Summary
The demand for HIV testing using dried blood spots (DBS) has increased recently. However, DBS is not an approved sample for HIV testing in Japan. This study examined the validation of HIV testing with DBS, prepared at the laboratory or remotely and mailed via postal service to the laboratory. DBS were punched out from a 5.5 mm diameter circle on filter paper, then eluted with 600 μL of phosphate buffered saline overnight at 4℃, and analyzed by Lumipulse S HIVAg/Ab (LUM). The mean LUM count of DBS was 237.4-times diluted compared to titrated plasma. Repeated sample testing showed that although LUM count of DBS decreased slightly with increase in sample storage time (up to one month), it did not affect the result of HIV testing with DBS. Based on testing of 50 HIV+ confirmed cases and 50 HIV- persons, the estimated sensitivity was 98% (49/50) with a specificity of 100% when the cut-off value is 0.5. The single false negative case was a patient with undetectable viral load over the last 10 years, resulting in a decrease of antibody titer below the cut-off level. In conclusion, although DBS cannot completely replace plasma in HIV testing because the sensitivity was a little lower than that of plasma, it can be potentially useful for a screening test by self-finger-prick and postal service use. This will allow people to receive HIV testing without visiting public health centers.
Keywords: HIV, dried blood spot, self-finger-prick, postal service
Introduction
In Japan and many other countries, HIV testing is conducted free of charge at local public health centers. However, some centers are not always convenient, for example, operating over limited hours during the week or closed over the weekends. For these reasons, the number of HIV tests conducted at centers has been on the decline by about 30% in the last decade according to the Ministry of Health, Labour and Welfare, Japan (1). Furthermore, during the second quarter of 2020 when the first wave of the COVID-19 pandemic hit Japan, HIV testing decreased steeply by 70% compared with the same quarter of the previous year (2) due to both the increased load on medical staff at public health facilities due to COVID-19 and lockdowns imposed on people. Under these circumstances, it is important to design alternative HIV testing procedures that can be performed without visiting public health centers (3).
In this regard, HIV testing using mailed via postal service samples has increased annually during the last several years, with an estimated rise in 2019 of 14.5% (according to Sudo K. 34th meeting of the Japanese Society for AIDS Research, 2020; P-S5-2). Many of them seem to use dried blood spot (DBS) samples because they are easy to collect by oneself, transport, and store (at ambient temperature), compared with fresh blood samples (4). However, DBS is currently not approved as a sample for HIV testing in Japan; only whole blood or serum or plasma samples are approved. Thus, validation and approval of HIV testing with DBS is urgently needed in Japan.
Many studies have described the use of DBS in HIV testing, however, these were often collected by medical staff who could obtain a sufficient volume of blood (5-10). In comparison, self-finger-prick sometimes yields a small volume of blood that is too inadequate to fill the circle on the designated area on the Whatman 903 card (11), which has been used in most HIV/DBS studies. Though these studies explored concordance rate of HIV testing between plasma and DBS, they did not focus on fundamental validation such as difference of filter papers, carry-over, accuracy, stability, or dilution effect in the elution step.
The purpose of this study was to validate HIV testing with DBS samples derived from self-finger-prick/postal service on the above fundamental points.
Materials and Methods
Participants and sample collection
For the fundamental research part of the study, 3 mL of venous blood was obtained from 50 HIV+ patients at AIDS Clinical Center and from 30 men who had sex with men (MSM) followed at the sexual health clinic and confirmed to be HIV-negative at the National Center for Global Health and Medicine (NCGM). Medical staff prepared DBS samples from these individuals by pipetting drops of collected venous blood onto filter paper. We also asked 20 healthy volunteers to prepare DBS by self-finger-prick using the BD Microtainer® contact-activated lancet Medium flow (Becton, Dickinson and Company, Franklin Lakes, NJ). After overnight drying, all DBS samples, including those prepared from the HIV+ and HIV- individuals, were mailed via postal service to our laboratory. All DBS samples were anonymously labelled and thus the laboratory staff was blinded to the clinical status of the individuals who provided the samples.
