Abstract
OBJECTIVES
The goal of the present study is to investigate changes in supra-aortic vessel perfusion after implantation of the non-covered Ascyrus Medical Dissection Stent (AMDS) for surgical treatment of acute type A aortic dissection.
METHODS
From 2017 to 2020, 16 consecutive patients treated with AMDS and involvement (dissection to total occlusion) of at least 1 supra-aortic vessel were included in the study. Centre-line based computed tomography measurements of true, false and total lumen area using Terarecon software were performed before and after surgery. Changes in the true lumen area were indexed to the entire vessel area. The paired sample t-test was used to assess the significance of the observed differences.
RESULTS
Analysis of supra-aortic vessels and the descending aorta showed significant improvement in true lumen perfusion after the AMDS was implanted. The indexed true lumen area increased postoperatively by 72%, 112% and 30% in the innominate, right and left common carotid arteries, respectively. Total occlusions of both common carotid arteries recovered completely after surgical treatment. The proximal- and the mid-descending aorta showed a 78% and 48% improvement of the indexed true lumen area, respectively.
CONCLUSIONS
Arch repair using AMDS shows promising results in the treatment of acute type A aortic dissection. Quantitative measurements of true and false lumen perfusion demonstrated a significant increase in true lumen area and a 100% regression of totally occluded supra-aortic branches. Further examination in a larger cohort of patients and comparison with isolated hemiarch repair are needed to confirm positive vascular remodelling after an AMDS implant.
Keywords: Type A aortic dissection, Arch repair, Cerebral malperfusion, DeBakey I, Ascyrus Medical Dissection Stent
INTRODUCTION
Acute type A aortic dissection (ATAAD) involving supra-aortic vessels represents an emergency in cardiac surgery with the need of urgent repair to reduce morbidity and mortality. Dissection of innominate and common carotid arteries with consecutive malperfusion syndrome can lead to brain injury, complicating postoperative care [1]. Despite continuous improvements in diagnosis, referral and surgical treatment, the management of supra-aortic vessel malperfusion remains challenging [2, 3]. Acute neurological impairment or stroke at presentation is a strong predictor of a poor perioperative outcome, especially in older patients [4, 5]. Adjustments regarding the re-establishment and monitoring of adequate cerebral perfusion were demonstrated to improve outcomes [6]. Innovations also involved surgical techniques: Besides the standard of care hemiarch repair, new prostheses were developed to perform more radical repairs, possibly reducing malperfusion complications and avoiding the need for reoperations [7, 8]. The Ascyrus Medical Dissection Stent (AMDS) was designed to upgrade the standard hemiarch procedure and to treat malperfusion: Sealing the distal anastomosis prevents antegrade false lumen flow and increases true lumen perfusion. The fate of dissected supra-aortic vessels after surgery for ATAAD remains unclear, and quantitative analyses of cerebral perfusion are lacking. Therefore, our goal was to investigate the effects of AMDS implants on true lumen perfusion in patients presenting with ATAAD and affected supra-aortic vessels.
METHODS
Patient population
The study was approved by the local ethics committee (No. EA2/096/20) and complies with the Declaration of Helsinki. Informed patient consent was waived.
Between February 2018 and March 2020, a total of 233 patients were operated on for ATAAD; 48 (21%) were given an AMDS implant for DeBakey I acute dissection, representing the primary study cohort. Patients in whom at least 1 supra-aortic vessel (n = 16) was affected, based on preoperative computed tomography (CT) scans, comprised the final study population. Two patients were excluded because their supra-aortic vessels were not fully captured in the preoperative CT scan.
Computed tomography analysis
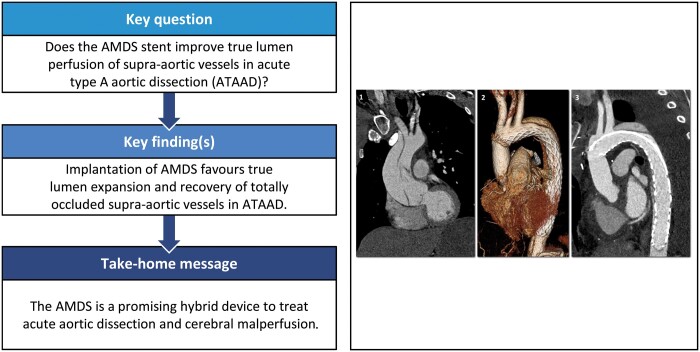
Quantitative measurements of true and false lumens and whole-vessel diameter and area were performed using the centre-line based Terarecon software (Aquarius Intuition Viewer, Durham, NC USA) in a standardized fashion either at the most stenotic level in the affected vessels or 20 mm above the respective origin in the non-affected vessels (Fig. 1.1 and 1.2). The most stenotic portion of a diseased branch was defined as ‘dissected’ in cases of dissection without perfusion impairment, as ‘sub-totally occluded’ in cases of 75–99% stenosis and as ‘totally occluded’ in cases with 100% stenosis. The true lumen area was indexed to the whole vessel area and compared before and after the AMDS was implanted (Fig. 1.3a and 1.3b). Similar quantitative measurements were performed on postoperative CT scans of the descending aorta at the half-length of the AMDS and 1 cm distal to the tip of the stent. The same landmarks were then identified in preoperative CT scans, starting 1 cm proximal to the origin of the innominate artery.
