We projected the impact of a Scaling Up Nutrition intervention policy, the Great Crusade, and found that increasing intervention coverage is unlikely to improve child stunting outcomes in Guatemala to meet Sustainable Development Goals by 2030.
Key Finding
Scaling-up coverage of evidence-based nutrition interventions recommended by the international community would only lead to a small improvement in child stunting in Guatemala and are unlikely to meet the ambitious national goals set for 2024 or the 2030 SDGs.
Our results support an increased focus on strategies that address the social determinants that contribute to stunting rather than a narrow focus on nutrition-specific interventions.
Key Implications
Large improvements in child stunting in some high-burden countries like Guatemala are unlikely to be achieved solely based on increases in nutrition intervention coverage.
Multisectoral nutritional and social policies are needed to address the structural drivers of stunting.
ABSTRACT
Background:
Child stunting is a critical global health issue. Guatemala has one of the world's highest levels of stunting despite the sustained commitment to international nutrition policy best practices endorsed by the Scaling Up Nutrition (SUN) movement. Our objective was to use Guatemala as a case study to project the impact of a recently published national nutrition policy, the Great Crusade, that is consistent with SUN principles.
Methods:
We used the Lives Saved Tool (LiST) to project the impact of scaling-up of nutrition interventions proposed in the Great Crusade and recommended by SUN. Our outcomes were changes in stunting prevalence, number of stunting cases averted, and number of cases averted by intervention in children under 5 years of age from 2020 to 2030. We considered 4 scenarios: (1) intervention coverage continues based on historical trends, (2) coverage targets in the Great Crusade are achieved, (3) coverage targets in the Great Crusade are achieved with reduced fertility risk, and (4) coverage reaches an aspirational level.
Results:
All scenarios led to modest reductions in stunting prevalence. In 2024, stunting prevalence was estimated to change by −0.1% (95% confidence interval [CI]= 0.0%,−0.2%) if historical trends continue, −1.1% (95% CI=−0.8%,−1.5%) in the Great Crusade scenario, and −2.2% (95% CI=−1.6%,−3.0%) in the aspirational scenario. In 2030, we projected a stunting prevalence of −0.4% (95% CI=−0.2%,−0.8%) and −3.7% (95% CI=−2.8%,−5.1%) in the historical trends and aspirational scenario, respectively. Complementary feeding, sanitation, and breastfeeding were the highest-impact interventions across models.
Conclusions:
Targeted reductions in child stunting prevalence in Guatemala are unlikely to be achieved solely based on increases in intervention coverage. Our results show the limitations of current paradigms recommended by the international nutrition community. Policies and strategies are needed to address the broader structural drivers of stunting.
Resumen en español al final del artículo.
BACKGROUND
More than 149 million children under 5 years of age worldwide have inadequate linear growth, which is also referred to as child stunting.1 Stunting is a crucial global child health issue given its negative impacts on health and well-being throughout the lifespan. In the short term, stunted children are more likely to experience morbidity, mortality, and developmental delays.2 In the long term, adults who were stunted as children are at risk for lower cognitive performance, educational achievement, and economic productivity.3–5 Stunting also may contribute to the development of adult cardiometabolic conditions such as diabetes, obesity, and hypertension.6,7 Sustainable Development Goal (SDG) target 2.2 aims to reduce the number of stunted children by 40% by 2025 and to eliminate all forms of malnutrition by 2030.
In response to the increasing recognition of malnutrition as a problem of global importance, the Scaling Up Nutrition (SUN) movement was founded in September 2010.8 The SUN movement works to end all forms of malnutrition by facilitating coordination between local, regional, and international stakeholders.8 More than 60 countries have joined the SUN movement by establishing national organizations of nutrition stakeholders that are supported by a high-level individual in the government.9 Increasing the coverage of evidence-based nutrition interventions is a central focus of the SUN agenda for improving nutrition in member countries. These interventions are targeted during the “first 1,000 days.” This period of time refers to the critical period of growth and development that occurs between conception and a child's second birthday.
The current study examines child nutrition policy in Guatemala, a Central American country of 16.9 million people. In 2005, Guatemala ratified the National Food and Nutrition Security System (SINASAN in Spanish), establishing a legal framework and political commitment to address stunting.10 In 2010, Guatemala joined the SUN movement, affirming many of its previous commitments while incorporating SUN principles into its subsequent national nutrition plans.11–13 In 2015, the SUN movement singled out Guatemala as a model case of a country that had made significant progress on addressing nutrition.14 In 2017, Guatemala was ranked number 1 of 45 countries in its political commitment to nutrition in the Hunger and Nutrition Commitment Index.15 However, despite more than a decade of political commitment to nutrition, Guatemala still has one of the world's highest levels of stunting.2,16 Overall, 47% of Guatemalan children under the age of 5 years are stunted.17 Within Guatemala, there are marked disparities in stunting according to wealth status, educational level, and ethnicity that have not substantially improved in the last 2 decades.16,18 Nearly 70% of poor indigenous children in Guatemala are stunted.19
Despite more than a decade of political commitment to nutrition, Guatemala still has one of the world's highest levels of stunting.
In February 2020, the new Guatemalan government led by President Alejandro Giammattei released the most recent national nutrition policy, the Gran Cruzada Nacional por la Nutrición (“Great National Crusade for Nutrition,” henceforth “Great Crusade”).13 Like previous national nutrition policies that were influenced by the SUN framework, the Great Crusade focuses on evidence-based interventions delivered during the 1,000-day window.11,12 Interventions in the Great Crusade include both nutrition-specific and nutrition-sensitive interventions. Nutrition-specific interventions address the immediate determinants of stunting through micronutrient supplementation, breastfeeding promotion, complementary feeding, and other strategies.20 Nutrition-sensitive interventions address the structural underpinnings of chronic malnutrition by targeting poverty, education, women's empowerment, environmental protection, social safety nets, and other targets.21 One of the Great Crusade's principal goals is to reduce the prevalence of child stunting by 7% by 2024.13
However, it is uncertain if the Great Crusade can reach this ambitious goal. Previous national policies have not met stunting prevalence targets.22 Nutrition-specific interventions form the backbone of the Great Crusade's recommendations, but these interventions tend to have small effect sizes on stunting in clinical trials.23 Nutrition-sensitive interventions put forth by the Great Crusade, while promising, have limited evidence of benefit on child growth.21 Finally, implementation of nutrition interventions in Guatemala historically has been inconsistent, which may limit the impact of national policies.24
The objective of our study was to project the impact of the 2020 Guatemalan national nutrition policy, the Great Crusade. We used a popular maternal and child health modeling program, the Lives Saved Tool (LiST), to carry out our projections. Realistic projections can show the potential impact and uncertainties of nutrition policy in Guatemala and assist in the policy's implementation. Finally, as a case study in the real-time application of a policy modeling tool, this study may assist policymakers in other low- and middle-income countries who wish to project the impact and uncertainty of maternal and child health policies in their own countries.
