Abstract
Background
Atrial fibrillation (AF) is associated with increased risk of sudden cardiac death (SCD) in humans, independent of secondary risk factors such as thrombogenic disorders. In dogs, SCD is described in a number of heart diseases, but an association between AF and SCD is unreported.
Hypothesis
(a) A higher proportion of dogs with AF will experience SCD, and (b) SCD will be associated with complex ventricular arrhythmias.
Animals
One‐hundred forty‐two dogs with AF, and 127 dogs without AF.
Methods
Retrospective, multicenter, case‐control study. Dogs included in the AF group were compared to a control group of dogs in sinus rhythm, matched for echocardiographic diagnosis. Descriptive statistics were used to identify proportions of each group suffering SCD, compared using chi‐squared testing. Risk factors for SCD in dogs with AF were evaluated at the univariable and multivariable level using binary logistic regression. Significance was P < .05.
Results
A significantly higher proportion of dogs with AF suffered SCD than dogs in the control group (14.8% vs 5.5%; P = .01). Younger age at diagnosis, larger left atrial size, and a history of syncope all were independent predictors of SCD in dogs with AF (χ 2, 16.3; P = .04).
Conclusions and Clinical Importance
Atrial fibrillation was associated with a higher prevalence of SCD in dogs. A history of syncope may be a useful predictor of SCD risk.
Keywords: cardiology, echocardiography, Holter analysis, ventricular arrhythmia
Abbreviations
- AF
atrial fibrillation
- DCM
dilated cardiomyopathy
- EF
ejection fraction
- LA : Ao
left atrium diameter to aortic root diameter ratio
- LVIDd
left ventricular internal diameter in diastole
- LVIDs
left ventricular internal diameter in systole
- MMVD
myxomatous mitral valve disease
- SCD
sudden cardiac death
- VT
ventricular tachycardia
1. INTRODUCTION
Atrial fibrillation (AF) is a common arrhythmia in dogs, associated with cardiac disease (causing structural and electrical remodeling of the atria) or with apparently normal cardiac morphology, so‐called isolated or “lone” AF. 1 , 2 , 3 , 4 , 5 , 6 , 7 Atrial fibrillation itself may trigger cardiac remodeling and, particularly at higher heart rates, systolic dysfunction in dogs. 8 The presence of worse structural heart disease identified using echocardiography and higher mean, Holter‐derived heart rate previously have been reported to be associated with shorter survival time after diagnosis, 1 , 2 and AF itself confers shorter survival time for dogs diagnosed with myxomatous mitral valve disease (MMVD) 9 and dilated cardiomyopathy (DCM). 10 Most dogs with AF are treated using antiarrhythmic medication to control ventricular response rate, such as digoxin, diltiazem, or both in combination. 2 , 11 Electrical cardioversion may be performed with the intent of restoring sinus rhythm, but has variable long‐term success rates. 12 , 13
In humans with AF, an increased risk of mortality 14 and, particularly, sudden cardiac death (SCD) 15 , 16 , 17 has been identified by large‐scale studies. In addition, people with AF have a higher risk of thrombogenic stroke, 18 presumably owing to decreased atrial function promoting a hypercoagulable state as well as various other tissue factors. 19 This may account for a proportion of the risk, as might complications associated with medication, or a predisposition to other arrhythmias. 16
In dogs with AF, sudden cardiac death is poorly described. One study reported that 4 of 46 (9%) dogs with AF died suddenly, 2 but further analysis was beyond the scope of that study.
Our aims were to identify the prevalence of SCD in a large population of dogs diagnosed with AF, comparing this prevalence to that of dogs without AF, matched for echocardiographic diagnosis. In addition, we aimed to evaluate possible risk factors for SCD in dogs with AF. Our hypotheses were: first, that dogs with AF would have a higher prevalence of SCD than the control group and, second, that complex ventricular arrhythmias on Holter ECG would be associated with SCD.
2. MATERIALS AND METHODS
The study was approved by the primary investigator's institutional ethical review board (reference VIN/18/054). Retrospective review of computerized medical records for dogs with AF was performed at 7 centers (Langford Vets, HeartVets, Royal Veterinary College, Southern Counties Veterinary Specialists. Pride Veterinary Centre, and Specialist Veterinary Cardiology Consultancy). To be included in the AF cohort, dogs diagnosed with AF must have undergone echocardiography and 24‐hour Holter ECG, both within 2 weeks of diagnosis. Outcome data, including date of last contact, status (alive/dead) at that time, and circumstances of death if appropriate. Patients were excluded if no outcome data were available, no echocardiographic or Holter data was available for review, or they were not in AF at the time of the Holter study.
Data recorded from dogs in the AF group were: signalment (age, sex, breed, body weight); heart failure signs (yes/no); history of syncope (yes/no); echocardiographic diagnosis, left atrial size, ejection fraction and left ventricular internal diameters normalized for body weight; Holter findings including heart rate parameters, number of ventricular ectopic beats, presence of complexity (couplets, triplets, bigeminy, or trigeminy), presence of ventricular tachycardia (VT; defined as a run ≥4 ventricular ectopic beats at a rate exceeding 200 beats/minute [bpm]) and maximum instantaneous ventricular rate; known comorbidities; antiarrhythmic treatment prescribed; and, date and circumstances of death.
