Abstract
Introduction:
Continuous neurological assessment in the pediatric intensive care unit (PICU) is challenging. Current electroencephalography (EEG) guidelines support monitoring status epilepticus, vasospasm detection, and cardiac arrest prognostication, but the scope of brain dysfunction in critically-ill patients is larger. We explore quantitative electroencephalography (QEEG) in PICU patients with neurological emergencies to identify QEEG changes preceding clinical detection.
Methods:
From 2017 to 2020, we identified PICU patients at a single quaternary children’s hospital with EEG recording near or during acute neurological deterioration. QEEG analysis was performed using Persyst P14 (Persyst Development Corporation). Included features were FFT, asymmetry, and rhythmicity spectrograms, “from-baseline” patient-specific versions of above features, and quantitative suppression ratio. Timing of QEEG changes was determined by expert review and prespecified QEEG alert thresholds. Clinical detection of neurologic deterioration was defined pre-hoc and determined by Electronic Medical Record documentation of exam change or intervention.
Results:
10 patients were identified, age 23 months to 27 years, 50% female. Of 10 patients, 6 died, 1 had new morbidity, and 3 had good recovery; the most common cause of death was cerebral edema and herniation. The fastest changes were on “from-baseline” FFT spectrograms, while persistent changes on asymmetry spectrograms and suppression ratio were most associated with morbidity and mortality. Median time from first QEEG change to clinical detection was 332 min (Interquartile range: 201 -- 456 min).
Conclusion:
Quantitative EEG is potentially useful in earlier detection of neurological deterioration in critically-ill PICU patients. Further work is required to quantify the predictive value, measure improvement in outcome, and automate the process.
Keywords: Electroencephalography, Neurological injury, Pediatrics, Early Diagnosis
Introduction
Continuous electroencephalography (cEEG) is a mainstay of non-invasive neuromonitoring in the Pediatric Intensive Care Unit (PICU) but the American Clinical Neurophysiology Society (ACNS) indications have primarily been focused on diagnosis and treatment monitoring of non-convulsive status epilepticus (NCSE) or seizures, identification of ischemia, and monitoring of level of consciousness for sedation (1, 2, 3, 4, 5). There is growing interest in the use of cEEG as a non-invasive monitor with high temporal resolution for detection of acute neurological injury, particularly with focus on neurovascular injury and elevated intracranial pressure (ICP) (6, 7).
Advantages of quantitative EEG (QEEG) include the ability to view trends over minutes or hours compressed on a single page and increased interpretability by non-neurology-trained bedside staff (8, 9). While manual review of the cEEG record is the mainstay of monitoring, clinical volume often dictates that it cannot be done real-time and must be done intermittently (at our institution, every two hours). A recent commercially-available development is “from-baseline” analysis, which allows the interpreter to select any portion of recording as the patient’s baseline and measure statistical deviations from that baseline. However, QEEG investigations have largely focused on seizure detection and recognition of delayed cortical ischemia following subarachnoid hemorrhage and not on other causes of acute neurological injury (10).
We explored QEEG analysis in a series of critically ill PICU patients with neurological deterioration of various etiologies that occurred during concurrent EEG monitoring. We hypothesized that QEEG analysis demonstrates earlier detection of pathology compared to clinical changes and conventional EEG review practices.
Patients and Methods
The University of Pittsburgh Institutional Review Board approved the study. The study cohort was collected by manual review of a convenience set of patients with acute neurological deterioration and concurrent cEEG monitoring in the PICU at a single quaternary care children’s hospital between 2017 and 2020. Neurological deterioration was defined as the development of a new injury with either permanent neuronal death or the likelihood of permanent cell death without intervention. Raw EEG recordings were comprehensively reviewed by a board-certified epileptologist (C.M.P.). Subsequent QEEG was reviewed by another board-certified electroencephalographer (M.L.S.) and two child neurologists (N.K.M, I.B.). Reported findings were agreed upon by reviewers with disagreement to be decided by majority vote. EEG technologists at our center review cEEG recordings at minimum every 2 hours, and timestamped notifications were extracted from the EEG record. All patients received continuous vital sign monitoring and hourly neurological checks.
