Abstract
Background
We report 3 cases of rescue transventricular off-pump mitral valve (MV) repair in high-risk patients with acute mitral regurgitation (MR) due to post–myocardial infarction (MI) papillary muscle rupture (PMR).
Methods
The 3 patients presented with acute inferior ST elevation myocardial infarction, cardiogenic shock, and pulmonary edema. Their preoperative peak troponin I levels were 1909 ng/L, 16,963 ng/L, and 8299 ng/L. All 3 patients underwent successful percutaneous intervention to the culprit coronary artery, and antiplatelet therapy was initiated. All patients required inotropic support and had an intra-aortic balloon pump inserted preoperatively. Transesophageal echocardiography (TEE) demonstrated severe eccentric MR due to the leaflet prolapse secondary to PMR. The patients’ estimated EuroSCORE II scores were 16.03%, 16.68%, and 7.81%, and their Society of Thoracic Surgeons scores were 14.77%, 18.24%, and 9.8%, respectively. All 3 patients underwent urgent transventricular off-pump MV repair using artificial chords, with 2 or 3 three neochords implanted. The duration of operation was <2 hours, and intraoperative and postoperative drainage was minimal in all cases. MV function was assessed by qualitative and semiquantitative TEE.
Results
Intraoperative MR reduction to a mild level was achieved in all 3 patients. All patients had moderate MR at discharge, likely due to left ventricular remodeling. Severe MR recurred in all patients, at 5, 4, and 2 months of follow-up, respectively. All 3 patients underwent an elective MV reoperation via conventional approach.
Conclusions
Off-pump transventricular MV repair may offer a safe and feasible alternative to stabilize high-risk patients with acute MR due to post-MI PMR. Although early MR recurrence is concerning, urgent transventricular MV repair may serve as a bridge to conventional surgery in such unstable patients.
Key Words: mitral valve, acute mitral regurgitation, papillary muscle rupture, cardiogenic shock, minimally invasive, off-pump, transventricular mitral repair, artificial chords
Abbreviations and Acronyms: AF, atrial fibrillation; ECG, electrocardiography; IABP, intra-aortic balloon pump; LAD, left anterior descending artery; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; MV, mitral valve; PCI, percutaneous coronary intervention; PMR, papillary muscle rupture; RCA, right coronary artery; STEMI, ST elevation myocardial infarction; STS, Society of Thoracic Surgeons; TEE, transesophageal echocardiography; TR, tricuspid regurgitation
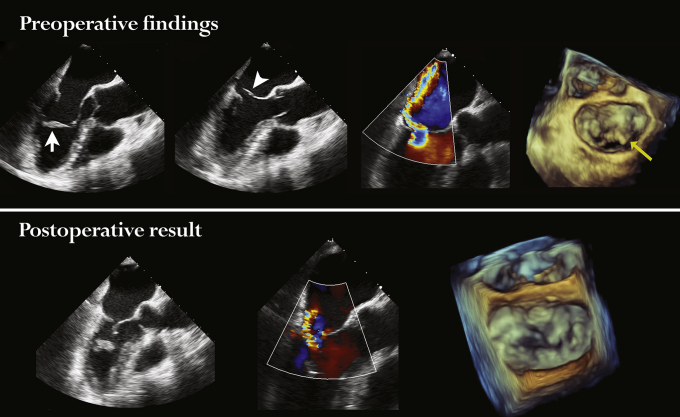
Graphical abstract
The first-in-human urgent transventricular mitral valve (MV) repair with artificial neochords was successfully performed in 3 high-risk patients with acute severe mitral regurgitation (MR) due to ischemic papillary muscle rupture, cardiogenic shock, and pulmonary edema. The primary intention of hemodynamic stabilization was achieved in all patients with intraoperative MR reduction to a mild level (1+). The NeoChord DS1000 device captured the bundle of native chords together with the ruptured portion of the papillary muscle during the procedure (schematic view provided). Predischarge echocardiography demonstrated moderate MR, likely related to left ventricular remodeling. Recurrent severe MR was noted in all patients at 2 to 5 months after the repair. All patients underwent a successful elective reoperation with MV repair or replacement. This proof-of-concept study shows that the urgent off-pump NeoChord procedure in unstable patients with acute severe MR may serve as a bridge to conventional surgery.


Ruptured papillary muscle and native chords are captured using the NeoChord device.
Perspective.
Papillary muscle rupture is a rare and potentially fatal complication of myocardial infarction causing acute severe mitral regurgitation and rapidly progressing to cardiogenic shock, pulmonary edema, and multiorgan failure. In such patients, urgent transventricular off-pump mitral valve repair may offer hemodynamic stabilization as a bridge to elective mitral valve surgery.
Central Message.
Transventricular off-pump repair of acute mitral regurgitation due to ischemic papillary muscle rupture may provide a safe and feasible alternative to bridge unstable patients to elective surgery.
See Commentary on page 243.
Papillary muscle rupture (PMR) is a rare complication of acute myocardial infarction (MI), occurring in 1% to 3% of all MIs.1, 2, 3 PMR with severe mitral regurgitation (MR) typically occurs within 5 days after MI4 and can result in severe acute MR, leading to cardiogenic shock and pulmonary edema. The abrupt onset of severe MR often causes pulmonary congestion and forward left ventricular (LV) failure. The right coronary artery (RCA) is often the culprit vessel; however, less commonly, an occlusion of the circumflex artery can cause PMR.5 These patients should be considered for emergency surgery, but surgical treatment carries a substantial perioperative morbidity and mortality rate, ranging between 19% and 53% with mitral valve (MV) repair or replacement as reported by multiple centers.6, 7, 8 However, nonoperative strategies carry a mortality of nearly 80% within the first week of rupture.9, 10, 11
We report 3 consecutive cases of rescue transventricular off-pump MV repair with artificial chords (4/0 Gore-Tex) using the NeoChord DS1000 Artificial Chordae Delivery System (NeoChord, St Louis Park, Minn) in high-risk patients with acute MR due to post-MI PMR. The surgery was performed via a small anterolateral thoracotomy under the guidance of real-time 3-dimensional transesophageal echocardiography (TEE), as previously reported by Ručinskas and colleagues.12 Rescue transventricular repair of a leaking MV allowed achievement of hemodynamic stability and bridged the patients to elective conventional MV surgery. This report provides proof-of-concept that transventricular off-pump MV repair is safe and feasible in patients with acute MR due to ischemic PMR (Figure 1).
Figure 1.
