Abstract
Background
Dietary protein and phosphorus (P) restriction is the mainstay for nutritional management of chronic kidney disease (CKD). However, adequate restriction levels for cats with early CKD remain unclear.
Objectives
To investigate responses in cats with early CKD to varying dietary protein, P, and calcium (Ca) : P ratio.
Animals
Nineteen research colony cats with International Renal Interest Society stages 1‐2 CKD.
Methods
In an opportunistic longitudinal case study, cats were fed a low protein (59 g/Mcal), low P (0.84 g/Mcal) dry diet (LP‐LP; Ca : P = 1.9) for 18 months and later transitioned onto a moderate protein (76‐98 g/Mcal), moderate P (1.4‐1.6 g/Mcal) dry‐wet diet regimen (MP‐MP; Ca : P = 1.4‐1.6) for 22 months. Fold‐changes in serum creatinine, total Ca (tCa) and P (primary outcomes) and fibroblast growth factor 23 (FGF23) were assessed by linear‐mixed models.
Results
While feeding LP‐LP, mean serum creatinine decreased (0.87‐fold, 95% confidence interval [CI] 0.81, 0.93, P < .001) to within reference range after 6 months, while increases in total Ca (tCa; 1.16‐fold, 95% CI 1.11, 1.22, P < .001) and FGF23 (2.72‐fold, 95% CI 1.72, 4.31, P < .001), but not in P (1.03‐fold, 95% CI 0.945, 1.124, P = .94), were observed after 17 months. On MP‐MP, mean creatinine, tCa and P remained within reference ranges and did not significantly change (P = .11, P = .98, and P = 1, respectively), while FGF23 significantly decreased (0.58‐fold, 95% CI 0.36, 0.95, P = .02) after 22 months.
Conclusions and Clinical Importance
Cats with early CKD developed hypercalcemia after long‐term feeding of a highly P‐restricted diet. Increasing dietary P and reducing Ca : P ratio maintained renal markers, while improving Ca‐P balance. Cats with early CKD could benefit from moderately protein‐ and P‐restricted diets.
Keywords: feline CKD, FGF23, hypercalcemia, IRIS 1‐2, phosphate
Abbreviations
- AAFCO
Association of American Feed Control Officials
- BUN
blood urea nitrogen
- CI
confidence interval
- CKD
chronic kidney disease
- FGF23
fibroblast growth factor 23
- GFR
glomerular filtration rate
- HCM
hypertrophic cardiomyopathy
- IRIS
International Renal Interest Society
- MAP
magnesium ammonium phosphate
- MER
maintenance energy requirement
- P
phosphorus
- PTH
parathyroid hormone
- RS
RIM sign
- RSS
relative super saturation
- SDMA
symmetric dimethylarginine
- UPC
urine protein to creatinine ratio
- USG
urine specific gravity
1. INTRODUCTION
Chronic kidney disease (CKD) is a common clinical condition in cats characterized by increased phosphorus (P) retention and protein catabolites. This can result in malaise, hypertension, anemia, and shortened life expectancy. The introduction of new diagnostic/screening approaches (eg, RenalTech, RenalDetect, Antech SDMA [www.antechdiagnostics.com/renaltech]) 1 has increased the ability to detect early CKD and improve the prognosis and welfare of cats by enabling measures to slow disease progression. The prevalence of International Renal Interest Society (IRIS) stages 1 (nonazotemic) and 2 CKD in the general cat population is 38% to 41% in cats up to 10 years, reaching 71% in cats >15 years. 2 Longitudinal studies evaluating healthy (nonazotemic) cats >9 years 3 , 4 over a 1‐year period indicate that 15% to 30% of cats had renal impairment before development of azotemic CKD. During early CKD, impaired function is compensated for by increased glomerular pressure and filtration rate (GFR) via activation of the renin‐angiotensin‐aldosterone system, 5 while calcium (Ca) and P balance is maintained through the stimulation of regulatory hormones fibroblast growth factor 23 (FGF23) and parathyroid hormone (PTH). 6 , 7 , 8 However, over the long term, these compensatory mechanisms might become mal‐adaptive, contributing to progressive renal damage in cats 5 and cardiovascular disease in humans. 9 Mineral disorders associated with CKD include both hypocalcemia, because of decreased renal synthesis of calcitriol, and hypercalcemia. 10 Total hypercalcemia occurs in up to 32% of cats with CKD 10 , 11 and is related to decreased GFR and increased tubular reabsorption in response to PTH, FGF23, calcitonin, and calcitriol in humans. 12 Idiopathic hypercalcemia occurs in healthy cats from 2 to 13 years (mean 6 years) 13 affecting 12.6% of cats diagnosed with ionized hypercalcemia. 14 In older cats (median 14 years) followed up over a 1‐year period there is a higher incidence rate of total hypercalcemia in cats with CKD compared with age‐matched apparently healthy cats (0.18 vs 0.03 per patient‐year). 11
Nutritional management of CKD relies on P and protein restriction in the azotemic stages of the disease. 15 Clinical renal diets for azotemic cats are effective in reducing the frequency of uremic crises, 16 , 17 reducing plasma FGF23, P, and PTH, 18 and increasing survival time. 19 However, the long‐term feeding effects of highly‐restricted protein and P renal diets on mineral homeostasis and renal function in early CKD remains uncertain. In azotemic cats (IRIS 2 and 3), 20 feeding a low P diet below the minimum of 1.25 g/Mcal established by the Association of American Feed Control Officials (AAFCO) 21 and containing high Ca : P ratios has been associated with development of total and ionized hypercalcemia in 13% of cats after 3 to 5 months. An improved understanding of Ca and P regulation across CKD stages could help refine dietary management of the condition at early stages.
After a previous study, 6 an opportunity presented itself to assess the long‐term effects of feeding 2 clinical diets varying in dietary protein, P, Ca, and Ca : P ratio on total Ca, P, and FGF23 in cats with IRIS stage 1 and 2 CKD as a longitudinal case study. The aim of this study was to present the effects of these 2 diets on CKD progression and biochemical markers of renal function and Ca‐P balance.
2. MATERIALS AND METHODS
This work was overseen and approved by the WALTHAM Animal Welfare and Ethical Review Body (Project Portfolio Management No. 55429) and conducted under the authority of the Animals (Scientific Procedures) Act 1986.
2.1. Cats
Nineteen domestic short‐haired neutered cats (9 males; 10 females) with a median age of 4.9 years (2.4‐9.8) were enrolled. Impaired renal function, characterized by altered renal ultrasonographic imaging (all cats) and >25% decrease in GFR (10/19) was observed after participation in another study investigating dietary P in healthy cats. Feeding an experimental diet containing 4.8 gP/Mcal (3.6 g/Mcal as inorganic P [iP] from sodium dihydrogen phosphate) and Ca : P = 0.6 for 4 weeks 6 caused changes consistent with CKD as confirmed by serum creatinine >1.6 mg/dL alongside (a) urine specific gravity (USG) <1.035 (10/19), (b) urine albumin to creatinine ratio (UAC) >17 mg/g, corresponding to urine protein to creatinine ratio (UPC) of 0.2‐0.4 22 (6/19), or (c) >40% decline in GFR (2/19). In 1 cat with serum creatinine <1.6 mg/dL renal proteinuria (UAC = 42 mg/g; corresponding to UPC of 0.4‐0.5) supported renal damage. Following diagnosis, cats were transitioned onto an experimental low protein (68 g/Mcal) dry diet with P and Ca <1.25 and <1.5 g/Mcal, respectively (prefeed diet; Table 1) for 5 months to allow clinical stabilization. International Renal Interest Society stage CKD was assessed based on serum creatinine and symmetric dimethylarginine (SDMA) measured after 2 and 3 months on the prefeed diet and substaged by proteinuria, systolic blood pressure, and renal ultrasonographic changes measured after 3 months (Table 2). Except for 1 cat with IRIS 1 CKD, the remaining 18 cats had IRIS 2 CKD (mean creatinine of 2.0 [1.6‐2.4] mg/dL and SDMA of 14.2 [9.5‐20.5] μg/dL), 2 of them diagnosed with renoliths. Cats were group housed, except during feeding or urine collection periods, during which they were individually housed in multilevel lodges (2 m high × 0.9 m wide × 1.8 m deep).
TABLE 1.
