Abstract
OBJECTIVES
This study aims to improve early detection of cardiac surgery-associated acute kidney injury (CSA-AKI) compared to classical clinical scores.
METHODS
Data from 7633 patients who underwent cardiac surgery between 2008 and 2018 in our institution were analysed. CSA-AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria. Cleveland Clinical Score served as the reference with an area under the curve (AUC) 0.65 in our cohort. Based on that, stepwise logistic regression modelling was performed on the training data set including creatinine (Cr), estimated glomerular filtration rate (eGFR) levels and deltas (ΔCr, ΔeGFR) at different time points and clinical parameters as preoperative haemoglobin, intraoperative packed red blood cells (units) and cardiopulmonary bypass time (min) to predict CSA-AKI in the early postoperative course. The AUC was determined on the validation data set for each model respectively.
RESULTS
Incidence of CSA-AKI in the early postoperative course was 22.4% (n = 1712). The 30-day mortality was 12.5% in the CSA-AKI group (n = 214) and in the no-CSA-AKI group 0.9% (n = 53) (P < 0.001). Logistic regression models based on Cr and its delta gained an AUC of 0.69; ‘Model eGFRCKD-EPI’ an AUC of 0.73. Finally, ‘Model DynaLab’ including dynamic laboratory parameters and clinical parameters as haemoglobin, packed red blood cells and cardiopulmonary bypass time improved AUC to 0.84.
CONCLUSIONS
Model DynaLab’ improves early detection of CSA-AKI within 12 h after surgery. This simple Cr-based framework poses a fundament for further endeavours towards reduction of CSA-AKI incidence and severity.
Keywords: Kidney injury, Renal function, Dialysis, Complications
INTRODUCTION
Cardiac surgery-associated acute kidney injury (CSA-AKI) occurs in up to 30% of the patients and remains a challenge, posing significant morbidity and mortality as well as enormous costs for the healthcare system [1–3]. Patients with preoperatively impaired renal function are at highest risk, but also patients with normal renal function may experience CSA-AKI [4]. Reasons are manifold ranging from patient-specific characteristics to procedure complexity along with various perioperative factors [1, 4, 5]. In the past, efforts have been made to develop scoring systems to predict the risk of CSA-AKI associated renal replacement therapy (RRT). The Cleveland Clinical Score (CCS) [6], the Simplified Renal Index for RRT [7] and the Mehta Score also predicting CSA-AKI [8] are among the most frequently used and validated.
Underlining the importance of clinical factors, these scores inherently lack incorporation of parameters mirroring dynamic changes of kidney function. Thus, the aim of the present study was to develop a new tool for enhanced early postoperative CSA-AKI detection.
METHODS
Study population
In total, 8173 consecutive patients who underwent cardiac surgery with cardiopulmonary bypass (CPB) at the Department of Cardiac Surgery at the Klinikum Nürnberg—Paracelsus Medical University (Nuremberg, Germany) were evaluated between April 2008 and January 2018. Patients with indication for heart transplantation, left ventricular assist device implantation or transcatheter aortic valve procedure were not included in the cohort. Pseudo anonymized data collection was done retrospectively via the clinical information system. From the initial 8173 patients, 540 were excluded to avoid potential biasing preconditions for CSA-AKI:
RRT preoperatively (n = 340)
impaired renal function defined as estimated Glomerular Filtration Rate (eGFR) < 30 ml in accordance with the Chronic Kidney Disease Epidemiology Collaboration Estimated Glomerular Filtration Rate [9] (eGFRCKD-EPI) (n = 144)
without the preoperative creatinine (Cr) value (n = 23)
without any postoperative value of Cr because they died during or shortly after the operation (n = 15)
after kidney transplantation (n = 10)
without information on preoperative RRT (n = 6)
dialysis intraoperatively (n = 2)
Totally 7633 patients were finally applicable for analyses.
Ethical statement
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The Institutional Review Board of the Paracelsus Medical University Nuremberg approved the study (IRB-2020–013) on 23 April 2020. Due to the retrospective character of the study using pre-existing, de-identified data, informed consent was waived.
Creatinine-based parameters
Plasma Cr was measured with a kinetic Jaffe method using the clinical chemistry systems from the AU-series (Beckman Coulter, Krefeld; Germany) or from the cobas c-Series (Roche Diagnostics, Mannheim, Germany).
Preoperative Cr and estimated glomerular filtration rate (eGFR) were taken within 3 days before surgery. Blood sampling was performed immediately at intensive care unit (ICU) arrival. Due to individual decisions of the attending clinicians and after careful revision of the data, we found at least one value for all patients within the time period of 6–12 h. In addition to absolute preoperative and early postoperative Cr and eGFR values, their respective changes (ΔCr and ΔeGFR) were calculated as:
ΔXpostop = (Xpostop—Xpreop)/Xpreop.
