Abstract
Background and Aims
Guillain Barré syndrome (GBS) could be triggered by an infectious disease but by vaccination as well. Thus, suffering GBS may influence patients' attitudes towards vaccination.
Methods
An anonymous questionnaire consisting of the Overall Neuropathy Limitations Score (ONLS), the short form‐36 health survey (SF‐36), and questions addressing patients' attitude towards vaccination was sent to members of a German GBS support group and to patients with GBS diagnosis who were treated at Jena University Hospital.
Results
Ninety‐seven questionnaires clearly stated GBS as a diagnosis and were included in the analysis. Although 19.6% of the GBS patients reported having no disability in the long‐time follow‐up, a considerable number of patients still had persistent neurological symptoms; 74.2% of the GBS patients reported being able to walk at least 10 m independently. However, 5.2% were restricted to wheelchair. The patients reached lower scores in all domains of quality of life compared to German controls. Moreover, patients showed a more critical attitude towards vaccination compared to a German representative survey. Fewer patients (58.8%) received a vaccination after suffering from GBS than before (77.3%). Every tenth patient believed that vaccination was the trigger for the GBS. 32% of the patients did not receive a vaccination in the last 5 years mainly because of the fear of adverse effects (32%) or disadvise of the general practitioners (25.8%).
Discussion
Although the risk of relapse following immunization may be rather low, uncertainties and fears still impair the counseling of these patients by their medical practitioner.
Keywords: attitude, Guillain Barré syndrome, outcome, quality of life, vaccination
1. INTRODUCTION
Guillain‐Barré syndrome (GBS) is an acute inflammatory disease of nerve roots and peripheral nerves. It is characterized by rapidly progressive, symmetrical weakness of the extremities. 1 , 2 , 3 However, several atypical variants exist. A worldwide epidemiological systematic review showed an overall incidence of GBS between 1.1 and 1.8/100 000/year. 4 Plasma exchange and intravenous immunoglobulin are proven effective treatments for GBS. 5 About 25% of patients develop respiratory insufficiency, 6 and many show signs of autonomic dysfunction 7 that mainly demands intensive care treatment. GBS is typically triggered by an infectious disease and may be associated with various pathogens (eg, campylobacter jejuni, cytomegalovirus, Epstein‐Barr virus, mycoplasma pneumonia, haemophilus influenzae, influenza A virus, and others). In addition, GBS was also found in patients with COVID‐19, 8 , 9 , 10 , 11 but also influenza vaccination, trauma, surgical intervention, and others may be a potential trigger to develop GBS.
Functional outcome is associated with the amount of axonal damage. 12 , 13 , 14 Incomplete recovery is mainly caused by residual neuropathy affecting various parts of the peripheral nervous system after the acute phase of GBS,15 and therefore, may cause significant impairment of quality of life. 16 However, functional recovery may be heterogeneous due to heterogeneity in pathophysiology, severity, duration of the disease, beginning of treatment, and individual comorbidities of the patients.
In addition, GBS has been generally considered to be a vaccine‐associated adverse event 17 as vaccination may potentially trigger GBS. Most data about vaccine‐associated GBS is available in the literature concerning seasonal flu vaccines. The controversial discussion about vaccination and GBS mainly is founded on the finding of an increased risk of vaccination‐associated GBS after the swine flu vaccinations in 1976. 18 In addition, a meta‐analysis 19 based on six adverse event monitoring systems with about 23 million vaccinated people showed that Influenza A (H1N1) 2009 vaccines were associated with a small increased risk of GBS. However, at the end of the 2009 pandemic, the cumulative GBS risk was less among the pH1N1vaccinated than the unvaccinated population, rather suggesting a benefit of vaccination as it relates to GBS. 20
Nevertheless, patients may fear potential recurrences of GBS following vaccination, 21 which, in turn, may influence patients' behavior and attitude relating to vaccination and introduce uncertainties in the counseling of these patients by the general practitioner. This becomes increasingly relevant in the focus of actual COVID‐19 vaccination programs, although COVID vaccination was not available at the timepoint of this survey. Here, we performed a survey focusing on the attitude towards vaccination of patients after suffering from GBS.
