Abstract
Background
ChAdOx1 nCoV-19 (AZD 1222) is the main vaccine planned for general administration in Thailand. This vaccine is stored in multiple-dose vials meant to be administered to 10 recipients with a volume of 0.5 mL for each dose. However, the vaccine vials were overfilled, which allows the administration of more than 10 doses per vial. We have stipulated the preparation and use of ChAdOx1 nCoV-19 vaccine using traditional 21 or 25G needles and planned to investigate the immune responses of participants who were administered the ChAdOx1 nCoV-19 vaccine using this technique.
Methods
We measured anti-SARS-CoV-2 anti-spike RBD IgG and neutralising antibody using a surrogate virus neutralising test (sVNT) among adults aged 18–72 years on average of 8.57 weeks (IQR 6.85–8.93) after the first dose of ChAdOx1 nCoV-19 vaccine. The primary outcome was the antibody level. The secondary outcomes included adverse events, factors affecting antibody levels, and incidence of COVID-19 infection.
Findings
In all, 60 participants comprised 25 males and 35 females. The mean age was 53.70 ± 17.48 years. BMI was 23.45 ± 3.69 kg/m2. Tests for the neutralising antibody were positive in 60% of the participants (71.4% among males and 44% among females). The median anti-SARS-CoV-2 QuantiVac (anti-spike IgG) level among male and female samples was 111.83 BAU/mL (IQR 73.48–196.74 BAU/mL) and 159.65 BAU/mL (IQR 100.39–371.81), respectively. The positive QuantiVac value of male and female samples was 88.00% and 98.44%, respectively (p-value = 0.382) .A good correlation was observed between neutralising Ab and anti-spike RBD IgG.
Conclusion
Patients receiving 12-dose per vial injections of ChAdOx1 nCoV-19 exhibited high levels of immunity without severe side effects. This technique can be adopted to maximise the number of doses per vial while preserving vaccine effectiveness.
Abbreviations: RBD, Receptor Binding Domain
Keywords: Neutralising antibody, ChAd0x1 nCoV-19, Covid-19, Immunogenicity, Anti-spike RBD
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in an unprecedented public health crisis. Although social distancing, universal use of masks, and hand washing were the initial measures to reduce the spread of this novel virus, still more than 3.8 million deaths have occurred worldwide. The urgent need for a vaccine prompted an international response with more than 200 COVID-19 candidate vaccines in development worldwide [1] and more than 30 vaccine candidates have entered clinical trials.
Recently, Thailand commenced its vaccine programme for COVID-19 with 2 candidate vaccines. The first is Sinovac (CoronaVac, Sinovac Life Science, Beijing, China) which is an inactivated COVID-19 vaccine that has shown good immunogenicity in mice, rats, and nonhuman primates [2]. The second is the ChAdOx-1 nCov-19 vaccine (AZD 1222), which is an adenoviral-vector-based vaccine. Thailand also produces the ChAdOx-1 nCOV-19 vaccine through Siam Bioscience, which acts as a manufacturing facility for AstraZeneca and has passed all requirements from the Department of Medical Sciences (DMS) to supply Southeast Asian populations.
The ChAdox-1 nCOV-19 vaccine requires two doses (0.5 mL each) administered intramuscularly, 10–12 weeks apart. Each vial (6.5 mL) meets the requirement of the European Pharmacopoeia to obtain 10 doses using a syringe with a capacity not exceeding the doses, multiplied by the dose volume to be measured and filled in a 21-G needle not less than 2.5 cm in length [3].
Recently, The European Medicines announced that an additional dose (six doses) could be extracted from each vial using low dead-volume syringes and/or needles regarding Pfizer-BioNTech COVID-19 vaccine. However, low dead space volume syringes are 6–10 times more expensive than normal syringes and less widely available. Therefore, our institution in collaboration with the Thailand University Hospital Network (UHosNET) implemented a method of preparing and withdrawing up to 12 doses from a single vial to optimise limited vaccine resources (data in supplemental file, Appendix 4). This study aimed to determine the immunogenicity of participants receiving vaccines prepared using this method by evaluating the rates and levels of anti-spike IgG and neutralising antibodies following one dose of ChAdOx-1 nCoV-19 vaccine.
