Abstract
Aims
Iron deficiency is common in heart failure with reduced ejection fraction (HFrEF) and negatively affects cardiac function and structure. The study the effect of ferric carboxymaltose (FCM) on cardiac reverse remodelling and contractile status in HFrEF.
Methods and results
Symptomatic HFrEF patients with iron deficiency and a persistently reduced left ventricular ejection fraction (LVEF <45%) at least 6 months after cardiac resynchronization therapy (CRT) implant were prospectively randomized to FCM or standard of care (SOC) in a double-blind manner. The primary endpoint was the change in LVEF from baseline to 3-month follow-up assessed by three-dimensional echocardiography. Secondary endpoints included the change in left ventricular end-systolic (LVESV) and end-diastolic volume (LVEDV) from baseline to 3-month follow-up. Cardiac performance was evaluated by the force–frequency relationship as assessed by the slope change of the cardiac contractility index (CCI = systolic blood pressure/LVESV index) at 70, 90, and 110 beats of biventricular pacing. A total of 75 patients were randomized to FCM (n = 37) or SOC (n = 38). At baseline, both treatment groups were well matched including baseline LVEF (34 ± 7 vs. 33 ± 8, P = 0.411). After 3 months, the change in LVEF was significantly higher in the FMC group [+4.22%, 95% confidence interval (CI) +3.05%; +5.38%] than in the SOC group (−0.23%, 95% CI −1.44%; +0.97%; P < 0.001). Similarly, LVESV (−9.72 mL, 95% CI −13.5 mL; −5.93 mL vs. −1.83 mL, 95% CI −5.7 mL; 2.1 mL; P = 0.001), but not LVEDV (P = 0.748), improved in the FCM vs. the SOC group. At baseline, both treatment groups demonstrated a negative force–frequency relationship, as defined by a decrease in CCI at higher heart rates (negative slope). FCM resulted in an improvement in the CCI slope during incremental biventricular pacing, with a positive force–frequency relationship at 3 months. Functional status and exercise capacity, as measured by the Kansas City Cardiomyopathy Questionnaire and peak oxygen consumption, were improved by FCM.
Conclusions
Treatment with FCM in HFrEF patients with iron deficiency and persistently reduced LVEF after CRT results in an improvement of cardiac function measured by LVEF, LVESV, and cardiac force–frequency relationship.
Keywords: Iron deficiency, Cardiac remodelling, Contractility, Heart failure, Randomized controlled trials
Graphical Abstract
See page 4915 for the editorial comment for this article ‘Intravenous iron supplementation: novel anti-remodelling therapy for patients with heart failure?’, by E.A. Jankowska and P. Ponikowski, https://doi.org/10.1093/eurheartj/ehab624.
Introduction
Iron deficiency is common in heart failure with a reduced ejection fraction (HFrEF) and associated with a reduced functional status, poor exercise performance, and increased risk for heart failure hospitalization and cardiovascular mortality.1–3 Iron is an essential co-factor in proteins of oxidative phosphorylation and anti-oxidative enzymes and as a result involved in the pathophysiology of progressive cardiac remodelling and failing cardiac and peripheral muscle energetics in heart failure.4,5 The negative impact of iron deficiency on cardiac contractility is more pronounced during exercise.6,7 In normal physiological conditions, contractility increases disproportionally to heart rate, a phenomenon called a positive force–frequency relationship.8 In HFrEF patients with iron deficiency, cardiac output increases less in comparison to non-iron-deficient counterparts during exercise.7 Animal models of iron deficiency indicate that during higher heart rates, the compensatory increase in ATP and phosphocreatine is blunted, resulting in a decrease in contractility at higher heart rates (negative force–frequency relationship).6 This feature is also found in HFrEF patients, when evaluating contractile status using an incremental biventricular pacing protocol.9 In addition, HFrEF patients with iron deficiency receiving cardiac resynchronization therapy (CRT) exhibit diminished cardiac reverse remodelling following CRT implant, documented by less improvement in left ventricular ejection fraction (LVEF).10,11
Ferric carboxymaltose (FCM) is recommended by heart failure guidelines to alleviate heart failure symptoms, to improve exercise capacity and quality of life.12 Recently, the AFFIRM-AHF (Study to Compare Ferric Carboxymaltose With Placebo in Patients with Acute Heart Failure and Iron Deficiency) has also demonstrated the beneficial impact of FCM on the recurrence of heart failure admissions in patients with recent acute heart failure.13 Nevertheless, little information is available about the effect of FCM on cardiac function and structure. The IRON-CRT trial (Effect of Intravenous Ferric Carboxymaltose on Reverse Remodelling Following Cardiac Resynchronization Therapy) was a prospective, double-blind randomized multi-centre trial specifically designed to determine if treatment with FCM (i) induces incremental reverse remodelling in CRT patients with iron deficiency and a persistently reduced LVEF and (ii) is capable of improving the force–frequency relationship in HFrEF.
Methods
Study population
Patients were eligible for the IRON-CRT trial if (i) aged ≥18 years, (ii) had stable heart failure (on maximal tolerated doses of all guideline-recommended medical heart failure therapies for at least 4 weeks, with the exception of loop diuretics), (iii) received CRT as part of their treatment plan for HFrEF (according to a guideline class IA, IB, IIa, or IIb indication) >6 months previously, (iv) had persistently reduced LVEF <45% at screening, (v) had ≥98% biventricular pacing the last 6 months before inclusion, (vi) had symptomatic heart failure defined as a New York Heart Association (NYHA) class ≥II, and (vii) had iron deficiency defined as a serum ferritin <100 ng/mL or serum ferritin between 100 and 300 ng/mL if transferrin saturation (TSAT) was <20%. A baseline LVEF up to 45% was allowed in this trial based on the data of previous trials with FCM in heart failure, which used this as LVEF entry criteria.14–16 Exclusion criteria are reported in Supplementary material online, Table S1. Ethical approval was obtained from each participating centre and all patients provided written informed consent before enrolment. The study followed the principles outlined in the Declaration of Helsinki and is in compliance with the standards of Good Clinical Practice. The manuscript was drafted according to the CONSORT guidelines for randomized controlled trials.17
Study design
The methods and design of IRON-CRT have been published previously.9 Briefly, the IRON-CRT trial was an investigator-initiated, double-blind, randomized, placebo-controlled trial conducted in two sites in Belgium (Ziekenhuis Oost-Limburg, Genk and Jessa Hospital, Hasselt). The aim of the IRON-CRT trial was to assess the impact of intravenous FCM compared to placebo on cardiac reverse remodelling and cardiac contractile status in HFrEF patients with iron deficiency who experienced incomplete reverse remodelling at least 6 months after CRT. If patients sufficed all inclusion and exclusion criteria and provided written informed consent, patients were randomized using a web-based randomization system (Castor EDC). Patients were assigned in balanced blocks assuring 1:1 randomization to either intravenous placebo [standard of care (SOC) group, with continuation of optimal medical therapy] or intravenous FCM. A block-randomization strategy without capping was used assuring an equal number of patients with a baseline LVEF <35% and LVEF ≥35% in both treatment arms. An LVEF of 35% was chosen to stratify into blocks of lower and higher baseline LVEF, based on the observation from previous trials illustrating a mean LVEF of on average 35% if an LVEF entry criteria of 45% was used.14,16,18 The study was designed and conducted by the first and last author of the manuscript. The study received an unrestricted research grant from Vifor Pharma. Vifor Pharma had no input in the design, collection, analysis or interpretation of the study. All analyses were performed according to a predefined statistical analysis plan, by an independent academic statistician (CenStat, University Hasselt). The IRON-CRT trial was registered at ClinicalTrials.gov (NCT03380520). Anonymized data will be made available upon reasonable request to the corresponding author.
