Abstract
OBJECTIVES
Lymph node dissection (LND) with robot-assisted thoracoscopic surgery (RATS) in lung cancer surgery has not been fully evaluated. The aim of this study was to compare LND surgical results between video-assisted thoracoscopic surgery (VATS) and RATS.
METHODS
We retrospectively compared perioperative parameters, including the incidence of LND-associated complications (chylothorax, recurrent and/or phrenic nerve paralysis and bronchopleural fistula), lymph node (LN) counts and postoperative locoregional recurrence, among 390 patients with primary lung cancer who underwent lobectomy and mediastinal LND by RATS (n = 104) or VATS (n = 286) at our institution.
RESULTS
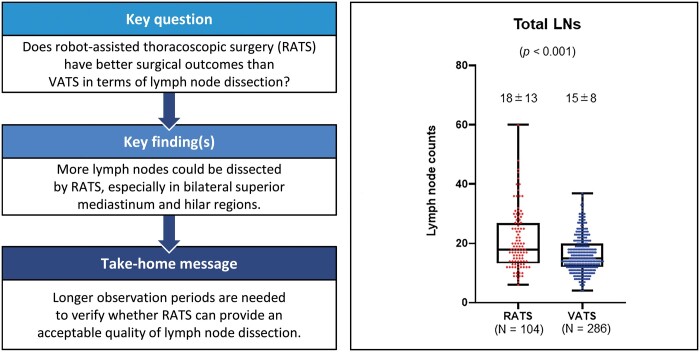
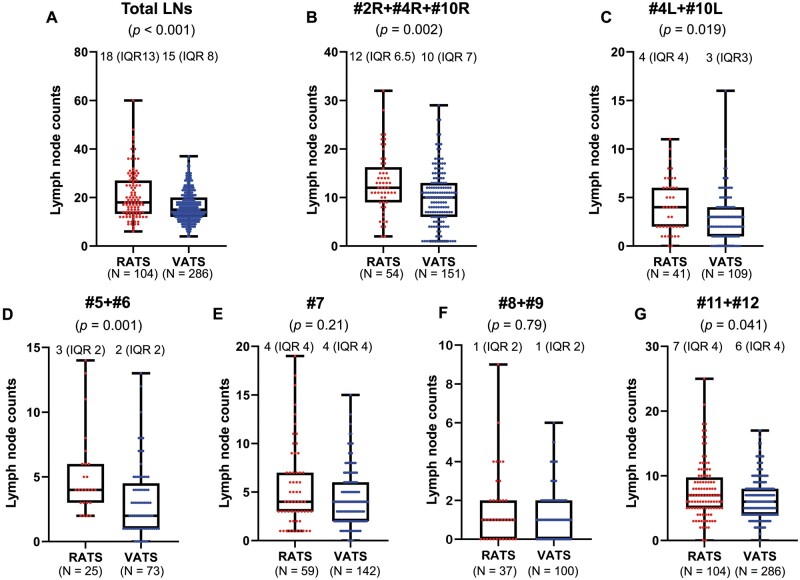
The median total dissected LN numbers significantly differed between the RATS and the VATS groups (RATS: 18, VATS: 15; P < 0.001). They also significantly differed in right upper zone and hilar (#2R + #4R + #10L) (RATS: 12, VATS: 10; P = 0.002), left lower paratracheal and hilar (#4L + #10L) (RATS: 4, VATS: 3; P = 0.019), aortopulmonary zone (#5 + #6) (RATS: 3, VATS: 2; P = 0.001) and interlobar and lobar (#11 + #12) LNs (RATS: 7, VATS: 6; P = 0.041). The groups did not significantly differ in overall nodal upstaging (P = 0.64), total blood loss (P = 0.69) or incidence of LND-associated complications (P = 0.77).
CONCLUSIONS
In this comparison, it was suggested that more LNs could be dissected using RATS than VATS, especially in bilateral superior mediastinum and hilar regions. Accumulation of more cases and longer observation periods are needed to verify whether RATS can provide the acceptable quality of LND and local control of lung cancer.
Keywords: Lung cancer, Robot-assisted thoracoscopic surgery, Video-assisted thoracoscopic surgery, Lymph node dissection, Nodal upstaging, LND-associated complication
INTRODUCTION
Systematic hilar and mediastinal lymph node dissection (LND) is an essential component of lung cancer surgery, which improves both prognosis and staging accuracy. Although some randomized controlled trials and meta-analyses have examined the prognostic significance of systematic LND for primary lung cancer [1–5], their results were inconsistent; as they evaluated different procedures and study designs, their conclusions regarding the prognostic impact of systematic LND for lung cancer were unclear. However, harvesting appropriate numbers of lymph nodes (LNs) in suitable regions is critical to accurate staging of primary lung cancer. In a non-randomized study by the Eastern Cooperative Oncology Group, multiple levels of mediastinal LN metastasis were documented in 30% of patients who underwent systematic LND, but only in 12% of patients who had systematic LN sampling [6]. In addition, in the largest randomized trial, ACOSOG Z0030, 4% of mediastinal LN metastases were underdiagnosed by systematic LN sampling, which suggests that systematic LND is superior for accurate disease staging [1]. For this reason, systematic LND has been adopted by many thoracic surgeons for lung cancer surgery.