2. Methods
2.1. Study design and participants
In this single-centre, prospective, observational study conducted at the Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand, participants receiving their first dose of the ChAdOx-1 nCOV-19 vaccine were recruited. Participants were eligible if they were more than 18 years old. The exclusion criteria included allergies to vaccine components, and risk of COVID-19 transmission in the previous 14 days before enrolment, i.e., close contact with index cases or a history of fever with upper respiratory tract infection. All participants underwent a screening visit where medical history was collected and physical examination was performed. At the time of this study, Thais had only received the first dose of ChAdOx-1 nCoV-19. Blood was collected for BUN, creatinine, and antibody measurement.
Sixty venous blood samples were collected from the 60 participants for antibody testing against SARS‐CoV‐2 after first dose of ChAdox-1 nCOV-19 vaccination at the Central Laboratory and Blood Bank, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University. Serum samples were tested using anti-SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) IgG (Lübeck, Germany), which quantitatively determines the levels of the S1 domain of the spike protein. All testing was performed according to manufacturer instructions including serum concentration, optical density, calibrator settings, and cut-off values on a fully automated ELISA analyser. Briefly, serum was applied at a 1:101 dilution on the provided antigen-coated 96-well ELISA plates and incubated with a peroxidase-linked anti-human IgG secondary antibody. Bound serum antibody was measured as the optical density (OD) at 450 nm after incubating with the substrate solution. Samples with concentrations less than 25.6 BAU/mL were interpreted as negative. Concentrations of 25.6–35.2 BAU/mL were considered borderline, and a concentration ≥35.2 BAU/mL was considered positive.
Personal and demographic information was obtained from clinical records. Written informed consent was obtained from all participants, and the experiment was performed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. This study was approved by the Vajira Institutional Review Board, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University. The vaccine used was authorised by the Thai FDA and the Department of Medical Sciences (DMS) (Appendix 1).
2.2. Antibody measurement
The IgG antibody levels were assessed using a standardised ELISA (Euroimmun, Hausen Bernstein, Germany) spike protein according to manufacturer instructions. ELISA provides semiquantitative in vitro determination of human antibodies of the immunoglobulin class IgG against SARS-CoV-2 S1/RBD spike protein, recombinantly producing I human cells (HEK293). The details of the test principles, assay procedure, and analytical and clinical performances are described in the supplemental files (Appendix 2).
2.3. Neutralising antibody (Nab) test
We used an ELISA-based surrogate virus neutralisation test based on antibody-mediated blockage of ACE-2 spike protein–protein interaction (SVnT) [4] (Euroimmune), which had high specificity and good correlation with the Euroimmun SARS-CoV-2 IgG ELISA [5]. The sVNT assay also showed a good correlation with conventional virus neutralisation tests [6], which detect neutralising antibodies in a patient’s blood and have the disadvantage of requiring the handling of live SARS-CoV-2 in a specialised biosafety level 3 (BSL3) laboratory. This type of test is also cumbersome and time-consuming, requiring 2–4 days to complete. The sNVT assay can also detect Nabs without the need for live viruses or cells and can be completed in 1–2 h in a BSL 2 laboratory. The sensitivity of the assay was determined to be 95.5% by testing samples collected from 124 convalescent COVID-19 patients and the specificity was 99.7% from 600 samples from blood donations before the SARs-CoV-2 pandemic occurred (before January 2020) (Appendix 3).
2.4. Statistical analysis
For continuous variables, the mean ± standard deviation was used for normally distributed data and the median with interquartile ranges (IQR) was used for nonnormally distributed data. Categorical outcomes were analysed using the Pearson chi-square test or the Fischer exact test with a 95% confidence interval. Comparisons of antibody levels between different variables such as age, sex, body weight, blood group, and underlying diseases were analysed using the Wilcoxon rank-sum test (Mann-Whitney U test) or Kruskal-Wall test. To assess the association between vaccination outcomes and different variables, we used multiple linear regression analyses. Antibody levels between males and females were compared using Fisher’s exact test. The correlation between anti-spike RBD IgG and nAb was analysed using Spearman's rank correlation coefficient. All statistical tests were two-sided and the significance level was set at a p-value of ≤0.05 for inferential analyses. All statistical analyses were conducted using STATA (Version 13.0, StataCorp, College Station, TX, USA).
