Abstract
Background and aims
Low HDL-cholesterol (HDLc) concentration is associated with a greater risk of infection-related mortality. We wanted to evaluate the relationship between pre-infection HDLc levels and mortality among older patients infected with SARS-Cov-2.
Methods
This is a population-based, cohort study, comprising all individuals residing in Madrid (Spain) born before 1 January 1945, and alive on 31 December 2019. Demographic, clinical, and analytical data were obtained from the primary care electronic clinical records. Confirmed SARS-CoV-2 infection was defined as a positive result in the RT-qPCR or in the antigen test. A death from COVID-19 was defined as that registered in the hospital chart, or as any death occurring in the 15 days following a confirmed SARS-CoV-2 infection. Data on infection, hospitalization, or death due to SAR-CoV-2 were collected from 1 March 2020 through 31 December 2020.
Results
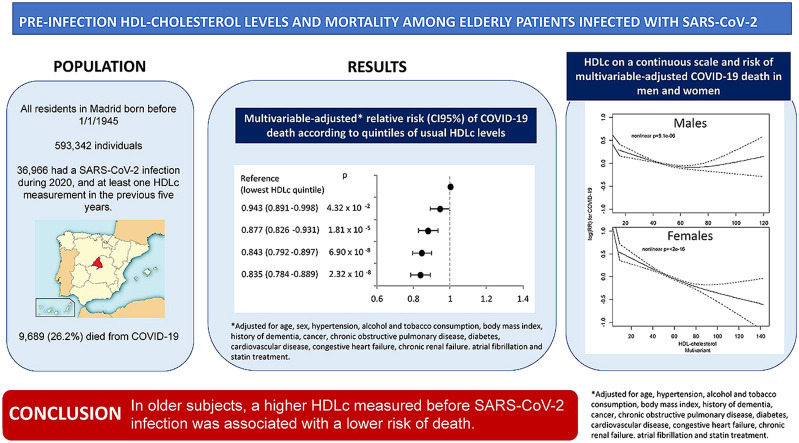
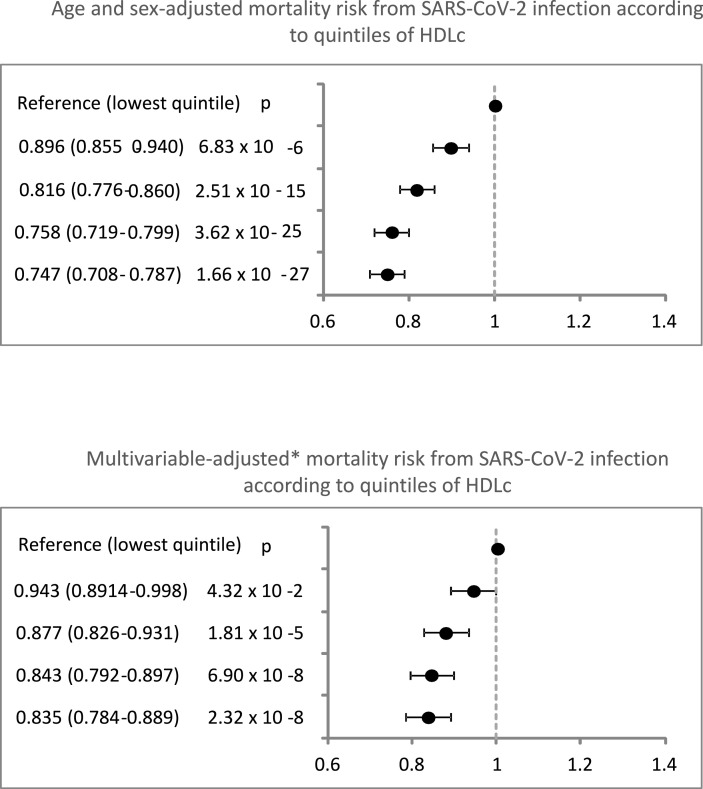
Of the 593,342 individuals comprising the cohort, 36,966 had a SARS-CoV-2 infection during 2020, and at least one HDLc measurement in the previous five years. Among them, 9689 (26.2%) died from COVID-19. After adjustment for age and sex, the relative risk (95% confidence interval) of COVID-19 death across increasing quintiles of HDLc was 1.000, 0.896 (0.855–0.940), 0.816 (0.776–0.860), 0.758 (0.719–0.799), and 0.747 (0.708–0.787). The association was maintained after further adjustment for comorbidities, statin treatment and markers of malnutrition. While in females this association was linear, in males it showed a U-shaped curve.
Conclusions
In older subjects, a higher HDLc measured before SARS-CoV-2 infection was associated with a lower risk of death.