Figure 1:
Centre-line definition of supra-aortic vessels. (1) 2-Dimensonal visualization of the centre line and recognition of the most stenotic level in the preoperative computed tomography scan. (2) 3-Dimensional reconstruction. (3) Comparison of the standardized centre-line measurements before (a) and after (b) the Ascyrus Medical Dissection Stent is implanted.
Figure 2:
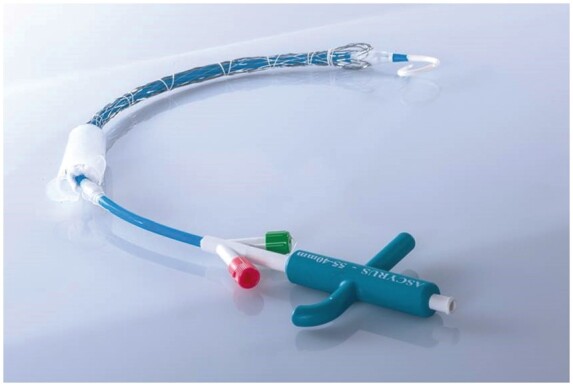
Overview of the Ascyrus Medical Dissection Stent.
How to use the AMDS
A detailed description of the AMDS device and of the sizing and implant procedures was published previously [9]. In brief, AMDS is a hybrid graft that comprises a 10-mm proximal polytetrafluoroethylene cuff for sealing the distal anastomosis and a super-helical non-covered nitinol stent for remodelling of the arch and the descending aorta (Fig. 2). The indication for an AMDS implant is a DeBakey I dissection with a primary entry tear in the ascending aorta and the absence of tears in the aortic arch or the proximal descending aorta. The stent is available in 2 shapes: tubular, with a fixed diameter along the stent, and tapered, with a decreasing diameter proximal to distal. Sizing is performed through multiplanar or centre-line reconstructions in the preoperative CT scan at 2 aortic landmarks: zone 1 of the aortic arch and the descending aorta at the level of the tracheal bifurcation. Based on obtained measurements, an appropriate stent can be selected according to tables provided on the Ascyrus Medical website (https://ascyrus.com/sizes-and-configurations/). The operative setting considers right axillary cannulation for the arterial line and right atrium or femoral access for venous drainage. After preparing the ascending aorta and the supra-aortic branches, aortic transection is performed in an orthogonal plane to the vessel, 1 cm proximal to the innominate artery. The device was implanted under unilateral selective cerebral perfusion (with 10 ml/kg/min flow) at moderate systemic hypothermia (core temperature, 28°C). Near infra-red spectroscopy is used during the operation to monitor cerebral perfusion. Placement of the AMDS in the true lumen can be assured either through direct view or using a femoral guidewire.
As soon as the stent is fully inserted, the protection sheath is removed, and the proximal cuff is unfolded and fixed in place through 4 cardinal stitches using a Teflon strip on the outer side of the aorta to avoid tension or tearing. The stent is released by unscrewing and pulling the green cap attached to the fixation suture along the delivery system. A circumferential 3–0 Prolene suture is performed using the sandwich technique to seal the distal anastomosis, readapting the aortic wall layers between the stent cuff and the outer felt strip. The ascending vascular graft is secured through a second running suture to the aorta-AMDS complex.
Assessment of neurological symptoms
Acute preoperative neurological symptoms were classified only if present on admission to our hospital. Postoperatively, the presence and quality of neurological symptoms were assessed for all patients by discriminating between loss of function (0 points out of 5 at neurological examination, Medical Research Council Muscle Scale) and impaired function (1–4 out of 5). Duration was divided into transient (symptom improves or recovers within days after surgery) and permanent (no recovery observed perioperatively).
Statistical analyses
Clinical and anatomical data are expressed as frequencies with corresponding percentages for categorical variables and as mean with standard deviation (SD) for continuous data. Statistical analyses were performed with the paired sample t-test using SPSS software Version 25 (IBM SPSS Statistics, Armonk, New York USA). A P-value of <0.05 was considered statistically significant. Statistical analyses and data reporting were in line with the statistical and data reporting guidelines of the European Journal of Cardio-thoracic Surgery and Interactive Cardiovascular and Thoracic Surgery [10].
