Abstract
Background
The optimal haemoglobin threshold for use of red blood cell (RBC) transfusions in anaemic patients remains an active field of research. Blood is a scarce resource, and in some countries, transfusions are less safe than in others because of inadequate testing for viral pathogens. If a liberal transfusion policy does not improve clinical outcomes, or if it is equivalent, then adopting a more restrictive approach could be recognised as the standard of care.
Objectives
The aim of this review update was to compare 30‐day mortality and other clinical outcomes for participants randomised to restrictive versus liberal red blood cell (RBC) transfusion thresholds (triggers) for all clinical conditions. The restrictive transfusion threshold uses a lower haemoglobin concentration as a threshold for transfusion (most commonly, 7.0 g/dL to 8.0 g/dL), and the liberal transfusion threshold uses a higher haemoglobin concentration as a threshold for transfusion (most commonly, 9.0 g/dL to 10.0 g/dL).
Search methods
We identified trials through updated searches: CENTRAL (2020, Issue 11), MEDLINE (1946 to November 2020), Embase (1974 to November 2020), Transfusion Evidence Library (1950 to November 2020), Web of Science Conference Proceedings Citation Index (1990 to November 2020), and trial registries (November 2020). We checked the reference lists of other published reviews and relevant papers to identify additional trials. We were aware of one trial identified in earlier searching that was in the process of being published (in February 2021), and we were able to include it before this review was finalised.
Selection criteria
We included randomised trials of surgical or medical participants that recruited adults or children, or both. We excluded studies that focused on neonates.
Eligible trials assigned intervention groups on the basis of different transfusion schedules or thresholds or 'triggers'. These thresholds would be defined by a haemoglobin (Hb) or haematocrit (Hct) concentration below which an RBC transfusion would be administered; the haemoglobin concentration remains the most commonly applied marker of the need for RBC transfusion in clinical practice. We included trials in which investigators had allocated participants to higher thresholds or more liberal transfusion strategies compared to more restrictive ones, which might include no transfusion. As in previous versions of this review, we did not exclude unregistered trials published after 2010 (as per the policy of the Cochrane Injuries Group, 2015), however, we did conduct analyses to consider the differential impact of results of trials for which prospective registration could not be confirmed.
Data collection and analysis
We identified trials for inclusion and extracted data using Cochrane methods. We pooled risk ratios of clinical outcomes across trials using a random‐effects model. Two review authors independently extracted data and assessed risk of bias. We conducted predefined analyses by clinical subgroups. We defined participants randomly allocated to the lower transfusion threshold as being in the 'restrictive transfusion' group and those randomly allocated to the higher transfusion threshold as being in the 'liberal transfusion' group.
Main results
A total of 48 trials, involving data from 21,433 participants (at baseline), across a range of clinical contexts (e.g. orthopaedic, cardiac, or vascular surgery; critical care; acute blood loss (including gastrointestinal bleeding); acute coronary syndrome; cancer; leukaemia; haematological malignancies), met the eligibility criteria. The haemoglobin concentration used to define the restrictive transfusion group in most trials (36) was between 7.0 g/dL and 8.0 g/dL. Most trials included only adults; three trials focused on children.
The included studies were generally at low risk of bias for key domains including allocation concealment and incomplete outcome data.
Restrictive transfusion strategies reduced the risk of receiving at least one RBC transfusion by 41% across a broad range of clinical contexts (risk ratio (RR) 0.59, 95% confidence interval (CI) 0.53 to 0.66; 42 studies, 20,057 participants; high‐quality evidence), with a large amount of heterogeneity between trials (I² = 96%).
Overall, restrictive transfusion strategies did not increase or decrease the risk of 30‐day mortality compared with liberal transfusion strategies (RR 0.99, 95% CI 0.86 to 1.15; 31 studies, 16,729 participants; I² = 30%; moderate‐quality evidence) or any of the other outcomes assessed (i.e. cardiac events (low‐quality evidence), myocardial infarction, stroke, thromboembolism (all high‐quality evidence)). High‐quality evidence shows that the liberal transfusion threshold did not affect the risk of infection (pneumonia, wound infection, or bacteraemia). Transfusion‐specific reactions are uncommon and were inconsistently reported within trials.
We noted less certainty in the strength of evidence to support the safety of restrictive transfusion thresholds for the following predefined clinical subgroups: myocardial infarction, vascular surgery, haematological malignancies, and chronic bone‐marrow disorders.
Authors' conclusions
Transfusion at a restrictive haemoglobin concentration decreased the proportion of people exposed to RBC transfusion by 41% across a broad range of clinical contexts. Across all trials, no evidence suggests that a restrictive transfusion strategy impacted 30‐day mortality, mortality at other time points, or morbidity (i.e. cardiac events, myocardial infarction, stroke, pneumonia, thromboembolism, infection) compared with a liberal transfusion strategy.
Despite including 17 more randomised trials (and 8846 participants), data remain insufficient to inform the safety of transfusion policies in important and selected clinical contexts, such as myocardial infarction, chronic cardiovascular disease, neurological injury or traumatic brain injury, stroke, thrombocytopenia, and cancer or haematological malignancies, including chronic bone marrow failure.
Further work is needed to improve our understanding of outcomes other than mortality. Most trials compared only two separate thresholds for haemoglobin concentration, which may not identify the actual optimal threshold for transfusion in a particular patient. Haemoglobin concentration may not be the most informative marker of the need for transfusion in individual patients with different degrees of physiological adaptation to anaemia. Notwithstanding these issues, overall findings provide good evidence that transfusions with allogeneic RBCs can be avoided in most patients with haemoglobin thresholds between the range of 7.0 g/dL and 8.0 g/dL. Some patient subgroups might benefit from RBCs to maintain higher haemoglobin concentrations; research efforts should focus on these clinical contexts.
Plain language summary
Is it safe to use lower blood counts (haemoglobin levels) as a trigger for blood transfusion in order to give fewer blood transfusions?
Key messages
•There is no evidence that giving blood transfusions to patients with lower blood counts (haemoglobin levels of 7.0 g/dL to 8.0 g/dL) compared to higher blood counts (9.0 g/dL to 10.0 g/dL) affects risks of death, heart attack, myocardial infarction, stroke, pneumonia, blood clots or infection.
• Giving blood only to patients with lower blood counts (7.0 g/dL to 8.0 g/dL) would reduce the amount of blood transfused substantially. It would also reduce the risk of unnecessary transfusions (transfusions can have harmful effects).
• More research is needed to:
‐ establish the blood count at which a blood transfusion is needed in people who have suffered a heart attack, brain injury, or have cancer; and to
‐ improve our understanding of outcomes other than death, including quality of life.
What happens in people who need blood transfusions?
Doctors and healthcare professionals often give blood transfusions to people who lose blood through surgery, bleeding, or illness. For example, blood transfusions may help patients with anaemia to recover after surgery, but they should only be given when they help people to get better from their medical condition. Blood is a limited resource and transfusion is not risk‐free, especially for people in low‐income countries where the blood used in transfusions may not be tested for harmful viruses such as HIV or hepatitis.
What did we want to find out?
The blood count measures the amount of haemoglobin in the blood. Haemoglobin is a protein that gives blood its red colour and carries oxygen around the body. A normal blood count is around 12 grams a decilitre (12 g/dL). We wanted to find out if it is safe to withhold blood transfusion until the blood count drops to between 7.0 g/dL to 8.0 g/dL, rather than transfusing sooner at higher blood counts of between 9.0 g/dL to 10.0 g/dL.
What did we do?
We examined the results of studies that allocated patients to one of two groups by chance (for example, by flipping a coin). In one group, the patients only received blood transfusions if their blood count fell below a higher threshold (typically, 9.0 g/dL to 10.0 g/dL). In the other group, the patients only received blood transfusions if their blood counts fell below a lower threshold (typically, 7.0 g/dL to 8.0 g/dL).
What did we find?
We found 48 studies that involved 21,433 patients. The patients had been hospitalised for a range of reasons including: bone (orthopaedic), heart (cardiac) or vascular surgery; critical care; acute blood loss (for example, through bleeding in the stomach or intestines); heart diseases; cancer and blood cancers. The studies compared higher or lower blood count thresholds for blood transfusion. (The ‘threshold’ is the blood count level that would need to be met before a transfusion would be given.)
Transfusion
We found that patients who received transfusions only at lower blood count thresholds were 41% less likely to receive a blood transfusion than those who received them only at higher blood count thresholds. If the lower threshold were applied routinely by medical staff, it would lead to a substantial reduction in the quantity of blood needed.
Death and harmful events
There was no clear difference in the risk of dying within 30 days of receiving, or not receiving, a transfusion for patients in the two different threshold groups.
There was also no clear difference between the low and high threshold groups for the number of serious harmful events that occurred after patients received, or did not receive, blood transfusions. The harmful events recorded included infection (pneumonia, wound infection, and blood poisoning), heart attacks, strokes, and problems with blood clots.
What are the limitations of the evidence?
We found that most of the studies provided a high quality of evidence; they were adequately conducted and used methods that minimised biases that could make the validity of the results uncertain.
We are confident in the evidence regarding likelihood of receiving a transfusion, death within 30 days of transfusion, heart attack, stroke and infection. We are moderately confident in the evidence for problems caused by blood clots, but too few occurred in either group for us to be more confident.
Too few studies evaluated quality of life for us to be able to see whether it varied between groups.
How up to date is this evidence?
This Cochrane Review updates our previous work on this subject (last published in 2016). Seventeen new studies are included. The evidence is up to date to November 2020.
Summary of findings
Summary of findings 1. Liberal compared with restrictive transfusion protocols for guiding red blood cell transfusion.
| Liberal compared with restrictive transfusion protocols for guiding red blood cell transfusion | ||||||
| Patient or population: adults and children (haemodynamically stable) with potential need for RBC transfusion Setting: inpatients Intervention: restrictive transfusion threshold Comparison: liberal transfusion threshold | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with liberal transfusion protocol | Risk with restrictive transfusion protocol | |||||
| Participants exposed to blood transfusion (all studies) | Study population | RR 0.59 (0.53 to 0.66) | 20,057 (42) | ⊕⊕⊕⊕ High | ||
| 815 per 1000 | 481 per 1000 (432 to 538) | |||||
| 30‐Day mortality | Study population | RR 0.99 (0.86 to 1.15) | 16,729 (31) | ⊕⊕⊕⊕ High | ||
| 83 per 1000 | 83 per 1000 (71 to 96) | |||||
| Myocardial infarction | Study population | RR 1.04 (0.87 to 1.24) | 14,370 (23) | ⊕⊕⊕⊕ High | ||
| 32 per 1000 | 33 per 1000 (28 to 40) | |||||
| Congestive heart failure | Study population | RR 0.83 (0.53 to 1.29) | 7247 (16) | ⊕⊕⊝⊝ Lowa | ||
| 35 per 1000 | 29 per 1000 (19 to 45) | |||||
| Cerebrovascular accident ‐ stroke | Study population | RR 0.84 (0.64 to 1.09) | 13,985 (19) | ⊕⊕⊕⊕ High | ||
| 17 per 1000 | 14 per 1000 (11 to 19) | |||||
| Rebleeding | Study population | RR 0.80 (0.59 to 1.09) | 3412 (8) | ⊕⊕⊕⊝ Moderateb | ||
| 158 per 1000 | 126 per 1000 (93 to 172) | |||||
| Thromboembolism | Study population | OR 1.11 (0.65 to 1.88) | 4201 (13) | ⊕⊕⊕⊝ Moderatec | ||
| 15 per 1000 | 17 per 1000 (10 to 28) | |||||
| Infection | Study population | RR 0.97 (0.88 to 1.07) | 17,104 (25) | ⊕⊕⊕⊕ High | ||
| 143 per 1000 | 139 per 1000 (126 to 153) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RBC: red blood cell; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded once for inconsistency, as there was no consistency in the direction of the effect (despite the relatively low statistical heterogeneity), and we downgraded once for imprecision, as there were very low numbers of events.
bDespite relatively low statistical heterogeneity, there was no consistency in the direction of the effect, hence we downgraded once for inconsistency.
cDowngraded once for imprecision, as there were few events (and hence a wide CI).
Background
Description of the condition
Patients who are ill in hospital are frequently anaemic, with low haemoglobin concentrations. The causes of anaemia are diverse, including loss of blood from surgery, bleeding, excessive blood sampling for laboratory tests, or as a consequence of illness. Additionally, patients with cancer may develop anaemia because the underlying disease, or chemotherapy, affects production of red cells in their bone marrow. Anaemia both decreases the oxygen content of blood supplied to the tissues, including the myocardial muscles of the heart, and increases myocardial oxygen demand by requiring higher cardiac output to maintain adequate oxygen delivery throughout the body (Sabatine 2005).
It is well known that anaemia is linked with multiple clinical symptoms; it is also associated with worse outcomes among patients who are anaemic before and after surgery or critical illness, or who have cardiovascular disease (Carson 1996; Kunz 2020; Shander 2014). However, it does not necessarily follow that correction of anaemia will improve outcomes, whether by red blood cell (RBC) transfusions (addressed in this review) or by alternative treatments such as intravenous iron (Richards 2020). Anaemia is generally well tolerated by many people, therefore, the benefits of administering potentially corrective treatments such as red cell transfusion need to be weighed against the risks.
Description of the intervention
The main treatment option for raising the haemoglobin concentration rapidly in patients with anaemia is RBC transfusion. RBCs for transfusion are collected from whole blood donations from blood donors. These are centrifuged to concentrate them before they are added to anticoagulant and storage solutions. Autologous transfusions, which are collected from and stored for the same individual, are not indicated for sicker hospitalised patients with anaemia.
Red cell transfusions are life‐saving for patients with major bleeding. Red cell transfusions will treat severe anaemia successfully and may reduce the risks of major complications related to severe anaemia, such as myocardial infarction and heart failure. Uncertainties about the role of red cell transfusions are less clear for patients with less severe degrees of anaemia, and this is the focus of this review.
There are recognised risks of blood transfusion, as with any medical intervention (Delaney 2016). These risks, and the general availability of RBC transfusion vary throughout the world. In countries with well‐regulated blood supplies and effective blood donor screening policies, the safety of allogeneic red cell transfusion has improved significantly over the past 30 years, and overall risks are very low. These risks continue to be well monitored through national haemovigilance systems (e.g. the UK's Serious Hazards of Transfusion; SHOT Annual Report 2019), which document very few cases of transfusion‐transmitted infection; these findings reinforce earlier data from many countries (Zou 2009; Zou 2010). In resource‐limited countries, the supply of blood remains inadequate, with highly variable rates of donation per 1000 individuals. Furthermore, blood may not be as safe in these countries as it is in resource‐rich countries because it is not tested rigorously, and countries may lack quality control for viral pathogens, specifically transfusion‐transmissible infections such as HIV, hepatitis B, hepatitis C, and syphilis (WHO 2016). In some resource‐limited countries, a significant proportion of the blood supply is collected from family or paid blood donors ‐ not from voluntary unpaid donors ‐ and donor screening policies may not be efficiently applied. The prevalence of diseases such as HIV can be higher in low‐income countries than in high‐income countries, which presents a risk for transfusion transmission. All these points are described in the latest report on Global Blood Safety and Availability produced by the Blood Transfusion Safety Programme in the World Health Organization (WHO) Department of Service Delivery and Safety (WHO 2016).
Other risks of transfusion that have been described include acute transfusion reactions, volume overload, and transfusion‐related acute lung injury (Delaney 2016; SHOT Annual Report 2019; Toy 2012). Less well‐defined, but potentially important, adverse effects include loss of red cell nitric oxide production, which is thought to induce local vasodilatation; pro‐thrombotic effects from factors in the supernatant or changes in blood viscosity following transfusions; and immunomodulatory (or pro‐inflammatory) effects of different cellular products in the red cell component (Youssef 2017). Such harmful effects of RBC transfusions may be manifested, for example, as increased risks of infection (Rohde 2014).
Blood transfusion is expensive when one considers that around two million components (of which 1.6 million are units of RBCs) are issued across the UK alone each year (www.shotuk.org). The direct cost of each collected bag of red cells fails to capture the many associated costs related to hospital blood‐banking practice and safe patient administration (Stokes 2018). In 2008, the mean payment for one unit of leuco‐reduced RBCs in the USA was USD 223 (Whitaker 2011). However, when costs of administration as well as acquisition expenses of RBC transfusion were considered, the estimated cost derived from four USA and European hospitals rose to USD 761 per unit (standard deviation ± USD 294) (Shander 2010).
The impact of the storage age of red cells has been addressed in other systematic reviews (Shah 2018; Steiner 2015; Trivella 2019). Treatment options other than red cell transfusions for anaemia include erythropoietin and oral, or intravenous, iron therapy, which have been the topics of other recent trials and reviews (Richards 2020; Roman 2020).
How the intervention might work
The main clinical rationale for transfusing RBCs in anaemic patients is to improve oxygen delivery to tissue beds and vital organs such as the myocardium and brain. Transfusions may reduce compensatory work done by the heart to increase cardiac output in the face of anaemia. These benefits may manifest as better functional activity in patients and, ultimately, improved survival. Red blood cell transfusion is one of the few readily available treatments that consistently raises haemoglobin concentration and may restore tissue oxygenation adequately when oxygen demand exceeds supply (Wang 2010).
There is a long history of randomised controlled trials that have compared outcomes for participants allocated to different policies or schedules of red cell transfusion; these have now been completed and reported (Mueller 2019; NIH 1988). These studies presented results after randomising participants to either 'restrictive' triggers (where, typically, participants are transfused only when their haemoglobin concentration falls below 7.0 g/dL to 8.0 g/dL) or 'liberal' triggers (where participants are transfused at a higher haemoglobin concentration of around 9.0 g/dL to 10.0 g/dL). Historically, the widely accepted clinical standard was to transfuse patients when haemoglobin level dropped below 10.0 g/dL or when haematocrit fell below 30% (Wang 2010). Many guidelines based on the evolving evidence base now recommend that a range of haemoglobin values between 6.0 g/dL to 10.0 g/dL can be safely used for directing transfusions, depending on the presence of serious comorbidity (AAGBI 2008; ASA 2006; Carson 2012a; Carson 2016a; Mueller 2019; Napolitano 2009).
Why it is important to do this review
Much of the earlier evidence comparing restrictive and liberal thresholds for red cell transfusion comes from trials based in critical care. In 1999, the landmark TRICC trial (transfusion requirements in critical care) reported similar mortality in participants transfused at a restrictive trigger less than 7.0 g/dL compared with a liberal trigger less than 10.0 g/dL (Hébert 1999). The number of randomised trials continues to expand, as has been reported in previous iterations of this Cochrane Review (Carless 2010b; Carson 2012b; Hill 2000; Hill 2002; Hill 2005). By 2012, the number of participants enrolled in trials had doubled from 6264 to 12,587 (Carson 2012b); this number rose to 19,049 participants in a targeted update published in 2018, which specifically focused on patients with cardiovascular disease (Carson 2018). As further new trials continue to be published, there remains an ongoing need to update this systematic review, to ensure that new and updated guidelines on the use of red cell transfusions are based on the most recent literature reports of the effectiveness and safety of RBC transfusion (Carson 2016a). In addition, new studies focus on relevant and specific clinical contexts, for which previous levels of evidence for supporting best practice were very limited. This allows this updated review to inform transfusion practice in relevant subpopulations of patients.
The purpose of this updated review was to identify, appraise, and summarise the data from all randomised controlled trials (RCTs) that studied the clinical impact of varying thresholds for transfusion with RBCs. We remain interested in whether results of RCTs support the trend for increasingly restrictive RBC transfusion practices across different trial settings without harm to patients and to what extent RBCs need to be given more liberally in selected patient subgroups.
Objectives
The aim of this review update was to compare 30‐day mortality and other clinical outcomes for participants randomised to restrictive versus liberal red blood cell (RBC) transfusion thresholds (triggers) for all clinical conditions. The restrictive transfusion threshold uses a lower haemoglobin concentration as a threshold for transfusion (most commonly, 7.0 g/dL to 8.0 g/dL), and the liberal transfusion threshold uses a higher haemoglobin concentration to direct transfusion (most commonly, 9.0 g/dL to 10.0 g/dL).
Methods
Criteria for considering studies for this review
Types of studies
To examine evidence for the effects of transfusion thresholds on the use of red blood cell (RBC) transfusions and evidence for any change in clinical outcomes, we included randomised controlled trials (RCTs) in which comparison groups were assigned on the basis of a transfusion 'threshold' (sometimes termed a 'trigger'), defined as haemoglobin concentration or haematocrit level (with or without a specified level of haemodynamic instability) that had to be reached before RBC transfusion was administered. We required trials in which groups of participants were transfused with RBCs at higher haemoglobin or haematocrit levels (transfusion threshold) than those in a lower transfusion group, or were compared to those transfused in accordance with current standard transfusion practices. We excluded trials that were not designed to include any clinical outcomes relevant to this review.
Types of participants
We included trials of surgical or medical participants, involving adults or children, or both. We excluded studies enrolling neonates, given the distinct pathophysiology and clinical features of neonate anaemia, which is the topic of a separate Cochrane Review (Whyte 2011).
Types of interventions
The intervention considered was use of transfusion thresholds ('triggers') as a means of guiding allogeneic or autologous RBC transfusion, or both. A liberal transfusion threshold most often refers to transfusion of blood when the haemoglobin level falls below 9.0 g/dL to 10.0 g/dL. A restrictive transfusion threshold most often refers to transfusion of blood when the haemoglobin level falls below 7.0 g/dL to 8.0 g/dL.
We also included trials that compared transfusion and no transfusion while defining the no transfusion group as the restrictive strategy. Such trials may define a second threshold as a lower limit under which participants' haemoglobin should not fall without initiation of transfusion; this is consistent with all other trials in which clinical discretion is allowed for severe symptomatic anaemia.
Types of outcome measures
We evaluated clinical outcomes for efficacy, and we assessed complications of transfusion for safety.
Primary outcomes
The primary outcome for the analysis was 30‐day mortality. Mortality is a clinically relevant outcome that is widely cited in studies including patients with acute illness, critical illness, and perioperative care.
Secondary outcomes
We examined three categories of secondary outcomes:
mortality at different time intervals;
morbidity outcomes;
subgroups for mortality and morbidity.
We recorded and analysed mortality at different time points, including during hospital admission, at 90 days, and over the long term (median follow‐up, 3.1 years).
We evaluated morbidity that occurred during hospitalisation, including cardiac events (both as a composite outcome that included myocardial infarction, cardiac arrhythmias, cardiac arrest, pulmonary oedema, and angina, and individually when feasible), non‐fatal and fatal myocardial infarction, congestive heart failure, cerebral vascular accident (stroke), rebleeding, infection, thromboembolism, renal failure, mental confusion, function, and fatigue.
Infection was defined in three ways: sepsis or bacteraemia, pneumonia alone, or pneumonia plus wound infection. For the 2021 update, we added a specific outcome of 'transfusion‐specific reactions', as defined and reported in included studies. These events are uncommon, but they are important.
We defined all morbidity outcomes according to the definitions provided in individual trials. We evaluated subgroups based on transfusion thresholds and clinical context.
We recorded information on quality of life and functional outcomes. We also compared use of RBC transfusion as a measure of implementation of the transfusion intervention between groups by proportions of participants exposed to transfusion, units of blood transfused, and mean haemoglobin levels.
As this review is an update, we have continued to include some of these secondary outcomes for historical reasons. As stronger evidence is accrued, we believe that in future updates of this review, reporting of some of these outcomes may need to be modified or omitted.
Search methods for identification of studies
Electronic searches
We searched the following databases and ongoing trial registries:
CENTRAL (Cochrane Central Register of Controlled Trials; 2020, Issue 11), in the Cochrane Library (www.cochranelibrary.com);
MEDLINE via OvidSP (from 1946 to 16 November 2020);
Embase via OvidSP (from 1974 to 16 November 2020);
PubMed (for e‐publications ahead of print only, on 16 November 2020);
Transfusion Evidence Library (www.transfusionevidencelibrary.com; 1950 to 16 November 2020);
Web of Science Conference Proceedings Citations Index (CPCI‐S, 1990 to 16 November 2020);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched to 16 November 2020);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched to 16 November 2020).
We combined searches in MEDLINE and Embase with adaptations of the Cochrane RCT search filter as detailed in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We did not restrict our search by date, language, or publication status. We present search strategies for the 2012 update in Appendix 1, for the 2016 update and trial registries in Appendix 2 and Appendix 3, respectively, and for the 2020 update in Appendix 4.
Searching other resources
We checked the references of all identified trials, relevant review articles, and current treatment guidelines for further literature. We limited these searches to 'first‐generation' reference lists (i.e. reference lists of papers retrieved directly by database searches).
We contacted experts in the field to identify information relevant to the review. When possible and when necessary, we contacted authors of published studies for clarification of trial methods and data. We emailed all authors of trials that did not report our primary outcome of 30‐day mortality, but this was not possible for older trials for which contact information was not available. We searched the reference lists of relevant reviews and transfusion trials.
Data collection and analysis
Selection of studies
Two review authors (JLC and SJS) independently screened the titles or abstracts of the search results, or both, and selected trials that met the inclusion criteria. We resolved disagreements by discussion until we reached consensus. We identified trials in which participants were randomised to a restrictive transfusion strategy (transfusion threshold or protocol, or both) or to a control group that was randomised to a liberal transfusion strategy.
Data extraction and management
JLC and Paul Carless (a prior review author) extracted all data for earlier versions of this review. For this 2021 update, JLC and SJS independently extracted study characteristics and outcomes of new trials added since the last review, using a data extraction form. Information recorded on the extraction form included study type, presence of a transfusion threshold, transfusion protocol, type of surgery involved, clinical setting, treatment outcomes, and general comments, as well as details relevant to assessment of risk of bias for key domains described below. JLC entered data into Review Manager 5.4; NR checked data; JD added new items into tables to meet contemporary MECIR (Methodological Expectations for Cochrane Intervention Reviews) standards, which were checked by both JLC and SJS. We contacted authors of trials to request missing data.
We used the data extraction form to record data on the following outcomes:
number of participants exposed to allogeneic blood;
amount of allogeneic blood transfused;
number of participants receiving any transfusion (allogeneic blood, autologous blood, or both).
For trials involving surgical participants, we recorded the following:
postoperative complications (infection, haemorrhage, non‐fatal myocardial infarction, cardiac events, renal failure, stroke, thromboembolism, pulmonary oedema, mental confusion);
mortality, blood loss, haemoglobin and haematocrit levels (on admission, pre‐ and post‐transfusion, and at discharge);
demographics (age, sex);
type of surgery; and
medical condition.
We extracted data for allogeneic blood transfusion if it was expressed as packed RBCs. We documented information regarding the use of fresh frozen plasma or platelets, or both.
Assessment of risk of bias in included studies
We used the Cochrane tool for assessing risk of bias as described in Section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
JLC, SJS, and JD assessed the following domains for each study:
sequence generation;
allocation concealment;
blinding (assessments were made separately with regard to objective (e.g. mortality) and subjective (e.g. self‐reported quality of life) outcomes);
incomplete outcome data;
selective outcome reporting; and
other potential sources of bias.
We completed a risk of bias table for each trial, incorporating a description of the trial's performance against each of the above domains and our overall judgement of the risk of bias for each entry as follows: 'low', 'unclear' (indicating unclear or unknown risk of bias), or 'high' risk of bias.
Measures of treatment effect
We obtained the risk ratio (RR) for allogeneic blood transfusion in the intervention group compared with the control group and corresponding 95% confidence intervals (CIs) for each trial. We adopted a similar approach for other outcomes of transfusion. When the event rate was low, we considered using the Peto odds ratio when criteria for this method were fulfilled. We also entered the mean number of units of RBCs transfused to each group and the corresponding standard deviations. We used the mean difference (MD) and 95% CI to express average mean reduction in the number of units of RBC administered to the intervention group compared with the control group.
Unit of analysis issues
The unit of analysis was the participant. In all trials except one (Jairath 2015), randomisation was done at the individual participant level. In this trial in people with gastrointestinal bleeding, randomisation was done at the level of the hospital (cluster), but analysis occurred at the level of the individual participant. The intraclass correlation coefficient (ICC) was very low (0.0001) for the outcome of mortality; therefore we included the data and considered the participant as the unit of randomisation and ignored the clustering. We performed a sensitivity analysis from which we excluded this trial, to see what effect, if any, this had on the analysis. We did not evaluate any outcomes with repeated measures.
Dealing with missing data
We performed all analyses on an intention‐to‐treat basis. We undertook no imputations for missing data. We received information on 30‐day mortality from three authors (DeZern 2016; Villanueva 2013; Webert 2008). Levels of missing data were never higher than 10%, which we consider acceptable.
Assessment of heterogeneity
We examined statistical heterogeneity using both the I² statistic and the Chi² test. The I² statistic describes the percentage of total variation across studies due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity; moderate or substantial heterogeneity is considered to exist when I² exceeds 50% or 85%, respectively (Higgins 2011). For the Chi² test, we used a P value < 0.10 to indicate the presence of statistically significant heterogeneity. Because of the anticipated significant clinical heterogeneity of trials, we analysed data using a random‐effects model. We also anticipated a high level of heterogeneity related to transfusion rates because practice in the different specialties of the trials would vary considerably according to specialty‐specific protocols. Therefore, as described later, we chose to provide a summary statistic for the outcomes of transfusion even when I² was very high, because of the clinically relevant information this provides.
Assessment of reporting biases
When more than 10 studies were available, we examined funnel plots for the primary outcome of 30‐day mortality and the proportion of participants transfused, to assess the potential for publication bias. We used the proportion of participants transfused because all trials reported this outcome, and this may reflect overall risk of publication bias better than 30‐day mortality, which was not reported in all of the trials. We sought evidence of selective outcome reporting by comparing plans from described registrations/protocols (when available) with final reports.
Data synthesis
We performed all analyses using Review Manager 5.4 software (Review Manager 5a). We entered data for numbers of participants exposed to red cell transfusions, anticipated to be allogeneic blood in most trials and patients. We present the results using haemoglobin concentration in grams per decilitre (g/dL). Based on study reporting, we converted haematocrit to haemoglobin concentration by dividing by three. When studies presented transfusion volume as millilitres (mL), we converted these amounts to units by dividing by 300 (as in most countries, a standard unit of red blood cells is 300 mL). We pooled data for all outcomes and presented data stratified by subgroups for the primary outcome of 30‐day mortality and proportion of participants transfused by using a random‐effects model (Der Simonian 1986), and we presented the pooled result along with its 95% CI. We used Peto odds ratios for outcomes with event rates less than 1%. For continuous variables, we estimated the pooled mean difference and the 95% CI by using the generic inverse variance method.
Subgroup analysis and investigation of heterogeneity
Prespecified subgroups, as established in prior reviews, consisted of the following clinical contexts:
acute blood loss/trauma;
cancer;
cardiac surgery;
critical care;
orthopaedic surgery;
myocardial infarction;
vascular surgery; and
haematological malignancy.
We examined 30‐day mortality and the proportion of participants exposed to transfusion stratified by the transfusion threshold (difference between liberal and restrictive transfusion thresholds: ≥ 2.0 g/dL and < 2.0 g/dL; and restrictive transfusion threshold < 7.0 g/dL versus one of 8.0 g/dL to 9.0 g/dL). We also examined a post hoc subgroup of enrolled participants with myocardial infarction compared with all other clinical specialties, and we combined cardiac surgery with myocardial infarction because of emerging evidence that participants with acute myocardial infarction might differ from other anaemic participants (Carson 2013).
For the primary outcome of 30‐day mortality, we also compared findings between prospectively registered trials and those that were unregistered, or were registered long after recruitment began. Blood components are not subject to the same legal requirements for prospective registration as medical devices or pharmaceutical interventions. As in prior versions of this review, we did not exclude unregistered trials published after 2010 (as per the Cochrane Injuries Group policy), and we did conduct analyses to consider differential impact of the results of all trials for which proof of prospective registration could (or could not) be confirmed.
Sensitivity analysis
We performed a sensitivity analysis to assess effects of studies with high risk of bias for allocation concealment for the primary outcome; however, as in earlier versions of the review, sensitivity analyses for secondary outcomes were not informative. We repeated the analysis while excluding the cluster randomised trial (Jairath 2015).
Summary of findings and assessment of the certainty of the evidence
We have presented judgements about the quality of evidence in a summary of findings table (according to guidelines developed by the GRADE Working Group) (Schünemann 2011). We rated the quality of evidence as 'high', 'moderate', 'low', or 'very low', according to the following five GRADE domain considerations of: risk of bias, inconsistency, indirectness, imprecision, and publication bias.
This table includes the following outcomes:
number of people receiving blood transfusions;
30‐day mortality;
myocardial infarction;
congestive heart failure;
cerebrovascular accident (stroke);
rebleeding; and
thromboembolism.
Results
Description of studies
Details of the selection process for, and characteristics of, the included studies are offered below, along with information about interventions and trial design.
Results of the search
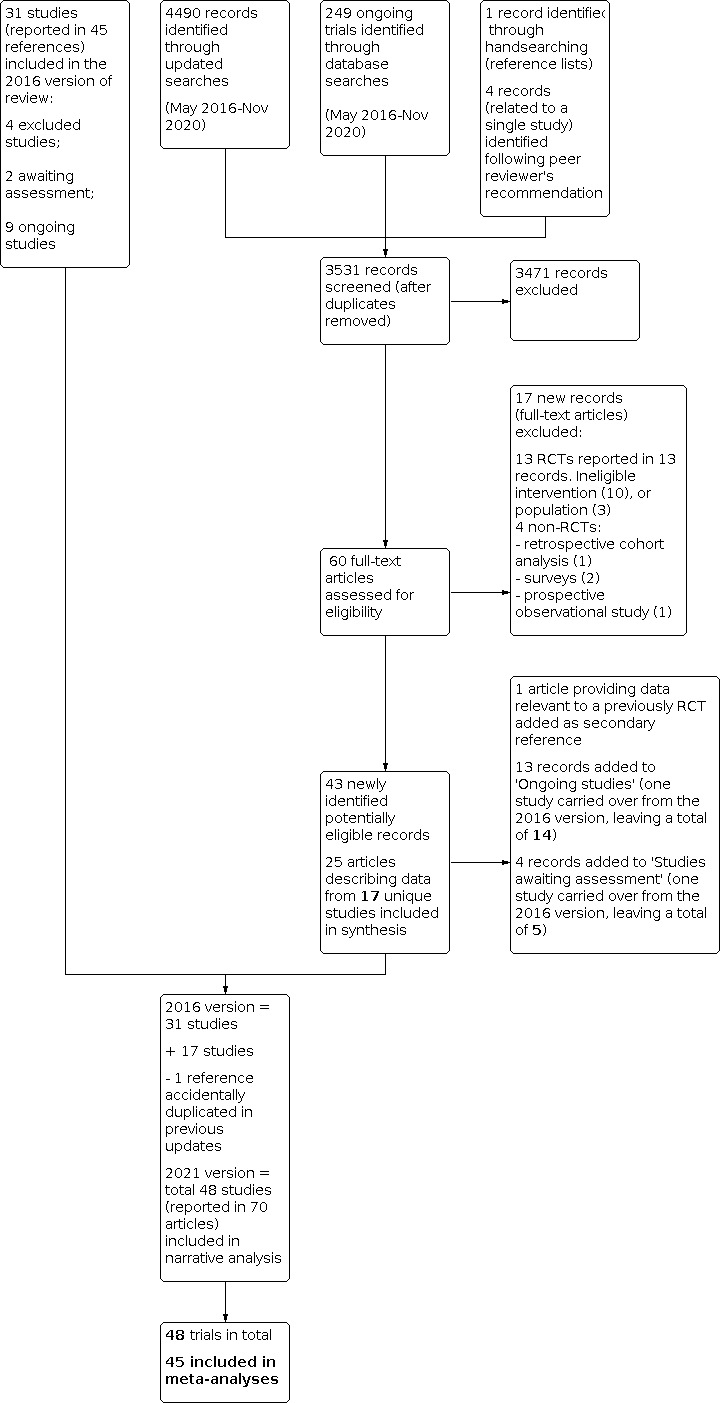
See Figure 1 for the PRISMA flowchart describing trial selection for the present update.
1.
Flow of studies for 2021 update
In the previous review, published in 2016, we included 31 studies.
For this 2021 update, we identified and analysed 17 additional trials (Akyildiz 2018; Bergamin 2017; Ducrocq 2021; Gillies 2020; Gobatto 2019; Hoff 2011; Jansen 2020; Koch 2017; Kola 2020; Laine 2018; Mazer 2017; Møller 2019; Palmieri 2017; Robitaille 2013; Stanworth 2020; Tay 2020; Yakymenko 2018) (Figure 1), leading to a total of 48. We identified one of these trials ‐ Ducrocq 2021 ‐ as an ongoing trial when we searched trials registers in November 2020; it has subsequently been published and is included in the analysis. We reviewed eligibility for one analysis after peer review and included data from it only in a narrative synthesis (Hoff 2011). This analysis treated the outcomes of two linked studies (DAHANCA 5 and DAHANCA 7) as a single trial (Hoff 2011a; Hoff 2011b; Overgaard 1998; Overgaard 2003).
Many of the included trials have been reported within multiple papers, which are included as secondary references. Whilst the focus of this review was the main (first) report of outcome data, reports of secondary or subgroup analyses (e.g. long‐term outcomes) occasionally offered complementary information useful for data extraction and assessment of bias.
Included studies
Participants
This updated systematic review includes a total of 21,433 trial participants (at baseline) across 48 trials described in 70 publications. By comparison, in the 2016 Cochrane review (Carson 2016b), we reported on an analysis of 31 trials that enrolled 12,587 participants.
The clinical context of the 48 trials was varied:
11 studies involved orthopaedic surgery (Carson 1998; Carson 2011; Fan 2014; Foss 2009; Gillies 2020; Gregersen 2015; Grover 2005; Lotke 1999; Nielsen 2014; Parker 2013; So‐Osman 2013);
seven involved critical care (de Almeida 2015; Gobatto 2019; Hébert 1995; Hébert 1999; Holst 2014; Palmieri 2017; Walsh 2013);
six examined acute blood loss (Blair 1986; Jairath 2015; Kola 2020; Prick 2014; Topley 1956; Villanueva 2013), four of which concerned gastrointestinal bleeding, one postpartum haemorrhage (Prick 2014), and one trauma (Topley 1956);
eight included cardiac surgery (Bracey 1999; Hajjar 2010; Johnson 1992; Koch 2017; Laine 2018; Mazer 2017; Murphy 2015; Shehata 2012);
three involved acute coronary syndrome (Carson 2013; Cooper 2011; Ducrocq 2021);
eight involved cancer, leukaemia, and haematological malignancies (Bergamin 2017; DeZern 2016; Hoff 2011; Jansen 2020; Stanworth 2020; Tay 2020; Webert 2008; Yakymenko 2018);
two were in vascular surgery (Bush 1997; Møller 2019);
three enrolled paediatric participants (Akyildiz 2018; Lacroix 2007; Robitaille 2013). Two trials were conducted in paediatric intensive care units (Akyildiz 2018; Lacroix 2007), and one trial involved bone marrow transplant recipients (Robitaille 2013).
Interventions
We noted variation in the definitions of transfusion strategies specified in the protocols, but most commonly, haemoglobin concentrations were used as 'triggers'. Four trials specified haematocrit values for the threshold (Cooper 2011; Hajjar 2010; Koch 2017; Johnson 1992). Four trials incorporated symptoms in addition to haemoglobin threshold in the restrictive transfusion strategy (Carson 2011; Carson 2013; Parker 2013; Prick 2014).
Transfusion thresholds by haemoglobin concentration in restrictive transfusion arms (44 trials) varied from 7.0 g/dL to 9.7 g/dL. The most common restrictive haemoglobin threshold for interventions was between 7.0 g/dL to 8.0 g/dL (35 trials). Two trials recruited patients in the outpatient chronic transfusion‐dependent population setting based on haemoglobin concentrations (Jansen 2020; Stanworth 2020), and thresholds for the intervention arms in these trials were higher, as might be expected for this population. Three trials defined a no‐transfusion strategy for the 'restrictive' arm (Hoff 2011; Parker 2013; Prick 2014), with provisions made for participants with clear signs of anaemia.
Restrictive haematocrit varied between 24% and 25% (equivalent to haemoglobin levels of around 8 g/dL) (Cooper 2011; Hajjar 2010; Johnson 1992; Koch 2017).
The most common transfusion threshold by haemoglobin concentration in the liberal transfusion arm was 9.0 g/dL to 10.0 g/dL. However, the liberal transfusion threshold varied and included:
100% of 'normal red cell volume' (Topley 1956);
two units of blood irrespective of clinical state (immediately in one trial (Blair 1986), postoperatively in another (Lotke 1999)); and
-
transfusion sufficient to maintain haemoglobin levels:
above 10 g/dL (Gregersen 2015; Hoff 2011; Jansen 2020; Robitaille 2013; Stanworth 2020; Webert 2008; Yakymenko 2018);
at 10 g/dL (Bush 1997; Carson 1998; Carson 2011; Carson 2013; Ducrocq 2021; Foss 2009; Grover 2005; Hajjar 2010; Hébert 1995; Hébert 1999; Jairath 2015; Parker 2013);
at 9.5 g/dL (Lacroix 2007; Shehata 2012);
at 9 g/dL (Bergamin 2017; Bracey 1999; de Almeida 2015; Gillies 2020; Gobatto 2019; Holst 2014; Murphy 2015; Tay 2020; Villanueva 2013; Walsh 2013);
at 8.9 g/dL (Prick 2014); and
at 8 g/dL (DeZern 2016; Kola 2020; Møller 2019).
Four trials used haematocrit levels when determining triggers (Cooper 2011 and Hajjar 2010 specified the liberal triggers as haematocrit levels of 30%; Koch 2017 specified a level of 28%, and Johnson 1992 a level of 32%).
Trial setting and design
See Table 2.
1. Trial setting details.
| Study ID | Number of participants at baseline | Country/Countries | Number of sites | Setting(s) | Year recruitment started |
| Mazer 2017 | 5092 | 19 countriesa | 73 | 73 sites ‐ varied | 2014 |
| Carson 2011 | 2016 | USA, Canada | 47 | 47 sites ‐ varied | 2004 |
| Murphy 2015 | 2003 | UK | 17 | 17 sites ‐ varied | 2009 |
| Holst 2014 | 1005 | Denmark, Sweden, Norway, Finland | 32 | 32 general ICUs | 2011 |
| Jairath 2015 | 936 | UK | 6 | University teaching hospitals | 2012 |
| Villanueva 2013 | 921 | Spain | 1 | General hospital | 2003 |
| Hébert 1999 | 838 | Canada | 25 | Tertiary (22), community ICU (3) | 1994 |
| Koch 2017 | 722 | USA (1), India (1) | 2 | 1 academic affiliated hospital in the USA, a private hospital in India | 2007 |
| Ducrocq 2021 | 668 | France, Spain | 35 | 35 sites ‐ varied | 2016 |
| Lacroix 2007 | 648 | Canada, Belgium, USA, UK | 19 | Tertiary paediatric ICU | 2001 |
| So‐Osman 2013 | 603 | Netherlands | 3 | Varied ‐ university and general hospitals | 2001 |
| Prick 2014 | 519 | Netherlands | 37 | Varied ‐ university and general hospitals | 2004 |
| Hajjar 2010 | 512 | Brazil | 1 | University teaching hospital | 2009 |
| Hoff 2011 | 466 | Denmark | ?? | Oncology centres | 1986 |
| Bracey 1999 | 428 | USA | 1 | University teaching hospital | 1997 |
| Palmieri 2017 | 345 | US (16 sites), Canada (1), New Zealand (1) | 18 | Specialist burn centres | 2010 |
| Tay 2020 | 300 | Canada | 4 | HCT sites | 2011 |
| Bergamin 2017 | 300 | Brazil | 1 | University teaching hospital | 2012 |
| Gregersen 2015 | 284 | Denmark | 1 | University teaching hospital | 2010 |
| Grover 2006 | 260 | UK | 3 | Acute hospitals | Not stated |
| Kola 2020 | 224 | India | 1 | Tertiary hospital | 2015 |
| Parker 2013 | 200 | UK | 1 | General hospital | 2002 |
| de Almeida 2015 | 198 | Brazil | 1 | Tertiary oncology university hospital | 2012 |
| Fan 2014 | 192 | China | 1 | University teaching hospital | 2011 |
| Akyildiz 2018 | 180 | Turkey | 1 | University teaching hospital | 2014 |
| Yakymenko 2018 | 133 | Denmark | 1 | University teaching hospital | 2010 |
| Lotke 1999 | 127 | USA | 1 | University teaching hospital | Not stated |
| Foss 2009 | 120 | Denmark | 1 | University teaching hospital | 2004 |
| Carson 2013 | 110 | USA | 8 | 8 sites ‐ varied | 2010 |
| Walsh 2013 | 100 | UK | 6 | Varied ‐ university and general hospitals | 2009 |
| Bush 1997 | 99 | USA | 1 | University teaching hospital | 1995 |
| DeZern 2016 | 89 | USA | 1 | Tertiary referral centre for oncology | 2014 |
| Carson 1998 | 84 | USA (3), UK (1) | 4 | University teaching hospitals | 1996 |
| Laine 2018 | 80 | Finland | 1 | University teaching hospital | 2014 |
| Hébert 1995 | 69 | Canada | 5 | Tertiary hospitals | 1993 |
| Nielsen 2014 | 66 | Denmark | 2 | University teaching hospital and general hospital | 2009 |
| Gillies 2020 | 62 | UK | 1 | University teaching hospital | 2017 |
| Webert 2008 | 60 | Canada | 4 | Tertiary oncology centres | 2003 |
| Møller 2019 | 58 | Denmark | 1 | General hospital | 2015 |
| Shehata 2012 | 50 | Canada | 1 | University teaching hospital | 2007 |
| Blair 1986 | 50 | UK | 1 | University teaching hospital | Not stated |
| Gobatto 2019 | 47 | Brazil | 1 | University teaching hospital | 2014 |
| Cooper 2011 | 45 | USA | 2 | Veterans' Affairs hospital centres | 2003 |
| Johnson 1992 | 39 | USA | 1 | University teaching hospital | Not stated |
| Stanworth 2020 | 38 | UK, Australia, New Zealand | 12 | 12 sites ‐ varied | 2015 |
| Topley 1956 | 22 | UK | 1 | 'Accident hospital' | Not stated |
| Januarysen 2020 | 19 | Netherlands | 3 | 1 university hospital, 2 general hospitals | 2002 |
| Robitaille 2013 | 6 | Canada | 1 | Not identified | 2009 |
aMazer 2017 (TRICS‐III): majority of sites in USA; sites also in Australia, Brazil, Canada, China, Colombia, Denmark, Egypt, Germany, Greece, India, Israel, Malaysia, New Zealand, Romania, Singapore, South Africa, Spain, and Switzerland.
The included studies were conducted at a total of nearly 400 sites within 26 countries. High‐income countries including Canada, the UK, and the USA contributed the bulk of both single‐site and multicentre studies, as well as co‐ordinating international multicentre studies. The next most common countries, in terms of providing settings for eligible trials, were Denmark, the Netherlands, Brazil, and France. Recruitment start dates for studies included within this review ran between 1955 and 2017, with a marked increase in the rate of new studies commencing recruitment from 2009 onwards.
A total of 24 of the 48 included studies were unregistered or were registered by investigators long after recruitment began. Although a majority of unregistered trials were relatively old, lack of prospective registration is a problem that persists to the present day.
In 47 of the 48 trials, the participant was the unit of randomisation and analysis. One trial used cluster randomisation by hospital (Jairath 2015). Sample sizes of included studies varied enormously (from 6 to 5092 participants randomised at baseline). Twenty‐six trials included 100 or more participants, and four trials included over 1000 participants each (Carson 2011; Holst 2014; Mazer 2017; Murphy 2015). Eleven of the included studies were described as pilot or feasibility studies (Carson 2013; DeZern 2016; Gillies 2020; Gobatto 2019; Hébert 1995; Jairath 2015; Møller 2019; Shehata 2012; Stanworth 2020; Webert 2008; Yakymenko 2018). We counted two linked studies in patients with head and neck squamous cell carcinoma before radiotherapy as one trial for the purpose of this review (Hoff 2011); the two component studies, DAHANCA 5 and DAHANCA 7, tested the same main trial intervention (nimorazole) and then applied a similar subrandomisation question to evaluate transfusion versus no transfusion, given concerns about poorer responses to radiation therapy due to a hypothesis of hypoxia‐induced radio‐resistance.
Excluded studies
In 2016, this review contained records of four excluded studies. In this 2021 update, we have excluded a further 17 studies; data for these studies were published in 22 publications (see Characteristics of excluded studies). Of the 21 excluded studies, 17 are ineligible RCTs (excluded largely on grounds of intervention or population); the remaining four are non‐randomised studies of different designs.
Studies awaiting classification
Brief details of five trials that are awaiting assessment are shown in the Studies awaiting classification section. Four have been completed but remain unpublished; we are considering how to handle data reported in the fifth (published) trial, which was of a complex, multifactorial design.
Ongoing studies
Brief details of 14 ongoing studies identified from searches of international trial registers are shown in the Ongoing studies section. When completed, and if eligibility criteria remain stable, results from these studies may add data from approximately 14,880 participants to this review, with five trials aiming to recruit over 1000 participants each. The latter (larger) studies are focusing on populations that are currently under‐represented in the studies included in this review, specifically, those with traumatic brain injury or cardiac/vascular disease.
Risk of bias in included studies
The risk of bias tables detail the assessment of studies for each domain and are summarised in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials. Forty‐eight trials are included in this review.
3.

