Abstract
OBJECTIVES
Assessment of early outcomes in patients with normal preoperative left ventricular ejection fraction (LVEF) in whom venoarterial extracorporeal membrane oxygenation (VA-ECMO) was implanted for postcardiotomy cardiogenic shock (PCCS) during the first postoperative 48 h.
METHODS
Retrospective single-centre analysis in adult patients with normal LVEF, who received VA-ECMO support for PCCS from May 1998 to May 2018. The primary outcome was 30-day perioperative mortality during the index hospitalization.
RESULTS
A total of 62 125 adult patients underwent cardiac surgery at our institution during the study period. Among them, 173 patients (0.3%) with normal preoperative LVEF required VA-ECMO for PCCS. Among them, 71 (41.1%) patients presented PCCS due to coronary malperfusion and in 102 (58.9%) patients, no evident cause was found for PCCS. Median duration of VA-ECMO support was 5 days (interquartile range 2–8 days). A total of 135 (78.0%) patients presented VA-ECMO-related complications and the overall 30-day perioperative mortality was 57.8%. Independent predictors of mortality were: lactate level just before VA-ECMO implantation [odds ratio (OR) 1.27; P < 0.001], major bleeding during VA-ECMO (OR 3.76; P = 0.001), prolonged cardiopulmonary bypass time (OR 1.01; P < 0.001) and female gender (OR 4.87; P < 0.001).
CONCLUSIONS
Mortality rates of VA-ECMO in PCCS patients are high, even in those with preoperative normal LVEF. Coronary problems are an important cause of PCCS; however, the aetiology remains unknown in the vast majority of the cases. The implantation of VA-ECMO before development of tissue hypoperfusion and the control of VA-ECMO-associated complications are the most important prognostic factors in PCCS patients. Lactate levels may help guide timing of VA-ECMO implantation and define the extent of therapeutic effort.
Keywords: Postcardiotomy cardiogenic shock, Extracorporeal life support, Extracorporeal membrane oxygenation
INTRODUCTION
Postcardiotomy cardiogenic shock (PCCS) occurs in 3–6% of patients undergoing routine cardiac procedures with reported mortality rates as high as 75% [1–3](1; 2; 3). Approximately 1% of the patients with PCCS require prolonged postoperative circulatory support due to refractory cardiac and/or pulmonary dysfunction [4] (4). In these cases, venoarterial extracorporeal membrane oxygenation (VA-ECMO) is a valuable therapeutic option that can save this group of very critical patients [4] (4). The decision whether or not to implant a VA-ECMO in PCCS patients can be difficult, especially in those with normal preoperative left ventricular ejection fraction (LVEF) in whom PCCS occurs unexpectedly. Additionally, haemodynamic worsening, severe complications or multiple organ dysfunction syndrome may still occur despite VA-ECMO circulatory support. This fact hinders decision-making and definition of the limits of the therapeutic effort during the early phase in the intensive care unit (ICU). Additionally, perioperative variables that may be useful to predict outcomes remain poorly defined; thus, determination of prognosis and likelihood of successful weaning from VA-ECMO is often clinically challenging and a difficult ethical issue [5] (5).
This study aims to assess early outcomes in patients with normal preoperative LVEF in whom VA-ECMO was implanted for PCCS during the first postoperative 48 h. Besides, we aimed to identify predictors of mortality which could facilitate decision-making both before the implantation of VA-ECMO and later during postimplantation management in the ICU. To the best of our knowledge, our study represents the first cohort of VA-ECMO patients with normal preoperative LVEF for PCCS.
PATIENTS AND METHODS
This study was approved by the ethics committee of the Faculty of Medicine at the University of Leipzig (protocol number 391/18-ek), and individual patient informed consent was waived.
Study design
Between May 1998 and May 2018, all adult patients with normal preoperative LVEF requiring VA-ECMO during the first postoperative 48 h due to PCCS were analysed. LVEF was registered in the preoperative echo the day before the intervention and was confirmed with the intraoperative transoesophageal echocardiography before cardiopulmonary bypass (CPB). Patients under 18 years of age, male patients with preoperative LVEF less than 52%, and female patients with less than 54% or those requiring VA-ECMO preoperatively were excluded from the study. The primary outcome was 30-day perioperative mortality. The secondary outcome was short-term outcomes and predictors of 30-day perioperative mortality in patients requiring VA-ECMO for PCCS. The definitions are in the Supplementary Material.
Extracorporeal membrane oxygenation circuit and implantation techniques
In the vast majority of cases, the VA-ECMO system was composed of 1 SCPC-centrifugal pump console (LivaNova PLC, London, UK), 1 Revolution centrifugal pump (LivaNova PLC), 1 A.L.ONE ECMO Oxygenator (EUROSETS, Milano, Italy), an HLS cannula set—arterial cannula 17–19 mm and venous cannula 19–23 mm (Maquet GmbH—Getinge AB, Rastatt, Germany) and standard heparin covered tubing set.
