Abstract
Background
T cell clonality assays in veterinary medicine currently target only the T cell receptor gamma (TRG) locus. Existing assays have suboptimal sensitivity because of insufficient primer coverage of all possible rearrangements.
Objective
Develop higher sensitivity clonality assays targeting the TRG, delta (TRD), and beta (TRB) loci in cats.
Animals
Cats with histopathologically confirmed lymphoma (n = 89), non‐lymphoma (n = 35), and possible hepatic small cell lymphoma (n = 31).
Methods
Molecular clonality assay development utilizing our recently reported topology and expressed repertoire data of the T cell receptor loci in cats. Determination of clonality status of lymphoma, non lymphoma, and possible hepatic small cell lymphoma samples, and calculation of assay sensitivity and specificity.
Results
The new multiplex TRG assay yielded the highest sensitivity (95.5%). All assays yielded 100% specificity except for the new multiplex TRG assay (97.3%). The combination of the new TRG and TRB assays yielded sensitivity of 98.9% and specificity of 97.0%. The new TRG assay detected clonality in 17/31 possible small cell lymphoma livers, whereas an existing TRG assay detected clonality in 6/31 livers.
Conclusions and Clinical Importance
The assessment of multiple T cell loci compensates for the potential shortcomings of individual assays. Using a combination of molecular clonality assays will increase the overall sensitivity for the diagnosis of T‐cell lymphoma in cats, especially intestinal, and hepatic small cell lymphoma. Hepatic small cell lymphomas detected by the new TRG assay utilized rarely expressed V and J genes not recognized by previous assays, likely indicating unique biology of hepatic small cell lymphoma in cats.
Keywords: cat, clonality assay, hepatic small cell lymphoma, lymphoma, multiplex PCR, T cell receptor
Abbreviations
- EATL
enteropathy‐associated T cell lymphoma
- FFPE
formalin‐fixed and paraffin‐embedded
- J gene
joining gene
- TR
T cell receptor
- TRB
T cell receptor beta
- TRD
T cell receptor delta
- TRG
T cell receptor gamma
- V gene
variable gene
1. INTRODUCTION
Hematopoietic neoplasms account for approximately one‐third of all tumors in cats, and among these, lymphoma is the most common. 1 Several studies have shown that the small intestine is a commonly affected site, and that small cell intestinal lymphoma is predominantly of T cell lineage. 2 , 3 , 4 , 5 Although histopathology has been regarded as the gold standard for the diagnosis of lymphoma, microscopic differentiation between lymphoid neoplasia and inflammatory conditions is difficult in some cases. This is especially true in distinguishing type II enteropathy‐associated (small) T‐cell lymphoma (EATL) from inflammatory bowel disease (IBD), or hepatic small cell lymphoma from lymphocytic cholangiohepatitis. 6 , 7 , 8
Lymphocyte antigen receptor gene rearrangement analyses (molecular clonality assays) have been used as adjunctive tests for the diagnosis of lymphoma in cats when routine morphologic assessment is equivocal. 9 , 10 Using PCR, the complementarity determining region 3 (CDR3) of lymphocyte antigen receptor genes is amplified and PCR products are size separated to visualize the antigen receptor gene rearrangement diversity of a lymphocyte population. A reactive lymphoid proliferation yields variably‐sized amplicons (polyclonal population) because of the heterogeneity of T cell receptor (TR) gene rearrangements, whereas lymphoma results in identically‐sized amplicons (clonal population) that are derived from a single neoplastic precursor cell. 11 The T cell receptor gamma (TRG) locus is widely used as a target for T cell clonality testing because of its restricted germline repertoire, which limits the number of PCR primers required, and because of the presence of TRG gene rearrangements in both αβ and γδ T cells. 12 , 13 Clonality testing of T cell proliferations targeting the TR beta (TRB) and delta (TRD) loci in addition to the TRG locus is routinely performed in human medicine to increase the detection rate of clonal rearrangements, a principle known as “complementarity of targets,” especially when the T cell lineage is unknown or the clinical presentation or histopathology is unusual or ambiguous. 14 In veterinary medicine, T cell clonality assays targeting loci other than the TRG locus have not been reported, and previously designed assays sometimes yield false negative results because of insufficient primer coverage of rearranged genes or obscuration of a clonal signal by a polyclonal background. 9 , 15 , 16 Recently, we reported the topology and expressed repertoire of the Felis catus T cell receptor loci (TR alpha [TRA], TRB, TRG, TRD). 17 Doing so enabled the design of TR clonality assays with complete coverage. The complete genomic annotation of all TR germline genes enables comprehensive candidate primer design. The expressed repertoire sequencing data informs the selection of PCR primer combinations which may improve assay performance.
Our objective was to utilize our recently published comprehensive description of the TR loci in cats to design novel multiplex primer sets targeting the feline TRG, TRD, and TRB loci. The combination of multiple gene targets increased the overall sensitivity of the diagnosis of T cell lymphomas in cats compared to our previous TRG assay. The new clonality assays proved to be superior for diagnosing small cell lymphoma in the intestine (EATL type II) and liver, which are important clinical entities in feline medicine.
2. MATERIAL AND METHODS
2.1. Feline lymphoma and non‐lymphoma samples
Our retrospective study was conducted on samples from cats retrieved from the case archive of the Pathology, Microbiology and Immunology (PMI) diagnostic service, School of Veterinary Medicine, University of California Davis (UCD) from 2000 to 2018. Cases that were submitted to the PMI Leukocyte Antigen Biology Laboratory (LABL) with a confirmed diagnosis of T cell lymphoma also were included as study samples. Histopathology and CD3 immunohistochemically (IHC)‐stained slides from all cases were reviewed by 2 pathologists (P.F.M., W.V.) to confirm morphologically unequivocal T cell lymphoma. 10 , 18 A total of 89 unequivocal T cell lymphoma samples were collected, consisting of formalin‐fixed and paraffin‐embedded (FFPE) biopsy specimens of small intestine (n = 72), lymph node (n = 4), liver (n = 3), skin (n = 2), eye (n = 1) and buccal mucosa (n = 1) as well as FFPE specimens from necropsy tissues of thymus (n = 2), liver (n = 2), lymph node (n = 1) and spinal cord (n = 1) obtained from cats euthanized with disseminated T cell lymphoma. Biopsies and necropsies were conducted with owner consent as part of the PMI diagnostic service.
