Abstract
Background
Symmetric dimethylarginine (SDMA) is a renal biomarker correlated with glomerular filtration rate (GFR).
Objectives
Describe changes in SDMA in clinically healthy foals and their mares during the first month postfoaling.
Animals
Convenience sampling of healthy periparturient Thoroughbred mares and their full‐term foals from a population of client‐owned horses.
Methods
Serum and EDTA whole blood samples were collected from mares in their last month of pregnancy and then from mares and foals at approximately <12 hours, 48 hours, 7 days, and 30 days postbirth. Samples were processed at a commercial reference laboratory for CBC and serum biochemistry, including SDMA concentrations.
Results
A total of 125 foals and 104 mares were included. Upper limits for SDMA concentrations in foals were above the adult horse reference interval for the first 20 or more days of life. Median SDMA concentrations decreased from 70 μg/dL (range, 7‐100 μg/dL) to 18 μg/dL (range, 6‐27 μg/dL) during the first 3 to 4 weeks of life. At birth, the SDMA concentration reference range was established as 0 to 100 μg/dL (upper limit of the assay); 0 to 85 μg/dL for 1 to 4 days old, 0 to 36 μg/dL for 5 to 10 days old, and 0 to 24 μg/dL for 20 to 30 days old. The upper reference limits for SDMA concentrations in mares did not differ from the general reference interval for adult horses. No correlation was identified between mare and foal SDMA concentrations (ρ = .06, P = .58).
Conclusions and Clinical Importance
Foal SDMA concentrations remained higher than the upper limit of the adult reference range and foals require a different reference range dependent on age.
Keywords: horse, kidney, neonate, renal
Abbreviations
- CI
confidence interval
- CKD
chronic kidney disease
- CSLI
Clinical and Laboratory Standards Institute
- GFR
glomerular filtration rate
- LC‐MS
liquid chromatography‐mass spectroscopy
- sCr
serum creatinine concentration
- SDMA
symmetric dimethylarginine
1. INTRODUCTION
Symmetric dimethylarginine (SDMA) is a structural isomer of asymmetric dimethylarginine and is eliminated by renal excretion. It was first isolated from human urine in 1970, which led to the 1997 discovery that concentrations of SDMA in both serum and urine correlated with the severity of renal insufficiency in nondialyzed patients with chronic kidney disease (CKD). 1 , 2 Dialysis‐dependent patients had even higher concentrations of circulating SDMA, which returned to baseline postkidney transplant. 2 As such, SDMA is highly correlated with multiple estimates of glomerular filtration rate (GFR). 3
In animal species, a retrospective study in cats found that an increase in SDMA concentrations above the reference interval corresponded with a 40% loss in GFR, or in some cases with even just a 25% decrease in GFR. 4 In this population, the increased SDMA concentration diagnosed CKD an average of 17 months earlier than did serum creatinine concentration (sCr). 4 Studies in dogs have produced similar results, with SDMA concentration increasing an average of 10 months earlier than sCr. 5 Other studies have shown that SDMA is an extremely specific biomarker of renal function, 6 , 7 unlike sCr which is much more dependent on muscle mass, as a major metabolite of muscle breakdown. On the contrary, SDMA appears to be minimally affected by muscle mass in dogs and cats. 8 , 9
A novel immunoassay has been developed that allows for quick and economical measurement of SDMA. 10 , 11 , 12 , 13 The assay for horses was validated in 2017 by comparison of results for 178 horses to the gold standard method, liquid chromatography‐mass spectrometry (LC‐MS). 14 This study confirmed that the IDEXX SDMA Test could accurately and specifically detect SDMA in equine blood. 14 After validation, a reference interval study on healthy adult horses was performed. IDEXX Laboratories, Inc followed the Clinical and Laboratory Standards Institute (CLSI) guidelines for determining the SDMA reference interval for horses. 15 Clinically healthy populations of adult horses of various breeds and sizes were enrolled into the study and the SDMA reference interval for horses was determined to be 0 to 14 μg/dL, 16 the same as seen in dog and cats. 10 Evaluation of 165 healthy draft horses also confirmed this reference interval for adult horses. 14 Measurement of SDMA has not yet been extensively evaluated in horses and a reference range has not been established for neonatal foals, although measurement in 17 foals aged 2 to 6 months using a nonspecies‐specific ELISA kit, yielded a median SDMA of 30.3 μg/dL (range, 15.15‐44.04 μg/dL), which is higher than observed in adults. 17 Our primary objective was to describe changes in SDMA concentrations in clinically healthy foals and their mares during the first month postfoaling. Although temporal changes in sCr in newborn healthy foals have been described previously, 18 , 19 sCr also was measured in our study to compare sCr with SDMA concentrations.
