Abstract
Background
Post-operative atrial fibrillation (POAF) is common after aortic valve replacement (AVR) and is associated with worse outcomes. We performed a meta-analysis of randomized controlled trials comparing Surgical Aortic Valve Replacement (SAVR) and Transcatheter Aortic Valve Replacement (TAVR) for incidence of POAF at 30 days.
Methods
We searched databases from 1/1/1990 to 1/1/2020 for randomized studies comparing TAVR and SAVR. POAF was defined as either worsening or new-onset atrial fibrillation. Random effects model was used to estimate the risk of POAF with TAVR vs SAVR in all trials, and in subgroups (low, intermediate, high risk, and in self-expandable vs balloon expandable valves). Sensitivity analysis was performed including only studies reporting new-onset atrial fibrillation.
Results
Seven RCTs were identified that enrolled 7,934 patients (3,999 to TAVR and 3,935 to SAVR). The overall incidence of POAF was 9.7% after TAVR and 33.3% after SAVR. TAVR was associated with a lower risk of POAF compared with SAVR (OR 0.21 [0.18-0.24]; P < 0.0001). Compared with SAVR, TAVR was associated with a significantly lower risk of POAF in the high-risk cohort (OR 0.37 [0.27-0.49]; P < 0.0001), in the intermediate-risk cohort (OR 0.23 [0.19-0.28]; P < 0.0001), low-risk cohort (OR 0.13 [0.10-0.16]; P < 0.0001). Sensitivity analysis of 4 trials including only new-onset POAF showed similar summary estimates (OR 0.21, 95% CI [0.18-0.25]; P< 0.0001).
Conclusions
TAVR is associated with a significantly lower risk of post-operative atrial fibrillation compared with SAVR in all strata. Further studies are needed to identify the contribution of post-operative atrial fibrillation to the differences in clinical outcomes after TAVR and SAVR.
Keywords: TAVR, SAVR, Atrial fibrillation, Meta-analysis
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as an alternative to surgical aortic valve replacement (SAVR) 1,2 for treatment of patients with symptomatic severe aortic stenosis (AS) in all risk cohorts 3-5 with short-term follow-up indicating durable results with TAVR. Recent trials comparing TAVR vs SAVR also showed that early and late outcomes are better in TAVR vs SAVR, and that stroke risk at one year is notably lower with TAVR vs SAVR 6.
Post-operative atrial fibrillation (POAF) is common after TAVR and SAVR7. The incidence of POAF has been reported in 20-40% of SAVR and 5-40% of TAVR patients and has been associated with risk of early and late stroke in both TAVR and SAVR 8-10. POAF is also associated with prolonged hospital stay as well as increased morbidity and mortality11.
While prior observational studies reported lower risk of POAF after TAVR vs SAVR, these studies are limited by design. We performed a meta-analysis of randomized trials to examine the incidence of POAF at 30 days after TAVR and SAVR.
Methods
Data sources and eligibility criteria
We conducted and reported this meta-analysis according to the Cochrane Central Register of Controlled Trials (CENTRAL). We performed a systematic computerized search through the MEDLINE, EMBASE, and COCHRANE databases from January 1st, 1990 to January 1st, 2020 using the following search terms separately and in combination: "randomized trial," "TAVR," "SAVR," "Atrial fibrillation," "Complications," and "Aortic stenosis." We performed the same search strategy for abstracts of the major scientific sessions, and ClinicalTrials.gov for any relevant studies. This was complemented with a manual search of PubMed and Google Scholar. Bibliographies of retrieved studies and review articles were searched to identify any additional relevant studies. Authors, country, study population, and affiliated institutions were screened to exclude duplicate studies. Our search was limited to manuscripts in English language. Only randomized studies comparing TAVR vs SAVR were included in this analysis. The search strategy, study selection, and analysis adhered to QUORUM (Quality of Reporting of Meta-Analysis) guidelines for meta-analyses.
Data extraction, outcome definition, and quality assessment
Two independent investigators (HA, RM) extracted the data, including baseline study characteristics, demographics, and outcomes of interest from the retrieved studies. There was good inter-rater agreement between the reviewers with respect to inclusion of studies, study quality, and data abstraction (κ > 0.85). The outcome was POAF, which was defined as new or worsening AF within 30 days post AVR (TAVR or SAVR). The definition of worsening AF was included in three clinical trials [Supplemental Table 1], while the remaining trials did not have a clear definition. Atrial fibrillation was diagnosed as any arrhythmia within hospitalization that has the ECG characteristics of atrial fibrillation (or flutter) and lasts sufficiently long to be recorded on a 12-lead ECG, or at least 30 seconds on a rhythm strip in some of these trials. No specific methods for AF detection were provided in other trials. [Supplemental Table 1] lists the definition of POAF in each trial. Cochrane risk-of-bias tool for randomized trials (RoB 2) was used to describe trial bias.
