Abstract
Introduction
Catheter ablation (CA) for atrial fibrillation (AF) can be associated with limited efficacy. Due to its autonomic innervation, the vein of Marshall (VOM) is an attractive target during AF ablation. In this meta-analysis, we aimed to evaluate the efficacy and safety of adjunctive ethanol infusion of VOM (VOM-EI) in AF ablation.
Methods
We performed a comprehensive literature search for studies that evaluated the efficacy and safety of VOM-EI in AF ablation compared to AF catheter ablation alone. The primary outcome of interest was late (≥3 months) AF or atrial tachycardia (AT) recurrence. The secondary outcomes included acute mitral isthmus bidirectional block (MIBB) and procedural complications (pericardial effusion, stroke, or atrio-esophageal fistula). Pooled relative risk (RR) and corresponding 95% confidence intervals (CIs) were calculated using the random-effects model.
Results
A total of four studies, including 804 AF patients (68.2% with persistent AF, the mean age of 63.5±9.9 years, 401 patients underwent VOM-EI plus CA vs. 403 patients who had CA alone), were included in the final analysis. VOM-EI group was associated with a lower risk of late AF/AT recurrence (RR:0.63; 95% CI:0.46-0.87; P = 0.005), and increased probability to achieve acute MIBB (RR:1.39; 95% CI:1.08-1.79; P = 0.009) without an increase in procedural complications (RR:1.05; 95% CI:0.57-1.94; P = 0.87).
Conclusions
Our meta-analysis demonstrated that adjunctive VOM-EI strategy is more effective than conventional catheter ablation with similar safety profiles.
Keywords: Ethanol infusion, Vein of Marshall, Ablation, Atrial fibrillation, Atrial tachycardia
Introduction
Vein of Marshall (VOM) was first described by the British surgeon John Marshall in 1850 1. VOM represents the persistent left horn of the sinus venosus, and it drains the posterior wall of the left atrium into the coronary sinus 1,2. It has unique electrophysiological properties as it is heavily supplied by both sympathetic and parasympathetic innervation. Thus, VOM can modulate the electrical potential of the atrial tissue and contribute to atrial fibrillation (AF) 1. Furthermore, VOM is located within the mitral isthmus, which is commonly ablated to manage peri-mitral atrial flutter (PMF) 3.
In 1972, Benjamin Scherlag was the first to describe the arrhythmogenic role of VOM by induction of an ectopic atrial rhythm with stimulation of the left cardiac sympathetic nerve 4. Since then, further studies have shown that the VOM is accountable for numerous atrial tachyarrhythmias in patients who underwent catheter ablation (CA) for AF 1,3,5.
Despite improved understanding of AF, the success rate for CA is still unsatisfactory, with only half of the patients attaining freedom from atrial tachyarrhythmias one year after the procedure 6. Vein of Marshal ethanol infusion (VOM-EI) is a potential strategy to induce scar formation around the pulmonary vein (PV), thus facilitating its isolation 7. In 2014, Yamashita et al. were the first to evaluate the use of adjunctive VOM-EI in AF-CA and found promising results 8. Since then, additional studies have been performed to assess the outcomes of adjunctive VOM-EI in AF-CA 9-12. However, the clinical use of VOM-EI in AF-CA remains controversial and has not been evaluated systematically. Therefore, we conducted this meta-analysis to evaluate all the available evidence to better assess the efficacy and safety of VOM-EI as an adjunct tool in AF ablation.
Methods
Data sources and search strategy
We performed a comprehensive search for published studies in PubMed/MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials from inception to January 22, 2021. We also performed a manual search for additional relevant studies using references of the included articles. The following search terms were used: (“Vein of Marshall” or “Ligament of Marshall”), (“Ethanol infusion” or “ablation”), and (“Atrial fibrillation” or “Atrial tachycardia”)”. The search was limited by the English language, but not to the study design, or country of origin. Online Supplementary Table 1 describes the full search term used in each database searched.
Study selection
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) and the meta-analysis of observational studies in epidemiology (MOOSE) guidelines to screen the studies 13,14. We included full texts of randomized controlled trials, cohort studies, and case-control studies. We excluded abstracts, single-arm studies, animal studies, case reports, case series, reviews, editorials, and letters to editors. Two investigators (MM and AB) independently screened and selected the studies for the final review. Discrepancies were resolved by a third investigator (PC).
Data extraction
We extracted the following data from the final studies: the last name of the first author, publication year, study design, country of origin, follow-up duration, sample size, efficacy endpoints (including recurrence of AF or Atrial tachycardia [AT] and acute mitral isthmus bi-directional block [MIBB]) and safety endpoints (including peri-procedural complications such as pericardial effusion, atrio-esophageal fistula, stroke, cerebrovascular accident [CVA], and death). Finally, we extracted data for the number of patients who underwent VOM-EI + CA or CA alone, their age, and baseline comorbidities (including diabetes mellitus, hypertension, stroke, coronary artery disease [CAD], and heart failure) and pre-procedural characteristics (including left ventricular ejection fraction [LVEF], left atrial [LA] diameter, and CHA2DS2‐VASc).
