Introduction
Persistent left superior vena cava (PLSVC) is a congenital venous developmental abnormality of the sinus venosus with an incidence of 0.47% in patients undergoing cardiac implantable electronic devices. The two variants include a double superior vena cava (right and left SVC, with or without an innominate vein connecting the two) or a single left sided SVC (without a rightSVC).1 Implantation of cardiac implantable electronic devices (CIED), especially cardiac resynchronization therapy (CRT), may be challenging in the presence of PLSVC.2The authors present two cases in which CRT was successfully implanted with PLSVC. The authors also provide a systematic approach that can be adopted during implantation of CIED in patients with PLSVC.
Case Examples
Case 1
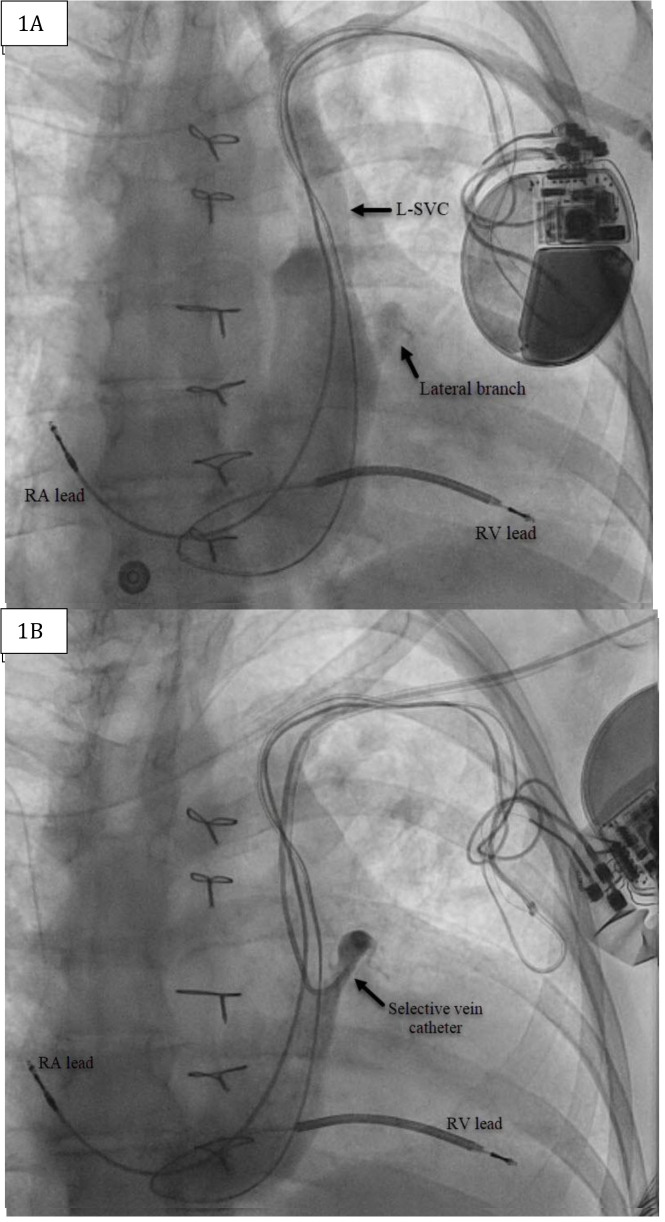
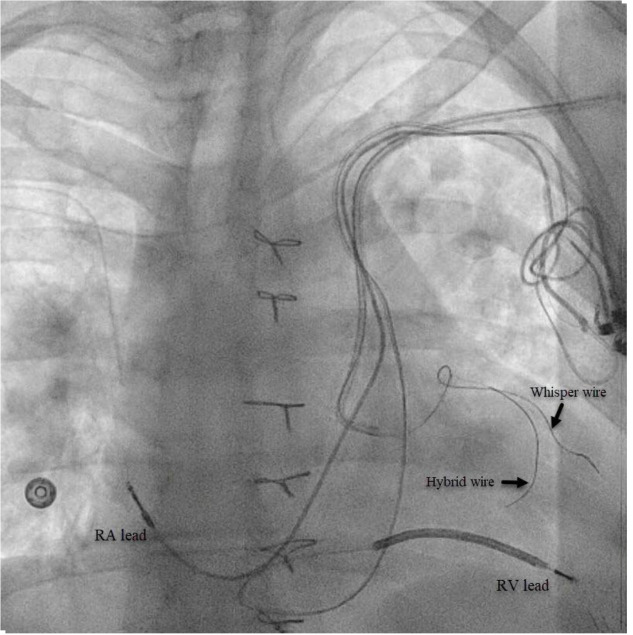
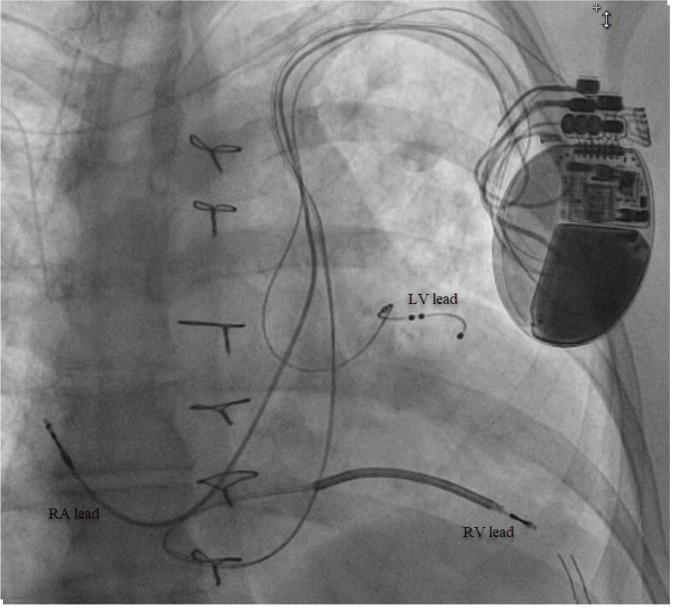
The patient is a59-year-old female with a history of atrial fibrillation status post atrioventricular node ablation, Tetralogy of Fallot status post repair and pulmonary valve replacement, ventricular tachycardia with implantable cardioverter-defibrillator (ICD), and heart failure (HF) with reduced ejection fraction (EF). She presented with decompensated heart failure with an EF of 35-40% in the setting of chronic right ventricular (RV) pacing. Given this finding, addition of left ventricular (LV) lead to provide CRT was recommended.3Left arm venogram demonstrated the PLSVC with a lateral branch off the coronary sinus (CS) [Figure 1].A selective venogram using the Worley™ lateral vein introducer (Merit Medical, Jordan UT, USA) demonstrated a small lateral tributary[Figure 