2. METHODS
An anonymous questionnaire was designed, which included the following subsets: General information of the patients were collected (age, gender, year of the disease onset, diagnosis).
Questions relating to the attitude towards vaccination were partly taken from a German representative survey of prevention of infection, 22 which is an opinion survey of 5054 interviewees to attitude, knowledge, and behavior regarding vaccination. In addition, some GBS‐specific questions were designed to evaluate a possible influence of the GBS towards the attitude concerning vaccination. The questions used in the questionnaire are collected in Table 1.
TABLE 1.
Questions concerning vaccination (English translation)
| Questions from the German representative survey of prevention of infection 22 |
|
| Questions related to GBS |
|
For evaluating the disability of the patients due to neuropathic symptoms, the ONLS (Overall Neuropathy Limitations Score) was used. 23 The ONLS is a scale that measures limitations in the everyday activities of the upper and lower limbs, and therefore, focuses on daily relevant activities. It is validated as an observed measure by clinicians watching patients perform the tasks. 23 In this study, the patients were asked to evaluate their abilities by themselves or by their relatives. Quality of life was evaluated using the short form‐36 health survey (SF‐36). 24 , 25 The SF‐36 is a validated patient‐reported survey of patient health and comprises eight domains (physical functioning, role physical, bodily pain, general health perception, vitality, social functioning, role emotional, mental health).
The study was approved by the local ethics committee (ethics committee of the Friedrich‐Schiller‐University Jena, number 2020‐1649‐Bef). The questionnaire was enclosed to the “GBS magazine” in March 2020, which is a German journal quarterly sent to the members of the Bundesverband deutsche GBS‐Vereinigung e.V. (German federation of GBS association) and Deutsche GBS‐Stiftung (German GBS foundation). The journal is sent to about 400 members of the self‐help group consisting of affected persons who have suffered from GBS, but also of relatives of GBS patients or other supporters. Therefore, it cannot be estimated how many former GBS patients finally received the questionnaire via the journal. In addition, the questionnaire was sent to 218 patients with GBS treated in the Department of Neurology at Jena Universital Hospital between 2010 and 2019.
All statistical analyses were performed using SPSS 27.0 (SPSS Inc., Chicago, IL, USA). Differences in self‐reported features between groups were calculated using the t‐test with a two‐sided significance level of P < .05%.
Missing values in the SF‐36 questionnaire were substituted with person‐specific estimates if the respondent answered at least 50% of the items in a domain according to the half‐scale rule from the SF‐36 developers. 26 The raw data of the SF‐36 were transformed to z‐scores based on a German normative sample. 24 A z‐score describes the position of a raw score in terms of its distance from the mean when measured in SD units. The z‐score is positive if the value lies above the mean and negative if it lies below the mean.
3. RESULTS
Totally 130 patients filled out and returned the questionnaires, but only in 97 questionnaires, GBS was clearly stated as a diagnosis and could finally be included in the analysis. CIDP was stated in 26 cases as diagnosis, and 7 did not state a diagnosis at all so that these questionnaires were excluded from the analysis. The basic characteristics of the patients can be seen in Table 2.
TABLE 2.
Baseline characteristics of the patients
| All GBS | Contacted via GBS magazine (patients in GBS support group) | Contacted via regular mail (former patients of Jena University Hospital) | |
|---|---|---|---|
| Number | 97 | 56 | 41 |
| Gender, male:female | 61.5%:38.5% | 58.2%:41.8% | 65%:35% |
| Age | 67.3 ± 14.3 | 66.6 ± 15.7 | 68.6 ± 12.3 |
| Years from onset of symptoms | 12.7 ± 9.5 | 17.0 ± 9.8 | 7.3 ± 5.5 |
3.1. Attitude towards vaccination
Totally 77.3% (n = 75) received a vaccination before suffering from GBS, but only 58.8% (n = 57) were vaccinated after GBS. 11.3% (n = 11) believe that a vaccination has triggered the GBS and 38.1% (n = 37) reported to be anxious that a vaccination may trigger a recurrence of the GBS.
