Abstract
Background
Infants born at term by elective caesarean section are more likely to develop respiratory morbidity than infants born vaginally. Prophylactic corticosteroids in singleton preterm pregnancies accelerate lung maturation and reduce the incidence of respiratory complications. It is unclear whether administration at term gestations, prior to caesarean section, improves the respiratory outcomes for these babies without causing any unnecessary morbidity to the mother or the infant.
Objectives
The objective of this review was to assess the effect of prophylactic corticosteroid administration before elective caesarean section at term, as compared to usual care (which could be placebo or no treatment), on fetal, neonatal and maternal morbidity. We also assessed the impact of the treatment on the child in later life.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov (20 January 2021) and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials comparing prophylactic antenatal corticosteroid administration (betamethasone or dexamethasone) with placebo or with no treatment, given before elective caesarean section at term (at or after 37 weeks of gestation). Quasi‐randomised and cluster‐randomised controlled trials were also eligible for inclusion.
Data collection and analysis
We used standard Cochrane Pregnancy and Childbirth methods for data collection and analysis. Two review authors independently assessed trials for inclusion, assessed risk of bias, evaluated trustworthiness (based on predefined criteria developed by Cochrane Pregnancy and Childbirth), extracted data and checked them for accuracy and assessed the certainty of the evidence using the GRADE approach. Our primary outcomes were respiratory distress syndrome (RDS), transient tachypnoea of the neonate (TTN), admission to neonatal special care for respiratory morbidity and need for mechanical ventilation.
We planned to perform subgroup analyses for the primary outcomes according to gestational age at randomisation and type of corticosteroid (betamethasone or dexamethasone). We also planned to perform sensitivity analysis, including only studies at low risk of bias.
Main results
We included one trial in which participants were randomised to receive either betamethasone or usual care. The trial included 942 women and 942 neonates recruited from 10 UK hospitals between 1995 and 2002. This review includes only trials that met predefined criteria for trustworthiness. We removed three trials from the analysis that were included in the previous version of this review.
The risk of bias was low for random sequence generation, allocation concealment and incomplete outcome data. The risk of bias for selective outcome reporting was unclear because there was no published trial protocol, and therefore it is unclear whether all the planned outcomes were reported in full. Due to a lack of blinding we judged there to be high risk of performance bias and detection bias. We downgraded the certainty of the evidence because of concerns about risk of bias and because of imprecision due to low event rates and wide 95% confidence intervals (CIs), which are consistent with possible benefit and possible harm
Compared with usual care, it is uncertain if antenatal corticosteroids reduce the risk of RDS (relative risk (RR) 0.34 95% CI 0.07 to 1.65; 1 study; 942 infants) or TTN (RR 0.52, 95% CI 0.25 to 1.11; 1 study; 938 infants) because the certainty of evidence is low and the 95% CIs are consistent with possible benefit and possible harm.
Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for respiratory complications, compared with usual care (RR 0.45, 95% CI 0.22 to 0.90; 1 study; 942 infants; moderate‐certainty evidence). The proportion of infants admitted to neonatal special care for respiratory morbidity after treatment with antenatal corticosteroids was 2.3% compared with 5.1% in the usual care group.
It is uncertain if antenatal steroids have any effect on the risk of needing mechanical ventilation, compared with usual care (RR 4.07, 95% CI 0.46 to 36.27; 1 study; 942 infants; very low‐certainty evidence). The effect of antenatal corticosteroids on the maternal development of postpartum infection/pyrexia in the first 72 hours is unclear due to the very low certainty of the evidence; one study (942 women) reported zero cases. The included studies did not report any data for neonatal hypoglycaemia or maternal mortality/severe morbidity.
Authors' conclusions
Evidence from one randomised controlled trial suggests that prophylactic corticosteroids before elective caesarean section at term probably reduces admission to the neonatal intensive care unit for respiratory morbidity. It is uncertain if administration of antenatal corticosteroids reduces the rates of respiratory distress syndrome (RDS) or transient tachypnoea of the neonate (TTN). The overall certainty of the evidence for the primary outcomes was found to be low or very low, apart from the outcome of admission to neonatal special care (all levels) for respiratory morbidity, for which the evidence was of moderate certainty. Therefore, there is currently insufficient data to draw any firm conclusions.
More evidence is needed to investigate the effect of prophylactic antenatal corticosteroids on the incidence of recognised respiratory morbidity such as RDS. Any future trials should assess the balance between respiratory benefit and potential immediate adverse effects (e.g. hypoglycaemia) and long‐term adverse effects (e.g. academic performance) for the infant. There is very limited information on maternal health outcomes to provide any assurances that corticosteroids do not pose any increased risk of harm to the mother.
Further research should consider investigating the effectiveness of antenatal steroids at different gestational ages prior to caesarean section. There are nine potentially eligible studies that are currently ongoing and could be included in future updates of this review.
Keywords: Child; Female; Humans; Infant; Infant, Newborn; Pregnancy; Adrenal Cortex Hormones; Adrenal Cortex Hormones/adverse effects; Betamethasone; Cesarean Section; Prenatal Care; Randomized Controlled Trials as Topic; Respiratory Distress Syndrome, Newborn; Respiratory Distress Syndrome, Newborn/prevention & control
Plain language summary
Corticosteroids for preventing serious breathing problems in the newborn after caesarean section at term
What is the issue?
Babies born at term (at or after 37 weeks of pregnancy) by planned (elective) caesarean section, before onset of labour, are more likely to develop breathing complications than babies born vaginally. Giving injections called corticosteroids to the mother has been shown to reduce the risk of breathing problems in babies born before 34 weeks of pregnancy, but it is not clear if they are also useful for babies born by caesarean section at term.
Why is this important?
Caesarean section increases the risk of a term newborn developing breathing problems, such as rapid breathing over the first few days (known as transient tachypnoea of the neonate) and the more serious respiratory distress syndrome (RDS). The affected babies may need treatment in special care units. This risk decreases from 37 weeks to 39 weeks of gestation, at which stage it is low. Most caesarean sections are performed after 39 weeks of gestation, but there are some instances when babies need to be born earlier. The aim of this review was to investigate if corticosteroids can reduce the rates of breathing problems prior to caesarean section, without causing problems for the mother or the infant.
How did we identify and evaluate the evidence?
We searched the medical literature for randomised controlled studies that met our criteria for being trustworthy and compared the effects of corticosteroids against a placebo (dummy) treatment or against usual care. We rated our confidence in the findings based on factors such as the number of studies, study methods, number of women and babies involved, the number of events and the variability of the findings.
What evidence did we find?
We included evidence up until 20 January 2021. We included one trial that involved 942 women and 942 babies recruited from 10 UK hospitals. The women in the treatment group received two doses of the corticosteroid betamethasone by injection into the muscle. The women in the control group received usual care. No blinding procedures were used therefore all the women, caregivers and investigators were aware of who received corticosteroids and who received usual care.
It is uncertain if corticosteroids reduce the risk of transient tachypnoea of the neonate (a mild breathing problem) or respiratory distress syndrome (i.e serious breathing problems) compared with usual care. Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for breathing complications compared with usual care.
It is uncertain if corticosteroids have any effect on the risk of the baby needing additional breathing support (mechanical ventilation) compared with usual care. It is uncertain if antenatal corticosteroids have any effect on women developing infection or high temperature within 72 hours of giving birth (there were no cases in the one study involving 942 women).
We did not find any evidence about the baby's risk of low blood sugar or about the woman's risk of serious illness, death or wound infection.
Certainty of evidence
The certainty of evidence from the included randomised trial was very low to moderate. This means that we can not be completely confident that future trials will come to the same conclusions about the treatment benefits for the babies of mothers receiving a course of antenatal corticosteroids prior to caesarean section at term.
What does this mean?
The risk of being admitted to neonatal special care because of breathing problems was reduced in one study. It is uncertain if corticosteroids have any effect on the risk of serious breathing problems (respiratory distress syndrome) or rapid breathing (transient tachypnoea) in the neonate, compared with usual care. Further studies are needed to investigate if antenatal corticosteroids do reduce the risk of serious respiratory problems (such as respiratory distress syndrome). Future trials need to make sure they assess for possible short‐ and long‐term harm to both mother and baby after receiving a course of antenatal corticosteroids prior to caesarean section at term. Further research could consider assessing whether any benefits or harms identified by giving a course of antenatal corticosteroids are affected by the gestational age at which the planned caesarean is performed.
There are nine potentially eligible studies that are currently ongoing and could be included in future updates of this review.
Summary of findings
Summary of findings 1. Effect of antenatal corticosteroids (betamethasone) compared to usual care prior to planned caesarean at term on maternal and neonatal outcomes.
| Antenatal corticosteroids compared to usual care prior to planned caesarean at term on maternal and neonatal outcomes | ||||||
| Patient or population: women undergoing planned elective caesarean section at 37 weeks' gestation and beyond for singleton pregnancy Setting: obstetric units from 10 UK hospitals Intervention: two intramuscular doses of 12 mg of betamethasone administered 24 hours apart Comparison: usual care without antenatal steroids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with Antenatal corticosteroids | |||||
| Respiratory distress syndrome (RDS) | Study population | RR 0.34 (0.07 to 1.65) | 942 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | It is uncertain if antenatal corticosteroids have any effect on risk of RDS compared with usual care. | |
| 11 per 1000 | 4 per 1000 (1 to 17) | |||||
| Transient tachypnoea of the neonate (TTN) | Study population | RR 0.52 (0.25 to 1.11) | 942 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | It is uncertain antenatal corticosteroids have any effect on risk of TTN compared with usual care. | |
| 40 per 1000 | 21 per 1000 (10 to 44) | |||||
| Admission to neonatal special care (all levels) for respiratory morbidity | Study population | RR 0.45 (0.22 to 0.90) | 942 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for respiratory complications compared with usual care. | |
| 51 per 1000 | 23 per 1000 (11 to 45) | |||||
| Need for mechanical ventilation | Study population | RR 4.07 (0.46 to 36.27) | 942 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 3 | It is uncertain if antenatal steroids have any effect on the risk of needing mechanical ventilation compared with usual care. | |
| 2 per 1000 | 9 per 1000 (1 to 76) | |||||
| Neonatal hypoglycaemia | Study population | Not estimable | 0 studies | ‐ | Outcome not reported in included trial | |
| 0 per 1000 | 0 per 1000 | |||||
| Maternal mortality and severe morbidity | Study population | Not estimable | 0 studies | ‐ | Outcome not reported in included trial | |
| 0 per 1000 | 0 per 1000 | |||||
| Maternal development of postpartum infection/pyrexia in the first 72 hours | Study population | Not estimable | 942 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1, 4 | It is uncertain if antenatal steroids have any effect on the risk of maternal development of postpartum infection/pyrexia. One trial reported zero cases of postpartum infection/pyrexia in the first 72 hours. | |
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level for serious risk of bias: lack of blinding could influence outcomes
2Downgraded one level for serious imprecision: low event rate and 95% confidence interval that spans possible benefit and possible harm
3Downgraded two levels for very serious imprecision: very low event rate and wide 95% confidence interval that spans possible benefit and possible harm
4Downgraded two levels for very serious imprecision: zero events
Background
Description of the condition
The rate of babies born by caesarean section is increasing globally, particularly in high‐ and middle‐income countries (Betrān 2016). Caesarean section is a risk factor for the development of neonatal respiratory complications, mostly respiratory distress syndrome (RDS) and transient tachypnoea of the neonate (TTN), both in term and preterm infants (Dani 1999; Gerten 2005; Levine 2001; Morrison 1995; Nielsen 1984; Reed 1978; White 1985). Neonates born at term by caesarean delivery are more likely to develop respiratory morbidity than those born vaginally; this risk increases further for the subgroup of neonates born after elective caesarean section, i.e. before the onset of labour (Bowers 1982; Gerten 2005; Hansen 2007; Morrison 1995), with potentially severe implications (Roth‐Kleiner 2003). The risk decreases with advancing gestational age, and neonates born between 37 weeks and zero days' gestation (37 + 0) and 37 + 6 are at 1.7 times greater risk for respiratory complications than those born between 38 + 0 and 38 + 6, which in turn are at 2.4 times greater risk than those born between 39 + 0 and 39 + 6 (Morrison 1995). This trend is particularly pronounced for RDS, where the risk decreases from about 39/1000 for the period between 37 + 0 to 37 + 6 to about 8/1000 for the period between 39 + 0 to 39 + 6, with the odds ratio in comparison to vaginal delivery similarly decreasing from 12.9 before 39 weeks to 1.1 from 39 + 0 onwards (Zanardo 2004). There is little evidence on how mode of anaesthesia (Nielsen 1984; Van den Berg 2001) and fetal gender (Dani 1999; Van den Berg 2001) further affect this risk.
In view of this evidence, it is currently recommended that elective caesarean section should be deferred to 39 weeks' gestation (NICE 2004). However, approximately 10% to 15% of women planned for caesarean may deliver before 39 weeks, and there may be concerns about waiting in the presence of specific clinical indications or previous history. Prophylactic corticosteroids in singleton preterm pregnancies accelerate lung maturation and reduce the incidence of RDS, and administration of steroids is currently recommended between 24 and 34 weeks in cases of threatened preterm labour, antepartum haemorrhage, preterm rupture of membranes or in any condition requiring elective preterm delivery (NICE 2017; RCOG 2004; WHO 2015).
Description of the intervention
In 1972, Liggins and Howie (Liggins 1972) were the first to demonstrate the efficacy of a single course of antenatal corticosteroids in reducing the incidence of RDS in the preterm infants enrolled in their randomised control trial. Subsequently, a number of systematic reviews have been completed and have demonstrated the benefits associated with administration of single (McGoldrick 2020) and repeat course/s (Crowther 2015) of antenatal corticosteroids in women at risk of preterm birth.
The evidence for administration of corticosteroids after 35 weeks' gestation is more controversial. The most recent version of the Cochrane Review on prophylactic corticosteroids for accelerating fetal lung maturation found, in subgroup analyses, no evidence that gestational age at trial entry led to different rates of death, RDS, intraventricular haemorrhage (IVH) or birthweight. This subgroup analysis compared women whose gestational age at trial entry was less than or equal to 35 weeks + 0 days or more than 34 weeks and 0 days (McGoldrick 2020). However, there was some overlap in the gestational age subgroups and the infants included in these randomised trials were born vaginally or by caesarean section.
