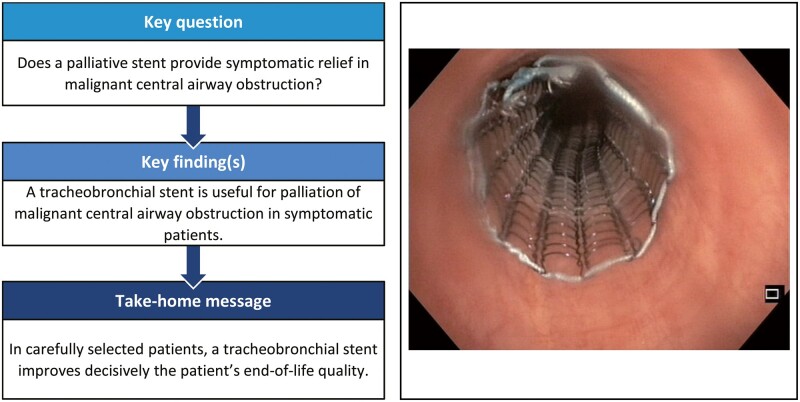
Abstract
OBJECTIVES
Tracheobronchial stenting has an established role in the palliation of malignant central airway obstruction (CAO). The purpose of this study is to describe the experience with self-expanding metal airway stents in 2 tertiary referral centres, covering a third of the population of Finland.
METHODS
Patients referred to and treated with airway stenting for malignant CAO using self-expanding metal-stents were identified from electronic patient records, and data were collected using a structured Endoscopic Lower Airway Management instrument. Statistical analysis to reveal factors affecting patient benefit and survival was carried out.
RESULTS
A total of 101 patients (mean age 65.8) and 116 procedures were identified. Procedure-related mortality was rare (3/101 patients) and complications infrequent. The median survival was 2.3 months [95% confidence interval (CI): 1.4–3.1). Stent benefit was not significantly affected by clinical characteristics. Survival was impacted by the use of adjunct procedures [hazard ratio (HR) 0.36, 95% CI: 0.23–0.58, P < 0.001), procedural urgency (HR 0.40; 95% CI: 0.23–0.71, P = 0.002) and post-treatment chemoradiotherapy (HR 0.29, 95% CI: 0.15–0.56, P < 0.001).
CONCLUSIONS
The beneficial impact observed supports the further use of tracheobronchial stenting in malignant CAO. The use of self-expanding metal stents is encouraged.
Keywords: Tracheal stents, Bronchial stents, Cancer
Tracheobronchial stents are inserted endoscopically mainly to provide symptom palliation in patients with central airway obstruction (CAO) due to either direct obstruction of the airway or compromised airway due to extrinsic compression.
INTRODUCTION
Tracheobronchial stents are inserted endoscopically mainly to provide symptom palliation in patients with central airway obstruction (CAO) due to either direct obstruction of the airway or compromised airway due to extrinsic compression. Malignant CAO may be caused by primary lung or oesophageal cancer, but also by metastatic cancer leading to mass in thoracic cavity [1]. A variety of endoscopic interventions are available for the treatment of malignant CAO, including balloon dilation, laser therapy and airway stents [2].
First introduced in 1987, endoscopically inserted tracheobronchial stents have proven useful in providing symptom palliation and, thus, improving the quality of life in patients with CAO [3]. Palliative airway stenting has been extensively studied in several patient cohorts [4–10]. Most previous studies have important limitations, however. They are relatively small, based on single-centre analysis, and have limited follow-up time. Thus, despite the obvious beneficial impact in patient quality of life with restoration of an obstructed airway, concerns regarding stent-associated complications and mortality have limited the application of airway stenting even in malignant obstruction. Recently, a U.S. Food and Drug Administration (FDA) black box warning was released, advising against treating benign airway obstruction with metal stenting [11, 12].
In this study, patients treated with tracheobronchial stenting for malignant CAO using self-expanding metal-stents in 2 tertiary referral centres of Turku and Oulu University Hospitals in 2002–2014 are described. The main objective is to evaluate the success rate and long-term symptomatic relief in this patient population. Furthermore, the benefit from concomitant endotracheal interventions such as lasertherapy is investigated. While endoscopic interventions in the treatment of malignant airway obstruction has previously been studied in Finland [13, 14], to our knowledge, this is the first study on exclusively tracheobronchial stenting in Finnish population.
