
Pulmonary allograft sewn in orthotopic position, double suture line.
Central Message.
Autograft harvest and right ventricular outflow tract reconstruction equally contribute to the long-term outcomes of the Ross procedure.
The Ross procedure (RP) radically addresses aortic valve pathology with a biologically sound double-valve operation. It essentially involves replacing the patient's diseased aortic valve with the native pulmonary valve and reconstructing the right ventricular outflow tract (RVOT) with a suitable conduit (Figure 1). This is generally offered to patients who are younger than 50 years, with congenital bicuspid aortic valve being the most common etiology. Important contraindications include active rheumatic etiology, connective tissue abnormalities such as Marfan syndrome, osteogenesis imperfecta, etc, and abnormalities of the pulmonary valve including cuspal asymmetry, leaflet fenestration, bicuspid/quadricuspid valves, and other congenital abnormalities. Patients with multivalvar etiologies and multivessel coronary artery disease are also generally excluded. Despite reports of excellent long-term outcomes across the world,1, 2, 3, 4 this procedure is not without its own list of criticisms. Complexity, long learning curve, lack of general reproducibility, as well as perceived high late reoperation rates on both the neoaortic root and pulmonary valve are a few worth mentioning.
Figure 1.

An artist's impression demonstrating completed right-sided reconstruction in the Ross procedure.
Reconstruction of the RVOT forms an integral part of the procedure. This has been described as the “Achilles’ heel,” a weak link surrounding the RP owing to concerns culminating in reinterventions in the late postoperative period.2 Certainly, the choice of prosthesis for RVOT reconstruction is important, as is the technique of insertion and the early and late results. Contemporary research has led to pulmonary allografts being preferred universally for RVOT reconstruction.5, 6, 7 Together with its superior performance, the pulmonary allograft, by virtue of its muscle skirt, fills the available space left after excision of the autograft perfectly and helps provide hemostasis in the autograft bed. We aim to present a detailed technical perspective on this very crucial right-sided reconstruction.
Preoperative Preparation
RP is a complex and lengthy procedure. A well-planned surgical operation guided by appropriate preoperative planning, strict adherence to institutional RP surgical protocol, meticulous dissection with great attention to detail, excellent tissue handling, and above all strict myocardial protection regimen contributes strongly to excellent perioperative, early-, and long-term results.
Imaging
A gated cardiac computed tomography scan of the chest with a computed tomography coronary angiogram is always performed. Analysis of the dimensions of the aortic root, ascending aortic dimension, and the pulmonary root (autograft) is important. It also gives an assessment of coronary ostial locations in relation to the commissures and any anomalous coronary anatomy. In particular, the authors would not offer a RP when the left coronary artery abnormally arises from the right coronary sinus or the right coronary artery.
Intraoperative Transesophageal Echocardiography
A good observation of the size of the pulmonary annulus in comparison with the aortic gradient across the pulmonary valve, grade of regurgitation if any, and cuspal anatomy is essential to support the decision to proceed with RP. Tricuspid valve with equal-sized leaflets and up to trivial to mild regurgitation are deemed acceptable. Our policy is not to proceed with a RP if the pulmonary valve is bicuspid (Figure 2), quadricuspid, or has cuspal asymmetry (up to 5% incidence in our experience).
Figure 2.