Compared to the results of the German representative study, 22 where 5054 people were interviewed for attitude, knowledge, and behavior with regard to vaccination, the GBS patients of our study showed a more critical attitude towards vaccination (Figure 1); 25% of the GBS patients reported being deprecatory or rather deprecatory against vaccination. Comparing the patients from the self‐help group with the former hospital patients showed a significant difference (Chi‐square test, P = .043) implying that the patients from the self‐help group showed the most critical attitude against vaccination (34% vs 15% deprecatory or rather deprecatory). Further results are shown in Figure 2.
FIGURE 1.
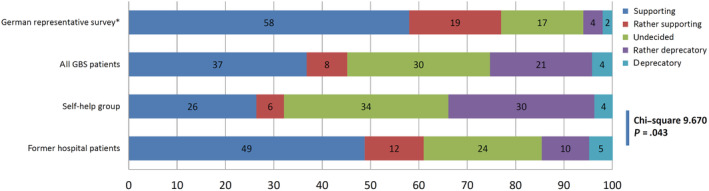
Attitude towards vaccination in the GBS patient group and in the German representative survey 22
FIGURE 2.

Additional questions regarding patients' attitude towards vaccination
Of the GBS patients, 36.1% (n = 35) reported having taken advice regarding vaccination in the last 2 years; 68% have been vaccinated in the last 5 years, in most cases, against tetanus or seasonal flu. There was no correlation with age (Table 3). Regional differences were not collected and could not be analyzed. Table 3 gives an overview of the reasons why GBS patients were not vaccinated. Patients reported as reasons not to receive a vaccination in 32% of cases about the fear of adverse effects and that in 25.8% of cases, the medical doctor has disadvised vaccination.
TABLE 3.
Vaccinations in the last 5 y
| 68% (n = 66) were vaccinated in the last 5 y | 32% were not vaccinated in the last 5 y |
|---|---|
Vaccination received:
|
Reasons:
|
| Age group | Vaccinated | Age group | Not vaccinated |
|---|---|---|---|
| <20 | 2 | <20 | 0 |
| 20‐39 | 3 | 20‐39 | 0 |
| 40‐49 | 1 | 40‐49 | 0 |
| 50‐59 | 14 | 50‐59 | 8 |
| 60‐69 | 13 | 60‐69 | 8 |
| 70‐79 | 22 | 70‐79 | 9 |
| 80‐89 | 10 | 80‐89 | 4 |
| ≥90 | 1 | ≥90 | 2 |
Note: Pearson's chi‐squared test 5.236; exact significance P = .679.
3.2. Disability and quality of life
Figure 3A shows the histograms of ONLS scores reached by the patients after GBS; 19.6% of the GBS patients were reported to have no disability (total score of 0); 21.6% reached a total score of 4, which represents a medium disability; 35.1% reported a leg scale of 2 (walks independently but gait looks abnormal), and 34% reported an arm scale of 2 (disability in one or both arms affecting but not preventing any of the functions listed); 12.5% reported a total score larger than 5 with rather significant disability.
FIGURE 3.

(A) Histograms of ONLS scores of the GBS patients. (B) Quality of life (SF‐36)
All patients scored lower in all items of the SF‐36 when compared with the normal German population. 24 No statistically significant differences (t‐test) in the SF‐36 scores were detected between patients from the support group and the former patients of the university hospital. Figure 3B shows the results of the SF‐36 survey. The ONLS score showed the highest Pearson correlation coefficients to physical functioning (−0.689, P < .001) and role physical (−0.595, P < .001).
3.3. Correlations
The semiquantitative scores for the attitude towards vaccination were weakly correlated to the ONLS score (Pearson correlation 0.219, P = .33, Figure 4), while no significance was found for a correlation to age or the SF‐36 scores.
FIGURE 4.

Attitude towards vaccination in correlation to the total ONLS score
4. DISCUSSION
In our study, 39% (n = 48) reported that they are anxious that vaccination may trigger a recurrence of the disease. GBS patients showed a more critical attitude towards vaccination compared to the German representative study; 22 25% of the patients were quoted to be deprecatory or rather deprecatory vs 6% in the representative study; 45% supported or rather supported vaccination vs 77% in the representative study. Consequently, the number of vaccinations was lower after GBS. Patients reported the reasons to not receive a vaccination, in 32% of cases, as the fear of adverse effects, and in 25.8% of cases that the medical doctor has disadvised vaccination. This is so far remarkable as there may apparently be a certain degree of uncertainty in medical doctors, too.
