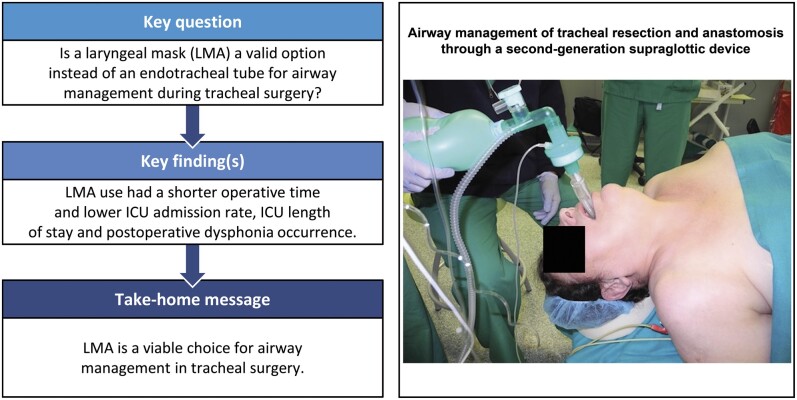
Abstract
OBJECTIVES
The endotracheal tube (ETT) and the laryngeal mask airway (LMA) are possible strategies for airway management during tracheal resection and reconstruction for tracheal and laryngotracheal stenosis. The goal of the study was to analyse and compare outcomes in the LMA and ETT groups.
METHODS
Between 2003 and 2020, a total of 184 patients affected by postintubation, post-tracheostomy and idiopathic stenosis who had tracheal or laryngotracheal resections and reconstructions via a cervicotomy were retrospectively enrolled in this single-centre study. In 29 patients, airway management was achieved through LMA during tracheal surgery, whereas in 155 patients, it was achieved through ETT. A case–control matching analysis was performed with a 1:1 ratio, according to age, gender, body mass index, aetiology and length of stenosis (1–4 cm), resulting in 22 patients managed through LMA (LMA group) matched with 22 patients managed through ETT (ETT group).
RESULTS
No significant differences were found in the reintubation rate, 30-day mortality and postoperative length of stay. Operative time was shorter in patients with LMA (96.23 ± 34.72 min in the ETT group vs 76.14 ± 26.94 min in the LMA group; P = 0.043). Intensive care unit (ICU) admission rate and stay were lower in the LMA group [18 in the ETT group vs 8 in the LMA group, odds ratio = 10.17, confidence interval (CI) 95% 1.79–57.79; P = 0. 009; 22.77 ± 16.68 h in ETT group vs 9.23 ± 13.51 h in LMA group; P = 0.005]. Dysphonia was more frequent in the ETT group than in the LMA group (20 in the ETT group vs 11 in the LMA group, odds ratio = 13.79, CI 95% 1.86–102; P = 0.010).
CONCLUSIONS
LMA is a feasible option for airway management in tracheal surgery, with lower operative time, ICU admission rate, ICU length of stay and postoperative dysphonia occurrence.
Keywords: Tracheal stenosis, Tracheal resection, Airway management, Laryngeal mask airway, Endotracheal tube
INTRODUCTION
Tracheal idiopathic stenosis is caused primarily by prolonged endotracheal intubation or tracheostomy [1]. Surgical tracheal resection and reconstruction are currently the definitive treatments of choice for tracheal stenosis with a low failure rate (9%) [2]. Tracheal surgery is a major therapeutic challenge and requires a highly specialized team of anaesthesiologists and thoracic surgeons. Airway management is characterized by a multidisciplinary approach. Close cooperation between the surgeon and the anaesthesiologist who are ‘sharing the airways’ is paramount. The goal is to ensure adequate surgical access to the trachea and, simultaneously, sufficient ventilation and gas exchanges. Several airway management tools (fibre-optic bronchoscope, video laryngoscope, laryngoscope) and different devices [supraglottic airway devices and mono- and double-lumen endotracheal tubes (ETTs)] may be required during surgery [3]. Numerous techniques to ensure ventilation and gas exchange can be chosen, based on the level of the stenosis: jet ventilation, high-frequency ventilation, cross-field ventilation, spontaneous ventilation, intermittent apnoea technique, one-lung ventilation and extracorporeal circulation techniques [4]. Airway management during tracheal surgery can be divided into 3 distinct phases: the induction of anaesthesia and surgical preparation of the trachea (dissection phase), resection and anastomosis of the airway (resection phase) and, lastly, primary wound closure of the surgical field (closure phase) [5].
Although the ETT has been widely considered the standard airway management approach during tracheal surgery, there is no generally accepted anaesthetic strategy. Several alternative techniques have been proposed [5]; the laryngeal mask airway (LMA) is the device arousing the most significant interest. This new approach involving the use of the LMA lacks clinical data that confirm its advantages over traditional techniques.
