Abstract
Aims
Recent clinical trials indicate that sodium-glucose cotransporter 2 (SGLT2) inhibitors improve cardiovascular outcomes in heart failure patients, but the underlying mechanisms remain unknown. We explored the direct effects of canagliflozin, an SGLT2 inhibitor with mild SGLT1 inhibitory effects, on myocardial redox signalling in humans.
Methods and results
Study 1 included 364 patients undergoing cardiac surgery. Right atrial appendage biopsies were harvested to quantify superoxide () sources and the expression of inflammation, fibrosis, and myocardial stretch genes. In Study 2, atrial tissue from 51 patients was used ex vivo to study the direct effects of canagliflozin on NADPH oxidase activity and nitric oxide synthase (NOS) uncoupling. Differentiated H9C2 and primary human cardiomyocytes (hCM) were used to further characterize the underlying mechanisms (Study 3). SGLT1 was abundantly expressed in human atrial tissue and hCM, contrary to SGLT2. Myocardial SGLT1 expression was positively associated with production and pro-fibrotic, pro-inflammatory, and wall stretch gene expression. Canagliflozin reduced NADPH oxidase activity via AMP kinase (AMPK)/Rac1signalling and improved NOS coupling via increased tetrahydrobiopterin bioavailability ex vivo and in vitro. These were attenuated by knocking down SGLT1 in hCM. Canagliflozin had striking ex vivo transcriptomic effects on myocardial redox signalling, suppressing apoptotic and inflammatory pathways in hCM.
Conclusions
We demonstrate for the first time that canagliflozin suppresses myocardial NADPH oxidase activity and improves NOS coupling via SGLT1/AMPK/Rac1 signalling, leading to global anti-inflammatory and anti-apoptotic effects in the human myocardium. These findings reveal a novel mechanism contributing to the beneficial cardiac effects of canagliflozin.
Keywords: SGLT2 inhibitor, SGLT1, Myocardial redox state, NADPH oxidase activity, NOS coupling, AMPK
Graphical Abstract
See page 4961 for the editorial comment for this article ‘Canagliflozin and myocardial oxidative stress: SGLT1 inhibition takes centre stage’, by G.S. Schiattarella and D. Bode, https://doi.org/10.1093/eurheartj/ehab519.
Translational perspective
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel calls of anti-diabetic drugs that have recently emerged as cardioprotective agents in heart failure, even in the absence of diabetes. Despite their clinical benefit, the underlying effects on the human myocardium remain largely unexplored. In this work, we demonstrate a novel, SGLT1-mediated effect of canagliflozin, a clinically used SGLT2 inhibitor, on myocardial biology affecting its redox state, inflammation, fibrosis, and apoptosis in humans. Our work suggests novel cellular mechanisms underlying the observed clinical benefits of SGLT2 inhibitors; further characterization of the mechanistic effects of the SGLT2 inhibitor drug class on the human myocardium will broaden their use and indications in cardiovascular disease more efficiently.
Introduction
Sodium-glucose cotransporter 2 (SGLT2) inhibitors comprise an anti-hyperglycaemic drug class regulating plasma glucose by inhibiting glucose reabsorption through SGLT2 blockade in the proximal renal tubules.1 Clinically available SGLT2 inhibitors also display variable affinity to SGLT1.2 While SGLT2 is the main responsible for glucose reabsorption from the glomerular filtrate, SGLT1, which is essential for the fast uptake of glucose and galactose in the intestine, plays a minor role in renal reabsorption. These two sodium-glucose cotransporters have different affinity and capacity for glucose transport and diverse expression patterns in the renal proximal tubule as well as in other organs.3 The cardioprotective effects of SGLT2 inhibitors have been demonstrated in recent clinical trials (EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58) in diabetic4–7 as well as non-diabetic patients with heart failure (DAPA-HF).8 , 9 Accordingly, the 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases recommend empagliflozin, canagliflozin, or dapagliflozin in patients with type 2 diabetes mellitus and cardiovascular disease, or at high/very high cardiovascular risk, to reduce cardiovascular events, as well as in diabetic patients to lower the risk of heart failure hospitalization.10 However, the mechanisms behind these effects remain unclear.
Limited evidence suggests that SGLT2 inhibitors have direct cardioprotective effects that extend beyond their systemic glucose-lowering function. Empagliflozin attenuates cardiac fibrosis in diabetic mice11 and preserves myocardial function in a mouse model of pressure overload-induced heart failure.12 Canagliflozin activates AMP kinase (AMPK), whose cardioprotective effects are well established, by inhibiting mitochondrial function and increasing cellular AMP levels in vitro. 13 However, several aspects of the direct effects of SGLT2 inhibition on the human heart remain unclear.14
Sustained oxidative stress is central to the pathogenesis of cardiac diseases, such as heart failure15 and atrial fibrillation.16 , 17 NADPH oxidases (Nox) are major sources of superoxide anions () in the human heart, and the activity of Nox1 and Nox2 isoforms is dependent on activation and membrane translocation of the GTPase Rac1 and the presence of p47phox.18 On the other hand, nitric oxide synthases (NOSs) are redox-related enzymes responsible for nitric oxide (NO) synthesis in the human heart.19 In disease states such as long-standing persistent atrial fibrillation, myocardial NOS appears to be uncoupled, therefore producing rather than NO as a result of oxidative depletion of its co-factor tetrahydrobiopterin (BH4).20 Similar NOS uncoupling is also observed in various diseases like diabetes and hypertension.21 However, despite the importance of myocardial redox state regulation in cardiac biology, the putative antioxidant effects on the human heart of different SGLT2 inhibitors have not been well investigated so far.14
In this study, we first explored the expression profiles of SGLT2 and SGLT1 in the human heart to determine the molecular target of direct SGLT1/2 inhibition in the human atrial myocardium. Secondly, we evaluated the direct effects of the SGLT2-specific inhibitor empagliflozin and the dual SGLT1/2 inhibitor canagliflozin on myocardial redox signalling in the human heart.
Methods
Study population
The study population consisted of patients undergoing cardiac surgery, all of whom were under the Oxford Heart Vessels and Fat (ox-HVF) programme (www.oxhvf.com) at Oxford University Hospitals NHS Foundation Trust, UK. The patient’s demographics, indication for surgery, and medication are presented in Table 1.
Table 1.
Demographic characteristics of the study participants
| Clinical study (Study 1) | Ex-vivo study (Study 2) | |
|---|---|---|
| Patients, n | 364 | 51 |
| Age (years) | 69 [60–75] | 66 [57.25–74.00] |
| Male sex | 301 (82.70) | 41 (80.40) |
| Hypertension | 265 (72.80) | 36 (70.60) |
| Hyperlipidaemia | 282 (77.50) | 34 (66.70) |
| T2DM | 69 (19.00) | 9 (17.65) |
| Smoking | ||
| Active | 193 (53) | 24 (47.10) |
| Past | 30 (8.2) | 3 (5.90) |
| CrCl (mL/min/1.73 m2) | 88.14 [25.11–108.99] | 97.93 [71.96–116.70] |
| BMI (kg/m2) | 27.59 [25.10–30.33] | 29.26 [26.03–32.44] |
| CABG | 279 (76.65) | 43 (84.31) |
| AVR due to AR/AS | 58 (15.93) | 6 (11.77) |
| MVR due to MR/MS | 27 (7.42) | 2 (3.92) |
| NYHA class | ||
| I | 133 (36.54) | 17 (33.34) |
| II | 154 (42.31) | 18 (35.29) |
| III | 64 (17.58) | 14 (27.45) |
| IV | 13 (3.57) | 2 (3.92) |
| Medication | ||
| Antiplatelet | 280 (76.90) | 36 (70.59) |
| ACEi/ARBs | 210 (57.70) | 28 (54.90) |
| Statins | 298 (81.90) | 34 (66.70) |
| β-blocker | 235 (64.60) | 21 (41.20) |
| CCB | 85 (23.40) | 12 (23.50) |
| Insulin | 24 (6.60) | 6 (11.80) |
| Oral anti-diabetics | 47 (12.90) | 4 (7.80) |
Values are presented as n (%) or median [25th–75th percentile].
ACEi, angiotensin-converting enzyme inhibitor; AR, aortic regurgitation; ARB, angiotensin receptor blocker; AS, aortic stenosis; AVR, aortic valve replacement; BMI, body mass index; CABG, coronary artery bypass grafting; CCB, calcium channel blocker; CrCl, creatinine clearance; MR, mitral regurgitation; MS, mitral stenosis; MVR, mitral valve replacement; T2DM, type 2 diabetes mellitus.
