Abstract
Lymphomas and plasma cell neoplasms are a heterogenous group of malignancies derived from lymphocytes. They are a significant cause of patient morbidity and mortality. Advances in morphologic, immunophenotypic and molecular techniques have led to better understanding of the pathogenesis and diagnosis of these neoplasms. Advances in treatment, particularly immune-based therapies, increasingly allow for targeted therapies of these diseases. Mechanistic studies using animal models and clinical trials have revealed the importance of the tumor microenvironment on disease pathogenesis, progression, and response to therapy in these malignancies. Simultaneous progress in diagnostic techniques has made it feasible to generate high-resolution, high-throughput data from the tumor microenvironment with spatial context. As the armamentarium of targeted therapies and diagnostic techniques grows, there is potential to harness these advances to better stratify patients for targeted therapies, including immune-based therapies, in hematologic malignancies.
Keywords: tumor microenvironment, immune based therapies, lymphomas, plasma cell neoplasms, biomarkers
Introduction
Lymphomas and plasma cell neoplasms are a heterogenous group of malignancies arising from lymphocytes at various stages of development. Depending on the cell of origin, morphology and immunophenotype, they are broadly categorized into non-Hodgkin lymphomas, Hodgkin lymphomas and plasma cell neoplasms (1). Non-Hodgkin lymphomas include the sub-categories of non-Hodgkin B-cell lymphomas (B-NHL) that comprise majority of cases in this group and T-cell/Natural-killer (T/NK) lymphomas. As a group, non-Hodgkin lymphomas are the seventh most common type of cancer in the United States and are expected to account for 81,560 new cases and 20,720 deaths in 2021 (2). Hodgkin lymphomas are rarer and are expected to account for 8,830 new cases and 960 deaths. For plasma cell neoplasms the corresponding numbers are 34,920 new cases and 12,410 deaths, respectively in 2021 (3, 4). The clinical course and prognosis for these heterogenous group of neoplasms is highly dependent on factors such as the specific type of disease, age, ethnicity and geographic location (1). Advances in our understanding of the molecular pathology of these diseases has resulted in considerable progress in the treatment of these diseases. Immune-based therapies using monoclonal antibodies have become part of treatment regimens for these diseases (discussed in greater detail below). More recently, greater understanding of the tumor microenvironment in these diseases has led to development of immune system modulatory agents with therapeutic potential (3, 4). Future treatment regimens are likely to rely on combinatorial strategies using these agents. These developments are going to necessitate the development of novel diagnostic and prognostic tools to facilitate optimal treatment.
Tumor Microenvironment in Lymphomas and Plasma Cell Neoplasms
Scientific research on molecular mechanisms has expanded our understanding of cancer pathogenesis leading to advances in diagnostics and therapy. More recently, there has been increasing focus in delineating the components of the tumor microenvironment in enabling tumorigenesis and progression (5). These have led to development of several immune based therapies that have shown encouraging results both in epithelial and hematologic malignancies (6).
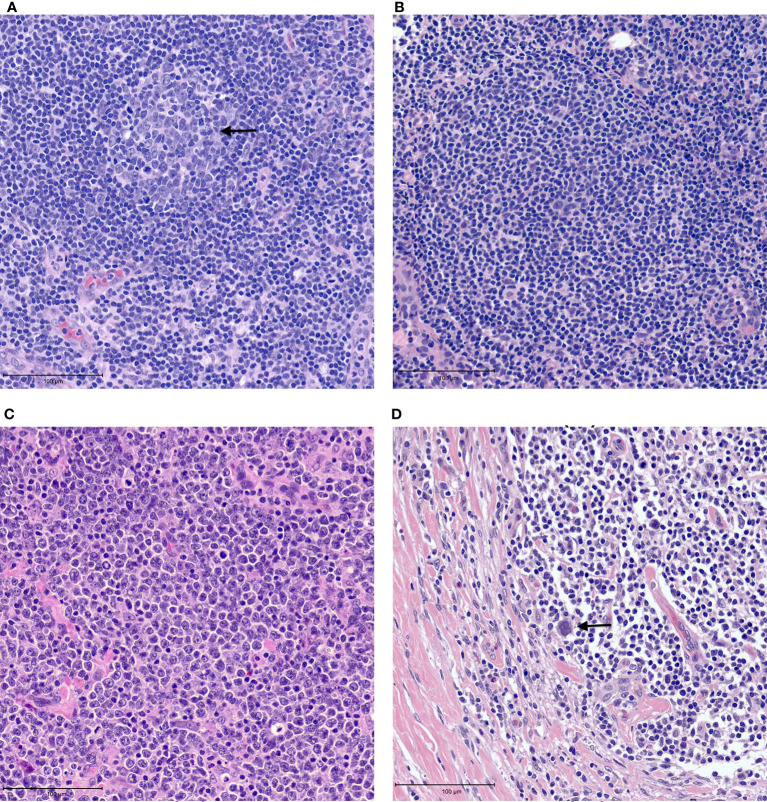
Tumor Microenvironment in Lymphomas
The tumor heterogeneity of non-Hodgkin lymphomas is reflected in the tumor microenvironment. The histologic architecture of lymph node and extra-nodal lymphoid organs is generally comprised of B-cell predominant follicles and interfollicular T-cells ( Figure 1A ). Naive B cells exposed to antigen home to follicles where they interact with follicular dendritic cells that are antigen presenting cells. Further maturation of B-cells occurs within germinal centers where they switch-off expression of the anti-apoptotic BCL2 protein rendering them vulnerable to apoptosis. Following the process of somatic mutation and affinity maturation, B-cells either undergo apoptosis or differentiate into memory/marginal zone B-cells found in the marginal zone around the germinal centers or into long-lived plasma cells (1). Macrophages present in the germinal centers remove the apoptotic cells giving rise to the characteristic tingible body macrophages seen in benign follicles ( Figure 1A ). Morphologically, lymphomas are characterized by architectural effacement of normal lymphoid tissue. The spatial distribution of tumor cells in lymphomas is variable. Their distribution may reflect the distribution of the cell of origin as in follicular lymphomas ( Figure 1B ) (7). Follicular lymphomas are derived from follicular germinal center B-cells. Typically, these tumors arise due to an IGH-BCL2 translocation leading to BCL2 overexpression in contrast to the BCL2 switch-off seen in normal follicles (8). Spatially the follicular lymphoma cells are seen within enlarged follicles. In contrast, marginal zone lymphomas that arise from post-germinal center B-cells show an expansion of the marginal zone around the follicles. The growth of neoplastic cells in marginal zone lymphomas is often due to continuous antigenic stimulation e.g., persistent Helicobacter pylori infection. While eradication of the underlying infection often leads to cure, over time marginal zone lymphomas can acquire translocations that lead to cell autonomous signaling independent of the microenvironment, leading to refractory disease. In contrast to follicular and marginal zone lymphomas, the tissue architecture is diffusely effaced in diffuse large B-cell lymphomas ( Figure 1C ). The cellular composition of lymphomas is also variable. In B-NHLs, the tumor is predominantly comprised of neoplastic B-cells with fewer admixed T-cells, macrophages and stroma that comprise the tumor microenvironment. In contrast, in Classic Hodgkin lymphoma (cHL) the neoplastic Hodgkin Reed-Sternberg (HRS) cells typically form only a minor subset of the neoplastic infiltrate. The substantial cellular component of the tumor is a characteristic polymorphous population of small lymphocytes, plasma cells, histiocytes and admixed granulocytes that constitutes a morphologically unique microenvironment ( Figure 1D ).
Figure 1.
Examples of tumor microenvironment in lymphomas. (A) Benign lymph node with a germinal center containing tangible body macrophages (arrow). (B) Follicular lymphoma with neoplastic follicle. (C) Diffuse large B cell lymphoma showing tissue effacement by large, neoplastic cells with very few admixed small lymphocytes. (D) Classic Hodgkin lymphoma showing rare Reed-Sternberg cells (arrow) in a background of small lymphocytes and histiocytes with fibrosis.
These morphologic pattens reflect different tumor microenvironments that result from genetic aberrations in these neoplasms and the external stimuli needed for survival, proliferation, and immune response evasion. Lymphoma cells have been shown to influence their microenvironment by their ability to use homing and trafficking mechanisms to spatially colonize milieus characteristic of their non-malignant counterparts. These include expression of various adhesion molecules as well as cytokines and cytokine receptors that regulate lymphocyte trafficking between various tissue compartments (9–13). After colonization, tumor cells can shift the tissue milieu to one that promotes cell survival and growth, and immune response evasion (14–17). Cell growth results from signaling through cell surface receptors such as the B-cell receptor and Toll-like receptor as well as cytokines released from stromal cells (18–21). The evasion of immune response occurs by various mechanisms including down-regulation of major histocompatibility complex molecules, recruitment of T-regulatory cells (T-regs) at the expense of T-helper cells, and expression of programmed cell death ligands PD-L1 and PD-L2 that bind to programmed cell death protein 1 (PD-1) on CD4-positive T cells and cytotoxic T lymphocytes to induce a state of T-cell exhaustion (22–28).