The first-in-human urgent transventricular mitral valve repairs with artificial neochords were successfully performed in 3 high-risk patients with acute severe mitral regurgitation (MR) due to ischemic papillary muscle rupture, cardiogenic shock, and pulmonary edema. The primary intention of hemodynamic stabilization was achieved in all patients, with intraoperative MR reduction to mild (1+). The NeoChord DS1000 device captured the bundle of native chords together with ruptured portion of the papillary muscle during the procedure (schematic view is provided). Predischarge echocardiography demonstrated moderate MR, likely related to LV remodeling. Recurrent severe MR was noted in all patients at 2 to 5 months after the repair. All patients underwent a successful elective reoperation with MV repair or replacement. This is a proof-of-concept that urgent off-pump NeoChord procedure in unstable patients with acute severe MR may serve as a bridge to conventional surgery.
All patients provided informed written consent for publication of their data. Institutional Review Board approval was not required for this case report.
Patient baseline characteristics and preoperative echocardiographic findings are provided in Table 1. Intraoperative details of transventricular off-pump MV repair, early outcomes, and postoperative complications are summarized in Table 2. An overview of elective reoperations is provided in Table 3.
Table 1.
Patient baseline characteristics
| Demographic data and comorbidities | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age, y, sex | 69, male | 74, female | 54, male |
| Comorbidities | COPD, extracardiac arteriopathy (profunda femoris 50%-70% stenosis) | Hypertension, left femoral osteosynthesis with screws and plates | Hypertension, dyslipidemia, right lung infection; short episode of SVT |
| Onset symptoms | Severe chest pain, shortness of breath | Acute onset of severe epigastrial pain, dyspnea | Chest discomfort |
| Cardiogenic shock | Yes | Yes | Yes |
| Pulmonary edema | Yes | Yes | Yes |
| Time from onset of symptoms to admission/PCI, h | 24 | 6 | 12 |
| Time from admission/PCI to shock, h | 1.5 | 0.5 | 12 |
| Time from admission/PCI to operation, h | 15.5 | 34 | 34.5 |
| Time from echocardiography to operation, h | 1.5 | 22 | 18 |
| Acute MI location | Inferior and RV STEMI | Inferior and RV STEMI | Inferior STEMI |
| Culprit vessel | RCA | RCA | RCx (OM3) |
| Coronary dominance | Right | Right | Left |
| Coronary lesions | RCA, s2, 100% | RCA, s2 50%; s3, 100%; LMS, 30%; proximal LAD, 30%; s7, 50%; s8, 50%; RCx, s14, 50% | LAD s6, 50%; s9, 50%; intermediate, 75%; RCx, s12,100% |
| Primary angioplasty | 2× DES to RCA | 2× DES to RCA | PCI: OM3 (not stented) |
| Post-PCI antiplatelet therapy | Clopidogrel 600 mg, aspirin 300 mg | Clopidogrel 600 mg, aspirin 300 mg | Ticagrelol 90 mg twice daily, aspirin 100 mg |
| Inotropes, dose, μg/kg/min | Norepinephrine, 0.1 | Norepinephrine, 0.1 | Norepinephrine, 0.2 |
| Preoperative IABP | Yes | Yes | Yes |
| Intubation/ventilation | Yes, on admission | No | Yes |
| LV assist device required | No | No | No |
| EuroSCORE II, % | 16.03 | 16.68 | 7.81 |
| STS predictive risk of mortality, % | 14.77 | 18.24 | 9.80 |
| Preoperative laboratory results | |||
| Peak troponin I, ng/L | 1909 | 9800→16,963 | 4229→5867→8299 |
| BNP, ng/L | N/A | 766 | 473 |
| Creatinine, μmol/L | 146 | 76 | 153 |
| Echocardiographic findings | |||
| LVEF, % | 55% | 55% | 50% |
| LVEDD, mm | 55 | 50 | 55 |
| LA dimensions, mm | 65 × 60 | 49 × 46 | 80 × 56 |
| RA dimensions, mm | 56 × 55 | 41 × 39 | 53 × 40 |
| MR grade | Severe, eccentric | Severe, eccentric | Severe, eccentric |
| MR mechanism | A2 prolapse due to thinning and elongation of anterior head of PMPM; P2 restriction; MV annulus not dilated | A3 prolapse due to ruptured anterior head of PMPM | P3 prolapse due to ruptured posterior head of PMPM |
| Papillary muscle involved | PMPM: anterior head elongated, thinned, not ruptured | PMPM: ruptured anterior head | PMPM: ruptured posterior head |
| TR grade | Moderate | Local | Mild-moderate |
| PAP, mm Hg | 54 | n/a | 45 |
BNP, Brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; DES, drug-eluting stent; IABP, intra-aortic balloon pump; LA, left atrium; LAD, left anterior descending coronary artery; LMS, left main stem; LV, left ventricle; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; MI, myocardial infarction; MV, mitral valve; MR, mitral regurgitation; OM, obtuse marginal coronary branch; PAP, pulmonary artery pressure; PCI, percutaneous coronary intervention; PMPM, posteromedial papillary muscle; RA, right atrium; RCA, right coronary artery; RCx, ramus circumflex coronary artery; RV, right ventricle; STS, Society of Thoracic Surgeons; STEMI, ST elevation myocardial infarction; SVT, supraventricular tachycardia; TR, tricuspid regurgitation; N/A, not applicable.
Table 2.
