Abstract
In the last 20 years, the diagnosis of pancreatitis has become more frequent as a result of improved diagnostic modalities such as abdominal ultrasound examination, advanced imaging, and immunoassays for the measurement of pancreatic lipase. Our aim is to provide a state‐of‐the‐art overview of the clinical diagnosis of acute pancreatitis (AP) in dogs with a particular focus on pancreatic lipase assay validation and clinical performance, in addition to advanced imaging modalities. We also discuss the potential indications for cytology and histopathology in dogs with suspected AP.
Keywords: acute pancreatitis, catalytic, CTA, cytology, DGGR, immunologic, lipase
Abbreviations
- 1,2 DiG
1,2 diglyceride
- AKI
acute kidney injury
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AP
acute pancreatitis
- AST
aspartate aminotransferase
- ASVCP
American Society for Veterinary Clinical Pathology
- AUC area
under curve
- AUS
abdominal ultrasound
- CEUS
contrast‐enhanced ultrasonography
- CHF
congestive heart failure
- CI
confidence interval
- CKD
chronic kidney disease
- CP
chronic pancreatitis
- cPLI
canine pancreatic lipase immunoreactivity
- CT
computed tomography
- CTA
computed tomographic angiography
- CV
coefficient of variation
- DGGR
1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric‐acid‐(6′methylresorufin) ester
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- EHBDO
extra hepatic bile duct obstruction
- EPI
exocrine pancreatic insufficiency
- FNA
fine needle aspirate
- GGT
γ‐glutamyl transferase
- HAC
hyperadrenocorticism
- ICC
intraclass correlation coefficient
- IL‐6
interleukin 6
- MMVD
myxomatous mitral valve disease
- MRI
magnetic resonance imaging
- RIA
radioimmunoassay
- ROC
receiver operator curve
- SDMA
symmetric dimethylarginine
- Tp
peak time
1. ABSENCE OF A WELL‐DEFINED CONSENSUS GOLD STANDARD
The diagnosis of AP in dogs is hampered by the lack of a universally‐accepted gold standard. 1 Traditionally, histopathology has been considered the gold standard for the diagnosis of pancreatitis and for distinguishing AP from chronic pancreatitis (CP). Acute pancreatitis is defined as inflammation of the exocrine pancreas that is not associated with permanent histopathologic changes, such as fibrosis and atrophy. 2 , 3 It is characterized primarily by neutrophilic inflammation, edema, and necrosis. 2 Chronic pancreatitis, in contrast, is characterized by fibrosis and acinar cell atrophy. 2 , 4 Lymphocytic or mixed mononuclear infiltrates commonly are reported. 2 , 5 , 6 The use of these definitions, although preferred by some authors, does not always reflect the clinical presentation of affected patients, and reliance on a histopathologic diagnosis for AP is questionable because lesions can be highly localized and the immediate clinical relevance of lesions is unclear. 6 , 7 , 8 , 9 Collection of histopathologic samples also is considered invasive, and dogs with severe AP may be poor anesthetic candidates. 10 Thus, many clinicians utilize a clinical reference standard, integrating the results of several diagnostic modalities. We utilize a combination of suggestive clinical history, physical examination findings, increased pancreatic lipase concentration and either suggestive ultrasonographic or advanced imaging findings to diagnose AP. This diagnostic strategy may select for only the more severe cases of AP, which may influence perceived test performance. This approach has its own limitations, including variation in diagnostic criteria among clinicians and resolution of discrepant findings (eg, imaging findings suggestive of pancreatitis in the absence of supportive clinicopathologic data), resulting in inconsistent diagnostic standards. 11 , 12 , 13 , 14 , 15 , 16 Consequently direct comparison among studies is often challenging. Agreement of diagnostic criteria, as with a consensus statement, could facilitate study design and thus progress in the study of pancreatitis. Of note, studies that compare multiple assays under the same study design may allow for better interassay comparisons and, alternatively, Bayesian latent class analysis, which allows for evaluation of test performance in the absence of a defined reference standard, could be valuable. 17 , 18 We will discuss diagnostic test performance in relation to a specified reference standard throughout this review.
2. CLINICAL SIGNS
No clinical sign or combination of signs has been identified as pathognomonic for AP in dogs. 10 Current paradigms suggest that AP has a more overt clinical presentation, which includes anorexia, vomiting, weakness, and abdominal pain, which is sometimes evidenced by the so‐called “prayer position”. 19 , 20 , 21 In contrast, CP often involves more subtle recurring gastrointestinal signs, but acute‐on‐chronic presentations also are recognized, and thus acute clinical presentations may not correlate to histopathologically acute disease. 22 Additional signs of AP may include diarrhea and evidence of nausea (eg, lip smacking or licking, ptyalism, eructation). 19 , 23 , 24 Additional details on clinical signs and the underlying pathophysiology are available in recent reviews. 8 , 25
3. PHYSICAL EXAMINATION FINDINGS
Similarly to clinical signs, physical examination findings in dogs with AP vary considerably, depending on the severity of AP, but may include dehydration, pain on abdominal palpation, increased rectal temperature, hypothermia, icterus, petechiation, ecchymosis, and ascites. 19 , 21 , 23 , 26 Additionally, signs of cardiovascular shock may be noted. Because of the nonspecific nature of clinical signs and physical examination findings, integration of this data with laboratory and imaging findings is essential to effectively diagnose AP. 27
4. ROUTINE CLINICOPATHOLOGICAL FEATURES
The CBC can reflect dehydration, characterized by increased PCV and total protein concentration. 19 Hypoproteinemia may occur as a result of a negative acute phase (albumin) response, loss of protein into inflammatory exudates, or secondary to fluid therapy. Thrombocytopenia can develop because of consumption secondary to inflammation, but when severe, thrombocytopenia can indicate disseminated intravascular coagulation. A left shift in neutrophils is relatively common and may occur in the absence of an increased white blood cell count. Thus, microscopic evaluation of a blood smear is important. 19 Serum concentrations of acute phase reactants, such as C‐reactive protein and inflammatory cytokines, such as interleukin‐6 (IL‐6) also may be increased. 28 , 29 , 30 , 31
The serum biochemistry profile may disclose azotemia, which can be prerenal or reflect acute kidney injury (AKI) secondary to pancreatitis. 19 , 28 , 32 , 33 , 34 A urinalysis before fluid therapy is necessary to differentiate these conditions. Additionally, serum symmetric dimethylarginine (SDMA) concentration may be a more sensitive biomarker for detection of AKI secondary to AP. 35 Increased serum liver enzyme activities (ie, alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase [ALP], and γ‐glutamyl transferase [GGT]) are frequent and likely reflect secondary reactive hepatic changes or posthepatic cholestasis from pancreatic inflammation compressing the common bile duct. Rarely, functional hepatic cholestasis secondary to a cytokine response or excess fatty acids can alter bilirubin metabolism. 19 , 26 , 36 , 37 , 38 Hypocalcemia is uncommon but may occur secondary to hypoalbuminemia or from formation of calcium salts with fatty acids in areas of necrosis. 36 , 39 Urine sediment examination may show cylindruria as with AKI or other nonspecific changes such as bilirubinuria, which may occur with extrahepatic bile duct obstruction (EHBDO). Proteinuria also may be present in dogs with AP. 40
4.1. Serum amylase activity
Amylase initially was proposed as a biomarker of AP after experimental studies in dogs, but subsequent studies indicated suboptimal performance, and alternate biomarkers were pursued. 41 Less than ideal specificity likely reflects the multiple tissues of origin of amylase. Indeed, dogs that have undergone total pancreatectomy still have considerable serum amylase activity. 42 In a study utilizing histopathology (22 samples) as the reference standard, serum amylase activity had a sensitivity of 18.2% for detection of pancreatitis, which was significantly lower relative to Spec cPL (Texas A&M University, Gastrointestinal Laboratory, College Station, Texas; 63.6%). 43 Dogs had both neutrophilic and lymphocytic pancreatic infiltrates on histopathology. Another study (84 dogs) utilizing a clinical reference standard comprised of history, physical examination findings, routine clinicopathologic data, and abdominal ultrasound (AUS), had a higher sensitivity of 52.4% to 54.6% and a specificity of 76.7% to 80.6%. 44 Yet another study (64 dogs) utilizing a similar clinical reference standard yielded a sensitivity and specificity of 68.9% and 81.8%, respectively. 45 Despite the moderate diagnostic performance of serum amylase activity in both of these studies, assays measuring pancreatic lipase performed more favorably in the same study designs when utilizing the recommended diagnostic cut‐offs. Additionally, other studies utilizing clinical signs and histopathology as reference standards have shown much lower sensitivities of 7% to 40.9%. 43 , 46 Amylase activity also has been shown to be increased in dogs with decreased renal function, which may complicate its interpretation. 47 Serum amylase activity therefore is no longer considered part of the routine diagnostic approach to AP in dogs. However, when serum amylase activity is substantially increased (>3‐5 times the upper reference interval) in a dog with suggestive clinical signs, pancreatitis should be considered a potential differential diagnosis.
5. LIPASE ASSAYS
Pancreatic lipase is derived from pancreatic acinar cells and, under normal physiologic conditions, very little enters the systemic circulation. 48 During pancreatic inflammation, large amounts of pancreatic lipase are released into the systemic circulation and can be used as a biomarker of AP. 44 , 49 , 50 Methodologically, lipase assays are either catalytic or immunologic. Catalytic assays reflect the enzymatic activity of a sample by quantification of substrate utilization or product accumulation, which for lipase typically is hydrolysis of a variety of substrates by multiple potential lipases. Colorimetric reactions are used as the detection mechanism and results are reported as enzyme activity. Assay specificity can be influenced by the substrate selected for the assay and additional factors such as the presence of cofactors such as bile acids or colipase, the pH of the assay, or wavelength used to measure light absorption. Immunological assays in contrast use antibodies to measure enzyme concentration. Selective assays leverage antibodies that are specific for canine pancreatic lipase. These assays are also dependent on specific methodologic conditions. One potential approach to evaluate the specificity of an assay is to measure the lipase concentration in dogs that are expected to have markedly decreased pancreatic lipase concentrations. Measurement of substantial amounts of detectable enzyme in such dogs would suggest detection of nonpancreatic sources of lipase. Historically, pancreatectomy was used as an experimental model, but more recent studies have utilized dogs with exocrine pancreatic insufficiency (EPI) as a naturally‐occurring model for assay specificity testing. Analytic validation is considered a prerequisite to clinical validation. Thus, in this review, we will discuss analytic validation for each assay before evaluation of clinical performance. Although quality assurance guidelines have been released by the American Society for Veterinary Clinical Pathology (ASVCP), not all analytic study designs are consistent with these guidelines. 51 The nature of validation techniques is discussed in the relevant sections below. Overviews are provided in Tables 1 and 2.
TABLE 1.