The study was approved by the Human Ethics Committee of the NCGM (#NCGM-G-2065), and each provider of blood samples also signed informed consent in accordance with the Declaration of Helsinki. The 20 healthy volunteers were considered to have consented to the study by mailing via postal service the DBS samples. These samples were collected from December 2017 to May 2018.
Preparation of DBS
For comparison of the filter papers used for DBS, we tested the following 9; Filter paper for blood collection (EIKEN CHEMICAL, Tokyo, Japan), DENTAL STICK II (SANRITSU, Chiba, Japan), 903 Protein Saver Card (GE Healthcare Life Sciences, Marlborough, MA), 903 Protein Saver Snap Apart Card (GE Healthcare Life Sciences), FTA™ DMPK-A Card (GE Healthcare Life Sciences), FTA™ DMPK-B Card (GE Healthcare Life Sciences), FTA™ DMPK-C Card (GE Healthcare Life Sciences), FTA™ Classic Card (GE Healthcare Life Sciences), and NucleoCard (MACHEREY-NAGEL, North Rhine-Westphalia, Germany).
HIV testing using DBS
A tool was used to punch out 5.5 mm diameter circles from the DBS filter paper (CARL, Tokyo, Japan). After punching out the DBS, the operator also punched out blank parts of the filter paper at three spots to avoid any contamination before using the punching tool again. The punched-out DBS were eluted overnight with 600 μL of phosphate-buffered saline (PBS) pH 7.2 (ThermoFisher Scientific, Waltham, MA) at 4°C. After vortexing and centrifugation, 100 μL of the supernatants were analyzed by Lumipulse S HIVAg/Ab (FUJIREBIO, Tokyo, Japan) (LUM). The LUM count represented the linear regression between 0.1 and 15.0, with a cutoff value for HIV positivity of ≥ 1.0 using the standard method. Samples determined as HIV-positive were tested again by the HISCL™5000 HIV Ag+Ab (Sysmex, Hyogo, Japan) (HIS) using 30 μL of the remaining supernatant. The HIS score represented the linear regression between 0.0 and 100, with a cutoff value for HIV positivity of ≥ 1.0 using the standard method. Both LUM and HIS were chemiluminescent enzyme immunoassay systems of the 4th generation HIV antigen and antibody testing approved in Japan for serum or plasma samples. In contrast, not only in Japan but also in other countries, DBS are not approved as a sample for LUM and HIS.
Statistical analysis
Bivariate correlation analysis was conducted by IBM SPSS Statistics version 23 (IBM, Armonk, NY) with a significance of p < 0.05. Simple linear regression was plotted by GraphPad Prism version 8.3.0 (GraphPad Software, San Diego, CA).
Results
Comparison of filter papers
First, we compared the performance of the nine filter papers using LUM. We prepared DBS using whole blood samples obtained from seven HIV+ patients (samples A-G, Figure 1). LUM count was similar for all filter papers, except the FTA™ DMPK-B card. We eventually adopted the Filter paper for blood collection (EIKEN CHEMICAL) throughout this study because of its design of four spots on the filter paper, each requiring 25 μL blood, which matches the volume of blood obtained from a self-finger-prick.
Figure 1.
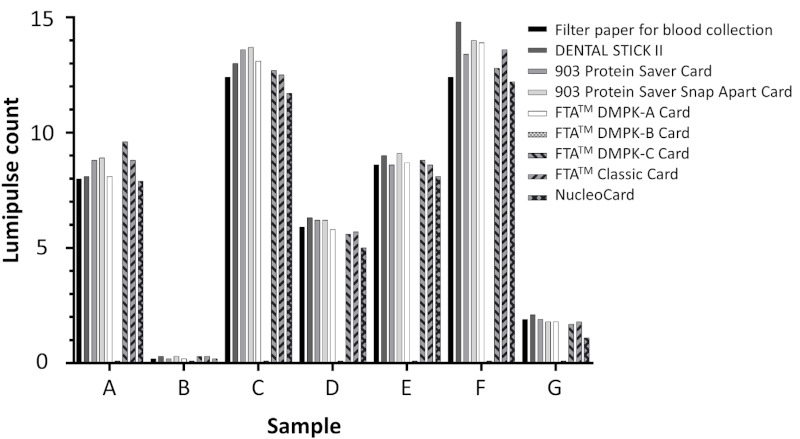
Comparison of Lumipulse S HIVAg/Ab among 9 filter papers. Blood samples were obtained from 7 HIV+ donors, then whole blood was placed on nine filter papers using a pipette. Each DBS was punched, eluted with PBS, and analyzed by Lumipulse S HIVAg/Ab.
