Summary
Interactions between intracellular bacteria and mononuclear phagocytes give rise to diverse cellular phenotypes that may determine the outcome of infection. Recent advances in single-cell RNA sequencing (scRNA-seq) have identified multiple subsets within the mononuclear population, but implications to their function during infection are limited. Here, we surveyed the mononuclear niche of intracellular Salmonella Typhimurium (S.Tm) during early systemic infection in mice. We described eclipse-like growth kinetics in the spleen, with a first phase of bacterial control mediated by tissue-resident red-pulp macrophages. A second phase involved extensive bacterial replication within a macrophage population characterized by CD9 expression. We demonstrated that CD9+ macrophages induced pathways for detoxificating oxidized lipids, that may be utilized by intracellular S.Tm. We established that CD9+ macrophages originated from non-classical monocytes (NCM), and NCM-depleted mice were more resistant to S.Tm infection. Our study defines macrophage subset-specific host-pathogen interactions that determine early infection dynamics and infection outcome of the entire organism.
Keywords: host-pathogen interactions, Salmonella, macrophages, single-cell RNA-seq, in-vivo infection, non-classical monocytes
Graphical abstract

Highlights
-
•
At early stages, Salmonella kinetics follows an eclipse-like dynamics
-
•
CD9 Macs are an intracellular replication niche for Salmonella during eclipse
-
•
CD9 Macs derive from non-classical monocytes and induce pathways to detoxify oxLDL
-
•
CD9 Macs depletion reduces Salmonella infection and prolongs mice survival
Macrophages are a well-studied heterogeneous population with a variety of functions, but the role of the different macrophage subsets during intracellular infection is presently limited. Hoffman et al. reveal that during early mice infection NCM give rise to CD9 macrophages that provide an intracellular replication niche for Salmonella.
Introduction
Encounters between pathogenic bacteria and mononuclear phagocytes during early systemic infection can determine the capacity of the host to eliminate the invading pathogen or the ability of bacteria to establish a successful infection (Mills and Avraham, 2017). One of the major sites for clearance of infection (Carter and Collins, 1974), the spleen is host to multiple mononuclear phagocyte populations: (1) tissue resident macrophages, consisting of red pulp, metallophilic, and marginal zone macrophages, are sessile and perform tissue-specific functions (De Jesus et al., 2008; Kirby et al., 2009). (2) Circulating classical monocytes (CM) from the blood constantly replenish the monocyte pool in the spleen and also convert into non-classical monocytes (NCM) (Yona et al., 2013). (3) A reservoir of undifferentiated splenic monocytes that rapidly respond to inflammatory events (Swirski et al., 2009). Early after infection, tissue-resident macrophages respond and engage invading pathogens followed by recruitment of CM from the blood that differentiate into effector cells and allow efficient clearance of the pathogen (Serbina et al., 2008). Within the tissue, these macrophages and monocytes are heterogeneous population; a taxonomy of discrete cell types with continuous transitions of cell differentiation and activation states (Ginhoux and Guilliams, 2016; Trzebanski and Jung, 2020). The heterogeneous nature of mononuclear phagocyte populations suggests that their interaction with intracellular bacteria is likely to result in a variety of different, complex phenotypes.
Studies using flow cytometry and microscopy indicate that intracellular infection outcome is dependent on the identity of the infected mononuclear phagocytes and the environmental context within the tissue (Italiani and Boraschi, 2014). In the lung, Mycobacterium tuberculosis is controlled by interstitial macrophages while resident alveolar macrophages are permissive to intracellular replication (Huang et al., 2018). In the gut, Salmonella enterica serovar Typhimurium (S.Tm) can survive within CD18+ phagocytes, which allow it to traverse the epithelial barrier and disseminate to the spleen (Vazquez-Torres et al., 1999). During systemic infection, S.Tm are found in the spleen within tissue-resident macrophages; red pulp macrophages (Rp Macs) identified as F4/80+ and marginal zone macrophages identified as Siglec1+ cells (Geddes et al., 2007; Salcedo et al., 2001). During chronic infection, S.Tm are found within CD11b+CD11c+Ly6C+ alternatively activated granuloma macrophages (Eisele et al., 2013; Pham et al., 2020). Streptococcus pneumonia bacteria are found replicating within resident CD169+ metallophilic macrophages (Ercoli et al., 2018), while Listeria monocytogenes induce the early necroptotic death of resident Kupffer cells in the liver (Blériot et al., 2015). Furthermore, recruitment of Ly6Chi inflammatory monocytes to the intestine restricts S.Tm infection (Tam et al., 2014), and during Listeria infection Ly6C+ monocytes differentiate into inducible nitric oxide synthase (NOS)-producing monocyte-derived cells (Menezes et al., 2016). Thus, the identity of mononuclear phagocytes can provide the basis for our understanding of the molecular details and outcome of intracellular infection. However, classification of the cell populations using a priori-defined cell-surface markers is often controversial, and the assignment of different cell types and their function remains challenging.
Recent advances in single-cell RNA sequencing (scRNA-seq) allow breakdown of complex tissues into cell types that have revolutionized our ability to characterize the variety of cells within the immune compartment (Hashimshony et al., 2012; Jaitin et al., 2014). Applied to mononuclear phagocyte populations, scRNA-seq revealed a rich biology of cellular subsets that far exceeds our knowledge of this cellular compartment (Guilliams et al., 2018). In the lung, two interstitial macrophage subsets have been identified based on Lyve-1 and MHCII expression (Chakarov et al., 2019), and patrolling NR4A1-dependent monocytes were reported to replenish the pool of one interstitial macrophages subset with features of antigen-presenting cells (Schyns et al., 2019). In the skin, three tissue resident macrophage subsets have been associated with regeneration and surveillance of local nerves (Kolter et al., 2019). In the heart, resident macrophages have been classified based on Tim4 expression, with monocytes contributing to replenish this population after cardiac infarction (Dick et al., 2019). Within adipose tissue, Trem2 defines lipid-associated macrophages that are activated upon tissue lipid loss (Jaitin et al., 2019). In the central nerve system, a monocyte subset activated by lipopolysaccharides (LPS) or interferon-γ have been classified by Cxcl10 and represent pathogenic effector cells (Giladi et al., 2020). Despite increasing knowledge of diverse mononuclear phagocyte populations, we currently lack an understanding of how these translate to their function during intracellular infection. What is needed is a functional analysis of the intracellular niche within the different subsets of monocytes and macrophages that will provide a fundamental understanding of infection biology.
Here, we utilized scRNA-seq to test whether and how mononuclear phagocyte populations in the spleen change and respond during systemic infection with S.Tm. We aimed to decipher the mechanistic underpinnings of different cellular subsets that determine the phenotype of intracellular infection and to link the diversity of mononuclear subsets to infection outcomes in vivo.
Results
Early systemic infection with S.Tm follows eclipse-like growth dynamics
To characterize the kinetics of early systemic infection, we inoculated 8-week-old female C57BL/6J mice intravenously (i.v.) with S.Tm (1 × 107 CFU). We collected spleens at different time points after infection and assessed bacterial load by plating the spleen homogenate on selective agar plates. Already 1 h post-infection (hpi), about 90% of the inoculum was found in the spleen (Figure 1A; Figure S1A). Subsequently, the bacterial load in the spleen followed eclipse-like infection dynamics (ELID) comprising two discrete time windows. In the first phase (<8 hpi), S.Tm infection was controlled and the bacterial load decreased. The second phase (>8 hpi) was characterized by rapid S.Tm replication. To investigate the mononuclear phagocyte diversity during ELID, we analyzed spleens from infected mice and stained with antibodies to exclude lymphocytes and neutrophils (Lin–) and gated using CD11b and F4/80 (Figure S1B). We detected three populations: CD11b– F4/80+ (Rp Macs), CD11b+ F4/80– (monocytes and dendritic cells), and CD11b+ F4/80+ (monocyte-derived macrophages) which appeared in infected mice only (Figure 1B). The observed changes in the mononuclear phagocyte populations mirrored ELID: the first phase of ELID involved reduction in Rp Macs, and an increase in monocyte-derived macrophages in the second phase. To test whether these cells contained intracellular bacteria, we infected mice with an S.Tm strain that constitutively express an episomal fluorescent protein (S.Tm-GFP). Flow cytometry analysis revealed that 24 hpi intracellular S.Tm was found mostly within macrophages (F4/80+ GFP+) but not monocytes (CD11b+F4/80–GFP+) (Figures 1C and 1D), indicating that these cells are the main cellular niche for S.Tm during systemic infection, as was previously shown (Geddes et al., 2007; Salcedo et al., 2001).
Figure 1.
Early systemic infection with S.Tm follows ELID
(A and B) Mice were infected i.v. with S.Tm, and CFU was measured from spleens at 1, 4, 8, 24, and 48 hpi (A); n = 2 per time point). Lines represents the median of two replicates. Using flow cytometry, cells were gated to exclude CD19, CD3e, NK1.1, and Ly6G+ positive cells (Lin–) and analyzed for CD11b and F4/80 expression (B).
(C and D) Mice were infected i.v. with S.Tm-GFP and spleens were harvested at 4 hpi (n = 4) and 24 hpi (n = 4). Infected cells were analyzed by flow cytometry using the GFP signal of the bacteria (C). Calculated ratios of GFP+ cells between F4/80+ and Cd11b+ F4/80– populations are shown (D).
(E) Mice were infected i.v. with either WT (black) or ΔSPI-2 (gray) S.Tm strains, and CFU was measured from spleens at 4, 8, and 24 hpi (n = 2 mice per condition).