Supplemental Table 1. Definition of postoperative atrial fibrillation in each study.
| Trial | Definition of POAF |
|---|---|
| PARTNER I | New onset atrial fibrillation determined by an electrocardiography core laboratory |
| Corevalve | New onset or worsening of atrial fibrillation |
| SURTAVI | New onset or worsening of atrial fibrillation |
| PARTNER 2 | New onset atrial fibrillation |
| PARTNER 3 | New onset atrial fibrillation |
| NOTION | New onset or worsening atrial fibrillation during the immediate post-procedure course |
| Evolut | New onset atrial fibrillation |
Data synthesis and statistical analysis
Continuous variables are expressed as means ± standard deviation and categorical variables as counts and percentages. Summary results are presented as mean differences. Random and fixed effects model was used to calculate Odd Ratios (OR) and corresponding 95% confidence interval (CI) given high heterogeneity (I2 > 50% in all analyses). Publication bias was examined by funnel plots and Egger's test.12 Subgroup analysis was conducted for high, intermediate and low risk cohorts, and valve type (balloon-expandable and self-expandable TAVR). Sensitivity analysis was performed by limiting analysis to trials that included only new-onset AF. Data collection, study selection, processing of the data, and reporting of results were performed according to accepted principles of systemic review and meta-analysis 13 All P-values were two-tailed, with statistical significance set at 0.05, and CIs were calculated at the 95% level. Review Manager software (Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used to perform the analysis.
Results
Search Results
The database search identified 203 relevant references or citations [Figure 1]. After duplicate removal and title and abstract reading, 182 records were excluded, and 8 were considered for full-text analysis. Of these, 1 article was excluded because it did not report POAF, and 7 studies were selected for the meta-analysis (PARTNER 114, CoreValve15, NOTION16, PARTNER 217, SURTAVI18, PARTNER 319, Evolut20).
Figure 1. CONSORT diagram.
Characteristics of the studies
A total of 7 randomized trials with 7,934 patients met our selection criteria. Of these, 3,999 patients were randomized to TAVR and 3,935 were randomized to SAVR. The baseline patient characteristics are listed in [Table 1]. Males constitute 59.0% of the cohort, 29.2% had a previous history of atrial fibrillation (AF), and 8% of patients had known prior history of a cerebrovascular accident. The Society of Thoracic Surgeons (STS) perioperative risk of mortality ranged from low (1.9%) in the “low-risk” TAVR trials to high (11.8%) in the “high-risk” studies. Transfemoral access was used in 90% of patients in the composite analysis.
Table 1. Baseline Characteristics of the 7 studies.
TAVR = Transcatheter aortic valve replacement; SAVR: Surgical aortic valve replacement; STS PROM= Society of Thoracic Surgery perioperative risk of mortality score; CVA = cerebrovascular accident; CKD = chronic kidney disease; PVD = Peripheral vascular disease; CABG = Coronary artery bypass grafting; PCI= percutaneous coronary intervention; AF= Atrial fibrillation; NA= Not applicable; NK= Not known.
