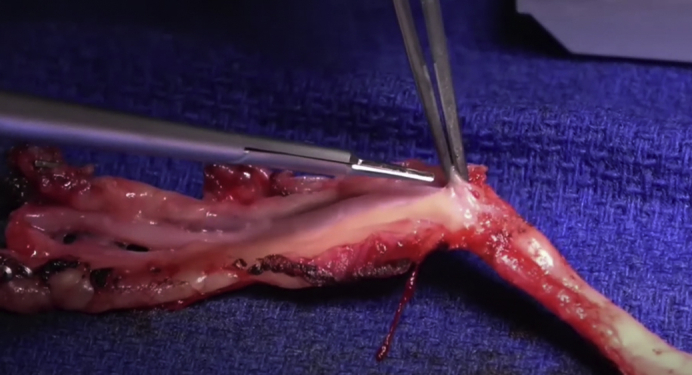
Preparation of the radial artery graft for coronary artery bypass surgery.
Central Message.
The use of the radial artery for coronary surgery is increasing. Patient profile, palmar arch patency, and coronary stenosis degree need to be evaluated before radial artery harvesting.
See Commentary on page 120.
More than 18 million adults in the United States have coronary artery disease (CAD), accounting for 7% of the entire American adult population.1 Coronary artery bypass grafting (CABG) is the most performed type of cardiac surgery with about 370,000 cases annually in the United States.2
The first ever left internal thoracic artery (LITA) to left anterior descending anastomosis took place in 1968, performed by George Green in New York. Today this procedure has become the gold standard and the patency of this anastomosis correlates most strongly with improved outcomes and better survival.3
Just few years later, in 1973, Carpentier and colleagues4 first described the radial artery (RA) as a conduit for CABG. However, he subsequently advised against its use because of a higher rate (35%) of occlusion or narrowing possibly attributed to spasm.5 Therefore, this graft quickly fell out of favor and would not be used again for approximately 20 years. In 1992, Acar and colleagues6 demonstrated that occluded or narrowed RA from the first Carpentier series were patent at 18-year follow-up. Furthermore, he studied 122 RA grafts and showed that the early patency was 100%, proving the RA was in fact suitable as a graft in CABG.6 Since then, the use of the RA has continued to increase for the treatment of patients with multivessel CAD undergoing CABG. Atraumatic harvesting technique, careful selection of the target vessel, and use of antispastic protocols are the likely reasons for the improved results of RA grafting in the cotemporary era.
Evidence of Superiority of the RA Over the Saphenous Vein for CABG
There are multiple options for choosing the second graft when performing CABG procedures in patients who require multiple bypass grafting. RA and saphenous vein (SV) are 2 of the most common conduits used along with the right internal thoracic artery (RITA). Multiple randomized clinical trials and meta-analyses along with observational studies have demonstrated the superiority of the RA over the SV when it comes to graft patency and clinical outcomes (Table 1).7, 8, 9, 10, 11 A network meta-analysis including 3651 grafts showed that among all the conduits used for CABG, the RA was the best conduit in terms of patency at 5-year follow-up (incidence rate ratio, 0.54; 95% confidence interval [CI], 0.35-0.82).7 A second meta-analysis of 14 studies and around 21,000 patients found decreased long-term mortality (incidence rate ratio, 0.74; 95% CI, 0.63-0.87; P < .001) in the RA group when compared with the SV group at 6.6 years of follow-up. The survival benefit was found to be independent of age, sex, diabetes status, and ventricular function.8 In the Radial Artery Database International Alliance, a patient-level meta-analysis including more than 1000 patients found lower risk of graft occlusion (hazard ratio [HR], 0.44; 95% CI, 0.28-0.70) along with decreased incidence of adverse cardiac events (HR, 0.67; 95% CI, 0.49-0.90) in the RA group compared with the SV group at 5 years of follow-up.9 In a subsequent report from the same database with 10-year follow-up, we found that patients who received the RA had also a lower incidence of the composite of death and myocardial infarction (HR, 0.77; 95% CI, 0.63-0.94), and a higher survival rate (HR, 0.73; 95% CI, 0.57-0.93).10 The Radial Artery Patency and Clinical Outcomes trial compared RITA versus RA in 394 patients and SV versus RA in 225 patients. In the RITA versus RA arm, the 10-year patency rate was 89% for RA versus 80% for RITA (HR, 0.45; 95% CI, 0.23-0.88) and the survival estimate was 90.9% versus 83.7%, respectively (HR, 0.53; 95% CI, 0.30-0.95). In the SV versus RA arm, the patency rates were 85% for RA and 71% for SV (HR, 0.40; 95% CI, 0.15-1.00) and 10-year survival estimate was 72.6% and 65.2%, respectively (HR, 0.76; 95% CI, 0.47-1.22).11
Table 1.