Outcomes
The primary outcome of our meta-analysis was AF or AT recurrence. Our secondary outcomes included acute mitral isthmus bidirectional block (MIBB) and peri-procedural complications (pericardial effusion, stroke, or atrio-esophageal fistula).
Statistical Analysis
The meta-analysis was performed using the Review Manager 5.3 (Cochrane Collaboration, Copenhagen, The Nordic Cochrane Centre). The random-effects model was used to calculate the weighted pooled risk ratio (RR) and corresponding 95% confidence intervals (CI). We also performed a subgroup analysis for the late AF/AT recurrence and acute MIBB outcomes based on the pathophysiology of the underlying atrial arrhythmia (de novo AF vs. post AF-AT (PMF)). A P-value <0.05 was considered statistically significant. Heterogeneity was assessed using the Higgins I2 index, where I2 values >50% implied the presence of substantial heterogeneity 15.
Quality assessment
We assessed the quality of the included studies using the Newcastle-Ottawa Scale for observational studies and the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) for RCTs 16,17. Two authors (MM and OS) independently assessed each study for bias. Discrepancies were resolved by consensus. We did not evaluate for publication bias in our study because of the limited number of included studies18.
Results
Study selection
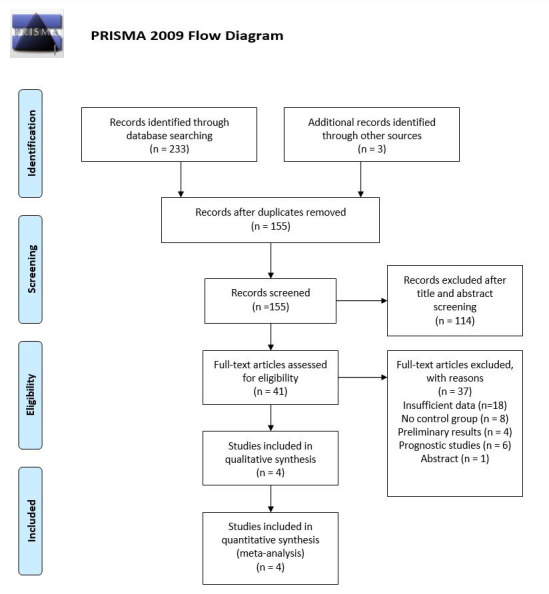
A total of 236 studies were retrieved by our search strategy. Among these, 155 were eligible for the systematic review. Subsequently, based on the titles and abstracts, we excluded 151 studies that were not relevant, had insufficient data, were preliminary studies, single-arm studies, or being a prognostic study. Finally, four studies met our inclusion criteria and were included in the meta-analysis 9-12. [Figure 1] shows the PRISMA flow chart that illustrates how the final studies were selected.
Figure 1. PRISMA flow diagram for the selection of studies.
Study characteristics
[Table 1] shows the characteristics of the four studies that were included in our meta-analysis. The studies included a total of 804 atrial fibrillation patients, of whom 401 underwent VOM-EI plus CA, and 403 underwent CA only. The studies were published between 2019 and 2020. Based on the country of origin, two studies originated from France, one from Taiwan, and one from the USA. Based on study design, one study was a randomized controlled trial12, and the rest were observational studies. All the included studies were full-text publications. The mean age was 63.5±9.9 years, males represented 78.5% of total patients, and 68.2% of patients presented with persistent or long-standing persistent AF. Hypertension and Diabetes Mellitus were more prevalent in the VOM-EI+CA group. Furthermore, patients in the VOM-EI+CA group were older when compared to the patients in the CA-only group. These differences in the baseline characteristics lead to a higher CHA2DS2‐VASc score in the VOM-EI+CA group when compared to the CA-only group. Both groups were similar regarding the rest of their baseline comorbidities. [Table 2] summarizes the baseline comorbidities and pre-procedural characteristics, including LVEF, LA diameter, and CHA2DS2‐VASc score.
Table 1. Characteristics of studies included in the meta-analysis.
Abbreviations: AF: Atrial fibrillation, AT: Atrial tachyarrhythmia, CVA: Cerebrovascular accident, MI: Mitral isthmus, N: sample size, NR: not reported, PMF: peri-mitral flutter, RCT: randomized controlled trial.
| Author, year | Study type | Country | AT subtype | Follow-up duration, months | VOM-EI + CA group, n | CA alone group, n | Efficacy endpoints | Safety endpoints |
|---|---|---|---|---|---|---|---|---|
| Liu, 2019 | Observational study. | Taiwan | post AF-AT | 12 | 32 | 64 | Recurrence of AF or any atrial arrhythmia | Periprocedural complications |
| Nakashima, 2020 | Observational study. | France | De novo AF | 9.7±5.6 | 152 | 110 | Acute MI block and MI reconnection | Periprocedural complications |
| Takigawa, 2020 | Observational study. | France | post AF-AT | 12 | 32 | 71 | Bidirectional conduction block | Periprocedural complications |
| Valderrábano, 2020 | RCT. | USA | De novo AF | 12 | 185 | 158 | Freedom from AF or atrial tachycardia | Acute procedural complications (Pericardial effusion, CVA and atrio-esophageal fistula) and total mortality |
Table 2. Baseline patients characteristics included in the meta-analysis.