Implantation of peripheral VA-ECMO was performed using a transcutaneous Seldinger technique with the puncture of the femoral artery and vein when the patient was haemodynamically extremely unstable. When the patient’s haemodynamic status was relatively stable, then peripheral VA-ECMO was instituted under direct surgical preparation of the femoral vessels followed by the Seldinger technique for venous cannulation and either direct cannulation of the femoral artery or indirect cannulation through an 8-mm Dacron prosthesis. If central VA-ECMO was chosen (i.e. in patients in whom the sternum was already opened or in those with a severe peripheral vascular disease), venous cannulation was performed via the femoral vein and arterial cannulation was performed via a Dacron prosthesis anastomosed either to the axillary artery or to the ascending aorta. The size of the ECMO cannulas was selected according to the patient’s body surface area. Transoesophageal echocardiography was always performed during the procedure to confirm the accurate placement of the cannulas.
Data collection and definitions
The demographic profile of patients, intraoperative data, postoperative outcomes and specific information related to ECMO (e.g. indication, cannulation site) were prospectively collected and entered into a computerized database from May 1998 to May 2018. Thereafter, data were retrospectively analysed.
Statistical analysis
To compare patients who survived or died, the Student’s t-test was used for normally distributed quantitative data (expressed as mean and standard deviation); otherwise, the Mann–Whitney U-test (shown as median and quartile range) was used. The χ2 or Fisher’s exact test, as appropriate, was performed for categorical variables (data are shown as a percentage). Bivariate odds ratios and 95% confidence intervals (CIs) also were estimated. The multiple logistic regression model with the variable ‘survival’ as the dependent variable was constructed using a backward stepwise selection procedure. Independent predictors were entered into the model if a significant association (P < 0.05) was identified on bivariate analysis and the correlation coefficient between them (collinearity) was <0.25. Potential predictors were removed if this exclusion did not result in a significant change in the log-likelihood ratio test. The cut-off for variable removal was set at a significance level of 0.05. Adjusted odds ratios and 95% CIs also were calculated.
To avoid overfitting and obtain reliable internal validation of the subset of factors, a bootstrap method was used, which derived 1000 computer-generated samples by random selection with replacement, each including the same number of patients. Within each bootstrap sample, the B-coefficient was calculated using all selected independent variables. The robustness of the model and thus the reliability of predictor variables in the final regression model were estimated by the 95% CI of the B-coefficient derived from the bootstrap samples. Statistical analyses were performed using the SPSS software package, version 25.0 (IBM Corp, Armonk, NY, USA). Bootstrapping was performed using R, version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
Between May 1998 and May 2018, a total of 62 125 adult patients (≥18 years old) underwent cardiac surgery procedures at our institution. Amongst them, 173 patients (0.3%) with normal preoperative LVEF required VA-ECMO during the first postoperative 48 h due to PCCS. These patients form the basis of the present study.
Demographic characteristics and intraoperative data
Patient demographic characteristics are depicted in Table 1. The type of procedures and Intraoperative data are shown in Table 2.
Table 1:
Baseline patient characteristics
| Variables | Survivors (n = 73) | Non-survivors (n = 100) | P-value |
|---|---|---|---|
| Age (years) | 65.4 (±11.0) | 65.3 (±10.5) | 0.933 |
| Female gender | 27 (36.9) | 54 (54.0) | 0.031 |
| BMI (kg/m2) | 27.5 (±4.8) | 28.2 (±5.4) | 0.392 |
| Diabetes mellitus | 20 (27.4) | 42 (42.0) | 0.035 |
| Arterial hypertension | 56 (76.7) | 80 (80.0) | 0.282 |
| Pulmonary hypertension | 14 (19.2) | 22 (22.0) | 0.510 |
| COPD | 2 (2.7) | 7 (7.0) | 0.306 |
| Smoker | 26 (35.6) | 29 (29.0) | 0.494 |
| Hyperlipidaemia | 39 (53.4) | 50 (50.0) | 0.789 |
| Peripheral vascular disease | 16 (21.9) | 37 (37.0) | 0.021 |
| Neurological dysfunction | 10 (13.7) | 11 (11.0) | 0.662 |
| Preoperative creatinine (mg/dl) | 1.2 (±1.1) | 1.2 (±0.5) | 0.917 |
| Preoperative GFR (ml/min) | 82.6 (±39.9) | 72.5 (±32.7) | 0.080 |
| Preoperative dialysis | 1 (1.4) | 4 (4.0) | 0.337 |
| Elective surgery | 41 (56.2) | 51 (51.0) | 0.501 |
| Urgent surgery | 19 (26.0) | 22 (22.0) | 0.538 |
| Emergency surgery | 13 (17.8) | 27 (27.0) | 0.157 |
| Left main stenosis | 8 (10.9) | 11 (11.0) | 0.683 |
| Prior PCI | 9 (12.3) | 17 (17.0) | 0.394 |
| Prior MI | 14 (19.2) | 21 (21.0) | 0.706 |
| MI <48 h | 4 (5.5) | 2 (2.0) | 0.683 |
| MI 2–21 days | 3 (4.1) | 3 (3.0) | 0.237 |
| MI 22–90 days | 2 (2.7) | 5 (5.0) | 0.425 |
| MI >91 days | 5 (6.8) | 11 (11.0) | 0.311 |
| Prior heart surgery | 12 (24.5) | 37 (37) | 0.003 |
| Months since prior heart surgery | 57 (7–141) | 48 (9–102) | 0.633 |
| Logistic EuroSCORE (%) | 10.9 (±13.3) | 17.5 (±18.1) | 0.009 |
| Active endocarditis | 6 (8.2) | 14 (14.0) | 0.240 |
| Type A aortic dissection | 0 (0) | 4 (4.0) | 0.139 |
Values are represented as number (percentage), mean (±standard deviation) or median (interquartile range). P values < 0.05 in bold are statistically significant.
