PURPOSE
Emerging evidence suggests a correlation between the tumor mutational burden (TMB) and the response to programmed cell death-1 protein (PD-1) monotherapy across multiple cancer types. In skin cancers, as high TMB is mostly because of ultraviolet (UV) exposure, we hypothesized a correlation between the primary melanoma cutaneous location according to sun exposure and response to anti–PD-1 monotherapy.
METHODS
The aim of this study was to analyze, in advanced melanoma, the relationship between TMB, locations according to sun exposure, and response to PD-1 inhibitors. We conducted a prospective multicentric analysis, by sequencing the most recent metastatic sample before PD-1 inhibitors using FoundationOne assay.
RESULTS
One hundred two patients were included, with TMB available for 94 cases. In univariate and multivariate linear regression, TMB was significantly associated with sun-exposed areas of the primary melanoma location and with age (coefficients of the association with log-TMB: non-UV location, –1.05; chronic sun-exposed area, 1.12; P value for the location, < 10–5; age, 0.021 per year, P value for age, .002). Molecular UV signature present on the metastatic site was associated with higher TMB (P = .003). Melanomas bearing a high TMB had a higher probability of response to PD-1 inhibitors compared with melanomas with a low TMB, with a dose-dependent effect following an exponential curve and a negative odds ratio of 0.40 (95% CI, 0.20 to 0.72, P = .004) between log-TMB and 6-month progression.
CONCLUSION
Cumulative sun exposure related to skin location and molecular UV signature present on the metastatic site appear to be relevant biomarkers directly linked to TMB. Because TMB is not yet available to all for routine clinical use, the location of the primary melanoma in a sun-exposed area may play an important role in clinical decisions regarding therapeutic choice.
INTRODUCTION
Immune checkpoint inhibitors (ICIs), including programmed cell death-1 protein (PD-1)/programmed cell death-ligand-1 and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) inhibitors, have profoundly changed the prognosis of patients in multiple cancer types, including advanced melanoma.1,2 PD-1 inhibitors (pembrolizumab and nivolumab) have been approved by the US Food and Drug Administration for metastatic melanoma, and recently in the adjuvant setting in stage III melanoma.3,4 Although response rates (RR) are durable and remarkable, only approximately 35%-45% of patients benefit from these costly drugs, but no positive or negative predictive biomarkers are available to date to guide clinicians in the choice of specific therapies.1,2,5 Tumor mutational burden (TMB, also known as mutation load) has emerged as a seemingly promising predictive biomarker for ICIs6-8 and is likely to be incorporated into future treatment algorithms for these agents: It reflects the measure of the number of somatic protein-coding base substitution and insertion or deletion mutations occurring in a tumor specimen.9 Several prior studies have reported a positive predictive value on the efficacy of ICIs in high TMB tumors across multiple cancers, supporting the fact that neoantigen burden influences sensitivity to ICIs, but this measure is not available worldwide for routine clinical use yet.10-12
CONTEXT
Key Objective
Immune checkpoint inhibitors have profoundly changed the prognosis of patients in multiple cancer types including advanced melanoma; however, predictive biomarkers are needed. Tumor mutational burden (TMB) has emerged as a seemingly promising predictive biomarker but is not accessible to everyone because of no standardized technique.
Knowledge Generated
This study showed that the TMB is associated with the primary site of melanoma according to sun exposure, which in turn is a biomarker of response to immune checkpoint inhibitors. Location of primary melanoma could thus be used as an easy-to-use proxy of the TMB criterion.
Relevance
As long as TMB is not more widely available, age and sun-exposure pattern of the location of primary melanoma can help to make decisions in conjunction with TMB assessment.
In this current study, we analyzed clinical, histologic, and mutational data from patients with advanced melanoma to analyze whether TMB is associated with primary melanoma sun-exposed location, molecular ultraviolet (UV) signature, and response to PD-1 inhibitors.
METHODS
Study Design and Participants
A multicenter prospective study was performed in six academic institutions, hospitals, and cancer centers in France. Patients with advanced melanoma, who underwent a somatic comprehensive genomic FoundationOne assay9 (Foundation Medicine, Cambridge, MA) were prospectively included. Patients' clinicopathologic characteristics, tumor genomics results, and outcome data were collected. According to the location of the known primary melanoma, we allocated each patient to a group of sun-exposure pattern including chronically sun-exposed area such as head and neck, intermittently sun-exposed area such as trunk, arms, and legs, and sun-protected areas such as feet, soles, toes, genitals, mucosal, and uveal areas. Progression and tumor response to PD-1 inhibitors were assessed according to the RECIST guidelines version 1.1.13 Informed consent was obtained from all patients. The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines.
Next-Generation Sequencing–Based Assessment of Genomic Characteristics
TMB, UV signature, and microsatellite instability status were analyzed on the most recent metastatic sample of the patient available before PD-1 inhibitors. DNA was extracted from formalin-fixed, paraffin-embedded tissue sections to perform comprehensive genomic profiling with FoundationOne Assay in a Clinical Laboratory Improvement Amendments–certified, College of American Pathologists–accredited, New York State–approved laboratory (Foundation Medicine).14 TMB was calculated by counting the number of synonymous and nonsynonymous mutations (muts) on up to 1.2 megabases (Mb) of sequenced DNA, according to an algorithm that extrapolated to the genome as a whole (muts/Mb).9 UV signature is dominated by C > T or CC > TT transitions and was assigned by analysis of the trinucleotide context and profiled using the Sanger COSMIC signatures of mutational processes in human cancer, as described by Zehir et al.15 Samples were considered positive for UV signature if there was a ≥ 40% fit. UV signature was not analyzed in samples with < 10 assessable alterations.
