Abstract
OBJECTIVES
A thymic epithelial tumour is the most common primary tumour in the anterior mediastinum of adults. A few retrospective studies compared the short-term outcomes between robotic-assisted thymectomy (RAT) and video-assisted thymectomy (VAT). So, it is necessary to conduct a meta-analysis to further compare these 2 surgical techniques.
METHODS
EMBASE, Medline and Web of Science were used. Thesaurus terms and medical subject headings were used in Medline and EMBASE, respectively. The Newcastle-Ottawa scale was used for grading because the included studies were all case-control studies.
RESULTS
Nine studies were included in the meta-analysis with a total of 723 patients, including 315 patients in the RAT group and 408 patients in the VAT group. The meta-analysis [odds ratio (OR) 0.24, 95% confidence interval (CI) 0.06–0.94; P = 0.041], indicating that RAT yielded a significantly lower rate of conversion compared with VAT. Duration of drainage with RAT was significantly less than that with VAT (weighted mean difference = −1.10; 95% CI −1.98 to −0.22; P = 0.014). The pooled analysis (weighted mean difference = −103.6; 95% CI −199.21 to −7.98; P = 0.034) suggested that patients in the RAT group had less drainage than those in the VAT group. The recurrence rates in both groups were comparable (OR 0.19, 95% CI 0.03–1.20; P = 0.078).
CONCLUSIONS
RAT has advantages over VAT in terms of short-term outcomes such as shorter duration of drainage, less total drainage and a lower rate of conversion. The recurrence rate was comparable between the 2 techniques. Therefore, RAT could be considered as an alternative treatment for diseases of the thymus.
Keywords: Robot-assisted thymectomy, Video-assisted thymectomy, Short-term outcomes
INTRODUCTION
The thymic epithelial tumour is the most common primary tumour in the anterior mediastinum of adults [1]. Thymomas are located mainly in the anterior mediastinum, originate in the thymus tissue and comprise 20–30% of mediastinal masses in adults [2]. A total of 10–30% of patients with myasthenia gravis (MG) suffer from thymoma and thymic neoplasia. MG was found in 50% of patients with thymomas [3]. Thymectomy is an effective treatment option for thymoma and for long-term remission of MG. To treat MG and anterior mediastinal tumours, thymectomy is a conventional surgical technique. It can be accomplished with various approaches, including a median sternotomy [4]. However, many less invasive surgical procedures are now possible. Minimally invasive surgery is used for the diagnosis, staging and treatment of mediastinal masses. Specifically, researchers have suggested that stage I and II thymomas can be resected using a minimally invasive technique such as video-assisted thymectomy (VAT) [5]. Compared with open thymectomy, minimally invasive surgical techniques have been associated with lower operative blood loss, shorter length of stay in the intensive care unit and in the hospital and decreased postoperative pain. However, VAT is limited by the decreased freedom of movement of the operators and by its 2-dimensional view, which may make elaborate dissections difficult. Furthermore, the high technical complexity of the VAT procedure can result in a complicated learning curve [6]. A relatively new kind of minimally invasive technique was applied in thoracic surgery called the robotic da Vinci Surgical System [7, 8]. It is also used in urological and rectal surgery. Compared with VAT, robotic-assisted thymectomy (RAT) provides 3-dimensional (3D) visualization and free articulation of the tips of the robotic arms and offers advantages when operating in narrow spaces such as the pelvis or the mediastinum [9]. In clinical practice, we found that the superior poles and upper border of the thymus and the lymph nodes can be removed more completely using the robotic technique [10]. Moreover, it can dissect thymic epithelial tumours more elaborately compared with the thoracoscopic technique. Therefore, it can better maintain membrane integrity.
However, whether RAT has advantages over VAT remains controversial. There are no clinical trials comparing the short-term outcomes between RAT and VAT. A few retrospective studies compared these 2 techniques. So, it is necessary to conduct a meta-analysis to further compare the short-term outcomes after RAT and VAT. In this meta-analysis, we included 9 studies and compared 10 clinical outcomes between RAT and VAT.
METHODS
Retrieval strategy
We used EMBASE (OVID, 1995 to 1 December 2020), Medline (PubMed, 1995 to 1 December 2020) and Web of Science (1995 to 1 December 2020). Thesaurus terms and medical subject heading (MeSH) were used in Medline and EMBASE, respectively. The keywords were identified and determined in the 2 databases (Medline and EMBASE) according to the PICO (population, intervention, control and outcomes) format: ((‘thymus’ OR ‘thymoma’ OR ‘thymectomy’) AND (‘video’ OR ‘video-assisted’ OR ‘video assisted’ OR ‘thoracoscopic’ OR ‘thoracoscopy’ OR ‘minimally invasive’ OR ‘VATS’) AND (‘robot’ OR ‘robotic’ OR ‘robot-assisted’ OR ‘robot assisted’ OR ‘da Vinci’ OR ‘daVinci’)). We used MeSH and free words as keywords. The PICO format was adopted to establish specific selection criteria, in which P referred to patients undergoing minimally invasive thymectomy, I referred to minimally invasive RAT, C referred to minimally invasive VAT and O included intraoperative estimated blood loss (ml), surgical time (min), conversion to open surgery, total amount of drainage (ml), duration of drainage (days), duration of hospital stay, postoperative complications, pneumonia, phrenic nerve paralysis and recurrence. No language restriction and no filters were applied. Studies meeting the following criteria were included: (i) the patients in the study had minimally invasive thymectomy; (ii) sufficient data were provided to estimate the odds ratio (OR) and the weighted mean difference (WMD) and their 95% confidence interval (CI); (iii) only the newest, largest or most informative articles were included if there were multiple articles based on similar populations. The exclusion criteria were as follows: (i) animal experiments or in vitro studies; (ii) case reports, meta-analyses, reviews, editorials or expert opinion.