2. MATERIALS AND METHODS
2.1. Mares and foals
Convenience sampling of healthy periparturient mares and their full‐term foals (≥330 days) from a population of client‐owned horses was performed during the 2018 and 2019 foaling seasons. The mares and foals were from 4 different Thoroughbred breeding farms. All samples were obtained with the consent of the horse owner or farm manager. To ensure privacy, only the name of the mare and foal were collected. The study was approved by the IDEXX Laboratories Animal Welfare Review Committee and complies with its guidelines.
Mares in their last month of pregnancy (31‐1 day before foaling) were identified and a complete physical examination was performed and blood was collected. All mares were sampled once during this period and samples analyzed. After parturition, foals and their mares were examined at <12 hours of life and a complete history and physical examination performed and recorded. Gross placental evaluations also were performed as standard practice after foaling. Immunoglobulin G concentrations were measured by ELISA (SNAP Foal IgG Test, IDEXX Laboratories, Westbrook, ME) to determine adequacy of transfer of passive immunity. Foals with neutrophilia, neutropenia, electrolyte disturbances, or inadequate passive transfer of immunity (IgG concentration <800 mg/dL) were excluded. Foals with azotemia without concurrent clinical abnormalities were not excluded because of the occurrence of spurious hypercreatininemia in a subset of healthy foals. Foals that were determined to be healthy and their mares were examined at 3 additional timepoints, approximately 1 to 4 days, 5 to 10 days, and 20 to 34 days after birth.
2.2. Hematology and serum biochemistry
At each time period, 7 mL blood was collected from both mares and foals and samples were submitted to a diagnostic laboratory for hematology, serum abiochemistry including SDMA concentration and sCr. The SDMA concentration was determined using a commercially available high‐throughput immunoassay validated for horses (IDEXX SDMA Test, IDEXX Laboratories, Inc, Westbrook, ME). Serum creatinine concentrations were determined by a colorimetric method with Jaffe's reaction using picrate at alkaline pH (Beckman Coulter, Inc, Diagnostics Division, Brea, CA).
2.3. Statistical analysis
Data were analyzed using R version 3.6.2. 20 Bootstrapped upper 95 percentiles were calculated in accordance with CLSI guidelines for reference intervals to inform expectations for this dynamic population. 15 These were calculated for mares and foals using Box Cox‐Transformed SDMA and sCr results at study time points, and 95% confidence intervals (95% CI) were calculated from the 100 000 bootstrapped samples. Outliers were examined using Tukey's fences, but this method suggested removal of 15% of foals and 25% of mares. A visual examination was used to identify extreme SDMA and sCr results that then were reviewed by a veterinarian, and aberrant results were removed. Primarily, individual observations, and not all results from the animal, were removed for foals and mares where the observed SDMA or sCr results were extreme outliers and for which incomplete data was collected, because these results could not be definitively determined to be from a healthy population. Correlation of foal‐to‐mare SDMA and sCr were assessed using Spearman's rank correlation (ρ) with a significance threshold of P < .05.
3. RESULTS
3.1. Mares and foals
A total of 125 individual Thoroughbred foals and 104 individual Thoroughbred mares were included in the study after outlier removal. There were 46 female and 49 male foals; sex was not listed for 30 foals. Each mare or foal was sampled at least once during the study period. Based on the availability of staff and the nature of foaling, some foals were not sampled at birth but were sampled at subsequent timepoints. No foals or mares in the study died, but foals and mares were lost to follow‐up because of movement of mares or foals to other farms or states. Poor sample quality and lack of sample volume also resulted in the loss of some samples. These losses occurred sporadically through the 2‐year study period.