Summary of the main studies comparing radial artery (RA) with saphenous vein (SV) grafts in coronary artery bypass grafting
| Study | Study period | No. of patients | Follow-up (y) | Main findings |
|---|---|---|---|---|
| Gaudino, 20217 | 1993-2014 | 3396 | 5 |
|
| Gaudino, 20198 | 1993-2012 | 20,931 | 7 |
|
| Gaudino, 20189 | 1996-2009 | 1036 | 5 |
|
| Gaudino, 202010 |
1997-2009 | 1036 | 10 |
|
| Buxton, 202011 | 1996-2005 | 416 | 10 |
|
RITA, Right internal thoracic artery; GEA, gastroepiploic artery; IRR, incidence rate ratio; CI, confidence interval; HR, hazard ratio.
Based on this findings, the 2018 European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularization conferred a Class I recommendation to the use of the RA for CABG.12 Because the evidence in support of the RA continues to grow stronger and stronger, it seems likely that when the new American College of Cardiology/American Heart Association guideline for myocardial revascularization will be published, the RA will have the same class of recommendation.
How to Harvest the RA
There are 2 main techniques used for harvesting the RA: open radial artery harvesting technique (ORAH) and endoscopic RA harvesting (ERAH). Until the early 2000s the only way to harvest the RA was ORAH, which requires a 15 to 18 cm incision in the patient's forearm (Figure 1). It wasn't until 2000 with the creation of the endoscopic vessel harvesting system that ERAH was possible. Using the ERAH technique, a 2 to 3 cm incision just above the radial styloid prominence is all that is necessary.13
Figure 1.
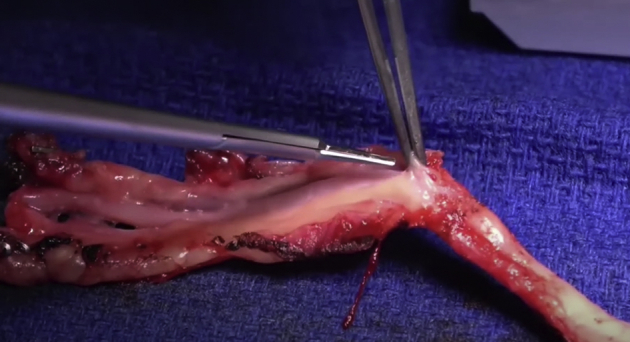
Preparation of the radial artery graft for coronary artery bypass surgery.
With the advancement in training and technologies, the endoscopic approach to harvest the RA for CABG continues to grow in popularity. In an analysis of the Society of Thoracic Surgeons National Database from 2008 to 2018 we found that 43.2% of surgeons use ERAH and 56.8% ORAH (unpublished data).14
The concern with ERAH is that it requires more manipulation of the artery than ORAH, with higher risk of mechanical injury particularly to the endothelium. Damage to the RA endothelium during ERAH may lead to graft spasm, thrombosis, and occlusion and potentially put patients at higher risk of cardiac events.15 In vitro studies comparing RA endothelial integrity and function between the 2 techniques have provided discordant results. Older studies have reported no difference between the 2 techniques.16 However, in a more contemporary organ bath study, our group showed that ORAH is associated with better preservation of endothelial function compared with ERAH.17
On the other hand, multiple studies have showed that ERAH produces significantly less arm complications compared with ORAH. Rates of wound infection, postoperative pain, neuropathy, and hematoma have all been demonstrated to be significantly lower with ERAH.18, 19, 20, 21, 22, 23 A meta-analysis of 4 small randomized controlled trials and 2 propensity-matched studies (743 patients; 324 ERAH) found that ERAH was associated with a lower incidence of wound complications (odds ratio, 0.33; 95% CI, 0.14-0.771), without significant difference in 30-day mortality, long-term mortality, and RA patency rate when compared with ORAH.20 Similar results were showed by Huang and colleagues22 in a recent meta-analysis of 24 studies (15 observational, 12 of them unadjusted).22
The main conclusion is that the available evidence suggests that ERAH may have improved arm outcomes with no significant differences in clinical outcome or RA patency.15,22 However, most of the published studies are underpowered or are observational studies with biases in patient selection. Further randomized trials are needed to better understand the differences in outcomes between the 2 techniques.
