Abstract
Anisakiasis is a gastrointestinal disease caused by infection with anisakid nematodes. Anisakis larvae have been listed as distinct food poisoning agents in the manual of Food Poisoning Statistics, Japan since 2013. The reported numbers of food poisoning cases caused by Anisakis larvae are gradually increasing. A total of 94.0% of the causative larvae species were identified as Anisakis simplex sensu stricto (A. simplex), and 4.4% were identified as Anisakis pegreffii, among human-isolated anisakid nematodes examined in Tokyo Metropolitan Institute of Public Health, Japan from 2011 to 2018. Anisakis species infecting fishes in Japanese waters differ depending on their habitat and depth. A. simplex mainly infects fishes in the Pacific side of Japan, and A. pegreffii mainly infects fishes in the East China Sea and Sea of Japan sides. Regarding the causative foods of anisakiasis, cases by ingestion of mackerel (Scomber spp.) have been the most common in Japan, and cases caused by eating “marinated mackerel” accounted for 32.8% of the total in Tokyo from 2011 to 2017. However, the number of reports of food poisoning caused by skipjack tuna (Katsuwonus pelamis) was highest in May 2018 in Japan. A parasitological surveys of Anisakis third-stage larvae in skipjack tuna in Japanese waters were conducted in 2018 and 2019, and it was confirmed that more A. simplex infections of skipjack tuna may have occurred in 2018 than usual due to the meandering flow of the Black Current. Moreover, a portion of A. simplex larvae migrated from visceral organs to the ventral muscle in live skipjack tuna before capture, suggesting that an extensive cold chain after capture cannot prevent anisakiasis. In fish species that were reported to be high frequency of causative food of anisakiasis, it is necessary to freeze or at least remove the ventral muscle.
Key words: food poisoning, parasitological survey, Anisakis simplex sensu stricto, Anisakis pegreffii, Katsuwonus pelamis, food safety
Introduction
Anisakid nematodes have been included as pathogenic agents in the Food Sanitation Law of Japan since 1999. Subsequently, in 2013, Anisakis larvae were listed as distinct food poisoning agents in the Manual of Food Poisoning Statistics, Japan, requiring medical doctors to report cases to a public health center. As a result, among the food poisoning incidents that have increased annually (Table 1), we have become to more clearly understand about symptoms, causative fishes and origins associated with food poisoning caused by Anisakis larvae (Anisakis food poisoning). Furthermore, anisakiasis is the most common form of food poisoning since 2017, having its case numbers exceeded Campylobacter jejuni/coli and Norovirus infections. These three food poisonings occupied 76.1% of the total case numbers in duration of 2015 through 2019. However, unlike many other food poisonings, 97.6% (1417/1452 cases) of food posoning anisakiasis cases were single patient cases. Moreover, the food poisoning cases caused by Anisakis spp. constitute only a part of total cases of anisakiasis, and the number of anisakiasis cases based on data from medical institutions is estimated to be 7,000 cases per year in Japan1). In this review, we will go over anisakiasis in Japan, mainly in Tokyo, and parasitological surveys of Anisakis third-stage (Anisakis L3) larvae in fish, especially skipjack tuna (Katsuwonus pelamis) and Pacific bluefin tuna (Thunnus orientalis) (PBT).
Table 1. Number of Anisakis food poisoning cases and patients in Japan from 2013 to 2020 based on food poisoning statistics from the MHLW.
| Year | No. of cases | No. of patients |
| 2013 | 88 | 89 |
| 2014 | 79 | 79 |
| 2015 | 127 | 133 |
| 2016 | 125 | 127 |
| 2017 | 234 | 246 |
| 2018 | 469 | 479 |
| 2019 | 330 | 338 |
| 2020 | 387 | 397 |
| Total | 1839 | 1888 |
1. Anisakis third-stage Larvae in Fish
Nematodes of the genus Anisakis are parasites, whose definitive hosts are marine mammals such as whales and dolphins, marine fish, and squid. They serve as paratenic hosts for Anisakis L3 larvae. Nine species of Anisakis nematodes are known2,3,4,5,6,7,8,9), and Anisakis L3 larvae are divided into Type I–IV based on their morphological differences10). The larvae of six species, namely, Anisakis simplex sensu stricto (A. simplex), Anisakis pegreffii (A. pegreffii), Anisakis berlandi, Anisakis typica (A. typica), Anisakis ziphidarum and Anisakis nascettii, are classified as Type I larvae because of their morphological similarity. It has been reported that A. pegreffii has a slightly shorter ventriculus length than A. simplex11). On the other hand, Anisakis Type II, Type III and Type IV larvae may be morphologically identified as Anisakis physeteris (A. physeteris), Anisakis brevispiculata and Anisakis paggiae, respectively12). Eight out of the nine Anisakis species, after excluding A. nascettii, can be detected in fish caught in Japanese waters, as has been confirmed in our survey. There is no report of A. nascettii being detected in Japan.
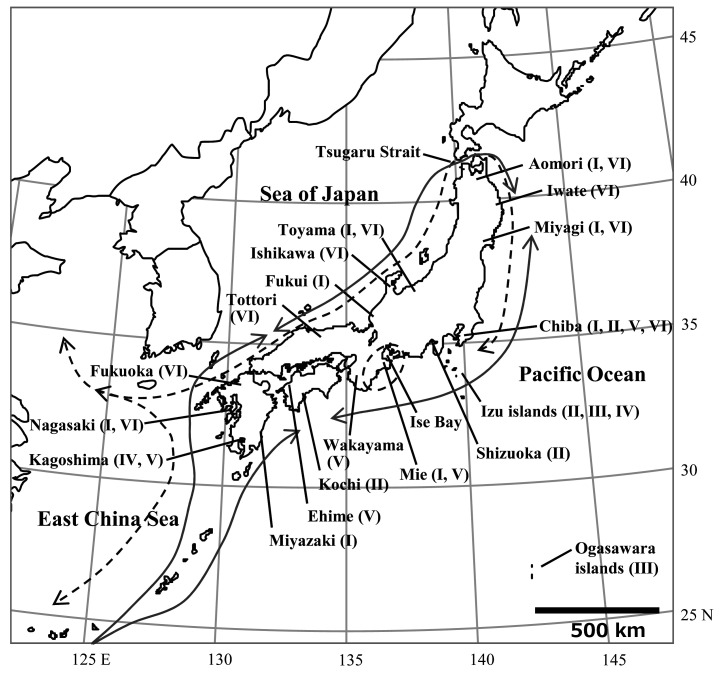
Anisakis species infecting fishes in Japanese waters differ depending on their habitat and depth. According to a survey of chub mackerel (Scomber japonicas), A. simplex mainly infects chub mackerel in the Pacific side of Japan, while A. pegreffii mainly infects chub mackerel in the East China Sea and the Sea of Japan sides13). This trend is often found in not only chub mackerel but also other fish species14,15). Fishing area and habitat depth as well as migration route affect on the Anisakis species in migratory fish. For example, in Japanese Spanish mackerel (Scomberomorus niphonius) caught in the Pacific Ocean side of Japan (details described in supplemental materials and methods), A. pegreffii was the main species detected (Table 2). This is because, although the fishes were caught on the Pacific side, they were originally born in the East China Sea and passed through the Tsugaru Strait from the Sea of Japan side (Fig. 1; dotted arrow line)16). A. simplex was the main species infecting Japanese Spanish mackerel shipped from Mie prefecture. They were thought to be born and grown around Ise Bay17) (Table 2). Among deep-sea fishes such as Beryx splendens, Etelis carbunculus and Etelis coruscans, A. physeteris was frequently detected (Table 3; methods for E. carbunculus and E. coruscans were described in supplemental materials and methods). A. typica was detected at a high rate in hairtail (Trichiurus lepturus) that were landed at sea ports of Kagoshima prefecture (Table 4; details described in supplemental materials and methods), and similarly reported as mainly collected from hairtail of Taiwan captures18). In most of the fish species in which Anisakis larvae were detected in the muscle, the muscular part in question was the ventral muscles. Detection of the larvae in the dorsal muscle was limited to species such as mackerel and natural salmon (Oncorhynchus keta), and almost all the larvae detected in the muscle were identified as A. simplex.