The LMA can be helpful, especially in patients with severe stenosis in whom positioning the ETT may be impossible due to extreme airway narrowing [6]. In addition, endotracheal intubation is burdened by mechanical trauma, swelling, bleeding and subsequent airway occlusion [7]. In contrast, the LMA does not cause mechanical stress to the freshly sutured airway and avoids impairing its blood supply [8]. The LMA also lowers the risk of coughing when the patient is emerging from the anaesthesia [9]. LMA allows control of the airway without interfering with the subglottic region and permits fibre-optic bronchoscopy to check the vocal cords [10].
There is no unanimous consensus about postoperative airway management after tracheal surgery. Some authors [11, 12] commonly leave an uncuffed nasal tube in situ, with the distal end beyond the anastomosis for 24 h postoperatively. Other authors [13] leave a small ETT in place for 48 h postoperatively only when laryngeal oedema is present at the moment of extubation. Protective tracheostomy can also be performed [2, 11]. Some surgeons prefer to extubate the patient in the operating room at the end of the tracheal reconstruction [2, 11].
According to the practice of each surgical centre, patients can remain intubated with an uncuffed nasal ETT for the first 24–48 h as a precaution, even though they exhibit spontaneous breathing [14]. The disadvantage of having the ETT in place during the early postoperative period is that it could impair evaluation of glottis function and patency, essential in very high resections of subglottic stenosis potentially involving the vocal fold [15]. The presence of the nasal tube can also cause patient discomfort, and intensive care unit (ICU) admission and postoperative sedation may be required to keep these patients comfortable [16]. Due to these concerns, different approaches using LMA as a primary airway device in tracheal surgery have been tried in some surgical centres [7, 8, 10, 15, 17–23].
In our centre, we began to use the LMA in 2018. The LMA was used as the primary device in tracheal resection and reconstruction in order to reduce airway manipulations during surgery and obviate the risk of failed intubation or complications such as mucosal trauma, bleeding or perforation; to avoid mechanical stress caused by the tube on the freshly sutured airway and to limit postoperative complications such as restenosis, anastomosis dehiscence or dysphonia; to increase the patient’s postoperative comfort by reducing airway irritation and the risk of coughing when the patient emerged from the anaesthesia and to avoid nasal tube positioning.
The goal of this study was to analyse and compare outcomes in the LMA and ETT groups after tracheal resection using a 1:1 case–control matched pairs analysis, according to age, gender, body mass index (BMI), aetiology and length of stenosis (1–4 cm).
METHODS
From June 2003 to May 2020, a total of 184 patients affected by postintubation, post-tracheostomy and idiopathic stenosis underwent tracheal or laryngotracheal resections and reconstructions via cervicotomy at Thoracic Surgery, Department of Clinical and Surgical Translational Medicine, Sant’ Andrea Hospital, Sapienza University of Rome, Rome, Italy. Exclusion criteria were age <18 years, malignant tracheal stenosis and all other tracheal surgery not including tracheal resection and reconstruction.
After institutional review board approval (CE/7868_2020), patient records were retrospectively analysed, including demographic data, intraoperative characteristics, perioperative complications and postoperative outcomes. A total of 22 patients who underwent laryngotracheal resection managed by endotracheal intubation (ETT group) were matched in a case–control approach with 22 patients undergoing tracheal surgery using the LMA approach (LMA group), with a 1:1 ratio according to according to age, gender, BMI, aetiology and length of stenosis (1–4 cm).
The patients in the ETT group were kept intubated for 24 h after surgery as a precaution while maintaining spontaneous breathing. In the LMA group, the supraglottic device was removed in the operating room at the end of the operation. All operations were performed by the same anaesthesiologist and surgical team.
Airway management
An institutional, standardized ventilation strategy was applied for all tracheal cases operated on during the study period. After establishment of standard practices for anaesthesia monitoring (electrocardiography, invasive blood pressure, peripheral oxygen saturation) and preoxygenation, general anaesthesia was induced intravenously using fentanyl 2 mcg/kg and propofol 2 mg/kg and rocuronium 0.8 mg/kg. Patients with a pre-existing tracheostomy were ventilated primarily through the tracheostomy. Then, an LMA or small nasal ETT was placed (Fig. 1A and B). Positive pressure ventilation was performed through the LMA or ETT until the airway was fully exposed. Ventilation was considered sufficient if the positive pressure ventilation was unimpaired (median peak airway pressures ≤ 25 cm H2O), and the peripheral oxygen saturation remained ≥95% with an end-tidal carbon dioxide level of ≤45 mmHg. After we exposed the cervical trachea, we performed cross-field ventilation with a second sterile armoured ETT (size 5 mm) inserted by the surgeon into the distal trachea (Fig. 2). For the resection and reconstruction period, we applied intermittent apnoea phases. At the end of the operation, flexible bronchoscopy was performed to check the anastomosis, free the distal airways from spilled blood and mucus and determine the width of the glottis.
