Abstract
Background:
β3-adrenoceptors (β3-AR) stimulate lipolysis and thermogenesis in white and brown adipose tissue. Obesity increases oxidative stress and inflammation that attenuate adipose tissue (AT) β3-AR signaling.
Objective:
To test the hypothesis that the combination of the β3-AR agonist CL-316,243 (CL) and the antioxidant alpha-lipoic acid (ALA) would lower inflammation in diet-induced obesity (DIO) and improve β3-AR function.
Methods:
A total of 40 DIO mice were separated into four groups: Control (per os [PO] and intraperitoneal [IP] vehicle); CL alone (0.01 mg/kg IP daily); ALA alone (250 mg/kg in drinking water); or ALA+CL combination, all for 5 weeks.
Results:
Food intake was similar in all groups, yet mice receiving ALA+CL showed improved body composition and inflammation: lower body weight [+1.7g Control vs. −2.5g ALA+CL (−7%); P<0.01] and % body fat (−9%, P < 0.001). Systemic and epididymal WAT (epiWAT) inflammation was lower with ALA+CL than all other groups, with enhanced recruitment of epiWAT anti-inflammatory CD206+ M2-macrophages. β3-AR signaling in WAT was enhanced in the combination-treatment group, with higher mRNA and protein levels of thermogenic uncoupling protein 1 (UCP1) and AT lipases.
Conclusions:
Chronic treatment with ALA and a β3-AR agonist reduces DIO-induced inflammation. AT immune modulation could be a therapeutic target in patients with obesity.
Keywords: Obesity, inflammation, adipose tissue, lipolysis, beta-3-adrenoceptor
Graphical Abstract

INTRODUCTION
The worldwide prevalence of obesity has nearly doubled over the past 40 years, and millions of people die each year throughout the world due to obesity or obesity-related adverse complications, such as hypertension, dyslipidemia, atherosclerosis, fatty liver disease, and type 2 diabetes (1). Obesity is a polygenic, multifactorial disease that begins an imbalance between caloric intake and energy expenditure that produces white adipose tissue (WAT) hypertrophy and hyperplasia (2). The substrate excess causes WAT metabolic stress and a maladaptive alteration of tissue-resident innate immunity (3,4) characterized by produciton of reactive oxygen species (ROS) and other mediators that trigger a switch from an anti-inflammatory, M2/IL4 macrophage milieu to an M1 macrophage-polarized pro-inflammatory environment (3). As the M1:M2 ratio increases in WAT, pro-inflammatory cytokines predominate such that fatty acids, ROS, and lipopolysaccharide drive activation of IκB kinases/IKKε, TBK1, and NF-κB and lead to chronic low-grade inflammation (3,5). This cascade contributes not only to insulin resistance and diabetes (6) but also activates phosphodiesterase 3B (PDE3B) to attenuate catecholamine signaling via β3-adrenergic receptors (ARs) in both WAT and brown adipose tissue (BAT) and leads to impaired catecholamine-mediated lipolysis and thermogenesis (5). Inversely, inhibitors of IκB kinases/IKKε restore β3-AR signal transduction and increase cAMP levels in adipocytes in response to sympathetic stimulation in vivo and in vitro, which may help reverse the metabolic dysregulation seen in obesity (5,7,8).
Signaling through the β-AR is essential to the proper catabolic function of both WAT and BAT, particularly lipolysis, which is the breakdown of stored triglycerides (TGs) to generate free fatty acids (FFA) and glycerol (9,10). The process begins when ligand-bound β-ARs trigger Gsα-mediated stimulation of adenylyl cyclase to generate cAMP and activate protein kinase A (PKA). PKA in turn phosphorylates and activates numerous intracellular enzymes, including hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) (10,11). Therapeutic strategies to reduce WAT TG content have therefore used sympathomimetics to increase lipolysis and subsequent fatty acid oxidation (12). Unfortunately, catecholamine-mediated lipolysis is reduced in obese patients compared to lean controls (9,13). For example, clinical trials using epinephrine to increases WAT lipolysis showed that obese individuals have a lower rate of glycerol release per unit fat mass than lean counterparts (13,14). This phenomenon of decreased sensitivity to adrenergic stimulation in people with obesity is known as catecholamine resistance (13,14), and it impairs the efficacy of interventions that utilize sympathetic stimulation. Reducing inflammation-associated catecholamine resistance is thus an appealing component of pharmacological treatments for obesity-related metabolic disease (15).
Alpha-lipoic acid (ALA) is a sulfur-containing compound present in all prokaryotic and eukaryotic cells (16). It is a potent antioxidant (17) that restores the age-related decline of mitochondrial lipoylation (18), and it also has anti-inflammatory activity (19) and may even promote weight loss in overweight adults (20). We hypothesized that combining ALA with a mouse-selective β3-AR agonist (CL-316,243) would mitigate the maladaptive immune state in mouse adipose tissue when fed a high-fat diet (HFD), enhancing β3-AR signaling. We report that in mice with diet-induced obesity (DIO), the combination of ALA+CL enhanced β3-AR signaling, which led to lower epiWAT inflammation, improved metabolic parameters, and reduced systemic inflammatory cytokines.
METHODS
Experimental Design
A total of 40 male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) 6 weeks old were given ad libitum access to water and high-fat diet (HFD) (D12492, 60% kcal fat, Research Diets, New Brunswick, NJ) with a 12:12-h dark-light cycle (lights on at 0600 h) and cellulose bedding (7099W White TEK-Fresh Cellulose Bedding, Harlan laboratories). At 15 weeks of age, DIO mice (43 ± 0.22 g) were randomly separated into 4 treatment groups (n=10): 1. CL-316,243 group received daily intraperitoneal injections of CL-316,243 (0.01 mg/kg; Wyeth, Princeton, NJ) and drinking water (ALA vehicle), changed weekly; 2. ALA group received ALA (250 mg/kg; cat# 62320, Sigma-Aldrich, MO) dissolved in drinking water (5 g x 2L first dissolved in 30 mL NaOH + 5N HCl titrated to pH 7.5 with 1N HCl) and daily IP saline injections; 3. CL-316,243+ALA group received daily IP injections of CL-316,243 (0.01 mg/kg) and 250 mg/kg ALA dissolved in drinking water; 4. The vehicle control (Control) group received IP injection of saline and drinking water (ALA vehicle) changed weekly for a total of 5 weeks. Both water and water with ALA had neutral pH. Welfare assessment was done daily to monitoring animals for signs of pain, suffering, and distress as per protocol.
