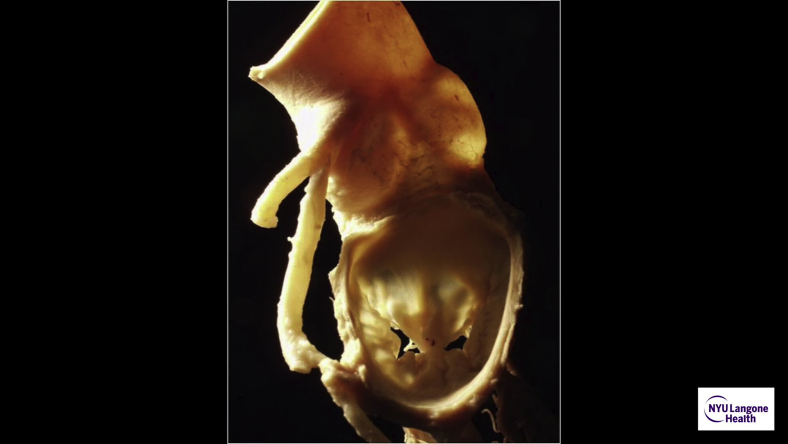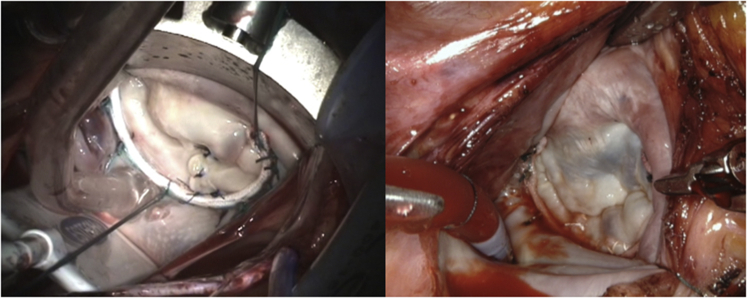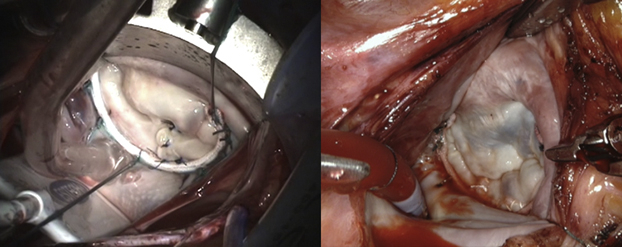
Left, Forme fruste, folding-plasty, Ht:W = 0.65. Right, Barlow's, sliding-plasty, Ht:W = 0.85.
Central Message.
The design characteristics of the semirigid posterior annuloplasty band are ideal for achieving a durable, physiologic mitral valve repair and allow flexibility in application.
When Carpentier introduced his breakthrough mitral valve (MV) reconstruction techniques in the 1970s, a key component was the placement of a rigid annuloplasty ring that would fix the MV into its kidney-shaped geometry and systolic dimensions.1, 2, 3 In 1978, after his initial experience with more than 200 repairs using the rigid annuloplasty ring, Duran introduced a flexible ring (Duran AnCore Annuloplasty System; Medtronic, Minneapolis, Minn).4 The flexible ring “corrected” MV annular dilation but did not provide fixed height/width (Ht:W) “remodeling” as advocated by Carpentier. The flexible ring, however, did allow some degree of MV annular movement throughout the cardiac cycle. In 1993, Cosgrove and colleagues5 introduced a totally flexible posterior annuloplasty band, primarily for the repair of degenerative disease, which allowed movement of the anterior annulus and the subaortic curtain and restricted posterior annular dilation but due to the complete flexibility did not provide predictable Ht:W annular remodeling.