Video-assisted thoracoscopic surgery (VATS) is widely used as a less-invasive procedure for primary lung cancer. Many thoracic surgeons appreciate its low invasiveness, which leads to less pain, shorter hospital stays and higher patient quality of life, as well as its straightforward manipulations with endoscopic instruments and creation of microstructural images. However, the effectiveness of using VATS for LND remains unclear. Although 1 randomized prospective study found no difference in numbers of dissected LNs by VATS versus thoracotomy, a recent meta-analysis showed that fewer total LNs were dissected by VATS [7, 8]. Because straight endoscopic instruments are mainly used in VATS, LND accuracy could conceivably decrease in narrow anatomical regions. The da Vinci Surgical System (DVSS; Intuitive Surgical Company, Sunnyvale, USA) has some advantages, including a three-dimensional visual field and an articulated joint forceps with 7 degrees of freedom. These innovative technologies may improve LND accuracy and quality; some reports found that RATS can dissect more LNs, with a higher rate of nodal upstaging, than VATS [9–12], whereas another report found that RATS is not superior to VATS with respect to LN yield or upstaging [13]. Moreover, few comparative studies of postoperative complications or locoregional recurrences after RATS versus VATS are available.
In this retrospective study, we reviewed consecutive lobectomies and mediastinal LNDs by RATS or VATS and compared perioperative parameters, including numbers of harvested LNs, incidence of LND-associated complications, nodal upstaging rates and postoperative locoregional recurrence between the 2 groups.
MATERIALS AND METHODS
Patient selection
This study was approved by our facility’s institutional review board in November 2019 (19A143), which waived the patients’ written informed consent requirement because of the study’s retrospective design. We reviewed 390 consecutive patients who underwent RATS or VATS lobectomy and mediastinal LND at our institution from January 2011 to April 2020. In principle, thoracoscopic surgery (RATS or VATS) were generally indicated for patients with clinical stage I–IIA disease; those with clinical stage IIB or higher were indicated for thoracoscopic surgery only when considered technically feasible. Patients who underwent induction therapy, incomplete resection or conversion from thoracoscopic surgery to thoracotomy were excluded from this study. During the period of this study, 2 lobectomy cases were finished with incomplete resection and 17 cases were converted from VATS to thoracotomy because of bleeding of intrathoracic major vessel, incomplete fissure, severe pleural adhesion, silicotic or metastatic LNs and longer operative time. These cases were excluded from this study. Patients who were indicated for thoracoscopic surgery gave informed consent regarding RATS, and patients who confirmed that medical treatment was possible at their own expense underwent RATS before March 2018. Since April 2018, both approaches (VATS or RATS) were offered to patients because the national health insurance system in our country began to cover RATS. All patients underwent preoperative contrast-enhanced computed tomography (CT) of the chest and upper abdomen within 1 month before their surgery. Primary tumours were evaluated by chest CT, and their sizes were determined by thin-section CT findings. For all tumours, we obtained the tumour’s maximum dimension (tumour) and its solid component (consolidation) using a lung window-level setting from thin-section CT images and then estimated the consolidation/tumour ratio (C/T ratio) for each tumour [14]. Magnetic resonance imaging of the brain and positron emission tomography/CT were routinely performed to evaluate LN status and to provide a systemic survey. LNs with short axes of >1.0 cm on chest CT that showed FDG uptake on positron emission tomography/CT were clinically suspected to be metastatic. Endobronchial ultrasonography-guided transbronchial needle aspiration was performed for patients with suspicious hilar and mediastinal LNs. Mediastinoscopy was not performed during the time of this study. Because the clinical and pathological stages were determined according to the 6th and 7th editions of the TNM classification in the initial period of this study, those cases were restaged according to the TNM 8th edition for the present study. Postoperative complication severity was graded according to the Clavien–Dindo classification system [15]. In this study, we specifically defined postoperative bronchopleural fistula, chylothorax, and recurrent and/or phrenic nerve paralysis as Clavien–Dindo grade ≥2 LND-associated complications. Locoregional recurrence was defined as occurrence in the (1) bronchial stump or lung staple lines, (2) ipsilateral pleura and/or chest wall or (3) ipsilateral hilar and/or mediastinal LNs, as described in our previous study [16].