Preventing Vasospasm of the RA
Due to the predominantly muscular wall of the RA and the consequent concerns of spasm, antispastic therapy with calcium-channel blockers (CCBs) is generally used postoperatively in patients with RA grafts. A survey of all Canadian cardiac surgery centers reported that some form of antispastic therapy is adopted in almost all institutions (25 out of 27) after RA grafting.24 However, the published evidence on the effect of CCB on the RA is controversial. In a small randomized trial, our group assigned 120 patients who received the RA for CABG to continue or suspend the CCBs after the first postoperative year and found no difference in graft patency, graft reactivity, myocardial ischemia, or clinical outcomes at 5-year follow-up.25 Subsequently, in another small trial, we randomized 100 patients to receive or not receive CCBs from the early postoperative period and did not find a difference in clinical and angiographic outcomes at 1 year.26 In a post hoc analysis of the Radial Artery Patency Study, among 440 RA patients, the incidence of string sign (the highest degree of RA graft spasm) was not influenced by the compliance with the prescribed postoperative CCBs.27 On the other hand, in a post hoc analysis of data in the Radial Artery Database International Alliance including 732 patients, we found that CCB therapy was associated with a significantly lower risk of major adverse cardiac events (HR, 0.52; 95% CI, 0.31-0.89) and RA graft occlusion (HR, 0.20; 95% CI, 0.08-0.49).28 This post hoc analysis shares the limitations of observational studies, especially in terms of indication biases and unmeasured confounders29 because it is likely that healthier patients were more compliant with the CCB therapy. Due to the lack of clear data supporting the use of CCBs following RA grafting, further randomized studies are necessary to better understand the real influence of CCBs on the RA grafting.
When and When Not to Use the RA
The key reasons not to use the RA are insufficient ulnar artery compensation to RA removal or contraindications to harvesting (Table 2). The latter includes history of major arm trauma or surgery, vasculitis, or Raynaud syndrome.30 Some autoimmune conditions, including scleroderma and rheumatoid arthritis, are also contraindications.31 Additionally, RA harvesting is contraindicated in patients who have advanced chronic kidney disease and are at risk for future dialysis because the RA may be needed for dialysis access.30 Furthermore, patients who have undergone coronary angiography via RA access should not have RA harvesting.30
Table 2.
Contraindications to the use of radial artery (RA) as a conduit for coronary artery bypass grafting
| When not to use RA |
| History of major arm trauma |
| Prior surgery on the arm |
| Vasculitis |
| Raynaud syndrome |
| Scleroderma |
| Advanced chronic kidney disease |
| Dialysis |
| Angiography via RA access |
| Insufficient ulnar artery compensation, evaluated with Allen test, Barbeau test, echo-Doppler, Doppler plethysmography |
| Mild-to-moderate stenosis of the coronary target vessel, evaluated with visual inspection, quantitative coronary angiography, fractional flow reserve |
The adequacy of ulnar compensation to RA removal must be carefully assessed before harvesting. The clinical Allen's test (AT) alone is not sufficient. Patients can have a normal AT but an incomplete arch, as shown by Agrifoglio and colleagues, where 8 out of 150 patients had incomplete arches despite a normal AT.32 Therefore, a second test should be done if the AT is normal. Among the simplest confirmatory tests is the Barbeau test, in which a pulse oximeter is placed on the index finger and both the ulnar artery and RA are manually occluded. Complete occlusion is confirmed by lack of a waveform on the pulse oximeter. The ulnar artery is then released; if the palmar arch is incomplete, the pulse oximeter waveform does not come back.33 Another option is echo-Doppler evaluation that can also determine the vessel size and identify plaques or calcifications. Patients are eligible for RA grafting if the vessel has a diameter >2.0 mm, with minimal evidence of plaque or calcifications. Other valuable information provided by Doppler ultrasound include the shape of the RA waveform and the evaluation of the ulnar collateral blood supply during manual compression of the RA.34,35 Doppler plethysmography assesses the palmar arch by visualization of pulsatile waveforms in each of the digits before and after RA compression. If the waveforms are either marginal or absent at baseline or after RA compression, the RA should not be used.36 Other modalities such as computed tomography angiography, and intraoperative RA pressure measurement are less commonly used.37 The RA should be harvested from the arm with the best ulnar compensation, without concerns related to hand dominance.