3. Results
3.1. Participant characteristics
Between April and May 2021, 60 individuals vaccinated with a single dose of ChAdOx1-CoV 19 were recruited. Blood samples from all volunteers were examined for vaccine-induced SARS-CoV-2 spike-specific IgG titres and SARS-CoV-2 neutralising antibodies. The mean age of the study population was 53.70 ± 17.48 years. The mean body mass index (BMI) was 23.45 ± 3.69 kg/m2. More females participated compared with males (35 females to 25 males); 46.7% (n = 28) had some underlying diseases; 5% (n = 3) had diabetes mellitus; 25% (n = 15) had dyslipidaemia; 18.3% (n = 11) had hypertension; and 3.3% (n = 2) had chronic kidney disease (CKD). The baseline characteristics are shown in Table 1 . None of the participants had a history of SARS-CoV-2. The ChAdOx-1 nCoV-19 vaccine was administered as a 0.5 mL injection in the deltoid muscle using either a 21G or 25G needle. The method of extracting the vaccine is shown in the supplemental files. The mean vaccine volume was 0.50 ± 1.01 using 21G needles as recorded in the preliminary result from 36 volunteers and the mean volume was 0.50 ± 0.01 using 25G needles (Table 2 ). A single vial of ChAdox-1 nCoV-19 vaccine contained 6.5 mL and was divided in 12 doses. The blood samples were collected on average at 8.57 weeks (IQR 6.85–8.93) after the participant received their first vaccination in early April 2021. The primary outcome was assessing immunogenicity using immunoassays for SARS-CoV-2S1/RBD spike protein and neutralising antibodies 7–8 weeks after vaccination with 12-dose vials. The secondary outcomes were the effects of other variables on the immune response and the presence of adverse events.
Table 1.
General characteristics (n = 60).
| Variables | ||
|---|---|---|
| Sex | ||
| Male | 25 | (41.7) |
| Female | 35 | (58.3) |
| Age (years), Mean ± SD | 53.70 ± 17.48 | |
| <60 | 25 | (41.7) |
| ≥60 | 35 | (58.3) |
| BMI (kg/m2), Mean ± SD | 23.45 ± 3.69 | |
| Normal weight | 33 | (55.0) |
| Overweight | 10 | (16.7) |
| Obesity | 17 | (28.3) |
| Blood group (ABO System) | ||
| A | 14 | (23.3) |
| B | 17 | (28.3) |
| AB | 6 | (10.0) |
| O | 20 | (33.3) |
| Unknown | 3 | (5.0) |
| Underlying diseases | 28 | (46.7) |
| DM | 3 | (5.0) |
| HT | 11 | (18.3) |
| DLP | 15 | (25.0) |
| CKD | 2 | (3.3) |
| COPD | 0 | (0.0) |
| Cancer | 1 | (1.7) |
| Other | 10 | (16.7) |
| Blood pressure | ||
| Systolic blood pressure (mmHg), Mean ± SD | 123.10 ± 1.15 | |
| Diastolic blood pressure (mmHg), Mean ± SD | 74.19 ± 11.07 | |
| Time (weeks) | 7.98 ± 0.99 | |
Data are presented as number (%) or mean ± standard deviation.
BMI; Body mass index, DM; Diabetes mellitus, HT; Hypertension, DLP; Dyslipidemia, CKD; Chronic kidney disease, COPD; Chronic obstructive pulmonary disease.
Table 2.
Weight and volume of vaccine in different sizes of needles.
| Descriptions | Needles with attached tips |
p-Value* | |
|---|---|---|---|
| 21 Gauge (N = 36) |
25 Gauge (N = 36) |
||
| Empty weights (g) | 2.9386 ± 0.0155 | 2.9393 ± 0.0138 | 0.837 |
| Full-filled weights (g) | 3.4778 ± 0.0169 | 3.4784 ± 0.0166 | 0.885 |
| Weights of vaccine (g) | 0.5393 ± 0.0116 | 0.5391 ± 0.0083 | 0.955 |
| Washed weights (g) | 2.9592 ± 0.0159 | 2.9603 ± 0.0151 | 0.768 |
| Weights of DS (g) | 0.0207 ± 0.0056 | 0.0210 ± 0.0076 | 0.813 |
| Weights of TC (g) | 0.5186 ± 0.0127 | 0.5181 ± 0.0079 | 0.839 |
| Volumes of TC (mL) | 0.5035 ± 0.0123 | 0.5030 ± 0.0076 | 0.839 |
Abbreviations: DS - dead space; TC -total capacity.
Data are presented as mean ± standard deviation.
P-value corresponds to Student's t-test.