| High risk | Intermediate risk | Low risk | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Partner 1 | CoreValve | Partner 2 | SURTAVI | Partner 3 | Evolut | Notion | ||||||||
| TAVR | SAVR | TAVR | SAVR | TAVR | SAVR | TAVR | SAVR | TAVR | SAVR | TAVR | SAVR | TAVR | SAVR | |
| Trials’ characteristics | ||||||||||||||
| Number of centers | 25 | 45 | 57 | 87 | 71 | 86 | 3 | |||||||
| Recruitment period | 2007-2009 | 2011-2012 | 2011-2013 | 2012-2016 | 2016-2017 | 2016-2018 | 2009-2013 | |||||||
| Longest follow-up, year | 5 | 3 | 2 | 2 | 1 | 2 | 2 | |||||||
| Design | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority and Superiority | Non-inferiority | Superiority | |||||||
| ITT patients | 348 | 351 | 394 | 401 | 1011 | 1021 | 864 | 796 | 503 | 497 | 734 | 734 | 145 | 135 |
| As-treated patients | 344 | 313 | 391 | 359 | 994 | 944 | 863 | 794 | 496 | 454 | 725 | 678 | 142 | 134 |
| Patients’ characteristics | ||||||||||||||
| Age, mean (SD) | 83.6 ± 6.8 | 84.5 ± 6.4 | 83.5 ± 7.1 | 83.5 ± 6.3 | 81.5 ± 6.7 | 81.7 ± 6.7 | 79.9 ± 6.2 | 79.7 ± 6.1 | 73.3 ± 5.8 | 73.6 ± 6.1 | 74.1 ± 5.8 | 73.6 ± 5.9 | 79.2 ± 4.9 | 79.0 ± 4.7 |
| Women, n (%) | 147 (42.2%) | 153 (43.6%) | 183 (46.4%) | 189 (47.1%) | 463 (45.8%) | 461 (45.2%) | 366 (42.4%) | 358 (45.0%) | 161 (32.0%) | 131 (26.4%) | 261 (35.6%) | 229 (31.2%) | 67 (46.2%) | 64 (47.4%) |
| STS, mean ± SD | 11.8± 3.3 | 11.7 ± 3.5 | 7.3 ± 3.0 | 7.5 ± 3.2 | 5.8 ± 2.1 | 5.8 ± 1.9 | 4.4 ± 1.5 | 4.5 ± 1.6 | 1.9 ± 0.7 | 1.9 ± 0.6 | 1.9 ± 0.7 | 1.9 ± 0.7 | 2.9 ± 1.6 | 3.1 ± 1.7 |
| CKD, n (%) | 38 (10.9%) | 24 (6.8%) | 48 (12.2%) | 52 (13.0%) | 51 (5.0%) | 53 (5.2%) | 14 (1.6%) | 17 (2.1%) | 1 (0.2%) | 1 (0.2%) | 3 (0.4%) | 1 (0.1%) | 2 (1.4%) | 1 (0.7%) |
| PVD, n (%) | 148 (42.5%) | 142 (40.5%) | 163 (41.4%) | 169 (42.1%) | 282 (27.9%) | 336 (32.9%) | 266 (30.8%) | 238 (29.9%) | 34 (6.8%) | 33 (6.6%) | 54 (7.4%) | 56 (7.6%) | 6 (4.1%) | 9 (6.7%) |
| Prior CVA, n (%) | 95 (27.3%) | 87 (24.8%) | 51 (12.9%) | 53 (13.2%) | NK | NK | 57 (6.6%) | 57 (7.2%) | 17 (3.4%) | 23 (4.6%) | 74 (10.1%) | 80 (10.9%) | 24 (16.6%) | 22 (16.3%) |
| Prior CABG, n (%) | 147 (42.2%) | 152 (43.3%) | 117 (29.7%) | 121 (30.2%) | 239 (23.6%) | 261 (25.6%) | 138 (16.0%) | 137 (17.2%) | NK | NK | 18 (2.5%) | 14 (1.9%) | NK | NK |
| Prior CAD, n (%) | 201 (57.8%) | 198 (56.4%) | 207 (52.5%) | 187 (46.6%) | 548 (54.2%) | 560 (54.8%) | 498 (57.6%) | 438 (55.0%) | 335 (66.6%) | 323 (65.0%) | 464 (63.2%) | 449 (61.2%) | 78 (53.8%) | 71 (52.6%) |
| Prior PCI, n (%) | 116 (33.3%) | 110 (31.3%) | 133 (33.8%) | 152 (37.9%) | 274 (27.1%) | 282 (27.6%) | 184 (21.3%) | 169 (21.2%) | NK | NK | 103 (14.0%) | 87 (11.9%) | 11 (7.6%) | 12 (8.9%) |
| Known AF or flutter, n (%) | 80 (23.0%) | 73 (20.8%) | 161 (40.9%) | 190 (47.4%) | 313 (31.0%) | 359 (35.2%) | 243 (28.1%) | 211 (26.5%) | 78 (15.5%) | 85 (17.1%) | 111 (15.1%) | 98 (13.4%) | 40 (27.6%) | 34 (25.2%) |
| Prior pacemaker, n (%) | 69 (19.8%) | 76 (21.7%) | 92 (23.4%) | 83 (20.7%) | 118 (11.7%) | 123 (12.0%) | 84 (9.7%) | 72 (9.0%) | 12 (2.4%) | 13 (2.6%) | NK | NK | 5 (3.4%) | 6 (4.4%) |
| Intervention’s characteristics | ||||||||||||||
| Valve Type | Edwards Sapien | NA | Corevalve | NA | Sapien XT | NA | CoreValve, Evolut R | NA | Sapien S3 | NA | Corevalve, Evolut R, Evolut Pro | NA | Corevalve | NA |