Throughout the 1980s and early 1990s, the classic rigid annuloplasty ring was the most widely adopted, and although both the rigid and the various flexible rings or bands had certain useful features, they each had clear limitations to their designs. Predictable flaws in the initial unidimensional characteristics of “remodeling” versus “flexibility” can be highlighted by noting the variations and disparities in leaflet and annular size seen in patients with fibroelastic deficiency or the spectrum of Barlow's pathology. Valve leaflets can be thin or excessive, small or large, and the systolic Ht:W ratio of the annulus can range from 0.6 to 0.8 or more, depending on pathology. While the annular diastolic Ht:W ratio is typically 1.0 for most valves, the overall diameter and actual height of the annulus can vary greatly, with Barlow's valves having excess leaflet tissue and a much larger diameter, compared with valves with fibroelastic deficiency. Moreover, neither the MV diameter nor shape are fixed throughout the cardiac cycle, with the orifice diameter varying by 20% to 25% between systole and diastole and the Ht:W ratios varying from 0.6 to 1.0. These anatomic and physiologic variations indicate that the ideal annuloplasty design for mitral valve repair (MVr) must consider multiple factors and allow for a degree of flexibility in their applications.
The Initial Era of MVr Results and Lessons Learned
By the late 1980s, long-term results had been published demonstrating the durability of MVr in terms of freedom from reoperation or recurrent mitral insufficiency, and the superiority of valve repair compared with valve replacement in terms of freedom for thromboembolic events and other valve-related complications.6,7 However, in the early 1990s a number of studies began to show mild-to-moderate postrepair transvalvular gradients, especially in patients who underwent MVr with smaller (ie, <30 mm) rigid annuloplasty rings. Likewise, reports arose of postoperative MV systolic anterior motion (SAM), which refers to the paradoxical anterior movement of the anterior mitral leaflet and/or chordae toward the interventricular septum during systole. Postoperative SAM after MVr was first reported in 1978 and unfortunately led some surgeons to abandon an otherwise-successful repair.8
Design of the Ideal Annuloplasty Device
With this perspective, none of the available annuloplasty devices seemed to result in an optimal repair. By design, the Carpentier-Edwards Classic ring (Edwards Lifesciences, Irvine, Calif) created a fixed Ht:W ratio of 0.65 throughout the cardiac cycle, and the subsequent Carpentier-Edwards Physio ring (Edwards Lifesciences) resulted in a fixed Ht:W ratio of 0.75. While the later was an improvement for valves with degenerative disease (eg, Barlow-type pathology), it was less favorable for patients with fibroelastic deficiency and for those in heart failure with a dilated annulus. Complete rigid annuloplasty rings do not permit physiologic movement of the mitral annulus, and diastolic flow remains fixed by the device diameter.
The ideal annuloplasty device would merge the best aspects of both annular remodeling and flexibility principles into a new design. The anterior/posterior (A:P) (Ht:W) remodeling characteristics of the rigid ring were important for predicting postrepair valve competency in patients with degenerative disease (Carpentier type II pathology). Similarly, A:P annular “remodeling” was also essential in patients who had heart failure and significant annular dilation.1 And predictable A:P remodeling was critical in patients with pure Carpentier type I annular dilation without prolapse. To achieve these design ideals, it was thought that a partial posterior band that would predictably remodel the annulus in the A:P dimension while simultaneously permitting physiologic movement of the anterior annulus and the subaortic curtain throughout the cardiac cycle.
These design characteristics were developed with industry, resulting in the C-G Future Annuloplasty Band (Medtronic). The device is constructed from a wire core overmolded with silicone and ensheathed in ironed polyester cloth. The wire core shape is longitudinally restricted to provide predictable annular A:P (Ht:W) remodeling but has deformational characteristics providing radial flexibility, which permit annular and intertrigonal flexion during systolic contraction.9 As a posterior-only device, it allows normal posterior and downward movement of the anterior mitral valve leaflet, annulus, and subaortic curtain during systole, in conjunction with systolic expansion of the left ventricular outflow track and aortic root. Likewise, in diastole the anterior annulus can flatten and move anteriorly and upward to provide maximal opening of the annular orifice.9, 10, 11, 12, 13 This design would also allow some degree of flexibility based on the site of anterior fixation (Video 1). When the band is placed commissure-to-commissure, as is often recommended in patients with Barlow's pathology with excessive leaflet tissue, the diastolic Ht:W ratio is increased up to 0.85 or higher (Figure 1; Table 1). The design allows predictable fixed A:P “remodeling” during systole, with a relatively higher (Ht:W) ratio in diastole, thus improving diastolic flow.
