Abstract
Background:
CDK4/6 inhibitors modulate immune response in breast cancer. This phase I/II trial was designed to test the safety and efficacy of palbociclib, pembrolizumab, and letrozole in women with hormone receptor positive (HR+) human epidermal growth factor receptor 2 negative (HER2−) metastatic breast cancer (MBC).
Patients and methods:
Women with stage IV HR+HER2−MBC were enrolled and treated with palbociclib, pembrolizumab, and letrozole. Primary endpoints were safety, tolerability, and efficacy.
Results:
Between November 2016 and July 2020, 23 patients were enrolled with 20 evaluable for response, including 4 patients in cohort 1 and 16 patients in cohort 2. Cohort 1 median age was 48 years (33–70) and cohort 2 median age was 55 (37–75). Cohort 1 closed early due to limited accrual. Grade 3–4 AEs were neutropenia (83%), leukopenia (65%), thrombocytopenia (17%) and elevated liver enzymes (17%). In cohort 1, 50% achieved a partial response and 50% had stable disease. In cohort 2, 31% achieved complete response (CR), 25% had PR, and 31% had SD by RECIST 1.1. Median progression free survival of was 25.2 months (95% CI 5.3, NR) and median overall survival was 36.9 months (95% CI, 36.9, NR) in cohort 2 with a median follow-up of 24.8 months (95% CI 17.1, NR). A correlative immune biomarker analysis was published separately.
Conclusion:
The combination of palbociclib, pembrolizumab, and letrozole is well tolerated, and a complete response rate of 31% was identified in HR+ MBC patients who received this combination as front line therapy. Confirmatory trials are required to better understand the immune-priming effects of CDK4/6 inhibitors.
Keywords: Immune check point inhibitor, CDK 4/6 inhibitor, hormone receptor positive, metastatic breast cancer
INTRODUCTION
CDK 4/6 inhibitors (CDK4/6i) halt cell cycle G1 to S phase transition and have shown promise in overcoming endocrine resistance1,2. The combination of CDK4/6i and aromatase inhibitor (AI) is standard-of-care therapy for patients with HR+ HER2- metastatic breast cancer (MBC). Currently three CDK4/6i were approved by the FDA as front line therapy for hormone receptor positive (HR+) metastatic breast cancer (MBC): palbociclib, ribociclib, and abemaciclib with endocrine therapy1,3,4. In PALOMA-2, palbociclib and letrozole demonstrated progression free survival (PFS) of 24.8 vs. letrozole alone (HR 0.58; 95% CI 0.46–0.72; p<0.001)1. Abemaciclib and ribociclib also demonstrated overall survival (OS) benefit5–7. Although CDK4/6 inhibitors have significantly improved clinical outcome in patients with HR+ MBC, intrinsic or acquired resistance develops over time and tumor progression is inevitable. In PALOMA-2 trial, the objective response rate (RR) was 42% in palbociclib-treated patients and complete response (CR) rate was only 2%1 (filed medical information). In PALOMA-3 study, no CR was observed8. Nearly all patients eventually progress on CDK 4/6i therapy after a prolonged PFS. Multiple studies are in progress to understand the mechanisms of intrinsic or acquired resistance to CDK 4/6i 9–11 and develop novel combination strategies in overcoming resistance12,13.
The immune checkpoint inhibitors (ICIs) atezolizumab and pembrolizumab in combination with chemotherapy were recently granted FDA approval for first-line treatment of metastatic triple negative breast cancer (TNBC)14–16. However, the role of ICIs is not well established in HR+ MBC, which are presumably “immune-cold” with low tumor infiltrating lymphocytes (TILs). Single agent pembrolizumab showed a RR of 12% in PD-L1 pre-selected HR+ MBC (N=25) and no CR was identified17. The combination of CTLA-4 inhibitor tremelimumab and exemestane showed 42% stable disease (SD) at 3 months in heavily pretreated HR+ MBC population18. This modest clinical activity may be explained by the low immunogenicity of hormone receptor positive and human epidermal growth factor receptor 2 negative metastatic breast cancer (HR+ HER2− MBC) compared with TNBC19,20. The modulation of host immune and tumor microenvironment (TME) may be the key to effective immune responses in HR+ HER2- MBC. Recent studies have demonstrated immune modulatory effects of CDK4/6i. Goel et al. showed CDK4/6i promoted anti-tumor immunity by producing interferons that enhanced tumor antigen presentation and suppression of regulatory T cell (Treg) proliferation21. Deng et al. reported that inhibition of CDK4/6 increased tumor infiltration and activation of effector T cells and enhanced the response to PD-1 blockade in a mouse model22. The current phase I/II trial was designed to evaluate the safety and efficacy of the combination of palbociclib, pembrolizumab, and letrozole.
METHODS
Study design:
This study is a single-center, open-label, phase I/II study designed to evaluate the safety and efficacy of palbociclib, pembrolizumab, and letrozole in patients with HR+ MBC (NCT02778685). Cohort 1 enrolled patients who were on letrozole and palbociclib for at least 6 with a primary efficacy endpoint of response rate (RR). Due to slow accrual, the study was re-designed as a Phase II study with a safety lead-in. Secondary objectives included safety and tolerability, PFS, and OS. Exploratory objectives were baseline tumor biopsy and serial peripheral blood sample analyses. This study was conducted with patient written informed consent, and in accordance with Good Clinical Practice standards, Declaration of Helsinki, US Department of Health and Human Services, AND the City of Hope Institutional Review Board.