A total of 35 non‐lymphoma samples were obtained from cats that were either healthy or had non‐lymphoproliferative diseases: (a) FFPE tissues of thymus (n = 3), mesenteric lymph node (n = 3) and spleen (n = 3) from 3 specific pathogen‐free (SPF) 4‐month‐old control cats from an unrelated study that operated under an Institutional Animal Care and Use Committee (IACUC) protocol 19 and (b) FFPE tissues from cats for which owner consented necropsies had been performed by the UCD diagnostic pathology service during the years 2003 to 2013. All cats were <5 years of age, were euthanized for nonhematopoietic neoplasia‐related causes, and histopathology was done to confirm the absence of lymphoma. Tissues were lymph nodes diagnosed as reactive hyperplasia (n = 14) and intestines with infectious enteritis confirmed by histopathology and PCR (n = 12).
Thirty‐one FFPE tissues of liver biopsy samples were obtained from the LABL archives (2016‐2018). The selected livers were morphologically ambiguous for T cell lymphoma vs lymphocytic cholangiohepatitis and clonality testing was necessary for differentiation. When additional tissue was available, CD3, CD79a, and CD20 IHC were performed. Additional details on age, sex, sampling location, and clinical diagnosis of T cell lymphoma, non‐lymphoma and inconclusive T cell lymphoma cases are provided (Tables S1, S2, and S3). Histopathologically normal FFPE liver samples also were collected from 10 cats submitted to the PMI diagnostic service for owner‐consented necropsies.
2.2. DNA extraction and quality control
Genomic DNA (gDNA) was isolated from FFPE sections mounted on glass slides using a commercial kit (DNeasy blood and tissue kit, Qiagen, CA). The DNA quality was assessed using an Ultraspec 2100 Pro spectrophotometer (Biochrom US, Harvard Bioscience Inc, MA). A 566 bp portion of the feline glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) gene was amplified by PCR to ensure adequate DNA integrity. Samples that did not yield the 566 bp PCR product were not included in the study to avoid false negativity. A reaction volume of 50 μL included 100 ng of gDNA, 10 pmol of each primer (forward primer sequence: 5′‐AGCAATGCCTCCTGCACCACC‐3′ and reverse primer sequence: 5′‐TACTCCTTGGAGGCCATGTGG‐3′), 1.25 units Taq enzyme (HotStarTaq, Qiagen, CA), 10× PCR buffer, 2 mM MgCl, 400 μM dNTP and PCR cycles as follows: 94°C for 1 minute; 35 cycles of 98°C for 10 seconds, 64°C for 15 seconds, 68°C for 30 seconds; 72°C for 3 minutes. The PCR products were visualized by capillary gel electrophoresis (eGene HDA‐GT12 analyzer, eGene Inc, Irvine, CA) equipped with BioCalculator software (Qiagen, CA) and using a High‐Resolution Cartridge (Qiaxcel, Qiagen, CA).
2.3. Primer design for multiplex PCR
The design and optimization of PCR primers and protocols for antigen receptor gene rearrangement analyses recommended by the EuroClonality/BIOMED‐2 consortium were applied to multiplex primer design and protocol optimization in our study. 12 Clonality assays targeting the TRG, TRD and TRB loci were established in separate reactions. The primer design aimed to balance a minimum number of primers per reaction with maximum coverage of relevant genes. Genes that were excluded from primer design were pseudogenes or genes that were found to be expressed at very low frequency in a previous study (<2% of TRG, 2% of TRD, and 0.5% of TRB repertoires). 17 Candidate genes were aligned, and forward and reverse primers were designed for variable (V) and joining (J) regions, respectively, using Geneious software (9.1.7; Biomatters Inc, San Diego, CA). Consensus primers had <2 nucleotide mismatches with any of the genes they covered, and any mismatches always were located at least 5 nucleotides away from the 3′ end of the primer. Primers were placed at least 12 nucleotides distant from the junctional region to avoid improper annealing because of nucleotide deletion or addition from the germline sequence. Expected product sizes were kept <200 bp to allow the routine use of FFPE material, which generally has some degree of compromised DNA integrity compared to other sample types. The software OLIGO v7.6 (Molecular Biology Insight Inc, Cascade, CO) was used for primer design and in silico multiplexing. 20 Primer combinations and PCR conditions for each clonality assay were optimized using polyclonal DNA from normal lymphoid tissues of SPF cats. All forward primers were tested individually using multiplexed reverse primers in a gradient PCR. If a forward primer produced a strong polyclonal signal without dimer formation, it was multiplexed with other forward primers. Annealing temperatures were chosen to accommodate optimal efficiency for all primers in the mix. Various primer combinations and PCR conditions were tested until the final selections met all criteria stated previously.
2.4. Assay testing and interpretation
The performance of the new multiplex assays was compared against a TRG clonality assay (TRG1) established previously. 21 The 3 new multiplex assays and the previous TRG1 assay were tested on the 89 lymphoma and 35 non‐lymphoma samples. Each reaction was run in triplicate, along with absent DNA template and polyclonal DNA controls. The PCR products were visualized using capillary electrophoresis as described previously.
Electrophoresis patterns were classified as follows: samples that yielded reproducible identical discrete, sharp peaks of the expected size (3/3 triplicate reactions) were defined as “clonal,” samples that yielded a Gaussian curve were defined as “polyclonal,” samples that yielded a discrete, sharp peak within a Gaussian curve, and in which the peak height of the dominant clone was at least twice the height of the polyclonal background were defined as “clonal in a polyclonal background,” samples that yielded different‐sized, and hence nonreproducible peaks in triplicate reactions were defined as “pseudoclonal” and samples that yielded neither a peak or a Gaussian curve were defined as “no amplification.” 9 Definitive classification of “clonal in a polyclonal background” rearrangement patterns required at least 2 out of 3 (triplicate) results for that sample having completely concordant discrete sharp peaks at least twice the height of the polyclonal background in their electrophoresis profiles.
2.5. Data analysis and statistical evaluation
Electrophoresis profiles classified as “clonal” or “clonal in a polyclonal background” were considered “test positive.” Electrophoresis profiles classified as “polyclonal,” “pseudoclonal,” or “no amplification” were considered “test negative.” Using unequivocal histopathology as the gold standard, true positives, true negatives, false positives, and false negatives were assigned in a 2 × 2 contingency table and test sensitivity and specificity including 95% confidence intervals (CI) were calculated. 22 In human medical hematopathology, testing TRG + TRB and TRG + TRD in parallel improves diagnostic sensitivity. 14 Therefore, in our study, sensitivity and specificity were determined for assays individually and for the assay combinations TRG + TRD and TRG + TRB done in parallel. 23
2.6. TRG clonality testing on morphologically ambiguous hepatic small T cell lymphoma
The new multiplex TRG assay (TRG2) and the previous TRG assay (TRG1) were tested on 31 liver biopsy samples that were histopathologically ambiguous for T cell lymphoma vs lymphocytic cholangiohepatitis, using a similar workflow and interpretation as above.