Transventricular off-pump mitral valve repair: Intraoperative details and postoperative outcomes
| Parameter | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Duration of operation, min | 110 | 110 | 120 |
| Number of artificial chords implanted | 3: 1× to A2; 1× to A3; 1× to subvalvar apparatus at the projection of A3 | 3: 1× to A2; 1× to A3; 1× to subvalvar apparatus at the projection of A3 | 2: 1× to P3; 1× to P3 chordae with ruptured papillary head |
| Intraoperative outcome | Mild MR | Mild MR | Trivial MR |
| Intraoperative blood loss, mL | 700 | 600 | 300 |
| Intraoperative inotropes, max dose, μg/kg/min | Norepinephrine, 0.15 | Norepinephrine, 0.12 | Norepinephrine, 0.25 |
| Intraoperative blood transfusions | 444 mL of platelets (3 units) | 410 mL of platelets (3 units), 546 mL of red blood cells (2 units) | None |
| Postoperative ventilation duration, h | 151 | 10 | 22 |
| Postoperative inotropic requirement, h | Norepinephrine, 216; dobutamine, 336 | Norepinephrine, 19; dobutamine, 27 | Norepinephrine, 24 |
| Postoperative 24-h bleeding, mL | 250 | 150 | 200 |
| Postoperative ICU stay, d | 13 | 7 | 3 |
| Postoperative in-hospital stay, d | 30 | 30 | 13 |
| Postoperative complications | COPD exacerbation, severe bronchial obstruction, prolonged ventilation, Candida albicans lung infection, paroxysmal AF, acute liver failure, sepsis, left posterior tibial artery thrombosis post-IABP (treated conservatively) | AF, pulmonary hypertension (sildenafil), anemia requiring blood transfusion, acute renal failure, urinary tract infection, right lung infection | Postprocedure apical MI, paroxysmal AF, right lung infection |
| Surgical outcomes | |||
| Intraoperative result | Mild MR | Mild MR | Trivial MR |
| MR at discharge | Moderate MR, moderate TR, dilated RV, pulmonary hypertension, LVEF 35% | Moderate MR caused by multiple jets around the ruptured PMPM muscle head entrapped at the leaflet coaptation line | Moderate MR |
| MR at 1-mo follow-up | Moderate MR, moderate TR, LVEF 35%; sinus rhythm | Moderate MR | Severe MR, LVEF 40% |
| MR at 5 mo | Severe recurrent MR, atrial flutter | At 4 mo: severe MR | N/A |
AF, Atrial fibrillation; COPD, chronic obstructive pulmonary disease; IABP, intra-aortic balloon pump; ICU, intensive care unit; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; PAP, pulmonary artery pressure; PMPM, posteromedial papillary muscle; RV, right ventricle; TR, tricuspid regurgitation; N/A, not applicable.
Table 3.
Reoperation details: Intraoperative findings and postoperative outcomes
| Parameter | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Time from transventricular MV repair to recurrent severe MR (4+) confirmed by echocardiography, mo | 5 | 4 | 2 |
| Preoperative echocardiography findings | Severe recurrent MR; moderate TR; dilated RV with severe systolic dysfunction; severe pulmonary hypertension (PAP 70 mm Hg); tiny ruptured native chord seen on the free edge of the A2 segment; restricted posterior leaflet; 3 eccentric, posteriorly directed regurgitant jets between the A2/A3 and P2/P3 segments. LVEF 40%-45% | Severe recurrent MR caused by the tip of the ruptured papillary muscle entrapped at the MV leaflet coaptation line at systole; no TR; LVEF 40% | Severe recurrent MR due to posterior leaflet prolapse; mild TR; pulmonary hypertension (PAP, 55 mm Hg); LVEF 40% |
| Redo surgery risk of mortality | |||
| EuroSCORE II, % | 13.04 | 8.3 | 4.49 |
| STS score, % | 6.44 | 5.17 | 1.21 |
| Reoperation | MVR (SJM 29-mm tissue valve), TV bicuspidization; IABP insertion | MVR (St Jude 31 mm tissue valve) with posterior leaflet preservation | MV repair (SJM Sequini 30- mm annuloplasty ring) and neochord to P2; suture closure of fissure between P2/P3; TV bicuspidization; LIMA to LAD graft; LV aneurysmectomy |
| Intraoperative findings | Ruptured native A2 chord; ruptured anterior head of posteromedial papillary muscle | A2/A3 prolapse, ruptured artificial chord, which was inserted to the subvalvar apparatus. The other 2 neochords secured at the leaflet edge appeared intact | Deep fissure between P2/P3, the tip of ruptured papillary muscle is tracked toward P2; many ruptured chords at P2; dilated TV annulus |
| Perioperative support (max inotropic dose, μg/kg/min) | IABP+; norepinephrine, 0.5; dobutamine, 10; milrinone, 0.1; adrenalin, 0.2; sildenafil | IABP–; norepinephrine, 0.05; dobutamine, 5 | IABP–; norepinephrine, 0.1; dobutamine, 5 |
| Postoperative complications | Paroxysmal AF, infection | Permanent pacemaker implantation for sick sinus syndrome | Atrial flutter, cardioversion |
| Postoperative 24-h drainage, mL | 100 | 250 | 200 |
| ICU stay, d | 9 | 2 | 6 |
| Postoperative in-hospital stay, d | 22 | 19 | 21 |
| Predischarge echocardiography | Normal MV prosthesis function, mild TR, LVEF 45% | Normal MV prosthesis function, mild TR, LVEF 30% | Competent MV and TV, LVEF 40% |
| Follow-up echocardiography | At 4 mo: normal MV prosthesis function (peak MVG, 12.8 mm Hg; Vmax, 1.79 m/s); local TR; enlarged atria and RV; LVEF ∼35% | At 1 mo: normal MV prosthetic function (peak MVG, 14.6 mm Hg; Vmax, 1.91 m/s); no TR; LVEF 35% | At 3 wk postoperatively: competent valves, LVEF 40% |
AF, Atrial fibrillation; IABP, intra-aortic balloon pump; ICU, intensive care unit; LA, left atrium; LAD, left anterior descending coronary artery; LIMA, left internal mammary artery; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MV, mitral valve; MR, mitral regurgitation; MR4+, severe mitral regurgitation; MVG, mitral valve gradient; MVR, mitral valve replacement; PAP, pulmonary artery pressure; RA, right atrium; RV, right ventricle; SJM, St Jude Medical; STS, Society of Thoracic Surgeons; TR, tricuspid regurgitation; TV, tricuspid valve; Vmax, maximum jet velocity (m/s).
Case Descriptions
Patient 1
This 69-year-old male presented to our tertiary care center with a 24-hour history of severe chest pain and shortness of breath. On admission, he was in cardiogenic shock and had pulmonary edema, necessitating prompt intubation due to rapidly progressing respiratory failure. Norepinephrine infusion was started at a rate of 0.1 μg/kg/min. Electrocardiography (ECG) revealed an inferior and right ventricular ST elevation myocardial infarction (STEMI) with a peak troponin I level of 1909 ng/L.