It remains unclear whether antenatal corticosteroid use at later gestational ages, and prior to planned caesarean section, provide discernable benefit without causing unnecessary harm. One of the randomised controlled trials included in the review on antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth (McGoldrick 2020) included a proportion of babies born at term (Gyamfi‐Bannerman 2017). This trial demonstrated a reduced rate of neonatal respiratory complications in infants who had been administered a course of antenatal corticosteroids prior to preterm delivery between 34 and 36 weeks of gestation (Gyamfi‐Bannerman 2017). However, the authors reported an increased rate of neonatal hypoglycaemia in the infants exposed to betamethasone compared to controls. The potential implications of this are concerning, given the association between prolonged symptomatic neonatal hypoglycaemia and brain injury (Burns 2008; Kerstjens 2012). There has also been some concern about the potential neurodevelopmental harms associated with antenatal corticosteroid exposure at late preterm and early term gestations (Jobe 2018; Jobe 2021; Melamed 2019; Raikkőnen 2020).
How the intervention might work
Respiratory morbidity in cases of term elective caesarean births appears to have a different pathophysiology than in preterm birth, the most likely reasons being fluid retention in the lungs (Avery 1966) and, especially, lack of the physiological catecholamine surge (Brown 1983; Irestedt 1984). Interestingly, recent evidence indicates that, apart from the traditional mechanical concept of 'vaginal squeeze', molecular mechanisms (predominantly lung epithelial sodium channels) promote alveolar fluid drainage, and these channels may be underactive in fetuses not exposed to the process of labour (Jain 2006). Glucocorticoids (a class of corticosteroids) appear to increase the number and function of sodium channels, as well as the responsiveness to catecholamines and thyroid hormones (Jain 2006), providing a rationale for their exogenous administration in cases of elective caesarean.
Why it is important to do this review
The practical impact of routine steroid administration in scheduled caesarean delivery at term is important to assess, given that caesarean births represent 30% to 40% of all births in some countries (McClure 2007), and approximately half of these are elective at term. After the publication of the Antenatal Steroids for Term Elective Caesarean Section (ASTECS) trial (Stutchfield 2005) and the original version of this Cochrane Review (Sotiriadis 2009), a recommendation for prophylactic corticosteroids before elective cesarean section at term was introduced in the now archived Royal College of Obstetricians and Gynaecologists (RCOG) Green‐top Guideline No. 7 (RCOG 2010). The recommendation was not repeated in the currently active National Institute for Health and Care Excellence (NICE) guidelines on preterm birth (NICE 2017) or caesarean section (NICE 2021). The only scientific body that currently considers this practice is the International Federation of Gynaecology and Obstetrics (FIGO) (FIGO 2019). Meanwhile, a follow‐up of the ASTECS study reported that children exposed to antenatal corticosteroids were slightly more likely to be in the lower quartile of academic ability at school than non‐exposed children (Stutchfield 2013), and much attention has been drawn to the potential of antenatal corticosteroids to cause neonatal hypoglycaemia in late‐preterm neonates (Gyamfi‐Bannerman 2017). Therefore, it is timely to update the evidence and reassess the overall effect of antenatal steroids before elective caesarean section at term.
Objectives
The objective of this review was to assess the effect of prophylactic corticosteroid administration before elective caesarean section at term, as compared to usual management (which includes placebo or no treatment), on fetal and neonatal morbidity and maternal morbidity. We also assessed the impact of the treatment on the child in later life.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials comparing prophylactic antenatal corticosteroid administration with placebo or with no treatment, given before elective caesarean section at term (at or after 37 + 0 weeks of gestation). Quasi‐randomised and cluster‐randomised controlled trials were also eligible for inclusion. We did not consider emergency caesarean deliveries at term, as steroid administration is not an appropriate intervention for these cases. Cross‐over trials were not eligible for inclusion.
Types of participants
We included women with singleton or twin pregnancies at term (37 + 0 weeks or more) who underwent elective caesarean section under general or regional anaesthesia. We did not consider triplet pregnancies due to their low prevalence and low likelihood to reach term.
Types of interventions
We included prophylactic maternal corticosteroid administration compared with placebo or no treatment.
Types of outcome measures
For the fetus/neonate
Respiratory distress syndrome (RDS) (as defined by the authors of primary reports)
Transient tachypnoea of the neonate (TTN) (as defined by the authors of primary reports)
Admission to neonatal special care for respiratory morbidity (all levels of care or neonatal intensive care unit (NICU))
Need for mechanical ventilation
Neonatal hypoglycaemia (defined as a blood glucose of less than 2.6 millimoles per litre (mmol/L))
For the woman
Maternal mortality and severe morbidity
Maternal development of postpartum infection/pyrexia in the first 72 hours
For the fetus/neonate
Admission to neonatal special care for any indication (all levels of special care or NICU)
Development of neonatal respiratory complications (pneumonia, air leak syndrome)
Neonatal infectious morbidity (e.g. systemic infection in the first 48 hours of life or whilst on the NICU)
Surfactant use
Perinatal death
Chronic lung disease (need for oxygen supplementation beyond 28 days of life)
Length of stay in the NICU
Duration of mechanical ventilation
Readmission for respiratory problems after initial discharge
Long‐term infantile morbidity
Survival free of neurodevelopmental disability (defined as one or more of the following: cerebral palsy, deafness, blindness, developmental delays/intellectual impairment (Mental Developmental Index (MDI) or Psychomotor Development Index (PDI) less than 70))
Cognitive impairment (as defined by authors)
Emotional and behavioural problems
For the woman
Adverse maternal effects of therapy
Different levels of neonatal special care may not be directly comparable in different reports. In the context of this review, we considered the definitions of the American Pediatric Association (specialty neonatal care ‐ level II (resuscitation and stabilisation of preterm and/or ill infants before transfer to a facility at which newborn intensive care is provided) versus subspecialty neonatal care ‐ level III (NICU)) (Stark 2004) and the British Association of Perinatal Medicine (special care versus high dependency and intensive care) (BAPM 2001).
Search methods for identification of studies
The following section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (20 January 2021). The Register is a database containing over 27,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register — including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service — please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (which includes the results of the centralised search of the WHO International Clinical Trials Registry Platform (ICTRP));
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections ('Included studies', 'Excluded studies', 'Studies awaiting classification' or 'Ongoing studies').
In addition, we searched ClinicalTrials.gov for unpublished, planned and ongoing trial reports (20 January 2021), using the search methods described in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Sotiriadis 2018. For this update, the following methods were used for assessing the new studies that were identified as a result of the updated search. The following section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
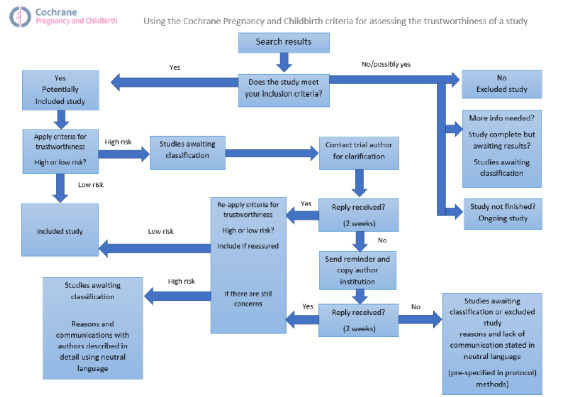
Screening eligible studies for trustworthiness
All studies meeting our inclusion criteria were evaluated by at least two review authors against predefined criteria to select studies that, based on available information, were deemed to be sufficiently trustworthy to be included in the analysis. The trustworthiness screening tool was developed by Cochrane Pregnancy and Childbirth and contains the following criteria.
Research governance
Was the study prospectively registered (for those studies published after 2010) If not, was there a plausible reason?
When requested, did the trial authors provide/share the protocol and/or ethics approval letter?
Did the trial authors engage in communication with the Cochrane Review authors within the agreed timelines?
Did the trial authors provide individual participant data upon request? If not, was there a plausible reason?.
Baseline characteristics
Is the study free from characteristics of the study participants that appear too similar (e.g. distribution of the mean and standard deviation (SD) excessively narrow or excessively wide, as noted by Carlisle 2017)?
Feasibility
Is the study free from characteristics that could be implausible (e.g. large numbers of women with a rare condition (such as severe cholestasis in pregnancy) recruited within 12 months)?
In cases with (close to) zero losses to follow‐up, is there a plausible explanation?
Results
Is the study free from results that could be implausible (e.g. massive risk reduction for main outcomes with small sample size)?
Do the numbers randomised to each group suggest that adequate randomisation methods were used (e.g. is the study free from issues such as unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods, if the authors say ‘no blocking was used’ but still end up with equal numbers, or if the authors say they used ‘blocks of 4’ but the final numbers differ by 6)?
Studies assessed as being potentially ‘high risk’ were not included in the review. Where a study was classified as ‘high risk’ we attempted to contact the study authors to address any possible lack of information/concerns. In cases where we could not obtain contact details for the study authors, or where adequate information remained unavailable, the study was categorised as ‘awaiting classification’ and the reasons and communications with the author (or lack of) were described in detail.
Abstracts
Data from abstracts will only be included if, in addition to the trustworthiness assessment, the study authors have confirmed in writing that the data to be included in the review have come from the final analysis and will not change. If such information is not available/provided, the study will remain in ‘awaiting classification’ (as above).
See Figure 1 for details of how we applied the trustworthiness screening criteria.
1.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors independently extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy. When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods used to blind outcome assessment as being at low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
We used the mean difference (MD) if outcomes were measured in the same way between trials. In future updates, should different instruments have been used to measure the same continuous outcome in different ways we will use the standardised mean difference (SMD) with 95% CIs, with the following interpretations.
SMD 0.8 or greater = large effect.
SMD greater than 0.49 and less than 0.8 = medium effect.
SMD greater than 0.19 and less than 0.5 = small effect.
SMD less than 0.2 = trivial or no effect.
Unit of analysis issues
The unit randomisation was per woman. In future updates, if we identify trials with twin pregnancies, we will use the number of babies as the denominator.
If we identify trials with more than two arms in future updates, we will take appropriate steps to include all possible pairwise comparisons in the analysis. For example, in a trial with two corticosteroids groups and one control group we would add the two intervention arms together to compare against the control arm for binary outcomes. To avoid double‐counting participants in an analysis of continuous data we would divide the denominator in the control arm by the number of different intervention arms and compare each control group to the separate intervention groups.
Cluster‐randomised trials
No cluster‐randomised trials were identified for inclusion. In future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We will adjust their standard errors (SEs) using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible) or from a similar trial. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
In cases where trial data were missing we contacted the trial authors to ask for missing data. For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We did not identify enough studies to combine in a meta‐analysis, but in future updates where more data are available we will assess heterogeneity in each meta‐analysis using the I² and Chi² statistics and visual inspection of forest plots. We will use the following guidance from the Cochrane Handbook of Systematic Reviewsof Interventions to interpret the I² statistic (Higgins 2020).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
If we identify substantial heterogeneity we will use a random‐effects model to conduct the analysis and attempt to explain possible sources of heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it using formal statistical tests.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We did not identify enough studies to carry out meta‐analysis, but in future updates we will use fixed‐effect analysis for combining data where it is reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar.
If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and the results will be presented as the average treatment effect with 95% CIs and the estimate of I².
Subgroup analysis and investigation of heterogeneity
We defined an a priori subgroup analysis to investigate if there are differences between different types of corticosteroids (betamethasone and dexamethasone) and gestational age at birth (37 + 0 to 37 + 6 weeks; 38 + 0 to 38 + 6 weeks; 39 + 0 weeks or later) in neonatal respiratory outcomes because of their different biologic mode of action. In this review, the antenatal corticosteroids were specifically administered 24 to 48 hours before scheduled birth. As the allocation to treatment or placebo was random throughout 37 to 39 weeks, we have no reason to expect bias in allocation according to gestational age. In this context, this subgroup comparison was not postrandomisation.
We did not identify enough studies to undertake subgroup analysis, but in future updates we will assess the following outcomes in subgroup analyses.
Respiratory distress syndrome (as defined by the authors of primary reports).
Transient tachypnoea of the neonate (as defined by the authors of primary reports).
Admission to neonatal special care for respiratory morbidity (all levels of care or NICU).
Need for mechanical ventilation.
We will assess subgroup differences by interaction tests available within Review Manager 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses by removing studies from the analysis which had high risk of bias in terms of random sequence generation, allocation concealment or incomplete outcome data. However, there were too few studies included in this update to carry out any meaningful sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
For this update we used the GRADE approach, as outlined in the GRADE handbook, in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparison, antenatal corticosteroids versus no steroids.
Respiratory distress syndrome (as defined by the authors of primary reports).
Transient tachypnoea of the neonate (as defined by the authors of primary reports).
Admission to neonatal special care for respiratory morbidity (all levels of care or NICU).
Need for mechanical ventilation.
Neonatal hypoglycaemia.
Maternal mortality and severe morbidity.
Maternal development of postpartum infection/pyrexia in the first 72 hours.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (RevMan 2014) in order to create a summary of findings table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
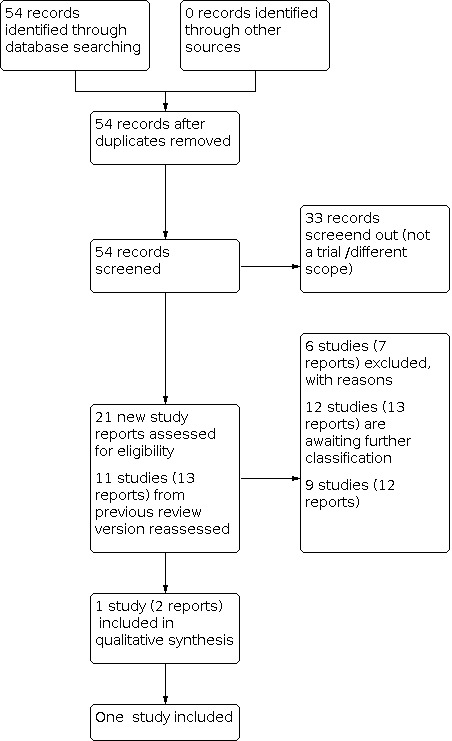
See Figure 2 for a full description of the study identification process.
2.
Study flow diagram
For this review update, we retrieved 21 new study reports to assess. We also reassesed all 11 studies (13 reports) in the previous version of the review. We included one study (two reports) and excluded six (seven reports). Nine studies (12 reports) are ongoing and 12 studies (13 reports) are awaiting classification.
Screening eligible studies for trustworthiness
We categorised 12 studies as awaiting classification because they did not fulfil our trustworthiness criteria (see Characteristics of studies awaiting classification). From the 13 eligible studies identified from our search and the four included studies in the previous version of the review, we judged that 12 studies did not meet our trustworthiness criteria for the following reasons.
