Abstract
REDOX mechanisms that induce biosynthesis of the Reactive Oxygen Species (ROS) have attracted considerable attention due to both the deleterious and beneficial responses elicited by the reactive radical. In several organisms including Drosophila melanogaster, modulation of ROS activity is thought to be crucial for the maintenance of cell fates in developmental contexts. Interestingly, REDOX mechanisms have been shown to be involved in maintaining progenitor fate of stem cells as well as their proliferation and differentiation. Here we have explored the possible functions of ROS during proper specification and developmental progression of embryonic Primordial Germ Cells (PGCs). Indicating its potential involvement in these processes, ROS can be detected in the embryonic PGCs, and the surrounding somatic cells from very early stages of embryogenesis. Using both ‘loss’ and ‘gain’ of function mutations in two different components of the REDOX pathway, we show that ROS levels are likely to be critical in maintaining germ cell behavior, including their directed migration. Altering the activity of a putative regulator of ROS also adversely influences the ability of PGCs to adhere to one another in cellular blastoderm embryos, suggesting potential involvement of this pathway in orchestrating different phases of germ cell migration.
Introduction:
In Drosophila melanogaster, primordial germ cells (PGCs) i.e. pole cells, which are formed very early during embryogenesis serve as the precursors of the germline stem cells (GSCs). This transition is crucial as subsequently the GSCs give rise to the adult germline (Wilson & Macdonald, 1993; Williamson & Lehmann, 1996; Wylie, 1999; Mahowald, 2001; Santos & Lehmann, 2004). After the successive and synchronous nuclear division cycles the embryo undergoes cellularization at the end of nuclear cycle 14 (Mavrakis et al., 2009). Even prior to cellularization of the somatic nuclei, around nuclear division cycle 9/10, a few nuclei migrate into specialized cytoplasm also referred to as pole plasm. Maternally deposited pole plasm is anchored to the posterior pole. The release of the cortically anchored pole plasm is initiated by the entry of the centrosomes associated with the nuclei, ultimately leading to precocious cellularization of the PGCs (Lerit and Gavis, 2011; Lerit et al, 2017). The pole plasm consists of maternally derived components required to confer and sustain PGC identity. Thus, proper development of the germline depends upon successful incorporation of the pole plasm components (proteins and RNAs) into these newly formed PGCs. oskar (osk) the master determinant of the germ cell fate is both necessary and sufficient to generate functional PGCs and is the principal component of the germ plasm (Ephrussi & Lehmann, 1992; reviewed in Seydoux & Brown, 2006). The downstream components, whose assembly Oskar orchestrates, are necessary for the recruitment, and maintenance of pole plasm during oogenesis and early embryogenesis. Different components of the pole plasm act, first to engineer PGC formation, and subsequently to specify their proper identity.
Distinct mechanisms are employed to preserve the identity of the germ cells, which insulate PGCs from the signaling events responsible for conferring the somatic identity on their neighboring cells. In flies, at least two different mechanisms protect the PGC identity and ensure their eventual progression to GSCs. The first mechanism involves precocious cellularization resulting in physical sequestration from the rest of the nuclei/cells. PGCs are formed by a budding process at the posterior surface of the embryo allowing physical segregation. Furthermore, posteriorly localized PGCs stay tightly adhered and exist as a group. The second is engineered by maternally deposited, cell-autonomous factors that impose transcriptional quiescence and limited mitotic potential. Global repression of Pol II has been implicated in the process of PGC specification in many organisms. In flies, three maternally deposited factors that attenuate messenger RNA production by downregulating Pol II activity have been identified: germ cell-less (gcl), polar granule component (pgc), and nanos (nos) (Kobayashi et al, 1996; Martinho et al., 2004; Asoaka et al, 1998; Deshpande et al., 1999; Deshpande et al., 2004; Leatherman et al, 2002). Supporting the conclusion that transcriptional quiescence is critical for PGC development, the inappropriate upregulation of RNA Pol II in mutant PGCs is accompanied by germ cell loss and migration defects likely due to aberrant specification.
While transcriptionally (and mitotically) quiescent, PGCs are competent to migrate towards the Somatic Gonadal Precursors (SGPs), which are mesodermal in origin (Jaglarz & Howard, 1994; Moore et al, 1998; reviewed in Santos & Lehmann, 2004). SGPs are specified under the control of the patterning gene hierarchy during embryogenesis and are located in parasegments 9–13 (Boyle & DiNardo, 1994). The generation of the embryonic gonad thus involves recognition, association, and coalescence between these two cell types. While SGPs are relatively stationary, the PGCs migrate from the posterior end of the embryo towards the SGPs that are located in the interior of the embryo. In order to migrate towards and ultimately coalesce with the SGPs, the PGCs must undergo a series of stereotypical migratory steps.
The process of PGC migration in Drosophila melanogaster is initiated during gastrulation. To begin with, the PGCs migrate away from the posterior end of the embryo, and move along the dorsal surface as a group where individual cells are adhered to their neighboring cells (reviewed in Kunwar et al., 2006). The PGCs are subsequently internalized via posterior midgut invagination as germ band extends. Upon migrating through the endodermal midgut cavity, PGCs reach the blind end of the posterior midgut. Subsequently, PGCs undergo a trans-epithelial migration governed by a number of germ cell-autonomous factors, including STAT and Tre-1, by stage 9–10 (Kunwar et al., 2003; Bausek, 2013). Germ cells orient towards the embryo’s dorsal side due to repulsion by the activity of wunen and wunen-2, both of which are expressed in the ventral region of the posterior midgut (reviewed in Santos and Lehmann, 2004; Kunwar et al., 2006). After entering the overlaying mesoderm, the germ cells then split into two clusters and migrate laterally through the mesoderm. Finally, the migrating PGCs specifically travel towards the gonadal mesoderm on both sides of the embryo and associate with the gonadal mesoderm. After linearly aligning with the SGPs located in para-segments 10–13, the PGCs coalesce with the SGPs to form the primitive embryonic gonad (Van Doren et al., 1998; Deshpande et al., 2001). Each of the two newly formed gonads incorporates approximately 10 to 15 germ cells, as Drosophila PGCs do not divide while migrating. As germ band retraction occurs, the PGCs coalesce with the SGPs to form the primitive embryonic gonad. Altogether, the complex specification and tightly orchestrated migration of the PGCs is dependent upon both germ cell intrinsic factors and extrinsic signals generated by the surrounding tissue such as the somatic mesoderm.
Germ cell specification must be tightly regulated throughout the course of embryonic development, as PGCs are the precursors of GSCs that are essential to yield fertile adults. We have been interested in analyzing the mechanisms involved in establishment and maintenance of PGC fate and their successful migration towards SGPs. Here we have focused our attention on the possible function(s) of Reactive Oxygen Species (ROS) during PGC development. We sought to investigate the ROS function in this regard as ROS activity has been shown to be crucial for the maintenance of progenitor state of stem cells in diverse developmental contexts (Owusu-Ansah and Banerjee, 2009; Labelle & Orozco, 2012, Atashi et al., 2015; Bigarella et al., 2014). Importantly, depending on the developmental and tissue context, the requirement of ROS in this regard seems to manifest in a distinctive fashion. Furthermore, supporting the possibility that ROS might play a role during embryonic PGC development, our initial observations indicated that ROS levels progressively increase in the young PGCs.
Here we have explored the possible requirement for ROS in establishing and/or maintaining some of the functional traits that impart unique identity and migratory potential to the PGCs in Drosophila. Thus, we have examined components that likely regulate PGC-specific ROS levels by autonomous and/or non-autonomous mechanisms. Investigating the involvement of ROS during PGC development will help us construct a more complete understanding of the mechanism for the establishment and maintenance of PGC fate in Drosophila.
Materials and Methods:
Fly Cultures and Stocks
Fly stocks were raised at 25°C, on standard medium, and were obtained from the Drosophila Flybase in Bloomington, Indiana. UAS and GAL4 constructs used (Brand & Perrimon, 1993) included UAS-Sod1-RNAi (Bloomington stock number 29389 and 32909), UAS-Pxt-RNAi (Bloomington stock numbers 28934 and 32382), Pxt partial loss of function allele (Bloomington stock number 15620), UAS-hop-GOF (Bloomington stock number 8492), Maternal-Tubulin-Gal4 (67.15), nos-VP16-Gal4.