Keywords: HDL-Cholesterol, SARS-CoV-2 infection, COVID-19, Lipoproteins, Infection
Graphical abstract
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection conveys a high risk of mortality. In Spanish subjects older than 80 years, the infection fatality risk based on confirmed Coronavirus Disease 2019 (COVID-19) deaths was 11.6% in males and 4.62% in females, increasing to 16.4% and 6.49%, based on excess all-cause deaths [1]. In hospitalized patients older than 75 years, the lethality rate approached 36% [2]. Mortality in SARS-CoV-2 infected patients is mainly driven by an exaggerated immune response that induces lung and vascular damage, conducting to acute respiratory insufficiency, and by an increased risk of associated bacterial infections [3,4].
Many studies have evaluated predictors of COVID-19 severity and death [5,6]. Among the most frequently reported are older age, male sex, obesity, diabetes, smoking, hypertension, and previous cardiovascular disease. All of them have in common their association with low levels of HDL-cholesterol (HDLc).
HDLs are a family of lipoproteins mainly involved in reverse cholesterol transport. In epidemiological studies, low HDLc concentration has been associated with higher total and cardiovascular mortality [7], greater risk of bacterial and viral infections [8], and increased risk of infectious disease-related death [9]. The potential causes linking HDLc concentration and risk of infection are not completely understood. HDLs may contribute to the protection against viral infections by inhibiting viral fusion and by decreasing viral uptake by the host cells through different receptors [9]. Also, a low HDLc level reduces the ability to neutralize and clear lipopolysaccharide (LPS), intensifying the inflammatory response to endotoxin [10], and increasing sepsis-related mortality [11,12].
Although HDLc concentration correlates with the risk of SARS-CoV-2 infection [13], and its severity [14,15], its association with COVID-19 mortality has not been investigated in large epidemiological studies. HDL could protect against COVID-19 mortality by interfering with viral infection, by reducing the rate of bacterial complications, or by neutralizing the exaggerated immune response characteristic of this disease.
Since 1 January 2015, we have followed a cohort of all residents in Madrid (Spain) born before 1 January 1945, to evaluate factors associated with cardiovascular morbidity and mortality. This allows us to assess the impact of COVID-19 mortality in the older subset of the population, which accounts for the larger number of deaths. The objective of the present study was to evaluate whether HDLc concentration, measured before SARS-CoV-2 infection, relates to COVID-19 mortality.
2. Patients and methods
2.1. Study design
On 1 January 2015, we set a population-based cohort study among all residents in the Community of Madrid (Spain), who were born before 1 January 1945. The Community of Madrid provides access-free healthcare coverage to 100% of the population. It provides primary care assistance through 3881 general practitioners working in 424 primary Health Centers. All residents have an electronic clinical record in primary care (AP-Madrid database). On 31 December 2019, there were data on 6,466,966 alive persons, of whom 593,342 were born before January 1945, and had at least one data entry during the preceding two years.
We collected data related to infection, hospitalization, or death due to SARS-CoV-2, from 1 March 2020, the beginning of the pandemic in Spain, until 31 December 2020.
The study protocol was approved by the Research Ethics Committee of the Ramon y Cajal University Hospital in Madrid.
2.2. Study variables
Data available in all participants included age, sex, cardiovascular risks factors, morbidities, and medication prescriptions until 31 December 2019, and were obtained from AP-Madrid Database. We recorded morbidities according to the International Classification of Primary Care (ICPC-2). We specifically registered the presence of any previous cardiovascular disease (either myocardial infarction, angina, stroke or peripheral artery disease), any cancer active during the previous five years (except non-melanoma skin cancer), and chronic renal failure, congestive heart failure, chronic obstructive pulmonary disease (COPD), atrial fibrillation, dementia, hypertension, and diabetes. We also gathered information about alcohol and tobacco consumption from AP-Madrid.
All blood analysis and anthropometric measurements performed from 1 January 2015 to 31 December 2019 were available for study. For each continuous variable, we used the average of all samples analyzed during that period. HDLc quintiles were calculated separately for men and women.
We considered a confirmed SARS-CoV-2 infection when a patient had a positive antigen or RT-qPCR test result. The results were obtained from AP-Madrid Database, provided by Madrid Public Health Office.
We considered a SARS-CoV-2 hospitalization, when recorded as such in the hospital discharge report, or any hospital admission occurring 15 days after a confirmed diagnosis of infection. In case of more than one admission with a SARS-CoV-2 infection diagnosis, we only considered the first one. Admissions were obtained from the Integrated Health Process Minimum Basic Dataset of hospitalized patients, provided by the Directorate General for integrated health process.