In contrast, large epidemiological studies have shown that GBS rates after the influenza vaccine have been less than one case per million vaccinated people. 27 In addition, influenza vaccine‐induced relapse of GBS also is evaluated as to be extremely seldom, so that prior GBS should not preclude influenza vaccination. 28 Even the risk of developing a GBS relapse is very low after mRNA COVID‐19 vaccine application. 29 Thus, it can be concluded that also post‐GBS patients can be vaccinated safely.
A comparison of a smallpox vaccination program in New York City in 1947 with the swine influenza immunization program in 1976 revealed that public compliance with mass immunization is strongly influenced by the perception of health threats and the fear of vaccine‐associated risks. 30
A meta‐analysis 31 of 39 studies of interest, published between 1981 and 2014, points to a small but statistically significant association between influenza vaccines and GBS. But a recent systematic review and meta‐analysis 17 based on 22 eligible epidemiological studies from 1981 to 2019 pointed to no risk of vaccine‐associated GBS, while an obvious high risk of GBS was observed in patients with previous influenza‐like illnesses. In this study, vaccination against seasonal influenza reduced the risk of developing influenza‐like illness‐associated GBS by about 88%.
It has been stated that the risk of GBS is 4‐7 times higher after influenza infection than after influenza vaccine. 32 Less than 1 case of GBS per million immunized persons might occur for these vaccines. 33 Weighing up the hypothesized risks of adverse events, such as GBS, and the beneficial effects of vaccination, it can be argued that the potential risk to develop GBS cannot be considered a valid reason to avoid the administration of currently recommended vaccines. 33
A major concern is that vaccination may trigger a relapse of GBS. Recently, a large population‐based nested case‐control study found no evidence that demonstrated an association of vaccines with an increased risk of GBS and its recurrence within the 180 days following vaccinations. 34
A questionnaire‐based survey demonstrated only 11 of 311 patients with GBS (3.5%) who had been immunized after having the disease reported a recurrence of symptoms. 35 This audit of patients with GBS and CIDP who have received vaccines suggests that the risk of relapse following immunization is low. A second study found that none of 106 GBS patients who received a flu vaccination (range 1‐37 times, in total 775 vaccinations) in the years after they experienced GBS reported a recurrence of GBS. 21 Moreover, Baxter et al 36 found no evidence that vaccination is associated with recurrent GBS based on an analysis of a large database with 550 identified GBS cases of over 33 million person‐years. Following their GBS diagnoses, 989 vaccines were given to 279 of these individuals.
Although the risk of vaccination‐associated relapse of GBS is very difficult to be assessed, all of the studies point to a very low risk of relapse caused by vaccination. Thus, the practical guideline regarding vaccination of patients with a history of GBS as used in the Netherlands 7 states that vaccination seems to be safe in patients who developed the GBS later than 3 months ago and when the onset of GBS was not shortly after vaccination.
This survey was done at a time point when the COVID‐19 pandemic had led to the first lockdown in Germany, but no COVID‐19 vaccine was available in Germany at that time. Nevertheless, the rapid development of the pandemic may have significantly influenced the general attitude towards vaccination. In the meantime, it is known that COVID‐19 infection may trigger GBS. 37 , 38 Also in addition, different kinds of COVID‐19 vaccines have been reported to have the potential to trigger GBS. 39 , 40 , 41 , 42
Although there is some uncertainty and controversy about this issue, the association between COVID‐19 and GBS is very low. In addition, the association between the COVID‐19 vaccine and GBS is even less. After mRNA COVID‐19 vaccine application to 702 previously diagnosed cases of GBS, only one patient showed a transient flare of symptoms leading to hospital admission. 29
However, it is most probable that similar concerns will also be discussed regarding COVID‐19 vaccination to trigger GBS. However, in the background of the threat of health and life by the COVID pandemic, the risk to develop GBS after COVID‐19 vaccination by far cannot outweigh its benefit. 43 Further follow‐up studies concerning COVID‐19 vaccine‐associated GBS remain to be seen.