Mice were single-housed at 22 °C with ad libitum access to HFD for the duration of the experiment (5 weeks). Body weight; food and water intake; and body composition were measured weekly. Consumption of food and of water were not different in any of the four groups throughout the study period. Body composition was measured by Echo MRI 3-in-1 in non-anesthetized mice (Echo Medical Systems, Houston, TX). Energy expenditure was estimated by measuring the caloric intake corrected for the change in caloric content of the mouse from the change in body composition over the measurement interval (21). All animal experiments were performed under ASP 141-MMC-19 approved by the NIDDK Animal Care and Use Committee.
Serum and tissue collection
At the conclusion of the study, mice were anesthetized using 100 mg/kg ketamine and 10 mg/kg xylazine IP, followed by cervical dislocation. Blood was then collected from the retro-orbital space at euthanasia. The serum was collected and frozen at −80 °C until thawed for assays. Interscapular brown adipose tissue (iBAT), inguinal white adipose tissue (ingWAT), epididymal white adipose tissue (epiWAT), liver, and skeletal muscle were dissected, weighed, frozen, and stored at −80 °C until analyzed.
Intraperitoneal glucose tolerance test (IPGTT)
Intraperitoneal glucose (1.0 g/kg) tolerance tests were performed following an overnight fast (~16 h). For fasting measures, water with ALA was not replaced with water without ALA. Blood was collected by tail bleed at t0, t15min, t30min, t90min, t120min. Glucose was measured with a Glucometer Contour (Bayer, Mishawaka, IN). Plasma insulin was measured at t0, t15min, and t120min by ELISA (Crystal Chem, Downers Grove, IL, #90080). HOMA-IR was calculated using the following equation [fasting insulin (mIU/L)*fasting glucose (mg/dL)]/405, where insulin (mLU/L)=insulin (ng/mL)*23.98. AUC (min*mg/dL) was calculated from 0 mg/dL and 0 U/dL for glucose and insulin excursion curves, respectively, using statistical software.
Plasma biochemical measurements
Fasting insulin was measured in plasma collected after an overnight fast (~16 h) during GTT. We used heparinized tubes for plasma collection during GTT and centrifuged samples for 1 minute at room temperature at ~12,000 × g. Insulin was measured using an ELISA kit (Crystal Chem Inc., Downers Grove, IL, #90080) by following the manufacturer’s instructions. Plasma leptin levels were measured using an ELISA kit (R&D Systems, Minneapolis, MN, #MOB00B). Plasma adipokine levels were measured using the Bio-Plex Multiplex Immunoassay System (Bio-Rad, #171F7001M)
Measurement of serum adipokine and cytokine levels
Blood was collected from mice fasted overnight (~16h) or from mice with free access to food. Serum adipokine levels were measured using the Bio-Plex Multiplex Immunoassay System (Bio-Rad, #171F7001M) following the manufacturer’s instructions. Serum cytokine levels were measured and analyzed by operators who were blinded to the study outcome using the LunarisTM technology for multiplex protein analysis (LMTH-10120S; AYOXXA Biosystems, MA, USA) as described by the manufacturer. Free fatty acids (FFA, Roche Diagnostics GmbH, Mannheim, Germany, #11383175001), triglycerides (Pointe Scientific Inc., Canton, MI, #23–666-411), and cholesterol (Thermo Scientific, Middletown, V, #TR13421) were measured using the indicated colorimetric assays. Leptin (R&D Systems, Minneapolis, MN, #MOB00B) and insulin (Crystal Chem, Downers Grove, I, #90080) were measured by ELISA.
Quantitative RT-PCR
RNA was extracted from frozen tissue n=6/ group (Qiagen RNeasy Plus Mini Kit, Germantown, MD), reverse transcribed (Roche Transcriptor High Fidelity cDNA Synthesis Kit, Indianapolis, IN), and quantified by real-time polymerase chain reaction (qRT-PCR, Applied Biosystems 7900HT, Foster City, CA) using SYBR green. Data were normalized to the housekeeping gene β-actin and are expressed as normalized to the vehicle Control as indicated in the figures.
Immunoblot analysis
Protein was prepared n=3/group using RIPA buffer, quantified (Pierce BCA Protein Assay Kit, Thermo Scientific, Rockford, IL), separated in 4–20% SDS-PAGE gels (10 mg/lane), transferred to PVDF membrane, and probed with anti-HSL p(660) (1:1000, #4126, Cell Signaling), anti-ATGL (1:1000 #2439, Cell Signaling), UCP-1 (Abcam, #Ab155177; 1:500 dilution), β-actin (1:5000 #A2228, Sigma). Bound antibodies were detected with horseradish peroxidase–conjugated linked anti-rabbit (1:10,000, #sc-2004, Cell Signaling) or anti-mouse (1:10,000, #7076P2, Cell Signaling) secondary antibodies and visualized by enhanced chemiluminescence (Bio-Rad).
Histology
Tissues (n=3/group) were randomly selected during dissection at study termination and were fixed in 10% formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and then examined by light microscopy. To minimize subjective bias, slides were reviewed by a pathologist blinded to study outcomes.
