Abstract
Objectives
Vitamin C has anti-inflammatory effects. This review aimed to investigate the therapeutic effect of high-dose intravenous vitamin C (HDIVC) in patients with coronavirus disease 2019 (COVID-19).
Methods
The following key phrases were searched for article inclusion: “Vitamin C OR ascorbic acid” AND “COVID-19 OR coronavirus disease 2019 OR severe acute respiratory syndrome coronavirus 2 OR SARS-CoV-2″. Articles that utilized HDIVC for the management of patients with COVID-19 were included, whereas review articles and case reports were excluded from this review. Moreover, we performed a meta-analysis to evaluate whether HDIVC can reduce the length of hospital stay and in-hospital mortality rate of patients with severe COVID-19.
Results
In total, eight articles were included in this review, and five studies were included in the meta-analysis. The length of hospital stay was not significantly different between the HDIVC and control groups. Also, although our meta-analysis showed a tendency for HDIVC to reduce the in-hospital mortality rate in patients with severe COVID-19, the in-hospital mortality rate was not significantly different between patients treated with HDIVC and those who did not receive HDIVC.
Conclusions
Evidence supporting the therapeutic use of HDICV in COVID-19 patients is lacking. Further studies are required for drawing a clear conclusion on this topic.
Keywords: Vitamin C, Intravenous, Treatment, COVID-19, Review
1. Introduction
Since the first confirmed case of the coronavirus disease 2019 (COVID-19) in late 2019, COVID-19 has rapidly spread worldwide in just 2–3 months and eventually became a global health issue.1 Majority of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have mild symptoms, such as cough, sore throat, runny nose, headache, body ache, and loss of taste, and do not require hospitalization.2, 3 However, pneumonia is a potential complication of COVID-19, and 10–20% of patients suffer from severe pneumonia and acute respiratory distress syndrome, which can lead to sepsis or multiple organ failure.4 Patients with mild to moderate COVID-19 require proper management to prevent disease aggravation. Those with severe symptoms or systemic complications need intensive care to reduce the risk of mortality. Thus far, despite much effort, effective therapeutic medications have not been developed and the main management for COVID-19 patients is supportive treatment.
In severe COVID-19 patients, pro-inflammatory cytokines are assumed to be activated.5 It is suggested that chronic inflammation is the main risk factor for increased COVID-19 morbidity and mortality.5 Vitamin C has a number of beneficial anti-inflammatory effects by modulating nuclear transcription factor kappa B, inhibiting proinflammatory cytokine production, neutralizing reactive oxygen species, and assisting immunomodulation as a cofactor of various biosynthetic pathways in the immune system.6, 7, 8 In addition, vitamin C protects neutrophils and phagocytes avoiding damage after oxidative burst and activates a caspase-dependent cascade that facilitates programmed apoptosis and inhibits necrosis.9, 10.
During the infectious state, especially lung infection or a critically ill state after the infection, oxidative stress becomes prominent.11, 12 Vitamin C has antioxidant properties that increases in patients with infection, which frequently reduces vitamin C levels, and in patients with pneumonia or critical illness, it suppresses inflammation and improves immunoregulatory function.13, 14., 15 In addition, vitamin C can shorten the duration of infection in the respiratory tract and regulate disease severity.16, 17. Several studies have recommended the use of vitamin C in patients with respiratory tract infections or critical illnesses for its therapeutic advantages,18., 19 in particular, high-dose intravenous vitamin C (HDIVC) that can reduce the severity of patients’ symptoms, shorten the length of hospitalization and duration of mechanical ventilation, and result in vasopressor sparing and reduction of in-hospital mortality rate.20, 21, 22, 23
Vitamin C can be supplied via oral or intravenous (IV) routes. However, the use of oral vitamin C is limited because of its low absorption rate.24, 25 Majority of orally administered vitamin C is flushed out of the body without being used, and its peak plasma concentration is very low,26 making it difficult to achieve its therapeutic plasma level. In contrast, the IV administration of vitamin C can reach the therapeutic level quickly with 30–70 times higher peak plasma concentration compared with that of oral vitamin C by bypassing the limits of intestinal transporters.27
HDIVC has also been administered in patients with COVID-19 in many previous studies that evaluated the effectiveness of HDIVC in patients with COVID-19 in different settings with various clinical findings and results.28, 29., 30, 31, 32., 33, 34, 35, 36 In 2021, Zhang et al.34 conducted a randomized controlled trial pilot study comparing the effects of HDIVC with placebo to determine whether HDIVC infusion was effective against severe COVID-19. HDIVC was not effective in reducing in-hospital mortality and improving invasive mechanical ventilation-free days in 28 days. However, HDIVC was effective in improving the arterial partial pressure of O2/fraction of inspired O2 ratio, suggesting that HDIVC can have an oxygenation benefit in patients with severe COVID-19. In addition, a meta-analysis protocol to investigate the therapeutic effect of vitamin C on COVID-19 has been published. According to a meta-analysis protocol by Huang et al. in 2021,36 vitamin C reduces the duration of ICU hospitalization in pneumonia patients by an average of 8% and shortens the duration of mechanical ventilation. Therefore, it was reported that HDIVC could be successfully applied to the treatment of patients with COVID-19-related pneumonia. Previous studies have yielded not only similar findings but also contradictory findings, and thus the therapeutic effects of HDIVC in COVID-19 patients remain unclear. This review aims to present the findings from recent studies on the possible role of high-dose vitamin C in the management of patients with COVID-19.
