Abstract
BACKGROUND:
This study aimed to understand the prevalence of prediabetes and diabetes in patients with cancer overall, by tumor site, by cancer treatment, and by time point in the cancer continuum.
METHODS:
This cohort study has been conducted at the Huntsman Cancer Institute, Salt Lake City, Utah. Patients with a first primary invasive cancer enrolled in the Total Cancer Care protocol between July 2016 and July 2018 were eligible for this analysis. The prevalence of prediabetes and diabetes is based on ICD code, laboratory tests for HbA1c (%), fasting plasma glucose (mg/dl), non-fasting blood glucose (mg/dl), or insulin prescription.
RESULTS:
The final cohort comprised 3,512 patients with cancer, with a mean age of 57.8 years at cancer diagnosis. Of all patients, 49.1% (n=1,724) were female. At cancer diagnosis, the prevalence of prediabetes was 6.0% (95% CI: 5.3–6.8) and of diabetes 12.2% (95% CI: 11.2–13.3). One year after diagnosis the prevalences were 16.6% for prediabetes (95% CI: 15.4–17.9) and 25.0% for diabetes (95% CI: 23.6–26.4). At the end of the observation period, the prevalence of prediabetes was 21.2% (95% CI: 20.4–23.0) and of diabetes was 32.6% (95% CI: 29.2–32.1). Patients with myeloma (39.2%; 95% CI: 32.6–46.2) had the highest prevalence of prediabetes and patients with pancreatic cancer the highest prevalence of diabetes (65.1%; 95% CI: 57.0–72.3). In patients who underwent chemotherapy (prediabetes: 29.1% vs 15.6%; diabetes: 37.6% vs 29.0%), radiation therapy (prediabetes: 24.3% vs. 19.9%; diabetes: 33.7% vs 32.1%), or immunotherapy (diabetes: 29.2% vs 20.4; prediabetes: 36.0% vs 32.2%), the prevalence of prediabetes and diabetes was higher compared to patients not undergoing those therapies.
CONCLUSIONS:
Every second patient with cancer suffers from prediabetes or diabetes. It is essential to foster interprofessional collaboration and to develop evidence-based practice guidelines. A better understanding of the impact of cancer treatment on the development of prediabetes and diabetes remains critical.
Background
Cancer and diabetes are challenging health systems worldwide. In the United States (US), it is estimated that 5% of the population (16.9 million people) have cancer.1 At the same time, 9.4% of the US population (30.3 million people) suffer from diabetes mellitus (DM). Additionally, 33.9% of American adults (84.1 million people) are living with prediabetes (preDM), raising their chances of developing DM.2 Overall, cancer and diabetes are often diagnosed simultaneously, independent of age, suggesting a possible common underlying mechanism.3
The co-occurrence of cancer and DM raises significant health challenges. In patients with cancer, DM is associated with higher rates of complications,4,5 a higher risk for hospitalization,6,7 increased mortality,8–10 increased psychological distress,11 and decreased Health Related Quality of Life (HRQoL).12–14 In fact, DM is a common cause of non-cancer mortality in patients with cancer.15
Research on the relationship between cancer and DM has long been focused on DM and its role as a risk factor in the development of several different types of cancer.16–19 However, a recent study by Hwangbo et al. has suggested that this relationship may be bi-directional as the diagnosis of diabetes not uncommonly occurs after cancer diagnoses.20 Still, the entire extent of the problem is not well understood. Just a few studies address the prevalence of DM in patients with cancer world-wide21–23, or the US.24–27 Evidence for the prevalence of preDM in patients with cancer remains scarce.21
Moreover, most studies addressing the prevalence of preDM or DM in patients with cancer are either retrospective analyses which did not include clinical data (e.g., HbA1c, glucose measures)24,26 or clinical studies with a small number of patients and a focus on one specific cancer. For both types of studies, the lack of cancer specifics (e.g., first primary cancer), timing of diabetes onset, and/or treatment characteristics limits the comparability and clinical utility of their results.
The aim of this study is to examine the prevalence of preDM and DM in patients with cancer, by utilizing a large, prospective cancer cohort. We incorporated clinical data to determine the prevalence of preDM and DM in patients with cancer overall, by tumor site, by cancer treatment, and by time point in the cancer continuum.
Methods
Design and population
This cohort study utilized the Total Cancer Care (TCC) protocol at the University of Utah (UofU) Huntsman Cancer Institute (HCI). TCC is the primary biobanking research protocol which serves as a large centralized clinical data and tissue repository. Participants enrolled under this protocol agree to be followed throughout their lifetime and release medical data for research, including cancer occurring before enrollment. The study population at the time of this project includes 5,865 individuals, men and women, who enrolled in TCC between July 1, 2016 and July 31, 2018. Participants were recruited at local sites in Salt Lake City, Utah, including the HCI as well as UofU hospitals and clinics. All patients provided informed consent. The HCI-TCC protocol was approved by the University of Utah (UofU) Institutional Review Board (IRB #89989).
Cancer characteristics
All patients included in this analysis have an ICD-O (International Classification of Diseases for Oncology) diagnosis.28 The determination of the tumor behavior is based on ICD-O morphology codes. Only patients with a tumor behavior code “3” (malignant, primary site) were included in this analysis. The determination of whether a tumor was the first tumor was based on the sequence number, coded in the cancer registry. In this analysis, we included patients with the sequence number “0” (first and only tumor) and “1” (first of more than one tumor). The onset of cancer was defined as the date of the first ICD-O diagnosis documented in the Huntsman Cancer Registry (HCR), even if this date is prior to TCC enrollment. The definition of tumor sites was based on the SEER Site Recode of ICD-O codes (ICD-O-3/WHO 2008 Definition).55
Determination of prediabetes and diabetes
The criteria for the determination of preDM and DM (Table 1) were based on the guidelines of the American Diabetes Association (ADA).29 The onset of preDM/DM was defined as the date of the first qualifying ICD code (preDM: ICD-9 790.xx, ICD-10 R73.xx; DM: ICD-9 250.xx, ICD-10 E8-E13), and/or laboratory results (preDM: HbA1c 5.7%−6.4%, fasting plasma glucose (FPG) 100–125 mg/dl, blood glucose (BG) 140–199 mg/dl; DM; HbA1c >6.4%, FPG >125 mg/dl, BG >199), and/or prescription for insulin. If the determination of DM or preDM was based solely on a laboratory result, a second test in the respective range (preDM or DM), at least three days after, was required to confirm the diagnosis.
Table 1:
Diagnostic indicators for prediabetes and diabetes
| Indicator | Prediabetes | Diabetes |
|---|---|---|
| ICD 9 | 790.xx | 250.XX |
| ICD 10 | R73.xx | E8 to E13 |
| HbA1c (%)* | 5.7–6.4 | ≥ 6.5 |
| Fasting plasma glucose (mg/dl)* | 100–125 | ≥ 126 |
| Blood glucose (mg/dl)* | 140–199 | ≥ 200 |
| Prescriptions | - | Insulin |
To confirm diagnosis, 2+ laboratory test results more than two days apart were required if no other indication of diagnosis was given (ICD code, insulin prescription)
Data collection
Patients in this study were identified through the TCC Cancer Clinical Research (CCR) database. The CCR contains study information and is linked with related research- and clinical information systems. For this analysis we included data form the Huntsman Cancer Registry and the University of Utah Health Enterprise Data Warehouse. Data from both sources (eTable 1) were available for the time between January 2000 to July 2019 (for patients recruited between July 2016 and July 2018), allowing at least one year of follow-up for each patient.