Patient population:
Eligibility criteria included age ≥18; histologically confirmed HR+HER2− metastatic breast cancer; measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1); no prior therapy (cohort 2) for metastatic disease; adequate organ and bone marrow function. Cohort 1 included patients previously on letrozole and palbociclib for > 6 months and had pembrolizumab added on cycle 1 day 1. Cohort 2 included patients who received up-front treatment with palbociclib, pembrolizumab, and letrozole.
Treatment and evaluation:
Patients received letrozole 2.5 mg daily, palbociclib at a starting dose of 125 mg daily 3 weeks on 1 week off, and pembrolizumab 200 mg iv every 3 weeks. Treatment cycles were defined as every 3 weeks per pembrolizumab cycles. Premenopausal patients received ovarian suppression. The adverse events (AE) were graded by CTCAE version 4.0 and responses were assessed by RECIST 1.1. Treatments were continued until unacceptable toxicity or disease progression. Baseline tumor biopsies were collected for immune biomarker analysis, tumor genomic analysis, and whole transcriptome analysis.
Tumor immune biomarkers:
Percentage of stromal TILs in tumor was evaluated using hematoxylin and eosin (H&E) diagnostic sections per International TIL Work Group guideline23. PD-L1 was determined by immunohistochemistry (IHC) using the PD-L1 IHC 22C3 antibody by QualTek Laboratory.
Genomic sequencing and gene deconvolution:
Genomic analysis was performed using Tempus xT sequencing technology; FoundationOne™ sequencing, or HopeSeq targeted solid tumor panel24. Tumor mutation burden (TMB) was reported as mutations per megabase (Mb) of the genome coding area of DNA. RNAseq was performed by Tempus and STAR (v2.7) was used to align the sequences to human genome24. HT-seq was used to quantify the gene expression from aligned reads. The raw counts were normalized by edgeR. Relative abundance of 18 immune cell types using CIBERSORT analysis of tumor microenvironment immune subsets in responders (CR+PR) vs. non-responders (SD+PD) were determined by Wilcoxon sign-ranked test.
Statistical methods:
Cohort 1 was designed as a safety lead-in using a three-at-risk design. The efficacy endpoint for cohort 1 required 2/18 (11%) responses to be deemed promising for the addition of pembrolizumab to distinguish between a discouraging 3% RR and an encouraging 20% RR (90% power, type I error 10%).
Cohort 2 enrolled patients in a staggered fashion for safety per the same three-at-risk design. For cohort 2, prior reports suggested a response (CR or PR by RECIST version 1.1) due to letrozole and palbociclib of 55% with rare complete responses. As a result, if ≥ 8/16 (50%) responses were observed, the triplet would be considered lacking promise (7.4% type II error if the true response rate was 70%). If 9/16 (56%) to 11/16 (69%) responses were observed, other considerations such as the observation of CRs, and the duration of responses or PFS would be needed, and if 12/16 (75%) responses were observed, the combination would be declared promising. The chance of a 55% RR resulting in 12 responses was less than 9% (type I error). Clinical outcomes including PFS and OS were calculated by Kaplan-Meier and median follow-up was calculated by reverse Kaplan-Meier.
RESULTS
Patient characteristics:
Patients received treatment between November 2016 and July 2020. A total of 23 patients were accrued, including 4 in cohort 1 and 16 in cohort 2, with 3 inevaluable patients (one was TNBC on repeat biopsy, one was lost to follow-up, and one was non-compliant) (Figure S1). Baseline patient characteristics are listed in Table 1. For cohort 1 (N=4), the median age was 48 years (33–70 years); 25% were Hispanic, 25% were non-Hispanic, and 50% were Asian; 50% had ductal carcinoma, 25% had lobular carcinoma, and 25% had adenocarcinoma not otherwise specified (NOS). For cohort 2 (N=16), the median age was 55 (37–75); 38% were Hispanic, 38% were non-Hispanic, and 25% were Asian; 50% had ductal carcinoma, 13% had lobular carcinoma, and 38% had adenocarcinoma (NOS).
Table 1.