2. Methods
2.1. Search strategy
In this study, the PICO (population, intervention, comparison, outcome) model for establishing the search strategy was set as follows: (1) patients or population, patients with severe COVID-19; (2) Intervention, HDIVC; (3) Comparison, placebo or usual care; (4) Outcome, in-hospital mortality rate and length of hospital stay. This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Articles published between January 1, 2019, and July 29, 2021, were searched in the PubMed, Cochrane, Embase, and Web of Science databases using the following key phrases: “Vitamin C OR ascorbic acid” AND “COVID-19 OR coronavirus disease 2019 OR severe acute respiratory syndrome coronavirus 2 OR SARS-CoV-2.”
2.2. Inclusion and exclusion criteria
The following studies were included in this study: (1) studies in which HDIVC (daily total dose ≥2 g) was administered to patients with severe COVID-19, (2) studies evaluating in-hospital mortality rate or length of hospital stay, (3) studies comparing HDIVC to placebo or control groups, (4) studies written in English. The exclusion criteria were as follows: (1) review articles, case report, protocol, and conference presentation.
2.3. Data extraction
Data for meta-analysis were independently investigated by two researchers (M.C.C and Y.J.C). Duplicate studies were excluded, and studies that met the eligibility criteria were selected. Studies were evaluated for eligibility by reviewing the title and abstract. After reading the full text, studies were finally selected for inclusion in the meta-analysis, and discrepancies were resolved through discussion.
2.4. Quality assessment
Quality assessment for randomized trials was conducted using the Cochrane Collaboration tool and for retrospective studies, using the Newcastle-Ottawa quality assessment scale (study quality: low (0−3), moderate (4−6), high (7−9)).37, 38.
2.5. Statistical analysis
The extracted data were analyzed using Comprehensive Meta-Analysis version 2 (Biostat Inc., Englewood, NJ, USA). A heterogeneity test was conducted using the I2 statistic to evaluate the extent of inconsistency in the obtained results. If the I2 value was > 50%, data were considered to have substantial heterogeneity, and a random-effects model was used for data analysis. In contrast, if the I2 value was ≤ 50%, then the pooled data were considered homogeneous, and a fixed-effects model was applied for data analysis. We analyzed the odds ratio (OR) for in-hospital mortality rate (with 95% confidence interval [CI]) and the standard mean difference (SMD) (with 95% CI) for length of hospital stay. Statistical significance was set at p < 0.05.
2.6. Publication bias
Funnel plot and Egger's test were used to confirm publication bias of studies included in meta-analysis. A funnel plot is a graph that shows the relationship between the sample size and the effect size, and it is easy to visually check whether the distribution is symmetrical with respect to the pooled estimate. Egger's test was performed to test whether the funnel plot is symmetric. The Comprehensive Meta-Analysis version 2 was used for funnel plot and Egger's test. In the result of Egger's test, when the p-value was > 0.05, it was considered that there was no publication bias.