Table 2. Number of Anisakis larvae detected in Japanese Spanish mackerel (Scomberomorus niphonius) from Japanese waters from 2007 to 2009.
| Catching area | District of fishby prefecture | No. of fish samples (positive) |
No. of Anisakis larvae | ||
| A. simplex sensu stricto | A. physeteris | Total | |||
| Pacific Ocean | Aomori | 2 (1) | 0 | 1 | 1 |
| Miyagi | 1 (1) | 0 | 1 | 1 | |
| Chiba | 10 (6) | 0 | 19 | 19 | |
| Mie | 3 (1) | 14 | 0 | 14 | |
| Sea of Japan and East China Sea |
Nagasaki | 2 (2) | 0 | 8 | 8 |
| Miyazaki | 2 (2) | 0 | 3 | 3 | |
| Fukui | 2 (1) | 0 | 4 | 4 | |
| Toyama | 12 (9) | 1 | 82 | 83 | |
| Total | 34 (23) | 15 | 118 | 133 | |
Fig. 1.
Localities of landing fishes in Japanese waters
I: Districts of Japanese Spanish mackerel (Scomberomorus niphonius), II: Districts of splendid alfonsino (Beryx splendens), III: District of deep-water red snapper (Etelis carbunculus), IV: District of deep-water longtail red snapper (Etelis coruscans), V: Districts of hairtail (Trichiurus lepturus), VI: Districts of juvenile PBT (Thunnus orientalis). Dotted arrow line indicate Japanese Spanish mackerel migration routes16), while arrowed lines indicate juvenile PBT migration routes45,46,47,48).
Table 3. Anisakis species detected in deep-sea fishes from Japanese waters.
| Fish species (examination periods) |
District of fish | Fish samples (positive) |
No. of Anisakis larvae (%) | Total larvae |
|||||
| A. simplex sensu stricto | A. berlandi | A. ziphidarum | A. physeteris | A. brevispiculata | A. paggiae | ||||
|
Beryx splendens12) (2007-2009) |
Tokyo (Izu islands) |
8 (8) | 11 | 1 | 0 | 107 | 2 | 2 | 123 |
| Chiba | 9 (9) | 4 | 0 | 0 | 31 | 1 | 1 | 37 | |
| Shizuoka | 19 (19) | 21 | 4 | 1 | 403 | 6 | 44 | 479 | |
| Kochi | 8 (8) | 6 | 0 | 0 | 83 | 1 | 1 | 91 | |
|
Etelis carbunculus (2012-2014) |
Tokyo (Izu-Ogasawara islands) |
12 (9) | 2 | 0 | 0 | 58 | 0 | 0 | 60 |
|
Etelis coruscans (2012-2014) |
Tokyo (Izu islands) |
17 (7) | 2 | 0 | 1 | 14 | 0 | 0 | 17 |
| Kagoshima | 2 (1) | 0 | 0 | 0 | 3 | 0 | 0 | 3 | |
| Total | 75 (62) |
46 (5.7) |
5 (0.6) |
2 (0.2) |
699 (86.3) |
10 (1.2) |
48 (5.9) |
810 (100) |
|
Table 4. Detection numbers of Anisakis larvae in hairtail (Trichiurus lepturus) from Japanese waters from 2013 to 2015.
| District of fish | No. of fish samples (positive) |
No. of Anisakis larvae | Total larvae | ||
| A. simplexsensu stricto | A. typica | A. physeteris | |||
| Chiba | 3 (3) | 13 | 0 | 0 | 13 |
| Mie | 5 (0) | 0 | 0 | 0 | 0 |
| Wakayama | 3 (0) | 0 | 0 | 0 | 0 |
| Ehime | 6 (0) | 0 | 0 | 0 | 0 |
| Kagoshima | 7 (3) | 0 | 47 | 7 | 54 |
| Total | 24 (6) | 13 | 47 | 7 | 67 |
2. Anisakiasis and Food Poisoning Caused by Anisakis larvae in Tokyo, Japan
Consumption of seafood prepared using raw fish and raw fish-alike (such as sashimi, sushi and marinated fish) containing Anisakis L3 larvae may let them invade into the stomach and intestinal walls to cause acute gastroenteritis in humans. This gastroenteritis is known as anisakiasis. Anisakiasis was for the first time reported in the Netherlands19) in 1960, and in Japan20) in 1965. Anisakiasis, as a result of Anisakis food poisoning, more frequently occur in households than most of other types of food poisonings based on food poisoning statistics in Japan. This tendency became clearer in year 2020 food poisoning statistics, possibly attributing to the requests issued by the Japanese government and local governments to refrain from eating out during the COVID-19 outbreak in Japan and people’s compliance during year 2020. In 2020, the number of food poisoning cases caused by Campylobacter jejuni/coli and Norovirus decreased by 43% and 58% year-on-year, respectively, but the number of Anisakis food poisoning cases increased by 17.3% from the previous year.
Anisakiasis clinical cases are divided into fulminant and mild forms according to their severity of symptoms. Mild form is often asymptomatic and rarely diagnosed. The fulminant form is further divided into two main types of gastric anisakiasis and intestinal anisakiasis, depending on the locations of Anisakis larvae. Moreover, there are also a few reports of gastro-allergic anisakiasis associated with A. simplex and A. pegreffii larvae21,22). Onset of clinical symptoms in acute gastric anisakiasis cases was reported to occur within 1 to 12 h after the ingestion of infected fish, with peaking at 6 h23). In accordance, onset of anisakiasis cases examined at the Tokyo Metropolitan Institute of Public Health from 2011 to 2017 showed, among 180 cases (179 gastric anisakiasis cases and an intestinal anisakiasis case), 91.2% (134/147 cases) with clearly known onset timing from the ingestion occurred within 12 h (Table 5). The main symptoms such as abdominal pain (100%), nausea (68.3%), vomiting (39.0%), diarrhea (19.5%), and urticarial (9.8%) were reported24).
