Supplemental Digital Content is available in the text.
Keywords: atherosclerotic plaque, coronary angiography, coronary artery disease, lipidomics, mass spectrometry
Abstract
Objective:
While the risk of acute coronary events has been associated with biological variability of circulating cholesterol, the association with variability of other atherogenic lipids remains less understood. We evaluated the longitudinal variability of 284 lipids and investigated their association with asymptomatic coronary atherosclerosis.
Approach and Results:
Circulating lipids were extracted from fasting blood samples of 83 community-sampled symptom-free participants (age 41–75 years), collected longitudinally over 6 months. Three types of coronary plaque volume (calcified, lipid-rich, and fibrotic) were quantified using computed tomography coronary angiogram. We first deconvoluted between-subject (CVg) and within-subject (CVw) lipid variabilities. We then tested whether the mean lipid abundance was different across groups categorized by Framingham risk score and plaques phenotypes (lipid-rich, fibrotic, and calcified). Finally, we investigated whether visit-to-visit variability of each lipid was associated with plaque burden. Most lipids (72.5%) exhibited higher CVg than CVw. Among the lipids (n=145) with 1.2-fold higher CVg than CVw, 26 species including glycerides and ceramides were significantly associated with Framingham risk score and the 3 plaque phenotypes (false discovery rate <0.05). In an exploratory analysis of person-specific visit-to-visit variability without multiple testing correction, high variability of 3 lysophospholipids (lysophosphatidylethanolamines 16:0, 18:0, and lysophosphatidylcholine O-18:1) was associated with lipid-rich and fibrotic (noncalcified) plaque volume while high variability of diacylglycerol 18:1_20:0, triacylglycerols 52:2, 52:3, and 52:4, ceramide d18:0/20:0, dihexosylceramide d18:1/16:0, and sphingomyelin 36:3 was associated with calcified plaque volume.
Conclusions:
High person-specific longitudinal variation of specific nonsterol lipids is associated with the burden of subclinical coronary atherosclerosis. Larger studies are needed to confirm these exploratory findings.
Highlights
Reference longitudinal variability dataset and deconvolution of sources of variability of 284 circulating lipids over a 6-month period in asymptomatic human participants across a spectrum of coronary artery disease risk.
Glycerides demonstrated the largest ratios of between-individual variability to within-individual variability, for which single time point measurement is sufficient for monitoring of a subject.
Among the lipids with comparable between- and within-individual variabilities, high person-specific fluctuation of lysophospholipids was associated with noncalcified coronary plaque burden in this study.
Many physiological parameters exhibit natural biological variability when measured repeatedly over time in the same individual.1,2 Altered visit-to-visit variability of several physiological parameters, including serum cholesterol, glucose, and blood pressure, may predict future adverse cardiovascular events.3–7 On a more mechanistic level, greater visit-to-visit variability of circulating cholesterol and triglycerides are associated with worse progression of coronary atherosclerosis in human imaging studies.8
Lipidomic profiling using mass spectrometry has uncovered many new lipids other than cholesterol and several of these nonsterol lipids have been associated with the development of atherosclerotic plaques.9–12 However, most of these discoveries are unidimensional and abundance-based, relying on measurement taken at a single time point and not taking the variation over time into consideration.13–16 Many nonsterol lipids, including diacylglycerols, triacylglycerols, phosphatidylcholines, phosphatidylethanolamine, lysophosphatidylethanolamine, and lysophosphatidylcholine, demonstrate unique patterns of biological variability when measured repeatedly in plasma over a 24-hour period.17,18 Although reports have emerged describing the long-term variability of these nonsterol lipids,19 studies linking altered long-term variability of nonsterol lipids with early disease states are scarce. Such longitudinal variability studies are challenging to conduct, but an understanding of longitudinal variation of lipid levels over a clinically meaningful time span may unravel additional atherogenic lipid markers of early onset coronary artery disease (CAD).
We, therefore, investigated the longitudinal variability of 284 plasma lipids over a period of 6 months among asymptomatic participants who are at risk of CAD, but with well-controlled cardiometabolic parameters. We first evaluated the between-subject variability and the within-subject variability of these lipids to understand the source of their longitudinal variability. With priority set on the lipids with 1.2-fold greater between-subject variability than within-subject variability, we investigated the association of longitudinal lipid measurements with Framingham risk score (FRS) and volumes of plaque burden measured via computed tomography coronary angiography (CTCA) as an instrumental measure of risk of CAD. We next evaluated the association between person-level visit-to-visit variability of lipid levels and 3 different compositions of plaque burden.
The overarching objective was to provide a plasma lipidomic reference of visit-to-visit variability in an at-risk population for the research community and test, as proof-of-principle, whether the visit-to-visit variability of an individual subject is associated with the risk of subclinical coronary atherosclerosis.
Materials and Methods
Detailed information on CTCA technique, CTCA image postprocessing and lipidomics profiling is available in the Expanded Materials and Methods. The lipid concentration data can be found in Table S1. Subject-level meta data, including CTCA and clinical variables, can be requested from the corresponding authors on reasonable request.
Study Design and Participants
Between June 2016 and September 2017, we screened 140 community-dwelling adults and recruited 87 of them aged 41 to 75 years (inclusive) to the study. A single ethnicity population was selected to minimize lipid variability caused by genetic and dietary differences.18 We included participants with and without controlled diabetes, hypertension, smoking, or obesity. Diabetes was defined as elevated fasting glucose of ≥7.0 mmol/L or hemoglobin A1c of ≥6.5% or current use of antiglycemic medication. Hyperlipidemia was defined as fasting total cholesterol or LDL-C (low-density lipoprotein cholesterol) concentrations ≥5.2 and ≥3.4 mmol/L, respectively, or current use of lipid-lowering treatment. Hypertension was defined by the presence of systolic and diastolic blood pressure ≥140 and ≥90 mm Hg, respectively, or current use of antihypertensive treatment. Exclusion criteria were self-reported chronic kidney disease or if estimated glomerular filtration rate was <60 mL/min because of the need to use of radio-iodinated contrast, a known history of cardiovascular or cerebrovascular disease, systolic and diastolic blood pressure higher than 150 and 100 mm Hg, respectively, if LDL-C>4.1 mmol/L and hemoglobin A1c >8.0%.
Body mass index (BMI) was calculated by dividing body weight (kg) by the height squared (m2). A 12-lead ECG was acquired on an electrocardiograph (MAC 5500 GE Medical systems, Chicago, IL) to exclude participants with unknown underlying cardiac abnormalities. Hematologic and biochemical assessments were performed at Quest laboratories Ptd Ltd, Singapore. The FRS was calculated20 and participants were stratified into low (<10%) and moderate- to high- (≥10%) risk groups.
All participants were asked to refrain from heavy exercise for 24-hour before study visits. Fasting blood samples were collected between 8 and 11 am at 6-weekly intervals (±2 weeks) and centrifuged within 1 hour at 4 °C for 10 minutes at 3550g. Plasma samples were then stored at −80 °C until use. The study protocol was approved by the Singapore National Healthcare Group Domain Specific Review Board (DSRB2016/00210). All participants gave written informed consent before study participation.
Statistical Analysis
Clinical Features
To test the differences in baseline characteristics of the study participants, we compared moderate- to high-risk individuals to low-risk individuals. We used 2-sample independent t tests for continuous variables to compare means between groups and Levene test to determine equal variances. For categorical variables, we used the χ2 test to test for association, and Fisher exact test when there were cell frequencies <5. Any findings with P<0.05 were considered statistically significant.
Analytical Variability, Within-Subject Variability, and Between-Subject Variability
For each lipid, we dissected the overall lipid variability into 3 components: (1) analytical variability, CVA, (2) within-subject variability, CVw, and (3) between-subject variability, CVg. CVA represents the contribution of technical variability. It is calculated using the 59 batch quality control samples, which are pooled samples that were subjected to the lipid extraction along with samples in each extraction batch, thus representing injections of the same biological sample across the extraction batches. CVw and CVg represent the overall variabilities when partitioned into within-subject and between-subject variability, respectively, accounting for the analytical variability.
