Abstract
Background
Early recognition of acute kidney injury (AKI) is hindered by current definitions and use of traditional, insensitive markers.
Hypothesis/Objectives
Urinary (u) activity of γ‐glutamyl transpeptidase (GGT) and alkaline phosphatase (ALP), and concentrations of heat‐shock protein 70 (HSP70) and interleukins (ILs) ‐6 and ‐18, are predictive biomarkers for AKI and survival.
Animals
Nonazotemic, hospitalized dogs (n = 118) and healthy controls (n = 20).
Methods
A prospective observational study. Nonazotemic dogs at risk of AKI were recruited and their urinary biomarker concentrations were measured at presentation. Serum creatinine (sCr) and symmetric dimethylarginine (sSDMA) were measured daily until discharge/death.
Results
The overall case fatality rate was 18.6%. Fifteen dogs (12.7%) developed AKI, which was associated with death (relative risk, 3.2; 95% confidence interval [CI], 1.57‐6.55). All 5 urinary biomarkers were significantly higher in hospitalized dogs compared to controls, with minimal overlap. uHSP70/uCr, uGGT/uCr, and uIL‐6/uCr at presentation were higher in dogs which later developed AKI. Areas under the receiver operator characteristic curve (AUROC) (95% CI) for the 3 biomarkers as predictors of AKI were 0.67 (0.51‐0.83), 0.68 (0.55‐0.81), and 0.78 (0.65‐0.91), respectively. When they were categorically classified as elevated/normal, each additional elevated biomarker increased the odds for AKI (OR, 2.83; 95% CI, 1.23‐6.52, P = .01). Agreement between sCr and sSDMA was poor (Cohen's kappa = .071). The AUROC of SDMA at presentation for AKI prediction was 0.73 (0.51‐0.95).
Conclusions and Clinical Importance
Kidney injury was common, irrespective of subsequent worsening of azotemia or death. The predictive value of individual urinary biomarkers was reduced by moderate sensitivities and specificities. SDMA showed moderate discriminatory utility for AKI prediction, and often displayed discordant results with sCr.
Keywords: alkaline phosphatase, heat shock protein, interleukin‐18, interleukin‐6, SDMA, γ‐glutamyl transpeptidase
1. INTRODUCTION
Acute kidney injury (AKI) is a potentially life‐threatening complication of various medical conditions in hospitalized dogs. 1 , 2 Its development, irrespective of the primary etiology, imparts a worse prognosis and markedly increases the case fatality rate. 3 , 4 , 5 , 6
Current definitions of AKI and its diagnosis rely on measuring serum creatinine concentration (sCr) and urine output, notwithstanding their inadequate sensitivity and specificity. 7 Furthermore, sCr is affected by extrarenal factors, such as muscle mass and the hydration status, and typically exceeds its reference interval (RI) only when >75% of kidney function is lost (depending on its baseline concentration). Serum creatinine concentration solely reflects changes in glomerular filtration rate (GFR) rather than tubular injury per se, rendering it unsuitable for detecting AKI when the injury is not associated with a measurable decrease in GFR. 8 , 9
Early recognition of a tubulopathy and International Renal Interest Society (IRIS) Grade‐I AKI might allow timely intervention and improve outcome. This can be achieved by utilizing urinary (u) biomarkers, whose activity or concentration in urine soon increase after renal tubular injury. 9 , 10 , 11
Readily available tubulopathy markers, including urine specific gravity and presence of glucosuria and cylinduria lack sensitivity or specificity. 10 Urinary enzyme activities can serve as early indicators of tubular injury, and demonstrate diagnostic and prognostic value in dogs and humans with AKI. 9 , 10 , 11 Among them, and unlike most other used urinary enzymes, measurement of urinary alkaline phosphatase (uALP) and γ‐glutamyl transpeptidase (uGGT) activity is simple, widely available and cost‐efficient.
Heat shock proteins (HSPs) are ubiquitous, cytoprotective cellular proteins, found in all organisms. 12 Urinary HSP72, a member of the HSP70 family, is increased in experimentally‐induced, murine models of renal tubular injury and has reno‐protective properties. 12 , 13 In dogs, uHSP72 has excellent diagnostic and prognostic performance in overt, azotemic AKI. 14 Furthermore, uHSP72 increases postoperatively in some dogs, especially in those with higher American Society of Anesthesiologists (ASA) status, and often in absence of any increase in sCr concentration. 15 Nevertheless, its predictive performance for AKI in nonazotemic, hospitalized dogs in general has not been investigated.
Interleukin (IL)‐18, a proinflammatory cytokine is expressed by macrophages/monocytes and renal tubular cells and increases early in AKI. 16 , 17 , 18 In humans, uIL‐18 concentration is a useful predictive marker of AKI and survival. 18 , 19 , 20 , 21 Interleukin‐6, another proinflammatory cytokine, is implicated in the progression of AKI in humans, 22 as well as in the development of renal fibrosis in dogs. 23
Urinary ALP, uGGT and uHSP72 concentrations, normalized to urinary creatinine (uCr) concentration, increase in naturally‐occurring AKI in dogs and show variable discriminatory ability in differentiating dogs with AKI from those with chronic kidney disease and healthy controls. 14 , 24 , 25 , 26 Nevertheless, most of these previous studies were retrospective or enrolled azotemic dogs, thus introducing a selection bias. Therefore, oftentimes the sensitivity and specificity of these urinary biomarkers for predicting AKI development could not be assessed. Additionally, the diagnostic utility of uIL‐6 and uIL‐18 to predict the development of AKI in hospitalized dogs is still unknown.
We hypothesized that uGGT/uCr, uALP/uCr, uHSP70/uCr, uIL‐6/uCr, and uIL‐18/uCr would prove useful, sensitive predictive biomarkers of AKI and the overall outcome in nonazotemic, hospitalized dogs at risk of AKI. A secondary objective was to examine the agreement between serum symmetric dimethylarginine (sSDMA) and sCr concentrations in such dogs during hospitalization.