Screening process, data extraction and quality assessment
Two authors (W.-J.W. and F.-Y.Z.) independently screened articles and extracted data from the included studies. To avoid bias, discrepancies were resolved by a third author (X.Q.). The Newcastle-Ottawa scale [11] was used for grading because the included studies were all case-control studies. The Newcastle-Ottawa scale comprises 3 factors: patient selection, comparability of the study groups and assessment of outcome. A score of 0–9 (allocated as stars) was assigned to each study, and a high-quality study was defined as a study with quality scores ≥7. Two reviewers assessed the risk of bias independently (W.-J.W. and F.-Y.Z.). A third reviewer (X.Q.) arbitrated unresolved disagreements.
Statistical analyses
Data were extracted and managed using EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) to delete duplicate references.
The effects of various dichotomous and continuous outcomes were evaluated using Stata 15.1 (StataCorp LP, College Station, TX, USA). Forest plots were generated to characterize the effects of outcome data for the studies. The WMD with the 95% CI was adopted to express continuous outcome variables if all studies were included with the same unit and magnitude; otherwise, standard mean difference was used. Dichotomous outcomes were assessed by the OR with the 95% CI [12]. In studies that only reported medians and ranges, the mean and standard deviation were assessed, using the means of the method provided by Hozo et al. [13]. Heterogeneity was assessed using the χ2 test and I2 test. If heterogeneity was significant (I2 < 50% or P > 0.05), the fixed-effect model was used as the pooling method; otherwise, a random-effects model was adopted. Publication bias was evaluated by the funnel plot with Egger’s weighted regression method and Begg’s rank correlation method [14]. If the P-value was <0.05, the difference was considered statistically significant.
RESULTS
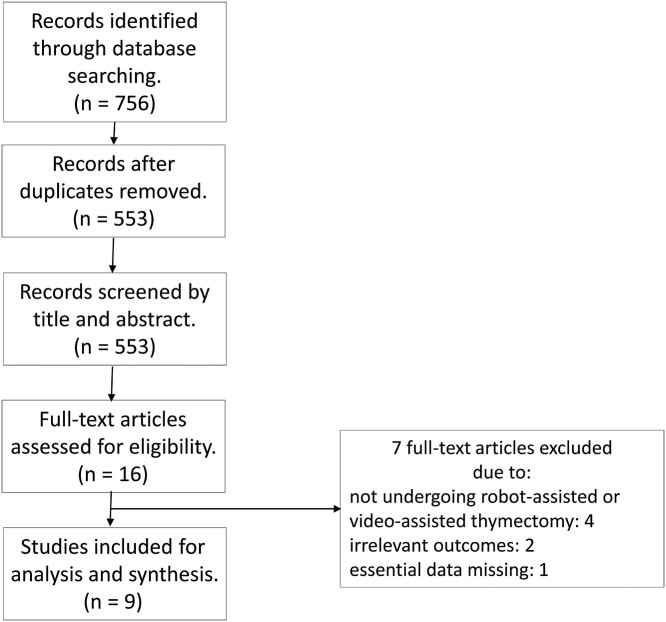
Figure 1 shows the literature research and selection protocol used in this meta-analysis. A total of 756 studies were initially selected from the 3 electronic databases. After removing 203 duplicates, 553 studies were extracted for screening. Then, 537 studies were removed after screening and reviewing titles and abstracts that did not fulfil the inclusion criteria. The remaining studies were eligible for further full-text screening: 7 of the articles did not meet the inclusion criteria. Finally, after excluding duplicates and studies that did not fulfil the inclusion criteria, 9 articles (Ruckert et al. [15], Ye et al. [16], Yi et al. [17], Rowse et al. [18], Suda et al. [19], Pfister et al. [20], Qian et al. [21], Şehitogullari et al. [22], Li et al. [23]) were included. The articles encompassed a total of 723 patients, including 315 patients in the RAT group and 408 patients in the VAT group. The basic characteristics of these studies are summarized in Table 1. Among the studies included in this meta-analysis, the studies reported by Ye et al. [17], Pfister et al. [20] and Şehitogullari et al. [22] only included patients with thymoma. Qian et al. [21] and Li et al. [23] both included thymoma and thymic carcinoma in their studies. However, the studies of Ruckert et al. [15], Jun et al. [17], Rowse et al. [18] and Suda et al. [19] included thymoma, thymic carcinoma, thymic cyst and other diseases of the thymus. Four studies were from Europe and the others were from Asia. All studies reported operating time. The majority of studies included intraoperative estimated blood loss, total amount of drainage and postoperative complications. All studies were rated as high-quality research based on the Newcastle-Ottawa scale. Table 1 and Supplementary Material, Table S1 show the characteristics and methodological quality assessment scores of the included studies.
Figure 1:
Flow chart.