In case of multiple samples in given timeframe, the higher value was taken. All values were evaluated with further clinical variables for the detection of outcomes. Regarding eGFR-calculation in Black-African or Afro-American people, we found only 34 patients with this etymological characteristics representing 0.45% of the entire cohort, without any influence on the statistical outcome.
Cardiac surgery-associated acute kidney injury definition
CSA-AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria [10] as an increase in Cr by ≥0.3 mg/dl or by ≥26.5 μmol/l from baseline within 48 h after cardiac surgery or increase in Cr to ≥1.5 times baseline within 7 days after cardiac surgery labelled AKI stage 1. AKI stage 2 was defined with an increase of Cr by 2.0–2.9 times baseline. AKI stage 3 was defined with an increase of Cr by ≥3.0 times baseline or with Cr of ≥353.6 μmol/l or with the initiation of RRT, excluding urine output criteria, respectively. CSA-AKI was further divided into transient, for those who recovered within 48 h and persistent, similar to the definition by Basu et al. [11].
According to recent findings, no consensus exists on the definition of CSA-AKI in the current literature with over 35 different definitions of CSA-AKI [8, 12]. However, most researchers have used the Acute Kidney Injury Network (AKIN) and/or the Risk, Injury, Failure, Loss, End Stage Kidney Disease (RIFLE) criteria to define AKI in the first 48 h after surgery. The application of AKIN and RIFLE criteria might lead to underdiagnosing of CSA-AKI. This is why ‘The Kidney Disease: Improving Global Outcomes’ (KDIGO) criteria exhibiting greater sensitivity to detect AKI with better prediction of associated in hospital mortality have been used in newer studies to better depict the underlying multifactorial processes in background [10]. These criteria, which essentially merge the RIFLE and AKIN criteria, have become the new consensus definition of AKI and can be used to diagnose CSA-AKI invariably including the whole period of 7 days after the surgery.
CCS established by Thakar et al. [6] to estimate the risk of dialysis after cardiac surgery and revalidated also for the risk of severe CSA-AKI was applied as basic clinical score [12–14].
Statistical analysis
The descriptive statistics of preoperative, intraoperative and postoperative data were performed to compare patients with and without CSA-AKI. Categorical variables were summarized as frequencies and percentages, while scalar variables were summarized as median with ranges from first to third quartiles, since the data were not normally distributed. The normality of data was tested with the Shapiro–Wilk test. Fisher’s exact test (in the case of categorical variables with frequencies below 5) or Chi square tests were used to compare patient characteristics, risk status and outcomes between CSA-AKI and non-CSA-AKI groups in the case of categorical variables, while Mann–Whitney U-tests were applied for scalar variables. Repeated-measures analysis of variance was performed to assess the differences in Cr and eGFR. The statistical results and modelling were reported on binary response of the AKI variable, i.e. AKI versus non-AKI. Additionally we divided it into 3 subtypes (stage 1–3) to draw attention to even mild kidney injury.
To evaluate Cr and ΔCr and eGFR and ΔeGFR for early detection of CSA-AKI, the multivariable logistic regression modelling was conducted. All logistic regression models were built by following a stepwise-forward approach with the Akaike Information Criteria for optimal model selection [15]. The models were estimated on the training set and validated on the validation set. Data sets were constructed by randomly divided patients in the ratio 0.8:0.2 between training data set, which included 6109 patients, and validation data sets, which included 1524 patients.
The logistic regression results were reported as odds with 95% confidence intervals, obtained on the training data set, while the receiver operating characteristic analyses were made on the validation data set, where the area under the receiver operating characteristic curve [area under the curve (AUC)], specificity and sensitivity at Youden cut-off points were computed for each model respectively.
All statistical analyses were performed by using R (https://www.R-project.org/, version 3.5.3, The R Foundation for Statistical Computing, Vienna, Austria) and a P-value of <0.05 was considered statistically significant.
RESULTS
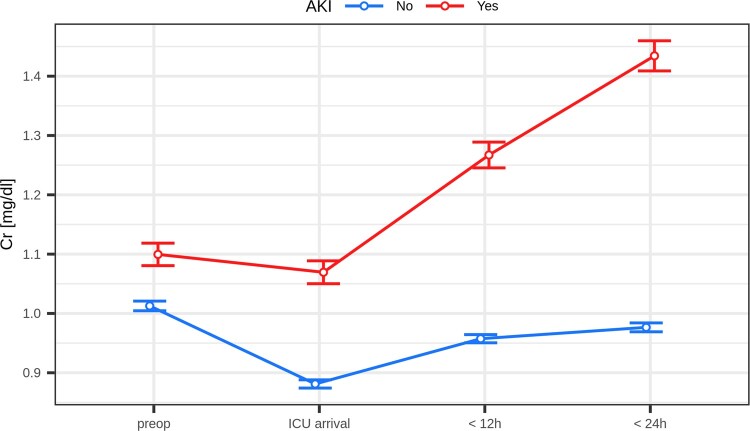
In the final study sample, 7633 patients were analysed. Demographics and preoperative characteristics are depicted in Table 1. Perioperative characteristics are shown in Table 2. The time course of Cr-Levels and eGFR is shown in Figs 1 and 2.