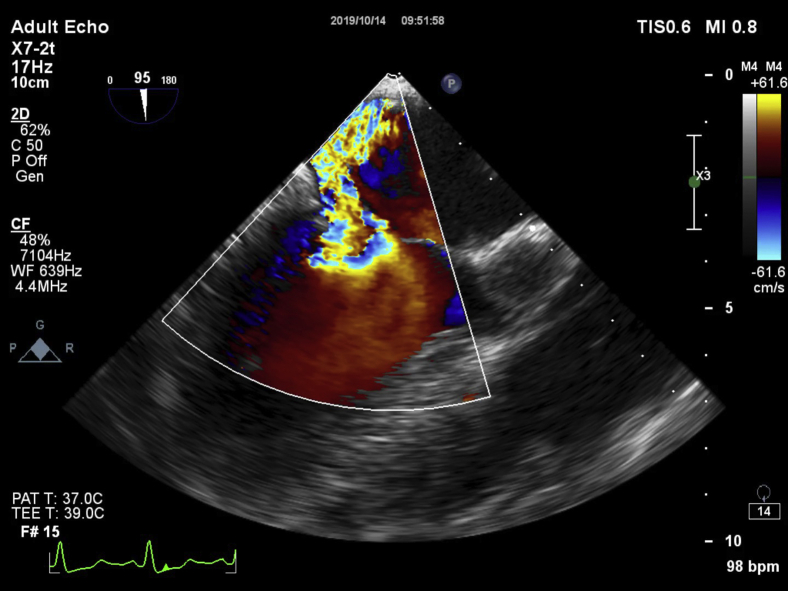
The patient was rushed to the catheterization laboratory, where an acutely occluded proximal RCA was successfully reopened. An intra-aortic balloon pump (IABP) was placed to improve hemodynamics, and dual antiplatelet therapy was initiated. TEE demonstrated severe eccentric MR due to A2 prolapse and restriction of the P2 segment. The A2 prolapse originated from the postinfarction thinning (3 mm) and elongation of the posteromedial papillary muscle (Figure 2). No PMR was observed. The pulmonary artery pressure was 54 mm Hg.
Figure 2.
Intraoperative 2- and 3-dimensional transesophageal echocardiograms demonstrating grade 4+ mitral valve (MV) regurgitation and immediate reduction to grade 1+ after the transventricular off-pump MV repair. Preoperatively, the posteromedial papillary muscle (white arrow) was hyperechogenic, elongated, and dysfunctional as a result of inferior ST elevation myocardial infarction. Because of relatively “loose” native chords, the leaflet coaptation was lost (arrowhead), and an eccentric regurgitant jet was noted. A cropped 3-dimensional view of the MV from the left atrial aspect demonstrated a significant prolapse of the A2 segment of the anterior MV leaflet (yellow arrow). Three artificial neochords were implanted to repair the prolapsing leaflet and stabilize the ischemic papillary muscle. An overall mitral regurgitation reduction to grade 1+ was achieved.
Given the patient's critical state, with a high risk of bleeding and mortality (EuroSCORE II, 16.03%; Society of Thoracic Surgeons [STS] risk score, 14.77%), a minimally invasive transventricular off-pump MV repair was offered. The procedure was performed the following morning. Three artificial neochords were implanted: one to A2, one to A3, and one to the subvalvar apparatus at the projection of the A3 segment. All neochords were secured at the external LV wall with an overall MR reduction to mild (Figure 2).
Postoperatively, the patient required prolonged inotropic support due to refractory cardiogenic shock. After removal of the IABP, he experienced exacerbation of chronic obstructive pulmonary disease, paroxysmal atrial fibrillation (AF), acute liver failure, sepsis, and left posterior tibial artery thrombosis, which resolved with conservative treatment. He had a 2-week recovery in the intensive care unit and gradually improved over the subsequent 2 weeks. He was discharged in stable condition at 1 month after the procedure. Discharge echocardiography demonstrated moderate MR, moderate tricuspid regurgitation (TR), and an LV ejection fraction (LVEF) of 35%.
At 5 months after the transventricular MV repair, the patient was readmitted in congestive heart failure with shortness of breath at rest. TEE revealed a ruptured native A2 chord and 3 eccentric posteriorly directed regurgitant jets between the A2/A3 and P2/P3 segments, resulting in an overall severe recurrent MR. Moderate TR and moderate LV impairment were noted. He underwent MV replacement with a St Jude 29-mm tissue valve and tricuspid bicuspidization. The anterior MV leaflet was excised, and the posterior leaflet was preserved. His postoperative course was compromised with paroxysmal AF and infection.
At 4 months after reoperation, the patient had improved remarkably. His echocardiogram demonstrated and LVEF of 35%, dilated atria, a well-functioning MV prosthesis, and trace TR.
Patient 2
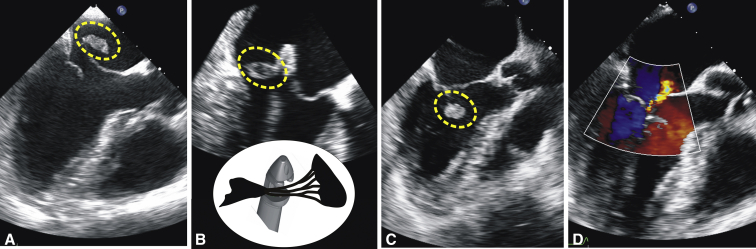
This 74-year-old female suffered an acute onset of severe epigastric pain and marked dyspnea at 2 weeks after undergoing left femoral osteosynthesis as an inpatient at the regional hospital. Her ECG demonstrated an inferior STEMI, and her initial troponin I level was 9800 ng/L. She was started on dual antiplatelet therapy and was urgently transferred to our tertiary center. On admission, she had severe chest pain and pulmonary edema and was in cardiogenic shock. She was immediately started on intravenous norepinephrine at 0.1 to 0.15 μg/kg/min. Coronary angiography revealed an acute occlusion of the culprit RCA, which was successfully reopened and stented. Echocardiography demonstrated good LV systolic function in the presence of a very severe (4+) eccentric MR due to A3 prolapse secondary to post-MI PMR. She was deemed suitable for an urgent minimally invasive transventricular off-pump MV repair with a high periprocedural mortality risk (EuroSCORE II, 16.68%; STS risk score, 18.24%). The procedure was performed the next morning. Intraoperative TEE confirmed a ruptured head of the posteromedial papillary muscle protruding into the left atrium at systole and resulting in a medial prolapse of the anterior mitral leaflet (A3 segment). Three artificial chordae were implanted: one onto the free edge of the A2 segment, one to A3, and one to the subvalvar apparatus at the projection of the A3. An immediate reduction of MR to minimal was confirmed (Figure 3).
Figure 3.