Exclusion criteria
Participants were excluded from analysis if they did not have a documented ICD-O diagnosis in the HCR, were not treated in the UofU Health system (HCR ‘class of case’ numbers: 30, 31, 33, 35, 37, 38, 40–43, 49, 99), were not diagnosed between January 2000 and December 2018, were not between 18 and 90 years old at cancer diagnosis or the cancer was not primary or not invasive (Figure 1).
Figure 1:
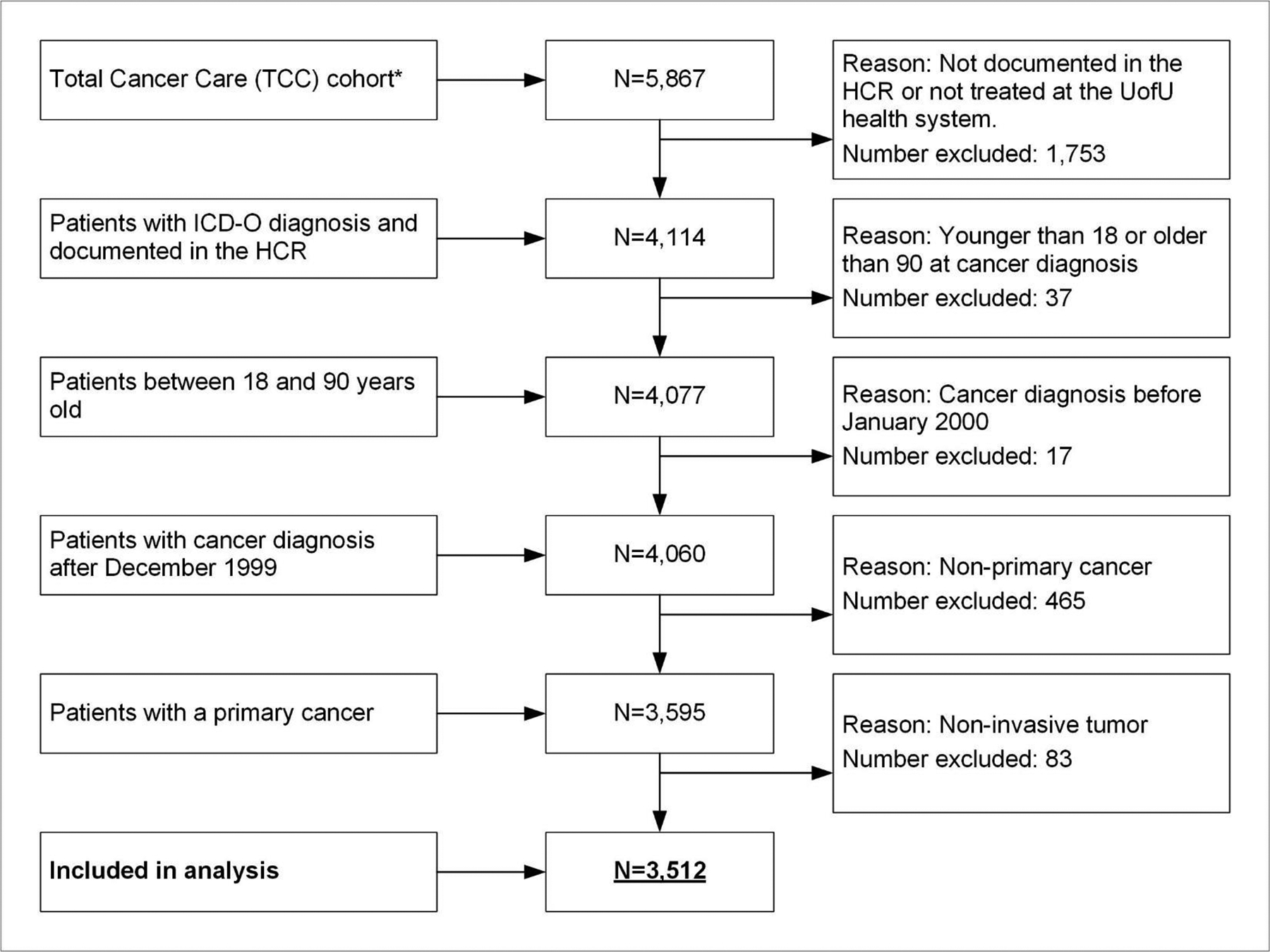
Flowchart with exclusion criteria
* Participants in the TCC cohort as of July 31, 2018
Statistical analysis
Clinicodemographic characteristics were examined by diabetes status (preDM, DM, and neither). Total counts and percentages among the diabetes status groups are displayed for categorical variables as well as means and standard deviations (SD) for quantitative variables. Differences in clinicodemographics by diabetes status were examined by chi-squared tests for categorical variables and one-way ANOVA for continuous variables. Pairwise comparisons for characteristics by diabetes status (pre-DM vs. neither, DM vs. neither, DM vs. pre-DM) were examined by chi-squared tests for categorical variables and t-tests for continuous variables. The p-values from these pairwise comparisons were adjusted for multiplicity using Hommel’s multiple comparison procedure. All statistical tests were two-sided, and p-values< 0.05 considered statistically significant.
The prevalence of preDM and DM with 95% confidence intervals is presented by clinicodemographics, tumor site, therapy (surgery, chemotherapy, radiation therapy, hormone therapy, immunotherapy, glucocorticoids), diagnostic indicator (ICD-code, laboratory test results, insulin prescription), as well as by the time point within the cancer continuum (at or before cancer diagnosis, after cancer diagnosis). A patient was considered as having DM or pre-DM at or before cancer diagnosis if they met DM or pre-DM criteria up to two weeks after cancer diagnosis. Complementarily, the time point ‘after cancer diagnosis’ started two weeks after cancer diagnosis and was subdivided in ‘One month after’ (up to 30 days), ‘Six month after’ (up to 182 days), ‘One year after’ (up to 365 days) and ‘End of the observation period’ (>365 days).
The length of follow-up was defined as the number of days between cancer diagnosis and the last documented date from data sources in the EDW (e.g. encounter, ICD-code, laboratory test). The median follow-up in months is provided. 95% CIs are constructed by the Wilson score confidence interval for binomial proportions.30 Analyses are conducted in R (R Foundation for Statistical Computation, Vienna, Austria), version 3.5.3.
Results
Description of the cohort
The HCI-TCC©-DM cohort (Figure 1) comprises 3,512 patients with cancer (median follow-up 28 months), with a mean age of 57.8 years at cancer diagnosis. Of all patients, 49.1% (n=1,724) were female, 90.5% (n=3,177) were non-Hispanic white, 60.4% (n=2,122) were overweight or obese, and 70.2% (n=2,464) were Utah residents (Table 2).