Figure 1:
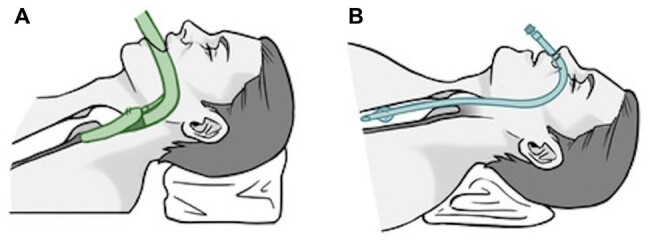
Airway management during tracheal resection and reconstruction through laryngeal mask airway (A) and nasotracheal tube (B).
Figure 2:
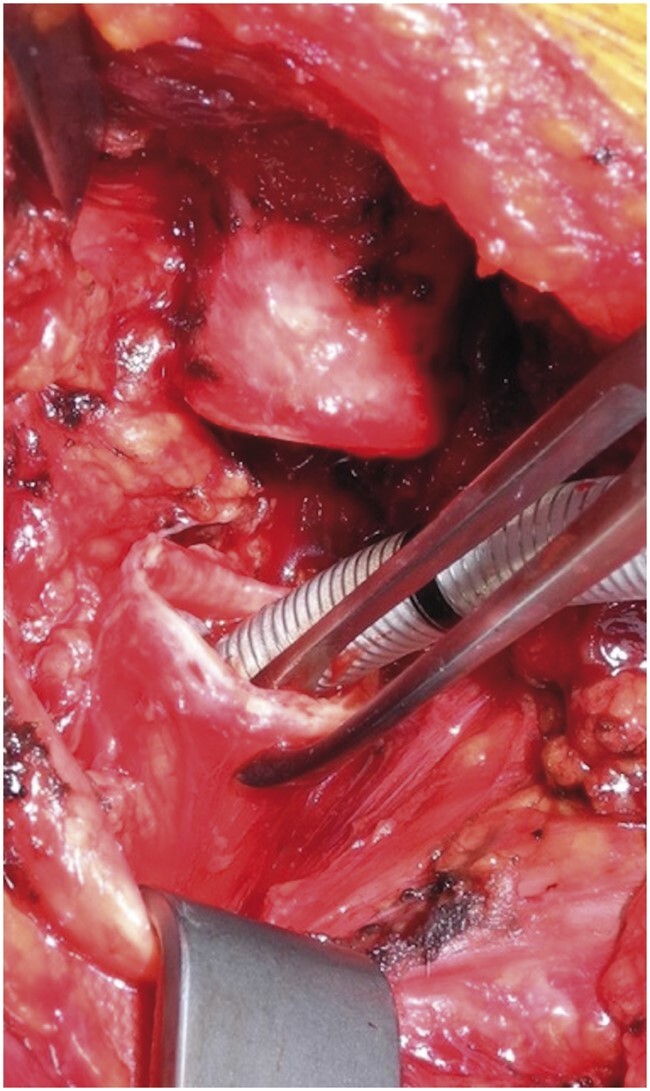
Cross-field ventilation established through an armoured endotracheal tube (size 5) inserted into the distal stump of the trachea during the resection phase of the operation.
Endotracheal intubation group
The ETT size was chosen according to the residual tracheal lumen (size 3–6.5 mm). Once the trachea was transected, the ETT was retired just below the vocal folds through bronchoscopy guidance, and cross-field ventilation was started through the surgical field. After completion of the anastomosis, patients were ventilated through the ETT again, which was kept in place during the entire operation. At the end of the operation, the ETT was changed using an exchange catheter (11 or 14-Fr Cook Airway Exchange Catheter; Cook Medical, Bloomington, IL USA) with a larger ETT, and the patients remained intubated with a deflated cuff. According to our experience, in order to prevent the patient’s inability to breathe spontaneously because of oedema of the glottis or the trachea, the ETT was left in place as a precaution in the awakened and spontaneously breathing patient for 24 h, then removed after a bronchoscopic check of the anastomosis and vocal cords. In patients with postoperative oedema of the glottis, the tube could be left for a longer time while we administered steroids.
The LMA group
The LMA was inserted after induction of anaesthesia. A second-generation supraglottic device (i-gel laryngeal mask, Intersurgical, Wokingham, UK) was used; the size was chosen according to a weight-based formula (size 4–5). As the anastomosis was completed, ventilation was changed back to LMA, and the patient was weaned directly from the ventilation in the operating room.