RESULTS
Baseline characteristics
Baseline characteristics are shown in Table 1. The mean age was 61 years (SD: 12 years); 5 (31.25%) patients were women. One patient (6.25%) was admitted in cardiogenic shock due to a massive pericardial effusion and with acute left-sided hemiparesis (PENN class Abc). The average German Registry for Acute Aortic Dissection Type A score was 20.47% (SD: 6.8%). Six patients (37.5%) presented with acute neurological symptoms: 5 of them had a left-sided hemiparesis, and 1 patient had isolated left leg paresis. Among these patients, preoperative CT scans showed involvement of 2 supra-aortic branches in 50% of cases, and 67% had at least 1 subtotally occluded vessel.
Table 1:
Baseline characteristics
| Baseline characteristics, mean (SD), n (%) | Total population, N = 16 |
|---|---|
| Age (years) | 61 (SD : 12) |
| Gender (female) | 5 (31.25) |
| BMI (kg/m2) | 25.8 (SD: 3.8) |
| BSA (m2) | 2.0 (SD: 0.3) |
| Hypertension | 11 (68.75) |
| Diabetes | 1 (6.25) |
| Smoker | 6 (37.5) |
| Chronic obstructive pulmonary disease | 1 (6.25) |
| Kidney insufficiency | 1 (6.25) |
| Coronary artery disease | 2 (12.5) |
| Previous aortic disease | 1 (6.25) |
| Intubation | 1 (6.25) |
| Acute shock | 1 (6.25) |
| Acute neurological deficit | 6 (37.5) |
| Malperfusion (any sign) | 6 (37.5) |
| Coronary | 1 (6.25) |
| Cerebral | 6 (37.5) |
| Peripheral | 2 (12.5) |
| Visceral | 1 (6.25) |
| PENN Classification | |
| Aa-no ischaemia | 10 (62.5) |
| Ab-localized ischaemia | 5 (31.25) |
| Ac-generalized ischaemia | 0 (0) |
| Abc-combined ischaemia | 1 (6.25) |
| GERAADA score | 20.47 (SD: 6.85) |
| Aortic regurgitation | |
| No-trace | 3 (18.75) |
| Mild | 2 (12.5) |
| Moderate | 10 (62.5) |
| Severe | 1 (6.25) |
| Left ventricular ejection fraction (%) | 52 (SD: 8) |
| Right ventricular ejection fraction (%) | 53 (SD: 4) |
| Pericardial effusion | 4 (25) |
BMI: body mass index; BSA: body surface area; GERAADA: German Registry for Acute Aortic Dissection Type A; SD: standard deviation.
Surgical data
Operative data are shown in Table 2. Time between onset of symptoms and the operation (pain-to-cut time) was 398 min (SD: 268 min) in the overall population and 270 min (SD: 110 min) in patients with acute neurological deficits. Mean caudal circulatory arrest time was 45 min (SD: 11 min). In 50% of cases, a tapered AMDS 55–40 was used. Moderate-to-severe aortic regurgitation was present in 69% of patients, with consecutive aortic valve replacement in 5 patients (31.25%).
Table 2:
Intraoperative data
| Operative data, mean (SD), n (%) | Total population, N = 16 |
|---|---|
| Pain-to-cut time (min) | 398 (SD: 268) |
| Operation time (min) | 370 (SD: 114) |
| Cardiopulmonary bypass time (min) | 194 (SD: 40) |
| Cross-clamp time (min) | 112 (SD: 33) |
| Reperfusion (min) | 56 (SD: 28) |
| Intraoperative transfusions (units) | |
| Red cell concentrate | 1.2 (SD: 1.9) |
| Platelets concentrate | 4.4 (SD: 2.3) |
| Fresh frozen plasma | 5.2 (SD: 5.8) |
| Hypothermia (°C) | 27.3 (SD: 1.2) |
| Selective cerebral perfusion time (min) | 45 (SD: 11) |
| Bicuspid valve | 1 (6.25) |
| Aortic valve surgery | |
| Repair | 11 (68.75) |
| Biological replacement | 4 (25) |
| Mechanical replacement | 1 (6.25) |
| Root operation | |
| Reconstruction | 9 (56.25) |
| Bentall | 5 (31.25) |
| David | 2 (12.5) |
| AMDS sizing | |
| 40 tubular | 4 (25) |
| 40–30 tapered | 3 (18.75) |
| 55 tubular | 1 (6.25) |
| 55–40 tapered | 8 (50) |
| Revision for bleeding | 2 (12.5) |
AMDS: Ascyrus Medical Dissection Stent; SD: standard deviation.