Table 5. Time to onset of clinical symptoms in anisakiasis cases at Tokyo Metropolitan Institute of Public Health from 2011 to 2017.
| Time | No. of cases (%) |
| Less than 4 h | 45 (25.0) |
| 4 to 8 h | 59 (32.8) |
| 8 to 12 h | 30 (16.7) |
| More than 12 h* | 13 (7.2) |
| Unknown** | 33 (18.3) |
| Total | 180 (100) |
*Maximum time was 39 h. **Cases where raw fish was eaten multiple times within 2 days or unanswered cases.
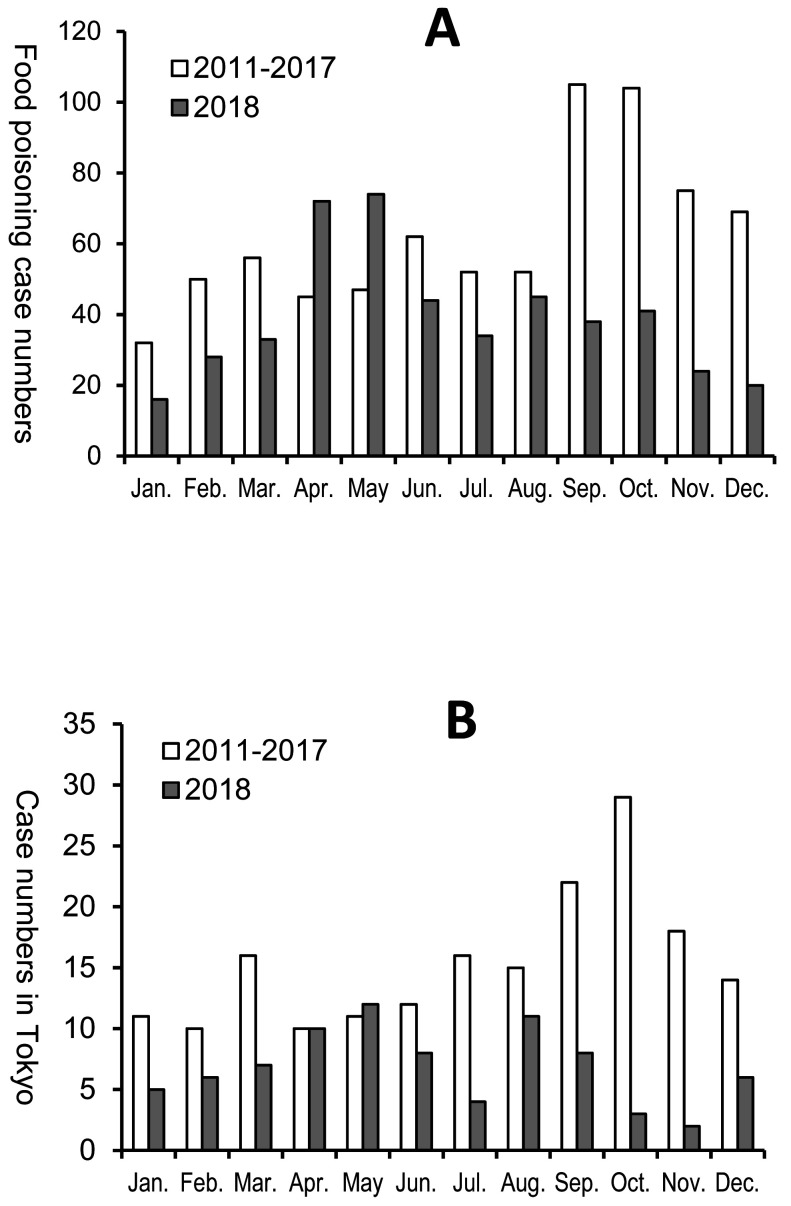
According to an interview-based survey conducted by public health centers’ staff in Tokyo from 2011 to 2017, mackerel was the most important causative food and accounted for 42.8% of anisakiasis cases’ reasons in Tokyo, followed by marinated mackerel accounting for 32.8%25). In the monthly statistics25) that were reported to Ministry of Health, Labour and Welfare of Japan (MHLW) regarding Anisakis food poisonings from 2011 to 2017, the case peak took place in September and October (Fig. 2), because of seasonally consumption saury (Cololabis saira) as a cause anisakiasis. It has been reported that A. simplex was detected in 13% of saury’s visceral organs and 1.8% of their muscle tissues26). This detection rate was lower than those in mackerel13) and natural salmon27), however, considering the number of Anisakis food poisoning cases by ingestion of saury, the detection rate in the muscle should not be ignored. In 2018, the number of Anisakis food poisoning cases in Japan peaked in April and May (Fig. 2), unlike in the regular trend. Moreover, in many of these cases, skipjack tuna was presumed to be the causative fish of Anisakis food poisoning. In cases where Anisakis spp. were identified at the Tokyo Metropolitan Institute of Public Health, 23 anisakiasis cases had a history of skipjack tuna ingestion in 2018, among which 13 cases were with a sole consumption of skipjack tuna25). This was only second to 33 cases confirmed to have ingested mackerel, among which 13 cases were with a sole consumption of mackerel (Table 6, numbers of food poisoning cases of mackerel; unpublished data).
Fig. 2.
Food poisoning cases caused by Anisakis spp. in Japan (A) and anisakiasis cases at the Tokyo Metropolitan Institute of Public Health (B).
A: Graph was created based on numbers of Anisakis food poisoning cases in the manual of Food Poisoning Statistics, Japan.
(https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/shokuhin/syokuchu/04.html), B: Anisakiasis case numbers examined at the Tokyo Metropolitan Institute of Public Health25).
Table 6. Numbers of anisakiasis cases with a history of skipjack tuna (Katsuwonus pelamis) and mackerel (Scomber spp.) ingestion in 2018, and the numbers of larvae detected per patient in Tokyo, Japan.
| No. of Anisakis larvae per patient | Skipjack tuna (ingestion of only skipjack tuna) |
Mackerel* (ingestion of only mackerel) |
| A. simplex sensu stricto | ||
| 1 | 19 (11) | 24 (8) |
| 2 | 2 (1) | 7 (4) |
| 3 | 1 (1) | 0 |
| 4 | 0 | 1 (0) |
| 5 | 1 (0) | 0 |
| A. pegreffii | ||
| 1 | 0 | 1 (1) |
| 2 | 0 | 0 |
| Total cases | 23 (13) | 33 (13) |
Anisakiasis cases were examined numbers at the Tokyo Metropolitan Institute of Public Health.