Surgical technique
The operations were performed in a single surgical centre by the same thoracic surgeon. All patients underwent preoperative assessment based principally on a laryngotracheal endoscopic examination. The goal was to evaluate the mobility and trophicity of the vocal cords, the severity and extent of the stricture, the grade of inflammation and the presence of oedema or malacia, thereby assessing the stability of the stenosis. Afterwards, a neck and chest computed tomography scan was performed to allow a more precise evaluation of the tracheal wall status (calcification, malacia) and of the extraluminal structures and tissues.
All patients with evidence of infection at the tracheostomy site were treated preoperatively with systemic and local antibiotics until sterilization was proved by microbiological analysis.
Before resecting the proximal portion, the trachea was sectioned below the stenotic segment, and the distal airway was intubated through the operative field by an armoured ETT. The anterior-proximal portion of the trachea, before the stenotic tract, was then divided. The tracheal reconstruction was performed through an end-to-end tracheal anastomosis, performed using interrupted sutures of 3–0 or 4–0 absorbable material (polydioxanone). A technical variation could include a running suture for the posterior membranous wall of the anastomosis.
Depending on the length of the resected segment and on the degree of tension of the anastomosis, 1 or 2 strong chin-chest sutures may be placed at the end of operation to maintain patient cervical flexion and were usually removed after 4–8 days [2].
Admission to the intensive care unit
The need for multiparametric monitoring postoperatively in the ICU was based on evaluation of the individual patient by the anaesthesiologist and was based on total operative time, patient stability and cooperation.
Statistical analysis
The 22 patients who underwent laryngotracheal resection managed by endotracheal intubation (ETT group) were matched in a case–control approach with 22 patients undergoing tracheal surgery using the LMA approach (LMA group), with a 1:1 ratio according to age, gender, BMI, aetiology and length of stenosis (1–4 cm), using a matching tolerance of ±0.2. In the matched sample, paired t-tests were used for continuous data whereas multinomial logit regression was used to compare categorical variables. Significance was accepted at P < 0.05. Data analysis was performed using SPSS 25 (SPSS Inc, Chicago, IL, USA).
RESULTS
Patient characteristics including gender, female/male ratio, age, BMI, American Society of Anesthestiology score, type of surgery (laryngotracheal/tracheal), length of stenosis, aetiology (postintubation, post-tracheostomy, idiopathic) are shown in Table 1. Intraoperative and postoperative outcomes are shown in Table 2.
Table 1:
Demographic, epidemiological and preoperative data in the endotracheal tube and the laryngeal mask airway groups
| Variable | ETT (N = 22) | LMA (N = 22) | P-valuea |
|---|---|---|---|
| Male gender (n/%) | 12/55 | 12/55 | 0.71 |
| Age (years), mean ± SD | 47.41 ± 16.48 | 47.18 ± 16.81 | 0.96 |
| BMI (kg/m2), mean ± SD | 24.05 ± 1.86 | 24.45 ± 3.14 | 0.62 |
| ASA (n/%) | |||
| II | 14/64 | 11/50 | 0.40 |
| III | 8/36 | 11/50 | |
| Aetiology (n/%) | |||
| Postintubation | 10/46 | 10/46 | 1.00 |
| Post-tracheostomy idiopathic | 6/27 | 6/27 | |
| Type of surgery (n/%) | |||
| Laryngotracheal | 13/59 | 6/27 | 0.52 |
| Tracheal | 9/41 | 16/73 | |
| Length of stenosis (mm), mean ± SD | 24.12 ± 5.65 | 23.61 ± 9.04 | 0.84 |
Data are expressed as mean ± SD or number of patients.
P-value determined by paired t-test or multinomial logit regression, as appropriate.
ASA: American Society of Anesthesiologists; BMI: body mass index; ETT: endotracheal intubation; LMA: laryngeal mask airway; M: male; SD: standard deviation.
Table 2:
Intraoperative and postoperative data in the endotracheal tube and the laryngeal mask airway groups
| Variable | ETT (N = 22) | LMA (N = 22) | OR (95%CI) | P-valuea |
|---|---|---|---|---|
| Total operative time (min), mean ± SD | 96.23 ± 34.72 | 76.14 ± 26.94 | 0.043 | |
| Postoperative length of stay (days), mean ± SD | 6.41 ± 1.79 | 6.18 ± 5.03 | 0.85 | |
| ICU admission rate (n/%) | 18/82 | 8/36 | 10.17 (1.79–57.79) | 0.009 |
| ICU stay (h), mean ± SD | 22.77 ± 16.68 | 9.23 ± 13.51 | 0.005 | |
| Postoperative complications (n/%) | ||||
| Restenosis | 1/4 | 0/0 | 1.00 | |
| Anastomotic dehiscence | 0/0 | 0/0 | NA | |
| Pneumonia | 0/0 | 1/4 | 1.00 | |
| Dysphonia | 20/91 | 11/50 | 13.79 (1.86–102) | 0.010 |
| Postoperative intubation rate (yes, n/%)b | 0/0 | 2/9 | 0.99 | |
| Postoperative deaths, 30 days (n/%) | 0/0 | 0/0 | NA |
The data are expressed as mean ± SD or number of patients.