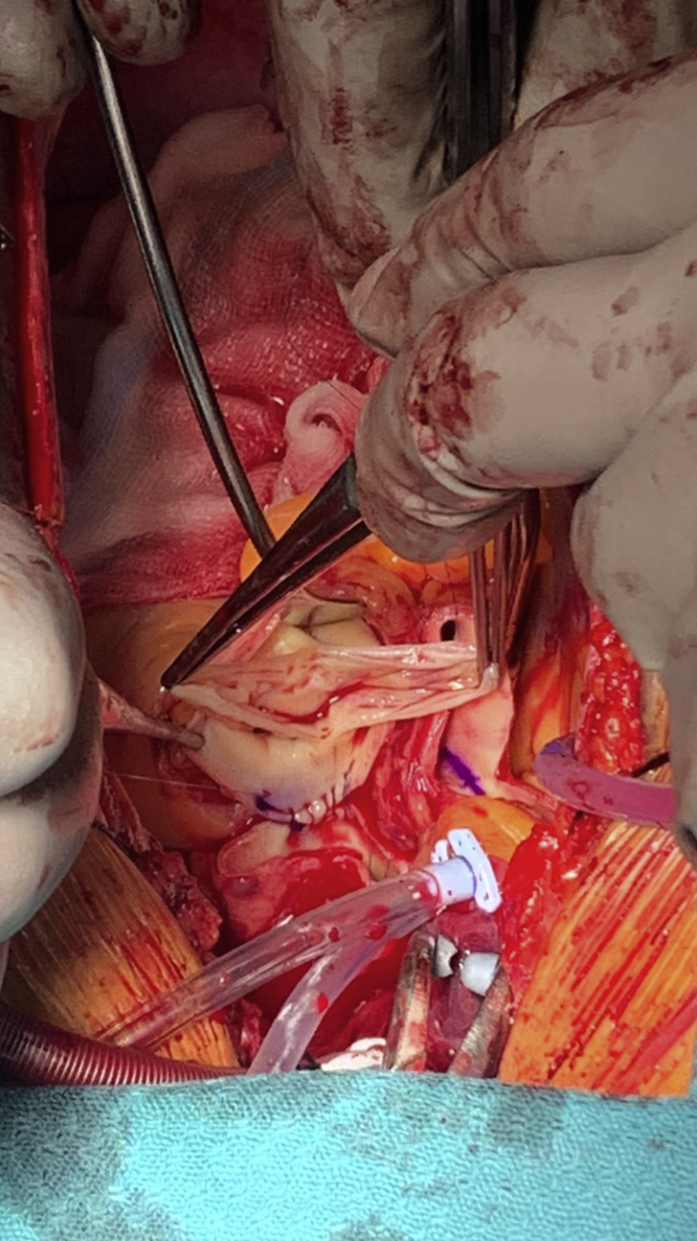
Intraoperative picture of a bicuspid pulmonary valve.
Choice of Prosthesis
Various prostheses have been used for RVOT reconstruction, which predominantly includes homograft (aortic or pulmonary) or xenograft. It has been widely published and accepted that pulmonary allografts are the preferred conduit owing to better durability than other available alternatives.5, 6, 7, 8, 9 The total calcium per gram of tissue in the pulmonary media is significantly less in contrast to the aortic homograft. Less elastic tissue and a lower calcium content contribute to better freedom from calcification in the long run for pulmonary allografts.10 The authors have always used pulmonary allografts for reconstructing the RVOT since the beginning of the Ross program. In our practice, a RP should not be offered if a suitable pulmonary allograft is not available.
Pulmonary Allograft Sizing
The availability of pulmonary allografts varies between different countries. In Australia, we have a paucity of pulmonary allografts and hence do not size them preoperatively. In adults, a size varying between 24 and 32 mm in male patients and 20 and 28 mm in female patients is acceptable in our experience. Generally, we are more inclined to request a large-sized allograft while planning for the RP (if available). This benefits the patient by potentially allowing a valve-in-valve procedure in future. However, we have not found differences in durability and postoperative gradients if smaller size pulmonary allografts are chosen in our patients.11 However, smaller size has been reported by some to be a risk factor for reintervention in pulmonary allografts.7,12,13
Cryopreserved Versus Decellularized
The natural history of the pulmonary allografts suggests conduit stenosis as a primary factor leading to the need for reintervention. Immunologic factors and excessive inflammatory response leading to perigraft fibrosis are considered to be important causes. The concept of decellularized (fresh or cryopreserved) allografts came into vogue to help prevent these major events and postulated to cause autologous cell repopulation while maintaining the extracellular conduit matrix.8 Mid-term results comparing decellularized versus conventional cryopreserved allograft haven't yielded any definite advantages for the former,13 and long-term results with decellularized pulmonary allografts are yet to be ascertained. The authors continue to use cryopreserved nondecellularized pulmonary allograft in routine practice.
