Abstract
Background
Measures to reduce spread of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) during the Covid-19 pandemic 2020–2021 may impact other microbiological agents. We aimed to investigate the incidence of infectious diseases and the incidence of viruses other than SARS-CoV-2 amongst children at The Department of Paediatric and Adolescent Medicine, Oslo University Hospital, Norway during 2020–2021 compared to previous years.
Methods
Data from April 1st 2020 – March 31st 2021 were compared to data from corresponding 12-months periods 2017–2020. ICD-10 infectious disease diagnoses were collected from the Hospital Diagnosis and Procedure Registry and results of virus PCR analyses of different specimens (mainly nasopharyngeal (NF) and faecal samples) were collected from the Laboratory System at the Department of Microbiology.
Results
The number of hospital contacts with acute bronchiolitis, viral pneumonia, gastroenteritis and viral central nervous system infections were reduced by 90% (p<0.0001), 89% (p<0.0001), 74% (p<0.0001) and 78% (p<0.01), respectively. Respiratory syncytial virus (RSV), influenza virus A and B and Human metapneumovirus (HMPV) were almost completely absent during the pandemic period. The proportions of rhinovirus positive NF samples were 31.7% vs. 34.9% (p<0.05), but not significantly different for adenovirus. The proportions of positive faecal samples were 1% vs. 10% for adenovirus (p<0.00001) and 3.3% vs. 12% for norovirus (p<0.00001), but not significantly different for rotavirus. The proportions of enterovirus positive samples were 3.5% vs. 21.6% (p<0.00001).
Conclusion
The incidence of several paediatric infectious diseases mainly of viral aetiology declined significantly during the Covid-19 pandemic. Some common respiratory viruses were almost completely absent.
Keywords: Paediatric infectious diseases; Epidemiology; Covid-19; Respiratory syncytial virus (RSV); influenza virus; Human metapneumovirus (HMPV); Rhinovirus, adenovirus; Enterovirus; Norovirus; Rotavirus <PE-FRONTEND>
1. Introduction
The first cases of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection were detected in Norway late February 2020. Several measures to reduce spread of the virus were introduced from early March 2020; handwashing, social distancing, stay home when sick, as well as a six-week nationwide lockdown from March 12th 2020. City of Oslo and surrounding areas reported the highest incidence of SARS-Cov-2 cases in Norway during the Covid-19 pandemic [1, 2]. In this region, infection control measures were maintained throughout most of the pandemic until late September 2021, except for facilitations of the measures for some periods mainly during the summer 2020.
Schools and kindergartens were closed six to eight weeks during the initial nationwide lock-down. In Oslo, schools were also closed for shorter periods from late autumn 2020 and throughout the pandemic, related to local outbreaks.
Severe disease from SARS-CoV-2 is rare in children and Covid-19 hospitalisation rates have been low compared to adult age groups [2], [3], [4]. The total number of all cause hospitalisations at The Department of Paediatric and Adolescent Medicine, Oslo University Hospital declined during 2020. We aimed to specifically investigate the incidence of selected infectious diseases as well as the incidence of different viruses other than SARS-CoV-2 amongst children admitted to our department during the Covid-19 pandemic 2020–2021 compared to previous years.
2. Material and methods
The Department of Paediatric and Adolescent Medicine, Oslo University Hospital is the primary hospital for most children in Oslo with a catchment area covering 107,570 individuals aged 0–18 years [5].
We collected data for the period April 1st 2020 – March 31st 2021 (the pandemic period) and for the corresponding 12 months periods during 2017–2018, 2018–2019 and 2019–2020, respectively (the pre-pandemic period).
The total number of hospital contacts (outpatient contacts and overnight hospitalisations) for patients aged 0–18 years with selected ICD-10 infectious disease diagnoses were collected from the Hospital Diagnosis and Procedure Registry.
Following results from PCR analyses of individuals aged 0–18 years were extracted from the Swisslab Laboratory System (Nexus Swisslab GmbH, Germany) at the Department of Microbiology: 11 common respiratory viruses (nasopharyngeal samples), enterovirus (CSF, blood, faeces, airway specimens, vesicles and other unspecified specimens), and rotavirus, adenovirus and norovirus (faecal samples).
Pearson's Chi-square test and Fischer exact test were used to compare categorical data (incidence of infectious disease diagnoses and proportion of positive virus test results).
Ethical board review and collection of informed consent were not required for this study because only anonymised register data were collected.
3. Results
The total number of hospital contacts at Oslo University Hospital for patients aged 0–18 years with selected ICD-10 infectious disease diagnoses during the pandemic period compared to the pre-pandemic period are shown in Table 1 .