(F) Mice were infected i.v. with either WT or ΔSPI-2 GFP-expressing strains. Twenty-four hpi, spleens were harvested and analyzed by flow cytometry and CD11b+F4/80+ macrophages were sorted and individually plated on LB agar (n = 94 in WT, n = 34 in ΔSPI-2) to determine the number of CFU per cell.
Results are representative of four (A) or three (B–F) independent experiments. (E) ∗∗p < 0.01 using two-way ANOVA.
See also Figure S1.
To assess whether ELID was a result of extracellular growth or intracellular replication of the pathogen within mononuclear phagocytes, we used a S.Tm mutant strain lacking the ssaV gene, encoding a structural protein of the needle complex used for type 3 secretion system located on the Salmonella pathogenicity island 2 (SPI-2). S.Tm mutants lacking ssaV are unable to secrete effectors through the SPI-2 apparatus (ΔSPI-2) (Hensel et al., 1998), which is essential for intracellular survival and replication of S.Tm within macrophages (Cirillo et al., 1998). We challenged mice with either wild-type (WT) or a ΔSPI-2 S.Tm and assessed bacterial load in the spleen. Unlike WT, ΔSPI-2 mutant had significantly reduced recovery and replication in the spleen between 8 and 24 hpi (Figure 1E; Figure S1C), suggesting that, during the second phase, ELID requires intracellular replication. To further support this observation, we sorted single infected macrophages to enumerate single cell CFU (scCFU). We found that more than 50% of WT infected cells contained multiple replicating bacteria, while the majority of ΔSPI-2 infected macrophages harbored a single intracellular bacterium (Figure 1F). Thus, S.Tm growth follows ELID, which involves replication within macrophages mediated by SPI-2 and changes in the mononuclear phagocyte populations.
scRNA-seq reveals three concomitant splenic macrophage populations during ELID of S.Tm
We next sought to decipher functional subsets of mononuclear phagocytes that are involved in ELID of S.Tm in the spleen. We infected mice with WT or ΔSPI-2 strains and gated for CD11b+F4/80– and CD11b–F4/80+ populations in naive mice, and CD11b+F4/80+ and CD11b–F4/80+ populations in mice infected with WT or ΔSPI2 (Figure 2A). We did not gate on CD11b+F4/80– in the infected mice as this population does not contain intracellular bacteria (Figure 1D). Using fluorescently activated cell sorting (FACS), we sorted single cells from the gated populations and applied scRNA-seq (Jaitin et al., 2014) to characterize mononuclear phagocyte populations before and after infection. A total of 1,932 cells from both uninfected (naive) and infected mice passed quality control filters, with a mean of 729 genes per cell (Figures S2A and S2B) (Keren-Shaul et al., 2019). To define cell types according to the scRNA-seq data, we applied a MetaCell (MC) algorithm that groups cells with similar transcriptional profiles (Baran et al., 2019), producing a map of 26 MCs (Figure 2B). To assign MCs to distinct cell types or activation states, we performed correlation analysis and identified MCs with similar transcriptional states (Figure S2C), which collapsed MCs to 11 cell types with distinct gene-expression program and marker genes (Figure 2C and Figure S2D). In naive mice, MCs of the CD11b+ F4/80– population were composed of dendritic cells (MHCII+Cd74+), CM (Ly6c2+Ccr2+), and NCM (Nr4a1+Ace+Spn+) that may derive from CM in the tissue (Olingy et al., 2017), and a small fraction of NK cells (Nkg7+Gzma+) (Figure S2E). MCs retrieved from the CD11b–F4/80+ population were annotated as Rp Macs in naive mice (Mertk+Mrc1+) and in infected mice (Marco+Cxcl9+). Among CD11b+ F4/80+ cells from infected mice, we identified two distinct MC populations. The first population, Ly6c2+Nos2+Chi3l3+ cells, is reminiscent of iNOS Macs derived from Ly6Chi CM (Menezes et al., 2016; Serbina et al., 2003). The second population identified by the expression of Ly6c2–Ly6i+Cd9+ was termed CD9 Macs (Figure 2D). Noteworthy, infection with either WT or ΔSPI-2 S.Tm did not alter the MC identities following infection, but only their activation state (Figures S2F and S2G), and were annotated as the same cell type for further analysis.
Figure 2.
scRNA-seq reveals three concomitant splenic macrophage populations during ELID of S.Tm
(A) Naive mice or mice infected i.v. with WT or ΔSPI-2 S.Tm were harvested 24 hpi and analyzed by flow cytometry using CD11b and F4/80 antibodies.
(B) Single cells from gated populations in (A) were sorted by flow cytometry and analyzed by scRNA-seq. Shown is a forced layout map of single cells (dots) and MCs (large circles), with infection conditions indicated (naive mice [white, n = 631 cells], mice infected with WT [black, n = 643 cells], or a ΔSPI-2 mutant [gray, n = 658 cells]). Cell-type annotations are indicated by outline color of the MC; infection status of the MC by fill color and similarity between MCs are indicated by connecting nodes.
(C) Marker gene annotations presented as the percentage of cells expressing the indicated genes in each MC (shown as the size of the circle) and the relative log2 fold change of the gene expression in each MC (shown as the color of the circle).
(D) Expression analysis across all MCs of genes used as markers for iNOS Macs (Ly6c2, Nos2) and CD9 Macs (Cd9, Ly6i).
(E) kNN classification of cell types presented as percentage of infected cells classified to cell types in the naive sample. The color bar indicates percentage of cells of each cell type in the infected mice classified to cell types from the naive mice.
See also Figure S2.
To align mononuclear populations before and after infection that can suggest a progeny-product relationship, we applied a k-nearest neighbor (kNN) classification algorithm to link single cells from the infected mice to the MCs in the naive mice. As expected, following infection, Rp Macs were matched mostly with Rp Macs in the naive sample, and iNOS Macs were aligned with CM in the naive sample. Importantly, CD9 Macs in the infected sample were assigned mostly to NCM (Figure 2E).
Thus, scRNA-seq analysis suggested that ELID of S.Tm is mirrored by changes in the macrophage landscape, underscored by the putative emergence of a CD9 Macs population from NCM.
Functional analysis reveals distinct inflammatory programs of iNOS Macs and reducing oxidized lipids programs of CD9 Macs
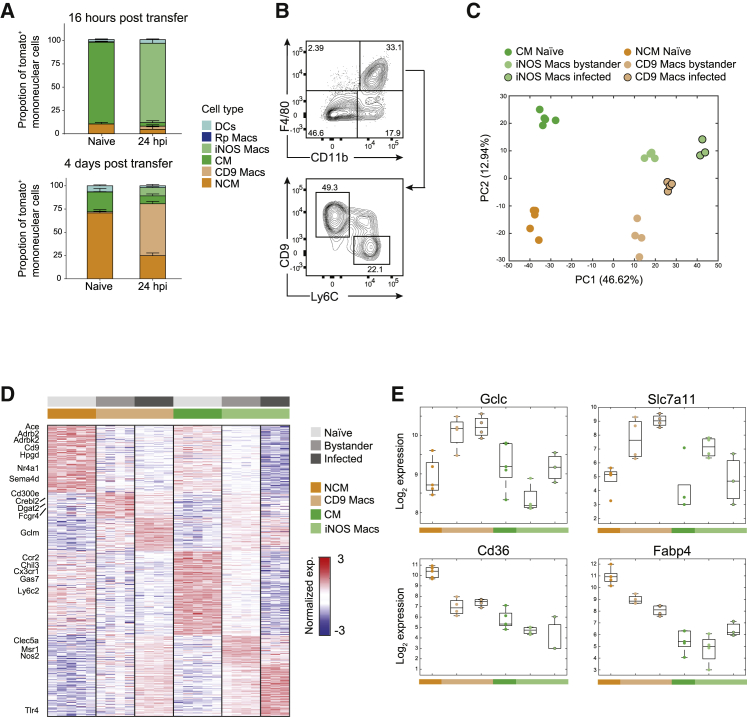
We next followed the dynamics of macrophage populations after S.Tm infection using flow cytometry by staining for the cell-surface molecules Ly6C and the tetraspanin receptor CD9 that are, according to our scRNA-seq analysis above, differentially expressed by iNOS Macs and CD9 Macs, respectively. Phenotypic characterization confirmed the existence of two distinct cell populations: iNOS Macs (Lin–CD11b+F4/80+CD64+Ly6C+CD69+SCA-1+MERTKmidCD206–MHCII–CD9–) and CD9 Macs (Lin–CD11b+F4/80+CD64+ CD9+Ly6C–CD69+SCA-1+MERTK–CD206–MHCII–) (Figures S3A and S3B). Both subsets downregulated monocytes markers CX3CR1 and CD115. To establish the origin of CD9 Macs, we isolated monocytes from the bone marrows of Ms4a3cre-RosaTdT mice and transferred them to WT mice. Mice were infected with S.Tm either 16 h or 4 days after adoptive transfer, as these timings were reported previously to allow for CM to convert to NCM (Yona et al., 2013), and tdTomato was used to mark transferred monocytes by flow cytometry (Figures S3C and S3D). Indeed, 16 h post-adoptive transfer, most tdTomato+ cells were CM in naive mice, that differentiate to iNOS Macs after infection (Figure 3A, top). Importantly, 4 days post-adoptive transfer, most tdTomato+ cells were NCM that differentiated to CD9 Macs after infection (Figure 3A, bottom), validating a precursor-product relationship of these cells.
Figure 3.
Functional analysis reveals distinct inflammatory programs of iNOS Macs and reducing oxidized lipids programs of CD9 Macs
(A) Monocytes from Ms4a3cre-RosaTdT chimera were transferred to naive mice and 16 h (top panel) or 4 days (bottom panel) post-transfer mice were infected with S.Tm or PBS (n = 5, per group per time point). Twenty-four hpi spleens were harvested and analyzed by FACS. Presented are calculated proportions of tdTomato+ of the mononuclear populations.
