Abstract
In this survey study of institutions across the US, marked variability in evaluation, treatment, and follow-up of adolescents 12 through 18 years of age with mRNA coronavirus disease 2019 (COVID-19) vaccine-associated myopericarditis was noted. Only one adolescent with life-threatening complications was reported, with no deaths at any of the participating institutions.
Keywords: mRNA COVID-19 vaccine-associated myopericarditis, survey, variability, outcomes
Abbreviations: COVID-19, Coronavirus disease 2019; IVIG, Intravenous immunoglobulin; NSAID, Nonsteroidal anti-inflammatory drug; PCR, Polymerase chain reaction; VAERS, Vaccine Adverse Event Reporting System; VAM, Vaccine-associated myopericarditis
Since April 2021, more than 1000 patients have been reported to the Vaccine Adverse Event Reporting System (VAERS) with presumed myopericarditis following administration of the mRNA coronavirus disease 2019 (COVID-19) (Pfizer-BioNTech, Moderna) vaccine.1 The afflicted patients predominantly have been male and <16 years of age, a vast majority of whom developed clinical features of myopericarditis within a few days after receiving the second mRNA COVID-19 vaccine (Pfizer-BioNTech, BNT162b2) dose.1 The overall reported incidence of myopericarditis after administration of mRNA COVID-19 vaccine has been estimated to be 4.2 and 32.4 per million doses administered in female and male adolescents 12 through 17 years of age, respectively.2 , 3
Case series4 and several other reports suggest wide variability in clinical evaluation and treatment of adolescents 12-18 years of age with mRNA COVID-19 vaccine-associated myopericarditis (VAM) across institutions within the US.5, 6, 7, 8, 9, 10 To better assess this variability, we conducted a cross-sectional survey of pediatric institutions across the US between July 9, 2021, and August 9, 2021. A secondary objective of this study was to determine the rate of serious, life-threatening complications (cardiopulmonary arrest requiring resuscitation, need for mechanical circulatory support [extracorporeal membrane oxygenation, Impella or ventricular assist device use], and death) in these adolescents.
Methods
After we obtained appropriate institutional review board approval, a questionnaire that inquired about the institutional practices regarding diagnosis, treatment, and follow-up of adolescents with VAM was emailed to pediatric cardiologists or pediatric infectious disease specialists at 107 institutions (the top 100 institutions in the US News ranking of pediatric cardiology programs and a few additional programs with which the authors were familiar) across the US between July 9, 2021, and August 9, 2021.11 The survey was forwarded to content experts for face validity.12 A follow-up email was sent 2 weeks later if there was no response to the initial email. Nonparametric statistical tests were used to evaluate differences in medians, and P values <.05 were considered significant. The details of the institutional review board process, the questionnaire, data analysis, and statistics are provided in Appendices 1 and 2 (available at www.jpeds.com).
Results
Participating Institutions
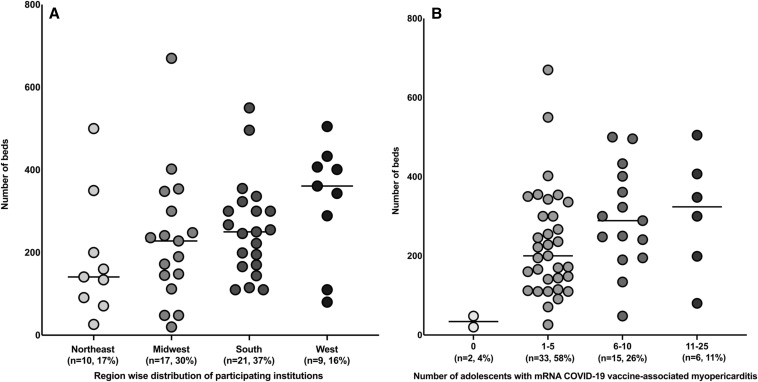
Fifty-seven institutions (53%) returned a completed survey. As per the US Census Bureau classification, approximately two-thirds of the participating institutions were located in either the Midwest or the South, and a majority had a bed capacity of >200 beds (Appendix 1, Figure 1, A and B).13 Protocols and guidelines for treatment of adolescents with VAM were developed at 15 (n = 26%) of the participating institutions. Eleven institutions gave permission to publish their protocol. One institution used their multisystem inflammatory syndrome in children protocol for evaluation and treatment of adolescents with VAM. The 9 different VAM protocols used by the remaining 10 institutions are included in Appendix 3 (available at www.jpeds.com [2 institutions located in different geographic regions shared a common protocol]).