'Risk of bias' summary: review authors' judgements about each methodological quality item for each included trial
Allocation
Sequence generation
We judged 41 trials to be at low risk of bias for this domain. We judged three trials to be at high risk of bias: one for basing the randomisation sequence on hospital record number, one for using coin‐tossing, and one because it mentioned using both a table of random numbers and odd/even (restrictive/liberal) allocation strategy. The remaining four trials presented insufficient information for us to be able to assess the adequacy of sequence generation, so we rated them as being at unclear risk.
Allocation concealment
We judged the risk of bias for this item to be low for 36 trials that used central allocation or sealed envelopes if appropriate safeguards (e.g. sequentially numbered envelopes) were used. We judged four trials to be at high risk of bias: one of these trials used a cluster design, so everyone in all hospitals knew to which group all participants had been assigned (Jairath 2015), one used a coin toss, one used hospital numbers that could be seen, and one used closed envelopes. We rated the risk for eight trials as unclear because the publications did not provide any information about how allocation was concealed.
Blinding
Performance bias
The nature of the intervention meant that blinding of clinicians involved in the care and administration of blood transfusions would not have been possible. Blinding of personnel for this intervention is also not feasible. In our view, for objective outcomes such as mortality (the primary outcome used within this review), it is appropriate to assess risk of bias as low.
Detection bias
Outcomes are assessed optimally when assessors are blinded to assignment. It is possible to blind the assessment of many outcomes by using, for example, an adjudication committee. In contrast, for some outcomes such as death, blinded assessment is less relevant. We classified risk of bias on the basis of the primary outcome of the trial (mortality) and on subjective outcomes, if reported, including functional measures and quality of life. We judged the risk of bias to be high for 11 trials for subjective outcomes.
Incomplete outcome data
We rated seven trials as being at high risk of bias for this domain, as data were missing for a large proportion of participants (20% to 45% of data for an outcome important to this review in six cases) or were missing disproportionately between arms (one trial).
Selective reporting
We rated 20 trials as being at unclear risk of bias for this domain, largely because evidence of prospective registration could not be confirmed. One trial (the oldest in the review, which recruited in the early 1950s) was assessed as being at high risk of bias for not reporting the groups in which deaths occurred. The remaining trials were assessed as having a low risk of bias for this domain.
Other potential sources of bias
We identified few other sources of bias. Small trials, including feasibility or pilot studies (which account for 20% of included trials), often reported small imbalances at baseline, as might be expected. Some trials were obliged to terminate prematurely due to slow recruitment. Only a limited number of trials described protocol violations for transfusions in detail, but these applied to both intervention arms. Overall, we assessed six of the 48 trials as having unclear risk of bias for this domain.
Effects of interventions
See: Table 1
Substantial variation in outcomes was reported in the included trials, which, in part, reflects their clinical settings.
Nearly all trials contributed to the analysis comparing the proportion of participants transfused in liberal and restrictive transfusion groups. Despite the heterogeneous methods and transfusion triggers reported in these RCTs, it was possible to pool data, to varying degrees, for each of the review outcomes. See Table 1.
Primary outcome
30‐Day mortality
The primary outcome of 30‐day mortality was reported by 31 trials (including 16,729 participants) in a form suitable for meta‐analysis. There was no difference in 30‐day mortality between restrictive and liberal transfusion strategies (risk ratio (RR) 0.99, 95% confidence interval (CI) 0.86 to 1.15; Analysis 1.1). Heterogeneity between these trials was not important (Chi² = 40.06, degrees of freedom (df) = 28 (P = 0.07); I² = 30%). The funnel plot demonstrates that the RR for 30‐day mortality is symmetrically distributed, which indicates there is not likely to be publication bias for this outcome (Figure 4).
1.1. Analysis.

Comparison 1: Mortality at 30 days, Outcome 1: 30‐Day mortality
4.

Funnel plot of comparison: 1 Mortality, outcome: 1.1 30‐Day mortality
Subgroup analysis of 30‐day mortality: restrictive threshold of 7.0 g/dL to 7.5 g/dL versus 8.0 g/dL to 9.0 g/dL
We examined 30‐day mortality and stratified it by the restrictive transfusion threshold used in the trials. Fifteen trials with 11,572 participants used a 7.0‐g/dL restrictive threshold. The RR for 30‐day mortality was 1.00 (95% CI 0.83 to 1.19; Analysis 1.2). Sixteen trials with 5157 participants used a restrictive threshold of 8.0 g/dL to 9.0 g/dL. The RR for 30‐day mortality was 0.97 (95% CI 0.75 to 1.24; Analysis 1.2). The test for subgroup differences did not show any differences between subgroups (Chi² = 0.04, df = 1 (P = 0.83), I² = 0%), indicating there was no difference in the mortality risk between the two thresholds.
1.2. Analysis.

Comparison 1: Mortality at 30 days, Outcome 2: 30‐Day mortality subgroup by restrictive haemoglobin level
Subgroup analyses of 30‐day mortality: clinical context
We examined 30‐day mortality and stratified it by the clinical context used in the trials: cardiac surgery, orthopaedic surgery, vascular surgery, acute blood loss or trauma (analyses for this grouping for 30‐day mortality included gastrointestinal (GI) bleeding only), critical care, acute myocardial infarction, and haematological malignancies. The overall RR for 30‐day mortality stratified by clinical specialty was 0.99 (95% CI 0.86 to 1.14; 31 trials, 16,729 participants; Analysis 1.3). There were no differences in 30‐day mortality between subgroups (Chi² = 6.73, df = 6 (P = 0.35); I² = 10.9%).
1.3. Analysis.

Comparison 1: Mortality at 30 days, Outcome 3: 30‐Day mortality subgroup analysis by clinical specialties
Cardiac surgery
Four trials conducted in 7411 patients undergoing cardiac surgery reported 30‐day mortality. The RR for 30‐day mortality for a restrictive compared to a liberal transfusion strategy was 0.99 (95% CI 0.74 to 1.33; Analysis 1.3.1).
Orthopaedic surgery
Eight trials of orthopaedic surgery contributed data from 3111 participants for 30‐day mortality. There was no clear effect of a restrictive compared to a liberal transfusion threshold (RR 1.16, 95% CI 0.75 to 1.79; Analysis 1.3.2).
Vascular surgery
Two trials contributed data from 157 participants for 30‐day mortality. The RR for 30‐day mortality was 0.98 (95% CI 0.30 to 3.25; Analysis 1.3.3).
Acute blood loss or trauma
Three trials reported mortality at 30 days among 1522 participants with acute blood loss or trauma (GI bleeding). Mortality was significantly lower when a restrictive strategy rather than a liberal strategy was used (RR 0.65, 95% CI 0.43 to 0.97; Analysis 1.3.4).
Critical care
Nine trials including 3529 participants receiving critical care for heterogeneous reasons contributed data for this outcome. The RR showed no clear effect of a restrictive compared to a liberal transfusion strategy (RR 1.06, 95% CI 0.85 to 1.32; 9 trials, 3529 participants; I² = 55%; Analysis 1.3.5).
Acute myocardial infarction
Three trials provided data from 820 participants with acute myocardial infarction and evaluated mortality; for this subgroup, mortality risk was higher in the restrictive strategy group than in the liberal strategy group (RR 1.61, 95% CI 0.38 to 6.88, Analysis 1.3.6). We carried out a post hoc subgroup analysis that compared 30‐day mortality for acute myocardial infarction participants versus all other participants but found no differences. The P value for subgroup differences was 0.50 (Chi² = 0.45, df = 1; I² = 0%; Analysis 1.4). Although we observed a moderately elevated RR for myocardial infarction participants (RR 1.61), the three included trials were modest in size, and hence, the pooled 95% confidence interval is very wide.
1.4. Analysis.

Comparison 1: Mortality at 30 days, Outcome 4: 30‐Day mortality by clinical specialties: myocardial infarction vs all others
Haematological malignancies
Two small trials provided data on 30‐day mortality among 149 participants. The 95% confidence interval was very wide, and no conclusions can be drawn for this subgroup (RR 0.37, 95% CI 0.07 to 1.95; 2 trials, 149 participants; I² = 0%; Analysis 1.3.7).
Mortality by cardiac surgery, vascular surgery, myocardial infarction, and all others
We examined 30‐day mortality and stratified it by the clinical context used in trials in a grouping comparing cardiac surgery, vascular surgery, myocardial infarction, and a group combining all other included trials. The overall RR for 30‐day mortality stratified by clinical specialty was (to repeat findings above) 0.99 (95% CI 0.86 to 1.14; 31 trials, 16,729 participants; Analysis 1.3). Again there were no differences in 30‐day mortality (test for subgroup differences: Chi² = 0.43, df = 3 (P = 0.93), I² = 0%; Analysis 1.5).
1.5. Analysis.

Comparison 1: Mortality at 30 days, Outcome 5: Mortality by cardiac surgery, vascular surgery, myocardial infarction, and all others
Subgroup analysis of 30‐day mortality: prospectively registered versus unregistered trials or trials for which registration was post hoc
We stratified 30‐day mortality according to whether or not trials were prospectively registered. Of the 31 trials that contributed data to our primary outcome, 18 (with 12,932 participants) were prospectively registered. The RR for 30‐day mortality provided by pooling data from these trials was 1.08 (95% CI 0.89 to 1.31). Pooling of the 13 unregistered trials (3797 participants) led to a RR of 0.81 (95% CI 0.66 to 1.00). The test for subgroup differences indicated a difference between subgroups: Chi² = 4.06, df = 1 (P = 0.04), I² = 75.4%; Analysis 2.1), but in neither group was there a clear effect for either transfusion strategy.
2.1. Analysis.

Comparison 2: Subgroup analysis by prospective registration, Outcome 1: 30‐Day mortality
Sensitivity analysis
There were no differences in 30‐day mortality between trials with low versus unclear or high risk of bias in one bias domain (i.e. allocation concealment) (Analysis 3.1). The RR was 1.01 (95% CI 0.87 to 1.18) in trials with low risk of bias for allocation concealment and 0.84 (95% CI 0.51 to 1.39) for trials with unclear or high risk of bias for allocation concealment. Testing for subgroup differences yielded the following: Chi² = 0.47, df = 1 (P = 0.49); I² = 0%.
3.1. Analysis.

Comparison 3: Sensitivity analysis by allocation concealment, Outcome 1: 30‐Day mortality
Secondary outcomes
As detailed below, none of the other analyses on mortality or morbidity showed differences between the groups compared.
Mortality at other time intervals
We analysed mortality at hospital discharge (15 trials; 6597 participants; Analysis 4.1), at 90 days (7 trials, 4143 participants; Analysis 4.2), and at six months or longer (2 trials, 4702 participants; Analysis 4.3).
4.1. Analysis.

Comparison 4: Mortality: other time intervals, Outcome 1: Hospital mortality
4.2. Analysis.

Comparison 4: Mortality: other time intervals, Outcome 2: 90‐Day mortality
4.3. Analysis.

Comparison 4: Mortality: other time intervals, Outcome 3: 6‐Month mortality
There were no differences in mortality between transfusion strategies at hospital discharge (RR 0.86, 95% CI 0.72 to 1.03; Chi² = 15.36, df = 13 (P = 0.29); I² = 15%), but the 90‐day mortality was higher for the restrictive strategy (RR 1.13, 95% CI 1.02 to 1.25; Chi² = 5.28, df = 6; P = 0.41; I² = 0%).
The two largest included trials (Carson 2011; Mazer 2017), reported mortality at six months or beyond in separate publications (Carson 2015; Mazer 2018). Results suggest no clear differences (RR 0.98, 95% CI 0.79 to 1.22; P = 0.84; Analysis 4.3). Both trials employed similar transfusion strategies.
The results of mortality analyses at hospital discharge, at 30 days, and at six months are consistent. The results of mortality analyses at 90 days were gathered from a smaller number of participants and are dominated by two particular trials (Bergamin 2017; Holst 2014), limiting interpretation.
Complex analysis in Hoff 2011 had the main purpose of defining a role for the drug nimorazole in patients receiving radiotherapy with head and neck squamous cell carcinoma (HNSCC); additional randomisation steps addressed the value of transfusion in participants who had low haemoglobin levels preradiation. This analysis combined data from a trial comparing the drug with placebo and from another comparing drug delivery at different intervals. We could not incorporate five‐year mortality data within our meta‐analysis, but investigators found that although transfusion improved haemoglobin levels before and during radiation treatment, it did not improve other outcomes for patients and may have had a negative impact on survival.
Clinical outcomes
We noted no differences in any groups compared for any of the clinical outcomes.
Cardiac events
Eleven trials reported data on post‐enrolment cardiac events in 5577 participants. Risks of cardiac events (myocardial infarction, cardiac arrhythmias, cardiac arrest, pulmonary oedema, and angina) were not increased by the use of restrictive transfusion strategies (RR 1.03, 95% CI 0.80 to 1.32; Analysis 5.1). Heterogeneity between these trials was moderate (Chi² = 24.09, df = 10 (P = 0.007); I² = 58%). It is possible that participants were counted in more than one category for this composite outcome because these disorders are clinically inter‐related (e.g. a participant could have angina that might lead to pulmonary oedema).
5.1. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 1: Cardiac events
Myocardial infarction
Twenty‐three trials reported data for myocardial infarction (fatal and non‐fatal) for 14,730 participants after random allocation to liberal or restrictive transfusion arms. There was no difference between restrictive and liberal transfusion strategies (RR 1.04, 95% CI 0.87 to 1.24; Analysis 5.2). We found no evidence of heterogeneity between trials (Chi² = 18.63, df = 21; P = 0.61; I² = 0%).
5.2. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 2: Myocardial infarction
Congestive heart failure
Sixteen trials reported data for congestive heart failure in 7247 participants. There was no difference between restrictive and liberal transfusion strategies (RR 0.83, 95% CI 0.53 to 1.29; Analysis 5.3). Heterogeneity between trials was moderate (Chi² = 22.06, df = 13; P = 0.05; I² = 41%).
5.3. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 3: Congestive heart failure
Cerebrovascular accident: stroke
Nineteen trials reported data for stroke in 13,985 participants. There was no difference between restrictive and liberal transfusion strategies (RR 0.84, 95% CI 0.64 to 1.09; Analysis 5.4). Heterogeneity between trials was not important (Chi² = 12.80, df = 18; P = 0.80; I² = 0%).
5.4. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 4: Cerebrovascular accident (CVA) ‐ stroke
Rebleeding
Eight trials reported data for rebleeding in 3412 participants. There was no difference between restrictive and liberal transfusion strategies (RR 0.80, 95% CI 0.59 to 1.09; Analysis 5.5). Heterogeneity between trials was not important (Chi² = 12.24, df = 7; P = 0.09; I² = 43%).
5.5. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 5: Rebleeding
Sepsis/bacteraemia
Nine trials reported data for sepsis/bacteraemia in 4352 participants. There was no difference between restrictive and liberal transfusion strategies (RR 1.06, 95% CI 0.86 to 1.30; Analysis 5.6). Heterogeneity between these trials was not important (Chi² = 8.56, df = 7; P = 0.29; I² = 18%).
5.6. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 6: Sepsis/bacteraemia
Pneumonia
Sixteen trials reported data for pneumonia in 6666 participants. There was no difference between restrictive and liberal transfusion strategies (RR 0.97, 95% CI 0.84 to 1.13; Analysis 5.7). Heterogeneity between these trials was not important (Chi² = 11.48, df = 15; P = 0.72; I² = 0.0%).
5.7. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 7: Pneumonia
Infection
Twenty‐five trials including 17,104 participants reported data for all infections defined as sepsis/bacteraemia, pneumonia, and wound infection. There was no difference between restrictive and liberal transfusion strategies (RR 0.97, 95% CI 0.88 to 1.07; Analysis 5.8). Heterogeneity between these trials was not important (Chi² = 21.42, df = 14; P = 0.09; I² = 35%).
5.8. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 8: Infection
Thromboembolism
Thirteen trials reported data for thromboembolism for 4201 participants. We calculated the odds ratio using the Peto method because the risk of thromboembolism was less than 1%. There was no difference between restrictive and liberal transfusion strategies (Peto odds ratio 1.11, 95% CI 0.65 to 1.88; Analysis 5.9). Heterogeneity between these trials was not important (Chi² = 14.48, df = 11; P = 0.21; I² = 24%).
5.9. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 9: Thromboembolism
Renal failure
Fifteen trials reported data on renal failure in 12,531 participants. There was no difference between restrictive and liberal transfusion strategies (RR 1.03, 95% CI 0.92 to 1.16; Analysis 5.10). Heterogeneity between these trials was not important (Chi² = 12.77, df = 14; P = 0.55%; I² = 0%).
5.10. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 10: Renal failure
Mental confusion
Nine trials reported data for mental confusion in 6442 participants. There was no difference between restrictive and liberal transfusion strategies (RR 1.11, 95% CI 0.88 to 1.40; Analysis 5.11). Heterogeneity between these trials was not important (Chi² = 10.29, df = 8; P = 0.24; I² = 22%).
5.11. Analysis.

Comparison 5: Morbidity: clinical outcomes, Outcome 11: Mental confusion
Functional recovery and fatigue
In total, 24 trials reported results showing differing scores for functional and mental outcomes and fatigue (Bracey 1999; Carson 1998; Carson 2011; de Almeida 2015; DeZern 2016; Fan 2014; Foss 2009; Gillies 2020; Gobatto 2019; Gregersen 2015; Hajjar 2010; Jairath 2015; Jansen 2020; Koch 2017; Lotke 1999; Murphy 2015; Nielsen 2014; Parker 2013; Prick 2014; So‐Osman 2013; Stanworth 2020; Tay 2020; Walsh 2013; Yakymenko 2018). However, there was considerable heterogeneity in the methods and questionnaires used, and in the timing of assessments, which precluded meta‐analysis. No larger trials reported significant differences in trial‐specific functional or mental outcomes, or fatigue. Exploratory findings for quality of life measures from two trials in a haematological setting showed possible beneficial effects of liberal transfusion, but these results need to be evaluated in larger trials (Stanworth 2020; Yakymenko 2018). Three trials reported functional outcomes for orthopaedic surgery participants, but assessment of these functional measures in different ways precluded pooling in a meta‐analysis.
Other outcomes ‐ blood transfusions and haemoglobin
Results for transfusion and haemoglobin data were presented across the included trials, and provide key information about the implementation of transfusion protocols in trials. We anticipated high levels of heterogeneity in the analysis of transfusion outcomes, for several reasons. In particular, standard 'control' rates of transfusion practice are highly variable across the clinical specialties in which trials were identified for this update. These differing policies regarding rates of transfusion reflect practice defined in specialty guidelines and recommendations. It is usually recommended that pooled estimates are not presented when heterogeneity is so high. However, we present the pooled results here, as there was consistency regarding the direction of effect; further justification for this is provided in the Discussion.
Proportion of participants transfused
This analysis demonstrates differences in the proportions of participants transfused with RBCs in the liberal and restrictive trial arms. Data on the proportions of transfused participants were available from 42 trials (20,057 participants). The implementation of a restrictive transfusion trigger across all trials reduced the relative risk of receiving at least one RBC transfusion by 41% (RR 0.59, 95% CI 0.53 to 0.66; Analysis 6.1). Heterogeneity between these trials was substantial (Chi² = 1104.24, df = 41 (P < 0.00001); I² = 96%); however, there was consistency in the direction of the effect.
6.1. Analysis.

Comparison 6: Blood transfusions, Outcome 1: Participants exposed to blood transfusion (all trials)
The proportions of participants transfused in liberal and restrictive trial arms were very different across different clinical contexts (Analysis 6.2); differences between subgroups were manifest (Chi² = 25.33, df = 6 (P = 0.0003); I² = 76.3%). There was a tendency for great variation within subgroups also (e.g. in the subgroup of critical care trials, heterogeneity was high (I² = 92%)). The acute blood loss/trauma subgroup included diverse underlying illnesses for haemorrhage, including comorbidities, leading to an I² of 96%. For example, Prick 2014 recruited young (otherwise healthy) women with postpartum haemorrhage, and Jairath 2015 enrolled older participants with gastrointestinal bleeding, characterised by many comorbidities. Prick 2014 contributed to a large extent to the high heterogeneity in this subgroup, and temporarily removing it from the analysis reduced heterogeneity to 77%. By contrast, participants enrolled in the subgroup of cardiac surgery trials demonstrated less variability in risk of transfusion across trials, and in this subgroup, we observed no important heterogeneity. This sensitivity analysis, although post hoc, highlights how transfusion policies in this setting differed from adult protocols in a critical care setting.
6.2. Analysis.

Comparison 6: Blood transfusions, Outcome 2: Participants exposed to blood transfusion by clinical specialties
When the difference in haemoglobin thresholds between restrictive and liberal arms was 2.0 g/dL or more, the RR of transfusion was 0.57 (95% CI 0.50 to 0.64; Analysis 6.3.1), which means there was a reduction in transfusion of 43% in the restrictive arm compared to the liberal arm. When the difference in haemoglobin transfusion thresholds between restrictive and liberal transfusion arms was less than 2.0 g/dL, the RR was 0.80 (95% CI 0.63 to 1.02; Analysis 6.3.2), which means there was a reduction in transfusion of 20% (test for subgroup differences: Chi² = 6.11, df = 1 (P = 0.01), I² = 83.6%).
6.3. Analysis.

Comparison 6: Blood transfusions, Outcome 3: Participants exposed to blood transfusion (by transfusion threshold)
There was no clear difference in the proportions of participants transfused between the 17 studies (11,919 partipants) that used a restrictive strategy of 7.5 g/dL or less as a threshold (RR 0.55, 95% CI 0.48 to 0.64) versus the 19 studies (6035 participants) that used a restrictive threshold of 8.0 g/dL to 9.0 g/dL (RR 0.59, 95% CI 0.48 to 0.72; test for subgroup differences: Chi² = 0.18, df = 1 (P = 0.67), I² = 0%; Analysis 6.4).
6.4. Analysis.

Comparison 6: Blood transfusions, Outcome 4: Participants exposed to blood transfusion by transfusion threshold
The forest plot for the proportions of participants transfused displays grouping of trials with a RR around 0.5 for receiving a transfusion in the restrictive transfusion arm (Figure 5), which is consistent with the overall observation that participants in the restrictive arm were transfused approximately half as often as those in the liberal arm. As expected, there were no trials in which participants in the restrictive arm were transfused more often than those in the liberal arm.
5.

Funnel plot of comparison: 2 Blood transfusions, outcome: 2.1 Participants exposed to blood transfusion (all trials)
Quantity of RBCs transfused
Seventeen trials reported the quantities of blood transfused. Most trials (40) provided some information on dose of red cells, or an algorithm or a target haemoglobin for transfusion, although the level of detail varied considerably. Among the three paediatric trials, two trials indicated a range for transfusion dose of 10 mL/kg or 10 mL/kg to 15 mL/kg (Akyildiz 2018; Robitaille 2013), and one trial reported a target haemoglobin of 8.5 g/dL to 9.5 g/dL in the restrictive arm, and 11.0 g/dL to 12.0 g/dL in the liberal arm (Lacroix 2007).
Use of a restrictive transfusion trigger resulted in an average saving of 1.21 units of RBCs per transfused participant (weighted mean difference (MD) ‐1.21, 95% CI ‐1.67 to ‐0.75; Analysis 6.5). Heterogeneity between these trials again was substantial (Chi² = 1173.58, df = 16 (P < 0.00001); I² = 91%); however, there was consistency in the direction of the effect.
6.5. Analysis.

Comparison 6: Blood transfusions, Outcome 5: Units of blood transfused
Transfusion‐specific reactions
Transfusion reactions appeared to be neither well nor consistently reported, and over half (26/48) of the trials provided no information (a further two trials mentioned collecting data on such reactions but did not report them). Of the remaining 20 trials, eight reported prospectively seeking transfusion‐related reactions, but found that none had occurred in either group. Twelve trials reported transfusion reactions in a heterogeneous manner that was not suited to quantitative pooling, given the variability in methods of reporting and the assigning of severity and causality assessment to transfusion.
Haemoglobin or haematocrit concentration
Nineteen trials reported the difference in haemoglobin or haematocrit levels between liberal and restrictive transfusion arms. Measures included averages (e.g. averages of different data points across a participant's stay in ICU) as well as single data points (e.g. the last measurement before discharge). When we pooled data (without regard to timing), participants assigned to a restrictive strategy had a lower haemoglobin concentration than participants assigned to a liberal transfusion strategy (mean difference ‐1.26, 95% CI ‐1.55 to ‐0.96; analysis not shown). Heterogeneity between these trials was substantial (Chi² = 914.39, df = 18 (P < 0.00001); I² = 98%), however, there was consistency in the direction of the effect.
Morbidity outcomes in participants undergoing cardiac surgery or vascular surgery and in those with myocardial infarction
We analysed morbidity outcomes for a subgroup of participants with underlying cardiovascular disease, defined as those undergoing cardiac surgery or vascular surgery and those with myocardial infarction. There was no difference between restrictive and liberal transfusion strategies for myocardial infarction (RR 1.01, 95% CI 0.81 to 1.26; 8 trials, 8219 participants; Analysis 7.1); renal failure (RR 1.07, 95% CI 0.89 to1.28; 7 trials, 9198 participants; Analysis 7.2); infection (RR 1.00, 0.79 to 1.28; 8 trials, 9219 participants; Analysis 7.3); thromboembolism (RR 1.02, 95% CI 0.11 to 9.55; 3 trials, 239 participants; Analysis 7.5); congestive heart failure (RR 0.77, 95% CI 0.24 to 2.43; 4 trials, 858 participants; Analysis 7.4); or cerebrovascular accident (RR 0.98, 95% CI 0.22 to 4.26; 4 trials, 905 participants; Analysis 7.6).
7.1. Analysis.

Comparison 7: Morbidity outcomes in participants undergoing cardiac surgery or vascular surgery, and with acute MI, Outcome 1: Myocardial infarction
7.2. Analysis.

Comparison 7: Morbidity outcomes in participants undergoing cardiac surgery or vascular surgery, and with acute MI, Outcome 2: Renal failure
7.3. Analysis.

Comparison 7: Morbidity outcomes in participants undergoing cardiac surgery or vascular surgery, and with acute MI, Outcome 3: Infection
7.5. Analysis.

Comparison 7: Morbidity outcomes in participants undergoing cardiac surgery or vascular surgery, and with acute MI, Outcome 5: Thromboembolism
7.4. Analysis.

Comparison 7: Morbidity outcomes in participants undergoing cardiac surgery or vascular surgery, and with acute MI, Outcome 4: Congestive heart failure
7.6. Analysis.