The assessment of long‐term outcomes and quality of life in our patients was performed in order to relate it to patients' attitudes towards vaccination. In our cross‐sectional survey time of follow‐up was heterogeneous with a mean of 12.7 years (and an SD of 9.7) after GBS, which is relatively long compared to previous studies. Here, 19.6% of the GBS patients reported to have no disability, and 21.6% reached a total ONLS score of 4, which represents a medium disability allowing for independent performance of daily activities. In addition, our patients scored lower in all items of the SF‐36 when compared with the normal German population.
In fact, there are many good population‐based studies available about disability 44 , 45 , 46 , 47 , 48 , 49 and quality of life 16 , 21 , 50 , 51 , 52 , 53 after GBS. The number of patients with poor functional outcomes differs between the studies 29 , 54 , 55 , 56 , 57 depending on the time interval after GBS, the definition of impaired function, symptoms analyzed, ICU treatment and necessity of mechanical ventilation, the studied population, and regional differences 44 as well.
Our study was partly based on questionnaires sent to patients who are members of a patient organization, which makes this study susceptible to recall and selection bias. However, no statistically significant differences have been found (t‐test) between patients contacted via patient organization (n = 71) and patients who were treated at Jena University Hospital (n = 48) neither in ONLS scores nor in SF‐36 scores. However, the patients from the self‐help group showed a more critical attitude towards vaccinations compared to the former hospital patients.
The questionnaires were sent to more than 800 persons, but less than 100 could be included in this study. This has led to a selection bias towards possibly more severe cases with unfavorable outcomes as the reported outcomes were more severe than in many of the population‐based outcome studies cited above. Thus, it has to be kept in mind that the data represent the attitude in a very selective group of patients.
5. CONCLUSION
Although 19.6% of the GBS patients were reported to have no disability in the long‐time follow‐up, a considerable number of patients still had persistent neurological symptoms. Moreover, the patients scored lower in all items of quality of life when compared with the normal German population.
In addition, the patients showed a more critical attitude towards vaccination compared to a German representative population; 32% of patients did not receive a vaccination in the last 5 years mainly because of the fear of adverse effects and disadvise of the general practitioner. However, the risk of a vaccination‐associated GBS, as well as the risk of a vaccination‐associated relapse of a GBS, is generally evaluated as rather low, and the benefit of the vaccination may outweigh its adverse effects.
Uncertainties and fears still impair the counselling of patients after GBS by their medical practitioner. In addition, functional disability and impaired quality of life still play important roles over a longer time and have to be addressed in the long‐time support of these patients.
FUNDING
No funding.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Conceptualization: Hubertus Axer, Ben‐Ole Holtz
Data Curation: Ben‐Ole Holtz
Formal Analysis: Hubertus Axer, Ben‐Ole Holtz
Investigation: Ben‐Ole Holtz
Methodology: Hubertus Axer, Ben‐Ole Holtz
Project Administration: Hubertus Axer
Resources: Hubertus Axer
Supervision: Hubertus Axer, Alexander Grimm
Writing – Original Draft: Hubertus Axer
Writing – Review and Editing: Ben‐Ole Holtz, Alexander Grimm
All authors have read and approved the final version of the manuscript.
Hubertus Axer has full access to all the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
We confirm that this manuscript is an honest, accurate, and transparent account of the study being reported, no important aspects of the study have been omitted, and all discrepancies from the study as planned have been explained.
ACKNOWLEDGEMENTS
The help of the Bundesverband deutsche GBS‐Vereinigung e.V. (German federation of GBS association) und Deutsche GBS‐Stiftung (German GBS foundation) is greatly acknowledged. Especially Peter Schmeißer and Andrea Funk have given great support to the project.