Surgical procedure
All patients underwent standard general anaesthesia with single-lung ventilation using a double-lumen endotracheal tube. The patient was placed in the lateral decubitus position. VATS was performed using previously described techniques [16]. In our institution, platforms used for RATS were the DVSS second- and third-generation systems (da Vinci S and Si, respectively) from January 2011 to December 2018, and DVSS fourth-generation systems (da Vinci X and Xi) from January 2019 to April 2020. RATS using da Vinci S and Si was performed with previously described settings and techniques [17]. In RATS using da Vinci X and Xi, procedures were performed by completely robotic portal lung resection with 4 robotic arms and a carbon dioxide (CO2) insufflation system. Linear port placement in the 8th intercostal space was adopted, and an assist window was placed in the 4th or 5th intercostal space at the anterior axillary line. The robotic instruments used were bipolar instruments (e.g. Long Bipolar Grasper, Fenestrated Bipolar Forceps, Maryland Bipolar Forceps) and Tip-Up Fenestrated Grasper. A vessel-sealing system (e.g. Vessel Sealer Extend) was occasionally used to seal and cut thick tissues and small blood vessel branches. Da Vinci staplers (e.g. EndoWrist® Staplers and SureForm™) were used to staple pulmonary arteries, veins and bronchi and divide the lung parenchyma.
Mediastinal LND procedures
During the time of this study, we performed systematic or lobe-specific mediastinal LND [18]. In principle, lobe-specific LND was used for patients with clinical stage IA disease in the right upper or lower lobe, left upper division or lower lobe. Systematic LND was used for patients with clinical stage ≥IB disease in the aforementioned lobes and divisions. Patients with disease at any clinical stage in the right middle lobe or left lingular segment were invariably candidates for systemic LND. We categorized and defined mediastinal and hilar LNs according to the 2009 International Association for the Study of Lung Cancer LN map [19].
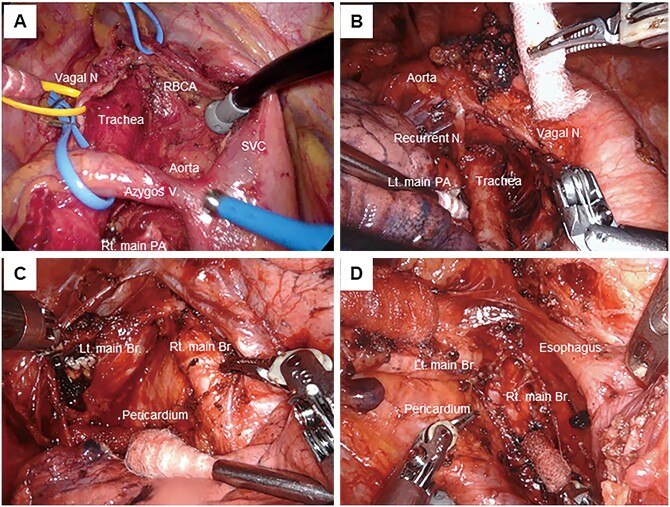
Detailed procedures and anatomical landmarks of LND were as follows. In the right upper mediastinal zone (stations #2R and #4R), all fat tissue with LNs between the phrenic nerve, vagus nerve, right innominate artery and right main bronchus were removed, exposing the superior vena cava, trachea, anterolateral aspect of the ascending aorta, right tracheobronchial angle, azygos vein and right main bronchus. In the left upper mediastinal zone (station #4L), all LN tissue on the distal left main bronchus was removed, up to and including the left tracheobronchial angle. In the aortopulmonary zone (stations #5 and #6), all fat tissues with LNs between the phrenic and vagus nerves were removed down to the left main pulmonary artery (PA), including the subaortic space. We did not divide the ligamentum arteriosum but identified the left recurrent laryngeal nerve along the ligament. In the subcarinal zone (station #7), all the subcarinal tissue was removed, exposing the right and left main bronchi, and posterior pericardium. In the lower mediastinal zone, the #8 and #9 nodes were removed by clearing all LNs around the inferior pulmonary vein, oesophagus and pulmonary ligament (Fig. 1). The interlobar (#11) and lobar (#12) LNs were dissected en bloc or separately; however, they were counted in the total because of their unclear dividing line.
Figure 1:
‘Skeletonized’ anatomic structures after lymph node dissection. (A) Right upper mediastinal and hilar zone. (B) Left upper mediastinal and hilar zone. Vagal N: vagal nerve; Recurrent N: recurrent nerve; Lt. main PA: left main pulmonary artery. (C) Right subcarinal zone. Rt. main Br: right main bronchus; Lt. main Br: left main bronchus. (D) Left subcarinal zone. SVC: superior vena cava; RBCA: right brachiocephalic artery; Vagal N: vagal nerve; Azygos V: azygos vein; Rt. main PA: right main pulmonary artery.
Statistical analysis
We used the Mann–Whitney U-test for continuous covariates, and Fisher’s exact test or the Χ2 test for categorical covariates when comparing the 2 groups. Recurrence-free survival was estimated with the Kaplan–Meier method. Difference between RATS and VATS was estimated with the log-rank test. Data were analysed using SPSS statistical software version 22 (IBM Corp., Armonk, NY, USA) and BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). P < 0.05 (two-sided) was considered significant.
RESULTS
Clinicopathological characteristics
Patient clinicopathological characteristics are shown in Table 1. We included 390 patients (RATS: n = 104; VATS: n = 286) in this study. The 2 groups significantly differed in body mass index (P = 0.022) and respiratory comorbidities (P = 0.003) but not in other clinical or pathological variables.