Ten studies published since 2010 did not provide a plausible reason for not having prospective trial registration (Ahmed 2015; Afzal 2019; Ammar 2013; Elewa 2020; Ismail 2017; Kurt 2019; Nada 2016; Nabil 2020; Nooh 2018; Sadiq 2019).
One study had authors who have not responded to our queries seeking clarification about their outcome data (Elbohoty 2020).
One study was published only as an abstract and the authors have not confirmed that the data were from the final analysis (Kholeif 2010).
Included studies
One study met our inclusion criteria and our trustworthiness criteria (Stutchfield 2005). In this study 998 women with singleton pregnancies were randomised; 942 women and 942 infants were included in the analysis. For further details see Characteristics of included studies.
Design
The study was a two‐arm, parallel‐group randomised controlled trial.
Setting
The study took place in ten hospitals the UK.
Participants
The study included women with singleton pregnancies, undergoing a planned elective cesarean section at or after 37 weeks' gestation.
Interventions and comparators
The women in the treatment group received two doses of 12 mg of betamethasone, administered intramuscularly 24 hours apart, 48 hours before delivery. The women in the comparator group received treatment as usual without antenatal steroids.
Outcomes
The study reported all four of our primary outcomes and several of our secondary outcomes. We did not identify any evidence relating to chronic lung disease, duration of mechanical ventilation, maternal development of postpartum infection/pyrexia, trauma infection, long‐term infantile morbidity or survival free of neurodevelopmental disability.
Dates of study
The study took place from February 1995 to December 2002.
Funding sources
The study received funding from the UK's National Health Service.
Declarations of interest
The authors of Stutchfield 2005 declared that they had no competing interests.
Ongoing studies
We identified nine ongoing studies (see Characteristics of ongoing studies).
Excluded studies
We excluded six studies (see Characteristics of excluded studies). In two of these, the women undergoing caesarean section were not at term gestations (Christofori 2011; Ontela 2018). Three studies had differences in gestational ages of participants among the treatment and control groups (Koch 2016; Sananes 2017). One study was a comparison between two different corticosteroids (Issa 2019). One study (Jain 2005) was terminated due to slow enrolment and lack of funding. No trial data were available to include in this review.
Risk of bias in included studies
Figure 3 illustrates the risks of bias, which are explained in more detail below.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We judged Stutchfield 2005 to be at low risk of bias for random sequence generation because it used a random number generator to allocate women to treatment groups.
Allocation concealment
We judged the included study to be at low risk of bias for allocation concealment because the list of treatment allocation was kept centrally and was concealed from all participants.
Blinding
Performance bias
We judged the included study to be at high risk of bias for blinding of participants and personnel because no placebo was used in the control group, therefore women and the people involved in their care were aware of their treatment allocation, which could have had an influence on outcomes.
Detection bias
We judged the study to be high risk of bias for blinding of outcome assessment because the assessors were not blinded to treatment allocation, which could have had an influence on outcomes.
Incomplete outcome data
We judged Stutchfield 2005 to be at low risk of bias for incomplete outcome data because attrition was low and non‐differential.
Selective reporting
We judged the included study to be at unclear risk of bias for selective reporting because there was no published trial protocol, and it is not clear which outcomes were prespecified and whether all the planned outcomes were reported in full.
Other potential sources of bias
We judged the study to be at low risk of other potential sources of bias because there was nothing in the trial report to suggest any other potential biases.
Effects of interventions
See: Table 1
This review includes one study and therefore meta‐analysis was not possible.
Antenatal corticosteroids (betamethasone) versus usual care
Primary outcomes
Respiratory distress syndrome (RDS) (as defined by the authors of primary reports)
It is uncertain if antenatal corticosteroids have any effect on the risk of RDS compared with usual care (risk ration (RR) 0.34, 95% CI 0.07 to 1.65; 1 study; 942 infants; low‐certainty evidence; Analysis 1.1; Table 1). The certainty of evidence is low and the 95% CI is consistent with possible benefit and possible harm.
1.1. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 1: Respiratory distress syndrome
Transient tachypnoea of the neonate (TTN) (as defined by the authors of primary reports)
It is uncertain if antenatal corticosteroids reduce the risk of TTN compared with usual care (RR 0.52, 95% CI 0.25 to 1.11; 1 study; 942 infants; low‐certainty evidence; Analysis 1.2; Table 1) because the certainty of evidence is low and the 95% CI is consistent with possible benefit and possible harm. The proportion of infants with TTN after treatment with antenatal corticosteroids was 2.1%, compared with 4% in the usual care group.
1.2. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 2: Transient tachypnoea of the neonate
Admission to neonatal special care for respiratory morbidity (all levels of care or neonatal intensive care unit (NICU))
Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for respiratory complications compared with usual care (RR 0.45, 95% CI 0.22 to 0.90; 1 study; 942 infants; moderate‐certainty evidence; Analysis 1.3; Table 1). The proportion of infants admitted to neonatal special care for respiratory morbidity after treatment with antenatal corticosteroids was 2.3%, compared with 5.1% in the usual care group.
1.3. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 3: Admission to neonatal special care (all levels) for respiratory morbidity
The included study (Stutchfield 2005) also reported a lower risk of admission to the NICU (RR 0.15, 95% CI 0.03 to 0.64, 1 study; 942 infants; Analysis 1.4).
1.4. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 4: Admission to neonatal intensive care unit for respiratory morbidity
Need for mechanical ventilation
It is uncertain if antenatal steroids have any effect on the risk of needing mechanical ventilation compared with usual care (RR 4.07, 95% CI 0.46 to 36.27; 1 study; 942 infants; very low‐certainty evidence; Analysis 1.5; Table 1), because the certainty of evidence is very low and the 95% CI is consistent with possible benefit and possible harm.
1.5. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 5: Need for mechanical ventilation
Hypoglycaemia (blood glucose less than 2.6 mmol/L)
This outcome was not reported.
Maternal mortality and severe morbidity
This outcome was not reported.
Maternal development of postpartum infection/pyrexia in the first 72 hours
We are uncertain about the effect of antenatal corticosteroids on the maternal development of postpartum infection/pyrexia in the first 72 hours; Stutchfield 2005 reported zero cases (942 women, very low‐certainty evidence; Analysis 1.6, Table 1).
1.6. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 6: Maternal development of postpartum infection
Secondary outcomes
Admission to neonatal special care for any indication (all levels of special care or NICU)
It is uncertain if antenatal steroids have any effect on the risk of admission to neonatal special care for any indication (RR 0.81, 95% CI 0.49 to 1.33; 1 study; 942 infants; Analysis 1.7, Table 1).
1.7. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 7: Admission to neonatal special care (all levels) for any indication
Development of neonatal respiratory complications (pneumonia, air leak syndrome)
This outcome was not reported.
Neonatal infectious morbidity
There were no cases of sepsis in either the corticosteroids group (467 infants) or the usual care group (475 infants); Analysis 1.8.
1.8. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 8: Neonatal infectious morbidity
Surfactant use
This outcome was not reported.
Perinatal death
There were no perinatal deaths in either the corticosteroids group (467 infants) or the usual care group (475 infants); Analysis 1.9.
1.9. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 9: Perinatal death
Chronic lung disease (need for oxygen supplementation beyond 28 days of life)
This outcome was not reported.
Length of stay in the neonatal intensive care unit
Antenatal corticosteroids may lead to a decrease in length of stay in the NICU (MD ‐2.14 days, 95% CI ‐2.50 to ‐1.78 days; 1 study; 942 infants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 10: Length of stay in neonatal intensive care unit (days)
Duration of mechanical ventilation
This outcome was not reported.
Readmission for respiratory problems after initial discharge
It is uncertain if antenatal corticosteroids have any effect on the risk of readmission for respiratory problems (RR 0.66, 95% CI 0.35 to 1.25; 1 study; 407 children; Analysis 1.11). These data are based on follow‐up questionnaires completed when the children in Stutchfield 2005 were between eight and 15 years old.
1.11. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 11: Readmission for respiratory problems after initial discharge
Long‐term infantile morbidity
This outcome was not reported.
Survival free of neurodevelopmental disability (defined as one or more of the following: cerebral palsy, deafness, blindness, developmental delays/intellectual impairment (Mental Developmental Index or Psychomotor Development Index less than 70))
This outcome was not reported.
Cognitive impairment (as defined by authors)
It is uncertain if antenatal corticosteroids have any effect on cognitive impairment, measured as learning difficulties (RR 0.81, 95% CI 0.49 to 1.35; Analysis 1.12; 1 study; 407 children). The learning difficulties measured were dyslexia, attention deficit hyperactivity disorder, severe learning difficulties, Asperger’s syndrome, autism, X‐linked mental retardation, global developmental delay with dysmorphic features, and Down's syndrome. These data are based on follow‐up questionnaires completed when the children in Stutchfield 2005 were between eight and 15 years old.
1.12. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 12: Cognitive impairment
Emotional and behavioural problems
It is uncertain if antenatal corticosteroids have any effect on emotional and behavioural problems, measured with the Strengths and Difficulties questionnaire (SDQ) score (ranging from zero to 25, where a higher score indicates greater difficulties) (MD 0.18, 95% CI ‐1.12 to 1.48; 1 study; 407 children; Analysis 1.13). These data are based on follow‐up questionnaires completed when the children in Stutchfield 2005 were between eight and 15 years old.
1.13. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 13: Emotional and behavioural problems: measured with Strengths and difficulties questionnaire
Adverse maternal effects of therapy
There were seven reports of adverse effects in the corticosteroid group compared to zero in the usual care group (RR 15.26, 95% CI 0.87 to 266.36; 1 study; 942 participants; Analysis 1.14). The adverse effects included generalised flushing, nausea, tenderness at the injection site and difficulty sleeping.
1.14. Analysis.

Comparison 1: Antenatal corticosteroids (betamethasone) versus usual care, Outcome 14: Adverse maternal effects of therapy
Discussion
Summary of main results
In this update we included only one trial, involving 942 women and 942 neonates. There are fewer trials in this update compared to the previous version of the review (Sotiriadis 2018) because we have assessed all the previously included trials against the Cochrane Pregnancy and Childbirth trustworthiness tool. Our results are therefore based only on data that we have judged to be trustworthy.
Antenatal corticosteroids probably reduce the risk of admission to neonatal special care for respiratory complications compared with usual care (Table 1).
Compared with usual care, it is uncertain if antenatal corticosteroids have any effect on the risk of RDS, TTN or the risk of needing mechanical ventilation because the certainty of the evidence is low (for the outcomes of RDS and TTN) or very low (for the outcome of mechanical ventilation) and the 95% CI is consistent with possible benefit and possible harm. It is uncertain if antenatal steroids have any effect on the risk of maternal development of postpartum infection/pyrexia (there were zero events) (Table 1).
In terms of our secondary outcomes, it is uncertain if antenatal corticosteroids have any effect on the risk of admission to neonatal special care, sepsis, perinatal death, readmission for respiratory problems, cognitive impairment, or emotional and behavioural problems. Antenatal corticosteroids may reduce the length of stay in the NICU. There is limited to no outcome data on substantial maternal health outcomes.
Overall completeness and applicability of evidence
Every effort was made to identify all published and unpublished randomised trial data for the use of prophylactic corticosteroids prior to elective caesarean section at term. Prophylactic antenatal corticosteroids may reduce the rates of admission to special care or NICU for respiratory complications after elective caesarean section (Stutchfield 2005). It is unclear if antenatal corticosteroids have any effect on the risk of RDS, TTN or mechanical ventilation. There is insufficient evidence to investigate if there are differences for gestational age at birth (37 + 0 to 37 + 6 weeks; 38 + 0 to 38 + 6 weeks; 39 + 0 weeks or later) in neonatal respiratory outcomes.
Currently, there are only a limited number of trials that evaluate the effects of prophylactic antenatal corticosteroid administration prior to caesarean section at term. Only one trial is included in this review (Stutchfield 2005). Therefore, there are insufficient data to draw any firm conclusions. Furthermore, this trial was undertaken over 16 years ago, and neonatal practice is likely to have changed, which could impact upon the clinical outcomes included in this review. This trial was a multicentre pragmatic study, and therefore the favourable results demonstrated may also reflect the true effect in regular clinical practice. However, as participants and outcome assessors were not blinded, and admission to a special care or intensive care unit is largely a subjective choice and may be affected by knowledge of treatment assignment, this outcome should be interpreted with caution.
Another systematic review published in 2016 included six trials comparing the use of antenatal corticosteroids with placebo or no treatment in women with a singleton pregnancy at 34 or more weeks' gestation (Saccone 2016). The population included both women expected to deliver in the late preterm period (34 + 0 to 36 + 6 weeks' gestation) and women before planned caesarean delivery at term (37 weeks' gestation or more). The authors found that administration of corticosteroids reduced neonatal respiratory morbidity. Interestingly, two of the three trials (which included women at 37 weeks' gestation or more) included in Saccone 2016 were considered for inclusion in our review, but unfortunately we were unable to confirm trustworthiness, therefore they remain in Studies awaiting classification.
Much larger numbers of participants are needed to adequately power clinical trials to evaluate the true impact of prophylactic antenatal corticosteroid administration on respiratory outcomes with associated morbidity (e.g. RDS). There is also a need to adequately assess for any maternal or neonatal harm associated with corticosteroid administration at term gestations. This is particularly important in view of the low rates of RDS and admission to NICU in this population, and therefore clinicians need to carefully consider the balance of statistical significance versus the clinical significance of the outcomes and the impact on the mother or baby.
The balance of risk is particularly important, as currently there is only limited evidence on any long‐term follow‐up of these infants. In the cohort of infants from the ASTECS trial (Stutchfield 2005), nearly twice as many betamethasone‐exposed children did not attain English proficiency (13% versus 7%), and they were twice as likely to be ranked in the lower quartile of academic ability by teachers (18% versus 9%) (Stutchfield 2013). Although this follow‐up study suffered from a high attrition rate (49%), it highlights the need for caution and for future trials to consider potential harms, both short‐ and long‐term, in their study outcomes. Further data on maternal health outcomes are also needed to provide assertions that the treatment is not harmful to women in terms of adverse effects or increased infectious morbidity.
There are currently nine studies that are ongoing and will be considered for inclusion in future updates. Encouragingly, one of the registered trials includes key clinical outcomes for both the infant and mother and has potential plans for long‐term follow‐up (Groom 2020).
Quality of the evidence
Current evidence comes from only one study. In an effort to ensure that all studies included in the review are sufficiently credible we have applied the trustworthy screening tool developed by the Cochrane Pregnancy and Childbirth Group (Figure 1). Therefore, three of the studies (Ahmed 2015; Nada 2016; Nooh 2018) included in the last version of this systematic review (Sotiriadis 2018) are awaiting classification and are not included in this update. We are awaiting further information from the trial authors particularly in relation to prospective registration, a requirement for all trials published after 2010.