Figure 1.
A, Bed capacity of institutions across the 4 geographic regions of the US. ∗One institution located in the Northeast admits to a center in the South. #The difference in bed capacity between the institutions in the Northeast (n = 10, median 141, range 26-500), Midwest (n = 17, median 228, range 20-670), South (n = 21, median 250, range 110-550), and West (n = 9, median 361, range 80-505) was not statistically significant (P = .11). B, Number of adolescents admitted with mRNA COVID-19 VAM vs bed capacity. ∗One institution did not provide data on the number of adolescents treated. #None of the institutions reported treating more than 25 adolescents. ˆNone (median 34, range 20-48), between 1 and 5 (median 200, 26-670), between 6 and 10 (median 289, 48-500), and between 11 and 25 adolescents (median 324 range 80-505). The difference in the bed capacity between the institutions that treated 1-5, 6-10, and 11-25 adolescents was not statistically significant (P = .14).
Clinical Symptoms of Concern Following Administration of mRNA COVID-19 Vaccine
All 31 institutions that responded to this question reported chest pain (100%) as a symptom of concern following administration of mRNA COVID-19 vaccine. Other concerning symptoms included shortness of breath (n = 27, 87%), chest tightness (n = 18, 56%), palpitations (n = 26, 81%), and fever (n = 16, 50%). The median duration of symptoms that prompted evaluation for VAM was 1 day (range 0-4 days). A majority of institutions reported symptoms after the second dose of mRNA COVID-19 vaccine (Appendix 1).
Initial Laboratory Evaluation of Patients with mRNA COVID-19 VAM
Cardiac Biomarkers (n Responding = 54)
Most institutions used cardiac troponin or creatine kinase myocardial band (98%), use of pro-N-terminal-brain type natriuretic peptide also was common (79%) (Appendix 1).
COVID-19 Antibody Testing (n = 54)
COVID-19 antibody testing was performed by a majority (98%) of the institutions on these adolescents; however, a positive antibody test was included in diagnostic criteria for VAM by only a few (22%, 7/32) (Appendix 1).
COVID-19 Polymerase Chain Reaction, Multiples Respiratory Viral Panel, and Viral Panel for Myocarditis (n = 54)
COVID-19 polymerase chain reaction (PCR) and respiratory viral panel were obtained by 45 (83%) of the institutions at the time of initial evaluation of these adolescents. Twenty-five (47%) of the institutions also obtained a comprehensive myocarditis viral panel during initial evaluation. Only 10 of 28 (36%) of the institutions included negative COVID-19 PCR in the diagnostic criteria for VAM.
Serum Inflammatory Markers, Serum Biochemistry, and D-Dimer Concentration (n = 54)
Most institutions (n = 50, 93%) measured serum C-reactive protein and erythrocyte sedimentation rate concentrations and obtained a complete blood count at admission. A comprehensive metabolic panel and serum D-dimer concentrations were obtained by 45 (83%) of the institutions during initial evaluation.
Initial Radiographic Evaluation of Patients with mRNA COVID-19 VAM
Chest Radiography, Echocardiography, and Cardiac Magnetic Resonance Imaging (n = 55)
Forty-two (79%) institutions obtained chest radiographs, 52 (95%) obtained echocardiograms, and 26 (47%) obtained a cardiac magnetic resonance imaging scan on adolescents with VAM at the time of initial evaluation.
Electrocardiography (n = 55)
Electrocardiograms were obtained by all 55 (100%) institutions at the time of the initial evaluation.
Abnormalities Considered Suggestive of mRNA COVID-19 VAM
There was marked variability in institutional criteria for diagnosis of VAM. The diagnostic criteria used by the 26 institutions are shown in Appendix 4 (available at www.jpeds.com). A majority of the institutions used a combination of elevated inflammatory and cardiac biomarkers, electrocardiographic and echocardiographic or CMR abnormalities for diagnosis (Appendix 4). A positive antispike COVID-19 antibody titer was included in diagnostic criteria by only 4 (15%) institutions (Table I ). The case definitions used by some of the institutions for diagnosis of VAM are shown in Appendix 5 (available at www.jpeds.com).