Nutrient composition of the prefeed and 2 commercial clinical renal diets containing low protein low phosphorus (LP‐LP) and moderate protein moderate phosphorus (MP‐MP)
| Prefeed | Commercial LP‐LP | Commercial MP‐MP | ||||||
|---|---|---|---|---|---|---|---|---|
| Dry | Dry a | Wet b | Dry c | Wet d | ||||
| Batch 1 | Batch 2 | Batch 1 | Batch 2 | Batch 1 | Batch 2 | |||
| Nutrients, per Mcal | ||||||||
| Moisture, g | 12.5 | 14.1 | 10.4 | 725 | 14.7 | 17.1 | 919 | 828 |
| Protein, g | 68.1 | 59.7 | 58.3 | 68.9 | 78 | 73 | 101 | 94.2 |
| Fat, g | 39.3 | 42.7 | 43.9 | 66.1 | 35.3 | 36.6 | 48.4 | 51.9 |
| Crude fiber, g | 5.5 | 10.9 | 10.7 | 8.36 | 12.9 | 12.1 | 15 | 15.9 |
| Calcium (Ca), g | 1.33 | 1.68 | 1.56 | 1.26 | 2.09 | 2.36 | 2.12 | 2.25 |
| Phosphorus (P), g | 1.23 | 0.96 | 0.72 | 0.81 | 1.35 | 1.45 | 1.6 | 1.59 |
| Inorganic P e , g | 0.06 | 0.32 | 0.32 | 0.25 | 0.48 | 0.48 | 0.17 | 0.17 |
| Ca : P ratio | 1.08 | 1.74 | 2.15 | 1.57 | 1.55 | 1.63 | 1.32 | 1.42 |
| Na, g | 3.11 | 1.02 | 0.99 | 0.93 | 1.05 | 1.12 | 1.2 | 1.15 |
| Mg, mg | 400 | 178 | 182 | 100 | 169 | 223 | 164 | 162 |
| Vitamin D3, IU | 254 | 277 | 275 | 389 | 214 | 235 | 474 | 541 |
| PME, kcal/100 g as fed | 399 | 394 | 403 | 104 | 380 | 380 | 86.8 | 94.5 |
Abbreviation: PME, predicted metabolizable energy by NRC (2006) equation based on proximate analysis and modified Atwater factors.
Maize flour, rice, animal fats, wheat gluten, soya protein isolate, vegetable fibers, maize, maize gluten, hydrolyzed animal proteins, minerals, chicory pulp, dehydrated poultry protein, fish oil, soya oil, fructo‐oligo‐saccharides, psyllium husks and seeds, marigold extract (source of lutein). Technological additives: 10 g/kg of Clinoptilolite of sediment origin.
Guaranteed nutrient analysis of Royal Canin Veterinary Feline Renal wet recipe.
Maize, wheat gluten, maize flour, dehydrated poultry protein, wheat, maize gluten, animal fats, rice, vegetable fibers, hydrolyzed animal proteins, chicory pulp, fish oil, soya oil, minerals, tomato, psyllium husks and seeds, fructo‐oligo‐saccharides, New Zealand green‐lipped mussel extract (0.3%), hydrolyzed yeast, hydrolyzed crustaceans, borage oil, marigold extract, hydrolyzed cartilage.
Pork and chicken meats, pork and chicken livers, wheat flour, cellulose, wheat gluten, minerals, sunflower oil, fish oil, tomato powder (source of lycopen), lecithin, egg white dried, taurine, gelling agents, yeast hydrolysate, marigold meal, L‐carnitine, hydrolyzed crustacean, hydrolyzed cartilage, vitamins.
Target levels for P coming from soluble salts.
TABLE 2.
Prevalence of clinical findings in cats staged as IRIS 1 or 2 before and at the end of each diet intervention a
| LP‐LP dry (months 1‐17) | MP‐MP dry‐wet (months 22‐43) | |||
|---|---|---|---|---|
| (Month 2) | (Month 17) | (Month 21) | (Month 43) | |
| Age, median (min, max), years | 4.9 (2.4‐9.8) | 7.0 (3.7‐11.1) | 7.3 (4.0‐11.4) | 9.2 (5.9‐13.3) |
| BCS 4‐5 | ND | 2 | 1 | 12 |
| BCS 6‐7/8‐9 | ND | 9/6 | 8/7 | 3/0 |
| IRIS 1 CKD | 1/19 | 6/17 | 5/16 | 11/15 |
| Adult | 0 | 4 | 3 | 7 |
| Senior (≥9 years) | 1 | 2 | 2 | 4 |
| IRIS 2 CKD | 18/19 | 11/17 | 11/16 | 4/15 |
| Adult | 15 | 6 | 6 | 0 |
| Senior (≥9 years) | 3 | 5 | 5 | 4 |
| Systolic hypertension >160 mm Hg b | 6/19 | 4/17 | 4/16 | 4/15 |
| IRIS 1 | 0 | 0 | 2 | 4 |
| IRIS 2 | 6 | 4 | 2 | 0 |
| Borderline proteinuria b , c | 2/19 | 4/17 | ND | 4/15 |
| IRIS 1 | 0 | 1 | 1 | |
| IRIS 2 | 2 | 3 | 3 | |
| Increased renal echogenicity | 4/19 | 5/17 | ND | 7/15 |
| IRIS 1 | 1 | 1 | 1 | |
| IRIS 2 | 3 | 4 | 6 | |
| RIM sign | 18/19 | 16/17 | ND | 15/15 |
| IRIS 1 | 1 | 1 | 5 | |
| IRIS 2 | 17 | 15 | 10 | |
| Altered shape/size | 1/19 | 1/17 | ND | 4/15 |
| IRIS 1 | 0 | 0 | 1 | |
| IRIS 2 | 1 | 1 | 3 | |
| Reduced cortico‐medullar definition | 2/19 | 2/17 | ND | 5/15 |
| IRIS 1 | 0 | 0 | 0 | |
| IRIS 2 | 2 | 2 | 5 | |
| Renoliths‐ureteroliths | 2‐0/19 | 2‐0/17 | ND | 1‐1/15 |
| IRIS 1 | 0 | 0 | 0 | |
| IRIS 2 | 2 | 2 | 2 | |
| Uroliths | 0/19 | 6/17 | ND | 1/15 |
| IRIS 1 | 0 | 0 | 0 | |
| IRIS 2 | 0 | 6 | 1 | |
| Total hypercalcemia, >11.8 mg/dL | 0/19 | 5/17 | 2/16 | 0/15 |
| IRIS 1 | 0 | 0 | 0 | 0 |
| IRIS 2 | 0 | 5 | 2 | 0 |
| Ionized hypercalcemia, >5.41 mg/dL | 0/19 | 13/17 | 7/16 | 3/15 |
| IRIS 1 | 0 | 1 | 1 | 2 |
| IRIS 2 | 0 | 12 | 6 | 1 |
| CaOx RSS > 12.5 | ||||
| In cats without renoliths | 0/17 | ND | ND | 0/12 |
| In cats with renoliths | 0/2 | 2/2 | ND | 0/2 |
| MAP RSS > 2.5 | ||||
| In cats without renoliths | 2/17 | ND | ND | 0/12 |
| In cats with renoliths | 1/2 | 0/2 | ND | 0/2 |
Abbreviations: BCS, body condition score; CaOx, calcium oxalate; IRIS, International Renal Interest Society; LP‐LP, low protein low P; ND, not determined; MAP, magnesium ammonium phosphate; MP‐MP, moderate protein moderate P; RSS, relative supersaturation; UAC, urine albumin to creatinine ratio; UPC, urine protein to creatinine ratio.
IRIS stage based on serum creatinine and SDMA and substaged by UPC, systolic blood pressure, and renal imaging.
Number of cases reported during each dietary intervention.
Based on UAC at prefeed (≥30 and <300 in 2 cats) 22 and on UPC (0.2‐0.3) during the study.
2.2. Study design
After the prefeed period, cats were clinically monitored over a 43‐month opportunistic longitudinal case study. Two dietary interventions were made in response to changes in clinical variables with a view to maintain their health. Routine hematology, biochemistry, urinalysis, and serial (n = 5) arterial blood pressure measurements along with GFR and Ca‐P regulatory hormones (PTH, FGF23, and vitamin D) were assessed regularly within each dietary intervention (Figure 1). Kidney ultrasonographic imaging and occurrence of urolithiasis/renoliths was assessed once a year. Urine relative super saturation (RSS) for calcium oxalate (CaOx) and magnesium ammonium phosphate (MAP) was determined at study start and end and monitored throughout the study in the 2 cats with renoliths. International Renal Interest Society stage of CKD was reassessed at the end of each dietary intervention. Body weight (BW) was measured weekly and body condition score (BCS) monthly from month 6 onwards by a 1 to 9 point algorithm. 23 Cats received a veterinary health check at least once every 6 months and were observed daily by experienced animal technicians.
FIGURE 1.
