Summary
Background
Aging influences COVID-19 severity and response to vaccination, but previous vaccine effectiveness (VE) analyzes lack the power to evaluate its role in subgroups within the elderly age group. Here we analyzed the impact of age on viral vector and inactivated virus vaccines' effectiveness, the main platforms used in low- and middle-income countries.
Methods
We report a retrospective longitudinal study of 75,919,840 Brazilian vaccinees from January 18 to July 24, 2021, evaluating documented infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), COVID-19-related hospitalisation, ICU admission, and death. Negative binomial regression models adjusted for sociodemographic characteristics were used for VE estimation.
Findings
The overall analyzes of full vaccination showed VE against hospitalisation, ICU admission, and death of 91·4% (95%CI:90·1–92·5), 91·1% (95%CI:88·9–92·9) and 92·3% (95%CI:90·5–93·7) for Vaxzevria and 71·2% (95%CI:70·0–72·4), 72·2% (95%CI:70·2–74·0) and 73·7% (95%CI:72·1–75·2) for CoronaVac, respectively. VE for all outcomes is progressively lower with age. In fully-Vaxzevria-vaccinated individuals aged <60 years, VE against death was 96.5% (95%CI:82.1–99.3) versus 68·5% (95%CI:40·0–83·4) in those ≥90 years. Among fully-CoronaVac-vaccinated individuals, VE against death was 84.8% (95%CI:77.1–89.9) in those <60 years compared to 63.5 (95%CI 58.7–67.7) for vaccinees aged 80–89 years and 48·6%; (95%CI:35·0–59·3) for individuals aged ≥90 years. Post-vaccination daily cumulative incidence curves for all outcomes showed increased risk from younger to elder decades of life. There was no increase in the incidence of hospitalisation for individuals <60 years vaccinated during the same period as those aged ≥90 years.
Interpretation
Although both vaccines have been effective in protecting against infection, hospitalization and death; Vaxzevria and CoronaVac demonstrated high effectiveness against severe outcomes for individuals up to 79 years of age. Our results reinforce the idea that booster doses should be carefully considered in elders.
Funding
This study was partially supported by a donation from the "Fazer o bem faz bem" program.
Keywords: COVID-19, Vaccine, Effectiveness, CoronaVac, Vaxzevria
Research in context.
Evidence before this study
Several COVID-19 vaccines have proved efficacious. While high-income countries preferentially administer mRNA-based vaccines, lower- and middle-income countries have employed vaccines based on viral vectors or inactivated virus technologies. As in observational effectiveness studies, real-life evaluations are elemental for the comprehension of an ever-changing pandemic scenario (e.g., the spread of SARS-CoV-2 variants, the use of different vaccines, and other biological, demographic, and socioeconomic components such as age). As age is a crucial element in COVID-19 severity, and elders might have a reduced response to vaccination to vaccines, particularly for influenza, a timely evaluation of the effectiveness of the currently available vaccines across different regions and age groups is essential in order to understanding vaccine impact. Additionally, used vaccines are based on distinct technological platforms, which influence some immune response characteristics, such as specificity, stimulation of T-cell responses, and immunological memory. A study in São Paulo (Brazil) evaluated CoronaVac vaccination in individuals ≥70 years of age and found the highest VE in the group of 70–74 years which were lower according to age. A study on the CoronaVac effectiveness in Chile analyzed age from 16 to 59 years and >60 years, not evaluating age range in elders.
Added value of this study
In the present study, we examine vaccine effectiveness in detail in age ranges of nearly 76 million individuals that have received two vaccines with distinct technological platforms – theviral-vector vaccine (Vaxzevria) and the inactivated virus vaccine (CoronaVac). By following individuals for up to six months, both vaccines were effective against COVID-19 severe outcomes in individuals up to 79 years of age, but both vaccines presented a markedly lower vaccine effectiveness, especially among those aged ≥90 years. Also, we were able to disentangle the effects of earlier vaccination of older individuals that occurred during the second wave of COVID-19 in Brazil from those of ageing. Ours is, to our knowledge, the first study to estimate the vaccine effectiveness of detailed age groups for both Vaxzevria and CoronaVac.
Briefly, this study has two important contributions to the understanding of VE of CoronaVac and Vaxzevria: 1. demonstration of a significant lowerVE in vaccinees aged 80–89 and ≥90 years; 2. evaluation of VE of Vaxzevria against death, especially for elderly patients mentioned above.
Implication of all the available evidence
Evaluating almost 76 million adults vaccinees for the first six months of an extensive national campaign protected against COVID-19 severe outcomes individuals up to 79 years of age, and both were markedly lower in individuals ≥90 years of age. Vaccines were from two distinct technological platforms. A viral-vector vaccine (Vaxzevria) experienced higher effectiveness rates in all age ranges than the inactivated virus vaccine (CoronaVac). Our results reinforce the idea that booster doses should be carefully considered in elders.