*Mackerels included in Scomber japonicus and S. australasicus.
3. Causative Anisakis species in Anisakiasis
Out of 318 anisakid nematodes from patients examined at the Tokyo Metropolitan Institute of Public Health from 2011 to 2018 period28), 299 larvae (94.0%) were identified as A. simplex and 14 larvae (4.4%) as A. pegreffii (Table 7). It has been reported that the causative Anisakis species of anisakiasis is A. simplex, even in Kyushu region, where the detection rate of A. pegreffii such as in mackerel is higher than that in Pacific coast regions29). The reason for this epidemiological trend was the migration rate of A. simplex from the visceral organs to the muscle of chub mackerel being 100 times faster than that of A. pegreffii13) shown in Table 8. A comparison of penetration abilities by A. simplex and A. pegreffii using agar confirmed that A. simplex had higher rate of agar penetration than A. pegreffii13,30). Moreover, a study with experimental infection in Wistar rats reported that the tissue penetration rate of A. simplex was 63% higher than that of A. pegreffii31). However, the clinical symptoms of fulminant anisakiasis caused by A. simplex and A. pegreffii are similar, characterized by epigastric pain, nausea, vomiting and abdominal fullness, and there is no significant difference in histopathological findings between the two species. Therefore, A. simplex is not greater pathogenic than A. pegreffii but migration capability in the fish muscle is greater. Because of increased opportunities to consume A. simplex, it is a major causative agent of anisakiasis. Anisakiasis caused by Anisakis species other than A. simplex and A. pegreffii in Japan was reported; in one case, A. typica was identified as a cause (The 86th Annual Meeting of the Japanese Society of Parasitology, 2017), and in two cases, A. physeteris was identified as causes32,33).
Table 7. Number of human isolate anisakid nematodes identified in Tokyo Metropolitan Institute of Public Health from 2011 to 2018.
| Larval species | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Total (%) |
| A. simplex sensu stricto | 11 | 30 | 30 | 24 | 23 | 27 | 56 | 98 | 299 (94.0) |
| A. pegreffii | 0 | 0 | 0 | 0 | 2 | 2 | 3 | 7 | 14 (4.4) |
| Pseudoterranova azarasi | 0 | 1* | 0 | 1* | 0 | 0 | 2 | 1 | 5 (1.6) |
| Total larvae | 11 | 31 | 30 | 25 | 25 | 29 | 61 | 106 | 318 (100) |
*Pseudoterranova sp. (genetic analysis not performed)
Table 8. Number of Anisakis larvae detected in chub mackerel (Scomber japonicus) from Japanese waters13).
| Catching area | Main Anisakis species |
No. of fish samples (positive) |
Total no. of Anisakis larvae (A) | No. of larvae in fish muscle (B) | Percentage of larvae in fish muscle (B/A) |
| Sea of Japan and East China Sea |
A. pegreffii | 86 (63) | 4073 | 4 | 0.1% (4/4073) |
| Pacific Ocean |
A. simplex sensu stricto |
132 (99) | 733 | 81 | 11.1% (81/733) |
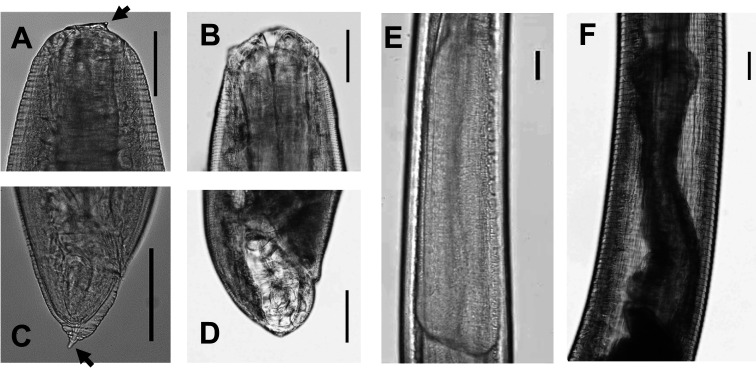
Among 208 Anisakis larvae detected in patients who were examined in our institute from 2011 to 2017, seven larvae were morphologically fourth-stage Anisakis (Anisakis L4) larvae, and 59 larvae (28.4%) were difficult to identify for their stages based because of damages for morphological examination. Morphological characteristics between Anisakis L3 and L4 in Type I larvae are very different34,35). Both the boring tooth at the anterior end and mucron at the posterior tip in morphological Type I larvae in the Anisakis L3 (Fig. 3-A and C) are absent in the Anisakis L4, and perioral lips are present at the anterior of L4 larvae (Fig. 3-B and D). L3 larvae possess a relatively long ventriculus with an oblique ventricular-intestinal junction, but the ventriculus of L4 larvae shows morphological changes in the internal structure36).Fig. 3-E and F: Table 1andFig. 3 display morphological L3 larvae. The maximum number of larvae detected in one patient was 10, and the suspected causative food was natural salmon. In 91.7% of cases (165/180), the number of larvae removed from per patient was one. The reporting of Anisakis food poisoning by each local government to the MHLW is often based on morphological examination of larvae, and a few local governments carry out molecular identification. Since the Anisakis species infecting fishes are closely associated with the catch area and habitat of the fishes, it is important to identify the larval species, and narrow down or determine the causative fish. It necessitates active conduct of molecular identification of larvae detected in humans.
Fig. 3.
Morphology of third-stage (L3) and fourth-stage (L4) larvae of Anisakis simplex sensu stricto
A, Cephalic end of L3 larva (arrowed line: boring tooth); B, Cephalic end of L4 larva; C, Caudal end of L3 larva (arrowed line: mucron); D, Caudal end of L4 larva; E, Ventricular part of L3; F, Ventricular part of L4. Bar: 100 μm.
4. Survey of Anisakis larvae from Skipjack Tuna, Katsuwonus pelamis
To investigate the cause of increase in Anisakis food poisoning cases by ingestion of skipjack tuna in 2018, a parasitological survey of Anisakis L3 larvae was conducted in skipjack tuna25) from May 2018 to October 2019.
In 2018, the survey investigated the timing of Anisakis migrates from visceral organs to the muscle of skipjack tuna, whether it took place before catching or after, a parasitological examination was conducted regarding Anisakis larvae between August and November by comparison among different timings of gutting before processing for sale. Anisakis larvae were detected not only in the muscle after landing, but also found in the muscle of skipjack tuna, in which the visceral organs were immediately removed after catching25). Anisakis larvae, when found in the muscle, were only in the ventral muscle. All the larvae in the ventral muscle were identified as A. simplex. These findings demonstrated, with a portion of A. simplex larvae migrated to the muscle in live skipjack tuna before capture, that an extensive cold chain after capture does not prevent anisakiasis.