(B) Mice were infected with S.Tm and 24 hpi spleens were harvested and analyzed by flow cytometry using CD11b, F4/80, CD9, and Ly6C antibodies. Presented are contour plots of iNOS Macs and CD9 Macs from the gated Lin–CD11b+F4/80+ population.
(C–E) Mice were infected as in (B), and CM or NCM from naive mice (n = 5) or bystander or infected CD9 Macs and iNOS Macs from S.Tm challenged mice (n = 4) were sorted by FACS and analyzed by bulk RNA-seq. Presented are PCA plots (C) and a heatmap (D) of the significant differentially expressed genes between all groups and log2 fold expression changes of selected genes (E).
Results are representative of two (A and C–E) or four (B) independent experiments. See also Figure S3 and Tables S1 and S2.
To further characterize these populations, we analyzed iNOS Macs and CD9 Macs by flow cytometry (Figure 3B) and performed bulk RNA-seq on 1,000 sorted cells from naive, bystander, and infected cells of each population. We curated a list of 2,018 differentially expressed genes (Figures 3C and 3D; Table S1, Q < 0.02). The expression signature of marker genes detected by the scRNA-seq matched the expression profiles of cells sorted using CD9 and Ly6C markers (genes indicated to the left of the heatmap), validating the identity of the populations. Gene ontology (GO) enrichment analysis of the differentially expressed genes revealed a distinct functional gene-expression program for each macrophage population (Table S2). iNOS Macs were enriched for a pro-inflammatory program (e.g., chemotaxis, positive regulation of the inflammatory process, including Nos2) and oxidative biosynthetic pathways (e.g., positive regulation of nitric oxide biosynthetic process, superoxide metabolic process). iNOS Macs were also enriched for factors that regulate phagocytosis (e.g., Gas7 and Msr1, Galectins) and receptors that recognize microbial ligands (e.g., Tlr4 and Clec5a), indicating that these cells can readily ingest and potentially eliminate invading pathogens. In contrast, CD9 Macs showed enrichment for anti-inflammatory processes including fatty acid and lipid metabolism (e.g., Dgat2, Crebl2, and Sema4d), beta-adrenergic signaling (e.g., Adrb2, Adrbk2) and prostaglandins dehydrogenation (Hpgd) (Ağaç et al., 2018; Batista-Gonzalez et al., 2020). Of note, infected and bystander CD9 Macs upregulated peroxisome proliferator activated receptor gamma (PPARγ)-regulated genes including the pathway for oxidized low-density lipoprotein (oxLDL) uptake (Cd36, Slc7a11) and glutathione detoxification (Gclc, Gclm) (Nagy et al., 1998) (Figure 3E). Further, CD9 Macs genes related to prostaglandin dehydrogenation and oxLDL provide ligands for activation of the transcription factor PPARγ (Forman et al., 1995). CD9 is suggested to associate with CD36 on macrophage surface and participate in signaling in response to oxLDL, further implicating the function of CD9 Macs in reducing ROS-mediated oxidative environment in the spleen during infection.
Next, we compared signatures of naive NCM to bystander and infected CD9 Macs and found that bystander and infected cells share similar transcriptional signatures, suggesting that bacterial PAMP recognition drives NCM to become CD9 Macs (Figures S3E and S3F). As we observed also an intermediate CD9+Ly6C+ population (Figure 3B), we sorted and sequenced this population and found that most of the genes differentially expressed between CD9 Macs and iNOS Macs are mutually expressed by this intermediate population (Figures S3G; Table S3) implying an intermediate differentiation state. Finally, CD9 was previously indicated in macrophages in the liver and adipose tissue that originate from CM (Jaitin et al., 2019; Ramachandran et al., 2019). We compared gene signatures from these studies to our CD9 Macs signature in the spleen and found they were not associated with CD9 Macs but were more similar to iNOS Macs, which is in line with their origin from CM (Figure S3H). Importantly, these distinct activation programs indicate that CD9 Macs and iNOS Macs may have different functional roles during intracellular S.Tm infection.
ELID is mirrored by a decline in Rp Macs and increase in CD9 Macs
We then characterized the dynamics of the macrophage populations using flow cytometry, as well as their intracellular bacterial load using S.Tm-GFP (Figures 4A and 4B; Figures S4A–S4C). In naive mice, ∼90% of the F4/80+ cells were Rp Macs. As infection progressed, Rp Macs abundance decreased and reached ∼10% at 24 hpi. Conversely, iNOS Macs and CD9 Macs were almost undetectable in naive mice and accumulated during the first hours until they reached at 24 hpi up to ∼30% and ∼55% of the splenic macrophage pool, respectively. Analysis of infected cells using the GFP signal of the intracellular bacteria revealed that during the first 8 h S.Tm was found mostly within Rp Macs. In contrast, by 24 hpi, most S.Tm resided within CD9 Macs and iNOS Macs. The numeric increase of iNOS Macs and CD9 Macs coincided with intracellular growth of S.Tm during the second phase of the eclipse. To directly examine intracellular bacterial replication, we infected mice with S.Tm-GFP and 24 hpi analyzed scCFU within CD9 Macs and iNOS Macs (Figure 4C). In iNOS Macs, we measured no more than a single bacterium per cell. In contrast, 30% of CD9 Macs contained more than a single bacteria, suggesting that these cells are permissive to S.Tm intracellular growth. Using microscopy, we confirmed that at 24 hpi 70% of the F4/80+CD9+ (CD9 Macs) contained more than three intracellular bacteria suggesting intracellular replication, compared to only 10% of the F4/80+CD9– (Figures 4D and 4E; Figure S4D). This higher number of intracellular bacteria in CD9 Macs as evident by microscopy compared to scCFU may underscore a deficiency of S.Tm to establish re-growth following intracellular infection (Helaine et al., 2014).
Figure 4.
ELID is mirrored by a decline of Rp Macs and increase of CD9 Macs
(A and B) Mice were infected with S.Tm-GFP (n = 3 per time point). At the indicated time points, spleens were harvested and analyzed by flow cytometry using antibodies to the indicated populations or the GFP signal of the bacteria (A). Bar graphs (left y axis) indicates mean ratio from the population of F4/80, and dots (right y axis) indicate cells containing intracellular bacteria (presented as ratio from F4/80+ GFP+ population) (B).
(C) Mice were infected as in (A), 24 hpi single GFP+ CD9 Macs and iNOS Macs were sorted, and scCFU was measured (n = 494, n = 553 respectively). Presented are the proportions of cells according to number of bacteria per cell.
(D and E) Mice were infected as in (A). Twenty-four hpi spleens were harvested, fixed, stained, and imaged with CD9 and F4/80 antibodies, DAPI, and GFP for intracellular bacteria (D). An example of CD9 Macs containing multiple bacteria. Number of intracellular bacteria were quantified across F4/80+CD9+ or F4/80+CD9– cells (n = 94 cells from 3 mice) (E).
Results are representative of four (A and B) or three (C–E) independent experiments. See also Figure S4.
Our results indicate that changes in the landscape of macrophage populations mirror the growth dynamics of S.Tm and support a model where in the first phase of ELID, Rp Macs control and eliminate intracellular S.Tm, resulting in Rp Macs decline. Bacterial growth in the second expansion phase of ELID coincides with intracellular replication of S.Tm within the newly arising CD9 Macs.
During the first phase of ELID, S.Tm growth is restricted by Rp Macs that undergo necroptotic cell death.
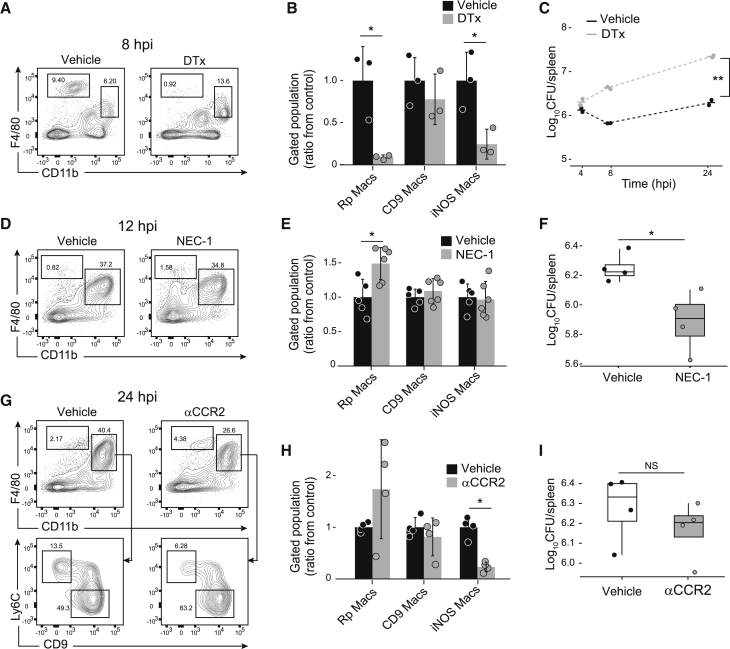
Our observation that the majority of S.Tm was found within Rp Macs during the first phase of ELID led us to hypothesize that these cells mediate intracellular pathogen clearance, as was previously proposed (Borges da Silva et al., 2015). To test this, we utilized mice that harbor a diphtheria toxin receptor (DTR) under the promoter of CD169 that is expressed by Rp Macs (Miyake et al., 2007). CD169DTR mice were treated with a single dose of either PBS (vehicle) or diphtheria toxin (DTx) to deplete Rp Macs, which was confirmed by flow cytometry analysis (p < 0.05, Figures 5A and 5B). DTx treatment also significantly reduced the abundance of iNOS Macs, probably due to their low, but detectable expression of CD169 (Figure S5A). Next, CD169DTR mice were treated with vehicle or DTx and infected with S.Tm for measurement of bacterial CFU (Figure 5C; Figure S5B). ELID of S.Tm was measured in vehicle-treated mice, but no CFU decline was evident in the DTx-treated animals in the first ELID phase (<8 h). Kinetics of the second ELID phase (>8 h) was similar between vehicle and DTx-treated mice. Collectively, these data suggest that Rp Macs eliminate intracellular bacteria during the first phase of ELID.