Alt-text: Unlabelled box
Introduction
Several COVID-19 vaccines have proved efficacious, and many are extensively being used around the world.1, 2, 3 While high-income countries preferentially administer mRNA-based vaccines, lower- and middle-income countries have mainly used vaccines based on viral vectors or inactivated virus technology. A timely evaluation of the effectiveness of the currently available vaccines across different regions is essential to the comprehensive understanding of vaccine impact. Age, a crucial element in COVID-19 severity, is likely related to immunosenescence and inflammaging, and is a known factor underlying the reduced response to vaccination in older individuals,4,5 particularly with respect to influenza.6
Brazil is one of the countries most affected by the pandemic. The Brazilian COVID-19 vaccination program initially relied on Vaxzevria/Fiocruz (previously Oxford-AstraZeneca or ChAdOx-1), and Sinovac's CoronaVac/Butantan.7 Brazil's recommended interdose interval for Vaxzevria is 12 weeks versus 2–4 weeks for CoronaVac.7 The period between doses determined for those receiving Vaxzevria has varied among several countries.8 CoronaVac has also been applied at divergent intervals,2,9 making direct comparisons difficult. Additionally, several early publications on vaccine effectiveness (VE) only considered the effects of the initial dose, or were limited to analysing effectiveness against symptomatic infection and hospitalisation; i.e., ICU admission and death were not addressed.10, 11, 12
Nationwide evaluations of the effectiveness of COVID-19 vaccines in Brazil offer advantages, such as the country's vast territory and large population size, with high-quality centralised and comprehensive data sources with which to perform countrywide VE evaluations. The COVID-19 vaccination campaign was initiated nationwide on January 18, 2021. By July 2021, most vaccinees had received either Vaxzevria/Fiocruz or CoronaVac/Butantan vaccines, allowing for precise evaluations of both vaccines’ effectiveness with respect to several outcomes across stratified age ranges.
A significant issue regarding the VE of vaccines against COVID-19 is the degree of circulation of distinct SARS-CoV-2 variants of concern (VOC) in different regions. During the course of the present study, the Gamma variant was the most frequent across all regions of Brazil.13 Importantly, the literature contains few reports on the VE of Vaxzevria and CoronaVac against the Gamma variant.2,12,14
The present study aimed to evaluate the influence of age on the effectiveness of Vaxzevria and Coronavac vaccines in nearly 76 million Brazilian vaccinees, concerning several different outcomes: SARS-CoV-2 infection, COVID-19-related hospitalisation, ICU admission, and death from the start of the nationwide vaccination campaign until July 24, 2021.
Methods
Study design and datasets
We conducted a retrospective longitudinal study using individual-level information on demographic, clinical characteristics, and SARS-COV-2 laboratory tests from Brazilian administrative datasets. The Brazilian Ministry of Health Department of Informatics provided unidentified datasets on the COVID-19 Vaccination Campaign (SI-PNI), Suspected Cases of Acute Respiratory Infection (e-SUS-Notifica), and Severe Acute Respiratory Infection/Illness (SIVEP-Gripe). A key-coded individual identification number present in each of the three datasets was used to perform deterministic linkage, and then removed from the resulting dataset used in our analyzes (Supplementary Figure S1). No personally identifiable data was accessed at any stage. The data dictionary and scripts used in this study have been made available at https://vigivac.fiocruz.br.
SI-PNI is a data warehouse containing information on all vaccine doses administered by Brazilian public health services. From SI-PNI, we extracted information on the type and date of COVID-19 vaccine administration. Brazilian population estimates for 2021, corrected by the all-cause deaths reported in 2020, were retrieved from a previous study.15 e-SUS-Notifica, a nationwide online health surveillance information system for registering acute respiratory infections and suspected/confirmed cases of COVID-19, has been used as a data source for epidemiological research.16
SIVEP-Gripe, the national system for the registry of SARI(Severe Acute Respiratory Infection/Illness)-related hospitalisations and deaths created during the H1N1 pandemic in 2009, has also been widely used as a source for epidemiological studies.17,18 All COVID-19 related SARI hospitalisations and deaths (regardless of prior hospitalisation) are registered in this system. Open versions of all datasets have been made available at: https://opendatasus.saude.gov.br.
The following information was extracted from both SIVEP-Gripe and e-SUS-Notifica: date of SARS-CoV-2 symptom onset, RT-PCR and/or antigen test results. From SIVEP-Gripe, we obtained data on hospitalization status, ICU admission, and hospitalisation outcome (discharge or death).
Study population
We included all individuals who received their first COVID-19 vaccine dose between January 18, 2021 and July 24, 2021. COVID-19 diagnosis was based on RT-PCR or antigen test results. Non-vaccinated individuals were not included in our analysis, since this data was not available.
The following were excluded: (i) individuals with confirmed COVID-19 infection prior to the date of vaccine administration; (ii) individuals with essential covariate information missing (sex and/or age); (iii) individuals receiving vaccines other than Vaxzevria or CoronaVac; (iv) individuals with inconsistent vaccine records (i.e., individuals who received a second dose without a record of the first, individuals who received different vaccines on their first and second doses, and individuals whose time interval between doses was <14 days). Individuals who had recently been infected with COVID-19 prior to vaccination were excluded due to a much lower risk of acquiring new infection compared to those without previous infection. The maintenance of recently infected individuals in the sample could have introduced selection bias.
Exposure and outcomes
Vaccination status, the exposure of interest, was defined for each vaccine based on the time elapsed since vaccine dose administration:
-
(1)
≤13 days after the first dose (reference period).
-
(2)
≥14 days after the first dose and prior to the second dose (partially vaccinated).
-
(3)
≥14 days after the second dose (fully vaccinated).