Intraoperative Considerations
Thawing of Pulmonary Allografts
In our practice, the allograft is thawed once the aortic root and the pulmonary autograft have been inspected and deemed suitable for a RP. A small portion of the trimmed pulmonary allograft muscle, storage solution, and rinsing solution are sent for bacteriological analysis.
Autograft Harvesting
A meticulously dissected autograft with great attention to detail is a harbinger for a successful pulmonary allografts implantation to reconstruct the RVOT. This initial surgical step avoids potential intraoperative complications such as damage to the pulmonary valve and its sinuses, injury to the left main coronary artery and the first septal perforator, bleeding from autograft harvest sites, lack of suturing margins in the posterior aspect of the proximal suture line, etc. These potential complications can be avoided by dissecting the autograft in the adventitial plane.
The main pulmonary artery is transected 5-8 mm above the level of the sinotubular junction of the pulmonary valve. The autograft is then dissected circumferentially downwards until the muscular infundibulum is reached in an adventitial plane. A right-angle forceps is passed through the valve and a transverse venticulotomy performed 5 mm proximal to the valve over the impression created by the forceps. The distal dissection is then carried out using scissors cutting across the previously performed transverse ventriculotomy. As the dissection proceeds posteriorly into the infundibular septum, a superficial transverse endocardial incision is performed with a scalpel and the autograft excised with the aid of scissors. This part of the dissection is tricky and leads to insufficient suturing margins for the pulmonary allografts if not done properly. It is also here, on the left posterior side where the first septal perforator artery runs at a variable depth from the endocardial surface. This needs to be looked out for and, if visualized, avoided (Video 1). Hemostasis of the area left behind can be achieved with diligent diathermy while administering antegrade blood cardioplegia down both left and right coronary ostia (Video 2).
Video 1.
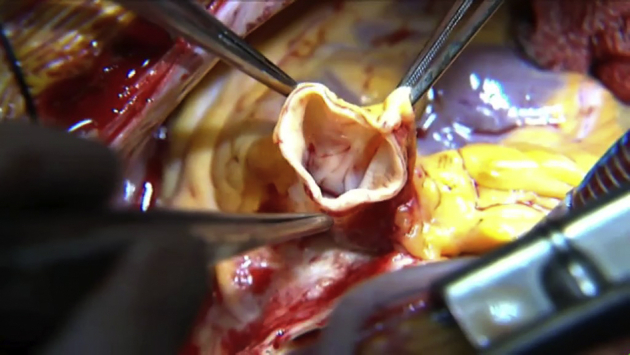
Technique of autograft harvest. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.
Video 2.

Technique of hemostasis of autograft bed while administering ostial cardioplegia. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.
RVOT Reconstruction
The pulmonary allograft is a perfect anatomical and physiologic fit to reconstruct the RVOT. Many small and important steps lead to its ultimate durability. Lack of convincing evidence regarding long-term durability, cost–benefit concerns, and availability are the reasons we continue to use cryopreserved nondecellularized over decellularized pulmonary allografts. In total, 96.6% freedom from reintervention at 20 years strongly supports this preference at our practice.11
Fashioning the pulmonary allograft
The muscle skirt underlying the pulmonary allografts valve is trimmed to a width of 3-4 mm. This ensures a proper anatomical sitting of the allograft. It must be highlighted that leaving less muscle hopefully leads to less immunologic reaction and thus presumably a lower transallograft gradient (Video 3).
Video 3.
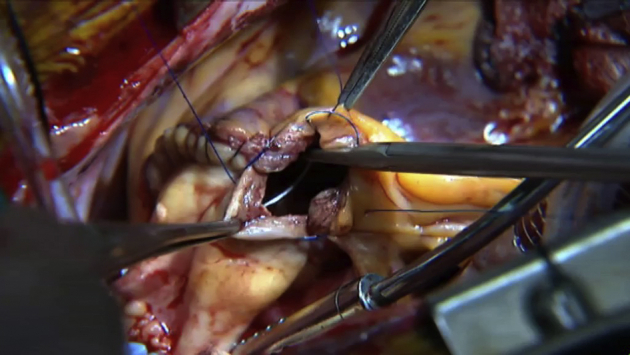
Technique of pulmonary allograft preparation and anastomosis. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.
