Abstract
OBJECTIVES
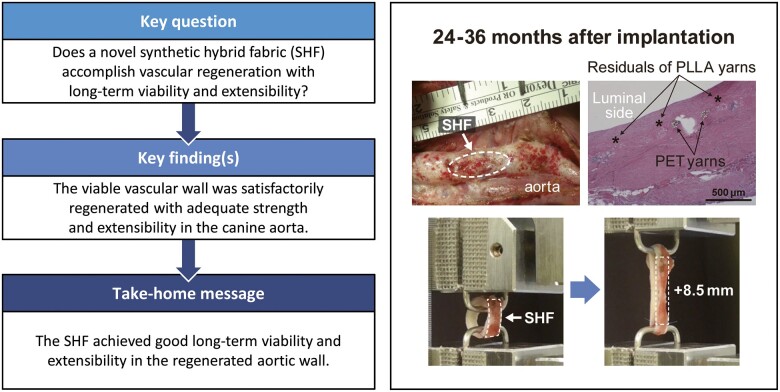
Many surgical materials promoting tissue regeneration have been explored for use in paediatric cardiac surgery. The aim of this study is to evaluate the long-term viability and extensibility of the canine aortic wall regenerated using a novel synthetic hybrid fabric.
METHODS
The sheet is a warp-knitted fabric of biodegradable (poly-l-lactic acid) and non-biodegradable (polyethylene terephthalate) yarns coated with cross-linked gelatine. This material was implanted as a patch to fill an oval-shaped defect created in the canine descending aorta. The tissue samples were explanted after 12, 24 or 36 months (N = 3, 2, 2, respectively) for histological examination and biomechanical testing.
RESULTS
There was no shrinkage, rupture or aneurysmal change after 24 months. The regenerated wall showed prototypical vascular healing without material degeneration, chronic inflammation, calcification or abnormal intimal overgrowth. Bridging tissue across the patch was well-formed and had expanded over time. The biodegradable yarns had completely degraded at 24 months after implantation, as scheduled, but the regenerated aortic wall demonstrated satisfactory levels of mechanical strength and extensibility in tensile strength tests.
CONCLUSIONS
The sheet achieved good long-term viability and extensibility in the regenerated aortic wall. These findings suggest that it is a promising surgical material for repairing congenital heart defects. Further developments of the sheet are required, including clinical studies.
Keywords: Congenital cardiac surgery, Surgical material, Tissue regeneration, Biodegradable polymer, Mechanical strength, Extensibility
INTRODUCTION
Xenobiological and conventional synthetic materials have been used worldwide for repairs in paediatric cardiac surgery. However, these non-viable materials have drawbacks of material degradation, calcification and excess foreign body reactions leading to haemodynamic disturbances in the affected vessels. These drawbacks have led to a focus on induction of autologous tissue regeneration, and several approaches have been developed for ideal tissue regeneration: (i) ‘classical’ in vitro tissue-engineering with cell-seeding, (ii) the in vivo fibrous membrane approach utilizing the foreign body reaction and (iii) the in situ tissue regeneration or restoration approach, which focuses exclusively on the use of biodegradable polymers.
Classical in vitro tissue-engineering [1] has been widely studied and was the earliest approach to be used clinically in paediatric cardiac surgery [2]. However, manufacturing costs and product specifications for physical properties remain practical problems. In the second approach, an in vivo fibrous membrane is created by a foreign body reaction through encapsulation of a mould implanted subcutaneously for several months. However, the encapsulation process varies, leading to unpredictable and non-uniform properties of the membrane. Clinical use as a surgical patch for pulmonary artery augmentation has been attempted [3], but the effectiveness of the membrane has not been verified.
The critical underlying problem of the 2 approaches is clinical practicality in terms of off-the-shelf availability. Thus, we shifted our interest towards in situ tissue restoration or a regeneration approach based exclusively on moulding techniques and biodegradable polymers. Animal studies have shown that autologous tissue gradually replaces the specially designed fabric in both tube grafts and cardiac valve leaflets [4, 5], and several clinical trials of the feasibility and safety of this method are ongoing in the area of congenital cardiac surgery [6, 7].
As a new option for in situ tissue regeneration, we developed synthetic hybrid fabric (SHF) sheets of a warp-knitted fabric composed of biodegradable and non-biodegradable yarns and coated with crosslinked gelatine. We observed successful in situ tissue regeneration on these sheets implanted in the canine aortic wall [8]. Following the proof of concept in a short-term follow-up study [8], the current study was designed to assess the long-term maturation and extensibility of the regenerated aortic wall, which are other important issues requiring proof of concept for paediatric use, after the biodegradable component has completely dissolved.