Patient baseline characteristics
| Characteristic | Cohort 1 (N=4) | Cohort 2 (N=16) |
|---|---|---|
|
| ||
| Age, median (range) years | 48 (33–70) | 55 (37–75) |
|
| ||
| Race/ethnicity, N (%) | ||
| Hispanic | 1 (25%) | 6 (38%) |
| Non-Hispanic | 1 (25%) | 6 (38%) |
| Asian | 2 (50%) | 4 (25%) |
|
| ||
| ECOG performance status, N (%) | ||
| 0 | 2 (50%) | 11 (69%) |
| 1 | 2 (50%) | 5 (31%) |
|
| ||
| Menopausal status, N (%) | ||
| Pre-menopausal1 | 0 (0%) | 3 (19%) |
| Post-menopausal | 4 (100%) | 13 (81%) |
|
| ||
| Histological grade, N (%) | ||
| 1 | 0 (0%) | 1 (6%) |
| 2 | 2 (50%) | 12 (75%) |
| 3 | 2 (50%) | 3 (19%) |
|
| ||
| Histology Type, N (%) | ||
| Ductal carcinoma | 2 (50%) | 8 (50%) |
| Lobular carcinoma | 1 (25%) | 2 (13%) |
| Adenocarcinoma (NOS) | 1 (25%) | 6 (38%) |
|
| ||
| Tumor stage at initial diagnosis, N (%) | ||
| I | 0 (0%) | 2 (13%) |
| II | 2 (50%) | 6 (38%) |
| III | 0 (0%) | 3 (19%) |
| IV | 2 (50%) | 5 (31%) |
|
| ||
| Prior Palbociclib | ||
| Median (range) months to treatment | 7.4 (6.0, 14.0) | --- |
|
| ||
| Prior surgery, N (%) | ||
| Lumpectomy | 0 (0%) | 6 (38%) |
| Mastectomy | 1 (25%) | 4 (25%) |
| None2 | 3 (75%) | 6 (38%) |
|
| ||
| Prior radiation, N (%) | ||
| Yes | 3 (75%) | 7 (44%) |
| No | 1 (25%) | 9 (56%) |
|
| ||
| Site of metastasis, N (%) | ||
| Bone3 | 3 (75%) | 7 (44%) |
| Lung | 1 (25%) | 8 (50%) |
| Liver | 1 (25%) | 5 (31%) |
| Lymph nodes | 1 (25%) | 2 (13%) |
| Chest wall | 0 (0%) | 1 (6%) |
All pre-menopausal patients were on Lupron therapy
Two patients were initially early stage, found to have bone or lung metastasis upon completion of neoadjuvant chemotherapy; hence, no surgery. Seven patients were de novo Stage IV;
Four patients (2-cohort 1, 2-cohort 2) had bone only metastasis and 16 patients (2-cohort 1, 14-cohort 2) had visceral metastasis (lung or liver). ECOG, Eastern Cooperative Oncology Group
Treatment follow-up:
Median follow-up was 37.9 months (95% CI 24.3, NR) in cohort 1, and 24.8 months (95% CI 17.1, 32.0) in cohort 2. Median number of cycles of pembrolizumab completed was 9.5 cycles (6–11) in cohort 1, and 8 cycles (2–37) in cohort 2. For cohort 2, 63% of patients had at least one dose delay, and 50% had at least one dose reduction, mostly due to neutropenia (Table 2). One patient in cohort 1 stopped pembrolizumab due to duodenal perforation. Three patients in cohort 2 stopped pembrolizumab due to pneumonitis (N=1) and elevated LFTs (N=2). All patients continued palbociclib and letrozole when pembrolizumab was discontinued.
Table 2.
Treatment follow-up and response based on RECIST v1.1
| Cohort 1 (N=4) | Cohort 2 (N=16) | |
|---|---|---|
|
| ||
| Median follow-up (95% CI) months | 37.9 (24.3, NR) | 24.8 (17.1, NR) |
|
| ||
| Median pembrolizumab cycles (range) | 9.5 (6 – 11) | 8 (2 – 37) |
|
| ||
| Median cycles delayed (range) | 1.5 (1 – 3) | 1.5 (0 – 7) |
| Patients with ≥ 1 cycle delayed | 4 (100%) | 10 (63%) |
|
| ||
| Median palbociclib dose reduction (range) | 0.5 (0 – 1) | 0.5 (1 – 3) |
| Patients with ≥ 1 dose reduction | 2 (50%) | 8 (50%) |
|
| ||
| Best overall response by RECIST1.1, n (%), 95% CI | ||
| CR | 0 (0%) | 5 (31%) |
| PR | 2 (50%) | 4 (25%) |
| SD | 2 (50%) | 5 (31%) |
| PD | 0 (0%) | 2 (13%) |
| Response rate (CR+PR) | 2 (50%) | 9 (56%) |
| Clinical benefit rate (CR+PR+SD) | 4 (100%) | 14 (88%) |
CR, complete response; PR, partial response; SD, stable disease; PD, progression of disease.