The data we present here come from the only trial that met our prespecified criteria for trial trustworthiness; therefore we can be confident that our findings are based on rigorous clinical trial evidence. Our decision not to include data from three trials that were included in the previous version of this review is based on concerns about the rigour and conduct of those trials. Similar concerns were raised in a recent article specifically examining the trustworthiness of the same trials (Mol 2021). Mol and colleagues have approached the investigators and their institutions and have received no documentation to confirm the findings presented in the published reports of the trials. Our own approaches to the study investigators have also failed to produce a response.
Furthermore, we decided not to include a further nine studies that met our inclusion criteria in terms of population, intervention, comparator and study design. The investigators of these studies have not yet responded to our attempts to seek clarification regarding various aspects of study conduct.
Unresolved queries about data integrity and lack of prospective trial registration are our main concerns about the trials whose data we have not used in this review. Where we have asked for further information about data presented in a conference abstract or in a full trial report, for example the cause of death of infants who died in a study, we are still waiting for a response. In the absence of adequate assurance about data provenance, we deemed it appropriate not to use these data in our analysis. Similarly, data from trials that were not registered before the investigators began to enrol participants may be problematic. Despite the long‐standing recognition of the important role of prospective trial registration in reducing the risk of selective outcome reporting (De Angelis 2004; Simes 1986), compliance with prospective registration remains at only 41.7% according to a recent analysis of 10,500 randomised controlled trials (Al‐Durra 2020). Without prospective registration it is very difficult to assess whether completed, published trials have reported their results in full. Systematic reviews and clinical guidelines that include evidence from trials whose integrity is uncertain can in turn lead to patients being put at risk if interventions are implemented without reliable evidence of their effectiveness.
The overall certainty of the evidence for the primary outcomes was found to be low and very low (Table 1), apart from the outcome of admission to neonatal special care (all levels) for respiratory morbidity, which was of moderate certainty. The main concern with the included trial is the high risk of performance bias, as both participants and health professionals were aware of their group allocation. Although all outcomes may be affected, it is likely that more subjective management decisions or assessments of outcome are more susceptible to bias arising from lack of blinding, as compared to more objective ones (Higgins 2008). Admission of neonates to special care is one such example; clinicians potentially biased in favour of the intervention may have been more likely to organise admission to NICU for babies not exposed to corticosteroids. Such bias was possible, but less likely, for the radiological diagnosis of RDS or TTN by independent specialists. Similarly, admission to lower levels of special care may be more prone to bias than referral to NICU, as the latter would require more clinical and laboratory findings.
The limitations of the evidence are reflected in its assessment according to GRADE. As shown in Table 1, for the outcomes of RDS and TTN, the certainty of the evidence was downgraded by one level for study limitations and one level for imprecision. Therefore, the certainty of the evidence for these outcomes was judged as low. For the outcome of NICU admission, we downgraded by one level for study limitations, therefore the certainty of the evidence is judged as moderate. We additionally downgraded by one level for imprecision for the outcome of need for mechanical ventilation, therefore the certainty for this outcome was judged as very low. In practical terms, this means that the true effect may be (or it is likely to be, for the outcome of need for mechanical ventilation) substantially different from our estimates, and the results should therefore be interpreted with caution.
A further potential concern is the lack of long‐term outcomes in the studies. This could result in preferentially favouring antenatal corticosteroid administration, when potential long‐term harms remain unknown.
Potential biases in the review process
We endeavoured to minimise the potential bias of the review process by ensuring an up‐to‐date search was undertaken to try and identify all relevant trials pertinent to the review. Two or more review authors independently appraised and extracted the trial data required and the data extraction process was complete and without missing data that would potentially exclude eligible studies. In the rare case where data were missing, or in order to adequately assess the risk of bias of the trial, we sought additional data from the trial authors who responded on average in a timely and clear manner.
Two of the review authors independently conducted the GRADE assessment of the certainty of evidence and four of the review authors independently applied the trustworthiness criteria to the studies that met our inclusion criteria. We acknowledge that there may be an element of subjectivity in both the GRADE assessment and in assessing trustworthiness, but we made every effort to minimise any risk of bias in this respect by ensuring that we carried out these steps independently and with referral to a Cochrane Pregnancy and Childbirth editor, where necessary, in order to reach consensus.
Agreements and disagreements with other studies or reviews
The favourable effect of antenatal corticosteroids on fetuses at risk for preterm birth has been reported in multiple trials and systematic reviews. Steroids were first tried as a means to accelerate fetal lung maturation and reduce perinatal respiratory morbidity; the latest update of the relevant Cochrane Review reaffirms that antenatal steroids reduce the incidence of RDS (RR 0.71, 95% CI 0.65 to 0.78) and moderate/severe RDS (RR 0.70, 95% CI 0.59 to 0.83) for preterm birth. In contrast, the evidence is uncertain with regard to the risk of chronic lung disease (RR 0.86, 95% CI 0.41 to 1.79) (McGoldrick 2020). It remains uncertain whether antenatal corticosteroids are equally effective in reducing neonatal respiratory morbidity in term infants delivered by elective caesarean section.
Apart from respiratory morbidity, antenatal corticosteroids in fetuses at risk for preterm birth also reduce neonatal mortality and severe neonatal morbidity, including necrotising enterocolitis and intraventricular haemorrhage), without increasing maternal and perinatal complications (McGoldrick 2020; Sotiriadis 2015). Moreover, there is some evidence, partly derived from non‐randomised trials, that steroids in fetuses at risk for preterm birth can also reduce severe neurological morbidity in childhood (Sotiriadis 2015; McGoldrick 2020).
In contrast, a recent population‐based cohort study has raised some concern that administration of antenatal corticosteroids at term gestations is associated with mental and behavioural disorders in the exposed children (Raikkőnen 2020). Despite some inherent limitations with this study (Raikkőnen 2020), this concurs with the cohort of infants from the ASTECS trial (Stutchfield 2005), who were followed up into childhood and were more likely to be ranked in the lower quartile of academic ability by their teachers (Stutchfield 2013). This evidence is certainly not conclusive and merits further investigation and consideration in any future randomised trials.
A common clinical question is whether either betamethasone or dexamethasone is superior to the other regarding their effectiveness and safety profile. The paradigm of fetuses at risk for preterm birth shows that there are no substantial differences between the two agents and the sample sizes for dexamethasone are usually much smaller than those for betamethasone, resulting in wide confidence intervals for the former drug (ASTEROID 2019). As only betamethasone was tested in our review, we cannot draw conclusions for comparative effectiveness. This comparison is also included in another Cochrane Review (Brownfoot 2013).
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to draw any definite conclusions. However, evidence from one randomised controlled trial suggests that prophylactic corticosteroids before elective caesarean section at term probably reduce the risk of admission to neonatal intensive care unit (NICU) for respiratory morbidity. It is uncertain if administration of corticosteroids has any impact on the rates of respiratory distress syndrome (RDS), transient tachypnoea of the neonate (TTN) or the need for mechanical ventilation.
There is limited evidence on maternal health outcomes to provide assurances that this treatment is not harmful to women in terms of adverse effects or increased risk of infectious morbidity.
Implications for research.
More evidence is needed to investigate the effect of prophylactic corticosteroids prior to caesarean section on the incidence of serious respiratory morbidity. It is important that any future trials include relevant maternal and neonatal outcomes, to facilitate an overall assessment of whether any potential benefit outweighs any serious harm to both the mother and the infant. Trials should also consider longer‐term follow‐up of infants into childhood, to provide reassurance about any neurodevelopmental effects.
Further research could consider assessing the effectiveness of antenatal corticosteroids at different gestational age thresholds at the time of caesarean section, to investigate if there is a differential effect.
There are nine studies that are currently ongoing and could be included in future updates.
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2022 | Amended | Edited the abstract main results section to correct a typograhpical error (changed severe mortality to severe morbidity) |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 20 January 2021 | New search has been performed | Search updated. All potentially eligible studies and studies already included in the review were assessed for trustworthiness. Three studies that were in the previous version of the review did not meet Pregnancy and Childbirth trustworthiness criteria and have not been included in this update. None of the studies identified in the search update met the trustworthiness criteria. No new studies added. |
| 22 June 2020 | New citation required but conclusions have not changed | Conclusions unchanged after removing data from studies that did not meet Pregnancy and Childbirth trustworthiness criteria. |
| 3 May 2011 | Amended | Corrected typo. |
Acknowledgements
The authors cordially thank Dr Peter Stutchfield of the ASTECS group for providing non‐published data on subgroup analysis by gestational age and clarifications on published data, and Dr Adel Nada for answering additional queries.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and Cochrane Pregnancy and Childbirth's Statistical Advisor. The authors are grateful to the following peer reviewers for their time and comments: Jim Thornton, University of Nottingham, UK; Joshua Vogel, Burnet Institute, Melbourne, Australia.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods for ClinicalTrials.gov
Advanced search
Interventional Studies | cesarean | Corticosteroid
Interventional Studies | cesarean | steroids
Interventional Studies | cesarean | Dexamethasone
Interventional Studies | cesarean | Betamethasone
Appendix 2. Correspondence with authors
AFZAL
Dear Dr Munawar Afzal,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term.
Your study; Effect of Prophylactic Antenatal Corticosteroid and Incidence of Neonatal Respiratory Morbidity after Elective Cesarean Section in Patients having Previous Cesarean Section, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Ethics approval: was ethical approval obtained prior to undertaking the trial? If so, please could you let us know from whom (i.e. National, Institutional; Hospital or University) and please send us a copy of the approval letter.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Kind regards,
Emails sent 7 February 2021, 21 February 2021
AHMED
ANMAR
Dear Dr Rabei,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term once again.
Your study; Dexamethasone in prevention of respiratory morbidity in elective caesarean section in term fetus. A randomized control trial, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Kind regards,
Emails sent 7 February 2021, 21 February 2021
Email to authors of the journal where the trial was published;
Dear Editor,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term.
In 2013 you published a study entitled; Dexamethasone in prevention of respiratory morbidity in elective caesarean section in term fetus. A randomized control trial, 2013,9(6) by Ahmed Rushdi Ammar et al. This trial meets our inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries. We are struggling to contact the corresponding author and wondered if you had any additional email/contact details please or if you could forward this email on.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Kind regards,
Email sent 21 February 2021
ELBOHOTY
Dear Dr. Ayman S. Dawood,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term once again.
Your study; The neonatal outcomes of Dexamethasone administration before scheduled cesarean delivery at term: a randomized clinical trial, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Study population: Would you be able to provide more detail of the baseline characteristics of the antenatal corticosteroid group and the control group please.
Kind regards,
Email sent 7 March 2021
Response from author;
Dear sir
Firstly, Thanks so much for your interest in our article
Secondly, its a great Honour to be included in cochrane review
Regarding the items you inquire about:
1‐ clinical trial protocol developed prior to the trial being undertaken as seen on the UMIN‐CTR on 2017/09/08 and a copy is available on the link: https://upload.umin.ac.jp/cgi-bin/ctr_e/ctr_view_reg.cgi?recptno=R000033247
2‐The corresponding registration number: UMIN000029070
3‐Each patient was given a concealed envelope containing the allocated group. This study was double blinded where both patients and researchers were not informed by the given drugs. Allocation was 1:1 ratio.
4‐Regarding patients lost from follow up were 25 patients with 12 in one group and 13 in the other group.
5‐Study population were selected according to inclusion and exclusion criteria. (refer to article)
At the end we hope to include our article in your respected review.
Thanks
Ayman Shehata Dawood
Corresponding author
Further correspondence;
Dr. Ayman S. Dawood,
Thank you very much for your reply. We had a few additional questions that we were hoping you could answer.
We would be very grateful if you could clarify what antenatal corticosteroid regimen you used as there seems to be a slight discrepancy between what is stated in the abstract and the regimen stated in the methods section of the manuscript. It would appear that the regimens used in the Dexamethasone and placebo groups differ. We would be grateful to know what the rationale behind this was please.
It would appear that unfortunately 3 infants in both the dexamethasone and the control group died. Do you have any further information about the cause of death in these infants.
Additionally, the rates of RDS and TTN appear to be quite high in both groups. Do the authors have any comments on this.
Finally, it appears that there are some differences between the study protocol and the trial with respect to inclusion and exclusion criteria and study outcomes. Do the authors have any comments on this.
Kind regards,
Email sent 14 March 2021
ELEWA
Dear Dr Al Saber,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term once again.
Your study; Does Corticosteroids Administration after 37 Weeks for Elective Lower Segment Cesarean Section Reduce Neonatal Respiratory Morbidity? a Randomized Controlled Trial, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Randomisation process: it appears that consent was also completed after randomisation; is this correct and what was the rationale behind this?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Kind regards,
emails sent 7 March 2021, 20 September 2021
GROOM
Dear Professor Groom
I am updating the Cochrane review “Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term” (https://doi.org/10.1002/14651858.CD006614.pub3). We have identified your trial “The C*STEROID Feasibility Study: Corticosteroids before planned caesarean section from 35+0 to 39+6 weeks” as eligible.
I realise that your trial includes women from 35+0 to 39+6 weeks but the focus of our review is only on women at 37+0 weeks and beyond. We would be grateful for any preliminary data you are able to provide us on the following outcomes:
Respiratory distress syndrome (RDS) (as defined by the authors of primary reports)
Transient tachypnoea of the neonate (TTN) (as defined by the authors of primary reports)
Admission to neonatal special care for respiratory morbidity (all levels of care or intensive care unit (NICU))
Need for mechanical ventilation
Any data you could give us would be enormously valuable to the Cochrane review.
Kind regards
Response from author;
Dear Roses
The C*STEROID Feasibility Study has now completed recruitment but we remain blinded to results by treatment group as clinical outcomes will also contribute to the main C*STEROID Trial. This was planned a priori. The main trial has commenced recruitment and we expect a 3‐4 year timeline to complete. We will be happy to share results once the trial is completed and results analysed.
Best wishes
Katie
ISMAIL
Dear Dr Akram Elkhier Hassan,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term.
Your study; Effect of Prophylactic Corticosteroid Therapy on Respiratory Morbidity in Infants Borne at Term by Elective Cesarean Section at Omdurman Maternity Hospital, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation: participants were allocated based on odd and even numbers. Who performed the randomisation, allocation to study group, administration of antenatal corticosteroid and were they involved in performing outcome assessments.