3. Results
3.1. Study selection and characteristics
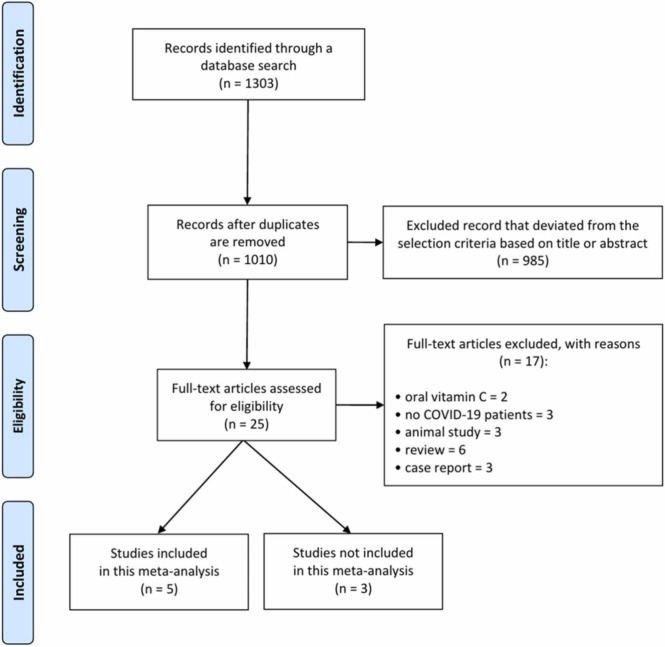
A total of 1303 articles were identified as potentially relevant articles in the primary literature search ( Fig. 1). After reviewing the titles and abstracts and assessing their eligibility based on the full text, eight articles were included in this review ( Table 1) (randomized controlled trials [RCT], 429., 30, 33, 34; retrospective studies, 428, 31, 32., 35). Of the included articles, five were used for meta-analysis. In the five studies included in the meta-analysis, 186 participants were included in the HDIVC group and 188 participants were included in the control group. In three studies not included in the meta-analysis, 256 participants in the HDIVC group and 275 participants in the control group were included. In all studies, the dose of HDIVC administered to patients with COVID-19 was 50 mg or more per day.
Fig. 1.
Flowchart showing the search results.
Table 1.
Key published studies on the role of high-dose vitamin C in the treatment of COVID-19.
| First author, year | Study design | Number of patients (E/C) | HDIVC protocol | Treatment other than HDIVC | Outcome measurement | Summary of outcome | Number of death case (E/C) |
|---|---|---|---|---|---|---|---|
| Gao, 2021 (27)a | Retrospective | 46/30 | 12 g/day for 1st day, 6 g/day for the 2nd to 5th days, for a total of 5 days | Antibiotics, corticosteroids, immunomodulators and other antivirals (e.g., Lopinavir/Ritonavir, Ribavirin) | Mortality rate, oxygen support status | Reduced mortality rate in severe COVID-19 patients after HDIVC. Improved oxygen support status after HDIVC | 1/5 |
| JamaliMoghadamSiahkali, 2021 (28)a | RCT | 30/30 | 6 g/day(1.5 g every 6 h) for 5 days | Lopinavir/Ritonavir 400/100 mg twice daily and single dose of oral hydroxychloroquine (400 mg) on the first day of hospitalization | Mortality rate, length of hospital stay, length of ICU stay, oxygen saturation | Longer hospital stay in the control group, and no significant difference in mortality rate, length of ICU stay, and oxygen saturation | 3/3 |
| Kumari, 2020 (29)a | RCT | 75/75 | 50 mg/kg/day; treatment duration was not described | Dexamethasone and prophylactic antibiotics | Mortality rate, length of hospital stay, need for mechanical ventilator | Shorter length of hospital stay in the HDIVC group; no difference in mortality rate and need for mechanical ventilator | 7/11 |
| Li, 2021 (30)a | Retrospective | 8/24 | 9 g/day (1.5 g every 6 h) for 4 days | Hydrocortisone 50 mg every 6 h and thiamine 200 mg every 12 h for a total course of 4 days | Mortality rate, length of hospital stay, length of ICU stay | Higher mortality rate in the HDIVC group; no difference in length of hospital stay and ICU stay | 7/19 |