In addition to the AF group, a control population of dogs in sinus rhythm was recruited in a similar manner, from 1 center only (Langford Vets). Inclusion criteria were the presence of signalment, echocardiographic and outcome data. There was no requirement for Holter data to be available in control dogs. Dogs surviving <7 days were excluded, so as to better match the control dogs to the AF dogs, which all were stable enough to undergo 24‐hour Holter ECG recording at home. Exclusion criteria were no outcome data available and insufficient echocardiographic data available to make a confident diagnosis of cardiac pathology, and otherwise as above. Recruitment to the control group was stratified to gather a population of dogs with a similar make‐up of heart disease diagnoses to the AF group, so as to more fairly assess SCD prevalence. After the AF group was collected, echocardiographic diagnoses were grouped as follows: DCM, MMVD, arrhythmogenic cardiomyopathy, congenital heart disease, and no detectable pathology using echocardiography (“normal” echocardiogram). Initially, control dogs were recruited and then the proportion of dogs in each of these groups was compared statistically between the AF and control groups. Where the overall group composition was significantly different, dogs were excluded from an overrepresented stratum of the control group (selected using a random number generator) so as to balance the group composition (Figure 1). No dogs from the AF group were excluded at this stage. The control group was not stratified for body weight, sex, or age.
FIGURE 1.
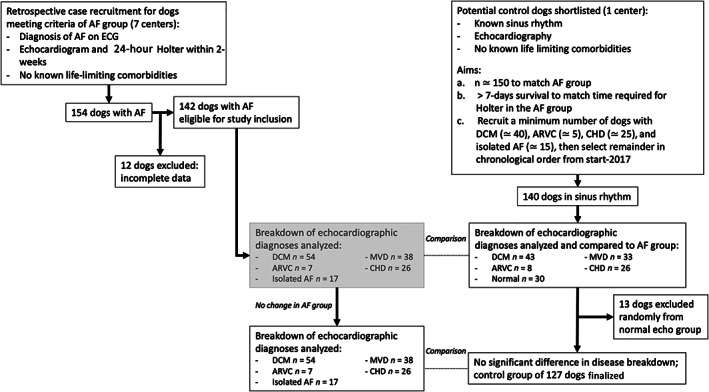
Flowchart to illustrate recruitment of eligible cases for inclusion in the study. DCM dilated cardiomyopathy; ARVC Arrhythmogenic right ventricular cardiomyopathy; MVD mitral valve disease; CHD congenital heart disease; AF atrial fibrillation
The definition of SCD used to classify status of death was as follows: if found dead, without cause and had been seen to be apparently well in the preceding 24 hours; or, if witnessed, had no apparent signs within the preceding hour. 20 , 21
2.1. Statistical analysis
Commercial software was used for analysis (IBM SPSS Statistics v.26 for MacOS Catalina, IBM Corp, Armonk, NY) and graphical representation (GraphPad Prism v.9 for MacOS Catalina, GraphPad Software, San Diego, CA) of data. Normality was assessed graphically and using a Shapiro Wilk test. Continuous, normally distributed variables were compared using an independent samples t‐test, and represented as mean ± SD. Nonnormally distributed continuous variables were compared using a Mann‐Whitney U test and represented as median (range). Categorical variables were compared using a chi‐squared or Fisher's exact test where appropriate. Survival was analyzed using Kaplan‐Meier curves and log‐rank tests. To evaluate risk factors for SCD, animals reported to be alive at last contact were excluded; this was done to eliminate the dogs that still may suffer SCD given sufficient time. Risk factors for SCD (vs other causes of death) in dogs with AF were evaluated at the univariable level, and then at the multivariable level using binary logistic regression. Factors tested at the univariable level were: age (years); weight (kilograms); history of congestive heart failure (yes/no), syncope (yes/no), antiarrhythmic drug treatment (yes/no), and ventricular ectopy detected in‐clinic (yes/no); in‐clinic ECG rate (bpm); echocardiographic measurements of left atrial‐to‐aortic root ratio (LA : Ao), left ventricular internal diameter at end‐diastole normalized for body weight (LVIDdN), left ventricular internal diameter at peak‐systole normalized for body weight (LVIDsN), and ejection fraction (%, using a 4‐chamber view, Simpson's method of discs); and Holter variables of mean 24‐hour heart rate (bpm), total ventricular premature complex (VPC) number, maximum instantaneous ventricular rate (bpm, based on interectopic R‐R interval), VT (yes/no), ventricular bigeminy or trigeminy (yes/no) and ventricular couplets or triplets (yes/no). Significance was set at P < .05 and all tests were 2‐tailed. Variables where P < .1 at the univariable level were included in multivariable binary logistic regression, using a forwards, stepwise method. A Hosmer and Lemshow goodness‐of‐fit test was performed for variables remaining significant in the final model.