Follow-up initiates the moment an individual gets their first vaccine dose. The reference period encompasses period between the day a subject receives the first dose and up to 13 days after (dependant on outcome). Persons who do not experience an outcome of interest during the reference period then progress to the “partially vaccinated” period, ranging from day 14 after the first dose up to the day of the second dose (or the date an outcome occurred, or the end of the study period). Finally, the “fully vaccinated” period starts on the 14th day after receiving the second dose, with follow-up ending on the date an outcome occurs or upon study termination. Not all studied subjects were considered across all three periods.
The reference period was defined based on results of a Phase III randomized controlled trial19 and three test-negative case-control studies.12,19,20 We further analyzed vaccine effectiveness during the period between days 1 to 13 after the second dose; these results are presented in Supplementary Table S2.
The primary outcome analyzed was COVID-19 infection, as documented by mild and severe cases registered in the SIVEP-Gripe and e-SUS-Notifica systems. Secondary endpoints included COVID-19 hospitalisation, admission to an intensive care unit (ICU), and death. To calculate total person-days of follow up, we considered the interval between the date the first or second dose was administrated until the day of symptom onset for each outcome or study termination (July 24, 2021). COVID-19 death was considered regardless of prior hospitalisation.
Statistical analyzes
Negative binomial regression was employed to estimate rate ratios (RR) for each outcome for partially and fully vaccinated individuals using log person-time as an offset, and cluster robust standard errors to account for individuals appearing multiple times and overdispersion. Subgroup analyzes were fitted separately. While Cox regression would have been our preferred choice, since the reference group had a restricted follow-up time (≤13 days after the first dose), it would not be possible to employ a comparison group during this period, which would likely result in biased hazard ratios.
The model was adjusted for the following baseline variables: age, sex, region of residence, socioeconomic status, the month during which the first dose was administered, and the SARS-CoV-2 (Rt) reproduction number measured at the State level on the day of the first dose, 14 days after the first dose, and day of the second dose. The Municipal Brazilian Deprivation Index was used as a proxy indicator of socioeconomic status.21 Vaccine effectiveness (VE) was calculated as 1-RR and reported as a percentage. Overall VE was calculated for the entire vaccinated population, as well as for each age subgroup (<60, 60–69, 70–79, 80–89, and ≥90 years).
We investigated rates of infection, hospitalisation, ICU admission, and death for each vaccine compared to the reference period (0–13 days after the first dose). Additionally, we used post-vaccination daily cumulative incidence curves for all outcomes to visually assess differences in risk following each dose of vaccine for different age strata. Cumulative incidence curves for the age groups of fully vaccinated groups were estimated using the Kaplan–Meier estimator.
We performed two sensitivity analyzes: (1) by repeating the principal analysis using the reference period of 0–9 days after the first dose; (2) by analysing VE for all outcomes including both laboratory-confirmed and clinically suspected cases.
We used the R statistical software and H2O packages and herein present descriptive statistics as frequencies and percentages.22 An estimated 95% confidence interval (95%CI) was employed for measures of association to interpret the findings. Missing data (less than 1%) was excluded from our analysis.
Ethical considerations