2. MATERIALS AND METHODS
2.1. Dogs and definitions
The study was conducted at a university hospital, between 2018 and 2020 and was approved by the Institutional Research Committee. Nonazotemic (sCr < 1.6 mg/dL) dogs, considered at risk of AKI, with the following conditions were deemed eligible for enrollment: acute pancreatitis (AP), heatstroke, gastric dilatation‐volvulus (GDV), left congestive heart failure (L‐CHF), systemic inflammatory response syndrome (SIRS), sepsis and dogs that had undergone surgery within 24 hours after collection of samples and were classified with ASA status ≥3. 27 Acute pancreatitis was diagnosed based on presence of compatible historical, clinical and characteristic ultrasonographic findings, 28 and 1,2‐o‐Dilauryl‐rac‐glycerol‐glutaric acid‐(6′‐methylresorufin) ester (DGGR)‐lipase activity (Cobas 6000, Roche, Mannheim, Germany, at 37°C) above RI. The diagnosis of SIRS required meeting ≥3 of the following criteria: tachypnea (respiratory rate, >40 breaths/min), tachycardia (heart rate, >120 bpm), leukocytosis or leukopenia (white blood cell count >18 000/μL or <5000/μL, respectively) and fever or hypothermia (rectal temperature, >39.5 or <37.5°C, respectively). The diagnosis of sepsis required fulfillment of the above SIRS criteria, along with cytological or bacteriological evidence of infection. Mandatory diagnostic criteria for L‐CHF included compatible clinical signs (eg, tachycardia, tachypnea, dyspnea, cyanosis, or crackles) and compatible echocardiographic and thoracic radiography findings. Current IRIS guidelines dictated the diagnosis of AKI. 7 The grade assigned to dogs that developed AKI was based on the maximum documented sCr concentration. Healthy, staff‐owned dogs comprised the control group. Control dogs were deemed healthy based on lack of history of disease, and normal physical examination, CBC and serum chemistry findings.
2.2. Collection of samples and laboratory methods
Surplus urine (of samples collected by cystocentesis during the routine diagnostic process) was stored at −80°C pending analyses and used for measuring the concentrations of urinary biomarkers and uCr. Urinary biomarker concentrations were normalized to uCr. Commercially available ELISAs were used to measure the concentrations of uHSP70 (HSP70‐ELISA kit, SKT‐108‐96, StressMarq Biosciences, Victoria, Canada), uIL‐6 (Canine IL‐6 ELISA kit, CA6000, R&D Systems, Minneapolis, Minnesota) and uIL‐18 (Canine IL‐18 ELISA kit, E0064d, EIAab, Wuhan, China) according to the manufacturers' instructions. The actual intraassay coefficients of variance of the above assays in the present study were 3.1%, 4.9%, and 6.5%, respectively. All measurements were done in duplicates, and their means were used for statistical analyses. Additional validation parameters were not performed independently by the authors but are available from the manufacturers. Urinary ALP and uGGT activities, as well as uCr and sCr concentrations were measured within 30 minutes of collection, using a wet chemistry autoanalyzer (Cobas 6000, Roche, Mannheim, Germany, at 37°C). For measurement of creatinine, a kinetic colorimetric assay, based on the Jaffe method, was used. A sCr > 1.6 μg/dL was considered elevated, according to IRIS guidelines. 7 Serum SDMA samples were stored at −80°C pending submission for batch analysis (IDEXX laboratories Ltd, Westbrook, Maine). The upper cutoff for serum SDMA was 14 μg/dL.
Urine samples for biomarker measurement were collected once, on the day of presentation. Blood samples for sCr measurement were collected on the day of presentation, and then daily, until discharge or death, as part of the routine follow‐up.
2.3. Statistical analysis
Based on preliminary results showing an increase of uHSP70/uCr from 0.2 ± 0.2 ng/mg in heathy dogs to 5‐10 ± ng/mg in dogs with AKI, 14 and based on previous veterinary studies where the incidence of hospital‐acquired AKI ranged from 12% to 64%, 5 , 29 , 30 we chose the more conservative estimates and assumed an increase of only 5 ng/mg of uHSP70/uCr and an incidence of only 12% for AKI development, thereby requiring the recruitment of a minimum of 108 dogs at risk of developing AKI to show a statistically significant difference at an alpha of .05 and power of 90%.
Normality was assessed by the Shapiro‐Wilk's test. Owing to data distribution, continuous parameters were described as medians and interquartile ranges (IQR) and the nonparametric Mann‐Whitney U‐test and Kruskal‐Wallis test were used to compare continuous variables between 2 and 3 outcome groups (ie, AKI and non‐AKI, survivors and nonsurvivors or with controls), respectively. The Fisher's exact test was applied for testing associations between categorical variables. The Cohen's kappa coefficient was computed to assess the agreement between sSDMA and sCr test results. The receiver operator characteristic (ROC) analysis was used to evaluate the different urinary biomarkers as predictors of AKI and of survival to discharge. The maximal point of the Youden index (Sen‐[1‐Spec]) was used as the optimal cutoff value for ROC analyses. The Mantel‐Haenszel test of trend and a logistic regression analysis were used to explore the additive effect of combining several urinary biomarkers. For that purpose, RIs for each urinary biomarker were constructed based on their concentrations in the healthy control group, using the maximal concentration of each marker as the upper reference limit (URL). 31 Subsequently, the urinary marker concentration was categorized as “normal” (ie, 0) if below its URL or “elevated” if >URL (ie, 1), and a new variable was produced in which either 0 of 3 urinary markers was elevated, 1 was elevated, 2 were elevated or all 3 were elevated. This new variable was entered into a logistic regression model to predict AKI. All tests were 2‐tailed, and P < .05 was considered significant. Statistical analyses were performed using statistical software packages (WinPEPI, Compare2, S6 Version 3.85; SPSS 25.0 for Windows, IBM, Armonk, New York).
3. RESULTS
The study and control groups comprised 118 dogs and 20 dogs, respectively. Their median ages were 120 months (IQR, 72) and 62.5 months (22.5), respectively (P < .001). The study population comprised 63 males (intact, 24; castrated, 39) and 55 females (intact, 24; spayed, 31), while the controls included 15 males (intact, 3; castrated, 12) and 5 spayed females. Most study dogs (65; 55.1%) were of mixed breed, and the most common of the 37 breeds were the German shepherd and the miniature Poodle (5 each), followed by the Cane Corso, Pekingese, Labrador retriever, golden retriever, Cocker Spaniel, and Doberman pinscher breeds (4 each). Excluding 1 Border Collie dog, all control dogs were mixed‐breed.
