Abstract
Only few patients with heart failure with preserved ejection fraction undergo durable mechanical circulatory support implantation. We identified three patients with heart failure with preserved ejection fraction who needed durable mechanical circulatory support as bridge-to-transplant therapy. In two patients with hypertrophic cardiomyopathy, the hypertrophic papillary muscles and myocardium were resected to allow for subsequent left ventricular assist device implantation. In one patient, all visible parts of the mitral valve were additionally resected. The third patient with restrictive cardiomyopathy underwent Berlin Heart Excor BVAD implantation with left atrial cannulation.
Keywords: LVAD, Berlin Heart, Heart failure with preserved ejection fraction
Durable mechanical circulatory support (d-MCS) is an established treatment for patients suffering from end-stage heart failure with reduced ejection fraction.
Durable mechanical circulatory support (d-MCS) is an established treatment for patients suffering from end-stage heart failure with reduced ejection fraction. Nevertheless, only around 2% of patients who undergo durable MCS implantation have end-stage heart failure with preserved ejection fraction (HFpEF, e.g. restrictive or hypertrophic cardiomyopathy) [1]. In HFpEF, reduced ventricular volume and compliance are associated with inflow cannula obstruction and suction events. In this report, we describe two different surgical approaches for patients with HFpEF needing d-MCS.
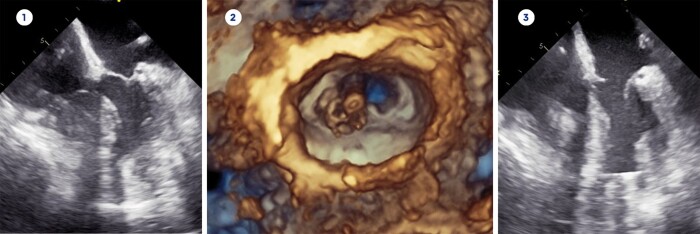
A 49-year-old female (patient 1) was diagnosed with hypertrophic cardiomyopathy in 1986 and underwent Morrow procedure and epicardial implantable cardioverter defibrillator (ICD) implantation. In 2019, the patient was hospitalized with decompensated heart and renal failure and required continuous inotropic support. She was subsequently listed for high-priority heart and kidney transplantation. While waiting for a suitable organ offer, she clincally deteriorated despite high-dose catecholamine therapy [Vasoactive-Inotropic Score (VIS) 43.9, heart rate (HR) 80 bpm, systolic blood pressure (sysBP) 84 mmHg, diastolic blood pressure (diaBP) 39 mmHg, central venous pressure (CVP) 22 mmHg] and d-MCS implantation was scheduled as a bailout procedure. Transoesophageal echocardiography (TEE) revealed a left ventricular end-diastolic diameter (LVEDD) of 44 mm and a left ventricular end-diastolic volume (LVEDV) of 40 ml with a left ventricular ejection fraction (LVEF) of 50%. The maximum extension of the interventricular septum (IVSd) was 16 mm with a left ventricular posterior wall thickness (LVPWd) of 12 mm (Fig. 1). We performed left lateral thoracotomy and initiation of cardiopulmonary bypass (CPB) as previously described [2]. Then, we identified the optimal site for inflow cannula placement via TEE and opened the left ventriclewith a round punch (component of the Heartware HVAD set). As we inspected the left ventricular cavity, we decided that standard d-MCSleft ventricular assist device (LVAD) inflow cannula implantation would pose a high risk for cannula obstruction. Therefore as first step, we carefully resected the hypertrophic septum close to the apex to prevent suction events, after that, we resected the papillary muscles including the chordae to increase left ventricular volume. In a third step, we aimed to resect as much of the mitral valve as possible to ensure sufficient cannula inflow at all times. Limited view of and access to the mitral valve resulted in resection of segments 2 and 3 of the anterior and posterior mitral leaflet (Fig. 1). We performed attachment of the apical ring and implantation of the Heartware HVAD pump (Medtronic, Minneapolis, Minnesota) as previously described [2]. The postoperative LVAD function was flawless with a pump flow of around 5 l/min at 2900 rpm, but the patient developed severe systemic infection necessitating vasoactive and inotropic therapy (VIS 3525) to obtain a satisfactory haemodynamic status (HR 100 bpm, sysBP 69 mmHg, diaBP 65 mmHg, CVP 10 mmHg). Since endoplastitis was suspected as the root cause of the infection, the transvenous ICD was explanted, but the patient subsequently developed multiple organ dysfunction syndrome and died six weeks after LVAD implantation.