Perioperative data and neurological outcome
Perioperative data are presented in Table 3. The mean intensive care unit stay was 10 days (SD: 8 days). Postoperative neurological deficits were present in 8 patients (50%): among the 6 patients with preoperative acute deficits, 5 (83%) had the deficits also after surgery and 1 recovered completely. Three new postoperative neurological deficits were diagnosed: 1 patient had an occluded right common carotid and a subtotally occluded left common carotid artery preoperatively, and 2 presented with subtotal occlusion of the right or left common carotid. Neurological deficits were transient in 62.5%, and 75% were classified as impaired function.
Table 3:
Perioperative data
| Perioperative data, mean ± SD, n (%) | Total population, N = 16 |
|---|---|
| ICU length of stay (days) | 11 ± 8 |
| Ventilation time (days) | 5 ± 6 |
| Open chest therapy | 1 (6.25) |
| Reintubation | 2 (12.5) |
| Tracheotomy | 2 (12.5) |
| Delirium | 6 (37.5) |
| Postoperative low cardiac output syndrome | 1 (6.25) |
| Dialysis | 2 (12.5) |
| Postoperative neurological deficit | 8 (50) |
| Neurological deficit duration | |
| Transient | 5 (62.5) |
| Permanent | 3 (37.5) |
| Neurological deficit quality | |
| Impaired function | 6 (75) |
| Loss of function | 2 (25) |
| Postoperative CT-diagnosed stroke | 6 (37.5) |
| 30-Day mortality | 3 (18.75) |
CT: computed tomography; ICU: intensive care unit; SD: standard deviation.
Impact of the AMDS on true lumen perfusion
Quantitative measurements of supra-aortic vessels and descending aorta are summarized in Tables 4 and 5; dissection characteristics of the supra-aortic vessels are provided in Fig. 3.
Table 4:
Centre-line-based measurements of dissected supra-aortic vessels and descending aorta before and after surgery
| Assessment of dissected vessels, mean (SD), n (%) | Preoperative, N = 16 | Postoperative, N = 16 |
|---|---|---|
| Innominate artery | ||
| Dissected | 12 (75) | 11 (68.75) |
| Subtotal occlusion | 4 (25) | 1 (6.25) |
| Total occlusion | 0 (0) | 0 (0) |
| True lumen diameter (mm) | 9.15 (SD: 1.81) | 11.51 (SD: 2.42) |
| True lumen area (mm2) | 68.04 (SD: 25.16) | 108.22 (SD: 44.92) |
| False lumen diameter (mm) | 13.67 (SD: 1.93) | 10.18 (SD: 6.25) |
| False lumen area (mm2) | 149.60 (SD: 41.78) | 110.53 (SD: 80.03) |
| Total area (mm2) | 214.82 (SD: 54.11) | 221.42 (SD: 78.00) |
| Measurement landmark (mm) | 18.82 (SD: 6.93) | 20.16 (SD: 8.34) |
| Right common carotid | ||
| Dissected | 4 (25) | 3 (18.75) |
| Subtotal occlusion | 6 (37.5) | 3 (18.75) |
| Total occlusion | 3 (18.75) | 0 (0) |
| True lumen diameter (mm) | 4.24 (SD: 2.34) | 5.98 (SD: 2.06) |
| True lumen area (mm2) | 18.21 (SD: 13.66) | 31.25 (SD: 15.87) |
| False lumen diameter (mm) | 7.04 (SD: 3.57) | 3.21 (SD: 4.56) |
| False lumen area (mm2) | 48.42 (SD: 28.59) | 23.48 (SD: 34.97) |
| Total area (mm2) | 67.12 (SD: 20.35) | 54.84 (SD: 25.82) |
| Measurement landmark (mm) | 30.74 (SD: 21.17) | 28.46 (SD: 17.20) |
| Left common carotid | ||
| Dissected | 4 (25) | 4 (25) |
| Subtotal occlusion | 2 (12.5) | 0 (0) |
| Total occlusion | 1 (6.25) | 0 (0) |
| True lumen diameter (mm) | 5.91 (SD: 2.12) | 7.51 (SD: 1.49) |
| True lumen area (mm2) | 30.92 (SD: 15.60) | 45.92 (SD: 18.39) |
| False lumen diameter (mm) | 3.89 (SD: 4.40) | 2.37 (SD: 4.01) |
| False lumen area (mm2) | 26.37 (SD: 33.09) | 16.28 (SD: 30.51) |
| Total area (mm2) | 57.13 (SD: 22.84) | 62.60 (SD: 31.87) |
| Measurement landmark (mm) | 40.30 (SD: 29.62) | 35.49 (SD: 20.00) |
| Proximal-descending aorta | ||
| True lumen diameter (mm) | 18.34 (SD: 4.42) | 23.91 (SD: 3.86) |
| True lumen area (mm2) | 278.59 (SD: 130.33) | 460.13 (SD: 144.61) |
| False lumen diameter (mm) | 24.30 (SD: 3.34) | 16.36 (SD: 8.45) |
| False lumen area (mm2) | 471.88 (SD: 134.24) | 262.81 (SD: 150.36) |
| Total area (mm2) | 749.75 (SD: 175.57) | 728.00 (SD: 193.56) |
| Measurement landmark (mm) | 105.56 (SD: 13.79) | 105.56 (SD: 13.79) |
| Mid-descending aorta | ||
| True lumen diameter (mm) | 16.13 (SD: 2.96) | 19.43 (SD: 2.82) |
| True lumen area (mm2) | 211.31 (SD: 80.47) | 303.00 (SD: 85.55) |
| False lumen diameter (mm) | 22.12 (SD: 3.24) | 19.34 (SD: 6.42) |
| False lumen area (mm2) | 392.06 (SD: 117.40) | 324.13 (SD: 158.53) |
| Total area (mm2) | 602.13 (SD: 166.26) | 621.63 (SD: 193.72) |
| Measurement landmark (mm) | 220.31 (SD: 28.44) | 220.31 (SD: 28.44) |
SD: standard deviation.