3.1. Diagnoses, incidence of AKI, and survival
The main diagnoses were grouped into 4 main categories: AP (n = 28), GDV (n = 12), L‐CHF (n = 6), and SIRS or sepsis (n = 72). The latter category included various diseases, including splenic torsion, septic peritonitis, immune‐mediated hemolytic anemia, aspiration and bacterial pneumonias, intestinal necrosis, pyometra, metritis, snakebite, heatstroke, pyothorax, and neoplasia‐associated hemoabdomen.
The incidence of AKI was 12.7% (15/118 dogs), including grade 1 (6 dogs; 40%), grade 2 (5 dogs; 33%), grade 3 (3 dogs; 20%), and grade 4 (1 dog; 7%) AKI. Median time between measurement of urinary biomarkers and development of AKI was 1 day (range, 1‐5 days). The overall case fatality rate was 18.6% (22/118 dogs). It was significantly (P = .002) higher in the AKI group (7/15 dogs; 47%) compared to the non‐AKI group (15/103 dogs; 14.5%). Correspondingly, the occurrence of AKI increased the risk of death (relative risk, 3.2; 95% confidence interval [CI] 1.57‐6.55). Neither the incidence of AKI nor the case fatality rate differed among the 4 disease categories. Furthermore, the incidence of AKI was not associated with age or sex. Median hospitalization period of the survivors was 3 days (IQR, 3).
3.2. Urinary biomarkers as predictors of AKI and death
All 5 urinary biomarkers were significantly higher in hospitalized dogs compared to the healthy dogs (P < .001; Table 1). Concentration/activity of uHSP70/uCr (P = .04), uGGT/uCr (P = .02), and uIL‐6/uCr (P = .001) differed between the AKI and non‐AKI groups (Table 1; Figure 1). The area under the ROC curve (AUROC) (95% CI) for these 3 biomarkers as predictors of AKI development was 0.67 (0.51‐0.83), 0.68 (0.55‐0.81), and 0.78 (0.65‐0.91), respectively (Table 2; Figure 2). Optimal cutoff points (concentration; sensitivity/specificity) were 3.8 ng/mg (79%/54%), 121 U/g (79%/54%), and 118 pg/mg (79%/73%) for uHSP70/uCr, uGGT/uCr, and uIL‐6/uCr, respectively. Additional cutoff points with their corresponding sensitivities/specificities are presented in Table 2. When the concentrations of these 3 biomarkers were categorically classified as elevated or normal, 31 a significant (P = .01), positive linear trend was found between the number of abnormally elevated biomarkers and AKI. The odds of AKI increased by a factor of 2.83 (95% CI, 1.23‐6.52, P = .01) over the previous odds with each additional elevated urinary biomarker. Only uALP/uCr differed between survivors and nonsurvivors (P = .02), with an AUROC of 0.66 (95% CI, 0.53‐0.79). Neither uIL‐6/uCr nor uIL‐18/uCr differed between the 4 etiological categories (ie, they were not increased in the SIRS/sepsis group).
TABLE 1.
Concentrations/activities of 5 urinary biomarkers, normalized to urinary creatinine concentration and measured at presentation, as predictors of AKI development or survival to discharge a
| Group | uALP/uCr (U/g) median (IQR) | uGGT/uCr (U/g) median (IQR) | uHSP70/uCr (ng/mg) median (IQR) | uIL‐6/uCr (pg/mg) median (IQR) | uIL‐18/uCr (pg/mg) median (IQR) |
|---|---|---|---|---|---|
| AKI (n = 15) | 57 (69) | 199 (130) | 8.5 (24.2) | 290.5 (1217) | 154 (397) |
| Non‐AKI (n = 103) | 31 (55) | 110 (119) | 3.6 (7.7) | 52.6 (112) | 64.3 (150) |
| Survivors (n = 96) | 30 (50) | 118 (112) | 3.7 (8.2) | 61.2 (164) | 78.3 (172) |
| Nonsurvivors (n = 22) | 67 (89) | 123 (264) | 5 (7.2) | 64.2 (203) | 60 (159) |
| Controls (n = 20) | 3.3 (4.4) | 28 (27.9) | 0.4 (0.7) | 7.3 (5.9) | 20.5 (22) |
Abbreviations: AKI, acute kidney injury; ALP, alkaline phosphatase; GGT, gamma‐glutamyl transpeptidase; HSP, heat shock protein; IL, interleukin; u, urinary.
Significantly different results are bolded. The study population significantly differed from the controls for all biomarkers (P < .001). The Mann‐Whitney U test was used to compare the AKI and non‐AKI groups, and the survivors and the nonsurvivors. For AKI development, uGGT/uCr, uHSP70/uCr, and uIL‐6/uCr significantly differed between groups (P = .02, P = .04, and P = .001, respectively).
FIGURE 1.

Box and whiskers plots for urinary (u) biomarkers (γ‐glutamyl transpeptidase [GGT], alkaline phosphatase [ALP], heat‐shock protein 70 [HSP70], and interleukin [IL] ‐6 and ‐18) as predictors of acute kidney injury (AKI) development during hospitalization. Five urinary biomarkers were measured at presentation in nonazotemic hospitalized dogs (n = 118; 15 dogs developed AKI during hospitalization and 103 without AKI). Healthy dogs constituted the control group (n = 20). The box extends from the 25th to the 75th percentiles, while the whiskers extend 1.5 times the interquartile range on either side of the box. The study population significantly differed from the controls for all biomarkers (P < .001). For AKI development, uGGT/uCr, uHSP70/uCr, and uIL‐6/uCr significantly differed between groups (P = .02, P = .04 and P = .001, respectively)
TABLE 2.
