomised: 212 adults. Gender split: intervention group 57% males, control group 55% males. Age: mean (SD) age intervention group 40.9 (19.6) years; control group 39.0 (16.4) years. Nutritional status: all but 4 participants were moderately or severely malnourished according to modified SGA. These 4 were randomised according to ITT principles, and allocated to intervention group (n = 1) or control group (n = 3). |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of 3 sessions of dietary counselling (administered at baseline, weeks 4 and 8) plus 2 servings of oral nutritional supplement per day for 12 weeks. Control: participants received dietary advice alone in the form of 3 sessions of dietary counselling (administered at baseline, weeks 4 and 8). Dietary counselling was provided to both groups by hospital dietitians who were trained in using a standardised methodology. In each dietary counselling session, energy and nutritional requirements were calculated for each group to achieve their individualised energy goals. The dietitians instructed the participants on the methods for improving their nutritional intake using home foods for the control group and home foods in conjunction with oral nutritional supplement for the intervention group. At hospital discharge and follow‐up visits at weeks 4 and 8, participants in the control group were given instructions on consuming small frequent meals and using protein rich foods and a high‐energy food‐source. In addition to being advised on using home foods in meal preparation, participants in the intervention group were instructed to consume the oral nutritional supplement between meals. |
|
| Outcomes |
Primary outcome: change in weight over 12 weeks. Secondary outcomes: change in BMI, modified SGA score, pre‐albumin, albumin, haemoglobin, total protein and C‐reactive protein over 12 weeks, change in dietary intake (energy intake and macronutrient intake) and functionality using handgrip strength. |
|
| Publication details |
Language: English. Funding: Abbott Nutrition provided funding for the study and was responsible for the study design, monitoring, data analysis, manuscript preparation and submission. Publication status: peer‐reviewed journal. |
|
| Notes | Outcomes are reported at 4 weeks, 8 weeks and 12 weeks. For meta‐analysis, only the 12 weeks outcomes were used. The authors were contacted to provide change data for weight, BMI, dietary intake and grip strength |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomised in a 1:1 ratio to the control or oral nutritional supplement group. Sealed envelopes containing the patient group assignment were prepared from randomisation schedules generated by Abbott Nutrition for each site." |
| Allocation concealment (selection bias) | Low risk | Quote: "As eligible patients were enrolled, they were assigned a subject number sequentially starting with the first envelope." |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Unknown whether the study was blinded or not. Nevertheless, this is unlikely to influence biochemical parameters. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk |
Quote: "Anthropometric measurements were performed by study staff trained in standardised methods of conducting the measurements." It is unknown whether these persons were aware of group allocation. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and unclear or high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Screened and randomised n = 212, allocated to each group n = 106, withdrew consent n = 5 (intervention n = 2, control n = 3), not completed n = 54 (intervention n = 30, control n = 24) ‐ most important reasons: lost to follow‐up, withdrew consent. No differences between groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol identified; all relevant outcomes reported. |
| Other bias | Unclear risk | Baseline characteristics comparable between the two groups, except for weight (mean (SD)) intervention group 46 (9.6) kg, control 48.5 (10.5) kg). |
Imes 1988.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 6 months. Location: Canada. |
|
| Participants |
Inclusion criteria: adults with Crohn's disease. Exclusion criteria: pregnancy, receiving parenteral nutrition, living too far from the hospital and age under 18 years or over 70 years. Number randomised: 137 participants (intervention group, n = 67; control group, n = 70). Gender split: 62 males and 75 females. Age: range, 17.5 ‐ 71.0 years. Disease status: CDAI, mean (SD) (range): 110 (96) (0 ‐ 463). Concomitant medication: prednisolone n = 42%, salazopyrin n = 45% or vitamin supplements n = 50% and with active and inactive disease included. Nutritional status: mean (SD) % ideal body weight, intervention group 103 (20); control group 105 (17). |
|
| Interventions |
Intervention: participants received dietary advice in the form of monthly dietary counselling sessions aiming to achieve the 'Canadian Recommended Dietary Allowances'. Control: participants received no dietary advice in the form of no dietary intervention. |
|
| Outcomes | Energy* and protein intake, vitamin and mineral intake, assessments of clinical condition, survival*, MAMC*, MUAC*, TSF*, hospital admissions*. | |
| Publication details |
Language: English. Funding: MSI Foundation and Alberta Heritage Foundation for Medical Research. Publication status: peer‐reviewed journal. |
|
| Notes | Additional outcomes and longer follow‐up reported in separate papers. Additional data and information on quality obtained from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author cannot recall how the sequence was generated therefore insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blinded assessment of clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional assessments made. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Assessment of nutritional outcomes not blinded and lack of blinding likely to influence assessment of some outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The outcome assessors were not blinded for assessment of nutritional status and lack of blinding might influence the outcome of the assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information obtained from the author. 137 participants randomised, 125 completed 6 months of study, 8/67 (12%) drop‐outs in the dietary advice group and 4/70 (6%) in the no dietary advice group. There were no deaths. Reasons for drop‐outs not reported therefore judged to be unclear. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. Data on mortality and hospital admissions could not be extracted from the papers and have been obtained from author. Additional outcomes and longer follow‐up reported in separate papers. |
| Other bias | Unclear risk | Baseline variables given, advice group were younger and had a lower CDAI that the no advice group which might have influenced the outcomes. |
Isenring 2004.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 12 weeks. Location: Australia. |
|
| Participants |
Inclusion criteria: adults receiving radiotherapy for cancers of head and neck (88%) or abdomen (12%). Exclusion criteria: under 18 years, hospitalised for 5 days or more, receiving enteral or parenteral nutrition. Number randomised: 60 participants (intervention group, n = 29; control group, n = 31). Attrition: 6 participants were lost to follow‐up (4 from the intervention group). 5 participants from the control group requested referral to a dietitian. Gender split: 51 males and 9 females. Age: mean (SD) 61.9 (14) years. Nutritional status: at baseline 65% of participants were well‐nourished and 35% malnourished (PG‐SGA). |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of individualised intensive nutritional counselling and nutritional supplements if appropriate. Control: participants received no dietary advice and no ONS in the form of a standard nutrition booklet and participants could request referral to a dietitian. |
|
| Outcomes | Survival*, weight*, grip strength*, fat‐free mass (BIA), QoL, change in PG‐SGA score, energy intake*. | |
| Publication details |
Language: English. Funding: Wesley Research Institute. Abbot Australia and Mead Johnson supplied the product. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details provided by the author, a random number table. |
| Allocation concealment (selection bias) | Low risk | Details provided by the author, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was assumed to be unblinded. However, this is unlikely to influence clinical outcomes |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was assumed to be unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was assumed to be unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because unclear risk for clinical, functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants were lost to follow‐up, details not given in the paper but provided by the author on request. 4 deaths (2 in the intervention group and 2 in the control group) and 2 others lost to follow‐up in the intervention group (1 as a result of deterioration in condition and 1 because participant discontinued treatment and withdrew from the study). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes reported in text and figures but not in a format usable for meta‐analysis. Data on mortality and mean change (SD) for weight and energy intake obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Jahnavi 2010.
| Study characteristics | ||
| Methods | RCT (randomised 1:1) Parallel design with 2 treatment arms. Duration: 3 months Location: multicentre, 30 community‐based government‐sponsored childcare centres in 16 villages in Andra Pradesh, India. |
|
| Participants |
Inclusion criteria: male and female, aged 18 ‐ 65 years, evidence of active tuberculosis, evidence of wasting (BMI < 20 kg/m2), started on Directly Observed Treatment Short Course (DOTS) within the past 2 weeks. Exclusion criteria: diabetes, positive human‐immunodeficiency antibody test or other severe underlying diseases. Number randomised: 100 participants. Gender split: intervention group 36 males, 14 females; control group 38 males, 12 females. Age: mean (SD) intervention group 41 (14.2) years; control group 39.5 (14.3) years. Nutritional status: BMI mean (SD), intervention group 17.1 (2.8) kg/m²; control group 17.9 (2.1) kg/m². |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of target intake calculated as 35kcal/kg/day at baseline, importance of meeting the target was explained to the patient. After 24 hour recall, advice was provided to meet this requirement + dietary plan + supplements (i.e. sweet balls every day ‐ 600kcal). Sweet balls had to be consumed in the presence of Anganwadi workers. Control: participants received no dietary advice and no ONS in the form of no dietary plan and no supplements, given general advice and instructed to increase food intake. |
|
| Outcomes | Weight*, quality of ife (SF‐36), functional status (handgrip)*; timed sit to stand test; bacterial conversion rate. | |
| Publication details |
Language: English. Funding: Padova University, Italy. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: The randomisation was 1:1 for the two groups and was performed by randomly shuffling the envelopes that contained the study codes. |
| Allocation concealment (selection bias) | Low risk | Study codes were concealed in envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | No blinding described however clinical outcome (sero‐conversion) unlikely to be affected by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No blinding described but functional outcome could be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | No blinding described but nutritional outcome could be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described and assessment of functional and nutritional outcomes could be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/100 (2 %) died (intervention group n = 0 (0%); control group n = 2 (4 %)). |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes mentioned in the methods are reported. |
| Other bias | Low risk | Baseline data reported and no differences between groups. |
Jensen 1997.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 110 days. Location: Denmark. |
|
| Participants |
Inclusion criteria: adults < 75 years. Exclusion criteria: not stated but individuals with diabetes and disseminated cancer were excluded. Diagnosis: post‐surgery for operable colon/rectum cancer n = 50, diverticulitis n = 15, ulcer n = 5 and other n = 17. Number randomised: 87 participants (intervention group, n = 40; control group, n = 47). Attrition: 28 dropouts (20 intervention group and 8 in control group). Gender split: 42 males and 45 females. Age: elective surgery intervention group 60 (12) years, control group 53 (14) years; acute surgery intervention group 53 (12) years, control group 74 (7) years. Nutritional status: unclear. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of dietary counselling to improve nutritional intake and aiming for a protein intake of 1.5 g/kg using oral nutritional supplements if required. Control: participants received no dietary advice and no ONS in the form of no nutritional advice. |
|
| Outcomes | Weight *, body composition (DEXA), energy intake*, appetite, fatigue assessments, handgrip strength*, work capacity, respiratory function*, QoL (not global). | |
| Publication details |
Language: English. Funding: the Health Insurance Foundation, the Danish Cancer Society, Onkologisk Forskningsfond and a European Society of Parenteral and Enteral Nutrition Fellowship donated by Abbott. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data awaited from authors. QoL could not be included because only subscores were available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of sequence generation reported. Randomisation was stratified. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded. However, this is unlikely to influence clinical outcomes. The paper states that the surgeon was blinded to intervention group. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 28 dropouts (20 (50%) dietary advice group and 8 (17%) in no advice group). Numbers of withdrawals not balanced between groups and reasons for withdrawals not given in paper and not provided by authors on request. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes not in a format usable for meta analysis, energy intake data presented as kcal/kg and weight change data described in text as mean at baseline and end of follow‐up. Additional data on mean change (SD) for weight and energy intake requested from authors but not provided. |
| Other bias | Unclear risk | Baseline variables given, control group (no advice) were significantly older and heavier than the treatment (advice plus supplements if required) group. |
Kalnins 2005.
| Study characteristics | ||
| Methods | Quasi‐RCT. Parallel design with 2 treatment arms. Duration: 6 months (intervention for 3 months and follow‐up to 6 months). Location: Canada. |
|
| Participants |
Inclusion criteria: adults and children with CF, under 90% weight for height or 5% reduction in weight for height over 3 months. Exclusion criteria: CF‐related diabetes, with a gastrostomy tube CF‐associated liver disease, FEV1 < 30%, oxygen dependence, already receiving routine ONS. Number randomised: 13 participants overall but mixed population of children and adults. Data obtained from authors on participants > 16 years of age (intervention group n = 2, control group n = 3). Attrition: No dropouts from the 5 adults. Gender split: not reported for the 5 adults included. Age: mean (SD) 27.4 (8.4) years. Nutritional status: not reported for the 5 adults included. |
|
| Interventions |
Intervention (intervention group 1): participants received dietary advice in the form of dietary counselling to increase food intake by 20% of predicted requirements. Intervention (intervention group 2): particpants received ONS in the form of a nutritional supplement to increase energy intake by 20% of predicted requirements. |
|
| Outcomes | Survival*, z scores for weight* and height, weight for height, pulmonary function*, energy* intake, REE, faecal balance studies. | |
| Publication details |
Language: English. Funding: Mead Johnson Canada. Publication status: peer‐reviewed journal. |
|
| Notes | Small trial of supplementation in a mixed population of children and adults with CF. Data obtained from the author on results for adults only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised using cards with advice or supplement written on them. The participant selected a card blind. Then the next participant randomised received the other intervention group. |
| Allocation concealment (selection bias) | High risk | Investigators used alternate allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | No blind assessment but unlikely that assessment of clinical outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | No blind assessment and likely that assessment of some functional outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | No blind assessment and likely that some nutritional outcomes would be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes were not assessed blinded to group allocation and it is likely that assessment of some outcomes would be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported 2 dropouts, 1 in each group. Study was of mixed ages, information obtained from authors indicated that dropouts were children and not adults, therefore no dropouts amongst the 5 adults included in this review. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. This paper reports outcomes for adults and children combined. Details of mean change (SD) weight and energy intake for the 5 adults have been obtained from the authors. |
| Other bias | Unclear risk | Baseline variables not given, unsure if groups similar at baseline. |
Kapoor 2017.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 6 months. Location: single centre in India. |
|
| Participants |
Inclusion criteria: adult women with cancer attending palliative clinics, with symptoms of cachexia, weight loss of more than 5% from pre‐treatment weight, BMI < 20 kg/m² along with haemoglobin level < 12 g/dL, and energy intake of < 1500 kcal/d. Exclusion criteria: GI tract disorders, on anabolic steroids, taking synthetic oral nutritional supplements, life expectancy less than 3 months. Number randomised: 123 adult women with advanced cancer were screened for eligibility. 63 participants randomised (intervention group n = 30; control group n = 33). Attrition: 39 participants lost to follow‐up (intervention group n = 13; control group n = 18). Gender split: all female Age: mean (SD) intervention 44 (13.2) years; control 47.8 (14.7) years. Nutritional status: not reported. |
|
| Interventions |
Intervention group: participants received dietary advice and ONS in the form of 30 minutes nutritional counselling* per fortnightly visit by a qualified nutritionist plus 100 g of IAtta (by RG)**, to be consumed in addition to their daily dietary intake. Control group: participants received dietary advice alone in the form of 30 minutes nutritional counselling* per fortnightly visit by a qualified nutritionist. *Participants were advised to increase the frequency of homemade meals, and the consumption of energy‐ and protein‐dense food products was encouraged during these sessions. Depending on the physical status of the participants, low levels of physical activity (walking and/or stairs) and participation in household activities was encouraged. **Each 100 g pack of IAtta contained a mixture of roasted bengal gram flour, roasted barley flour, roasted soybean flour, flaxseed powder, and dried Amaranthus spinosus powder. Each pack was labelled with use by date and batch number. The caregiver was advised to make unleavened flat breads (chapatis) by adding spices from the dispensed IAtta pack and to discard leftover supplement at the end of the day. On average 3 flat breads could be prepared from each pack, which provides approximately 400 kcal. Each 400 kcal consists of 50% daily protein requirement, 75% daily fat requirement, and 30% to 50% of iron, calcium, and vitamin A as part of an Indian sedentary woman’s recommended dietary allowance. |
|
| Outcomes | Anthropometric measures (body weight, MUAC, sum of four skin folds), nutritional status (PG‐SGA), dietary intake (one‐on‐one interview sessions between the nutritionist and the participant, 2‐day 24‐hour dietary recall data and the Indian Migrant Study Food Frequency Questionnaire for portion size estimation, daily energy, carbohydrate, protein, and fat intake), physical activity levels (Indian Migrant Study Physical Activity Questionnaire, metabolic equivalent unit), QoL (EORTC‐QLQ‐C30). Adherence to dietary supplement was recorded. Anthropometric measurements, dietary intake, physical activity level and quality of life parameters were assessed at baseline, after 3 months, and at the end of 6 months. |
|
| Publication details |
Language: English Funding: the study was self‐funded Publication status: peer‐reviewed journal |
|
| Notes | The author provided change scores for body weight, MUAC, energy intake and protein intake for inclusion in this review. Energy intake and protein were measured in two different ways, by dietary recall and by FFQ. We used the data collected by dietary recall. The clinical trial record seems to be for this study, although the full text by Kapoor indicates that the clinical trial number is NCT 02350855, so this does not match. I think the trial number listed in the manuscript might be an error. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Eligible patients were asked for consent and enrolled in the study. They were allocated study codes, and a randomization sheet was generated by using nQuery software (7.0 version) by RG |
| Allocation concealment (selection bias) | Unclear risk | Quote: Eligible patients were asked for consent and enrolled in the study. They were allocated study codes, and a randomization sheet was generated by using nQuery software (7.0 version) by RG. Unclear whether the person recruiting the participants could foresee which group the next participant would be in. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk |
Quote: The patients in control groups were unaware of the IAtta intervention in the other group. Although the study was not a blinded study, it is unlike that the outcome was influenced by lack of blinding, as participants were unaware of the interventions in the other group |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | NK performed recruitment and measurements, but was aware of group assignment. Functional outcomes could have been influenced by lack of blinding |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | NK performed recruitment and measurements, but was aware of group assignment. Nutritional outcomes could have been influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 63 participants were included; 13 of 30 (43%) in the intervention group and 18 of 33 (55%) in the control group were lost to follow‐up (intervention group n = 13, control group n = 18) because of travelling difficulties, financial causes, being bedridden and death. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol identified; some relevant data not reported i.e. mid arm circumference and skinfold thickness measurements |
| Other bias | Low risk | There were differences in baseline characteristics for body fat, energy intake, protein intake, PG‐SGA score and several QoL scores. Baseline parameters ‐ body weight, body fat, MUAC, energy intake, physical activity level, global health quality of life, and fatigue domain ‐ were adjusted to observe the overall difference between the groups using a generalized estimating equation. |
Kendell 1982.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 6 weeks. Location: single centre in the USA. |
|
| Participants |
Inclusion criteria: adults awaiting elective orthognathic surgery. Exclusion criteria: not specified. Number randomised: 24 participants (intervention group n = 12; control group n = 12). Attrition: 100% follow‐up. Gender split: 5 males and 19 females. Age: mean (SD) 25 (8.1) years. Nutritional status: 12 out of 24 participants had a weight below IBW at inclusion. |
|
| Interventions |
Intervention group: participants received dietary advice and ONS in the form of dietary instruction and an oral nutritional supplement (1.5 kcal/mL) to provide 50% of calculated energy requirements. Control group: participants received dietary advice alone in the form of dietary instruction given verbally and in writing. |
|
| Outcomes | Survival*, body weight*, MAUC, MAMC*, TSF*, serum chemistry and creatinine height index, macro and micronutrient intake, length of hospital stay. | |
| Publication details |
Language: English Funding: Publication status: peer‐reviewed journal |
|
| Notes | Data not available from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | High risk | All outcomes reported but using general statements e.g. 'at each time interval, there were no statistically significant differences in body weight, MAC, TSF and creatinine height index between the experimental and control groups'. Data presented in table as % deficit and data not available from the authors, therefore risk of bias due to partial reporting. |
| Other bias | Unclear risk | Baseline variables not given, no information available from authors, not sure if groups similar at baseline. |
Kiss 2016.
| Study characteristics | ||
| Methods | Pilot RCT. Parallel design with 2 treatment arms. Duration: 3 months following completion of radiation therapy. Location: Australia. |
|
| Participants |
Inclusion criteria: adults undergoing radical (chemo) radiation therapy for a primary diagnosis of non‐small cell lung cancer or small cell lung cancer. Exclusion criteria: palliative intent radiation therapy, induction chemotherapy (with the exception of people with small cell lung cancer in which this is standard care), small peripheral tumours or no mediastinal disease, hyperfractionated radiation therapy, non‐English speaking, or cognitive impairment or psychiatric illness. Number randomised: 24 participants. Attrition: overall attrition was 37%. Gender split: 12 males and 12 females. Age: mean (SD) intervention group 62.6 (12.8) years; control group 64.3 (12.0) years. Nutritional status: (PG‐SGA) intervention group well‐nourished n = 7, moderately malnourished n = 5; control group well‐nourished n = 6, moderately malnourished n = 6. BMI, mean (SD): 27.8 (7.7). |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of intensive, individualized dietary counselling tailored to manage participants' specific symptoms and accommodate food preferences and social circumstances so as to meet nutritional requirements and maintain nutritional status. Dietary counselling was provided 1 week prior to radiation therapy, weekly during radiation therapy, and fortnightly for 6 weeks post‐therapy. The intervention was delivered by three dietitians, in person during treatment and over the phone during pre‐treatment and post‐treatment. Control: participants received no dietary advice and no ONS in the form of standard care of the centre at that time, consisting of assessment and review by a dietitian fortnightly during radiation therapy and at 4 weeks post therapy including symptom assessments, weight changes and dietary intake, and provision of dietary counselling. However, this differed from the intervention group in that they were not structured or defined. |
|
| Outcomes | PG‐SGA score at 4 weeks post‐radiation therapy, weight, fat‐free mass, fatigue, functional status, and global QoL. Outcomes measured at week 1 and end of radiotherapy and 4 weeks and 3 months following completion of radiation therapy. |
|
| Publication details |
Language: English. Funding: PhD scholarship from the Victorian Cancer Agency. Publication status: peer‐reviewed journal. |
|
| Notes | Not all participants were malnourished according to the SGA. Additional data on weight change was provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not measured. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Outcome measures were determined by the research dietitian who was not blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Outcome measures were determined by the research dietitian who was not blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome measures were determined by the research dietitian who was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 37%, equally distributed between groups, mostly due to disease progression and inability to contact the participant. |
| Selective reporting (reporting bias) | Low risk | Study protocol identified ANZCTR ACTRN12612000180819 and all planned outcomes were reported. |
| Other bias | Low risk | Baseline characteristics similar between groups. |
Kunvik 2018.
| Study characteristics | ||
| Methods | RCT. Duration: 6 months (data collection at baseline and 6 months). Location: Finland. |
|
| Participants |
Inclusion criteria: caregivers, age > 65 years, living at home, normal cognition (MMSE < 25 geriatric assessment). Exclusion critieria: non‐specified. Number randomised: 79 (intervention group, n = 28, control group, n = 27). Attrition: 10/79 (18%) intervention group n = 6, control group n = 4 (data reported on 55 participants with protein intake < 1.2 g/kg BW/d. 24 participants therefore not included in this analysis. Gender split: 45.5% male. Age: mean (SD) years 73.5 (6.0) years. Nutritional status: assessed using MNA, 85.5% good nutritional status (> 23.5, 12.7% at risk (MNA 17 ‐ 23.5), 1.8% malnourished (< 17 points). |
|
| Interventions |
Intervention: participants received dietary advice in the form of a nutritional care plan from nutritionist plus group discussion and cooking classes( (provided by the nutritionist) plus booklet. Control: participants received no dietary advice in the form of a booklet plus usual care community care as needed. |
|
| Outcomes | Energy and protein intake, BMI, usefulness and satisfaction with intervention. | |
| Publication details |
Language: English. Funding: National Institute for Health and Welfare (THL) Finland. Publication status: unpublished (but submitted manuscript). |
|
| Notes | Email to authors to confirm study numbers and request data for change in protein intake in g and change in BMI per group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate randomisation. Quote: "a computer‐generated, blocked randomization list". |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment. Quote: "a person unrelated to the investigation and unfamiliar with the procedure performed the randomisation" "a person unrelated to the investigation and unfamiliar with the procedure performed the randomisation". |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Blinding not described but no clinical outcomes assessed. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Blinding not described but no functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Blinding not described. BMI and nutritional intake assessed. Judged to be high risk as nutritional intake assessment might be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described and likely that assessment of some outcomes would be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 out of 79 (intervention group n = 6 out of 35 (17%); control group n = 4 out of 35 (11%) participants withdrew. Reasons reported (for 9 participants the caring role ended and 1 other reasons). Data reported only on 55 participants with protein intake below 1.2 g/kg body weight per day. Judged to be unclear because the data from the 24 participants with protein intake over 1.2 g/kg body weight per day not reported and so not possible to judge the impact of this decision. |
| Selective reporting (reporting bias) | High risk | Protocol identified and indicates 4 aims, including data collection on QoL, well‐being, coping and effect of nutritional status of caregivers on those receiving care. This manuscript reports nutritional intake and BMI of caregivers only and judged to be evidence of selective reporting or reporting of findings in multiple manuscripts. |
| Other bias | Low risk | Baseline characteristics compared and similar for all elements. |
Le Cornu 2000.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: variable (from pre‐transplant assessment to liver transplant). Location: single centre in the UK. |
|
| Participants |
Inclusion criteria: adults with end‐stage liver disease accepted for orthotopic liver transplantation, MAMC below 25th percentile. Exclusion criteria: MAMC greater than 25th percentile, fulminant or subacute hepatic failure or malignant disease, requiring acute transplantation, fluid restriction < 500 mL/day, re‐grafts, multiple organ grafts or celiac disease. Number randomised: 82 participants (intervention group, n = 42; control group, n = 40). Gender split: 83% males, 27% females. Age: range 24 ‐ 68 years. Nutritional status: median (range) MAMC (cm) intervention 22.15 (17.6 ‐ 26.2); control 23.2 (17.2 ‐ 26.8). |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of a supplement of a calorie‐dense enteral feed taken daily (in addition to standard dietary advice) until transplantation. Control: participants received dietary advice alone in the form of standard dietetic advice. Standard dietary advice consisted of a dietary recall after which patients were advised on how to adapt their usual dietary intake to increase energy intake and achieve or maintain a moderate protein intake. |
|
| Outcomes | Nutritional status (upper arm anthropometric measurements and handgrip strength), dietary intake was calculated from 5‐day food diaries. Post‐transplant: 30‐day mortality, 6‐month mortality, length of stay in ICU, time spent on ventilator, septic complications, major non‐infectious complications, frequency and severity of rejections. |
|
| Publication details |
Language: English. Funding: no funding, products were supplied by Nutricia. Publication status: peer‐reviewed journal. |
|
| Notes | Authors report that, for the supplemented group, there was a statistically significant increase in MAC, grip strength and MAMC between trial entry and the appointment nearest transplant or death. For the control group, there was also a statistically significant improvement in MAC and MAMC, but no change in grip strength. We emailed the authors to ask for the data, but they replied that they do not have the data anymore. Dietary intake was only measured in a few 10 randomly selected (5 in each group) participants. Dietary intake increased from 1840 kcal at entry to the trial to 2395 kcal at transplant in 5 randomly selected participants in the supplemented group and from 2473 tot 2718 in 5 participants in the control group. Data on dietary intake not entered in the review due to small numbers. Authors report pre‐transplant mortality, post‐transplant mortality, and total mortality. As the primary goal of the study was to study the effects of nutritional support on mortality, we report combined mortality. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by using sealed envelopes selected by a person other than the trial coordinator. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded, but is unlikely that this will have influenced primary endpoints such as mortality. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded, functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded, nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mortality (primary outcome) was n = 7 (17.5%) in the control group and n = 2 (5%) in the intervention group. Reasons for death are clearly described. |
| Selective reporting (reporting bias) | High risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Unable to interpret outcomes, as results were presented as global description. Quote: "when all diagnoses were considered together, most of the biochemical parameters measured at the appointment before transplantation or death were similar for the two groups" (except for serum phosphate). Outcome data are missing; although described as improvement this can not be verified as no data are given in the manuscript. |
| Other bias | Low risk | Similar baseline characteristics at inclusion in the study. |
Locher 2013.
| Study characteristics | ||
| Methods | RCT (stratified by gender and BMI). Duration: 60 days. Location: USA. |
|
| Participants |
Inclusion: older adults receiving Medicare home health services, > 65 years, homebound, able to communicate, living in a private residence, experiencing either an acute or chronic illness, undereating. Exclusion: cognitive impairment (< 8/10 Short Portable MSQ), terminal illness, cancer diagnosis within past 5 years, end‐stage renal disease, tube feed, dependence on ventilator. Diagnosis: acute or chronic illness. Number randomised: 40 participants, but 34 included in analyses (intervention group n = 18, control group n = 16). Attrition: 6/40 but reasons not described. Gender split: 6/34 (18%) male, 28/34 (82%) female. Age: mean (SD) years 81.4 (8.2). Nutritional status: 15.2% BMI < 18.5. |
|
| Interventions |
Intervention: participants received dietary advice in the form of B‐NICE (behavioural nutrition intervention), self management education approaches to guide participants and carers to improve caloric intake. Control: participants received no dietary advice in the form of standard care. |
|
| Outcomes | Body weight, caloric intake. | |
| Publication details |
Language: English. Funding: National Institutes of Health/National Institute on Aging. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "randomly assigned…using stratified blocked randomisation". Judgement, insufficient information on method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | No clinical outcomes assessed. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Quote: "research interviewers collecting outcomes data were blinded to group assignment". Absence of performance blinding might have influenced assessment of nutritional intake. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was a social behavioural intervention therefore not possible to blind participants or study personnel to group assignment." Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Knowledge of group allocation might have influenced collection of food intake data, therefore some outcome assessment might have been influenced by absence of performance bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "40 participants randomised and 34 included in the analyses". Group allocation and reasons for attrition not described therefore insufficient information to make a judgement. |
| Selective reporting (reporting bias) | Unclear risk | Published protocol identified. Planned outcomes, energy intake and weight at 60 days and 6 months, and fidelity outcomes. Only data at 60 days reported and fidelity outcomes not reported. Outcome data are reported without SDs, therefore data requested from authors. |
| Other bias | Unclear risk | Baseline characteristics not presented but in the text "the randomisation schedule was successful in balancing for both gender and BMI". Judged as unclear because insufficient information on all characteristics likely to influence differences in outcomes. |
Lovik 1996.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 6 weeks. Location: Norway. |
|
| Participants |
Inclusion criteria: adults who had received radiotherapy for cancers of head and neck. Exclusion criteria: serious liver or kidney disease, other cancers, diseases presumed to affect nutritional status, poor ability to cooperate or poor general condition. Number randomised: 52 participants (intervention group, n = 28; control group, n = 24). Attrition: 3 deaths (group not reported, analysed 49 participants). Gender split: 40 males, 9 females. Age: range 34 ‐ 86 years. Nutritional status: at study entry unclear, 10% reported weight loss and BMI ranged from 18 ‐ 37kg/m². |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of intensive dietary instruction from a dietitian including advice to use nutritional supplements if required. Control: participants received no dietary advice and no ONS in the form of a standard information sheet "for patients receiving radiation therapy for cancer" providing information on all aspects of treatment and including advice to eat a nutritious diet. |
|
| Outcomes | Body weight*, BMI, TSF, MAMC, MUAC, energy intake*, survival*, serum chemistry, albumin and transferrin. | |
| Publication details |
Language: Norwegian. Funding: Meddinova's Research Fund. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information on quality obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details from author, sequence generation using a random number list. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was assumed to be unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was assumed to be unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was assumed to be unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 deaths, group not reported. All participants were analysed. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Data on change in weight extracted from the paper, but clarification needed for mortality data. Data on TSF, MAMC presented as number of participants with values below 85% of the normal limit and so not included. Data on energy intake is expressed according to expected intake, therefore not usable. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Macia 1991a.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: not reported. Location: Spain. |
|
| Participants |
Inclusion criteria: adults receiving radiotherapy for cancers of head and neck, breast and abdominopelvic area. Exclusion criteria: Karnofsky score < 50, previous diet therapy for diabetes, hypercholesterolemia or other conditions. Number randomised: 92 participants (intervention group, n = 30; control group, n = 62). Numbers of withdrawals and deaths not reported. Gender split: not reported. Age: not reported. Nutritional status: unclear. |
|
| Interventions |
Intervention: participants received dietary advice in the form of dietary instructions on appropriate alimentation during radiotherapy given verbally and in writing. Control: participants received no dietary advice in the form of ad lib food intake and no dietary instruction. |
|
| Outcomes | Weight*, TSF*, MAUC*, MAMC*, BMI*, total protein, albumin, transferrin, total lymphocyte count, iron, cholesterol, triglycerides, clinical observations. | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Dietary advice given by doctors from nutrition and dietetic unit. The ID Macia 1991a has been used to identify the head and neck cancer group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used coin toss to randomise participants. |
| Allocation concealment (selection bias) | Unclear risk | No details provided however unlikely that lack of concealment would influence group allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Paper states that clinical variables were assessed by doctors unaware of group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not stated and likely that lack of blinding would influence the assessment of some nutritional outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described for all outcomes and absence of blinding of some outcomes might influence the results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of withdrawals and deaths not reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes in the methods reported but as mean change at baseline and end of follow‐up according to site of tumour therefore change scores were calculated and SDs imputed. No response received from author to requests for data. |
| Other bias | Unclear risk | Baseline variables not reported, not sure if groups similar at baseline. |
Macia 1991b.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: not reported. Location: Spain. |
|
| Participants |
Inclusion criteria: adults receiving radiotherapy for cancers of head and neck, breast and abdominopelvic area. Exclusion criteria: Karnofsky score < 50, previous diet therapy for diabetes, hypercholesterolemia or other conditions. Number randomised: 92 participants (intervention group, n = 30; control group, n = 62). Numbers of withdrawals and deaths not reported. Gender split: not reported. Age: not reported. Nutritional status: unclear. |
|
| Interventions |
Intervention: participants received dietary advice in the form of dietary instructions on appropriate alimentation during radiotherapy given verbally and in writing. Control: participants received no dietary advice in the form of ad lib food intake and no dietary instruction. |
|
| Outcomes | Weight*, TSF*, MAUC*, MAMC*, BMI*, total protein, albumin, transferrin, total lymphocyte count, iron, cholesterol, triglycerides, clinical observations. | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Dietary advice given by doctors from nutrition and dietetic unit. The ID Macia 1991b has been used to identify the breast cancer group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used coin toss to randomise participants. |
| Allocation concealment (selection bias) | Unclear risk | No details provided however unlikely that lack of concealment would influence group allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Paper states that clinical variables were assessed by doctors unaware of group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not stated and likely that lack of blinding would influence the assessment of some nutritional outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described for all outcomes and absence of blinding of some outcomes might influence the results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of withdrawals and deaths not reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes in the methods reported but as mean change at baseline and end of follow‐up according to site of tumour therefore change scores were calculated and SDs imputed. No response received from author to requests for data. |
| Other bias | Unclear risk | Baseline variables not reported, not sure if groups similar at baseline. |
Macia 1991c.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: not reported. Location: Spain. |
|
| Participants |
Inclusion criteria: adults receiving radiotherapy for cancers of head and neck, breast and abdominopelvic area. Exclusion criteria: Karnofsky score < 50, previous diet therapy for diabetes, hypercholesterolemia or other conditions. Number randomised: 92 participants (intervention group, n = 30; control group, n = 62). Numbers of withdrawals and deaths not reported. Gender split: not reported. Age: not reported. Nutritional status: unclear. |
|
| Interventions |
Intervention: participants received dietary advice in the form of dietary instructions on appropriate alimentation during radiotherapy given verbally and in writing. Control: participants received no dietary advice in the form of ad lib food intake and no dietary instruction. |
|
| Outcomes | Weight*, TSF*, MAUC*, MAMC*, BMI*, total protein, albumin, transferrin, total lymphocyte count, iron, cholesterol, triglycerides, clinical observations. | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Dietary advice given by doctors from nutrition and dietetic unit. The ID Macia 1991c has been used to identify the abdominopelvic cancer group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used coin toss to randomise participants. |
| Allocation concealment (selection bias) | Unclear risk | No details provided however unlikely that lack of concealment would influence group allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Paper states that clinical variables were assessed by doctors unaware of group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not stated and likely that lack of blinding would influence the assessment of some nutritional outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described for all outcomes and absence of blinding of some outcomes might influence the results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of withdrawals and deaths not reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes in the methods reported but as mean change at baseline and end of follow‐up according to site of tumour therefore change scores were calculated and SDs imputed. No response received from author to requests for data. |
| Other bias | Unclear risk | Baseline variables not reported, not sure if groups similar at baseline. |
Manguso 2005.
| Study characteristics | ||
| Methods | RCT. Cross‐over design with 2 treatment arms. Duration: 6 months in total but only results from the first 3 months will be considered. Location: Naples, Italy. |
|
| Participants |
Inclusion criteria: adults admitted to a specialist unit for the management of liver cirrhosis (Child A or B class). Exclusion criteria: HBV infection, autoimmune liver disease, drug or alcohol use, hepatocellular carcinoma, HIV infection, liver transplantation, impaired renal function, sepsis, or throid dysfunction, following specific diets, ascites and in current or previous treatment with albumin. Number randomised: 90 participants (intervention group, n = 45; control group, n = 45). Attrition: 3 withdrawals, but information from authors, no deaths. Gender split: 52 males and 38 females. Age: mean (IQR) 60 (9) years. Nutritional status: Mean (SD) BMI, intervention 27.8 (2.1) kg/m2; control 28.5 (3.2) kg/m2. |
|
| Interventions |
Intervention: participants received dietary advice in the form of a controlled diet consisting of specific prescription for macronutrients and calcium. Control: participants received no dietary advice in the form of spontaneous diet. |
|
| Outcomes | Survival*, weight*, MAMC*, TSF*, energy intake*, Childs Score, biochemistry profile. | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Weight may be inappropriate in analysis due to possible presence of ascites. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque sealed envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blinding not described but assessment of mortality unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | None measured. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Information from author, the assessor was blinded to intervention group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The main outcomes assessed were nutritional intake and body composition and information from the author indicated that these were assessed by a researcher blinded to the intervention. Judged unclear as lack of blinding of performance might have influenced nutritional intake. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information from author: no deaths occurred in the study, 1/45 participant withdrew from the dietary advice group and 2/45 from the no intervention group. 3 additional participants not included because they developed ascites. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. Results on all outcomes specified in the methods reported but as mean (SD) at baseline and end of intervention. Mean change (SD) for weight, energy intake, TSF and MAMC and additional information obtained from authors. |
| Other bias | Low risk | Baseline characteristics comparable between groups. |
McCarthy 1999.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 4 weeks. Location: single centre in the USA. |
|
| Participants |
Inclusion criteria: adults beginning a first course of curative radiotherapy for stage 1 or 2 cancer. Exclusion criteria: head and neck cancer. Number randomised: 40 participants (intervention group, n = 19; control group, n = 18). Attrition: 32 completed the 4‐week data collection period, 8 participants dropped out, 6 in the intervention group and 2 in the control group. Gender split: 23 males, 9 females. Age: mean (SD) intervention group 59.6 (9.6) years; control group 55.6 (14) years. Nutritional status: unclear. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of weekly nutritional counselling to maintain recommended dietary intake of calories and protein plus 8 oz of 1.0 kcal/mL nutritional supplement. Control: participants received dietary advice alone in the form of weekly nutritional counselling. |
|
| Outcomes | Energy intake*. | |
| Publication details |
Language: English. Funding: Mead Johnson and Abbott provided some of the nutritional supplements. Publication status: peer‐reviewed journal. |
|
| Notes | Data obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss used to randomise participants. |
| Allocation concealment (selection bias) | Low risk | Not reported but unlikely to have been influenced. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | None measured. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | None measured. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Paper states that assessments were made by the nurse and dietitian that implemented the intervention. Outcomes may have been influenced by knowing groups assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Paper states that assessments were made by the nurse and dietitian that implemented the intervention. Outcomes may have been influenced by knowing groups assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 participants lost to follow‐up, 6 of 20 (30%) in the experimental group (disliked the supplements) and 2 of 20 (10%) in the control group. These 8 were not analysed. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Outcomes reported are presented in a figure and so not in a format usable for meta‐analysis. Mean change (SD) in energy intake obtained from authors. |
| Other bias | Unclear risk | Baseline variables given, the supplement group weighed less and received less radiotherapy. |
Moloney 1983.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: unclear, intervention given for 3 weeks, survival reported to 1 year; outcomes reported at different time points. Location: Ireland. |
|
| Participants |
Inclusion criteria: consecutive adults with cancer (various sites) undergoing radiotherapy. Exclusion criteria: clinically poor and considered unethical to withhold adjunctive feeding. Number randomised: 84 participants (intervention group, n = 42; control group, n = 42). Attrition: no information. Gender split: 50 males and 34 females. Age: mean intervention group 63 years; control group 55 years. Nutritional status: no information given. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of dietary counselling and supplements. Control: participants received no dietary advice and no ONS in the form of no advice. |
|
| Outcomes | Survival*, energy intake*, macronutrient and micronutrient intake. | |
| Publication details |
Language: English. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | Data for survival given at 9 months for dietary advice and supplement group and at 11 months for no advice group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was assumed to be unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not measured. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on attrition. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Mortality data obtained from the paper. Data on change in energy intake is expressed as mean (SD) at baseline and end of intervention, therefore change scores were calculated and SDs imputed. No response received from author. |
| Other bias | Unclear risk | Baseline variables given, treatment group were older. |
Murphy 1992.
| Study characteristics | ||
| Methods | Quasi‐RCT. Parallel design with 2 treatment arms. Duration: 16 weeks. Location: single centre in Canada. |
|
| Participants |
Inclusion criteria: adults with HIV infection who had involuntary weight loss of more than 5%. Exclusion criteria: impaired phagia or gut function or previous dietary counselling from a dietitian. Number randomised: 22 participants (intervention group, n = 11; control group, n = 11). Attrition: 6 dropouts, 1 in the intervention group and 5 in the control group. Gender split: all males. Age: mean (SD) 37.3 (6.7) years. Nutritional status: mean(SD) % weight loss, intervention 10.1 (4.3) %; control 10.7 (3.0) %. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of dietary counselling verbally and in writing to consume a calculated amount of energy and protein per day and 2x 235 mL of a supplement (1.5 kcal/mL). Control: participants received dietary advice alone in the form of dietary counselling verbally and in writing to consume a calculated amount of energy and protein per day. |
|
| Outcomes | Survival*, body weight*, BMI*, MUAC*, serum albumin, energy* and protein intake. | |
| Publication details |
Language: English. Funding: nothing mentioned. Publication status: peer‐reviewed journal. |
|
| Notes | Outcomes not assessed blind. Additional data and information on quality obtained from authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were selected consecutively as they presented with weight loss (alternate allocation). No details of the group for the first participant. |
| Allocation concealment (selection bias) | High risk | Investigators used alternate allocation. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 dropouts, 5 (45%) in the dietary counselling group and 1 (9%) in the dietary counselling and supplement group. 5 dropouts because of subsequent GI disease, and 1 due to self exclusion. Significantly more participants dropped out from the dietary counselling group compared with counselling plus supplement group and the imbalance might have influenced outcome assessment. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All specified outcomes reported but continuous variables presented as mean (SD) at baseline and end of intervention. Mean change with SDs for weight has been imputed. Data on change in energy intake are presented with precise P values and so mean change (SD) obtained by calculation. Additional data and information on quality obtained from authors. |
| Other bias | Unclear risk | Baseline variables given, dietary counselling group weighed 5 kg less than the dietary counselling group and supplement group. |
Neelemaat 2011.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 3 months post hospital discharge. Location: single centre (University Medical Centre) in Amsterdam, The Netherlands. |
|
| Participants |
Inclusion criteria: malnourished elderly adults (> 60 years) admitted to hospital, expected length of stay > 2 days. Malnourished (5% unintentional weight loss 3/12 OR 10% unintentional weight loss 6/12, BMI < 20 kg/m²). Exclusion criteria: senile dementia, could not understand Dutch language, not willing or able to give consent, already received nutritional support. Number randomised: 210 (intervention group, n = 105, control group, n = 105). Attrition: intervention group n = 30 (16 withdrew and 14 died), control group n = 30 (19 withdrew and 11 died). Gender split: intervention group 56% female, 44% male; control group 60% female, 40% male. Age: mean (SD) years, intervention group 74.6 (9.7); control group 74.4 (9.3). Nutritional status: all malnourished as above. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of energy and protein enriched diet, 2 x oral nutritional supplements (Nutridrink, Nutricia: expected increase in intake of 600 kcal/day and 24 g protein/day during the entire study period), 400 IE vitamin D3, 500 mg calcium (Calci‐Chew D3, Nycomed) per day during the entire study period, telephone counselling by a dietitian to give advice and to stimulate compliance (every other week after discharge, 6 in total). Control: participants received no dietary advice and no ONS in the form of usual care i.e. nutritional support only on prescription by their treating physician, which means that in general they did not receive post‐discharge nutritional support. |
|
| Outcomes |
Primary outcomes: changes in activities of daily living (functional limitations, LASA Functional Limitation Questionnaire and LASA Physical Activity Questionnaire); functional status (Short Physical Performance Battery) and muscle strength (handgrip strength)*. Secondary outcomes: changes in body weight*, body composition (measured by Bioelectro‐impedance analysis). |
|
| Publication details |
Language: English. Funding: The Netherlands Organisation for Health Research and Development. Publication status: peer‐reviewed journal. |
|
| Notes | Data included from 2 subsequent publications (Neelemaat 2012 and Neelemaat 2017). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computerised random number generator was used to assign patients in blocks of 10 to the intervention or control groups. |
| Allocation concealment (selection bias) | Low risk | Quote: "...opened a consecutively numbered opaque envelope containing the patients' group assignment". |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not described but unlikely to affect assessment of clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not described and lack of blinding might have influenced the assessment of some functional outcomes. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not described and lack of blinding might have influenced the assessment of some nutritional outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not described and lack of blinding might have influenced the assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition fully reported and reasons similar in each group: 60/210 (29%). Intervention group 30/105 (29%); control group 30/105 (29%). |
| Selective reporting (reporting bias) | Unclear risk | Study protocol identified. (http://www.trialregister.nl (candidate number 1660, NTR number NTR476, ISRCTN ISRCTN29617677, date ISRCTN created 27 Jan 2006). Most outcomes specified in the protocol were reported. Complication rate is not reported in any of the three publications identified. |
| Other bias | Low risk | Baseline characteristics were similar in both groups. |
Norman 2008b.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 3 months from hospital discharge. Location: single centre in Germany. |
|
| Participants |
Inclusion criteria: adults with benign gastrointestinal disorders admitted to hospital and classified as malnourished according to SGA criteria. Exclusion criteria: malignant disease, renal insufficiency (serum creatinine 41.3mg/dl), and life expectancy < 3 months or age < 18 years. 2008: Number randomised: 101 participants (intervention group, n = 48, control group, n = 48). Attrition: 21 dropouts, 10 intervention group (withdrew before baseline) and 11 lost to follow‐up in the control group. Gender split: not stated. Age: mean (SD), intervention group 52.2 (16.5) years; control group 53.6 (16.8) years. Nutritional status: all malnourished according to SGA (grade B or C). 2011: Number randomised: 160 participants, 114 completed the study (intervention group, n = 60; control group, n = 54). Gender split: 57 males, 57 females. Age: mean (SD) total cohort 50.6 (16.1) years. Nutritional status: all malnourished according to SGA (grade B or C). |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of dietary counselling from a dietitian to increase energy and protein intake from food and up to 3 x 200 mL Fresubin protein energy drinks. Control: participants received dietary advice alone in the form of dietary counselling to increase energy and protein intake from food. |
|
| Outcomes |
2008: Energy intake*, weight*, height, BMI*, TSF*, MUAC*, body composition (BIA), handgrip strength*, length of stay, number of readmissions*, number of prescribed drugs on discharge, peak expiratory flow. 2011: QoL, cost. |
|
| Publication details |
Language: English. Funding: grant from Fresenius Kabi, Bad Homburg. Publication status: peer‐reviewed journal. |
|
| Notes | A first study was published in 2008; new data were published in 2011. Quality‐adjusted life years were calculated by adopting the area under the curve method. QoL was assessed with Short‐Form (SF)‐36 Health Survey and SF‐36 values were transformed into health‐status utilities. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised according to a computer‐generated randomisation list kept by a co‐worker not involved in the study. |
| Allocation concealment (selection bias) | Low risk | Central allocation, web‐based by a co‐worker not involved in the study. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not reported, in the 2008 paper the main outcome was number of readmissions. It is unlikely that this may have been influenced by knowing the group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Although not reported, intervention participants received supplements and control participants did not. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Although not reported, intervention participants received supplements and control participants did not. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
2008: 21 dropouts:
Dietary counselling and supplement group: 10 withdrew before baseline;
Dietary counselling alone: 11 lost to follow‐up. Also, in the dietary counselling and supplement group 8 known to not take the supplement, but included in the ITT analysis. In the dietary counselling group, 4 reported consuming nutritional supplements during the study period. 2011: 160 participants were recruited for the study, of which 120 completed the study. Dietary counselling and supplement group: 12 withdrew before the start, 8 lost to follow‐up. Dietary counselling alone: 20 lost to follow‐up, 6 did not complete SF 36 QoL questionnaires. ITT analyses for 60 participants in intervention group and 54 participants in control group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Data on mean change (SD) for weight and grip strength were extracted from the paper. Data on TSF and MUAC were not presented but were assessed and so have been obtained from author. Details of hospital admissions are not reported clearly and therefore have been clarified with the authors. For the 2011 manuscript 6 participants did not complete SF36 correctly and all were assigned to the control group. |
| Other bias | Low risk | Baseline characteristics described in text as not different and data given for some variables. |
Olejko 1984.