Table 1:
Patient clinicopathological characteristics
| RATS |
VATS |
P-value | |||
|---|---|---|---|---|---|
| N = 104 (% or range) | N = 286 (% or range) | ||||
| Age (years), median (IQR) | 69.5 (11) | (39–84) | 69 (11) | (33–84) | 0.65 |
| Gender | |||||
| Male | 59 | (57) | 175 | (61) | 0.43 |
| Female | 45 | (43) | 111 | (39) | |
| Smoking status | |||||
| Ever | 59 | (57) | 181 | (63) | 0.24 |
| Never | 45 | (43) | 105 | (37) | |
| BMI, median (IQR) | 23.2 (3.6) | (16.9–35.8) | 22.2 (4.1) | (15.7–33.7) | 0.022 |
| CEA (ng/ml), median (IQR) | 2.7 (3.4) | (0.9–90.1) | 2.9 (3.2) | (0.8–103.1) | 0.73 |
| Cardiovascular comorbidities | |||||
| No | 76 | (73) | 230 | (80) | 0.12 |
| Yes | 28 | (27) | 56 | (20) | |
| Respiratory comorbidities | |||||
| No | 91 | (88) | 210 | (73) | 0.003 |
| Yes | 13 | (13) | 76 | (27) | |
| %VC (%), median (IQR) | 106.3 (20.4) | (66.4–145.1) | 108.1 (22.9) | (71.5–168.6) | 0.36 |
| FEV1.0% (%), median (IQR) | 75.7 (8.6) | (42.0–94.3) | 74.7 (10.6) | (44.9–100.0) | 0.12 |
| C/T ratio, median (IQR) | 1.0 (0.3) | (0.2–1.0) | 1.0 (0.3) | (0–1.0) | 0.55 |
| Tumour laterality | |||||
| Right | 63 | (61) | 177 | (62) | 0.81 |
| Left | 41 | (39) | 109 | (38) | |
| Primary lobe | |||||
| Upper and middle | 67 | (64) | 186 | (65) | 0.91 |
| Lower | 37 | (36) | 100 | (35) | |
| Clinical stage | |||||
| IA | 76 | (73) | 209 | (73) | 0.070 |
| IB | 14 | (13) | 48 | (17) | |
| IIA | 6 | (6) | 9 | (3) | |
| IIB | 3 | (3) | 17 | (6) | |
| IIIA or more | 5 | (5) | 3 | (1) | |
| pT | |||||
| pT1 | 64 | (62) | 181 | (63) | 0.75 |
| pT2 | 33 | (32) | 91 | (32) | |
| pT3 | 5 | (5) | 12 | (4) | |
| pT4 | 2 | (2) | 2 | (1) | |
| pN | |||||
| pN0 | 91 | (88) | 240 | (84) | 0.65 |
| pN1 | 8 | (8) | 26 | (9) | |
| pN2 | 5 | (5) | 20 | (7) | |
| Pathological stage | |||||
| IA | 61 | (59) | 170 | (59) | 0.65 |
| IB | 22 | (21) | 57 | (20) | |
| IIA | 5 | (5) | 7 | (2) | |
| IIB | 6 | (6) | 26 | (9) | |
| IIIA or more | 10 | (10) | 26 | (9) | |
| Histology | |||||
| Adenocarcinoma | 89 | (86) | 234 | (82) | 0.56 |
| Squamous cell carcinoma | 12 | (12) | 37 | (13) | |
| Others | 3 | (3) | 15 | (5) | |
| Pleural invasion | |||||
| Positive | 18 | (17) | 62 | (22) | 0.35 |
| Negative | 86 | (83) | 224 | (78) | |
| Lymphatic invasion | |||||
| Positive | 27 | (26) | 99 | (35) | 0.11 |
| Negative | 77 | (74) | 187 | (65) | |
| Vascular invasion | |||||
| Positive | 27 | (26) | 94 | (33) | 0.19 |
| Negative | 77 | (74) | 192 | (67) | |
BMI: body mass index; CEA: carcinoembryonic antigen; C/T ratio: consolidation–tumour ratio; IQR: interquartile range; FEV: forced expiratory volume; RATS: robot-assisted thoracoscopic surgery; VATS: video-assisted thoracoscopic surgery; VC: vital capacity.
Surgical outcomes
Surgical outcomes are summarized in Table 2. The extent of LND was not different between 2 groups (P = 0.36). Total operative time was significantly longer for RATS than for VATS (P < 0.001). The 2 groups did not significantly differ in blood-loss volume, chest tube duration or prevalence of total postoperative complications, including LND-associated postoperative complications. Twelve patients suffered LND-associated complications (RATS: n = 3, including 2 BPF and 1 chylothorax; VATS: n = 9, including 1 BPF, 2 chylothorax and 6 recurrent nerve paralysis). No hospital death occurred in either group.