Table 1:
Demographics and preoperative clinical status
| Characteristic | Missing data | No AKI (n = 5921) | AKI (n = 1712) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (year) | 0 | 69.0 [61.0–75.0] | 73.0 [65.0–77.0] | <0.001 |
| Female sex | 0 | 1559 (26.3%) | 500 (29.2%) | 0,02 |
| BMI | 0 | 27.4 [24.9–30.4] | 27.4 [24.8–31.0] | 0,55 |
| Preoperative information | ||||
| Medical history | ||||
| Hypertension | 11 | 5446 (92.0%) | 1577 (92.5%) | 0.575 |
| Hyperlipidaemia | 20 | 4924 (83.2%) | 1332 (78.5%) | <0.001 |
| NIDDM | 11 | 1773 (30.0%) | 601 (35.2%) | <0.001 |
| IDDM | 11 | 525 (8.87%) | 221 (13.0%) | <0.001 |
| Current or ex-smoker | 28 | 3107 (52.6%) | 833 (49.0%) | 0.009 |
| COPD | 4 | 764 (12.9%) | 304 (17.8%) | <0.001 |
| Compensated kidney disease | 0 | 610 (10.3%) | 384 (22.4%) | <0.001 |
| Preoperative infection | 6 | 254 (4.29%) | 199 (11.6%) | <0.001 |
| Infectious endocarditis | 6 | 162 (2.74%) | 148 (8.66%) | <0.001 |
| Catheter to surgery ≤2 days | 284 | 442 (7.67%) | 177 (11.2%) | <0.001 |
| Preoperative laboratory values | ||||
| Haematocrit [%] | 16 | 40.0 [38.0–43.0] | 39.0 [36.0–42.0] | <0.001 |
| Haemoglobin [g/dl] | 7 | 13.8 [12.7–14.8] | 13.1 [11.6–14.3] | <0.001 |
| Cr (last value before operation) [mg/dl] | 0 | 0.95 [0.83–1.11] | 1.01 [0.85–1.21] | <0.001 |
| eGFR [ml/min/1.73 m²] | 0 | 77.2 [62.8–88.5] | 69.0 [54.6–84.6] | <0.001 |
| ≥60 ml/min/1.73 m² | 0 | 4707 (79.5%) | 1118 (65.3%) | <0.001 |
| <60 ml/min/1.73 m² | 0 | 1214 (20.5%) | 594 (34.7%) | <0.001 |
| Preoperative cardiac status | ||||
| NYHA class III+IV | 1 | 3277 (55.3%) | 1117 (65.3%) | <0.001 |
| Atrial fibrillation | 2 | 625 (10.6%) | 381 (22.3%) | <0.001 |
| Ejection fraction [%] | 13 | 60.0 [50.0–65.0] | 60.0 [47.0–65.0] | <0.001 |
| Myocardial infarction within last 3 weeks | 1 | 970 (16.4%) | 340 (19.9%) | 0.001 |
| Cardiogenic shock within last 48 h—acute cardiogenic shock | 1 | 97 (1.64%) | 103 (6.02%) | <0.001 |
| IABP preoperatively | 0 | 96 (1.62%) | 77 (4.50%) | <0.001 |
| Previous cardiac surgery ≥1 | 0 | 317 (5.35%) | 142 (8.29%) | <0.001 |
| Preoperative risk scores | ||||
| Euroscore | 2 | 4.43 [2.27–8.96] | 8.83 [4.45–17.7] | <0.001 |
| Cleveland Clinical Score | 51 | 2.00 [1.00–3.00] | 3.00 [2.00–4.00] | <0.001 |
| 0–2 | 51 | 3765 (63.9%) | 702 (41.6%) | <0.001 |
| 3–5 | 51 | 1974 (33.5%) | 833 (49.3%) | <0.001 |
| 6–8 | 51 | 149 (2.53%) | 143 (8.47%) | <0.001 |
| >9 | 51 | 5 (0.08%) | 11 (0.65%) | <0.001 |
Values are presented as n (%) for categorical data or as median with first and third quartiles for scalar values. P-value for comparison of outcomes between groups. For Clevland Clinic Score a point system was utilized: Female gender: 1; Congestive heart failure: 1; Left ventricular ejection fraction <35%: 1; Preoperative use of IABP: 2; COPD: 1; Insulin-dependent diabetes mellitus: 1; Previous cardiac surgery: 1; Emergency surgery: 2; Surgery type: CABG: 0, Valve only: 1, CABG + Valve: 2, Other cardiac surgeries: 2; Preoperative Cr: 1.2 to <2.1 mg/dl: 2, ≥2.1 mg/dl: 5 REF THAKAR + KIES.