| Study characteristics | ||
| Methods | RCT. Parallel design with 3 treatment arms. Duration: 6 weeks. Location: single centre in the USA. |
|
| Participants |
Inclusion criteria: adults awaiting elective orthognathic surgery. Exclusion criteria: not specified. Number randomised: 24 participants (intervention group 1, n = 8; intervention group 2, n = 8; control group, n = 8). 100% follow‐up. Gender split: 12 males, 12 females. Age: mean (SD), 22.8 (6.1) years. Nutritional status: 12 of 24 participants had a weight below IBW at study inclusion. Mean BMI can be calculated from individual participant data (mean BMI 22). |
|
| Interventions |
Intervention (intervention group 1): participants received dietary advice and ONS in the form of dietary instruction and an oral nutritional supplement (1.5 kcal/mL) to provide 50% of energy requirements. Intervention (intervention group 2): participants received dietary advice and ONS in the form of dietary instruction, an oral nutritional supplement (1.5 kcal/mL) to provide 50% of energy requirements and a nutritional supplement to take preoperatively. Control: participants received dietary advice alone in the form of dietary instruction given verbally and in writing. |
|
| Outcomes | Survival*, body weight*, MUAC*, MAMC*, TSF*, serum chemistry and creatinine height index, macro and micronutrient intake. | |
| Publication details |
Language: English. Funding: not reported. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not reported, but the nature of the study suggests that the study was unblinded. However, it is unlikely that knowing groups assignment would have influenced clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not reported, but the nature of the study suggests that the study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not reported, but the nature of the study suggests that the study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because of low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | High risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. All outcomes were reported but using general statements about change rather than numerical presentation e.g. 'the pre‐load group reported an average weight gain of 3.1% during the one month pre‐operative period, which was significantly greater (P < 0.05) than that of the other two groups'. No data are available from the authors therefore unclear risk of bias due to partial reporting. |
| Other bias | Unclear risk | Baseline variables not given, not sure if groups similar at baseline. |
Ollenschlager 1992.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: mean of 25.5 weeks. Location: Germany. |
|
| Participants |
Inclusion criteria: adults undergoing chemotherapy for acute leukaemia who had undesired weight loss > 5% or weight 90% < IBW. Exclusion criteria: metabolic diseases, renal or liver insufficiency, the need for artificial nutrition. Number randomised: 29 participants (intervention group, n = 15; control group, n = 16). Attrition: 2 deaths in the intervention group. Gender split: not possible to work out from the information provided. Age: aged 17 ‐ 59 years. Nutritional status: not possible to work out from the information provided. |
|
| Interventions |
Intervention: participants received dietary advice in the form of daily dietary instruction and modification of diet. Control: participants received no dietary advice in the form of ad libitum intake. |
|
| Outcomes | Weight*, survival*, number of complete remissions and days temperature >38.5 C, nutrient intake (intervention gp only) *, subjective well‐being (intervention gp only). | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Data given for mean study period. Data on nutrient intake and subjective well‐being only collected for intervention group so not used. Additional data and information obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blinding not described but assessment of mortality unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described and likely that lack of blinding might influence assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully described with reasons. (2/15 deaths in the dietary advice group, 0/16 deaths in the routine care group; 2 participants in the dietary advice group excluded from the analysis because only 1 weight obtained in week 1). |
| Selective reporting (reporting bias) | High risk | No protocol identified. Data given for mean study period. Data on nutrient intake and subjective well‐being only collected for intervention group so not used. Data on weight change presented as % of ideal body weight, mean change (SD) not available from authors and so not included in the review. Additional data on mortality and information on some outcomes obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Ovesen 1993.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 5 months. Location: Denmark. |
|
| Participants |
Inclusion criteria: adults receiving chemotherapy for small‐cell lung cancer, ovarian cancer or breast cancer. Exclusion criteria: prior chemotherapy, current or planned hormonal therapy, major surgery within the last month, present of ascites, oedema, malabsorption or other diseases that necessitated dietary intervention, CNS metastases. Number randomised: 137 participants (intervention group, n = 74; control group, n = 63). Attrition: 30 deaths, 20 in the intervention group and 10 in the control group; 19 withdrawals, 9 in the intervention group and 10 in the control group. Gender split: 75% males and 25% females. Age: combined range 22 ‐ 80 years. Nutritional status: 50% of participants malnourished at study entry defined as > 5% weight loss in the previous 3 months. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of dietary instruction given 2x monthly to exceed the Nordic recommended allowances using supplements if indicated. Control: participants received no dietary advice and no ONS in the form of no dietary advice. |
|
| Outcomes | Survival*, weight*, TSF, MAMC, MUAC, energy intake*, FFM*, QoL*, tumour response. | |
| Publication details |
Language: English. Funding: the Danish Cancer Society. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was reported as using a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was reported as using sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was assumed to be unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 deaths: 20 in the dietary advice group and 10 in the no advice group. 19 withdrawals: 9 in the dietary advice group and 10 in the no advice group. Amounts similar between groups but no reasons given. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. outcomes reported at baseline and monthly to five months after the intervention. The data on mortality (up to 1400 days ~ 3.8 years) at interim time‐points is unclear and it has not been possible to clarify with authors, therefore only data from baseline to 5 months have been used in meta‐analysis. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Parsons 2016.
| Study characteristics | ||
| Methods | RCT. Parallel design with two treatment arms. Duration: 12 weeks. Location: UK. |
|
| Participants |
Inclusion criteria: care home resident, > 50 years of age, medium/high risk of malnutrition (MUST), able to give consent and able to eat and drink safely with adequate cognitive function (not tested, judgement of nursing staff or info in medical notes). Exclusion criteria: receiving enteral or parenteral feeding or oral nutritional supplements (or in the 4 weeks prior to the study), chronic kidney disease requiring dialysis, liver failure, malignancy, receiving palliative care for terminal illness. Diagnosis: multiple problems, 45% nursing care, 55% residential care. Number randomised: 104 participants. Attrition: 34 of 104 (33%) (intervention group, n = 20; control group, n = 14). Gender split: 145 males, 86% females. Age: mean (SD) 88.5 (7.9) years. Nutritional status: assessed using MUST, 46% medium risk, 54% high risk. |
|
| Interventions |
Intervention (intervention group 1): particpants received ONS in the form of a specially designed diet sheet encouraging intake of high energy foods, drinks and snacks. Intervention (intervention group 2): participants received dietary advice in the form of access to a range of oral nutritional supplements to take ad lib according to choice and guidance to staff and participants on using ONS. Target intake 600 kcal/day. Both interventions discussed at baseline and week 6 by dietitian. |
|
| Outcomes | Recorded at baseline, 6 & 12 weeks; malnutrition risk (MUST), QoL (EQ5D), dietary intake (24 h recall), appetite on VAS. Mortality also recorded. | |
| Publication details |
Language: English. Funding: educational grant from Nutricia. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: undertaken independently of the researchers using random number tables produced by EXCEL. Codes were generated prior to commencement of the trial. |
| Allocation concealment (selection bias) | Low risk | Quote: ..using opaque, sealed envelopes labelled with the random numbers containing the designated interventions. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not reported beyond inclusion into the trial, but unlikely that assessment of clinical outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not reported beyond inclusion into the trial, and likely that assessment of functional outcomes would be influenced by lack of blinding. Quote: "at the point of randomisation, both the residents and researchers were blinded to the designated intervention". |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not reported beyond inclusion into the trial, and likely that assessment of clinical outcomes would be influenced by lack of blinding. Quote: "at the point of randomisation, both the residents and researchers were blinded to the designated intervention". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported and likely that assessment of some outcomes would be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition fully reported. 34/104 (33%) participants lost to follow‐up (dietary advice group n = 20/51 (39 %), including 4 deaths; ONS group n = 14/53 (26 %), including 2 deaths). Reasons similar for both groups and mainly related to decline in health or memory. |
| Selective reporting (reporting bias) | Unclear risk | Protocol published on Clinical Trial Registry which records only the primary outcome of QoL. All other outcomes mentioned in the methods are reported in the results but data for quality of life, energy and protein intake reported as baseline value (SD) for the combined group and end value (SD) for the group allocation. Data have been requested from authors. |
| Other bias | Unclear risk | Baseline characteristics compared between groups and no significant differences other than the visual analogue score component of EQ5D. VAS scores significantly higher at baseline in the comparison group receiving ONS and unclear what influence this would have on outcomes. |
Paton 2004.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 24 weeks (intervention for 6 or 12 weeks and follow‐up to 24 weeks). Location: single centre (Hospital‐based TB Control Unit, Singapore). |
|
| Participants |
Inclusion criteria: males and females aged 18 ‐ 69 years old, evidence of active tuberculosis (symptoms of fever or cough + sputum smear ‐ acid‐fast bacilli or chest x‐ray), evidence of wasting (BMI < 20 kg/m2), started on combination antituberculous chemotherapy within the previous 2 weeks. Exclusion criteria: diabetes mellitus, severe underlying disease, concomitant corticosteroid or immunosuppressive therapy, positive HIV test or considered at risk of HIV infection, past history of noncompliance to tuberculosis therapy, unable to tolerate conventional treatment, required inpatient treatment for their disease. Number randomised: 36 participants (intervention group, n = 15; control group, n = 13). Attrition: 10 participants lost to follow‐up (4 in intervention group and 6 in control group). Gender split: intervention group 8 (47%) males and 11 (53%) females; control group 8 (42%) males and 9 (58%) females. Age: mean (SD), intervention group 39.5 (14.3) years; control group 38.4 (19.3) years. Nutritional status: mean(SD) BMI, intervention 16.7 (1.5) kg/m2; control 17.9 (1.9) kg/m2. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of advice to increase intake, aim 35 kcal/kg body weight/day; high energy oral nutritional supplements (2 ‐ 3 packets/day), contacted by telephone to assess progress, target of 35 kcal/kg and explained why important to meet, also phoned at 2 ‐ 3 weeks, 6 weeks and 12 weeks. Control: participants received no dietary advice and no ONS in the form of general advice to address any major dietary imbalance identified, instructed to increase intake as they could but no specific instructions. |
|
| Outcomes | Weight*, BMI, body composition (DEXA)*, energy intake*, grip strength*, QoL, physical function (sit‐to‐stand test). | |
| Publication details |
Language: English. Funding: none detailed. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data requested from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: sequence generated by a member of staff not otherwise involved in the study. |
| Allocation concealment (selection bias) | Low risk | Described as shuffling of sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not stated but assessment of mortality unlikely to be affected by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated but assessment of functional outcomes may have been affected by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated but assessment of nutritional outcomes may have been affected by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated and assessment of some outcomes may have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition fully reported and reasons balanced between groups. 10/36 (28%) participants lost to follow‐up at participants' request, 4/19 (21 %) in dietary advice and supplement group and 6/17 (35 %) in dietary advice group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All specified outcomes reported but some in unusable format. Data on change in energy intake was presented in the text and not suitable for entry into meta‐analysis therefore obtained from author. Other data were extracted from the paper although 'n' for weight and grip strength were clarified with the authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Payette 2002.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 16 weeks. Location: multicentre at 7 local community service centres, Quebec, Canada. |
|
| Participants |
Inclusion criteria: older adults (aged > 65 years) who were at high nutritional risk (unintentional weight loss > 5% in previous month, > 7.5% in previous 3 months or > 10% in previous 6 months AND BMI < 27 OR BMI < 24), orientated to time and place. Exclusion criteria: palliative care, alcoholic, active cancer or illness requiring a therapeutic diet incompatible with oral nutritional supplements. Number randomised: 83 (intervention group, n = 41; control group, n = 42). Attrition: 5 participants refused the assignment and declined participation before the start of the study (intervention group n = 1, control group n = 4); 1 participant (intervention group) was lost to follow‐up due to cancer diagnosis. Gender split: intervention group 12 (29%) male, 29 (71%) female; control group 12 (29%) male, 30 (71%) female. Age: mean (SD) years, intervention group 81.6 (7.5), control group 78.6 (6.1). Nutritional status: mean (SD) BMI, intervention 20.1 (2.7) kg/m2; control 20.1 (3.0) kg/m2. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of 2 x 235 mL/day oral nutritional supplements (Ensure or Ensure Plus, Abbott Laboratories), clearly instructed not to replace their usual meals with the drinks, encouraged to use oral nutritional supplements to supplement diet; contacted by phone every 2 weeks by a dietitian and given nutritional counselling and encouragement to improve their food and supplement intake. Control: participants received no dietary advice and no ONS in the form of no nutritional treatment and no oral nutritional supplements, visited each month and, to control for any effect of greater attention to 1 group, were given a small gift. |
|
| Outcomes | Energy intake, body weight, handgrip strength, AMC. | |
| Publication details |
Language: English. Funding: none detailed. Publication status: peer‐reviewed journal. |
|
| Notes | Author contacted to request change data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | 2 research dietitians collected data: 1 was responsible for recruitment and collection of baseline and follow‐up nutritional data and the other (blinded to participants' treatment) completed measurements of functional and health status. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | See comment above. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | See comment above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment of some outcomes may have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition fully reported and reasons balanced between groups. 6/89 (7 %); intervention group 2/43 (5%) and control group 4/46 (9 %). |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. |
| Other bias | Low risk | Baseline characteristics presented in Table 1. Study groups similar at baseline. Although control group was significantly younger than intervention group (mean (SD) age 78.6 (6.1) versus 81.6 (7.5) years respectively) this is unlikely to affect outcomes. |
Pedersen 2016a.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 intervention groups and 1 control group. Duration: 8 weeks (randomization took place upon discharge from hospital and follow‐up interventions lasted 8 weeks). Location: single centre in Denmark. |
|
| Participants |
Inclusion criteria: malnourished or at risk of malnutrition, 75 years and older, living independently and alone in the area served by the hospital, able to speak the Danish language, and to communicate over the telephone. Exclusion criteria: nursing home residents, terminal illnesses or cognitive impairment. Number randomised: 208 participants (intervention group 1, n = 73; intervention group 2, n = 68; control group, n = 67). Gender split: intervention group 1, 57% females; intervention group 2, 51% females; control group, 65% females. Age: mean 86.1 years. Nutritional status: mean (SD) MNA score, intervention 1 17.1 (3.2); intervention 2 17.3 (3.7); control 17.0 (3.9). |
|
| Interventions |
Intervention (group 1): participants received dietary advice plus ONS if required in the form of standard care during hospital stay followed by nutritional counselling* during home visits (45 min duration) by a clinical dietician at 1, 2 and 4 weeks post hospital discharge. Intervention (group 2): participants received dietary advice plus ONS if required in the form of standard care during hospital stay followed by nutritional counselling via telephone consultations (15 min duration) by a clinical dietician at 1, 2 and 4 weeks post hospital discharge. Control: participants received no dietary advice and no ONS in the form of standard care during hospital stay and no follow‐up care from the hospital after discharge. *based on nutritional needs identified during the hospital stay, and tailored to the individual’s preferences and circumstances; since reduced appetite and low food intake had become normal, the intervention focused on nutritional and meal behaviour that improve appetite and increase nutritional intake. The counselling sessions were attended by the participant’s home carer, who holds a key position in supporting the participant on a daily basis. |
|
| Outcomes |
Primary outcome: change in ADL (Barthel‐100 score) at discharge and 8 weeks later. Secondary outcomes: change in physical performance (handgrip strength*, 30‐sec. chair stand test, CAS), QoL and depression measurements (SF‐36 (not global), Depression List, Geriatric Depression Score), and Avlund mobility‐tiredness score (Mob‐T). |
|
| Publication details |
Language: English. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | This study is a duplicate of Pederson 2016b. The study has 2 intervention arms and a single control group. Therefore the number of participants in the control group is divided by 2. This study ID describes the data of intervention group 'home visit' versus control, whereas Pederson 2016b describes 'telephone counselling' versus control. The primary aim of the study was to compare the effects of 2 nutritional follow‐up intervention strategies (home visit and telephone consultation) with no follow‐up, with regard to preventing short‐term deterioration in ADL, and the second, to compare the effect of the interventions on physical function, health‐related QoL, and emotional health. QoL data could not be used because only subscores were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation executed electronically via the web‐based, clinical‐trial‐ support‐system, ‘TrialPartner’ (Public Health and Quality Improvement, Central Denmark Region). This central computer program used permuted block‐sizes and stratified the randomisation according to nutritional status. |
| Allocation concealment (selection bias) | Low risk | Randomisation took place upon discharge and was executed electronically via the web‐based, clinical‐trial‐ support‐system, ‘TrialPartner’ (Public Health and Quality Improvement, Central Denmark Region). This central computer program used permuted block‐sizes and stratified the randomisation according to nutritional status. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not measured. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Owing to the nature of the interventions, it was not possible to blind the participants to the intervention. The principal investigator obtained baseline characteristics before randomisation, and was not in contact with the participants after that. The research assistant who conducted the baseline and follow‐up measurements (week 0 and week 8) was not informed of the results of the randomisation, but it cannot be ruled out that the participants may have mentioned their assignment. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Owing to the nature of the interventions, it was not possible to blind the participants to the intervention. The principal investigator obtained baseline characteristics before randomisation, and was not in contact with the participants after that. The research assistant who conducted the baseline and follow‐up measurements (week 0 and week 8) was not informed of the results of the randomisation, but it cannot be ruled out that the participants may have mentioned their assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research assistant who conducted the baseline and follow‐up measurements (week 0 and week 8) was not informed of the results of the randomisation, but it cannot be ruled out that the participants may have mentioned their assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 8 weeks after discharge, 157/208 (75%) completed the follow‐up (home visit n = 52, telephone consultation n = 51, and control group n = 54). Drop‐outs (25%) were equally divided across groups but the reasons for drop‐out not mentioned. |
| Selective reporting (reporting bias) | Low risk | The study protocol was published in 2015 (Journal of Ageing Reasearch & Clinical Practice) with primary outcome: ADL and secondary outcomes: physical performance (handgrip strength, chair stand), QoL, depression measurements, and tiredness score. All specified outcomes reported in 2 papers |
| Other bias | Low risk | Baseline characteristics given and similar between groups |
Pedersen 2016b.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 intervention groups and 1 control group. Duration: 8 weeks (randomization took place upon discharge from hospital and follow‐up interventions lasted 8 weeks). Location: single centre in Denmark. |
|
| Participants |
Inclusion criteria: malnourished or at risk of malnutrition, 75 years and older, living independently and alone in the area served by the hospital, able to speak the Danish language, and to communicate over the telephone. Exclusion criteria: nursing home residents, terminal illnesses or cognitive impairment. Number randomised: 208 participants (intervention group 1, n = 73; intervention group 2, n = 68; control group, n = 67). Gender split: intervention group 1, 57% females; intervention group 2, 51% females; control group, 65% females. Age: mean 86.1 years. Nutritional status: mean (SD) MNA score, intervention group 1 17.1 (3.2); intervention group 2 17.3(3.7); control group 17.0 (3.9). |
|
| Interventions |
Intervention group 1: participants received dietary advice plus ONS if required in the form of standard care during hospital stay followed by nutritional counselling* during home visits (45 min duration) by a clinical dietician at 1, 2 and 4 weeks post hospital discharge. Intervention group 2: participants received dietary advice plus ONS if required in the form of standard care during hospital stay followed by nutritional counselling via telephone consultations (15 min duration) by a clinical dietician at 1, 2 and 4 weeks post hospital discharge. Control group: participants received no dietary advice and no ONS in the form of standard care during hospital stay and no follow‐up care from the hospital after discharge. *based on nutritional needs identified during the hospital stay, and tailored to the individual’s preferences and circumstances; since reduced appetite and low food intake had become normal, the intervention focused on nutritional and meal behaviour that improve appetite and increase nutritional intake. The counselling sessions were attended by the participant’s home carer, who holds a key position in supporting the participant on a daily basis. |
|
| Outcomes |
Primary outcome: change in ADL (Barthel‐100 score) at discharge and 8 weeks later. Secondary outcomes: change in physical performance (handgrip strength*, 30‐sec. chair stand test, CAS), QoL and depression measurements (SF‐36, Depression List, Geriatric Depression Score), and Avlund mobility‐tiredness score (Mob‐T). |
|
| Publication details |
Language: English. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | This study is a duplicate of Pederson 2016a. The study has 2 intervention arms and a single control group. Therefore the number of participants in the control group is divided by 2. This study ID describes the data of intervention group 'telephone counselling' versus control, whereas Pederson 2016a describes 'home visits' versus control. The primary aim of the study was to compare the effects of 2 nutritional follow‐up intervention strategies (home visit and telephone consultation) with no follow‐up, with regard to preventing short‐term deterioration in ADL, and the second, to compare the effect of the interventions on physical function, health‐related QoL, and emotional health. QoL data could not be used because only subscores were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation executed electronically via the web‐based, clinical‐trial‐ support‐system, ‘TrialPartner’ (Public Health and Quality Improvement, Central Denmark Region). This central computer program used permuted block‐sizes and stratified the randomisation according to nutritional status. |
| Allocation concealment (selection bias) | Low risk | Randomisation took place upon discharge and was executed electronically via the web‐based, clinical‐trial‐ support‐system, ‘TrialPartner’ (Public Health and Quality Improvement, Central Denmark Region). This central computer program used permuted block‐sizes and stratified the randomisation according to nutritional status. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Not measured. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Owing to the nature of the interventions, it was not possible to blind the participants to the intervention. The principal investigator obtained baseline characteristics before randomisation, and was not in contact with the participants after that. The research assistant who conducted the baseline and follow‐up measurements (week 0 and week 8) was not informed of the results of the randomisation, but it cannot be ruled out that the participants may have mentioned their assignment. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Owing to the nature of the interventions, it was not possible to blind the participants to the intervention. The principal investigator obtained baseline characteristics before randomisation, and was not in contact with the participants after that. The research assistant who conducted the baseline and follow‐up measurements (week 0 and week 8) was not informed of the results of the randomisation, but it cannot be ruled out that the participants may have mentioned their assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research assistant who conducted the baseline and follow‐up measurements (week 0 and week 8) was not informed of the results of the randomisation, but it cannot be ruled out that the participants may have mentioned their assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 8 weeks after discharge, 157/208 (75%) completed the follow‐up (home visit n = 52, telephone consultation n = 51, and control group n = 54). Drop‐outs (25%) were equally divided across groups but the reasons for drop‐out not mentioned. |
| Selective reporting (reporting bias) | Low risk | The study protocol was published in 2015 (Journal of Ageing Reasearch & Clinical Practice) with primary outcome: ADL and secondary outcomes: physical performance (handgrip strength, chair stand), QoL, depression measurements, and tiredness score. |
| Other bias | Low risk | Baseline characteristics given and similar between groups |
Persson 2002.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 24 months. Location: Sweden. |
|
| Participants |
Inclusion criteria: adults who were newly diagnosed with colorectal or gastric cancer. Exclusion criteria: requiring constant hospital care (Karnofsky score < 40), with an earlier cancer diagnosis or those who did not speak or understand Swedish. Number randomised: 142 participants but 5 dropouts immediately after randomisation (intervention group, n = 3; control group, n = 2) so 137 participants included (intervention group, n = 67; control group, n = 70). Attrition: at 24 months there were 74 drop outs (intervention group, n = 33; control group, n = 41). Gender split: 63% males, 37% females. Age: range 42 ‐ 89 years. Nutritional status: number with weight loss, intervention 52/67; control 48/70. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of nutritional counselling to increase food intake to Nordic Nutrition Recommendations and a prescription for nutritional supplements if wanted. Control: participants received no dietary advice and no ONS in the form of standard care. |
|
| Outcomes | Survival*, weight*, BMI*, energy intake*. | |
| Publication details |
Language: English. Funding: the Swedish Cancer Society. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from the author, random sequence generated on computer by independent centre. |
| Allocation concealment (selection bias) | Low risk | Information from the author, allocation performed by independent centre, allocation concealed until participant recruited. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not stated but unlikely to affect assessment of clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated and lack of blinding likely to influence assessment of some functional outcomes |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not stated and lack of blinding likely to influence assessment of some nutritional outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described and lack of blinding might have influenced assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information from author: 137 participants randomised in the study, at 24 months there were 74 drop outs (intervention group: 25 deaths, 5 withdrawals and 3 exclusions; control group: 26 deaths, 14 withdrawals and 1 exclusion). Amounts and reasons similar between groups. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported.The data on mortality is unclear in the paper. The data on weight change is presented partly in text and partly in figures and not suitable for direct entry into meta‐analysis. The data on energy intake is presented as % recommendations. All data included in the review has been obtained from the author including data at 4 time‐points. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Persson 2007.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: median 4.3 months, range 3.6 ‐ 6.9 months. Location: Sweden. |
|
| Participants |
Inclusion criteria: elderly people admitted to hospital for trauma or acute illness, at risk of malnutrition defined by MNA score < 10. Exclusion criteria: malignant disorders, terminal illness or with severe cognitive dysfunction. Number randomised: 108 participants (intervention group, n = 51; control group, n = 57). Attrition: 54 dropouts. Gender split: not reported. Age: mean (SD), intervention group 85 (5.9) years, control group 85 (6.1) years. Nutritional status: mean (SD) BMI, intervention 19.8 (1.9); control 20.6 (3.0). |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of individualised counselling to increase food intake, plus a nutritional supplement and a multivitamin supplement. Control: participants received no dietary advice and no ONS in the form of brief written dietary advice. |
|
| Outcomes | Weight *, BMI*, handgrip strength*, energy intake*, activities of daily living, cognitive function, peak expiratory flow, QoL. | |
| Publication details |
Language: English. Funding: the Swedish Research Council, Karolinska Institutet and by grants from S. Persson Family Foundation and Sempers Foods AB. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: randomisation performed by drawing files from a sealed box. |
| Allocation concealment (selection bias) | Low risk | Quote: randomisation performed by drawing files from a sealed box. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not blinded but clinical outcomes unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not blinded but functional outcomes may have been affected by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not blinded but nutritional outcomes may have been affected by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not blinded and some outcomes likely to have been affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis using data obtained at inclusion carried forward and used at follow‐up for those who were still alive but not examined. Treated as protocol analysis also included. Attrition fully reported. 54 (50%) participants did not complete the study; 22/51 (43%) in the intervention group and 32/57 (56%) in the control group including 6/51 (12%) deaths in the intervention group and 12/57 (21%) deaths in the control group. The remaining participants withdrew consent or declined follow‐up; 8 participants in the control group had the intervention prescribed during the study. The high attrition rate and imbalance between groups might have influenced outcome assessment. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported.Outcomes reported but not in a format suitable for meta‐analysis. Data on change in weight and handgrip strength are presented as mean (SD) at the start and end of the intervention and have therefore been obtained from authors. Data on mortality extracted from the paper. |
| Other bias | Unclear risk | Stated in text that baseline characteristics not different but data not shown. |
Pivi 2011.
| Study characteristics | ||
| Methods | RCT. Parallel design. Duration: 6 months. Location: Brazil. |
|
| Participants |
Inclusion criteria: > 65 years old with probable Alzheimer's disease. Exclusion criteria: other forms of dementia; receiving tube feeding; diabetes or renal disease; Diagnosis: Alzheimer's disease. Number randomised: 90 (intervention groups, dietary advice n = 29 and oral nutritional supplements n = 30; control group, n = 31). Attrition: fully described ‐ dietary advice group: 4/29 (14%); oral nutritional supplements group: 4/30 (13%); control group: 4/31 (13%). Gender split: (32%) male, (68%) female. Age: mean (SD) 75.2 years. Nutritional status: not reported. |
|
| Interventions |
Intervention (intervention group 1): participants received dietary advice in the form of 10x classroom interactive education program delivered to caregivers and participants. Intervention (intervention group 2): particpants received ONS in the form of oral nutritional supplements twice daily for 6 months. Control: participants received no dietary advice in the form of monthly assessments with no intervention. |
|
| Outcomes | Mortality; weight; BMI; arm circumference and MAMC; TSF thickness; total protein; total lymphocyte count. | |
| Publication details |
Language: English. Funding: commercial / non‐commercial funding ‐ Ministry of Education; Abbott Laboratories. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "subjects were randomised into three groups.....". Judged as insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blinding not described but assessment of mortality unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Blinding not described and assessment of nutritional status unlikely to be influenced by lack of blinding. Nutritional intake not assessed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described but unlikely that the outcomes assessed would be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully described and similar amounts in each group. Overall 13% attrition. Dietary advice: 4/29 (14%); oral nutritional supplements group: 4/30 (13%); control group: 4/31 (13%). |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes described in the methods reported but not possible to judge overall. |
| Other bias | Unclear risk | Baseline characteristics compared and no differences between groups but nutritional status not an inclusion and not reported at baseline, therefore not possible to judge the influence of baseline nutritional status on outcomes. |
Rabeneck 1998.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 treatment arms. Duration: 6 weeks. Location: multicentre trial in the USA. |
|
| Participants |
Inclusion criteria: adults with HIV infection who were < 90% ideal weight or who had > 10% weight loss in previous 6 months. Exclusion criteria: dysphagia, severe diarrhoea, cytomegalovirus, or mycobacterium avium complex infection, suspected untreated infection, or a diagnosis of infection or hospitalisation within the previous 2 weeks. Number randomised: 118 participants (intervention group, n = 50; control group, n = 52). Attrition: 28 dropouts (intervention group, n = 16; control group, n = 12). Gender split: all males. Age: mean (SD), intervention group 39.3 (8.8) years; control group 41.1 (9.7) years. Nutritional status: mean (SD) BMI, intervention 20.6 (3.0) kg/m2; control 21.0 (2.6) kg/m2. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of nutritional counselling to achieve target and an oral nutritional supplement. Control: participants received dietary advice alone in the form of nutritional counselling to achieve specific energy target. |
|
| Outcomes | Weight*, MUAC*, skinfold measurements at all sites*, body composition (BIA)*, grip strength*, cognitive function, QoL, energy intake*. | |
| Publication details |
Language: English. Funding: the study was supported by Mead Johnson. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | No objective clinical outcomes measured. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was unblinded. Functional and nutritional outcomes could have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 dropouts in intervention group (dietary counselling and supplements) and 12 dropouts in control group (dietary counselling); reasons for dropouts reported for the 19 participants who failed to complete at least 4 weeks of the 6‐week treatment period. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Specified outcomes extracted from paper. No data obtained from the author. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Ravasco 2005a.
| Study characteristics | ||
| Methods | RCT. Parallel design with 3 arms. Duration: 42 days intervention plus 3 months follow‐up. Location: Portugal. |
|
| Participants |
Inclusion criteria: adults with colorectal cancer undergoing radiotherapy. Exclusion criteria: renal disease or diabetes. Number randomised: 111 participants (intervention group 1, n = 37; intervention group 2, n = 37; control group, n = 37). Attrition: no participants lost to follow‐up. Gender split: 66 males and 45 females. Age: mean (SD) 58 (15) years. Nutritional status: at baseline 42/111 participants were 'malnourished' (identified by PG‐SGA); 15 in Intervention group 1, 14 in Group 2, 13 in Group 3). |
|
| Interventions |
Intervention (intervention group 1): participants received dietary advice in the form of individualised dietary counselling to achieve calculated energy and protein requirements. Intervention (intervention group 2): particpants received ONS in the form of 2x 200 mL cans of nutritional supplement. Control group: participants received no dietary advice in the form ad libitum food intake. |
|
| Outcomes | Survival*, weight*, energy intake*, protein intake, symptom‐induced morbidity, QoL. | |
| Publication details |
Language: English. Funding: Nucleo Regional do Sul da Liga Portuguesa contra o Cancro‐Terry Fox Foundation. Publication status: peer‐reviewed journal. |
|
| Notes | Data will be used in 2 parts of the review dietary advice versus no advice and dietary advice versus nutritional supplements. Additional data and information obtained from authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blinding not described but assessment of mortality unlikely to be influenced by lack of blinding |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated and likely that some outcomes might be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated and likely that some outcomes might be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described and likely that lack of blinding might influence assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up. Author confirmed that no deaths occurred in the 3‐month study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All specified outcomes reported in text and figures but not in a format or sufficient detail to make them usable for meta‐analysis. Much of the data reported as medians (IQR). Additional data on mean change (SD) for weight, energy intake and QOL obtained from author. |
| Other bias | Unclear risk | Baseline variables not given, not sure if groups similar at baseline. |
Ravasco 2005b.
| Study characteristics | ||
| Methods | RCT. Parallel design with 3 arms. Duration: 42 days intervention plus 3 months follow‐up. Location: Portugal. |
|
| Participants |
Inclusion criteria: adults receiving radiotherapy for head and neck cancer. Exclusion criteria: renal disease or diabetes. Number randomised: 75 participants (intervention group 1, n = 25; intervention group 2, n = 25; control group, n = 25). Attrition: no participants lost to follow‐up. Gender split: 60 males, 15 females. Age: mean (range) 60 years (36 ‐ 79 years). Nutritional status: at baseline 45/75 participants were 'malnourished' (identified by PG‐SGA); intervention group 1, n = 16 ; intervention group 2, n = 14; control group, n = 15). |
|
| Interventions |
Intervention (intervention group 1): participants received dietary advice in the form of individualised dietary counselling to achieve calculated energy and protein requirements. Intervention (intervention group 2): particpants received ONS in the form of 2x 200 mL cans of nutritional supplement. Control group: participants received no dietary advice in the form ad libitum food intake. |
|
| Outcomes | Survival*, weight*, energy intake*, nutritional status (PG‐SGA), symptom‐induced morbidity, QoL. | |
| Publication details |
Language: English. Funding: Nucleo Regional do Sul da Liga Portuguesa contra o Cancro‐Terry Fox Foundationn. Publication status: peer‐reviewed journal. |
|
| Notes | Data will be used in 2 parts of the review dietary advice versus no advice and dietary advice versus nutritional supplements. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using computer‐generated random assignments. |
| Allocation concealment (selection bias) | Low risk | Concealed in numbered opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blinding not described but assessment of mortality unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not stated and likely that some outcomes might be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Not stated and likely that some outcomes might be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described and likely that lack of blinding might influence assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up. Author confirmed that no deaths occurred in the 3‐month study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All specified outcomes reported in text and figures but not in a format or sufficient detail to make them usable for meta‐analysis. Much of the data reported as medians (IQR). Additional data on mean change (SD) for weight, energy intake and QoL obtained from author. |
| Other bias | Unclear risk | Baseline variables not given, not sure if groups similar at baseline. |
Rogers 1992.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 4 months. Location: USA. |
|
| Participants |
Inclusion criteria: adults with COPD and weight < 90% of IBW and FEV1/FVC < 0.6. Exclusion criteria: recent (within 8 weeks) exacerbation of COPD, diabetes, thyroid dysfunction, malabsorption, alcoholism, myopathic disease or neoplastic disease, received nutritional supplements in the previous 3 months, corpulmonale, congestive heart failure. Number randomised: 28 participants (intervention group, n = 15; control group, n = 12). Attrition: 1 withdrawal in the control (no advice) group. Gender split: not reported. Age: mean (SE), 62 (2.0) years. Nutritional status: mean (SE) % IBW, intervention 77.8 (1.6) %; control 78.6 (2.0) %. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of nutritional counselling to achieve a balanced meal plan plus supplements as needed; advice provided during 4‐week inpatient phase and then at each outpatient visit. Control: participants received no dietary advice and no ONS in the form of no dietary advice. |
|
| Outcomes | Weight*, TSF*, MUAC*, grip strength*, respiratory function*, QoL* (Sickness Impact Profile (SIP)). | |
| Publication details |
Language: English. Funding: the National Heart, Lung and Blood Institute, the American Lung Association of Southwestern Pennsylvania, Clinical Research Unit of Presbyterian‐University Hospital and Ross Laboratories. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information awaited from authors. No data on QoL was presented, only that the difference was not significant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | No nutritional outcomes reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for functional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal in the no advice group. Reason not given. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. All specified outcomes were reported in a format not suitable for entry into meta‐analysis. Change in weight, TSF, MAMC and handgrip strength are reported as mean (SD) at the start and end of intervention with a P value. No data obtained from authors therefore mean change (SD) derived using data in the paper. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Rydwik 2008.
| Study characteristics | ||
| Methods | RCT. Parallel design with 4 arms. Duration: 12 weeks intervention, and a further 6 months follow‐up. Location: Sweden. |
|
| Participants |
Inclusion criteria: frail adults, aged over 75 years with unintentional weight loss > 5% or BMI < 20 kg/m² (or both) and low physical activity level. Exclusion criteria: under 75 years, BMI > 30 kg/m2, non‐walkers, people with recent cardiac problems requiring hospital care, recent hip fracture or surgery with previous 6 months, CVA within the previous 2 years, score below 7 points on the SF‐MMSE or institutionalisation. Diagnosis: older people living in the community. Number randomised: 96 participants (intervention group 1, n = 25; intervention group 2, n = 25; intervention group 3, n = 23; control group, n = 23). Attrition: 32 dropouts (intervention group 1, n = 7; intervention group 2, n = 11; intervention group 3, n = 4; control group, n = 10). Gender split: 38/96 (40%) males, 58/96 (60%) females. Age: mean (SD) years intervention group 1, 83.1 (4.5); intervention group 2, 83.1 (4); intervention group 3, 83.5 (3.7); control group, 82.9 (4). Nutritional status: BMI kg/m2 intervention group 1, 21.8 (3.4); intervention group 2, 21.9 (3.8); intervention group 3, 21.9 (3.8); control group, 21.6 (3.6). |
|
| Interventions |
Intervention 1: participants received dietary advice in the form of dietary counselling to increase energy intake. Intervention 2: dietary counselling plus exercise training. Intervention 3: exercise training alone. Control group: participants received no dietary advice in the form of general physical training advice (30 min per day) and general diet advice (3x main courses and 2 ‐ 3 in‐between meal snacks daily). |
|
| Outcomes | Weight*, TSF*, fat free mass, energy intake*, muscle strength, physical performance (balance, time‐to‐up‐and‐go, walking speed, chair to stand etc), self‐efficacy. | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Data on dietary counselling group and control group will be used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "subjects were randomised consecutively in batches.....The randomisation procedure was conducted in an open manner". Insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Blinding not described but assessment of mortality unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not stated and likely that some outcomes might be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described and likely that lack of blinding might influence assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully described 32/96 (33%) dropouts overall: 7/25 (28%) in the dietary counselling group, 11/25 in the dietary counselling plus exercise, 4/23 in the exercise alone group and 10/23 (43%) in the control group. Judged as low risk because amount of attrition imbalanced between intervention groups (dietary counselling 28% versus control 43%) but the difference not greater than 20%. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All specified outcomes reported apart from TSF. The data were requested from the authors but unavailable. Data on mean change in weight and energy intake were reported without SD and so have been obtained from the author. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Salva 2011.
| Study characteristics | ||
| Methods | Cluster RCT. Duration: 12 months. Location: Spain. |
|
| Participants |
Inclusion criteria: diagnosis of mild‐moderate dementia; MMSE < 26; living at home; ambulatory with identified caregiver. Exclusion criteria: MMSE > 26; residents in an institution; nasogastric feeding; terminal care; already participating in a nutrition intervention study. Diagnosis: dementia (SSM IV criteria). Number randomised: 946 (intervention group, n = 448; control group, n = 498). Attrition: fully described; intervention group 4/29 (14%), control group 4/31 (13%). Gender split: 302/646 (32%) male, 644/946 (68%) female. Age: mean (SD) years 79 (7.3). Nutritional status: MNA (intervention group 7.8% malnourished and 51.5% at risk; control group 2.8% malnourished and 34.5% at risk). |
|
| Interventions |
Intervention: participants received dietary advice in the form of standardised protocol for feeding and nutrition delivered as education to caregivers, participants and relatives; Control: participants received no dietary advice in the form of usual care (detail not described). |
|
| Outcomes | Activities of daily living; MNA; caregiver burden scale; nutritional status (weight, MAC, calf circumference); cognitive function (MMSE, Clinical Dementia Rating scale, Eating Behaviour Scale, neuropsychiatric inventory questionnaire, depression, instrumental activities of daily living, healthcare costs, caregiver burden (Zarit scale), mortality. | |
| Publication details |
Language: English. Funding: commercial funding ‐ Nestec Limited. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from paper: "The unit of randomisation was the medical centres..." Comment: insufficient detail of the method provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not described but judged to be low risk as assessment of clinical outcomes is unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Not described but judged to be high risk as assessment of some functional outcomes is likely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Not described but judged to be low risk as no nutritional intake outcomes assessed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described and likely that some outcomes might be influenced by lack of blinding of assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants lost to follow‐up/deaths/dropouts fully described: intervention group 155/448(35%) (43 deaths,7 lost to follow‐up, dropouts 105); control group 135/498 (27%) (29 deaths,5 lost to follow‐up, dropouts 101). Attrition high but numbers and reasons similar in each group. |
| Selective reporting (reporting bias) | Low risk | Published protocol identified. All outcomes fully reported. |
| Other bias | High risk | Baseline characteristics were compared. The intervention group were frailer at baseline (MMSE score Clinical Dementia Rating score, NPI‐Q score and activities of daily living score and had a higher caregiver burden (Zarit scale). More participants in the intervention group were malnourished or at risk of being malnourished intervention group 7.8% malnourished and 51.5% at risk; control group 2.8% malnourished and 34.5% at risk. All of these factors have the potential to influence outcomes assessed. Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: no (2) Baseline imbalance: frail status (3) Loss of clusters: no (4) Incorrect analysis: no (5) Comparability with individually randomised trials / different types of clusters: different types of clusters |
Schilp 2013.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 6 months. Location: the Netherlands. |
|
| Participants |
Inclusion criteria: adults aged > 65 years, home‐dwelling, non‐institutionalized, undernourished (SNAQ65+). Exclusion criteria: MMSE < 18, unable to stand on the weighing scale. Number randomised: 146 participants (intervention group,n = 72; control group, n = 74). Attrition: 127 participants completed the 6 months examination: intervention group, n = 62 (86 %); control group, n = 65 (88 %). The reasons for dropout were withdrawal (intervention group n = 5; control group n = 6), death (intervention group n = 3; control group n = 0) and health problems (intervention group n = 2; control group n = 3). Gender split: 52 males, 94 females. Age: mean (SD), intervention 80.6 (7.5) years; control 80.5 (7.5) years. Nutritional status: n (%) meeting SNAQ criteria for undernutrition, intervention weight loss ≥ 4 kg/6 months 23(32), MUAC < 25 cm 35 (49), both 14 (19); control weight loss ≥ 4 kg/6 months 26 (35), MUAC < 25 cm 37 (50), both 11 (15). |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of dietary counselling from a team of 18 qualified trained dietitians, aiming to achieve adequate protein and energy intake, preferably by regular foods and beverages. Control: participants received no dietary advice and no ONS in the form of usual care, no referral to a dietitian but provided with a standard brochure of the Netherlands Nutrition Centre with general information about healthy eating habits. |
|
| Outcomes | Body weight*, physical performance, handgrip strength*, energy intake*, protein intake*, fat‐free mass* as costs* were assessed at baseline, after 3 months and 6 months and QoL* after 6 months. | |
| Publication details |
Language: English. Funding: the Ministry of Health, Welfare and Sports of the Netherlands. Publication status: peer‐reviewed journal. |
|
| Notes | To avoid bias of potential prescription of vitamin D as part of the dietetic treatment, all participants were prescribed a combined calcium (1000 mg calcium carbonate) plus vitamin D (800 IU cholecalciferol) supplement by their general practitioner if this was not already used. Analyses were derived from GEE; mean changes were re‐calculated from the data set by one of researchers of the group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Block randomisation by the primary investigator within 1 day of baseline examination using the website www.randomization.com. Participants recruited at an outpatient clinic department were randomised with a separate scheme, because they were expected to be more severely undernourished. |
| Allocation concealment (selection bias) | Low risk | Random allocation to either the intervention group or the control group was individually performed in blocks of 4 and 6 by using the website www.randomization.com |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Quote: Participants, researcher and research assistants were no longer blinded for the intervention assignment from this point [=after randomization]. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Quote: Participants, researcher and research assistants were no longer blinded for the intervention assignment from this point [=after randomisation]. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After 3 months 8 participants in the intervention group and 9 participants in the control group were lost to follow‐up (withdrawn, health problems or death), after months another 2 participants in the intervention group (withdrawn, health problems) and 0 participants in the control group were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol identified Dutch Trial Register NTR 1808, all planned outcomes with the exception of the secondary outcomes MUAC and supplementation with calcium and vitamin D were reported. |
| Other bias | Low risk | Baseline characteristics were similar between groups and there were no statistically significant differences in baseline characteristics between participants who discontinued early and study completers. |
Schwenk 1999.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 8 weeks. Location: Germany. |
|
| Participants |
Inclusion criteria: HIV positive adults who had lost > 5% of usual weight or who were actively losing weight, > 3% in last month. Exclusion criteria: unable to swallow usual food, severe lactose intolerance, prescription of any ONS, nutritional counselling, hormonal or appetite stimulants, enteral or parenteral nutrition during the previous 3 months. Number randomised: 50 participants (intervention group, n = 26; control group, n = 24). Attrition: 5 drop outs (intervention group, n = 2; control group, n = 3). Gender split: 47 males, 3 females. Age: mean (SD) intervention group 39.4 (9.2) years; control group 39.5 (10.2) years). Nutritional status: mean (SD) BMI, intervention group 1 19.6 (2.3) kg/m2; intervention group 2 19.9 (2.1) kg/m2. |
|
| Interventions |
Intervention (intervention group 1): particpants received ONS in the form of oral nutritional supplements (0.6 ‐ 1.5 kcal/mL) to increase energy intake by 600 kcal. Intervention (intervention group 2): participants received dietary advice in the form of dietary counselling to increase food intake by 600 kcal using household food items. |
|
| Outcomes | Survival*, change in body cell mass and change in weight*, change in energy intake*, hospital admissions*, Cost‐effectiveness planes and cost‐effectiveness acceptability curves, QALYs. | |
| Publication details |
Language: English. Funding: Nestle Clinical Nutrition, Germany. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from author, block randomisation derived using random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared by a person not involved in the study. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Information from author indicates that the study was not blinded but unlikely that lack of blinding would influence assessment of clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Information from author indicates that the study was not blinded and likely that lack of blinding would influence assessment of functional outcomes. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Information from author indicates that the study was not blinded and likely that lack of blinding would influence assessment of nutritional outcomes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Incomplete blinding and likely that assessment of some outcomes could be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully reported and similar in each group. 3/24 (12%) dropouts in control (dietary counselling) group and 2/26 (8%) dropouts in intervention (supplement) group. Reasons for drop out were opportunistic infections n = 4 and change of residence n = 1. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All specified outcomes reported but data were not in a form usable for meta analysis. Data on weight change were reported as % change in area under the curve and data on energy intake was reported as mean calories per kg, therefore mean change (SD) obtained from authors. Data on number of hospital admissions confirmed with the author. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Sharma 2002a.
| Study characteristics | ||
| Methods | RCT. Parallel group with 3 arms. Duration: 1 month. Location: single centre in India. |
|
| Participants |
Inclusion criteria: adults with renal disease receiving maintenance dialysis 3x weekly for more than 1 month, BMI < 20 kg/m2, and serum albumin < 4.0 g/dL. Exclusion criteria: diabetes, presence of intercurrent illness. Number randomised: 47 participants (intervention group 1, n = 10; intervention group 2, n = 16; control group, n = 14 control). Attrition: 40 participants analysed; 7 dropouts (intervention groups n = 5; control group n = 2). Gender split: 35 males, 5 females. Age: mean intervention group 1 (commercial supplement) 29.6 years; intervention group 2 (home blend) 32.7 years; control group 31.9 years. Nutritional status: mean (SD) BMI, intervention 1, 17.9 (1.3) kg/m2; intervention 2, 17.2 (1.9) kg/m2 control 17.1 (1.9) kg/m2. |
|
| Interventions |
Intervention (group 1): participants received dietary advice and ONS in the form of dietary counselling to increase intake in line with current recommendations for renal disease plus 300 mL of commercial supplement (500 kcal, 15 g protein). Intervention (group 2): participants received dietary advice and ONS in the form of dietary counselling to increase intake in line with current recommendations for renal disease plus 300 mL of home‐produced blend providing similar kcal and protein. Control: participants received dietary advice alone in the form of dietary counselling to increase intake but in line with current recommendations for renal disease. |
|
| Outcomes | Weight*, biochemistry, energy intake*, protein intake*, appetite, Karnofski index, supplementation acceptability questionnaire. | |
| Publication details |
Language: English. Funding: the study was supported by Baxter and by a hospital fund. Publication status: peer‐reviewed journal. |
|
| Notes | This study is a duplicate of Sharma 2002b. The study has 2 intervention arms and a single control group. Therefore the number of participants in the control group is divided by 2. This study ID describes the data of intervention group 'home blend' versus control, whereas Sharma 2002b describes 'commercial supplement' versus control. Study is eligible for inclusion on the basis of the intervention; however, due to the high number of control participants that crossed over to the intervention, data cannot be included without further analysis. Participants were randomised 1:2 into control and intervention, and in turn the experimental group was randomised to receive the commercial nutritional supplement or home‐prepared supplement blend. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Is is unlikely that biochemistry measurements were influenced by knowing the group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 dropouts; 5 of 26 (19%) in the intervention group and 2 of 14 (14%) in the control group. Reasons not given. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Data not all in usable format and not available from author, therefore mean change (SD) has been derived by calculation from the data in Table 2. |
| Other bias | High risk | Baseline data only presented on participants that completed the study (n = 40). 5 participants crossed over from the control to the supplement group. |
Sharma 2002b.
| Study characteristics | ||
| Methods | RCT. Parallel group with 3 arms. Duration: 1 month. Location: single centre in India. |
|
| Participants |
Inclusion criteria: adults with renal disease receiving maintenance dialysis x3 weekly for more than 1 month, BMI < 20 kg/m2, and serum albumin < 4.0 g/dL. Exclusion criteria: diabetes, presence of intercurrent illness. Number randomised: 47 participants (intervention group 1, n = 10; intervention group 2, n = 16; control group, n = 14 control). Attrition: 40 participants analysed; 7 dropouts (intervention groups n = 5; control group n = 2). Gender split: 35 males, 5 females. Age: mean intervention group 1 (commercial supplement) 29.6 years; intervention group 2 (home blend) 32.7 years; control group 31.9 years. Nutritional status: mean (SD) BMI, intervention 1, 17.9 (1.3) kg/m2; intervention 2, 17.2 (1.9) kg/m2 control 17.1 (1.9) kg/m2. Nutritional status: all participants had a BMI < 20 kg/m² and serum albumin < 4.0 g/dL. |
|
| Interventions |
Intervention (group 1): participants received dietary advice and ONS in the form of dietary counselling to increase intake in line with current recommendations for renal disease plus 300 mL of commercial supplement (500 kcal, 15 g protein). Intervention (group 2): participants received dietary advice and ONS in the form of dietary counselling to increase intake in line with current recommendations for renal disease plus 300 mL of home‐produced blend providing similar kcal and protein. Control: participants received dietary advice alone in the form of dietary counselling to increase intake but in line with current recommendations for renal disease. |
|
| Outcomes | Weight*, biochemistry, energy intake*, protein intake*, appetite, Karnofski index, supplementation acceptability questionnaire. | |
| Publication details |
Language: English. Funding: the study was supported by Baxter and by a hospital fund. Publication status: peer‐reviewed journal. |
|
| Notes | This study is a duplicate of Sharma 2002a. The study has 2 intervention arms and a single control group. Therefore the number of participants in the control group is divided by 2. This study ID describes the data of intervention group 'commercial supplement' versus control, whereas Sharma 2000a describes 'home blend' versus control. Study is eligible for inclusion on the basis of the intervention; however, due to the high number of control participants that crossed over to the intervention, data cannot be included without further analysis. Participants were randomised 1:2 into control and intervention, and in turn the experimental group was randomised to receive the commercial nutritional supplement or home‐prepared supplement blend. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Is is unlike that biochemistry measured were influenced by knowing the group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The trial was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The trial was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 dropouts; 5 of 26 (19%) in the intervention group and 2 of 14 (14%) in the control group. Reasons not given. |
| Selective reporting (reporting bias) | High risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Data not all in usable format and not available from author, therefore mean change (SD) has been derived by calculation from the data in Table 2. |
| Other bias | High risk | Baseline data only presented on participants that completed the study (n = 40). 5 participants crossed over from the control to the supplement group. |
Sharma 2017.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 3 months. Location: single centre in Australia. |
|
| Participants |
Inclusion criteria: 60 years and over presenting to the General Medicine Department of the Flinders Medical Centre between November 2014 and June 2016, malnourished according to PG‐SGA (classes B or C). Exclusion criteria: receiving palliative care, resident in rural areas, indigenous Australians, non‐English speaking and unable to give informed consent. Number randomised: 148 participants (intervention group, n = 78; control group, n = 70). Attrition: 103 analysed (intervention group, n = 57; control group, n = 46), 45 lost to follow‐up (intervention group, n = 21; control group, n = 24). Gender split: intervention group 29.7% males, control group 32.9% males. Age: mean (range), intervention group 82.0 (80.0 ‐ 83.9), control group 81.6 (79.5 ‐ 83.6). Nutritional status: n (%) PG‐SGA score, intervention PG‐SGA B 67 (90.5), PG‐SGA C 7 (9.5); control PG‐SGA B 60 (87), PG‐SGA C 9 (13). |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of individualized nutrition care plan plus monthly post‐discharge telehealth follow‐up. Control: participants received no dietary advice and no ONS in the form of intervention only upon referral by their treating clinicians. |
|
| Outcomes | Length of stay, skinfolds*, MUAC*, grip strength* complications, QoL (EQ‐5D)*, mortality*, readmission rate*, nutritional status by PG‐SGA score. | |
| Publication details |
Language: English. Funding: not declared. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: An independent biostatistician prepared the randomisation schedule using random blocks of 8; treatment allocations were randomly permuted and balanced within blocks. |
| Allocation concealment (selection bias) | Low risk | Quote: After obtaining written informed consent, the researcher contacted central office to open these sealed envelopes to allocate participants to either control or intervention groups. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Quote: After group allocation the participants and the ward dietitian, who provided nutrition intervention, were not blinded to group allocation but the research dietitian who conducted the final outcome assessment was blinded to participants' group allocation. In addition, the research person overseeing data entry and the biostatistician were blinded. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Quote: After group allocation the participants and the ward dietitian, who provided nutrition intervention, were not blinded to group allocation but the research dietitian who conducted the final outcome assessment was blinded to participants' group allocation. In addition, the research person overseeing data entry and the biostatistician were blinded. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Quote: After group allocation the participants and the ward dietitian, who provided nutrition intervention, were not blinded to group allocation but the research dietitian who conducted the final outcome assessment was blinded to participants' group allocation. In addition, the research person overseeing data entry and the biostatistician were blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by a research dietitian who was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 148 participants randomised (intervention group n = 78; control group n = 70), complete data were available for analysis for 103 participants (intervention group n = 57; control group n = 46 participants). The main reasons for participants being lost to follow‐up were loss of contact n = 7 (intervention group n = 1; control group n = 6), consent withdrawal n = 12 (intervention group n = 8; control group n = 4) and death n = 26 (intervention group n = 12; control group n = 14). Numbers and reasons similar between groups |
| Selective reporting (reporting bias) | High risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Low risk | Baseline characteristics similar between groups, with both groups having a higher number of females, the majority of participants residing at home pre‐admission, a similar number of co‐morbidities, and similar Charlson Co‐morbidity Index and other baseline characteristics. There was no difference in the severity of malnutrition at baseline. |
Silvers 2014.