To assess the contribution of technical variability to our estimates of true biological variability, we computed the analytic variability (CVA) of each lipid using the measurements of each lipid in the 59 batch quality control samples, evenly distributed across the mass spectrometry analysis sequence and lipid extraction batches as follows:
where σ and μ represent the SD and mean of the batch quality control samples, respectively.
The within-subject variability (CVw) and between-subject variability (CVg) for each lipid were calculated by partitioning the total variances into within-subject and between-subject variances using the raw lipid concentration values in a linear mixed-effects model with random intercepts with unstructured correlation assuming that the longitudinal lipid profile is independent of time. The coefficients-of-variations are calculated as follows:
where and represent the between-subject and within-subject SD of lipid i, respectively, and is the overall mean of the measurement of lipid i.
Lipids with a high CVg and low CVw were defined such that their ratio CVg/CVw is at least 1.2-fold and the difference (CVg−CVw) is >10% (arbitrarily chosen). These are the lipids that vary more from one individual to another with stable longitudinal measurements, making them ideal candidate markers in a clinical setting. Using these lipids, we investigated their association with Framingham risk and volume of plaque burden.
Test of Association With FRS and Plaque Burden
For FRS, individuals were categorized into 2 risk groups, low risk (FRS<10%) and moderate to high risk (FRS≥10%). To categorize individuals based on the volume of plaque burden, we first computed a distance matrix using Gower’s distance on 3 plaque indices: lipid-rich volume index, fibrotic volume index, and calcified plaque index. Then, we performed partition around medoids clustering analysis on the distance matrix to separate the individuals into 3 groups with distinct plaque burden.
Next, linear mixed-effects model was used to test for differences in mean lipid profile between the 2 FRS risk groups and across the 3 categories of plaque burden. In each linear mixed-effects model, time was included as a covariate to test for time-effect and a random intercept with unstructured correlation was specified. Additional models adjusting for age and sex were also fitted. The resulting P values were adjusted using the Benjamini-Hochberg (BH) correction to control false discovery rates21 at 10% to establish statistical significance. Post hoc analyses were performed for statistically significant lipids by performing pairwise comparisons between groups using t test and Bonferroni’s correction was used for multiple-testing correction. BH correction was used for multiple-testing correction on all the P values, and the resulting values are referred to as BH-adjusted P. All statistical analyses were performed in R (version 4.0.2).22
Visit-to-Visit Lipid Variability
Visit-to-visit variability was calculated for each participant by computing the SD of each standardized lipid measurement across the time points. Due to the skewness of the distribution of noncalcified plaque indices, we classified the participants into quartiles of fibrotic plaque index and lipid-rich plaque index. For calcified plaque, we categorized the participants into 3 categories: No calcified plaque, calcified plaque index ≤0.05, and calcified plaque >0.05. Then, we fitted an ordinal regression model for the visit-to-visit variability with plaque categories for each of the 3 plaque types, that is, (noncalcified) fibrotic plaque, (noncalcified) lipid-rich plaque, and calcified plaque, to test for association between the burden of each plaque phenotype and the visit-to-visit variability. As the variability of a lipid is likely to be positively correlated with the mean lipid measurement, we adjusted for each participant’s baseline lipid measurement in the model. Also, we further included for age and sex in the adjusted models.
Specifically, we fitted 2 types of models for each plaque phenotype:
where SDi is the vector of visit-to-visit variabilities of lipid i, ei is the vector of residuals, and plaque phenotype is treated as an ordinal independent variable.
Results
Baseline Characteristics of the Study Participants
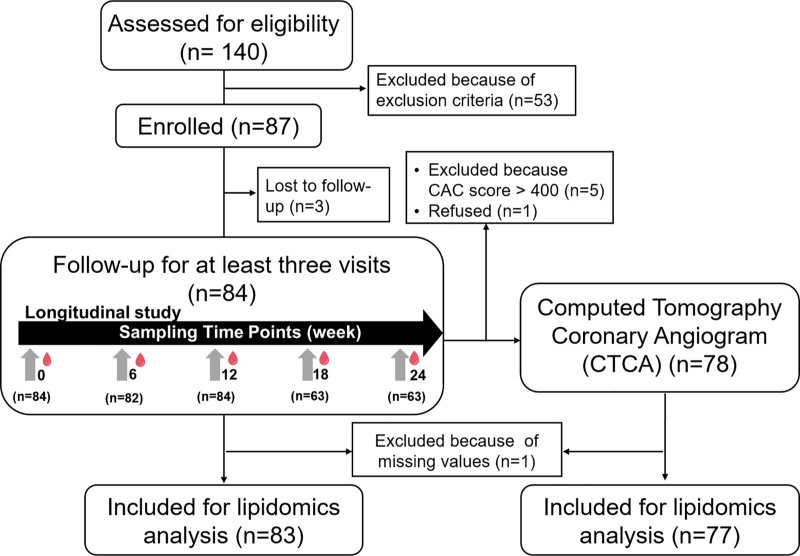
Among 140 screened participants, 87 met the eligibility criteria and were enrolled (Figure 1). Three participants withdrew, which led to 84 participants completing 3 or more study visits over 6 months. A majority of participants (72.6%) completed all 5 visits, 2 (2.4%) completed 4 visits and 21 (25.0%) completed 3 study visits. One participant was removed as one of the samples had failed QC in the lipidomics profiling, resulting in measurements available only at 2 time points. Thus, lipid measurements quantified from 83 individuals with at least 3 visits over 6 months were used in the downstream analysis. Coronary artery calcium scoring was performed on all 83 participants except one who refused; 5 participants had coronary artery calcium score above 400 Agatston Units and were, therefore, excluded from CTCA scanning.
Figure 1.
Flow diagram of the screening, recruitment, follow-up, and computed tomography coronary angiography (CTCA). Fasting blood samples were collected during prescreening (week 0) and at 6-weekly over 6 mo. CTCA was performed between visits 3 and 4. CAC indicates coronary artery calcium.
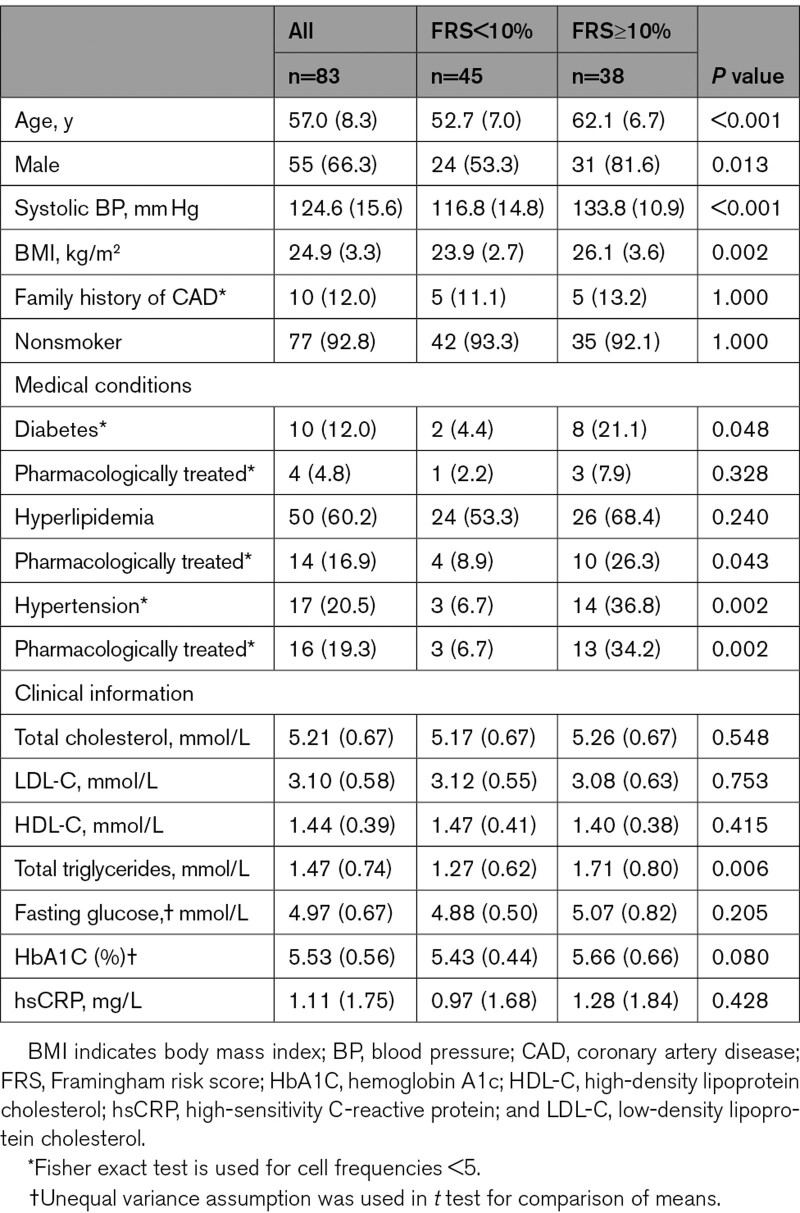
The baseline characteristics and clinical parameters of the 83 study participants are shown in the Table. In this study, there were more male (66.3%) than female participants, and the median age was 57 years (range 41–75 years), a sex and age distribution that is representative of the population at-risk of CAD in the community.20 Mean concentrations of total cholesterol, total triglycerides, HDL-C (high-density lipoprotein cholesterol), and LDL-C were 5.21, 1.47, 1.44, and 3.10 mmol/L, respectively. Forty-five (54.2%) and 38 (45.8%) participants were classified, based on the FRS, as FRS<10% (low risk) and FRS≥10% (moderate to high risk), respectively. Participants with FRS<10% were younger and had lower blood pressure and BMI. Regardless of FRS categories, participants had similar lipid and glycemic profiles except for total triacylglycerols (P<0.01). This was expected given the intention to enroll only participants with well-controlled cardiometabolic parameters.