BMI: body mass index; COPD: chronic obstructive pulmonary disease; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention.
Table 2:
Intraoperative data
| Variables | Survivors (n = 73) | Non-survivors (n = 100) | P-value |
|---|---|---|---|
| Type of surgery | |||
| CABG | 17 (23.3) | 16 (16.0) | 0.228 |
| OPCAB | 4 (5.5) | 5 (5.0) | 0.888 |
| CABG + valve surgery | 10 (13.7) | 16 (16.0) | 0.676 |
| Isolated AVR | 6 (8.2) | 5 (5.0) | 0.530 |
| AVR + AAR | 7 (9.6) | 8 (8.0) | 0.714 |
| Bentall | 7 (9.6) | 18 (18.0) | 0.120 |
| Aortic arch surgery | 3 (4.1) | 8 (8.0) | 0.360 |
| MVR ± TVR | 16 (21.9) | 19 (19.0) | 0.637 |
| AVR + MVR/TVR | 3 (4.1) | 5 (5.0) | 1.000 |
| Intraoperative variables | |||
| Length of surgery (min) | 255 (180–384) | 342 (194–423) | 0.092 |
| Bypass time (min) | 150 (101–220) | 191 (112–264) | 0.019 |
| Cross-clamp time (min) | 81 (58–110) | 93 (65–139) | 0.114 |
| Antegrade CP | 52 (71.2) | 80 (80.0) | 0.576 |
| Antegrade + retrograde CP | 15 (20.5) | 15 (15.0) | 0.287 |
| Surgery without CP | 6 (8.2) | 5 (5.0) | 0.530 |
| Blood CP | 13 (17.8) | 25 (25.0) | 0.438 |
| Crystalloid CP | 54 (73.9) | 70 (70.0) | |
| Cardioplegia volume (ml) | 1800 (1050–2550) | 1800 (1500–2775) | 0.725 |
| Minimal temperature (°C) | 34 (32–34) | 34 (32–34) | 0.207 |
Values are represented as number (percentage) or median (interquartile range). P values < 0.05 in bold are statistically significant.
AAR: ascending aortic replacement; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; CP: cardioplegia; MVR: mitral valve replacement; OPCAB: off-pump coronary artery bypass; TVR: tricuspid valve replacement.
Venoarterial extracorporeal membrane oxygenation implantation
In 56 (32.4%) patients, VA-ECMO was implanted intraoperatively due to failure to wean from CPB or cardiogenic shock immediately after CPB weaning. In the remaining 117 (67.6%) patients, PCCS was diagnosed postoperatively in the ICU. In a total of 71 (41.1%) patients, coronary malperfusion was identified as the cause of PCCS. Among those patients, 17 (9.8%) patients required a bypass revision during the same surgery, but in 54 (31.2%) patients, the problem was detected afterwards in the cardiac catheterization laboratory: 13 (7.5%) patients were stented and the remaining 41 (23.7%) patients underwent re-do surgery. In a total of 102 (58.9%) patients, no evident cause was found for PCCS. All indications for VA-ECMO implantation are listed in Table 3. Parameters immediately before ECMO implantation are listed Table 4. VA-ECMO-related aspects, time delay from CPB weaning to VA-ECMO implantation, duration of VA-ECMO and most frequent complications are listed in Table 5. There was no statistically significant difference in mortality rates according to the kind of surgery, the cause of PCCS or the elapsed time until VA-ECMO implantation (Tables 3 and 5). Variables during VA-ECMO are listed in Supplementary Material, Table S1.