Carry-over
To assess the probability of carry-over, the DBS and the three blank circles of the filter paper were punched out and subjected to LUM. In this test, we used 6 HIV+ samples, and no carry-over was observed even in the first punched out blank filter paper (Table 1). Subsequent sampling for analyses used all 3 blank punches after each DBS.
Table 1. Carry-over in continuous punch of DBS with Lumipulse S HIVAg/Ab.
| Sample | Lumipulse count |
|||
|---|---|---|---|---|
| DBS | Blank 1 | Blank 2 | Blank 3 | |
| H | 15.0 | 0.1 | 0.1 | 0.1 |
| I | 15.0 | 0.1 | 0.1 | 0.1 |
| J | 2.2 | 0.1 | 0.1 | 0.1 |
| K | 4.0 | 0.1 | 0.1 | 0.1 |
| L | 15.0 | 0.1 | 0.1 | 0.1 |
| M | 15.0 | 0.1 | 0.1 | 0.1 |
DBS: dried blood spot.
Comparison of blood volume
To determine the appropriate amount of whole blood necessary, 1, 2, 4, 8, 10, and 20 μL of whole blood were pipetted on the filter paper discs prior to the LUM measurement procedure. In this part of the study, we used samples from 7 HIV+ individuals. LUM count increased with increasing amounts of blood up to 8 μL but reached a plateau thereafter (Figure 2). These results indicated that 8 μL of whole blood was sufficient to spread throughout the entire 5.5 mm circle on the filter card. Since less than 8 μL of blood may be collected in real sampling situations, we decided that testing criteria should exclude samples that appear to contain < 8 μL of blood (as judged by DBS diameter).
Figure 2.
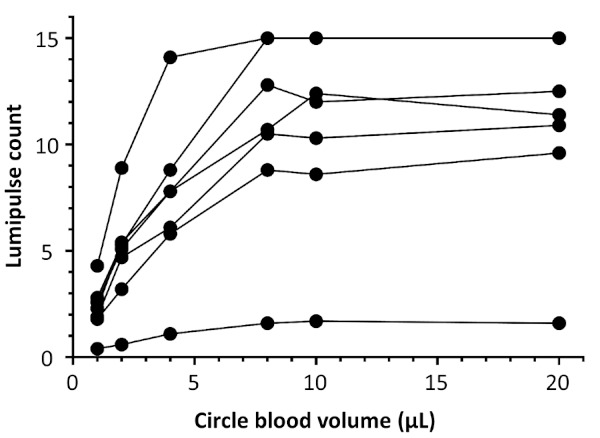
Comparison of Lumipulse S HIVAg/Ab among different volumes of blood placed on the filter paper using a pipette. The blood samples were obtained from 7 HIV+ donors, then 1, 2, 4, 8, 10, and 20 μL of whole blood were placed on the filter papers using a pipette. Each DBS was punched, eluted with PBS, and analyzed by Lumipulse S HIVAg/Ab.
Comparison between DBS and plasma
To determine the difference in sensitivity between DBS and plasma, we compared the LUM count of DBS with that obtained from plasma samples after serial titrations. First, whole blood was used to create DBS, then plasma was prepared from the same blood sample. The samples were serially diluted 100, 300, 1000, 3000, and 10000 times with PBS. We used 5 HIV+ samples in this experiment. LUM counts of DBS corresponded to 265.5, 216.8, 238.8, 257.8, and 208.3 times dilution (mean of 237.4) of the titrated plasma samples (Table 2).