Study drug administration and blinding
The active treatment intervention consisted of intravenous iron in the form of intravenous FCM (Injectafer®/Ferinject ®—Glattbrugg, Switzerland) diluted into 250 mL NaCl 0.9%. The placebo intervention consisted of the same 250 mL NaCl 0.9% without FCM. The required dose of FCM was calculated according to the regulatory-approved dosing scheme and can be found in Supplementary material online, Table S2. The dose needed was calculated based on screening weight and screening haemoglobin. Based on this dosing scheme, patients would require a dose of FCM ranging between 500 and 2000 mg. Because the maximal allowed dose of FCM during one intravenous administration is 1000 mg per week, patients who required a dose of either 1500 or 2000 mg received a follow-up appointment after 1–2 weeks to receive the remaining dose. To assure maximal blinding, patients assigned to the placebo group who would also require an additional dose based on their body weight and haemoglobin levels also received a second dosing appointment with the infusion of placebo at that time. As the collection of the endpoints in this study occur on a relative short basis (3 months), no additional maintenance doses of FCM were administered. Because FCM is a dark-brown solution, additional measures were undertaken to assure patient and investigator blinding.14,16,18 Both the placebo and FCM solutions were covered in non-see-through white bags. In addition, all infusion lines were made of non-see-through white plastic, avoiding the detection of the colour of the infusate (Supplementary material online, Figure S1). A study member from the Clinical Trial Unit (CTU) of the Ziekenhuis Oost-Limburg (Genk, Belgium), not involved in endpoint assessment, was unblinded and responsible for randomization, preparation, and administration of the blinded study infusate (FCM of placebo). In addition, all post-baseline iron and haematological indices were only made available by the central laboratory of Ziekenhuis Oost-Limburg (Genk, Belgium) to the unblinded study member of the CTU.
Echocardiographic endpoints
The objective of this study was to determine if treatment with FCM (i) induces incremental reverse remodelling and (ii) is capable of improving cardiac contractile function. The primary endpoint was the change in LVEF from baseline to 3-month follow-up. Secondary endpoints of reverse remodelling were the change from baseline in left ventricular end-systolic (LVESV) and end-diastolic volume (LVEDV) to 3 months. LVEF, LVEDV, and LVESV were measured using three-dimensional (3D) transthoracic echocardiography by an experienced blinded sonographer to alleviate inter-observer variability (one observer) and minimize intra-observer variability (3D echocardiography).19 The second objective of this study was to determine if FCM treatment is capable of improving cardiac contractility by assessing the force-frequency relationship. The gold standard for measuring the force–frequency relationship is by plotting invasively measured contractility against heart rate. Previous studies however have used a non-invasive surrogate (cardiac contractility index, CCI).8,20 This CCI is the ratio between systolic blood pressure divided by LVESV index (LVESV/body surface area). To determine the force–frequency relationship, all patients underwent a previously validated pacing protocol.8,9 A detailed description of all echocardiographic measurements and this pacing protocol can be found in the Supplementary material online, methods. Briefly, patients underwent biventricular pacing in DDD mode (or VVI in case of atrial fibrillation) at different lower rates of pacing (70, 90, and 110 b.p.m.). The lower rate was first programmed to 70 b.p.m. After a 5-min adaptation period in decubitus, blood pressure measurements (the arm with the highest pressure) were repeated three times and averaged. Patients were then positioned in left lateral decubitus, and after a 5-min adaptation period, echocardiographic measurements were taken to calculate the CCI. The R-mode of the CRT device was programmed off during this image protocol to avoid movement inducing surges in heart rate above the programmed lower rate. This protocol was afterward repeated for a pacing rate of 90 and 110 b.p.m. The impact of FCM on cardiac performance was assessed as the change in the slope of CCI at different heart rates (force-frequency relationship), with a negative slope indicating a negative force–frequency relationship and a positive slope a positive relationship.
Other endpoints
Other supporting endpoints included the change from baseline to 3-month follow-up in: (i) cardiopulmonary exercise test variables of peak oxygen consumption (VO2) and the slope of minute ventilation/carbon dioxide production (VE/VCO2) ratio, (ii) Kansas City Cardiomyopathy Questionnaire (KCCQ) score, and (iii) N-terminal pro B-type natriuretic peptide (NT-proBNP) level. In addition, safety and tolerability of the study drug was assessed by collecting adverse events. A detailed description of the methods on these supporting endpoints can be found in the Supplementary material online, methods.
Statistics
The IRON-CRT trial was powered for the primary endpoint, change in LVEF from baseline to 3-month follow-up. The sample size calculation was based on a previous study illustrating in 40 patients (n = 20 iron sucrose) a mean improvement in LVEF of 4.4% in favour of iron sucrose.21 Considering a type I error rate α = 0.05 and a type II error rate β = 0.10 (statistical power of 90%), we calculated a total sample size of 66 patients to detect a mean 2.4% difference in LVEF, using a 3% absolute difference in LVEF standard deviation. In addition, to account for potential drop-out and to allow for more power for the secondary endpoint of force-frequency relationship, the sample size was rounded to 100 patients. Due to poor enrolment in one of the centres (no patients were enrolled at Jessa Hospital as this site was activated just before the COVID-19 pandemic), it was decided to close the study prematurely at 75 patients in December 2020. Because only three patients were lost to follow-up, this was well above the calculated sample size of 66 patients. Data analysis for the efficacy endpoints were assessed for a full analysis set (FAS). The FAS consists of patients randomly assigned to treatment that received trial medication and had at least one post-baseline assessment. Because three patients died (all in the SOC group) before the post-baseline assessment, these patients were excluded from this analysis. As predefined in the analysis plan, the effect of missing data on the primary endpoint was assessed in a sensitivity analysis. Missing not at random imputation was done by creating control-based pattern imputation (regression model created using baseline age, gender and baseline LVEF, LVESV, and LVEDV).9 The change in primary (LVEF), secondary (LVESV and LVEDV) and tertiary endpoints (peakVO2, VE/VCO2, KCCQ, and NT-proBNP) from baseline to 3-month follow-up were assessed using analysis of covariance adjusted for the baseline value. Squared transformation was performed in case of none normal distributions. The force–frequency relationship was assessed using linear mixed models including a fixed effect of randomization group, heart rate (linear assumption), time (baseline or follow-up), and an interaction term of the aforementioned fixed effects. Categorical safety endpoints and investigator reported adverse events were assessed at 3 months using Fisher’s exact test. Risk differences were calculated and presented with 95% confidence intervals (CIs), using the Clopper–Pearson method. Predefined subgroup analyses for the primary endpoint were performed without correction for multiple testing. Categorical baseline values were reported as numbers and percentages and group difference tested with Fisher’s exact test. Continuous variables were reported as mean ± standard deviation if normally distributed or medians and 25–75th interquartile range, with group difference testing using chi-square or Mann–Whitney U-test when appropriate. Correlations were assessed using Pearson correlation coefficient. All analyses were performed using SAS software (SAS Institute Inc., Cary, NC, USA). A two-sided P-value of 0.05 was considered statistically significant for all analysis.