1]. A “buddy wire” technique was employed using the AcuityTMWhisper wire (Boston Scientific, Marborough, MA, USA) and an Attain™ hybrid guide wire (Medtronic, Minneapolis, MN, USA) in order to gain adequate wire stability into the target vein [Figure 2]. A quadripolar LV lead was successfully advanced to this branch with adequate lead parameters. [Figure 3]
Figure 1. (A) Anteroposterior (AP) fluoroscopic projection of left-sided venogram with current ICD in place. (B) AP fluoroscopic projection of selective venogram using Worley™ lateral vein introducer.
L-SVC=Left superior vena cava; RA=Right atrial; RV=Right ventricle.
Figure 2. AP fluoroscopic projection of “buddy wire” technique with Acuity™ Whisper wire and Attain™ hybrid guide wire in our target vessel.
RA=Right atrial; RV=Right ventricle.
Figure 3. Final projection of implanted CRT-D with adequate positioning of LV lead.
RA=Right atrial; RV=Right ventricle; LV=Left ventricle.
Case 2
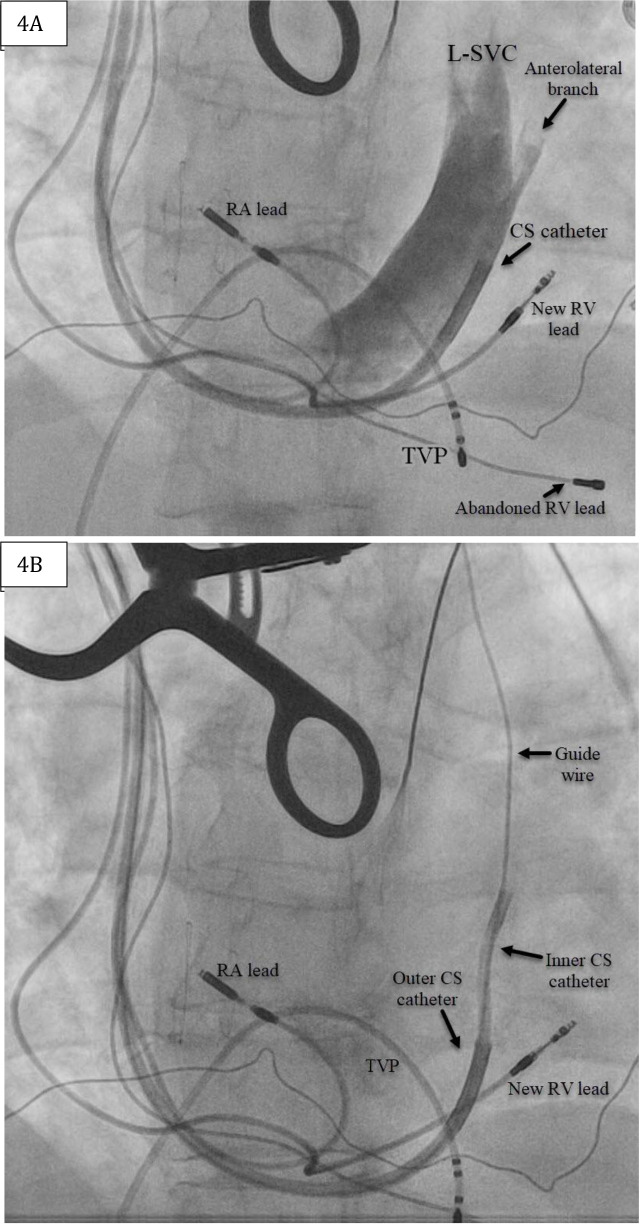
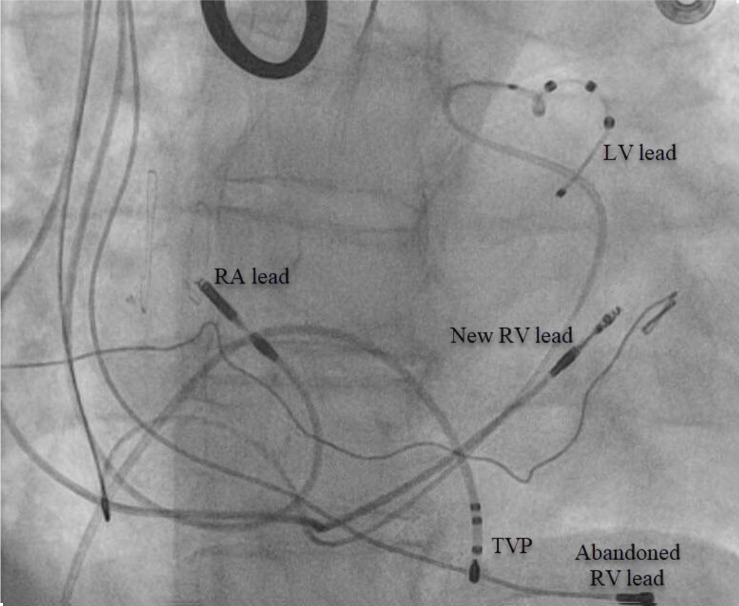
The patient is a 71-year-old female with a history of rheumatic mitral valve disease with mitral valve repair, ventricular septal defect repair, and complete heart block implanted with a dual-chamber pacemaker. Reportedly, she had a right-sided pacemaker implanted due to “vascular issues” during a prior left-sided attempt. She presented with decompensated HF, and transthoracic echo (TTE) demonstrated left ventricular EF of 40-45% in setting of chronic RV pacing. LV lead placement to her current pacing system was recommended given her chronic RV pacing, HF and the clinical benefits of CRT in this patient population.3Her chronically