METHODS
This modeling study used observational data and LiST to project the policy impact of the Great Crusade. LiST is a publicly available maternal and child health modeling tool within the Spectrum software package version 5.761 (Avenir Health). Previous publications have detailed LiST's methodology.25–28 LiST is a linear and deterministic model that uses publicly available data to project maternal and child outcomes including stunting.29,30 Although not a probabilistic model, LiST contains an uncertainty analysis. LiST can assess uncertainty by randomly sampling distributions around the model's inputs.31 We used LiST's uncertainty analysis tool by running 250 iterations with plausibility bounds set to 95%. Our projections are included in a Supplement. LiST was developed in 2003 and is maintained by the Johns Hopkins Bloomberg School of Public Health with funding from the Bill and Melinda Gates Foundation.25 Since its development, LiST has been used in more than 110 peer-reviewed research publications.32 Many LiST publications have been seminal contributions in the field of global maternal and child health such as Disease Control Priorities 3rd Edition33 and the 2008 and the 2013 Lancet Maternal and Child Undernutrition Series papers.23
Data Sources
Data inputs necessary to project stunting in LiST cover 4 broad categories: (1) demographics, (2) baseline child and maternal health characteristics, (3) intervention coverage levels, and (4) intervention effectiveness.
National-level default input data for most countries including Guatemala are available for download through Spectrum. The default data sources include demographic surveys, academic research, and estimates produced by international bodies such as the World Health Organization (WHO), United Nations Children's Fund (UNICEF), United Nations Population Fund (UNFPA), World Bank, and United Nations. Default data are updated either annually or as often as national surveys are released.34
We carefully reviewed each default input for Guatemala, and, using our knowledge of local data sources, updated inputs with more recent or appropriate values. With one exception, data on intervention effectiveness were not changed from the default values estimated from systematic reviews, meta-analyses, Delphi methods, and randomized control trials.34 In May 2020, after consulting with a member of the LiST team at the Johns Hopkins Bloomberg School of Public Health, we were informed of new intervention effectiveness estimates for water, sanitation, and hygiene (WASH) interventions. We then manually updated these estimates in the model. A complete list of data sources and input parameters used in this study can be found in the Supplement.
Outcomes
We defined policy impact through our primary outcomes of changes in stunting prevalence, number of stunting cases averted, and number of stunting cases averted by intervention. Stunting is defined in LiST as children with a height-for-age that is 2 standard deviations or more below the median of the WHO Child Growth Standards.35
Intervention Variables
We first reviewed the interventions that impact child stunting in LiST and cross-referenced them with the interventions recommended in the Great Crusade. Of the 15 stunting-related interventions included in LiST, 14 were proposed in the Great Crusade. We subsequently excluded 2 interventions described in the Great Crusade that are not epidemiologically significant in Guatemala and 2 additional interventions for which data were not available. Our final model included 10 interventions (Table and Supplement). Of note, the Great Crusade includes interventions, such as education and conditional-cash transfer programs, that are not included in LiST because there is no high-level evidence that they are effective.
TABLE.
Stunting-Related Interventions Included in the Models
| Interventions in the Lives Saved Tool That Affect Stunting | Interventions Covered in the Great Crusade | Interventions Included in the Models | Reason Not Included in Models | Baseline (2020) Coverages, % |
|---|---|---|---|---|
| Calcium supplementation in pregnant women | X | Data not available | ||
| Multiple micronutrient supplementation in pregnancy | X | X | 82.3 | |
| Breastfeeding (early initiation) | X | X | 66.2 | |
| Complementary feeding education | X | X | 62.6 | |
| Complementary feeding education and supplementary feeding | X | X | 62.6 | |
| Vitamin A supplementation | X | X | 26.0 | |
| Zinc supplementation | X | X | 86.1 | |
| Basic sanitation | X | X | 65.4 | |
| Point-of-use water filter or piped in water | X | X | 89.2 | |
| Handwashing with soap | X | X | 76.9 | |
| Rotavirus (2 doses) | X | X | 88.4 | |
| Kangaroo mother care | X | Data not available | ||
| Balanced energy supplementation | Not described in the Great Crusade | |||
| Insecticide-treated nets and indoor residual spraying for malaria control | X | Not epidemiologically significant | ||
| Intermittent preventive treatment of malaria in pregnancy | X | Not epidemiologically significant |
Scenarios
We modeled 4 scenarios of intervention coverage change from 2020 to 2030. We chose this period because it encompasses both the Great Crusade's targets in 2024 as well as the SDG targets in 2030. Each scenario started with the same inputs for the baseline year of 2020 and continued over time based upon the below assumptions. Baseline, 2024, and 2030 coverage levels can be found in the Supplement.
Four different scenarios of intervention coverage change from 2020 to 2030 were modeled to determine the potential impact of intervention.
Scenario 1: Historical Trends
Our baseline scenario estimated future coverage levels based on past historical trends. As described below and similar to prior LiST analyses,36 we estimated future trends in intervention coverage based on regression models of historical survey data. The key assumption of this scenario is that past intervention coverage trends predict future coverage.
Scenario 2: Great Crusade
Intervention coverage levels were set to the goals detailed in the Great Crusade.13 From 2024 to 2030, coverage levels remained static.
Scenario 3: Great Crusade With Decreased Fertility Risk
Intervention coverage levels were set to the goals detailed in the Great Crusade. Fertility risk was lowered by eliminating pregnancies before 18 years of age and birth intervals of less than 24 months by 2030. This scenario assumed a hypothetical implementation of policies and interventions to eliminate teen pregnancy and short interpregnancy intervals. These measures have been associated with improved child stunting in international and national surveys but were not otherwise included as an intervention in the models.37,38
Scenario 4: Aspirational Coverage
Intervention coverage levels were set to reach 90% by 2024. From 2024 to 2030, coverage levels remained static.
Analyses
Data Preparation
Given that the majority of survey data available in Guatemala were at least 3 years old, we updated model input parameters for the baseline year of 2020. We estimated baseline levels by fitting a logistic regression curve fixed to pass through the last available data point of population-averaged survey data. Shifting the trendline in this manner has been done in a prior LiST study and reflects our greater confidence in more recent survey estimates in Guatemala.36 Stata version 16.1 was used in these analyses.
Consistent with previous LiST studies, increases in intervention coverage over time were assumed to increase linearly from the base year.39,40 In instances in which coverage inputs exceeded 90%, we fixed values at 90% for the remaining years to reflect a reasonable upper bound for coverage. We did not fix the upper bound of WASH interventions, as we believe that these interventions will follow a meaningful trend of improvement beyond this level.