| Study characteristics | ||
| Methods | RCT (pilot study). Parallel design with 2 arms. Duration: 6 months. Location: Australia. |
|
| Participants |
Inclusion criteria: histologically proven new diagnosis of primary oesophageal or stomach cancer, due to undergo surgery and/or chemotherapy, aged 19 years and over. Exclusion criteria: recurrent disease or physical, cognitive, language or emotional problems that would prevent participation, treatment was planned at another health care facility. Number randomised: 21 participants (intervention group, n = 10; control group, n = 11). Attrition: 6 deaths (intervention group, n = 1; control group, n = 5). Gender split: intervention group 50% males, control group 64% males. Age: mean (SD) intervention group 72 (12) years; control group 64 (14) years. Nutritional status: mean (SD) BMI: intervention group 28 (6) kg/m2, control group 26 (5) kg/m2. |
|
| Interventions |
Intervention group: participants received dietary advice plus ONS if required in the form of intensive nutritional counselling commenced immediately after diagnosis via weekly telephone call by a research dietitian (nutritional assessment and advice using a tailored, symptom‐directed treatment approach) with face‐to‐face interviews scheduled if the participant was attending the hospital; weight, nutrition‐impact symptoms and oral intake monitored and oral nutritional supplements supplied if clinically indicated. Control group: participants received no dietary advice and no ONS in the form of no planned dietetic input until participants admitted for surgery or chemotherapy leading to an anticipated delay of approximately 6 ‐ 10 weeks before initial contact with a dietitian; contact with the dietitian only if nursing or medical staff made a referral. |
|
| Outcomes |
Primary outcome: health‐related QoL*. Secondary outcomes: change in weight*, and patient‐generated SGA. |
|
| Publication details |
Language: English. Funding: Southern Melbourne Integrated Cancer Service (SMICS). Publication status: peer‐reviewed journal. |
|
| Notes | Additional data on weight change were obtained from the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was constructed using a computer random number generator. Randomisation was stratified by diagnosis (oesophageal or stomach cancer). |
| Allocation concealment (selection bias) | Low risk | The method of concealment was through use of opaque, consecutively numbered, sealed envelopes with the group allocation written on a piece of paper inside. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The study was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data unbalanced in numbers across intervention groups; 5 (45%) deaths in control group, 1(10%) death in intervention group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Unclear risk | Participants in the intervention group tended to be older and have a higher BMI. |
Singh 2008.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 3 months. Location: India. |
|
| Participants |
Inclusion criteria: adults with chronic pancreatitis and undernourished, BMI < 18.5 kg/m² or > 10% weight loss in previous 6 months. Exclusion criteria: clinically apparent steatorrhoea, pancreatic cancer, biliary obstruction, undergoing endoscopic or surgical therapy, uncontrolled diabetes, acute exacerbation of pancreatitis, large pseudocysts, currently consuming alcohol, opioid addicts, comorbid condititions eg. chronic liver disease. Number randomised: 60 participants (intervention group, n = 31; intervention group 2, n = 29). Attrition: 6 dropouts (intervention group, n = 4; intervention group 2, n = 2). Gender split: 50 males, 10 females. Age: mean (SD), intervention group 1, 28 (10) years; intervention group 2, 32 (10) years. Nutritional status: mean (SD) BMI: intervention group 1, 16.7 (1.6) kg/m2, intervention group 2, 17.2 (1.7) kg/m2. |
|
| Interventions |
Intervention (intervention group 1): particpants received ONS in the form of a nutritional supplement enriched with medium chain triglycerides to meet predicted energy requirements. Intervention (intervention group 2): participants received dietary advice in the form of dietary advice from a dietitian to meet predicted energy requirements. |
|
| Outcomes | BMI*, weight* TSF*, MAMC*, energy and protein intake, nitrogen balance, faecal fat, pain score. | |
| Publication details |
Language: English. Funding: Indian Council for Medical Research. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Carried out by individual not otherwise involved in the study. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Outcome assessment blinded to treatment. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Outcome assessment blinded to treatment. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Low risk | Outcome assessment blinded to treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessment was assessed blinded to group allocation. Quote: "the person assessing outcome was blinded to the treatment the patient was receiving". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fully reported and similar between groups: 4/31 (13%) intervention group at 1.5 months, 2/29 (7%) control group at 1.5 months. But all included in the final analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes reported but not in a format usable for meta analysis. Data on change in weight, TSF, MAC and energy intake were reported as mean (SD) at baseline and mean (SD) at end of intervention, therefore mean change (SD) obtained from authors. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Starke 2011.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: intervention 5 days to maximum 28 days, follow‐up for 6 months. Location: Switzerland. |
|
| Participants |
Inclusion criteria: adults consecutively admitted to the general medical ward at “Kantonsspital Liestal” hospital at nutritional risk using the NRS‐2002 questionnaire (NRS score 3 or above; participants with a score below 3 were re‐evaluated weekly during hospitalisation and asked to participate if a nutritional risk developed). Exclusion criteria: no informed consent, terminal condition, expected stay less than 5 days (judged by physician), previous participation in this study, participant on starvation, on parenteral nutrition, and/or being on dialysis. Number randomised: 132 participants (intervention group, n = 66; control group, n = 66). Protocol violations: 8 participants in each groups. Data analysed, based in ITT analyses, all 132 participants. Gender split: not reported. Age: mean (SD) intervention group 70 (16) years; control group 75 (11) years. Nutritional status: mean (SD) BMI, intervention group 24.6 (5.3) kg/m2, control group 24.1 (4.9) kg/m2. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of individual nutritional care to meet the daily energetic requirement*, including a detailed nutritional assessment, individual food supply, fortification of meals with maltodextrin, rapeseed oil, cream and/or protein powder, in‐between snacks and oral nutritional supplements. Complications influencing feeding (e.g. nausea) were reported to the ward physician and treatment was optimised (e.g. medication). If less than 75% of the portion (i.e. served food at one meal with known energy/protein content) was consumed, energy and protein intake was compensated on a daily basis by either oral nutritional supplements (Nestlé Nutrition) or in‐between meals. Control: participants received no dietary advice and no ONS in the form of standard nutritional care, including the prescription of oral nutritional supplements and nutritional therapy prescribed by the physician independently of this study and according to the routine ward management. *daily energetic requirement according to the individual total energy expenditure (calculated from resting energy expenditure corrected by an individual factor for physical activity level and disease (stress factor, SF12); protein intake was set at 1.0 g/kg body weight. |
|
| Outcomes |
Primary outcomes: average daily energy* and protein* intake. Secondary outcomes: change in body weight* during hospitalisation, number of complications, number of antibiotic therapies due to infectious complications, length of hospital stay*, QoL* Short Form 36 Questions Score, hospital readmission* (after 6 months), mortality* (hospital and 6 months after discharge), compliance with oral nutrition standard supplement consumption and plasma concentrations of 25‐OH‐D3, ascorbic acid and glutathione. |
|
| Publication details |
Language: English. Funding: no financial or personal interest in any company/organisation sponsoring the study (JS, HS, PS). JS received grants from the Exchange Organisation StudEx/ Switzerland and the German Acadamic Exchange Service (DAAD)/ Germany at different time intervals during the study. Further study support was received by Nestlé Nutrition/Switzerland for biochemical analyses. None of the mentioned organisations and enterprises participated in protocol preparation, analysis nor interpretation of data, writing the manuscript and publication matters. RM received grants for clinical studies and education from Nestlé. Publication status: peer‐reviewed journal. |
|
| Notes | The aim of the present study was to develop and evaluate a routinely manageable concept for an improved nutritional care of malnourished hospitalised patients. Some results are only described in the text, but no data given. The corresponding author was contacted and asked to provide change scores energy intake and protein, but he answered that he could not provide these data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: computer‐generated randomization. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Quote: except energy and protein intake, all outcome data were blinded in terms of that physicians and nurses who were responsible for the outcome did not have access to group allocation. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | Quote: except energy and protein intake, all outcome data were blinded in terms of that physicians and nurses who were responsible for the outcome did not have access to group allocation. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Quote: except energy and protein intake, all outcome data were blinded in terms of that physicians and nurses who were responsible for the outcome did not have access to group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical and functional outcomes but high risk for nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Discharged or death before minimum intervention period occurred in 8 participants in each group. 1 participant in each group was excluded from ITT analyses due to withdrawal or death. Numbers and reasons for attrition balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. Protocol violations. |
| Other bias | Low risk | Baseline parameters were comparable between groups. |
Stow 2015.
| Study characteristics | ||
| Methods | RCT (feasibility study). Cluster randomised. Duration: 6 months. Location: UK. |
|
| Participants |
Inclusion criteria: MUST score > 1, able to eat and drink, registered with a GP and eligible for treatment by the UK National Health Service, living in residential care home. Exclusion criteria: receiving enteral or parenteral nutrition, receiving nutritional support (advice or oral nutritional supplements), known eating disorder or condition not compatible with receiving the intervention, non‐native English speaker, lacking capacity. Number randomised: 93 participants (intervention group 1, n = 29; intervention group 2, n = 32; control group, n = 32). Attrition: 63 of 93 residents (36%). Gender split: 20 males, 73 females. Age: mean (SD) years not reported. Nutritional status: 50/91 (55%) high nutritional risk, 41/91 (45%) medium nutritional risk (MUST)*. |
|
| Interventions |
Intervention (intervention group 1): particpants received ONS in the form of standard care* plus 2x liquid oral nutritional supplements providing 600 kcal, 24 g protein daily. Intervention (intervention group 2): participants received dietary advice in the form of face‐to‐face instruction from the primary researcher to increase intake by approximately 600 kcal and 20 ‐ 25g protein daily. Control: participants received no dietary advice and no ONS in the form of standard care* plus instructions to care and catering staff on increasing residents' daily intake by 600 kcal, 20 ‐ 25g protein per day. *provision of a calorie‐dense diet, small frequent, energy‐enriched meals in a family‐style dining room with prompting and assistance. |
|
| Outcomes | Nutritional intake, weight, BMI, handgrip strength, MUAC, TSF, VAS, QoL (EQ‐5D, CO‐OP). | |
| Publication details |
Language: English. Funding: undertaken by an MRes student funded by NIHR Clinical Academic Training Programme for AHPs. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: sequence was generated using a computer‐generated random number list. Homes were randomised once eligible residents had been identified. |
| Allocation concealment (selection bias) | Low risk | Quote: sequence was generated using a computer‐generated random number list. Homes were randomised once eligible residents had been identified. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | No blinding, but unlikely that assessment of clinical outcomes would be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | No blinding, and likely that assessment of functional outcomes would be influenced by lack of blinding. Quote: 1 primary researcher communicated intervention allocation … and conducted outcome assessments. It was therefore impossible to blind the researcher to intervention. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | No blinding, and likely that assessment of nutritional outcomes would be influenced by lack of blinding. Quote: 1 primary researcher communicated intervention allocation…and conducting outcome assessments. It was therefore impossible to blind the researcher to intervention. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: residents were recruited prior to random allocation of care homes to the treatment/control arms. Individual residents were not told of the care home intervention assignment. …due to the nature of the interventions, ... it was not possible to blind the staff delivering them. Judgement, incomplete blinding and lack of blinding likely to have influenced outcome assessments. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Researcher collected all data; it was impossible to blind the researcher to group. No blinding and lack of blinding might have influenced the assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition amount fully reported but group allocation unclear. 63/93 (36%) participants completed the study. 23 deaths, 6 in the control (dietary advice) group, 6 in the intervention (supplement) group and 11 in the routine care group (information provided by author). Numbers and reasons for withdrawal reported but not according to group allocation. |
| Selective reporting (reporting bias) | Low risk | Protocol published on Current Controlled Clinical Trials. All outcomes specified reported or commented on, e.g. qualitative outcomes to be reported in a separate paper and QoL not analysed because of completion by too few residents. |
| Other bias | High risk | Baseline characteristics presented, the group receiving the control (food‐based intervention) were heavier than the intervention (supplement) group and had a higher energy, protein and fluid intake at baseline as well as a higher EQ5D VAS score. Judged to be likely that these differences would influence outcome assessment. Assessment of risk of bias in cluster‐randomised trials (1) Recruitment bias: yes (care home residents recruited after recruitment of care homes) (2) Baseline imbalance: weight, energy, protein & fluid intake, QoL (detail above) (3) Loss of clusters: no (4) Incorrect analysis: ? (feasibility trial and so not carried out for analyses in this paper, but planned for subsequent trial) (5) Comparability with individually randomised trials / different types of clusters: inclusion in meta‐analyses results in heterogeneity for some outcomes |
Suominen 2015.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 1 year. Location: Finland. |
|
| Participants |
Inclusion criteria: individuals with Alzheimer's disease living with a spouse, aged > 64 years, able to reach the study place by taxi, able to stand on a scale, resident in the Helsinki metropolitan area, absence of terminal disease, an estimated life expectancy of at least half a year (confirmed by medical records). Exclusion criteria: not mentioned. Number randomised: 99 couples (intervention group, n = 50; control group, n = 49). Attrition: 78 couples completed the study (intervention group, n = 40; control group, n = 38). Gender split: 31% males, 69% females. Age: mean (SD), intervention 78.2 years; control 76.8 (5.9) years. Nutritional status: assessed by MNA. Intervention % with score < 17: 0%; 17 ‐ 23.5: 43%; > 23.5: 57%. Control % with score < 17: 0%; 17 ‐ 23.5: 37%; > 23.5: 63%. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of tailored nutritional guidance on the basis of the food diaries, results of weight measurement, home visits and discussions with the participants and their caregivers held every 3 months; oral nutritional supplements provided according to participants' needs. Control: participants received no dietary advice and no ONS in the form of a written guide on nutrition of older people. |
|
| Outcomes |
Primary outcome: change in weight* of individuals with Alzheimer's disease. Secondary outcomes: changes in protein intake* and other nutrients, health‐related QoL* and rate of falls. |
|
| Publication details |
Language: English. Funding: support from Finland’s Slot Machine Association (RAY) and Nutricia provided the protein supplements. The funders had no role in the design, analysis or interpretation of data or in writing, reporting or deciding whether to submit this article for publication. The authors are independent researchers unassociated with the funders. Publication status: peer‐reviewed journal. |
|
| Notes | Study aimed to examine the effect of tailored nutritional guidance on nutrition, health‐related QoL and falls in people with Alzheimer disease. The authors report no change in weight between the groups, however they cannot provide the data. They also emailed that they collected data on energy intake, but these were not reported in the manuscript and were not received from the authors either. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participating couples were randomly allocated according to a computer‐generated, blocked randomisation list. The block size was six, and the randomisation took place between August 2010 and January 2011. A person unrelated to the investigation and unfamiliar with the procedure performed the randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: A person unrelated to the investigation and unfamiliar with the procedure performed the randomisation. It remains unclear how, and at which time‐point this was done. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | It is unlikely that participants were unaware of group assignment. Thus assume that the trial was unblinded. However, this is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The trial was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not descrobed and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of 99 participating couples, 78 completed the study (intervention group n = 40; control group n = 38). Reasons for drop out are well‐explained (moving to another city, moving to long‐term care, death, food records not received). |
| Selective reporting (reporting bias) | High risk | The data for change in weight (primary outcome) are not available. Authors only report that there were no differences. As data are not available, this is judged as high risk. The study protocol is available, Jyvakorpi SK, Trials 2012, and all outcomes are reported in different papers. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Terp 2018.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 3 months. Location: Denmark. |
|
| Participants |
Inclusion criteria: geriatric patients in a university hospital and in the primary healthcare sector in Copenhagen, > 65 years, at nutritional risk at admission (NRS‐2002), BMI < 20.5 kg/m2 and/or weight loss within the last three months and/or a reduced dietary intake in the previous week and/or severely ill). Exclusion criteria: terminal illness, active cancer diagnosis, permanently living in a nursing home, and not willing or able to give an informed consent. Number randomised: 144 participants. Gender split: 32 males, 112 females. Age: mean (SD), intervention group 87 (6) years; control group 88 (6) years. Nutritional status: mean (SD) BMI, intervention group 19.6 (2.0) kg/m2; control group 19.7 (2.8) kg/m2. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of an individual dietary plan, based on everyday food, if relevant combined with oral nutritional supplements, by a dietitian for each participant including advice on nutritional intake after discharge. After discharge, participants received a prescription for oral nutritional supplements, funded by the participants and 3 follow‐up visits conducted by a district nurse or a healthcare assistant were scheduled at 1, 4, and 8 weeks after discharge. Control: participants received no dietary advice and no ONS in the form of usual care i.e. weekly monitoring of nutritional status. The nursing staff completed the screening for nutrition risk at admission and the clinical dietician was involved in the process if the participant had specific needs and gave dietary advice and prepared a dietary plan for nutrition intake while they were hospitalized. At discharge, any nutritional problems were documented in the discharge summary, but no follow‐up on those who were at nutritional risk was planned. |
|
| Outcomes | Change in body weight*, Barthel Index, handgrip strength* and self‐rated health from baseline (discharge) to 3 months after discharge, readmission*, and mortality* (90 and 120 days). | |
| Publication details |
Language: English. Funding: work was supported by the Capital Region of Denmark. Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomization was performed using a computer‐generated randomization table. An equal distribution among intervention and control was achieved by blocks. |
| Allocation concealment (selection bias) | Low risk | Quote: The treatment allocation was written and stored in sequentially numbered opaque envelopes and was opened by a study personnel immediately after the participant had given informed consent. The randomization was performed before collection of baseline data. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Comment: Not blinded, clinical outcomes were unlikely to have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Comment: Not blinded, functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Comment: Not blinded, nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear because low risk for clinical outcomes and high risk for functional and nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 103 (72%) participants completed the 2nd data collection; 21 participants died (12 in the intervention group and 9 in the control group); 8 participants withdrew (4 in the intervention group and 4 in the control group); 12 participants were unable to participate (6 in the intervention group and 6 in the control group). Numbers and reasons for attrition balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol identified CliniclTrials NCT03131856 all planned outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Tu 2013.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: not clear. Location: Taiwan. |
|
| Participants |
Inclusion criteria: cancer patients on discharge from hospital. Exclusion criteria: multiple types of cancer, metastases, over 80 years of age. Diagnosis: cancer (breast 30%, colon 34.8%, other 35.2%). Number randomised: 537 participants (intervention group, n = 279; control group, n = 258). Attrition: not reported. Gender split: not reported. Age: mean (SD) 64.2 (10.3) years. Nutritional status: assessed using SGA, but difficult to ascertain baseline status. |
|
| Interventions |
Intervention group: participants received dietary advice in the form of on average 30 minutes of nutrition counselling and meal planning during the 1st week post discharge. Control group: participants received no dietary advice in the form of no nutrition counselling or meal planning. |
|
| Outcomes | SGA, weight, food intake. Analysed as composites: weight loss < 5% in 1 month + food intake > 75% of usual = "pass"; weight loss > 5% + food intake < 75% of usual = "fail". |
|
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Numerous questions about this study in terms of risk of bias and data. No response from authors. At present the method of data presentation means that it is not possible to extract data on any outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: patients… were randomly divided into experimental and control groups. Insufficient information to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk |
Quote: patients… were randomly divided into experimental and control groups. Insufficient information to make a judgement. |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | No clinical outcomes assessed. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not described and likely that assessment of some outcomes would be affected by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described and likely that lack of blinding might influence assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | High risk | No protocol identified. The 3 outcomes specified are reported, but only as a composite score used to assess arbitrary cut‐offs 'pass' and 'fail'. No original data presented. |
| Other bias | Unclear risk | Baseline characteristics not presented or described. |
Um 2014.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: duration of radiotherapy (3 weeks) with 1 month follow‐up. Location: single centre, hospital‐based radiotherapy unit, Seoul, South Korea. |
|
| Participants |
Inclusion criteria: individuals (> 15 years of age) with cancer of the head and neck, thorax or abdomen whose treatment plan included radiotherapy over 3 weeks. Exclusion criteria: people who had received nutrition counselling within 3 months, age > 80 years, poor tolerance of radiotherapy. Number randomised: 87 participants recruited (intervention group, n = 44; control group, n = 43). Attrition: 3 participants died (intervention group, n = 1; control group, n = 2). Gender split: intervention group 33 males (75%), 11 females (25%); control group 23 males (53%), 20 females (47%). Age: mean (SD) intervention group males 58.3 (11.8) years, females 56.8 (20.5) years; control group males 64.0 (11.4) years, 58.8 (11.8) years females. Nutritional status: BMI, mean (SD): intervention group 24.1 (2.6) kg/m² males, 23.8 (4.1) kg/m² females; control group 24.7 (2.7) kg/m² males, 22.7 (2.6) kg/m² females. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of intensive nutrition intervention following the standard protocol of the Academy of Nutrition and Dietetics for the duration of radiotherapy; information offered "to help them achieve adequate energy and protein intake"; 3 nutritional counselling sessions of 15 ‐ 30 minutes each (1) educated to meet goals using "a standard formula from the hospital" (2) educational materials provided to help minimise radiotherapy‐related side effects (3) calorie‐dense foods or quick, easy cooking ideas provided and individualised to each participant's environment. Educational materials and recipe suggestions for incorporating various antioxidant foods were provided. Started within 4 days of starting radiotherapy and repeated weekly or every other week during radiotherapy. Control: participants received no dietary advice and no ONS in the form of one routine 20 minute education session by a dietitian within 4 days of starting radiotherapy, plus samples of an oral nutritional supplement and a cancer survival booklet. |
|
| Outcomes | Nutritional status (PG‐SGA scores, weight*, BMI), dietary intake (energy* and protein*), QoL (EORTC‐QLQ‐C30)*, laboratory results. | |
| Publication details |
Language: English. Funding: not stated. Publication status:peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly placed into either nutritional intervention or control groups". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Unlikely to be blinded but assessment of mortality unlikely to be affected by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Unlikely to be blinded but assessment of functional outcomes likely to be affected by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | Unclear risk | Unlikely to be blinded but assessment of nutritional outcomes likely to be affected by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment of some outcomes may have been influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition fully reported. 3 participants (3.5%) died; 1/44 (2%) in the intervention group and 2/43 (5%) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified. |
| Other bias | Low risk | Baseline characteristics described and intervention and control groups similar at baseline. |
Uster 2013.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 3 months. Location: Switzerland. |
|
| Participants |
Inclusion criteria: adult cancer outpatients, undernourished or at high risk for undernutrition by the NRS 2002 tool. Exclusion criteria: estimated survival < 6 months (as judged by the treating physician), on enteral tube feeding or parenteral nutrition, ongoing nutritional counselling or interventions (e.g. intake of oral nutritional supplements), adjuvant chemotherapy, impaired cognition and inability to give consent. Number randomised: 58 participants (intervention group, n = 30; control group, n = 28). Attrition n = 29 (intervention group, n = 15; control group, n = 14). Gender split: 46 males, 12 females. Age: mean (SD), intervention group 66.2 (8.9) years; control group 63.8 (13.3) years. Nutritional status: mean (SD) BMI, intervention group 23.1 (2.4) kg/m2, control group 22.6 (2.8) kg/m2. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form ofstandardized individual nutritional therapy, including counselling by a dietitian, food fortification, and oral nutritional supplements if required. Control: participants received no dietary advice and no ONS in the form of standard care without specific nutritional intervention or fixed prescription of oral nutritional supplements; if participants had questions concerning nutrition, they were advised by the cancer centre's attending physician or the nurses but not by professional dietitians. |
|
| Outcomes | Dietary intake (3‐day dietary record)*, nutritional status (body weight)*, physical functioning* (performance status, handgrip strength) and QoL (EORTC‐C30)*. | |
| Publication details |
Language: English. Funding: grant from the Krebsliga Schweiz (Swiss Cancer Foundation), Nestle Healthcare Medical Nutrition (Vevey, Switzerland) contributed to the funding of the study. Publication status: peer‐reviewed journal. |
|
| Notes | The authors provided additional change scores for weight, energy intake, protein and handgrip strength. Additional change scores for QoL not available at the moment but will become available later, therefore final scores and SD for QoL read from the graphs. Due to a low recruitment of patients, the study was terminated after 2.5 years. A sample size of 200 participants (100 per arm) was calculated, 67 participants were included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The description of the study strongly suggests it was unblinded and that both participants and the treating dietitian were aware of group allocation. Unclear who performed the clinical or functional outcome measures. However, knowing group allocation is unlikely to influence clinical outcomes. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | The study was unblinded. Functional outcomes could have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | The study was unblinded. Nutritional outcomes could have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Due to a low recruitment of participants, the study was terminated after 2.5 years. A sample size of 200 participants (100 per arm) was calculated, 67 participants were included. Attrition n = 29 (intervention group n = 15; control group n = 14), 16 (8 in each group) of these participants died. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Unclear risk | The tumour types were comparable in the two groups, except for head and neck cancer, which happened to be randomised only the intervention group. The performance status in the intervention group was significantly lower than in the usual care group. |
Vivanti 2015.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: starting at the emergency department with follow‐up at 12 weeks. Location: Australia. |
|
| Participants |
Inclusion criteria: malnourished adults (MST, confirmed with SGA) aged 60 years or over, presenting to the emergency department. Exclusion criteria: unable to provide informed consent, triage category 1 (highest priority), already receiving dietetic care, admitted from a healthcare facility (including nursing home). Number randomised: 24 participants (intervention group, n = 10, control group, n = 14). Attrition: 5 participants (intervention group, n = 1; control group, n = 4). Gender split: 10 males, 14 females. Age: mean (SD) 79.0 (7.7) years. Nutritional status: assessed using the MST and confirmed with SGA, 21/24 (88 %) participants diagnosed with malnutrition. |
|
| Interventions |
Intervention: participants received dietary advice plus ONS if required in the form of individualised dietary counselling at baseline when nutrition goals and strategies were made in collaboration with the dietitian in the emergency department following standard medical nutrition therapy practice; follow‐up at a minimum of weeks 4 and 8, by telephone review or home visit or both. Strategies included both food‐based advice and use of ONS depending on the needs of the patient*. Control: participants received no dietary advice and no ONS in the form of regular treatment through community hospital interface programme nursing staff and community support. |
|
| Outcomes | Weight change, length of stay, QoL (EQ‐5D), depression, further decline in nutritional status, number of falls (self‐recorded). | |
| Publication details |
Language: English. Funding: Queensland Health allied health grants. Publication status: peer‐reviewed journal. |
|
| Notes | *information on nutrition strategies obtained from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician‐generated randomised sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed in sequentially numbered opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | The intervention group received usual care + individualised dietary care. This suggests that both treating dietitians and participants were aware of group allocation. The emergency department dietitian provided dietary counselling to the participants in the intervention groups and performed the following follow‐up measurements in both groups: malnutrition screening tool and subjective global assessment. Data on death, malnutrition diagnosis, number of presentations to the emergency department, days of unplanned hospital admissions and pressure ulcers were recorded from health information management and emergency department information systems. Clinical outcomes were unlike to be influenced by knowing group assignment. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | Blinding of assessment of depression not described and the outcome might have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and the treating dietitian were aware of group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judged to be unclear, as low risk for clinical outcomes and high risk for nutritional outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition: intervention group n = 1; control group n = 4, due to withdrawal and death. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. |
| Other bias | Unclear risk | No description of baseline characteristics. |
Weekes 2009.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 12 months (6 months intervention and follow‐up to 12 months). Location: UK. |
|
| Participants |
Inclusion criteria: adult outpatients with COPD and at risk of malnutrition using a validated nutritional screening tool. Exclusion criteria: conditions likely to compromise nutritional status further (diabetes, disseminated malignancy, congestive cardiac failure and untreated thyroid disease). Number randomised: 66 participants; but only 59 at baseline assessment (intervention group n = 31; control group n = 28). Attrition: 37 participants completed (7 deaths; 15 participants withdrew mainly because of deteriorating health). Gender split: (at baseline n = 59), 30 males, 29 females. Age: mean (range): 69 (47 ‐ 85) years for 37 participants completing the study; 69.1 (46 ‐ 89) years for 22 withdrawals. Nutritional status: mean (SD) BMI, intervention 19.9 (1.4) kg/m²; control 19.5 (1.9) kg/m². Mean (SD) unintentional change from usual weight, intervention ‐8.0 (5.2) kg; control ‐9.2 (6.2) kg. |
|
| Interventions |
Intervention: participants received dietary advice in the form of dietary counselling to increase intake and advice on food fortification. Control: participants received no dietary advice in the form of usual care consisting of a leaflet. |
|
| Outcomes | Survival*, weight*, fat‐free mass, fat mass, triceps skinfold*, MAC*, MAMC*, handgrip strength*, energy intake*, cost*, respiratory function, respiratory muscle function, QoL. | |
| Publication details |
Language: English. Funding: Research Training Fellowship London Regional NHS Executive & Guy's and St Thomas' Hospital Charitable Foundation. Publication status: peer‐reviewed journal. |
|
| Notes | Some data have been obtained from the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | All assessments made by the lead investigator who was not blinded to group allocation, but clinical assessments unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | High risk | All assessments made by the lead investigator who was not blinded to group allocation and some assessments of physical function might have been influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | All assessments made by the lead investigator who was not blinded to group allocation and assessments of nutritional intake might have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described and likely that lack of blinding might influence assessment of some outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 66 randomised, 59 at baseline and 37 completed study. Attrition: intervention group 16/36 (44%); control group 13/30 (43%). 7 deaths ‐ 4 in the intervention group and 3 in the control group. 15 participants withdrew during the study, reasons fully reported; 11% dropped out before baseline assessment. Attrition high but numbers and reasons similar in each group. |
| Selective reporting (reporting bias) | Low risk | No protocol identified but study author also an author of this review. All specified outcomes reported, but not as mean change (SD) at 6 months (end of intervention) for the outcomes of interest therefore additional data and information obtained from the author. |
| Other bias | Low risk | Baseline characteristics reported and no differences between groups. |
Wilson 2001.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 9 months (6 months treatment and 3 months follow‐up). Location: multicentre in the USA. |
|
| Participants |
Inclusion criteria: adults with hypoalbuminaemia (serum albumin 3.5 ‐ 3.7 g/dL) receiving haemodialysis. An additional group is included with severe hypoalbuminaemia (serum albumin 2.5 ‐ 3.4 g/dL) who received intervention according to current practice. Exclusion criteria: < 18 years or > 80 years of age, hospitalisation for longer than 1 week in the past 3 months, major surgery or sepsis in the past 3 months, urea reduction ratio less than 65% for 2 of the past 3 months, unintentional weight loss > 10% in the past 6 months, HIV infection, malignancy, use of appetite stimulanting medication, on haemodialysis for less than 3 months. Number randomised: 32 participants (intervention group, n = 16; control group, n = 16). Attrition: 5 participants were not included in the analysis but details of the group allocation are unclear. Gender split: intervention group 39% males, 61% females; control group 14% males, 86% females. Age: mean (SD) intervention group 64 (10) years; control group 58 (8.6) years. Nutritional status: not reported. |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of dietary counselling to increase energy and protein intakes and 1 ‐ 2 cans of supplement (250 calories per serving). Control: participants received dietary advice alone in the form ofdietary counselling to increase energy and protein intake. |
|
| Outcomes | Time to nutritional repletion, number of days spent in hospital*. | |
| Publication details |
Language: English. Funding: grant from the council on renal nutrition from the National Kidney Foundation. Publication status: peer‐reviewed journal. |
|
| Notes | No usable data from this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not stated, but it is unlikely that knowing group allocation would influence number of days spent in hospital. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | Not measured. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not blinded and lack of blinding may have influenced assessemnt of achieving nutritional repletion. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment of some outcomes may have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants were not included in the analysis but details of the group allocation is unclear, therefore risk of bias. |
| Selective reporting (reporting bias) | High risk | No study protocol identified, thus unable to judge whether all planned outcomes were reported. The methods section of the paper states that height, weight and weight history and serum albumin are collected at baseline, The results section reports % achieving nutritional repletion defined by improvement in serum albumin and length of hospital stay but no data on weight change. No response received from authors. |
| Other bias | Unclear risk | Baseline variables given, the dietary counselling and supplement group were significantly older than the dietary group, therefore risk of bias. |
Wong 2004.
| Study characteristics | ||
| Methods | RCT. Parallel design with 2 arms. Duration: 4 months. Location: Hong Kong. |
|
| Participants |
Inclusion criteria: adults presenting with osteoporotic fractures. Exclusion criteria: not described. Number randomised: 189 participants (intervention group, n = 73; control group, n = 77). Attrition: 39 participants lost to follow‐up (intervention group, n = 18; control group, n = 21). Gender split: intervention group 18% males, 82% females; control group, 15% males, 85% females. Age: mean (SD), intervention group 75.8 (9.5) years; control group 73.8 (11.6) years. Nutritional status: no details of numbers malnourished. BMI, mean (SD): intervention 22.6 (3.9) kg/m2; control 22.6 (3.5) kg/m2 . |
|
| Interventions |
Intervention: participants received dietary advice in the form of tailored dietary advice with recipes and specific goals for energy, protein and calcium plus 500 mg calcium and anti‐resorptive agent. Control: participants received no dietary advice in the form of no advice plus 500 mg calcium and anti‐resorptive agent. |
|
| Outcomes | Mortality*, weight*, BMI*, energy intake* protein intake*. | |
| Publication details |
Language: English. Funding: none declared. Publication status: peer‐reviewed journal. |
|
| Notes | Additional data and information requested from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information from author indicated that a computer‐generated list of random numbers was used. |
| Allocation concealment (selection bias) | Low risk | Information from author indicated that allocation concealment was achieved by an independent person managing this aspect of the trial. |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not described but judged to be low risk as assessment of clinical outcomes is unlikely to be influenced by lack of blinding. |
| Blinding (performance bias and detection bias) Functional outcomes | Unclear risk | No functional outcomes assessed. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Not described but judged to be high risk as assessment of some nutritional outcomes is likely to be influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described and likely that assessment of some outcomes would be affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 39 participants lost to follow‐up. 18/73 (25%) in the intervention group and 21/77 (27%) in the control group but reasons not given, therefore insufficient information to make a judgement. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. All outcomes mentioned in methods reported. Mortality data confirmed with authors. Additional information on study quality obtained from authors. Lack of protocol means insufficient information to make a judgement. |
| Other bias | Low risk | Baseline variables given, groups similar at baseline. |
Wyers 2013.