Table 2:
Clinicodemographic characteristics by diabetes status among patients with cancer (n=3,512)
| Totala | Prediabetes | Diabetes | Neither | p-value | |
|---|---|---|---|---|---|
| Study Population n (%) | 3,512 (100) | 745 (21.2) | 1,145 (32.6) | 1,622 (46.2) | |
| Age (years) mean (SD)b | 57.8 (14.0) | 57.4 (14.1) | 60.4 (13.1) | 56.2 (14.2) | <0.001 |
| Age categorized n (%)b | <0.001 | ||||
| <50 years | 854 (24.3) | 194 (26.0) | 206 (18.0) | 454 (28.0) | |
| 50 to 64 years | 1,440 (41.0) | 293 (39.3) | 463 (40.4) | 684 (42.2) | |
| 65 to 79 years | 1,091 (31.1) | 238 (31.9) | 423 (36.9) | 430 (26.5) | |
| 80+ years | 127 (3.6) | 20 ( 2.7) | 53 ( 4.6) | 54 ( 3.3) | |
| Sex n (%) | 0.02 | ||||
| Female | 1,724 (49.1) | 360 (48.3) | 529 (46.2) | 835 (51.5) | |
| Male | 1,788 (50.9) | 385 (51.7) | 616 (53.8) | 787 (48.5) | |
| Race and Ethnicity n (%) | <0.001 | ||||
| Non-Hispanic White | 3,177 (90.5) | 678 (91.0) | 1,006 (87.9) | 1,493 (92.0) | |
| Non-Hispanic Black | 18 (0.5) | 10 (1.3) | 2 ( 0.2) | 6 ( 0.4) | |
| Non-Hispanic Asian | 38 (1.1) | 7 ( 0.9) | 15 (1.3) | 16 (1.0) | |
| Non-Hispanic Otherc | 142 (4.0) | 26 (3.5) | 54 (4.7) | 62 (3.8) | |
| Hispanic | 137 (3.9) | 24 (3.2) | 68 (5.9) | 45 (2.8) | |
| BMI kg/m2 mean (SD)d | 29.1 (6.6) | 28.1 (5.8) | 31.3 (7.7) | 28.0 (5.8) | <0.001 |
| BMI kg/m2 category n (%)d | <0.001 | ||||
| Underweight (<18.5) | 33 (0.9) | 7 (0.9) | 9 (0.8) | 17 (1.0) | |
| Normal (18.5 – 24.99) | 814 (23.2) | 198 (26.6) | 185 (16.2) | 431 (26.6) | |
| Overweight (25.0 – 29.99) | 1,004 (28.6) | 222 (29.8) | 281 (24.5) | 501 (30.9) | |
| Obese (>=30) | 1,118 (31.8) | 210 (28.2) | 482 (42.1) | 426 (26.3) | |
| Unknown | 543 (15.5) | 108 (14.5) | 188 (16.4) | 247 (15.2) | |
| Population n (%)e | 0.31 | ||||
| Rural | 1,125 (32) | 223 (29.9) | 362 (31.6) | 540 (33.3) | |
| Non-Rural | 2,357 (67.1) | 515 (69.1) | 770 (67.2) | 1,072 (66.1) | |
| Unknown | 30 (0.9) | 7 (0.9) | 13 (1.1) | 10 (0.6) | |
| State of Residence n (%)f | <0.001 | ||||
| Utah | 2,464 (70.2) | 557 (74.8) | 818 (71.4) | 1,089 (67.1) | |
| Idaho | 425 (12.1) | 65 (8.7) | 146 (12.8) | 214 (13.2) | |
| Wyoming | 274 (7.8) | 45 (6.0) | 86 (7.5) | 143 (8.8) | |
| Nevada | 168 (4.8) | 39 (5.2) | 54 (4.7) | 75 (4.6) | |
| Other | 181 (5.2) | 39 (5.2) | 41 (3.6) | 101 (6.2) | |
| Cancer Stage n (%) | <0.001 | ||||
| Stage 0-I | 872 (24.8) | 137 (18.4) | 226 (19.7) | 509 (31.4) | |
| Stage II | 638 (18.2) | 107 (14.4) | 208 (18.2) | 323 (19.9) | |
| Stage III | 553 (15.7) | 94 (12.6) | 167 (14.6) | 292 (18.0) | |
| Stage IV | 543 (15.5) | 153 (20.5) | 199 (17.4) | 191 (11.8) | |
| Unknown/ Not Applicableg | 906 (25.8) | 254 (34.1) | 345 (30.1) | 307 (18.9) | |
| Cancer treatment n (%) | |||||
| Surgery | 2,957 (84.2) | 594 (79.7) | 945 (82.5) | 1,418 (87.4) | <0.001 |
| Chemotherapy | 1,458 (41.5) | 425 (57.0) | 548 (48.0) | 485 (29.9) | <0.001 |
| Radiation | 1,035 (29.5) | 251 (33.7) | 349 (30.5) | 435 (26.8) | 0.002 |
| Hormone therapy | 731 (20.8) | 169 (22.7) | 204 (17.8) | 358 (22.1) | 0.01 |
| Immunotherapy | 339 (9.7) | 99 (13.3) | 122 (10.7) | 118 (7.3) | <0.001 |
| Glucocorticoids | 2,938 (83.7) | 656 (88.1) | 972 (84.9) | 1,310 (80.8) 1.5) | <0.001 |
| Cancer Sequence Number n (%) | <0.001 | ||||
| 00 – only one primary cancer | 3,084 (87.8) | 632 (84.8) | 977 (85.3) | 1,475 (90.9) | |
| 01 – multiple primary cancer | 428 (12.2) | 113 (15.2) | 168 (14.7) | 147 (9.1) |
Not all %s add up to 100 because of rounding decimal places;
At cancer diagnosis;
American Indian/Alaska Native, Hawaiian/Other Pacific Islander, Other, or Unknown;
Body Mass Index, at cancer diagnosis (90 days window before and after cancer diagnosis);
Determined from RUCA score on zip code;
Determined from last known residence;
Brain and nervous system cancers are not routinely staged; n=number; SD=standard deviation; BMI=body mass index
Cancers of the breast (13.8%; n=484) and prostate (14.1%; n=494) were the most common in this cohort (Table 3). Regarding cancer severity and treatment, 31.2% (n=1,096) of all patients were stage III/IV, 84.2% (n=2,957) had undergone surgery and 41.5% (n=1,458) had undergone chemotherapy. 83.7% (n=2,938) of patients were treated with glucocorticoids (Table 2).