Figure 1:
Transoesophageal echocardiography before mechanical circulatory support implantation (1), intraoperative 3D reconstruction of the partially resected mitral valve (2) and echocardiography after resection of septum, papillary muscles and mitral valve with implanted LVAD (3).
A 33-year-old male (patient 2) with hypertrophic obstructive cardiomyopathy was transferred to our clinic for MCS implantation. At admission, he was in cardiogenic shock (HR 123 bpm, sysBP 75 mmHg, diaBP 68 mmHg, CVP 24 mmHg, cardiac index 1.0 l/min/m2) and received vasoactive therapy (VIS 26). As first step, we percutaneously implanted peripheral veno-arterial extracorporeal life support (ECLS) in the spontaneously breathing patient to restore sufficient haemodynamic status. Echocardiography showed a small left ventricular cavity (LVEDD 47 mm, IVSd 16 mm and LVPWd 17 mm) with severe systolic anterior motion of the mitral valve resulting in a reduced LVEF of 25%. Since end-organ function soon recovered, LVAD implantation was scheduled. After performing median sternotomy, we used the ECLS cannulas and an additional cannula in the superior vena cava for CPB. Then, we performed right atriotomy to seal the persistent foramen ovale and opened the apex for LVAD inflow cannula as outlined above. An inspection of the LV cavity revealed a hypertrophy of the ventricular wall, the septum and the papillary muscles. Against this backdrop, we performed myectomy and resection of the anterior papillary muscle. After completion of LVAD implantation, a pump flow of 4.5 l/min at 2900 rpm was achieved. ECLS was explanted and a stable haemodynamic status with low-dose inotropic support (VIS 5, HR 89 bpm, sysBP 61 mmHg, diaBP 57 mmHg, CVP 6 mmHg) was accomplished. Latent right ventricular failure complicated weaning of inotropic support and the patient was listed for heart transplantation. Eventually intropic support was terminated and the patient was discharged home. Back at home, he developed severe driveline infection, was upgraded to high-urgency transplantation status and was successfully transplanted two years after LVAD implantation.
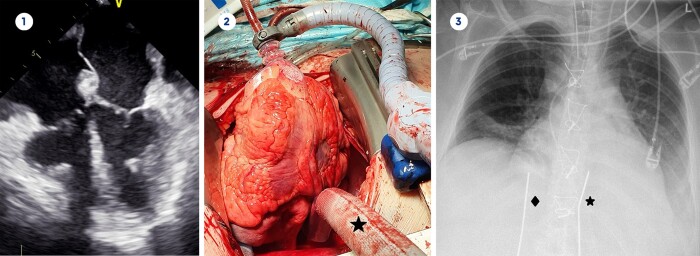
A 31-year-old female (patient 3) with restrictive cardiomyopathy had been receiving treatment in our outpatient clinic for 10 years. A myocardial biopsy did not reveal a specific cause for her cardiomyopathy. As her heart function gradually declined and no further treatment options existed, we started the patient on inotropic support and listed her for high-priority heart transplantation. TEE showed an LVEDD of 32 mm, an LVEDV of 33 ml and a preserved LVEF of 64% (Fig. 2). The IVSd was 15 mm and the LVPWd was 16 mm. Despite inotropic and vasoactive therapy (VIS 32), she developed low cardiac output syndrome (HR 100 bpm, sysBP 92 mmHg, diaBP 52 mmHg, CVP 11 mmHg) with anuria. Since ventricular volume and function were impaired on both sides, Berlin Heart Excor BVAD (Berlin Heart GmbH, Berlin, Germany) implantation was scheduled. After performing median sternotomy and initiating CPB, we performed lateral right atrial cannulation and end-to-side anastomoses of the outflow grafts to the ascending aorta and pulmonary artery as previously described [3]. The large right atrium precluded lateral left atrial cannulation for the inflow cannula of the Excor, so the posterior left atrial wall was the only appropriate spot for cannulation. A cardiac positioner (Starfish®, Medtronic) was used to ensure persistent anterior cardiac displacement of 90 degrees. (Fig. 2). We performed left atrial cannulation in the posterior wall between left and right inferior pulmonary vein, as this was the easiest location for the anastomosis. After successful implantation of Berlin Heart Excor BVAD, a stable haemodynamic status (HR 78 bpm, sysBP 130 mmHg, diaBP 74 mmHg, CVP 10 mmHg) was quickly achieved with norepinephrine 0.05 μg/kg/min (VIS 5). Apart from thrombosis-related pump exchanges, the function of the Berlin Heart Excor BVAD function has been impeccable for the last 16 months (Fig. 2). The patient is now at home awaiting heart transplantation.