Clinical activity:
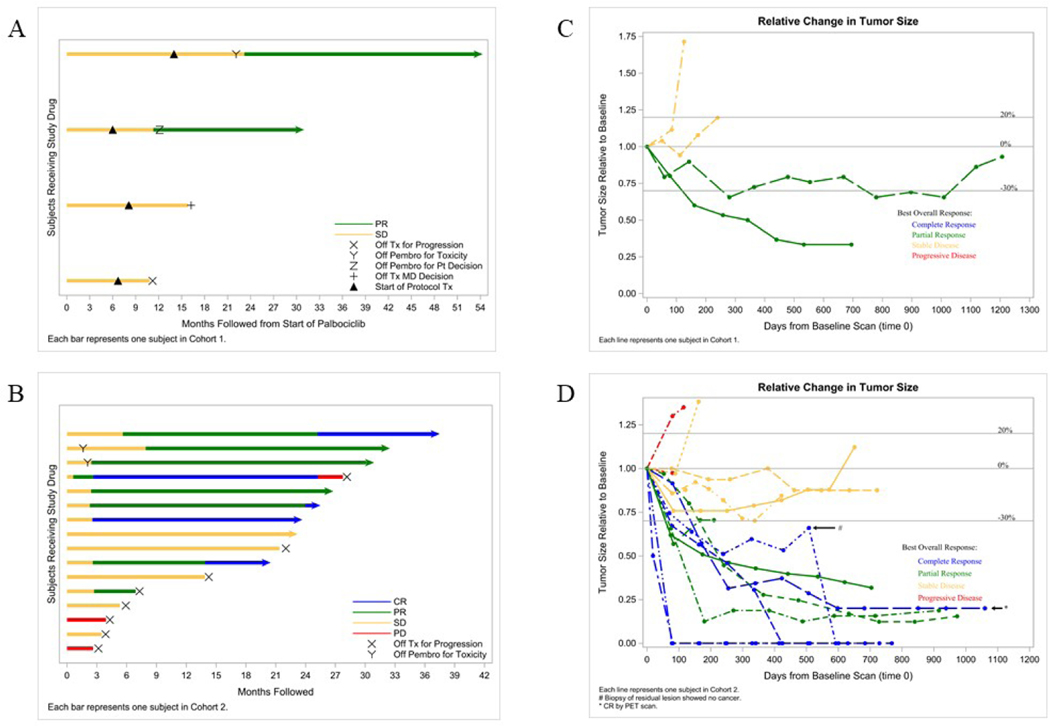
In cohort 1, 2/4 (50%) patients achieved a PR and 2/4 (50%) had SD, which met the pre-specified criteria for activity for that cohort. In cohort 2, a total of 5/16 (31%) evaluable patients achieved CR, 4/16 (25%) had PR, and 5/16 (31%) had SD by RECIST 1.1 (Table 2, Figure 1). The bar plots show PFS over time (Figure 1A and 1C), and the spider plots show relative changes in tumor size from baseline over time (Figure 1B and 1D). Of note, 2/3 (67%) patients with lobular carcinoma had CR. For cohort 2, after median follow-up of 24.8 months, the median PFS was 25.2 months (95% CI 5.3, NR) and median OS was 36.9 months (95% CI 36.9, NR). (Figure 2).
Figure 1. Response to therapy.
A) Cohort 1 bar plot (N=4) showing patient response to study drug over time starting with palbociclib and letrozole treatment on cycle 1 day 1 (C1D1) and the black triangle representing protocol therapy with the addition of pembrolizumab; each bar represents one patient; B) Cohort 1 spider plot (N=4) showing relative change in tumor size from baseline over time starting at time of protocol therapy with pembrolizumab; C) Cohort 2 bar plot (N=16); and D) Cohort 2 spider plot (N=16). CR (blue), complete response; PR (green), partial response; SD (orange), stable disease; PD (red), progression of disease; X, off trial for progression; Y, off pembrolizumab for toxicity; Z, off pembrolizumab per patient decision; +, off treatment per physician decision.
Figure 2. Kaplan Meier survival analysis for cohort 2 (N=16).
A) Median PFS was 25.2 months (95% CI 5.3, NR) from start of treatment; B) Median OS was 36.9 months (95% CI 36.9, NR). PFS, progression free survival; OS, overall survival; NR, not reached.
Safety:
Of the 23 patients that received treatment, 20/23 (87%) had grade 3 toxicity and 7/23 (30%) had grade 4 toxicity related to treatment (Table 3). Grade 3–4 hematological toxicities were neutropenia (83%), leukopenia (65%), lymphocytopenia (26%), thrombocytopenia (17%), anemia (9%) and febrile neutropenia (4%). Grade 3–4 non-hematological toxicities were elevated LFTs (17%), musculoskeletal pain (9%), sepsis (8%), pruritis (4%), diarrhea (4%), nausea or vomiting (4%), abdominal pain (4%), duodenal perforation (4%), and pneumonitis (4%). Of these, grade 3–4 immune-related AEs (irAEs) included elevated LFT (17%), pruritus (4%), diarrhea (4%), pneumonitis (4%), and duodenal perforation (4%) (Table 3). AEs for each cohort are included in Table S1 and Table S2.
Table 3.