Abbreviations: CAD: Coronary artery disease, LA: Left atrium, LVEF: Left ventricular ejection fraction, and Pers/LSPers AF: Persistent or long standing persistent atrial fibrillation.
| Number of studies | All patients (n = 804), (% or mean ± SD) | VOM-EI + CA group (n = 401) (% or mean ± SD) | CA only group (n = 403) (% or mean ± SD) | P value | |
|---|---|---|---|---|---|
| Age (years) | 4 | 63.5±9.9 | 64.5±9.8 | 62.6±9.9 | <0.01 |
| Male | 4 | 78.5% (631/804) | 76.3% (306/401) | 80.6% (325/403) | 0.14 |
| Hypertension | 3 | 65.1% (353/542) | 73.1% (182/249) | 58.4 (171/293) | <0.01 |
| Diabetes mellitus | 3 | 19.5% (106/542) | 25.7% (64/249) | 14.3% (42/293) | <0.01 |
| CAD | 3 | 24.9% (135/542) | 26.9% (67/249) | 23.2% (68/293) | 0.32 |
| Stroke | 3 | 10% (54/542) | 10% (25/249) | 9.9% (29/293) | 0.96 |
| Heart failure | 3 | 22.1% (120/542) | 22.1% (55/249) | 22.2% (65/293) | 0.98 |
| CHA2DS2‐VASc | 4 | 2.2±1.6 | 2.4±1.6 | 2.1±1.7 | 0.01 |
| LVEF, % | 4 | 55.3±9.3 | 55.3±10.2 | 55.4±8.4 | 0.88 |
| LA diameter, mm | 2 | 44.9±7.3 | 44.4±7.8 | 45.3±6.9 | 0.18 |
| Pers/LSPers AF | 3 | 68.2% (478/701) | 71.2% (263/369) | 64.7% (215/332) | 0.07 |
Primary outcome
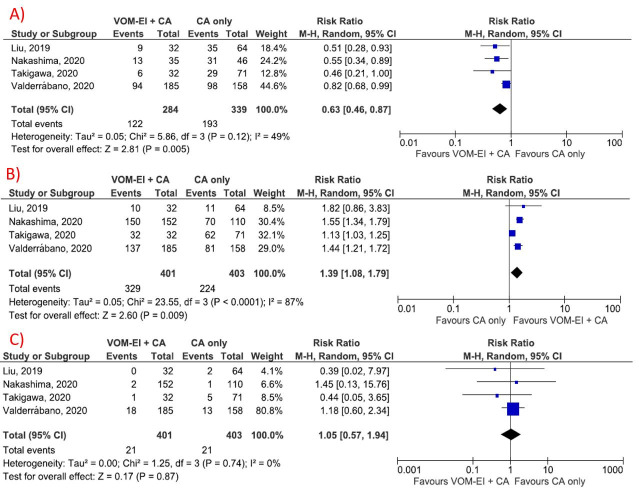
All the included studies reported the rate of late atrial arrhythmia recurrence (after three months of the index procedure) in the form of atrial fibrillation or atrial tachycardia. VOM-EI group was associated with a lower risk of late AF/AT recurrence (RR:0.63; 95% CI:0.46-0.87; P = 0.005). No significant heterogeneity was found in the measurement of AF/AT recurrence rate (I2 = 49%, P = 0.12) [Figure 1A].
Secondary outcomes
The rate of MIBB attained was higher in the VOM-EI group compared to CA only group (RR:1.39; 95% CI:1.08-1.79; P = 0.009) [Figure 2B]. The rate of periprocedural complications was not statistically different between the two groups (RR:1.05; 95% CI:0.57-1.94; P = 0.87) [Figure 2C]. Substantial heterogeneity was seen in the measurement of acute MIBB (I2 = 87%, P<0.01). A sensitivity analysis was conducted by removing one study at a time to reduce heterogeneity and found no significant heterogeneity after removal of the Takigawa et al. study (I2 = 0%, P heterogeneity = 0.73), with the results of the acute MIBB success rate continuing to be significantly different between the two groups (RR 1.51, 95% CI 1.36-1.69, P < 0.01) (Online Supplementary figure 1). No significant heterogeneity was found in the measurement of the risk of periprocedural complications (I2 = 0%, P = 0.88).