3. RESULTS
3.1. Primer design and PCR conditions
For each locus, all primers were successfully multiplexed in a single tube. All assays were tested with normal thymic DNA and produced a Gaussian curve without primer dimers larger than 50 bp. The reactions were established for a final reaction volume of 50 μL and 100 ng of gDNA template. Reaction conditions (Table 1) and cycling conditions (Table 2) were optimized for each TR locus individually.
TABLE 1.
Optimized master mix conditions of the TRG2, TRD, and TRB multiplex PCR
| TRG2 | TRD | TRB | |
|---|---|---|---|
| Primer a | 6 pmol | 5 pmol | 10 pmol |
| Taq b | 1.25 units | 1.25 units | 2 units |
| MgCl2 b | 2 mM | 3 mM | 2 mM |
| dNTP b | 0.4 mM | 0.6 mM | 0.6 mM |
Note: The master mix was optimized in a 50 μL reaction. 10× PCR buffer was used following the HotStartTaq DNA polymerase manufacturer's protocol.
Amount of individual primer irrespective of total numbers of primers in each multiplex PCR tube.
Final concentration in 50 μL reaction.
TABLE 2.
PCR for cycling conditions of CDR3 amplification of TRG, TRD, and TRB loci
| Step | Temperature | Time | Number of cycles | |
|---|---|---|---|---|
| Preactivation | 95°C | 15 min | 1 | |
| Denaturation | 94°C | 30 s |

|
|
| Annealing | 65°C a /61°C b /64°C c | 60 s | 35 | |
| Extension | 72°C | 60 s | ||
| Final extension | 72°C | 7 min | 1 |
Note: Number of PCR cycles of all assays is 35.
TRG2 multiplex primers.
TRD multiplex primers.
TRB multiplex primers.
Six TRG primers were multiplexed to detect 5 V genes and 6 J genes, resulting in an expected product size of approximately 75‐135 bp. The multiplex primers are anticipated to detect at least 98% of known TRG rearrangements based on large‐scale expressed repertoire data. 17 The TRG multiplex primer set developed in our study (TRG2) covers TRGV7‐1, TRGJ5‐1, and TRGJ6‐1, which are not covered by the previously used TRG1 assay (Figure 1A). Although the TRGV5‐3 gene is rearranged, the primer specific to the TRGV5‐3 gene was omitted because, during preliminary testing, an invariant rearrangement with the TRGJ5‐1 gene produced a reproducible and recurrent distinct 98 bp spike, mimicking a clonal rearrangement in both inflamed and 10 normal liver samples (Figure S1).
FIGURE 1.

Feline T cell receptor locus representation and primer sequences. (A) Feline TRG locus. (B) Feline TRD locus. (C) Feline TRB locus. Rearranged V genes and J genes that are detected by a primer are depicted in green and yellow boxes, respectively. V and J genes that are not covered by any primer in an assay are depicted in empty boxes. Genes not related to the assay are depicted in black boxes. Gray arrows indicate genes that are detected by the previously used TRG1 PCR assay (A). Black arrow indicates inverse orientation of genes on the chromosome. Box size is not to‐scale. Primer sequences are shown next to their detected genes. The relative position of the V and J primers is indicated according to their most 5′ nucleotide upstream (−) or downstream (+) of the involved recombination signal sequences. Schematic locus representations adopted from www.IMGT.org
For the TRD assay, 3 forward and 2 reverse primers were designed to detect 7 rearranged V genes and 2 rearranged J genes, respectively (Figure 1B). The expected product size range was approximately 95‐170 bp and primer dimerization occasionally was seen outside of the expected size range (approximately 45 bp) when a sample contained low target T cell DNA.
Because of the extensive combinatorial repertoire and low nucleotide homology of feline TRB genes, 17 forward primers were designed to detect 20 rearranged V genes and 10 reverse primers were designed for each of the rearranged TRBJ genes except TRBJ2‐5 because it is a pseudogene with very low usage (Figure 1C). In total, 27 primers were multiplexed to yield an expected product size of approximately 125‐200 bp with minimal primer dimerization.
3.2. Testing of new multiplex assays in lymphoma and non‐lymphoma samples
The new, optimized TRG2, TRD and TRB multiplex assays and the original TRG1 assay developed previously were tested on a series of lymphoma and nonlymphoma samples. The electrophoresis profiles of the TRG1 and the new multiplex TRG2 assays yielded various patterns including “clonal,” “clonal with polyclonal background,” and “polyclonal.” Both TRG assays frequently showed multiple clonal rearrangements in lymphoma samples, and up to 6 clonal rearrangements were observed with the new multiplex TRG2 assay, consistent with the multicassette structure of the feline TRG locus. 17 The multiplex TRG2 assay produced clonal results in 85/89 (95.5%) lymphomas whereas the previously used TRG1 assay was clonal in 79/89 (88.8%) lymphomas. The TRG assays were concordant in 81/89 (91.0%) of cases. The new multiplex TRG2 assay yielded clonal results in 7/89 (8%) lymphomas whereas the original TRG1 assay did not (Figure 2). The multiplex TRG2 assay yielded a negative test result in 1 lymphoma when the original TRG1 assay was positive (Table S1). The original TRG1 assay produced a polyclonal result in all nonlymphoma cases (0/35) whereas the multiplex TRG2 assay produced a clonal rearrangement in a single non‐lymphoma case (1/35); (Table S2).
FIGURE 2.
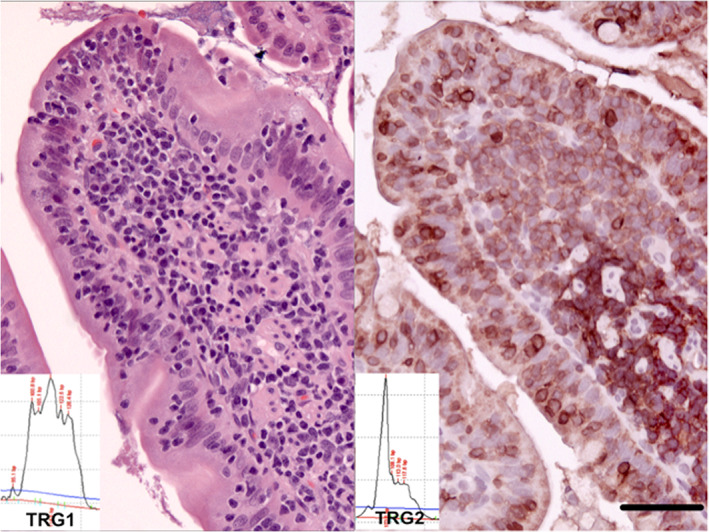
An intestinal small T‐cell lymphoma that is not clonal with the previous TRG1 assay but clearly clonal with the new multiplex TRG2 assay. HE stain left panel. CD3 immunoperoxidase stain; Vector NovaRed substrate; right panel. Bar = 50 μm. Molecular clonality PCR electropherograms insets—all PCR assays were performed in triplicate
The multiplex TRD assay produced a clonal result in 56/89 (62.9%) lymphomas. The TRD assay produced a clonal result in 1 case of hepatic lymphoma that failed to produce a clonal result with all of the other clonality assays (Figure 3). More than 1 clonal rearrangement frequently was observed with the TRD assay. Negative results with the TRD assay in the lymphoma group were frequently “no amplification” rather than “polyclonal” electrophoresis patterns (Table S1). The TRD assay was negative in all non‐lymphoma samples (0/35), 25 cases being “polyclonal” and 10 cases resulting in no amplification (Table S2).