Table 2:
Surgical outcomes
| RATS |
VATS |
P-value | |||
|---|---|---|---|---|---|
| N = 104 (% or range) | N = 286 (% or range) | ||||
| Extent of mediastinal LND | |||||
| Systematic LND | 26 | (25) | 59 | (21) | 0.36 |
| Lobe-specific LND | 78 | (75) | 227 | (79) | |
| Total operation time (min), median (IQR) | 236.0 (77) | (133–362) | 189.5 (67) | (83–320) | < 0.001 |
| Robotic console time (min), median (IQR) | 174.0 (56) | (90–275) | – | – | – |
| Bleeding amount (ml), median (IQR) | 7.5 (15) | (5–350) | 10 (25) | (5–620) | 0.65 |
| Duration of chest drain (days), median (IQR) | 2 (0.3) | (1–11) | 2 (0) | (2–21) | 0.46 |
| Total postoperative complications | |||||
| No | 80 | (77) | 228 | (80) | 0.55 |
| Yes | 24 | (23) | 58 | (20) | |
| LND-associated postoperative complications (G2 or more) | |||||
| No | 101 | (97) | 277 | (97) | 0.90 |
| Yes | 3 | (3) | 9 | (3) | |
| Bronchopleural fistula | 2 | (2) | 1 | (0) | |
| Chylothorax | 1 | (1) | 2 | (1) | |
| Recurrent and/or phrenic nerve paralysis | 0 | (0) | 6 | (2) | |
IQR: interquartile range; LND: lymph node dissection; RATS: robot-assisted thoracoscopic surgery; VATS: video-assisted thoracoscopic surgery.
Analysis of dissected LN counts
Figure 2 shows results of dissected LN counts. Numbers of total dissected LNs significantly differed between the 2 groups (P < 0.001; Fig. 2A); and more specifically, in the right upper zone and hilar (#2R + #4R + #10R) LNs (P = 0.002; Fig. 2B), left lower paratracheal and hilar (#4L + #10L) LNs (P = 0.019; Fig. 2C), aortopulmonary zone (#5 + #6) LNs (P = 0.001; Fig. 2D), and interlobar and lobar (#11 + #12) LNs (P = 0.041; Fig. 2G). However, the 2 groups did not significantly differ for the subcarinal zone (#7) LNs (P = 0.21; Fig. 2E) and lower mediastinal zone (#8 + #9) LNs (P = 0.79; Fig. 2F).
Figure 2:
Comparison of median and interquartile range (IQR) of dissected lymph nodes between robot-assisted thoracoscopic surgery (RATS) and video-assisted thoracoscopic surgery (VATS) groups. (A) Total dissected lymph nodes [RATS: 18 (IQR 13), VATS: 15 (IQR 8)]. (B) Right upper zone and hilar (#2R + #4R + #10R) lymph nodes [RATS: 12 (IQR 6.5); VATS: 10 (IQR 7)]. (C) Left lower paratracheal and hilar (#4L + #10L) lymph nodes [RATS: 4 (IQR 4); VATS: 3 (IQR 3)]. (D) Aortopulmonary zone (#5 + #6) lymph nodes [RATS: 3 (interquartile range 2); VATS: 2 (IQR 2)]. (E) Subcarinal zone (#7) lymph nodes [rRATS: 4 (IQR 4); VATS: 4 (IQR 4)]. (F) Lower mediastinal zone (#8 + #9) lymph nodes [RATS: 1 (IQR 2); VATS: 1 (IQR 2)]. (G) Interlobar and lobar (#11 + #12) lymph nodes [RATS: 7 (IQR 4); VATS: 6 (IQR 4)].
Nodal upstaging and incidence of postoperative recurrence
Table 3 shows nodal upstaging, downstaging and postoperative recurrences in both groups. Upstaging rates in the RATS group were clinical (c-) N0 → pathological (p-) N1: 7.7%, cN0 → pN2: 3.8% and cN1 → pN2: 1.0%; and those in the VATS group were cN0 → pN1: 8.0%, cN0 → pN2: 5.9% and cN1 → pN2: 0.3%. Their overall nodal upstaging rates did not significantly differ (RATS: 12.5%, VATS: 14.3%; P = 0.64). The incidence of postoperative recurrence between the groups did significantly differ (P = 0.034), except for ipsilateral hilar and/or mediastinal LNs (RATS: 4.8%, VATS: 5.6%). There were no patients with the recurrence at the bronchial stump or lung staple lines in both groups. The incidence of ipsilateral pleural recurrence was not different between the groups (P = 0.42).
Table 3:
Nodal upstaging and incidence of postoperative recurrences
| RATS |
VATS |
P-value | |||
|---|---|---|---|---|---|
| N = 104 | (%) | N = 286 | (%) | ||
| Nodal upstages | 13 | (12.5) | 41 | (14.3) | 0.64 |
| cN0 to pN1 | 8 | (7.7) | 23 | (8.0) | |
| cN0 to pN2 | 4 | (3.8) | 17 | (5.9) | |
| cN1 to pN2 | 1 | (1.0) | 1 | (0.3) | |
| Nodal downstages | 1 | (1.0) | 3 | (1.0) | |
| No changes | 90 | (86.5) | 242 | (84.6) | |
| Postoperative recurrence | |||||
| No | 96 | (92.3) | 240 | (83.9) | 0.034 |
| Yes | 8 | (7.7) | 46 | (16.1) | |
| Locoregional/locoregional + distant | 5 | (4.8) | 28 | (9.8) | 0.12 |
| Ipsilateral hilar and/or mediastinal lymph nodes | 5 | (4.8) | 16 | (5.6) | 0.76 |
| Bronchial stump or lung staple lines | 0 | (0) | 0 | (0) | — |
| Ipsilateral pleura and/or chest wall | 3 | (2.9) | 15 | (5.2) | 0.42 |
RATS: robot-assisted thoracoscopic surgery; VATS: video-assisted thoracoscopic surgery.