Immunohistochemistry
Tissue from fat pads fixed with 10% formalin (n=3/group, 10 μm thick for BAT and WAT) was treated in 10 mM sodium citrate, 0.05% Tween 20 at 85 °C for 20, and then in 3% hydrogen peroxide for 10 min. After blocking in 5% BSA for 20 min, we incubated the sections with anti-F4/80 antibody (BioRad, #MCA497; 1:100 dilution) or anti-UCP1 antibody (Abcam, #Ab10983; 1:500 dilution) anti-CD11c antibody (Abcam, #Ab219799, 1:100 dilution) and anti CD206 antibody (Abcam, # Ab64693, 1:500 dilution) at 4 °C overnight, and then incubated with biotinylated goat anti-rabbit IgG secondary antibody (Agilent DAKO, #E043201–6; 1:500 dilution) at room temperature for 1 h. The protein signals were detected using streptavidin horseradish peroxidase (Vector Laboratories) and visualized with diaminobenzidine tetrahydrochloride (Sigma). The sections were counterstained with hematoxylin. To minimize subjective bias, slides were reviewed by a pathologist blinded to study outcomes.
Statistical methods
This study’s primary outcome was inflammation measured by analyzing both the serum concentration of inflammatory cytokines and molecular tissue markers of tissue inflammation. The secondary outcomes were body weight, body fat composition, and insulin sensitivity. Values are expressed as mean ± SEM. Comparison among the four groups on measures collected during the 5th week of treatment were done using 2-way ANOVA with Tukey’s multiple comparisons test. The interaction between ALA and CL was determined via ANOVA with two factors and their interaction term. Comparison of blood glucose and plasma insulin levels during IPGTT across the 5 weeks in the four groups was made via repeated-measures ANOVA. Comparisons among the four groups of measures collected on the 5th week of treatment were done with single measure AVOVA. All reported P values are 2-tailed, corrected to multiple comparison, with P ≤ 0.05 considered significant. Statistical analyses were conducted using the statistical software GraphPad Prism version 9.0.0 for Windows (GraphPad Software Inc).
RESULTS
ALA+CL lowered fat mass
To test the hypothesis that the combination of ALA and CL would improve the metabolic profile in both BAT and WAT, we separated 40 DIO male C57BL/6 mice treated with HFD for 10 weeks into 4 groups of n=10, treated for 5 weeks with the following: vehicle control (Control), CL alone, ALA alone, or ALA+CL. Diet, water intake, body composition, and body weight were measured weekly, with baseline characteristics summarized in Table 1. After five weeks, calorie intake was indistinguishable among all four treatment groups (Figure 1A), but the estimated energy expenditure (EEE) was higher in the CL and ALA+CL groups compared to the Control (Figure 1B). The Control group gained weight eating the HFD (P = 0.0005), while CL and ALA each slowed weight gain in DIO mice but did not cause weight loss. In contrast, weight loss in the ALA+CL group, compared to Control, started at week two and continued through week 5 (44.0 ± 4.2 g vs. 40.0 ± 4.8 g; − 4.0 g; −9.6%). Weight loss over the whole treatment period was also significant (mean difference: −2.7 g; P = 0.0001) (Figure 1C). The body weight loss in the ALA+CL group was mainly from loss of fat mass (Figure 1D) (−2.8 ± 0.6 g P < 0.0001) with preservation of lean mass (Figure 1D). Serum leptin was lower in the CL and ALA+CL groups (P=0.01 and P=0.001 respectively; Figure 1E) and positively correlated with fat mass (R=0.64, P<0.0001; Figure 1F). Serum triglycerides and total cholesterol were similar in all groups (Figure 1G).
Table 1.
Baseline characteristics of all study groups
| Study groups | Body weight (g) | Fat mass (g) | Lean mass (g) | Food intake (kcal/mouse/day) | Energy expenditure (kcal/mouse/day) | Water intake (mL/mouse/day) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Control | 42.8 | 1.3 | 16.1 | 0.9 | 26.5 | 0.6 | 15.7 | 0.4 | 14.5 | 0.6 | 4.1 | 0.2 |
| ALA | 43.3 | 1.2 | 16.5 | 0.9 | 26.6 | 0.6 | 15.9 | 0.3 | 14.1 | 0.5 | 4.4 | 0.2 |
| CL | 42.8 | 1.3 | 16.2 | 1.0 | 26.4 | 0.7 | 15.9 | 0.4 | 14.6 | 0.3 | 4.3 | 0.2 |
| ALA+CL | 43.0 | 1.2 | 16.1 | 0.9 | 26.6 | 0.6 | 15.9 | 0.5 | 14.7 | 0.4 | 4.2 | 0.2 |
SEM: Standard error of the mean
Control: Vehicle Control group received an intraperitoneal injection (IP) of saline and drinking water
ALA: Alpha-lipoic acid (ALA, 250 mg/kg orally in drinking water)
CL: CL-316,243 (CL; 0.01 mg/kg/day, IP daily)
ALA+CL: CL-316,243 (0.01 mg/kg, IP daily) and ALA (250 mg/kg in drinking water)
Figure 1.
The combination of CL-316,243 (CL) and alpha-lipoic acid (ALA) for 5 weeks caused fat weight loss in mice with DIO. (A) Cumulative energy intake; (B) cumulative estimated energy expenditure (EE); (C) the change in body weight; (D) the change in body composition. (E) The change in serum leptin and (F) its correlation with fat mass in grams. (G) Serum triglycerides and total cholesterol. Values are expressed as mean ± SEM. Comparisons among the four groups (n=10/group) of measures collected after the 5th week of treatment were done by two-way ANOVA with Tukey’s multiple comparisons test. P-values are two-tailed with P < 0.05 considered significant. Control: Veh-PO/ IP (saline), ALA: Alpha-lipoic acid PO + Veh-IP, CL: Veh-PO + CL-316,243, ALA + CL: ALA(PO) + CL(IP). DIO: Diet-Induced Obesity, HFD: High Fat Diet, Veh: vehicle; PO: Per os; IP: intraperitoneal * P < 0.05, ** P ≤ 0.01, *** P ≤ 0.001
Insulin sensitivity improved with CL, but adding ALA had no further effect
An intraperitoneal glucose tolerance test [IPGTT; 1.0 g/kg] showed an AUCglucose that was lower in both CL and ALA+CL groups compared to Control (P = 0.001, P = 0.0008, respectively) (Figure 2A–2B). Serum fasting plasma insulin levels were also lower in both CL and ALA+CL compared to Control (Control 2.8 ± 1.2 μg/mL vs. CL 1.5 ± 0.7 μg/mL; P=0.02; and ALA+CL 1.2±0.8 μg/mL; P=0.01) (Figure 2C). Analysis of the insulin response to a given glucose load revealed lower insulin levels in the CL and ALA+CL compared to the Control (P < 0.0001 for both), also evident by a lower AUCinsulin in both CL and ALA+CL groups (Figure 2D). HOMA-IR indicated that insulin sensitivity was higher in CL and ALA +CL than Control (22.9 ± 11.3 vs. 10.6± 6.8; P = 0.01; and 9.0±6.3; P = 0.001), though CL and ALA+CL groups had a similar response (P = 0.45, Figure 2E). Insulin Receptor Substrate 1 (Irs1) mRNA expression was higher in epiWAT in response to ALA+CL than Control (P = 0.0004). There were no differences with Control seen with ALA or CL in any other tissue. Therefore, while CL improved insulin sensitivity, ALA, either alone or in combination with CL, did not have an additional effect on glucose homeostasis or insulin signaling.