Holtz B‐O, Grimm A, Axer H. Patients' attitude towards vaccination after Guillain Barré syndrome. Health Sci Rep. 2021;4:e469. doi: 10.1002/hsr2.469
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Shahrizaila N, Lehmann HC, Kuwabara S. Guillain‐Barré syndrome. Lancet (Lond Engl.). 2021;397(10280):1214‐1228. [DOI] [PubMed] [Google Scholar]
- 2. Malek E, Salameh J. Guillain‐Barre Syndrome. Semin Neurol. 2019;39(5):589‐595. [DOI] [PubMed] [Google Scholar]
- 3. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain‐Barré syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain‐Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32(2):150‐163. [DOI] [PubMed] [Google Scholar]
- 5. Verboon C, Doets AY, Galassi G, et al; IGOS Consortium. Current treatment practice of Guillain‐Barré syndrome. Neurology. 2019;93(1):e59‐e76. [DOI] [PubMed] [Google Scholar]
- 6. de Boisanger L. Outcomes for patients with Guillain‐Barré syndrome requiring mechanical ventilation: a literature review. Ir J Med Sci. 2016;185(1):11‐15. [DOI] [PubMed] [Google Scholar]
- 7. van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain‐Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469‐482. [DOI] [PubMed] [Google Scholar]
- 8. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19(9):767‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alberti P, Beretta S, Piatti M, et al. Guillain‐Barré syndrome related to COVID‐19 infection. Neurol Neuroimmunol Neuroinflammation. 2020;7(4):e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toscano G, Palmerini F, Ravaglia S, et al. Guillain‐Barré Syndrome Associated with SARS‐CoV‐2. N Engl J Med. 2020;382(26):2574‐2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Svačina MKR, Kohle F, Sprenger A, Lehmann HC. Could symptom overlap of COVID‐19 and Guillain‐Barré syndrome mask an epidemiological association? J Neurol. 2021;268(10):3595‐3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shang P, Zhu M, Wang Y, et al. Axonal variants of Guillain‐Barré syndrome: an update. J Neurol. 2021;268(7):2402‐2419. [DOI] [PubMed] [Google Scholar]
- 13. Vedeler CA, Wik E, Nyland H. The long‐term prognosis of Guillain‐Barré syndrome. Evaluation of prognostic factors, including plasma exchange. Acta Neurol Scand. 1997;95(5):298‐302. [DOI] [PubMed] [Google Scholar]
- 14. Petzold A, Hinds N, Murray NMF, et al. CSF neurofilament levels: a potential prognostic marker in Guillain‐Barré syndrome. Neurology. 2006;67(6):1071‐1073. [DOI] [PubMed] [Google Scholar]
- 15. Dornonville de la Cour C, Jakobsen J. Residual neuropathy in long‐term population‐based follow‐up of Guillain‐Barré syndrome. Neurology. 2005;64(2):246‐253. [DOI] [PubMed] [Google Scholar]
- 16. Rudolph T, Larsen JP, Farbu E. The long‐term functional status in patients with Guillain‐Barré syndrome. Eur J Neurol. 2008;15(12):1332‐1337. [DOI] [PubMed] [Google Scholar]
- 17. Petráš M, Lesná IK, Dáňová J, Čelko AM. Is an increased risk of developing Guillain‐Barré syndrome associated with seasonal influenza vaccination? a systematic review and meta‐analysis. Vaccine. 2020;8(2):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schonberger LB, Bregman DJ, Sullivan‐Bolyai JZ, et al. Guillain‐Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110(2):105‐123. [DOI] [PubMed] [Google Scholar]
- 19. Salmon DA, Proschan M, Forshee R, et al; H1N1 GBS Meta‐Analysis Working Group. Association between Guillain‐Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta‐analysis. Lancet (Lond Engl). 2013;381(9876):1461‐1468. [DOI] [PubMed] [Google Scholar]