Timing of clinical suspicion of neurological deterioration was determined by chart review of the Electronic Medical Record (EMR). Clinical metrics included worrisome changes in vital signs or neurological exam. This timing was defined as the first 1) documentation of acute change in neurologic exam, 2) STAT neuroimaging, or 3) medication administration for intervention of neurologic morbidity, either anti-epileptic or hyperosmolar agent. This definition represents the earliest EMR-documented time of concern and was chosen due to the challenges of determining timing of clinician suspicion through chart review, though may reflect a delay in the exact timing the clinical manifestation was discovered either through imprecision in the timing of documentation or delay from the time the imaging is requested to its acquisition.
QEEG tracings included bihemispheric rhythmicity spectrograms, fast Fourier transform (FFT) spectrograms, asymmetry spectrograms, aEEG, suppression ratio index, and “from-baseline” FFT analysis. “From-baseline” analysis measures the statistical deviation in z-scores of the power at each frequency of the FFT spectrograms and asymmetry spectrograms from an empirical null distribution determined for a baseline period set as the first 5 minutes of artifact-free recording using the artifact-detection trendline. It represents the degree of deviation of the spectrogram of each epoch compared to this baseline.
QEEG notifications, or alerts, were set as standard thresholds of persistent deviation longer than 120 seconds of at least 2 z-scores from the patient’s own baseline mean. There have been no prior validated parameters published for the notifications. These values were empirically determined from prior analysis to balance the conflicting goals of precision and sensitivity. Alerts were set for hemispheric and quadrant (anterior/posterior) analyses of the from-baseline FFT and asymmetry spectrograms, as well as the suppression ratio. Because the alerts were set using the patient’s baseline, we used the same parameters for all patients, regardless of age. We compared the timing of first QEEG change (as determined by expert review) to documented clinical change, as well as the timing of the first QEEG alert to the timestamped EEG technologist discovery.
Software versions were Natus NeuroWorks 7.1.1 for raw EEG, and Persyst versions 12 and 14. All features used are commercially available at the time of publication.
Data not published here will be anonymized and shared upon request from qualified investigators.
Results
We identified 10 PICU patients (age 1–27 years, 50% female) on CEEG during or immediately preceding neurologic deterioration during the study period. Six (75%) patients died in the ICU, one had major new morbidity (large left hemisphere infarct), and three made a complete clinical recovery. The most common etiology of deterioration was CNS infection (4 patients). The most common cause of death was herniation (4 patients). Six (60%) were intubated and sedated at the time of the deterioration. Two (20%) patients had seizures on CEEG in the 24 hours preceding the event.
Median time from first QEEG signal change to clinical change was 332 minutes (Interquartile range [IQR]: 201 -- 456 min). For 6 (60%) of patients the QEEG change as determined by expert review and automated QEEG notification occurred within 2 minutes of each other.
Corresponding raw EEG changes were noted in 70% of cases by the EEG technologist, with a median delay of 309 minutes (IQR: 94 -- 481 min) from the time of “from-baseline” automated alert. All documented changes on raw EEG analysis were seen by automated QEEG notification, and all of the earliest QEEG notifications were triggered on “from-baseline” features.
Table 1 lists clinical and QEEG findings for each patient. On QEEG analysis (Figure 1), 10/10 (100%) patients were noted to have changes in the FFT spectrogram, and this was consistently the earliest finding on QEEG. This was generally shown to be a dropout in power in the higher, then lower, frequencies. “From-baseline” analysis demonstrated subtle change of these trends earlier than visual inspection of the FFT spectrogram alone. Four (40%) of the patients also had important findings on asymmetry measures, and all four were related to initially unilateral injury. Six (60%) of the patients had a rise in suppression ratio in one or more hemispheres immediately preceding clinical recognition (Figure 2), which was associated with either diffuse cerebral injury or global anoxia in each case. Two of the 3 patients with full recovery had pre-deterioration EEG captured and demonstrated QEEG features that approached the baseline in the recovery phase; the third patient (patient 9) showed nearly resolved asymmetry after surgical intervention.
