IVUS allows immediate evaluation of the distal aortic configuration and branch vessels.
Central Message.
Intraoperative antegrade intravascular ultrasound is a feasible and safe procedure to assess detailed configuration of the true lumen and visceral branches after central repair.
See Commentary on page 188.
Visceral malperfusion remains a devastating complication of acute type A aortic dissection (ATAD).1 Although most malperfusions spontaneously resolve following central aortic repair for ATAD,2 some may require an additional intervention for persistent ischemia. However, the configuration of the distal aorta and perfusion status of the visceral branches following the repair are unpredictable. Intraoperative assessment for malperfusion following the central repair has not been established. Since 2013, we have used intraoperative antegrade intravascular ultrasound (IVUS) via ascending graft for patients with type A aortic dissection with suspected visceral malperfusion. In this report, we describe our technique and its safety and efficacy.
Patients and Methods
This is a retrospective review between January 2013 and December 2017; 15 of 192 patients with type A aortic dissection (8%) underwent intraoperative IVUS examination at our institution. Clinical data were collected and analyzed retrospectively. The suspicion for visceral malperfusion was based on clinical symptoms, laboratory (eg, elevated serum lactate level), or computed tomography findings (eg, compressed true lumen at the level of the visceral portion, and nonperfused visceral branches). Data collection and analysis were approved by The McGovern Medical School at UTHealth's Committee for the Protection of Human Subjects (IRB: HSC-MS-03-077).
Operative Procedure
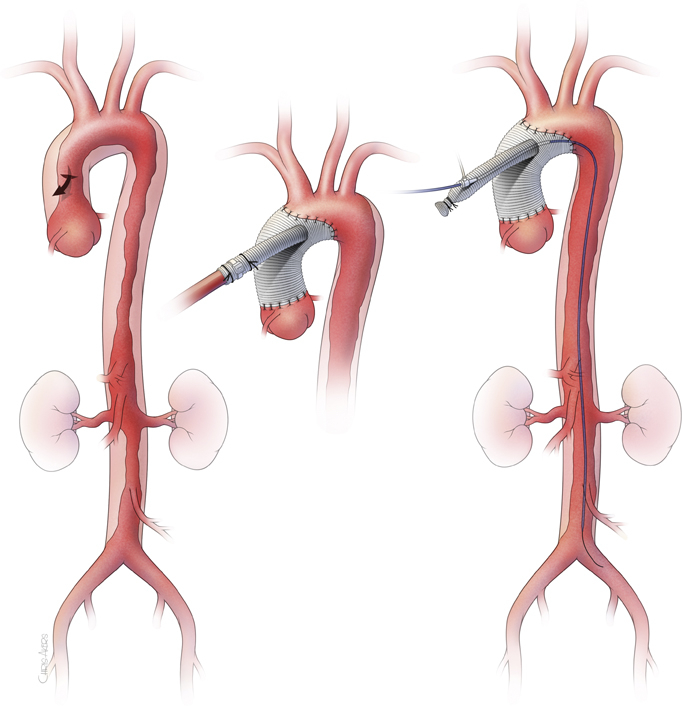
Our approach for ATAD has been reported previously.3 To summarize, the dissected ascending aorta and proximal arch are replaced and entry tear is resected under profound hypothermia circulatory arrest with retrograde cerebral perfusion, using a single, sidearm Dacron graft. The systemic circulation is re-established via the perfusion sidearm branch after the distal anastomosis. Then, the proximal anastomosis is performed during warming. After the patient is weaned off the cardiopulmonary bypass, pulsatile flow to the body is regained. An 8-French sheath is inserted to the sidearm perfusion branch by the Seldinger technique. Transesophageal echocardiogram is used to guide the 0.035-inch guidewire into the true lumen of the descending thoracic aorta. Then, an IVUS catheter is advanced over the wire—all the way to the iliac artery in antegrade fashion. IVUS imaging is obtained using a Volcano s5 Imaging System and a Visions PV .035 Digital IVUS Catheter (Philips North America Co, Cambridge, Mass). True lumen configuration and flap extension to visceral arteries are evaluated. Based on the IVUS finding, additional procedures are performed, if required (Figure 1).
Figure 1.