Overview of analytic and clinical validation data for common immunologic pancreatic lipase assays in dogs
| Assay | Analytic evaluation | Clinical evaluation | ||||
|---|---|---|---|---|---|---|
| Analytic validation | Measurement in EPI dogs a | Effect of heparinization b | Effect of lipemia, icterus, and/or hemolysis c | Histopathologic reference standard | Clinical reference standard | |
| Spec cPL/cPLI |
Laboratory: Inter‐ and intra‐assay CV <12% 52 Clinical: mean intra‐assay CV 5.5% 56 |
Median: 0.1 μg/L (RI: <400 μg/L) 57 98% of dogs had lipase concentration within lower 20% of RI (≤40 μg/L) 78 100% of dogs had lipase concentration within lower 25% of RI (≤50 μg/L) 78 |
No significant effect 74 | No significant effect 52 | ||
| SNAP cPL |
Laboratory: 96%‐100% agreement between SNAP cPL and Spec cPL, when Spec cPL lipase was within RI 63 88%‐92% agreement between SNAP cPL and Spec cPL when Spec cPL lipase > RI 63 Clinical agreement with Spec cPL k = 0.78 49 ICC = 0.92 50 |
– | – | No significant effect 63 | – | |
| VetScan cPL |
Laboratory: interassay CV 31.8%, intra‐assay CV 25.1% 65 Clinical: mean intra‐assay CV 17.0% 56 Clinical agreement with Spec cPL ICC = 0.96 50 |
– | – | No significant effect 56 | – |
Sensitivity: 73.9%‐83.3% 50 Specificity: 76.9%‐83.8% 50 |
| Vcheck cPL |
No laboratory evaluation Clinical: mean intra assay CV 23.7% 56 Clinical agreement with Spec cPL Unknown |
– | – | No significant effect 56 | – | – |
Dogs with EPI should have negligible serum concentrations of pancreatic lipase.
Heparinization leads to release of lipoprotein lipase and hepatic lipase.
Lipemia and icterus are commonly seen in dogs with suspected pancreatitis.
TABLE 2.
Overview of analytic and clinical validation data for common catalytic pancreatic lipase assays in dogs
| Assay | Analytic evaluation | Clinical evaluation | ||||
|---|---|---|---|---|---|---|
| Analytic validation | Measurement in EPI dogs a | Effect of heparinization b | Effect of lipemia, icterus, and/or hemolysis c | Histopathologic reference standard | Clinical reference standard | |
| DGGR lipase |
Laboratory: interassay CV <3% and intra‐assay CV ≤14% 69 Linearity: R 2 .98 69 Clinical agreement with Spec cPL k = 0.43‐0.68 69 , 75 ICC = 0.89 50 |
Median 34 U/L (RI: 20‐94 U/L) Lipase within RI in 33/48 dogs 73 |
Significant increases in lipase detected postheparinization 74 | No significant effect 69 | – | |
| v‐LIP‐P |
Laboratory: Inter‐ and intra‐assay CV < 5% 77 Clinical agreement with Spec cPL r = .91 79 |
58% of dogs had lipase concentration within lower 20% of RI (≤32 U/L) 78% of dogs had lipase concentration within lower 25% of RI (≤40 U/L) 78 |
– |
Intralipid administration increases v‐LIP‐P activity Naturally occurring hyperlipidemia had a lesser effect on v‐LIP‐P activity 78 Influenced by hemolysis 78 |
– |
Sensitivity: 100% 45 Specificity: 89.5% 45 |
Dogs with EPI should have negligible serum activities of pancreatic lipase.
Heparinization leads to release of lipoprotein lipase and hepatic lipase.
Lipemia and icterus are commonly seen in dogs with suspected pancreatitis.
5.1. Immunologic assays
5.1.1. Canine pancreatic lipase immunoreactivity
-
1Laboratory assays
-
1.1Original canine pancreatic lipase immunoreactivity assays and Spec cPL
-
1.1.1Development and validation studies
-
1.1.1
-
1.1
Although immunologic assays originally utilized radioisotopes, they were quickly replaced by a sandwich ELISA. 52 , 53 This assay was refined for commercial use, utilizing 2 monoclonal antibodies, each recognizing a specific epitope of pancreatic lipase (Spec cPL; Texas A&M University, Gastrointestinal Laboratory). 52 This assay was shown to be linear and have repeatability across the reportable range of the assay (30‐1000 μg/L; inter‐ and intra‐assay coefficient of variation [%CV] <12%) and lacked interference from lipid, hemoglobin, or bilirubin. 52 Linearity plots for Spec cPL (Texas A&M University, Gastrointestinal Laboratory) had an R 2 = .99. 52 The reference interval for the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay is 0 to 200 μg/L, with an equivocal zone between 201 and 399 μg/L, which suggests the need for repeat testing as clinically indicated. 54 Concentrations ≥400 μg/L are highly suggestive of pancreatitis. 10 , 54 The cut‐offs were determined based on a case series of sick dogs with histopathologic evaluation of the pancreas (personal communication JMS), but have been utilized and verified in several clinical studies. 46 , 49 , 50 , 55 A recent study also evaluated the repeatability of the Spec cPL assay (Texas A&M University, Gastrointestinal Laboratory) in a clinical setting and compared the results to 2 patient‐side pancreatic lipase assays. 56 In this study, serum samples from 12 dogs with clinical signs of gastrointestinal disease underwent repeat testing across the reportable range of each assay (6 replicates per assay) using the Spec cPL (Texas A&M University, Gastrointestinal Laboratory), VetScan cPL Rapid Test (Abaxis, Inc, Union City, California), and the Vcheck cPL (Bionote, Hwaseong‐si, Gyeonggi‐do, Republic of Korea). The Spec cPL (Texas A&M University, Gastrointestinal Laboratory) had the lowest %CV (5.5%; range, 2.9%‐5.2%) of the 3 assays and thus the highest repeatability. 56 Additionally, transportation variables (samples had to be mailed to a diagnostic laboratory) did not have significant effects on the results of the Spec cPLassay (Texas A&M University, Gastrointestinal Laboratory). 56
The specificity of the original sandwich canine pancreatic lipase immunoreactivity (cPLI) ELISA was evaluated in 25 dogs with EPI. Serum cPLI concentrations were low and the median concentration was 0.1 μg/L (range, 0.1‐1.4 μg/L) compared to 16.3 μg/L (range, 1.4‐270.6 μg/L) in the control group, suggesting that measurement of cPLI does not detect nonpancreatic lipases. 57 This also was shown to be the case with the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay, but this information has not been published in the peer‐reviewed literature to date. 58
-
1.1.2Clinical performance
-
1.1.2.1Utilizing histopathology as the assigned reference standard
-
1.1.2.1
An early study evaluating the clinical performance of the original cPLI assay evaluated its sensitivity in a population of 22 dogs with macroscopic evidence of pancreatitis at necropsy. 43 These dogs underwent necropsy for a variety of causes. Neutrophilic and lymphocytic inflammatory change, edema, necrosis, and pancreatic atrophy were scored separately. 43 All dogs had histologic evidence of low‐grade pancreatic inflammation as determined by a previously published grading scheme. 59 Twenty of the 22 dogs (90.9%) had clinical signs of pancreatitis and 6/9 (66.7%) had ultrasonographic evidence of pancreatitis. Sixteen of 22 dogs (72.3%) had cPLI concentrations ≥200 μg/L and 14/22 (63.4%) had cPLI concentrations ≥400 μg/L. Six of 22 (27.3%) dogs did not have increased cPLI concentrations. Of these 6 dogs, 1 had no clinical signs of AP, 1 had no ultrasonographic evidence of pancreatitis, 3 had clinical signs consistent with AP but did not have AUS performed, and 1 dog had both clinical signs and AUS findings suggestive of AP. 43 Because of study design, specificity was not determined. A subsequent study evaluated the specificity of the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay. In this study, 20 dogs with macroscopic evidence of pancreatitis and an additional 44 dogs surrendered for euthanasia underwent physical examination and serum Spec cPL (Texas A&M University, Gastrointestinal Laboratory) measurement before necropsy and histopathologic assessment of the pancreas. 60 Forty dogs had no histologic evidence of pancreatitis and the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentration was within reference intervals in 38/40, within the equivocal zone in 1/40, and 1/40 dogs had increased concentrations, indicating a specificity of 97.5%. 60 The equivocal zone result was not included in the calculation of specificity. Another study evaluated the sensitivity and specificity of the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay in 70 dogs comparing results from samples collected within 24 hours of death with semiquantitative histopathologic assessments of the severity of pancreatitis. Sixty‐three of 70 dogs (90%) had histopathologic features of pancreatitis. 46 The Spec cPLassay (Texas A&M University, Gastrointestinal Laboratory) had a sensitivity of 21% for histopathologically mild and 71% for histopathologically moderate to severe pancreatitis, with a corresponding specificity of 100%. 46 Histopathology identified features of AP and CP concurrently in 58/63 (92.1%) of samples, with only 5/63 (7.9%) having features of AP alone. A third study found sensitivity of 33% and specificity of 90%. 7 Different reported sensitivities could be related to suspected differences between the sensitivity of the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay for detection of AP vs CP, because of decreased enzyme leakage in a fibrotic or atrophied pancreas. 22
-
1.1.2.2
Clinical performance compared to a defined clinical reference standard or Bayesian analysis
The sensitivity of the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay reported in clinical diagnosis study designs tends to be higher than that reported in some histopathologic studies, possibly reflecting the frequent detection of histopathologic lesions in the absence of clinical pancreatitis. 6 , 9 In 1 of the first studies utilizing a clinical diagnosis, 38 dogs with clinical signs of acute abdominal disease had an SNAP canine pancreatic lipase (SNAP cPL; IDEXX Laboratories, Inc, Westbrook, Maine) and a Spec cPL (Texas A&M University, Gastrointestinal Laboratory) performed and results were compared to a clinical reference standard diagnosis based on patient history, physical examination findings, clinicopathologic data, and AUS. 49 Sensitivity, specificity, and accuracy for the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) were 70%, 77%, and 75%, respectively. 49 Agreement between Spec cPL (Texas A&M University, Gastrointestinal Laboratory) and clinical diagnosis had a kappa score of 0.43. 49 An additional study in 50 dogs presented for clinical signs of gastrointestinal disease documented sensitivity of 81.0% to 90.9% and specificity of 74.1% to 81.1% when compared to a clinical reference standard including AUS. 50 Agreement between Spec cPL (Texas A&M University, Gastrointestinal Laboratory) and clinical diagnosis had an intraclass correlation coefficient (ICC) of 0.68. 50 Another study utilized a latent class model to assess performance of the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay in 84 dogs. 44 In this study, the estimated sensitivity and specificity of the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay was 71.7% to 77.8% and 80.5% to 88.0%, respectively. 44
-
1.1.3
Discrepancies between abdominal ultrasound and Spec cPL assay results
A retrospective study evaluated medical records from 157 dogs that had AUS and Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay performed within 30 hours of each other. Abdominal ultrasound findings were weakly correlated with the results of the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay (r s = .0178, P = .03). 14 An additional study noted that changes in AUS may not occur until later in the disease, which may help explain some of the apparent differences documented in the aforementioned study. 16
-
2Patient‐side tests for cPLI
-
2.1SNAP canine pancreatic lipase
-
2.1.1Development and validation studies
-
2.1.1
-
2.1
The SNAP cPL (IDEXX Laboratories, Inc) is a patient‐side assay that utilizes antibodies against specific epitopes of canine pancreatic lipase. 61 , 62 A validation study utilized 49 serum samples distributed across the testable range of the assay, finding a 96% to 100% agreement with the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay when pancreatic lipase concentrations were within the reference interval and a 88% to 92% agreement when pancreatic lipase concentrations were above the reference interval. 63 Samples classified as normal by SNAP cPL (IDEXX Laboratories, Inc) but abnormal by Spec cPL (Texas A&M University, Gastrointestinal Laboratory) had pancreatic lipase concentrations within the diagnostic equivocal zone. 63 Assay precision was evaluated by performing 10 replicates per sample, replicated across 3 days with visually normal and abnormal results recorded. A single discrepant result occurred and was classified as normal despite having a Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentration of 337 μg/L (diagnostic equivocal zone). 63 No interference from bilirubin (up to 17‐19 mg/dL), lipids (up to 7.4 optical density units at 660 nm), or hemoglobin (up to 490‐510 mg/dL) was observed. 63
-
2.1.2
Clinical performance
Bayesian latent class analysis was used to estimate the sensitivity and specificity of the SNAP cPL (IDEXX Laboratories, Inc) assay in 84 dogs, and were found to be 91.5% to 94.1% and 71.1% to 77.5%, respectively. 44 Another study evaluated the clinical performance of the SNAP cPL (IDEXX Laboratories, Inc) assay in 38 client‐owned dogs presented to an emergency department with clinical signs of acute abdominal disease. 49 In that study, sensitivity and specificity of the SNAP cPL (IDEXX Laboratories, Inc) assay relative to a clinical reference standard that included AUS were 82% and 59%, respectively. 49 A false positive rate of 41% was reported. 49 The manufacturer states that a positive SNAP test (IDEXX Laboratories, Inc) must be confirmed by measuring Spec cPL (Texas A&M University, Gastrointestinal Laboratory) because the SNAP test (IDEXX Laboratories, Inc) is abnormal for dogs in the equivocal zone of 200 to 400 μg/L. 61 Agreement between SNAP cPL (IDEXX Laboratories, Inc) and clinical diagnosis had kappa score of 0.33. 49 In a third study, the sensitivity and specificity of the SNAP cPL (IDEXX Laboratories, Inc) assay were found to be 73.9% to 100% and 71.1% to 77.8% utilizing a clinical reference standard that included AUS. 50 Agreement between the SNAP cPL (IDEXX Laboratories, Inc, Westbrook, Maine) and Spec cPL (Texas A&M University, Gastrointestinal Laboratory) has been reported to have a kappa score of 0.78 and an ICC of 0.92. 49 , 50
-
2.2VetScan cPL rapid test
-
2.2.1Development and validation studies
-
2.2.1
The VetScan cPL Rapid Test (Abaxis, Inc) is a quantitative point‐of‐care immunoassay for the measurement of canine pancreatic lipase. 56 , 64 A partial analytical validation study of the VetScan cPL (Abaxis, Inc) assay was performed utilizing serum samples to assess linearity (6 samples), repeatability (10 samples) and reproducibility (3 samples) of results. 65 The linearity of the assay was evaluated using dilutional parallelism with observed‐to‐expected ratios of 119.3 ± 28.7%. Observed‐to‐expected ratios of 80% to 120% generally are considered acceptable. 65 The mean intra‐assay variability (assay runs were performed in succession) was 25.1% (range, 16.9%‐36.7%) and the mean interassay variability (assay runs were performed on consecutive days) was 31.8% (range, 14.1%‐51.2%). 65 Statistical methods for assessment of correlation were not performed. This study was performed in a research environment using stored serum samples. 66 A subsequent study was performed using serum from 12 dogs with clinical signs of gastrointestinal disease at the point of care. 56 The mean within‐day CV for the VetScan cPL (Abaxis, Inc) was 17.0% (range, 4.7%‐32.6%). 56 This study also noted that the least square‐mean cPLI concentration as measured for the VetScan cPL (Abaxis, Inc) assay was lower (558.5 μg/L) than that of the Spec cPL (Texas A&M University, Gastrointestinal Laboratory; 807.9 μg/L), suggesting that utilization of reference intervals designed for the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay, as outlined in the manufacturer's product guidelines, may not be suitable. 56 A comparative least‐square mean approach was performed to account for additional variations, such as different working ranges of each assay.