3.2. Removal of outliers
Seven SDMA and sCr results from 6 different animals were removed from the analysis. Samples removed and the justification for removal are presented in Table S1 and illustrated in Figure S1. An a posteriori approach to outlier removal was considered appropriate for the study. Little was known about the population expected results, and exclusion criteria were established after data collection for the reference interval. 15 , 21
3.3. SDMA and sCr expected ranges
Timepoint‐specific sample sizes, median concentrations of SDMA, and sCr and reference upper limits with their 95% CI are presented in Table 1. Changes in foal and mare SDMA concentrations and sCr over time are presented in Figures 1 and 2. Upper limits for foal SDMA concentrations were above the adult horse reference interval (>14 μg/dL) for the first 20 or more days of life. Median SDMA concentrations decreased from 70 to 18 μg/dL during the first 3 to 4 weeks of life (Figure 1A). Upper limits of sCr in foals were above the adult horse reference interval (>1.8 mg/dL) only on samples collected within 12 hours of birth. Most sCr results rapidly decreased to within the adult horse reference interval within the first 4 days of life (Figure 1B). Unlike foals, the upper reference limits for mare SDMA and sCr did not differ appreciably from the general adult horse reference intervals (Figure 2).
TABLE 1.
Timepoint‐specific sample sizes, median concentrations of SDMA and sCr, and reference interval upper limits with their 95% CI for mares and foals
| Time point | Foal | Mare | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median concentrations | Upper limit of RI | N | Median concentrations | Upper limit of RI | |||||
| SDMA μg/dL (range) | sCr mg/dL (range) | SDMA μg/dL (95% CI) | sCr mg/dL (95% CI) | SDMA μg/dL (range) | sCr mg/dL (range) | SDMA μg/dL (95% CI) | sCr mg/dL (95% CI) | |||
| −14 to − 31 d | – | – | – | – | – | 33 | 10 (8‐17) | 1.2 (1.0‐1.9) | 15 (12‐17) | 1.7 (1.5‐1.9) |
| −13 to − 7 d | – | – | – | – | – | 28 | 10 (6‐20) | 1.2 (0.8‐1.8) | 14 (11‐20) | 1.6 (1.3‐1.8) |
| −6 to − 1 d | – | – | – | – | – | 18 | 9 (5‐19) | 1.2 (0.7‐1.5) | 14 (12‐19) | 1.5 (1.3‐1.5) |
| Birth | 112 | 70 (7‐100) | 1.8 (1‐7.9) | 100 (100‐100) | 2.9 (2.6‐3.9) | 79 | 10 (3‐20) | 1.0 (0.6‐2.2) | 14 (13‐19) | 1.6 (1.3‐1.9) |
| 1‐4 d | 87 | 49 (5‐100) | 1.1 (0.6‐3.3) | 85 (74‐96) | 1.4 (1.3‐1.9) | 61 | 9 (2‐20) | 0.8 (0.6‐1.4) | 13 (11‐15) | 1.1 (1.0‐1.3) |
| 5‐10 d | 80 | 26 (5‐37) | 0.9 (0.5‐1.5) | 36 (33‐37) | 1.1 (1.1‐1.2) | 61 | 8 (3‐17) | 0.8 (0.6‐1.6) | 12 (10‐16) | 1.2 (1.0‐1.2) |
| 20+ d | 78 | 18 (6‐27) | 1.0 (0.6‐2.0) | 24 (22‐27) | 1.5 (1.3‐1.7) | 54 | 7 (1‐15) | 0.8 (0.6‐1.1) | 11 (9‐13) | 1.0 (0.9‐1.1) |
Abbreviations: CI, confidence interval; RI, reference interval; sCr, serum creatinine; SDMA, symmetric dimethylarginine.
FIGURE 1.