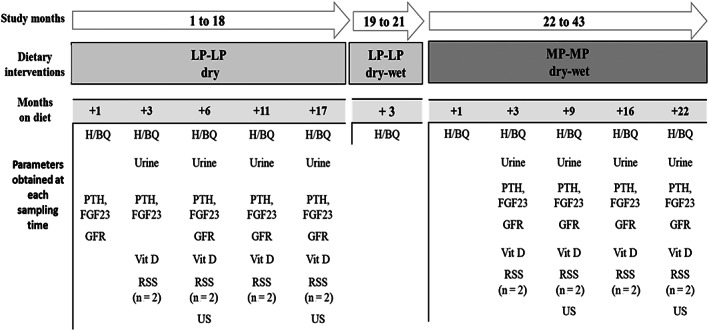
Study scheme showing dietary interventions and clinical and research measurements obtained. H/BQ, hematology/biochemistry; FGF23, fibroblast growth factor 23; GFR, glomerular filtration rate; LP‐LP, low protein low phosphorus diet provided on a dry and a dry‐wet regimen; MP‐MP, moderate protein moderate phosphorus diet provided on a dry‐wet regimen; PTH, parathyroid hormone; RSS, relative super saturation; US, ultrasound; Vit D, vitamin D
2.3. Dietary interventions
Dietary nutrient compositions are shown in Table 1. The protein and P contents of the diets were above the recommended allowance established by the National Research Council for adult cats (50 gprotein/Mcal and 0.64 gP/Mcal). 24 During the first intervention (months 1‐21), all cats received a commercial clinical renal diet (Royal Canin [RC], Aimargues, France) low in protein and P (LP‐LP), within renal diet PARNUTS (feed intended for PARticular NUTritional purposes [Commission Regulation (EU) 2020/354 of March 4, 2020 establishing a list of intended uses of feed intended for particular nutritional purposes, http://data.europa.eu/eli/reg/2020/354/oj]), containing higher levels of iP than the prefeed diet, which was specifically formulated to contain negligible amounts of iP. The dry format (LP‐LP‐dry: RC Veterinary Diet Feline Renal Dry) was fed during the first 18 months, while from months 19 to 21 the wet format was introduced (LP‐LP‐wet: RC Veterinary Feline Renal), each format providing approximately 50% of the maintenance energy requirement (MER). Because of the clinical status of cats following 17 months on the LP‐LP‐dry diet, decisions were made to introduce (a) the dry‐wet LP‐LP regimen, and subsequently (b) the second dietary intervention (months 22‐43), during which all cats were fed a commercial clinical senior diet containing a higher but moderate amount of protein and P (MP‐MP diet: RC Senior Consult Stage 2 diet [Royal Canin Senior Consult Stage 2 was relaunched in September 2020 as Royal Canin Early Renal]), both nutrients above AAFCO minima. This diet was provided as a dry‐wet regimen throughout the feeding period. Throughout the trial, diets were fed twice daily, split equally on an energy basis. Tap water was provided ad libitum. Daily food amounts were estimated by intake records from the 2 previous months.
2.4. Measures and analyses
2.4.1. Blood‐based measurements
Hematology was measured in EDTA plasma via a Mythic cell counter (Woodley Veterinary Diagnostics, Horwich, UK). Serum samples were analyzed for biochemistry (VetTest Chemistry analyzer), electrolytes (VetLyte Electrolyte analyzer), and SDMA (IDEXX SDMA test). Ionized Ca (iCa) was measured in heparinized blood with a blood gas‐analyzer (Stat Profile Prime Critical Care Analyser, Woodley Equipment Company Ltd, Horwich, UK from prefeed to month 24, and ABL‐90 FLEX analyzer, Radiometer Medical ApS, Brønshøj, Denmark from months 30 onwards). Intact FGF23 and PTH were measured in EDTA plasma by ELISA and immunoradiometric assays previously validated for cats. 8 , 25 Calcitriol was measured in serum by a chemiluminescent immunoassay (DiaSorin LIASON XL1,25 Dihydroxy Vitamin D). Additional vitamin D metabolites were determined by LC/MS‐MS. 26 For GFR determination, iohexol concentration was analyzed with high performance capillary electrophoresis in serum samples taken at 120, 180, and 240 minutes after IV administration of 647 mg iohexol/kgBW (Omnipaque 300, Amersham Health, New Jersey). Glomerular pressure and filtration rate (mL × min−1 × kgBW−1) was calculated by a corrected slope‐intercept iohexol clearance method. 27
2.4.2. Urinalysis
Urinary protein, glucose, ketones, leucocytes, red blood cells, creatinine, microalbumin, and UAC were assessed within 30 minutes of urine collection with InSight MS‐11 and MS‐2 Vet urine strips (Woodley Equipment Company Ltd, Bolton, UK). During the study, UPC, pH, and USG were analyzed at IDEXX Laboratories (Wetherby, West Yorkshire, UK). For urine RSS measurement, urine collected over a 3‐days period was analyzed for oxalate, citrate, potassium, Ca, sodium, ammonium, chloride, magnesium, sulfate, P, and uric acid by high performance liquid chromatography at Royal Canin European Regional Laboratory (Aimargues, France). Values were entered in SUPERSAT software 28 to calculate the RSS (activity product/solubility product) for MAP and CaOx.
2.4.3. Renal ultrasounds
Ultrasonographic changes were assessed by a diplomate of the European College of Veterinary Diagnostic Imaging with an ultrasound scan (GE Healthcare LOGIC, Little Chalfont, UK) with cats under sedation (0.01 mg/kgBW acepromazine, Acecare, IM, 0.3 mg/kgBW methadone, Synthadon, IM, and 8 mg/kg propofol, PropoFlo Plus, IV). From month 20 onwards 1 of the cats diagnosed with hypertrophic cardiomyopathy (HCM) underwent ultrasound while awake with 100 mg (18 mg/kgBW) gabapentin (Summit Pharmaceuticals) as anxiolytic.
2.5. Statistical analysis
Data from cats after +1, +3, +6, +11, and + 17 months on LP‐LP‐dry (months 1‐17) and after +1, +3, +9, +16, and + 22 months on MP‐MP‐dry‐wet (months 22‐43) were log10 transformed for fold‐change estimation and a linear mixed model was then fitted with diet, timepoint, and their interaction as the fixed effects and individual cat as the random effect. Within LP‐LP‐dry and MP‐MP‐dry‐wet diets, differences in clinical and research parameters over time were established relative to levels after 1 month on each diet (acclimation period). Estimated means with 95% confidence intervals (CIs) and P‐values were obtained for all comparisons made. These were calculated with a family‐wise adjusted 5% level. A statistically significant difference was defined as P ≤ .05. The effect of dietary transition from prefeed to LP‐LP‐dry, from LP‐LP‐dry to LP‐LP‐dry‐wet, and from LP‐LP‐dry‐wet to MP‐MP‐dry‐wet was also analyzed. All analyses were performed by R version 4.0.0. A descriptive analysis is provided for cats presenting with plasma PTH and vitamin D concentration below the lower reference range.
3. RESULTS
3.1. Body condition score, energy, and nutrient intake
During dietary intervention with LP‐LP, mean [range] BW was 5.13 [3.58‐6.64] kg at month 1 and 5.29 [4.02‐6.27] kg at month 21 and mean BCS remained ≥7 (Figure 2A). A 10% to 20% restriction in daily food amounts was applied during intervention with MP‐MP to maintain BCS <7. This resulted in a decrease in mean [range] energy intake from 65.3 [54.3‐74.9] kcal/kgBW0.711 with LP‐LP to 58.9 [52.8‐68.5] kcal/kgBW0.711 with MP‐MP. Consequently, mean [range] BW decreased from 5.28 [3.95‐6.24] kg at month 22 to 4.27 [4‐5.50] kg at month 43, all cats having a BCS of 4‐6 by the study end. Transitioning cats from LP‐LP‐dry to MP‐MP‐dry‐wet resulted in increased mean [range] daily intake of protein from 3.96 [3.28‐4.45] to 5.05 [4.72‐5.62] g/kgBW0.711), P from 56 [48.1‐65.5] to 88 [79.8‐104] mg/kgBW0.711, and Ca from 105 [89‐118] to 124 [108‐158] mg/kgBW0.711 (Figure 2B).
FIGURE 2.