Comparison 7: Morbidity outcomes in participants undergoing cardiac surgery or vascular surgery, and with acute MI, Outcome 6: Cerebrovascular accident
Economic and costing analyses
The protocol for this review did not include plans for formal economic analysis: findings from included trials are reported as a narrative summary only. Many trials included within this review discussed the potential cost implications of favouring a restrictive strategy or recommended cost‐effectiveness analysis in future research without providing data (e.g. DeZern 2016; Lotke 1999; Stanworth 2020).
Investigators in three trials went further, making estimates based on their own data when different transfusion strategies were compared for patients with severe burns (Palmieri 2017), requiring hematopoietic cell transplantation (HCT) (Tay 2020), or with cardiac issues (Koch 2017). The former two reported that a restrictive strategy would reduce costs considerably; the latter stated only that "health care costs were similar between groups".
Of the six RCTs in which formal economic analysis was specified as an outcome, two were conducted in elective surgery (cardiac or infrainguinal) (Bush 1997; Murphy 2015). The smaller, older trial (n = 99) found a substantial difference favouring the restrictive group; the latter trial included more than 2000 participants and reported, "Total costs did not differ significantly between the groups". One trial on older, critically ill patients requiring mechanical ventilation reported increased costs for this population within the group treated with a restrictive strategy, but this was a small trial, and there was a small difference in survival outcomes (Walsh 2013).
A large trial considering outcomes of women with acute anaemia after postpartum haemorrhage concluded that intervention was more expensive per woman than non‐intervention, with only a small improvement in health‐related quality of life after RBC transfusion (Prick 2014).
A trial on upper GI bleeding was a feasibility trial and did report results, confirming that transfusions were an important driver of costs alongside inpatient stay and endoscopy (Campbell 2015; Jairath 2015). A large trial on myocardial infarction concluded that significant savings were likely with the use of a restrictive strategy, although a formal publication is still pending (Ducrocq 2021). For this trial, the cost‐effectiveness endpoint was the incremental cost‐effectiveness ratio (ICER) at 30 days. It is reported that "the restrictive strategy had an 84% probability of being cost‐saving while improving clinical outcomes, i.e. "dominant" from a medico‐economic standpoint" (conference proceeding; https://www.eurekalert.org/news-releases/775135).
Discussion
Summary of main results
When compared with liberal transfusion strategies, restrictive transfusion strategies did not increase or decrease the risk of 30‐day mortality (risk ratio (RR) 0.99, 95% confidence interval (CI) 0.86 to 1.15; 31 trials, 16,729 participants; I² = 30%; moderate‐quality evidence) or of any of the other outcomes assessed (i.e. myocardial infarction, stroke (high‐quality evidence), thromboembolism (moderate‐quality evidence), and congestive heart failure (low‐quality evidence)). Restrictive transfusion strategies led to a reduction of 41% in the number of participants who received at least one unit of blood; an overall red blood cell (RBC) transfusion requirement that was approximately 1.2 units lower per participant; and a mean haemoglobin concentration that was around 1.26 g/dL lower than in liberal transfusion groups.
These findings are based on an analysis of 48 randomised controlled trials (RCTs) in this updated review that compared outcomes for participants allocated to receive transfusions of RBCs at different haemoglobin concentration thresholds. These trials enrolled 20,967 participants across diverse patient populations; most participants were adults. Since our previous 2016 review, we have included an additional 17 randomised trials.
Meta‐analyses provided no evidence that restrictive transfusion policies harmed participants, or that participants benefited from the use of liberal transfusion policies, within the parameters defined in these trials. Put another way, there was no evidence of an impact on clinically important outcomes when a restrictive RBC transfusion policy rather than a liberal RBC transfusion policy was followed. Results indicate that transfusion strategy did not influence the risk of cardiovascular events, including myocardial infarction, congestive heart failure, or stroke, although statistical heterogeneity was observed in trials that evaluated congestive heart failure (P = 0.01; I² = 57%).
In this updated review, only three trials enrolled children and the results in paediatrics were dominated by findings from a single, large pragmatic trial (Lacroix 2007), which observed no benefit for liberal transfusion in critically ill children. Another very small randomised trial recruited only six children undergoing bone marrow transplantation and was stopped because of concerns about an excess of veno‐occlusive disease in the liberal arm (Robitaille 2013); we await the results of a further ongoing trial in this setting (ISRCTN17438123; see Ongoing studies).
Subgroup analyses
With regard to our predefined clinical subgroups, results indicate that risk of death and other adverse events were not impacted by liberal or restrictive transfusion thresholds for most analyses. This is important because there are pathophysiological reasons to postulate why transfusion might impact clinical outcomes differently in different patient populations as the result of factors such as duration of anaemia (short‐term transfusion dependence in critical illness versus long‐term transfusion dependence in bone marrow failure) or presence or absence of an underlying restriction in cardiac function (Docherty 2016).
However, for patients with acute blood loss and for those with acute myocardial infarction, mortality may be influenced by a liberal or restrictive transfusion strategy, although the test for differences in 30‐day mortality between subgroups showed no differences (P = 0.13; I² = 41.2%). In three trials (1522 participants) including people with gastrointestinal bleeding (included in the acute blood loss or trauma grouping), a restrictive transfusion strategy was associated with a 35% lower risk of 30‐day mortality compared with a liberal transfusion strategy. The mechanism responsible for this significantly reduced risk of death may be lower risk of rebleeding under restrictive transfusion regimens (RR 0.65, 95% CI 0.43 to 0.97; Analysis 1.3.4). The reason for this effect is not known, but it may reflect higher vascular pressures following transfusion in the liberal transfusion group compared with the restrictive transfusion group.
Patients with acute myocardial infarction are another important patient subpopulation. In the 2016 update (Carson 2016b), two small trials included people with myocardial infarction (154 participants) for whom 30‐day mortality was 3.88 times higher in the restrictive transfusion group than in the liberal transfusion group (95% CI 0.83 to 18.13). In one trial, 12.7% of participants (n = 14) undergoing cardiac catheterisation had stable coronary artery disease but did not have acute myocardial infarction (Carson 2013). These results have been extended by inclusion in the meta‐analysis of Ducrocq 2021, which enrolled 666 patients and reported results for a composite major adverse cardiac events (MACE) outcome of all‐cause mortality, stroke, recurrent myocardial infarction, or emergency revascularisation in 11.1% of the restrictive group and in 14.2% of the liberal group. Deaths from any cause occurred in 5.6% of the restrictive group and in 7.7% of the liberal group. With the addition of this trial, the relative risk for 30‐day mortality was closer to 1.0, with wide confidence intervals (RR 1.61, 95% CI 0.38 to 6.88). A 3500‐patient trial in acute myocardial infarction, called 'MINT', is currently under way and will inform this subgroup further (NCT02981407).
The nature of the restrictive transfusion intervention
Around half of the trials identified applied a restrictive threshold of 7.0 g/dL; the other half used 8.0 g/dL. The largest trial including cardiac surgery patients used a 7.5‐g/dL threshold (Mazer 2017). Most participants in the 7.0 g/dL restrictive transfusion threshold trials were based in critical care and acute settings of anaemia. Clinical specialties were more varied in trials that tested an 8.0 g/dL restrictive transfusion threshold and included orthopaedic and cardiac surgery, gastrointestinal bleeding, and acute myocardial infarction. However, there was no apparent difference in risk of death at 30 days between the two strata.
We compared 30‐day mortality in trials where the difference between liberal and restrictive transfusion thresholds in the trial protocol was at least 2.0 g/dL versus trials where the difference was less than 2.0 g/dL. Again, there was no evidence of a dose effect on clinical outcomes of RBC transfusion by different threshold levels of haemoglobin concentration.
Risks of infection and outcomes of recovery
In view of potential immunomodulatory effects of blood transfusion, we compared the risk of infection in three ways. We did not find evidence of an increased risk of infection associated with liberal transfusion. We combined all infections and also examined sepsis or bacteraemia and pneumonia (alone); comparative risks of infection between the two transfusion strategies were nearly identical for all of these analyses. These results varied from prior analyses in another systematic review, which reported an elevated risk of infection in the liberal transfusion group (Rohde 2014). However, they are consistent with later analyses (Carson 2016b; Carson 2018), which were based on a substantially larger number of trials.
Although 23 trials assessed functional recovery or quality of life and fatigue, these trials applied different measures or tools for assessment; therefore quantitative meta‐analysis could not be supported for these outcomes.
Overall completeness and applicability of evidence
As the number of trials expands, the completeness of evidence continues to increase. Clinical trials have now evaluated many of the most common clinical specialties in which RBCs are transfused. Thus, the findings of this review are widely applicable to most clinical contexts. However, we continue to lack knowledge and sufficient precision about the safety of different transfusion thresholds for some groups of patients who frequently receive transfusion, as an insufficient number of trials with adequate power have been published for these groups. These understudied clinical contexts include people with myocardial infarction, neurological injury/traumatic brain injury, acute neurological disorder, stroke, cancer/haematological malignancy, and chronic bone marrow failure. We anticipate some of these gaps may be filled relatively soon as new trials are completed, for which details and recruitment targets are listed in Characteristics of ongoing studies.
A core rationale for RBC transfusion is to improve tissue and cellular oxygenation, but technologies for monitoring this directly, or at a cellular level, are not available routinely. Therefore, haemoglobin concentration continues to be applied as the main surrogate marker of need for transfusion in our included trials, but it may not be a reliable biomarker and it may not support a more precise or personalised approach to transfusion therapy (Baek 2019). Trials that evaluate mechanistic and physiological variables alongside haemoglobin concentration are required (Møller 2019).
Quality of the evidence
Overall, the quality of evidence across trials is good and continues to show improvement over time. The number of trials and enrolled participants has increased substantially, and the precision of the estimate of the effect of transfusion improves with updates of this review. We found relatively little heterogeneity for each clinical outcome across all analyses.
Risk of bias evaluations revealed a variety of methodological issues between trials. For more recent trials, including those reporting on larger sample sizes, evaluations of risk of bias remained generally low risk. We applied Cochrane methods for defining high or low risk of bias to all trials, but we acknowledge a number of challenges, including how to assign a single level of bias for multiple outcomes, for example, incomplete data or blinding (masking). We therefore considered risk of bias for objective (mortality) and subjective (functional and quality of life) outcomes separately. We will explore this further in future updates of this review, possibly by employing the new Cochrane 'Risk of bias 2' tool. We recognise that blinding the use of transfusion at the bedside is difficult to achieve unless trial personnel are assigned to each participant, which would be an expensive procedure. The importance of blinding will differ according to the choice of primary trial outcome; mortality is a hard endpoint (as in this review) that is less open to bias than other functional outcomes.
Outcome assessment by observers who are blind to the treatment group is probably the most rigorous practical approach for transfusion threshold trials, but this is less relevant for outcomes such as mortality. We judged the risk of bias to be high for 11 trials for subjective outcomes, including functional outcomes and quality of life. This issue of detection bias for subjective outcomes will be explored in greater detail in a further update of this review, and informed by additional trial data (see Characteristics of ongoing studies; Characteristics of studies awaiting classification). Maintaining the integrity of the randomisation process becomes important if the trial is not to overestimate the benefit of the intervention (Schulz 1995). We judged the risk of bias for allocation concealment ‐ a key methodological domain ‐ to be low for 36 trials. Only a few trials in this review did not report the methods used to conceal the allocation sequence from treating clinicians.
We recognise a number of further limitations to the quality of trial evidence, beyond those considered by the Cochrane risk of bias assessment. As described by authors of other reviews, these include variable degrees of difference in numbers of transfusions between arms, and variable degrees of actual separation of haemoglobin concentration achieved between trial arms, which often is less than defined thresholds for trial interventions stated in the protocol (Trentino 2020a; Trentino 2020b). Reasons for protocol violations, whether given as extra transfusions in the restrictive arm, or for lack of transfusions in the liberal arm, are often not reported in sufficient detail.
We considered the policy of the Cochrane Injuries Group for research integrity (Roberts 2015). We have explored possible reasons for subgroup differences between trials that were prospectively registered and those not prospectively registered (Chi² = 4.06, df = 1 (P = 0.04), I² = 75.4%). First, we created a funnel plot and found little evidence to support selection bias (Figure 6). Second, it is possible that this statistically significant finding is a chance observation given the large number of statistical tests performed in this review. Third, we note that of the 13 trials that were not prospectively registered, seven were conducted before 2000 or 2001 when legislation regarding prospective registration was introduced in the USA and European Union, respectively, and nine of the trials were conducted before September 2007 when it was mandated by the Food and Drug Administration (FDA).
6.