Embryo Fixation
Embryos were collected after being allowed to age at room temperature for 0–14 or 0–4 hours. Embryos were washed and dechorionated by submerging the embryos in a commercially available bleach solution for 1.5 minutes. Embryos rinsed of bleach were then placed in 4% formaldehyde based fixative solution (0.54 mL of 37% formaldehyde, 4.46 mL of phosphate buffered saline (1x PBS), and 5 mL of n-heptane). After ~20 minutes, the formaldehyde/PBS layer was removed from the solution and replaced with 5 mL of methanol. After vitelline membrane was removed by vigorous shaking embryos that fell to the bottom of the vial were collected and stored in methanol at −20°C before staining.
Whole Mount Fluorescent Antibody Staining
Fixed embryos stored at −20°C were gradually transferred from methanol to PBST (1x PBS, 0.1% Triton X-100). Embryos were incubated for 1.5 hours in permeabilization solution PAT, (1x PBS, 1% Triton, 5% BSA), and then incubated for 30 minutes in blocking solution (5% BSA in PBST) before subjecting them to primary antibody treatment (anti-Vasa raised in rabbit; 1:1000). To visualize the germ cells, fluorescently labeled secondary antibodies were used (Invitrogen; 1:500). DNA marker dye Hoechst was used at 1: 500 dilution. To visualize levels of ROS, Dihydroethidium (DHE) staining protocol published by Owusu-Ansan E., et al. 2008 was followed. However instead of live imaging as prescribed, we employed a modified embryo fixation protocol. The time that embryos are in the 4% formaldehyde based fixative solution was shortened from 22 minutes to 10 minutes. The fixed embryo samples were treated with DHE after anti-Vasa antibody staining. Following staining with DHE embryos were washed 3X in PBS and subsequently mounted in AquaPolymount (Polysciences, Inc.) to be imaged using confocal microscopy. Images were processed using photoshop. Fluorescence intensities were compared using Image-J using projections. For ROS signal comparison, mean pixel intensities were estimated using control and experimental samples (n=7) between the embryos of similar stage. Embryos were initially classified using single slices, as the differences in the signal intensities were clear-cut.
Immunohistochemical DAB Staining
To visualize germ cell migration defects, 3,3’ Diaminobenzidine (DAB) staining was used with anti-Vasa to stain the PGCs. Either 0–4 or 0–16 hour old, paraformaldehyde fixed embryos were used for staining. As a control, white1 or Oregon-R embryos were stained simultaneously. In UAS-GAL4 based experiments embryos derived from either just the GAL4 or UAS strain were also used as additional controls. The histochemical staining was performed as described before. Embryos were mounted in Permount and visualized using NIKON Microphot.
Results:
Basal levels of ROS are present in the blastoderm embryos including the PGCs.
To examine possible function(s) of ROS during PGC development, we decided to assess if quantifiable levels of ROS are present in the early PGCs. ROS are extremely unstable reactive molecules, making it difficult to image them directly. As a result, detection of ROS levels has depended mainly on assaying the end products that are formed through reaction with ROS. Dihydroethidium (DHE) has been used extensively in tissue culture experiments to assess ROS production (Wang et al., 2013). Recent studies have suggested that the product of reaction between DHE and superoxide anions is 2-hydroxyethidium (Zielonka et al., 2008). DHE can detect superoxide radicals, as it is retained by cells, and also tolerates mild fixation. As a result, it offers the advantage of being able to detect real-time ROS production in live as well as fixed tissues, and it allows for detection of different levels of ROS production among cells within the same tissue. To determine if ROS is present in young embryonic PGCs, we stained 0–4 hour old embryos with antibodies against germ cell specific marker Vasa and subsequently treated these embryos with DHE as described before (Owusu-Ansah et al, 2008; see Methods for a detailed protocol).
Our initial findings indicate that ROS levels progressively build up in the PGCs during early stages of embryogenesis. The DHE labeling showed that in stage 3 embryos when nuclei are migrating towards the periphery, PGCs in the WT embryo have barely detectable accumulation of ROS (Figure 1B). By stage 4, ROS levels begin to rise (Figure 1D) and in late syncytial blastoderm WT embryos, PGCs show a marked increase in ROS levels as compared to earlier stages (Figure 1F). By cellular blastoderm stage, PGCs accumulate readily detectable levels of ROS. (Figure 1H). It is also apparent that ROS levels also increase in the somatic nuclei and the gradual accumulation of ROS observed in the PGCs through syncytial blastoderm formation is mirrored in the somatic nuclei/cells.
FIGURE 1. ROS levels steadily increase in early stage Drosophila embryos.
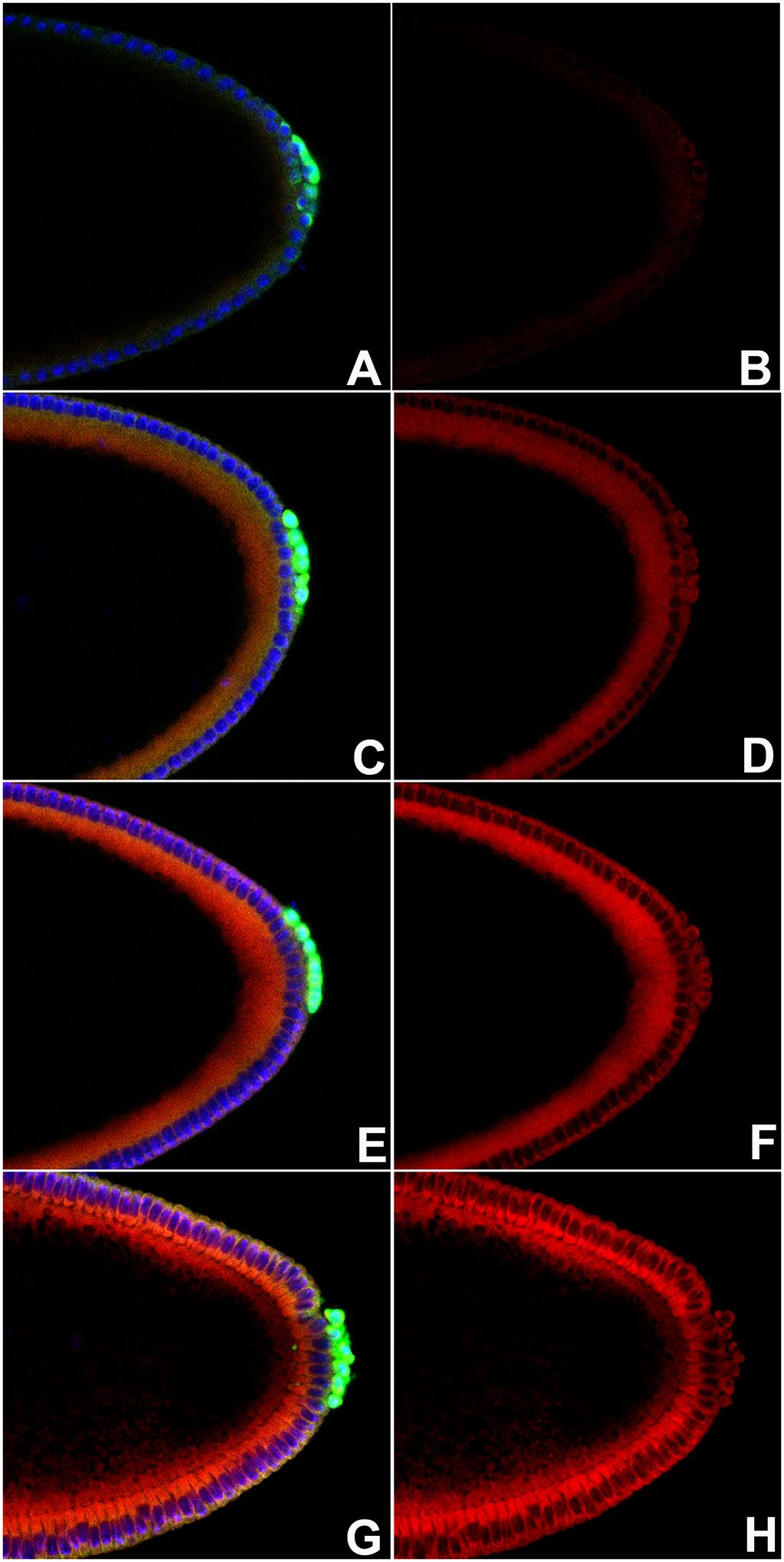
WT embryos were stained with anti-Vasa antibody (green), DNA marker Hoechst (blue). Left-hand panels (Composite panels: A, C, E, G) and ROS detector dihydroethidium DHE (red). Right-hand panels (B, D, F, H) show just the DHE signal. Embryos are oriented anterior to the left and dorsal side on top in all panels.