3.2. Quantitative SARs-CoV-2 spike IgG levels
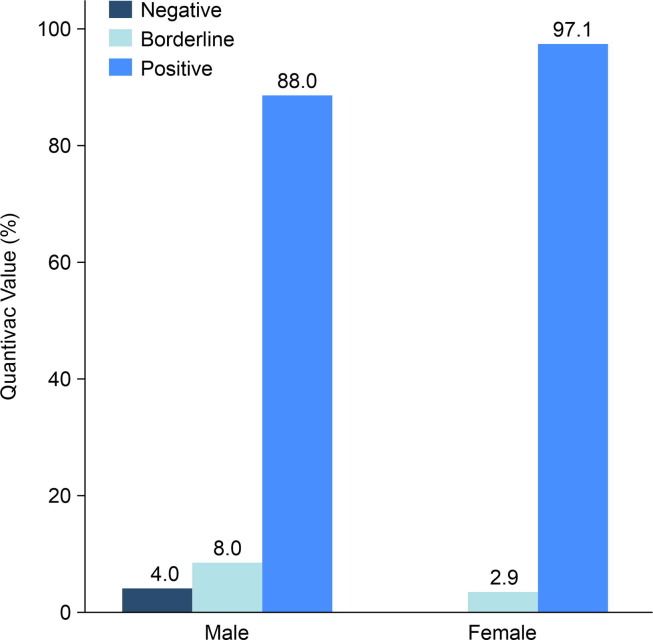
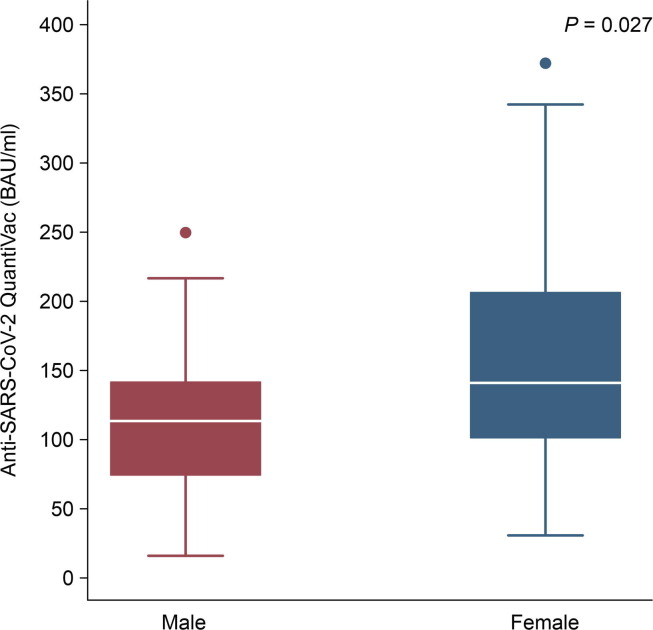
After the first dose, the preliminary results showed that anti-SARS-CoV-2 (anti-spike IgG) levels rapidly increased with a seroconversion rate of 93.3%. The seroconversion rate was higher among males than females but without significance (97.1% among females vs. 88.0% among males, p = 0.382) (Fig. 1 ). The anti-SARS-CoV Quantivac levels among females were significantly higher than those among males (median 159.65 IQR100.39–371.81 BAU/Ml among female vs. 111.83 IQR73.48–196.72, p = 0.027) (Fig. 2 ). The median level of anti-spike IgG (Quantivac) was 111.83 BAU/mL (IQR 73.48–196.74) among males and 159.65 BAU/mL (IQR 100.39–371.81) among females, with a p-value of 0.027. The overall median magnitude of antibody titre was 140.4 (IQR 82.97–308.83) BAU/mL. The level of immunity did not correlate with age, comorbidity, BMI, blood group, or blood pressure (Table 3 ) (Appendix).
Fig. 1.
Seroconversion rate after first dose of ChAdOx1 vaccine.
Fig. 2.
Anti-spike protein IgG antibody titres (BAU/ml) after the first dose of vaccination were higher among females than males (N = 25 among males and 35 among females). Box plots indicated the median and interquartile range (IQR) of anti-spike protein IgG antibody titres.
Table 3.