Funnel plot of comparison: 2 Subgroup analysis by prospective registration, outcome: 2.1 30‐Day mortality
Whilst we cannot exclude the presence of selection bias, we are confident we did not miss any large trials published before 2007 that would have impacted our inferences. Indeed, the overall RR for an analysis including all trials was 0.99 (95% CI 0.86 to 1.15), while an analysis limited to the trials with prospective registration was RR 1.08 (95% CI 0.89 to 1.31).
We observed a substantial amount of statistical heterogeneity in analyses evaluating the proportions of participants transfused, the quantity of RBCs transfused, and differences in haemoglobin/haematocrit concentrations. It is conventional practice not to pool data from studies in which there is a large amount of heterogeneity, however, we chose to present pooled results for these transfusion outcomes for several reasons. First, the impact of restrictive transfusion on the proportion of participants transfused varied only by the magnitude of the reduction in transfusion ‐ not the direction. In all trials, participants in the restrictive transfusion group received fewer transfusions, although the number varied because transfusion protocols were different and clinical contexts required different frequencies of transfusion. Second, we expected this heterogeneity because of the variety of contexts for clinical trials, including participant age, degrees of comorbidity, and policies for standard transfusion practice, which, in turn, reflect specialty‐specific guidelines and recommendations. At one extreme, nearly all participants, if not all, with leukaemia and cancer were transfused (DeZern 2016; Stanworth 2020). Transfusion risk in participants in critical care (Hébert 1995; Hébert 1999; Lacroix 2007), or with acute blood loss (Villanueva 2013), was about 50% at the time of the trials.
In summary, we have chosen to present pooled results for outcomes of transfusion because we are evaluating the effects of restrictive transfusion practice, and because all trial estimates for changes in transfusion are consistently in the same direction. The substantial heterogeneity, therefore, reflects diversity in the strength of estimates, rather than efficacy of the policy. Reasons for diversity in the strength of trial estimates include known and expected clinical contexts and different practice guidelines used by different specialties. Subgroup explorations for transfusion outcomes reported earlier demonstrated these differences.
Potential biases in the review process
We performed extensive searches in an attempt to identify all eligible trials irrespective of publication status or language. Inspection of funnel plots did not reveal a major risk of publication bias (Figure 4; Figure 5).
Other trial limitations apply to the findings of this review. Timing of mortality reporting varied between trials, in part as a consequence of the clinical context. To address this issue, an initiative to undertake an individual patient data analysis has been commenced, including contact with all trial investigators to explore willingness to share trial data.
Randomised trials in this review may not have evaluated important clinical outcomes adequately that are specifically relevant to the use of RBC transfusions, such as quality of life. The identified trials evaluated the effects of transfusion only in hospitalised patients, and only two small trials have tested different thresholds in an outpatient population (Jansen 2020; Stanworth 2020), for whom function and fatigue would be more important endpoints.
Different grades of severity of cardiovascular events, such as myocardial infarction, congestive heart failure, or stroke, and different risks of overall infection will occur in patients; these events may present in ways that are not always clinically overt and so are more subjective in interpretation. This is important because RBC transfusions may have both harmful and beneficial effects on the risk of these outcomes, for example, balancing prothrombotic tendencies against protective mechanisms to limit restrictions in myocardial oxygen delivery. Future trials need to establish robust definitions of all outcomes (Docherty 2016). Despite the large number of participants included in these trials, there remains inadequate power for many outcomes.
Agreements and disagreements with other studies or reviews
The results of this review are consistent with previous published systematic reviews and guidance documents (Carson 2012a; Docherty 2016; Holst 2015; Meybohm 2020). A review of reviews reported no evidence that a difference in mortality exists between patients assigned to a restrictive or a liberal transfusion strategy (Trentino 2020a). These overall findings provide no evidence that restrictive transfusion policies harm patients within the limits defined by the trials.
Multiple reviews have addressed outcomes in selected subgroups or subpopulations of patients. A review by Fominskiy aimed to assess effects of liberal and restrictive RBC transfusion strategies on mortality in perioperative and critically ill adult patients through a meta‐analysis of relevant trials (Fominskiy 2015); a more recent review focused on cardiac surgery (Shehata 2019). A meta‐analysis of trials in gastrointestinal bleeding reported evidence of harm with application of liberal thresholds in this patient group (Odutayo 2017), as found in our review, although our review has identified one further trial in gastrointestinal bleeding (Kola 2020). We suggest some caution in interpretation of systematic reviews that report only separate subpopulations of the wider trial literature. Anaemia is the common presenting clinical problem for all patients when a red cell transfusion is considered, irrespective of clinical setting. There is a risk that the patterns of findings in different clinical contexts are inappropriately selective to a small subpopulation. The clinical decision process for transfusion in one clinical context may need to draw on findings of safety as reported across all randomised trials in different clinical settings, for a common intervention of RBC.
The results of our meta‐analyses need to be viewed against studies or reviews of large observational studies that have reported comparisons of clinical outcomes at varying haemoglobin levels in transfused and non‐transfused patients. Publications of the observational literature have reported findings at variance with the randomised trial literature (Carson 1998; Hébert 1997; Hébert 1999; Patel 2015; Spiess 1998; Wu 2001; Wu 2007; Wu 2010). Reviews of observational data have reported an increase in risk of death associated with transfusion (Chatterjee 2013; Marik 2008). However, a limitation of observational studies is that there may be residual confounding by indication, despite extensive statistical adjustment of the results. It is possible that differences in patient characteristics between those who were transfused and those who were not transfused may not have been identified or adjusted for adequately. In contrast, results of the meta‐analysis of clinical trials performed in this review update show no increase in risk of death for liberal transfusion thresholds compared with restrictive transfusion thresholds. Despite assertions to the contrary (Benson 2000; Concato 2000), we continue to believe there is a need for adequately powered, rigorously performed, randomised trials to provide the highest level of evidence when effects of different transfusion policies are tested, as the way of overcoming these limitations.
The transfusion policies reviewed here represent fairly small but significant modifications to routine clinical practice. They are consistent with the recommendations of published clinical practice guidelines (AAGBI 2008; ASA 2006; BCTMAG 2003; Carson 2012a; Carson 2016b; Mueller 2019; Napolitano 2009; NBUGI 2001; Retter 2013; STSBCGTF 2011). Transfusion triggers (in terms of haemoglobin levels) were most often in the range of 7.0 g/dL to 10.0 g/dL. In fact, the 'restrictive' transfusion triggers in some trials were equivalent to the 'liberal' triggers used in other trials. Nevertheless, trials documented significant reduction in exposure of patients to unnecessary RBC transfusion. Our findings for red cell transfusion strategies should be interpreted alongside findings for the use of 'alternative' agents to red cells or blood‐sparing techniques, such as intravenous iron (Richards 2020), cell salvage/blood conservation (Carless 2010a; STSBCGTF 2011), and antifibrinolytic drugs (Henry 2011). Adoption of a conservative transfusion threshold appears to be as effective, if not more effective, in the context of Patient Blood Managment implementation, and is likely to cost less (Roman 2020).
Some guidelines have recommended RBC transfusion for symptoms or haemodynamic instability, rather than for a specific trigger haemoglobin level (AAGBI 2008; ASA 2006; Napolitano 2009; NBUGI 2001). Three studies tested this approach to transfusion: a pilot study involving 84 participants (Carson 1998), a trial involving 2016 participants (Carson 2011), and a 110‐participant trial for acute myocardial infarction (Carson 2013), in which patients could be transfused if they exhibited symptoms or had a haemoglobin concentration less than 8.0 g/dL. These studies found no differences in functional recovery, mortality, or morbidity among patients in the restrictive (symptomatic) transfusion group in orthopaedic surgery trials (Carson 1998; Carson 2011), although in the trial involving patients with acute myocardial infarction (Carson 2013), there was a tendency towards worse outcomes in the restrictive transfusion group.
Authors' conclusions
Implications for practice.
Analysis of published evidence reveals that transfusing at a restrictive strategy of 7.0 g/dL to 8.0 g/dL, compared with a liberal haemoglobin threshold of 9.0 g/dL to 10.0 g/dL, across a broad range of hospitalised patients does not have an adverse effect on clinical outcomes, including 30‐day mortality, myocardial infarction, congestive heart failure, and infection.
Given there is no evidence of additional benefit of red blood cell (RBC) transfusion at higher haemoglobin concentration thresholds (9.0 g/dL to 10.0 g/dL), and that blood for transfusion is a costly and scarce biological resource with finite risks, a restrictive transfusion trigger policy (7.0 g/dL to 8.0 g/dL) could be widely adopted. A restrictive transfusion policy is not associated with increased adverse events and reduces both risk of exposure to RBC transfusion and the total number of units transfused.
Trial interventions varied on the haemoglobin concentration used to define the restrictive transfusion group. About half of the trials used a 7.0‐g/dL threshold, and the other half used a threshold of 8.0 g/dL to 9.0 g/dL. However, within each clinical subgroup, the number of clinical trials (and the total numbers of enrolled patients) testing restrictive thresholds at 7.0 g/dL varied. The exact implications for transfusion practice regarding the nature of restrictive haemoglobin thresholds will, therefore, vary by clinical group.
In critical care trials, a 7.0‐g/dL threshold was used most frequently and shown to have a similar safety profile to higher thresholds for mortality (RR 1.06, 95% CI 0.85 to 1.32). Similarly, a restrictive threshold of 7.0 g/dL was used in trials including patients with acute blood loss from gastrointestinal bleeding; evidence indicates that these patients have lower risk of 30‐day mortality with restrictive transfusion that uses a 7.0‐g/dL threshold.
In patients undergoing cardiac surgery, a restrictive threshold of 7.5 g/dL (rather than 7.0 g/dL) was used in the largest trials and shown to have a risk for mortality which was similar to that of higher thresholds.
In trials of orthopaedic surgery, the restrictive strategy used most frequently was 8.0 g/dL, which had a similar risk profile for mortality as higher transfusion thresholds. In this clinical subgroup, it is not possible to conclude that 7.0 g/dL is as efficacious as 8.0 g/dL, without testing lower thresholds in trials.
In other clinical subgroups, the results do not provide adequate evidence to conclude which specific restrictive transfusion threshold should be applied. These subgroups include vascular surgery and haematological malignancies, where trials are insufficient in number or recruit only small numbers of participants.
The analysis does provide some evidence that a restrictive strategy might be appropriate for patients with underlying cardiovascular disease. The REALITY trial conducted in patients with acute myocardial infarction found fewer deaths and fewer major adverse cardiac events (MACE) with a restrictive threshold of 8.0 g/dL (Ducrocq 2021). However, pooled analysis of all three trials in people with acute myocardial infarction (820 participants) reveals that the risk ratio of 1.61 and very wide 95% confidence intervals (0.38 to 6.88) are also consistent with the possibility of significant benefit for more liberal transfusion policies.
In summary, it is not possible to suggest a single restrictive transfusion threshold across all clinical groups and patients with anaemia. While it is possible that a 7.0‐g/dL threshold could be used in most adult patients, in some settings trial data for thresholds of 7.0 g/dL do not exist. Without these data, it is impossible to be certain of the effects of higher or lower thresholds in these settings. Trials that should clarify the optimal threshold in some of the most important subgroups that currently lack data are now underway (Ongoing studies).
Evidence is insufficient to evaluate the effects of different strategies on functional recovery. Quality of life is an important outcome in many trial settings, for example, people who are transfusion dependent and are managed in outpatient settings. In our review, most included randomised trials were based on patients hospitalised for the management of 'acute' anaemia. In contrast, patients with chronic bone marrow failure, such as myelodysplasia, are transfusion dependent for prolonged periods of time at home, and this may persist for years, yet our understanding of the impact of different transfusion policies on quality of life and functional outcomes for these patients is incomplete.
For countries where there are concerns about microbiological screening and the safety of donated blood, the data in this updated review constitute a strong basis for avoiding liberal RBC transfusion in many clinical settings. The benefits of minimising allogeneic RBC transfusion are likely to be greatest when there is doubt about the safety of the blood supply (WHO 2016). There is a need for practice and research to implement our review findings, with support for education and training and updating of robustly constructed guidelines (Kwan 2020; Pavenski 2018; Smith 2020; Vlaar 2020).
Implications for research.
The totality of research evidence now allows us to make firmer recommendations for research priorities. Further randomised trials should be targeted to address specific research questions when the strength of evidence‐based recommendations has significant uncertainty, rather than repeating trials in clinical settings or at haemoglobin thresholds for which the evidence base is better defined. Acute cardiovascular disease is a high‐priority area, for which a restrictive approach may not be as safe, but ongoing larger trials have the potential to provide additional evidence (NCT02981407; Shah 2020). Limited data are available for participants in clinical contexts of acute coronary syndrome, myocardial infarction, neurological injury/traumatic brain injury, acute neurological disorders, and stroke. Areas of uncertainty in cancer and haematological malignancy include chronic bone marrow failure and the role of transfusion at different thresholds for patients receiving radiotherapy (Hoff 2011). Liberal thresholds for red cell transfusion could provide important additive benefits for important outcomes such as fatigue and quality of life for elderly patients with chronic bone marrow failure, who may be transfusion dependent for many years (Stanworth 2020).
Although one large pragmatic trial has been undertaken in critically ill children (Lacroix 2007), many children with anaemia, although eligible, were not recruited into this trial, and further research is warranted to examine the generalisability of these trial findings for all groups of sick infants and children, including those with cardiac disorders. Patients with severe burn injuries who require large volumes of red cells may also present in a clinical context that requires further research.
In summary, we believe that in these selected clinical contexts, clinical goals and pathophysiology preclude generalisation from the completed trials included in this review to date, and there remains uncertainty regarding optimal transfusion practices in these subgroups.
There is a need to continue to update this review, given the large number of ongoing trials, which reflects an active programme of research in the field of red cell transfusion research. All new trials should be adequately powered and apply consistent definitions for clinical outcomes, such as infection, myocardial infarction and ischaemic heart disease (Docherty 2018). Outcomes of importance in trials will continue to include mortality, along with outcomes that are more specific and relevant to the clinical setting, such as function and quality of life measures and bleeding endpoints in transfusion‐dependent patients with cancer and haematological malignancy. All new trials should be prospectively registered, to assist researchers in assessing risk of selective outcome reporting and other matters related to research integrity.
Trials are also needed to evaluate haemoglobin concentrations below 7.0 g/dL, such as 6.0 g/dL (Yao 2020), which may be especially relevant in countries with suboptimal blood safety and inadequate blood supply (Maitland 2019). One randomised feasibility trial identified as ongoing in children undergoing allogeneic haematopoietic stem cell transplantation is comparing restrictive versus liberal red cell transfusion strategies using haemoglobin concentrations of < 6.5 g/dL and < 8.0 g/dL respectively (ISRCTN17438123). Further research should recognise the need for gender‐specific reference ranges for haemoglobin concentration (Butcher 2017).
A limitation of this trial‐level meta‐analysis is the difficulty in analysing subgroups of patients with varying underlying diseases, age, and clinical settings. This is especially important when considering transfusion thresholds because there are pathophysiological and clinical data to suggest that the optimal transfusion threshold could differ according to the patient's underlying co‐morbidity (e.g. cardiovascular disease), gender and age. One approach to address this limitation would be to conduct individual patient data meta‐analysis by obtaining and analysing detailed information on each patient enrolled in the trials.
Research is needed to identify methods used to measure oxygen delivery to vital organs directly, and to define the need for red cell transfusion more precisely. This recognises the challenge of applying haemoglobin concentration as an imperfect surrogate marker of transfusion requirements (Baek 2019; Mueller 2019; Ochocinska 2020). Although it is beyond the scope of this review, further research should explore factors related to the red cell product used for transfusion, including differences in processing and characteristics of the product. As one example of a donor‐specific factor that may be highly relevant for interpretation of trial results, our analysis revealed that only 16 trials clearly specified use of leuco‐depleted red cells in the trial protocol (Turner 2018).
Consideration should be given to aspects of trial design in the future. The most common design identified in eligible studies in our review remains the parallel two‐arm trial, in which two (arguably arbitrarily defined) thresholds for haemoglobin concentration are compared. A two‐arm trial design, while simple and pragmatic, may be an inefficient approach for identifying the exact optimal threshold for transfusion in a setting ‐ it may indeed be a different threshold than those tested in the trial. We and other groups have highlighted the fact that the actual separation of haemoglobin concentrations attained in trials varies considerably (Trentino 2020a; Trentino 2020b), and this separation rarely approaches the hoped for differences defined by protocol trial interventions.
Allied research is needed to address the optimal target haemoglobin concentration post transfusion, which will depend on the dose of transfusion given. Doses of red cells in adults are increasingly recommended as single‐unit transfusions in non‐bleeding patients, but the evidence base is limited (Shih 2018). The question of optimal dose for transfusion is particularly relevant for children and infants, given that a common paediatric dose is 10 mL/kg to 15 mL/kg, which is much greater proportionately by weight than the single unit dose (or bag) commonly used in adults (New 2016).
Ultimately, the optimal threshold for transfusion is likely to vary between patients, and new trial designs are needed that can test and evaluate targeted or personalised approaches to the need for transfusion, possibly incorporating individual physiological parameters. Indeed, it has been argued that current trials do not allow a genuine 'standard of care' arm (Wang 2010), which would mirror current clinical practice by clinicians at the bedside.
What's new
| Date | Event | Description |
|---|---|---|
| 25 January 2022 | Amended | Typographical errors corrected |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 16 December 2021 | New search has been performed | The review has been updated to 16 November 2020. Two new review authors joined the team: Marialena Trivella in November 2020, and Jane Dennis in July 2021. |
| 16 December 2021 | New citation required and conclusions have changed | Update of 2016 review; 17 new trials are included. We added six‐month (long‐term) mortality as an outcome and clarified the outcome "blood transfusion as a measure of implementation". |
| 13 November 2017 | New search has been performed | We updated the search ‐ the review was published elsewhere. |
| 27 May 2016 | New citation required and conclusions have changed | The conclusions of the review changed; the search date is now 27 May 2016. |
| 26 May 2016 | New search has been performed | We made the following changes • We added 16 new trials • We used 30‐day mortality as the primary outcome because mortality is a more clinically relevant outcome and the number of participants enrolled in the trials provided sufficient power to examine this outcome • We added sensitivity analyses to evaluate heterogeneity for transfusion outcomes between trials • We made changes to the author line |
| 20 December 2011 | New citation required and conclusions have changed | We updated searches to February 2011. We included data from two new trials and amended the results accordingly. We identified one trial through the updated search; the other was previously included as an ongoing trial, and the results became available recently. We updated the Background section of the review. The overall conclusions of the review remain similar, but we have extended the clinical specialties for which the results can be generalised. As part of this update, we replaced the assessment of methodological quality used in earlier versions of this review with an assessment of risk of bias. This amendment is in accordance with a change in Cochrane's methodological guidance. The authors of the review have changed. |
| 1 February 2011 | New search has been performed | We updated the search for studies to February 2011. |
| 9 September 2008 | Amended | We converted the review to new review format. |
| 17 November 2004 | New search has been performed | We conducted an updated search for new trials in November 2004. We identified no new trials eligible for inclusion. |
Acknowledgements
In this 2021 update, we acknowledge the contributions of co‐authors to the Carson 2018 review, which focused on patients with cardiovascular disease. We also thank Drs DeZern, Villaneuva, and Webert for providing additional data. We thank peer reviewers Jeannie Callum (Queen’s University, Kingston, Canada) and Paul M Ness MD (Professor of Pathology and Medicine, Johns Hopkins University School of Medicine, USA) for their constructive comments, which we believe improved this manuscript profoundly. We gratefully acknowledge the work of Dr Catriona Gilmour‐Hamilton (Oxford, UK) and that of the patient engagement group she co‐ordinates (Oxford Blood Group) in the development of the Plain Language Summary. We thank statisician Dr Kerry Dwan (Cochrane Editorial & Methods Department) for valuable comments and assistance. The review authors are especially thankful for the work and enthusiasm of Dr Elizabeth Royle at all steps of this 2021 review update, including extensive editorial assistance. We also acknowledge administrative support from Kim Lacey for this 2021 update.
We acknowledge the contributions of a number of individuals to prior editions, all of which have informed subsequent updates. These include Suzanne Hill (World Health Organization), the first author of the original version, Hill 2000, and the 2005 update of the review, and Paul Carless, who was first author on the update published in 2010 (Carless 2010b). We also acknowledge the contributions of Kim Henderson to the original review first published in 2000.
We acknowledge David Henry (Institute for Clinical Evaluative Sciences) and Brian McClelland, who co‐wrote reviews up to 2010; Katharine Ker (London School of Hygiene & Tropical Medicine), who undertook the following tasks for the 2010 update: screened search output, obtained articles, applied inclusion/exclusion criteria to retrieved papers, assessed risk of bias, extracted data, performed data analysis, and revised the text of the review. We thank Karen Blackhall (former Injuries Group Trials Search Co‐ordinator), who ran electronic database searches in 2009 and 2011.
For the 2016 update, we thank Deirdre Beecher (former Injuries Group Information Specialist), who ran the searches in 2015, and Sarah Dawson (former Injuries Group Information Specialist), who updated the searching section in 2016. We are especially appreciative to Marialena Trivella, Emma Sydenham, Elizabeth Royle, and the other editors at Cochrane Injuries for their extensive assistance in the analysis and presentation of this version of the review.
This project is independent research funded by the National Institute for Health Research (Systematic Reviews Infrastructure Funding, NIHR129465‐Cochrane Injuries Group). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care.
Appendices
Appendix 1. Search strategies 2011 (for 2012 update)
Cochrane Injuries Group's Specialised Register (searched 1 February 2011)
(Blood or "Red blood cell" or "Red blood cells" or RBC) and (therap* or transfus*) and (polic* or practice or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard* or restrict* or liberal* or management or program*)
Cochrane Central Register of Controlled Trials (CENTRAL; 2011, Issue 1), in the Cochrane Library
#1 MeSH descriptor Blood Transfusion, this term only with qualifiers: MT,ST #2 transfus* near5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*) #3 (Red blood cell* or RBC) near5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*) and (therap* or transfus*) #4 (H?emoglobin or h?emocrit or HB or HCT) near5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*) #5 transfus* near5 (restrict* or liberal*) #6 (blood transfus*) near3 (management or program*) #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6)
MEDLINE (Ovid) 1948 to January Week 3 2011
1. *Blood Transfusion/ 2. ((Red blood cell* or RBC) adj3 (therap* or transfus*)).mp. 3. 1 or 2 4. exp Reference Standards/ 5. standards.fs. 6. methods.fs. 7. 4 or 5 or 6 8. 3 and 7 9. (transfus* adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 10. ((Red blood cell* or RBC) adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 11. ((H?emoglobin or h?emocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 12. (transfus* adj5 (restrict* or liberal*)).mp. 13. ((blood or transfus*) adj3 (management or program*)).mp. 14. 8 or 9 or 10 or 11 or 12 or 13 15. randomi?ed.ab,ti. 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. placebo.ab. 19. clinical trials as topic.sh. 20. randomly.ab. 21. trial.ti. 22.15 or 16 or 17 or 18 or 19 or 20 or 21 23. (animals not (humans and animals)).sh. 24. 22 not 23 25. 24 and 14
Embase (Ovid) 1980 to Week 4 2011
1. *Blood Transfusion/ 2. ((Red blood cell* or RBC) adj3 (therap* or transfus*)).mp. 3. 1 or 2 4. exp standard/ 5. 3 and 4 6. (transfus* adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 7. ((Red blood cell* or RBC) adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 8. ((H?emoglobin or h?emocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 9. (transfus* adj5 (restrict* or liberal*)).mp. 10. ((blood or transfus*) adj3 (management or program*)).mp. 11. 5 or 6 or 7 or 8 or 9 or 10 12. exp Randomized Controlled Trial/ 13. exp controlled clinical trial/ 14. randomi?ed.ab,ti. 15. placebo.ab. 16. *Clinical Trial/ 17. randomly.ab. 18. trial.ti. 19. 12 or 13 or 14 or 15 or 16 or 17 or 18 20. exp animal/ not (exp human/ and exp animal/) 21. 19 not 20 22. 11 and 21
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to February 2011) and ISI Web of Science: Conference Proceedings Citation Index ‐ Science (CPCI‐S) (1990 to February 2011)
#1 TS=((Blood or "Red blood cell" or "Red blood cells" or RBC or Hemoglobin* or haemoglobin* or haemocrit or hemocrit or HB or HCT) SAME transfus*) #2 TS=(polic* or practice or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard* or restrict* or liberal* or management or program*) #3 #1 and #2 #4 TS=(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial) OR Topic=(controlled clinical trial OR controlled trial OR clinical trial OR placebo) #5 TS=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)) #6 #2 or #3 #7 #3 and #6 #8 Topic=(human*) #9 #7 and #8
Appendix 2. Search strategies May 2016
CENTRAL, in the Cochrane Library #1 MeSH descriptor: [Blood Transfusion] this term only and with qualifier(s): [Methods ‐ MT, Standards ‐ ST, Trends ‐ TD] #2 MeSH descriptor: [Erythrocyte Transfusion] this term only and with qualifier(s): [Methods ‐ MT, Standards ‐ ST] #3 ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) near/5 (trigger* or thresh?old* or target* or restrict* or liberal* or aggressive* or conservative* or prophylactic* or limit* or protocol* or policy or policies or practic* or indicat* or strateg* or regimen* or criteri* or standard* or management or program*)) #4 ((h?emoglobin or h?ematocrit or HB or HCT) near/5 (polic* or practic* or protocol* or trigger* or threshold* or maintain* or indicator* or strateg* or criteri* or standard*)) #5 (blood near/3 (management or program*)) #6 ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) and (critical* or intensive* or h?emorrhag* or bleed*)):ti #7 #1 or #2 or #3 or #4 or #5 or #6
MEDLINE (OvidSP) 1. *Blood Transfusion/ad, mt, st, td or *Erythrocyte Transfusion/mt, st, td 2. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) adj5 (trigger* or thresh?old* or target* or restrict* or liberal* or aggressive* or conservative* or prophylactic* or limit* or protocol* or policy or policies or practic* or indicat* or strateg* or regimen* or criteri* or standard* or management or program*)).tw. 3. ((h?emoglobin or h?ematocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or maintain* or indicator* or strateg* or criteri* or standard*)).tw. 4. (blood adj3 (management or program*)).mp. 5. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) and (critical* or intensive* or h?emorrhag* or bleed*)).ti. 6. or/1‐5 7. randomized controlled trial.pt. 8. controlled clinical trial.pt. 9. randomi*.tw. 10. placebo.ab. 11. clinical trials as topic.sh. 12. randomly.ab. 13. groups.ab. 14. trial.tw. 15. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. exp animals/ not humans/ 17. 15 not 16 18. 6 and 17
Embase (OvidSP) 1. *Blood Transfusion/ or Erythocyte Transfusion/ 2. ((red blood cell* or red cell* or RBC* or PRBC*) adj5 (therap* or transfus*)).mp. 3. 1 or 2 4. Standard/ or Gold Standard/ 5. 3 and 4 6. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) adj5 (trigger* or thresh?old* or target* or restrict* or liberal* or aggressive* or conservative* or prophylactic* or limit* or protocol* or policy or policies or practic* or indicat* or strateg* or regimen* or criteri* or standard* or management or program*)).tw. 7. ((h?emoglobin or h?ematocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or maintain* or indicator* or strateg* or criteri* or standard*)).tw. 8. (blood adj3 (management or program*)).mp. 9. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) and (critical* or intensive* or h?emorrhag* or bleed*)).ti. 10. or/5‐9 11. Randomized Controlled Trial/ 12. Randomization/ 13. Single Blind Procedure/ 14. Double Blind Procedure/ 15. Crossover Procedure/ 16. Placebo/ 17. exp Clinical Trial/ 18. Prospective Study/ 19. (randomi* or double‐blind* or single‐blind* or RCT*).tw. 20. (random* adj2 (allocat* or assign* or divid* or receiv*)).tw. 21. (crossover* or cross over* or cross‐over* or placebo*).tw. 22. ((treble or triple) adj blind*).tw. 23. or/11‐22 24. Case Study/ 25. case report*.tw. 26. (note or editorial).pt. 27. or/24‐26 28. 23 not 27 29. limit 28 to embase 30. 10 and 29
PubMed (for E‐publications ahead of print only) #1 ((transfus*[TI] OR red cell*[TI] OR red blood cell*[TI] OR RBC*[TI] OR PRBC*) AND (trigger*[TI] OR threshold*[TI] OR target*[TI] OR restrict*[TI] OR liberal*[TI] OR aggressive*[TI] OR conservative*[TI] OR prophylactic*[TI] OR limit*[TI] OR protocol*[TI] OR policy[TI] OR policies[TI] OR practic*[TI] OR indicat*[TI] OR strateg*[TI] OR regimen*[TI] OR criteri*[TI] OR standard*[TI] OR management[TI] OR program*[TI])) #2 ((hemoglobin[TI] OR haemoglobin[TI] OR hematocrit[TI] OR haematocrit[TI] OR HB[TI] OR HCT[TI]) AND (polic*[TI] OR practic*[TI] OR protocol*[TI] OR trigger*[TI] OR threshold*[TI] OR maintain*[TI] OR indicator*[TI] OR strateg*[TI] OR criteri*[TI] OR standard*[TI])) #3 (blood[TI] AND (management[TI] OR program*[TI])) #4 ((transfus*[TI] OR red cell*[TI] OR red blood cell*[TI] OR RBC*[TI] OR PRBC*[TI]) and (critical*[TI] OR intensive*[TI] OR hemorrhag*[TI] OR haemorrhage*[TI] OR bleed*[TI])) #5 #1 OR #2 OR #3 OR #4 #6 (random* OR blind* OR "control group" OR placebo* OR controlled OR groups OR trial* OR "systematic review" OR "meta‐analysis" OR metaanalysis OR "literature search" OR medline OR cochrane OR embase) AND (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb]) #7 #5 AND #6
Transfusion Evidence Library Subject Area: Red Cells AND (trigger OR threshold OR target OR restrict OR restrictive OR liberal OR aggressive OR aggressively OR conservative OR prophylactic OR limit OR limits OR protocol OR policy OR policies OR practice OR indicator OR strategy OR strategies OR regimen OR criteria OR standard OR management OR program OR programme) OR Subject Area: Red Cells AND title:(critical OR critically OR intensive OR intensively OR hemorrhage OR haemorrhage OR hemorrhaging OR haemorrhaging OR bleed OR bleeding)
Web of Science Conference Proceedings Citation Index ‐ Science (CPCI‐S) ((TOPIC: ((transfus* OR "red cell*" OR "red blood cell*" OR RBC* OR PRBC*) NEAR/5 (trigger* OR threshold* OR target* OR restrict* OR liberal* OR aggressive* OR conservative* OR prophylactic* OR limit* OR protocol* OR policy OR policies OR practic* OR indicat* OR strateg* OR regimen* OR criteri* OR standard* OR management OR program*)) OR (TOPIC: ((hemoglobin OR haemoglobin OR hematocrit OR haematocrit OR HB OR HCT) NEAR/5 (polic* OR practic* OR protocol* OR trigger* OR threshold* OR maintain* OR indicator* OR strateg* OR criteri* OR standard*))) OR (TOPIC: (blood NEAR/3 (management OR program*))) AND (TOPIC: (random* OR blind* OR "control group" OR placebo* OR "controlled trial" OR "controlled study" OR "controlled clinical trial" OR groups OR trials OR systematic review OR meta‐analysis OR metaanalysis OR "literature search" OR medline OR cochrane OR embase))
Appendix 3. Search strategies ongoing trial registries May 2016
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform(ICTRP)
Title/Intervention= (transfusion and (liberal or restrictive or threshold or Hb or haemoglobin or hemoglobin or haemaglobin or hemaglobin))
We also conducted an earlier search on the international trial registries in December 2015.
US National Institutes of Health Ongoing Trials RegisterClinicaltrials.gov INFLECT EXACT "Interventional" [STUDY‐TYPES] AND ( "blood transfusion" OR "hemoglobin threshold" OR "haemoglobin threshold" OR "red blood cell transfusion" ) [TREATMENT] AND ( "01/02/2011": "12/09/2015" ) [FIRST‐RECEIVED‐DATE]
World Health Organization International Clinical Trials Registry Platform(ICTRP) Intervention: "blood transfusion" OR "red blood cell transfusion" OR "hemoglobin threshold" OR "haemoglobin threshold" Recruitment status: ALL Date of registration: 01/02/2011 to 09/12/2015
ISRCTN Registry Intervention: "blood transfusion" Date applied: 01/02/2011 to 09/12/2015
Appendix 4. Search strategies 2020 (for 2021 update)
CENTRAL #1 MeSH descriptor: [Blood Transfusion] this term only and with qualifier(s): [Methods ‐ MT, Standards ‐ ST, Trends ‐ TD] #2 MeSH descriptor: [Erythrocyte Transfusion] this term only and with qualifier(s): [Methods ‐ MT, Standards ‐ ST] #3 ((transfus* OR "red cell" OR "red cells" OR "red blood cell" OR "red blood cells" OR RBC* OR PRBC*) and (trigger* OR thresh?old* OR target* OR restrict* OR liberal* OR aggressive* OR conservative* OR prophylactic* OR limit* OR protocol* OR policy OR policies OR practic* OR indicat* OR strateg* OR regimen* OR criteri* OR standard* OR management OR program*)):ti,ab #4 ((h?emoglobin OR h?ematocrit OR HB OR HCT) and (polic* OR practic* OR protocol* OR trigger* OR threshold* OR maintain* OR indicator* OR strateg* OR criteri* OR standard*)):ti #5 (blood near/3 (management OR program*)):ti #6 ((transfus* OR "red cell" OR "red cells" OR "red blood cell" OR "red blood cells" OR RBC* OR PRBC*) and (critical* OR intensive* OR h?emorrhag* OR bleed*)):ti #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
MEDLINE 1. *Blood Transfusion/ad, mt, st, td or *Erythrocyte Transfusion/mt, st, td 2. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) adj5 (trigger* or thresh?old* or target* or restrict* or liberal* or aggressive* or conservative* or prophylactic* or limit* or protocol* or policy or policies or practic* or indicat* or strateg* or regimen* or criteri* or standard* or management or program*)).tw. 3. ((h?emoglobin or h?ematocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or maintain* or indicator* or strateg* or criteri* or standard*)).tw. 4. (blood adj3 (management or program*)).mp. 5. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) and (critical* or intensive* or h?emorrhag* or bleed*)).ti. 6. or/1‐5 7. (Randomized Controlled Trial or Controlled Clinical Trial or Clinical Trial Protocol).pt. 8. (randomi* or randomly or placebo).tw,kf. 9. trial.ti,kf. 10. Clinical Trials as Topic/ 11. Clinical Trial, Phase III/ or ("phase 3" or "phase3" or "phase III" or P3 or "PIII").tw,kf. 12. or/7‐11 13. (exp Animals/ or exp Animal Experimentation/ or exp Models, Animal/) not Humans/ 14. 12 not 12 15. 6 and 14
Embase 1. *Blood Transfusion/ or Erythocyte Transfusion/ 2. ((red blood cell* or red cell* or RBC* or PRBC*) adj5 (therap* or transfus*)).mp. 3. 1 or 2 4. Standard/ or Gold Standard/ 5. 3 and 4 6. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) adj5 (trigger* or thresh?old* or target* or restrict* or liberal* or aggressive* or conservative* or prophylactic* or limit* or protocol* or policy or policies or practic* or indicat* or strateg* or regimen* or criteri* or standard* or management or program*)).tw. 7. ((h?emoglobin or h?ematocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or maintain* or indicator* or strateg* or criteri* or standard*)).tw. 8. (blood adj3 (management or program*)).mp. 9. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) and (critical* or intensive* or h?emorrhag* or bleed*)).ti. 10. or/5‐9 11. crossover‐procedure/ or double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 12. (random* or factorial* or crossover* or (cross adj1 over*) or placebo* or (doubl* adj1 blind*) or (singl* adj1 blind*) or assign* or allocat* or volunteer*).tw. 13. 11 or 12 14. (animal* or cat or cats or dog or dogs or pig or pigs or sheep or rabbit* or mouse or mice or rat or rats or feline or canine or porcine or ovine or murine or model*).ti. 15. 13 not 14 16. 10 and 15 17. limit 16 to (conference abstracts or embase)
PubMed #1 ((transfus*[TI] OR red cell*[TI] OR red blood cell*[TI] OR RBC*[TI] OR PRBC*) AND (trigger*[TI] OR threshold*[TI] OR target*[TI] OR restrict*[TI] OR liberal*[TI] OR aggressive*[TI] OR conservative*[TI] OR prophylactic*[TI] OR limit*[TI] OR protocol*[TI] OR policy[TI] OR policies[TI] OR practic*[TI] OR indicat*[TI] OR strateg*[TI] OR regimen*[TI] OR criteri*[TI] OR standard*[TI] OR management[TI] OR program*[TI])) #2 ((hemoglobin[TI] OR haemoglobin[TI] OR hematocrit[TI] OR haematocrit[TI] OR HB[TI] OR HCT[TI]) AND (polic*[TI] OR practic*[TI] OR protocol*[TI] OR trigger*[TI] OR threshold*[TI] OR maintain*[TI] OR indicator*[TI] OR strateg*[TI] OR criteri*[TI] OR standard*[TI])) #3 (blood[TI] AND (management[TI] OR program*[TI])) #4 ((transfus*[TI] OR red cell*[TI] OR red blood cell*[TI] OR RBC*[TI] OR PRBC*[TI]) and (critical*[TI] OR intensive*[TI] OR hemorrhag*[TI] OR haemorrhage*[TI] OR bleed*[TI])) #5 #1 OR #2 OR #3 OR #4 #6 (random* OR blind* OR "control group" OR placebo* OR controlled OR trial OR "systematic review" OR "meta‐analysis" OR metaanalysis OR "literature search" OR medline OR cochrane OR embase) AND (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb]) #7 #5 AND #6
TRANSFUSION EVIDENCE LIBRARY Subject Area: Red Cells AND (trigger OR threshold OR target OR restrict OR restrictive OR liberal OR aggressive OR aggressively OR conservative OR prophylactic OR limit OR limits OR protocol OR policy OR policies OR practice OR indicator OR strategy OR strategies OR regimen OR criteria OR standard OR management OR program OR programme) OR Subject Area: Red Cells AND title:(critical OR critically OR intensive OR intensively OR hemorrhage OR haemorrhage OR hemorrhaging OR haemorrhaging OR bleed OR bleeding)
WEB OF SCIENCE ‐ Conference Proceedings Citation Index ‐ Science (CPCI‐S) #1 TS=((transfus* OR "red cell*" OR "red blood cell*" OR RBC* OR PRBC*) NEAR/5 (trigger* OR threshold* OR target* OR restrict* OR liberal* OR aggressive* OR conservative* OR prophylactic* OR limit* OR protocol* OR policy OR policies OR practic* OR indicat* OR strateg* OR regimen* OR criteri* OR standard* OR management OR program*)) #2 TS=((hemoglobin OR haemoglobin OR hematocrit OR haematocrit OR HB OR HCT) NEAR/5 (polic* OR practic* OR protocol* OR trigger* OR threshold* OR maintain* OR indicator* OR strateg* OR criteri* OR standard*)) #3 TS= (blood NEAR/3 (management OR program*)) #4 #1 OR #2 OR #3 #5 TS=(random* OR blind* OR "control group" OR placebo* OR "controlled trial" OR "controlled study" OR "controlled clinical trial" OR groups OR trials OR systematic review OR meta‐analysis OR metaanalysis OR "literature search" OR medline OR cochrane OR embase) #6 #4 AND #5
ClinicalTrials.gov Other Terms: trigger OR threshold OR target OR restrict OR liberal OR aggressive OR conservative OR prophylactic OR limit OR protocol Intervention: blood transfusion OR red cell transfusion OR RBC transfusion OR red blood cell transfusion WHO ICTRP transfusion AND trigger OR transfusion AND triggers OR transfusion AND threshold OR transfusion AND thresholds OR transfusion AND restrictive OR transfusion AND liberal OR transfusion AND aggressive OR transfusion AND conservative OR transfusion AND prophylactic OR transfusion protocol OR transfusion protocols OR transfusions AND trigger OR transfusions AND triggers OR transfusions AND threshold OR transfusions AND thresholds OR transfusions AND restrictive OR transfusions AND liberal OR transfusions AND aggressive OR transfusions AND conservative OR transfusions AND prophylactic OR transfusions protocol OR transfusions protocols
Data and analyses
Comparison 1. Mortality at 30 days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 30‐Day mortality | 31 | 16729 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.15] |
| 1.2 30‐Day mortality subgroup by restrictive haemoglobin level | 31 | 16729 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.14] |
| 1.2.1 Restrictive 7.0 g/dL to 7.5 g/dL | 15 | 11572 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.19] |
| 1.2.2 Restrictive < 8.0 g/dL to 9.0 g/dL | 16 | 5157 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.75, 1.24] |
| 1.3 30‐Day mortality subgroup analysis by clinical specialties | 31 | 16729 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.14] |
| 1.3.1 Cardiac surgery | 4 | 7441 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 1.3.2 Orthopaedic surgery | 8 | 3111 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.75, 1.79] |
| 1.3.3 Vascular | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.30, 3.25] |
| 1.3.4 Acute blood loss/trauma | 3 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.97] |
| 1.3.5 Critical care | 9 | 3529 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 1.3.6 Acute myocardial infarction | 3 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.38, 6.88] |
| 1.3.7 Haematological malignancies | 2 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.07, 1.95] |
| 1.4 30‐Day mortality by clinical specialties: myocardial infarction vs all others | 31 | 16729 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.14] |
| 1.4.1 Myocardial infarction | 3 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.38, 6.88] |
| 1.4.2 All but myocardial infarction | 28 | 15909 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.15] |
| 1.5 Mortality by cardiac surgery, vascular surgery, myocardial infarction, and all others | 31 | 16729 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.14] |
| 1.5.1 Cardiac surgery | 4 | 7441 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 1.5.2 Myocardial infarction | 3 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.38, 6.88] |
| 1.5.3 Vascular surgery | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.30, 3.25] |
| 1.5.4 Others | 22 | 8311 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.83, 1.19] |
Comparison 2. Subgroup analysis by prospective registration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 30‐Day mortality | 31 | 16729 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.15] |
| 2.1.1 Prospectively registered trials | 18 | 12932 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 2.1.2 Trials without prospective registration | 13 | 3797 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 1.00] |
Comparison 3. Sensitivity analysis by allocation concealment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 30‐Day mortality | 31 | 16729 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.15] |
| 3.1.1 Low risk of bias | 26 | 15764 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.87, 1.18] |
| 3.1.2 Unclear or high risk of bias | 5 | 965 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.39] |
Comparison 4. Mortality: other time intervals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Hospital mortality | 15 | 6597 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.03] |
| 4.2 90‐Day mortality | 7 | 4143 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.02, 1.25] |
| 4.3 6‐Month mortality | 2 | 4702 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
Comparison 5. Morbidity: clinical outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Cardiac events | 11 | 5577 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.80, 1.32] |
| 5.2 Myocardial infarction | 23 | 14370 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.24] |
| 5.3 Congestive heart failure | 16 | 7247 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.29] |
| 5.4 Cerebrovascular accident (CVA) ‐ stroke | 19 | 13985 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.09] |
| 5.5 Rebleeding | 8 | 3412 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.59, 1.09] |
| 5.6 Sepsis/bacteraemia | 9 | 4352 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.86, 1.30] |
| 5.7 Pneumonia | 16 | 6666 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.13] |
| 5.8 Infection | 25 | 17104 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 5.9 Thromboembolism | 13 | 4201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.65, 1.88] |
| 5.10 Renal failure | 15 | 12531 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.16] |
| 5.11 Mental confusion | 9 | 6442 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.88, 1.40] |
Comparison 6. Blood transfusions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Participants exposed to blood transfusion (all trials) | 42 | 20057 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.53, 0.66] |
| 6.2 Participants exposed to blood transfusion by clinical specialties | 41 | 19977 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.53, 0.66] |
| 6.2.1 Cardiac surgery | 7 | 8598 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.66, 0.73] |
| 6.2.2 Orthopaedic surgery | 11 | 3969 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.38, 0.65] |
| 6.2.3 Vascular surgery | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.57, 1.08] |
| 6.2.4 Acute blood loss/trauma | 5 | 2416 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.23, 0.67] |
| 6.2.5 Critical care | 9 | 3529 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.77] |
| 6.2.6 Acute myocardial infarction | 3 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.28, 0.53] |
| 6.2.7 Haematological malignancies | 4 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.26] |
| 6.3 Participants exposed to blood transfusion (by transfusion threshold) | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.3.1 Difference between liberal and restrictive haemoglobin thresholds ≥ 2.0 g/dL | 27 | 15072 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.64] |
| 6.3.2 Difference between liberal and restrictive haemoglobin thresholds < 2.0 g/dL | 6 | 2966 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.02] |
| 6.4 Participants exposed to blood transfusion by transfusion threshold | 36 | 17954 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.51, 0.64] |
| 6.4.1 Restrictive 7.0 g/dL to 7.5 g/dL | 17 | 11919 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.48, 0.64] |
| 6.4.2 Restrictive < 8.0 g/dL to 9.0 g/dL | 19 | 6035 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.48, 0.72] |
| 6.5 Units of blood transfused | 17 | 6253 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.67, ‐0.75] |
Comparison 7. Morbidity outcomes in participants undergoing cardiac surgery or vascular surgery, and with acute MI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Myocardial infarction | 8 | 8219 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| 7.2 Renal failure | 7 | 9198 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 7.3 Infection | 8 | 9219 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.79, 1.28] |
| 7.4 Congestive heart failure | 4 | 858 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.24, 2.43] |
| 7.5 Thromboembolism | 3 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.11, 9.55] |