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Kind regards,
Emails sent 7 February 2021, 21 February 2021
KURT
Dear Dr Begum Kurt,
Your study; Antenatal betamethasone administration before elective caesarean section in term pregnant women, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Study population: 1 in 5 women in the study suffered with chronic disease. What were these conditions?
Kind regards,
Emails sent 7 February 2021, 21 February 2021
Email to authors of the journal where the trial was published;
Dear Editor,
You published a study in your journal in December 2019 entitled; Antenatal betamethasone administration before elective caesarean section in term pregnant women (http://dx.doi.org/10.7197/cmj.vi.633752). This trial meets the inclusion criteria for our cochrane review entiltled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term, and we would very much like to include it in our analysis but we have some queries for the authors. Unfortunately we have been unable to contact them and were wondering if you have another contact email or if you could forward this email onto them please.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Study population: 1 in 5 women in the study suffered with chronic disease. What were these conditions?
Kind regards,
Email sent 21 February 2021
MIRZAMORADI
Dear Dr Zheidar,
Your study; Evaluation of the effect of antenatal betamethasone on neonatal respiratory morbidity in early‐term elective cesarean, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Trial registration: we noticed that trial registration was done after all the participants were enrolled. Can you explain the reason why the trial was not registered before enrolment of participants began?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Baseline data unexpectedly similar: The study groups were identical with respect to Race. What was done to stratify patients to facilitate comparison?
Study population; There is a significant difference in the age and gestational ages of participants between the betamethasone and control group. Is there any potential explanation to account for this or is it purely due to chance.
Kind regards,
Emails sent 7 February 2021, 21 February 2021
Email to authors of the journal where the trial was published;
Dear colleague,
We are attempting to contact Dr Zahra Heidar regarding their study; Evaluation of the effect of antenatal betamethasone on neonatal respiratory morbidity in early‐term elective cesarean. It meets the inclusion criteria for the Cochrane review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Trial registration: we noticed that trial registration was done after all the participants were enrolled. Can you explain the reason why the trial was not registered before enrolment of participants began?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Baseline data unexpectedly similar: The study groups were identical with respect to Race. What was done to stratify patients to facilitate comparison?
Study population; There is a significant difference in the age and gestational ages of participants between the betamethasone and control group. Is there any potential explanation to account for this or is it purely due to chance.
Kind regards,
Email sent 21 February 2021
NABIL
Dear Professor Nabil,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term once again.
Your study; Does antenatal dexamethasone before full term planned cesarean section affect the incidence or severity of neonatal jaundice. A randomized control trial, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Study population: it would appear that no participants withdrew from the study following randomisation and that there were no participants lost to follow up. Is this correct?
Kind regards,
Emails sent 7 February 2021, 21 February 2021
Email to the authors of the journal where the trial was published;
Dear Professor Dr Akhtar Sherin,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term.
In February 2019 your journal published an article entitled; Effects of Prophylactic Maternal Dexamethasone administration on the neonatal respiratory outcomes at term after elective caesarean section: Randomised Controlled trial. This trial meets the inclusion criteria for the Cochrane review entitled; Corticosteroids for preventing respiratory complications in the newborn after caesarean section at term and we would very much like to include it in our analysis but we have some queries. Unfortunately, we have struggled to get in contact with the lead author and wondered whether you would have any additional contact information or would be able to forward this email.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Kind regards,
Email sent 21 February 2021
Response received from editor:
Thanks for your email.
Will try to contact author for the relevant queries and get back to you soon.
Regards
Email sent 21 February 2021
NADA
Dear Dr Nada
I am one of the authors in the process of updating the Cochrane systematic review entitled ‘Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term.’
Your study ‘Antenatal corticosteroid administration before elective caesarean section at term to prevent neonatal respiratory morbidity’ was included in the last published version of the review but we have some outstanding queries about the trial.
We would be very grateful if you could answer the following:
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Randomisation process: there is an equal number of participants in both groups; what methods did you use (e.g. blocking) to ensure an equal number of women were allocated to each group?
Baseline data unexpectedly similar for age, parity and gestational age: What was done to stratify patients to facilitate comparison?
Many thanks and regards
Email sent 18 January 2021
Response from authors;
Dear Fiona
1‐ we did not registered the study but we just registered it as a thesis in AIN SHAMS UNVERSITY
2‐ WE USED BLOCKING AND COMPUTER PROGRAM FOR RANDOMISATION BY EXPERT STATICIAN
THANK YOU FOR YOUR Email
NOOH
Dear Dr Nooh,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term once again.
Your study; Does implementing a regime of dexamethasone before planned cesarean section at term reduce admission with respiratory morbidity to neonatal intensive care unit? A randomized controlled trial. A randomized control trial, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Emails sent 2018, 18 January 2021
SADIQ
Dear Dr Sadiq,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term.
Your study; Effects of Prophylactic Maternal Dexamethasone administration on the neonatal respiratory outcomes at term after elective caesarean section: Randomised Controlled trial, meets the inclusion criteria for the review and we would very much like to include it in our analysis but we have some queries.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Kind regards,
Emails sent 7 February 2021, 21 February 2021
Email to authors of the journal where the trial was published;
Dear Professor Dr Akhtar Sherin,
We are in the process of updating the Cochrane systematic review entitled; Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term.
In February 2019 your journal published an article entitled; Effects of Prophylactic Maternal Dexamethasone administration on the neonatal respiratory outcomes at term after elective caesarean section: Randomised Controlled trial. This trial meets the inclusion criteria for the Cochrane review entitled; Corticosteroids for preventing respiratory complications in the newborn after caesarean section at term and we would very much like to include it in our analysis but we have some queries. Unfortunately, we have struggled to get in contact with the lead author and wondered whether you would have any additional contact information or would be able to forward this email.
We would be very grateful if you could answer the following:
Clinical trial protocol: was a clinical trial protocol developed prior to the trial being undertaken? If so, please can you provide us with a copy?
Trial registration: was the trial prospectively registered? If so, please could you let us know where the trial was registered and the corresponding registration number.
Kind regards,
Email sent 21 February 2021
STUTCHFIELD
Email sent for 2018 update and response received.
Data and analyses
Comparison 1. Antenatal corticosteroids (betamethasone) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Respiratory distress syndrome | 1 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.65] |
| 1.1.1 Birth before 38 + 0 weeks | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.09] |
| 1.1.2 Birth 38 + 0 to 38 + 6 weeks | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.80] |
| 1.1.3 Birth at or after 39 + 0 weeks | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.96] |
| 1.2 Transient tachypnoea of the neonate | 1 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.25, 1.11] |
| 1.2.1 Birth before 38 + 0 weeks | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.45, 34.45] |
| 1.2.2 Birth 38 + 0 to 38 + 6 weeks | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.61] |
| 1.2.3 Birth at or after 39 + 0 weeks | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.28, 9.78] |
| 1.3 Admission to neonatal special care (all levels) for respiratory morbidity | 1 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.90] |
| 1.3.1 Birth before 38 + 0 weeks | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.16, 1.57] |
| 1.3.2 Birth 38 + 0 to 38 + 6 weeks | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.17, 1.14] |
| 1.3.3 Birth at or after 39 + 0 weeks | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.50] |
| 1.4 Admission to neonatal intensive care unit for respiratory morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Need for mechanical ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 Maternal development of postpartum infection | 1 | 942 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7 Admission to neonatal special care (all levels) for any indication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 Birth before 38 + 0 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.2 Birth 38 + 0 to 38 + 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.3 Birth at or after 39 + 0 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8 Neonatal infectious morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.9 Perinatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Length of stay in neonatal intensive care unit (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 Readmission for respiratory problems after initial discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.12 Cognitive impairment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.13 Emotional and behavioural problems: measured with Strengths and difficulties questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14 Adverse maternal effects of therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Stutchfield 2005.
| Study characteristics | ||
| Methods | Parallel randomised control trial | |
| Participants | Location: 10 hospitals in the UK Timeframe: February 1995 to December 2002 Eligibility: women with a singleton pregnancy undergoing a planned elective cesarean section at or after 37 weeks' gestation Exclusion: women with severe maternal hypertension, history of peptic ulceration, severe fetus rhesus sensitisation and evidence of intrauterine infection were excluded Number randomised: 998 (A 503, B 495) Number analysed: 942 (A 467, B 475) Stutchfield 2013: follow‐up of participants (now aged 8‐15 years) from the 4 largest recruiting centres from the original trial |
|
| Interventions | Intervention (n = 503): 2 doses of 12 mg of betamethasone administered intramuscularly 24 hours apart, 48 hours before delivery Control (n = 495): treatment as usual (without antenatal corticosteroids) |
|
| Outcomes | Primary outcome: admission to special care baby unit with respiratory distress Secondary outcomes
Stutchfield 2013: assessment of long‐term behavioural, cognitive or developmental outcome, and the risk of asthma or atopic disease using questionnaires and general health and school performance |
|
| Notes | Funding source of study: "This study was partially funded by the North Wales Small Grant Committee and Welsh Children and Young People’s Research Network (CYPRN)." Declarations of interest: "All authors have the support of Betsi Cadwaladr University Health Board for the submitted work but no other competing interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation through a random number generator in MS Excel at the trial centre |
| Allocation concealment (selection bias) | Low risk | The list of treatment allocation (centrally kept) was concealed from all participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel were not blinded to the participants group allocation potentially influencing outcomes. Participants were not blinded to their group allocation, which could impact on maternal outcomes reported but is unlikely to influence other outcomes. Stutchfield 2013: unclear risk of bias as no comment on whether professionals (teachers) were aware of treatment allocation. Participants (mothers) were aware of group allocation, which could influence the outcome (survey response). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the participants' group allocation, potentially influencing outcomes. Participants were not blinded to their group allocation, which could impact on maternal outcomes reported but is unlikely to influence other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rate of loss between allocation and delivery: 7% in treatment group and 4% in controls; total losses or deviations from protocol 17%, described in detail by authors |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were prespecified. However, no maternal outcomes were included and adverse maternal outcomes/events were reported. Additional outcomes were reported including: Apgar scores at 1 and 5 minutes, time in special care, time on oxygen and maximum inspired oxygen. |
| Other bias | Low risk | Nothing to indicate any other source of bias |
ACS: antenatal corticosteroids IM: intramuscular NICU: neonatal intensive care unit PPROM: preterm prelabour rupture of the membranes RDS: respiratory distress syndrome TTN: tachypnoea of the newborn
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Christofori 2011 | This trial was conducted in women from 34 to 36 weeks of gestation. |
| Issa 2019 | Comparison between two different corticosteroids |
| Jain 2005 | This trial was terminated due to slow enrolment and lack of funding. |
| Koch 2016 | Ineligible comparator: gestational ages are different in the two groups |
| Ontela 2018 | Ineligible population: women not at term |
| Sananes 2017 | Comparison groups are of different gestational age. |
Characteristics of studies awaiting classification [ordered by study ID]
Afzal 2019.
| Methods | Design: RCT Setting: Department of Gynaecology and Obstetrics Sughra, Shafi Medical Complex Narowal, Pakistan |
| Participants | Inclusion criteria: women delivered by El‐LSCS between 37‐39 weeks of pregnancy having previous cesarean section, singleton pregnancies and only women with confirmed dates (by earliest USG or sure of LMP) Exclusion criteria: women with diabetes, IUGR, preterm babies, multiple pregnancies and congenital malformed babies. Gestational age (weeks): mean (SD) 37.83 (0.77) and 37.38 (4.56) Number randomised: A 60, B 60 Number analysed: A 60, B 60 |
| Interventions | Intervention (n = 60): dexamethasone intramuscularly in two doses of 12 mg, 12 hours apart 48 hours prior to date of cesarean section Control (n = 60): 2 mL normal saline 0.9% every 12 hours for 4 doses with the last dose at least 24 hours before scheduled CD |
| Outcomes |
|
| Notes | Study dates: January to June 2018 Funding sources: not reported Declarations of interest: “The study has no conflict of interest to declare by any author.” We emailed the trial authors 7 February 2021 to ask for information regarding prospective trial registration, ethical approval, randomisation and number of women lost to follow‐up. Awaiting reply. Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Ahmed 2015.
| Methods | Parallel randomised control trial |
| Participants | Location: 1 hospital in Egypt Timeframe: July 2012 to December 2013 Eligibility: 452 women with a singleton pregnancy (228 intervention, 224 control) undergoing an elective caesarean section from 37 + 0 weeks' gestation to 39 + 6 weeks' gestation. Exclusion: women with multiple gestations or a fetus with a major congenital anomaly and those with other medical or obstetrical conditions warranting early or immediate delivery and/or women who had received prophylactic dexamethasone during the current pregnancy were excluded. |
| Interventions | Intervention: 2 doses of 12 mg IM dexamethasone administered 24 hours apart (n = 228) Control group: usual care (without antenatal steroids) (n = 224) |
| Outcomes |
|
| Notes | All participants in the intervention group received the antenatal corticosteroids at 37 weeks' gestation irrespective of gestational age at delivery. * severity of RDS was only assessed for the NICU admitted cases. Funding source of study and declarations of interest: "The authors report no conflict of interest or financial support." We contacted the trial authors in January 2021 to ask for more information about their trial registration and randomisation processes. Awaiting reply. Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Ammar 2013.
| Methods | Parallel randomised control trial |
| Participants | Location: Ain Shams Maternity Hospital, Egypt Eligibility: women scheduled to undergo elective caesarean section at or after 37 completed weeks (based on the last menstrual period) Exclusion criteria: women with obstetric complications (including pre‐eclampsia, antepartum haemorrhage, fetal anomaly, hypertensive disease), chronic disease (including diabetes mellitus and renal disease) or preoperative infection were excluded. |
| Interventions | Intervention: 2 doses of 12 mg dexamethasone administered intramuscularly 12 hours apart, 48 hours before delivery (n = 300) Control: usual care (without antenatal corticosteroids) (n = 300) |
| Outcomes |
|
| Notes | Study dates: March 2010 to March 2011 Funding sources: not reported Declarations of interest: "There is no relationship for any author that may influence the objectivity of the paper." We emailed the trial authors on 7 February 2021 to ask for information regarding prospective trial registration, randomisation and number of women lost to follow‐up. Awaiting reply. Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Elbohoty 2020.