Table.
Baseline Characteristics and Clinical Parameters of the Study Participants
CTCA Analysis
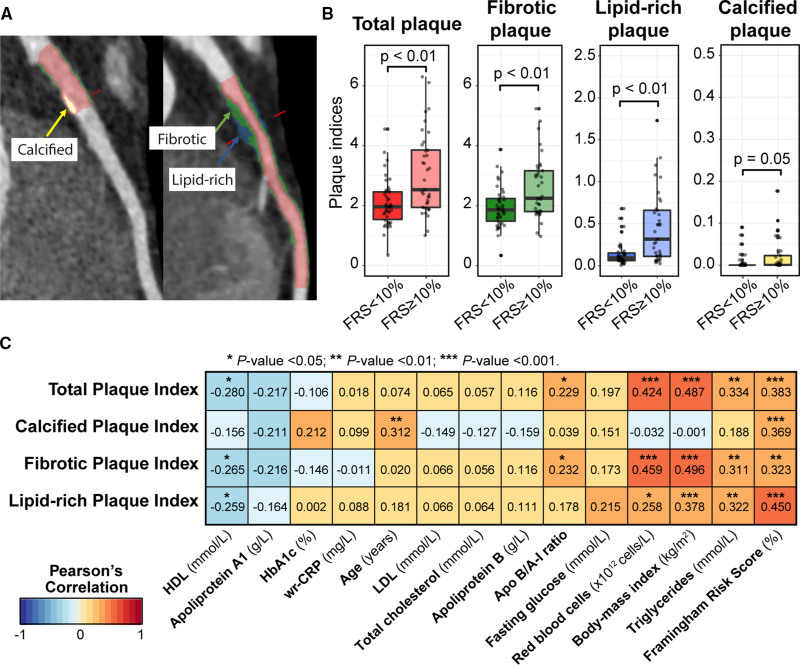
We classified atherosclerotic plaques in the coronary arteries shown in Figure 2A as calcified plaques (yellow), noncalcified fibrotic plaques (green), and noncalcified lipid-rich plaques (blue). Expectedly, participants in the FRS≥10% group had higher plaque indices compared with participants in the FRS<10% group (Figure 2B). Although all the plaque indices were positively correlated with FRS, the magnitude of correlation was modest (r ranging from 0.323 to 0.450, P<0.01; Figure 2C), suggesting that other factors may be influencing the onset of subclinical coronary atherosclerosis. Only calcified plaque was significantly correlated to age (r=0.312, P<0.01), pointing to a build-up of calcified plaque in the coronary arteries over time. However, noncalcified fibrotic and lipid-rich plaque was positively correlated with triglycerides, BMI, red blood cells, Apo B/A-I ratio, and negatively correlated to HDL. BMI was relatively strongly correlated with both fibrotic plaque index (r=0.496, P<0.01) and lipid-rich plaque index (r=0.378, P<0.01), suggesting that diet and lifestyle may play an important role in the build-up of noncalcified plaques.
Figure 2.
Coronary plaque characterization and association with Framingham risk score and clinical parameters. A, Representative curved multiplanar reformat images, with lumen in red, fibrotic plaque in green, lipid-rich plaque in blue and calcified plaque in yellow. B, Comparative boxplots of plaque indices between participants with low (n=42) and moderate- to high-Framingham risk scores (FRS; n=35). Wilcoxon rank-sum test was used to compare the difference in median plaque indices between the 2 groups. C, Pearson correlation coefficient between clinically measurable risk factors and plaque indices across 77 participants. Negative and positive correlations are marked in blue and red, respectively. Color intensities reflect the strength of correlation and the asterisks are placed above significant P values. HbA1c indicates hemoglobin A1c; HDL, high-density lipoprotein; and LDL, low-density lipoprotein.
To better understand the burden of coronary plaque, we clustered the 77 participants who underwent CTCA into 3 groups with distinct plaque burden using their lipid-rich, fibrotic, and calcified plaque indices (Figure S1A and S1B). The largest cluster (n=49) was formed with individuals with overall low plaque burden, second cluster (n=20) composed of individuals with higher noncalcified lipid-rich and fibrotic plaque indices and the third cluster consisted of individual with high-calcified plaque (Figure S1C). The 5 participants with coronary artery calcium scores >400 were grouped with the third cluster and regarded as individuals with high-calcified plaque burden.
