Biologic disease-modifying anti-rheumatic drugs (DMARDs) represent a potent treatment option for patients with immune-mediated inflammatory diseases.1 Yet, infections make up the largest proportion of serious adverse events associated with biological DMARD therapy.2 Throughout the COVID-19 pandemic, patients receiving immunosuppressive treatment were shown to be at higher risk of severe disease outcomes.3 Vaccination could prevent these outcomes, but the efficacy of COVID-19 vaccines in these patients is incompletely understood.4
Although studies investigating the effect of biological DMARDs on the development of immune responses to COVID-19 vaccines are slowly accumulating, most of our current knowledge relies on studies evaluating the serological responses to COVID-19 vaccines.5 However, the serological response to COVID-19 vaccines is highly variable between individuals, in terms of both antibody titres and kinetics.6, 7 There is also no cutoff value of antibody titres that clearly reflects protection from SARS-CoV-2 infection.7
Vaccine-elicited cellular immune responses were shown to be crucially important for the development of humoral immune response and for the clearance of SARS-CoV-2.6, 8 For that reason, more data on vaccine-elicited SARS-CoV-2-reactive T cells in patients treated with biological DMARDs are urgently needed. Recent studies by Mahil and colleagues3, 5 show encouraging results on COVID-19 vaccine immunogenicity in patients with psoriasis treated with biological DMARDs. These studies showed that patients receiving these drugs were able to mount both serological and cellular responses to both the first and second dose of the BNT162b2 (tozinameran, Pfizer–BioNTech) mRNA vaccine.3, 5
We aimed to evaluate the immunogenicity of two doses of the BNT162b2 vaccine in individuals receiving monotherapy with tumour necrosis factor (TNF) inhibitors or interleukin(IL)-17A inhibitors. We evaluated antibody and T-cell responses in 17 patients with axial spondyloarthritis who had not been exposed to SARS-CoV-2 and who were being treated with either the TNF inhibitor adalimumab (n=10) or the IL-17A inhibitor secukinumab (n=7), compared with six healthy individuals. 11 (65%) of the patients were treated for radiographic axial spondyloarthritis, 15 (88%) were men. The mean age of the patients was 39·8 (SD 9·4) years, and the mean disease duration was 97·3 (80·8) months (appendix p 1). All patients were managed according to treatment guidelines; secukinumab (150 mg) was administered every four weeks, and adalimumab (40 mg) was administered either every 2 weeks (n=7) or every three weeks (n=3; appendix p 1). Adherence to biological DMARD treatment was confirmed by all study participants. The healthy control cohort had a mean age of 48·2 (10·5) years and included three men and three women. Patients who were previously exposed to SARS-CoV-2 infection, as indicated by positivity for SARS-CoV-2-specific IgG or IgA antibodies before vaccination, and patients with a history of acute respiratory tract infection symptoms 6 months before study initiation, were excluded.
All study participants received two standard doses of the BNT162b2 vaccine with a 21-day interval between doses. The humoral immune response was evaluated by measuring titres of anti-SARS-CoV-2-specific IgA and IgG antibodies, and the cellular immune response was assessed as the proportion of SARS-CoV-2-reactive CD4+ and CD8+ T cells producing interferon γ, TNF, or IL-17A after ex vivo stimulation with spike glycoprotein-derived peptides (PepMix SARS-CoV-2) and co-stimulation with CD28 and CD49d antibodies. CD107a expression was assessed as an indicator of CD8+ T-cell degranulation. Cellular immune responses were analysed by flow cytometry.
The study was approved by the Ethics Committee for Multi-Centric Clinical Trials of the University Hospital Motol and 2nd Faculty of Medicine, Charles University in Prague (reference EK-753.1.3/21). All study participants provided written informed consent.
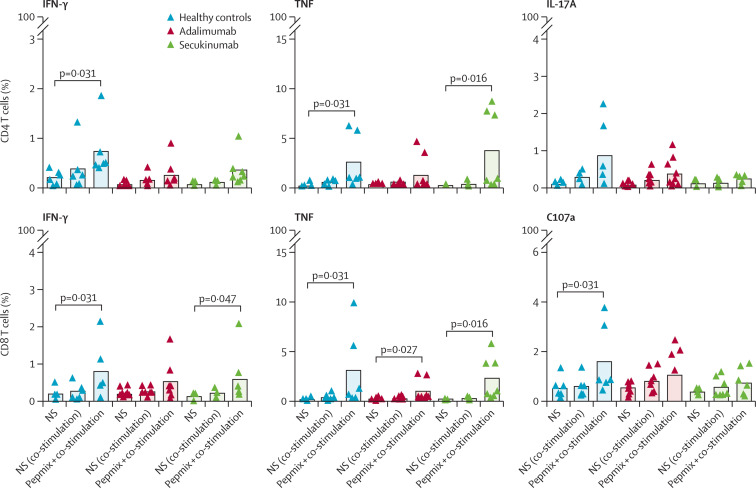
We observed a robust seroconversion in all study participants (appendix p 2). Vaccination also generated proinflammatory cytokine-producing CD4+ and CD8+ T cells, with no significant differences in the proportion of SARS-CoV-2-reactive T cells between the study groups (figure ). The second dose of the BNT162b2 vaccine did not elicit CD8+ T-cell degranulation in patients treated with adalimumab nor in those treated with secukinumab.