Surgical approach of intraoperative antegrade intravascular ultrasound. Intraoperative intravascular ultrasound through the perfusion sidearm branch allows immediate evaluation of the distal aortic configuration and branch vessel status following central repair of the aortic dissection in patients suspected for malperfusion syndrome to guide further treatment. The access through the sidearm requires no additional arterial access and allows reliable engagement to the true lumen of the dissected aorta without technical difficulties.
Results
Patient characteristics are shown in Table 1. Technical success of the IVUS examination was seen in all patients. After the central repair, true lumen was expanded in 11 patients (73%). Three patients (Nos. 5, 6, and 15) had compressed true lumen at the level of the visceral aorta and underwent thoracic endovascular aortic repair after upsizing the sheath in the sidearm. One patient (No. 4) received axillary–bifemoral bypass due to collapsed true lumen limited to the infrarenal abdominal aorta (Figure 2). All 4 had improved true lumen expansion and peripheral perfusion after the additional interventions (Figure 3), which was also confirmed by IVUS. One patient (No. 9) received exploratory laparotomy with left colectomy, despite good pulsation on visceral vessels. This patient had a left low anterior resection for colon cancer, which likely contributed to the low reserve to the remaining left colon. The damage was irreversible at the time of presentation. Operative mortality was seen in 2 patients (13%), whereas 12 patients recovered and were discharged home and 1 (No. 4) was discharged to hospice care due to being comatose.
Table 1.
Patient demographics and outcomes
| Patient | Age, y | Sex | Malperfusion symptoms | Occlusion/compression of the true lumen | Preoperative |
ET | IVUS findings | Additional interventions | Ex Lap | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFR, mL/min/1.73 m2 | Lactic acid, mg/dL | ||||||||||
| #1 | 64 | M | None | R renal | 22 | 5.3 | – | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #2 | 48 | M | Abdominal pain | R renal | 59 | 3.7 | – | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #3 | 39 | M | Abdominal pain, diminished distal pulses | Celiac, SMA L renal | 77 | 6.5 | – | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #4 | 50 | M | Paraplegia with palpable femoral pulses | Descending aorta | 54 | 7.2 | – | Compressed true lumen at the level of the abdominal aorta, patent visceral branches | Ax-biFem bypass | – | Discharged to hospice care |
| #5 | 71 | F | Metabolic acidosis, diminished distal pulses | Descending aorta | 33 | >12 | – | Compressed true lumen in the visceral portion of abdominal aorta, compressed visceral branches | TEVAR | – | Death due to cardiogenic shock on POD 1 |
| #6 | 44 | M | Abdominal and left leg pain | Lower extremities | 52 | >12 | – | Compressed true lumen of left common iliac artery, patent visceral branches | TEVAR + iliac stents + fasciotomy | + | Discharged home |
| #7 | 50 | F | Abdominal and right leg pain | Celiac | 58 | 3.6 | – | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #8 | 86 | M | Abdominal pain | Celiac | 25 | 8.5 | – | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #9 | 71 | M | Abdominal pain | SMA, L renal | 28 | 6.8 | – | Expanded true lumen of descending aorta, patent visceral branches | Left colectomy | + | Death from MOF on POD#13 |
| #10 | 62 | M | Abdominal pain | Celiac, L renal | 64 | 5 | + | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #11 | 64 | M | Abdominal pain | Celiac | 90 | 7.8 | – | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #12 | 57 | M | Abdominal pain | L renal | 74 | 6.2 | + | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #13 | 58 | M | Abdominal pain | Celiac | 83 | 3.2 | – | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #14 | 65 | M | None | L renal | 72 | 3.2 | – | Expanded true lumen of descending aorta, patent visceral branches | None | – | Discharged home |
| #15 | 57 | M | Abdominal pain, numbness in left leg | Celiac, SMA | 50 | 8.2 | – | Compressed true lumen in the visceral portion of abdominal aorta, compressed visceral branches | TEVAR | – | Discharged home |
eGFR, Estimated glomerular filtration rate; ET, conventional elephant trunk; IVUS, intravascular ultrasound; Ex Lap, exploratory laparotomy; M, male; R, right; SMA, superior mesenteric artery; L, left; Ax-biFem, axillary–bifemoral bypass; F, female; TEVAR, thoracic endovascular aortic repair; POD, postoperative day; MOF, multiorgan function.