A, B. Stage 3 WT embryos. Somatic nuclei and pole buds show little to no accumulation of ROS.
C, D. Early stage 4 WT embryos show modest enhancement in ROS levels across the embryo compared to earlier stage.
E, F. Somatic nuclei and PGCs of late syncytial blastoderm stage WT embryos show progressive increase in the ROS levels as compared to early stage 4 WT embryos.
G, H. Somatic cells and PGCs of cellular blastoderm WT embryos show readily detectable increase in ROS levels.
ROS accumulation increases in PGCs and somatic cells during later stage development
ROS levels appear to increase uniformly in the somatic cells as well as in the PGCs by cellular blastoderm formation. We wondered if in older embryos, ROS levels continue to remain high across the embryo including in the PGCs or if the accumulation is spatially restricted in the somatic cells. To examine the pattern of ROS accumulation during later stages of embryogenesis, we performed a similar DHE labeling on overnight embryo collection (0–14 hour), which included later stage embryos (stages 5–14). We identified and tracked the PGCs by co-labelling embryos with anti-Vasa antibodies. The embryo samples were also labeled with a DNA-dye Hoechst, allowing us to assess the developmental stage.
As gastrulation is initiated, PGCs collectively migrate on the dorsal surface before entering the posterior midgut cavity. PGCs continue to move through the midgut pocket until they reach the blind end of the midgut. By stage 10 of embryogenesis, PGCs undergo trans-epithelial migration that ensures the exit of the PGCs from the midgut cavity. PGCs continue to migrate on the endodermal surface and enter the mesodermal layer by stage 11. Interestingly, we observe accumulation of ROS in the PGCs at this stage (Figure 2C).
FIGURE 2. ROS accumulation increases in PGCs and surrounding somatic cells during later stage development.
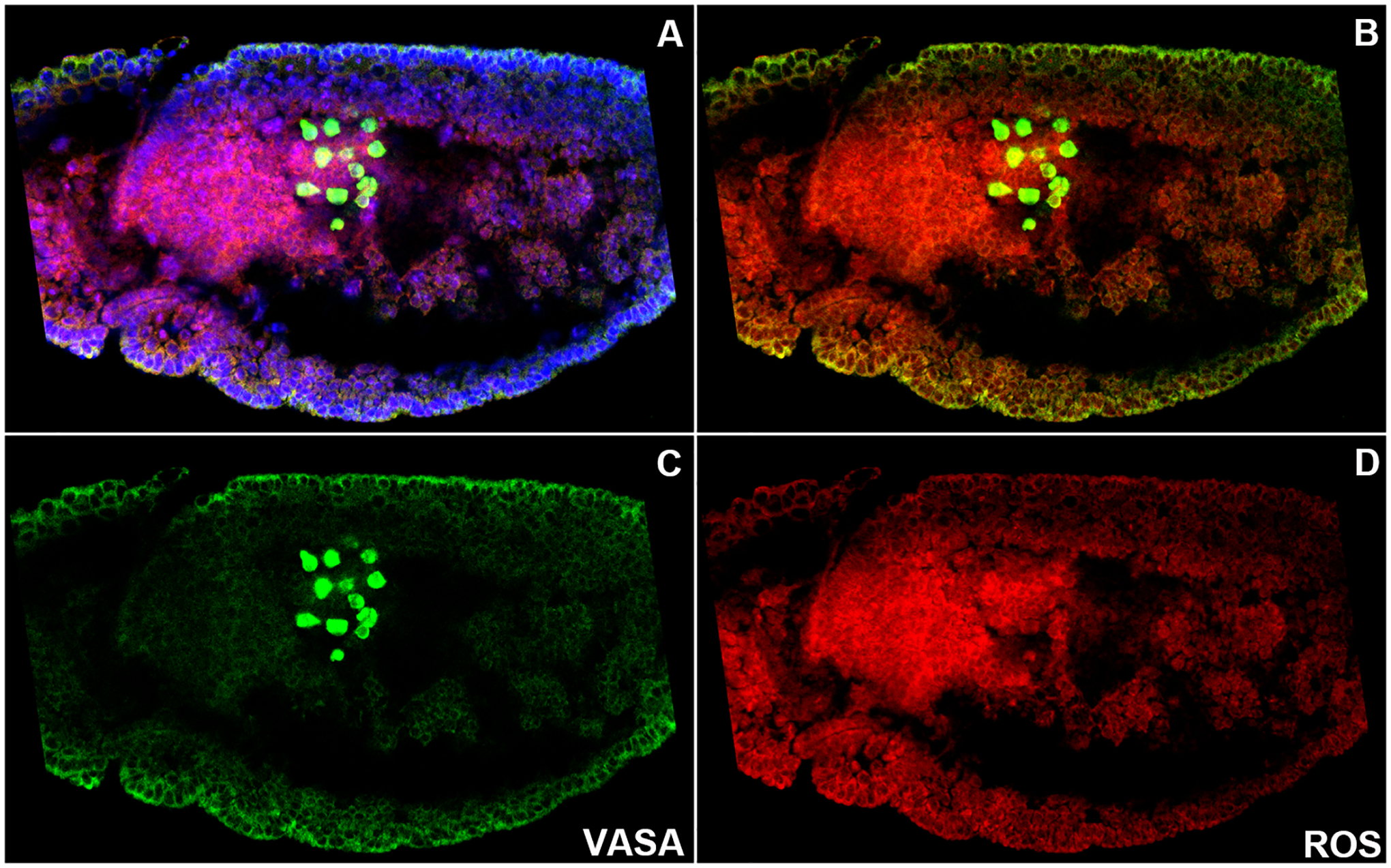
WT embryos (0–12 hour old) were stained with anti-Vasa antibody (green), DNA marker Hoechst (blue), and ROS detector dihydroethidium DHE (red). Embryos are oriented anterior to the left and dorsal side on top in all panels.
A–D. Each panel shows the same germ-band extended WT embryo at stage 9. A: Composite panel showing all three labels. B: Composite showing Vasa and ROS. C: Vasa stained PGCs (Green). D: ROS levels are accumulated in a spatiotemporally restricted manner (Red). As PGCs exit the midgut primordium, ROS levels seem to be elevated compared to the earlier stages. ROS levels are maintained in the PGCs and the surrounding midgut primordial cells show high levels of ROS accumulation (D).
PGCs migrate through the mesodermal layer under the influence of chemo-attractive guidance signal(s) from the gonadal mesoderm. PGCs cease to migrate upon reaching the SGPs situated in parasegments 10–13. Upon recognition and sustained association during stages 13–15, the primitive embryonic gonad forms by the coalescence of the two cell types. As can be seen from the figure, ROS appears to be present in the PGCs across all the stages of development (Figure 2). Taken together these data indicate that basal levels of ROS are present in young PGCs and the levels progressively rise and stabilize as PGCs are coalesced into the gonad.
Detection of ROS across different developmental stages also revealed that ROS accumulates in a regionally restricted manner in the somatic cells of the developing embryos. By stage 9 of embryogenesis, ROS is specifically detected in the midgut primordium. Curiously, PGCs are placed in the midgut pocket and as a result, are in the close physical proximity of cells that are ROS-positive (see Supplementary Figure 1). As in the case of PGCs, elevated levels of ROS are also detected in the midgut primordium until later stages of embryogenesis (Supplementary figure 1).
Expression of ROS scavenger Sod1 in early embryos coincides with ROS.
As ROS was specifically detected in the PGCs and the midgut primordium across early embryonic development, we wondered if this accumulation has any functional role. This seemed pertinent as in other developmental contexts modulation of ROS levels was shown to be essential to maintain stem cell fate (Atashi et al., 2015; Bigarella et al., 2014). Moreover, there are several scavenger proteins that control ROS levels, including three different isoforms of superoxide dismutase (Sod; Sod1-3). It is noteworthy that individual knockdown of the three genes results in either partial or complete lethality which indicates essential and non-redundant function(s) (Mukherjee et al., 2011). Furthermore, knockdown of Sod2, one of the three members of the family, in the hematopoietic stem cells (HSCs) resulted in increased ROS production, which correlated with precocious differentiation. In order to experimentally manipulate ROS levels, we first decided to determine if any of the Sod variants are expressed in the early embryos. Scanning the publicly available expression data from the Fly base (Bloomington) Sod1 emerged as a suitable candidate.