Video 1.
Movement of the sub aortic curtain after repair with semirigid posterior annuloplasty band. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00687-8/fulltext.
Figure 1.
Left, Forme fruste, folding-plasty, Ht:W = 0.65. Right, Barlow's, sliding-plasty, Ht:W = 0.85.
Table 1.
Annuloplasty device Ht:W ratio
| Annuloplasty | Ht:W ratio |
|---|---|
| Carpentier-Edwards Classic ring | Ht:W = 0.65 (fixed) |
| Carpentier-Edwards Physio ring | Ht:W = 0.75 (fixed) |
| Semirigid posterior band | Ht:W = 0.65 (systole) |
| When fixed at trigones | Ht:W = 0.78 (diastole) |
| When fixed at commissures | Ht:W = 0.85 (diastole) |
Ht:W, Height/width.
Repair Durability With the Semirigid Posterior Annuloplasty Band: New York University (NYU) Results
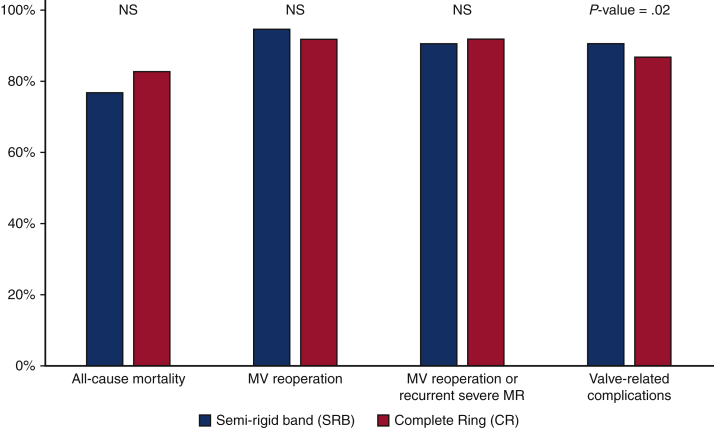
At NYU School of Medicine, between 1993 and 2010, a total of 1612 patients with degenerative mitral regurgitation underwent MVr with either a semirigid posterior annuloplasty band (n = 1101) or annuloplasty ring (n = 511).11 Hospital mortality did not differ between the posterior band (1.9%; 21/1101) and the ring (1.8%; 9/511) (P = .8). The median follow-up was 55 months, with no differences noted in cumulative freedom from all-cause mortality, MV reoperation, or the composite outcome of MV reoperation or recurrent severe mitral regurgitation between the devices at 8 years (Figure 2).11 Patients who received the posterior band demonstrated greater freedom from valve-related complications compared with patients who received an annuloplasty ring (91% vs 87%, P = .02). These results confirmed the durability of repair with the semirigid posterior band in terms of freedom from reoperation or recurrent mitral insufficiency. At NYU, the semirigid posterior band annuloplasty has been the standard device for repair of degenerative mitral insufficiency for all patients regardless of surgical approach.14
Figure 2.
Intraoperative transesophageal echocardiography change in semirigid posterior band versus rigid ring mitral valve orifice area (cm2). Data analysis was performed at Dr Galloway's institution. NS, Not significant; MV, mitral valve; MR, mitral regurgitation.