| 7.6 Cerebrovascular accident | 4 | 905 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.22, 4.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akyildiz 2018.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site, non‐inferiority, 'per‐protocol' approach Setting: PICU, tertiary (university‐affiliated hospital), Kayseri, Turkey Recruitment: January 2014 to December 2015 Maximum follow‐up: in‐hospital stay |
|
| Participants | 180 children in paediatric ICU randomised; 160 completed follow‐up
|
|
| Interventions |
|
|
| Outcomes |
Primary outcomes: cardiac output and other haemodynamic measures Secondary outcome: in‐hospital mortality |
|
| Notes |
Trial registration: not confirmed. Internal (university) documentation: registration TSA‐2014‐5299 Trial funding/Sponsor: Erciyes University Scientific Research Unit Conflict of interest: study authors declare they have none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was provided by a coin toss" (Akyildiz 2018 p 2) |
| Allocation concealment (selection bias) | High risk | No information was provided beyond the statement above |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we will grade risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | "Cardiologists who assessed the cardiac output measurements were blinded to treatment allocations" (Akyildiz 2018 p 2) No other subjective outcomes were used in this review |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a differential loss to follow‐up, with more losses in the restrictive arm (1 loss in the liberal arm and 19 in the other) |
| Selective reporting (reporting bias) | Unclear risk | No evidence of prospective registration; trial protocol unavailable; information insufficient to make a judgement |
| Other bias | Low risk | None apparent |
Bergamin 2017.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: ICU, tertiary (university teaching hospital), São Paulo, Brazil Recruitment: June 2012 to May 2014 Maximum follow‐up: 90 days |
|
| Participants | 300 participants 18 years of age or older with a diagnosis of solid cancer and septic shock in ICU
|
|
| Interventions | Liberal transfusion at Hb < 9 g/dL Restrictive transfusion at Hb < 7 g/dL |
|
| Outcomes |
Primary outcome: 28‐day mortality Secondary outcomes: need for advanced organ support (invasive mechanical ventilation, inotropic therapy, or renal replacement therapy), cerebral ischaemia (diagnosed by imaging and new focal deficit), acute myocardial infarction, mesenteric ischaemia, limb ischaemia, and serious adverse reactions (haemolytic transfusion reactions, anaphylaxis, transfusion‐associated lung injury, or transfusion‐associated circulatory overload) in the 28 days after randomisation; ICU and hospital length of stay; ICU re‐admission; death by 60 and 90 days after randomisation |
|
| Notes |
Trial title: Transfusion Requirements in Critically Ill Oncologic Patients (TRICOP) Trial registration: NCT01648946. Study start date was June 2012. Registration first submitted 19 July 2012; posted 25 July 2012 Trial funding: not reported. Sponsor was Instituto do Cancer do Estado de São Paulo, Brasil COI statement by investigators: "Dr. Park disclosed government work. The remaining authors have disclosed that they do not have any potential conflicts of interest" (Bergamin 2017 p 766) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomly assigned to the liberal or restrictive RBC transfusion strategy by means of an Internet based system that concealed assignments" (Bergamin 2017 p 767) |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | "Two blinded investigators assessed primary and secondary outcomes by patient records review or by telephone call (long‐term survival). There was no identification of transfusion strategy group on patients, patient records, or patient beds. Patients and investigators who collected outcomes had no access to transfusion data and were unaware of the group assignment" (Bergamin 2017 p 767) "Transfusion decisions were not performed blindly ... Physicians and nurses of the ICU were aware of the groups of treatment ... Patients were unaware of the group assignment" (Bergamin 2017 p 767) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up; data analysed on intention‐to‐treat model |
| Selective reporting (reporting bias) | Low risk | Outcomes as specified in the trial registration (NCT01648946) appear in full in the published paper |
| Other bias | Low risk | None identified |
Blair 1986.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: tertiary (university teaching hospital), London, UK Recruitment: not specified; pre‐1987 Maximum follow‐up: in‐hospital stay |
|
| Participants | 50 consecutive participants with severe upper GI haemorrhage were randomised to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Blood usage (units), rebleeding, mortality, clotting times, Hct on admission/discharge, kaolin cephalin clotting time after 24 hours, impedance clotting time after 24 hours | |
| Notes |
Trial registration: not confirmed Trial funding: Crawley and Jersey Research Fund (UK) COI statement by investigators: none provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Investigators reported no information regarding this domain |
| Allocation concealment (selection bias) | Unclear risk | Investigators reported no information regarding this domain |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we grade risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data (all data were collected during hospital stay) |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent, but in the absence of prospective registration or a trial protocol, assessment must remain 'unclear' |
| Other bias | Low risk | No other biases identified |
Bracey 1999.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: non‐profit research/treatment centre, Houston, TX, USA Recruitment: February to November 1997 Maximum follow‐up: in‐hospital stay |
|
| Participants | 428 consecutive participants undergoing elective primary coronary artery bypass graft surgery, randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Mortality, length of hospital stay, blood usage (units), blood loss, complications, infection rates, cardiac events | |
| Notes |
Trial registration: not confirmed Trial funding: not reported COI statement by investigators: none provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants "were randomly assigned on the basis of the last digit of their medical record number" (Bracey 1999 p 1071) Review authors' judgement: this is not a genuinely random method of allocation. High risk of bias |
| Allocation concealment (selection bias) | High risk | Sequence generation (record number) obviated concealment of any kind |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we grade risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Investigators provided no information regarding the survey questionnaire "When analyzed by Hb content, FACT‐F and FACT‐An scores were significantly different on postoperative Day 5, when patients with a Hb <9 g per dL were compared with patients with a Hb level >10 g per dL (p = 0.004). However, the same groups had similar survey scores on postoperative Day 3. Because the goal of our study was to maintain Hb content at >8 g per dL, we reanalyzed the subgroup and excluded patients with lowest Hb <8 g per dL. Then, survey scores did not differ significantly between low and high Hb groups...." (Bracey 1999 p 1075) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial used intention‐to‐treat analysis. Mortality data were reported for all participants, but for analysis of transfusion, participants who died during hospitalisation were excluded from analysis, as "early death precludes observation of transfusion and morbidity rate" (Bracey 1999 p 1075) |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent, but in the absence of prospective registration or a trial protocol, assessment must remain 'unclear' |
| Other bias | Low risk | None identified |
Bush 1997.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: university teaching hospital, Sacramento CA, USA Recruitment: August 1995 to November 1996 Maximum follow‐up: in‐hospital stay |
|
| Participants | 99 participants undergoing elective aortic or infrainguinal arterial reconstruction were randomised to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes |
Primary endpoints: myocardial ischaemia, myocardial infarction, death during hospitalisation Secondary endpoints: length of ICU stay, hospital stay, graft patency |
|
| Notes |
Trial registration: none confirmed Trial funding: not reported COI statement by investigators: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sealed envelopes were chosen at random for participant assignment" (Bush 1997 p 144) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were chosen at random for participant assignment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we have graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data on subjective outcomes (e.g. function) Review authors' judgement: low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data appeared to be complete |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent, but in the absence of prospective registration or a trial protocol, assessment must remain 'unclear' |
| Other bias | Low risk | No other biases identified |
Carson 1998.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre, international trial Setting: 4 university‐affiliated hospitals (3 in the USA; 1 in the UK) Recruitment: March 1996 to March 1997 Maximum follow‐up: 60 days |
|
| Participants | 84 hip fracture participants undergoing surgical repair who had postoperative Hb < 10.0 g/dL were randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Mortality, length of hospital stay, blood usage (units), complications, pneumonia, stroke, thromboembolism | |
| Notes |
Trial registration: not reported Trial funding: supported by Richard C Reynolds Chair COI statement by investigators: none identified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules were stratified by clinical site and cardiovascular disease state. Randomisation was designed in blocks of 2 to 8 participants to avoid imbalance within a site (Carson 1998 p 524) |
| Allocation concealment (selection bias) | Low risk | Study personnel at clinical sites randomly assigned participants by contacting the data co‐ordinating centre's 24‐hour automated telephone service (Carson 1998 p 523) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | There was no blinding of participants or personnel. The primary outcome of mortality allowed a judgement of low risk of bias |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | "Study nurses, blind to the transfusion status of the patient, obtained information from patients or proxies on survival, place of residence, and functional status" (Carson 1998 p 524) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 84 enrolled patients, 3 refused to comply with the study protocol after randomization, although follow‐up was completed at 60 days. The assigned transfusion strategy was successfully implemented in 93.8 percent (76/81) of the remaining patients. One patient in the threshold transfusion group did not receive a transfusion, and four patients in the symptomatic transfusion group received a transfusion in violation of the protocol (i.e., they did not have symptoms of anemia or a Hb of 8 gldL). Sixty‐day follow‐up was obtained in all patients" (Carson 1998 p 525) Data were analysed by the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent, but in the absence of prospective registration or a trial protocol, assessment must remain 'unclear' |
| Other bias | Low risk | No other biases identified |
Carson 2011.
| Study characteristics | ||
| Methods |
Design: Randomised, unblinded, parallel 2‐group, multicentre, international trial Setting: 47 clinical sites in the USA and Canada Recruitment: Juyl 2004 to February 2009 Maximum follow‐up: up to 5 years (mean of 3.1 years) |
|
| Participants | 2016 participants aged 50 years or older who were undergoing surgical repair of a hip fracture, with Hb < 10.0 g/dL within 3 days after surgery, and who had clinical evidence of cardiovascular disease (original protocol, valid until December 2005) or cardiovascular risk factors (from December 2005 onwards, following change to expand criteria to enhance recruitment)
|
|
| Interventions |
|
|
| Outcomes |
Primary outcomes: inability to walk 10 feet (or across a room) without human assistance, death prior to closure of the window for 60‐day mortality Other outcomes: Hb, ACS, in‐hospital myocardial infarction, unstable angina or death, disposition on discharge, survival, functional measures, fatigue/energy, re‐admission to hospital, pneumonia, wound infection, thromboembolism, stroke or transient ischaemic attack, cognition (Gruber‐Baldini), mortality at 30 days Results for long‐term mortality were published after the main trial report (Carson 2015) |
|
| Notes |
Trial title: FOCUS Trial registration: NCT00071032. Protocol available on journal website Trial funding: National Heart, Lung, and Blood Institute (USA) COI statement by investigators: apart from participating as investigators in the trial, the paper reported, "No other potential conflict of interest relevant to this article was reported" (Carson 2011 p 1). NCT record states all PIs employed by sponsoring organisation (Rutgers) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Data co‐ordinating centre staff prepared randomisation schedules for each site using randomly ordered block sizes of 2, 4, 6, or 8 (Carson 2011 p 2) |
| Allocation concealment (selection bias) | Low risk | The trial used an automated telephone randomisation system (Carson 2011 p 2) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | "After randomization, clinical‐site staff members, clinicians, and patients were aware of study‐group assignments" (Carson 2011 p 2454) "The primary and secondary outcomes were assessed blinded to treatment assignment in hospital and later, during telephone follow‐up" "Nurses at the clinical coordinating center who were not involved with study implementation and were unaware of study‐group assignments telephoned patients or proxies at or close to 30 days and 60 days after randomization to ascertain outcomes after hospital discharge. They spoke directly to patients who were accessible by telephone or to proxies if patients were cognitively impaired or could not talk on the telephone" (Carson 2011 p 2455) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | Functional measures, fatigue, and myocardial infarction were assessed blinded to treatment assignment. Other in‐hospital morbidity (i.e. pneumonia) was assessed by clinical staff with knowledge of assignment "Nurses at the clinical coordinating center who were not involved with study implementation and were unaware of study‐group assignments tele‐ phoned patients or proxies at or close to 30 days and 60 days after randomization to ascertain outcomes after hospital discharge. They spoke directly to patients who were accessible by telephone or to proxies if patients were cognitively impaired or could not talk on the telephone" (Carson 2011 p 2455) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for primary outcomes and most secondary outcomes were nearly complete: "there were 14 withdrawals, 2 losses to follow‐up, and 1 incomplete follow‐up ascertainment; follow‐up for the primary analysis was obtained in 99.2% of the patients. Of the 1999 patients included in the primary analysis, we directly interviewed 1075 (53.8%) and obtained data on 923 (46.2%) by proxy; the source of information was missing for 1 patient" (Carson 2011 p 5) |
| Selective reporting (reporting bias) | Low risk | Reporting was complete (ascertainable by comparison of publications with trial registration and protocol) |
| Other bias | Low risk | No other biases identified. It is noted that there was a change to inclusion criteria and a reduction in the recruitment target. In the follow‐up trial of long‐term outcomes for mortality, 10% of cases could not be linked to death registries |
Carson 2013.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre pilot trial Setting: 8 US hospitals Recruitment: March 2010 to May 2012 Maximum follow‐up: 6 months |
|
| Participants | Participants with acute myocardial infarction or undergoing cardiac catheterisation with Hb < 10 g/dL
|
|
| Interventions |
|
|
| Outcomes |
Primary outcomes: death, myocardial infarction, unscheduled revascularisation Secondary outcomes: 30‐day and 6‐month mortality, long‐term mortality, myocardial infarction, congestive heart failure, stroke, thromboembolism, pneumonia |
|
| Notes | Intention was to recruit 200 participants; the Data Safety Monitoring Committee approved early termination of recruitment at 110 participants 12.7% of patients had stable coronary artery disease, were undergoing a cardiac catheterisation, and did not have acute myocardial infarction Trial registration: NCT01167582 Trial funding: National Heart, Lung, and Blood Institute COI statement by investigators: COI statement not made available in publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme generated allocation sequence, implementing a permuted block randomisation process stratified by clinical site and clinical diagnosis |
| Allocation concealment (selection bias) | Low risk | Trial used an automated, centralised telephone system for allocation |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' "We were unable to blind the treating physician or patient to the transfusion strategy. However, we did classify outcomes blinded to treatment assignment. We do not know if process of care differed between the two groups of patients although adherence to the protocol was similar" |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' "Outcome adjudications and even Sample t classifications were performed by a committee composed of two cardiologists or infectious disease specialist (for infections) masked to the assignment group. Disagreements were settled by consensus" "We contacted all surviving patients discharged from the hospital by telephone at 30 days and 6 months after randomization to learn of their vital status and repeated hospital admissions. Follow‐up telephone calls were performed centrally by the Clinical Coordinating Center. If a patient was admitted to the hospital, copies of medical records were obtained" |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) All outcomes adjudicated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 of 110 participants was lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Trial registration: NCT01167582. Feasibility study: all clinical outcomes were reported |
| Other bias | Low risk | Intention was to recruit 200; the Data Safety Monitoring Committee approved early termination of recruitment at 110 participants. We assess this as low risk of bias given the independence of the Committee |
Cooper 2011.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, 2‐site trial Setting: 2 VA hospital centres, DC/Virginia, USA Recruitment: May 2003 to October 2009 Maximum follow‐up: 30 days |
|
| Participants | 45 participants with acute myocardial infarction and Hct < 30%
|
|
| Interventions |
|
|
| Outcomes | Primary clinical safety measurements: in‐hospital death, recurrent myocardial infarction, new or worsening congestive heart failure | |
| Notes |
Trial registration (prospective): NCT00126334 Trial funding: the trial was supported by the Cardiovascular Research Institute of the Washington Hospital Center and received no external funding COI statement by investigators: COI statement not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned in a 1:1 ratio to 1 of 2 treatment groups by the coordinating center using consecutively numbered opaque envelopes" (Cooper 2011 p 1108) |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | "Patients were followed daily by study personnel during their hospitalization and contacted by telephone at 30 days from randomization. Events were determined by the local study investigator" (Cooper 2011 p 1108) Howver, blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In‐hospital follow‐up was complete; 3 of 45 participants were lost to follow‐up at 30 days |
| Selective reporting (reporting bias) | Low risk |
Trial registration (prospective): NCT00126334 One outcome planned in the trial registration was not reported, but it was not a main outcome, viz in‐hospital acute renal insufficiency (increase in serum creatinine ≥ 0.5 mg/dL) |
| Other bias | Low risk | No other biases identified |
de Almeida 2015.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site, superiority trial Setting: ICU, tertiary oncology university teaching hospital, São Paulo, Brazil Recruitment: January 2012 to July 2012 Maximum follow‐up: 30 days |
|
| Participants | Adult participants who underwent a major surgical procedure for abdominal cancer and required postoperative care in the ICU
|
|
| Interventions | While in the ICU:
|
|
| Outcomes |
Primary outcome: a composite of all‐cause mortality or severe clinical complications within 30 days. Severe clinical complications included major cardiovascular complications, septic shock, acute kidney injury requiring renal replacement therapy, ARDS, and reoperation Secondary measures: length of stay in ICU, length of stay in hospital, health‐related QOL, hospital costs |
|
| Notes |
Trial name: Transfusion Requirements in Surgical Oncologic Patient (TRISOP) Trial registration: (prospective) NCT01502215 Trial funding: "Support was provided solely from institutional and/or departmental sources" (de Almeida 2015 p 37) COI statement by investigators: "the authors declare no competing interests" (de Almeida 2015 p 37) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... patients were randomized in a 1:1 ratio to one of two erythrocyte strategies ... After consent, the medical staff contacted the study randomization center to register the patient and to be told which group the patient was allocated to ..." (de Almeida 2015 p 30) "Allocation numbers were derived from a random number table prepared by the chief statistician and were placed in opaque envelopes and opened sequentially to determine the treatment group of the patient" (de Almeida 2015 p 30) |
| Allocation concealment (selection bias) | Low risk | "To avoid loss of concealment, the group to which the patient was allocated could only be accessed after registration in the study randomization center. Allocation numbers were derived from a random number table prepared by the chief statistician and were placed in opaque envelopes and opened sequentially to determine the treatment group of the patient" (de Almeida 2015 p 30) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) was not relevant, and we graded risk of bias as 'low' "The participants and the study investigators who classified outcomes and those who conducted the follow‐up telephone assessments were blinded to the study‐group assignments and had no access to transfusion data" (de Almeida 2015 p 30) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | "The participants and the study investigators who classified outcomes and those who conducted the follow‐up telephone assessments [including function] were blinded to the study‐group assignments and had no access to transfusion data" (de Almeida 2015 p 30) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals or exclusions. There were 20 protocol deviations (13 in the liberal group, 7 in the restrictive group). Analysis was done by intention to treat. "If the treating clinicians transfused an additional erythrocyte unit outside the protocol, it was recorded as a protocol deviation. After ICU discharge, the decision to transfuse was left to the discretion of the physician in charge of the patient clinical care. During the 30‐day follow‐up period, if a patient returned to the ICU for any reason, the allocated transfusion strategy was maintained. An intention‐to‐treat analysis was performed and considered the patients in their originally assigned groups" (de Almeida 2015 p 30) |
| Selective reporting (reporting bias) | Low risk |
Trial registration: (prospective) NCT01502215 No reporting bias was apparent |
| Other bias | Low risk | No other biases identified |
DeZern 2016.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site pilot study Setting: tertiary referral centre for oncology, Baltimore, MD, USA Recruitment: April 2014 to July 2015 Maximum follow‐up: 60 days |
|
| Participants | Acute leukaemia participants (acute myeloid leukaemia, acute lymphoblastic leukaemia/lymphoma, acute promyelocytic leukaemia, treatment‐related myeloid neoplasm, high‐grade myelodysplastic syndrome) over 18 years of age admitted to inpatient leukaemia services with plans for inpatient myelosuppressive chemotherapy
|
|
| Interventions |
Participants randomised in 2:1 ratio favouring the restrictive group |
|
| Outcomes |
Primary outcomes: feasibility defined a priori as achieving the following criteria:
Secondary outcomes: fatigue, bleeding, response to therapy, vital status on day 60, length of hospital stay (days), numbers of units of RBCs and PLTs transfused per participant Trial author provided 30‐day mortality by telephone to Dr Carson |
|
| Notes |
Trial registration: (prospective) NCT02086773 Trial funding: "... supported by a grant from the Society for the Advancement of Blood Management (SABM) sponsored by Haemonetics Corp. (Braintree, MA [USA])" [to first author of study] (DeZern 2016 p 1) COI statement by investigators: trial authors disclosed none (DeZern 2016 p 8) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random‐number sequence was generated using computer software (JMP Version 9.0, SAS Institute)" "Treatment assignment was done with a 2:1 ratio, for the LOW:HIGH Hb trigger groups, respectively. Blocking was used to specify a 2:1 ratio ... for each group of 18 consecutive patients" |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered envelopes were opened upon determination of inclusion for each participant in the trial. An investigator who did not enrol or provide consent for participants performed randomisation sequence and creation and numbering of the envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we grade risk of bias as 'low' Detection bias was therefore low risk for the outcome of mortality (data not in published paper, supplied to current review authors by personal contact); other secondary outcomes had high risk (see below) |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | "Lack of blinding could theoretically have influenced some outcome measures. For example, the fatigue scores may have been falsely low in the LOW group, resulting in an overestimation of fatigue difference between groups; however, the similarity in fatigue scores suggests this potential bias was not a concern. Finally, there was initial inherent bias among nurses and physicians who were concerned about withholding transfusions from patients who need them, which may have increased the incidence of crossovers from the LOW to the HIGH group. This bias, however, appeared to decrease over time, suggesting that the change in practice to a restrictive transfusion strategy is one that can be accomplished with clinical providers adapting well to the change" (DeZern 2016 p 7) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 participants enrolled; 80 were judged to be evaluable. Of these, 75 were transfused at goal; 3 died (deaths not attributable to transfusion); 1 stopped for cardiac ischaemia; 2 were transfused off protocol trigger; 2 withdrew consent There was an imbalance between clinician withdrawals (5 in the low arm compared with 1 in the high arm), but we judged that there was no important attrition bias |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT02086773). No reporting bias was apparent |
| Other bias | Low risk | No other bias was apparent |
Ducrocq 2021.
| Study characteristics | ||
| Methods |
Design: RCT, open‐label, multicentre, international, non‐inferiority trial Setting: 35 hospitals in France and Spain Recruitment: March 2016 to September 2019 Maximum follow‐up: 30 days (investigators plan 12 month followup; results unpublished at the time of this update) |
|
| Participants | 666 participants with myocardial infarction within 48 hours of onset of symptoms, Hb between 7 g/dL and 10 g/dL, with healthcare insurance. Excluded were those with cardiogenic shock, post PCI or CABG, transfusion in previous 30 days, any known haematological disease, or massive bleeding
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome: cost‐effectiveness Secondary outcome: the outcome of greatest clinical interest was MACE, defined as the composite of all cause 30‐day mortality, recurrent myocardial infarction, stroke, or emergency revascularisation for ischaemia |
|
| Notes |
Trial title: Effect of a Restrictive vs Liberal Blood Transfusion Strategy on Major Cardiovascular Events Among Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Clinical Trial Trial registration: NCT02648113 Trial funding: "... the trial was designed by the French Alliance for Cardiovascular Trials and was funded via a grant from the Programme de Recherche Médico‐Economique (PRME) 2015 from the French Ministry of Health and a grant from the Instituto de Salud Carlos III (Spanish Ministry of Economy and Competitiveness: grant PI15/01543). There was no industry support. ... The funders and sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication" (Ducrocq 2021 p 559) COI statement by investigators: Dr. Danchin reported receiving personal fees from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Intercept, MSD, Novo Nordisk, Pfizer, Sanofi, Servier, UCB, and Vifor outside the submitted work. Dr. Ducrocq reported receiving personal fees from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen, Sanofi, and Terumo outside the submitted work. Dr. Durand‐Zaleski reported receiving grants from the Ministry of Health during the conduct of the study and personal fees from Vifor outside the submitted work, and being the chair of the scientific committee of the French Blood Establishment. Dr. Lemesle reported receiving personal fees from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, MSD, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi Aventis, and Servier outside the submitted work. ... " (for further disclosures: see Ducrocq 2021 p 559) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Centralized blocked randomization list will be prepared by the statisticians of the clinical research Unit (URC‐EST). The investigators at each site ...obtain[ed] the randomized strategy allocation using Internet (CleanWeb, Telemedecin Technologies, S.A.S). CleanWeb will assign the patient a unique randomization number that corresponds to one of the two transfusion strategies" |
| Allocation concealment (selection bias) | Low risk | Web‐based with varying block sizes |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' "The choice of an open label design is justified by the difficulty in blinding the intervention (blood transfusion) and by the fact that the clinical outcomes are "hard" outcomes (mortality and non‐fatal major cardiac events) adjudicated by a critical event committee, blinded to randomization, and the Hb levels" (Ducrocq 2021, Supplement 1, p 19) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 668 were randomised; 2 were excluded due to lack of consent/re‐consent; 666 were randomised with consent. All participants were included in as‐randomised analysis; 327/342 were included in as‐treated analysis for restrictive strategy arm; 322/324 were included in as‐treated analysis |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT02648113), and both the protocol and the trial statistical analysis plan were made publicly available. No reporting bias was apparent |
| Other bias | Low risk | None detected |
Fan 2014.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: tertiary (university teaching hospital), Nanjing, China Recruitment: October 2011 to May 2013 Maximum follow‐up: in‐hospital stay |
|
| Participants | 192 randomised 186 participants analysed, 65 years of age or older, undergoing elective unilateral total hip replacement
|
|
| Interventions |
|
|
| Outcomes | Postoperative delirium, cerebrovascular accident, cardiac failure, myocardial infarction, pulmonary embolism, pneumonia, superficial wound infection, urinary tract infection, acute renal failure | |
| Notes |
Trial registration: none ascertainable Trial funding: work was supported by grants from the National Natural Science Foundation of China (No. 81300946) and the Natural Science Foundation of Jiangsu Province (BK2012778) COI statement by investigators: "there is no conflict of interest to be stated" (Fan 2014 p 184) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to the restrictive or liberal transfusion strategy group using a random number table and a sealed envelope technique" (Fan 2014 p 182) |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned to the restrictive or liberal transfusion strategy group using a random number table and a sealed envelope technique" (Fan 2014 p 182) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of outcome assessment was not described. Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low'. We have also considered outcomes such as pneumonia, requiring radiological investigations as a hard outcome |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | Blinding of outcome assessment was not described for assessments of cognition and delirium. We considered risk of bias as low, given the structured assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 192 participants were randomised. Data were included for 94/96 in the restrictive group (2 excluded as declined transfusion). Data were included for 92/96 in the liberal group (4 excluded as declined transfusion) |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent, but in the absence of prospective registration or a trial protocol, assessment must remain 'unclear' |
| Other bias | Low risk | No other bias was apparent. Study sample size might be considered small for the outcomes |
Foss 2009.
| Study characteristics | ||
| Methods |
Design: RCT, parallel, single‐site trial Setting: university teaching hospital, Denmark Recruitment: February 2004 to July 2006 Maximum follow‐up: 30 days (for mortality); ambulatory capacity (the primary outcome) was assessed at 3 days |
|
| Participants | 120 hip fracture participants were randomly allocated:
|
|
| Interventions |
|
|
| Outcomes | Ambulatory capacity (measured by Cumulated Ambulatory Score), mortality, length of stay, cardiac complications, infectious complications | |
| Notes |
Trial registration: NCT00162617 Trial funding: received financial support from IMK‐Almene fond (Denmark) COI statement by investigators: "the authors have no conflicts of interest" (Foss 2009 p 227) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was done via a computer generated list by a person not affiliated with the project" (Foss 2009 p 228) |
| Allocation concealment (selection bias) | Low risk | "Upon inclusion, the sealed envelope, containing the transfusion threshold and with the patient’s study number on it, was placed in the patient charts next to the transfusion papers concealing the allocation to both the patient and the physiotherapists conducting the ambulation assessments, making the study double‐blind" (Foss 2009 p 228) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention was not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' "....30‐day mortality were registered, the latter established via the Danish civil registry" (Foss 2009 p 229) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | The physiotherapist who performed ambulation assessment was blinded ".... concealing the allocation to both the patient and the physiotherapists conducting the ambulation assessments, making the study double‐blind" (Foss 2009 p 228) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13 of 100 participants did not have ambulation assessment (54/60 included in per‐protocol analysis of liberal transfusion; 53/60 in restrictive transfusion) |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT00162617). No reporting bias was apparent |
| Other bias | Low risk | No other biases were apparent |
Gillies 2020.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, feasibility trial Setting: acute university teaching hospital (Scotland, UK) Recruitment: November 2017 to January 2019 Maximum follow‐up: 30 days |
|
| Participants | 62 participants > 50 years of age with hip fracture admitted to single centre within 48 hours before surgery
|
|
| Interventions |
Participants were transfused with 1 unit of RBCs at a time, and Hb was re‐checked until within the target range |
|
| Outcomes |
Primary outcomes: related to feasibility of the trial; included proportion of eligible patients recruited; protocol compliance; Hb distribution within 3 and 30 days of surgery (including nadir haemoglobin); red blood cell use within 5 days of anaemia, and within 30 days of surgery or hospital discharge Primary clinical outcome: postoperative myocardial injury, defined as elevated high‐sensitivity troponin concentration above upper reference limit during the trial period Secondary clinical outcomes: mortality at 30 and 60 days, acute kidney injury (KDIGO definition), delirium, myocardial infarction (universal definition), major adverse cardiac events (MACE), postoperative complications |
|
| Notes |
Trial title: RESULT‐NOF Trial registration:NCT03407573 (deemed prospective: record first submitted February 2017; trial began October 2017; record first posted January 2018) Trial funding: British Journal of Anaesthesia/Royal College Anaesthetics Grant (ID WKRO 2016 0018) COI statement by investigators: 1 trial author: "NLM [sic] has received consultancy, research grants, and speaker fees from manufacturers of cardiac troponin testing including Abbott Diagnostics, Roche, and Singulex. All other authors declare they have no conflicts of interest" (Gillies 2020 p 9) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned 1:1 to either a restrictive or liberal transfusion strategy for the duration of their hospital stay or 30 days, whichever was sooner. A secure electronic Internet‐based randomisation system was used with a dynamically allocated block list (block sizes 4 and 6) ..." (Gillies 2020 p 3) |
| Allocation concealment (selection bias) | Low risk | "Secure Internet‐based system ... The patient’s clinical team and the hospital blood transfusion laboratory were informed of the patient’s study status by the study team, having been concealed until that point, and that they were to be transfused according to the study protocol" (Gillies 2020 p 3) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' "The clinical and research teams were not blinded to the intervention but were blinded to the primary outcome. The study statistician analysed data blinded from group allocation. The adjudicators who assessed ECGs and other data used to assess cardiovascular outcomes were also blinded" (Gillies 2020 p 3) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | "The clinical and research teams were not blinded to the intervention but were blinded to the primary outcome. The study statistician analysed data blinded from group allocation. The adjudicators who assessed ECGs and other data used to assess cardiovascular outcomes were also blinded" (Gillies 2020 p 3) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up was complete for 26/26 in the liberal transfusion group; for 34/36 in the restrictive group |
| Selective reporting (reporting bias) | Low risk | Trial registration: NCT03407573. Record deemed prospective (see 'notes' above). All prespecified outcomes reported in the paper |
| Other bias | Low risk | Protocol violations noted ‐ 81% liberal compliance, 64% restrictive compliance "There was a higher prevalence of cardiovascular risk factors in participants who became anaemic compared with those who did not" (Gillies 2020 p 5), but this was considered acceptable for a feasibility trial |
Gobatto 2019.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: 2 ICUs within a university teaching hospital, Brazil Recruitment: August 2014 to June 2016 Maximum follow‐up: 6 months |
|
| Participants | Participants over 18 years of age admitted to the ICU with moderate or severe traumatic brain injury (Glasgow Coma Scale (GCS) score ≤ 12 at hospital admission) with Hb < 9 g/dL within 7 days of hospital admission 47 participants randomised; 44 in analysis
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome: difference in mean Hb between liberal and restrictive groups during the 14 days after hospital admission Secondary outcomes: ICU mortality; hospital mortality; mortality at 6 months after hospital discharge; adverse events; presence of elevated intracranial pressure and intensity of intracranial hypertension treatment; cerebral haemodynamic findings on sequential transcranial Doppler ultrasound analysis; lengths of ICU and hospital stay; ICU‐free days; duration of mechanical ventilation; mechanical ventilation‐free days; neurological status at hospital discharge and 6 months after hospital discharge (GOS) |
|
| Notes |
Trial name: Transfusion Requirements After Head Trauma (TRAHT) Trial registration (prospective): NCT0220329 Trial funding: "the study was not financially supported by any funding source. The design, collection, analysis and the interpretation of data, plus the writing and the publication of the manuscript, were done by the authors without participation or influence from any funding source" (Gobatto 2019 p 9) COI statement by investigators: "the authors declare that they have no competing interests" (Gobatto 2019 p 9) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Enrolled patients were randomized in a 1:1 ratio to the restrictive or the liberal arm in random permuted blocks. The randomization was performed using an automated third party Internet‐based service (Sealed Envelope, London, UK) in order to maintain allocation concealment" (Gobatto 2019 p 2) |
| Allocation concealment (selection bias) | Low risk | "The randomization was performed using an automated third party Internet‐based service (Sealed Envelope, London, UK) in order to maintain allocation concealment" (Gobatto 2019 p 2) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low'. In this trial, the primary outcome was change in haemoglobin ‐ a laboratory measure and a hard outcome "None of the investigators or ICU staff members was aware of the randomization list prior to group allocation, or of the block numbers or block sizes, at any time ... be blinded to the treatment assignments" (Gobatto 2019 p 2) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | "None of the investigators or ICU staff members was aware of the randomization list prior to group allocation, or of the block numbers or block sizes, at any time ... Given the nature of the intervention, the ICU staff could not be blinded to the treatment assignments" (Gobatto 2019 p 2) Primary outcome was change in haemoglobin level, but neurological outcomes were also assessed; we consider risk of bias to be low due to the above measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47 participants were randomised; in the restrictive strategy arm, there was 1 exclusion due to a 'randomization error'; in the liberal strategy arm, there were 2 exclusions due to withdrawal of consent and to 1 randomisation [sic] (error) (Gobatto 2019 p 4) |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT02203292). No reporting bias was apparent (all prespecified outcomes reported in the paper) |
| Other bias | Unclear risk | Some baseline imbalances (e.g. pupil alterations, computed tomography changes, use of blood prerandomisation) |
Gregersen 2015.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: orthopaedic ward in a university teaching hospital, Denmark Recruitment: January 2010 to June 2013 Maximum follow‐up: 12 months |
|
| Participants | 284 participants aged 65 years or older undergoing hip fracture surgery who had postoperative Hb between 9.7 g/dL and 11.3 g/dL during the first 6 days postoperatively
|
|
| Interventions |
|
|
| Outcomes |
Primary outcomes: recovery from physical disabilities (3 tools were used to measure physical performance: the Modified Barthel index, New Mobility score, and Cumulated Ambulation Score); total number of infections (pneumonia, urinary tract infection, other); cognition; delirium (10 days); depression; quality of life; modified Barthel Index; comprehensive frailty index Death within 30 days provided (together with causes, e.g. sepsis, cardiovascular disease, pneumonia) |
|
| Notes | Four publications reported results Trial title: Transfusion Requirements In Frail Elderly (TRIFE) Trial registration: NCT01102010 Trial funding: "we have received grants from the Helga and Peter Korning Foundation for medical equipment (HemoCue portable photometer). Costs of data collection, analyses, and article writing were borne by the Department of Geriatrics at Aarhus University Hospital" (Gregersen 2015 p 7) COI statement by investigators: "the authors declare that there is no conflict of interest" (Gregersen 2015 p 7) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization procedure was provided by an allocation concealment process and conducted electronically in the web‐based clinical‐trial‐support‐system ‘TrialPartner’ from Public Health and Quality Improvement in Central Denmark Region. This central computer program using permuted block‐sizes stratified the randomization according to gender and type of residence. Results of randomization were available at the electronic patient record for the hospital staff in the Orthopedic and Geriatric wards since the staff should administer the transfusions" (Gregersen 2015 p 2) |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | "The participants, their relatives, and the endpoint assessors were blinded to the result of randomization" (Gregersen 2015 p 2) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 284 participants, there were 8 dropouts (4 from each group ‐ reasons cited as refusing transfusion and 1 acute bleeding ulcer) and 8 further protocol deviations per transfusion group |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT01102010). No reporting bias was apparent |
| Other bias | Low risk | No other biases were apparent |
Grover 2006.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre equivalence trial Setting: 3 acute hospitals in southeast England, UK Recruitment: not stated Maximum follow‐up: in‐hospital stay |
|
| Participants | 260 participants undergoing elective lower limb joint replacement surgery randomly allocated to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Ischaemic load, blood load, Hb, number of units transfused, length of hospital stay, adverse events, new infections requiring antibiotic therapy | |
| Notes |
Trial registration: none ascertainable Trial funding: NHS/NBS National Research Review Committee (UK) COI statement by investigators: nothing reported in the published paper concerning this issue |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized preoperatively using permuted blocks that were derived from random number tables" (Grover 2006 p 107) |
| Allocation concealment (selection bias) | Low risk | "Envelopes containing the number and allocation sequence remained sealed until the patient was assigned to intervention" (Grover 2006 p 107) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' "The patient and technician analysing the Holter tapes were unaware of treatment allocation. The anaesthetists and surgical team responsible for the patient were informed of treatment allocation" (Grover 2006 p 107) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 260 participants recruited (from a planned target of 660), outcome data were presented for 218. The missing 42 participants did not have analysable Holter tape recordings |
| Selective reporting (reporting bias) | Unclear risk | Evidence of prospective registration/trial protocol unavailable, so insufficient information on which to make a judgement |
| Other bias | Unclear risk | "Unfortunately, the study recruited only 260 participants, from a target of 660 to achieve sufficient statistical power. As recruitment commenced it became clear that the strict exclusion criteria, specifically the ECG criteria, meant that the proportion of patients eligible to participate in the study was much lower than anticipated. This, in turn, prolonged the time during which recruitment took place. With a fixed amount of funding and time available to research fellows in subspecialty training in England, the study had to be curtailed after 2 years" |
Hajjar 2010.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre, non‐inferiority trial Setting: ICU at university hospital cardiac surgery referral centre in Brazil Recruitment: February 2009 to February 2010 Maximum follow‐up: 30 days |
|
| Participants | 512 adult participants who underwent cardiac surgery with cardiopulmonary bypass
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome composite endpoint: 30‐day all‐cause mortality and severe morbidity (cardiogenic shock; ARDS or acute renal injury requiring dialysis or haemofiltration; respiratory, cardiac, neurological, and infectious complications; inflammatory complications; bleeding; ICU and hospital lengths of stay, RBC transfusions) Confusion assessment scale was used daily in those who presented with delirium |
|
| Notes |
Trial title: Transfusion Requirements After Cardiac Surgery: The TRACS Randomized Controlled Trial Trial registration (not prospective; recruitment began in February, registration posted in November 2009): NCT01021631 Trial funding: none mentioned in main trial publication nor in NCT record COI statement by investigators: "financial disclosures: none reported" (Hajjar 2010 p 1567) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to a liberal or a restrictive transfusion strategy. Opaque envelopes arranged using a random‐number table were prepared by the chief statistician and opened sequentially to determine the patient’s treatment group" (Hajjar 2010 p 1560) |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' "The patient and outcome assessors were blinded to group assignment" (Hajjar 2010 p 1560) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | "Information about the treatment strategy was given to the anesthesiologist and to health care workers in the intensive care unit (ICU). The patient and outcome assessors were blinded to group assignment" (Hajjar 2010 p 3) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was undertaken. Follow‐up was high (512 randomised/502 in analysis; losses relatively equal ‐ 4/257 vs 6/255 ‐ between groups) |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent, but the trial was not prospectively registered (recruitment began 9 months before study registration (NCT01021631)) Confusion Assessment Scale was briefly described on pp 1563‐4 in section on 'neurologic complications' data |
| Other bias | Low risk | No other biases were apparent |
Hébert 1995.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre pilot trial Setting: 5 tertiary‐level ICUs, in Canada Recruitment: March 1993 to January 1994 Maximum follow‐up: in‐hospital stay |
|
| Participants | 69 normovolaemic critically ill participants admitted to 1 of 5 tertiary‐level ICUs with Hb values < 9.0 g/dL within 72 hours of admission were randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Mortality, length of hospital stay, length of ICU stay, blood usage (units), complications, Hb | |
| Notes |
Trial title: Transfusion Requirements in Critical Care (TRICC) Pilot Study Trial registration: none ascertainable Trial funding: this work was supported by the Canadian Red Cross Society, Blood Services, Ottawa, Ontario, and the Physicians' Services Incorporated, North York, Ontario COI statement by investigators: none referred to in published paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned to 1 of 2 groups by consecutive allocation from a random listing stratified by centre and disease severity ... blocked by balanced groups of 10" (Hébert 1995 p 1440) |