Table 2. Count of Lumipulse S HIVAg/Ab with diluted plasma and DBS.
| Sample | Lumipulse count of diluted plasma |
Lumipulse count of DBS | DBS/plasma ratio | ||||
|---|---|---|---|---|---|---|---|
| 1/100 | 1/300 | 1/1000 | 1/3000 | 1/10000 | |||
| N | (15.0)* | 7.8 | 2.8 | 0.8 | 0.2 | 8.9 | 1/265.5 |
| O | 3 | 1.3 | 0.5 | 0.2 | 0.1 | 1.5 | 1/216.8 |
| P | (15.0)* | 9 | 3.3 | 0.9 | 0.3 | 11.4 | 1/238.8 |
| Q | (15.0)* | 8.9 | 2.9 | 0.8 | 0.3 | 10.4 | 1/257.8 |
| R | (15.0)* | 9.6 | 3.3 | 0.9 | 0.3 | 13.9 | 1/208.3 |
The DBS/plasma ratio was calculated by linear regression of Lumipulse count with diluted plasma. *We excluded these from linear regression analysis because 15.0 was the upper limit of Lumipulse count. DBS: dried blood spot.
Accuracy and stability
To evaluate the accuracy of our method, we tested the within-run accuracy. First, we prepared 10 DBS samples from a single whole blood sample, then analyzed them with LUM. Five HIV+ samples were used in this experiment. The mean coefficient of variance was 6.69% (range 4.22-10.14 %) (Table 3). Second, we tested the between-run accuracy. We prepared 5 DBS samples from a single whole blood sample, stored them at room temperature and analyzed them by LUM on days 0, 6-16, 13-18, 21-30 and 28-37. The mean coefficient of variance was 8.74% (range, 4.38-12.28%), which was similar to that of the within-run accuracy experiment (Table 4). Although a small but gradual decrease in LUM count was noted in two samples (Figure 3), LUM could be counted in all samples up to day 28.
Table 3. Within-run accuracy of Lumipulse S HIVAg/Ab with DBS.
| Sample | n | Mean Lumipulse count | Range | Standard deviation | Coefficient of variance (%) |
|---|---|---|---|---|---|
| S | 10 | 4.91 | 4.5 - 5.3 | 0.251 | 5.11 |
| T | 10 | 8.76 | 8.3 - 9.4 | 0.369 | 4.22 |
| U | 10 | 0.90 | 0.8 - 1.0 | 0.045 | 4.97 |
| V | 10 | 1.54 | 1.3 - 1.9 | 0.156 | 10.14 |
| W | 10 | 6.84 | 6.0 - 8.0 | 0.617 | 9.02 |
DBS: dried blood spot.
Table 4. Between-run accuracy of Lumipulse S HIVAg/Ab with DBS.
| Sample | n | Mean Lumipulse count | Range | Standard deviation | Coefficient of variance (%) | Slope (Lumipulse count/day) | p value of slope |
|---|---|---|---|---|---|---|---|
| X | 5 | 13.46 | 12.6 - 15.0 | 0.967 | 7.18 | -0.0685 | 0.0379 |
| Y | 5 | 9.76 | 9.2 - 10.4 | 0.427 | 4.38 | -0.0145 | 0.5153 |
| Z | 5 | 0.82 | 0.7 - 0.9 | 0.075 | 9.13 | -0.0012 | 0.8016 |
| AA | 5 | 1.08 | 0.9 - 1.3 | 0.133 | 12.28 | -0.0093 | 0.0472 |
| AB | 5 | 5.54 | 4.8 - 6.3 | 0.595 | 10.74 | -0.0387 | 0.1014 |
The slopes and p values of the slope were calculated by linear regression analysis. DBS: dried blood spot.
Figure 3.