Table 3:
Indications for VA-ECMO implantation
| Variables | Survivors (n = 73) | Non-survivors (n = 100) | P-value |
|---|---|---|---|
| IntraOP RV failure after CPB weaning | 6 (8.2) | 6 (6.0) | 0.570 |
| IntraOP LV failure after CPB weaning | 16 (21.9) | 9 (9.0) | 0.017 |
| IntraOP biventricular failure after CPB weaning | 1 (1.4) | 6 (6.0) | 0.124 |
| PostOP RV failure | 4 (5.5) | 8 (8.0) | 0.563 |
| PostOP LV failure | 1 (1.4) | 9 (9.0) | 0.034 |
| PostOP biventricular failure | 3 (4.1) | 14 (14.0) | 0.031 |
| PostOP cardiopulmonary resuscitation | 0 (0) | 2 (2.0) | 0.509 |
| PostOP cardiac arrhythmias | 7 (9.6) | 10 (57.8) | 0.929 |
| PostOP coronary malperfusion, n (%) | 35 (47.9) | 36 (36.0) | 0.115 |
Values are represented as number (percentage). P values < 0.05 in bold are statistically significant.
CPB: cardiopulmonary bypass; IntraOP: intraoperative; LV: left ventricular; PostOP: postoperative; RV: right ventricular; VA-ECMO: venoarterial extracorporeal membrane oxygenation.
Table 4:
Variables immediately before VA-ECMO implantation
| Variables | Survivors (n = 73) | Non-survivors (n = 100) | P-value |
|---|---|---|---|
| Lactate (mmol/l) | 5.5 (±3.2) | 10.1 (±5.9) | <0.001 |
| pH | 7.3 (±0.08) | 7.3 (±0.11) | 0.341 |
| Glucose (mmol/l) | 9.4 (±3.2) | 10.4 (±10.8) | 0.496 |
| Haematocrit (%) | 30.6 (±4.0) | 31.4 (±4) | 0.795 |
| Systolic blood pressure (mmHg) | 81.0 (±10.0) | 72.9 (±10.1) | <0.001 |
| Diastolic blood pressure (mmHg) | 45.6 (±6.0) | 39.9 (±5.7) | <0.001 |
| Mean blood pressure (mmHg) | 57.4 (±6.1) | 50.9 (±5.9) | <0.001 |
| CK before VA-ECMO (µmol/l) | 17.6 (±25.4) | 22.3 (±30.0) | 0.515 |
| CKMB before VA-ECMO (µmol/l) | 2.2 (±2.3) | 3.1 (±2.8) | 0.194 |
| ASAT (µmol/l) | 3.3 (±4.4) | 10.6 (±28.2) | 0.324 |
| ALAT (µmol/l) | 1.3 (±0.9) | 2.2 (±2.4) | 0.353 |
| INR | 1.5 (±0.3) | 1.8 (±1.0) | 0.087 |
| Creatinine (mg/dl) | 1.8 (±1.1) | 2.2 (±0.9) | 0.018 |
| GFR (ml/min) | 55.9 (±29.9) | 41.3 (±22.8) | 0.001 |
| Epinephrine (ml/h) | 32.3 (±29.5) | 43.8 (±33.3) | 0.056 |
| Norepinephrine (ml/h) | 36.8 (±30.5) | 52.9 (±36.0) | 0.012 |
| Telipressin (ml/h) | 4.1 (±11.6) | 8.7 (±17.2) | 0.106 |
| Dobutamine (ml/h) | 1.4 (±2.4) | 1.1 (±2.1) | 0.452 |
| Milrinone (ml/h) | 1.5 (±2.5) | 1.9 (±2.4) | 0.455 |
| IABP implantation | 32 (43.8) | 46 (46.0) | 0.778 |
Values are represented as mean (±standard deviation) or number (percentage). P values < 0.05 in bold are statistically significant.
ALAT: alanine transaminase; ASAT: aspartate transaminase; CK: creatine kinase; CKMB: creatine kinase-MB; IABP: intra-aortic balloon pump; INR: international normalized ratio; VA-ECMO: venoarterial extracorporeal membrane oxygenation.
Table 5:
VA-ECMO implantation-related aspects, duration of VA-ECMO and complications among survivors and non-survivors
| Variables | Survivors (n = 73) | Non-survivors (n = 100) | P-value |
|---|---|---|---|
| Antegrade cannulation | 39 (53.4) | 63 (63.0) | 0.206 |
| Retrograde cannulation | 34 (46.6) | 37 (37.0) | 0.206 |
| Time until VA-ECMO implantationa (min) | 240 (30–507) | 283 (41–840) | 0.509 |
| Days on VA-ECMO (days) | 5 (3–7) | 5 (2–8) | 0.282 |
| ECMO-related complications | 49 (67.1) | 86 (86.0) | 0.003 |
| Bleeding | 36 (49.3) | 71 (71.0) | 0.004 |
| Re-exploration for bleeding | 24 (32.9) | 31 (31.0) | 0.793 |
| Acute renal failure | 28 (38.4) | 53 (53.0) | 0.057 |
| Acute liver failure | 1 (1.4) | 12 (12.0) | 0.009 |
| Sepsis | 7 (9.6) | 5 (5.0) | 0.241 |
Values are represented as number (percentage) or median (interquartile range). P values < 0.05 in bold are statistically significant.
