Abstract
How the nervous system regulates bone remodeling is an exciting area of emerging research in bone biology. Accumulating evidence suggest that neurotransmitter-mediated inputs from neurons may act directly on osteoclasts. Dopamine is a neurotransmitter that can be released by hypothalamic neurons to regulate bone metabolism through the hypothalamic-pituitary-gonadal axis. Dopamine is also present in sympathetic nerves that penetrate skeletal structures throughout the body. It has been shown that dopamine suppresses osteoclast differentiation via a D2-like receptors (D2R)-dependent manner, but the intracellular secondary signaling pathway has not been elucidated. In this study, we found that cAMP-response element binding protein (CREB) activity responds to dopamine treatment during osteoclastogenesis. Considering the critical role of CREB in osteoclastogenesis, we hypothesize that CREB may be a critical target in dopamine’s regulation of osteoclast differentiation. We confirmed that D2R is also present in RAW cells and activated by dopamine. Binding of dopamine to D2R inhibits the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway which ultimately decreases CREB phosphorylation during osteoclastogenesis. This was also associated with diminished expression of osteoclast markers that are downstream of CREB. Pharmacological activation of adenylate cyclase (to increase cAMP production) and PKA reverses the effect of dopamine on CREB activity and osteoclastogenesis. Therefore, we have identified D2R/cAMP/PKA/CREB as a candidate pathway that mediates dopamine’s inhibition of osteoclast differentiation. These findings will contribute to our understanding of how the nervous and skeletal systems interact to regulate bone remodeling. This will enable future work toward elucidating the role of the nervous system in bone development, repair, aging, and degenerative disease.
Keywords: dopamine, dopamine receptor, osteoclasts, cAMP-response element binding protein (CREB), cyclic adenosine monophosphate (cAMP), protein kinase A (PKA)
1. Introduction
Bone remodeling is a lifelong dynamic process in which old bone is resorbed by osteoclasts (OC) and subsequently replaced with new bone synthesized by osteoblasts [1]. An imbalance between bone resorption and formation often leads to metabolic bone diseases. For example, pathological bone loss due to increased resorption is associated with osteopenia, osteoporosis, or Paget’s disease of bone [2]. How this delicate balance is regulated is not fully understood, but is likely orchestrated by intracellular molecular pathways in bone cells in response to hormones, growth factors, or mechanical stimuli [3, 4]. How the nervous system regulates bone remodeling is an exciting area of emerging research in bone biology, supported by accumulating evidence that inputs from the central and peripheral nervous system may regulate bone remodeling [5, 6]. A number of neurotransmitters and their receptors have also been demonstrated to regulate the activity of bone cells [7–9].
Dopamine (DA) is a member of the catecholamine family of neurotransmitters found in diverse neuronal subtypes in the central nervous system [10]. DA has two categories of receptors: D1-like receptors (D1R) family (Drd1 and Drd5) and D2-like receptors (D2R) family (Drd2, Drd3, Drd4) that differentially regulate intracellular level of second messenger cyclic adenosine monophosphate (cAMP) [11]. DA can be released by hypothalamus neurons and regulates bone metabolism through the hypothalamic-pituitary-gonadal axis [12]. Accumulating clinical evidence have suggested the close relationship of DA with bone health and diseases. For example, the high risk of osteoporosis observed in Parkinson’s disease patients as well as the increased fracture risk associated with antipsychotic medications [13–15]. In these examples, low DA level from dopaminergic degeneration or pharmacological DA receptor blockade leads to excessive pituitary prolactin secretion and hence a reduction of gonadal steroid hormones, which adversely impacts bone metabolism.
In the peripheral nervous system, DA is stored in sympathetic nerves and can directly act on bone cells to regulate bone metabolism. We previously discovered that DA enhances osteoblast differentiation and mineralization in vitro [16, 17]. which can enhance bone formation. For bone resorption, Hanami et al discovered that DA or D2R agonist but not D1R agonist inhibits OC differentiation in vitro, suggesting a D2R-signal-dependent regulatory effect on osteoclastogenesis [18]. The D2R-signal-dependent anti-osteoclastogenic effect has also been demonstrated in a peri-implant osteolysis model [19].
However, the intracellular pathway by which D2R signal regulates the expression of OC-specific genes has not been fully elucidated. In addition, effect of D1R blockade on in vitro OC differentiation has also been reported [20], which further demonstrates that the mechanism by which dopaminergic signal regulates osteoclastogenesis is very complex and remains to be elucidated. In the present study, we found that cAMP-response element binding protein (CREB) activity responds to DA treatment in osteoclastogenesis. Considering the critical role of CREB in osteoclastogenesis--CREB alone or in cooperation with other transcription factors may transactivate many OC-specific genes [21], we hypothesize that CREB may be a critical target in DA’s regulation of OC differentiation. By testing the above hypothesis, we have identified an intracellular signaling pathway (D2R/cAMP/PKA/CREB) as a molecular mechanism by which DA mediates OC differentiation.
2. Materials and methods
2.1. Cell culture and OC differentiation
RAW 264.7 (ATCC® TIB-71) cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 10% FBS and penicillin/streptomycin at 37°C, 5% CO2. RAW cells were seeded at a density of 4 × 104 cells/cm2 and received receptor activator of nuclear factor kappa-B ligand (RANKL) (10 ng/mL, R&D systems) stimulation for 3d ~ 4d to become mature OC, as indicated in specific experiments. Then the cells were fixed with 4% paraformaldehyde, permeabilized with Triton X-100, and stained with tartrate-resistant acid phosphatase (TRAP) staining solution [22]. Images were captured by Nikon Eclipse Ti-U inverted microscope (Nikon) and TRAP positive, multinucleated (≥3 nuclei) cells were counted as OC. For bone resorption pit visualization, RAW cells were seeded on bone slices (BioVendor). After 3~5 days RANKL treatment, cells were removed from bone slices by sonicating 10 minutes and then the bone slices were stained with 1% toluidine blue for 4 minutes [23]. Images were captured by Nikon SMZ18 stereo microscope (Nikon).
Primary OC differentiation were performed as previously described [22]. Femurs were harvested from 8-week-old C57BL/6J male mice and bone marrow cells were flushed out into α-MEM supplemented with 10% FBS and penicillin/streptomycin. Non-adherent cells were removed overnight and re-plated at a density of 1.5 × 105 cells/cm2 with 30 ng/mL M-CSF (R&D Systems) for growing OC precursor cells. After 3 days, OC precursor cells were treated with 30ng/mL M-CSF and 10 ng/mL RANKL (R&D Systems) for 4 days to differentiate to OC. All animal procedures were approved by Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill and the Ohio State University, and were conducted in accordance with the NIH guidelines.