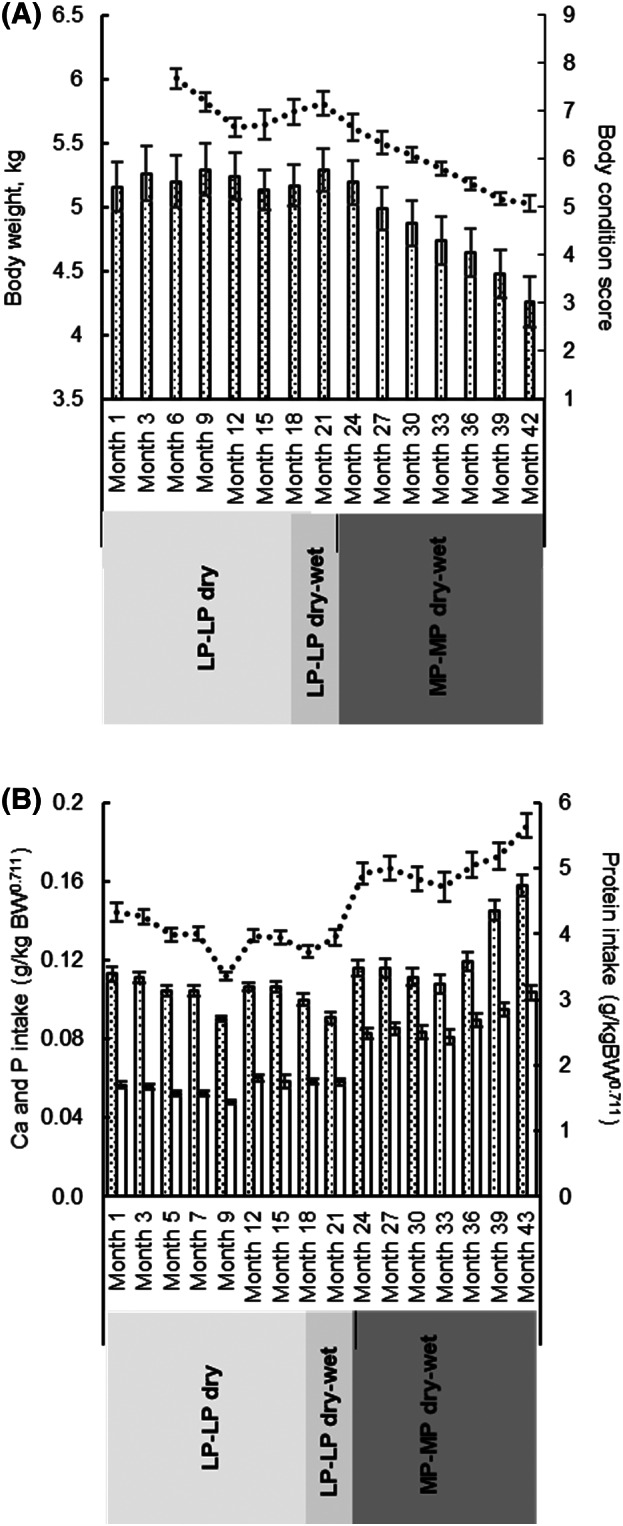
Mean (A) body weight and body condition score and (B) daily protein ( ), phosphorus (P;
), phosphorus (P;  ) and calcium (Ca;
) and calcium (Ca;  ) intake of cats with each dietary intervention. Error bars denote SE of the mean. LP‐LP, low protein low P diet; MP‐MP, moderate protein moderate P diet
) intake of cats with each dietary intervention. Error bars denote SE of the mean. LP‐LP, low protein low P diet; MP‐MP, moderate protein moderate P diet
3.2. General health and IRIS stage development
Fifteen of the 19 cats completed the study. Four cats were euthanized during the study, 3 during the first 21 months, and a fourth cat during month 40. At month 12, 1 cat (IRIS 2; 3 years) developed an acute thromboembolism accompanied by hindlimb paralysis. Postmortem examination confirmed HCM and thrombosis of the abdominal aorta. Histopathologic investigation of the kidneys revealed chronic inflammation with interstitial fibrosis and dystrophic calcification in the medullary tubules. At month 15, a second cat (IRIS 2, 4 years) developed acute and nonrespondent uremic crisis (creatinine = 7.3 mg/dL; BUN = 84.9 mg/dL); postmortem histopathologic examination identified tubulointerstitial nephritis and HCM. At month 20, a third cat (IRIS 1; 7 years) was euthanized because of recurrent urinary tract obstruction linked to a bladder stone analyzed as 100% silica. The last cat (IRIS 1, 13 years) was euthanized at month 40 because of an invasive adrenal tumor. The other 15 cats remained clinically stable, 11 of them displaying an improvement in renal markers moving from IRIS 2 to IRIS 1 CKD over the 43‐month study (Table 2). One of the cats (IRIS 2; 3.5 years) was diagnosed with HCM after a heart scan at month 20 because of pulse arrhythmia. An ECG confirmed supraventricular rhythm and the cat started treatment with atenolol (6.25 mg per day, Summit Pharmaceuticals). Another cat (IRIS 2; 10.3 years) reported with 5% weekly BW loss over 2 consecutive weeks was diagnosed with hyperthyroidism at month 40 (total thyroxine: 43.8 nmol/L; free thyroxine: 36.8 pmol/L; upper reference 60 nmol/L and 33.5 pmol/L, respectively) and started treatment with carbimazole (10 mg per 48 hours, Vidalta, MSD Animal Health). Total thyroxin levels assessed in the remaining 14 cats were <30 nmol/L. During the study, 4 cats presented with recurrent episodes of cystitis and received 1 or 2 meals as wet while on LP‐LP‐dry, or both meals as wet while on MP‐MP‐dry‐wet (n = 3) for a minimum of 3 months as advised by the attending veterinarian.
3.3. Clinical and research measurements of renal function
During the study, hematology remained within reference ranges. Main biochemical findings (including iCa) within each dietary intervention are summarized in Table 3, while GFR and Ca‐P regulatory hormone responses (including tCa) are illustrated in Figures 3 and 4, respectively.
TABLE 3.
Biochemistry variables in cats with early CKD fed a low protein, low P (LP‐LP) and subsequently a moderate protein, moderate P (MP‐MP) diet
| Prefeed | LP‐LP dry (months 1‐18) | LP‐LP dry‐wet (months 19‐21) | MP‐MP dry‐wet (months 22‐43) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item [reference] | Mean 95% CI | Time a | Mean | 95% CI | P b | Time a | Mean | 95% CI | P c | Time a | Mean | 95% CI | P b |
| Albumin, g/dL [25−45] | 34.8 (33.3, 36.3) | +1 | 32.6 | 31.1, 34.3 | <.001 | +3 | 29 | 27.7, 30.3 | .003 | +1 | 29.8 | 28.4, 31.3 | .14 |
| +3 | 31.1 | 29.7, 32.6 | <.001 | +3 | 31.4 | 29.9, 32.9 | <.001 | ||||||
| +6 | 31.8 | 30.3, 33.3 | .14 | +9 | 31.6 | 30.1, 33.2 | <.001 | ||||||
| +11 | 29.6 | 28.2, 31 | <.001 | +16 | 31.1 | 29.6, 32.6 | .007 | ||||||
| +17 | 30.3 | 28.9, 31.8 | <.001 | +22 | 30.9 | 29.4, 32.4 | .04 | ||||||
| Creatinine, mg/dL [0.91‐2.02] | 2.03 (1.87, 2.2) | +1 | 1.84 | 1.7, 2 | .001 | +3 | 1.73 | 1.59, 1.88 | .74 | +1 | 1.49 | 1.37, 1.62 | <.001 |
| +3 | 1.62 | 1.49, 1.76 | <.001 | +3 | 1.49 | 1.36, 1.62 | 1 | ||||||
| +6 | 1.6 | 1.47, 1.73 | <.001 | +9 | 1.41 | 1.3, 1.53 | .33 | ||||||
| +11 | 1.64 | 1.51, 1.78 | <.001 | +16 | 1.47 | 1.35, 1.6 | 1 | ||||||
| +17 | 1.68 | 1.54, 1.83 | .005 | +22 | 1.39 | 1.28, 1.51 | .11 | ||||||
| BUN, mg/dL [7.00‐27.7] | 24 (22.4, 25.7) | +1 | 23.8 | 22, 25.7 | .96 | +3 | 22.2 | 20.6, 23.8 | .07 | +1 | 24 | 22.2, 26.1 | .01 |
| +3 | 20.6 | 19.1, 22.3 | <.001 | +3 | 22.8 | 21, 23.5 | .38 | ||||||
| +6 | 19.3 | 17.9, 20.9 | <.001 | +9 | 22.5 | 20.7, 24.4 | .13 | ||||||
| +11 | 19.6 | 18.1, 21.2 | <.001 | +16 | 21.8 | 20.1, 23.6 | .004 | ||||||
| +17 | 20.9 | 19.2, 22.6 | <.001 | +22 | 23 | 21.2, 25 | .58 | ||||||
| SDMA, μg/dL | 14.2 (12.5, 16.2) | +1 | 15.5 | 13.2, 18.1 | .22 | +3 | 14.2 | 12.4, 16.3 | .01 | +1 | 10.6 | 9.03, 12.5 | <.001 |
| +3 | 12.9 | 11, 15 | .008 | +3 | 11.3 | 9.55, 13.2 | .95 | ||||||
| +6 | 11.2 | 9.57, 13.1 | <.001 | +9 | 11.8 | 9.99, 13.9 | .48 | ||||||
| +11 | 11.9 | 10.1, 13.9 | <.001 | +16 | 13.1 | 11.1, 15.4 | .005 | ||||||
| +17 | 16.4 | 13.9, 19.2 | .93 | +22 | 11 | 9.35, 13 | 1 | ||||||
| P, mg/dL [2.79‐6.88] | 4.15 (3.88, 4.46) | +1 | 4.43 | 4.15, 4.74 | .06 | +3 | 4.68 | 1.4, 1.62 | .88 | +1 | 4.34 | 4.03, 4.68 | .05 |
| +3 | 4.99 | 4.65, 5.33 | .002 | +3 | 4.19 | 3.91, 4.53 | .92 | ||||||
| +6 | 4.96 | 4.65, 5.33 | .002 | +9 | 4.25 | 3.94, 4.59 | 1 | ||||||
| +11 | 4.37 | 4.12, 4.71 | 1 | +16 | 4.37 | 4.09, 4.71 | 1 | ||||||
| +17 | 4.59 | 4.25, 4.93 | .94 | +22 | 4.28 | 3.97, 4.59 | 1 | ||||||
| iCa, mg/dL [4.85‐5.41] | 5.01 (4.85, 5.17) | +1 | 5.01 | 4.73, 5.31 | 1 | +3 | 5.47 | 5.31, 5.65 | .005 | +1 | 5.27 | 4.95, 5.6 | .02 |
| +3 | ND | +3 | 5.16 | 4.84, 5.49 | .95 | ||||||||
| +6 | ND | +9 | 5.31 | 5.98, 5.65 | 1 | ||||||||
| +11 | ND | +16 | 5.08 | 4.78, 5.41 | .68 | ||||||||
| +17 | 5.71 | 5.38, 6.07 | <.001 | +22 | 5.19 | 4.88, 5.52 | .98 | ||||||
| Ca × P, mg2/dL2 | 38 (34.9, 41.4) | +1 | 42.8 | 39.1, 46.9 | .002 | +3 | 47.8 | 43.6, 52.4 | .14 | +1 | 42.6 | 38.7, 47 | .02 |
| +3 | 47.4 | 43.4, 51.8 | .06 | +3 | 43.2 | 39.2, 47.6 | 1 | ||||||
| +6 | 50.2 | 45.9, 54.9 | <.001 | +9 | 41.9 | 38.1, 46.1 | 1 | ||||||
| +11 | 45.3 | 41.4, 49.6 | .64 | +16 | 41.5 | 34.1, 50.5 | 1 | ||||||
| +17 | 51.4 | 48.9, 56.5 | <.001 | +22 | 41.5 | 37.7, 45.7 | 1 | ||||||
Abbreviations: BUN, blood urea nitrogen; CI, confidence interval; CKD, chronic kidney disease; ND, not determined; P, phosphorus; SDMA, symmetric dimethylarginine.