| Suna, 2021 (31) | Retrospective | 153/170 | 2 g/day; treatment duration was not described | No information | Mortality rate, length of hospital stay, readmission rate, ICU admission, and advanced oxygen support | No significant difference | 17/24 |
| Thomas, 2021 (32) | RCT | 48 (HDIVC)/58 (zinc)/58 (HDIVC+zinc)/50 (control) | 8 g/day (divided over 2–3 times a day) for 10 days | If necessary, glucocorticoid | Symptoms related to COVID-19, mortality rate, hospitalization rate | No significant difference | 1(HDIVC)/0(zinc) 2(HDIVC+zinc)/ 0(control) |
| Zhang, 2021 (33)a | RCT | 27/29 | 12 g/day for 7 days | Oseltamivir + azithromycin (if necessary, Piperacillin/tazobactam or hydrocortisone (1 mg/kg/day)) | Mortality rate, length of hospital stay, length of ICU stay | No significant difference | 6/11 |
| Zhao, 2021 (34) | Retrospective | 55/55 | 100 mg/kg for 7 days | Antiviral therapy (if necessary, glucocorticoid) | Rate of transition to severe state, duration of systematic inflammatory response | Lower rate of transition to severe state and shorter duration of systematic inflammatory response in the HDIVC group | No information |
E, experimental group; C, control group; HDIVC, high-dose intravenous vitamin C; COVID-19, coronavirus disease 2019; ICU, intensive care unit; RCT, randomized controlled trial
Studies that included for meta-analysis
3.2. Results of the meta-analysis
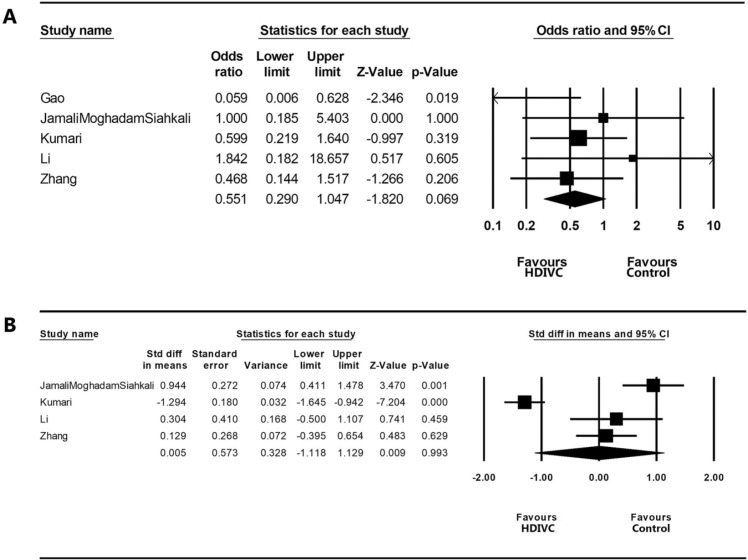
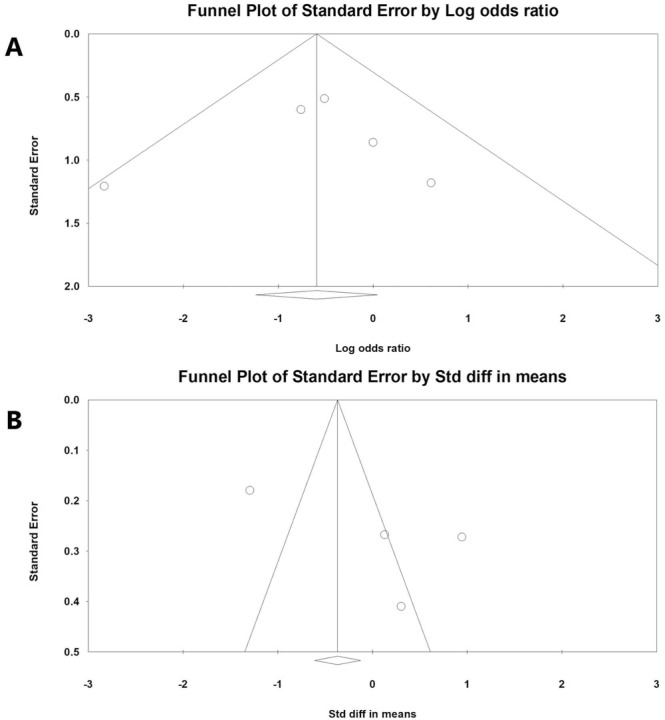
In the meta-analysis to analyze the effectiveness of HDIVC in patients with severe COVID-19, 186 patients who received treatment with HDIVC and 184 patients who received standard care only without HDIVC were recruited from five studies (three RCTs29., 30, 34 and two retrospective studies28, 31). For the meta-analysis of in-hospital mortality rate, a fixed-effects model was adopted (I2 = 20.877) ( Fig. 2A). In-hospital mortality rate was not significantly different between the HDIVC and control groups (OR = 0.551 [95% CI = 0.290–1.047], p = 0.069). However, the in-hospital mortality rate of patients treated with HDIVC tended to be lower than that of patients who did not receive HDIVC. In the meta-analysis of the length of hospital stay, a random-effects model was used (I2 = 94.638) (Fig. 2B). The length of hospital stay was not significantly different between the HDIVC and control groups (SMD = 0.005 [95% CI = −1.118 to 1.129], p = 0.993). The risk of publication bias was determined using a funnel plot and Egger’s test. Funnel plots of the in-hospital mortality rate and length of hospital stay were visually symmetrical ( Fig. 3). In Egger’s test, p-values were 0.404 and 0.118 in the analyses of in-hospital mortality rate and length of hospital stay, respectively. Therefore, a significant publication bias was unlikely to occur.