Consistent with previous LiST studies, increases in intervention coverage over time were assumed to increase linearly from the base year.39,40 In instances where coverage inputs exceeded 90%, we fixed values at 90% for the remaining years to reflect a reasonable upper bound for coverage. We did not fix the upper bound of WASH interventions, as we believe that these interventions will follow a meaningful trend of improvement beyond this level.
Ethics and Preregistration
This study uses de-identified data and did not require approval by the Institutional Review Board of the University of Michigan (UM00177760). We preregistered our analysis at the Open Science Foundation on April 13, 2020.41
RESULTS
Changes in Stunting Prevalence
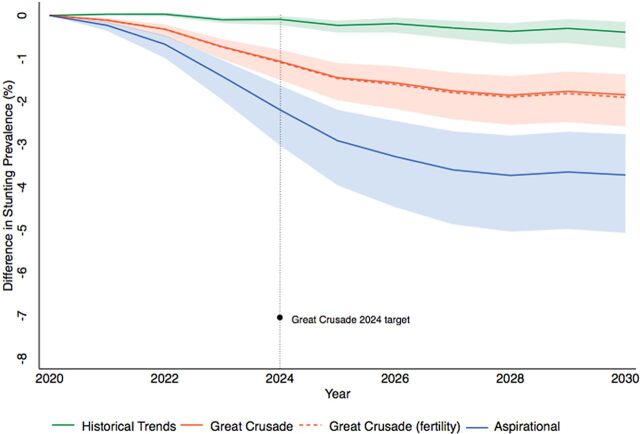
The projected impact of scaling up intervention coverage for each scenario is depicted in Figure 1. The shaded area represents 95% confidence intervals (CIs), which for the Great Crusade (fertility) model are omitted to simplify the figure given overlapping uncertainty bands.
FIGURE 1.
Projected Change in Stunting Prevalence in Children Under 5 Years From the Baseline Year by Scenario
2020–2024 Outcomes
If intervention coverage levels increase at historical levels stunting prevalence is projected to change in 2024 by −0.1% (95% CI=0.0%,−0.2%). The projected change in stunting prevalence in the Great Crusade model by 2024 is a −1.1% (95% CI=−0.8%,−1.5%) change. The projected change in stunting prevalence in the Great Crusade with reduced fertility risk model is nearly identical at −1.1% (95% CI=−0.8%,−1.6%). Our aspirational model projects a −2.2% (95% CI=−1.6%,−3.0%) change in stunting prevalence by 2024.
2020–2030 Outcomes
In the Great Crusade model that assumes coverage goals are attained in 2024 and then maintained from 2024 to 2030, the model projected a change in the prevalence of stunting of −1.8% (95% CI=−1.4%,−2.6%). The historical trends and aspirational scenarios projected a change in stunting from 2020 to 2030 of −0.4% (95% CI=−0.2%,−0.8%) and −3.7% (95% CI=−2.8%,−5.1%), respectively.
Stunting Cases Averted in 2024 and 2030
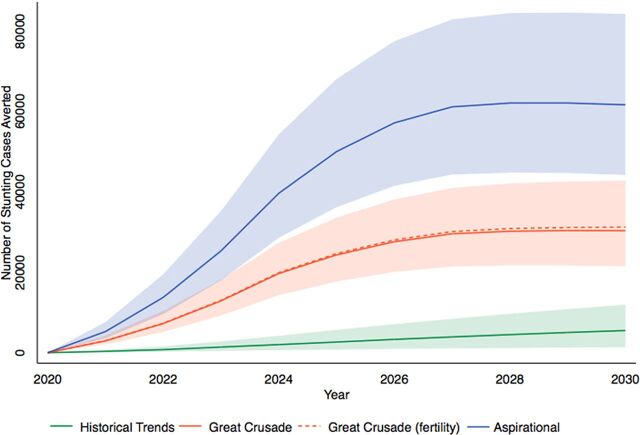
Figure 2 shows the estimated number of stunted cases that would be averted by scenario per year. The shaded area represents 95% CIs, which for the Great Crusade (fertility) model are omitted to simplify the figure given overlapping uncertainty bands.
FIGURE 2.
Projected Number of Stunting Cases Averted in Children Under 5 Years per Year From the Baseline Year by Scenario
2020–2024 Outcomes
The estimated number of cumulative stunting cases averted in the historical trends model from 2020 to 2024 is 4,463. The Great Crusade is projected to avert 42,754 total cases from 2020 to 2024, and implementation of the Great Crusade along with decreasing fertility risk would avert 43,208 total cases over this period. The aspirational model is estimated to avert 83,970 total cases of stunting from 2020 to 2024.
2020–2030 Outcomes
From 2020 to 2030, if intervention coverage continues to rise based on historical trends, we have estimated that 29,307 total cases of stunting will be averted. The Great Crusade plan is projected to avert 215,033 total cases during this time, and the Great Crusade model along with decreasing fertility risk would avert 219,167 total cases. The aspirational model is estimated to avert 437,958 total cases of stunting from 2020 to 2030.
Stunting Cases Averted by Intervention
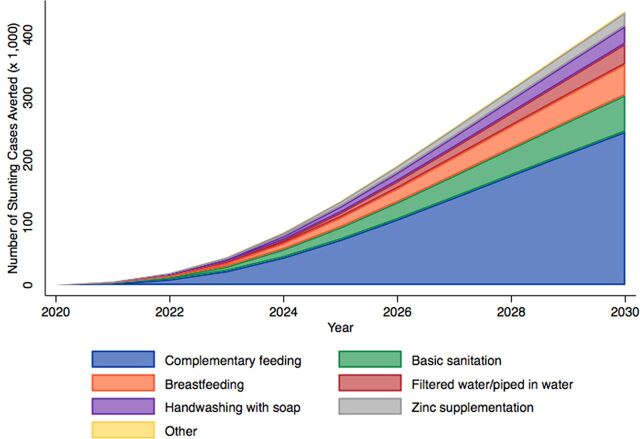
Figure 3 elaborates the contribution of each intervention to the cumulative number of stunting cases averted from 2020 to 2024 (panel A) and from 2020 to 2030 (panel B). In the historical trends model, changes in coverage of point-of-use water filter or piped water primarily contribute to the number of stunting cases averted. Differences in stunting cases averted between the historical trends and the Great Crusade models are driven largely by differential coverage of complementary feeding and sanitation. Differences between the Great Crusade models and aspirational model are largely driven by differential coverage in complementary feeding and breastfeeding promotion.
FIGURE 3.
Contribution of Interventions on Cumulative Stunting Cases Averted by Scenario (A) 2020–2024 (B) 2020–2030a
a Only interventions that contribute at least 1.0% of the total are included in colored subsegments.