Grade 2–4 AEs related to treatment with at least two patients experiencing a Grade ≥ 2 AE per CTCAE 4.0 (N=23)
| Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Hematological AEs | 18 (78%) | 19 (83%) | 5 (22%) |
| Anemia | 7 (30%) | 2 (9%) | |
| Neutropenia | 16 (70%) | 3 (13%) | |
| Febrile neutropenia | 1 (4%) | ||
| Leukopenia | 6 (26%) | 12 (52%) | 3 (13%) |
| Lymphocytopenia | 12 (52%) | 4 (17%) | 2 (9%) |
| Thrombocytopenia | 3 (13%) | 4 (17%) | |
| Non-hematological AEs | 19 (83%) | 9 (39%) | 2 (9%) |
| Fatigue | 7 (30%) | ||
| Pain1 | 2 (9%) | ||
| Upper respiratory infection | 3 (13%) | ||
| Urinary tract infection | 1 (4%) | ||
| Sepsis | 1 (4%) | 1 (4%) | |
| Arthralgia | 3 (13%) | ||
| Pruritus | 1 (4%) | ||
| Diarrhea | 2 (9%) | 1 (4%) | |
| Nausea/vomiting | 2 (9%) | 1 (4%) | |
| Abdominal pain | 1 (4%) | 1 (4%) | |
| Duodenal perforation | 1 (4%) | ||
| Pneumonitis | 1 (4%) | ||
| Hypertension | 4 (17%) | ||
| Elevated LFTs | 2 (9%) | 3 (13%) | 1 (4%) |
| Hypophosphatemia | 2 (9%) | ||
| Immune Related AEs (irAEs) | 4 (17%) | 5 (22%) | 2 (9%) |
| Pruritus | 1 (4%) | ||
| Diarrhea | 2 (9%) | 1 (4%) | |
| Duodenal perforation | 1 (4%) | ||
| Pneumonitis | 1 (4%) | ||
| Elevated LFTs | 2 (9%) | 3 (13%) | 1 (4%) |
Extremity, hip
Tumor biomarkers:
Stromal TILs and PD-L1 expression were evaluated in pretreatment tumor biopsies. TILs ranged from 2 to 20% but did not show correlation with response (Figure 3A). 5/19 (26%) patients scored positive for PD-L1 expression, but PD-L1 was a poor predictor of response (Figure 3B). TMB was low in all tumors and ranged from 0.4 to 4.2 mutations/mega base (m/MB) (Figure 3C). Microsatellite stability status was stable for all tumors in this study.
Figure 3. Pre-treatment tumor stromal TILs, PD-L1, and TMB.
A) % Stromal TILs vs. response (N=19); B) % PD-L1 vs. response (N=19); and C) TMB (m/MB) vs. response (N=14). Tumor mutation burden (TMB), progression of disease (PD), stable disease (SD), partial response (PR), complete response (CR).
Genomic analysis:
15/20 (75%) of patients had sufficient pre-treatment tumor tissues for genomic analysis. Genomic alterations in responders vs. non-responders were analyzed and the gene alterations were reported (Figure S2). No significant differences in genomic alterations between responders (R, CR+ PR) and non-responders (NR, SD+PD) were identified. The most commonly observed genomic mutations were: 4/15 (27%) PIK3CA mutations; 3/15 (20%) CDH1 mutations, and 3/15 (20%) GATA3 mutations. Other alterations present in 2/15 (13%) patients were ARID1A, FAT1, FGFR1, MLL3, PALB2, and PTEN. Genomic alterations identified in this study are consistent with published breast cancer genomic profiles25,26.
Tumor mRNA profiling:
Gene expression patterns in pre-treatment tumor tissues were assessed by whole transcriptome analysis (N=11). Principle Component Analysis (PCA) was performed. Heat map clustering of top 20 variants for responders (R, CR+PR) vs. non-responders (NR, SD+PD) is shown in Figure S3A. PCA did not identify distinct subgroups of patients, and there were no noticeable differences in response to therapy (Figure S3B). Gene expression related to CDK4/6 resistance including CCND1, CCNE1, CCNE2, CDKN2C, ESR1, PGR, and RB127–29 showed no significant differences between responders and non-responders (Figure S3C). Gene set enrichment analysis of E2F and RB between responders and non-responders did not show significant difference30 (Figure S3D).
DISCUSSION
The combination of palbociclib, pembrolizumab and letrozole as front-line therapy in HR+ MBC showed a PFS of 25.2 months which is comparable with 24.8 months of historical control seen in PALOMA-2 trial. A CR of 31% was observed in cohort 2 compared with 0–2% of historical control1 (filed medical information). In addition, despite limited sample size, 2/4 (50%) of cohort 1 patients demonstrated a “deepened” response when pembrolizumab was added to palbociclib after initial plateaued response to palbociclib and letrozole, which suggests an immune priming effect of palbociclib. Our results suggest that adding immune checkpoint inhibition at time of disease resistance from CDK4/6i therapy could be utilized as a strategy to change the trajectory of tumor response. In a recent preclinical study conducted by Pandey et al., deregulated immune pathway is a mechanism of CDK4/6i resistance in addition to activation of cyclin E-CDK2 pathway and loss of RB, etc31. The finding from our study support immune suppression pathway targeting as a strategy to overcome CDK4/6i resistance in ER+ metastatic breast cancer. In a preclinical model of small cell lung cancer, transient CDK4/6 inhibition by trilaciclib was sufficient to enhance and prolong the duration of the antitumor response by chemotherapy/ICI combinations, suggesting a role for the transient cell cycle arrest of tumor immune infiltrates in remodeling the tumor microenvironment32. Collectively, these results provide a rationale for combining CDK 4/6 inhibitor with ICI regimens to improve antitumor efficacy in patients with breast cancer and such trials are current ongoing including ImmunoADAPT (NCT03573648) and PACE (NCT03147287). In PACE trial, fulvestrant, palbociclib and avelumab combination are tested in participants with metastatic ER+ breast cancer that has previously stopped responding to prior palbociclib and endocrine therapy.