Intraoperative transesophageal echocardiography, long-axis views. A, Preoperatively, the ruptured tip (traced by the oval contour) of the papillary muscle is seen flailing into the left atrium together with a large portion of the anterior mitral valve (MV) leaflet, causing severe mitral regurgitation. B, The NeoChord DS1000 device captured the bundle of native chords together with the ruptured portion of the papillary muscle during the procedure; a schematic view of this moment is provided. C, The position of the ruptured papillary muscle after neochord tensioning and fixation at the apex resulted in a good coaptation of the MV leaflets. D, Only a small residual mitral regurgitant jet was noted on color Doppler after the procedure.
Predischarge TEE demonstrated multiple eccentric regurgitant jets around the papillary muscle head entrapped at the leaflet coaptation line at systole, resulting in moderate MR. The patient was discharged at 30 days after the procedure. Although mobilizing with crutches, she was no longer limited by dyspnea.
At 4 months postoperatively, the patient complained of rapid fatigue and shortness of breath on minimal exertion. Echocardiography demonstrated recurrent severe MR caused by the tip of the ruptured papillary muscle entrapped at the MV leaflet coaptation line at systole. She underwent MV replacement with a St Jude 31-mm tissue valve. She had an intensive care unit stay of 2 days and was discharged at 3 weeks after the reoperation. Her postoperative course was complicated by sick sinus syndrome, and a pacemaker was implanted. At 4 months after MV replacement, she was doing remarkably well, limited only by mild dyspnea on exertion.
This case report is supplemented with echocardiographic images in an associated video (Video 1).
Video 1.
This video with echocardiographic images provides insight into these case reports of transventricular off-pump mitral valve repair in a setting of acute mitral regurgitation due to ischemic papillary muscle rupture. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00678-7/fulltext.
Patient 3
This 54-year-old male patient was brought in with a 12-hour history of chest discomfort and an acute inferior STEMI. On admission, he was lethargic and developed cardiogenic shock and pulmonary edema. His medical history was obtained from family members. Admission ECG showed non-specific ST/T changes, and his troponin I level was 4229 ng/L. Coronary angiography demonstrated left coronary arterial dominance and an acutely occluded distal OM3 branch, which was successfully reopened. No stenting was attempted due to the small caliber of the vessel.
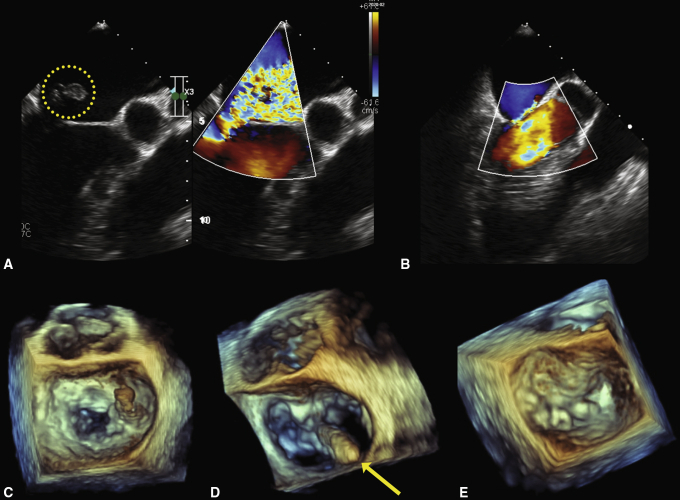
The patient was started on ticagrelor. Owing to rapidly progressing respiratory failure, he was intubated and ventilated. Large amounts of purulent bronchial discharge were aspirated. An episode of supraventricular tachycardia at 190 bpm led to severe cardiogenic shock. He was started on intravenous norepinephrine at 0.2 μg/kg/min and intravenous amiodarone. Furosemide infusion was required for developing acute renal failure. A chest X-ray demonstrated marked pulmonary edema, right pleural effusion, and right basal infiltration. TEE revealed severe MR secondary to the ruptured posterior head of the posteromedial papillary muscle (Figure 4). Mild LV systolic impairment was noted.
Figure 4.
Cropped views of intraoperative 2- and 3-dimensional transesophageal echocardiograms demonstrating the outcome of transventricular mitral valve (MV) repair. The ruptured posterior head of the posteromedial papillary muscle (A, circled contour) together with the P3 segment (C) protruding into the left atrium at systole caused a very severe anteriorly directed mitral regurgitation (MR). D, The tip of the NeoChord DS 1000 device (yellow arrow) crossing the MV plane, as seen from the left atrial aspect. B and E, Two artificial neochords were implanted and reduced the grade of MR to trivial.
The patient was scheduled for minimally invasive transventricular off-pump MV repair the next day, with an estimated EuroSCORE II of 7.79% and STS risk score of 9.8%. Two artificial neochords were inserted; their length was adjusted to achieve optimal repair, and they were then secured at the external LV wall.
The morning after the procedure, a rise in troponin I level to 99,432 ng/L was noted, and an ST elevation of 2 to 3 mm in leads I and V2 to V4 was seen on ECG. A repeat coronary angiogram revealed kinking of the distal left anterior descending artery (LAD). During the procedure, it became clear that the site of transventricular access had been chosen inappropriately (too anterior and too close to the LAD), and closure of the entry site caused cockling of epicardial tissues and consequent kinking of the LAD. The LAD blood flow was restored by stenting. Echocardiography revealed minimal MR, normal LV systolic function, and impaired contraction of the LV apex with no significant hemodynamic impact. Because of abundant tracheal discharge, extubation was postponed to postoperative day 2. Further recovery was complicated with pneumonia and AF. Moderate MR with good overall LV systolic function were noted at discharge.
The patient became symptomatic at 2 months after the transventricular off-pump MV repair. Repeat coronary angiogram demonstrated no restenosis. Ventriculography revealed a dilated LV with an apical aneurysm and severe MR. He underwent a redo MV repair with a St Jude Medical Sequini 30-mm annuloplasty ring and neochordae to P2, fissure closure between P2 and P3, tricuspid valve bicuspidization, a left internal mammary artery–to–LAD graft, and LV aneurysmectomy. Postoperatively, the MV and tricuspid valve were competent.
Discussion
PMR is a rare and potentially fatal complication of MI that carries a high mortality rate due to the development of acute life-threatening cardiogenic shock, leading to acute pulmonary edema and subsequent multiorgan dysfunction and resulting in rapid deterioration and death within hours of diagnosis. Nearly 80% of PMRs occur within 7 days of an MI and can be diagnosed during the index hospitalization10; however, some patients can present in a delayed fashion.