Table I.
Laboratory, electrocardiographic, and imaging abnormalities considered suggestive of mRNA COVID-19 VAM (n = 26)
| Variables | Number of institutions |
|---|---|
| ↑CRP/ESR | 12 (46%) |
| ↑BNP or pro-NT-BNP | 14 (54%) |
| ↑Troponin I or T | 26 (100%) |
| Abnormal electrocardiogram | 23 (88%) |
| Abnormal echocardiogram | 21 (81%) |
| Abnormal cardiac MRI | 15 (58%) |
| + COVID-19 antispike antibody | 4 (15%) |
| – COVID-19 antinucleocapsid antibody | 2 (8%) |
| ↑ D-dimer | 3 (11.5%) |
BNP, brain-type natriuretic peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; pro-NT-BNP, pro-N-terminal-brain type natriuretic peptide.
Common electrocardiographic abnormalities thought to be indicative of VAM included isolated diffuse ST-segment elevation (10%) or diffuse ST-segment elevation and T-wave inversion (58%), in addition to other nonspecific T-wave abnormalities (23%).
Common echocardiographic and CMR findings that were considered potentially consistent with a diagnosis of VAM are shown in Table II (available at www.jpeds.com). Global ventricular dysfunction, pericardial effusion, and atrioventricular valve regurgitation were the 3 most common echocardiographic abnormalities. A combination of late gadolinium enhancement, myocardial edema, and pericardial effusion was considered suggestive of VAM by 75% of the institutions.
Management
Pediatric cardiologists were involved in management of these adolescents, most of whom were hospitalized (Appendix 1).
First-Line Therapy (n = 54)
There was wide variability in the first-line medications used to treat adolescents with VAM. A majority (35/54, 66%) of institutions initially treated these adolescents with nonsteroidal anti-inflammatory drugs (NSAIDs). The initial treatment consisted of intravenous immunoglobulin (IVIG) at a few institutions (n = 2, 4%). Other institutions treated with a combination of drugs (Appendix 1). One institution reported only conservative treatment without any specific medication. The criteria used to determine response to therapy were highly variable (Appendix 1).
Although corticosteroids and IVIG were indicated as the most common second- and third-line therapeutic agents, there was marked variability (Appendix 1).
Hospitalization (n = 49)
The median upper limit of duration of hospitalization for the illness was 4 days (range 1-21 days) while the median of the lower limit of hospitalization for the illness was 1 day (range a few hours to 5 days) across 49 institutions.
Respiratory Support
Only one institution cared for an adolescent with VAM who required ventilatory support. Eight institutions indicated using oxygen via nasal cannula and 4 indicated using high flow nasal cannula.
Arrhythmias (n = 50)
Seventeen (34%) institutions reported patients with heart rhythm abnormalities. Six institutions reported at least 1 patient with premature ventricular contractions, 5 institutions reported at least one patient with nonsustained ventricular tachycardia, 2 institutions reported patients with atrioventricular block, and 3 institutions reported a patient with sustained ventricular tachycardia 1 of whom was treated with a beta-blocker. One institution reported a patient with electrocardiographic changes suggestive of ischemia.
Adverse Events
One institution reported a patient who had cardiac arrest and required cardiopulmonary resuscitation. This patient was supported with extracorporeal membrane oxygenation for 6 days and had normalized cardiac size and systolic function but persistent diastolic dysfunction and diffuse late gadolinium enhancement in the epicardium and mid-myocardium of the left ventricle on CMR by day 10 of hospitalization. No deaths were reported by any of the institutions, and no patient was reportedly supported by Impella or a ventricular assist device.
Recommendations Regarding Activity (n = 47)
Four institutions recommended return to full activity in <4 weeks, 5 institutions between 4 and 12 weeks, 14 institutions at 12 weeks, and 24 institutions between 12 and 24 weeks. Thirty-two (66%) of the institutions recommended a stress test before return to full activity (Appendix 1).