| Study characteristics | ||
| Methods | RCT. Parallel design with two treatment arms. Duration: 3 months. Location: multicentre in Maastricht, Heerlen and Sittard, The Netherlands. |
|
| Participants |
Inclusion criteria: adults (> 55 years) admitted for surgical treatment of hip fracture. Exclusion criteria: pathological/periprosthetic fracture, disease of bone metabolism, estimated life expectancy < 1 year, oral nutritional supplements before hospital admission, unable to speak Dutch, outside region or bedridden by fracture, dementia or cognitively impaired (Abbreviated Mental Test score < 7). Number randomised: 152 participants: intervention group, n = 73; control group, n = 79. Attrition: 7 participants died (intervention group, n = 3; control group, n = 4), 7 participants withdrew (intervention group, n = 3; control group, n = 4). Gender split: intervention female 54, male 19; control female 54, male 25. Age: mean(SEM), intervention 77 (1.2) years; control 76 (1.1) years. Nutritional status: assessed by MNA, intervention number (%) with no malnutrition 46 (63), number at risk of malnutrition 27 (37); control number (%) with no malnutrition 41 (52), number at risk of malnutrition 38 (48). |
|
| Interventions |
Intervention: participants received dietary advice and ONS in the form of frequent dietary counselling (2x during hospital stay and 3x at home (1, 2 and 6 weeks after discharge) plus calls at home at 3, 4, 5, 8 and 10 weeks after discharge) and consumption of 2x oral nutritional supplement each day (Cubitan, Nutricia: providing 500 kcal and 40 g protein per 500 mL) for 3 months. Control: participants received no dietary advice and no ONS in the form of usual care i.e. oral nutritional supplements only if doctor provided them (13%) and 28% received dietetic counselling. |
|
| Outcomes | Weight, QALYs (EQ‐5D‐3L), cost (Euros). | |
| Publication details |
Language: English. Funding: The Netherlands Organisation for Health Research and Development; oral nutritional supplements provided by Nutricia Advanced Medical Nutrition (Danone Research, Wageningen, The Netherlands). Publication status: peer‐reviewed journal. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: randomized according to a concealed computer‐generated random‐number sequence list after pre‐stratification for hospital, gender and age (55 ‐ 74 years versus 75 years and above) with an allocation ratio of 1:1. |
| Allocation concealment (selection bias) | Unclear risk | Blinded for the reseacher, according to a computer generated random‐numbersequence list |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Study dietitian was not blinded but assessment of mortality unlikely to be affected. |
| Blinding (performance bias and detection bias) Functional outcomes | Low risk | The study did not address functional outcomes. |
| Blinding (performance bias and detection bias) Nutritional outcomes | High risk | Study dietitian was not blinded. Assessment of weight may have been influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and likely that researchers and participants were aware of group allocation as this was a nutritional intervention. It is possible that assessment of some outcomes was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment of some outcomes likely to be affected by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition fully reported and reasons were similar for both groups. There were 4/73 (5.5%) deaths in the intervention group and 3/79 (4%) in the control group. 3/73 (4%) withdrew from the intervention group and 4/79 (5%) withdrew from the control group. |
| Selective reporting (reporting bias) | High risk | Published protocol identified (Wyers et al 2010); however, not all stated outcomes have been reported. |
| Other bias | Low risk | Baseline characteristics reported and groups similar at baseline. |
* outcomes included in this review if data usable
ADL: activities of daily living ANZCTR: Australia and New Zealand Clinical Trials Registry BASDEC: brief assessment schedule depression cards BIA: bioelectric impedence analysis BMI: body mass index CDAI: Crohn's disease activity index CF: cystic fibrosis COPD: chronic obstructive pulmonary disease CVD: cardiovascular disease DEXA: dual energy X‐ray absorptiometry DRAQ: Disease‐Related Appetite Questionnaire EORTC: European Organisation for Research and Treatment of Cancer FAACT: Functional Assessment of Anorexia/Cachexia Therapy FEV1: forced expiratory volume in 1 second FVC: forced vital capacity GDS: geriatric depression score GEE: generalized estimating equation GFR: glomular filtration rate GI: gastro‐intestinal Hb: haemoglobin HIV: human immunodeficiency virus IADL: instrumental activities of daily living IBW: ideal body weight IDDM: insulin‐dependent diabetes mellitus IQR: interquartile range ITT: intention‐to‐treat MAC: mid‐arm circumference MAMA: mid‐arm muscle area MAMC: mid‐arm muscle circumference MCT: medium chain triglycerides MMSE: mini mental state examination MNA: mini nutritional assessment MUAC: mid‐upper arm circumference NRS: nutritional risk screening PG‐SGA: patient‐generated subjective global assessment PU: pressure ulcer PUSH: Pressure Ulcer Scale for Healing QALY: quality adjusted life year QoL: quality of life RCT: randomised controlled trial REE: resting energy expenditure SD: standard deviation SE: standard error SGA: subjective global assessment SNAQ65+:short nutritional assessment questionnaire for home living older persons TIBC: total iron binding capacity TSF: triceps skinfold thickness VAS: visual analogue score vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12613000518763 | Not a RCT. This is a prospective observational study of usual nutritional management in malnourished adults in rural Australia. |
| Ahnfeldt‐Mollerup 2015 | Comparison does not meet inclusion criteria: no dietary counselling. |
| Aleman‐Mateo 2014 | Comparison does not meet inclusion criteria: no dietary counselling. |
| Amlogu 2016 | Comparison does not meet inclusion criteria: this is a public health intervention, not a health care intervention. |
| Antila 1993 | The included participants are well‐nourished and advice is given to maintain normal nutritional status. |
| Apovian 2017 | Comparison does not meet the inclusion criteria: the intervention is readymade home delivered meals and no dietary counselling component |
| Arija 2012 | Comparison does not meet inclusion criteria: no dietary counselling. |
| Arnarson 2013 | Comparison does not meet inclusion criteria: no dietary counselling. |
| Arutiunov 2009 | Not a RCT: this was an observational study and therefore did not meet the inclusion criteria of randomised controlled trial design. |
| Bachmann 1998 | Not a randomised controlled trial. |
| Badia 2015 | Comparison does not meet inclusion criteria: multifactorial intervention. |
| Baradzina 2013 | Comparison does not meet inclusion criteria: multifactorial intervention. |
| Bauer 1994 | Not disease‐related malnutrition and not adults, participants are adolescent weight lifters. |
| Beange 1995 | Not a randomised controlled trial, no control group, the 88 participants were "chosen" from 550 residents. |
| Beck 2008 | Comparison does not meet the inclusion criteria: the intervention was nutrition plus exercise compared with a control group receiving neither. |
| Beck 2016 | Comparison does not meet inclusion criteria, the intervention is multidisciplinary and therefore not possible to assess the contribution of the dietetic component alone. |
| Beddhu 2015 | Not a randomised controlled trial; this is an observational study. |
| Beelen 2017a | Comparison does not meet the inclusion criteria: the intervention is provision of protein‐enriched foods with no dietary counselling |
| Beelen 2017b | Not a RCT: this is a pilot feasibility study of incorporation of protein‐enriched foods into the diet |
| Bello 2019 | The comparison does not meet the inclusion criteria. The intervention is not focused on managing malnutrition but seems to have a health promotion focus and therefore seems more consistent with a public health intervention rather than clinical nutrition. |
| Benzekri 2019 | Comparison does not meet the inclusion criteria. This study compares a basket of local foods with a ready to use therapeutic food and there is no mention of dietary advice. |
| Bernoth 2014 | Not a randomised controlled trial; this is a qualitative study. |
| Bhattacharjee 2015 | Not a randomised controlled trial; this is a prospective observational study. |
| Bills 1993 | Not a randomised controlled trial, a questionnaire survey of nutritional practices in a nursing home. |
| Bolton 1990 | Not a randomised controlled trial, but a palatability study of nutritional supplements. |
| Bories 1994 | Not a randomised controlled trial. |
| Botella‐Carretero 2008 | Comparison does not meet inclusion criteria, this is a 3‐arm trial which compares 2 different oral nutritional supplements with routine care. |
| Botella‐Carretero 2010 | Comparison does not meet the inclusion criteria: this is a study of ONS versus usual care with no dietary counselling |
| Boulos 2016 | Not a randomised controlled trial; this is a cross‐sectional study. |
| Bozzetti 1998 | Not a randomised controlled trial, a letter with no data. |
| Braunschweig 2015 | Comparison does not meet inclusion criteria: no dietary counselling. |
| Bugge 1997 | Not a randomised controlled trial. |
| Bunout 1989 | Comparison does not meet inclusion criteria, this trial compares an enhanced calorie‐ and protein‐based diet plus a specialized nutritional supplement with a standard hospital diet. |
| Burger 1993 | Not a randomised controlled trial, a 6‐month prospective follow‐up of nutritional counselling in malnourished individuals with HIV infection. |
| Buys 2017 | Comparison does not meet the inclusion criteria; the intervention is home‐delivered meals and not dietary advice. |
| Caccialanza 2006 | Not a randomised controlled trial. |
| Caetano 2017 | Not a randomised controlled trial. This is a 2‐group study with no indication of how participants were selected for groups |
| Capozzi 2012 | Comparison does not meet the inclusion criteria: this is a study of nutritional intervention combined with physical activity and so not possible to determine the effect of nutritional intervention alone. |
| Carlos Candido 2016 | Not a randomised controlled trial; This study is published in Portuguese and was translated using Google translate. It is a cross‐sectional study of diet and nutritional status in older healthy Portuguese attending a gym. |
| Carlsson 2005 | Comparison does not meet inclusion criteria, this is a 3‐arm trial comparing an oral nutritional supplement with an oral nutritional supplement plus nandrolone (appetite stimulant) with routine care. |
| Cereda 2018 | Comparison does not meet inclusion criteria: use of immunomodulating oral nutritional supplements. |
| Charlton 2012 | Not a randomised controlled trial: this is a retrospective analysis |
| Chen 2017 | Not a randomised controlled trial: this is a pre‐test/post‐test study. |
| Chew 2021 | Comparison does not the meet the inclusion criteria. The intervention includes an ONS with beta‐hydroxy‐beta‐methylbutyrate which is a novel ingredient and therefore excluded. |
| ChiCTR1900020807 | Comparison does not meet the inclusion criteria. The intervention is enteral feeding and not dietary advice. |
| ChiCTR1900021167 | Comparison does not meet inclusion criteria. The intervention is a nutritious formula food versus no formula food with no mention of dietary advice. This looks more like an ONS supplementation study. |
| ChiCTR‐INR‐17012826 | Comparison does not meet the inclusion critieria. The intervention includes an ONS with a novel ingredient HMB. |
| Choi 2013 | Not a randomised controlled trial; this is a cross‐sectional study. |
| Della Valle 2018 | Not a RCT, this is a one group observational study. |
| DeLuis 2010 | Comparison does not meet the inclusion criteria: this is a study of a standard ONS versus an n‐3 fatty acid enriched ONS with no dietary counselling |
| Demeny 2015 | Not a randomised controlled trial; this is a questionnaire‐based survey of practice. |
| Deutz 2016 | Comparison does not meet the inclusion criteria; the intervention is an oral nutritional supplement with beta‐hydroxy‐beta methylbutyrate versus placebo. |
| De Waele 2015 | Comparison does not meet inclusion criteria: participants in the control group (usual care) also received intensive, personalized nutritional counselling by an oncology dietitian and this does not fit our definition of 'no supplementation, usual diet'. |
| Ding 2016 | Comparison does not meet inclusion criteria; the intervention is a comprehensive intervention including dietary advice, symptom management and psychological support. |
| Dizon 2016 | Comparison does not meet inclusion criteria: the intervention was dietary counselling plus high‐protein meal boxes versus low‐energy and low‐protein meal boxes. |
| Dorner 2013 | Comparison does not meet inclusion criteria: dietary counselling is combined with exercise, therefore not possible to examine the effects of dietary care alone. |
| DRKS00016661 | Comparison does not meet the inclusion critieria. From the trial report this seems to be an ONS versus placebo study. |
| Duncan 2006 | Comparison does not meet inclusion criteria, the intervention is help with eating from a dietetic assistant compared with routine care. |
| Dupuis 2017 | Comparison does not meet inclusion criteria: the intervention is about training protocols for nutritional management rather than specifically dietary advice. |
| Efthimiou 1988 | Comparison does not meet inclusion criteria, this is a 3‐arm trial which compares an oral nutritional supplement with routine care. The 3rd group are individuals that are normally nourished and receiving usual diet. |
| Ekramzadeh 2015 | Comparison does not meet the inclusion criteria. This is a study of dietary advice versus an anti‐inflammatory diet. No routine care arm. |
| Elbanna 1996 | Not a randomised controlled trial, comparison of preoperative nutritional support in 2 groups, but the control group are purposively recruited before the intervention group. |
| Elkort 1981 | Comparison does not meet inclusion criteria, comparison of an oral nutritional supplement with routine care, both groups are given encouragement to eat a balanced diet which was considered not to constitute dietary advice. |
| Elmstaahl 1987 | Comparison does not meet inclusion criteria, this study has 3 arms comparing 3 different oral nutritional supplements. |
| Eneroth 1997 | Not a randomised controlled trial and comparison does not meet inclusion criteria. This study compares supplementary nutrition, which can consist of an oral nutritional supplement, enteral or parenteral feeding with hospital food. |
| Engel 1995 | Not a randomised controlled trial. |
| Evans 2013 | Comparison does not meet the inclusion criteria: this is a study of supplementation with FutureLife porridge versus usual care with no dietary counselling |
| Faber 2015 | Comparison does not meet inclusion criteria: use of immunomodulating oral nutritional supplement which is outside the scope of this review. |
| Faccio 2021 | Comparison does not meet the inclusion criteria. The ONS includes novel ingredients, leucine and zinc IMMAX for nutritional recovery. |
| Fietkau 2013 | Comparison does not meet the inclusion criteria: this is a study of a standard ONS with a disease‐specific ONS with no dietary counselling |
| Flynn 1987 | Comparison does not meet inclusion criteria, this study compares individualised nutritional counselling to an oral nutritional supplement with standard nutritional. |
| Forli 2006 | Not a randomised controlled trial. |
| Franzoni 1996 | Not a randomised controlled trial. |
| Gil Gregorio 2003 | Comparison does not meet inclusion criteria: oral nutritional supplements with no dietary advice. |
| Glimelius 1992 | Not a randomised controlled trial, an historical control group was used. |
| Gomez Sanchez 2010 | Comparison does not meet the inclusion criteria: this is a study of an immune‐enhancing ONS versus usual care with no dietary counselling |
| Gurgun 2013 | Comparison does not meet the inclusion criteria: Participants received an exercise intervention in addition to dietary interventions (dietary advice +ONS) versus exercise versus routine care and so it is not possible to isolate the effects of dietary counselling. |
| Hamirudin 2017 | Not a randomised controlled trial; this is a one group prospective study with no comparison group. |
| Hammersley 2015 | Not a randomised controlled trial; this is an observational study with a multi‐component intervention. |
| Hansra 2017 | The participants do not have illness‐related malnutrition. The intervention is dietary counseling to manage weight gain. |
| Hashmi 2016 | Comparison does not meet inclusion criteria: no dietary counselling |
| Hatsu 2014 | Not a randomised controlled trial and comparison does not meet inclusion criteria: no dietary counselling. |
| Hayashi 2014 | Not a randomised controlled trial; this is a matched case‐controlled study. Comparison does not meet inclusion criteria: no dietary counselling. |
| Heberer 1984 | Comparison does not meet the inclusion criteria: this is a study of parenteral nutrition. |
| Henquin 1989 | Not a randomised controlled trial. |
| Hickson 2004 | Comparison does not meet inclusion criteria, the intervention is help with eating from a dietetic assistant compared with routine care. |
| Hogan 1997 | Not a randomised controlled trial. |
| Holder 2003 | Not a randomised controlled trial, a review article. |
| Huisman 2012 | Comparison does not meet the inclusion criteria: this is a study of preventative nutritional support where participants were monitored and received the intervention if weight loss >5% developed. Not comparable with other studies in the review were intervention is initiated at the start of the study. |
| Hulsewe 1997 | Not a randomised controlled trial, a discussion of perioperative nutritional interventions. |
| Huppertz 2020 | The comparison does not meet the inclusion criteria. The comparison in this trial will be between a pre‐thickened ONS and an ONS thickened using conventional additives. |
| Idilman 2009 | Comparison does not meet inclusion criteria, this is a retrospective review of nutrition intervention and outcomes in individuals with alcoholic liver disease. |
| Ingadottir 2019 | Comparison does not meet inclusion criteria, the intervention is snacks which were provided versus ONS with no mention of dietary advice. |
| Ireton 1995 | Not a randomised controlled trial, an observational study. |
| ISRCTN11132850 | Other: It looks like this trial was completed and is relevant to comparison 3 but we can’t find evidence of publication. Excluded because of lack of information. |
| ISRCTN56882109 | Comparison does not meet the inclusion criteria. This is a trial of a specialised ONS Renilon 7.5 vs standard treatment in people with end stage renal disease |
| Jacka 2017 | Participants are not malnourished or at risk of malnutrition. |
| Jamieson 1997 | Not a randomised controlled trial, a retrospective audit. |
| Jancey 2017 | Comparison does not meet the inclusion criteria; the intervention group receive dietary counselling plus physical activity advice. |
| Jang 2018 | Comparison does not meet the inclusion critieria, the intervention is multicomponent (including nutritional supplementation) with no dietary advice. |
| Jie 2009 | Comparison does not meet inclusion criteria, this study compares enteral and parenteral feeding. |
| Johnson 1993 | Not a randomised controlled trial and comparison does not meet inclusion criteria, this is a comparison of oral nutritional supplements with no nutritional supplement. Both groups follow their usual diet, therefore there is no counselling component. |
| Kang 2016 | Comparison does not meet the inclusion criteria. Although the abstract mentions individualised counselling, the authors have confirmed that they received enteral and parenteral nutrition. |
| Karavetian 2016 | Comparison does not meet the inclusion criteria. This is a study of individualised education versus usual care, but the education relates to the management of hypophosphataemia. |
| Keller 1995 | Not a randomised controlled trial, a retrospective survey of outcomes in malnourished and normally nourished participants. |
| Kirkil 2012 | Comparison does not meet the inclusion criteria: this is a study of immune enhancing enteral and parenteral nutrition with no dietary counselling |
| Kiss 2014 | Malnourished individuals were excluded and both the intervention and control group received nutritional support. |
| Knowles 1988 | Comparison does not meet inclusion criteria, a comparison of an oral nutritional supplement with no nutritional supplement. |
| Kondrup 1998 | Not a randomised controlled trial, a retrospective survey of outcomes in malnourished individuals. |
| Kong 2018 | Comparison does not meet the inclusion criteria. This is an ONS versus no ONS study and there is no dietary advice. |
| Krasnoff 2006 | Comparison does not meet inclusion criteria, a comparison of nutritional counselling plus exercise compared with routine care. |
| Kristensen 2020 | Comparison does not meet the inclusion criteria. The intervention is multidisciplinary and so it is not possible to determine whether any benefits relate to the nutritional intervention. |
| Kruizenga 2005 | Not a randomised controlled trial, a controlled study using historical controls. |
| La Torre 2018 | Comparison does not meet the inclusion criteria, it is focussed on achieving a healthy lifestyle and not on improving nutritional intake in nutritionally vulnerable patients. |
| Lee 2013 | Comparison does not meet the inclusion criteria: this is a study of ONS versus usual care with no dietary counselling |
| Lee 2016 | Comparison does not meet the inclusion criteria; a comparison of oral nutritional supplements with no oral nutritional supplements. |
| Leedo 2017 | Comparison does not meet the inclusion criteria; the intervention consisted of meals and snacks but no dietary counselling. |
| Lehtisalo 2017 | Comparison does not meet the inclusion criteria; this is a multi‐component intervention consisting of a dietary component, physical activity, cognitive training and metabolic and cardiovascular management. |
| Lejeune 2005 | Comparison does not meet inclusion criteria, a comparison of dietary advice to achieve weight loss in moderately overweight individuals. |
| Leslie 2013 | Comparison does not meet the inclusion criteria: this is a study of dietary fortification versus usual care with no dietary counselling |
| Levine 1982 | Comparison does not meet inclusion criteria, comparison of standard diet with parenteral nutrition. |
| Li 2017 | Comparison does not meet the inclusion criteria; the intervention is enteral nutrition |
| Lipschitz 1985 | Not a randomised controlled trial. |
| Lonbro 2013 | Not a randomised controlled trial: this is a retrospective data analysis |
| Luppino 2015 | Not a randomised controlled trial. This is a two group study with a group receiving an intervention and a matched historical control group |
| Lynch 1983 | Not a randomised controlled trial, a prospective study of an oral carbohydrate supplement. |
| Manders 2009 | Comparison does not meet inclusion criteria, this is a study of oral nutritional supplements compared with no nutritional supplement. |
| Margare 2002 | This paper was identified during searching but the full manuscript has remained unavailable on the journal website for a number of years. The study was excluded at the 2018/9 update as it was judged unlikely that the paper will be identified. |
| Maurya 2019 | This is not a RCT it is a two group observational study with no random allocation to groups. |
| Mazzuca 2019 | Comparison does not meet the inclusion criteria. This is an ONS versus no ONS study and there is no dietary advice. |
| McCarter 2018 | Comparison does not meet the inclusion criteria: the intervention is an enhanced management protocol and not specifically dietary counselling. |
| McCormack 2013 | Comparison does not meet the inclusion criteria: this is a study of two different ONS containing beta‐alanine with no dietary counselling |
| McWhirter 1996 | Comparison does not meet the inclusion criteria: this is a study of oral versus naso‐gastric supplementation. |
| Mendenhall 1993 | Comparison does not meet inclusion criteria, comparison of a nutritional supplement with a placebo nutritional supplement. |
| Mendenhall 1995 | Comparison does not meet inclusion criteria, this study compares hospital diet plus an oral nutritional supplement and a vitamin and mineral supplement with hospital diet plus a vitamin and mineral supplement plus a placebo nutritional supplement. |
| Monnin 1993 | Not a randomised controlled trial, a report of the findings from a questionnaire on nutritional counselling in breast cancer. |
| Montoya 2014 | Not a randomised controlled trial. Following translation, this is a quasi‐experimental study, the control arm recruited first and then the intervention group. |
| Morasutti 2012 | Not a randomised controlled trial; this is a 2‐group study, where the control group is historic control. |
| Munck 1998 | Not a randomised controlled trial, a review of dietary counselling. |
| Munk 2014 | Comparison does not meet the inclusion criteria: this is a study of fortified additional hospital dishes versus usual care with no dietary counselling |
| Myint 2013 | Comparison does not meet the inclusion criteria: this is a study of ONS versus usual care with no dietary counselling |
| NCT00136253 | Other: This looks relevant (?comparison 1) but we can’t find evidence of it being published. There is a retrospective study of nutritional intervention by the primary investigator Sehgal AR and an RCT but of clinical barriers. Excluded because of insufficient information. |
| NCT00417508 | Other: This looks like a group 3 study but there is no evidence of publication either from the trial report or doing and author and title search. Excluded because of lack of information. |
| NCT00769652 | Other: This trial looks relevant to include in comparison 1 or 4 but there is no evidence of publication. Excluded because of lack of information. |
| NCT01116947 | Comparison does not meet the inclusion criteria. All participants receive dietary counselling and the intervention is Fosrenol versus a conventional phosphate binder. |
| NCT01190969 | The trial seems to be relevant for inclusion in comparison 2 but on Clinical Trials is listed as withdrawn because of difficulty recruiting. |
| NCT02681601 | Comparison does not meet the inclusion criteria. The intervention is dietary advice plus an oral nutritional supplement which contains omega‐3 fatty acids. |
| NCT03488511 | Comparison does not meet the inclusion criteria. The intervention is home‐delivered meals versus routine care and there is no dietary advice. |
| NCT03649698 | Comparison does not meet the inclusion criteria. The intervention is supplementation with a protein powder, there is no dietary advice. |
| NCT03741283 | Comparison does not meet the inclusion criteria. The intervention is multimodal and it would not be possible to determine whether any benefits were related to the nutritional components. |
| NCT03774953 | Comparison does not meet the inclusion criteria. This is a trial of a protein supplement versus no supplement with no dietary advice. |
| NCT03792711 | Comparison does not meet inclusion criteria. The intervention is dietary advice versus ONS but the ONS contains a novel ingredient (β‐hydroxy‐β‐methylbutyrate) which is excluded. |
| NCT03807310 | Comparison does not meet the inclusion criteria. This is lifestyle counselling plus a novel ONS versus lifestyle counselling and an isocaloric ONS. |
| NCT03924089 | Comparison does not meet the inclusion criteria, the intervention is dietary advice versus ONS, but the ONS contains novel ingredients (probiotics) which are excluded. |
| NCT04027413 | Comparison does not meet the inclusion criteria. The intervention is exercise plus an ONS versus exercise plus a placebo ONS with no dietary advice. |
| NCT04036825 | Comparison does not meet the inclusion criteria, the intervention is a liquid ONS versus a placebo ONS with no dietary advice. |
| NCT04109495 | Comparison does not meet the inclusion criteria. The intervention is a Smartphone App which provides feedback on intake. There is no dietary advice component. |
| NCT04175769 | Comparison does not meet the inclusion criteria. The intervention involves capsules containing a novel ingredient (fish oils) and there is no dietary advice. |
| NCT04218253 | Comparison does not meet the inclusion criteria. The intervention includes an oral nutritional supplement that contains branched chain amino acids which is judged to be a novel ingredient. |
| Neidich 1985 | Not a randomised controlled trial, the intervention is a high‐nitrogen food supplement and the participants are mainly children. |
| Newmark 1981 | Not a randomised controlled trial. |
| Neyman 1996 | Not a randomised controlled trial and comparison does not meet inclusion criteria. This study compares outcomes in participants in a congregate‐site meals programme with people not participating in the programme. |
| Ng 2015 | Comparison does not meet inclusion criteria: no dietary counselling. |
| Nijs 2006 | The comparison does not meet the inclusion criteria, this study compares family‐style dining versus traditional dining. |
| NTR6713 | The personalised dietary advice is given to improve healthy eating and not to manage illness‐related malnutrition |
| NTR7506 | Comparison does not meet the inclusion criteria. The intervention is ONS versus routine care. There is no dietary advice. |
| Nyamathi 2018 | Comparison does not meet the inclusion criteria. The food‐based intervention is given with a health promotion focus rather than to manage a clinical problem. |
| Nykanen 2014 | Comparison does not meet the inclusion criteria, this is a multi‐component intervention and therefore not possible to assess the individual contribution of the nutrition component. |
| Nykanen 2018 | Comparison does not meet the inclusion criteria. The intervention is a local berry‐based supplement and there is no dietary advice. |
| Olofsson 2007 | Comparison does not meet the inclusion criteria: the intervention included many aspects of medical care in addition to a nutritional intervention that may have accounted for any reported benefits. |
| Ommundsen 2017 | Comparison does not meet the inclusion criteria. The intervention is geriatric assessment including nutrition and tailored management versus usual care and so it would not be possible to separate the effects of nutrition. |
| Openbrier 1984 | Not a randomised controlled trial, this is a prospective evaluation of nutritional intervention in malnourished participants with emphysema. |
| Orell 2019 | The routine care arm is not consistent with other studies in the review. The intervention is an individualised nutritional intervention but the comparison arm is on‐demand nutritional counselling (requested by the managing clinician). |
| Otsuki 2020 | The comparison does not meet the inclusion criteria. Although participants received individual dietary counselling there is an escalation of intervention to enteral feeding and around 20% of participants received enteral feeding. |
| Ottery 1996 | Not a randomised controlled trial, a description of improvements following nutritional intervention. |
| Ottestad 2017 | Comparison does not meet the inclusion criteria: participants received a protein‐enriched milk drink or an isocaloric carbohydrate drink so no dietary advice component. |
| Palar 2015 | Comparison does not meet the inclusion criteria. The food‐based intervention is given in the context of public health and not a healthcare context |
| Parrott 2006 | The comparison does not meet the inclusion criteria, this study compares a snack‐type supplement provided to people with Alzheimer's disease in a nursing home which is not the same as dietary advice. |
| Patel 1998 | Not a randomised controlled trial and comparison does not meet inclusion criteria; it examines the efficacy of dietary advice to avoid weight gain. |
| Paulsen 2020 | Comparison does not meet the inclusion criteria. The intervention is a computerised decision support system to enhance nutritional intake and not individualised dietary advice. |
| Pedersen 2005 | Not a randomised controlled trial, this is a quasi‐experimental study of nurse‐facilitated patient involvement in care. |
| Penalva 2009 | Comparison does not meet the inclusion criteria: this is a RCT of oral nutritional supplements versus "oral cooking supplements". Following translation, there is no mention of the use of dietary advice. |
| Pietersma 2003 | Comparison does not meet inclusion criteria, a comparison of individual selection of 1 meal a day from the food cart compared with receiving the usual plated meal. |
| Planas 2005 | Comparison does not meet inclusion criteria, comparison of 2 groups both receiving dietary advice and supplements but the target energy intake varied between the groups. |
| Plank 2008 | Comparison does not meet the inclusion criteria, comparison of oral nutritional supplements with no nutritional supplements. |
| Poulsen 2014 | Comparison does not meet inclusion criteria: use of immunomodulating oral nutritional supplements. |
| PrayGod 2012 | Comparison does not meet the inclusion criteria: this is a study of nutritionally‐enriched biscuits versus usual care with no dietary counselling |
| Rabinovitch 2006 | Not a randomised controlled trial, a re‐analysis of data. |
| Rassmussen 2006 | Not a randomised controlled trial and comparison does not meet inclusion criteria, as the intervention does not aim to increase nutritional intake. |
| Reinders 2018 | Not a RCT. This is a systematic review with pooled analysis. |
| Rizk 2017 | Comparison does not meet the inclusion criteria. This is a study of a trained dedicated dietitian versus hospital dietitian in managing patients with renal disease. |
| Rollo 2020 | Participants are not malnourished, they are relatively young people on a university campus. |
| Roussel 2016 | Comparison does not meet the inclusion criteria, around 20% of participants in each group received enteral feeding |
| Rozentryt 2010 | Comparison does not meet the inclusion criteria: this is a study of ONS versus usual care with no dietary counselling |
| Rüfenacht 2010 | Comparison does not meet inclusion criteria; comparison of hospital diet plus oral nutritional supplement with dietary counselling plus oral nutritional supplement as required. |
| Salas‐Salvado 2005 | Comparison does not meet the inclusion criteria: the comparison is unclear but appears to be dietary advice plus provision of puree diet and inclusion of a snack‐type supplement based on natural lypolysed food compared with dietary advice plus provision of a puree diet. |
| Sankhaanurak 2021 | Comparison does not meet the inclusion criteria. All participants received nutrition counselling and were then randomised to a simplified protein counting tool versus control. |
| Sartorelli 2005 | Participants do not have illness‐related malnutrition. The intervention relates to promotion of healthy eating rather than management of malnutrition. |
| Saudny‐Unterberger 1997 | Comparison does not meet inclusion criteria, comparison of hospital diet and a supplement or extra food with hospital diet only. No dietary counselling. |
| Shamoto 2020 | Comparison does not meet the inclusion criteria. The intervention is a multidisciplinary comprehensive care and so not possible to isolate the effects of dietary advice. |
| Shan 2001 | Comparison does not meet the inclusion criteria. The intervention is described as parenteral nutrition or no parenteral nutrition. |
| Simmons 2008 | Comparison does not meet the inclusion criteria: this is a study of feeding assistance versus usual care with no dietary counselling. Potential for bias in participant selection because to be eligible for inclusion, the nursing home residents had to demonstrate that they were responsive to one of the feeding assistance interventions. |
| Simmons 2010 | Comparison does not meet the inclusion criteria: this is a study of ONS versus snacks versus usual care with no dietary counselling |
| Skaarud 2016 | Comparison does not meet the inclusion criteria: information from the authors indicates that all patients in the intervention group received tube feeding. |
| Skaarud 2018 | Comparison does not meet the inclusion criteria. A high proportion of participants receive an escalation of intervention to enteral nutrition. |
| Smoliner 2008 | Comparison does not meet the inclusion criteria, comparison of the provision of fortified food with routine care in a nursing home which does not meet the definition of dietary advice. |
| Sohrabi 2016 | Comparison does not meet the inclusion criteria: comparison of an oral nutritional supplement with and without a vitamin E supplement and there is no dietary advice. |
| Solerte 2008 | Comparison does not meet inclusion criteria, comparison of an amino acid mixture with a placebo. |
| Solomon 1978 | Comparison does not meet inclusion criteria, comparison of a combination of pre‐operative and post‐operative diets including a hypo‐caloric, carbohydrate‐free, protein‐containing diet with normal diet. |
| Somanchi 2011 | Not a randomised controlled trial; this is a 2‐arm non‐randomised study with the two wards "being chosen at random". |
| Sridar 1994 | Not a randomised controlled trial, a prospective study of 12 individuals with COPD after nutritional intervention. |
| Stack 1996 | Not a randomised controlled trial, a prospective, descriptive trial with no control group. |
| Stark 1990 | Comparison does not meet inclusion criteria, all participants were children. |
| Stevenson 2019 | Comparison does not meet the inclusion criteria. The dietary advice given relates to the management of renal disease rather than to improve nutritional intake. |
| Stewart 2017 | Comparison does not meet the inclusion criteria: this is a protocol for a trial of nutritional intervention combined with physical activity. |
| Suzuki 2019 | The dietary advice given in this study is based on healthy eating rather than an intervention to increase energy, protein and nutrient intake. |
| Swaminathan 2010 | This is a cluster intervention study but the method of assignment of clusters is not described and there is no mention of randomisation to clusters. |
| Swanenburg 2007 | Comparison does not meet inclusion criteria, comparison of exercise plus an oral nutritional supplement with no exercise and no supplement. |
| Tandon 1984 | Comparison does not meet the inclusion criteria. The intervention is enteral feeding versus standard oral diet |
| Tatsumi 2009 | Comparison does not meet the inclusion criteria, the intervention is "Hochuekkito" which is a herbal medicine. |
| Taylor 2006 | Comparison does not meet the inclusion criteria, comparison of meal frequency (5 meals versus 3 meals) on nutritional outcomes. |
| Trabal 2010 | Comparison does not meet the inclusion criteria; the oral nutritional supplement used is an immuno‐nutrition supplement which is outside of the scope of this review. |
| Turic 1998 | Comparison does not meet the inclusion criteria, intervention involves the provision of a nutritional supplement or snacks to nursing home residents which does not meet the definition of dietary advice. |
| Turnock 2013 | Comparison does not meet the inclusion criteria: this is a study of an immune‐enhancing ONS versus standard ONS with no dietary counselling |
| Unosson 1992 | Comparison does not meet inclusion criteria, the intervention is hospital diet plus a nutritional supplement compared with hospital diet. |
| van Beers 2020 | Comparison does not meet the inclusion criteria. The intervention is dietary counselling plus ONS versus no counselling and a placebo but the ONS contains a novel ingredients. |
| van Blarigan 2020 | Participants and comparison don't meet the inclusion criteria. The participants are people with CRC but who have completed treatment and have stable disease. The intervention is a healthy eating intervention. The study is not about illness‐related malnutrition. |
| van den Berg 2010 | Comparison does not meet inclusion criteria: participants in the control group (usual care) also received intensive, personalized nutritional counselling by a nurse and this does not fit our definition of 'no supplementation, usual diet'. |
| van den Heuvel 2017 | Comparison does not meet the inclusion criteria. The intervention consists of supplementation with eggs, there is no dietary counselliing. |
| van der Meij 2010 | Comparison does not meet the inclusion criteria: this is a study of an immune‐enhancing ONS versus standard ONS with no dietary counselling |
| van der Pols‐Vijlbrief 2017 | Comparison does not meet inclusion criteria; the study had a multifactorial approach in which participants could choose which problem they wanted to address, meaning that dietary counselling was not the primary intervention and also a number of participants in the intervention group did not choose dietary counselling as the main approach and thus never received any dietary counselling or advice. |
| Vargas 1995 | Comparison does not meet inclusion criteria, 4 arms comparing different combination of nutritional supplements and training. |
| Vazquez Martinez 2015 | Not a randomised trial; on translation, this seems to be an editorial. |
| Volkert 1996 | Comparison does not meet inclusion criteria, the intervention is hospital diet plus a nutritional supplement compared with hospital diet. |
| Watson 2008 | Comparison does not meet the inclusion criteria: the intervention consists of both dietetic and educational and psychological motivation; it would be difficult to attribute any reported benefits to nutrition alone. |
| Williams 1989a | Participants were mainly children with some adults; no participants over 16 years of age in control group therefore no comparison group. |
| Williams 1989b | Comparison does not meet inclusion criteria, the intervention is hospital diet plus a nutritional supplement compared with hospital diet. |
| Woo 1994 | Comparison does not meet inclusion criteria, the intervention is hospital diet plus a nutritional supplement compared with hospital diet. |
| Wouters‐Wessling 2005 | Comparison does not meet the inclusion criteria, comparison of a nutritional supplement with routine care. |
| Wright 2008 | Not a randomised trial, a prospective observational study with retrospective control group examining feeding assistance to increase intake. |
| Wu 2020 | The comparison does not meet the inclusion criteria. The intervention is not a dietary intervention but seems to be exercises for the oral cavity and oral hygiene in nutritionally at risk participants. |
| Xie 2017 | Comparison does not meet inclusion criteria: participants in the control group (usual care) also received intensive, personalized nutritional counselling and this does not fit our definition of 'no supplementation, usual diet'. |
| Yoneda 1992a | Comparison does not meet inclusion criteria, a report on the clinical course of individuals with asthma. |
| Yoneda 1992b | Comparison does not meet inclusion criteria, Japanese study that appears to be an intervention to reduce psychological stress in individuals with respiratory disease. |
| Yuvaraj 2016 | Comparison does not include dietary advice; a comparison of an oral nutritional supplement with "kitchen feeding" implying the provision of additional food. |
| Zhang 2018a | Comparison does not meet the inclusion criteria. The intervention is descripted as priphylactic enteral nutrition powder formula. There is no mention of dietary advice and no description of whether the formula is taken orally or via a tube. |
| Zhao 1995 | Other: This reference might be incorrect. Is there a journal ‘parenteral and enteral nutrition’. I have checked JPEN and this paper is not there. Nothing identified on title and author search. Excluded because of insufficient information. |
| Zweers 2020 | The majority (95%) of participants are not malnourished, therefore this is not illness‐related malnutrition. |
HMB: beta‐hydroxy‐beta‐methylbutyrate
ONS: oral nutritional supplement
Characteristics of studies awaiting classification [ordered by study ID]
Abdelsalam 2019.
| Methods | Randomised study |
| Participants | 100 adults with end stage renal disease on hemodiaylsis. |
| Interventions | "nutritional support educational program" |
| Outcomes | SGA score, anthropometry, nutritional knowledge |
| Notes | Currently published in an abstract only and insufficient details of the intervention to evaluate eligibility. Assess against inclusion critieria when a full‐text publication is available. |
Abdollahi 2019.
| Methods | RCT |
| Participants | 150 women with breast cancer receiving chemotherapy. |
| Interventions | Nutrition education by a dietitian to reduce the severity of side‐effects of treatment. |
| Outcomes | Primary: chemotherapy induced nausea, vomiting and diarrhoea. Secondary: weight, constipation, reflux, dyspepsia, anorexia, gastritis and chest pain. |
| Notes | This published study seems to be relevant to include in comparison 1. |
Banda 2017.
| Methods | Randomised factorial design. |
| Participants | 81 patients with tuberculosis. |
| Interventions | "Multivitamin and/or high calorie versus control". |
| Outcomes | Weight change, BMI, performance status, treatment success. |
| Notes | Currently published as an abstract only. It is impossible to fully evaluate eligibility from the details available. Assess once a full‐text publication is available. |
Britton 2019.
| Methods | A stepped‐wedge RCT. |
| Participants | 307 participants with head and neck cancer undergoing chemoradiation or radiotherapy with curative intent. |
| Interventions | A motivational interviewing and cognitive behavioural therapy‐based intervention delivered by oncology dieitians to support usual dietetic management. |
| Outcomes | Primary outcome: nutritional status at the end of treatment using PG‐SGA. Secondary outcomes: SGA, percentage weight loss, weight loss >10%, depression, treatment interruptions, unplanned hospital admission, QoL. |
| Notes | This study looks eligible for inclusion but further details needed of the nature of the dietetic intervention to determine comparison group. |
Camere 2016.
| Methods | RCT. |
| Participants | 95 participants with chronic obstructive pulmonary disease with or at risk of malnutrition. |
| Interventions | Personalised dietary advice versus oral nutritional ONS for 3 months. |
| Outcomes | Nutritional status (BMI, weight, FFMI), numbers improving nutritional status, lung function (FEV1), handgrip strength, exercise endurance. |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 2 of the review but needs full assessment once the final paper is available. |
Cawood 2017.
| Methods | RCT. |
| Participants | 308 older people (over 50 years) attending GP services and at risk of malnutrition according to MUST. |
| Interventions | Dietary advice and low volume oral nutritional supplement versus dietary advice alone. |
| Outcomes | Total food intake (macronutrient and micronutrient intake). |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 3 of the review but needs full assessment once the final paper is available. |
Cereda 2019.
| Methods | RCT. |
| Participants | 166 participants with advanced cancer and malnutrition undergoing chemotherapy. |
| Interventions | Individualised nutritional counselling with1 ‐ 2 cans of whey protein ONS versus individualised nutritional counselling alone. |
| Outcomes | Primary outcome: change in phase angle at 3 months. Secondary outcomes: change in phase angle at 1 month, change in standardized phase angle, FFMI, body weight, muscle strength, treatment toxicities. |
| Notes | Consider for inclusion in comparison 3 of the review (dietary advice versus dietary advice + ONS). |
Chewaskulyong 2015.
| Methods | RCT. |
| Participants | 50 people with locally advanced unresectable or metastatic cancer undergoing chemotherapy. |
| Interventions | Dietary counselling versus routine care. |
| Outcomes | Weight, BMI, PG‐SGA score, QoL, energy intake, chemotherapy outcome and biochemistry. |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 1 of the review but needs full assessment once the final paper is available. |
ChiCTR1800014842.
| Methods | 2‐group trial (likely RCT but not specified) |
| Participants | Individuas with intra‐abdominal infection. |
| Interventions | Nutrition management versus routine treatment. |
| Outcomes | Clinical outcome after treatement, post‐op complications, inflammatory markers, immunologic function, APACHE II score, SOFA score, NUTRIC score. |
| Notes | It is not possible to determine from the description in the abstract what nutritional management entails to determine eligibility. Assess once a full‐text publication is available. |
ChiCTR‐IOR‐17013151.
| Methods | Not specified. |
| Participants | Outpatients with tuberculosis. |
| Interventions | "dietary intervention versus dietary intervention plus ONS". |
| Outcomes | Malnutrition risk (NRS‐2002) weight, arm muscle circumference, TSF, albumin, prealbumin, transferrin, haemoglobin, total lymphocyte count, 25‐hydroxy vitamin D status. |
| Notes | The WHO International Register was not available at the time of scrutiny. There are insufficient details in the trial report to understand what the intervention is. This needs to be evaluated once there is a full text paper. |
Collins 2014.
| Methods | RCT. |
| Participants | 200 clinically stable outpatients with chronic obstructive pulmonary disease at risk of malnutriiton (MUST). |
| Interventions | Oral nutritional ONS versus dietary advice. |
| Outcomes | Global QoL (St George's Respiratory Questionnaire), handgrip strength, weight, nutritional intake. |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 2 of the review but needs full assessment once the final paper is available. |
Cong 2016.
| Methods | RCT. |
| Participants | 80 participants with oesophageal cancer undergoing radiotherapy. |
| Interventions | Nutritional counseling combined with oral nutritional supplements (Ensource) versus nutritional counselling alone. |
| Outcomes | Energy intake, nutritional status, incidence of complications. |
| Notes | This paper is in Chinese and needs translation to allow full assessment. It looks to be eligible for inclusion in comparison 3 (dietary advice +ONS versus dietary advice alone). |
Cramon 2019.
| Methods | RCT. |
| Participants | 40 hospitalised geriatric participants (over 65 years of age). |
| Interventions | Individualised nutritional intervention consisting of dietary counselling and a nutrition plan at hospital discharge + 2 home visits. |
| Outcomes | 30‐day hospital admissions/re‐admission rate, percent of energy and protein target achieved, ADL, handgrip strength and QoL. |
| Notes | This is currently published as an abstract. The full‐text paper needs to be evaluated once available to determine the comparison group (1, 4 or 5). |
CTRI/2012/05/002698.
| Methods | RCT. |
| Participants | People with HIV infection beginning anti‐retroviral therapy. |
| Interventions | Oral protein supplementation (16 gm/day for 6 months) versus nutritional counselling "according to 6 modules" for a period of 6 months versus a combination of nutritional counselling and oral protein supplementation (16 gm/day) for a period of 6 months. |
| Outcomes | Nutritional status, immunological status, dietary profile, clinical status, mortality, QoL. |
| Notes | The International Trial Register was not functioning at the time of assessment. We need to see the full trial record to identify the authors and to identify a full‐text publication. A title search and trial ID search do not yield anything obvious. |
CTRI/2018/10/015882.
| Methods | RCT. |
| Participants | Participants with gastrointestinal disease planned for surgery. |
| Interventions | Preoperative nutrition based on nutritional status plus 50 g of soya protein versus preoperative nutrition only. |
| Outcomes | Not specified. |
| Notes | The details of the preoperative nutrition and soya protein supplement need to be evaluated once there is a full‐text publication to determine whether this study meets the inclusion criteria. It could meet the criteria for comparison 3. |
CTRI/2018/11/016369.
| Methods | Controlled trial. |
| Participants | Patients diagnosed with CHILD B & C chronic liver disease. |
| Interventions | Telephonic reinforcement of nutritional counselling vs. standard care with no telephonic counselling. |
| Outcomes | Primary outcome: reduction in mortality. Secondary outcomes: improvement of sarcopenia, reducation of upper GI bleed, reduction hepatorenal syndrome, reduction of hepatic encephalopathy. |
| Notes | Full trial record not available. This trial needs assessment against the inclusion criteria when a full‐text publication is available. |
Cui 2017.
| Methods | Single‐blind RCT. |
| Participants | 25 people with gastric cancer (postoperative and receiving neo‐adjuvant chemotherapy). |
| Interventions | Dietary advice and oral nutritional ONS versus personalised dietary advice. |
| Outcomes | BMI, haemoglobin, prealbumin, albumin, gastrointestinal function score, QoL. |
| Notes | This study is in Chinese. Limited translation indicated that it meets the inclusion criteria for Group 3. Full translation awaited. |
Gaitan 2017.
| Methods | RCT. |
| Participants | 100 participants with chronic renal failure and protein‐ energy wasting on haemodialysis. |
| Interventions | Nutritional counselling to meet daily requirements versus ONS. |
| Outcomes | BMI, weight, serum cholesterol, serum albumin and handgrip strength. |
| Notes | Currently published as an abstract only but looks eligible for inclusion in comparison 3 (dietary advice versus ONS). Once a full‐text publication is available evaluate against the inclusion criteria. |
Ha 2010.
| Methods | RCT. |
| Participants | 170 participants with acute stroke. |
| Interventions | Individualised nutritional care (including ONS and enteral feeding) versus routine care. |
| Outcomes | Primary outcome: % of participants with weight loss. Secondary outcomes: QoL, handgrip strength, length of stay. |
| Notes | 17 of 170 participants receive enteral feeding. This study should be considered for inclusion in comparison 4 of the review (dietary advice plus ONS versus routine care). |
Hansen 2020.
| Methods | RCT. |
| Participants | Participants over 65 years with commuity‐acquired pneumonia. |
| Interventions | Individualised nutrition guidance and oral supplementation versus standard care. |
| Outcomes | Weight, FFM, handgrip strength, QoL, ADL. |
| Notes | Currently available as an abstract only. It looks to be eligible for inclusion in comparison 5 (dietary advice plus ONS versus routine care but needs to be assessed once a full‐text publication is available. |
Hebuterne 2019.
| Methods | Multicentre RCT. |
| Participants | 173 participants with metastatic colorectal cancer receiving chemotherapy. |
| Interventions | Early & active individualised dietary counselling by a dietitian with ONS and enteral & parenteral feeding prescribed according to guidelines versus no dietary counselling. |
| Outcomes | Primary outcome: grade >3 toxicity. Secondary outcomes: treatment delay, weight. |
| Notes | Currently available as an abstract only. Full details of the intervention needed as well as numbers of participants going on to receive EN and PN needed to judge eligibility. |
Hoekstra 2005.
| Methods | Pilot RCT. |
| Participants | 30 participants with COPD. |
| Interventions | An individual nutritional intervention carried out by a dietitian versus standard nutritional advice from a pulmonologist and 3x 125 mL of an ONS. |
| Outcomes | FFMI, QoL, compliance. |
| Notes | This seems to be published as an abstract only. A full‐text publication is needed to assess fully but looks to meet the inclusion criteria for comparison 2 (dietary advice versus ONS). Also seems to be published in Aktuelle Ernahrungsmedizin 2005;30‐24. |
Hsieh 2019.
| Methods | RCT (4‐arm). |
| Participants | 319 frail or pre‐frail older adults attending outpatient clinics at Miaoli General Hospital, Taiwan. |
| Interventions | Interventions were exercise, nutrition intervention, exercise+nutrition intervention and control. The nutritional intervention consisted of advice based around the Taiwanese Food Guide six food groups to maintain a desirable body weight plus a food supplement of 25 g of skim milk powder and 10 g of mixed nuts. |
| Outcomes | Primary outcome: frailty score, handgrip strength, gait speed, physical activity. Secondary outcomes: physical performance, mental health status. Dietary compliance |
| Notes | Two groups relevant to include in comparison 1 ( nutrition intervention versus control). |
Hubbard 2009.
| Methods | RCT. |
| Participants | 45 community‐based older adults at risk of malnutrition (MUST). |
| Interventions | 400 kcal/d from an oral nutritional supplement (Calogen Extra) or dietary advice. |
| Outcomes | Energy and nutrient intake, appetite, acceptability, tolerance, compliance with supplementation. |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 2 of the review but needs full assessment once the final paper is available. |
Jabbour 2019.
| Methods | RCT. |
| Participants | 46 adults admitted to the bone marrow transplantation unit (Beirut). |
| Interventions | At hospital discharge dietary advice tailored to individual requirements plus ONS if required versus no tailored advice. Both groups were advised on food safety guidelines. |
| Outcomes | Nutritional status, dietary intake, body compositiion, muscle strength. |
| Notes | This seems to be relevant for inclusion in comparison 4 (dietary advice plus ONS if required versus no dietary advice). |
Jia 2019.
| Methods | RCT. |
| Participants | 160 participants with COPD. |
| Interventions | Nutritional support versus usual medical care. |
| Outcomes | Lung function, nutritional index, blood gas index, length of stay. |
| Notes | This study is published in Chinese and needs translation before the details of the nutritional intervention can be assessed against the inclusion criteria. |
Kalal 2016.
| Methods | RCT |
| Participants | 104 malnourished individuals with alcoholic liver cirrhosis. |
| Interventions | Standard nutritional counselling versus nutritional counselling and ONS. |
| Outcomes | Nutritional, clinical and biochemical status. |
| Notes | Published as an abstract only but looks to be eligible for inclusion in comparison 3. Evaluate against the inclusion criteria once a full‐text publication is available. |
Kandel 2014.
| Methods | RCT. |
| Participants | Frail adults. |
| Interventions | Dietary intervention versus dietary intervention and Nordic walking training versus dietary counselling for 8 weeks. |
| Outcomes | Gait speed, handgrip strength, frailty score. |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 3 of the review but needs full assessment once the final paper is available. Contact with the senior author failed to clarify questions about the intervention groups. |
Kang 2013.
| Methods | RCT. |
| Participants | 60 hospitalised adults aged over 65 years following surgery for hip fracture. |
| Interventions | Dietary counselling and oral nutritional ONS + trace element supplement for 2 weeks postoperatively versus usual care. |
| Outcomes | Change in MNA score, energy and protein intake, handgrip strength. |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 1 of the review but needs full assessment once the final paper is available. |
Kuhlmann 1999.
| Methods | To be determined, participants were "assigned to three groups". |
| Participants | 18 participants with renal disease on maintenance haemodiaylsis. |
| Interventions | 45 kcal/kg/d and 1.5 g protein /kg/d versus 35 kcal/kg/d and 1.2 g protein /kg/d versus spontaneous intake. Participants in the first 2 groups received food supplements at appropriate dosing to reach the target intake. |
| Outcomes | Nutritional intake, compliance, tolerance, weight, serum albumin, pre‐albumin, cholesterol. |
| Notes | Full‐text paper not available. Further details of the intervention needed including whether dietary counselling was used to implement the intervention. |
Kwon 2004.
| Methods | Not clear from the abstract. |
| Participants | Urban dwelling older women in Korea. |
| Interventions | Weekly home‐visit nutrition education by a dietitian. |
| Outcomes | Nutritional knowledge, attitude, and dietary habits, nutrient intake, anthropometric and biochemical status. |
| Notes | This article is in Japanese and not possible to assess fully against the inclusion criteria. It looks as if the intervention might be a healthy eating intervention but translation needed to determine eligibility. |
Limwannata 2021.
| Methods | RCT. |
| Participants | 80 participants with renal disease receiving haemodialysis. |
| Interventions | Nutrition counselling plus ONS (ONCE Dialyze) versus nutrition counselling plus ONS (NEPRO) versus nutrition counselling alone. |
| Outcomes | Nutriitonal status, body composition, serum albumin and pre‐albumin. |
| Notes | This looks eligible for inclusion in comparison 3 (dietary advice plus ONS versus dietary advice alone). |
Lin 2017.
| Methods | Prospective clinical trial. |
| Participants | 110 adults with colorectal cancer undergoing chemotherapy. |
| Interventions | Individualised nutritional support (education, encouragement and recipes) and "afterwards doctors administered parenteral or enteral nutrition at 70‐80% of requirements" versus normal diet. |
| Outcomes | Body weight, serum albumin and prealbumin. |
| Notes | The description of the intervention is confusing in that it describes recipes but also indicates that the doctors administered enteral and parenteral nutrition. Clarification has been sought from authors but no reply received. |
Liu 2018.
| Methods | RCT. |
| Participants | 170 hospitalised patients with Alzheimer's disease. |
| Interventions | Nurse‐led intensive nutritional intervention compared with routine nutritional management. |
| Outcomes | Nutritional risk (NRS‐2002) and QoL. |
| Notes | The full‐text article is in Chinese and so needs translation to determine the detail of the nutritional intervention and eligibility. |
Liu 2019.
| Methods | RCT. |
| Participants | 101 older hospital inpatients with Alzheimer's disease. |
| Interventions | "nutritional support based on clinical nursing pathway" versus routine nutritional management. |
| Outcomes | Nutritional risk (NRS‐2002) and QoL. |
| Notes | This article is in Chinese and needs translation to enable judgement about eligibility. **note this seems to be very similar to Liu 2018. Evaluate the two studies together** |
Loser 2021.
| Methods | RCT. |
| Participants | 61 participants with squamous cell carcinoma of the head and neck referred for adjuvant or definitive treament with curative intent. |
| Interventions | Individualised nutritional counselling from a dietitian with individualised recommendations versus no nutritional counselling. |
| Outcomes | Primary outcome: amount of malnutrition (assessed using a range of tools). Secondary outcomes: therapy‐related side‐effects, biochemical status. |
| Notes | This study meets the inclusion criteria for comparison 1 (dietary advice versus no advice). |
Maharshi 2016.
| Methods | RCT. |
| Participants | 120 participants with cirrhosis and minimal hepatic encephalopathy. |
| Interventions | Nutritional education to achieve energy and protein targets +2 g salt/day versus routine diet +2g salt/d. |
| Outcomes | Primary outcomes: improvement or worsening of minimal hepatic encephalopathy, QoL. Secondary outcomes: nutritional status, change in Child‐Turcotte‐Pugh score, change in Model for End‐Stage Liver disease score, change in critical flicker frequency, arterial ammonia, developement of HE, hospital admission, complications, death. |
| Notes | This looks relevant to include in comparison 1 (dietary advice versus no advice). |
Meng 2021.
| Methods | RCT. |
| Participants | 353 adults post surgery for (i) gastric cancer and at nutritional risk. Participants with (ii) colorectal cancer, (iii) head and neck cancer included in related clinical trial reports. |
| Interventions | Dietary advice and oral intake of Nutren Optimum versus dietary advice alone. |
| Outcomes | Nutritional outcomes, prevalence of sarcopenia, tolerance to chemotherapy, 90‐day readmission, QoL. |
| Notes | This group of trials listed in the WHO International register all look the same in terms of intervention but involve participants with different sites of cancer. Two trials are now published and look to be relevant to comparison 3 (dietary advice plus ONS versus dietary advice alone). |
Molassiotis 2021.
| Methods | Pilot RCT. |
| Participants | Adults with advanced cancer and family care‐givers. |
| Interventions | A family‐centred individualised nutritional intervention plus ONS if required versus usual care. |
| Outcomes | Feasibility outcomes: recruitment, consent rate, retention rate, acceptability of assessment tools. Nutritional intake, PG‐SGA, QoL, self‐efficacy, carer distress, anxiety and depression. |
| Notes | This looks eligible for inclusion in comparison 4 (dietary advice plus ONS if required versus usual care). |
Movahed 2020.
| Methods | RCT. |
| Participants | 100 participants with oesophageal cancer. |
| Interventions | Medical Nutrition Therapy consisting of an individualised plan with dietary education, escalation to use of ONS, enteral or parenteral feeding if participants failed to meet requirements versus general nutrition advice (possible to receive ONS, enteral and parenteral nutrition if prescribed by oncologists. |
| Outcomes | PG‐SGA, anthropometry, body composition, dietary intake, biochemical status, nutrition‐related complications. |
| Notes | More details are needed of the numbers of participants receiving enteral and parenteral feeding in order to determine eligibility. |
NCT01171495.
| Methods | RCT |
| Participants | 120 people with HIV who are asymptomatic and anti‐retroviral therapy naiive. |
| Interventions | Dietary counselling + multivitamin + ONS versus dietary counselling + multivitamin. |
| Outcomes | Nutritional status, BMI, clinical status, immune function and antioxidant status. |
| Notes | This looks relevant to include, possibly in comparison 3 (dietary advice plus ONS versus dietary advice alone) and is listed as completed but no full‐text paper identified. If a full‐text publication is identified assess against the inclusion criteria. |
NCT02051777.
| Methods | RCT. |
| Participants | 120 adults (> 50 years) at nutritional risk and living in the community. |
| Interventions | Dietary advice plus ONS1 versus dietary advice plus ONS2 versus dietary advice alone. |
| Outcomes | Nutrient intake. |
| Notes | Listed as completed on Clinical Trials but no publication identified. Contact the sponsor Nutricia for further details. |
NCT02975089.
| Methods | RCT. |
| Participants | 40 frail older adults. |
| Interventions | Multiple nutrients supplementations versus multiple nutrients plus isolated soy protein supplementation versus individualized nutrition education with designed dishware for balanced diet as well as food supplementations (mixed nuts and milk powder) versus no intervention. |
| Outcomes | Primary outcomes: change in nutritional intake. Secondary outcomes: frailty score, geriatric depression score, nutritional status, urinary nitrogen and creatinine. |
| Notes | Currently available as a clinical trial record only and impossible to fully assess the intervention for eligibility. Assess against the inclusion criteria once a full‐text paper is available. |
NCT03631537.
| Methods | RCT. |
| Participants | 200 participants with advanced cancer receiving chemotherapy. |
| Interventions | Nutritional intervention from the nutrition support team including patient education, dietary supplements and parenteral solutions versus clinicians deciding how and whether to give nutritional support. |
| Outcomes | Treatment toxicities, number of chemotherapy cycles completed, progression‐free survival, adverse events. |
| Notes | Only available as a clinical trial record and not possible to evaluate eligibility. Once a full‐text publication is available the intervention should be examined against the inclusion criteria. |
NCT03632200.
| Methods | RCT. |
| Participants | 60 participants with head and neck cancer undergoing surgery. |
| Interventions | Individualised dietetic consultation post operatively for six months versus routine care. |
| Outcomes | Primary outcome: nutritional status (weight change). Secondary outcome: PG‐SGA score. |
| Notes | Full details of the dietetic consultation needed to allow assessment against inclusion criteria. Assess for eligibility once there is a full‐text publication. |
NCT03944161.
| Methods | RCT. |
| Participants | Participants with malnutrition. |
| Interventions | ONS versus nutrition advice from the clinician. |
| Outcomes | BMI, change in nutritional status, number with malnutrition, QoL, strength and endurance, number of hospital admissions, number of consultations with clinician. |
| Notes | From the trial record this looks to be relevant to comparison 2. I note that the advice is not described as dietary counselling but nutrition advice from a clinician and so will need to be evaluated once the full study is published. |
NCT04217564.
| Methods | RCT. |
| Participants | 120 participants with multiple sclerosis. |
| Interventions | Nutritional counselling versus no nutritional counselling. |
| Outcomes | Primary outcomes: change in QoL, change in nutritional status. Secondary outcome: change in disease progression. |
| Notes | The intervention and nutritional status of participants needs assessment against the inclusion criteria once a full‐text publication is available. It is not possible to determine the content and goals of the nutritional counselling from the clinical trial record. |
Norshariza 2018.
| Methods | RCT. |
| Participants | 40 outpatients with head and neck cancer undergoing radiotherapy. |
| Interventions | Dietary counselling from a dietitian plus ONS versus dietary counselling from a dietitian. |
| Outcomes | Nutritional outcomes (weight change, BMI, body composition, dietary intake, biochemical status), functional outcomes (handgrip strength), side effects of treatment. |
| Notes | This is currently published as an abstract of The Asian Congress of Nutrition and there is a clincal trials record that seems to relate to the same study. The trial was registered in September 2016 with a projected completion of September 2018. There is no full‐text publication identified other than Neoh 2020 which is not an RCT but a prospective observational study. From the trial report it looks to be relevant to include in comparison 3 (dietary advice plus ONS versus dietary advice alone) but needs assessment against further information. |
Nyguyen 2020.
| Methods | RCT. |
| Participants | 120 malnourished individuals with COPD. |
| Interventions | Tailored nutritional counselling versus routine care (a booklet). |
| Outcomes | Nutritional intake, change in body weight, nutritional status, SGA score, muscle strength, QoL. |
| Notes | This study meets the inclusion criteria for comparison 1 (dietary advice versus routine care). |
Otten 2016.
| Methods | RCT. |
| Participants | 71 frail older adults with malnutrition (MNA). |
| Interventions | Dietary counselling versus oral nutritional ONS for 3 months. |
| Outcomes | Mobility (TUG), handgrip strength, knee extension, QoL (EQ‐VAS), functional limitations (LASA). |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 2 of the review but needs full assessment once the final paper is available. |
Park 2012.
| Methods | Comparison of 2 groups (unclear whether participants were randomly allocated to groups). |
| Participants | 40 people with colorectal cancer receiving chemotherapy. |
| Interventions | Individualised nutrition counselling versus control group who received counselling after completion of data collection. |
| Outcomes | Energy and protein intake, weight, serum albumin. |
| Notes | This paper is in Korean and it has not been possible to obtain a translation to date. |
Pinto 2021.
| Methods | RCT. |
| Participants | 80 patients post surgery for oesophagectomy for cancer. |
| Interventions | Individualised nutrition counselling plus ONS if patients failed to achieve 75% of estimated energy requirements versus standard care. |
| Outcomes | Primary outcomes: dyspnoea, appetite loss and Global QoL after 1 month. Secondary outcomes: eating, dyspnoea, and Global QoL after 3 months. |
| Notes | This study looks eligible for inclusion in comparison 4 (dietary advice plus ONS if required versus routine care). Note escalation of intervention to enteral feeding. Check numbers receiving enteral feeding to determine eligibility. |
Qui 2020.
| Methods | RCT. |
| Participants | 96 participants with oesophageal cancer receiving concurrent chemotherapy. |
| Interventions | Whole course nutritional intervention developed by a dietitian and oncologist which could include ONS, enteral and parenteral feeding versus routine care. |
| Outcomes | Nutritional risk (NRS‐2002), nutritional status, nutritional intake, biochemical status, QoL, psychological condition, complications, completion of therapy, efficacy of intervention, length of stay and costs. |
| Notes | It is not possible to determine from the paper the number of patients that received enteral and parenteral feeding. This needs to be evaluated to determine eligibility for inclusion. |
RBR‐35kjvg.
| Methods | RCT. |
| Participants | Patients with head and neck cancer receiving radiotherapy. |
| Interventions | Intensive nutritional counselling versus routine care. |
| Outcomes | Primary outcomes: change in nutritional status, QoL. Secondary outcomes: PG‐SGA, food intake, FFM, complications, hospital admissions, treatment interruptions. |
| Notes | The full trial record is not available. This trial needs to be assessed against the inclusion criteria following full‐text publication. |
RBR‐3shhxs.
| Methods | RCT. |
| Participants | 62 participants with breast cancer planned to receive neoadjuvant chemotherapy. |
| Interventions | "dietary guidelines regarding her intervention group". |
| Outcomes | Primary outcome: QoL. Secondary outcomes: nutritional intake, biochemical status, anthropometry, PG‐SGA, treatment toxicity. |
| Notes | Not currently possible to access the trial registry and so information from the abstract only. This looks relevant to include but the intervention needs to be assessed against the inclusion criteria once more information are available. |
Reinders 2020.
| Methods | RCT. |
| Participants | 264 community dwelling older adults (>65 years) with an habitual low protein intake. |
| Interventions | Personalised dietary advice to increase protein intake to 1.2 kg/body weight/day using regular foods and protein‐enriched products versus personalised dietary advice to increase protein intake to 1.2 kg/body weight/day plus exercise versus no intervention. All groups receive a standard brochure about healthy eating. |
| Outcomes | Primary outcome: change in walk time (400 m walk test). Secondary outcomes: Change in dietary intake, prevalence of malnutrition, physical performance, mobility limitations, muscle strength, body weight, body composition, frailty status, QoL, healthcare costs. |
| Notes | This looks like it can be included in comparison 1 (dietary advice versus no advice). |
Sahathevan 2018.