Receiver operator characteristic analyses for uIL‐6/uCr, uGGT/uCr, and uHSP70/uCr at presentation to the hospital as predictors of development of acute kidney injury during hospitalization
| uIL‐6/uCr (pg/mg) | uGGT/uCr (U/g) | uHSP/uCr (ng/mg) | |
|---|---|---|---|
| AUROC (95% CI) | 0.78 (0.65‐0.91) | 0.68 (0.55‐0.81) | 0.67 (0.51‐0.83) |
| Optimal cutoff value a (sensitivity/specificity) | 118 (79%/73%) | 121 (79%/54%) | 3.8 (79%/54%) |
| Additional cutoffs (sensitivity/specificity) | 30 (93%/32%) | 90 (93%/41%) | 1.4 (93%/26%) |
| 204 (64%/82%) | 171 (57%/73%) | 10.1 (50%/79%) | |
| 1478 (29%/96%) | 281 (21%/85%) | 32.3 (29%/92%) |
Abbreviations: AUROC, area under the receiver operator characteristic curve; CI, confidence interval; GGT, gamma‐glutamyl transpeptidase; HSP, heat shock protein; IL, interleukin; u, urinary.
The maximal point of the Youden index (Sen‐[1‐Spec]) was used as the optimal cutoff value.
FIGURE 2.
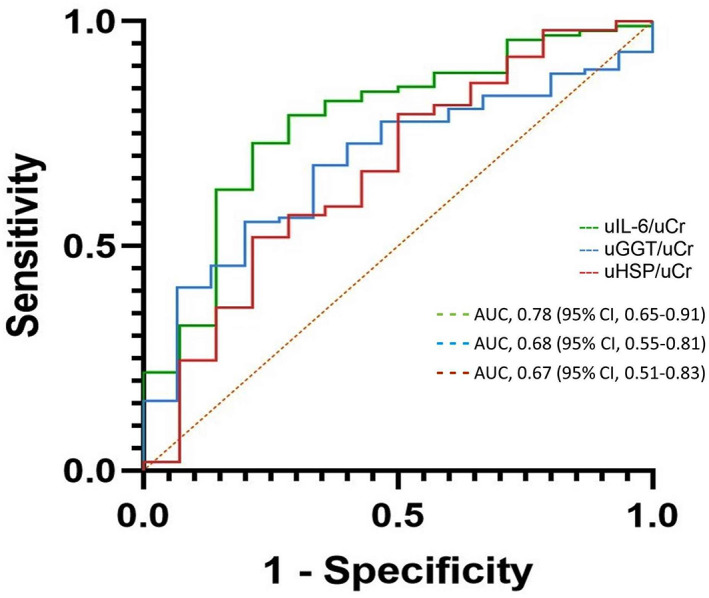
Receiver operator characteristic curves for uIL‐6/uCr, uGGT/uCr, and uHSP70/uCr at presentation as predictors of acute kidney injury development during hospitalization. The refence line is indicated by orange
3.3. Agreement between sCr and sSDMA concentrations
Serum SDMA was measured concurrently with sCr in 40 hospitalized dogs at presentation, and in 71, 26, 5, and 2 dogs on days 2, 3, 4, and 5 of hospitalization, respectively. Additionally, sSDMA and sCr were measured concurrently in 20 healthy control dogs. Consequently, 164 concurrent measurements of both sSDMA and sCr were available, of which 104 were repeat measurements from the same dog during hospitalization. The overall, agreement between sCr and sSDMA was poor (Cohen's kappa = .071). In 114 samples, concentrations of both were within their RIs. Serum SDMA concentration was increased above RI (>14 μg/dL) in 46 samples, while sCr concentration was within RI (<1.4 mg/dL). Conversely, sCr concentration was increased above RI (>1.6 mg/dL) in only 1 control dog (1.7 mg/dL), which was of large‐breed, while its sSDMA concentration was within RI (5.8 μg/dL). sCr and SDMA concentrations were both increased in 3 dogs with AKI. In 6 dogs from the AKI group, sCr concentration was within the RI on their first or second day of hospitalization, while their concurrent sSDMA concentration was above RI. The AUROC for SDMA at presentation as a predictor of AKI was 0.73 (95% CI; 0.51‐0.95). Corresponding sensitivities/specificities for the traditional cutoff value of SDMA (14 μg/dL) and the optimal cutoff value (11.4 μg/dL) were 72%/63% and 59%/88%, respectively.
4. DISCUSSION
In the present study, 5 urinary biomarkers at presentation were prospectively tested in a cohort of nonazotemic, hospitalized dogs, with various systemic diseases, at risk of developing AKI, which were subsequently monitored throughout hospitalization for occurrence of AKI. Urinary activities or concentrations of all 5 biomarkers differed significantly between the hospitalized dog cohort and the healthy controls, with minimal overlap. Moreover, uIL‐6/uCr, uGGT/uCr, and uHSP70/uCr concentrations at presentation were increased in dogs that later developed AKI, while uALP/uCr was significantly higher in nonsurvivors compared to survivors. Their prognostic utility was hindered by moderate sensitivities and specificities, but higher cutoff points improved their specificities, albeit at the expense of sensitivity. Lastly, a potential additive value of measuring several biomarkers concurrently was observed, as the odds of developing AKI during hospitalization increased with each additional elevated urinary biomarker.