3. RESULTS
3.1. Population characteristics
Seven centers submitted cases for the AF group, with a total of 154 dogs with AF. Twelve dogs were excluded because of incomplete clinical or outcome data, leaving 142 suitable dogs in the AF group. The control group was made up of 127 dogs where cardiac diagnosis was stratified to match the AF group as closely as possible (Table 1). Dogs in the AF group were presented over the period March 2013 to March 2018, and those in the control group were presented January 2013 to March 2020. Dogs in the AF group were significantly heavier than those in the control group (weight 38 kg [range, 6‐88 kg] vs 23.4 kg [range, 1.8‐89 kg]; P < .001). Heart failure signs were more common in the AF group (66.2% vs 44.9%; P < .001). On echocardiography, median left atrial size was larger than controls (LA : Ao 2.2 [1‐3.9] vs 1.9 [0.9‐2.9]; P < .05).
TABLE 1.
Population characteristics and comparison between dogs with atrial fibrillation and the control group of dogs with sinus rhythm
| Variable | Control group | Atrial fibrillation | P value |
|---|---|---|---|
| Number of dogs | 127 | 142 | n/a |
| Age (y) | 7.2 (±3.9) | 7.4 (±3.1) | .67 |
| Weight (kg) | 23.4 (1.8‐89) | 38 (6‐88) | <.001 |
| Male (number/%) | 88/69.3% | 98/69.0% | 1 |
| Syncope history | 17 (13.4%) | 27 (19%) | .21 |
| Current or previous heart failure | 57 (44.9%) | 94 (66.2%) | <.001 |
| Ventricular arrhythmia detected in clinic | 14 (11%) | 28 (19.7%) | .12 |
| Disease | |||
| Dilated cardiomyopathy | 43 (33.9%) | 54 (38%) | .87 |
| Mitral valve disease | 33 (26%) | 38 (26.7%) | |
| Arrhythmogenic cardiomyopathy | 8 (6.3%) | 7 (4.9%) | |
| Congenital heart disease | 26 (20.5%) | 26 (18.3%) | |
| Normal echocardiogram | 17 (13.4%) | 17 (12%) | .85 |
| Status | |||
| Cardiac euthanasia | 26 (20.5%) | 35 (24.6%) | .47 |
| Sudden cardiac death | 7 (5.5%) | 21 (14.8%) | .01 |
| Surgery/procedure related | 1 (0.8%) | 3 (2.1%) | .62 |
| Noncardiac death | 7 (5.5%) | 18 (12.7%) | .15 |
| Alive at last contact | 86 (67.7%) | 65 (45.8%) | <.001 |
| Normalized LVIDd | 2.1 (0.9‐3.1) | 1.9 (1‐3.1) | .01 |
| Normalized LVIDs | 1.3 (1.0‐2.6) | 1.3 (0.7‐2.3) | .98 |
| Ejection fraction (%) | 36 (±18.4) | 44 (±17) | .1 |
| LA : Ao ratio | 1.9 (0.9–2.9) | 2.2 (1‐3.9) | .05 |
Abbreviations: LA : Ao, left atrium diameter to aortic root diameter ratio; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole.
3.2. Dogs with atrial fibrillation
Most common breeds in dogs with AF were German shepherd (n = 13), Labrador retriever (n = 13), Newfoundland (n = 11), Dogue de Bordeaux (n = 10), Boxer (n = 9), Irish wolfhound (n = 7), Rotweiller (n = 7), and Golden retriever (n = 6). Thirty‐one other breeds were represented by ≤5 individuals each. Comorbidities were present in 30/142 dogs: neoplasia (8 dogs), endocrine disorders (6 dogs), renal disease (6 dogs), musculoskeletal disease (4 dogs), neurological disease (3 dogs), and laryngeal paralysis, immune‐mediated polyarthritis, and dermatologic disease (1 dog each).
At the time of Holter recording, the most commonly prescribed drugs were diltiazem and digoxin: as a combination in 53 dogs, diltiazem alone in 24 dogs, and digoxin alone in 11 dogs. No medication was being received by 26 dogs, and the remainder were being treated using a variety of other protocols (Table 2). In comparison, none of the dogs in the control group were receiving antiarrhythmic drug treatment.
TABLE 2.