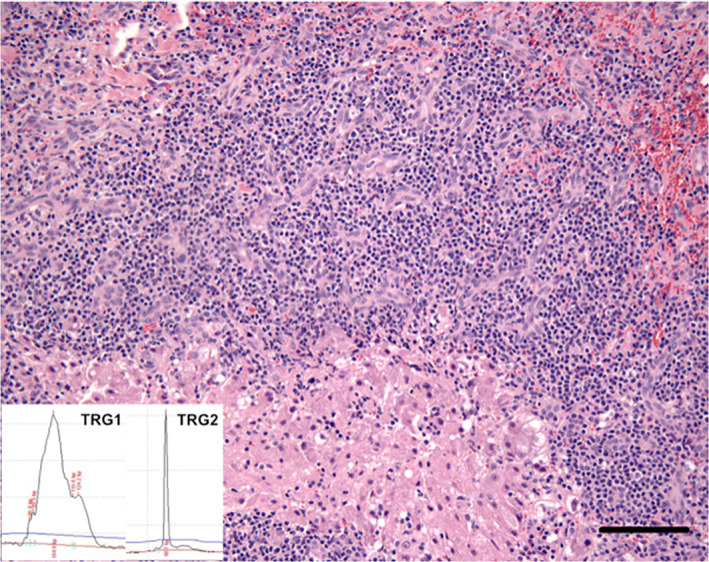
FIGURE 3.

A large T‐cell lymphoma confirmed by a clonal TRD result that is not clonal with the TRG1, TRG2, or TRB assays. HE base image. Bar = 40 μm. Molecular clonality PCR electropherograms insets—all PCR assays were performed in triplicate
The TRB assay was clonal in 71/89 (79.8%) lymphomas. Biclonal rearrangements and multiple clonal rearrangements were observed frequently in the samples that also yielded multiple rearrangements with the TRD assay. Additional PCR products at approximately 350‐400 bp occasionally were detected, presumably because of concurrent priming of a downstream TRBJ gene. Most negative test results in the lymphoma group were classified as “polyclonal” (Table S1). All non‐lymphoma samples produced negative results (0/35). Of those, 32 cases had polyclonal rearrangements and 3 cases showed no amplification (Table S2).
3.3. Evaluation of clonality assay performance characteristics
True positive, true negative, false positive and false negative percentages were calculated for each assay, along with diagnostic sensitivity and specificity and their 95% CI (Table 3). The new multiplex TRG2 assay yielded the highest sensitivity (95.5%) and the multiplex TRD assay yielded the lowest sensitivity (62.9%). The multiplex TRB assay had an intermediate sensitivity (79.8%). All assays yielded 100% specificity except for the multiplex TRG2 assay (97.3%). The sensitivity and specificity of the combined multiplex TRG2 assay and the TRB assay were 99.1% and 97.1%, respectively. The combination of the multiplex TRG2 assay and the TRD assay yielded a sensitivity of 98.3% and a specificity of 97.1%.
TABLE 3.
Clonality testing results of the TRG1 clonality assay and 3 novel multiplex clonality assays targeting the TRG, TRD, and TRB loci using lymphoma and non‐lymphoma cases
| TRG1 | TRG2 | TRD | TRB | TRG2 + TRD | TRG2 + TRB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ve+ | ve− | ve+ | ve− | ve+ | ve− | ve+ | ve− | |||
| Lymphoma | 89 | 79 | 10 | 85 | 4 | 56 | 33 | 71 | 18 | ||
| Nonlymphoma | 35 | 0 | 35 | 1 | 34 | 0 | 35 | 0 | 35 | ||
| Sensitivity | 88.8% (80.3‐94.5%) a | 95.5% (88.9‐98.8%) | 62.9% (52.0‐72.9%) | 79.8% (69.9‐87.5%) | 98.3% | 99.1% | |||||
| Specificity | 100% (90.0‐100%) a | 97.1% (85.0‐99.9%) | 100% (90.0‐100%) | 100% (90.0‐100%) | 97.1% | 97.1% | |||||
Note: Test ve+: electrophoresis profiles had a clonal rearrangement or a clonal rearrangement with a polyclonal background. Test ve−: electrophoresis profiles had a polyclonal rearrangement, a pseudoclonal rearrangement or no amplification. Sensitivity = true positive/ (true positive + false negative). Specificity = true negative/(true negative + false positive).
Abbreviations: TRG1, original TRG assay; TRG2, new multiplex TRG assay.
95% confidence interval.
3.4. TRG clonality analysis of morphologically ambiguous hepatic small T cell lymphoma samples
The multiplex TRG2 assay detected clonality in 17/31 (54.8%) livers with a diagnosis of possible small cell lymphoma based on histopathology, whereas the original TRG1 assay detected clonality in 6/31 (19.3%) livers (Figure 4 and Table S3).
FIGURE 4.