Months on diet with LP‐LP dry, LP‐LP dry‐wet, and MP‐MP dry‐wet. Prefeed variables measured at month −2.
For LP‐LP dry and MP‐MP dry‐wet, comparisons vs +1 are performed; P‐values at +1 denote changes from prefeed and from LP‐LP dry‐wet (+3), respectively.
For LP‐LP dry‐wet, comparison vs LP‐LP dry (+17) is performed.
FIGURE 3.
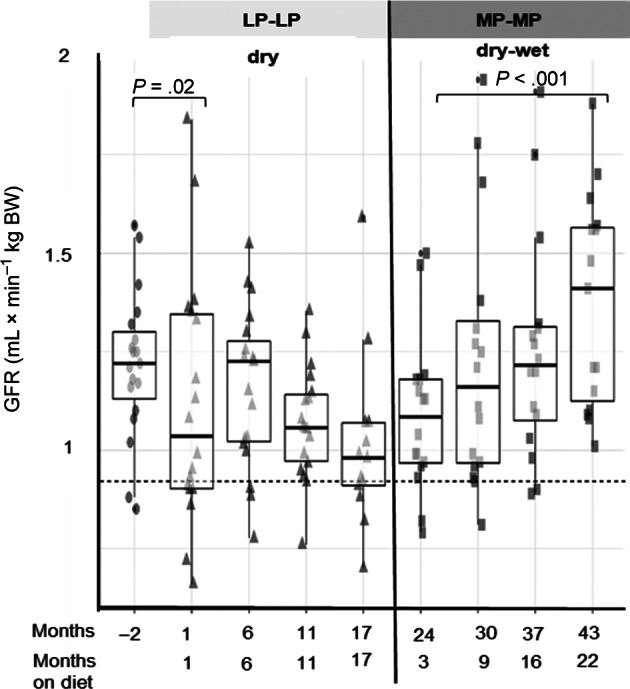
Boxplots with whiskers with maximum 1.5 IQR showing the progression of glomerular filtration rate (GFR) with each dietary intervention. LP‐LP, low protein low phosphorus; MP‐MP, moderate protein moderate phosphorus. Dashed line represents the lower reference for GFR at 0.92 mL × min−1 × kgBW−1
FIGURE 4.
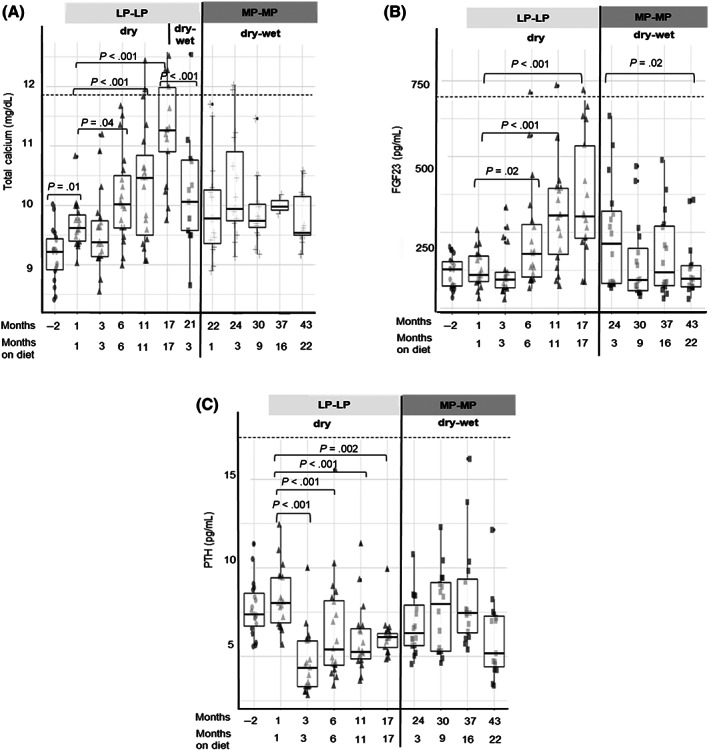
Boxplots with whiskers with maximum 1.5 IQR showing the evolution of (A) serum total calcium, (B) plasma FGF23, and (C) PTH with each dietary intervention. LP‐LP, low protein low phosphorus; MP‐MP, moderate protein moderate phosphorus. Dashed lines represent the physiological upper reference
Mean serum albumin remained within the reference range with both dietary interventions. During dietary intervention with LP‐LP‐dry decreases (P < .001) in mean creatinine (0.87‐fold, 95% CI 0.81, 0.93), BUN (0.81‐fold, 95% CI 0.76, 0.87), and SDMA (0.72‐fold, 95% CI 0.62, 0.84) were observed from +1 to +6 months. After 17 months, creatinine and BUN remained below +1 month values, whereas SDMA increased to starting values (+17 vs +1, P > .1). However, feeding LP‐LP‐dry‐wet was associated with decreased (0.87‐fold, 95% CI 0.77, 0.97, P = .01) SDMA (+17 vs +3 on LP‐LP‐dry‐wet). Further decreases (P < .001) in SDMA (0.75‐fold, 95% CI 0.67, 0.84) and creatinine (0.86‐fold, 95% CI 0.81, 0.93) were observed after transitioning cats onto MP‐MP‐dry‐wet (+1 vs +3 on LP‐LP‐dry‐wet), levels remaining <1.6 mg/dL and < 14 μg/dL, respectively throughout the feeding period. Blood urea nitrogen remained within the reference range despite an increase (1.08‐fold, 95% CI 1.01, 1.15, P = .01) after transitioning onto MP‐MP‐dry‐wet.
Dietary transition from prefeed (−2) to LP‐LP‐dry (+1) was associated with decreased GFR (0.90‐fold, 95% CI 0.82, 0.99, P = .02), which did not significantly change over the following months (Figure 3). While on MP‐MP‐dry‐wet an increase (1.28‐fold, 95% CI 1.10, 1.48, P < .001) in GFR from +3 to +22 months was observed.
3.4. Ca, P, FGF23, PTH, and vitamin D
At study entry, cats were normo‐phosphatemic (mean 4.15, 95% CI 3.82, 4.46 mg/dL) and normo‐calcemic (mean tCa 9.18, 95% CI 8.42, 10 mg/dL). Mean PTH (7.69, 95% CI 5.67, 11.4 pg/mL) and FGF23 (116; 95% CI 34.8203 pg/mL) were within levels reported in healthy cats. 7 , 29
Serum P remained within reference range throughout the study, although increases >4.5 mg/dL (IRIS target) were observed at +3, +6, and +17 months on LP‐LP‐dry. Total Ca increased (P < .01) after 6 months on LP‐LP‐dry up to 1.17‐fold (95% CI 1.11, 1.22) at +17 (vs +1) (Figure 4A). This coincided with increased (P < .001) iCa (1.14‐fold, 95% CI 1.06, 1.23) and FGF23 (2.7‐fold, 95% CI 1.72, 4.31; Figure 4B) at +17 vs +1 month and with development of total and ionized hypercalcemia in 5/17 and 13/17 cats, respectively, by month 17 (Table 2). Parathyroid hormone decreased (0.53‐fold, 95% CI 0.43, 0.65, P < .001) from +1 to +3 months on LP‐LP‐dry, 14/19 cats presenting with PTH <5 pg/mL during the feeding period (Figure 4C). Consistent changes in vitamin D metabolites were not observed while on LP‐LP‐dry (Table 4). Feeding the LP‐LP‐dry‐wet diet was associated with decreased (P < .01) tCa (0.91‐fold, 95% CI 0.86, 0.95) and iCa (0.95‐fold, 95% CI 0.92, 0.99) at +3 vs +17 months on LP‐LP‐dry. The occurrence of total/ionized hypercalcemia was also reduced (Table 2). During dietary intervention with MP‐MP‐dry‐wet, total hypercalcemia was resolved and the frequency of ionized hypercalcemia was further reduced. A decrease in FGF23 (0.58‐fold, 95% CI 0.35, 0.95, P = .02) from +3 to +22 months was also observed. Vitamin D metabolites did not consistently change while on MP‐MP‐dry‐wet, although calcitriol was below the limit of detection (12 pmol/L) in 7/15 cats at the end of the feeding period.
TABLE 4.