| Transfemoral access, n (%) | 244 (70.1%) | NA | 394 (100.0%) | NA | 775 (76.7%) | NA | 809 (93.6%) | NA | 490 (97.4%) | NA | 727 (99.0%) | NA | 145 (100.0%) | NA |
| Transthoracic access, n (%) | 104 (29.9%) | NA | 0 (0.0%) | NA | 236 (23.3%) | NA | 55 (6.4%) | NA | 0 (0.0%) | NA | 7 (1.0%) | NA | 0 (0.0%) | NA |
Outcomes
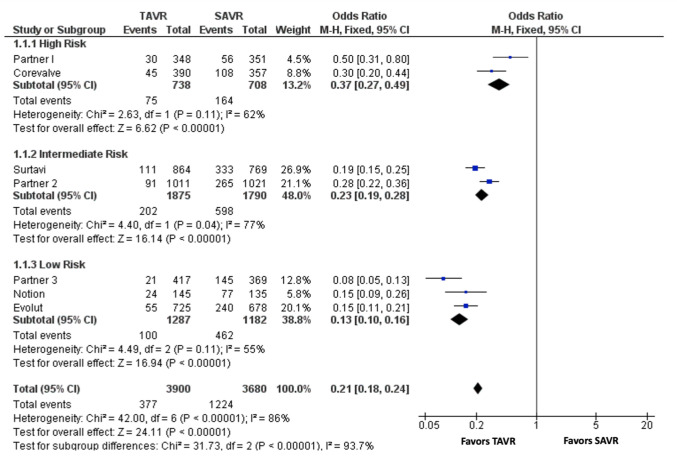
POAF at 30 days was defined as either new onset AF or worsening of AF. The composite incidence of POAF at 30 days was 9.7% (377/3900) after TAVR and 33.3% (1224/3680) after SAVR [Figure 2]. In a fixed-effect meta-analysis including all 7 trials, TAVR was associated with a lower risk of 30-day POAF compared with SAVR (OR 0.21, 95% CI [0.18-0.24]; P < 0.0001, I2 = 86%) [Figure 3]. The lower incidence of POAF at 30 days was noted across different risk strata: high-risk cohort (23.2% vs 10.2%, OR 0.37, 95%CI [0.27-0.49]; P < 0.0001, I2 = 62%), intermediate-risk cohort (33.4% vs 10.8%, OR 0.23, 95% CI [0.19-0.28]; P < 0.0001, I2 = 77%), and low-risk cohort (39.1% vs 7.8%, OR 0.13, 95% CI [0.10-0.16]; P < 0.0001, I2 = 55%). Similarly, the lower incidence in 30-day POAF with TAVR was significant in trials using both self-expanding valves (41.1% vs 12.9%, OR 0.21 [0.17-0.25], P<0.0001, I2=62%) and balloon-expandable valves (29.2% vs 7.9%, OR 0.21 [0.18-0.25], P<0.0001, I2=92%). The reported incidence of stroke, mortality and readmission at 30 days in TAVR vs SAVR are showed in [Supplemental Table 2].
Figure 2. Incidence of post-operative atrial fibrillation at 30 days with TAVR and SAVR Across the Randomized Clinical Studies.
Figure 3. Forrest plot of studies comparing post-operative atrial fibrillation at 30 days in TAVR versus SAVR in high, intermediate, and low risk cohort. CI = confidence interval; TAVR = transcatheter aortic valve replacement; SAVR = surgical aortic valve replacement.
Supplemental Table 2. Incidence of stroke, early mortality and readmission post AVR.
| TAVR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Early stroke | Late stroke | Early mortality | Early readmission | POAF | Early stroke | Late stroke | Early mortality | Early readmission | POAF |
| PARTNER 1 | 5.5% | 8.3% | 3.4% | 4.4% | 9% | 2.4% | 4.3% | 6.5% | 3.7% | 16% |
| Corevalve | 4.9% | 8.8% | 3.3% | 12% | 6.2% | 12.6% | 4.5% | 30% | ||
| PARTNER 2 | 6.4% | 10.1% | 3.9% | 6.5% | 9% | 6.5% | 9.7% | 4.1% | 6.5% | 26% |
| SURTAVI | 3.4% | 5.4% | 2.2% | 2.9% | 13% | 5.6% | 6.9% | 1.7% | 4.2% | 43% |
| PARTNER 3 | 0.6% | 1.3% | 0.4% | 3.2% | 5% | 2.3% | 3.1% | 1% | 6.4% | 21% |
| NOTION | 1.4% | 2.9% | 2.1% | 17% | 3% | 3.6% | 3.7% | 57% | ||
| Evolut | 3.4% | 4% | 0.5% | 0.90% | 8% | 3.4% | 4.2% | 1.3% | 1.1% | 35% |
Sensitivity analysis
Four trials [Evolut20, PARTNER 114, PARTNER 217, PARTNER 319] reported incidence of “new-onset” AF. The risk of new onset AF at 30 days in TAVR group was lower than SAVR group (OR 0.21, 95% CI [0.18-0.25]; P< 0.0001, I2 = 92%), [Figure 4]. Calculation of summary effects with fixed- and random-effects yielded similar results [Supplemental Table 3].
Figure 4. Forest plot of studies comparing the risk of new onset atrial fibrillation at 30 days in TAVR versus SAVR. TAVR = transcatheter aortic valve replacement; SAVR = surgical aortic valve replacement.