Discussion
The findings of our study, which included 57 institutions across the US, show that there is marked variability in diagnosis and management of VAM in adolescents. Although the Centers for Disease Control and Prevention has established a case definition for this condition, most of the institutions had formulated their own diagnostic criteria, which included (in addition to receipt of mRNA COVID-19 vaccine) clinical symptoms such as chest pain, shortness of breath or palpitations, laboratory abnormalities such as elevated inflammatory or cardiac biomarkers, electrocardiographic abnormalities, and noninvasive cardiovascular imaging abnormalities following administration of mRNA COVID-19 vaccine.14 Most of the institutions reported 1 day as the threshold duration of symptoms after onset following administration of mRNA COVID-19 vaccine to consider evaluation for VAM; however, there was marked variability in responses (range 0-4 days). Similar variability was noted in the initial diagnostic evaluation. Cardiac biomarkers, inflammatory biomarkers, electrocardiograms, and echocardiograms were obtained by most of the institutions during initial diagnostic evaluation. However, there was more variability in using cardiac magnetic resonance imaging, evaluating for other causes of myocarditis, and measuring serum COVID-19–specific antispike and antinucleocapsid antibodies.
The treatment of these patients was also widely variable. A pediatric cardiologist was central to management of these patients, with additional support from pediatric infectious disease and other specialists at some institutions. Although NSAIDs were the most commonly used first-line agents for treatment of these patients, steroids and IVIG were the most likely to be used second- and third-line agents, respectively. However, marked variability was noted in the first-line, second-line, and third-line therapeutic regimens, with a number of institutions using drug combinations. Most institutions admitted these adolescents for evaluation and management; however, a few managed these adolescents in the outpatient setting with reported good outcomes.
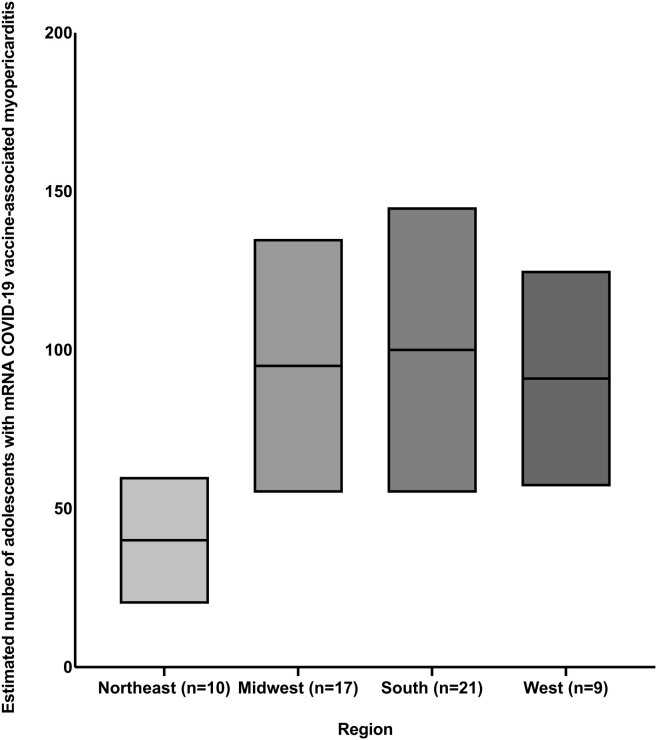
Heart rhythm problems were common in these patients, with one-third of the institutions reporting patients with new-onset ectopy, heart block, or arrhythmia. The findings of this survey study are consistent with the results of previous studies, which have shown a high arrhythmia burden in adolescents hospitalized with acute myocarditis.15 Approximately 20% of the institutions also reported adolescents with VAM who required respiratory support via nasal cannula. However, serious adverse outcomes were rare with only one institution reporting an adolescent who required extracorporeal membrane oxygenation support. The overall reported incidence of myopericarditis after administration of mRNA COVID-19 vaccine has been estimated to be 4.2 and 32.4 per million doses administered in female and male patients 12 through 17 years of age, respectively.2 , 3 Although survey-based studies are not optimal to calculate incidence or rate, among an estimated 189-465 adolescents with VAM treated at 57 participating institutions (Figure 2; available at www.jpeds.com),19 a single adolescent was reported to have life-threatening complications. Between December 2020 and mid-July of 2021, 9 million adolescents aged 12-17 years received Pfizer-BioNTech’s mRNA COVID-19 vaccine. Postvaccination adverse effects were reported in 9246 adolescents (1/1000 recipients); however, more than 90% reports filed in VAERS were for nonserious symptoms such as dizziness, nausea, headache, and fever. Of the 863 serious adverse events, the most common reported conditions were chest pain, elevated serum troponin concentration, and myocarditis. Myocarditis was listed in 397 reports, representing 4.3% of all VAERS reports. Fourteen deaths were reported, the cause of 6 of which is still under investigation. No direct linkage to vaccination was reported in the rest.16
Figure 2.