Results
Study population
A total of 75 patients were enrolled between November 2017 and June 2019. A consort flowchart of the patients screened, randomized, and used for the efficacy analysis is provided in Figure 1. Three patients randomized to the SOC group died before the collection of the follow-up echocardiography data. In not a single case treatment, allocation was unblinded before dataset lock. Baseline characteristics of the patients included are shown in Table 1. Patient groups were well balanced in age, gender, heart failure severity, cardiac remodelling indices, and background medical heart failure therapies. Supplementary material online, Table S3 describes patient characteristics historically before CRT implant and potential confounders of reverse remodelling, illustrating two well-balanced treatment groups. Based on their weight and haemoglobin levels, patients in the FCM group received a mean dose of 959 ± 380 mg FCM (median 1000 mg). All patients received their first dose on the day of randomization. Patients requiring an additional dose >1000 mg received their second dose on average 9 days later (range 8–14 days).
Figure 1.
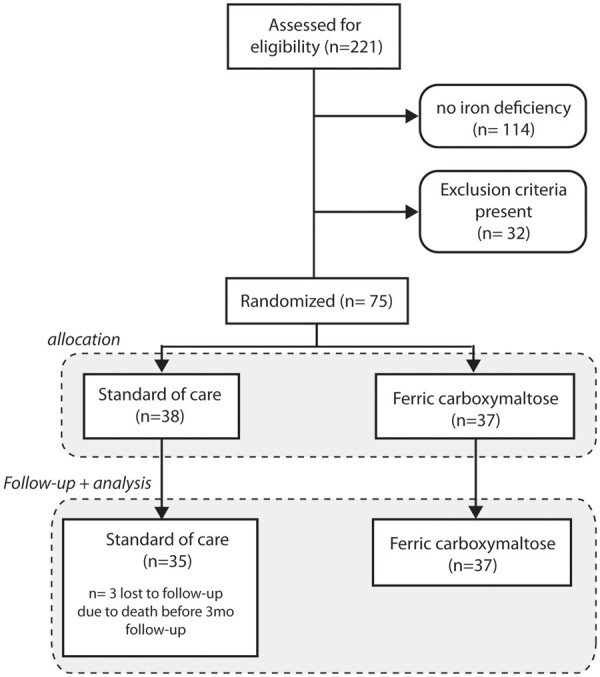
CONSORT flow chart of patients screened, randomized and followed up. A total of 221 met the inclusion criteria (numbers 1–6) mentioned in the Methods section and were screened for the presence of iron deficiency, which was present in 48%. Exclusion criteria included haemoglobin >15 g/dL, C-reactive protein >20 mg/L, insufficient image quality to assure three-dimensional echocardiography, and active inclusion in another randomized controlled trial or recent (<30 days) completion of another randomized controlled trial.
Table 1.
Baseline characteristics of the study population
| Parameter | Standard of care (n = 38) | Ferric carboxymaltose (n = 37) | P-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 73 ± 9 | 72 ± 12 | 0.594 |
| Male sex | 25 (66) | 26 (70) | 0.677 |
| Medical history | |||
| Hypertension | 37 (97) | 32 (87) | 0.082 |
| Diabetes | 19 (50) | 17 (46) | 0.725 |
| Stroke | 4 (11) | 1 (3) | 0.174 |
| COPD | 7 (18) | 5 (14) | 0.562 |
| Malignancy | 4 (11) | 2 (5) | 0.414 |
| Valve surgery | 6 (16) | 5 (14) | 0.781 |
| Peripheral artery disease | 3 (8) | 5 (14) | 0.431 |
| Former/active smoking | 21 (55) | 20 (44) | 0.684 |
| Heart failure features | |||
| Ischaemic etiology | 24 (63) | 19 (51) | 0.301 |
| NYHA class II | 19 (50) | 22 (59) | 0.411 |
| NYHA class III | 19 (50) | 15 (41) | 0.411 |
| Baseline LVEF, % | 34 ± 7 | 33 ± 8 | 0.411 |
| Baseline LVESV, mL | 129 ± 60 | 133 ± 62 | 0.845 |
| Baseline LVEDV, mL | 191 ± 74 | 195 ± 75 | 0.739 |
| Baseline peak VO2, mL/kg/min | 10.86 ± 3.51 | 10.99 ± 4.96 | 0.906 |
| Physical features | |||
| Body mass index, kg/m2 | 27 ± 5 | 27 ± 5 | 0.779 |
| Systolic blood pressure, mmHg | 115 ± 15 | 121 ± 15 | 0.074 |
| Laboratory parameters | |||
| NT-proBNP, pg/mL | 1604 [767–2204] | 2227 [299–2967] | 0.485 |
| eGFR, mL/min/1.73 m2 | 51 ± 22 | 56 ± 25 | 0.339 |
| Haemoglobin, g/dL | 13.1 ± 1.3 | 13.3 ± 1.2 | 0.522 |
| Ferritin, µg/L | 81 [43–99] | 82 [38–106] | 0.565 |
| Transferrin saturation, % | 19.4 ± 7.0 | 18.8 ± 6.0 | 0.611 |
| C-reactive protein, mg/L | 2.0 [1.2–4.0] | 1.7 [0.95–7.8] | 0.903 |
| Heart failure therapies | |||
| ACEi/ARB/ARNi | 33 (87) | 34 (92) | 0.475 |
| ARNi | 18 (47) | 20 (54) | 0.563 |
| Βeta-blocker | 37 (97) | 37 (100) | 0.321 |
| MRA | 29 (76) | 30 (81) | 0.615 |
| Loop diuretics | 21 (55) | 20 (54) | 0.916 |
| CRT-D | 19 (50) | 23 (62) | 0.289 |
Values are given as mean ± standard deviation, n (%), or median [interquartile range].
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; COPD, chronic obstructive pulmonary disease; CRT-D, cardiac resynchronization therapy-defibrillator; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume; NYHA, New York Heart Association; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro B-type natriuretic peptide; VO2, oxygen consumption.