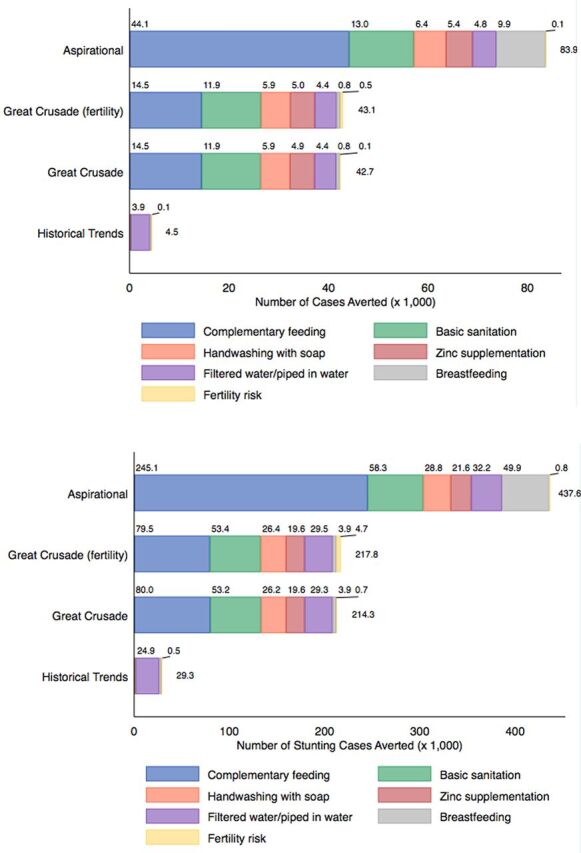
Figure 4 shows the cumulative contribution of each intervention on stunting cases averted in the aspirational model from 2020 to 2030. This model projected that 56% (245,129 of 437,579) of averted stunting cases would be attributable to increasing coverage of complementary feeding.
FIGURE 4.
Contribution of Interventions on Cumulative Stunting Cases Averted in the Aspirational Modela
a “Other” category includes multiple micronutrient supplementation, fertility risk, and rotavirus vaccination.
We include the complete output data of our models in the Supplement.
DISCUSSION
This study used LiST to project the impact of the 2020 Guatemalan national nutrition policy, the Great Crusade. We found that increases in intervention coverage proposed in the Great Crusade are unlikely to improve child stunting outcomes to a sufficient degree to meet 2024 national targets or 2030 SDG targets. We also described the uncertainty of our projections and the relative impact of different interventions. Our study has implications for the optimization of nutrition in Guatemala and other low- and middle-income countries.
Increased intervention coverage as proposed in the Great Crusade are unlikely to improve child stunting outcomes enough to meet 2024 national targets or 2030 SDG targets.
Our modeling study of nutrition policy in Guatemala reveals limitations in the SUN framework that focuses on scaling up evidence-based nutrition interventions. Despite strong political commitment to the SUN movement since 2010, Guatemala has not met historical stunting targets and, as our study shows, may have difficulty meeting future goals. In the 2013 Lancet series on Maternal and Child Health, it was estimated that increasing the coverage of 10 evidence-based interventions to 90% in 34 high-burden countries would result in a 20% relative reduction in stunting prevalence.23 Our models suggest that increasing intervention coverage to 90% in Guatemala would result in a 4.7% relative reduction in stunting in 2024 and 8.0% relative reduction in 2030. The modest impact of nutrition coverage expansion in Guatemala compared with other countries may be explained by the relatively high baseline intervention coverage in Guatemala. Taken together, our findings imply that there may be substantial heterogeneity in impact between countries that implement evidence-based nutrition stunting interventions endorsed within the SUN framework. In other country case studies, contextual differences and variations in investments both within and outside the health sector have been proposed as explanatory factors for improvements in stunting.42
Our findings also reveal the limitations of currently available evidence to prevent stunting. Except for WASH, all the stunting interventions incorporated in LiST and included in our models are nutrition-specific interventions. These interventions are supported by high-level evidence, but their effect sizes are generally modest. As an example, complementary food supplementation in food-insecure populations causes a 0.10 (95% CI=0.03,0.17) improvement in length-for-age Z-scores.43 Nutrition-sensitive interventions focusing on the broader context of nutrition including poverty, agriculture, social safety nets, education, and other areas have been proposed as a potential way to generate more sizeable reductions in stunting.44,45 In Peru, for example, large improvements in child stunting observed from 2000 to 2013 were likely attributable to economic growth, increased societal participation, poverty-reduction strategies, and increased health spending.46,47 Furthermore, a retrospective review of 5 exemplar countries that made significant improvements in stunting found that nutrition-sensitive strategies accounted for 50% of the total stunting reduction.42 Despite compelling ecological evidence, nutrition-sensitive interventions have not consistently shown improvements in linear growth in randomized controlled trials. Future research is urgently needed in this area.21
Our study supports the emerging criticism of SUN's emphasis on technical solutions to address stunting. An independent review of the SUN movement has acknowledged that (1) there is limited evidence that SUN has improved nutrition outcomes of member countries, (2) some countries including Guatemala that closely adhere to SUN have not observed meaningful improvements in stunting, and (3) SUN's standardized approach does not sufficiently account for local country factors.48 Other critics have argued that SUN emphasizes short-term technical solutions with limited consideration of the structural causes of stunting.49 In this view, even SUN's nutrition-sensitive interventions primarily serve to benefit commercial food systems that disrupt indigenous food cultures, decrease confidence in local foods, and reduce biodiversity.49 In Guatemala and elsewhere, critics of SUN have pointed out conflicts of interest,50 human-rights concerns among adolescent mothers,51,52 and detachment from communities affected by malnutrition.49 Although our study does not address these broader critiques, our results buttress criticisms against SUN's emphasis on narrow technical interventions and call for increased focus on strategies that address the social determinants that give rise to stunting.
The current study supports the emerging criticism of SUN's emphasis on technical solutions to address stunting.
With respect to our study's implications in Guatemala, a principal target of the Great Crusade is a 7% reduction in stunting prevalence from 2020 to 2024. Our study suggests that this target is very ambitious and unlikely to be achieved solely based on scaling-up interventions recommended by SUN and outlined in the Great Crusade. According to our projections, even aspirational levels of coverage would only reduce stunting prevalence by −2.2% (95% CI=−1.6%,−3.0%) in 2024, −0.6% per year. Previous reviews of countries that made significant strides in improving stunting have shown that per year reductions in stunting greater than 1% are possible.46,53 The previous Guatemalan national nutrition policies from 2012 to 201611 and from 2016 to 202012 were also unable to reach targets of a 10% absolute reduction in stunting. Importantly, while the Great Crusade confers only a small absolute reduction in stunting prevalence in our models, we also project that the policy would avert over 42,000 total stunting cases from 2020 to 2024 and 217,000 total cases from 2020 to 2030. This absolute number of cases averted may be viewed as substantial by policy makers and nutrition stakeholders in a country with a total population of 16.9 million people.