2.2. Chemicals
The concentrations of DA hydrochloride (Acros Organics) were indicated in specific experiments. D1R agonist SKF38393 (Sigma-Aldrich) and D2R agonist quinpirole (Sigma-Aldrich) was used at a concentration of 100 nM and 100 nM, respectively. D1R antagonist SCH23390 (10nM, Sigma-Aldrich), D2R antagonist haloperidol (10nM, Sigma-Aldrich), protein kinase A (PKA) inhibitor H89 (10 μM, Sigma-Aldrich), extracellular signal-related kinase 1/2 (ERK1/2) inhibitor SCH772984 (300 nM, Selleckchem), Akt 1/2/3 inhibitor MK-2206 (5 μM, Selleckchem), Ca2+/calmodulin-dependent protein kinase II (CaMKII) inhibitor KN-93 (10 μM, Selleckchem) were applied on the cells for 30 minutes before the DA treatment. Adenylate cyclase (AC) agonist Forskolin (10 μM, Sigma-Aldrich) and cAMP analog 8-Br-cAMP (50 μM, Selleckchem) that activates PKA was used together with DA treatment.
2.3. Cell viability assay
Cell viability in response to various concentrations of DA was detected by the RealTime-Glo™ MT cell viability assay (Promega) according to the manufacturer protocol. The luminescence (relative light unit, RLU) was read by the Cytation 5 imaging reader (Bio-Tek).
2.4. RT-qPCR
Cells were harvested by RNAzol (Molecular Research Center) for total mRNA extraction and cDNA reverse-transcription was performed by using iScript™ cDNA Synthesis Kit (Bio-Rad). RT-qPCR was performed on StepOnePlus Real-time PCR system (Applied Biosystems) using the iTaq™ Universal SYBR Green Supermix reagent (Bio-Rad). The primers are listed in Supplementary Table 1. The relative expression of target genes was normalized to housekeeping gene β-2-microglobulin (B2m) based on the comparative CT (ΔΔCT) method.
2.5. Western blot
Cells were lysed by RIP A buffer plus phosphatase & protease inhibitor cocktail. The total protein was separated by using XCell SureLock Mini-Cell (Invitrogen) sodium dodecyl sulfate-polyacrylamide gel electrophoresis system and transferred onto a nitrocellulose membrane by using Trans-Blot Cell system (Bio-Rad). Target protein was immunodetected using primary antibodies phospho-CREB (Serl33) (Cell Signaling, #9198), CREB (Cell Signaling, #9197), PKA-Cα (Cell Signaling, #4782), β-Actin (Santa Cruz, #sc-47778), and corresponding peroxidase-conjugated secondary antibodies (Merck Millipore). Protein was visualized by enhanced chemiluminescence solution (Thermo Fisher). Band images were captured by ImageQuant LAS 4000 camera system (GE Healthcare) and quantified by NIH ImageJ software.
2.6. CREB luciferase reporter assay
CREB transcriptional activity was determined by measuring the luciferase reporter activity in cells transfected with p-CREB-Luc reporter vector (Signosis, #LR-2008), which contains CREB cis-acting enhancer element upstream of a minimal TA promoter. 1 × 105 cells seeded in 24-well plate were transfected with 5μg plasmid using Lipofectamine 2000 (Invitrogen), according to the manufacturer protocol. 24 hours post transfection, the cells were washed off before being treated with indicated agents, and lysed for luciferase activity detection using Nano-Glo luciferase assay system (Promega). The luminescence (RLU) was read by the Cytation 5 imaging reader (Bio-Tek).
2.7. cAMP immunoassay detection
Intracellular cAMP level was detected by a fluorometric competitive ELISA kit (Abeam, #abl38880) according to manufacturer’s instructions. RAW cells were treated with 100 μM DA in the presence of 10 ng/mL RANKL and lysed at indicated time points. HRP-labeled cAMP and free cAMP within the cell lysates were loaded into anti-cAMP antibody-coated plates. The activity of HRP-cAMP conjugate was measured by the Cytation 5 imaging reader (Bio-Tek). Free cAMP concentrations in the samples were calculated using a calibration curve.
2.8. Statistical analyses
Experiments were performed in triplicate and repeated three times. Data was presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software). The comparisons among multiple groups were assessed using one-way ANOVA followed by Bonferroni post-hoc test. P < 0.05 was set for significance.
3. Results
3.1. DA suppresses OC differentiation and CREB activity, which is dose-dependent
It has been reported that DA inhibits in vitro OC differentiation from human monocyte or murine bone marrow macrophages [18, 19]. Here we demonstrated that DA also suppresses OC differentiation from RAW 264.7 cells in concentration-dependent manner. RAW cells represent an OC precursor cell line that can be chemically induced by RANKL exposure to differentiate into mature OCs that can functionally resorb bone. The bone resorbing capacity of the RAW-derived OCs in our system was verified by the formation of resorption pit (Supplementary Fig. 1). In the following experiments, RAW cells were treated with various concentration of DA in the presence of RANKL for 4 days. Differentiation of RAW cells into OC was inhibited by DA treatment, and 100 μM DA showed the strongest inhibitory effects (Fig. 1A). TRAP staining showed decreasing number of OC with increasing DA concentration (Fig 1A, right panel). Expression of osteoclastic markers (c-Fos, Nfatc1, Trap, Ctsk) also generally exhibited an inverse relationship with DA concentration, demonstrating DA-mediated inhibition of osteoclastogenesis (Fig. 1B). We also investigated whether DA might be toxic to RAW cells through the MT cell viability assay. Within the concentrations used for treatment (10μM~100μM), DA did not show significant toxicity on RAW cells after 24, 72, and 96 hours of incubation (Supplementary Fig. 2). Therefore, the decrease in OC formation is not due to non-specific DA toxicity, but represents a direct inhibitory effect on the differentiation process.
Fig. 1. DA inhibits OC differentiation from RAW cells.
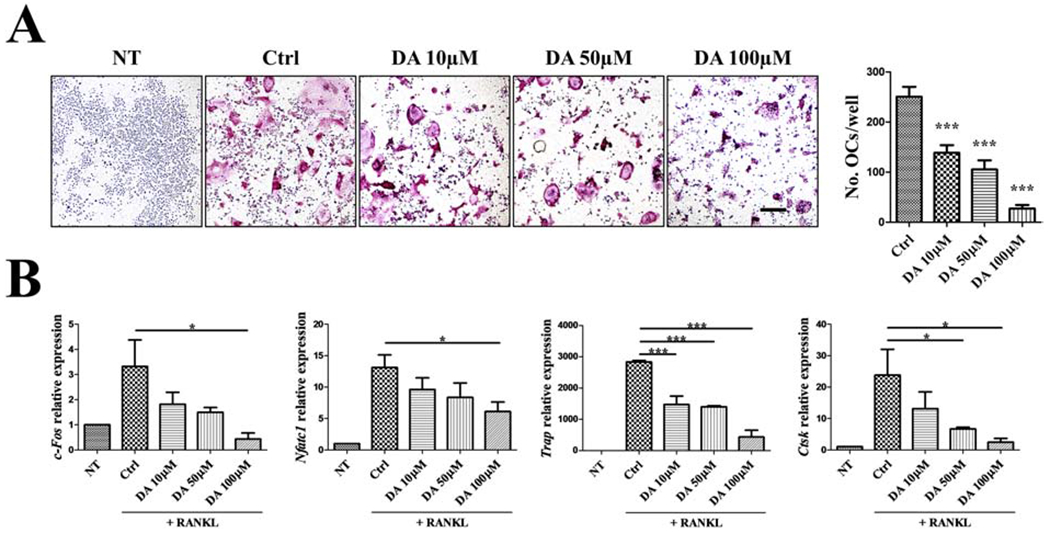
RAW cells were treated with indicated concentration of DA in the presence of 10 ng/mL RANKL for 4 days. (A) TRAP staining and quantification of OC. TRAP positive, multinucleated OC in red were counted. n=4. Scale bar is 200μm. (B) Relative expression of osteoclastic genes (c-Fos, Nfatc1, Trap, Ctsk) on day 4 after DA+RANKL treatment were determined by RT-qPCR; normalized to B2m. n=3. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Ctrl group. Data shown as mean ± SEM.