Of note, 2/3 (67%) patients with lobular carcinoma achieved a CR, regardless of PD-L1 status. Du et al. reported top pathways enriched in invasive lobular carcinoma (ILC) in contrast with invasive ductal carcinoma (IDC) were related to immune response. ILC exhibited a higher activity of almost all types of immune cells based on cell type-specific signatures compared to IDC33. In contrast, Desmedt et al. reported statistically significantly lower TILs in ILC compared to IDC34. Further research is needed to understand the association between lobular histology and immune features.
In the current study, grade 3–4 neutropenia rate was 83%, which resulted in palbociclib dose reduction in 50% of patients. Our relatively high neutropenia rate may be attributed to 30% Asian ethnicity included in the study. In a recent analysis of Asian population from PALOMA-2, the grade3–4 neutropenia rate in Asians vs. non-Asians were 87.5% vs. 66.6%35 respectively. Immune checkpoint inhibitors were associated with unique immune-related hematological toxicities such as immune thrombocytopenic purpura (ITC), hemolytic anemia (HA), hemophagocytic lymphohistiocytosis, aplastic anemia, and pure red cell aplasia36, but there have been no report showing precipitated neutropenia or leukopenia in the literature. Grade 3–4 neutropenia of 3 weeks on and 1 week off palbociclib is very common (68%) and alternative dosing schedules have been studied. For example, a 5-days on and 2-days off schedule showed a reduced grade 3 neutropenia rate of 21% and ORR of 48% (N=42) (NCT03007979) 37. Considering high grade 3–4 neutropenia rate in the current study, feasibility of alternating dosing schedule and preclinical evidence of immune-priming effect of low dose CDK 4/6i, a lower dose or alternative dosing schedule of CDK 4/6 inhibitor with the combination of ICI study is warranted.
In the current study, grade 3–4 elevated LFTs (likely reflecting immune hepatitis) was 17% and all patients were successfully managed with steroids and had full recovery of liver function. The safety and tolerability profiles of this combination largely reflect known toxicity profiles of palbociclib and pembrolizumab, with no additional safety concerns. Rugo et al. reported the safety and efficacy of abemaciclib plus pembrolizumab in patients with endocrine resistant HR+ HER2- MBC38. The median PFS was 8.9 months (95% CI [3.9, 11.1]) and RR was 29%. Grade 3 or 4 increased alanine aminotransferase (8 pts, 31%) and increased aspartate aminotransferase (6 pts, 23%) were observed; 2 (7.7%) fatal events of pneumonitis occurred. Due to significant toxicities and discouraging efficacy, further development of abemaciclib plus pembrolizumab combination was halted. The results of our study indicate that the combination of palbociclib and pembrolizumab appears to be safe and efficacious, and therefore warrants further development.
CDK4/6i has been adopted as the mainstay of treatment in the metastatic setting for HR+ MBC39. However, intrinsic or acquired resistance limits long-term utilization of CDK4/6i. Recent studies have discovered multiple mechanisms of resistance: loss of RB, increased CDK4/6 and D-cyclin complexes (FAT1 loss), FGFR amplification, ESR1 mutation, de-regulation of cyclin E, E2F amplification, CDK4 or CDK6 amplification, CDK7 overexpression, WEE1 overexpression, MDM2 overexpression, and HDAC activation10,11,31,40. Due to limited sample size, genomic analysis of baseline tumors in the current study needs to be interpreted with caution and a future trial with a larger sample size is required for further confirmation.
The response to ICIs is associated with a complex tumor microenvironment41,42. High TILs, increased PD-L1 expression, and high TMB are established biomarkers predicting response to ICIs43,44. In our exploratory immune biomarker analysis, we observed a low level of TILs, PD-L1, and TMB in this cohort of patients, in contrast with the robust response to this ICI combination. This observation supports the hypothesis that immune response can be elicited by palbociclib and pembrolizumab combination, regardless of pre-treatment stromal TILs, PD-L1, or TMB level. Several preclinical studies have supported the immune-priming effects of CDK 4/6 inhibitors. Goel et al. reported CDK4/6 inhibitors enhances immune response of breast cancer cells through several mechanisms: activate tumor cell expression of endogenous retroviral elements, increase intracellular levels of double-stranded RNA and stimulates production of type III interferons and hence enhances tumor antigen presentation; markedly suppress the proliferation of regulatory T cells (Tregs); reduced activity of the E2F target, DNA methyltransferase 1; promote cytotoxic T cell-mediated clearance of tumor cells21. In a preclinical model of small cell lung cancer, transient CDK4/6 inhibition by trilaciclib was sufficient to enhance and prolong the duration of the antitumor response by chemotherapy/ICI combinations, suggesting a role for the transient cell cycle arrest of tumor immune infiltrates in remodeling the tumor microenvironment32. An in-depth analysis of peripheral blood mononuclear cells (PMBC) and baseline tumor multi-color IHC was published separately45. We showed that CDK4/6i has an immune priming effect. In a combined analysis of peripheral blood flow cytometry of PBMCs, patients treated with palbociclib + pembrolizumab + letrozole (palbo+pembro+AI) from the current trial vs. pembrolizumab + aromatase inhibitor (pembro+AI) from a separate trial were studied. Over the course of treatment, significant shifts in peripheral blood, myeloid composition and phenotype were observed in palbo+pembro+AI treated patients, but not in those treated with pembro+AI. We identified increased fractions of type 1 dendritic cells within circulating dendritic cells and decreased classical monocytes within circulating monocytes only in patients treated with palbo+pembro+AI, but not in those treated with pembro+AI. In addition, preclinical evidence has shown that the immune priming effect of CDK4/6i can occur at a low dose. Charles et al., showed low-dose treatment of breast cancer cells with CDK4/6 inhibitors abemaciclib and palbociclib induced marked changes in presentation of HLA ligands46. Based on these findings, the low dose priming effect of CDK4/6i in combination with ICI should be further investigated in future trials. This approach may alleviate the high neutropenia rate seen in the current trial. This study was limited by the small number of patients and two different cohorts. Cohort 1 included four patients previously on letrozole and palbociclib for > 6 months for “immune priming”. We observed a deepened response in 2/4 (50%) of the patients in cohort 1 which warrants further investigation. Based on the current findings, our group will further investigate the combination as first-line therapy with a palbociclib lead-in and a mandatory on-treatment tumor biopsy.