We found dramatic differences in impact among interventions recommended by SUN and included in the Great Crusade. Complementary feeding and basic sanitation contribute to more than half of the stunting cases averted in most of our models. In LiST, complementary feeding is defined as the percentage of mothers who received counseling on complementary feeding practices and continuing breastfeeding after 6 months.54 Complementary feeding has a particularly large impact in the aspirational models, and we estimate that increasing complementary feeding intervention coverage to 90% by 2024 would avert 245,000 total stunting cases from 2020 to 2030. After complementary feeding, the intervention contributing the greatest impact on stunting reduction between the Great Crusade and aspirational models was breastfeeding promotion. Interventions such as complementary feeding and breastfeeding promotion are “double-duty actions” that also may confer beneficial impacts on obesity and other diet-related noncommunicable diseases.55 Notably, some interventions such as multiple micronutrient supplementation in pregnancy, rotavirus vaccine, and vitamin A supplementation had minimal impact on stunting due to high levels of coverage at baseline, small effect sizes, or limited need. Previous research has suggested an association between family planning and child stunting,37 but our models found that decreasing fertility risks had a very small impact on stunting. Our results suggest that efforts to reduce stunting should focus resources on scaling up coverage of specific interventions (complementary feeding, basic sanitation, and breastfeeding) over others (multiple micronutrient supplementation in pregnancy, rotavirus vaccine, and vitamin A supplementation) during the Great Crusade's implementation phase.
Efforts to reduce stunting should focus resources on scaling up coverage of specific interventions over others during the Great Crusade's implementation phase.
Our study makes a useful methodological contribution to the broader literature that uses LiST and other modeling tools to project maternal and child health outcomes. To our knowledge, this is the first study that uses LiST to project the impact of a national health policy as the policy is launched.
The strengths of this study include our choice of a well-established modeling tool (LiST) and our detailed assessments of uncertainty. Policy analyses often report exact predictions without assessments of uncertainty, a practice criticized in the literature as “incredible certitude.”56 We agree that uncertainty should be transparently and appropriately communicated in policy analyses including maternal and child health models such as LiST.57 We depict the uncertainty of our models in Figures 2 and 3 where greater changes in intervention coverage and time were accompanied by larger uncertainty. We also updated default inputs with new and nonpublic data sources. Finally, we addressed the gap between the last Demographic and Health Survey in Guatemala (2014–2015) and the first year of our projection (2020) by estimating baseline 2020 inputs using a methodology from the literature.
Limitations
Our study has several limitations. First, our study assesses only a single country, Guatemala. We justify our focus on Guatemala given the country's unique position of extraordinary stunting prevalence and sustained commitment to SUN. Furthermore, country case studies have a rich tradition in informing global policy within the maternal and child health field.46 Second, LiST was designed to estimate the impact of evidence-based interventions with known effect sizes. We are unable to estimate the impact of nutrition-sensitive interventions in the Great Crusade that have uncertain effect sizes such as education and conditional-cash transfer programs. We also are unable to model complex secular trends in stunting prevalence in Guatemala that are likely to be occurring as the country is becoming wealthier and more educated. Third, our study focuses on linear growth—represented by stunting—as a central metric of early child health. However, there has been increasing criticism of the causal assumptions between linear growth in children and long-term developmental outcomes.58 Pioneering studies in Jamaica59–61 and meta-analyses62,63 show that early childhood education and stimulation interventions can improve child development without improving growth. In Guatemala, community-based interventions targeting the determinants of child development (and not solely growth) are important but are not considered in our study.64,65
A final limitation involves the uncertainty of nutrition policy in Guatemala during the COVID-19 pandemic. The Great Crusade was published in February 2020 before the first documented case of COVID-19 in Guatemala. Undoubtedly, COVID-19 will have direct and indirect effects on nutrition in Guatemala. Reports of increased food insecurity and acute malnutrition have emerged,66,67 while funds destined for nutrition programs are being rerouted to fight COVID-19.68 We chose not to consider COVID-19 in our analysis for 3 reasons: (1) our primary goal was to assess the Great Crusade as a policy document, (2) the lack of currently available COVID-19 data in Guatemala make any long-term projections impractical, and (3) modeling health system shocks in LiST requires different modeling techniques and assumptions.69
CONCLUSION
This modeling study using LiST found that that the most recent Guatemalan national nutrition policy, the Great Crusade, will have difficulty achieving 2024 national targets or 2030 SDG targets solely based on increases in intervention coverage. Our results show the limitations of technical solutions to nutrition put forth by the international nutrition community. Despite more than a decade of political commitment on the issue of nutrition, Guatemala may continue to have exceptionally high prevalence of stunting over the coming decade. We recommend the prioritization of interventions that are projected to confer the largest impact such as complementary feeding, breastfeeding, and basic sanitation. Policies and strategies are needed to address the broader social structures that predispose children to stunting.
Supplementary Material
Author contributions
ST and DF designed the study and executed the analysis. AC, GA, MM, MFK, BJ, and PR all made contributions to the study design, data collection, and data analysis. All authors contributed to the production of the manuscript.
Competing interests
None declared.
Translation
En Español
Proyección del Impacto de La Política Nutricional para Mejorar el Retraso del Crecimiento en Niños: Un Estudio de Caso en Guatemala Usando la Herramienta Lives Saved Tool
Hallazgos Clave
La ampliación de la cobertura de las intervenciones nutricionales basadas en evidencia, recomendadas por la comunidad internacional, solamente contribuirán a una pequeña mejora en el retraso del crecimiento de los niños en Guatemala, y es muy poco probable que se cumpla la ambiciosa meta nacional fijada para el 2024 o con los ODS para el 2030.
Nuestros resultados apoyan un enfoque para aumentar las estrategias que abordan los determinantes sociales del retraso del crecimiento en lugar de un enfoque estrecho enfocado en intervenciones específicas de nutrición.
Implicaciones Clave
Es poco probable que se logren grandes mejoras en el retraso del crecimiento de los niños en algunos países con alta prevalencia como Guatemala, solamente con ampliar la cobertura de las intervenciones específicas de nutrición.
Se necesitan políticas nutricionales y sociales multisectoriales para abordar los determinantes estructurales del retraso del crecimiento.