Despite similar spatial localization of neoplastic cells in some lymphomas when compared to their non-malignant counterparts, there can be significant differences in their respective microenvironments. For example, the tumor microenvironment in the neoplastic follicles in follicular lymphoma has been shown to be significantly different than normal germinal centers with respect to T-cells and macrophages. Specifically, there appears to be an increase in T-regs and immune-suppressive monocytes at the expense of T-helper cells in neoplastic follicles (14, 15, 17). In cHL, the secretion of cytokines produced by and in response to HRS cells is thought to result in the characteristic polymorphous milieu that also promotes cell survival (13). Cytokines such as CCL5, CCL17 and CCL22 recruit CD4+ T-cells that constitute the major population of the tumor milieu. CSF1 and CX3CL1 recruit eosinophils and macrophages. Fibrosis, most prominently seen in the nodular sclerosis variant of cHL, is attributed to activation and proliferation of fibroblasts by IL-13, TNF-α and fibroblast growth factors by HRS cells (13). Interaction between membrane-bound or secreted ligands from the microenvironment and cell surface receptors on HRS cells results in activation of canonical signaling pathways such as the JAK-STAT, NF-kB and BCR pathways. These ligand-based mechanisms are complimented by gene mutations that cause constitutional activation of these signaling pathways (29). In highly aggressive tumors such as Burkitt lymphoma, the tumor cells often acquire mutations that result in cell autonomous growth signals (30). Most cases of Burkitt lymphoma harbor translocations of the MYC gene, typically juxtaposing it with enhancer genes of the IGH locus, and less frequently the IGK and IGL loci resulting in upregulation of c-myc, its target genes and target microRNAs. These and other mutations result in cell-autonomous growth signaling. These tumors are, therefore, less likely to need stromal signals for survival resulting in a characteristically sparse tumor microenvironment (31–34).
A better understating of expression, distribution, and interaction of PD-1 and PD-L1 in the tumor microenvironment has been critical in the development of immune checkpoint blockade therapy (CBT) in cancer therapy. CBT involves blockade of the PD-1/PD-L1 axis to enhance the therapeutic effects of anti-tumor immune response (35). PD-1 is expressed predominantly on activated T cells, natural killer cells, dendritic cells, macrophages, and B-cell subsets. PD-L1 is expressed on a wide variety of hematopoietic and non-hematopoietic cells including stromal cells and can also be expressed by tumor cells. In the context of lymphomas, PD-1 expression is seen in tumor infiltrating lymphocytes, as well as the neoplastic cells in some types of B- and T-cell lymphomas (36). PD-L1 expression is seen in a wide variety of neoplastic cells in various lymphomas including cHL, Primary mediastinal large B cell lymphoma, and extra-nodal NK-T cell lymphomas among others (37). PD-L1 expression is also seen in some immune cells within the tumor microenvironment including tumor infiltrating lymphocytes as well as tumor associated macrophages (TAMs).
Tumor Microenvironment in Plasma Cell Neoplasms
Unlike lymphomas that often present in nodal tissues, plasma cell neoplasms are primarily diseases of the bone marrow. The marrow stromal niche in plasma cell neoplasm is occupied by a variety of cells including T-regs, NK cells, macrophages, dendritic cells, bone marrow stromal cells, endothelial cells, osteoblasts, and osteoclasts embedded in an extracellular matrix (38–40). The stromal niche has been shown to influence homing and adhesion of plasma cells by expression of adhesion molecules and cytokines (41–43). It also appears to aid immune evasion by mechanisms similar to those seen in lymphomas. For example, increased T-regs have been reported in the peripheral blood of neoplasms patients with plasma cell neoplasms (44). Interaction between myeloma cells and T-regs results T-reg expansion by a type 1IFN-dependent positive feedback loop (45). In response to stromal signals, plasma cell neoplasms cells express PD-L1 that interacts with PD-1 protein in T-cells, leading to immune exhaustion (46, 47). A caveat to these observations is that they are based on qRT-PCR and flow cytometry studies on small sample sets that include both patient samples and cell lines; larger studies are needed to validate these observations.
Immune-Based Therapy in Lymphomas and Plasma Cell Neoplasms
Current therapy for non-Hodgkin lymphomas is dependent on various factors including the pathologic subtype of lymphoma, stage at presentation, and performance status of the patients. The treatment can range from watchful waiting to combination modality regimens followed by stem cell transplant. Salvage regimens are used to treat relapsed disease (48). Therapy for Hodgkin lymphoma is also influenced by stage and risk factors and can range from chemotherapy alone to combination modality treatment, and salvage therapy for progressive disease (49). Treatment for symptomatic myeloma typically involves immunomodulatory drugs and proteasome inhibitors (50, 51). For all three entities, immune based therapies are increasingly part of the therapeutic armamentarium in treatment regimens (3, 40).
Immune-Based Therapy in Lymphomas
Immune based therapies have been used for treatment of lymphomas for more than two decades (3). The earliest success was with rituximab, a chimeric monoclonal antibody targeting the B-cell marker CD20 (52–55). Since then, several other antibodies targeting B- and T-cell markers including CD30, CD19 and CCCR4 have been evaluated in lymphoma treatment with many such drugs receiving regulatory approval (56–59). Initial targeted monoclonal antibodies used complement dependent cytotoxicity or antibody-dependent cytotoxicity for anti-tumor effect. More recently, attempts have been made to increase their efficacy by conjugating them to cytotoxic drugs (60, 61). Bispecific T cell engager antibodies (BiTEs) that are designed to target both tumor antigens and T-cells to bring the tumor cells in close physical proximity to the T cells for enhanced anti-tumoral effects are also being evaluated (62, 63). There is mechanistic evidence that the tumor microenvironment can impact response to therapy. In cell-culture based studies CXCR-4 dependent interaction of lymphoma cells with stromal cells has been shown to protect lymphoma cells from anti-CD20 monoclonal antibody induced apoptosis (64). Studies have also shown induction of microRNAs impacting levels of proapoptotic proteins and upregulation of cell survival signals (65). As in solid organ epithelial tumors, there is intense interest in harnessing the power of CBT in lymphoid malignancies. Downstream signaling from the PD-1 receptor in T cells due to PDL-1 overexpression in tumor cells leads to immune exhaustion and helps tumors evade immune response. Blockade of this signaling pathway with monoclonal antibodies targeting either the PD-1 receptor or its ligand is predicted to enhance antitumor immune response (35). The results of immune checkpoint blockade in lymphomas have been mixed. The strongest response has been seen in lymphomas associated with high PDL-1 expression including Hodgkin lymphoma, primary mediastinal large B cell lymphoma and EBV-associated lymphoproliferative disorders (66–71). In contrast, the efficacy of CBT in other lymphomas such as DLBCL and chronic lymphocytic leukemia (CLL) have been less impressive (72, 73). Other strategies targeting the host immune response to lymphoma are being evaluated e.g., antibodies to the CD47 molecule that suppresses macrophage-induced phagocytosis by binding to signal regulatory protein-alpha (74).
The data with engineered adoptive cell therapies is promising in lymphoma therapy. Details of chimeric antigen receptor T cells (CAR-T) based therapy have been reviewed elsewhere (75). Briefly, CAR-Ts are created by transducing genetic material into patient’s own T-cells using lentiviral or retroviral vectors. The chimeric receptor contains an antigen binding extracellular domain that targets a tumor antigen coupled to an intracellular signaling domain, including chimeric domains derived from costimulatory proteins. This design allows the CAR-T cells to respond to tumor antigen without MHC presentation. The CAR-T cells are infused after lymphodepletion chemotherapy. Upon antigen recognition, the receptors cluster together to trigger a T-cell activating signaling cascade. CAR-T therapy targeting CD19 has been approved for the treatment of relapsed refractory aggressive B-NHLs and acute lymphoblastic leukemia following impressive and sustained response in clinical trials (76–78). Prospective clinical trials to evaluate expanded use of CAR-T as an alternative to stem cell transplant and in other B-NHLs are ongoing including combinatorial strategies with CBT and immunomodulatory therapy (3). Lack of long-term response due to emergence of target antigen negative cells (also known as antigen escape) has led to combinatorial antigen targeting including CD19 and CD20 or CD19 and CD22 (79–81). In contrast to CBT, CAR-T responses in Hodgkin lymphoma and T-cell lymphomas have not been impressive (82, 83).