Distal anastomosis
It is constructed using running 5-0 polypropylene sutures ensuring that the pulmonary allograft is inserted in an orthotopic fashion. The authors believe that this contributes to long-term durability.
Proximal anastomosis
A 4-0 running polypropylene suture is used with an intention to anastomosing the nadir of the scallop adjoining the posterior leaflet of the pulmonary allograft to the center of the posterior anastomotic margin of the RV. The right heart should be deaired before completion of this anastomosis. Twisting and torsion of the allograft is a real concern and must be avoided at all cost (Video 3).
Timing of reconstruction
The RVOT in our practice is reconstructed under crossclamp. This step at our practice is often performed after the proximal anastomosis of the autograft but before reimplantation of the coronaries and performing the distal anastomosis especially if the allograft has not completely thawed after harvesting the autograft.
Swan–Ganz advancement
The authors routinely advance a Swan–Ganz catheter, which preoperatively is parked in the superior vena cava until coming off bypass.
Postoperative Considerations
Role of Perioperative Antibiotics
We advocate for the administration of 48 hours of antibiotics (vancomycin, cephazolin, and ceftriaxone) or until the homograft tissue cultures have returned negative. Positive cultures are rare but not impossible. Positive bacterial cultures from pulmonary allograft muscle entail intravenous antibiotics for up to 4 weeks. Positive cultures of the rinsing solution may be ignored as they usually are contaminants only and would have been covered by the perioperative antibiotics.
Anti-inflammatory Drugs
Inflammation has been described as an important etiology for pulmonary allograft failures.13 It is known to occur at valvar level, on the proximal, distal suture line, and through the entire conduit.11,13 This is a well-recognized problem, and many centers advocate 6 months of nonsteroidal anti-inflammatory drugs to minimize this problem.3,13
Early and Late Pulmonary Allograft Dysfunction/Failure
Pulmonary allograft dysfunction may happen at the level of the valve (structural), conduit, and/or the proximal and distal suture line (nonstructural). The structural changes include increase in gradients, grade of regurgitation, or a combination of both. Etiologies reported include inflammation, immune-mediated reaction leading to fibrosis, conduit calcification, structural degeneration of the valve, proximal and distal suture line stenosis, and also infective endocarditis.8,12,13 This leads to right ventricular (RV) dysfunction/failure necessitating reintervention and significantly affects the surgical results surrounding the RP. There is no universal definition for dysfunction of pulmonary allograft. Different criteria are used for reporting pulmonary allograft valvar dysfunction in the large series describing long-term outcomes governing the RP.1,8,11,13 This makes interpretation and standardization difficult.
The dysfunction of the pulmonary allografts is a continuous process but reintervention rates remain extremely low,11, 12, 13, 14, 15 albeit with a lifetime risk of less than 20%.9 This dysfunction normally follows a biphasic pattern with an early risk phase which sets in 6 to 12 months postsurgery, progresses for the first 2 years postimplantation. This is followed by a low and constant risk phase.3,13 Analysis of late results from individual series and larger registries like the Canadian and German-Dutch registries, which look at various factors that could influence pulmonary allograft longevity such as blood group and human leucocyte antigen compatibility between donor and recipients, donor sex, age of donor and recipient, sex, allograft size (length and diameter), allograft quality (sclerosis, fibrosis, fenestrations), and surgical adjustments of the pulmonary allograft, haven't yielded conclusive evidence to predict allograft failure.13, 14, 15 Moreover, the paucity of available pulmonary allografts makes it extremely difficult to incorporate limited evidence into surgical practice.