Table 3:
Prevalence of prediabetes and diabetes among patients with cancer by diabetes indicator, time frame and treatment (n=3,512)
| Total | Prediabetes | Diabetes | |||
|---|---|---|---|---|---|
| No | No | % (95% CI) | No | % (95% CI) | |
| Study Population | 3,512 | 745 | 21.2 (19.9–22.6) | 1,145 | 32.6 (31.1–34.2) |
| By time point c | 3,512 | ||||
| At cancer diagnosisd | 211 | 6.0 (5.3–6.8) | 429 | 12.2 (11.2–13.3) | |
| After cancer diagnosis | |||||
| One month after | 306 | 8.7 (7.8–9.7) | 590 | 16.8 (15.6–18.1) | |
| Six months after | 536 | 15.3 (14.1–16.5) | 824 | 23.5 (22.1–24.9) | |
| One year after | 584 | 16.6 (15.4–17.9) | 877 | 25.0 (23.6–26.4) | |
| End of OP (>365 days) | 745 | 21.2 (19.9–22.6) | 1,145 | 32.6 (31.1–34.2) | |
| By diagnostic indicator a,e | 3,512 | ||||
| Based on ICD codes | 147 | 4.2 (3.6–4.9) | 742 | 21.1 (19.8–22.5) | |
| DM Type 1 | - | - | 56 | 1.6 (1.2–2.1) | |
| DM Type 2 | - | - | 700 | 19.9 (18.6–21.3) | |
| DM secondary | - | - | 36 | 1.0 (0.7–1.4) | |
| DM other | - | - | 232 | 6.6 (5.8–7.5) | |
| Based on lab valuesb | 725 | 20.6 (19.3–22.0) | 781 | 22.2 (20.9–23.6) | |
| HbA1c (%) | 184 | 5.2 (4.5–6.0) | 314 | 8.9 (8.0–9.9) | |
| Fasting plasma glucose (mg/dl) | 25 | 0.7 (0.5–0.1.0) | 42 | 1.2 (0.9–1.6) | |
| Blood glucose (mg/dl) | 745 | 21.2 (19.9–22.6) | 732 | 20.8 (19.5–22.2) | |
| Based on insulin prescription | - | - | 768 | 21.9 (20.5–23.3) | |
| By cancer treatment e | |||||
| Chemotherapy | |||||
| Yes | 1,458 | 425 | 29.1 (26.9–31.5) | 548 | 37.6 (35.1–40.1) |
| No | 2,050 | 320 | 15.6 (14.1–17.2) | 594 | 29.0 (27.1–31.0) |
| Surgery | |||||
| Yes | 2,957 | 594 | 20.1 (18.7–21.6) | 945 | 32.0 (30.3–33.7) |
| No | 555 | 151 | 27.2 (23.7–31.1) | 200 | 36.0 (32.2–40.1) |
| Radiation | |||||
| Yes | 1,035 | 251 | 24.3 (21.7–27.0) | 349 | 33.7 (30.9–36.7) |
| No | 2,477 | 494 | 19.9 (18.4–21.6) | 796 | 32.1 (30.3–34.0) |
| Hormone therapy | |||||
| Yes | 731 | 169 | 23.1 (20.2–26.3) | 204 | 27.9 (24.8–31.3) |
| No | 2,777 | 576 | 20.7 (19.3–22.3) | 939 | 33.8 (32.1–35.6) |
| Immunotherapy | |||||
| Yes | 339 | 99 | 29.2 (24.6–34.3) | 122 | 36.0 (31.1–41.2) |
| No | 3,172 | 646 | 20.4 (19.0–21.8) | 1,022 | 32.2 (30.6–33.9) |
| Glucocorticoids | |||||
| Yes | 2,938 | 656 | 22.3 (20.9–23.9) | 972 | 33.1 (31.4–34.8) |
| No | 574 | 89 | 15.5 (12.8–18.7) | 173 | 30.1 (26.5–34.0) |
OP=Observation period;
Numbers are not mutually exclusive. For example, a patient can have an ICD code for DM and a HbA1c in the DM range;
preDM and DM diagnoses based solely on lab values were valid only if a patient had at least 2 lab values greater than 2 days apart to more fully establish robust diagnosis;
Sum of patients with preDM by ‘time frame’ is 1,343. This number reflects the number of all patients who had pre-DM over time (‘Lifetime’ prevalence’). Because 598 patients transitioned to DM, at end of the observation period 745 patients have preDM;
Prevalence as of two weeks after cancer diagnosis including all cases before cancer diagnosis;
At the end of the observation period
Compared to patients with neither preDM nor DM, patients with preDM or DM were older (preDM: P=0.04; DM: P<0.001), more often underwent chemotherapy (PreDM/DM: P<0.001), or immune-therapy (PreDM/DM: P<0.001), were more often treated with glucocorticoids (preDM: P<0.001; DM: P=0.01) and less frequently underwent surgery (PreDM/DM: P<0.001). Compared to patients with neither preDM nor DM, patients with DM were more often male (P=0.02) and more often Hispanic (DM: P=0.001); patients with preDM more frequently received radiation therapy (P<0.01) (Table 2; eTable 2).
Prevalence by clinicodemographics
Compared to female patients (30.7%; 95% CI: 28.6–32.9), the prevalence of DM was higher in male patients (34.5%; 95% CI: 32.3–36.7). Across BMI categories, the prevalence of DM for patients with normal weight was 22.7% (95% CI: 20.0–25.7), the prevalence of diabetes for patients who were overweight was 28.0% (95% CI: 25.3–30.8) and the prevalence of diabetes for obese patients was 43.1% (95% CI: 40.2–46.0) (eTable 3).
Prevalence by time point
At cancer diagnosis, the prevalence of preDM was 6.0% (95% CI: 5.3–6.8) and of DM was 12.2% (95% CI: 11.2–13.3). One year after diagnosis, the prevalence of preDM was 16.6% (95% CI: 15.4–17.9) and of diabetes was 25.0% (95% CI: 23.6–26.4). At the end of the observation period, the prevalence of prediabetes was 21.2% (95% CI: 19.9–22.6) and of diabetes was 32.6% (95% CI: 31.1–34.2) (Table 3).
Prevalence by diagnostic indicator
Solely based on ICD codes, the prevalence of preDM was 4.2% (95% CI: 3.6–4.9) and of DM 21.1% (95% CI: 19.8–22.5) at the end of the observation period. Based lab values, the prevalence of preDM was 20.6% (95% CI: 19.3–22.0) and the prevalence of DM was 22.2% (95% CI: 20.9–23.6). With respect to insulin prescription, we observed a prevalence of 21.9% (95% CI: 20.5–23.3) for DM (Table 3).
Prevalence by cancer treatment
Patients undergoing chemotherapy had a higher prevalence of preDM (29.1% vs 15.6%) and DM (37.6% vs 29.0%) compared to patients who did not receive chemotherapy. The same was true in patients undergoing radiation therapy for preDM (24.3% vs 19.9%) and DM (33.7% vs 32.1%), patients undergoing immunotherapy for preDM (29.2% vs 20.4%) and DM (36.0% vs 32.2%), as well as patients receiving glucocorticoids for preDM (22.3% vs 15.5%) and DM (33.1% vs 30.1%).
Prevalence by tumor site
There were vast differences in the prevalence of preDM and DM between tumor sites (Table 4), with the highest prevalence for preDM in patients with myeloma (39.2%) and DM in patients with pancreatic cancer (65.1%), respectively. The lowest prevalence of preDM and DM was observed among patients with melanoma of the skin (preDM: 11.0%; DM: 20.1%) (Table 4; Figure 2).