MATERIALS AND METHODS
Ethical statement
The study was approved by the Animal Research Committee of Osaka Medical College (approval IDs #27019, #28086, #29075, #30062, #2019-042). The animals received humane care based on the guidelines of this committee and the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Design and fabrication of the synthetic hybrid fabric
The fabric was designed and fabricated as described previously [8]. Briefly, SHF is an extensible warp-knitted fabric composed of non-biodegradable [polyethylene terephthalate (PET)] and biodegradable [poly-l-lactic acid (PLLA)] yarns coated with crosslinked gelatine to seal its porous structure. The non-biodegradable yarn was designed to stretch up to 4 times the original surface area after the biodegradable portion dissolves. The warp structure was designed and knitted using software connected to a customized industrial warp-knitting machine. The PLLA has a high molecular weight that was designed to gradually decrease to almost zero by hydrolysis 24 months after implantation, without loss of mechanical strength. The SHF was manufactured under a quality management system stipulated by the International Organization of Standardization 13485 protocol and was sterilized and packaged to meet standards for clinical use. Fukui Tateami Co., Ltd., Fukui, Japan manufactured the warp-knit fabric. Sample quality was also confirmed through strict spot inspection, following clinical product regulations in Japan.
Implantation of the sheet in the descending aorta and explantation of samples for assessment
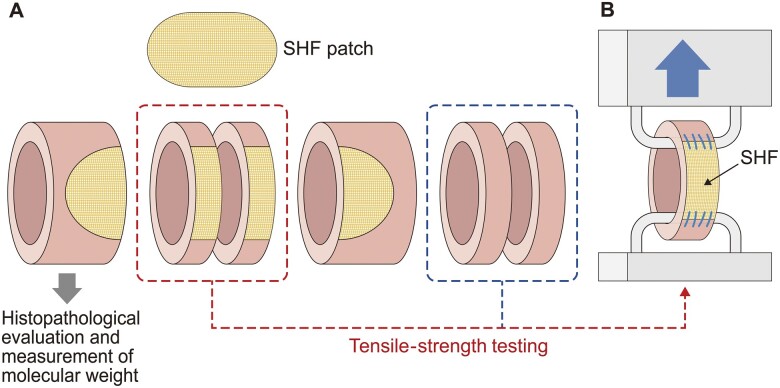
The sheet was implanted in 7 adult male beagles (age 7–19 months, bodyweight 7–10 kg, Hamaguchi Laboratories LLC, Osaka, Japan), as we have described previously [8]. Briefly, an oval-shaped defect was made in the descending aorta and filled with a patch (20 × 10 mm) trimmed from the SHF using a 6–0 polypropylene running suture (Prolene®, Ethicon Inc., Somerville, NJ, USA). All care required for animal welfare was provided throughout the study. No antiplatelet agents or anticoagulants were given after implantation. All beagles had similar backgrounds and were bred and raised under similar conditions to minimize inter-animal variation. At 12, 24 or 36 months after implantation, the beagles were euthanized with general anaesthesia followed by a fatal dose of an intravenous barbiturate. The patch was explanted en bloc with the connecting native aortic wall as a tube-shaped sample. Each excised tissue sample was divided into sections for histopathological evaluation, tensile strength testing and measurement of remaining PLLA (Fig. 1A).
Figure 1:
Schematic representation of the materials and methods. (A) Sampling from explanted tissue specimens. (B) Tensile strength test of a ring sample. SHF: synthetic hybrid fabric.
Histological examination
Histological examinations were performed as described in our previous study [8]. Sample slices were stained with haematoxylin and eosin to examine the general status of the SHF patch and inflammatory response. Collagen fibres, elastic fibres and calcium deposition in regenerative tissue were evaluated using Masson’s trichrome, Elastica van Gieson and Alizarin red staining, respectively. Endothelial cells were detected by immunohistochemistry with von Willebrand factor, and myofibroblasts and smooth muscle cells were detected by immunohistochemistry with alpha-smooth muscle actin. Tissue regeneration, infiltration of inflammatory cells at the patch implant site, and status of the implanted patch were scored semi-quantitatively: 0: none, 1: very slight, 2: mild, 3: moderate, 4: marked (Supplementary Material, Table S1).
The thickness of the regenerated tissue was measured in photographic images of the SHF sheet at the implanted sites taken under 150× magnification with a digital microscope (VHX-5000, Keyence Corp., Osaka, Japan). The thickness of the intimal layer on the luminal side of the SHF yarns was then assessed by 2-point measurement at 10 sites on samples at 12, 24 and 36 months after implantation.
Evaluation of polymer remnants
To evaluate the extent of PLLA degradation, the weight-averaged molecular weight of each polymer in the excised SHF samples at 12, 24 and 36 months was analysed by gel permeation chromatography using a modified version of a previously reported method [9]. Samples collected at 2 weeks, 3 months and 6 months after implantation [8] were similarly evaluated.