PATIENTS AND METHODS
Patient population
All patients treated with tracheobronchial stenting for malignant airway disease between 2002 and 2014 in Turku University Hospital and Oulu University Hospital were identified. These 2 tertiary care university hospitals provide care for about 1.5 million people, representing a third of Finnish population. Altogether 101 patients were identified for the study. Patient charts were analysed, and relevant data were extracted using a structured data collection instrument, the Endoscopic Lower Airway Management form, including patient age, gender, treatment intent, site of the lesion, procedure details and follow-up data including complications. Patient data were handled in accordance with local research council permit (Dnro T81/2015). The retrospective chart review was not subject to an ethics committee approval.
Overall survival was calculated from stent placement to death or end of follow-up. Immediate benefit was assessed at 24 h after insertion and long-term palliation at the end of follow-up, based on patient electronic chart review. Benefit was defined as clear subjective patient-reported and objective physician-assessed improvement in shortness of breath, recorded to electronic patient charts at the time of treatment by the endoscopist or attending physician. Symptom palliation at the end of follow-up was extracted from the electronic patient charts as evaluated and recorded at the end of follow-up. The possible benefit was analysed separately for early benefit during hospitalization and long-term palliation during the follow-up period (median follow-up 2.3 months; range 0–27 months).
Operative complications occurring during procedure including stent insertion problems, early complications occurring within 3 days and late complications occurring after 3 days of stent insertion were recorded. Procedure urgency was assessed prior to stenting and was categorized as urgent, semi-urgent and elective. Urgent and semi-urgent procedures could not be postponed for >24 h or for >7 days, respectively, whereas elective procedures were scheduled to the normal workflow of the operating rooms.
Insertion of tracheobronchial stents
All patients were referred to endoscopic procedures by the attending pulmonologist or oncologist. Stenting was used only when other procedures such as laser resection did not prove sufficient to dilate the compromised airway. Final decision to use a stent was made by the endoscopist and informed consent was obtained. Stents were placed with a rigid bronchoscope under general anaesthesia and under local anaesthesia using a flexible videobronchoscope. The stents used were self-expanding nitinol stents (Boston Scientific Corporation or Olympus Medical Systems). Fully covered, half-covered and uncovered stents were used. The stents characteristics including the size of the stent were selected according to the clinical view of the intervention site acquired by a preoperative computed tomography (CT) and an intraoperative evaluation. Fluorography evaluation was not required intraoperatively and no problems in stent insertion were encountered.
Statistical analysis
Patient data were entered into SPSS 25 software (SPSS, IBM). A logistic regression model was used for the assessment of factors contributing to stent benefit and patient discharge status. Survival estimates were calculated using Kaplan–Meier method for overall survival after stent placement. For both univariable and multivariable survival analyses, Cox proportional hazards models were used.
For all multivariable models, step-wise backward LR method was applied for the identification of significant factors, setting exclusion P-value at 0.10. For two-step models investigating the impact of, for example stent details or oncological treatments, identified factors were entered into the first block of the model as indicated. For all analysis, P-value <0.05 was considered significant.
RESULTS
Patients and procedures
Overall, tracheobronchial stents were applied to 101 symptomatic patients for the palliation of malignant CAO. In addition to stenting, laser resection or dilatation was used when appropriate. Stenting was indicated only when other endoscopic procedures were successful in clearing the compromised airway and the distal airway was patent.
The stenting procedure was classified as urgent (scheduled within 24 h) in 18% of cases. Almost half of the stents were placed in trachea or carina (Table 1). A total of 31/101 patients were admitted to procedure from home. Four patients were admitted to procedure from intensive care unit, and 64 patients from regular wards. The mean and median follow-up times were 5.4 and 2.3 months (range 0–27 months), respectively.