Criteria and Methods for Reintervention
Reintervention rates for pulmonary autografts are low. We reported 96% freedom from aortic valve reintervention at 18 years4 and 99% for those with predominant aortic stenosis.16 Rates of pulmonary allografts reinterventions in RP are lower in comparison with what is seen when reconstructing the RVOT in congenital heart disease.5,8,12 In our experience, analyzed from the initial 443 patients, the incidence of freedom from mild-to-moderate pulmonary allograft dysfunction was 78.3% (stenosis and/or regurgitation), and freedom from reintervention was 96.6% at 20 years.11
In general, our thresholds for reintervention are moderately high and guided by a multitude of factors. Mean pulmonary systolic gradient ≥36 mm Hg or severe pulmonary allograft valvar regurgitation (associated with symptoms, signs of RV dysfunction, RV end-diastolic volume index greater than 140 mL/m2, or evidence of RV volume index being 1.8 times greater than that of the left ventricle on cardiac magnetic resonance imaging)11 form part of the authors' criteria for reintervention. Cardiac catheterization is often avoided.
The method in which the pulmonary allografts fail differs. Percutaneous interventions including balloon valvuloplasty, transcatheter valve implantation, and stenting for distal suture line stenosis are in vogue and preferred in most of the centers worldwide.8 Redo surgeries for RVOT reconstruction can be offered with very low surgical risk in the current era. Surgical reintervention is reserved for cases involving conduit shrinkage, left main coronary artery to pulmonary allograft distance less than 3 mm,13 heavy calcification of the conduit, and endocarditis.
Technique for Redo Surgery
Closing the pericardium during the primary surgery or covering the heart with a synthetic membrane helps proceed with a quick re-entry. Surgery is carried out with aortic and double-stage right atrial cannulation on a beating heart. The RVOT conduit is freed from adhesions while on bypass and removed. We advocate the use of a stentless porcine root prosthesis for reintervention except in the setting of endocarditis, where pulmonary allografts are preferred.
Conclusions
Greater than 5 decades have passed, and there still remains a lot to master in RP. The RP has always been about perfecting the techniques.17 Various units have reported excellent long-term results, which include survival, freedom from reintervention, freedom from major adverse cardiac and cerebrovascular events, quality of life, and hemodynamic performance beyond 20 years.1, 2, 3, 4 The main focus after RP has been on the durability of the autograft. Dysfunction of the pulmonary allografts leading to need for future reinterventions has been recognized as an important and a potentially preventable problem. This article looks at the multitude of factors that contribute to the durability of the pulmonary allografts and culminates in adding to the growing list of advantages of the RP.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Technique of autograft harvest. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.
Technique of hemostasis of autograft bed while administering ostial cardioplegia. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.
Technique of pulmonary allograft preparation and anastomosis. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.
References
- 1.Romeo J.L.R., Papageorgiou G., da Costa F.F.D. Long-term clinical and echocardiographic outcomes in young and middle-aged adults undergoing the Ross procedure. JAMA Cardiol. 2021;65:539–548. doi: 10.1001/jamacardio.2020.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David T.E., David C., Woo A., Manhlhoit C. The Ross procedure: outcomes at 20 years. J Thorac Cardiovasc Surg. 2014;147:85–94. doi: 10.1016/j.jtcvs.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 3.El-Hamamsy I., Eryigit Z., Stevens L.-M., Sarang Z., George R., Clark L., et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376:524–531. doi: 10.1016/S0140-6736(10)60828-8. [DOI] [PubMed] [Google Scholar]