A third key reason not to use the RA is related to the coronary target vessel. The RA has a more muscular wall than RITA and LITA and SV and as such is more prone to spasm in case of mild-to-moderate coronary stenosis and chronic competitive flow.38 If the target vessel has mild or moderate stenosis, a greater shear force produced by the oscillating flow between the conduit and native circulations may impair endothelial function predisposing to an anastomotic occlusion.39 In an analysis of 123 postoperative angiographies, the RA patency rate for target vessel with moderate stenosis (50% to 74%) was 78.9% compared with 84.9% for severe stenosis (75% to 89%) and 98% for critical stenosis (≥90%) (P = .001). At multivariable analysis, anastomosis to a vessel with stenosis <90% was an independent predictor of graft occlusion (HR, 14.9; 95% CI, 2.6-83.2).40 Furthermore, in an angiographic evaluations of RA grafts after 20 years of follow-up, we found a patency rate of the RA similar to that of LITA when anastomosed to target vessels with stenosis ≥90%.41
Visual inspection, quantitative coronary angiography, and fractional flow reserve (FFR) have all been used to estimate the severity of the coronary stenosis and the potential for competitive flow. It is important to note that target vessel stenosis is a relatively inaccurate surrogate for chronic competitive flow because it does not account for the minimal residual lumen diameter or the functional assessment of the stenosis, which may be better indicators of chronic competitive flow. FFR is the only direct method to assess the hemodynamic effect of a stenosis. Based on the most recent evidence, a FFR cutoff of less than 0.75 to 0.80 is used to distinguish functionally and nonfunctionally significant stenosis.42 In the Impact of Preoperative FFR on Arterial Bypass Graft Function trial, Glineur and colleagues43 found a significant association between the preoperative FFR measurement of the target vessel and the anastomotic functionality at 6 months (P < .001), with a cutoff of 0.78.43 It must be noted that the majority of grafts in are LITA or RITA, not RA. In an analysis of 164 patients, Botman and colleagues44 found a graft occlusion rate of 8.9% when FFR was >0.75 and 21.4% when FFR was <0.75 (P < .001). We have been using the ratio between the diameter of the RA and the diameter of the target vessel for decision making, with a cutoff of 1.3.
The RA can be proximally anastomosed to the ascending aorta or to the LITA and RITA. It seems that when RA is proximally anastomosed to the ascending aorta, the higher pressure in the aorta helps with eventual competitive flow, whereas the anastomosis with the ITAs makes the RA more vulnerable to the negative effect of chronic native competitive flow. In an analysis of 228 consecutive, the use of ITA-anastomosed RA, compared with aorta-anastomosed RA, was associated with higher rate of graft failure when the coronary target vessel had a 70% to 90% stenosis (25.0% vs 2.4%; P = .02). No difference was found when the coronary stenoses were >90%.45
With the exception of the higher risk of failure of ITA anastomosed RA grafts in situations of competitive flow, there are no data to support difference in patency rate of RA grafts based on target vessels location or graft configuration.