Out of 10 skipjack tunas caught off the east coast of Chiba prefecture in May 2018, 13 Anisakis L3 larvae were detected in the ventral muscle of 2 fishes. In a total of 680 Anisakis L3 larvae were collected from 88 out of 90 skipjack tunas from August to November 2018 period25), where, 47 larvae were detected in the muscle of 14 fishes (Table 9). Out of 21 skipjack tunas caught off the coasts of Mie and Shizuoka prefectures between April and June 2019, a total of 115 Anisakis L3 larvae were detected in 19 fishes, and 2 larvae were detected in the ventral muscle of two fishes. Out of 30 skipjack tunas caught off the coasts of Chiba and Miyagi prefectures between July and October 2019, 494 Anisakis L3 larvae were detected from 29 fishes and 8 larvae from the ventral muscle of six fishes25) as in Table 9. In the present survey from May 2018 to October 2019, the most common Anisakis species was A. simplex (1016/1421 larvae), and all the larvae detected in the muscle were identified as A. simplex. The second most frequently detected species was A. physeteris in this survey. It was recently reported that skipjack tuna prey on fish even at a depth of 200 meters37), explaining why A. physeteris was detected in skipjack tuna.
Table 9. Number of Anisakis larvae detected in skipjack tuna (Katsuwonus pelamis) from May 2018 to October 201925).
| Period | Fish samples (A) |
Positive samples (in muscle) |
No. of Anisakis larvae | %, larvae in fish muscle (B/C) |
Mean abundance of larvae in fish muscle (B/A) |
|
| In muscle* (B) |
Total (C) |
|||||
| May, 2018 | 10 | 8 (2) | 13 | 132 | 9.80% | 1.3 |
| (13/132) | (13/10) | |||||
| Aug to Nov, 2018 | 90 | 88 (14) | 47 | 680 | 6.90% | 0.52 |
| (47/680) | (47/90) | |||||
| Apr to Jun, 2019 | 21 | 19 (2) | 2 | 115 | 1.70% | 0.1 |
| (2/115) | (2/21) | |||||
| Jul to Oct, 2019 | 30 | 29 (6) | 8 | 494 | 1.60% | 0.27 |
| (8/494) | (8/30) | |||||
*All larvae in fish muscle were Ansiakis simplex sensu stricto.
Current status of anisakiasis and Anisakis larvae in Tokyo, Japan (Jun Suzuki, Rie Murata, Yukihiro Kodo)
In May 2018, the mean abundance and percentage of A. simplex larvae in the ventral muscle of skipjack tuna were 1.30 (13 larvae/10 fishes) and 9.8% (13 larvae/132 larvae), respectively25), and these values were higher than those in the other study periods (Table 9). Because the skipjack tuna was obtained from a single locality (off Chiba) in May 2018 and was caught in an area of the ocean approximately 500 km away from the main area of fish catch in April and May 2018, when Anisakis food poisoning cases caused by the ingestion of skipjack tuna were reported, there was insufficient evidence supporting that the skipjack tuna examined in April and May 2018 were infected with A. simplex. However, because the number of A. simplex larvae in the ventral muscle of skipjack tuna in 2018 was higher than that in 2019, it was considered that Anisakis food poisoning caused by ingestion of skipjack tuna was reported more frequently than usual in 2018.
The difference in the number of Anisakis larvae in “Hatsugatsuo” (the season’s first skipjack tuna in April and June) between 2018 and 2019 may be related to the host migratory patterns. As skipjack tuna inhabit sea waters at temperatures of >18°C, those around Japan may migrate north via three major routes as the ocean water temperature increases: along with the Black Current, around the Izu-Ogasawara Islands, and more extensively, in the Pacific Ocean on the east side of the Izu Islands38,39,40). The fishing area of “Hatsugatsuo” is usually the sea around the Ogasawara Islands in April and around the Izu Islands in May. In 2018, the Black Current took a large meandering path along the Pacific coasts and increased the temperature of the sea around Miyakejima Island41), the main fishing area of skipjack tuna in April 2018, to above 18°C, which is relative to the sea temperature (18°C) in May 2017. Some schools of skipjack tuna may have migrated north earlier than usual in 2018. This may have changed their feeding habits, causing an increase in Anisakis infection. Anisakis food poisoning was reported in Tokyo, Fukushima and Miyazaki prefectures, as well as elsewhere, at the same time, which was thought to be a result of skipjack tuna being caught in the same sea area and distributed across Japan.
As part of an investigation into the cause of the increase in anisakiasis by the ingestion of skipjack tuna in 2018, a questionnaire survey of 102 fish and shellfish distributors and 339 restaurant businesses in Tokyo was conducted by the public health center staff in Tokyo between October and December 2018, where they were asked whether skipjack tuna had been frozen25,42). Among the fish and shellfish distributors (102 facilities), 47 (46.1%) and 35 (34.3%) facilities distributed only either fresh chilled or frozen skipjack tuna, respectively, whereas 20 (19.6%) distributed both. Of restaurant businesses (339 facilities), 254 (74.9%) and 30 (8.8%) facilities served only either fresh chilled or frozen skipjack tuna, respectively, whereas 30 (9.0%) served both. Moreover, of 67 fish and shellfish distributors trading in chilled raw or semi-raw skipjack tuna, the survey revealed that 45 of the 67 facilities (67.2%) removed the ventral muscle. On the other hand, among 284 restaurant businesses that served chilled raw or semi-raw skipjack tuna, 165 facilities (58.1%) removed the ventral muscle. From the questionnaire survey, it was revealed that fish and shellfish distributors had paid more attention to prevention of Anisakis food poisoning by skipjack tuna than restaurant businesses.
5. Anisakis larvae in Juvenile PBT, Thunnus orientalis
It is known that Thunnus spp. and skipjack tuna (Katsuwonus pelamis) are migratory fish and belong to the same clade in the morphological phylogeny of the family Scombridae43). PBT (Thunnus orientalis) is called “Honmaguro” in Japan, and the muscle of adult PBT is an expensive seafood used for sushi and sashimi. On the other hand, juvenile PBT are often accidentally caught in Japanese waters and can generally be purchased in markets at lower prices. Ingestion of tuna has sometimes been reported as a cause of anisakiasis in interview-based surveys conducted by public health center staff in Tokyo, because tuna is included in many sashimi platters and not only in single dishes such as tuna bowls and sushi. However, there are few reports in Japan of Anisakis larvae in PBT, and we had only two cases thus far, in which Anisakis larvae were detected from patients or tuna leftovers.