| Methods | RCT. |
| Participants | 126 participants receiving peritoneal dialysis. |
| Interventions | Dietary counselling and 2x 15 g sachets of a whey protein supplement versus dietary counselling alone. |
| Outcomes | Anthropometry, body composition, biochemical assessment, dietary intake and appetite, nutritional status, QoL and handgrip strength. |
| Notes | Full‐text publication available. Consider for inclusion in comparison 3 (dietary advice versus dietary advice + ONS). |
Salem 2020.
| Methods | RCT. |
| Participants | 100 particpants receiving haemodialysis. |
| Interventions | Nutrition education including tailored dietary counselling from a renal dieitian versus usual care. |
| Outcomes | Nutritional intake (diet history), malnutrition inflammation score, anthropometry and biochemical status. |
| Notes | Published as an abstract only. It is not possible to fully assess the eligibility of the nutrition education. Assess against inclusion criteria once a full‐text publication is available. |
Sathiaraj 2020.
| Methods | Pilot RCT. |
| Participants | 103 participants with cancer receiving chemotherapy. |
| Interventions | Nutrition interventions using the Nutrition Care Process. |
| Outcomes | Primary outcome: change in fatigue. Secondary outcomes: change in nutritional status, BMI, body weight, depression. |
| Notes | Published as a conference abstract only and not possible to fully evaluate the nutrition intervention. Assess against the inclusion criteria once a full‐text publication is available. |
Schuetz 2019.
| Methods | RCT. |
| Participants | 2088 medical patients who were in hospital and at nutritional risk. |
| Interventions | Protocol‐guided individualised nutritional support to reach protein and calorie goals versus standard hospital food. |
| Outcomes | Primary endpoint: any adverse clinical outcome (all‐cause mortality), admission to intensive care, non‐elective hospital readmission, major complications and decline in functional status at 30 days. |
| Notes | This study meets the inclusion criteria for comparison 4 (dietary advice plus ONS if required versus routine care). |
Shadid 2019.
| Methods | RCT. |
| Participants | 60 participants with histologically proven squaumous cell carcinoma for tratment with radical radiotherapy or radical concurrent chemoradiation with curative intent. |
| Interventions | Nutrition counselling following the American Dietetic Association Medical Nutrition Therapy protocol for radiation oncology versus a talk on general nutrition and a booklet. |
| Outcomes | Dietary intake, treatment interruption and health‐related QoL. |
| Notes | Currently reported as an abstract only, needs further assessment once the full‐text paper is available to determine the appropriate comparison group. |
Shatenstein 2017.
| Methods | Quasi‐experimental (3 clinics intervention, the remainder control). |
| Participants | 67 elderly people (aged >70 years) with early stage dementia recruited with their carers. |
| Interventions | Tailored nutritional intervention versus usual care. |
| Outcomes | Nutrient intake. |
| Notes | 2008 paper reported the following outcomes would be measured: questionnaire (socio‐demographic, general health information, medication use, health perception and physical activity), VAS for hunger and appetite, functional status (ADL and IADL), weight, height, grip strength, nutrient intake. Need to contact the authors to determine if group allocation was randomised. |
Smith 2020.
| Methods | RCT. |
| Participants | 308 free‐living older people (over 50 years) attending GP services and at risk of malnutrition (MUST). |
| Interventions | Dietary advice plus low volume ONS (Fortisip Compact) versus dietary advice alone. |
| Outcomes | Energy and protein intake. |
| Notes | This study seems to meet the inclusion criteria for comparison 3 of the review. |
Soderstrom 2020.
| Methods | Multicentre RCT. |
| Participants | 671 older participants (> 65 years) malnourished on hospital admission. |
| Interventions | Individualised dietary advice versus ONS versus individualised dietary advice + ONS versus no intervention. |
| Outcomes | Survival. |
| Notes | This trial has four arms and could be included in comparison 1 (dietary advice versus no advice), comparison 2 (dietary advice versus ONS), comparison 3 (dietary advice +ONS versus dietary advice) and comparison 5 (dietary advice+ONS versus no intervention). |
Stratton 2007.
| Methods | RCT. Duration to be confirmed. |
| Participants | 50 adults (42 females, 8 males) with hip fractures. Mean age 82 years (range 46 ‐ 97 years). All participants at risk of malnutrition assessed by MUST. |
| Interventions | Intervention (n = 26): oral nutritional ONS (300 kcal/carton). Control (n = 24): readily available snacks (300 kcal/portion). |
| Outcomes | Mortality, number of participants with complications, energy, protein and micronutrient intake. |
| Notes | Full paper not published. Available as an abstract only. This seems to meet the inclusion criteria for comparison 2 of the review but needs full assessment once the final paper is available. |
Sudarsanam 2011.
| Methods | Pilot RCT. |
| Participants | 103 participants with tuberculosis alone or tuberculosis‐HIV coinfectionx receiving anti‐tuberculous therapy. |
| Interventions | Dietary advice from a dietitian plus a locally made macronutrient supplement versus dietary advice alone. |
| Outcomes | Primary outcome: treatment outcome (cure, completion or failure, interruption) . Secondary outcomes: body composition, compliance and treatment outcome at 1 year. |
| Notes | This study looks eligible for inclusion in comparison 3 (dietary advice plus ONS versus dietary advice alone). |
Sui 2020.
| Methods | RCT. |
| Participants | 60 older people with squamous cell carcinoma of the oesophagus. |
| Interventions | "multidisciplinary team nutrition intervention" versus routine nutritional intervention. |
| Outcomes | BMI, nutritional status, adverse events, treatment time. |
| Notes | Article in Chinese and needs translation before it can be fully evaluated. |
Tharun 2020.
| Methods | RCT. |
| Participants | 109 participants with cirrhosis of the liver. |
| Interventions | Dietary counselliing to reinforce nutritional goals supported by telephone calls every 2 weeks versus standard care. |
| Outcomes | Mortality, complications, anthropometry. |
| Notes | Published as an abstract only. The details of the dietary counselling needs to be assessed against the inclusion criteria once a full‐text publication is available. |
Torbergsen 2019.
| Methods | RCT. |
| Participants | 113 participants admitted to hospital with hip fracture. |
| Interventions | An individualised plan made by a clinical nutritionist on improving food intake plus 2 protein‐enriched nutrition drinks daily plus a multivitamin supplement versus routine care. |
| Outcomes | Micronutrient status, bone turnover markers, body weight. |
| Notes | Seems to be eligible for inclusion in comparison 5 (dietary advice +ONS versus routine care). |
Touger Decker 1997.
| Methods | Randomised trial |
| Participants | 48 people who were edentate, with limited natural dentition or old dentures planned for denture insertion. |
| Interventions | Individualized dietary counselling versus no counselling |
| Outcomes | Nutrition risk status, dietary intake, percieved biting and chewing problems |
| Notes | This study is currently identifed as an abstract only. A full publication needs to be identified to enable assessment against the inclusion criteria for the review. |
UMIN000032234.
| Methods | Not described. |
| Participants | People on dialysis, over 65 years of age. |
| Interventions | Nutritional guidance aimed at adequate intake of energy and protein using nutrient supplement versus conventional treatment. |
| Outcomes | Primary outcomes: dietary intake, survival. Secondary outcomes: biochemical status, body composition, bone density, complications, hospital admissions. |
| Notes | It has not been possible to see the trial record and so not possible to evaluate against the inclusion criteria. Once a full‐text publication is available assessment against the inclusion criteria needed. |
van der Werf 2020.
| Methods | RCT. |
| Participants | 107 participants with metastatic colorectal cancer receiving first‐line chemotherapy. |
| Interventions | Individualised nutritional counselling by a dietitian to include ONS and EN as needed versus usual care. |
| Outcomes | Primary outcome: proportion of participants with a clinically relevant decrease in skeletal muscle area. Secondary outcomes: body weight, QoL, treatment toxicity, progression free and overall survival. |
| Notes | Only 8% of participants received EN and so relevant for inclusion in comparison 4 (dietary advice plus ONS if required versus routine care). |
Vazquez‐Sanchez 2019.
| Methods | RCT. |
| Participants | 106 hospitalised participants with malnutrition. |
| Interventions | Nutritional counselling by case manager nurses versus standard healthcare. |
| Outcomes | Nursing Outcomes Classification criteria ‐ compliance behaviour, knowledge, nutritional status and nutritional risk (M|UST), degree of dependence (Barthel's Functional Independence). |
| Notes | This study meets the inclusion criteria. The details of the nutritional counselling need to be clarified in order to determine the comparison group. |
Verho 2017.
| Methods | RCT. |
| Participants | Independently living older adults after discharge from hospital. |
| Interventions | Personalised nutrition care plan with ONS when required versus usual care. |
| Outcomes | Nutritional intake. |
| Notes | Currently available as an abstract only. When a full‐text publication is available this needs assessment against the inclusion criteria. |
Wills 2019.
| Methods | RCT. |
| Participants | 78 participants with amyotrophic lateral sclerosis |
| Interventions | Nutritional couseling in person from a registered dietitian versus nutritional counseling supported by an mHealth application versus standard care (general counseling on balanced nutrition from a doctor or nurse). |
| Outcomes | Primary outcomes: weight change, safety and tolerability, compliance with the dietary intervention. Secondary outcomes: dietary intake Tertiary outcomes: patient‐reported outcomes measurement information system short form (PROMIS SF), QoL. |
| Notes | This meets the criteria for inclusion in comparison 1 (dietary advice versus no dietary advice). |
Wu 2018.
| Methods | 5‐group RCT. |
| Participants | 40 frail or pre‐frail participants attending the Miaoli General Hospital, Taiwan. |
| Interventions | (1) control group (leaflet 'The Daily Food Guide') versus (2) Multinutrient group + leaflet 'The Daily Food Guide' versus (3) multinutrient, soy protein and multivitamin group + leaflet 'The Daily Food Guide' versus (4) individualised nutrition education from a dietitian to consume a nutritious diet plus 10 g/d mixed nuts and 25 g/d milk powder. |
| Outcomes | Nutritional status, dietary intake, anthropometry, frailty, depression, urinary nitrogen and creatinine. |
| Notes | Group 4 versus group 1 could be considered for inclusion in comparison 1 (dietary advice versus no advice). More details needed on what the multinutrient intervention consists of to fully evaluate. |
Yang 2019.
| Methods | RCT. |
| Participants | 82 older participants (> 65 years) with pneumonia and malnutrition. |
| Interventions | Individualised dietary advice versus standard nutritional supplements with no dietary advice. |
| Outcomes | Primary outcome: malnutrition risk score (MNA‐SF). Secondary outcomes: anthropometry, biochemical status, nutritional requirements, nutritional intake, adherence to dietary prescription, length of stay and hospital admissions. |
| Notes | This study seems to be eligible for comparison 2 (dietary advice versus and oral nutritional supplement). Check the details of the "standard nutritional supplements". |
Yang 2020.
| Methods | RCT. |
| Participants | 120 participants with oesophageal cancer undergoing radiotherapy. |
| Interventions | Individualised nutritional counselling plus ONS versus individualised nutritional counselling. |
| Outcomes | BMI, patient‐generated SGA, serum albumin, haemoglobin, white blood cell count, prealbumin, platelets, adverse events. |
| Notes | This study looks eligible for inclusion in comparison 3 (dietary advice plus ONS versus dietary advice alone). |
Zhang 2018b.
| Methods | RCT. |
| Participants | 64 participants with GI malignancy admitted to hospital. |
| Interventions | States "added with nutritional support". |
| Outcomes | Short‐term efficacy, adverse events, change in nutritional status. |
| Notes | This study is published in Chinese and needs translation to allow evaluation of what "nutritional support" entails. |
Zhou 2011.
| Methods | RCT. |
| Participants | 104 adults with end‐stage renal disease receiving peritoneal dialysis. |
| Interventions | Nutritional intervention and individualized nursing versus self‐diet and routine nursing for 6 months. |
| Outcomes | Nutritional risk, handgrip strength, anthropometry (TSF, MAC, MAMC), QoL, status of renal disease and general clinical condition. |
| Notes | Paper published in Chinese and need translation to determine whether it meets the inclusion criteria for any of the comparison groups. |
Zhu 2019.
| Methods | RCT. |
| Participants | 114 participants with gastrointestinal cancer and at nutritional risk treated with surgery . |
| Interventions | Dietary guidance from a physician plus ONS versus dietary guidance from a physician alone. |
| Outcomes | Anthropometry, biochemical status, infections, complications, GI functional status, QoL. |
| Notes | This study meets the inclusion criteria for comparison 3 (dietary advice plus ONS versus dietary advice alone). |
ADL: activities of daily living APACHE: Acute Physiology and Chroic Health Evaluation BMI: body mass index COPD: chronic obstructive pulmonary disease EN: enteral nutrition FEV1: forced expiratory volume in one second FFMI: fat‐free mass index GI: gastrointestinal GP: general practitioner IADL: instrumental activities of daily living HE: hepatic encephalopathy LASA: longitudinal Amsterdam aging study questionnaire MAC: mid‐arm circumference MAMC: mid‐arm muscle circumference MNA: mini nutritional assessment MUST: malnutrition universal screening tool NUTRIC: nutrition risk in critically ill ONS: oral nutritional supplement PG‐SGA: patient‐generated subjective global assessment PN: parenteral nutrition QoL: quality of life RCT: randomised controlled trial SGA: subjective global assessment SOFA: sequential organ failure assessment TSF: triceps skinfold thickness TUG: timed up and go VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612001253897.
| Study name | Porvoo Sarcopenia & Nutrition Trial. |
| Methods | RCT. |
| Participants | Community‐dwelling older adults in Finland (target recruitment 250 participants). |
| Interventions | Written information on dietary protein and nutrition in old age + instruction on simple exercises + encouragement to use vitamin D supplementation for all participants. Randomisation to no supplementation, protein supplementation, placebo supplement. |
| Outcomes | Physical performance (physical performance battery), handgrip strength, gait speed, balance, chair stand test, 2‐minute step test, compliance to interventions, patient‐reported benefits and adverse events, MNA, diet quality questionnaire, dietary intake, body composition (BMI, BIA), cognition, QoL, use of health and social care services, number of falls, mortality, biochemical status. |
| Starting date | April 2012. |
| Contact information | Mikko Bjorkman (mikko.bjorkman@helsinki.fi). |
| Notes | Seems to meet the inclusion criteria for comparison 3 but needs full assessment following publication. |
ChiCTR2000028963.
| Study name | |
| Methods | RCT. |
| Participants | Patients with head and neck cancer planned to undergo radiotherapy. |
| Interventions | Individualized and professional nutritional dietary counseling, as well as oral nutritional supplements (ONS) with an intervention duration of 12 weeks. The ONS was a 500 ml total nutrition formula (containing 500 kcal energy, 21 g protein, and corresponding amounts of other nutrients) given daily verus routine nutrition education by a clinician. |
| Outcomes | Primary outcome: body weight. Secondary outcomes: BMI, anthropometry, handgrip strength, calf circumference, PG‐SGA, QoL, depression, biochemical status, performance status, body composition, stand‐walk test. |
| Starting date | |
| Contact information | |
| Notes | Not possible to access the full trial report but this looks like it is eligible for inclusion in comparison 5 (dietary advice + ONS versus routine care). |
CTRI/2019/05/019387.
| Study name | CTRI/2019/05/019387 |
| Methods | 2‐group trial (no details of whether randomised). |
| Participants | Individuals with cirrhosis and frailty and sarcopenia. |
| Interventions | Stated as "home‐based intensive nutrition therapy" versus standard medical management. |
| Outcomes | Primary outcomes: frailty score, physical performance, prognostic score. Secondary outcomes: unplanned hospitalisation, death, need for liver transplantation, anthropometry. |
| Starting date | |
| Contact information | |
| Notes | Not possible to access the trial registry report and so full details no available. The intervention needs to be evaluated against the inclusion critieria once a full‐text publication is available. |
Munk 2020.
| Study name | |
| Methods | RCT |
| Participants | 200 oncology, gastrointestinal and medical patients from Herlev Gentofte Hospital, Denmark |
| Interventions | Individualised dietary counseling and provision of a package at hospital discharge containing energy and protein‐rich meals and snacks plus protein‐rich ONS and recommendation to take a daily multivitamin tablet |
| Outcomes | Primary outcomes: hospital admissions & LoS Secondary outcomes: 30‐day readmissions & LoS, QoL, Appetite, physical performance status, dietary intake, energy & protein requirements, nutritional status, mortality and evaluation of the intervention. |
| Starting date | |
| Contact information | |
| Notes | Once the full trial is published assess for inclusion in comparison 4 or comparison 5. |
NCT02440165.
| Study name | Basic Care Revisited: Early nutrition intervention for outpatients (BCR_N_). |
| Methods | Multi‐centre cluster‐RCT. |
| Participants | 150 participants attending surgical outpatient clinics. |
| Interventions | Early nursing intervention (malnutrition screening + standardised nutrition care plan (individualised advice+energy and protein rich meals and ONS) versus usual care for 6 months. |
| Outcomes | BMI, nutritional intake, length of stay, QoL, patient satisfaction, costs, process evaluation. |
| Starting date | January 2015. |
| Contact information | Getty Huisman ‐ de Waal (getty.huisman‐deaal@radboudumc.nl); Maud Heinen (maud.heinen@radboudumc.nl). |
| Notes | Seems to meet the inclusion criteria for comparison 4 but needs full assessment following publication. |
NCT02763904.
| Study name | Systematic oral nutritional support in hospitalized, moderately hypophagic patients at nutritional risk. |
| Methods | RCT. |
| Participants | 286 hospitalised individuals at nutritional risk. |
| Interventions | Intensive nutritional counselling + ONS versus intensive nutritional counselling. |
| Outcomes | Body composition (phase angle BIA), function (handgrip strength), nutritional intake, infections, adverse events (GI intolerance). |
| Starting date | July 2016. |
| Contact information | Emanuelle Cereda (elcereda@smatteo.pv.it). |
| Notes | Seems to meet the inclusion criteria for comparison 3 but needs full assessment following publication. |
NCT02892747.
| Study name | Dietetics education focused on malnutrition prevention (NUTRICOEUR). |
| Methods | RCT. |
| Participants | 295 adults with chronic heart failure, BMI ≥ 18.5. |
| Interventions | Personalised education program for the prevention of malnutrition versus usual follow‐up by cardiologist and nutritionist. |
| Outcomes | Number of hospitalisations, length of hospital stay, reason for hospitalisation, nutritional intake (sodium, energy, protein), QoL, Minnesota Living With Heart Failure Questionnaire, burden of diet questionnaire, compliance, unplanned hospitalisation for cardiac complications, mortality. |
| Starting date | September 2016. |
| Contact information | Veronique Benedyga (veronique.benedyga@aphp.fr). |
| Notes | Seems to meet the inclusion criteria for comparison 1 but needs full assessment following publication. |
NCT03075189.
| Study name | |
| Methods | RCT. |
| Participants | 38 individuals recruited from a geriatric ward in a Danish hospital. |
| Interventions | Protein‐enriched snacks/dishes in the morning and late evening, before bedtime and on discharge individualised dietary counseling focusing on choosing protein‐rich foods and on protein rich meals versus normal hospital food without enrichment and no dietary counseling at discharge. |
| Outcomes | Primary outcome: recorded protein intake Secondary outcomes: anthropometric measurements (weight, height, body composition estimated with bioimpedance), functional ability (De Morton Mobility Index) and Barthels ADL‐index), hand grip strength, sarcopenic status (SARC‐F), quality of life (EQ‐5D‐3L), length of stay and 30‐day readmissions after discharge. |
| Starting date | March 2017 |
| Contact information | Lars Holm, Associate professor, University of Copenhagen |
| Notes | The trial is listed as completed in 2017 but no results available. This looks to be eligible for comparison 1 (dietary advice versus no dietary advice). Evaluate once more detail and results available |
NCT03114202.
| Study name | NCT03114202 |
| Methods | RCT. |
| Participants | 90 participants with head and neck cancers undergoing radiotherapy. |
| Interventions | Individualised nutrition counselling from a dietitian versus . |
| Outcomes | Nutrition counselling by hospital nurses. |
| Starting date | April 2017. |
| Contact information | Jose Eluf Neto+55(11)3061‐8278 jose.eluf@hc.fm.usp.br Sheilla Faria+55(35)3701‐9745 shefaria@hotmail.com |
| Notes | This looks like it should be included in comparison 1 (dietary advice versus no advice). Assess once a full publication is available. Note in 2020 this group published a retrospective study on this population Faria et al Cancer Treatment & Research Communications. Need to check whether this is the same study? |
NCT03191253.
| Study name | Changing Health Through Food Support (CHEFS) program. |
| Methods | RCT. |
| Participants | 200 HIV positive adults living on a low income. |
| Interventions | Individualised nutritional counselling, provision of medically‐appropriate food support and group‐based nutrition education versus routine care from Project Open Hand. |
| Outcomes | Viral load, QoL, severity of depression (Patient Health Questionnaire PHQ‐9), adherence to antiretroviral therapy, diet quality, food security (HFSS). |
| Starting date | July 2016. |
| Contact information | None provided. |
| Notes | Seems to meet the inclusion criteria for comparison 4 but needs full assessment following publication. |
NCT03315195.
| Study name | Preoperative oral nutritional supplement vs conventional dietary advice in major gastrointestinal surgery. |
| Methods | RCT. |
| Participants | 268 adults undergoing major gastrointestinal surgery. |
| Interventions | Dietary advice versus oral nutritional supplement and dietary advice. |
| Outcomes | Postoperative complications. |
| Starting date | November 2017. |
| Contact information | Nattapanee Sukphol (nattapanee_benz@hotmail.com); Narongsak Rungsakulkji (narongsak.run@mahidol.ac.th). |
| Notes | Seems to meet the inclusion criteria for comparison 3 but needs full assessment following publication. |
NCT03352388.
| Study name | Effects of dairy and berry‐based snacks on nutritional and functional status and quality of life in older people (MAVIRE1). |
| Methods | RCT. |
| Participants | 85 older people (over 70 years) receiving home‐care services. |
| Interventions | High‐protein dairy‐based products and energy‐enriched berry products versus no additional snacks for 3 months. |
| Outcomes | Nutritional status (MNA), serum albumin, serum prealbumin, handgrip strength, BMI, MAMC, haemoglobin, C‐reactive protein, activity and sleep (ActiGraph monitoring), QoL. |
| Starting date | September 2015. |
| Contact information | Senior researchers, Riitta Torronen and Irma Nykanen. |
| Notes | Seems to meet the inclusion criteria for comparison 1 but needs full assessment following publication. |
NCT03519139.
| Study name | |
| Methods | RCT. |
| Participants | 40 older medical inpatients (> 65 years) with at least two comorbidities. |
| Interventions | Individualised dietary counselling versus routine care. |
| Outcomes | Primary outcome: 30 day hospital readmission. Secondary outcomes: hospital readmission up to 60 days, time between admissions, nutritional status, functional status, muscle strength, QoL, mortality, adverse events. |
| Starting date | February 2018. |
| Contact information | Jens Rikardt Andersen, Associate Professor, University of Copenhagen |
| Notes | This meets the inclusion criteria for comparison 1 (dietary advice versus routine care). Assess once a full publication is available. |
NCT03540784.
| Study name | Effects of WB‐EMS and High Protein Diet in IBD Patients. |
| Methods | RCT. |
| Participants | Outpatients with inflammatory bowel disease. |
| Interventions | High protein nutritional intervention (dietary counselling from a dietitian) versus WB‐EMS supervised exercise training and high protein nutritional intervention versus routine care. |
| Outcomes | Primary outcome: skeletal muscle mass. Secondary outcomes: body composition, physical function, QoL, physical activity, disease activity, inflammation status. |
| Starting date | 01 October 2013. |
| Contact information | University of Erlangen‐Nürnberg Medical School. |
| Notes | Comparison group to be determined once the full trial report is available. |
NCT03995303.
| Study name | HOMEFOOD Study. |
| Methods | RCT. |
| Participants | 200 older adults at nutritional risk who have been discharged from hospital. |
| Interventions | An individualised nutriton care plan based on estimated requirements and nutritional goals plus ONS if required and home delivered meals versus routine care at hospital discharge. |
| Outcomes | Body weight, body composition, nutritional status, function (sit to stand, balance test), nutritional intake, handgrip strength, cognitive function, QoL. |
| Starting date | June 2018. |
| Contact information | Professor Alfons Ramel. |
| Notes | This seems to meet the inclusion criteria for comparison 4 (dietary advice plus ONS if required). Evaluate fully when a full‐text publication is available. |
NCT04628117.
| Study name | Effect of Oral Nutritional Supplementation on Oxidative Stress in Protein‐energy Wasting Patients With Peritoneal Dialysis |
| Methods | RCT. |
| Participants | 22 participants with end‐stage kidney disease receiving continuous peritoneal dialysis. |
| Interventions | Nutritional counselling plus 237 ml/day of ONS for kidney disease versus nutritional counselling alone. |
| Outcomes | Change in oxidative stress levels, change in protein‐energy wasting, change in dietary intake. |
| Starting date | October 2021. |
| Contact information | Francisco Gerardo Yanowsky Escatell, Principal Investigator, Hospital Civil Juan I. Menchaca, Mexico. |
| Notes | The intervention looks to be eligible for inclusion in comparison 3 (dietary advice plus ONS versus dietary advice alone). Assess against the inclusion criteria once a full‐text report is available. |
PACTR201108000303396.
| Study name | Assessing the impact of a food supplement on the nutritional status and body composition of HIV‐infected Zambian women on ARVs. |
| Methods | Random assignment to intervention or control group by the sister in charge at the clinic ‐ implies quasi‐randomised. |
| Participants | 200 HIV infected females commencing antiretroviral therapy at 1 of 2 urban clinics in Lusaka, Zambia. |
| Interventions | Advice on good nutrition + a food supplement versus advice on good nutrition. |
| Outcomes | Nutritional status (weight, height, 4‐site skinfold measurements, body composition (BIA), total body water, HIV‐related clinical outcomes (viral load, CD4 count), dietary intake. |
| Starting date | Not available. |
| Contact information | Author for correspondence on published protocol Andrew Hills (ahills@mmri.mater.org.au). |
| Notes | Might meet the inclusion criteria for comparison three of the review but needs full assessment following publication. |
BIA: bioelectrical impedance analysis BMI: body mass index GI: gastro‐intestinal HFSS: US Household Food Security Survey MAMC: mid‐arm muscle circumference ONS: oral nutritional supplement PG‐SGA: patient‐generated Subjective Global Assessment QoL: quality of life RCT: randomised controlled trial
Differences between protocol and review
The authors added the intervention 'dietary advice plus ONS if required compared with no advice and no ONS' post hoc as a result of an additional group of studies they identified during searching and study identification. They considered these studies relevant to this review as they examine dietary advice compared with no advice, but the dietary advice includes information on using ONS if considered 'necessary'.
2021 update
The review authors added the comparison 'dietary advice and prescription of an ONS compared with no advice and no ONS' as a result of closer scrutiny of the studies of dietary advice plus ONS if required. It became clear that studies for this comparison were falling into two distinct groups, those where ONS were sometimes used in addition to dietary advice in only some participants, with the phrase "if judged appropriate" often being used. The review authors recognised a second group of studies, where dietary advice and ONS were given to all participants from the start and so they added this fifth comparison to the review.
The authors have added 'complications' as a measure of morbidity (Primary outcome 2).
The authors have used the Egger Test to look for publication bias and described this in the methods. They have also been able to examine the overall quality of evidence using the GRADE process and complete summary of findings tables.
Contributions of authors
Until January 2007, the original review and all updates were prepared by Christine Baldwin and Tessa Parsons with Stuart Logan acting in a senior author capacity. Christine Baldwin, Tessa Parsons and Stuart Logan contributed to the development of the protocol and preparation of the review. Christine Baldwin and Tessa Parsons conducted the searches, selected studies for inclusion, entered data and prepared the analyses. Any questions or disagreements were resolved in discussion with Stuart Logan. After this date, both Tessa Parsons and Stuart Logan stepped down as authors on this review.
In 2007 a new co‐author, Elizabeth Weekes was recruited and has contined to contribute to all versions up to the current the update.
For the 2021 update two new co‐authors joined the team, Marian de van der Scheuren and Hinke Kruizenga. All four authors have contributed equally to the process of selection of studies for inclusion, data entry, preparation of the analyses and interpretation of findings.
Christine Baldwin acts as guarantor of the review.
Sources of support
Internal sources
-
Systematic Reviews Training Unit (funded by the London Regional Health Authority), UK
The Systematic Reviews Training Unit at University College London provided a fully funded place on a one year scheme (1998 to 1999) which provided training and support in undertaking a Cochrane systematic review.
External sources
-
British Dietetic Association, UK
Through a grant from the General and Education Trust, the British Dietetic Association funded some of the work in the first two years of undertaking this review.
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
The first year of work on the protocol for this review was funded by the British Dietetic Association.
The authors of this review are all authors of studies included in the review, but have not extracted data or assessed risk of bias for any studies they have authored.
MdvdS declares her institution received honoraria for independent lectures which she gave at educational and scientific events organized by Fresenius Kabi, Nutricia; she has also received a grant (paid to her institution) from the Dutch cancer society to perform a nutritional intervention trial.
LW declares receipt of funds from Abbott Nutrition for an educational webinar on the estimation of nutritional requirements and from Nutricia UK for serving on the expert panel of the recently published COPD Malnutrition Pathway; neither of these had any direct bearing on the preparation and completion of this Cochrane Review.
CB and HMK declare no potential conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Akpele 2004 {published data only}
- Akpele L, Bailey JL. Nutrition counselling impacts serum albumin levels. Journal of Renal Nutrition 2004;14(3):143-8. [DOI] [PubMed] [Google Scholar]
Alo 2014 {published data only}
- Alo C, Ogbonnaya LU, Azuogu BN. Effects of nutrition counselling and monitoring on the weight and hemoglobin of patients receiving antiretroviral therapy in Ebonyi State, Southeast Nigeria. HIV/AIDS - Research and Palliative Care 2014;6:91-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anbar 2014 {published data only}
- Anbar R, Beloosesky Y, Cohen J, Madar Z, Weiss A, Theilla M, et al. Tight calorie control in geriatric patients following hip fracture decreases complications: a randomized, controlled study. Clinical Nutrition 2014;33(1):23-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Andersson 2017 {published data only}
- Andersson J, Hulander E, Rothenberg E, Iversen PO. Effect on body weight, quality of life and appetite following individualized, nutritional counselling to home-living elderly after rehabilitation - an open randomized trial. Journal of Nutrition, Health & Aging 2017;21(7):811-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01962272. The effect of nutritional counseling for cancer patients. clinicaltrials.gov/show/NCT01962272 (date first posted 14 October 2013).
Arnold 1989 {published data only}
- Arnold C, Richter MP. The effect of oral nutritional supplements on head and neck cancer. International Journal Of Radiation Oncology, Biology, Physics 1989;16(6):1595-9. [DOI] [PubMed] [Google Scholar]
Baldwin 2011 {published and unpublished data}
- Baldwin C, Spiro A, McGough C, Norman AR, Gillbanks A, Thomas K, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. Journal of Human Nutrition and Dietetics 2011;24:431-40. [DOI] [PubMed] [Google Scholar]
- Baldwin C, Spiro A, McGough C, Norman AR, O'Brien M, Cunningham DC, et al. The NUT study: the effect of dietetic and oral nutritional interventions on survival and quality of life in patients with weight loss undergoing palliative chemotherapy for gastrointestinal or lung malignancy, a randomised controlled trial. Proceedings of the Nutrition Society 2008;67(OCE4):E136. [Google Scholar]
- Bonney AH, Baldwin C, Whelan K, Andreyev HJ. The NUT study: what effect does nutritional intervention have on energy intake in patients with gastrointestinal cancer receiving chemotherapy. Proceedings of the Nutrition Society 2008;67(OCE4):E141. [Google Scholar]
Banks 2016 {published data only}
- Bank MD, Ross LJ, Webster J, Mudge A, Stankiewicz M, Dwyer K, et al. Pressure ulcer healing with an intensive nutrition intervention in an acute setting: a pilot randomised controlled trial. Journal of Wound Care EPUAP supplement 2020;29(9):S10-7. [DOI] [PubMed] [Google Scholar]
- Banks MD, Ross LJ, Webster J, Mudge A, Stankiewicz M, Dwyer K, et al. Pressure ulcer healing with an intensive nutrition intervention in an acute setting: a pilot randomised controlled trial. Journal of Wound Care 2016;25(7):384-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beattie 2000 {published data only}
- Beattie A, Prach AT, Baxter JP, Pennington CR. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut 2000;46(6):813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2012 {published data only}
- Beck AM, Kjær S, Hansen BS, Storm RL, Thal-Jantzen K, Bitz C. Follow-up home visits with registered dietitians have a positive effect on the functional and nutritional status of geriatric medical patients after discharge: a randomized controlled trial. Clinical Rehabilitation 2012;27(6):483–93. [DOI] [PubMed] [Google Scholar]
- NCT01249716. Follow-up home visits with nutrition. clinicaltrials.gov/show/NCT01249716 (date first posted 30 Nov 2010).
Beck 2015 {published data only}
- Beck A, Andersen UT, Leedo E, Jensen LL, Martins K, Quvang M, et al. Does adding a dietician to the liaison team after discharge of geriatric patients improve nutritional outcome: a randomised controlled trial. Clinical Rehabilitation 2015;29(11):1117-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Belqaid K, Brandt CF, Lugnet K, Rasmussen NML, Beck AM. Adding a dietitian to a discharge follow-home team is cost-effective for geriatric patients at nutritional risk. In: Clinical Nutrition. Vol. 35, Suppl 1. 2016:S160.
- NCT01776762. Nutritional intervention in a cross-sector model for the rehabilitation of geriatric patients. clinicaltrials.gov/show/NCT01776762 (date first posted 28 Jan 2013).
- Pohju A, Belqaid K, Brandt C, Lugnet K, Nielsen AL, Højgaard Rasmussen H, et al. Adding a dietitian to a Danish liaison-team after discharge of geriatric patients at nutritional risk may save health care costs. Journal of Aging Science 2016;4(3):1000159. [Google Scholar]
Berneis 2000 {published data only}
- Berneis K, Battegay M, Bassetti S, Nuesch R, Leisibach A, Bilz S, et al. Nutritional supplements combined with dietary counselling diminish whole body protein catabolism in HIV-infected patients. European Journal of Clinical Investigation 2000;30(1):87-94. [DOI] [PubMed] [Google Scholar]
Bonilla‐Palomas 2016 {published data only}
- Bonilla-Palomas JL, Gámez-López AL, Castillo-Domínguez JC, Moreno-Conde M, López-Ibáñez MC, Anguita-Sánchez M . Does nutritional intervention maintain its prognostic benefit in the long term for malnourished patients hospitalised for heart failure. Revista clinica espanola 2018;218(2):58-60. [DOI] [PubMed] [Google Scholar]
- Bonilla-Palomas JL, Gamez-Lopez AL, Castillo-Dominguez JC, Moreno-Conde M, Lopez Ibanez MC, Alhambra Exposito R, et al. Nutritional intervention in malnourished hospitalized patients with heart failure. Archives of Medical Research 2016;47(7):535-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bonilla Palomas JL, Gamez Lopez AL, Moreno Conde MC, Lopez Ibanez MC, Ramiro Ortega E, Castellano Garcia P, Pimentel Quezada Y, Villar Raez AL. Nutritional intervention in malnourished hospitalized patients with heart failure and hypoalbuminemia: subanalysis of PICNIC study. In: European Journal of Heart Failure. Vol. 18. 2016:37-38.
- Gámez-López AL, Bonilla-Palomas JL, Anguita-Sánchez M, Moreno-Conde M, López-Ibáñez C, Alhambra-Expósito R, et al. Rationale and design of PICNIC study: nutritional intervention program in hospitalized patients with heart failure who are malnourished [Justificación y diseño del estudio PICNIC:Programa de IntervenCión Nutricional en pacientes hospitalizados por Insuficiencia Cardiaca desnutridos]. Revista Española de Cardiología 2014;67(4):277–82. [DOI: 10.1016/j.recesp.2013.07.014] [DOI] [PubMed] [Google Scholar]
- Ramiro-Ortega E, Bonilla-Palomas JL, Gamez-Lopez AL, Moreno-Conde M, Lopez-Ibanez MC, Alhambra-Exposito R, et al. Nutritional intervention in acute heart failure patients with undernutrition and normalbuminemia: a subgroup analysis of PICNIC study. Clinical Nutrition 2018;37(5):1762-4. [DOI: 10.1016/j.clnu.2017.07.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bourdel‐Marchasson 2014 {published data only}
- Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, Germain C, Blanc JF, Dauba J, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PloS One 2014;9(9):e108687. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdel-Marchasson I, Durrieu J, Doussau A, Blanc-Bisson C, Fonck M on behalf of INOGAD investigators. The effects of nutritional advices in older patients at risk for malnutrition during chemotherapy for cancer on the quality of intakes. Multicentre inogad study. In: Clinical Nutrition. Vol. 32. 2013:S64, PP111.
- NCT00459589. Nutritional intervention in geriatric oncology. clinicaltrials.gov/show/NCT00459589 (date first posted 12 Apr 2007).
Burden 2011 {published data only}
- Burden ST, Hill J, Shaffer JL, Campbell M, Todd C. An unblinded randomised controlled trial of preoperative oral supplements in colorectal cancer patients. Journal of Human Nutrition and Dietetics 2011;24(5):441-8. [DOI: 10.1111/j.1365-277X.2011.01188.x.] [DOI] [PubMed] [Google Scholar]
Burden 2017 {published data only}
- Burden ST, Gibson DJ, Lal S, Hill J, Pilling M, Soop M, et al. Pre-operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight-losing patients with colorectal cancer: single-blind randomized controlled trial. Journal of Cachexia, Sarcopenia and Muscle 2017;8(3):437-46. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caccialanza 2015 {published data only}
- Caccialanza R, Palladini G, Cereda E, Bonardi C, Milani P, Cameletti B, et al. Nutritional counseling in systemic immunoglobulin light-chain (AL) amyloidosis: a prospective randomized, controlled trial. Clinical Nutrition 2014;33:S56-7, PP100. [Google Scholar]
- Caccialanza R, Palladini G, Cereda E, Bonardi C, Milani P, Cameletti B, et al. Nutritional counselling improves quality of life and preserves body weight in systemic immunoglobulin light-chain (AL) amyloidosis. Nutrition (Burbank, Los Angeles County, Calif.) 2015;31(10):1228-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Calegari 2011 {published data only}
- Calegari A, Barros EG, Veronese FV, Thome FS. Malnourished patients on hemodialysis improve after receiving a nutritional intervention [Pacientes desnutridos em hemodialise melhoram apos receber intervencao nutricional]. Brazilian Journal of Nephrology 2011;33(4):394-401. [PubMed] [Google Scholar]
Campbell 2008 {published data only}
- ACTRN12606000493549. Structured nutrition intervention in pre-dialysis chronic kidney disease. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12606000493549 2006.
- Campbell KL, Ash S, Bauer JD. The impact of nutrition intervention on quality of life in pre-dialysis chronic kidney disease patients. Clinical Nutrition 2008;27(4):537-44. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Ash S, Davies PS, Bauer JD. Randomized controlled trial of nutritional counselling on body composition and dietary intake in severe CKD. American Journal of Kidney Diseases 2008;51(5):748-58. [DOI] [PubMed] [Google Scholar]
Cano‐Torres 2017 {published data only}
- Cano-Torres EA, Simental-Mendía LE, Morales-Garza LA, Ramos-Delgado JM, Reyes-Gonzalez MM, Sánchez-Nava VM, et al. Impact of nutritional intervention on length of hospital stay and mortality among hospitalized patients with malnutrition: a clinical randomized controlled trial. Journal of American College of Nutrition 2017;36(4):235-9. [DOI: 10.1080/07315724.2016] [DOI] [PubMed] [Google Scholar]
Carey 2013 {published data only}
- ACTRN12609000219280. Individualised nutritional counselling for patients who have undergone major upper gastrointestinal surgery - a randomised controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12609000219280 (date first registered 30 Apr 2009).
- Carey S, Ferrie S, Ryan R, Beaton J, Young J, Allman-Farinelli M. Long-term nutrition intervention following major upper gastrointestinal surgery: a prospective randomized controlled trial. European Journal of Clinical Nutrition 2013;67(4):324–9. [DOI: 10.1038/ejcn.2013.17] [DOI] [PubMed] [Google Scholar]
Casals 2015 {published data only}
- Casals C, Garcia-Agua-Soler N, Vazquez-Sanchez MA, Requena-Toro MV, Padilla-Romero L, Casals-Sanchez JL. Randomized clinical trial of nutritional counselling for malnourished hospital patients [Ensayo clinico aleatorizado del asesoramiento nutricional en pacientes desnutridos hospitalizados]. Revista Española de Cardiología 2015;215(6):308-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chandra 1985 {published data only}
- Chandra RK, Puri S. Nutritional support improves antibody response to influenza virus vaccine in the elderly. BMJ 1985;291(6497):705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Luis 2003 {published and unpublished data}
- Luis D, Aller R, Bachiller P, Gonzalez-Sagrado M, deLuis J, Izaola O, et al. Isolated dietary counselling program versus supplement and dietary counselling in patients with human immunodeficiency virus infection [Consejo nutricional aislado frente a suplemento y consejo nutricionales en pacientes con infeccion por el virus do la inmunodeficiencia humana]. Medicina Clinica 2003;120(15):565-7. [DOI] [PubMed] [Google Scholar]
de Sousa 2012 {published data only}
- Sousa OL, Amaral TF. Three-week nutritional supplementation effect on long-term nutritional status of patients with mild Alzheimer disease. Alzheimer Disease and Associated Disorders 2012;26(2):119-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Diouf 2016 {published data only}
- Diouf A, Badiane A, Manga NM, Idohou-Dossou N, Sow PS, Wade S. Daily consumption of ready-to-use peanut-based therapeutic food increased fat free mass, improved anemic status but has no impact on the zinc status of people living with HIV/AIDS: a randomized controlled trial. BMC Public Health 2016;16:1. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dixon 1984 {published data only}
- Dixon J. Effect of nursing interventions on nutritional and performance status in cancer patients. Nursing Research 1984;33(6):330-5. [PubMed] [Google Scholar]
Endevelt 2011 {published data only}
- Endevelt R, Lemberger J, Bregman J, Kowen G, Berger-Fecht I, Lander H, et al. Intensive dietary intervention by a dietitian as a case manager among community dwelling older adults: the EDIT study. Journal of Nutrition, Health & Aging 2011;15(8):624-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Evans 1987 {published data only}
- Evans WK, Nixon DW, Daly JM, Ellenberg SS, Gardner L, Wolfe E, et al. A randomized study of oral nutritional support versus ad lib nutritional intake during chemotherapy for advanced colorectal and non-small-cell lung cancer. Journal of Clinical Oncology 1987;5(1):113-24. [DOI] [PubMed] [Google Scholar]
- Foltz A, Besser P, Ellenberg S, Carty C, Mitchell S, Paul K, et al. Effectiveness of nutritional counselling on caloric intake, weight change, and percent protein intake in patients with advanced colorectal and lung cancer. Nutrition 1987;3(4):263-71. [Google Scholar]
Feldblum 2011 {published data only}
- Feldblum I, German L, Castel H, Harman-Boehm I, Shahar DR. Individualized nutritional intervention during and after hospitalization: the nutrition intervention study clinical trial. Journal of American Geriatric Society 2011;59:10–7. [DOI] [PubMed] [Google Scholar]
Fernandez‐Barres 2017 {published data only}
- Fernandez-Barres S, Garcia-Barco M, Basora J, Martinez T, Pedret R, Arija V. The efficacy of a nutrition education intervention to prevent risk of malnutrition for dependent elderly patients receiving Home Care: a randomised controlled trial. International Journal of Nursing Studies 2017;70:131-41. [DOI: 10.1016/j.ijnurstu.2017.02.020] [DOI] [PubMed] [Google Scholar]
Forli 2001 {published and unpublished data}
- Forli L, Bjortuft O, Vatn M, Kofstad J, Boe J. A study of intensified dietary support in underweight candidates for lung transplantation. Annals of nutrition and metabolism 2001;45(4):159-68. [DOI] [PubMed] [Google Scholar]
Forster 2012 {published data only}
- Forster SE, Powers HJ, Foulds GA, Flower DJ, Hopkinson K, Parker SG, et al. Improvement in nutritional status reduces the clinical impact of infections in older adults. Journal of American Geriatric Society 2012;60(9):1645-54. [DOI] [PubMed] [Google Scholar]
Fuenzalida 1990 {published data only}
- Fuenzalida CE, Petty TL, Jones ML, Jarrett S, Harbeck RJ, Terry RW, et al. The immune response to short-term nutritional intervention in advanced chronic obstructive pulmonary disease. American Review of Respiratory Disease 1990;142(1):49-56. [DOI] [PubMed] [Google Scholar]
Ganzoni 1994 {published and unpublished data}
- Ganzoni A, Heilig P, Schonenberger K, Hugli O, Fitting JW, Brandli O. High-caloric nutrition in chronic obstructive lung disease [Hochkalorische Ernahrung bei chronischer obstruktiver Lungenkrankheit]. Schweizerische Rundschau Fuer Medizin Praxis 1994;83(1):13-6. [PubMed] [Google Scholar]
Gonzalez‐Espinoza 2005 {published data only}
- Gonzalez-Espinoza L, Gutierrez-Chavez J, Campo FM, Martinez-Ramirez HR, Cortez-Sanabria L, Rojas-Campos E, et al. Randomized, open-label, controlled clinical trial of oral administration of an egg albumen-based protein supplement to patients on continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 2005;25(2):173-80. [PubMed] [Google Scholar]
Gray‐Donald 1995 {published and unpublished data}
- Gray-Donald K, Payette H, Boutier V. Randomized clinical trial of nutritional supplementation shows little effect on functional status among free-living frail elderly. Journal of Nutrition 1995;125(12):2965-71. [DOI] [PubMed] [Google Scholar]
Gu 2015 {published data only}
- Gu B, Zhang R, Wang Yi, Yao J. Effect of nutritional support on nutritional status and clinical outcome of patients in internal medical departments. Chinese Journal of Clincal Nutrition 2015;23(3):137-41. [Google Scholar]
Hampson 2003 {published and unpublished data}
- Hampson G, Martin FC, Moffat K, Vaja S, Sankaralingam S, Cheung J, et al. Effects of dietary improvement on bone metabolism in elderly underweight women with osteoporosis: a randomised controlled trial. Osteoporosis international 2003;14(9):750-6. [DOI] [PubMed] [Google Scholar]
Hernandez 2014 {published data only}
- Hernandez Morante JJ, Sanchez-Villazala A, Canavate-Cutillas R, Conesa-Fuentes MC. Effectiveness of a nutrition education program for the prevention and treatment of malnutrition in end-stage renal disease. Journal of Renal Nutrition 2014;24(1):42-9. [DOI] [PubMed] [Google Scholar]
Holyday 2012 {published data only}
- Holyday M, Daniells S, Bare M, Caplan GA, Petocz P, Bolin T. Malnutrition screening and early nutrition intervention in hospitalised patients in acute aged care: a randomised controlled trial. Journal of Nutritional Health and Aging 2012;16(6):562-8. [DOI] [PubMed] [Google Scholar]
Huynh 2015 {published data only}
- Huynh DT, Devitt AA, Paule CL, Reddy BR, Marathe P, Hegazi RA, et al. Effects of oral nutritional supplementation in the management of malnutrition in hospital and post-hospital discharged patients in India: a randomised, open-label, controlled trial. Journal of Human Nutrition and Dietetics 2015;28(4):331-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01641770. Oral nutritional supplementation in hospital patients. clinicaltrials.gov/show/NCT01641770 (date first posted 17 Jul 2012).
Imes 1988 {published and unpublished data}
- Imes S, Pinchbeck B, Dinwoodie A, Walker K, Thomson AB. Effect of Ensure, a defined formula diet, in patients with Crohn's disease. Digestion 1986;35(3):158-69. [DOI] [PubMed] [Google Scholar]
- Imes S, Pinchbeck B, Thompson AB. Diet counselling improves the clinical course of patients with Crohn's disease. Digestion 1988;39(1):7-19. [DOI] [PubMed] [Google Scholar]
- Imes S, Pinchbeck BR, Thomson AB. Diet counselling modifies nutrient intake of patients with Crohn's disease. Journal of the American Dietetic Association 1987;87(4):457-62. [PubMed] [Google Scholar]
Isenring 2004 {published and unpublished data}
- Isenring EA, Bauer JD, Capra S. Nutrition support using the American Dietetic Association medical nutrition therapy protocol for radiation oncology patients improves dietary intake compared with standard practice. Journal of American Dietetic Association 2007;107(3):404-12. [DOI] [PubMed] [Google Scholar]
- Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. British Journal of Cancer 2004;91(3):447-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jahnavi 2010 {published data only}
- Jahnavi G, Sudha CH. Randomised controlled trial of food supplements in patients with newly diagnosed tuberculosis and wasting. Singapore Medical Journal 2010;51(12):957-62. [PMID: ] [PubMed] [Google Scholar]
Jensen 1997 {published data only}
- Jensen MB, Hessov I. Dietary supplementation at home improves the regain of lean body mass after surgery. Nutrition 1997;13(5):422-30. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Hessov I. Randomization to nutritional intervention at home did not improve postoperative function, fatigue or well-being. British Journal of Surgery 1997;84(1):113-8. [PubMed] [Google Scholar]
Kalnins 2005 {published and unpublished data}
- Kalnins D, Corey M, Ellis L, Pencharz PB, Tullis E, Durie PR. Failure of conventional strategies to improve nutritional status in malnourished adolescents and adults with cystic fibrosis. Journal of Pediatrics 2005;147(3):399-401. [DOI] [PubMed] [Google Scholar]
- Kalnins D, Durie P. Oral supplements vs normal food intake in children and adults. Israel Journal of Medical Sciences 1996;32 Suppl:S120-1. [Google Scholar]
- Kalnins D, Durie PR, Corey M, Ellis L, Pencharz P, Tullis E. Are oral dietary supplements effective in the nutritional management of adolescents and adults with CF? Pediatric Pulmonology 1996;381 Suppl 13:314-5. [Google Scholar]
Kapoor 2017 {published data only}
- Kapoor N, Naufahu J, Tewfik S, Bhatnagar S, Garg R, Tewfik I. A prospective randomized controlled trial to study the impact of a nutrition-sensitive intervention on adult women with cancer cachexia undergoing palliative care in India. Integrative Cancer Therapies 2017;16(1):74-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02561143. Effectiveness of nutritional counselling and "Improved Atta" supplementation in cachexic adult Indian cancer patients. clinicaltrials.gov/show/NCT02561143 (date first posted 25 Sep 2015).
Kendell 1982 {published data only}
- Kendell BD, Fonseca RJ, Lee M. Postoperative nutritional supplementation for the orthognathic surgery patient. Journal of Oral and Maxillofacial Surgery 1982;40(4):205-13. [DOI] [PubMed] [Google Scholar]
Kiss 2016 {published data only}
- Kiss N, Isenring E, Gough K, Wheeler G, Wirth A, Campbell BA, et al. Early and intensive dietary counselling in lung cancer patients receiving (chemo)radiotherapy-a pilot randomized controlled trial. Nutrition and Cancer 2016;68(6):958-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kunvik 2018 {unpublished data only}
- Kunvik S, Valve R, Salonoja M, Suominen MH. Does tailored nutritional guidance increase protein intake among older caregivers? The CareNutrition trial (RCT). Journal of Aging Research & Lifestyle 2018;7:136-42. [Google Scholar]
Le Cornu 2000 {published data only}
- Le Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation 2000;69(7):1364-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Locher 2013 {published data only}
- Locher JL, Bales CW, Ellis AC, Lawrence JC, Newton L, Ritchie CS, et al. A theoretically based behavioral Nutrition Intervention for community elders at high risk: the B-NICE randomized controlled clinical trial. Journal of Nutrition in Gerontology and Geriatrics 2011;30(4):384-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher JL, Vickers KS, Buys DR, Ellis AC, Lawrence JC, Newton L, et al. A randomised controlled trial of a theoretically based behavioral nutrition intervention for community elders: lessons learned from the Behavioral Nutrition Intervention for Community Elders Study. Journal of the Academy of Nutrition and Dietetics 2013;113(12):1675-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01197768. A multi-component behavioral nutrition intervention for homebound elderly. clinicaltrials.gov/show/NCT01197768 (date first posted 09 Sep 2010).