Study 1 was used for cohort-wide associations and consisted of 364 patients. Myocardial biopsies were obtained intraoperatively from the right atrial appendage cannulation site, transferred in ice-cold buffer, and processed for quantification or gene expression studies as described.22 The exclusion criteria were any active inflammatory disease (e.g. autoimmune disease in active/relapse phase) or active infectious disease, advanced liver failure (known or suspected cirrhosis) or end-stage renal disease (estimated glomerular filtration rate <15 mL/min), active malignancy (untreated or under treatment) and systemic use of nonsteroidal anti-inflammatory drugs (i.e. cyclooxygenase-2 inhibitors) or antioxidant vitamins. As chronic obstructive pulmonary disease (COPD) has not been previously associated with changes in myocardial oxidative stress, COPD patients were not excluded if they were not receiving systemic anti-inflammatory treatments.
Study 2 included patient samples used for mechanistic ex vivo experiments and consisted of 51 prospectively recruited patients undergoing cardiac surgery with the same exclusion criteria as above. Atrial myocardium specimens were collected during surgery, transferred to the lab, and used for ex vivo experiments as described.22
Hyperlipidaemia and hypertension were defined according to the current European Society Cardiology guidelines.23 , 24 Diabetes mellitus was defined according to the American Diabetes Association guidelines.25 Study protocols were in agreement with the Declaration of Helsinki and all participants had provided written informed consent. The collection of human ventricle biopsy was approved by the Istituto Europeo di Oncologia and Centro Cardiologico IRCCS—Ethics Committee (R1020/19-94 CCM1072). Participant demographic characteristics are reported in Table 1.
Cell culture models
The effects of SGLT1/2 on cardiomyocytes were first screened in the rat cardiac myocyte-derived cell line, H9c2, and the key findings were replicated in human cardiomyocytes (hCM, PromoCell, Heidelberg, Germany). H9c2 cells were differentiated to cardiac myocytes in Dulbecco’s Modified Eagle Medium (glucose 5.5 mM, Sigma-Aldrich, cat. number D6046) supplemented with 1% horse serum (Sigma-Aldrich). Primary hCM isolated from adult heart ventricles were purchased and differentiated to cardiac myocytes in dedicated Myocyte Growth Medium (glucose 5.5 mM) with SupplementMix (PromoCell) for 60 days after reaching 100% confluence. Troponin I expression and myotube formation were used to confirm efficient cardiomyocyte differentiation (Supplementary material online, Figure S1).
Both hCM and H9c2 cells were cultured in a high-glucose medium (25 mM) for 72 h before in vitro incubations to mimic the human ex vivo experimental environment and the in vivo diabetes environment, as 25 mM is the most prevalent concentration of glucose used in the literature to mimic diabetic hyperglycaemia in H9c2 cells.26 In selected experiments, glucose was replaced by mannitol 25 mM to study the role of the canagliflozin-induced glucose transfer. Cells were treated with canagliflozin 10 μmol/L (Cayman) ± Compound C (CC, an AMPK inhibitor, 10 μmol/L) or carrier (DMSO) for 60 min or 24 h and used for chemiluminescence experiments, gene expression studies and downstream signalling experiments. The specificity of CC as an AMPK inhibitor was confirmed by tracking downstream phosphorylation of its validated surrogate target, acetyl-CoA carboxylase (ACC).
Human tissue harvesting
Human myocardial biopsies were processed as described (see Supplementary material online, Data supplement) .22
Superoxide quantification
production was measured in human atrial myocardium homogenates and cell lysates using lucigenin (5 μmol/L)-enhanced chemiluminescence evaluating NADPH oxidase activity and NOS coupling status as described (see Supplementary material online, Data supplement).17 , 22 , 27
RNA isolation and quantitative real time-polymerase chain reaction
RNA was purified in a semi-automated way and processed for cDNA generation and gene expression studies as described (see Supplementary material online, Data supplement).28
Western blotting
Western immunoblotting was performed on myocardium homogenates and cell lysates (see Supplementary material online, Data supplement).
Rac1 activation assay
Rac1 activation was detected in myocardial homogenates and cell lysates using a commercial kit (Cell Signalling, see Supplementary material online, Data supplement).
Evaluation of myocardial Rac1 and p47phox membrane translocation
Membrane translocation of Rac1 and p47phox in human myocardial tissue was estimated by differential centrifugation of myocardial homogenates to isolate membrane proteins as described (see Supplementary material online, Data Supplement).22 , 29
Biopterin measurements
BH4, dihydrobiopterin (BH2), and biopterin levels were determined using high-performance liquid chromatography as described (see Supplementary material online, Data Supplement).27
Oxidative fluorescent microtopography
In situ production was determined in human atrial myocardium cryosections and hCMs with an oxidative fluorescent dye (see Supplementary material online, Data Supplement).22
TUNEL assay
Apoptotic cells in human myocardial sample sections were detected with the in situ terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) method using a commercially available kit (see Supplementary material online, Data Supplement).
JC-10 mitochondrial membrane potential assay
The mitochondrial function of samples derived from human atrial myocardium was analysed by means of a commercially available kit (see Supplementary material online, Data Supplement).
Intracellular ADP and ATP measurement
Adenosine triphosphate/Adenosine diphosphate (ATP/ADP) ratio was quantified in hCM by a commercial kit (see Supplementary material online, Data Supplement).
SGLT1 siRNA transfection studies
SGLT1 siRNA and negative control siRNA (Thermo Fisher Scientific) were used to knock down SGLT1 in hCMs and in differentiated H9c2 cells by lipofectamine RNAiMax and BlockiT Alexa Fluor Red Fluorescent control as a positive transfection control (see Supplementary material online, Data Supplement).
Transcriptome profiling of canagliflozin-treated human cardiomyocytes
Treatments and RNA extraction
Human cardiomyocytes were incubated 24 h with 10 μmol/L canagliflozin or DMSO. RNA was extracted with the RNeasy Micro/Mini kit (Qiagen), and samples were purified with an RNA purification kit (ReliaPrep RNA Clean-up and Concentration System, Promega), achieving 260/280 ratio > 2, 260/230 ratio > 1.8.
Clariom™ S Assay-HT, Human Array Plate
Genome-wide expression profiling was done using the GeneChip© WT PLUS assay kit and processed on the GeneTitan using Human Clariom S Assay-HT 16-Array Plate at the High-Throughput Genomics Wellcome Trust Centre for Human Genetics (Oxford, UK). RNA quality was controlled using Agilent Tape Station. The latest version of Affymetrix Genechip Command Console software for GeneTitan was used for quality check and data analyses.
Statistical analysis
For Study 1, continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Non-normally distributed variables are presented as median [25th–75th percentile], whereas normally distributed variables are presented as mean ± SD. The correlation of two continuous variables was evaluated with Pearson’s r or Spearman’s rank correlation coefficients, as appropriate. Continuous variable comparisons were performed using Student’s t-test or Mann–Whitney U-test (for two groups, as appropriate) or by ANOVA (for >2 groups), followed by Bonferroni correction for multiple comparisons. Power calculations were based on myocardial NADPH oxidase activity and we estimated that with 363 patients we could detect a difference of 0.25 in log(NADPH-stimulated ) between high vs. low myocardial SGLT1 expression with power 0.9 [assuming a standard deviation of 1.04 in log(NADPH-stimulated )].
To test whether the association of myocardial NADPH-stimulated with myocardial SGLT1 expression was independent of other risk factors, we performed multivariable linear regression analysis where myocardial NADPH-stimulated was used as a dependent variable and myocardial SGLT1 expression, age, sex, diabetes, hypertension, hypercholesterolaemia, and NYHA class were used as independent variables. The standardized betas (Bstand) are presented for each covariate.
In order to assess the independent effect of myocardial SGLT1 expression on NADPH-stimulated and Vas2870-inhibitable as well as myocardial TNFα, IL6, ANP, BNP, and COL1A1 expression, each of the aforementioned variables was used as a dependent variable in individual multivariable linear regression analyses, and myocardial SGLT1 expression was used as an independent variable. Age, sex, diabetes, hypertension, smoking (categorized as previous/active smoker vs. never-smoked) and NYHA class (class IV vs. lower classes) were used as covariates.
For Study 2, we estimated that with a minimum of five pairs of samples we would be able to identify a change of log(NADPH-stimulated ) by 0.37 with α = 0.05, power 0.9 and SD for paired response difference of 0.30 upon canagliflozin treatments. Paired comparisons between two groups were performed using Wilcoxon signed-rank tests. When the changes between two paired analyses were compared that was performed using two-way ANOVA with interaction terms as stated in the respective figure legends. All tests were two-sided and alpha (α) was set at 0.05. Statistical analysis was performed using SPSS version 20.0 and R version 3.6.0.