Table 1.
Quantitative EEG versus clinical and raw EEG detection of neurological deterioration
| id | Clinical vignette | Continuous EEG Background (pre-event) | First QEEG finding | Subsequent QEEG findings | Clinical findings | Time from first QEEG change to clinical recognition | Time from QEEG alert to raw EEG recognition | Outcome |
|---|---|---|---|---|---|---|---|---|
| Mortality | ||||||||
| 1 | 23-month-old female with history of spells, presents with encephalopathy of unclear etiology, herniation | Disorganized mixture of delta activity, no sleep architecture. No seizures. | Bilateral 6–12 Hz attenuation | Global 0–20 Hz attenuation, rising suppression ratio | Tachycardia, “vital sign instability” | 187 minutes | No notable changes recognized in EEG waveforms | Death by neurological criteria |
| 2 | 2-year-old male with history of TAPVR, sinus venous thrombosis, presents with adenoviral septic shock, progressive hemodynamic instability, in-hospital cardiac arrest | Disorganization, generalized (high amplitude delta activity), initially, leading to discontinuity, generalized, progressive. No sleep architecture. No seizures. | Bilateral anterior-predominant 0–4 Hz increase, 4–20 Hz decrease vs baseline | Progressive global 0–20 Hz attenuation and rise to high suppression ratio | Tachycardia, hypotension, wide-complex arrhythmia | 250 minutes | 310 minutes | Death after cardiac arrest |
| 3 | 3-year old male with remote TBI, presents with sepsis, S. pneumoniae meningitis, seizures, herniation | Poorly developed / modulated polymorphic delta slowing, partially developed sleep architecture. Tonic seizures >24 hours prior to event. | Asymmetry change, decrease in 8–12 Hz power L > R, increased R 0–4 Hz vs baseline | L then R rapid attenuation at all frequencies, rise in suppression ratio to 100% | Tachycardia, hypotension, disordered breathing | 462 minutes | 500 minutes | Death after comfort care pursued |
| 4 | 9-year old male with out-of-hospital cardiac arrest, cerebral edema, herniation | Diffuse delta frequency slowing, bilateral posterior voltage attenuation. No sleep architecture. No seizures. | Increased 0–6 Hz vs baseline | Rapid global 0–20 Hz attenuation, rise in suppression ratio to 100% | Absent brainstem reflexes on day 2 | 845 minutes | 460 minutes | Death after comfort care pursued |
| 5 | 17-year old female with metastatic rhabdomyosarcoma, leptomeningeal metastases, spontaneous subarachnoid hemorrhage, herniation | Disorganization, generalized, mixed frequencies; symmetric sleep spindles. No seizures. | Asymmetry change, increased power L 14–20 Hz, decreased R 0–10 Hz vs baseline | Increased asymmetry, L decreasing power 0–20 Hz, R 0–20 Hz moderate/severe attenuation, suppression ratio beginning to rise | Dilated, non-reactive pupils | 449 minutes | No notable changes recognized in EEG waveforms | Death after comfort care pursued |