Table 5:
Paired t-test comparison of indexed true lumen area in the supra-aortic vessels and descending aorta after an Ascyrus Medical Dissection Stent implant
| Indexed true lumen area, mean (SD) | Preoperative | Postoperative | P-value |
|---|---|---|---|
| Indexed INA true lumen (%) | 32 (SD: 9) | 55 (SD: 28) | 0.002 |
| Indexed RCC true lumen (%) | 34 (SD: 33) | 72 (SD: 40) | 0.01 |
| Indexed LCC true lumen (%) | 64 (SD: 40) | 83 (SD: 29) | 0.13 |
| Indexed proximal-descending true lumen (%) | 36.53 (SD: 12.49) | 64.31 (SD: 18.91) | <0.001 |
| Indexed mid-descending true lumen (%) | 35.09 (SD: 8.66) | 50.74 (SD: 16.41) | 0.002 |
INA: innominate artery; LCC: left common carotid artery; RCC: right common carotid artery; SD: standard deviation.
Figure 3:
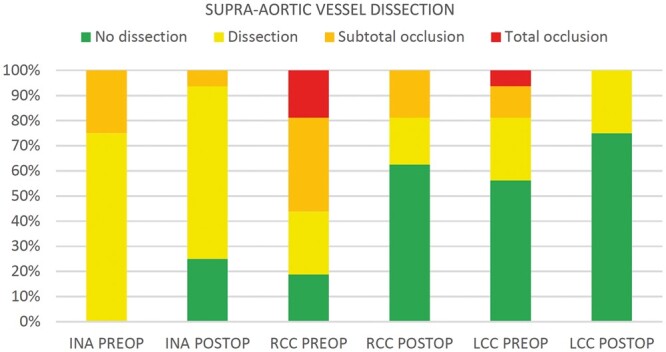
Dissection morphology of supra-aortic vessels before and after the Ascyrus Medical Dissection Stent is implanted. INA: innominate artery; LCC: left common carotid artery: preop: preoperative; postop: postoperatively; RCC: right common carotid artery.
The innominate artery was affected in all patients, presenting either dissection without impaired perfusion (75%) or subtotal occlusion (25%). Mean total vessel area was 214.82 mm2 (SD: 54.11 mm2) whereas the false lumen occupied most of vessel surface with 149.60 mm2 (SD: 41.78 mm2). The right common carotid had the worst involvement, showing 6 subtotal occlusions (37.5%) and 3 total occlusions (18.75%); also in this branch, false lumen covered most of the vessel area and decreased postoperatively. The left common carotid was diseased in 7 cases (43.75%), with 2 (12.5%) subtotal occlusions and 1 (6.25%) total occlusion.
The comparison between dissection morphology in the supra-aortic vessels before and after surgery shows complete regression of total occlusions in both common carotid arteries and an increase in dissection-free vessels: 0–25% for the innominate artery, 18.75–62.5% for the right common carotid and 56.25–75% for the left common carotid artery.
After indexing the true lumen as a percentage of the whole vessel area, perfusion improvement was achieved in all supra-aortic branches. Indexed innominate artery true lumen increased from 32% to 55% (P = 0.002) and indexed right common carotid true lumen doubled from 34% to 72% (P = 0.01). No statistical relevance was reached by the left common carotid (P = 0.13), despite true lumen increases from 64% to 83%.
Full AMDS expansion was confirmed in all patients postoperatively, without any device-related complications. In 87.5% of cases, elimination of antegrade false lumen perfusion in the aortic arch was achieved, with partial or full false lumen thrombosis of the descending aorta in 68.75% of patients.