A plethora of medical conditions engender changes in systemic and renal hemodynamics, inflammation, and oxidative stress, which synergistically culminate in tubulopathy, glomerulopathy, and occasionally in azotemic AKI. 3 , 5 , 29 , 32 , 33 , 34 , 35 In humans, the incidence of hospital‐acquired AKI increases from 5% to 7% in the general population to 36% to 67% in ICU patients. 11 , 35 The incidence in the present study (12.7%) is comparable to previous publications, 3 , 5 , 6 excluding 1 study of hospitalized dogs, which showed an exceptionally high (64%) incidence of IRIS Grade‐I AKI. 30 In agreement with previous studies, development of AKI herein significantly increased the risk of death. 3 , 4 , 5 , 6
Traditionally, AKI is defined, and often recognized by documenting incremental increases in sCr concentration, a GFR marker. However, renal tubular damage may be an early marker of kidney injury that precedes a decrease in GFR. 10 The increased mortality rate in face of AKI possibly also results from loss of beneficial renal tubular cell functions, such as clearance of inflammatory cytokines, as observed in nephrectomized dogs with endotoxin‐induced sepsis. 36 Since traditional markers of tubulopathy (eg, cylinduria and glucosuria) are insensitive, more sensitive urinary biomarkers of renal tubular cells can improve early recognition of tubulopathy, and potentially aid in predicting AKI and initiating timely therapy. Most veterinary studies tested urinary biomarkers in dogs with experimentally‐induced AKI, 10 or retrospectively enrolled dogs with overt AKI, but only few have reported their clinical utility for predicting AKI and death prospectively and longitudinally. 15 , 30
Measurement of uALP and uGGT is simple and widely available. Moreover, their activities are unaffected by hematuria, bacteriuria or hemoglobinuria, yet urine pH outside the 6.5 to 8 range drastically decreases their reliability. 37 Lastly, their high molecular weight precludes their glomerular filtration in absence of glomerular injury, and their presence in the urine is primarily of renal proximal tubular origin. 38 Both uALP and uGGT increase in experimentally‐induced (eg, gentamicin, mercuric‐acid) 39 , 40 and naturally‐occurring tubulopathy and AKI (secondary to pyometra, snake envenomation, heartworm disease, AP, and proteinuria). 41 , 42 , 43 , 44 Urinary GGT and uALP concentrations show moderate discriminatory ability between overt AKI and CKD in dogs. 24 , 26 In a small cohort of elderly ICU patients, uALP and uGGT upon admission were highly predictive of subsequent AKI (AUROC, 0.95 and 0.86, respectively). 45 Only 1 study investigated uGGT/uCr in nonazotemic, hospitalized dogs at risk of AKI, with comparable results (AUROC, 0.78) 30 to the present study. This latter study which had an exceptionally high incidence of AKI (64%), included only dogs that had developed Grade‐I AKI during the first 48 hours of hospitalization, while in 16% of the cases, AKI was diagnosed solely based on urine production, which possibly accounted for the different performance of uGGT compared to the present study. Unlike uGGT/uCr, in the present study uALP/uCr failed to predict later development of AKI, possibly owing to inadequate sample size. Nevertheless, it was the only urinary biomarker significantly associated with death, independently of AKI development.
After gentamicin administration or in dogs with pyometra, the magnitude of uALP and uGGT activities is positively correlated with severity of renal tubular cell histological changes. 40 , 42 Furthermore, uGGT is increased in dogs with Leishmania‐associated protein‐losing nephropathy, 41 pyometra 42 and AP, 44 irrespective of presence of azotemia. In the present study, both biomarkers were significantly increased in hospitalized dogs, compared to healthy controls, with minimal overlap. Their high molecular weight often precludes glomerular filtration, even in face of glomerulopathy, 10 and the findings herein likely reflect a high occurrence of tubulopathy in this cohort of dogs, which was often unassociated with subsequent development of AKI. Nevertheless, in absence of urine protein‐to‐creatinine ratio or renal histology, a systemic origin of either biomarker cannot be ruled‐out.
While uALP and uGGT mostly serve as markers of tubular injury, ILs actively participate in propagation of kidney injury through neutrophil recruitment and induction of inflammation and fibrosis. 16 , 17 , 22 , 46 , 47 In experimental murine AKI models, interfering with IL‐6 and IL‐18 activity or their expression mitigates kidney inflammation and injury. 17 , 22 , 47 , 48 Although both ILs might be filtered through the glomeruli, increases in their urinary concentrations often reflect increased secretion from inflammatory cells within the kidney, and from podocytes (secreting IL‐6) and renal tubular cells (secreting IL‐6 and IL‐18). 22 , 46 In septic AKI human patients, uIL‐18 concentration is higher compared to those with AKI of other etiologies, unlike the findings herein, where uIL‐18/uCr concentration was unassociated with the etiology of AKI. 19 , 21 In humans, reported AUROC for uIL‐18 as a predictor of AKI highly vary, ranging from 0.55 to 0.73. 46 In dogs with cisplatin‐induced AKI, neither uIL‐18 nor uIL‐6 concentration is increased, 49 while renal IL‐6 expression is increased in dogs with CKD, correlating with the magnitude of renal fibrosis, as assessed by immunohistochemical staining. 23 However, in the clinical setting, both uIL‐6 and uIL‐18 have not been prospectively evaluated as predictive biomarkers of naturally‐occurring AKI in hospitalized dogs. In this study, only uIL‐6/uCr and not IL‐18/uCr at presentation had discriminatory ability to predict occurrence of AKI during hospitalization, notwithstanding significant differences for both urinary markers between hospitalized dogs and the healthy controls. The discrepancy between the findings herein and the study of dogs with cisplatin‐induced AKI might stem from the significantly lower number of dogs (n = 17) in the latter, and the heterogeneity of etiologies for AKI in the former. Consonant with the results regarding uALP/uCr and uGGT/uCr herein, this finding might reflect the occurrence of kidney injury in systemically ill dogs, irrespective of subsequent development of overt AKI with worsening azotemia. Additionally, uIL‐6/uCr had the best predictive performance for AKI compared to the other tested individual biomarkers.
Heat shock proteins possess an assortment of cytoprotective, anti‐apoptotic, anti‐fibrotic, and anti‐inflammatory properties, and participate in cellular protein repair and degradation processess. 12 , 50 Expressed in macrophages and renal tubular cells, the HSP70 subfamily includes 4 isoforms, of which HSP72 is highly inducible during AKI. 18 They actively protect against cellular injury, and are amenable to pharmacological interventions, which increase their expression. 12 , 50 In humans, markedly disparate results exist concerning their ability to predict AKI. In 1 study of 56 critically‐ill patients, a cutoff value of 0.5 ng/mL for uHSP70 (values were not normalized to uCr) had sensitivity and specificity of 100% and 90%, respectively, when measured 2 days before the onset of AKI. 20 Furthermore, its accuracy was corroborated prospectively. Nevertheless, a subsequent study, using the same methodology, failed to find differences in uHSP70 concentrations between critically‐ill patients with or without AKI. 51 In the only clinical study in dogs with AKI, uHSP70 significantly differed between those with AKI and healthy controls, and was predictive of death. Nevertheless, that study enrolled dogs with overt AKI, thus precluding investigation of its predictive performance for AKI. 14 Recently, uHSP70/uCr was prospectively evaluated in 80 dogs undergoing elective or emergency surgery, and was significantly increased, while sCr significantly decreased postoperatively. 15 Furthermore, the proportion of dogs with elevated uHSP70/uCr increased with increase in their ASA status, which served as a surrogate marker of disease severity and anesthetic risk. Overall, uHSP70/uCr increased post‐op in 33/80 dogs, although only 5 dogs fulfilled the IRIS AKI criteria. Correspondingly, in the present study, while uHSP70/uCr significantly differed between hospitalized dogs in general and the healthy controls, and was higher in dogs which later developed AKI compared to those which did not, it had poor predictive performance for AKI when assessed by ROC analysis.