RESUMEN
Contexto: El retraso del crecimiento en niños es un asunto crítico de salud a nivel mundial. Guatemala tiene una de las prevalencias más altas de retraso del crecimiento en niños en el mundo, a pesar de su compromiso sostenido con las intervenciones nutricionales respaldadas por el Movimiento para el Fomento a la Nutrición (SUN por sus siglas en inglés). Nuestro objetivo fue utilizar a Guatemala como un estudio de caso y proyectar el impacto de la política nacional de nutrición recientemente publicada, la Gran Cruzada por la Nutrición, que es consistente con los principios de SUN.
Métodos:Usamos la herramienta Lives Saved Tool (LiST) para proyectar el impacto de ampliar la cobertura de las intervenciones nutricionales propuestas en la Gran Cruzada por la Nutrición y recomendadas por SUN. Nuestros resultados fueron cambios en la prevalencia de retraso del crecimiento, el número de casos evitados de retraso del crecimiento, y el número de casos evitados de retraso del crecimiento por cada intervención en niños menores de 5 años de edad del 2020 al 2030. Consideramos cuatro escenarios: (1) cobertura de las intervenciones basada en las tendencias históricas, (2) metas de cobertura de la Gran Cruzada por la Nutrición alcanzadas, (3) metas de cobertura de la Gran Cruzada por la Nutrición alcanzadas al disminuir la tasa de fertilidad y (4) cobertura ampliada a un nivel aspiracional.
Resultados: Todos los escenarios resultaron en reducciones modestas de la prevalencia de retraso del crecimiento. En 2024, estimamos que la prevalencia de retraso del crecimiento cambiaría en −0.1% (intervalo de confianza 95% [IC]= 0.0%,−0.2%) si las tendencias históricas continúan, −1.1% (IC 95%=−0.8%,−1.5%) en el escenario de la Gran Cruzada por la Nutrición, y −2.2% (IC 95%= −1.6%,−3.0%) en el escenario aspiracional. En 2030, proyectamos una prevalencia de retraso del crecimiento de −0.4% (95% CI=−0.2%,−0.8%) y −3.7% (95% CI=−2.8%,−5.1%) en el escenario de tendencias históricas y en el escenario aspiracional, respectivamente. La alimentación complementaria, saneamiento y la lactancia materna fueron las intervenciones de mayor impacto en todos los modelos.
Conclusiones: Es poco probable que la reducción en la prevalencia de retraso del crecimiento propuesto en Guatemala sea lograda solamente con el aumento de la cobertura de las intervenciones. Nuestros resultados muestran las limitaciones de los paradigmas actuales recomendados por la comunidad internacional de nutrición. Se necesitan políticas y estrategias que aborden los determinantes estructurales del retraso del crecimiento.
Peer Reviewed
First published online: October 26, 2021.
Cite this article as: Tschida S, Cordon A, Asturias G, et al. Projecting the impact of nutrition policy to improve child stunting: a case study in Guatemala using the Lives Saved Tool. Glob Health Sci Pract. 2021;9(4):752-764. https://doi.org/10.9745/GHSP-D-20-00585
REFERENCES
- 1. Development Initiatives. 2020 Global Nutrition Report: Action on Equity to End Malnutrition. Development Initiatives; 2020. Accessed August 27, 2021. https://globalnutritionreport.org/reports/2020-global-nutrition-report/ [Google Scholar]
- 2. Black RE, Victora CG, Walker SP, et al.; Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 3. Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70. 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ford ND, Behrman JR, Hoddinott JF, et al. Exposure to improved nutrition from conception to age 2 years and adult cardiometabolic disease risk: a modelling study. Lancet Glob Health. 2018;6(8):e875–e884. 10.1016/S2214-109X(18)30231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adair LS, Fall CHD, Osmond C, et al.; COHORTS group. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–534. 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeBoer MD, Lima AAM, Oría RB, et al. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev. 2012;70(11):642–653. 10.1111/j.1753-4887.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Lucia Rolfe E, de França GVA, Vianna CA, et al. Associations of stunting in early childhood with cardiometabolic risk factors in adulthood. PLoS One. 2018;13(4):e0192196. 10.1371/journal.pone.0192196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scaling Up Nutrition. An Introduction to the Scaling Up Nutrition Movement. Scaling Up Nutrition; 2014. Accessed August 27, 2021. http://scalingupnutrition.org/wp-content/uploads/2015/06/Orange_Internal_InOutline_ENG_20140415_web.pdf [Google Scholar]
- 9. The vision and principles of SUN. Scaling Up Nutrition. Accessed August 27, 2021. https://scalingupnutrition.org/about-sun/the-vision-and-principles-of-sun/
- 10. Gobierno de Guatemala. Secretaría de Seguridad Alimentaria y Nutricional. Accessed September 9, 2020. http://www.siinsan.gob.gt/siinsan/wp-content/uploads/Ley_de-SAN.pdf
- 11. Gobierno de Guatemala. El Plan Del Pacto Hambre Cero. 2012. Accessed August 27, 2021. https://www.transparencia.gob.gt/wp-content/uploads/2017/07/INF-2012-002.pdf
- 12. Secretaría de Seguridad Alimentaria y Nutricional. Estrategia Nacional Para La Prevención de La Desnutrición Crónica 2016–2020. Comisión Nacional para la Reducción de la Desnutrición Crónica; 2016. Accessed August 27, 2021. http://www.sesan.gob.gt/wordpress/wp-content/uploads/2017/07/Estrategia-para-la-Prevencion-de-la-Desnutricion-Cronica.pdf [Google Scholar]
- 13. Gobierno de Guatemala. Gran Cruzada Nacional Por La Nutrición. 2020. Accessed August 27, 2021. http://www.sesan.gob.gt/wordpress/wp-content/uploads/2020/04/Documento-tecnico-Gran-Cruzada-Nacional_17HD.pdf
- 14. Mokoro Limited. Independent Comprehensive Evaluation of the Scaling Up Nutrition Movement: Final Report—Main Report and Annexe. Mokoro Limited; 2015. Accessed August 27, 2021. http://scalingupnutrition.org/wp-content/uploads/2015/05/SUN_ICE_FullReport-All(1-5-15).pdf [Google Scholar]
- 15. HANCI Global. Hunger and Nutrition Commitment Index. HANCI; 2020. Accessed August 27, 2021. http://www.hancindex.org/hanci/ [Google Scholar]
- 16. Kinyoki DK, Osgood-Zimmerman AE, Pickering BV, et al.; Local Burden of Disease Child Growth Failure Collaborators. Mapping child growth failure across low- and middle-income countries. Nature. 2020;577(7789):231–234. 10.1038/s41586-019-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ministerio de Salud Pública y Asistencia Social (MSPAS), Instituto Nacional de Estadística (INE), Secretaría de Planificación y Programación de la Presidencia (Segeplán). Encuesta Nacional de Salud Materno Infantil 2014–2015. MSPAS, INE, Segeplán; 2017. Accessed August 27, 2021. https://www.ine.gob.gt/images/2017/encuestas/ensmi2014_2015.pdf [Google Scholar]