Fig. 2.
Results of the meta-analysis on the difference of (A) in-hospital mortality rate and (B) length of hospital stay between the high-dose intravenous vitamin C (HDIVC) group and control group. Meta-analysis was performed with Comprehensive Meta-Analysis version 2 (Biostat Inc., Englewood, NJ, USA).
Fig. 3.
Graphic funnel plots of the included studies. Comparison of (A) in-hospital mortality rate and (B) length of hospital stay between the high-dose intravenous vitamin C (HDIVC) and control groups.
3.3. Review of the studies that were not included in the meta-analysis
Summarizing the results of three studies that were not included in the meta-analysis, two studies (Thomas et al. and Suna et al.’s studies) evaluated the effectiveness of HDIVC in COVID-19 patients without considering disease severity.32., 33 In-hospital mortality and length of hospital stay (hospitalization rates) were not significantly improved after the treatment with HDIVC. The other study (Zhao et al.’s study) analyzed the effectiveness of HDIVC in patients with moderate COVID-19.35 Fifty-five patients treated with HDIVC showed significantly shorter duration and lower occurrence of systemic inflammatory response syndrome compared to that of 55 patients in the control group.
3.4. Adverse effects
In all eight included studies,28, 29., 30, 31, 32., 33, 34, 35 the rate of occurrence of adverse effects after the treatment of COVID-19 between the HDIVC and control groups was not significantly different. In addition, specific adverse events related to HDIVC treatment, such as headache, nausea, bloating, or abdominal discomfort, have not been reported.
3.5. Assessment of the study quality
In the assessment of quality of included studies, of four RCTs,29., 30, 33, 34 all studies were rated as having a low risk of bias in the random sequence, incomplete outcome data, and selective reporting domains ( Table 2). However, only one RCT (Zhang et al.’s study34) was rated as having a low risk of bias in the domains of allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. All four retrospective studies28, 31, 32., 35 were rated as nine stars, which are considered of high quality.
Table 2.
Results of the quality assessment using of the Cochrane Collaboration tool (A) and the Newcastle-Ottawa quality assessment scale (B).
| Randomized trials (A) | Quality criteria | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | |||||||||
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | ||||||||||
| JamaliMoghadamSiahkali, 2021 (28) | Low risk | Unclear | High risk | High risk | Low risk | Low risk | |||||||||
| Kumari, 2020 (29) | Low risk | Unclear | Unclear | High risk | Low risk | Low risk | |||||||||
| Thomas,2021 (32) | Low risk | Unclear | Unclear | High risk | Low risk | Low risk | |||||||||
| Zhang, 2021 (33) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | |||||||||
| Retrospective studies (B) | Quality criteria | Selection | Comparability | Exposure | Total (9) | ||||||||||
| Is case definition adequate? (1) | Representativeness of the cases (1) | Selection of controls (1) | Definition of controls (1) | Comparability on basis of design or analysis (2) | Ascertainment of exposure (1) | Same method of ascertainment for cases and controls (1) | Nonresponse rate (1) | ||||||||
| Gao, 2021 (27) | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||||||
| Li, 2021 (30) | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||||||
| Suna, 2021 (31) | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||||||
| Zhao, 2021 (34) | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | ||||||
4. Discussion
Although theoretical evidence supporting the use of HDIVC in patients with infectious disease is sufficient and some previous studies showed positive therapeutic effect of HDIVC, in our review, we hardly found the prominent effectiveness of HDIVC in COVID-19 patients.