P-value determined by paired t-test or multinomial logit regression, as appropriate.
In the ETT group, intubation occurred after the extubation.
CI: confidence interval; ETT: endotracheal intubation; ICU: intensive care unit; LMA: laryngeal mask airway; OR: odds ratio; SD: standard deviation.
Sufficient ventilation was achieved in all patients. No intraoperative anaesthesiology-related adverse events or complications occurred. Total operative time (time between start of anaesthesia induction and the emergence from anaesthesia) was shorter in patients with the LMA (96.23 ± 34.72 min in the ETT group vs 76.14 ± 26.94 min in the LMA group; P = 0.043). The admission rate to and the stay in the ICU were lower in the LMA group [18 in the ETT group vs 8 in the LMA group, odds ratio = 10.17, 95% confidence interval (CI) 1.79–57.79, P = 0. 009; 22.77 ± 16.68 h in the ETT group vs 9.23 ± 13.51 h in the LMA group, P = 0.005]. Among possible complications, dysphonia was more frequent in the ETT group than in the LMA group (20 in the ETT group vs 11 in the LMA group; odds ratio = 13.79, 95% CI 1.86–102, P = 0.010). One patient in the ETT group developed restenosis, and 1 patient in the LMA group contracted pneumonia.
No significant differences were found in terms of postoperative length of stay, intubation rate and 30-day mortality.
DISCUSSION
In this retrospective study, the LMA compared to the ETT approach demonstrated lower operative time, ICU admission rate and ICU length of stay. The LMA also reduced the occurrence of postoperative dysphonia.
To the best of our knowledge, no studies have compared the use of LMA versus ETT in tracheal resection in terms of early and long-term outcomes. A recent meta-analysis studying new approaches to airway management during tracheal resection also reported on conventional tube management and concluded that it is currently not possible to compare the various airway techniques described in the literature and to reliably determine their safety profile, feasibility and outcomes [5].
Historically, the preferred technique for airway management during general anaesthesia in these patients was endotracheal intubation using a narrow-diameter ETT placed above or below the stenotic segment [24]. However, this management technique is not free from difficulties and complications, such as the risk of failed intubation and hypoxia or difficulty passing the tube due to subglottic stenosis and trauma to the tracheal mucosa, which can lead to bleeding or even perforation at the level of the stenosis [25]. Some previous studies and case reports assessed LMA use in patients having tracheal surgery (Table 3).
Table 3:
Previous published studies and case reports on laryngeal mask airway use in tracheal resection and reconstruction
| Author and year of publication | Study type | Number of patients | Type of stenosis | Type of LMA | Ventilation strategy | Main results |
|---|---|---|---|---|---|---|
| Menna and Fiorelli et al., 2021 |
Single-centre retrospective study Case–control matching analysis with 1:1 ratio |
n = 184 n = 22 patients managed through LMA (LMA group) matched with n = 22 patients managed through ETT (ETT group) |
Laryngotracheal stenosis and tracheal stenosis | i-gel | PPV |
LMA provided sufficient ventilation in all patients. Operative time was shorter in patients with LMA. ICU admission rate and stay were lower in the LMA group. Dysphonia was more frequent in ETT group than in LMA group. |
| Schweiger et al. [15], 2020 | Single-centre retrospective study | n = 108 | Laryngotracheal stenosis | Classical LMA | PPV and HFJV in complex subglottic resections or laryngotracheal reconstructions |
Sufficient ventilation using a laryngeal mask was possible in 107 of 108 patients (99.1%). In 1 patient with severe retrognathism, correct positioning of the LMA was not feasible. Postoperatively, 2 patients (1.9%) developed pneumonia. |
| Krecmerova et al. [17], 2017 | Single-centre retrospective study | n = 54 | Tracheal stenosis | Laryngeal mask LarySeal | VCV |
LMA was successfully inserted in 53 (98.1%) patients. One patient developed dislocation, and repositioning was not feasible due to anatomical changes caused by radiotherapy and prior surgical resections. |
| Schieren et al. [8], 2017 | Single-centre retrospective study | n = 10 | Laryngotracheal stenosis and tracheal stenosis | Classical LMA | PPV and HFJV |
LMA insertion and subsequent PPV were successful in all patients. One patient with preoperative respiratory failure had persistent hypercarbia. Six patients (60%) had an uneventful postoperative course. Postoperative complications (i.e. vocal cord oedema, postoperative haemorrhage, pneumonia) occurred in 4 patients (40%). |
| Zardo et al. [23], 2016 | Case report | n = 1 | Laryngotracheal stenosis | Classical LMA | PPV | Unexpected higher stenosis and conventional intubation were impossible. |
| Caronia et al. [20], 2016 | Case report | n = 1 | Tracheal stenosis | Classical LMA | Spontaneous breathing | Successful management without complications. |
| Donaldson et al. [19], 2010 | Case report | n = 1 | Laryngotracheal stenosis | i-gel | VCV | i-gel supraglottic airway was inserted without difficulty, provided a good seal and allowed for controlled ventilation with acceptable peak pressures throughout the operation. |
| Biro et al. [7], 2001 | Case report | n = 1 | Tracheal stenosis | Classical LMA | PPV and HFJV | Successful management without complications. |
| Adelsmayr et al. [10], 1998 | Case report | n = 1 | High tracheal stenosis | Classical LMA | PPV and HFJV |
Peak airway pressure was limited to 15 cm H2O. End-tidal CO2 in the normal range (33-40 mmHg). |
| Asai et al. [22], 1993 | Case report | n = 1 | Tracheal stenosis | Classical LMA | Spontaneous breathing | End-expiratory carbon dioxide tension and arterial oxygen saturation were within normal limits. |
| Asai et al. [21], 1991 | Case report | n = 1 | Laryngotracheal congenital stenosis | Classical LMA | PPV | Increase of airway pressure and hypercarbia during surgery. |
ETT: endotracheal intubation; HFJV: high-frequency jet ventilation; ICU: intensive care unit; LMA: laryngeal mask airway; PPV: positive pressure ventilation; VCV: volume-controlled ventilation.
