Abstract
Background
The efficacy of posterior wall isolation (PWI) on top of pulmonary vein isolation (PVI) in patients affected by persistent atrial fibrillation (AF) is still controversial and little is known about the impact of contact-force (CF) technology.
Objective
In this retrospective study, we present our experience with PWI using CF sensing catheters and its efficacy and safety as an adjunctive ablation strategy on top of PVI for management of patients with persistent and longstanding persistent AF.
Methods
A total of 73 consecutive patients (20.5% female) affected by persistent atrial fibrillation (10.9% long-standing) underwent PWI as an adjunctive therapy to PVI using CF sensing catheters. Outcomes were reported as incidence of atrial arrhythmic recurrences (ARs) lasting >30 seconds at follow up and in addition, in patients provided with insertable cardiac monitors (ICM), as burden of AF or atrial tachycardias (AT) at relevant time points.
Results
PWI was successfully achieved in 65 (89.0%) patients. Two (2.7%) minor vascular procedural complications were observed. At 1 and 2-year follow-up, ARs free survival was observed in 80.5% and 64.1% of patients, respectively with 75.3% of patients off antiarrhythmic drugs at the last follow-up. Ten patients underwent repeat ablations during the follow-up. At multivariate analysis, early ARs within 3 months after procedure, were associated with a two-fold increased risk of late ARs at follow-up. Among patients provided with ICM, PWI on top of PVI was able to reduce the mean AT/AF burden of more than 50% compared with pre-ablation time, reporting very low levels (≤ 5%) over 2 years.
Conclusions
In persistent atrial fibrillation, PWI on top of PVI using CF sensing catheters is safe and effective, providing great reduction of burden of ARs. Early ARs are associated with a greater risk of late recurrences.
Keywords: Persistent Atrial Fibrillation, Posterior Wall Isolation, Contact Force, Early Recurrence, AF Burden
Introduction
Pulmonary vein isolation (PVI) is currently recommended for paroxysmal atrial fibrillation (AF) catheter ablation, but persistent AF remains a clinical challenge 1-3. In this setting, guidelines recommend that substrate modification should be considered on top of PVI, but the technical approach is not univocally defined and various strategies have been proposed 1,2,4. Among strategies to achieve atrial compartmentalization and de-bulking, posterior wall isolation (PWI) allows the reduction of LA critical mass and also the suppression of AF triggers and drivers 5. Percutaneous PWI derives from the Cox maze IV surgical procedure 6 and it may be achieved by creating a roof line together with a line, close to the floor of the LA, joining the lower borders of the inferior pulmonary veins on top of PVI 7. PWI seems to be an alternative option in persistent AF treatment, though technically demanding, uncertain in terms of arrhythmic recurrences (ARs) 8,9 and burdened by a great incidence of reconnections at follow-up 10,11. Contact-force (CF) technology was not, however, routinely used in previous prospective studies on percutaneous PWI, even if it provides deeper and more durable lesions when integrated in ablation catheters either in paroxysmal or in persistent AF ablations 12-14. In this retrospective study, we present our experience with PWI using CF sensing catheters and its efficacy and safety as an adjunctive ablation strategy on top of PVI for management of patients with persistent and longstanding persistent AF.
Methods
Study population
Consecutive patients with symptomatic and drug-refractory persistent or long-standing persistent AF who underwent CF supported PWI on top of PVI between October 2012 and October 2018 at Istituto Clinico Sant’Ambrogio (Milan, Italy) were retrospectively analysed. All data were obtained by review of the electronic medical records and any incongruent data was appropriately evaluated by two independent reviewers. Persistent AF was defined as AF episodes lasting longer than 7 days but less than 1 year. Longstanding persistent AF was defined as AF lasting longer than 1 year 1. Prior catheter ablations did not represent an exclusion criterion, unless ablation strategies other than PVI were included in their prior procedures. To assess the confounding impact of previous ablations on the interpretation of our results, we additionally performed sub-analyses of our data dividing our cohort in two groups according to whether PWI was performed on first or repeat ablation and reported results as appropriate. Patients who had less than 3 months of follow-up post procedure were excluded from the analysis. Demographic data and baseline characteristics were collected in all patients. The study protocol adhered to the Declaration of Helsinki and was approved by our institutional Review Board.