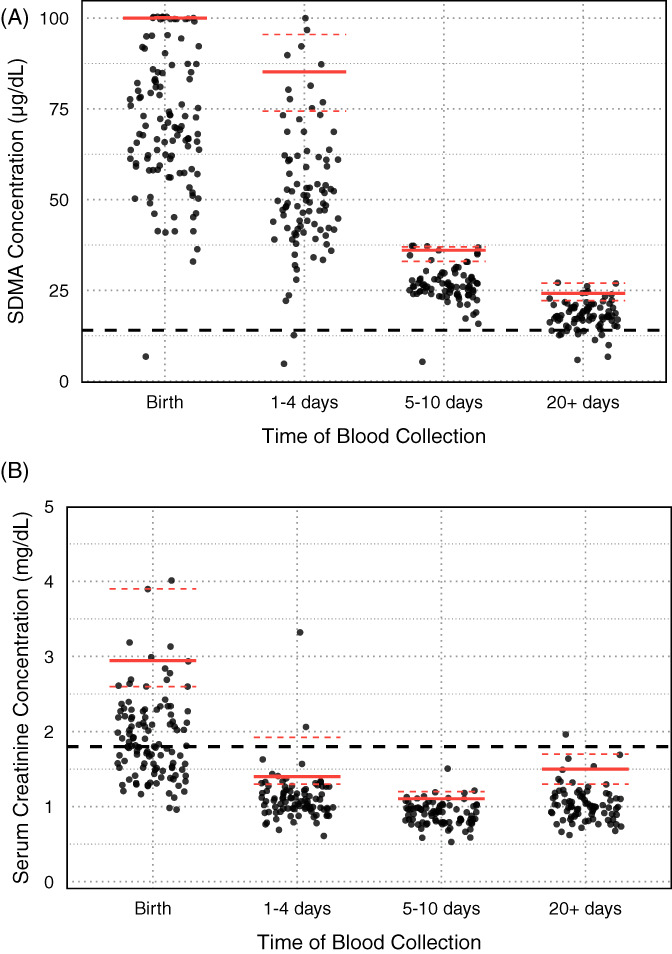
Foal symmetric dimethylarginine (SDMA) and serum creatinine concentrations (sCr) at the different age ranges throughout the study period. The upper reference limits are illustrated with the red lines for each age range, with the dashed red lines representing the 95% confidence intervals. (A) Foal SDMA concentrations. The dashed black line represents the upper limit of the adult reference range (14 μg/dL). (B) Foal sCr. The dashed black line represents the upper limit of the adult reference range (1.8 mg/dL)
FIGURE 2.
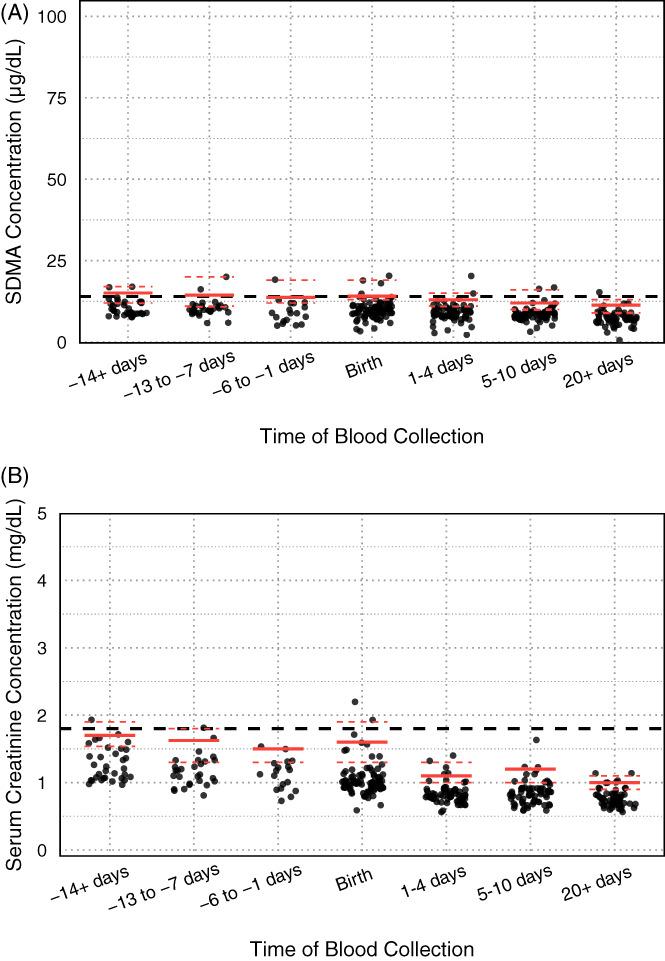
Mare symmetric dimethylarginine (SDMA) and serum creatinine concentrations (sCr) at the different age ranges throughout the study period. The upper reference limits are illustrated with the red lines for each age range, with the dashed red lines representing the 95% confidence intervals. (A) Mare SDMA concentrations. The dashed black line represents the upper limit of the adult reference range (14 μg/dL). (B) Mare sCr. The dashed black line represents the upper limit of the adult reference range (1.8 mg/dL)
Correlations between paired mare and foal SDMA and sCr are presented in Figure 3. In this population, no correlation was seen between mare and foal SDMA (ρ = .06, P = .58) or between mare and foal sCr (ρ = .13, P = .27).