Statistical Analysis
Multiple linear regression models were fit to estimate the TMB according to a set of characteristics of the melanoma and the subject, including the location according to sun exposure. To make the distribution of the TMB consistent with a normal distribution, the TMB was log-transformed (log-TMB). Two patients were excluded because of a TMB equal to zero mut/Mb. Univariate logistic regression analyses were performed to estimate the association between the 3-month and the 6-month progression and the log-TMB. Multivariate analysis was conducted to estimate the association between the 6-month progression and the log-TMB after adjustment for potential confounders. We added a sensitivity analysis excluding mucosal and uveal melanomas or adding combination of anti–CTLA-4 and anti–PD-1. TMB was compared between progressors and nonprogressors using a Mann-Whitney U test. For all statistical analyses, the type 1 error has been set at 5%. All data were analyzed using Rstudio Version 1.0.136 (Rstudio, Inc, Boston, MA).
RESULTS
Patients' Characteristics
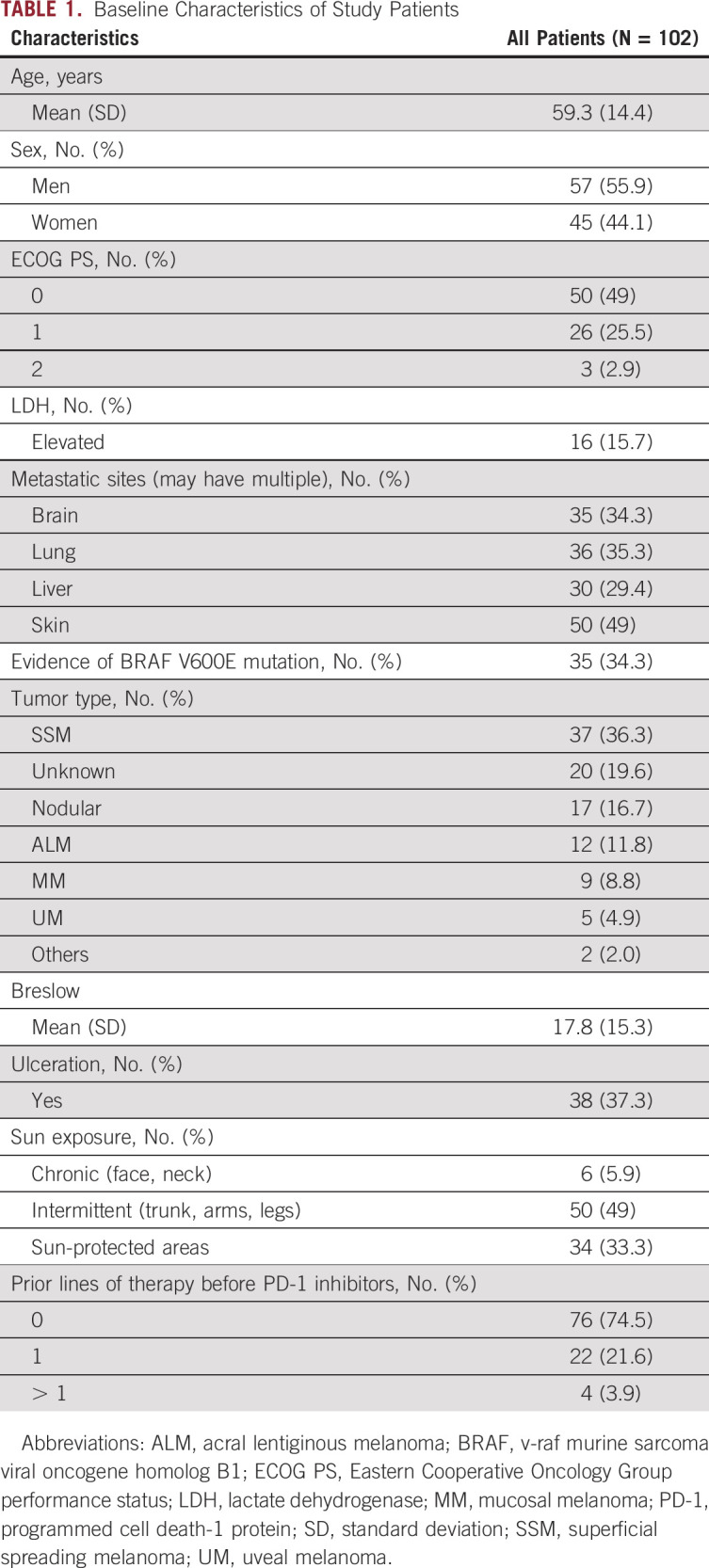
One hundred two patients were prospectively included from six French treatment centers: University Hospital of Bordeaux (n = 32), Gustave Roussy Institute (n = 21), University Hospital of Rennes (n = 18), University Hospital of Clermont-Ferrand (n = 17), Saint-Louis Hospital-University of Paris (n = 11), and University of Lille (n = 3), between October 2017 and June 2019 (Table 1). Median age at diagnosis was 59.3 years (standard deviation [SD] 14.4), with 57% of patients being male. Most melanomas were superficial spreading melanomas (n = 37; 36%), followed by unknown primary (n = 20; 19.6%) and nodular types (n = 17; 16.8%). Twelve (11.8%) were acral lentiginous melanomas, nine (8.8%) mucosal, five (5%) uveal, one naevocytoid, and one desmoplastic. The v-raf murine sarcoma viral oncogene homolog B1 (BRAF) V600E alterations were detected in 34 cases (34%). 80 patients were treated with anti–PD-1 monotherapy, which was the first-line treatment for 72.5% of them (n = 58). TMB was assessed in 94 cases (DNA extraction failure in 8 cases). Median TMB was 12.4 muts/Mb (SD, 12.6; range, 0-60 muts/Mb). Its distribution is depicted in the Data Supplement. Corresponding metastatic sites are presented in the Data Supplement. All melanomas were considered microsatellite instability–stable.