Figure 2. Forest plot showing the study outcomes (A) Late AF/AT recurrence, (B) Acute mitral isthmus bidirectional block, and (C) procedural complications. Results showed that VOM-EI group was associated with a lower risk of late AF/AT recurrence and increased probability to achieve acute MIBB without an increase in procedural complications. Abbreviation: AF: Atrial fibrillation, AT: Atrial tachycardia, CA: catheter ablation, CI: confidence interval, VOM-EI: vein of Marshall ethanol infusion.
Subgroup analysis for late atrial arrhythmia recurrence
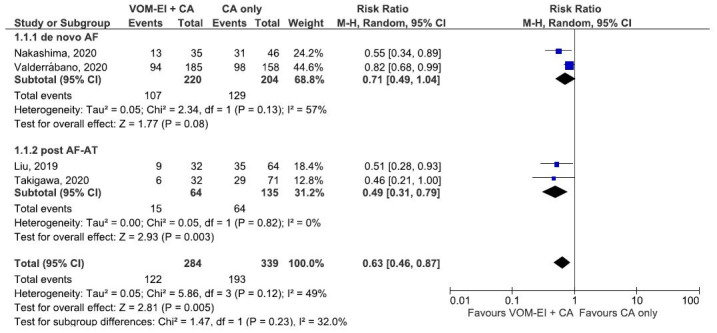
[Figure 3] shows the forest plot that compares VOM-EI plus CA and CA only groups regarding late AF/AT recurrence based on the underlying pathophysiology of the atrial arrhythmia. The two groups had similar late AF/AT recurrence rates regardless of whether the studies were conducted for a de novo AF or for a post AF-AT (PMF) (test of subgroup difference: Chi2: 1.47, df= 1, P-value (0.23), I2: 32%) [Figure 3].
Figure 3. Forest plot showing subgroup analysis comparing VOM-EI + CA and CA only regarding late atrial arrhythmia recurrence rate based on the underlying pathophysiology of the atrial arrythmia. The two groups had similar rates regardless of whether the studies were conducted for a de novo AF or for a post AF-AT. Abbreviation: AF: Atrial fibrillation, AT: Atrial tachycardia, CA: catheter ablation, CI: confidence interval, VOM-EI: vein of Marshall ethanol infusion.
Subgroup analysis for late atrial arrhythmia recurrence
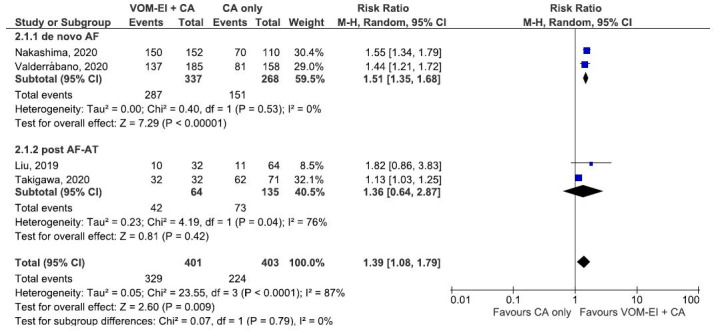
[Figure 4] shows the forest plot that compares VOM-EI plus CA and CA only groups regarding the acute MIBB success rate based on the underlying pathophysiology of the atrial arrhythmia. The two groups had similar acute MIBB rates regardless of whether the studies were conducted for a de novo AF or for a post AF-AT (PMF) (test of subgroup difference: Chi2: 0.07, df= 1, P-value (0.79), I2: 0%) [Figure 4].
Figure 4. Forest plot showing subgroup analysis comparing VOM-EI + CA and CA only regarding acute mitral isthmus bidirectional block rate based on the underlying pathophysiology of the atrial arrythmia. The two groups had similar rates regardless of whether the studies were conducted for a de novo AF or for a post AF-AT. Abbreviation: AF: Atrial fibrillation, AT: Atrial tachycardia, CA: catheter ablation, CI: confidence interval, VOM-EI: vein of Marshall ethanol infusion.
Quality assessment
We assessed the quality of the included studies by using the Newcastle-Ottawa Scale for cohort studies and the Revised Cochrane risk-of-bias tool for randomized controlled trials, as shown in Online Supplementary Tables 2 and Supplementary Tables 3. All studies scored low to moderate on the scales.
Discussion
This study was a systematic review and meta-analysis of published studies that investigated the efficacy and safety of the VOM-EI adjunctive strategy compared to conventional catheter ablation strategy in the management of AF. Our meta-analysis demonstrated that the VOM-EI adjunctive strategy significantly reduced the risk of AF/AT recurrence and increased the rate of acute mitral isthmus bidirectional block achieved. Furthermore, there were no safety concerns regarding periprocedural complications.
AF continues to be the most common cardiac arrhythmia, with an estimated 12.1 million people are expected to have AF by 2030 in the United States 19. AF is associated with increased all-cause mortality, with death often attributed to CAD or CVA 20. Treatment for AF aims to alleviate symptoms, reduce the risk of stroke, and prevent tachycardia-related cardiomyopathy 21. The decision to pursue rhythm control depends on several factors, mainly to prevent the irreversible electrical and structural remolding in longstanding AF 21. Several data reported comparable outcomes of either rate or rhythm control; however, in a secondary analysis of the AFFIRM trial of rhythm versus rate control in AF, rhythm control was associated with a statistically significant reduction in mortality (hazard ratio 0.53) 22. Similar results were observed in the DIAMOND trial that compared Dofetilide to placebo in patients with reduced ejection fraction (RR 0.44) 23.