Acute MR may develop as a result of papillary muscle dysfunction or PMR. Acute ischemic MR without PMR may lead to severe MR due to leaflet tethering from a sudden onset of regional or global LV dysfunction.13 Data are sparse regarding treatment options for patients with post-MI PMR, who often are not included in registries or randomized trials. Moreover, successful outcomes are much more likely to be reported than failures.
Until recently, cardiac surgery was the only option available for treating this condition. In a recent review of all published series, the pooled 30-day mortality was 19%, with some showing a mortality of approximately 39%.14
PMR can be addressed with either MV repair in selected patients or MV replacement.2 MV repair may be extremely challenging because of fragile necrotic tissue and uncertainties related to LV wall remodeling after acute MI.15 Chordal transfer may be an option in these patients, but long-term data in the setting of PMR are lacking. Conventional MV repair may provide a distinct advantage in these patients, with freedom from endocarditis and anticoagulation in patients with partial PMR.16, 17, 18
Tavakoli and colleagues15 have suggested that acute PMR is better managed by MV replacement with preservation of chordae tendineae of the posterior leaflet. Sultan and colleagues2 reviewed 24 patients with acute MR due to PMR who underwent emergency MV surgery with an overall STS predicted risk of mortality of 17.5%. In their cohort, 71% of the patients underwent MV replacement and 29% had MV repair; 54.2% underwent concomitant coronary artery bypass grafting. MV repair was attempted in patients with a partial PMR, in which the head of the papillary muscle was not completely detached from the body of the muscle. Repair was performed using chordal translocation and/or placement of artificial chords. The authors reported an operative mortality of 12.5% and estimated 3-year freedom from mortality, reoperation, and readmission of 78.9%, 95%, and 54.6%, respectively.2
Bouma and colleagues16 presented 9 patients who underwent MV repair for partial PMR and reported a 90% repair rate and a 5-year survival rate of 83%. All patients had a partial PMR and were not in cardiogenic shock at the time of surgery.
In this era of evolving minimally invasive surgical techniques, several novel approaches to correcting acute ischemic MR have been proposed. Transcatheter MitraClip implantation (Abbott Vascular, Santa Clara, Calif) has been suggested by professional societies as an alternative treatment option for patients with significant symptomatic MR and prohibitive surgical risk,19 and its use in correcting acute ischemic MR has been reported.20 One of the largest studies of acute post-MI MR treated with the MitraClip in Europe has been reported by Estévez-Loureiro and colleagues,21 also known as the EREMMI group (European Registry of MitraClip in Acute Mitral Regurgitation following Acute Myocardial Infarction). The authors presented data on a prospective registry of 44 consecutive high-risk patients (median EuroSCORE II, 15.1%) with severe MR that developed early after an acute transmural MI, but with intact papillary muscles. All patients underwent percutaneous MV repair at 11 highly experienced centers across Europe. The median time from MI to treatment was 18 days (range, 13-36.8 days), and that from diagnosis of MR to treatment was 12.5 days (range, 4.5-18 days). Technical success was obtained in 86.6% of cases, with a median of 2 clips per case. The median length of stay after the procedure was 16 days. The EREMMI investigators concluded that edge-to-edge MV repair with the MitraClip device was feasible in selected patients with acute MR after MI.21 In our series, all patients were operated on within 48 hours after onset of initial symptoms.
Data on consecutive patients treated specifically for acute MR due to post-MI PMR are lacking, and the issue remains understudied; however, a literature review yielded several isolated case reports of acute MR due to PMR that were treated successfully with the MitraClip device.22, 23, 24, 25, 26, 27, 28, 29
In a case of acute severe MR secondary to ischemic partial rupture of the posteromedial papillary muscle reported by Valle and colleagues,26 3 MitraClips implanted in a zipper fashion essentially served a dual purpose of MR reduction from 4+ to 1+ and papillary muscle stabilization. The authors argued that whether or not to perform surgical intervention for valvular disease after initial stabilization with a percutaneous approach must be addressed on a patient-to-patient basis until more definitive data exist to guide management.26
Wolff and colleagues23 reported the successful use of the MitraClip procedure for PMR in an extremely high-risk patient with acute MI and cardiogenic shock (STS score, 64%; EuroSCORE II, 75%). This 68-year-old man with a 3-day history of increasing retrosternal discomfort and shortness of breath, in cardiogenic shock and pulmonary edema, had an IABP placed and inotropic support initiated soon after admission. He had sustained posterolateral STEMI due to OM1 occlusion, an LVEF of 25%, and a very severe MR owing to rupture of the anterolateral papillary muscle and flail of the A2 segment. Having performed the procedure within 24 hours after admission, they managed to reduce grade 4+ MR to grade 2+ with 2 clips deployed at the central A2–P2 interface. Although the initial intention was to stabilize the patient in preparation for eventual surgical repair, the patient responded extremely well and did not require further intervention, remaining in New York Heart Association class II for at least 6 months postoperatively.
Previous studies have shown that concomitant coronary artery bypass grafting has short- and long-term benefits for survival in patients with acute ischemic MR and the need for coronary revascularization.30,31 Chevalier and colleagues30 reported their experience in 37 patients who underwent MV surgery for ischemic PMR. Twenty-five of these patients had a complete PMR. Operative mortality was significantly reduced when concomitant coronary revascularization was performed (9% vs 34%). In this respect, transventricular off-pump MV repair has access limitations if concomitant coronary revascularization is required.
To the best of our knowledge, the NeoChord MV repair technique has not yet been attempted or reported in the setting of acute MR secondary to post-MI PMR. This case series represents the first-in-human transventricular off-pump MV repair in high-risk patients with acute ischemic MR. Our patients had relatively well-preserved LV systolic function, and thus a reduction in MR severity had a good chance of changing their clinical course and stabilizing hemodynamics. In our opinion, these patients otherwise might have died due to clinical instability and the detrimental effects of progressive cardiogenic shock and multiorgan failure. The transventricular off-pump MV repair made it possible to avoid the perils of initiating cardiopulmonary bypass in such unstable patients, as well as to mitigate the risk of bleeding in these patients with post–percutaneous coronary intervention (PCI) antiplatelet therapy. Moreover, it provided a bridge to a subsequent elective MV surgery. Patients who develop multiorgan failure and approach the need for intubation are in danger of further rapid deterioration. A strategy of stabilization with extracorporeal membrane oxygenation might have been tried, but we felt that treating the primary cause of the patients’ deterioration had the best chance of success.