Tensile strength tests of the regenerated aortic wall
Tensile strength tests were performed with a texture analyser (EZ-SX, Shimadzu Corp., Kyoto, Japan) on ring-shaped test specimens containing the SHF (Fig. 1) [10, 11]. As a comparative control, a ring-shaped specimen was prepared from the autologous native aortic wall. The internal diameters of the ring-shaped tissue samples were calculated by measuring the distance between the 2 hooks on which the samples were hung and stretched. The sample was pulled starting from its original diameter at a speed of 2 mm/min with a tensile strength of 0.01 N and a rupture setting of 1% of %MAX. The border between the SHF and native aortic wall was marked. The sample stretching process was videotaped and the amount of extension of the SHF portion was determined off-site.
RESULTS
Macroscopic findings
Findings for the SHF at implantation and after explantation are shown in Fig. 2. Cross-sections of the aortic lesion showed no stenosis, rupture or aneurysm formation at any of the study time points after implantation (Fig. 2A and B). Interestingly, at 24 months after implantation, the examination of the luminal side of the surgical site revealed that the warp-knitted structure of the non-degradable PET portion was visible from inside the neovascular wall (Fig. 2C).
Figure 2:
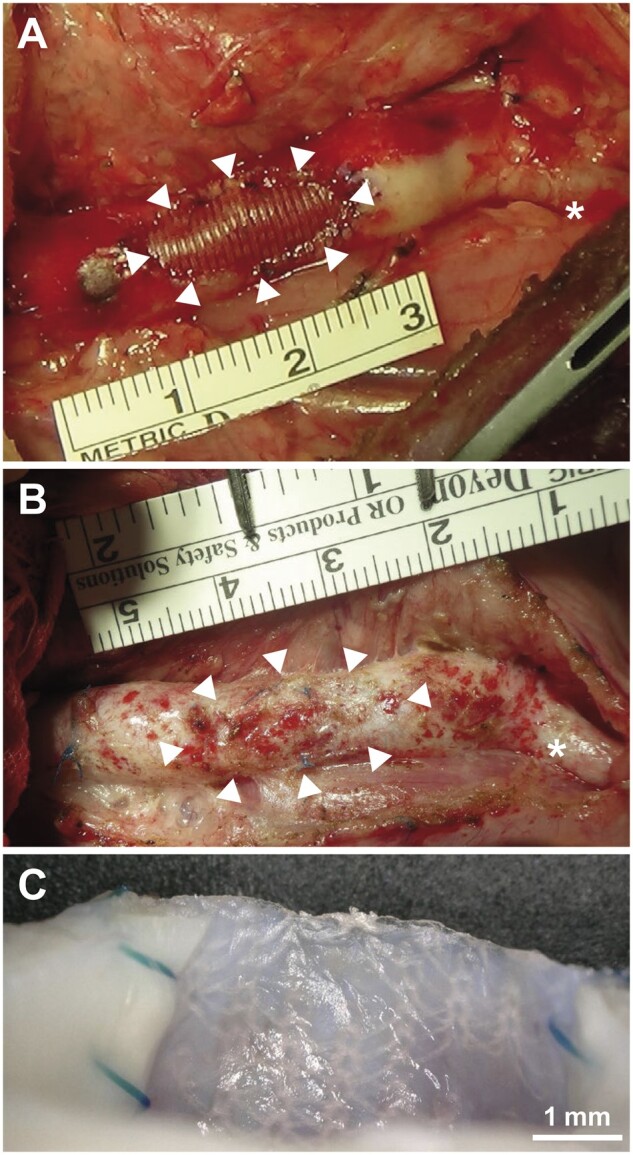
Macro photographs of the synthetic hybrid fabric after implantation. (A) At implantation. (B) At explantation after 24 months. White arrowheads: synthetic hybrid fabric. Asterisks (*) indicate left subclavian artery. (C) Cross-section of synthetic hybrid fabric patch shown in (B), showing polyethylene terephthalate yarns visible from inside the vessel.