implanted 31-year-old unipolar RV lead was abandoned and replaced by a new RV without difficulty. CS venogram revealed a markedly enlarged PLSVC [Figure 4]. The only suitable target for LV lead placement was an anterolateral branch best seen in the left anterior oblique (LAO) view [Figure 4].Given the large caliber size of this side-branch within a persistent L-SVC, a Boston Scientific Acuity™ spiral quadripolar LV lead was selected as this was felt to impart the greatest probability of lead stability.Pacing from this location yielded a QRS complex of inferior axis with negative Rwave in leads I and aVL. Final fluoroscopy revelaed adequate lead positioning and pacing parameters[Figure 5].
Figure 4. (A) Fluoroscopy in the left anterior oblique (LAO) projection depicting coronary sinus venogram revealing the left superior vena cava (L-SVC) and an anterolateral side-branch. (B)Fluoroscopy in the left anterior oblique (LAO) projection depicting guidewire probing that was easily advanced past the cardiac silhouette.
RA=Right atrial; RV=Right ventricle; LV=Left ventricle.
Figure 5. Final LV lead positioning during procedure.
RA=Right atrial; RV=Right ventricle; LV=Left ventricle.
Strategies for Device Implantation in Patients With PLSPV
PLSPV is an uncommon anomaly. Typically, the implanting physician encounters the abnormality incidentally during the procedure while attempting to advance the leads into cardiac chambers. Often, left pre-pectoral pocket is already made when this anomaly is identified. It is often not desirable to abandon the left side at this point. The following strategies can be helpful during lead implantation in the presence of PLSPV.