Sources of Variability in the Longitudinal Lipid Profiles
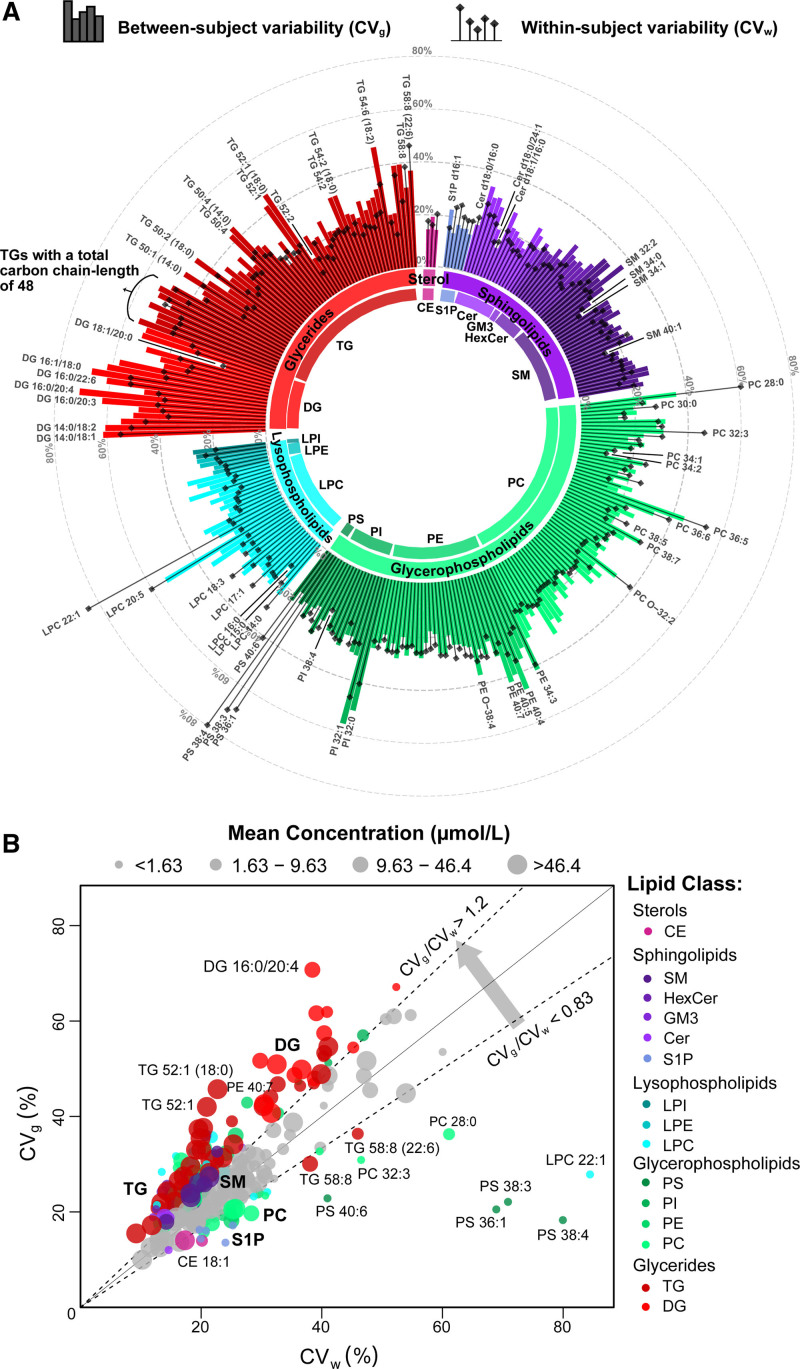
The median analytical variability, CVA, of all 284 quantified lipids was 17.4% (Table S2). The within-subject variability, CVw (represented by lollipop icons) and between-subject variability, CVg (represented by bars) of the profiled lipids are shown in Figure 3A. Overall, 29 lipids exhibit low variabilities, with both CVw and CVg smaller than 20%, making them good candidates for single time point biomarkers. These lipids include 2 cholesterol esters, 3 glycerides, 13 sphingolipids, 10 glycerophospholipids, and lysophosphatidylcholine 16:0. By contrast, 19 lipids (lysophosphatidylcholine 20:5, phosphatidylcholine 36:5, phosphatidylethanolamine 34:3, phosphatidylinositol 32:0, phosphatidylinositol 32:1, and 7 triacylglycerols and 7 diacylglycerols) showed higher variabilities with both CVw and CVg >40%.
Figure 3.
Decomposition of total biological variability of plasma lipidome into within- and between-subject variability. A, Circular plot of lipid variabilities over a 6-mo period for 83 participants. The lollipops represent the within-subject variabilities and the bars represent the between-subject variabilities of 284 lipids. The length of bars indicates the magnitude of coefficients-of-variation and the colors represent the respective lipid classes. B, Scatter plot of the within-subject (CVw) against the between-subject variability (CVg) for 284 lipids. The lipids with ratios of CVw to CVg >1.2-fold are colored by their respective lipid classes and all remaining lipids are colored in gray. The size of the circles was categorized by quartiles of mean concentrations. CE indicates cholesteryl esters; Cer, ceramide; DG, diacylglycerol; GM3, monosialodihexosylganglioside; HexCer, hexosylceramide; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; S1P, sphingosine-1-phosphate; SM, sphingomyelin; and TGs, triacylglycerols.
Among the lipid classes, lysophosphatidylcholines and phosphatidylcholines demonstrated a wide range of variabilities within the same class. For example, lysophosphatidylcholine 16:0 has both CVw and CVg smaller than 15% but lysophosphatidylcholine 20:5 had both CVw and CVg >50%. Although most lipids (72.5%) exhibited lower within-subject variability than between-subject variability, lysophosphatidylcholine 22:1 (cyan) and 3 phosphatidylserine species (green; phosphatidylserine 36:1, phosphatidylserine 38:3, and phosphatidylserine 38:4) showed 2-fold or greater within-subject variability compared with between-subject variability (Figure 3B). A total of 145 lipids had at least 1.2-fold higher CVg compared with CVw, including 47 glycerides (13 diacylglycerols and 34 triacylglycerols), 33 glycerophospholipids (25 phosphatidylcholines, 4 phosphatidylethanolamines, and 3 phosphatidylinositol), 22 lysophospholipids (17 lysophosphatidylcholines, 4 lysophosphatidylethanolamines, and 1 lysophosphatidylinositol), 43 sphingolipids (13 ceramides, 1 monosialodihexosylganglioside, 5 hexosylceramides, 3 dihexosylceramides, 2 trihexosylceramides, 18 sphingomyelin, and 1 sphingosine-1-phosphate). However, 31 lipids (cholesteryl esters/ceramide/lysophosphatidylcholine/phosphatidylcholine/phosphatidylethanolamine/phosphatidylinositol/phosphatidylserine/sphingosine-1-phosphate/triacylglycerol) showed 1.2-fold higher CVw compared with CVg (Full results presented in Table S2).
Association of Lipid Levels With FRS and Coronary Plaque Burden
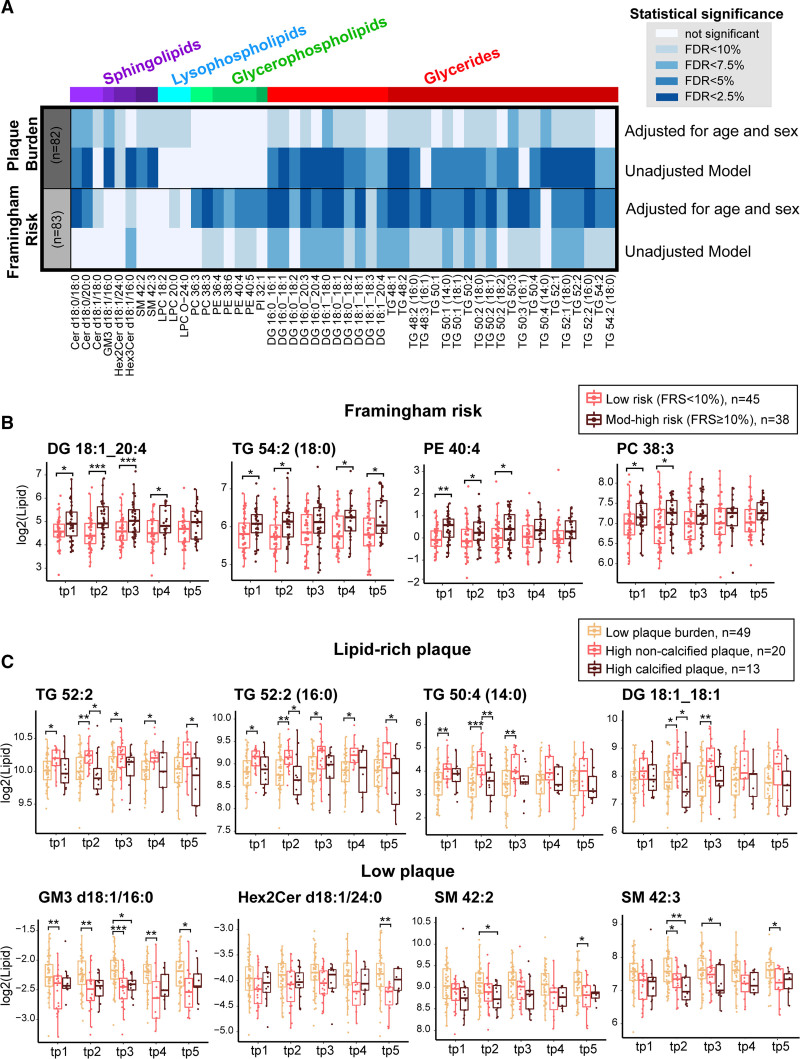
Prioritizing the 145 lipids with 1.2-fold higher between-subject variability than within-subject variability, we compared the mean lipid profile across the 2 FRS group and the 3 stratified groups of plaque burden. Figure 4A illustrates the overall statistical significance of the 2 comparisons, where 50 lipids showed a significant difference (BH-adjusted P<0.1) either between the 2 FRS groups or across the 3 categories of stratified plaque burden, adjusting for age and sex (Table S3). None of the lipids showed statistically significant time-effect, suggesting that the overall lipid profile remained stable over the time scale of this study.