Table 1:
Characteristics of the included studies
| Name | Year | Study type | Data source | Country | Design | Centre | Randomization | Matching | Group | Number | Sex (M/F) | Age | Tumour size (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ruckert | 2011 | RO | OD | Germany | CS | Single | No | No | RAT | 74 | 42/32 | NA | NA |
| VAT | 79 | 56/23 | NA | NA | |||||||||
| Ye | 2013 | RO | OD | China | CS | Single | No | No | RAT | 21 | 9 (42.9%)/12 (57.1%) | 52.7 ± 7.8 | 29.1 ± 7.7 |
| VAT | 25 | 13 (52.0%)/12 (48.0%) | 53.4 ± 5.4 | 30.4 ± 7.9 | |||||||||
| Yi | 2014 | RO | OD | China | CS | Single | No | No | RAT | 55 | 25/30 | 41.38 | NA |
| VAT | 60 | NA | 43.53 | NA | |||||||||
| Rowse | 2015 | RO | OD | Germany | CS | Single | No | No | RAT | 11 | 6/5 | 52.2 (23–74) | 3.5 |
| VAT | 45 | 19/26 | 50.6 (23–87) | 2.6 | |||||||||
| Suda | 2016 | RO | OD | Japan | CS | Single | No | No | RAT | 8 | 5/3 | 55.5 ± 9.9 | 4.1 ± 2.1 |
| VAT | 72 | 31/41 | 53.4 ± 14.8 | 3.2 ± 1.7 | |||||||||
| Pfister | 2017 | RO | OD | France | CS | Single | No | No | RAT | 14 | 4 (28.6%)/10 (71.4%) | 64 (39–76) | 18.5 (7–22) |
| VAT | 8 | 3 (37.5%)/5 (62.5%) | 42 (30–56) | 12.2 (6–20) | |||||||||
| Qian | 2017 | RO | OD | China | CS | Single | No | No | RAT | 51 | 21/30 | 48.8 ± 13.3 | 3.8 ± 1.1 |
| VAT | 35 | 19/16 | 50.3 ± 13.1 | 3.9 ± 1.1 | |||||||||
| Şehitogullari | 2019 | RO | OD | Turkey | CS | Single | No | No | RAT | 21 | 13/8 | 41.3 ± 7.1 | 25.8 ± 5.6 |
| VAT | 24 | 14/10 | 42.5 ± 7.5 | 27.3 ± 5.7 | |||||||||
| Li | 2020 | RO | OD | China | CS | Single | No | Yes | RAT | 60 | 30/30 | 53.7 ± 13.1 | 5.5 ± 2.4 |
| VAT | 60 | 30/30 | 51.2 ± 12.2 | 5.3 ± 2.9 |
CS: cohort study; F: female; M: male; NA: not available; OD: original data; RAT: robotic-assisted thoracotomy; RO: retrospective observational; VAT: video-assisted thoracotomy.
Surgical outcomes
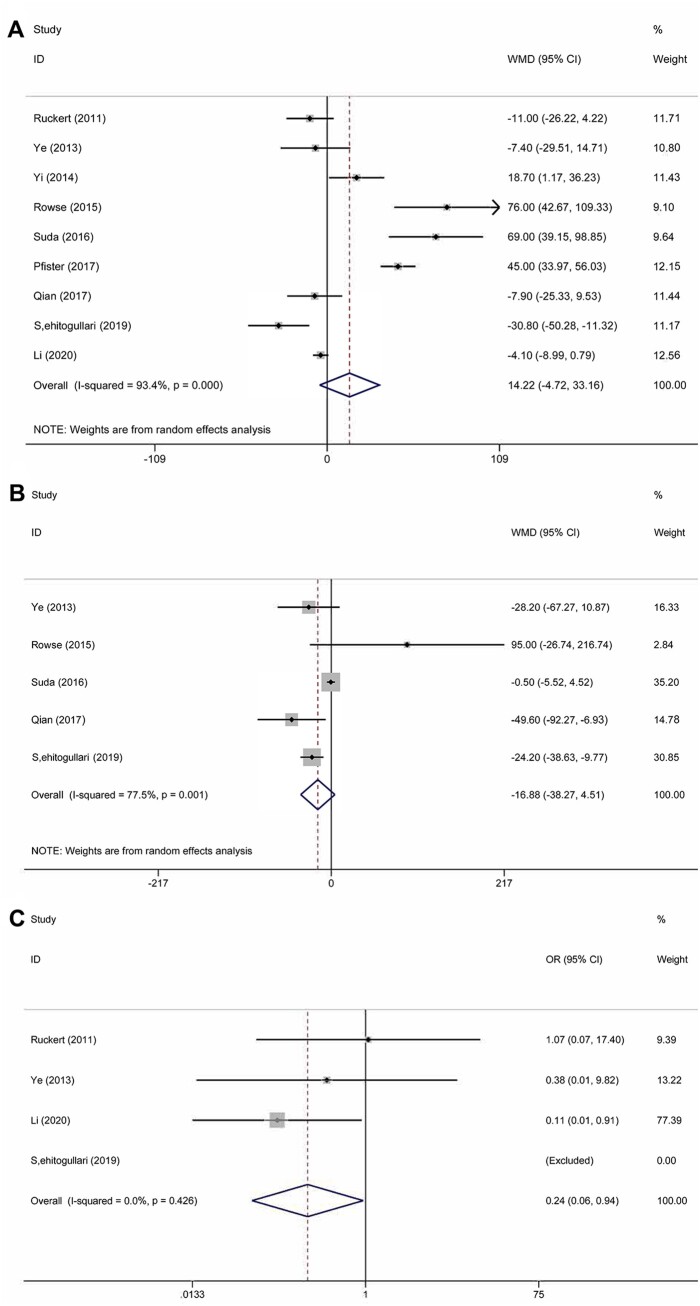
The meta-analysis of the surgical outcomes is shown in Fig. 2A–C. All 9 studies, which included 723 patients, reported the duration of the operation. Statistical heterogeneity was significant (χ2 = 121.15, P < 0.001, I2 = 93.4%); therefore, a random effects model was used for the meta-analysis, and there was no significant difference between the 2 groups (WMD = 14.22; 95% CI −4.72 to 33.16; P = 0.141). The funnel plot is shown in Fig. 3; no publication bias was identified using the Egger test (P = 0.330) and the Begg test (P = 0.251). The amount of intraoperative estimated blood loss was provided in 5 studies that included 313 patients. Heterogeneity was obvious (χ2 = 17.79, P = 0.001, I2 = 77.5%); therefore, a