| 6 | 27-year old female with leukemia status post bone marrow transplant with multi-organ failure, seizures, who develops massive air embolism | Diffuse generalized slowing and voltage attenuation; no sleep architecture. Focal electrographic seizures. | Global 0–20 Hz decreased power vs baseline | Rapid global 0–20 Hz severe attenuation and rise to high suppression ratio | Dilated, non-reactive pupils | 140 minutes | 77 minutes | Death after comfort care pursued |
| Morbidity | ||||||||
| 7 | 11-year-old female with metastatic CNS tumor status post shunt placement who presents with H. influenzae sepsis, meningitis, hemispheric infarction | Mild background slowing, 7 Hz posterior dominant rhythm; well-devleoped sleep architecture. Myoclonic and focal motor seizures. | On-and-off “stuttering” L 8–12 Hz attenuation vs baseline, rising asymmetry | Severe 0–20 Hz asymmetry due to L 0–20 Hz attenuation vs baseline | GCS dropped from 15 to 12 by bedside nurse exam. Patient not appropriately awake 3 days later | 244 minutes | 88 minutes | Survival with global developmental injury, right hemiparesis, aphasia |
| Survival with full recovery | ||||||||
| 8 | 6-year-old male with acute disseminated encephalomyelitis, Chiari I malformation | Inconsistent 5–8 Hz posterior dominant rhythm; moderately developed sleep architecture. No seizures. | 0–4 Hz increased signal, state fluctuations with some brief periods resembling baseline | More frequent epochs resembling baseline. Remote: Similar to baseline with some posterior increase in 10–20 Hz | Encephalopathy, no focal deficits, followed by gradual improvement over days | 1200 minutes | 670 minutes | Chiari decompression, immunotherapy, survival with inpatient rehabilitation and full recovery |
| 9 | 9-year-old male with traumatic left subdural hematoma | Diffuse delta frequency slowing, left hemisphere voltage attenuation. No sleep architecture. No seizures. | Baseline asymmetry (L decreased 0–20 Hz) | Post-evacuation decrease in asymmetry, improvement in 8–20 Hz power, decrease in 0–8 Hz power | Decreased mental status | 74 minutes | No notable changes recognized in EEG waveforms | Subdural evacuation, full clinical recovery |
| 10 | 11-year-old female with culture-negative meningoencephalitis | Diffuse theta/delta frequency slowing; poorly developed sleep spindles noted. One focal electrographic seizure. | Asymmetry change, R 4–10 Hz decreased power | R 4–20 Hz attenuation, bilateral 0–4 Hz increased power | Decreased mental status, poor airway protective reflexes | 420 minutes | 99 minutes | Full clinical recovery |
Abbreviations: TBI = Traumatic Brain Injury; QEEG = Quantitative Electroencephalography; GCS = Glasgow Coma Scale; TAPVR = Total Anomalous Pulmonary Venous Return
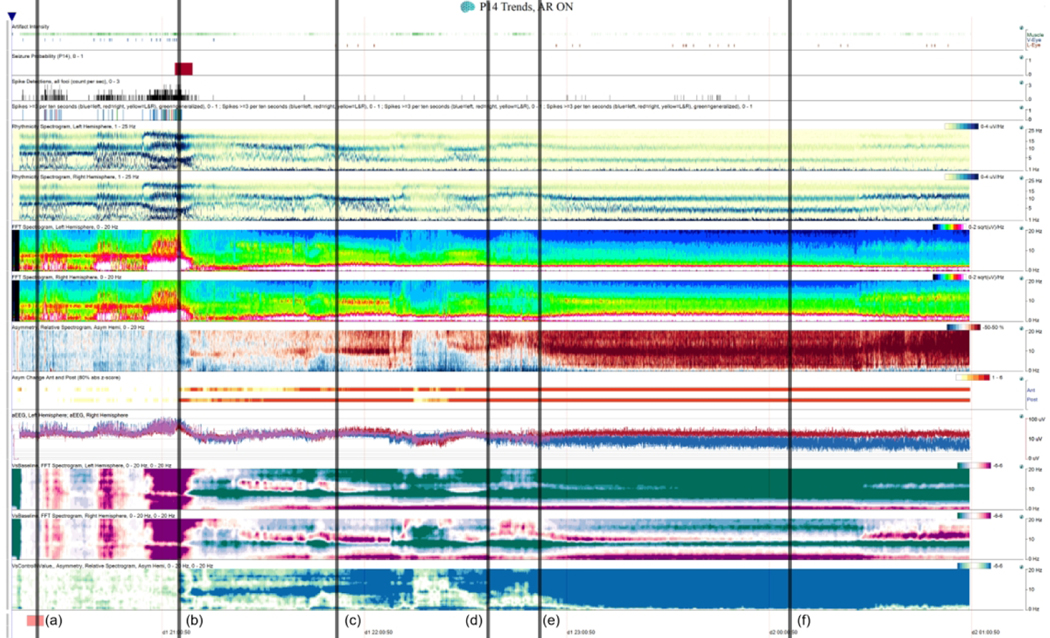
Figure 1. Patient 7 Quantitative EEG Display.