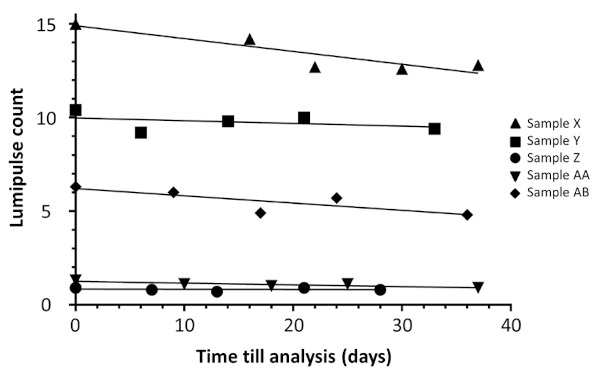
Stability test of Lumipulse S HIV Ag/Ab with DBS samples. Blood samples were obtained from 5 HIV+ donors. Five DBS were prepared from each using whole blood. They were stored at room temperature and analyzed by Lumipulse S HIV Ag/Ab after 0, 6-16, 13-18, 21-30 and 28- 37 days.
Sensitivity and specificity
To determine the sensitivity and specificity of our method, we tested 50 samples from HIV+ and HIV-persons. HIV was normally considered positive using a LUM cutoff value of 1.0 for plasma samples. In this study, however, we lowered the cutoff value to 0.5 because we found that DBS samples were diluted around 150 times with PBS due to DBS processing. Next, we re-tested all samples with LUM value ≥ 0.5 by HIS using a lower cutoff value of 0.5 (standard cutoff value = 1.0). Among 50 HIV+ DBS samples, 49 samples were judged as HIV positive (sensitivity: 98%). The LUM counts ranged from 0.2 to 15.0 (Figure 4A). HIS counts of the LUM-positive samples ranged from 0.1 to 111.4 (Figure 4B), resulting in 3 samples being considered negative for HIV by HIS. These three samples with HIS counts of 0.1, 0.2, and 0.3 corresponded to LUM counts of 0.2, 2.6, and 0.8, respectively. None of 50 HIV- DBS samples were judged as HIV positive by LUM (specificity: 100%). LUM count was 0.1 in all HIV-negative DBS samples. One HIV+ patient whose LUM count was 0.2 has been on anti-retroviral treatment (ART) for more than 10 years with persistently undetectable viral load during the same period. We tested a plasma sample from this false-negative patient by western blotting (NEW LAV BLOT 1, Bio-Rad Laboratories, Tokyo). The results showed absence of several bands, including anti-GP41 antibody, which is one of the targets of LUM.
Figure 4.
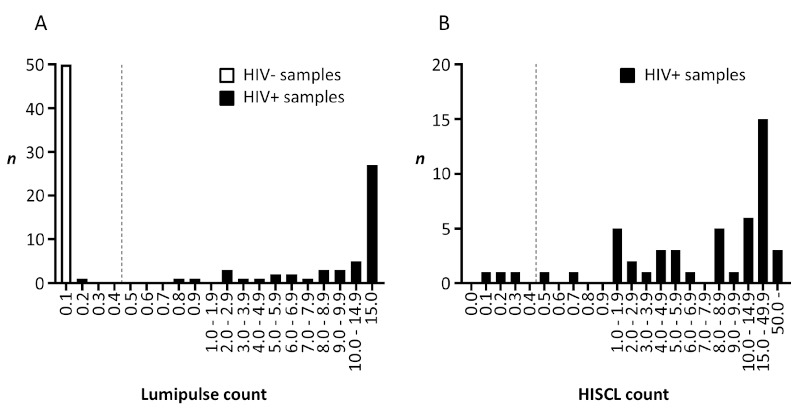
Specificity and sensitivity test of Lumipulse S HIVAg/Ab and HISCL™-5000 HIV Ag+Ab using DBS samples. The DBS samples were obtained from 50 HIV+ and 50 HIV- donors. (A) 100 DBS samples were analyzed by Lumipulse S HIVAg/Ab. Dotted line: cutoff value. (B) 50 DBS samples from HIV+ donors were analyzed by HISCL™-5000 HIV Ag+Ab. Dotted line: cutoff value.