Table 1:
Clinical characteristics, procedural details and survival impacta of the patient cohort
| Patient cohort |
OS impact |
|||
|---|---|---|---|---|
| n | % | HR (95% CI) | P-value | |
| Age | ||||
| <65 | 52 | 51 | 1.02 (1.00–1.04)/year | 0.034 |
| >65 | 49 | 49 | – | – |
| Gender | ||||
| Male | 54 | 53 | 0.89 (0.59–1.33) | 0.57 |
| Female | 47 | 47 | 1 | – |
| Malignancy | ||||
| Lung cancer | 62 | 61 | 1.00 (0.66–1.51) | 0.99 |
| Others | 39 | 39 | 1 | – |
| Treatment intent | ||||
| Palliative | 93 | 92 | 1 | – |
| Curative | 8 | 8 | 0.12 (0.04–0.34) | <0.001 |
| Oncological treatment | ||||
| None | 26 | 26 | 1 | – |
| Prior | 38 | 38 | 0.55 (0.33–0.92) | 0.022 |
| After | 21 | 21 | 0.31 (0.17–0.58) | <0.001 |
| Both | 16 | 16 | 0.48 (0.25–0.89) | 0.021 |
| Urgency | ||||
| Non-urgent | 22 | 22 | 1 | – |
| Semi-urgent | 61 | 60 | 0.85 (0.51–1.40) | 0.52 |
| Urgent | 18 | 18 | 1.73 (0.92–3.28) | 0.09 |
| Procedure type | ||||
| Stent only | 62 | 61 | 1 | – |
| Stent + laser | 34 | 34 | 0.60 (0.39–0.93) | 0.021 |
| Stent + dilation | 5 | 5 | 0.40 (0.14–1.10) | 0.076 |
| Site | ||||
| Upper trachea | 18 | 18 | 1 | – |
| Lower trachea | 17 | 17 | 1.49 (0.74–3.00) | 0.26 |
| Carina | 10 | 10 | 2.16 (0.95–4.91) | 0.065 |
| Right main bronchus | 23 | 23 | 1.66 (0.87–3.16) | 0.12 |
| Left main bronchus | 31 | 31 | 1.36 (0.74–2.49) | 0.33 |
| Both main bronchi | 2 | 2 | 2.53 (0.57–12.24) | 0.22 |
| Stent type | ||||
| Coated | 75 | 74 | 1 | – |
| Non-coated | 11 | 11 | 0.78 (0.40–1.52) | 0.47 |
| No data | 15 | 15 | – | – |
Impact of each variable on OS was tested in a univariable analysis using Cox proportional hazards model. HRs with 95% CIs are reported.
CI: confidence interval; HR: hazard ratio; OS: overall survival.
Stent insertion problems occurred in 5 patients. Two of these patients did not receive any benefit from stenting, 1 dying on the day of the procedure. One patient developed severe dyspnoea after the procedure, leading to death on the day of the procedure, while another death occurred after an unproblematic procedure. The only early-onset complication after procedure was dyspnoea, occurring in 6 patients within 24 h of procedure. Late complications occurring during the follow-up period were rare events, with 2 patients developing granulation and 1 patient experiencing stent migration.
Twenty-five of the 31 home-admitted patients were discharged by day 3, whereas only 6 of the 68 previously hospitalized patients were discharged by the 3rd postoperative day. Discharge of patients by the third postoperative day was strongly dependent on admission to procedure from home [odds ratio (OR) 44.4, 95% confidence interval (CI) 13.1–150.9, P < 0.001], but not patient age, gender or other clinical variables.
Symptomatic relief and follow-up treatments
Stent application had a favourable impact on symptoms (as evaluated by a significant subjective and objective improvement of breathing) for most patients, which lasted throughout the follow-up period in 75/101 patients. Importantly, no patients died of complications after a primarily successful procedure.
Stent benefit was not strongly dependent on clinical variables (Table 2). In a multivariable analysis, admission to procedure from home was the strongest predictor of stent benefit (OR 7.50; 95% CI: 0.94–69.8, P = 0.057). Univariable logistic regression did not reveal strong association of clinical variables with long-term stent benefit, although patient gender was included in multivariable logistic regression (OR 0.41; 95% CI: 0.16–1.06, P = 0.065). Notably, early benefit from stenting was not associated with long-term benefit.