- 20. Vellozzi C, Iqbal S, Stewart B, Tokars J, DeStefano F. Cumulative risk of Guillain‐Barré syndrome among vaccinated and unvaccinated populations during the 2009 H1N1 influenza pandemic. Am J Public Health. 2014;104(4):696‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuitwaard K, Bos‐Eyssen ME, Blomkwist‐Markens PH, van Doorn PA. Recurrences, vaccinations and long‐term symptoms in GBS and CIDP. J Peripher Nerv Syst. 2009;14(4):310‐315. [DOI] [PubMed] [Google Scholar]
- 22. Horstkötter N, Müller U, Ommen O, et al. Einstellungen, Wissen Und Verhalten von Erwachsenen Und Eltern Gegenüber Impfungen – Ergebnisse Der Repräsentativbefragung 2018 Zum Infektionsschutz. BZgA‐Forschungsbericht. Köln, Germany: Bundeszentrale für gesundheitliche Aufklärung; 2019. [Google Scholar]
- 23. Graham RC, Hughes RAC. A modified peripheral neuropathy scale: the Overall Neuropathy Limitations Scale. J Neurol Neurosurg Psychiatry. 2006;77(8):973‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morfeld M, Kirchberger I, Bullinger M. SF‐36 ‐ Deutsche Version Des Short Form‐36 Health Survey: Manual. 2nd ed. Göttingen, Germany: Hogrefe; 2011. [Google Scholar]
- 25. Ware JJ, Kosinski M, Gandek B. SF‐36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Inc; 2005. [Google Scholar]
- 26. Ware JJ, Kosinski M, Bjorner J, Turner‐Bowket D, Gandek B, Maruish M. User's Manual for the SF‐36v2 Health Survey. Lincoln, RI: Quality Metric Inc; 2007. [Google Scholar]
- 27. Lehmann HC, Hartung H‐P, Kieseier BC, Hughes RAC. Guillain‐Barré syndrome after exposure to influenza virus. Lancet Infect Dis. 2010;10(9):643‐651. [DOI] [PubMed] [Google Scholar]
- 28. Wijdicks EF, Fletcher DD, Lawn ND. Influenza vaccine and the risk of relapse of Guillain‐Barré syndrome. Neurology. 2000;55(3):452‐453. [DOI] [PubMed] [Google Scholar]
- 29. Shapiro Ben David S, Potasman I, Rahamim‐Cohen D. Rate of recurrent Guillain‐Barré syndrome after mRNA COVID‐19 vaccine BNT162b2. JAMA Neurol. 2021;78(11):1409‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imperato PJ. Reflections on New York City's 1947 Smallpox Vaccination Program and Its 1976 Swine Influenza Immunization Program. J Community Health. 2015;40(3):581‐596. [DOI] [PubMed] [Google Scholar]
- 31. Martín Arias LH, Sanz R, Sáinz M, Treceño C, Carvajal A. Guillain‐Barré syndrome and influenza vaccines: a meta‐analysis. Vaccine. 2015;33(31):3773‐3778. [DOI] [PubMed] [Google Scholar]
- 32. Poland GA, Jacobsen SJ. Influenza vaccine, Guillain‐Barré syndrome, and chasing zero. Vaccine. 2012;30(40):5801‐5803. [DOI] [PubMed] [Google Scholar]
- 33. Principi N, Esposito S. Vaccine‐preventable diseases, vaccines and Guillain‐Barre' syndrome. Vaccine. 2019;37(37):5544‐5550. [DOI] [PubMed] [Google Scholar]
- 34. Chen Y, Zhang J, Chu X, Xu Y, Ma F. Vaccines and the risk of Guillain‐Barré syndrome. Eur J Epidemiol. 2020;35(4):363‐370. [DOI] [PubMed] [Google Scholar]
- 35. Pritchard J, Mukherjee R, Hughes RAC. Risk of relapse of Guillain‐Barré syndrome or chronic inflammatory demyelinating polyradiculoneuropathy following immunisation. J Neurol Neurosurg Psychiatry. 2002;73(3):348‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP, CISA Network . Recurrent Guillain‐Barre syndrome following vaccination. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2012;54:800‐804. doi: 10.1093/cid/cir960 [DOI] [PubMed] [Google Scholar]
- 37. Sansone P, Giaccari LG, Aurilio C, et al. Post‐infectious Guillain‐Barré syndrome related to SARS‐CoV‐2 infection: a systematic review. Life (Basel Switz). 2021;11(2):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khan F, Sharma P, Pandey S, et al. COVID‐19‐associated Guillain‐Barre syndrome: postinfectious alone or neuroinvasive too? J Med Virol. 2021;93(10):6045‐6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maramattom BV, Krishnan P, Paul R, et al. Guillain‐Barré Syndrome following ChAdOx1‐S/nCoV‐19 vaccine. Ann Neurol. 2021;90(2):312‐314. [DOI] [PubMed] [Google Scholar]