The recurrence rate of AF after CA varies significantly among different studies; early recurrences (within the first three months) occur in almost half of the patients after CA24. Late recurrence (after three months) was observed in more than 40% of patients as detected by continuous rhythm monitoring in the CIRCA-DOSE trial 6. Thus, repeated procedures and the use of maintenance antiarrhythmic medications are usually necessary to achieve acceptable success rates 25. The rate of repeated ablation procedures may reach up to 80% 26. Despite added strategies beyond the isolation of PV, the success rate did not improve remarkably 27.
Given that VOM has dual sympathetic and parasympathetic innervation and its unique anatomical position by locating within the mitral isthmus, VOM is implicated in the generation and maintenance of AF 3,28. Therefore, ablation of VOM can potentially eliminate its intrinsic arrhythmogenic properties by eliminating AF trigger in the mitral isthmus and the surrounding atrial tissue, and its PV connections 28. Valderrabano et al.7 conducted an animal study showing that VOM-EI resulted in eliminating vagally-mediated decrease in the left atrial effective refractory period by forming scar around the pulmonary veins. The use of VOM-EI in human was initially assessed by a small study on 14 patients; VOM-EI led to the formation of a low voltage area in the left atrium (LA) and isolation of the left inferior PV in 4 of 10 patients without acute complications 29. Furthermore, a larger study on 61 patients demonstrated that VOM signals are consistently present in recurrent AF, and EI successfully eliminated PV reconnections with no reportable complications30. Kitamura et al.31 showed that VOM was accessible in 92.5% of patients, and 56% of Marshall bundle-related atrial tachycardia (MB-AT) were terminated only by ethanol infusion without CA, and the low-voltage area was significantly larger after ethanol infusion (12.7 ± 8.3 versus 6.6 ± 5.3 cm2, p < 0.001).
Regarding the late AF/AT recurrence risk after ablation, our meta-analysis showed that VOM-EI adjunctive therapy was associated with a significantly lower risk of recurrence compared to the non-VOM-EI group; 57% of patients of the VOM-EI group attained late AF/AT freedom compared to 43% in the CA-only group (P-value = 0.005). Previous studies showed similar results to our meta-analysis 9,11. Liu et al. showed that VOM-EI was an independent predictor of freedom from AF recurrence 9. In addition, Takigawa et al. showed that VOM-EI was significantly associated with less AT recurrence by multivariate analysis (hazard ratio = 0.35, P = 0.018) 11. Previous studies have shown that CHA2DS2-VASc score was an independent predictor of AF recurrence after ablation32. In our study, despite having a higher CHA2DS2-VASc score, the VOM-EI group had a lower AF/AT recurrence when compared to the CA-only group. This further emphasizes the effectiveness of the adjunctive VOM-EI to the standard CA in patients with AF.
Our meta-analysis demonstrated a pooled success rate of acute bidirectional MIBB of 82% with the VOM-EI strategy, which is consistent with previous studies. Acute MIBB success achieved by CA in previous studies varied between 32%-92% based on various technical approaches33. Interestingly, adjunctive VOM-EI increased the success rate of acute MIBB up to 100% 34. However, substantial heterogeneity was seen for acute MIBB in our study, which might be attributed to the limited number of studies. In addition, subgroup analysis showed that the heterogeneity was contributed more by Takigawa’s study; this might be explained as that study was conducted amongst patients treated for post-AF demonstrated peri-mitral flutter rather than for AF demonstrated rhythm.
Concerning the safety of VOM-EI, our study showed that VOM-EI is a safe procedure with no significant periprocedural complications with a comparable incidence of adverse events between the two groups. Adverse events that may occur during VOM-EI include pericardial effusion (due to contrast leakage out of VOM), CVA, and atrio-esophageal fistula 9,12. The most recent study by Valderrábano et al.12 showed that adverse events were comparable between VOME-EI and non-VOM-EI groups.
Our study may represent heterogeneous populations of patients; Takigawa et al. enrolled patients with PMF, Nakashima et al. enrolled patients mostly with persistent AF (the number with peri-mitral flutter was not specified), and Valderrábano et al. enrolled persistent AF patients. However, we conducted subgroup analyses that showed no subgroup differences in the effectiveness of the adjunctive VOM-EI in terms of prevention of AF/AT recurrence or attaining acute MIBB whether the underlying arrhythmia was a de novo AF or a post-AF ablation demonstrated atrial tachycardia in the form of peri-mitral block.