Survival analysis
The median follow-up period for the entire cohort was 34 months (VATS group: 42 months; RATS group: 12 months). The 2-year recurrence-free survival rates were 87.9% in the RATS group and 87.6% in the VATS group, respectively. There was no significant difference in the recurrence-free survival between 2 groups (P = 0.35) (Supplementary Material, Fig. S1).
DISCUSSION
In this study, we retrospectively reviewed the records of lung cancer patients who underwent RATS or VATS lobectomies and mediastinal LNDs and compared their perioperative outcomes and LND-related results, including postoperative complications, dissected LN counts, nodal upstaging and locoregional recurrences between the RATS and VATS groups. We found that significantly more LNs were dissected in the RATS group than in the VATS group. However, we saw no significant differences in LND-associated complications, overall nodal upstaging rate or recurrence rates for ipsilateral hilar and/or mediastinal LNs between the groups.
Worldwide, VATS is the most widely performed minimally invasive approach in lung cancer surgery and, in recent years, has accounted for >70% of surgeries for primary lung cancer in Japan [20]. It has evolved over the decades as various technical, visual and instrumental improvements have been introduced. However, VATS may have a steep learning curve that requires lengthy, comprehensive training because of some drawbacks that include counterintuitive hand movements, instrument fulcrum effect and tremor amplification [21]. Robotic surgery can ameliorate these drawbacks. The articulated robotic forceps joints enable delicate and accurate dissection in any direction without shaking, which may be most effective in hilar and mediastinal LND. Although our previous study showed that 3-year recurrence-free survival rates of lung cancer patients did not significantly differ between RATS and VATS groups after propensity-score matching, the sample size was small and lacked detailed analyses of LND-related parameters [22].
Several large-scale retrospective studies have shown that fewer LNDs could be associated with poor prognosis through inaccurate staging, whereas greater numbers of dissected LNs were associated with more accurate node staging and better long-term survival from resected non-small-cell lung cancer (NSCLC) [23, 24]. As these results are widely accepted among thoracic surgeons, removal of more mediastinal and hilar LNs is considered important in lung cancer surgery. Some studies have compared LN counts and nodal upstaging rates between RATS and other approaches (VATS and/or open thoracotomy) [12, 13, 25–27]. In most of those studies, numbers of dissected LNs and nodal upstaging rates were equivalent for RATS and other approaches; however, few studies analysed the zones or stations of dissected LNs separately among these approaches. Kneuertz et al. [12] analysed patients with clinical stage N0/N1 NSCLC who underwent lobectomies and assessed the effectiveness of intraoperative LN staging by comparing upstaging between robotic, VATS and open thoracotomy after propensity-score matching. They reported no differences in numbers of harvested N1 and/or N2 LNs between robotic, VATS and open groups; although the estimated rate of LN upstaging with robotic lobectomy was lower than with open thoracotomy and higher than with VATS, it did not significantly differ among these approaches. They credited the relative ease of RATS LND to the high-definition robotic camera and the wristed robotic instrumentation with increased freedom of motion and manoeuvrability in the chest. Toker et al. [9] also compared open, VATS and RATS techniques in dissecting hilar and mediastinal LNs during lung cancer surgery and demonstrated RATS to dissect more N1 (#10, #11 and #12) LNs. They speculated that RATS allows accurate, sharp dissection of the vascular sheath around pulmonary vessels and LNs, unlike blunt dissection in VATS; and robotic instruments provide sharp surgical techniques and prevent violation of the LN capsules and may thus increase the numbers of harvested nodes. In our study, more LNs were dissected by RATS than by VATS in hilar regions, and all mediastinal zones except for the subcarinal zone. We share the speculation of Toker et al. because we also think that articulated robotic instruments enable more precise and elaborate handling and dissection in any direction, without shaking around hilar anatomical structures, such as pulmonary major vessels and bronchi, but also around mediastinal structures, such as superior vena cava, azygos vein, trachea and oesophagus; complete removal of all tissues including LNs and surrounding fatty tissue within those anatomical landmarks is possible with RATS [28].