In the meta-analysis, although the in-hospital mortality rate tended to be reduced after HDIVC in severe COVID-19 patients, the in-hospital mortality rate was not significantly different between patients treated with HDIVC and those who did not receive HDIVC. Also, the length of hospital stay was not significantly different between the HDIVC and control groups. In addition, the studies that were conducted without considering disease severity showed no better prognosis after the treatment with HDIVC. Although Zhao et al. showed the positive therapeutic effect in moderate COVID-19 after the treatment with HDIVC, its reliability is low due to lack of studies that showed positive therapeutic effect of HDIVC.
In patients with moderate to severe COVID-19, pro-inflammatory cytokines and systemic inflammation are largely increased, which damages the alveolar capillaries and multiple organs.39, 40 It was reported that the anti-inflammatory effect of HDIVC might reduce inflammation in the body of COVID-19 patients and might prevent the transition to a severe state or death.6, 7, 8 Despite some positive therapeutic findings in COVID-19 patients, the main conclusion of our review including that of our meta-analysis is that the use of HDIVC is not helpful for treating patients with COVID-19. Conversely, combinatorial use of steroids or tocilizumab with HDIVC can help to modulate inflammation in patients with COVID-19.41
5. Conclusions
Currently, there is no evidence to support the role of high-dose intravenous vitamin C in the management of patients with COVID-19 due to infection with SARS-CoV-2. There have been few controlled studies on this topic. With the recent developments in vaccines to prevent COVID-19 and the ongoing clinical trials to investigate specific antiviral therapies, it is unclear whether there will be future controlled clinical studies on the therapeutic role of high-dose intravenous vitamin C in COVID-19. To reach an accurate conclusion on this issue, further clinical trials with large sample sizes should be performed. In addition, our review of the literature indicates that heterogeneous doses of vitamin C can affect the results after the treatment. Thus, studies to evaluate the most appropriate dose for patients with COVID-19 should be conducted. Furthermore, the term “severe COVID-19″ was not clearly defined in the; therefore, the severity of COVID-19 cases included in our analysis is heterogeneous. Severity of COVID-19 cases may change the effectiveness of HDIVC; hence, this variable should also be evaluated in the future.
Sources of funding
This study wassupported by the National Research Foundationof Korean Grant funded by the Korean govern-ment (NRF-2021R1A2C1013073).
CRediT authorship contribution statement
Sang Gyu Kwak: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing, Yoo Jin Choo: Project administration, Visualization, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing, Min Cheol Chang: Supervision, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing.
Acknowledgments
None.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Chang M.C., Baek J.H., Park D. Lessons from South Korea regarding the early stage of the COVID-19 outbreak. Healthcare. 2020;8:229. doi: 10.3390/healthcare8030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baj J., Karakuła-Juchnowicz H., Teresiński G., et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9:1753. doi: 10.3390/jcm9061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J., Kim S.Y., Sung H., Choe Y.J., Hong K.H. Letter to the editor: the interpretation of COVID-19 seroprevalence study should be cautious. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang M.C., Park Y.K., Kim B.O., Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis. 2020;20:445. doi: 10.1186/s12879-020-05144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariyanto T.I., Japar K.V., Kwenandar F., et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am J Emerg Med. 2021;41:110–119. doi: 10.1016/j.ajem.2020.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Luo G., Yuan J., et al. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat Inflamm. 2014;2014 doi: 10.1155/2014/426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Härtel C., Strunk T., Bucsky P., Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27:101–106. doi: 10.1016/j.cyto.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Cerullo G., Negro M., Parimbelli M., et al. The long history of vitamin C: from prevention of the common cold to potential aid in the treatment of COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.574029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vissers M.C.M., Wilkie R.P. Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1α. J Leukoc Biol. 2007;81:1236–1244. doi: 10.1189/jlb.0806541. [DOI] [PubMed] [Google Scholar]