Serum vitamin D and urine variables in cats with early CKD fed a low protein, low P (LP‐LP) and subsequently a moderate protein, moderate P (MP‐MP) diet
| Prefeed dry | LP‐LP dry (months 1‐18) | MP‐MP dry‐wet (months 22‐43) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) a | Time b | Mean | 95% CI | P c | Time b | Mean | 95% CI | P c | |
| Calcidiol 25(OH)D3, nmol/L | ND | +3 | 121 | 98.5, 148 | +3 | 114 | 92.2, 142 | ||
| +6 | 116 | 94.9, 143 | 1 | +9 | 105 | 84.4, 130 | .86 | ||
| +11 | 89.8 | 73.2, 111 | <.001 | +16 | 99.6 | 80.3, 126 | .46 | ||
| +17 | 101 | 82.1, 125 | .17 | +22 | 107 | 86.1, 134 | .97 | ||
| C3‐Epi‐25(OH)D3, nmol/L | ND | +3 | 25.3 | 20.9, 30.6 | +3 | 19.4 | 15.9, 23.7 | ||
| +6 | 25.9 | 21.4, 31.4 | 1 | +9 | 25.7 | 21.1, 31.5 | .001 | ||
| +11 | 22.7 | 18.7, 27.4 | .49 | +16 | 18.9 | 15.4, 23.1 | 1 | ||
| +17 | 20.4 | 16.7, 24.8 | .02 | +22 | 17.7 | 14.4, 21.7 | .76 | ||
| Calcitriol 1,25 (OH)2D, pmol/L | ND | +3 | 20.3 | 14.8, 27.7 | +3 | 35.3 | 25.3, 49.4 | ||
| +6 | 21.2 | 15.5, 28.9 | 1 | +9 | 27.1 | 19.9, 36.8 | .15 | ||
| +11 | 14.3 | 9.52, 21.6 | .14 | +16 | 40.2 | 9.6, 54.7 | .83 | ||
| +17 | 28.6 | 20.3, 40.3 | .05 | +22 | 25.7 | 17.8, 37.1 | .14 | ||
| 24,25(OH)2D2, nmol/L | ND | +3 | 29.8 | 22.1, 40.2 | +3 | 25.2 | 18.4, 34.6 | ||
| +6 | 22.8 | 6.9, 30.8 | .14 | +9 | 28.9 | 21, 39.6 | .85 | ||
| +11 | 15.2 | 11.3, 20.5 | <.001 | +16 | 24.8 | 18.1, 34.1 | 1 | ||
| +17 | 16.2 | 11.9, 22.1 | <.001 | +22 | 29.3 | 21.2, 40.5 | .79 | ||
| Urine specific gravity | 1.037 (1.033, 1.044) | +3 | 1.042 | 1.035, 1.049 | +3 | 1.03 | 1.023, 1.037 | ||
| +6 | 1.043 | 1.036, 1.05 | 1 | +9 | 1.03 | 1.023, 1.038 | 1 | ||
| +11 | 1.041 | 1.034, 1.048 | 1 | +16 | 1.036 | 1.028, 1.043 | .4 | ||
| +17 | 1.036 | 1.03, 1.04 | .42 | +22 | 1.039 | 1.032, 1.047 | .05 | ||
| Urine pH | 6.31 (5.87, 6.78) | +3 | 5.94 | 5.36, 6.59 | +3 | 5.74 | 5.14, 6.4 | ||
| +6 | 7.67 | 6.94, 8.49 | <.001 | +9 | 6.59 | 5.88, 7.38 | .1 | ||
| +11 | 7.16 | 6.45, 7.94 | .004 | +16 | 6.64 | 5.95, 7.41 | .06 | ||
| +17 | 7.36 | 6.61, 8.18 | <.001 | +22 | 6.81 | 6.06, 7.65 | .02 | ||
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; ND, not determined; P, phosphorus.
Urine parameters measured 2 months before feeding LP‐LP dry.
Months on diet with LP‐LP dry and MP‐MP dry‐wet. Prefeed variables measured at month‐2.
Within each dietary intervention, P‐values denote changes from levels after 3 months (+3) on each diet.
3.5. Urinary tract health measures
While on LP‐LP‐dry, mean USG remained >1.035 while urine pH increased (P < .001) >7 after 6 months. While on MP‐MP‐dry‐wet, USG remained <1.035 during the first 9 months, increasing >1.035 thereafter, while urine pH remained <7 (Table 4). Transient borderline proteinuria (UPC 0.2‐0.3) was observed in 4/17 cats while on LP‐LP‐dry after 11 months (Table 2). This condition reoccurred in 2 of these cats and was identified in 3 other cats at various timepoints while on MP‐MP‐dry‐wet. Borderline proteinuria (UPC 0.4‐0.5) was identified in 1 cat with a developing adrenal tumor. In the 2 cats presenting with renoliths, CaOx RSS increased above a formation product value of 12.5 28 after 11 months on LP‐LP‐dry. Mean [range] CaOx RSS was 3.7 [0.61‐6.5] when reassessed in all cats at the end of the MP‐MP‐dry‐wet feeding period (Table 2). The occurrence of frequent CaOx crystals increased from 0 to 47% (8/17) while on LP‐LP‐dry (+17 vs +3), whereas frequent MAP crystal occurrence increased from 32% (6/19) to 41% (7/15). At the end of the MP‐MP‐dry‐wet feeding period, the occurrence decreased to 7% (1/15) for both CaOx and MAP.
3.6. Kidney and urinary tract imaging
Ultrasonographic imaging after 17 months on LP‐LP‐dry showed bladders stones in 6/17 cats, all 6 also presenting with ionized hypercalcemia, while 2 of the 6 also with total hypercalcemia. Affected cats had various small stones, except for 1 cat with a single bladder stone (4 × 5 mm). A follow‐up scan after 9 months on MP‐MP dry‐wet showed urolith resolution in 5/6 affected cats, although a bladder stone (1 × 1 mm) had developed in another cat (IRIS 2; 13 years), with this being the only case at the end of the study. An ureterolith was identified in 1 of the 2 cats with renoliths at study entry, while renoliths persisted in the other cat. On LP‐LP‐dry, increased renal echogenicity and reduced cortico‐medullar (CM) definition was identified in 1 cat presenting with renoliths. While feeding MP‐MP‐dry‐wet, 6 cats (5 IRIS 2, 1 IRIS 1; 8‐13 years) had increased echogenicity and/or reduced CM definition. In 1 of the cats and in 2 additional ones (1 IRIS 1, 2 IRIS 2; 8‐13 years) smaller kidneys were identified.
4. DISCUSSION
In this study, the impact of varying dietary protein and P contents and Ca : P ratios in the long‐term maintenance of cats with early CKD was assessed. Feeding LP‐LP‐dry, a highly protein (59 [58‐60] g/Mcal) and P (0.84 [0.72‐0.96] g/Mcal) restricted commercial diet with a high Ca : P ratio (1.9 [1.7‐2.1]), reduced serum creatinine, SDMA, and BUN over the first 6 months, but was associated with total and ionized hypercalcemia development after 17 months. Increasing dietary protein (76 [73‐78] and 98 [94‐101] g/Mcal) and P (1.4 [1.35‐1.45] and 1.6 g/Mcal) and decreasing Ca : P ratio (1.4 [1.32‐1.42] and 1.6 [1.55‐1.63]) when transitioned to dry and wet formats of LP‐LP and later MP‐MP commercial diets, resolved total hypercalcemia while maintaining renal function. This supports moderate inclusion levels of these nutrients for long‐term management of cats with early CKD.
Some particularities of this study concern the nature of CKD and the dietary management of these cats before study entry. As the etiology of CKD was because of P overload, dietary P was quickly restricted before study entry by feeding the experimental diet specifically formulated to contain low protein, low P, and negligible amounts of iP. Both the etiology and the early dietary management of these cats with such a P‐restricted diet might differ from the general cat population, possibly making the outcomes of the current study somewhat unique. In fact, after the 5‐month stabilization period with the experimental diet, plasma PTH and FGF23 had reverted to normal values. Most of the cats that completed the 43‐month study (15/19; 79%), displayed improvement in clinical parameters, with the majority of cases (11/15; 73%) finishing at IRIS 1 CKD, which apparently differs from the outcome of older cats with idiopathic CKD (IRIS 2‐4) maintained on a renal diet. 15 , 19 Therefore, despite commonalities in pathophysiological and possibly histopathological changes between our cats and cats with idiopathic CKD, 30 differences in the clinical progression should be considered. This might be indicated by 2 of 4 cats being euthanized at 15 and 20 months because of urinary tract and renal disease, which is similar to the median survival time of 16 to 21 months observed in azotemic cats maintained on clinical renal diets. 15 , 19
In keeping with earlier studies, 17 , 19 , 31 initial feeding of the LP‐LP‐dry diet was associated with decreased BUN and creatinine. The increased serum P reported over the first 6 months could be partly attributed to the comparatively higher iP levels in LP‐LP than in the prefeed diet. Although the LP‐LP diet contained less than 0.5 giP/Mcal from sodium tripolyphosphate, that level has been shown to induce a postprandial peak in PTH, but not in plasma P after a single meal (50% MER) exposure in cats. 32 Thus the transient increase in fasted serum P observed with LP‐LP‐dry could have occurred as an adaptive response to increased dietary iP. An increase in fasted serum P concentration associated with intake of food additives containing iP has been shown in humans. 33
After 17 months of feeding LP‐LP‐dry, total and ionized hypercalcemia was observed in 29% and 76% of cats, respectively. Whereas the occurrence of total hypercalcemia is in line with the 8% to 32% prevalence observed in cats with CKD, 10 , 11 the ionized hypercalcemia occurrence is comparatively greater than the prevalence reported in cats with azotemic CKD (up to 9%), 10 which suggests the interplay of other factors in this response. An overall decrease in GFR, which has been related to lower renal Ca clearance leading toward an increased Ca retention in humans, 12 was not observed here. Blood concentration of PTH and calcitriol, both of which are involved in upregulation of Ca retention, remained within reference ranges and did not show a consistent increase over time, ruling out any obvious cause of hypercalcemia. An effect of dietary P restriction on this response associated with high Ca : P was therefore hypothesized. Dietary P restriction has been related to increased Ca absorption in humans 12 and pigs 34 because of a lower Ca binding by P in the intestine. The higher relative amount of iP in LP‐LP‐dry (0.32/0.84; 38%) compared with the prefeed (0.06/1.23; 5%) and MP‐MP‐dry‐wet diets (0.32/1.5; 21%) could have also contributed to this response, as iP would have been more rapidly absorbed. Development of hypercalcemia has previously been described in cats with azotemic CKD fed a P‐restricted diet (0.73 and 0.8 gP/Mcal) containing a high Ca : P ratio of 1.9. 20 , 31 An improvement also occurred after transitioning the cats to a moderately phosphate‐restricted diet (1.5 gP/Mcal and Ca : P = 1.3), 31 which approximates levels in the MP‐MP diet. An increased frequency of ionized hypercalcemia (19 vs 3.5%) has also been reported in healthy older cats (≥9 years) fed a diet containing 1.6 gP/Mcal and Ca : P = 1.2 for 18 months compared with cats fed a diet containing 2.6 gP/Mcal and Ca : P = 1.1. 35 However, in that study, iCa increased in all cats and outcomes might not be truly comparable to that of cats with CKD.