After quantitative assessment of the descending aorta, the true lumen area increased from 278.59 mm2 (SD: 130.33 mm2) to 460.13 mm2 (SD: 144.61mm2) at the proximal descending landmark [105 mm (SD: 13.79 mm) at the half-length of the AMDS], with an increase in indexed true lumen area of 77.8% (P < 0.001). Similarly, the indexed true lumen area increased from 35% to 50% (P-value = 0.002) at the mid-descending landmark (220 mm [SD: 28.44 mm] 1 cm distal to the tip of the stent).
DISCUSSION
ATAAD involving the supra-aortic branches have been object of intensive study in past years due to the poor prognosis and high morbidity rates. Preoperative cerebral malperfusion was demonstrated as a relevant risk factor for hospital death and as an independent predictor of postoperative cerebral injuries [3, 11]. In 2011, Tsukube et al. showed a favourable cut-off of 5 h between the onset of symptoms and the operation, a treatment window that granted low hospital mortality (14%), remarkable postoperative neurological improvement and a 71.8% cumulative survival rate at 3 years [12]. These results show that preoperative neurological deficits and cerebral malperfusion do not represent contraindications for surgery in patients with ATAAD, above all if patients reach the operating room within a few hours after the onset of the dissection and sufficient cerebral perfusion is re-established. The improvements in cerebral protection led to the development of numerous surgical strategies for arch repair in ATAAD. Although procedures like hemiarch repair or the frozen elephant trunk technique have been analysed extensively [13, 14], showing comparable and reproducible results in terms of mortality and freedom from reoperation, a wide consensus about which strategy should be used is currently not available. The AMDS was properly designed to address these issues: reduce complexity in the surgery for ATAAD, prolonging by only a few min the hemiarch procedure, treat malperfusion and promote true lumen expansion, which should lead to positive remodelling and prevent the need for reinterventions. The proximal AMDS cuff prevents the formation of the distal anastomotic new entry, an insufficiency of the distal anastomosis that was shown to be present in up to 70% of postoperative CT scans from patients who developed aortic growth after isolated hemiarch repair [15]. Due to its low radial force, the non-covered AMDS is not intended to relaminate the aorta but just to readapt the intima against the media and adventitia; the subsequent expansion of the true lumen will drive the resolution of malperfusion and the depressurization of the false lumen. It is well known that the involvement of supra-aortic vessels is a great challenge and might need complex repairs, but not all occlusions in supra-aortic branches can or should be radically treated [16]. Our CT assessment showed that the location of the most stenotic segment was at 30.7 and 40.3 mm from vessel’s origin for the right and left common carotid arteries, respectively, meaning a level often difficult to reach during open surgery. Moreover, the AMDS enables the treatment of supra-aortic vessel occlusions, as underlined by our findings. Considering all mentioned arguments, the AMDS is an arch repair device that represents an upgrade of the hemiarch procedure and should not be primarily compared to total arch replacement techniques, due to the profound differences in concept, implant technique and mechanism of function.
Initial data about the AMDS device were published as a result of a multicentre trial, showing encouraging results, especially regarding the treatment of acute malperfusion [17, 18]. A cohort of 26 patients experienced a 95.5% resolution rate of vessel malperfusion, with no device-related adverse events. A previous report described positive aortic arch remodelling with elimination of antegrade false lumen flow and resolution of cerebral malperfusion involving supra-aortic vessels in 85.7% of patients [19].
The recently proposed German Registry for Acute Aortic Dissection Type A score and other malperfusion classifications confirm the high risk of our cohort [20, 21]. Also the novel TEM classification, which considers dissection type, entry location and the presence of clinical or radiological signs of malperfusion, may be a valuable tool to shift the treatment paradigm to a better risk stratification and a more comprehensive assessment of dissection complexity [22].
The present analysis underlines the fact that supra-aortic branch malperfusion and preoperative neurological deficit represent a severe condition, with 5 patients out of 6 still presenting neurological symptoms after the operation, although the AMDS improved cerebral perfusion relieving carotid artery occlusions. The 3 additional patients presenting with postoperative stroke had no preoperative neurological deficits but had at least a subtotal occlusion in 1 carotid artery. Despite full postoperative recovery of cerebral perfusion and no signs of embolic events, these 3 patients suffered from brain injury, probably due to prolonged dynamic malperfusion. These outcomes indirectly underline the importance of fast referral in case of supra-aortic vessel involvement, enabling a prompt surgical repair to restore cerebral perfusion. This goal is fully achieved with the AMDS device, with a remarkable increase in dissection-free vessels, especially for carotid arteries (18.75–62.5% for the right common carotid and 56.25–75% for the left common carotid artery).