Figure 5.
During the first phase of ELID, S.Tm growth is restricted by Rp Macs
(A and B) Mice expressing diphtheria toxin receptor under Siglec-1 promoter were treated with diphtheria toxin (DTx; n = 3) or PBS (vehicle; n = 3). Twenty-four hours later, mice were infected with S.Tm, and 8 hpi spleens were harvested. Spleens were analyzed by flow cytometry using antibodies to mark the indicated populations (A) and presented as the ratio of the population from vehicle-treated mice (B).
(C) Mice were treated as in (A), spleens were harvested at the indicated time points, and CFU was measured (n = 2 per treatment in each time point).
(D–F) Mice were injected intravenously with either NEC-1 (n = 5) or vehicle (n = 4). One hour later, mice were infected with S.Tm, and 12 hpi spleens were harvested, and analyzed by flow cytometry using antibodies to mark the indicated populations (D), presented as the ratio of the population from vehicle-treated mice (E) and plated for CFU (n = 4 per treatment) (F).
(G–I) Mice were injected intraperitoneally with αCCR2 antibodies or vehicle (n = 4 per treatment). Twenty-four hours later, mice were infected with S.Tm, and 24 hpi spleens were harvested. Spleens were analyzed by flow cytometry using antibodies to mark the indicated populations (G), presented as the ratio of the population from vehicle-treated mice (H), and plated for CFU (I).
Results are representative of four (A)–(C) or three (D)–(I) independent experiments. (B, E, and H) Error bars indicate standard deviation. (B) ∗p < 0.05, using Student’s t test. (E, F, and H) ∗p < 0.05, using Mann-Whitney U test. (C) ∗∗p < 0.01 using two-way analysis of variance. See also Figure S5.
Previously, it has been shown that Listeria monocytogenes infection results in cell death of resident Kupffer cells in the liver through necroptosis (Blériot et al., 2015). We hypothesized that this may also be the mechanism of Rp Macs decline we observe in the first ELID phase (Figure 4B). We therefore treated mice with the necroptosis inhibitor Necrostatin-1 (NEC-1) 1 h before infection with S.Tm. At 12 hpi, we measured significantly higher numbers of Rp Macs in NEC-1-treated mice compared to vehicle-treated mice but not in other cell populations (Figures 5D and 5E; Figure S5C). At 24 hpi, we measured significantly lower bacterial load in NEC-1-treated mice compared to vehicle-treated mice (p < 0.05, Figure 5F). These results suggest that S.Tm infection in the first phase of ELID is controlled by Rp Macs that then undergo necroptotic cell death.
During gut infection, inflammatory monocytes promote S.Tm expansion in the lumen of the inflamed intestine (McLaughlin et al., 2019). We turned to test the involvement of these cells in the second ELID phase. CM migration from the bone marrow to infected tissues is dependent on CCR2 (Boring et al., 1997), whereupon they differentiate to iNOS-producing cells and contribute to infection control (Tam et al., 2014). We specifically depleted CM by intraperitoneal injection of αCCR2 antibody (Brühl et al., 2007). Flow cytometry analysis of the spleen confirmed that αCCR2 treatment resulted in significantly reduced numbers of CM, as well as iNOS Macs, corroborating the precursor-product relationship of these cells (p < 0.05, Figures 5G and 5H; Figures S5D and S5E). CD9 Macs and Rp Macs numbers remained unchanged. Moreover, treatment with αCCR2 did not significantly change bacterial CFU (Figure 5I), suggesting that iNOS Macs have a limited contribution to S.Tm control during the second ELID phase.
CD9 Macs originate from NCM and provide an intracellular replication niche for S.Tm during the second phase of ELID
The above data suggested a critical role of CD9 Macs in S.Tm expansion. Fate-mapping experiments indicated that CD9 Macs arose from NCM (Figure 3A), suggesting a possible approach for in vivo manipulation of this population. Specifically, ablation of NCM can be achieved by a deletion of a super enhancer domain that controls NCM expression of the survival factor Nr4a1, termed E2, without affecting Nr4a1 expression in other tissue macrophages (Thomas et al., 2016). Analysis of WT and E2−/− mice validated a significant decrease of NCM in naive mice (p < 0.05, Figures 6A and 6B). We next infected WT and E2−/− mice with S.Tm. Compared to WT, E2−/− mice displayed a significant decrease of CD9 Macs (p < 0.05, Figures 6C and 6D). This provides another experimental confirmation that CD9 Macs indeed originate from NCM. In contrast and in line with their derivation from Ly6Chi CM, iNOS Macs were unaffected. Finally, we infected WT or E2−/− mice with a low dose of S.Tm (1,000 CFU) to test extended infection and survival of mice. Analysis of CFU measurements revealed that E2−/− mice displayed a significantly reduced splenic bacterial load, compared to WT mice (Figure 6E). Testing for survival, mutant mice lacking NCM and CD9 Macs lost significantly less weight than WT (p < 0.01, Figure 6F) and displayed significantly extended survival compared to WT mice (p < 0.01, Figure 6G). Collectively, the lower CFU and extended survival in E2−/− mice indicate that CD9 Macs are an intracellular niche required for S.Tm expansion, which has implications for the survival of the entire organism.
Figure 6.
CD9 Macs provide a niche for intracellular S.Tm replication during the second phase of ELID
(A and B) Spleens of E2+/+ (WT; n = 3) and E2−/− (n = 4) were harvested and analyzed by flow cytometry using antibodies to mark the indicated populations (A) and presented as the ratio of the population from WT mice (B).
(C and D) WT (n = 5) and E2−/− mice (n = 5) were infected with S.Tm. Twenty-four hpi spleens were harvested and analyzed by flow cytometry using antibodies to mark the indicated populations (C) and presented as the ratio of the population from WT mice (D).
(E) WT and E2−/− mice were infected with 1,000 CFU of S.Tm. Three days post-infection, spleen were harvested and plated for CFU (n = 5 per condition).
(F and G) WT and E2−/− mice were infected with 1,000 CFU of S.Tm (n = 7 per condition). Mice were weighed (F) and monitored for survival for 5 days, presented on a Kaplan-Meier plot (G).
Results are representative of three independent experiments in all panels. (B and D) Error bars indicate standard deviation. (F) Error bars indicate standard error. (B) ∗p < 0.05 Student’s t test. (D)∗p < 0.05, Mann-Whitney U test. (E) p < 0.01, Mann-Whitney U test. (F) ∗∗p > 0.01 two-way analysis of variance. (G) ∗∗p < 0.01 log-rank test.
Discussion
Mononuclear phagocytes can derive from several sources, including tissue-resident populations and infiltrating monocytes that differentiate into effector cells. At sites of infection, macrophages engage invading pathogens and induce effector processes to kill the invading agents. Intracellular bacterial pathogens present a paradox, as they preferably replicate within macrophages, the very cells that aim to destroy them (Price and Vance, 2014). Mostly, this duality is explained by differential signaling and gene-expression programs of macrophages toward an inflammatory state (M1-like that eliminates the pathogen) or an anti-inflammatory state (M2-like that is more permissive for intracellular replication) (Pham et al., 2020; Saliba et al., 2016). Here, using scRNA-seq, we identified co-existing macrophage populations during early infection, each with unique molecular activation features, that influenced distinct outcomes of intracellular S.Tm infection. According to our results, during in vivo infection, activation phenotypes of macrophages arise not from bifurcation of a macrophage population but rather a “division of labor” between different macrophage progeny that determines their activation state, as we show here for iNOS Macs from CM (Pro-inflammatory) and CD9 Macs from NCM (alternatively activated).
During early systemic infection, ELID was mediated by dispersal of S.Tm within three functionally distinct populations of F4/80 macrophages: tissue-resident Rp Macs, CM that give rise to iNOS Macs and CD9 Macs originated from NCM. In the first phase of ELID, S.Tm was controlled by Rp Macs, at the expense of their demise. Most tissue resident macrophages are known to have limited microbicidal activity (Davies et al., 2013). It has been suggested that rather than directly eliminating invading bacteria, tissue-resident macrophages undergo necroptotic cell death that triggers chemokine and cytokine release and recruitment of monocytes that eliminate the bacteria (Ginhoux et al., 2017). Indeed, inhibition of Rp Macs necroptotic cell death resulted in lower bacterial survival, which is in accordance with better control of S.Tm infection of Rip3−/− mice, that cannot undergo necroptotic cell death (Robinson et al., 2012).