The agreement between sCr and sSDMA was poor in this study. Weak to moderate correlations and agreement between these 2 GFR markers were previously reported in dogs and cats, 52 , 53 , 54 , 55 but seemed to improve when tested in a homogeneous group of dogs with AKI. 56 In a study of nonazotemic dogs, sCr proved superior to sSDMA in predicting decreases in GFR when the traditional 14 μg/dL cutoff for SDMA was used. A higher, 18 μg/dL cutoff, only improved the ability of SDMA to detect ≥40% decrease in GFR, but not smaller ones. 53 Notwithstanding the above, in the present study, sSDMA concentration was a moderate predictor for the development of AKI during hospitalization, with comparable performance to the tested urinary biomarkers.
This study has several limitations. First, consistent urine production measurements or investigation of abnormalities in other urinary markers (eg, glucosuria and urinary granular cylinduria) were not performed. Therefore, some dogs might have presented with AKI upon admission. Second, SDMA concentration could not be measured in a large proportion of dogs because of insufficient surplus sera, limiting statistical analyses. Third, urinary constituents and chemical characteristics might interfere with accurate urinary measurement of certain biomarkers. Specifically, urinary pH might interfere with accurate uGGT measurement, 36 while urinary matrix proteins, carbohydrates, and specific gravity interfere with uIL‐6 measurement. 57 While uALP, uGGT, and uCr were measured immediately after collection, measurement of uIL‐6, uIL‐18 uHSP72, and sSDMA was performed on stored samples. Storage time at −80°C differed between samples, which had been collected over the course of 2 years. Several studies in humans and rats demonstrate the stability of uIL‐18 58 and sSDMA 59 at −20°C/−80°C for more than 2 years, and of uHSP72 60 for 9 months. Serum IL‐6 61 is also stable for several years when stored at −20°C, However, similar information regarding urinary stability of each marker in dogs is lacking, and degradation might have affected results. Fourth, the control group was considerably smaller than the group of hospitalized dogs and had a significantly lower median age. The small sample size of the controls also necessitated using the maximal concentration of each marker as the URL, which was inferior to conventional methods of RI estimation which require a minimum of 120 dogs. 31 Fifth, the effects of age, sex and breed on urinary biomarkers is unknown in dogs and might have affected results. Sixth, urine protein to creatinine ratio was not measured in all dogs, precluding assessment of concurrent glomerulopathy and renal proteinuria. Consequently, the presence of ILs and uHSP72 in the urine, and possibly uALP or uGGT, might have been of systemic origin, rather than tubular origin alone. Lastly, the relatively low incidence of AKI and the large heterogeneity in etiologies might have weakened the statistical power.
In conclusion, development of AKI significantly increased the risk of death in hospitalized dogs with various systemic conditions. Urinary concentrations/activities of the 5 biomarkers tested herein were significantly elevated in the study sample of hospitalized dogs compared to controls, with minimal overlap, reflecting the high occurrence of kidney injury in that cohort of dogs, which was often unassociated with subsequent development of overt AKI with worsening azotemia or with death. Despite significant differences between the AKI and non‐AKI groups, none of the tested biomarkers showed satisfactory predictive performance for AKI and death. Nevertheless, combining uGGT/uCr, uHSP70/uCr, and uIL‐6/uCr had an additive value for AKI prediction and might improve their clinical utility, as the odds of developing AKI during hospitalization increased with each additional elevated urinary biomarker. Lastly, the agreement between sSDMA and sCr was poor, but increased sSDMA concentration at presentation, when concurrent sCr concentration was within RI, showed moderate predictive performance for AKI.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by The Hebrew University of Jerusalem Internal Ethics Review Committee, reference number KSVM‐VH/03_2018.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by the European College of Veterinary Internal Medicine Companion Animals (ECVIM‐CA) Clinical Studies Fund 2018.
Nivy R, Chaim N, Hanael E, et al. Prospective evaluation of 5 urinary biomarkers as predictors of acute kidney injury in nonazotemic, hospitalized dogs. J Vet Intern Med. 2021;35(6):2812-2820. doi: 10.1111/jvim.16308
Funding information European College of Veterinary Internal Medicine Companion Animals (ECVIM‐CA) Clinical Studies Fund 2018
REFERENCES
- 1. Behrend EN, Grauer GF, Mani I, Groman RP, Salman MD, Greco DS. Hospital‐acquired acute renal failure in dogs: 29 cases (1983‐1992). J Am Vet Med Assoc. 1996;208:537‐541. [PubMed] [Google Scholar]
- 2. Grauer GF. Prevention of acute renal failure. Vet Clin North Am Small Anim Pract. 1996;26:1447‐1459. [DOI] [PubMed] [Google Scholar]
- 3. Gori E, Lippi I, Guidi G, Perondi F, Pierini A, Marchetti V. Acute pancreatitis and acute kidney injury in dogs. Vet J. 2019;245:77‐81. [DOI] [PubMed] [Google Scholar]
- 4. Harison E, Langston C, Palma D, Lamb K. Acute azotemia as a predictor of mortality in dogs and cats. J Vet Intern Med. 2012;26:1093‐1098. [DOI] [PubMed] [Google Scholar]
- 5. Thoen ME, Kerl ME. Characterization of acute kidney injury in hospitalized dogs and evaluation of a veterinary acute kidney injury staging system. J Vet Emerg Crit Care (San Antonio). 2011;21:648‐657. [DOI] [PubMed] [Google Scholar]
- 6. Kenney EM, Rozanski EA, Rush JE, et al. Association between outcome and organ system dysfunction in dogs with sepsis: 114 cases (2003‐2007). J Am Vet Med Assoc. 2010;236:83‐87. [DOI] [PubMed] [Google Scholar]
- 7. International Renal Interest Society . Grading of AKI . http://www.iris-kidney.com/guidelines/grading.html. Accessed February 1, 2021.