A hepatic small T‐cell lymphoma that is not clonal with the previous TRG1 assay but clearly clonal with the new multiplex TRG2 assay. HE base image. Bar = 100 μm. Molecular clonality PCR electropherograms insets—all PCR assays were performed in triplicate
4. DISCUSSION
The first PCR‐based T cell clonality assay for cats was developed based on Sanger sequencing data of TRG transcripts from normal lymphoid tissues nearly 2 decades ago, and utilized 1 pair of degenerate consensus primers. 21 After additional V and J genes of the feline TRG locus had been identified, clonality assays using more complex primer mixtures were developed to expand the number of rearranged genes detected. 24 , 25 Nevertheless, the development of earlier clonality assays for cats was hindered because of the knowledge gaps in high‐quality genome assembly and high‐throughput sequencing data in the cat. Recently, we reported a comprehensive description of all T cell receptor loci in cats and a high‐throughput dataset of expressed antigen receptor gene sequences. 17 Knowledge of the locus structure and expressed repertoire facilitates primer design and aids in the interpretation of clonality results. Given the large number of antigen receptor genes in some loci, knowledge of V/J usage helps limit primer design to genes that are rearranged at an appreciable rate, which in turn minimizes the complexity of the PCR mixture and improves assay robustness. 9 In human medicine, T cell clonality testing initially was confounded by false negative results because laboratories originally targeted only the TRG locus because of its rearrangement in both major T cell lineages and its restricted germline repertoire that simplified primer design. Increased detection rates of neoplastic clones were accomplished not only by optimized primer design, but also by incorporation of additional gene targets. 14 The combination of 2 independent clonality assays has been shown to significantly improve overall test accuracy. 26 , 27 In our study, we successfully developed multiplex PCR assays targeting the TRG, TRD and TRB loci in cats that are based on the knowledge of the expressed V/J repertoire and also gene topology. The assessment of multiple T cell loci can compensate for the potential shortcomings of individual assays and increase the overall sensitivity of diagnostic testing for T cell lymphoma in cats. Our multiplex assays can be used with a variety of sample types, including FFPE tissues, which are the lowest quality routine samples for performing PCR.
Unlike TR clonality assays in humans where multiple clonal rearrangements frequently are detected only in TRB clonality assays, the TR clonality assays for cats frequently amplified multiple clonal rearrangements in all 3 target loci in a given T cell lymphoma. 28 The fact that more clonal rearrangements per case were seen with the TRG primer set than with the TRB or TRD primer sets can be explained by the multicassette structure of the feline TRG locus. 17 A similar situation exists in the dog, where the TRG locus topology consists of 8 cassettes, and multiple clonal rearrangements are commonly detected in lymphomas in dogs. 29 , 30 , 31 This phenomenon affords multiplex clonality assays that target the TRG locus increased sensitivity because there is redundancy if 1 rearrangement is missed by a given primer set. However, it is unknown if multiple clonal rearrangements in any given lymphoma are caused by the simultaneous rearrangement of multiple cassettes in a single neoplastic clone (most likely) or the presence of multiple neoplastic clones. The TRGV7‐1, TRGJ5‐1 and TRGJ6‐1 genes are not recognized by primers of the original TRG1 assay. This likely results in failure to detect some subsets of clonal lymphocyte populations and would explain the lower sensitivity compared to the new multiplex TRG2 assay. The increased sensitivity of the multiplex TRG2 assay reported here is similar to a previous study that reported 72.7% sensitivity of a singleplex assay and 90.9% sensitivity of another multiplex assay. 16 Some TRG assays utilizing multiplex primers have been developed by others, but the sensitivity of those assays potentially is decreased because nonrearranged pseudogenes were unnecessarily included in the PCR primer mixture, and TRGV7‐1, which is the most second most utilized TRGV, is not covered. 25 , 32
During preliminary multiplex TRG assay optimization using DNA from non‐neoplastic lymphoid tissue, an invariant junctional rearrangement was unexpectedly detected when a primer for the TRGV5‐3 gene was included in the multiplex V gene primer mix. The fact that this 98 bp rearrangement was found in multiple liver samples without neoplasia, or in those that were histologically normal, suggests it is a “canonical rearrangement.” A canonical rearrangement refers to the preferential use of a particular V and J gene combination with a nondiversified CDR3 region. Canonical rearrangements can be found in normal lymphocyte populations. 33 The feline TRGV5‐3 is the rarest rearranged gene and exclusively pairs with TRGJ5‐1. 17 Hence, we concluded that the 98 bp rearrangement is most likely a canonical rearrangement and excluded the TRGV5‐3 primer from the final primer set to avoid falsely identifying a canonical rearrangement as a clonal rearrangement. The exclusion of primers to detect a rearranged gene possibly compromises assay sensitivity, but given the interpretational consequences, we prioritized minimizing the occurrence of false positive results over maximizing sensitivity. It is conceivable that rare lymphomas in cats utilize this particular rearrangement, because both TRG assays in our study failed to recognize a neoplastic clone in 2 confirmed T cell lymphomas (Table S1, cases 77 and 86), both of which interestingly involved the liver. A similar phenomenon has been reported in the multiplex primer design phase of a TRG clonality assay for humans, in which the Jγ1.2 primer was omitted owing to the occurrence of a Vγ9‐Jγ1.2 canonical rearrangement. 12 In humans, the invariant Vγ9‐Jγ1.2 rearrangement is preferentially utilized by normal γδT cells (approximately 1% of peripheral blood T cells). 34 , 35 A recent study of clonality testing in cats reported an invariant rearrangement detected by a primer mixture consisting of TRGV‐f1 and TRGJ‐r2 primers that recognizes the TRGV5‐3 and TRGJ5‐1 genes, respectively. This rearrangement was found in non‐lymphoma samples, hence it was considered a canonical rearrangement. 32 We confirmed that these primers also resulted in amplification of a canonical PCR product when used with DNA extracted from normal livers in our study (data not shown). A potential canonical rearrangement was reported in the J region subgroup 2 genes of the feline TRG locus that is presumably consistent with TRGJx‐1 genes according to the feline TR germline. 17 , 24 The invariant junctional rearrangements of lymphocyte antigen receptor genes have not been systematically investigated in domestic animals.
The TRD locus is potentially a good target for clonality testing because it has a limited number of rearranged V and J genes and possesses a diverse CDR3 region. In our study, the sensitivity of the TRD assay was considerably lower compared to the situation in humans. 12 Most of the false negative results encountered with the TRD assay for cats were a consequence of no amplification. The most probable explanation of this finding is that the majority of lymphomas in cats are of αβ T cell lineage and αβ T cells commonly do not retain rearranged TRD genes. 36 The TRD segments are located within the TRA locus and many TRD rearrangements are deleted in αβ T cells during recombination of the TRA locus, although TRD rearrangements have been reported in αβ T cell neoplasia. 36 , 37 In humans, most types of T cell lymphoma express an αβ TR and only distinct entities commonly express a γδ TR. 27 Alternatively, and less likely, false negative results could be caused by insufficient primer coverage of the novel TRD assay for cats. The evaluation of TRD rearrangements would be most helpful in γδ T cell tumors, especially those with an αβ T cell inflammatory background that could obscure a clonal rearrangement when assessed by TRG and TRB assays.