Effect on left ventricular ejection fraction, left ventricular end-systolic volume, and end-diastolic volume
At baseline the two treatment groups were well balanced for LVEF, LVESV, and LVEDV (Table 1). The least square mean (LSM) change from baseline to 3-month follow-up in LVEF, LVESV, and LVEDV is shown in Figure 2. After 3 months, the LSM change in LVEF from baseline was significantly higher in the FCM group (+4.22%, 95% CI +3.05%; 5.38%) compared to the SOC group (−0.23%, 95% CI −1.44%; +0.97%, P < 0.001). Similarly, in the predefined sensitivity analysis assessing the impact of missing data (n = 3), treatment with FCM resulted in more improvement of LVEF after 3 months (P < 0.001). In addition, the LSM change in LVESV from baseline to follow-up was more pronounced in the FCM group vs. the SOC group (−9.72 mL, 95% CI −13.5 mL; −5.93 vs. −1.83 mL, 95% CI −5.7 mL; 2.1 mL, P = 0.001). However, the change in LVEDV did not differ between the two treatment groups (FCM: −2.5 mL, 95% CI −5.3 mL; +0.3 vs. −1.9 mL, 95% CI −4.7 mL; +1.0 mL, P = 0.748).
Figure 2.
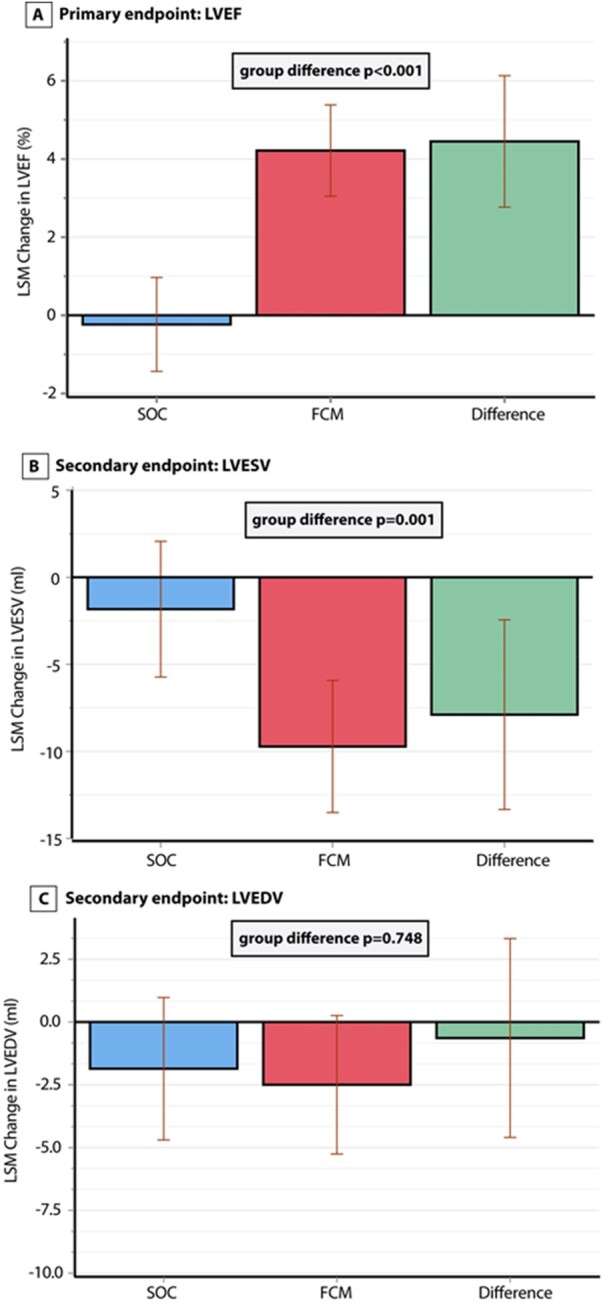
Change in primary and secondary endpoints. Change at 3 months in (A) left ventricular ejection fraction, (B) left ventricular end-systolic volume, and (C) left ventricular end-diastolic volume. FCM, ferric carboxymaltose; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; SOC, standard of care. P-values are from an ANCOVA (analysis of covariance) model with correction for baseline values.
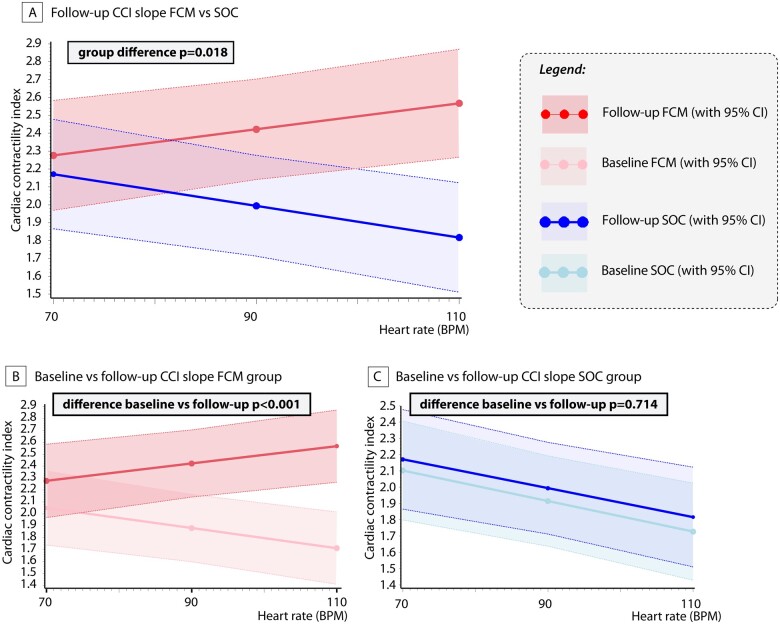
Effect on the force–frequency relationship
Figure 3 illustrates the slopes of CCI according to treatment assignment for both baseline and follow-up. At baseline patients with iron deficiency exhibited a negative force-frequency relationship, illustrated by the negative slope (decrease in CCI per 10 b.p.m.; SOC: −0.089, P < 0.001; FCM: −0.084, P = 0.007). Patients randomized to FCM treatment had a significant improvement in the slope of CCI vs. heart rate in comparison to patients in the SOC group (Figure 3A, group difference P = 0.018) or in comparison to their baseline slope (Figure 3B, P < 0.001 for follow-up vs. baseline). Furthermore, the slope improved in patients assigned to FCM (CCI change per 10 b.p.m. +0.073, P < 0.001) but not in patients assigned to SOC (Figure 3C, CCI change per 10 b.p.m. −0.008, P = 0.714). The change in CCI was correlated with the improvement in LVEF (Supplementary material online, Table S4).
Figure 3.
Effect on the cardiac force-frequency relationship. (A) Difference in cardiac contractility index slope between ferric carboxymaltose and standard of care at follow-up. (B) Cardiac contractility index slope at baseline and follow-up in the ferric carboxymaltose group. (C) Cardiac contractility index slope at baseline and follow-up in the standard of care group. Slope evaluation was analysed using linear mixed models as described in the statistics section. FCM, ferric carboxymaltose; CCI, cardiac contractility index; CI, confidence interval; SOC, standard of care.