TABLE 1.
Baseline Characteristics of Study Patients
High TMB Is Associated With Older Age
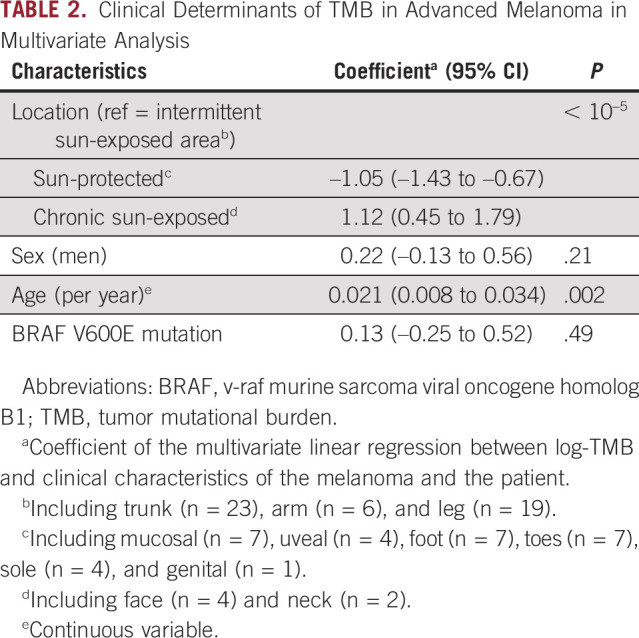
Advanced-age patients had higher TMB. The coefficient of age with log-TMB in a multivariate regression model was 0.021 (95% CI, 0.008 to 0.034, P = .002; Table 2). Sex was not associated with TMB (P = .21). BRAF V600E alterations were not statistically correlated with TMB (P = .38).
TABLE 2.
Clinical Determinants of TMB in Advanced Melanoma in Multivariate Analysis
High TMB Is Associated With Sun-Exposed Location
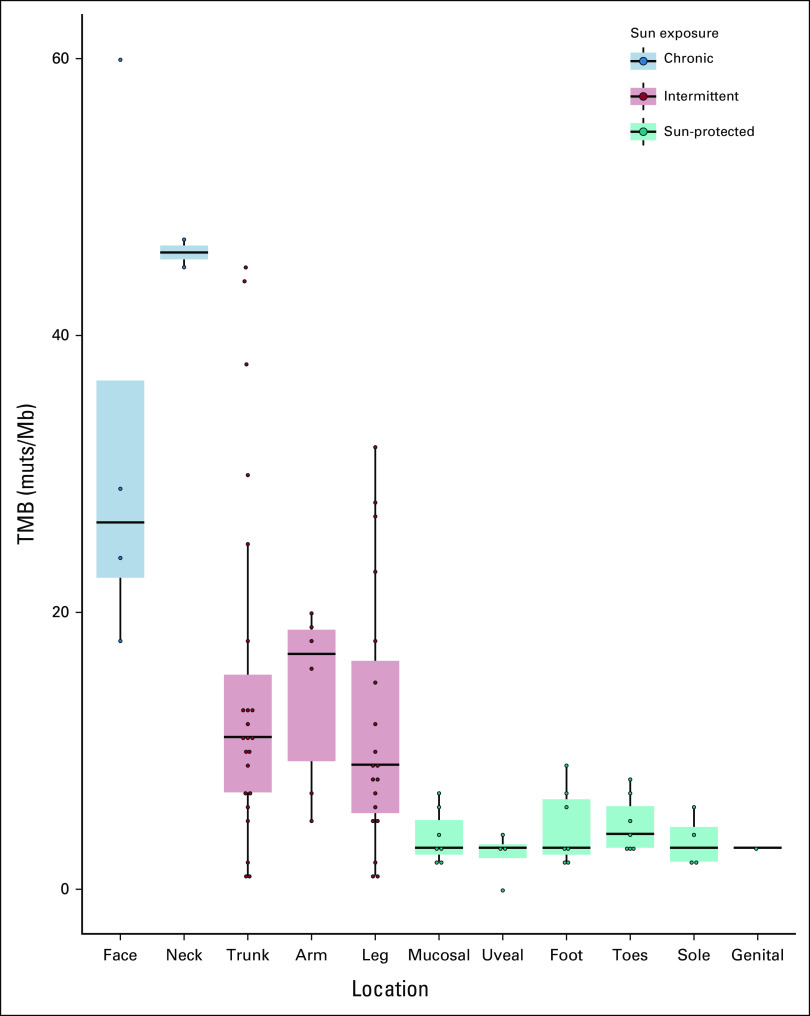
The distribution of TMB according to the melanoma location is shown in Figure 1. The mean TMB was 4 muts/Mb (SD, 2.1) for sun-protected areas, 13.6 muts/Mb (SD, 10.9) for intermittently sun-exposed areas, and 37.2 muts/Mb (SD, 16.0) for chronically sun-exposed areas. In univariate analysis, log-TMB was associated with sun-protected areas with a –0.95 coefficient (95% CI, –1.32 to –0.57; P < 10–5) and chronically sun-exposed areas with a 1.28 coefficient (95% CI, 0.59 to 1.98; P < 10–5). After adjustment on age, sex, and BRAF mutational status in a multivariate analysis, log-TMB was significantly associated with location according to sun exposure (sun-protected areas: coefficient = –1.05, 95% CI, –1.43 to –0.67; chronically sun-exposed areas: coefficient = 1.12, 95% CI, 0.45 to 1.79; P < 10–5, compared with intermittently sun-exposed areas; Table 2).