Supplemental Table 3. Fixed and Random effect calculation of the odd ratio.
| Study | M-H, Random, 95% CI | M-H, Fixed, 95% CI |
|---|---|---|
| High Risk | ||
| Partner I | 0.50 [0.31,0.80] | 0.50 [0.31,0.80] |
| Corevalve | 0.30 [0.20,0.44] | 0.30 [0.20,0.44] |
| Subtotal (95%CI) | 0.38 [0.23,0.62] | 0.37 [0.27,0.49] |
| Intermediate Risk | ||
| Surtavi | 0.19 [0.15,0.35] | 0.19 [0.15,0.25] |
| Partner II | 0.28 [0.22,0.36] | 0.28 [0.22,0.36] |
| Subtotal (95%CI) | 0.23 [0.16,0.34] | 0.23 [0.19,0.38] |
| Low Risk | ||
| Partner III | 0.08 [0.05,0.13] | 0.08 [0.05,0.13] |
| Notion | 0.15 [0.09,0.26] | 0.15 [0.09,0.26] |
| Evolut | 0.15 [0.11,0.21] | 0.15 [0.11,0.21] |
| Subtotal (95%CI) | 0.38 [0.23,0.62] | 0.13 [0.10,0.16] |
| Total (95% CI) | 0.21 [0.14,0.30] | 0.21 [0.18,0.24] |
Discussion
This study demonstrates a lower overall cumulative incidence of worsening and new-onset atrial fibrillation after TAVR compared with SAVR. The lower risk of POAF extends to all risk categories and TAVR valve types. Similarly, we also demonstrate a lower incidence of new onset post-operative atrial fibrillation after TAVR compared with SAVR.
The incidence of POAF is reported to be between 20% and 50%, and it varies by the procedure type 21-23. Prior single-center studies consistently showed that TAVR is associated with a lower risk of AF compared with SAVR. In a single center study of 170 patients undergoing AVR (84 TAVR, 86 SAVR), a lower risk of new onset AF was noted in the TAVR group compared with SAVR group (6% vs 33.7%, P<0.05) 24. Non-femoral access routes used in TAVR seemed to be associated with higher risk of POAF. In a retrospective single-center cohort study of 231 patients undergoing TAVR, POAF occurred in 53% after transapical TAVR, 33% after transaortic TAVR, and 14% after transfemoral TAVR. AVR without pericardiotomy was associated with a lower risk of AF, with an adjusted OR (adjusted OR: 0.18; 95% CI: 0.05 to 0.59) similar to the summary effect observed in our analysis. These studies, however, are limited by the different baseline characteristics of patients undergoing TAVR vs SAVR, and the non-randomized nature of the analyses predisposing to significant bias. Only randomized studies were included in the current metanalysis, therefore minimizing bias and balancing confounders. 25
Studying the incidence of POAF is important as it is associated with worse clinical outcomes after AVR (TAVR and SAVR). In a study of 24,076 patients undergoing TAVR in the US, post-TAVR AF was associated with increased risk of stroke and readmission 26. Similarly, other studies showed that new-onset AF after TAVR is associated with increased risks of systemic embolization and stroke 27, and short-term 10 and long-term mortality 25.
Our findings have important clinical implications. The key differences in complications rates of TAVR and SAVR, including the risk of POAF, should be part of the shared-decision making process between the patient and the Integrated Heart Team. Furthermore, differences in the incidence of POAF between the two AVR approaches may provide some insight into POAF mechanism. POAF is generally thought to be multifactorial: inflammatory response triggered by pericardiectomy and cardiopulmonary bypass, catecholamine surge, pericardial inflammation, and the overall hemodynamic burden of surgery. Patients with preexisting atrial remodeling seem to have higher risk of POAF after TAVR28. Transfemoral TAVR is a less invasive procedure, with a significantly reduced hemodynamic burden, no compromise of the pericardium, and a reduced inflammation and sympathetic tone. Our findings further confirm the importance of these contributing factors and procedure type on POAF.
Notably, there is uncertainty on the role of anticoagulation in post-operative AF. POAF has been linked with early and late stroke after both TAVR and SAVR8,29. Most patients with POAF after TAVR receive anticoagulation whereas anticoagulation for SAVR is much less common30. Whether POAF and/or anticoagulation mediates short and long-term stroke risk and other outcomes after TAVR vs SAVR remains to be elucidated.
In the current meta-analysis, the odds ratio of POAF with TAVR vs SAVR is lower with decreasing surgical risk: (high risk OR 0.37 [0.27-0.49]; intermediate risk OR 0.23 [0.19-0.28]; and low risk OR 0.13 [0.10-0.16]). The lower rate of POAF with TAVR vs SAVR may be due to the decreased utilization of “alternative” access use (ex. trans-apical or trans-aortic) in the lower risk population, in addition to the improved overall TAVR techniques in the lower risk studies. As such, differences in POAF between TAVR and SAVR could be magnified. Additionally, patients in the higher risk trials have higher prevalence of comorbidities including cardiomyopathy and atrial remodeling, and peri-procedural AF may reflect paroxysmal AF that was not detected, and thus diluting the effect of post-AVR pericardial inflammation. The contribution of reduced POAF burden to the improved outcomes (especially stroke and rehospitalization) noted with low-risk TAVR compared with SAVR should needs further investigation.