In humans, assessment of the TRB locus has been used extensively in the diagnosis of T cell lymphoproliferative disorders and in minimal residual disease monitoring because of its extensive combinatorial repertoire. 38 Clonality testing targeting the TRB locus has been slightly more informative than targeting the TRG locus, and hence is used as the first‐line target. 27 , 39 Interestingly, our TRB assay for cats yielded a lower sensitivity than the multiplex TRG assay. The TRB primer sets used in humans also detect incomplete D‐J rearrangements in addition to complete V‐D‐J rearrangements, which might contribute to the better performance of TRB over TRG primer sets in humans. 26 The fact that 2 clonal rearrangements frequently were found in our study using the TRB primer set could be attributed to biallelic rearrangements. Additionally, multiple clonal rearrangements could arise from the configuration of the feline TRB locus where TRBJ genes are spaced as close as 42 bp, which could allow for additional priming of a downstream J gene. 28
The new multiplex TRG2 assay detected more clonal rearrangements in suspected hepatic lymphoma samples than the original TRG1 assay. This finding suggests that T lymphocyte subsets utilizing TRGV7‐1, TRGJ5‐1 or TRGJ6‐1 genes are more common in the liver than in the intestine. Further investigation into hepatic T cell subsets may identify V/J gene usage distinct from that found in the intestine, similar to human γδ T cells, in which the Vδ1 subset is predominant in gut epithelium whereas the Vδ3 subset preferentially localizes to the liver. 40 The complex mixture of intrahepatic lymphocyte populations in rodents and humans is comprised of natural killer (NK) T cells, NK cells, αβ T cells, γδ T cells and fewer B cells. 41 , 42 In mice, the expressed TRG chain of tissue‐resident γδ T cells typically is associated with their location and effector functions, whereas in humans the TRD chain serves that purpose. 43 , 44 For instance, human Vδ3+ γδ T cells are found exclusively in normal livers. Liver infiltrating Vδ2− γδ T cells were phenotypically and functionally distinct from peripheral blood subsets, which dominantly express Vδ2. 45 , 46
Lymphocytic cholangitis/cholangiohepatitis, a common liver disorder in cats, is a predominantly T‐cell mediated disease. 47 A previous study reported that 17% of cases of lymphocytic cholangitis in cats had clonal rearrangements, whereas only 63% of morphologically diagnosed hepatic T cell lymphomas had clonal rearrangements using a previously established TRG clonality assay. 8 These results reflect the World Small Animal Veterinary Association (WSAVA) guidelines for lymphocytic cholangitis, which indicate the difficulty in differentiating it from small cell lymphoma morphologically. 48 The high sensitivity and specificity of the new multiplex TRG2 assay suggests it will be useful for distinguishing lymphocytic cholangitis from hepatic lymphoma in cats when histologic morphology is inconclusive. Furthermore, the preferential usage of TRGV7‐1, TRGJ5‐1 and TRGJ6‐1 genes in hepatic small T cell lymphomas suggests they arise in the liver and are not metastatic from the intestine, as is commonly believed.
T cell receptor gene target selection is important for assay performance as well as result interpretation. The EuroClonality/BIOMED‐2 guidelines recommend that lineage assignment of the suspected neoplastic population should be done by IHC or flow cytometry in order to select the clonality targets. In addition, the guidelines recommend that suspected T cell neoplasms should be investigated by evaluating TRB and TRG clonality in parallel. Assessment of the TRD locus in combination with the TRG locus should only be used as a target for suspected γδ T cell lymphomas or immature T cell proliferations. 14 Unfortunately, differentiation of T cell receptor lineages in cats currently is not possible because antibodies against the feline αβ TR and γδ TR are not available. Given our findings, simultaneous use of assays targeting the TRG and TRB loci would maximize the sensitivity of diagnosis of suspected T‐cell lymphomas in cats. The TRD clonality assay could be performed after other assays have failed to detect a clone and suspicion of T cell lymphoma remains high.
The performance of a new diagnostic test generally is determined in relation to a gold standard. Histopathology currently is considered the gold standard for diagnosing lymphoproliferative disorders, but a reliable diagnosis can be hampered by concurrent inflammation. For this reason, only morphologically unequivocal lymphomas without a marked inflammatory component were selected to represent true positives in our study. The selection of non‐lymphoma cases is more challenging because histopathology cannot always completely exclude a neoplastic lymphocyte population existing among inflammatory cells. Hence, signalment, patient history and clinical data, including follow‐up, were incorporated into selection criteria to exclude subclinical lymphoma.
5. CONCLUSION
We developed novel multiplex molecular clonality PCR assays targeting the TRG, TRD, and TRB loci for the diagnosis of T cell lymphoid neoplasia in cats. Performance of the new assays was tested by investigating a large number of histologically‐confirmed T cell lymphomas of diverse origins as well as inflammatory lesions and normal lymphoid tissues. The combination of clonality assays targeting the TRG, TRD, and TRB loci has high sensitivity and specificity and is a useful ancillary tool for the detection of T cell clonality in lymphoproliferative diseases of cats, especially those arising in the gastrointestinal tract and liver. Furthermore, our results refute the contention of 2 recent studies that questioned the specificity of molecular clonality PCR for the assessment of intestinal disease in cats. 49 , 50 That said, PCR‐based clonality assays are not stand‐alone tests. The clinical and morphologic context in which clonality is observed is extremely important, and although clonality is strongly predictive of neoplasia, it is not synonymous with malignancy. Benign clonal expansions may occur in certain situations, including very focused immune responses. Accurate interpretation of molecular clonality PCR assays requires integration with clinical, morphologic, and immunophenotypic data.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The care of cats used in this study operated under an IACUC protocol. Animal care at University of California Davis is regulated by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) (Accredited Number 000029) and Public Health Service (PHS) Animal Assurance (Animal Welfare Assurance Number A3433‐01).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1. Multiplex primer optimization phase. Electropherograms of multiplex TRG (TRG2) clonality PCR assays tested with DNA from 2 normal livers with unexpected invariant/canonical rearrangement mimicking a clonal rearrangement at approximately 98 bp (arrow) when the TRGV5‐3 gene primer was pooled with multiplex J gene primers (upper panels). The suspected canonical rearrangements without polyclonal background were found at 98 bp when the primer pair of TRGV5‐3 and TRGJ5‐1 genes were used in isolation as a singleplex assay (lower panels). Triangles indicate 50 and 500 bp markers.
Table S1. Signalment and electrophoresis profiles of TR clonality in lymphoma samples from 89 cats.
Table S2. Signalment and electrophoresis profiles of TR clonality in non‐lymphoma samples from 35 cats.