FIGURE 3.
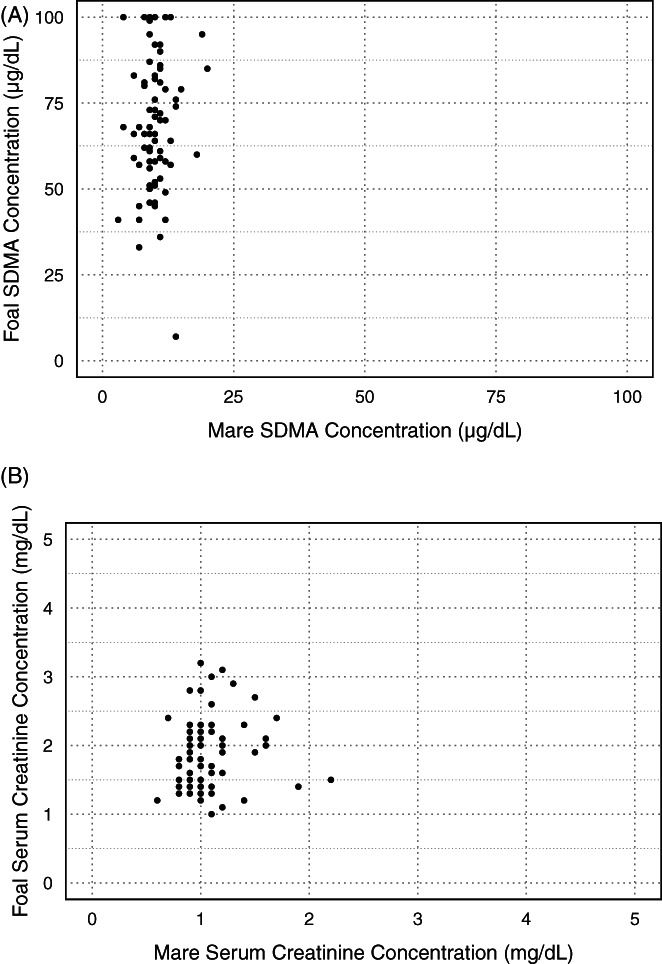
Correlation between paired mare and foal symmetric dimethylarginine (SDMA) and serum creatinine concentrations (sCr) at birth. (A) SDMA, illustrating no evidence of correlation (ρ = .06, P = .58). (B) SCr, illustrating no evidence of correlation (ρ = .13, P = .27)
4. DISCUSSION
With samples collected from 125 foals, the reported results can be used to establish a reference range for foals over the first month of life. Our results suggest that healthy neonatal foals have higher SDMA concentrations than adult horses throughout the study period, although the upper limit of the reference range appears to decrease with age during that time. The change over time in the calculated upper reference limit from our study defines an expectation for how SDMA concentrations should decrease in the first month of life, and foals with SDMA concentrations above these limits might require increased monitoring or treatment. At birth, the upper limit of the reference range is reported as 100 μg/dL, which is the upper limit of the assay. Measurement above this concentration is not commercially available, but it would be interesting to establish a more accurate upper limit for this timepoint. The upper limit of the reference range for foals from 20 to 30 days old was established as 24 μg/dL. Samples from older foals are required to determine when SDMA concentrations decrease to the adult reference concentration of 14 μg/dL. No correlation was found between mare and foal SDMA concentrations, and it does not appear that placental or colostral transfer of SDMA occurs.
The sCr in our study suggest that sCr in neonatal foals can be higher than the reference upper limit for adult horses. This finding has been reported previously, and is most commonly a result of spurious hypercreatininemia, which is associated with placental insufficiency or fetal stress. 19 In these cases, sCr usually decreases by approximately 50% within 24 hours and normalizes by 72 hours. 19 Furthermore, as foals usually do not urinate until they are between 6 and 12 hours old, the clearance of endogenous creatinine is delayed. Our findings support previous findings, that within the first few days of life sCr should be within the adult reference range.