CONCLUSION
The combination of palbociclib, pembrolizumab, and letrozole is well tolerated, and a CR rate of 31% was identified in HR+ MBC patients who received this combination as front-line therapy. Confirmatory trials are required to confirm the current finding and elucidate the immune-priming effect of CDK4/6 inhibitors.
Supplementary Material
Highlights.
Palbociclib, pembrolizumab, and AI is safe in HR+ metastatic BC patients.
Median PFS is 25.2 months.
Combination has a CR rate of 31% in HR+ metastatic BC patients.
Acknowledgements:
The authors gratefully acknowledge Merck for sponsoring the study and providing funding support for the clinical trial enrollment and follow up, and for providing drug supply of pembrolizumab and PD-L1 testing. We also gratefully acknowledge Pfizer for providing study drug palbociclib. The authors thank the STOP Cancer Foundation (PI Yuan Yuan), NCI K-12 Career Development Award (K12CA001727, PI Joanne Mortimer), the National Institutes of Health (P30CA033572, and the patients who participated this study and their families. This study was also supported by COH Pathology Research Services Core, Biostatistics and Mathematical Modeling Core, Integrative Genomics Core, Bioinformatics Core, and Immune-Oncology (National Cancer Institute of the National Institutes of Health under award number P30CA033572). YY was supported by STOP Cancer Foundation (PI Yuan Yuan) and NCI K-12 Career Development Award (K12CA001727, PI Joanne Mortimer).
Dr. Yuan has contracted research sponsored by Merck, Eisai, Novartis, Puma, Genentech, Celgene, and Pfizer; is a consultant for Puma, Pfizer, and Immunomedics; and is on the Speakers Bureau for Eisai, Genentech, AstraZeneca, Daiichi Sankyo, Pfizer, Merck, and Immunomedics.
Footnotes
Ethics approval and consent to participate: The protocol was approved by City of Hope IRB. All procedures were performed in accordance with the ethical standards of the institution, national research committee, and the 1964 Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice and later amendments. Written informed consent was obtained from all participants of this study. ClinicalTrials.gov NCT02778685.
Declaration of Interest: The other authors declare they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Finn RS, Martin M, Rugo HS, et al. : Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 375:1925–1936, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Finn RS, Dering J, Conklin D, et al. : PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 11:R77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hortobagyi GN, Stemmer SM, Burris HA, et al. : Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 375:1738–1748, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Goetz MP, Toi M, Campone M, et al. : MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 35:3638–3646, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Sledge GW, Toi M, Neven P, et al. : The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. 6:116–124, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Neven P, Chia S, et al. : Overall survival with ribociclib plus fulvestrant in advanced breast cancer. 382:514–524, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Im S-A, Lu Y-S, Bardia A, et al. : Overall survival with ribociclib plus endocrine therapy in breast cancer. 381:307–316, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Iwata H, Im SA, Masuda N, et al. : PALOMA-3: Phase III Trial of Fulvestrant With or Without Palbociclib in Premenopausal and Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer That Progressed on Prior Endocrine Therapy-Safety and Efficacy in Asian Patients. J Glob Oncol 3:289–303, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wander SA, Cohen O, Gong X, et al. : The Genomic Landscape of Intrinsic and Acquired Resistance to Cyclin-Dependent Kinase 4/6 Inhibitors in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancer Discov 10:1174–1193, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner NC, Liu Y, Zhu Z, et al. : Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer. J Clin Oncol 37:1169–1178, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa C, Wang Y, Ly A, et al. : PTEN Loss Mediates Clinical Cross-Resistance to CDK4/6 and PI3Kα Inhibitors in Breast Cancer. Cancer Discov 10:72–85, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Clark AS, McAndrew NP, Troxel A, et al. : Combination Paclitaxel and Palbociclib: Results of a Phase I Trial in Advanced Breast Cancer. Clin Cancer Res 25:2072–2079, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Bardia A, Hurvitz SA, DeMichele A, et al. : Triplet therapy (continuous ribociclib, everolimus, exemestane) in HR+/HER2− advanced breast cancer postprogression on a CDK4/6 inhibitor (TRINITI-1): Efficacy, safety, and biomarker results. Journal of Clinical Oncology 37:1016–1016, 2019 [Google Scholar]
- 14.PD-1 Inhibitor promising in treatment of triple-negative breast cancer. Hum Vaccin Immunother 11:1298, 2015 [Google Scholar]
- 15.Schmid P, Adams S, Rugo HS, et al. : Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 379:2108–2121, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Cortes J, Cescon DW, Rugo HS, et al. : Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396:1817–1828, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Rugo HS, Delord J-P, Im S-A, et al. : Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer. Clinical Cancer Research, 2018 [DOI] [PubMed]