- 11.Chernyak B.V., Popova E.N., Prikhodko A.S., Grebenchikov O.A., Zinovkina L.A., Zinovkin R.A. COVID-19 and oxidative stress. Biochemistry. 2020;85:1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forcados G.E., Muhammad A., Oladipo O.O., Makama S., Meseko C.A. Metabolic implications of oxidative stress and inflammatory process in SARS-CoV-2 pathogenesis: therapeutic potential of natural antioxidants. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.654813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakaev V.V., Duntau A.P. Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. Int J Tube Lung Dis. 2004;8:263–266. [PubMed] [Google Scholar]
- 14.Carr A.C., Rosengrave P.C., Bayer S., Chambers S., Mehrtens J., Shaw G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21:300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorah C.J., Downing C., Piripitsi A., et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr. 1996;63:760–765. doi: 10.1093/ajcn/63.5.760. [DOI] [PubMed] [Google Scholar]
- 16.Dykes M.H., Meier P. Ascorbic acid and the common cold. Evaluation of its efficacy and toxicity. J Am Med Assoc. 1975;231:1073–1079. [PubMed] [Google Scholar]
- 17.Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci USA. 1971;68:2678–2681. [DOI] [PMC free article] [PubMed]
- 18.Hemilä H., Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD005532.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Holford P., Carr A.C., Jovic T.H., et al. Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients. 2020;12 doi: 10.3390/nu12123760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler Iii A.A., Kim C., Lepler L., et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Crit Care Med. 2017;6:85–90. doi: 10.5492/wjccm.v6.i1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler A.A., 3rd, Syed A.A., Knowlson S., et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowler A.A., 3rd, Truwit J.D., Hite R.D., et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. J Am Med Assoc. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii T., Luethi N., Young P.J., et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. J Am Med Assoc. 2020;323:423–431. doi: 10.1001/jama.2019.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lykkesfeldt J. On the effect of vitamin C intake on human health: how to (mis)interprete the clinical evidence. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakanishi K., Hiramoto K., Ooi K. High-dose vitamin C exerts its anti-cancer effects in a xenograft model of colon cancer by suppressing angiogenesis. Biol Pharm Bull. 2021;44:884–887. doi: 10.1248/bpb.b21-00089. [DOI] [PubMed] [Google Scholar]
- 26.Ohno S., Ohno Y., Suzuki N., Soma G., Inoue M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009;29:809–815. [PubMed] [Google Scholar]
- 27.LeBlanc J.G. Vitamin C. An Update on Current Uses and Functions: BoD–Books on Demand. Vol. 16. 2019. Implementation of nurse-driven HIV screening targeting key populations in emergency departments: a multilevel analysis from the DICI-VIH trial; pp. 444–453. [DOI] [PubMed] [Google Scholar]
- 28.Gao D., Xu M., Wang G., et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study. Aging. 2021;13:7020–7034. doi: 10.18632/aging.202557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.JamaliMoghadamSiahkali S., Zarezade B., Koolaji S., al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021;26:20. [DOI] [PMC free article] [PubMed]
- 30.Kumari P., Dembra S., Dembra P., et al. The role of vitamin C as adjuvant therapy in COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M., Ching T.H., Hipple C., Lopez R., Sahibzada A., Rahman H. Use of intravenous vitamin C in critically Ill patients with COVID-19 infection. J Pharm Pract. 2021 doi: 10.1177/08971900211015052. [DOI] [PubMed] [Google Scholar]
- 32.Suna K., Melahat U.Ş., Murat Y., Figen Ö.E., Ayperi Ö. Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia. Med Clin. 2021 doi: 10.1016/j.medcli.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas S., Patel D., Bittel B., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., Rao X., Li Y., et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11:5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao B., Liu M., Liu P., et al. High dose intravenous vitamin C for preventing the disease aggravation of moderate COVID-19 pneumonia. A retrospective propensity matched before-after study. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.638556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L., Wang L., Tan J., Liu H., Ni Y. High-dose vitamin C intravenous infusion in the treatment of patients with COVID-19: a protocol for systematic review and meta-analysis. Medicine. 2021;100 doi: 10.1097/MD.0000000000025876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 39.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivan Hariyanto T., Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021;93:1832–1836. doi: 10.1002/jmv.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]