FIG 1.
TMB (muts/Mb) measure according to sun exposure of the site of primary melanoma. The median, and the first and third quartiles of the TMB are depicted in boxplots according to the sun exposure of the site of primary melanoma. Patients with unknown site of primary melanoma (n = 10) were excluded. muts/Mb, mutations per megabases; TMB, tumor mutational burden.
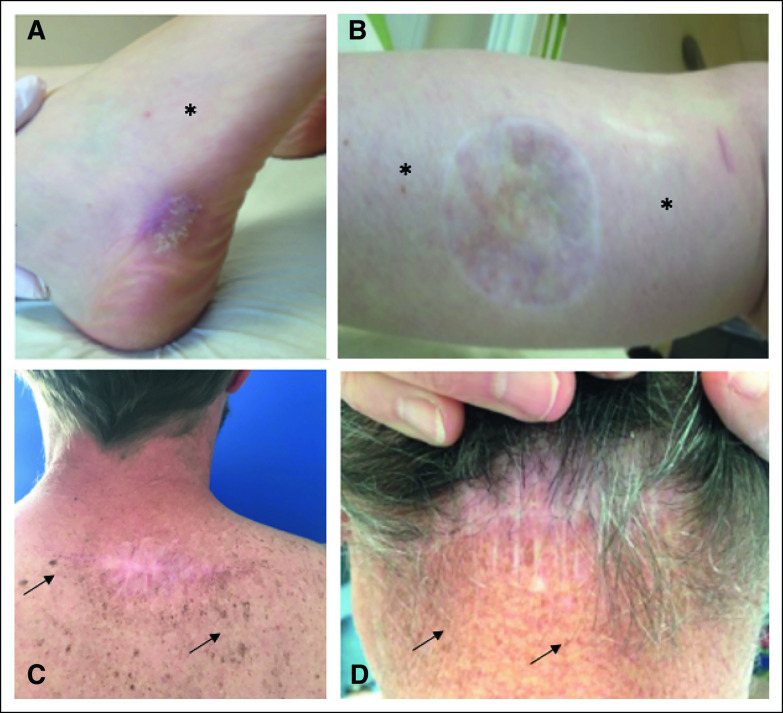
When a clinical picture of the melanoma scar was available (Fig 2), we observed various signs of past UV exposure, presumably involved in melanoma genesis: pigmentation heterogeneities, sun-induced freckling (lentigines), wrinkles, sagging, and presence of actinic keratoses. Notably, the skin surrounding low TMB melanoma scars were typically devoid of sun damage (Figures 2A and 2B v 2C and 2D).
FIG 2.
TMB and clinical signs of sun exposure. Photographs of the observable clinical signs of sun damage surrounding the scars of primary melanoma excision of four patients: (A) melanoma of the external side of the right foot; TMB 2 muts/Mb DNA; progression disease at 6-month follow-up, (B) melanoma of posterior side of the right calf; TMB 1 mut/Mb DNA; progression disease at 6-month follow-up, (C) melanoma of the upper back; TMB 45 muts/Mb; complete response at 6-month follow-up, and (D) melanoma of the posterior part of the neck; TMB 44 muts/Mb DNA; complete response at 6-month follow-up. Absence of sun-damaged skin (*). Sun-induced freckling (black arrow). muts/Mb, mutations per megabases; TMB, tumor mutational burden.
TMB Is Associated With Molecular UV Signature
The tumor DNA canonical mutational signature secondary to UV-exposure was available for 30 melanomas whose TMB reached at least 10 muts/Mb, as shown in the Data Supplement. UV signature was associated with higher log-TMB (coefficient 1.29, 95% CI, 0.48 to 2.10, P = .003).