AKI: acute kidney injury; BMI: body mass index; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; COPD: chronic obstructive pulmonary disease ; Cr: creatinine; eGFR: estimated glomerular filtration rate; IABP: intra-aortic balloon pump; IDDM: insulin-dependent diabetes mellitus; NIDDM: non-insulin-dependent diabetes mellitus; NYHA: New York Heart Association; pRBC: packed red blood cells; RRT: renal replacement therapy.
Table 2:
Perioperative course
| Characteristic | Missing data | No AKI (n = 5921) | AKI (n = 1712) | P-value |
|---|---|---|---|---|
| Intraoperative information | ||||
| Type of surgery: CABG | 0 | 3683 (62.2%) | 780 (45.6%) | <0.001 |
| Valve | 0 | 1412 (23.8%) | 499 (29.1%) | <0.001 |
| CABG + Valve | 0 | 629 (10.6%) | 306 (17.9%) | <0.001 |
| Valved conduit aorta ascendens | 0 | 197 (3.33%) | 127 (7.42%) | <0.001 |
| Priority: elective | 24 | 4252 (72.0%) | 1121 (65.7%) | <0.001 |
| Urgent | 24 | 1439 (24.4%) | 408 (23.9%) | 0.719 |
| Emergent | 24 | 212 (3.59%) | 177 (10.4%) | <0.001 |
| CPB time [min] | 58 | 80.0 [62.0–101] | 96.0 [72.0–132] | <0.001 |
| Cross clamp time [min] | 71 | 47.0 [36.0–64.0] | 57.0 [42.0–84.0] | <0.001 |
| Deepest temperature [°C] | 69 | 34.5 [34.0–35.0] | 34.2 [33.5–34.8] | <0.001 |
| Circulatory arrest | 0 | 15 (0.25%) | 29 (1.69%) | <0.001 |
| pRBC intraoperatively (number of units) | 0 | 0.00 [0.00–0.00] | 0.00 [0.00–2.00] | <0.001 |
| Postoperative information | ||||
| IABP postoperatively | 0 | 131 (2.21%) | 131 (7.65%) | <0.001 |
| Revision | 14 | 221 (3.74%) | 253 (14.8%) | <0.001 |
| Postoperative laboratory values | ||||
| Cr ICU arrival [mg/dl] | 36 | 0.84 [0.72–0.98] | 0.98 [0.81–1.21] | <0.001 |
| Cr <12 h postop [mg/dl] | 21 | 0.91 [0.79–1.06] | 1.17 [0.96–1.43] | <0.001 |
| Cr <24 h postop | 28 | 0.93 [0.79–1.09] | 1.32 [1.06–1.62] | <0.001 |
| First ΔCr preop/ICU arrival [%] | 36 | −12.50 [−20.34–4.24] | −4.29 [−14.29–8.75] | <0.001 |
| Second ΔCr preop/12 h [%] | 21 | −4.53 [−12.50–4.35] | 12.9 [0.00–31.0] | <0.001 |
| eGFR ICU arrival [ml/min/1.73 m²] | 36 | 87.0 [74.6–95.1] | 71.7 [57.1–85.4] | <0.001 |
| eGFR <12 h postop [ml/min/1.73 m²] | 21 | 80.7 [67.2–90.7] | 58.3 [45.6–72.8] | <0.001 |
| eGFR <24 h [ml/min/1.73 m²] | 28 | 79.2 [64.2–91.3] | 50.3 [38.3–64.2] | <0.001 |
| First ΔeGFR preop/ICU arrival [%] | 36 | 10.2 [2.76–22.3] | 3.88 [−7.06–16.2] | <0.001 |
| Second ΔeGFR preop/6–12 h [%] | 21 | 3.53 [−3.44–13.0] | −12.14 [−25.98–0.00] | <0.001 |
| Outcomes | ||||
| Last Cr before discharge from clinic [mg/dl] | 0 | 0.95 [0.82–1.12] | 1.18 [0.93–1.57] | <0.001 |
| Last eGFR before discharge [ml/min/1.73 m²] | 0 | 76.8 [62.0–89.8] | 57.2 [40.0–77.2] | <0.001 |
| Last eGFR before discharge ≥60 ml/min/1.73 m² N/Y | 0 | 4604 (77.8%) | 793 (46.3%) | <0.001 |
| <60 ml/min/1.73 m² | 0 | 1317 (22.2%) | 919 (53.7%) | <0.001 |
| <15 ml/min/1.73 m² | 0 | 2 (0.03%) | 41 (2.39%) | <0.001 |
| RRT postop | 9 | 37 (0.63%) | 355 (20.8%) | <0.001 |
| Any postoperative infection | 1 | 751 (12.7%) | 627 (36.6%) | <0.001 |
| 30-day mortality | 0 | 53 (0.90%) | 214 (12.5%) | <0.001 |
Values are presented as n (%) for categorical data or as median with first and third quartiles for scalar values. P-value for comparison of outcomes between groups. For Clevland Clinic Score a point system was utilized: Female gender: 1; Congestive heart failure: 1; Left ventricular ejection fraction <35%: 1; Preoperative use of IABP: 2; COPD: 1; Insulin-dependent diabetes mellitus: 1; Previous cardiac surgery: 1; Emergency surgery: 2; Surgery type: CABG: 0, Valve only: 1, CABG + Valve: 2, Other cardiac surgeries: 2; Preoperative Cr: 1.2 to <2.1 mg/dl: 2, ≥2.1 mg/dl: [6, 14].