Figure 4.
Plasma lipids and coronary plaque burden. A, Heatmap shows the significance score (−log10 [Benjamini-Hochberg (BH)-adjusted P]) of the 50 lipids that were significantly associated with either low (n=45) and mod-to-high Framingham risk score (FRS; n=38) or the 3 stratified plaque groups: low plaque burden (n=49), high noncalcified plaque (n=20), and high-calcified plaque (n=13), after adjustments for age and sex. The intensity of the colors represents the strength of association. B, Boxplots of 4 lipids that are unique to the association with FRS, with mean lipid profile higher among individuals with moderate- to high-risk scores. C, Boxplots of 8 lipids that are significantly different across the 3 plaque categories where 4 showed higher concentration levels among individuals with high lipid-rich plaque (top) and 4 showed higher concentration levels among those with low plaque burden (bottom). For the pairwise comparisons, 2-sample t test was used and Bonferroni’s correction was performed. Cer indicates ceramide; DG, diacylglycerol; GM3, monosialodihexosylganglioside; HexCer, hexosylceramide; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; S1P, sphingosine-1-phosphate; SM, sphingomyelin; and TGs, triacylglycerols. *P<0.05, **P<0.01, and *** P<0.001.
In the comparison of FRS risk groups, 37 lipids (trihexosylceramide d18:1/16:0, lysophosphatidylcholine 20:3, phosphatidylcholine 38:3, phosphatidylethanolamine 36:4, phosphatidylethanolamine 40:4, phosphatidylethanolamine 40:5, sphingomyelin 34:0, and 20 triacylglycerols and 10 diacylglycerols) were significantly different between low FRS and moderate- to high-FRS groups. After adjusting for age and sex, 43 lipids were significant at the same false discovery rate threshold, where 34 lipids overlapped with the unadjusted results. The clinical adjustments resulted in saturated ceramides (ceramide d18:0/18:0, ceramide d18:0/20:0, and ceramide d18:1/18:0) becoming statistically significant. Comparing across the categories of plaque burden, 43 lipids (79.5%) had mean lipid levels significantly different (BH-adjusted P<0.1) in at least one of the 3 groups (Table S4). Thirty-three lipids were significantly different after adjusting for the effects of age and sex, among which 27 lipids were significant in the unadjusted results. These 33 lipids include monosialodihexosylganglioside d18:1/16:0, dihexosylceramide d18:1/24:0, 3 ceramides (ceramide d18:0/18:0, ceramide d18:0/20:0, and ceramide d18:1/18:0), 3 lysophosphatidylcholines (lysophosphatidylcholine 18:2, lysophosphatidylcholine 20:0, and lysophosphatidylcholine O-24:0), 2 sphingomyelin (sphingomyelin 42:2 and sphingomyelin 42:3), 16 triacylglycerols, and 7 diacylglycerols.
Overall, glycerides showed the largest difference in lipid levels when considering FRS and plaque burden. In both comparisons, 26 common lipids included 15 triacylglycerols, 7 diacylglycerols, 3 ceramides (ie, ceramide d18:0/18:0, ceramide d18:0/20:0, ceramide d18:1/18:0), and lysophosphatidylcholine 20:0. Seventeen lipids were uniquely associated with FRS, including trihexosylceramide d18:1/16:0, 4 diacylglycerols, 5 triacylglycerols, and 7 glycerophospholipids (ie, 2 phosphatidylcholines, 4 phosphatidylethanolamines, and 1 phosphatidylinositol). Figure 4B demonstrates the abundance of 4 of the lipids (ie, phosphatidylcholine 38:3, phosphatidylethanolamine 40:4, diacylglycerol 18:1_20:4, and triacylglycerol 54:2 [18:0]), that were higher among the moderate- to high-risk group. Figure 4C shows the boxplot of 4 selected glycerides (upper) with higher mean concentration levels among the individuals presented with greater lipid-rich plaque burden. Out of 7 lipids that were uniquely associated with the 3 groups of stratified plaque burden, 4 were sphingolipids (ie, monosialodihexosylganglioside d18:1/16:0, dihexosylceramide d18:1/24:0, sphingomyelin 42:2, and sphingomyelin 42:3) and 2 were lysophospholipids (ie, lysophosphatidylcholine 18:2 and lysophosphatidylcholine O-24:0). Unexpectedly, all 4 sphingolipids were present at higher levels among individuals with lower plaque burden (Figure 4C, lower).
Visit-to-Visit Variability Associated With Individual Plaque Phenotype
Next, we tested the association of plaque phenotype with the visit-to-visit variability of longitudinal clinical characteristics and lipid measurements collected. Unlike within-subject variability, visit-to-visit variability (SD over 6 months) is a person-specific parameter that only measures longitudinal lipid variability of an individual.3,18 In addition to lipid measurements, we also collected longitudinal measurements of BMI, systolic and diastolic blood pressure, heart rate (sitting), fasting blood glucose, and high-sensitivity troponin-I during the follow-up visits. The visit-to-visit variability of all 6 clinical parameters was not significantly associated with the 3 types of plaque burden, except for systolic blood pressure that showed higher visit-to-visit variability among individuals with high-calcified plaque burden (Figure S2).
Then, we reclassified participants by ordinal groups of plaque burden and tested the associations of the 3 individual phenotypes of plaque burden with the visit-to-visit lipid variability. For noncalcified lipid-rich and fibrotic plaque indices, we categorized them into quartiles and for calcified plaque index, we categorized them into 3 groups: No detectable plaque, plaque index ≤0.05 and plaque index >0.05.
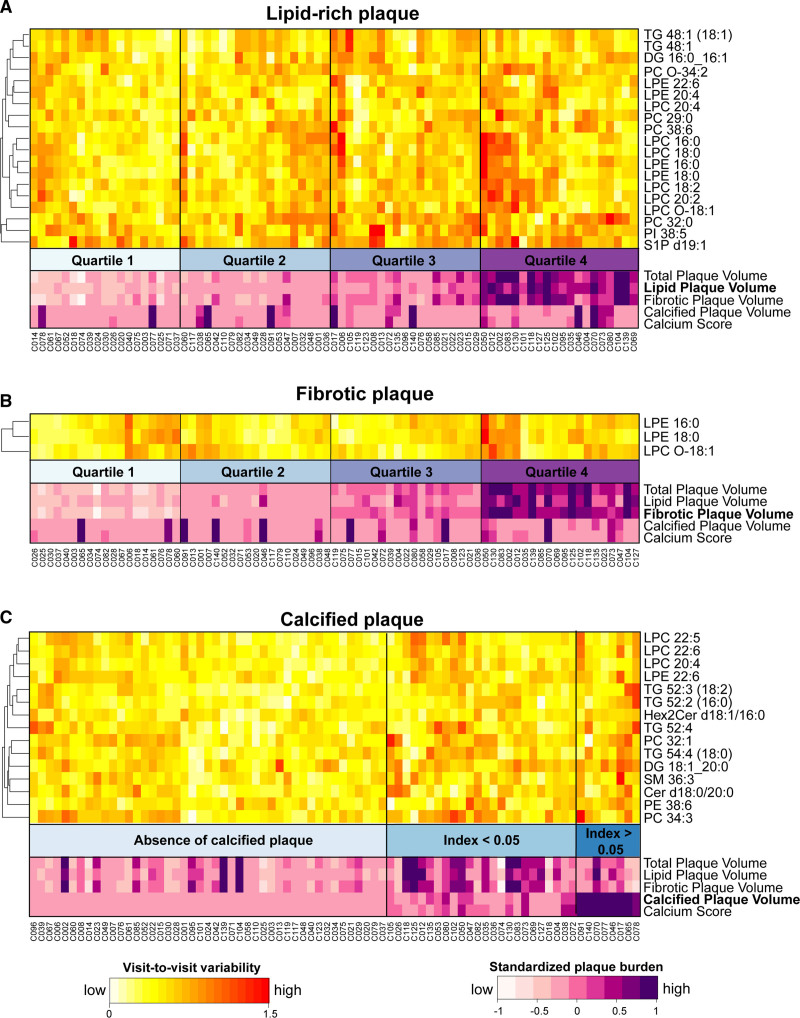
None of the lipids showed significant association after multiple-testing correction, which is largely due to the flat distribution of the P values, suggesting that the total number of significant findings is small and the current sample size does not offer the statistical power to detect the mild effect sizes with the adjustment (Tables S5 and S7). Thus, all subsequent reports are based on unadjusted P values as we expect the total number of false discoveries will be small, and we refrained from labeling the results in this section as statistically significant findings. Nineteen lipids exhibited higher visit-to-visit variability among participants with increasing lipid-rich plaque indices (P<0.05), adjusting for age, sex, and baseline lipid measurement (Figure 5A, Table S5). The heatmap clearly shows the visit-to-visit variability of those lipids in participants is associated with increasing lipid-rich plaque index in this data. These lipids include lysophospholipids (4 lysophosphatidylethanolamines and 6 lysophosphatidylcholines), glycerophospholipids (4 phosphatidylcholines and 1 phosphatidylinositol), 3 glycerides (diacylglycerol 16:0_16:1, triacylglycerol 48:1, triacylglycerol 48:1 [18:1]), and sphingosine-1-phosphate d19:1. Out of these 19 lipids, the visit-to-visit variability of 3 lipids (lysophosphatidylethanolamine 16:0, lysophosphatidylethanolamine 18:0, and lysophosphatidylcholine O-18:1), were at the same time associated with increasing fibrotic plaque index (Figure 5B) in the adjusted model (P<0.05). However, 15 lipids showed greater visit-to-visit variability in the high-calcified plaque group (Figure 5C) in the adjusted model (P<0.05). These lipids include 5 glycerides (triacylglycerol 52:2 [16:0], triacylglycerol 52:3 [18:2], triacylglycerol 52:4, triacylglycerol 54:4 [18:0], and diacylglycerol 18:1_20:0) and 3 sphingolipids (ceramide d18:0/20:0, dihexosylceramide d18:1/16:0, and sphingomyelin 36:3).