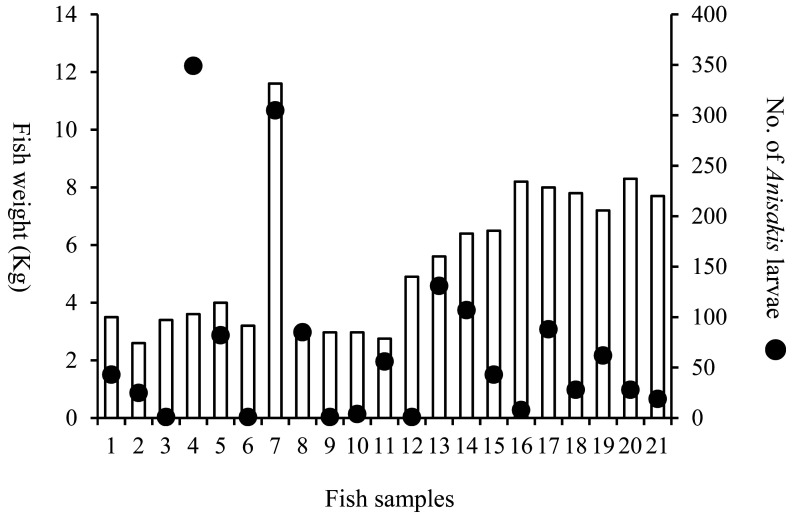
A parasitological survey of Anisakis larvae from 39 juvenile PBT (1.4 kg to 11.6 kg) was conducted in 2005 to 2006, and 1,467 Anisakis larvae detected in 21 fishes were genetically identified. No correlation was found between the weight of juvenile PBT and the number of larvae in this study44) (Pearson’s correlation test, P > 0.05) (Fig. 4). Moreover, a parasitological survey of Anisakis larvae from 104 juvenile PBT (1.5 kg to 12.7 kg) was conducted from 2011 to 2013 (Fig. 1), and Anisakis larvae detected in 30 fishes were genetically identified. In this parasitological survey, a total of 302 larvae were detected in 16 juvenile PBT caught in the Sea of Japan and the East China Sea, of which 295 larvae (97.7%) were identified as A. pegreffii. A total of 143 larvae were detected in 14 juvenile PBT caught in the Pacific Ocean side, of which 130 larvae (90.9%) were also identified as A. pegreffii, and there was no difference in Anisakis species depending on the catch areas (manuscripts in preparation). PBT spawn around the Nansei Islands, grow in the Sea of Japan side (off Shimane prefecture to off Toyama prefecture) while migrating around Japanese waters (Fig. 1), and eventually cross the Pacific Ocean and reach the west coast of North America45,46,47,48). PBT larvae are reported to prey primarily on plankton such as copepods49). As PBT grows, it begins to prey on small fish and grows rapidly50,51). The number of Anisakis larvae in juvenile PBT was expected to increase as a fish grew; however, no correlation was found between the weight of juvenile PBT and the number of larvae in this study. Since 90.9% of the Anisakis larvae detected in juvenile PBT caught on the Pacific side of Japan were identified as A. pegreffii and the number of larvae did not increase with fish weight, it was suggested that the juvenile PBT may have been infected with Anisakis spp. larvae during the fish larval period. The risk of anisakiasis due to ingestion of juvenile PBT is considered to be lower than that of skipjack tuna because more than 90% of the Anisakis larvae detected in juvenile PBT were A. pegreffii and no larvae were detected in the fish muscle.
Fig. 4.
Association between the number of Anisakis larvae and the weight of juvenile PBT in Japanese waters from 2005 to 200644)
6. Concluding Remarks
It is recommended by the MHLW that Anisakis larvae are killed by freezing in fish at < –20°C for 24 h or heating at > 60°C for 1 minute. The Food and Drug Administration (2020) recommends freezing fish at −20°C or below for 7 days or −35°C or below until solid, then storing at −35°C for 15 h, −35°C or below until solid, or at −20°C or below for 24 h or by cooking adequately to an internal temperature risen at least to > 63°C52). Appropriate freezing and cooking are the most reliable measures to prevent Anisakis food poisoning; however, there are some fish species whose commercial values are significantly reduced by such measures. Therefore, it will be necessary to monitor not only A. simplex infection but also the larvae migrating in fish muscle, which is frequently reported as a source of infection. We recommend removal of the ventral muscle to prevent anisakiasis according to the fish species and the fishing area when a high frequency of A. simplex is detected in fish. Furthermore, it is necessary to pay close attention to the trends of anisakiasis and the causative foods, and to alert not only fish and shellfish distributors and restaurant businesses but also the general public regarding measures to prevent anisakiasis.
Acknowledgements
We are very grateful to Dr. Kazuo Ogawa and the staff of the Meguro Parasitological Museum for jointly conducting the survey of skipjack tuna. We would like to sincerely thank Dr. Hiromu Sugiyama, National Institute of Infectious Diseases, Japan for constructive comments on the survey of skipjack tuna. We are very grateful to the staff of the Tokyo Metropolitan Institute of Public Health, Tama Branch Institute, for providing fish samples.
Footnotes
Declarations of interest: The authors declare no conflict of interest.