We next investigated how DA modulates RAW differentiation into OCs by examining intracellular signaling pathways associated with both DA receptor activation and OC differentiation. At different stages of osteoclastogenesis (24h, 48h after RANKL treatment), a concentration-dependent inhibition of CREB phosphorylation by DA was observed (Fig. 2A and B). Consistently, DA also showed a concentration-dependent inhibition on the expression of osteoclastic genes (Fig. 2C). 100μM of DA treatment showed the strongest inhibitory effect. CREB actually plays an important role in the transactivation of osteoclastic genes during differentiation [21]. At the same time, CREB is a downstream target of dopaminergic signaling pathways [24]. Taken together, our data demonstrated that CREB activity is involved in the regulatory effect of DA on osteoclastogenesis, suggesting that CREB may be a critical node for connecting dopaminergic signal to OC differentiation.
Fig. 2. CREB activity is inhibited by DA in osteoclastogenesis.
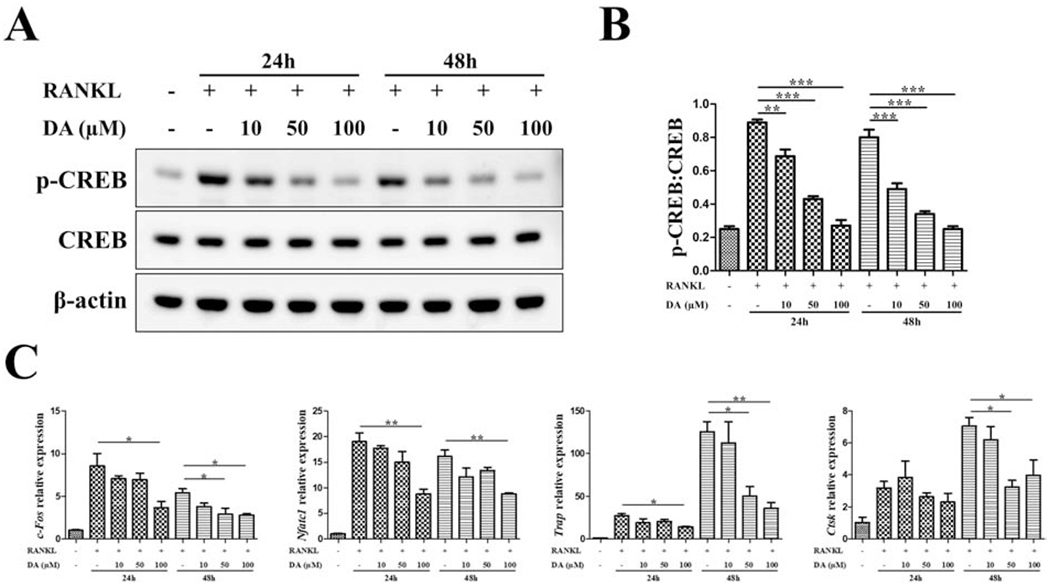
RAW cells were treated with indicated concentration of DA in the presence of 10 ng/mL RANKL. At different time points of osteoclastogenesis (24h, 48h), p-CREB and CREB level was detected by western blot (A) and quantitated as a ratio of p-CREB/CREB (B). (C) Relative expression of osteoclastic genes (c-Fos, Nfatc1, Trap, Ctsk) were determined by RT-qPCR; normalized to B2m. n=3 for all experiments; *P < 0.05, **P < 0.01, ***P < 0.001. Data shown as mean ± SEM.
3.2. D2R but not D1R signaling regulates CREB activity in osteoclastogenesis
DA receptors are seven-transmembrane G protein-coupled receptors that couple to different G proteins [25]. Upon binding to DA, Gαs-coupled D1R family activates AC and increases cAMP production. In contrast, Gαi/o-coupled D2R family suppresses AC activity and reduces cAMP production. It has been demonstrated that DA suppresses osteoclastogenesis via D2R signaling, and D2R agonist significantly inhibits OC differentiation from monocytes or macrophages [18, 19]. However, which intracellular signaling pathway and transcription factor is activated remains to be elucidated.
To determine the dopamine receptor subtype and signaling pathway involved, we first checked basal gene expression of DA receptors in RAW cells and RAW-derived OC by RT-qPCR. While all five subtypes of DA receptors were expressed, there were no differences in expression levels (Supplementary Fig. 3). We next investigated whether DA inhibited p-CREB through D1R or D2R. Subtype-specific agonists (SKF38393 for D1R and quinpirole for D2R) and antagonists (SCH23390 for D1R and haloperidol for D2R) were used. We found that D2R agonist reduced the p-CREB during osteoclastogenesis, which was similar to DA’s effect. In contrast D1R agonist did not have any effect on the level of p-CREB (Fig. 3A and B). Consistently, DA’s inhibitory effect on p-CREB was abolished when D2R was blocked with the D2R-specific antagonist, but the effect was not observed with D1R antagonist (Fig. 3A and B). These findings showed that DA inhibited p-CREB during osteoclastogenesis specifically through D2R.
Fig. 3. D2R-dependent inhibition on CREB activity in osteoclastogenesis.