| Allocation concealment (selection bias) | Unclear risk | Trial reported no information regarding this domain |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All randomized patients completed the study" (Hébert 1995 p 1440) |
| Selective reporting (reporting bias) | Unclear risk | Evidence of prospective registration/trial protocol unavailable; insufficient information available to make a judgement |
| Other bias | Low risk | No other biases were apparent |
Hébert 1999.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre trial Setting: 22 tertiary‐level and 3 community ICUs in Canada Recruitment: November 1994 to November 1997 Maximum follow‐up: 30 days |
|
| Participants | 838 critically ill participants with euvolaemia after initial treatment and with Hb < 9.0 g/dL within 72 hours after admission to the ICU were randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Mortality, length of hospital stay, length of ICU stay, blood usage (units), complications, infection rates, cardiac events, pulmonary oedema, pneumonia | |
| Notes |
Trial registration: none ascertainable Trial funding: supported by the Medical Research Council of Canada and by an unrestricted grant from Bayer COI statement by investigators: published paper included no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random order was computer generated (Hébert 1999 p 410) |
| Allocation concealment (selection bias) | Low risk | "The data coordinating centre prepared sealed opaque envelopes, which they distributed to each participating institution where they were opened up sequentially to determine the participants treatment assignment. The envelopes were returned periodically to the coordinating centre for auditing" (Hébert 1999 p 410) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' "It was not feasible to mask the assigned transfusion strategy from health care providers" Participants were ICU patients. Most outcomes were based on laboratory measures |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 838 participants randomised, there were 4 withdrawals from the liberal transfusion arm and 5 from the restrictive arm |
| Selective reporting (reporting bias) | Unclear risk | Evidence of prospective registration/trial protocol unavailable; insufficient information available to make a judgement |
| Other bias | Low risk | No other biases were apparent |
Hoff 2011.
| Study characteristics | ||
| Methods |
Design: 2 RCTs, parallel 2‐arm, multicentre trial (DAHANCA 7 is a follow‐on trial from DAHANCA 5, undertaken to extend its usefulness) Setting: multiple oncology departments in Denmark Recruitment: January 1986 to September 1990 (DAHANCA 5) and January 1992 and October 1997 (DAHANCA 7) Maximum follow‐up: 5 years or until death |
|
| Participants | People with diagnosed invasive squamous cell carcinoma of the supraglottic larynx or pharynx and oral cavity, with no evidence of distant metastases Participants were stratified before randomisation according to sex, institution, site of tumour, tumour status, and haemoglobin status DAHANCA 5 assessed effects of the hypoxic radiosensitiser nimorazole plus radiotherapy vs placebo plus radiotherapy, and contained a subrandomised group of trial participants presenting with condition‐specific low Hb to either transfusion or no transfusion DAHANCA 7 assessed effects of the same dose of nimorazole given with 5 fractions of radiotherapy/week vs 6 fractions of radiotherapy/week. In this trial, again, all participants presenting with a condition‐specific low Hb were subrandomised to transfusion or no transfusion Trial flow: within the original DAHANCA 5 trial (n = 414), 171 participants with low pre‐irradiation Hb (women < 13 g/dL; men < 14.5 g/dL) were randomised to receive or not receive transfusion before final randomisation to nimazorole vs placebo. Within the DAHANCA 7 trial (n = 786), 321 participants with low haemoglobin (as above) were randomised to different regimens of administration of nimazorole (5 vs 6 radiotherapy fractions per week) Of the 492 participants from both trials, 26 were not randomised (no reason given); 1 participant in the no‐transfusion group was 'in the wrong strata' and was not considered evaluable Analysis included:
|
|
| Interventions |
For those who met the criteria for low Hb, transfusions were given with packed RBCs to achieve Hb in the 'high' value range (1 unit at a time; without leucocyte depletion as standard). If during treatment, Hb fell below the values indicated above, transfusion was repeated. Haemoglobin level was measured every fortnight |
|
| Outcomes |
Primary outcome (both trials): locoregional control after radiotherapy (defined as complete and persistent disappearance of disease in the primary tumour site and also the regional lymph nodes) Secondary outcomes: local and regional control (with and without salvage surgery), overall survival, early and late treatment‐related morbidity The influence of haemoglobin on tumour response to irradiation was also a stated goal (Overgaard 1998 p 136) |
|
| Notes |
Trial registration: none confirmed Trials (original trials and analyses) funding: sources of support included the Danish Cancer Society; Legatstiftelsen, Pedersholm and the Danish Cancer Society; Clinical Resarch Unit at Aarhus Oncological Centre; the Lundbeck Foundation Center for Interventional Reseach in Radiation Oncology; and the Danish Council for Strategic Research COI statement by investigators: no conflicts of interest reported by trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "In patients where all eligibility criteria were fulfilled, patient data was entered into a local computer which generated the correct strata and randomization number and at the same time printed a confirmation letter which was sent to the data center ... " (Overgaard 1998 p 136) Procedures for the later trial (DAHANCA 7) are described as similar, although here, it is noted that randomisation confirmation was made by telephone, not by letter (Overgaard 2003) |
| Allocation concealment (selection bias) | Low risk | "Each center was supplied with sealed envelopes indicating the randomization code. These envelopes were kept outside the radiotherapy department (at the hospital pharmacy) and could only be reached in the case of a clinical situation where knowledge of the presence of active drug was crucial for the further treatment of the patient. This did not happen in the present study and all envelopes were returned intact to the data center after completion of the trial. The trial has been maintained blinded during followup and the involved institutions are still unaware of which drug treatment the individual patients received" (Overgaard 1998 p 136) No details of allocation concealment were given for the later trial, but as was already mentioned, procedures were described as similar |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for the pharmacological component of DAHANCA 5 was manifestly met (see above) and could not be met for DAHANCA 7 (where the intervention involved number of doses). Blinding of the transfusion component of both trials was, as ever, not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), risk of bias remains 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant. In addition, we note that "all diagnostic, therapeutic and follow‐up data were validated and processed by the DAHANCA data center. To optimize the data quality, the events recorded were crosschecked with the hospital records to ensure correct registration of the site or sites of failure and course of death" (Overgaard 1998 p 138) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from 465 of 466 participants randomised were used within the analysis |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent, but in the absence of prospective registration or a trial protocol, assessment must remain 'unclear' |
| Other bias | Unclear risk | DAHANCA 5: no other biases noted DAHANCA 6: "when the recruitment number was reached in October 1997, it became apparent that the number of patients with glottic tumours would not express enough events to secure a conclusive outcome in this groups of patients. We closed the DAHANCA 7 protocol ..." (Overgaard 2003) |
Holst 2014.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre trial Setting: 32 general ICUs in Denmark, Sweden, Norway, and Finland Recruitment: December 2011 to December 2013 Maximum follow‐up: 12 months |
|
| Participants | 1005 participants with septic shock and Hb < 9 g/dL were randomised
|
|
| Interventions |
The intervention period was the entire ICU stay, to a maximum of 90 days after randomisation |
|
| Outcomes | Primary outcome: 90‐day mortality | |
| Notes |
Trial registration: NCT01485315 Trial funding: supported by a grant (09‐066938) from the Danish Strategic Research Council and by Copenhagen University Hospital, Rigshospitalet, the Scandinavian Society of Anaesthesiology and Intensive Care Medicine (ACTA Foundation), and Ehrenreich’s Foundation COI statement by investigators: "Dr. Johansson reports receiving grant support from Pharmacosmos; and Dr. Perner, receiving grant support from CSL Behring, Fresenius Kabi, Cosmed, and Bioporto Diagnostics, and lecture fees from LFB. No other potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org" (Holst 2014 p 10) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A centralised computer generated the assignment sequence. Randomization was performed with the use of a centralized computer generated assignment sequence, with stratification according to study site and the presence or absence of active hematologic cancer ... Patients with septic shock were randomly assigned in a 1:1 ratio, with the use of permuted blocks of varying sizes of 6, 8, or 10, to blood transfusion at the higher haemoglobin threshold or the lower haemoglobin threshold" (Holst 2014 p 10) |
| Allocation concealment (selection bias) | Low risk | Use of a centralised computer ensured allocation concealment. See above |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' "Treatment assignments were concealed from the investigators assessing mortality, the data and safety monitoring committee, and the trial statistician" (Holst 2014 p 10) |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 99.8% (998) included in all analyses of mortality; (977) 97.7% included in analyses of all outcomes |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT01485315). Reporting was comprehensive for 90‐day outcomes in Holst 2014; longer‐term data appear to have been fully reported in Rygård 2016 |
| Other bias | Low risk | No other sources of bias were apparent |
Jairath 2015.
| Study characteristics | ||
| Methods |
Design: RCT, pragmatic, open‐label, feasibility, cluster design trial Setting: 6 university teaching hospitals in the UK Recruitment: September 2012 to March 2013 Maximum follow‐up: 28 days |
|
| Participants | 6 clusters including 936 participants. This trial did not require participants to meet a haemoglobin threshold for enrolment Participants were 18 years of age or older and were admitted to 1 of the participating hospitals with upper GI bleeding Participants with exsanguinating haemorrhage were excluded. Participants consented to permit data collection and follow‐up
|
|
| Interventions |
|
|
| Outcomes |
Feasibility outcomes included: recruitment rates, adherence to transfusion policy, difference in Hb, RBC exposure, evidence for selection bias Clinical outcomes included: further bleeding, thromboembolic and ischaemic events, number of infections, mortality, serious adverse events, health‐related quality of life |
|
| Notes |
Trial title: Transfusion in Gastrointestinal Bleeding (TRIGGER) Trial registration (prospective): ISRCTN85757829 Trial funding: NHS Blood and Transplant Research and Development COI statement by investigators: "we declare no competing interests" (p 144) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly allocated (using a computer‐generated randomisation sequence) centres to a transfusion policy using a random permuted block of six (three hospitals per policy), without stratification or matching (randomisation done by BCK)" |
| Allocation concealment (selection bias) | High risk | "All clinicians, patients, and outcome assessors were unmasked to treatment allocation" (Jairath 2015 p 2) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' "TRIGGER was an open‐label cluster randomised trial whose primary outcome was further bleeding. Because of the cluster randomisation, all researchers in a hospital were aware of treatment allocation and so could not perform a blinded assessment. A blinded adjudication committee was also not feasible as it was impossible to compile relevant information to send to the committee in a blinded manner. Therefore, the definition of further bleeding was modified to exclude subjective aspects (such as whether symptoms like vomiting blood were severe enough to indicate the outcome had been met), leaving only objective aspects (the presence versus absence of active bleeding in the upper gastrointestinal tract confirmed by an internal examination)" |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | Quality of life measures were reported. As described above, blinding was not possible for a cluster trial, and it is unclear how assessors could use this information in a selective way |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 533 were enrolled in the liberal arm, 403 in the restrictive arm. All participants were analysed for feasibility outcomes. 512/533 of one arm and 393/403 were analysed for further bleeding at 28 days. The intraclass correlation coefficient (ICC) was very low (0.0001) for the outcome of mortality; we therefore included data considering the participant as the unit of randomisation and ignored clustering, but we performed a sensitivity analysis excluding this trial to see what effect, if any, it had on the analysis Rates of return for EQ‐5D data at 28 days (by telephone) were low (237/533 and 267/403), but these results were as expected for this patient population |
| Selective reporting (reporting bias) | Unclear risk | Trial was prospectively registered. No reporting bias was apparent |
| Other bias | Low risk | No other biases noted |
Jansen 2020.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre, non‐inferiority trial Setting: 3 sites (1 university and 2 general hospitals) in the Netherlands Recruitment: July 2002 to August 2004 Maximum follow‐up: 12 months |
|
| Participants | 19 adult participants (≥ 18 years of age) with diagnosed myelodysplasia according to the French‐American‐British classification and dependent on RBC transfusion (e.g. who had ≥ 1 RBC transfusion recently)
|
|
| Interventions |
|
|
| Outcomes | Primary endpoint: physical fatigue, measured with the Multidimensional Fatigue Inventory | |
| Notes |
Trial registration (post hoc, 2005): ISRCTN43616311 Trial funding: no information COI statement by investigators: "the authors have no conflict of interest to declare" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Participants were blinded but physicians and nurses were not |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Participants were reported to be blinded, but this was not tested. Physicians and nurses were not blinded. Quality of life outcomes including fatigue were reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Insufficient data. 1 death in restrictive arm, 2 in liberal arm, but timing not provided. Follow‐up was incomplete "After randomization 6 out of 10 patients (60%) from the restrictive and 5 out of 9 patients (55.5%) from the liberal arm completed or still participated when the study terminated. Reasons of study withdrawal were withdrawal of informed consent (two in the restrictive and one in the liberal arm), death (one in the restrictive and two in the liberal arm) and usage of growth factors (one patient in each arm)" Thus, the trial's primary outcome data were available for 11/19 participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data; trial registration was done post hoc, and trial report was provided only in the form of a letter |
| Other bias | Unclear risk | Terminated prematurely "The Temple study was terminated prematurely due to the slow recruitment rate with only 21 patients in three hospitals in 2 years. Patients who were still participating when the study ended, received transfusion therapy according to the guidelines of the local hospital" (Jansen 2020 p 879) |
Johnson 1992.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐centre trial Setting: university teaching hospital, Boston (USA) Recruitment: not specified Maximum follow‐up: in‐hospital stay |
|
| Participants | 39 autologous blood donors undergoing elective myocardial revascularisation were randomised to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Cardiac events, complications, postoperative blood loss, blood use (total units), allogeneic blood use (units), autologous blood use (units), all product blood use (units), number of participants receiving transfusions, mean cardiac index, mean systemic resistance, exercise capacity, Hct levels, length of ICU stay, length of hospital stay 5 days after surgery, all participants were asked to complete an exercise treadmill test. A second test was performed the following day |
|
| Notes | Funding not stated Trial registration: none ascertainable Trial funding: none specified for the trial, but 1 investigator was supported in part by Transfusion Medicine Academic Award K07HL02033 from the National Institutes of Health (Johnson 1992 p 307) COI statement by investigators: none appears in the publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "All patients giving informed consent were randomized (with the aid of a table of random numbers and an odd‐even [conservative strategy‐liberal strategy] designation) to one of two postoperative transfusion strategies" (Johnson 1992 p 308) |
| Allocation concealment (selection bias) | Unclear risk | It is unclear if assignment was concealed before randomisation |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Surgeons and anaesthesiologists were said to be blinded to the group of randomisation until the participant reached the ICU |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | No data from subjective outcomes (e.g. function). Exercise tolerance was assessed and reported, and risk of bias is considered unclear without more information on the assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Data were complete for 38/39 participants (18/18 in the liberal group; 20/21 in the restrictive group)" |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent, but in the absence of prospective registration or a trial protocol, assessment must remain 'unclear' |
| Other bias | Low risk | No other biases were apparent |
Koch 2017.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre trial Setting: 2 hospitals (1 in Cleveland, OH, USA; 1 in Ahmedabad, Gujerat, India) Recruitment: March 2007 to August 2014 Maximum follow‐up: 3 months |
|
| Participants | 722 participants 18 years of age and older scheduled for elective isolated heart valve procedures, CABG surgery with or without valve procedures, and ascending aorta replacement performed on cardiopulmonary bypass
|
|
| Interventions |
|
|
| Outcomes | A multi‐disciplinary Data and Safety Monitoring Board adjudicated clinical events Primary outcome: a composite of in‐hospital postoperative morbidity and mortality, as defined for the Society of Thoracic Surgeons National Cardiac Database, which included:
Secondary outcomes included:
|
|
| Notes |
Trial registration: registered ‐ NCT00651573 ‐ but not prospectively, because trial started in March 2007 and record was first submitted in March 2008 Trial funding: "this study was supported in part by the Gus P. Karos Registry Fund, the Kenneth Gee and Paula Shaw, PhD, Chair in Heart Research (EHB), and the Sheikh Hamdan bin Rashid Al Maktoum Distinguished Chair in Thoracic and Cardiovascular Surgery (JFS). These persons and funding organizations played no role in the collection of data or analysis and interpretation of the data, and had no right to approve or disapprove publication of the finished manuscript" (Koch 2017 p 1249) COI statement by investigators: none reported in the publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Consenting patients meeting inclusion criteria were randomly assigned at time of surgery to a hematocrit trigger of either 24% or 28% for the duration of hospitalization. Randomization was stratified by site, using within each site randomly sized blocks of 6, 8, 10, and 12 so that at any given time, approximately equal numbers of patients were randomized into each transfusion trigger group" (Koch 2017 p 124) |
| Allocation concealment (selection bias) | Unclear risk | See above; no further information available |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Surgeon and patient were blinded. Personnel assessing patient outcomes were blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | SF‐12 mental or physical scores; personnel assessing patient outcomes were blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for 717/722 participants analysed |
| Selective reporting (reporting bias) | Unclear risk | Registration undertaken a year after enrolment began |
| Other bias | Low risk | No other biases were apparent |
Kola 2020.
| Study characteristics | ||
| Methods |
Design: RCT, single‐centre, prospective, open‐label, parallel‐arm, non‐inferiority trial Setting: tertiary care, university teaching hospital (Pondicherry, India) Recruitment: June 2015 to May 2017 Maximum follow‐up: 45 days |
|
| Participants | Patients 18 years of age or older who presented to the emergency surgical unit with a diagnosis of upper GI bleeding
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome: mortality. The time period was not designated, but deaths were reported as in‐hospital, during follow‐up, and overall mortality within follow‐up of 45 days Secondary outcomes: number of days from admission to death, rebleeding episodes (in‐hospital bleeding and rebleeding during 45‐day follow‐up), Hb value before death, number of sessions of endoscopic treatment, requirement for banding/sclerosant treatment, requirement of SB tube placement and duration, incidence of transfusion reaction (major/minor), dose, duration of octreotide infusion, length of hospital stay |
|
| Notes |
Trial registration: CTRI/2017/09/009682, but not prospective. Trial appears to have been registered only in May 2017, when, it is reported, recruitment had closed. Maximum follow‐up was 45 days. Study was submitted for publication in April 2020 and was accepted in June Trial funding: "nil"(Kola 2020 p 19) COI statement by investigators: "there are no conflicts of interest" (Kola 2020 p 19) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified permuted block randomization was done using a computer program with randomly selected unequal block sizes of 4 and 6. Stratification was done based on variceal bleeding vs. non‑variceal bleeding" (Kola 2020 p 14) |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was performed using a serially numbered opaque sealed envelope (SNOSE) technique. The envelopes were opened by the residents on duty and allocation was carried out at the time of admission of the patient" (Kola 2020 p 14) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low'. Primary outcome is mortality and risk of bias is low |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported. No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial first recruited in 2015; finished in May 2017; registration also in May 2017 |
| Other bias | Low risk | Investigators reported some baseline imbalances (e.g. cirrhosis), but they were not statistically significant |
Lacroix 2007.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre trial Setting: 19 tertiary‐care paediatric ICUs in 4 countries (Canada, Belgium, USA, UK) Recruitment: November 2001 to August 2005 Maximum follow‐up: 30 days |
|
| Participants | 648 (637 following withdrawals) stable critically ill children with Hb < 9.5 g/dL within 7 days after admission to an ICU were randomly allocated to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | 28‐day mortality, sepsis, transfusion reactions, infection, length of stay | |
| Notes |
Trial name: Transfusion Requirements in the Pediatric Intensive Care Unit (TRIPICU) study Trial registration (not prospective): ISRCTN37246456 Trial funding: supported by grants (84300 and 130770) from the Canadian Institutes of Health Research and by grants (3348 and 3568) from the Fonds de la Recherche en Santé du Québec COI statement by investigators: "Drs. Lacroix and Hébert report receiving consulting fees and grant support from Johnson & Johnson; Dr. Hébert also reports receiving consulting fees and unrestricted funds from Novo Nordisk and Amgen serving as a Career Scientist of the Ontario Ministry of Health (1994–2004), and receiving unrestricted training funds from Canadian Blood Services; Dr. Hume reports being employed by the Canadian Blood Services; and Dr. Peters reports receiving consulting fees from Baxter, Xoma, and Eli Lilly. No other potential conflict of interest relevant to this article was reported" (Lacroix 2007 p 1609) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was centralized, with assignment data posted on the Internet. Patients were assigned to the study groups in blocks of 2 or 4 that were randomly distributed and stratified according to center and three age groups (≤28 days, 29 to 364 days, and >364 days)" (Lacroix 2007 p 1610) |
| Allocation concealment (selection bias) | Low risk | Allocation was Internet based and central |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' "Clinical staff and parents of the participants were aware of the assignments to study groups, but physicians, nurses, and research staff were unaware of the block‐randomization strategy" (Lacroix 2007 p 1610) |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Mortality was the primary outcome. Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full data for 626/648; withdrawals were more common in the restrictive than the liberal arm (7 vs 4); protocol violations were more common in the liberal than the restrictive arm (10 vs 1). Both ITT and per‐protocol analyses were conducted |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent; however, trial was retrospectively registered (June 2004; when recruitment began in November 2001). ISRCTN37246456 |
| Other bias | Low risk | No other biases were apparent |
Laine 2018.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm trial Setting: university teaching hospital (Finland) Recruitment: June 2014 to December 2015 Maximum follow‐up: 7 days after hospital |
|
| Participants | Patients undergoing non‐emergency CABG, simple 1‐valve (aortico mitral) replacement, or both, requiring cardiopulmonary bypass (CPB)
|
|
| Interventions |
|
|
| Outcomes | ROTEM was performed at 3 predetermined time points: before anaesthesia induction, immediately after CPB/surgery, and on the first postoperative morning | |
| Notes |
Trial registration: after trial was registered at the Hospital District of Helsinki and Uusimaa (§94,9.05.2014) [sic] and receiving approval from the institutional Ethics Committee for Surgery in Helsinki University Hospital 2014 (D‐number 58/13/03/02/2014) Trial funding: this work was supported by a government grant for medical research and by the Finnish Angiological Society COI statement by investigators: "Dr. Laine has received congress related travel reimbursement from MSD Finland Oy. Dr. Schramko has received congress related travel reimbursement from TEM InternationalGmBH" (Laine 2018 p 132) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was done in blocks of 20" (Laine 2018 p 133) ‐ no further information given |
| Allocation concealment (selection bias) | High risk | "... using closed envelopes" ‐ no further information given (Laine 2018 p 133) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Clinical staff blinded to ROTEM results |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low'. Laboratory outcomes including ROTEM results are also considered hard outcomes |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All 80 patients were included in the analyses" (Laine 2018 p 134) |
| Selective reporting (reporting bias) | Unclear risk | Evidence of prospective registration/trial protocol unavailable; insufficient information to make a judgement |
| Other bias | Low risk | None evident |
Lotke 1999.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: university hospital (Pennsylvania, USA) Recruitment: not specified Maximum follow‐up: 30 days |
|
| Participants | 127 participants undergoing primary TKA who elected to pre‐donate blood were randomly assigned to 1 of 2 groups
|
|
| Interventions |
|
|
| Outcomes | Complications, cardiac events, Hb, blood usage (units), mental confusion, lethargy, orthostatic hypotension, number of participants transfused | |
| Notes |
Trial registration: none ascertainable Trial funding: none reported COI statement by investigators: no information reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial used a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Blinding of participants and personnel was not specified |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Assessments were made by a person blinded to the group to which the participant was assigned |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | Assessments were made by a person blinded to the group to which the participant was assigned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data appear to have been complete |
| Selective reporting (reporting bias) | Low risk | Evidence of prospective registration/trial protocol unavailable; insufficient information to make a judgement |
| Other bias | Low risk | No other biases were apparent |
Mazer 2017.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre, open‐label, non‐inferiority trial Setting: 73 sites in 19 countries (Australia, Brazil, Canada, China, Colombia, Denmark, Egypt, Germany, Greece, India, Israel, Malaysia, New Zealand, Romania, Singapore, South Africa, Spain, Switzerland, USA) Recruitment: January 2014 to March 2017 Maximum follow‐up: 28 days |
|
| Participants | People 18 years of age or older who were scheduled to undergo cardiac surgery with CPB and who had a preoperative EuroSCORE I ≥ 6 5035 participants were randomised in the main trial; 208 had been randomised in the multicentre pilot trial 5092 participants were used in modified ITT analyses; numbers below are as for per‐protocol analysis
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome: a composite of death from any cause, non‐fatal myocardial infarction, stroke, or new‐onset renal failure with dialysis, occurring during the index hospitalisation from the start of surgery until either hospital discharge or 28 days after surgery, whichever occurred first Secondary outcomes included: components of the primary outcome, blood‐product (including red‐cell) transfusion, lengths of stay in the ICU and in the hospital, duration of mechanical ventilation, prolonged state of low cardiac output, infection, bowel infarction, acute kidney injury, seizure, delirium, encephalopathy |
|
| Notes | Primary results were presented per protocol rather than by intention to treat. This prespecified analysis included all participants except those who had protocol adherence < 90%, those who were withdrawn from the trial by the treating physician at any time, and those who withdrew consent Trial title: The Transfusion Requirements in Cardiac Surgery ‐ TRICS III Trial registration: NCT02042898 Trial funding: supported by grants (232416 and 301852) from the Canadian Institutes of Health Research, by a grant (Kenneth J. Fyke Award, to Dr. Shehata) from the Canadian Blood Services – Health Canada, by a grant (1085942) from the National Health and Medical Research Council of Australia, and by a grant (16/353) from the Health Research Council of New Zealand COI statement by investigators: on file in supplementary forms at https://www.nejm.org/doi/suppl/10.1056/NEJMoa1711818/suppl_file/nejmoa1711818_disclosures.pdf |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random permuted blocks randomly assigned to one of two red‐cell transfusion strategies, in a 1:1 ratio with the use of a concealed centralized, Web‐based system, stratified according to center, with computer‐generated random permuted blocks of varying sizes from two to six" (Mazer 2017 p 2) |
| Allocation concealment (selection bias) | Low risk | Concealed centralised web‐based system (as above) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Patients and clinical teams were not blinded |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Outcomes were adjudicated by committee blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not apparent. Losses to follow‐up low (5092 from 5243 patients) |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT02042898). No evidence of selective reporting |
| Other bias | Low risk | None evident |
Murphy 2015.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre, open‐label, non‐inferiority trial Setting: 17 centres (UK) Recruitment: July 2009 to February 2013 Maximum follow‐up: 90 days |
|
| Participants | Participants > 16 years of age who were undergoing non‐emergency cardiac surgery with Hb < 9 g/dL 2007 randomised; 4 withdrew
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome: a composite of serious infection (sepsis or wound infection) or an ischaemic event (permanent stroke, myocardial infarction, infarction of the gut, or acute kidney injury) within 3 months after randomisation Secondary outcomes included: units transfused, infection, ischaemic events, acute kidney injury, hospital stay and ICU stay, general quality of life (using the EQ‐5D), cost |
|
| Notes |
Trial title: TITRe2 Trial registration (prospective): ISRCTN70923932 Trial funding: "supported by the NIHR Health Technology Assessment program ... Dr. Reeves and the research nurse team in Bristol were supported in part by the NIHR Bristol Biomedical Research Unit in Cardiovascular Disease, and Drs. Murphy, Angelini, and Rogers were supported by the British Heart Foundation" (Murphy 2015 p 1007) COI statement by investigators: "no potential conflict of interest relevant to this article was reported" (Murphy 2015 p 1007) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to either the liberal transfusion‐threshold group (threshold hemoglobin level, 9 g per deciliter) or the restrictive transfusion‐threshold group (threshold hemoglobin level, 7.5 g per deciliter) by means of a secure Internet‐based system that concealed assignments and used cohort minimization to balance assignments according to center and type of surgery" (Murphy 2015 p 998) |
| Allocation concealment (selection bias) | Low risk | Trial used an Internet‐based system that concealed assignments and used cohort minimisation to balance assignments according to centre and type of surgery (Murphy 2015 p 998) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Physicians and nurses were aware of group assignments |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Outcomes were adjudicated |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | For the subjective measure of quality of life (EQ‐5D), we rated risk of bias to be low, as participants were unaware of group assignment. Furthermore, investigators reported that they "tested our success in keeping the study groups blinded by asking the patients if they were aware of the group they were in" "At discharge, 15.1% of patients believed they knew treatment and 75.6% were correct" (Murphy 2015 p 1002); however, "3 months after surgery, a greater number of patients (27.5%) thought that they knew which treatment they had received, but fewer (56.6%) were correct" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were missing for the primary outcome analysis (composite as above) for 4.8% of the sample; overall data including for mortality were missing for only 1.2% at 3 months |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered, and all prespecified outcomes appear to have been reported in full |
| Other bias | Low risk | No other biases were apparent |
Møller 2019.
| Study characteristics | ||
| Methods |
Design: RCT, single‐site, 2‐arm parallel trial Setting: "vascular unit servicing a population of 820,000"; Denmark Recruitment: 2015 to 2017 Maximum follow‐up: 90 days |
|
| Participants | 58 participants over 40 years of age undergoing elective open infrarenal abdominal aortic aneurysm repair or lower limb bypass surgery or femoro‐femoral cross‐over surgery
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome: mean postoperative Hb between days 0 and 15 Secondary outcomes: units of RBCs transfused, randomisation rate, proportions of patients with protocol suspensions, adherence to Hb concentrations used for transfusion triggers, intraoperative tissue oxygenation determined by near‐infrared spectroscopy, severe adverse events within 30 days of surgery |
|
| Notes |
Trial title: Transfusion in Vascular surgery (TV) trial Trial registration: NCT02465125 Trial funding: Local Research Fund of Region Zealand, Næstved, Slagelse, Ringsted Hospital (2015‐01‐26) (A. Møller), and Region Sjaelland Health Research Fund (Denmark). The funders of this trial were reported to be public organisations with no role in trial design, collection, management, analysis, and interpretation of data, writing of the report, or the decision to submit the report for publication COI statement by investigators: "the authors declare no competing financial interests" (Møller 2019 p 2648) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was computer generated in a 1:1 ratio with fixed block sizes of 6 stratified for type of surgery: open abdominal aortic aneurism operation vs lower limb bypass |
| Allocation concealment (selection bias) | Low risk | External, centralised, web‐based system |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Patients, statisticians, and outcome assessor were blinded to group assignment |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Outcome assessors were blinded to group assignment |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) Near‐infrared spectroscopy done by blinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 58 participants randomised. There were 8 protocol suspensions in the restrictive group and 4 in the liberal group. 10 participants in the restrictive group 'avoided' RBC transfusion; none did in the liberal group All participants were included in an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Trial reports 90 days' mortality, not 30 days (as stated on Clinicaltrials.gov) 30‐Day outcomes were presented (e.g. laboratory values) and data on mortality were presented in a supplement (1 death both arms) prepublished protocol was reported |
| Other bias | Unclear risk | Protocol suspensions for RBC transfusion were recorded, but there was a good discussion about the definitions of protocol adherence, and this would be expected as an issue for trials in this patient population. Overall rates of non‐adherence were 28% and 34% of patients in the 2 arms, which seems quite high |
Nielsen 2014.
| Study characteristics | ||
| Methods |
Design: RCT, parallel, 2‐centre trial. Trial authors wrote, "our study may be considered a feasibility study" (Nielsen 2014 p 8) Setting: orthopaedic departments at 1 university teaching hospital and 1 general hospital, Copenhagen, Denmark Recruitment: June 2009 to May 2011 Maximum follow‐up: 30 days |
|
| Participants | 66 participants at least 18 years of age scheduled for elective hip revision surgery
|
|
| Interventions |
Target levels of Hb were > 8.9 g/dL in the liberal group and 7.3 g/dL to 8.9 g/dL in the restrictive group |
|
| Outcomes |
Primary outcome: 'Timed up and go' test Other outcomes included: pneumonia, wound infection, GI complications, dizziness, hypotension, fatigue, deep vein thrombosis, falls |
|
| Notes |
Trial registration (prospective): NCT00906295 Trial funding: "the first author has received an unrestricted research grant from the TrygFonden foundation, Denmark. TrygFonden has not taken any part in designing the study, analyzing the data or approving the manuscript" (Nielsen 2014 p 8) COI statement by investigators: "the authors declare that they have no competing interests" (Nielsen 2014 p 8) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A dedicated computer program (Idefix) was used after participants' baseline data were entered. Allocation was written on a form, which was kept in the investigator's office, and allocation could be accessed only by the investigator in charge of administrating RBCs |
| Allocation concealment (selection bias) | Low risk | Only 1 investigator had access to the programme. Investigators at the other hospital had to call this investigator to randomise |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Allocation and Hb during the testing period were concealed from participants, but the investigator, staff in the operating room, and staff at the ward could not be blinded |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' The physiotherapist testing the participant was blinded, but it is not stated who reviewed medical records for other outcomes |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | Timed up and go test, assessed by blinded physiotherapist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias was apparent, but patient flow identified losses to primary analysis (8 in restrictive arm, 5 in liberal arm) |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered. All prespecified outcomes were reported. No deaths were reported in either group |
| Other bias | Unclear risk | Transfusion threshold was never reached in around half of recruited patients |
Palmieri 2017.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, phase 3, multicentre, international trial Setting: 18 burn centres located in 3 countries (USA (16), Canada (1), New Zealand (1)) Recruitment: August 2010 to August 2015 Maximum follow‐up: mortality reported at and after 30 days; 'after' not specified |
|
| Participants | 345 participants 19 years of age or older admitted to burn unit within 96 hours of injury with a burn of 20% or more; need for burn excision and grafting was anticipated
|
|
| Interventions |
Transfusion was administered 1 unit at a time |
|
| Outcomes |
Primary outcome: bloodstream infection Secondary outcomes included: mortality, number of infectious episodes (urinary tract infections, pneumonia, wound infection), burn ICU length of stay, hospital length of stay, duration of mechanical ventilation, organ dysfunction (MODS), time to 90% burn wound healing (defined as 7 days after last excision and grafting procedure) |
|
| Notes |
Trial registration (prospective): NCT01079247 Trial funding: "this study was supported by the American Burn Association and funded by USAMRMC Award W81XWH‐08‐1‐0760 with support from the National Center for Research Resources, National Institutes of Health, through grant UL1 RR024146, the National Center for Advancing Translational Sciences, National Institutes of Health, through grant TR 000002, and the National Center for Advancing Translational Sciences, National Institutes of Health through grant UL1 TR001860" (Palmieri 2017 p 1) COI statement by investigators: "Dr. Holmes: equity positions in Abbott Labs, AbbVie, and Permeaderm Inc. Dr. Tredget: contract research, Scar X, KLOX Therapeutics, and Exciton (ExSALT), collaborative research British Canadian BioSciences Corp (novel antifibrotic agent). The remaining authors report no conflicts of interest" (Palmieri 2017 p 1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not specified but adaptive random allocation procedure used to balance groups with respect to screening prognostic variables using a "biased coin" procedure, which creates low risk of bias |
| Allocation concealment (selection bias) | Low risk | Not specified but adaptive random allocation procedure used to balance groups with respect to screening prognostic variables using a "biased coin" procedure, which creates low risk of bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Blinding of participants and personnel was not addressed |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Blinding of outcome assessment was not addressed |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29 (8%) patients withdrew |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT01079247). No reporting bias was apparent |
| Other bias | Low risk | No other biases were apparent |
Parker 2013.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site trial Setting: general hospital, Peterborough, UK Recruitment: not stated in paper; ISRCT states trial ran from 2002 to 2012 Maximum follow‐up: 12 months |
|
| Participants | 200 participants 60 years of age or older with hip fracture whose postoperative Hb on postoperative day 1 or 2 was 8.0 g/dL to 9.5 g/dL
|
|
| Interventions | Participants had postoperative Hb in a prescribed range (8.0 g/dL to 9.5 g/dL)
For the purposes of this review, the trial author's definitions of 'transfusion' group = liberal, and 'no transfusion' or 'symptomatic' = restrictive
|
|
| Outcomes | Mobility, mental agility, physical status using American Society of Anesthesiologists grades | |
| Notes |
Trial registration (not prospective): ISRCTN61328173 Trial funding: "there was no external funding for this study" (Parker 2013 p 1918) COI statement by investigators: "the author does not have any conflict of interest with this article" (Parker 2013 p 1918) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was undertaken using numbered, sealed, opaque envelopes that were prepared before the start of the study" |
| Allocation concealment (selection bias) | Low risk | Trial used opaque numbered envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Blinding of participants and personnel was not addressed |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Blinding of outcome assessment was not addressed |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Blinding of outcome assessor was not addressed for mobility |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Mobility score was missing for 94 of 200 participants |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent; however, trial was retrospectively registered (ISRCTN61328173), and in the absence of a protocol, assessment must remain 'unclear' |
| Other bias | Low risk | No other biases were apparent |
Prick 2014.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre trial Setting: 37 university and general hospitals in the Netherlands Recruitment: May 2004 to February 2011 Maximum follow‐up: 6 weeks |
|
| Participants | Participants with postpartum haemorrhage (blood loss ≥1000 mL or decrease in Hb ≥1.9 g/dL, or both) and Hb between 4.8 g/dL and 7.9 g/dL 12 to 24 hours after delivery 521 randomised, 2 excluded from analysis (included 3 days postpartum), 519 remained
40 "did not comply" with allocated intervention |
|
| Interventions |
|
|
| Outcomes | Primary outcome: physical fatigue 3 days postpartum using the Multidimensional Fatigue Inventory Scale | |
| Notes |
Trial title: Well‐Being of Obstetric Patients on Minimal Blood Transfusions (WOMB) Trial registration: NCT00335023 Trial funding: Landsteiner Foundation for Blood Transfusion Research (file number 0904) and Stichting Vrienden van de Bloedtransfusie (file number 1201‐995). "The funders had no role in study design, data collection and analysis, data interpretation, decision to publish or preparation of the manuscript" (Prick 2014 p 1013) COI statement by investigators: "all authors confirm no conflicts of interest with regards to the data reported" (Prick 2014 p 1013) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After informed consent, women were randomly allocated in a 1:1 ratio to receive RBC transfusion or no intervention, using a web‐based application for block randomisation with a variable block size of 2 to 8. Randomisation was stratified for mode of delivery and participating hospital |
| Allocation concealment (selection bias) | Low risk | Trial used a web‐based application with block randomisation of variable block size |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Participants were not blinded |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Primary outcome was based on a questionnaire for fatigue; it is unclear how assessors were blinded and participants were not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 521 randomised, 2 excluded from analysis (included 3 days postpartum), 519 remained. 40 "did not comply" with allocated intervention (imbalanced numbers: 33 in 'non intervention' arm; 7 in RBC arm) 20% of data for the primary outcome was missing. Health‐related quality of life data were available for 78% and 81% of patients in the 2 arms |
| Selective reporting (reporting bias) | Low risk | NCT00335023l. Trial was preregistered and mortality was never planned to be an outcome, which was clinically appropriate for this population |
| Other bias | Low risk | No other biases were apparent. 11% of women in the non‐intervention (restrictive) arm received transfusions |
Robitaille 2013.
| Study characteristics | ||
| Methods |
Design: RCT, intended to be multicentre trial Setting: 1 institution (not identified), Canada Recruitment: June 2009 Maximum follow‐up: intended to be 5 years (overall survival) |
|
| Participants | Children aged 1 to 18 years who were undergoing an allogeneic BMT for malignant or benign disease (except sickle cell disease) were eligible Recruitment target was 62; enrolment was 6 3 ‐ liberal threshold trigger transfusion strategy 3 ‐ restrictive threshold trigger transfusion strategy |
|
| Interventions |
|
|
| Outcomes | Time to neutrophil engraftment, time to platelet engraftment, transfusions given, length of stay, immune reconstitution, mortality/overall survival (2 to 5 years), graft vs host disease, relapse, chimerism | |
| Notes | Trial closed early due to an excess of cases of veno‐occlusive disease in 3 out of 6 cases, all in the intervention (liberal) arm Trial registration: NCT00937053 Trial funding: Fonds de laRecherche en Santé du Québec (grants 9967 and 24460) COI statement by investigators: "the authors declare no conflict of interest to report" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of patients was done on the first day of their conditioning regimen using a web‐based randomization system" (Robitaille 2013 p 469) |
| Allocation concealment (selection bias) | Low risk | As above ‐ no further information supplied |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Primary outcome of veno‐occlusive diease is also a hard outcome |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented for small cohort |
| Selective reporting (reporting bias) | Low risk | All data presented for small cohort; 1 death at 6 months in experimental arm. Small sample size rendered many predefined outcomes inappropriate |
| Other bias | Low risk | Trial closed early due to an excess of cases of veno‐occlusive disease in 3 out of 6 cases, all in the intervention (liberal) arm Overall considered at low risk of bias based on limited data available for analysis within this review |
Shehata 2012.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, open‐label, single‐site pilot trial Setting: university teaching hospital, Toronto, Canada Recruitment: January 2007 to June 2010 Maximum follow‐up: 30 days |
|
| Participants | 50 adult participants undergoing cardiac surgery with a CARE score (a score for cardiac surgery participants used to predict morbidity and mortality) of 3 or 4, or participants of advanced age defined as ≥ 80 years