Table 2:
The association of clinical variablesa with early symptomatic relief after stent placement (primary success, right columns) and long-term symtomatic relief (left columns)
| Overall, n = 101 | Primary success |
Long-term success |
||||
|---|---|---|---|---|---|---|
|
n = 86 |
n = 75 |
|||||
| n | OR (95% CI) | P-value | n | OR (95% CI) | P-value | |
| Age | ||||||
| <65 | 43 | 0.99 (0.94–1.03)/year | 0.57 | 38 | 0.99 (0.95–1.03) | 0.65 |
| >65 | 43 | – | – | 37 | 1 | – |
| Gender | ||||||
| Male | 43 | 1 | – | 36 | 0.41 (0.16–1.06) | 0.065 |
| Female | 43 | 2.75 (0.81–9.31) | 0.10 | 39 | 1 | – |
| Malignancy | ||||||
| Lung cancer | 52 | 0.77 (0.24–2.43) | 0.65 | 45 | 0.79 (0.31–2.01) | 0.63 |
| Others | 34 | 1 | – | 30 | 1 | – |
| Oncological treatment | ||||||
| None | 19 | 1 | – | 18 | 1 | – |
| Prior | 31 | 1.63 (0.50–5.38) | 0.42 | 26 | 0.96 (0.33–2.83) | 0.95 |
| After | 20 | 7.37 (0.83–65.66) | 0.074 | 16 | 1.42 (0.39–5.24) | 0.60 |
| Both | 16 | NA | 0.99 | 15 | 6.67 (0.75–59.50) | 0.089 |
| Admission to procedure | ||||||
| Home | 30 | 1 | – | 26 | 1 | – |
| Hospital | 56 | 0.13 (0.017–1.06) | 0.057 | 49 | 0.45 (0.15–1.33) | 0.15 |
| Procedure type | ||||||
| Stent only | 50 | 1 | – | 45 | 1 | – |
| Stent + laser/dilation | 36 | 2.88 (0.76–10.95) | 0.12 | 30 | 0.79 (0.31–2.01) | 0.63 |
| Urgency | ||||||
| Non-urgent | 19 | 1 | – | 15 | 1 | – |
| Semi-urgent | 53 | 1.05 (0.25–4.36) | 0.95 | 47 | 1.57 (0.53–4.60) | 0.41 |
| Urgent | 14 | 0.55 (0.11–2.87) | 0.48 | 13 | 1.21 (0.31–4.76) | 0.78 |
| Stent site | ||||||
| Upper trachea | 16 | 1 | – | 12 | 1 | – |
| Lower trachea | 17 | NA | 1.00 | 15 | 3.75 (0.64–22.04) | 0.14 |
| Carina | 5 | 0.13 (0.018–0.86) | 0.034 | 4 | 0.33 (0.067–1.65) | 0.18 |
| Right main bronchus | 19 | 0.59 (0.096–3.68) | 0.58 | 17 | 1.42 (0.37–5.47) | 0.61 |
| Left main bronchus | 27 | 0.84 (0.14–5.14) | 0.85 | 25 | 2.08 (0.55–7.83) | 0.28 |
| Both main bronchi | 2 | NA | 1.00 | 2 | NA | 1.00 |
The association of clinical variables with treatment success was analysed using a univariable logistic regression analysis. ORs with 95% CIs are reported.
CI: confidence interval; OR: odds ratio; NA: not applicable.
For 54 patients, repeat endoscopic procedures were required (mean 2.09, median 1, range 1–23). The highest rates of re-procedures (6 and 23 re-interventions) were observed in the 2 patients experiencing stent-related granulation. Re-stenting was, however, less common, with 15 repeated stent applications for 11 patients. Re-procedures and re-stenting did not impact stent benefit or patient discharge status. Stent removal was used in 6 patients, all of whom received postprocedural oncological treatment. No complications occurred during stent removal.