Figure 2.
Adding alpha-lipoic acid (ALA) to CL-316,243 (CL) did not alter the effect of CL alone in improving insulin sensitivity. (A) Blood glucose levels during IP glucose tolerance test [IPGTT; 1 g/kg; n = 10/group)] and (B) AUC of glucose. (C) Plasma insulin levels during IPGTT (D), AUC insulin and (E) HOMA-IR. (F) Irs1 expression in liver, skeletal muscle, inguinal white adipose tissue (ingWAT), and epididymal white adipose tissue (epiWAT) (n = 6/group). Values are expressed as mean ± SEM. Comparisons across weeks in the 4 groups were made with repeated measures ANOVA, while comparisons among the 4 groups of measures collected after the 5th week of treatment were done with single-factor AVOVA. P-values are two-tailed with P < 0.05 considered significant. The area under the curve (AUC) was calculated from 0 mg/dL and 0 ng/mL for glucose and insulin excursion curves respectively. * P < 0.05, ** P ≤ 0.01, ***P ≤ 0.001.
ALA+CL lowered HFD-induced epiWAT inflammation
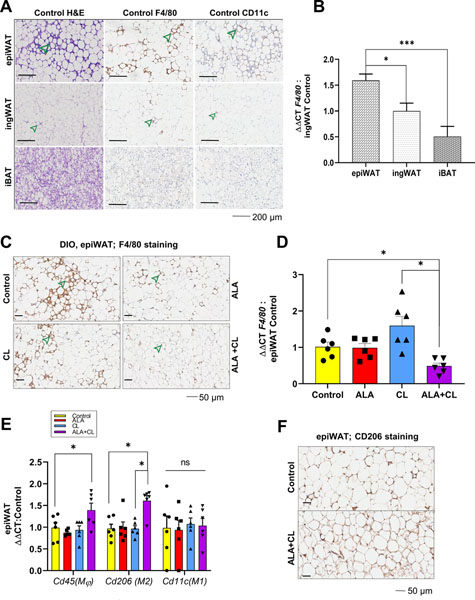
After 15 weeks of eating a HFD, the Control group mice developed AT inflammation, mainly in the epiWAT (Figure 3A), as demonstrated by the numerous crown-like structures (CLS). The CLS were in greater abundance in the epiWAT compared to ingWAT or iBAT. For corroboration, we found that the macrophage marker, F4/80, showed more evidence for CLS in epiWAT compared to ingWAT and iBAT of the Control group (Figure 3A). Also, F4/80 mRNA gene expression in epiWAT was 45% higher than ingWAT (P = 0.04) and 145% higher than in iBAT (P = 0.0008) (Figure 3B). We found that ALA or CL alone did not affect epiWAT inflammation compared to Control. However, ALA+CL lowered epiWAT inflammation, as demonstrated by fewer CLS (Figure 3C) and lower f4/80 expression (Figure 3D).
Figure 3.
The combination of CL-316,243 (CL) and alpha-lipoic acid (ALA) reduced HFD-induced epiWAT inflammation on local adipose tissue (AT) inflammation. (A) Histology (H&E and F4/80 staining) of epididymal white adipose tissue (epiWAT), inguinal white adipose tissue (ingWAT), and interscapular brown adipose tissue (iBAT) in Control after 15 weeks of HFD showing crown-like structures (CLS, green arrows) n = 3/group. (B) mRNA levels of F4/80 in different AT depots n = 6/group and (C) F4/80 staining of epiWAT showing CLS (green arrows) in each treatment group n = 3/group. (D) epiWAT mRNA levels of F4/80; (E) epiWAT mRNA of Cd45, Cd206 (M2 markers), and Cd11c (M2 markers). (F) CD 206 staining of epiWAT. mRNA levels are normalized to vehicle Control mice n = 6/group. Values are expressed as mean ± SEM. Comparisons among the 4 groups on measures collected after the 5th week of treatment were done using two-way ANOVA with Tukey’s multiple comparisons test. P-values are two-tailed with P < 0.05 considered significant. * P < 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Given that macrophages have different functions in adipose tissue based on their polarization, where M2 macrophages (CD206+) are anti-inflammatory and M1 macrophages (CD11c+) are pro-inflammatory, we measured the expression of different macrophage markers in epiWAT. There was higher CD206 staining in the ALA+CL group compared to Control (Figure 3E), and mRNA expression of cd206 was >50% higher in the ALA+CL group compared to Control, ALA alone, or CL alone (Figure 3F). There was a higher expression of the general macrophage marker cd45 in the epiWAT in the ALA+CL group than the other three groups, while there were no differences in cd11c expression. This difference in expression could indicate that the higher numbers of M2 macrophages in epiWAT of ALA+CL-treated mice might have resulted from M2 macrophage recruitment rather than an M1-M2 phenotype switch.