Among 50 HIV+ donors, 36 were on ART. Next, we investigated the relationship between the duration of ART and LUM or HIS counts. The results showed a significant negative correlation with LUM count (Spearman's correlation factor: -0.430, p = 0.009) and HIS count (-0.620, p < 0.001) (Figure 5). Finally, we compared LUM and HIS counts of DBS and plasma samples obtained from patients with either LUM or HIS count of < 1.0 from DBS samples (Table 5). Although LUM and/or HIS counts of the DBS were below 1.0, those from plasma were high enough to consider the samples HIV-positive. These results clearly demonstrated the low sensitivity of DBS samples compared to those of plasma samples.
Figure 5.
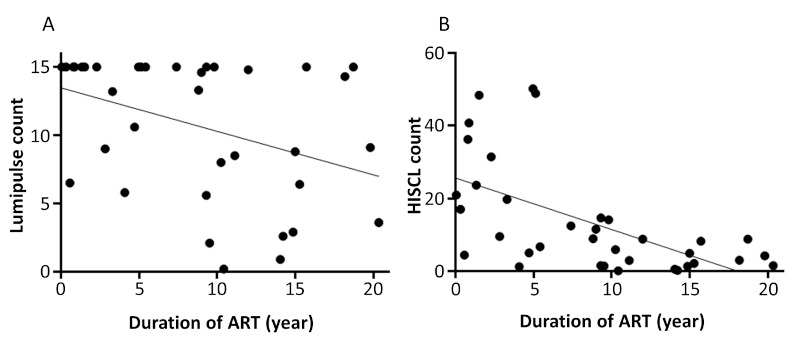
Correlation between duration of ART and (A) Lumipulse S HIVAg/Ab and (B) HISCL™-5000 HIV Ag+Ab using DBS samples. DBS samples were obtained from HIV+ patients on ART. (A) 36 DBS samples were analyzed by Lumipulse S HIVAg/Ab. (B) 36 DBS samples were analyzed by HISCL™-5000 HIV Ag+Ab.
Table 5. Comparison between DBS and plasma with low count Lumipulse S HIVAg/Ab or HISCL™-5000 HIV Ag+Ab samples.
| Sample | Sample status | DBS |
Plasma |
||
|---|---|---|---|---|---|
| Lumipulse count | HISCL count | Lumipulse count | HISCL count | ||
| AC | on ART | 0.2 | 0.1 | 8.7 | 33.6 |
| AD | acute infection | 0.8 | 0.3 | 11.9 | 20.8 |
| AE | acute infection | 4.7 | 0.7 | 15.0 | 44.1 |
| AF | on ART | 0.9 | 0.5 | 15.0 | 52.8 |
| AG | on ART | 2.6 | 0.2 | 15.0 | 43.6 |
ART: anti-retroviral treatment, DBS: dried blood spot.
Discussion
The aim of this study was to validate HIV testing with DBS samples derived from self-finger-prick and mailed via postal service. The main finding of the study was that the use of DBS is feasible for the screening of HIV infection, and that mailing via postal service self-collected DBS to the laboratory does not jeopardize the test reliability.
In this study, it is true that the use of DBS resulted in misdiagnosis of one HIV+ case whereas all cases were diagnosed correctly using the plasma samples. However, 36 of our HIV+ patients were on ART and their viral loads were undetectable. Although our analysis of the relationship between antibody titer and duration of ART was based on a limited number of patients, the results demonstrated a clear correlation between decrease in antibody titers and length of ART use. Other studies also reported low titers of anti-HIV antibodies in patients receiving ART (12,13). Thus, it was recommended that data of HIV+ patients on ART should be excluded from evaluation of sensitivity and specificity in HIV testing (14). This is one of the limitations of our study. Thus, we consider that HIV+ patients on ART should not be tested by DBS (3) in order to avoid misdiagnosis.