Figure 5.
Visit-to-visit variability with coronary plaque burden. The visit-to-visit variabilities of 77 individuals showed ordinal trends across the quartiles of lipid-rich plaque index (A) and fibrotic plaque index (B), and across 3 categories of calcified plaque index (C), adjusting for age and sex. None of the associations was statistically significant after multiple-testing correction. Instead, the lipids were selected controlling type I error individually (P<0.05). Cer indicates ceramide; DG, diacylglycerol; GM3, monosialodihexosylganglioside; HexCer, hexosylceramide; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; S1P, sphingosine-1-phosphate; SM, sphingomyelin; and TGs, triacylglycerols.
Overall, we observed that the visit-to-visit variability of lysophospholipids was positively associated with noncalcified plaque burden, particularly lipid-rich plaque, whereas the visit-to-visit variabilities of a few sphingolipids and triacylglycerols were associated with increased calcified plaque burden. However, the number of individuals with high-calcified plaque index was small (8 participants with calcified plaque index >0.05), and therefore, this observed association warrants further verification. Two lysophospholipids, lysophosphatidylcholine 20:4 and lysophosphatidylethanolamine 22:6, showed positive associations with both lipid-rich plaque (P=0.039 and P=0.039, respectively) and calcified plaque index (P=0.013 and P=0.023, respectively), further validation studies are required to investigate them as candidates of circulating lipid markers of increased overall plaque burden.
Discussion
This study characterized the biological variability of the plasma lipidome over a 6-month period among 83 asymptomatic participants across a spectrum of cardiovascular risks (Figure 5). We first examined 2 measures that summarize the overall variability of a lipid: variability across individuals (between-subject variability) and fluctuation within individuals over time (within-subject variability). The use of a biological analyte as clinical biomarker is based on the premise that the between-subject variability of the molecule is greater than the within-individual variability and a reliable reference range can be established. Our exploratory data (n=83) suggest that, regardless of the range of total variability, glycerides (triacylglycerol and diacylglycerol) meet this criterion the best among all lipid species (Figure 3B) in the fasting state. Glyceride levels remain stable over 6 months and a single time point measurement would suffice for the purpose of clinical diagnosis or association studies. Most of these circulating lipids (72.5%) exhibited mildly greater between-subject variability than within-subject variability, consistent with earlier findings,19 with the exception of phosphatidylserine, phosphatidylcholine, and sphingosine-1-phosphate species. Our subsequent association analysis between each individual’s longitudinal fluctuation (visit-to-visit variability) and plaque phenotypes from CTCA shows that some lipids are well-positioned for variability-based scoring, particularly the lysophospholipids that were associated with noncalcified (fibrotic and lipid-rich) plaque phenotypes.
The FRS provides robust estimate of future CAD events,23 and in our study, it showed modest correlations with coronary plaque indices, suggesting that factors other than FRS parameters influence the early coronary plaque formation. While FRS and coronary plaque imaging are 2 overlapping yet distinct instruments for measuring CAD risk, the former quantifies global cardiometabolic risk and the latter quantifies the actual presence of early coronary atherosclerosis. It is, therefore, of great interest that we identified lipids that were independently correlated with either FRS or coronary plaque burden but not both (mutually exclusive); 7 glycerophospholipids (phosphatidylcholine/phosphatidylethanolamine/phosphatidylinositol) were significantly associated with FRS only but not plaque burden, while 4 sphingolipids (monosialodihexosylganglioside/dihexosylceramide/sphingomyelin) were significantly different among the 3 stratified groups of plaque phenotypes but did not correlate with FRS. Sphingolipids have been implicated in the development of atherosclerotic plaque and are suggested to be both proatherogenic and antiatherogenic.24 Our study highlights that the decreased levels of 4 sphingolipids may be useful in clinical diagnosis for early detection of subclinical CAD. Out of the 3 phenotypes of coronary plaque, the calcified plaque phenotype was significantly correlated with age, suggesting the accrual of calcified plaque is a function of time. In contrast, only lipid-rich and fibrotic plaque volumes were significantly correlated with BMI. This suggests that the build-up of noncalcified plaque may be more relevant to dietary or lifestyle behavior that is potentially modifiable.
Some study participants (26.5%) were consuming medications for metabolic diseases during the course of the study, including agents for treating hypertension and type 2 diabetes. Some of these medications, particularly statins, are known to alter lipid levels. To this end, we performed secondary analyses to determine whether the consumption of any medication, as well as statin alone, might influence the overall lipid levels. We used linear mixed-effects model to compare the longitudinal lipid profile between participants on medications (n=22) and those not on any medication (n=61), and between participants on statin (n=14) and those not on statin (n=69). Time was included as a covariate in the model to account for the changes across time points and the resulting P values were adjusted using the BH’s method to control false discovery rate. The results (Table S6) showed that none of the lipids, except lysophosphatidylinositol 20:4, were significantly increased with the use of medications (BH-adjusted P<5%). However, the analysis for the use of statins showed statistically significant results for 38 lipids (BH-adjusted P<0.05), including 2 lysophospholipids (1 lysophosphatidylethanolamine and 1 lysophosphatidylinositol), 12 glycerophospholipids (7 phosphatidylcholines, 4 phosphatidylethanolamines, and 1 phosphatidylserine), 6 sphingolipids (3 hexosylceramides, 1 dihexosylceramide, and 2 sphingomyelin), and 18 glycerides (14 triacylglycerols and 4 diacylglycerols). It remains unclear if statin use affected the levels of these lipids in this study as statins are known to primarily reduce cholesterol levels.25 Of the lipids with significant association with coronary plaque phenotypes in our study, the mean levels of diacylglycerol 16:0_20:3, diacylglycerol 18:1_18:1 and triacylglycerol 50:4 were higher among statin users compared with nonusers. Lysophosphatidylethanolamine 22:6, phosphatidylethanolamine 38:6, and phosphatidylcholine 38:6, for which the visit-to-visit variability was associated with greater plaque burden, were also significantly higher among statin users.