Antiarrhythmic medication received by the atrial fibrillation group at the time of Holter recording
| Antiarrhythmic medicine prescribed | Number of dogs |
|---|---|
| Digoxin and diltiazem | 53 |
| No antiarrhythmic medication | 26 |
| Diltiazem alone | 24 |
| Digoxin alone | 11 |
| Amiodarone and diltiazem | 5 |
| Amiodarone, diltiazem, and digoxin | 3 |
| Amiodarone alone | 3 |
| Atenolol alone | 3 |
| Mexiletine alone | 2 |
| Sotalol alone | 2 |
| Amiodarone and atenolol | 1 |
| Amiodarone and digoxin | 1 |
| Amiodarone, digoxin, diltiazem | 1 |
| Amiodarone, digoxin, diltiazem, atenolol | 1 |
| Amiodarone, digoxin, diltiazem, mexiletine | 1 |
| Amiodarone and mexiletine | 1 |
| Amiodarone and diltiazem | 1 |
| Digoxin and sotalol | 1 |
| Diltiazem and mexiletine | 1 |
| Omega‐3 fatty acid supplements alone | 1 |
On Holter recordings, mean 24‐hour heart rate was 132 ± 38 bpm. Number of ventricular premature complexes was highly variable (median, 1591; range, 0‐42 921). Ventricular couplets or triplets were detected in 80 dogs (56.3%), with ventricular bigeminy or trigeminy in 56 dogs (39.4%). Ventricular tachycardia was identified in 19 dogs (13.4%). Two dogs had paroxysmal third‐degree atrioventricular block detected.
3.3. Survival analysis
In the AF group, 77/142 dogs (54.2%) were dead from all causes at the time of analysis, compared to a death rate of 41/127 (32.3%) in the control group (Table 1; P < .001). A cardiac cause of death (including peri‐anesthetic mortality, n = 3) was identified in 59 dogs with AF, whereas 18/77 (23.4%) died because of noncardiac causes. In the control group, 7/41 deaths (17.1%) were defined as noncardiac. Sudden cardiac death was significantly more common in dogs with AF; prevalence was 14.8% compared to 5.5% in the control group (P = .01).
Median survival time to all‐cause mortality for dogs with AF was 492 days (95% CI, 363‐621 days) and shorter than dogs in sinus rhythm (median, 593 days; 95% CI, 343‐717 days; P = .02; Figure 2). Median survival time to SCD in dogs with AF was 389 days (95% CI, 50‐728 days).
FIGURE 2.
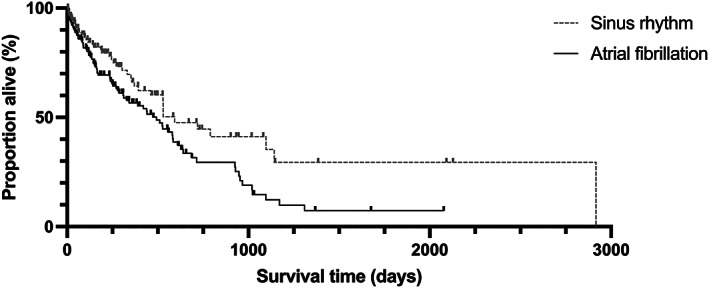
Kaplan‐Meier curve to show survival to all‐cause mortality for dogs with atrial fibrillation compared to a control group of dogs in sinus rhythm. Median survival time for dogs with atrial fibrillation was 492 days (95% CI, 363‐621 days), significantly shorter dogs in sinus rhythm, at 593 days (95% CI, 343‐717 days; P = .0165)
3.4. Factors associated with sudden cardiac death in atrial fibrillation
There was no significant difference between the time from diagnosis to SCD (median, 338 days; range, 22‐1142 days) or nonsudden cardiac death (median, 327 days; range, 7‐1097 days; P = .83). Dogs that experienced SCD were younger at diagnosis than those that suffered nonsudden cardiac death (6.6 ± 1.6 years vs 8.1 ± 2.3 years; P < .05). No historical factors (heart failure signs, drug treatment, syncope), echocardiographic variables or Holter variables were significantly associated with SCD at the univariable level; these included left atrial and ventricular diameter (systole and diastole), systolic function indices, echocardiographic diagnosis, 24‐hour heart rate (minimum, mean and maximum), total number of VPCs, presence of complexity (≥1 of couplets, triplets, bigeminy, trigeminy, or VT), presence of VT, and maximum instantaneous ventricular rate (Table 3).
TABLE 3.