Another important aspect of our study is the use of a low cut-off value of 0.5. To elute HIV antigen/ antibody from the punched-out DBS containing 8 μL of whole blood (corresponding to 4 μL of plasma), we suspended DBS into 600 μL of PBS and used 100 μL of the supernatant for HIV testing (equivalent to 0.67 μL of plasma). Standard HIV testing requires 100 μL of plasma samples, i.e., 150 times dilution. Our titration study indicated that the LUM count of HIV testing with DBS was 237-times less compared with that of plasma. In our analysis of 50 HIV+ samples, the LUM counts of 3 samples (0.2, 0.8, and 0.9) and HIS counts of 5 samples (0.1, 0.2, 0.3, 0.5. and 0.7) were actually less than 1.0. Therefore, we tentatively lowered the cut-off value to 0.5. Based on additional analysis, we were able to estimate the appropriate cut-off value for DBS HIV testing. Based on the information supplied by the manufacturer, the actual sample volume used in each analysis is 100 μL by LUM and 30 μL by HIS, and the upper limit of LUM count is 15 and HIS 100, indicating that LUM seems to focus on the lower count. We believe that LUM could be better than HIS when the HIV testing system is applied using the DBS samples. Also, we used only two commercially available HIV testing systems, which is another limitation of our study, thus a larger study is needed to select the best system for the DBS HIV testing.
According to the manufacturer, the FTA™ DMPK cards are designed for analysis of drug metabolism and pharmacokinetics. The recommended procedure includes elution of DBS with methanol. In this study, DBS was eluted with PBS, which could have caused inefficient elution from the FTA™ DMPK-B Card based on its low LUM count. Although the FTA™ Classic Card and NucleoCard are intended to preserve nucleic acids, these filter papers seem to perform well with our method. In this study, we adopted Filter paper for blood collection (EIKEN CHEMICAL). The performance of this paper is equivalent to that of 903 Protein Saver Card, which had been used previously in many studies on HIV testing (5-11).
In a previous study, we examined the usefulness of DBS collected from MSM and mailed via postal service to the laboratory for HIV testing (15). The results demonstrated that the time between DBS collection and HIV testing was around 1 week (15). In the present study, the between-run experiment confirmed that LUM counts of samples stored at room temperature were relatively stable at least up to 28 days. Therefore, we believe that DBS tested by LUM is potentially suitable for mailed via postal service samples collected by self-finger-prick and that such a protocol could be a useful alternative HIV testing system.
In conclusion, the sensitivity of HIV testing with DBS was slightly lower compared to that of plasma. Therefore, DBS is not a replacement for plasma in standard HIV testing. In our study, a single unusual HIV+ case was misdiagnosed by the DBS/LUM system. Nonetheless, HIV testing with DBS can be potentially useful as a screening test in the situation of self-finger-prick and mailing via postal service. Applying that, people can receive HIV testing without visiting public health centers which will help in informing them of their HIV status easily and early.
Acknowledgements
We thank study participants who generously gave their blood for our study.
Funding:
This work was supported by Grants-in Aid for AIDS research from the Ministry of Health, Labor and Welfare (#H29-AIDS-G-001).
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. AIDS Prevention Information Network, Japan Foundation for AIDS Prevention. Number of HIV testing in local health centers, annual report for trends in AIDS. https://api-net.jfap.or.jp/status/japan/data/2019/nenpo/kensa.pdf (accessed June 26, 2021). (in Japanese) .
- 2. Ejima K, Koizumi Y, Yamamoto N, Rosenberg M, Ludema C, Bento AI, Yoneoka D, Ichikawa S, Mizushima D, Iwami S. HIV testing by public health centers and municipalities and new HIV cases during the COVID-19 pandemic in Japan. J Acquir Immune Defic Syndr. 2021; 87:e182-e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Consolidated guidelines on HIV testing services 2019. https://www.who.int/publications/i/item/978-92-4-155058-1 (accessed June 25, 2021).