Patient survival
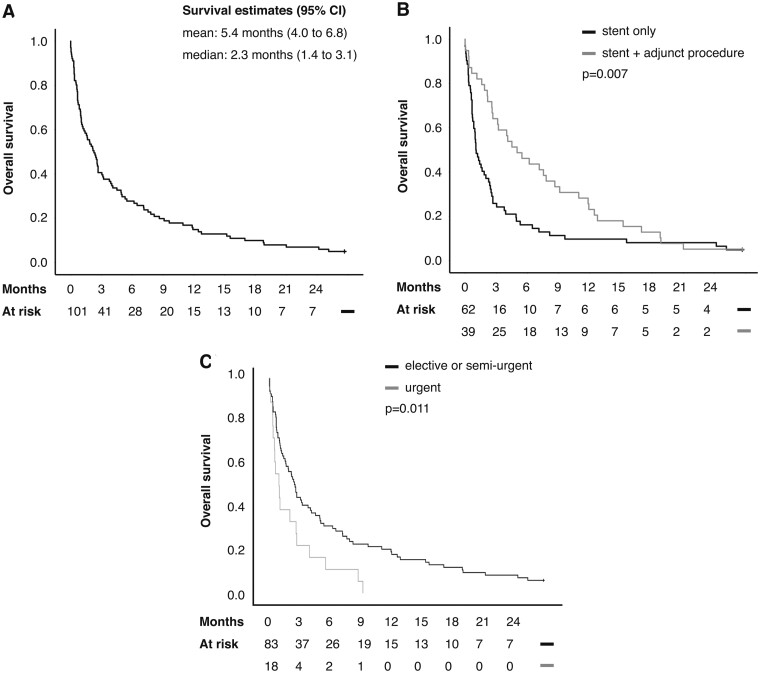
The overall survival of patients was poor, with median survival <3 months (Fig. 1A). The number of patients alive at 1, 3 and 12 months after procedure were 66 (65%), 41 (41%) and 15 (15%), respectively. Five patients (5%) survived until the end of follow-up, which is accounted for by the palliative nature of the treatment. Two of the survivors required repeat interventions, and 4 had been given curative therapy.
Figure 1:
(A) Kaplan–Meier estimates of overall survival in patients treated with airway stents for malignant airway obstruction. (B) A significant benefit was associated with the use of adjunct procedures in addition to airway stenting. (C) The need for urgent airway stenting was associated with a poor prognosis. Censoring (the end of follow-up) is indicated by a stick.
The univariable survival effects of clinical variables used in multivariable modelling are shown in Table 1. In a multivariable survival analysis of clinical variables, favourable survival after stent placement was associated curative treatment intent, and the use of postprocedural oncological treatment. In addition, adjunct procedures were associated with a favourable prognosis in both univariable (Fig. 1B) and multivariable analyses [hazard ratio (HR) 0.36, 95% CI: 0.23–0.58, P < 0.001). Adjunct procedures were associated with a favourable prognosis independently of the primary tumour site.
Procedure urgency
There was a trend for lesser likelihood of discharge within 3 days in the semi-urgently (scheduled within 1 week) treated patients (n = 61, OR 0.42; 95% CI: 0.1–1.14, P = 0.088), reaching significance in the urgently treated patients (n = 18, OR 0.13; 95% CI: 0.023–0.68, P = 0.016), compared to the 22 electively treated patients. Similarly, urgently treated patients had a poor survival (Fig. 1C), remaining significant in multivariable adjustment for treatment intent and oncological therapy (HR 0.40; 95% CI: 0.23–0.71, P = 0.002). Somewhat surprisingly, procedure urgency was however not a significant predictor of either early or long-term success from stenting.
Impact of oncological treatment
Majority of patients were treated oncologically either prior to stent placement or after airway stenting (Table 3). Discharge of patients by the third postoperative day was more likely, when prior oncological treatment was used. However, in a multivariable model adjusting for admission from home this trend did not reach significance (OR 2.14; 95% CI: 0.60–7.62, P = 0.242). Oncological treatments prior to airway stenting were not associated with patient survival. Furthermore, stent benefit was not affected by prior oncological treatment.
Table 3:
The use of oncological treatments prior or after airway stenting and their associationa with survival
| n | HR (95% CI) | P-value | |
|---|---|---|---|
| Prior oncological treatment | |||
| None | 47 | 1 | – |
| Radiotherapy | 13 | 0.59 (0.29–1.17) | 0.13 |
| Chemoradiotherapy | 23 | 1.31 (0.79–2.19) | 0.29 |
| Chemotherapy | 18 | 1.10 (0.63–1.89) | 0.746 |
| Oncological treatment after stenting | |||
| None | 57 | 1 | – |
| Radiotherapy | 19 | 0.79 (0.47–1.34) | 0.38 |
| Chemoradiotherapy | 15 | 0.36 (0.19–0.66) | 0.001 |
| Chemotherapy | 3 | 0.54 (0.17–1.73) | 0.30 |
| No data | 7 | 0.76 (0.34–1.67) | 0.49 |
The associations between oncological treatments and overall survival were analysed using Cox proportional hazards model. HRs and 95% CIs are reported.