Histological findings
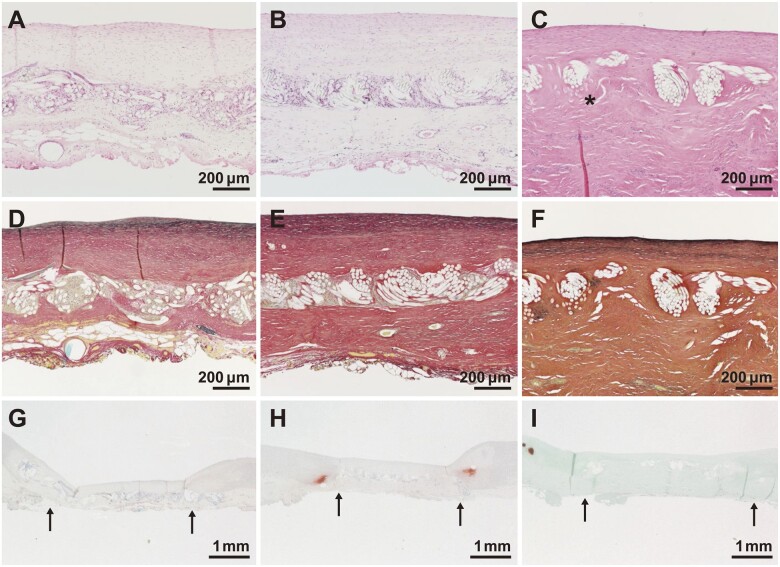
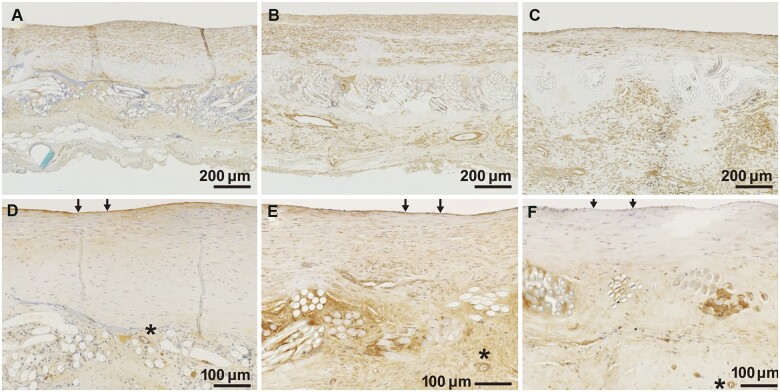
Quantitative scores for histological findings are shown in Table 1. Regenerated tissue on the luminal and external sides of the patch consisted mainly of collagen fibres and smooth muscle cells (Table 1, Figs 3A–C and 4A–C). Subintimal elastic fibres were slightly observed in tissues harvested at 12 months but were found under the regenerating intima on the luminal side of the vessel at 24 months after implantation (Table 1, Fig. 3D–F). For all samples at all time points, endothelial cells had formed on the most superficial layer of the luminal surface (Table 1, Fig. 4F) and inflammatory reactions were minimal (Table 1, Fig. 3A–C). There was no calcium deposition in regenerative tissue and slight calcium deposition in native tissue at the suture sites (Table 1, Fig. 3G–I).
Table 1:
Quantitative scoring of histological findings
| Evaluation items | Implantation period |
||
|---|---|---|---|
| 12 months (n = 3) | 24 months (n = 2) | 36 months (n = 2) | |
| Regenerated wall, maturity | |||
| Tissue regeneration | |||
| Granulation tissue | 1, 0, 1 | 0, 0 | 0, 0 |
| Smooth muscle cells | 1, 2, 2 | 3, 3 | 2, 2 |
| Collagen fibres | 4, 2, 2 | 2, 3 | 2, 2 |
| Endothelial cells (luminal surface) | 3, 3, 3 | 3, 3 | 3, 3 |
| Elastic fibres | 0, 2, 0 | 1, 2 | 1, 1 |
| Bridge formation | 1, 3, 4 | 4, 3 | 4, 4 |
| Inflammatory cells infiltration | |||
| Neutrophils | 0, 0, 0 | 0, 0 | 0, 0 |
| Lymphocytes | 0, 1, 1 | 1, 1 | 1, 0 |
| Plasma cells | 0, 0, 0 | 0, 0 | 0, 0 |
| Macrophages/giant cells | 1, 1, 3 | 1, 1 | 1, 0 |
| Material condition (SHF) | |||
| Gelatine remaining | 2, 1, 1 | 0, 0 | 0, 0 |
| Calcium deposition | 0, 0, 0 | 0, 0 | 0, 0 |
Grading: 0 none; 1 very slight; 2 mild; 3 moderate; 4 marked (Supplementary Material, Table S1). Raw data are shown as, for example, ‘2, 1’, which means that the scores were 2 and 1 in the 2 animals. SHF: synthetic hybrid fabric.
Figure 3:
Changes over time in representative histological images. (A–C) Haematoxylin and eosin staining. Asterisks (*) indicate poly-l-lactic acid fibres. (D–F) Elastica van Gieson staining. (G–I) Alizarin red staining. Arrows (→) indicate the suturing border. (I) Counterstained with light green. (A, D, G) 12 months, (B, E, H) 24 months, and (C, F, I) 36 months after implantation.
Figure 4:
Changes over time in representative immunohistochemical images. Immunohistochemistry with (A–C) alpha smooth muscle actin and (D–F) von Willebrand factor. Asterisks (*) indicate small blood vessels in bridging tissue. Arrows (→) indicate endothelial cells. (A, D) 12 months, (B, E) 24 months and (C, F) 36 months after implantation.