Tertiary endpoints and safety
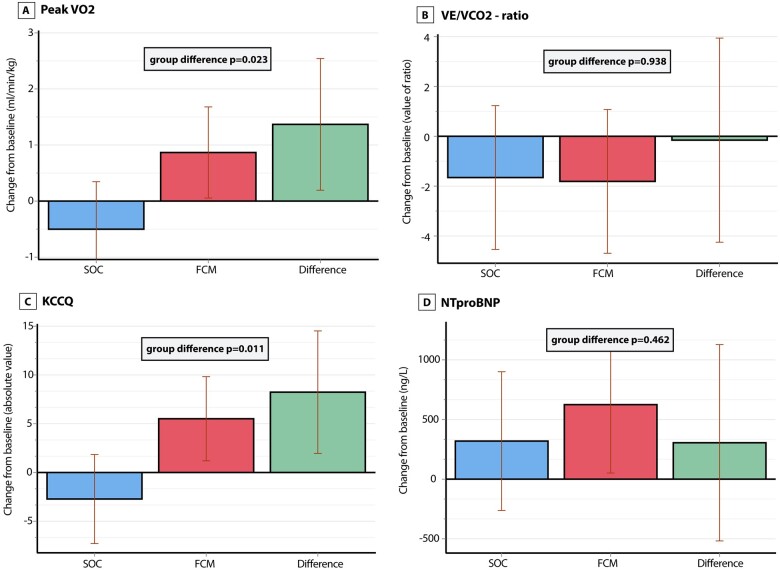
Figure 4 illustrates the change in tertiary endpoints between the two treatment groups. At baseline, peak VO2 was similar in both groups. After 3 months, the LSM change in peak VO2 in the FCM group (+0.87 mL/kg/min, 95% CI 0.05; 1.68) was significantly higher than in the SOC group (−0.50 mL/kg/min, 95% CI −1.35; +0.35, P = 0.023). The VE/VCO2 slope was similar at baseline as was the LSM change after 3 months between the two treatment groups (P = 0.938). The KCCQ score was similar between both treatment groups at baseline, while the LSM change in absolute value of the KCCQ score was higher in the FCM group (+5.51, 95% CI +1.20; +9.82) than in the SOC group (−2.72, 95% CI −7.29; 1.84, P = 0.011). The correlation between change in KCCQ score and CCI is reported in Supplementary material online, Table S4. The LSM change in log transformed NT-proBNP between the SOC and FCM groups was not significantly different (P = 0.462). FCM was well tolerated in comparison to SOC and was not associated with a higher risk for adverse events (Table 2). Dose and percentage of patients taking neurohormonal blockers did not change during the study.
Figure 4.
Change in tertiary endpoints. Change at 3 months in (A) peak VO2, (B) VE/VCO2 ratio, (C) Kansas City Cardiomyopathy Questionnaire, and (D) N-terminal pro B-type natriuretic peptide. P-values are from an ANCOVA model with correction for baseline values. FCM, ferric carboxymaltose; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; NT-proBNP, N-terminal pro B-type natriuretic peptide; SOC, standard of care; VE/VCO2, slope of minute ventilation/carbon dioxide production; VO2, oxygen consumption.
Table 2.
Death, hospitalization, and adverse events according to treatment assignment
| Parameter | Standard of care (n = 38) | Ferric carboxymaltose (n = 37) | Risk difference (95% CI) | P-value |
|---|---|---|---|---|
| All | ||||
| Death, hospitalization or adverse event | 12 (32%) | 11 (30%) | −0.019 | 0.862 |
| (−0.229, 0.197) | ||||
| Deaths | ||||
| Death | 3 (8%) | 0 (0%) | −0.079 | 0.081 |
| (−0.214, 0.024) | ||||
| Cardiovascular death | 2 (5%) | 0 (0%) | −0.053 | 0.157 |
| (−0.178, 0.047) | ||||
| Heart failure death | 2 (5%) | 0 (0%) | −0.053 | 0.157 |
| (−0.178, 0.047) | ||||
| Hospitalization | ||||
| Cardiovascular hospitalization | 7 (18%) | 4 (11%) | −0.076 | 0.352 |
| (−0.25, 0.094) | ||||
| Heart failure hospitalization | 4 (11%) | 1 (3%) | − 0.078 | 0.174 |
| (−0.022, 0.050) | ||||
| Adverse events | ||||
| Serious adverse event | 0 (0%) | 0 (0%) | NA | NA |
| Adverse events total | 3 (8%) | 7 (19%) | 0.110 | 0.160 |
| (−0.052, 0.282) | ||||
| Adverse event general | 1 (3%) | 2 (5%) | 0.028 | 0.540 |
| (−0.093, 0.158) | ||||
| Adverse event cutaneous | 1 (3%) | − | 0.028 | 0.540 |
| (−0.093, 0.158) | ||||
| Adverse event neurological | 0 (0%) | 0 (0%) | NA | NA |
| Adverse event gastro-intestinal | 1 (3%) | 3 (8%) | 0.055 | 0.291 |
| (−0.067, 0.197) | ||||
| Adverse event cardiac | 0 (0%) | 1 (3%) | 0.027 | 0.308 |
| (−0.069, 0.145) | ||||
P-values are from Fisher’s exact test/chi-square test. Negative estimate of risk difference indicates lower risk in the ferric carboxymaltose vs. standard of care group. None of the risk differences reached statistical significance as indicated by the 95% CI.
CI, confidence interval; NA, not available.
Subgroup analysis
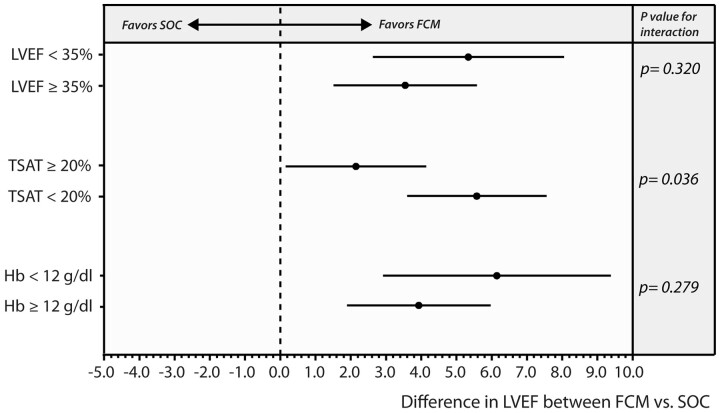
Figure 5 illustrates the results of the three predefined subgroup analyses. In all three subgroups, FCM resulted in a significantly higher (all P < 0.05) improvement in LVEF in comparison to SOC. Testing for interaction showed that the subgroup of patients with TSAT ≥20% had less improvement in LVEF than patients with a TSAT of <20% (P-value for interaction = 0.0362); however, both subgroups still demonstrated an improvement in LVEF in comparison to the SOC subgroup (LSM difference FCM vs. SOC, TSAT ≥20%: 2.15 [0.15–4.14], P = 0.0363; TSAT <20%: 5.58 [3.60–7.56], P < 0.001). No heterogeneity in the treatment effect of FCM was found for patients with vs. without a low haemoglobin (P-value for interaction = 0.279) or low vs. higher baseline LVEF (P-value for interaction = 0.320). Similarly, using LVEF 40% as cut-off to define high vs. low LVEF did not indicate significant interaction in the treatment effect of FCM (P-value for interaction = 0.2996).
Figure 5.
Forest plot of subgroup analysis for the effect of ferric carboxymaltose on the primary endpoint. FCM, ferric carboxymaltose; Hb, haemoglobin; LVEF, left ventricular ejection fraction; SOC, standard of care; TSAT, transferrin saturation.