As can be seen from the composite assembled from the available data in Supplemental figure 2, maternally deposited Sod1 RNA is uniformly distributed across the embryo and as a result, RNA is detected in all the embryonic nuclei at cellular blastoderm stage (stage 5). However, as embryogenesis proceeds, expression of Sod1 RNA becomes locally restricted and high level of expression is retained only in the PGCs and the surrounding somatic endodermal cells by stage 7. By stage 9, even greater restriction of Sod1 RNA is observed and it is detected almost exclusively in the PGCs and midgut primordial cells that surround the PGCs (see Supplemental Figure 2).
Sod1 modulates levels of ROS in PGCs and soma
Our observations thus far indicate that basal levels of ROS are uniformly distributed in the early embryos. As embryonic development progresses, ROS levels increase discernibly just in the PGCs and the surrounding midgut primordial cells. Intriguingly, the same two cells types, i.e. PGCs, and midgut cells, also display Sod1 specific transcripts that encode the scavenger protein. We thus sought to explore possible influence of Sod1 on the ROS accumulation in the developing embryo. As the loss of function mutations in Sod1 are homozygous lethal, we decided to use UAS-GAL4 system to compromise Sod1 RNA levels (Brand and Perrimon, 1993). We employed a maternal-tubulin-GAL4 driver to express UAS-Sod1-RNAi. We expected that if Sod1 expression is diminished upon expression of RNAi, it should result in increased ROS production. Moreover, as Sod1 expression is local, and primarily restricted to just two cell types during mid embryogenesis, ROS levels would be elevated only in the PGCs and the surrounding midgut cells. Indeed, knockdown of Sod1 resulted in increased ROS production in the PGCs and the adjacent midgut cells at stage 13 embryos (Figure 3). Comparison with the WT embryos thus indicated that ROS levels are elevated upon knockdown of Sod1. While the increase in the ROS levels was seen in the post-gastrulation stage embryos (7/8), it remained apparent until later stages (12/13) when PGCs align against the SGPs. Two independent Sod1-RNAi lines were used to confirm the observations (for quantitation of ROS accumulation see Supplementary Figure 3). Altogether, these data suggest that compromising Sod1 levels/activity results in increased ROS production in the migrating PGCs and the midgut primordial cells.
FIGURE 3. Sod regulates levels of ROS in PGCs and soma during embryogenesis.
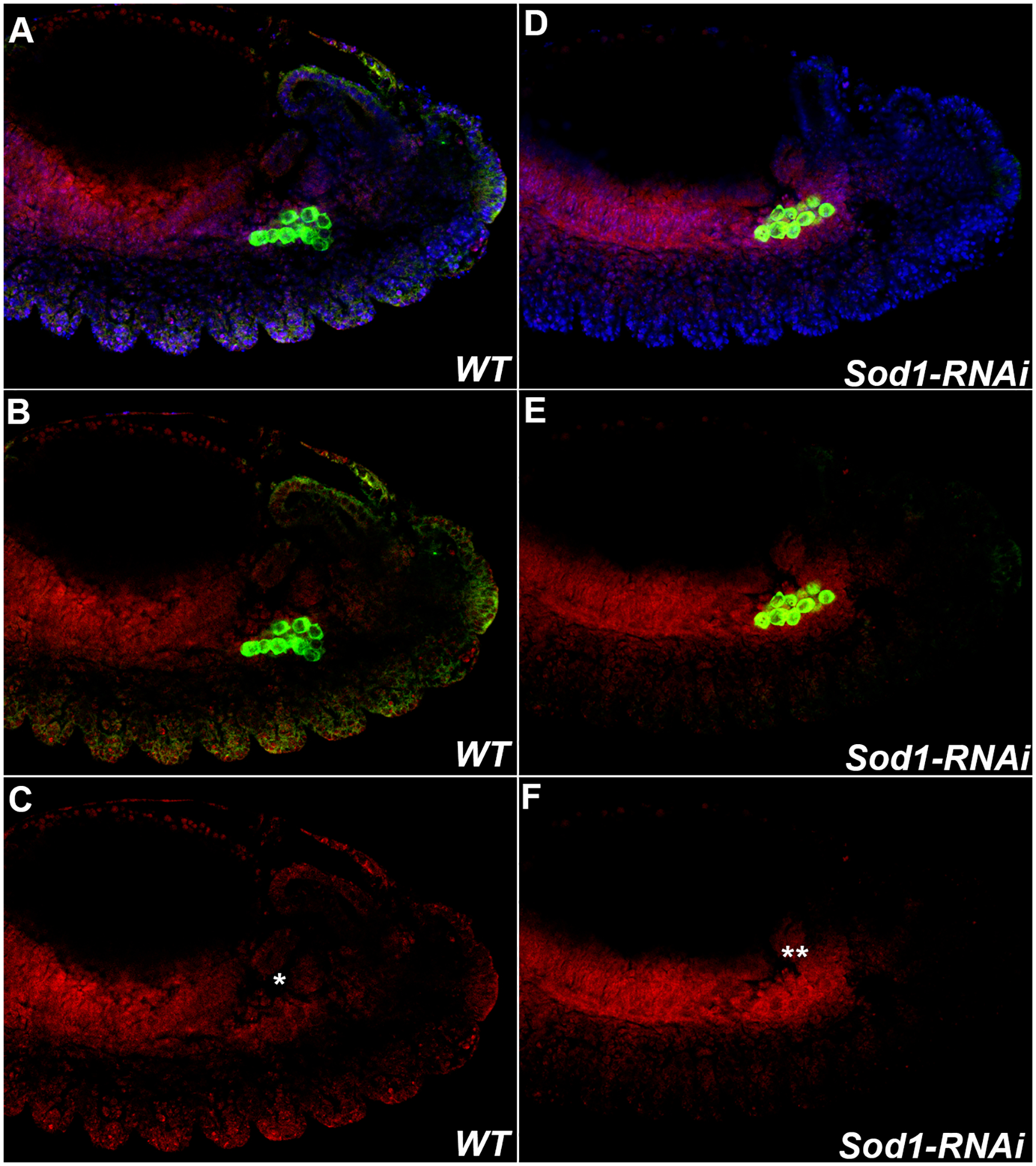
Levels of ROS in PGCs of Stage 13 germ-band extended embryos increase when levels of the scavenger protein Sod are compromised. WT and MAT-Gal4/UAS-Sod1-RNAi embryos were stained with anti-Vasa antibody (Green), DNA marker Hoechst (Blue), and ROS detector dihydroethidium DHE (Red). A, D: All three markers. B, E: Vasa and DHE. C, F: DHE. Embryos are oriented anterior to the left and dorsal side on top in all panels.
WT. Levels of ROS in PGCs in Stage 13 wild type embryos are still detected (arrow).
Each panel (A-C) on the left shows the same stage 13 WT embryo. During stage 13, as PGCs in the mesoderm align against the SGPs (not shown), detectable levels of ROS are still observed (marked with *).
Sod1-RNAi. Levels of ROS in PGCs and soma are markedly increased in Stage 13 MAT-Gal4/UAS-Sod1-RNAi embryos (PGCs marked with two **).
Increase in ROS levels upon compromising Sod1 correlates with germ cell migration defects
As the ROS levels increased upon maternal knockdown of Sod1, we wondered if there are also any functional consequences. To examine the influence of elevated ROS levels on germ cell behavior, we stained MAT-GAL4/UAS-Sod1-RNAi embryos using anti-vasa antibodies. In these embryos the early stages of PGC development seemed comparable to those of control embryos, as a similar number of PGCs entered the midgut pocket as seen in the UAS-Sod1-RNAi control embryos. However, by stage 13 when PGCs align against the SGPs, their migration behavior was visibly aberrant (Figure 4, Sod-RNAi). MAT-GAL4/UAS-Sod1-RNAi PGCs at this stage remained scattered on several instances and presumably did not effectively associate with the SGPs. As a result, many PGCs were seen in the posterior of the embryo at ectopic locations (2/17 control embryos showed >5 mis-migrating PGCs as opposed to 11/18 MAT-GAL4/UAS-Sod1-RNAi embryos displayed >5 mispositioned PGCs). Curiously, the experimental embryos also display a modest increase in the total number of PGCs (Figure 4, bottom right panel). These results thus indicated that increase in ROS levels, induced by compromising Sod1, could possibly influence PGC migration. It should be noted that Maternal-Gal4 is relatively uniformly distributed in the early embryos and in MAT-GAL4/UAS-Sod1-RNAi embryos, the midgut cells situated adjacent to PGCs also showed increased ROS levels. Consistently, nos-Gal4/UAS-Sod1-RNAi embryos also exhibit comparable defects in germ cell migration supporting the conclusion that ROS levels within the PGCs may be important for their proper migration and gonad coalescence. These data however do not rule out possible additional, non-autonomous role for ROS derived from the neighboring mid-gut primordium.