All work presented here used anonymized secondary data in accordance with the Brazilian General Personal Data Protection Law (LGPD). The study coordinator (MB-N) signed a term of responsibility for the use of each database made available by the Brazilian Ministry of Health (MoH). Each member of the research team signed a term of confidentiality prior to being granted data access. Data were manipulated in a secure computing environment, ensuring protection against data leakage. The Brazilian National Commission on Research Ethics approved the present research protocol (CONEP approval number 4.921.308).
Role of the funding source
All funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
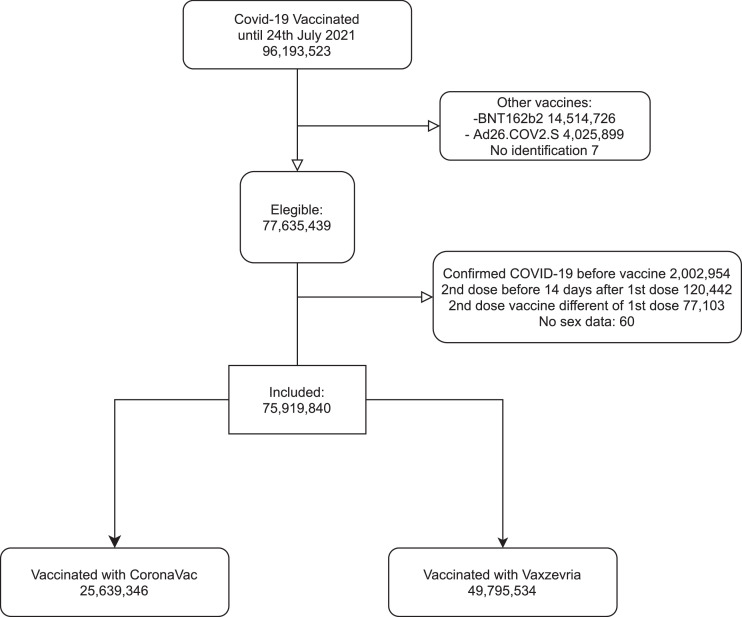
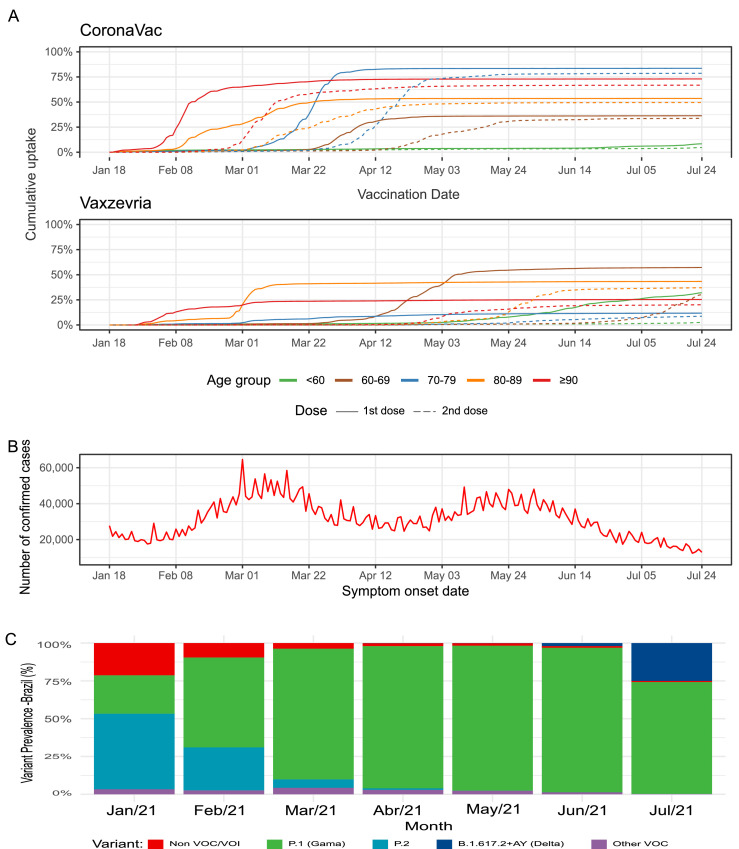
From January 18 to July 24, 2021, a total of 96,193,523 individuals received at least one dose of one of the two COVID-19 vaccines analyzed in this study, and 77,635,439 (80·7%) met the initial eligibility criteria. After excluding individuals diagnosed with COVID-19 before vaccination, those presenting problematic registries of vaccine dose administration or lacking information on sex, 75,919,840 individuals were included in the analysis. The majority (66·2%, n = 49,795,534 individuals) received at least one dose of Vaxzevria and the remaining (33·8%, n = 25,639,346 individuals) received at least one dose of CoronaVac (Figure 1). Throughout the study period, the gamma variant predominated among genotypes reported in Brazil.13 The Delta variant was first identified in June, and by July corresponded to 27% of the sequences analyzed (Figure 2C). Vaccination with CoronaVac occurred mainly from January to April 2021, while Vaxzevria was administered predominantly after March 2021 (Figure 2A). The majority of our cohort was comprised of women (54·9%) and individuals aged 60 years or older (36·7%). Compared to individuals receiving CoronaVac, those who received Vaxzevria were younger (75·8% vs 38·3% of individuals <60 years old), and fewer had a complete vaccine schedule (20·2% vs. 79·1%). Among those who received the second dose, the median time between doses was 84 days (IQR 82–90) for Vaxzevria and 27 days (IQR 21–28) for CoronaVac (Supplementary Table S1).
Figure 1.
Flowchart of the selection of the study individuals vaccinated between January 18 and July 24, 2021. Eligible participants received at least one dose of CoronaVac or Vaxzevria vaccine between January 18 and July 24, 2021. We excluded persons with confirmed COVID-19 diagnosis in 2021 before the first dose and all persons with different vaccines from CoronaVac or Vaxzevria.
Figure 2.
(A) Coverage of first and second dose of CoronaVac and Vaxzevria in Brazil during the study period, the solid line represents 1st dose and dashed line 2nd dose. (B) Number of daily confirmed cases by date of symptom onset. (C) Prevalence of variant of concern (VOC) in Brazil during the study period.
Table 1 shows the COVID-19 VE analysis results, including the number of events and incidence rate per 100 person-years; Supplementary Table S2 shows crude and adjusted VE analyzes. Individuals fully vaccinated (≥14 days after the second dose) with Vaxzevria had a 78·1% (95% CI 77·2 to 79·0), 91·4% (95% CI 90·1 to 92·5), 91·1% (95% CI 88·9 to 92·9), and 92·3% (95% CI 90·5 to 93·7) lower risk of infection, hospitalisation, ICU admission, and death, respectively. Partial vaccination (i.e., ≥14 days after the first dose up to the second dose) with Vaxzeria was associated with at least 50% lower risk of infection (50·4%; 95% CI 49·6.2 to 51·1), hospitalisation (70·9%; 95% CI 69·7 to 72·1), ICU admission (71·0%; 95% CI 69·0 to 73·0), and death (69·7%; 95% CI 67·5 to 71·8). Complete vaccination with CoronaVac was associated with lower risk of infection (53·2%, 95% CI 52·4–54·1), hospitalisation (71·2%, 95% CI 70·0 to 72·4), ICU admission (72·2%, 95% CI 70·2 to 74·0) and death (73·7%, 95% CI 72·1 to 75·2). Partial vaccination with CoronaVac was associated with a slight reduction in the risk of infection (28·7%; 95% CI 27·1 to 30·2), hospitalisation (38·4%; 95% CI 35·5 to 41·2), ICU admission (39·6%; 95% CI 34·8 to 44·0), and death (39·0%; 95% CI 34·9 to 42·9).
Table 1.