| Methods | Design: RCT Setting: Tanta University, Tanta, Egypt |
| Participants | Inclusion criteria: age 20 to 40 years, singleton pregnancy, gestational age ≥ 37 weeks, scheduled cesarean section Exclusion criteria: multiple gestation, obstetric complications such as pre‐eclampsia, diabetes mellitus, antepartum haemorrhage, PROM and preterm delivery, malformed baby, patients in labour, patients with contraindication to spinal anaesthesia, refusal to participate in the study. |
| Interventions | Intervention (n = 200): dexamethasone 6 mg every 12 hours for 4 doses with the last dose at least 24 hours before scheduled caesarean section Control (n = 200): 2 mL normal saline 0.9% every 12 hours for 4 doses with the last dose at least 24 hours before scheduled caesarean section |
| Outcomes |
|
| Notes |
Not included in 2021 update because we are awaiting a response from the trial authors to our query seeking clarification about the data in the trial report Study dates: October 2017 to March 2019 Funding sources: “No funding sources” Declarations of interest: “None declared” |
Elewa 2020.
| Methods | Design: RCT Setting: Benha University Hospital and Benha Teaching Hospital, Egypt |
| Participants | Inclusion criteria: age 18 to 40 years, gestational age of 37 to 39 weeks, primigravida or multipara, singleton pregnancy Exclusion criteria: fetuses with major congenital anomalies, medical problems that can affect fetal well‐being, medical or obstetric conditions that warrant early or immediate delivery; women who had received prophylactic dexamethasone during the current pregnancy and those who developed a spontaneous labour |
| Interventions | Corticosteroid (n = 200): two intramuscular doses of 12 mg dexamethasone 12 hours apart Control (n = 200): IM saline as a placebo in the same regimen as the steroid group |
| Outcomes |
|
| Notes | Study dates: March 2018 to September 2019 Funding sources: not reported Declarations of interest: not reported We emailed the trial authors on 7 March 2021 to ask for information regarding prospective trial registration, randomisation and number of women lost to follow‐up. Awaiting reply Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Ismail 2017.
| Methods | Design: RCT Setting: Omdurman Maternity Hospital, Sudan |
| Participants | Inclusion criteria: women with singleton pregnancies at term (complete 37+0‐39 weeks); under regional anaesthesia, and only women with confirmed dates (early ultrasound scan 1st and 2nd trimester, or sure about LMP) Exclusion criteria: women with DM, women with congenital malformed babies, IUGR, gestation less than 37 weeks, multiple pregnancies, women who received dexamethasone, two doses during pregnancy due to other causes, and women who refused this intervention Number randomised: 560 (281 and 279) Number analysed: A 281, B 279 |
| Interventions | Intervention (n = 281): dexamethasone 12 mg, 12 hours apart 48 hours before elective caesarean section Control (n = 279): no treatment |
| Outcomes |
|
| Notes | Study dates: July 2013 to January 2014 Funding sources: not reported Declarations of interest: not reported We emailed the trial authors on 7 February 2021 to ask for information regarding prospective trial registration, randomisation and number of women lost to follow‐up. Awaiting reply. Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Kholeif 2010.
| Methods | Design: RCT Setting: Faculty of Medicine, Alexandria University, Alexandria, Egypt |
| Participants | Inclusion criteria: pregnant women at 37 completed weeks or more, scheduled for elective CS Exclusion criteria: women with medical disorders affecting the neonatal outcome Number randomised: 400 Number analysed: not reported |
| Interventions | Intervention: betamethasone 7 mg, 2 IM doses separated by 24 hours and 48 hours prior the operation Control: placebo, 2 IM doses separated by 24 hours and 48 hours prior the operation |
| Outcomes | RDS |
| Notes | Study dates: not reported Funding sources: not reported Declarations of interest: not reported Conference abstract only, no useable data reported Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial only published as abstract with no confirmation that the data are from the final analysis) Contact details: dr_ tamer_hosny@yahoo.com |
Kurt 2019.
| Methods | Design: RCT Setting: Obstetrics and Gynecology, Clinic of Haydarpaşa Numune Research Hospital, Sivas, Turkey |
| Participants | Inclusion criteria: pregnant women scheduled for elective caesarean sections over 37 weeks Exclusion criteria: "In 24 hours after antenatal betamethasone administration, the patients who had to be taken by cesarean section for urgent reasons before the efficacy of the drug was formed and the patients with side effects due to corticosteroids. These patients were not included in the study." Number randomised: 50 (25 and 25) Number analysed: 50 (25 and 25) |
| Interventions | Intervention: 6 mg intramuscular injection; 2 dosage in the morning and 2 dosage in the evening, was administered, at least 24 hours before the caesarean was performed Control: no treatment |
| Outcomes |
|
| Notes | Study dates: January 2007 to March 2008 Funding sources: not reported Declarations of interest: not reported We emailed the trial authors on 7 February 2021 to ask for information regarding prospective trial registration, randomisation, number of women lost to follow‐up and data for women with chronic disease. Awaiting reply. Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Nabil 2020.
| Methods | Design: RCT Setting: Mansoura University Hospitals, Egypt |
| Participants | Inclusion criteria: pregnant women having elective CS at 37 to 39 completed weeks of gestation Exclusion criteria: multiple pregnancies; premature rupture of membranes; presence of fetal congenital malformations or intrauterine growth restriction; women with diabetes, hypertensive disorders, cardiac disease, viral or non‐viral hepatitis, history of neonatal jaundice in previous deliveries; |
| Interventions | Intervention (n = 200): single course of dexamethasone (dose and timing are not reported) Control (n = 200): no treatment |
| Outcomes | Neonatal jaundice |
| Notes | Study dates: January 2016 to December 2017 Funding sources: not reported Declarations of interest: not reported We emailed the trial authors on 7 March 2021 to ask for information regarding prospective trial registration, number of women lost to follow‐up, and. Awaiting reply. Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Nada 2016.
| Methods | Parallel randomised control trial |
| Participants | Location: 1 hospital in Egypt Timeframe: November 2011 to December 2014 Eligibility criteria: women with a singleton pregnancy (as calculated from the first day of their last menstrual period) between 38 and 38 + 6 weeks' of pregnancy, aged between 20 and 40 years of age undergoing an elective caesarean section Exclusion criteria: women with multiple gestations or any medical problems that could affect fetal well‐being, or a fetus with a major congenital anomaly, intrauterine growth restriction, oligohydramnios or hydramnios. Women who developed spontaneous labour or had uterine contractions or tenderness were excluded. |
| Interventions | Intervention: 4 doses of 8 mg of dexamethasone administered intramuscularly 12 hours apart, 48 hours prior to elective caesarean section (n = 645) Placebo: in the placebo group: women received IM saline prior to elective caesarean section (n = 645) |
| Outcomes | Primary outcome: rates of NICU admissions with respiratory distress. Secondary outcomes
|
| Notes | Consent: verbal informed consent Funding source of study: not reported Declarations of interest: "None declared." We contacted the trial authors in January 2021 to ask for more information about their trial registration and randomisation processes. They replied that they did not register the study prospectively. Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Nooh 2018.
| Methods | Randomised controlled trial |
| Participants | Location: 1 hospital in Egypt Timeframe: September 2012 to August 2016 Eligibility criteria: women with a singleton pregnancy in the cephalic presentation planned to have a cesarean section at 38 weeks' gestation of pregnancy or beyond Exclusion criteria: women with multiple gestations or any obstetric disorders (such as pre‐eclampsia, antepartum haemorrhage, intrauterine growth restriction, breech presentation etc) or medial disorders (such as asthma, hypertension, diabetes, intrauterine infection, etc.) or history of smoking or peptic ulceration were excluded |
| Interventions | Intervention: 3 doses of 8 mg/2mL of dexamethasone administered intramuscularly 8 hours apart, 24 hours after the last dose (n = 636) Placebo: in the placebo group: usual care (without antenatal steroids) (n = 636) |
| Outcomes | Primary: admission to NICU with respiratory morbidity Secondary: incidence of TTN, RDS and need for mechanical ventilation |
| Notes | Additional outcomes including neonatal death and neonatal sepsis were reported on. Funding source of study: "The authors received no financial support for the research, authorship, or publication of this article." Declarations of interest: "The authors report no conflicts of interest." We contacted the trial authors in January 2021 to ask for more information about their trial registration. Awaiting reply. Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
Sadiq 2019.
| Methods | Design: RCT Setting: Kahuta Research Laboratories (KRL), Hospital, Islamabad, Pakistan |
| Participants | Inclusion criteria: pregnant women with singleton alive fetus between gestational age of 37 + 6 to 38 + 6 weeks, admitted for elective LSCS under spinal anaesthesia, gestational age confirmed by the date of last menstrual period and in cases of unsure of dates, by the first trimester dating scan, patients with uncomplicated gestational diabetes mellitus, pregnancy induced hypertension, anaemia and asthma Exclusion criteria: women with type 1 or 2 DM, chronic hypertension, preeclampsia, pre‐labour rupture of membranes, multiple pregnancies, infections including tuberculosis, congenital anomalous fetus, intrauterine growth restriction (IUGR) fetuses and emergency LSCS or operated under general anaesthesia Number randomised: 320 (A 158, B 162) Number analysed: 304 (A 152, B 152) |
| Interventions | Intervention (n = 158): one course of dexamethasone within 7 days of LSCS. Two doses of 12 mg, each given intramuscularly to the pregnant woman, 12 hours apart Control (n = 162): no treatment |
| Outcomes |
|
| Notes | Dates of study: September 2017 to September 2019 Funding sources: "Grant support and financial disclosure ‐ NIL" Declarations of interest: "Authors declared no conflict of interest" We contacted the trial authors in March 2021 to ask for more information the prospective trial registration status. The trial authors replied "a clinical protocol was designed before conducting the study that was presented to the institutional review committee. But the clinical trial was not registered except with the local hospital body and after reviewing they gave us the ethical permission and approval for conducting study." Not included in 2021 update because of lack of response from trial authors regarding our trustworthiness criteria (trial published since 2010 without prospective registration) |
BPD: bronchopulmonary dysplasia CPAP: continuous positive airway pressure CS: caesarean section DM: diabetes mellitis ECMO: extracorporeal membrane oxygenation El‐LSCS: lower (uterine) segment caesarean section IM: intramuscular IUGR: intrauterine growth restriction LMP: last menstrual period NICU: neonatal intensive care unit PROM: premature rupture of membranes RDS: respiratory distress syndrome TTN: tachypnoea of the newborn
Characteristics of ongoing studies [ordered by study ID]
Ahmadpour‐kacho 2015.
| Study name | Effect of antenatal steroid before elective cesarean section (C/S) on prevention of respiratory morbidity of term neonates |
| Methods | Parallel randomised control trial Setting: 2 hospitals in Iran |
| Participants | Timeframe: August 2015‐February 2015 Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group: 2 doses of 12 mg of betamethasone administered 24 hours apart, 48 hours prior to caesarean section. Control group: usual care (without antenatal corticosteroids) |
| Outcomes |
|
| Starting date | 3 August 2015 |
| Contact information | Dr Mousa Ahmadpour‐Kacho Babol University of Medical Sciences Gangafrooz Babol Mazandaran Iran 00981132197667/00989111122855 mousa_ahmadpour@hotmail.com |
| Notes | Recruitment completed IRCT registration number: IRCT2015090923963N1 Authors contact February 2020, awaiting response. |
Custo 2007.
| Study name | Use of antenatal dexamethasone in late pregnancy and its effect on incidence of neonatal respiratory distress after elective caesarean sections |
| Methods | Randomised controlled trial |
| Participants | Women planned to deliver by caesarean section after 37 completed gestational weeks |
| Interventions | Intervention: dexamethasone, 12 mg x 2 Control: usual care (no steroids) |
| Outcomes |
|
| Starting date | Not known |
| Contact information | Custo R
Obstetrics and Gynaecology Department
St. Luke's Hospital
Malta jean@waldonet.net.mt Contacted investigator February 2021 to ask for further details. Awaiting reply. |
| Notes |
Dawood 2018.
| Study name | The neonatal outcomes of Dexamethasone administration before scheduled cesarean delivery at term: a randomized clinical trial |
| Methods | Randomised controlled trial Setting: Egypt |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group: receive dexamethasone 8mg IM/12 hours for 3 doses with the last dose at least 24 hours before elective caesarean Control group: receive saline 0.9% 2 mL IM/12 hours for 4 doses with the last dose at least 24 hours before elective caesarean |
| Outcomes | Primary outcomes: neonatal
Secondary outcomes: maternal
|
| Starting date | 1 January 2018 |
| Contact information | Tanta University Elgeish st., Tanta, Egypt +201020972067 qau@med.tanta.edu.eg |
| Notes | Authors contact February 2020, awaiting response. |
Groom 2020.
| Study name | The C*STEROID Trial: Corticosteroids before planned caesarean section from 35+0 to 39+6 weeks of pregnancy |
| Methods | Randomised controlled trial Setting: New Zealand Blinding: participants, care provider, outcome assessor and investigator |
| Participants | Inclusion criteria
Exclusion criteria
"We will recruit 2548 infants (1274 per group)" |
| Interventions | Intervention group: two doses of 11.4 mg betamethasone by intramuscular injection into the thigh, arm or buttock, 24 hours apart given within seven days of planned caesarean section. Control group: placebo ‐ 0.9% NaCl in a visually matching syringe |
| Outcomes | Co‐primary outcomes
* > 60 minutes selected to eliminate short‐term support which may be subject to variation by clinician. Secondary outcomes
|
| Starting date | 4 June 2019 |
| Contact information | A/Prof Katie Groom Associate Professor of Maternal and Perinatal Health Hugo Charitable Trust Research Fellow Maternal Fetal Medicine Subspecialist The University of Auckland, 85 Park Road, Grafton Private Bag 92019, Auckland 1142, New Zealand +64 9 373 7599 ext 89823 k.groom@auckland.ac.nz |
| Notes | Contacted principal investigator asking for data for women at 37 weeks and beyond. Reply from Prof Groom: "The C*STEROID Feasibility Study has now completed recruitment but we remain blinded to results by treatment group as clinical outcomes will also contribute to the main C*STEROID Trial. This was planned a priori. The main trial has commenced recruitment and we expect a 3‐4 year timeline to complete. We will be happy to share results once the trial is completed and results analysed." |
Haroun 2018.
| Study name | Antenatal corticosteroid in elective cesarean section |
| Methods | Randomised controlled trial Blinding: participant, care provider, investigator |
| Participants | Females aged 18 to 35 years Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group: dexamethasone 6 mg, 48 hours before cesarean section Control group: placebo 6 mg, 48 hours before cesarean section |
| Outcomes | Incidence of respiratory complications after cesarean section |
| Starting date | February 2018 |
| Contact information | Ahmed Fayek, Professor 01005211819 ahmedamen1@yahoo.com Hisham Abo Taleb, Lecturer 01003332139 hishamaboutaleb1@yahoo.com |
| Notes | Authors contacted February 2020, awaiting response. |
Hubesih 2020.