Table 1.
The total number of hospital contacts (outpatient contacts and hospitalisations) for patients 0–18 years with selected infectious disease diagnoses during 12 months periods (April 1st - March 31st) 2017–2018, 2018–2019 and 2019–2020 (the pre-pandemic period) and 2020–2021 (the Covid-19 pandemic period).
| ICD-10 diagnoses | Female sex | Mean (SD) age (years) | 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 | Difference 2017–2020 vs. 2020–2021 | p-value |
| J21 Acute bronchiolitis | 38.6% | 0.9 (1.7) | 357 | 513 | 274 | 40 | -90% | p<0.00001 |
| J12 Viral pneumonia | 42.5% | 3.1 (3.3) | 151 | 117 | 145 | 15 | -89% | p<0.00001 |
| J13+J14+J15 Bacterial pneumonia | 48.9% | 6.5 (5.4) | 50 | 63 | 108 | 35 | -53% | p<0.001 |
| A08 + A09 Gastroenteritis* | 40.6% | 3.6 (4.2) | 422 | 357 | 298 | 92 | -74% | p<0.00001 |
| A85+A86+A87 Viral encephalitis/ meningitis* | 50.9% | 8.6 (6.6) | 15 | 23 | 17 | 4 | -78% | p<0.01 |
| N10 Pyelonephritis | 62.4% | 3.0 (4.4) | 190 | 141 | 153 | 119 | -26% | p<0.05 |
| M86 Osteomyelitis | 66.3% | 10.3 (4.9) | 48 | 82 | 102 | 99 | +28% | p = 0.10 |
| A69.2 Lyme Borreliosis* | 53.9% | 10.8 (4.5) | 23 | 28 | 39 | 41 | +37% | p = 0.19 |
| R56.0 Febrile seizures | 45.9% | 2.9 (2.4) | 106 | 95 | 101 | 45 | -55% | p<0.00001 |
* Either primary or secondary diagnosis code.
The proportion of nasopharyngeal samples positive for selected airway viruses and faecal samples positive for gastrointestinal viruses during the same periods are shown in Table 2 .
Table 2.
Proportion of nasopharyngeal samples positive for selected airway viruses, faecal samples positive for gastrointestinal viruses and samples from multiple specimens positive for enteroviruses during 12 months periods (April 1st - March 31st) 2017–2018, 2018–2019 and 2019–2020 (the pre-pandemic period) and 2020–2021 (the Covid-19 pandemic period).
| Sample specimen | 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 | p-value* | |
| RSV | NF | 322/3032 (10.6%) | 570/3072 (18.6%) | 247/2730 (9.0%) | 10/2102 (0.5%) | p<0.0001 |
| Influenza virus A/B | NF | 316/3032 (10.4%) | 202/3072 (6.6%) | 213/2730 (7.8%) | 4/2102 (0.2%) | p<0.0001 |
| HMPV | NF | 226/3032 (7.5%) | 32/3072 (1.0%) | 195/2730 (7.1%) | 0/2102 | p<0.00001 |
| Rhinovirus | NF | 979/3032 (32.3%) | 1116/3072 (36.3%) | 992/2730 (36.3%) | 666/2102 (31.7%) | p<0.05 |
| Adenovirus | NF | 324/3032 (10.6%) | 349/3072 (11.3%) | 265/2730 (9.7%) | 190/2102 (9.0%) | p = 0.06 |
| Rotavirus | F | 51/462 (11%) | 26/387 (6.7%) | 33/413 (8%) | 18/296 (6.1%) | p = 0.18 |
| Adenovirus | F | 50/462 (10.8%) | 59/387 (15.2%) | 16/414 (3.9%) | 3/295 (1%) | p<0.00001 |
| Norovirus | F | 69/571 (12.1%) | 63/540 (11.7%) | 66/537 (12.3%) | 11/332 (3.3%) | p<0.00001 |
| Enterovirus | B, F, A, V, C, O | 131/653 (20.0%) | 185/775 (23.8%) | 200/956 (20.9%) | 20/569 (3.5%) | p<0.00001 |
* 2017-2020 vs. 2020-2021. NF: nasopharyngeal, F: faecal, B: blood, A: airways, V: vesicles, C: cerebrospinal fluid.
O: other unspecified specimens, RSV: Respiratory syncytial virus, HMPV: Human metapneumovirus.