Time elapsed from cardiopulmonary bypass stop to VA-ECMO implantation.
VA-ECMO: venoarterial extracorporeal membrane oxygenation.
Postoperative outcomes
From a total of 173 patients, 64 (37%) patients could not be weaned off from VA-ECMO [median time in VA-ECMO 5 days (interquartile range, IQR 1–9 days)]. Additionally, during the first 30 days, out of 109 (63%) patients who were weaned off from VA-ECMO, 36 (21%) patients died while 73 (42.2%) patients were alive [median days in VA-ECMO 5 (IQR 2–8 days) vs 5 days (IQR 3–7 days) P = 0.458]. Overall 30-day perioperative hospital mortality was 57.8%. The median hospital stay was 12 days (5–24 days). The causes of death are depicted in Supplementary Material, Table S2. Non-survivors had a median hospital stay of 7 days (2–11 days). Survivors had a median hospital stay of 24 days (18–35 days). A total of 135 (78.0%) patients had at least one VA-ECMO-related complication, as summarized in Table 5 and Supplementary Material, Table S3. VA-ECMO-related complications were more often observed in patients with longer mechanical circulatory support: among survivors, the median duration of VA-ECMO support in those patients who presented complications was 6 days (3–9 days) and in those patients who did not present complications, it was 4 days (2–5 days) (P = 0.014). From the total of 73 (42.2%) patients who survived the initial 30 postoperative days, 16 (9.2%) patients were lost to follow-up, 18 (10.4%) patients died [median survival days 91 (IQR 62–126 days)] and 39 patients were alive after the first follow-up year, leading to an estimated 1-year mortality of 68.2%.
Significant association with 30-day mortality was initially identified on bivariate analysis for 15 different variables (i.e. variables with significant P-values on Tables 1–5 and Supplementary Material, Table S1). These variables were then entered into the logistic regression model. The resulting independent predictors of mortality are shown in Table 6.
Table 6:
Independent predictors of mortality defined in the multiple logistic regression analysis
| Preoperative parameters | Odds ratio | 95% CI | P-value | Bootstrapped 95% CI |
|---|---|---|---|---|
| Female gender | 4.87 | 1.94–13.26 | <0.001 | 1.62–14.38 |
| Bleeding during VA-ECMO | 3.76 | 1.41–10.83 | 0.001 | 1.34–9.62 |
| Lactate level just before VA-ECMO | 1.27 | 1.15–1.44 | <0.001 | 1.15–1.40 |
| CPB time | 1.01 | 1.00–1.01 | <0.001 | 1.00–1.01 |
P values < 0.05 in bold are statistically significant.
CI: confidence interval; CPB: cardiopulmonary bypass; VA-ECMO: venoarterial extracorporeal membrane oxygenation.
We identified a cut-off point of lactate level immediately before VA-ECMO implantation of 6.45 mmol/l with a fair receiver operating characteristic curve (0.73) that predicts higher mortality. This cut-off point was accompanied by a significantly higher dose of vasopressin and lower mean blood pressure in the bivariate analysis, although none of them was significant in multivariate analysis. Estimated survival according to lactate cut-off point is shown in Fig. 1. Lactate level decreased during VA-ECMO support in patients who could be weaned off, but it increased further in patients who could not be weaned off (mean of maximal lactate under VA-ECMO 8.7 ± 6.4 mmol/l vs 17.2 ± 4.1 mmol/l; P < 0.001).
Figure 1:
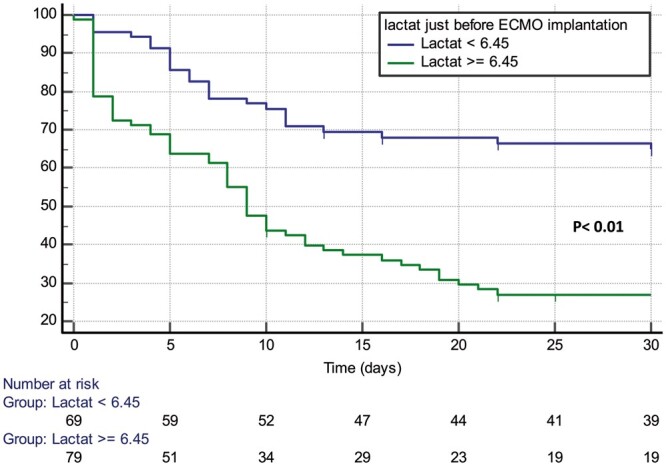
Kaplan–Meier curve displaying the estimated survival probability (as percentage) as per lactate cut-off point immediately before veno-arterial extracorporeal membrane oxygenation implantation. ECMO: extracorporeal membrane oxygenation.