Discussion
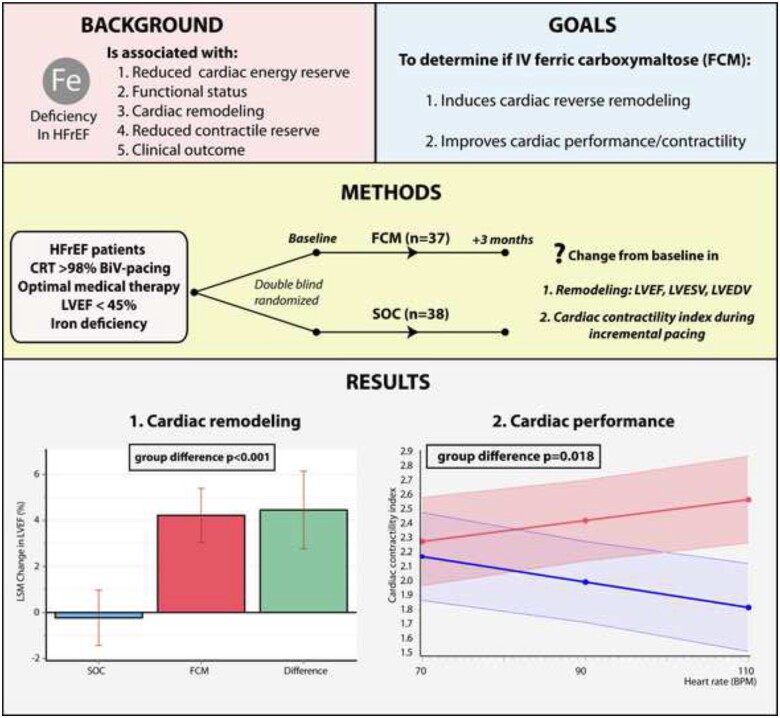
This prospective, randomized double-blind trial offers novel important information regarding the effect of FCM on cardiac function and structure in HFrEF patients with persistently reduced LVEF despite optimal medical and device management. The main findings are that intravenous iron repletion (i) results in an improvement of LVEF and LVESV, (ii) results in force–frequency amplification and therefore at least partially restores cardiac contractile performance, and (iii) improves functional status (KCCQ) and maximal exercise capacity measured by peak VO2 (Graphical abstract).
Overview of study design and main findings.
Iron is an essential co-factor for anti-oxidative enzymes and is part of the iron-sulfur clusters of the first three elements of the electron transport chain in mitochondria.4,22 As such, iron deficiency is implicated as an important comorbidity in the pathophysiology of heart failure.4,22–27 Several trials have shown the beneficial effect of FCM on functional status, maximal exercise capacity, and risk for heart failure readmissions.13,14,16,18 However, little data are available about the effect on cardiac reverse remodelling of the guideline-recommended intravenous iron formulation, being FCM.12 Animal models indicate that iron deficiency is associated with progressive cardiac remodelling.6,22 In addition, previous studies have suggested that treatment of iron deficiency with iron sucrose is associated with an improvement in LVEF, but these studies predate the use of contemporary heart failure therapies and used a different iron formulation (iron sucrose), currently not endorsed by the European Society of Cardiology heart failure guidelines.28,29 More recently, an elegant magnetic resonance imaging study illustrated that in HFrEF patients with iron deficiency, treatment with FCM is capable of replenishing intra-myocardial iron content as measured by T2* mapping cardiac magnetic resonance sequences.30 Interestingly, changes in myocardial iron content (T2*) were correlated with changes in LVEF, indicating that myocardial iron repletion might improve left ventricular function.30,31 In HFrEF patients receiving CRT, several studies have illustrated that the presence of iron deficiency at the time of CRT implant is associated with less left ventricular reverse remodelling in comparison to CRT recipients without iron deficiency.10,11 However, such observational studies are not capable of answering the question of causation beyond the observed correlations between reduced remodelling and presence of iron deficiency. Persistent left ventricular dysfunction and symptomatic disease is present in up to 30% of CRT recipients.32 We are the first to demonstrate in this double-blind trial that treatment of iron deficiency with FCM results in an improvement of LVEF (LSM change +4.22%), reduces LVESV, and improves functional status as measured by the KCCQ and exercise capacity measured by peak VO2. By nature, randomized controlled trials are capable of answering the question of causality, hereby showing that FCM offers incremental reverse remodelling. Importantly, although all patients were on guideline-recommended optimal medical treatment consisting of neurohormonal blockers—including 50% on angiotensin receptor–neprilysin inhibitor—and CRT, they remained to have symptomatic severe heart failure with cardiac remodelling as defined by their NYHA class, LVEF, left ventricular volumes, and NT-proBNP. Because we selected heart failure patients with a persistently reduced LVEF after CRT implant, we are not able to answer the question whether iron deficiency should be treated before CRT implant. Nevertheless, iron-deficient heart failure patients with a guideline indication for CRT (by nature of selection) also have a guideline indication for treatment with FCM.12 Although we specifically targeted an HFrEF population demonstrating persistent heart failure progression despite current guideline-recommended device and medical therapy, there is no reason to believe that FCM would not have similar effects in other iron-deficient HFrEF patients.
Careful elucidation of the beneficial effects of iron repletion in improving myocardial contractile performance may hold an important key to understanding the role of iron deficiency in the failing heart. By performing force–frequency relationship assessment, we illustrate the impact of reversing iron deficiency in improvement of myocardial performance.20 Pre-clinical studies have shown that incubation of cardiomyocytes with an iron chelator results in a 74% reduction in ATP content leading to diminished cardiomyocyte shortening.4 Haddad et al.6 demonstrated that this energetic crisis induced by iron deficiency becomes more relevant at higher heart rates. We have previously shown that HFrEF patients with iron deficiency have a diminished contractile reserve during exercise.9 We now demonstrate that FCM is capable of improving cardiac contractile performance. Indeed, treatment with FCM did reverse the negative down-sloping relation between non-invasive CCI and heart rate towards a positive upsloping relation.
In line with previous trials of HFrEF patients with iron deficiency, FCM also improved functional status as measured by the KCCQ.14,18 Similarly, in line with the EFFECT-HF (Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Iron Deficiency and Chronic Heart Failure) trial,16 we show that FCM improves maximal exercise capacity as measured by peak VO2. While the EFFECT-HF trial mainly demonstrated a prevention in further decline of peak VO2 in comparison to the control group, we document a positive improvement in peak VO2 (+0.87 mL/kg/min, 95% CI 0.05; 1.68). A potential reason might be the fact that all our patients had a CRT device and that rate adaptive pacing with a high upper rate was provided if necessary, thereby overcoming chronotropic incompetence.