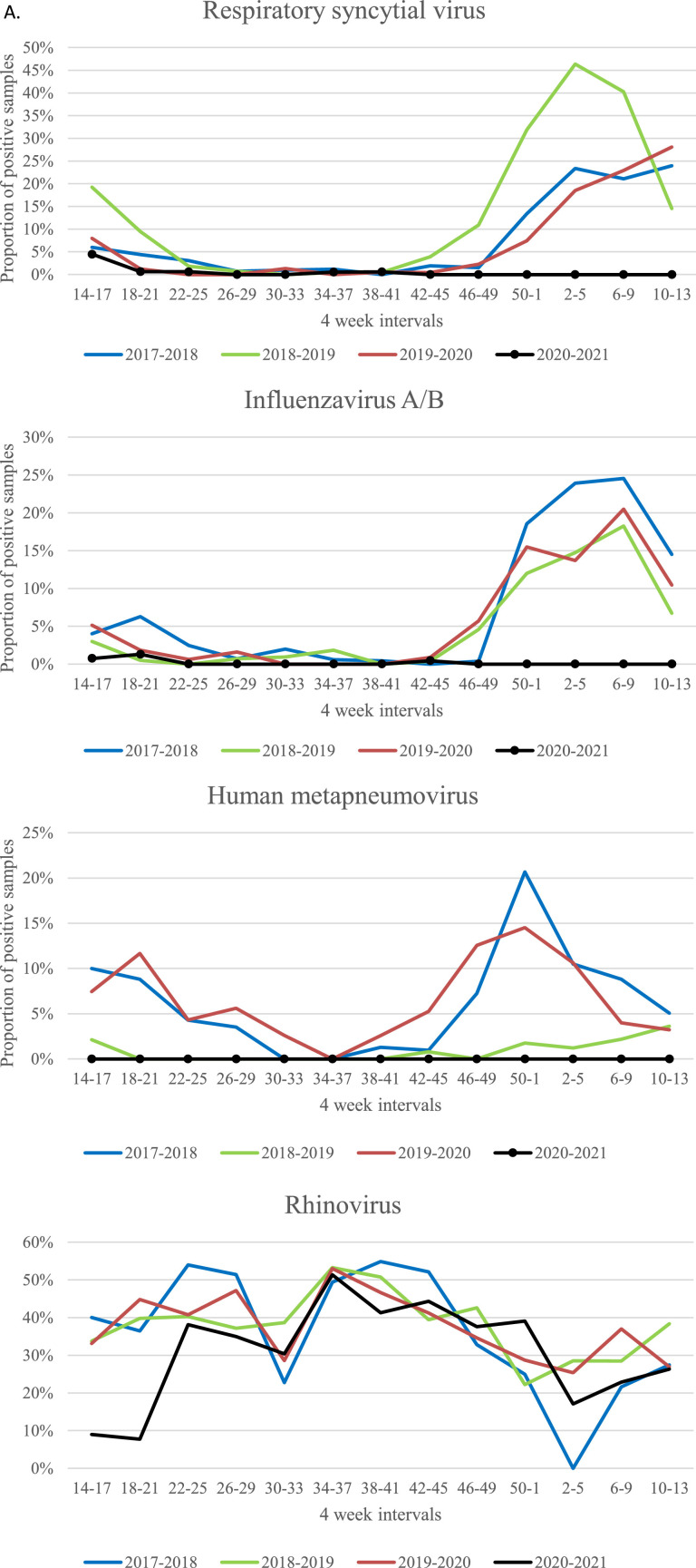
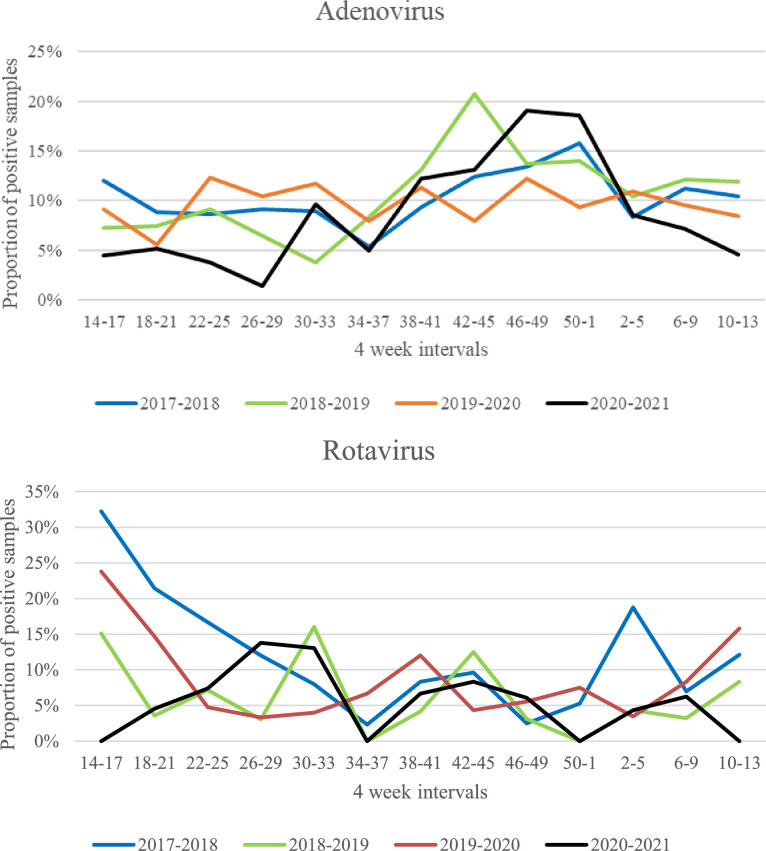
The proportion of clinical samples of different origin positive for enterovirus is also shown in Table 2. The seasonality of the proportions of positive samples for selected viruses are shown in Fig. 1 .