Table 4:
Prevalence of prediabetes and diabetes by tumor site at the end of the observation period (n=3,512)a
| Total | Prediabetes | Diabetes | |||
|---|---|---|---|---|---|
| No (%) | No | % (95% CI) | No | % (95% CI) | |
| Study Population | 3,512 (100) | 745 | 21.2 (19.9–22.6) | 1,145 | 32.6 (31.1–34.2) |
| Oral Cavity and Pharynx | 116 (3.3) | 28 | 24.1 (17.3–32.7) | 44 | 37.9 (29.6–47.0) |
| Digestive System | 397 (11.3) | 81 | 20.4 (16.7–24.6) | 184 | 46.3 (41.5–51.3) |
| Colon and Rectum | 186 (5.3) | 43 | 23.1 (17.6–29.7) | 54 | 29.0 (23.0–35.9) |
| Pancreas | 146 (4.2) | 24 | 16.4 (11.3–23.3) | 95 | 65.1 (57.0–72.3) |
| Otherb | 65 (1.9) | 14 | 21.5 (13.3–33) | 35 | 53.8 (41.9–65.4) |
| Respiratory System | 235 (6.7) | 75 | 31.9 (26.3–38.1) | 101 | 43.0 (36.8–49.4) |
| Lung and Bronchus | 209 (6.0) | 69 | 33.0 (27.0–39.6) | 91 | 43.5 (37.0–50.3) |
| Otherc | 26 (0.7) | 6 | 23.1 (11.0–42.1) | 10 | 38.5 (22.4–57.5) |
| Skind | 295 (8.4) | 32 | 10.8 (7.8–14.9) | 60 | 20.3 (16.1–25.3) |
| Melanoma of the skin | 283 (8.1) | 31 | 11.0 (7.8–15.1) | 57 | 20.1 (15.9–25.2) |
| Othere | 12 (0.3) | 1 | 8.3 (0.4–35.4) | 3 | 25.0 (8.9–53.2) |
| Breast | 484 (13.8) | 88 | 18.2 (15–21.9) | 98 | 20.2 (16.9–24.1) |
| Female Genital System | 278 (7.9) | 47 | 16.9 (13–21.8) | 104 | 37.4 (31.9–43.2) |
| Corpus and Uterus | 162 (4.6) | 27 | 16.7 (11.7–23.2) | 62 | 38.3 (31.1–45.9) |
| Ovary | 82 (2.3) | 19 | 23.2 (15.4–33.4) | 31 | 37.8 (28.1–48.6) |
| Otherf | 34 (1.0) | 1 | 2.9 (0.2–14.9) | 11 | 32.4 (19.1–49.2) |
| Male Genital System | 518 (14.7) | 69 | 13.3 (10.7–16.5) | 105 | 20.3 (17.0–23.9) |
| Prostate | 494 (14.1) | 66 | 13.4 (10.6–16.6) | 100 | 20.2 (16.9–24.0) |
| Otherg | 24 (0.7) | 3 | 12.5 (4.3–31.0) | 5 | 20.8 (9.2–40.5) |
| Urinary system | 194 (5.5) | 46 | 23.7 (18.3–30.2) | 79 | 40.7 (34.1–47.8) |
| Bladder | 94 (2.7) | 30 | 31.9 (23.4–41.9) | 41 | 43.6 (34.0–53.7) |
| Kidney and Renal Pelvis | 95 (2.7) | 14 | 14.7 (9.0–23.2) | 37 | 38.9 (29.8–49.0) |
| Otherh | 5 (0.1) | 2 | 40.0 (11.8–76.9) | 1 | 20.0 (1.0–62.4) |
| Brain-nervous system | 244 (6.9) | 84 | 34.4 (28.7–40.6) | 88 | 36.1 (30.3–42.3) |
| Endocrine system | 114 (3.2) | 18 | 15.8 (10.2–23.6) | 33 | 28.9 (21.4–37.9) |
| Thyroid | 108 (3.1) | 17 | 15.7 (10.1–23.8) | 31 | 28.7 (21.0–37.9) |
| Otheri | 6 (0.2) | 1 | 16.7 (0.9–56.4) | 2 | 33.3 (9.7–70.0) |
| Lymphoma | 61 (1.7) | 19 | 31.1 (20.9–43.6) | 22 | 36.1 (25.2–48.6) |
| Myeloma | 194 (5.5) | 76 | 39.2 (32.6–46.2) | 89 | 45.9 (39.0–52.9) |
| Leukemia | 246 (7.0) | 53 | 21.5 (16.9–27.1) | 91 | 37.0 (31.2–43.2) |
| Miscellaneousj | 136 (3.9) | 29 | 21.3 (15.3–28.9) | 47 | 34.6 (27.1–42.9) |
Using row percentages;
Esophagus, Stomach, Small Intestine, Liver, Intra Bile Duct, Anus, Gallbladder Other Biliary, Retroperitoneum, Peritoneum, Other Digestive Organs;
Nose, Larynx, Pleura, Trachea, Other;
excluding Basal and Squamous;
Other Non-Epithelial, Squamous Cell Carcinoma;
Cervix Uteri, Vagina, Vulva, Other Genital Organs;
Testis, Penis, Other Genital Organs;
Ureter, Other Urinary Organs;
Other Endocrine System Organs;
Kaposi Sarcoma, Mesothelioma, Eye Orbit, Soft tissue, Bone Joints, Other Miscellaneous
Figure 2:
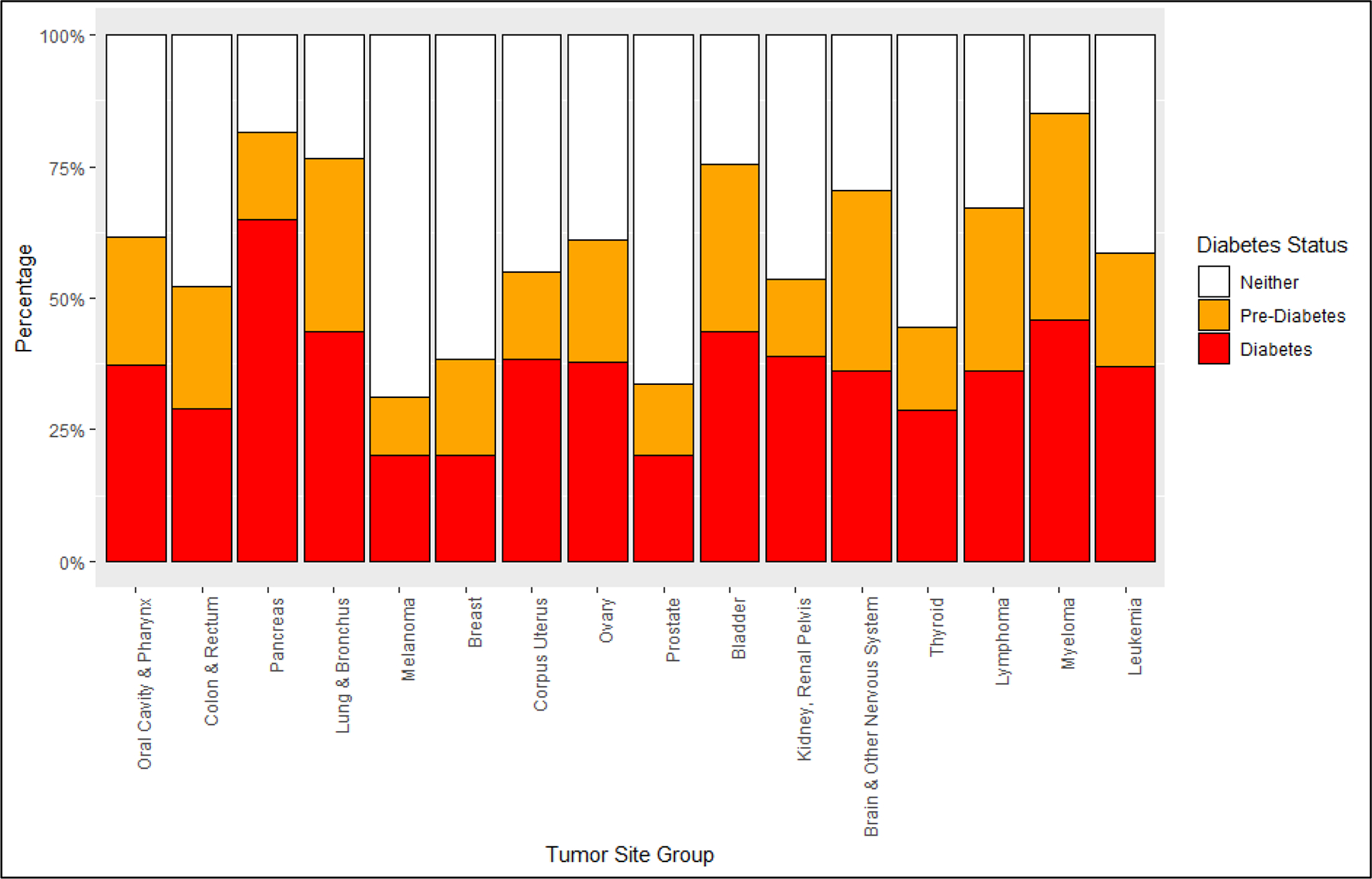
Patterns of diabetes and pre-diabetes among selected cancer types
Discussion
Our analysis has three major findings: First, in patients with cancer, preDM/DM are highly prevalent, and every second patient is affected. Second, the prevalence of preDM/DM varies widely with respect to the tumor site and cancer treatment. For example, in patients with pancreas cancer as well as in patients undergoing chemotherapy, the prevalence of DM is markedly higher than in the whole population. Third, the prevalence of preDM/DM increases substantially following cancer diagnosis. Consequently, there may be the potential to prevent the new onset of preDM/DM, as well as the transition from preDM to DM.