In the second phase of ELID, intracellular S.Tm resided within iNOS Macs and CD9 Macs. A direct comparison between iNOS Macs and CD9 Macs indicated disparate molecular details and distinct phenotypic outcome of intracellular infection. Our understanding of the contribution of NCM to tissue macrophage populations is limited. It is suggested that NCM differentiate into alternatively activated macrophages (Narasimhan et al., 2019). Importantly, S.Tm growth during the second phase of the eclipse was mediated by replication within CD9 Macs and mice deficient of CD9 Macs alter the course of infection of the entire organism. Gene expression of infected CD9 Macs implicated their role in reducing ROS-mediated oxLDL, an event that was suggested to occur during chronic infection, the formation of TB granulomas and to support intracellular bacterial growth (Vrieling et al., 2019). Thus, a plausible model of ELID is that CD9 Macs are recruited to the necroptotic environment of Rp Macs, where they may uptake S.Tm or engulf dead Rp Macs which contain intracellular S.Tm. While S.Tm may create a replication niche in CD9 Macs by infecting its precursor NCM, our data suggest that NCM recognition of bacterial ligands drives their differentiation to CD9 Macs. Further experimental work is required to discern between these alternatives. Finally, further experiments can discern the contribution of incoming NCM during infection to the expansion of CD9 Macs.
We foresee that CD9 Macs may be relevant to other models of infection with S.Tm, including chronic infection and oral route administration. During chronic infection, S.Tm resides within macrophages recruited to granulomas (Goldberg et al., 2018) that are actively directed by S.Tm toward an anti-inflammatory phenotype (Pham et al., 2020). It remains to be tested whether CD9 Macs are involved in the anti-inflammatory phenotype of macrophages during chronic infection. During oral infection, S.Tm can traverse the epithelial barrier of the gut to the bloodstream and disseminate to the spleen either through invasion of epithelial cells (Frost et al., 1997; Takeuchi, 1967) or transported via CD18-expressing phagocytes (Vazquez-Torres et al., 1999). NCM are also found within the gut (Schleier et al., 2020), and their contribution to macrophages in the gut mucosa is yet to be determined.
In summary, our work describes that, at early systemic infection, intracellular S.Tm is distributed within three distinct macrophage populations in the spleen, with diverse progeny and activation programs, that determines an eclipse-like growth dynamics. Beyond S.Tm infection, we established the fate and function of NCM-derived CD9 Macs with implications to infection in other organs and other pathogens.
Limitations of the study
Although we demonstrated that NCM-derived CD9 Macs provided a replication niche for S.Tm in rodent models of disease, examining this population and corroboration in humans remains to be established. Furthermore, in this study we used intravenous inoculation of S.Tm in an acute model of infection. Further studies utilizing oral infection and chronic models will help establish S.Tm-CD9 Macs interactions in other tissues (e.g., gut mucosal barrier) and their role during persistent infection.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Monoclonal anti-mouse CD16/CD32, clone 93 | BioLegend | Cat#101301; RRID:AB_312800 |
| Monoclonal anti-mouse NK1.1-APC clone PK136 | BioLegend | Cat#108710; RRID:AB_313397 |
| Monoclonal anti-mouse CD19-FITC clone 6D5 | BioLegend | Cat#115506; RRID:AB_313641 |
| Monoclonal anti-mouse CD19-APC clone 6D5 | BioLegend | Cat#115512; RRID:AB_313647 |
| Monoclonal anti-mouse CD3ε-FITC clone 145-2C11 | BioLegend | Cat#100306; RRID:AB_312671 |
| Monoclonal anti-mouse CD3-APC clone 17A2 | BioLegend | Cat#100236; RRID:AB_2561456 |
| Monoclonal anti-mouse Ly6G-APC clone 1A8 | BioLegend | Cat#127614; RRID:AB_2227348 |
| Monoclonal anti-mouse Ly6G-FITC clone 1A8 | BioLegend | Cat#127606; RRID:AB_1236494 |
| Monoclonal anti-mouse Ly6C- Alexa Fluor® 700 clone HK1.4 | BioLegend | Cat#128024; RRID:AB_10643270 |
| Monoclonal anti-mouse Ly6C- Brilliant Violet 605 clone HK1.4 | BioLegend | Cat#128036; RRID:AB_2562353 |
| Monoclonal anti-mouse CD11b- PerCP-eFluor 710 clone M1/70 | Invitrogen | Cat#46-0112-82; RRID:AB_2866430 |
| Monoclonal anti-mouse CD11b- APC/Cy7 clone M1/70 | BioLegend | Cat#101226; RRID:AB_830642 |
| Monoclonal anti-mouse CD11c- PE/Cyanine5.5 clone N418 | Invitrogen | Cat#35-0114-82; RRID:AB_469709 |
| Monoclonal anti-mouse CD11c-FITC clone N418 | BioLegend | Cat#117306; RRID:AB_313775 |
| Monoclonal anti-mouse CD64- Brilliant Violet 605 clone X54-5∖7.1 | BioLegend | Cat#139323; RRID:AB_2629778 |
| Monoclonal anti-mouse CD9-Biotin clone EM-04 | GeneTex | Cat#GTX80172; RRID:AB_11177678 |
| Monoclonal anti-mouse CD9- PE/Cy7 clone MZ3 | BioLegend | Cat#124816; RRID:AB_2783075 |
| Monoclonal anti-mouse F4/80- PE clone BM8 | BioLegend | Cat#123110; RRID:AB_893486 |
| Monoclonal anti-mouse F4/80- Brilliant Violet 421 clone BM8 | BioLegend | Cat#123137; RRID:AB_2563102 |
| Monoclonal anti-mouse F4/80- Alexa Fluor® 647 clone BM8 | BioLegend | Cat#123121; RRID:AB_893492 |
| Monoclonal anti-mouse CD68- Pacific Blue clone FA-11 | BioLegend | Cat#137028; RRID:AB_2800644 |
| Monoclonal anti-mouse CD204- PerCP/Cyanine5.5 clone 1F8C33 | BioLegend | Cat#154716; RRID:AB_2892313 |
| Monoclonal anti-mouse I-A/I-E (MHC-II)- Brilliant Violet 650 clone M5/114/15.2 | BioLegend | Cat#107641; RRID:AB_2565975 |
| Monoclonal anti-mouse CD69-PE clone H1.2F3 | BioLegend | Cat#104508; RRID:AB_313111 |
| Monoclonal anti-mouse CX3CR1-PE/Dazzle 594 clone SA011F11 | BioLegend | Cat#149013; RRID:AB_2565697 |
| Monoclonal anti-mouse Ly-6A/E (Sca-1)- PE/Cyanine5 clone D7 | BioLegend | Cat#108114; RRID:AB_493596 |
| Monoclonal anti-mouse MERTK (Mer)-APC clone 2B10C42 | BioLegend | Cat#151508; RRID:AB_2650739 |
| Monoclonal anti-mouse CD206- Alexa Fluor® 647 clone C068C2 | BioLegend | Cat#141711; RRID:AB_10900240 |
| Monoclonal anti-mouse CD43- Alexa Fluor® 700 clone S11 | BioLegend | Cat#143214; RRID:AB_2800661 |
| Monoclonal anti-mouse CD115 (CSF-1R)- APC/Cy7 clone AFS98 | BioLegend | Cat#135532; RRID:AB_2632740 |
| Monoclonal anti-mouse CD115 (CSF-1R)- Biotin clone AFS98 | BioLegend | Cat#135507; RRID:AB_2028401 |
| Monoclonal anti-mouse CD45-APC/Fire 810 clone 30-F11 | BioLegend | Cat#103173; RRID:AB_2860599 |
| Streptavidin- Cy3 | Jackson immunoresearch | Cat#016-160-084; RRID:AB_2337244 |
| Polyclonal anti-mouse | Prof. Steffen Jung | N/A |
| Experimental models: Mice strains | ||
| C57BL/6JOlaHsd | ENVIGO | Order code:057 |
| Nr4a1se_2 (E2−/−) | the jaxon laborory | RRID:IMSR_JAX:030204 |
| CD169-DTR | RIKEN | RRID:IMSR_RBRC04395 |
| MS4A3cre-ROSATdT | Prof. Florent Ginhoux | N/A |
| Experimental models: Bacterial strains | ||
| Salmonella typhimurium (strain SL1344) | Prof. Denise Monack | N/A |
| GFP- Salmonella typhimurium (strain SL1344) | Prof. Denise Monack | N/A |
| ΔssaV - Salmonella typhimurium (strain SL1344) | Prof. Denise Monack | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Diphtheria Toxin (unnicked) from Corynebacterium diphtheriae | Cayman Chemical | Cat#19657 |
| Necrostatin-1 | Sigma-Aldrich | Cat#N9037 |
| Brilliant Stain Buffer | BD Biosciences | Cat#563794 |
| Red Blood cell Lysing buffer Hybri-Max™ | Sigma-Aldrich | Cat#R7767 |
| EDTA disodium salt | Sigma-Aldrich | Cat#E7889 |
| AMP | Fisher Bioreagents | BP1760 |
| Kanamycin Sulfate | Caisson Labs | Cat#K003 |
| Gentamycin Sulfate | Sigma-Aldrich | G1272 |
| Streptomycin sulfate | Acros Organics B.V.B.A. | Cat#455340250 |
| BD Liquid Counting Beads | BD Biosciences | Cat#335925 |
| Helix NP Blue | BioLegend | Cat#425305 |
| RNA Fragmentation Reagents | Invitrogen | Cat#AM8740 |
| RNaseOUT | Invitrogen | Cat#10777019 |
| SuperScript II | Invitrogen | Cat#18064 |
| RNasin® Plus | Promega | Cat#N2615 |
| ExoSAP-IT | Applied Biosystems | Cat#78201.1.ML |
| Agencourt AMPure XP | Beckman Coulter | Cat#A63881 |
| RNAClean XP | Beckman Coulter | Cat#A63987 |
| Phusion® High-Fidelity PCR Master Mix with HF Buffer | Thermo Scientific | Cat#F531 |
| Second Strand Buffer | Invitrogen | Cat#10812014 |
| dNTP Set | Thermo Scientific | Cat#R0181 |
| E. coli DNA ligase | Thermo Scientific | Cat#18052019 |
| DNA Polymerase I | Thermo Scientific | Cat#EP0041 |
| RNaseH | Invitrogen | Cat#AM2293 |
| MEGAscript T7 | Invitrogen | Cat#AM1334 |
| SuperScript III | Invitrogen | Cat#18080 |
| NEBNext® Ultra II Non-Directional RNA Second Strand Synthesis Module | New England Biolabs | Cat#E6111 |
| Exonuclease I | New England Biolabs | Cat#M0293 |
| AffinityScript cDNA Synthesis Kit | Agilent Technologies | Cat#200436 |
| T4 RNA Ligase 1 | New England Biolabs | Cat#M0204 |
| Tissue-Tek® O.C.T. Compound | Sakura Finetek | REF#4583 |
| Streptavidin MicroBeads | Miltenyi Biotec | Cat#130-048-101 |
| Ficoll® Paque Plus | GE Healthcare | GE17-1440-02 |
| Triton X-100 | Sigma-Aldrich | Cat#T9284 |
| Software and algorithms | ||
| FlowJo V10.7 | FlowJo LLC | https://www.flowjo.com |
| Imaris 9.5 | Bitplane | https://imaris.oxinst.com/ |
| SlideViewer V2.5 | 3DHISTECH Ltd | https://www.3dhistech.com/research/software-downloads/ |
| Cel-seq pipeline | Yanai lab | https://github.com/yanailab/CEL-Seq-pipeline |
| MARS-Seq pipeline | Tanay lab | http://compgenomics.weizmann.ac.il/tanay/?page_id=672 |
| MetaCell | Tanay lab | https://github.com/tanaylab/metacell/ |
| Deposited data | ||
| Monocytes Single-cell sequencing data | This paper | GEO: GSE164254 |
| Monocytes bulk sequencing data (related to Figure 3) | This paper | GEO: GSE164255 |
| Monocytes bulk sequencing data (related to Figure S3) | This paper | GEO: GSE164253 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Roi Avraham (roi.avraham@weizmann.ac.il).