DISCUSSION
PCCS is a devastating complication after cardiac surgical operations. Our preference of treatment for this group of patients has been the use of VA-ECMO to provide short-term cardiopulmonary support. Our cohort presented a 30-day perioperative mortality after VA-ECMO implantation of 57.8% and an estimated 1-year mortality of 68.2%. The decision to implant a VA-ECMO can be very challenging in the setting of PCCS, as patients have a preoperative normal LVEF, and there are many serious questions to be answered in a very short time frame. Perioperative variables that may be useful to predict outcomes remain poorly defined [5] (5). We tried to find information that helps in the decision whether or not to initiate VA-ECMO therapy and whether to interrupt the therapy in case of further deterioration on the ICU. If the decision to institute VA-ECMO support is taken, it is reasonable not to wait until secondary organ damage or profound metabolic acidosis has occurred. We found an elevated lactate level before ECMO implantation (cut-off in our cohort 6.45 mmol/l) to be an independent predictor of mortality. The duration of VA-ECMO should be individualized and prolonged until cardiopulmonary parameters are fully optimized [6]. Two factors should be considered in order to continue or interrupt the VA-ECMO therapy: the possibility of myocardial recovery and the complications associated with VA-ECMO. We identified higher mortality in patients who did not show a fall in lactate levels under VA-ECMO support. This is a sign of continued shock state in spite of VA-ECMO. The patients with a longer duration of VA-ECMO support showed higher morbidities due to higher incidence of complications. Once multiple organ dysfunction syndrome or other serious complications occur, the decision to interrupt VA-ECMO should be considered, as prolonging mechanical support does not provide survival benefits [7]. In our ICU setting, VA-ECMO therapy is evaluated daily using transoesophageal echocardiography after stepwise reduction of VA-ECMO support, which together with a close monitoring of organ perfusion markers, provides an assessment of the degree of myocardial recovery and organ dysfunction. VA-ECMO support is terminated after at least 3 unsuccessful weaning attempts without evidence of myocardial recovery and if the patient is not candidate for left ventricular assist device and/or transplant, or in the case of severe irreversible complications. Additionally, the decision to discontinue VA-ECMO for PCCS support, especially in patients with normal preoperative LVEF, is also based on individual patient factors. The median duration of VA-ECMO support in our study was 5 days. This is comparable with other studies where the prolongation of VA-ECMO therapy to up to 7 days has shown no improvement in mortality, with the exception of post-transplant patients [8]. On the contrary, prolonged VA-ECMO support has been associated with increased mortality rates due to high occurrence of VA-ECMO-related complications [9].
An important aspect of VA-ECMO therapy is the type of cannulation. Central cannulation avoids complications such as limb ischaemia, and provides antegrade flow which does not compete with the heart’s ejection, thus avoiding hypoxia of the upper body (North-South syndrome) and allowing better left ventricular venting [4, 10]. Nonetheless, central cannulation is not the first option in emergency situations when the patient needs VA-ECMO implantation in the ICU and the chest is closed. Two previous meta-analyses reported central cannulation to be associated with greater in-hospital mortality than peripheral cannulation, probably due to increased bleeding and associated consequences (i.e. pericardial tamponade, reoperation and mass transfusion) [11, 12]. Although patients with central cannulation more frequently presented with bleeding (P = 0.028) and required more often re-exploration for bleeding during VA-ECMO therapy (P = 0.029) in our study, there were no statistically significant differences in mortality according to cannulation type (P = 0.206).
We found elevated lactate levels just before VA-ECMO implantation, bleeding during VA-ECMO, prolonged CPB time, and female gender to be independent predictors of mortality after implantation of VA-ECMO for PCCS.
Lactate level just before implantation is the only independent predictor, which can be considered before the institution of VA-ECMO. Similarly to our findings, other groups have also identified lower lactate levels before initiation of VA-ECMO to be associated with improved hospital survival [6, 10, 13], which reflects earlier recognition of shock and more prompt effective resuscitation. We found a cut-off point of lactate level just before VA-ECMO implantation of 6.45 mmol/l. This value, along with higher doses of terlipresin, lower mean blood pressure and further increase in lactate level under VA-ECMO support, reflects a continued shock state and predicts higher mortality.
Bleeding during VA-ECMO, CPB time and female gender have little influence on the decision to initiate VA-ECMO therapy, but become more important during the postimplantation period. Bleeding during VA-ECMO was found to be an independent predictor of mortality in our cohort. This predictor can help in the decision-making process during the ICU stay, but not as a parameter to decide whether or not to implant VA-ECMO. Prolonged CPB time was significantly longer in those patients who died, but as cross-clamp time was not significantly different between both groups, it is probably just a surrogate of difficult CPB weaning. One could hypothesize that a longer and complicated CPB weaning with the need of an immediate VA-ECMO implantation should reflect a more severe myocardial damage leading to a higher mortality, but we could not find any relation between the time delay of VA-ECMO implantation and mortality.