Results
SGLT1/2 expression profile in the human heart
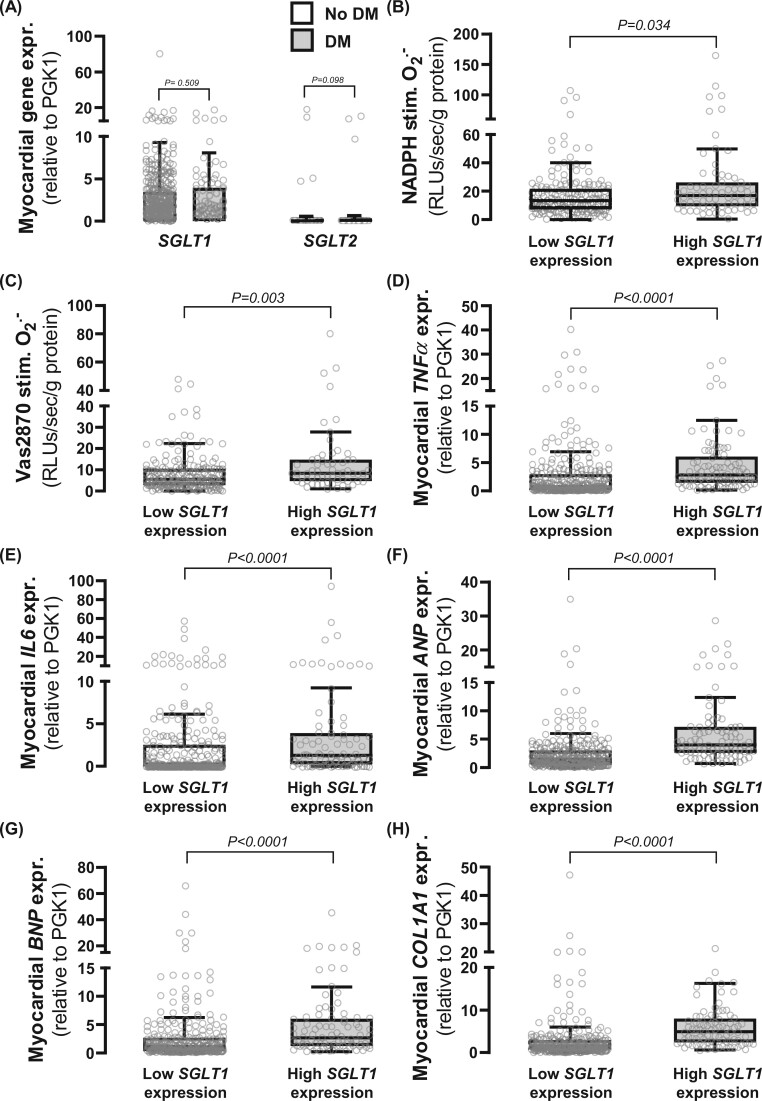
We first evaluated the gene expression levels of SGLT1 and SGLT2 in the human myocardium (Study 1). SGLT2 was undetectable in ∼96% of tested human myocardial samples whilst SGLT1 was abundantly expressed (Figure 1A and Supplementary material online, Figure S2). SGLT1 expression in atrial myocardium was positively associated with NADPH-stimulated (Figure 1B) and Vas2870-inhibitable (Figure 1C) in 244 biopsies. Importantly, myocardial SGLT1 expression was positively related to NADPH oxidase activity, independently of traditional cardiac risk factors (Table 2). In a multivariable linear regression analysis (log-transformed values), SGLT1 expression in the human atrial myocardium was positively associated with basal and NADPH oxidase activity independently of left ventricular ejection fraction and NYHA class (markers of heart-pumping function), suggesting an independent redox association with SGLT1 expression (Supplementary material online, Table S1). A multivariable linear regression analysis also revealed that the association between atrial SGLT1 expression and NADPH-stimulated (stand.β: 0.449, P < 0.001) was independent of medical treatment with hypoglycaemic agents (stand.β: 0.202, P = 0.031), insulin (stand.β: 0.025, P = 0.791), statins (stand.β: −0.007, P = 0.940), angiotensin-converting enzyme inhibitor/angiotensin receptor blockers (stand.β: 0.081, P = 0.388), calcium channel blockers (stand.β: −0.117, P = 0.2), diuretics (0.092, P = 0.301), beta-blockers (stand.β: −0.048, P = 0.603), nitrates (stand.β: −0.026, P = 0.780), and antiplatelet therapy (stand.β: 0.046, P = 0.618).
Figure 1.
Sodium-glucose cotransporter (SGLT)1/2 expression in human atrial myocardium and relations with myocardial redox state and inflammation biomarkers. (A) SGLT1 was abundantly expressed in the human atrial myocardium, contrary to SGLT2. n = 357 [290 non-diabetic patients (no DM); 67 diabetic patients (DM)]. SGLT1 expression was positively correlated with NADPH-stimulated (B) and Vas2870-inhibitable (C) as well as tumour necrosis factor-α (TNFα, D), interleukin-6 (IL6, E), atrial natriuretic peptide (ANP, F), brain natriuretic peptide (BNP, G), and collagen 1A1 (Col1A1, H) expression. P-values by Mann–Whitney U-test for no DM vs. DM (A) and highest quartile of SGLT1 expression vs. rest (B–H). Data are presented as mean ± SD (A) and median [25th–75th percentile] (B–H).
Table 2.
Table Multivariable regression model of myocardial NADPH oxidase activity
| Covariate | Standardized β | Adjusted P-value |
|---|---|---|
| Myocardial SGLT1 expression | 0.339 | 0.001 |
| Age (years) | 0.152 | 0.021 |
| NYHA class | 0.137 | 0.032 |
| Smoking | 0.067 | 0.284 |
| Diabetes | 0.054 | 0.378 |
| Hypertension | 0.022 | 0.737 |
| Sex | −0.052 | 0.401 |
| Hypercholesterolaemia | −0.034 | 0.601 |
SGLT1, sodium-glucose cotransporter 1. Statistically significant values are in bold.
SGLT1 expression in atrial myocardium was also strongly related with markers of myocardial inflammation [tumour necrosis factor-α (TNFα), Figure 1D and interleukin-6 (IL6), Figure 1E], wall stretch [atrial natriuretic peptide (ANP), Figure 1F and brain natriuretic peptide (BNP), Figure 1G], as well as fibrosis [collagen 1A1 (COL1A1) expression, Figure 1H]. We also ran multivariable regression analyses to explore whether SGLT1 gene expression was related to each one of these readouts independently of the baseline demographic characteristics. Indeed, SGLT1 expression was related to myocardial IL6 expression (stand.β: 0.110, P = 0.042), myocardial TNFα expression (stand.β: 0.133, P = 0.014), myocardial ANP expression (stand.β: 0.342, P < 0.001), myocardial BNP expression (stand.β: 0.161, P = 0.003), myocardial COL1A1 expression (stand.β: 0.290, P < 0.001), NADPH-stimulated (stand.β: 0.196, P = 0.003), and Vas2870-delta (stand.β: 0.163, P = 0.014), independently of age, sex, smoking, diabetes, hypertension, and NYHA class.