| Study name | Effect of single dose antenatal betamethasone on neonatal respiratory morbidity after elective cesarean |
| Methods | Randomised controlled trial Setting: One General Hospital, Lebanon Blinding: participant, care provider, investigator |
| Participants | Females age 17 to 45 years Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group: 14 mg (2 mL) intramuscular betamethasone at least 24 hours befor undergoing cesarean section. Control group: an equivalent volume of normal saline in a single injection |
| Outcomes | Percentage of patients with respiratory morbidity Admission to Neonatal Intensive Care Unit |
| Starting date | 29 May 2020 |
| Contact information | Manal Hubeish, MD, +9611636297 drmanalhubeishhusari@gmail.com |
| Notes |
IRCT20120918010876N4 2018.
| Study name | |
| Methods | Design: RCT Setting: Mahdieh hospital, Tehran, Iran |
| Participants | Inclusion criteria: women with singleton pregnancy with elective cesarean section at week 37 to 38 weeks and 6 days will enrol in the study Exclusion criteria: multiple pregnancies, fetus with major congenital anomalies, fetus with other medical or midwifery conditions that give birth earlier, receive dexamethasone or betamethasone during pregnancy, maternal severe hypertension, maternal history of peptic ulcer, and evidences of intrauterine infection Target sample size: 220 |
| Interventions | Treatment group: intramuscular injection of 12 mg betamethasone in two doses between 24 hours, 48 hours before cesarean section Control group: cesarean section without betamethasone injection |
| Outcomes | Neonatal respiratory outcomes
|
| Starting date | |
| Contact information | |
| Notes | Study dates: registered 11 January 2018 Funding sources: "Shahid Beheshti University of Medical Sciences" Declarations of interest: not reported We contacted the investigators on 8 March 2020 to ask if they had any outcome data we could include. Contact details: z.joshaghani@hotmail.com |
IRCT20121224011862N3 2019.
| Study name | |
| Methods | Design: RCT Setting: Tbriz Alzahra hospital, Iran |
| Participants | Inclusion criteria: women with singleton pregnancy with elective cesarean section at week 37 to 38 weeks and 6 days will enrol in the study Exclusion criteria include multiple pregnancies, fetus with major congenital anomalies |
| Interventions | Treatment group: intramuscular injection of 12 mg betamethasone in two doses between 24 hours, 48 hours before cesarean section Control group: no treatment Target sample size: 200 |
| Outcomes |
|
| Starting date | |
| Contact information | |
| Notes | Study dates: registered June 2019, expected recruitment start date June 2019 and expected end date July 2020 Funding sources: "Vice chancellor for Research,Tabriz University Of Medical Sciences" Declarations of interest: not reported We contacted the investigators on 8 March 2021 to ask if they had any data they could share with us. Contact details: Farnaz Sahaf sahaf@tbzmed.ac.ir, lahroudin@gmail.com |
Said 2019.
| Study name | PRECeDe Pilot: Prevention of neonatal Respiratory distress with antenatal corticosteroids prior to Elective Caesarean section in women with Diabetes ‐ A Feasibility Randomised Trial |
| Methods | Randomised controlled trial Setting: one hospital in Australia Blinding: participants, investigator |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group: 11.4 mg of Celestone Chrondose in 2 mL (betamethasone 11.4 mg, as betamethasone sodium phosphate and betamethasone acetate). Two injections will be administered intramuscularly, 24 hours apart, within 7 days prior to elective caesarean section, to participants randomised to receive the investigational product. Control group: placebo. Normal saline 2 mL in an identical appearing syringe. Two injections will be administered intramuscularly, 24 hours apart, within 7 days prior to elective caesarean section, to participants randomised to receive the placebo. |
| Outcomes |
|
| Starting date | Not yet recruiting although anticipated start date is 2 March 2020 |
| Contact information | A/Prof Joanne Said Maternal Fetal Medicine Unit Joan Kirner Women's & Children's, Sunshine Hospital 176 Furlong Rd St Albans, Victoria, 3021, Australia +61 3 9055 2400 jsaid@unimelb.edu.au |
| Notes |
ANZNN: Australian and New Zealand Neonatal Network
CPAP: comtinuous positive airway pressure
ECMO: Extracorporeal membrane oxygenation
NICU: neonatal intensive care unit
NNU: neontal unit
RDS: respiratory distress syndrom
TTN: tachypnoea of the newborn
WHO: World Health Organisation
Differences between protocol and review
In the 2018 update of the review, the original outcome "Admission to the neonatal special care unit or above" was split into a primary outcome, "Admission to neonatal special care for respiratory morbidity (all levels of care or intensive care unit (NICU))", and a secondary outcome, "Admission to neonatal special care for any indication (all levels of care or intensive care unit (NICU))". This was done because, in the case of non‐blinded trials, there is a possibility of bias, as infants in the treatment group may have been less likely to have had their reason for admission classified as 'respiratory' because they were known to have received corticosteroids. Furthermore, special care was split into two categories "special care ‐ all levels" and "special care ‐ NICU", as different levels of care may reflect different severity of respiratory distress.
The review included three additional outcomes which looked at the long‐term outcomes of administration of antenatal corticosteroids to the infant as a child. The authors felt that these outcomes were important to include in view of concerns regarding long‐term effects of antenatal corticosteroid administration.
A summary of findings table was incorporated for this update.
2021 update
We used the Cochrane Pregnancy and Childbirth Study Trustworthiness Screening tool to assess the trustworthiness of studies that otherwise meet the review's inclusion criteria. Using these predefined criteria, we categorised any studies that were assessed as untrustworthy as "awaiting classification" and did not include them in the review.
Contributions of authors
For the protocol: Alexandros Sotiriadis (AS) prepared the first draft and revised it in response to editorial comments. George Makrydimas (GM) and John PA Ionannidis (JI) commented on the drafts.
For the 2009 review: AS and GM assessed potential studies. Stefania Papatheodorou (SP) and AS extracted the data, entered them into Review Manager, assessed studies for risk of bias, and tabulated the results. All authors contributed in preparation of the review.
For the 2018 update: AS and EM assessed potential studies, extracted the data and entered the data into Review Manager. AS and EM assessed studies for risk of bias, tabulated results and completed the GRADE assessment and summary of findings table. All authors commented on drafts.
For this update: AS, EM, FS and RP assessed all eligible studies for trustworthiness. EM and FS also assessed studies for risk of bias, tabulated the results and completed the GRADE assessment and summary of findings table. All authors commented on drafts.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Alexandros Sotiriadis: I am Associate Professor of Obstetrics, Gynaecology and Maternal Fetal Medicine at Aristotle University of Thessaloniki, Greece. I am also a private practicing physician at EMVRYO PCC.
George Makrydimas: none known.
Stefania Papatheodorou: none known.
John PA Ioannidis: none known.
Emma McGoldrick: I am an author of another Cochrane Review entitled 'Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth' (McGoldrick 2020). I am also an author on an overview relating to antenatal corticosteroids (McGoldrick 2016). The present review is potentially eligible for inclusion in the overview. I will not be involved in any assessment or data extraction and two independent review authors will assess this review.
Roses Parker: none known.
Fiona Stewart: I am an author of the Cochrane Review entitled 'Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth' (McGoldrick 2020).
Edited (no change to conclusions)
References
References to studies included in this review
Stutchfield 2005 {published and unpublished data}
- Stutchfield P, Whitaker R, Russell I, Antenatal Steroids for Term Elective Caesarean Section (ASTECS) Research Team.Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ 2005;331(7518):662. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ.Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Archives of Disease in Childhood Fetal & Neonatal Edition 2013;98(3):F195-F200. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Christofori 2011 {published data only}
- EUCTR2011-002919-28-IT.Antenatal corticosteroids and respiratory distress syndrome in late preterm infants born by elective cesarean section. Multicentric randomized controlled clinical trial. clinicaltrialsregister.eu/ctr-search/trial/2011-002919-28/IT (first received: 5 December 2011).
Issa 2019 {published data only}
- Issa AK, Taha WS, Elsayeh AA, Elhadad HK.Comparative study between betamethasone and dexamethasone as a prophylactic corticosteroids therapy on neonatal outcome in elective cesarean section at 37 weeks. The Egyptian Journal of Hospital Medicine 2019;74(2):420-7. [Google Scholar]
Jain 2005 {published data only}
- NCT00139256.Randomized controlled trial of antepartum betamethasone treatment for prevention of respiratory distress in infants born by elective cesarean section. clinicaltrials.gov/show/NCT00139256 (first received 29 August 2005).
Koch 2016 {published data only}
- Koch A, Sananes N, Kuhn P, Escande B, Aissi G, Fritz G, et al.Elective caesarean section at 38 weeks of gestation after corticosteroid versus elective caesarean section at 39 weeks: a randomized controlled trial. European Journal of Obstetrics and Gynecology 2016;206:e160. [DOI] [PubMed] [Google Scholar]
- NCT00446953.Caesarean and corticotherapy. clinicaltrials.gov/show/NCT00446953 (first received: 12 March 2007.
Ontela 2018 {published data only}
- Ontela V, Dorairajan G, Bhat VB, Chinnakali P.Effect of antenatal steroids on respiratory morbidity of late preterm newborns: a randomized controlled trial. Journal of Tropical Pediatrics 2018;64(6):531-8. [CENTRAL: CN-01991657] [EMBASE: 626300500] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sananes 2017 {published data only}
- Sananes N, Koch A, Escande B, Aissi G, Fritz G, Roth E, et al.Pilot randomised controlled trial comparing the risk of neonatal respiratory distress in elective caesarean section at 38 weeks' gestation following a course of corticosteroids versus caesarean at 39 weeks. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;212:54-9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Afzal 2019 {published data only}
- Afzal M, Bilal H, Hayat T.Effect of prophylactic antenatal cortecosteroid and incidence of neonatal respiratory morbidity after elective cesarean section in patients having previous cesarean section. Medical Forum Monthly 2019;30(4):88-91. [CENTRAL: CN-01936415] [EMBASE: 2001966640] [Google Scholar]
Ahmed 2015 {published data only}
- Ahmed MR, Ahmed WAS, Mohammed TY.Antenatal steroids at 37 weeks, does it reduce neonatal respiratory morbidity? A randomized trial. Journal of Maternal-Fetal & Neonatal Medicine 2015;28(12):1486-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ammar 2013 {published data only}
- Ammar AR, Rabei NH, Gad HA.Dexamethasone in prevention of respiratory morbidity in elective cesarean section in term fetus. A randomized controlled trial. Journal of American Science 2013;9(6):286-9. [Google Scholar]
Elbohoty 2020 {published data only}
- Elbohoty SB, Dawood AS, Abbas AM, Elgergawy AE.The neonatal outcomes of Dexamethasone administration before scheduled cesarean delivery at term: a randomized clinical trial. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2020;9(3):1222-7. [CENTRAL: CN-02217949] [Google Scholar]
Elewa 2020 {published data only}
- Elewa A, Saad A, Soliman A, Al Saber A.Does corticosteroids administration after 37 weeks for elective lower segment cesarean section reduce neonatal respiratory morbidity? A randomized controlled trial. Benha Medical Journal 2020;37(3):607-18. [Google Scholar]
Ismail 2017 {published data only}
- Ismail KS, Mahgoub AA, Abdulilah K, Elkheir HA, Kholoud AM, Mohamed O, et al.Effect of prophylactic corticosteroid therapy on respiratory morbidity in infants borne at term by elective cesarean section at Omdurman maternity hospital. Family Medicine and Medical Science Research 2017;6:215. [CENTRAL: CN-02129568] [Google Scholar]
Kholeif 2010 {published data only}
- Kholeif A, Galal H, Hosny T, Hammad B.Betamethasone intramuscularly and neonatal respiratory distress in cases of term elective Cesarean section: alexandria experience. Prenatal Diagnosis 2010;30 Suppl 1:S29. [CENTRAL: CN-02129364] [EMBASE: 70430204] [Google Scholar]
Kurt 2019 {published data only}
- Kurt B, Kose G.Antenatal betamethasone administration before elective caesarean section in term pregnant women [Cumhuriyet Tip Dergisi]. Cumhuriyet Medical Journal 2019;42:747-53. [Google Scholar]
Nabil 2020 {published data only}
- Nabil H, Talkhan HM.Does antenatal dexamethasone before full term planned cesarean section affect the incidence or severity of neonatal jaundice? A randomized controlled trial. Egyptian Journal of Fertility and Sterility 2020;24(2):22-6. [Google Scholar]
Nada 2016 {published data only (unpublished sought but not used)}
- Nada AM, Shafeek MM, El Maraghy MA, Nageeb AH, Salaheldine AS, Awad MH.Antenatal corticosteroid administration before elective caesarean section et term to prevent neonatal respiratory morbidity: a randomized controlled trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;199:88-91. [DOI] [PubMed] [Google Scholar]
- NCT01772381.Dexamethasone in prevention of respiratory morbidity in elective caesarean. clinicaltrials.gov/show/NCT01772381 (first received 31 December 2012).
Nooh 2018 {published data only}
- Nooh AM, Abdeldayem HM, Arafa E, Shazly SA, Elsayed H, Mokhtar WA.Does implementing a regime of dexamethasone before planned cesarean section at term reduce admission with respiratory morbidity to neonatal intensive care unit? A randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2018;31(5):614-20. [DOI] [PubMed] [Google Scholar]
Sadiq 2019 {published data only}
- Sadiq H, Sohail I.Effects of prophylactic maternal dexamethasone administration on the neonatal respiratory outcomes at term after elective caesarean section: randomized controlled trial. Khyber University Medical Journal 2019;11(1):6-11. [CENTRAL: CN-02118735] [Google Scholar]
References to ongoing studies
Ahmadpour‐kacho 2015 {published data only}
- IRCT2015090923963N1.Effect of antenatal steroid before elective cesarean section(c/s) on prevention of respiratory morbidity of term neonates. en.search.irct.ir/view/25584 (first received 13 November 2015).
Custo 2007 {published data only}
- Custo R, Zammit K, Calleja Agius J, Grech H, Brincat M.Use of antenatal dexamethasone in late pregnancy and its effects on incidence of neonatal respiratory distress after elective caesarean sections. In: 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4-6; London, UK. 2007:97.