Lovik 1996 {published data only}
- Lovik A, Almendingen K, Dotterud M, Forli L, Boysen M, Omarhus M, et al. Dietary information after radiotherapy of head and neck cancer [Kostveiledning etter stralebehandling for kreft i hode- og halsregionen]. Tidsskrift For Den Norske Laegeforening 1996;116(19):2303-6. [PubMed] [Google Scholar]
Macia 1991a {published data only}
- Macia E, Moran J, Santos J, Blanco M, Mahedero G, Salas J. Nutritional evaluation and dietetic care in cancer patients treated with radiotherapy: prospective study. Nutrition 1991;7(3):205-9. [PubMed] [Google Scholar]
Macia 1991b {published data only}
- Macia E, Moran J, Santos J, Blanco M, Mahedero G, Salas J. Nutritional evaluation and dietetic care in cancer patients treated with radiotherapy: prospective study. Nutrition 1991;7(3):205-9. [PubMed] [Google Scholar]
Macia 1991c {published data only}
- Macia E, Moran J, Santos J, Blanco M, Mahedero G, Salas J. Nutritional evaluation and dietetic care in cancer patients treated with radiotherapy: prospective study. Nutrition 1991;7(3):205-9. [PubMed] [Google Scholar]
Manguso 2005 {published data only}
- Manguso F, D'Ambra G, Menchise A, Sollazzo R, D'Agostino L. Effects of an appropriate oral diet on the nutritional status of patients with HCV-related liver cirrhosis: a prospective study. Clinical Nutrition 2005;24(5):751-9. [DOI] [PubMed] [Google Scholar]
McCarthy 1999 {published and unpublished data}
- McCarthy D, Weihofen D. The effect of nutritional supplements on food intake in patients undergoing radiotherapy. Oncology Nursing Forum 1999;26(5):897-900. [PubMed] [Google Scholar]
Moloney 1983 {published data only}
- Moloney M, Moriarty M, Daly L. Controlled studies of nutritional intake in patients with malignant disease undergoing treatment. Human Nutrition: Applied Nutrition 1983;37A:30-5. [PubMed] [Google Scholar]
Murphy 1992 {published data only}
- Murphy J, Cameron DW, Garber G, Conway B, Denomme N. Dietary counselling and nutritional supplementation in HIV infection. Journal of the Canadian Dietetic Association 1992;53(3):205-8. [Google Scholar]
Neelemaat 2011 {published data only}
- Neelemaat F, Bosmans JE, Thijs A, Seidell JC, Bokhorst-de van der Schueren MA. Oral nutritional support in malnourished elderly decreases functional limitations with no extra costs. Clinical Nutrition 2012;31(2):183-90. [DOI] [PubMed] [Google Scholar]
- Neelemaat F, Bosmans JE, Thijs A, Seidell JC, Bokhorst-de van der Schueren MA. Post-discharge nutritional support in malnourished elderly individuals improves functional limitations. Journal of the American Directors Association 2011;12:295-301. [DOI] [PubMed] [Google Scholar]
- Neelemaat F, Lips P, Bosmans J, Thijs A, Seidell J, Bokhorst-de van der Schueren M. Fall incidents decrease after short-term oral nutritional intervention in malnourished elderly patients: a randomized controlled trial. In: Clinical Nutrition. Vol. 6, Issue 1. 2011:32.
- Neelemaat F, Lips P, Bosmans JE, Thijs A, Seidell JC, Bokhorst-de van der Schueren MA. Short-term oral nutritional intervention with protein and vitamin D decreases falls in malnourished older adults. Journal of the American Geriatrics Society 2012;60(4):691-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Neelemaat F, Thijs A, Seidell JC, Bosmans JE, Bokhorst-de van der Schueren MA. Study protocol: cost-effectiveness of transmural nutritional support in malnourished elderly patients in comparison with usual care. Nutrition Journal 2010;9:6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelemaat F, Keeken S, Langius JA, Bokhorst-de van der Schueren MA, Thijs A, Bosmans JE. Survival in malnourished older patients receiving post-discharge nutritional support: long-term results of a randomized controlled trial. Journal of Nutrition Health and Aging 2017;21(8):855-60. [DOI] [PubMed] [Google Scholar]
- Neelemaat F, Keeken S, Languis J, Thijs A, van Bokhorst-de van der Schueren M, Bosmans JE. Survival of malnourished elderly patients receiving post-discharge nutritional support; A randomized controlled study. Clinical Nutrition 2014;33:S10. [Google Scholar]
Norman 2008b {published data only}
- NCT02470013. Medical and health economic benefit of oral nutritional supplements (ONS) in ambulant patients. clinicaltrials.gov/show/NCT02470013 (date first posted 12 Jun 2015).
- Norman K, Kirchner H, Freudenreich M, Ockenga J, Lochs H, Pirlich M. Three-month intervention with protein and energy rich supplements improved muscle function and quality of life in malnourished patients with none neoplastic gastrointestinal disease - a randomized controlled trial. Clinical Nutrition 2008;27(1):48-56. [DOI] [PubMed] [Google Scholar]
- Norman K, Pirlich M, Smoliner C, Kilbert A, Schulzke JD, Ockenga J, et al. Cost-effectiveness of a 3-month intervention with oral nutritional supplements in disease-related malnutrition: a randomised controlled pilot study. European Journal of Clinical Nutrition 2011;65(6):735-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Olejko 1984 {published data only}
- Olejko TD, Fonseca RJ. Preoperative nutritional supplementation for the orthognathic surgery patient. Journal of Oral and Maxillofacial Surgery 1984;42(9):573-7. [DOI] [PubMed] [Google Scholar]
Ollenschlager 1992 {published data only}
- Ollenschlager G, Thomas W, Konkol K, Diehl V, Roth E. Nutritional behaviour and quality of life during oncological polychemotherapy: results of a prospective study on the efficacy of oral nutrition therapy in patients with acute leukaemia. European Journal Of Clinical Investigation 1992;22(8):546-53. [DOI] [PubMed] [Google Scholar]
- Ollenschlager G et al. Supportive nutritional treatment during induction therapy of acute leukaemia: efficiency and influence on the quality of living. Klinische Wochenschrift 1990;68 (Suppl 19):320-1. [Google Scholar]
Ovesen 1993 {published data only}
- Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. Journal of Clinical Oncology 1993;11(10):2043-9. [DOI] [PubMed] [Google Scholar]
Parsons 2016 {published data only}
- Elia M, Parsons EL, Cawood AL, Smith TR, Stratton RJ. Cost-effectiveness of oral nutritional supplements in older malnourished care home residents. Clinical Nutrition 2018;37:651-658. [DOI] [PubMed] [Google Scholar]
- NCT00515125. Community oral nutrition support trial. clinicaltrials.gov/show/NCT00515125 (date first posted 13 Aug 2007).
- Parsons EL, Elia M, Cawood AL, Smith TR, Warwick H, Stratton RJ. Randomized controlled trial shows greater total nutritional intakes with liquid supplements than dietary advice in care home residents. In: Clinical Nutrition Supplements. Vol. 6. 2011:31, Abstract PP022.
- Parsons EL, Stratton RJ, A Cawood, Jackson M. Oral nutritional supplements are more cost effective than dietary advice in improving quality adjusted life years (QALYS) in malnourished care home residents. In: Clinical Nutrition Supplement. Vol. 7(1). 2012:44, abstract OC-039. [DOI] [PubMed]
- Parsons EL, Stratton RJ, Cawood AL, Smith TR, Elia M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clinical Nutrition (Edinburgh, Scotland) 2017;36(1):134-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Parsons EL, Stratton RJ, Cawood AL, Smith TR, Warwick H, Elia M. Randomized controlled trial in care home residents shows improved quality of life (QOL) with oral nutritional supplements. In: Clinical Nutrition Supplements. Vol. 6, Issue 1. 2011:31.
- Parsons EL, Stratton RJ, Jackson M, Elia M. Oral nutritional supplements are cost effective in improving quality adjusted life years in malnourished care home residents. In: Gut. Vol. 61, Issue Suppl 2. 2012:A17, abstract OC-039.
Paton 2004 {published and unpublished data}
- Paton NI, Chua YK, Earnest A, Chee CB. Randomised controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. American Journal of Clinical Nutrition 2004;80(2):460-5. [DOI] [PubMed] [Google Scholar]
Payette 2002 {published data only}
- Payette H, Boutier V, Coulombe C, Gray-Donald K. Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: A prospective randomised community trial. Journal of American Dietetic Association 2002;102(8):1088-95. [PubMed] [Google Scholar]
Pedersen 2016a {published data only}
- Lindegaard Pedersen J, Pedersen PU, Damsgaard EM. Nutritional follow-up after discharge prevents readmission to hospital - a randomized clinical trial. Journal of Nutrition, Health & Aging 2017;21(1):75-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lindegaard-Pedersen J, Pedersen PU, Damsgaard EM. Nutritional follow-up in malnourished geriatric patients after discharge: does the follow-up method influence the number of readmissions to hospital. In: European Geriatric Medicine. Vol. 7. 2016:S3.
- NCT01345032. The effect of nutrition follow up after hospital discharge in undernourished elderly. clinicaltrials.gov/show/NCT01345032 (date first posted 29 Apr 2011).
- Pedersen JL, Pedersen PU, Damsgaard EM. Early nutritional follow-up after discharge prevents deterioration of ADL functions in malnourished, independent, geriatric patients who live alone - a randomized clinical trial. Journal of Nutrition, Health & Aging 2016;20(8):845-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pedersen 2016b {published data only}
- Lindegaard Pedersen J, Pedersen PU, Damsgaard EM. Nutritional follow-up after discharge prevents readmission to hospital - a randomized clinical trial. Journal of Nutrition, Health & Aging 2017;21(1):75-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01345032. The effect of nutrition follow up after hospital discharge in undernourished elderly. clinicaltrials.gov/show/NCT01345032 (date first posted 29 Apr 2011).
- Pedersen JL, Pedersen PU, Damsgaard EM. Early nutritional follow-up after discharge prevents deterioration of ADL functions in malnourished, independent, geriatric patients who live alone - a randomized clinical trial. Journal of Nutrition, Health & Aging 2016;20(8):845-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Persson 2002 {published and unpublished data}
- Persson CR, Johansson BB, Sjoden PO, Glimelius BL. A randomized study of nutritional support in patients with colorectal and gastric cancer. Nutrition and Cancer 2002;42(1):48-58. [DOI] [PubMed] [Google Scholar]
Persson 2007 {published data only}
- Persson M, Hytter-Landahl A, Brismar K, Cederholm T. Nutritional supplementation and dietary advice in geriatric patients at risk of malnutrition. Clinical Nutrition 2007;26(2):216-24. [DOI] [PubMed] [Google Scholar]
Pivi 2011 {published data only}
- Pivi GA, da Silva RV, Juliano U, Novo NF, Okamoto IH, Brnat CQ, et al. A prospective study of nutrition education and oral nutritional supplementation in patients with Alzheimer's disease. Nutrition Journal 2011;10:98. [DOI: 10.1186/1475-2891-10-98] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabeneck 1998 {published data only}
- Rabeneck L, Palmer A, Knowles JB, Seidehamel RJ, Harris CL, Merkel KL, et al. A randomized controlled trial evaluating nutrition counselling with or without oral supplementation in malnourished HIV-infected patients. Journal of the American Dietetic Association 1998;98(4):434-8. [DOI] [PubMed] [Google Scholar]
Ravasco 2005a {published and unpublished data}
- Ravasco P, Monteiro-Grillo I, Camilo M. Individualised nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. American Journal of Clinical Nutrition 2012;96(6):1346-53. [DOI] [PubMed] [Google Scholar]
- Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME. Dietary counselling improves patient outcomes: a prospective, randomised, controlled trial in colorectal cancer patients undergoing radiotherapy. Journal of Clinical Oncology 2005;23(7):1431-8. [DOI] [PubMed] [Google Scholar]
Ravasco 2005b {published data only}
- Ravasco P, Monteiro Grillo I, Marques Vidal P, Camilo M. Nutritional counselling vs commercial supplements vs ad lib: a prospective randomised controlled trial in head-neck cancer patients undergoing radiotherapy. In: European Journal of Cancer. Vol. 1, issue 5. 2003:S272.
- Ravasco P, Monteiro Grillo I, Marques Vidal P, Camilo M. Nutritional counselling vs supplements: a prospective randomised controlled trial in head-neck cancer patients undergoing radiotherapy. In: Clinical Nutrition. Vol. 22 (Suppl 1). 2003:S63-S63.
- Ravasco P, Monteiro-Grillo I, Vidal PM, Camillo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head and Neck 2005;27(8):659-68. [DOI] [PubMed] [Google Scholar]
Rogers 1992 {published data only}
- Rogers RM, Donahoe M, Costantino J. Physiologic effects of oral supplemental feeding in malnourished patients with chronic obstructive pulmonary disease. A randomized control study. American Review of Respiratory Disease 1992;146(6):1511-7. [DOI] [PubMed] [Google Scholar]
Rydwik 2008 {published data only}
- Rydwik E, Lammes E, Frandin K, Ackner G. Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomised controlled pilot treatment trial. Aging Clinical and Experimental Research 2008;20(2):159-70. [DOI] [PubMed] [Google Scholar]
Salva 2011 {published data only}
- Salva A, Andrieu S, Fernandez E, Schiffrin EJ, Moulin J, Decarli B, et al. Health and nutritional promotion program for patients with dementia (NutriAlz Study): design and baseline data. Journal of Nutrition, Health & Aging 2009;13(6):522-7. [DOI] [PubMed] [Google Scholar]
- Salva A, Andrieu S, Fernandez E, Schiffrin EJ, Moulin J, Decarli B, et al. Health and nutrition promotion program for patients with dementia (Nutrialz): cluster randomised trial. Journal of Nutrition Health & Aging 2011;15(10):822-30. [DOI] [PubMed] [Google Scholar]
Schilp 2013 {published data only}
- Schilp J, Bosmans JE, Kruizenga HM, Wijnhoven HA, Visser M. Is dietetic treatment for undernutrition in older individuals in primary care cost-effective? Journal of the American Medical Directors Association 2014;15(3):226.e7-226.e13. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schilp J, Kruizenga HM, Wijnhoven HA, Binsbergen JJ, Visser M. Effects of a dietetic treatment in older, undernourished,community-dwelling individuals in primary care: a randomized controlled trial. European Journal of Nutrition 2013;52(8):1939-48. [DOI: 10.1007/s00394-013-0495-9] [DOI] [PubMed] [Google Scholar]
Schwenk 1999 {published and unpublished data}
- Schwenk A, Steuck H, Kremer G. Oral supplements as adjunctive treatment to nutritional counseling in malnourished HIV-infected patients: randomized controlled trial. Clinical Nutrition 1999;18(6):371-4. [DOI] [PubMed] [Google Scholar]
Sharma 2002a {published data only}
- Sharma M, Rao M, Jacob S, Jacob CK. A controlled trial of intermittent enteral nutrient supplementation in maintenance haemodialysis patients. Journal of Renal Nutrition 2002;12(4):229-37. [DOI] [PubMed] [Google Scholar]
Sharma 2002b {published data only}
- Sharma M, Rao M, Jacob S, Jacob CK. A controlled trial of intermittent enteral nutrient supplementation in maintenance haemodialysis patients. Journal of Renal Nutrition 2002;12(4):229-37. [DOI] [PubMed] [Google Scholar]
Sharma 2017 {published data only}
- Sharma Y, Thompson C, Kaambwa B, Shahi R, Hakendorf P, Miller M. Investigation of the benefits of early malnutrition screening with telehealth follow up in elderly acute medical admissions. QJM: monthly journal of the Association of Physicians 2017;110(10):639-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sharma Y, Thompson C, Miller M, Shahi R, Hakendorf P, Horwood C, Kaambwa B. Economic evaluation of an extended nutritional intervention in older Australian hospitalized patients: a randomized controlled trial. BMC Geriatrics 2018;18(41):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silvers 2014 {published data only}
- Furness K, Silvers MA, Savva J, Huggins CE, Truby H, Haines T. Long-term follow-up of the potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: a pilot randomised trial. Supportive Care in Cancer 2017;25(11):3587-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Silvers MA, Savva J, Huggins CE, Truby H, Haines T. Potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: a pilot randomised trial. Supportive Care in Cancer 2014;22(11):3035-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Singh 2008 {published data only}
- Singh S, Midha S, Singh N, Joshi YK, Garg PK. Dietary counselling versus dietary supplements for malnutrition in chronic pancreatitis: a randomised controlled trial. Clinical Gastroenterology and Hepatology 2008;6(3):353-9. [DOI] [PubMed] [Google Scholar]
Starke 2011 {published data only}
- Starke J, Schneider H, Alteheld B, Stehle P, Meier R. Short-term individual nutritional care as part of routine clinical setting improves outcome and quality of life in malnourished medical patients. Clinical Nutrition 2011;30(2):194-201. [DOI] [PubMed] [Google Scholar]
Stow 2015 {published data only}
- Stow R, Ives N, Smith C, Rick C, Rushton A. A cluster randomised feasibility trial evaluating nutritional interventions in the treatment of malnutrition in care home adult residents. Trials 2015;16:433:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow R, Rushton A, Ives N, Smith C, Rick C. A cluster randomised feasibility trial evaluating six-month nutritional interventions in the treatment of malnutrition in care home-dwelling adults: recruitment, data collection and protocol. Pilot and Feasibility Studies 2015;1(3):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow RE, Smith CH, Rushton AB. Care home resident and staff perceptions of the acceptability of nutrition intervention trial procedures: a qualitative study embedded within a cluster randomised feasibility trial. BMJ Open 2018;8(7):e022307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suominen 2015 {published data only}
- Jyvakorpi SK, Puranen T, Pitkala KH, Suominen MH. Nutritional treatment of aged individuals with Alzheimer disease living at home with their spouses: study protocol for a randomized controlled trial. Trials 2012;13:66. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranen TM, Jyvakorpi SK, Pitkala KG, Eloniemi-Sulkava U, Raivio MM, Suominen MH. Nutritional intervention of patients with Alzheimer's disease living at home with their spouse: a randomized controlled trail. Baseline findings and feasibility. Journal of Aging Research & Clinical Practice 2013;2(2):236-41. [Google Scholar]
- Puranen TM, Pietila SE, Pitkala KH, Kautiainen H, Raivio M, Eloniemi-Sulkava U, et al. Caregivers' male gender is associated with poor nutrient intake in AD families (NuAD-trial). Journal of Nutrition, Health & Aging 2014;18(7):672-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Puranen TM, Pitkala KH, Suominen MH. Tailored nutritional guidance for home-dwelling AD families: the feasibility of and elements promoting positive changes in diet (NuAD-Trial). Journal of Nutrition, Health & Aging 2015;19(4):454-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Suominen MH, Puranen TM, Jyvakorpi SK, Eloniemi-Sulkava U, Kautiainen H, Siljamaki-Ojansuu U, et al. Nutritional guidance improves nutrient intake and quality of life, and may prevent falls in aged persons with Alzheimer disease living with a spouse (NuAD Trial). Journal of Nutrition, Health & Aging 2015;19(9):901-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Terp 2018 {published data only}
- NCT03131856. Nutritional intervention in geriatric patients. clinicaltrials.gov/show/NCT03131856 (date first posted 27 Apr 2017).
- Terp R, Jacobsen KO, Kannegaard P, Larsen AM, Madsen OR, Noiesen E. A nutritional intervention program improves the nutritional status of geriatric patients at nutritional risk-a randomized controlled trial. Clinical rehabilitation 2018;32(7):930-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tu 2013 {published data only}
- Tu MY, Chien TW, Lin HP, Liu MY. Effects of an intervention on nutrition consultation for cancer patients. European Journal of Cancer Care 2013;22(3):370-6. [DOI] [PubMed] [Google Scholar]
Um 2014 {published data only}
- UM MH, Choi MY, Lee SM, Lee IJ, Lee CG, Park YK. Intensive nutritional counseling improves PG-SGA scores and nutritional symptoms during and after radiotherapy in Korean cancer patients. Supportive Care in Cancer 2014;22(11):2997-3005. [DOI] [PubMed] [Google Scholar]
Uster 2013 {published data only}
- Uster A, Ruefenacht U, Ruehlin M, Pless M, Siano M, Haefner M, et al. Influence of a nutritional intervention on dietary intake and quality of life in cancer patients: a randomized controlled trial. Nutrition 2013;29(11-2):1342-9. [DOI: 10.1016/j.nut.2013.05.004] [DOI] [PubMed] [Google Scholar]
Vivanti 2015 {published data only}
- Vivanti A, Isenring E, Baumann S, Powrie D, O'Neill M, Clark D, et al. Emergency department malnutrition screening and support model improves outcomes in a pilot randomised controlled trial. Emergency medicine journal : EMJ 2015;32(3):180-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weekes 2009 {published and unpublished data}
- Weekes CE, Emery PW, Elia M. Dietary counselling and food fortification in stable COPD: a randomised controlled trial. Thorax 2009;64(4):326-31. [DOI] [PubMed] [Google Scholar]
Wilson 2001 {published data only}
- Wilson B, Fernandez-Madrid A, Hayes A, Hermann K, Smith J, Wassell A. Comparison of the effects of two early intervention strategies on the health outcomes of malnourished hemodialysis patients. Journal of Renal Nutrition 2001;11(9):166-71. [DOI] [PubMed] [Google Scholar]
Wong 2004 {published and unpublished data}
- Wong SY, Lau EM, Lau WW, Lynn HS. Is dietary counselling effective in increasing dietary calcium, protein and energy intakes in patients with osteoporotic fractures? A randomised controlled clinical trial. Journal of Human Nutrition & Dietietics 2004;17(4):359-64. [DOI] [PubMed] [Google Scholar]
Wyers 2013 {published data only}
- NCT00523575. Nutritional intervention in hip fracture patients. clinicaltrials.gov/show/NCT00523575 (date first posted 31 Aug 2007).
- Wyers CE, Breedveld-Peters JJ, Reijven PL, Helden S, Guldemond NA, Severens JL, et al. Efficacy and cost-effectiveness of nutritional intervention in elderly after hip fracture: design of a randomized controlled trial. BMC Public Health 2010;10:212. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers CE, Reijven PL, Breedveld-Peters JJ, Denissen KF, Schotanus MG, Dongen MC, et al. Efficacy of nutritional intervention in elderly after hip fracture: a multicenter randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2018;73(10):1429-37. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers CE, Reijven PL, Evers SM, Willems PC, Heyligers IC, Verburg AD, et al. Cost-effectiveness of nutritional intervention in elderly subjects after hip fracture. A randomized controlled trial. Osteoporosis International 2013;24(1):151-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12613000518763 {published data only}
- ACTRN12613000518763. Malnutrition in the Australian rural rehabilitation community: a prospective observational study observing usual care in older adults admitted for rehabilitation. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364079 2013.
Ahnfeldt‐Mollerup 2015 {published data only}
- Ahnfeldt-Mollerup P, Hey H, Johansen C, Kristensen S, Brix Lindskov J, Jensahnfeldt-Mollerupen C. The effect of protein supplementation on quality of life, physical function, and muscle strength in patients with chronic obstructive pulmonary disease. European Journal of Physical and Rehabilitation Medicine 2015;51(4):447-56. [PMID: ] [PubMed] [Google Scholar]
Aleman‐Mateo 2014 {published data only}
- Aleman-Mateo H, Carreon VR, Macias L, Astiazaran-Garcia H, Gallegos-Aguilar AC, Enriquez JR. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: a single-blind randomized clinical trial. Clinical Interventions in Aging 2014;9:1517-25. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amlogu 2016 {published data only}
- Amlogu AM, Tewfik S, Wambebe C, Tewfik I. A compartive study: long and short term effect of a nutrition sensitive approach to delay the progression of HIV to AIDS among people living with HIV (PLWH) in Nigeria. Functional Foods in Health and Disease 2016;6(2):79-90. [Google Scholar]
Antila 1993 {published data only}
- Antila H, Salo M, Nanto V, Forssell D, Salonen M, Kirvela O. The effect of intermaxillary fixation on leucocyte zinc, serum trace elements and nutritional status of patients undergoing maxillofacial surgery. Clinical Nutrition 1993;12(4):223-9. [DOI] [PubMed] [Google Scholar]
Apovian 2017 {published data only}
- Apovian CM, Singer MR, Campbell WW, Bhasin S, McCarthy AC, Shah M, et al. Development of a novel six-month nutrition intervention for a randomized trial in older men with mobility limitations. Journal of Nutrition, Health & Aging 2017;21(10):1081-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arija 2012 {published data only}
- Arija V, Martin N, Canela T, Anguera C, Castelao AI, Garcia-Barco M, et al. Nutrition education intervention for dependent patients: protocol of a randomized controlled trial. BMC Public Health 2012;12:373. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnarson 2013 {published data only}
- Arnarson A, Gudny Geirsdottir O, Ramel A, Briem K, Jonsson PV, Thorsdottir I. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: double blind, randomised controlled trial. European Journal of Clinical Nutrition 2013;67(8):821-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arutiunov 2009 {published data only}
- Arutiunov GP, Kostiukevich OI, Bylova NA. Prevalence and clinical significance of malnutrition and effectiveness of nutritional support for patients suffering from chronic heart failure. Eksperimental' Naia i Klinicheskaia Gastroenterologiia 2009;2:22-33. [PubMed] [Google Scholar]
Bachmann 1998 {published data only}
- Bachmann P, Gordiani B, Ranchere JY, Lallemand Y, Combret D, Latour JF, et al. Assessment of indication and quality of nutritional support in medical oncology. Nutrition Clinique et Metabolisme 1998;12(1):3-12. [Google Scholar]
Badia 2015 {published data only}
- Badia T, Formiga F, Ferrer A, Sanz H, Hurtos L, Pujol R. Multifactorial assessment and targeted intervention in nutritional status among the older adults: a randomized controlled trial: the Octabaix study. BMC Geriatrics 2015;15:45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baradzina 2013 {published data only}
- Baradzina HL. Shot and long-term effects of pulmonary rehabilitation program in sarcoidosis. European Respiratory Journal 2013;42:3788. [Google Scholar]
Bauer 1994 {published data only}
- Bauer S, Jakob E, Berg A, Keul J. Energy and nutrient intake in adolescent weight-lifters before and after nutritional counselling. Schweizerische Zeitschrift fur Medizin und Traumatologie 1994;3:35-42. [PubMed] [Google Scholar]
Beange 1995 {published data only}
- Beange H, Gale L, Stewart L. Project re nourish: a dietary intervention to improve nutritional status in people with multiple disabilities. Australian and New Zealand Journal of Developmental Disability 1995;20(3):165-74. [Google Scholar]
Beck 2008 {published data only}
- Beck AM, Damkjaer K, Beyer N. Multifacted nutritional intervention among nursing-home residents has a positive influence on nutrition and function. Nutrition 2008;24(11-12):1073-80. [DOI] [PubMed] [Google Scholar]
Beck 2016 {published data only}
- Beck AM, Christensen AG, Hansen BS, Damsbo-Svendsen S, Moller TK. Multidisciplinary nutritional support for undernutrition in nursing home and home-care: a cluster randomized controlled trial. Nutrition (Burbank, Los Angeles County, Calif.) 2016;32(2):199-205. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Beck AM, Gogsig Christensen A, Stenbaek Hansen B, Damsbo-Svendsen S, Kreinfeldt Skovgaard Moller T, Boll Hansen E, et al. Study protocol: cost effectiveness of multidisciplinary nutritional support for undernutrition in older adults in nursing home and home-care: cluster randomized controlled trial. Nutrition Journal 2014;13:86. [DOI: 10.1186/1475-2891-13-86] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck Docent AM, Christensen AG, Hansen BS, Damsbo-Svendsen S, Møller TK. Author response "Rehabilitation nutrition for undernourished participants in nursing home and home care: cluster randomized controlled study". Nutrition 2016;32(4):503-4. [DOI] [PubMed] [Google Scholar]
Beddhu 2015 {published data only}
- Beddhu S, Filipowicz R, Chen X, Neilson JL, Wei G, Huang Y, et al. Supervised oral protein supplementation during dialysis in patients with elevated C-reactive protein levels: a two phase, longitudinal, single center, open labeled study. BMC Nephrology 2015;16:87. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beelen 2017a {published data only}
- Beelen J, Roos NM, Groot LC. A 12-week intervention with protein-enriched foods and drinks improved protein intake but not physical performance of older patients during the first 6 months after hospital release: a randomised controlled trial. British Journal of Nutrition 2017;117(11):1541-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beelen 2017b {published data only}
- Beelen J, Roos NM, Groot LC. Protein enrichment of familiar foods as an innovative strategy to increase protein intake in institutionalized elderly. Journal of Nutrition, Health & Aging 2017;21(2):173-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bello 2019 {published data only}
- Bello TK, Gericke GJ, MacIntyre UE, Becker P. A nutrition education programme improves quality of life but not anthropometric status of adults living with HIV in Abeokuta, Nigeria. Public Health Nutrition 2019;22(12):2290-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzekri 2019 {published data only}
- Benzekri NA, Sambou JF, Tamba IT, Diatta JP, Sall I, Cisse O, et al. Nutrition support for HIV-TB co-infected adults in Senegal, West Africa: a randomized pilot implementation study. PLoS ONE 2019;14(7):e0219118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernoth 2014 {published data only}
- Bernoth MA, Dietsch E, Davies C. 'Two dead frankfurts and a blob of sauce': the serendipity of receiving nutrition and hydration in Australian residential aged care. Collegian (Royal College of Nursing, Australia) 2014;21(3):171-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhattacharjee 2015 {published data only}
- Bhattacharjee A, Bahar I, Saikia A. Nutritional assessment of patients with head and neck cancer in north-east India and dietary intervention. Indian Journal of Palliative Care 2015;21(3):289-95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bills 1993 {published data only}
- Bills DJ, Spangler AA. Impact of dietitians on quality of care in 16 nursing homes in east central Indiana. Journal Of The American Dietetic Association 1993;93(1):73-5. [DOI] [PubMed] [Google Scholar]
Bolton 1990 {published data only}
- Bolton J, Shannon L, Smith V, Abbott R, Bell SJ, Stubbs L, et al. Comparison of short-term and long-term palatability of six commercially available oral supplements. Journal of Human Nutrition and Dietetics 1990;3(5):317-21. [Google Scholar]
Bories 1994 {published data only}
- Bories PN, Campillo B. One-month regular oral nutrition in alcoholic cirrhotic patients. Changes of nutritional status, hepatic function and serum lipid pattern. British Journal of Nutrition 1994;72(6):937-46. [DOI] [PubMed] [Google Scholar]
Botella‐Carretero 2008 {published data only}
- Botella-Carretero JI, Iglesias B, Balsa JA, Zamarron I, Arrieta F, Vazquez C. Effects of oral nutritional supplements in normally nourished or mildly undernourished geriatric patients after surgery for hip fracture: a randomised clinical trial. Journal of Parenteral and Enteral Nutrition 2008;32(2):120-8. [DOI] [PubMed] [Google Scholar]
Botella‐Carretero 2010 {published data only}
- Botella-Carretero JI, Iglkesias B, Balsa JA, Arrieta F, Zamarron I, Vazquez C. Perioperative oral nutritional supplements in normally or mildly undernourished geriatric patients submitted to surgery for hip fracture: a randomised clinical trial. Clinical Nutrition 2010;29(5):574-9. [DOI] [PubMed] [Google Scholar]
Boulos 2016 {published data only}
- Boulos C, Salameh P, Barberger-Gateau P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clinical Nutrition 2016;35(1):138-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bozzetti 1998 {published data only}
- Bozzetti F. Comments on: Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies, [comment on: Andreyev et al., European Journal of Cancer 1998, 34, pp. 503-9] (multiple letters). European Journal of Cancer 1998;34(13):2132-3. [DOI] [PubMed] [Google Scholar]
Braunschweig 2015 {published data only}
- Braunschweig CA, Sheean PM, Peterson SJ, Gomez Perez S, Freels S, Lateef O, et al. Intensive nutrition in acute lung injury: a clinical trial (INTACT). Journal of Parenteral and Enteral Nutrition 2015;39(1):13-20. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bugge 1997 {published data only}
- Bugge KE, Tschudi MC. Developing a research-based innovation protocol on nutritional support to patients with cancer in the gastrointestinal tract. Vard I Norden Nursing Science and Research in the Nordic Countries 1997;17:24-7. [Google Scholar]
Bunout 1989 {published data only}
- Bunout D, Aicardi V, Hirsch S, Petermann M, Kelly M, Silva G, et al. Nutritional support in hospitalized patients with alcoholic liver disease. European Journal of Clinical Nutrition 1989;43(9):615-21. [PubMed] [Google Scholar]
Burger 1993 {published data only}
- Burger B, Ollenschlager G, Schrappe M, Stute A, Fischer M, Wessel D, et al. Nutrition behavior of malnourished HIV-infected patients and intensified oral nutritional intervention. Nutrition 1993;9(1):43-4. [PubMed] [Google Scholar]
Buys 2017 {published data only}
- Buys DR, Campbell AD, Godfryd A, Flood K, Kitchin E, Kilgore ML, et al. Meals enhancing nutrition after discharge: findings from a pilot randomised controlled trial. Journal of the Academy of Nutrition and Dietetics 2017;117(4):599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caccialanza 2006 {published data only}
- Caccialanza R, Palladini G, Klersy C, Cena H, Vagia C, Cameletti B, et al. Nutritional status of outpatients with systemic immunoglobulin light-chain amyloidosis. American Journal of Clinical Nutrition 2006;83(2):350-4. [DOI] [PubMed] [Google Scholar]
Caetano 2017 {published data only}
- Caetano C, Valente A, Silva FJ, Antunes, J, Garagaza C. Effect of an intradialytic proein-rich meal intake in nutritional and body composition parameters on hemodialysis patients. Clinical Nutrition ESPEN 2017;20:29-33. [DOI] [PubMed] [Google Scholar]
- Garagaza C, Silva J, Valente A, Antunes J, Caetano C. Effect of a protein-rich meal intake during haemodialysis treatment. Nephrology Dialysis Transplantation 2016;31 Suppl 1:298-305. [ABSTRACT NO: SP612] [Google Scholar]
Capozzi 2012 {published data only}
- Capozzi LC, Lau H, Reimer RA, McNeely M, Giese-Davis J, Culos-Reed SN. Exercise and nutrition for head and neck cancer patients: a patient orientated, clinic supported randomized controlled trial. BMC Cancer 2012;12:446. [DOI: 10.1186/1471-2407-12-446.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlos Candido 2016 {published data only}
- Carlos Candido AP, Soares Torres Melo A. Evaluation of the nutritional aspects of health conditions and the sociodemographic profile of elderly people attending the elderly health department [Avaliacao dos aspectos nutricionais das condicoes de saude e do perfil sociodemografico de idosos atendidos no departamento de saude do idoso]. Revista de Atencao Primaria a Saude 2016;19(4):533-45. [Google Scholar]
Carlsson 2005 {published data only}
- Carlsson P, Tidermark J, Ponzer S, Soderqvist A, Cederholm T. Food habits and appetite of elderly women at the time of femoral neck fracture and after nutritional and anabolic support. Journal of Human Nutrition and Dietetics 2005;18(2):117-20. [DOI] [PubMed] [Google Scholar]
Cereda 2018 {published data only}
- Cereda E, Cappello S, Colombo S, Klersy C, Imarisio I, Turri A, et al. Counseling with or without systematic use of oral supplements in head-neck cancer patients undergoing radiotherapy. Nutrition 2018;50:e1. [DOI] [PubMed] [Google Scholar]
- Cereda E, Cappello S, Colombo S, Klersy C, Imarisio I, Turri A, et al. Nutritional counselling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiotherapy and Oncology 2018;126(1):81-8. [DOI: 10.1016/j.radonc.2017.10.015] [DOI] [PubMed] [Google Scholar]
- NCT02055833. Intensive nutritional counseling in head-neck cancer patients undergoing radiotherapy. clinicaltrials.gov/show/NCT02055833 (date first posted 05 Feb 2014).
Charlton 2012 {published data only}
- Charlton K, Nichols C, Bowden S, Milosavljevic M, Lambert K, Barone L, et al. Poor nutritional status of older subacute patients predicts clinical outcomes and mortality at 18 months of follow-up. European Journal of Clinical Nutrition 2012;66(11):1224-8. [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen SH, Huang YP, Shao JH. Effects of a dietary self-management programme for community-dwelling older adults: a quasi-experimental design. Scandinavian Journal of Caring Sciences 2017;31(3):619-29. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chew 2021 {published data only}
- Chew ST, Tan NC, Cheong M, Oliver J, Baggs G, Choe Y, et al. Impact of specialized oral nutritional supplement on clinical, nutritional, and functional outcomes: a randomized, placebo-controlled trial in community-dwelling older adults at risk of malnutrition. Clinical Nutrition 2021;40(4):1879-92. [DOI] [PubMed] [Google Scholar]
ChiCTR1900020807 {unpublished data only}
- ChiCTR1900020807. Randomized controlled trial for early ONS treatment vs standard nutrition treatment in patients with nasopharyngeal carcinoma undergoing concurrent chemoradiotherapy. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900020807 2019.
ChiCTR1900021167 {published data only}
- ChiCTR1900021167. Clinical application of Yukunlun nutritious formula food in improving the nutritional status of patients with COPD. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900021167 2019.
ChiCTR‐INR‐17012826 {published data only}
- ChiCTR-INR-17012826. Study of the effect of HMB on sarcopenia in the community-dwelling elderly. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-INR-17012826 2017.
Choi 2013 {published data only}
- Choi OJ, Cho YG, Kang JH, Park HA, Kim KW, Hur YI, et al. Weight control attempts in underweight Korean adults: Korea national health and nutrition examination survey, 2007-2010. Korean Journal of Family Medicine 2013;34(6):393-402. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Della Valle 2018 {published data only}
- Della Valle S, Colatruglio S, La Vela V, Tagliabue E, Mariani L, Gavazzi C. Nutritional intervention in head and neck cancer patients during chemo-radiotherapy. Nutrition 2018;51-2:95-7. [DOI] [PubMed] [Google Scholar]
DeLuis 2010 {published data only}
- De Luis DA, Bachiller P, Palacios T, Izaola O, Eiros Bouza JM, Aller R. Nutritional treatment for ambulatory patients with acquired immunodeficiency virus infection and previous weight loss using a formula enriched with n3 fatty acids. a randomized prospective trial. European Review for Medical and Pharmacological Sciences 2010;14(5):449-54. [PubMed] [Google Scholar]
Demeny 2015 {published data only}
- Demeny D, Jukic K, Dawson B, O’Leary F. Current practices of dietitians in the assessment and management of malnutrition in elderly patients. Nutrition & Dietetics 2015;72(3):254–60. [Google Scholar]
Deutz 2016 {published data only}
- Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clinical Nutrition 2016;35(1):18-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
De Waele 2015 {published data only}
- De Waele E, Mattens S, Honore PM, Spapen H, De Greve J, Pen JJ. Nutrition therapy in cachectic cancer patients. The Tight Caloric Control (TiCaCo) pilot trial. Appetite 2015;91:298-301. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ding 2016 {published data only}
- Ding J, Cai X, Chen M. The effect of a comprehensive intervention on the quality of life of oseophageal cancer patients treated with radiotherapy. Chinese Journal of Clinical Nutrition 2016;24(4):230-5. [Google Scholar]
Dizon 2016 {published data only}
- Dizon F, Wang L, Parsons T, Gray V, Reiboldt W. Impact of modified dietary education combined with high energy/protein meals during hemodialysis treatment on protein and phosphorus intake and nutritional status in hypoalbuminemic hemodialysis patients. FASEB Journal 2016;30(1 Supplement):1159.5-1159.5. [ABSTRACT NO.: 1159.5] [DOI: 10.1096/fasebj.30.1_supplement.1159.5] [DOI] [Google Scholar]
Dorner 2013 {published data only}
- Dorner TE, Lackinger C, Haider S, Luger E, Kapan A, Luger M, et al. Nutritional intervention and physical training in malnourished frail community-dwelling elderly persons carried out by trained lay "buddies": study protocol of a randomized controlled trial. BMC Public Health 2013;13:1232. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00016661 {unpublished data only}
- DRKS00016661. The influence of oral supplement nutrition for geriatric patients with fractures of the proximal femur and malnutrition. http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00016661.
Duncan 2006 {published data only}
- Duncan DG, Beck SJ, Hood K, Johansen A. Using dietetic assistants to improve the outcome of hip fracture: a randomised controlled trial of nutritional support in an acute trauma ward. Age and Ageing 2006;35(2):148-53. [DOI] [PubMed] [Google Scholar]
Dupuis 2017 {published data only}
- Dupuis M, Kuczewski E, Villeneuve L, Bin-Dorel S, Haine M, Falandry C, et al. Age Nutrition Chirugie (ANC) study: impact of a geriatric intervention on the screening and management of undernutrition in elderly patients operated on for colon cancer, a stepped wedge controlled trial. BMC Geriatrics 2017;17(1):10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Efthimiou 1988 {published data only}
- Efthimiou J, Fleming J, Gomes C, Spiro SG. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1988;137(5):1075-82. [DOI] [PubMed] [Google Scholar]
Ekramzadeh 2015 {published data only}
- Ekramzadeh M, Jafari P, Ayatollahi M, Saghev M. Anti-inflammatory diet alleviates malnutrition, inflammation and oxidative stress in hemodialysis patients: a randomized trial. Annals of Nutrition and Metabolism 2015;67(suppl 1):371-2. [Google Scholar]
Elbanna 1996 {published data only}
- Elbanna HM, Tolba KG, Darwish OA. Dietary management of surgical patients: effects on incisional wound healing. Eastern Mediterranean Health Journal 1996;2:243-54. [Google Scholar]
Elkort 1981 {published data only}
- Elkort RJ, Baker FL, Vitale JJ, Cordano A. Long-term nutritional support as an adjunct to chemotherapy for breast cancer. Journal of Parenteral and Enteral Nutrition 1981;5(5):385-90. [DOI] [PubMed] [Google Scholar]
Elmstaahl 1987 {published data only}
- Elmstahl S, Steen B. Hospital nutrition in geriatric long-term care medicine: II. Effects of dietary supplements. Age and Ageing 1987;16(2):73-80. [DOI] [PubMed] [Google Scholar]
Eneroth 1997 {published data only}
- Eneroth M, Apelqvist J, Larsson J, Persson BM. Improved wound healing in transtibial amputees receiving supplementary nutrition. International Orthopaedics 1997;21(2):104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engel 1995 {published data only}
- Engel B, Kon P, Raftery MJ. Strategies to identify and correct malnutrition in hemodialysis patients. Journal of Renal Nutrition 1995;5:62-6. [Google Scholar]
Evans 2013 {published data only}
- Evans D, McNamara L, Maskew M, Selibas K, Amsterdam D, Baines N, et al. Impact of nutritional supplementation on immune response, body mass index and bioelectrical impedance in HIV-positive patients starting antiretroviral therapy. Nutrition Journal 2013;12:111. [DOI: 10.1186/1475-2891-12-111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faber 2015 {published data only}
- Faber J, Uitdehaag MJ, Spaander M, Steenbergen-Langeveld S, Vos P, Berkhout M, et al. Improved body weight and performance status and reduced serum PGE2 levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro-esophageal junction. Journal of Cachexia, Sarcopenia and Muscle 2015;6(1):32-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faccio 2021 {published data only}
- Faccio AA, Peinado de Sampaio Mattos CH, Dos Santos EA, Meto NR, Moreira RP, Batella LT, et al. Oral nutritional supplementation in cancer patients who were receiving chemo/chemoradiation therapy: a multicenter, randomized phase II study. Nutrition Cancer 2021;73(3):442-9. [DOI] [PubMed] [Google Scholar]
Fietkau 2013 {published data only}
- Fietkau R, Lewitzki V, Kuhnt T, Holscher T, Hess CF, Berger B, et al. A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: results of a randomized controlled multicenter trial. Cancer 2013;119(18):3343-53. [DOI] [PubMed] [Google Scholar]
Flynn 1987 {published data only}
- Flynn MB, Leightty FF. Preoperative outpatient nutritional support of patients with squamous cancer of the upper aerodigestive tract. American Journal of Surgery 1987;154(4):359-62. [DOI] [PubMed] [Google Scholar]
Forli 2006 {published data only}
- Forli L, Moum T, Bjortuft O, Vatn M, Boe J. The influence of underweight and dietary support on well-being in lung transplant candidates. Respiratory Medicine 2006;100(7):1239-46. [DOI] [PubMed] [Google Scholar]
Franzoni 1996 {published data only}
- Franzoni S, Frisoni GB, Boffelli S, Rozzini R, Trabucchi M. Good nutritional oral intake is associated with equal survival in demented and non-demented very old patients. Journal of the American Geriatrics Society 1996;44(11):1366-70. [DOI] [PubMed] [Google Scholar]
Gil Gregorio 2003 {published data only}
- Gil Gregorio P, Ramirez Diaz SP, Ribera Casado JM. Dementia and Nutrition. Intervention study in institutionalized patients with Alzheimers Disease. Journal of Nutrition, Health & Aging 2003;7(5):304-8. [PubMed] [Google Scholar]
Glimelius 1992 {published data only}
- Glimelius B, Birgegard G, Hoffman K, Hagnebo C, Hogman G, Kvale G, et al. Improved care of patients with small cell lung cancer. Nutritional and quality of life aspects. Acta Oncologica 1992;31(8):823-31. [DOI] [PubMed] [Google Scholar]
Gomez Sanchez 2010 {published data only}
- Gomez Sanchez MB, Garcia-Talavera Espin NV, Sanchez Alvarez C, Zomeno Ros AI, Hernandez MN, Gomez Ramos MJ, et al. Perioperative nutritional support in patients with colorectal neoplasms. Nutricion Hospitalaria 2010;25(5):797-805. [PubMed] [Google Scholar]
Gurgun 2013 {published data only}
- Gurgun A, Deniz S, Argin M, Karapolat H. Effects of nutritional supplementation combined with conventional pulmonary rehabilitation in muscle-wasted chronic obstructive pulmonary disease: a prospective, randomized and controlled study. Respirology 2013;18(3):495-500. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hamirudin 2017 {published data only}
- Hamirudin AH, Walton K, Charlton K, Carrie A, Tapsell L, Milosavljevic M, et al. Feasibility of home-based dietetic intervention to improve the nutritional status of older adults post-hospital discharge. Nutrition & Dietetics 2017;74(3):217-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hammersley 2015 {published data only}
- Hammerley ML, Cann VR, Parrish AM, Jones RA, Holloway DA. Evaluation of the effects of a telephone-delivered health behaviour change program on weight and physical activity. Nutrition & Dietetics 2015;72(4):356-62. [Google Scholar]
Hansra 2017 {published data only}
- Hansra D, Norman-Tiner C, Diaz C, Randolph C, Hugo G. Effect of dietary counseling on chemotherapy induced weight gain in patients with hematologic and oncologic malignancies. Blood 2017;130(suppl 1):5664. [Google Scholar]
- Hansra DM, Diaz C, Camp S, Randolph C, Norman-Tiner C, Antunez de Mayolo J. Miami NICE trial: nutritional support for patients incurring chemotherapy side effects. Journal of Clinical Oncology 2017;35(15):6568. [Google Scholar]
Hashmi 2016 {published data only}
- Hashmi B, Fones S, Holbrook P. Effects of protein intake on physical functioning and muscle strengthening, when consumed after an exercise program, in seniors living in residential care. Canadian Journal of Dietetic Practice & Research 2016;77(3):pe3-e3. 1/3p. [Google Scholar]
Hatsu 2014 {published data only}
- Hatsu I, Johnson P, Baum M, Huffman F, Thomlison B, Campa A. Association of Supplemental Nutrition Assistance Program (SNAP) with health related quality of life and disease state of HIV infected patients. AIDS and Behavior 2014;18(11):2198-206. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayashi 2014 {published data only}
- Hayashi F, Okuyama M, Adachi Y, Takemi Y. Efficacy of the diet-lifestyle support guide on body weight in the specific health guidance program. Journal of the Academy of Nutrition & Dietetics 2014;114(9 Suppl):A51. [Google Scholar]
Heberer 1984 {published data only}
- Heberer M, Durig M, Harder F. Oropharyngoesophageal carcinoma - aspects of the nutritional status and immunoresistance [Die oro-pharyngo-osophagealen Karzinome - Aspekt des Ernahrungszustandes und der Immunabwehr]. Helvetica Chirurgica Acta 1984;50(5):493-503. [PubMed] [Google Scholar]
Henquin 1989 {published data only}
- Henquin N, Havivi E, Reshef A, Barak F, Horn Y. Nutritional monitoring and counselling for cancer patients during chemotherapy. Oncology 1989;46(3):173-7. [DOI] [PubMed] [Google Scholar]
Hickson 2004 {published data only}
- Hickson M, Bulpitt C, Nunes M, Peters R, Cooke J, Nicholl C, et al. Does additional feeding support provided by health care assistants improve nutritional status and outcome in acutely ill older in-patients?-a randomised control trial. Clinical Nutrition 2004;23(1):69-77. [DOI] [PubMed] [Google Scholar]
- ISRCTN43554088. Does intensive feeding improve nutritional status and outcome in acutely ill older in-patients? www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN43554088 2004.
Hogan 1997 {published data only}
- Hogan SE, Evers SE. A nutritional rehabilitation program for persons with severe physical and developmental disabilities. Journal of the American Dietetic Association 1997;97(2):162-6. [DOI] [PubMed] [Google Scholar]
Holder 2003 {published data only}
- Holder H. Nursing management of nutrition in cancer and palliative care. British Journal of Nursing 2003;12(11):667-8. [DOI] [PubMed] [Google Scholar]
Huisman 2012 {published data only}
- Huisman EF, Hoek B, Soest H, Nierwkerk KM, Arends JE, Siersema PD, et al. Preventive versus "on-demand" nutritional support during antiviral treatment for hepatitis C: a randomised controlled trial. Journal of Hepatology 2012;57(5):1069-75. [DOI] [PubMed] [Google Scholar]
Hulsewe 1997 {published data only}
- Hulsewe KW, Meijerink WJ, Soeters PB, Meyenfeldt MF. Assessment of outcome of perioperative nutritional interventions. Nutrition 1997;13(11-12):996-8. [DOI] [PubMed] [Google Scholar]
Huppertz 2020 {published data only}
- Huppertz VA, Wijk N, Baijens LW, Groot LC, Halfens RJ, Schols JM, et al. Design of the DYNAMO study: a multi-center randomized controlled trial to investigate the effect of pre-thickened oral nutritional supplements in nursing home residents with dysphagia and malnutrition (risk)." . BMC Geriatrics 2020;20:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Idilman 2009 {published data only}
- Idilman R, Seval G, Cinar K, Mizrak D, Bozkus Y, Arsaln M, et al. The effect of dietary intervention on the long-term clinical outcome of non-alcoholic fatty liver disease. Journal of Hepatology 2009;50:1027. [Google Scholar]
Ingadottir 2019 {published data only}
- Ingadottir AR, Beck AM, Baldwin C, Weekes CE, Geirsdottir OG, Ramel A, et al. Oral nutrition supplements and between-meal snacks for nutrition therapy in patients with COPD identified as at nutritional risk: a randomised feasibility trial. BMJ Open Respiratory Research 2019;6:e000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ireton 1995 {published data only}
- Ireton JC, Garritson B, Kitchens L. Nutrition intervention in cancer patients: does the registered dietitian make a difference? Topics in Clinical Nutrition 1995;10:42-8. [Google Scholar]
ISRCTN11132850 {published data only}
- ISRCTN11132850. Efficacy of oral intra-dialysis supplement in haemodialysis patients with malnutrition. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN11132850. 2007.