Table S3. Signalment and electrophoresis profiles of TRG clonality in livers from 31 cats with histopathology and immunophenotyping indicative of possible small T cell lymphoma.
ACKNOWLEDGMENT
This work was supported by funds from a Center for Companion Animal Health (CCAH) grant (No. 2016‐27‐F), School of Veterinary Medicine, University of California, Davis. Araya Radtanakatikanon received PhD support from the Centenary Fund, Chulalongkorn University, Thailand.
Radtanakatikanon A, Moore PF, Keller SM, Vernau W. Novel clonality assays for T cell lymphoma in cats targeting the T cell receptor beta, T cell receptor delta, and T cell receptor gamma loci. J Vet Intern Med. 2021;35(6):2865-2875. doi: 10.1111/jvim.16288
Funding information Center for Companion Animal Health (CCAH), School of Veterinary Medicine, University of California, Davis, Grant/Award Number: 2016‐27‐F; Centenary Fund, Chulalongkorn University, Thailand
REFERENCES
- 1. Vail DM. Hematopoietic tumors. In: Withrow SJ, Vail DM, Page RL, eds. Small Animal Clinical Oncology. 5th ed. St Louis, MO: Saunders Elsevier; 2012:608‐678. [Google Scholar]
- 2. Chino J, Fujino Y, Kobayashi T, et al. Cytomorphological and immunological classification of feline lymphomas: clinicopathological features of 76 cases. J Vet Med Sci. 2013;75:701‐707. [DOI] [PubMed] [Google Scholar]
- 3. Louwerens M, London CA, Pedersen NC, et al. Feline lymphoma in the post‐feline leukemia virus era. J Vet Intern Med. 2005;19:329‐335. [DOI] [PubMed] [Google Scholar]
- 4. Rissetto K, Villamil JA, Selting KA, et al. Recent trends in feline intestinal neoplasia: an epidemiologic study of 1,129 cases in the veterinary medical database from 1964 to 2004. J Am Anim Hosp Assoc. 2011;47:28‐36. [DOI] [PubMed] [Google Scholar]
- 5. Roccabianca P, Vernau W, Caniatti M, et al. Feline large granular lymphocyte (LGL) lymphoma with secondary leukemia: primary intestinal origin with predominance of a CD3/CD8(alpha)(alpha) phenotype. Vet Pathol. 2006;43:15‐28. [DOI] [PubMed] [Google Scholar]
- 6. Briscoe KA, Krockenberger M, Beatty JA, et al. Histopathological and immunohistochemical evaluation of 53 cases of feline lymphoplasmacytic enteritis and low‐grade alimentary lymphoma. J Comp Pathol. 2011;145:187‐198. [DOI] [PubMed] [Google Scholar]
- 7. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2008. [Google Scholar]
- 8. Warren A, Center S, McDonough S, et al. Histopathologic features, immunophenotyping, clonality, and eubacterial fluorescence in situ hybridization in cats with lymphocytic cholangitis/cholangiohepatitis. Vet Pathol. 2011;48:627‐641. [DOI] [PubMed] [Google Scholar]
- 9. Keller SM, Vernau W, Moore PF. Clonality testing in veterinary medicine: a review with diagnostic guidelines. Vet Pathol. 2016;53:711‐725. [DOI] [PubMed] [Google Scholar]
- 10. Moore PF, Rodriguez‐Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol. 2012;49:658‐668. [DOI] [PubMed] [Google Scholar]
- 11. Hodges E, Krishna MT, Pickard C, et al. Diagnostic role of tests for T cell receptor (TCR) genes. J Clin Pathol. 2003;56:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T‐cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED‐2 concerted action BMH4‐CT98‐3936. Leukemia. 2003;17:2257‐2317. [DOI] [PubMed] [Google Scholar]
- 13. Mahe E, Pugh T, Kamel‐Reid S. T cell clonality assessment: past, present and future. J Clin Pathol. 2018;71:195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Langerak AW, Groenen PJ, Bruggemann M, et al. EuroClonality/BIOMED‐2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia. 2012;26:2159‐2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gress V, Wolfesberger B, Fuchs‐Baumgartinger A, et al. Characterization of the T‐cell receptor gamma chain gene rearrangements as an adjunct tool in the diagnosis of T‐cell lymphomas in the gastrointestinal tract of cats. Res Vet Sci. 2016;107:261‐266. [DOI] [PubMed] [Google Scholar]
- 16. Hammer SE, Groiss S, Fuchs‐Baumgartinger A, et al. Characterization of a PCR‐based lymphocyte clonality assay as a complementary tool for the diagnosis of feline lymphoma. Vet Comp Oncol. 2017;15:1354‐1369. [DOI] [PubMed] [Google Scholar]
- 17. Radtanakatikanon A, Keller SM, Darzentas N, et al. Topology and expressed repertoire of the Felis catus T cell receptor loci. BMC Genomics. 2020;21:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiupel M, Smedley RC, Pfent C, et al. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet Pathol. 2011;48:212‐222. [DOI] [PubMed] [Google Scholar]
- 19. Woo JC, Dean GA, Pedersen NC, et al. Immunopathologic changes in the thymus during the acute stage of experimentally induced feline immunodeficiency virus infection in juvenile cats. J Virol. 1997;71:8632‐8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rychlik W. OLIGO 7 primer analysis software. Methods Mol Biol. 2007;402:35‐60. [DOI] [PubMed] [Google Scholar]
- 21. Moore PF, Woo JC, Vernau W, et al. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Vet Immunol Immunopathol. 2005;106:167‐178. [DOI] [PubMed] [Google Scholar]
- 22. Altman DG. Diagnostic tests. In: Douglas Altman DM, Bryant T, Gardner M, eds. Statistics with Confidence: Confidence Intervals and Statistical Guidelines. 2nd ed. London, UK: BMJ Books; 2000. [Google Scholar]
- 23. Sergeant E, Perkins N. Epidemiology for Field Veterinarians: An Introduction. Wallingford, UK: CABI; 2015. [Google Scholar]
- 24. Weiss AT, Klopfleisch R, Gruber AD. T‐cell receptor gamma chain variable and joining region genes of subgroup 1 are clonally rearranged in feline B‐ and T‐cell lymphoma. J Comp Pathol. 2011;144:123‐134. [DOI] [PubMed] [Google Scholar]
- 25. Mochizuki H, Nakamura K, Sato H, et al. GeneScan analysis to detect clonality of T‐cell receptor gamma gene rearrangement in feline lymphoid neoplasms. Vet Immunol Immunopathol. 2012;145:402‐409. [DOI] [PubMed] [Google Scholar]
- 26. van Krieken JH, Langerak AW, Macintyre EA, et al. Improved reliability of lymphoma diagnostics via PCR‐based clonality testing: report of the BIOMED‐2 concerted action BHM4‐CT98‐3936. Leukemia. 2007;21:201‐206. [DOI] [PubMed] [Google Scholar]