Asai et al. [21] in 1991 first published a case detailing the successful use of a classical LMA in a child with tracheal stenosis, despite an increase of airway pressure and hypercarbia during the operation. These authors also reported a patient with tracheal stenosis in whom the laryngeal mask was successfully used by maintaining spontaneous breathing and adequate ventilation during the whole procedure [22].
Schweiger et al. [15], in a single-centre retrospective study, evaluated LMA as the primary airway device in 108 patients. They found that LMA was feasible and safe in patients undergoing laryngotracheal surgery even in cases with high-grade stenosis; they achieved sufficient ventilation in 99.1% of cases. Krecmerova et al. [17] reported the use of LMA in 54 patients who had laryngotracheal surgery; ventilation was feasible in 96.4% of patients. Schieren et al. [8] showed no perioperative anaesthesia-related complications in a series of 10 patients receiving cervical tracheal resection using LMA. Six patients (60%) had an uneventful postoperative course. Postoperative complications (i.e. vocal cord oedema, postoperative haemorrhage, pneumonia) occurred in 4 patients (40%).
In contrast to ETT, LMA cannot bypass tracheal stenosis, but it may have several advantages that make it the preferable choice. First, LMA may avoid the need for a potentially difficult intubation, especially in cases of subglottic stenosis where secure ETT placement is not feasible because of the lack of distance to the vocal cords. In severe stenosis, given the small diameter and the tortuous lumen of the stenosis, an ETT even using a small tube is burdened by several risks, such as bleeding, perforation of the trachea or loss of control of the airway [7, 23]. Using LMA can also obviate the need for an eventual tube exchange at the end of the surgical procedure, with a decrease in airway management complexity with related possible complications, thus reducing the operative time. LMAs can also defer stenosis manipulations until surgical exposure of the trachea is complete. Once the anastomosis is performed, the LMA does not cause mechanical stress to the freshly sutured airway and reduces airway irritation and the risk of coughing as the patient emerges from the anaesthesia, thus protecting the anastomosis from dehiscence and resulting in a lower rate of postoperative dysphonia. Several studies previously reported a lower incidence of sore throat, hoarseness, dysphagia, dysphonia, laryngospasm and cough using LMA rather than ETT as a primary airway device [9, 18]. These findings suggest that, compared with ETT, LMA positioning causes less trauma to the vocal cords and trachea. In tracheal surgery, less manipulation of the stenosis before surgery, thereby avoiding mechanical stress on the anastomosis and limited irritation of the vocal cords, is paramount.
In addition, the supraglottic device allows control of the airway without interfering with the subglottic region and permits the use of fibre-optic bronchoscopy to check the vocal cords and the anastomosis while the patient is ventilated. Donaldson and Michalek [19] first successfully used an i-gel supraglottic device for airway management of a patient with subglottic stenosis and a difficult intubation during a previous procedure. An i-gel is a supraglottic device that has an integrated bite block without an inflatable cuff. Advanced supraglottic airway devices such as the i-gel may be more advantageous in tracheal surgery because of the higher seal pressure, the wide breathing lumen that also allows bronchoscopic examination and the presence of an additional channel for insertion of the gastric tube. These features make i-gel a more suitable airway device in situations in which a narrow ETT is inadvisable or too challenging to be used.