To account for the impact of statin use on these associations, we removed 14 statin users and reanalyzed the associations of visit-to-visit variability with the different plaque phenotypes on the remaining 69 participants. In this subanalysis, the visit-to-visit variability remained significantly correlated with the quartiles of lipid-rich plaque in the model adjusted for baseline lipid measurement, age and sex in 5 of the 19 lipids (lysophosphatidylethanolamine 16:0, lysophosphatidylethanolamine 18:0, lysophosphatidylcholine O-18:1, phosphatidylcholine 32:0, and diacylglycerol 16:0_16:1). Both lysophosphatidylethanolamine 22:6 and phosphatidylcholine 38:6 became not significant after removing statin users. For the association of visit-to-visit variability with fibrotic plaque, including all 3 lipids initially found to be significant, 6 additional lipids (lysophosphatidylcholine 20:2, lysophosphatidylcholine O-24:1, lysophosphatidylcholine 16:0, lysophosphatidylcholine O-16:0, phosphatidylcholine 40:6, and diacylglycerol 18:1_20:4) became significant (P<0.05) after excluding statin-taking participants, resulting in stronger association between the high visit-to-visit variability of lysophosphatidylcholine and fibrotic plaque. However, the lipids with visit-to-visit variability associated with calcified plaque remained largely the same, adjusting for baseline lipid measurement, age and sex, and excluding the statin users. For example, the associations of the variation of lysophosphatidylethanolamine 22:6 and phosphatidylethanolamine 38:6 (higher among statin users) and dihexosylceramide d18:1/16:0 (lower among statin users) with calcified plaque appear to be independent of statin use. Overall, this subanalysis showed that the visit-to-visit variabilities of 5 lipids (diacylglycerol 16:0_16:1, phosphatidylcholine 32:0, lysophosphatidylcholine O-18:1, lysophosphatidylethanolamine 16:0, and lysophosphatidylethanolamine 18:0) were robustly correlated with increased noncalcified plaque, regardless of statin use. The full analysis results are found in Table S7.
Longitudinal variability of plasma cardiac biomarkers, such as cardiac troponin and B-type cardiac natriuretic peptides, can profoundly influence diagnostic and prognostic thresholds.26 Our study underscores the importance of using lipid concentrations measured over several time points instead of a single measurement at the point of risk stratification. While other lipidomic studies employing single time point measurements have highlighted increased concentrations of 4 ceramides and 3 phosphatidylcholines as risk factors for future coronary events,9,27,28 our study of lipid variability across multiple time points over 6 months revealed that not only the nominal level of lipid concentrations are associated with CAD risk but also their variation unveils novel insights into specific coronary plaque phenotypes.
Lysophosphatidylcholine species were prominently associated with the high-risk plaque phenotype in ours and other studies.29 Lysophosphatidylcholine is a phospholipid derived from phosphatidylcholines, among other pathways, via the action of lipoprotein-associated phospholipase A2 or lecithin-cholesterol acyltransferase.30 Lysophosphatidylcholine 16:0, 18:0, and 18:1 not only play important roles in plaque inflammation and vulnerability31 but also mediate the survival of macrophages, which maintain a central role throughout atherosclerosis onset and progression even after their transformation into foam cells.32 Moreover, preclinical studies have shown that reducing Lp-PLA2 activity decreases circulating lysophosphatidylcholine concentration, leading to plaque regression.33,34 Taken together with our findings, altered lysophosphatidylcholine variability may be an important risk factor leading to a greater burden of high-risk lipid-rich plaques during the early stages of atherosclerosis, as observed in this asymptomatic cohort.
Our study has several strengths. First, we intentionally enrolled participants with low-to-high FRS but well-controlled metabolic parameters, including cholesterol, blood pressure, and glycemic parameters. The objective was to maximally test the potential of a broader class of lipids, beyond sterol lipids, as early indicators of asymptomatic coronary atherosclerosis. Second, we used high-resolution CT coronary imaging and high-definition imaging analysis capabilities to differentiate between lower-risk (calcified and noncalcified fibrotic) and higher-risk (noncalcified lipid-rich) plaques. Third, other studies have assessed the ability of circulating lipids measured at a single time point to predict noncalcified plaque burden35; by assessing the variability of lipids sampled at multiple time points over a 6-month period, we have added a new dimension to the relation between circulating lipid profiles and the presence of asymptomatic coronary atherosclerosis.
Our study also has limitations. First, despite the laborious study process, our study cohort is modestly sized (n=83) and a group of individuals with high-calcified plaque burden was underrepresented. Second, while participants were informed to maintain consistent dietary habits and physical activity over the 6-month period, we were neither able to prescribe a uniform dietary and physical regimen to all participants nor to control participants’ level of adherence. Hence, to what extent different lifestyles, dietary habits or physical activity might influence the variability of lipids warrants further investigation. Last, we recruited only participants of Chinese ethnicity to minimize the potential for genetic variability to influence our study results. Therefore, our results may not be directly generalizable to other ethnic groups because dietary habits alone, for instance, may differ substantially between ethnic groups.
In conclusion, this report annotated the biological variability of 284 lipids over a 6-month period in asymptomatic human participants across a spectrum of cardiovascular risk and underscores how profiling of personalized visit-to-visit lipidomic variability can deliver new insights into the relationship between circulating lipids and different plaque phenotypes in subclinical atherosclerosis. Future larger studies are needed to confirm this finding and better understand the underlying causes of this lipid variability and its potential role in detecting subclinical atherosclerosis.
Article Information
Acknowledgments
We thank the study participants for their effort and time. We also thank the staff from Department of Diagnostic Imaging at National University of Hospital as well as colleagues from Cardiovascular Research Institute, especially Sock-Cheng Poh for study coordination, Zhen-Long Teo for study support. We also thank Dr Guoshou Teo for his assistance with peak integration and normalization of the lipidomics data.
Sources of Funding
This work was supported by NMRC/CSA-INV/0001/2016 from the National Medical Research Council, Singapore to M.Y. Chan, and NMRC/CG/M009/2017 and MOE/T2/2/084/2013 to H. Choi. A.M. Richards receives salary support from NMRC/STaR/0022/2014. Work in the Wenk laboratory is supported by grants from the National University of Singapore via the Life Sciences Institute (LSI), the National Research Foundation (NRFI2015-05, NRFSBP-P4) and A*STAR Grant I1901E0040.
Disclosures
None.
Supplemental Material
Expanded Materials and Methods
Figures S1–S2
Excel Files S1–S7
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BH
- Benjamini-Hochberg
- BMI
- body mass index
- CAD
- coronary artery disease
- CTCA
- computed tomography coronary angiography
- FRS
- Framingham risk score
- HDL-C
- high-density lipoprotein cholesterol
- LDL-C
- low-density lipoprotein cholesterol
S.H. Tan and H.W.L. Koh are joint first authors.
M.R. Wenk and M.Y. Chan are joint senior authors.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.121.316847.
For Sources of Funding and Disclosures, see page 111.
Contributor Information
Sock Hwee Tan, Email: mdctshw@nus.edu.sg.
Hiromi W.L. Koh, Email: mdckwlh@nus.edu.sg.
Jing Yi Chua, Email: jinggyyi@gmail.com.
Bo Burla, Email: bo.burla@nus.edu.sg.
Ching Ching Ong, Email: ching_ching_ong@nuhs.edu.sg.
Li San Lynette Teo, Email: lynette_ls_teo@nuhs.edu.sg.
Xiaoxun Yang, Email: mdcyx@nus.edu.sg.
Peter I. Benke, Email: bchbpi@nus.edu.sg.
Hyungwon Choi, Email: hyung_won_choi@nus.edu.sg.