Materials availability
This study did not generate new unique reagents.
Mice and bacteria strains
C57BL/6 mice, 7-9 weeks old, were purchased from ENVIGO, housed at the Weizmann Institute pathogen-free facility and provided with standard food and water without restriction. Cd169-DTR KO mice were purchased from RIKEN (RBRC No. RBRC04395, (Miyake et al., 2007). Nr4a1se_2 (E2−/−) were purchased from Jackson laboratory (stock No. 030204, (Thomas et al., 2016)). MS4A3creROSATdT mice were kindly provided by Prof. Florent Ginhoux (Liu et al., 2019). All experiments were performed in accordance to the guidelines outlined by the Weizmann Institute Committee on Animal Care.
All S.Tm strains used in this study were derived from the wild-type strain SL1344, harboring a constitutive-GFP expressing plasmid (pFPV25.1; Addgene). ΔssaV SL1344 mutant strain (ΔSPI-2) was a generous gift from Prof. Denise Monack (Stanford).
Mice infection
Cultures of S.Tm were grown in Luria-Bertani (LB) medium at 37°C for 16 hours to a stationary phase. The cultures were washed in PBS and counted using OD600. Mice were injected intravenously (i.v.) with 200 μL containing either 1Χ107 (high dose) or 1000 (low dose) CFU of bacteria or PBS (as controls). Injected bacterial load was verified by CFU. Mice were euthanized by CO2, spleens were harvested and CFU numbers were evaluated by plating serial 10-fold dilutions of homogenized spleens on selective agar plates.
Splenocytes isolation and staining
Spleens were extracted and stored in cooled FACS Buffer (PBS, 10 mM EDTA, 5% FBS) until splenocytes isolation. The spleens were dissected, squashed against 70 μm cell strainer and washed with cold FACS Buffer. Cells were then centrifuged twice (3 min, 500g, 4°C). The pellet was re-suspended with red blood lysis buffer (Sigma-Aldrich, Israel), incubated for 3 min at room temperature, centrifuged (3 min, 500g) and re-suspended with FACS buffer containing 50 μg/ml gentamycin to eliminate extracellular bacteria or with Brilliant stain buffer (BD biosciences) (several brilliant violet fluorophores were introduced in one panel). Single cell suspensions were incubated with CD16/CD32 blocking antibodies (Biolegend, Israel) for 15 minutes on ice. Subsequently fluorophore conjugated antibodies cocktails were introduced and incubated for 30 minutes on ice. Cells were washed, re-suspended with 500-1000 mL FACS buffer and passed through a cell strainer. Prior to analysis, live/dead stain was added to the samples (final concentration of 30 nM, Helix NP Blue, BioLegend, Israel). For absolute quantification, 10-50 μL of BD Liquid Counting Beads (BD Biosciences) were added to the sample prior analysis to a fixed final concentration.
Immunohistochemistry
Spleens were extracted and fixed in 4% paraformaldehyde (PFA) overnight at 4°C, transferred to 30% sucrose in PBS overnight at 4°C, then embedded in OCT for 1 hour, flash frozen on dry ice (−80°C) and stored at −80°C for further processing. 8-10 μm thick slices were sectioned using a cryostat (Leica CM 1950) and stored at −80°C until staining. Upon staining, slides were fixed with ice cold methanol for 20 minutes, washed 3 times with washing buffer (PBS, 1% BSA, 0.1% tween), permeabilized with permeabilization buffer (PBS, 1% BSA, 0.25% Triton X-100) for 10 minutes, washed three times with washing buffer and blocked with blocking buffer (PBS, 1% BSA, 0.1% tween, 5% goat serum) for one hour. Staining with primary antibodies (F4/80 conjugated to Alexa Fluor® 647 (BM8, BioLegend,1:100) and CD9 conjugated to Biotin (EM-04, GeneTex,1:150) was done in blocking buffer and incubated overnight at 4°C. Next, slides were washed three times with PBS, incubated with streptavidin conjugated to Cy3 (1:200) in PBS for 1.5 hours at room temperature and washed 3 times with PBS. Slides were mounted in mounting buffer (Shandon immu-Mount, Thermo Scientific) supplemented with with DAPI (1:1000) and stored in 4°C until imaging. Imaging was performed using Zeiss LSM780 upright confocal microscope (Carl Zeiss, Jena, Germany) equipped with X60 NA 1.0 objective lens. Fluorescent proteins were excited sequentially with single-photon lasers (405nm,488nm, 561nm and 633nm). Slide was also scanned using PANORAMIC MIDI II (3DHISTECH Ltd.) for gross morphology imaging.
Necrostatin-1 treatment
Necrostatin-1 (Sigma-Aldrich, Israel) was dissolved in 90% PBS and 10% DMSO to a concentration of 0.5 mg/ml. Mice were administered with 100 μg by i.v. injection one hour prior to infection.
Cd169 macrophages and monocyte depletion
DTX (Cayman chemical) was dissolved in PBS to a concentration of 2 mg/ml. Mice expressing DTX receptor under Cd169 promotor (Cd169-DTR) were i.v. injected with 200n g of DTX, twenty-four hours prior to infection. Classical monocytes were depleted systematically using αCCR2 antibody (clone MC21, (Mack et al., 2001; Mildner et al., 2007)) generously provided by Prof. Steffen Jung. Intraperitoneal injection of 200 μL containing 280 μg of antibody was administered twenty-four hours prior to infection. Both classical monocytes and CD169 macrophages depletion were verified by FACS analysis for at least 48 hours after administration.
Mice survival assay
Mice were injected i.v. with 1000 CFU of S.Tm or PBS (control). Mice weight and overall wellbeing were monitored twice a day. Once the mice lost > 10% of their initial weight, subcutaneous injection of saline was applied daily (0.1 ml/g) to avoid dehydration for the duration of the experiment. Mice were sacrificed upon reaching 20% of body weight loss.
MS4A3creROSATdT chimera generation
For generation of BM chimeras, wild-type C57BL/6 J mice (Envigo) were used as recipients and MS4A3creROSATdT were used as donors. Recipient mice were lethally irradiated with a single dose of 950 cGy using an XRAD 320 machine (Precision X-Ray (PXI)) and reconstituted the next day by i.v. injection of 5Χ106 donor bone marrow cells per mouse.
Isolation of monocytes for adoptive transfers
Bone marrow cells of MS4A3creROSATdT chimera were harvested from the femora, tibiae, coxal and humerus; mononuclear cells were enriched by Ficoll density gradient and filtered through 70 μM wire mesh. Monocytes were isolated by MACS enrichment using biotinylated anti-CD115 antibody and streptavidin coupled magnetic beads (Miltenyi Biotec). Isolated monocytes were suspended to 15Χ106 monocytes/ml in sterile PBS and 100 μl injected i.v. into wild-type C57BL/6J mice (Envigo).
Flow cytometry and sorting for RNA sequencing
Cell acquisition was done on either BD FACSAria III (BD Biosciences) or by Cytec Aurora (Cytek Biosciences). Cell sorting was performed using a BD FACSAria III (BD Biosciences), using gating of live/dead (Helix NP Blue, Biolegend) and doublet cells. Single cells were sorted into 384 wells plate containing 2 μL of solution containing barcoded poly T reverse transcription primers as described (Jaitin et al., 2014). For bulk cell capture, 1000 cells from each population was sorted into tubes containing 50 μL lysis buffer (0.1% Triton-100, 0.5U/μl RNasin+ (promega). Immediately after sorting, plates were spun down, flash frozen in a mixture of dry ice and ethanol and stored in −80°C until processing. Flow cytometry data was analyzed using the FlowJo Software.