-
2.2.2
Clinical performance
The clinical performance of the VetScan cPL rapid test (Abaxis, Inc) was evaluated in a prospective study (50 dogs) comparing 4 pancreatic lipase assays to a clinical reference standard diagnosis including AUS. 50 The sensitivity of the VetScan cPL Rapid Test (Abaxis, Inc) was 73.9% to 83.3% and the specificity was 76.9% to 83.8%. 50 Agreement between the VetScan cPL (Abaxis, Inc, Union City) and Spec cPL (Texas A&M University, Gastrointestinal Laboratory) was reported to have an ICC of 0.96. 50
-
2.3Vcheck canine pancreas‐specific lipase assay
-
2.3.1Development and validation studies
-
2.3.1
The Vcheck cPL (Bionote) is a point‐of‐care immunoassay that utilizes antibodies that bind canine pancreatic lipase. 67 To our knowledge, no validation studies have been published, although the package insert notes a measurable range of 50 to 2000 μg/L and no interference by triglycerides or bilirubin. 67 This assay was included in a comparative repeatability study and the mean CV between replicates (performed on the same day) was 23.7% (range, 4.6%‐40.8%). 56 In that study, serum samples above the detectable limit of the assay were excluded from analysis, resulting in 9/12 dogs being available for assessment of repeatability. Serum samples from 5/9 dogs had a CV >20%. 56 Prior studies have reported a CV of ≤20% as acceptable. 65 This study also confirmed a lack of interference from gross hemolysis and lipemia on the results of the Vcheck cPL (Bionote) assay. 56 The VCheck cPL 2.0 (Bionote) subsequently has been developed, and further study is required to determine whether precision, linearity, and accuracy have been improved.
-
2.3.2
Clinical performance
To our knowledge, no peer‐reviewed publications have evaluated the clinical performance of the Vcheck cPL (Bionote) assay or its agreement with the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay.
5.2. Catalytic (activity) assays
-
1
1,2‐Diglyceride assays
Original lipase activity assays were based on hydrolysis of 1,2 diglyceride (1,2 DiG) resulting in a color change. 68 Although these assays were an improvement over other early lipase activity assays, 1,2 DiG assays also were shown to lack specificity with increased lipase activities noted postlaparotomy, and in dogs with renal and hepatic disease. 69 , 70 , 71 Given substantial increase in the use of 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester (DGGR) and triolein‐based assays in recent years, our review will focus on these methodologies.
-
2DGGR assays
-
2.1Validation and development
-
2.1
In 2001, a pancreatic lipase assay utilizing DGGR as a substrate was developed. 72 The assay first was studied in dogs in 2005, utilizing a Hitachi 911 automated analyzer (Boehringer Mannheim Corp, Laval, Quebec, Canada) and a commercially available DGGR reagent (Coloripase, NuClin Diagnostics, Inc, Northbrook, Illinois). 69 This validation study utilized serum from 30 dogs, 15 of which had a clinical diagnosis of AP based on history, clinical signs, and AUS. Low within‐run %CVs (<3%) and low to moderate day‐to‐day %CVs (≤14%) were found. The assay was linear, precise, and lacked interference by hemolysis (hemolytic index up to 1000 units) and lipemia (lipemia index up to 950 units). 69 The mean R 2 for linearity plots was .98. 69 Comparison of the catalytic rate constants for 1,2 DiG assays and DGGR assays suggested that DGGR assays were more selective than 1,2 DiG assays and may be more specific for pancreatic lipase. 69 Thus, utilization has shifted from 1,2 DiG assays to DGGR‐based assays over the past several years. However, a study published in abstract form noted that serum lipase concentrations, measured by a DGGR lipase assay, were within reference limits in 33/48 dogs with EPI, in which pancreatic lipase concentrations should be undetectable. 73 This observation and significant postheparinization increases in serum lipase activity in dogs using these assays suggest that DGGR is not exclusively hydrolyzed by pancreatic lipase. 74 In 2018, another DGGR‐based assay (DiaSys Lipase DC FS, Holzheim, Germany) underwent analytic validation. 75 The assay was linear (R 2 = 0.998; lipase, 51‐246 nmol/L), and low interassay (0.3%‐1.1%) and intra‐assay (0.7%‐0.9%) %CVs were reported. 75
-
2.2
Clinical performance
In 2005, the performance of the DGGR lipase assay (automated analyzer: Hitachi 911, Boehringer Mannheim Corp, DGGR substrate: Coloripase, NuClin Diagnostics, Inc) was compared to a clinical reference standard based on patient history, clinical signs, and AUS findings. 69 The sensitivity of the assay with a diagnostic cut‐off of 120 U/L was determined to be 93%, with a corresponding specificity of 53%. Using a cut‐off of 180 U/L improved the specificity to 66% with a corresponding sensitivity of 73%. 69 Receiver operator curve (ROC) analysis identified similar areas under the curve (AUCs) for both cut‐offs. In 2014, the clinical performance of a DGGR‐based lipase assay (Lipase Colorimetric, Roche Diagnostics, Switzerland) in 142 dogs was compared to the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay, and showed kappa scores 0.55 to 0.79, depending on the cut‐off utilized. 76 The kappa coefficient was 0.55 (0.43‐0.67) when utilizing the recommended reference intervals. Although this finding was reported as a high level of agreement, Cohen's kappa statistic was mainly designed to assess agreement or disagreement of operators of subjective diagnostic tests (eg, abdominal ultrasound, cytology, histopathology) and for 2 assays measuring the same analyte, strong agreement >0.9 would be expected. In 2018, another study evaluated the clinical performance of a DGGR lipase assay produced by a different manufacturer (DiaSys Lipase DC FS). The assay was analytically validated and the clinical performance of the assay was evaluated in 18 dogs. 75 The DGGR lipase assay showed agreement with the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay (k = 0.68). 75 In 2018, the clinical performance of a different DGGR lipase‐based assay was studied (Precision PSL; Antech Diagnostics, Irvine, California) in 50 dogs with clinical signs of gastrointestinal disease. 50 The sensitivity and specificity of the assay compared to a clinical reference standard, that included AUS were 85.7% to 90.9% and 64.0% to 74.3%, respectively. 50 Agreement with Spec cPL (Texas A&M University, Gastrointestinal Laboratory) had an ICC of 0.89. 50 The decreased specificity compared to the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) in the same population and using the same study design was suspected to be caused by a lack of substrate specificity of DGGR for pancreatic lipase. 74 To our knowledge, no studies have directly compared the performance of different DGGR lipase assays. Given the rapid and cost‐effective nature of DGGR lipase assays, several laboratories have incorporated these tests into routine serum biochemistry panels. As with other analytes, the results must be interpreted in light of the complete clinicopathologic picture.
-
2.3
Discrepancies between abdominal ultrasound and DGGR assay results
Similarly to the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay, discrepancies have been noted between the results of DGGR lipase assays and AUS in dogs with AP. 76 A 2014 study found that agreement between a DGGR lipase assay and AUS was fair (k = 0.29‐0.35). 76 The authors subsequently evaluated factors influencing correlation between AUS and pancreatic lipase, reporting that radiology submission forms containing the words “suspicion of pancreatitis” or “increased lipase” were more likely to generate a final AUS interpretation of pancreatitis compared to evaluation in the absence of these descriptors. 11 Blinded evaluation of AUS images is recommended when utilizing a clinical reference standard in research.