Estimated number of adolescents with mRNA COVID-19 VAM treated in different geographic regions of the US. ∗Northeast (mean 40, range 20-60), Midwest (mean 95, range 55-135), South (mean 100, range 55-145), and West (mean 91, range 57-125). #The numbers within parentheses indicate the number of institutions in that region. ˆ As of September 9, 2021, the total number of adolescents 12 through 17 years of age vaccinated in the 4 geographic regions is as follows: Northeast: 2 053 241, Midwest: 1 990 101, South: 3 655 317, and West: 2 905 148.19
Thus, the findings of this multi-institutional study are consistent with recently published VAERS data and affirm the findings of previous smaller reports and case-series which suggest excellent short-term outcomes in these patients.4, 5, 6, 7, 8, 9, 10 , 16 In addition, because the VAERS is critically dependent on reporting by practitioners and institutions, adverse events can be missed due to a lack of reporting. Our data, gathered from a large number of institutions across the US, provides an independent affirmation of the fact that serious life-threatening adverse events are extremely rare in adolescents with VAM.
Previous reports have shown that electrocardiographic abnormalities are common in adolescents with VAM at the time of initial diagnosis4; however, only a handful were noted to have persistent abnormalities on 12-lead electrocardiogram or Holter monitoring suggesting that the electrocardiographic abnormalities noted in the acute phase are very likely to resolve within a few weeks. A majority of institutions indicated obtaining cardiac magnetic resonance imaging and an echocardiogram at follow-up visits; however, there was marked variability in the timing of these investigations.
As per the current guidelines from the American College of Cardiology, athletes diagnosed with myocarditis should be restricted from exercise for 3-6 months to promote resolution of inflammation, especially if extensive inflammation is noted on cardiac magnetic resonance imaging.17 Previous studies have shown extensive myocardial involvement in adolescents with VAM in the absence of significant echocardiographic abnormalities.4 , 8 , 9 However, marked variability in return to activity recommendations was noted in our study; almost one-half of the institutions indicated allowing these adolescents to return to full activity by 12 weeks. Only two-thirds of the institutions indicated considering a stress test before returning to full activity.
Despite the observed variability in diagnosis and management, the short-term outcomes in adolescents with VAM were good and the majority recovered completely. Although treatment regimens differed, there was no significant difference in the lower (1.69 ± 0.84 vs 2.29 ± 1.3 days; P = 14) or the upper (5.41 ± 3.7 vs 4.1 ± 1.6 days; P = .27) limit of duration of hospitalization between those adolescents who were treated with only NSAIDs vs those who were treated with multidrug regimens. The variability highlighted by our study is reflective of the challenges faced by clinicians who were using their best judgement in the absence of robust guiding data. In the short-term, our data, which shows that life-threatening complications are extremely rare in adolescents with VAM, is quite encouraging and should motivate the population to receive COVID-19 vaccination.
Our study had several limitations. Because this was a survey study, data for individual patients were not sought; therefore, the overall accuracy of data may not be comparable with an observational study. However, to enhance the quality of the data, the surveys were directed to physicians who directly participated in the care of adolescents with VAM. Several institutions had no or only a few adolescents with VAM. The responses from these centers might indicate diagnostic and treatment modalities which are likely to be used than the ones that were used. A majority of the participating centers were located in either the Midwest or the South. The responses could therefore reflect the prevailing practices in these geographic regions. Nevertheless, given the large number of centers that participated, our study does provide an estimate of various diagnostic and therapeutic aspects of VAM in adolescents across the US. If the responses of the institutions that responded were fundamentally different from the institutions that did not respond, this could bias the results. The response rate, however, is comparable with other survey studies that were conducted across a large geographic region.18 Because it is difficult to survey all providers, it is possible that patients with mild VAM could have been under-reported. Lastly, if the survey was completed by an infectious disease physician, the recognition of cardiology protocols may have been incomplete.