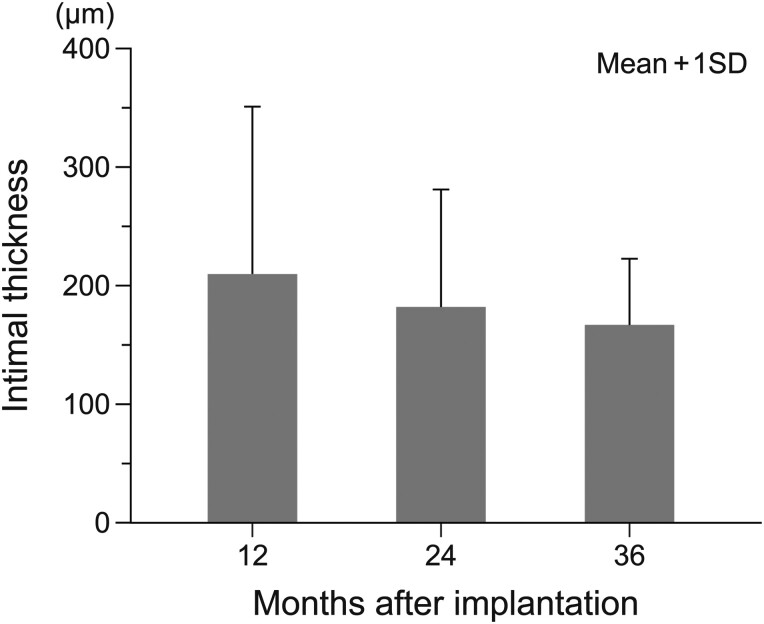
At 12 months, gelatine had almost disappeared (Table 1). PLLA had almost entirely disappeared by 36 months (Fig. 3C). Over time, there were increases in the amount and width of regenerative tissue growing across the patch and forming new bridging tissue (Table 1, Fig. 3A–C). Von Willebrand Factor staining showed the formation of small blood vessels in the bridging tissue (Fig. 4D and E). The regenerative intima on the luminal side of the SHF yarns was stable in the samples at 12, 24 and 36 months, with a thickness of approximately 190 ± 111 µm and without further thickening or growth (Table 1, Fig. 5). The results of changes over time and during the acute stage [8] and haematoxylin and eosin-stained images are shown in the Supplementary Material, Table S2 and Fig. S1.
Figure 5:
Thickness of regenerated intima on the luminal side of the patch. The bars show means of all measurement results for each implantation period (10-point measurements for each animal). Error bars are standard deviations. Number of animals: 12 months (3), 24 months (2), 36 months (2).
Degradation of poly-l-lactic acid
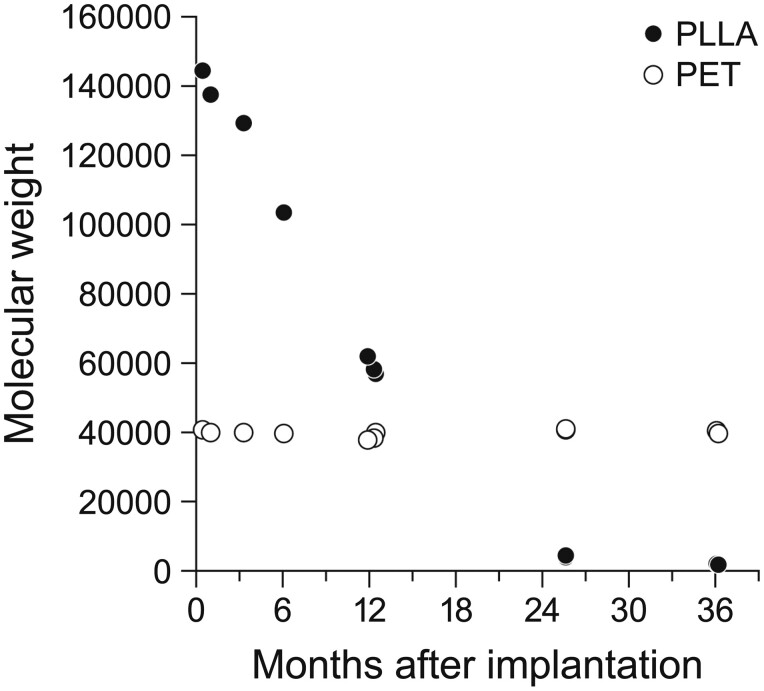
PLLA degraded to 50% of its initial level at 12 months after implantation, using the weight-averaged molecular weight. By 24 months, the PLLA molecular weight in the regenerated wall had decreased to <4000, resulting in complete loss of PLLA-related mechanical strength. The molecular weight of PET remained stable, with no changes after implantation (Fig. 6).