The apparent positive effect of feeding LP‐LP‐dry‐wet and MP‐MP‐dry‐wet in reducing hypercalcemia compared with LP‐LP‐dry indicate benefits of differing dietary constituents and format. For example, the higher moisture and protein content and the lower Ca : P ratio in LP‐LP and MP‐MP diets fed as a dry‐wet regimen might have promoted diuresis, decreased Ca absorption, and minimized CaOx urolith formation. 36
The suppression of plasma PTH after 3 months on LP‐LP‐dry alongside the parallel increase in plasma FGF23 after 6 months on this diet might indicate a compensatory mechanism to overcome hypercalcemia. FGF23 is considered a predictor for the development of azotemia and CKD progression in azotemic cats 7 , 37 and its effect on Ca‐P homeostasis in humans and mice is well described. 38 , 39 In response to increased serum phosphate, FGF23 inhibits phosphate reabsorption in the proximal tubule. 38 An effect of FGF23 on suppressing the synthesis of calcitriol 40 and PTH 41 has been demonstrated in rodents, which is in keeping with the positive relationship between FGF23 and total Ca reported in cats. 8 In addition, the overall stability of vitamin D metabolites over the feeding period with LP‐LP‐dry, which remained within reference ranges reported in healthy adult cats, 6 could have been because of a FGF23‐mediated inhibitory effect to reduce serum Ca. The decline in FGF23 and improvement of hypercalcemia observed after transitioning onto MP‐MP‐dry‐wet, containing comparatively higher P levels, support a hypercalcemia‐mediated stimulation of FGF23 while on LP‐LP‐dry.
Although mean GFR did not significantly change from month 1 onwards while on LP‐LP dry, the increase in SDMA and FGF23, which are negatively correlated with GFR, 7 observed after 6 months could be an early indicator of declining kidney function as a result of the development of hypercalcemia and urolithiasis. On this note, hypercalcemia has been shown to decrease GFR and renal blood flow in rats 42 and an early increase in SDMA has been reported in cats with renoliths. 43 Although there are numerous factors involved in the formation of uroliths, hypercalcemia was likely a contributing factor, considering the increase of serum Ca × P > 55 mg2/dL2 in cats with total hypercalcemia, which is the advisory level used for humans with CKD to prevent soft tissue calcification, 44 as well as the increased CaOx RSS in cats with persistent renoliths. Although not directly linked with urolithiasis, all cats with total hypercalcemia and most (4/6 cats) with uroliths had frequent CaOx crystals at month 17. The nature of the uroliths was only confirmed in 1 of the cats euthanized, this revealing a 100% silica stone. This was an unexpected finding and a causative factor could not be directly linked. At the end of the feeding period with MP‐MP‐dry‐wet, resolution of total hypercalcemia and the reduced frequency of ionized hypercalcemia to 3/15 cats was accompanied by a reduced frequency of urolithiasis (1/15), which had frequent CaOx crystals. It could therefore be speculated that affected cats had CaOx uroliths and that these were voided during the study because of their small size. In addition to the apparent control of hypercalcemia, feeding MP‐MP‐dry‐wet was associated with serum creatinine <1.6 mg/dL (IRIS stage 2 cut off value) and P < 4.5 mg/dL (IRIS target), which is in keeping with the increased GFR observed with this diet. The increased BUN, and potentially GFR, after 1 month could be partly explained by the higher protein content of this diet. In human studies, GFR upregulation by dietary protein has been reported within maintenance levels, increasing above a threshold equivalent to the recommended daily allowance for protein intake. 45
The prognosis of CKD in these cats at the end of the study was considered positive based on the decline of and further stabilization of plasma FGF23 and the apparent improvement in both hypercalcemia and GFR observed while on MP‐MP‐dry‐wet. Nevertheless, there was also a deterioration in some parameters that could be indicative of subtle CKD progression. These included a drop in calcitriol <12 pmol/L in 47% of cats, as well as increased renal echogenicity, reduced CM definition and altered kidney size/shape in 47%, 33% and 27% of cats, respectively, by the end of the study. The above changes were not directly linked to IRIS staging of CKD and could have been ascribed to a natural progression of CKD, associated with cats becoming older in addition to the primary insult.
One of the main limitations of this study is the lack of a control group, which prevents attribution of the effects to diet alone. Diet intervention was dictated by the clinical status of cats, justifying the initial transition to the LP‐LP diet with a view to control azotemia. As different diets were fed subsequently, a carry‐over effect cannot be fully ruled out. This risk was partly mitigated by evaluating the long‐term feeding effects and by using values after 1 month on each diet as the first timepoint. Equipment failure prevented measuring iCa throughout the LP‐LP‐dry feeding period, limiting our ability to determine the onset of ionized hypercalcemia. Overall, the clinical data reported here support the use of a moderate protein and phosphate diet for the long‐term management of normo‐phosphatemic cats with early CKD. Extrapolation of these findings to cats from the general population with idiopathic CKD warrants further investigation.
5. CONCLUSION
Feeding a dry diet with highly restricted protein (59 g/Mcal), total P (0.84 g/Mcal), and high Ca : P (1.9 : 1) to cats with early stage CKD (IRIS 1 and 2) controlled azotemia, but was associated with the development of ionized hypercalcemia in the majority of cats after 17 months. Increased plasma FGF23, SDMA, and frequency of urolithiasis were also observed. Increasing dietary moisture, protein, and P content and reducing the Ca : P ratio by providing the same diet on a dry‐wet regimen for 3 months improved these conditions. Transitioning cats onto a less protein and P‐restricted diet (76‐98 g protein/Mcal; 1.4‐1.6 gP/Mcal; Ca : P 1.4‐1.6), with P inclusion above the range seen in most commercially‐available renal diets, decreased the occurrence of hypercalcemia and urolithiasis, while maintaining serum creatinine, P, PTH, and FGF23 within reference ranges over the long term.
CONFLICT OF INTEREST DECLARATION
Sofia Schauf, Jennifer C. Coltherd, Jujhar Atwal, Matthew Gilham, Laura J. Carvell‐Miller, Phillip Watson, and Anne Marie Bakke are employees of Mars Petcare at WALTHAM Petcare Science Institute and Denise Elliott, Esther S. Bijsmans, and Vincent C. Biourge are employees of Royal Canin, a division of Mars Petcare. Helen Renfrew and Jonathan Elliott acted as paid independent consultants for Mars Petcare.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the WALTHAM Animal Welfare and Ethical Review Body and conducted under the authority of the Animals (Scientific Procedures) Act 1986. The study was authorized and registered under Project Portfolio Management No. 55429.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by Mars Petcare. The authors acknowledge all the personnel at WALTHAM Petcare Science Institute involved in the care of the cats and in the collection and analysis of the study samples. We thank Jonathan Stockman for his contribution to the set‐up of the study and Janet Alexander for sharing her expertise throughout. We are also thankful to Yann Queau and Delphine Moniot for providing scientific advice in the interpretation of the results.