We considered a SARS-CoV-2 death when registered as such in the clinical chart of a hospitalized patient, or when the death recorded in the National Death Spanish Index (INDEF) occurred 15 days from a first confirmed diagnosis of SARS-CoV-2 infection. INDEF collects all deaths occurring in Spain but it does not record their cause.
Statin prescriptions were obtained from the Electronic Pharmacy Database (Módulo Unico de Prescripción) of Madrid, integrated in AP-Madrid.
We have validated the quality of the electronic clinical records in primary care for research use [16], and the database has been widely employed to study the epidemiology of cardiovascular risk factors in older patients [17].
2.3. Statistical analysis
The continuous variables are presented as mean ± standard deviation, and the categorical variables as percentages. Comparisons between continuous variables were performed by ANOVA, and between categorical variables by the χ2 test. To study factors associated with COVID-19 mortality, we used a Poisson log-linear model with robust ‘sandwich’ standard errors. Three models with sequential adjustments for covariates were fit: model 1, adjusted for age and sex; model 2, further adjusted for covariates associated with HDLc concentration in univariate analysis, and for covariates previously reported to be associated with COVID-19 mortality, and model 3, further adjusted for albumin, hemoglobin and lymphocyte count as markers of poor nutrition. These three latter variables were only available in 25,240 infected subjects. In addition, we performed exploratory adjustments for LDLc and triglycerides as continuous variables.
We tested for interactions by incorporating two-factor interaction terms into the multivariate model 2. p-values for interaction were obtained using the Wald test.
Non-linear associations between HDLc and COVID-19 mortality were tested using a likelihood ratio test, comparing a model with the exposure fitted on a spline against a model assuming a linear exposure-outcome relationship. We chose spline smoothness after proving two, three and four degrees of freedom. p-value for nonlinearity <0.05 suggested evidence against the linearity assumption. Two-tailed p-values <0.05 were considered statistically significant.
We conducted the analyses with the package ‘mgcv’ (mgcv_1.8–33), in R Statistical Software V4.0.4 (2021-02-15).
3. Results
Of the 593,342 participants in the cohort, 41,643 (7.02%) had a confirmed SARS-CoV-2 infection during 2020, and 36,996 had at least one HDLc measurement in the previous 5 years. The number of HDLc determinations during the preceding 5 years was one in 4780 (12.9%) subjects, two in 5341 (14.4%), three in 5770 (15.6%), four in 5689 (15.4%), five in 5054 (13.7%), and six or more in 10,362 (28.4%) individuals. Mean HDLc across increasing quintiles was, 0.95 ± 0.12, 1.17 ± 0.10, 1.33 ± 0.11, 1.52 ± 0.13 and 1.89 ± 0.27 mmol/L.