Finally, we did not find a statistical interaction between the treatment effect of FCM and the presence of anaemia or a lower or higher baseline LVEF. Although guidelines position FCM for the treatment of symptomatic iron-deficient HFrE, defined as LVEF <40%, our study is consistent with the CONFIRM-HF and AFFIRM-AHF trials in showing no significant interaction if LVEF was above 40%, suggesting that FCM works equally well in patients with an LVEF 40–45%.13,18 Similarly to the meta-analysis of Anker et al.,33 we did observe significant interaction between the treatment effect of FCM and the presence of a high TSAT ≥20% (also referred to as patients with isolated hypoferritinaemia). While these patients still had an improvement in LVEF, the effect was less pronounced than if TSAT was <20%. Nevertheless, the recent AFFIRM-AHF trial (the largest published trial to date with FCM in heart failure) did not observe a treatment interaction with low or high TSAT, showing that the effect of FCM on endpoints such as heart failure hospitalization is perhaps more than just an improvement in LVEF or cardiac contractility but also relates to the effects of FCM on peripheral muscles and global heart failure status.13,24,27
Limitations
Several limitations should be acknowledged. First, we used 3D echocardiography and not magnetic resonance imaging to determine LVEF and left ventricular volumes because all patients had a CRT. Nevertheless, 3D echocardiography has shown excellent correlation with magnetic resonance measurements of LVEF and left ventricular volumes. The semi-automated algorithms used offer high reproducibility and low test–retest variability. Studies have shown that the source of the largest variability between 3D echocardiography and magnetic resonance imaging stems from poor image quality.34 Therefore, to optimize the accuracy of our analysis, poor two-dimensional image quality on screening evaluation was an exclusion criteria. Second, we did not invasively measure cardiac contractility during incremental pacing. Third, we studied CRT patients to determine changes in the force-frequency relationship; therefore, results might not apply to all HFrEF patients. Fourth, the sample size is relatively small compared to other trials with FCM. Fifth, due to the COVID-19 pandemic, no patients were included from the Jessa Hospital. As such, the generated results might not be applicable to broader range of practice settings.
Conclusion
Ferric carboxymaltose improves cardiac function as measured by LVEF, LVESV, and the force–frequency relationship in HFrEF patients with persistently reduced LVEF despite optimal medical and device management.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
P.M. is supported by a doctoral fellowship by the Research Foundation—Flanders (FWO, grant-number: 1127917N). P.M., J.D., and W.M. are researchers for the Limburg Clinical Research Program (LCRP) UHasselt-ZOL-Jessa, supported by the foundation Limburg Sterk Merk (LSM), Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital. The IRON-CRT trial was supported by an unrestricted research grant from Vifor Pharma.
Conflict of interest: P.M. has received consultancy fees, speaker fees, travel grants, and an unrestricted research grant from Vifor Pharma. M.D. declares no conflict of interest related to this work. J.D. has received travel grants from Servier, Abbott, and Boehringer-Ingelheim. P.N. has received travel grants from Vifor pharma and Novartis. L.H. declares no conflict of interest related to this work. P.D. declares no conflict of interest related to this work. P.V. declares no conflict of interest related to this work. L.B. declares no conflict of interest related to this work. W.H.W.T. has received research grants from the National Institutes of Health and received consultancy or speaker fees from Sequana Medical AG, Owkin Inc, PreCardia Inc, Relypsa Inc, CardioRx Inc, ABIM, and Springer Nature. W.M. declares no conflict of interest related to this work.
Supplementary Material
References
- 1. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010;31:1872—1880. [DOI] [PubMed] [Google Scholar]
- 2. Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013;165:575—582. [DOI] [PubMed] [Google Scholar]
- 3. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol 2018;73:115—123. [DOI] [PubMed] [Google Scholar]
- 4. Hoes MF, Grote BN, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018;20:910—919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martens P, Dupont M, Mullens W. Cardiac iron deficiency-how to refuel the engine out of fuel. Eur J Heart Fail 2018;20:920—922. [DOI] [PubMed] [Google Scholar]
- 6. Haddad S, Wang Y, Galy B, Korf-Klingebiel M, Hirsch V, Baru AM, Rostami F, Reboll MR, Heineke J, Flogel U, Groos S, Renner A, Toischer K, Zimmermann F, Engeli S, Jordan J, Bauersachs J, Hentze MW, Wollert KC, Kempf T. Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J 2017;38:362—372. [DOI] [PubMed] [Google Scholar]
- 7. Martens P, Verbrugge FH, Nijst P, Dupont M, Mullens W. Limited contractile reserve contributes to poor peak exercise capacity in iron-deficient heart failure. Eur J Heart Fail 2018;20:806—808. [DOI] [PubMed] [Google Scholar]
- 8. Mullens W, Bartunek J, Tang WH, Delrue L, Herbots L, Willems R, de BB, Goethals M, Verstreken S, Vanderheyden M. Early and late effects of cardiac resynchronization therapy on force-frequency relation and contractility regulating gene expression in heart failure patients. Heart Rhythm 2008;5:52—59. [DOI] [PubMed] [Google Scholar]
- 9. Martens P, Dupont M, Dauw J, Somers F, Herbots L, Timmermans P, Verwerft J, Mullens W. Rationale and design of the IRON-CRT trial: effect of intravenous ferric carboxymaltose on reverse remodelling following cardiac resynchronization therapy. ESC Heart Fail 2019;6:1208—1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lacour P, Dang PL, Morris DA, Parwani AS, Doehner W, Schuessler F, Hohendanner F, Heinzel FR, Stroux A, Tschoepe C, Haverkamp W, Boldt LH, Pieske B, Blaschke F. The effect of iron deficiency on cardiac resynchronization therapy: results from the RIDE-CRT study. ESC Heart Fail 2020;7:1072—1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martens P, Verbrugge F, Nijst P, Dupont M, Tang WH, Mullens W. Impact of iron deficiency on response to and remodeling after cardiac resynchronization therapy. Am J Cardiol 2017;119:65—70. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891—975. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Kirwan B-A, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual-Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin-Colet J, von Haehling S, Cohen-Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA, Azize G, Fernandez A, Zapata GO, Garcia Pacho P, Glenny A, Ferre Pacora F, Parody ML, Bono J, Beltrano C, Hershson A, Vita N, Luquez HA, Cestari HG, Fernandez H, Prado A, Berli M, García Durán R, Thierer J, Diez M, Lobo Marquez L, Borelli RR, Hominal MÁ, Metra M, Ameri P, Agostoni P, Salvioni A, Fattore L, Gronda E, Ghio S, Turrini F, Uguccioni M, Di Biase M, Piepoli M, Savonitto S, Mortara A, Terrosu P, Fucili A, Boriani G, Midi P, Passamonti E, Cosmi F, van der Meer P, Van Bergen P, van de Wetering M, Al-Windy NYY, Tanis W, Meijs M, Groutars RGEJ, The HKS, Kietselaer B, van Kesteren HAM, Beelen DPW, Heymeriks J, Van de Wal R, Schaap J, Emans M, Westendorp P, Nierop PR, Nijmeijer R, Manintveld OC, Dorobantu M, Darabantiu DA, Zdrenghea D, Toader DM, Petrescu L, Militaru C, Crisu D, Tomescu MC, Stanciulescu G, Rodica Dan A, Iosipescu LC, Serban DL, Drozdz J, Szachniewicz J, Bronisz M, Tycińska A, Wozakowska-Kaplon B, Mirek-Bryniarska E, Gruchała M, Nessler J, Straburzyńska-Migaj E, Mizia-Stec K, Szelemej R, Gil R, Gąsior M, Gotsman I, Halabi M, Shochat M, Shechter M, Witzling V, Zukermann R, Arbel Y, Flugelman M, Ben-Gal T, Zvi V, Kinany W, Weinstein JM, Atar S, Goland S, Milicic D, Horvat D, Tušek S, Udovicic M, Šutalo K, Samodol A, Pesek K, Artuković M, Ružić A, Šikić J, McDonagh T, Trevelyan J, Wong Y-K, Gorog D, Ray R, Pettit S, Sharma S, Kabir A, Hamdan H, Tilling L, Baracioli L, Nigro Maia L, Dutra O, Reis G, Pimentel Filho P, Saraiva JF, Kormann A, dos Santos FR, Bodanese L, Almeida D, Precoma D, Rassi S, Costa F, Kabbani S, Abdelbaki K, Abdallah C, Arnaout MS, Azar R, Chaaban S, Raed O, Kiwan G, Hassouna B, Bardaji A, Zamorano J, del Prado S, Gonzalez Juanatey JR, Ga Bosa Ojeda FI, Gomez Bueno M, Molina BD, Pascual Figal DA, Sim D, Yeo TJ, Loh SY, Soon D, Ohlsson M, Smith JG, Gerward S, Khintibidze I, Lominadze Z, Chapidze G, Emukhvari N, Khabeishvili G, Chumburidze V, Paposhvili K, Shaburishvili T, Khabeishvili G, Parhomenko O, Kraiz I, Koval O, Zolotaikina V, Malynovsky Y, Vakaliuk I, Rudenko L, Tseluyko V, Stanislavchuk M. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet 2020;396:1895—1904. [DOI] [PubMed] [Google Scholar]
- 14. Anker SD, Comin CJ, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, V, Eisenhart RB, Pocock SJ, Poole-Wilson PA, Ponikowski P; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436—2448. [DOI] [PubMed] [Google Scholar]
- 15. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh TA, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Rationale and design of the CONFIRM-HF study: a double-blind, randomized, placebo-controlled study to assess the effects of intravenous ferric carboxymaltose on functional capacity in patients with chronic heart failure and iron deficiency. ESC Heart Fail 2014;1:52—58. [DOI] [PubMed] [Google Scholar]
- 16. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Bohm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen-Solal A; EFFECT-HF Investigators. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017;136:1374—1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;63:834—840. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD; for the CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J 2015;36:657—668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten Cate FJ, Vannan MA, Zamorano JL, Zoghbi WA; European Association of Echocardiography. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012;13:1—46. [DOI] [PubMed] [Google Scholar]
- 20. Bombardini T, Correia MJ, Cicerone C, Agricola E, Ripoli A, Picano E. Force-frequency relationship in the echocardiography laboratory: a noninvasive assessment of Bowditch treppe? J Am Soc Echocardiogr 2003;16:646—655. [DOI] [PubMed] [Google Scholar]
- 21. Toblli JE, Lombrana A, Duarte P, Di GF. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007;50:1657—1665. [DOI] [PubMed] [Google Scholar]
- 22. Dong F, Zhang X, Culver B, Chew HG Jr., Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond) 2005;109:277—286. [DOI] [PubMed] [Google Scholar]
- 23. Celsing F, Blomstrand E, Werner B, Pihlstedt P, Ekblom B. Effects of iron deficiency on endurance and muscle enzyme activity in man. Med Sci Sports Exerc 1986;18:156—161. [PubMed] [Google Scholar]
- 24. Charles-Edwards G, Amaral N, Sleigh A, Ayis S, Catibog N, McDonagh T, Monaghan M, Amin-Youssef G, Kemp GJ, Shah AM, Okonko DO. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency. Circulation 2019;139:2386—2398. [DOI] [PubMed] [Google Scholar]
- 25. Jankowska EA, Ponikowski P. Molecular changes in myocardium in the course of anemia or iron deficiency. Heart Fail Clin 2010;6:295—304. [DOI] [PubMed] [Google Scholar]
- 26. Melenovsky V, Petrak J, Mracek T, Benes J, Borlaug BA, Nuskova H, Pluhacek T, Spatenka J, Kovalcikova J, Drahota Z, Kautzner J, Pirk J, Houstek J. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail 2017;19:522—530. [DOI] [PubMed] [Google Scholar]
- 27. Melenovsky V, Hlavata K, Sedivy P, Dezortova M, Borlaug BA, Petrak J, Kautzner J, Hajek M. Skeletal muscle abnormalities and iron deficiency in chronic heart failure. An exercise (31)P magnetic resonance spectroscopy study of calf muscle. Circ Heart Fail 2018;11:e004800. [DOI] [PubMed] [Google Scholar]
- 28. Bolger AP, Bartlett FR, Penston HS, O’Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol 2006;48:1225—1227. [DOI] [PubMed] [Google Scholar]
- 29. Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol 2008;21:236—242. [PubMed] [Google Scholar]
- 30. Nunez J, Minana G, Cardells I, Palau P, Llacer P, Facila L, Almenar L, Lopez-Lereu MP, Monmeneu JV, Amiguet M, Gonzalez J, Serrano A, Montagud V, Lopez-Vilella R, Valero E, Garcia-Blas S, Bodi V, Espriella-Juan R, Lupon J, Navarro J, Gorriz JL, Sanchis J, Chorro FJ, Comin-Colet J, Bayes-Genis A. Noninvasive imaging estimation of myocardial iron repletion following administration of intravenous iron: the myocardial-IRON trial. J Am Heart Assoc 2020;9:e014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Núñez J, Monmeneu JV, Mollar A, Núñez E, Bodí V, Miñana G, García-Blas S, Santas E, Agüero J, Chorro FJ, Sanchis J, López-Lereu MP. Left ventricular ejection fraction recovery in patients with heart failure treated with intravenous iron: a pilot study. ESC Heart Fail 2016;3:293—298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mullens W, Auricchio A, Martens P, Witte K, Cowie MR, Delgado V, Dickstein K, Linde C, Vernooy K, Leyva F, Bauersachs J, Israel CW, Lund L, Donal E, Boriani G, Jaarsma T, Berruezo A, Traykov V, Yousef Z, Kalarus Z, Nielsen JC, Steffel J, Vardas P, Coats A, Seferovic P, Edvardsen T, Heidbuchel H, Ruschitzka F, Leclercq C. Optimized Implementation of cardiac resynchronization therapy: a call for action for referral and optimization of care: a joint position statement from the Heart Failure Association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology. Eur J Heart Fail 2020;22:2349—2369. [DOI] [PubMed] [Google Scholar]
- 33. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, Luscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018;20:125—133. [DOI] [PubMed] [Google Scholar]
- 34. Tsang W, Salgo IS, Medvedofsky D, Takeuchi M, Prater D, Weinert L, Yamat M, Mor-Avi V, Patel AR, Lang RM. Transthoracic 3D echocardiographic left heart chamber quantification using an automated adaptive analytics algorithm. JACC Cardiovasc Imaging 2016;9:769—782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.