Postrepair Physiologic Results and Functional Mitral Stenosis (FMS)
In 2004, Gorman and colleagues15 performed ovine studies designed to evaluate the normal movement of the MV annulus throughout the cardiac cycle and the impact of a positive inotropic or chronotropic state on annular dynamics. Changes in the diameter of the MV annular orifice were measured in the resting state and with subsequent pacing and exercise as simulated by isoproterenol infusion. The results confirmed the expected 20% to 25% change in MV annular diameter between systole and diastole while the heart was in its resting state. However, when the contractile state of the heart was enhanced with isoproterenol, the annular orifice underwent much greater variation in size throughout the cardiac cycle. This finding is not unexpected, as this is the normal physiologic mechanism that allows the MV to accommodate increased cardiac output during exercise. The study highlights that some degree of physiologic movement of the mitral annulus would be necessary after MVr for patients to have the physiologic capability to increase and sustain cardiac output.
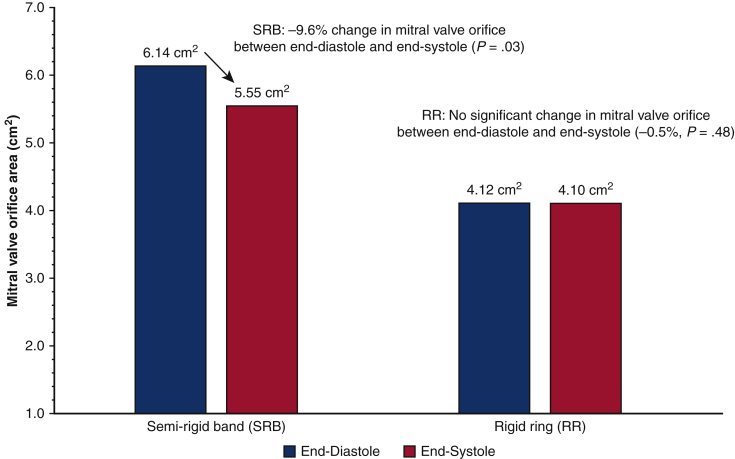
In the same year, Sharony and colleagues9 used 3-dimensional transesophageal echocardiography to evaluate the annular dynamics and post-MVr transvalvular gradients of patients who received the semirigid posterior annuloplasty band versus those who received a rigid annuloplasty ring. These results demonstrated that patients who received a posterior band had lower resting mean and peak transvalvular gradients compared with patients who received a rigid ring (mean gradients, 4.0 ± 0.3 mm Hg vs 5.0 ± 0.3 mm Hg; P = .02; peak gradients, 8.9 ± 0.5 mm Hg vs 11.1 ± 0.5 mm Hg; P = .01), regardless of device size.9 The study also evaluated changes in MV orifice area throughout the cardiac cycle, demonstrating that in posterior band patients, the MV orifice area changed by –9.6% (P = .01) between diastole and systole with the intertrigonal distance decreasing by –5.2% (P = .03) in systole. As expected, patients who had a rigid ring experienced no significant change in the MV orifice area between diastole and systole (–0.5%, P = .48) (Figure 3).9
Figure 3.
Eight-year cumulative freedom from adverse events. Data analysis was performed at Dr Galloway's institution.
In 2013, Mesana and colleagues16 performed stress echocardiography in degenerative mitral patients 5 years postrepair with either a flexible annuloplasty ring or a semirigid posterior annuloplasty band. Their results demonstrated that patients with a ring had greater postrepair mean transvalvular gradients and greater postrepair pulmonary artery pressures than patients who received a posterior band. This FMS was more pronounced at peak exercise and was most significant in patients who had smaller annuloplasties (ie, <30 mm). Importantly, these differences were associated with worse long-term functional status, especially in patients with smaller ring devices.
In terms of the incidence and clinical significance of late FMS after MVr, a recent study by Kim and colleagues17 evaluated 792 consecutive patients who underwent MVr for degenerative disease between 1990 and 2015, with a late follow-up of 20 years. Approximately 90% of the patients received a rigid annuloplasty ring and 10% received a flexible ring. Within the study, 24.2% developed late FMS, and these patients were propensity matched against those who did not develop FMS. A small left ventricular end-diastolic diameter (ie, <57 mm) and the use of a small annuloplasty ring (ie, <30 mm) were found to be independent risk factors for the development of late FMS. In addition, patients who developed postrepair FMS had a significantly increased incidence of developing late atrial fibrillation and late tricuspid valve insufficiency, required more late reoperations for valve replacement, and had worse long-term survival. These findings illustrate the high clinical cost associated with late postrepair FMS. It is therefore imperative that the surgeon pay close attention to intraoperative technique to avoid late FMS, which relates to the diastolic flow characteristics intrinsic to the selected annuloplasty device.