Table 1 shows the overall population characteristics and according to HDLc quintiles on 31 December 2019. The mean age was 84.6 ± 5.9 years (75–108 years), and 58.2% were females. The characteristics of the population divided by sex and according to sex specific HDLc quintiles can be seen in Supplementary Tables 1 and 2 As expected, HDLc was associated with age, and with different cardiovascular risks factors and morbidities.
Table 1.
Clinical characteristics of 36,996 participants in the cohort before infection by SARS-CoV-2, according to sex-specific quintiles of HDL-cholesterol.
| Total | Q1 lowest |
Q2 | Q3 | Q4 | Q5 highest |
p | |
|---|---|---|---|---|---|---|---|
| HDL-cholesterol (mmol/L) | 0.95 ± 0.12 | 1.17 ± 0.10 | 1.33 ± 0.11 | 1.52 ± 0.13 | 1.89 ± 0.27 | <0.001 | |
| Age (years) | 84.6 ± 5.9 | 85.6 ± 6.2 | 85.0 ± 6.0 | 84.5 ± 6.0 | 84.2 ± 5.7 | 83.9 ± 5.6 | <0.001 |
| Any cancer during the preceding 5 years (%) | 5.7 | 5.6 | 5.7 | 5.9 | 5.5 | 5.7 | 0.865 |
| Hypertension (%) | 65.3 | 64.9 | 67.1 | 66.7 | 65.9 | 61.8 | <0.001 |
| Diabetes (%) | 29.2 | 42.2 | 34.2 | 28.5 | 23.5 | 17.6 | <0.001 |
| Atrial fibrillation (%) | 18.9 | 23.2 | 20.0 | 18.6 | 17.1 | 15.5 | <0.001 |
| Chronic renal failure (%) | 7.9 | 10.3 | 9.0 | 7.7 | 6.4 | 5.9 | <0.001 |
| Congestive heart failure (%) | 9.3 | 13.5 | 10.2 | 8.9 | 7.1 | 6.7 | <0.001 |
| Chronic obstructive pulmonary disease (%) | 13.5 | 12.6 | 13.9 | 13.5 | 13.3 | 14.3 | 0.031 |
| Alzheimer's disease (%) | 13.8 | 18.1 | 14.8 | 13.5 | 11.4 | 11.2 | <0.001 |
| Cardiovascular disease (%): | 21.9 | 30.7 | 23.9 | 20.9 | 17.9 | 16.2 | <0.001 |
| Acute coronary syndrome (%) | 5.7 | 9.0 | 6.6 | 5.4 | 4.5 | 3.2 | <0.001 |
| Angina (%) | 5.6 | 8.1 | 6.3 | 4.9 | 4.9 | 3.9 | <0.001 |
| Stroke (%) | 8.1 | 12.1 | 8.7 | 7.9 | 5.9 | 5.7 | <0.001 |
| Peripheral artery disease (%) | 6.0 | 7.4 | 6.3 | 5.8 | 5.0 | 5.4 | <0.001 |
| Alcohol consumption (%) | 1.4 | 1.2 | 1.4 | 1.2 | 1.2 | 1.9 | <0.001 |
| Tobacco consumption (%) | 4.9 | 5.2 | 4.9 | 4.8 | 5.0 | 4.7 | 0.598 |
| Body Mass Index (kg/m2) | 28.6 ± 4.7 | 29.5 ± 4.8 | 29.1 ± 4.7 | 28.9 ± 4.5 | 28.2 ± 4.6 | 27.3 ± 4.5 | <0.001 |
| LDL-cholesterol (mmol/L) | 2.65 ± 0.7 | 2.36 ± 0.7 | 2.63 ± 0.7 | 2.71 ± 0.7 | 2.79 ± 0.7 | 2.78 ± 0.7 | <0.001 |
| Triglycerides (mmol/L) | 1.34 ± 0.6 | 1.68 ± 0.7 | 1.45 ± 0.6 | 1.32 ± 0.5 | 1.20 ± 0.4 | 1.03 ± 0.3 | <0.001 |
| Albumin (mmol/L) | 0.604 ± 0.07 | 0.579 ± 0.07 | 0.598 ± 0.07 | 0.609 ± 0.06 | 0.617 ± 0.06 | 0.627 ± 0.06 | <0.001 |