Our study informs the need for consensus guidelines to help facilitate prompt diagnosis and optimal management of these adolescents. In addition, the findings of our study affirm that serious life-threatening complications due to VAM remain rare in adolescents.
Data Statement
Data sharing statement available at www.jpeds.com.
Footnotes
Affiliation information is available at www.jpeds.com.
A.H. is supported by a Sub-agreement from the Johns Hopkins University with funds provided by R61HD105591 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the Office of the Director, National Institute of Health (OD). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the Office of the Director, National Institutes of Health (OD), the National Institutes of Health, the National Institute of Biomedical Imaging and Bioengineering, the National Heart, Lung, and Blood Institute, or the Johns Hopkins University. The authors declare no conflicts of interest.
Appendix
Table II.
Echocardiographic and cardiac MRI abnormalities indicative of mRNA COVID-19 VAM (n = 29)
| Abnormalities | Number of institutions, n (%) |
|---|---|
| Echocardiography | |
| Diminished ventricular function (global) | 27 (93%) |
| Regional wall motion abnormality | 5 (17%) |
| Abnormal strain imaging | 3 (10%) |
| Pericardial effusion | 21 (72%) |
| Atrioventricular valve regurgitation | 8 (28%) |
| Diastolic dysfunction | 3 (10%) |
| Coronary artery dilation | 2 (7%) |
| Cardiac MRI | |
| Late gadolinium enhancement | 29 (100%) |
| Myocardial edema | 28 (96%) |
| Pericardial effusion | 22 (76%) |
MRI, magnetic resonance imaging.
In total, 75% of institutions considered a combination of late gadolinium enhancement, myocardial edema, and pericardial effusion to be diagnostic of mRNA COVID-19 VAM.
Appendix
From the 1Division of Pediatric Cardiology, Department of Pediatrics, West Virginia University School of Medicine and West Virginia University Medicine Children’s Hospital, Morgantown, WV; 2Children’s Hospital of Michigan, Division of Pediatric Infectious Diseases, Department of Pediatrics, Detroit, MI; 3Central Michigan University, College of Medicine, Mt Pleasant, MI; 4Cardiology Care for Children, Nemours Children's Hospital, Wilmington, DE; 5Division of Pediatric Cardiology, Department of Pediatrics, Children’s National Hospital, Washington, DC; 6The George Washington University School of Medicine & Health Sciences, Washington, DC; 7Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s National Hospital, Washington, DC; 8Division of Pediatric Cardiology, Department of Pediatrics, Phoenix Children’s Hospital, Phoenix, AZ; 9Department of Pediatrics, Children’s Medical Center Dallas, UTSW Medical Center, Dallas, TX; 10Department of Cardiology, Nicklaus Children’s Hospital, Miami, FL; 11Division of Pediatric Cardiology, Department of Pediatrics, Monroe Carell Jr Children's Hospital, Vanderbilt University Medical Center, Nashville, TN; 12Division of Pediatric Cardiology, Department of Pediatrics, Children’s Hospital Los Angeles, Los Angeles, CA; 13Division of Pediatric Cardiology, Department of Pediatrics, Riley Children’s Hospital, Indianapolis, IN; 14Division of Pediatric Cardiology, Department of Pediatrics, Oregon Health & Science University Doernbecher Children’s Hospital, Portland, OR; 15Beaumont Children’s Hospital, Royal Oak, MI; 16Oakland University William Beaumont School of Medicine, Rochester, MI; 17Division of Pediatric Cardiology, Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Children’s Hospital of Philadelphia, Philadelphia, PA; 18Division of Pediatric Cardiology, Department of Pediatrics, Rady Children’s Hospital, University of California San Diego, San Diego, CA; 19Division of Pediatric Cardiology, Department of Pediatrics, C.S. Mott Children’s Hospital, Ann Arbor, MI; 20Division of Pediatric Cardiology, Department of Pediatrics, Lucile Packard Children’s Hospital Stanford, Palo Alto, CA; 21Division of Pediatric Cardiology, Department of Pediatrics, Nemours Children's Hospital, Wilmington, DE; 22Division of Pediatric Cardiology, Department of Pediatrics, University of Pittsburgh Medical Center (UPMC), Pittsburgh, PA; 23Division of Pediatric Cardiology, Department of Pediatrics, Lurie Children’s Hospital, Chicago, IL; 24Division of Pediatric Cardiology, Department of Pediatrics, Duke Children’s Hospital, Durham, NC; 25Division of Pediatric Cardiology, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock, AR; 26Division of Pediatric Cardiology, Department of Pediatrics, University of Utah/Primary Children's