There are certain limitations to our meta-analysis. First, the number of included studies was limited, with relatively small sample size, therefore, our outcomes should be interpreted with caution; future RCTs are needed to validate our findings. However, a recently initiated MARS-AF trial evaluating the outcomes of adjunctive VOM-EI in the ablative therapy of patients with persistent AF is undergoing and expected to provide more evidence on the effectiveness of VOM-EI (NCT 01898221). Second, our study included only a single randomized controlled trial. Third, the included trials were of a single-blinded design. Therefore, investigator bias cannot be excluded. Additionally, we could not perform publication bias due to the small number of included studies.
However, there are several strengths to our meta-analysis. First, to our knowledge, this is the first meta-analysis to compare the clinical outcomes of adjunctive VOM-EI strategy with conventional CA strategy in terms of efficacy and safety. Second, we performed a subgroup analysis for the late atrial arrhythmia recurrence rate and acute MIBB success rate based on the pathophysiology of the underlying rhythm, adjunctive VOM-EI was effective for both de novo AF and post AF-AT (PMF). In addition, no heterogeneity was found in the measurement of our primary outcome (late AF/AT recurrence risk).
Conclusions
In conclusion, our meta-analysis demonstrated that the VOM-EI adjunctive strategy reduced the risk of atrial arrhythmia recurrence and increased the success rate of acute mitral isthmus bidirectional block. In addition, the VOM-EI strategy had a similar safety profile compared to the conventional catheter ablation. However, further long-term RCT with larger sample sizes are needed to testify the clinical application value of VOM-EI guided strategy in the ablative treatment of AF.
References
- 1.Kim D T, Lai A C, Hwang C, Fan L T, Karagueuzian H S, Chen P S, Fishbein M C. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000 Oct;36 (4):1324–7. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- 2.J. VI Marshall. On the development of the great anterior veins in man and mammalia; including an account of certain remnants of fætal structure found in the adult, a comparative view of these great veins the different mammalia, and an analysis of their occasional peculiarities in the human subject. Philosophical Transactions of the Royal Society of London. 0;1850:0–0. [Google Scholar]
- 3.Valderrábano Miguel. Ligament of Marshall arrhythmogenesis and vein of Marshall ethanol: A problem with a solution. Heart Rhythm. 2018 Jan;15 (1):25–27. doi: 10.1016/j.hrthm.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Scherlag B J, Yeh B K, Robinson M J. Inferior interatrial pathway in the dog. Circ Res. 1972 Jul;31 (1):18–35. doi: 10.1161/01.res.31.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Han Seongwook, Joung Boyoung, Scanavacca Mauricio, Sosa Eduardo, Chen Peng-Sheng, Hwang Chun. Electrophysiological characteristics of the Marshall bundle in humans. Heart Rhythm. 2010 Jun;7 (6):786–93. doi: 10.1016/j.hrthm.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade Jason G, Champagne Jean, Dubuc Marc, Deyell Marc W, Verma Atul, Macle Laurent, Leong-Sit Peter, Novak Paul, Badra-Verdu Mariano, Sapp John, Mangat Iqwal, Khoo Clarence, Steinberg Christian, Bennett Matthew T, Tang Anthony S L, Khairy Paul. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation. 2019 Nov 26;140 (22):1779–1788. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 7.Valderrábano Miguel, Chen Harvey R, Sidhu Jasvinder, Rao Liyun, Ling Yuesheng, Khoury Dirar S. Retrograde ethanol infusion in the vein of Marshall: regional left atrial ablation, vagal denervation and feasibility in humans. Circ Arrhythm Electrophysiol. 2009 Feb;2 (1):50–6. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M Yamashita, K Okishige, S Iwai. Clinical study of the long-term effects of ethanol infusion into marshall vein in patients with atrial fibrillation. Heart Rhythm. 2014;11:0–0. [Google Scholar]