-
3Triolein‐based assays
-
3.1Fuji DRI‐CHEM v‐LIP‐P assay (V‐LIP‐P)
-
3.1.1Validation and development
-
3.1.1
-
3.1
An analytical validation of the V‐LIP‐P (Fujifilm Corporation, Minato‐ku, Tokyo, Japan) was published in abstract form in 2019. In this study, serum samples from 73 dogs with clinical signs of gastrointestinal disease were evaluated using the V‐LIP‐P assay (Fujifilm Corporation) in addition to DGGR‐based and 1,2 DiG‐based assays. 77 Intra‐ and interassay variabilities were low (<5%) and dilutional parallelism indicated high linearity (correlation coefficient close to 1). This data was published in abstract form and an exact R 2 value was unavailable. The ROC analysis also indicated a higher AUC for the V‐LIP‐P assay (Fujifilm Corporation; 0.90) compared to DGGR‐based (0.84), and 1,2 DiG based (0.84) assays. 77 Eight serum samples spiked with Intralipid (Fresenius Kabi, Clayton, North Carolina; ≥300 mg/dL) had significant increases in serum lipase concentration and were above the working range of the V‐LIP‐P assay (Fujifilm Corporation). Hemolysis also was reported to affect lipase activity. This marked increase in lipase activity because of lipemia however was likely a consequence of the model used for evaluating the effect of hyperlipidemia. The standard for such studies uses the addition of Intralipid to serum, which has been reported to falsely amplify the effects of lipids on lipase activity assays. In 8 dogs with naturally‐occurring hyperlipidemia, the effects of lipid on V‐LIP‐P (Fujifilm Corporation) results were lower. 78 This study also evaluated V‐LIP‐P (Fujifilm Corporation) lipase concentrations in 50 dogs with EPI. The study found significant differences between lipase concentrations measured by the V‐LIP‐P (Fujifilm Corporation) assay when compared to healthy dogs. However, overlap existed and 42% of dogs had lipase concentrations above the lower 20% of the reference interval, indicating that many dogs had substantial detection of lipase by the V‐LIP‐P (Fujifilm Corporation) assay. Thus, triolein does not appear to be specific for the measurement of lipase of pancreatic origin. 78
In 2011, serum lipase concentrations as measured by the V‐LIP‐P assay (Fujifilm Corporation) were compared to lipase concentrations measured by the Spec cPL (Texas A&M University, Gastrointestinal Laboratory) assay in 65 dogs. 79 Given that 12 dogs had serum lipase concentrations outside the detection limits, these were excluded from analysis. There was good correlation (r = .91) between the V‐LIP‐P (Fujifilm Corporation) and Spec cPL (Texas A&M University, Gastrointestinal Laboratory). Evaluation of the correlation coefficient curves indicates increasing variation at higher lipase activities or concentrations, beginning at a Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentration of approximately 400 μg/L. 79
-
3.1.2
Clinical performance
In 2016, a study of 64 dogs evaluated the clinical utility of various assays including the V‐LIP‐P assay (Fujifilm Corporation) in a primary care hospital setting. 45 Serum amylase activity and the V‐LIP‐P assay (Fujifilm Corporation) were compared to a clinical reference standard of compatible clinical signs, AUS findings, and increased Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentration. This study reported sensitivity and specificity of 100% (95% CI, 87.7%‐100%) and 89.5% (95% CI, 66.9%‐98.7%) respectively when using a cut‐off of 160 U/L. 45 This study also reported a higher diagnostic performance of amylase activity in the diagnosis of AP than previously reported, suggesting that case selection may have impacted the sensitivities and specificities reported in the study. 45
6. PANCREATIC LIPASE CONCENTRATIONS IN DOGS WITH PRIMARY DISEASES OTHER THAN PANCREATITIS
6.1. Renal disease
Early studies of surgically‐induced chronic renal failure noted increases in serum lipase activity as measured by a functional assay, suggesting that hyperlipasemia was a poor biomarker of AP in dogs with chronic kidney disease (CKD). 47 However, a subsequent study, using similar methodologies, reported no clinically relevant increases in lipase or serum cPLI concentrations, thus questioning the results of the previous experiment. 80 Recent studies also have investigated the relationship AKI and lipase concentration. In 2016, 2/5 young purpose‐bred dogs with gentamicin‐induced AKI developed increases in Spec cPL (Texas A&M University, Gastrointestinal Laboratory), and 4/52 serially measured Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations were consistent with a diagnosis of pancreatitis. 55 Additionally, Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations did not consistently correlate with serum creatinine concentrations, and the mechanism for the increases therefore is unlikely to be attributed to glomerular filtration rate. 55 This finding is not unexpected because pancreatic lipase is negatively charged and fairly large, with a molecular mass of approximately 50.7 kDa. 81 A retrospective study of 864 serum samples at a commercial laboratory found that azotemic dogs had higher median amylase activity, lipase activity, and cPLI concentrations when compared to nonazotemic dogs. 82 Likewise, a retrospective study documented that renal disease was the most common nonpancreatic cause of increased DGGR lipase activity, but the increases were poorly correlated with plasma creatinine concentrations, suggesting a complex interaction. 83 An additional study noted a high prevalence of increased Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations in dogs with hemodialysis‐dependent AKI. 84 The combined interpretation of these studies suggests that renal function may have an impact on some lipase assays. However, they also point to a complex interplay between the kidneys and the pancreas in naturally‐occurring disease, the mechanism of which requires further study.
6.2. Cardiac disease
Increased Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations have been reported in dogs with myxomatous mitral valve disease (MMVD). 85 In 42 dogs with congestive heart failure (CHF), Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations were correlated with severity of heart failure and given that CHF is associated with occult tissue hypoperfusion, we suspect that this correlation may reflect subclinical secondary pancreatic injury in CHF. 85 , 86 Alternatively, the increased pancreatic lipase concentrations may be secondary to pancreatic edema in the absence of inflammation, which recently was reported in a pilot study of dogs with portal hypertension. 87 Lipase activity as measured by a catalytic assay also has been shown to be increased in dogs with MMVD. 88
6.3. Endocrine disease
Increased Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations are reported in 73% of dogs with diabetic ketoacidosis (DKA). 89 In this study of 119 dogs, an increased Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentration did not affect clinical outcome or duration of hospitalization. 89 Diabetes mellitus (DM) is associated with a 12.4× increased risk of pancreatitis in dogs, and these diseases may occur concurrently with overlapping clinical signs. 90 Dogs with DM and increased lipase concentrations should be evaluated for abdominal discomfort and have AUS performed. They also should be evaluated for hypertriglyceridemia because it may mediate the relationship between these diseases. 90 Increased Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations have been reported in dogs with hyperadrenocorticism (HAC), 35% of which had no clinical signs of AP in 1 study. 91 Increased DGGR lipase and 1,2 DiG lipase concentrations also have been reported in 22 dogs with HAC and no clinical or ultrasonographic signs of AP. Given that exogenous glucocorticoids cause only minor increases in Spec cPL (Texas A&M University, Gastrointestinal Laboratory) and DGGR‐lipase, we suspect that increased lipase concentrations in dogs with HAC reflect subclinical pancreatic injury of unknown clinical relevance. 92 , 93 , 94 This occult pancreatitis hypothesis is supported by a study reporting pancreatic hyperechogenicity in dogs with HAC. 95 Although a hyperechoic pancreas is not a feature of AP, hyperechogenicity is a suspected ultrasonographic feature of CP in dogs based on anecdotal reports, data from a case series, and experimental models. 96 , 97 , 98 , 99 Regions of pancreatic hyperechogenicity also have been reported in humans with CP. 100 , 101 Additional studies are needed to further substantiate the current limited understanding of AUS features of naturally‐occurring CP in dogs before definitive conclusions are made. Some studies also note clinically relevant increases in pancreatic lipase in dogs treated with exogenous glucocorticoids. In 1 study, Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations ≥400 μg/L were noted in 5/10 dogs with immune‐mediated disease treated with prednisolone. 102 Additionally, another study noted no histopathologic evidence of pancreatitis in dogs on glucocorticoids. 94 Thus, the contrasting results of these studies suggest that more research is needed. Until then, clinicians should utilize clinical signs and diagnostic imaging to determine whether these dogs require analgesics and other supportive management for AP.
6.4. Other diseases
Pancreatic lipase concentrations are increased in several infectious diseases including parvovirus gastroenteritis, babesiosis, and monocytic ehrlichiosis. 103 , 104 , 105 , 106 Pancreatic lipase concentrations also may be increased in dogs with intervertebral disc disease, foreign bodies and gastric‐dilatation and volvulus. 49 , 107 , 108 , 109 These increases in pancreatic lipase concentration may be secondary effects on the exocrine pancreas associated with the primary disease condition. A complete clinical evaluation including history, physical examination, and diagnostic imaging is required to assist in interpretation of lipase concentrations in these conditions.
7. ADDITIONAL DIAGNOSTIC MARKERS
7.1. Micro‐RNA biomarkers
The diagnostic potential of microRNAs in the detection of pancreatic injury was evaluated using a caerulein infusion model of AP. 110 In this study, miR‐148a, miR‐216a, miR‐216b, miR‐217, and miR‐375 were increased coincident with histopathological evidence of acinar cell injury. 110 These micro‐RNAs had higher peak concentrations and a wider dynamic range than traditional serum amylase and lipase activities. 110 A subsequent study reported that miR‐126a and miR‐375 concentrations reflected the extent of pancreatic injury in dogs with experimentally‐induced pancreatitis. 111
8. DIAGNOSTIC IMAGING
8.1. Abdominal radiography
Radiographic findings associated with AP include decreased serosal detail within the cranial abdomen, a focal mass effect between the pyloroduodenal angle and colon, and mild gas dilatation of the duodenum. 19 Radiographs are also useful in ruling out other differential diagnoses in dogs with acute abdominal disease.
8.2. Abdominal ultrasound findings
Abdominal ultrasound findings consistent with AP include pancreatic enlargement, hypoechoic parenchyma (focal or diffuse), hyperechoic surrounding mesenteric fat, peri‐pancreatic fluid, EHBDO, or some combination of these (Figure 1). 19 , 26 , 37 , 112 , 113 Sequelae, such as pancreatic and peri‐pancreatic fluid accumulations (eg, abscesses, cysts, pseudocysts) and gastric wall edema are also readily identified using ultrasonography. 114 , 115 Despite its frequent use, B‐mode AUS can be affected by gas within the stomach or duodenum, patient discomfort, and operator inexperience. 116 Clinical information provided to the sonographer and disease severity also may influence interpretation. 11 Lastly, hypoalbuminemia, portal hypertension, and other physiologic conditions may result in pancreatic edema and similar ultrasonographic abnormalities. 117 , 118
FIGURE 1.
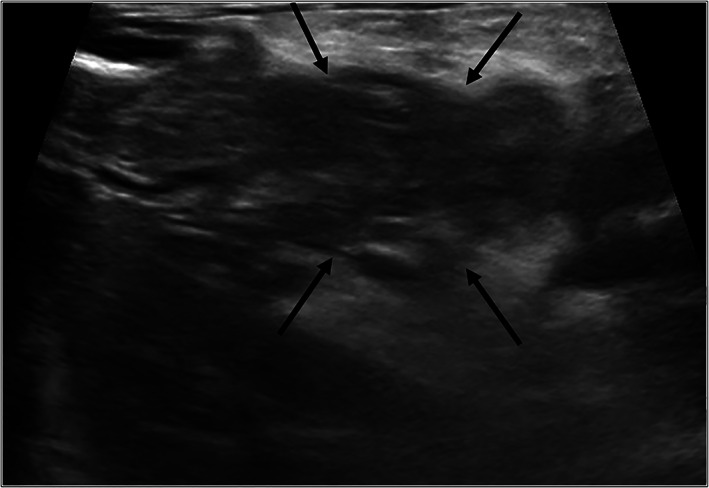
Ultrasonographic findings. Transverse plane B mode ultrasound image of the midbody of the pancreas (arrows). Note the hypoechoic pancreatic parenchyma with hyperechoic surrounding mesentery
Studies have indicated variable sensitivities and specificities for sonographic detection of AP in dogs, and these likely reflect differences in operator skill and the reference standard utilized. One study documented a sensitivity of 68% for ultrasonographic detection of fatal acute pancreatitis (based on clinical signs and histopathologic findings). 19 In a retrospective study of 157 dogs using a clinical reference standard, with AUS and Spec cPL (Texas A&M University, Gastrointestinal Laboratory) measured within 30 hours of each other, AUS had high sensitivity when utilizing a single ultrasonographic abnormality whereas 3 abnormalities were required to obtain high specificity. 14 Specifically, when only 1 of pancreatic enlargement, altered pancreatic echogenicity or hyperechoic mesentery was required for a diagnosis of AP, sensitivity was high at 89% (95% CI, 71.8‐97.7) but specificity was low at 43% (95% CI, 34.0‐51.6). 14 In contrast when all 3 changes were required, sensitivity and specificity were 43% (95% CI, 24.5‐62.8) and 92% (95% CI, 85.3‐95.7), respectively. 14 B‐mode ultrasonography also may be utilized for monitoring dogs with AP. In 1 study, changes in sonographic severity of pancreatitis were not correlated with changes in pancreatic lipase concentration, but the number of animals with repeat ultrasonographic evaluations was limited (12 dogs). 14 A recent study of 38 dogs utilized AUS repeated within approximately 2 days in patients suspected of having AP based on clinical signs and a point‐of care pancreatic lipase assay (SNAP cPL; IDEXX Laboratories, Inc). This study concluded that ultrasonographic signs of AP may not be present at the time of presentation and may only become observable later during hospitalization. 16 Thus, previously noted diagnostic discrepancies between AUS and pancreatic lipase may reflect different sampling timelines or alternatively different rates of resolution of AUS as compared to pancreatic lipase after an acute pancreatic insult.