Single cell CFU
Infected cells (GFP+) single cells were sorted into a 96-well plate containing 10 μL DDW with 0.2% Triton X-100 (Sigma-Aldrich, Israel) to release intracellular bacteria. Immediately after sorting, plates were spun down, and each well was plated on LB agar plate with the appropriate antibiotics as a single drop. The plates were incubated for 12 hours and the CFU per drop were counted manually.
Single cell RNA-seq library preparation
Single cell libraries were prepared as described (Jaitin et al., 2014). Briefly, mRNA from cells was converted to cDNA alongside barcoding and UMI addition. The cDNA of each plate was pooled followed by second DNA strand synthesis and T7 in vitro transcription. Amplified RNA was fragmented, followed by ligation of partial P5 Illumina sequence and converted to cDNA. Full sequences of P5 and barcoded P7 were added by PCR for a sequence ready library.
Bulk RNA-seq library preparation
RNA was extracted and cleaned using PCR purification beads (Ampure, Beckman Coulter). Beads were resuspended in 1.2 μL of primer mix (4 ng/μl Cel-seq primers, 1.66 nM dNTPs). The mRNA was processed as described in (Hashimshony et al., 2016)
Bioinformatic analysis
Sequencing and pre-processing
Single cell RNA-seq libraries were sequenced using an Illumina NextSeq 500 to a sequencing depth of ∼50,000 reads per cell or a minimum sequencing depth of ∼1 million reads per bulk sample. Single cell RNA-seq libraries sequencing reads were filtered, demultiplexed and mapped to the mouse genome (mm9) as described (Jaitin et al., 2014).
Bulk samples were sequenced to a depth of at least 1M reads per sample. The reads were filtered, demultiplexed and mapped to the mouse genome (mm9) as described (Hashimshony et al., 2016).
Saturation evaluation
Saturation was evaluated by random deduction of number of sequencing reads to 4, 20, 40, 100, 160 and 200M reads followed by determination of the number of detected transcripts. Data obtained was fitted to logarithmic curve and 100% saturation was determined as 1M reads per cell.
Meta-cell analysis
Single cell data was modeled and analyzed using the MetaCell pipeline (Baran et al., 2019). Genes with poor relevance to cell type and cell function were removed (Mitochondrial, cell cycle and ribosomal genes). Cells with low UMI number (< 200) were also removed. Thresholds were set using transcripts per million of 0.2 and a minimum of 50 UMI in order to select the features genes resulting in ∼586 feature genes. Computation of the balanced MetaCell (MC) similarity graph was used with K = 95. Otherwise all default parameters were used. Different subsets were defined using hierarchal clustering and supervised annotation of each MC.
Projection maps visualization
Forced layout maps were generated using the ggplot2 package in R.
Coordinates of cells and MC were extracted from the mc2d_id variable calculated using the mcell_mc2d_force_knn command from the R package “MetaCell.”
Dotplots
Dotplots were generated using geom_point function from R package ggplot2. Log2 enrichment of the gene expression of each MC compared to all MCs was scaled to mean 0 and unit standard deviation 1 before visualization. Percentage of expressing cells was calculated as the number of cells expressing each gene (> 0) divided by the number of cells in the MC. The following color scale were used: scale_fill_gradient2(low = “blue,” mid = “white,” high = “red4,” midpoint = 0)
k-nearest neighbor classification
K-nearest neighbor (kNN) classification was performed using the kNN function (k = 100) (Venables and Ripley, 2002). Cells obtained from naive mice (training set) and infected mice (test set) were classified to the naive MC by the footprint and feature genes. The classification percentage of cells from each infected cell to the naive MC was calculated.
Bulk RNA-seq data pre-processing and normalization
Data were normalized to a library size factor. Factors were calculated by dividing the total number of reads from each sample to the median of the total number of reads across all samples followed by log 2 transformation of the data and minimal expression threshold was set to 3. Five replicates of the Naive conditions and four replicates of bystander and infected conditions were used. One infected sample was excluded (iNOS infected Macs from mouse 1) due to low coverage (∼200K exonic reads) and therefore three replicated were used for iNOS infected Macs.
Bulk RNA-seq data analysis
To identify genes that are significantly differentially expressed between cell types and/or conditions we performed one way ANOVA. Differential expressed genes (2%FDR) were centered and scaled for mean and standard deviation and plotted using heatmap sorted based on mean expression peak. Principal component analysis was performed on scale and centered significantly expressed genes (2% FDR).
Similarity analysis to previously reported CD9+ macrophages
To calculate the similarity of cell types from other datasets (Jaitin et al., 2019; Ramachandran et al., 2019) to our CD9 Macs and iNOS Macs populations we extracted the marker genes of each cell type, and calculated its mean expression signature in our data. We correlated these signatures to our CD9 and iNOS Macs marker genes (Cd9 and Nos2, respectively). As a reference we correlated the significant genes from ANOVA that are associated with CD9 Macs cells and iNOS Macs cells.
Acknowledgments
We thank Steffen Jung and Lynn Hedrick for their valuable advice. We thank Florent Ginhoux, Masato Tanaka, and Kenji Kohno for providing mice strains. This work was supported by the European Research Council (ERC grant No. 756653), the Israel Science Foundation (grant No. 1890/17), Sagol Weizmann-MIT Bridge Program, Pasteur-Weizmann Delegation, the Merle S. Cahn Foundation, the Estate of Zvia Zeroni, and the Estate of Leah Arbel. R.A. is the Incumbent of Philip Harris and Gerald Ronson career development chair.
Author contributions
D.H. and R.A. designed the study and experiments and wrote the manuscript. D.H., Y.T., S.T., G.R., L.V., A.S., and S.H.-A. performed the experiments. D.H. and N.B.B.-M. performed data analysis.
Declaration of interests
The authors declare no competing interests.
Published: November 16, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.immuni.2021.10.015.
Supplemental information
Data and code availability
RNA-seq data have been deposited at GEO and is publicly available. Accession numbers are listed in the Key Resources Table. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request
References
- Ağaç D., Estrada L.D., Maples R., Hooper L.V., Farrar J.D. The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav. Immun. 2018;74:176–185. doi: 10.1016/j.bbi.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran Y., Bercovich A., Sebe-Pedros A., Lubling Y., Giladi A., Chomsky E., Meir Z., Hoichman M., Lifshitz A., Tanay A. MetaCell: analysis of single-cell RNA-seq data using K-nn graph partitions. Genome Biol. 2019;20:206. doi: 10.1186/s13059-019-1812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Gonzalez A., Vidal R., Criollo A., Carreño L.J. New Insights on the Role of Lipid Metabolism in the Metabolic Reprogramming of Macrophages. Front. Immunol. 2020;10:2993. doi: 10.3389/fimmu.2019.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blériot C., Dupuis T., Jouvion G., Eberl G., Disson O., Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42:145–158. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Borges da Silva H., Fonseca R., Pereira R.M., Cassado A. dos A., Álvarez J.M., D’Império Lima M.R. Splenic Macrophage Subsets and Their Function during Blood-Borne Infections. Front. Immunol. 2015;6:480. doi: 10.3389/fimmu.2015.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L., Gosling J., Chensue S.W., Kunkel S.L., Farese R.V., Jr., Broxmeyer H.E., Charo I.F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl H., Cihak J., Plachý J., Kunz-Schughart L., Niedermeier M., Denzel A., Rodriguez Gomez M., Talke Y., Luckow B., Stangassinger M., Mack M. Targeting of Gr-1+,CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum. 2007;56:2975–2985. doi: 10.1002/art.22854. [DOI] [PubMed] [Google Scholar]
- Carter P.B., Collins F.M. The route of enteric infection in normal mice. J. Exp. Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarov S., Lim H.Y., Tan L., Lim S.Y., See P., Lum J., Zhang X.-M., Foo S., Nakamizo S., Duan K., et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363:eaau0964. doi: 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- Cirillo D.M., Valdivia R.H., Monack D.M., Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat. Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus M., Park C.G., Su Y., Goldman D.L., Steinman R.M., Casadevall A. Spleen deposition of Cryptococcus neoformans capsular glucuronoxylomannan in rodents occurs in red pulp macrophages and not marginal zone macrophages expressing the C-type lectin SIGN-R1. Med. Mycol. 2008;46:153–162. doi: 10.1080/13693780701747182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick S.A., Macklin J.A., Nejat S., Momen A., Clemente-Casares X., Althagafi M.G., Chen J., Kantores C., Hosseinzadeh S., Aronoff L., et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019;20:29–39. doi: 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele N.A., Ruby T., Jacobson A., Manzanillo P.S., Cox J.S., Lam L., Mukundan L., Chawla A., Monack D.M. Salmonella require the fatty acid regulator PPARδ for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe. 2013;14:171–182. doi: 10.1016/j.chom.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercoli G., Fernandes V.E., Chung W.Y., Wanford J.J., Thomson S., Bayliss C.D., Straatman K., Crocker P.R., Dennison A., Martinez-Pomares L., et al. Intracellular replication of Streptococcus pneumoniae inside splenic macrophages serves as a reservoir for septicaemia. Nat. Microbiol. 2018;3:600–610. doi: 10.1038/s41564-018-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B.M., Tontonoz P., Chen J., Brun R.P., Spiegelman B.M., Evans R.M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR γ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Frost A.J., Bland A.P., Wallis T.S. The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet. Pathol. 1997;34:369–386. doi: 10.1177/030098589703400501. [DOI] [PubMed] [Google Scholar]