At the end of the operation, an uncuffed nasal ETT may be placed with the distal end beyond the anastomosis in order to allow adequate toilette of the airway and to protect the anastomosis and is commonly removed on the first postoperative day, after bronchoscopic review of the anastomosis. Other authors leave a small uncuffed ETT in place for a few days postoperatively only when laryngeal oedema is present at the moment of extubation [26]. If airway management is performed through the LMA, this ‘protective’ tube is obviously avoided, and consequently the need for ICU and sedation in the postoperative period is reduced. Although postoperative intubation as a precaution was not performed in the LMA group, the reintubation rate was not higher in this population.
LMA is associated with some disadvantages. First, use of the LMA should be avoided in patients with a high risk of aspiration, morbid obesity and conditions that could lead to bleeding [27]. In addition, the application of LMA could be limited in the presence of intraoral anatomical changes following radiotherapy, stiff tissue, oedema of the upper airway and critical tracheal stenosis [17]. The safety of LMAs for airway management in tracheal resection and reconstruction, especially with regard to patient selection and the risk for ventilation difficulties, has not been fully assessed [8]. Fatalities after failed ventilation, because of increased airway resistance across LMAs in patients with tracheal stenosis during resuscitations, have been described previously [28]. Moreover, some sporadic reports showed increased airway pressure during tracheal surgery and hypercarbia [8, 21]. For these possible hazards, rescue airway techniques must always be available in case of LMA failure.
LMA dislocation due to neck extension and surgical manipulations can also occur. Although LMA could be easily reinserted, usually without adverse consequences, rescue airway management could be necessary [17].
This study has some potential weaknesses. First, its retrospective nature does not allow a wide assessment of LMA use in tracheal surgery. A complete prospective, randomized trial comparing LMA use with conventional ETT would be desirable, defining accurate criteria for LMA indications in tracheal surgery. However, obtaining a sample size adequate for a prospective study could be difficult because of the relative rarity of tracheal resections, even in highly specialized surgical centres. Another limitation of this study is the small sample size.
CONCLUSION
In conclusion, this retrospective study demonstrates that LMA is a viable choice for airway management in tracheal surgery and can contribute to a favourable postoperative outcome by facilitating lower operative times, ICU admission rate, ICU length of stay and postoperative dysphonia occurrence compared to the ETT approach.
ACKNOWLEDGEMENTS
We would like to thank Alessia Valentini for providing the drawings and Marco Giustini for statistical support.
Conflict of interest: none declared.
ABBREVIATIONS
- BMI
Body mass index
- CI
Confidence interval
- ETT
Endotracheal tube
- ICU
Intensive care unit
- LMA
Laryngeal mask airway
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
Author contributions
Cecilia Menna: Conceptualization; Investigation; Methodology; Supervision; Writing—original draft; Writing—review & editing. Silvia Fiorelli: Data curation; Methodology; Software; Writing—original draft. Domenico Massullo: Investigation; Supervision. Mohsen Ibrahim: Conceptualization; Validation. Monica Rocco: Data curation; Writing—review & editing. Erino Angelo Rendina: Supervision; Validation; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Alexander Kogan, Georg Trummer and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Grillo HC, Donahue DM.. Post intubation tracheal stenosis. Semin Thorac Cardiovasc Surg 1996;8:370–80. [PubMed] [Google Scholar]
- 2. Andrilli AD, Maurizi G, Andreetti C, Maria A, Ibrahim M, Poggi C. et al. Long-term results of laryngotracheal resection for benign stenosis from a series of 109 consecutive patients. Eur J Cardiothorac Surg 2016;50:105–9. [DOI] [PubMed] [Google Scholar]
- 3. Wilkey BJ, Alfille P, Weitzel NS, Puskas F.. Anesthesia for tracheobronchial surgery. Semin Cardiothorac Vasc Anesth 2012;16:209–19. [DOI] [PubMed] [Google Scholar]
- 4. Wiedemann K, Männle C.. Anesthesia and gas exchange in tracheal surgery. Thorac Surg Clin 2014;24:13–25. [DOI] [PubMed] [Google Scholar]
- 5. Schieren M, Böhmer A, Dusse F, Koryllos A, Wappler F, Defosse J.. New approaches to airway management in tracheal resections—a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2017;31:1351–8. [DOI] [PubMed] [Google Scholar]