Conclusions
The RA has been showed to be superior to the SV in CABG and its use is increasing for the treatment of patients with multivessel CAD undergoing CABG. Patient profile, patency of the palmar arch, and stenosis of the target vessel must be considered in the decision-making process to use the RA, but in principle the conduit should be used every time it is not contraindicated. The optimal technique for harvesting the RA and the use of CCBs need further evaluation.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Fryar C.D. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief. 2012;103:1–8. [PubMed] [Google Scholar]
- 2.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Green G.E., Stertzer S.H., Reppert E.H. Coronary arterial bypass grafts. Ann Thorac Surg. 1968;5:443–450. doi: 10.1016/s0003-4975(10)66377-1. [DOI] [PubMed] [Google Scholar]
- 4.Carpentier A., Guermonprez J.L., Deloche A., Frechette C., DuBost C. The aorta-to-coronary radial artery bypass graft: a technique avoiding pathological changes in grafts. Ann Thorac Surg. 1973;16:111–121. doi: 10.1016/s0003-4975(10)65825-0. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier A., Geha A.S., Krone R.J., McCormick J.R., Baue A.E. Selection of coronary bypass: anatomic, physiological, and angiographic considerations of vein and mammary artery grafts. J Thorac Cardiovasc Surg. 1975;70:414–431. [PubMed] [Google Scholar]
- 6.Acar C., Jebara V.A., Portoghese M., Beyssen B., Pagny J.Y., Grare P., et al. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg. 1992;54:652–659. doi: 10.1016/0003-4975(92)91007-v. [DOI] [PubMed] [Google Scholar]
- 7.Gaudino M., Hameed I., Robinson N.B., Ruan Y., Rahouma M., Naik A., et al. Angiographic patency of coronary artery bypass conduits: a network meta-analysis of randomized trials. J Am Heart Assoc. 2021;10:e019206. doi: 10.1161/JAHA.120.019206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudino M., Rahouma M., Abouarab A., Leonard J., Kamel M., Di Franco A., et al. Radial artery versus saphenous vein as the second conduit for coronary artery bypass surgery: a meta-analysis. J Thorac Cardiovasc Surg. 2019;157:1819–1825. doi: 10.1016/j.jtcvs.2018.08.123. [DOI] [PubMed] [Google Scholar]
- 9.Gaudino M., Benedetto U., Fremes S., Biondi-Zoccai G., Sedrakyan A., Puskas J.D., et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med. 2018;378:2069–2077. doi: 10.1056/NEJMoa1716026. [DOI] [PubMed] [Google Scholar]
- 10.Gaudino M., Benedetto U., Fremes S., Ballman K., Biondi-Zoccai G., Sedrakyan A., et al. Association of radial artery graft vs saphenous vein graft with long-term cardiovascular outcomes among patients undergoing coronary artery bypass grafting: a systematic review and meta-analysis. JAMA. 2020;324:179–187. doi: 10.1001/jama.2020.8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buxton B.F., Hayward P.A., Raman J., Moten S.C., Rosalion A., Gordon I., et al. Long-term results of the RAPCO trials. Circulation. 2020;142:1330–1338. doi: 10.1161/CIRCULATIONAHA.119.045427. [DOI] [PubMed] [Google Scholar]
- 12.Sousa-Uva M., Neumann F.-J., Ahlsson A., Alfonso F., Banning A.P., Benedetto U., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2019;55:4–90. doi: 10.1093/ejcts/ezy289. [DOI] [PubMed] [Google Scholar]
- 13.Blitz A., Osterday R.M., Brodman R.F. Harvesting the radial artery. Ann Cardiothorac Surg. 2013;2:533–542. doi: 10.3978/j.issn.2225-319X.2013.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudino M., Rahouma M., Habib R.H., Hameed I., Robinson N.B., Farrington W.J., et al. Surgeons' coronary bypass practice patterns in the United States. J Am Coll Cardiol. 2020;76:1714–1715. doi: 10.1016/j.jacc.2020.07.064. [DOI] [PubMed] [Google Scholar]