Catheter ablation procedure
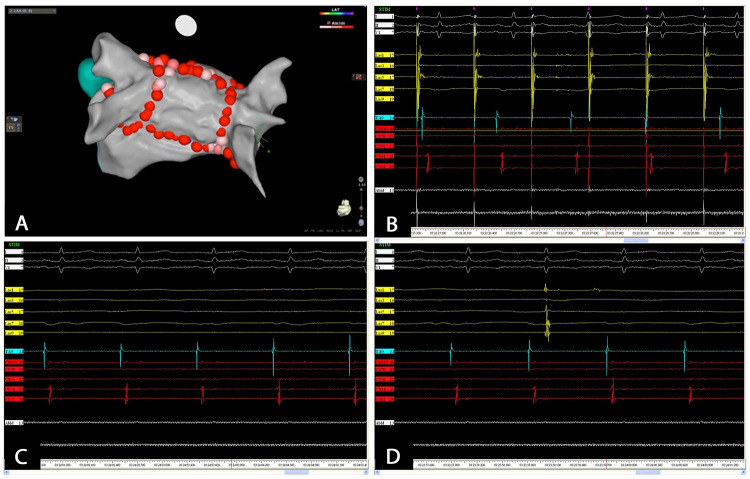
AF radiofrequency catheter ablations were performed by 4 experienced cardiac electrophysiologists using standard protocols. All procedures were undertaken under general anaesthesia and after the exclusion of the presence of an intracardiac thrombus with an intraoperative trans-oesophageal echocardiographic exam. All patients received intravenous unfractioned heparin with a target Activated Coagulation Time (ACT) of 350-400 sec. Using a single transfemoral venous access and a single transseptal puncture, a circular mapping catheter (Lasso® Catheter, Biosense Webster or Reflexion SpiralTM, Abbot Laboratories) and a 3.5-mm open-irrigated-tip CF supported ablation catheter (Thermo Cool® Smart Touch® Biosense Webster or TactiCathTM Abbot Laboratories) were positioned in the LA. Catheter ablation was assisted by 3D mapping with image integration (NavX velocity, Abbot Laboratories or CARTO3, Biosense Webster). In all patients PVI was performed by creating a wide antral ablation line around each pair of ipsilateral pulmonary veins with point-by-point RF delivery. Ablation in the carina segments was optional. In case of repeat procedures, only pulmonary veins with evidence of electrical re-connections were isolated. Following PVI, PWI was performed with ‘roof line’ at the most cranial aspect of the LA, followed by ‘floor line’ joining the most inferior margin of the inferior pulmonary veins [Figure 1A]. Ablations were performed with point-by-point tags until separation or attenuation of the local electrogram limiting power to 30-35 W for anterior wall and 25-30 W for posterior wall. Ablation targets were points showing local CF values comprised between 10 g and 40 g. In patients with persistent AF at the end of the procedure, sinus rhythm was restored using external cardioversion. Electrical disconnection between pulmonary veins and LA was validated with circular mapping catheter positioned at the ostia of the respective PVs. Endpoint of the procedure was complete isolation of the posterior wall that was validated by positioning the circular mapping catheter in the midposterior wall of LA. Electrical block was validated by pacing from posterior wall showing local capture and exit block to the remainder of the atrium [Figure 1B] or by showing absence of local potential and/or the appearance of a dissociated potential ([Figure 1C] and [Figure 1D]). If further atrial flutter or focal atrial tachycardia (AT) were observed, mapping and ablation were performed as appropriate. Persistency of PVI and PWI was confirmed at 30 minutes from the beginning of the ablation. Atrial bursts aimed at testing AF inducibility were performed at the end of the procedure. Cavo-tricuspid isthmus ablation was performed in patients who had history of typical atrial flutter. An oesophageal temperature probe (Esotherm, FIAB Spa, Firenze, Italy) was used in all patients and radiofrequency delivery was interrupted for temperature elevations greater than 40 °C. Radiofrequency, fluoroscopy and procedural times and incidence of complications were collected for each procedure.
Figure 1. Following PVI, PWI is performed with roof and floor lines (panel A). PWI is validated with pacing maneuvers within the posterior wall showing electrical dissociation from left atrium (panel B). Electrical block is also validated by evidence of absence of local potential (panel C) and/or appearance of a dissociated potential within the posterior wall (panel D).
Follow-up and clinical outcomes
Patients received a prescription of pantoprazole (40 mg daily) for 4 weeks after discharge to avoid gastroparesis. Oral anticoagulants were stopped at 3-month of follow-up based on CHA2DS2 VASC-score, while antiarrhythmic drugs (AAD) were withdrawn at 3 months (if prescribed at discharge) or continued at discretion of physician. Routine follow-up assessments were conducted in accordance with a standard protocol at our centre. Follow-up consultations in the outpatient clinic were scheduled at 3, 6, 12 and 24 months after procedure and in any case if needed based on AF-related symptoms. Each follow-up focused on assessment of ARs and AF-related symptoms. AR was defined as any documented episode of atrial arrhythmia (including AF, atrial flutter or AT) lasting longer than 30 seconds and occurring after 90 days after ablation (blanking period). Any AR observed within 3-month after ablation was defined as early AR.
Assessment of ARs was based on 24-h Holter ECG monitoring that was routinely ordered at 3-6-12 and 24 months post procedure and as needed thereafter to assess symptoms. In patients provided with insertable cardiac monitors (ICMs) implanted before procedure, the AR detection was based on continuous monitoring. ICMs included implantable electronic devices (CIEDS) with atrial leads or implantable loop recorders (ILRs). ILRs were most commonly Reveal XT (Medtronic, Minneapolis, MN). The ILRs are able to detect episodes of atrial arrhythmias lasting at least 2 minutes, while for CIEDS individual manufacturer provides specific atrial arrhythmia detection algorithms. ICM was able to provide, over time, the number of arrhythmia episodes, their duration, and, when all durations of AF/AT episodes were added, the AF/AT burden. The AF/AT burden was calculated as the percentage of time in AF/AT between each follow-up visit, based on manually adjudicated episodes. During follow-up, repeat ablations or electrical cardioversions and/or drug adjustment were performed based on documented and clinically relevant ARs beyond the ‘blanking period’ and on clinical judgement.