| Haemoglobin (g/dL) | 13.6 ± 1.5 | 13.1 ± 1.6 | 13.5 ± 1.5 | 13.7 ± 1.5 | 13.8 ± 1.4 | 13.8 ± 1.4 | <0.001 |
| Lymphocytes (x103/mm3) | 2.50 ± 3.1 | 2.58 ± 4.1 | 2.46 ± 2.5 | 2.48 ± 2.5 | 2.49 ± 2.6 | 2.47 ± 3.3 | 0.132 |
| Statin treatment (%) | 50.2 | 49.8 | 51.0 | 51.0 | 51.0 | 48.4 | 0.003 |
Comparison between continuous variables were performed with ANOVA, and between categorical variables with the χ2 test.
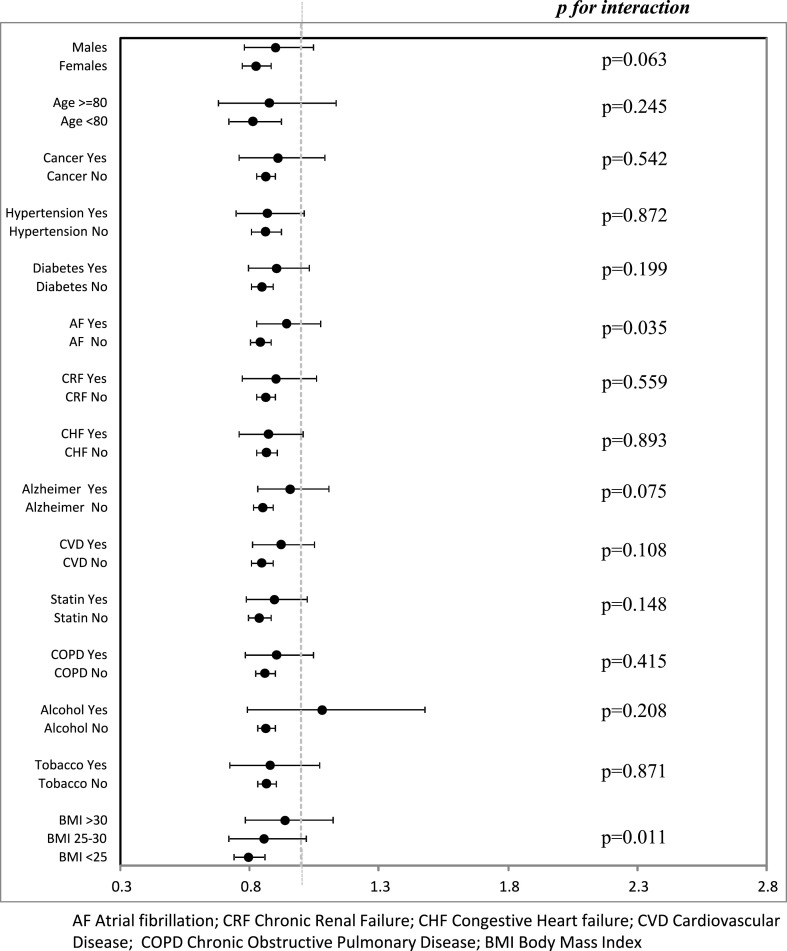
A total of 20,152 subjects (55.5% of the infected population) were hospitalized, and 9689 (26.2%) died. COVID-19 mortality decreased with increasing HDLc quintiles. There were 32.4%, 28.3%, 25.1%, 22.9% and 22.3% deaths, across quintiles 1 to 5. Higher HDLc was associated with a lower risk of death after adjustment for sex and age, and this association held after additional adjustment for comorbidities, and statin treatment (Fig. 1 ). The multivariable-adjusted association between an HDLc below or above the median and mortality did not differ after stratification for age, sex, cardiovascular risk factors and morbidities (Fig. 2 ), except for a weaker association in subjects with obesity or atrial fibrillation. When the analysis were performed separately for men and women (Supplementary Figs. 1 and 2), the results were similar to the ones obtained for the overall population.
Fig. 1.
Quintiles of HDLc and relative risk (CI 95%) of COVID-19 death, among older patients infected with SARS-CoV-2.
Fig. 2.
Multivariable-adjusted relative risk of COVID-19 death according to HDLc above vs below the median among older patients infected by SARS-CoV-2 across strata defined by age, sex, BMI, morbidities, and statin treatment.
We additionally adjusted the analyses for markers of malnutrition, including hemoglobin, albumin, and lymphocyte count; the association attenuated but persisted so the RR (95%CI) of death across increasing quintiles of HDLc were 1, 0.954 (0.890–1.021), 0.906 (0.842–0.975), 0.867 (0.802–0.936), and 0.881 (0.815–0.951). We also introduced in the multivariable-adjusted model, the concentration of LDLc and triglycerides. While the association of HDLc and SARS-CoV-2 mortality remained unchanged, we found no association between SARS-CoV-2 mortality and triglyceride levels (RR 1.00; 0.999–1.001), or low-density lipoprotein-cholesterol (LDLc) concentration (RR 0.999; 0.998–0.999).
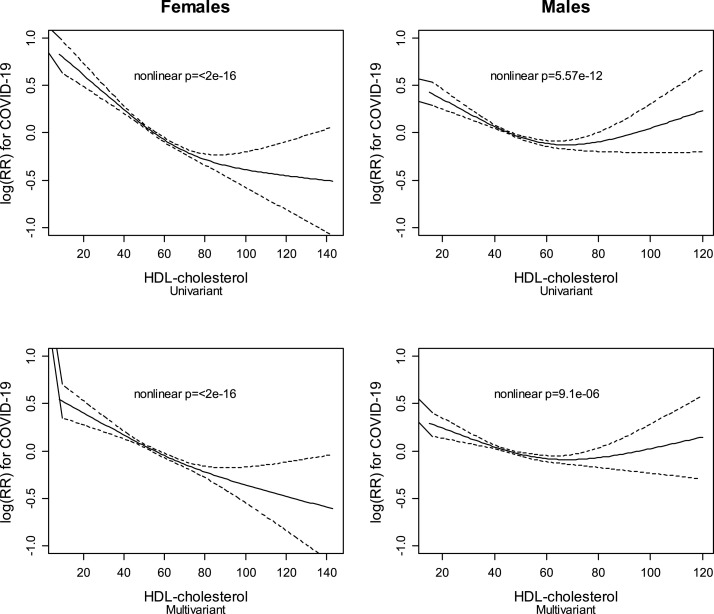
The association between HDLc levels and COVID-19 mortality was inversely linear in women but showed a U-shaped relationship in men (Fig. 3 ).
Fig. 3.
HDLc on a continuous scale and risk of COVID-19 death, in men and women, age and multivariable-adjusted.