- 18. Mazariegos M, Kroker-Lobos MF, Ramírez-Zea M. Socio-economic and ethnic disparities of malnutrition in all its forms in Guatemala. Public Health Nutr. 2020;23(S1):s68–s76. 10.1017/s1368980019002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gatica-Domínguez G, Victora C, Barros AJD. Ethnic inequalities and trends in stunting prevalence among Guatemalan children: an analysis using national health surveys 1995–2014. Int J Equity Health. 2019;18(1):110. 10.1186/s12939-019-1016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. United Nations Children's Fund (UNICEF). Improving Child Nutrition: The Achievable Imperative for Global Progress. UNICEF; 2013. Accessed August 27, 2021. https://data.unicef.org/resources/improving-child-nutrition-the-achievable-imperative-for-global-progress/ [Google Scholar]
- 21. Ruel MT, Alderman H; Maternal and Child Nutrition Study Group. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382(9891):536–551. 10.1016/S0140-6736(13)60843-0. [DOI] [PubMed] [Google Scholar]
- 22. International Food Policy Research Institute (IFPRI). Informe Final de Evaluación de Impacto Del Plan Del Pacto Hambre Cero. IFPRI; 2016. Accessed August 27, 2021. http://www.siinsan.gob.gt/siinsan/wp-content/uploads/Informe-Final-Evaluacion-Impacto-PPH0.pdf [Google Scholar]
- 23. Bhutta ZA, Das JK, Rizvi A, et al.; Lancet Nutrition Interventions Review Group, the Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–477. 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 24. Fundación para el Desarrollo de Guatemala (FUNDESA). Alianza por la Nutrición. Informe 6to. Monitoreo de la Ventana de los Mil Días: Informe con los resultados completos del análisis cuantitativo de las distintas bases de datos colectadas. FUNDESA; 2019. Accessed August 27, 2021. https://www.fundesa.org.gt/content/files/publicaciones/20196to_Informe_final_6to_Monitoreo_Ventana_Mil_Dias_-_FUNDESA-APN-comprimido.pdf [Google Scholar]
- 25. Walker N, Tam Y, Friberg IK. Overview of the lives saved tool (LiST). BMC Public Health. 2013;13(Suppl 3):S1. 10.1186/1471-2458-13-S3-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet. 2011;378(9790):515–525. 10.1016/S0140-6736(10)61505-X. [DOI] [PubMed] [Google Scholar]
- 27. Cousens S, Perin J, Christian P, et al. Modelling stunting in LiST: the effect of applying smoothing to linear growth data. BMC Public Health. 2017;17(S4)(Suppl 4):778. 10.1186/s12889-017-4744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clermont A, Walker N. Nutrition Interventions in the Lives Saved Tool (LiST). J Nutr. 2017; 147(11):2132S–2140S. 10.3945/jn.116.243766. [DOI] [PubMed] [Google Scholar]
- 29. Alderman H, Nguyen PH, Menon P. Progress in reducing child mortality and stunting in India: an application of the Lives Saved Tool. Health Policy Plan. 2019;34(9):667–675. 10.1093/heapol/czz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mishra NR, Mohanty SK, Mittra D, Shah M, Meitei WB. Projecting stunting and wasting under alternative scenarios in Odisha, India, 2015–2030: a Lives Saved Tool (LiST)-based approach. BMJ Open. 2019;9(5):e028681. 10.1136/bmjopen-2018-028681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. The Lives Saved Tool. LiST sensitivity and uncertainty description of methods 042820. [Google Scholar]
- 32. LiST in peer-reviewed journals. The Lives Saved Tool. Accessed August 27, 2021. https://www.livessavedtool.org/list-in-peerreviewed-journals
- 33. Black RE, Levin C, Walker N, Chou D, Liu L, Temmerman M. Reproductive, maternal, newborn, and child health: key messages from Disease Control Priorities 3rd Edition. Lancet. 2016;388(10061):2811–2824. 10.1016/s0140-6736(16)00738-8. [DOI] [PubMed] [Google Scholar]
- 34. About LiST. The Lives Saved Tool. Accessed August 27, 2021. https://www.livessavedtool.org/about-1
- 35. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 36. Júnior JM, Cane RM, Gonçalves MP, et al. Projecting the lives saved by continuing the historical scale-up of child and maternal health interventions in Mozambique until 2030. J Glob Health. 2019;9(1):011102. 10.7189/jogh.09.011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flood D, Petersen A, Martinez B, Chary A, Austad K, Rohloff P. Associations between contraception and stunting in Guatemala: secondary analysis of the 2014–2015 Demographic and Health Survey. BMJ Paediatr Open. 2019;3(1):e000510. 10.1136/bmjpo-2019-000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fink G, Sudfeld CR, Danaei G, Ezzati M, Fawzi WW. Scaling-up access to family planning may improve linear growth and child development in low and middle income countries. PLoS One. 2014;9(7):e102391. 10.1371/journal.pone.0102391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chou VB, Friberg IK, Christian M, Walker N, Perry HB. Expanding the population coverage of evidence-based interventions with community health workers to save the lives of mothers and children: an analysis of potential global impact using the Lives Saved Tool (LiST). J Glob Health. 2017;7(2):020401. 10.7189/jogh.07.020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Higgins-Steele A, Yousufi K, Sultana S, Ali AS, Varkey S. Ending preventable child deaths from pneumonia and diarrhoea in Afghanistan: an analysis of intervention coverage scenarios using the Lives Saved Tool. J Trop Med. 2017;2017:3120854. 10.1155/2017/3120854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Open Science Framework (OSF). Simulating the impact of the Gran Cruzada using the Lives Saved Tool (LiST). OSF; 2020. Accessed August 27, 2021. https://osf.io/2nu87 [Google Scholar]
- 42. Bhutta ZA, Akseer N, Keats EC, et al. How countries can reduce child stunting at scale: lessons from exemplar countries. Am J Clin Nutr. 2020;112(Supplement_2):894S–904S. 10.1093/ajcn/nqaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Panjwani A, Heidkamp R. Complementary feeding interventions have a small but significant impact on linear and ponderal growth of children in low- and middle-income countries: a systematic review and meta-analysis. J Nutr. 2017;147(11):jn243857. 10.3945/jn.116.243857. [DOI] [PubMed] [Google Scholar]