Statistical Analysis
Continuous variables are summarized as mean ± standard deviation (SD), whereas categorical variables as count and percentages. Statistical differences between the two groups were assessed with a t-test for continuous variables and a Chi-squared or Fisher’s exact test for categorical variables, respectively. Incidence of ARs were estimated using the Kaplan–Meier method for the entire cohort and log-rank test was performed to present and compare survivals in first and repeat ablation groups. A Cox hazard regression analysis was performed to determine predictors of ARs. All parameters that had a suggested association with recurrence by univariate analysis (P <0.1) were included in a stepwise regression analysis, and the results were reported as hazard ratios (HR) with 95% confidence intervals (CIs). All data were analysed using SPSS statistical software, version 22.0 (IBM Corp., Chicago, IL, USA). A two-sided P value ≤ 0.05 was considered statistically significant.
Results
Study population
After exclusion of 8 patients who had undergone previous either surgical o percutaneous PWI, 10 patients who experienced previous linear ablations in LA (roof and anterior lines) and 1 patient with less than 3 months of follow-up, 73 patients were included in this study. Baseline characteristics of the study population are presented in [Table 1]. The mean age was 60.9±10.0 years and 8 (10.9) patients presented longstanding persistent AF. PWI was performed on a repeat procedure in 48 patients (65.7%), who had previously undergone PVI. AF duration prior PWI was longer in patients with history of previous ablation than in those at first procedure (2.8 ± 1.3 vs 1.5 ± 1.2, p=0.04). ICMs, which included 29 loop recorders, 2 pacemakers and 1 cardiac defibrillator, were present in 32 (43.8%) patients.
Table 1. Baseline characteristics.
All values represent mean±standard deviation or number and (percentage). AF: atrial fibrillation; AAD: anti arrhythmic drugs; CAD: coronary artery disease; ICM: insertable cardiac monitor; LA: left atrium. LVEF: left ventricular ejection fraction; PVI: pulmonartìy vein isolation; TIA: transient ischemic attack.
| Study population (n = 73) | |
|---|---|
| Mean age (yr) | 60.9 ± 10.0 |
| Females, n (%) | 15 (20.5) |
| AF duration (years from diagnosis) | 2.1 ± 1.3 |
| Longstanding persistent AF, n (%) | 8 (10.9) |
| Previous ablation in LA (PVI), n (%) | 48 (65.7) |
| Number of tested AAD | 1.4± 0.6 |
| Hypertension, n (%) | 41 (69.8) |
| Diabetes, n (%) | 10 (13.6) |
| CAD, n (%) | 7 (9.6) |
| Previous stroke or TIA, n (%) | 4 (5.5) |
| LVEF (%) | 52.9 ± 8.5 |
| LA diameter (mm) | 45.6 ± 5.5 |
| ICM, n (%) | 32 (43.8) |
Catheter ablation procedure
[Table 2] shows procedural characteristics for the entire population and additionally for two groups divided according to the history of previous ablation (first and repeat ablation groups). PVI was acutely achieved in all patients. PWI was acutely completed at index procedure in 65 (89.0%) patients, while remaining patients showed insuperable oesophageal temperatures (greater than 40°C). Reversion to sinus rhythm during ablation occurred in 8 (10.9%) patients. In repeat ablation group, 9 out of 48 (18.7%) patients showed reconnections in at least one pulmonary vein that were re-isolated as appropriate. Average CF per ablation lesions at all sites was 17.4 ± 3.7 g. Average CF per ablation lesion was 13.2 ± 3.7, 18.2 ± 4.7, 18.2 ± 4.2 and 20.2 ± 2.7 g at the anterior aspect of the right and left pulmonary veins sites, at roof and posterior wall sites, respectively. The average procedure duration was 178.1±40.9 min. No differences in procedural characteristics were observed between first and repeat ablation groups. With respect to complications, we observed 1 femoral artero-venous fistula and 1 femoral pseudoaneurysm conservatively treated and equally distributed in first and repeat ablation groups.
Table 2. Procedural variables.
All values represent mean±standard deviation or number and (percentage). PWI: posterior wall isolation. *Includes transesophageal echocardiography and administration of general anesthesia and mechanical ventilation.
| Study population Overall (n = 73) | First ablation group (n=25) | Repeat ablation group (n =48) | p-value | |
|---|---|---|---|---|
| Completion of PWI, n (%) | 65 (89.0%) | 23 (92.0) | 42 (87.5) | 0.29 |
| Restoration of sinus rhythm during ablation, n (%) | 8 (10.9%) | 3 (12.0) | 7 (14.5) | 0.45 |
| CARTO 3/Navx, n | 49/24 | 16/9 | 33/15 | 0.86 |
| Cavo-tricuspid isthmus ablation, n (%) | 30 (41.1%) | 11 (44.0) | 17 (35.0) | 0.12 |
| Procedure time (min)* | 178.1 ± 40.9 | 169.1 ± 38.9 | 152.1 ± 42.9 | 0.13 |
| Ablation time (min) | 44.1 ± 12.4 | 49.1 ± 18.6 | 36.2 ± 15.4 | 0.12 |
| Fluoroscopy time (min) | 26.6 ± 13.8 | 28.3 ± 11.0 | 22.4 ± 11.7 | 0.23 |
Follow-up and clinical outcomes
Patients were followed for a mean follow-up of 28.2 ± 2.7 months. At the end of follow-up period, total ARs were 27 (30.1%), of which 21 were AF, 5 were atrial flutter and 1 was AT. In 55 patients (75.3%) AAD were withdrawn at the end of the 90-day blanking period. Among patients experiencing ARs, 8 patients required elective cardioversion outside the blanking period and 10 patients underwent a repeat ablation during the follow-up (1 for AT, 4 for atrial flutter, 5 for AF). Of them, 4 patients presented incompleteness of PWI at endocardial mapping. Among 8 patients with incomplete PWI at index procedure, 3 patients experienced ARs at follow up, with 2 patients treated with repeat ablations and 1 with drug adjustment.