Schauf S, Coltherd JC, Atwal J, et al. Clinical progression of cats with early‐stage chronic kidney disease fed diets with varying protein and phosphorus contents and calcium to phosphorus ratios. J Vet Intern Med. 2021;35(6):2797-2811. doi: 10.1111/jvim.16263
Funding information Mars Petcare
REFERENCES
- 1. Bradley R, Tagkopoulos I, Kim M, et al. Predicting early risk of chronic kidney disease in cats using routine clinical laboratory tests and machine learning. J Vet Intern Med. 2019;33(6):2644‐2656. 10.1111/jvim.15623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg. 2014;16(6):465‐472. 10.1177/1098612X13511446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finch NC, Syme HM, Elliott J. Risk factors for development of chronic kidney disease in cats. J Vet Intern Med. 2016;30(2):602‐610. 10.1111/jvim.13917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med. 2009;23(4):806‐813. 10.1111/j.1939-1676.2009.0339.x [DOI] [PubMed] [Google Scholar]
- 5. Ames MK, Atkins CE, Pitt B. The renin‐angiotensin‐aldosterone system and its suppression. J Vet Intern Med. 2019;33(5):363‐382. 10.1111/jvim.15454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander J, Stockman J, Atwal J, et al. Effects of the long‐term feeding of diets enriched with inorganic phosphorus on the adult feline kidney and phosphorus metabolism. Br J Nutr. 2019;121:1‐21. 10.1017/s0007114518002751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finch NC, Geddes RF, Syme HM, et al. Fibroblast growth factor 23 (FGF‐23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med. 2013;27(2):227‐233. 10.1111/jvim.12036 [DOI] [PubMed] [Google Scholar]
- 8. Geddes RF, Finch NC, Elliott J, et al. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med. 2013;27:234‐241. 10.1111/jvim.12044 [DOI] [PubMed] [Google Scholar]
- 9. Fujii H. Association between parathyroid hormone and cardiovascular disease. Ther Apher Dial. 2018;22(3):236‐241. 10.1111/1744-9987.12679 [DOI] [PubMed] [Google Scholar]
- 10. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39(3):108‐116. 10.1093/jn/124.suppl_12.2660S [DOI] [PubMed] [Google Scholar]
- 11. van den Broek DH, Chang YM, Elliott J, et al. Chronic kidney disease in cats and the risk of total hypercalcemia. J Vet Intern Med. 2017;31:465‐475. 10.1111/jvim.14643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5(suppl 1):S23‐S30. 10.2215/CJN.05910809 [DOI] [PubMed] [Google Scholar]
- 13. Midkiff AM, Chew DJ, Randolph JF, et al. Idiopathic hypercalcemia in cats. J Vet Intern Med. 2000;14(6):619‐626. 10.1111/j.1939-1676.2000.tb02286.x [DOI] [PubMed] [Google Scholar]
- 14. Coady M, Fletcher DJ, Goggs R. Severity of ionized hypercalcemia and hypocalcemia is associated with etiology in dogs and cats. Front Vet Sci. 2019;6:276. 10.3389/fvets.2019.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plantinga EA, Everts H, Kastelein AM, et al. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec. 2005;157:185‐187. 10.1136/vr.157.7.185 [DOI] [PubMed] [Google Scholar]
- 16. Harte JG, Markwell PJ, Moraillon RM, et al. Dietary management of naturally occurring chronic renal failure in cats. J Nutr. 1994;124:2660s‐2662s. 10.1093/jn/124.suppl_12.2660S [DOI] [PubMed] [Google Scholar]
- 17. Ross SJ, Osborne CA, Kirk CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc. 2006;229(6):949‐957. 10.2460/javma.229.6.949 [DOI] [PubMed] [Google Scholar]
- 18. Geddes RF, Elliott J, Syme HM. The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med. 2013;27(6):1354‐1361. 10.1111/jvim.12187 [DOI] [PubMed] [Google Scholar]
- 19. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract. 2000;41(6):235‐242. 10.1111/j.1748-5827.2000.tb03932.x [DOI] [PubMed] [Google Scholar]
- 20. Barber PJ, Rawlings JM, Markwell PJ, et al. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract. 1999;40(2):62‐70. 10.1111/j.1748-5827.1999.tb03039.x [DOI] [PubMed] [Google Scholar]
- 21. Association of American Feed Control Officials (AAFCO) . Official Publication; 2016.
- 22. Kuwahar Y, Nishii N, Takasu M, et al. Use of urine albumin/creatinine ratio for estimation of proteinuria in cats and dogs. J Vet Med Sci. 2008;70(8):865‐867. 10.1292/jvms.70.865 [DOI] [PubMed] [Google Scholar]
- 23. German AJ, Holden SL, Moxham GL, et al. A simple, reliable tool for owners to assess the body condition of their dog or cat. J Nutr. 2006;136(7 suppl):2031s‐2033s. 10.1093/jn/136.7.2031S [DOI] [PubMed] [Google Scholar]
- 24. National Research Council (NRC) . Nutrient Requirements of Dogs and Cats. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 25. Williams TL, Elliott J, Syme HM. Calcium and phosphate homeostasis in hyperthyroid cats—associations with development of azotaemia and survival time. J Small Anim Pract. 2012;53(10):561‐571. 10.1111/j.1748-5827.2012.01253.x [DOI] [PubMed] [Google Scholar]
- 26. Tang JCY, Nicholls H, Piec I, et al. Reference intervals for serum 24,25‐dihydroxyvitamin D and the ratio with 25‐hydroxyvitamin D established using a newly developed LC‐MS/MS method. J Nutr Biochem. 2017;46:21‐29. 10.1016/j.jnutbio.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 27. Finch NC, Syme HM, Elliott J, et al. Glomerular filtration rate estimation by use of a correction formula for slope‐intercept plasma iohexol clearance in cats. Am J Vet Res. 2011;72(12):1652‐1659. 10.2460/ajvr.72.12.1652 [DOI] [PubMed] [Google Scholar]
- 28. Robertson WG, Jones JS, Heaton MA, et al. Predicting the crystallization potential of urine from cats and dogs with respect to calcium oxalate and magnesium ammonium phosphate (struvite). J Nutr. 2002;132(6 suppl 2):1637S‐1641S. 10.1093/jn/132.6.1637S [DOI] [PubMed] [Google Scholar]
- 29. Coltherd JC, Alexander JE, Pink C, et al. Towards establishing no observed adverse effect levels (NOAEL) for different sources of dietary phosphorus in feline adult diets: results from a 7‐month feeding study. Br J Nutr. 2021;1‐16. 10.1017/S0007114521000477 [DOI] [PubMed] [Google Scholar]
- 30. Lawson J, Elliott J, Wheeler‐Jones C, et al. Renal fibrosis in feline chronic kidney disease: known mediators and mechanisms of injury. Vet J. 2015;203(1):18‐26. 10.1016/j.tvjl.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 31. Geddes RF, van den Broek DHN, Chang Y‐M, et al. The effect of attenuating dietary phosphate restriction on blood ionized calcium concentrations in cats with chronic kidney disease and ionized hypercalcemia. J Vet Intern Med. 2021;35(2):997‐1007. 10.1111/jvim.16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coltherd JC, Staunton R, Colyer A, et al. Not all forms of dietary phosphorus are equal: an evaluation of postprandial phosphorus concentrations in the plasma of the cat. Br J Nutr. 2019;121(3):270‐284. 10.1017/S0007114518003379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore LW, Nolte JV, Gaber AO, et al. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am J Clin Nutr. 2015;102(2):444‐453. 10.3945/ajcn.114.102715 [DOI] [PubMed] [Google Scholar]
- 34. Fox J, Pickard DW, Care AD, et al. Effect of low phosphorus diets on intestinal calcium absorption and the concentration of calcium‐binding protein in intact and parathyroidectomized pigs. J Endocrinol. 1978;78(2):379‐387. 10.1677/joe.0.0770225 [DOI] [PubMed] [Google Scholar]
- 35. Geddes RF, Biourge V, Chang Y, et al. The effect of moderate dietary protein and phosphate restriction on calcium‐phosphate homeostasis in healthy older cats. J Vet Intern Med. 2016;30(5):1690‐1702. 10.1111/jvim.14563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lekcharoensuk C, Osborne CA, Lulich JP, et al. Association between dietary factors and calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. J Am Vet Med Assoc. 2001;219(9):1228‐1237. 10.2460/javma.2001.219.1228 [DOI] [PubMed] [Google Scholar]
- 37. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor‐23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med. 2015;29(6):1494‐1501. 10.1111/jvim.13625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91‐104. 10.1146/annurev.med.051308.111339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quinn SJ, Thomsen AR, Pang JL, et al. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am J Physiol Endocrinol Metab. 2013;304(3):E310‐E320. 10.1152/ajpendo.00460.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khuituan P, Teerapornpuntakit J, Wongdee K, et al. Fibroblast growth factor‐23 abolishes 1,25‐dihydroxyvitamin D3‐enhanced duodenal calcium transport in male mice. Am J Physiol Endocrinol Metab. 2012;302(8):E903‐E913. 10.1152/ajpendo.00620.2011 [DOI] [PubMed] [Google Scholar]
- 41. Ben‐Dov IZ, Galitzer H, Lavi‐Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117(12):4003‐4008. 10.1172/JCI32409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin‐angiotensin system, and calcium. J Clin Invest. 1983;71(6):1624‐1632. 10.1172/jci110918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hall JA, Yerramilli M, Obare E, Li J, Yerramilli M, Jewell DE. Serum concentrations of symmetric dimethylarginine and creatinine in cats with kidney stones. PLoS One. 2017;12(4):e0174854. 10.1371/journal.pone.0174854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hawley C. Calcium x phosphate product. The CARI Guidelines—Caring for Australasians With Renal Impairment; 2015.
- 45. Cirillo M, Zingone F, Lombardi C, et al. Population‐based dose‐response curve of glomerular filtration rate to dietary protein intake. Nephrol Dial Transplant. 2015;30(7):1156‐1162. 10.1093/ndt/gfv026 [DOI] [PubMed] [Google Scholar]