Vaccine effectiveness in adults partially and fully vaccinated+ with Vaxzevria and CoronaVac for COVID-19 infection, hospitalization, ICU admission, and death. Brazil, 2021.
| Vaxzevria/Fiocruz |
CoronaVac/Butantan |
|||||||
|---|---|---|---|---|---|---|---|---|
| Person-years | Events | Incidence per 100 person-years | VE% (95% CI)* | Person-years | Events | Incidence per 100 person-years | VE% (95% CI)* | |
| Infection | ||||||||
| Reference period | 1 662 565·5 | 130,302 | 7·84 | Ref | 855,542·2 | 68,126 | 7·96 | Ref |
| Partially vaccinated | 5 550 664·6 | 247,799 | 4·46 | 50·4 (49·6–51·1) | 1,290,469·1 | 74,895 | 5·80 | 28·7 (27.1–30.2) |
| Fully vaccinated | 572 003·4 | 14,771 | 2·58 | 78·1 (77·2–79·0) | 4,574,691·4 | 194,864 | 4·26 | 53·2 (52·4–54·1) |
| Hospitalization | ||||||||
| Reference period | 1,664,388·0 | 22,449 | 1·35 | Ref | 856,418·3 | 16,289 | 1·90 | Ref |
| Partially vaccinated | 5,587,966·0 | 28,713 | 0·51 | 70.9 (69·7–72·1) | 1,303,567·1 | 15,076 | 1·16 | 38·4 (35·5–41·2) |
| Fully vaccinated | 580,979·1 | 1292 | 0·22 | 91.4 (90·1–92·5) | 4,624,347·6 | 28,810 | 0·62 | 71.2 (70·0–72·4) |
| ICU admission | ||||||||
| Reference period | 1,664,660·2 | 7558 | 0·45 | Ref | 856,597·5 | 6008 | 0·70 | Ref |
| Partially vaccinated | 5,592,952·5 | 9907 | 0·18 | 71·0 (69·0–73·0) | 1,307,124·7 | 5560 | 0·43 | 39·6 (34·8–44·0) |
| Fully vaccinated | 581,594·0 | 477 | 0·08 | 91·1 (88·9–92·9) | 4,629,831·8 | 10,364 | 0·22 | 72·2 (70·2 – 74·0) |
| Death | ||||||||
| Reference period | 1,664,670·8 | 7037 | 0·42 | Ref | 856,563·2 | 7852 | 0·92 | Ref |
| Partially vaccinated | 5,592,331·8 | 10,579 | 0·19 | 69·7 (67·5–71·8) | 1,305,706·9 | 7203 | 0·55 | 39·0 (34·9–42·9) |
| Fully vaccinated | 581,648·9 | 564 | 0·10 | 92·3 (90·5–93·7) | 4,629,255·8 | 13,166 | 0·28 | 73·7 (72·1–75·2) |
Reference period: ≤13 days after the first dose; Partially vaccinated: ≥14 days after the first dose and without the second dose; Fully vaccinated: ≥14 days after the second dose. ICU denotes intensive care unit.
Negative binomial model adjusted for age, sex, region of residence, month of administration of first dose, municipal deprivation level and Effective Reproductive Number (Rt).
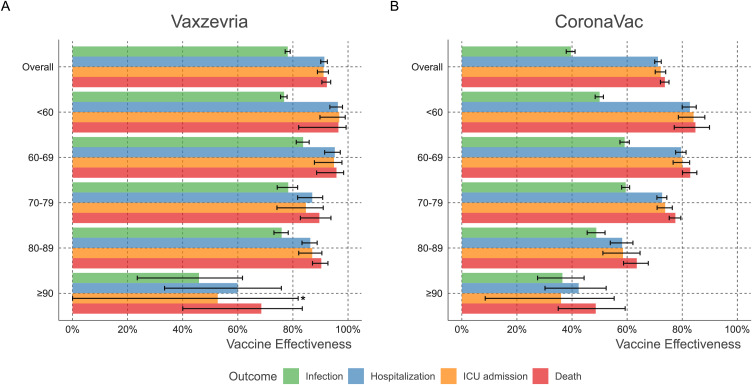
When the analysis was stratified by age, complete vaccination with Vaxzevria or CoronaVac conferred differing degrees of protection. Vaxzevria-induced VE was around 90% in the analyzed outcomes for those aged up to 89 years. In individuals aged 90 years or older, complete vaccination with Vaxzevria granted a VE of 68·5% against death. CoronaVac VE reached around 75% protection in individuals aged ≤ 79 years, versus 63·5% in individuals aged 80–89 years and 48·6% among those over 90 years of age. Partial vaccination with either vaccine conferred no protection among individuals ≥90 years (Table S3, Figure 3).
Figure 3.
Vaccine effectiveness of Vaxzevria and CoronaVac in Brazil by age group. VE (1-Rate Ratio) was obtained through Negative binomial regression adjusted for age, sex, region of residence, the month of administration of the first dose, Effective Reproductive Number at State level (Rt), and municipal deprivation level (IBP). *The point estimate and confidence interval for ICU admission in ≥90 years. are 52.7 (95%CI −23.9 to 81.9%).