random effects model was used. The pooled analysis (WMD = −16.88, 95% CI −38.27 to 4.51; P = 0.122) suggested that the amount of blood loss in the RAT group was less than that in the VAT group, but the difference was not significant. The rate of conversion to open surgery was reported in 3 studies that included 319 patients, the statistical heterogeneity of which was not significant (χ2 = 1.71, P = 0.426, I2 = 0%); therefore, a fixed-effect model was chosen. The meta-analysis (OR 0.24, 95% CI 0.06–0.94; P = 0.041) indicated that RAT yielded a significantly lower rate of conversion than VAT. The R0 rate was reported in 2 studies conducted by Rowse et al. [18] and Pfister et al. [20]. In the study by Rowse et al. [18], all patients with thymomas underwent R0 resection with negative surgical margins. The results of the study reported by Pfister et al. [20] showed that only 1 patient underwent an R1 resection, and the others underwent an R0 resection.
Figure 2:
(A) Comparison of the surgical time between robotic-assisted thymectomy and video-assisted thymectomy. (B) Comparison of intraoperative blood loss between robotic-assisted thymectomy and video-assisted thymectomy. (C) Comparison of the conversion rate between robotic-assisted thymectomy and video-assisted thymectomy. CI: confidence interval; OR: odds ratio; WMD: weighted mean difference.
Figure 3:
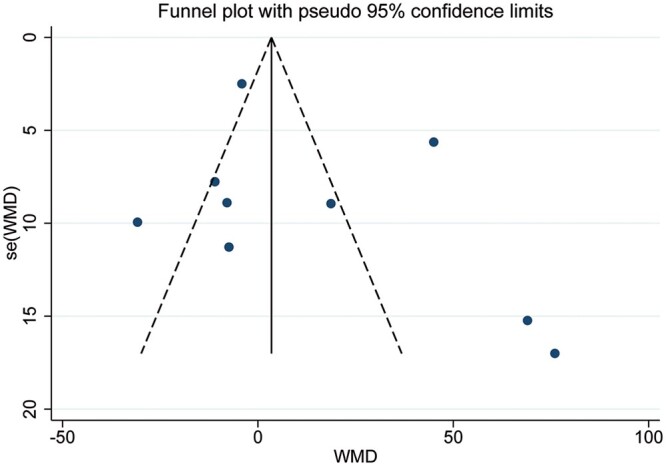
The funnel plot of the comparison of the surgical time between robotic-assisted thymectomy and video-assisted thymectomy. WMD: weighted mean difference.
Short-term outcomes
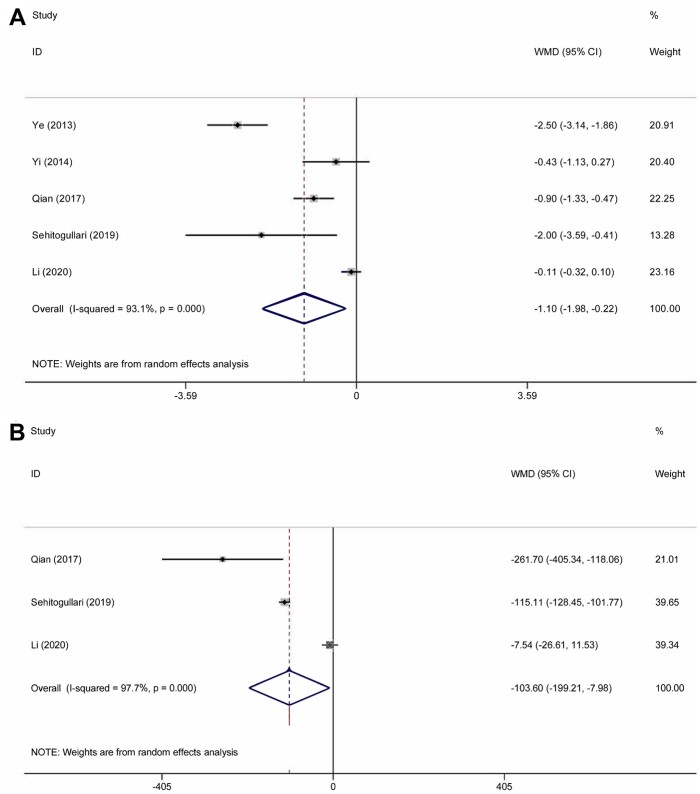
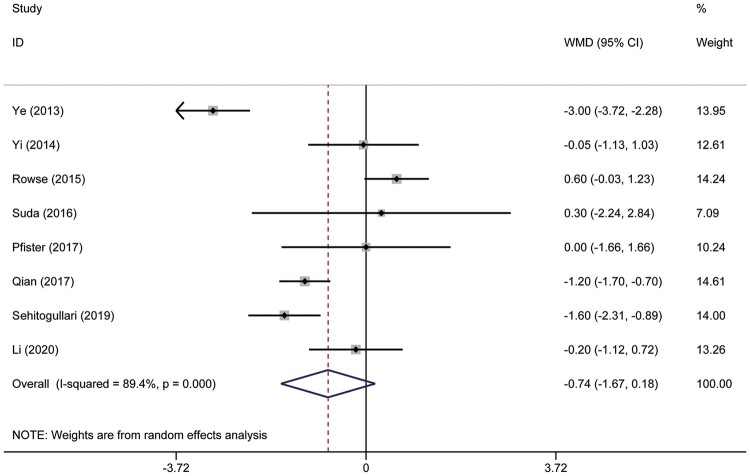
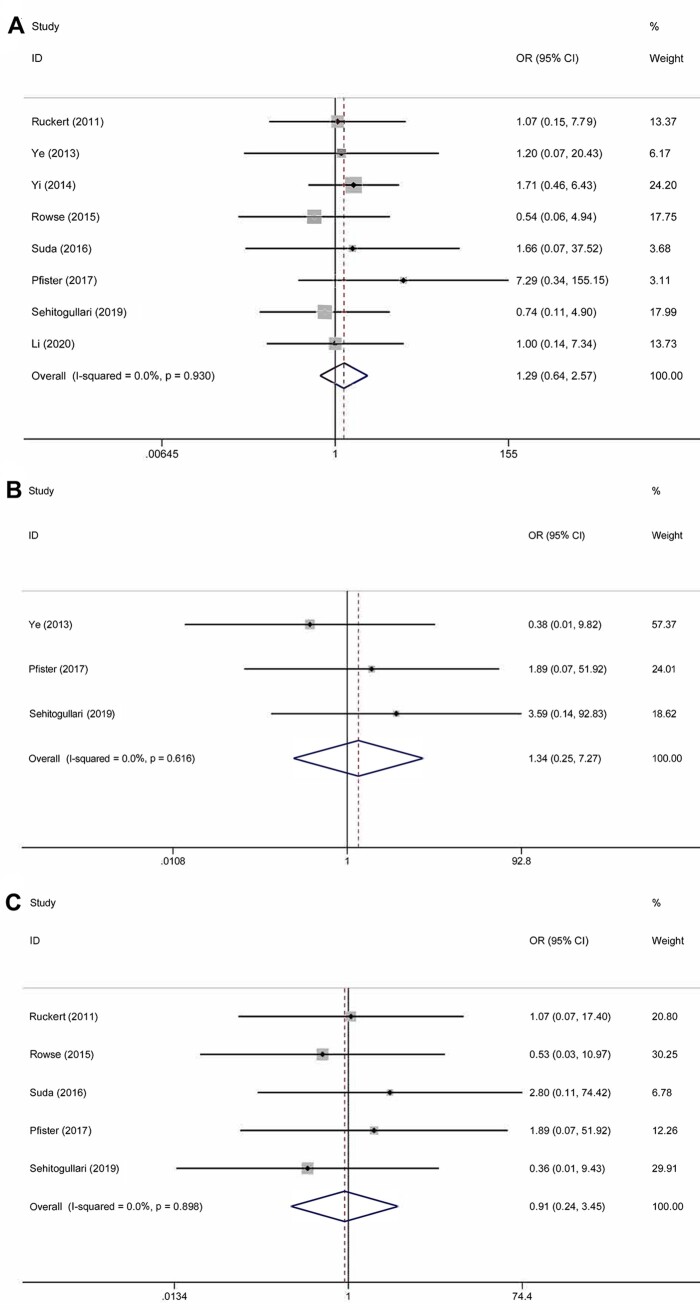
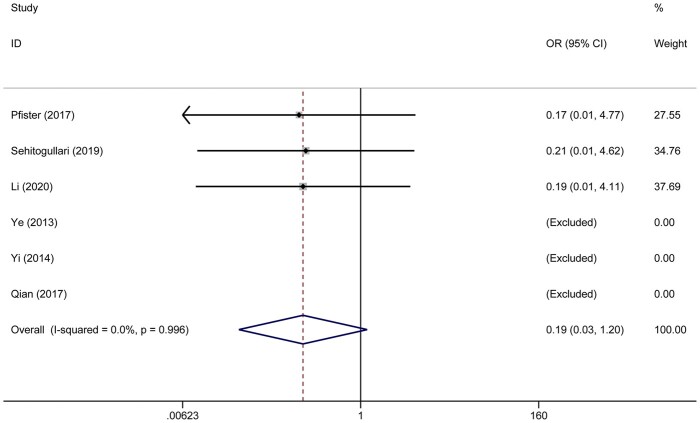
In the meta-analysis, duration of drainage was reported in 5 studies that included 412 patients. Heterogeneity was deemed to be significant (χ2 = 58.15, P < 0.001, I2 = 93.1%); therefore, a random effects model was used. Duration of drainage in the RAT group was significantly less than that in the VAT group (WMD = −1.10; 95% CI −1.98 to −0.22; P = 0.014) (Fig. 4A). A total of 251 patients in 3 studies were evaluated to determine the total amount of drainage. Significant heterogeneity was found in this analysis (χ2 = 88.18, P < 0.001, I2 = 97.7%); thus, the random-effect model was applied. The pooled analysis (WMD = −103.6; 95% CI −199.21 to −7.98; P = 0.034) suggested that patients in the RAT group had less drainage than those in the VAT group (Fig. 4B). The duration of the hospital stay was reported in 8 studies that included 570 patients. The heterogeneity was identified (χ2 = 65.75, P < 0.001, I2 = 89.4%). The random-effect model was applied for pooled analysis. The results showed that there was no significant difference between patients in the RAT and VAT groups (WMD = −0.74; 95% CI −1.67 to 0.18; P = 0.114) (Fig. 5). There was no publication bias assessed by the Egger test (P = 0.597) and the Begg test (P = 1.000). Postoperative complications were reported in 8 studies that included 637 patients. Heterogeneity was identified (χ2 = 2.46, P = 0.930, I2 = 0.0%). However, there was still no significant difference between the RAT group and the VAT group (OR 1.29, 95% CI 0.64–2.57; P = 0.476) (Fig. 6A). There was no publication bias assessed by the Egger test (P = 0.745) and the Begg test (P = 0.536). The incidence of pneumonia was reported in 3 studies that included 56 patients in the RAT group and 57 patients in the VAT group. Heterogeneity was deemed to be non-significant (χ2 = 0.97, P = 0.616, I2 = 0.0%). A significant difference could not be found between the 2 groups (OR 1.34, 95% CI 0.25–7.27; P = 0.735) (Fig. 6B). Five studies that included 356 patients reported data regarding the rate of phrenic nerve paralysis. Statistical heterogeneity was not significant (χ2 =1.08, P = 0.898, I2 = 0.0%), so the fixed effect-model was used, and no significant difference was found between the 2 groups (OR 0.91, 95% CI 0.24–3.45; P = 0.893) (Fig. 6C). Five studies reported the incidence of tumour recurrence. However, the incidence of tumour recurrence in the studies reported by Ye et al. [16], Yi et al. [17] and Qian et al. [21] was 0 in each group; these studies were excluded from the meta-analysis. No heterogeneity was seen (χ2 = 0.01, P = 0.996, I 2 =0.0%). The incidence of recurrence in the RAT group tended to be less than that in the VAT group (OR 0.19, 95% CI 0.03–1.20; P = 0.078) (Fig. 7). The recurrence rate was reported in 6 studies. However, the incidence of tumour recurrence in the studies reported by Ye et al. [16], Yi et al. [17] and Qian et al. [21] was 0 in each group; these studies were excluded from the meta-analysis.
Figure 4:
(A) Comparison of the duration of drainage after robotic-assisted thymectomy and video-assisted thymectomy. (B) Comparison of the total amount of drainage after robotic-assisted thymectomy and video-assisted thymectomy. CI: confidence interval; ID: identification; WMD: weighted mean difference.
Figure 5:
Comparison of the duration of hospital stay after robotic-assisted thymectomy and video-assisted thymectomy. CI: confidence interval; ID: identification; WMD: weighted mean difference.
Figure 6:
(A) Comparison of the rate of postoperative complications after robotic-assisted thymectomy and video-assisted thymectomy. (B) Comparison of the rate of pneumonia after robotic-assisted thymectomy and video-assisted thymectomy. (C) Comparison of the rate of phrenic nerve paralysis after robotic-assisted thymectomy and video-assisted thymectomy. CI: confidence interval; ID: identification; OR: odds ratio.
Figure 7:
Comparison of the rate of recurrence after robotic-assisted thymectomy and video-assisted thymectomy. CI: confidence interval; ID: identification; OR: odds ratio.
DISCUSSION
A thymic epithelial tumour is a rare epithelial neoplasm of the thymus gland, and it is the most common tumour of the anterior superior mediastinum, comprising 20–30% of mediastinal masses in adults [2]. In the early 1990s, Roviaro first reported using a video-assisted technique to resect thymomas. A comparison of VAT and sternotomy for the treatment of MG indicates shown that VAT is typically associated with reduced blood loss and a shorter operative time and hospital stay, whereas VAT may also yield a lower incidence of postoperative complications by reducing tissue damage, postoperative pain and the risk of infection [24]. With the advantages of minimal invasiveness and rapid recovery, VAT has been adopted more often in recent years to treat early-stage thymic epithelial tumours [25, 26].
Berman et al. [27] reported their first robotic-assisted surgery of an anterior mediastinal mass with the Zeus robotic surgical system, which lacked 3D technology. Since then, the role of robotic-assisted surgery in the treatment of thymic epithelial tumours has grown. When comparing RAT (the newest surgical therapy for MG) and sternotomy for the treatment of MG, it has been demonstrated that RAT has favourable outcomes in terms of surgical complications and hospital stay [28]. Some surgeons favour using an open upper sternal split or cervical approach for large tumours or in those patients in whom the preoperative imaging appears to have invasive characteristics. In our clinical practice, upper sternal splits or cervical approaches could yield less surgical damage and accelerate recovery of patients postoperatively compared with VAT. However, these 2 techniques are not widely used. With the development of the latest robotic technology, a robotic system offers 3D vision with multiarticulated arm movement, which can reduce the surgeon’s tremor, enable better visualization and provide meticulous dissection of the anterior mediastinal structures. Several studies demonstrated that RAT is superior to VAT in treating anterior mediastinal masses [29].