Nevertheless, transventricular off-pump MV repair with artificial chords may offer several potential advantages over conventional valve surgery for this condition. First, following successful repair of MR, rapidly decreased LV, left atrial, and pulmonary artery pressures and increased cardiac output may lead to rapid recovery, similar to what has been observed in studies with the MitraClip.32 Such effects may rescue the patient from disastrous sequelae of cardiogenic shock and respiratory insufficiency. Second, a considerable benefit is avoidance of the LV damage induced by the systemic inflammatory response, free radical injury, and myocardial oxidative stress associated with cardiopulmonary bypass.33 Third, patients on antiplatelet therapy post-PCI are at increased risk of bleeding, and thus a minimally invasive alternative is appealing in an acute clinical setting.
Although the risk of MR recurrence remains, transventricular beating-heart MV repair may offer hemodynamic stabilization to help the patient survive life-threatening acute cardiogenic shock. Recurring severe MR may be corrected via a conventional approach at a later stage once the patient becomes a stable surgical candidate with lower operative risk. However, for these patients, transventricular off-pump MV repair should be performed only by highly experienced surgeons in a dedicated cardiac center.
This report serves as a proof-of-concept of the feasibility of treating patients with acute MR due to ischemic PMR by expanding the applicability of the transventricular beating-heart MV repair. This case series is limited by its retrospective descriptive design. The sample size of patients was small, as in other reports in the literature addressing PMR.
Conclusions
Off-pump transventricular MV repair may offer a safe and feasible alternative for stabilizing high-risk patients with acute MR due to post-MI PMR. Although early MR recurrence is concerning, urgent transventricular MV repair may serve as a bridge to conventional surgery in such unstable patients.
Conflict of Interest Statement
Drs Zakarkaitė and Aidietis have served as proctors for Neochord Inc. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Accepted for the 100th Annual Meeting of The American Association for Thoracic Surgery.
Supplementary Data
This video with echocardiographic images provides insight into these case reports of transventricular off-pump mitral valve repair in a setting of acute mitral regurgitation due to ischemic papillary muscle rupture. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00678-7/fulltext.
References
- 1.Braunwald E. In: Heart Disease: A Textbook of Cardiovascular Medicine. 4th ed. Braunwald E., editor. WB Saunders; Philadelphia: 1992. Valvular heart disease. [Google Scholar]
- 2.Sultan I., Aranda-Michel E., Gleason T.G., Navid F., Kilic A. Mitral valve surgery for acute papillary muscle rupture. J Card Surg. 2018;33:484–488. doi: 10.1111/jocs.13773. [DOI] [PubMed] [Google Scholar]
- 3.Wei J.Y., Hutchins G.M., Bulkley B.H. Papillary muscle rupture in fatal acute myocardial infarction: a potentially treatable form of cardiogenic shock. Ann Intern Med. 1979;90:149–152. doi: 10.7326/0003-4819-90-2-149. [DOI] [PubMed] [Google Scholar]
- 4.Austen W.G., Sokol D.M., DeSanctis R.W., Sanders C.A. Surgical treatment of papillary-muscle rupture complicating myocardial infarction. N Engl J Med. 1968;278:1137–1141. doi: 10.1056/NEJM196805232782102. [DOI] [PubMed] [Google Scholar]
- 5.Figueras J., Calvo F., Cortadellas J., Soler-Soler J. Comparison of patients with and without papillary muscle rupture during acute myocardial infarction. Am J Cardiol. 1997;80:625–627. doi: 10.1016/s0002-9149(97)00435-9. [DOI] [PubMed] [Google Scholar]
- 6.DiSesa V.J., Cohn L.H., Collins J.J., Jr., Koster J.K., Jr., VanDevanter S. Determinants of operative survival following combined mitral valve replacement and coronary revascularization. Ann Thorac Surg. 1982;34:482–489. doi: 10.1016/s0003-4975(10)62992-x. [DOI] [PubMed] [Google Scholar]
- 7.Czer L.S., Gray R.J., DeRobertis M.A., Bateman T.M., Stewart M.E., Chaux A., et al. Mitral valve replacement: impact of coronary artery disease and determinants of prognosis after revascularization. Circulation. 1984;70(3 Pt 2):I198–I207. [PubMed] [Google Scholar]
- 8.Schroeter T., Lehmann S., Misfeld M., Borger M., Subramanian S., Mohr F.W., et al. Clinical outcome after mitral valve surgery due to ischemic papillary muscle rupture. Ann Thorac Surg. 2013;95:820–824. doi: 10.1016/j.athoracsur.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura R.A., Schaff H.V., Shub C., Gersh B.J., Edwards W.D., Tajik A.J. Papillary muscle rupture complicating acute myocardial infarction: analysis of 17 patients. Am J Cardiol. 1983;51:373–377. doi: 10.1016/s0002-9149(83)80067-8. [DOI] [PubMed] [Google Scholar]
- 10.Thompson C.R., Buller C.E., Sleeper L.A., Antonelli T.A., Webb J.G., Jaber W.A., et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK trial registry. SHould we use emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol. 2000;36(Suppl A):1104–1109. doi: 10.1016/s0735-1097(00)00846-9. [DOI] [PubMed] [Google Scholar]
- 11.Sanders R.J., Neubuerger K.T., Ravin A. Rupture of papillary muscles: occurrence of rupture of the posterior muscle in posterior myocardial infarction. Dis Chest. 1957;31:316–323. doi: 10.1378/chest.31.3.316. [DOI] [PubMed] [Google Scholar]
- 12.Rucinskas K., Janusauskas V., Zakarkaite D., Aidietiene S., Samalavicius R., Speziali G., et al. Off-pump transapical implantation of artificial chordae to correct mitral regurgitation: early results of a single-center experience. J Thorac Cardiovasc Surg. 2014;147:95–99. doi: 10.1016/j.jtcvs.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Sannino A., Grayburn P.A. Ischemic mitral regurgitation after acute myocardial infarction in the percutaneous coronary intervention era. Circ Cardiovasc Imaging. 2016;9:e005323. doi: 10.1161/CIRCIMAGING.116.005323. [DOI] [PubMed] [Google Scholar]