4. Discussion
Our results demonstrate that HDLc concentration measured before SARS-CoV-2 infection is associated with COVID-19 mortality, independently of age, sex, comorbidities, or statin treatment. Previous studies have demonstrated that low HDLc levels were related to a higher risk of SARS-CoV-2 infection and a more severe disease [14,15] but, to our knowledge, this is the first study linking HDLc to COVID-19 mortality. After further adjustment for albumin, total lymphocyte count, and hemoglobin as markers of poor nutrition, the association of HDLc with COVID-19 mortality remained significant, ruling out malnutrition as the factor influencing the association.
The HDLc-related protection was observed in different groups of patients, but it was somewhat weaker in patients with obesity or atrial fibrillation.
The results are in agreement with studies evaluating other types of infections, where HDLc levels also showed an inverse association with the risk of infections, and infectious-related mortality. Although some studies have focused on the correlation between HDLc level during the acute phase of the infection and its prognosis [14], our study evaluated the relation between HDLc concentration before SARS-CoV-2 infection, and COVID-19 mortality. This aspect is important as would denote that HDLc is a marker of a more severe acute infection, and a risk factor for the development and aggravation of an infection.
A low HDLc concentration may imply a reduced capacity of protection against infectious diseases, and/or may be a marker of a disproportionate inflammatory response, being this a feature of COVID-19 infection. HDLs inhibit viral fusion and compete with viral uptake [9], protecting against infection [8]. It has been proposed that HDLs modulate the membrane properties of cells implicated in the immune response and the innate humoral response, influencing complement activation [9] that could increase resistance to viral infections. Some proteins carried by HDL particles may have a critical role in the protection against infectious diseases [9]. As many patients with COVID-19 die because of superimposed bacterial infections, a high HDLc may protect from these secondary infections that obscure prognosis. HDLs neutralize and clear LPS and lipoteicoic acid, reducing the inflammatory response induced by LPS [18]. Apo A1 knock out mice have a reduced capacity to neutralize LPS, while mice overexpressing Apo A1 have lower mortality after infection [19]. In fact, infusion of reconstituted HDLs inhibits LPS induced cytokine release in vitro [20] and increases the LPS neutralizing capacity reducing the inflammatory response [21]. Injection of 4F, an ApoA1 mimetic peptide, improves HDL quality and function, inhibits the expression of inflammatory mediators induced by the injection of LPS in mice, reduces lung and vascular damage, and increases survival [[22], [23], [24]]. These actions are mediated by the LPS neutralizing effect, and by down-regulation of the nuclear factor-[kappa]B pathway [22], a pathway directly activated by SARS-Cov-2 [25] that could be partially responsible for the cytokine storm induction.
We have observed an inverse continuous linear relationship between HDLc and mortality in females. However, males sustained a curvilinear relationship, with a higher risk of SARS-CoV-2 mortality with extremely high or low HDLc concentrations (Fig. 3). The association between extremely high HDLc levels and risk of infection has been controversial. In the Copenhagen General Population Study, subjects with a very high HDLc had an increased risk of infections, a finding that could not be replicated in the analysis of the population of the Copenhagen City Heart Study [8]. Also, in the UK database, a genetically higher HDLc concentration did not associate with a higher risk of SARS-CoV-2 infection [15]. Although an extremely high HDLc has been associated with increased all-cause mortality in both sexes, the effect was more prominent in males [26]. Moreover, in females the increase in mortality was restricted to cardiovascular causes. Women with very high HDLc concentration, as opposing to men, did not have increased cancer mortality or mortality for other causes [26]. The differential sex-related association between very high HDLc levels and mortality observed in our study requires further investigation. Women have a lower susceptibility to and mortality from infection than men [27] and are also less likely to die from COVID-19 [1], which has been explained by a differential immune response between both sexes [27]. Whether this sex-difference in the immune response, the advanced age of our population, or any other circumstance may alter the U-shaped association of HDLc and COVID-19 mortality needs additional studies.
Extreme high levels of HDL are associated with dysfunctional HDLs that may have lost some properties that protect against severe infections. In the ILLUMINATE study [28], treatment with torcetrapib, a cholesterol ester transfer protein (CETP) inhibitor, dramatically increased the concentration of HDLc, changed particle composition, and was associated with increased infection-related mortality.
Although we have found a strong relation between HDLc and COVID-19 mortality, genetic scores associated with a higher HDLc concentration do not correlate with a higher risk of SARS-CoV-2 infection [15], but they may have a modest association with the risk of hospitalization [29]. It is not known, however, whether this relationship also exists for COVID-19-related mortality.
These results suggest that HDLc prior to SARS-CoV-2 infection could be causally related to a poorer prognosis and open a research field to evaluate which aspects of HDL metabolism are responsible for their protection against infection.