Ruckert et al. [15] was the first to compare RAT and VAT and reported that the conversion rate with RAT and VAT was 1.4% (1/74) and 1.3% (1/79), respectively. The overall postoperative complication rate was 2.7% (2/74) and 2.5% (2/79) for RAT and VAT, respectively. There was 1 bleed and 1 phrenic nerve resection due to thymoma involvement in each group. Qian et al. [21] reported shorter duration of postoperative pleural drainage (RAT: 2.9 ± 0.8 vs VAT: 3.8 ± 1.1, P < 0.001), less volume of pleural drainage (RAT: 352.2 ± 193.4 vs VAT: 613.9 ± 402.9; P < 0.001) and shorter hospital stays in the RAT group compared with the VAT group (RAT: 4.3 ± 1.1 vs VAT: 5.5 ± 1.2; P < 0.001). The study reported by Şehitogullari et al. [22] demonstrated that the RAT group had better surgical outcomes than the VAT group. The duration of the operation (RAT: 75.7 ± 38.08 min vs VAT: 106.52 ± 26.68 min; P < 0.001), amount of total drainage from chest tubes (RAT: 210.34 ± 20.22 ml vs VAT: 325.45 ± 25.38 ml; P < 0.001), duration of postoperative pleural drainage (RAT: 3.10 ± 2.2 days vs VAT: 5.10 ± 3.21 days, P < 0.001) and duration of hospital stay (RAT: 4.16 ± 1.15 days vs VAT: 5.76 ± 1.27 days; P < 0.001) in the RAT group were significantly less than those in the VAT group. Only 1 study reported by Li et al. [23] explored the survival data in patients undergoing RAT and VAT. The results showed that there was no significant difference in progression-free survival between the 2 groups (5-year progression-free survival rate: RAT: 81.5% vs VAT: 75.4%, respectively; log-rank P = 0.095). Only the meta-analysis of Buentzel et al. [30] compared RAT and VAT. However, it only included 5 studies and compared 4 short-term outcomes including duration of the operation, length of hospitalization, intraoperative blood loss and postoperative complications, but none of these had significant differences between the 2 groups. Therefore, it is necessary to conduct an updated meta-analysis to further compare the short-term outcomes after RAT and VAT.
The types of diseases of the thymus differed among the studies included in this meta-analysis. As we all know, TNM (tumour-node-metastasis) stage and histological classification are associated with recurrence, overall survival and disease-free survival. However, the goal of this study was to compare the short-term outcomes and the postoperative recovery of patients treated with RAT and VAT for diseases of thymus. Therefore, the characteristics of the patient populations are comparable among the included studies.
The results of the meta-analysis showed that intraoperative blood loss was less with the RAT compared with VAT procedure, and the surgical time was longer with the RAT procedure than with the VAT procedure. The rate of conversion to open surgery with RAT was significantly less than that with VAT. The potential reason for the longer surgical time with RAT could be that the surgeon requires more time to prepare for the Da Vinci surgical system before the operation. Less intraoperative blood loss and fewer conversions indicated that the RAT yielded a more precise dissection and reduced the risk of surgery. The pooled analysis showed that RAT yielded a significantly shorter duration of drainage and a smaller amount of drainage. These 2 short-term outcomes were associated with the accuracy of the operation because the damage to the peripheric tissue such as capillaries and pleura could prolong the duration and increase the amount of drainage. Therefore, RAT could offer better manipulation and a clearer surgical view, which might reduce injury to the peripheric tissue. Furthermore, RAT yielded a shorter hospital stay compared with VAT, although the difference was not significant. There was no heterogeneity among the remaining studies, and the results showed that RAT yielded a lower rate of recurrence of thymoma compared with VAT. In conclusion, RAT is superior to VAT in terms of short-term clinical outcomes because it can provide 3D visualization and free articulation of the tips of the robotic arms when the surgeon operates in narrow spaces such as the mediastinum. Therefore, a surgeon using the robotic technique may dissect the thymoma more elaborately and better maintain the membrane integrity compared with the thoracoscopic technique.
Limitations
High heterogeneity could be found in some of the meta-analyses. However, we did not apply sensitivity analysis because the number of included studies was <10; neither was a subgroup analysis conducted. Meanwhile, all the included studies were retrospective, and no blinding method was used in these studies, resulting in observer bias that could not be diminished. The variability of the same data collected in the 9 studies makes one suspicious of the conclusion. Therefore, more high-quality studies, especially randomized controlled studies comparing RAT and VAT are urgently warranted.