At the time of cancer diagnosis, the prevalence of DM in our cohort (mean age 58 years) was 12.2%, a value very similar to the 12.7% DM prevalence reported by the CDC for adults age 45–64.2 However, the 2.5-fold increase in DM prevalence up to 32.6% by the end of the observation period suggests very strongly that the cancer treatment markedly, and pathologically, increased the risk for the development of diabetes. This prevalence rate of 32.6% was much higher than previously reported DM prevalences in patients with cancer.24 Interestingly, the prevalence of preDM in our cohort at the time of cancer diagnosis was 6.0%, a value much lower than the expected preDM prevalence of 40.9% reported by the CDC for adults age 45–64.2 Reasons for our likely underestimation of the prevalence of prediabetes are listed below. However, consistent with finding that the prevalence of DM increased substantially following cancer diagnosis, the 2.6-fold increase in prediabetes prevalence up to 21.2% by the end of the observation period is also consistent with the notion that cancer treatment markedly raises blood sugar levels and increases the risk for the development of preDM.
Regarding specific cancer types, we observed a high prevalence of DM in patients with pancreatic cancer, bladder cancer, as well as cancer of the respiratory system. Patients with breast cancer, melanoma of the skin, and prostate cancer had the lowest prevalence of DM in our sample. Previous studies have pointed in the same direction.21,27,31,32 In contrast to earlier studies, our results provide a systematic overview of the prevalence of preDM and DM for various cancer types.33 For most of these cancer types the prevalence of preDM has never been reported before.
Cancer and diabetes are closely linked and share several risk factors. For example, common risk factors are male sex, older age, and obesity.34,35 Also in our study, patients with DM tend to be older, more often men and more often obese compared to patients with cancer without DM. However, shared risk factors alone cannot explain the rapid rise of DM after cancer diagnosis. For example, in the study of Hwangbo et al., the risk of developing DM after cancer diagnosis was similar in women and men and younger patients were at higher risk compared to older patients.36,20
Instead, increasing evidence supports the impact of cancer treatment on the development of DM. In our study, patients undergoing chemotherapy, radiation therapy, immunotherapy, or treated with glucocorticoids had a higher prevalence of DM compared to patients not receiving those treatments. Previous research has indicated that treatment with antineoplastics is linked to hyperglycemia and DM, by interfering with insulin production and secretion (e.g., L-Asparaginase,37,38 immune checkpoint inhibitors39–41), reducing insulin sensitivity (e.g., nucleoside metabolic inhibitors,42,43 mTOR inhibitors44,45) or both (e.g., selective estrogen receptor modulators46). Glucocorticoids, as part of the cancer treatment or used to treat cancer treatment side effects,47,48 are also related to hyperglycemia in patients with cancer.49–51
Still, diagnosing and managing DM in patients with cancer remains a challenge. Glycemic control is frequently insufficient26 and preDM as well as DM is frequently undiagnosed in patients with cancer.21 Often, providers and patients prioritize treatment of cancer over managing DM.52,53 In the absence of specific guidelines, specific roles, and responsibilities of managing DM and other chronic diseases remain unclear.54
Strengths and limitations
This study has several strengths. The basis for this analysis is the HCI-TCC-DM cohort, a large real-world patient with cancer population from the Western US. Our population was restricted to patients diagnosed with a pathologically-confirmed first primary invasive cancer. Through the linkage of the HCI-TCC clinical data repository, the Huntsman Cancer Registry, and the University of Utah Health Enterprise Data Warehouse (EDW) a wealth of clinical and study-related data was available for this analysis. To the best of our knowledge, this is the first study to systematically analyze the prevalence of preDM and DM across cancer types.
This study also has several limitations. The prevalence of preDM and DM was based on clinical data and had been assessed based on ADA guidelines.29 However, even if glucose is measured several times during cancer treatment (e.g., before surgery), systematic glucose monitoring is neither standard of care nor part of the HCI-TCC protocol. Therefore, the measurement of fasting or fed blood glucose levels alone may underestimate the true prevalence of preDM and DM.