ALA+CL augmented lipolytic enzymes and UCP-1 activation in epiWAT
Targeting the post-transcriptional NF-kB pathway in obese mouse and human models has been shown to alleviate adipose tissue catecholamine resistance mediated by obesity-induced inflammation (3,5,7,13). Having shown that the interaction between ALA and CL reduced local inflammation, we next determined whether this combination would increase cAMP signaling in adipose tissue of DIO mice by measuring markers of browning and lipolytic enzyme activation downstream of PKA. The mRNA expression of Pcg-1α and Ucp1, two genes upregulated by β3-AR signaling, were higher in the CL and ALA+CL groups in epiWAT, while no differences were observed between groups in either ingWAT or iBAT (Figure 4A–4B). However, UCP-1 protein levels were higher in epiWAT in the ALA+CL group compared to the other three groups when measured by either immunohistochemistry (Figure 4C) or immunoblotting (Figure 4D–4E). Moreover, the phosphorylation of lipolytic enzymes in epiWAT downstream of cAMP, ATGL, and HSL were higher in the ALA+CL compared to all three groups (Figure 4D). These results suggest that the anti-inflammatory effect of combining ALA+CL augments catecholamine signaling in the epiWAT of DIO mice. The selective rise in UCP-1 protein in the ALA+CL group compared to CL suggests that the effect of ALA+CL on UCP-1 is mediated via a translational or post-translational process.
Figure 4.
The combination of CL-316,243 (CL) and alpha-lipoic acid (ALA) augmented lipolytic enzyme activation and epididymal white adipose tissue (epiWAT) browning. (A) mRNA levels of Pcg1α and (B) Ucp-1 in different adipose tissues n = 6/group normalized to iBAT. (C) Immunohistochemistry of UCP-1 in epiWAT (AT) n = 3/group. (D) Western blotting (WB) of UCP-1, ATGL, HSL, and HSL-p660, n = 3/group, with one representative image shown for each. (E) Quantification of WB band intensity. Values are expressed as mean ± SEM; comparisons among the 4 groups of measures collected after the 5th week of treatment was done using a two-way ANOVA with Tukey’s multiple comparisons test. P-values are two-tailed with P < 0.05 considered significant. Scale bar indicates 50 μm. Gene expression data were normalized to Control (n = 6). * P< 0.05, ** P ≤ 0.01, *** P≤ 0.001
Systemic inflammation reduced by ALA+CL
Models of mouse obesity have shown a connection between adipose tissue inflammation and systemic inflammation. Therefore, we next determined if the reduction in epiWAT inflammation seen in the ALA+CL group, especially the enhanced M2 macrophage polarization, was associated with similar changes in systemic inflammation. Using a Luminex multiplex ELISA, we measured serum cytokines that have been shown to predispose obese mice toward cardiovascular and metabolic complications (Table 2 and Figure 5A–5E). We found that overall, treatment with either CL or ALA alone did not change either pro- or anti-inflammatory systemic cytokines compared to Control. However, the combination of ALA+CL led to a consistent reduction in serum pro-inflammatory cytokines compared to Control [IL-1β (−22%; P = 0.03), IL-23 (−46%; P = 0.003), IL-6 (−21%; P = 0.04), IL-17A (−45%; P = 0.02); Figure 5A–5D]. Consistent with the overall physiological pattern, the serum concentration of anti-inflammatory IL-10 was higher after treatment with ALA+CL compared to Control (+30%, P = 0.03; Figure 5E). Given that we had seen a reduction in epiWAT inflammation after treatment with ALA+CL (Figure 3C–3D), we used ANOVA with an interaction term to determine if the two compounds also interacted to affect serum cytokine levels. We found that ALA and CL interacted to lower IL-1β, IL-23, IL-6, and IL-17A (all P ≤ 0.001, Table 2). Moreover, ALA and CL interacted to raise IL-10 (P < 0.002, Table 2). These results suggest that ALA and CL interact to produce an overall anti-inflammatory effect in the epiWAT and in the serum, distinct from the effects of either ALA or CL alone.
Table 2.
Changes in serum cytokines between treatment groups
| Control | ALA | CL | ALA+CL | P-Value for ALA+CL interaction | |
|---|---|---|---|---|---|
| pg/ml ± SD | pg/mL ± SD (P-value vs ALA+CL) | pg/mL ± SD (P-value vs ALA+CL) | pg/mL ± SD (P-value vs Control) | ||
| Pro-inflammatory | |||||
| IL-1β | 105.9 ± 17.7 | 84.0 ± 11.3 (0.89) | 105.9 ± 17.8 (0.15) | 85.1 ± 10.1 (0.03) | 0.001 |
| IL-23 | 141.6 ± 24.2 | 173.3 ± 35.5 (0.41) | 182.7 ± 29.3 (0.001) | 89.4 ± 36.1 (0.003) | 0.0004 |
| IL-6 | 19.4 ± 2.7 | 13.3 ± 3.7 (0.02) | 18.3 ± 3.5 (0.29) | 15.8 ± 3.5 (0.04) | 0.0002 |
| IL-17A | 16.2 ± 4.0 | 14.6 ± 2.6 (0.11) | 13.9 ± 2.9 (0.20) | 10.2 ± 0.6 (0.02) | 0.0005 |
| Anti-Inflammatory | |||||
| IL-10 | 38.7 ± 6.4 | 40.6 ± 6.1 (0.69) | 40.2 ± 6.6 (0.06) | 52.6 ± 8.9 (0.03) | 0.002 |
SD: Standard deviation
Control: Vehicle Control group received an intraperitoneal injection (IP) of saline and drinking water
ALA: Alpha-lipoic acid (ALA, 250 mg/kg orally in drinking water)
CL: CL-316,243 (CL; 0.01 mg/kg/day, IP daily)
ALA+CL: CL-316,243 (0.01 mg/kg, IP daily) and ALA (250 mg/kg in drinking water)
Figure 5.