Comparison of Anti-SARS-CoV-2 QuantiVac according to various parameters.
| Variables | Quantivac level (BAU/ml) |
p-Valuea | Quantivac Value |
p-Valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative |
Borderline |
Positive |
||||||||
| Median | (IQR) | n | (%) | n | (%) | n | (%) | |||
| Sex | ||||||||||
| Male | 111.83 | (73.48–196.74) | 0.027 | 1 | (4.00) | 2 | (8.00) | 22 | (88.00) | 0.382 |
| Female | 159.65 | (100.39–371.81) | 0 | (0.00) | 1 | (2.86) | 34 | (97.14) | ||
| Age (years) | ||||||||||
| <60 | 216.29 | (100.39–384.00) | 0.062 | 0 | (0.00) | 0 | (0.00) | 25 | (100.00) | 0.258 |
| ≥60 | 120.05 | (79.76–243.10) | 1 | (2.86) | 3 | (8.57) | 31 | (88.57) | ||
| BMI (kg/m2) | 1 | (3.03) | 1 | (3.03) | 31 | (93.94) | ||||
| Normal weight | 159.04 | (100.39 - 371.81) | 0.424 | |||||||
| Overweight | 107.71 | (57.66–196.74) | 0 | (0.00) | 1 | (10.00) | 9 | (90.00) | 0.857 | |
| Obesity | 135.66 | (79.76–252.77) | 0 | (0.00) | 1 | (5.88) | 16 | (94.12) | ||
| Blood group (ABO System) | 0 | (0.00) | 0 | (0.00) | 14 | (100.00) | ||||
| A | 147.35 | (80.03–384.00) | 0.942 | |||||||
| B | 139.89 | (86.17–243.10) | 0 | (0.00) | 1 | (5.88) | 16 | (94.12) | 0.116 | |
| AB | 140.20 | (79.76–327.62) | 0 | (0.00) | 1 | (16.67) | 5 | (83.33) | ||
| O | 170.63 | (97.12–248.94) | 0 | (0.00) | 1 | (5.00) | 19 | (95.00) | ||
| Underlying diseases | ||||||||||
| No | 151.29 | (90.77–271.40) | 0.624 | 0 | (0.00) | 1 | (3.13) | 31 | (96.88) | 0.402 |
| Yes | 128.21 | (78.58–362.92) | 1 | (3.57) | 2 | (7.14) | 25 | (89.29) | ||
| Time (weeks) | ||||||||||
| <8 | 210.65 | (95.37–371.81) | 0.497 | 0 | (0.00) | 1 | (4.55) | 21 | (95.45) | 1.000 |
| ≥8 | 140.40 | (80.45–327.62) | 1 | (3.85) | 2 | (7.69) | 23 | (88.46) | ||
Data are n (%) or median (IQR).Data shown are level of anti-spike protein IgG according to various parameters.
BMI; Body mass index.
Wilcoxon rank-sum test or Kruskal- Wallis test.
Fisher's exact test.
3.3. Neutralising antibodies (Nabs)
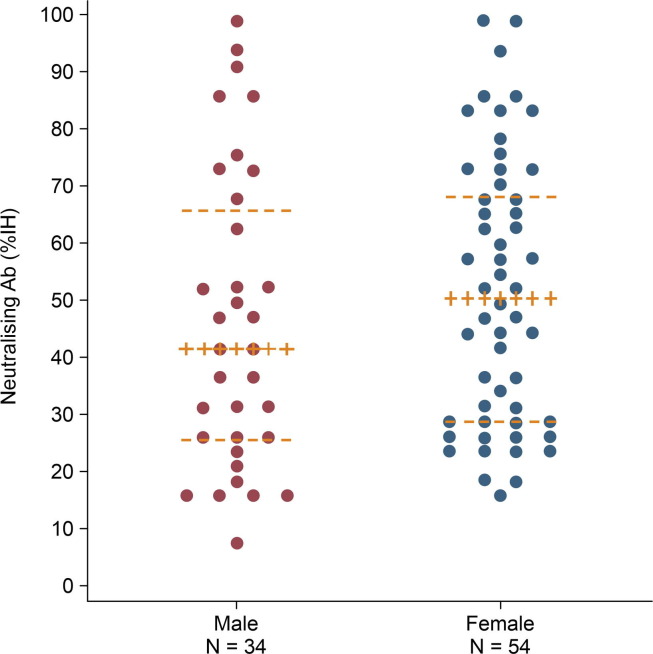
After the first dose, sVNT responses were detected in 60% of the participants (71.4% among females, 44% among males, p = 0.048) and 26.7% exhibited borderline levels (Table 4 ). A dose–effect was seen in 32% of the males (IQR 21.48–47.10) (% inhibition ≥ 35%) and 49.81% (IQR 29.20–66.08) of females, p = 0.048) (Fig. 3 ).
Table 4.