- 27. Bruggemann M, White H, Gaulard P, et al. Powerful strategy for polymerase chain reaction‐based clonality assessment in T‐cell malignancies report of the BIOMED‐2 concerted action BHM4 CT98‐3936. Leukemia. 2007;21:215‐221. [DOI] [PubMed] [Google Scholar]
- 28. Langerak AW, van Dongen JJM. Multiple clonal Ig/TCR products: implications for interpretation of clonality findings. J Hematopathol. 2012;5:35‐43. [Google Scholar]
- 29. Massari S, Bellahcene F, Vaccarelli G, et al. The deduced structure of the T cell receptor gamma locus in Canis lupus familiaris. Mol Immunol. 2009;46:2728‐2736. [DOI] [PubMed] [Google Scholar]
- 30. Keller SM, Moore PF. Rearrangement patterns of the canine TCRgamma locus in a distinct group of T cell lymphomas. Vet Immunol Immunopathol. 2012;145:350‐361. [DOI] [PubMed] [Google Scholar]
- 31. Keller SM, Moore PF. A novel clonality assay for the assessment of canine T cell proliferations. Vet Immunol Immunopathol. 2012;145:410‐419. [DOI] [PubMed] [Google Scholar]
- 32. Rout ED, Burnett RC, Yoshimoto JA, et al. Assessment of immunoglobulin heavy chain, immunoglobulin light chain, and T‐cell receptor clonality testing in the diagnosis of feline lymphoid neoplasia. Vet Clin Pathol. 2019;48(suppl 1):45‐58. [DOI] [PubMed] [Google Scholar]
- 33. Delfau MH, Hance AJ, Lecossier D, et al. Restricted diversity of V gamma 9‐JP rearrangements in unstimulated human gamma/delta T lymphocytes. Eur J Immunol. 1992;22:2437‐2443. [DOI] [PubMed] [Google Scholar]
- 34. Kallemeijn MJ, Kavelaars FG, van der Klift MY, et al. Next‐generation sequencing analysis of the human TCRgammadelta+ T‐cell repertoire reveals shifts in Vgamma‐ and Vdelta‐usage in memory populations upon aging. Front Immunol. 2018;9:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Porcelli S, Brenner MB, Band H. Biology of the human gamma delta T‐cell receptor. Immunol Rev. 1991;120:137‐183. [DOI] [PubMed] [Google Scholar]
- 36. Krangel MS. Mechanics of T cell receptor gene rearrangement. Curr Opin Immunol. 2009;21:133‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joachims ML, Chain JL, Hooker SW, et al. Human alpha beta and gamma delta thymocyte development: TCR gene rearrangements, intracellular TCR beta expression, and gamma delta developmental potential—differences between men and mice. J Immunol. 2006;176:1543‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruggemann M, van der Velden VH, Raff T, et al. Rearranged T‐cell receptor beta genes represent powerful targets for quantification of minimal residual disease in childhood and adult T‐cell acute lymphoblastic leukemia. Leukemia. 2004;18:709‐719. [DOI] [PubMed] [Google Scholar]
- 39. Assaf C, Hummel M, Dippel E, et al. High detection rate of T‐cell receptor beta chain rearrangements in T‐cell lymphoproliferations by family specific polymerase chain reaction in combination with the GeneScan technique and DNA sequencing. Blood. 2000;96:640‐646. [PubMed] [Google Scholar]
- 40. Rajoriya N, Fergusson JR, Leithead JA, et al. Gamma delta T‐lymphocytes in hepatitis C and chronic liver disease. Front Immunol. 2014;5:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights. Gastroenterology. 2001;120:250‐260. [DOI] [PubMed] [Google Scholar]
- 42. Nemeth E, Baird AW, O'Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol. 2009;31:333‐343. [DOI] [PubMed] [Google Scholar]
- 43. Fichtner AS, Ravens S, Prinz I. Human gammadelta TCR repertoires in health and disease. Cell. 2020;9(4):800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836‐840. [DOI] [PubMed] [Google Scholar]
- 45. Hunter S, Willcox CR, Davey MS, et al. Human liver infiltrating gammadelta T cells are composed of clonally expanded circulating and tissue‐resident populations. J Hepatol. 2018;69:654‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kenna T, Golden‐Mason L, Norris S, et al. Distinct subpopulations of gamma delta T cells are present in normal and tumor‐bearing human liver. Clin Immunol. 2004;113:56‐63. [DOI] [PubMed] [Google Scholar]
- 47. Day MJ. Immunohistochemical characterization of the lesions of feline progressive lymphocytic cholangitis/cholangiohepatitis. J Comp Pathol. 1998;119:135‐147. [DOI] [PubMed] [Google Scholar]
- 48. van den Ingh TSGAM, Cullen JM, Twedt DC, et al. Chapter 5—Morphological classification of biliary disorders of the canine and feline liver. In: Rothuizen J, Bunch SE, Charles JA, et al., eds. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Edinburgh: W.B. Saunders; 2006:61‐76. [Google Scholar]
- 49. Marsilio S, Ackermann MR, Lidbury JA, et al. Results of histopathology, immunohistochemistry, and molecular clonality testing of small intestinal biopsy specimens from clinically healthy client‐owned cats. J Vet Intern Med. 2019;33:551‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chow B, Hill SL, Richter KP, et al. Comprehensive comparison of upper and lower endoscopic small intestinal biopsy in cats with chronic enteropathy. J Vet Intern Med. 2021;35:190‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Multiplex primer optimization phase. Electropherograms of multiplex TRG (TRG2) clonality PCR assays tested with DNA from 2 normal livers with unexpected invariant/canonical rearrangement mimicking a clonal rearrangement at approximately 98 bp (arrow) when the TRGV5‐3 gene primer was pooled with multiplex J gene primers (upper panels). The suspected canonical rearrangements without polyclonal background were found at 98 bp when the primer pair of TRGV5‐3 and TRGJ5‐1 genes were used in isolation as a singleplex assay (lower panels). Triangles indicate 50 and 500 bp markers.
Table S1. Signalment and electrophoresis profiles of TR clonality in lymphoma samples from 89 cats.
Table S2. Signalment and electrophoresis profiles of TR clonality in non‐lymphoma samples from 35 cats.
Table S3. Signalment and electrophoresis profiles of TRG clonality in livers from 31 cats with histopathology and immunophenotyping indicative of possible small T cell lymphoma.