Also, since pre-DM as a formal diagnosis is often not coded (and certainly under-reported), and the fact that glucose tolerance tests were not performed, the prevalence of pre-DM may have been underestimated. Additionally, since pre-DM and DM are often treated with various glucose lowering drugs, restricting the diagnosis criteria to only one drug (insulin) may have also led to an underestimation of the prevalence of preDM and DM. Also, the ADA guidelines have not been specifically developed for patients with cancer. Whether elevated lab results, even if they are two days apart, are actually diagnostic for DM, perhaps only stress-induced hyperglycemia, reflect the impact of cancer treatment (e.g., glucocorticoid-induced preDM or DM), or other factors, cannot be determined conclusively.
However, the diagnosis of DM was determined solely by lab values only in 8.9% (n=102) of all patients with DM (n=1,145). In all other cases, patients had at least an insulin prescription or a DM-related ICD code. Moreover, only 2,5% (n=29) of all DM patients received glucocorticoids on the same both days as their elevated glucose levels (in the DM range) were measured.
Because coding for preDM is often omitted, the portion of patients in which preDM was determined solely based on lab values was higher. In 80.3% (n=598) of all patients with preDM (n=745), the definition of preDM was based solely on lab values. Out of all patients with preDM, 27,2% (n=203) patients received glucocorticoids on the same days as when their elevated glucose levels (in the preDM range) were measured. Overall, more detailed analyses, examining the impact of glucocorticoids and chemotherapeutic agents on the development of new-onset DM and preDM after a cancer diagnosis, are needed.
Conclusions
In patients with cancer, prediabetes and diabetes are highly prevalent and this prevalence increases markedly after diagnosis. In order to enable strategies for the prevention and management of diabetes during and after cancer treatment, it is essential, (1) to recognize that patients with cancer are at high risk for developing diabetes and related complications (e.g., hyperglycemia), (2) to foster interprofessional collaboration between cancer treatment, endocrinology, and primary care, and (3) to develop and implement evidence-based practice guidelines.
Supplementary Material
Funding/Support
This work was supported by a grant from the ‘Driving out Diabetes: A Larry H. Miller Family Wellness Initiative’. This work was also supported by grants from the National Institutes of Health/National Cancer Institute (U01 CA206110, R01 CA189184, and R01 CA207371 to Ulrich) and the Huntsman Cancer Foundation. Holowatyi was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute. The research reported in this publication was also supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA042014.
Moreover, the research reported in this publication utilized the Cancer Biostatistics Shared Resource at Huntsman Cancer Institute at the University of Utah and was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA042014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Role of the Funder/Sponsor
The ‘Driving out Diabetes: A Larry H. Miller Family Wellness Initiative’ had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest Disclosures
Dr. Ulrich has as cancer center director oversight over research funded by several pharmaceutical companies but has not received funding directly herself.
References
- 1.National Cancer Institute, Division of Cancer Control and Population Science. Office of Cancer Survivorship: Statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html#ref1. Accessed October 5, 2019.
- 2.American Diabetes Association. National Diabetes Statistics Report, 2017. Atlanta: GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed September 2, 2019. [Google Scholar]
- 3.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: A consensus report. Diabetes Care. 2010;33(7):1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaorsky NG, Shaikh T, Ruth K, et al. Prostate Cancer Patients With Unmanaged Diabetes or Receiving Insulin Experience Inferior Outcomes and Toxicities After Treatment With Radiation Therapy. Clin Genitourin Cancer. 2017;15(2):326–335.e3. doi: 10.1016/j.clgc.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raikundalia MD, Fang CH, Spinazzi EF, et al. Impact of Diabetes Mellitus on Head and Neck Cancer Patients Undergoing Surgery. Otolaryngol Head Neck Surg. 2016;154(2):294–299. doi: 10.1177/0194599815607852. [DOI] [PubMed] [Google Scholar]
- 6.Karlin NJ, Kosiorek HE, Castro JC, Cook CB. Risk of hospitalization in patients with diabetes mellitus who have solid-organ malignancy. Future Sci OA. 2016;2(3):FSO129. doi: 10.4155/fsoa-2016-0020. [DOI] [Google Scholar]
- 7.Dąbrowski M, Grondecka A. Diabetes as a risk factor of hospitalization in the surgical ward due to cancer in the elderly and middle-aged population. Arch Med Sci. 2017;13(5):1025–1030. doi: 10.5114/aoms.2016.58666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barone BB, Yeh H-C, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barone BB, Yeh H-C, Snyder CF, et al. Postoperative mortality in cancer patients with preexisting diabetes: Systematic review and meta-analysis. Diabetes Care. 2010;33(4):931–939. doi: 10.2337/dc09-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranc K, Jørgensen ME, Friis S, Carstensen B. Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia. 2014;57(5):927–934. doi: 10.1007/s00125-014-3186-z. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman KE, McCarthy EP, Recklitis CJ, Ng AK. Psychological distress in long-term survivors of adult-onset cancer: results from a national survey. Arch Intern Med. 2009;169(14):1274–1281. doi: 10.1001/archinternmed.2009.179. [DOI] [PubMed] [Google Scholar]
- 12.Thong MSY, van de Poll-Franse L, Hoffman RM, et al. Diabetes mellitus and health-related quality of life in prostate cancer: 5-year results from the Prostate Cancer Outcomes Study. BJU Int. 2011;107(8):1223–1231. doi: 10.1111/j.1464-410X.2010.09861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96(17):1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 14.Bowker SL, Pohar SL, Johnson JA. A cross-sectional study of health-related quality of life deficits in individuals with comorbid diabetes and cancer. Health Qual Life Outcomes. 2006;4:17. doi: 10.1186/1477-7525-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin DW, Ahn E, Kim H, Park S, Kim YA, Yun YH. Non-cancer mortality among long-term survivors of adult cancer in Korea: national cancer registry study. Cancer Causes Control. 2010;21(6):919–929. doi: 10.1007/s10552-010-9521-x. [DOI] [PubMed] [Google Scholar]
- 16.Renehan A, Smith U, Kirkman MS. Linking diabetes and cancer: A consensus on complexity. Lancet. 2010;375(9733):2201–2202. doi: 10.1016/S0140-6736(10)60706-4. [DOI] [PubMed] [Google Scholar]
- 17.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 18.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Cai X, Qiu M, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57(11):2261–2269. doi: 10.1007/s00125-014-3361-2. [DOI] [PubMed] [Google Scholar]