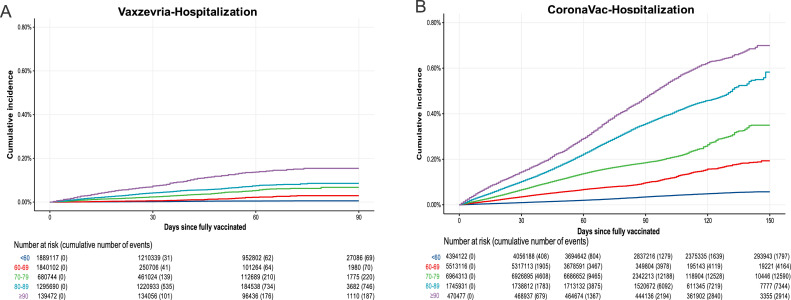
To further explore the impact of age on the duration of protection, we analyzed the cumulative incidence of all outcomes after full vaccination in each age strata. Figure 4 shows that the cumulative hospitalisation incidence (or rates) curves for Vaxzevria-vaccinated individuals increased slowly over time since full vaccination, reaching around 0·1% at 90 days after the second dose across almost all age groups. For individuals ≥90 years, cumulative hospitalisation reached 0·2% after 90 days. CoronaVac vaccinees presented different rates of increase for each age group, and individuals aged 80–89 years reached almost 0·6% cumulative hospitalization incidence by 150 days after the second dose (around 0·35% at 90 days), while those ≥90 years had a cumulative hospitalisation incidence of approximately 0·7% at day 150 (about 0·45% at day 90). Analyzes of ICU admission and death followed similar patterns. Regarding infection, the younger group exhibited a higher incidence than older groups (Supplementary Figs. S1–S3).
Figure 4.
Cumulative Incidence of new cases requiring hospitalisation by age group during the period after fully vaccinated (≥14 days after the second dose) up to 150 days. (A) Vaxzevria/Fiocruz vaccine. (B) CoronaVac/Butantan vaccine.
Considering that most individuals ≥80 years old were vaccinated in the first two months of the campaign, we hypothesized that the lower VE effectiveness observed in older individuals could be related to waning immunity rather than the effects of ageing. To address this issue, we analyzed all individuals regardless of age who were immunised between January and February, and then compared these results to the overall VE for the entire period (i.e., those immunised from January-July). Similar VE patterns were observed for both groups, indicating that reduced protection is most likely associated with ageing regardless of the vaccine administrated (Supplementary Table S4)
No differences were seen in the RR of outcomes (infection, hospitalisation, ICU admission, and death) in the reference period for each vaccine (Table S5). Modifying the reference period to 0–9 days after the first dose, we identified VE values and interval estimates similar to those in the main analysis for both Vaxzevria and CoronaVac vaccines (Table S6). Additionally, the calculated VE for all COVID-19 cases (i.e., adding the clinically suspected cases to the laboratory-confirmed case pool) was similar to that for laboratory-confirmed cases only (Table S7).
Discussion
After analysing data from almost 76 million Brazilian adults vaccinated between January and July 2021, our results demonstrate that complete vaccination with Vaxzevria and CoronaVac offered around 90% and 75% overall protection, respectively, regardless of age or hospitalisation, ICU admission or COVID-19-related death outcome. Importantly, individuals ≥90 years who received CoronaVac reached a VE of 48·6% against death. Regartding infection, overall vaccine effectiveness was 78% for Vaxzevria and 53% for CoronaVac. These findings reinforce the age-related VE reduction of CoronaVac in Brazil19 and provide new, more granular data on the age-stratified VE of Vaxzevria.20 We also investigated whether lower protection in the elderly resulted from waning immunity, considering that individuals ≥90 years were among the first immunised in Brazil. Compared individuals aged <60 years vaccinated around the same calendar date, individuals ≥90 years exhibited higher rates of COVID-19-related hospitalisation. Considering the entire post-vaccination period, hospitalisation rates among individuals aged <60 years remained low despite higher SARS-CoV-2 exposure, in contrast to steadily increasing rates among individuals aged ≥90 years.
Our observation on the marked effect of age is consistent with findings demonstrating the elderly exhibit poorer responses to the trivalent inactivated influenza vaccine.23 Additionally, the curves indicate a rise of hospitalisation, in each age range, circa two months after the second dose of CoronaVac. The short follow-up period in the present dataset after the second Vaxzevria dose does not yet allow us to conduct a similar analysis on waning immunity with respect to this vaccine.
It is reasonable to attribute the observed reduction in VE to immunosenescence, which is associated with a higher frequency of comorbidities and may imply higher death rates. In the context of limited vaccine availability, the precise identification of age limits, i.e., at which point immune protection becomes impaired, can provide valuable evidence to inform public health decisions. In that regard, our results support the careful consideration of booster doses for the elders.