Although some groups have described specific catecholamine doses as a prerequisite for VA-ECMO institution (e.g. epinephrine 8–10 μg/min, norepinephrine 6–14 μg/min, dobutamine 5–10 μg/kg/min) [14], we prefer a patient-specific approach where several clinical factors are considered before initiation of VA-ECMO. In general, however, we treat nearly all patients with preoperative normal LVEF, who develop refractory PCCS with VA-ECMO, as time and haemodynamic support are required to try to identify a reversible cause for this catastrophic complication.
Interestingly, we did not find any association between age and increased mortality. This correlates with the findings of other studies which concluded that an ECMO-based approach can be justifiable and has acceptable results in older patients [15, 16]. Although advanced age should not be considered an absolute contraindication for VA-ECMO, older patients present a higher risk of early mortality and increased scrutiny is therefore required before implementing VA-ECMO [10, 17, 18].
Our cohort included only patients with normal preoperative LVEF. Nevertheless, the mortality in our cohort was high in comparison to other series without differentiation of LVEF, some with observed mortality rates up to 39% [10, 19]. This demonstrates the high lethality of PCCS, which depends on a complex composite of putative prognostic factors and not only on the preoperative LVEF per se. Hence, despite technological advances and increased clinical experience with VA-ECMO therapy over the years, the overall mortality of patients undergoing VA-ECMO after PCCS continues to be high [20]. We did not observe a decrease in mortality during our study period.
Initiating VA-ECMO in patients with PCCS is indeed a salvage intervention in very critical patients with poor prognosis. Given the tremendous resource burden related to VA-ECMO, recognition of predictors is therefore of utmost importance to identify patients with a higher likelihood of survival.
Limitations
The main limitation of this study is to be a single-centre experience with retrospective nature. Therefore, it is subject to inherent biases. There is no uniformity in the employed selection criteria related to the implantation technique and the cannulation site, as this decision-making process was left at the discretion of the operating surgeon and has varied during the 20-year observation period. Being a tertiary referral centre that performs large volumes of surgeries in adult patients, our current results may not apply to all institutions, especially those that have very limited experience with VA-ECMO therapy. Finally, we do not have data regarding long-term follow-up.
CONCLUSION
Mortality rates of VA-ECMO in PCCS patients are high, even in those with preoperative normal LVEF. Coronary problems are an important cause of PCCS; however, the aetiology remains unknown in the vast majority of the cases.
The implantation of VA-ECMO before development of tissue hypoperfusion and the control of VA-ECMO-associated complications are the most important prognostic factors in PCCS patients. Lactate level may help guide timing of VA-ECMO implantation and define the extent of therapeutic effort.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Author contributions
Priya R. Menon: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. Anna Flo Forner: Formal analysis; Methodology; Supervision; Writing—review & editing. Mateo Marin-Cuartas: Conceptualization; Formal analysis; Writing—review & editing. Sven Lehmann: Methodology. Diyar Saeed: Writing—review & editing. André Ginther: Resources. Michael A. Borger: Supervision; Writing—review & editing. Jörg Ender: Conceptualization; Methodology; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Dominique Shum-Tim, Jarle Vaage and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- ICU
Intensive care unit
- IQR
Interquartile range
- LVEF
Left ventricular ejection fraction
- OR
Odds ratio
- PCCS
Postcardiotomy cardiogenic shock
- VA-ECMO
Venoarterial extracorporeal membrane oxygenation
REFERENCES
- 1. Sylvin EA, Stern DR, Goldstein DJ.. Mechanical support for postcardiotomy cardiogenic shock: has progress been made? J Card Surg 2010;25:442–54. [DOI] [PubMed] [Google Scholar]
- 2. Unosawa S, Sezai A, Hata M, Nakata K, Yoshitake I, Wakui S. et al. Longterm outcomes of patients undergoing extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. Surg Today 2013;43:264–70. [DOI] [PubMed] [Google Scholar]