Direct effect of SGLT inhibitors on human myocardial redox state
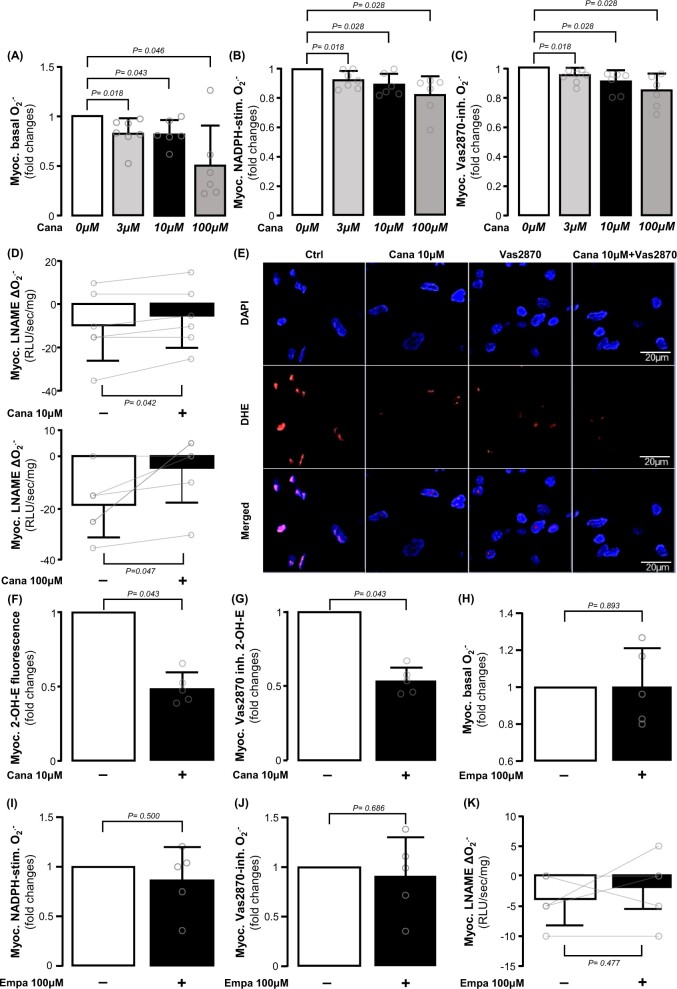
Given the previous associations of myocardial SGLT1 expression with oxidative stress, we explored the direct effects of canagliflozin, an SGLT2 inhibitor with significant affinity to SGLT1,30 on the redox state in the human heart. Incubation of human atrial myocardium with canagliflozin ex vivo reduced basal (Figure 2A), NADPH-stimulated (Figure 2B) and Vas2870-inhibitable (Figure 2C) generation; importantly, all canagliflozin concentrations (3, 10, or 100 μM) had significant effects on these readings of myocardial redox state. In addition, canagliflozin reduced production from uncoupled NOS, as estimated by the L-NAME-induced reduction in (Figure 2D). Canagliflozin reduced oxidative stress in myocardial tissue from patients with or without diabetes mellitus, but it showed stronger effects on the myocardial tissue from diabetic patients (Supplementary material online, Figure S3). Canagliflozin reduced total and Vas2870-inhibitable dihydroethidium fluorescence in human atrial myocardium (Figure 2E–G), similar to the chemiluminescence experiments. In contrast, treatment with the SGLT2 inhibitor empagliflozin, which has little affinity to SGLT1,31 did not affect myocardial generation, NADPH oxidase activity, or NOS coupling (Figure 2H–K). This finding could be explained by the very low affinity of empagliflozin to SGLT1, which is the main SGLT isoform in the human atrial and ventricular myocardium, and implies that clinically used SGLT2 inhibitors may exert direct myocardial effects depending on the degree of SGLT1 affinity. Therefore, we focused our next experiments on evaluating the direct effects of canagliflozin on myocardial redox signalling in humans, due to its dual SGLT1/SGLT2 affinity.
Figure 2.
Direct effects of canagliflozin and empagliflozin on human myocardial redox state. Ex vivo canagliflozin (3, 10, and 100 μM) treatment for 1 h reduced basal (A), NADPH-stimulated (B), and Vas2870 inhibitable (C) and increased L-NAME-(delta ) (D) dose-dependently in human atrial myocardium. Canagliflozin decreased the intensity of basal and Vas2870 inhibitable 2-hydroxyethidium (2-OH-ethidium) fluorescence (E–G) in human atrial biopsies stained with dihydroethidium (DHE). Empagliflozin had non-significant direct effect on either of these measures (H–K). n = 5–7 in panels A–L. Data are presented as mean ± SD. P-values are calculated by Wilcoxon signed-rank test (A–C, F–J) and paired t-test (D, K).
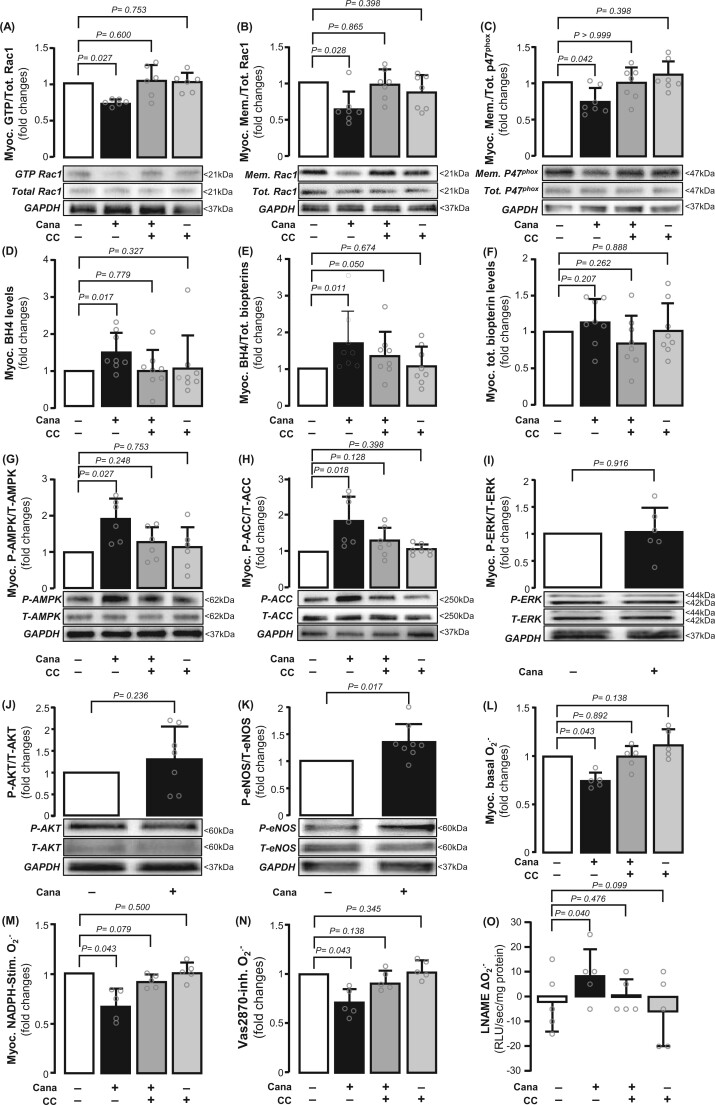
We first investigated the mechanism by which canagliflozin affects NADPH oxidase activity and NOS coupling status. To understand how canagliflozin reduced NADPH oxidase activity, we explored which subunits of the enzyme are mostly affected. Canagliflozin prevented the GTP activation of Rac1 (an important Nox1/2 subunit, Figure 3A) and its membrane translocation (Figure 3B), which lead to Nox1/2 isoform activation, while preventing the membrane translocation of the p47phox subunit of Nox2 (Figure 3C). To assess whether NADPH oxidase activity increased generation, not only directly, but also indirectly, we evaluated the oxidation of the NOS co-factor BH4. Canagliflozin increased BH4 levels in the atrial myocardium without affecting total biopterin content (Figure 3D–F), suggesting that it decreased BH4 oxidation (e.g. as a result of NADPH oxidase inhibition) rather than increased the biopterin biosynthetic pathway. Indeed, we also observed a reduction in BH2, the direct oxidation product of BH4, while biopterin, the terminal oxidation product of BH2 oxidation, was borderline decreased (Supplementary material online, Figure S4A–F). This would lead to improved enzymatic coupling of NOS and to a reduction in NOS-derived .
Figure 3.
Effects of canagliflozin on myocardial NADPH oxidase activity and nitric oxide synthase (NOS) coupling status in the human atrial myocardium. Canagliflozin (10 μM for 1 h) inhibited GTP activation (A) and membrane translocation (B) of Rac1, as well as the membrane translocation of p47phox (C). Canagliflozin increased myocardial BH4 but not total biopterin content (D–F). Canagliflozin induced AMPK Thr172 phosphorylation (G) and downstream acetyl-coA carboxylase (ACC) Ser79 phosphorylation (H). These were prevented by the AMPK inhibitor, compound C (CC) (G and H). Canagliflozin did not affect ERK or AKT phosphorylation (I and J). Canagliflozin induced NOS Ser1177 phosphorylation (K). Compound C prevented the effects of canagliflozin on Rac1 activation, BH4 bioavailability (A-F), generation (L–N), and NOS coupling (O). n = 5–8 pairs in panels A–O. Data are presented as mean ± SD. P-values are calculated by Wilcoxon signed-rank test (A–N) and paired t-test (O).