- Geddes K., Cruz F., Heffron F. Analysis of cells targeted by Salmonella type III secretion in vivo. PLoS Pathog. 2007;3:e196. doi: 10.1371/journal.ppat.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi A., Wagner L.K., Li H., Dörr D., Medaglia C., Paul F., Shemer A., Jung S., Yona S., Mack M., et al. Cxcl10+ monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat. Immunol. 2020;21:525–534. doi: 10.1038/s41590-020-0661-1. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Bleriot C., Lecuit M. Dying for a Cause: Regulated Necrosis of Tissue-Resident Macrophages upon Infection. Trends Immunol. 2017;38:693–695. doi: 10.1016/j.it.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Goldberg M.F., Roeske E.K., Ward L.N., Pengo T., Dileepan T., Kotov D.I., Jenkins M.K. Salmonella Persist in Activated Macrophages in T Cell-Sparse Granulomas but Are Contained by Surrounding CXCR3 Ligand-Positioned Th1 Cells. Immunity. 2018;49:1090–1102.e7. doi: 10.1016/j.immuni.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M., Mildner A., Yona S. Developmental and Functional Heterogeneity of Monocytes. Immunity. 2018;49:595–613. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Hashimshony T., Wagner F., Sher N., Yanai I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2012;2:666–673. doi: 10.1016/j.celrep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Hashimshony T., Senderovich N., Avital G., Klochendler A., de Leeuw Y., Anavy L., Gennert D., Li S., Livak K.J., Rozenblatt-Rosen O., et al. CEL-Seq2: sensitive highly-multiplexed single-cell RNA-Seq. Genome Biol. 2016;17:77. doi: 10.1186/s13059-016-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helaine S., Cheverton A.M., Watson K.G., Faure L.M., Matthews S.A., Holden D.W. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science. 2014;343:204–208. doi: 10.1126/science.1244705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel M., Shea J.E., Waterman S.R., Mundy R., Nikolaus T., Banks G., Vazquez-Torres A., Gleeson C., Fang F.C., Holden D.W. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- Huang L., Nazarova E.V., Tan S., Liu Y., Russell D.G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018;215:1135–1152. doi: 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiani P., Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitin D.A., Kenigsberg E., Keren-Shaul H., Elefant N., Paul F., Zaretsky I., Mildner A., Cohen N., Jung S., Tanay A., Amit I. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343:776–779. doi: 10.1126/science.1247651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitin D.A., Adlung L., Thaiss C.A., Weiner A., Li B., Descamps H., Lundgren P., Bleriot C., Liu Z., Deczkowska A., et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell. 2019;178:686–698.e14. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H., Kenigsberg E., Jaitin D.A., David E., Paul F., Tanay A., Amit I. MARS-seq2.0: an experimental and analytical pipeline for indexed sorting combined with single-cell RNA sequencing. Nat. Protoc. 2019;14:1841–1862. doi: 10.1038/s41596-019-0164-4. [DOI] [PubMed] [Google Scholar]
- Kirby A.C., Beattie L., Maroof A., van Rooijen N., Kaye P.M. SIGNR1-negative red pulp macrophages protect against acute streptococcal sepsis after Leishmania donovani-induced loss of marginal zone macrophages. Am. J. Pathol. 2009;175:1107–1115. doi: 10.2353/ajpath.2009.090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter J., Feuerstein R., Zeis P., Hagemeyer N., Paterson N., d’Errico P., Baasch S., Amann L., Masuda T., Lösslein A., et al. A Subset of Skin Macrophages Contributes to the Surveillance and Regeneration of Local Nerves. Immunity. 2019;50:1482–1497. doi: 10.1016/j.immuni.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Liu Z., Gu Y., Chakarov S., Bleriot C., Kwok I., Chen X., Shin A., Huang W., Dress R.J., Dutertre C.-A., et al. Fate Mapping via Ms4a3-Expression History Traces Monocyte-Derived Cells. Cell. 2019;178:1509–1525.e19. doi: 10.1016/j.cell.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Mack M., Cihak J., Simonis C., Luckow B., Proudfoot A.E., Plachý J., Brühl H., Frink M., Anders H.J., Vielhauer V., et al. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- McLaughlin P.A., Bettke J.A., Tam J.W., Leeds J., Bliska J.B., Butler B.P., van der Velden A.W.M. Inflammatory monocytes provide a niche for Salmonella expansion in the lumen of the inflamed intestine. PLoS Pathog. 2019;15:e1007847. doi: 10.1371/journal.ppat.1007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes S., Melandri D., Anselmi G., Perchet T., Loschko J., Dubrot J., Patel R., Gautier E.L., Hugues S., Longhi M.P., et al. The Heterogeneity of Ly6Chi Monocytes Controls Their Differentiation into iNOS+ Macrophages or Monocyte-Derived Dendritic Cells. Immunity. 2016;45:1205–1218. doi: 10.1016/j.immuni.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A., Schmidt H., Nitsche M., Merkler D., Hanisch U.-K., Mack M., Heikenwalder M., Brück W., Priller J., Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Mills E., Avraham R. Breaking the population barrier by single cell analysis: one host against one pathogen. Curr. Opin. Microbiol. 2017;36:69–75. doi: 10.1016/j.mib.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Miyake Y., Asano K., Kaise H., Uemura M., Nakayama M., Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J. Clin. Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L., Tontonoz P., Alvarez J.G.A., Chen H., Evans R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- Narasimhan P.B., Marcovecchio P., Hamers A.A.J., Hedrick C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019;37:439–456. doi: 10.1146/annurev-immunol-042617-053119. [DOI] [PubMed] [Google Scholar]
- Olingy C.E., San Emeterio C.L., Ogle M.E., Krieger J.R., Bruce A.C., Pfau D.D., Jordan B.T., Peirce S.M., Botchwey E.A. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci. Rep. 2017;7:447. doi: 10.1038/s41598-017-00477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.H.M., Brewer S.M., Thurston T., Massis L.M., Honeycutt J., Lugo K., Jacobson A.R., Vilches-Moure J.G., Hamblin M., Helaine S., Monack D.M. Salmonella-Driven Polarization of Granuloma Macrophages Antagonizes TNF-Mediated Pathogen Restriction during Persistent Infection. Cell Host Microbe. 2020;27:54–67.e5. doi: 10.1016/j.chom.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.V., Vance R.E. The macrophage paradox. Immunity. 2014;41:685–693. doi: 10.1016/j.immuni.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Ramachandran P., Dobie R., Wilson-Kanamori J.R., Dora E.F., Henderson B.E.P., Luu N.T., Portman J.R., Matchett K.P., Brice M., Marwick J.A., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N., McComb S., Mulligan R., Dudani R., Krishnan L., Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat. Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo S.P., Noursadeghi M., Cohen J., Holden D.W. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell. Microbiol. 2001;3:587–597. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- Saliba A.-E., Li L., Westermann A.J., Appenzeller S., Stapels D.A.C., Schulte L.N., Helaine S., Vogel J. Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat. Microbiol. 2016;2:16206. doi: 10.1038/nmicrobiol.2016.206. [DOI] [PubMed] [Google Scholar]
- Schleier L., Wiendl M., Heidbreder K., Binder M.-T., Atreya R., Rath T., Becker E., Schulz-Kuhnt A., Stahl A., Schulze L.L., et al. Non-classical monocyte homing to the gut via α4β7 integrin mediates macrophage-dependent intestinal wound healing. Gut. 2020;69:252–263. doi: 10.1136/gutjnl-2018-316772. [DOI] [PubMed] [Google Scholar]
- Schyns J., Bai Q., Ruscitti C., Radermecker C., De Schepper S., Chakarov S., Farnir F., Pirottin D., Ginhoux F., Boeckxstaens G., et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat. Commun. 2019;10:3964. doi: 10.1038/s41467-019-11843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina N.V., Salazar-Mather T.P., Biron C.A., Kuziel W.A., Pamer E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Serbina N.V., Jia T., Hohl T.M., Pamer E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F.K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.-L., Kohler R.H., Chudnovskiy A., Waterman P., et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 1967;50:109–136. [PMC free article] [PubMed] [Google Scholar]
- Tam J.W., Kullas A.L., Mena P., Bliska J.B., van der Velden A.W.M. CD11b+ Ly6Chi Ly6G- immature myeloid cells recruited in response to Salmonella enterica serovar Typhimurium infection exhibit protective and immunosuppressive properties. Infect. Immun. 2014;82:2606–2614. doi: 10.1128/IAI.01590-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G.D., Hanna R.N., Vasudevan N.T., Hamers A.A., Romanoski C.E., McArdle S., Ross K.D., Blatchley A., Yoakum D., Hamilton B.A., et al. Deleting an Nr4a1 Super-Enhancer Subdomain Ablates Ly6Clow Monocytes while Preserving Macrophage Gene Function. Immunity. 2016;45:975–987. doi: 10.1016/j.immuni.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzebanski S., Jung S. Plasticity of monocyte development and monocyte fates. Immunol. Lett. 2020;227:66–78. doi: 10.1016/j.imlet.2020.07.007. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A., Jones-Carson J., Bäumler A.J., Falkow S., Valdivia R., Brown W., Le M., Berggren R., Parks W.T., Fang F.C. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D. Springer-Verlag; 2002. Modern Applied Statistics with S. [Google Scholar]
- Vrieling F., Wilson L., Rensen P.C.N., Walzl G., Ottenhoff T.H.M., Joosten S.A. Oxidized low-density lipoprotein (oxLDL) supports Mycobacterium tuberculosis survival in macrophages by inducing lysosomal dysfunction. PLoS Pathog. 2019;15:e1007724. doi: 10.1371/journal.ppat.1007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S., Kim K.-W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data have been deposited at GEO and is publicly available. Accession numbers are listed in the Key Resources Table. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request