Hospital, Salt Lake City, UT; 27Division of Pediatric Cardiology, Department of Pediatrics, University of Pittsburgh Medical Center (UPMC), Harrisburg, Harrisburg, PA; 28Division of Pediatric Cardiology, Department of Pediatrics, University of Iowa Stead Family Children’s Hospital, Iowa City, IA; 29Division of Pediatric Cardiology, Department of Pediatrics, University of Florida, Gainesville, Gainesville, FL; 30Division of Pediatric Cardiology, Department of Pediatrics, Children’s Hospital of Richmond at VCU, Richmond, VA; 31Department of Pediatrics, Children’s of Mississippi Heart Center, University of Mississippi Medical Center, Jackson, MS; 32Division of Pediatric Cardiology, Department of Pediatrics, Kentucky Children’s Hospital, Lexington, KY; 33University of Kentucky College of Medicine, Lexington, KY; 34Division of Pediatric Cardiology, Department of Pediatrics, Seattle Children’s Hospital, Seattle, WA; 35Division of Pediatric Cardiology, Department of Pediatrics, Mayo Clinic Children’s Center, Rochester, MN; 36Division of Pediatric Cardiology, Department of Pediatric, Penn State Health Children’s Hospital, Hershey, PA; 37Division of Pediatric Infectious Diseases, Department of Pediatrics, UC Davis Children’s Hospital, Sacramento, CA; 38Division of Pediatric Cardiology, Department of Pediatrics, Oklahoma Children’s Hospital, Oklahoma City, OK; 39Division of Pediatric Cardiology, Department of Pediatrics, West Virginia University School of Medicine and CAMC Women’s and Children’s Hospital, Charleston, WV; 40Division of Pediatric Cardiology, Department of Pediatrics, Children’s Memorial Hermann Hospital, Houston, TX; 41Division of Pediatric Cardiology, Department of Pediatrics, Rush University Medical Center, Chicago, IL; 42Division of Pediatric Cardiology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH; 43Division of Pediatric Cardiology, Department of Pediatrics, Children’s Mercy Hospital, Kansas City, MO; 44Division of Pediatric Cardiology and Pediatric Infectious Diseases, Department of Pediatrics, Upstate Golisano Children’s Hospital, Syracuse, NY; 45Division of Pediatric Cardiology, Department of Pediatrics, Helen DeVos Children’s Hospital, Grand Rapids, MI; 46Division of Pediatric Cardiology, Department of Pediatrics, Dell Children’s Medical Center, Austin, TX; 47Division of Pediatric Cardiology, Department of Pediatrics, Children’s Hospital of New Orleans, New Orleans, LA; 48Division of Pediatric Cardiology, Department of Pediatrics, Loma Linda University Children’s Hospital, Loma Linda, CA; 49Division of Pediatric Cardiology, Department of Pediatrics, Jack and Lucy Clark Department of Pediatrics at the Icahn School of Medicine at Mount Sinai Children’s Hospital, New York, NY; 50Division of Pediatric Cardiology, Department of Pediatrics, Cleveland Clinic Children’s Hospital, Cleveland, OH; 51Division of Pediatric Cardiology, Department of Pediatrics, UNC Children’s Hospital, Chapel Hill, NC; 52Division of Pediatric Cardiology, Department of Pediatrics, Rainbow Babies and Children’s Hospital, Cleveland, OH; 53Division of Pediatric Cardiology, Department of Pediatrics, Children’s Hospital at Montefiore, Bronx, NY; 54Division of Pediatric Cardiology, Department of Pediatrics, Children’s Hospital and Medical Center, Omaha, NE; 55Division of Infectious Diseases, Department of Pediatrics, Le Bonheur Children’s Hospital, Memphis, TN; 56Division of Infectious Diseases, Department of Pediatrics, Weill Cornell Medical Center, New York, NY; 57Division of Infectious Diseases, Department of Pediatrics, University of Chicago Comer Children’s Hospital, Chicago, IL; 58Division of Pediatric Cardiology, Department of Pediatrics, Columbia University Medical Center, New York, NY; 59Division of Pediatric Cardiology, Department of Pediatrics, Norton Children’s Hospital, Louisville, KY; 60Division of Pediatric Cardiology, Department of Pediatrics, Washington University School of Medicine, St Louis, MO; 61Division of Pediatric Cardiology, Department of Pediatrics, Lutheran Hospital, Fort Wayne, IN; and 62Division of Pediatric Infectious Diseases, Department of Pediatrics, Ascension St John Hospital, Detroit, MI; 63Division of Pediatric Critical Care, Department of Pediatrics, West Virginia University School of Medicine and West Virginia University Medicine Children's Hospital, Morgantown, WV
References
- 1.Centers for Disease Control and Prevention (CDC) Myocarditis and Pericarditis After mRNA COVID-19 Vaccination. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html Accessed September 10, 2021.