8.3. Contrast‐enhanced ultrasonography
Contrast‐enhanced ultrasonography (CEUS) utilizes microbubble contrast agents to characterize focal lesions and organ perfusion. Regions of interest on the captured images are manually indicated, and dedicated quantification software assists in interpretation of the captured images. This modality recently was evaluated in dogs with AP, given its correlation with perfusion and ability to distinguish inflammatory (hyperechoic enhancement) and necrotic (nonenhancement) lesions in humans. 119 , 120 , 121 , 122 As in humans, CEUS was able to detect changes in pancreatic perfusion in dogs with naturally‐occurring AP and selected parameters could distinguish dogs with AP from control dogs. 122 , 123 In a prospective study of 23 dogs with pancreatitis, dogs with AP had a more prolonged peak time (Tp), a higher peak intensity, and higher AUC than did control dogs. When compared to a clinical reference standard composed of compatible clinical signs, an increased Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentration and standard B‐mode ultrasonography, a Tp ≥48 seconds had a sensitivity and specificity of 90% (95% CI, 70‐97) and 83% (95% CI, 55‐95), respectively. 122 Currently, CEUS is not widely available in veterinary medicine.
8.4. Elastography
Elastography is a noninvasive imaging technique that uses sound waves to assess tissue mechanical properties such as stiffness. 124 Diseased tissues can have different stiffness values when compared to healthy tissues, and specific software packages can detect these differences. Elastography recently was investigated in 25 dogs, including 9 with suspected AP based on clinical signs, abdominal discomfort and clinicopathologic data. 125 Shear wave velocity was higher in dogs with suspected AP than in the control group. 125
8.5. Computed tomography angiography
The speed of image acquisition using multislice computed tomography (CT) has increased dramatically, allowing for rapid image collection. 126 Computed tomography angiography (CTA) utilizes iodinated contrast material to better evaluate blood flow in organs and surrounding tissues. Iodinated contrast agents are excreted by the kidneys, which should be considered before using them in patients that are dehydrated or have concurrent kidney disease. 127 The normal pancreas is iso‐ to hypoattenuating relative to the spleen and liver, and uniformly undergoes contrast enhancement. 128 In AP, the pancreas is enlarged, hypoattenuating, and may show homogenous or heterogenous contrast enhancement (Figure 2A,B). 126 , 129 In a study of 26 dogs, heterogenous contrast enhancement of the pancreas, which likely indicates decreased vascularity, was associated with longer hospitalization, increased risk of relapse, higher likelihood of portal vein thrombosis (Figure 2A,B) and increased serum Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentrations. 129 When comparing CTA to AUS, CTA was found to be superior at identifying severe AP and portal vein thrombosis (10/26 dogs had thrombi on CT vs 1/26 dogs using AUS). 129 Although CTA allowed for better visualization of the pancreas, accuracy of detection of AP was not different between CTA and AUS. 129 Computed tomography angiography allows for rapid and complete evaluation of the pancreas and may detect imaging features and sequelae that affect clinical management. A recent study evaluated the role of repeat abdominal CTA in 11 dogs with AP and found that repeat CTA examinations were unlikely to be helpful in disease monitoring in the absence of worsening clinical signs. 130
FIGURE 2.
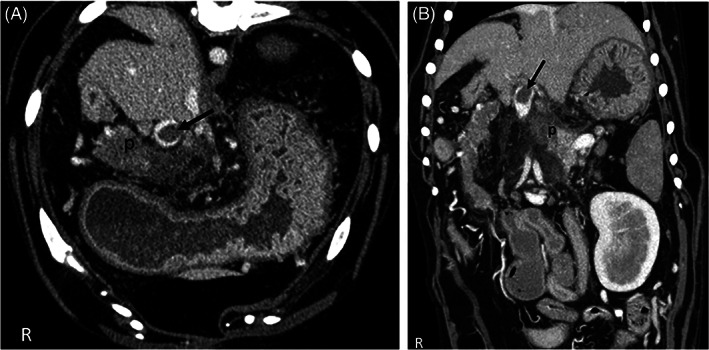
Transverse (A) and dorsal (B) CTA image. (A) CTA venous phase transverse plane image of the cranial abdomen. Note the heterogeneously contrast enhancing pancreas (p) and oval thrombus in the portal vein (arrow). There is a large amount of fat stranding within the mesenteric fat surrounding the pancreas, indicating edema and inflammation. (B) CTA venous phase dorsal plane image of the cranial abdomen. Note the heterogeneously contrast enhancing pancreas (p) and oval thrombus in the portal vein (arrow)
8.6. Magnetic resonance imaging
On magnetic resonance imaging (MRI), the normal pancreas is uniformly hyperintense relative to the liver on T1‐weighted images and is iso‐ to hypointense on T2 fat‐saturated images. When evaluating for pancreatitis, in both humans and cats, MRI images are assessed for pancreatic parenchymal hypersensitivity (on T2 fat saturated images), contrast enhancement, pancreatic duct dilatation, and peri‐pancreatic abnormalities. 131 , 132 Currently, no peer‐reviewed publications are available on the use of MRI in dogs with suspected AP.
9. PANCREATIC CYTOLOGY AND HISTOPATHOLOGY
Many clinicians are hesitant to sample the pancreas, but the complication rate for doing so is reported to be very low. 133 , 134 A study of 27 healthy dogs undergoing pancreatic fine needle aspiration (FNA) and surgical biopsy identified no increase in serum Spec cPL (Texas A&M University, Gastrointestinal Laboratory) concentration after sampling. 133 Additionally, a study of 92 dogs documented no adverse effects in 92.6% of dogs, and those that did have complications often had concurrent sampling of other tissues performed and life‐threatening comorbidities. 134 Complications included fever (3 dogs), hemoabdomen (1 dog), and seizures (1 dog). Four dogs had cardiac arrest between 3 hours and 4 days after the procedure. Given the nature of the study and the presence of clinically relevant comorbidities, a relationship could not be determined between these complications and pancreatic aspiration. In dogs with AP, cytologic findings include degenerate or nondegenerate neutrophils, cellular and necrotic debris, lipid mineralization, and clusters of normal to hyperplastic exocrine pancreatic cells (Figure 3). 135 The diagnostic yield of pancreatic cytology is similar to that of other abdominal organs at approximately 73.5%. 134 In a study that evaluated the diagnostic yield and complication rate of pancreatic FNA in dogs, cytologic findings were shown to correlate with additional testing in 90.1% of cases, but only 11 dogs had confirmatory testing performed. 134 Cytology may be most useful when pancreatic neoplasia is considered a likely differential diagnosis.
FIGURE 3.
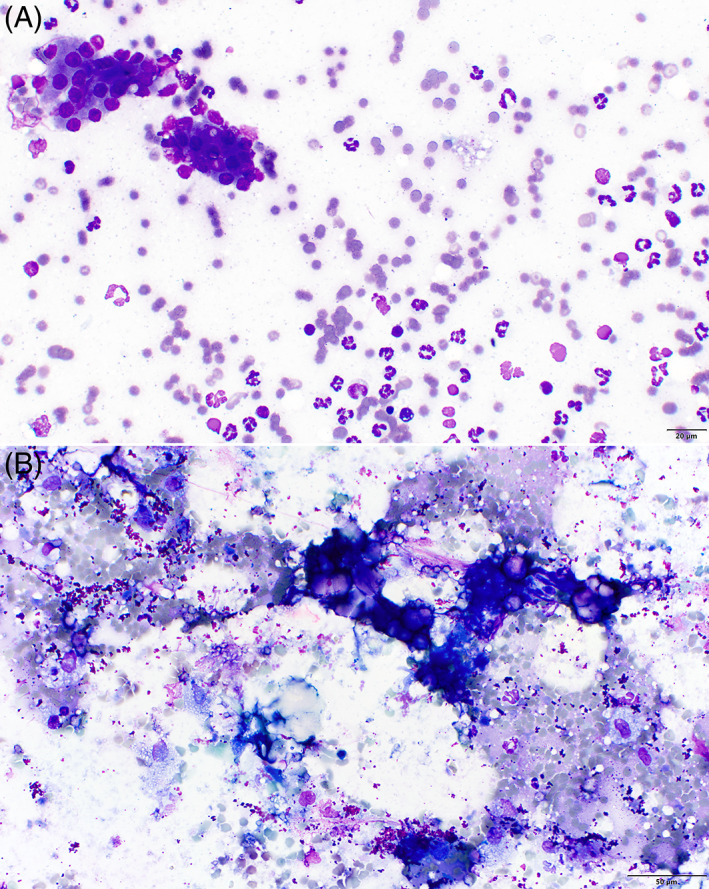
Cytologic findings. (A) Microscopic image of pancreatitis with mature exocrine pancreatic cells in the upper left, accompanied by red blood cells and increased numbers of nondegenerate neutrophils in a coarsely granular proteinaceous background (500×, Wright Giemsa Stain, image provided by Francisco O. Conrado). (B) Microscopic image of pancreatitis containing mineralized material (hyperchromatic purple refractile material), red blood cells, neutrophils, and scattered vacuolated macrophages that likely contain lipid from fat degradation. The background also contains purple granular material consistent with lubricant ultrasound coupling gel (400×, Wright Giemsa Stain, image provided by Francisco O. Conrado)
Although histopathology is rarely performed in the diagnostic approach to AP, it may be considered in cases of suspected neoplasia or when a patient with pancreatitis must undergo exploratory laparotomy for another reason. 118 , 136 , 137 Pancreatic carcinomas, although rare in dogs and cats, can lead to nonspecific clinical signs, and concurrent inflammation can limit the detection of pancreatic carcinomas in the absence of histopathologic evaluation. 136 , 137 Both cytology and histopathology are considered highly specific for a diagnosis of AP in that the presence of acinar cells and inflammatory cells identifies the presence of pancreatitis. In a study of 47 nontargeted pancreatic necropsy samples, 16% had suppurative inflammation in just 1 region, whereas 17% had necrosis in just 1 region and 55% had lymphocytic inflammation within in just 1 area of the pancreas. 9 Thus, a single surgical biopsy sample likely would have low and variable sensitivity for detection of pancreatic inflammation and necrosis. Therefore, a negative finding should not be considered definitive. 9 Finally, the diagnostic agreement between cytologic and histopathologic samples of the exocrine pancreas is poorly documented, and more studies are required in this area, including investigations incorporating clinical follow‐up.