AKI: acute kidney injury; BMI: body mass index; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass; eGFR: estimated glomerular filtration rate; IABP: intra-aortic balloon pump; ICU: intensive care unit; IDDM: insulin-dependent diabetes mellitus; NIDDM: non-insulin-dependent diabetes mellitus; NYHA: New York Heart Association; RRT: renal replacement therapy; Cr: creatinine; pRBC: packed red blood cells.
Figure 1:
Creatinine over time: mean value (error bars represent 95% CI of mean value); P < 0.001 at any time point. AKI: acute kidney injury; Cr: creatinine; ICU: intensive care unit.
Figure 2:
Estimated glomerular filtration rate over time: mean value (error bars represent 95% CI of mean value); P < 0.001 at any time point. AKI: acute kidney injury; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; eGFR: estimated glomerular filtration rate; ICU: intensive care unit.
Overall 30-day mortality was 3.5% (267). EuroSCORE estimated mortality was 4.43% [2.27–8.96] for no-AKI and 8.8% [4.45–17] for CSA-AKI. Overall Incidence of CSA-AKI was 22.4% (n = 1712). The 30-day mortality in no-CSA-AKI patients was 0.9% (n = 53) and in CSA-AKI patients, 12.5% (n = 214) (P < 0.001). Occurrence and severity of CSA-AKI are shown for ICU arrival, 24 and 48 h compared to the third to seventh day as well as newly diagnosed CSA-AKI patients in Fig. 3. Over time, stage 1 patients progressed to higher stages of AKI in 33.4%, persisted in the same stage in 37.8% and transient regarding to Basu et al. [11] in 28.8%.
Figure 3:
Occurrence and severity of cardiac surgery-associated acute kidney injury; dark line represents absolute number of patients with first diagnosis of cardiac surgery-associated acute kidney injury. AKI: acute kidney injury; CSA-AKI: cardiac surgery-associated acute kidney injury; ICU: intensive care unit.
Modelling for cardiac surgery-associated acute kidney injury prediction
Models are shown in Table 3. First using the CCS, ‘Model CCS’ served as reference point with an AUC 0.66 (95% CI 0.62–0.69).
Table 3:
All models for cardiac surgery-associated acute kidney injury-detection
| Model | Variables in the model (units or classification) | AUC [95% CI] | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| ‘Model CCS’ | CCS | Train Set (n = 6109) | 0.658 [0.642, 0.674] | 0.803 | 0.425 |
| Test Set (n = 1524) | 0.657 [0.625, 0.690] | 0.580 | 0.628 | ||
| ‘Model eGFRCKD-EPI’ | eGFR preop (ml/min/1.73 m²) + eGFR ICU arrival + ΔeGFR preop/ICU arrival (%) | Train Set (n = 6109) | 0.735 [0.720, 0.751] | 0.617 | 0.733 |
| Validation Set (n = 1524) | 0.727 [0.694, 0.760] | 0.552 | 0.798 | ||
| ‘Model DynaLab’ | eGFR preop (ml/min/1.73 m²) + eGFR ICU arrival + ΔeGFR preop/ICU arrival (%) + eGFR 12 h + ΔeGFR preop/12 h (%) + haemoglobin + pRBC intraop (units) + CPB time (min) | Train Set (n = 6109) | 0.844 [0.832, 0.857] | 0.730 | 0.805 |
| Test Set (n = 1524) | 0.836 [0.807, 0.865] | 0.735 | 0.816 |
The results are presented in terms of AUC with 95% CI for training and validation sets and with optimal sensitivity and specificity values at Youden cut-off points.
For Cleveland Clinical Score a point system was utilized: Female gender: 1; Congestive heart failure: 1; Left ventricular ejection fraction <35%: 1; Preoperative use of IABP: 2; COPD: 1; Insulin-dependent diabetes mellitus: 1; Previous cardiac surgery: 1; Emergency surgery: 2; Surgery type: CABG: 0, Valve only: 1, CABG + Valve: 2, Other cardiac surgeries: 2; Preoperative Cr: 1.2 to <2.1 mg/dl: 2, ≥2.1 mg/dl: 5 [6, 14].