6-hour Quantitative EEG (QEEG) recording for patient 7 with Artifact Reduction On. QEEG features (top to bottom): Artifact Intensity, Seizure Probability, Spike Detection, Spike >=3 / 10 s, Rhythmicity Spectrograms (Left/Right), FFT Spectrogram (Left/Right), Asymmetry Spectrogram, Asymmetry changes (Anterior/Posterior), amplitude-integrated EEG, from-baseline FFT spectrogram (Left, Right), from-baseline Asymmetry Spectrogram. (a) Recording baseline (represented by the pink patch at the bottom) from which all “versus baseline” comparisons are being made. (b) Epoch of bihemispheric increased power leading up to a L hemispheric seizure detected by the Seizure Detector (top, panel 2–3) after which there is decreased bilateral (L > R) 8–12 Hz signal versus baseline (“VsControlNValue”). (c) Beginning of striking stuttering 8–20 Hz asymmetry. (d) EEG technologist first documents hemispheric attenuation on review. (e) Rapid progressive persistent asymmetry with L 0–20 Hz attenuation vs baseline. (f) Bedside nurse documents a change in Glasgow Coma Scale from 15 to 12, the first clinical change noted.
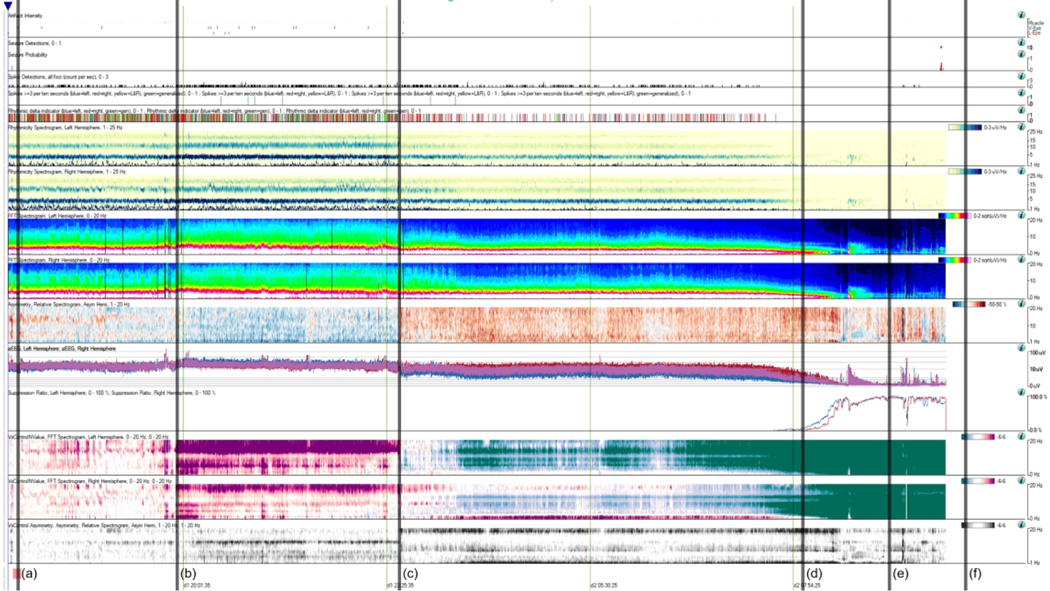
Figure 2. Patient 3 Quantitative EEG Display.