Considering the different results between carotid arteries, right axillary cannulation and recovery of innominate artery may have played an additional role in terms of perfusion gain in the right common carotid as well as the lower involvement rate of the left common carotid. Also, the descending aorta shows improved perfusion of the true lumen along and distal to the stent: the sealing of the distal anastomosis and the elimination of antegrade false lumen flow in 87.5% of patients induce thrombosis and positive vascular remodelling. Contemporarily, the non-covered stent allows for full perfusion of the supra-aortic branches and for expansion of the descending true lumen, without increasing the risk of paraplegia. Implanting an AMDS does not add complexity to the standard hemiarch procedure and shows a high safety profile, with no device-related complications observed in this cohort. Details regarding surgical treatment in case of aneurysmatic arch dilatation following an AMDS implant cannot be provided since no case was observed during the currently available follow-up period.
Resolution of vessel occlusions, postoperative improvement in dissection-free supra-aortic branches and a relevant gain in the indexed true lumen area in the descending aorta are the main achievements of the AMDS concept and the key of malperfusion treatment.
CONCLUSION
This study represents a confirmation of the encouraging results achieved by the AMDS device in the treatment of ATAAD, especially in cases of vessel malperfusion. The remodelling of the aortic arch, driven by the sealing of the distal anastomosis and by the antegrade expansion of the true lumen, produces notable benefits in the supra-aortic vessels and downstream aorta, with significant increase of the true lumen area.
Limitations
This analysis is limited by its retrospective nature and the small sample size. Moreover, the analysis refers to the perioperative course only. A longer follow-up period is required to provide valuable information regarding positive vascular remodelling. The collected quantitative measurements are derived from the CT scan, which is known to provide a static flow pattern only. A dynamic analysis using carotid Doppler may have been a more precise tool to assess cerebral perfusion. Due to the lack of availability of dynamic measurements for most patients, we were not able to provide meaningful results regarding this topic.
Further investigation on a larger cohort and the comparison to hemiarch repair alone are necessary to confirm the present results, but the AMDS already appears to be a safe and reproducible adjunct to the standard of care in case of malperfusion, the goal being to effect a single-stage hybrid restoration of perfusion of the supra-aortic vessels.
Funding
Matteo Montagner and Jörg Kempfert have received travel grants and speaker fees from Ascyrus Medical (Boca Raton, FL, USA).
Conflict of interest: none declared.
ABBREVIATIONS
- ATAAD
Acute type A aortic dissection
- AMDS
Ascyrus Medical Dissection Stent
- CT
Computed tomography
- SD
Standard deviation
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
Author contributions
Matteo Montagner: Conceptualization; Data curation; Formal analysis; Methodology; Writing—original draft. Markus Kofler: Conceptualization; Formal analysis; Methodology; Writing—review & editing. Roland Heck: Data curation; Investigation. Semih Buz: Investigation; Software; Supervision. Christoph Starck: Investigation; Supervision; Writing—review & editing. Stephan Kurz: Data curation; Investigation. Volkmar Falk: Conceptualization; Methodology; Supervision; Writing—review & editing. Jörg Kempfert: Conceptualization; Methodology; Supervision; Validation; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Roman Gottardi, Martin Grabenwöger and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Dumfarth J, Kofler M, Stastny L, Plaikner M, Krapf C, Semsroth S. et al. Stroke after emergent surgery for acute type A aortic dissection: predictors, outcome and neurological recovery. Eur J Cardiothorac Surg 2018;53:1013–20. [DOI] [PubMed] [Google Scholar]
- 2. Sultan I, Bianco V, Patel HJ, Arnaoutakis GJ, Di Eusanio M, Chen EP. et al. Surgery for type A aortic dissection in patients with cerebral malperfusion: results from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg 2019;S0022-5223(19)32762-X. [DOI] [PubMed] [Google Scholar]
- 3. Czerny M, Schoenhoff F, Etz C, Englberger L, Khaladj N, Zierer A. et al. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA Registry. J Am Coll Cardiol 2015;65:2628–35. [DOI] [PubMed] [Google Scholar]