Comparison of Anti-SARS-CoV-2 QuantiVac between sexes.
| Variables | Total (n = 60) |
Male (n = 25) |
Female (n = 35) |
||
|---|---|---|---|---|---|
| Quantivac level, (RU/ml) | 43.88 | (25.93–96.51) | 34.95 | (22.96–61.48) | 49.89 |
| Quantivac level, (BAU/ml) | 140.4 | (82.97–308.83) | 111.83 | (73.48–196.74) | 159.65 |
| Quantivac Value | |||||
| Negative | 1 | (1.7) | 1 | (4.0) | 0 |
| Borderline | 3 | (5.0) | 2 | (8.0) | 1 |
| Positive | 56 | (93.3) | 22 | (88.0) | 34 |
| Neutralising Ab, (%IH) | 42.67 | (26.69–62.55) | 32.06 | (21.48–47.10) | 49.81 |
| Negative | 8 | (13.3) | 6 | (24.0) | 2 |
| Borderline | 16 | (26.7) | 8 | (32.0) | 8 |
| Positive | 36 | (60.0) | 11 | (44.0) | 25 |
Values are number of patients (%) or median (interquartile range, 25th and 75th percentiles).
Data are n (%) or median (IQR).Data shown are level of anti-spike protein IgG according to sex.
aWilcoxon rank-sum test.
bFisher's exact test.
Fig. 3.
Neutralizing antibody titres measured among males and females. Datapoints are median (IQR).
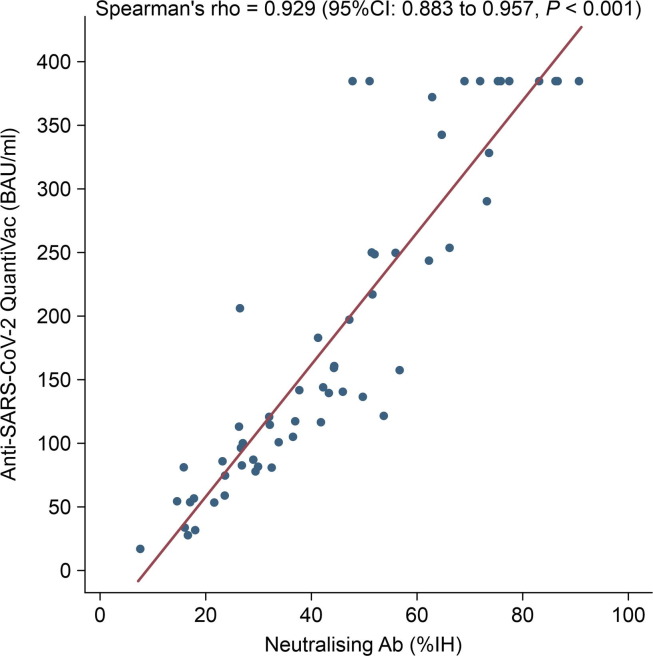
A good correlation was noted between anti-spike IgG and neutralising antibody levels (r = 0.929, 95% CI 0.88–0.96, p > 0.001) (Fig. 4 ).
Fig. 4.
Correlation between neutralising activity and anti-spike protein IgG antibody titres. Spearman’s correlation test was performed to statistically analyse matched pairs.
3.4. Side effects
The first dose was generally well-tolerated without serious toxicity, and systemic events were mild or moderate. The most reported symptom was fever (45%), which was the highest on the day after vaccination. The other reported symptoms included fatigue (35%), pain at the injection site (33.37%) (Table 5 ), nausea and vomiting (6.67%), dizziness (10%), and diarrhoea (3.35%). None of the participants had been infected with COVID-19 after the first dose of the vaccine until the time of study (average 8 weeks).
Table 5.
Side effects of vaccine (n = 60).