Postrepair SAM: The NYU Experience
In 1979 Stephen Colvin performed the first Carpentier-type MVr with a rigid annuloplasty ring at NYU, and in 1985 Frank Spencer reported results from the first 103 repairs.6 In 1983, postrepair SAM with dynamic left ventricular outflow tract (LVOT) obstruction was recognized and described in 2 reports.18,19 Subsequent studies were forthcoming, by Grossi in 1992, and most recently by Loulmet.8,20,21 We have therefore had the opportunity to sequentially evaluate postrepair SAM for over 30 years.
While multiple factors have subsequently been identified related to the risk of postrepair SAM, much of the early understanding was derived from knowledge of hypertrophic cardiomyopathy, whose physiology parallels postrepair SAM. In patients with hypertrophic cardiomyopathy, subaortic septal hypertrophy narrows the LVOT. These patients also have significant papillary muscle and MV leaflet pathology, typically with a very large, elongated anterior MV leaflet. During mid-systole, flow in the LVOT results in acute bending of the elongated anterior mitral leaflet, pushing the leaflet into the outflow tract, where it abuts the septum, leading to obstruction of flow. This mechanism is similar to that of postrepair SAM, except for the etiology of the systolic narrowing of the LVOT.8 In 1992, Grossi and colleagues8 observed that postrepair SAM was associated with a hyperdynamic left ventricle, a large “sail-like” anterior mitral leaflet, extensive quadrangular resection of the posterior leaflet, and “overcorrection” with a ring annuloplasty. It was hypothesized that patients who had MVr with a rigid annuloplasty ring had a smaller systolic LVOT diameter without posterior movement of the anterior annulus and subaortic curtain. This would lead to an increased risk of SAM when the other predisposing leaflet conditions were present.
Due to an improved understanding of MV physiology over the years, as well as refinements in surgical technique, such as the introduction of leaflet height reducing procedures, the incidence of SAM has decreased over time, from an initial incidence of 9% in 1983, to 6.4% in 1992, to 4.0% in the most recent experience.8,18,21, 22, 23 Loulmet and colleagues21 found that the odds ratio for postrepair SAM was increased by the severity of preoperative mitral insufficiency and by left ventricular ejection fraction greater than 60%. The use of a semirigid posterior annuloplasty band and the application of posterior leaflet height reduction procedures were negative predictors of post-repair SAM (Table 2).21
Table 2.
Preoperative and procedural independent risk factors for the development of postrepair SAM
| Factors | Odds ratio (P value) |
|---|---|
| Preoperative | |
| Left ventricular ejection faction >60% | 2.7 (P = .04) |
| Severe mitral regurgitation | 2.5 (P = .08) |
| Procedural | |
| Posterior band annuloplasty∗ | 0.52 (P = .02) |
| Posterior leaflet height reduction procedure† | 0.62 (P = .10) |
Compared with ring annuloplasty.
Posterior leaflet folding plasty or posterior papillary muscle sliding plasty.
The Next Frontier: A Durable, Physiologically Normal Repair
In the current era of mitral valve repair the gold standard should be both durability, in terms of freedom from reoperation and recurrent mitral insufficiency, and a physiologically normal valve without FMS or SAM. The design characteristics of a semirigid posterior annuloplasty band can increase the likelihood of achieving this goal. The surgeon should always consider the height of the repaired leaflets, the diameter of the annulus, and the Ht:W ratio of the annuloplasty device used. Depending on the patient's pathology, height-reduction techniques such as sliding-plasty or folding-plasty for excessively tall posterior leaflets may be beneficial. Data reviewed in this manuscript suggest that the characteristics of a posterior annuloplasty band may mitigate the risks of postrepair FMS and SAM, by allowing physiologic posterior movement of the subaortic curtain and the anterior MV annulus and leaflet during systole, and by flexibility in the surgical placement of the device. Yet while device characteristics can be helpful and important, ultimately achieving a durable and physiologically normal MVr is most dependent on the vigilant observations and technical excellence of the surgeon.