Figure.
Proportion of SARS-CoV-2 reactive T cells between study groups
T-cell responses were measured 30 days after the second dose of the BNT162b2 vaccine in healthy donors and patients receiving therapy with adalimumab or secukinumab. T cells were stimulated with a mixture of SARS-CoV-2 spike protein peptides, and production of interferon γ, interleukin(IL)-17A, and tumour necrosis factor (TNF) was measured by flow cytometry in CD4+ T cells and CD8+ T cells. Expression of CD107a was also measured in CD8+ T cells. Statistical analysis was done between cell samples from one group of patients or healthy controls stimulated with costimulatory antibody and costimulatory antibody in combination with PepMix. A correlation was also made in cells stimulated by a combination of co-stimulation and PepMix within the groups of patients and healthy controls. NS=not stimulated.
Cytokine-producing CD8+ T cells and CD107a-expressing CD8+ T cells presumably represent anti-viral effector immunity, whereas vaccine-elicited CD4+ T cells have been shown to contribute to the efficient production of neutralising antibodies.9 Our data also showed individual differences in the cellular immune responses to full vaccination with the BNT162b2 vaccine, with T-cell responses undetectable in up to 20% (appendix p 3) of vaccinees in each group (figure).
COVID-19 vaccines were shown in clinical trials to substantially reduce the severity of COVID-19 and also to reduce the risk of SARS-CoV-2 transmission.4, 10 However, patients receiving immunosuppressants were excluded from these trials and few published studies have evaluated vaccine responses in patients treated with biological DMARDs.5
Our data indicated that neither adalimumab nor secukinumab substantially affected the immunogenicity of the BNT162b2 mRNA COVID-19 vaccine. Patients receiving these biological DMARDs were able to develop cellular immune responses after vaccination with the BNT162b2 vaccine, and the overall humoral and cellular immune response did not differ significantly between patients and healthy individuals. By contrast to the data presented by Mahil and colleagues,3, 5 our findings did not indicate a significant disparity between humoral and cellular immune responses in individual patients. However, although all study participants had high levels of anti-SARS-CoV-2-specific IgA and IgG antibodies, cellular responses against the SARS-CoV-2 spike glycoprotein could not be identified in all participants.
Our study has several limitations worth noting, in particular the small number of participants studied. Although we initially enrolled 21 patients with axial spondyloarthritis receiving biological DMARDs, we had to exclude three patients because of previous exposure to COVID-19 and one patient because of concomitant treatment with methotrexate. We also did not assess neutralising antibody titres. In addition, our study reflects the immunogenicity of the BNT162b2 mRNA COVID-19 vaccine at a single timepoint. We presume that the magnitude of cellular and humoral immune responses might change over time and thus further investigations are needed to fully understand the consequences of biological DMARD therapy in the era of COVID-19.
This online publication has been corrected. The corrected version first appeared at thelancet.com/rheumatology on February 20, 2023
Acknowledgments
JS, ZS, AS, TM, and RH contributed to study design. JS, ZS, TM, and RH contributed to the conceptualisation of the study protocol and the data curation. ZS and RH contributed to the formal analysis and administration of the project. ZS, RH, and AS supervised the project. JS, ZS, and TM did the main study investigation. JS and TM contributed to the validation of the study and software analyses. JS contributed to the methodology and provided the visualisation of the obtained data. JS, AS, TM, and RH contributed to the funding acquisition and AS, TM, and RH provided the resources for the study. ZS, JS, and TM contributed to the writing of the original draft. JS, ZS, AS, TM, and RH reviewed and edited the manuscript.
We declare no competing interests.
The authors are happy to share data on request to the corresponding author.
The study was supported by a NU20-05-00320 grant issued by the Czech Health Research Council and Ministry of Health (Czechia), Institutional support issued by the Motol University Hospital (Prague, Czechia), the Ministry of Health (00064203; University Hospital Motol, Prague, Czech Republic).
Supplementary Material
References
- 1.Her M, Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol. 2016;137:19–27. doi: 10.1016/j.jaci.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Ramiro S, Sepriano A, Chatzidionysiou K, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2017;76:1101–1136. doi: 10.1136/annrheumdis-2016-210708. [DOI] [PubMed] [Google Scholar]
- 3.Mahil SK, Bechman K, Raharja A, et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00333-7. published online Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Lancet Rheumatology COVID-19 vaccine data provide reassurance. Lancet Rheumatol. 2021;3:e605. doi: 10.1016/S2665-9913(21)00255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paces J, Strizova Z, Smrz D, Cerny J. COVID-19 and the immune system. Physiol Res. 2020;69:379–388. doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun. 2021;12 doi: 10.1038/s41467-021-22958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauer K, Harris T. An effective COVID-19 vaccine needs to engage T cells. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.581807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.