ISRCTN56882109 {published data only}
- ISRCTN56882109. The efficacy of disease-specific nutritional support compared with usual treatment in hemodialysis patients. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN56882109 2006.
- NTR698. The efficacy of disease specific nutritional support compared with usual treatment in hemodialysis (HD) patients. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR698 2006.
Jacka 2017 {published data only}
- Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the 'SMILES' trial). BMC Medicine 2017;15(1):23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamieson 1997 {published data only}
- Jamieson CP, Norton B, Day T, Lakeman M, Powell TJ. The quantitative effect of nutrition support on quality of life in outpatients. Clinical Nutrition 1997;16(1):25-8. [Google Scholar]
Jancey 2017 {published data only}
- Jancey J, Holt AM, Lee A, Kerr D, Robinson S, Tang L, et al. Effects of a physical activity and nutrition program in retirement villages: a cluster randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2017;14(1):92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2018 {published data only}
- Jang I-Y, Jung H-W, Park H, Lee CK, Yu SS, Lee YS, et al. A multicomponent frailty intervention for socioeconomically vulnerable older adults: a designed-delay study. Clinical Interventions in Aging 2018;13:1799-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jie 2009 {published data only}
- Jie B. The impact of nutritional support on clinical outcome in patients at nutritional risk: multicenter study in Baltimore and Beijing teaching hospitals. Annals of Nutrition and Metabolism 2009;55:131-2. [DOI] [PubMed] [Google Scholar]
Johnson 1993 {published data only}
- Johnson LE, Dooley PA, Gleick JB. Oral nutritional supplement use in elderly nursing home patients. Journal of the American Geriatrics Society 1993;41(9):947-52. [DOI] [PubMed] [Google Scholar]
Kang 2016 {published data only}
- Kang WX, Li W, Huang SG, Dang Y, Gao H. Effects of nutritional intervention in head and neck cancer patients undergoing radiotherapy: a prospective randomized clinical trial. Molecular and Clinical Oncology 2016;5(3):279-82. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karavetian 2016 {published data only}
- Karavretian M, Elzein H, Rizk R, Jibai R, Vries N. Nutritional education for management of osteodystrophy: impact on serum phosphorus, quality of life and malnutrition. Hemodialysis International. International Symposium on Home Hemodialysis 2016;20(3):432-40. [DOI] [PubMed] [Google Scholar]
Keller 1995 {published data only}
- Keller HH. Weight gain impacts morbidity and mortality in institutionalized older persons. Journal of the American Geriatrics Society 1995;43(2):165-9. [DOI] [PubMed] [Google Scholar]
Kirkil 2012 {published data only}
- Kirkil C, Bulbuller N, Aygen E, Basbug M, Ayten R, Ilhan N, et al. The effect of preoperative nutritional supports on patients with gastrointestinal cancer: prospective randomized study. Hepatogastroenterology 2012;59(113):86-9. [DOI] [PubMed] [Google Scholar]
Kiss 2014 {published data only}
- Kiss N, Seymour JF, Prince HM, Dutu G. Challenges and outcomes of a randomized study of early nutrition support during autologous stem-cell transplantation. Current Oncology 2014;21(2):e334-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knowles 1988 {published data only}
- Knowles JB, Fairbarn MS, Wiggs BJ, Chan-Yan C, Pardy RL. Dietary supplementation and respiratory muscle performance in patients with COPD. Chest 1988;93(5):977-83. [DOI] [PubMed] [Google Scholar]
Kondrup 1998 {published data only}
- Kondrup J, Bak L, Hansen BS, Ipsen B, Ronneby H. Outcome from nutritional support using hospital food. Nutrition 1998;14(3):319-21. [DOI] [PubMed] [Google Scholar]
Kong 2018 {published data only}
- Kong S-H, Lee H-J, Na J-R, Kim WG, Han D-S, Park S-H, et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: a prospective randomized trial. Surgery 2018;164:1263-70. [DOI] [PubMed] [Google Scholar]
Krasnoff 2006 {published data only}
- Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Paul SM, Dodd MJ, et al. A randomised trial of exercise and dietary counselling after liver transplantation. American Journal of Transplantation 2006;6(8):1896-905. [DOI] [PubMed] [Google Scholar]
Kristensen 2020 {published data only}
- Kristensen MB, Wessel I, Beck AM, Dieperink KB, Mikkelsen TB, Moller JJ, et al. Effects of a multidisciplinary residential nutritional rehabilitation program in head and neck cancer survivors—results from the nutri-hab randomized controlled trial. Nutrients 2020;12:2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen MB, Wessel I, Beck AM, Dieperink KB, Mikkelsen TB, Moller JJ, et al. Rationale and design of a randomised controlled trial investigating the effect of multidisciplinary nutritional rehabilitation for patients treated for head and neck cancer (the NUTRI-HAB trial). Nutrition Journal 2020;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kruizenga 2005 {published data only}
- Kruizenga HM, Van Tulder MW, Seidell JC, Thijs A, Ader HJ, Bokhorst-de van de Scheuren MA. Effectiveness and cost-effectiveness of early screening and treatment of malnourished patients. American Journal of Clinical Nutrition 2005;82(5):1082-9. [DOI] [PubMed] [Google Scholar]
La Torre 2018 {published data only}
- La Torre G, Cocchiara RA, Lo Sordo E, Chiarini M, Siliquini R, Firenze A, et al. Counseling intervention to improve quality of life in patients with pre-existing acute myocardial infarction (AMI) or chronic obstructive pulmonary disease (COPD): a pilot study. Journal of Preventive Medicine and Hygiene 2018;59:E153-8. [PMC free article] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee LC, Tsai AC, Wang JY, Hurng BS, Hsu, HC, Tsai HJ. Need-based intervention is an effective strategy for improving the nutritional status of older people living in a nursing home: a randomized controlled trial. International Journal of Nursing Studies 2013;50(12):1580-8. [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee HJ, Kong SH. Randomized controlled trial evaluating postoperative oral nutritional supplementation after major gastrointestinal surgery. Clinical Nutrition 2016;35 Suppl 1:S39. [DOI] [PubMed] [Google Scholar]
Leedo 2017 {published data only}
- Leedo E, Gade J, Granov S, Mellemgaard A, Klausen TW, Rask K, et al. The effect of a home delivery meal service of energy- and protein-rich meals on quality of life in malnourished outpatients suffering from lung cancer: a randomized controlled trial. Nutrition and Cancer 2017;69(3):444-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lehtisalo 2017 {published data only}
- Lehtisalo J, Ngandu T, Valve P, Antikainen R, Laatikainen T, Strandberg T, et al. Nutrient intake and dietary changes during a 2-year multi-domain lifestyle intervention among older adults: secondary analysis of the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) randomised controlled trial. British Journal of Nutrition 2017;118(4):291-302. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lejeune 2005 {published data only}
- Lejeune MP, Kovacs EM, Westerterp-Plantenga MS. Additional protein intake limits weight regain after weight loss in humans. British Journal of Nutrition 2005;93(2):281-9. [DOI] [PubMed] [Google Scholar]
Leslie 2013 {published data only}
- Leslie WS, Woodward M, Lean ME, Theobald H, Watson L, Hankey CR. Improving the dietary intake of under nourished older people in residential care homes using an energy-enriching food approach: a cluster randomised controlled study. Journal of Human Nutrition and Dietetics 2013;26(4):387-94. [DOI] [PubMed] [Google Scholar]
Levine 1982 {published data only}
- Levine AS, Brennan MF, Ramu A, Fisher RI, Pizzo PA, Glaubiger DL. Controlled clinical trials of nutritional intervention as an adjunct to chemotherapy, with a comment on nutrition and drug resistance. Cancer Research 1982;42 Suppl 2:774s-81s. [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li T, Lv J, Zhu G, Li G, Zhu S, Wang J, et al. Enteral nutrition to improve nutritional status, treatment tolerance, and outcomes in patients with esophageal cancer undergoing concurrent chemoradiotherapy (CCRT): results of a prospective, randomized, controlled, multicenter trial. Journal of Clinical Oncology 2017;35(15):4033. [Google Scholar]
Lipschitz 1985 {published data only}
- Lipschitz DA, Mitchell CO, Steele RW, Milton KY. Nutritional evaluation and supplementation of elderly subjects participating in a 'meals on wheels' program. Journal of Parenteral Enteral Nutrition 1985;9(3):343-7. [DOI] [PubMed] [Google Scholar]
Lonbro 2013 {published data only}
- Lonbro S, Dalgas U, Primdahl H, Johansen J, Nielsen JL, Overgaard J, et al. Lean body mass and muscle function in head and neck cancer patients and healthy individuals - results from the DAHANCA 25 study. Acta Oncologica 2013;52(7):1543-51. [DOI] [PubMed] [Google Scholar]
Luppino 2015 {published data only}
- Luppino I, Isabella R, De Marco R, Paese P, Leo P. Effects of early nutritional intervention on hospital malnutrition. Digestive and Liver Disease 2015;47:e203-4. [Google Scholar]
Lynch 1983 {published data only}
- Lynch K, Henry DA, Roberts C, Coburn JW. Clinical trial with oral carbohydrate supplement in hemodialysis patients: a nutritional evaluation. Dialysis and Transplantation 1983;12(8):566-8. [Google Scholar]
Manders 2009 {published data only}
- Manders M, De Groot LC, Hoefnagels WH, Dhonukshe-Rutten RA, Wouters-Wessling W, Mulders AJ, et al. The effect of a nutrient dense drink on mental and physical function in institutionalized elderly people. Journal of Nutrition, Health and Aging 2009;13(9):760-7. [DOI] [PubMed] [Google Scholar]
Margare 2002 {published data only}
- Margare N, Lymperopoulos N, Iordanou P, Noula M, Gesoule-Voltyrake E, Bellow-Milona P. The role of dietary counselling on food intake in lung cancer patients undergoing chemotherapy. ICUs & Nursing Web Journal 2002;11.
Maurya 2019 {published data only}
- Maurya NK, Arya P, Sengar NS. Effect of dietary counseling in chronic renal failure patients on hemodialysis. Asian Journal of Pharmaceutics 2019;13(1):33-6. [Google Scholar]
Mazzuca 2019 {published data only}
- Mazzuca F, Roberto M, Arrivi G, Sarfati E, Schipilliti FM, Crimini E, et al. Clinical impact of highly purified, whey proteins in patients affected with colorectal cancer undergoing chemotherapy: preliminary results of a placebo-controlled study. Integrative Cancer Therapies 2019;18:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuca F, Roberto M, Botticelli A, Marchetti L, Sarfati E, Crimini E, et al. Effect of nutritional support with highly purified, whey proteins for malnutrition and sarcopenia in patients affected with stage IIIII colorectal or breast cancer: a blind, placebo controlled, randomized clinical trial. Journal of Clinical Oncology 2018;36(15 suppl):TPS10129. [Google Scholar]
- Roberto M, Sarfati E, Di Girolamo M, Schipilliti FM, Crimini E, Botticelli A, et al. Clinical impact of whey protein and nutritional counseling in gastrointestinal cancer patients. Annals of Oncology 2018;29:viii619‐20. [ABSTRACT NO.: 1469] [Google Scholar]
McCarter 2018 {published data only}
- Britton B, McCarter K, Baker A, Wolfenden L, Wratten C, Bauer J, et al. Eating As Treatment (EAT) study protocol: a stepped-wedge, randomised controlled trial of a health behaviour change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiotherapy. BMJ Open 2015;5(7):e008921. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter K, Baker AL, Britton B, Beck AK, Carter G, Bauer J, et al. Effectiveness of clinical practice change strategies in improving dietitian care for head and neck cancer patients according to evidence-based clinical guidelines: a stepped-wedge, randomized controlled trial. Translational Behavioral Medicine 2018;8(2):166-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
McCormack 2013 {published data only}
- McCormack WF, Stout JR, Emerson NS, Scanlon TC, Warren AM, Wells AJ, et al. Oral nutritional supplement fortified with beta-alanine improves physical working capacity in older adults: a randomized placebo controlled study. Experimental Gerontology 2013;48(9):933-9. [DOI] [PubMed] [Google Scholar]
McWhirter 1996 {published data only}
- McWhirter JP, Pennington CR. A comparison between oral and nasogastric nutritional supplements in malnourished patients. Nutrition 1996;12(7-8):502-6. [DOI] [PubMed] [Google Scholar]
Mendenhall 1993 {published data only}
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology 1993;17(4):564-76. [DOI] [PubMed] [Google Scholar]
Mendenhall 1995 {published data only}
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. Journal of Parenteral and Enteral Nutrition 1995;19(4):258-65. [DOI] [PubMed] [Google Scholar]
Monnin 1993 {published data only}
- Monnin S, Schiller MR. Nutrition counselling for breast cancer patients. Journal of the American Dietetic Association 1993;93(1):72-3. [DOI] [PubMed] [Google Scholar]
Montoya 2014 {published data only}
- Montoya S, Munera Garcia NE. Effect of early nutritional intervention in the outcome of patients at risk of clinical nutrition [Efecto de la intervención nutricional temprana en el resultado clínico de pacientes en riesgo nutricional]. Nutricion Hospitalaria 2014;29(2):427-36. [DOI] [PubMed] [Google Scholar]
Morasutti 2012 {published data only}
- Morasutti I, Giometto M, Baruffi C, Marcon ML, Michieletto S, Giometto B, et al. Nutritional intervention for amyotrophic lateral sclerosis. Minerva Gastroenterologica e Dietologica 2012;58(3):253-60. [PubMed] [Google Scholar]
Munck 1998 {published data only}
- Munck A, Le-Bourgeois M, Gerardin M, Navarro J. Cystic fibrosis: Dietary counselling and nutritional support. Nutrition Clinique et Metabolisme 1998;12(4):283-8. [Google Scholar]
Munk 2014 {published data only}
- Munk T, Beck AM, Holst M, Rosenbom E, Rasmussen HH, Nielsen MA, et al. Positive effect of protein-supplemented hospital food on protein intake in patients at nutritional risk: a randomised controlled trial. Journal of Human Nutrition and Dietetics 2014;27(2):122-32. [DOI] [PubMed] [Google Scholar]
Myint 2013 {published data only}
- Myint MW, Wu J, Wong E, Chan SP, To TS, Chau MW, et al. Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: a single blind randomised controlled trial. Age Ageing 2013;42(1):39-45. [DOI] [PubMed] [Google Scholar]
- NCT01088139. Nutritional intervention for geriatric hip fracture patients. clinicaltrials.gov/show/NCT01088139 (date first posted 17 Mar 2010).
NCT00136253 {published data only}
- NCT00136253. Overcoming nutritional barriers in hemodialysis patients. clinicaltrials.gov/show/NCT00136253 (date first posted 29 Aug 2005).
NCT00417508 {published data only}
- NCT00417508. Nutritional intervention in malnourished elderly patients. clinicaltrials.gov/show/NCT00417508 (date first posted 01 Jan 2006).
NCT00769652 {published data only}
- NCT00769652. Medical nutrition therapy or standard care in treating patients with lung cancer, pancreatic cancer, or stage III or stage IV prostate cancer. clinicaltrials.gov/show/NCT00769652 (date first posted 09 Oct 2008).
NCT01116947 {published data only}
- NCT01116947. Fosrenol for enhancing dietary protein intake in hypoalbuminemic dialysis patients (FrEDI) study. clinicaltrials.gov/show/NCT01116947 (date first posted 05 May 2010).
NCT01190969 {published data only}
- NCT01190969. A trial of oral nutritional supplements on nutritional status and clinical outcomes. clinicaltrials.gov/show/NCT01190969 (date first posted 30 Aug 2010).
NCT02681601 {published data only}
- NCT02681601. Nutrition support to improve outcomes in patients with unresectable pancreatic cancer. clinicaltrials.gov/ct2/show/NCT02681601 (date first posted 12 Feb 2016).
NCT03488511 {published data only}
- NCT03488511. The impact of preoperative FoodforCare at Home on functional and clinical outcomes. clinicaltrials.gov/ct2/show/NCT03488511 (date first posted 05 Apr 2018).
NCT03649698 {published data only}
- NCT03649698. Exercise and nutrition for healthy ageing (ENHANce). clinicaltrials.gov/show/NCT03649698 (date first posted 28 Aug 2018).
NCT03741283 {unpublished data only}
- NCT03741283. Optimisation of nutrition and medication for acutely admitted older medical patients. clinicaltrials.gov/show/NCT03741283 (date first posted 14 Nov 2018).
NCT03774953 {unpublished data only}
- NCT03774953. The effect of protein supplement on lean body mass in patients with pneumonia. clinicaltrials.gov/show/NCT03774953 (date first posted 13 Dec 2018).
NCT03792711 {unpublished data only}
- NCT03792711. Th effects of the oral nutritional supplement with β-hydroxy-β-methylbutyrate in lean body mass among Taiwan elderly. clinicaltrials.gov/show/NCT03792711 (date first posted 03 Jan 2019).
NCT03807310 {unpublished data only}
- NCT03807310. Targeted nutrient supplement in COPD (NUTRECOVER-trial). clinicaltrials.gov/show/NCT03807310 (date first posted 16 Jan 2019).
NCT03924089 {unpublished data only}
- NCT03924089. Oral nutritional supplement on nutritional and functional status, and biomarkers in malnourished hemodialysis patients. clinicaltrials.gov/show/NCT03924089 (date first posted 23 Apr 2019).
NCT04027413 {unpublished data only}
- NCT04027413. Protein supplementation to enhance exercise capacity in COPD. clinicaltrials.gov/show/NCT04027413 (date first posted 22 Jul 2019).
NCT04036825 {unpublished data only}
- NCT04036825. Liquid nutritional supplement on malnutrition hospitalized patient. clinicaltrials.gov/show/NCT04036825 (date first posted 30 Jul 2019).
NCT04109495 {published data only}
- NCT04109495. Usefulness of smartphone application for improving nutritional status of pancreatic cancer patients. clinicaltrials.gov/show/NCT04109495 (date first posted 30 Sep 2019).
NCT04175769 {unpublished data only}
- NCT04175769. EPA+DHA for non-small cell lung cancer patients. clinicaltrials.gov/show/NCT04175769 (date first posted 25 Nov 2019).
NCT04218253 {published data only}
- NCT04218253. Clinical application of nutrition support package before hepatectomy. clinicaltrials.gov/show/NCT04218253 (date first posted 06 Jan 2020).
Neidich 1985 {published data only}
- Neidich G, Schissel K, Sharp HL. Noninvasive outpatient nutritional therapy in inflammatory bowel disease. Journal of Parenteral and Enteral Nutrition 1985;9(3):350-2. [DOI] [PubMed] [Google Scholar]
Newmark 1981 {published data only}
- Newmark SR, Simpson S, Daniel P, Sublett D, Black J, Geller R. Nutritional support in an inpatient rehabilitation unit. Archives of Physical Medicine and Rehabilitation 1981;62(12):634-7. [PubMed] [Google Scholar]
Neyman 1996 {published data only}
- Neyman MR, Zidenberg CS, McDonald RB. Effect of participation in congregate-site meal programs on nutritional status of the healthy elderly. Journal of the American Dietetic Association 1996;96:475-82. [DOI] [PubMed] [Google Scholar]
Ng 2015 {published data only}
- Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. American Journal of Medicine 2015;128(11):1225-36.e1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nijs 2006 {published data only}
- Nijs KA, Graaf C, Kok FJ, Staveren WA. Effect of family style mealtimes on quality of life, physical performance, and body weight of nursing home residents: cluster randomised controlled trial. BMJ 2006;332(7551):1180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
NTR6713 {published data only}
- NTR6713. Personalized dietary advice. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR6713 2017.
NTR7506 {published data only}
- NTR7506. Protein intake in patients with colorectal or lung cancer when receiving a nutritional supplement. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR7506 2018.
Nyamathi 2018 {published data only}
- Nyamathi AM, Carpenter CL, Ekstrand ML, Yadav K, Garfin DR, Muniz M, et al. Randomized controlled trial of a community-based intervention on HIV and nutritional outcomes at six months among women living with HIV/AIDS in rural India. AIDS 2018;32(18):2727-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nykanen 2014 {published data only}
- Nykanen I, Rissanen TH, Sulkava R, Hartikainen S. Effects of individual dietary counselling as part of a comprehensive geriatric assessment (CGA) on nutritional status: a population-based intervention study. Journal of Nutrition, Health & Aging 2014;18(1):54-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nykanen 2018 {published data only}
- Nykanen I, Torronen R, Schwab U. Dairy-based and energy-enriched berry-based snacks improve or maintain nutritional and functional status in older people in home care. Journal of Nutrition Health Aging 2018;22(10):1205-10. [DOI] [PubMed] [Google Scholar]
- Nykanen I. Insufficient reporting of randomization procedures and unexplained unequal allocation: a commentary on “Dairy-based and energy-enriched berry-based snacks improve or maintain nutritional and functional status in older people in home care". Journal of Nutrition Health & Aging 2019;23(4):397 (letter). [DOI] [PubMed] [Google Scholar]
Olofsson 2007 {published data only}
- Olofsson B, Stenvall M, Svensson O, Gustafson Y. Malnutrition in hip fracture patients: an intervention study. Journal of Clinical Nursing 2007;16(11):2027-38. [DOI] [PubMed] [Google Scholar]
Ommundsen 2017 {published data only}
- Ommundsen N, Wyller TB, Nesbakken A, Bakka AO, Jordhoy MS, Skovlund E, et al. Preoperative geriatric assessment and tailored interventions in frail older patients with colorectal cancer: a randomized controlled trial. Colorectal Disease 2017;20:16-25. [DOI] [PubMed] [Google Scholar]
Openbrier 1984 {published data only}
- Openbrier DR, Irwin MM, Dauber JH, Owens G, Rogers RM. Factors affecting nutritional status and the impact of nutritional support in patients with emphysema. Chest 1984;85(6 Suppl):67S-69S. [Google Scholar]
Orell 2019 {published data only}
- NCT02159508. Intensive nutrition counselling in patients with head and neck cancer. clinicaltrials.gov/show/NCT02159508 (date first posted 10 Jun 2014).
- Orell H, Schwab U, Saarilahti K, Osterlund Pia, Ravasco P, Makitie A. Nutritional counselling for head and neck cancer patients undergoing (chemo) radiotherapy - A prospective randomized trial. Frontiers in Nutrition 2019;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otsuki 2020 {published data only}
- Otsuki I, Himuro N, Tatsumi H, Mori M, Niiya Y, Kumeta Y, Yamakage M. Individualized nutritional treatment for acute stroke patients with malnutrition risk improves functional independence measurement: a randomized controlled trial. Geriatrics and Gerontology International 2020;20(3):176-82. [DOI] [PubMed] [Google Scholar]
- UMIN-CTR 000023954. Efficacy of nutrition management for acute stroke patients with malnutrition risk: randomized controlled study. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000027580 2019.
Ottery 1996 {published data only}
- Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12(1 Suppl):S15-S19. [DOI] [PubMed] [Google Scholar]
Ottestad 2017 {published data only}
- Ottestad I, Lovstad AT, Gjevestad GO, Hamarsland H, Saltyte Benth J, Andersen LF, et al. Intake of a protein-enriched milk and effects on muscle mass and strength. A 12-Week randomized placebo controlled trial among community-dwelling older adults. Journal of Nutrition, Health & Aging 2017;21(10):1160-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Palar 2015 {published data only}
- Palar K, Derose KP, Linnemayr S, Smith A, Farias H, Wagner G, et al. Impact of food support on food security and body weight among HIV antiretroviral therapy recipients in Honduras: a pilot intervention trial. AIDS Care 2015;27(4):409-15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parrott 2006 {published data only}
- Parrott MD, Young KW, Greenwood CE. Energy-containing nutritional supplements can affect usual energy intake post-supplementation in institutionalised seniors with probable Alzheimer's disease. Journal of American Geriatrics Society 2006;54(9):1382-7. [DOI] [PubMed] [Google Scholar]
Patel 1998 {published data only}
- Patel MG. The effect of dietary intervention on weight gains after renal transplantation. Journal of Renal Nutrition 1998;8(3):137-41. [DOI] [PubMed] [Google Scholar]
Paulsen 2020 {published data only}
- Paulsen MM, Paur I, Gjestland J, Henriksen C, Varsi C, Tangvik RJ, et al. Effects of using the MyFood decision support system on hospitalized patients' nutritional status and treatment: a randomized controlled trial. Clinical Nutrition 2020;39:3607-17. [DOI] [PubMed] [Google Scholar]
Pedersen 2005 {published data only}
- Pedersen PU. Nutritional care: the effectiveness of actively involving older patients. Journal of Clinical Nursing 2005;14(2):247-55. [DOI] [PubMed] [Google Scholar]
Penalva 2009 {published data only}
- Penalva A, San Martin A, Rossello J, Perez-Portabella C, Palacios A, Planas JY. Oral nutritional supplementation in hematologic patients [Suplementacion oral nutricional en pacientes hemotologicos]. Nutricion Hospitalaria 2009;24(1):10-6. [PubMed] [Google Scholar]
Pietersma 2003 {published data only}
- Pietersma P, Follett-Bick S, Wilkinson B, Guebert N, Fisher K, Pereira J. A bedside food cart as an alternate food service for acute and palliative oncological patients. Supportive Care in Cancer 2003;11(9):611-4. [DOI] [PubMed] [Google Scholar]
Planas 2005 {published data only}
- Planas M, Alvarez J, Garcia-Peris PA, la Cuerda C, Lucas P, Castella M, et al. Nutritional support and quality of life in stable chronic obstructive pulmonary disease (COPD) patients. Clinical Nutrition 2005;24(3):433-41. [DOI] [PubMed] [Google Scholar]
Plank 2008 {published data only}
- Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, et al. Noctural nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12 month trial. Hepatology 2008;48(2):557-66. [DOI] [PubMed] [Google Scholar]
Poulsen 2014 {published data only}
- Poulsen GM, Pedersena LL, Østerlind K, Bæksgaard L, Andersen JR. Randomized trial of the effects of individual nutritional counseling in cancer patients. Clinical Nutrition 2014;33(5):749-53. [DOI] [PubMed] [Google Scholar]
PrayGod 2012 {published data only}
- PrayGod G, Range N, Faurholt-Jepsen D, Jermiah K, Faurholt-Jepsen M, Aabye MG, et al. The effect of energy-protein supplementation on weight, body composition and handgrip strength among pulmonary tuberculosis HIV-co-infected patients: randomised controlled trial in Mwanza Tanzania. British Journal of Nutrition 2012;107(2):263-71. [DOI] [PubMed] [Google Scholar]
Rabinovitch 2006 {published data only}
- Rabinovitch R, Grant B, Berkey BA, Raben D, Ang KK, Fu KK, et al. Impact of nutrition support on treatment outcome in patients with a locally advanced head and neck squamous cell cancer treated with definitive radiotherapy: a secondary analysis of RTOG trial 90-03. Head and Neck 2006;28(4):287-96. [DOI] [PubMed] [Google Scholar]
Rassmussen 2006 {published data only}
- Rasmussen HH, Kondrup J, Staun M, Ladefoged K, Lindorff K, Jorgensen LM, et al. A method for implementation of nutrition therapy in hospitals. Clinical Nutrition 2006;25(3):515-23. [DOI] [PubMed] [Google Scholar]
Reinders 2018 {published data only}
- Reinders I, Volkert K, Groot LC, Beck AM, Feldblum I, Jobse I, et al. Effectiveness of nutritional interventions in older adults at risk of malnutrition across different health care settings: pooled analyses of individual participant data from nine randomized controlled trials. Clinical Nutrition 2019;38:1791-806. [DOI] [PubMed] [Google Scholar]
Rizk 2017 {published data only}
- Karavetian M, Abboud S, Elzein H, Haydar S, Vries N. Nutritional education for management of osteodystrophy (NEMO) trial: design and patient characteristics, Lebanon. Nutrition Research and Practice 2014;8(1):103-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavetian M, Vries N, Elzein H, Rizk R, Bechwaty F. Effect of behavioral stage-based nutrition education on management of osteodystrophy among hemodialysis patients, Lebanon. Patient Education and Counselling 2015;98:1116-22. [DOI] [PubMed] [Google Scholar]
- Karavetian M, Vries N, Elzein H. Nutritional intervention for management of osteodystrophy (NIMO) program in hemodialysis patients, Lebanon and baseline data. Kidney Research and Clinical Practice 2012;31(2):A43. [ABSTRACT NO.: 107] [Google Scholar]
- Rizk R, Karavetian M, Hiligsmann M, Evers SMAA. Effect of stage-based education provided by dedicated dietitians on hyperphosphataemic haemodialysis patients: results from the Nutrition Education of Management of Osteodystrophy randomised controlled trial. Journal of Human Nutrition and Dietetics 2017;30:554-62. [DOI] [PubMed] [Google Scholar]
- Saade A, Karavetian M, Elzein H, Vries N. Nutritional education for management of osteodystrophy (NEMO) trial: impact on quality of life. Nephrology Dialysis Transplantation 2013;28:i119. [Google Scholar]
Rollo 2020 {published data only}
- Rollo ME, Haslam RL, Collins CE. Impact on dietary intake of two levels of technology-assisted personalized nutrition: a randomized trial. Nutrients 2020;12:3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roussel 2016 {published data only}
- NCT02510859. Nutrition and life quality patients with head and neck cancers. https://clinicaltrials.gov/ct2/show/NCT02510859 (date first posted 29 Jul 2010).
- Roussel L-M, Micault E, Peyronnet D, Blanchard D, Guarnieri S, Choussy O, et al. Intensive nutritional care for patients treated with radiotherapy in head and neck cancer: a randomized study and meta-analysis. European Archives of Oto-Rhino-Laryngology 2016;274:977-87. [DOI] [PubMed] [Google Scholar]
Rozentryt 2010 {published data only}
- Rozentryt P, Haehling S, Lainscak M, Nowak JU, Kalantar-Zadeh K, Polonski L, et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition and inflammation markers: a randomized, double-blind pilot study. Journal of Cachexia, Sarcopenia and Muscle 2010;1(1):35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rüfenacht 2010 {published data only}
- Rüfenacht U, Ruhlin M, Wegmann M, Imoberdorf R, Ballmer PE. Nutritional counselling improves quality of life and nutrient intake in hospitalized undernourished patients. Nutrition 2010;26(1):53-60. [DOI] [PubMed] [Google Scholar]
Salas‐Salvado 2005 {published data only}
- Salas-Salvado J, Torres M, Planas M, Altimir S, Pagan C, Gonzalez ME, et al. Effect of oral administration of a whole formula diet on nutritional and cognitive status in patients with Alzheimer's disease. Clinical Nutrition 2005;24(3):390-7. [DOI] [PubMed] [Google Scholar]
Sankhaanurak 2021 {published data only}
- Sankhaanurak A, Thongsawat S, Leerapun A. Effect of simplifying protein counting tool and educational intervention on the nutritional status in patient with cirrhosis: a randomized clinical trial. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2021;103(12):S113‐20. [Google Scholar]
- Sankhaanurak A, Thongsawat S, Leerapun A. Effect of simplifying protein counting tool and educational intervention on the nutritional status of patient with cirrhosis: a randomized clinical trial. Hepatology International 2020;14(Suppl 1):S380. [ABSTRACT NO.: 454] [Google Scholar]
Sartorelli 2005 {published data only}
- Sartorelli DS, Sciarra EC, Franco LJ, Cardoso MA. Beneficial effects of short-term nutritional counselling at primary health care level among Brazillian adults. Public Health Nutrition Journal 2005;8(7):820-5. [DOI] [PubMed] [Google Scholar]
Saudny‐Unterberger 1997 {published data only}
- Saudny UH, Martin JG, Gray DK. Impact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):794-9. [DOI] [PubMed] [Google Scholar]
- Saudny-Unterberger H. Impact of nutritional support on changes in functional status during an acute exacerbation of chronic obstructive pulmonary disease (COPD). Thesis https://escholarship.mcgill.ca/concern/theses/p8418q23t 1995. [DOI] [PubMed]
Shamoto 2020 {published data only}
- Shamoto H, Koyama T, Momosaki R, Maeda K, Wakabauashi H. The effects of promoting oral intake using the Kuchi-kara Taberu index, a comprehensive feeding assistant tool, in older pneumonia patients: a cluster randomized controlled trial. BMC Geriatrics 2020;20:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shan 2001 {published data only}
- Shan L. Short-term nutritional support in treating COPD and respiratory insufficiency with severely malnutrition. Fudan University Journal of Medical Sciences 2001;28(6):512-5. [Google Scholar]
Simmons 2008 {published data only}
- Simmons SF, Keeler E, Zhuo X, Hickey KA, Sato H, Schnelle JF. Prevention of unintentional weight loss in nursing home residents: a controlled trial of feeding assistance. Journal of the American Geriatric Society 2008;56(8):1466-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 2010 {published data only}
- Simmons SF, Zhuo X, Keeler E. Cost-effectiveness of nutrition interventions in nursing home residents: a pilot intervention. Journal of Nutrition, Health & Aging 2010;14(5):367-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skaarud 2016 {published and unpublished data}
- Skaarud KJ, Hjermstad MJ, Bye A, Veierød MB, Gudmundstuen AM, Lundin KE, et al. No effect of individualized nutrition on quality of life, acute graft-versus-host disease or oral mucositis after allogeneic stem cell transplantation: a randomized controlled trial. Blood 2016;128:2211. [Google Scholar]
Skaarud 2018 {published data only}
- Skaarud KJ, Hjermstad MJ, Bye A, Veierod MB, Gudmundstuen AM, Lundin KEA, et al. Effects of individualized nutrition after allogeneic hematopoietic stem cell transplantation following myeloablative conditioning; a randomized controlled trial. Clinical Nutrition ESPEN 2018;28:59-66. [DOI] [PubMed] [Google Scholar]
- Skaarud KJ, Veierod MG, Lergenmuller S, Bye A, Iversen PO, Tjonnfjord GE. Body weight, body composition and survival after 1 year: follow-up of a nutritional intervention trial in allo-HSCT recipients. Bone Marrow Transplantation 2019;54:2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smoliner 2008 {published data only}
- Smoliner C, Norman K, Scheufele R, Hartig W, Pirlich M, Lochs H. Effects of food fortification on nutritional and functional status in frail elderly nursing home residents at risk of malnutrition. Nutrition 2008;24(11-12):1139-44. [DOI] [PubMed] [Google Scholar]
Sohrabi 2016 {published data only}
- Sohrabi Z, Eftekhari MH, Eskandari MH, Rezaianzadeh A, Sagheb MM. Intradialytic oral protein supplementation and nutritional and inflammation outcomes in hemodialysis: a randomized controlled trial. American Journal of Kidney Diseases 2016;68(1):122-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Solerte 2008 {published data only}
- Solerte SE, Gazzaruso C, Bonacasa R, Rondanelli M, Zamboni M, Basso C, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. American Journal of Cardiology 2008;101(Suppl):69E-77E. [DOI] [PubMed] [Google Scholar]
Solomon 1978 {published data only}
- Solomon MJ, Smith MF, Dowd JB, Bistrian BR, Blackburn GL. Optimal nutritional support in surgery for bladder cancer: preservation of visceral protein by amino acid infusions. Journal of Urology 1978;119(3):350-4. [DOI] [PubMed] [Google Scholar]
Somanchi 2011 {published data only}
- Somanchi M, Tao X, Mullin GE. The facilitated early enteral and dietary management effectiveness trial in hospitalized patients with malnutrition. Journal of Parenteral and Enteral Nutrition 2011;35(2):209-16. [DOI] [PubMed] [Google Scholar]
Sridar 1994 {published data only}
- Sridhar MK, Galloway A, Lean ME, Banham SW. An out-patient nutritional supplementation programme in COPD patients. European Respiratory Journal 1994;7(4):720-4. [DOI] [PubMed] [Google Scholar]
Stack 1996 {published data only}
- Stack JA, Bell SJ, Burke PA, Forse A. High-energy, high-protein, oral, liquid, nutrition supplementation in patients with HIV infection: effect on weight status in relation to incidence of secondary infection. Journal of the American Dietetic Association 1996;96:337-41. [DOI] [PubMed] [Google Scholar]
Stark 1990 {published data only}
- Stark LJ, Bowen AM, Tyc VL, Evans S, Passero MA. A behavioral approach to increasing calorie consumption in children with cystic fibrosis. Journal of Pediatric Psychology 1990;15(3):309-26. [DOI] [PubMed] [Google Scholar]
Stevenson 2019 {published data only}
- Stevenson J, Campbell KL, Brown M, Craig J, Howard K, Howell M, et al. Targeted, structured text messaging to improve dietary and lifestyle behaviours for people on maintenance haemodialysis (KIDNEYTEXT): study protocol for a randomised controlled trial. BMJ Open 2019;9(5):e023545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2017 {published data only}
- Stewart JL, Besenyi GB, Williams LB, Burt V, Anglin JC, Ghamande SA, et al. Healthy lifestyle intervention for African American uterine cancer survivors: study protocol. Contemporary Clinical Trials Communications 2017;8:11-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 2019 {published data only}
- Suzuki H, Kanazawa M, Komagamine Y, Iwaki M, Amagai N, Minakuchi S. Changes in the nutritional statuses of edentulous elderly patients after new denture fabrication with and without providing simple dietary advice. Journal of Prosthodontic Research 2019;63(3):288-92. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kanazawa M, Komagamine Y, Iwaki M, Amagai N, Minakuchi S. Influence of simplified dietary advice combined with new complete denture fabrication on masticatory function of complete denture wearers. Journal of Oral Rehabilitation 2019;46:1100-6. [DOI] [PubMed] [Google Scholar]
Swaminathan 2010 {published data only}
- Swaminathan S, Padmapriyadarsini C, Yoojin L, Sukumar B, Iliayas S, Karthiprya J, et al. Nutritional supplementation in HIV-infected individuals in South India: a prospective interventional study. Clinical Infectious Diseases 2010;51(1):51-7. [DOI] [PubMed] [Google Scholar]
Swanenburg 2007 {published data only}
- Swanenburg J, Stauffacher M, Mulder T, Uebelhart D. Effects of exercise and nutrition on postural balance and risk of falling in elderly people with decreased bone mineral density: randomised controlled trial pilot study. Clinical Rehabilitation 2007;21(6):523-34. [DOI] [PubMed] [Google Scholar]
Tandon 1984 {published data only}
- Tandon SP, Gupta SC, Sinha SN, Naithani YP. Nutritional support as an adjunct therapy of advanced cancer patients. Indian Journal of Medical Research 1984;80:180-8. [PubMed] [Google Scholar]
Tatsumi 2009 {published data only}
- Tatsumi K, Shinozuka N, Nakayama K, Sekiya N, Kuriyama T, Fukuchi Y. Hochuekkito improves systemic inflammation and nutritional status in elderly patients with chronic obstructive pulmonary disease. Journal of the American Geriatrics Society 2009;57(1):169-72. [DOI] [PubMed] [Google Scholar]
Taylor 2006 {published data only}
- Taylor KA, Barr SI. Provision of small frequent meals does not improve energy intake of elderly residents with dysphagia who live in an extended-care facility. Journal of American Dietetic Association 2006;106(7):1115-8. [DOI] [PubMed] [Google Scholar]
Trabal 2010 {published data only}
- Trabal J, Leyes P, Forga M, Maurel J. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutricion Hospitalaria 2010;25(5):736-40. [PubMed] [Google Scholar]
Turic 1998 {published data only}
- Turic A, Gordon KL, Craig LD, Ataya DG, Voss AC. Nutrition supplementation enables elderly residents of long-term-care facilities to meet or exceed RDAs without displacing energy or nutrient intakes from meals. Journal of American Dietetic Association 1998;98(12):1457-9. [DOI] [PubMed] [Google Scholar]
Turnock 2013 {published data only}
- Turnock A, Calder PC, West AL, Izzard M, Morton RP, Plank LD. Perioperative immunonutrition in well-nourished patients undergoing surgery for head and neck cancer: evaluation of inflammatory and immunologic outcomes. Nutrients 2013;5(4):1186-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unosson 1992 {published data only}
- Unosson M, Larsson J, Ek AC, Bjurulf P. Effects of dietary supplement on functional condition and clinical outcome measured with a modified Norton scale. Clinical Nutrition 1992;11(3):134-9. [DOI] [PubMed] [Google Scholar]
van Beers 2020 {published data only}
- Beers M, Rutten-van Molken MP, de Bool C, Boland M, Kremers SP, Franssen FM, et al. Clinical outcome and cost-effectiveness of a 1-year nutritional intervention programme in COPD patients with low muscle mass: the randomized controlled NUTRAIN trial. Clinical Nutrition 2020;39:405-13. [DOI] [PubMed] [Google Scholar]
van Blarigan 2020 {published data only}
- Blarigan EL, Kenfield SA, Chan JM, Van Loon K, Paciorek A, Zhang L, et al. Feasibility and acceptability of a web-based dietary intervention with text messages for colorectal cancer: a randomized pilot trial. Cancer Epidemiology Biomarkers & Prevention 2020;29(4):752-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Berg 2010 {published data only}
- den Berg MG, Rasmussen-Conrad EL, Wei KH, Lintz-Luidens H, Kaanders JH, Merkx MA. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. British Journal of Nutrition 2010;104(6):872-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
van den Heuvel 2017 {published data only}
- den Heuvel E, Murphy JL, Appleton KM. Evaluating individual differences in a food based approach to increase egg and protein intake in older adults. In: Proceedings of the Nutrition Society. Vol. 79. 2020:OCE2.
- den Heuvel E, Murphy JL, Appleton KM. Increasing dietary protein intake in community dwelling older adults: protocol for a randomised controlled trial and baseline data. In: Proceedings of the Nutrition Society. Vol. 76. 2017:OCE4.
van der Meij 2010 {published data only}
- Meij BS, Langius JA, Smit EF, Spreeuwenberg MD, Blomberg BM, Heijboer AC, et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. Journal of Nutrition 2010;40(10):1774-80. [DOI] [PubMed] [Google Scholar]
van der Pols‐Vijlbrief 2017 {published data only}
- Pols-Vijlbrief R, Wijnhoven HA, Bosmans JE, Twisk JW, Visser M. Targeting the underlying causes of undernutrition. Cost-effectiveness of a multifactorial personalized intervention in community-dwelling older adults: a randomized controlled trial. Clinical Nutrition 2017;36(6):1498-508. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vargas 1995 {published data only}
- Vargas M, Puig A, la Maza M, Morales P, Vargas D, Bunout B, et al. Patients with chronic airflow limitation: effects of the inspiratory muscle training with threshold load valve, built with appropriate technology, associated to nutritional support. Revista Medica De Chile 1995;123(10):1225-34. [PubMed] [Google Scholar]
Vazquez Martinez 2015 {published data only}
- Vazquez Martinez C. Nutritional counselling: an effective tool in the fight against malnutrition. Revista Clinica Espanola 2015;5(6):322-3. [DOI] [PubMed] [Google Scholar]
Volkert 1996 {published data only}
- Volkert D, Hubsch S, Oster P, Schlierf G. Nutrition support and functional status in undernourished geriatric patients during hospitalization and 6-month follow-up. Aging: Clinical and Experimental Research 1996;8(6):386-95. [DOI] [PubMed] [Google Scholar]
Watson 2008 {published data only}
- Walton H, Bilton D, Truby H. A randomised controlled trial of a behavioural nutrition education programme "Eat Well with CF" for adults with CF. Journal of Cystic Fibrosis 2006;5 Suppl 1:S73. [Google Scholar]
- Watson H, Bilton D, Truby H. A randomized controlled trial of a new behavioral home-based nutrition education program, "Eat Well with CF" in adults with cystic fibrosis. Journal of the American Dietetic Association 2008;108(5):847-52. [DOI] [PubMed] [Google Scholar]
- Watson H, Bilton D, Truby H. Evaluating the process of a behavioural nutrition education programme "Eat Well with CF" in adults with cystic fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl 1:S73. [Google Scholar]
- Watson H, Truby H, Haworth CS, Bilton D. Pilot study to evaluate a home based behavioural nutrition education programme for adults. Journal of Cystic Fibrosis 2004;3 Suppl 1:S75. [Google Scholar]
Williams 1989a {published data only}
- Williams J, Handy DJ, Weller PH. Improving oral energy intake in older children with cystic fibrosis. Journal of Human Nutrition and Dietetics 1989;2(4):279-85. [Google Scholar]
Williams 1989b {published data only}
- Williams CM, Driver LT, Older J, Dickerson JW. A controlled trial of sip-feed supplements in elderly orthopaedic patients. European Journal of Clinical Nutrition 1989;43(4):267-74. [PubMed] [Google Scholar]
Woo 1994 {published data only}
- Woo J, Ho SC, Mak YT, Law LK Cheung A. Nutritional status of elderly patients during recovery from chest infection and the role of nutritional supplements assessed by a prospective randomised single-blind trial. Journal of Applied Nutrition 1994;46(3):93. [DOI] [PubMed] [Google Scholar]
Wouters‐Wessling 2005 {published data only}
- Wouters-Wesseling W, Slump E, Kleijer CN, Groot LC, Staveren WA. Early nutritional supplementation immediately after diagnosis of infectious disease improves body weight in psychogeriatric nursing home residents. Aging and Clinical Experimental Research 2006;18(1):70-4. [DOI] [PubMed] [Google Scholar]
Wright 2008 {published data only}
- Wright L, Cotter D, Hickson M. The effectiveness of targeted feeding assistance to improve the nutritional intake of elderly dysphagic patients in hospital. Journal of Human Nutrition and Dietetics 2008;21(6):555-62. [DOI] [PubMed] [Google Scholar]
Wu 2020 {published data only}
- Wu S-J, Shieh S-H, Lai Y-J, Shih Y-T, Hwu Y-J. Effects of an eating ability promotion program for community-dwelling older adults. Journal of the American Medical Directors Association 2020;21:1336-40. [DOI] [PubMed] [Google Scholar]
Xie 2017 {published data only}
- ChiCTR-IOQ-16010276. The effect of whole-course educational and nutritional interventions on the nutritional status and compliance with chemotherapy of gastric cancer patients. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IOQ-16010276 2016.
- Xie FL, Wang YQ, Peng LF, Lin FY, He YL, Jiang ZQ. Beneficial effect of educational and nutritional intervention on the nutritional status and compliance of gastric cancer patients undergoing chemotherapy: a randomized trial. Nutrition and Cancer 2017;69(5):762-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yoneda 1992a {published data only}
- Yoneda T, Yoshikawa M, Tsukaguchi K, Fu A, Tokuyama T, Cho S, et al. Supplementary nutrition therapy is effective in patients with chronic obstructive pulmonary disease. Japanese Journal of Thoracic Diseases 1992;30(10):1807-13. [PubMed] [Google Scholar]
Yoneda 1992b {published data only}
- Yoneda T, Yoshikawa M, Tsukaguchi K, Fu A, Tomoda K, Egawa S, et al. Nutritional assessment and the effect of supplementary oral nutrition in patients with pulmonary emphysema. Japanese Journal of Thoracic Diseases 1992;30(8):1483-87. [PubMed] [Google Scholar]
Yuvaraj 2016 {published data only}
- Yuvaraj A, Vijayan M, Alex M, Abraham G, Nair S. Effect of high-protein supplemental therapy on subjective global assessment of CKD-SD patients. Hemodialysis International 2016;20(1):56-62. [DOI] [PubMed] [Google Scholar]
Zhang 2018a {published data only}
- Zhang P, Xie K, Huang X, Li C, Song Y, Li B, et al. Early nutrition support therapy to improve the nutrition status of head and neck cancer patients accepted concurrent chemoradiotherapy (NSTIP): interim analysis from a prospective randomized controlled clinical study. International Journal of Radiation Oncology Biology Physics 2018;102(3S):E744, TU 42_3740. [Google Scholar]
Zhao 1995 {published data only}
- Zhao HC, Zhang R. The observation and analysis for nutritional support prolonging survival in patients with middle and advanced non-small-cell lung cancer. Parenteral & Enteral Nutrition 1995;2(1):39-40. [Google Scholar]
Zweers 2020 {published data only}
- Zweers H, Smit D, Leij S, Wanten G, Janssen MC. Individual dietary intervention in adult patients with mitochondrial disease due to the m.3243A>G mutation: the DINAMITE study. Journal of Inherited Metabolic Disease 2018;41(Issue S1):P46. [ABSTRACT NO.: P-062] [DOI] [PubMed] [Google Scholar]
- Zweers H, Smit D, Leij S, Wanten G, Janssen MC. Individual dietary intervention in adult patients with mitochondrial disease due to the m.3243 A>G mutation. Nutrition 2020;69:110544. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdelsalam 2019 {published data only}
- Abdelsalam M, El Aty NA, Tawfik M, Mohamed H, Hassanin A. Efficacy of nutritional support program on anthropometric measurement and subjective global assessment score among hemodialysis patients. In: Nephrology Dialysis Transplantation. Vol. 34, supplement 1. 2019:a490;SP391.
Abdollahi 2019 {published data only}
- Abdollahi R, Majafi S, Razmpoosh E, Shoormasti RS, Haghighat S, Lahiji MR, et al. The effect of dietary intervention along with nutritional education on reducing the gastrointestinal side effects caused by chemotherapy among women with breast cancer. Nutrition and Cancer 2019;71(6):922-30. [DOI] [PubMed] [Google Scholar]
Banda 2017 {published data only}
- Banda M, Kalimbira A, Kalumizika Z, Mbendera K. Efficacy of nutrition support amongst tuberculosis patients at selected referral hospitals in Malawi. In: Annals of Nutrition and Metabolism. Vol. 71. 2017:1012.