FIGURE 4. Embryos maternally compromised for Sod1 exhibit defects in PGC migration.
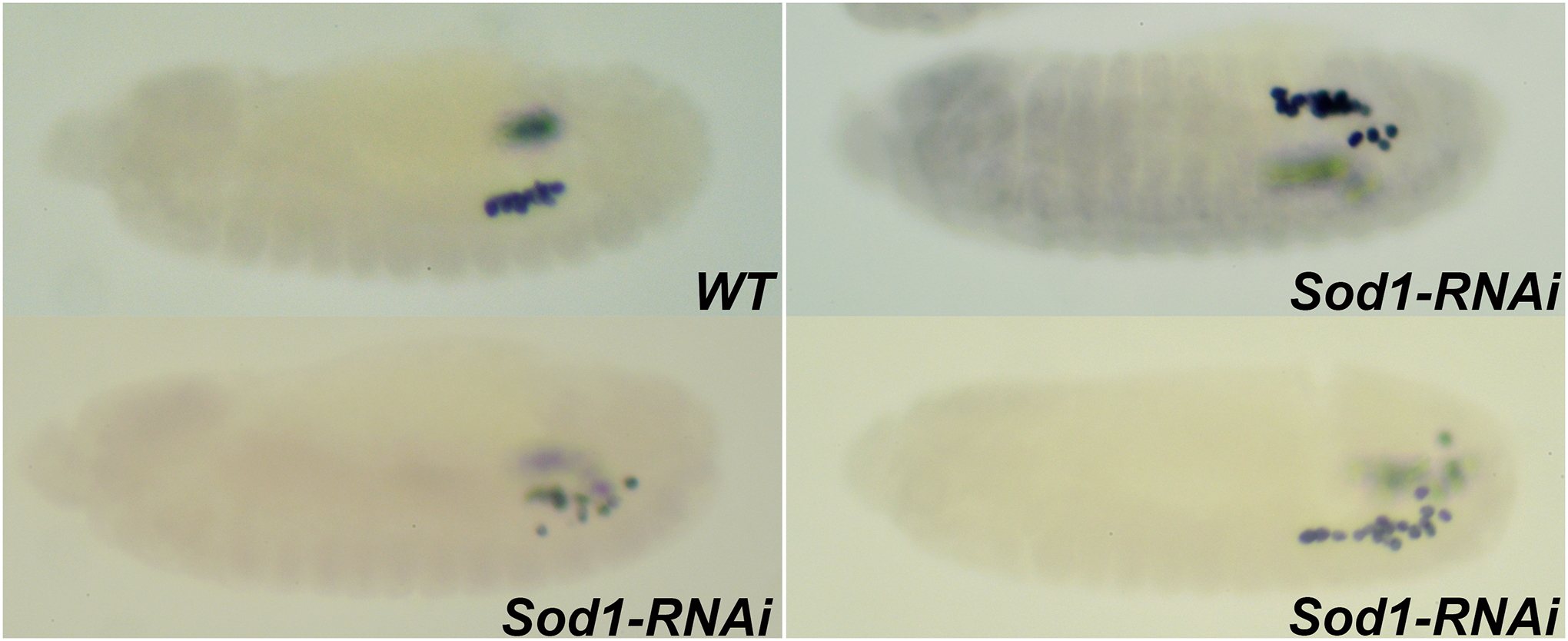
Stage 13 embryos compromised for Sod1 using RNAi show defective PGC migration as assessed by anti-Vasa labeling. Embryos are oriented anterior to the left and dorsal side on top in all the panels.
WT. At Stage 13, PGCs cluster and align against the SGPs situated in the para-segments 12 and 13.
Sod1-RNAi. MAT-Gal4/UAS-Sod1-RNAi embryos at stage 13 exhibit defects in PGC migration resulting in scattering of PGCs in the posterior of the embryo
PGCs of Pxt mutant embryos exhibit defects in PGC adhesion and migration.
Emboldened by the germ cell migration defects observed by compromising Sod1 we decided to explore other components known to modulate ROS levels/activity. In this context, we sought to examine possible regulatory role of Peroxinectin-like or Pxt, which encodes a Drosophila cyclooxygenase and, mutations in Pxt induce oogenesis defects resulting in eggshell defects (Tootle et al, 2011). Moreover, cyclooxygenases have been shown to be involved in ROS production/metabolism (Takahashi et al., 2004; Yoon et al., 2010). Interestingly, Pxt also seemed like an ideal candidate to analyze its potential involvement during the embryonic germ cell development due to its PGC specific expression as revealed by the publicly available RNA expression data base (Supplementary Figure 2). We decided to compare the expression pattern of Pxt with Sod1. Maternally deposited Pxt RNA is protected only in the PGCs by cellular blastoderm stage while the somatic RNA is likely degraded. Furthermore, the germ cell-specific expression is retained as the embryo undergoes gastrulation by stage 7. Importantly, unlike Sod1 RNA, Pxt expression appears to be restricted to the PGCs, and appears to be eliminated from all other somatic tissues including the midgut primordium (see Supplemental Figure 2, panel showing stage 7 and 9 embryos).
The selective retention of Pxt RNA in the pole cells, followed by specific expression in the PGCs made Pxt an attractive candidate to analyze during embryonic PGC development. We generated maternal-Gal4/UAS-Pxt-RNAi embryos using two different RNAi lines and stained these embryos with Anti-Vasa antibodies to assess PGC formation and specification in early embryos. Interestingly, a substantial proportion of embryos displayed characteristic defects by late stage 4/early stage 5 of embryonic development. In wild type embryos at this stage, PGCs are tightly adhered to one another, and exist as a group. By late stage 5, they initiate a collective migration on the dorsal surface coincident with the initiation of gastrulation. In contrast to the PGCs from either wild type or control embryos, PGCs from the maternal-Gal4/UAS-Pxt-RNAi embryos showed severe defects in the ability to cluster (Figure 5, Pxt-RNAi). On many occasions, PGCs migrated on the ‘wrong’ surface towards the ventral side of the embryo (5/14 embryos as opposed to 0/15 control embryos). In some instances, MAT-Gal4/UAS-Pxt-RNAi PGCs also displayed precocious invasive migration into the interior of the embryo (Supplementary Figure 4). Further supporting a role for Pxt in early adhesion among the PGCs, homozygous mutant embryos derived from a partially viable allele also displayed similar defects. Importantly the germ cell behavior defects seen in early embryos also persisted in later stage embryos (stages 13–14) compromised for Pxt. MAT-Gal4/UAS-Pxt-RNAi (9/19 embryos with >5 lost PGCs) or homozygous mutant embryos (11/24 embryos with >5 lost PGCs) derived from partial loss of function allele of Pxt showed scattering of germ cells as compared to the wild type control (3/21; >5 lost PGCs). Moreover, as in the case of Sod1, PGCs from the embryos compromised for Pxt, showed elevated levels of ROS accumulation (Supplementary figure 3).
FIGURE 5. PGCs from embryos compromised for Pxt display defects in PGC adhesion and migration at cellular blastoderm stage.