AKI: acute kidney injury; AUC: area under the curve; CABG: coronary artery bypass grafting; CCS: Cleveland Clinical Score; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CPB: cardiopulmonary bypass; eGFR: estimated glomerular filtration rate; Cr: creatinine; ICU: intensive care unit; pRBC: packed red blood cells.
In the second step, we took pre- and postoperative Cr and eGFR values at ICU arrival similar to Karkouti et al. [1] and Ho et al. [16], and the corresponding deltas. ‘Model eGFRCKD-EPI’ was found to be more precise in predicting CSA-AKI with an AUC of 0.73 (95% CI 0.69–0.76) than a model based on Cr and its delta alone with an AUC of 0.70 (95% CI 0.67–0.74).
In the final model, ‘DynaLab’, the simplest modifiable factors from our demographic findings as preoperative haemoglobin, intraoperative packed red blood cells (units) and cardiopulmonary bypass time (min) were combined with dynamic laboratory findings based on eGRF within 12 h after surgery. Early CSA-AKI detection improved AUC to 0.84 (95% CI 0.81–0.86). Detailed logistic regression is shown in Table 4.
Table 4:
Logistic regression ‘Model DynaLab’ for detection of cardiac surgery-associated acute kidney injury
| Logistic regression for ‘Model DynaLab’ | Odds ratio | 95% CI [,] | P-value |
|---|---|---|---|
| eGFRCKD-EPI preop [ml/min/1.73 m²] | 1.088 | [1.058, 1.119] | <0.001 |
| eGFRCKD-EPI ICU arrival [ml/min/1.73 m²] | 0.973 | [0.938, 1.009] | 0.135 |
| eGFRCKD-EPI Δpreop/ICU arrival [ml/min/1.73 m²] | 1.029 | [1.005, 1.053] | 0.018 |
| Hb preop [g/dl] | 0.869 | [0.827, 0.913] | <0.001 |
| eGFRCKD-EPI 12 h postop [ml/min/1.73 m²] | 0.911 | [0.876, 0.947] | <0.001 |
| eGFRCKD-EPI Δpreop/12 h postop [ml/min/1.73 m²] | 0.969 | [0.943, 0.995] | 0.022 |
| pRBC intraop [number of units] | 1.188 | [1.107, 1.278] | <0.001 |
| CPB time [min] | 1.001 | 1.000, 1.003] | 0.064 |
CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CPB: cardiopulmonary bypass; eGFR: estimated glomerular filtration rate; Hb: haemoglobin; ICU: intensive care unit; pRBC: packed red blood cells.
DISCUSSION
In our study, we found that more than one-fifth of patients developed any form of CSA-AKI. Of those, 35.0% progressed and 37.1% maintained the assumed stage of CSA-AKI in the first 48 h after surgery.
The discrepancy between expected mortality (EuroSCORE) and observed mortality among patients with CSA-AKI was wide, but still within the CI: 8.83 [4.45–17.7]. Nevertheless, the observed notion just underlines the fact that any, even milder form of CSA-AKI contributes to worse outcomes that are not accounted in the current risk models.
Validation of CCS for RRT and severe AKI compared well to the reported values by Thakar and Englberger [6, 17]. However, identically to Birnie et al. [2], CCS alone performed poorly in discrimination of patients who developed CSA-AKI of any grade. In contrast, preoperative kidney function combined with single Cr value at arrival to ICU predicted CSA-AKI in the modest-to-good range. Finally, incorporating dynamic Cr and eGFR changes within 12 h after surgery allowed for good-to-excellent CSA-AKI detection before the stand-alone rise of Cr-values is obvious.
The older studies anticipated that the absolute increase of Cr, used as standard for the diagnosis of AKI, has a delay of 24–48 h [18]. This delivers late information on the extent of kidney injury, especially under the circumstances of perioperative haemodilution [19, 20]. Recent studies increasingly show that detection of postoperative even subclinical Cr changes could enhance early CSA-AKI diagnosis [16, 21]. Karkouti et al. [1] used the threshold of ΔCr postoperative to preoperative ratio of 1.3 to identify postoperative AKI. Further, Ho et al. [16] demonstrated that a 10% early postoperative Cr increase could significantly predict a higher AKI risk compared to a 10% decrease of Cr predicting a lower CSA-AKI risk. Further, an even minimal increase of Cr was associated with a substantial decrease in survival [20].
Our present study extends the previous findings by demonstrating that multiple low threshold ΔCr within the first 12 h carry more prognostic information in contrast to single sampling. In contrast to Karkouti et al. [1], with reported sensitivity of 20%, our model with sensitivity of 73% and specificity of 82% could also be used as an early clinical detection tool.
Subclinical rise in Cr after cardiac surgery has been originally used to demonstrate higher mortality in the affected groups [22]. The present study offers an earlier insight into the critical period for CSA-AKI development, demonstrating that already less pronounced early Cr decrease and reciprocal eGFR change due to haemodilution carry important prognostic information.