10. CONCLUSIONS
Substantial advances have been made in the diagnostic approach to AP in dogs over the past several years, predominantly in the areas of diagnostic imaging and lipase assays. We utilize a combination of suggestive clinical signs and physical examination findings, in conjunction with increased pancreatic lipase concentration and consistent findings on diagnostic imaging to make a clinical diagnosis of AP. Analytic validation is a prerequisite to clinical investigation when considering the use of lipase assays. Additionally, several diseases other than AP may result in increases in serum amylase or lipase activities. Although AUS frequently is used in the diagnosis of AP, the number of abnormalities detected likely influences test performance, with more abnormalities resulting in higher diagnostic specificity. Diseases other than AP also may result in similar ultrasonographic changes. It is likely that CTA and other advanced imaging modalities such as CEUS will play a larger role in the diagnosis of AP in dogs in the future. Given the lack of a single diagnostic gold standard and limited agreement between any single diagnostic modality and clinical reference standard, integration of clinical findings, imaging results, lipase assays, and cytologic or histopathologic findings, where available, will provide optimal diagnostic results. Enzyme activities or diagnostic imaging should not be utilized in isolation. Future studies evaluating the diagnostic performance of any assay or imaging modality in AP should utilize blinded assessment of data and should specify in detail the nature of the clinical reference standard utilized, including the sampling timeline and duration of clinical findings. Given the limitations of retrospective studies, well‐designed prospective studies are needed to investigate the performance of various diagnostic assays, imaging modalities and agreement between tests. Studies utilizing histopathology should clearly distinguish analysis of performance for AP vs CP. Furthermore, integration of diagnostic criteria should facilitate progress in the study of pancreatitis in dogs.
CONFLICT OF INTEREST DECLARATION
Dr. Steiner is affiliated with the Gastrointestinal Laboratory at Texas A&M University, which offers measurement of cPLI testing on a fee‐for‐service basis. Dr. Steiner also serves as a paid consultant and speaker for IDEXX Laboratories, the manufacturer of the Spec cPL and SNAP cPL assays. Dr. Cridge has published research funded by Abaxis, Inc and the Gastrointestinal Laboratory at Texas A&M University.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no‐off label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC, or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. The authors of this review thank Andrea Kepsel of the Michigan State University library service who assisted in performance of a comprehensive literature search in this area.
Cridge H, Twedt DC, Marolf AJ, Sharkey LC, Steiner JM. Advances in the diagnosis of acute pancreatitis in dogs. J Vet Intern Med. 2021;35(6):2572-2587. doi: 10.1111/jvim.16292
REFERENCES
- 1. Lidbury JA, Suchodolski JS. New advances in the diagnosis of canine and feline liver and pancreatic disease. Vet J. 2016;215:87‐95. [DOI] [PubMed] [Google Scholar]
- 2. Watson P. Pancreatitis in dogs and cats: definitions and pathophysiology. J Small Anim Pract. 2015;56(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 3. Lack E. Pathology of the Pancreas, Gallbladder, Extrahepatic Biliary Tract and Ampullary Region. New York: Oxford University Press; 2003. [Google Scholar]
- 4. Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682‐707. [DOI] [PubMed] [Google Scholar]
- 5. Bostrom BM, Xenoulis PG, Newman SJ, Pool RR, Fosgate GT, Steiner JM. Chronic pancreatitis in dogs: a retrospective study of clinical, clinicopathological, and histopathological findings in 61 cases. Vet J. 2013;195(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 6. Watson PJ, Roulois AJA, Scase T, Johnston PEJ, Thompson H, Herrtage ME. Prevalence and breed distribution of chronic pancreatitis at post‐mortem examination in first‐opinion dogs. J Small Anim Pract. 2007;48(11):609‐618. [DOI] [PubMed] [Google Scholar]
- 7. Mansfield CS, Anderson GA, O'Hara AJ. Association between canine pancreatic‐specific lipase and histologic exocrine pancreatic inflammation in dogs: assessing specificity. J Vet Diagn Investig. 2012;24(2):312‐318. [DOI] [PubMed] [Google Scholar]
- 8. Mansfield C. Acute pancreatitis in dogs: advances in understanding, diagnostics, and treatment. Top Companion Anim Med. 2012;27(3):123‐132. [DOI] [PubMed] [Google Scholar]
- 9. Newman S, Steiner J, Woosley K, Barton L, Ruaux C, Williams D. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med. 2004;18(4):488‐493. [DOI] [PubMed] [Google Scholar]
- 10. Xenoulis PG. Diagnosis of pancreatitis in dogs and cats. J Small Anim Pract. 2015;56(1):13‐26. [DOI] [PubMed] [Google Scholar]
- 11. Kook P, Hammes K. Evaluation of anamnestic and clinicopathologic factors that might explain the poor correlation between pancreatic lipase concentrations (DGGR‐lipase and Spec cPL) and ultrasonographic evidence of pancreatitis in dogs. Paper presented at: ECVIM‐CA Online Congress; 2020:19.
- 12. Kim MJ, Song JH, Hwang TS, Lee HC, Jung DI. Comparison between SNAP canine pancreas‐specific lipase (cPL) test results and pancreatic ultrasonographic findings in dogs with pancreatitis. J Vet Clin. 2017;34(4):229‐233. [Google Scholar]
- 13. Paran E, Hygonnard M. Agreement of feline and canine pancreas‐specific lipase with pancreatic ultrasonographic findings in 62 cats and 54 dogs with suspicion of pancreatitis: a retrospective study (2007‐2013). J Vet Intern Med. 2017;31(1):261‐262. [Google Scholar]
- 14. Cridge H, Sullivant AM, Wills RW, Lee AM. Association between abdominal ultrasound findings, the specific canine pancreatic lipase assay, clinical severity indices, and clinical diagnosis in dogs with pancreatitis. J Vet Intern Med. 2020;34(2):636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kook PH, Kranjc A, Dennler M, Glaus TM. Pancreatitis associated with clomipramine administration in a dog. J Small Anim Pract. 2009;50(2):95‐98. [DOI] [PubMed] [Google Scholar]
- 16. Puccini Leoni F, Pelligra T, Citi S, et al. Ultrasonographic monitoring in 38 dogs with clinically suspected acute pancreatitis. 2020;7(4):180. doi: 10.3390/vetsci7040180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Branscum AJ, Gardner IA, Johnson WO. Estimation of diagnostic‐test sensitivity and specificity through Bayesian modeling. Prev Vet Med. 2005;68(2–4):145‐163. [DOI] [PubMed] [Google Scholar]
- 18. Enøe C, Georgiadis MP, Johnson WO. Estimation of sensitivity and specificity of diagnostic tests and disease prevalence when the true disease state is unknown. Prev Vet Med. 2000;45(1–2):61‐81. [DOI] [PubMed] [Google Scholar]
- 19. Hess R, Saunders M, Van Winkle T, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986‐1995). J Am Vet Med Assoc. 1998;213(5):665‐670. [PubMed] [Google Scholar]
- 20. Fabrès V, Dossin O, Reif C, et al. Development and validation of a novel clinical scoring system for short‐term prediction of death in dogs with acute pancreatitis. J Vet Intern Med. 2019;33(2):499‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weatherton LK, Streeter EM. Evaluation of fresh frozen plasma administration in dogs with pancreatitis: 77 cases (1995‐2005): retrospective study. J Vet Emerg Crit Care. 2009;19(6):617‐622. [DOI] [PubMed] [Google Scholar]
- 22. Watson P. Chronic pancreatitis in dogs. Top Companion Anim Med. 2012;27(3):133‐139. [DOI] [PubMed] [Google Scholar]
- 23. Simpson K. Update on the diagnosis & management of canine pancreatitis. Paper presented at: OVMA Conference Proceedings. 2015:94–97.
- 24. Linklater A. Canine pancreatitis. Clin Brief. 2013;83‐86. [Google Scholar]
- 25. Mansfield C. Pathophysiology of acute pancreatitis: potential application from experimental models and human medicine to dogs. J Vet Intern Med. 2012;26:875‐887. [DOI] [PubMed] [Google Scholar]
- 26. Palermo SM, Brown DC, Mehler SJ, Rondeau MP. Clinical and prognostic findings in dogs with suspected extrahepatic biliary obstruction and pancreatitis. J Am Anim Hosp Assoc. 2020;56(5):270‐279. [Google Scholar]
- 27. Romanita M, Niculae T, Cosmin M, Poliana T, Constantin V. Pancreatitis in dogs—clinical and laboratory evaluation. Curr Opin Biotechnol. 2013;24:S54. [Google Scholar]
- 28. Sato T, Ohno K, Tamamoto T, et al. Assesment of severity and changes in C‐reactive protein concentration and various biomarkers in dogs with pancreatitis. J Vet Med Sci. 2017;79(1):35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holm JL, Rozanski EA, Freeman LM, Webster CRL. C‐reactive protein concentrations in canine acute pancreatitis. J Vet Emerg Crit Care. 2004;14(3):183‐186. [Google Scholar]
- 30. Gori E, Pierini A, Lippi I, Ceccherini G, Perondi F, Marchetti V. Evaluation of C‐reactive protein/albumin ratio and its relationship with survival in dogs with acute pancreatitis. N Z Vet J. 2020;68(6):345‐348. [DOI] [PubMed] [Google Scholar]
- 31. Kuzi S, Mazaki‐Tovi M, Suchodolski JS, et al. Protease inhibitors, inflammatory markers, and their association with outcome in dogs with naturally occurring acute pancreatitis. J Vet Intern Med. 2020;34(5):1801‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gori E, Lippi I, Guidi G, Perondi F, Pierini A, Marchetti V. Acute pancreatitis and acute kidney injury in dogs. Vet J. 2019;245:77‐81. [DOI] [PubMed] [Google Scholar]
- 33. Nassar TI, Qunibi WY. AKI associated with acute pancreatitis. Clin J Am Soc Nephrol. 2019;14(7):1106‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marchetti V, Gori E, Lippi I, Luchetti E, Manca ML, Pierini A. Elevated serum creatinine and hyponatraemia as prognostic factors in canine acute pancreatitis. Aust Vet J. 2017;95(11):444‐447. [DOI] [PubMed] [Google Scholar]
- 35. Gori E, Pierini A, Lippi I, Meucci V, Perondi F, Marchetti V. Evaluation of symmetric dimethylarginine (SDMA) in dogs with acute pancreatitis. Vet Sci. 2020;7(2):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steiner JM. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract. 2003;33(5):1181‐1195. [DOI] [PubMed] [Google Scholar]
- 37. Wilkinson AR, DeMonaco SM, Panciera DL, et al. Bile duct obstruction associated with pancreatitis in 46 dogs. J Vet Intern Med. 2020;34(5):1794‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trauner M, Fickert P, Stauber RE. Inflammation‐induced cholestasis. J Gastroenterol Hepatol. 1999;14(10):946‐959. [DOI] [PubMed] [Google Scholar]
- 39. Feldman B, Attix E, Strombeck D, O'Neill S. Biochemical and coagulation changes in a canine model of acute necrotizing pancreatitis. Am J Vet Res. 1981;42(5):805‐809. [PubMed] [Google Scholar]
- 40. Gori E, Pierini A, Lippi I, Boffa N, Perondi F, Marchetti V. Urinalysis and urinary GGT‐to‐urinary creatinine ratio in dogs with acute pancreatitis. Vet Sci. 2019;6(1):27. doi: 10.3390/vetsci6010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brobst D, Ferguson AB, Carter J. Evaluation of serum amylase and lipase activity in experimentally induced pancreatitis in the dog. J Am Vet Med Assoc. 1970;157(11):1697‐1702. [PubMed] [Google Scholar]
- 42. Simpson KW, Simpson JW, Lake S, Morton DB, Batt RM. Effect of pancreatectomy on plasma activities of amylase, isoamylase, lipase and trypsin‐like immunoreactivity in dogs. Res Vet Sci. 1991;51(1):78‐82. [DOI] [PubMed] [Google Scholar]
- 43. Steiner J, Newman S, Xenoulis P, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9(4):263‐273. [PubMed] [Google Scholar]
- 44. McCord K, Morley PS, Armstrong J, et al. A multi‐institutional study evaluating the diagnostic utility of the Spec cPL and SNAP cPL in clinical acute pancreatitis in 84 dogs. J Vet Intern Med. 2012;26(4):888‐896. [DOI] [PubMed] [Google Scholar]
- 45. Yuki M, Hirano T, Nagata N, et al. Clinical utility of diagnostic laboratory tests in dogs with acute pancreatitis: a retrospective investigation in a primary care hospital. J Vet Intern Med. 2016;30(1):116‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas‐specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med. 2011;25(6):1241‐1247. [DOI] [PubMed] [Google Scholar]
- 47. Polzin D, Osborne C, Stevens J, Hayden D. Serum amylase and lipase activities in dogs with chronic primary renal failure. Am J Vet Res. 1983;44(3):404‐410. [PubMed] [Google Scholar]
- 48. Jasdanwala S, Babyatsky M. A critical evaluation of serum lipase and amylase as diagnostic tests for acute pancreatitis. Integr Mol Med. 2015;2(3):189‐195. [Google Scholar]
- 49. Haworth MD, Hosgood G, Swindells KL, Mansfield CS. Diagnostic accuracy of the SNAP and Spec canine pancreatic lipase tests for pancreatitis in dogs presenting with clinical signs of acute abdominal disease. J Vet Emerg Crit Care. 2014;24(2):135‐143. [DOI] [PubMed] [Google Scholar]
- 50. Cridge H, MacLeod AG, Pachtinger GE, et al. Evaluation of SNAP cPL, Spec cPL, VetScan cPL rapid test and precision PSL assays for the diagnosis of clinical pancreatitis in dogs. J Vet Intern Med. 2018;32(2):658‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Flatland B, Freeman KP, Friedrichs KR, et al. ASVCP quality assurance guidelines: control of general analytical factors in veterinary laboratories. Vet Clin Pathol. 2010;39(3):264‐277. [DOI] [PubMed] [Google Scholar]
- 52. Huth SP, Relford R, Steiner JM, Strong‐Townsend MI, Williams DA. Analytical validation of an ELISA for measurement of canine pancreas‐specific lipase. Vet Clin Pathol. 2010;39(3):346‐353. [DOI] [PubMed] [Google Scholar]
- 53. Steiner JM, Williams DA. Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res. 2003;64(10):1237‐1241. [DOI] [PubMed] [Google Scholar]
- 54.Pancreatic Lipase Immunoreactivity (PLI) [Internet]. Texas A&M University. https://vetmed.tamu.edu/gilab/service/assays/pli/. Accessed January 27, 2021.