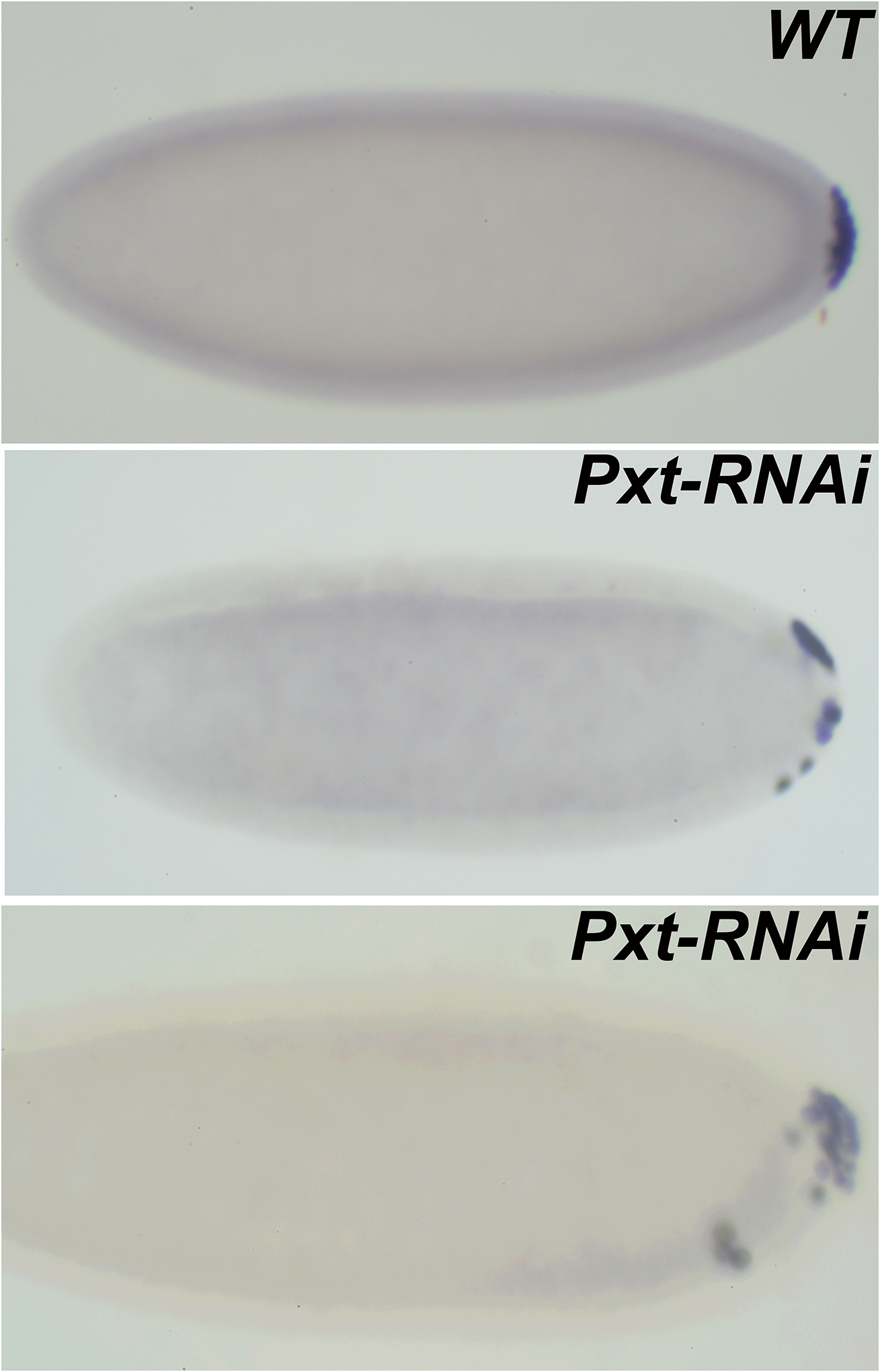
Embryos labeled with Vasa antibodies are oriented anterior to the left and dorsal side on top in all panels.
WT. PGCs in a cellular blastoderm stage embryo remain clustered at the posterior pole. Pxt-RNAi. In MAT-Gal4/UAS-Pxt-RNAi embryos pole cells lose adhesion and migrate away from the posterior pole. In more severe cases, PGCs not only migrate away from the pole but also invade the embryo. Interestingly, these pole cells move away from dorsal side and instead migrate on the ventral surface (bottom panel). Similar defects are also observed in Pxt mutant embryos collected from a viable, partial loss of function allele.
Some of the phenotypes we observed in the embryos compromised for Pxt including invasive migration are reminiscent of hyperactivation of JAK-STAT pathway (Amoyel et al., 2014) (Bausek, 2019) (Li et al, 2003) (Li, 2004). We thus wondered whether PGC behavior defects induced by the loss of Pxt resemble those induced by the constitutive activation of JAK/STAT pathway also in slightly older embryos. hopscotch (hop) encodes a non-receptor tyrosine kinase of the JAK family. Expectedly, expression of a constitutively active form of hop resulted in aberrant PGC migration from stage 5 onwards during embryogenesis. Importantly, PGCs from the Mat-Gal4/UAS-Hop-(GOF) embryos at stage 5 migrated in the opposite direction towards the ventral side. This defective migration was observed in >40% of the total embryos (9/21 experimental embryos as opposed to 0/12 control embryos). Interestingly, the defects were similar to those seen either in Pxt mutant or Maternal-Gal4/UAS-Pxt-RNAi embryos (see Figure 5). In both instances, in the germ-band extended embryos PGCs lingered on the dorsal side of the embryo, failed to reach the midgut pocket, and on several occasions remained trapped in the midgut (Figure 6).
FIGURE 6. Constitutive activation of JAK/STAT pathway results in defects that resemble those in Pxt-RNAi PGCs.
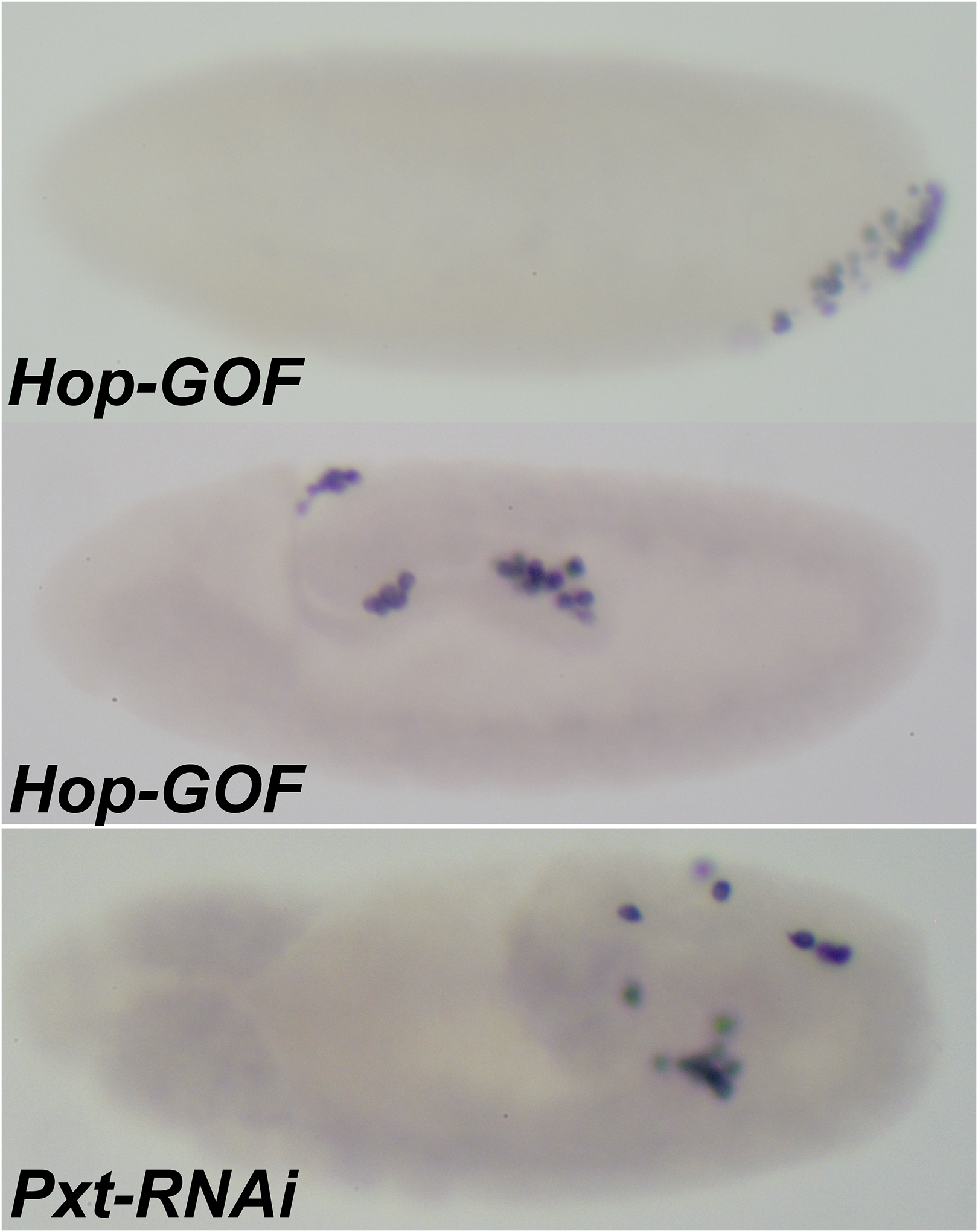
Expression of a constitutively active form of hopscotch, a JAK family tyrosine kinase, results in aberrant PGC migration defects in Stage 10 embryos that are phenotypically similar to Mat-Gal4/Pxt-RNAi embryos. To assess the behavior of the PGCs embryos were stained with anti-Vasa antibody. Embryos are oriented anterior to the left and dorsal side on top in all panels.
hop GOF: Stage 10 MAT-Gal4/UAS-hop-GOF germ-band-extended embryo shows that PGCs linger on the dorsal side of the embryo, fail to reach the midgut pocket, and remain trapped in the midgut.