- 20.Hwangbo Y, Kang D, Kang M, et al. Incidence of Diabetes After Cancer Development: A Korean National Cohort Study. JAMA Oncol. 2018;4(8):1099–1105. doi: 10.1001/jamaoncol.2018.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roeyen G, Jansen M, Chapelle T, et al. Diabetes mellitus and pre-diabetes are frequently undiagnosed and underreported in patients referred for pancreatic surgery. A prospective observational study. Pancreatology. 2016;16(4):671–676. doi: 10.1016/j.pan.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Lohmann AE, Ennis M, Taylor SK, Goodwin PJ. Metabolic factors, anthropometric measures, diet, and physical activity in long-term breast cancer survivors: change from diagnosis and comparison to non-breast cancer controls. Breast Cancer Res Treat. 2017;164(2):451–460. doi: 10.1007/s10549-017-4263-z. [DOI] [PubMed] [Google Scholar]
- 23.Cetin M, Colak R, Bayram F, Altinbas M, Unal A, Kelestimur F. High prevalence of diabetes in patients with pancreatic cancer in central Anatolia, Turkey. Diabetes Res Clin Pract. 2002;58(2):97–100. doi: 10.1016/S0168-8227(02)00130-4. [DOI] [PubMed] [Google Scholar]
- 24.Edwards BK, Noone A-M, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulus YM, Riedel ER, Sabra MM, Tuttle RM, Kalin MF. Prevalence of diabetes mellitus in patients with newly evaluated papillary thyroid cancer. Thyroid Res. 2014;7:7. doi: 10.1186/1756-6614-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlin NJ, Dueck AC, Cook CB. Cancer with diabetes: prevalence, metabolic control, and survival in an academic oncology practice. Endocr Pract. 2012;18(6):898–905. doi: 10.4158/EP12128.OR. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal G, Kamada P, Chari ST. Prevalence of Diabetes Mellitus in Pancreatic Cancer Compared to Common Cancers. Pancreas. 2013;42(2):198–201. doi: 10.1097/MPA.0b013e3182592c96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fritz AG. International classification of diseases for oncology: ICD-O. Third edition, First revision. Geneva: World Health Organization; 2013. [Google Scholar]
- 29.2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 30.Agresti A, Coull BA. Approximate is Better than “Exact” for Interval Estimation of Binomial Proportions. The American Statistician. 1998;52(2):119–126. doi: 10.1080/00031305.1998.10480550. [DOI] [Google Scholar]
- 31.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roujun C, Yanhua Y, Bixun L. High prevalence of diabetes mellitus and impaired glucose tolerance in liver cancer patients: A hospital based study of 4610 patients with benign tumors or specific cancers. F1000Res. 2016;5:1397. doi: 10.12688/f1000research.8457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SEER site recodes. https://seer.cancer.gov/siterecode/. Accessed September 2, 2019.
- 34.Garg SK, Maurer H, Reed K, Selagamsetty R. Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab. 2014;16(2):97–110. doi: 10.1111/dom.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkins ML, Blackburn BE, Rowe K, et al. Endocrine and Metabolic Diseases among Colorectal Cancer Survivors in a Population-Based Cohort. J Natl Cancer Inst. 2019. doi: 10.1093/jnci/djz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho J, Kang D, Hwangbo Y, Guallar E. Reply to: Risk of Diabetes Associated With Cancer Development. JAMA Oncol. 2019;5(3):429. doi: 10.1001/jamaoncol.2018.6619. [DOI] [PubMed] [Google Scholar]
- 37.Flores-Calderón J, Exiga-Gonzaléz E, Morán-Villota S, Martín-Trejo J, Yamamoto-Nagano A. Acute pancreatitis in children with acute lymphoblastic leukemia treated with L-asparaginase. J Pediatr Hematol Oncol. 2009;31(10):790–793. doi: 10.1097/MPH.0b013e3181b794e8. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H, Imamura T, Saito AM, et al. Protracted Administration of L-Asparaginase in Maintenance Phase Is the Risk Factor for Hyperglycemia in Older Patients with Pediatric Acute Lymphoblastic Leukemia. PLoS ONE. 2015;10(8):e0136428. doi: 10.1371/journal.pone.0136428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes. 2018;67(8):1471–1480. doi: 10.2337/dbi18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzoulis P, Corbett RW, Ponnampalam S, et al. Nivolumab-induced fulminant diabetic ketoacidosis followed by thyroiditis. Endocrinol Diabetes Metab Case Rep. 2018;2018. doi: 10.1530/EDM-18-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godwin JL, Jaggi S, Sirisena I, et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer. 2017;5:40. doi: 10.1186/s40425-017-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michie CO, Sakala M, Rivans I, Strachan MWJ, Clive S. The frequency and severity of capecitabine-induced hypertriglyceridaemia in routine clinical practice: a prospective study. Br J Cancer. 2010;103(5):617–621. doi: 10.1038/sj.bjc.6605807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng J-P, Yuan X-L, Li M, et al. Secondary diabetes associated with 5-fluorouracil-based chemotherapy regimens in non-diabetic patients with colorectal cancer: results from a single-centre cohort study. Colorectal Dis. 2013;15(1):27–33. doi: 10.1111/j.1463-1318.2012.03097.x. [DOI] [PubMed] [Google Scholar]
- 44.Milluzzo A, Tumminia A, Vella V, et al. Short-term adverse effects of anticancer drugs in patients with type 2 diabetes. J Chemother. 2019;31(3):150–159. doi: 10.1080/1120009X.2019.1572297. [DOI] [PubMed] [Google Scholar]
- 45.Gallo M, Muscogiuri G, Felicetti F, et al. Adverse glycaemic effects of cancer therapy: indications for a rational approach to cancer patients with diabetes. Metab Clin Exp. 2018;78:141–154. doi: 10.1016/j.metabol.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Lipscombe LL, Fischer HD, Yun L, et al. Association between tamoxifen treatment and diabetes: a population-based study. Cancer. 2012;118(10):2615–2622. doi: 10.1002/cncr.26559. [DOI] [PubMed] [Google Scholar]
- 47.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31(25):3076–3082. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 48.Wooldridge JE, Anderson CM, Perry MC. Corticosteroids in advanced cancer. Oncology. 2001;15(2):225–34. [PubMed] [Google Scholar]
- 49.Hwangbo Y, Lee EK. Acute Hyperglycemia Associated with Anti-Cancer Medication. Endocrinol Metab (Seoul). 2017;32(1):23–29. doi: 10.3803/EnM.2017.32.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon S, Hermayer KL, Hermayer K. Glucocorticoid-induced hyperglycemia. Am J Med Sci. 2013;345(4):274–277. doi: 10.1097/MAJ.0b013e31828a6a01. [DOI] [PubMed] [Google Scholar]
- 51.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469–474. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- 52.Goebel J, Valinski S, Hershey DS. Improving Coordination of Care Among Healthcare Professionals and Patients With Diabetes and Cancer. Clin J Oncol Nurs. 2016;20(6):645–651. doi: 10.1188/16.CJON.645-651. [DOI] [PubMed] [Google Scholar]
- 53.Piette JD, Kerr EA. The Impact of Comorbid Chronic Conditions on Diabetes Care. Diabetes Care. 2006;29(3):725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 54.Walsh J, Young JM, Harrison JD, et al. What is important in cancer care coordination? A qualitative investigation. Eur J Cancer Care (Engl). 2011;20(2):220–227. doi: 10.1111/j.1365-2354.2010.01187 [DOI] [PubMed] [Google Scholar]
- 55.Surveillance, Epidemiology, and End Results (SEER) Program. Site Recode ICD-O-3/WHO 2008 Definition. https://seer.cancer.gov/siterecode/. Accessed March 25, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