Right ventricular lead
The authors recommend using a long lead. It should be attempted to have the RV lead prolapse and form a big loop in the right atrium prior to entry into the right ventricle. As demonstrated in first case, RV lead has a large loop in the right atrium.
Right atrial lead
Right atrial lead is often easy to deploy in the presence of PLSPV. After exiting the ostium of CS, the RA lead is directed towards the latera wall of the atrium. At this point a stylet it with a tighter Curve should be used to direct the lead towards the RA appendage. Implantation of lead into lateral wall is associated with a higher rate of dislodgment and often cardiac perforation.
Coronary sinus lead
Implantation of LV lead is the most challenging part during implantation of devices in patients with PLSPV. Cardiac venous system is not developed in the usual fashion in presence of PLSPV. Typically, there are no cardiac veins entering into the main body of CS. Often, lateral branches entering into great cardiac vein are selected as target location. Cannulation of smaller branches requires small catheters with acute bend. After the vein is cannulated with smaller caliber inner sheath or vein selectors, it is easier to advance the lead over the wire. As demonstrated in the first case, a second wire can be used to provide stability.
Discussion
Our cases highlight the challenges associated with implantation of an LV lead in patients with PLSVC. We demonstrated successful implantation from both a right and left-sided approach. In case 1 the important factors increasing the complexity of lead placement from the left side included the tendency for the RV lead to be deflected away from the tricuspid annulus, torrential CS blood flow, acute angulation at the point where the PLSVC joins the CS and other anomalies making lead placement extremely difficult. In case 2 the major difficulty was maintaining stability within the CS given the vigorous blood flow from the PLSVC into the CS.4-6 In both cases we were able to overcome these difficulties with equipment that is routinely used in our EP lab. The presence of PLSVC can be inferred from the presence of dilated CS on TTE, which is typically done prior to CIED implantation. If this is found and suspected the presence of a PLSVC cardiac imaging (CT/MRI/3-D echo) can be used to understand superior venous anatomy. This may assist the operator in selecting the most appropriate approach and the necessary tools (stylets, leads, cannulation catheters and guidewires) for CIED implantation, possibly increasing the chance of success.
Conclusions
Superior venous anomalies such as PLSVC can make CIED implantation technically challenging. However, with increasing operator experience, cardiac imaging and appropriate tools successful CIED implantation is possible in almost all cases.
References
- 1.Biffi M, Boriani G, Frabetti L, Bronzetti G, Branzi A. Left superior vena cava persistence in patients undergoing pacemaker or cardioverter-defibrillator implantation: a 10-year experience. Chest. 2001 Jul;120 (1):139–44. doi: 10.1378/chest.120.1.139. [DOI] [PubMed] [Google Scholar]
- 2.Guenther M, Kolschmann S, Rauwolf T P, Christoph M, Sandfort V, Strasser R H, Wunderlich C. Implantable cardioverter defibrillator lead implantation in patients with a persistent left superior vena cava--feasibility, chances, and limitations: representative cases in adults. Europace. 2013 Feb;15 (2):273–7. doi: 10.1093/europace/eus287. [DOI] [PubMed] [Google Scholar]
- 3.Beck Hiroko, Curtis Anne B. Right Ventricular Versus Biventricular Pacing for Heart Failure and Atrioventricular Block. Curr Heart Fail Rep. 2016 Oct;13 (5):230–236. doi: 10.1007/s11897-016-0299-3. [DOI] [PubMed] [Google Scholar]
- 4.Worley Seth Joseph, Gohn Douglas Charles, Pulliam Robert Ward. Interventional approach to CRT in a patient with drainage of the superior vena cava into the coronary sinus. Pacing Clin Electrophysiol. 2008 Apr;31 (4):506–8. doi: 10.1111/j.1540-8159.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- 5.Cagin Charles, Barsness Gregory W, Friedman Paul A, Cha Yong-Mei. Coronary venoplasty-assisted implantation of cardiac resynchronization device in a patient with persistent left superior vena cava. Heart Rhythm. 2010 Jan;7 (1):141–2. doi: 10.1016/j.hrthm.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Anselmino Matteo, Marocco Maria Cristina, Amellone Claudia, Massa Riccardo. Hybrid right-left cardiac resynchronization therapy defibrillator implantation in persistent left superior vena cava. Europace. 2009 Apr;11 (4):533–4. doi: 10.1093/europace/eun371. [DOI] [PubMed] [Google Scholar]