Study limitations
We acknowledge the limitations inherent to meta-analyses. The validity of our results is dependent on the validity of the studies included. We do not have access to patient level data in order to perform individual-level meta-analysis. Nevertheless, by limiting included studies to RCTs we minimize variability in burden of comorbidities between TAVR and SAVR groups and as a result decrease the possibility that differences could be due to confounding. The included studies did not entirely differentiate between new onset and provoked AF, and none of the included studies reported time to and duration of AF. Finally, not all the patients in these trials had continuous monitoring such as implantable loop recorders or pacemakers, and methods for AF detection may not have been standardized across studies, which may have led to underdiagnosis of AF. While the overall implications for post-procedural management may be similar, there are different implications for underlying mechanism and for the role of long-term anticoagulation. The definition of POAF was not standardized across trials, and thus a level of heterogeneity is expected due to different definitions. We address this shortcoming by conducting a sub-analysis with only the studies that reported new onset POAF specifically and demonstrate that the results are consistent with our overall findings.
Conclusions
TAVR is associated with a significantly lower risk of post-operative atrial fibrillation compared with SAVR in all risk strata and valve types. Further studies are needed to identify the contribution of post-operative atrial fibrillation to the clinical outcomes observed after TAVR and SAVR.
References
- 1.Chen Shmuel, Redfors Bjorn, Ben-Yehuda Ori, Crowley Aaron, Greason Kevin L, Alu Maria C, Finn Matthew T, Vahl Torsten P, Nazif Tamim, Thourani Vinod H, Suri Rakesh M, Svensson Lars, Webb John G, Kodali Susheel K, Leon Martin B. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Prior Cardiac Surgery in the Randomized PARTNER 2A Trial. JACC Cardiovasc Interv. 2018 Nov 12;11 (21):2207–2216. doi: 10.1016/j.jcin.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Frerker C, Baldus S, Nitschmann S. [Transcatheter aortic valve replacement : PARTNER 3 trial and Evolut Low Risk Trial]. Internist (Berl) 2019 Nov;60 (11):1221–1224. doi: 10.1007/s00108-019-00663-5. [DOI] [PubMed] [Google Scholar]
- 3.Mack Michael J, Leon Martin B, Thourani Vinod H, Makkar Raj, Kodali Susheel K, Russo Mark, Kapadia Samir R, Malaisrie S Chris, Cohen David J, Pibarot Philippe, Leipsic Jonathon, Hahn Rebecca T, Blanke Philipp, Williams Mathew R, McCabe James M, Brown David L, Babaliaros Vasilis, Goldman Scott, Szeto Wilson Y, Genereux Philippe, Pershad Ashish, Pocock Stuart J, Alu Maria C, Webb John G, Smith Craig R. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019 May 02;380 (18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 4.Mack Michael J, Leon Martin B, Thourani Vinod H, Makkar Raj, Kodali Susheel K, Russo Mark, Kapadia Samir R, Malaisrie S Chris, Cohen David J, Pibarot Philippe, Leipsic Jonathon, Hahn Rebecca T, Blanke Philipp, Williams Mathew R, McCabe James M, Brown David L, Babaliaros Vasilis, Goldman Scott, Szeto Wilson Y, Genereux Philippe, Pershad Ashish, Pocock Stuart J, Alu Maria C, Webb John G, Smith Craig R. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019 May 02;380 (18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura Rick A, Otto Catherine M, Bonow Robert O, Carabello Blase A, Erwin John P, Fleisher Lee A, Jneid Hani, Mack Michael J, McLeod Christopher J, O'Gara Patrick T, Rigolin Vera H, Sundt Thoralf M, Thompson Annemarie. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017 Jun 20;135 (25):e1159–e1195. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 6.Kapadia Samir R, Huded Chetan P, Kodali Susheel K, Svensson Lars G, Tuzcu E Murat, Baron Suzanne J, Cohen David J, Miller D Craig, Thourani Vinod H, Herrmann Howard C, Mack Michael J, Szerlip Molly, Makkar Raj R, Webb John G, Smith Craig R, Rajeswaran Jeevanantham, Blackstone Eugene H, Leon Martin B. Stroke After Surgical Versus Transfemoral Transcatheter Aortic Valve Replacement in the PARTNER Trial. J Am Coll Cardiol. 2018 Nov 13;72 (20):2415–2426. doi: 10.1016/j.jacc.2018.08.2172. [DOI] [PubMed] [Google Scholar]