CI: confidence interval; HR: hazard ratio.
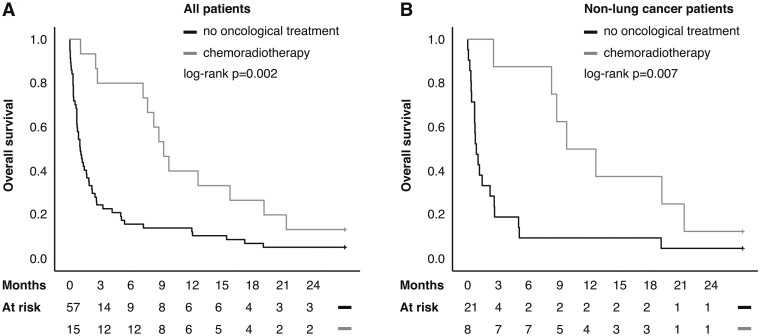
Postprocedural oncological treatment was a favourable prognostic indicator. Patient discharge status was understandably not affected by the use of postprocedural treatments. The use of chemoradiotherapy after application of airway stent was associated with a favourable patient prognosis in both univariable (Fig. 2A) and multivariable analyses (HR 0.29, 95% CI: 0.15–0.56, P < 0.001). The survival effect was more pronounced in patients with non-lung cancer (Fig. 2B). The use of radiotherapy alone afforded no survival benefit in neither univariable nor multivariable survival analyses. Interestingly, neither early- nor long-term stent benefit was associated with postprocedural oncological treatments. However, in 6 cases, postprocedural oncological treatment using chemoradiotherapy led to stent removal. Two of these patients were alive at the end of follow-up, while 4 died after a median follow-up of 64 months (range 8–21 months).
Figure 2:
(A) The use of chemoradiotherapy after stent application was associated with improved survival overall and (B) in non-lung cancer patients. Censoring (the end of follow-up) is indicated by a stick.
DISCUSSION
Our data support the benefit of tracheobronchial stenting in the context of malignant CAO. Most importantly, the goal of long-term airway patency was achieved in the majority of patients, leading to palliation for the rest of the patients’ life. In accordance with prior studies, pulmonary symptoms associated with malignant CAO can feasibly be palliated with tracheobronchial stenting resulting in long-term central airway patency [15]. The benefits associated with bronchoscopy-guided airway stenting to prevent suffocation include the direct observation of the airway and possibility for adjunct procedures such as laser therapy [16]. In suitable patients, fast-track discharge after airway stenting was possible, in concordance with previous studies [8, 17].
Limitations
A major limitation of retrospective studies into airway interventions is the lack of objective evaluation of stent benefit. The lack of reliable objective measurements or validated Finnish language questionnaires makes it impossible to absolutely determine the impact procedures have on patient quality of life even in prospective settings. At our institutions, however, as a part of usual clinical practice, the stent benefit is carefully evaluated after the procedure and recorded on electronic patient charts. In addition, we were able to retrospectively follow-up patients due to the extensive electronic medical records in use in Finland. Due to the palliative nature of the treatment, no routine follow-up imaging or bronchoscopic assessment was used in asymptomatic patients.
The overall prognosis of malignant CAO is dismal [18]. However, the significant improvement attainable in both the quality of end-of-life and life-expectancy supports the use of tracheobronchial stenting [15, 18, 19]. Prognostic factors associated with therapeutic success have previously been identified as distal airway patency in direct observation or imaging studies [20] and oncological treatment for the obstructing malignancy [21, 22]. A requirement for palliative tracheobronchial stenting at our institutions is the presence of a patent distal airway. In this study, improved survival was observed in patients treated with chemoradiotherapy after stenting, but not if oncological treatments were used prior to stent application. However, due to possible confounding bias associated with the ability to undergo oncological treatments, an observational study such as ours cannot confirm the relation between survival, stents and oncological treatments.