Evaluation of factors for an association with sudden cardiac death in dogs with atrial fibrillation at the univariable level
| Factor | Death in other circumstances | Sudden cardiac death | P value |
|---|---|---|---|
| Signalment and history | |||
| Age (y) | 8.1 ± 2.3 | 6.6 ± 1.6 | .05 |
| Weight (kg) | 35.6 (24‐88) | 29.5 (19‐73) | .56 |
| Heart failure; number (%) | 42/56 (75%) | 13/21 (62%) | .36 |
| Syncope; number (%) | 10/56 (18%) | 8/21 (38%) | .08 |
| Antiarrhythmic drug treatment; number (%) | 48/56 (86%) | 16/21 (76%) | .32 |
| Antiarrhythmics including digoxin; number (%) | 31/48 (65%) | 11/16 (69%) | 1 |
| Ventricular ectopy in‐clinic; number (%) | 10/56 (18%) | 4/21 (19%) | 1 |
| In‐clinic ECG rate (bpm) | 200 (130‐280) | 205 (120‐220) | .8 |
| Echocardiographic variables | |||
| LA : Ao ratio | 2.5 (1.4‐1.9) | 3.1 (2‐3.9) | .1 |
| LVIDdN | 1.97 ± 0.27 | 1.95 ± 0.47 | .94 |
| LVIDsN | 1.39 (0.89‐1.76) | 1.41 (0.84‐2.25) | .94 |
| Ejection fraction (%) | 43 ± 15 | 39 ± 16 | .75 |
| Holter variables | |||
| Holter mean 24 h heart rate | 160 (97‐184) | 139 (116‐200) | .94 |
| Total VPC number | 643 (17‐18 898) | 110 (51‐8278) | .6 |
| Maximum instantaneous ventricular rate | 230 ± 76 | 267 ± 40 | .12 |
| All complex ventricular ectopy; number (%) | 35/56 (63%) | 12/21 (57%) | .79 |
| Ventricular tachycardia; number (%) | 6/56 (11%) | 3/21 (14%) | .7 |
| Ventricular bigeminy/trigeminy; number (%) | 21/56 (38%) | 5/21 (24%) | .29 |
| Ventricular couplets/triplets; number (%) | 30/56 (54%) | 12/21 (57%) | 1 |
Note: Dogs alive or lost to follow‐up were excluded. “All complex ventricular ectopy” refers to all dogs with one or more of ventricular couplets/triplets, ventricular bigeminy/trigeminy, or ventricular tachycardia.
Abbreviations: LA : Ao, left atrium diameter to aortic root diameter ratio; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; VPC, ventricular premature complex.
Variables with P < .1 at the univariable level were included the multivariable analysis. These were age, history of syncope, and echocardiographic LA : Ao ratio. The logistic regression model was statistically significant including all 3 variables (χ 2, 16.3; P = .04). It explained 34.5% of the variability in outcome (Nagelkerke R 2) and correctly classified 82.2% of cases. Dogs with a history of syncope were 4.3 times more likely to experience SCD than those without, and younger age and increased left atrial size were associated with a higher risk of SCD (Table 4).
TABLE 4.
Factors independently associated with an increased risk of sudden cardiac death after multivariable binary logistic regression
| Factor | Exp(B) | 95% CI Exp(B) | P value |
|---|---|---|---|
| Age (y) | 0.75 | 0.6‐0.94 | .013 |
| LA : Ao ratio | 6.53 | 1.95‐21.9 | .002 |
| Syncope (yes) | 4.31 | 1.1‐16.9 | .04 |
Note: Overall, the model was statistically significant (χ 2, 16.3; P = .04) and correctly classified 82.2% of cases.
Abbreviation: LA : Ao, left atrium diameter to aortic root diameter ratio.
4. DISCUSSION
In this retrospective study, the prevalence of SCD in dogs with AF was significantly higher than in a control group of dogs in sinus rhythm, matched for echocardiographic diagnoses (14.8% vs 5.5%). Younger age at diagnosis, larger left atrial size and history of syncope were independently associated with increased risk of SCD in dogs with AF. Analysis did not support our hypothesis that ventricular arrhythmias detected on 24‐hour Holter ECG would be associated with SCD.
The reason for SCD in dogs AF is unknown. In humans, >20% of people with established AF treated using anticoagulants die suddenly, 16 and the association is independent of other risk factors such as heart failure, hypertension, or QT‐prolonging antiarrhythmic medication. 22 Similar to our finding in dogs, some studies report that younger people with AF are at higher risk of SCD. 23 Dogs diagnosed with heart disease and AF at a younger age may have a more aggressive disease phenotype and therefore faster progression of disease (leading to earlier death). In addition, younger dogs may be exercised more or behave more excitably, thus increasing the risk from catecholamine surges, which could potentiate underlying arrhythmic foci or detrimentally alter vascular resistance.
The relationship between AF and SCD in humans may represent an intrinsic electrophysiologic link, where AF itself somehow predisposes to VT or ventricular fibrillation. Ventricular ectopy was certainly common among dogs with AF in our population, but none of the dogs died while wearing a Holter monitor, so we have no information to suggest that it relates to death. In fact, our results did not identify any association between Holter arrhythmia variables and SCD. However, the independent association between syncope and SCD in dogs with AF suggests that a relationship may exist between episodic arrhythmias, alterations in vascular tone, or activation of cardiac reflexes, or some combination of these, and increased risk. In humans with dual‐chamber implantable cardioverter defibrillator devices, atrial tachyarrhythmias (including AF) may increase susceptibility to ventricular arrhythmias. 24 An experimental study in dogs also supports this hypothesis. Programmed ventricular stimulation did not induce VT in dogs in sinus rhythm, but when AF was induced, 25/26 dogs did develop VT in response to the same stimulus. 25 This may be the case in dogs with naturally occurring cardiac disease, but we did not design our study to evaluate this factor.