- 3. Hernandez AF, Grab JD, Gammie JS, O’Brien SM, Hammill BG, Rogers JG. et al. A decade of short-term outcomes in postcardiac surgery ventricular assist device implantation: data from the Society of Thoracic Surgeons National Cardiac Database. Circulation 2007;116:606–12. [DOI] [PubMed] [Google Scholar]
- 4. Doll N, Kiaii B, Borger M, Bucerius J, Krämer K, Schmitt DV. et al. Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg 2004;77:151–7. [DOI] [PubMed] [Google Scholar]
- 5. Elsharkawy HA, Li L, Esa WA, Sessler DI, Bashour CA.. Outcome in patients who require venoarterial extracorporeal membrane oxygenation support after cardiac surgery. J Cardiothorac Vasc Anesth 2010;24:946–51. [DOI] [PubMed] [Google Scholar]
- 6. Fux T, Holm M, Corbascio M, Lund LH, van der Linden J.. Venoarterial extracorporeal membrane oxygenation for postcardiotomy shock: risk factors for mortality. J Thorac Cardiovasc Surg 2018;156:1894–902.e3. [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Wang H, Hou X.. Clinical outcomes of adult patients who receive extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2018;32:2087–93. [DOI] [PubMed] [Google Scholar]
- 8. Fiser SM, Tribble CG, Kaza AK, Long SM, Zacour RK, Kern JA. et al. When to discontinue extracorporeal membrane oxygenation for postcardiotomy support. Ann Thorac Surg 2001;71:210–4. [DOI] [PubMed] [Google Scholar]
- 9. Slottosch I, Liakopoulos O, Kuhn E, Deppe A-C, Scherner M, Madershahian N. et al. Outcomes after peripheral extracorporeal membrane oxygenation therapy for postcardiotomy cardiogenic shock: a single-center experience. J Surg Res 2013;181:e47–55. [DOI] [PubMed] [Google Scholar]
- 10. Biancari F, Perrotti A, Dalén M, Guerrieri M, Fiore A, Reichart D. et al. Meta-analysis of the outcome after postcardiotomy venoarterial extracorporeal membrane oxygenation in adult patients. J Cardiothorac Vasc Anesth 2018;32:1175–82. [DOI] [PubMed] [Google Scholar]
- 11. Mariscalco G, Salsano A, Fiore A, Dalén M, Ruggieri VG, Saeed D. et al. Peripheral versus central extracorporeal membrane oxygenation for postcardiotomy shock: multicenter registry, systematic review, and meta-analysis. J Thorac Cardiovasc Surg 2020;160:1207–16.e44. [DOI] [PubMed] [Google Scholar]
- 12. Raffa MG, Kowalewski M, Brodie D, Ogino M, Whitman G, Meani P. et al. Meta-analysis of peripheral or central extracorporeal membrane oxygenation in postcardiotomy and non-postcardiotomy shock. Ann Thorac Surg 2019;107:311–21. [DOI] [PubMed] [Google Scholar]
- 13. Hu RTC, Broad JD, Osawa EA, Ancona P, Iguchi Y. et al. 30-day outcomes post veno-arterial extra corporeal membrane oxygenation (VA-ECMO) after cardiac surgery and predictors of survival. Heart Lung Circ 2020;29:1217–25. [DOI] [PubMed] [Google Scholar]
- 14. Musiał R, Moncznik P, Śmiałek P, Stoliński J, Sadowski J, Drwiła R.. Veno-arterial extracorporeal membrane oxygenation for short-term mechanical circulation support in adults with cardiogenic shock: a single centre experience. Kardiol Pol 2016;74:1477–84. [DOI] [PubMed] [Google Scholar]
- 15. Smith C, Bellomo R, Raman JS, Matalanis G, Rosalion A, Buckmaster J. et al. An extracorporeal membrane oxygenation-based approach to cardiogenic shock in an older population. Ann Thorac Surg 2001;71:1421–7. [DOI] [PubMed] [Google Scholar]
- 16. Biancari F, Saeed D, Fiore A, Dalén M, Ruggieri VG, Jónsson K. et al. Postcardiotomy venoarterial extracorporeal membrane oxygenation in patients aged 70 years or older. Ann Thorac Surg 2019;108:1257–64. [DOI] [PubMed] [Google Scholar]
- 17. Guihaire J, Dang Van S, Rouze S, Rosier S, Roisne A, Langanay T. et al. Clinical outcomes in patients after extracorporeal membrane oxygenation support for post-cardiotomy cardiogenic shock: a single-centre experience of 92 cases. Interact CardioVasc Thorac Surg 2017;25:363–9. [DOI] [PubMed] [Google Scholar]
- 18. Wu MY, Lin PJ, Lee MY, Tsai FC, Chu JJ, Chang YS. et al. Using extracorporeal life support to resuscitate adult postcardiotomy cardiogenic shock: treatment strategies and predictors of short-term and midterm survival. Resuscitation 2010;81:1111–6. [DOI] [PubMed] [Google Scholar]
- 19. Sauer CM, Yuh DD, Bonde P.. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J 2015;61:31–6. [DOI] [PubMed] [Google Scholar]
- 20. Bardia A, Schonberger RB.. Postcardiotomy venoarterial extracorporeal membrane oxygenation (VA ECMO) in adult patients—many questions, few answers, and hard choices. J Cardiothorac Vasc Anesth 2018;32:1183–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.