- 4. Di Eusanio M, Patel HJ, Nienaber CA, Montgomery DM, Korach A, Sundt TM. et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg 2013;145:S213–21.e1. [DOI] [PubMed] [Google Scholar]
- 5. Dumfarth J, Peterss S, Luehr M, Etz CD, Schachner T, Kofler M. et al. Acute type A dissection in octogenarians: does emergency surgery impact in-hospital outcome or long-term survival? Eur J Cardiothorac Surg 2017;51:472–7. [DOI] [PubMed] [Google Scholar]
- 6. Rylski B, Urbanski PP, Siepe M, Beyersdorf F, Bachet J, Gleason TG. et al. Operative techniques in patients with type A dissection complicated by cerebral malperfusion. Eur J Cardiothorac Surg 2014;46:156–66. [DOI] [PubMed] [Google Scholar]
- 7. Sultan I, McGarvey J, Vallabhajosyula P, Desai ND, Bavaria JE, Szeto WY.. Routine use of hemiarch during acute type A aortic dissection repair. Ann Cardiothorac Surg 2016;5:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Yu C, Yang X, Sun X, Qiu J, Jiang W. et al. Hybrid and frozen elephant trunk for total arch replacement in DeBakey type I dissection. J Thorac Cardiovasc Surg 2019;158:1285–92. [DOI] [PubMed] [Google Scholar]
- 9. Montagner M, Heck R, Kofler M, Buz S, Starck C, Sündermann S. et al. New hybrid prosthesis for acute type A aortic dissection. Surg Technol Int 2020;36:95–7. [PubMed] [Google Scholar]
- 10. Hickey GL, Dunning J, Seifert B, Sodeck G, Carr MJ, Burger HU. et al. ; EJCTS and ICVTS Editorial Committees. Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur J Cardiothorac Surg 2015;48:180–93. [DOI] [PubMed] [Google Scholar]
- 11. Dumfarth J, Kofler M, Stastny L, Gasser S, Plaikner M, Semsroth S. et al. Immediate surgery in acute type A dissection and neurologic dysfunction: fighting the inevitable? Ann Thorac Surg 2020;110:5–12. [DOI] [PubMed] [Google Scholar]
- 12. Tsukube T, Hayashi T, Kawahira T, Haraguchi T, Matsukawa R, Kozawa S. et al. Neurological outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. Circulation 2011;124:S163–7. [DOI] [PubMed] [Google Scholar]
- 13. Rylski B, Milewski RK, Bavaria JE, Vallabhajosyula P, Moser W, Szeto WY. et al. Long-term results of aggressive hemiarch replacement in 534 patients with type A aortic dissection. J Thorac Cardiovasc Surg 2014;148:2981–5. [DOI] [PubMed] [Google Scholar]
- 14. Berger T, Weiss G, Voetsch A, Arnold Z, Kreibich M, Rylski B. et al. Multicentre experience with two frozen elephant trunk prostheses in the treatment of acute aortic dissection†. Eur J Cardiothorac Surg 2019;56:572–8. [DOI] [PubMed] [Google Scholar]
- 15. Rylski B, Hahn N, Beyersdorf F, Kondov S, Wolkewitz M, Blanke P. et al. Fate of the dissected aortic arch after ascending replacement in type A aortic dissection. Eur J Cardiothorac Surg 2017;51:1127–34. [DOI] [PubMed] [Google Scholar]
- 16. Luehr M, Etz CD, Nozdrzykowski M, Lehmkuhl L, Misfeld M, Bakhtiary F. et al. Extra-anatomic revascularization for preoperative cerebral malperfusion due to distal carotid artery occlusion in acute type A aortic dissection. Eur J Cardiothorac Surg 2016;49:652–8. discussion 658–9. [DOI] [PubMed] [Google Scholar]
- 17. Bozso SJ, Nagendran J, MacArthur RGG, Chu MWA, Kiaii B, El-Hamamsy I. et al. Dissected aorta repair through stent implantation trial: Canadian results. J Thorac Cardiovasc Surg 2019;157:1763–71. [DOI] [PubMed] [Google Scholar]
- 18. Bozso SJ, Nagendran J, Chu MWA, Kiaii B, El-Hamamsy I, Ouzounian M. et al. Single-stage management of dynamic malperfusion using a novel arch remodeling hybrid graft. Ann Thorac Surg 2019;108:1768–75. [DOI] [PubMed] [Google Scholar]
- 19. Bozso SJ, Nagendran J, Chu MWA, Kiaii B, El-Hamamsy I, Ouzounian M. et al. Midterm outcomes of the dissected aorta repair through stent implantation trial. Ann Thorac Surg 2020;S0003-4975(20)31134-6. [DOI] [PubMed] [Google Scholar]
- 20. Czerny M, Siepe M, Beyersdorf F, Feisst M, Gabel M, Pilz M. et al. Prediction of mortality rate in acute type A dissection: the German Registry for Acute Type A Aortic Dissection score. Eur J Cardiothorac Surg 2020;58:700–6. [DOI] [PubMed] [Google Scholar]
- 21. Augoustides JG, Geirsson A, Szeto WY, Walsh EK, Cornelius B, Pochettino A. et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med 2009;6:140–6. [DOI] [PubMed] [Google Scholar]
- 22. Sievers HH, Rylski B, Czerny M, Baier ALM, Kreibich M, Siepe M. et al. Aortic dissection reconsidered: type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact CardioVasc Thorac Surg 2020;30:451–7. [DOI] [PubMed] [Google Scholar]