Other immune based therapeutics in lymphoma include immunomodulators and small molecule inhibitors such as lenalidomide and ibrutinib. Lenalidomide and other thalidomide analogs exhibit immune modulatory effects by altering cytokine production, regulating T cell co-stimulation and augmenting NK cell cytotoxicity (84, 85). Ibrutinib and acalabrutinib are small molecule inhibitors that inhibit Bruton tyrosine kinase (BTK) that is part of the B cell receptor signaling pathway (86–89). In addition, ibrutinib can modulate immune response by increasing Th1 T cell response at the expense of Th2 T cell response by blocking IL-2 inducible kinase (90). Finally, observations in non-Hodgkin lymphomas have shown that treatment with these kinase inhibitors leads to the mobilization of lymphoma cells from their stromal niches into the bloodstream which may be an added component of their efficacy (91).
Immune-Based Therapy in Plasma Cell Neoplasms
Several immunotherapy approaches are being used in treatment of plasma cell neoplasms, both as part of standard-of-care treatments and in clinical trials (92). Lenalidomide is used in first line treatment of both standard-risk and high-risk plasma cell neoplasms (51). Targeted antibodies are now FDA-approved for treatment of plasma cell neoplasms. Anti-CD38 antibodies (daratumumab) in combination with immunomodulators has shown improved outcomes in relapsed refractory plasma cell neoplasms. The mechanism of action includes complement- and antibody-mediated cytotoxicity, and suppression of T-regs and regulatory myeloid populations (93). In contrast to B-NHLs results of CBT therapy in plasma cell neoplasms have been less impressive (94, 95). Combination immunotherapy approaches after autologous stem cell transplant are being evaluated based on encouraging data from preclinical trials (96). Immune modulatory drugs are now routinely used in treatment of symptomatic myeloma in combination regimens where they induce plasma cell apoptosis in addition to immune stimulatory effects. Other immunomodulatory regimens are being evaluated in early clinical trials. CAR-T therapy targeting B cell maturation antigen (BCMA) has shown promise (97, 98). BCMA is expressed primarily in plasmablasts and plasma cells in the bone marrow with no detectable expression in naïve B cells and hematopoietic cells (99, 100). The BCMA expression levels are much higher in neoplastic plasma cells compared to normal plasma cells. In response to signaling from its ligands APRIL and BAFF, BCMA signaling leads to activation of pro-survival pathways (38). Despite impressive early response in trials, persistent and durable response has not been seen, likely due to antigen escape and immunosuppressive effect of the bone marrow tumor microenvironment (101). Other immunomodulatory techniques targeting BCMA including targeted antibodies, BiTE antibodies, and CAR-T therapies to other antigens such as CD138 are under clinical development (102, 103).
Impact on Diagnostics, Prognostics, and Biomarkers
The diagnostic work-up of lymphomas and plasma cell neoplasms incorporates morphologic evaluation, immunophenotyping by tissue immunohistochemistry and multiparametric flow cytometry, and ancillary studies. The ancillary studies include cytogenetics, fluorescence in situ hybridization (FISH), molecular studies such as PCR for immunoglobulin heavy chain rearrangement, and targeted mutation detection for specific disease entities (104). While substantial information about disease behavior and prognosis can be obtained by these studies, they may provide only limited information regarding the tumor microenvironment and the potential for response to immune based therapies. There is a need to broaden the diagnostic and prognostic modalities to better predict disease response to immune-based therapies in lymphomas and plasma cell neoplasms. Some specific diagnostic modalities and their potential applications are briefly discussed below. While some techniques can be applied routinely in clinical diagnostic labs at the current time, other techniques exist currently more in the realm of clinical research that with time will probably supplant the current techniques.
Immunophenotyping
Immunohistochemistry (IHC) which involves interrogation of formalin-fixed paraffin embedded (FFPE) tissue with specific antibodies for specific antigens, and multiparametric flow cytometry are indispensable tools in clinical hematopathology diagnostic labs (104). These methods have potential utilities in assessing tissue for response to immune-based therapy. In solid organ tumors involving the lung and genitourinary tract for example, an FDA-approved immunohistochemistry based diagnostic assay for PD-L1 is used to predict response to CBT (105, 106). There are currently no FDA-approved IHC diagnostic assays to identify patients that will respond to CBT in hematologic malignancies. Analysis of PD-1 and PD-L1 expression patterns in hematologic malignancies show promising albeit sometimes contradictory results. For example, high proportion of PD-L1 positive macrophages or PD-1-positive T-cells is associated with favorable outcomes in primary testicular lymphoma (107). Similarly in a cohort of de novo DLBCL, increased myeloid derived PD-L1 cells correlated with STAT3 and macrophage gene expression, and improved outcomes in a subset of patients (108). A study utilizing 3-marker fluorescent multiplex immunohistochemistry coupled with automated immunofluorescent analysis concluded that the PD-1/PD-L1 expression and interaction is associated with adverse prognosis in DLBCLs with significant T cell infiltration (109). The variability of results in some of these studies may be attributable to the different antibody clones and experimental methods used in these studies illustrating the need for standardization of these assays across clinical labs prior to widespread diagnostic use. The fact that many of the antigens targeted by immune-based therapies such as CD20, CD19 and CD30 are evaluated during routine lymphoma diagnostic workup is fortuitous. However, the utility of IHC assays to predict disease response to CBT and targeted immunotherapy in hematologic neoplasms is limited in the absence of prospective studies. While it is logical to assume that immunopositivity and the level of antigen expression as assessed by immunophenotyping will correlate with disease response, one should be cautious with such assumptions. Individual studies looking at the correlation of antigen expression by IHC and response to targeted therapy have sometimes shown variable correlation as in the case of CD30 (110, 111). Additionally, lab-to-lab variability in the individual clones of antibody used and in staining protocols can lead to subjective interpretation and poor reproducibility of IHC and flow cytometry studies (112–114). On the other hand, immunomodulatory techniques themselves have potential to impact diagnostic assessment by these techniques. For example, CD20 staining can yield negative IHC results after treatment with rituximab due to antigen masking and down-regulation of CD20 expression (115). Bright CD38 expression is often used in assessing plasma cells by flow cytometry; treatment by daratumumab can lead to interference and artifactual results with laboratory assays for monitoring disease (116, 117). As the arsenal of targeted antibodies grows, diagnosticians need to be keenly aware of such iatrogenic artifacts during diagnostic and prognostic work up. Documentation of increased macrophages, cytotoxic T cells and NK cells by IHC has been shown to correlate with outcomes in cHL (118–121). Current immunohistochemical workup in clinical labs typically uses one antibody on one slice of tissue at a time on glass slides. The development of high-throughput, multiplex immunohistochemical methods opens the possibility of simultaneous evaluation of multiple markers on a single slice of tissue. The individual markers can be evaluated for their expression status and spatial distribution facilitating assessment of the tumor microenvironment by immunophenotypic methods (122–124). Immunophenotypic evaluation may also have utility in predicting response in CAR-T therapy. For example, a population of CD27+PD-1-CD8+ cells expressing high levels of the IL-6 receptor has been shown to correlate with therapeutic response (125).
Digital and Computational Pathology
IHC assays performed on FFPE tissue are easy to adopt in anatomic pathologic labs. However, interlaboratory variability in protocols and subjective variation in manual interpretation can lead to poor reproducibility of assays with impact on treatment and clinical course. The evolution of digital pathology where whole slide imaging scanners are used to digitize glass slides and render images in digital formats has aided the development of automated, reproducible, computer aided diagnostic tools that promise to be the next frontier in tissue-based diagnostics (126). WSI technology builds images of whole slides by stitching together multiple images of tissue sections on slides (127). WSI scanners have now been approved by the FDA for purposes of rendering anatomic pathology diagnoses (128). The College of American Pathology has published guidelines for validation and adoption of digital pathology techniques in clinical settings (129). Digital analyses tools including machine learning algorithms can be applied on digitized histology images for reproducible and quantitative assessment of tumors and their microenvironment. Multiplex approaches can yield high-complexity data with regard to spatial expression of multiple markers in the tumor microenvironment (130). In cHL for example, multiplex IF has shown the association of tumor microenvironment with CTLA-4-positive T cells that are PD-1 negative (131). Data shows that multiplex methods may be better at predicting response to CBT than standard IHC or gene expression profiling methods (132). These analyses can be extended to assess response to immunotherapy by assessing for distribution of components of the immune system such as regulatory T cells and macrophages. A limiting factor in the development and application of machine learning algorithms in computer aided diagnosis is the need for large, high quality data sets to train these algorithms (133). The performance metrics and portability of these algorithms across datasets can also be impacted by pre-analytic variables such as slide scan quality, need for additional image processing and input from human pathologists for accurate interpretation (134, 135). While multiplex IF provides high-resolution data about the tumor microenvironment, complexity of analyses, cost and time considerations currently limit the applicability of this technique in clinical settings. For practical purposes, gene expression analysis is currently utilized to assess characteristic signatures in diagnostic settings.