- 55. Hulsebosch SE, Palm CA, Segev G, Cowgill LD, Kass PH, Marks SL. Evaluation of canine pancreas‐specific lipase activity, lipase activity, and trypsin‐like immunoreactivity in an experimental model of acute kidney injury in dogs. J Vet Intern Med. 2016;30(1):192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cridge H, Mackin AJ, Lidbury JA, Suchodolski JS, Steiner JM. Comparative repeatability of pancreatic lipase assays in the commercial and in‐house laboratory environments. J Vet Intern Med. 2020;34(3):1150‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steiner JM, Rutz GM, Williams DA. Serum lipase activities and pancreatic lipase immunoreactivity concentrations in dogs with exocrine pancreatic insufficiency. Am J Vet Res. 2006;67(1):84‐87. [DOI] [PubMed] [Google Scholar]
- 58. Steiner JM, Lidbury JA & Suchodolski JS The GI Lab: Promoting Gastrointestinal Health in Companion Animals; Spring 2016 [Newsletter]. https://vetmed.tamu.edu/gilab-dev/wp-content/uploads/sites/12/2019/11/newsletter_2016e_without-submission.pdf. Accessed April 8, 2021.
- 59. Newman SJ, Steiner JM, Woosley K, Williams DA, Barton L. Histologic assessment and grading of the exocrine pancreas in the dog. J Vet Diagn Investig. 2006;18(1):115‐118. [DOI] [PubMed] [Google Scholar]
- 60. Neilson‐Carley SC, Robertson JE, Newman SJ, et al. Specificity of a canine pancreas‐specific lipase assay for diagnosing pancreatitis in dogs without clinical or histologic evidence of the disease. Am J Vet Res. 2011;72(3):302‐307. [DOI] [PubMed] [Google Scholar]
- 61. IDEXX SNAP cPL Test—reference laboratory accuracy pet‐side [Internet]; 2016. https://www.idexx.com/files/snap-cpl-accuracy-white-paper.pdf. Accessed January 6, 2021.
- 62. Beall M, Huth S, Krah E. Antibodies that bind canine pancreatic lipase. US patent 7,722,875.
- 63. Beall MJ, Cahill R, Pigeon K, Hanscom J, Huth SP. Performance validation and method comparison of an in‐clinic enzyme‐linked immunosorbent assay for the detection of canine pancreatic lipase. J Vet Diagn Investig. 2011;23(1):115‐119. [DOI] [PubMed] [Google Scholar]
- 64. VetScan Canine Pancreatic Lipase Test Kit [Package Insert]. Union City, CA: Abaxis, Inc; 2017.
- 65. Steiner JM, Guadiano P, Gomez RR, Suchodolski JS, Lidbury JA. Partial analytical validation of the VetScan cPL rapid test. Vet Clin Pathol. 2019;48(4):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cridge H, Mackin AJ, Sullivant AM, et al. Response from Dr. Cridge, et al. to Dr. Steiner, et al. letter to editor regarding JVIM_15039. J Vet Intern Med. 2018;32:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vcheck cPL [Package Insert]; 2019. Hwaseong‐si, Gyeonggi‐do 18449, Republic of Korea.
- 68. Mackenzie AL, Burton SA, Olexson DW, et al. Evaluation of an automated colorimetric assay for the measurement of lipase activity in canine sera. Can J Vet Res. 1996;60(3):205‐209. [PMC free article] [PubMed] [Google Scholar]
- 69. Graca R, Messick J, McCullough S, Barger A, Hoffmann W. Validation and diagnostic efficacy of a lipase assay using the substrate 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′methyl resorufin)‐ester for the diagnosis of acute pancreatitis in dogs. Vet Clin Pathol. 2005;34(1):39‐43. [DOI] [PubMed] [Google Scholar]
- 70. Strombeck D, Farver T, Kaneko J. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. Am J Vet Res. 1981;42(11):1966‐1970. [PubMed] [Google Scholar]
- 71. Bellah J, Bell G. Serum amylase and lipase activities after exploratory laparotomy in dogs. Am J Vet Res. 1989;50(9):1638‐1641. [PubMed] [Google Scholar]
- 72. Panteghini M, Bonora R, Pagani F. Measurement of pancreatic lipase activity in serum by a kinetic colorimetric assay using a new chromogenic substrate. Ann Clin Biochem. 2001;38(4):365‐370. [DOI] [PubMed] [Google Scholar]
- 73. Steiner J, Suchodolski J, Gomez R. DGGR is not a specific substrate for pancreatic lipase. Paper presented at: WSAVA World Congress [Internet]; 2015:2. https://www.vin.com/doc/?id=7259064 Accessed January 6, 2021. [Google Scholar]
- 74. Lim SY, Xenoulis PG, Stavroulaki EM, et al. The 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase assay in cats and dogs is not specific for pancreatic lipase. Vet Clin Pathol. 2020;49(4):607‐613. [DOI] [PubMed] [Google Scholar]
- 75. Goodband EL, Serrano G, Constantino‐Casas F, Archer J, Watson PJ, Williams TL. Validation of a commercial 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′‐methylresorufin) ester lipase assay for diagnosis of canine pancreatitis. Vet Rec Open. 2018;5(1):6‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kook PH, Kohler N, Hartnack S, Riond B, Reusch CE. Agreement of serum spec cPL with the 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase assay and with pancreatic ultrasonography in dogs with suspected pancreatitis. J Vet Intern Med. 2014;28(3):863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Escribano D, Nicolas‐Barcelo P, Vivo‐Romero N, et al. Analytical and clinical validation of a point‐of‐care assay for lipase activity determination in canine serum (Abstract: International Society for Animal Clinical Pathology 2018). Vet Clin Pathol. 2019;48:220‐221. [Google Scholar]
- 78. Steiner JM, Gomez R, Suchodolski JS, Lidbury JA. Specificity of, and influence of hemolysis, lipemia, and icterus on serum lipase activity as measured by the v‐LIP‐P slide. Vet Clin Pathol. 2017;46(3):508‐515. [DOI] [PubMed] [Google Scholar]
- 79. Ishioka K, Hayakawa N, Nakamura K, et al. Patient‐side assay of lipase activity correlating with pancreatic lipase immunoreactivity in the dog. J Vet Med Sci. 2011;73(11):1481‐1483. [DOI] [PubMed] [Google Scholar]
- 80. Steiner J, Finco D, Williams D. Serum lipase activity and canine pancreatic lipase immunoreactivity (cPLI) concentrations in dogs with experimentally induced chronic renal failure. Vet Res. 2010;3(3):58‐63. [Google Scholar]
- 81. Steiner JM, Williams DA. Purification of classical pancreatic lipase from dog pancreas. Biochimie. 2002;84(12):1243‐1251. [DOI] [PubMed] [Google Scholar]
- 82. Jaensch S. Associations between serum amylase, lipase and pancreatic specific lipase in dogs. Comp Clin Pathol. 2012;21(2):157‐160. [Google Scholar]
- 83. Prümmer JK, Howard J, Grandt LM, Aguilar R, Meneses F, Peters LM. Hyperlipasemia in critically ill dogs with and without acute pancreatitis: prevalence, underlying diseases, predictors, and outcome. J Vet Intern Med. 2020;34(6):2319‐2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Takada K, Palm CA, Epstein SE, Cowgill LD. Assessment of canine pancreas‐specific lipase and outcomes in dogs with hemodialysis‐dependent acute kidney injury. J Vet Intern Med. 2018;32(2):722‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Han D, Choi R, Hyun C. Canine pancreatic‐specific lipase concentrations in dogs with heart failure and chronic mitral valvular insufficiency. J Vet Intern Med. 2015;29(1):180‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Soares FB, Pereira‐Neto GB, Rabelo RC. Assessment of plasma lactate and core‐peripheral temperature gradient in association with stages of naturally occurring myxomatous mitral valve disease in dogs. J Vet Emerg Crit Care. 2018;28(6):532‐540. [DOI] [PubMed] [Google Scholar]
- 87. Serrano G, Paepe D, Williams T, Watson P. Increased canine pancreatic lipase immunoreactivity (cPLI) and 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase in dogs with evidence of portal hypertension and normal pancreatic histology: a pilot study. J Vet Diagn Investig. 2021;33(3):548‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Park JS, Park JH, Seo KW, Song KH. Correlation between NT‐proBNP and lipase levels according to the severity of chronic mitral valve disease in dogs. J Vet Sci. 2019;20(4):e43. doi: 10.4142/jvs.2018.20.e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bolton TA, Cook A, Steiner JM, Fosgate GT. Pancreatic lipase immunoreactivity in serum of dogs with diabetic ketoacidosis. J Vet Intern Med. 2016;30(4):958‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kim H, Kang JH, Heo TY, et al. Evaluation of hypertriglyceridemia as a mediator between endocrine diseases and pancreatitis in dogs. J Am Anim Hosp Assoc. 2019;55(2):92‐100. [DOI] [PubMed] [Google Scholar]
- 91. Mawby DI, Whittemore JC, Fecteau KA. Canine pancreatic‐specific lipase concentrations in clinically healthy dogs and dogs with naturally occurring hyperadrenocorticism. J Vet Intern Med. 2014;28(4):1244‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mendoza B, Dias MJ, Hernandez J, Leal RO. Effect of prednisolone therapy on serum levels of 1,2‐O‐dilauryl‐rac‐glycero glutaric acid‐(6′‐methylresorufin) ester lipase in dogs. J Vet Intern Med. 2020;43(6):2330‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Steiner JM, Teague SR, Lees GE, Willard MD, Williams DA, Ruaux CG. Stability of canine pancreatic lipase immunoreactivity concentration in serum samples and effects of long‐term administration of prednisone to dogs on serum canine pancreatic lipase immunoreactivity concentrations. Am J Vet Res. 2009;70(8):1001‐1005. [DOI] [PubMed] [Google Scholar]
- 94. Ohta H, Kojima K, Yokoyama N, et al. Effects of immunosuppressive prednisolone therapy on pancreatic tissue and concentration of canine pancreatic lipase immunoreactivity in healthy dogs. Can J Vet Res. 2018;82(4):278‐286. [PMC free article] [PubMed] [Google Scholar]