- 14.Alajaji W.A., Akl E.A., Farha A., Jaber W.A., AlJaroudi W.A. Surgical versus medical management of patients with acute ischemic mitral regurgitation: a systematic review. BMC Res Notes. 2015;8:712. doi: 10.1186/s13104-015-1704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavakoli R., Weber A., Brunner-La Rocca H., Bettex D., Vogt P., Pretre R., et al. Results of surgery for irreversible moderate to severe mitral valve regurgitation secondary to myocardial infarction. Eur J Cardiothorac Surg. 2002;21:818–824. doi: 10.1016/s1010-7940(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 16.Bouma W., Wijdh-den Hamer I.J., Klinkenberg T.J., Kuijpers M., Bijleveld A., van der Horst I.C.C., et al. Mitral valve repair for post-myocardial infarction papillary muscle rupture. Eur J Cardiothorac Surg. 2013;44:1063–1069. doi: 10.1093/ejcts/ezt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo A., Suri R.M., Grigioni F., Roger V.L., Oh J.K., Mahoney D.W., et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation. 2008;118:1528–1534. doi: 10.1161/CIRCULATIONAHA.107.747949. [DOI] [PubMed] [Google Scholar]
- 18.Kilic A., Shah A.S., Conte J.V., Baumgartner W.A., Yuh D.D. Operative outcomes in mitral valve surgery: combined effect of surgeon and hospital volume in a population-based analysis. J Thorac Cardiovasc Surg. 2013;146:638–646. doi: 10.1016/j.jtcvs.2012.07.070. [DOI] [PubMed] [Google Scholar]
- 19.O'Gara P.T., Calhoon J.H., Moon M.R., Tommaso C.L. Transcatheter therapies for mitral regurgitation: a professional society overview from the American College of Cardiology, The American Association for Thoracic Surgery, Society for Cardiovascular Angiography and Interventions Foundation, and The Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2014;147:837–849. doi: 10.1016/j.jtcvs.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Estévez-Loureiro R., Arzamendi D., Freixa X., Cardenal R., Carrasco-Chinchilla F., Serrador-Frutos A., et al. Percutaneous mitral valve repair for acute mitral regurgitation after an acute myocardial infarction. J Am Coll Cardiol. 2015;66:91–92. doi: 10.1016/j.jacc.2015.03.597. [DOI] [PubMed] [Google Scholar]
- 21.Estevez-Loureiro R., Adamo M., Arzamendi D., Denti P., Freixa X., Nombela-Franco L., et al. Transcatheter mitral valve repair in patients with acute myocardial infarction: insights from the European Registry of MitraClip in Acute Mitral Regurgitation following an acute myocardial infarction (EREMMI) EuroIntervention. 2020;15:1248–1250. doi: 10.4244/EIJ-D-19-00653. [DOI] [PubMed] [Google Scholar]
- 22.Bilge M., Alemdar R., Yasar A.S. Successful percutaneous mitral valve repair with the MitraClip system of acute mitral regurgitation due to papillary muscle rupture as complication of acute myocardial infarction. Catheter Cardiovasc Interv. 2014;83:E137–E140. doi: 10.1002/ccd.24960. [DOI] [PubMed] [Google Scholar]
- 23.Wolff R., Cohen G., Peterson C., Wong S., Hockman E., Lo J., et al. MitraClip for papillary muscle rupture in patient with cardiogenic shock. Can J Cardiol. 2014;30:1461.e13–1461.e14. doi: 10.1016/j.cjca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Bahlmann E., Frerker C., Kreidel F., Thielsen T., Ghanem A., van der Schalk H., et al. MitraClip implantation after acute ischemic papillary muscle rupture in a patient with prolonged cardiogenic shock. Ann Thorac Surg. 2015;99:e41–e42. doi: 10.1016/j.athoracsur.2014.09.075. [DOI] [PubMed] [Google Scholar]
- 25.Horstkotte J.C., Horstkotte M., Beucher H., Felderhoff T., Boekstegers P. Percutaneous mitral valve repair as rescue procedure after post–myocardial infarction papillary muscle rupture and acute cardiogenic shock. Clin Res Cardiol. 2015;104:275–278. doi: 10.1007/s00392-014-0789-9. [DOI] [PubMed] [Google Scholar]
- 26.Valle J.A., Miyasaka R.L., Carroll J.D. Acute mitral regurgitation secondary to papillary muscle tear: is transcatheter edge-to-edge mitral valve repair a new paradigm? Circ Cardiovasc Interv. 2017;10:e005050. doi: 10.1161/CIRCINTERVENTIONS.117.005050. [DOI] [PubMed] [Google Scholar]
- 27.Yasin M., Nanjundappa A., Annie F.H., Tager A., Farooq A., Bhagat A., et al. Use of MitraClip for postmyocardial infarction mitral regurgitation secondary to papillary muscle dysfunction. Cureus. 2018;10:e3065. doi: 10.7759/cureus.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komatsu I., Cohen E.A., Cohen G.N., Czarnecki A. Transcatheter mitral valve edge-to-edge repair with the new MitraClip XTR system for acute mitral regurgitation caused by papillary muscle rupture. Can J Cardiol. 2019;35:1604.e5–1604.e7. doi: 10.1016/j.cjca.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos K., Chrissoheris M., Nikolaou I., Spargias K. Edge-to-edge mitral valve repair for acute mitral valve regurgitation due to papillary muscle rupture: a case report. Eur Heart J Case Rep. 2019;3:ytz001. doi: 10.1093/ehjcr/ytz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier P., Burri H., Fahrat F., Cucherat M., Jegaden O., Obadia J.F., et al. Perioperative outcome and long-term survival of surgery for acute post-infarction mitral regurgitation. Eur J Cardiothorac Surg. 2004;26:330–335. doi: 10.1016/j.ejcts.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Kishon Y., Oh J.K., Schaff H.V., Mullany C.J., Tajik A.J., Gersh B.J. Mitral valve operation in postinfarction rupture of a papillary muscle: immediate results and long-term follow-up of 22 patients. Mayo Clin Proc. 1992;67:1023–1030. doi: 10.1016/s0025-6196(12)61116-1. [DOI] [PubMed] [Google Scholar]
- 32.Siegel R.J., Biner S., Rafique A.M., Rinaldi M., Lim S., Fail P., et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. 2011;57:1658–1665. doi: 10.1016/j.jacc.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 33.van Boven W.J.P., Gerritsen W.B., Driessen A.H., Morshuis W.J., Waanders F.G., Haas F.J., et al. Myocardial oxidative stress, and cell injury comparing three different techniques for coronary artery bypass grafting. Eur J Cardiothorac Surg. 2008;34:969–975. doi: 10.1016/j.ejcts.2008.07.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video with echocardiographic images provides insight into these case reports of transventricular off-pump mitral valve repair in a setting of acute mitral regurgitation due to ischemic papillary muscle rupture. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00678-7/fulltext.