Molecular Techniques
Ancillary molecular techniques are a significant part of current diagnostic workflow in lymphoid and plasma cell malignancies (104). The presence of specific cytogenetic abnormalities, specific gene mutations, and other molecular findings such as IGH hypermutation status impact disease prognosis in various lymphomas and plasma cell neoplasms, and factor into the calculation of risk stratification scores of individual entities (1). These studies reveal little about the effect of tumor microenvironment on disease course since the spatial context is often lost with these techniques. However, studies have shown the potential utility of gene expression profiles that appear to reflect the tumor microenvironment in predicting response to immune based therapies. For example, in DLBCL gene expression profiles derived from non-malignant cells have shown association with response to R-CHOP therapy. A signature enriched for genes associated with components of the extracellular matrix deposition and histiocytic infiltration was associated with good behavior whereas a signature associated with angiogenesis was associated with poor prognosis (136). Similarly in follicular lymphoma, gene expression profiles associated with macrophages are correlated with different prognoses. Expression of a set of genes enriched for T-cell markers and genes highly expressed in macrophages was associated with better prognosis while a signature enriched for genes highly expressed in dendritic cells, macrophages or both was associated with worse prognosis (137). The refinement of techniques that allow for spatial single cell sequencing from FFPE tissue with the potential to deliver high-resolution molecular data of the tumor microenvironment with spatial context are particularly exciting developments in this realm (138). Techniques such as CODEX (for CO-Detection by indEXing) that utilizes DNA barcodes, fluorescent dNTP analogs and an in-situ polymerization based indexing procedure to iteratively detect antibody binding events have recently been described (139). This methodology allows for single cell antigen quantification in tissue sections, and unlimited levels of multiplexing to map cell types in tissues. The continued development of multiplexed and high-dimensional imaging methods, and their application in translational research are likely to lead to a better understanding of the tumor microenvironment in malignancies and their impact on response to therapy (140).
The power of using a combinatorial approach to dissect tumor microenvironment in lymphoid malignancies is demonstrated by a recent study that used FISH, chromogenic IHC, and multiplex immunofluorescence microscopy with cell phenotyping followed by spatial analyses of the cell phenotypic data to characterize the PD1/PD-L1 pathway in the tumor microenvironment of a multi-institutional cohort of T-cell/histiocyte-rich large B-cell lymphomas (THRLBCL) (141). The authors found frequent PD-L1/PD-L2 copy gain or amplification in the large malignant B-cells of THRLBCL. Using sophisticated spatial image analyses to characterize the distribution of immune cells and their PD1/PDL1 expression status, the authors were able to develop spatially resolved immune signatures that distinguish TCRLBCL from cHL and DLBCL.
Conclusion
Recent advances in our understanding of the tumor microenvironment have led to better understanding of pathogenesis of lymphomas and plasma cell neoplasms. Concurrent advances in immune based therapies have highlighted the importance on the tumor microenvironment on disease course and response to therapy. Advances in diagnostic modalities are likely to lead to better biomarker identification, patient risk stratification and theranostic prediction in hematologic malignancies.
Author Contributions
The author researched the topic and wrote the manuscript.
Funding
Salary support for the author was provided by the Department of Pathology, Yale School of Medicine, Yale University.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Steven H, Swerdlow EC, Lee Harris N, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. 4th Edition. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, editors. Lyon: IARC; (2017). [Google Scholar]
- 2. National Cancer Institute . Surveillance, Epidemiology, and End Results Program. In: Cancer Stat Facts: Non-Hodgkin Lymphoma (2021). Available at: https://seer.cancer.gov/statfacts/html/nhl.html (Accessed September 13, 2021). [Google Scholar]
- 3. Ansell SM, Lin Y. Immunotherapy of Lymphomas. J Clin Invest (2020) 130(4):1576–85. doi: 10.1172/JCI129206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scott DW, Gascoyne RD. The Tumour Microenvironment in B Cell Lymphomas. Nat Rev Cancer (2014) 14(8):517–34. doi: 10.1038/nrc3774 [DOI] [PubMed] [Google Scholar]
- 5. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 6. Waldman AD, Fritz JM, Lenardo MJ. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat Rev Immunol (2020) 20(11):651–68. doi: 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su W, Spencer J, Wotherspoon AC. Relative Distribution of Tumour Cells and Reactive Cells in Follicular Lymphoma. J Pathol (2001) 193(4):498–504. doi: 10.1002/path.820 [DOI] [PubMed] [Google Scholar]
- 8. Choi SM, Betz BL, Perry AM. Follicular Lymphoma Diagnostic Caveats and Updates. Arch Pathol Lab Med (2018) 142(11):1330–40. doi: 10.5858/arpa.2018-0217-RA [DOI] [PubMed] [Google Scholar]
- 9. Pals ST, de Gorter DJ, Spaargaren M. Lymphoma Dissemination: The Other Face of Lymphocyte Homing. Blood (2007) 110(9):3102–11. doi: 10.1182/blood-2007-05-075176 [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Giral S, Quintana NE, Cabrerizo M, Alfonso-Perez M, Sala-Valdes M, De Soria VG, et al. Chemokine Receptors That Mediate B Cell Homing to Secondary Lymphoid Tissues Are Highly Expressed in B Cell Chronic Lymphocytic Leukemia and Non-Hodgkin Lymphomas With Widespread Nodular Dissemination. J Leukoc Biol (2004) 76(2):462–71. doi: 10.1189/jlb.1203652 [DOI] [PubMed] [Google Scholar]
- 11. Rehm A, Anagnostopoulos I, Gerlach K, Broemer M, Scheidereit C, Johrens K, et al. Identification of a Chemokine Receptor Profile Characteristic for Mediastinal Large B-Cell Lymphoma. Int J Cancer (2009) 125(10):2367–74. doi: 10.1002/ijc.24652 [DOI] [PubMed] [Google Scholar]
- 12. Rehm A, Mensen A, Schradi K, Gerlach K, Wittstock S, Winter S, et al. Cooperative Function of CCR7 and Lymphotoxin in the Formation of a Lymphoma-Permissive Niche Within Murine Secondary Lymphoid Organs. Blood (2011) 118(4):1020–33. doi: 10.1182/blood-2010-11-321265 [DOI] [PubMed] [Google Scholar]
- 13. Steidl C, Connors JM, Gascoyne RD. Molecular Pathogenesis of Hodgkin's Lymphoma: Increasing Evidence of the Importance of the Microenvironment. J Clin Oncol (2011) 29(14):1812–26. doi: 10.1200/JCO.2010.32.8401 [DOI] [PubMed] [Google Scholar]
- 14. Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. CD70+ Non-Hodgkin Lymphoma B Cells Induce Foxp3 Expression and Regulatory Function in Intratumoral CD4+CD25 T Cells. Blood (2007) 110(7):2537–44. doi: 10.1182/blood-2007-03-082578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B Cells Skew the Balance of Regulatory T Cells and TH17 Cells in B-Cell Non-Hodgkin's Lymphoma. Cancer Res (2009) 69(13):5522–30. doi: 10.1158/0008-5472.CAN-09-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ Regulatory T-Cell-Mediated Suppression of Infiltrating CD4+ T Cells in B-Cell Non-Hodgkin Lymphoma. Blood (2006) 107(9):3639–46. doi: 10.1182/blood-2005-08-3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ai WZ, Hou JZ, Zeiser R, Czerwinski D, Negrin RS, Levy R. Follicular Lymphoma B Cells Induce the Conversion of Conventional CD4+ T Cells to T-Regulatory Cells. Int J Cancer (2009) 124(1):239–44. doi: 10.1002/ijc.23881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuppers R. Mechanisms of B-Cell Lymphoma Pathogenesis. Nat Rev Cancer (2005) 5(4):251–62. doi: 10.1038/nrc1589 [DOI] [PubMed] [Google Scholar]