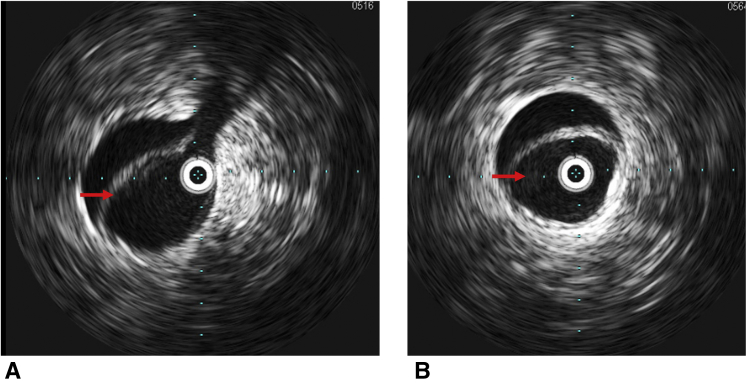
Figure 2.
Intravascular ultrasound images of compressed true lumen. A and B, Intravascular ultrasound images of compressed true lumen (red arrows) at the abdominal aorta after central repair of type A aortic dissection.
Figure 3.
Intravascular ultrasound images of expanded true lumen. A and B, Intravascular ultrasound images of expanded true lumen (red arrows) at the abdominal aorta after central repair of type A aortic dissection.
Discussion
The use of IVUS in aortic dissection was first described more than 30 years ago4 and with the growth of endovascular applications, has gained in importance in treating patients with dissection. Transesophageal echocardiogram is routinely used during ATAD repair and may be used to evaluate the proximal descending thoracic aorta but not the distal descending or thoracoabdominal aorta. The advantages of IVUS examination in aortic dissection are the real-time assessment of the true and false lumen configurations throughout the aorta and iliac arteries5 and visualization of the visceral branches. Antegrade approach after the central repair provides easy and reliable access to the true lumen of the dissected aorta, allowing us to inspect the true lumen expansion, but also can be used in cases of antegrade placement of a stent graft in the descending aorta to confirm deployment in the true lumen. We believe that patients with high suspicion for malperfusion at presentation, based on clinical, laboratory, and imaging, may benefit from an antegrade IVUS study if additional interventions, such as thoracic endovascular aortic repair or exploratory laparotomy, are needed. Antegrade IVUS allows us to avoid the use of contrast agent and additional arterial access. The limitation of the study is the IVUS system we used currently does not allow Doppler imaging, so we could not directly evaluate the flow. The use of a Doppler-available device may further expedite the evaluation, but further study is required.
Conclusions
Our study demonstrates the feasibility and safety of intraoperative IVUS examination through the side branch of the ascending Dacron graft. An immediate evaluation of the residual dissection using IVUS provides useful information for our decision-making in treating patients with suspected malperfusion syndrome.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Tanaka A., Estrera A.L. Mesenteric malperfusion: the insidious, dreadful enemy. Semin Thorac Cardiovasc Surg. 2017;29:179–180. doi: 10.1053/j.semtcvs.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Charlton-Ouw K.M., Sritharan K., Leake S.S., Sandhu H.K., Miller C.C., III, Azizzadeh A., et al. Management of limb ischemia in acute proximal aortic dissection. J Vasc Surg. 2013;57:1023–1029. doi: 10.1016/j.jvs.2012.10.079. [DOI] [PubMed] [Google Scholar]
- 3.Estrera A.L., Miller C.C., III, Lee T.Y., Shah P., Safi H.J. Ascending and transverse aortic arch repair: the impact of retrograde cerebral perfusion. Circulation. 2008;118:S160–S166. doi: 10.1161/CIRCULATIONAHA.107.757419. [DOI] [PubMed] [Google Scholar]
- 4.Belkin N., Jackson B.M., Foley P.J., Damrauer S.M., Kalapatapu V., Golden M.A., et al. The use of intravascular ultrasound in the treatment of type B aortic dissection with thoracic endovascular aneurysm repair is associated with improved long-term survival. J Vasc Surg. 2020;72:490–497. doi: 10.1016/j.jvs.2019.10.073. [DOI] [PubMed] [Google Scholar]
- 5.Koschyk D.H., Nienaber C.A., Knap M., Hofmann T., Kodolitsch Y.V., Skriabina V., et al. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation. 2005;112:I260–I264. doi: 10.1161/CIRCULATIONAHA.104.525972. [DOI] [PubMed] [Google Scholar]