The impact of the obstruction site on the therapeutic benefit is less clear [23]. In our patients, there was a non-significant trend for poor survival in carina level obstruction, while otherwise the stent application site had no impact on patient survival. In our study, the complication rate of stent application was low (6/101), similarly to other observational studies. Especially since no deaths related to airway stents were observed during the follow-up, the use of tracheobronchial stenting in the palliation of symptomatic malignant CAO is supported, even when the patient has a relatively long prognosis. Importantly, based on prior studies as well as our experience, stenting should not be postponed when deemed necessary [7, 15]. This is highlighted by the observation that procedure urgency did not impact patient benefit, while being associated with poorer survival and lesser likelihood of discharge. While procedure urgency may indicate more acute symptoms of dyspnoea and thus a more pronounced need for the procedure, it may also indicate a more serious background illness. Finally, as the use of adjunct procedures during the stent application procedure was associated with a favourable prognosis, such procedures may well be beneficial, when an endobronchial tumour can be debulked to better accommodate for stent placement.
In this study, either coated or uncoated self-expanding metal stents were used. Traditional silicone stents have several issues, most importantly stent migration, which are overcome by the use of self-expanding metal stents [1]. The burying of the stent in the airway mucosal or the ingrowth of granulation tissue or cancer through the uncoated surface of metal stent can be overcome by the use of polyester coating on the surface of stents [24]. In this study, uncoated stents were used for a minority of patients with short prognosis, while coating was not associated with a favourable prognosis. Overall, metal stenting cannot generally be preferred, when only short-term dilation of airway is attempted, as the removal of metal stent is more difficult and prone to complications. In this study, however, the removal of metal stents was not associated with complications, when performed in carefully selected situations. Future prospects include investigations into bioabsorbable and 3D-printed stents, with potential to overcome several problematic issues.
CONCLUSION
In conclusion, this study supports the practice of applying expandable, endoscopically inserted stents for malignant tracheobronchial obstruction. Further investigation into earlier intervention in patients with symptomatic malignant airway obstruction is encouraged, as stent-related complications are rare, and no clinical factors were associated with an unequivocally poor prognosis.
Funding
This research was funded by the Finnish State Research funding.
Conflict of interest: none declared.
Author contributions
Johannes Routila: Formal analysis; Visualization; Writing—original draft; Writing—review & editing. Eino Herhi: Data curation; Investigation. Jarkko Korpi: Data curation; Investigation. Jaakko Pulkkinen: Project administration; Supervision; Writing—review & editing. Petri Koivunen: Conceptualization; Project administration; Supervision; Writing—review & editing. Jami Rekola: Conceptualization; Project administration; Resources; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Paula Moreno, Paolo Scanagatta and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- CAO
Central airway obstruction
- CI
Confidence interval
- CT
Computed tomography
- FDA
Food and Drug Administration
- HR
Hazard ratio
- NA
Not applicable
- OR
Odds ratio
REFERENCES
- 1. Mudambi L, Miller R, Eapen GA.. Malignant central airway obstruction. J Thorac Dis 2017;9:S1087–S1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorden JA, Ernst A.. Endoscopic management of central airway obstruction. Semin Thorac Cardiovasc Surg 2009;21:263–73. [DOI] [PubMed] [Google Scholar]
- 3. Dutau H, Dumon J-F.. Airway stenting revisited: 30 years, the age of reason? J Bronchol Interv Pulmonol 2017;24:257–9. [DOI] [PubMed] [Google Scholar]
- 4. Tojo T, Iioka S, Kitamura S, Maeda M, Otsuji H, Uchida H. et al. Management of malignant tracheobronchial stenosis with metal stents and Dumon stents. Ann Thorac Surg 1996;61:1074–8. [DOI] [PubMed] [Google Scholar]