Negative Association Between TMB and Progression
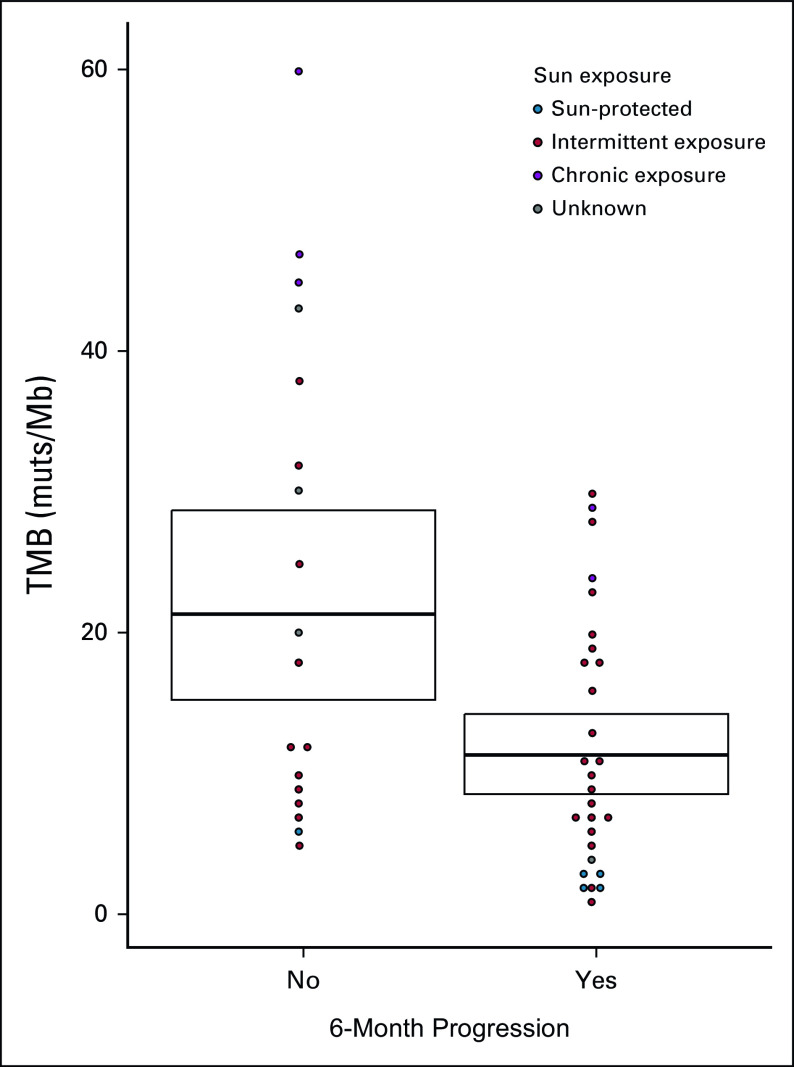
For response to PD-1 inhibitors analysis, 22 patients were excluded. Nine did not receive any systemic treatment, two received PD-1 inhibitors as an adjuvant treatment, and 11 received a combination of CTLA-4 and PD-1 inhibitors. Low TMB was strongly associated with progression to PD-1 inhibitors at 3 and 6 months in cutaneous melanomas (3-month mean TMB from progressors v nonprogressors: 10.2 v 18.32 muts/Mb, P = .02; 6-month mean TMB from progressors v nonprogressors: 10.6 v 19.6 muts/Mb, P = .01, Fig 3). An inversely exponential relationship was observed when plotting the estimated association between the 6-month progression and the TMB against the centers of the quartiles of the TMB (Data Supplement).
FIG 3.
Dotplot comparing TMB for patients receiving PD-1 inhibitors who progressed or not after 6 months of treatment (restricted to cutaneous melanomas). The sun exposure of the site of primary melanoma is represented. Mean TMB and SD are provided (crossbars). muts/Mb, mutations per megabases; PD-1, programmed cell death-1 protein; SD, standard deviation; TMB, tumor mutational burden.
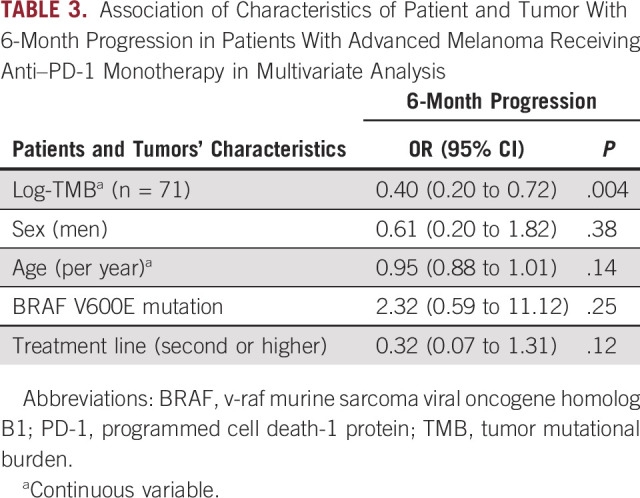
A unit increase in log-TMB, ie, a multiplication of TMB by 2.7 (because exp(1) ≈ 2.7), changed the odds of 3-month progression by 0.47( 95% CI, 0.26 to 0.79, P = .007) and 6-month progression by 0.45 (95% CI, 0.24 to 0.77, P = .006) in a univariate analysis. These results were confirmed by multivariate analysis, after adjusting for sex, age, BRAF mutation, and treatment line, with a significant negative association of log-TMB with 6-month progression (OR = 0.40, 95% CI, 0.20 to 0.72, P = .004), as shown in Table 3. When excluding uveal and mucosal melanoma from the nonexposed group, we found similar results (OR = 0.36, 95% CI, 0.16 to 0.69, P = .005, Data Supplement). When including patients receiving a combination of CTLA-4 and PD-1 inhibitors, the negative association was even stronger (P = .0009). A direct association between 6-month progression and location according to sun exposure was then analyzed. The association did not reach significance, with an OR of 0.21, 95% CI, 0.02 to 1.38 for sun-protected sites, and 1.15, 95% CI, 0.37 to 3.73 for UV-chronic areas compared with intermittently sun-exposed areas.
TABLE 3.
Association of Characteristics of Patient and Tumor With 6-Month Progression in Patients With Advanced Melanoma Receiving Anti–PD-1 Monotherapy in Multivariate Analysis
DISCUSSION
Our study identifies a strong association between TMB and cumulative sun exposure related to skin location in advanced melanoma tumors. The TMB in cutaneous melanoma is high compared with other nonmelanoma tumors because of the mutagenic effects of UV exposure.16-19 We found, to our knowledge, for the first time that both the UV molecular signature present on the metastatic site and the sun exposure on the primary location of the melanoma were associated with higher TMB. This suggests that melanoma metastases carry the UV molecular signature present at their primary site of origin, which is a matter of importance. Similarly, BRAF or neuroblastoma RAS viral oncogene homolog mutations are preserved between primary and metastatic melanoma, explaining why the initial melanoma is used for BRAF analysis before treatment.20 Therefore, one may hypothesize that when TMB measure is not available, the molecular UV signature on the primary melanoma or various signs of past UV exposure have to be considered.