Britton 2019 {published data only}
- Britton B, Baker A, Wolfenden L, Wratten C, Bauer J, Beck A, McElduff P, Carter G. Eating As Treatment (EAT): a stepped-wedge, randomised controlled trial of a health behaviour intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiotherapy. In: Psycho-oncology. Vol. 25, Issue S3. 2016:65 (abstract 197). [DOI] [PMC free article] [PubMed]
- Britton B, Baker AL, Wolfenden L, Wratten C, Bauer J, Beck AK, et al. Eating as treatment (EAT): a stepped-wedge, randomized controlled trial of a health behavior change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiation therapy (TROG 12.03). International Journal of Radiation Oncology, Biology and Physics 2019;103(2):353-62. [DOI] [PubMed] [Google Scholar]
Camere 2016 {published data only}
- Camere MA, Benito P, Camere MD, La Serna JE, Guarro M, Morera M. An oral nutritional supplement reduces malnutrition in chronic obstructive pulmonary disease patients. Clinical Nutrition 2016;35 Suppl 1:S187-8. [Google Scholar]
Cawood 2017 {published data only}
- Cawood AL, Smith TR, Guildford N, Wood C, Ashbolt K, Walters ER, et al. Low volume energy dense oral nutritional supplements improve micronutrient intakes in free living malnourished older people - a randomised trial. Clinical Nutrition 2017;36:S175-6. [Google Scholar]
Cereda 2019 {published data only}
- Cereda E, Turri A, Klersy C, Cappello S Ferrari A, Filippi AR, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Medicine 2019;8:6923-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chewaskulyong 2015 {published data only}
- Chewaskulyong B, Sukaraphat N, Buranapin S. Dietary counseling outcome in locally advanced unresectable or metastatic cancer patients undergoing chemotherapy. European Journal of Cancer 2015;51 Suppl 3:S229. [PubMed] [Google Scholar]
ChiCTR1800014842 {published data only}
- ChiCTR1800014842. Nutritional management in the application of abdominal infection and efficacy: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800014842 2018.
ChiCTR‐IOR‐17013151 {published data only}
- ChiCTR-IOR-17013151. The effect of nutrition support therapy on nutrition status and clinical outcome of outpatients with tuberculosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IOR-17013151 2017.
Collins 2014 {published data only}
- Collins PF, Stratton RJ, Elia M. Nutritional support in chronic obstructive Pulmonary Disease (COPD) a randomised trial. Clinical Nutrition 2014;33 Suppl 1:S65. [Google Scholar]
Cong 2016 {published data only}
- Cong M, Li S, Liu X, Liu W, Cheng G, Yu L. Effect of nutritional counseling combined with oral nutritional supplements on clinical outcome of esophageal cancer patients under radiotherapy treatment. Chinese Journal of Clinical Nutrition 2016;24(2):86-90. [Google Scholar]
Cramon 2019 {published data only}
- Cramon MO, Raben I, Beck AM, Andersen JR. Individual nutritional intervention during 4 weeks for the prevention of re-admissions among geriatric patients - RCT. In: Clinical Nutrition. Vol. 38. 2019:S14 OR28.
CTRI/2012/05/002698 {published data only}
- CTRI/2012/05/002698. A randomised control trial on the effect of nutritional counseling and supplementation on HIV patients initiating Anti Retroviral Therapy. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2012/05/002698 2012.
CTRI/2018/10/015882 {published data only}
- Effect of structured preoperative nutritional protocol on postoperative recovery in patients undergoing gastrointestinal surgeries in JIPMER - randomized controlled trial. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=28149&EncHid=&modid=&compid=%27,%2728149det%27 2018.
CTRI/2018/11/016369 {published data only}
- CTRI/2018/11/016369. Will nutitional counselling through telephone reduce death and complications of chronic liver disease. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/11/016369. 2018.
Cui 2017 {published data only}
- Cui H, Yang X, Tang D, Zhou X, Ding R, Zhu M, et al. Effect of oral nutritional supplementation on nutritional status and quality of life in patients with gastric cancer after operation (23 cases RCT observations). Chinese Journal of Clinical Nutrition 2017;25(3):183-8. [Google Scholar]
Gaitan 2017 {published data only}
- Gaitan S, Hernandez, L, Santos A, Hernandez J, Zavaleta G. Effectiveness of specialized nutritional supplement in hemodialysis patients with protein energy wasting. Nephrology Dialysis Transplantation 2017;32(suppl 3):iii88.
Ha 2010 {published data only}
- Ha L, Hauge T, Spenning AB, Ole Iversen P. Individual nutritional support prevents undernutrition, increases muscle strength and improves QoL among elderly at nutritional risk hospitalized for acute stroke: a randomized controlled trial. Clinical Nutrition 2010;29(5):567-73. [DOI] [PubMed] [Google Scholar]
- NCT00163007. Nutritional therapy for stroke patients. clinicaltrials.gov/show/NCT00163007 (date first posted 13 Sep 2005).
Hansen 2020 {published data only}
- Hansen NM, Faurholt-Jepsen D, Ryrso CK, Hegelund MH, Dungu AM, Seijdic A, et al. The effect of protein-based nutritional supplementation during and after hospital stay in patients with community acquired pneumonia. Clinical Nutrition ESPEN 2020;40:428-9. [Google Scholar]
Hebuterne 2019 {published data only}
- Hebuterne X, Besnard I, François E, Bachmann P, Mahamat A, Assenat E, et al. Impact of an early active dietary counseling on grade ≥ 3 toxicity in patients receiving firs t-line chemotherapy for metastatic colorectal cancer. United European Gastroenterology Journal 2019;7(8):948. [Google Scholar]
Hoekstra 2005 {published data only}
- Hoekstra JC, Uil SM, den Berg JW, Elvers JW. Nutritional intervention versus standard nutritional advice in malnourished COPD patients. European Respiratory Journal 2005;26 Suppl 49:721s. [Google Scholar]
Hsieh 2019 {published data only}
- Hsieh J-T, Su S-C, Chen C-W, Kang Y-W, Hu M-H, Hsu L-L, et al. Individualised home-based exercise and nutrition interventions improve frailty in older adults: a randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2019;16(119):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hubbard 2009 {published data only}
- Hubbard G, Holdoway A, Kerr A, Robertson D, Stratton R. A randomised controlled trial comparing the use of a very dense nutritional supplement with dietary advice in malnourished community-based elderly patients. Clinical Nutrition Supplements 2009;4(2):121. [Google Scholar]
Jabbour 2019 {published data only}
- Jabbour J, Manana B, Sakr M, Zahreddine Z, Tamim H, Bazarbachi A, Blaise D, El-Cheikh J. The impact of counseling on nutritional status among hematopoietic stem cell recipients: results of a randomized controlled trial. Bone Marrow Transplantation 2019;54:752-756. [DOI] [PubMed] [Google Scholar]
- NCT02791347. Nutrition intervention among stem cell recipients: post hospital discharge. clinicaltrials.gov/show/NCT02791347 (first posted 06 Jun 2016).
Jia 2019 {published data only}
- Jia Z, Fang Y, Ting Ting W, BiQing Y, Jing Z, Ni JJ. Effect of nutrition support on the chronic obstructive pulmonary disease in acute episode. Progress in Modern Biomedicine 2019;19(5):899-902. [Google Scholar]
Kalal 2016 {published data only}
- Kalal C, Benjamin J, Shasthry V, Philips CA, Vyas TS, Premkumar M, et al. Effect of long term aggressive nutritional therapy on survival in patients with alcoholic liver cirrhosis-interim analysis of a randomized controlled trial. Hepatology 2016;64(Issue S1):705A. [ABSTRACT NO.: 1409] [Google Scholar]
- Kalal CR, Benjamin J, Shashthry V, Joshi YK, Kumar G, Sarin SK. Effect of long-term aggressive nutritional therapy on survival in patients with alcoholic liver cirrhosis- Interim analysis of a randomized controlled trial. Indian Journal of Gastroenterology 2016;35(1):A107-8. [DOI] [PubMed] [Google Scholar]
Kandel 2014 {published data only}
- Kandel R, Chatterjee P, Kumar V, Gopalan V, Ambashtha A, Jathar S, et al. Impact of nutritional supplementation and Nordic walking in frail older patients in geriatrics department of tertiary care hospital in India. European Geriatric Medicine 2014;5:S127. [Google Scholar]
Kang 2013 {published data only}
- Kang JH, Shin DW, Baik HW, Hong J. Short-term oral nutritional supplements and nutrition intervention in elderly patients after hip fracture surgery: a randomised controlled clinical trial. Clinical Nutrition Supplements 2012;7(1):280. [LB039-MON] [Google Scholar]
Kuhlmann 1999 {published data only}
- Kuhlmann MK, Schmidt F, Kohler H. High protein/energy vs. standard protein/energy nutritional regimen in the treatment of malnourished hemodialysis patients. Mineral Electrolyte Metabolism 1999;25(4-6):306-10. [DOI] [PubMed] [Google Scholar]
- Kuhlmann MK et al. Effects of a high protein/calorie diet on nutritional indices in malnourished maintenance hemodialysis (MHD) patients. Journal of the American Society of Nephrology 1998;9 (Program & Abstracts):75A. [Google Scholar]
Kwon 2004 {published data only}
- Kwon J, Suzuki T, Kim H, Yoon H, Lee S. Effects of home-visit nutrition education on nutritional status improvement of an urban community-swelling elderly women in Korea. Nihon Koshu Eisei Zasshi (Japanese Journal of Public Health) 2004;51(6):391-402. [PubMed] [Google Scholar]
Limwannata 2021 {published data only}
- Limwannata P, Satirapoj B, Chotsriluecha S, Thimachai P, Supasyndh O. Effectiveness of renal‐specific oral nutritional supplements compared with diet counseling in malnourished hemodialysis patients. International Urology and Nephrology 2021;53:1675-87. [DOI] [PubMed] [Google Scholar]
- TCTR20200801001. The Nutritional Effect between ONCE Dialyze compare with diet counseling on malnourished hemodialysis patients. www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20200801001 2020.
Lin 2017 {published data only}
- Lin JX, Chen XW, Chen ZH, Huang XY, Yang JJ, Xing YF, et al. A multidisciplinary team approach for nutritional interventions conducted by specialist nurses in patients with advanced colorectal cancer undergoing chemotherapy: a clinical trial. Medicine 2017;96(26):e7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J-X, Fang H-Y, Chen Z-H, Duan M-Q, Lin Q. Single-center experience with nutrition intervention on patients with advanced colorectal cancer undergoing chemotherapy. Journal of Clinical Oncology 2016;34(15 suppl):e21615. [Google Scholar]
- Lin J-X, Yang JJ, Duan M-Q, Huang XY, Lin ZX. A study of nutritional improvement in colorectal cancer patients with chemotherapy after nutritional therapy leading by specialized nurse teams. Supportive Care in Cancer 2016;24(Suppl 1):S142-3. [Google Scholar]
Liu 2018 {published data only}
- Liu X, Du H, Huo X, Jiang H. Effect of nurse-led intensive nutritional intervention on nutritional risk, undernutrition and quality of life in hospitalized patients with Alzheimer disease: A randomized controlled study. Chinese Journal of Clinical Nutrition 2018;26(4):197-201. [Google Scholar]
- NCT03488329. Effect of nutritional efforts on discharged elderly patients. clinicaltrials.gov/show/NCT03488329 (date first posted 05 Apr 2018).
Liu 2019 {published data only}
- Liu C, Du H, Liu X. Effect of nutritional support based on clinical nursing pathway on nutritional status and quality of life among elderly inpatients with Alzheimer disease. Chinese Journal of Clinical Nutrition 2019;27(5):287-92. [Google Scholar]
Loser 2021 {published data only}
- DRKS00016862. Nutritional Aspects in Head and Neck Cancer Patients undergoing Radio(chemo)therapy. www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00016862.
- Loser A, Abel J, Kutz LM, Krause L, Finger A, Greinert F, et al. Head and neck cancer patients under (chemo-) radiotherapy undergoing nutritional intervention: results from the prospective HEADNUT-trial. Radiotherapy and Oncology 2021;159:82-90. [DOI] [PubMed] [Google Scholar]
Maharshi 2016 {published data only}
- Maharshi S, Sharma BC, Sachdeva S, Srivastava S, Sharma P. Efficacy of nutritional therapy for patients with cirrhosis and minimal hepatic encephalopathy in a randomised trial. Clinical Gastroenterology and Hepatology 2016;14:454-60. [DOI] [PubMed] [Google Scholar]
- Maharshi S, Sharma BC, Singh JP, Sriastava S, Mantri AK. Effects of nutritional therapy on complications and outcome in cirrhosis-randomized trial. Hepatology International 2016;10(1):S387. [Google Scholar]
Meng 2021 {published data only}
- ChiCTR1900026630. Application of oral nutrition supplements in patients at nutritional risk after surgery for gastric cancer: a prospective, randomized, control clinical trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900026630 2019.
- ChiCTR2000029322. Impact of oral nutritional supplements in post-discharge patients at nutritional risk after surgery for colorectal cancer: a randomised clinical trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000029322 2020.
- ChiCTR2000029708. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000029708 2020.
- ChiCTR-IIR-17011243. Nutrition support treatment by Nutren to improve the nutritional status of head and neck cancer patients accepted chemoradiotherapy and the influence to patients’ psychological status, quality of life and the prognosis: a prospective randomized controlled clinical study. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IIR-17011243 2017.
- Meng Q, Tan S, Jiang Y, Xi JHQ, Zhuang Q, Wu G. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clinical Nutrition 2021;40:40-46. [DOI] [PubMed] [Google Scholar]
- Tan S, Meng Q, Jiang U, Zhuang Q, Xu QXJ, Xhoa J, Sui X, Wu G. Impact of oral nutriitonal supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: a randomised clinical trial. Clinical Nutrition 2021;40:47-53. [DOI] [PubMed] [Google Scholar]
Molassiotis 2021 {published data only}
- ACTRN12618001352291. A patient-family-centered intervention to promote nutrition In advanced cancer patients: a pilot randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375642 2018.
- Molassiotis A, Brown T, Cheng HL, Byrnes A, Chan RJ, Wyld D, et al. The effects of a family-centered psychosicial-based nutriiton intervention in patients with advanced cancer: the PiCNIC2 pilot randomised controlled trial. Nutrition Journal 2021;20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Movahed 2020 {published data only}
- Movahed S, Toussi MS, Pahlavani N, Motlagh AF, Eslami S, Nematy M, et al. Effects of medical nutrition therapy compared with general nutritional advice on nutritional status and nutrition-related complications in esophageal cancer patients receiving concurrent chemoradiation: a randomized controlled trial. Mediterranean Journal of Nutrition and Metabolism 2020;13:265-76. [Google Scholar]
NCT01171495 {published data only}
- NCT01171495. Impact of nutrition intervention on HIV/AIDS infected patients. clinicaltrials.gov/show/NCT01171495 (date first posted 28 Jul 2010).
NCT02051777 {published data only}
- NCT02051777. Hospital discharge oral nutrition support trial. clinicaltrials.gov/show/NCT02051777 (date first posted 31 Jan 2014).
NCT02975089 {published data only}
- NCT02975089. A pilot study comparing effects of nutrients supplements and dietary approach in frailty management. clinicaltrials.gov/show/NCT02975089 (date first posted 29 Nov 2016).
NCT03631537 {published data only}
- NCT03631537. Nutrition-support-team based intervention in patients with advanced gastrointestinal cancer (NSTBIPAGC). clinicaltrials.gov/ct2/show/NCT03631537 (date first posted 15 Aug 2018).
NCT03632200 {published data only}
- NCT03632200. Nutritional course of care after surgical treatment at the patients affected by a cancer of the head and by the neck (NUTRIMAX). clinicaltrials.gov/show/NCT03632200 (date first posted 15 Aug 2018).
NCT03944161 {published data only}
- NCT03944161. Effectiveness and cost-effectiveness study of medical nutrition in malnourished patients in Spain. clinicaltrials.gov/show/NCT03944161 (date first posted 09 May 2019).
NCT04217564 {published data only}
- NCT04217564. Nutritional counselling for patients at Kasr AlAiny Multiple Sclerosis Clinic: an intervention study. clinicaltrials.gov/show/NCT04217564 (date first posted 03 Jan 2020).
Norshariza 2018 {published data only}
- NCT03688646. Efficacy of ONS supplementation in HNC outpatient under treatment. clinicaltrials.gov/show/NCT03688646 (date first posted 28 Sep 2018).
- Neoh MK, Zaid ZA, Daud ZAM, Yusop NBM, Ibrahim Z, Rahman ZA, Jamhuir N. Changes in nutrition impact symptoms, nutritional and functional status during head and neck cancer treatment. Nutrients 2020;12:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norshariza J, Zuriati I, Salina AZ, Azuan MDZ, Baizura MYN, Sharina MH, et al. Effectiveness of oral nutrition supplement on nutritional outcomes among head and neck cancer patients attending radiotherapy clinic. In: Annals of Nutrition & Metabolism. Vol. 75. 2019:56-6.
Nyguyen 2020 {published data only}
- Nguyen HT, Pavey TG, Collins PF, Nguyen NV, Pham TD, Gallegos D. Effectiveness of tailored dietary counseling in treating malnourished outpatients with chronic obstructive pulmonary disease: a randomized controlled trial. Journal of the Academy of Nutrition & Dietetics 2020;120(5):778-91. [DOI] [PubMed] [Google Scholar]
Otten 2016 {published data only}
- Otten L, Kiselev J, Franz K, Steinhagen-Thiessen E, Müller-Werdan U, Eckardt R, et al. Effect of a three month post-hospital nutritional intervention on functional performance in frail and malnourished older adults - a randomized controlled study. Clinical Nutrition 2016;35(1 Suppl):S161. [Google Scholar]
Park 2012 {published data only}
- Park KO, Choi-Kwon, S. Effects of individualised nutritonal education programs on the level of nutrient intake and nutritional status of colorectal cancer patients undergoing palliative chemotherapy. Journal of Korean Academy of Nursing 2012;42(6):799-809. [DOI] [PubMed] [Google Scholar]
Pinto 2021 {published data only}
- Pinto E, Nardi MT, Marchi R, Cavallin F, Alfieri R, Saadeh L, et al. QOLEC2: a randomized controlled trial on nutritional and respiratory counseling after esophagectomy for cancer. Supportive Care in Cancer 2021;29:1025-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qui 2020 {published data only}
- ChiCTR1900020543. Effect of whole-course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: a randomized control trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900020543. [DOI] [PubMed]
- Qui U, You J, Wang K, Cao Y, Hu Y, Zhang H, et al. Effect of whole course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: a randomised control trial. Nutrition 2020;69:110558. [DOI] [PubMed] [Google Scholar]
RBR‐35kjvg {published data only}
- RBR-35kjvg. Effects of intensive nutritional counseling on nutritional status and quality of life of patients with head and neck cancer undergoing radiation therapy. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-35kjvg 2017.
RBR‐3shhxs {published data only}
- RBR-3shhxs. Dietary action on the side effects resulting from chemotherapy and quality of life in patients with breast cancer during chemotherapy. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-3shhxs 2018.
Reinders 2020 {published data only}
- NCT03712306. The (cost)effectiveness of increasing protein intake on physical funtioning in older adults. clinicaltrials.gov/show/NCT03712306 (date first posted 19 Oct 2018).
- NTR6849. PILOT STUDY 2 'Prevention Of Malnutrition In Senior Subjects (PROMISS)'. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR6849 2017.
- NTR6915. PILOT STUDY 1 'Prevention Of Malnutrition in Senior Subjects (PROMISS)'. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR6915 2017.
- Reinders E, Visser M, Wijnhoven HAH. Two dietary advice strategies to increase protein intake among community-dwelling older adults: a feasibility study. Clinical Nutrition ESPEN 2020;37:157-67. [DOI] [PubMed] [Google Scholar]
- Reinders I, Wijnhoven HA, Jyvakorpi SK, Suominen MH, Niskanen R, Bosmans JE, et al. Effectivenss and cost effectiveness of personalised dietary advice aiming at increasing protein intake on physical functioning in community-dwelling older adults with lower habitual protein intake: rationale and design of the PROMISS randomised controlled trial. BMJ Open 2020;10:e040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahathevan 2018 {published data only}
- Sahathevan S, Se C-H, Ng S, Khor B-H, Chinna K, Goh BL, et al. Clinical efficacy and feasibility of whey protein isolates supplementation in malnourished peritoneal dialysis patients: A multicenter, parallel, open-label randomized controlled trial. Clinical Nutrition ESPEN 2018;25:68-77. [DOI] [PubMed] [Google Scholar]
Salem 2020 {published data only}
- Salem KM, Nassar MK, El-Savakhawi DH, Elshahat O, Amin MN, Sayed-Ahmed N, et al. The impact of nutritional education on phosphorus control in hemodialysis patients. Nephrology Dialysis Transplantation 2020;35(3 Supplement 3):Abstract no. P0927. [Google Scholar]
Sathiaraj 2020 {published data only}
- Sathiaraj E, Afshan K, Rangaraj S, Biswas S, Mufti SS, Naik R. Effects of nutrition intervention using nutrition care process on fatigue in patients undergoing chemotherapy – a randomized controlled pilot study. Clinical Nutrition ESPEN 2020;40:P423. [Google Scholar]
Schuetz 2019 {published data only}
- Bargetzi L, Schutz P, Muller B, Bargetzi A, Hersberger L on behalf of EFFORT study group. Association of different cancer types and benefit from nutritional support in patients at nutritional risk: secondary analysis of a prospective randomized trial. In: Clinical Nutrition ESPEN. Vol. 40. 2020:P559.
- Hersberger L, Burgler H, Dietz A, Bargetzi L, Bargetzi A on behalf of the EFFORT study group. Individualized nutritional support in congestive heart failure inpatients at nutritional risk secondary analysis of a randomized clinical trial. In: Clinical Nutrition ESPEN. Vol. 40. 2020:P414.
- Kaigi-Braun N, Tribolet P, Gomes F, Fehr R, Baechli V, Geiser M, Deiss M, Kutz A, Bregenzer T, Hoess C, Pavlicek V, Schmid S, Bilz S, Sigrist S, Brandle M, Benz C, Henzen C, Mattmann S, Thomann R, Rutishauser J, Aujesky D, Rodondi N, Donze J, Stanga Z, Bueller B, Schuetz P. Six-month outcomes after individualized nutritional support during the hospital stay in medical patients at nutritional risk: secondary analysis of a prospective randomized tria. Clinical Nutrition 2021;40:812-819. [DOI] [PubMed] [Google Scholar]
- Merker M, Felder M, Gueissaz L. Does inflammation influence the response to nutritional therapy in patients with disease-related malnutrition? - secondary analysis of a randomized multicenter trial. Critical Care 2019;23(Suppl 2):117. [ABSTRACT NO.: P271] [Google Scholar]
- NCT02517476. Effect of Early Nutritional Therapy on Frailty, Functional Outcomes and Recovery of Undernourished Medical Inpatients Trial. https://ClinicalTrials.gov/show/NCT02517476 2014.
- Schuetz P, Fehr R, Vaechli V, Geiser M, Diess M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet 2109;393:2312-21. [DOI] [PubMed] [Google Scholar]
- Schuetz P, Sulo S, Walzer S, Vollmer L, Stanga Z, Gomes F, et al. Economic evaluation of individualised nutritional support in medical inpatients: Secondary analysis of the EFFORT Trial. Clinical Nutrition 2019;38:S310-1. [DOI] [PubMed] [Google Scholar]
- Schuetz P, Sulo S, Walzer S, Vollmer L, Stanga Z, Gomes F, Rueda R, Partridge J. PAM3 INDIVIDUALIZED NUTRITIONAL SUPPORT IN MEDICAL INPATIENTS RESULTS IN COST SAVINGS: ECONOMIC EVALUATION OF THE EFFORT TRIAL. In: Value in Health. Vol. 22, Suppl 3. 2019:S415.
Shadid 2019 {published data only}
- Shadid T, Kalyani N, Modak Das S, Mukherjee M, Battacharya J, De A, et al. To compare outcome of Intensive nutritional support with standard practise in head ands neck cancer. Radiotherapy and Oncology 2019;133:S642. [Google Scholar]
Shatenstein 2017 {published data only}
- Shatenstein B, Kergoat M, Reid I, Chicoine ME. Dietary intervention in older adults with early-stage Alzheimer dementia: early lessons learned. Journal of Nutrition, Health and Aging 2008;12(7):461-9. [DOI] [PubMed] [Google Scholar]
- Shatenstein B, Kergoat MJ, Reid I. Outcome of a targeted nutritional intervention among older adults with early-stage Alzheimer's disease: the Nutrition Intervention Study. Journal of Applied Gerontology 2017;36(7):782-807. [DOI] [PubMed] [Google Scholar]
- Silva P, Kergoat MJ, Shatenstein B. Challenges of managing the diet of older adults with early-stage Alzheimer dementia: a care-giver perspective. Journal of Nutrition, Health and Aging 2013;17(2):142-7. [DOI] [PubMed] [Google Scholar]
Smith 2020 {published data only}
- ISRCTN26004104. Oral nutritional supplements and dietary advice versus dietary advice alone on clinical outcomes in malnourished community dwelling individuals. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN26004104 2012.
- Smith T, Cawood AL, Guildford N, Stratton RJ. Randomised trial shows low volume energy dense oral nutritional supplements improve total nutritional intake with little suppression of food intake in malnourished free living older people. Clinical Nutrition 2017;36 Suppl 1:S7-8. [Google Scholar]
- Smith TR, Cawood AL, Walter ER, Guildford N, Stratton RJ. Ready-made oral nutritional supplements improve nutritional outcomes and reduce health care use - a randomised trial in older malnourished people in primary care. Nutrients 2020;12:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soderstrom 2020 {published data only}
- NCT01057914. Effects of nutritional supplementation and dietary advice in elderly patients after hospital discharge. clinicaltrials.gov/show/NCT01057914 (date first posted 28 Jan 2010).
- Soderstrom L, Rosenblad A, Bergkvist L, Frid H, Adolfsson ET. Dietary advice and oral nutritional supplements do not increase survival in older malnourished adults: a multicentre randomised controlled trial. Upsala Journal of Medical Sciences 2020;125(3):240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stratton 2007 {published data only}
- Stratton RJ, Bowyer G, Elia M. Fewer complications with liquid supplements that food snacks in fracture patients at risk of malnutrition. Clinical Nutrition 2007;9 Suppl 2:9 (0014). [Google Scholar]
- Stratton RJ, Bowyer G, Elia M. Food snacks or liquid oral nutritional supplements as a first line treatment for malnutrition in post operative patients. In: Nutrition Society. Vol. 65. 2006:Abstract 4a.
- Stratton RJ, Bowyer G, Elia M. Greater total energy and protein intakes with liquid supplements than food snacks in patients at risk of malnutrition. In: Proceedings of ESPEN XXVIIIth Congress; 2006 Oct 19-22; Istanbul, Turkey. 2006:158.
Sudarsanam 2011 {published data only}
- CTRI/2010/091/006112. Randomized trial of diet supplementation in HIV and tuberculosis. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2010/091/006112 2010.
- Sudarsanam TD, John J, Kang G, Mahendri V, Gerrior J, Franciosa M, et al. Pilot randomized trial of nutritional supplementation in patients with tuberculosis and HIV-tuberculosis coinfection receiving directly observed short-course chemotherapy for tuberculosis. Tropical Medicine International Health 2011;16(6):699-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sui 2020 {published data only}
- Sui J, Zhang H, Wang Z, Li X. Effects of whole- course MDT nutrition management on radiotherapy in elderly patients with esophageal cancer. Chinese Journal of Clinical Oncology 2020;47(1):29-33. [Google Scholar]
Tharun 2020 {published data only}
- Tharun O, David M, Jijo V, Atul H, Premaletha N, Krishnadas D. The effectiveness of telephonic reinforcement of nutritional counselling in reducing mortality and complications of decompensated chronic liver disease. a randomised control trial. Hepatology International 2020;14(suppl 1):S428-9. [Google Scholar]
- Tharun T, Krishnadas D. The role of telephonic reinforcement of nutritional counselling as an intervention to reduce mortality and complications of cirrhosis. a randomized control trial. Hepatology 2020;72(Suppl 1):1085A. [Google Scholar]
Torbergsen 2019 {published data only}
- Torbergsen AC, Watne LO, Frojagem F Wyller TB, Mowe M. Effects of nutritional intervention upon bone turnover in elderly hip fracture patients. Randomized controlled trial. Clinical Nutrition ESPEN 2018;29:52-8. [DOI] [PubMed] [Google Scholar]
Touger Decker 1997 {published data only}
- Touger Decker R, Schaeffer M, Flinton R, Steinberg L. Impact of diet counseling and diet and nutrition status post-denture. Journal of Dental Research 1997;76 (IADR Abstracts):Special Abstract ue 1. [ABSTRACT NO.: 1267] [Google Scholar]
UMIN000032234 {published data only}
- UMIN000032234. Utility of nutritional intervention for elderly dialysis patients. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000032234 2018.
van der Werf 2020 {published data only}
- NCT01998152. Individualized nutritional counselling during chemotherapy for colorectal cancer (COLONUT). clinicaltrials.gov/show/NCT01998152 (date fist posted 28 Nov 2013).
- Werf A, Blauwhoff-Buskermolen S, Lanjius JA, Berkhoff J, Verheul HM, Schueren MA. The effect of individualized NUTritional counseling on muscle mass and treatment outcome in patients with metastatic COLOrectal cancer undergoing chemotherapy: a randomized controlled trial protocol. BMC Cancer 2015;15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werf A, Langius JA, Beeker A, ten Tije AJ, Vulnik AF, Haringhuizen A, et al. The effect of nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: a randomized controlled trial. Clinical Nutrition 2020;39:3005-13. [DOI] [PubMed] [Google Scholar]
Vazquez‐Sanchez 2019 {published data only}
- Vazquez-Sanchez MA, Valero-Cantero I, Carrion-Velasco Y, Castro-Lopez P, Suarez-adenas E, Casals C. Applicability and clinical validity of nursing outcomes classification in a nursing intervention of nutritional counseling for patients with malnutrition. International Journal of Nursing Knowledge 2019;30(3):168-72. [DOI] [PubMed] [Google Scholar]
Verho 2017 {published data only}
- Verho J, Puranen T, Suominen M. Tailored nutritional guidance has positive effect on energy and protein intake of geriatric patients after discharge: a randomized controlled trial. European Geriatric Medicine 2017;8:S28. [Google Scholar]
Wills 2019 {published data only}
- Wills AM, Garry J, Hubbard J, Mezoian T, Breen CT, Ortiz-Miller C, et al. Nutritional counseling with or without mobile health technology: a randomized open-label standard-of-care-controlled trial in ALS. BMC Neurology 2019;19(104):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills AM, Garry J, Hubbard J, Mezoian T, Zie J, Breen C, et al. Effects of dietary counseling on nutrient consumption from the electronic health application to measure outcomes remotely (EAT MORE) study. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2017;18(Suppl 2):300. [ABSTRACT NO.: RNM-20] [Google Scholar]
Wu 2018 {published data only}
- Wu S-Y, Hsu L-L, Hsu C-C, Hsieh T-J, Su S-C, Peng Y-W, et al. Dietary education with customised dishware and food supplements can reduce frailty and improve mental well-being in elderly people: A single-blind randomized controlled study. Asia Pacific Journal of Clinical Nutrition 27;5:1018-30. [DOI] [PubMed] [Google Scholar]
Yang 2019 {published data only}
- NCT04160819. The effects of individualized nutritional intervention program in malnutrition elderly with pneumonia. clinicaltrials.gov/show/NCT04160819 (first posted 13 Nov 2019).
- Yang P-H, Ln M-C, Liu Y-Y, Lee C-L, Chang N-J. Effect of nutritional intervention programs on nutritional status and readmission rate in malnourished older adults with pneumonia: a randomized control trial. Environmental Research and Public Health 2019;16:4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2020 {published data only}
Zhang 2018b {published data only}
- Zhang T, Huang H, Hu Q, Yin F. Clinical efficacy of FOLFOX chemotherapy combined with nutritional support in the treatment of gastrointestinal malignancies. Anti Tumour Pharmacy 2018;8(4):635-8. [Google Scholar]
Zhou 2011 {published data only}
- Zhou X-R, Yu K, Tang QQ. Effects of nutritional intervention and individualized nursing on nutritional risk, undernutrition, and quality of life in end-stage renal disease patients with peritoneal dialysis: a randomized controlled study. Chinese Journal of Clinical Nutrition 2011;19(4):222-6. [Google Scholar]
Zhu 2019 {published data only}
- Zhu M-W, Yang X, Xiu D-R, Yang Y, Li G-X, Hu W-G, et al. Effect of oral nutritional supplementation on the post-discharge nutritional status and quality of life of gastrointestinal cancer patients after surgery: a multi-center study. Asia Pacific Journal of Clinical Nutrition 2019;28(3):450-6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12612001253897 {published data only}
- Bjorkman MP, Suominen MH, Pitkala KH, Finne-Soveri HU, Tilvis RS. Porvoo sarcopenia and nutrition trial: effects of protein supplementation on functional performance in home-dwelling sarcopenic older people - study protocol for a randomized controlled trial. Trials 2013;14:387. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR2000028963 {published data only}
- ChiCTR2000028963. Prospective, randomized, controlled clinical trial for personalized nutritional intervention for head and neck cancer patients undergoing radiotherapy. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000028963 2020.
CTRI/2019/05/019387 {published data only}
- CTRI/2019/05/019387. Role of diet in patients with liver cirrhosis with muscle loss. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/05/019387.CTRI Trial Data 2019.
Munk 2020 {published data only}
- Munk T, Svendsen JA, Thomsen T, Rasmussen HH, Beck AM. A multimodal nutritional intervention after discharge improves qality of life and physical function in older patients. Clinical Nutrition ESPEN 2020;40:P425-6. [DOI] [PubMed] [Google Scholar]
- Munk T, Swendsen JA, Knudsen AW, Ostergaard TB, Beck AM. Effect of nutritional interventions on discharged older patients: a study protocol for a randomized controlled trial. Trials 2020;21(365):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03488329. Effect of nutritional efforts on discharged elderly patients. clinicaltrials.gov/show/NCT03488329 (date first posted 05 Apr 2018).
NCT02440165 {unpublished data only}
- NCT02440165. Basic care revisited: early nutrition intervention for outpatients [Basic care revisited: an early nursing nutrition intervention for outpatients in need of surgery]. clinicaltrials.gov/ct2/show/NCT02440165 (first posted 12 May 2015).
NCT02763904 {unpublished data only}
- NCT02763904. Systematic oral nutritional support in hospitalized, moderately hypophagic patients at nutritional risk [Systematic oral nutritional support in hospitalized, moderately hypophagic patients at nutritional risk receiving dietary counseling: a randomized, controlled trial]. clinicaltrials.gov/ct2/show/NCT02763904 (first posted 05 May 2016).
NCT02892747 {unpublished data only}
- NCT02892747. Dietetics education focused on malnutrition prevention (NUTRICOEUR) [Impact of dietetics education focused on prevention of malnutrition, on reducing morbidity and improving life quality of patients with chronic heart failure (CHF): a randomized controlled multicenter clinical trial]. clinicaltrials.gov/ct2/show/NCT02892747 (first posted 08 September 2016).
NCT03075189 {published data only}
- NCT03075189. Increased focus on protein intake among geriatric patients. clinicaltrials.gov/show/NCT03075189 (date first posted 09 Mar 2017).
NCT03114202 {published data only}
- NCT03114202. Effects of nutritional counseling on nutritional status and quality of life of head and neck cancer patients. clinicaltrials.gov/show/NCT03114202 (date first posted 14 Apr 2017).
NCT03191253 {unpublished data only}
- NCT03191253. Changing health through food support (CHEFS) Program [Changing Health Through Food Support (CHEFS): pilot RCT to Identify Potential Health Impacts of Providing Comprehensive, Medically-appropriate Food Support to Low-income Urban Adults Living With HIV (PLHIV)]. clinicaltrials.gov/ct2/show/NCT03191253 (first posted 19 June 2017).
NCT03315195 {unpublished data only}
- NCT03315195. Preoperative oral nutritional supplement versus conventional dietary advice in major gastrointestinal surgery [A randomized controlled trial comparing postoperative complication between preoperative oral nutritional supplement with dietary advice and conventional dietary advice alone in patients undergoing elective major gastrointestinal surgery]. clinicaltrials.gov/ct2/show/NCT03315195 (first posted 20 October 2017).
NCT03352388 {unpublished data only}
- NCT03352388. Effects of dairy- and berry-based snacks on nutritional and functional status and quality of life in older people (MAVIRE1) [Tailored food solutions for improving nutrition and well-being in older people. Effects on nutritional and functional status and quality of life in older people at home care]. clinicaltrials.gov/ct2/show/NCT03352388 (first posted 24 November 2017).
NCT03519139 {published data only}
- NCT03519139. Individual nutritional intervention for the prevention of readmission among geriatric patients. clinicaltrials.gov/show/NCT03519139 (date first posted 08 May 2018).
NCT03540784 {published data only}
- NCT03540784. Effects of WB-EMS and high protein diet in IBD patients. clinicaltrials.gov/ct2/show/NCT03540784 (date first posted 30 May 2018).
NCT03995303 {published data only}
- NCT03995303. HOMEFOOD Study - home delivered food and nutrition therapy for discharged geriatric hospital patient. clinicaltrials.gov/ct2/show/NCT03995303 (date first posted 24 Jun 2019).
NCT04628117 {published data only}
- NCT04628117. Effect of oral nutritional supplementation on oxidative stress in protein-energy wasting patients with peritoneal dialysis. clinicaltrials.gov/show/NCT04628117 (date first posted 13 Nov 2020).
PACTR201108000303396 {unpublished data only}
- Zulu RM, Byrne NM, Munthali GK, Chipeta J, Handema R, Musonda M, et al. Assessing the impact of a food supplement on the nutritional status and body composition of HIV-infected Zambian women on ARVs. BMC Public Health 2011;11:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Age Concern 2006
- Age Concern. Hungry to be heard. www.ageconcern.org.uk/ (accessed prior to 21 July 2020).
Avenell 2001
- Avenell A, Handoll HH, Grant AM. Lessons for search strategies from a systematic review, in The Cochrane Library, of nutritional supplementation trials in patients after hip fracture. American Journal of Clinical Nutrition 2001;73(3):505-10. [DOI] [PubMed] [Google Scholar]
Baldwin 2021
- Baldwin C, Smith R, Gibbs M, Weekes CE, Emery PW. What is the quality of the evidence supporting the role of oral nutritional supplements in the management of malnutrition? An overview of systematic reviews and meta-analyses.. Advances in Nutrition 2021;12(2):nmaa108. [DOI: 10.1093/advances/nmaa108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bavelaar 2008
- Bavelaar JW, Otter CD, Bodegraven AA, Thijs A, Bokhorst-de van der Schueren MA. Diagnosis and treatment of (disease-related) malnutrition: the performance of medical and nursing staff. Clinical Nutrition 2008;27:431-8. [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088-101. [PubMed] [Google Scholar]
Braunschweig 1999
- Braunschweig CA. Creating a clinical nutrition registry: prospects, problems and preliminary results. Journal American Dietetic Association 1999;99(4):467-70. [DOI] [PubMed] [Google Scholar]
Bruce 2003
- Bruce D, Laurance I, McGuiness M, Ridley M. Nutritional supplements after hip fracture: poor compliance limits effectiveness. Clinical Nutrition 2003;22(5):497-500. [DOI] [PubMed] [Google Scholar]
Carr 2001
- Carr AJ, Higginson IJ. Are quality of life measures patient-centred? BMJ 2001;322:1357-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cederholm 2016
- Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clinical Nutrition 2016;36(1):49-64. [DOI: 10.1016/j.clnu.2016.09.004] [DOI] [PubMed] [Google Scholar]
Cederholm 2019
- Cederholm T, Jensen GL, Correia MI, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clinical Nutrition 2019;38(1):1-9. [DOI: 10.1016/j.clnu.2018.08.002] [DOI] [PubMed] [Google Scholar]
DeSalvo 2006
- DeSalvo KB, Bloser N, Reynolds K, He J, Muntner PJ. Mortality prediction with a single, general self-rated health question among older adults: a meta-analysis. Journal of General Internal Medicine 2006;21(3):267-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
de van der Schueren 2019
- Bokhorst de van der Schueren MAE, Soeters PB, Reijven PLM, Allison SP, Kondrop J. 1.3 Diagnosis of malnutrition - screening and assessment. In Basics in Clinical Nutrition. 5th edition. Český Těšín, Czech Republic: Galén, 2019. [Google Scholar]
Dominick 2002
- Dominick KL, Ahern FM, Gold CH, Heller DA. Relationship of health-related quality of life to healthcare utilisation and mortality among older adults. Aging Clinical and Experimental Research 2002;14(6):499-508. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elia 2009
- Elia M, Russell CA. Nutrition screening survey in the UK in 2008. Hospitals, care homes and mental health units. www.bapen.org.uk/pdfs/nsw/nsw_report2008-09.pdf (accessed prior to 09 October 2018).
Elia 2015
- Elia M on behalf of the British association for Parenteral and Enteral Nutrition (BAPEN) and the National Institute for Health Research Southampton Biomedical Research Centre. The cost of malnutrition in England and potential cost-savings from nutritional interventions. A report on the cost of disease-related malnutrition in England and a budget impact analysis of implementing the NICE clinical guidelines/quality standard on nutritional support in adults; November 2015. Available at www.uhs.nhs.uk/Media/Southampton-Clinical-Research/BRCdownloads/ECONOM-REPORT-FULL-18.11.15.pdf.
Feinberg 2017
- Feinberg J, Nielsen EE, Korang SK, Halberg Engell K, Nielsen M, Zhang K, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD011598. [DOI: 10.1002/14651858.CD011598.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferris 2009
- Ferris GD, Bruera E, Cherny N, Cummings C, Currow D, Dudgeon D, et al. Palliative cancer care a decade later: accomplishments, the need and next steps - from the American Society of Clinical Oncology. Journal of Clinical Oncology 2009;27:3052-8. [DOI] [PubMed] [Google Scholar]
Gosney 2003
- Gosney M. Are we wasting our money on food supplements in elder care wards? Journal of Advanced Nursing 2003;43(3):275-80. [DOI] [PubMed] [Google Scholar]
Guest 2011
- Guest JF, Panca M, Baeyens JP, Man F, Ljungqvist O, Pichard C, Wait S, Wilson L. Health economic impactr of managing patients following a community-based diagnosis of malnutrition in the UK. Clinical Nutrition 2011;30:422-429. [DOI] [PubMed] [Google Scholar]
Guigoz 1997
- Guigoz Y, Vellas BJ. Malnutrition in the elderly: the Mini Nutritional Assessment (MNA). Therapeutische Umschau. Revue therapeutique 1997;54(6):345-50. [PubMed] [Google Scholar]
Hanger 1999
- Hanger HC, Smart EJ, Merrilees MJ, Frampton CM. The prevalence of malnutrition in elderly hip fracture patients. New Zealand Medical Journal 1999;112(1084):88-90. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hubbard 2012
- Hubbard GP, Elia M, Holdoway A, Stratton R. A systematic review of compliance to oral nutritional supplements. Clinical Nutrition 2012;31(3):293-312. [DOI] [PubMed] [Google Scholar]
Jensen 2010
- Jensen GL, Mirtallo J, Compher C, Dhailval R. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clinical Nutrition 2010;29(2):151-3. [DOI] [PubMed] [Google Scholar]
Jensen 2019
- Jensen GL, Cederholm T, Correia IT, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the Global Clinical Nutrition Community. Journal of Parenteral and Enteral Nutrition 2019;43(1):32-40. [DOI] [PubMed] [Google Scholar]
Keele 1997
- Keele AM, Bray MJ, Emery PW, Duncan HD, Silk DB. Two phase randomised controlled clinical trial of postoperative oral dietary supplements in surgical patients. Gut 1997;40(3):393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keys 1950
- Keys A, Brozek J, Henschel A. The Biology of Human Starvation. Mineapolis: University of Minnesota Press, 1950. [Google Scholar]
Khalatbari‐Soltani 2018
- Khalatbari-Soltani S, Marques-Vidal P. Adherence to hospital nutritional status monitoring and reporting guidelines. PLoS ONE 2018;13(9):e0204000. [DOI: 10.1371/journal.pone.0204000] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 1996
- King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Quality of Life Research 1996;5(6):555-67. [DOI] [PubMed] [Google Scholar]
Kubrak 2007
- Kubrak C, Jensen L. Malnutrition in acute care patients: a narrative review. International Journal of Nursing Studies 2007;44(6):1036-54. [DOI] [PubMed] [Google Scholar]
Kvamme 2011
- Kvamme JM, Olsen JA, Florholmen J, Jacobsen BK. Risk of malnutrition and health-related quality of life in community-living elderly men and women: The Tromso study.. Qual Life Res 2011;20:575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leij‐Halfwerk 2019
- Leij-Halfwerk S, Verwijs MH, Houdt S, Borkent JW, Guaitoli PR, Pelgrim T, et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults > 65 years: a systematic review and meta-analysis. Maturitas 2019;126:80-9. [DOI] [PubMed] [Google Scholar]
Ljungqvist 2010
- Ljungqvist O, Possum A, Sanz ML, Man F. The European fight against malnutrition. Clinical Nutrition 2010;29(2):149-50. [DOI] [PubMed] [Google Scholar]
McCormack 1997
- McCormack P. Undernutrition in the elderly population living at home in the community: a review of the literature. Journal of Advanced Nursing 1997;26(5):856-63. [DOI] [PubMed] [Google Scholar]
McWhirter 1994
- McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ 1994;308(6934):945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2018
- Mills SR, Wilcox CR, Ibrahim K, Roberts HC. Can fortified foods and snacks increase the energy and protein intake of hospitalised older patients? a systematic review. Journal of Human Nutrition and Dietetics 2018;31:379-89. [DOI] [PubMed] [Google Scholar]
Munk 2016
- Munk T, Tolstrup U, Beck AM, Holst M, Rasmussen HH, Hovhannisyan K, et al. Individualised dietary counselling for nutritionally at risk older patients following discharge from acute hospital to home: a systematic review and meta-analysis. Journal of Human Nutrition and Dietetics 2016;29:196-208. [DOI] [PubMed] [Google Scholar]
Munro 1998
- Munro J, Passey C, Brosman S, Margetts B, Rivers H (published in association with BAPEN 1998). Value-added benefits. Are nutritional supplements used effectively. Nursing Times 1998;94(4):59-60. [Google Scholar]
Naber 1997
- Naber TH, Schermer T, de-Bree A, Nusteling K, Eggink L, Kruimel JW, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. American Journal of Clinical Nutrition 1997;66(5):1232-9. [DOI] [PubMed] [Google Scholar]
NHS BSA
- NHS Business Services Authority. ePACT2. https://www.nhsbsa.nhs.uk/epact2.
NICE 2006
- National Collaborating Centre for Acute Care. Nutrition support in adults: oral supplements, enteral and parenteral feeding. www.nice.org.uk (accessed prior to 09 October 2018).
NICE 2012
- NICE National Institute for Health and Care Excellence. Nutrition support in adults. Quality standard [QS24]. Available at www.nice.org.uk/guidance/qs24/resources 2012.
Norman 2008a
- Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clinical Nutrition 2008;27(1):5-15. [DOI] [PubMed] [Google Scholar]
Pawson 2005
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review - a new method of systematic review designed for complex policy interventions. Journal of Health Services Research & Policy 2005;10 Suppl 1:21-34. [DOI] [PubMed] [Google Scholar]
Peake 1998a
- Peake HJ, Evans WC, Maltby AA, Bartram J, Frost G. Determining the incidence of malnutrition to plan and target nutritional support strategies. British Association of Parenteral and Enteral Nutrition (BAPEN) 1998. [ABSTRACT: OC28]
Peake 1998b
- Peake HJ, Evans S, Chambers A, Riches C, Frost CG. Nutritional supplementation: how much do people drink. In: Proceedings of the Nutrition Society. Vol. 57. 1998:94a.
Philipson 2013
- Philipson TJ, Snider JT, Lakdawalla DN, Stryckman B, Goldman DP. Impact of oral nutritional supplementation on hospital outcomes. American Journal of Managed Care 2013;19(2):121-8. [PubMed] [Google Scholar]
Prieto 1996
- Prieto RM, Garcia YC, Gordon-del RA, Redel-del PJ, Arevalo JE. Incidence of malnutrition in surgical departments of the Reina Sofia hospital of Cordoba. Nutrition Hospital 1996;11:286-90. [PubMed] [Google Scholar]
Ravasco 2012
- Ravasco P, Monteiro-Grillo I, Camilo M. Individualized nutrition intervention is of major benefit to coloretal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. American Journal of Clinical Nutrition 2012;96(6):1346-53. [DOI] [PubMed] [Google Scholar]
Reinders 2019
- Reinders I, Volkert D, Groot LC, Beck AM, Feldblum I, Jobse I, et al. Effectiveness of nutritional interventions in older adults at risk of malnutrition across different healthcare settings: pooled analyses of individual participant data from nine randomised controlled trials. Clinical Nutrition 2019;38:1797-1806. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schilp 2012
- Schilp J, Kruizenga HM, Wifnhoven HA, Leistra E, Evers AM, Binsbergen JJ, et al. High prevalence of undernutrition in Dutch community-dwelling older individuals. Nutrition 2012;28(11-12):1151-6. [DOI] [PubMed] [Google Scholar]
Schuetz 2019
- Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet 2019;393(10188):2312-21. [DOI: 10.1016/S0140-6736(18)32776-4] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schunemann 2006
- Schunemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 13. Applicability, transferability and adaptation. Health Research Policy & Systems 2006;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snider 2014
- Snider JT, Linthicum MT, Wu Y, LaVallee C, Lakdawalla DN, Hegazi R, et al. Economic burden of community-based disease-associated malnutrition in the United States.. Journal of Parenteral and Enteral Nutrition 2014;38(2 Suppl):77s-85s. [DOI] [PubMed] [Google Scholar]
Temel 2010
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New England Journal of Medicine 2010;363:733-42. [DOI] [PubMed] [Google Scholar]
Volkert 2010
- Volkert D, Saeglitz C, Gueldenzoph H, Sieber CC, Stehle P. Undiagnosed malnutrition and nutrition-related problems in geriatric patients. Journal of Nutrition Health and Aging 2010;14(5):387-92. [DOI] [PubMed] [Google Scholar]
Watson 1998
- Watson JL. The prevalence of malnutrition in patients admitted to Care of the Elderly wards. In: British Association of Parenteral and Enteral Nutrition (BAPEN). 1998:PC50.
Weekes 1998
- Weekes E. The incidence of malnutrition in medical patients admitted to a hospital in south London. In: British Association of Parenteral and Enteral Nutrition (BAPEN). 1998:PC2.
Wilson 2013
- Wilson L. A review and summary of the impact of malnutrition in older people and the reported costs and benefits of interventions. A report from The Malnutrition Task Force and International Longevity Centre https://ilcuk.org.uk/wp-content/uploads/2019/01/Costs-Benefits_Report_Jun13.pdf May 2013.
References to other published versions of this review
Baldwin 2001
- Baldwin C, Parsons T, Logan S. Dietary advice for illness-related malnutrition in adults. Cochrane Database of Systematic Reviews 2001, Issue 2. Art. No: CD002008. [DOI: 10.1002/14651858.CD002008] [DOI] [PubMed] [Google Scholar]
Baldwin 2006
- Baldwin C, Parsons T, Logan S. Dietary advice for illness-related malnutrition in adults. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD002008. [DOI: 10.1002/14651858.CD002008] [DOI] [PubMed] [Google Scholar]
Baldwin 2008
- Baldwin C, Weekes CE. Dietary advice for illness-related malnutrition in adults. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD002008. [DOI: 10.1002/14651858.CD002008.pub3] [DOI] [PubMed] [Google Scholar]
Baldwin 2009
- Baldwin C, Weekes CE. Dietary advice for illness-related malnutrition in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD002008. [DOI: 10.1002/14651858.CD002008.pub3] [DOI] [PubMed] [Google Scholar]
Baldwin 2011
- Baldwin C, Weekes CE. Dietary advice with or without oral nutritional supplements for disease-related malnutrition in adults. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD002008. [DOI: 10.1002/14651858.CD002008.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