Pxt-RNAi. The phenotypic consequences of gain of function of hop are mimicked in the MAT-Gal4/UAS-Pxt-RNAi embryo. Decreased levels of Pxt also result in lagging behind of PGCs, ultimately showing similar defects.
Discussion
It is widely believed that the presence and enrichment of ROS are indicative of undifferentiated cells in several biological contexts. Moreover, increasing ROS levels is thought to induce precocious differentiation ultimately resulting in an excess number of differentiated cells. By contrast, decreasing ROS results in a reduction in the total number of undifferentiated cells. Thus, we reasoned that REDOX mechanisms could be involved in PGC specification, development, and behavior. The stage-specific increase in ROS further prompted us to explore the possible functional involvement of ROS in the development of Drosophila embryonic Primordial Germ Cells, as ROS levels only increased gradually in the quiescent PGCs.
Our results also demonstrate that changes in ROS levels are spatially restricted from mid-embryogenesis, as ROS specifically accumulates in the PGCs and surrounding midgut primordial cells. Supporting the conclusion that ROS might perform a specific function during PGC development, we found that experimentally manipulating ROS levels resulted in functional consequences including lack of adhesion and invasive migration in early embryos as well as aberrant migration in later stages.
These data dovetail nicely with multiple recent findings in which involvement of ROS in stem cell fate maintenance, proliferation and differentiation was investigated. For instance, Bailey et al., (2015) demonstrated that the glial cell niches modulate ROS levels by employing lipid droplets to regulate development of neural stem cells, to prevent oxidative damage and premature differentiation. These elegant studies demonstrate how oxidative stress stimulates lipid droplet biosynthesis, presumably as a protective measure against detrimental peroxidation chain reactions which could otherwise induce precocious proliferation of the neural stem cells. Similarly, Xu et al. (2017) found that increased levels of ROS in intestinal stem cells (ISC) are associated with different forms of tissue damage. In this context, oxidative stress was shown to be necessary and sufficient to trigger ISC proliferation through activation of the Ras/MAPK pathway via Ca2+ signaling.
The most pertinent of these findings is the one reported by Owusu-Ansah et. al., These studies showed that ROS acts in a cell autonomous manner in the progenitor stem cells to maintain their fate. Using ‘loss’ and ‘gain’ of function analysis of ROS pathway components including Sod2, these authors showed that changes in ROS levels exert reciprocal influence on the stem cell fate i.e. inadvertent increase leads to excess proliferation whereas decrease in the ROS levels results in premature differentiation. Taken together our study raises interesting questions about what processes ROS accumulation can control, ultimately altering cell behavior. By manipulating Sod1 and Pxt, we were able to alter ROS levels, resulting in precocious scattering and invasive migration of PGCs. The inability of Pxt mutant PGCs to adhere tightly is reminiscent of the phenotype observed in embryos compromised for hydrogen peroxide-degrading enzyme Jafrac1. These authors also proposed a defective cell adhesion-dependent mechanism involving alterations in the levels of E-Cadherin to explain the defect in peroxiredoxin mutant embryos (DeGennaro et al., 2011). Our study demonstrates that decreasing Pxt levels also leads to similar defects in PGC adhesion and migration. Interestingly, enhancing activity of JAK/STAT pathway also mimicked some of these phenotypes. Curiously PGCs from embryos expressing constitutively active form of hop did not display a reliably elevated accumulation of ROS (not shown). As this negative result indicates that ROS signaling may regulate JAK-STAT pathway in PGCs, future studies will focus on whether the two pathways work together or in parallel to influence germ cell behavior and specification.
Supplementary Material
Supplementary Figure 2: Expression of Sod1 and Pxt in early and later stage WT embryos coincides with ROS expression and eventually only with PGCs and associated cells. The panels shown in this figure were selected from Berkeley Drosophila Genome Project (BDGP) expression data sets that are publicly available. Embryos are oriented anterior to the left and dorsal side on top in all panels.
Right: Sod1 RNA
Stage 5. Sod1 RNA is maternally deposited and appears to be uniformly distributed all through the embryo.
Stage 7. As embryogenesis proceeds, higher levels of Sod1 RNA are detected in the PGCs, and the midgut primordium.
Stage 9. Sod1 RNA appears to be selectively present just in the two cell types: PGCs and midgut.
Left: Pxt RNA
Stage 5, 7 and 9. Pxt RNA is detected specifically only in the PGCs from early to mid-embryogenesis.
Supplementary Figure 1: ROS accumulation in PGCs and midgut primordial cells during mid-embryogenesis. ROS levels (red) specifically rise in PGCs (stained with anti-Vasa: green) and surrounding mid-gut primordial cells as seen in the magnified view (100X) of the embryo shown in Figure 2. MGP: Midgut primordial cells; GC: Germ cells.
Supplementary Figure 3: Quantitation of ROS accumulation in embryos compromised for Sod1 and Pxt Lightly fixed embryos were stained with anti-Vasa antibodies (not shown) followed by DHE specific labeling (red) to detect ROS. Wild type control embryos show moderate accumulation of ROS in PGCs from stage 13 embryos. PGCs from embryos of the same stage compromised for Sod1 and Pxt display elevated levels of ROS as compared to the control. The graph in the lower panel shows increase in the ROS levels is statistically significant and was seen consistently (n=7). Level of signal was estimated by measuring pixel intensities using fixed area and the mean signal intensities were plotted.
Supplementary Figure 4: Embryos compromised for Pxt display PGCs that initiate precocious invasive migration into the embryo Embryos of specified genotype stained with anti-Vasa antibodies. Unlike WT control embryo, MAT-Gal4/UAS-Sod1-RNAi embryo shows PGCs that are not closely adhered to one another and a few PGCs have moved inappropriately in the interior.
Acknowledgements:
This work was supported by a grant from National Institute of Health (NICHD:093913). Paul Lasko and Eric Wieschaus kindly provided Anti-Vasa antibodies and Maternal Gal4 strain respectively. Authors thank Paul Schedl for stimulating discussions. We thank Dr. Gary Laevsky and the Molecular Biology Confocal Microscopy Facility which is a Nikon Center of Excellence. Gordon Grey provided fly media. We gratefully acknowledge the Bloomington stock center for different fly lines. We thank the Berkeley Drosophila Genome Project (BDGP) for the ‘In situ’ hybridization data sets. These were used to assemble the supplemental figure showing temporal pattern of RNA accumulation for Sod1 and Pxt.