References
- 1.Sugiyama H,Morishima Y,Ohmae H,Yamasaki H,Kimura S. Anisakis food poisoning: the annual number of cases noticed by ordinance for enforcement of the food sanitation act and estimated through healthcare claim data analysis [in Japanese]. Clinical Parasitology. 2013; 24: 44–46. [Google Scholar]
- 2.Nascetti G,Paggi L,Orecchia P,Smith JW,Mattiucci S,Bullini L. Electrophoretic studies on the Anisakis simplex complex (Ascaridida: Anisakidae) from the Mediterranean and North-East Atlantic. Int J Parasitol. 1986; 16(6): 633–640. 10.1016/0020-7519(86)90032-9 [DOI] [PubMed] [Google Scholar]
- 3.Mattiucci S,Garcia A,Cipriani P,Santos MN,Nascetti G,Cimmaruta R. Metazoan parasite infection in the swordfish, Xiphias gladius, from the Mediterranean Sea and comparison with Atlantic populations: implications for its stock characterization. Parasite. 2014; 21: 35. 10.1051/parasite/2014036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattiucci S,Paggi L,Nascetti G,et al. Genetic markers in the study of Anisakis typica (Diesing, 1860): larval identification and genetic relationships with other species of Anisakis Dujardin, 1845 (Nematoda: Anisakidae). Syst Parasitol. 2002; 51(3): 159–170. 10.1023/A:1014554900808 [DOI] [PubMed] [Google Scholar]
- 5.Paggi L,Nascetti G,Webb SC,Mattiucci S,Cianchi R,Bullini L. A new species of Anisakis Dujardin, 1845 (Nematoda, Anisakidae) from beaked whales (Ziphiidae): allozyme and morphological evidence. Syst Parasitol. 1998; 40(3): 161–174. 10.1023/A:1006093201920 [DOI] [Google Scholar]
- 6.Mattiucci S,Paoletti M,Webb SC. Anisakis nascettii n. sp. (Nematoda: Anisakidae) from beaked whales of the southern hemisphere: morphological description, genetic relationships between congeners and ecological data. Syst Parasitol. 2009; 74(3): 199–217. 10.1007/s11230-009-9212-8 [DOI] [PubMed] [Google Scholar]
- 7.Mattiucci S,Nascetti G,Bullini L,Orecchia P,Paggi L. Genetic structure of Anisakis physeteris, and its differentiation from the Anisakis simplex complex (Ascaridida: Anisakidae). Parasitology. 1986; 93(2): 383–387. 10.1017/S0031182000051544 [DOI] [PubMed] [Google Scholar]
- 8.Mattiucci S,Paggi L,Nascetti G,et al. Genetic divergence and reproductive isolation between Anisakis brevispiculata and Anisakis physeteris (Nematoda: Anisakidae)s. I Int J Parasitol. 2001; 31(1): 9–14. 10.1016/S0020-7519(00)00125-9 [DOI] [PubMed] [Google Scholar]
- 9.Mattiucci S,Nascetti G,Dailey M,et al. Evidence for a new species of Anisakis Dujardin, 1845: morphological description and genetic relationships between congeners (Nematoda: Anisakidae). Syst Parasitol. 2005; 61(3): 157–171. 10.1007/s11230-005-3158-2 [DOI] [PubMed] [Google Scholar]
- 10.Shiraki T. Larval nematodes of family Anisakidae (Nematoda) in the northern sea of Japan as a causative agent of eosinophilic phlegmone or granuloma in the human gastro-intestinal tract. Acta Medica et Biologica. 1974; 22: 57–98. [Google Scholar]
- 11.Quiazon KMA,Yoshinaga T,Ogawa K,Yukami R. Morphological differences between larvae and in vitro-cultured adults of Anisakis simplex (sensu stricto) and Anisakis pegreffii (Nematoda: Anisakidae). Parasitol Int. 2008; 57(4): 483–489. 10.1016/j.parint.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Murata R,Suzuki J,Sadamasu K,Kai A. Morphological and molecular characterization of Anisakis larvae (Nematoda: Anisakidae) in Beryx splendens from Japanese waters. P Parasitol Int. 2011; 60(2): 193–198. 10.1016/j.parint.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 13.Suzuki J,Murata R,Hosaka M,Araki J. Risk factors for human Anisakis infection and association between the geographic origins of Scomber japonicus and anisakid nematodes. Int J Food Microbiol. 2010; 137(1): 88–93. 10.1016/j.ijfoodmicro.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 14.Quiazon KMA,Yoshinaga T,Ogawa K. Distribution of Anisakis species larvae from fishes of the Japanese waters. Parasitol Int. 2011; 60(2): 223–226. 10.1016/j.parint.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 15.Gomes TL,Quiazon KMA,Kotake M,Itoh N,Yoshinaga T. Anisakis spp. in fishery products from Japanese waters: Updated insights on host prevalence and human infection risk factors. Parasitol Int. 2020; 78. 10.1016/j.parint.2020.102137 [DOI] [PubMed] [Google Scholar]
- 16.Tojima T,Oota T,Kodama K,Kidokoro H,Fujiwara K. Migration and distribution patterns of Spanish mackerel Scomberomorus niphonius in the Sea of Japan, estimated from catch data and tagging experiments in recent years [in Japanese]. Bull. Kyoto Inst. Ocean. Fish. Sci. 2013; 35: 1–11. [Google Scholar]
- 17.Sasaki D, Okada M, Tsumoto K. Age and growth of the Japanese Spanish mackerel Scomberomorus niphonius in Mie prefecture, Japan [in Japanese]. Fisheries biology and oceanography in the Kuroshio. 2018; 19: 55–58.
- 18.Umehara A,Kawakami Y,Ooi HK,Uchida A,Ohmae H,Sugiyama H. Molecular identification of Anisakis type I larvae isolated from hairtail fish off the coasts of Taiwan and Japan. Int J Food Microbiol. 2010; 143(3): 161–165. 10.1016/j.ijfoodmicro.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 19.van Thiel P,Kuipers FC,Roskam RT. A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop Geogr Med. 1960; 12: 97–113. [PubMed] [Google Scholar]
- 20.Asami K,Okamoto R,Sakai H,Imano H,Watanuki T. Two cases of stomach granuloma caused by Anisakis-like larval nematodes in Japan. Am J Trop Med Hyg. 1965; 14(1): 119–123. 10.4269/ajtmh.1965.14.119 [DOI] [PubMed] [Google Scholar]
- 21.Daschner A,Alonso-Gómez A,Cabañas R,Suarez-de-Parga JM,López-Serrano MC. Gastroallergic anisakiasis: Borderline between food allergy and parasitic disease—Clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J Allergy Clin Immunol. 2000; 105(1): 176–181. 10.1016/S0091-6749(00)90194-5 [DOI] [PubMed] [Google Scholar]
- 22.Mattiucci S,Fazii P,De Rosa A,et al. Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infection, Italy. Emerg Infect Dis. 2013; 19(3): 496–499. 10.3201/eid1903.121017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakanari JA,McKerrow JH. Anisakiasis. Clin Microbiol Rev. 1989; 2(3): 278–284. 10.1128/CMR.2.3.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtaki H, Ohtaki R. Clinical manifestation of gastric anisakiasis. In Ishikura H, Namiki M, eds. Gastric Anisakiasis in Japan. Tokyo, Japan: Springer Tokyo; 1989: 37–4. [Google Scholar]