- 19. Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. Oncogenically Active MYD88 Mutations in Human Lymphoma. Nature (2011) 470(7332):115–9. doi: 10.1038/nature09671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan Q, Huang Y, Watkins AJ, Kocialkowski S, Zeng N, Hamoudi RA, et al. BCR and TLR Signaling Pathways Are Recurrently Targeted by Genetic Changes in Splenic Marginal Zone Lymphomas. Haematologica (2012) 97(4):595–8. doi: 10.3324/haematol.2011.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwaller J, Went P, Matthes T, Dirnhofer S, Donze O, Mhawech-Fauceglia P, et al. Paracrine Promotion of Tumor Development by the TNF Ligand APRIL in Hodgkin's Disease. Leukemia (2007) 21(6):1324–7. doi: 10.1038/sj.leu.2404627 [DOI] [PubMed] [Google Scholar]
- 22. Riemersma SA, Jordanova ES, Schop RF, Philippo K, Looijenga LH, Schuuring E, et al. Extensive Genetic Alterations of the HLA Region, Including Homozygous Deletions of HLA Class II Genes in B-Cell Lymphomas Arising in Immune-Privileged Sites. Blood (2000) 96(10):3569–77. doi: 10.1182/blood.V96.10.3569 [DOI] [PubMed] [Google Scholar]
- 23. Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, et al. MHC Class II Transactivator CIITA Is a Recurrent Gene Fusion Partner in Lymphoid Cancers. Nature (2011) 471(7338):377–81. doi: 10.1038/nature09754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Challa-Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV, et al. Combined Genetic Inactivation of Beta2-Microglobulin and CD58 Reveals Frequent Escape From Immune Recognition in Diffuse Large B Cell Lymphoma. Cancer Cell (2011) 20(6):728–40. doi: 10.1016/j.ccr.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkinson ST, Vanpatten KA, Fernandez DR, Brunhoeber P, Garsha KE, Glinsmann-Gibson BJ, et al. Partial Plasma Cell Differentiation as a Mechanism of Lost Major Histocompatibility Complex Class II Expression in Diffuse Large B-Cell Lymphoma. Blood (2012) 119(6):1459–67. doi: 10.1182/blood-2011-07-363820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rimsza LM, Roberts RA, Miller TP, Unger JM, LeBlanc M, Braziel RM, et al. Loss of MHC Class II Gene and Protein Expression in Diffuse Large B-Cell Lymphoma Is Related to Decreased Tumor Immunosurveillance and Poor Patient Survival Regardless of Other Prognostic Factors: A Follow-Up Study From the Leukemia and Lymphoma Molecular Profiling Project. Blood (2004) 103(11):4251–8. doi: 10.1182/blood-2003-07-2365 [DOI] [PubMed] [Google Scholar]
- 27. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative Analysis Reveals Selective 9p24.1 Amplification, Increased PD-1 Ligand Expression, and Further Induction via JAK2 in Nodular Sclerosing Hodgkin Lymphoma and Primary Mediastinal Large B-Cell Lymphoma. Blood (2010) 116(17):3268–77. doi: 10.1182/blood-2010-05-282780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diepstra A, Poppema S, Boot M, Visser L, Nolte IM, Niens M, et al. HLA-G Protein Expression as a Potential Immune Escape Mechanism in Classical Hodgkin's Lymphoma. Tissue Antigens (2008) 71(3):219–26. doi: 10.1111/j.1399-0039.2008.01005.x [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Abdul Razak FR, Terpstra M, Chan FC, Saber A, Nijland M, et al. The Mutational Landscape of Hodgkin Lymphoma Cell Lines Determined by Whole-Exome Sequencing. Leukemia (2014) 28(11):2248–51. doi: 10.1038/leu.2014.201 [DOI] [PubMed] [Google Scholar]
- 30. Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, et al. Burkitt Lymphoma Pathogenesis and Therapeutic Targets From Structural and Functional Genomics. Nature (2012) 490(7418):116–20. doi: 10.1038/nature11378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greenough A, Dave SS. New Clues to the Molecular Pathogenesis of Burkitt Lymphoma Revealed Through Next-Generation Sequencing. Curr Opin Hematol (2014) 21(4):326–32. doi: 10.1097/MOH.0000000000000059 [DOI] [PubMed] [Google Scholar]
- 32. Salaverria I, Martin-Guerrero I, Wagener R, Kreuz M, Kohler CW, Richter J, et al. A Recurrent 11q Aberration Pattern Characterizes a Subset of MYC-Negative High-Grade B-Cell Lymphomas Resembling Burkitt Lymphoma. Blood (2014) 123(8):1187–98. doi: 10.1182/blood-2013-06-507996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, et al. The Genetic Landscape of Mutations in Burkitt Lymphoma. Nat Genet (2012) 44(12):1321–5. doi: 10.1038/ng.2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richter J, Schlesner M, Hoffmann S, Kreuz M, Leich E, Burkhardt B, et al. Recurrent Mutation of the ID3 Gene in Burkitt Lymphoma Identified by Integrated Genome, Exome and Transcriptome Sequencing. Nat Genet (2012) 44(12):1316–20. doi: 10.1038/ng.2469 [DOI] [PubMed] [Google Scholar]
- 35. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie M, Huang X, Ye X, Qian W. Prognostic and Clinicopathological Significance of PD-1/PD-L1 Expression in the Tumor Microenvironment and Neoplastic Cells for Lymphoma. Int Immunopharmacol (2019) 77:105999. doi: 10.1016/j.intimp.2019.105999 [DOI] [PubMed] [Google Scholar]
- 37. Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 Expression Is Characteristic of a Subset of Aggressive B-Cell Lymphomas and Virus-Associated Malignancies. Clin Cancer Res (2013) 19(13):3462–73. doi: 10.1158/1078-0432.CCR-13-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia-Ortiz A, Rodriguez-Garcia Y, Encinas J, Maroto-Martin E, Castellano E, Teixido J, et al. The Role of Tumor Microenvironment in Multiple Myeloma Development and Progression. Cancers (Basel) (2021) 13(2):217. doi: 10.3390/cancers13020217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawano Y, Moschetta M, Manier S, Glavey S, Gorgun GT, Roccaro AM, et al. Targeting the Bone Marrow Microenvironment in Multiple Myeloma. Immunol Rev (2015) 263(1):160–72. doi: 10.1111/imr.12233 [DOI] [PubMed] [Google Scholar]
- 40. Lomas OC, Tahri S, Ghobrial IM. The Microenvironment in Myeloma. Curr Opin Oncol (2020) 32(2):170–5. doi: 10.1097/CCO.0000000000000615 [DOI] [PubMed] [Google Scholar]
- 41. Pellegrino A, Ria R, Di Pietro G, Cirulli T, Surico G, Pennisi A, et al. Bone Marrow Endothelial Cells in Multiple Myeloma Secrete CXC-Chemokines That Mediate Interactions With Plasma Cells. Br J Haematol (2005) 129(2):248–56. doi: 10.1111/j.1365-2141.2005.05443.x [DOI] [PubMed] [Google Scholar]
- 42. Roccaro AM, Sacco A, Purschke WG, Moschetta M, Buchner K, Maasch C, et al. SDF-1 Inhibition Targets the Bone Marrow Niche for Cancer Therapy. Cell Rep (2014) 9(1):118–28. doi: 10.1016/j.celrep.2014.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roccaro AM, Mishima Y, Sacco A, Moschetta M, Tai YT, Shi J, et al. CXCR4 Regulates Extra-Medullary Myeloma Through Epithelial-Mesenchymal-Transition-Like Transcriptional Activation. Cell Rep (2015) 12(4):622–35. doi: 10.1016/j.celrep.2015.06.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feyler S, von Lilienfeld-Toal M, Jarmin S, Marles L, Rawstron A, Ashcroft AJ, et al. CD4(+)CD25(+)FoxP3(+) Regulatory T Cells Are Increased Whilst CD3(+)CD4(-)CD8(-)alphabetaTCR(+) Double Negative T Cells Are Decreased in the Peripheral Blood of Patients With Multiple Myeloma Which Correlates With Disease Burden. Br J Haematol (2009) 144(5):686–95. doi: 10.1111/j.1365-2141.2008.07530.x [DOI] [PubMed] [Google Scholar]
- 45. Kawano Y, Zavidij O, Park J, Moschetta M, Kokubun K, Mouhieddine TH, et al. Blocking IFNAR1 Inhibits Multiple Myeloma-Driven Treg Expansion and Immunosuppression. J Clin Invest (2018) 128(6):2487–99. doi: 10.1172/JCI88169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, et al. Marrow Stromal Cells Induce B7-H1 Expression on Myeloma Cells, Generating Aggressive Characteristics in Multiple Myeloma. Leukemia (2013) 27(2):464–72. doi: 10.1038/leu.2012.213 [DOI] [PubMed] [Google Scholar]