- 2.Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Coronavirus disease 2019 (COVID-19) vaccines. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html
- 3.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das B.B., Kohli U., Ramachandran P., Nguyen H.H., Greil G., Hussain T., et al. Myopericarditis after messenger RNA coronavirus disease 2019 vaccination in adolescents 12 to 18 years of age. J Pediatr. 2021;238:26–32.e1. doi: 10.1016/j.jpeds.2021.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dionne A., Sperotto F., Chamberlain S., Baker A.L., Powell A.J., Prakash A., et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021:e213471. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. e2021052478. [DOI] [PubMed] [Google Scholar]
- 7.Snapiri O., Rosenberg Danziger C., Shirman N., Weissbach A., Lowenthal A., Ayalon I., et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine. Pediatr Infect Dis J. 2021;40:e360–e363. doi: 10.1097/INF.0000000000003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schauer J., Buddhe S., Colyer J., Sagiv E., Law Y., Chikkabyrappa S.M., et al. Myopericarditis after the Pfizer mRNA COVID-19 vaccine in adolescents. J Pediatr. 2021;238:317–320. doi: 10.1016/j.jpeds.2021.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S.S., Steele J.M., Fonseca B., Huang S., Shah S., Maskatia S.A., et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148 doi: 10.1542/peds.2021-053427. e2021053427. [DOI] [PubMed] [Google Scholar]
- 10.Tano E., San Martin S., Girgis S., Martinez-Fernandez Y., Sanchez Vegas C. Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19 vaccine. J Pediatr Infect Dis Soc. 2021;10:962–966. doi: 10.1093/jpids/piab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US News and World Report Best Children's Hospitals for Cardiology & Heart Surgery. https://health.usnews.com/best-hospitals/pediatric-rankings/cardiology-and-heart-surgery
- 12.Harahsheh A.S., Ottolini M., Lewis K., Blatt B., Mitchell S., Greenberg L. An innovative pilot curriculum training pediatric residents in referral and communication skills on a cardiology rotation. Acad Pediatr. 2016;16:700–702. doi: 10.1016/j.acap.2016.05.146. [DOI] [PubMed] [Google Scholar]
- 13.US Census Bureau 2010 Census Regions and Divisions of the United States. https://www.census.gov/geographies/reference-maps/2010/geo/2010-census-regions-and-divisions-of-the-united-states.html
- 14.Centers for Disease Control and Prevention Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7027e2-H.pdf [DOI] [PMC free article] [PubMed]
- 15.Miyake C.Y., Teele S.A., Chen L., Motonaga K.S., Dubin A.M., Balasubramanian S., et al. In-hospital arrhythmia development and outcomes in pediatric patients with acute myocarditis. Am J Cardiol. 2014;113:535–540. doi: 10.1016/j.amjcard.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Kuehn B.M. Adolescent myocarditis after COVID-19 vaccination is rare. JAMA. 2021;326:902. doi: 10.1001/jama.2021.14237. [DOI] [PubMed] [Google Scholar]
- 17.American College of Cardiology Sport Recommendations for Athletes With Cardiomyopathies, Myocarditis, Pericarditis. https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2019/07/19/08/37/recommendations-for-participation-in-competitive
- 18.Madaan P., Chand P., Linn K., Wanigasinghe J., Mynak M.L., Poudel P., et al. Management practices for West syndrome in South Asia: a survey study and meta-analysis. Epilepsia Open. 2020;5:461–474. doi: 10.1002/epi4.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention COVID-19 Vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.