We also analyzed TMB according to histologic subtypes. Although acral, uveal, or mucosal melanomas had different genetic alterations, and tumor behaviors, TMB values were similar between subgroups varying between 0 and 9 muts/Mb. These results are in accordance with those reported in the literature,21-24 as uveal melanomas had the lowest TMB and the poorest RR to PD-1 inhibitors (overall survival 3.6% and median progression-free survival 2.6 months).25 In acral and mucosal melanomas, where the RR is lower compared with other melanoma subtypes,26 higher degrees of aneuploidy and lower numbers of mutagenic drivers may allow a biologic situation where TMB does not need to be high for tumorigenesis and propagation.27 In our study, TMB was higher in chronically sun-exposed areas compared with intermittently sun-exposed sites. Recent works showed that desmoplastic melanoma, known to be a specific UV-related melanoma, had a high mutational burden and a significant clinical benefit of PD-1 inhibitors with strong UV signature.11,28,29 Our cohort confirms these associations across all melanoma subtypes: UV signature was exponentially and significantly associated with higher TMB. Not surprisingly, the BRAF V600E mutation was not associated with a higher TMB in our results, in accordance with the literature as only V 600K mutants are typically UV-induced and associated with higher mutational loads.30 To go even further, we recently proposed pericicatricial sun-exposure pattern of the skin around the primary melanoma, as a directly accessible surrogate marker for TMB, to predict response to systemic treatments, in two independent studies including more than 900 patients.31,32
Another important finding of this study is the positive association between advanced age and higher TMB (P = .002). A few studies reported an association between age and TMB, but these studies encompassed smaller cohorts and covered multiple solid tumors.12,33 In cancer biology, chronic sun exposure over years permits the accumulation of sun damage, and this may correlate with age of melanoma diagnosis.
Therefore, one may suggest the use of multiple criteria, including age, pattern of sun-exposure area, and signs of past UV exposure around melanoma scar, to clinically approach TMB value.
We also confirmed TMB as a predictive biomarker for response to PD-1 inhibitors, with a significant negative association of log-TMB with 6-month progression in the multivariate analysis, even when excluding uveal and mucosal melanomas from the nonexposed group of patients. These results are in accordance with several studies showing a significant correlation between TMB and the objective response rate.7,34-36 Furthermore, several authors sought to estimate a TMB cutoff in the metastatic melanoma population.37,38 A universal definition of high TMB is difficult to propose, as TMB cutoff points vary across cancer types, and may also differ as no standardized technique is available.38-41 In our study, we showed an inversely exponential relationship between TMB and progression under PD-1 inhibitors, with a dose-dependent proportional effect rather than a threshold effect. This finding is relevant because it means that suggesting a TMB cutoff value has no statistical and biologic rationale in patients with melanoma. Another study highlighted an exponential relationship between TMB and objective response rate to PD-1 inhibitors, but the correlation was analyzed across different types of cancers only.42 This can be applied to individual patient care or counseling: although a high TMB should favor single anti–PD-1 immunotherapy, an intermediate TMB may favor combined immunotherapies (anti–PD-1 plus anti–CTLA-4 or anti–lymphocyte activation gene-3), whereas a low TMB may predict a prolonged response to targeted therapy as shown for lung cancer43 because of fewer subclones able to lead to clinical resistance under selective pressure. Thus, TMB will be of great help in optimizing the choice of first-line treatment, which is currently restricted to administration mode (oral v intravenous delivery) or side-effect preferences. This predictive biomarker might even one day avoid wide re-excisions in patients with thick melanomas who are predicted long-term responders to adjuvant or neoadjuvant anti–PD-1 therapy.
In conclusion, we showed a specific and significant relationship between the location of the melanoma according to sun exposure and TMB, turning the location into an easy-to-use proxy of the TMB criterion. Furthermore, our study suggests that melanomas bearing a high TMB have a higher probability of response to immunotherapy compared with melanomas with a low TMB, with a dose-dependent effect following an exponential curve. Although it remains to be confirmed on larger settings, clinical criteria such as signs of sun exposure around the initial scar, or UV molecular signature, may be valuable for clinical decision making in conjunction with TMB assessment.
ACKNOWLEDGMENT
The authors gratefully acknowledge the patients in this study and their families, as well as the participating study teams, especially Guillaume Villechenoux, for making this study possible. They thank Roche laboratory for providing Foundation Medicine analysis, and Dr Heinz Arnheiter for his invaluable help in editing this manuscript.