Reasons for the higher SDMA concentrations in foals are not currently understood, but might be a result of a difference in arginine metabolism. Alternatively, foals might have incomplete nephrogenesis (which would be supported by the higher sCr also noted at birth), but previously renal function of neonatal foals, as assessed by GFR, has been shown to be similar to that of adult horses. 22 Fractional electrolyte clearance results in foals are similar to those of adult horses, supporting that renal tubular function in foals is similar to that of adult horses.
The upper limit of the SDMA reference range for puppies and kittens is 16 μg/dL (although 85%‐90% of results lie within the adult reference range of <14 μg/dL). 23 The slightly higher upper reference limit in younger animals of these species may be associated with the physiological needs for increased arginine methylation in growing animals, resulting in increased SDMA generation. Puppies achieve the adult SDMA reference interval by approximately 1 year of age although the exact age depends on breed and size of the animal (small breed dogs reach the adult reference range at approximately 6 months of age but it may take up to 2 years in larger breeds). 23
Unfortunately, it was not practical in this field setting to collect urine samples or measure GFR, which are limitations of our study. Not all foals or mares were sampled at each timepoint because of movement of the mares or foals to other farms or states, and because of the nature of clinical practice, sampling times varied resulting in a range of days for each sampling period. Data for our study do not constitute a rigorous reference interval determination. Study foals and mares were all a single breed and from 4 breeding farms, which may limit the generalizability of our findings. However, every effort was taken to select for a healthy sample of mares and foals, and analytical methods followed the CLSI recommendations for the establishment of reference intervals. In the absence of other temporal reference intervals, our findings can serve as guidelines for evaluation of renal function in neonatal foals and mares.
In conclusion, our results suggest that SDMA reference intervals for foals are higher than those of adults, are age‐dependent, and do not reach the adult reference range during the first month of life. In contrast, upper limits of foal sCr were only above the adult horse reference interval for the first 12 hours after birth and rapidly decreased thereafter. Additional studies are required to sample older foals and determine when SDMA concentrations reach the adult reference interval as well as to assess how SDMA concentrations differ in foals with kidney injury.
CONFLICT OF INTEREST DECLARATION
Rebecca Mack, Michael Coyne, Rachel Murphy, Evan Hegarty have an affiliation with the commercial funders of this research as current employees of IDEXX Laboratories, Inc (https://www.idexx.com/en/about-idexx/).
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approval from IDEXX Laboratories Animal Welfare Review Committee.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1 Samples removed following outlier analysis. (A) Foal symmetric dimethylarginine (SDMA) concentration. (B) Foal serum creatinine concentration (sCr). (C) Mare SDMA concentration.
Table S1 Samples removed following outlier analysis.
ACKNOWLEDGMENT
Funding provided by IDEXX Laboratories, Inc. The data was presented at the 2019 American College of Veterinary Internal Medicine Forum in Phoenix, AZ; and at the 2019 American Association of Equine Practitioners Forum in Denver, CO.
Bozorgmanesh R, Thornton J, Snyder J, et al. Symmetric dimethylarginine concentrations in healthy neonatal foals and mares. J Vet Intern Med. 2021;35(6):2891-2896. doi: 10.1111/jvim.16295
Funding information IDEXX Laboratories, Inc
Contributor Information
Rana Bozorgmanesh, Email: rb@hagyard.com.
Michael Coyne, Email: Michael-Coyne@idexx.com.