CONCLUSION
RAT has advantages over VAT in terms of short-term outcomes such as shorter duration of drainage, less total drainage and fewer conversions. The recurrence rate was comparable between the 2 techniques. Therefore, RAT could be considered as an alternative treatment for diseases of the thymus.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Author contributions
Wen-Jie Wu: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing—original draft. Fu-Yu Zhang: Investigation; Software; Writing—original draft. Xiao Qin: Conceptualization; Validation; Visualization; Writing—review & editing. Xiao-Kun Li: Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Clemens Aigner, Olgun Kadir Aribas, Frank A. Baciewicz Jr., Michelle Demetres and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- 3D
3-Dimensional
- CI
Confidence interval
- MG
Myasthenia gravis
- OR
Odds ratio
- RAT
Robotic-assisted thymectomy
- VAT
Video-assisted thymectomy
- WMD
Weighted mean difference
References
- 1. Engels EA, Pfeiffer RM.. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546–51. [DOI] [PubMed] [Google Scholar]
- 2. Levine GD, Rosai J.. Thymic hyperplasia and neoplasia: review of current concepts. Hum Pathol 1978;9:495–515. [DOI] [PubMed] [Google Scholar]
- 3. Kondo K, Monden Y.. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878–84; discussion 884–5. [DOI] [PubMed] [Google Scholar]
- 4. Mao ZF, Mo XA, Qin C, Lai YR, Hackett ML.. Incidence of thymoma in myasthenia gravis: a systematic review. J Clin Neurol 2012;8:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng YJ, Hsu JS, Kao EL.. Characteristics of thymoma successfully resected by videothoracoscopic surgery. Surg Today 2007;37:192–6. [DOI] [PubMed] [Google Scholar]
- 6. Seong YW, Kang CH, Choi JW, Kim HS, Jeon JH, Park IK. et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68–73. discussion e73. [DOI] [PubMed] [Google Scholar]
- 7. Li XK, Cong ZZ, Xu Y, Zhou H, Wu WJ, Wang GM. et al. Clinical efficacy of robot-assisted thoracoscopic surgery for posterior mediastinal neurogenic tumors. J Thorac Dis 2020;12:3065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu Y, Li XK, Cong ZZ, Zhou H, Wu WJ, Qiang Y. et al. Long-term outcomes of robotic-assisted versus thoraco-laparoscopic McKeown esophagectomy for esophageal cancer: a propensity score-matched study. Dis Esophagus 2020;doi:10.1093/dote/doaa114. [DOI] [PubMed] [Google Scholar]
- 9. Hartwich J, Tyagi S, Margaron F, Oitcica C, Teasley J, Lanning D.. Robot-assisted thoracoscopic thymectomy for treating myasthenia gravis in children. J Laparoendosc Adv Surg Tech A 2012;22:925–9. [DOI] [PubMed] [Google Scholar]
- 10. Brown LM, Louie BE.. Robot-assisted total thymectomy: how I teach it. Ann Thorac Surg 2017;103:369–72. [DOI] [PubMed] [Google Scholar]
- 11. Lo CK, Mertz D, Loeb M.. Newcastle-Ottawa scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hickey GL, Dunning J, Seifert B, Sodeck G, Carr MJ, Burger HU. et al. Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive Cardiovascular and Thoracic Surgery. Eur J Cardiothorac Surg 2015;48:180–93. [DOI] [PubMed] [Google Scholar]
- 13. Hozo SP, Djulbegovic B, Hozo I.. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buccheri S, Sodeck GH, Capodanno D.. Statistical primer: methodology and reporting of meta-analyses. Eur J Cardiothorac Surg 2018;53:708–13. [DOI] [PubMed] [Google Scholar]
- 15. Ruckert JC, Swierzy M, Ismail M.. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673–7. [DOI] [PubMed] [Google Scholar]
- 16. Ye B, Tantai JC, Li W, Ge XX, Feng J, Cheng M. et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jun Y, Hao L, Demin L, Guohua D, Hua J, Yi S.. Da Vinci robot-assisted system for thymectomy: experience of 55 patients in China. Int J Med Robot 2014;10:294–9. [DOI] [PubMed] [Google Scholar]
- 18. Rowse PG, Roden AC, Corl FM, Allen MS, Cassivi SD, Nichols FC. et al. Minimally invasive thymectomy: the Mayo Clinic Experience. Ann Cardiothorac Surg 2015;4:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suda T, Kaneda S, Hachimaru A, Tochii D, Maeda R, Tochii S. et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfister AW, Baste Piton JMN, Bubenheim M, Melki J, Wurtz A. et al. [Thymomectomy by minimally invasive surgery. Comparative study videosurgery versus robot-assisted surgery]. Rev Mal Respir 2017;34:544–52. [DOI] [PubMed] [Google Scholar]
- 21. Qian L, Chen X, Huang J, Lin H, Mao F, Zhao X. et al. A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis 2017;9:1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Şehitogullari A, Nasir A, Anbar R, Erdem K, Bilgin C.. Comparison of perioperative outcomes of videothoracoscopy and robotic surgical techniques in thymoma. Asian J Surg 2020;43:244–50. [DOI] [PubMed] [Google Scholar]
- 23. Li XK, Xu Y, Cong ZZ, Zhou H, Wu WJ, Shen Y.. Comparison of the progression-free survival between robot-assisted thymectomy and video-assisted thymectomy for thymic epithelial tumors: a propensity score matching study. J Thorac Dis 2020;12(8):4033–43. doi:10.21037/jtd-20-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yim AP. Thoracoscopic thymectomy: which side to approach? Ann Thorac Surg 1997;64:584–5. [PubMed] [Google Scholar]
- 25. Yim AP. Video-assisted thoracoscopic resection of anterior mediastinal masses. Int Surg 1996;81:350–3. [PubMed] [Google Scholar]
- 26. Roviaro G, Varoli F, Nucca O, Vergani C, Maciocco M.. Videothoracoscopic approach to primary mediastinal pathology. Chest 2000;117:1179–83. [DOI] [PubMed] [Google Scholar]
- 27. Berman M, Stamler A, Vidne BA, Saute M.. Computer-enhanced thoracoscopic thymectomy with the Zeus telemanipulation surgical system. Interact CardioVasc Thorac Surg 2003;2:262–4. [DOI] [PubMed] [Google Scholar]
- 28. Pennathur A, Qureshi I, Schuchert MJ, Dhupar R, Ferson PF, Gooding WE. et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694–701. [DOI] [PubMed] [Google Scholar]
- 29. Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653–6. [DOI] [PubMed] [Google Scholar]
- 30. Buentzel J, Heinz J, Hinterthaner M, Schondube FA, Straube C, Roever C. et al. Robotic versus thoracoscopic thymectomy: the current evidence. Int J Med Robot 2017;13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.