We next explored the upstream mechanism of the effects of canagliflozin on the myocardial redox state. Canagliflozin induced rapid phosphorylation of AMPKα2 (the main isoform in the heart) at the activation site Thr172 (Figure 3G), resulting in increased AMPKα2 activity documented by phosphorylation of its downstream target ACC at Ser79 (Figure 3H). Canagliflozin did not affect ERK phosphorylation at Thr202/Tyr204 or AKT at Ser473 (Figure 3I and J). Interestingly, canagliflozin also induced endothelial NOS phosphorylation at the activation site Ser1177 (Figure 3K), whereas it did not affect inducible NOS phosphorylation (Supplementary material online, Figure S4G and H). We next used CC, as an allosteric modulator of AMPK activity,32 to prove the concept that downstream SGLT1 effects are prevented by inhibiting AMPK activity. Compound C inhibited ACC phosphorylation (Figure 3G and H) as well as the ability of canagliflozin to inhibit Rac1 activation or increase BH4 bioavailability (Figure 3A–E). Compound C prevented the canagliflozin-induced reduction of myocardial generation (Figure 3L–N) and of the increase in NOS coupling (Figure 3O), identifying AMPKα2 as a link between canagliflozin, NADPH oxidase activity, and NOS coupling. Canagliflozin could also reduce apoptosis and mitochondrial dysfunction through AMPKα2 (Supplementary material online, Figure S5).
Direct effects of canagliflozin on primary human cardiomyocytes
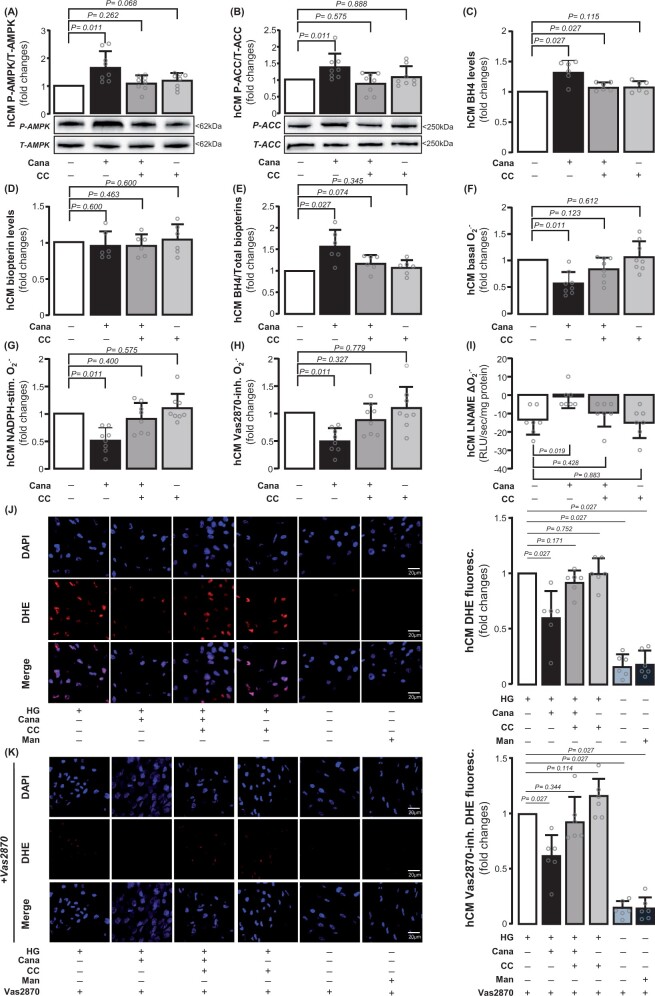
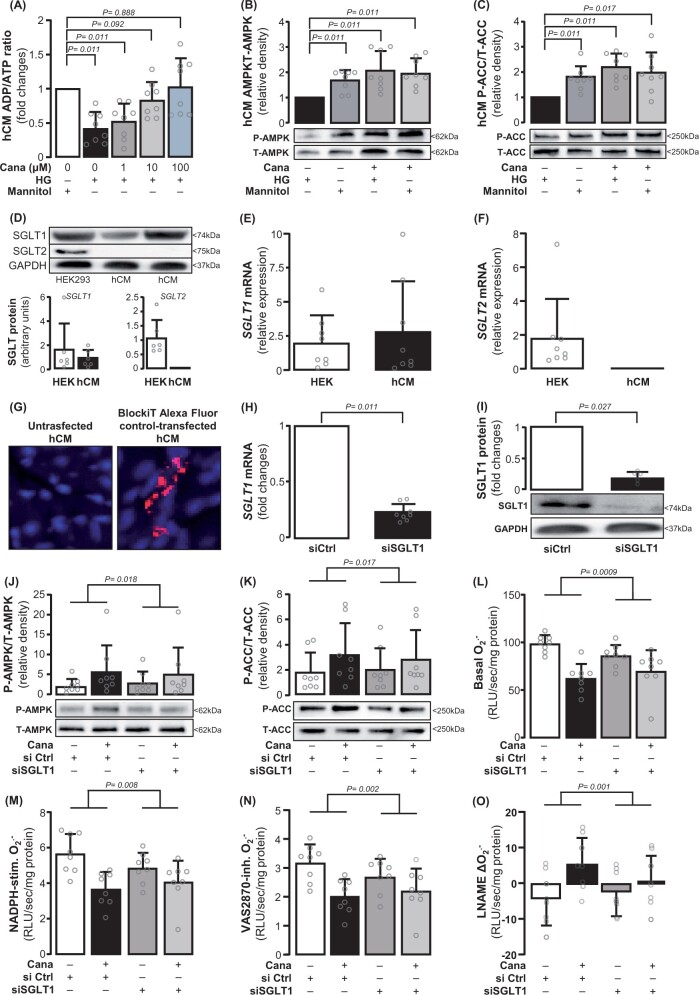
We next studied the effects of canagliflozin specifically on primary terminally differentiated hCM (Supplementary material online, Figure S1). To mimic the ex vivo human atrial myocardium experimental conditions as well as a clinically relevant diabetic environment, hCM were cultured in high-glucose medium. Osmolality changes associated with high glucose had no effect on myocardial redox state using mannitol as osmolality control (Supplementary material online, Figure S6). As observed in the human atrial myocardium, canagliflozin increased AMPKα2 Thr172 phosphorylation (Figure 4A) leading to AMPKα2 activation, as assessed by the phosphorylation status of ACC at Ser79 (Figure 4B). In addition, canagliflozin increased BH4 bioavailability, without altering total biopterins in hCM, in a CC-reversible way (Figure 4A–E). Similarly to the human atrial myocardium, exogenous canagliflozin reduced basal generation (Figure 4F) and NADPH oxidase activity (Figure 4G and H) and improved NOS coupling (Figure 4I) in an AMPKα2-dependent manner (since all those effects were reversed by CC) in hCM. Dihydroethidium staining confirmed the effect of canagliflozin on NADPH oxidase-derived O2.− in hCM (Figure 4J–L). Canagliflozin also induced NOS phosphorylation at its activation site Ser1177 in hCM (Supplementary material online, Figure S7). These findings were replicated in H9c2 cells (Supplementary material online, Figures S8 and S9), where the results were more striking after culture in a high-glucose medium.
Figure 4.
Direct effects of canagliflozin on human cardiomyocytes (hCM). Canagliflozin (10 μΜ) induced phosphorylation of AMPK and acetyl-coA carboxylase (ACC) in high glucose (HG)-treated human cardiomyocytes (A and B, n = 8) and increased BH4 levels without affecting total biopterin levels (C–E, n = 8). Canagliflozin decreased basal, NADPH-stimulated and Vas2870-inhibitable and increased the value of L-NAME delta() in human cardiomyocytes (F–I, n = 7). Dihydroethidium (DHE) staining combined with Vas2870 confirmed these (J–L, n = 8). Data are presented as mean ± SD. P-values are calculated by Wilcoxon signed-rank test (A–H, J, K) and paired t-test (I).
The role of SGLT1 in myocardial redox regulation by canagliflozin
To explore how canagliflozin affects the upstream phosphorylation of AMPKα2, we next evaluated the intracellular ADP-to-ATP ratio in hCM, which can allosterically regulate AMPKα2 activity and auto-phosphorylation. Canagliflozin increased ADP/ATP in a dose-dependent manner (Figure 5A). Depleting glucose from the culture media resulted in increased ADP/ATP (Figure 5A) and activated AMPKα2, evidenced by ACC phosphorylation. Adding canagliflozin to glucose-starved hCM did not lead to further change on either AMPKα2 or ACC (Figure 5B and C). These indicate that the effect of canagliflozin on hCM is dependent on glucose uptake, which then affects intracellular ADP/ATP and subsequent AMPKα2 activation.
Figure 5.