|
|
| Interventions |
|
|
| Outcomes | Primary outcome: enrolment rate and overall adherence to transfusion strategies (pilot study). Clinical outcomes were assessed | |
| Notes |
Trial title: Transfusion Triggers in Cardiac Surgery Trial registration (not prospective; record posted 6 months after trial start): NCT00470444 Trial funding: this trial was supported by Canadian Blood Services SPF XT00070. Canadian Blood Services as a funding agency had no role in the design and conduct of the trial COI statement by investigators: "the authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION" (Shehata 2012 p 97) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician generated the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sequential sealed envelopes were opened at the start of surgery |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Clinicians and participants were not blinded |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data appeared complete for all 50 participants |
| Selective reporting (reporting bias) | Low risk | This pilot trial was registered 6 months after recruitment began. All expected outcomes for a pilot were reported |
| Other bias | Low risk | No other biases were apparent |
So‐Osman 2013.
| Study characteristics | ||
| Methods |
Design: RCT, parallel, multiple‐arm, multiple‐site trial Setting: 3 hospitals in the Netherlands Recruitment: 2001 to 2003 Maximum follow‐up: 14 days |
|
| Participants | 603 participants eligible for elective orthopaedic surgery
|
|
| Interventions | Restrictive transfusion was compared with liberal transfusion regimens, which varied among the 3 hospitals of the original trial ‐ So‐Osman 2010 ‐ to such an extent that amongst the 3 hospitals involved, investigators' planned so‐called "new" policy (protocol A (restrictive)) " .... was more restrictive than the standard policy (B) for two hospitals and the patients randomised to protocol A were labelled as 'restrictive' and those randomised to protocol B as 'liberal'" However, in the third hospital, "the new transfusion trigger was in fact more liberal than the hospital's existing standard policy. As a consequence, patients randomised to the new policy (protocol A) actually received more RBC transfusions, and this group was now labelled as "liberal", whereas the group randomised to the standard policy (protocol B) was labelled as "restrictive" In brief (and with emphasis added to show the overlap): The protocol intended to be the 'liberal' arm (standard care aka Protocol B) varied between hospitals, thus:
The protocol intended to be the 'restrictive' arm (Protocol A) entailed stratifying participants into 3 risk categories (low, intermediate, and high risk). Risk was determined based on age (> 50 years, 50 to 70 years, > 70 years) and/or any one of 11 conditions (e.g. unstable cardiac ischaemia, heart failure, insulin‐dependent diabetes). Within risk categories, triggers differed depending on length of time since surgery (within 4 hours of surgery, or later) At the lowest end possible of the range for Protocol A (low‐risk patients within 4 hours of surgery), patients received no transfusion if Hb remained at or above 6.4 g/dL At the highest end (high‐risk patients > 4 hours after surgery), patients received no transfusion if Hb remained at or above 9.7 g/dL Hospital 2 had a more restrictive policy as standard care than that employed in the 'new', supposedly restrictive, Protocol A; therefore for the purposes of analysis, we used data from So‐Osman 2013 ‐ in which the arms of the latter trial were reversed and all data were thus appropriate for our comparison |
|
| Outcomes | Primary outcome variable: RBC use (originally) Secondary outcomes included: postoperative complications, quality of life | |
| Notes | Review authors (JC and SS) re‐analysed the prior report (So‐Osman 2010), comparing restrictive vs liberal transfusion. It should be noted that whilst details of the thresholds applied in this trial are provided in the papers, there were challenges in implementing the protocol at all sites; significant differences between trial arms were not apparent Trial registration: none ascertainable Trial funding: this trial was fully supported by a grant from the LUMC, Leiden (Doelmatigheidsstudie P01.065) COI statement by investigators: "the authors declare no conflicts of interest" (So‐Osman 2013 p 6) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "For each stratum, a separate randomization list was created, using blocks of variable length to avoid predictability of the random treatment assignment towards the end of each block. Treatment allocation was random using a uniform distribution for a pregenerated list of sufficient length, based on the maximum expected sample size in each stratum. For each subject to be randomized, a sheet of paper with all relevant stratification and group‐allocation information was produced and placed in a sealed opaque envelope. Batches were created according to the stratification factors" (So‐Osman 2010 p 57) |
| Allocation concealment (selection bias) | Low risk | A research nurse opened sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Clinicians caring for participants were aware of allocation status. There was no blinding information on participants |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low'. This also relates to transfusion requirements as the trial outcome |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Quality of life measures were reported for an unblinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No trial registration identified |
| Other bias | Low risk | No other biases were apparent, apart from a slight baseline imbalance (history of COPD more prevalent in restrictive group than in liberal group) |
Stanworth 2020.
| Study characteristics | ||
| Methods |
Design: RCT, 2‐arm parallel, multicentre international feasibility trial Setting: 12 sites (UK, Australia, New Zealand) Recruitment: 2015 to 2017 Maximum follow‐up: 3 months |
|
| Participants | 38 participants:
|
|
| Interventions |
|
|
| Outcomes | Primary outcomes of this feasibility study, from day 0 to day 84, were:
For the review primary outcome of mortality, 1 patient was reported to have died (restrictive arm), but the timing was not clear |
|
| Notes |
Trial registration: ISRCTN26088319 Trial funding: "funding was provided by grants awarded by NHSBT R&D, the Australian and New Zealand Society of Blood Transfusion (ANZSBT) and the Wellington Division of the New Zealand Cancer Society" (Stanworth 2020 p 289) "The sponsor and funders had no role in the collection, analysis and interpretation of data, the writing of the report or the decision to submit" (p 283) COI statement by investigators: "there are no conflicts of interest" (Stanworth 2020 p 289) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule, stratified block design (blocks of 2 and 4) |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes (prepared from the schedule by an independent member of the clinical trials unit) were opened in sequential order |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Patients were blinded to treatment arm and haemoglobin, but not to transfusions; investigators and clinicians were unblinded to treatment allocation |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Investigators and clinicians were unblinded to treatment allocation, and multiple quality of life measures were reported. Transfusion measures could be altered in response to perceived changes in quality of life measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes were reported; all participants were included in analyses |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered, and all data on all outcomes are reported (ISRCTN26088319) |
| Other bias | Low risk | Some imbalances between arms were noted, but the trial was small. "Two patients were randomised in error as not red cell transfusion dependent" (Stanworth 2020 p 283); four patients were not transfused despite being a study of transfusion‐dependency (p 287) |
Tay 2020.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre, non‐inferiority trial Setting: 4 HCT centres, Canada Recruitment: March 2011 to February 2016 Maximum follow‐up: 100 days |
|
| Participants | 300 participants over 18 years of age undergoing autologous or allogeneic HCT for any haematological malignancy randomised There was one late exclusion
|
|
| Interventions |
|
|
| Outcomes | Primary outcome: health‐related quality of life (HRQOL) measured by FACT‐BMT score at day 100 | |
| Notes |
Trial registration (prospective): NCT01237639 Trial funding: supported by grants from the Canadian Institute of Health Research and the Canadian Blood Services COI statement by investigators: "Jason Tay: Honoraria: Celgene, Takeda, Sanofi Canada, Amgen. David S. Allan: Other Relationship: Canadian Blood Services. Mohamed Elemary: Consulting or Advisory Role: Jazz Pharmaceuticals, Amgen, Roche Canada. Adrienne Fulford: Honoraria: Teva Pharmaceutical Industries; Consulting or Advisory Role: Amgen. Irwin Walker: Honoraria: Jazz Pharmaceuticals; Consulting or Advisory Role: Jazz Pharmaceuticals; Research Funding: Sanofi Canada;Travel, Accommodations, Expenses: Jazz Pharmaceuticals. Anargyros Xenocostas: Honoraria: Novartis (Inst), Amgen (Inst), Apobiologix (Inst), Pfizer (Inst); Research Funding: Novartis (Inst)" (Tay 2020 p 1474) No other potential conflicts of interest were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Secure online electronic randomisation platform was used |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Not possible to blind patients or caregivers |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Health‐related outcomes were self‐reported and were trial primary outcomes. Patients were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 300 participants were randomised. There was 1 late exclusion in the restrictive group and 2 in each group were lost to full follow‐up. 299 participants were included from ITT analysis |
| Selective reporting (reporting bias) | Low risk | Trial was prospectively registered (NCT01237639). All outcomes appear to be reported |
| Other bias | Low risk | None was evident. A small difference in storage age at transfusion between the 2 arms was noted. Overall transfusion adherence rate was 95% |
Topley 1956.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐centre trial Setting: an 'accident hospital', Birmingham, UK Recruitment: not reported. Pre‐1957 Maximum follow‐up: 3 months |
|
| Participants | 22 trauma participants randomly allocated to 1 of 2 groups
NB: no demographic data were reported |
|
| Interventions |
|
|
| Outcomes | Blood usage (units), blood loss, wound healing, elevated temperature, number of participants transfused, Hb. Deaths were reported, but timing and transfusion strategy were not provided | |
| Notes |
Trial registration: none ascertainable Trial funding: none reported COI statement by investigators: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Trial used sealed envelopes. When participants were considered eligible for the trial, they were placed in a severity grade and an envelope was opened to decide which transfusion schedule was to be used |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trial did not report in which group the deaths occurred; therefore we classified this as high risk of bias |
| Selective reporting (reporting bias) | High risk | Trial did not report in which group the deaths occurred; therefore we classified this as high risk of bias |
| Other bias | Low risk | No other biases were apparent |
Villanueva 2013.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, single‐site, phase 4 trial Setting: large general hospital, Barcelona, Spain Recruitment: June 2003 to December 2009 Maximum follow‐up: 45 days |
|
| Participants | Participants > 18 years of age who had haematemesis or melena, or both (due to upper GI bleeding) 921 randomised
|
|
| Interventions |
In both groups, 1 unit of RBCs was transfused initially |
|
| Outcomes |
Primary outcome: death at 45 days Secondary outcomes included: rate of further bleeding, rate of in‐hospital complications |
|
| Notes |
Trial registration (December 2006 to not prospective): NCT00414713 Trial funding: Fundació Investigació Sant Pau COI statement by investigators: "Dr. Guarner reports receiving consulting fees from Sequana Medical. No other potential conflict of interest relevant to this article was reported" (Villanueva 2013 p 20) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was computer generated |
| Allocation concealment (selection bias) | Low risk | Trial used sealed consecutively numbered, opaque envelopes |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Clinicians and participants were not blinded |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' Mortality was the primary trial outcome. Assessors of other outcomes were not documented to be blinded |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 15 withdrawals from the liberal arm (of 460 participants) and 17 withdrawals from the restrictive arm (of 461 participants) |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered post hoc (3 years after recruitment began). The primary outcome changed from death at 30 days (December 2006) to death at 45 days (November 2007) (all data were obtained from primary author of trial report by JC) |
| Other bias | Low risk | No other biases were apparent |
Walsh 2013.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre, pilot study Setting: 6 ICUs in university teaching and general hospitals (UK) Recruitment: August 2009 to December 2010 Maximum follow‐up: 6 months |
|
| Participants | 100 ICU participants aged ≥ 55 years, Hb < 9 g/dL, mechanical ventilation for ≥ 96 hours, who were expected to require ≥ 24 hours of further mechanical ventilation when assessed
|
|
| Interventions |
|
|
| Outcomes | Primary feasibility outcome: difference in mean Hb among groups. Clinical outcomes were assessed, including SF‐12 (with physical function scale) | |
| Notes |
Trial name: Restrictive versus Liberal Transfusion Strategies for Older Mechanically Ventilated Critically Ill Patients (RELIEVE) Trial registration (prospective): NCT00944112 Trial funding: supported, in part, by the Chief Scientists Office, Scotland (CZB/4/698); the Scottish National Blood Transfusion Service, the NHS Lothian Academic Health Science Centre; and the Transfusion Medicine Education and Research Foundation COI statement by investigators: no statement of interest reported in the paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Minimisation by centre and the presence of IHD, including a random element, was used" (Walsh 2013 p 2355) |
| Allocation concealment (selection bias) | Low risk | Trial used telephone randomisation |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Clinicians were not blinded. Most surviving participants stated that they were unaware of group allocation at 180 days (restrictive group: 67%; liberal group: 78%); 23% of participants in the restrictive group and 9% in the liberal group correctly stated their treatment group |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Researchers concealed from group allocation collected questionnaire‐based measures at 60 and 180 days post randomisation. Clinical outcomes were not documented to have been done blindly; however, investigators did ask surviving patients at 180 days which group they thought they had been allocated to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were rare at long‐term follow‐up At 60 days, of surviving participants in the restrictive group (37/51), there were 29/37 complete RMIs (Rivermead Mobility Index) and 23/37 completed SF‐12s. At 180 days, of surviving participants in this group (33), there were 29 complete RMIs and 28 SF‐12s and 29 HE questionnaires At 60 days, of surviving participants in the restrictive group (27/49), there were 26/27 complete RMIs and 23/27 completed SFIs. At 180 days, of surviving participants in this group (22), there were 21 complete RMIs and 21 SF‐12s and 20 HE questionnaires |
| Selective reporting (reporting bias) | Low risk | Trial registration was prospective. No reporting bias was apparent |
| Other bias | Low risk | No other biases were apparent |
Webert 2008.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, multicentre feasibility study Setting: 4 tertiary haematology centres (Canada) Recruitment: March 2003 to October 2004 Maximum follow‐up: 30 days |
|
| Participants | 60 adult participants with acute leukaemia were randomly allocated to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Transfusions, bleeding risk, 30‐day mortality provided by trial authors | |
| Notes |
Trial registration: none ascertainable Trial funding: "This study was funded by a grant from Canadian Blood Services and a CIHR Canada Research Chair. KEW was supported by a Canadian Blood Services/Novo Nordisk Research Fellowship in Hemostasis. RJC is a Canada Research Chair" (Webert 2008 p 81) COI statement by investigators: no information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was computer generated |
| Allocation concealment (selection bias) | Low risk | Allocation was Internet based and central |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Participants and clinicians were not blinded |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | Low risk | No data from subjective outcomes (e.g. function) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data were missing |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent, but in the absence of prospective registration or a trial protocol, assessment must remain 'unclear' |
| Other bias | Low risk | No other biases were apparent |
Yakymenko 2018.
| Study characteristics | ||
| Methods |
Design: RCT, parallel 2‐arm, open‐label, single‐site, phase 2 (feasibility) trial Setting: oncology department, university teaching hospital, Copenhagen, Denmark Recruitment: March 2010 to March 2013 Maximum follow‐up: follow‐up during chemotherapy cycles |
|
| Participants | 133 participants undergoing chemotherapy at the Department of Oncology
|
|
| Interventions |
|
|
| Outcomes |
Primary objective (authors stated): to establish correlation between quality of life and Hb
Secondary objectives: to compare relief of symptoms and improvement in quality of life between randomisation arms, to provide data to plan a larger randomised trial (e.g. safety, completion rate, clinician compliance with protocol) Outcome data were not presented by transfusion group |
|
| Notes |
Trial registration: NCT 01116479 (submitted in April 2010; trial had already begun in March 2010, but registration was deemed prospective) Trial funding: not reported COI statement by investigators: "the authors have no competing interests" (Yakymenko 2018 p 214) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "When included, patients were randomly assigned to one of two transfusion trigger levels through a computer programme" (Yakymenko 2018 p 209) |
| Allocation concealment (selection bias) | Unclear risk | As above ‐ no information other than ‐ "When included, patients were randomly assigned to one of two transfusion trigger levels through a computer programme" (Yakymenko 2018 p 209) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding of personnel for this intervention is not feasible, but in our view, for objective outcomes such as mortality (the primary outcome used within this review), we graded risk of bias as 'low' Unblinded |
| Blinding of outcome assessment (detection bias) Objective measures | Low risk | Blinding of mortality (the primary outcome used within this review) is not relevant, and we graded risk of bias as 'low' |
| Blinding of outcome assessment (detection bias) Subjective measures | High risk | Self‐reported questionnaires were applied in an unblinded trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 65 participants in the restrictive arm, 28 received RBC transfusion. Reasons for 37 who did not included termination of chemotherapy (20); patient request (1); death (1); unknown (2); 'end of study period' (13). 5 questionnaires from the remaining 28 were missing, so 23/28 questionnaires were analysed Of 68 participants in the liberal arm, 58 received RBC transfusion. 10 did not, because of termination of chemotherapy (3); patient request (4); death (2); 'end of study period' (1). 12 questionnaires of the remaining 28 were missing, so 46/58 questionnaires were analysed |
| Selective reporting (reporting bias) | Low risk | Paper does not appear to be explicit about timing of deaths (3); however, the questionnaires appear to have been administered within 7 days of any transfusions delivered, so in our view (since deaths are reported as reasons for not getting questionnaires filled in), these deaths must have occurred within a 7‐day period Trial registration close to prospective; investigators submitted registration on 28 April for a trial that began in March |
| Other bias | Low risk | Trial was stopped early due to 'low accrual'. Investigators aimed to recruit 180 participants |
Abbreviations
ACS: acute coronary syndrome ARDS: acute respiratory distress syndrome ASA: American Society of Anaestheologists BMT: bone marrow transplant CABG: coronary artery bypass grafting CARE: Cardiac Anesthesia Risk Evaluation CMML: chronic myelomonocytic leukaemia COPD: chronic obstructive pulmonary disease CPB: cardiopulmonary bypass DSMB: Data Safety Monitoring Board ECG: electrocardiogram GI: gastrointestinal Hb: haemoglobin concentration Hct: haematocrit HCT: hematopoietic cell transplantation HE: health economics ICU: intensive care unit IHD: ischaemic heart disease IQR: interquartile range ITT: intention‐to‐treat MACE: major adverse cardiac events MDS: myelodysplastic syndrome M/F: male/female MODS: multiple organ dysfunction syndrome MPN: myeloproliferative neoplasm PAB: preoperatively donated autologous blood PCI: percutaneous coronary intervention PI: principal investigator PLTs: platelets QOL: quality of life RBCs: red blood cells RCT: randomised controlled trial RMI: Rivermead Mobility Index ROTEM: rotational thromboelastometry SB: Sengstaken‐Blakemore SD: standard deviation SF‐12: Short Form Health Survey TKA: total knee arthroplasty VA: US Department of Veterans Affairs WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atilla 2017 | RCT. Ineligible intervention (haemoglobin content‐based vs standard (unit‐based) transfusion policy, rather than threshold)) |
| Baron‐Stefaniak 2019 | RCT. Ineligible intervention; not haemoglobin threshold |
| Cholette 2017 | RCT. Ineligible participants (infants (cardiac)) |
| de Bruin 2019 | Ineligible design. Survey amongst ICU physicians reviewing transfusion practices |
| Elshinawy 2020 | RCT. Ineligible intervention; not haemoglobin threshold |
| Fogagnolo 2020 | Ineligible design. Prospective observational study |
| Fortune 1987 | RCT (reported only in an abstract) appeared to measure only oxygen utilisation. Investigators planned no clinical outcomes of interest |
| Franz 2020 | RCT. Ineligible participants (neonates (low birth weight)) |
| Haensig 2019 | RCT. Ineligible intervention (not red cell transfusion but thrombelastometry guided blood‐component therapy) |
| Hamm 2020 | RCT. Ineligible intervention (trial assessed effects of single‐unit vs multiple‐unit transfusion, rather than threshold) |
| Jain 2019 | RCT. Ineligible intervention (trial assessed effects of known haemoglobin content of packed red blood cell units vs standard transfusion practice in thalassaemia major) |
| Kirpalani 2020 | RCT. Ineligible participants: neonates |
| Koster 2016 | Ineligible design. Ineligible intervention. This single‐centred retrospective cohort study looked at coronary artery bypass patients who received either 1 to 2 or no units of blood |
| Kumar 2019 | RCT. Ineligible intervention (not red cell transfusion but thrombelastometry guided blood‐component therapy) |
| Leal‐Noval 2017 | RCT. Ineligible intervention (trial assessed effects of using a transcranial oxygen saturation threshold, as measured by near‐infrared spectroscopy, in neurocritically ill patients) |
| Osawa 2016 | RCT. Ineligible intervention (trial assessed effects of cardiac output‐guided haemodynamic therapy algorithm (goal‐directed therapy group) or usual care) |
| Robertson 2014 | RCT. Ineligible intervention/comparator. The trial employed a 2 × 2 factorial design in which participants received erythropoietin or transfusion at different thresholds. We were unable to isolate the effects of transfusion from erythropoietin |
| Vichinsky 1995 | RCT. Ineligible intervention. The transfusion trigger was based on the level of sickle haemoglobin, not the haemoglobin or haematocrit level |
| Voorn 2017 | RCT. Ineligible intervention (trial focused on educating clinicians to cease to use preoperative erythropoietin and perioperative autologous blood salvage, rather than transfusion thresholds) |
| Yamada 2020 | Ineligible design. Survey of details on antigen‐positive RBC transfusions (826 cases from 45 institutions) |
| Zygun 2009 | RCT. Eligible intervention. Ineligible focus ‐ this trial measured oxygen utilisation and planned no clinical outcomes of interest |
Abbreviations
ICU: intensive care unit RBCs: red blood cells RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
IRCT20190209042660N1.
| Methods | RCT (Iran) |
| Participants | Patients > 18 years of age with > 20% body surface thermal burns |
| Interventions | Intervention: blood transfusion when patient's blood haemoglobin is below 8 mg [sic]/dL Control group: blood transfusion when patient's blood haemoglobin is below 10 mg [sic]/dL |
| Outcomes | Total number of transfused blood bags, mortality during hospitalisation, inpatient morbidity (including wound infection; positive blood culture; sepsis; cardiac, pulmonary, neurological, and compartment syndrome); total episodes of infection (each infection); duration of time in hospital |
| Notes | Trial registration: IRCT20190209042660N1 Not prospectively registered. Trial began recruitment in 2018. Ethics approval reported as 2019. Trial registered 2020 |
ISRCTN26088319.
| Methods | RCT (UK, Australia, New Zealand) |
| Participants | Participants with MDS, transfusion dependent, life expectancy > 6 months |
| Interventions | Liberal protocol: to maintain haemoglobin concentration Restrictive protocol: to maintain haemoglobin level between 8.5 g/dL and 10 g/dL |
| Outcomes | Percentage of compliance of pre‐transfusion haemoglobin levels with achievement of at least 2 g/dL difference between liberal and restrictive transfusion |
| Notes | Trial registration (prospective): ISRCTN26088319 |
Maitland 2019.
| Methods | RCT (multicentre; Uganda, Malawi) |
| Participants | Children aged 2 months to 12 years (median 37 months) with haemoglobin level < 6 g/dL and severity features |
| Interventions | Complex. The main intervention involved receipt of immediate blood transfusion with 20 mL/kg or 30 mL/kg. Three other randomised analyses also investigated:
|
| Outcomes | Primary outcome: 28‐day mortality |
| Notes | Trial registration (prospective): ISRCTN84086586 |
Morton 2020.
| Methods | RCT (feasibility) (UK) |
| Participants | Adults with acute myeloid leukaemia |
| Interventions | Red cell transfusions |
| Outcomes | Feasibility, quality of life |
| Notes | Trial registration (prospective): ISRCTN96390716 |
NCT02099669.
| Methods | RCT (feasibility) (Canada) |
| Participants | All participants with MDS ≥ 18 years of age, transfusion dependent: at least 1 transfusion per month in the last 8 weeks, haemoglobin < 10 g/dL |
| Interventions | Liberal transfusion strategy: to maintain Hb between 11 g/dL and 12 g/dL Restrictive transfusion strategy: to maintain Hb between 8.5 g/dL and 10 g/dL |
| Outcomes | Percentage compliance |
| Notes | Trial registration (prospective): NCT02099669 |
Abbreviations
Hb: haemoglobin concentration MDS: myelodysplastic syndrome RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619001053112.
| Study name | REDDS2: Red Cell Transfusion Schedule in Melodysplastic Syndrome |
| Methods | RCT (feasibility) |
| Participants | Patients with transfusion‐dependent MDS Estimated enrolment: 30 |
| Interventions | Red cell transfusions: liberal weekly policy (Hb < 11 g/dL) vs standard policy |
| Outcomes | Feasibility |
| Starting date | 2020 |
| Contact information | Allisson Mo; allison.mo1@monash.edu |
| Notes | Trial registration (prospective): ACTRN12619001053112 Universal trial identifier: U11112316575 (UTN) Funding: Australian & New Zealand Society of Blood Transfusion (ANZSBT); National Blood Authority; Monash Haematology Research fund (Australia) |
Hayakawa 2020.
| Study name | Restrictive Transfusion Strategy for Critically Injured Patients (RESTRIC) |
| Methods | RCT (multicentre; cluster) |
| Participants | Severe trauma Estimated enrolment: 22 hospitals; n = 400 |
| Interventions | Transfusions to target Hb 7 g/dL to 9 g/dL vs 10 g/dL to 12 g/dL |
| Outcomes | Survival at 28 days |
| Starting date | 2019 |
| Contact information | Mineji Hayakawa; mineji@dream.com |
| Notes | Trial registration (prospective): UMIN000034405 Funding: supported in part by research grants from The General Insurance Association of Japan and The Marumo Emergency Medical Research Promotion Fund (Japan) |
ISRCTN17438123.
| Study name | RePAST: Red cell transfusion in Paediatric Allogeneic HSCT ‐ a feasibility randomised controlled trial comparing restrictive versus liberal RBC transfusion strategies in children undergoing allogeneic HSCT |
| Methods | RCT (feasibility) |
| Participants | Children (aged 1 to 17 years) undergoing allogeneic HSCT Estimated enrolment: 34 |
| Interventions | Restrictive (Hb < 6.5 g/dL) vs liberal red cell transfusion (Hb < 8.0 g/dL) policies |
| Outcomes | Feasibility outcomes |
| Starting date | 2019 |
| Contact information | Val Hopkins; valerie.hopkins@nhsbt.nhs.uk |
| Notes | Trial registration (prospective): ISRCTN17438123 Funding: NHSBT Trust Fund Grant (UK) |
Meybohm 2019.
| Study name | Liberal Transfusion Strategy to Prevent Mortality and Anaemia‐associated, Ischaemic Events in Elderly NOn‐cardiac Surgical Patients ‐ the LIBERAL Trial |
| Methods | RCT (multicentre) |
| Participants | Elderly (≥ 70 years) surgical patients for non‐cardiac surgery Estimated enrolment: 2470 |
| Interventions | Liberal vs restrictive transfusions (9 g/dL vs 7.5 g/dL) |
| Outcomes | All‐cause mortality, myocardial infarction, stroke, kidney injury |
| Starting date | 2018 |
| Contact information | Patrick Meybohm; patrick.meybohm@kgu.de |
| Notes | Trial registration (prospective): NCT03369210 Funding: support from the German Research Foundation (Deutsche Forschungsgemeinschaft; grant no. ME 3559/3–1) (Germany) |
NCT02981407.
| Study name | Myocardial Ischemia and Transfusion (MINT) |
| Methods | RCT (multicentre; international) |
| Participants | Adults with STEMI or non‐STEMI consistent with the 3rd Universal Definition of Myocardial Infarction criteria (Thygesen 2012), as assessed on admission or during the index hospitalisation Estimated enrolment: 3500 (including participants enrolled in earlier pilot conducted in Canada (NCT0248335)). The pilot (intended to recruit 60 participants) is complete; participants' results will be published when the main trial reports, not separately |
| Interventions | Red cell transfusion liberal (10 g/dL) vs restrictive (7 g/dL) |
| Outcomes | Mortality and non‐fatal myocardial reinfarction |
| Starting date | 2017 |
| Contact information | Jeff Carson; jeffrey.carson@rutgers.edu; Helaine Noveck; Helaine.Noveck@Rutgers.edu |
| Notes | Trial registration (prospective): NCT02981407 Funding: NLHBI (USA) |
NCT03229941.
| Study name | Transfusion Trigger after Operations in High Cardiac Risk Patients (TOP) |
| Methods | RCT (multicentre) |
| Participants | The trial will include people having:
Estimated enrolment: 3070 |
| Interventions |
|
| Outcomes | A composite endpoint of all‐cause post‐randomisation mortality, myocardial infarction, coronary revascularization, acute renal failure, or post‐randomisation ischaemic stroke up to 90 days after randomisation |
| Starting date | 2018 |
| Contact information | Panagiotis Kougias; panagiotis.kougias@va.gov Sherene Sharath; sherene,sharath@va.gov |
| Notes | Trial registration (prospective): NCT03229941 Sponsor: Veterans Affairs Office of Research and Development (USA) |
NCT03260478.
| Study name | HEMOglobin Transfusion Threshold in Traumatic Brain Injury OptimizatioN: The HEMOTION Trial (HEMOTION) |
| Methods | RCT (multicentre) |
| Participants | Adults with acute moderate or severe traumatic brain injury Estimated enrolment: 712 |
| Interventions | Liberal group: RBC transfusion if Hb ≤ 10 g/dL Restrictive group: RBC transfusion if Hb < 7 g/dL |
| Outcomes | Extended Glasgow Outcome Scale (GOSe) (time frame: 6 months) |
| Starting date | 2017 |
| Contact information | Lucy Clayton; hemotion@crchudequebec.ulaval.ca Alexis Turgeon; alexis.turgeon@fmed.ulaval.ca |
| Notes | Trial registration (prospective): NCT03260478 Funding: CIHR (Canada) |
NCT03309579.
| Study name | Aneurysmal SubArachnoid Hemorrhage ‐ red blood cell transfusion and outcome (SAHaRA) |
| Methods | RCT (multicentre; international; pragmatic) |
| Participants | Adults with aneurysmal subarachnoid haemorrhage Estimated enrolment: 740 (including participants enrolled in earlier pilot (NCT02483351)) NCT02483351 is now complete; participants' results will be published when the main trial reports, not separately |
| Interventions | Liberal vs restrictive threshold (10 g/dL vs 8 g/dL) |
| Outcomes | Primary outcome: modified Rankin scale (for measuring functional outcome in stroke) Mortality will be reported at 12 months |
| Starting date | 2018 |
| Contact information | Shane English; senglish@toh.ca |
| Notes | Trial registration (prospective): NCT03309579 Funding: supported by a Transfusion Science research grant awarded by a Canadian Blood Services and Health Canada in partnership with CIHR Institute of Circulatory and Respiratory Health (Canada) |
NCT03871244.
| Study name | Pilot Optimising Transfusion Thresholds in Critically Ill Children with Anaemia (pOpTTICCA) |
| Methods | RCT (feasibility for a large pragmatic trial) |
| Participants | Children admitted to paediatric ICU Estimated enrolment: 120 |
| Interventions | Red cell transfusion: restrictive (Hb < 7 g/dL) vs standard of care |
| Outcomes | Feasibility measures including recruitment |
| Starting date | 2019 |
| Contact information | Jacques Lacroix; jacques.lacroix.hsj@ssss.gouv.qc.ca |
| Notes | Trial registration (prospective): NCT03871244 Funding: CIHR (Canada) |
NCT04506125.
| Study name | Liberal versus Restrictive Transfusion Threshold in High‐risk Oncologic Surgery: a multicenter, randomised, controlled, pilot study |
| Methods | RCT (multicentre, pilot) |
| Participants | Cancer surgery Estimated enrolment: 30 |
| Interventions | Liberal arm: Hb 9.5 g/dL threshold for transfusion Restrictive arm: Hb 7.5 g/dL threshold for transfusion |
| Outcomes | Listed as: method of the pilot study, epidemiological data of the pilot study |
| Starting date | 2021 |
| Contact information | Cécile Aubron; cecile.aubron@chu-brest.fr Xavier Chapalain; xavier.chapalain@chu-brest.fr |
| Notes | Trial registration (prospective): NCT04506125 Funding: none specified. Sponsor is University Hospital, Brest (France) |
NCT04591574.
| Study name | Anaemia Management with Red Blood Cell Transfusion to Improve Post‐Intensive Care Disability: a randomised controlled trial (the ABC Post‐intensive Care Trial) |
| Methods | RCT (multicentre ‐ acute hospitals throughout the UK) |
| Participants | Patients > 16 years old considered ready for ICU discharge with Hb < 9.4 g/dL Estimated enrolment: 305 |
| Interventions | Liberal group: transfusion at Hb 10 g/dL threshold Restrictive group: transfusion at Hb 7.0 g/dL threshold |
| Outcomes | Health‐related quality of life (SF‐36) |
| Starting date | 2020 |
| Contact information | Timothy Walsh; ABC.Trial@ed.ac.uk |
| Notes | Trial registration (prospective): NCT04591574 Funding: Moulton Grant (UK) |
NCT04754022.
| Study name | TRICSIV: an international, multicentre, randomised controlled trial to assess transfusion thresholds in younger patients undergoing cardiac surgery |
| Methods | RCT (multicentre) |
| Participants | Adults (≥ 18 and ≤ 65 years of age) undergoing planned cardiac surgery Estimated enrolment: 1440 |
| Interventions | Liberal group: transfusion at Hb < 9.5 g/dL threshold Restrictive group: transfusion at Hb < 7.5 g/dL threshold |
| Outcomes | Composite score of any 1 of the following events occurring 6 months after cardiac surgery: all‐cause mortality, myocardial infarction, new‐onset renal failure requiring dialysis, new focal neurological deficit (stroke) |
| Starting date | 2021 |
| Contact information | David Mazer; david.mazer@unityhealth.to Nadine Shehata; Mount Sinai Hospital, NY, USA |
| Notes | Trial registration (prospective): NCT04754022 Funding: not specified, but sponsor/collaborator is listed as Unity Health Toronto (Canada) |
NIHR 130875.
| Study name | The impact of REstrictive versus LIberal Transfusion strategy on cardiac injury and death in patients undergoing surgery for Hip Fracture (RESULT‐Hip) |
| Methods | RCT |
| Participants | Adults aged 60 years or older with hip fracture who become anaemic (< Hb 9.0 g/dL) during the 7 days following surgery Estimated enrolment: 1964 |
| Interventions | Liberal group: transfusion threshold of Hb 9.0 g/dL (target Hb 9.0 g/dL to 11.0 g/dL) for duration of acute hospital stay Restrictive group: transfusion threshold of Hb 7.5 g/dL (target Hb 7.5 g/dL to 9.0 g/dL) for duration of acute hospital stay |
| Outcomes | Primary outcome: death or MACE within 30 days of surgery. MACE will be defined as any combination of the following: death, myocardial Infarction, new arrhythmia, cardiac or respiratory arrest, cardiogenic pulmonary oedema |
| Starting date | 2022 |
| Contact information | Michael Gillies; michael.gillies@ed.ac.uk |
| Notes | Funding: National Institute for Health Research (NIHR) ‐ Health Technology Assessment (UK) |
NL3090 NTR3244.
| Study name | Perioperative Transfusion Study (PETS): does a liberal transfusion protocol improve outcome in high‐risk cardiovascular patients undergoing non‐cardiac surgery? |
| Methods | RCT |
| Participants | Elective high‐risk cardiac surgery participants Estimated enrolment: 100 |
| Interventions | Liberal group: 11 g/dL transfusion threshold Restrictive group: 9.7 g/dL transfusion threshold |
| Outcomes | Troponin elevation above 99th percentile |
| Starting date | August 2015 |
| Contact information | Felix van Lier; f.vanlier@erasmusmc.nl |
| Notes | Trial registration (prospective): NTR3244 (https://www.trialregister.nl/trial/3090) Funding: not specified other than 'initiator' (presumed, investigator) |
Abbreviations
CIHR: Canadian Institutes of Health Research Hb: haemoglobin concentration HSCT: haematopoietic stem cell transplantation ICU: intensive care unit IHD: ischaemic heart disease MACE: major adverse cardiac events MDS: myelodysplastic syndromes NHSBT: National Health Service Blood and Transplant (UK) PAD: peripheral arterial disease RBCs: red blood cells RCT: randomised controlled trial STEMI: ST‐segment elevation myocardial infarction
Differences between protocol and review
The following differences were applied in this 2021 version of the review.
We changed the title of the review from "Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion" to "Transfusion thresholds for guiding red blood cell transfusion".
The authors of the review have changed ‐ two new authors have been added (Marialena Trivella and Jane Dennis).
We clarified the outcome of blood transfusion as a measure of treatment implementation and reported and analysed these elements more clearly.
We added a new secondary outcome (transfusion‐specific reactions).
We added quality of life (QOL) to data items collected under 'function'.
Our summary of findings table now reports on infections as a composite category, rather than 'pneumonia' as a specific one.
We have added details of random‐effects analysis into the data synthesis section.
We no longer include an estimate of optimal information size (sample size calculations for trials to yield reliable information on mortality assuming baseline mortality at 30 days), given the breadth of clinical contexts included within this review.
We have divided risk of bias assessment for outcome assessment by objective (mortality) and subjective (function, QOL) measures.
We have omitted sensitivity analysis on the basis of outcome assessment for mortality, considering that the objective nature of the outcome makes blinding unimportant. Should future versions of the review include data suitable for meta‐analysis for the outcomes of function and fatigue, we will conduct sensitivity analyses on the basis that blinding is relevant in such cases.
We reviewed our ratings for bias under the domain of 'Selective outcome reporting'.
The following differences were applied in the 2016 version of the review.
The primary outcome changed from "the proportion of patients 'at risk' who were transfused with red blood cells", to "30‐day mortality". Previously, 30‐day mortality was a secondary outcome. The proportion of participants 'at risk' who were transfused with red blood cells became a secondary outcome. The primary outcome was changed because mortality is a more clinically relevant outcome and the number of participants enrolled in trials provided sufficient power to examine this outcome.
Sample size calculations that assumed a baseline 30‐day mortality of 9% for restrictive transfusion, 90% power, alpha level of 0.05, indicated that to detect a 15%, 20%, or 25% relative decrease in mortality with the use of liberal transfusion, a trial would need to enrol 17,500, or 9600, or 6000 participants, respectively.
We added one new exclusion criterion: we excluded trials that were not designed to include any clinical outcomes relevant to this review.
We added a new sensitivity analysis: registered trials versus unregistered trials.
We separated blinding of participants and personnel from blinding of outcome assessment.
Contributions of authors
For the 2021 update
Carson, Stanworth and Dennis contributed equally to this review update. Carson and Stanworth continue to provide overall leadership of the review updates with clinical input; they screened abstracts and titles identified in the searches, and applied inclusion and exclusion criteria. Carson, Stanworth and Dennis extracted data, and assessed risk of bias of trials. Carson entered the data into Review Manager 5.4 and, with Trivella and Dennis, performed the initial analyses. Dennis and Trivella provided methodological and statistical input and assurance (Review Manager 5a). Stanworth and Carson prepared the first draft of the manuscript. Nareg Roubinian reviewed the accuracy of data and assisted with preparation of the manuscript. Fergusson provided methodological and statistical expertise and assisted with final drafts of the of the manuscript. Triulzi and Hébert reviewed the manuscript and provided content expertise. Doree performed additional literature searches.
For the 2016 update
Carson and Stanworth screened abstracts and titles identified in the searches, applied inclusion and exclusion criteria, and assessed the quality of trials. Stanworth led the quality review, and Carson entered the data into Review Manager 5.3, performed initial analyses, and prepared the first draft of the manuscript (Review Manager 5a). Roubinian checked the accuracy of data and assisted with preparation of the manuscript. Fergusson provided methodological and statistical expertise and assisted with preparation of the manuscript. Triulzi and Hébert reviewed the manuscript and provided content expertise. Doree performed additional literature searches.
For the 2012 review
Paul Carless performed original database literature searches, screened abstracts and titles for relevant articles, obtained relevant papers, applied inclusion/exclusion criteria to retrieved papers, extracted data from trials, quality‐assessed trials, entered data into Meta‐View 4.1, entered all study details into Review Manager 5.1, and co‐wrote the review (Review Manager 5b). Jeffrey Carson screened abstracts and titles for relevant articles, obtained relevant papers, applied inclusion/exclusion criteria to retrieved papers, extracted data from trials, quality‐assessed trials, entered data and all study details into Review Manager 5.1, and co‐wrote the review. Paul Hébert reviewed the manuscript and provided expertise with analysis and content expert opinion.
Sources of support
Internal sources
-
National Institute for Health Research (NIHR), UK
Provides support for the Cochrane Injuries Group, which employs authors MHT and JD
External sources
No sources of support provided
Declarations of interest
We have considered disclosures relevant to this review.
Jeffrey Carson reports being the chief investigator on three trials included in this review (Carson 1998; Carson 2011 (FOCUS); Carson 2013). He has received multiple grants supporting his institution from the US National Institutes of Health. He has been involved with guideline development for red cell transfusions in the Association for the Advancement of Blood & Biotherapies (AABB). He has received a grant from the US National Institutes of Health to evaluate transfusion thresholds in patients with acute myocardial infarction in an ongoing trial (MINT ‐ NCT02981407).
Carolyn Dorée: nothing to declare.
Dean Fergusson reports being an author on three completed trials included within this review (Hébert 1999; Mazer 2017 (TRICS III); Shehata 2012). He was a Co‐Principal Investigator on the TRICSIII trial (Mazer 2017); he is a member of the Steering Committee for the ongoing MINT trial (NCT02981407).
Paul Hébert reports being an author on three completed trials identified in this review (Hébert 1995; Hébert 1999; Lacroix 2007 (TRIPICU). He is the lead investigator on the study NCT02619136, the Canadian pilot study for MINT (NCT02981407, for which he is a member of the Executive Committee); he has published six non‐Cochrane reviews in this area. He serves on a guideline panel for the American College of Chest Physicians (ACCP) and has written several editorials for leading journals concerning transfusion triggers.
Nareg Roubinian: nothing to declare.
Simon Stanworth reports being the chief investigator on one trial included in this review (Stanworth 2020); a coauthor on another (Gillies 2020 (RESULT‐NOF)); and is a co‐investigator on four ongoing trials (ISRCTN17438123; ACTRN12619001053112; NCT03871244; Morton 2020). He has received funding for four RBC transfusion trials, including three in patients with haematological malignancy. He has published three non‐Cochrane reviews in this area.
Darrell Triulzi is a member of the Steering Committee for the ongoing MINT trial (NCT02981407) and a member of the scientific advisory board for Fresenius‐Kabi.
Jane Dennis was employed by Cochrane Injuries during her involvement in development of the review.
Marialena Trivella was employed by Cochrane Injuries during her involvement in development of the review.
Data extraction for all trials was checked by NR. Final decisions on risk of bias assessments were made by review authors not involved in trials as researchers.
JLC, SJS and JAD contributed equally to this work
JLC, SJS and JAD contributed equally to this work
JLC, SJS and JAD contributed equally to this work
Edited (no change to conclusions)
References
References to studies included in this review
Akyildiz 2018 {published data only}TSA‐2014‐5299
- Akyildiz B, Ulgen Tekerek N, Pamukcu O, Dursun A, Karakukcu M, Narin N, et al.Comprehensive analysis of liberal and restrictive transfusion strategies in pediatric intensive care unit. Journal of Tropical Pediatrics 2018;64(2):118-25. [DOI] [PubMed] [Google Scholar]
Bergamin 2017 {published data only}
- Bergamin FS, Almeida JP, Landoni G, Galas F, Fukushima JT, Fominskiy E, et al.Liberal versus restrictive transfusion strategy in critically ill oncologic patients: the transfusion requirements in critically ill oncologic patients: randomized controlled trial. Critical Care Medicine 2017;45(5):766-73. [DOI] [PubMed] [Google Scholar]
Blair 1986 {published data only}
- Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM.Effect of early blood transfusion on gastrointestinal haemorrhage. British Journal of Surgery 1986;73(10):783-5. [DOI] [PubMed] [Google Scholar]
Bracey 1999 {published data only}
- Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, et al.Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion 1999;39(10):1070-7. [DOI] [PubMed] [Google Scholar]
Bush 1997 {published data only}
- Bush RL, Pevec WC, Holcroft JW.A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. American Journal of Surgery 1997;174(2):143-8. [DOI] [PubMed] [Google Scholar]
Carson 1998 {published data only}
- Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al.A pilot randomized trial comparing symptomatic vs. hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion 1998;38(6):522-9. [DOI] [PubMed] [Google Scholar]
Carson 2011 {published data only}
- Carson JL, Sieber F, Cook DR, Hoover DR, Noveck H, Chaitman BR, et al.Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet 2015;385(9974):1183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al.Liberal or restrictive transfusion in high-risk patients after hip surgery. New England Journal of Medicine 2011;365(26):2453-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber-Baldini AL, Marcantonio E, Orwig D, Magaziner J, Terrin M, Barr E, et al.Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. Journal of the American Geriatrics Society 2013;61(8):1286-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2013 {published data only}
- Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al.Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. American Heart Journal 2013;165(6):964-71. [CTG: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2011 {published data only}
- Cooper HA, Rao SV, Greenberg MD, Rumsey MP, Mckenzie M, Alcorn KW, et al.Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT randomized pilot study). American Journal of Cardiology 2011;108(8):1108-11. [DOI] [PubMed] [Google Scholar]
de Almeida 2015 {published data only}
- Almeida JP, Vincent JL, Galas FR, Almeida EP, Fukushima JT, Osawa EA, et al.Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology 2015;122(1):29-38. [DOI] [PubMed] [Google Scholar]
DeZern 2016 {published and unpublished data}
- DeZern AE, Williams K, Zahurak M, Hand W, Stephens RS, King KE, et al.Red blood cell transfusion triggers in acute leukemia: a randomized pilot study. Transfusion 2016;56(7):1750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducrocq 2021 {published data only}
- Ducrocq G, Gonzalez-Juanatey JR, Puymirat E, Lemesle G, Cachanado M, Durand-Zaleski I, et al.Effect of a restrictive vs liberal blood transfusion strategy on major cardiovascular events among patients with acute myocardial infarction and anemia: the REALITY randomized clinical trial. JAMA 2021;325(6):552-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2014 {published data only}
- Fan YX, Liu FF, Jia M, Yang JJ, Shen JC, Zhu GM, et al.Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total hip replacement: a preliminary study. Archives of Gerontology and Geriatrics 2014;59(1):181-5. [DOI] [PubMed] [Google Scholar]
Foss 2009 {published data only}
- Foss NB, Kristensen MT, Jensen PS, Palm H, Krasheninnikoff M, Kehlet H.The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion 2009;49(2):227-34. [DOI] [PubMed] [Google Scholar]
Gillies 2020 {published data only}