Dawood 2018 {published data only}
- UMIN000029070.Antenatal steroids before full term elective cesarean section: randomized controlled study. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000029070 2018. [CENTRAL: CN-01899282]
Groom 2020 {published data only}
- ACTRN12618002028280.The C*STEROID Feasibility Study: a two-site, randomised placebo controlled trial of corticosteroids before planned caesarean section delivery at 35+0 to 39+6 weeks of pregnancy to determine feasibility for recruitment to the C*STEROID Trial [The C*STEROID Feasibility Study: Corticosteroids before planned caesarean section from 35+0 to 39+6 weeks]. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12618002028280 2018. [CENTRAL: CN-01948945]
- ACTRN12620000914965.The C*STEROID Trial: corticosteroids before planned caesarean section from 35+0 to 39+6 weeks of pregnancy [The C*STEROID Trial. Antenatal corticosteroids prior to planned caesarean section delivery from 35+0 to 39+6 weeks gestation; a randomised controlled trial assessing the effects on neonatal respiratory morbidity and glycaemic control]. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12620000914965 2020. [CENTRAL: CN-02184429]
- Groom K.Trial protocol: the C*STEROID Trial: corticosteroids before planned caesarean section from 35+0 to 39+6 weeks of pregnancy. cdn.auckland.ac.nz/assets/liggins/docs/CSTEROID_Protocol_V2_9Sept2020.pdf 2020.
Haroun 2018 {published data only}
- NCT03396107.Antenatal corticosteroid in elective cesarean section [Role of antenatal corticosteroid use in elective term cesarean section]. clinicaltrials.gov/show/nct03396107 5 January 2018. [CENTRAL: CN-01578593]
Hubesih 2020 {published data only}
- NCT04407975.Effect of single dose antenatal betamethasone on neonatal respiratory morbidity after elective cesarean [Effect of single dose antenatal betamethasone on the incidence of neonatal respiratory morbidity after elective cesarean section at term: a prospective double blinded placebo controlled trial]. Https://clinicaltrials.gov/show/NCT04407975 29 May 2020. [CENTRAL: CN-02118516]
IRCT20120918010876N4 2018 {published data only}
- IRCT20120918010876N4.Effect of steroid on neonatal respiratory morbidity in elective cesarean section in 37 weeks or later [Assessment of effect of antenatal betamethasone administration on neonatal respiratory morbidity in early term (37 to 38 weeks and 6 days) elective cesarean section]. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20120918010876N4 2018. [CENTRAL: CN-01898196]
- Mirzamoradi M, Joshaghani Z, Hasani Nejhad F, Vafaeenia M, Heidar Z.Evaluation of the effect of antenatal betamethasone on neonatal respiratory morbidity in early-term elective cesarean. Journal of Maternal-Fetal and Neonatal Medicine 2020;33(12):1994-9. [CENTRAL: CN-01917141] [EMBASE: 626663421] [DOI] [PubMed] [Google Scholar]
IRCT20121224011862N3 2019 {published data only}
- IRCT20121224011862N3.The effect of antenatal Betamethasone on prevention of neonatal respiratory distress syndrome before elective cesarean section at term. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20121224011862N3 2019. [CENTRAL: CN-01969231]
Said 2019 {published data only}
- ACTRN12619001475134.PRECeDe Pilot: prevention of neonatal Respiratory distress with antenatal corticosteroids prior to Elective Caesarean section in women with Diabetes - A Feasibility Randomised Trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619001475134 2019. [CENTRAL: CN-02064898] [DOI] [PubMed]
Additional references
Al‐Durra 2020
- Al-Durra M, Nolan RP, Seto E, Cafazzo JA.Prospective registration and reporting of trial number in randomised clinical trials: global cross sectional study of the adoption of ICMJE and Declaration of Helsinki recommendations. The BMJ 2020;369:m982. [DOI] [PMC free article] [PubMed] [Google Scholar]
ASTEROID 2019
- Crowther CA, Ashwood P, Andersen CC, Middleton PF, Tran T, Doyle L, et al, ASTEROID Study Group†.Maternal intramuscular dexamethasone versus betamethasone before preterm birth (ASTEROID): a multicentre, double-blind, randomised controlled trial. The Lancet Child & Adolescent Health 2019;3(11):769-80. [DOI] [PubMed] [Google Scholar]
Avery 1966
- Avery ME, Gatewood OB, Brumley G.Transient tachypnea of newborn. Possible delayed resorption of fluid at birth. American Journal of Diseases of Children 1966;111:380-5. [DOI] [PubMed] [Google Scholar]
BAPM 2001
- British Association of Perinatal Medicine.Standards for hospitals providing neonatal intensive and high dependency care (second edition) and categories of babies requiring neonatal care. http://www.bapm.org/media/documents/publications/hosp_standards.pdf (accessed 2009).
Betrān 2016
- Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR .The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PloS one 2016;11(2):e0148343. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowers 1982
- Bowers SK, MacDonald HM, Shapiro ED.Prevention of iatrogenic neonatal respiratory distress syndrome: elective repeat cesarean section and spontaneous labor. American Journal of Obstetrics and Gynecology 1982;143(2):186-9. [DOI] [PubMed] [Google Scholar]
Brown 1983
- Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV.Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. Journal of Physiology 1983;344:137-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brownfoot 2013
- Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA.Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD006764. [DOI: 10.1002/14651858.CD006764.pub3] [DOI] [PubMed] [Google Scholar]
Burns 2008
- Burns CM, Rutherford MA, Boardman JP, Cowan FM.Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycaemia. Pediatrics 2008;122(1):65-74. [DOI] [PubMed] [Google Scholar]
Carlisle 2017
- Carlisle JB.Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trial in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. [DOI] [PubMed] [Google Scholar]
Crowther 2015
- Crowther CA, McKinlay CJ, Middleton P, Harding JE.Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD003935. [DOI: 10.1002/14651858.CD003935.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dani 1999
- Dani C, Reali MF, Bertini G, Wiechmann L, Spagnolo A, Tangucci M, et al.Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Italian Group of Neonatal Pneumology. European Respiratory Journal 1999;14(1):155-9. [DOI] [PubMed] [Google Scholar]
De Angelis 2004
- De Angelis C, Drazen JM, Frizelle FA.Clinical trial registration: a statement from the International Committee of Medical Journal Editors. New England Journal of Medicine 2004;351:1250-1. [DOI] [PubMed] [Google Scholar]
FIGO 2019
- FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine.Good clinical practice advice: antenatal corticosteroids for fetal lung maturation. International Journal of Gynaecology and Obstetrics 2019;144(3):352-5. [PMID: 30710360 ] [DOI] [PubMed] [Google Scholar]
Gerten 2005
- Gerten KA, Coonrod DV, Bay RC, Chambliss LR.Cesarean delivery and respiratory distress syndrome: does labor make a difference? American Journal of Obstetrics and Gynecology 2005;193(3 Pt 2):1061-4. [DOI] [PubMed] [Google Scholar]
Gyamfi‐Bannerman 2017
- Gyamfi-Bannerman C.Antenatal corticosteroids: it's all about timing. BJOG: an International Journal of Obstetrics and Gynaecology 2017;124(10):1575. [DOI] [PubMed] [Google Scholar]
Hansen 2007
- Hansen AK, Wisborg K, Uldbejerg N, Henriksen TB.Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstetricia et Gynecologica Scandinavica 2007;86(4):389-94. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors.Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from training.cochrane.org/handbook/archive/v5.0.0/.
Higgins 2011
- Higgins JPT, Green S, editors.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors).Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Available from www.training.cochrane.org/handbook: Cochrane, 2020. [Google Scholar]
Irestedt 1984
- Irestedt L, Lagercrantz H, Belfrage P.Causes and consequences of maternal and fetal sympathoadrenal activation during parturition. Acta Obstetricia et Gynecologica Scandinavica 1984;118:111-5. [DOI] [PubMed] [Google Scholar]
Jain 2006
- Jain L, Dudell GG.Respiratory transition in infants delivered by cesarean section. Seminars in Perinatology 2006;30(5):296-304. [DOI] [PubMed] [Google Scholar]
Jobe 2018
- Jobe AH, Goldenberg RL.Antenatal corticosteroids: an assessment of anticipated benefits and potential risks. American Journal of Obstetrics and Gynecology 2018;219(1):62-74. [DOI] [PubMed] [Google Scholar]
Jobe 2021
- Jobe AH, Schmidt AF.Chapter for antenatal steroids - treatment drift for a potent therapy with unknown long term safety seminars in fetal and neonatal medicine. Seminars in Fetal and Neonatal Medicine 2021;26(2):101231. [DOI: 10.1016/j.siny.2021.101231] [DOI] [PubMed] [Google Scholar]
Kerstjens 2012
- Kerstjens JM, Bocca-Tjeertes IF, Winter AF, Reijneveld SA, Bos AF.Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics 2012;130(2):e265-72. [DOI] [PubMed] [Google Scholar]
Levine 2001
- Levine EM, Ghai V, Barton JJ, Strom CM.Mode of delivery and risk of respiratory diseases in newborns. Obstetrics & Gynecology 2001;97(3):439-42. [DOI] [PubMed] [Google Scholar]
Liggins 1972
- Liggins GC, Howie RN.A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants.. Pediatrics 1972;50:515-25. [PubMed] [Google Scholar]
McClure 2007
- McClure EM, Goldenberg RL, Bann CM.Maternal mortality, stillbirth and measures of obstetric care in developing and developed countries. International Journal of Gynecology & Obstetrics 2007;96(2):139-46. [DOI] [PubMed] [Google Scholar]
McGoldrick 2016
- McGoldrick E, Brown J, Middleton P, McKinlay CJD, Haas DM, Crowther CA.Antenatal corticosteroids for fetal lung maturation: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD012156. [DOI: 10.1002/14651858.CD012156] [DOI] [Google Scholar]
McGoldrick 2020
- McGoldrick E, Stewart F, Parker R, Dalziel SR.Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD004454. [DOI: 10.1002/14651858.CD004454.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melamed 2019
- Melamed N, Asztalos E, Murphy K, Zaltz A, Redelmeier D, Shah BR, et al.Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: a population-based study. BMJ Open 2019;9(9):e031197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mol 2021
- Mol BW, Li W, Lai S, Stock S.Effectiveness of antenatal corticosteroids at term: can we trust the datathat ‘inform’ us? European Journal of Obstetrics & Gynecology and Reproductive Biology 2021;261:144-7. [DOI] [PubMed] [Google Scholar]
Morrison 1995
- Morrison JJ, Rennie JM, Milton PJ.Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. British Journal of Obstetrics and Gynaecology 1995;102(2):101-6. [DOI] [PubMed] [Google Scholar]
NICE 2004
- National Collaborating Centre for Women's and Children's Health.Caesarean Section. London: National Institute for Clinical Excellence, 2004. [DOI] [PubMed] [Google Scholar]
NICE 2017
- NICE.Preterm Labour and Birth Guideline (NG25). National Institute for Health and Care Excellence, 2015. [PubMed] [Google Scholar]
NICE 2021
- NICE.Caesarean birth (NG192). National Institute for Health and Care Excellence, 2021. [Google Scholar]
Nielsen 1984
- Nielsen TF, Hokegard KH.The incidence of acute neonatal respiratory disorders in relation to mode of delivery. Acta Obstetricia et Gynecologica Scandinavica 1984;63(2):109-14. [DOI] [PubMed] [Google Scholar]
Raikkőnen 2020
- Raikkőnen K, Gissler M, Kajantie E.Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA 2020;323(19):1924-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
RCOG 2004
- Royal College of Obstetricians and Gynaecologists.Antenatal Corticosteroids to Prevent Respiratory Distress Syndrome. Clinical Green Top Guidelines. London: Royal College of Obstetricians and Gynaecologists, 2004. [Google Scholar]
RCOG 2010
- RCOG.Green-Top Guideline 7. Antenatal Corticosteroids to Reduce Neonatal Morbidity and Mortality. /www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg7/: Royal College of Obstetricians and Gynaecologists, 2010. [DOI] [PubMed] [Google Scholar]
Reed 1978
- Reed DM, Bakketeig LS, Nugent RP.The epidemiology of respiratory distress syndrome in Norway. American Journal of Epidemiology 1978;107(4):299-310. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan).Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth‐Kleiner 2003
- Roth-Kleiner M, Wagner BP, Bachmann D, Pfenninger J.Respiratory distress syndrome in near-term babies after caesarean section. Swiss Medical Weekly 2003;133(19-20):283-8. [DOI] [PubMed] [Google Scholar]
Saccone 2016
- Saccone G, Berghella V.Antenatal corticosteorids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. The BMJ 2016;355:i5044. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simes 1986
- Simes, RJ.Publication bias: the case for an international registryof clinical trials. Journal of Clinical Oncology 1986;4:1529-41. [DOI] [PubMed] [Google Scholar]
Sotiriadis 2015
- Sotiriadis A, Tsiami A, Papatheodorou S, Baschat AA, Sarafidis K, Makrydimas G.Neurodevelopmental outcome after a single course of antenatal steroids in children born preterm: a systematic review and meta-analysis. Obstetrics and Gynecology 2015;125(6):1385-96. [DOI] [PubMed] [Google Scholar]
Stark 2004
- Stark AR, American Academy of Pediatrics Committee on Fetus and Newborn.Levels of neonatal care. Pediatrics 2004;114(5):1341-7. [DOI] [PubMed] [Google Scholar]
Stutchfield 2013
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ.Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Archives of Disease in Childhood Fetal & Neonatal Edition 2013;98(3):F195-F200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Berg 2001
- Van den Berg A, Van Elburg RM, Van Geijn HP, Fetter WP, Van Elburg RM, Van Geijn HP, et al.Neonatal respiratory morbidity following elective caesarean section in term infants. A 5-year retrospective study and a review of the literature. European Journal of Obstetrics & Gynecology and Reproductive Biology 2001;98(1):9-13. [DOI] [PubMed] [Google Scholar]
White 1985
- White E, Shy KK, Daling JR.An investigation of the relationship between cesarean section birth and respiratory distress syndrome of the newborn. American Journal of Epidemiology 1985;121(5):651-63. [DOI] [PubMed] [Google Scholar]
WHO 2015
- WHO recommendations on interventions to improve preterm birth outcomes. World Health Organization. [ISBN: 978 92 4 150898 8] [PubMed]
Zanardo 2004
- Zanardo V, Simbi AK, Franzoi M, Solda G, Salvadori A, Trevisanuto D.Neonatal respiratory morbidity risk and mode of delivery at term: influence of timing of elective caesarean delivery. Acta Paediatrica 2004;93(5):643-7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sotiriadis 2007
- Sotiriadis A, Makrydimas G, Ioannidis JPA.Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD006614. [DOI: 10.1002/14651858.CD006614] [DOI] [PubMed] [Google Scholar]
Sotiriadis 2009
- Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JPA.Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD006614. [DOI: 10.1002/14651858.CD006614.pub2] [DOI] [PubMed] [Google Scholar]
Sotiriadis 2018
- Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JPA, McGoldrick E.Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD006614. [DOI: 10.1002/14651858.CD006614.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