We found no significant differences in surgical outcomes between RATS and VATS, including LND-associated postoperative complications. No previous studies have compared those complications in detail between the approaches. With RATS, resection of more LNs without damaging the bronchial arteries or the thoracic duct or large lymphatic vessels may be feasible because of the remarkable 3D visual field and high-quality instrument manoeuvrability. Regarding nerve complications, no recurrent and/or phrenic nerve paralysis was observed in the RATS group during the period of this study, which suggested that RATS may provide accuracy and meticulousness around those nerves. We believe that high-quality RATS procedures will enable dissection of more LNs without increasing the incidence of postoperative complications. However, further case accumulation and large-scale analysis are needed to verify these conclusions. We also analysed the incidences of postoperative recurrences in both groups and clarified recurrence patterns, especially locoregional recurrence rates. Although recurrence rates for ipsilateral hilar and/or mediastinal LNs did not significantly vary between the groups, the RATS group had more recent cases and shorter observational periods than the VATS group. Reviewing the patterns of postoperative recurrence is important, especially locoregional recurrence after minimally invasive surgery, to evaluate oncological efficacy and surgical quality [16]. Whether dissecting more LNs is directly linked to local cancer control should be evaluated with a longer observational period.
Limitations
This study had several limitations. First, this is a retrospective, single-institution study so the sample size was small and its statistical power might be insufficient for definitive conclusions. Second, surgeon bias was evident. In this study, 7 surgeons were involved in the RATS group and 13 surgeons were in the VATS group, and the RATS and VATS surgeons were not entirely the same people. Third, selection bias was undoubtedly present, as only ‘simple cases’ were selected for the RATS group and ‘complex cases’ were avoided during the initial RATS period. Fourth, the observational period was shorter in the RATS group than in the VATS group because the use of RATS increased during the later period of this study. The analyses of postoperative recurrence might be unreliable due to this bias. Fifth, there were some learning curve effects in this study because RATS cases were done later than most of the VATS cases. Finally, the number of removed LNs depended on both the surgeon and the pathologist and is more challenging, as nodes may be removed in pieces or fragments. Removed LN blocks were subdivided by the surgeon in the operating room and submitted for pathological examination. The counting of LN numbers was completely dependent on the pathologists, and it was thought to be difficult to perfectly distinguish between LNs and LN fragments in some cases.
CONCLUSION
In conclusion, our results suggest that the RATS approach has some possibility to be superior to VATS in terms of numbers of dissected LNs in hilar and mediastinal regions, except for the subcarinal zone. These approaches also do not differ in overall nodal upstaging and their incidences of LND-associated complications. Accumulation of more RATS cases and discussion of long-term outcomes are important, especially with regard to locoregional recurrence, as the essential significance of surgery as ‘local control of cancer’ cannot be overlooked.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
ACKNOWLEDGEMENT
We thank Marla Brunker, from Edanz Group (https://en-author-services.edanzgroup.com/ac), for editing a draft of this manuscript.
Author contributions
Tomohiro Haruki: Conceptualization; Data curation; Methodology; Writing—original draft. Yuzo Takagi: Data curation; Writing—review & editing. Yasuaki Kubouchi: Data curation; Writing—review & editing. Yoshiteru Kidokoro: Data curation; Writing—review & editing. Atsuyuki Nakanishi: Data curation; Writing—review & editing. Yuji Nozaka: Data curation; Writing—review & editing. Yuki Oshima: Data curation; Writing—review & editing. Shinji Matsui: Data curation; Writing—review & editing. Hiroshige Nakamura: Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Biruta Witte, Marcin Zielinski, Andrea Zuin and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- CT
Computed tomography
- DVSS
da Vinci Surgical System
- LNs
Lymph nodes
- LND
Lymph node dissection
- RATS
Robot-assisted thoracoscopic surgery
- VATS
Video-assisted thoracoscopic surgery
REFERENCES
- 1. Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI. et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Izbicki JR, Passlick B, Pantel K, Pichlmeier U, Hosch SB, Karg O. et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu N, Yan S, Lv C, Feng Y, Wang Y, Zhang L. et al. Comparison of systematic mediastinal lymph node dissection versus systematic sampling for lung cancer staging and completeness of surgery. J Surg Res 2011;171:e169-73–e173. [DOI] [PubMed] [Google Scholar]
- 4. Sugi K, Nawata K, Fujita N, Ueda K, Tanaka T, Matsuoka T. et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290–4. [DOI] [PubMed] [Google Scholar]
- 5. Meng D, Zhou Z, Wang Y, Wang L, Lv W, Hu J.. Lymphadenectomy for clinical early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:597–604. [DOI] [PubMed] [Google Scholar]