Fig. 1.
Proportion of nasopharyngeal samples positive for Respiratory syncytial virus, influenza virus, Human metapneumovirus, rhinovirus and adenovirus, and proportion of faecal samples positive for rotavirus shown for four-week intervals during 12 months periods (April 1st - March 31st) 2017–2018, 2018–2019 and 2019–2020 (the pre-pandemic period) and 2020–2021 (the Covid-19 pandemic period).
4. Discussion
The incidences of several common infectious diseases amongst children admitted to Oslo University Hospital were greatly reduced during the Covid-19 pandemic 2020–2021 compared to the pre-pandemic period 2017–2020. The largest reduction was noticed for airway infections like acute bronchiolitis and viral pneumonia, known to be caused by respiratory viruses. Respiratory syncytial virus (RSV), the most common cause of acute bronchiolitis in infants, as well as influenza virus and Human metapneumovirus (HMPV) were almost completely absent during the fall and winter season 2020–2021. Similar observations have been reported from several other countries [6, 7].
The most likely explanation of this great reduction is that societal infection control measures against SARS-CoV-2 also effectively reduce spread of other viruses that are transmitted by contact, droplets or aerosols. Another theory is that interaction between SARS-CoV-2 and other respiratory virus may possibly have played a role. Immune-mediated interference has been described for influenza virus and other respiratory viruses that may possibly explain why common cold-like virus diminish during flu seasons [8].
The proportion of rhinovirus positive nasopharyngeal samples was only modestly reduced during the pandemic period (31.7% positive samples) compared to the pre-pandemic period (34.9% positive samples), and the cumulative incidence of adenovirus positive samples was not significantly reduced. However, the proportions of rhinovirus and adenovirus positive samples were low during the lock-down period in the beginning of the pandemic period (Fig. 1). Later on the level of rhinovirus and adenovirus increased despite continuation of several infection control measures. Both rhinovirus and adenovirus show less seasonal variation than other respiratory viruses and are normally present all year around as shown in the pre-pandemic periods (Fig. 1). They are non-enveloped viruses that are not so quickly inactivated by commonly used hand sanitizers as enveloped viruses like RSV and influenza virus [9]. A study by Leung et al. showed that surgical facemasks significantly reduced detection of influenza virus RNA in respiratory droplets and coronavirus RNA in aerosols. For rhinovirus however, there were no significant differences between detection of virus with or without facemasks, neither in respiratory droplets nor in aerosols [10]. Additionally, children are known to be a major reservoir for rhinovirus and physical distancing may not effectively prevent transmission of the rhinovirus [11].
The proportion of adenovirus positive faecal samples differed significantly between the three pre-pandemic 12 months periods, with the lowest level in 2019–2020 (3.9%). But adenovirus was almost completely absent from faecal samples during the pandemic period. The proportion of norovirus positive faecal samples was also significantly lower during the pandemic period (3,3%) compared to the pre-pandemic period (12%). In contrast, the level rotavirus did not significantly decline during the pandemic. A live attenuated rotavirus vaccine was introduced in the Norwegian national immunisation programme in October 2014, and the incidence of rotavirus gastroenteritis declined significantly the following four years period [12]. The immunisation programme was maintained throughout the Covid-19 pandemic, and we speculate that rotavirus vaccine strains may have contributed to a stable level of rotavirus positive faecal samples during the pandemic period.