Léa Dousset
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: MSD Oncology
Caroline Robert
Consulting or Advisory Role: Roche, Bristol Myers Squibb, Novartis, Pierre Fabre, MSD, Sanofi, AstraZeneca, Pfizer
Sandrine Mansard
Consulting or Advisory Role: Novartis, Sanofi
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pierre Fabre, Novartis
Laurent Mortier
Honoraria: BMS France, MSD Oncology, Novartis
Research Funding: MSD Oncology (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Bristol Myers Squibb, Roche/Genentech
Alain Dupuy
Consulting or Advisory Role: Sanofi
Travel, Accommodations, Expenses: Sanofi, UCB
Maxime Battistella
Honoraria: Takeda, LEO Pharma, Kyowa Kirin International
Consulting or Advisory Role: Innate Pharma, Bristol Myers Squibb France, Kyowa Kirin International
Research Funding: Takeda, Kyowa Kirin International
Travel, Accommodations, Expenses: Roche
Marie-Dominique Galibert
Honoraria: Pierre Fabre, AstraZeneca, Roche/FMI, Amgen
Clara Allayous
Travel, Accommodations, Expenses: Bristol Myers Squibb, Amgen
Caroline Dutriaux
Honoraria: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Alexa B. Schrock
Employment: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine, Roche
Jessica Lee
Employment: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine, Roche
Siraj M. Ali
Employment: Foundation Medicine, EQRX
Leadership: Incysus, Elevation Oncology, Pillar Biosciences
Stock and Other Ownership Interests: Exelixis, Merus NV, Pfizer
Consulting or Advisory Role: Azitra (Inst), Princeptx (Inst), ArcherDx
Patents, Royalties, Other Intellectual Property: Patents via Foundation Medicine, Patents via Seres Health on microbiome stuff in non neoplastic disease
Travel, Accommodations, Expenses: Merck Sharp Domme
Céleste Lebbé
Honoraria: Roche, Bristol Myers Squibb, Novartis, Amgen, MSD, Pierre Fabre, Pfizer, Incyte
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Novartis, Amgen, Roche, Merck Serono, Sanofi, Pierre Fabre
Speakers' Bureau: Roche, Bristol Myers Squibb, Novartis, Amgen, MSD
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Novartis, Sanofi, Pierre Fabre
Other Relationship: Avantis Medical Systems
Marie Beylot-Barry
Travel, Accommodations, Expenses: Kyowa Kirin International
Lise Boussemart
Consulting or Advisory Role: BMS, Pierre Fabre, Novartis, MSD, Pfizer
Patents, Royalties, Other Intellectual Property: Application PCT/EP2014/059797, published on 2014-11-20 (WO2014184211): Prognosis and predictive biomarkers and biological applications thereof
No potential conflicts of interest were reported.
Footnotes
L.D. and F.P. contributed equally as first authors.
M.B.-B. and L.B. contributed equally as last senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Léa Dousset, Florence Poizeau, Jean-Philippe Merlio, Lise Boussemart
Provision of study materials or patients: Caroline Robert, Laurent Mortier, Charline Caumont, Jacques Rouanet, Maxime Battistella, David Cappellen, Inès Kerneuzet, Jean-Philippe Merlio, Solène-Florence Kammerer-Jacquet, Marie Beylot-Barry
Collection and assembly of data: Léa Dousset, Florence Poizeau, Sandrine Mansard, Charline Caumont, Émilie Routier, Jacques Rouanet, Maxime Battistella, Anna Greliak, David Cappellen, Clara Allayous, Alexandra Lespagnol, Émilie Gerard, Inès Kerneuzet, Séverine Roy, Caroline Dutriaux, Jean-Philippe Merlio, Alexa B. Schrock, Jessica Lee, Lise Boussemart
Data analysis and interpretation: Léa Dousset, Florence Poizeau, Caroline Robert, Laurent Mortier, Charline Caumont, Alain Dupuy, David Cappellen, Marie-Dominique Galibert, Jean-Philippe Merlio, Beatrice Vergier, Siraj M. Ali, Solène-Florence Kammerer-Jacquet, Céleste Lebbé, Marie Beylot-Barry, Lise Boussemart
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Léa Dousset
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: MSD Oncology
Caroline Robert
Consulting or Advisory Role: Roche, Bristol Myers Squibb, Novartis, Pierre Fabre, MSD, Sanofi, AstraZeneca, Pfizer
Sandrine Mansard
Consulting or Advisory Role: Novartis, Sanofi
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pierre Fabre, Novartis
Laurent Mortier
Honoraria: BMS France, MSD Oncology, Novartis
Research Funding: MSD Oncology (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Bristol Myers Squibb, Roche/Genentech
Alain Dupuy
Consulting or Advisory Role: Sanofi
Travel, Accommodations, Expenses: Sanofi, UCB
Maxime Battistella
Honoraria: Takeda, LEO Pharma, Kyowa Kirin International
Consulting or Advisory Role: Innate Pharma, Bristol Myers Squibb France, Kyowa Kirin International
Research Funding: Takeda, Kyowa Kirin International
Travel, Accommodations, Expenses: Roche
Marie-Dominique Galibert
Honoraria: Pierre Fabre, AstraZeneca, Roche/FMI, Amgen
Clara Allayous
Travel, Accommodations, Expenses: Bristol Myers Squibb, Amgen
Caroline Dutriaux
Honoraria: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre
Alexa B. Schrock
Employment: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine, Roche
Jessica Lee
Employment: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine, Roche
Siraj M. Ali
Employment: Foundation Medicine, EQRX
Leadership: Incysus, Elevation Oncology, Pillar Biosciences
Stock and Other Ownership Interests: Exelixis, Merus NV, Pfizer
Consulting or Advisory Role: Azitra (Inst), Princeptx (Inst), ArcherDx
Patents, Royalties, Other Intellectual Property: Patents via Foundation Medicine, Patents via Seres Health on microbiome stuff in non neoplastic disease
Travel, Accommodations, Expenses: Merck Sharp Domme
Céleste Lebbé
Honoraria: Roche, Bristol Myers Squibb, Novartis, Amgen, MSD, Pierre Fabre, Pfizer, Incyte
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Novartis, Amgen, Roche, Merck Serono, Sanofi, Pierre Fabre
Speakers' Bureau: Roche, Bristol Myers Squibb, Novartis, Amgen, MSD
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Novartis, Sanofi, Pierre Fabre
Other Relationship: Avantis Medical Systems
Marie Beylot-Barry
Travel, Accommodations, Expenses: Kyowa Kirin International
Lise Boussemart
Consulting or Advisory Role: BMS, Pierre Fabre, Novartis, MSD, Pfizer
Patents, Royalties, Other Intellectual Property: Application PCT/EP2014/059797, published on 2014-11-20 (WO2014184211): Prognosis and predictive biomarkers and biological applications thereof
No potential conflicts of interest were reported.