- 4. Bertagnolio S, Parkin NT, Jordan M, Brooks J, Garcia- Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing. A review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010; 12:195-208 [PubMed] [Google Scholar]
- 5. Chang J, de Sousa A, Sabatier J, Assane M, Zhang G, Bila D, Vaz P, Alfredo C, Cossa L, Bhatt N, Koumans EH, Yang C, Rivadeneira E, Jani I, Houston JC. Performance characteristics of finger-stick dried blood spots (DBS) on the determination of human immunodeficiency virus (HIV) treatment failure in a pediatric population in Mozambique. PLoS One. 2017; 12:e0181054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernandez McPhee C, Alvarez P, Prieto L, Obiang J, Avedillo P, Vargas A, Rojo P, Abad C, Ramos JT, Holguin A. HIV-1 infection using dried blood spots can be confirmed by Bio-Rad Geenius™ HIV 1/2 confirmatory assay. J Clin Virol. 2015; 63:66-69. [DOI] [PubMed] [Google Scholar]
- 7. Grüner N, Stambouli O, Ross RS. Dried blood spots - preparing and processing for use in immunoassays and in molecular techniques. J Vis Exp. 2015; 97:52619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kania D, Bekale AM, Nagot N, Mondain AM, Ottomani L, Meda N, Traore M, Ouedraogo JB, Ducos J, Van de Perre P, Tuaillon E. Combining rapid diagnostic tests and dried blood spot assays for point-of-care testing of human immunodeficiency virus, hepatitis B and hepatitis C infections in Burkina Faso, West Africa. Clin Microbiol Infect. 2013; 19:E533-E541. [DOI] [PubMed] [Google Scholar]
- 9. Lofgren SM, Morrissey AB, Chevallier CC, Malabeja AI, Edmonds S, Amos B, Sifuna DJ, von Seidlein L, Schimana W, Stevens WS, Bartlett JA, Crump JA. Evaluation of a dried blood spot HIV-1 RNA program for early infant diagnosis and viral load monitoring at rural and remote healthcare facilities. AIDS. 2009; 23:2459-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo W, Davis G, Li L, Shriver MK, Mei J, Styer LM, Parker MM, Smith A, Paz-Bailey G, Ethridge S, Wesolowski L, Owen SM, Masciotra S. Evaluation of dried blood spot protocols with the Bio-Rad GS HIV Combo Ag/Ab EIA and Geenius™ HIV 1/2 Supplemental Assay. J Clin Virol. 2017; 91:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacGowan RJ, Chavez PR, Gravens L, Wesolowski LG, Sharma A, McNaghten AD, Freeman A, Sullivan PS, Borkowf CB, Michele Owen S, eSTAMP Study Group. Pilot evaluation of the ability of men who have sex with men to self-administer rapid HIV tests, prepare dried blood spot cards, and interpret test results, Atlanta, Georgia, 2013. AIDS Behav. 2018; 22:117-126. [DOI] [PubMed] [Google Scholar]
- 12. Stefic K, Novelli S, Mahjoub N, Seng R, Molina J-M, Cheneau C, Barin F, Chaix M-L, Meyer L, Delaugerre C, French National Agency for Research on AIDS and Viral Hepatitis (ANRS) PRIMO Study Group. Nonreactive human immunodeficiency virus type 1 rapid tests after sustained viral suppression following antiretroviral therapy initiation during primary infection. J Infect Dis. 2018; 217:1793-1797. [DOI] [PubMed] [Google Scholar]
- 13. Hayashida T, Gatanaga H, Tanuma J, Oka S. Effects of low HIV type 1 load and antiretroviral treatment on IgG-capture BED-enzyme immunoassay. AIDS Res Hum Retroviruses. 2008; 24:495-498. [DOI] [PubMed] [Google Scholar]
- 14. Johnson CC, Fonner V, Sands A, Ford N, Obermeyer CM, Tsui S, Wong V, Baggaley R. To err is human, to correct is public health: A systematic review examining poor quality testing and misdiagnosis of HIV status. J Int AIDS Soc. 2017; 20:21755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takano M, Iwahashi K, Satoh I, Araki J, Kinami T, Ikushima Y, Fukuhara T, Obinata H, Nakayama Y, Kikuchi Y, Oka S; HIV Check Study Group. Assessment of HIV prevalence among MSM in Tokyo using self-collected dried blood spots delivered through the postal service. BMC Infect Dis. 2018; 18:627. [DOI] [PMC free article] [PubMed] [Google Scholar]