- 95. Granger LA, Hilferty M, Francis T, Steiner JM, Gaschen L. Variability in the ultrasonographic appearance of the pancreas in healthy dogs compared to dogs with hyperadrenocorticism. Vet Radiol Ultrasound. 2015;56(5):540‐548. [DOI] [PubMed] [Google Scholar]
- 96. Watson PJ. Exocrine pancreatic insufficiency as an end stage of pancreatitis in four dogs. J Small Anim Pract. 2003;44(7):306‐312. [DOI] [PubMed] [Google Scholar]
- 97. Nyland TG, Mattoon JS, Herrgesel ET, et al. Pancreas. In: Nyland TG, Mattoon JS, eds. Small Animal Diagnostic Ultrasound. Philadelphia, PA: Saunders; 2002:144‐157. [Google Scholar]
- 98. Huynh E, Berry C. Small animal abdominal ultrasonography: the pancreas. Todays Vet Pract. 2018;8(3):58‐69. [Google Scholar]
- 99. Morita Y, Takiguchi M, Yasuda J, Eom KD, Hashimoto A. Endoscopic ultrasonographic findings of the pancreas after pancreatic duct ligation in the dog. Vet Radiol Ultrasound. 1998;39(6):557‐563. [DOI] [PubMed] [Google Scholar]
- 100. Engjom T, Sangnes DA, Havre RF, et al. Diagnostic accuracy of transabdominal ultrasound in chronic pancreatitis. Ultrasound Med Biol. 2017;43(4):735‐743. [DOI] [PubMed] [Google Scholar]
- 101. Irisawa A, Mishra G, Hernandez L, et al. Quantitative analysis of endosonographic parenchymal echogenicity in patients with chronic pancreatitis. J Gastroenterol Hepatol. 2004;19(10):1199‐1205. [DOI] [PubMed] [Google Scholar]
- 102. Ohta H, Morita T, Yokoyama N, et al. Serial measurement of pancreatic lipase immunoreactivity concentration in dogs with immune‐mediated disease treated with prednisolone. J Small Anim Pract. 2017;58(6):342‐347. [DOI] [PubMed] [Google Scholar]
- 103. Kalli IV, Adamama‐Moraitou KK, Patsika MN, et al. Prevalence of increased canine pancreas‐specific lipase concentrations in young dogs with parvovirus enteritis. Vet Clin Pathol. 2017;46(1):111‐119. [DOI] [PubMed] [Google Scholar]
- 104. Möhr AJ, Lobetti RG, Van Der Lugt JJ. Acute pancreatitis: a newly recognised potential complication of canine babesiosis. J S Afr Vet Assoc. 2000;71(4):232‐239. [DOI] [PubMed] [Google Scholar]
- 105. Masuda M, Otsuka‐Yamasaki Y, Shiranaga N, et al. Retrospective study on intercurrent pancreatitis with Babesia gibsoni infection in dogs. J Vet Med Sci. 2019;81(11):1558‐1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mylonakis ME, Xenoulis PG, Theodorou K, et al. Serum canine pancreatic lipase immunoreactivity in experimentally induced and naturally occurring canine monocytic ehrlichiosis (Ehrlichia canis). Vet Microbiol. 2014;169:198‐202. [DOI] [PubMed] [Google Scholar]
- 107. Spinella G, Dondi F, Grassato L, et al. Prognostic value of canine pancreatic lipase immunoreactivity and lipase activity in dogs with gastric dilatation‐volvulus. PLoS One. 2018;13(9):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cochran L, Hill S, Suchodolski JS, et al. Serum pancreatic lipase immunoreactivity in dogs with gastric foreign bodies (Abstract: 2016 ACVIM forum). J Vet Intern Med. 2016;30:1453. [Google Scholar]
- 109. Schueler RO, White G, Schueler RL, Steiner JM, Wassef A. Canine pancreatic lipase immunoreactivity concentrations associated with intervertebral disc disease in 84 dogs. J Small Anim Pract. 2018;59(5):305‐310. [DOI] [PubMed] [Google Scholar]
- 110. Rouse R, Rosenzweig B, Shea K, et al. MicroRNA biomarkers of pancreatic injury in a canine model. Exp Toxicol Pathol. 2017;69(1):33‐43. [DOI] [PubMed] [Google Scholar]
- 111. Lee HB, Park HK, Choi HJ, et al. Evaluation of circulating microRNA biomarkers in the acute pancreatic injury dog model. Int J Mol Sci. 2018;19(10):3048. doi: 10.3390/ijms19103048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hecht S, Henry G. Sonographic evaluation of the normal and abnormal pancreas. Clin Tech Small Anim Pract. 2007;22(3):115‐121. [DOI] [PubMed] [Google Scholar]
- 113. Nyland T, Mulvany M, Strombeck D. Ultrasonographic features of experimentally induced, acute pancreatitis in the dog. Vet Radiol. 1983;24(6):260‐266. [Google Scholar]
- 114. Murakami M, Heng HG, Lim CK, et al. Ultrasonographic features of presumed gastric wall edema in 14 dogs with pancreatitis. J Vet Intern Med. 2019;33(3):1260‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Anderson JR, Cornell KK, Parnell NK, Salisbury SK. Pancreatic abscess in 36 dogs: a retrospective analysis of prognostic indicators. J Am Anim Hosp Assoc. 2008;44(4):171‐179. [DOI] [PubMed] [Google Scholar]
- 116. Washabau R. Pancreas. In: Washabau R, Day M, eds. Canine and Feline Gastroenterology. St. Louis, MO: Elsevier Saunders; 2013:799‐848. [Google Scholar]
- 117. Wilkinson R, Giannitrapani I, Griffin B, et al. Letter regarding “Association between abdominal ultrasound findings, the specific canine pancreatic lipase assay, clinical severity indices, and clinical diagnosis in dogs with pancreatitis”. J Vet Intern Med. 2020;34(5):1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cridge H, Sullivant A, Lee AM. Response to letter regarding “Association between abdominal ultrasound findings, the specific canine pancreatic lipase assay, clinical severity indices, and clinical diagnosis in dogs with pancreatitis”. J Vet Intern Med. 2020;34(5):1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. D'Onofrio M, Zamboni G, Faccioli N, Capelli P, Pozzi Mucelli R. Ultrasonography of the pancreas. 4. Contrast‐enhanced imaging. Abdom Imaging. 2007;32(2):171‐181. [DOI] [PubMed] [Google Scholar]
- 120. Golea A, Badea R, Socaciu M, Diaconu B, Iacob D. Quantitative analysis of tissue perfusion using contrast‐enhanced transabdominal ultrasound (CEUS) in the evaluation of the severity of acute pancreatitis. Med Ultrason. 2010;12(3):198‐204. [PubMed] [Google Scholar]
- 121. Badea R, Seicean A, Diaconu B, et al. Contrast‐enhanced ultrasound of the pancreas—a method beyond its potential or a new diagnostic standard? J Gastrointest Liver Dis. 2009;18(2):237‐242. [PubMed] [Google Scholar]
- 122. Lim SY, Nakamura K, Morishita K, et al. Quantitative contrast‐enhanced ultrasonographic assessment of naturally occurring pancreatitis in dogs. J Vet Intern Med. 2015;29(1):71‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rademacher N, Schur D, Gaschen F, Kearney M, Gaschen L. Contrast‐enhanced ultrasonography of the pancreas in healthy dogs and in dogs with acute pancreatitis. Vet Radiol Ultrasound. 2016;57(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 124. Sigrist RMS, Liau J, El Kaffas A, et al. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7(5):1303‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Avante LM, Rossi FMA, Ramirez URA, et al. Pancreatic evaluation in dogs using different ultrasonographic techniques—preliminary results. Acta Vet Brno. 2020;70(2):255‐266. [Google Scholar]
- 126. Adrian AM, Twedt DC, Kraft SL, Marolf AJ. Computed tomographic angiography under sedation in the diagnosis of suspected canine pancreatitis: a pilot study. J Vet Intern Med. 2015;29(1):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Marolf AJ. Computed tomography and MRI of the hepatobiliary system and pancreas. Vet Clin North Am Small Anim Pract. 2016;46(3):481‐497. [DOI] [PubMed] [Google Scholar]
- 128. Cáceres AV, Zwingenberger AL, Hardam E, et al. Helical computed tomographic angiography of the normal canine pancreas. Vet Radiol Ultrasound. 2006;47(3):270‐278. [DOI] [PubMed] [Google Scholar]
- 129. French JM, Twedt DC, Rao S, Marolf AJ. Computed tomographic angiography and ultrasonography in the diagnosis and evaluation of acute pancreatitis in dogs. J Vet Intern Med. 2019;33(1):79‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. French JM, Twedt DC, Rao S, Marolf AJ. CT angiographic changes in dogs with acute pancreatitis: a prospective longitudinal study. Vet Radiol Ultrasound. 2020;61(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 131. Balci NC, Bieneman BK, Bilgin M, Akduman IE, Fattahi R, Burton FR. Magnetic resonance imaging in pancreatitis. Top Magn Reson Imaging. 2009;20(1):25‐30. [DOI] [PubMed] [Google Scholar]
- 132. Marolf AJ, Kraft SL, Dunphy TR, Twedt DC. Magnetic resonance (MR) imaging and MR cholangiopancreatography findings in cats with cholangitis and pancreatitis. J Feline Med Surg. 2013;15(4):285‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cordner AP, Armstrong PJ, Newman SJ, Novo R, Sharkey LC, Jessen C. Effect of pancreatic tissue sampling on serum pancreatic enzyme levels in clinically healthy dogs. J Vet Diagn Investig. 2010;22(5):702‐707. [DOI] [PubMed] [Google Scholar]
- 134. Cordner AP, Sharkey LC, Armstrong PJ, McAteer KD. Cytologic findings and diagnostic yield in 92 dogs undergoing fine‐needle aspiration of the pancreas. J Vet Diagn Investig. 2015;27(2):236‐240. [DOI] [PubMed] [Google Scholar]
- 135. Sharkey LC, Crain S. Pancreas. In: Sharkey LC, Radin M, Seeling D, eds. Veterinary Cytology. Hoboken, NJ: Wiley Blackwell; 2021:445‐453. [Google Scholar]
- 136. Aupperle‐Lellbach H, Törner K, Staudacher M, et al. Histopathological findings and canine pancreatic lipase immunoreactivity in normal dogs and dogs with inflammatory and neoplastic diseases of the pancreas. J Vet Intern Med. 2020;34(3):1127‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Aupperle‐Lellbach H, Törner K, Staudacher M, Müller E, Steiger K, Klopfleisch R. Characterization of 22 canine pancreatic carcinomas and review of literature. J Comp Pathol. 2019;173:71‐82. [DOI] [PubMed] [Google Scholar]