A. Mark Richards, Email: mark.richards@cdhb.health.nz.
References
- 1.Widjaja A, Morris RJ, Levy JC, Frayn KN, Manley SE, Turner RC. Within- and between-subject variation in commonly measured anthropometric and biochemical variables. Clin Chem. 1999; 45:561–566 [PubMed] [Google Scholar]
- 2.Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008; 52:1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620 [DOI] [PubMed] [Google Scholar]
- 3.Messerli FH, Hofstetter L, Rimoldi SF, Rexhaj E, Bangalore S. Risk factor variability and cardiovascular outcome: JACC review topic of the week. J Am Coll Cardiol. 2019; 73:2596–2603. doi: 10.1016/j.jacc.2019.02.063 [DOI] [PubMed] [Google Scholar]
- 4.Kim MK, Han K, Kim HS, Park YM, Kwon HS, Yoon KH, Lee SH. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based study. Eur Heart J. 2017; 38:3560–3566. doi: 10.1093/eurheartj/ehx585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezende P, Hueb W, Hlatky M, Garcia R, Garzillo C, Scudeler T, Boros GAB, Ribas FF, Dallazen AR, Favarato D, et al. Variability in glycated hemoglobin values and cardiovascular events in patients with type 2 diabetes and multivessel coronary artery disease. J Am Coll Cardiol. 2019; 73:108 [Google Scholar]
- 6.Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016; 68:1375–1386. doi: 10.1016/j.jacc.2016.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coviello I, Pinnacchio G, Laurito M, Stazi A, Battipaglia I, Barone L, Mollo R, Russo G, Villano A, Sestito A, et al. Prognostic role of heart rate variability in patients with ST-segment elevation acute myocardial infarction treated by primary angioplasty. Cardiology. 2013; 124:63–70. doi: 10.1159/000345779 [DOI] [PubMed] [Google Scholar]
- 8.Clark D, 3rd, Nicholls SJ, St John J, Elshazly MB, Kapadia SR, Tuzcu EM, Nissen SE, Puri R. Visit-to-visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. Eur Heart J. 2018; 39:2551–2558. doi: 10.1093/eurheartj/ehy209 [DOI] [PubMed] [Google Scholar]
- 9.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016; 37:1967–1976. doi: 10.1093/eurheartj/ehw148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma ceramides: a novel predictor of major adverse cardiovascular events after coronary angiography. Arterioscler Thromb Vasc Biol. 2018; 38:1933–1939. doi: 10.1161/ATVBAHA.118.311199 [DOI] [PubMed] [Google Scholar]
- 11.Mukhin DN, Chao FF, Kruth HS. Glycosphingolipid accumulation in the aortic wall is another feature of human atherosclerosis. Arterioscler Thromb Vasc Biol. 1995; 15:1607–1615. doi: 10.1161/01.atv.15.10.1607 [DOI] [PubMed] [Google Scholar]
- 12.Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014; 129:1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500 [DOI] [PubMed] [Google Scholar]
- 13.Meikle PJ, Barlow CK, Mellett NA, Mundra PA, Bonham MP, Larsen A, Cameron-Smith D, Sinclair A, Nestel PJ, Wong G. Postprandial plasma phospholipids in men are influenced by the source of dietary fat. J Nutr. 2015; 145:2012–2018. doi: 10.3945/jn.115.210104 [DOI] [PubMed] [Google Scholar]
- 14.Malik VS, Guasch-Ferre M, Hu FB, Townsend MK, Zeleznik OA, Eliassen AH, Tworoger SS, Karlson EW, Costenbader KH, Ascherio A, et al. Identification of plasma lipid metabolites associated with nut consumption in US men and women. J Nutr. 2019; 149:1215–1221. doi: 10.1093/jn/nxz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa M, Maekawa K, Saito K, Senoo Y, Urata M, Murayama M, Tajima Y, Kumagai Y, Saito Y. Plasma and serum lipidomics of healthy white adults shows characteristic profiles by subjects’ gender and age. PLoS One. 2014; 9:e91806. doi: 10.1371/journal.pone.0091806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maekawa K, Okemoto K, Ishikawa M, Tanaka R, Kumagai Y, Saito Y. Plasma lipidomics of healthy Japanese adults reveals gender- and age-related differences. J Pharm Sci. 2017; 106:2914–2918. doi: 10.1016/j.xphs.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Chua EC, Shui G, Lee IT, Lau P, Tan LC, Yeo SC, Lam BD, Bulchand S, Summers SA, Puvanendran K, et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc Natl Acad Sci U S A. 2013; 110:14468–14473. doi: 10.1073/pnas.1222647110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begum H, Li B, Shui G, Cazenave-Gassiot A, Soong R, Ong RT, Little P, Teo YY, Wenk MR. Discovering and validating between-subject variations in plasma lipids in healthy subjects. Sci Rep. 2016; 6:19139. doi: 10.1038/srep19139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tebani A, Gummesson A, Zhong W, Koistinen IS, Lakshmikanth T, Olsson LM, Boulund F, Neiman M, Stenlund H, Hellström C, et al. Integration of molecular profiles in a longitudinal wellness profiling cohort. Nat Commun. 2020; 11:4487. doi: 10.1038/s41467-020-18148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008; 117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodological). 1995; 57:289–300 [Google Scholar]
- 22.Team RC. R: A language and environment for statistical computing. 2020, R; Foundation for; Statistical; Computing [Google Scholar]
- 23.Chia YC, Gray SY, Ching SM, Lim HM, Chinna K. Validation of the Framingham general cardiovascular risk score in a multiethnic Asian population: a retrospective cohort study. BMJ Open. 2015; 5:e007324. doi: 10.1136/bmjopen-2014-007324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edsfeldt A, Dunér P, Ståhlman M, Mollet IG, Asciutto G, Grufman H, Nitulescu M, Persson AF, Fisher RM, Melander O, et al. Sphingolipids contribute to human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol. 2016; 36:1132–1140. doi: 10.1161/ATVBAHA.116.305675 [DOI] [PubMed] [Google Scholar]
- 25.Scirica BM, Cannon CP. Treatment of elevated cholesterol. Circulation. 2005; 111:e360–e363. doi: 10.1161/CIRCULATIONAHA.105.539106 [DOI] [PubMed] [Google Scholar]
- 26.Klinkenberg LJ, van Dijk JW, Tan FE, van Loon LJ, van Dieijen-Visser MP, Meex SJ. Circulating cardiac troponin T exhibits a diurnal rhythm. J Am Coll Cardiol. 2014; 63:1788–1795. doi: 10.1016/j.jacc.2014.01.040 [DOI] [PubMed] [Google Scholar]
- 27.Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol. 2016; 36:2424–2430. doi: 10.1161/ATVBAHA.116.307497 [DOI] [PubMed] [Google Scholar]
- 28.Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J. 2020; 41:371–380. doi: 10.1093/eurheartj/ehz387 [DOI] [PubMed] [Google Scholar]
- 29.Stegemann C, Drozdov I, Shalhoub J, Humphries J, Ladroue C, Didangelos A, Baumert M, Allen M, Davies AH, Monaco C, et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet. 2011; 4:232–242. doi: 10.1161/CIRCGENETICS.110.959098 [DOI] [PubMed] [Google Scholar]
- 30.Gauster M, Rechberger G, Sovic A, Hörl G, Steyrer E, Sattler W, Frank S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res. 2005; 46:1517–1525. doi: 10.1194/jlr.M500054-JLR200 [DOI] [PubMed] [Google Scholar]
- 31.Gonçalves I, Edsfeldt A, Ko NY, Grufman H, Berg K, Björkbacka H, Nitulescu M, Persson A, Nilsson M, Prehn C, et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol. 2012; 32:1505–1512. doi: 10.1161/ATVBAHA.112.249854 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem. 2007; 14:3209–3220. doi: 10.2174/092986707782793899 [DOI] [PubMed] [Google Scholar]
- 33.Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Ohkawa R, Nakamura K, Yanagisawa N, Tsuboi S, Ogita M, Yokoyama K, et al. Decreased circulating lipoprotein-associated phospholipase A2 levels are associated with coronary plaque regression in patients with acute coronary syndrome. Atherosclerosis. 2011; 219:907–912. doi: 10.1016/j.atherosclerosis.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 34.Wilensky RL, Shi Y, Mohler ER, 3rd, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008; 14:1059–1066. doi: 10.1038/nm.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellims AH, Wong G, Weir JM, Lew P, Meikle PJ, Taylor AJ. Plasma lipidomic analysis predicts non-calcified coronary artery plaque in asymptomatic patients at intermediate risk of coronary artery disease. Eur Heart J Cardiovasc Imaging. 2014; 15:908–916. doi: 10.1093/ehjci/jeu033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.