- 47. Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma Cells From Multiple Myeloma Patients Express B7-H1 (PD-L1) and Increase Expression After Stimulation With IFN-{Gamma} and TLR Ligands via a MyD88-, TRAF6-, and MEK-Dependent Pathway. Blood (2007) 110(1):296–304. doi: 10.1182/blood-2006-10-051482 [DOI] [PubMed] [Google Scholar]
- 48. Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Bartlett NL, Caimi PF, et al. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J Natl Compr Canc Netw (2019) 17(6):650–61. doi: 10.6004/jnccn.2019.0029 [DOI] [PubMed] [Google Scholar]
- 49. Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Armand P, Bello CM, et al. Hodgkin Lymphoma, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2020) 18(6):755–81. doi: 10.6004/jnccn.2020.0026 [DOI] [PubMed] [Google Scholar]
- 50. Rajkumar SV, Kumar S. Multiple Myeloma Current Treatment Algorithms. Blood Cancer J (2020) 10(9):94. doi: 10.1038/s41408-020-00359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rajkumar SV. Multiple Myeloma: 2020 Update on Diagnosis, Risk-Stratification and Management. Am J Hematol (2020) 95(5):548–67. doi: 10.1002/ajh.25791 [DOI] [PubMed] [Google Scholar]
- 52. McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab Chimeric Anti-CD20 Monoclonal Antibody Therapy for Relapsed Indolent Lymphoma: Half of Patients Respond to a Four-Dose Treatment Program. J Clin Oncol (1998) 16(8):2825–33. doi: 10.1200/JCO.1998.16.8.2825 [DOI] [PubMed] [Google Scholar]
- 53. Colombat P, Salles G, Brousse N, Eftekhari P, Soubeyran P, Delwail V, et al. Rituximab (Anti-CD20 Monoclonal Antibody) as Single First-Line Therapy for Patients With Follicular Lymphoma With a Low Tumor Burden: Clinical and Molecular Evaluation. Blood (2001) 97(1):101–6. doi: 10.1182/blood.V97.1.101 [DOI] [PubMed] [Google Scholar]
- 54. Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline Therapy With Rituximab Added to the Combination of Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) Significantly Improves the Outcome for Patients With Advanced-Stage Follicular Lymphoma Compared With Therapy With CHOP Alone: Results of a Prospective Randomized Study of the German Low-Grade Lymphoma Study Group. Blood (2005) 106(12):3725–32. doi: 10.1182/blood-2005-01-0016 [DOI] [PubMed] [Google Scholar]
- 55. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP Chemotherapy Plus Rituximab Compared With CHOP Alone in Elderly Patients With Diffuse Large-B-Cell Lymphoma. N Engl J Med (2002) 346(4):235–42. doi: 10.1056/NEJMoa011795 [DOI] [PubMed] [Google Scholar]
- 56. Salles G, Duell J, Gonzalez Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab Plus Lenalidomide in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (L-MIND): A Multicentre, Prospective, Single-Arm, Phase 2 Study. Lancet Oncol (2020) 21(7):978–88. doi: 10.1016/S1470-2045(20)30225-4 [DOI] [PubMed] [Google Scholar]
- 57. Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, et al. Defucosylated Anti-CCR4 Monoclonal Antibody (KW-0761) for Relapsed Adult T-Cell Leukemia-Lymphoma: A Multicenter Phase II Study. J Clin Oncol (2012) 30(8):837–42. doi: 10.1200/JCO.2011.37.3472 [DOI] [PubMed] [Google Scholar]
- 58. Ogura M, Ishida T, Hatake K, Taniwaki M, Ando K, Tobinai K, et al. Multicenter Phase II Study of Mogamulizumab (KW-0761), a Defucosylated Anti-Cc Chemokine Receptor 4 Antibody, in Patients With Relapsed Peripheral T-Cell Lymphoma and Cutaneous T-Cell Lymphoma. J Clin Oncol (2014) 32(11):1157–63. doi: 10.1200/JCO.2013.52.0924 [DOI] [PubMed] [Google Scholar]
- 59. Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, et al. Mogamulizumab Versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC): An International, Open-Label, Randomised, Controlled Phase 3 Trial. Lancet Oncol (2018) 19(9):1192–204. doi: 10.1016/S1470-2045(18)30379-6 [DOI] [PubMed] [Google Scholar]
- 60. Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients With Relapsed or Refractory Hodgkin's Lymphoma. J Clin Oncol (2012) 30(18):2183–9. doi: 10.1200/JCO.2011.38.0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, et al. Brentuximab Vedotin With Chemotherapy for Stage III or IV Hodgkin's Lymphoma. N Engl J Med (2018) 378(4):331–44. doi: 10.1056/NEJMoa1708984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 Study of the Bispecific T-Cell Engager (BiTE) Antibody Blinatumomab in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood (2016) 127(11):1410–6. doi: 10.1182/blood-2015-06-651380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Budde LE, Wu D, Martin DB, Philip M, Shustov AR, Smith SD, et al. Bendamustine With Rituximab, Etoposide and Carboplatin (T(R)EC) in Relapsed or Refractory Aggressive Lymphoma: A Prospective Multicentre Phase 1/2 Clinical Trial. Br J Haematol (2018) 183(4):601–7. doi: 10.1111/bjh.15585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mraz M, Zent CS, Church AK, Jelinek DF, Wu X, Pospisilova S, et al. Bone Marrow Stromal Cells Protect Lymphoma B-Cells From Rituximab-Induced Apoptosis and Targeting Integrin Alpha-4-Beta-1 (VLA-4) With Natalizumab can Overcome This Resistance. Br J Haematol (2011) 155(1):53–64. doi: 10.1111/j.1365-2141.2011.08794.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lwin T, Lin J, Choi YS, Zhang X, Moscinski LC, Wright KL, et al. Follicular Dendritic Cell-Dependent Drug Resistance of Non-Hodgkin Lymphoma Involves Cell Adhesion-Mediated Bim Down-Regulation Through Induction of microRNA-181a. Blood (2010) 116(24):5228–36. doi: 10.1182/blood-2010-03-275925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zinzani PL, Ribrag V, Moskowitz CH, Michot JM, Kuruvilla J, Balakumaran A, et al. Safety and Tolerability of Pembrolizumab in Patients With Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma. Blood (2017) 130(3):267–70. doi: 10.1182/blood-2016-12-758383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 Blockade With Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. N Engl J Med (2015) 372(4):311–9. doi: 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol (2016) 34(31):3733–9. doi: 10.1200/JCO.2016.67.3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol (2018) 36(14):1428–39. doi: 10.1200/JCO.2017.76.0793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol (2017) 35(19):2125–32. doi: 10.1200/JCO.2016.72.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for Classical Hodgkin's Lymphoma After Failure of Both Autologous Stem-Cell Transplantation and Brentuximab Vedotin: A Multicentre, Multicohort, Single-Arm Phase 2 Trial. Lancet Oncol (2016) 17(9):1283–94. doi: 10.1016/S1470-2045(16)30167-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J Clin Oncol (2019) 37(6):481–9. doi: 10.1200/JCO.18.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ding W, LaPlant BR, Call TG, Parikh SA, Leis JF, He R, et al. Pembrolizumab in Patients With CLL and Richter Transformation or With Relapsed CLL. Blood (2017) 129(26):3419–27. doi: 10.1182/blood-2017-02-765685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's Lymphoma. N Engl J Med (2018) 379(18):1711–21. doi: 10.1056/NEJMoa1807315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An Introduction to Chimeric Antigen Receptor (CAR) T-Cell Immunotherapy for Human Cancer. Am J Hematol (2019) 94(S1):S3–9. doi: 10.1002/ajh.25418 [DOI] [PubMed] [Google Scholar]
- 76. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric Antigen Receptor T-Cell Therapy - Assessment and Management of Toxicities. Nat Rev Clin Oncol (2018) 15(1):47–62. doi: 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med (2019) 380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 78. Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-Term Follow-Up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med (2018) 378(5):449–59. doi: 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bukhari A, El Chaer F, Koka R, Singh Z, Hutnick E, Ruehle K, et al. Rapid Relapse of Large B-Cell Lymphoma After CD19 Directed CAR-T-Cell Therapy Due to CD-19 Antigen Loss. Am J Hematol (2019) 94(10):E273–E5. doi: 10.1002/ajh.25591 [DOI] [PubMed] [Google Scholar]