Interestingly, the incidence of central nervous system infections of viral aetiology was significantly reduced during the pandemic period, although the total number of patients is small. Detection of enterovirus was significantly reduced, indicating that infection control measures also effectively reduce transmission of this group of viruses.
The incidence of Lyme Borreliosis increased, although not statistically significant. This tick-borne disease is endemic in most parts of southern Norway and we speculate that an increased incidence may be due to more outdoor activities in woods and fields during the pandemic.
Finally, a higher threshold to contact the health services during the pandemic due to fears of contracting SARS-CoV-2 or to overburden the services may have contributed to lower detection rates of infectious diseases. However, the total number of contacts of children with pyelonephritis only modestly decreased during the pandemic. Looking at the subgroup of hospitalised children with pyelonephritis, the incidence did not significantly decrease. The number of cases with osteomyelitis increased in the pandemic period compared to the pre-pandemic periods, although not statistically significant. Pyelonephritis and osteomyelitis are endogenous infections caused by bacteria colonising the patient. The occurrence of such infections is not likely to be substantially affected by infection control measures directed against SARS-CoV-2.
Lack of exposure to common viruses like RSV and influenza virus amongst children, may have led to depleted immunity, which may result in larger outbreaks with such viruses when infection control measures are abolished. Large non-seasonal outbreaks of RSV infections are reported from different parts of the world when societies have re-opened after the Covid-19 pandemic [13, 14].
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Great thanks to Ingrid Lundeby for data extraction.
References
- 1.Norwegian Institute of Public Health . 2021. Daily Report and Statistics About Coronavirus and COVID-19.https://www.fhi.no/en/id/infectious-diseases/coronavirus/daily-reports/daily-reports-COVID19/ [October 24th 2021]. Available from: [Google Scholar]
- 2.Størdal K., Ruiz P.L.-.D., Greve-Isdahl M., Surén P., Knudsen P.K., Løvdal Gulseth H., et al. Risk factors for SARS-CoV-2 infection and hospitalisation in children and adolescents in Norway: a nationwide population-based study. medRxiv. 2021 doi: 10.1136/bmjopen-2021-056549. 2021.07.01.21259887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swann O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M., et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.City of Oslo . 2021. Statistics Bank City of Oslo.https://statistikkbanken.oslo.kommune.no/webview/ [cited 2019. Available from: [Google Scholar]
- 6.Van Brusselen D., De Troeyer K., Ter Haar E., Vander Auwera A., Poschet K., Van Nuijs S., et al. Bronchiolitis in COVID-19 times: a nearly absent disease? Eur. J. Pediatr. 2021;180(6):1969–1973. doi: 10.1007/s00431-021-03968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polcwiartek L.B., Polcwiartek C., Andersen M.P., Østergaard L., Broccia M.D., Gislason G.H., et al. Consequences of coronavirus disease-2019 (COVID-19) lockdown on infection-related hospitalizations among the pediatric population in Denmark. Eur. J. Pediatr. 2021;180(6):1955–1963. doi: 10.1007/s00431-021-03934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickbakhsh S., Mair C., Matthews L., Reeve R., Johnson P.C.D., Thorburn F., et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. U S A. 2019;116(52):27142–27150. doi: 10.1073/pnas.1911083116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kampf G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018;98(4):331–338. doi: 10.1016/j.jhin.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.H., McDevitt J.J., Hau B.J.P., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020;26(5):676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole S., Brendish N.J., Tanner A.R., Clark T.W. Physical distancing in schools for SARS-CoV-2 and the resurgence of rhinovirus. Lancet Respiratory Med. 2020;8(12):e92–ee3. doi: 10.1016/S2213-2600(20)30502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruun T., Salamanca B.V., Bekkevold T., Døllner H., Gibory M., Gilje A.M., et al. Impact of the rotavirus vaccination program in norway after four years with high coverage. Pediatr. Infect. Dis. J. 2021;40(4):368–374. doi: 10.1097/INF.0000000000003020. [DOI] [PubMed] [Google Scholar]
- 13.Foley D.A., Yeoh D.K., Minney-Smith C.A., Martin A.C., Mace A.O., Sikazwe C.T., et al. The interseasonal resurgence of respiratory syncytial virus in australian children following the reduction of coronavirus disease 2019-related public health measures. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciaa1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ujiie M., Tsuzuki S., Nakamoto T., Iwamoto N. Resurgence of respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021;27(11):2969–2970. doi: 10.3201/eid2711.211565. [DOI] [PMC free article] [PubMed] [Google Scholar]