- 9.Liu Chih-Min, Lo Li-Wei, Lin Yenn-Jiang, Lin Chin-Yu, Chang Shih-Lin, Chung Fa-Po, Chao Tze-Fan, Hu Yu-Feng, Tuan Ta-Chuan, Liao Jo-Nan, Chen Yun-Yu, Kuo Ling, Chang Ting-Yung, Hoang Quang Minh, Salim Simon, Vicera Jennifer Jeanne B, Wu Cheng-I, Chuang Chieh-Mao, Huang Ting-Chung, Chen Shih-Ann. Long-term efficacy and safety of adjunctive ethanol infusion into the vein of Marshall during catheter ablation for nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2019 Aug;30 (8):1215–1228. doi: 10.1111/jce.13969. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima Takashi, Pambrun Thomas, Vlachos Konstantinos, Goujeau Cyril, André Clémentine, Krisai Philipp, Ramirez F Daniel, Kamakura Tsukasa, Takagi Takamitsu, Nakatani Yosuke, Kitamura Takeshi, Takigawa Masateru, Roux Jean-Rodolphe, Cheniti Ghassen, Tixier Romain, Chauvel Remi, Welte Nicolas, Duchateau Josselin, Sacher Frédéric, Cochet Hubert, Hocini Mélèze, Haïssaguerre Michel, Jaïs Pierre, Derval Nicolas. Impact of Vein of Marshall Ethanol Infusion on Mitral Isthmus Block: Efficacy and Durability. Circ Arrhythm Electrophysiol. 2020 Dec;13 (12) doi: 10.1161/CIRCEP.120.008884. [DOI] [PubMed] [Google Scholar]
- 11.Takigawa Masateru, Vlachos Konstantinos, Martin Claire A, Bourier Felix, Denis Arnaud, Kitamura Takeshi, Cheniti Ghassen, Lam Anna, Martin Ruairidh, Frontera Antonio, Thompson Nathaniel, Massoullié Grégoire, Wolf Michael, Escande William, André Clémentine, Zeng Li-Jun, Nakatani Yosuke, Nakashima Takashi, Pillois Xavier, Ramirez Daniel, Duchateau Josselin, Pambrun Thomas, Sacher Frederic, Cochet Hubert, Hocini Mélèze, Haïssaguerre Michel, Jaïs Pierre, Derval Nicolas. Acute and mid-term outcome of ethanol infusion of vein of Marshall for the treatment of perimitral flutter. Europace. 2020 Aug 01;22 (8):1252–1260. doi: 10.1093/europace/euaa137. [DOI] [PubMed] [Google Scholar]
- 12.Valderrábano Miguel, Peterson Leif E, Swarup Vijay, Schurmann Paul A, Makkar Akash, Doshi Rahul N, DeLurgio David, Athill Charles A, Ellenbogen Kenneth A, Natale Andrea, Koneru Jayanthi, Dave Amish S, Giorgberidze Irakli, Afshar Hamid, Guthrie Michelle L, Bunge Raquel, Morillo Carlos A, Kleiman Neal S. Effect of Catheter Ablation With Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation: The VENUS Randomized Clinical Trial. JAMA. 2020 Oct 27;324 (16):1620–1628. doi: 10.1001/jama.2020.16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati Alessandro, Altman Douglas G, Tetzlaff Jennifer, Mulrow Cynthia, Gøtzsche Peter C, Ioannidis John P A, Clarke Mike, Devereaux P J, Kleijnen Jos, Moher David. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009 Jul 21;339 () doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup D F, Berlin J A, Morton S C, Olkin I, Williamson G D, Rennie D, Moher D, Becker B J, Sipe T A, Thacker S B. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr 19;283 (15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Higgins Julian P T, Thompson Simon G, Deeks Jonathan J, Altman Douglas G. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 06;327 (7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins Julian P T, Altman Douglas G, Gøtzsche Peter C, Jüni Peter, Moher David, Oxman Andrew D, Savovic Jelena, Schulz Kenneth F, Weeks Laura, Sterne Jonathan A C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011 Oct 18;343 () doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeks J J, Dinnes J, D'Amico R, Sowden A J, Sakarovitch C, Song F, Petticrew M, Altman D G. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7 (27):iii–x, 1-173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 18.Dalton Jarrod E, Bolen Shari D, Mascha Edward J. Publication Bias: The Elephant in the Review. Anesth Analg. 2016 Oct;123 (4):812–3. doi: 10.1213/ANE.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colilla Susan, Crow Ann, Petkun William, Singer Daniel E, Simon Teresa, Liu Xianchen. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013 Oct 15;112 (8):1142–7. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 20.Chen Lin Y, Sotoodehnia Nona, Bůžková Petra, Lopez Faye L, Yee Laura M, Heckbert Susan R, Prineas Ronald, Soliman Elsayed Z, Adabag Selcuk, Konety Suma, Folsom Aaron R, Siscovick David, Alonso Alvaro. Atrial fibrillation and the risk of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med. 2013 Jan 14;173 (1):29–35. doi: 10.1001/2013.jamainternmed.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padanilam Benzy J, Prystowsky Eric N. Atrial fibrillation: goals of therapy and management strategies to achieve the goals. Cardiol Clin. 2009 Feb;27 (1):189–200, x. doi: 10.1016/j.ccl.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Corley Scott D, Epstein Andrew E, DiMarco John P, Domanski Michael J, Geller Nancy, Greene H Leon, Josephson Richard A, Kellen Joyce C, Klein Richard C, Krahn Andrew D, Mickel Mary, Mitchell L Brent, Nelson Joy Dalquist, Rosenberg Yves, Schron Eleanor, Shemanski Lynn, Waldo Albert L, Wyse D George. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004 Mar 30;109 (12):1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen O D, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001 Jul 17;104 (3):292–6. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 24.Andrade Jason G, Khairy Paul, Macle Laurent, Packer Doug L, Lehmann John W, Holcomb Richard G, Ruskin Jeremy N, Dubuc Marc. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):69–75. doi: 10.1161/CIRCEP.113.000586. [DOI] [PubMed] [Google Scholar]