12-hour Quantitative EEG (QEEG) recording (with one 4-hour break) for patient 1 with Artifact Reduction On. Patient has a remote history of a severe penetrating Traumatic Brain Injury. (a) Recording baseline (represented by the pink patch at the bottom) from which all “versus baseline” comparisons are being made. (b) Bilateral increase in power vs baseline (“VsControlNValue”) L > R. (c) Disconnected for 4 hours. Interval head CT at 2230 with no change from baseline. On reconnection, asymmetry change noted, with L decreased power 4–20 Hz, R increased power 0–4 Hz. (d) 0730: Rapid progressive bilateral global attenuation of power 0–20 Hz, also seen in the amplitude-integrated EEG (aEEG). Rapid sustained rise in suppression ratio to 100%, near complete suppression. (e) 0800: First clinical change noted, persistent tachycardia, hypotension, and disordered breathing. (f) 1030: STAT Head CT done with no change from baseline. External Ventricular Drain (EVD) placed 6 hours later with markedly elevated intracranial pressure (>50 mmHg), and follow-up CT showed malignant cerebral edema and herniation.
Discussion
In this case series of PICU patients who experienced neurological deterioration, we report two primary findings. First, we described common QEEG features in these patients, including “from-baseline” analysis. Second, we demonstrated that carefully-set QEEG alerts might have produced earlier warning than conventional CEEG and standard care alone, sometimes by hours.
The underlying pathologies were heterogeneous, but several QEEG themes were evident. Decreased power at various frequencies in the FFT spectrogram was the most consistent and early change associated with acute neurological deterioration. However, historically these signals are poorly specific and can be seen transiently in benign situations such as normal state change (11). More specific for morbidity or mortality was sustained or intensifying new asymmetry, which is not expected in benign change of state or sedation administration and was only seen in this cohort in those who died or had a hemispheric infarct. The most specific QEEG pattern change for mortality, but latest to arise, was a spontaneous rise in suppression ratio of one or both hemispheres, which universally portended a poor outcome if sustained.
Advances in QEEG monitoring software now allow one to set a patient baseline, create trends showing deviations of QEEG parameters from that baseline, and set specific automated notification thresholds for “from-baseline” analyses. This process utilizes the patient’s own EEG patterns as control, allowing recognition of QEEG changes despite EEG abnormalities present at the start of recording, as seen with sedative drug effects or focal brain pathologies. While the baseline can be set automatically, it can also be set manually if a change in baseline is observed. In this case series most of the earliest signal changes were most clearly visible on these spectrograms relative to patient baseline, rather than in the absolute change on the FFT or asymmetry spectrograms. Given the heterogeneity in the patient population and EEG backgrounds, there is no set of pre-specified alarm thresholds that would have captured the deterioration in all of these patients on the absolute spectrograms.
Persistent QEEG findings were noted to precede clinical findings by multiple hours in most cases. Many of the patients were sedated and/or paralyzed, limiting the utility of the clinical neurological examination; additionally, even with hourly neurological checks an acute change may have delayed recognition. QEEG notifications universally preceded the EEG technologist’s recognition of deterioration on CEEG; these findings were confirmed by EEG experts to be challenging to detect on CEEG by real-time review. Even in the studies where QEEG and raw EEG findings were retrospectively felt to be simultaneous, the nature of intermittent CEEG review leads to delays in review by a physician or EEG technologist. A judiciously placed set of QEEG change notification triggers have the potential to provide earlier notification to the clinical team. In 60% of cases the first QEEG changes and the QEEG alert occurred almost simultaneously. In the remaining 40% the initial findings were subtle enough not to cross the QEEG alert threshold. The optimization of this threshold is non-trivial, as the cost of false-positives will need to be studied.
QEEG can also be used as a measure of reassurance. In the right clinical scenario, such as after initial treatment, epochs closer to baseline suggest lack of ongoing decline or possible improvement. Combined with other clinical evidence this could represent a source of reassurance for family and clinician alike and potentially prevent unnecessary testing or intervention.