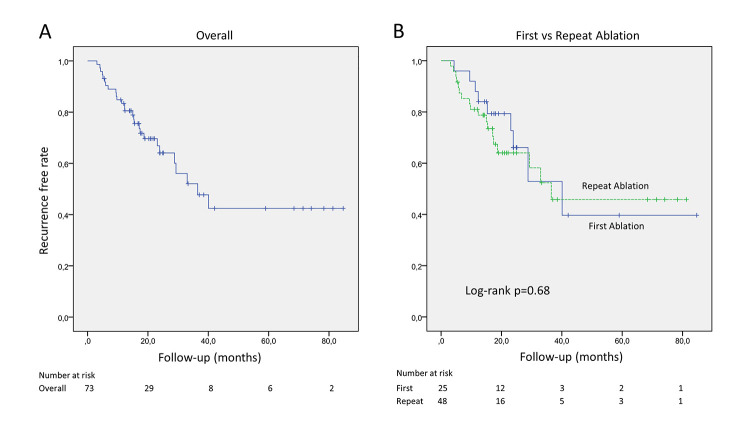
At 1 and 2-year follow-up, freedom from ARs was observed in 80.5% and 64.1% of patients, respectively. The Kaplan-Meier estimates of freedom from any ARs across the entire cohort is shown in [Figure 2A]. At sub analysis of the data according to the history of previous ablations, 9 (36.0%) and 18 (37.5%) ARs were observed in first and repeat ablation groups, at the end of follow-up, respectively. AR free survival curves are shown for the two groups in [Figure 2B], reporting no differences between groups at one-year (84.0% vs 81.0%) and two-years (66.1% vs 64.0%) of follow-up, log-Rank p = 0.68 [Figure 2B].
Figure 2. Kaplan-Meier curves show arrhythmia-free survivals associated with PWI on top of PVI in overall population (panel A) and in patients grouped according to the history of previous ablations (PVI) (panel B).
[Table 3] shows results of Cox hazard regression analyses concerning potential factors associated with risk of ARs after blanking period at follow-up. Previous ablation was not associated with risk of recurrence, while occurrence of early ARs during blanking period was independently associated with incidence of ARs at follow-up (HR 2.47, CI 0.99-6.15, p=0.04). In addition, the achievement of complete PWI during the index procedure demonstrated a favorable effect in averting recurrences at follow up (p=0.08), however statistical significance was not reached.
Table 3. Cox hazard regression analyses of the risks of arrhythmic recurrences.
AR: arrhythmic recurrence; LA: left atrium; LVEF: left ventricular ejection fraction; PVI: pulmonary vein isolation; PWI: posterior wall isolation; HR: hazard ratio; CI: confidence interval
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 0.99 (0.95-2.79) | 0.91 | ||
| Females | 1.09 (0.41-2.91) | 0.85 | ||
| Previous ablation in LA (PVI) | 0.50 (0.21-1.19) | 0.12 | ||
| LVEF < 40% | 2.73 (0.64-7.6) | 0.17 | ||
| LA diameter > 40 mm | 1.19 (0.47-2.99) | 0.70 | ||
| Complete PWI | 0.42 (0.16-1.11) | 0.08 | 0.41 (0.15-1.16) | 0.08 |
| Early AR | 2.45 (0.98-6.10) | 0.05 | 2.47 (0.99-6.15) | 0.04 |
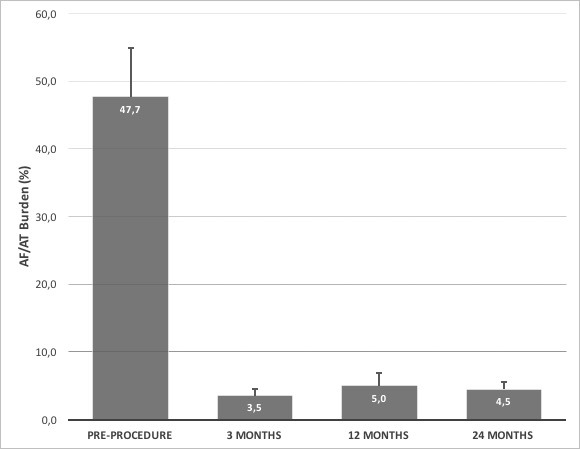
Among 32 patients provided with ICMs, PWI on top of PVI was able to significantly reduce the mean burdens of ARs (more than 50%) compared with time prior PWI+PVI procedure, reporting levels ≤ 5% at 3, 12 and 24 months after index procedure [Figure 3].