- 15.Kinlay S., Libby P., Ganz P. Endothelial function and coronary artery disease. Curr Opin Lipidol. 2001;12:383–389. doi: 10.1097/00041433-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Shapira O.M., Eskenazi B.R., Anter E., Joseph L., Christensen T.G., Hunter C.T., et al. Endoscopic versus conventional radial artery harvest for coronary artery bypass grafting: functional and histologic assessment of the conduit. J Thorac Cardiovasc Surg. 2006;131:388–394. doi: 10.1016/j.jtcvs.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Gaudino M.F., Lorusso R., Ohmes L.B., Narula N., McIntire P., Gargiulo A., et al. Open radial artery harvesting better preserves endothelial function compared to the endoscopic approach. Interact Cardiovasc Thorac Surg. 2019;29:561–567. doi: 10.1093/icvts/ivz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamim M., Alexiou C., Al-Hassan D., Al-Faraidy K. Prospective randomized trial of endoscopic vs open radial artery harvest for CABG: clinical outcome, patient satisfaction, and midterm RA graft patency. J Card Surg. 2020;35:2147–2154. doi: 10.1111/jocs.14706. [DOI] [PubMed] [Google Scholar]
- 19.Kiaii B.B., Swinamer S.A., Fox S.A. A prospective randomized study of endoscopic versus conventional harvesting of the radial artery. Innovations. 2017;12:231–238. doi: 10.1097/IMI.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 20.Rahouma M., Kamel M., Benedetto U., Ohmes L.B., Franco A.D., Lau C., et al. Endoscopic versus open radial artery harvesting: a meta-analysis of randomized controlled and propensity matched studies. J Card Surg. 2017;32:334–341. doi: 10.1111/jocs.13148. [DOI] [PubMed] [Google Scholar]
- 21.Cao C., Tian D.H., Ang S.C., Peeceeyen S., Allan J., Fu B., et al. A meta-analysis of endoscopic versus conventional open radial artery harvesting for coronary artery bypass graft surgery. Innovations. 2014;9:269–275. doi: 10.1097/IMI.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 22.Huang T.-Y., Huang T.-S., Cheng Y.-T., Wang Y.-C., Chen T.-P., Yin S.-Y., et al. Radial artery harvesting in coronary artery bypass grafting surgery—endoscopic or open method? A meta-analysis. PLoS One. 2020;15:e0236499. doi: 10.1371/journal.pone.0236499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimitrova K.R., Hoffman D.M., Geller C.M., DeCastro H. Endoscopic radial artery harvest produces equivalent and excellent midterm patency compared with open harvest. Innovations. 2010;5:265–269. doi: 10.1097/IMI.0b013e3181ee93f0. [DOI] [PubMed] [Google Scholar]
- 24.Myers M.G., Fremes S.E. Prevention of radial artery graft spasm: a survey of Canadian surgical centres. Can J Cardiol. 2003;19:677–681. [PubMed] [Google Scholar]
- 25.Gaudino M., Glieca F., Luciani N., Alessandrini F., Possati G. Clinical and angiographic effects of chronic calcium channel blocker therapy continued beyond first postoperative year in patients with radial artery grafts: results of a prospective randomized investigation. Circulation. 2001;104:164–167. doi: 10.1161/hc37t1.094819. [DOI] [PubMed] [Google Scholar]
- 26.Gaudino M., Luciani N., Nasso G., Salica A., Canosa C., Possati G. Is postoperative calcium channel blocker therapy needed in patients with radial artery grafts? J Thorac Cardiovasc Surg. 2005;129:532–535. doi: 10.1016/j.jtcvs.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 27.Miwa S., Desai N., Koyama T., Chan E., Cohen E.A., Fremes S.E., et al. Radial artery angiographic string sign: clinical consequences and the role of pharmacologic therapy. Ann Thorac Surg. 2006;81:112–118. doi: 10.1016/j.athoracsur.2005.06.076. [DOI] [PubMed] [Google Scholar]
- 28.Gaudino M., Benedetto U., Fremes S.E., Hare D.L., Hayward P., Moat N., et al. Effect of calcium-channel blocker therapy on radial artery grafts after coronary bypass surgery. J Am Coll Cardiol. 2019;73:2299–2306. doi: 10.1016/j.jacc.2019.02.054. [DOI] [PubMed] [Google Scholar]
- 29.Gaudino M., Di Franco A., Rahouma M., Tam D.Y., Iannaccone M., Deb S., et al. Unmeasured confounders in observational studies comparing bilateral versus single internal thoracic artery for coronary artery bypass grafting: a meta-analysis. J Am Heart Assoc. 2018;7:e008010. doi: 10.1161/JAHA.117.008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaudino M., Fremes S., Schwann T.A., Tatoulis J., Wingo M., Tranbaugh R.F. Technical aspects of the use of the radial artery in coronary artery bypass surgery. Ann Thorac Surg. 2019;108:613–622. doi: 10.1016/j.athoracsur.2018.10.066. [DOI] [PubMed] [Google Scholar]