SGLT1 mediates the effects of canagliflozin on myocardial redox state. Canagliflozin increased ADP/ATP dose-dependently in human cardiomyocytes, while glucose deprivation from the culture medium (NG) induced similar changes in ADP/ATP ratio (A, n = 8). There were no differences in AMPK and ACC phosphorylation among the NG-incubated cells with or without canagliflozin (10μΜ), and high glucose (HG)-incubated cells with canagliflozin (panels B and C, n = 8). SGLT1 expression was detected, while SGLT2 was not detected in human cardiomyocytes (D–F, n = 8). SGLT1 was knocked down using siRNA (transfection low toxicity and efficiency was confirmed by transfecting BlockiT Alexa Fluor Red Fluorescent control, G), resulting into ∼76% down-regulation of SGLT1 mRNA (panel H, n = 6), and ∼85% protein down-regulation (panel I, n = 6). Canagliflozin-induced AMPK and ACC phosphorylation was attenuated in SGLT1 knocked down human cardiomyocytes compared with siRNA ctrl human cardiomyocytes (J and K, n = 8). SGLT1 deletion diminished canagliflozin-induced decrease of basal (L), NADPH-stimulated (M), and Vas2870-inhibitable production (N) and increased L-NAME delta (O). n = 8 in L–O. Data are presented as mean ± SD. P-values are calculated by Wilcoxon signed-rank test (A–C, H, I). Comparisons in canagliflozin responses between siControl and siSGLT1 cells were performed with two-way ANOVA with treatment (canagliflozin) × cell type (siControl or siSGLT1) interaction (J–O).
Consistent with our findings in human cardiac samples, hCM SGLT2 expression was undetectable (Figure 5D–F). SGLT1 expression was confirmed by qPCR and Western immunoblotting using HEK293 cells as positive control (Figure 5D and E). To further explore the dependency of the effects of canagliflozin on SGLT1, we knocked down the expression of SGLT1 in hCM using siRNA (Figure 5G), achieving down-regulation of SGLT1 mRNA by ∼76% and protein by ∼85% (Figure 5H and I). This prevented canagliflozin-induced AMPK activation and downstream ACC phosphorylation in hCM (Figure 5J and K). SGLT1 knock down prevented the ability of canagliflozin to reduce basal, NADPH-stimulated and Vas2870-inhibitable (Figure 5L–N), supporting that canagliflozin suppresses myocardial NADPH oxidase activity by inhibiting SGLT1. SGLT1 knock down in hCM also prevented the ability of canagliflozin to improve NOS coupling (Figure 5O). SGLT1 knock down did not lead to a compensatory increase in SGLT2 expression (Supplementary material online, Figure S10). Interestingly, knock down of SGLT1 did not fully mimic the effects observed with canagliflozin, and this could be explained either by the incomplete SGLT1 knock down technically achieved in these cells, or even by the presence of off-target effects of canagliflozin, independent of SGLT1/2 inhibition.
Direct effects of canagliflozin on redox-sensitive pro-inflammatory signalling in the human myocardium
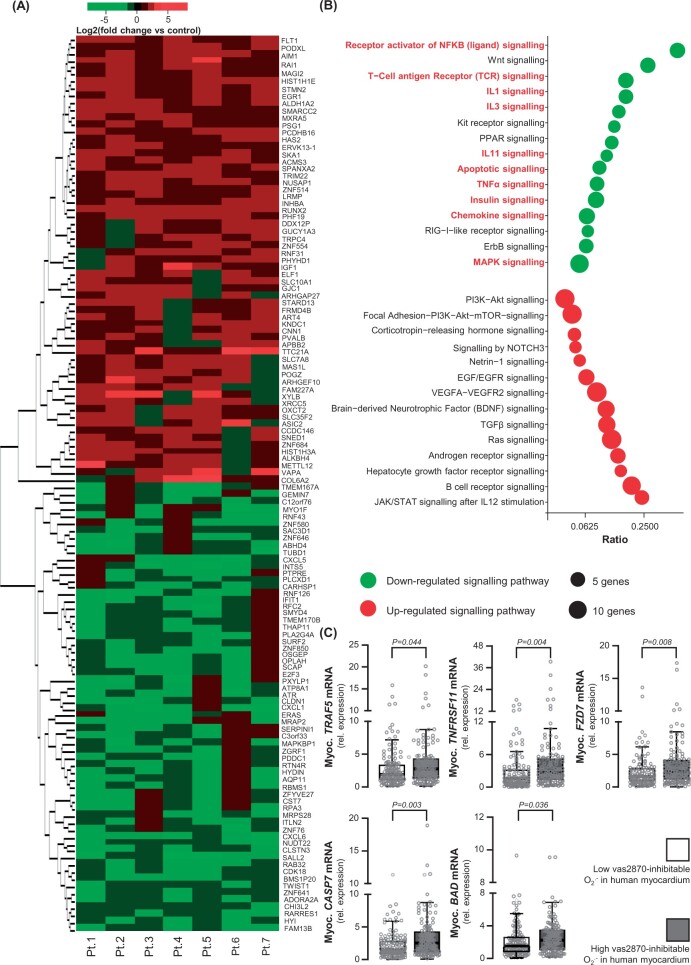
After establishing a role for canagliflozin as a regulator of the myocardial redox state, we explored its downstream effects on the transcriptomic profile of hCM. Canagliflozin (10 μM) treatment for 24 h altered the expression of 946 differentially expressed genes (DEGs, 466 up-regulated and 480 down-regulated) in hCM, with 127 DEGs demonstrating an effect size of >50% or `−50% (Figure 6A). Pathway analysis revealed that canagliflozin down-regulated a number of pro-inflammatory pathways such as those of IL1, IL3, TNFα, chemokines, MAPK signalling, and others, as well as apoptotic signalling pathways (Figure 6B). Notably, the nuclear factor kappa beta (NFkB) signalling pathway, a major redox-sensitive inflammatory transcriptional regulator, was one of the most down-regulated targets by canagliflozin, suggesting that it may contribute to the antioxidant effect of canagliflozin in hCM. Cell survival pathways such as PI3K-Akt, NOTCH3, and Ras signalling were also up-regulated by canagliflozin. Key genes of the NFkB, TNF-α, and apoptotic pathways mostly affected by canagliflozin included TNFRSF11, TRAF5, FZD7, CASP7, and BAD. The expression of these genes, quantified in atrial biopsies of 364 patients from Study 1, was positively associated with myocardial NADPH oxidase activity (Figure 6C).
Figure 6.
Canagliflozin has a global anti-inflammatory and anti-apoptotic effect on human cardiomyocytes. In this experiment, human cardiomyocytes were cultured in high-glucose medium (25 mM) for 72 h and then treated with canagliflozin (10 μM) or DMSO for 24 h. Heat map of 127 down- or up-regulated genes by canagliflozin (fold change >1.5 or `−1.5, P < 0.05) in canagliflozin-treated human cardiomyocytes from n = 7 patients (A). NFkB, Wnt, IL1, IL3, TNFα, chemokine, MAPK pathways, and apoptotic pathways (highlighted by red font) were down-regulated by canagliflozin (more than 50% of pathway genes down-regulated by canagliflozin, B). TNFRSF11, TRAF5, FZD7, CASP7, and BAD were the most down-regulated individual genes upon canagliflozin treatment, implicated in NFkB, TNFα, and apoptosis pathways. The mRNA expression of these genes was positively correlated with myocardial NADPH oxidase activity (i.e. high Vas2870-inhibitable signal) in 240 atrial biopsies from Study 1 (C). Data are presented as median [25th–75th percentile]. P-values are calculated by Mann–Whitney U-test for high (above median) vs. low (below median).
Discussion
In this work, we identify SGLT1 as the main SGLT isoform in the human myocardium, its expression being positively related with myocardial oxidative stress. As the main objective of this study was to evaluate the direct effects of SGLT1 vs. SGLT2 inhibition on the myocardium and SGLT2 is not expressed in human myocardium and cardiac myocytes, we focused on canagliflozin, which has significant affinity for SGLT1. We further demonstrate that a clinically relevant concentration of canagliflozin directly suppresses NADPH oxidase activity, increases BH4 bioavailability, and improves NOS coupling in isolated hCM and myocardial tissue via an SGLT1/AMPK/Rac1-dependent mechanism. These suppress redox-sensitive pro-inflammatory and pro-apoptotic signalling in the human cardiomyocyte, providing a novel mechanism contributing to the beneficial effects of canagliflozin on cardiac adverse events (Graphical abstract).