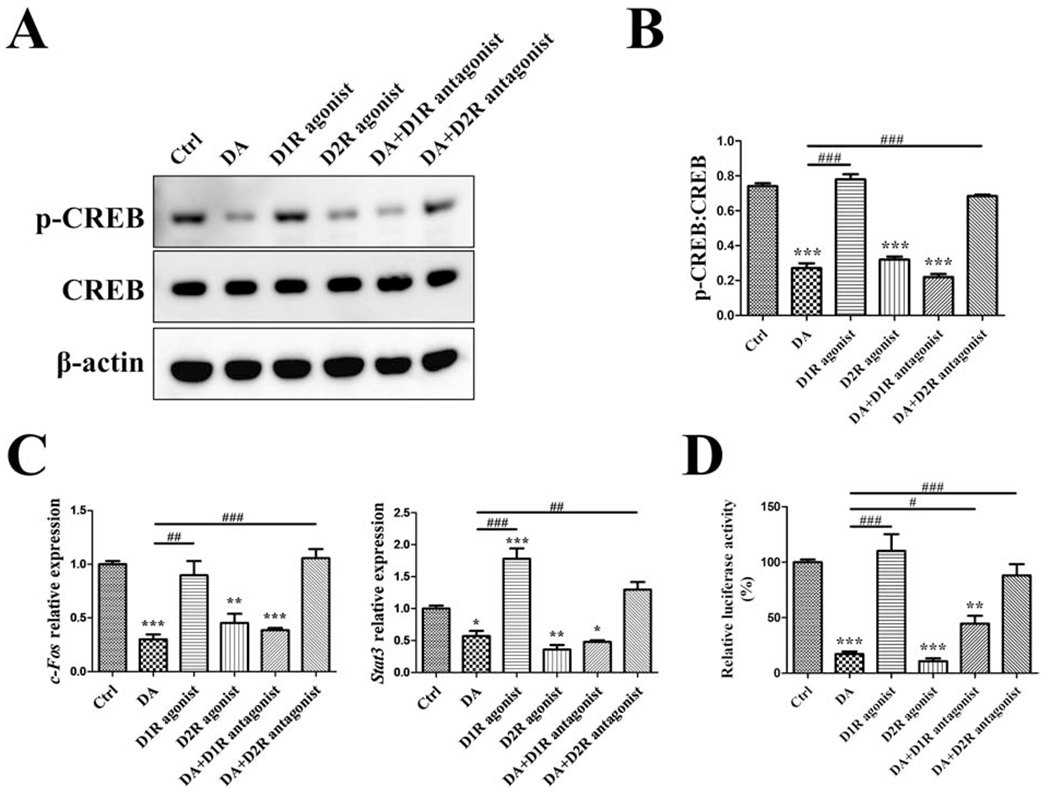
RAW cells were treated with vehicle (Ctrl), 100 μM DA, 100 nM D1R agonist SKF38393, 100 nM D2R agonist quinpirole, 100 μM DA plus 10nM D1R antagonist SCH23390 (after 30min SCH23390 pretreatment), or 100 μM DA plus l0nM D2R antagonist haloperidol (after 30min haloperidol pretreatment), in the presence of 10 ng/mL RANKL for 1 day. (A, B) Western blot detection of p-CREB/CREB. (C) RT-qPCR detection of c-Fos and Stat3 genes; normalized to B2m. (D) For p-CREB luciferase activity assay, p-CREB-Luc reporter-transfected RAW cells were used to receive the treatment same as above. n=3 for all experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Ctrl group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. DA group. Data shown as mean ± SEM.
We next checked their effects on CREB-mediated transcriptional activity by examining the expression of c-Fos and Stat3. These genes contain a cAMP response element (CRE) region in the promoter region so they can be transactivated by CREB [24]. Furthermore, we also assayed CREB transcriptional activity through a luciferase reporter assay containing CRE in the promoter. As anticipated, D2R agonist decreased the expression of c-Fos and Stat3, as well as p-CREB luciferase activity during osteoclastogenesis, comparable to DA’s effects (Fig. 3C and D). Further, blocking D2R signaling successfully abolished DA’s inhibitory effects while blocking D1R generally had no effect (Fig. 3C and D). We also repeated these experiments utilizing primary mouse bone marrow mononuclear cells and observed similar effects on OC differentiation (Supplementary Fig. 4). Taken together, these results demonstrated that D2R mediated DA’s ability to modulate CREB activity during osteoclastogenesis.
3.3. DA mediates CREB activity through cAMP/PKA pathway during osteoclastogenesis
A variety of protein kinases are capable of phosphorylating CREB in its kinase-inducible domain, which enables CREB a convergent target for multiple signaling cascades. Among DA-mediated signaling network, in addition to the canonical cAMP/PKA pathway, activation of Akt [26], PLCβ/Ca2+/CaMKII [27], or cAMP/Epac/ERK pathway [28] have also been reported to activate CREB. Thus, we next investigated which of these signaling pathways might mediates anti-osteoclastogenic activity of DA. We combined DA with PKA inhibitor H89, or with the kinase inhibitor for other pathways, to determine their impact on CREB activity. DA combined with PKA inhibitor aggravated inhibition of both p-CREB level and CRE-luciferase activity during osteoclastogenesis (Fig. 4A–C). In contrast, DA combined with ERK, Akt, or CaMKII inhibitors showed little effect. We next checked the levels of cAMP, PKA, and p-CREB in response to DA during osteoclastogenesis. At various time points (10min, 30min, and 60min), level of cAMP, PKA, and p-CREB were coincidentally decreased in response to DA (Fig. 4D–F). Taken together, our data suggest that the cAMP/PKA signaling pathway mediates DA’s inhibition of CREB activity during osteoclastogenesis.
Fig. 4. DA mediates CREB activity through cAMP/PKA pathway during osteoclastogenesis.
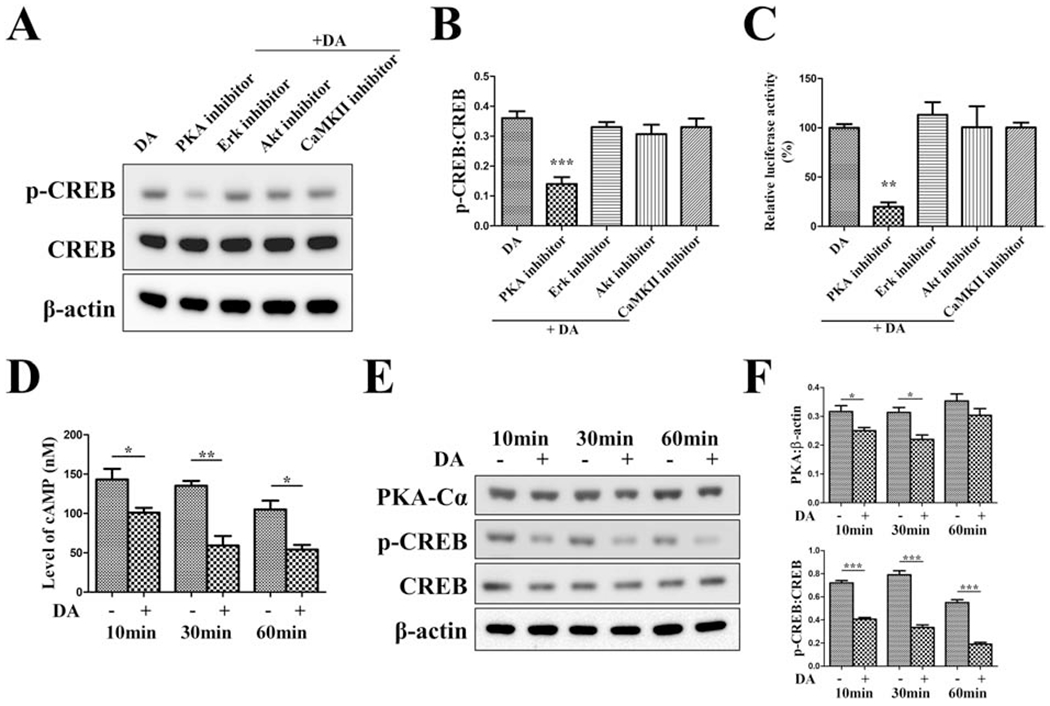
For experiments in (A-C), RAW cells were pretreated with PKA inhibitor H89 (10 μM), ERK1/2 inhibitor SCH772984 (300 nM), Akt1/2/3 inhibitor MK-2206 (5 μM), or CaMKII inhibitor KN-93 (10 μM) for 30min and then stimulated with 100 μM DA plus indicated inhibitor in the presence of 10 ng/mL RANKL for 1 day. (A, B) Western blot detection of p-CREB/CREB. (C) For p-CREB luciferase activity assay, p-CREB-Luc reporter-transfected RAW cells were used to receive the treatment same as above. n=3 for all experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. DA group. For experiments in (D-F), RAW cells were treated with 100 μM DA in the presence of 10 ng/mL RANKL and lysed at indicated time points. (D) intracellular cAMP concentrations were detected by a fluorometric competitive ELISA method. (E, F) Western blot detection of PKA and p-CREB/CREB. n=3 for all experiments. *P < 0.05, **P < 0.01, ***P < 0.001. Data shown as mean ± SEM.