- 7.Vavuranakis Manolis, Kolokathis Angelos-Michail, Vrachatis Dimitrios A, Kalogeras Konstantinos, Magkoutis Nikolaos A, Fradi Sabi, Ghostine Saïd, Karamanou Marianna, Tousoulis Dimitrios. Atrial Fibrillation During or After TAVI: Incidence, Implications and Therapeutical Considerations. Curr Pharm Des. 2016;22 (13):1896–903. doi: 10.2174/1381612822666151208123050. [DOI] [PubMed] [Google Scholar]
- 8.Indja Ben, Woldendorp Kei, Vallely Michael P, Grieve Stuart M. New Onset Atrial Fibrillation Following Transcatheter and Surgical Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2020 Oct;29 (10):1542–1553. doi: 10.1016/j.hlc.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Doshi Rajkumar, Pisipati Sailaja, Taha Mohamed, Dave Mihir, Shah Jay, Adalja Devina, Gullapalli Nageshwara. Incidence, 30-day readmission rates and predictors of readmission after new onset atrial fibrillation who underwent transcatheter aortic valve replacement. Heart Lung. 2019 Nov 7;49 (2):186–192. doi: 10.1016/j.hrtlng.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Kalra Rajat, Patel Nirav, Doshi Rajkumar, Arora Garima, Arora Pankaj. Evaluation of the Incidence of New-Onset Atrial Fibrillation After Aortic Valve Replacement. JAMA Intern Med. 2019 Jun 03; () doi: 10.1001/jamainternmed.2019.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maisel W H, Rawn J D, Stevenson W G. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001 Dec 18;135 (12):1061–73. doi: 10.7326/0003-4819-135-12-200112180-00010. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315 (7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egger M, Smith G D, Phillips A N. Meta-analysis: principles and procedures. BMJ. 1997 Dec 06;315 (7121):1533–7. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith Craig R, Leon Martin B, Mack Michael J, Miller D Craig, Moses Jeffrey W, Svensson Lars G, Tuzcu E Murat, Webb John G, Fontana Gregory P, Makkar Raj R, Williams Mathew, Dewey Todd, Kapadia Samir, Babaliaros Vasilis, Thourani Vinod H, Corso Paul, Pichard Augusto D, Bavaria Joseph E, Herrmann Howard C, Akin Jodi J, Anderson William N, Wang Duolao, Pocock Stuart J. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011 Jun 09;364 (23):2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 15.Adams David H, Popma Jeffrey J, Reardon Michael J, Yakubov Steven J, Coselli Joseph S, Deeb G Michael, Gleason Thomas G, Buchbinder Maurice, Hermiller James, Kleiman Neal S, Chetcuti Stan, Heiser John, Merhi William, Zorn George, Tadros Peter, Robinson Newell, Petrossian George, Hughes G Chad, Harrison J Kevin, Conte John, Maini Brijeshwar, Mumtaz Mubashir, Chenoweth Sharla, Oh Jae K. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014 May 08;370 (19):1790–8. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 16.Thyregod Hans Gustav Hørsted, Steinbrüchel Daniel Andreas, Ihlemann Nikolaj, Nissen Henrik, Kjeldsen Bo Juel, Petursson Petur, Chang Yanping, Franzen Olaf Walter, Engstrøm Thomas, Clemmensen Peter, Hansen Peter Bo, Andersen Lars Willy, Olsen Peter Skov, Søndergaard Lars. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol. 2015 May 26;65 (20):2184–94. doi: 10.1016/j.jacc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Mack Michael J, Leon Martin B, Thourani Vinod H, Makkar Raj, Kodali Susheel K, Russo Mark, Kapadia Samir R, Malaisrie S Chris, Cohen David J, Pibarot Philippe, Leipsic Jonathon, Hahn Rebecca T, Blanke Philipp, Williams Mathew R, McCabe James M, Brown David L, Babaliaros Vasilis, Goldman Scott, Szeto Wilson Y, Genereux Philippe, Pershad Ashish, Pocock Stuart J, Alu Maria C, Webb John G, Smith Craig R. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019 May 02;380 (18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 18.Reardon Michael J, Van Mieghem Nicolas M, Popma Jeffrey J, Kleiman Neal S, Søndergaard Lars, Mumtaz Mubashir, Adams David H, Deeb G Michael, Maini Brijeshwar, Gada Hemal, Chetcuti Stanley, Gleason Thomas, Heiser John, Lange Rüdiger, Merhi William, Oh Jae K, Olsen Peter S, Piazza Nicolo, Williams Mathew, Windecker Stephan, Yakubov Steven J, Grube Eberhard, Makkar Raj, Lee Joon S, Conte John, Vang Eric, Nguyen Hang, Chang Yanping, Mugglin Andrew S, Serruys Patrick W J C, Kappetein Arie P. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017 Apr 06;376 (14):1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 19.Mack Michael J, Leon Martin B, Thourani Vinod H, Makkar Raj, Kodali Susheel K, Russo Mark, Kapadia Samir R, Malaisrie S Chris, Cohen David J, Pibarot Philippe, Leipsic Jonathon, Hahn Rebecca T, Blanke Philipp, Williams Mathew R, McCabe James M, Brown David L, Babaliaros Vasilis, Goldman Scott, Szeto Wilson Y, Genereux Philippe, Pershad Ashish, Pocock Stuart J, Alu Maria C, Webb John G, Smith Craig R. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019 May 02;380 (18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 20.Popma Jeffrey J, Deeb G Michael, Yakubov Steven J, Mumtaz Mubashir, Gada Hemal, O'Hair Daniel, Bajwa Tanvir, Heiser John C, Merhi William, Kleiman Neal S, Askew Judah, Sorajja Paul, Rovin Joshua, Chetcuti Stanley J, Adams David H, Teirstein Paul S, Zorn George L, Forrest John K, Tchétché Didier, Resar Jon, Walton Antony, Piazza Nicolo, Ramlawi Basel, Robinson Newell, Petrossian George, Gleason Thomas G, Oh Jae K, Boulware Michael J, Qiao Hongyan, Mugglin Andrew S, Reardon Michael J. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019 May 02;380 (18):1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 21.Creswell L L, Schuessler R B, Rosenbloom M, Cox J L. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993 Sep;56 (3):539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 22.Aranki S F, Shaw D P, Adams D H, Rizzo R J, Couper G S, VanderVliet M, Collins J J, Cohn L H, Burstin H R. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996 Aug 01;94 (3):390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 23.Mathew J P, Parks R, Savino J S, Friedman A S, Koch C, Mangano D T, Browner W S. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996 Jul 24;276 (4):300–6. [PubMed] [Google Scholar]
- 24.Motloch Lukas J, Reda Sara, Rottlaender Dennis, Khatib Rosa, Müller-Ehmsen Jochen, Seck Catherine, Strauch Justus, Madershahian Navid, Erdmann Erland, Wahlers Thorsten, Hoppe Uta C. Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg. 2012 Jan;93 (1):124–31. doi: 10.1016/j.athoracsur.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 25.Biviano Angelo B, Nazif Tamim, Dizon Jose, Garan Hasan, Fleitman Jessica, Hassan Dua, Kapadia Samir, Babaliaros Vasilis, Xu Ke, Parvataneni Rupa, Rodes-Cabau Josep, Szeto Wilson Y, Fearon William F, Dvir Danny, Dewey Todd, Williams Mathew, Mack Michael J, Webb John G, Miller D Craig, Smith Craig R, Leon Martin B, Kodali Susheel. Atrial Fibrillation Is Associated With Increased Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights From the Placement of Aortic Transcatheter Valve (PARTNER) Trial. Circ Cardiovasc Interv. 2016 Jan;9 (1) doi: 10.1161/CIRCINTERVENTIONS.115.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doshi Rajkumar, Pisipati Sailaja, Taha Mohamed, Dave Mihir, Shah Jay, Adalja Devina, Gullapalli Nageshwara. Incidence, 30-day readmission rates and predictors of readmission after new onset atrial fibrillation who underwent transcatheter aortic valve replacement. Heart Lung. 2019 Nov 7;49 (2):186–192. doi: 10.1016/j.hrtlng.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Yoon Yong-Hoon, Ahn Jung-Min, Kang Do-Yoon, Ko Euihong, Lee Pil Hyung, Lee Seung-Whan, Kim Ho Jin, Kim Joon Bum, Choo Suk Jung, Park Duk-Woo, Park Seung-Jung. Incidence, Predictors, Management, and Clinical Significance of New-Onset Atrial Fibrillation After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2019 Apr 01;123 (7):1127–1133. doi: 10.1016/j.amjcard.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 28.Amat-Santos Ignacio J, Rodés-Cabau Josep, Urena Marina, DeLarochellière Robert, Doyle Daniel, Bagur Rodrigo, Villeneuve Jacques, Côté Mélanie, Nombela-Franco Luis, Philippon François, Pibarot Philippe, Dumont Eric. Incidence, predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J Am Coll Cardiol. 2012 Jan 10;59 (2):178–88. doi: 10.1016/j.jacc.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 29.Nuis Rutger-Jan, Van Mieghem Nicolas M, Schultz Carl J, Moelker Adriaan, van der Boon Robert M, van Geuns Robert Jan, van der Lugt Aad, Serruys Patrick W, Rodés-Cabau Josep, van Domburg Ron T, Koudstaal Peter J, de Jaegere Peter P. Frequency and causes of stroke during or after transcatheter aortic valve implantation. Am J Cardiol. 2012 Jun 01;109 (11):1637–43. doi: 10.1016/j.amjcard.2012.01.389. [DOI] [PubMed] [Google Scholar]
- 30.Brennan J Matthew, Edwards Fred H, Zhao Yue, O'Brien Sean, Booth Michael E, Dokholyan Rachel S, Douglas Pamela S, Peterson Eric D. Early anticoagulation of bioprosthetic aortic valves in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. J Am Coll Cardiol. 2012 Sep 11;60 (11):971–7. doi: 10.1016/j.jacc.2012.05.029. [DOI] [PubMed] [Google Scholar]