4.1. Limitations of the study
In the first pandemic wave, an unidentified number of patients died from respiratory causes that could not be attributed to SARS-CoV-2 infection as PCR tests were not widely available. So, mortality by COVID-19 was probably underestimated. Moreover, in that pandemic moment, many subjects with non-severe clinical symptoms were not evaluated with a PCR test for potential SARS-CoV-2 infection, so the total number of infected subjects could also be underestimated.
For each patient, HDLc levels were measured at different times during the preceding 5 years. Furthermore, the number of HDLc measurements was also different for each subject.
Although we cannot conclude that HDL is a causal risk factor for COVID-19 mortality, the large size of the cohort, the inclusion of all the residents in Madrid, the multiple pre-infection HDLc measurements, the consistency of the data, and the strong association even after multiple adjustments suggest that HDLc or any particle present at their surface may protect against mortality.
In conclusion, HDLc levels measured before SARS-CoV-2 infection are independently associated with lower COVID-19 mortality. Thus, our data suggest that HDL may play a role in the protection against mortality risk of this infection.
Financial support
Sociedad Española de Arteriosclerosis and Regional Ministry of Health of the Community of Madrid through non-refundable grants from the credits awarded to the Community of Madrid by the Spanish Government Fund COVID-19, included in Order HAC/667/2020, dated 17 July, which provides extraordinary credit in budget application 32.01.941O.459.00 “Fondo COVID-19".
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Jose M. Mostaza: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, Supervision, Project administration. Miguel A. Salinero-Fort: Conceptualization, Methodology, Investigation, Writing – review & editing, Funding acquisition. Juan Cardenas-Valladolid: Investigation, Resources, Writing – review & editing. Fernando Rodriguez-Artalejo: Conceptualization, Methodology, Writing – review & editing. Mariana Díaz-Almiron: Formal analysis, Writing – review & editing. Pilar Vich-Pérez: Investigation, Data curation, Writing – review & editing. F.Javier San Andres-Rebollo: Investigation, Data curation, Writing – review & editing. Ignacio Vicente: Investigation, Data curation, Writing – review & editing. Carlos Lahoz: Conceptualization, Methodology, Investigation, Writing – review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2021.12.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pastor-Barriuso R., Pérez-Gómez B., Hernán M.A., Pérez-Olmeda M., Yotti R., Oteo-Iglesias J., et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: Nationwide seroepidemiological study. BMJ. 2020;3710:m4509. doi: 10.1136/bmj.m4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mostaza J.M., García-Iglesias F., González-Alegre T., Blanco F., Varas M., Hernández-Blanco C., et al. Clinical course and prognostic factors of COVID-19 infection in an elderly hospitalized population. Arch. Gerontol. Geriatr. 2020;91:104204. doi: 10.1016/j.archger.2020.104204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bengoechea J.A., Bamford C.G. SARS ‐CoV‐2, bacterial co‐infections, and AMR : the deadly trio in COVID ‐19? EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge E., Li Y., Wu S., Candido E., Wei X. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: a population-based cohort study. PLoS One. 2021 Oct 5;16 doi: 10.1371/journal.pone.0258154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Almeida-Pititto B., Dualib P.M., Zajdenverg L., Dantas J.R., de Souza F.D., Rodacki M., Bertoluci M.C., Brazilian Diabetes Society Study Group (SBD) Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol. Metab. Syndrome. 2020 Aug 31;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson P.W.F., Abbott R.D., Castelli W.P. High density lipoprotein cholesterol and mortality. The Framingham heart study. Arteriosclerosis. 1988;8:737–741. doi: 10.1161/01.atv.8.6.737. [DOI] [PubMed] [Google Scholar]
- 8.Madsen C.M., Varbo A., Tybjærg-Hansen A., Frikke-Schmidt R., Nordestgaard B.G. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur. Heart J. 2018;39:1181–1190. doi: 10.1093/eurheartj/ehx665. [DOI] [PubMed] [Google Scholar]
- 9.Pirillo A., Catapano A.L., Norata G.D. Handbook of Experimental Pharmacology. Springer New York LLC; 2015. HDL in infectious diseases and sepsis; pp. 483–508. [DOI] [PubMed] [Google Scholar]