REFERENCES
- 1.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. : Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19:1480-1492, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Ribas A, Schachter J, et al. : Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20:1239-1251, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Weber J, Mandala M, Del Vecchio M, et al. : Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824-1835, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AMM, Blank CU, Mandala M, et al. : Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378:1789-1801, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Snyder A, Makarov V, Merghoub T, et al. : Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371:2189-2199, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugo W, Zaretsky JM, Sun L, et al. : Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 165:35-44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riaz N, Havel JJ, Makarov V, et al. : Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171:934-949.e15, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalmers ZR, Connelly CF, Fabrizio D, et al. : Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 9:34, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGranahan N, Furness AJS, Rosenthal R, et al. : Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351:1463-1469, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eroglu Z, Zaretsky JM, Hu-Lieskovan S, et al. : High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553:347-350, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Xu J, Du C, et al. : The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: A systematic review and meta-analysis. Front Oncol 9:1161, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Frampton GM, Fichtenholtz A, Otto GA, et al. : Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 31:1023-1031, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martincorena I, Campbell PJ: Somatic mutation in cancer and normal cells. Science 349:1483-1489, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. : Signatures of mutational processes in human cancer. Nature 500:415-421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodis E, Watson IR, Kryukov GV, et al. : A landscape of driver mutations in melanoma. Cell 150:251-263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagore E, Roeck K, Budden T, et al. : Chronic UV damage of the stroma improves melanoma survival. Cancer Res 79, 2019. (13 suppl; abstr 2022) [Google Scholar]
- 20.Boursault L, Haddad V, Vergier B, et al. : Tumor homogeneity between primary and metastatic sites for BRAF status in metastatic melanoma determined by immunohistochemical and molecular testing. PLoS One 8:e70826, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi K, Zhang B, Kong BY, et al. : Distinct genomic features in a retrospective cohort of mucosal, acral and vulvovaginal melanomas. J Am Acad Dermatol 10.1016/j.jaad.2019.07.017 [epub ahead of print on July 12, 2019] [DOI] [PubMed] [Google Scholar]
- 22.Hayward NK, Wilmott JS, Waddell N, et al. : Whole-genome landscapes of major melanoma subtypes. Nature 545:175-180, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Furney SJ, Turajlic S, Stamp G, et al. : Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol 230:261-269, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Furney SJ, Turajlic S, Stamp G, et al. : The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res 27:835-838, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Algazi AP, Tsai KK, Shoushtari AN, et al. : Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 122:3344-3353, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mignard C, Deschamps Huvier A, Gillibert A, et al. : Efficacy of immunotherapy in patients with metastatic mucosal or uveal melanoma. J Oncol 2018:1908065, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turajlic S, Furney SJ, Lambros MB, et al. : Whole genome sequencing of matched primary and metastatic acral melanomas. Genome Res 22:196-207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shain AH, Garrido M, Botton T, et al. : Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat Genet 47:1194-1199, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boussemart L, Johnson A, Schrock AB, et al. : Tumor mutational burden and response to programmed cell death protein 1 inhibitors in a case series of patients with metastatic desmoplastic melanoma. J Am Acad Dermatol 80:1780-1782, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Da Silva IP, Wang KYX, Wilmott JS, et al. : Distinct molecular profiles and immunotherapy treatment outcomes of V600E and V600K BRAF-mutant melanoma. Clin Cancer Res 25:1272-1279, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo D, Poizeau F, Dinulescu M et al. Skin photoaging around the site of occurrence of primary melanoma as a clinical predictive biomarker of response to PD-1 inhibitors. Ann Oncol 31, 2020. (suppl 4; abstr 1133P) [Google Scholar]
- 32.Russo D, Dalle S, Dereure O, et al. Differential gradients of efficacy of immunotherapy according to the sun-exposure pattern of the site of occurrence of primary melanoma: A multicenter prospective cohort study (MELBASE). J Clin Oncol 39, 2020. (suppl 15; abstr e21545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman AM, Kato S, Bazhenova L, et al. : Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 16:2598-2608, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JS, Ruppin E: Multiomics prediction of response rates to therapies to inhibit programmed cell death 1 and programmed cell death 1 ligand 1. JAMA Oncol 5:1614-1618, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cristescu R, Mogg R, Ayers M, et al. : Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362:eaar3593, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riaz N, Morris L, Havel JJ, et al. : The role of neoantigens in response to immune checkpoint blockade. Int Immunol 28:411-419, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forschner A, Battke F, Hadaschik D, et al. : Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma-results of a prospective biomarker study. J Immunother Cancer 7:180, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samstein RM, Lee CH, Shoushtari AN, et al. : Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51:202-206, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addeo A, Banna GL, Weiss GJ: Tumor mutation burden—From hopes to doubts. JAMA Oncol 5:934-935, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Addeo A, Banna GL, Weiss GJ: Tumor mutation burden-From doubts to concerns-In reply. JAMA Oncol 5:1809, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Parikh K, Huether R, White K, et al. : Tumor mutational burden from tumor-only sequencing compared with germline subtraction from paired tumor and normal specimens. JAMA Netw Open 3:e200202, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarchoan M, Hopkins A, Jaffee EM: Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377:2500-2501, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Offin M, Rizvi H, Tenet M, et al. : Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res 25:1063-1069, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]