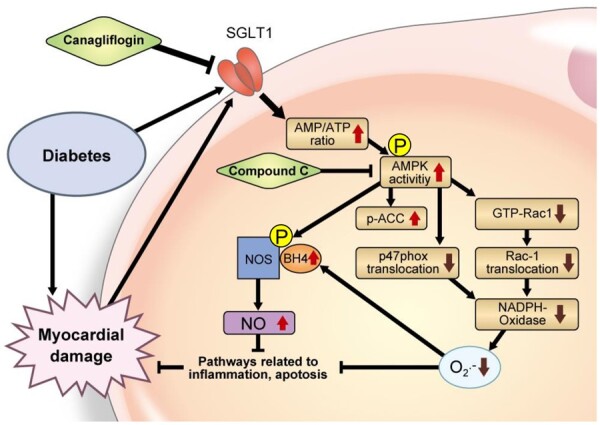
Proposed mechanism of canagliflozin-induced improvement of myocardial redox state. Canagliflozin increases intracellular AMP/ATP ratio through inhibition of SGLT1, which can activate AMPK/NOS signalling and increase NO that suppresses pro-inflammatory signalling. AMPK activation also inhibits activation of Rac1 and membrane translocation of Rac1 and p47phox, which decrease NADPH oxidase activity and superoxide () production, attenuates inflammatory and apoptotic pathways and increasing the bioavailability of tetrahydrobiopterin (BH4), a key factor for NOS coupling.
The role of SGLT isoforms in myocardial physiology is controversial.33 , 34 SGLT2 expression has been detected in hCM after exposure to high glucose; however, other studies have shown no SGLT2 expression in human hearts. In contrast, SGLT1 was found in hCM 34 , 35 while functionally damaging variants in SGLT1 have been associated with reduced death/heart failure risk.36 We definitively demonstrate, using a large number of human myocardial biopsies and hCM, that SGLT1 is abundantly expressed in the human heart, whereas SGLT2 is barely detectable.
Clinically used SGLT2 inhibitors have variable SGLT1 affinities. Canagliflozin displays higher SGLT1 affinity than other SGLT2 inhibitors,30 , 31 , 37 whilst empagliflozin is the most selective SGLT2 inhibitor.31 Evidence has suggested differing cellular responses to canagliflozin vs. empagliflozin, e.g. canagliflozin is a stronger inducer of AMPK phosphorylation in HEK293 cells or human vascular endothelial cells.13 , 38 Importantly, we demonstrate for the first time that canagliflozin (in concentrations comparable to in vivo pharmacological levels39 , 40) directly inhibits NADPH oxidase-derived oxidative stress in human myocardial redox signalling via SGLT1/AMPK/Rac1 signalling, which may be more potent than empagliflozin. Furthermore, canagliflozin could reverse the decrease in ADP/ATP ratio due to a hyperglycaemic environment by inhibiting glucose uptake in hCM.
Myocardial oxidative stress facilitates apoptosis and inflammation, leading to cardiac fibrosis and promoting cardiac dysfunction.41 Myocardial inflammation is influenced by redox-sensitive transcriptional factors such as NFkB.42 Our transcriptome analysis demonstrated that canagliflozin suppresses several redox-sensitive pro-inflammatory and pro-apoptotic pathways in hCM, such as those of TNF-α and IL-1 as well as, crucially, on NFkB activity. The link of canagliflozin with myocardial redox regulation was further validated in ∼400 human atrial biopsies, by demonstrating a clear relationship between myocardial NADPH oxidase-derived and the expression of key genes regulated by canagliflozin.
Certain aspects of the interplay between SGLT2 inhibitors and myocardial disease are not explored in our work. SGLT2 inhibitors with little SGLT1 selectivity (e.g. empagliflozin) also demonstrate cardioprotective effects despite lacking SGLT1 affinity. A very recent study from Kolijn et al.14 described an antioxidant effect of empagliflozin, in humans with heart failure with preserved ejection fraction as well as in ZDF obese rats, evaluated by lipid peroxidation levels, which was accompanied by improved NO-sGC-PKG signalling. Although in our study empagliflozin did not affect NADPH oxidase-derived superoxide production in human atrial myocardium, these two pieces of work may describe distinct mechanisms, which could be complementary and the discrepancies in results may be due to different participant characteristics, use of different (indirect) readings of redox state (we specifically targeted NADPH oxidase-derived superoxide, whilst Kolijn et al. 14 targeted lipid peroxidation, which is influenced by reactive oxygen species levels, antioxidant capacity, and cell metabolism) and tissue type differences (atrial vs. ventricular tissue). Furthermore, this suggests that systemic SGLT2 inhibition has also indirect effects on the human heart, most likely by affecting kidney function as well as the secretome of other tissues with indirect, endocrine effects on cardiac function, which is not explored here. However, some SGLT2 inhibitors (like canagliflozin) may well have chronic direct effects on the heart via cardiac SGLT1 inhibition, and this direct effect on the human myocardium was the objective of our study. This concept warrants further validation in appropriate in vivo animal models, such as Sglt1 loss- and gain-of-function mouse models.
Of note is the fact that the ex vivo experiments were performed on myocardial tissue of atrial origin, whereas our mechanistic experiments were carried out in cardiomyocytes derived from ventricles. The fact that our proposed mechanism is shown in a similar way in all models (human atrial myocardium, human ventricular cardiomyocytes, and terminally differentiated H9c2 cells) further strengthens the validity of the proposed mechanisms by which canagliflozin affects myocardial biology. In this study, we evaluate the membrane translocation of NADPH oxidase subunits by measuring their levels on isolated membranes vs. the cytosolic phase using an ultra-centrifugation protocol, rather than electron microscopy, and this is a methodological limitation that needs to be acknowledged. Finally, in translational studies like this one, surgical human tissue availability varies, and this has led to variability in sample sizes across the various experiments in this study.
In conclusion, we demonstrate for the first time that canagliflozin inhibits NADPH oxidase activity and improves NOS coupling via SGLT1/AMPKα2/Rac1 signalling, whilst suppressing several pro-inflammatory and pro-apoptotic pathways in the human myocardium. Our work describes an important SGLT1-mediated mechanism that could contribute to the cardioprotective effects of the SGLT2 drug class.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
H.K. conceived and performed experiments, performed data analysis, and drafted the manuscript; I.A. conceived and performed experiments, contributed to participant recruitment, performed data analysis, and contributed to the writing of the manuscript; I.B. performed experiments and data analysis and reviewed the manuscript; N.A. performed data analysis and reviewed the manuscript; C.P.K. contributed to participant recruitment and human sample collection, conceived and performed experiments, performed data analysis, and contributed to the writing of the manuscript; M.P. performed experiments and data analysis; I.S. performed experiments and data analysis; E.S. contributed to biopsy collection and performed data analysis; A.S.A. performed experiments, contributed to participant recruitment, and performed data analysis; M.C.C. conceived and performed experiments; E.K.O. contributed to the project design; E.M.R. contributed to participant recruitment; R.S., G.K., V.S., and S.F. contributed to surgical specimen collection; S.C. performed experiments; C.S. and K.M.C. contributed to the project design and reviewed the manuscript; B.C. contributed to the project design, provided experimental resources and expertise and reviewed the manuscript; C.A. conceived the project, secured funding, oversaw the implementation of individual experiments, performed data analysis, and corrected the manuscript.
Funding
This study was supported by the British Heart Foundation (FS/16/15/32047, and RG/F/21/110040 to C.A., CH/16/1/32013 to K.C., and Centre of Research Excellence award RG/13/1/30181), the National Institute for Health Research Oxford Biomedical Research Centre. H.K. acknowledges support by the Japanese Heart Rhythm Society-European Heart Rhythm Association fellowship grant sponsored by Biotronik. The authors thank the High-Throughput Genomics Group at The Wellcome Trust Centre for Human Genetics (funded by Wellcome Trust grant reference 090532/Z/09/Z) for the generation of the microarray data.
Contributor Information
Hidekazu Kondo, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK; Department of Cardiology and Clinical Examination, Faculty of Medicine, Oita University, 1-1 Idaigaoka, Hasama, Yufu, Oita 879-5593, Japan.
Ioannis Akoumianakis, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Ileana Badi, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Nadia Akawi, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK; Department of Genetics and Genomics, College of Medicine and Health Sciences, United Arab Emirates University, Khalifa Ibn Zayed Street, Al Maqam, Al-Ain, P.O. Box 17666, United Arab Emirates.
Christos P Kotanidis, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Murray Polkinghorne, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Ilaria Stadiotti, Unit of Vascular Biology and Regenerative Medicine, Centro Cardiologico Monzino IRCCS, via Carlo Parea 4, 20138, Milan, Italy.
Elena Sommariva, Unit of Vascular Biology and Regenerative Medicine, Centro Cardiologico Monzino IRCCS, via Carlo Parea 4, 20138, Milan, Italy.
Alexios S Antonopoulos, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Maria C Carena, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Evangelos K Oikonomou, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Elsa Mauricio Reus, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Rana Sayeed, Oxford University Hospitals NHS Trust, Headley Way, Oxford OX3 9DU, UK.
George Krasopoulos, Oxford University Hospitals NHS Trust, Headley Way, Oxford OX3 9DU, UK.
Vivek Srivastava, Oxford University Hospitals NHS Trust, Headley Way, Oxford OX3 9DU, UK.