Figure 6:
Changes over time in molecular weight of PLLA and PET. PET: polyethylene terephthalate; PLLA: poly-l-lactic acid.
Mechanical strength and extensibility
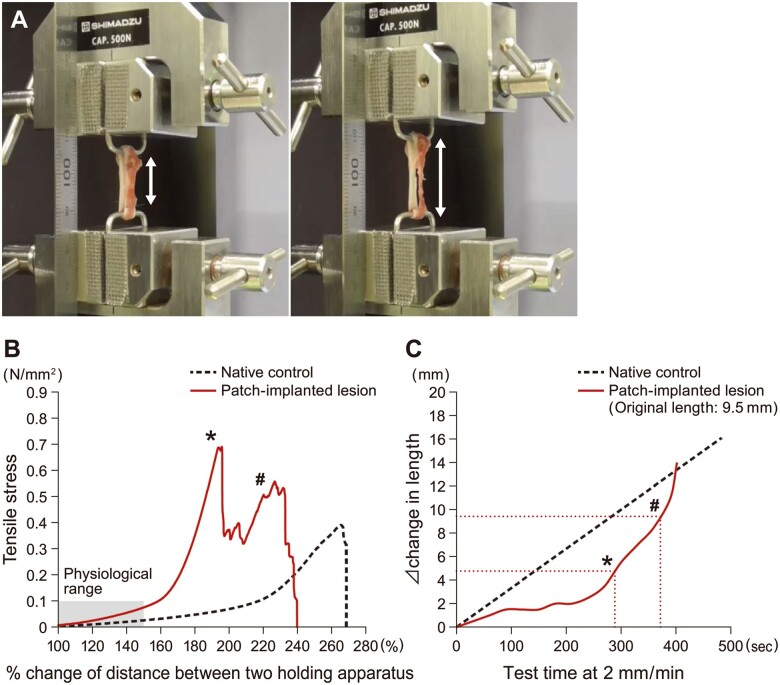
The equipment used to assess the mechanical strength and extensibility of tissue specimens is shown in Fig. 7A. The % changes in entire specimen lengths and tensile stress curves are shown in Fig. 7B. Within the extensibility-tensile stress range under normal physiological conditions of the aorta [12, 13], the tensile stress curve showed continuity without breakage (Fig. 7B). As shown in the photos in Fig. 7A and the curve in Fig. 7B, continued extension beyond the physiological range led first to rupture of the luminal neointimal layer, followed by complete rupture of the neovascular wall containing the SHF with a greater extension (2-phase transition). The SHF portion was extended to 1.5 times the original length at neointimal rupture (Fig. 7C, asterisk) and twice the original length at complete rupture (Fig. 7C, hashtag).
Figure 7:
Assessment of mechanical strength and extensibility of the patch at 24 months after implantation. (A) Testing unit and extent of expansion. Left: at rupture of neointima, right: at rupture of entire vessel. Double head arrows indicate the length of the regenerated wall. (B) Stress-extensibility curve [shaded area: the estimated physiological range (force approximately 0.1 N/mm2, expansion approximately 150%)]. (C) Change in length of the regenerated wall. In (B) and (C), the asterisk (*) indicates the point of neointimal rupture on the luminal side and the hashtag (#) indicates the point of complete rupture of the entire specimen.
DISCUSSION
There were 2 major findings in this study of the long-term efficacy of a novel PLLA/PET warp knitted SHF for in situ regeneration of the canine aortic wall. First, the regenerated aortic wall continued to mature and remain viable around the remaining non-degradable yarn at 24 months after implantation. The regenerated wall was not fully normal but was consistent with an endothelial cell layer, aligned collagen and muscle fibres, vasa vasorum formation and small amounts of elastin fibres. There was no inflammation, calcification or excess intimal thickening on the luminal side. PLLA had degraded to near zero in molecular weight at 24 months after implantation, as designed. Second, the regenerated wall maintained sufficient strength to prevent aneurysmal or stenotic deformity, even after the loss of mechanical strength associated with PLLA degradation, and the regenerated walls remained extensible by 50–100% in length.
A critical element of in situ tissue engineering is to determine the optimal combination of the implant structure and the biodegradable material component. A non-woven fabric formed by electrospinning in combination with a rapidly degradable polymer has attracted attention, and the tissue-regenerating capabilities of this fabric have been assessed in large animal models in pulmonary arteries [14] and pulmonary valve leaflets [4, 5]. Since the fabric was non-porous with poor cell attachment, and polymer degradation depended on inflammatory cell phagocytosis, the biological breakdown of the polymer was inhomogeneous and unpredictably sustained over several years, despite the use of a rapidly biodegradable polymer [14]. The intrinsic nature of the electro-spun scaffold results in abnormal histology of regenerated tissue (i.e. islands of undegraded polymer, persistent inflammatory reactions and dense collagen bands) and the effects are far from the prototypical vascular healing in classical in vitro tissue engineering using sponge [11] or mesh fabric [15]. In contrast, our use of a computationally designed porous warp-knit in combination with slowly hydrolysed PLLA yarns achieved prototypical vascular healing. Maturation of regenerated tissue progressed continuously and consistently without abnormal intimal thickening in the acute phase [8] or the late phase after almost all PLLA yarn was degraded, as scheduled at 24 months after implantation.