- 44. Stewart CP, Iannotti L, Dewey KG, Michaelsen KF, Onyango AW. Contextualising complementary feeding in a broader framework for stunting prevention. Matern Child Nutr. 2013;9(Suppl 2):27–45. 10.1111/mcn.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hossain M, Choudhury N, Adib Binte Abdullah K, et al. Evidence-based approaches to childhood stunting in low and middle income countries: a systematic review. Arch Dis Child. 2017;102(10):903–909. 10.1136/archdischild-2016-311050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huicho L, Segura ER, Huayanay-Espinoza CA, et al.; Peru Countdown Country Case Study Working Group. Child health and nutrition in Peru within an antipoverty political agenda: a Countdown to 2015 country case study. Lancet Glob Health. 2016;4(6):e414–e426. 10.1016/S2214-109X(16)00085-1. [DOI] [PubMed] [Google Scholar]
- 47. Huicho L, Vidal-Cárdenas E, Akseer N, et al. Drivers of stunting reduction in Peru: a country case study. Am J Clin Nutr. 2020;112(Suppl_2):816S–829S. 10.1093/ajcn/nqaa164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strategic Review of the Scaling Up Nutrition (SUN) Movement, 2019–2020. 2020. Accessed August 27, 2021. https://scalingupnutrition.org/wp-content/uploads/2020/04/SUN-Strategic-Review-Final-Report_ENG.pdf
- 49. Michéle L, Prato S, Rundall P, Valente F. When the SUN Casts a Shadow—The Human Rights Risks of Multi-Stakeholder Partnerships: The Case of Scaling up Nutrition (SUN). FIAN International, IBFAN, Society for International Development; 2019. Accessed August 27, 2021. https://fian.org/files/files/WhenTheSunCastsAShadow_En.pdf [Google Scholar]
- 50. Lie AL. ‘We are not a partnership’—constructing and contesting legitimacy of global public-private partnerships: the Scaling Up Nutrition (SUN) Movement. Globalizations. 2020;18(2):237–255. 10.1080/14747731.2020.1770038 [DOI] [Google Scholar]
- 51. Flood D, Chary A, Colom A, Rohloff P. Adolescent rights and the “First 1,000 days” global nutrition movement: a view from Guatemala. Health Hum Rights. 2018;20(1):295–301. [PMC free article] [PubMed] [Google Scholar]
- 52. Colom A. Forced motherhood in Guatemala: an analysis of the Thousand Days Initiative. In: Chary A, Rohloff P, eds. Privatization and the New Medical Pluralism: Shifting Healthcare Landscapes in Maya Guatemala. Lexington Books; 2015:35–49. [Google Scholar]
- 53. Zanello G, Srinivasan CS, Shankar B. What explains Cambodia's success in reducing child stunting—2000–2014? PLoS One. 2016;11(9):e0162668. 10.1371/journal.pone.0162668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Avenir Health. Spectrum Manual: Spectrum System of Policy Models. Avenir Health; 2021. Accessed August 27, 2021. https://avenirhealth.org/Download/Spectrum/Manuals/SpectrumManualE.pdf [Google Scholar]
- 55. Hawkes C, Ruel MT, Salm L, Sinclair B, Branca F. Double-duty actions: seizing programme and policy opportunities to address malnutrition in all its forms. Lancet. 2020;395(10218):142–155. 10.1016/S0140-6736(19)32506-1. [DOI] [PubMed] [Google Scholar]
- 56. Manski CF. Communicating uncertainty in policy analysis. Proc Natl Acad Sci USA. 2019;116(16):7634–7641. 10.1073/pnas.1722389115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hodgins S. Modeling outputs can be valuable when uncertainty is appropriately acknowledged, but misleading when not. Glob Health Sci Pract. 2017;5(4):530–533. 10.9745/GHSP-D-17-00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leroy JL, Frongillo EA. Perspective: what does stunting really mean? A critical review of the evidence. Adv Nutr. 2019;10(2):196–204. 10.1093/advances/nmy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gertler P, Heckman J, Pinto R, et al. Labor market returns to an early childhood stimulation intervention in Jamaica. Science. 2014;344(6187):998–1001. 10.1126/science.1251178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Walker SP, Chang SM, Vera-Hernández M, Grantham-McGregor S. Early childhood stimulation benefits adult competence and reduces violent behavior. Pediatrics. 2011;127(5):849–857. 10.1542/peds.2010-2231. [DOI] [PubMed] [Google Scholar]
- 61. Walker SP, Chang SM, Powell CA, Grantham-McGregor SM. Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-stunted Jamaican children: prospective cohort study. Lancet. 2005;366(9499):1804–1807. 10.1016/S0140-6736(05)67574-5. [DOI] [PubMed] [Google Scholar]
- 62. Aboud FE, Yousafzai AK. Global health and development in early childhood. Annu Rev Psychol. 2015;66(1):433–457. 10.1146/annurev-psych-010814-015128. [DOI] [PubMed] [Google Scholar]
- 63. Prado EL, Larson LM, Cox K, Bettencourt K, Kubes JN, Shankar AH. Do effects of early life interventions on linear growth correspond to effects on neurobehavioural development? A systematic review and meta-analysis. Lancet Glob Health. 2019;7(10):e1398–e1413. 10.1016/S2214-109X(19)30361-4. [DOI] [PubMed] [Google Scholar]
- 64. Durao S, Visser ME, Ramokolo V, et al. Community-level interventions for improving access to food in low- and middle-income countries. Cochrane Database Syst Rev. 2020;7(7):CD011504. 10.1002/14651858.cd011504.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Engle PL, Fernald LCH, Alderman H, et al.; Global Child Development Steering Group. Strategies for reducing inequalities and improving developmental outcomes for young children in low-income and middle-income countries. Lancet. 2011;378(9799):1339–1353. 10.1016/S0140-6736(11)60889-1. [DOI] [PubMed] [Google Scholar]
- 66. La COVID-19 duplica el número de personas sin alimentos en Guatemala. Acción Contra El Hambre. June 15, 2020. Accessed August 27, 2021. https://www.accioncontraelhambre.org/es/te-contamos/actualidad/la-covid-19-duplica-el-numero-de-personas-sin-alimentos-en-guatemala
- 67. Lucía Ola A. Crisis del coronavirus: Desnutrición y el hambre que agobia a varias familias del Corredor Seco. Prensa Libre. May 4, 2020. Accessed August 27, 2021. https://www.prensalibre.com/guatemala/comunitario/antes-del-coronavirus-en-el-corredor-seco-la-gente-lucha-contra-el-hambre/ [Google Scholar]
- 68. Lucía Ola A. Salud reduce presupuesto a programas contra la desnutrición y traslada los fondos al combate del covid-19. Prensa Libre. June 23, 2020. Accessed August 27, 2021. https://www.prensalibre.com/guatemala/comunitario/salud-reduce-presupuesto-a-programas-contra-la-desnutricion-y-traslada-los-fondos-al-combate-del-covid-19/
- 69. Roberton T, Carter ED, Chou VB, et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(7):e901–e908. 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.