3.4. Activation of cAMP/PKA/CREB pathway reverses DA-mediated inhibition of osteoclastogenesis
Given the critical role of cAMP/PKA/CREB pathway in the anti-osteoclastogenic effect of DA, we next investigated whether increasing cAMP/PKA/CREB activity reverses DA’s inhibition of OC differentiation. To increase cAMP levels, we will utilize forskolin which is a known AC activator that increases cAMP production. Similarly, we will use 8-Br-cAMP, a cAMP analog, to activate PKA. When RAW cells were treated with forskolin or 8-Br-cAMP, a significantly increased phosphorylation of CREB and CREB transcriptional activity was observed, when compared to DA treatment (Fig. 5A–C). Further, DA-impaired OC differentiation was significantly improved by Forskolin or 8-Br-cAMP treatment by TRAP staining and quantitation of the number of mature OCs (Fig. 5D). Expression of most of the osteoclastic genes (c-Fos, Nfatc1, Trap, Ctsk) was also enhanced by Forskolin or 8-Br-cAMP as compared to DA inhibition (Fig. 5E). DA’s inhibition of c-Fos expression was completely reversed by Forskolin or 8-Br-cAMP to normal control levels. Similar findings were also demonstrated using primary mouse OC precursor cells (Supplementary Fig. 5). Collectively, activating cAMP/PKA/CREB pathway reverses DA’s inhibition of osteoclastogenesis, further demonstrating that DA regulates OC differentiation via the D2R/cAMP/PKA/CREB pathway.
Fig. 5. Activation of AC/PKA partly abolishes DA’s anti-osteoclastogenic effect.
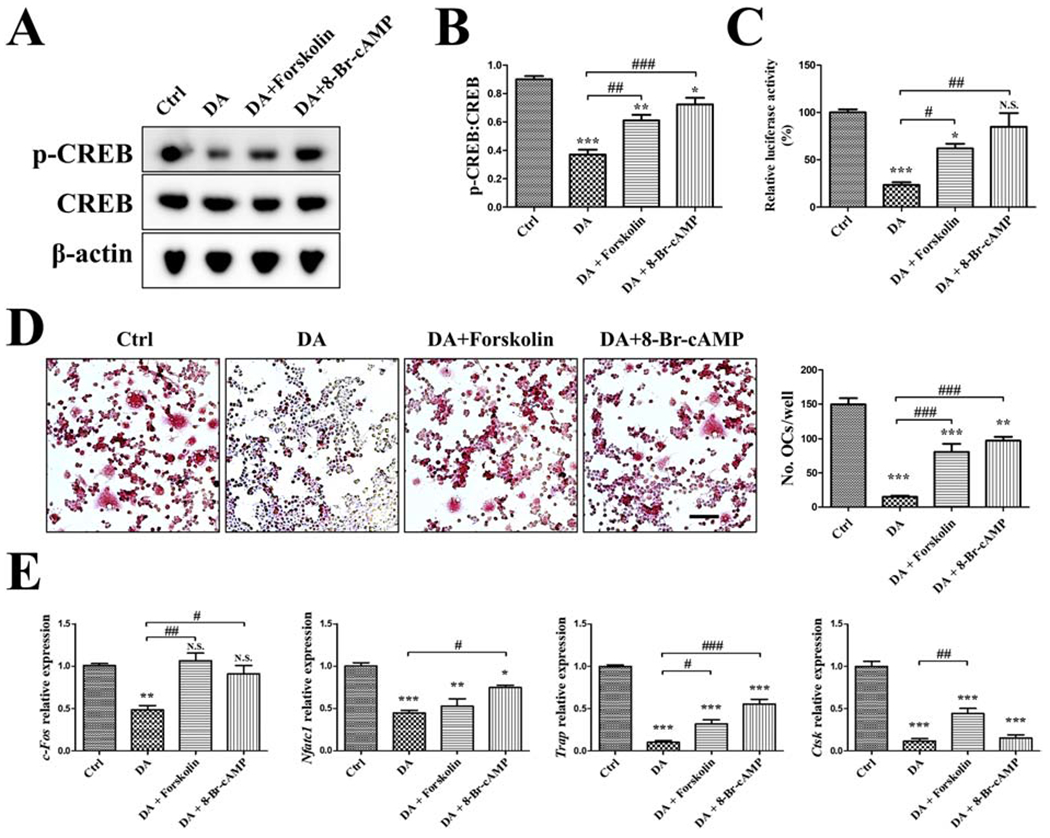
RAW cells were treated with vehicle (Ctrl), 100 μM DA, 10 μM AC agonist Forskolin, or 50 μM PKA agonist 8-Br-cAMP as indicated, in the presence of 10 ng/mL RANKL for 3 days. (A, B) Western blot detection of p-CREB/CREB. (C) For p-CREB luciferase activity assay, p-CREB-Luc reporter-transfected RAW cells were used to receive the treatment same as above. (D) TRAP staining and quantification of OCs. TRAP positive, multinucleated OC in red were counted. Scale bar is 200μm. (E) RT-qPCR detection of osteoclastic genes (c-Fos, Nfatc1, Trap, Ctsk); normalized to B2m. n=3 for all experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Ctrl group; *P < 0.05, ##P < 0.01, ###P < 0.001 vs. DA group. Data shown as mean ± SEM.