- 6. Stoelben E, Koryllos A, Beckers F, Ludwig C.. Benign stenosis of the trachea. Thorac Surg Clin 2014;24:59–65. [DOI] [PubMed] [Google Scholar]
- 7. Biro P, Hegi TR, Weder W, Spahn DR.. Case reports laryngeal mask airway and high-frequency jet ventilation for the resection of a high-grade upper tracheal stenosis. J Clin Anesth 2001;13:141–3. [DOI] [PubMed] [Google Scholar]
- 8. Schieren M, Egyed E, Hartmann B, Aleksanyan A, Stoelben E, Wappler F. et al. Airway management by laryngeal mask airways for cervical tracheal resection and reconstruction: a single-center retrospective analysis. Anesth Analg 2018;126:1257–61. [DOI] [PubMed] [Google Scholar]
- 9. Park SK, Ko G, Choi GJ, Ahn EJ, Kang H.. Comparison between supraglottic airway devices and endotracheal tubes in patients undergoing laparoscopic surgery: a systematic review and meta-analysis. Medicine 2016;95:e4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adelsmayr E, Keller C, Erd G, Brimacombe J.. The laryngeal mask and high-frequency resection of high tracheal stenosis. Anesth Analg 1998;86:907–8. [DOI] [PubMed] [Google Scholar]
- 11. D'Andrilli A, Ciccone AM, Venuta F, Ibrahim M, Andreetti C, Massullo D. et al. Long-term results of laryngotracheal resection for benign stenosis. Eur J Cardiothorac Surg 2008;33:440–3. [DOI] [PubMed] [Google Scholar]
- 12. Couraud L, Jougon JB, Velly JF.. Surgical treatment of nontumoral stenoses of the upper airway. Ann Thorac Surg 1995;60:250–60. [DOI] [PubMed] [Google Scholar]
- 13. Mathisen DJ. Surgery of the trachea. Curr Probl Surg 1998;35:453–542. [DOI] [PubMed] [Google Scholar]
- 14. Pinsonneault C, Fortier J, Donati F.. Tracheal resection and reconstruction. Can J Anesth/J Can Anesth 1999;46:439–55. [DOI] [PubMed] [Google Scholar]
- 15. Schweiger T, de Faria Soares Rodrigues I, Roesner I, Schneider-Stickler B, Evermann M, Denk-Linnert D-M. et al. Laryngeal mask as the primary airway device during laryngotracheal surgery: data from 108 patients. Ann Thorac Surg 2020;110:251–7. [DOI] [PubMed] [Google Scholar]
- 16. Fiorelli S, Creazzola F, Massullo D, Defraia V, Maggi L, Rocco M. et al. Dexmedetomidine sedation after tracheal surgery: a prospective pilot study. Ann Thorac Surg 2019;108:256–61. [DOI] [PubMed] [Google Scholar]
- 17. Krecmerova M, Schutzner J, Michalek P, Johnson P, Vymazal T.. Laryngeal mask for airway management in open tracheal surgery—a retrospective analysis of 54 cases. J Thorac Dis 2018;10:2567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luce V, Harkouk H, Brasher C, Michelet D, Hilly J, Maesani M. et al. Supraglottic airway devices vs tracheal intubation in children: a quantitative meta-analysis of respiratory complications. Paediatr Anaesth 2014;24:1088–98. [DOI] [PubMed] [Google Scholar]
- 19. Donaldson W, Michalek P.. The use of an i-gel supraglottic airway for the airway management of a patient with subglottic stenosis: a case report. Minerva Anestesiol 2010;76:369–72. [PubMed] [Google Scholar]
- 20. Caronia FP, Loizzi D, Nicolosi T, Castorina S, Fiorelli A.. Tubeless tracheal resection and reconstruction for management of benign stenosis. Head Neck 2017;39:E114–7. [DOI] [PubMed] [Google Scholar]
- 21. Asai T, Fujise K, Uchida M.. Use of the laryngeal mask in a child with tracheal stenosis. Anesthesiology 1991;75:903–4. [DOI] [PubMed] [Google Scholar]
- 22. Asai T, Fujise K, Uchida M.. Laryngeal mask and tracheal stenosis. Anaesthesia 1993;48:81. [DOI] [PubMed] [Google Scholar]
- 23. Zardo P, Kreft T, Hachenberg T.. Airway management via laryngeal mask in laryngotracheal resection. Thorac Cardiovasc Surg Rep 2016;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grillo HC, Donahue DM, Mathisen DJ, Wain JC, Wright CD.. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:483–6. [DOI] [PubMed] [Google Scholar]
- 25. Donaldson W. MP. The use of an i-gel supraglottic airway for the airway management of a patient with subglottic stenosis: a case report. Minerva Anestesiol 2010;76:369–72. [PubMed] [Google Scholar]
- 26. Douglas J, Mathisen M.. Surgery of the trachea. Curr Probl Surg 1998;35:453–542. [DOI] [PubMed] [Google Scholar]
- 27. Hernandez MR, Klock PAJ, Ovassapian A.. Evolution of the extraglottic airway: a review of its history, applications, and practical tips for success. Anesth Analg 2012;114:349–68. [DOI] [PubMed] [Google Scholar]
- 28. Kokkinis K, Papageorgiou E.. Failure of the laryngeal mask airway (LMA) to ventilate patients with severe tracheal stenosis. Resuscitation 1995;30:21–2. [DOI] [PubMed] [Google Scholar]