Data from animal studies indicate that the therapeutic window for renal injury is limited to the first 24–48 h after the onset [1, 23, 24]. In our cohort, we found that as high as 72.1% of all patients developing CSA-AKI could benefit from such an early detection and diagnosis followed by preventive interventions like volume management, maintenance of adequate blood pressure, and judicious avoidance of nephrotoxins [25]. Preoperative anaemia as well as intraoperative blood transfusions increase the risk of CSA-AKI [1, 26, 27]. In line with the previous observations, transfusion of more than 2 units of packed red blood cells significantly increased the odds of CSA-AKI in our study. Anticipating that preoperative haemoglobin per se is a significant independent factor for CSA-AKI, the timing of transfusion and optimal entering haemoglobin remains open dilemma for future considerations.
Endeavours to identify novel biomarkers for early kidney injury detection have recognized neutrophil gelatinase-associated lipocalin and combination of tissue inhibitor of metalloproteinases-2 (TIMP-2) with insulin-like growth factor-binding protein 7 [TIMP-2]•[IGFBP7] among the most promising [28–30], yet both biomarkers perform inferiorly with regard to our ‘DynaLAb’ Model. We strongly believe that a combination of Cr changes and injury biomarker is the way to go in further implementation of the diagnostic paradigm for CSA-AKI [29].
Limitations
Data of our study were collected retrospectively from a single centre. External validation is mandatory and needs to be performed in the future. The broad study period could be a confounding because of change in anaesthesiological or clinical protocols over 10 years. Finally, we excluded patients with eGFR <30 ml/min/1.73 m2 and therefore our results may not be applicable to patients with stages 4 and 5 of chronic kidney disease undergoing heart surgery.
CONCLUSIONS
In cardiac surgery patients, determination of immediate sequential Cr and eGFR implemented with assessment of corresponding subclinical changes facilitates early identification of CSA-AKI, better than the widely known Cleveland Clinical Score. The appealing simplicity, specificity and sensitivity of our enriched ‘DynaLab’ model could be implemented easily at most departments worldwide. The model, especially if integrated in the laboratory medical software as an alert system or provided as Apps, might represent a viable, inexpensive clinical tool.
Funding: This research received no external funding.
Conflict of interest: none declared.
Author contributions
Ferdinand Aurel Vogt: Conceptualization; Formal analysis; Methodology; Validation; Visualization; Writing—original draft; Writing—review & editing. Janez Zibert: Data curation; Formal analysis; Software; Validation; Visualization. Alenka Bahovec: Data curation; Formal analysis; Investigation; Project administration; Validation; Visualization. Francesco Pollari: Data curation; Formal analysis; Resources; Visualization. Joachim Sirch: Data curation; Resources; Software; Validation. Matthias Fittkau: Supervision; Writing—review & editing. Thomas Bertsch: Methodology; Resources; Supervision; Writing—review & editing. Martin Czerny: Supervision; Writing—review & editing. Giuseppe Santarpino: Conceptualization; Writing—review & editing. Theodor Fischlein: Supervision; Writing—review & editing. Jurij M. Kalisnik: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Heyman Luckraz, Milan Milojevic and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- AUC
Area under the curve
- CCS
Cleveland Clinical Score
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- Cr
Creatinine
- CSA-AKI
Cardiac surgery-associated acute kidney injury
- eGFR
Estimated glomerular filtration rate
- Hb
Haemoglobin
- ICU
Intensive care unit
- KDIGO
Kidney Disease: Improving Global Outcomes
- pRBC
Packed red blood cells
- RRT
Renal replacement therapy
REFERENCES
- 1. Karkouti K, Rao V, Chan CT, Wijeysundera DN, the TACS Investigators. Early rise in postoperative creatinine for identification of acute kidney injury after cardiac surgery. Can J Anesth 2017;64:801–9. [DOI] [PubMed] [Google Scholar]
- 2. Birnie K, Verheyden V, Pagano D, Bhabra M, Tilling K, Sterne JA. et al. Predictive models for kidney disease: improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care 2014;18:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouma HR, Mungroop HE, de Geus AF, Huisman DD, Nijsten MWN, Mariani MA. et al. Acute kidney injury classification underestimates long-term mortality after cardiac valve operations. Ann Thorac Surg 2018;106:92–8. [DOI] [PubMed] [Google Scholar]
- 4. Axtell AL, Fiedler AG, Melnitchouk S, D'Alessandro DA, Villavicencio MA, Jassar AS. et al. Correlation of cardiopulmonary bypass duration with acute renal failure after cardiac surgery. J Thorac Cardiovasc Surg 2019; Jan 31:S0022-5223(19)30286-7. [DOI] [PubMed] [Google Scholar]