CL-316,243 (CL) and alpha-lipoic acid (ALA) interact to lowered systemic inflammation in DIO mice eating a HFD. Pro-inflammatory serum cytokines (A) IL-1B, (B) IL-23, (C) IL-6, (D) IL-17A, and immune-modulatory serum cytokine (E) IL-10. Mice were studied after 5 weeks of treatment with vehicle, ALA (250 mg/kg), CL-316,243 (CL 0.01 mg/kg), or ALA + CL. Cytokine analysis was done on n=5/group. Values are expressed as mean ± SEM. Comparisons among the 4 groups of measures collected after the 5th week of treatment were done using ANOVA with Tukey’s multiple comparisons test. The interaction between ALA and CL was analyzed using ANOVA with 2 factors. P-values are two-tailed with P < 0.05 considered significant. * P < 0.05, **P ≤ 0.01, ***P ≤ 0.001.
DISCUSSION
Previous studies have shown that obesity-induced inflammation attenuates the ability of catecholamines to stimulate lipolysis and thermogenesis (5,14). ALA is an antioxidant and an inhibitor of the NF-κB pro-inflammatory signaling (16), making it a promising agent to restore catecholamine-mediated lipolysis. This study shows that when ALA and CL were combined, there was a restoration of β3-AR downstream signaling; lower inflammation in epiWAT; and reduced systemic inflammatory cytokines in mice with DIO. The changes in inflammation were likely through enhanced recruitment of M2 macrophages.
CL is a selective β3-AR agonist whose effects depend on the dose. When given to mice chronically IP at doses ranging from 0.5–1.0 mg/kg, CL improves glucose homeostasis, activates BAT thermogenesis, and increases serum FFA (23). When CL is given acutely at 1% of this dose, it increases EE (24), but the effect of chronic use at 0.01 mg/kg on metabolic parameters has not been reported. Our study shows that treating DIO mice chronically with CL at 0.01 mg/kg IP improves insulin sensitivity and increases lipolytic enzyme activation and energy expenditure. When combined with ALA, we found no additional improvement in insulin sensitivity compared to CL alone, but there was higher expression in epiWAT of lipolytic enzymes; higher mRNA levels of ucp1, pgc1α; and more UCP-1 protein.
It is well-established that HFD leads to WAT inflammation. Visceral WAT in particular is more prone to inflammation compared to ingWAT (28), which is likely due to depot-specific up-regulation of toll-like receptor (TLR) genes and related pro-inflammatory signaling cascades in obesity (29,30). We showed that F4/80+/CD11c+ positive macrophages and CLS are seen more in epiWAT than ingWAT or iBAT. TLR4 is a primary activator of the innate immune system in adipose tissue, and its preferential upregulation in epiWAT might explain the association between central obesity, inflammation, and obesity complications (30). Here, we saw no evidence of macrophage infiltration into iBAT. One explanation is that since iBAT may be protected from obesity-induced inflammation because it expresses Programmed Death-Ligand 1 (PDL-1), a ligand for Programmed Cell Death Protein 1 (PD-1) that leads to attenuation of innate immune signal activation (31)(32).
ALA is an antioxidant that can help recycle other antioxidants, repair oxidized proteins, and directly scavenge ROS in both the reduced and oxidized form (33). Little is known about the direct effect of ALA on adipose tissue inflammation. We found that ALA alone did not lower the inflammation or change macrophage polarization in epiWAT, nor did it lower obesity-induced systemic inflammation. On the other hand, CL is known to promote adipose tissue inflammation when used in higher doses (≥1mg/kg) (24), resulting in neutrophil infiltration and higher expression of pro-inflammatory adipose tissue cytokines (24,34). In this study, CL did not raise the expression of pro-inflammatory markers in epiWAT compared to Control, which implies that the pro-inflammatory effect of CL may be dose dependent. However, when adding ALA to CL, there was a reduction in F4/80+/CLS structures associated with higher M2 macrophage expression and detection, demonstrating that the interaction between ALA and CL enhanced the recruitment of anti-inflammatory macrophages epiWAT.
The alteration of the tissue M1/M2 ratio after HFD leads to dynamic changes in fate-specifying cytokines in adipose tissue environments that favor Th1/Th17 CD4+ cell activation with an increase in systemic pro-inflammatory cytokines (35). Dendritic cells (DCs) in an M1-rich environment may drive the differentiation of Th17 cells, which produce high amounts of IL-17 to the circulation (35). When counterregulatory M2 macrophages increase, a shift towards an immune environment characteristic of lean adipose tissue occurs, that would eventually decrease systemic inflammation. In the ALA+CL group, we found lower systemic inflammation, as evidenced by lower IL-6, IL-1β, and IL-17, which have been shown to cause coronary heart disease (36), atherosclerosis, hypertension, and cardiovascular disease (37,38). This could imply that increasing M2 macrophages in adipose tissue in the obese host could reduce pro-inflammatory cytokines in the circulation. The mechanism by which ALA and CL interact is not fully understood and is an area of future investigation.
We showed that ALA and CL, each alone, protected DIO mice from weight gain under HFD. Consistent with other animal and human studies, we saw that each drug also did not cause any significant weight loss (39–42). ALA+CL, however, led to a loss of fat mass with lean mass preservation, all without a reduction in food intake. We hypothesize that ALA+CL increases lipid mobilization, coupled with improved lipid fuel utilization. ALA+CL augmented epiWAT lipolysis enzyme activation and UCP-1 protein production. Both lipolysis enzyme activation (9) and UCP-1 expression are regulated either partially or entirely by β-AR signaling transduction via cAMP and PKA, and a relative defect in lipolysis is associated with obesity (9), and weight regain (43). It has been shown that the NF-κB pathway regulates cAMP-mediated function in adipocytes (5,7,8) and that blockage of this pathway reverses adipose tissue dysfunction (17). We saw that lipolytic enzyme activation and UCP-1 expression were higher in epiWAT of the ALA+CL than in all other groups. Therefore, ALA+CL, by modulating AT inflammation, led to enhanced responsiveness to β-AR stimulation in epiWAT.