The loss of mechanical strength that accompanies polymer breakdown is a major concern in tissue engineering using this type of polymer. This strength depends on a favourable balance between the rates of polymer breakdown and tissue development. An imbalance in these rates became a major complication in a clinical trial using classical in vitro tissue engineering. Tube grafts of the scaffold were created from a mixture of ε-caprolactone/PLLA on a polylactic acid or polyglycolic acid backbone, seeded with mononuclear cells from the patient’s bone marrow, and implanted in 25 patients as an extracardiac conduit in Fontan surgery [2]. Unfortunately, significant graft stenosis developed in 7 patients (28%) and some required catheter intervention. The stenosis occurred in the early phase, 1.5 years after implantation, and in the long term at 9 years after implantation [2]. We used a different implantation site in a canine model, so no direct comparison is possible. However, our SHF maintained satisfactory mechanical strength for 3 years after implantation, similarly to electro-spun fabric with a rapidly biodegradable polymer in in situ tissue regeneration described by Brugmans et al. [14]. Thus, the current combination of SHF design with a skeleton of non-degradable yarns and slowly biodegradable polymer may offer a safe and practical choice for obtaining a favourable balance between the rates of polymer breakdown and tissue development.
Regenerated tissue induced in this approach is required to have growth potential when used in a paediatric population. This potential is recognized practically as extensibility that occurs with somatic growth or is introduced by catheter angioplasty. A regenerated vascular wall formed by classical in vitro tissue engineering has been found to expand by 45% and 40% in length in the long term when implanted as a graft in the pulmonary artery of lambs [15] and in the inferior vena cava of lambs [16] and children [2], respectively. Therefore, although it remains unclear whether expansion occurred by somatic growth or simple mechanical stretching, a regenerated vascular wall with less mechanical strength is likely to have greater expandability. The expandability of a regenerated vascular wall induced by in situ tissue engineering has yet to be demonstrated in animal models that allow observation of defined somatic growth. Because of limitations on the experimental use of lambs in Japan, the SHF extensibility after complete PLLA degradation during somatic growth could not be assessed. However, the regenerated canine aortic wall along the SHF that was explanted 2 years after implantation expanded by 50–100% in length when tensile force was applied. This expandability of the SHF might be beneficial in clinical settings such as balloon angioplasty, even though there are some limitations.
Limitations
Although in situ tissue engineering has strong practical advantages for commercial production, it is difficult to determine the best combination of the moulding method for the device and the biodegradable polymer as materials for targeted tissue regeneration. This is because the extent and speed of regeneration depend on the location and dimensions of the implantation site, the age of the recipient and the biomechanism of degradation after implantation. This study adds another option of the reported combination of electro-spun fabric using a rapidly biodegradable polymer; however, this may need to be developed further through adjustments to yarns, patterns of warp knitting and the optimal choice of polymer to obtain the desired level of tissue regeneration. Since our current long-term data are derived from only 4 adult canines, further investigation of the extensibility of the sheet with somatic growth is required. However, we proved, at least in part, that the sheet has positive extensibility with sustainable mechanical strength. A prospective multicentre single-arm clinical study to evaluate the efficacy and safety of SHF in patients with congenital heart disease was initiated in Japan in May 2019 [17]. Upcoming findings and results from this study will provide additional supportive data for in situ tissue regeneration based on our selection of polymers and structure.
CONCLUSION
The regenerated canine aortic wall along the SHF continued to mature over the long term, and the histology of the wall was identical to that for typical vascular healing. There was no material degeneration, chronic inflammation, calcification or intimal overgrowth in the regenerated wall. The regenerated aortic wall had sufficient mechanical strength and extensibility after the complete biodegradation of the polymer. These findings suggest that the SHF is a promising surgical material for the repair of congenital heart defects. We believe that commercialization of the SHF could provide an off-the-shelf surgical product to introduce tissue regeneration therapy as a practical tool in daily practice.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENTS
Atsuko Ueda coordinated animal studies and data collection. Kayoko Miyata handled data collection. Takafumi Ogawa (Kyodo Byori Inc., Kobe, Japan) prepared histological specimens. Medical writing support was provided by EDIT, Inc., Tokyo, Japan and PALABRA, Co. Ltd., Kyoto, Japan.