- 40. Hasan T, Khan M, Khan F, Hamza G. Case of Guillain‐Barré syndrome following COVID‐19 vaccine. BMJ Case Rep. 2021;14(6):e243629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Finsterer J. Exacerbating Guillain‐Barré syndrome eight days after vector‐based COVID‐19 vaccination. Case Rep Infect Dis. 2021;2021:3619131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID‐19: Guillain‐Barre syndrome following pfizer COVID‐19 vaccine. Cureus. 2021;13(2):e13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koike H, Chiba A, Katsuno M. Emerging infection, vaccination, and Guillain‐Barré syndrome: a review. Neurol Ther. 2021;10(2):523‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doets AY, Verboon C, van den Berg B, et al. Regional variation of Guillain‐Barré syndrome. Brain: J Neurol. 2018;141(10):2866‐2877. [DOI] [PubMed] [Google Scholar]
- 45. Fletcher DD, Lawn ND, Wolter TD, Wijdicks EF. Long‐term outcome in patients with Guillain‐Barré syndrome requiring mechanical ventilation. Neurology. 2000;54(12):2311‐2315. [DOI] [PubMed] [Google Scholar]
- 46. Cheng B‐C, Chang W‐N, Chen J‐B, et al. Long‐term prognosis for Guillain‐Barré syndrome: evaluation of prognostic factors and clinical experience of automated double filtration plasmapheresis. J Clin Apher. 2003;18(4):175‐180. [DOI] [PubMed] [Google Scholar]
- 47. Chiò A, Cocito D, Leone M, et al; Piemonte and Valle d'Aosta Register for Guillain‐Barré Syndrome. Guillain‐Barré syndrome: a prospective, population‐based incidence and outcome survey. Neurology. 2003;60(7):1146‐1150. [DOI] [PubMed] [Google Scholar]
- 48. Hiraga A, Mori M, Ogawara K, et al. Recovery patterns and long term prognosis for axonal Guillain‐Barré syndrome. J Neurol Neurosurg Psychiatry. 2005;76(5):719‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dhar R, Stitt L, Hahn AF. The morbidity and outcome of patients with Guillain‐Barré syndrome admitted to the intensive care unit. J Neurol Sci. 2008;264(1‐2):121‐128. [DOI] [PubMed] [Google Scholar]
- 50. Merkies ISJ, Schmitz PIM, van der Meché FGA, Samijn JPA, van Doorn PA, Inflammatory Neuropathy Cause and Treatment (INCAT) group . Quality of life complements traditional outcome measures in immune‐mediated polyneuropathies. Neurology. 2002;59(1):84‐91. [DOI] [PubMed] [Google Scholar]
- 51. Djordjevic G, Stojanov A, Bozovic I, et al. Six‐month prospective study of quality of life in Guillain‐Barre syndrome. Acta Neurol Scand. 2020;141(3):236‐241. [DOI] [PubMed] [Google Scholar]
- 52. Berisavac I, Arsenijevic M, Bozovic I, et al. Disability and quality of life in Guillain‐Barré syndrome ‐ Longitudinal study. J Clin Neurosci. 2020;78:185‐188. [DOI] [PubMed] [Google Scholar]
- 53. Witsch J, Galldiks N, Bender A, et al. Long‐term outcome in patients with Guillain‐Barré syndrome requiring mechanical ventilation. J Neurol. 2013;260(5):1367‐1374. [DOI] [PubMed] [Google Scholar]
- 54. van den Berg B, Storm EF, Garssen MJP, Blomkwist‐Markens PH, Jacobs BC. Clinical outcome of Guillain‐Barré syndrome after prolonged mechanical ventilation. J Neurol Neurosurg Psychiatry. 2018;89(9):949‐954. [DOI] [PubMed] [Google Scholar]
- 55. Martic V, Bozovic I, Berisavac I, et al. Three‐year follow‐up study in patients with Guillain‐Barré syndrome. Can J Neurol Sci. 2018;45(3):269‐274. [DOI] [PubMed] [Google Scholar]
- 56. Koeppen S, Kraywinkel K, Wessendorf TE, et al. Long‐term outcome of Guillain‐Barré syndrome. Neurocrit Care. 2006;5(3):235‐242. [DOI] [PubMed] [Google Scholar]
- 57. Bersano A, Carpo M, Allaria S, Franciotta D, Citterio A, Nobile‐Orazio E. Long term disability and social status change after Guillain‐Barré syndrome. J Neurol. 2006;253(2):214‐218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