One case report of a dog with AF that experienced SCD while wearing a Holter ECG has been published. In this report, the dog developed complex ventricular arrhythmias, followed by an episode of R‐on‐T phenomenon, which was followed by ventricular fibrillation and asystole. 26 This observation supports the idea that ventricular arrhythmias underlie SCD in dogs with AF. In contrast, a recent retrospective study reporting dogs that experienced transient loss of consciousness while undergoing ambulatory ECG described 7 cases of AF (out of 230 episodes; 3.3%) where loss of consciousness was associated with progressive slowing of AF rate followed by ventricular arrest. 27 No episodes of VT were associated with the reported episodes. If we consider transient loss of consciousness as an abortive episode of sudden death (a “near‐miss”), this finding suggests that dogs with AF may be at risk of SCD because of inappropriate vagal reflexes, such as extreme bradycardia and vasodilatation triggered by inappropriate activation of ventricular mechanoreceptors during an initiating tachycardia. This hypothesis is supported by a case in which a dog died spontaneously because of ventricular arrest after rapid AF. 28 In reality, of course, ventricular arrhythmias and inappropriate vagal reflexes are not mutually exclusive and may not represent the entire spectrum of SCD triggers.
A secondary link between AF and SCD also may be present where AF acts via another factor to cause SCD. Examples would include thrombogenic complications (rare in dogs with cardiac disease) or adverse events associated with drugs used to treat AF. Again, this possibility cannot be reliably evaluated from our data, and it would be difficult to separate out the effect of AF from shared risk factors without large, longitudinal studies of standardized treatment groups.
In humans, the use of or requirement for antiarrhythmic drug treatment has been associated with increased risk of mortality in patients with AF for over 30 years. 29 Because use of digoxin is associated with a higher risk of hospitalization 30 and death in humans with AF, 31 and a high proportion of the dogs with AF in our population were receiving treatment with digoxin, we performed some basic analysis to compare the proportion of dogs receiving digoxin in the SCD group vs dogs dead from other causes, but no significant difference was found. Class III antiarrhythmic drug use may be another potential risk factor for QT prolongation and a proarrhythmic effect, triggering malignant ventricular ectopy which could be associated with SCD. 32 Both sotalol and amiodarone have been associated with increased risk of death in human patients when used to maintain sinus rhythm after successful cardioversion. 33 We chose not to analyze any association between class III drugs and SCD in dogs with AF owing to a relatively small number of dogs receiving amiodarone or sotalol in our cohort.
Our group of dogs with AF had larger left atrial size and a higher proportion of dogs with a history of congestive heart failure. This association was not addressed by our method of control group recruitment matching, and other factors associated with more advanced heart disease (eg, pulmonary hypertension) may contribute to the risk of SCD observed in dogs with AF. Alterations in myocardial energetics, abnormal calcium loading, myocardial ischemia and fibrosis all may be contributing factors to arrhythmias that trigger SCD, and these do not relate to the presence of AF itself.
To further evaluate the relationship between AF and SCD, a prospective, collaborative study would be required. An online registry, recording various clinical, echocardiographic, ECG and Holter variables at the time of AF diagnosis, in addition to follow‐up visits (ideally at predefined timepoints, such as every 6 months) and long‐term outcome would help lay the foundation for future study. Ideally for a prospective study, treatment should follow a standardized protocol, and it may be useful to evaluate the Holter findings before antiarrhythmic treatment or the recording at which attending clinicians considered the dogs to be stabilized. Here, we simply took the first Holter data available within 2 weeks of diagnosis, whether or not the dog was on treatment with antiarrhythmic drugs already. This approach may have included a wide range of stable and unstable cases, thus blurring the distinction between dogs more at risk of SCD and those less at risk.
The higher prevalence of SCD in dogs with AF compared to a suitable control group should be used to inform communication with owners of dogs that have AF. Sudden cardiac death occurred with a frequency of approximately 1 in 7 dogs in our population, and this is a clinically relevant complication of which owners should be aware.
Other important findings of our study were a potential relationship between younger age at diagnosis and increased risk of SCD in dogs with AF, and shorter survival time in dogs with AF compared to the control group of dogs in sinus rhythm. The relationship between younger age at diagnosis and risk of SCD has been reported in a number of studies in humans, both in people with AF and those with sinus rhythm. 23 , 34 , 35 , 36 It may be that SCD in dogs is simply more common in younger animals, rather than age being a particular association with AF, or perhaps catecholamine surges during exercise may be more frequent or exaggerated in younger dogs, leading to increased arrhythmia risk. The shorter survival time to all‐cause mortality in dogs with AF as compared to that in dogs in sinus rhythm supports previous data in dogs suggesting AF indicates a poor prognosis. 9 , 10
One limitation of our study is that the control and AF groups were not perfectly matched, specifically the proportion of dogs with current or previous heart failure signs was higher in the AF group. In humans, heart failure itself is associated with an increased risk of SCD in AF. 17 Although we did our best to match the control group for disease process, it was impossible to also match for heart failure status with the available data.