- 18.Vonderheide RH, LoRusso PM, Khalil M: Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 16:3485–3494, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Sabatier R, Finetti P, Mamessier E, et al. : Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6:5449–64, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barroso-Sousa R, Jain E, Kim D, et al. : Determinants of high tumor mutational burden (TMB) and mutational signatures in breast cancer. Journal of Clinical Oncology 36:1010–1010, 2018 [Google Scholar]
- 21.Goel S, DeCristo MJ, Watt AC, et al. : CDK4/6 inhibition triggers anti-tumour immunity. Nature 548:471, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng J, Wang ES, Jenkins RW, et al. : CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov 8:216–233, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salgado R, Denkert C, Demaria S, et al. : The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–71, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaubier N, Tell R, Lau D, et al. : Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget 10:2384–2396, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burstein MD, Tsimelzon A, Poage GM, et al. : Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 21:1688–98, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann BD, Jovanovic B, Chen X, et al. : Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS One 11:e0157368, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Li Z, Bhatt T, et al. : Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36:2255–2264, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spring LM, Wander SA, Zangardi M, et al. : CDK 4/6 Inhibitors in Breast Cancer: Current Controversies and Future Directions. Curr Oncol Rep 21:25, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCartney A, Migliaccio I, Bonechi M, et al. : Mechanisms of Resistance to CDK4/6 Inhibitors: Potential Implications and Biomarkers for Clinical Practice. Front Oncol 9:666, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller TW, Balko JM, Fox EM, et al. : ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov 1:338–51, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey K, An HJ, Kim SK, et al. : Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int J Cancer 145:1179–1188, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai AY, Sorrentino JA, Dragnev KH, et al. : CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy. J Immunother Cancer 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du T, Zhu L, Levine KM, et al. : Invasive lobular and ductal breast carcinoma differ in immune response, protein translation efficiency and metabolism. Sci Rep 8:7205, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desmedt C, Salgado R, Fornili M, et al. : Immune Infiltration in Invasive Lobular Breast Cancer. J Natl Cancer Inst 110:768–776, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukai H, Shimizu C, Masuda N, et al. : Palbociclib in combination with letrozole in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int J Clin Oncol 24:274–287, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis EJ, Salem JE, Young A, et al. : Hematologic Complications of Immune Checkpoint Inhibitors. Oncologist 24:584–588, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnamurthy J, Luo J, Ademuyiwa F, et al. : Abstract P1–19-13: A phase II trial assessing the safety of an alternative dosing schedule of palbociclib (palbo) in hormone receptor positive (HR+), HER2 negative (HER2−) metastatic breast cancer (MBC): Alt Dose Palbo. Cancer Research 80:P1-19-13-P1-19-13, 2020 [Google Scholar]
- 38.Rugo HS, Kabos P, Beck JT, et al. : A phase Ib study of abemaciclib in combination with pembrolizumab for patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2−) locally advanced or metastatic breast cancer (MBC) (NCT02779751): Interim results. Journal of Clinical Oncology 38:1051–1051, 2020 [Google Scholar]
- 39.Ingham M, Schwartz GK: Cell-Cycle Therapeutics Come of Age. Journal of Clinical Oncology 35:2949–2959, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Condorelli R, Spring L, O’Shaughnessy J, et al. : Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol 29:640–645, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Giraldo NA, Sanchez-Salas R, Peske JD, et al. : The clinical role of the TME in solid cancer. Br J Cancer 120:45–53, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitt JM, Marabelle A, Eggermont A, et al. : Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol 27:1482–92, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Bense RD, Sotiriou C, Piccart-Gebhart MJ, et al. : Relevance of Tumor-Infiltrating Immune Cell Composition and Functionality for Disease Outcome in Breast Cancer. J Natl Cancer Inst 109, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dushyanthen S, Beavis PA, Savas P, et al. : Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med 13:202, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egelston C, Guo W, Yost S, et al. : Pre-existing effector T-cell levels and augmented myeloid cell composition denote response to CDK4/6 inhibitor palbociclib and pembrolizumab in hormone receptor-positive metastatic breast cancer. J Immunother Cancer 9, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charles A, Bourne C, Aretz ZE, et al. : Low-Dose CDK4/6 Inhibitors Induce Presentation of Pathway Specific MHC ligands as Targets for Cancer Immunotherapy. bioRxiv:2020.06.18.157800, 2020 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.