Lipolytic enzyme activation increases FFA release (10). Simultaneously, higher FFA may increase adipose tissue inflammation, insulin resistance, and NAFLD (44). While ALA+CL improved lipolytic enzyme activation in the current study, there was no loss in CL-mediated improvement in insulin sensitivity. This preservation of insulin signaling suggests that catecholamine-mediated lipolysis in a healthier adipose tissue microenvironment does not have the lipotoxic effect seen in dysregulated lipolysis in inflamed dysfunctional adipose tissue. While the latter is dysfunctional and thus a precursor for metabolic complications, the former is necessary for fat mobilization during fasting and response to exercise (9,43,45).
A limitation of this study is the absence of lean Control for each treatment group; this would have ideally shown the effect of obesity on catecholamine sensitivity and illustrated the effect of immune manipulation on reversing catecholamine resistance. The use of this large number of mice was not possible logistically. However, the effect of obesity-induced inflammation on blunting catecholamine signaling in DIO mice has been reproduced by multiple investigators. Second, housing mice at room temperature, which is considered thermal stress, could have mitigated the intervention’s effect on BAT activation. Studying the effect of ALA+CL under different thermal conditions could be done in a subsequent study. Lastly, although we found an association between the reduction in tissue inflammation and body fat reduction, we cannot establish causation. Future studies are needed to show the temporal relationship between changes in inflammation and body weight.
CONCLUSIONS
Our study identifies an interaction between lipolytic stimulation by chronic treatment with a β3-AR agonist and the antioxidant, anti-inflammatory activity conferred by ALA. Together, these two pathways have a substantial immunomodulatory effect on obesity-induced adipose tissue inflammation, leading to augmented catecholamine/cAMP signaling in epiWAT and reducing systemic cytokines associated with metabolic syndrome and cardiovascular disease. These findings indicate that a combination of catecholamine stimulation and adipose tissue immunomodulation may be an approach to treat metabolic disease in people with obesity.
STUDY IMPORTANCE QUESTIONS:
What is already known about this subject?
Obesity leads to chronic low-grade inflammation.
CL-316,243 is a β3-adrenergic receptor agonist with known lipolytic, thermogenic, and insulin-sensitizing properties that could be attenuated by obesity-induced inflammation.
Alpha-lipoic acid (ALA) is an antioxidant with anti-inflammatory properties.
Reversal of obesity-induced inflammation may lead to enhanced catecholamine signaling in adipose tissue.
What are the new findings in your manuscript?
The combination of CL-316,243 and ALA interacts to lower obesity-induced inflammatory changes in the epididymal white adipose tissue (epiWAT) and systemically.
Lowering adipose tissue inflammation was associated with enhanced β3-adrenergic receptor function in epiWAT.
How might your results change the direction of research or the focus of clinical practice?
In clinical trials, β3-adrenergic receptor (AR) agonists have been shown to reduce obesity-induced insulin resistance. Combining ALA with a β3-AR in humans may lessen obesity-induced inflammation and potentiate the lipolytic and thermogenic capacity of β3-AR agonists.
ACKNOWLEDGMENTS
We thank Peter Walter and Hongyi Cai for serum ALA measurement, F. Rinaldi and A. Kiraly from AYOXXA Biosystems for serum cytokine measurement, and Paul Wakim for assistance in data analysis, and Marc Reitman and Lee Weinstein for comments on the data and manuscript.
FUNDING:
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health grants DK-075115.
Footnotes
CONFLICT OF INTEREST: All authors declare no conflict of interest
REFERENCES
- 1.Haslam DW, WP J Obesity. Lancet. 2005;366(9492):1197–1209. [DOI] [PubMed] [Google Scholar]
- 2.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252. [DOI] [PubMed] [Google Scholar]
- 3.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–643. [DOI] [PubMed] [Google Scholar]
- 4.Chouchani ET, Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat Metab. 2019;1(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowers J, Uhm M, Reilly SM, Simon J, Leto D, Chiang SH, Chang L, Saltiel AR. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKKepsilon and TBK1. Elife. 2013;2:e01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S49–52. [DOI] [PubMed] [Google Scholar]
- 7.Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, Downes M, Yu RT, Liddle C, Evans RM, Oh D, Li P, Olefsky JM, Saltiel AR. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19(3):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oral EA, Reilly SM, Gomez AV, Meral R, Butz L, Ajluni N, Chenevert TL, Korytnaya E, Neidert AH, Hench RJCm. Inhibition of IKKɛ and TBK1 improves glucose control in a subset of patients with type 2 diabetes. Cell metab. 2017;26(1):157–170. e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luglio HF, Sulistyoningrum DC, Susilowati R. The role of genes involved in lipolysis on weight loss program in overweight and obese individuals. J Clin Biochem Nutr. 2015;57(2):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweiger M, Eichmann TO, Taschler U, Zimmermann R, Zechner R, Lass A. Measurement of lipolysis. Meth Enzymol. 2014;538:171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavrilova O, Marcus-Samuels B, Reitman ML. Lack of responses to a beta3-adrenergic agonist in lipoatrophic A-ZIP/F-1 mice. Diabetes. 2000;49(11):1910–1916. [DOI] [PubMed] [Google Scholar]
- 12.Tulp OL, Buck CL. Caffeine and ephedrine stimulated thermogenesis in LA-corpulent rats. Comp Biochem Physiol C. 1986;85(1):17–19. [DOI] [PubMed] [Google Scholar]
- 13.Bougneres P, Stunff CL, Pecqueur C, Pinglier E, Adnot P, Ricquier D. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J Clin Investig. 1997;99(11):2568–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arner PJIJoO. Catecholamine-induced lipolysis in obesity. 1999;23(1):S10–S13. [DOI] [PubMed] [Google Scholar]