Limitations of our study are related to its retrospective, observational nature. Cases were managed in a nonstandardized manner by numerous clinicians at several different centers. Although all dogs in the AF group underwent Holter monitoring, it was not an inclusion criterion for the control group. Dogs with AF were heavier and had larger left atrial size, which means that the control and AF groups were not perfectly matched, but this difference may be more related to the pathophysiology of AF than to the study methods. Some dogs were euthanized because of poor quality of life, and some of these dogs may have gone on to suffer SCD had they lived longer. We did not evaluate causes of SCD in the control group as a comparison. In addition, the circumstances of death were not always clear, and some cases of SCD may have been mis‐classified. No necropsies were performed. We have tried to be conservative and use previously reported criteria for SCD in veterinary studies, 20 , 21 which are comparable to those used in humans. The recruitment of a control group from only 1 center, as compared to AF dogs from 7 separate centers may be considered a weakness, but because all clinics were located within 200 miles of each other, the extent of genetic, environmental or geographic variation among populations is likely minimal and likely does not represent an important confounding factor. Finally, the lack of a standard treatment protocol by veterinarians managing the cases means that the effects of treatment cannot be evaluated, and the study was not designed to evaluate possible treatment effects.
Despite its weaknesses, this multicenter retrospective case‐control study identified that a significantly higher proportion of dogs with AF died suddenly than dogs with sinus rhythm, matched for heart disease type. Younger age at diagnosis, larger left atrial size and history of syncope were independent risk factors for SCD in dogs with AF in our study. Prospective studies will be required to better understand these risk factors and any potential benefit of treatment.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Langford Vets, University of Bristol, institutional regulatory body approval: VIN/18/054.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by a grant from The Langford Trust. We thank Dave Dickson (HeartVets) for helping to facilitate data collection.
Borgeat K, Pack M, Harris J, et al. Prevalence of sudden cardiac death in dogs with atrial fibrillation. J Vet Intern Med. 2021;35(6):2588-2595. doi: 10.1111/jvim.16297
Funding information The Langford Trust
REFERENCES
- 1. Menaut P, Belanger MC, Beauchamp G, et al. Atrial fibrillation in dogs with and without structural or functional cardiac disease: a retrospective study of 109 cases. J Vet Cardiol. 2005;7:75‐83. [DOI] [PubMed] [Google Scholar]
- 2. Pedro B, Dukes‐McEwan J, Oyama MA, Kraus MS, Gelzer AR. Retrospective evaluation of the effect of heart rate on survival in dogs with atrial fibrillation. J Vet Intern Med. 2018;32:86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vollmar AC, Fox PR. Long‐term outcome of Irish wolfhound dogs with preclinical cardiomyopathy, atrial fibrillation, or both treated with pimobendan, benazepril hydrochloride, or methyldigoxin monotherapy. J Vet Intern Med. 2016;30:553‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brundel BJ, Melnyk P, Rivard L, et al. The pathology of atrial fibrillation in dogs. J Vet Cardiol. 2005;7:121‐129. [DOI] [PubMed] [Google Scholar]
- 5. Guglielmini C, Goncalves Sousa M, Baron Toaldo M, et al. Prevalence and risk factors for atrial fibrillation in dogs with myxomatous mitral valve disease. J Vet Intern Med. 2020;34:2223‐2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baron Toaldo M, Mazzoldi C, Romito G, et al. Echocardiographic predictors of first onset of atrial fibrillation in dogs with myxomatous mitral valve disease. J Vet Intern Med. 2020;34:1787‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vollmar C, Vollmar A, Keene B, Fox PR, Reese S, Kohn B. Irish wolfhounds with subclinical atrial fibrillation: progression of disease and causes of death. J Vet Cardiol. 2019;24:48‐57. [DOI] [PubMed] [Google Scholar]
- 8. Dosdall DJ, Ranjan R, Higuchi K, et al. Chronic atrial fibrillation causes left ventricular dysfunction in dogs but not goats: experience with dogs, goats, and pigs. Am J Physiol Heart Circ Physiol. 2013;305:H725‐H731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jung SW, Sun W, Griffiths LG, Kittleson MD. Atrial fibrillation as a prognostic indicator in medium to large‐sized dogs with myxomatous mitral valvular degeneration and congestive heart failure. J Vet Intern Med. 2016;30:51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friederich J, Seuss AC, Wess G. The role of atrial fibrillation as a prognostic factor in Doberman pinschers with dilated cardiomyopathy and congestive heart failure. Vet J. 2020;264:105535. [DOI] [PubMed] [Google Scholar]