- 8. Braun JP, Lefebvre HP, Watson AD. Creatinine in the dog: a review. Vet Clin Pathol. 2003;32:162‐179. [DOI] [PubMed] [Google Scholar]
- 9. Hokamp JA, Nabity MB. Renal biomarkers in domestic species. Vet Clin Pathol. 2016;45:28‐56. [DOI] [PubMed] [Google Scholar]
- 10. De Loor J, Daminet S, Smets P, et al. Urinary biomarkers for acute kidney injury in dogs. J Vet Intern Med. 2013;27:998‐1010. [DOI] [PubMed] [Google Scholar]
- 11. Amaral Pedroso L, Nobre V, Dias Carneiro de Almeida C, et al. Acute kidney injury biomarkers in the critically ill. Clin Chim Acta. 2020;508:170‐178. [DOI] [PubMed] [Google Scholar]
- 12. Chebotareva N, Bobkova I, Shilov E. Heat shock proteins and kidney disease: perspectives of HSP therapy. Cell Stress Chaperones. 2017;22:319‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang PL, Lun M, Schworer CM, et al. Heat shock protein expression is highly sensitive to ischemia‐reperfusion injury in rat kidneys. Ann Clin Lab Sci. 2008;38:57‐64. [PubMed] [Google Scholar]
- 14. Bruchim Y, Avital Y, Horowitz M, Mazaki‐Tovi M, Aroch I, Segev G. Urinary heat shock protein 72 as a biomarker of acute kidney injury in dogs. Vet J. 2017;225:32‐34. [DOI] [PubMed] [Google Scholar]
- 15. Kavkovsky A, Avital Y, Aroch I, Segev G, Shipov A. Perioperative urinary heat shock protein 72 as an early marker of acute kidney injury in dogs. Vet Anaesth Analg. 2020;47:53‐60. [DOI] [PubMed] [Google Scholar]
- 16. Leslie JA, Meldrum KK. The role of interleukin‐18 in renal injury. J Surg Res. 2008;145:170‐175. [DOI] [PubMed] [Google Scholar]
- 17. Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt‐Ott KM. Biomarkers in acute kidney injury—pathophysiological basis and clinical performance. Acta Physiol (Oxf). 2017;219:554‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrera‐Chimal J, Bobadilla NA. Are recently reported biomarkers helpful for early and accurate diagnosis of acute kidney injury? Biomarkers. 2012;17:385‐393. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Guo W, Zhang J, et al. Urinary interleukin 18 for detection of acute kidney injury: a meta‐analysis. Am J Kidney Dis. 2013;62:1058‐1067. [DOI] [PubMed] [Google Scholar]
- 20. Morales‐Buenrostro LE, Salas‐Nolasco OI, Barrera‐Chimal J, et al. Hsp72 is a novel biomarker to predict acute kidney injury in critically ill patients. PLoS One. 2014;9:e109407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bagshaw SM, Langenberg C, Haase M, Wan L, May CN, Bellomo R. Urinary biomarkers in septic acute kidney injury. Intensive Care Med. 2007;33:1285‐1296. [DOI] [PubMed] [Google Scholar]
- 22. Su H, Lei CT, Zhang C. Interleukin‐6 signaling pathway and its role in kidney disease: an update. Front Immunol. 2017;8:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yhee JY, Yu CH, Kim JH, Sur JH. Effects of T lymphocytes, interleukin‐1, and interleukin‐6 on renal fibrosis in canine end‐stage renal disease. J Vet Diagn Invest. 2008;20:585‐592. [DOI] [PubMed] [Google Scholar]
- 24. Nivy R, Avital Y, Aroch I, Segev G. Utility of urinary alkaline phosphatase and γ‐glutamyl transpeptidase in diagnosing acute kidney injury in dogs. Vet J. 2017;220:43‐47. [DOI] [PubMed] [Google Scholar]
- 25. Lippi I, Perondi F, Meucci V, Bruno B, Gazzano V, Guidi G. Clinical utility of urine kidney injury molecule‐1 (KIM‐1) and gamma‐glutamyl transferase (GGT) in the diagnosis of canine acute kidney injury. Vet Res Commun. 2018;42:95‐100. [DOI] [PubMed] [Google Scholar]
- 26. Heiene R, Biewenga WJ, Koeman JP. Urinary alkaline phosphatase and gamma‐glutamyl transferase as indicators of acute renal damage in dogs. Journal of Small Animal Practice. 1991;32:521‐524. [Google Scholar]
- 27. Portier K, Ida KK. The ASA physical status classification: what is the evidence for recommending its use in veterinary anesthesia?—a systematic review. Front Vet Sci. 2018;5:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xenoulis PG. Diagnosis of pancreatitis in dogs and cats. J Small Anim Pract. 2015;56:13‐26. [DOI] [PubMed] [Google Scholar]
- 29. Keir I, Kellum JA. Acute kidney injury in severe sepsis: pathophysiology, diagnosis, and treatment recommendations. J Vet Emerg Crit Care (San Antonio). 2015;25:200‐209. [DOI] [PubMed] [Google Scholar]
- 30. Perondi F, Lippi I, Ceccherini G, Marchetti V, Guidi G. Evaluation of urinary γ‐glutamyl transferase and serum creatinine in non‐azotaemic hospitalised dogs. Vet Rec. 2019;185:52. [DOI] [PubMed] [Google Scholar]
- 31. Le Boedec K. Reference interval estimation of small sample sizes: a methodologic comparison using a computer‐simulation study. Vet Clin Pathol. 2019;48:335‐346. [DOI] [PubMed] [Google Scholar]
- 32. Segev G, Daminet S, Meyer E, et al. Characterization of kidney damage using several renal biomarkers in dogs with naturally occurring heatstroke. Vet J. 2015;206:231‐235. [DOI] [PubMed] [Google Scholar]
- 33. Nicolle AP, Chetboul V, Allerheiligen T, et al. Azotemia and glomerular filtration rate in dogs with chronic valvular disease. J Vet Intern Med. 2007;21:943‐949. [DOI] [PubMed] [Google Scholar]
- 34. Legatti SAM, El Dib R, Legatti E, et al. Acute kidney injury in cats and dogs: a proportional meta‐analysis of case series studies. PLoS One. 2018;13:e0190772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2:1303‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fissell WH, Dyke DB, Weitzel WF, et al. Bioartificial kidney alters cytokine response and hemodynamics in endotoxin‐challenged uremic animals. Blood Purif. 2002;20:55‐60. [DOI] [PubMed] [Google Scholar]