- 6. Keller SM, Adak S, Wagner H, Johnson DH.. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358–65. [DOI] [PubMed] [Google Scholar]
- 7. Palade E, Passlick B, Osei-Agyemang T, Günter J, Wiesemann S.. Video-assisted vs open mediastinal lymphadenectomy for Stage I non-small-cell lung cancer: results of a prospective randomized trial. Eur J Cardiothorac Surg 2013;44:244–9. [DOI] [PubMed] [Google Scholar]
- 8. Zhang W, Wei Y, Jiang H, Xu J, Yu D.. Video-assisted thoracoscopic surgery versus thoracotomy lymph node dissection in clinical stage I lung cancer: a meta-analysis and system review. Ann Thorac Surg 2016;101:2417–24. [DOI] [PubMed] [Google Scholar]
- 9. Toker A, Ozyurtkan MO, Demirhan O, Ayalp K, Kaba E, Uyumaz E.. Lymph node dissection in surgery for lung cancer: comparison of open vs. video-assisted vs. robotic-assisted approaches. ATCS 2016;22:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toosi K, Velez-Cubian FO, Glover J, Ng EP, Moodie CC, Garrett JR. et al. Upstaging and survival after robotic-assisted thoracoscopic lobectomy for non-small cell lung cancer. Surgery 2016;160:1211–8. [DOI] [PubMed] [Google Scholar]
- 11. Velez-Cubian FO, Rodriguez KL, Thau MR, Moodie CC, Garrett JR, Fontaine JP. et al. Efficacy of lymph node dissection during robotic-assisted lobectomy for non-small cell lung cancer: retrospective review of 159 consecutive cases. J Thorac Dis 2016;8:2454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kneuertz PJ, Cheufou DH, D'Souza DM, Mardanzai K, Abdel-Rasoul M, Theegarten D. et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1457–66. [DOI] [PubMed] [Google Scholar]
- 13. Hennon MW, DeGraaff LH, Groman A, Demmy TL, Yendamuri S.. The association of nodal upstaging with surgical approach and its impact on long-term survival after resection of non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;57:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haruki T, Aokage K, Miyoshi T, Hishida T, Ishii G, Yoshida J. et al. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: possibility of rational lymph node dissection. J Thorac Oncol 2015;10:930–6. [DOI] [PubMed] [Google Scholar]
- 15. Dindo D, Demartines N, Clavien PA.. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haruki T, Miwa K, Araki K, Taniguchi Y, Nakamura H.. Distribution and prevalence of locoregional recurrence after video-assisted thoracoscopic surgery for primary lung cancer. Thorac Cardiovasc Surg 2016;64:526–32. [DOI] [PubMed] [Google Scholar]
- 17. Taniguchi Y, Nakamura H, Miwa K, Haruki T, Araki K, Takagi Y. et al. Initial results of robotic surgery for primary lung cancer: feasibility, safety and learning curve. Yonago Acta Med 2017;60:162–6. [PMC free article] [PubMed] [Google Scholar]
- 18. Hishida T, Miyaoka E, Yokoi K, Tsuboi M, Asamura H, Kiura K. et al. ; Japanese Joint Committee of Lung Cancer Registry. Lobe-specific nodal dissection for clinical stage I and II NSCLC: Japanese multi-institutional retrospective study using a propensity score analysis. J Thorac Oncol 2016;11:1529–37. [DOI] [PubMed] [Google Scholar]
- 19. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P; Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568–77. [DOI] [PubMed] [Google Scholar]
- 20. Shimizu H, Okada M, Tangoku A, Doki Y, Endo S, Fukuda H. et al. ; Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Thoracic and cardiovascular surgeries in Japan during 2017: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2020;68:414–49. [DOI] [PubMed] [Google Scholar]
- 21. Huang L, Shen Y, Onaitis M.. Comparative study of anatomic lung resection by robotic vs. video-assisted thoracoscopic surgery. J Thorac Dis 2019;11:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haruki T, Kubouchi Y, Takagi Y, Kidokoro Y, Matsui S, Nakanishi A. et al. Comparison of medium-term survival outcomes between robot-assisted thoracoscopic surgery and video-assisted thoracoscopic surgery in treating primary lung cancer. Gen Thorac Cardiovasc Surg 2020;68:984–92. [DOI] [PubMed] [Google Scholar]
- 23. Samayoa AX, Pezzi TA, Pezzi CM, Greer Gay E, Asai M, Kulkarni N. et al. Rationale for a minimum number of lymph nodes removed with non-small cell lung cancer resection: correlating the number of nodes removed with survival in 98,970 patients. Ann Surg Oncol 2016;23:1005–11. [DOI] [PubMed] [Google Scholar]
- 24. Liang W, He J, Shen Y, Shen J, He Q, Zhang J. et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a Chinese Multi-Institutional Registry. JCO 2017;35:1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee BE, Shapiro M, Rutledge JR, Korst RJ.. Nodal upstaging in robotic and video assisted thoracic surgery lobectomy for clinical n0 lung cancer. Ann Thorac Surg 2015;100:229–33. [DOI] [PubMed] [Google Scholar]
- 26. Zirafa C, Aprile V, Ricciardi S, Romano G, Davini F, Cavaliere I. et al. Nodal upstaging evaluation in NSCLC patients treated by robotic lobectomy. Surg Endosc 2019;33:153–8. [DOI] [PubMed] [Google Scholar]
- 27. Tang A, Raja S, Bribriesco A, Raymond D, Sudarshan M, Murthy SC. et al. Robotic approach offers similar nodal upstaging to open lobectomy for clinical stage I NSCLC. Ann Thorac Surg 2020;110:424–33. [DOI] [PubMed] [Google Scholar]
- 28. Watanabe S. Lymph node dissection for lung cancer: past, present, and future. Gen Thorac Cardiovasc Surg 2014;62:407–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.