- 5. Mizuguchi KA, Huang CC, Shempp I, Wang J, Shekar P, Frendl G.. Predicting kidney disease progression in patients with acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 2018;155:2455–63.e5. [DOI] [PubMed] [Google Scholar]
- 6. Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP.. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 2005;16:162–8. [DOI] [PubMed] [Google Scholar]
- 7. Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT. et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA 2007;297:1801–9. [DOI] [PubMed] [Google Scholar]
- 8. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, workgroup ADQI. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singbartl K, Kellum JA.. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 2012;81:819–25. [DOI] [PubMed] [Google Scholar]
- 11. Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS. et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014;64:2753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Bellomo R.. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol 2017;13:697–711. [DOI] [PubMed] [Google Scholar]
- 13. Kristovic D, Horvatic I, Husedzinovic I, Sutlic Z, Rudez I, Baric D. et al. Cardiac surgery-associated acute kidney injury: risk factors analysis and comparison of prediction models. Interact CardioVasc Thorac Surg 2015;21:366–73. [DOI] [PubMed] [Google Scholar]
- 14. Kiers HD, van den Boogaard M, Schoenmakers MC, van der Hoeven JG, van Swieten HA, Heemskerk S. et al. Comparison and clinical suitability of eight prediction models for cardiac surgery-related acute kidney injury. Nephrol Dial Transplant 2013;28:345–51. [DOI] [PubMed] [Google Scholar]
- 15. Pan W. Akaike's information criterion in generalized estimating equations. Biometrics 2001;57:120–5. [DOI] [PubMed] [Google Scholar]
- 16. Ho J, Reslerova M, Gali B, Nickerson PW, Rush DN, Sood MM. et al. Serum creatinine measurement immediately after cardiac surgery and prediction of acute kidney injury. Am J Kidney Dis 2012;59:196–201. [DOI] [PubMed] [Google Scholar]
- 17. Englberger L, Suri RM, Li Z, Dearani JA, Park SJ, Sundt TM. et al. Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. Am J Kidney Dis 2010;56:623–31. [DOI] [PubMed] [Google Scholar]
- 18. Star RA. Treatment of acute renal failure. Kidney Int 1998;54:1817–31. [DOI] [PubMed] [Google Scholar]
- 19. Takaki S, Shehabi Y, Pickering JW, Endre Z, Miyashita T, Goto T.. Perioperative change in creatinine following cardiac surgery with cardiopulmonary bypass is useful in predicting acute kidney injury: a single-centre retrospective cohort study. Interact CardioVasc Thorac Surg 2015;21:465–9. [DOI] [PubMed] [Google Scholar]
- 20. Moore E, Tobin A, Reid D, Santamaria J, Paul E, Bellomo R.. The impact of fluid balance on the detection, classification and outcome of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth 2015;29:1229–35. [DOI] [PubMed] [Google Scholar]
- 21. McIlroy DR, Farkas D, Matto M, Lee HT.. Neutrophil gelatinase-associated lipocalin combined with delta serum creatinine provides early risk stratification for adverse outcomes after cardiac surgery: a prospective observational study. Crit Care Med 2015;43:1043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P. et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004;15:1597–605. [DOI] [PubMed] [Google Scholar]
- 23. Bonventre JV, Weinberg JM.. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 2003;14:2199–210. [DOI] [PubMed] [Google Scholar]
- 24. Fu Y, Lin Q, Gong T, Sun X, Zhang ZR.. Renal-targeting triptolide-glucosamine conjugate exhibits lower toxicity and superior efficacy in attenuation of ischemia/reperfusion renal injury in rats. Acta Pharmacol Sin 2016;37:1467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J. et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017;43:1551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth 2012;109:i29–38. [DOI] [PubMed] [Google Scholar]
- 27. Khan UA, Coca SG, Hong K, Koyner JL, Garg AX, Passik CS. et al. Blood transfusions are associated with urinary biomarkers of kidney injury in cardiac surgery. J Thorac Cardiovasc Surg 2014;148:726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Geus HR, Ronco C, Haase M, Jacob L, Lewington A, Vincent JL.. The cardiac surgery-associated neutrophil gelatinase-associated lipocalin (CSA-NGAL) score: a potential tool to monitor acute tubular damage. J Thorac Cardiovasc Surg 2016;151:1476–81. [DOI] [PubMed] [Google Scholar]
- 29. Kalisnik JM, Fischlein T, Santarpino G.. Cardiac surgery-associated neutrophil gelatinase-associated lipocalin score for postoperative acute kidney injury: what is the clinical implication? J Thorac Cardiovasc Surg 2017;154:938. [DOI] [PubMed] [Google Scholar]
- 30. Guzzi LM, Bergler T, Binnall B, Engelman DT, Forni L, Germain MJ. et al. Clinical use of [TIMP-2]•[IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: guidance from an expert panel. Crit Care 2019;23:225. [DOI] [PMC free article] [PubMed] [Google Scholar]