- 25.Murata R,Suzuki J,Kodo Y,et al. Probable association between Anisakis infection in the muscle of skipjack tuna (Katsuwonus pelamis) and human anisakiasis in Tokyo, Japan. Int J Food Microbiol. 2021; 337. 10.1016/j.ijfoodmicro.2020.108930 [DOI] [PubMed] [Google Scholar]
- 26.Ogawa K,Iwaki T,Araki J,Itoh N. Anisakis larvae in the fillet of the Pacific saury Cololabis saira [in Japanese]. Bulletin of the Japanese Society of Scientific Fisheries. 2012; 78(6): 1193–1195. 10.2331/suisan.78.1193 [DOI] [Google Scholar]
- 27.Sugiyama H,Morishima Y,Yamazaki H. Detection and identification of anisakid nematodes from salmon, Oncorhynchus keta and Oncorhynchus masou, caught off the coast of the northern Japan [in Japanese]. Clinical Parasitology. 2017; 28: 32–35. [Google Scholar]
- 28.Suzuki J. Food poisoning caused by Anisakis larvae and its causative foods in Japan [in Japanese]. Japanese Journal of Food Microbiology. 2020; 37(3): 122–125. 10.5803/jsfm.37.122 [DOI] [Google Scholar]
- 29.Umehara A,Kawakami Y,Araki J,Uchida A. Molecular identification of the etiological agent of the human anisakiasis in Japan. Parasitol Int. 2007; 56(3): 211–215. 10.1016/j.parint.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 30.Arizono N,Yamada M,Tegoshi T,Yoshikawa M. Anisakis simplex sensu stricto and Anisakis pegreffii: biological characteristics and pathogenetic potential in human anisakiasis. Foodborne Pathog Dis. 2012; 9(6): 517–521. 10.1089/fpd.2011.1076 [DOI] [PubMed] [Google Scholar]
- 31.del Carmen Romero M,Valero A,Navarro-Moll MC,Martín-Sánchez J. Experimental comparison of pathogenic potential of two sibling species Anisakis simplex s.s. and Anisakis pegreffii in Wistar rat. Trop Med Int Health. 2013; 18(8): 979–984. 10.1111/tmi.12131 [DOI] [PubMed] [Google Scholar]
- 32.Kagei N,Sano M,Takahashi Y,Takahashi Y. A case of acute abdominal syndrome caused by Anisakis Type-II larva. Japanese Journal of Parasitology. 1978; 27: 427–431. [Google Scholar]
- 33.Asato R,Wakuda M. Sueyoshi, T. A case of human infection with Anisakis physeteris larvae in Okinawa, Japan. Japanese Journal of Parasitology. 1991; 40: 181–183. [Google Scholar]
- 34.Iglesias R,Leiro J,Ubeira FM,Santamarina MT,Sanmartín ML. Anisakis simplex: stage-specific antigens recognized by mice. J Helminthol. 1995; 69(4): 319–324. 10.1017/S0022149X00014899 [DOI] [PubMed] [Google Scholar]
- 35.Ishii Y, Fujino T, Weerasooriya MV. Morphology of Anisakine Larvae. In Ishikura H, Namiki M, eds. Gastric Anisakiasis in Japan. Tokyo, Japan: Springer Tokyo; 1989: 37–46. [Google Scholar]
- 36.Suzuki J,Murata R. A Review of anisakiasis and Anisakis larvae in Japan: From the prevalence and risk of Anisakis infections to the identification of Anisakis larvae [in Japanese]. Annual report of Tokyo Metropolitan Institute of Public Health. 2011; 62: 13–24. [Google Scholar]
- 37.Kiyofuji H,Aoki Y,Kinoshita J,et al. Northward migration dynamics of skipjack tuna (Katsuwonus pelamis) associated with the lower thermal limit in the western Pacific Ocean. Progress in Oceanography. 2019; 175: 55–67. 10.1016/j.pocean.2019.03.006 [DOI] [Google Scholar]
- 38.Barkley RA,Neil WH,Gooding RM. Skipjack tuna, Katsuwonus pelamis, habitat based on temperature and oxygen requirements. Fishery Bulletin. 1978; 76: 653–662. [Google Scholar]
- 39.Fujino K. Range of the skipjack tuna subpopulation in the western Pacific Ocean. Proceeding of the 2nd CSK Symposium. 1972; 373–384.
- 40.Mugo R,Saitoh SI,Nihira A,Kuroyama T. Habitat characteristics of skipjack tuna (Katsuwonus pelamis) in the western North Pacific: a remote sensing perspective. Fisheries Oceanography. 2010; 19(5): 382–396. 10.1111/j.1365-2419.2010.00552.x [DOI] [Google Scholar]
- 41.Sugimoto S,Qiu B,Kojima A. Marked coastal warming off Tokai attributable to Kuroshio large meander. Journal of Oceanography. 2020; 76(2): 141–154. 10.1007/s10872-019-00531-8 [DOI] [Google Scholar]
- 42.Suzuki J. Current status and countermeasures of anisakisis in Japan [in Japanese]. Modarn Media. 2020; 66: 165–170. [Google Scholar]
- 43.Graham JB,Dickson KA. Tuna comparative physiology. Journal of Experimental Biology. 2004; 207(23): 4015–4024. 10.1242/jeb.01267 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki J,Murata R,Yanagawa Y. Anisakiasis caused by raw fish of Thunnus thynnus and detection rate of Ansiakis simplex from Thunnus thynnus and Seriola dumerili [in Japanese]. Clinical Parasitology. 2007; 18: 18–20. [Google Scholar]
- 45.Ohshimo S,Tawa A,Ota T,et al. Horizontal distribution and habitat of Pacific bluefin tuna, Thunnus orientalis, larvae in the waters around Japan. Bulletin of Marine Science. 2017; 93(3): 769–787. 10.5343/bms.2016.1094 [DOI] [Google Scholar]
- 46.Itoh T,Tsuji S,Nitta A. Migration patterns of young Pacific bluefin tuna (Thunnus orientalis) determined with archival tags. Fishery Bulletin. 2003; 101: 514–534. [Google Scholar]
- 47.Furukawa S,Fujioka K,Fukuda H,Suzuki N,Tei Y,Ohshimo S. Archival tagging reveals swimming depth and ambient and peritoneal cavity temperature in age-0 Pacific bluefin tuna, Thunnus orientalis, off the southern coast of Japan. Environmental Biology of Fishes. 2017; 100(1): 35–48. 10.1007/s10641-016-0552-3 [DOI] [Google Scholar]
- 48.Inagake D,Yamada H,Segawa K,Okazaki M,Nitta A,Itoh T. Migration of young bluefin tuna, Thunnus orientalis Temminck et Schlegel, through archival tagging experiments and its relation with oceanographic condition in the western North Pacific. Bull. Natl. Res. Inst. Far Seas Fish. 2001; 38: 53–81. [Google Scholar]
- 49.Uotani I,Saito T,Hiranuma K,Nishikawa Y. Feeding habit of bluefin tuna Tunnus thynnus larvae in the western North Pacific Ocean [in Japanese]. Nippon Suisan Gakkaishi. 1990; 56(5): 713–717. 10.2331/suisan.56.713 [DOI] [Google Scholar]
- 50.Tanaka Y,Minami H,Ishihi Y,et al. Relationship between prey utilization and growth variation in hatchery-reared Pacific bluefin tuna, Thunnus orientalis (Temminck et Schlegel), larvae estimated using nitrogen stable isotope analysis. Aquaculture Research. 2014; 45(3): 537–545. 10.1111/j.1365-2109.2012.03258.x [DOI] [Google Scholar]
- 51.Shimose T,Watanabe H,Tanabe T,Kubodera T. Ontogenetic diet shift of age-0 year Pacific bluefin tuna Thunnus orientalis. J Fish Biol. 2013; 82(1): 263–276. 10.1111/j.1095-8649.2012.03483.x [DOI] [PubMed] [Google Scholar]
- 52.Food and Drug Administration. Fish and Fishery Products, Hazards and Controls Guidance 4th ed. 2020; 91–98. https://www.fda.gov/food/seafood-guidance-documents-regulatory-information/fish-and-fishery-products-hazards-and-controls. Accessed on February 15, 2021.