4. Discussion
In our present study, we have identified a specific signaling pathway that mediates DA’s inhibition of osteoclastogenesis (summarized in Fig. 6). We showed that D2R is the DA receptor subtype that is activated by DA in RAW cells, and not D1R. Downstream from D2R, cAMP modulates PKA activity which ultimately act upon the transcriptional factor CREB. Phosphorylation of CREB is associated with the expression of downstream genes, specifically OC markers, that are transcriptionally controlled by CREB. Pharmacological activation of cAMP and PKA reverses the effect of DA on osteoclastogenesis. Therefore, we show for the first time that DA triggers the D2R/cAMP/PKA/CREB pathway to inhibit OC differentiation.
Fig. 6. Working model.
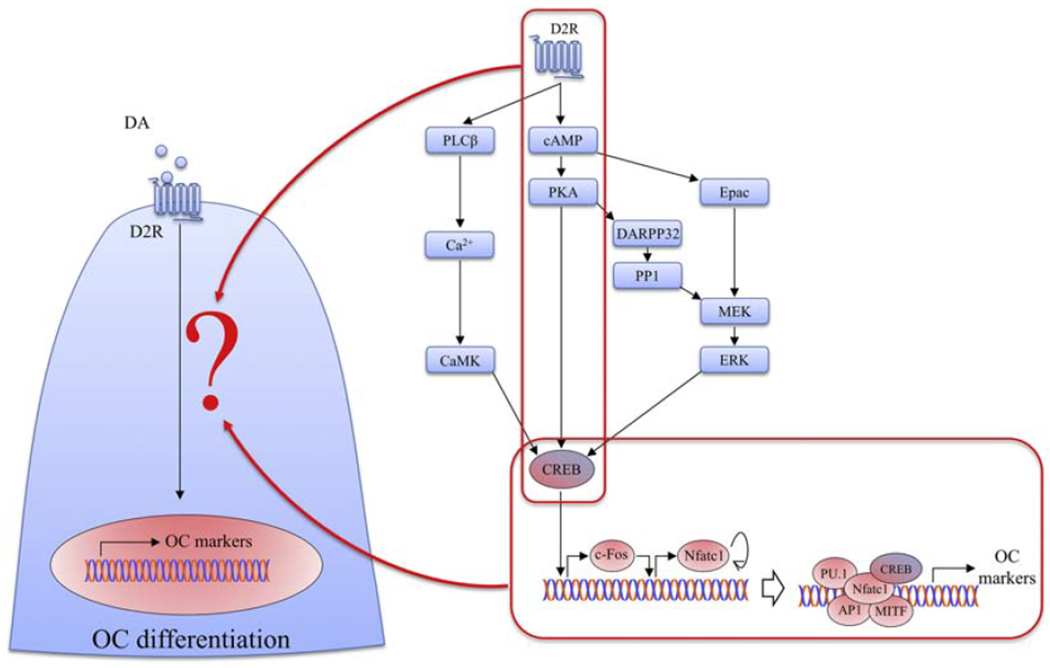
Our present study demonstrated DA regulates CREB activity via D2R/cAMP/PKA/CREB signaling pathway during osteoclastogenesis, which serves as a molecular mechanism by which DA modulates OC differentiation. DA, dopamine; D2R, D2-like receptors; OC, osteoclasts; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; CREB, cAMP-response element binding protein; PLCβ, phospholipase C-β; CaMK, Ca2+/calmodulin-dependent protein kinase; DARPP32, dopamine and cAMP regulated phosphoprotein 32kDa; PP1, protein phosphatase-1; Epac, exchange protein activated by cAMP; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-related kinase; Nfatc1, nuclear factor of activated T-cells, cytoplasmic 1; API, activator protein 1; MITF, melanocyte inducing transcription factor.
Peripheral DA is present throughout the body in sympathetic nerves and plays a critical role in the regulation of hormone secretion, blood pressure, immune response, circulation, as well as lung, kidney and gastrointestinal functions [29]. Bone tissues are highly innervated by sympathetic nerve fibers, where high level of DA is stored [30]. The presence of endogenous DA in bone marrow (10−9 ~ 10−5 M) has also been reported [31, 32]. Moreover, mRNA and protein expression of five DA receptor subtypes in human or mouse OC precursors have been verified by recent studies, which is even higher in mature OCs [14, 18]. These evidences form the physiological foundation for peripheral DA to directly act on OC precursors and hence influences OC formation. In our experiments, the concentration of DA required to show effective inhibition of RAW cells-derived OC differentiation is higher than reported in mouse primary OCs [33]. This difference in DA sensitivity might be due to the relatively lower DA receptors expression in RAW cells (Supplementary Fig. 3). Despite this differential DA sensitivity, key results of our RAW cell experiments were confirmed using mouse primary OC precursor cells (Supplementary Fig. 4 and 5).
Previous reports suggested that D1R-signaling appears to have little impact on OC differentiation [18, 19], which is consistent with our finding that D1R agonist SKF38393 did not affect CREB activity or c-Fos expression (Fig. 3). However, D1R seems to have diverse effects on osteoclastogenesis under different conditions. D1R antagonist is reported to shows therapeutic potential on collagen-induced arthritis partly due to its anti-osteoclastogenic effect [20]. In contrast, selective Drd1 agonist A77636 inhibits osteoclastogenesis by downregulating NFATc1 through increased phosphorylation of eIF2α, and prevents osteolytic lesions in a bone model of tumor metastasis [34]. The diverse responses of OC differentiation to pharmacological D1R manipulation suggest that OC dopaminergic signaling is quite complex and requires further elucidation.
Recent reports have demonstrated D2R-dependent suppression of osteoclastogenesis [18, 19], but how D2R stimulation lead to downstream effectors that trigger osteoclastic genes expression has been unclear. A potential downstream signaling molecule is cAMP, which was described in a study by Hanami et al [18]. Our study expanded this study by identifying a complete pathway, D2R/cAMP/PKA/CREB axis, which acts as a molecular mechanism by which DA mediates osteoclastogenesis. Our data is also consistent with recent reports that pramipexole and quinpirole can also effectively inhibits OC differentiation from monocytes or macrophages [18, 19].
cAMP/PKA is thought to negatively regulate osteoclastogenesis by phosphorylating and deactivating Nfatc1, with crosstalk with Wnt signal or Ca2+/CaMK [35, 36]. Moreover, Ramaswamy et al indicated that paternal allele deletion of Gnas (Gnas+/p−) showed decreased cAMP/PKA activity, decreased p-CREB, elevated Nfatc1, and enhanced osteoclastogenesis, which can all be reversed by pharmacological treatment that activates AC [37]. This is contrast to our finding that cAMP/PKA/CREB activity, Nfatc1, and osteoclastogenesis were uniformly decreased when responding to DA stimulation (Fig. 4 and 5). In view of the pro-osteoclastogenic effect of CREB--transactivating osteoclastogenic transcription factor c-Fos [38], the anti- or pro-osteoclastogenic findings for cAMP/PKA appears paradoxical and is in need of further investigation. Our data is consistent with Hanami et al study [18] which reported a reduction in cAMP level, along with decreased c-Fos and Nfatc1 gene expression as well as decreased OC formation when D2R was activated. Moreover, as Fig. 5 showed, pharmacological activation of cAMP/PKA using Forskolin or 8-Br-cAMP exhibited pro-osteoclastogenic effects, implying that cAMP/PKA might mediate osteoclastogenesis preferentially by activating CREB rather than by deactivating Nfatc1, when responding to DA signal.