- 80. Shalabi H, Kraft IL, Wang HW, Yuan CM, Yates B, Delbrook C, et al. Sequential Loss of Tumor Surface Antigens Following Chimeric Antigen Receptor T-Cell Therapies in Diffuse Large B-Cell Lymphoma. Haematologica (2018) 103(5):e215–8. doi: 10.3324/haematol.2017.183459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific Anti-CD20, Anti-CD19 CAR T Cells for Relapsed B Cell Malignancies: A Phase 1 Dose Escalation and Expansion Trial. Nat Med (2020) 26(10):1569–75. doi: 10.1038/s41591-020-1081-3 [DOI] [PubMed] [Google Scholar]
- 82. Ramos CA, Ballard B, Zhang H, Dakhova O, Gee AP, Mei Z, et al. Clinical and Immunological Responses After CD30-Specific Chimeric Antigen Receptor-Redirected Lymphocytes. J Clin Invest (2017) 127(9):3462–71. doi: 10.1172/JCI94306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang CM, Wu ZQ, Wang Y, Guo YL, Dai HR, Wang XH, et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin Cancer Res (2017) 23(5):1156–66. doi: 10.1158/1078-0432.CCR-16-1365 [DOI] [PubMed] [Google Scholar]
- 84. Davies FE, Forsyth PD, Rawstron AC, Owen RG, Pratt G, Evans PA, et al. The Impact of Attaining a Minimal Disease State After High-Dose Melphalan and Autologous Transplantation for Multiple Myeloma. Br J Haematol (2001) 112(3):814–9. doi: 10.1046/j.1365-2141.2001.02530.x [DOI] [PubMed] [Google Scholar]
- 85. Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, et al. Immunomodulatory Drug Lenalidomide (CC-5013, IMiD3) Augments Anti-CD40 SGN-40-Induced Cytotoxicity in Human Multiple Myeloma: Clinical Implications. Cancer Res (2005) 65(24):11712–20. doi: 10.1158/0008-5472.CAN-05-1657 [DOI] [PubMed] [Google Scholar]
- 86. Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-Term Efficacy and Safety of First-Line Ibrutinib Treatment for Patients With CLL/SLL: 5 Years of Follow-Up From the Phase 3 RESONATE-2 Study. Leukemia (2020) 34(3):787–98. doi: 10.1038/s41375-019-0602-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med (2016) 374(4):323–32. doi: 10.1056/NEJMoa1509981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-Year Follow-Up of Treatment-Naive and Previously Treated Patients With CLL and SLL Receiving Single-Agent Ibrutinib. Blood (2015) 125(16):2497–506. doi: 10.1182/blood-2014-10-606038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shanafelt TD, Wang XV, Kay NE, Hanson CA, O'Brien S, Barrientos J, et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med (2019) 381(5):432–43. doi: 10.1056/NEJMoa1817073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib Is an Irreversible Molecular Inhibitor of ITK Driving a Th1-Selective Pressure in T Lymphocytes. Blood (2013) 122(15):2539–49. doi: 10.1182/blood-2013-06-507947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chang BY, Francesco M, De Rooij MF, Magadala P, Steggerda SM, Huang MM, et al. Egress of CD19(+)CD5(+) Cells Into Peripheral Blood Following Treatment With the Bruton Tyrosine Kinase Inhibitor Ibrutinib in Mantle Cell Lymphoma Patients. Blood (2013) 122(14):2412–24. doi: 10.1182/blood-2013-02-482125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Minnie SA, Hill GR. Immunotherapy of Multiple Myeloma. J Clin Invest (2020) 130(4):1565–75. doi: 10.1172/JCI129205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med (2016) 375(8):754–66. doi: 10.1056/NEJMoa1606038 [DOI] [PubMed] [Google Scholar]
- 94. Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol (2016) 34(23):2698–704. doi: 10.1200/JCO.2015.65.9789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. D'Souza A, Hari P, Pasquini M, Braun T, Johnson B, Lundy S, et al. A Phase 2 Study of Pembrolizumab During Lymphodepletion After Autologous Hematopoietic Cell Transplantation for Multiple Myeloma. Biol Blood Marrow Transplant (2019) 25(8):1492–7. doi: 10.1016/j.bbmt.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 96. Jing W, Gershan JA, Weber J, Tlomak D, McOlash L, Sabatos-Peyton C, et al. Combined Immune Checkpoint Protein Blockade and Low Dose Whole Body Irradiation as Immunotherapy for Myeloma. J Immunother Cancer (2015) 3(1):2. doi: 10.1186/s40425-014-0043-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol (2018) 36(22):2267–80. doi: 10.1200/JCO.2018.77.8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B Cell Maturation Antigen-Specific CAR T Cells Are Clinically Active in Multiple Myeloma. J Clin Invest (2019) 129(6):2210–21. doi: 10.1172/JCI126397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA Is Essential for the Survival of Long-Lived Bone Marrow Plasma Cells. J Exp Med (2004) 199(1):91–8. doi: 10.1084/jem.20031330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Friedman KM, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL, et al. Effective Targeting of Multiple B-Cell Maturation Antigen-Expressing Hematological Malignances by Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum Gene Ther (2018) 29(5):585–601. doi: 10.1089/hum.2018.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sidana S, Shah N. CAR T-Cell Therapy: Is It Prime Time in Myeloma? Hematol Am Soc Hematol Educ Program (2019) 2019(1):260–5. doi: 10.1182/hematology.2019000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sun C, Mahendravada A, Ballard B, Kale B, Ramos C, West J, et al. Safety and Efficacy of Targeting CD138 With a Chimeric Antigen Receptor for the Treatment of Multiple Myeloma. Oncotarget (2019) 10(24):2369–83. doi: 10.18632/oncotarget.26792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gogishvili T, Danhof S, Prommersberger S, Rydzek J, Schreder M, Brede C, et al. SLAMF7-CAR T Cells Eliminate Myeloma and Confer Selective Fratricide of SLAMF7(+) Normal Lymphocytes. Blood (2017) 130(26):2838–47. doi: 10.1182/blood-2017-04-778423 [DOI] [PubMed] [Google Scholar]
- 104. Kroft SH, Sever CE, Bagg A, Billman B, Diefenbach C, Dorfman DM, et al. Laboratory Workup of Lymphoma in Adults. Am J Clin Pathol (2021) 155(1):12–37. doi: 10.1093/ajcp/aqaa191 [DOI] [PubMed] [Google Scholar]
- 105. Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, et al. A Prospective, Multi-Institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol (2017) 3(8):1051–8. doi: 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Davis AA, Patel VG. The Role of PD-L1 Expression as a Predictive Biomarker: An Analysis of All US Food and Drug Administration (FDA) Approvals of Immune Checkpoint Inhibitors. J Immunother Cancer (2019) 7(1):278. doi: 10.1186/s40425-019-0768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pollari M, Bruck O, Pellinen T, Vahamurto P, Karjalainen-Lindsberg ML, Mannisto S, et al. PD-L1(+) Tumor-Associated Macrophages and PD-1(+) Tumor-Infiltrating Lymphocytes Predict Survival in Primary Testicular Lymphoma. Haematologica (2018) 103(11):1908–14. doi: 10.3324/haematol.2018.197194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McCord R, Bolen CR, Koeppen H, Kadel EE, 3rd, Oestergaard MZ, Nielsen T, et al. PD-L1 and Tumor-Associated Macrophages in De Novo DLBCL. Blood Adv (2019) 3(4):531–40. doi: 10.1182/bloodadvances.2018020602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li L, Sun R, Miao Y, Tran T, Adams L, Roscoe N, et al. PD-1/PD-L1 Expression and Interaction by Automated Quantitative Immunofluorescent Analysis Show Adverse Prognostic Impact in Patients With Diffuse Large B-Cell Lymphoma Having T-Cell Infiltration: A Study From the International DLBCL Consortium Program. Mod Pathol (2019) 32(6):741–54. doi: 10.1038/s41379-018-0193-5 [DOI] [PubMed] [Google Scholar]
- 110. Xu ML, Gabali A, Hsi ED, Fedoriw Y, Vij K, Salama ME, et al. Practical Approaches on CD30 Detection and Reporting in Lymphoma Diagnosis. Am J Surg Pathol (2020) 44(2):e1–e14. doi: 10.1097/PAS.0000000000001368 [DOI] [PubMed] [Google Scholar]
- 111. Xu ML, Acevedo-Gadea C, Seropian S, Katz SG. Expression of CD30 as a Biomarker to Predict Response to Brentuximab Vedotin. Histopathology (2016) 69(1):155–8. doi: 10.1111/his.12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol (2018) 13(9):1302–11. doi: 10.1016/j.jtho.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat Rev Clin Oncol (2021) 18(6):345–62. doi: 10.1038/s41571-021-00473-5 [DOI] [PubMed] [Google Scholar]