Figure 3. The graphic shows AF/AT burdens detected by ICMs at different time points: prior to ablation, at 3,12 and 24 months following PWI on top of PVI. All values represent mean + standard deviation.
Discussion
In this study, we reported efficacy and safety at 1 and 2 years followup of PWI on top of PVI on persistent and long standing persistent AF patients when performed with CF sensing catheters. We showed that early recurrences within 90 days after procedure were associated with a two-fold increased risk of ARs at follow-up. Furthermore, we reported that CF supported PWI +PVI provided great and persisting reduction of burden of ARs over time.
The posterior wall or ‘pulmonary venous component’ of the LA seems an attractive target for ablation, since there is anatomic and electrophysiological evidence that it contributes to the genesis and maintenance of AF 5. Furthermore, the posterior wall is a part of the critical mass necessary to maintain AF and so debulking the LA through PWI can reduce AF burden 15. Despite PWI has not been shown to have additional benefit to PVI in paroxysmal AF 16, it can reduce arrhythmia in persistent AF, as reported in previous studies, however data are still conflicting 8,9. CF sensing catheters are known to be associated with deeper and more durable lesions either in paroxysmal 12,13 or, as recently reported, in persistent AF ablations 14 and are nowadays advisable also for safety concerns. Actually, real-tim CF assessment helps in detection of excessive pressures during ablation, thus avoiding dangerous overtreatments.
The role of CF technology in efficacy and safety of PWI on top of PVI has not been deeply investigated in prospective trials, but a retrospective study demonstrated the superior efficacy of PVI plus PWI using CF sensing compared with standard radiofrequency ablation catheters for patients with persistent AF 17. In this study, the reported 12 months rates of freedom from ARs were 85% vs 70% in CF vs non-CF group, respectively. However, the authors investigated the role of non-PV triggers ablation in addition to PWI on top of PVI, providing a potential bias in the analysis of the results. In our study, we analysed only procedures where PWI was firstly attempted on top of PVI, without targeting additional ablation strategies. Furthermore, we evaluated incidence of ARs at longer follow-up period (2 years). In our study, arrhythmias-free survivals at 1 and 2-year follow-up of PWI on top of PVI performed with CF sensing catheters were 80.5% and 64.1% respectively, with 75.3% of patients free from AAD at follow up. A recent randomized trial reported a 73.5% freedom from ARs on and off AAD after PWI in addition to PVI after a mean follow-up of 16.2±8.8 months without the routinely use of CF sensing 18. Based on our results, routine use of CF sensing catheters while performing PWI might ameliorate efficacy of PWI in terms of incidence of ARs at follow up.
With respect to predictors of recurrence, we found that occurrence of early ARs during blanking period was independently associated with risk of ARs at follow-up. This association was previously reported in literature for different ablation strategies in AF 19,20. In our series, we reported for the first time that detection of early ARs during blanking period is associated with a 2-fold increased risk of late recurrences after PWI+PVI. It is possible that inflammatory response related to ablation might favor early irritability in LA after ablations and, consequently, the incidence of early ARs soon after the ablation. Based on that, it could be speculated that PWI on top of PVI is associated with more extensive myocardial damage and greater inflammatory response than PVI alone, influencing the incidence of early ARs. However, in a randomized study, Kim et al. showed that levels of hs-CRP, CK-MB and troponin-T were similar in PVI alone and PVI+PWI groups after ablation, proving that inflammatory response and myocardial injury are not increased PWI on top of PVI procedures compared with PVI alone (21). The relatively high proportion of patients (43.8%) provided with continuous monitoring in our cohort favored a more accurate detection of arrhythmic events, reinforcing this result.
Furthermore, we reported that complete PWI during the index procedure provided a favorable effect in averting recurrences at follow up, despite on our small sample size statistical significance was not reached. Similarly, Kim et al. previously reported that acutely achievement of PWI is a strong predictor of ARs at follow up in a randomized study including patients undergoing PVI+PWI 21. However, in another observational study PWI incompleteness at index procedure did not impact the outcome 22. Nevertheless, documented complete posterior wall electrical dissociation is generally the main endpoint of the procedure. It is out of doubt, however, that completeness of PWI is often challenging and, even with meticulous point by point linear ablations, residual gaps often persist 10,11. In our study, the incompleteness of PWI was observed only in cases where radiofrequency power delivery was reduced or stopped due to excessive temperatures detected at the intraluminal oesophageal probe and not for insuperable technical difficulties. Based on our retrospective analysis, CF sensing catheters supported the operator in achieving complete PWI, however larger and prospective studies are needed to evaluate the role of CF sensing catheters on such procedural endpoint during PWI execution.
With respect to patients who underwent previous PVI before performing PWI, repeat ablation was not associated with risk of ARs at follow up, as previously reported 22. As expected, patients undergoing PWI on a repeat ablation showed longer AF history compared with patients who underwent ablation for the first time. On the other hand, procedural times and complication rates were similar in the two groups. The inclusion of patients with history of previous ablation in our study can be questionable, as it introduces a possible source of confounding factors. However, the aim of our study was to describe the routine clinical practice of our centre between 2012 and 2018, where persistent AF patients underwent PWI as an adjunctive strategy more frequently after failure of previous PVI strategy than at a first ablation procedure.