Conflict of Interest Statement
Drs Galloway and Grossi have intellectual property and receive royalties from Medtronic for valve repair devices and have intellectual property and receive royalties from Edwards Lifesciences. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Movement of the sub aortic curtain after repair with semirigid posterior annuloplasty band. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00687-8/fulltext.
References
- 1.Carpentier A. Cardiac valve surgery—the “French correction.”. J Thorac Cardiovasc Surg. 1983;86:323–337. [PubMed] [Google Scholar]
- 2.Carpentier A., Deloche A., Dauptain J., Soyer R., Blondeau P., Piwnica A., et al. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg. 1971;61:1–13. [PubMed] [Google Scholar]
- 3.Carpentier A. Reconstructive valvuloplasty. A new technique of mitral valvuloplasty. Presse Med. 1969;77:251–253. [in French] [PubMed] [Google Scholar]
- 4.Duran C.M., Pomar J.L., Cucchiara G. A flexible ring for atrioventricular heart valve reconstruction. J Cardiovasc Surg (Torino) 1978;19:417–420. [PubMed] [Google Scholar]
- 5.Cosgrove D.M., III, Arcidi J.M., Rodriguez L., Stewart W.J., Powell K., Thomas J.D. Initial experience with the Cosgrove-Edwards Annuloplasty System. Ann Thorac Surg. 1995;60:499–503. doi: 10.1016/0003-4975(95)00458-W. discussion 503-504. [DOI] [PubMed] [Google Scholar]
- 6.Spencer F.C., Colvin S.B., Culliford A.T. Isom OW. Experiences with the Carpentier techniques of mitral valve reconstruction in 103 patients (1980-1985) J Thorac Cardiovasc Surg. 1985;90:341–350. [PubMed] [Google Scholar]
- 7.Galloway A.C., Colvin S.B., Baumann F.G., Esposito R., Vohra R., Harty S., et al. Long-term results of mitral valve reconstruction with Carpentier techniques in 148 patients with mitral insufficiency. Circulation. 1988;78:I97–I105. [PubMed] [Google Scholar]
- 8.Grossi E.A., Galloway A.C., Parish M.A., Asai T., Gindea A.J., Harty S., et al. Experience with twenty-eight cases of systolic anterior motion after mitral valve reconstruction by the Carpentier technique. J Thorac Cardiovasc Surg. 1992;103:466–470. [PubMed] [Google Scholar]
- 9.Sharony R., Saunders P.C., Nayar A., McAleer E., Galloway A.C., Delianides J., et al. Semirigid partial annuloplasty band allows dynamic mitral annular motion and minimizes valvular gradients: an echocardiographic study. Ann Thorac Surg. 2004;77:518–522. doi: 10.1016/j.athoracsur.2003.06.005. discussion 522. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz C.F., Gulkarov I., Bohmann K., Colvin S.B., Galloway A.C. The role of annuloplasty in mitral valve repair. J Cardiovasc Surg (Torino) 2004;45:419–425. [PubMed] [Google Scholar]
- 11.Yaffee D.W., Loulmet D.F., Zias E.A., Ursomanno P.A., Rabinovich A.E., Galloway A.C., et al. Long-term results of mitral valve repair with semi-rigid posterior band annuloplasty. J Heart Valve Dis. 2014;23:66–71. [PubMed] [Google Scholar]
- 12.Fasol R., Meinhart J., Deutsch M., Binder T. Mitral valve repair with the Colvin-Galloway Future Band. Ann Thorac Surg. 2004;77:1985–1988. doi: 10.1016/j.athoracsur.2003.11.023. discussion 1988. [DOI] [PubMed] [Google Scholar]