Our findings regarding the CoronaVac/Butantan vaccine protection against symptomatic COVID-19 stand in agreement with a previous Brazilian efficacy study, but are lower than those reported by a Turkish efficacy trial.9,19 A cohort study in Chile presented higher VE than our findings on infection and hospitalisation, but these differences may be partially explained by the higher frequency of younger individuals in the Chilean study.2 During the vaccination campaign, Brazil experienced periods of health system collapse, contributing to more severe clinical presentations and increased death rates.24 Additionally, the incidence of the Gamma variant has been estimated at 28·6% in Chile and 69·6% in Brazil during the study periods,2,13 and plasma samples obtained from fully vaccinated CoronaVac vaccinees have shown a reduced capacity to neutralize the Gamma variant.2 Furthermore, considering the reduced territorial area and population, vaccine deployment and complete immunization in Chile was achieved more quickly than in Brazil, which may have resulted in differences in viral transmission.2
For Vaxzevria, our finding of 78·1% effectiveness against infection exceeded the level of 66·7% previously reported in a combined analysis of four clinical trials conducted in the UK, South Africa, and Brazil.8 Effectiveness against hospitalisation was consistent with the 80% and 88% protection observed in studies in Scotland and England, respectively.1,11 Additionally, our findings support the high level of protection offered by Vaxzevria despite the abundant circulation of the Gamma variant in Brazil during this period. Other studies have reported on the VE of Vaxzevria in populations infected by VOCs, mainly focused on protection against symptomatic infection or hospitalisation.10,12,14,20 The findings reported herein, combined with data in the literature, confirm a consistently high rate of protection against moderate to severe COVID-19 despite a large circulation of VOCs. Nevertheless, the observed differences in effectiveness between Vaxzevria and CoronaVac may be related to the distinct technologies used in these two products and the resulting influence on immunogenicity.25
In several countries, including Brazil, the second dose of Vaxzevria has been administered 12 weeks after the first dose, despite the four-week interval used in efficacy trials.8 While limited data has suggested an increase in efficacy resulting from an extended between-dose interval,8 effectiveness in large populations has yet to be evaluated. We observed that during the 12-week interval between first and second doses, individuals aged ≥90 years exhibited no protection against severe outcomes and there was a significant VE increase after the second dose (68.5% after the second dose). Considering the difference between VE after first and second dose of Vaxzevria, a shorter interval between doses should be evaluated in circumstances of high viral transmission.
Our study has several strengths. The large sample size allowed us to identify the age limits in which immune protection starts to decrease. We used State-level Rt values and period of vaccine administration in the model to circumvent the challenge of vaccination at distinct pandemic periods. The elimination of missing or underreported information in our dataset further increases the quality of the data and confidence of our analysis. However, our study is also subject to some limitations. VE was estimated using routinely collected data, making our analysis dependant on data availability. Importantly, no data is available on unvaccinated individuals, making it impossible to evaluate the effects of possible selection bias. Nevertheless, Brazil has a record of high adherence to immunization campaigns, and the coverage of the COVID-19 vaccination has been high across prioritized groups, which may serve to mitigate this bias. Second, our analyzes were not controlled for comorbidities, a recognized risk factor for severe COVID-19. Several comorbidities are intrinsically related to age and are likely to be relevant in the causal pathway between age and COVID-19 severity. As a consequence, the lower VE observed among the elderly may also be partially related to comorbidities in addition to immunosenescence. However, our analysis indirectly, or at least partially, controls for comorbidities by controlling for age.26 Still, residual confounding by not including comorbidities in the model could nevertheless exist, and our results should be interpreted in light of this limitation. Unfortunately, we were unable to dissociate the impact of comorbidities on the VE of CoronaVac and Vaxzevria. Analyzes lacking adjustments for comorbidities will potentially lead to imprecise estimations of VE, and it is recommended that further studies address this limitation, which has been neglected in most vaccine efficacy or effectiveness studies.27,28 Third, in contrast to many VE studies, the reference period used herein for comparison purposes was 0–13 days after vaccination, which did not allow us to use the Cox regression model, a common technique in follow-up studies in which the force of infection changes over time. Immunity levels tend to increase over time since the administration of a vaccine dose.9,11 In this sense, recently vaccinated persons are likely to present low levels of immunity, perhaps even similar to unvaccinated persons depending on the length of time elapsed.9,11 Although using early post-vaccination as a reference may underestimate VE, previous studies have used a similar approach and obtained results similar to those found in clinical trials.29,30 Additionally, our sensitivity analysis demonstrated similar results when a 0–9-day reference period was used. Moreover, the effectiveness results detailed in the present report are similar, across the pertinent age ranges, to previously reported information on both vaccines using distinct approaches.2,19,20 Comparisons between the effectiveness of CoronaVac and Vaxzevria vaccines should be made with caution, considering the follow-up time after full vaccination presented here is longer for CoronaVac than that of Vaxzevria.
Another possible bias concerns case definition. In the main analysis, a SARS-CoV-2 infection case was defined as a person who had a positive RT-PCR or rapid antigen test result for SARS-CoV-2. This case definition may have been a source of misclassification bias, as cases may be asymptomatic, and also due to a general shortage of tests for confirming/discarding cases in early stages of the pandemic.31 However, to mitigate the possible misclassification of cases, we performed a sensitivity analysis considering as cases also those who were only clinically suspected of SARS-CoV-2 infection; this sensitivity analysis produced results similar to those of the main analysis.
Using the data available in Brazil, we estimated the overall VE for each vaccine evaluated as well as VE specific to each age group. Vaxzevria/Fiocruz and CoronaVac/Butantan were both shown to be highly protective against severe COVID-19 in the adult population aged up to 80 years. We were further able to pinpoint those age ranges associated with substantially lower VE soon after receiving the second dose. Due to lower VE associated with age and the pronounced reduction in VE over time after receiving the second dose, an early booster dose should be considered for those over 80 years of age who received CoronaVac, and especially for individuals over 90 years regardless of which of these two vaccines were administered.