Figure 2:
Transoesophageal echocardiography before mechanical circulatory support implantation (1), 90° anterior cardiac displacement and atrial cannula (★) between left and right inferior pulmonary vein (2), chest X-ray after Berlin Heart Excor BVAD implantation with right (♦) and left (★) atrial cannulation (3).
In our small case series, we demonstrated that technical adjustments during MCS implantation can lead to a sufficient pump flow without cannula obstruction or clinically significant suction events. As patients with an LVEDD of <50 mm generally experience the worst outcome with a 6-month mortality of >50%, our results are promising, with one patient who underwent heart transplantation after 2 years on LVAD support and a second patient who has been supported with Berlin Heart Excor BVAD for over a year [1, 4]. However, our third patient died due to septic shock six weeks after MCS implantation.
Although most patients with restrictive or hypertrophic cardiomyopathy develop some ventricular dilation as heart failure progresses, patients with HFpEF have a significantly smaller LVEDD and LVEDV compared to patients with HfrEF [1]. Therefore, heart transplantation is the treatment of choice, but d-MCS should be considered in patients with progressive HfpEF as bridge to heart transplantation [5].
In patients with small LV cavities and hypertrophic papillary muscles, it may be advisable to perform myectomy and to resect the papillary muscles and chordae via the apex to maximize the LV dimension. Excision of mitral valve to create a tube between left atrium and cannula may be advisable in certain cases. With proper VAD function, mitral regurgitation should not represent a problem.
For patients with only minimal residual LV cavities (LVEDV <40 ml), the Berlin Heart Excor with left atrial cannulation may present the best treatment option.
Total artificial heart (TAH) implantation would be another option, especially in patients with systemic disease and involvement of both ventricular chambers. TAH implantation was either not possible in any of our patients due to the limited thoracic dimensions or not necessary due to mainly left ventricular failure.
Conflict of interest: Evgenij Potapov is a proctor for and receives institutional travel grants from Medtronic. Volkmar Falk is a member of the advisory board of Berlin Heart GmbH. Other authors report no conflict of interest.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Jason M. Ali, David Schibilsky and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Patel SR, Saeed O, Naftel D, Myers S, Kirklin J, Jorde UP. et al. Outcomes of restrictive and hypertrophic cardiomyopathies after LVAD: an INTERMACS analysis. J Card Fail 2017;23:859–67. [DOI] [PubMed] [Google Scholar]
- 2. Schulz A, Stepanenko A, Krabatsch T.. HeartMate 3 implantation via left lateral thoracotomy with outflow graft anastomosis to the descending aorta. J Heart Lung Transplant 2016;35:690–2. [DOI] [PubMed] [Google Scholar]
- 3. Hetzer R, Hennig E, Schiessler A, Friedel N, Warnecke H, Adt M.. Mechanical circulatory support and heart transplantation. J Heart Lung Transplant 1992; 11:S175–81. [PubMed] [Google Scholar]
- 4. Grupper A, Park SJ, Pereira NL, Schettle SD, Gerber Y, Topilsky Y. et al. Role of ventricular assist therapy for patients with heart failure and restrictive physiology: improving outcomes for a lethal disease. J Hear Lung Transplant 2015;34:1042–9. [DOI] [PubMed] [Google Scholar]
- 5. Topilsky Y, Pereira NL, Shah DK, Boilson B, Schirger JA, Kushwaha SS. et al. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail 2011;4:266–75. [DOI] [PubMed] [Google Scholar]