Shakil Farid, Oxford University Hospitals NHS Trust, Headley Way, Oxford OX3 9DU, UK.
Surawee Chuaiphichai, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Cheerag Shirodaria, Caristo Diagnostics, 1st Floor, New Barclay House, 234 Botley Rd, Oxford OX2 0HP, UK.
Keith M Channon, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK; Oxford University Hospitals NHS Trust, Headley Way, Oxford OX3 9DU, UK.
Barbara Casadei, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Charalambos Antoniades, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, L6 West Wing, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK; Oxford University Hospitals NHS Trust, Headley Way, Oxford OX3 9DU, UK; Acute Vascular Imaging Centre, University of Oxford, Headley Way, Oxford OX3 9DU, UK.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 2012;8:495—502. [DOI] [PubMed] [Google Scholar]
- 2. Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther 2013;139:51—59. [DOI] [PubMed] [Google Scholar]
- 3. Danne T, Biester T, Kordonouri O. Combined SGLT1 and SGLT2 inhibitors and their role in diabetes care. Diabetes Technol Ther 2018;20:S269—S277. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117—2128. [DOI] [PubMed] [Google Scholar]
- 5. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644—657. [DOI] [PubMed] [Google Scholar]
- 6. Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018;137:323—334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE-TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347—357. [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJV, DeMets DL, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjostrand M, Solomon SD; DAPA-HF Committees and Investigators. The Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail 2019;21:1402—1411. [DOI] [PubMed] [Google Scholar]
- 9. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995—2008. [DOI] [PubMed] [Google Scholar]
- 10. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Juni P, Lettino M, Marx N, Mellbin LG, Ostgren CJ, Rocca B, Roffi M, Sattar N, Seferovic PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J 2020;41:255—323. [DOI] [PubMed] [Google Scholar]
- 11. Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, Yu X, Sun B, Chen L. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 2019;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byrne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker DL, Masson G, Fedak PWM, Verma S, Dyck JRB. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. JACC Basic Transl Sci 2017;2:347—354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 2016;65:2784—2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolijn D, Pabel S, Tian Y, Lódi M, Herwig M, Carrizzo A, Zhazykbayeva S, Kovács Á, Fülöp GÁ, Falcão-Pires I, Reusch PH, Linthout SV, Papp Z, van Heerebeek L, Vecchione C, Maier LS, Ciccarelli M, Tschöpe C, Mügge A, Bagi Z, Sossalla S, Hamdani N. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res 2021;117:495—507. [DOI] [PubMed] [Google Scholar]
- 15. Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 2007;93:903—907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, Alp NJ, Schotten U, Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation 2011;124:1107—1117. [DOI] [PubMed] [Google Scholar]
- 17. Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, Marinou K, Nahar K, Jayaram R, Tousoulis D, Bakogiannis C, Sayeed R, Triantafyllou C, Koumallos N, Psarros C, Miliou A, Stefanadis C, Channon KM, Casadei B. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol 2012;59:60—70. [DOI] [PubMed] [Google Scholar]
- 18. Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal 2014;20:2794—2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 2012;33:829—837, 837a—837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roe ND, Ren J. Nitric oxide synthase uncoupling: a therapeutic target in cardiovascular diseases. Vascul Pharmacol 2012;57:168—172. [DOI] [PubMed] [Google Scholar]
- 21. Carnicer R, Crabtree MJ, Sivakumaran V, Casadei B, Kass DA. Nitric oxide synthases in heart failure. Antioxid Redox Signal 2013;18:1078—1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, Psarros C, Nasrallah H, Coutinho P, Akoumianakis I, Brewer AC, Sayeed R, Krasopoulos G, Petrou M, Tarun A, Tousoulis D, Shah AM, Casadei B, Channon KM, Antoniades C. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circ Res 2016;118:842—855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT; ESC Scientific Document Group. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999—3058. [DOI] [PubMed] [Google Scholar]
- 24.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [Google Scholar]
- 25. Garza L, Dols J, Gillespie M. An initiative to improve primary prevention of cardiovascular disease in adults with type II diabetes based on the ACC/AHA (2013) and ADA (2016) guidelines. J Am Assoc Nurse Pract 2017;29:606—611. [DOI] [PubMed] [Google Scholar]
- 26. Tuncay E, Bitirim VC, Durak A, Carrat GR, Taylor KM, Rutter GA, Turan B. Hyperglycemia-induced changes in ZIP7 and ZnT7 expression cause Zn2+ release from the sarco (endo) plasmic reticulum and mediate ER stress in the heart. Diabetes 2017;66:1346—1358. [DOI] [PubMed] [Google Scholar]
- 27. Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 2013;127:2209—2221. [DOI] [PubMed] [Google Scholar]
- 28. Akoumianakis I, Sanna F, Margaritis M, Badi I, Akawi N, Herdman L, Coutinho P, Fagan H, Antonopoulos AS, Oikonomou EK, Thomas S, Chiu AP, Chuaiphichai S, Kotanidis CP, Christodoulides C, Petrou M, Krasopoulos G, Sayeed R, Lv L, Hale A, Naeimi Kararoudi M, McNeill E, Douglas G, George S, Tousoulis D, Channon KM, Antoniades C. Adipose tissue-derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1-mediated activation of NADPH oxidases. Sci Transl Med 2019;11:eaav5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Sanna F, De Silva R, Petrou M, Sayeed R, Krasopoulos G, Lee R, Digby J, Reilly S, Bakogiannis C, Tousoulis D, Kessler B, Casadei B, Channon KM, Antoniades C. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 2015;64:2207—2219. [DOI] [PubMed] [Google Scholar]
- 30. Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, Du F, Liu Y, Xu J, Conway B, Conway J, Polidori D, Ways K, Demarest K. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One 2012;7:e30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anker SD, Butler J. Empagliflozin, calcium, and SGLT1/2 receptor affinity: another piece of the puzzle. ESC Heart Fail 2018;5:549—551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Handa N, Takagi T, Saijo S, Kishishita S, Takaya D, Toyama M, Terada T, Shirouzu M, Suzuki A, Lee S, Yamauchi T, Okada-Iwabu M, Iwabu M, Kadowaki T, Minokoshi Y, Yokoyama S. Structural basis for compound C inhibition of the human AMP-activated protein kinase α2 subunit kinase domain. Acta Crystallogr D Biol Crystallogr 2011;67:480—487. [DOI] [PubMed] [Google Scholar]
- 33. Ng KM, Lau YM, Dhandhania V, Cai ZJ, Lee YK, Lai WH, Tse HF, Siu CW. Empagliflozin ammeliorates high glucose induced-cardiac dysfuntion in human iPSC-derived cardiomyocytes. Sci Rep 2018;8:14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Franco A, Cantini G, Tani A, Coppini R, Zecchi-Orlandini S, Raimondi L, Luconi M, Mannucci E. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: a new potential pharmacological target. Int J Cardiol 2017;243:86—90. [DOI] [PubMed] [Google Scholar]
- 35. Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res 2009;84:111—118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seidelmann SB, Feofanova E, Yu B, Franceschini N, Claggett B, Kuokkanen M, Puolijoki H, Ebeling T, Perola M, Salomaa V, Shah A, Coresh J, Selvin E, MacRae CA, Cheng S, Boerwinkle E, Solomon SD. Genetic variants in SGLT1, glucose tolerance, and cardiometabolic risk. J Am Coll Cardiol 2018;72:1763—1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilding JP. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 2014;63:1228—1237. [DOI] [PubMed] [Google Scholar]
- 38. Mancini SJ, Boyd D, Katwan OJ, Strembitska A, Almabrouk TA, Kennedy S, Palmer TM, Salt IP. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep 2018;8:5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen X, Hu P, Vaccaro N, Polidori D, Curtin CR, Stieltjes H, Sha S, Weiner S, Devineni D. Pharmacokinetics, pharmacodynamics, and safety of single-dose canagliflozin in healthy chinese subjects. Clin Ther 2015;37:1483—1492.e1. [DOI] [PubMed] [Google Scholar]
- 40. Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy J, Rusch S, Rothenberg PL. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol 2013;53:601—610. [DOI] [PubMed] [Google Scholar]
- 41. Kondo H, Takahashi N, Gotoh K, Fukui A, Saito S, Aoki K, Kume O, Shinohara T, Teshima Y, Saikawa T. Splenectomy exacerbates atrial inflammatory fibrosis and vulnerability to atrial fibrillation induced by pressure overload in rats: possible role of spleen-derived interleukin-10. Heart Rhythm 2016;13:241—250. [DOI] [PubMed] [Google Scholar]
- 42. Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal 2005;7:395—403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.