- 114. Brunnstrom H, Johansson A, Westbom-Fremer S, Backman M, Djureinovic D, Patthey A, et al. PD-L1 Immunohistochemistry in Clinical Diagnostics of Lung Cancer: Inter-Pathologist Variability Is Higher Than Assay Variability. Mod Pathol (2017) 30(10):1411–21. doi: 10.1038/modpathol.2017.59 [DOI] [PubMed] [Google Scholar]
- 115. Hiraga J, Tomita A, Sugimoto T, Shimada K, Ito M, Nakamura S, et al. Down-Regulation of CD20 Expression in B-Cell Lymphoma Cells After Treatment With Rituximab-Containing Combination Chemotherapies: Its Prevalence and Clinical Significance. Blood (2009) 113(20):4885–93. doi: 10.1182/blood-2008-08-175208 [DOI] [PubMed] [Google Scholar]
- 116. van de Donk NW, Otten HG, El Haddad O, Axel A, Sasser AK, Croockewit S, et al. Interference of Daratumumab in Monitoring Multiple Myeloma Patients Using Serum Immunofixation Electrophoresis can be Abrogated Using the Daratumumab IFE Reflex Assay (DIRA). Clin Chem Lab Med (2016) 54(6):1105–9. doi: 10.1515/cclm-2015-0888 [DOI] [PubMed] [Google Scholar]
- 117. Kleinot W, Aguilera N, Courville EL. Daratumumab Interference in Flow Cytometry Producing a False Kappa Light Chain Restriction in Plasma Cells. Lab Med (2020) 52(4):403–9. doi: 10.1093/labmed/lmaa107 [DOI] [PubMed] [Google Scholar]
- 118. Chetaille B, Bertucci F, Finetti P, Esterni B, Stamatoullas A, Picquenot JM, et al. Molecular Profiling of Classical Hodgkin Lymphoma Tissues Uncovers Variations in the Tumor Microenvironment and Correlations With EBV Infection and Outcome. Blood (2009) 113(12):2765–3775. doi: 10.1182/blood-2008-07-168096 [DOI] [PubMed] [Google Scholar]
- 119. Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-Associated Macrophages and Survival in Classic Hodgkin's Lymphoma. N Engl J Med (2010) 362(10):875–85. doi: 10.1056/NEJMoa0905680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, et al. Outcome in Hodgkin's Lymphoma Can Be Predicted From the Presence of Accompanying Cytotoxic and Regulatory T Cells. Clin Cancer Res (2005) 11(4):1467–73. doi: 10.1158/1078-0432.CCR-04-1869 [DOI] [PubMed] [Google Scholar]
- 121. Kelley TW, Pohlman B, Elson P, Hsi ED. The Ratio of FOXP3+ Regulatory T Cells to Granzyme B+ Cytotoxic T/NK Cells Predicts Prognosis in Classical Hodgkin Lymphoma and Is Independent of Bcl-2 and MAL Expression. Am J Clin Pathol (2007) 128(6):958–65. doi: 10.1309/NB3947K383DJ0LQ2 [DOI] [PubMed] [Google Scholar]
- 122. Dixon AR, Bathany C, Tsuei M, White J, Barald KF, Takayama S. Recent Developments in Multiplexing Techniques for Immunohistochemistry. Expert Rev Mol Diagn (2015) 15(9):1171–86. doi: 10.1586/14737159.2015.1069182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Blom S, Paavolainen L, Bychkov D, Turkki R, Maki-Teeri P, Hemmes A, et al. Systems Pathology by Multiplexed Immunohistochemistry and Whole-Slide Digital Image Analysis. Sci Rep (2017) 7(1):15580. doi: 10.1038/s41598-017-15798-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed Immunohistochemistry, Imaging, and Quantitation: A Review, With an Assessment of Tyramide Signal Amplification, Multispectral Imaging and Multiplex Analysis. Methods (2014) 70(1):46–58. doi: 10.1016/j.ymeth.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 125. Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of Response and Resistance to CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy of Chronic Lymphocytic Leukemia. Nat Med (2018) 24(5):563–71. doi: 10.1038/s41591-018-0010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Parwani AV. Next Generation Diagnostic Pathology: Use of Digital Pathology and Artificial Intelligence Tools to Augment a Pathological Diagnosis. Diagn Pathol (2019) 14(1):138. doi: 10.1186/s13000-019-0921-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zarella MD, Bowman D, Aeffner F, Farahani N, Xthona A, Absar SF, et al. A Practical Guide to Whole Slide Imaging: A White Paper From the Digital Pathology Association. Arch Pathol Lab Med (2019) 143(2):222–34. doi: 10.5858/arpa.2018-0343-RA [DOI] [PubMed] [Google Scholar]
- 128. Evans AJ, Bauer TW, Bui MM, Cornish TC, Duncan H, Glassy EF, et al. US Food and Drug Administration Approval of Whole Slide Imaging for Primary Diagnosis: A Key Milestone Is Reached and New Questions Are Raised. Arch Pathol Lab Med (2018) 142(11):1383–7. doi: 10.5858/arpa.2017-0496-CP [DOI] [PubMed] [Google Scholar]
- 129. Evans AJ, Brown RW, Bui MM, Chlipala EA, Lacchetti C, Milner DA, et al. Validating Whole Slide Imaging Systems for Diagnostic Purposes in Pathology: Guideline Update From the College of American Pathologists in Collaboration With the American Society for Clinical Pathology and the Association for Pathology Informatics. Arch Pathol Lab Med (2021). doi: 10.5858/arpa.2020-0723-CP [DOI] [Google Scholar]
- 130. Heindl A, Nawaz S, Yuan Y. Mapping Spatial Heterogeneity in the Tumor Microenvironment: A New Era for Digital Pathology. Lab Invest (2015) 95(4):377–84. doi: 10.1038/labinvest.2014.155 [DOI] [PubMed] [Google Scholar]
- 131. Patel SS, Weirather JL, Lipschitz M, Lako A, Chen PH, Griffin GK, et al. The Microenvironmental Niche in Classic Hodgkin Lymphoma Is Enriched for CTLA-4-Positive T Cells That Are PD-1-Negative. Blood (2019) 134(23):2059–69. doi: 10.1182/blood.2019002206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-Analysis. JAMA Oncol (2019) 5(8):1195–204. doi: 10.1001/jamaoncol.2019.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tizhoosh HR, Pantanowitz L. Artificial Intelligence and Digital Pathology: Challenges and Opportunities. J Pathol Inform (2018) 9:38. doi: 10.4103/jpi.jpi_53_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pantanowitz L, Quiroga-Garza GM, Bien L, Heled R, Laifenfeld D, Linhart C, et al. An Artificial Intelligence Algorithm for Prostate Cancer Diagnosis in Whole Slide Images of Core Needle Biopsies: A Blinded Clinical Validation and Deployment Study. Lancet Digit Health (2020) 2(8):e407–16. doi: 10.1016/S2589-7500(20)30159-X [DOI] [PubMed] [Google Scholar]
- 135. Perincheri S, Levi AW, Celli R, Gershkovich P, Rimm D, Morrow JS, et al. An Independent Assessment of an Artificial Intelligence System for Prostate Cancer Detection Shows Strong Diagnostic Accuracy. Mod Pathol (2021) 34(8):1588–95. doi: 10.1038/s41379-021-00794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal Gene Signatures in Large-B-Cell Lymphomas. N Engl J Med (2008) 359(22):2313–23. doi: 10.1056/NEJMoa0802885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al. Prediction of Survival in Follicular Lymphoma Based on Molecular Features of Tumor-Infiltrating Immune Cells. N Engl J Med (2004) 351(21):2159–69. doi: 10.1056/NEJMoa041869 [DOI] [PubMed] [Google Scholar]
- 138. Liu Y, Yang M, Deng Y, Su G, Enninful A, Guo CC, et al. High-Spatial-Resolution Multi-Omics Sequencing via Deterministic Barcoding in Tissue. Cell (2020) 183(6):1665–81 e18. doi: 10.1016/j.cell.2020.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Goltsev Y, Samusik N, Kennedy-Darling J, Bhate S, Hale M, Vazquez G, et al. Deep Profiling of Mouse Splenic Architecture With CODEX Multiplexed Imaging. Cell (2018) 174(4):968–81.e15. doi: 10.1016/j.cell.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Decalf J, Albert ML, Ziai J. New Tools for Pathology: A User's Review of a Highly Multiplexed Method for in Situ Analysis of Protein and RNA Expression in Tissue. J Pathol (2019) 247(5):650–61. doi: 10.1002/path.5223 [DOI] [PubMed] [Google Scholar]
- 141. Voorhees PM, Rodriguez C, Reeves B, Nathwani N, Costa LJ, Lutska Y, et al. Daratumumab Plus RVd for Newly Diagnosed Multiple Myeloma: Final Analysis of the Safety Run-in Cohort of GRIFFIN. Blood Adv (2021) 5(4):1092–6. doi: 10.1182/bloodadvances.2020003642 [DOI] [PMC free article] [PubMed] [Google Scholar]