Finally, in patients provided with ICMs, our data reported an important reduction of AF/AT burden after PWI on top of PVI compared with burden at time prior to ablation and this data persisted over time. Our data showed that AF/AT burden was reduced at very low levels (≤5%), if compared with CABANA trial and STAR-AF II trial sub-studies, where reported AF/AT burdens at 12 months after AF ablations were 6.3% and 6.8%, respectively 23,24. AF/AT burden after ablation seems to provide better assessment of outcome of AF catheter ablation, when compared with conventional definition of AF ablation success (occurrence of any AF/AT or flutter lasting >30 seconds), because it is based on clinically relevant recurrences compared with the absolute freedom from recurrence 25. In this regard, patients undergoing PWI are generally the most challenging ones, presenting with persistent AF, multiple previous ablations and disabling AF related symptoms for many years. In this context, as AF ablation success rate is currently unsatisfactory 3, AF/AT burden reduction could be a desirable target and, based on our results, PWI could play an important role. However, further studies are needed aimed at evaluating the success rate of PWI on top of PVI based on continuous monitoring in all patients.
Limitations of the study
The major limitation of this study is the absence of a comparator group. Although our data suggest that CF sensing catheters can achieve reduction in ARs and durably low AF/AT burden in patients with persistent AF, we are not able to comment on whether similar outcomes may be achievable without CF supported PWI. Furthermore, the retrospective nature of the study and the small sample size limit the power of the analysis. Another limitation is that a great number of patients (65.7%) underwent CF guided PWI after previous ablation in LA, that could be a confounding factor in efficacy and safety assessment of PWI on top of PVI. However, only PVI-based ablations performed before the PWI index procedure were included in the analysis. To assess the weight of this possible confounding factor on our results, we performed sub-analyses of our data dividing our cohort in two groups according to the history of previous ablations and we found no significant differences in procedural results and follow-up outcomes. Another limitation of this study is that AF burden data, provided at ICMs analysis, were derived from less than 50% of patients of our population, reducing the power of our conclusions.
Conclusions
CF supported PWI on top of PVI in persistent AF ablation was as effective as safe at 1 and 2 years of follow-up. Early ARs during blanking period predicted the incidence of late recurrences at follow-up. PWI on top of PVI provided significant and persistent AF/AT burden reduction over time. Prospective studies aimed at evaluating the efficacy of PWI on top of PVI using CF sensing catheters with lesion detecting tools and ICMs as monitoring systems in all patients are advisable.
References
- 1.Calkins Hugh, Hindricks Gerhard, Cappato Riccardo, Kim Young-Hoon, Saad Eduardo B, Aguinaga Luis, Akar Joseph G, Badhwar Vinay, Brugada Josep, Camm John, Chen Peng-Sheng, Chen Shih-Ann, Chung Mina K, Nielsen Jens Cosedis, Curtis Anne B, Davies D Wyn, Day John D, d'Avila André, de Groot N M S Natasja, Di Biase Luigi, Duytschaever Mattias, Edgerton James R, Ellenbogen Kenneth A, Ellinor Patrick T, Ernst Sabine, Fenelon Guilherme, Gerstenfeld Edward P, Haines David E, Haissaguerre Michel, Helm Robert H, Hylek Elaine, Jackman Warren M, Jalife Jose, Kalman Jonathan M, Kautzner Josef, Kottkamp Hans, Kuck Karl Heinz, Kumagai Koichiro, Lee Richard, Lewalter Thorsten, Lindsay Bruce D, Macle Laurent, Mansour Moussa, Marchlinski Francis E, Michaud Gregory F, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Okumura Ken, Packer Douglas, Pokushalov Evgeny, Reynolds Matthew R, Sanders Prashanthan, Scanavacca Mauricio, Schilling Richard, Tondo Claudio, Tsao Hsuan-Ming, Verma Atul, Wilber David J, Yamane Teiichi. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017 Oct;14 (10):e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink Thomas, Schlüter Michael, Heeger Christian-Hendrik, Lemes Christine, Maurer Tilman, Reissmann Bruno, Riedl Johannes, Rottner Laura, Santoro Francesco, Schmidt Boris, Wohlmuth Peter, Mathew Shibu, Sohns Christian, Ouyang Feifan, Metzner Andreas, Kuck Karl-Heinz. Stand-Alone Pulmonary Vein Isolation Versus Pulmonary Vein Isolation With Additional Substrate Modification as Index Ablation Procedures in Patients With Persistent and Long-Standing Persistent Atrial Fibrillation: The Randomized Alster-Lost-AF Trial (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation). Circ Arrhythm Electrophysiol. 2017 Jul;10 (7) doi: 10.1161/CIRCEP.117.005114. [DOI] [PubMed] [Google Scholar]