- 25.Rostock Thomas, Salukhe Tushar V, Steven Daniel, Drewitz Imke, Hoffmann Boris A, Bock Karsten, Servatius Helge, Müllerleile Kai, Sultan Arian, Gosau Nils, Meinertz Thomas, Wegscheider Karl, Willems Stephan. Long-term single- and multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm. 2011 Sep;8 (9):1391–7. doi: 10.1016/j.hrthm.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Verma Atul, Jiang Chen-yang, Betts Timothy R, Chen Jian, Deisenhofer Isabel, Mantovan Roberto, Macle Laurent, Morillo Carlos A, Haverkamp Wilhelm, Weerasooriya Rukshen, Albenque Jean-Paul, Nardi Stefano, Menardi Endrj, Novak Paul, Sanders Prashanthan. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015 May 07;372 (19):1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 27.Fink Thomas, Schlüter Michael, Heeger Christian-Hendrik, Lemes Christine, Maurer Tilman, Reissmann Bruno, Riedl Johannes, Rottner Laura, Santoro Francesco, Schmidt Boris, Wohlmuth Peter, Mathew Shibu, Sohns Christian, Ouyang Feifan, Metzner Andreas, Kuck Karl-Heinz. Stand-Alone Pulmonary Vein Isolation Versus Pulmonary Vein Isolation With Additional Substrate Modification as Index Ablation Procedures in Patients With Persistent and Long-Standing Persistent Atrial Fibrillation: The Randomized Alster-Lost-AF Trial (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation). Circ Arrhythm Electrophysiol. 2017 Jul;10 (7) doi: 10.1161/CIRCEP.117.005114. [DOI] [PubMed] [Google Scholar]
- 28.Báez-Escudero José L, Keida Takehiko, Dave Amish S, Okishige Kaoru, Valderrábano Miguel. Ethanol infusion in the vein of Marshall leads to parasympathetic denervation of the human left atrium: implications for atrial fibrillation. J Am Coll Cardiol. 2014 May 13;63 (18):1892–901. doi: 10.1016/j.jacc.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valderrábano Miguel, Liu Xiushi, Sasaridis Christine, Sidhu Jasvinder, Little Stephen, Khoury Dirar S. Ethanol infusion in the vein of Marshall: Adjunctive effects during ablation of atrial fibrillation. Heart Rhythm. 2009 Nov;6 (11):1552–8. doi: 10.1016/j.hrthm.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dave Amish S, Báez-Escudero José L, Sasaridis Christine, Hong Thomas E, Rami Tapan, Valderrábano Miguel. Role of the vein of Marshall in atrial fibrillation recurrences after catheter ablation: therapeutic effect of ethanol infusion. J Cardiovasc Electrophysiol. 2012 Jun;23 (6):583–91. doi: 10.1111/j.1540-8167.2011.02268.x. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura Takeshi, Vlachos Konstantinos, Denis Arnaud, Andre Clementine, Martin Ruairidh, Pambrun Thomas, Duchateau Josselin, Frontera Antonio, Takigawa Masateru, Thompson Nathaniel, Cheniti Ghassen, Martin Claire A, Lam Anna, Bourier Felix, Sacher Frederic, Hocini Meleze, Haissaguerre Michel, Jais Pierre, Derval Nicolas. Ethanol infusion for Marshall bundle epicardial connections in Marshall bundle-related atrial tachycardias following atrial fibrillation ablation: The accessibility and success rate of ethanol infusion by using a femoral approach. J Cardiovasc Electrophysiol. 2019 Sep;30 (9):1443–1451. doi: 10.1111/jce.14019. [DOI] [PubMed] [Google Scholar]
- 32.Letsas Konstantinos P, Efremidis Michael, Giannopoulos Georgios, Deftereos Spyridon, Lioni Louiza, Korantzopoulos Panagiotis, Vlachos Konstantinos, Xydonas Sotirios, Kossyvakis Charalampos, Sideris Antonios. CHADS2 and CHA2DS2-VASc scores as predictors of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace. 2014 Feb;16 (2):202–7. doi: 10.1093/europace/eut210. [DOI] [PubMed] [Google Scholar]
- 33.Choi Jong-Il, Pak Hui-Nam, Park Jae Hyung, Choi Eun Jeong, Kim Sook Kyoung, Kwak Jae Jin, Jang Jin Kun, Hwang Chun, Kim Young-Hoon. Clinical significance of complete conduction block of the left lateral isthmus and its relationship with anatomical variation of the vein of Marshall in patients with nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2009 Jun;20 (6):616–22. doi: 10.1111/j.1540-8167.2008.01408.x. [DOI] [PubMed] [Google Scholar]
- 34.Báez-Escudero José L, Morales Percy Francisco, Dave Amish S, Sasaridis Christine M, Kim Young-Hoon, Okishige Kaoru, Valderrábano Miguel. Ethanol infusion in the vein of Marshall facilitates mitral isthmus ablation. Heart Rhythm. 2012 Aug;9 (8):1207–15. doi: 10.1016/j.hrthm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]