- 5. Wilson GE, Walshaw MJ, Hind CR.. Treatment of large airway obstruction in lung cancer using expandable metal stents inserted under direct vision via the fibreoptic bronchoscope. Thorax 1996;51:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood DE, Liu Y-H, Vallières E, Karmy-Jones R, Mulligan MS.. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167–74. [DOI] [PubMed] [Google Scholar]
- 7. Razi SS, Lebovics RS, Schwartz G, Sancheti M, Belsley S, Connery C. et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90:1088–93. [DOI] [PubMed] [Google Scholar]
- 8. McGrath EE, Warriner D, Anderson P.. The insertion of self expanding metal stents with flexible bronchoscopy under sedation for malignant tracheobronchial stenosis: a single-center retrospective analysis. Arch Bronconeumol 2012;48:43–8. [DOI] [PubMed] [Google Scholar]
- 9. Inchingolo R, Sabharwal T, Spiliopoulos S, Krokidis M, Dourado R, Ahmed I. et al. Tracheobronchial stenting for malignant airway disease: long-term outcomes from a single-center study. Am J Hosp Palliat Care 2013;30:683–9. [DOI] [PubMed] [Google Scholar]
- 10. Tjahjono R, Chin RY-K, Flynn P.. Tracheobronchial stents in palliative care: a case series and literature review. BMJ Support Palliat Care 2018;8:335–9. [DOI] [PubMed] [Google Scholar]
- 11.FDA. FDA Public Health Notification: Complications from Metallic Tracheal Stents in Patients with Benign Airway Disorders. 2005
- 12. Folch E, Keyes C.. Airway stents. Ann Cardiothorac Surg 2018;7:273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hujala K, Sipilä J, Grenman R.. Endotracheal and bronchial laser surgery in the treatment of malign and benign lower airway obstructions. Eur Arch Otorhinolaryngol 2003;260:219–22. [DOI] [PubMed] [Google Scholar]
- 14. Sipilä J, Pulkkinen J, Hujala K, Grenman R.. Endoscopic lasersurgery in obstructive tracheal and bronchial tumors. Otolaryngol Pol 2004;58:187–90. [PubMed] [Google Scholar]
- 15. Ong P, Grosu HB, Debiane L, Casal RF, Eapen GA, Jimenez CA. et al. Long-term quality-adjusted survival following therapeutic bronchoscopy for malignant central airway obstruction. Thorax 2019;74:141–56. [DOI] [PubMed] [Google Scholar]
- 16. Mohan A, Shrestha P, Madan K, Hadda V, Pandey R, Upadhyay A. et al. A prospective outcome assessment after bronchoscopic interventions for malignant central airway obstruction. J Bronchol Interv Pulmonol 2020;27:95–105. [DOI] [PubMed] [Google Scholar]
- 17. Yerushalmi R, Fenig E, Shitrit D, Bendayan D, Sulkes A, Shitrit D. et al. Endobronchial stent for malignant airway obstructions. Isr Med Assoc J 2006;8:615–7. [PubMed] [Google Scholar]
- 18. Stratakos G, Gerovasili V, Dimitropoulos C, Giozos I, Filippidis F, Gennimata S. et al. Survival and quality of life benefit after endoscopic management of malignant central airway obstruction. J Cancer 2016;7:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amjadi K, Voduc N, Cruysberghs Y, Lemmens R, Fergusson DA, Doucette S. et al. Impact of interventional bronchoscopy on quality of life in malignant airway obstruction. Respiration 2008;76:421–8. [DOI] [PubMed] [Google Scholar]
- 20. Giovacchini CX, Kessler ER, Merrick C, Gao J, Wang X, Wahidi M. et al. Clinical and radiographic predictors of successful therapeutic bronchoscopy for the relief of malignant central airway obstruction. BMC Pulm Med 2019;19:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemaire A, Burfeind WR, Toloza E, Balderson S, Petersen R, Harpole D. et al. Outcomes of tracheobronchial stents in patients with malignant airway disease. Ann Thorac Surg 2005;80:434–8. [DOI] [PubMed] [Google Scholar]
- 22. Guibert N, Mazieres J, Lepage B, Plat G, Didier A, Hermant C.. Prognostic factors associated with interventional bronchoscopy in lung cancer. Ann Thorac Surg 2014;97:253–9. [DOI] [PubMed] [Google Scholar]
- 23. Mahmood K, Wahidi MM, Thomas S, Argento A, Ninan N, Smathers E. et al. Therapeutic bronchoscopy improves spirometry, quality of life, and survival in central airway obstruction. Respiration 2015;89:404–13. [DOI] [PubMed] [Google Scholar]
- 24. Menna C, Poggi C, Ibrahim M, D'Andrilli A, Ciccone AM, Maurizi G. et al. Coated expandable metal stents are effective irrespective of airway pathology. J Thorac Dis 2017;9:4574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]