| Variables | N (%) |
|---|---|
| Total | 37 (61.67) |
| Fever | 27 (45.00) |
| Fatique | 21 (35.00) |
| Site pain | 20 (33.33) |
| Weakness | 2 (3.33) |
| Nausea vomiting | 4 (6.67) |
| Vertigo | 6 (10.00) |
| Diarrhoea | 2 (3.33) |
4. Discussion
The COVID-19 pandemic has led to the development of various vaccines, which have been made available to the public by emergency use authorisation. The vaccination programme in Thailand employs two types of vaccines (ChAdox-1 n COV-19 and Sinovac), which were planned to be used in vulnerable groups, such as frontline workers, senior citizens, and those with comorbidities, such as obesity, CKD, diabetes mellitus, cancer, chronic lung disease, and coronary heart disease. The ChAdox-1 nCOV-19 vaccine was overfilled with an excess of 5 mL to compensate for the residual vaccine in the syringe and needle during the process of preparing and vaccinating ten recipients. This additional volume was the same amount as in the case of any other brand where the vaccine is administered to more than one recipient (multiple doses) [7]. For instance, the COVID-19 Pfizer vaccine vial can be used to vaccinate six recipients. For this reason, many countries, where maximising the number of doses per vial is authorised, allowed for the use of low dead space syringes and needles, reducing the residual volume in the syringe and needle tip, to avoid vaccine wastage [8]. However, the costs of the low dead space syringes and needles are 6–10 times higher than those commonly used for vaccinations in Thailand. Additionally, the production and importation of low dead space syringes have been hindered by a global shortage problem. Therefore, UHosNet and the Faculty of Medicine, Vajira Hospital, Navamindradhiraj University jointly stipulated that preparation and vaccination using the ChAdOx1 nCoV-19 vaccine (AstraZeneca) could be performed using 11–12 doses per vial to increase the vaccine supply. This could address the problem of vaccine inaccessibility in numerous countries due to the shortage of low dead space syringes. The cost is minimal and materials are easily accessible. Thus, it would be convenient to implement in areas experiencing vaccine shortage.
We extracted 12 doses from each vial with an acceptable volume and weight of vaccine in each syringe. Herein, we report the preliminary results of the immunogenicity of the ChAdOx1 nCoV-19 vaccine when 12 doses per vial were used. The candidate vaccine was safe and well-tolerated despite some minor adverse events such as fever and fatigue. A single dose of vaccine elicited an increase in spike-specific IgG antibodies after an average of 55.86 ± 6.93 days in nearly 93% of all participants. High levels of neutralising antibodies were observed in 60% of participants and were negative in only 13.3%. After completing the second dose, the spike protein and neutralising antibody titres were expected to be higher. In a phase 1/2 randomised controlled trial, Folegatti et al. [9] showed that among 1077 participants, the anti-spike IgG response increased by day 28 (median 157 ELISA units (EU]. Neutralising antibody responses were detected in 91% of participants after a single dose when measured using a microneutralisation assay and in 100% of the participants when measured using a plaque reduction neutralisation assay (PRNT). The participants in this study received ChAdOx1 nCoV-19 at the standard dose of 5 × 1010 viral particles (0.5 mL). Their study population consisted of healthy adults aged 18–55 years in the UK. Subsequently, the same group conducted a phase 2/3 trial among healthy adults [10] aged 18 to more than 70 years. The vaccine doses ranged from 3.5 to 6.5 × 1010 virus particles. The investigators reported 89% neutralising antibody positivity 14 days after the booster dose. Voysey et al. [11] presented data from three single-blind randomised controlled trials in a phase 1/2 study in the UK (COV 001) [5], one phase 2/3 study in the UK (COV002), a phase 3 study in Brazil (COV 003), and one double-blind phase 1/2 study in South Africa (COV 005) [12]. Exploratory analyses showed that vaccine efficacy after a single standard dose of the vaccine was 76.0% (55.3–85.5) on days 22–90 after vaccination. Their modelling analysis indicated that protection was still active during the initial 3-month period. The antibody levels were maintained during this period (geometric mean ratio, 0.66, 95%, CI 0.59–0.75). The efficacy was higher in the participants who received two standard doses.
In our study, the humeral response after the first vaccination, as assessed by standardised total IgG ELISA against SARS-CoV-2 spike RBD IgG, was robust. Moreover, the neutralising antibodies levels were above the standard goals, 60% (≥35% inhibition level, Euroimmune test kit). Notably, the correlation between the spike-specific antibodies and neutralising antibodies was significant (r = 0.929, p > 0.001).
In a short term survey, we did not identify new COVID-19 transmission among the vaccinated individuals, indicating excellent vaccine efficacy. Our hospital has practiced withdrawing 12 doses from a single vaccine vial since the beginning of the vaccination programme in Thailand, and our results will confirm the efficacy of the technique used and the accuracy of the extraction method. The advantage of this technique is that it helps optimise the limited vaccine resources and maximise yields.
We used the SNVT technique for the neutralising antibody assay, which correlated well with the PRNT technique [4], [13]. Robust associations between nAb titres and reactivity with the ELISA test demonstrated their possible use in serving as an alternative to the microneutralisation assay that requires virus constructs containing SARS-CoV-2 specimens and PRNT that uses live viruses.
We detected several adverse events, which mostly disappeared within a day or two. No association was detected between age, sex, BMI, underlying diseases, and the immunogenicity of the vaccine. Further follow-up studies are required to assess the immunogenicity and safety of the vaccine after two doses.