- 31.Budillon A.M., Nicolini F., Agostinelli A., Beghi C., Pavesi G., Fragnito C., et al. Complications after radial artery harvesting for coronary artery bypass grafting: our experience. Surgery. 2003;133:283–287. doi: 10.1067/msy.2003.43. [DOI] [PubMed] [Google Scholar]
- 32.Agrifoglio M., Dainese L., Pasotti S., Galanti A., Cannata A., Roberto M., et al. Preoperative assessment of the radial artery for coronary artery bypass grafting: is the clinical Allen test adequate? Ann Thorac Surg. 2005;79:570–572. doi: 10.1016/j.athoracsur.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 33.Barbeau G.R., Arsenault F., Dugas L., Simard S., Larivière M.M. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen's test in 1010 patients. Am Heart J. 2004;147:489–493. doi: 10.1016/j.ahj.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 34.Jarvis M.A., Jarvis C.L., Jones P.R., Spyt T.J. Reliability of Allen's test in selection of patients for radial artery harvest. Ann Thorac Surg. 2000;70:1362–1365. doi: 10.1016/s0003-4975(00)01551-4. [DOI] [PubMed] [Google Scholar]
- 35.Pola P., Serricchio M., Flore R., Manasse E., Favuzzi A., Possati G.F. Safe removal of the radial artery for myocardial revascularization: a Doppler study to prevent ischemic complications to the hand. J Thorac Cardiovasc Surg. 1996;112:737–744. doi: 10.1016/S0022-5223(96)70060-0. [DOI] [PubMed] [Google Scholar]
- 36.Kohonen M., Teerenhovi O., Terho T., Laurikka J., Tarkka M. Is the Allen test reliable enough? Eur J Cardiothorac Surg. 2007;32:902–905. doi: 10.1016/j.ejcts.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Gaudino M., Crea F., Cammertoni F., Mazza A., Toesca A., Massetti M. Technical issues in the use of the radial artery as a coronary artery bypass conduit. Ann Thorac Surg. 2014;98:2247–2254. doi: 10.1016/j.athoracsur.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 38.van Son J.A., Smedts F., Vincent J.G., van Lier H.J., Kubat K. Comparative anatomic studies of various arterial conduits for myocardial revascularization. J Thorac Cardiovasc Surg. 1990;99:703–707. [PubMed] [Google Scholar]
- 39.Maniar H.S., Sundt T.M., Barner H.B., Prasad S.M., Peterson L., Absi T., et al. Effect of target stenosis and location on radial artery graft patency. J Thorac Cardiovasc Surg. 2002;123:45–52. doi: 10.1067/mtc.2002.118686. [DOI] [PubMed] [Google Scholar]
- 40.Yie K., Na C.-Y., Oh S.S., Kim J.-H., Shinn S.-H., Seo H.-J. Angiographic results of the radial artery graft patency according to the degree of native coronary stenosis. Eur J Cardiothorac Surg. 2008;33:341–348. doi: 10.1016/j.ejcts.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Gaudino M., Tondi P., Benedetto U., Milazzo V., Flore R., Glieca F., et al. Radial artery as a coronary artery bypass conduit: 20-year results. J Am Coll Cardiol. 2016;68:603–610. doi: 10.1016/j.jacc.2016.05.062. [DOI] [PubMed] [Google Scholar]
- 42.Spadaccio C., Glineur D., Barbato E., Di Franco A., Oldroyd K.G., Biondi-Zoccai G., et al. Fractional flow reserve-based coronary artery bypass surgery: current evidence and future directions. JACC Cardiovasc Interv. 2020;13:1086–1096. doi: 10.1016/j.jcin.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glineur D., Grau J.B., Etienne P.-Y., Benedetto U., Fortier J.H., Papadatos S., et al. Impact of preoperative fractional flow reserve on arterial bypass graft anastomotic function: the IMPAG trial. Eur Heart J. 2019;40:2421–2428. doi: 10.1093/eurheartj/ehz329. [DOI] [PubMed] [Google Scholar]
- 44.Botman C.J., Schonberger J., Koolen S., Penn O., Botman H., Dib N., et al. Does stenosis severity of native vessels influence bypass graft patency? A prospective fractional flow reserve-guided study. Ann Thorac Surg. 2007;83:2093–2097. doi: 10.1016/j.athoracsur.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 45.Gaudino M., Alessandrini F., Pragliola C., Cellini C., Glieca F., Luciani N., et al. Effect of target artery location and severity of stenosis on mid-term patency of aorta-anastomosed vs. internal thoracic artery-anastomosed radial artery grafts. Eur J Cardiothorac Surg. 2004;25:424–428. doi: 10.1016/j.ejcts.2003.11.027. [DOI] [PubMed] [Google Scholar]