Clinical evidence regarding skeletal effects of DA, such as in Parkinson’s disease patients or side effects of antipsychotic medications, mainly focus on hypothalamic-pituitary-gonadal axis as a potential mechanism [13, 15]. Our present work yields novel insight into how bone might be directly regulated by the nervous system, which in turn has possible clinical applications. First, dysregulated OC formation or activity can cause abnormal bone homeostasis. Excessive OC formation and activity are associated with osteoporosis, peri-implant osteolysis, and Paget’s disease of bone. Our finding that D2R can inhibit osteoclastogenesis highlight the potential of using D2R agonists as novel drug therapies for these metabolic bone diseases. Secondly, the same D2R-CREB signaling pathway has been delineated for the regulation of synaptic function in neurons and suggest that cells in the nervous and skeletal systems utilize similar pathways to achieve different biological functions. This helps explain how abnormal bone loss might result as side-effects from psychiatric or neurological medications that modulate DA in the brain. Thirdly, because various resident cell types of the skeletal system (OCs, osteoblasts, chondrocytes, etc.) express receptors for neurotransmitters [39], our findings also support a hypothesis that the skeletal system is under tight regulation from the nervous system during growth, repair, remodeling, and aging. As such, this hypothesis predicts the existence of possible chemical mediators from bone, such as calcium released from bone matrix, that conversely act on neurons as part of a feedback system. Lastly, because DA and CREB also play critical roles in neuronal function, our data may help delineate a possible role for bone in maintaining normal function in the nervous system. Therefore, in addition to treating bone diseases, targeting bone dysfunction might constitute a novel approach to treating neurodegenerative diseases in the brain and eye.
5. Conclusion
In our study, we have identified D2R/cAMP/PKA/CREB as a candidate pathway that mediates DA’s regulation of OC differentiation. These findings will promote our understanding of how the nervous and skeletal systems interact to regulate bone remodeling by characterizing the effect of the neurotransmitter DA on OCs. Finally, our findings support a clinical role of using DA receptors agonists/antagonists in the treatment of bone metabolic diseases.
Supplementary Material
HIGHLIGHTS.
We identified a candidate pathway (D2R/cAMP/PKA/CREB) that mediates dopamine’s regulation of osteoclast differentiation.
Pharmacological activation of cAMP/PKA/CREB pathway reverses dopamine’s inhibition of osteoclastogenesis.
Dopamine and CREB may be promising mediators of neuronal regulation of bone remodeling.
Acknowledgements
This work was supported by Ohio State University College of Dentistry and the NIH/NIDCR grant (R01DE022816 to CCK).
Definition of Abbreviation
- CREB
cAMP-response element binding protein
- cAMP
cyclic adenosine monophosphate
- PKA
protein kinase A
- OC
osteoclasts
- DA
Dopamine
- D1R
D1-like receptors
- D2R
D2-like receptors
- RANKL
receptor activator of nuclear factor kappa-B ligand
- TRAP
tartrate-resistant acid phosphatase
- ERK1/2
extracellular signal-related kinase 1/2
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- AC
Adenylate cyclase
- RLU
relative light unit
- CRE
cAMP response element
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors have no conflicts of interest to disclose.
References
- [1].Zaidi M, Skeletal remodeling in health and disease, Nat Med 13(7) (2007) 791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- [2].Xiao W, Li S, Pacios S, Wang Y, Graves DT, Bone Remodeling Under Pathological Conditions, Front Oral Biol 18 (2016) 17–27. doi: 10.1159/000351896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rachner TD, Coleman R, Hadji P, Hofbauer LC, Bone health during endocrine therapy for cancer, Lancet Diabetes Endocrinol 6(11) (2018) 901–910. doi: 10.1016/S2213-8587(18)30047-0. [DOI] [PubMed] [Google Scholar]
- [4].Dirckx N, Moorer MC, Clemens TL, Riddle RC, The role of osteoblasts in energy homeostasis, Nat Rev Endocrinol 15(11) (2019) 651–665. doi: 10.1038/s41574-019-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maryanovich M, Takeishi S, Frenette PS, Neural Regulation of Bone and Bone Marrow, Cold Spring Harb Perspect Med 8(9) (2018). doi: 10.1101/cshperspect.a031344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosen CJ, Bone remodeling, energy metabolism, and the molecular clock, Cell Metab 7(1) (2008) 7–10. doi: 10.1016/j.cmet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [7].Elefteriou F, Regulation of bone remodeling by the central and peripheral nervous system, Arch Biochem Biophys 473(2) (2008) 231–236. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zengin A, Zhang L, Herzog H, Baldock PA, Sainsbury A, Neuropeptide Y and sex hormone interactions in humoral and neuronal regulation of bone and fat, Trends Endocrinol Metab 21 (7) (2010) 411–418. doi: 10.1016/j.tem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- [9].Huang S, Li Z, Liu Y, Gao D, Zhang X, Hao J, Yang F, Neural regulation of bone remodeling: Identifying novel neural molecules and pathways between brain and bone, J Cell Physiol 234(5) (2019) 5466–5477. doi: 10.1002/jcp.26502. [DOI] [PubMed] [Google Scholar]
- [10].Berke JD, What does dopamine mean?, Nat Neurosci 21(6) (2018) 787–793. doi: 10.1038/s41593-018-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beaulieu JM, Espinoza S, Gainetdinov RR, Dopamine receptors - IUPHAR Review 13, Br J Pharmacol 172(1) (2015) 1–23. doi: 10.111/bph.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iacovazzo D, De Marinis L, Treatment of hyperprolactinemia in post-menopausal women: pros, Endocrine 48(1) (2015) 76–78. doi: 10.1007/s12020-014-0377-9. [DOI] [PubMed] [Google Scholar]