REFERENCES
- 1. Kakimoto Y, Akazawa S. Isolation and identification of N‐G,N‐G‐ and N‐G,N′‐G‐dimethyl‐arginine, N‐epsilon‐mono‐, di‐, and trimethyllysine, and glucosylgalactosyl‐ and galactosyl‐delta‐hydroxylysine from human urine. J Biol Chem. 1970;245(21):5751‐5758. [PubMed] [Google Scholar]
- 2. Fleck C, Schweitzer F, Karge E, Busch M, Stein G. Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in patients with chronic kidney diseases. Clin Chim Acta. 2003;336(1–2):1‐12. [DOI] [PubMed] [Google Scholar]
- 3. Kielstein JT, Salpeter SR, Bode‐Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function—a meta‐analysis. Nephrol Dial Transplant. 2006;21(9):2446‐2451. [DOI] [PubMed] [Google Scholar]
- 4. Hall JA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med. 2014;28(6):1676‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall JA, Yerramilli M, Obare E, Yerramilli M, Almes K, Jewell DE. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J Vet Intern Med. 2016;30(3):794‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall JA, Yerramilli M, Obare E, Yerramilli M, Yu S, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L‐carnitine, and medium‐chain triglycerides. Vet J. 2014;202(3):588‐596. [DOI] [PubMed] [Google Scholar]
- 7. Yerramilli M, Yerramilli M, Obare E, Jewell DE, Hall JA. Prognostic value of symmetric dimethylarginine (SDMA) to creatinine ratio in dogs and cats with chronic kidney disease (CKD). J Vet Intern Med. 2015;29(4):1274. [Google Scholar]
- 8. Hall JA, Yerramilli M, Obare E, Yerramilli M, Melendez LD, Jewell DE. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med. 2015;29(3):808‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szlosek D, Robertson J, Quimby J, et al. A retrospective evaluation of the relationship between symmetric dimethylarginine, creatinine and body weight in hyperthyroid cats. PLoS One. 2020;15(1):e0227964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Relford R, Robertson J, Clements C. Symmetric dimethylarginine: improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin Small Anim Pract. 2016;46(6):941‐960. [DOI] [PubMed] [Google Scholar]
- 11. Patch D, Obare E, Prusevich P, et al. High throughput immunoassay for kidney function biomarker symmetric dimethylarginine (SDMA) [abstract]. Clin Chem. 2015;16:S135. [Google Scholar]
- 12. Prusevich P, Patch D, Obare E, et al. Validation of a novel high throughput immunoassay for the quantitation of symmetric dimethylarginine (SDMA) [abstract]. Clin Chem. 2015;16:S135. [Google Scholar]
- 13. Nabity MB, Lees GE, Boggess MM, et al. Symmetric dimethylarginine assay validation, stability, and evaluation as a marker for the early detection of chronic kidney disease in dogs. J Vet Intern Med. 2015;29(4):1036‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schott HC, Gallant LR, Coyne M, et al. Symmetric dimethylarginine and creatinine concentrations in serum of healthy draft horses. J Vet Intern Med. 2021;35(2):1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horowitz GL. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory: Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 16. SDMA Test Available for Equine Patients . IDEXX US [Internet]. https://www.idexx.com/en/equine/equine-reference-laboratories/test-menu/sdma-equine/. Accessed April 28, 2021.
- 17. Siwinska N, Zak A, Slowikowska M, Niedzwiedz A, Paslawska U. Serum symmetric dimethylarginine concentration in healthy horses and horses with acute kidney injury. BMC Vet Res. 2020;16(1):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards D, Brownlow M, Hutchins D. Indices of renal function: values in eight normal foals from birth to 56 days. Aust Vet J. 1990;67(7):251‐254. [DOI] [PubMed] [Google Scholar]
- 19. Chaney KP, Holcombe SJ, Schott HC, Barr BS. Spurious hypercreatininemia: 28 neonatal foals (2000‐2008). J Vet Emerg Crit Care. 2010;20(2):244‐249. [DOI] [PubMed] [Google Scholar]
- 20. R Core Team . R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R-project.org/. Accessed 2019. [Google Scholar]
- 21. Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem Med. 2016;26:5‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holdstock NB, Ousey JC, Rossdale PD. Glomerular filtration rate, effective renal plasma flow, blood pressure and pulse rate in the equine neonate during the first 10 days post partum. Equine Vet J. 1998;30(4):335‐343. [DOI] [PubMed] [Google Scholar]
- 23. Pediatric reference interval for IDEXX SDMA Test . IDEXX Canada [Internet]. https://ca.idexx.com/en-ca/veterinary/reference-laboratories/sdma/sdma-reference-interval-update/. Accessed April 28, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Samples removed following outlier analysis. (A) Foal symmetric dimethylarginine (SDMA) concentration. (B) Foal serum creatinine concentration (sCr). (C) Mare SDMA concentration.
Table S1 Samples removed following outlier analysis.