- 13.Lange R., Guenther T., Kiefer B., Noebauer C., Goetz W., Busch R., et al. Mitral valve repair with the new semirigid partial Colvin-Galloway Future annuloplasty band. J Thorac Cardiovasc Surg. 2008;135:1087–1093. doi: 10.1016/j.jtcvs.2007.11.037. 1093.e1-4. [DOI] [PubMed] [Google Scholar]
- 14.Loulmet D.F., Ranganath N.K., Neuburger P.J., Nampiaparampil R.G., Galloway A.C., Grossi E.A. Can complex mitral valve repair be performed with robotics? An institution's experience utilizing a dedicated team approach in 500 patients. Eur J Cardiothorac Surg. 2019;56:470–478. doi: 10.1093/ejcts/ezz029. [DOI] [PubMed] [Google Scholar]
- 15.Gorman J.H., III, Jackson B.M., Moainie S.L., Enomoto Y., Gorman R.C. Influence of inotropy and chronotropy on the mitral valve sphincter mechanism. Ann Thorac Surg. 2004;77:852–857. doi: 10.1016/j.athoracsur.2003.08.050. discussion 857-858. [DOI] [PubMed] [Google Scholar]
- 16.Mesana T.G., Lam B.K., Chan V., Chen K., Ruel M., et al. Clinical evaluation of functional mitral stenosis after mitral valve repair for degenerative disease: potential affect on surgical strategy. J Thorac Cardiovasc Surg. 2013;146:1418–1423. doi: 10.1016/j.jtcvs.2013.08.011. discussion 1423-1425. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.H., Lee S.H., Joo H.C., Youn Y.N., Yoo K.J., Chang B.C., et al. Long-term clinical impacts of functional mitral stenosis after mitral valve repair. Ann Thorac Surg. 2021;111:1207–1215. doi: 10.1016/j.athoracsur.2020.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Kronzon I., Cohen M.L., Winer H.E., Colvin S.B. Left ventricular outflow obstruction: a complication of mitral valvuloplasty. J Am Coll Cardiol. 1984;4:825–828. doi: 10.1016/s0735-1097(84)80413-1. [DOI] [PubMed] [Google Scholar]
- 19.Gallerstein P.E., Berger M., Rubenstein S., Berdoff R.L., Goldberg E. Systolic anterior motion of the mitral valve and outflow obstruction after mitral valve reconstruction. Chest. 1983;83:819–820. doi: 10.1378/chest.83.5.819. [DOI] [PubMed] [Google Scholar]
- 20.Grossi E.A., Steinberg B.M., LeBoutillier M., III, Ribacove G., Spencer F.C., Galloway A.C., et al. Decreasing incidence of systolic anterior motion after mitral valve reconstruction. Circulation. 1994;90:II195–II197. [PubMed] [Google Scholar]
- 21.Loulmet D.F., Yaffee D.W., Ursomanno P.A., Rabinovich A.E., Applebaum R.M., Galloway A.C., et al. Systolic anterior motion of the mitral valve: a 30-year perspective. J Thorac Cardiovasc Surg. 2014;148:2787–2793. doi: 10.1016/j.jtcvs.2014.07.076. [DOI] [PubMed] [Google Scholar]
- 22.Mihaileanu S., Marino J.P., Chauvaud S., Perier P., Forman J., Vissoat J., et al. Left ventricular outflow obstruction after mitral valve repair (Carpentier's technique). Proposed mechanisms of disease. Circulation. 1988;78:I78–I84. [PubMed] [Google Scholar]
- 23.Grossi E.A., Galloway A.C., Kallenbach K., Miller J.S., Esposito R., Schwartz D.S., et al. Early results of posterior leaflet folding plasty for mitral valve reconstruction. Ann Thorac Surg. 1998;65:1057–1059. doi: 10.1016/s0003-4975(98)00088-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movement of the sub aortic curtain after repair with semirigid posterior annuloplasty band. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00687-8/fulltext.