- 11. Gelzer AR, Kraus MS, Rishniw M, et al. Combination therapy with digoxin and diltiazem controls ventricular rate in chronic atrial fibrillation in dogs better than digoxin or diltiazem monotherapy: a randomized crossover study in 18 dogs. J Vet Intern Med. 2009;23:499‐508. [DOI] [PubMed] [Google Scholar]
- 12. Bright JM, Martin JM, Mama K. A retrospective evaluation of transthoracic biphasic electrical cardioversion for atrial fibrillation in dogs. J Vet Cardiol. 2005;7:85‐96. [DOI] [PubMed] [Google Scholar]
- 13. Bright JM, Zumbrunnen J. Chronicity of atrial fibrillation affects duration of sinus rhythm after transthoracic cardioversion of dogs with naturally occurring atrial fibrillation. J Vet Intern Med. 2008;22:114‐119. [DOI] [PubMed] [Google Scholar]
- 14. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946‐952. [DOI] [PubMed] [Google Scholar]
- 15. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 16. Marijon E, Le Heuzey JY, Connolly S, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing‐risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation. 2013;128:2192‐2201. [DOI] [PubMed] [Google Scholar]
- 17. Waldmann V, Jouven X, Narayanan K, et al. Association between atrial fibrillation and sudden cardiac death: pathophysiological and epidemiological insights. Circ Res. 2020;127:301‐309. [DOI] [PubMed] [Google Scholar]
- 18. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373:155‐166. [DOI] [PubMed] [Google Scholar]
- 19. Kamel H, Okin PM, Elkind MS, et al. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Payne JR, Borgeat K, Brodbelt DC, et al. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol. 2015;17(suppl 1):S318‐S328. [DOI] [PubMed] [Google Scholar]
- 21. Wilkie LJ, Smith K, Luis FV. Cardiac pathology findings in 252 cats presented for necropsy; a comparison of cats with unexpected death versus other deaths. J Vet Cardiol. 2015;17(suppl 1):S329‐S340. [DOI] [PubMed] [Google Scholar]
- 22. Bardai A, Blom MT, van Hoeijen DA, van Deutekom HWM, Brouwer HJ, Tan HL. Atrial fibrillation is an independent risk factor for ventricular fibrillation: a large‐scale population‐based case‐control study. Circ Arrhythm Electrophysiol. 2014;7:1033‐1039. [DOI] [PubMed] [Google Scholar]
- 23. Eisen A, Ruff CT, Braunwald E, et al. Sudden cardiac death in patients with atrial fibrillation: insights from the ENGAGE AF‐TIMI 48 trial. J Am Heart Assoc. 2016;5:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stein KM, Euler DE, Mehra R, et al. Do atrial tachyarrhythmias beget ventricular tachyarrhythmias in defibrillator recipients? J Am Coll Cardiol. 2002;40:335‐340. [DOI] [PubMed] [Google Scholar]
- 25. Somberg JC, Torres V, Keren G, et al. Enhancement of myocardial vulnerability by atrial fibrillation. Am J Ther. 2004;11:33‐43. [DOI] [PubMed] [Google Scholar]
- 26. Gunasekaran T, Sanders RA. Sudden cardiac death in a dog during Holter recording‐R on T phenomenon. J Vet Cardiol. 2017;19:455‐461. [DOI] [PubMed] [Google Scholar]
- 27. Perego M, Porteiro Vazquez DM, Ramera L, et al. Heart rhythm characterisation during unexplained transient loss of consciousness in dogs. Vet J. 2020;263:105523. [DOI] [PubMed] [Google Scholar]
- 28. Santilli R, Saponaro V, Carlucci L, Perego M, Battaia S, Borgarelli M. Heart rhythm characterization during sudden cardiac death in dogs. J. Vet. Cardiol. 10.1016/j.jvc.2021.09.005 [DOI] [PubMed] [Google Scholar]
- 29. Flaker GC, Blackshear JL, McBride R, Kronmal RA, Halperin JL, Hart RG. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The stroke prevention in atrial fibrillation investigators. J Am Coll Cardiol. 1992;20:527‐532. [DOI] [PubMed] [Google Scholar]
- 30. Freeman JV, Reynolds K, Fang M, et al. Digoxin and risk of death in adults with atrial fibrillation: the ATRIA‐CVRN study. Circ Arrhythm Electrophysiol. 2015;8:49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vamos M, Erath JW, Benz AP, Lopes RD, Hohnloser SH. Meta‐analysis of effects of digoxin on survival in patients with atrial fibrillation or heart failure: an update. Am J Cardiol. 2019;123:69‐74. [DOI] [PubMed] [Google Scholar]
- 32. Frommeyer G, Eckardt L. Drug‐induced proarrhythmia: risk factors and electrophysiological mechanisms. Nat Rev Cardiol. 2016;13:36‐47. [DOI] [PubMed] [Google Scholar]
- 33. Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:CD005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096‐2101. [DOI] [PubMed] [Google Scholar]
- 35. Zipes DP, Camm AJ, Borggrefe M, et al. Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Executive summary. Rev Esp Cardiol. 2006;59:1328. [PubMed] [Google Scholar]
- 36. Krahn AD, Connolly SJ, Roberts RS, Gent M, ATMA Investigators . Diminishing proportional risk of sudden death with advancing age: implications for prevention of sudden death. Am Heart J. 2004;147:837‐840. [DOI] [PubMed] [Google Scholar]