- [13].Torsney KM, Noyce AJ, Doherty KM, Bestwick JP, Dobson R, Lees AJ, Bone health in Parkinson’s disease: a systematic review and meta-analysis, J Neurol Neurosurg Psychiatry 85(10) (2014) 1159–1166. doi: 10.1136/jnnp-2013-307307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Handa K, Kiyohara S, Yamakawa T, Ishikawa K, Hosonuma M, Sakai N, Karakawa A, Chatani M, Tsuji M, Inagaki K, Kiuchi Y, Takami M, Negishi-Koga T, Bone loss caused by dopaminergic degeneration and levodopa treatment in Parkinson’s disease model mice, Sci Rep 9(1) (2019) 13768. doi: 10.1038/s41598-019-50336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Calarge CA, Ivins SD, Motyl KJ, Shibli-Rahhal AA, Bliziotes MM, Schlechte JA, Possible mechanisms for the skeletal effects of antipsychotics in children and adolescents, Ther Adv Psychopharmacol 3(5) (2013) 278–293. doi: 10.1177/2045125313487548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee DJ, Tseng HC, Wong SW, Wang Z, Deng M, Ko CC, Dopaminergic effects on in vitro osteogenesis, Bone Res 3 (2015) 15020. doi: 10.1038/boneres.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen S, Bai B, Lee DJ, Diachina S, Li Y, Wong SW, Wang Z, Tseng HC, Ko CC, Dopaminergic enhancement of cellular adhesion in bone marrow derived mesenchymal stem cells (MSCs), J Stem Cell Res Ther 7(8) (2017). doi: 10.4172/2157-7633.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hanami K, Nakano K, Saito K, Okada Y, Yamaoka K, Kubo S, Kondo M, Tanaka Y, Dopamine D2-like receptor signaling suppresses human osteoclastogenesis, Bone 56(1) (2013) 1–8. doi: 10.1016/j.bone.2013.04.019. [DOI] [PubMed] [Google Scholar]
- [19].Yang H, Xu Y, Zhu M, Gu Y, Zhang W, Shao H, Wang Y, Ping Z, Hu X, Wang L, Geng D, Inhibition of titanium-particle-induced inflammatory osteolysis after local administration of dopamine and suppression of osteoclastogenesis via D2-like receptor signaling pathway, Biomaterials 80 (2016) 1–10. doi: 10.1016/j.biomaterials.2015.11.046. [DOI] [PubMed] [Google Scholar]
- [20].Nakashioya H, Nakano K, Watanabe N, Miyasaka N, Matsushita S, Kohsaka H, Therapeutic effect of D1-like dopamine receptor antagonist on collagen-induced arthritis of mice, Mod Rheumatol 21(3) (2011) 260–266. doi: 10.1007/s10165-010-0387-2. [DOI] [PubMed] [Google Scholar]
- [21].Koga Y, Tsurumaki H, Aoki-Saito H, Sato M, Yatomi M, Takehara K, Hisada T, Roles of Cyclic AMP Response Element Binding Activation in the ERK1/2 and p38 MAPK Signalling Pathway in Central Nervous System, Cardiovascular System, Osteoclast Differentiation and Mucin and Cytokine Production, Int J Mol Sci 20(6) (2019). doi: 10.3390/ijms20061346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wong SW, Huang BW, Hu X, Ho Kim E, Kolb JP, Padilla RJ, Xue P, Wang L, Oguin TH 3rd, Miguez PA, Tseng HC, Ko CC, Martinez J, Global deletion of Optineurin results in altered type I IFN signaling and abnormal bone remodeling in a model of Page’s disease, Cell Death Differ 27(1) (2020) 71–84. doi: 10.1038/s41418-019-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vesprey A, Yang W, Pit Assay to Measure the Bone Resorptive Activity of Bone Marrow-derived Osteoclasts, Bio Protoc 6(12) (2016). doi: 10.21769/BioProtoc.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang H, Xu J, Lazarovici P, Quirion R, Zheng W, cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia, Front Mol Neurosci 11 (2018) 255. doi: 10.3389/fnmol.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bibb JA, Decoding dopamine signaling, Cell 122(2) (2005) 153–155. doi: 10.1016/j.cell.2005.07.011. [DOI] [PubMed] [Google Scholar]
- [26].Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J, Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation, J Neurosci 22(20) (2002) 8911–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yan Z, Feng J, Fienberg AA, Greengard P, D(2) dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons, Proc Natl Acad Sci U S A 96(20) (1999) 11607–11612. doi: 10.1073/pnas.96.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grimes MT, Powell M, Gutierrez SM, Darby-King A, Harley CW, McLean JH, Epac activation initiates associative odor preference memories in the rat pup, Learn Mem 22(2) (2015) 74–82. doi: 10.1101/lm.037101.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Matt SM, Gaskill PJ, Where Is Dopamine and how do Immune Cells See it?: Dopamine-Mediated Immune Cell Function in Health and Disease, J Neuroimmune Pharmacol 15(1) (2020) 114–164. doi: 10.1007/s11481-019-09851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Serre CM, Farlay D, Delmas PD, Chenu C, Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers, Bone 25(6) (1999) 623–629. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- [31].Maestroni GJ, Cosentino M, Marino F, Togni M, Conti A, Lecchini S, Frigo G, Neural and endogenous catecholamines in the bone marrow. Circadian association of norepinephrine with hematopoiesis?, Exp Hematol 26(12) (1998) 1172–1177. [PubMed] [Google Scholar]
- [32].Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S, Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization, J Clin Invest 118(4) (2008) 1380–1389. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Motyl KJ, Beauchemin M, Barlow D, Le PT, Nagano K, Treyball A, Contractor A, Baron R, Rosen CJ, Houseknecht KL, A novel role for dopamine signaling in the pathogenesis of bone loss from the atypical antipsychotic drug risperidone in female mice, Bone 103 (2017) 168–176. doi: 10.1016/j.bone.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Minami K, Liu S, Liu Y, Chen A, Wan Q, Na S, Li BY, Matsuura N, Koizumi M, Yin Y, Gan L, Xu A, Li J, Nakshatri H, Yokota H, Inhibitory Effects of Dopamine Receptor D1 Agonist on Mammary Tumor and Bone Metastasis, Sci Rep 7 (2017) 45686. doi: 10.1038/srep45686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yoon SH, Ryu J, Lee Y, Lee ZH, Kim HH, Adenylate cyclase and calmodulin-dependent kinase have opposite effects on osteoclastogenesis by regulating the PKA-NFATc1 pathway, J Bone Miner Res 26(6) (2011) 1217–1229. doi: 10.1002/jbmr.310. [DOI] [PubMed] [Google Scholar]
- [36].Weivoda MM, Ruan M, Hachfeld CM, Pederson L, Howe A, Davey RA, Zajac JD, Kobayashi Y, Williams BO, Westendorf JJ, Khosla S, Oursler MJ, Wnt Signaling Inhibits Osteoclast Differentiation by Activating Canonical and Noncanonical cAMP/PKA Pathways, J Bone Miner Res 31(1) (2016) 65–75. doi: 10.1002/jbmr.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ramaswamy G, Kim H, Zhang D, Lounev V, Wu JY, Choi Y, Kaplan FS, Pignolo RJ, Shore EM, Gsalpha Controls Cortical Bone Quality by Regulating Osteoclast Differentiation via cAMP/PKA and beta-Catenin Pathways, Sci Rep 7 (2017) 45140. doi: 10.1038/srep45140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T, Kodama T, Chatila TA, Bito H, Takayanagi H, Regulation of osteoclast differentiation and function by the CaMK-CREB pathway, Nat Med 12(12) (2006) 1410–1416. doi: 10.1038/nm1515. [DOI] [PubMed] [Google Scholar]
- [39].Grassel SG, The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology, Arthritis Res Ther 16(6) (2014) 485. doi: 10.1186/s13075-014-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