- 10.Birjmohun R.S., van Leuven S.I., Levels J.H.M., van ’T Veer C., Kuivenhoven J.A., Meijers J.C.M., et al. High-density lipoprotein attenuates inflammation and coagulation response on endotoxin challenge in humans. Arterioscler. Thromb. Vasc. Biol. 2007;27:1153–1158. doi: 10.1161/ATVBAHA.106.136325. [DOI] [PubMed] [Google Scholar]
- 11.Barlage S., Gnewuch C., Liebisch G., Wolf Z., Audebert F.X., Glück T., et al. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009;35:1877–1885. doi: 10.1007/s00134-009-1609-y. [DOI] [PubMed] [Google Scholar]
- 12.Chien J.Y., Jerng J.S., Yu C.J., Yang P.C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit. Care Med. 2005;33:1688–1693. doi: 10.1097/01.ccm.0000171183.79525.6b. [DOI] [PubMed] [Google Scholar]
- 13.Ho F.K., Celis-Morales C.A., Gray S.R., Katikireddi S.V., Niedzwiedz C.L., Hastie C., et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masana L., Correig E., Ibarretxe D., Anoro E., Arroyo J.A., Jericó C., et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021 Mar 30;11:7217. doi: 10.1038/s41598-021-86747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilser J.R., Han Y., Biswas S., Gukasyan J., Cai Z., Zhu R., et al. Association of serum HDL-cholesterol and apolipoprotein A1 levels with risk of severe SARS-CoV-2 infection. J. Lipid Res. 2021;62:100061. doi: 10.1016/j.jlr.2021.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Burgos-Lunar C., Salinero-Fort M.A., Cárdenas-Valladolid J., Soto-Díaz S., Fuentes-Rodríguez C.Y., Abánades-Herranz J.C., et al. Validation of diabetes mellitus and hypertension diagnosis in computerized medical records in primary health care. BMC Med. Res. Methodol. 2011;11:146. doi: 10.1186/1471-2288-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostaza J.M., Lahoz C., Salinero-Fort M.A., Cardenas J. Cardiovascular disease in nonagenarians: prevalence and utilization of preventive therapies. Eur J Prev Cardiol. 2019;26:356–364. doi: 10.1177/2047487318813723. [DOI] [PubMed] [Google Scholar]
- 18.Levine D.M., Parker T.S., Donnelly T.M., Walsh A., Rubin A.L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Nat Acad Sci USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo L., Ai J., Zheng Z., Howatt D.A., Daugherty A., Huang B., et al. High density lipoprotein protects against polymicrobe-induced sepsis in mice. J. Biol. Chem. 2013;288:17947–17953. doi: 10.1074/jbc.M112.442699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker T.S., Levine D.M., Chang J.C.C., Laxer J., Coffin C.C., Rubin A.L. Reconstituted high-density lipoprotein neutralizes gram-negative bacterial lipopolysaccharides in human whole blood. Infect. Immun. 1995;63:253–258. doi: 10.1128/iai.63.1.253-258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pajkrt D., Doran J.E., Koster F., Lerch P.G., Arnet B., van der Poll T., et al. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J. Exp. Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta H., Dai L., Datta G., Garber D.W., Grenett H., Li Y., et al. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ. Res. 2005;97:236–243. doi: 10.1161/01.RES.0000176530.66400.48. [DOI] [PubMed] [Google Scholar]
- 23.Kwon W.Y., Suh G.J., Kim K.S., Kwak Y.H., Kim K. 4F, apolipoprotein AI mimetic peptide, attenuates acute lung injury and improves survival in endotoxemic rats. J Trauma Acute Care Surg. 2012;72:1576–1583. doi: 10.1097/TA.0b013e3182493ab4. [DOI] [PubMed] [Google Scholar]
- 24.White C.R., Datta G., Mochon P., Zhang Z., Kelly O., Curcio C., et al. Vasculoprotective effects of apolipoprotein mimetic peptides: an evolving paradigm in HDL therapy. Vasc. Dis. Prev. 2009;6:122–130. doi: 10.2174/1567270000906010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su C.M., Wang L., Yoo D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 2021;11:13464. doi: 10.1038/s41598-021-92941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madsen C.M., Varbo A., Nordestgaard B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur. Heart J. 2017;38:2478–2486. doi: 10.1093/eurheartj/ehx163. [DOI] [PubMed] [Google Scholar]
- 27.Fischer J., Jung N., Robinson N., Lehmann C. Sex differences in immune responses to infectious diseases. Infection. 2015 Aug;43(4):399–403. doi: 10.1007/s15010-015-0791-9. Epub 2015 May 9. PMID: 25956991. [DOI] [PubMed] [Google Scholar]
- 28.Barter P.J., Caulfield M., Eriksson M., Grundy S.M., Kastelein J.J.P., Komajda M., et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 29.Trinder M., Walley K.R., Boyd J.H., Brunham L.R. Causal inference for genetically determined levels of high-density lipoprotein cholesterol and risk of infectious disease. Arterioscler. Thromb. Vasc. Biol. 2020;40:267–278. doi: 10.1161/ATVBAHA.119.313381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.