Grant #: HD093913 (NIH)
References:
- Amoyel M, Anderson AM, & Bach EA (2014). JAK/STAT pathway dysregulation in tumors: A Drosophila perspective. Seminars in Cell and Developmental Biology, 28, 96–103. 10.1016/j.semcdb.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asoaka M, Sano H, Obara Y, Kobayashi S (1998). Maternal Nanos regulates zygotic gene expression in germline progenitors of Drosophila melanogaster. Mech Dev. 78 :153–8. [DOI] [PubMed] [Google Scholar]
- Atashi F, Modarressi A, & Pepper MS (2015). The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells and Development, 24(10), 1150–1163. 10.1089/scd.2014.0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Koster G, Guillermier C, Hirst EMA, MacRae JI, Lechene CP, … Gould AP (2015). Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell, 163(2), 340–353. 10.1016/j.cell.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausek N (2013). JAK-STAT signaling in stem cells and their niches in Drosophila. Jak-Stat, 2(3), e25686. 10.4161/jkst.25686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigarella CL, Liang R, & Ghaffari S (2014). Stem cells and the impact of ROS signaling. Development (Cambridge), 141(22), 4206–4218. 10.1242/dev.107086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, & DiNardo S (1995). Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development (Cambridge, England), 121(6), 1815–1825. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7600996 [DOI] [PubMed] [Google Scholar]
- Brand AH, & Perrimon N (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118(2), 401–415. [DOI] [PubMed] [Google Scholar]
- DeGennaro M, Hurd TR, Siekhaus DE, Biteau B, Jasper H, & Lehmann R (2011). Peroxiredoxin stabilization of DE-cadherin promotes primordial germ cell adhesion. Developmental cell, 20(2), 233–243. doi: 10.1016/j.devcel.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Calhoun G, & Schedl P (2004). Overlapping mechanisms function to establish transcriptional quiescence in the embryonic Drosophila germline. Development, 131(6), 1247–1257. 10.1242/dev.01004 [DOI] [PubMed] [Google Scholar]
- Deshpande G, Calhoun G, Yanowitz JL, & Schedl PD (1999). Novel functions of nanos in downregulating mitosis and transcription during the development of the Drosophila germline. Cell, 99(3), 271–281. 10.1016/S0092-8674(00)81658-X [DOI] [PubMed] [Google Scholar]
- Deshpande G, Swanhart L, Chiang P, & Schedl P (2001). Hedgehog signaling in germ cell migration. Cell, 106(6), 759–769. 10.1016/S0092-8674(01)00488-3 [DOI] [PubMed] [Google Scholar]
- Deshpande G, Willis E, Chatterjee S, Fernandez R, Dias K, & Schedl P (2014). BMP signaling and the maintenance of primordial germ cell identity in drosophila embryos. PLoS ONE, 9(2). 10.1371/journal.pone.0088847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A, & Lehmann R (1992). Induction of germ cell formation by oskar. Nature, 358(6385), 387–392. 10.1038/358387a0 [DOI] [PubMed] [Google Scholar]
- Jaglarz MK, & Howard KR (1994). Primordial germ cell migration in Drosophila melanogaster is controlled by somatic tissue. Development, 120(1), 83–89. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yamada M, Asaoka M, and Kitamura T (1996). Essential role of the posterior morphogen nanos for germline development in Drosophila. Nature. 280: 708–11. [DOI] [PubMed] [Google Scholar]
- Kunwar PS, Starz-Gaiano M, Bainton RJ, Heberlein U, & Lehmann R (2003). Tre1, a G protein-coupled receptor, directs transepithelial migration of Drosophila germ cells. PLoS biology, 1(3), E80. doi: 10.1371/journal.pbio.0000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar PS, Siekhaus DE, & Lehmann R (2006). In Vivo Migration: A Germ Cell Perspective. Annual Review of Cell and Developmental Biology, 22(1), 237–265. 10.1146/annurev.cellbio.22.010305.103337 [DOI] [PubMed] [Google Scholar]
- LaBelle JE, & Orozco NM (2012). Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner., 8(1), London Calling Conference. 10.1016/j.stem.2010.11.028.Proliferative [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xia F, & Li WX (2003). Coactivation of STAT and Ras is required for germ cell proliferation and invasive migration in Drosophila. Developmental cell, 5(5), 787–798. doi: 10.1016/s1534-5807(03)00328-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WX (2004). Receptor tyrosine kinase signaling and primordial germ cell development. Cell Cycle, 3(3), 249–251. 10.4161/cc.3.3.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrakis M, Rikhy R, & Lippincott-Schwartz J (2009). Plasma membrane polarity and compartmentalization are established before cellularization in the fly embryo. Developmental cell, 16(1), 93–104. doi: 10.1016/j.devcel.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinho RG, Kunwar PS, Casanova J, Lehmann R. (2004). A noncoding RNA is required for the repression of RNApolII-dependent transcription in primordial germ cells. Curr. Biol 14: 159–165. [DOI] [PubMed] [Google Scholar]
- Mahowald AP (2001). Assembly of the Drosophila germ plasm. Int Rev Cytol. 203:187–213. [DOI] [PubMed] [Google Scholar]
- Mukherjee Subhas et al. “SOD2, the principal scavenger of mitochondrial superoxide, is dispensable for embryogenesis and imaginal tissue development but essential for adult survival.” Fly vol. 5,1 (2011): 39–46. doi: 10.4161/fly.5.1.14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Yavari A, Banerjee U. A protocol for in vivo detection of Reactive Oxygen Species. Nature Protocol Network. (published online 3 February 2008) [Google Scholar]
- Owusu-Ansah E, & Banerjee U (2009). Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature, 461(7263), 537–541. 10.1038/nature08313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AC, & Lehmann R (2004). Germ cell specification and migration in Drosophila and beyond. Current Biology, 14(14), 578–589. 10.1016/j.cub.2004.07.018 [DOI] [PubMed] [Google Scholar]
- Seydoux G, & Braun RE (2006). Pathway to Totipotency: Lessons from Germ Cells. Cell, 127(5), 891–904. 10.1016/j.cell.2006.11.016 [DOI] [PubMed] [Google Scholar]
- Takahashi T, et al. (2004). The role of selective COX-2 inhibitors in reactive oxygen species formation in osteosarcoma cells after X-ray irradiation. Int J Mol Med. 13(5), 661–664. [PubMed] [Google Scholar]
- Tootle TL, Williams D, Hubb A, Frederick R, & Spradling A (2011). Drosophila eggshell production: Identification of new genes and coordination by Pxt. PLoS ONE, 6(5). 10.1371/journal.pone.0019943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. (2013). Imaging ROS signaling in cells and animals. J Mol Med (Berl). 91 (8), 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, & Lehmann R (1996). Germ Cell Development in Drosophila. Annual Review of Cell and Developmental Biology, 12(1), 365–391. 10.1146/annurev.cellbio.12.1.365 [DOI] [PubMed] [Google Scholar]
- Wilson JW, and Macdonald PM (1993). Formation of germ cells in Drosophila. Curr Opin Genet Dev. 4: 562–5. [DOI] [PubMed] [Google Scholar]
- Wylie C (1999). Germ cells. Cell. 96:165–74. [DOI] [PubMed] [Google Scholar]
- Xu Chiwei et al. “Oxidative stress induces stem cell proliferation via TRPA1/RyR-mediated Ca2+ signaling in the Drosophila midgut.” eLife vol. 6 e22441. 31 May. 2017, doi: 10.7554/eLife.22441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Lim TG, Lee KM, Jeon AJ, Kim SY, & Lee KW (2011). Tangeretin reduces ultraviolet B (UVB)-induced cyclooxygenase-2 expression in mouse epidermal cells by blocking mitogen-activated protein kinase (MAPK) activation and reactive oxygen species (ROS) generation. Journal of Agricultural and Food Chemistry, 59(1), 222–228. 10.1021/jf103204x [DOI] [PubMed] [Google Scholar]
- Zielonka J, Vasquez-Vivar J & Kalyanaraman B Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3, 8–21 (2008) doi: 10.1038/nprot.2007.473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2: Expression of Sod1 and Pxt in early and later stage WT embryos coincides with ROS expression and eventually only with PGCs and associated cells. The panels shown in this figure were selected from Berkeley Drosophila Genome Project (BDGP) expression data sets that are publicly available. Embryos are oriented anterior to the left and dorsal side on top in all panels.
Right: Sod1 RNA
Stage 5. Sod1 RNA is maternally deposited and appears to be uniformly distributed all through the embryo.
Stage 7. As embryogenesis proceeds, higher levels of Sod1 RNA are detected in the PGCs, and the midgut primordium.
Stage 9. Sod1 RNA appears to be selectively present just in the two cell types: PGCs and midgut.
Left: Pxt RNA
Stage 5, 7 and 9. Pxt RNA is detected specifically only in the PGCs from early to mid-embryogenesis.
Supplementary Figure 1: ROS accumulation in PGCs and midgut primordial cells during mid-embryogenesis. ROS levels (red) specifically rise in PGCs (stained with anti-Vasa: green) and surrounding mid-gut primordial cells as seen in the magnified view (100X) of the embryo shown in Figure 2. MGP: Midgut primordial cells; GC: Germ cells.
Supplementary Figure 3: Quantitation of ROS accumulation in embryos compromised for Sod1 and Pxt Lightly fixed embryos were stained with anti-Vasa antibodies (not shown) followed by DHE specific labeling (red) to detect ROS. Wild type control embryos show moderate accumulation of ROS in PGCs from stage 13 embryos. PGCs from embryos of the same stage compromised for Sod1 and Pxt display elevated levels of ROS as compared to the control. The graph in the lower panel shows increase in the ROS levels is statistically significant and was seen consistently (n=7). Level of signal was estimated by measuring pixel intensities using fixed area and the mean signal intensities were plotted.
Supplementary Figure 4: Embryos compromised for Pxt display PGCs that initiate precocious invasive migration into the embryo Embryos of specified genotype stained with anti-Vasa antibodies. Unlike WT control embryo, MAT-Gal4/UAS-Sod1-RNAi embryo shows PGCs that are not closely adhered to one another and a few PGCs have moved inappropriately in the interior.