Despite high population adherence, the vaccination rollout is evolving unevenly throughout Brazil. SARS-CoV-2 variants have been a cause for concern given the risk of emerging variants capable of escaping the protective effect of a given vaccine. Therefore, the continuous monitoring of VE in the current context may provide sound evidence to inform public health decision-making. As shown in the present analysis, the data available in Brazil offer strong potential to monitor the VE of different vaccines across the diverse and evolving contexts of the COVID-19 pandemic within the country.
Declaration of interests
VO, VB, MB, and MB-N are employees of Fiocruz, a federal public institution, which manufactures Vaxzevria in Brazil, through a full technology transfer agreement with AstraZeneca. Fiocruz allocates all manufactured products to the Brazilian Ministry of Health for public health service (SUS) use. All other authors report no potential competing interests.
Acknowledgments
Funding
This study was partially supported by a donation from the “Fazer o bem faz bem” program.
Data sharing
Our agreement with the Brazilian MoH for accessing the referenced databases patently denies authorization of access to any third parties. All requests to access these databases must be addressed to the MoH. Unlinked data is available at https://opendatasus.saude.gov.br. Data dictionaries, linkage scripts and analyzes scripts are available at https://vigivac.fiocruz.br.
Acknowledgments
The authors thank DATASUS for its excellent work in providing unidentified databases. GLW, MLB, and MB-N are research fellows from CNPq, the Brazilian National Research Council. The authors would also like to thank Andris K. Walter for English language revision and manuscript copyediting assistance.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2021.100154.
Appendix. Supplementary materials
References
- 1.Vasileiou E., Simpson C.R., Shi T., et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jara A., Undurraga E.A., González C., et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. New Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moghadas S.M., Vilches T.N., Zhang K., et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab079. published online Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrobon A.J., Teixeira F.M.E., Sato M.N. I mmunosenescence and inflammaging: risk factors of severe COVID-19 in older people. Front Immunol. 2020;11:2728. doi: 10.3389/fimmu.2020.579220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciabattini A., Nardini C., Santoro F., Garagnani P., Franceschi C., Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 7.da Saúde M. 2021. Plano Nacional de Operacionalização da Vacinação Contra a COVID-19.https://www.gov.br/saude/pt-br/coronavirus/publicacoes-tecnicas/guias-e-planos/plano-nacional-de-vacinacao-covid-19 published online May 7. (accessed July 31, 2021) [Google Scholar]
- 8.Voysey M., Clemens S.A.C., Madhi S.A., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanriover M.D., Doğanay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheikh A., McMenamin J., Taylor B., Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung H., He S., Nasreen S., et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374:n1943. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rede Genômica Fiocruz. Dashboard Rede Genômica. Genomahcov - Fiocruz. http://www.genomahcov.fiocruz.br/dashboard/ (accessed July 31, 2021).
- 14.Bernal J.L., Andrews N., Gower C., et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. Engl J Med. 2021 doi: 10.1056/NEJMoa2108891. published online July 21. [DOI] [PubMed] [Google Scholar]
- 15.Victora P.C., Castro P.M.C., Gurzenda S., Medeiros A.C., França G.V.A., Barros P.A.J.D. Estimating the early impact of vaccination against COVID-19 on deaths among elderly people in Brazil: analyzes of routinely-collected data on vaccine coverage and mortality. EClinicalMedicine. 2021;38:10103. doi: 10.1016/j.eclinm.2021.101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lima F.E.T., Albuquerque N.L.S., Florencio S.S.G., et al. Time interval between onset of symptoms and COVID-19 testing in Brazilian state capitals, August 2020. Epidemiol Serv Saude. 2020;30 doi: 10.1590/S1679-4974202100010002. [DOI] [PubMed] [Google Scholar]
- 17.Ranzani O.T., Bastos L.S.L., Gelli J.G.M., et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9:407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira E.A., Colosimo E.A., Silva A.C.S.e, et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021;5:559–568. doi: 10.1016/S2352-4642(21)00134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranzani O.T., Hitchings M.D.T., Dorion M., et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374:n2015. doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hitchings MDT, Lewnard JA, Dean NE, Ko AI, Ranzani OT, Andrews JR, et al. Use of recently vaccinated individuals to detect bias in test-negative case-control studies of COVID-19 vaccine effectiveness. medRxiv. DOI: 2021.06.23.21259415. [DOI] [PMC free article] [PubMed]
- 21.Allik M., Ramos D., Agranonik M., et al. Small-area Deprivation Measure for Brazil: Data Documentation. University of Glasgow. 2020 doi: 10.5525/gla.researchdata.980. [DOI] [Google Scholar]
- 22.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 23.Wilkinson K., Wei Y., Szwajcer A., et al. Efficacy and safety of high-dose influenza vaccine in elderly adults: a systematic review and meta-analysis. Vaccine. 2017;35:2775–2780. doi: 10.1016/j.vaccine.2017.03.092. [DOI] [PubMed] [Google Scholar]
- 24.Silva S.J.R., Pena L. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frater J., Ewer K.J., Ogbe A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8:e474–e485. doi: 10.1016/S2352-3018(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhi S.A., Baillie V., Cutland C.L., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. Engl J Med. 2021 doi: 10.1056/NEJMoa2102214. published online March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chodick G., Tene L., Rotem R.S., et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab438. published online May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chodick G., Tene L., Patalon T., et al. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medidas de distanciamento social no controle da pandemia de COVID-19: potenciais impactos e desafios no Brasil. Ciênc Saúde Coletiva. 2020;25:2423–2446. doi: 10.1590/1413-81232020256.1.10502020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.