- 3.Nyong Jonathan, Amit Guy, Adler Alma J, Owolabi Onikepe O, Perel Pablo, Prieto-Merino David, Lambiase Pier, Casas Juan Pablo, Morillo Carlos A. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016 Nov 22;11 () doi: 10.1002/14651858.CD012088.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voskoboinik Aleksandr, Moskovitch Jeremy T, Harel Nadav, Sanders Prashanthan, Kistler Peter M, Kalman Jonathan M. Revisiting pulmonary vein isolation alone for persistent atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm. 2017 May;14 (5):661–667. doi: 10.1016/j.hrthm.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Elbatran Ahmed I, Anderson Robert H, Mori Shumpei, Saba Magdi M. The rationale for isolation of the left atrial pulmonary venous component to control atrial fibrillation: A review article. Heart Rhythm. 2019 Sep;16 (9):1392–1398. doi: 10.1016/j.hrthm.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Cox J L, Schuessler R B, D'Agostino H J, Stone C M, Chang B C, Cain M E, Corr P B, Boineau J P. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991 Apr;101 (4):569–83. [PubMed] [Google Scholar]
- 7.Sugumar Hariharan, Thomas Stuart P, Prabhu Sandeep, Voskoboinik Aleksandr, Kistler Peter M. How to perform posterior wall isolation in catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018 Feb;29 (2):345–352. doi: 10.1111/jce.13397. [DOI] [PubMed] [Google Scholar]
- 8.Salih Mohsin, Darrat Yousef, Ibrahim Abdisamad M, Al-Akchar Mohammad, Bhattarai Mukul, Koester Cameron, Ayan Mohamed, Labedi Mohamed, Elayi Claude S. Clinical outcomes of adjunctive posterior wall isolation in persistent atrial fibrillation: A meta-analysis. J Cardiovasc Electrophysiol. 2020 Jun;31 (6):1394–1402. doi: 10.1111/jce.14480. [DOI] [PubMed] [Google Scholar]
- 9.Thiyagarajah Anand, Kadhim Kadhim, Lau Dennis H, Emami Mehrdad, Linz Dominik, Khokhar Kashif, Munawar Dian A, Mishima Ricardo, Malik Varun, O'Shea Catherine, Mahajan Rajiv, Sanders Prashanthan. Feasibility, Safety, and Efficacy of Posterior Wall Isolation During Atrial Fibrillation Ablation: A Systematic Review and Meta-Analysis. Circ Arrhythm Electrophysiol. 2019 Aug;12 (8) doi: 10.1161/CIRCEP.118.007005. [DOI] [PubMed] [Google Scholar]
- 10.Markman Timothy M, Hyman Matthew C, Kumareswaran Ramanan, Arkles Jeffrey S, Santangeli Pasquale, Schaller Robert D, Supple Gregory E, Frankel David S, Riley Michael P, Lin David, Garcia Fermin, Dixit Sanjay, Callans David J, Marchlinski Francis E, Nazarian Saman. Durability of posterior wall isolation after catheter ablation among patients with recurrent atrial fibrillation. Heart Rhythm. 2020 Oct;17 (10):1740–1744. doi: 10.1016/j.hrthm.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Bai Rong, Di Biase Luigi, Mohanty Prasant, Trivedi Chintan, Dello Russo Antonio, Themistoclakis Sakis, Casella Michela, Santarelli Pietro, Fassini Gaetano, Santangeli Pasquale, Mohanty Sanghamitra, Rossillo Antonio, Pelargonio Gemma, Horton Rodney, Sanchez Javier, Gallinghouse Joseph, Burkhardt J David, Ma Chang-Sheng, Tondo Claudio, Natale Andrea. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2016 Jan;13 (1):132–40. doi: 10.1016/j.hrthm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Reddy Vivek Y, Dukkipati Srinivas R, Neuzil Petr, Natale Andrea, Albenque Jean-Paul, Kautzner Josef, Shah Dipen, Michaud Gregory, Wharton Marcus, Harari David, Mahapatra Srijoy, Lambert Hendrik, Mansour Moussa. Randomized, Controlled Trial of the Safety and Effectiveness of a Contact Force-Sensing Irrigated Catheter for Ablation of Paroxysmal Atrial Fibrillation: Results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation. 2015 Sep 08;132 (10):907–15. doi: 10.1161/CIRCULATIONAHA.114.014092. [DOI] [PubMed] [Google Scholar]
- 13.Kautzner Josef, Neuzil Petr, Lambert Hendrik, Peichl Petr, Petru Jan, Cihak Robert, Skoda Jan, Wichterle Dan, Wissner Erik, Yulzari Aude, Kuck Karl-Heinz. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015 Aug;17 (8):1229–35. doi: 10.1093/europace/euv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansour Moussa, Calkins Hugh, Osorio Jose, Pollak Scott J, Melby Daniel, Marchlinski Francis E, Athill Charles A, Delaughter Craig, Patel Anshul M, Gentlesk Philip J, DeVille Brian, Macle Laurent, Ellenbogen Kenneth A, Dukkipati Srinivas R, Reddy Vivek Y, Natale Andrea. Persistent Atrial Fibrillation Ablation With Contact Force-Sensing Catheter: The Prospective Multicenter PRECEPT Trial. JACC Clin Electrophysiol. 