Limitations
There are multiple limitations to this study. As a retrospective convenience sample of notable cases, further study will be needed to determine the incidence of false positive findings. The broad set of underlying illnesses may help with generalizability but makes it hard to draw conclusions about any specific illness. The specific set of features and the parameters for QEEG alert also need to be validated in prospective studies, as this series does not capture the false-positive rate for the alerts. Importantly, the optimal threshold of alert will need to be determined: too low and there will be much unnecessary and potentially harmful testing, but too high and some cases may be missed. Additionally, EEG features vary significantly across the age spectrum of pediatric patients, and though the from-baseline feature allows comparison to the same patient, the use of the same parameters to study neonates and teenagers or adults will need to be evaluated. Finally, further study will be needed to determine the effects of benign interventions on EEG background, such as repositioning, sedative medications, and re-setting EEG leads.
Future Directions
While some cases had irreversible causes of injury, further study will be essential to determine the degree to which early reversible brain injury can be rescued by a lead time of minutes to hours. Additionally, given the retrospective nature of patient selection, further work is needed to find the incidence of conditions that would benefit from QEEG monitoring, determine sensitivity and specificity of these findings, and demonstrate their predictive values.
Conclusion
QEEG and “from-baseline” notifications may have utility as a real-time monitoring tool to detect neurologic deterioration in critically ill PICU patients. Further work is necessary to quantify the predictive value of these markers, determine if timeliness of diagnosis or outcomes may be improved, perform cost/benefit analysis, and automate interpretation of the output.
Acknowledgments
Conflicts of Interest and Source of Funding: Neil Munjal receives support from NIH NICHD 5T32HD040686, University of Pittsburgh, Pittsburgh, PA, USA. Mark Scheuer is the Chief Medical Officer of Persyst Development Corporation. No funding was provided by Persyst Development Corporation. For the remaining authors, none were declared.
References
- 1.Herman ST, Abend NS, Bleck TP, et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part I: Indications. J Clin Neurophysiol. 2015; 32: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. EEG and Clin Neurophysiol. 1997; 103: 607–615. [DOI] [PubMed] [Google Scholar]
- 3.Scheuer ML. Continuous EEG Monitoring in the Intensive Care Unit. Epilepsia. 2002; 43: 114–127. [DOI] [PubMed] [Google Scholar]
- 4.Van Putten MJ, Hofmeijer J. EEG Monitoring in Cerebral Ischemia: Basic Concepts and Clinical Applications. J Clin Neurophysiol. 2016; 33(3): 203–210. [DOI] [PubMed] [Google Scholar]
- 5.Forman B, Claassen J. Quantitative EEG for the Detection of Brain Ischemia. 2012. Annual Update in Intensive Care and Emergency Medicine. Springer. 2012: 746–758. [Google Scholar]
- 6.Huguenard AL, Guerriero RM, Tomko SR, et al. Immediate Postoperative Electroencephalography Monitoring in Pediatric Moyamoya Disease and Syndrome. Pediatr Neurol. 2021; 118:40–45. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh ZB, Maciel CB, Dhakar MB, et al. Nonepileptic Electroencephalographic Correlates of Episodic Increases in Intracranial Pressure. J Clin Neurophysiol. 2020; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Dericioglu N, Yetim E, Bas DF, et al. Non-expert use of quantitative EEG displays for seizure identification in the adult neuro-intensive care unit. Epilepsy Res. 2015;109:48–56. [DOI] [PubMed] [Google Scholar]
- 9.Steward CP, et al. Seizure identification in the ICU using quantitative EEG displays. Neurology. 2010; 75 (17): 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer AH, Kromm J. Quantitative Continuous EEG: Bridging the Gap Between the ICU Bedside and the EEG Interpreter. Neurocrit Care. 2019; 30: 499–504. [DOI] [PubMed] [Google Scholar]
- 11.Kaskinoro K, Maksimow A, Georgiadis S, et al. Electroencephalogram reactivity to verbal command after dexmedetomidine, propofol and sevoflurane-induced unresponsiveness. Anaesthesia. 2014; 70(2), 190–204. [DOI] [PubMed] [Google Scholar]