- Gillies MA, Ghaffar S, Moppett IK, Docherty AB, Clarke S, Rea N, et al.A restrictive versus liberal transfusion strategy to prevent myocardial injury in patients undergoing surgery for fractured neck of femur: a feasibility randomised trial (RESULT-NOF). British Journal of Anaesthesia 2020;126:77-86. [DOI] [PubMed] [Google Scholar]
Gobatto 2019 {published data only}
- Gobatto AL, Link MA, Solla DJ, Bassi E, Tierno PF, Paiva W, et al.Transfusion requirements after head trauma: a randomized feasibility controlled trial. Critical Care 2019;23(1):89. [CTG: NCT02203292 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregersen 2015 {published data only}
- Blandfort S, Gregersen M, Borris LC, Damsgaard EM.Blood transfusion strategy and risk of postoperative delirium in nursing homes residents with hip fracture. A post hoc analysis based on the TRIFE randomized controlled trial. Aging Clinical and Experimental Research 2017;29(3):459-66. [DOI] [PubMed] [Google Scholar]
- Gregersen M, Borris LC, Damsgaard EM.Blood transfusion and overall quality of life after hip fracture in frail elderly patients - the transfusion requirements in frail elderly randomized controlled trial. Journal of the American Medical Directors Association 2015;16(9):762-6. [DOI] [PubMed] [Google Scholar]
- Gregersen M, Borris LC, Damsgaard EM.Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture: the TRIFE randomized controlled trial. Acta Orthopaedica 2015;86(3):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen M, Damsgaard EM, Borris LC.Blood transfusion and risk of infection in frail elderly after hip fracture surgery: the TRIFE randomized controlled trial. European Journal of Orthopaedic Surgery & Traumatology 2015;25(6):1031-8. [DOI] [PubMed] [Google Scholar]
Grover 2006 {published data only}
- Grover M, Talwalkar S, Casbard A, Boralessa H, Contreras M, Boralessa H, et al.Silent myocardial ischaemia and haemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sanguinis 2006;90(2):105-12. [DOI] [PubMed] [Google Scholar]
- Grover M, Talwalker SA, Contreras, Soni N.Blood transfusion threshold and silent myocardial ischemia after lower limb arthroplasty: a randomized controlled trial of transfusion strategy. Transfusion Alternatives in Transfusion Medicine 2005;7(1 (Suppl)):62-3. [Google Scholar]
Hajjar 2010 {published data only}
- Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al.Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304(14):1559-67. [DOI] [PubMed] [Google Scholar]
Hébert 1995 {published data only}
- Hébert PC, Wells G, Marshall J, Martin C, Tweeddale M, Pagliarello G, et al.Transfusion requirements in critical care. A pilot study. Canadian Critical Care Trials Group [published erratum appears in JAMA 1995 Sep 27;274(12):944]. JAMA 1995;273(18):1439-44. [DOI] [PubMed] [Google Scholar]
Hébert 1999 {published data only}
- Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al.A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. New England Journal of Medicine 1999;340(6):409-17. [DOI] [PubMed] [Google Scholar]
- McIntyre L, Hébert PC, Wells G, Fergusson D, Marshall J, Yetisir E, et al, Canadian Critical Care Trials Group.Is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients? Journal of Trauma 2004;57(3):563-8. [DOI] [PubMed] [Google Scholar]
Hoff 2011 {published data only}
- Hoff CM, Hansen HS, Overgaard M, Grau C, Johansen J, Bentzen O, et al.The importance of haemoglobin level and effect of transfusion in HNSCC patients treated with radiotherapy - results from the randomized DAHANCA 5 study. Radiotherapy and Oncology 2011;1998:28-33. [DOI: 10.1016/j.radonc.2010.09.024] [DOI] [PubMed] [Google Scholar]
- Hoff CM, Lassen P, Eriksen JG, Hansen HS, Specht L, Overgaard M, et al.Does transfusion improve the outcome for HNSCC patients treated with radiotherapy? Results from the randomized DAHANCA 5 and 7 trials. Acta Oncologica 2011;50(7):1006-14. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al.A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma: results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiotherapy and Oncology 1998;46:135-46. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Anderson E, et al.Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 & 7 randomised controlled trial. Lancet 2003;362:933-40. [DOI] [PubMed] [Google Scholar]
Holst 2014 {published and unpublished data}
- Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al.Lower versus higher hemoglobin threshold for transfusion in septic shock. New England Journal of Medicine 2014;371(15):1381-91. [DOI] [PubMed] [Google Scholar]
- Rygård SL, Holst LB, Wetterslev J, Winkel P, Johansson PI, Wernerman J, et al, TRISS Trial Group, Scandinavian Critical Care Trials Group.Long-term outcomes in patients with septic shock transfused at a lower versus a higher haemoglobin threshold: the TRISS randomised, multicentre clinical trial. Intensive Care Medicine 2016;42(11):1685-94. [DOI] [PubMed] [Google Scholar]
Jairath 2015 {published data only}ISRCTN85757829
- Campbell HE, Stokes EA, Bargo D, Logan RF, Mora A, Hodge R, et al.Costs and quality of life associated with acute upper gastrointestinal bleeding in the UK: cohort analysis of patients in a cluster randomised trial. BMJ Open 2015;5(4):e007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, et al.Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet 2015;386(9989):137-44. [ISRCTN: ISRCTN85757829] [DOI] [PubMed] [Google Scholar]
Jansen 2020 {published data only}
- Jansen AJ, den Bosch J.Results of the prematurely terminated TEMPLE randomized controlled trial in patients with myelodysplastic syndrome: liberal versus restrictive red blood cell transfusion threshold [Letter to the Editor]. Transfusion 2020;60(4):879-81. [ISRCTN: ISRCTN43616311] [DOI] [PubMed] [Google Scholar]
Johnson 1992 {published data only}
- Johnson RG, Thurer RL, Kruskall MS, Sirois C, Gervino EV, Critchlow J, et al.Comparison of two transfusion strategies after elective operations for myocardial revascularization. Journal of Thoracic and Cardiovascular Surgery 1992;104(2):307-14. [PubMed] [Google Scholar]
Koch 2017 {published data only}
- Koch CG, Sessler DI, Mascha EJ, Sabik JF 3rd, Li L, Duncan AI, et al.A randomized clinical trial of red blood cell transfusion triggers in cardiac surgery. Annals of Thoracic Surgery 2017;104(4):1243-50. [DOI] [PubMed] [Google Scholar]
Kola 2020 {published data only}JIP/IEC/2015/615
- Kola G, Sureshkumar S, Mohsina S, Sreenath GS, Kate V.Restrictive versus liberal transfusion strategy in upper gastrointestinal bleeding: a randomized controlled trial. Saudi Journal of Gastroenterology 2020;27(1):13-9. [DOI: 10.4103/sjg.SJG_152_20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacroix 2007 {published data only}
- Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, et al.Transfusion strategies for patients in pediatric intensive care units. New England Journal of Medicine 2007;356(16):1609-19. [DOI] [PubMed] [Google Scholar]
Laine 2018 {published data only}
- Laine, A, Niemi, T, Schramko Al.Transfusion threshold of hemoglobin 80g/L is comparable to 100g/L in terms of bleeding in cardiac surgery: a prospective randomized study. Journal of Cardiothoracic and Vascular Anesthesia 2018;32(1):131-9. [DOI] [PubMed] [Google Scholar]
Lotke 1999 {published data only}
- Lotke PA, Barth P, Garino JP, Cook EF.Predonated autologous blood transfusions after total knee arthroplasty: immediate versus delayed administration. Journal of Arthroplasty 1999;14(6):647-50. [DOI] [PubMed] [Google Scholar]
Mazer 2017 {published data only}
- Galan J, Mateo E, Carmona P, Gajate L, Mazer CD, Martinez-Zapata MJ.Restrictive or liberal transfusion for cardiac surgery: Spanish TRICCS results of a randomized multicenter international parallel open-label clinical trial [Transfusión Restrictiva o liberal en cirugía cardiaca: Resultados españoles de un ensayo clínico aleatorizado, multicéntrico, internacional, paralelo y abierto]. Medicina Intensiva (English edition) 2020;In press/epub: no volume:Epub: no pagination. [DOI: 10.1016/j.medin.2020.07.011] [DOI] [Google Scholar]
- Garg AX, Badner N, Bagshaw SM, Cuerden MS, Fergusson DA, Gregory AJ, et al, TRICS Investigators and Perioperative Anesthesia Clinical Trials Group.Safety of a restrictive versus liberal approach to red blood cell transfusion on the outcome of AKI in patients undergoing cardiac surgery: a randomized clinical trial. Journal of the American Society of Nephrology: JASN 2019;30(7):1294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazer CD, Whitlock RP, Fergusson DA, Belley-Cote E, Connolly K, Khanykin B, et al, TRICS Investigators and Perioperative Anesthesia Clinical Trials Group.Restrictive or liberal red-cell transfusion for cardiac surgery. New England Journal of Medicine 2017;377(22):2133-44. [DOI] [PubMed] [Google Scholar]
- Mazer CD, Whitlock RP, Fergusson DA, Belley-Cote E, Connolly K, Khanykin B, TRICS Investigators and Perioperative Anesthesia Clinical Trials Group.Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. New England Journal of Medicine 2018;379(13):1224-33. [DOI] [PubMed] [Google Scholar]
Murphy 2015 {published data only}70923932
- Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al.Liberal or restrictive transfusion after cardiac surgery. New England Journal of Medicine 2015;372:997-1008. [DOI] [PubMed] [Google Scholar]
- Stokes EA, Wordsworth S, Bargo D, Pike K, Rogers CA, Brierley RC, et al.Are lower levels of red blood cell transfusion more cost-effective than liberal levels after cardiac surgery? Findings from the TITRe2 randomised controlled trial. BMJ Open 2016;6(8):e011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Møller 2019 {published data only}
- Møller A, Nielsen HB, Wetterslev J, Pedersen OB, Hellemann D, Winkel P, et al.Low vs high hemoglobin trigger for transfusion in vascular surgery: a randomized clinical feasibility trial. Blood 2019;133(25):2639-50. [DOI] [PubMed] [Google Scholar]
- Møller A, Shahidi S, Wetterslev J, Helleman D, Pedersen OB, Marcussen KV, et al.Cardiac output in the transfusion triggers in vascular surgery trial. Acta Anaesthesiologica Scandinavica 2019;63:e12. [Google Scholar]
Nielsen 2014 {published data only}
- Nielsen K, Johansson PI, Dahl B, Wagner M, Frausing B, Børglum J, et al.Perioperative transfusion threshold and ambulation after hip revision surgery - a randomized trial. BMC Anesthesiology 2014;14:89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmieri 2017 {published data only}
- Palmieri TL, Holmes JH, Arnoldo B, Peck M, Cochran A, King BT, et al.Restrictive transfusion strategy is more effective in massive burns: results of the TRIBE multicenter prospective randomized trial. Military Medicine 2019;184 (Suppl 1):11-5. [DOI: doi: 10.1093/milmed/usy279] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri TL, Holmes JH, Arnoldo B, Peck M, Potenza B, Cochran A, et al.Transfusion requirement in burn care evaluation (TRIBE). A multicenter randomized prospective trial of blood transfusion in major burn injury. Annals of Surgery 2017;266:595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2013 {published data only}61328173
- Parker MJ.Randomised trial of blood transfusion versus a restrictive transfusion policy after hip fracture surgery. Injury 2013;44(12):1916-8. [DOI] [PubMed] [Google Scholar]
Prick 2014 {published data only}
- Prick BW, Duvekot JJ, Moer PE, Gemund N, Salm PC, Jansen AJ, et al.Cost-effectiveness of red blood cell transfusion vs. non-intervention in women with acute anaemia after postpartum haemorrhage. Vox Sanguinis 2014;107(4):381-8. [DOI: 10.1111/vox.12181] [DOI] [PubMed] [Google Scholar]
- Prick BW, Jansen AJ, Steegers EA, Hop WC, Essink-Bot ML, Uyl-de Groot CA, et al.Transfusion policy after severe postpartum haemorrhage: a randomised non-inferiority trial. BJOG 2014;121(8):1005-14. [DOI] [PubMed] [Google Scholar]
Robitaille 2013 {published data only}
- Robitaille N, Lacroix J, Alexandrov L, Clayton L, Cortier M, Schultz KR, et al.Excess of veno-occlusive disease in a randomized trial on a higher trigger for red cell transfusion after bone marrow transplantation: a Canadian blood and marrow transplant group trial. Biology of Blood and Marrow Transplantation 2013;19:468-73. [DOI] [PubMed] [Google Scholar]
Shehata 2012 {published data only}
- Shehata N, Burns LA, Nathan H, Hebert P, Hare GM, Fergusson D, et al.A randomized controlled pilot study of adherence to transfusion strategies in cardiac surgery. Transfusion 2012;52(1):91-9. [DOI] [PubMed] [Google Scholar]
So‐Osman 2013 {published data only}
- So-Osman C, Nelissen R, Brand R, Faber F, Slaa RT, Stiggelbout A, et al.The impact of a restrictive transfusion trigger on post-operative complication rate and well-being following elective orthopaedic surgery: a post-hoc analysis of a randomised study. Blood Transfusion (Trasfusione del sangue) 2013;11(2):289-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So-Osman C, Nelissen R, Te Slaa R, Coene L, Brand R, Brand A.A randomized comparison of transfusion triggers in elective orthopaedic surgery using leucocyte-depleted red blood cells. Vox Sanguinis 2010;98(1):56-64. [DOI] [PubMed] [Google Scholar]
- So-Osman C.A restrictive transfusion trigger is a method for blood saving in elective orthopaedic surgery. Vox Sanguinis 2004;87(Suppl 3):52. [Google Scholar]
Stanworth 2020 {published data only}26088319
- Stanworth SJ, Killick S, McQuilten ZK, Karakantza M, Weinkove R, Smethurst H, et al, REDDS Investigators.Red cell transfusion in outpatients with myelodysplastic syndromes: a feasibility and exploratory randomised trial. British Journal of Haematology 2020;189(2):279-90. [ISRCTN26088319] [DOI] [PubMed] [Google Scholar]
Tay 2020 {published data only}
- Tay J, Allan DS, Chatelain E, Coyle D, Elemary M, Fulford A, et al.Liberal versus restrictive red blood cell transfusion thresholds in hematopoietic cell transplantation: a randomized, open label, phase III, noninferiority trial. Journal of Clinical Oncology 2020;38(13):1463-73. [DOI] [PubMed] [Google Scholar]
Topley 1956 {published data only}
- Fisher MR, Topley ET.The illness of trauma. British Journal of Clinical Practice 1956;10(11):770-6. [PubMed] [Google Scholar]
Villanueva 2013 {published and unpublished data}
- Colomo A, Hernandez-Gea V, Muniz-Diaz E, Madoz P, Aracil C, Alvarez-Urturi C.Transfusion strategies in patients with cirrhosis and acute gastrointestinal bleeding. Hepatology 2008;48(4 Suppl):413A. [Google Scholar]
- Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al.Transfusion strategies for acute upper gastrointestinal bleeding. New England Journal of Medicine 2013;368(1):11-21. [DOI] [PubMed] [Google Scholar]
Walsh 2013 {published data only}
- Walsh TS, Boyd JA, Watson D, Hope D, Lewis S, Krishan A, et al.Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Critical Care Medicine 2013;41(10):2354-63. [DOI] [PubMed] [Google Scholar]
Webert 2008 {published and unpublished data}
- Webert KE, Cook RJ, Couban S, Carruthers J, Lee KA, Blajchman MA, et al.A multicenter pilot-randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukemia or stem cell transplantation. Transfusion 2008;48(1):81-91. [DOI] [PubMed] [Google Scholar]
Yakymenko 2018 {published data only}
- Yakymenko D, Frandsen KB, Christensen IJ, Norgaard A, Johansson PI, Daugaard G, et al.Randomised feasibility study of a more liberal haemoglobin trigger for red blood cell transfusion compared to standard practice in anaemic cancer patients treated with chemotherapy. Transfusion Medicine 2018;28(3):208-15. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atilla 2017 {published data only}
- Atilla E, Toprak SK, Civriz Bozdag S, Topcuoglu P, Arslan O.A randomized comparison of hemoglobin content-based versus standard (unit-based) red blood cell transfusion policy. Turkish Journal of Haematology 2017;34(244):244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baron‐Stefaniak 2019 {published data only}
- Baron-Stefaniak J, Leitner GC, Kuntzel NKI, Meyer EL, Hiesmayr MJ, Ullrich R, et al.Transfusion of standard-issue packed red blood cells induces pulmonary vasoconstriction in critically ill patients after cardiac surgery - a randomized,double-blinded, clinical trial. PLOS One 2019;14(3):e0213000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cholette 2017 {published data only}
- Cholette JM, Swartz MF, Rubenstein J, Henrichs KF, Wang H, Powers KS.Outcomes using a conservative versus liberal red blood cell transfusion strategy in infants requiring cardiac operation. Annals of Thoracic Surgery 2017;103(1):206-14. [DOI] [PubMed] [Google Scholar]
de Bruin 2019 {published data only}
- Bruin S, Scheeren TW, Bakker J, Bruggen R, Vlaar AP, Cardiovascular Dynamics Section and Transfusion Guideline Task Force of theESICM.Cardiovascular Dynamics Section and Transfusion Guideline Task Force of the ESICM. Transfusion practice in the non-bleeding critically ill: an international online survey - the TRACE survey. Critical Care 2019;23:309. [DOI: 10.1186/s13054-019-2591-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elshinawy 2020 {published data only}
- Elshinawy M, Al Marhoobi N, Al Abri R, Nazir HF, Khater D, Maktoom M, et al.Preoperative transfusion versus no transfusion policy in sickle cell disease patients: a randomized trial. Transfusion 2020;60(Suppl 1):S22-7. [DOI] [PubMed] [Google Scholar]
Fogagnolo 2020 {published data only}
- Fogagnolo A, Taccone FS, Vincent JL, Benetto G, Cavalcante E, Marangoni E, et al.Using arterial-venous oxygen difference to guide red blood cell transfusion strategy. Critical Care 2020;24:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortune 1987 {published data only}
- Fortune JB, Feustel PJ, Saifi J, Stratton HH, Newell JC, Shah DM.Influence of hematocrit on cardiopulmonary function after acute hemorrhage. Journal of Trauma 1987;27(3):243-9. [DOI] [PubMed] [Google Scholar]
Franz 2020 {published data only}
- Franz AR, Engel C, Bassler D, Rüdiger M, Thome UH, Maier RF, et al, the ETTNO Investigators.Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO randomized clinical trial. JAMA 2020;324:560-70. [CTG: ] [EUCTR: EUCTR2010-021576-28-DE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haensig 2019 {published data only}
- Haensig M, Kempfert J, Kempfert PM, Girdauskas E, Borger MA, Lehmann S.Thrombelastometry guided blood-component therapy after cardiac surgery: a randomized study. BMC Anesthesiology 2019;19(1):201. [DOI: 10.1186/s12871-019-0875-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamm 2020 {published data only}
- Hamm RF, Perelman S, Wang EY, Levine LD, Srinivas SK.Single-unit vs multiple-unit transfusion in hemodynamically stable postpartum anemia: a pragmatic randomized controlled trial. American Journal of Obstetrics & Gynecology 2021;224(84):e1-7.0002-9378/. [CTG: ] [DOI] [PubMed] [Google Scholar]
Jain 2019 {published data only}
- Jain A, Raja A, Marwaha N, Trehan A.Comparison of efficacy of packed red blood cell transfusion based on its hemoglobin content versus the routine transfusion practice in thalassemia major patients. Vox Sanguinis 2019;114:213. [DOI] [PubMed] [Google Scholar]
- Raja A, Jain A, Marwaha N, Trehan A.Comparison of efficacy of packed red blood cell transfusion based on its hemoglobin content versus the standard transfusion practice in thalassemia major patients (HEMOCON study). Transfusion and Apheresis Science 2020;59:6 (Article ID: 102736). [DOI: ] [DOI] [PubMed] [Google Scholar]
Kirpalani 2020 {published data only}
- Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al .Higher or lower hemoglobin transfusion thresholds for preterm infants . New England Journal of Medicine 2020;383(27):2639-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koster 2016 {published data only}
- Koster A, Zittermann A, Borgermann J, Knabbe C, Diekmann J, Schirmer U, et al.Transfusion of 1 and 2 units of red blood cells does not increase mortality and organ failure in patients undergoing isolated coronary artery bypass grafting. European Journal of Cardio-thoracic Surgery 2016;49:931-6. [DOI] [PubMed] [Google Scholar]
Kumar 2019 {published data only}
- Kumar M, Ahmad J, Maiwall R, Choudhury A, Bajpai M, Mitra LG, et al.Thromboelastography-guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology 2019;71(1):235-46. [DOI] [PubMed] [Google Scholar]
Leal‐Noval 2017 {published data only}
- Leal-Noval SR, Arellano-Orden V, Maestre-Romero A, Muñoz-Gómez M, Fernández-Cisneros V, Ferrándiz-Millón C, et al.Red blood cell transfusion guided by near nfrared spectroscopy in neurocritically ill patients with moderate or severe anemia: a randomized, controlled trial. Journal of Neurotrauma 2017;34(17):2553-9. [DOI] [PubMed] [Google Scholar]
Osawa 2016 {published data only}
- Osawa EA, Rhodes A, Landoni G, Galas FR, Fukushima JT, Park CH, et al.Effect of perioperative goal-directed hemodynamic resuscitation therapy on outcomes following cardiac surgery: a randomized clinical trial and systematic review. Critical Care Medicine 2016;44(4):724-33. [DOI: 10.1097/CCM.0000000000001479] [DOI] [PubMed] [Google Scholar]
Robertson 2014 {published data only}
- Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al.Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA 2014;312(1):36-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vichinsky 1995 {published data only}
- Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, et al.A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. New England Journal of Medicine 1995;333(4):206-13. [DOI] [PubMed] [Google Scholar]
Voorn 2017 {published data only}
- Voorn VM, Marang-van de Mheen PJ, Hout A, Hofstede SN, So-Osman C, den Akker-van Marle ME, et al.The effectiveness of a de-implementation strategy to reduce low-value blood management techniques in primary hip and knee arthroplasty: a pragmatic cluster-randomized controlled trial. Implementation Science 2017;12(1):72. [DOI: 10.1186/s13012-017-0601-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamada 2020 {published data only}
- Yamada C, Takeshita A, Ohto H, Ishimaru K, Kawabata K, Nomaguchi Y, et al.A Japanese multi-institutional collaborative study of antigen-positive red blood cell (RBC) transfusions in patients with corresponding RBC antibodies. Vox Sanguinis 2020;115(5):456-65. [DOI] [PubMed] [Google Scholar]
Zygun 2009 {published data only}
- Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK.The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Critical Care Medicine 2009;37(3):1074-8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
IRCT20190209042660N1 {unpublished data only}20190209042660N1
- IRCT20190209042660N1.A clinical trial on comparing morbidity and mortality of restrictive blood transfusion strategy with a traditional strategy in patients with a thermal burn. Iranian Registry of Clinical Trials - Beta version. https://en.irct.ir/trial/43402 (first posted 15 June 2020).
ISRCTN26088319 {unpublished data only}26088319
- ISRCTN26088319.Red cell transfusion and QoL in myelodysplastic syndromes [Red blood cell transfusion thresholds and quality of life in myelodysplastic syndromes: a pilot, feasibility study]. www.isrctn.com/ISRCTN26088319 (first received 13 November 2014).
Maitland 2019 {published data only}
- Maitland K, Kiguli S, Olupot-Olupot, Engoru C, Mallewa M, Saramago Goncalves P, et al, the TRACT Group.Immediate transfusion in African children with uncomplicated severe anaemia. New England Journal of Medicine 2019;381(5):407-19. [DOI: 10.1056/NEJMoa1900105] [ISRCTN: ISRCTN84086586] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland K, Polupot-Olupot P, Kiguli S, Chagaluka G, Alaroker F, Opoka RO, et al, TRACT Group.Transfusion volume for children with severe anemia in Africa. New England Journal of Medicine 2019;381(5):420-31. [DOI: 10.1056/NEJMoa1900100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morton 2020 {published data only}
- Morton S, Sekhar M, Smethurst H, Mora S, Hodge R, Hudson C, et al.Results for the REAL pilot randomised trial of red cell transfusion in acute myeloid leukaemia: is there sufficient evidence of equipoise to support a definitive study? British Journal of Haematology 2020;189 (Suppl 1):36-7. [ISRCTN: ISRCTN96390716 ] [Google Scholar]
NCT02099669 {unpublished data only}
- Buckstein RJ, Prica A, Leber B, Heddle N, Yee KW, Stanworth SJ, et al.RBC-Enhance: a randomized pilot feasibility trial of red cell transfusion thresholds in myelodysplastic syndromes. Blood 2020;136(Suppl 1):3-4. [CTG: ] [Google Scholar]
- NCT02099669.Red blood cell transfusion thresholds and QOL in MDS (EnhanceRBC): a pilot, feasibility study. www.clinicaltrials.gov/ct2/show/NCT02099669 2014 (last accessed 6/7/21). [CTG: ]
References to ongoing studies
ACTRN12619001053112 {published data only}
- ACTRN12619001053112.Red blood cell transfusion schedule in myelodysplastic syndromes: study 2 (REDDS-2) [A randomised feasibility n-of-1 trial of weekly-interval red cell transfusion myelodysplastic syndromes]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=377401&isReview=true (first received 4 June 2019). [ACTRN: ACTRN12619001053112]
Hayakawa 2020 {published data only}
- Hayakawa M, Tagami T, IIjima H, Kudo D, Sekine K, Ogura T, et al.Restrictive transfusion strategy for critically injured patients (RESTRIC) trial: a study protocol for a cluster-randomised, crossover non-inferiority trial. BMJ Open 2020 2020;10(9):e037238. [UMIN000034405] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN17438123 {unpublished data only}
- ISRCTN17438123.A small pilot feasibility study for a possible randomised control trial comparing clinical outcomes and quality of life following two different transfusion strategies in children undergoing allogeneic hematopoietic stem cell transplant (HSCT) [RePAST: Red cell transfusion in Paediatric Allogeneic HSCT - A feasibility randomised controlled trial comparing restrictive versus liberal red cell (RBC) transfusion strategies in children undergoing allogeneic haematopoietic stem cell transplant (HSCT)]. www.isrctn.com/ISRCTN17438123 (first posted 23 May 2019). [ISRCTN: ISRCTN17438123]
Meybohm 2019 {published data only}
- Meybohm P, Lindau S, Treskatsch S, Francis R, Spies C, Velten M, et al, LIBERAL Collaboration Group.Liberal transfusion strategy to prevent mortality and anaemia-associated, ischaemic events in elderly non-cardiac surgical patients - The study design of the LIBERAL-Trial. Trials 2019;20(1):101. [CTG: ] [DOI: 10.1186/s13063-019-3200-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02981407 {published data only}
- NCT02619136.Myocardial ischemia and transfusion (MINT) [Myocardial ischemia and transfusion: a pilot, multi-centre, open-label randomized controlled trial of two commonly used transfusion strategies in patients with myocardial infarction]. www.clinicaltrials.gov/ct2/show/NCT02619136 (first posted 2 December 2015). [CTG: ]
- NCT02981407.Myocardial Ischemia and Transfusion (MINT). clinicaltrials.gov/ct2/show/NCT02981407 (first posted 5 December 2016). [CTG: ]
NCT03229941 {published data only}
- NCT03229941.Transfusion trigger after operations in high cardiac risk patients (TOP) [CSP #599 - Transfusion trigger after operations in high cardiac risk patients (TOP)]. clinicaltrials.gov/ct2/show/NCT03229941 (first posted 26 July 2017). [CTG: ]
NCT03260478 {published data only}
- NCT03260478.HEMOglobin Transfusion Threshold in Traumatic Brain Injury OptimizatioN: The HEMOTION Trial (HEMOTION). clinicaltrials.gov/ct2/show/NCT03260478 (first posted 24 August 2017).
NCT03309579 {published data only}
- English SW, Fergusson D, Chassé M, Turgeon AF, Lauzier F, Griesdale D, et al, Canadian Critical Care Trials Group.Aneurysmal SubArachnoid Hemorrhage - Red Blood Cell Transfusion And Outcome (SAHaRA): a pilot randomised controlled trial protocol. BMJ Open 2016;6(12):e012623. [CTG: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02483351.Aneurysmal subarachnoid hemorrhage: red blood cell transfusion and outcome (SAHaRA Pilot) [Aneurysmal subarachnoid hemorrhage: red blood cell transfusion and outcome - a pilot randomized controlled trial]. www.clinicaltrials.gov/ct2/show/NCT02483351 (first received 26 June 2015). [CTG: ]
- NCT3309579.SAHaRA: a randomized controlled trial [Aneurysmal subarachnoid hemorrhage - red blood cell transfusion and outcome (SAHaRA): a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03309579 (first received 13 October 2017). [CTG: ]
NCT03871244 {published data only}
- NCT03871244.Pilot Optimizing Transfusion Thresholds in Critically-ill Children with Anaemia (P-OpTTICCA) [Pilot Optimizing Transfusion Thresholds in Critically-ill Children with Anaemia (P-OpTTICCA): a pilot trial for a large pragmatic international parallel open-label non-inferiority randomised controlled trial]. clinicaltrials.gov/ct2/show/NCT03871244 (first posted 12 March 2019). [CTG: ]
NCT04506125 {published data only}
- NCT04506125.Liberal versus restrictive transfusion threshold in oncologic surgery (LIBERTY1) [Liberal versus restrictive transfusion threshold in oncologic surgery: a multicenter, randomized, controlled, pilot study]. clinicaltrials.gov/ct2/show/NCT04506125 (first posted 10 August 2020). [CTG: ]
NCT04591574 {published data only}
- NCT04591574.ABC - a post intensive care anaemia management trial [Anaemia management with red blood cell transfusion to improve post-intensive care disability: a randomised controlled trial (the ABC post-intensive care trial)]. https://clinicaltrials.gov/ct2/show/NCT04591574 (first accepted 19 October 2020).
NCT04754022 {published data only}
- NCT04754022.Transfusion requirements in younger patients undergoing cardiac surgery (TRICS-IV) [An international, multi-centre, randomized controlled trial to assess transfusion thresholds in younger patients undergoing cardiac surgery]. www.clinicaltrials.gov/ct2/show/NCT04754022 (first posted 15 February 2021). [CTG: ]
NIHR 130875 {published data only}
- The impact of REstrictive versus LIberal Transfusion strategy on cardiac injury and death in patients undergoing surgery for Hip Fracture (RESULT-Hip). Ongoing study.2022. Contact author for more information.
NL3090 NTR3244 {unpublished data only}https://www.trialregister.nl/trial/3090
- NL3090 NTR3244.Blood transfusion study in patients at risk for cardiac complications after non-cardiac surgery [Perioperative Transfusion Study (PETS): does a liberal transfusion protocol improve outcome in high-risk cardiovascular patients undergoing non-cardiac surgery?]. www.trialregister.nl/trial/3090 (first posted 17 January 2012).
Additional references
AAGBI 2008
- Association of Anaesthetists of Great Britain and Ireland.Blood transfusion and the anaesthetist - red cell transfusion. www.aagbi.org/publications/guidelines/docs/red_cell_08.pdf 2008 (accessed 11 October 2016).
ASA 2006
- American Society of Anesthesiologists (ASA) Task Force on Perioperative Blood Transfusion and Adjuvant Therapies.Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology 2006;105(1):198-208. [DOI] [PubMed] [Google Scholar]
Baek 2019
- Baek JH, Buehler PW.Can molecular markers of oxygen homeostasis and the measurement of tissue oxygen be leveraged to optimize red blood cell transfusions? Current Opinion in Hematology 2019;26(6):453-60. [DOI: 10.1097/MOH.0000000000000533] [DOI] [PubMed] [Google Scholar]
BCTMAG 2003
- British Columbia Transfusion Medicine Advisory Group (BCTMAG).Guidelines for red blood cell transfusion. British Columbia Transfusion Medicine Advisory Group 2003;November:1-3.
Benson 2000
- Benson K, Hartz AJ.A comparison of observational studies and randomized, controlled trials. New England Journal of Medicine 2000;342(25):1878-86. [DOI] [PubMed] [Google Scholar]
Butcher 2017
- Butcher A, Richards T, Stanworth SJ, Klein AA.Diagnostic criteria for pre-operative anaemia - time to end sex discrimination. Anaesthesia 2017;72(7):811-4. [DOI] [PubMed] [Google Scholar]
Campbell 2015
- Campbell HE, Stokes EA, Bargo D, Logan RF, Mora A, Hodge R, et al.Costs and quality of life associated with acute upper gastrointestinal bleeding in the UK: cohort analysis of patients in a cluster randomised trial. BMJ Open 2015;5(4):e007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carless 2010a
- Carless PA, Henry DA, Moxey AJ, O'Connell D, Brown T, Fergusson DA.Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No: CD001888. [DOI: 10.1002/14651858.CD001888.pub4] [DOI] [PubMed] [Google Scholar]
Carson 1996
- Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, et al.Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet 1996;348(9034):1055-60. [DOI] [PubMed] [Google Scholar]
Carson 2012a
- Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al, Clinical Transfusion Medicine Committee of theAABB.Red blood cell transfusion: a clinical practice guideline from the AABB. Annals of Internal Medicine 2012;157(1):49-58. [DOI] [PubMed] [Google Scholar]
Carson 2015
- Carson JL, Sieber F, Cook DR, Hoover DR, Noveck H, Chaitman BR, et al.Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet 2015;385(9974):1183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2016a
- Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al.Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016;316:2025-35. [DOI] [PubMed] [Google Scholar]
Carson 2018
- Carson JL, Stanworth SJ, Alexander JH, Roubinian N, Fergusson DA, Triulzi DJ, et al.Clinical trials evaluating red blood cell transfusion thresholds: an updated systematic review and with additional focus on patients with cardiovascular disease. American Heart Journal 2018;200:96-101. [DOI] [PubMed] [Google Scholar]
Chatterjee 2013
- Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D.Association of blood transfusion with increased mortality in myocardial infarction: a meta-analysis and diversity-adjusted study sequential analysis. JAMA Internal Medicine 2013;173(2):132-9. [DOI] [PubMed] [Google Scholar]
Concato 2000
- Concato J, Shah N, Horwitz RI.Randomized, controlled trials, observational studies, and the hierarchy of research designs. New England Journal of Medicine 2000;342(25):1887-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delaney 2016
- Delaney M, Wendel S, Bercovitz RS, Cid J, Cohn C, Dunbar NM, et al, Biomedical Excellence for Safer Transfusion (BEST) Collaborative.Transfusion reactions: prevention, diagnosis, and treatment. Lancet 2016;388(10061):2825-36. [DOI: 10.1016/S0140-6736(15)01313-6] [DOI] [PubMed] [Google Scholar]
Der Simonian 1986
- Der Simonian R, Laird N.Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Docherty 2016
- Docherty AB, O'Donnell R, Brunskill S, Trivella M, Doree C, Holst L, et al.Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non-cardiac surgery setting: systematic review and meta-analysis. BMJ 2016;352:i1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Docherty 2018
- Docherty AB, Alam S, Shah AS, Moss A, Newby DE, Mills NL, et al, TROPICCAL Investigators.Unrecognised myocardial infarction and its relationship to outcome in critically ill patients with cardiovascular disease. Intensive Care Medicine 2018;44(12):2059-69. [DOI] [PubMed] [Google Scholar]
Fominskiy 2015
- Fominskiy E, Putzu A, Monaco F, Scandroglio AM, Karaskov A, Galas FR, et al.Liberal transfusion strategy improves survival in perioperative but not in critically ill patients. A meta-analysis of randomised trials. British Journal of Anaesthesia 2015;115(4):511-9. [DOI] [PubMed] [Google Scholar]
Hébert 1997
- Hébert PC, Wells G, Tweeddale M, Martin C, Marshall J, Pham B, et al.Does transfusion practice affect mortality in critically ill patients? Transfusion Requirements in Critical Care (TRICC) Investigators and the Canadian Critical Care Trials Group. American Journal of Respiratory and Critical Care Medicine 1997;155(5):1618-23. [DOI] [PubMed] [Google Scholar]
Henry 2011
- Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, et al.Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD001886. [DOI: 10.1002/14651858.CD001886.pub4] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JT, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hoff 2011a
- Hoff CM, Lassen P, Eriksen JG, Hansen HS, Specht L, Overgaard M, et al.Does transfusion improve the outcome for HNSCC patients treated with radiotherapy? - results from the randomized DAHANCA 5 and 7 trials. Acta Oncologica 2011;50(7):1006-14. [DOI] [PubMed] [Google Scholar]
Hoff 2011b
- Hoff CM, Hansen HS, Overgaard M, Grau C, Johansen J, Bentzen O, et al.The importance of haemoglobin level and effect of transfusion in HNSCC patients treated with radiotherapy - results from the randomized DAHANCA 5 study. Radiotherapy and Oncology 2011;1998:28-33. [DOI: 10.1016/j.radonc.2010.09.024] [DOI] [PubMed] [Google Scholar]
Holst 2015
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J.Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 2015;350:h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunz 2020
- Kunz JV, Spies CD, Bichmann A, Sieg M, Mueller A.Postoperative anaemia might be a risk factor for postoperative delirium and prolonged hospital stay: a secondary analysis of a prospective cohort study. PLOS ONE 2020;15(2):e0229325. https://doi.org/10.1371/journal.pone.0229325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwan 2020
- Kwan JL, Lo L, Ferguson J, Goldberg H, Diaz-Martinez JP, Tomlinson G, et al.Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. BMJ 2020;370:m3216:1-10. [DOI: 10.1136/bmj.m3216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J.Chapter 6. Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Marik 2008
- Marik PE, Corwin HL.Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Critical Care Medicine 2008;36(9):2667-74. [DOI] [PubMed] [Google Scholar]
Mazer 2018
- Mazer CD, Whitlock RP, Fergusson DA, Belley-Cote E, Connolly K, Khanykin B, et al, TRICS Investigators and Perioperative Anesthesia Clinical Trials Group.Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. New England Journal of Medicine 2018;379(13):1224-33. [DOI] [PubMed] [Google Scholar]
Meybohm 2020
- Meybohm P, Straub N, Füllenbach C, Judd L, Kleinerüschkamp A, Taeuber I, et al.Health economics of Patient Blood Management: a cost-benefit analysis based on a meta-analysis. Vox Sanguinis 2020;115(2):182-8. [DOI: 10.1186/s13063-019-3200-3] [DOI] [PubMed] [Google Scholar]
Mueller 2019
- Mueller MM, Van Remoortel H, Meybohm P, Aranko K, Aubron C, Burger R, et al, Group LCC Pbm Frankfurt.Patient blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA 2019;321(10):983-97. [DOI] [PubMed] [Google Scholar]
Napolitano 2009
- Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, et al.Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Critical Care Medicine 2009;37(12):3124-57. [DOI] [PubMed] [Google Scholar]
NBUGI 2001
- National Blood Users Group Ireland (NBUGI).A guideline for transfusion of red blood cells in surgical patients. www.dohc.ie/publications/transfusion_of_red_blood_cells.html (accessed 11 October 2016).
New 2016
- New HV, Berryman J, Bolton-Maggs PH, Cantwell C, Chalmers EA, Davies T, et al, British Committee for Standards in Haematology .Guidelines on transfusion for fetuses, neonates and older children. . British Journal of Haematology 2016 ;175(5):784-828. [DOI] [PubMed] [Google Scholar]
NIH 1988
- National Institutes of Health (NIH).Perioperative red blood cell transfusion. JAMA 1988;260(18):2700-3. [PubMed] [Google Scholar]
Ochocinska 2020
- Ochocinska MJ, Spitalnik SL, Abuhamad A, Bennett-Guerrero E, Carlo WA, Cherukuri M, et al.NIH Workshop 2018: towards minimally-invasive or non-invasive approaches to assess tissue oxygenation pre- and post-transfusion. Transfusion Medicine Reviews 2020;S0887-7963(20):30074-2. [DOI: doi: 10.1016/j.tmrv.2020.12.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Odutayo 2017
- Odutayo A, Desborough MJ, Trivella M, Stanley AJ, Dorée C, Collins GS, et al.Restrictive versus liberal blood transfusion for gastrointestinal bleeding: a systematic review and meta-analysis of randomised controlled trials. Lancet Gastroenterology and Hepatology 2017;2(5):354-60. [DOI: 10.1016/S2468-1253(17)30054-7] [DOI] [PubMed] [Google Scholar]
Overgaard 1998
- Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al.A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma: results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiotherapy and Oncology 1998;46:135-46. [DOI] [PubMed] [Google Scholar]
Overgaard 2003
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Anderson E, et al.Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 & 7 randomised controlled trial. Lancet 2003;362:933-40. [DOI] [PubMed] [Google Scholar]
Patel 2015
- Patel NN, Avlonitis VS, Jones HE, Reeves BC, Sterne JA, Murphy GJ.Indications for red blood cell transfusion in cardiac surgery: a systematic review and meta-analysis. Lancet Haematology 2015;2(12):e543-53. [DOI: doi: 10.1016/S2352-3026(15)00198-2] [DOI] [PubMed] [Google Scholar]
Pavenski 2018
- Pavenski K, Stanworth S, Fung M, Wood EM, Pink J, Murphy MF, et al, International Collaboration for Transfusion Medicine Guidelines (ICTMG).Quality of evidence-based guidelines for transfusion of red blood cells and plasma: a systematic review. Transfusion Medicine Reviews 2018;S0887-7963(18):30017-8. [DOI] [PubMed] [Google Scholar]
Retter 2013
- Retter A, Wyncoll D, Pearse R, Carson D, McKechnie S, Stanworth S, et al.Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. British Journal of Haematology 2013;160(4):445-64. [DOI] [PubMed] [Google Scholar]
Review Manager 5a [Computer program]
- Review Manager 5 (RevMan 5).Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Review Manager 5b [Computer program]
- Review Manager 5 (RevMan 5).Version 5.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Richards 2020
- Richards T, Baikady RR, Clevenger B, Butcher A, Abeysiri S, Chau M, et al.Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet 2020;396:1353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2015
- Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E.The knowledge system underpinning healthcare is not fit for purpose and must change. BMJ 2015;350:h2463. [DOI] [PubMed] [Google Scholar]
Rohde 2014
- Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al.Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014;311(13):1317-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roman 2020
- Roman MA, Abbasciano RG, Pathak S, Oo S, Yusoff S, Wozniak M, et al.Patient blood management interventions do not lead to important clinical benefits or cost-effectiveness for major surgery: a network meta-analysis. British Journal of Anaesthesia 2020;S0007-0912(20):30342-1. [DOI: 10.1016/j.bja.2020.04.087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rygård 2016
- Rygård SL, Holst LB, Wetterslev J, Winkel P, Johansson PI, Wernerman J, et al, TRISS Trial Group, Scandinavian Critical Care Trials Group.Long-term outcomes in patients with septic shock transfused at a lower versus a higher haemoglobin threshold: the TRISS randomised, multicentre clinical trial. Intensive Care Medicine 2016;42(11):1685-94. [DOI] [PubMed] [Google Scholar]
Sabatine 2005
- Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, et al.Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 2005;111(16):2042-9. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DJ.Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled clinical trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Flasziou P, Guyatt GH.Chapter 11. Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Review of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Shah 2018
- Shah A, Brunskill SJ, Desborough MJ, Doree C, Trivella M, Stanworth SJ.Transfusion of red blood cells stored for shorter versus longer duration for all conditions. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD010801. [DOI: 10.1002/14651858.CD010801.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2020
- Shah A, Stanworth SJ, Docherty AB.Restrictive blood transfusion - is less really more? Anaesthesia 2020;75(4):433–7. [DOI] [PubMed] [Google Scholar]
Shander 2010
- Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR.Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50(4):753-65. [DOI] [PubMed] [Google Scholar]
Shander 2014
- Shander A, Javidroozi M, Naqvi S, Aregbeyen O, Caylan M, Demir S, et al.An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME). Transfusion 2014;54(10 Pt 2):2688-95. [DOI] [PubMed] [Google Scholar]
Shehata 2019
- Shehata N, Mistry N, da Costa BR, Pereira TV, Whitlock R, Curley GF, et al.Restrictive compared with liberal red cell transfusion strategies in cardiac surgery: a meta-analysis. European Heart Journal 2019;40(13):1081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shih 2018
- Shih AW, Liu A, Elsharawi R, Crowther MA, Cook RJ, Heddle NM.Systematic reviews of guidelines and studies for single versus multiple unit transfusion strategies. Transfusion 2018;58(12):2841-60. [DOI] [PubMed] [Google Scholar]
SHOT Annual Report 2019
- Narayan S (Ed), Poles D, et al, on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group.The 2019 Annual SHOT Report (2020). https://www.shotuk.org/wp-content/uploads/myimages/SHOT-REPORT-2019-Final-Bookmarked-v2.pdf.
Smith 2020
- Smith JD, Li DH, Rafferty MR.The implementation research logic model: a method for planning, executing, reporting, and synthesizing implementation projects. Implementation Science 2020;15(84):doi.org/10.1186/s13012-020-01041-8. [DOI: 10.1186/s13012-020-01041-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
So‐Osman 2010
- So-Osman C, Nelissen R, Te Slaa R, Coene L, Brand R, Brand A.A randomized comparison of transfusion triggers in elective orthopaedic surgery using leucocyte-depleted red blood cells. Vox Sanguinis 2010;98(1):56-64. [DOI] [PubMed] [Google Scholar]
Spiess 1998
- Spiess BD, Ley C, Body SC, Siegel LC, Stover EP, Maddi R, et al.Hematocrit value on intensive care unit entry influences the frequency of Q-wave myocardial infarction after coronary artery bypass grafting. The Institutions of the Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Journal of Thoracic & Cardiovascular Surgery 1998;116(3):460-7. [DOI] [PubMed] [Google Scholar]
Steiner 2015
- Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, et al.Effects of red-cell storage duration on patients undergoing cardiac surgery. New England Journal of Medicine 2015;372(15):1419-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stokes 2018
- Stokes EA, Wordsworth S, Staves J, Mundy N, Skelly J, Radford K, et al.Accurate costs of blood transfusion: a microcosting of administering blood products in the United Kingdom National Health Service. Transfusion 2018;58(4):846-53. [DOI] [PubMed] [Google Scholar]
STSBCGTF 2011
- Society of Thoracic Surgeons Blood Conservation Guideline Task Force (STSBCGTF), Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al.2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Annals of Thoracic Surgery 2011;91(3):944-82. [DOI] [PubMed] [Google Scholar]
Thygesen 2012
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction.Third universal definition of myocardial infarction. Circulation 2012;126(16):2020-35. [DOI: 10.1161/CIR.0b013e31826e1058] [DOI] [PubMed] [Google Scholar]
Toy 2012
- Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, et al.Transfusion-related acute lung injury: incidence and risk factors. Blood 2012;119(7):1757-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trentino 2020a
- Trentino KM, Farmer SL, Leahy MF, Sanfilippo FM, Isbister JP, Mayberry R, et al.Systematic reviews and meta-analyses comparing mortality in restrictive and liberal haemoglobin thresholds for red cell transfusion: an overview of systematic reviews. BMC Medicine 2020;18:154; https://doi.org/10.1186/s12916-020-01614-w. [DOI: 10.1186/s12916-020-01614-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trentino 2020b
- Trentino KM, Farmer SL, Isbister JP, Sanfilippo FM, Leahy MF, Hofmann A, et al.Restrictive versus liberal transfusion trials: are they asking the right question? Anesthesia and Analgesia 2020;131(6):1950-5. [DOI: 10.1213/ANE.0000000000005227] [DOI] [PubMed] [Google Scholar]
Trivella 2019
- Trivella M, Stanworth SJ, Brunskill S, Dutton P, Altman DG.Can we be certain that storage duration of transfused red blood cells does not affect patient outcomes? BMJ 2019;365:l2320. [DOI] [PubMed] [Google Scholar]
Turner 2018
- Turner ML.Safety of blood, blood derivatives, and plasma-derived products,. Handbook of Clinical Neurology 2018;153:463-72. [DOI] [PubMed] [Google Scholar]
Vlaar 2020
- Vlaar AP, Oczkowski S, Bruin S, Wijnberge M, Antonelli M, Aubron C, et al.Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Medicine 2020;46:673-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010
- Wang JK, Klein HG.Red blood cell transfusion in the treatment and management of anaemia: the search for the elusive transfusion trigger. Vox Sanguinis 2010;98(1):2-11. [DOI] [PubMed] [Google Scholar]
Whitaker 2011
- US Department of Health and Human Services, Office of the Assistant Secretary for Health.Report of the US Department of Health and Human Services. The 2009 National Blood Collection and Utilization Survey Report. www.hhs.gov/ophs/bloodsafety/2007nbcus_survey.pdf (accessed 11 October 2016).
WHO 2016
- WHO.Global status report on blood safety and availability. www.who.int/worldblooddonorday/media/who_blood_safety_factsheet_2011.pdf (accessed 17 January 2021);Fact Sheet 279:1-173.
Whyte 2011
- Whyte R, Kirpalani H.Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD000512. [DOI: 10.1002/14651858.CD000512.pub2] [DOI] [PubMed] [Google Scholar]
Wu 2001
- Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM.Blood transfusion in elderly patients with acute myocardial infarction. New England Journal of Medicine 2001;345(17):1230-6. [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, et al.Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA 2007;297(22):2481-8. [DOI] [PubMed] [Google Scholar]
Wu 2010
- Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, et al.Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Annals of Surgery 2010;252(1):11-7. [DOI] [PubMed] [Google Scholar]
Yao 2020
- Yao RQ, Ren C, Zhang ZC, Zhu YB, Xia ZF, Yao YM.Is haemoglobin below 7.0 g/dL an optimal trigger for allogenic red blood cell transfusion in patients admitted to intensive care units? A meta-analysis and systematic review. BMJ Open 2020;10:e030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Youssef 2017
- Youssef LA, Spitalnik SL.Transfusion-related immunomodulation: a reappraisal. Current Opinion in Hematology 2017;24(6):551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zou 2009
- Zou S, Stramer SL, Notari EP, Kuhns MC, Krysztof D, Musavi F, et al.Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion 2009;49(8):1609-20. [DOI] [PubMed] [Google Scholar]
Zou 2010
- Zou S, Dorsey KA, Notari EP, Foster GA, Krysztof DE, Musavi F, et al.Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion 2010;50(7):1495-504. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Carless 2010b
- Carless PA, Henry DA, Carson JL, Hebert PC, McClelland B, Ker K.Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD002042. [DOI: 10.1002/14651858.CD002042.pub2] [DOI] [PubMed] [Google Scholar]
Carson 2012b
- Carson JL, Carless PA, Hebert PC.Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD002042. [DOI: 10.1002/14651858.CD002042.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2016b
- Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al.Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD002042. [DOI: 10.1002/14651858.CD002042] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 2000
- Hill S, Carless PA, Henry DA, Carson JL, Hebert PPC, Henderson KM, et al.Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2000, Issue 1. Art. No: CD002042. [DOI: 10.1002/14651858.CD002042] [DOI] [PubMed] [Google Scholar]
Hill 2002
- Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DB, et al.Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD002042. [DOI: 10.1002/14651858.CD002042] [DOI] [PubMed] [Google Scholar]
Hill 2005
- Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DB, et al.Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD002042. [DOI: 10.1002/14651858.CD002042] [DOI] [PubMed] [Google Scholar]