2020 Aug;6 (8):958–969. doi: 10.1016/j.jacep.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Lee Anson M, Aziz Abdulhameed, Didesch Jacob, Clark Kal L, Schuessler Richard B, Damiano Ralph J. Importance of atrial surface area and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. J Thorac Cardiovasc Surg. 2013 Sep;146 (3):593–8. doi: 10.1016/j.jtcvs.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Tae-Hoon, Park Junbeom, Park Jin-Kyu, Uhm Jae-Sun, Joung Boyoung, Hwang Chun, Lee Moon-Hyoung, Pak Hui-Nam. Linear ablation in addition to circumferential pulmonary vein isolation (Dallas lesion set) does not improve clinical outcome in patients with paroxysmal atrial fibrillation: a prospective randomized study. Europace. 2015 Mar;17 (3):388–95. doi: 10.1093/europace/euu245. [DOI] [PubMed] [Google Scholar]
- 17.Takamiya Tomomasa, Nitta Junichi, Inaba Osamu, Sato Akira, Ikenouchi Takashi, Murata Kazuya, Inamura Yukihiro, Takahashi Yoshihide, Goya Masahiko, Hirao Kenzo. One-year outcomes after pulmonary vein isolation plus posterior wall isolation and additional non-pulmonary vein trigger ablation for persistent atrial fibrillation with or without contact force sensing: a propensity score-matched comparison. J Interv Card Electrophysiol. 2020 Dec;59 (3):585–593. doi: 10.1007/s10840-019-00700-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee Jung Myung, Shim Jaemin, Park Junbeom, Yu Hee Tae, Kim Tae-Hoon, Park Jin-Kyu, Uhm Jae-Sun, Kim Jin-Bae, Joung Boyoung, Lee Moon-Hyoung, Kim Young-Hoon, Pak Hui-Nam. The Electrical Isolation of the Left Atrial Posterior Wall in Catheter Ablation of Persistent Atrial Fibrillation. JACC Clin Electrophysiol. 2019 Nov;5 (11):1253–1261. doi: 10.1016/j.jacep.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Vaishnav Aditi S, Levine Evan, Coleman Kristie M, Beldner Stuart J, Chinitz Jason S, Bhasin Kabir, Bernstein Neil E, Skipitaris Nicholas T, Mountantonakis Stavros E. Early recurrence of atrial fibrillation after pulmonary vein isolation: a comparative analysis between cryogenic and contact force radiofrequency ablation. J Interv Card Electrophysiol. 2020 Jan;57 (1):67–75. doi: 10.1007/s10840-019-00639-3. [DOI] [PubMed] [Google Scholar]
- 20.Andrade Jason G, Macle Laurent, Khairy Paul, Khaykin Yaariv, Mantovan Roberto, De Martino Giuseppe, Chen Jian, Morillo Carlos A, Novak Paul, Guerra Peter G, Nair Girish, Torrecilla Esteban G, Verma Atul. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J Cardiovasc Electrophysiol. 2012 Dec;23 (12):1295–301. doi: 10.1111/j.1540-8167.2012.02399.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim Jin-Seok, Shin Seung Yong, Na Jin Oh, Choi Cheol Ung, Kim Seong Hwan, Kim Jin Won, Kim Eung Ju, Rha Seung-Woon, Park Chang Gyu, Seo Hong Seog, Oh Dong Joo, Hwang Chun, Lim Hong Euy. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation?: A prospective randomized clinical trial. Int J Cardiol. 2015 Feb 15;181 ():277–83. doi: 10.1016/j.ijcard.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Kumar Prabhat, Bamimore Ayotunde M, Schwartz Jennifer D, Chung Eugene H, Gehi Anil K, Kiser Andy C, Hummel James P, Mounsey J Paul. Challenges and Outcomes of Posterior Wall Isolation for Ablation of Atrial Fibrillation. J Am Heart Assoc. 2016 Sep 23;5 (9) doi: 10.1161/JAHA.116.003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole Jeanne E, Bahnson Tristram D, Monahan Kristi H, Johnson George, Rostami Hoss, Silverstein Adam P, Al-Khalidi Hussein R, Rosenberg Yves, Mark Daniel B, Lee Kerry L, Packer Douglas L. Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial. J Am Coll Cardiol. 2020 Jun 30;75 (25):3105–3118. doi: 10.1016/j.jacc.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti Sergio, Jiang Chen-Yang, Betts Timothy R, Chen Jian, Deisenhofer Isabel, Mantovan Roberto, Macle Laurent, Morillo Carlos A, Haverkamp Wilhelm, Weerasooriya Rukshen, Albenque Jean-Paul, Nardi Stefano, Menardi Endrj, Novak Paul, Sanders Prashanthan, Verma Atul. Effect of Different Cutpoints for Defining Success Post-Catheter Ablation for Persistent Atrial Fibrillation: A Substudy of the STAR AF II Trial. JACC Clin Electrophysiol. 2017 May;3 (5):522–523. doi: 10.1016/j.jacep.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Wechselberger Simon, Kronborg Mads, Huo Yan, Piorkowski Judith, Neudeck Sebastian, Päßler Ellen, El-Armouche Ali, Richter Utz, Mayer Julia, Ulbrich Stefan, Pu Liying, Kirstein Bettina, Gaspar Thomas, Piorkowski Christopher. Continuous monitoring after atrial fibrillation ablation: the LINQ AF study. Europace. 2018 Nov 01;20 (FI_3):f312–f320. doi: 10.1093/europace/euy038. [DOI] [PMC free article] [PubMed] [Google Scholar]