ABBREVIATIONS
- PET
Polyethylene terephthalate
- PLLA
Poly-l-lactic acid
- SHF
Synthetic hybrid fabric
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
Funding
This work was partially supported by the Japan Agency for Medical Research and Development, AMED [JP19he1302009].
Conflict of interest: Shintaro Nemoto has received research grant from the Japan Agency for Medical Research and Development, AMED [JP19he1302009]. Satomi Osawa is an employee of Teijin Pharma Limited. Ayuko Yamaguchi is an employee of Teijin Limited. The other authors declared no conflicts of interest.
Author contributions
Shintaro Nemoto: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—review & editing. Hayato Konishi: Investigation. Tatsuya Suzuki: Investigation. Ryo Shimada: Investigation. Takahiro Katsumata: Supervision; Validation. Satomi Osawa: Investigation. Ayuko Yamaguchi: Investigation.
Reviewer Information
Reviewer information Interactive CardioVascular and Thoracic Surgery thanks Vito Domenico Bruno and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Langer R, Vacanti JP.. Tissue engineering. Science 1993;260:920–6. [DOI] [PubMed] [Google Scholar]
- 2. Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C. et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 2010;139:431–6. 436.e1-2. [DOI] [PubMed] [Google Scholar]
- 3. Kato N, Yamagishi M, Kanda K, Miyazaki T, Maeda Y, Yamanami M. et al. First successful clinical application of the in vivo tissue-engineered autologous vascular graft. Ann Thorac Surg 2016;102:1387–90. [DOI] [PubMed] [Google Scholar]
- 4. Kluin J, Talacua H, Smits AIPM, Emmert MY, Brugmans MCP, Fioretta ES. et al. In situ heart valve tissue engineering using a bioresorbable elastometric implant-From material design to 12 months follow-up in sheep. Biomaterials 2017;125:101–17. [DOI] [PubMed] [Google Scholar]
- 5. Stassen OMJA, Muylaert DEP, Bouten CVC, Hjortnaes J.. Current challenges in translating tissue-engineered heart valves. Curr Treat Options Cardiovasc Med 2017;19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bockeria LA, Svanidze O, Kim A, Shatalov K, Makarenko V, Cox M. et al. Total cavopulmonary connection with a new bioabsorbable vascular graft: first clinical experience. J Thorac Cardiovasc Surg 2017;153:1542–50. [DOI] [PubMed] [Google Scholar]
- 7.Xeltis Bioabsorbable Pulmonary Valved Conduit Early Feasibility Study (Xplore 2). ClinicalTrials.gov ID: NCT03022708. https://clinicaltrials.gov/ct2/show/NCT03022708 (21 August 2020, date last accessed).
- 8. Nemoto S, Konishi H, Shimada R, Suzuki T, Katsumata T, Yamada H. et al. In situ tissue regeneration using a warp-knitted fabric in the canine aorta and inferior vena cava. Eur J Cardiothorac Surg 2018;54:318–27. [DOI] [PubMed] [Google Scholar]
- 9. Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, García-García HM. et al. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation 2010;122:2288–300. [DOI] [PubMed] [Google Scholar]
- 10.International Organization for Standardization. ISO 7198:2016. Cardiovascular Implants and Extracorporeal Systems—Vascular Prostheses—Tubular Vascular Grafts and Vascular Patches. Switzerland: ISO, 2016. [Google Scholar]
- 11. Matsumura G, Ishihara Y, Miyagawa-Tomita S, Ikada Y, Matsuda S, Kurosawa H. et al. Evaluation of tissue-engineered vascular autografts. Tissue Eng 2006;12:3075–83. [DOI] [PubMed] [Google Scholar]
- 12. Greenfield JC Jr, Patel DJ.. Relation between pressure and diameter in the ascending aorta of man. Circ Res 1962;10:778–81. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi K, Naiki T.. Adaptation and remodeling of vascular wall; biomechanical response to hypertension. J Mech Behav Biomed Mater 2009;2:3–19. [DOI] [PubMed] [Google Scholar]
- 14. Brugmans M, Serrero A, Cox M, Svanidze O, Schoen FJ.. Morphology and mechanisms of a novel absorbable polymeric conduit in the pulmonary circulation of sheep. Cardiovasc Pathol 2019;38:31–8. [DOI] [PubMed] [Google Scholar]
- 15. Hoerstrup SP, Cummings Mrcs I, Lachat M, Schoen FJ, Jenni R, Leschka S. et al. Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation 2006;114:I159–66. [DOI] [PubMed] [Google Scholar]
- 16. Brennan MP, Dardik A, Hibino N, Roh JD, Nelson GN, Papademitris X. et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg 2008;248:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OFT-G1-301 study: A Prospective, Multi-Center, Single-Arm Study in Congenital Heart Disease (JapicCTI-194765). https://www.clinicaltrials.jp/cti-user/trial/Show.jsp (25 January 2021, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.