- 4.Skillington P.D., Mokhles M., Takkenberg J.J.M., Larobina M., O'Keefe M., Wynne R., et al. The Ross procedure using autologous support of the pulmonary autograft: techniques and late results. J Thorac Cardiovasc Surg. 2015;147:S46–S52. doi: 10.1016/j.jtcvs.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 5.Miskovic A., Monsefi N., Doss M., Özaslan F., Karimian A., Moritz A. Comparison between homografts and freestyle bioprosthesis for right ventricular outflow tract replacement in Ross procedure. Eur J Cardiothorac Surg. 2012;149:927–933. doi: 10.1093/ejcts/ezs185. [DOI] [PubMed] [Google Scholar]
- 6.Homann M., Haehnel J.C., Mendler N., Paek S.U., Holper K., Meisner H., et al. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardiothorac Surg. 2000;17:624–630. doi: 10.1016/s1010-7940(00)00414-0. [DOI] [PubMed] [Google Scholar]
- 7.Forbess J.M., Shah A.S., St Louis J.D., Jaggers J.J., Ungerleider R.M. Cryopreserved homografts in the pulmonary position: determinants of durability. Ann Thorac Surg. 2001;71:54–60. doi: 10.1016/s0003-4975(00)01788-4. [DOI] [PubMed] [Google Scholar]
- 8.Etnel J.R.G., Grashuis P., Huygens S.A. The Ross procedure: a systematic review, meta-analysis, and microsimulation. Circ Cardiovasc Qual Outcomes. 2018;11:e004748. doi: 10.1161/CIRCOUTCOMES.118.004748. [DOI] [PubMed] [Google Scholar]
- 9.Brown J.W., Ruzmetov M., Fukui T., Rodefeld M.D., Mahomed Y., Turrentine M.W. Fate of autograft and homograft following Ross aortic valve replacement: reoperative frequency, outcome and management. J Heart Valve Dis. 2006;15:253–259. [PubMed] [Google Scholar]
- 10.Urist M.R., Adams J.M. Localization mechanism of calcification in transplants of aorta. Ann Surg. 1967;166:1–18. doi: 10.1097/00000658-196707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fricke T.A., Skillington P.D., Shi W., Buratto E., Wynne R., Larobina M., et al. Pulmonary valve function late after Ross procedure in 443 adult patients. Ann Thorac Surg. 2020;109:1127–1131. doi: 10.1016/j.athoracsur.2019.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Boethig D., Goerler H., Westhoff-Bleck M., Ono M., Daiber A., Haverich A., et al. Evaluation of 188 consecutive homografts implanted in pulmonary position after 20 years. Eur J Cardiothorac Surg. 2007;32:133–142. doi: 10.1016/j.ejcts.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Chauvette V., Bouhout I., Tarabzoni M., Pham M., Wong D., Whitlock R., et al. Pulmonary homograft dysfunction after the Ross procedure using decellularized homografts—a multicentre study. J Thorac Cardiovasc Surg. July 22, 2020 doi: 10.1016/j.jtcvs.2020.06.139. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Carr-White G.S., Kilner P.J., Hon J.K.F. Incidence, location, pathology, and significance of pulmonary homograft stenosis after the Ross operation. Circulation. 2001;104:I16–I20. doi: 10.1161/hc37t1.094545. [DOI] [PubMed] [Google Scholar]
- 15.Mokhles M.M., Charitos E.I., Stierle U., Rajeswaran J., Blackstone E.H., Bogers A.J., et al. The fate of pulmonary conduits after the Ross procedure: longitudinal analysis of the German-Dutch Ross Registry experience. Heart. 2013;99:1857–1866. doi: 10.1136/heartjnl-2013-304425. [DOI] [PubMed] [Google Scholar]
- 16.Skillington P.D., Mokhles M.M., Wilson W., Grigg L., Larobina M., O'Keefe M., et al. Inclusion cylinder method for aortic valve replacement utilising the Ross operation in adults with predominant aortic stenosis—99% freedom from re-operation on the aortic valve at 15 years. Glob Cardiol Sci Pract. 2013;2013:383–394. doi: 10.5339/gcsp.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazine A., Ghoneim A., El-Hamamsy I. The Ross procedure: how I teach it. Ann Thorac Surg. 2018;105:1294–1298. doi: 10.1016/j.athoracsur.2018.01.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technique of autograft harvest. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.
Technique of hemostasis of autograft bed while administering ostial cardioplegia. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.
Technique of pulmonary allograft preparation and anastomosis. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00698-2/fulltext.