- 37. Ilchyshyn NP, Villiers E, Monti P. Validation of a spectrophotometric method for GGT measurement in canine urine and determination of the urine GGT‐to‐creatinine ratio reference interval and biological variation in 41 healthy dogs. J Vet Diagn Invest. 2019;31:33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guder WG, Ross BD. Enzyme distribution along the nephron. Kidney Int. 1984;26:101‐111. [DOI] [PubMed] [Google Scholar]
- 39. Grauer GF, Greco DS, Behrend EN, Mani I, Fettman MJ, Allen TA. Estimation of quantitative enzymuria in dogs with gentamicin‐induced nephrotoxicosis using urine enzyme/creatinine ratios from spot urine samples. J Vet Intern Med. 1995;9:324‐327. [DOI] [PubMed] [Google Scholar]
- 40. Clemo FA. Urinary enzyme evaluation of nephrotoxicity in the dog. Toxicol Pathol. 1998;26:29‐32. [DOI] [PubMed] [Google Scholar]
- 41. de Oliveira Frazilio F, de Almeida Borges F, de Souza AI, et al. Biomarkers and renal arterial resistive index in dogs naturally infected with Leishmania infantum . Parasitol Res. 2018;117:3399‐3405. [DOI] [PubMed] [Google Scholar]
- 42. Heiene R, Moe L, Mølmen G. Calculation of urinary enzyme excretion, with renal structure and function in dogs with pyometra. Res Vet Sci. 2001;70:129‐137. [DOI] [PubMed] [Google Scholar]
- 43. Palviainen M, Raekallio M, Vainionpää M, Lahtinen H, Vainio O. Evaluation of renal impairment in dogs after envenomation by the common European adder (Vipera berus berus). Vet J. 2013;198:723‐724. [DOI] [PubMed] [Google Scholar]
- 44. Gori E, Pierini A, Lippi I, Boffa N, Perondi F, Marchetti V. Urinalysis and urinary GGT‐to‐urinary creatinine ratio in dogs with acute pancreatitis. Vet Sci. 2019;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Westhuyzen J, Endre ZH, Reece G, et al. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant. 2003;18:543‐551. [DOI] [PubMed] [Google Scholar]
- 46. Beker BM, Corleto MG, Fieiras C, Musso CG. Novel acute kidney injury biomarkers: their characteristics, utility and concerns. Int Urol Nephrol. 2018;50:705‐713. [DOI] [PubMed] [Google Scholar]
- 47. LaSpina M, Tripathi S, Gatto LA, Bruch D, Maier KG, Kittur DS. An interleukin‐6‐neutralizing antibody prevents cyclosporine‐induced nephrotoxicity in mice. J Surg Res. 2008;148:121‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017;55:1074‐1089. [DOI] [PubMed] [Google Scholar]
- 49. McDuffie JE, Sablad M, Ma J, et al. Urinary parameters predictive of cisplatin‐induced acute renal injury in dogs. Cytokine. 2010;52:156‐162. [DOI] [PubMed] [Google Scholar]
- 50. O'Neill S, Harrison EM, Ross JA, et al. Heat‐shock proteins and acute ischaemic kidney injury. Nephron Exp Nephrol. 2014;126:167‐174. [DOI] [PubMed] [Google Scholar]
- 51. Vaara ST, Lakkisto P, Immonen K, et al. Urinary biomarkers indicative of apoptosis and acute kidney injury in the critically ill. PLoS One. 2016;11:e0149956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sargent HJ, Elliott J, Jepson RE. The new age of renal biomarkers: does SDMA solve all of our problems? J Small Anim Pract. 2021;62:71‐81. [DOI] [PubMed] [Google Scholar]
- 53. McKenna M, Pelligand L, Elliott J, Cotter D, Jepson R. Relationship between serum iohexol clearance, serum SDMA concentration, and serum creatinine concentration in non‐azotemic dogs. J Vet Intern Med. 2020;34:186‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Torrent E, Planellas M, Ordeix L, Pastor J, Rodon J, Solano‐Gallego L. Serum symmetric dimethylarginine as an early marker of excretory dysfunction in canine Leishmaniosis (L. infantum) induced nephropathy. Vet Med Int. 2018;2018:7517359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu L, Lacorcia L, Finch S, Johnstone T. Assessment of serum symmetric dimethylarginine and creatinine concentrations in hyperthyroid cats before and after a fixed dose of orally administered radioiodine. J Vet Intern Med. 2020;34:1423‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gori E, Pierini A, Lippi I, Meucci V, Perondi F, Marchetti V. Evaluation of symmetric dimethylarginine (SDMA) in dogs with acute pancreatitis. Vet Sci. 2020;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wood MW, Nordone SK, Vaden SL, Breitschwerdt EB. Assessment of urine solute and matrix effects on the performance of an enzyme‐linked immunosorbent assay for measurement of interleukin‐6 in dog urine. J Vet Diagn Invest. 2011;23:316‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schuh MP, Nehus E, Ma Q, et al. Long‐term stability of urinary biomarkers of acute kidney injury in children. Am J Kidney Dis. 2016;67:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Teerlink T. HPLC analysis of ADMA and other methylated L‐arginine analogs in biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:21‐29. [DOI] [PubMed] [Google Scholar]
- 60. Ortega‐Trejo JA, Perez‐Villalva R, Barrera‐Chimal J, et al. Heat shock protein 72 (Hsp72) specific induction and temporal stability in urine samples as a reliable biomarker of acute kidney injury (AKI). Biomarkers. 2015;20:453‐459. [DOI] [PubMed] [Google Scholar]
- 61. Kenis G, Teunissen C, De Jongh R, et al. Stability of interleukin 6, soluble interleukin 6 receptor, interleukin 10 and CC16 in human serum. Cytokine. 2002;19:228‐235. [PubMed] [Google Scholar]