In this interim analysis, we were unable to assess the duration of protection or vaccine efficacy in the long term. Further evidence will be required to determine the pending data regarding the duration of protection after the booster dose. Cellular immunity was not determined as the vaccine was expected to activate both humoral and cellular immunity. Additional laboratory analyses, involving more varied study populations, should be conducted in future. The other limitation was the absence of a control group vaccinated with 10 doses per vial as the use of the 12-dose technique was already widely practiced in Thailand at the time of the clinical trial design.
5. Conclusion
Vaccination with 12 doses per vial elicited a good humoral response against SARS-CoV-2. Nearly 93.3% of the participants achieved acceptable antibody levels while more than 50% exhibited high levels of neutralising antibodies. The side effects were few. Considering many countries are still facing a third wave of the pandemic, our recommended method to maximise vaccine doses can be employed safely with good immunogenicity and clinical outcomes. The ChAdOx1 nCoV-19 vaccine is safe, well-tolerated, and immunogenic.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This work was supported by a grant from Navamindradhiraj. The Hausen Company (Germany) provided the Euroimmun Quantivac ELISA and Neutralisa Test Kits and oversaw all trial experiments. Data were collected by clinical site research staff, managed by a research coordinator, and monitored by a safety monitoring board. The analysis was performed by an independent statistician who was not involved in the trial after the data were collected and checked.
Contributors
The study was designed by AM, TT, and UP, and TT collected the study data and oversaw participant visits. AM provided project management. JM recruited the participants analysed the safety data and interpretation was performed by TT and UP. Immunogenicity testing was conducted and analysed by UP, YC, WJ, and AP. PP prepared and wrote the extraction methods. TT and UP wrote the article. All authors contributed to the review and editing processes, and all approved the final version. The article was prepared by all study authors and the decision to submit the article for publication was made by all study authors.
Data sharing
The individual participant data will be made available to others after de-identification. Additional related documents will be available (text, figures, tables, study protocol, preparation and vaccination of hAdOx1 nCoV-19 (AstraZeneca) with ten doses per vial, 0.5 ml each. The data will be available immediately after publication and finalisation of the complete clinical study protocol for at least 6 months. The detail of the laboratory protocol will be made available to a Mendeley Researcher providing a scientifically sound proposal so the de-identified individual participant data can be accessed. Proposals should be sent to the corresponding authors, at thananda@nmu.ac.th. The proposals will be reviewed and approved by the investigator, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.12.023. These data include Google maps of the most important areas described in this article.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
GoogleMap
The following KML file contain the Google maps of the most important areas described in this article.
References
- 1.World Health Organization. Draft landscape of COVID-19 candidate vaccines [Accessed July 30, 2020]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 2.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Pharmacopoeia (Ph. Eur) 10th Edition|EDQM-European Directorate for the Quality of Medicines. https://www.edqm.eu/en/eu ropean-pharmacopoeia-ph-eur-10th-edition [21 February 2021, date last accessed].
- 4.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.-C., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nature Biotech. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 5.Walker G.J., Naing Z., Ospina Stella A., Yeang M., Caguicla J., Ramachandran V., et al. SARS Coronavirus-2 Microneutralisation and commercial serological assays correlated closely for some but not all enzyme immunoassays. Viruses. 2021;13(2):247. doi: 10.3390/v13020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvala H., Robb M.L., Watkins N., Ijaz S., Dicks S., Patel M., et al. Convalescent plasma therapy for the treatment of patients with COVID-19: assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus Med. 2021;31(3):167–175. doi: 10.1111/tme.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Daré B., Bacle A., Lhermitte R., Lesourd F., Lurton Y. Maximizing number of doses drawn from multi-dose COVID-19 vaccines by minimizing dead-volume. J Travel Med. 2021;28(4):taab049. doi: 10.1093/jtm/taab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kesten J.M., Ayres R., Neale J., et al. Acceptability of low dead space syringes and implications for their introduction: a qualitative study in the West of England. Int J Drug Policy (Internet) 2017;39:99–108. doi: 10.1016/j.drugpo.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramasamy M.N., Minassian A.M., Ewer K.J., et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Oxford COVID Vaccine Trial Group. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valcourt E.J., Manguiat K., Robinson A., Chen J.-Y., Dimitrova K., Philipson C., et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Diagn Microbiol Infect Dis. 2021;99(4):115294. doi: 10.1016/j.diagmicrobio.2020.115294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.