- 15.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Investig. 2011;121(6):2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790(10):1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illesca P, Valenzuela R, Espinosa A, Echeverria F, Soto-Alarcon S, Ortiz M, Videla LA. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-gamma and NF-kappaB. Biomed Pharmacother. 2019;109:2472–2481. [DOI] [PubMed] [Google Scholar]
- 18.Tajima K, Ikeda K, Chang HY, Chang CH, Yoneshiro T, Oguri Y, Jun H, Wu J, Ishihama Y, Kajimura S. Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat Metab. 2019;1(9):886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki YJ, Aggarwal BB, Packer L. α-Lipoic acid is a potent inhibitor of NF-κB activation in human T cells. Biochem Biophys Res Commun. 1992;189(3):1709–1715. [DOI] [PubMed] [Google Scholar]
- 20.Bobe G, Michels AJ, Zhang W-J, Purnell JQ, Woffendin C, Pereira C, Vita JA, Thomas NO, Traber MG, Frei B. A randomized controlled trial of long-term (r)-α-lipoic acid supplementation promotes weight loss in overweight or obese adults without altering baseline elevated plasma triglyceride concentrations. J Nutr. 2020;150(9):2336–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravussin Y, Gutman R, LeDuc CA, Leibel RL. Estimating energy expenditure in mice using an energy balance technique. Int J Obes (Lond). 2013;37(3):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedossa P Histological Assessment of NAFLD. Dig Dis Sci. 2016;61(5):1348–1355. [DOI] [PubMed] [Google Scholar]
- 23.Xiao C, Goldgof M, Gavrilova O, Reitman MLJO. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22° C. Obesity. 2015;23(7):1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto Y, Hashimoto O, Shindo D, Sugiyama M, Tomonaga S, Murakami M, Matsui T, Funaba M. Metabolic changes in adipose tissues in response to beta3 -adrenergic receptor activation in mice. J Cell Biochem. 2019;120(1):821–835. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Pennisi PA, Gavrilova O, Pack S, Jou W, Setser-Portas J, East-Palmer J, Tang Y, Manganiello VC, Leroith D. Effect of adipocyte beta3-adrenergic receptor activation on the type 2 diabetic MKR mice. Am J Physiol Endocrinol. 2006;290(6):E1227–1236. [DOI] [PubMed] [Google Scholar]
- 26.Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, Klein S, Kahn BB. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484(7394):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park KG, Min AK, Koh EH, Kim HS, Kim MO, Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology. 2008;48(5):1477–1486. [DOI] [PubMed] [Google Scholar]
- 28.Caspar-Bauguil S, Cousin B, Bour S, Casteilla L, Penicaud L, Carpene C. Adipose tissue lymphocytes: types and roles. J Physiol Biochem. 2009;65(4):423–436. [DOI] [PubMed] [Google Scholar]
- 29.Kim SJ, Choi Y, Choi YH, Park T. Obesity activates toll-like receptor-mediated pro-inflammatory signaling cascades in the adipose tissue of mice. J Nutr Biochem. 2012;23(2):113–122. [DOI] [PubMed] [Google Scholar]
- 30.Dodson MV, Du M, Wang S, Bergen WG, Fernyhough-Culver M, Basu U, Poulos SP, Hausman GJ. Adipose depots differ in cellularity, adipokines produced, gene expression, and cell systems. Adipocyte. 2014;3(4):236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingram JR, Dougan M, Rashidian M, Knoll M, Keliher EJ, Garrett S, Garforth S, Blomberg OS, Espinosa C, Bhan A. PD-L1 is an activation-independent marker of brown adipocytes. Nat Commun. 2017;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TP F, S K, M A, GM H, J S, MP C. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301(4):H1425–H1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdali D, Samson SE, Grover A. How effective are antioxidant supplements in obesity and diabetes? Med Princ Pract. 2015;24(3):201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mottillo EP, Shen XJ, Granneman J. β3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochim Biophys Acta. 2010;1801(9):1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R, Nikolajczyk BS. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front Immunol. 2019;10:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. [DOI] [PubMed] [Google Scholar]
- 37.Egeberg A, Gisondi P, Carrascosa JM, Warren RB, Mrowietz U. The role of the interleukin-23/Th17 pathway in cardiometabolic comorbidity associated with psoriasis. J Eur Acad Dermatol Venereol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orejudo M, Garcia-Redondo AB, Rodrigues-Diez RR, Rodrigues-Diez R, Santos-Sanchez L, Tejera-Munoz A, Egido J, Selgas R, Salaices M, Briones AM, Ruiz-Ortega M. Interleukin-17A induces vascular remodeling of small arteries and blood pressure elevation. Clin Sci (1979). 2020;134(5):513–527. [DOI] [PubMed] [Google Scholar]
- 39.Park S, Karunakaran U, Jeoung NH, Jeon JH, Lee IK. Physiological effect and therapeutic application of alpha lipoic acid. Curr Med Chem. 2014;21(32):3636–3645. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida T, Yoshioka K, Hiraoka N, Umekawa T, Sakane N, Kondo M. Effect of CL 316,243, a novel beta 3-adrenoceptor agonist, on insulin secretion in perfused mouse pancreas. Endocr J. 1994;41(6):671–675. [DOI] [PubMed] [Google Scholar]
- 41.Namazi N, Larijani B, Azadbakht L. Alpha-lipoic acid supplement in obesity treatment: A systematic review and meta-analysis of clinical trials. Clin Nutr. 2018;37(2):419–428. [DOI] [PubMed] [Google Scholar]
- 42.O’Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA, Fink YA, Kapuria D, Cassimatis TM, Kelsey N, Cero C, Abdul-Sater Z, Piccinini F, Baskin AS, Leitner BP, Cai H, Millo CM, Dieckmann W, Walter M, Javitt NB, Rotman Y, Walter PJ, Ader M, Bergman RN, Herscovitch P, Chen KY, Cypess AM. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Invest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasher-Meron M, Youn DY, Zong H, Pessin JE. Lipolysis defect in white adipose tissue and rapid weight regain. Am J Physiol Endocrinol. 2019;317(2):E185–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boden G Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111(3):121–124. [DOI] [PubMed] [Google Scholar]
- 45.Jocken JW, Goossens GH, van Hees AM, Frayn KN, van Baak M, Stegen J, Pakbiers MT, Saris WH, Blaak EE. Effect of beta-adrenergic stimulation on whole-body and abdominal subcutaneous adipose tissue lipolysis in lean and obese men. Diabetologia. 2008;51(2):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]